Abstract
The 5xFAD mouse model of Alzheimer's disease (AD) rapidly develops AD‐related neuro‐behavioral pathology. Learning and memory impairments in 5xFAD mice, however, are not always replicated and the size of impairments varies considerably across studies. To examine possible sources of this variability, we analyzed the effects of age, sex, albinism due to background genes (Tyr c , Oca2 p ) and motor impairment on learning and memory performance of wild type and 5xFAD mice on the Morris water maze, from 3 to 15 months of age. The 5xFAD mice showed impaired learning at 6–9 months of age, but memory impairments were not detected with the test procedure used in this study. Performance of 5xFAD mice was profoundly impaired at 12–15 months of age, but was accompanied by slower swim speeds than wild‐type mice and a frequent failure to locate the escape platform. Overall female mice performed worse than males, and reversal learning impairments in 5xFAD mice were more pronounced in females than males. Albino mice performed worse than pigmented mice, confirming that albinism can impair performance of 5xFAD mice independently of AD‐related transgenes. Overall, these results show that 5xFAD mice have impaired learning performance at 6–9 months of age, but learning and memory performance at 12–15 months is confounded with motor impairments. Furthermore, sex and albinism should be controlled to provide an accurate assessment of AD‐related transgenes on learning and memory. These results will help reduce variability across pre‐clinical experiments with 5xFAD mice, and thus enhance the reliability of studies developing new therapeutics for AD.
Keywords: 5xFAD, aging, Alzheimer's disease, dementia, learning and memory, motor ability, neurodegeneration, sex differences, visual ability, visuo‐spatial
The 5xFAD mouse model of Alzheimer's disease shows impaired visual‐spatial learning on the Morris water maze (MWM). These learning impairments are influenced by the age of mice, sex, motor dysfunction, and albinism. Controlling for these factors will help improve the quality of pre‐clinical research with the 5xFAD mouse.
1. INTRODUCTION
Alzheimer's disease (AD) is an age‐related neurodegenerative disorder that causes profound impairments in cognitive function. 1 , 2 , 3 Among the cognitive domains affected, memory and visuo‐spatial navigation are markedly impaired in AD. 4 , 5 These impairments are associated with reduced quality of life in AD, and increase the risk of AD patients becoming lost in novel and once familiar environments. 6 , 7 Impairments in memory and spatial navigation have been proposed as cognitive bio‐markers for AD, as they are present during the pre‐clinical phase of mild cognitive impairment and help to predict which patients will develop AD. 8 , 9
Transgenic mouse models are commonly used to examine the neurobiological basis of memory loss in AD and to assess the efficacy of novel therapeutics to ameliorate these memory impairments. 10 , 11 , 12 The double‐transgenic 5xFAD mouse is a valuable animal model, given it rapidly develops many AD‐related pathologies, including widespread Aβ‐plaque deposition, 13 , 14 neuron loss, 15 inflammatory microgloisis, 16 , 17 astrogliosis 18 and impaired cerebral glucose uptake. 19 The 5xFAD mice also develop age‐related impairments in many forms of learning and memory, including cued and/or contextual associative fear, 20 , 21 object and spatial recognition, 22 conditioned taste aversion 23 and visuo‐spatial navigation and reference memory. 24 , 25
Visuo‐spatial learning and memory in the 5xFAD mouse is commonly assessed with the Morris water maze (MWM), wherein mice use extra‐maze cues to learn the spatial location of a hidden platform that provides escape from water. Learning and memory impairments of 5xFAD mice on the MWM have generally been reported between 4 and 10 months of age, but the size of these impairments varies considerably across studies. For example, some studies show modest deficits in the learning performance of 5xFAD mice, 25 whereas other studies show large learning impairments, 26 , 27 or an inability to improve performance with training. 28 , 29 , 30 , 31 This variability in the extent of visuo‐spatial learning and memory impairment in 5xFAD mice in the MWM makes it difficult to establish a baseline level of “cognitive impairment” with which to judge the efficacy of novel therapeutics, because the age of the 5xFAD mice and sample size (statistical power) needed to reliably detect learning and memory impairments are not clear (given in Table S1). Nevertheless, the MWM has been used with some success to detect therapeutic effects of novel drugs in 5xFAD mice and continues to be used in pre‐clinical research with 5xFAD mice. 30 , 31 , 32 , 33 , 34 , 35 , 36
The variability of learning and memory performance in 5xFAD mice across studies is likely due to multiple factors associated with the cognitive and sensori‐motor ability of mice, as well as experimental procedure. One of the most important determinants of cognitive dysfunction in mouse models of AD is the age of the mice being tested. Importantly, age‐related changes within AD‐mouse models differ depending on the genotype of the mouse model used 12 , 37 , 38 and the behavior being measured. For example, 5xFAD mice show a significant impairment in acoustic startle response as early as 4 months of age, 39 but show profound age‐related deterioration of motor ability between 9 and 16 months of age. 40 On the other hand, the 5xFAD mice show no signs of age‐related decline in olfactory sensitivity or conditioned olfactory memory between 3 and 15 months of age. 41 , 42 , 43 The age‐related decline in visuo‐spatial memory in 5xFAD mice has not been systematically examined as previous studies have generally only tested mice at one or two ages. Therefore, the first aim of the present study was to examine the age‐related changes in visuo‐spatial learning and memory in 5xFAD mice from 3 to 15 months of age.
The variability in cognitive performance previously observed in 5xFAD mice on the MWM might be explained by sex differences and by the development of sensori‐motor dysfunction, due to AD‐related transgenes and/or background genes. For example, AD is more prevalent in females than males, and females can have more severe presentation of symptoms than males. 44 , 45 , 46 , 47 Despite known sex differences in AD, the use of both sexes in studies using 5xFAD mice or other AD mouse models is rare. Nevertheless, sex differences have been reported in 5xFAD mice, including higher levels of amyloid precursor protein (APP), Aβ‐42 peptides, Aβ plaque pathology and altered hippocampal expression of immune‐related genes in females than males. 13 , 48 , 49 , 50 Female 5xFAD mice show earlier onsets of age‐related weight loss and motor impairment than males at 12–16 months of age, 40 , 51 but do not differ from males in memory for learned odor preferences. 41 Moreover, since female C57BL/6J mice show greater age‐related deficits in learning and memory in the MWM than males, 52 one would expect a similar sex difference in 5xFAD mice, and this difference may become more pronounced with age. Therefore, the second aim of this study was to determine if there are sex differences in the age‐related development of visuo‐spatial learning and memory impairment in 5xFAD mice.
The background strains of AD mouse models can influence the expression of AD‐related pathology and behavioral dysfunction. 53 , 54 , 55 Inbred mice used as the background strains for transgenic AD mouse models differ in their genotype, their propensity to develop diseases of aging, their lifespan, and their ability to clear amyloid beta from their brains. 37 , 56 , 57 , 58 These background strain effects can influence behavioral phenotypes by acting independently or through interactions with AD‐related transgenes (epistasis) in mouse models of aging and AD. 59 , 60 , 61 , 62 Thus, our third aim was to investigate the effects of background genes on learning and memory in the 5xFAD mouse in the MWM.
The 5xFAD mouse was originally created on a hybrid C57BL/6J x SJL/J background, 14 and is still commonly used with this background. The SJL/J strain, however, carries mutant alleles that cause functional blindness due to retinal degeneration (Pde6b rd1 ) or partially impaired vision due to albinism (Tyr c ) and pink‐eyed dilution (Oca2 p ) (Refs. 56, 63; see SJL/J strain information [Jackson Laboratory, https://www.jax.org/strain/000686] for other known polymorphisms). Because these mutant alleles are autosomal recessive, a subset of 5xFAD mice will be homozygous for each mutant allele, thus creating a heterogeneous sample with varying degrees of visual impairment. Inbred mouse strains with retinal degeneration and albinism generally perform worse than strains with normal vision on the MWM, confirming that impaired visual function confounds assessment of learning and memory in visuo‐spatial tasks. 64 Indeed, retinal degeneration has been shown to impair MWM performance of inbred strains 65 and AD mouse models 66 , 67 and thus is expected to impair performance of 5xFAD mice. In addition, retinal degeneration interacts with AD‐related transgenes, altering whisking behavior in 5xFAD and WT mice. 68 Although we screened for retinal degeneration within mice used in this study, the albino‐related alleles of the SJL/J strain were expressed in many mice. Since albinism reduces visual acuity 69 , 70 we examined the effects of albinism on the performance of 5xFAD and WT mice on the MWM.
Due to the development of AD‐related pathology in motor systems of the brain and spinal cord, the 5xFAD mice exhibit motor impairments beginning at 9 months of age. 15 These motor impairments increase with age and affect multiple domains of motor function including locomotor activity, gait, motor coordination and grip strength as measured by the open‐field, foot‐print analysis, rota‐rod, balance beam and forelimb wire‐hang tests. 29 , 40 Motor impairment in 5xFAD mice at 12 months of age is sufficient to impair swimming ability and reduce swim speeds on the MWM, thus confounding measurement of learning and memory performance. 71 The degree to which the age‐related decline in motor function in the 5xFAD mice affects swim speed and other measures of learning and memory is not known, thus the fourth aim of the present study was to examine the confounding effects of motor dysfunction on measures of learning and memory in the MWM.
In the present study, we examined visuo‐spatial learning and memory of 5xFAD and WT mice from 3 to 15 months of age in the hidden platform version of the MWM. We examined four factors proposed to influence performance of 5xFAD mice on tests of visuo‐spatial learning and memory, and thus confound the effects of AD‐transgenes on measures of learning and memory in the MWM. First, we examined the effects of age on the size of learning and memory impairments in 5xFAD mice and second, we tested both male and female mice to determine sex differences during aging. Third, we controlled for blindness due to retinal degeneration (Pde6b rd1 ) and analyzed our data to determine if albinism (Tyr c , Oca2 p ) influenced performance of WT and 5xFAD mice on the MWM. Finally, we included behavioral measures (i.e., swim speed, visible platform training) to identify the effects of age‐related motor dysfunction on the performance of 5xFAD mice on the MWM. Thus, this study provides an in‐depth analysis of visuo‐spatial learning and memory in 5xFAD mice and examines how learning and memory is influenced by age, sex, sensory (albinism), and motor dysfunction.
2. MATERIAL AND METHODS
2.1. Subjects
The male and female 5xFAD (B6SJL‐Tg [APPSwFILon, PSEN1*M146L*L286V] 6799Vas/Mmjax) and wild‐type C57BL/6J x SJL/J mice tested were the offspring of hemizygous 5xFAD (JAX #34840) males and wild‐type females (B6SJLF1/J; JAX #100012), which were obtained from Jackson Laboratories (Bar Harbor, Maine) and bred in‐house. Poor breeding occurred in some females, and therefore 5xFAD males were bred with wild‐type F2 females to increase sample size. After weaning at 21 days of age, mice were housed in same sex groups of 2–4, in polyethylene cages (30 × 19 × 13 cm3) with wood‐chip bedding and a small polyethylene tube for enrichment. Cages were covered with a metal wire top and micro‐isolator filters. Food (Purina rodent chow, #5001) and tap‐water were available ad‐libitum. Some 12–15 month old 5xFAD mice showed severe motor impairment, including difficulty rearing to obtain food from the hoppers on the top of the home‐cage. For these mice, powdered food mixed with water was placed on the floor of the home cage. Mice were housed under a 12:12 h reversed light–dark cycle with lights off from 09:30 to 21:30, and behavioral testing was completed during the dark phase. The Dalhousie University Committee on Animal Care approved experimental procedures. Mice were genotyped for the APP and PS1 transgenes by Dr. Chris Sinal (Pharmacology Department, Dalhousie University) by PCR, using tissue samples obtained from ear punches taken from mice at weaning (PND 21). To reduce the frequency of phosphodiesterase‐6b retinal degeneration (Pde6b rd1 ) alleles, 5xFAD and wild‐type mice without a Pde6b rd1 allele were selected for breeding whenever possible. We also genotyped all mice for the Pde6b rd1 allele, and mice that were homozygous for Pde6b rd1 were excluded from the experiment. Identification of mice with albinism (Tyr c ) was determined by a white coat color, and identification of mice with pink‐eyed dilution (Oca2 p ) with yellow (agouti: A/A or A/a) and gray (non‐agouti black: a/a) coat colors.
2.2. General experimental design
Mice completed a behavioral test battery at 3, 6, 9, 12, or 15 months of age in a cross‐sectional experimental design. Mice were tested on the open‐field and rota‐rod and their body‐weight was obtained prior to testing on the MWM (typically 1–3 days between tests, data presented in Ref. 40). Swim‐speed data for female 5xFAD and WT mice at 12 months of age have been summarized in O'Leary et al., 71 and are included here with additional measures of performance to permit analyses at each age. The number of mice tested in each group at each age is given in Table S2 and the number of pigmented and albino mice tested at 6–9 and 12–15 months of age is given in Tables S3 and S4.
2.3. Morris water maze
The MWM consisted of a black circular pool (110 cm diameter) with 25 cm high walls. 65 The pool was filled with water (21°C) to a depth of 15 cm, and the water was made opaque with white non‐toxic liquid tempera paint (Schola). A circular escape platform (9 cm diameter) was placed 1 cm beneath the surface of the water. To assist older motor‐impaired 5xFAD mice in climbing onto the platform, small grooves were etched on the top and a rubber tube was placed around the escape platform (1 cm below the top). Extra‐maze visual cues consisted of furniture in the test room (two tables, a computer and monitor, a large door, a series of cabinets) various cues adhered to the room's walls, the experimenter and the geometric layout of the test room (5.2 × 2.4 m2). The test room was diffusely lit by two 100 W lights oriented towards the ceiling. The WaterMaze (Actimetrics, Wilmette, IL) tracking system recorded movement of mice in the MWM (5 samples/s) using a camera located above the pool.
Mice were tested in groups of 3–6, and were placed into individual holding cages with a bedding of paper towel during testing. Mice completed 3 days of acquisition training with four trials per day. For each trial, mice were released into the pool using a 500 ml plastic container at one of four positions around the pool's edge. The release location across trials was determined by a Latin Square design, so that the sequence was not repeated on multiple days. Mice were given a maximum of 60 s to locate the escape platform, after which they were guided to the platform. Following every trial, mice remained on the escape platform for 10 s before being returned to the holding cages. Learning performance was measured with latency and distance traveled to locate the escape platform. Cumulative search error 72 was also calculated using distance from the escape platform, summed across all recorded samples of the mouse's location in the pool. To control for different release locations, the time required to reach the escape platform given an optimal swim path from the release point was calculated using the average swim speed for the trial. The time for the optimal swim path was then omitted from the beginning of each trial in the final calculation of cumulative search error. Swim speed was measured to assess swimming ability, and general ability to complete the test was measured with the percentage of failed trials on days 2–3 of acquisition and reversal training (unable to find escape platform in 60 s). The inter‐trial interval was 5–10 min, but was extended to 10–15 min at 12 and 15 months of age to prevent hypothermia given the slower swim speeds of 5xFAD mice.
Following acquisition training, mice completed reversal training (3 days, 4 trials/day) where the escape platform was moved to the opposite side of the pool. The effect of moving the escape platform location was measured with difference scores using averages of the last two trials of acquisition training and the first two trials of reversal training. A probe trial was completed 24 h after the last day of reversal training to assess memory for the spatial location of the escape platform. In the probe trial, the escape platform was removed and the mouse was released into the pool for 60 s. The time spent in each of the four quadrants, and the average distance to the reversal escape platform location was recorded. The day following the probe trial, visible platform training (four trials) was completed to determine if performance was influenced by non‐cognitive confounds, including poor visual ability, motor control problems, or reduced motivation to escape from the water. In visible platform training, the escape platform was placed in a new location, and the escape platform had a brightly colored top and flag that extended above the water's surface.
2.4. Statistical analyses
All statistical analyses were completed separately for each age of testing. For measures of learning performance on the MWM, mixed design ANOVAs (2 × 2 × 3) were completed with genotype and sex as between‐subject factors, and day of training as a within‐subject factor. Measures of memory in the MWM were analyzed with between‐subject ANOVAs (2 × 2) for genotype and sex. For the effects of albinism and pigment dilution, analyses were completed separately for each sex, using mixed design ANOVAs (genotype × albinism × day) for measures of learning and between‐subject ANOVAs for measures of memory (genotype × albinism). Post‐hoc tests were completed with paired or unpaired t‐tests and simple effects analyses. One‐tail single sample t‐tests were used to determine if groups of mice spent significantly more time in the correct quadrant during the probe trial than expected by chance (25%). Interactions are reported only when significant. Relationships between motor function and swim‐speed were completed with Pearson product moment correlations. All statistical analyses were completed with the SPSS (IBM version 20) or GraphPad Prism (version 8.0.1) software.
In a minority of trials, data were lost due to equipment error (<1% of trials), and for measures of learning, averages for each day were obtained with the remaining trials for the respective day. For visible platform trials, a minority of mice showed an unexpected impairment in performance relative to previous reversal training, likely due to difficulty switching from spatial to cued navigational strategies. Therefore, mice with an average distance to reach the visible platform that was >500 cm above the average for the last day of reversal training were excluded from analyses (N = 2, 3 m WT females; N = 1, 9 m 5xFAD female; N = 3, 15 m 5xFAD females). Finally, some mice showed abnormal swimming patterns during the probe trial, with little exploration of the pool away from the release location. Therefore, mice that traveled less than 500 cm during the probe trial, or spent more than 80% of the time in a single quadrant were excluded from analyses of memory performance (Males: N = 2, 12 m 5xFAD) (Females: N = 1, 3 m WT; N = 1, 6 m 5xFAD; N = 1, 9 m WT; N = 3, 12 m 5xFAD; N = 1, 15 m WT; N = 1, 15 m 5xFAD).
3. RESULTS
The learning and memory performance for male and female 5xFAD and WT mice are first presented separately for each age of testing. This represents how data are presented in most studies using the 5xFAD mouse, which often test mice at one or two ages and do not account for background gene effects such as albinism. We then re‐analyzed the data from mice at 6–9 and 12–15 months of age to determine the effects of albinism on learning and memory performance. At each age, performance on measures of learning (latency to reach escape platform, distance, cumulative search error) are described first, followed by memory probe trial performance (time in correct quadrant, average distance to platform), and finally control measures to gauge the influence of sensori‐motor dysfunction on performance (swim‐speed, distance to reach visible platform, percentage of failed trials).
3.1. MWM performance of 5xFAD and WT mice at 3 months of age
At 3 months of age, 5xFAD and WT mice showed improved performance on all measures of learning (latency, distance traveled and cumulative search error) across both acquisition and reversal training (Figure 1A–F, all p < 0.0001). Performance of male and female 5xFAD mice did not differ significantly from WT mice on any measure of learning. The effect of reversing the escape platform location produced a larger impairment in performance in male than female mice across all measures of learning (all p < 0.005; Figure S1A–C). Moreover, a sex by day interaction was present during reversal training for latency (F[2, 76] = 6.79, p < 0.005), distance traveled (F[2, 76] = 4.21, p < 0.05) and cumulative search error (F[2, 76] = 6.70, p < 0.005), as male mice showed worse performance on the first day of reversal training (R1) than female mice. Overall, 5xFAD and WT mice did not differ on measures of memory in the probe trial (Figure 1G,H, Figure S2A). There was, however, a sex × genotype interaction (F[1, 37] = 4.34, p < 0.05) wherein male 5xFAD mice spent more time in the correct quadrant than male WT mice, whereas no difference was found between female 5xFAD and WT mice (Figure 1G,H). Male and female mice of both genotypes spent more time in the correct quadrant than expected by chance. Among control measures that gauge sensori‐motor confounds, no genotype differences were found in swim speed, but female mice swam significantly faster than males in both acquisition (F[1, 38] = 5.06, p < 0.05) and reversal training (F[1, 38] = 4.67, p < 0.05; Figures 1J and S2B). No genotype differences were found in distance traveled to the visible platform or in percentage of failed trials (Figures 1I,J and S2C).
FIGURE 1.
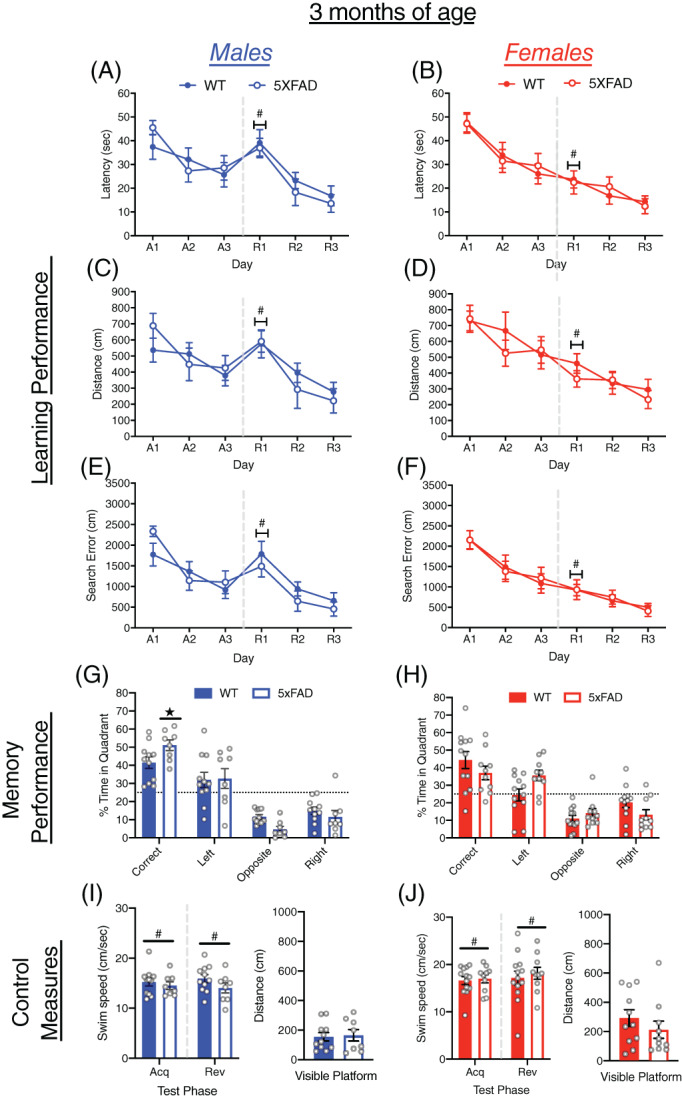
Learning and memory performance of male and female 5xFAD mice at 3 months of age. (A–F) Performance of WT and 5xFAD mice on measures of learning (latency, distance, and cumulative search error), shown separately for males (left) and females (right). Performance is shown for both acquisition (A1‐3) and reversal training (R1‐3), separated by a dashed line. (G,H) Memory performance during the probe trial for 5xFAD and WT mice as measured by percent time in pool quadrants. Dashed line represents time in quadrants expected by chance (25%). Swim‐speed during acquisition and reversal training, along with distance traveled during visible platform training for (I) male and (J) female WT and 5xFAD mice. #, sex difference, p < 0.05;  , genotype difference, p < 0.05
, genotype difference, p < 0.05
3.2. MWM performance of 5xFAD and WT mice at 6 months of age
At 6 months of age both male and female 5xFAD and WT mice showed improved performance over acquisition and reversal training (all p < 0.0001; Figure 2A–F). In acquisition training, 5xFAD mice took longer to locate the escape platform (F[1, 39] = 6.46, p < 0.05) and had larger cumulative search errors (F[1, 39] = 6.012, p < 0.05) than WT mice, but the difference in distance traveled was not significant. At the onset of reversal training, 5xFAD and WT mice did not differ in the size of the reversal effect difference scores (Figure S1A–C). During reversal training, the 5xFAD mice took longer to locate the escape platform (F(1, 39) = 7.27, p < 0.05), traveled farther (F(1, 39) = 5.73, p < 0.05) and had larger cumulative search error (F(1, 39) = 4.37, p < 0.05) than WT mice. During the probe trial, no differences were found due to genotype or sex (Figures 2G,H and S2A), and all groups spent more time in the correct quadrant than expected by chance. Among control measures, neither male nor female 5xFAD mice differed from WT mice in swim speed (Figures 2I,J and S2B). The 5xFAD mice, however, traveled farther in the visible platform test (F[1, 39] = 5.83, p < 0.05), and failed to reach the escape platform more often than WT mice (F[1, 39] = 8.58, p < 0.01; Figures 2I,J and S2C). Female mice of both genotypes also traveled farther to reach the visible escape platform than males (F[1, 39] = 6.42, p < 0.05).
FIGURE 2.
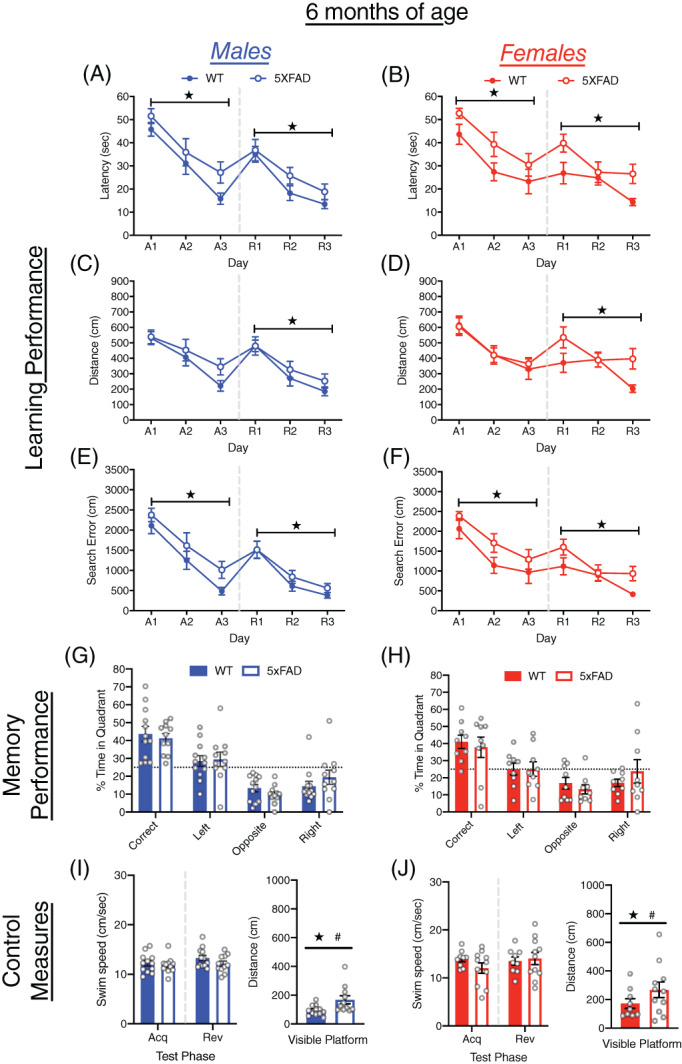
Learning and memory performance of male and female 5xFAD mice at 6 months of age. (A–F) Performance of WT and 5xFAD mice on measures of learning (latency, distance and cumulative search error), shown separately for males (left) and females (right). Performance is shown for both acquisition (A1‐3) and reversal training (R1‐3), separated by a dashed line. (G,H) Memory performance during the probe trial for 5xFAD and WT mice as measured by percent time in pool quadrants. Dashed line represents time in quadrants expected by chance (25%). Swim‐speed during acquisition and reversal training, along with distance traveled during visible platform training for (I) male and (J) female WT and 5xFAD mice. #, sex difference, p < 0.05;  , genotype difference, p < 0.05
, genotype difference, p < 0.05
3.3. MWM performance of 5xFAD and WT mice at 9 months of age
At 9 months of age, both male and female 5xFAD and WT mice improved on all measures of performance over acquisition and reversal training (all p < 0.0001; Figure 3A–F). In acquisition training, the 5xFAD mice took longer to locate the escape platform (F[1, 43] = 9.92, p < 0.005), traveled a farther distance (F[1, 43] = 4.56, p < 0.05) and had larger cumulative search error (F[1, 43] = 12.03, p < 0.001) than WT mice. At the onset of reversal training, the 5xFAD and WT mice did not differ in the size of reversal effect difference scores (Figure S1A–C). During reversal training, 5xFAD and WT mice did not differ significantly on any measures of learning performance. Overall male mice tended to locate the escape platform faster (F[1, 43] = 4.02, p = 0.051) and had smaller cumulative search error (F[1, 43] = 3.84, p = 0.057) than female mice during reversal training, but these differences did not reach significance. The 5xFAD mice did not differ from the WT mice on measures of memory during the probe trial (Figures 3G,H and S2A). The 5xFAD female mice, however, did not spend more time in the correct quadrant than expected by chance. Male mice spent more time in the correct quadrant (F[1, 42] = 6.76, p < 0.05) and had a shorter average proximity to the escape platform than females (F[1, 42] = 8.02, p < 0.01); Figure S2A). The 5xFAD and WT mice did not differ in swim‐speed or other control measures (Figures 3I,J and S2B). Female mice failed to reach the escape platform on more trials than males (F[1, 43] = 4.15, p < 0.05; Figure S2C).
FIGURE 3.
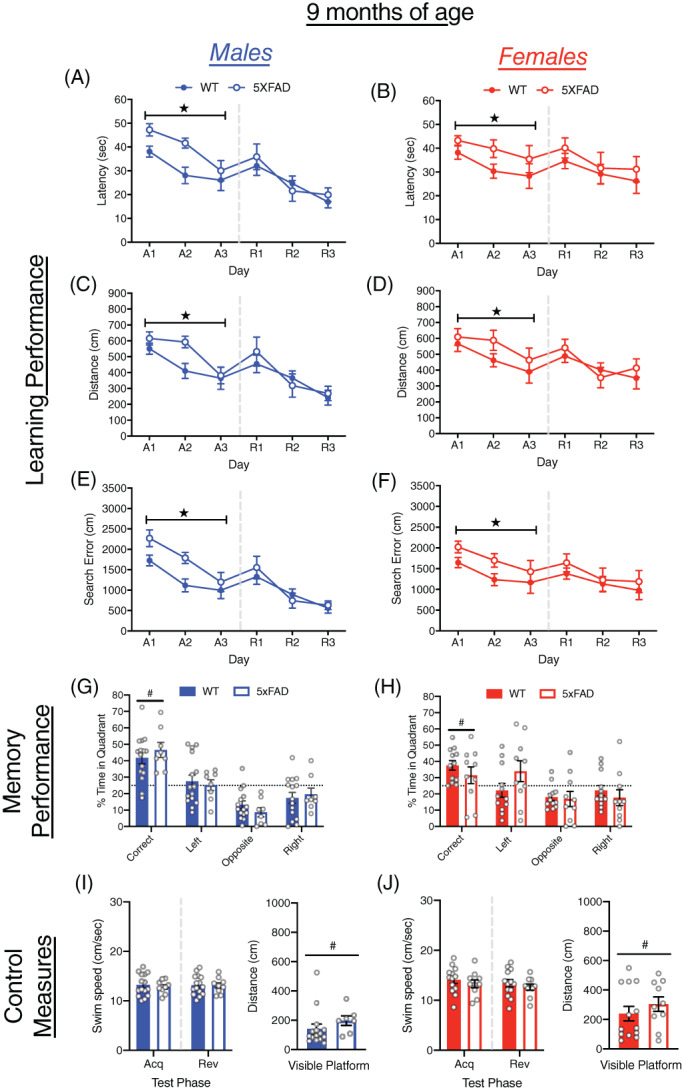
Learning and memory performance of male and female 5xFAD mice at 9 months of age. (A–F) Performance of WT and 5xFAD mice on measures of learning (latency, distance and cumulative search error), shown separately for males (left) and females (right). Performance is shown for both acquisition (A1‐3) and reversal training (R1‐3), separated by a dashed line. (G,H) Memory performance during the probe trial for 5xFAD and WT mice as measured by percent time in pool quadrants. Dashed line represents time in quadrants expected by chance (25%). Swim‐speed during acquisition and reversal training, along with distance traveled during visible platform training for (I) male and (J) female WT and 5xFAD mice. #, sex difference, p < 0.05;  , genotype difference, p < 0.05
, genotype difference, p < 0.05
3.4. MWM performance of 5xFAD and WT mice at 12 months of age
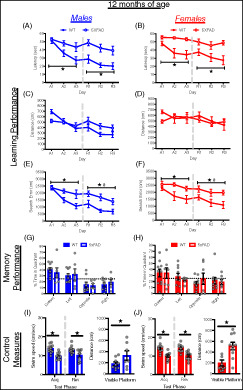
At 12 months of age, mice improved their performance over days in both acquisition and reversal training (all p < 0.005; Figure 4A–F). During acquisition training, the extent of improvement clearly differed between 5xFAD and WT mice (day × genotype interactions, p < 0.05), with WT mice showing robust improvements across all measures of learning, whereas 5xFAD mice showed little or no improvement. The 5xFAD mice were slower to locate the escape platform than WT mice (F[1, 37] = 19.49, p < 0.0001) and had larger cumulative search errors (F[1, 37] = 13.45, p < 0.005) than WT mice, but did not differ in distance traveled. At the onset of reversal training, 5xFAD and WT mice did not differ in the size of reversal effect difference scores (Figure S1A–C). During reversal training, WT mice located the escape platform faster (F[1, 37] = 24.98, p < 0.0001) and had smaller cumulative search error (F[1, 37] = 17.52, p < 0.0005) than 5xFAD mice, but again no difference was observed in distance traveled. Overall, male mice performed better than female mice on cumulative search error (F[1, 37] = 4.2, p < 0.05), but this difference was not quite significant for distance traveled (F[1, 37] = 3.98, p = 0.053). The 5xFAD mice did not differ significantly from WT mice in probe trial performance, nor were there differences due to sex (Figures 4G,H and S2A). However, both male and female 5xFAD mice, and female WT mice failed to spend more time in the correct quadrant than expected by chance. Clear differences were observed on all control measures which identify potential sensori‐motor confounds on performance. Male and female 5xFAD mice swam significantly slower in acquisition (F[1, 37] =34.90, p < 0.0001), reversal (F[1, 37] = 23.34, p < 0.0001; Figure 4I,J) and visible platform training (F[1, 37] = 22.53. p < 0.0001), traveled farther to reach a visible escape platform (F[1, 37] = 15.22 p < 0005) and failed to reach the escape platform more often than WT mice (F[1, 37] = 36.92, p < 0.0001; Figure S2B,C).
FIGURE 4.
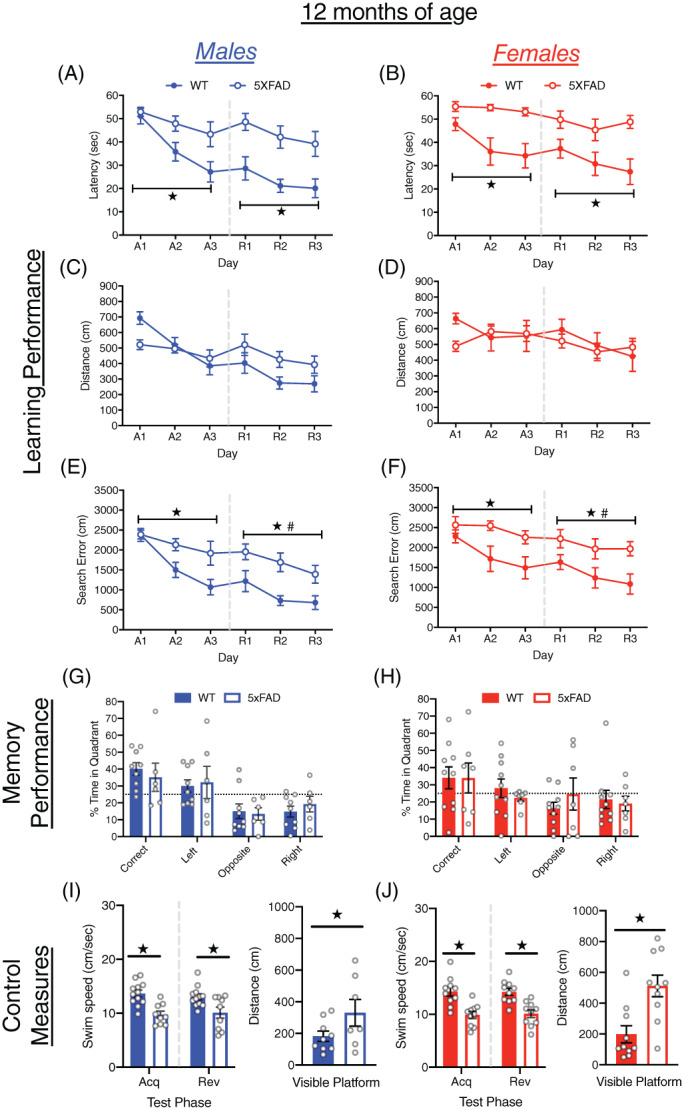
Learning and memory performance of male and female 5xFAD mice at 12 months of age. (A–F) Performance of WT and 5xFAD mice on measures of learning (latency, distance and cumulative search error), shown separately for males (left) and females (right). Performance is shown for both acquisition (A1‐3) and reversal training (R1‐3), separated by a dashed line. (G,H) Memory performance during the probe trial for male and female 5xFAD and WT mice as measured by percent time in pool quadrants. Dashed line represents time in quadrants expected by chance (25%). Swim‐speed during acquisition and reversal training, along with distance traveled during visible platform training for (I) male and (J) female WT and 5xFAD mice. #, sex difference, p < 0.05;  , genotype difference, p < 0.05
, genotype difference, p < 0.05
3.5. MWM performance of 5xFAD and WT mice at 15 months of age
At 15 months of age, all mice improved their performance over days in both acquisition and reversal training (all p < 0.05; Figure 5A–F). However, similar to 12 months of age, the extent of improvement in acquisition training clearly differed between WT and 5xFAD mice (day × genotype interactions, all p < 0.05). The WT mice showed improvements across all measures of learning, whereas 5xFAD mice showed little or no improvement. The 5xFAD mice were slower to locate the escape platform (F[1, 34] = 45.58, p < 0.0001), and had larger cumulative search errors than WT mice (F[1, 34] = 40.23, p < 0.0001) but did not differ in distance traveled. At the onset of reversal training, the 5xFAD mice had smaller reversal effect difference scores than the WT mice on all measures of learning (all p < 0.0005; Figure S1A–C). During reversal training, the 5xFAD mice were slower to locate the escape platform (F[1, 34] = 27.119, p < 0.0001) traveled farther (F[1, 34] = 10.11, p < 0.005) and had larger cumulative search errors (F[1, 34] = 16.27, p < 0.0005) than WT mice. Overall, male mice performed better than female mice on latency to reach the escape platform (F[1, 34] = 5.09, p < 0.05), distance traveled (F[1, 34] = 9.31, p < 0.005) and cumulative search error (F[1, 34] = 7.22, p < 0.01) during reversal training. During the probe trial, both male and female 5xFAD mice spent less time in the correct quadrant (F[1, 32] = 12.75, p < 0.005) and had a larger average distance to the escape platform (F[1, 32] = 15.51, p < 0.0005) than WT mice (Figure 5G,H, Figure S2A). Both male and female 5xFAD mice failed to spend more time in the correct quadrant than expected by chance. Clear genotype differences were observed on all control measures as 5xFAD mice swam significantly slower in acquisition (F[1, 34] = 17.85, p < 0.0005), reversal (F[1, 34] = 14.34, p < 0.005), and visible platform training (F[1, 31] = 10.91, p < 0.005), traveled farther to reach a visible escape platform F(1, 31) = 43.51, p < 0.0001) and failed to reach the escape platform more often than WT mice (F[1, 34] = 39.50, p < 0.0001; Figures 5I,J and S2B,C).
FIGURE 5.
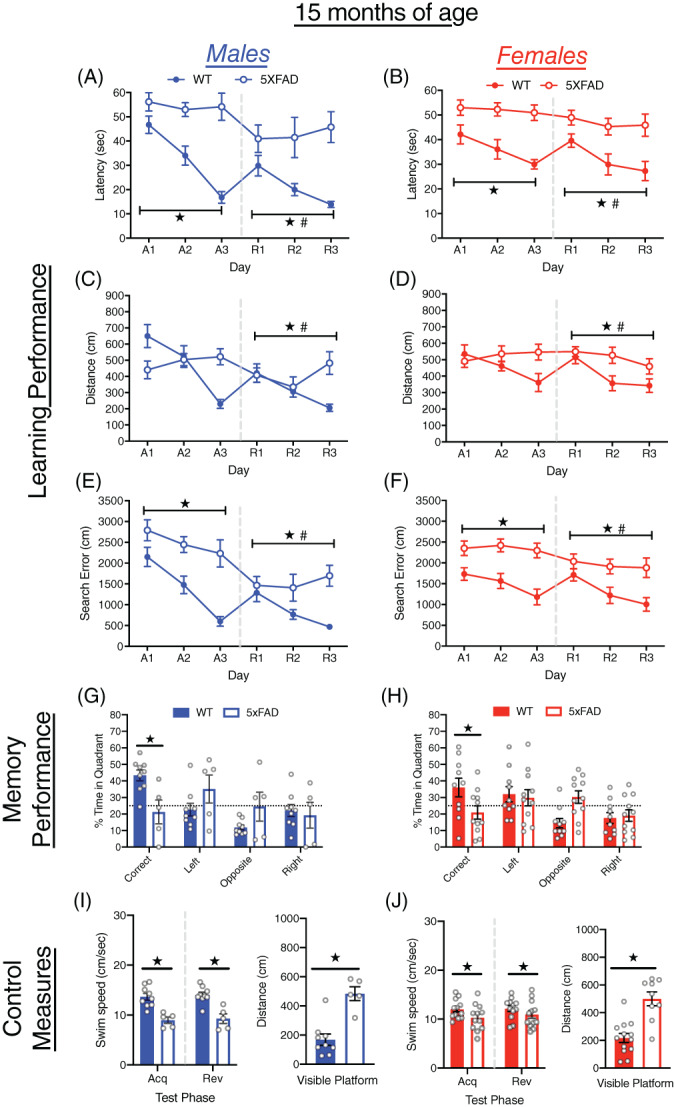
Learning and memory performance of male and female 5xFAD mice at 15 months of age. (A–F) Performance of WT and 5xFAD mice on measures of learning (latency, distance and cumulative search error), shown separately for males (left) and females (right). Performance is shown for both acquisition (A1‐3) and reversal training (R1‐3), separated by a dashed line. (G,H) Memory performance during the probe trial for 5xFAD and WT mice as measured by percent time in pool quadrants. Dashed line represents time in quadrants expected by chance (25%). Swim‐speed during acquisition and reversal training, along with distance traveled during visible platform training for (I) male and (J) female WT and 5xFAD mice. #, sex difference, p < 0.05;  , genotype difference, p < 0.05
, genotype difference, p < 0.05
3.6. Age related changes in learning and memory performance in 5xFAD and WT mice
Although the analysis of learning and memory scores at each age is informative for comparing results to previous studies that tested 5xFAD mice at one or two specific ages, it does not provide an overall picture of age‐related cognitive decline in 5xFAD mice. Therefore, we compared the performance of 5xFAD and WT mice from 3 to 15 months of age on each measure used so that the performance at each age could be compared to baseline performance at 3 months of age (Figure 6). There was a slight improvement in measures of learning at 6 months of age, but neither male nor female WT mice showed an age‐related decline in learning performance (Figure 6A–F). On the other hand, both male and female 5xFAD mice showed an age‐related decline in each measure of learning which became significantly different from the 3 month old mice at 12 and 15 months of age in males and at 9, 12, and 15 months of age in females. During the probe trial, neither male nor female WT mice showed a significant decline in memory performance with age, whereas male 5xFAD mice showed impaired memory performance at 12 and 15 months of age, and female 5xFAD mice were significantly impaired at 15 months of age (Figure 6G,H). Finally, all mice showed an age‐related decline in swim speed (Figure 6I,J). From 6 to 15 months of age both male and female WT mice swam slower than at 3 months of age. Male and female 5xFAD mice also showed an age‐related decline in swim speed, which was more pronounced than that shown by WT mice.
FIGURE 6.
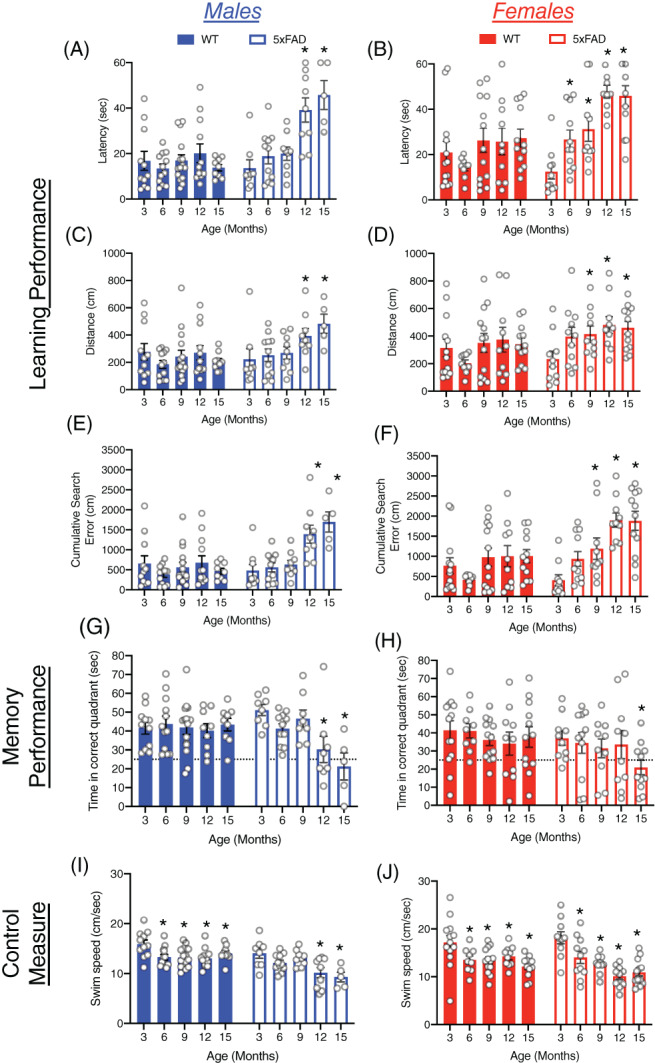
Age‐related changes in learning and memory performance of male and female 5xFAD and WT mice on the MWM. (A–F) Age‐related changes in learning performance of WT and 5xFAD mice (latency, distance and cumulative search error), shown separately for males (left) and females (right). Performance is shown for the last day of reversal training. (G,H) Memory performance during the probe trial for male and female 5xFAD and WT mice from 3 to 15 months of age, as measured by percent time in the correct pool quadrant. Dashed line represents time in quadrants expected by chance (25%). Swim‐speed during reversal training, for (I) male and (J) female WT and 5xFAD from 3 to 15 months of age. *p < 0.05, compared to 3 months of age
3.7. Relationships between motor function and body weight on swim‐speed in the MWM
In order to determine the potential contribution of non‐cognitive phenotypes on swimming‐ability, we correlated previous data on motor function and body‐weight of mice (presented in 40 ), with swim‐speed on the MWM presented here. Measures of motor function (Figure S3) on the rota‐rod and open‐field, were positively associated with swimming‐ability (faster swim speeds) in 5xFAD mice (all p < 0.05; Figure S3B,D,F). Interestingly, these correlations were not significant in WT mice. Body‐weight was negatively associated with swim‐speed in WT mice, but this correlation was not significant in 5xFAD mice (Figure S3G,H).
3.8. The effect of albinism on learning and memory in 5xFAD and WT mice at 6–9 months of age
We next examined the effects of albinism on measures of learning and memory performance. We pooled data from mice with pigment dilution (Oca2 p ) and albinism (Tyr c ) to create groups of male and female albino/pigment‐diluted mice (subsequently referred to as albino) to compare with pigmented mice. To increase statistical power to detect effects of albinism, data were pooled into two age groups: 6–9 months of age, and 12–15 months of age (see Tables S3 and S4 for sample sizes). At 12–15 months of age there were very few albino 5xFAD males and thus only data for females were analyzed. Since genotype differences are described in previous analyses, only main effects or interactions including albinism are reported here.
Albino males did not differ from pigmented males on any measure of performance during acquisition training but showed significant impairments during reversal training (Figure 7A,B). Albino males took longer to reach the escape platform (F[1, 43] = 10.15, p < 0.005) traveled farther (F[1, 43] = 6.07, p < 0.05) and had larger cumulative search errors (F[1, 43] = 6.61, p < 0.05) than pigmented mice during reversal learning. Albino males did not differ from pigmented males on measures of memory during the probe trial (Figures 7E and S4A), nor were there differences in swim speed or other control measures (Figures 7G and S4D).
FIGURE 7.
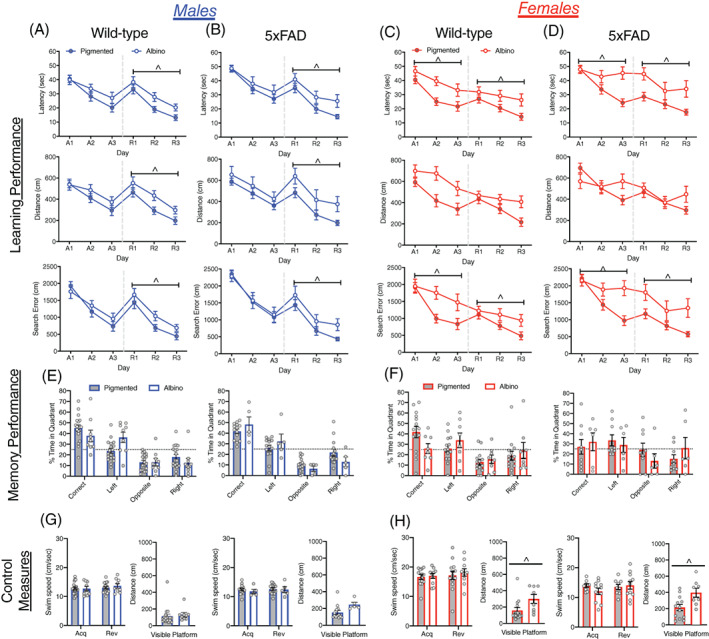
Learning and memory performance for pigmented and albino mice, within both 5xFAD and WT mice at 6–9 months of age. (A–D) Learning performance (latency, distance, and cumulative search error) of albino and pigmented mice, within both WT and 5xFAD mice. Data are graphed and analyzed separately for males (A,B) and females (C,D). Performance is shown for both acquisition (A1‐3) and reversal training (R1‐3), separated by a dashed line. (E and F) Memory performance during the probe trial for male and female albino and pigmented mice as measured by percent time in each pool quadrant. Dashed line represents time in quadrants expected by chance (25%). (G) Swim‐speed during acquisition and reversal training, along with distance traveled during visible platform training for (G) male and (H) female pigmented and albino mice. ^, albinism difference, p < 0.05
Female albino mice took longer to locate the escape platform (F[1, 39] = 10.695, p < 0.005), and had larger cumulative search errors (F[1, 39] = 7.14, p < 0.05) than pigmented females in acquisition training (Figure 7C,D) but did not differ in distance traveled (F[1, 39] = 2.97, p = 0.09). Similarly, in reversal training, female albino mice took longer to locate the escape platform (F[1, 39] = 14.85, p < 0.0005) and had larger cumulative search errors than pigmented females (F[1, 39] = 12.17, p < 0.005) but the difference in distance traveled did not reach significance (F[1, 39] = 3.67, p = 0.063). In the memory probe trial the performance of albino and pigmented mice did not differ. However, albino 5xFAD females failed to spend more time in in the correct quadrant than expected by chance (Figures 7F and S4B). Among control measures, swim‐speed did not differ between albino and pigmented mice in acquisition or reversal training. Albino females of both genotypes, however, traveled farther to reach the visible escape platform (F[1, 39] = 13.18, p < 0.005), and failed to reach the escape platform on more trials than pigmented females (F[1, 39] = 26.84 p < 0.0001; Figures 7H and S4E).
3.9. The effect of albinism on learning and memory in 5xFAD and WT mice at 12–15 months of age
At 12–15 months of age, albino females did not differ from pigmented females on any measure of learning during acquisition training, but during reversal training the albino females were slower to locate the hidden platform (F[1, 40] = 4.71, p < 0.05) and had greater cumulative search errors than the pigmented females (F[1, 40] = 5.75, p < 0.05). There was no difference between albino and pigmented females on distance traveled (Figure S5A,B). In memory performance on the probe trial, only the pigmented WT females spent a significant amount of time in the correct quadrant; albino WT females and both pigmented and albino 5xFAD females did not spend more time than chance in the correct quadrant (Figure S5C). There was no difference between albino and pigmented females in average distance from the escape platform location on the probe trial (Figure S5C). Albino mice swam significantly slower than pigmented mice during acquisition training (F[1, 40] = 5.49, p < 0.05), but not in reversal training. Albino and pigmented mice did not differ in distance traveled during visible platform training (Figure S5D). An albinism by genotype interaction was also found for percentage of failed trials (F[1, 40] = 4.43, p < 0.05), where 5xFAD albino mice failed to reach the escape platform more than 5xFAD pigmented mice (Figure S4F).
4. DISCUSSION
The results of this study show that visuo‐spatial learning and memory deficits in 5xFAD mice on the MWM are determined by the age and sex of the mouse, background genes which affect vision, and age‐related motor impairments. Here we interpret these findings, propose directions for future research, and describe strategies for disentangling sensori‐motor and cognitive dysfunction in 5xFAD mice.
4.1. 5xFAD mice show an age‐related decline in visuo‐spatial learning and memory on the Morris water maze
There was a clear age‐related decline in visuo‐spatial learning performance in male and female 5xFAD mice on the MWM, which was not shown by WT mice. Learning impairments were first observed in 5xFAD mice at 6 months of age during acquisition training, and were also detected at older ages (9–15 months). Although less reliable than impairments in acquisition training, impaired reversal learning performance was found in 5xFAD mice at 6, 12, and 15 months of age. Learning impairments were most pronounced at 12–15 months, with a 3–4 fold decrease in performance in male and female 5xFAD mice from 3–15 months of age (see Figure 6). These results are consistent with previous work using the MWM, which does not report learning impairments prior to 4 months of age, 26 and more frequently shows learning impairments between 4 and 10 months of age. 24 , 25 , 30 , 31 Thus, our findings suggest visuo‐spatial learning impairment begins as early as 6 months of age in 5xFAD mice, with the most significant impairments co‐occurring with motor dysfunction after 9 months age. 40
Impaired memory performance of 5xFAD mice, however, did not coincide with impairments in learning on the MWM, and was detected only at 12–15 months of age. Memory impairments on the MWM have been previously shown in 5xFAD mice, 24 , 25 , 31 , 34 , 73 but in some cases impaired learning can occur without impairments in memory performance. 27 , 35 , 74 This pattern of results suggests modest memory impairments in 5xFAD mice on the MWM, which may require specific test procedures to be reliably detected. However, increased variability in memory performance was observed in female 5xFAD mice at 9–12 months of age, which may have reduced sensitivity to detect differences in memory performance at these ages. We should note that in the present study the probe trial occurred at the end of reversal training, and thus mice could show preference for both the acquisition (opposite quadrant) and/or reversal (correct quadrant) escape platform locations. Memory performance for the acquisition escape platform location was poor in both 5xFAD and WT mice, and thus memory for the acquisition platform did not appear to persist through reversal training nor influence memory for the reversal escape platform location during the probe trial.
The 5xFAD mouse develops a range of AD‐related neuropathology that likely contributes to age‐related impairments in visuo‐spatial learning. Aβ‐plaque deposition in the cortex and hippocampus begins as early as 2–3 months and increases rapidly with age. 15 , 22 Within the hippocampus, plaque deposition is most pronounced in the subiculum, and is accompanied by neuron loss at 9 months of age. 14 Elevated micro‐gliosis has been shown at 5 and 9 months of age throughout the hippocampus, 16 , 17 as well as increased astrogliosis at 6 months of age. 18 Reduced levels of adult neurogenesis in the dentate gyrus occurs at 3–6 months of age. 75 , 76 Impaired basal synaptic transmission, and reduced long‐term potentiation is present in CA1 following stimulation of the Schaffer collateral pathway at 6–7 months of age. 21 , 77 Altered neural connectivity is also present, as the hippocampus of 5xFAD mice receives fewer projections from the entorhinal cortex, raphe nucleus, medial septum and substantia nigra than WT mice. 78 Further research is needed to establish the contribution of these different neural pathologies to learning and memory impairments in 5xFAD mice after 6 months of age.
Our pattern of behavioral results suggests dysfunction within specific hippocampal circuits/sub‐regions in 5xFAD mice, rather than gross hippocampal dysfunction. Overall, the 5xFAD mice had difficulty efficiently navigating to the escape platform, but by the end of reversal training could recognize/remember the escape platform location once reached. This pattern of results is consistent with dysfunction within the ventral hippocampus, given lesions of the ventral hippocampus can produce deficits in learning and not memory. 79 Furthermore, lesions and pharmacological deactivations of the ventral hippocampus generally produce smaller impairments on learning and memory than respective manipulations in the dorsal hippocampus, 80 , 81 , 82 consistent with the moderate learning and memory deficits reported here at 6–9 months. However, given the widespread AD‐related pathology in 5xFAD mice, we cannot preclude the possibility that learning and memory impairments are due to dysfunction within other brain regions. For example, 5xFAD mice develop Aβ‐pathology within the dorsal striatum, 83 and mice with lesions of the dorsomedial striatum are severely impaired in learning to find the platform in the MWM, fail to develop a spatial search strategy, and show no spatial memory during the probe trial. 84 However, prediction of neural dysfunction from behavioral performance is likely obscured in 5xFAD mice, given our results show large individual differences in the age‐related decline of learning and memory, depending on the sex of the mice, the background genes and the age‐related decline in motor function (discussed below).
4.2. Why were memory impairments in 5xFAD mice not replicated in this study?
Relative to previous work, our results diverged most noticeably in memory performance of 5xFAD mice. We did not detect memory impairments in 5xFAD mice from 6 to 9 months of age, whereas previous studies show memory impairments in 5xFAD mice on the MWM, 25 , 30 , 32 , 33 , 34 and in other tests of learning and memory at these ages. 20 , 22 , 24 Although we identified multiple phenotypic factors that influence the behavior of 5xFAD mice on the MWM, there are likely multiple factors associated with apparatus design and test procedure, which also contribute to discrepancies across studies. Indeed, our data was idiosyncratic in that mice generally preferred to spend more time in the left non‐target quadrant, compared to the other non‐target quadrants (i.e., mice should show equal preference across non‐target quadrants). This could conceivably be due to idiosyncrasies in the extra‐maze environment, including the distribution and/or salience of extra‐maze visual cues. Therefore, we cannot preclude the possibility that memory impairments in 5xFAD mice can be detected with more optimal, and thus more sensitive experimental designs. Thus, we propose that memory performance of 5xFAD mice is likely governed by a complex interaction of both behaviorally‐relevant phenotypic traits and experimental parameters. Therefore, we stress the importance for each laboratory to optimize their experimental design, and establish their own baseline levels of cognitive performance in 5xFAD mice.
4.3. Sex differences in visuo‐spatial reversal learning in WT and 5xFAD mice
Sex‐differences are present in AD patients, with females having increased prevalence of AD, greater levels of neuropathology and cognitive impairment, and a stronger association between neuropathology and symptom severity than males. 44 , 45 , 46 , 47 Female 5xFAD mice also have greater levels of AD‐related neuropathology than males, including increased levels of APP, Aβ‐42, and Aβ‐plaque deposition. 13 , 14 , 50 , 85 , 86 Female 5xFAD mice also have a greater number of immune‐related genes with altered expression than males. 49 Sex differences in sensori‐motor and cognitive impairment have been sporadically reported in 5xFAD mice 22 , 40 but generally rates of age‐related decline are similar in males and females. The extent that these sex differences are due to sex‐dependent vulnerability to AD‐related pathology, rather than sex differences in the level of mutant APP transgene expression, however, is not clear. 49 , 50 , 54
In the present study, the majority of sex differences in learning and memory were found in both WT and 5xFAD mice, independent of transgenes. Although somewhat sporadically detected across ages, these sex differences consistently showed improved performance of males compared to females. For example, at 9 months of age memory performance of females was worse than males, and females more frequently failed to locate the escape platform during training. Sex differences were most reliable in reversal training, wherein female mice showed impaired reversal learning performance at 12 and 15 months of age (and trending significance at 9 months) and failed to show a reversal effect at 3 months of age. Although the absence of a reversal effect in female mice at 3 months may indicate poor memory, it is also possible that male and female mice use different search strategies at the onset of reversal training. We suspect that when the escape platform is moved, male mice perseverately search at the previous platform location, whereas females quickly adapt to searching other areas of the maze.
Evidence for sex‐dependent modulation of AD‐transgene effects on learning and memory was relatively less common than overall sex differences. For example, male 5xFAD mice showed better memory performance than WT mice at 3 months, but this difference was not found at older ages. Perhaps the most consistent sex‐dependent effect of AD‐transgenes was present in reversal training. As shown in Figure 6, male 5xFAD mice show significant age‐related deficits in reversal training at 12 and 15 months of age, whereas females show deficits as early as 6–9 months of age. In addition, genotype differences on measures of reversal learning at 6 months of age were driven largely by deficits in female 5xFAD rather than male 5xFAD mice. This female specific vulnerability to age‐related decline in 5xFAD mice is consistent with the elevated AD‐related pathology in females than males. We should reiterate, however, that 5xFAD mice at 12–15 months showed little improvement in performance across both acquisition and reversal training, and thus poor reversal learning performance is likely due to motor dysfunction, rather than impaired reversal learning. Nevertheless, it appears that reversal learning is somewhat more informative for understanding sex differences in learning and memory and sex‐specific vulnerability to AD‐related pathology than acquisition training. 87
4.4. Background genes (albinism) increase visuo‐spatial learning and memory deficits in 5xFAD mice, and affect females more than males
The 5xFAD mouse is often bred on a C57BL/6J x SJL/J hybrid genetic background, and the SJL/J mouse confers mutant alleles (Pde6b rd1 , Tyr c , Oca2 p ) that influence visual ability. Generally inbred mouse strains with albinism (Tyr c ) have poorer visual acuity than pigmented strains, 70 , 88 and this visual impairment confounds learning and memory performance on the MWM 65 and in the Barnes maze. 89 We controlled for blindness due to the retinal degeneration‐1 mutant allele (Pde6b rd1 ) in this experiment, but when this is not done, the effects of Pde6b rd1 on visual ability and thus MWM performance are expected to be even larger than those observed in albino animals. 65 , 66 Thus, a random selection of 5xFAD mice will consist of a heterogeneous sample, some of which will have normal vision, some with moderate visual impairment (Tyr c , Oca2 p ), and others with functional blindness (Pde6b rd1 ). If mice are not screened for the genes that cause visual deficits, the effects of poor vision will overwhelm the effects of the AD transgenes. Thus one must beware of breeding AD‐model mice on genetic backgrounds with albinism and retinal degeneration alleles, as these alleles may confound the effect of gene manipulations. 90
Reanalysis of our MWM data revealed that albinism impaired learning performance in both sexes of 5xFAD and WT mice at 6–9 months of age, and the effect of albinism was at least as large as that of the AD‐related transgenes. At 6–9 months of age, the effects of albinism were noticeably larger in female mice than in males, and this difference was most pronounced in female 5xFAD mice (Figures 7 and S3). We subsequently replicated many of the effects of albinism in female WT and 5xFAD mice at 12–15 months of age, although deficits were somewhat smaller. Albinism produces multiple abnormalities within the visual system that may contribute to reduced visual acuity, including a reduction in the number of photoreceptors, 91 an abnormal decussation of the optic nerve, 92 abnormal innervation of the lateral geniculate nucleus 93 and nystagmus. 94 The Oca 2p mutant allele results in oculocutaneous albinism 95 and is associated with cataract formation, which may also contribute to visual impairments in these mice. Moreover, light‐induced retinal degeneration occurs in albino mouse strains, and may be exacerbated in aging studies due to long‐term cumulative exposure to light. 96 , 97 Therefore age‐related albinism‐induced impairments in vision may exaggerate the performance deficits in the 5xFAD mice in visuo‐spatial tasks, such as the MWM.
4.4.1. The problem of dissociating the effects of transgenes from background genes on learning and memory processes
Since the first genetically modified mice were generated, the question has arisen as to whether the behavioral phenotypes of these mice were due to the genetic modification or due to the background genes. 98 , 99 , 100 The confounding of background gene effects with the effects of gene manipulations has serious repercussions for the reliability and validity of mouse models of neurodegenerative disorders. 101 , 102 A number of strategies have been proposed for dissociating the effects of gene manipulations on behavioral phenotypes from those of background genes. 54 , 100 , 103 , 104 , 105 First, the effects of mutant alleles with deleterious effects on sensori‐motor function or cognition can be removed. This is accomplished by screening mice for the mutant alleles and removal of mice that carry these alleles from analyses. Mutant alleles can also be bred out by selecting breeders with the non‐mutant allele, or by backcrossing mice onto an alternative genetic background without mutant alleles. Second, the robustness of the genetic modification on learning and memory, independent of genetic background, can be established by testing the same genetic modification on different background strains. Phenotypes that differ across genetic backgrounds may reveal epsistatic interactions, or the masking/exaggeration of behavioral phenotypes due to pre‐existing differences in the behavior of background strains. 54 , 55 , 106 , 107 Therefore, it is important to conduct comprehensive analyses of behavioral phenotypes within background strains used. 53 , 57 , 62 , 108 , 109 , 110 , 111 However, it should be noted that APP/PS1 transgenes in 5xFAD mice are not inserted within endogenous APP and PS1 loci, and thus the relative effects of endogenous and transgenic APP and PS1 genes cannot be dissociated in 5xFAD mice.
Among these strategies, the influence of background genes from SJL/J mice is commonly removed by backcrossing the 5xFAD transgenes for multiple generations onto an alternative genetic background. Although, the 5xFAD mice are now available on a congenic C57BL/6J background (B6.CgTg[APPSwFlLon,PSEN1*M146L*L286V]6799Vas/Mmjax), 5xFAD mice are still commonly used on a hybrid C57BL6/J x SJL/J background. It is possible that the continued use of the 5xFAD mice on the C57BL/6J x SJL/J hybrid background is because disease related phenotypes are more severe than on a C57BL/6J background. Indeed, Aβ‐pathology in 5xFAD mice is reduced 112 and cognitive impairments are less severe on a C57BL/6J background, suggesting that C57BL/6J mice confer alleles that protect against the development of AD‐related phenotypes. 54 Although, the variability in AD‐related phenotypes across genetic backgrounds in 5xFAD mice reduces reliability across studies, it has been successfully leveraged with recombinant BxD lines to identify genes that confer resilience against the development of AD‐related phenotypes. 54 , 55 Because of the complicated interactions between transgenes and background genes in the 5xFAD mice, it may be necessary to evaluate the performance of individual mice to dissociate the interaction of transgenes and background genes. 113 , 114
4.5. Motor impairments in aged 5xFAD mice confound measurement of learning and memory on the MWM and exaggerate performance impairments
The 5xFAD mice showed reduced swim speed at 12 and 15 months accompanied by a frequent inability to locate the escape platform during training. The 5xFAD mice also develop profound motor impairments in balance, grip strength, locomotor activity, and motor coordination starting at 9 months of age, which increase in severity from 12 to 15 months of age. 40 Therefore, it is likely that these motor impairments exacerbate cognitive impairment on visuo‐spatial tasks such as the MWM, in which measures of learning and memory depend on motor performance. These motor deficits in 5xFAD mice are due to the development of AD‐related neuropathology within the pyramidal and extra‐pyramidal systems of the brain and spinal cord. 15 , 115 We previously demonstrated that at 12 months of age, motor dysfunction in female 5xFAD mice is sufficient to impair swimming ability and reduce swim speed on the MWM. 71 The age‐related development of impaired swimming ability in 5xFAD mice, however, has not yet been characterized. Here, we show that swimming speed of 5xFAD mice in the MWM is unimpaired until 9 months of age, but at 12 and 15 months 5xFAD mice have reduced swim speeds. In addition, poor performance on measures of motor function 40 was associated with reduced swim speeds in 5xFAD mice. This supports previous work showing normal swim‐speed in 5xFAD mice up until 10 months of age. 24 , 25 , 26 Although less common, 5xFAD mice have been tested on the MWM after 12 months of age, 27 , 29 but these studies do not report swim speed, so the extent that impaired swimming influenced learning and memory performance is not known (however, see Ref. 28, for a non‐significant reduction in swim speed in 5xFAD mice at 12 months of age).
The impaired swimming ability in 5xFAD mice at 12 and 15 months of age confounds measures of performance on the MWM (latency, cumulative search error), and thus caution must be taken when interpreting these measures of MWM performance in 5xFAD mice after 12 months of age. Furthermore, 5xFAD mice showed impaired performance in visible platform training at 12–15 months, a non‐hippocampal dependent and less cognitively demanding version of the water maze, providing further evidence for sensori‐motor confounds effecting performance in 5xFAD mice. Performance impairments of 5xFAD mice at 12–15 months of age, however, were not replicated with the measure of distance traveled. Although distance traveled is considered largely independent of swim‐speed, the maximum swim distance possible for a given trial is constrained by swim speed and the maximum trial length (i.e., 60 s) when the escape platform is not found. We suspect that the differences between 5xFAD and WT mice were smaller for distance traveled than other behavioral measures at 12 and 15 months, because 5xFAD mice swam slower and often failed to reach the escape platform.
Although the impaired swimming ability may be viewed as a limitation of the 5xFAD mouse as an animal model of AD, motor dysfunction in 5xFAD mice may be an important behavioral phenotype in these mice 15 , 40 and a target for future drug studies. 116 Therefore, future pre‐clinical research with 5xFAD mice should assess age‐related decrements in motor behavior as well as cognitive function, particularly after 9 months of age.
4.5.1. The problem of dissociating age‐related cognitive and performance deficits in mouse models of AD
Learning and memory in mice are not measured directly but are inferred from performance on behavioral tasks. When mouse models of AD perform worse than WT control mice, we claim (infer) that they have a learning/memory deficit. 117 , 118 However, impaired performance in mouse models of AD on tests of learning and memory may be due to sensory or motor deficits, rather than cognitive deficits. 102 , 119 As stated succinctly by Cahill et al. 117 : “We ignore the learning/performance distinction at our peril”. Given that age‐related motor impairment and learning and memory impairment both occur between 12 and 15 months of age in 5xFAD mice, how can these motor and cognitive impairments be dissociated?
There are a number of possible approaches to dissociate cognitive and motor impairments. First, use cognitive tasks that do not rely heavily on motor performance. For example, although 5xFAD mice are not cognitively impaired in a conditioned olfactory digging task, they can complete this test up to 15 months of age in the presence of profound motor impairment. 41 For the MWM, it may be best to include visible platform training prior to hidden platform training, permitting a clearer picture of how swimming ability and learning of general task demands influences subsequent visuo‐spatial learning performance with a hidden platform. It may also be possible to use operant tasks 120 ; fear conditioning 121 , 122 or other learning and memory tasks that do not rely on swimming to dissociate motor and cognitive function in 5xFAD mice. 123 , 124 , 125 Second, pharmacological interventions can be used to dissociate motor from cognitive deficits. In this case, drugs can be used to selectively treat motor function to determine if cognitive performance is also restored. 126 , 127 , 128 Alternatively, agents to treat cognitive function can be used to determine if cognition is restored without affecting impairments in motor function. 129 , 130 Third, a number of multivariate statistical techniques can be potentially used to dissociate cognitive from motor deficits. 119 , 131 , 132
Finally, it is possible that the age‐related decline in motor and cognitive impairment in the 5xFAD mice are intricately intertwined and caused by the degeneration of common neurochemical pathways and can not be dissociated: they are two aspects of the progression of AD. 133 Similar combinations of motor and cognitive deficits occur in Parkinson's disease 134 , 135 , 136 and Huntington's disease, 137 and it is possible that effective drug treatments will improve both motor and cognitive functions in the 5xFAD mice. 138
4.6. General conclusions
This study provides a detailed analysis of learning and memory impairments in the 5xFAD mouse on the MWM from 3 to 15 months of age. We identified factors that influence the size of learning and memory impairments in 5xFAD mice including sex, albinism and motor dysfunction. These factors should be controlled in future pre‐clinical research with 5xFAD mice, to permit a more accurate assessment of transgene and treatment effects on behavior. We suspect that many other factors related to test procedure also contribute to performance of 5xFAD mice on the MWM, and thus warrant further study. Overall, we predict that reducing variability in performance unrelated to AD‐related transgenes will increase statistical power and improve the reliability of detecting learning impairments in 5xFAD mice across studies. Thus the results of this study will help better optimize the design of pre‐clinical research using 5xFAD mice on the MWM, and ultimately reduce the frequency of false‐positive or false‐negative findings concerning treatment efficacy.
Supporting information
Table S1: Experimental details and results for studies that test 5xFAD mice on the MWM.
Table S2: The numbers of male and female, 5xFAD and WT mice tested on the MWM at 3, 6, 9, 12 and 15 months of age.
Table S3: The number of pigmented and albino/pigment diluted, 5xFAD and WT mice tested on the MWM at 6–9 months of age.
Table S4: The number of pigmented and albino/pigment diluted, 5xFAD and WT mice tested on the MWM at 12–15 months of age.
Figure S1: The effect of reversing the escape platform location for 5xFAD and WT mice on the MWM. (A) Reversal difference scores for latency to locate the escape platform at 3–15 months of age (left to right). (B) Reversal difference scores for distance traveled and (C) cumulative search error. # = sex difference, p < 0.05,  = genotype difference, p < 0.05.
= genotype difference, p < 0.05.
Figure S2: Additional measures of performance for 5xFAD and WT mice on the MWM. (A) Memory performance of male and female 5xFAD and WT mice during the probe trial as measured by average distance from the escape platform location used in reversal training. Data are shown for male and female mice at 3, 6, 9, 12 and 15 months of age (left to right). (B) Swim‐speed of male and female 5xFAD and WT mice during visible‐platform training. (C) Percentage of trials on which male and female 5xFAD and WT mice failed to reach the escape platform across acquisition and reversal training. # = sex difference, p < 0.05,  = genotype difference, p < 0.05.
= genotype difference, p < 0.05.
Figure S3: Correlations between swim‐speed in the MWM, and measures of motor function and body‐weight in 5xFAD and WT mice. (A‐B) Correlations between swim‐speed in the MWM and latency to fall on the Rota‐rod, shown separately for WT and 5xFAD mice. Correlations are also shown between swim‐speed on the MWM and distance traveled in the open‐field (C‐D), rears in the open‐field (E‐F) and body weight (G‐H). * = p < 0.05. OFM = Open‐field maze.
Figure S4: Additional measures of performance for pigmented and albino 5xFAD and WT mice at 6–9 and 12–15 months of age. (A) Memory performance of pigmented and albino mice during the probe trial as measured by average distance from the escape platform location used in reversal training. Data are analyzed separately for males (A) and females (B) at 6–9 months of age. (C) Memory performance for albino and pigmented female mice at 12–15 months of age. Percentage of trials in which albino and pigmented (D) male and (E) female mice failed to reach the escape platform at 6–9 months of age. (F) Percentage of trials in which albino and pigmented female mice failed to reach the escape platform at 12–15 months of age. ^=albinism difference, p < 0.05.
Figure S5: Learning and memory performance for female 5xFAD and WT pigmented and albino mice at 12–15 months of age. (A‐B) Learning performance (latency, distance and cumulative search error) of female albino and pigmented WT and 5xFAD mice. Performance is shown for both acquisition (A1‐A3) and reversal training (R1‐R3), separated by a dashed line. (C) Memory performance during the probe trial for albino and pigmented mice as measured by percent time in pool quadrants. (D) Swim‐speed and distance traveled in visible platform training during acquisition and reversal trials for female pigmented and albino mice. ^=albinism difference, p < 0.05.
ACKNOWLEDGMENTS
This research was supported by the Natural Sciences and Engineering Council (NSERC) and Alzheimer's Association to Richard E. Brown, and Alzheimer Society of Canada to Timothy P. O'Leary. We would like to thank Rhian Gunn for assistance with mouse breeding and husbandry, and Kurt Stover & Hector Mantolino for assistance with data collection.
O'Leary TP, Brown RE. Visuo‐spatial learning and memory impairments in the 5xFAD mouse model of Alzheimer's disease: Effects of age, sex, albinism, and motor impairments. Genes, Brain and Behavior. 2022;21(4):e12794. doi: 10.1111/gbb.12794
Funding information Alzheimer Society of Canada; Alzheimer's Association; Natural Sciences and Engineering Research Council of Canada
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author (Richard E. Brown) upon reasonable request.
REFERENCES
- 1. Khan ZU, Martín‐Montañez E, Navarro‐Lobato I, Muly EC. Memory deficits in aging and neurological diseases. Prog Mol Biol Transl Sci. 2014;122:1‐29. [DOI] [PubMed] [Google Scholar]
- 2. Lane CA, Hardy J, Schott JM. Alzheimer's disease. Eur J Neurol. 2018;25:59‐70. [DOI] [PubMed] [Google Scholar]
- 3. Scheltens P, Blennow K, Breteler MMB, et al. Alzheimer's disease. Lancet. 2016;388:505‐517. [DOI] [PubMed] [Google Scholar]
- 4. Toepper M. Dissociating normal aging from Alzheimer's disease: a view from cognitive neuroscience. J Alzheimer's Dis. 2017;57:331‐352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Weintraub S, Wicklund SH, Salmon DP. The neuropsychological profile of Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2:a006171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pai M‐C, Jacobs WJ. Topographical disorientation in community‐residing patients with Alzheimer's disease. Int J Geriatr Psychiatry. 2004;19:250‐255. [DOI] [PubMed] [Google Scholar]
- 7. Zanco M, Plácido J, Marinho V, et al. Spatial navigation in the elderly with Alzheimer's disease: a cross‐sectional study. J Alzheimers Dis. 2018;66:1683‐1694. [DOI] [PubMed] [Google Scholar]
- 8. Allison SL, Fagan AM, Morris JC, Head D. Spatial navigation in preclinical Alzheimer's disease. J Alzheimers Dis. 2016;52:77‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Coughlan G, Laczó J, Hort J, Minihane A‐M, Hornberger M. Spatial navigation deficits ‐ overlooked cognitive marker for preclinical Alzheimer disease? Nat Rev Neurol. 2018;14:496‐506. [DOI] [PubMed] [Google Scholar]
- 10. Götz J, Bodea LG, Goedert M. Rodent models for Alzheimer disease. Nat Rev Neurosci. 2018;19:583‐598. [DOI] [PubMed] [Google Scholar]
- 11. Jankowsky JL, Zheng H. Practical considerations for choosing a mouse model of Alzheimer's disease. Mol Neurodegen. 2017;12:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Scearce‐Levie K, Sanchez PE, Lewcock JW. Leveraging preclinical models for the development of Alzheimer disease therapeutics. Nat Rev Drug Discov. 2020;19:447‐462. [DOI] [PubMed] [Google Scholar]
- 13. Darvesh S, Reid GA. Reduced fibrillar β‐amyloid in subcortical structures in a butyrylcholinesterase‐knockout Alzheimer disease mouse model. Chem Biol Interact. 2016;259:307‐312. [DOI] [PubMed] [Google Scholar]
- 14. Oakley H, Cole SL, Logan S, et al. Intraneuronal beta‐amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer's disease mutations: potential factors in amyloid plaque formation. J Neurosci. 2006;26:10129‐10140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jawhar S, Trawicka A, Jenneckens C, Bayer TA, Wirths O. Motor deficits, neuron loss, and reduced anxiety coinciding with axonal degeneration and intraneuronal Aβ aggregation in the 5XFAD mouse model of Alzheimer's disease. Neurobiol Aging. 2012;33:29‐40. [DOI] [PubMed] [Google Scholar]
- 16. Ardestani PM, Evans A, Yi B, Nguyen T, Coutellier L, Shamloo M. Modulation of neuroinflammation and pathology in the 5XFAD mouse model of Alzheimer's disease using a biased and selective beta‐1 adrenergic receptor partial agonist. Neuropharmacology. 2017;116:371‐386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sosna J, Philipp S, Albay R, et al. Early long‐term administration of the CSF1R inhibitor PLX3397 ablates microglia and reduces accumulation of intraneuronal amyloid, neuritic plaque deposition and pre‐fibrillar oligomers in 5XFAD mouse model of Alzheimer's disease. Mol Neurodegen. 2018;13:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Girard SD, Jacquet M, Baranger K, et al. Onset of hippocampus‐dependent memory impairments in 5XFAD transgenic mouse model of Alzheimer's disease. Hippocampus. 2014;24:762‐772. [DOI] [PubMed] [Google Scholar]
- 19. Macdonald IR, DeBay DR, Reid GA, et al. Early detection of cerebral glucose uptake changes in the 5XFAD mouse. Curr Alzheimer Res. 2014;11:450‐460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kaczorowski CC, Sametsky E, Shah S, Vassar R, Disterhoft JF. Mechanisms underlying basal and learning‐related intrinsic excitability in a mouse model of Alzheimer's disease. Neurobiol Aging. 2011;32:1452‐1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kimura R, Ohno M. Impairments in remote memory stabilization precede hippocampal synaptic and cognitive failures in 5XFAD Alzheimer mouse model. Neurobiol Dis. 2009;33:229‐235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Creighton SD, Mendell AL, Palmer D, et al. Dissociable cognitive impairments in two strains of transgenic Alzheimer's disease mice revealed by a battery of object‐based tests. Science Reports. 2019;9:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Devi L, Ohno M. Genetic reductions of beta‐site amyloid precursor protein‐cleaving enzyme 1 and amyloid‐beta ameliorate impairment of conditioned taste aversion memory in 5XFAD Alzheimer's disease model mice. Eur J Neurosci. 2010;31:110‐118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Flanigan TJ, Xue Y, Kishan Rao S, Dhanushkodi A, McDonald MP. Abnormal vibrissa‐related behavior and loss of barrel field inhibitory neurons in 5xFAD transgenics. Genes Brain Behav. 2014;13:488‐500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ohno M, Chang L, Tseng W, et al. Temporal memory deficits in Alzheimer's mouse models: rescue by genetic deletion of BACE1. Eur J Neurosci. 2006;23:251‐260. [DOI] [PubMed] [Google Scholar]
- 26. Kanno T, Tsuchiya A, Nishizaki T. Hyperphosphorylation of tau at Ser396 occurs in the much earlier stage than appearance of learning and memory disorders in 5XFAD mice. Behav Brain Res. 2014;274:302‐306. [DOI] [PubMed] [Google Scholar]
- 27. Paesler K, Xie K, Hettich MM, et al. Limited effects of an eIF2αS51A allele on neurological impairments in the 5xFAD mouse model of Alzheimer's disease. Neural Plast. 2015;2015:825157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bouter Y, Kacprowski T, Weissmann R, et al. Deciphering the molecular profile of plaques, memory decline and neuron loss in two mouse models for Alzheimer's disease by deep sequencing. Front Aging Neurosci. 2014;6:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hüttenrauch M, Walter S, Kaufmann M, Weggen S, Wirths O. Limited effects of prolonged environmental enrichment on the pathology of 5XFAD mice. Mol Neurobiol. 2017;54:6542‐6555. [DOI] [PubMed] [Google Scholar]
- 30. Sawmiller D, Li S, Mori T, et al. Beneficial effects of a pyrroloquinoline quinone‐containing dietary formulation on motor deficiency, cognitive decline and mitochondrial dysfunction in a mouse model of Alzheimer's disease. Heliyon. 2017;3:e00279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Urano T, Tohda C. Icariin improves memory impairment in Alzheimer's disease model mice (5xFAD) and attenuates amyloid β‐induced neurite atrophy. Phytother Res. 2010;24:1658‐1663. [DOI] [PubMed] [Google Scholar]
- 32. Gee MS, Son SH, Jeon SH, et al. A selective p38α/β MAPK inhibitor alleviates neuropathology and cognitive impairment, and modulates microglia function in 5XFAD mouse. Alzheimers Res Ther. 2020;12:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kong Y, Peng Q, Lv N, et al. Paeoniflorin exerts neuroprotective effects in a transgenic mouse model of Alzheimer's disease via activation of adenosine A1 receptor. Neurosci Lett. 2020;730:135016. [DOI] [PubMed] [Google Scholar]
- 34. Lebois EP, Schroeder JP, Esparza TJ, et al. Disease‐modifying effects of M1 muscarinic acetylcholine receptor activation in an Alzheimer's disease mouse model. ACS Chem Nerosci. 2017;8:1177‐1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schneider F, Baldauf K, Wetzel W, Reymann KG. Effects of methylphenidate on the behavior of male 5xFAD mice. Pharmacol Biochem Behav. 2015;128:68‐77. [DOI] [PubMed] [Google Scholar]
- 36. Wu Y, Gong Y, Luan Y, et al. BHBA treatment improves cognitive function by targeting pleiotropic mechanisms in transgenic mouse model of Alzheimer's disease. FASEB J. 2020;34:1412‐1429. [DOI] [PubMed] [Google Scholar]
- 37. Rae EA, Brown RE. The problem of genotype and sex differences in life expectancy in transgenic AD mice. Neurosci Biobehav Rev. 2015;57:238‐251. [DOI] [PubMed] [Google Scholar]
- 38. Webster SJ, Bachstetter AD, Nelson PT, Schmitt FA, Van Eldik LJ. Using mice to model Alzheimer's dementia: an overview of the clinical disease and the preclinical behavioral changes in 10 mouse models. Front Genet. 2014;5(88):88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. O'Leary TP, Shin S, Fertan E, et al. Reduced acoustic startle response and peripheral hearing loss in the 5xFAD mouse model of Alzheimer's disease. Genes Brain Behav. 2017;16:554‐563. [DOI] [PubMed] [Google Scholar]
- 40. O'Leary TP, Mantolino HM, Stover KR, Brown RE. Age‐related deterioration of motor function in male and female 5xFAD mice from 3 to 16 months of age. Genes Brain Behav. 2020a;19:e12538. [DOI] [PubMed] [Google Scholar]
- 41. O'Leary TP, Stover KR, Mantolino HM, Darvesh S, Brown RE. Intact olfactory memory in the 5xFAD mouse model of Alzheimer's disease from 3 to 15 months of age. Behav Brain Res. 2020b;393:112731. [DOI] [PubMed] [Google Scholar]
- 42. Roddick KM, Schellinck HM, Brown RE. Olfactory delayed matching to sample performance in mice: sex differences in the 5XFAD mouse model of Alzheimer's disease. Behav Brain Res. 2014;270:165‐170. [DOI] [PubMed] [Google Scholar]
- 43. Roddick KM, Roberts AD, Schellinck HM, Brown RE. Sex and genotype differences in odor detection in the 3×Tg‐AD and 5XFAD mouse models of Alzheimer's disease at 6 months of age. Chem Senses. 2016;41:433‐440. [DOI] [PubMed] [Google Scholar]
- 44. Alzheimer's Association . 2020 Alzheimer's disease facts and figures. Alzheimers Dement. 2020;16:391‐460. [Google Scholar]
- 45. Barnes LL, Wilson RS, Bienias JL, Schneider JA, Evans DA, Bennett DA. Sex differences in the clinical manifestations of Alzheimer disease pathology. Arch Gen Psychiatry. 2005;62:685‐691. [DOI] [PubMed] [Google Scholar]
- 46. Henderson VW, Buckwalter JG. Cognitive deficits of men and women with Alzheimer's disease. Neurology. 1994;44:90‐96. [DOI] [PubMed] [Google Scholar]
- 47. Irvine K, Laws KR, Gale TM, Kondel TK. Greater cognitive deterioration in women than men with Alzheimer's disease: a meta analysis. J Clin Exp Neuropsychol. 2012;34:989‐998. [DOI] [PubMed] [Google Scholar]
- 48. Bhattacharya S, Haertel C, Maelicke A, Montag D. Galantamine slows down plaque formation and behavioral decline in the 5XFAD mouse model of Alzheimer's disease. PloS One. 2014;9:e89454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bundy JL, Vied C, Badger C, Nowakowski RS. Sex‐biased hippocampal pathology in the 5XFAD mouse model of Alzheimer's disease: a multi‐omic analysis. J Comp Neurol. 2019;527:462‐475. [DOI] [PubMed] [Google Scholar]
- 50. Sadleir KR, Eimer WA, Cole SL, Vassar R. Aβ reduction in BACE1 heterozygous null 5XFAD mice is associated with transgenic APP level. Mol Neurodegen. 2015;10:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gendron WH, Fertan E, Pelletier S, et al. Age related weight loss in female 5xFAD mice from 3 to 12 months of age. Behav Brain Res. 2021;406:3214. [DOI] [PubMed] [Google Scholar]
- 52. Benice TS, Rizk A, Kohama S, Pfankuch T, Raber J. Sex‐differences in age‐related cognitive decline in C57BL/6J mice associated with increased brain microtubule‐associated protein 2 and synaptophysin immunoreactivity. Neuroscience. 2006;137(2):413‐423. [DOI] [PubMed] [Google Scholar]
- 53. Kim DH, Jang YS, Jeon WK, Han JS. Assessment of cognitive phenotyping in inbred, genetically modified mice, and transgenic mouse models of Alzheimer's disease. Exp Neurobiol. 2019;28:146‐157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Neuner SM, Heuer SE, Huentelman MJ, O'Connell KMS, Kaczorowski CC. Harnessing genetic complexity to enhance translatability of Alzheimer's disease mouse models: a path toward precision medicine. Neuron. 2019;101:399‐411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. O'Connell KMS, Ouellette AR, Neuner SM, Dunn AR, Kaczorowski CC. Genetic background modifies CNS‐mediated sensorimotor decline in the AD‐BXD mouse model of genetic diversity in Alzheimer's disease. Genes Brain Behav. 2019;18:e12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Brayton CF, Treuting PM, Ward JM. Pathobiology of aging mice and GEM: background strains and experimental design. Vet Pathol. 2012;49:85‐105. [DOI] [PubMed] [Google Scholar]
- 57. Qosa H, Kaddoumi A. Effect of mouse strain as a background for Alzheimer's disease models on the clearance of amyloid‐β. J Syst Integr Neurosci. 2016;2:135‐140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sundberg JP, Annerose Berndt A, Sundberg BA, et al. The mouse as a model for understanding chronic diseases of aging: the histopathologic basis of aging in inbred mice. Pathobiol Aging Age‐Relat Dis. 2011;1:7179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chin J. Selecting a mouse model of Alzheimer's disease. Methods Mol Biol. 2011;670:169‐189. [DOI] [PubMed] [Google Scholar]
- 60. Esquerda‐Canals E, Montoliu‐Gaya L, Güell‐Bosch J, Villegas S. Mouse models of Alzheimer's disease. J Alzheimers Dis. 2017;57:1171‐1183. [DOI] [PubMed] [Google Scholar]
- 61. Kõks S, Dogan S, Tuna BG, González‐Navarro H, Potter P, Vandenbroucke RE. Mouse models of ageing and their relevance to disease. Mech Ageing Dev. 2016;160:41‐53. [DOI] [PubMed] [Google Scholar]
- 62. Sultana R, Ogundele OM, Lee CC. Contrasting characteristic behaviours among common laboratory mouse strains. R Soc Open Sci. 2019;6:190574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Brayton C. Spontaneous diseases in commonly used mouse strains. The Mouse in Biomedical Research. History, Wild Mice, and Genetics. American College of Laboratory Animal Medicine. Vol 2. 2nd ed. Elsevier; 2007:623‐717. [Google Scholar]
- 64. Clapcote SJ, Lazar NL, Bechard AR, Roder JC. Effects of the rd1 mutation and host strain on hippocampal learning in mice. Behav Genet. 2005;35:591‐601. [DOI] [PubMed] [Google Scholar]
- 65. Brown RE, Wong AA. The influence of visual ability on learning and memory performance in 13 strains of mice. Learn Mem. 2007;14:134‐144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Garcia MF, Gordon MN, Hutton M, et al. The retinal degeneration (rd) gene seriously impairs spatial cognitive performance in normal and Alzheimer's transgenic mice. Neuroreport. 2004;15:73‐77. [DOI] [PubMed] [Google Scholar]
- 67. Yassine N, Lazaris A, Dorner‐Ciossek C, et al. Detecting spatial memory deficits beyond blindness in tg2576 Alzheimer mice. Neurobiol Aging. 2013;34:716‐730. [DOI] [PubMed] [Google Scholar]
- 68. Grant RA, Wong AA, Fertan E, Brown RE. Whisker exploration behaviours in the 5xFAD mouse are affected by sex and retinal degeneration. Genes Brain Behav. 2020;19:e12532. [DOI] [PubMed] [Google Scholar]
- 69. Prusky GT, Harker KT, Douglas RM, Whishaw IQ. Variation in visual acuity within pigmented, and between pigmented and albino rat strains. Behav Brain Res. 2002;136:339‐348. [DOI] [PubMed] [Google Scholar]
- 70. Wong AA, Brown RE. Visual detection, pattern discrimination and visual acuity in 14 strains of mice. Genes Brain Behav. 2006;5:389‐403. [DOI] [PubMed] [Google Scholar]
- 71. O'Leary TP, Robertson A, Chipman PH, Rafuse VF, Brown RE. Motor function deficits in the 12 month‐old female 5xFAD mouse model of Alzheimer's disease. Behav Brain Res. 2018;337:256‐263. [DOI] [PubMed] [Google Scholar]
- 72. Gallagher M, Burwell R, Burchinal M. Severity of spatial learning impairment in aging: development of a learning index for performance in the Morris water maze. Behav Neurosci. 1993;107:618‐626. [DOI] [PubMed] [Google Scholar]
- 73. Lee M, Lee HJ, Jeong YJ, et al. Age dependency of mGluR5 availability in 5xFAD mice measured by PET. Neurobiol Aging. 2019;84:208‐216. [DOI] [PubMed] [Google Scholar]
- 74. Schneider F, Baldauf K, Wetzel W, Reymann KG. Behavioral and EEG changes in male 5xFAD mice. Physiol Behav. 2014;135:25‐33. [DOI] [PubMed] [Google Scholar]
- 75. Aytan N, Choi J‐K, Carreras I, et al. Protective effects of 7,8‐dihydroxyflavone on neuropathological and neurochemical changes in a mouse model of Alzheimer's disease. Eur J Pharmacol. 2018;828:9‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Fiol‐deRoque MA, Gutierrez‐Lanza R, Terés S, et al. Cognitive recovery and restoration of cell proliferation in the dentate gyrus in the 5XFAD transgenic mice model of Alzheimer's disease following 2‐hydroxy‐DHA treatment. Biogerontology. 2013;14:763‐775. [DOI] [PubMed] [Google Scholar]
- 77. Lee K, Kim H, An K, et al. Replenishment of microRNA‐188‐5p restores the synaptic and cognitive deficits in 5XFAD mouse model of Alzheimer's disease. Sci Rep. 2016;6:34433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Jeon SG, Kim YJ, Kim KA, Mook‐Jung I, Moon M. Visualization of altered hippocampal connectivity in an animal model of Alzheimer's disease. Mol Neurobiol. 2018;55:7886‐7889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. McDonald RJ, Balog RJ, Lee JQ, Stuart EE, Carrels BB, Hong NS. Rats with ventral hippocampal damage are impaired at various forms of learning including conditioned inhibition, spatial navigation, and discriminative fear conditioning to similar contexts. Behav Brain Res. 2018;351:138‐151. [DOI] [PubMed] [Google Scholar]
- 80. Bannerman DM, Yee BK, Good MA, Heupel MJ, Iversen SD, Rawlins JNP. Double dissociation of function within the hippocampus: a comparison of dorsal, ventral, and complete hippocampal cytotoxic lesions. Behav Neurosci. 1999;113:1170‐1188. [DOI] [PubMed] [Google Scholar]
- 81. Moser E, Moser MB, Andersen P. Spatial learning impairment parallels the magnitude of dorsal hippocampal lesions, but is hardly present following ventral lesions. J Neurosci. 1993;13:3916‐3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Pothuizen HHJ, Zhang WN, Jongen‐Rêlo AL, Feldon J, Yee BK. Dissociation of function between the dorsal and the ventral hippocampus in spatial learning abilities of the rat: a within‐subject, within‐task comparison of reference and working spatial memory. Eur J Neurosci. 2004;19:705‐712. [DOI] [PubMed] [Google Scholar]
- 83. Cho WH, Park JC, Chung C, Jeon WK, Han JS. Learning strategy preference of 5XFAD transgenic mice depends on the sequence of place/spatial and cued training in the water maze task. Behav Brain Res. 2014;273:116‐122. [DOI] [PubMed] [Google Scholar]
- 84. Pooters T, Gantois I, Vermaercke B, D'Hooge R. Inability to acquire spatial information and deploy spatial search strategies in mice with lesions in dorsomedial striatum. Behav Brain Res. 2016;298:134‐141. [DOI] [PubMed] [Google Scholar]
- 85. Devi L, Ohno M. Effects of BACE1 haploinsufficiency on APP processing and Aβ concentrations in male and female 5XFAD Alzheimer mice at different disease stages. Neuroscience. 2015;307:128‐137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Reid GA, Darvesh S. Butyrylcholinesterase‐knockout reduces brain deposition of fibrillar β‐amyloid in an Alzheimer mouse model. Neuroscience. 2015;298:424‐435. [DOI] [PubMed] [Google Scholar]
- 87. Izquierdo A, Brigman JL, Radke AK, Rudebeck PH, Holmes A. The neural basis of reversal learning: an updated perspective. Neuroscience. 2017;345:12‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Yeritsyan N, Lehmann K, Puk O, Graw J, Löwel S. Visual capabilities and cortical maps in BALB/c mice. Eur J Neurosci. 2012;36:2801‐2811. [DOI] [PubMed] [Google Scholar]
- 89. O'Leary TP, Savoie V, Brown RE. Learning, memory and search strategies of inbred mouse strains with different visual abilities in the Barnes maze. Behav Brain Res. 2011;216:531‐542. [DOI] [PubMed] [Google Scholar]
- 90. Baker KB, Wray SP, Ritter R, Mason S, Lanthorn TH, Savelieva KV. Male and female Fmr1 knockout mice on C57 albino background exhibit spatial learning and memory impairments. Genes Brain and Behavior. 2010;9:562‐574. [DOI] [PubMed] [Google Scholar]
- 91. Donatien P, Jeffery G. Correlation between rod photoreceptor numbers and levels of ocular pigmentation. Investigative Ophthalmology and Visual Science. 2002;43:1198‐1203. [PubMed] [Google Scholar]
- 92. Rice DS, Williams RW, Goldowitz D. Genetic control of retinal projections in inbred strains of albino mice. J Comp Neurol. 1995;354:459‐469. [DOI] [PubMed] [Google Scholar]
- 93. Rebsam A, Bhansali P, Mason CA. Eye‐specific projections of retinogeniculate axons are altered in albino mice. J Neurosci. 2012;32:4821‐4826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Traber GL, Chen CC, Huang YY, et al. Albino mice as an animal model for infantile nystagmus syndrome. Invest Opthalmol Visual Sci. 2012;53:5737‐5747. [DOI] [PubMed] [Google Scholar]
- 95. Shoji H, Kiniwa Y, Okuyama R, Yang M, Higuchi K, Mori M. A nonsense nucleotide substitution in the oculocutaneous albinism II gene underlies the original pink‐eyed dilution allele (Oca2p) in mice. Exp Anim. 2015;64:171‐179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Bell BA, Kaul C, Boniha VL, Rayborn ME, Shadrach K, Hollyfield JG. The BALB/c mouse: effect of standard vivarium lighting on retinal pathology during aging. Exp Eye Res. 2015;135:192‐205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Leinonen H, Tanila H. Vision in laboratory rodents –tools to measure it and implications in research. Behav Brain Res. 2018;352:172‐182. [DOI] [PubMed] [Google Scholar]
- 98. Gerlai R. Gene‐targeting studies of mammalian behavior: is it the mutation or the background genotype? Trends Neurosci. 1996;19:177‐181. [DOI] [PubMed] [Google Scholar]
- 99. Wolfer DP, Lipp HP. Dissecting the behaviour of transgenic mice: is it the mutation, the genetic background, or the environment? Exp Physiol. 2000;85:627‐634. [PubMed] [Google Scholar]
- 100. Wolfer DP, Crusio WE, Lipp HP. Knockout mice: simple solutions to the problems of genetic background and flanking genes. Trends Neurosci. 2002;25:336‐340. [DOI] [PubMed] [Google Scholar]
- 101. Kafkafi N, Agassi J, Chesler EJ, et al. Reproducibility and replicability of rodent phenotyping in preclinical studies. Neurosci Biobehav Rev. 2018;87:218‐232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Schellinck HM, Cyr DP, Brown RE. How many ways can mouse behavioral experiments go wrong? Confounding variables in mouse models of neurodegenerative diseases and how to control them. Advan Study Behav. 2010;41:255‐366. [Google Scholar]
- 103. Gerlai R. Gene targeting: technical confounds and potential solutions in behavioral brain research. Behav Brain Res. 2001;125:13‐21. [DOI] [PubMed] [Google Scholar]
- 104. Lathe R. Mice, gene targeting and behaviour: more than just genetic background. Trends Neurosci. 1996;19:183‐186. [DOI] [PubMed] [Google Scholar]
- 105. Sellers RS. The gene or not the gene—that is the question: understanding the genetically engineered mouse phenotype. Vet Pathol. 2012;49:5‐15. [DOI] [PubMed] [Google Scholar]
- 106. Fertan E, Wong AA, Purdon MK, Weaver ICG, Brown RE. The effect of background strain on the behavioral phenotypes of the MDGA2 +/− mouse model of autism spectrum disorder. Genes Brain Behav. 2020;18:e12696. [DOI] [PubMed] [Google Scholar]
- 107. Lassalle JM, Halley H, Daumas S, Verret L, Francés B. Effects of the genetic background on cognitive performances of TG2576 mice. Behav Brain Res. 2008;191:104‐110. [DOI] [PubMed] [Google Scholar]
- 108. Fisher EMC, Bannerman DM. Mouse models of neurodegeneration: know your question, know your mouse. Sci Transl Med. 2019;11(493):1818. [DOI] [PubMed] [Google Scholar]
- 109. Moy SS, Nadler JJ, Young NB, et al. Mouse behavioral tasks relevant to autism: phenotypes of 10 inbred strains. Behav Brain Res. 2007;176:4‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Moy SS, Nadler JJ, Young NB, et al. Social approach and repetitive behavior in eleven inbred mouse strains. Behav Brain Res. 2008;191:118‐129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Võikar V, Kõks S, Vasar E, Rauvala H. Strain and gender differences in the behavior of mouse lines commonly used in transgenic studies. Physiol Behav. 2001;72:271‐281. [DOI] [PubMed] [Google Scholar]
- 112. Mutant Mouse Resource Center . (2020). B6. CgTg(APPSwFlLon, PSEN1*M146L*L286V) 6799Vas/Mmjax: Strain Detail Sheet. https://www.jax.org/strain/008730.
- 113. Gallistel CR, Fairhurst S, Balsam P. The learning curve: implications of a quantitative analysis. Proc Natl Acad Sci U S A. 2004;101:13124‐13131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Schauwecker PE. The relevance of individual genetic background and its role in animal models of epilepsy. Epilepsy Res. 2011;97:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Chu T‐H, Cummins K, Sparling JS, et al. Axonal and myelinic pathology in 5xFAD Alzheimer's mouse spinal cord. PloS One. 2017;12:e0188218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Wagner JM, Sichler ME, Schleicher EM, et al. Analysis of motor function in the Tg4‐42 mouse model of Alzheimer's disease. Front Behav Neurosci. 2019;13:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Cahill L, McGaugh JL, Weinberger NM. The neurobiology of learning and memory: some reminders to remember. Trends Neurosci. 2001;24:578‐581. [DOI] [PubMed] [Google Scholar]
- 118. Sarter M. Animal cognition: defining the issues. Neurosci Biobehav Rev. 2004;28:645‐650. [DOI] [PubMed] [Google Scholar]
- 119. Wolfer DP, Stagljar‐Bozicevic M, Errington ML, Lipp HP. Spatial memory and learning in transgenic mice: fact or artifact. News Physiol Sci. 1998;13:118‐123. [DOI] [PubMed] [Google Scholar]
- 120. Yhnell E, Heuer A. Automated operant assessments of Huntington's disease mouse models. Methods Mol Biol. 2018;1780:143‐162. [DOI] [PubMed] [Google Scholar]
- 121. Holahan MR, White NM. Conditioned memory modulation, freezing, and avoidance as measures of amygdala‐mediated conditioned fear. Neurobiol Learn Mem. 2002;77:250‐275. [DOI] [PubMed] [Google Scholar]
- 122. Maren S. Pavlovian fear conditioning as a behavioral assay for hippocampus and amygdala function: cautions and caveats. Eur J Neurosci. 2008;28:1661‐1666. [DOI] [PubMed] [Google Scholar]
- 123. Carvalho RC, Patti CC, Takatsu‐Coleman AL, et al. Effects of reserpine on the plus‐maze discriminative avoidance task: dissociation between memory and motor impairments. Brain Res. 2006;1122:179‐183. [DOI] [PubMed] [Google Scholar]
- 124. De Coninck M, Van Dam D, Van Ginneken C, De Deyn PP. Adapted Morris water maze protocol to prevent interference from confounding motor deficits on cognitive functioning. Somatosens Mot Res. 2017;34:172‐178. [DOI] [PubMed] [Google Scholar]
- 125. Kantak SS, Winstein CJ. Learning‐performance distinction and memory processes for motor skills: a focused review and perspective. Behav Brain Res. 2012;228:219‐231. [DOI] [PubMed] [Google Scholar]
- 126. Duty S, Jenner P. Animal models of Parkinson's disease: a source of novel treatments and clues to the cause of the disease. Br J Pharmacol. 2011;164:1357‐1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Massaquoi MS, Liguore WA, Churchill MJ, Moore C, Melrose HL, Meshul CK. Gait deficits and loss of striatal tyrosine hydroxlase/Trk‐B are restored following 7,8‐dihydroxyflavone treatment in a progressive MPTP mouse model of Parkinson's disease. Neuroscience. 2020;433:53‐71. [DOI] [PubMed] [Google Scholar]
- 128. Zhang Y, Qin L, Xie J, Li J, Wang C. Eupatilin prevents behavioral deficits and dopaminergic neuron degeneration in a Parkinson's disease mouse model. Life Sci. 2020;253:117745. [DOI] [PubMed] [Google Scholar]
- 129. Stanciu GD, Luca A, Rusu RN, et al. Alzheimer's disease pharmacotherapy in relation to cholinergic system involvement. Biomolecules. 2019;10:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Wang H, Zhang H. Reconsideration of anticholinesterase therapeutic strategies against Alzheimer's disease. ACS Chem Nerosci. 2019;10:852‐862. [DOI] [PubMed] [Google Scholar]
- 131. Castilla‐Ortega E, Sánchez‐López J, Hoyo‐Becerra C, et al. Exploratory, anxiety and spatial memory impairments are dissociated in mice lacking the LPA1 receptor. Neurobiol Learn Mem. 2010;94:73‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Martin LA, Goldowitz D, Mittleman G. The cerebellum and spatial ability: dissection of motor and cognitive components with a mouse model system. Eur J Neurosci. 2003;18:2002‐2010. [DOI] [PubMed] [Google Scholar]
- 133. Wirths O, Bayer TA. Motor impairment in Alzheimer's disease and transgenic Alzheimer's disease mouse models. Genes Brain Behav. 2008;7(1):1‐5. [DOI] [PubMed] [Google Scholar]
- 134. Tan SH, Karri V, Tay NWR, et al. Emerging pathways to neurodegeneration: dissecting the critical molecular mechanisms in Alzheimer's disease, Parkinson's disease. Biomed Pharmacother. 2019;111:765‐777. [DOI] [PubMed] [Google Scholar]
- 135. Wang YX, Zhao J, Li DK, et al. Associations between cognitive impairment and motor dysfunction in Parkinson's disease. Brain Behav. 2017;7:e00719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Xie A, Gao J, Xu L, Meng D. Shared mechanisms of neurodegeneration in Alzheimer's disease and Parkinson's disease. Biomed Res Int. 2014;2014:648740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Van Raamsdonk JM, Pearson J, Slow EJ, Hossain SM, Leavitt BR, Hayden MR. Cognitive dysfunction precedes neuropathology and motor abnormalities in the YAC128 mouse model of Huntington's disease. J Neurosci. 2005;25:4169‐4180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Liu H, Li S, Yang C, et al. D‐serine ameliorates motor and cognitive impairments in β‐amyloid 1‐42 injected mice by inhibiting JNK signaling pathway. J Chem Neuroanat. 2020;109:101852. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Experimental details and results for studies that test 5xFAD mice on the MWM.
Table S2: The numbers of male and female, 5xFAD and WT mice tested on the MWM at 3, 6, 9, 12 and 15 months of age.
Table S3: The number of pigmented and albino/pigment diluted, 5xFAD and WT mice tested on the MWM at 6–9 months of age.
Table S4: The number of pigmented and albino/pigment diluted, 5xFAD and WT mice tested on the MWM at 12–15 months of age.
Figure S1: The effect of reversing the escape platform location for 5xFAD and WT mice on the MWM. (A) Reversal difference scores for latency to locate the escape platform at 3–15 months of age (left to right). (B) Reversal difference scores for distance traveled and (C) cumulative search error. # = sex difference, p < 0.05,  = genotype difference, p < 0.05.
= genotype difference, p < 0.05.
Figure S2: Additional measures of performance for 5xFAD and WT mice on the MWM. (A) Memory performance of male and female 5xFAD and WT mice during the probe trial as measured by average distance from the escape platform location used in reversal training. Data are shown for male and female mice at 3, 6, 9, 12 and 15 months of age (left to right). (B) Swim‐speed of male and female 5xFAD and WT mice during visible‐platform training. (C) Percentage of trials on which male and female 5xFAD and WT mice failed to reach the escape platform across acquisition and reversal training. # = sex difference, p < 0.05,  = genotype difference, p < 0.05.
= genotype difference, p < 0.05.
Figure S3: Correlations between swim‐speed in the MWM, and measures of motor function and body‐weight in 5xFAD and WT mice. (A‐B) Correlations between swim‐speed in the MWM and latency to fall on the Rota‐rod, shown separately for WT and 5xFAD mice. Correlations are also shown between swim‐speed on the MWM and distance traveled in the open‐field (C‐D), rears in the open‐field (E‐F) and body weight (G‐H). * = p < 0.05. OFM = Open‐field maze.
Figure S4: Additional measures of performance for pigmented and albino 5xFAD and WT mice at 6–9 and 12–15 months of age. (A) Memory performance of pigmented and albino mice during the probe trial as measured by average distance from the escape platform location used in reversal training. Data are analyzed separately for males (A) and females (B) at 6–9 months of age. (C) Memory performance for albino and pigmented female mice at 12–15 months of age. Percentage of trials in which albino and pigmented (D) male and (E) female mice failed to reach the escape platform at 6–9 months of age. (F) Percentage of trials in which albino and pigmented female mice failed to reach the escape platform at 12–15 months of age. ^=albinism difference, p < 0.05.
Figure S5: Learning and memory performance for female 5xFAD and WT pigmented and albino mice at 12–15 months of age. (A‐B) Learning performance (latency, distance and cumulative search error) of female albino and pigmented WT and 5xFAD mice. Performance is shown for both acquisition (A1‐A3) and reversal training (R1‐R3), separated by a dashed line. (C) Memory performance during the probe trial for albino and pigmented mice as measured by percent time in pool quadrants. (D) Swim‐speed and distance traveled in visible platform training during acquisition and reversal trials for female pigmented and albino mice. ^=albinism difference, p < 0.05.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author (Richard E. Brown) upon reasonable request.


