Abstract
To explore the role of intestinal microbiota on the occurrence of depression‐like behavior. Twenty male adult Wistar rats were randomly divided into control and experimental groups. Depression‐like behavior of the rats was validated using sucrose preference test (SPT) and forced swimming test (FST) after chronic unpredictable mild stress (CUMS) for 3 weeks. Fecal microbiota was analyzed through 16S rRNA sequence analysis. The levels of 5‐HT and inflammatory factors in the colon, brain and sera were measured using enzyme‐linked immunosorbent assay (ELISA), quantitative PCR (qPCR) and western blotting analyses. The percentage of different types of immune cells in the peripheral blood was determined through flow cytometry. CUMS caused depression‐like symptoms, including anhedonia and desperate behavior. Significant differences were found in the structure and abundance of intestinal microbiota. CUMS intervention significantly increased the levels of 5‐HT and Tph1 in the colon and decreased the level of Scl6a4. The concentrations of 5‐HT and Tph2 in the prefrontal and hippocampal tissues were lower, while IDO1 was higher. Certain cytokines, such as IL‐6, IL‐1 and TNF‐ɑ, were significantly elevated in peripheral blood, while the percentage of CD3+ CD4+ double‐positive cells and CD4+/CD8+ ratio were downregulated in the CUMS group. Pearson correlation analysis showed that intestinal microbiota was significantly associated with not only the metabolism of 5‐HT in intestinal and brain tissues, but also with the proportion of immune cells and certain cytokines. Stress can lead to disturbances in the intestinal microbial structure, which may contribute to depression by interfering with 5‐HT metabolism and immune inflammatory responses.
Keywords: cytokines, depression, gut microbiota, immunity, serotonin, stress
1. BACKGROUND
Depression is a serious neuropsychiatric disorder characterized by a lack of pleasure or interest in daily activities, and involves neurological, endocrine and immune dysfunction. At the molecular level, depression is associated with a deficiency of the neurotransmitter, 5‐hydroxytryptamine (5‐HT) 1 , 2 and a significant increase in the levels of inflammatory cytokines. 3 Studies have increasingly shown the involvement of the gut microbiota in the pathogenesis of various neurological and psychiatric disorders, including depression. For instance, 95% of the mood regulating neurotransmitter, serotonin, is synthesized, storedand released from the intestine. Although intestinal 5‐HT cannot directly affect brain 5‐HT levels, intestinal tryptophan can cross the blood–brain barrier and participate in brain 5‐HT synthesis. 4 An increase in the synthesis of 5‐HT in the gut may lead to a decrease in the amount of tryptophan entering the brain, thus interfering with the synthesis of 5‐HT in the brain. Indoleamine 2,3‐dioxygenase1 (IDO1) is the only rate‐limiting enzyme outside the liver that catalyzes the epoxidation of indoles in tryptophan molecules, resulting in catabolism along the canine uric acid pathway. The expression of IDO1 is only weakly expressed in normal healthy tissues, but in some special or pathological conditions, the expression of IDO1 is significantly enhanced, and participates in the mediation of local immunosuppression. 5 Therefore, intestinal microbes may also affect depression directly or indirectly by regulating tryptophan metabolism. 5
In addition, the gut microbiota plays a key role in the intestinal barrier function and innate immunity as it modulates the expansion, maturation and activity of immune cells. 6 The interactions between the intestinal bacteria and mucosal cells regulate the production of various inflammatory cytokines, 7 , 8 , 9 such as tumor necrosis factor (TNF‐α) and interleukin‐1β (IL‐1β), 10 which can cross the blood–brain barrier and significantly affect mood and behavior. Intestinal microbiota may be an important regulatory mechanism of intestinal inflammatory response and 5‐HT metabolism, thereby remotely regulating mood changes and depressive states in the brain. 16S rDNA gene sequencing is a commonly used research method to study the changes in intestinal microbial structure and abundance. Studies have used 16S rDNA gene sequencing to analyze the changes in the microbiota of depressed patients and healthy people. There have also been studies to conduct metagenomic and metabolomic analysis of depressed‐like behavior changes in rats by observation of microbiota transplantation. 11 , 12 Intestinal flora is inextricably linked with serotonin metabolism and immune inflammatory response, which might be closely related to the occurrence of depression. This study focused on intestinal microbiota associated with 5‐HT metabolism, intestinal immune cells and inflammatory factors, to explore the pathogenesis of depression.
2. MATERIALS AND METHODS
2.1. Experimental animals
Twenty adult male specific‐pathogen‐free (SPF) Wistar rats weighing 200–220 g, 7 weeks old, were raised at the Animal Experimental Research Center of the Medical Department of Qingdao University. The rats were housed five per cage inside polycarbonate cages, measuring 545 × 395 × 200 mm3 (FENGSHI Group, China) under standard laboratory conditions with constant temperature (22 ± 2°C), humidity (55 ± 5%), and standard ventilation system. The rats were maintained in 12:12 light/dark (LD) cycles with lights on from 7 a.m. to 7 p.m. if not otherwise stated, clean drinking water and food pellets (Jiangsu Medicience Biological Medicine Co. Ltd., China) were provided ad libitum. Drinking water and food were added daily. Rat padding (Jiangsu Medicience Biological Medicine) were replaced according to the pollution level, usually every 2 to 3 days. This study protocol was approved by the Ethics Committee of Qingdao University Affiliated Hospital (Qingdao, China).
2.2. Experimental scheme
All rats were normally fed for 1 week under the above standard laboratory conditions to adapt the environment and no abnormality were observed. Then the rats were weighed and randomly distributed into control group (n = 10) and experimental group (n = 10) and their tails were marked. The rats were housed five per cage in the above polycarbonate cages. The rats in the experimental group were subjected to chronic unpredictable mild stress (CUMS) 13 intervention for 3 weeks, as previously described. 14 , 15 In brief, the stimuli included 45° cage tilt for 12 h (hard to get food and water, 7 a.m. to 7 p.m.), tail pinching for 3 min with a clip (just whine without skin damage), cage shaking for 5 min, swimming in 4 °C cold water or 45 °C hot water for 5 min (using 50 cm high plastic drum, 20 cm in diameter, the water depth was determined by the rats' toes reaching the bottom of the container), reversed light/dark cycle for 24 h (lights on from 7 p.m. to 7 a.m.), maintained in an empty squirrel cage with no padding for 15 h (7 a.m. to 10 p.m.), damp bedding for 15 h (7 a.m. to 10 p.m.) and lack of food or water for 24 h (7 a.m. to 7 a.m. the next day). Normally, the rats of both groups were fed in the same room, but the experimental group was placed in another laboratory with the above standard temperature and humidity during the diurnal reversal intervention. The specific interventions used are listed in Table 1. CUMS is an effective and reliable strategy used to stimulate depression‐like behavior in animal models. 13 , 16 The resulting depression can be evaluated in terms of anhedonia determined through sucrose preference, 17 , 18 or prolonged periods of relatively restless behavior using the forced swimming test. 19
TABLE 1.
CUMS intervention schedule
| First week | Second week | Third week | |
|---|---|---|---|
| Monday | Clip tail for 3 min | Shake cage for 5 min | Shake cage for 5 min |
| Tuesday | Shake cage for 5 min | Empty cage for 15 h | Deprive water for 24 h |
| Wednesday | Tilt cage 45° for 12 h | Cold water bath for 5 min | Hot water bath for 5 min |
| Thursday | Wet cage for 15 h | Clip tail for 3 min | Clip tail for 3 min |
| Friday | Cold water bath for 5 min | Reverse day and night | Reverse day and night |
| Saturday | Reverse day and night | Tilt cage 45° for 12 h | Tilt cage 45° for 12 h |
| Sunday | Deprive water for 24 h | Deprive diet for 24 h | Deprive diet for 24 h |
2.2.1. Forced swim test (FST)
An altered type of the FST defined earlier by Cryan et al 20 was implemented here. Concisely, rats were kept into a Perspex cylinder comprising 30 cm of water heated at 25 °C for a 15 min before the test on day one. The next day, test periods lasting 5 min were noted. The immovability time was recorded when the animals were floating in the water with no struggle at all and they only moved to maintain their heads above the water level.
2.2.2. Sucrose Preference Test (SPT)
SPT was carried out as explained with slight alterations after the CUMS. 21 All rats were taught to acclimate to the 1% (w/v) sucrose solution: First they were exposed for 24 h to two bottles of sucrose solution. Next, they were exposed to one bottle of sucrose solution plus one bottle of water for another 24 h. Then the rats were not given food and water for 12 h. SPT was carried out for 12 h, in this course of duration, the rats were kept in separate cages with easy access to two bottles (1% sucrose solution and water bottle). The locations of the bottles in the cage were interchanged after 6 h to evade probable side‐preference impacts. The intakes of the sucrose solution, water as well as total consumption of liquids were assessed by weighing the bottles. The inclination for sucrose was noted as a fraction of the ingested sucrose liquid comparative to the entire volume of liquid consumption. Following equation was used to calculate the sucrose preference value: Preference value (%) = sucrose intake/ (sucrose intake + water intake) × 100%.
Once depression was established, the animals were anesthetized through the intraperitoneal injection of 3% pentobarbital sodium at 20 mg/kg body weight, and blood samples were collected from the inferior vena cava into coagulation and anticoagulation tubes. The blood samples were centrifuged and the supernatants were put in storage at −20°C for consequent serum serotonin (5‐HT), dopamine (DA), IL‐6, IL‐1 and TNF‐α by enzyme‐linked immunosorbent assay (ELISA) kit (MultiSciences Biotech, PRC). The former were centrifuged, and the serum supernatant was aspirated and subjected to ELISA assay to measure levels of the blood samples collected into anticoagulation tubes were treated with a red blood cell lysis buffer and centrifuged, and the resulting pellets were fixed using formalin at 4 °C. The number of CD3+, CD4+ and CD8+ cells and the CD4+/CD8+ ratio were determined using routine immunostaining and flow cytometry analyses. Beckman FC500 was used, CD3‐PC5, CD4‐FITC and CD8‐PE mAb (Thermo Fisher Scientific, China) were added for fluorescent antibody staining.
After the drawing of blood, the rats were quickly decapitated, and the medulla were cut at the foramen magnum. The skulls were then removed to completely expose the brain tissue, and the hippocampal and prefrontal tissues were isolated on pre‐chilled dishes, and snap frozen in liquid nitrogen. Total RNA and protein were extracted from the frozen tissues, and the expression levels of Tph1 and TH at both mRNA and protein levels were analyzed using fluorescence quantitative PCR (qPCR) and western blotting analysis, respectively. Total RNA (1 μg) was reverse transcribed into cDNA by TIANScript RT KIT (TIANGEN, Cat# KR104‐02) as per the supplier's protocol. List of primers is mentioned in Table 2 (TaKaRa Biotechnology Co., Ltd., China). IDO1 protein expression in hippocampus and forebrain was measured by Western blot using rabbit anti‐IDO1 monoclonal antibody purchased from Zymed Laboratories, Shanghai YUBO Biotechnology Co., Ltd agent, PRC. The content of 5‐HT in the hippocampus and prefrontal lobe were measured using HPLC‐MS.
TABLE 2.
Primers used for RT‐PCR
| Gene | Sequence (sense, antisense: 5′–3′) | Size (bp) |
|---|---|---|
| Tph2 |
CTTGGGGTGTTGTGTTTCG TACTTGGTCAGCAGGGGGA |
91 |
| IDO1 |
GGAGCUACCAUCUGCAAAUTT AUUUGCAGAUGGUAGCUCCTT |
568 |
| β‐Actin |
CTTGCATCCCTCAGCACCTT TCCTGTGGACAATGGATGGA |
140 |
| Tph1 |
GGCGCGATCAGGATCACTG ACTTTTTTCAAACATACGT |
263 |
| SLC6A4 |
GGGTACAGGAGAGAGGATTG GTGCAATTTAAACCTTATAC |
108 |
| GAPDH |
GGGGCTGGGAAGGAACCACG CGGTAAGGACTATATAATGT |
72 |
The colon tissues were dissected, and feces contained were collected for 16S rRNA gene sequencing. Total genome DNA from the samples was extracted using the CTAB/SDS method. 16S rRNA genes were amplified using specific primers with the barcode, 16S V4: 515F‐806R. The colon tissues were then cut along the mesangial margin, washed using ice‐cold saline, and the 5‐HT content in the supernatant of the homogenate was determined by ELISA assay as per the supplier's protocol (Cloud Clone Corp., Hubei, PRC). The experimental scheme followed is shown in Figure 1.
FIGURE 1.
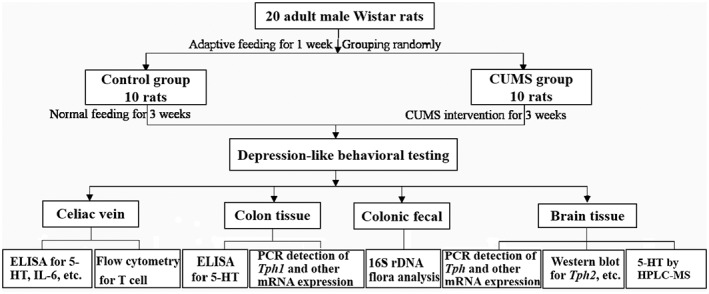
Flowchart of the experimental scheme
2.3. Microbiota analysis
Intestinal fecal specimens were entrusted to Beijing Nuohe Zhiyuan Technology Co. Ltd. PRC, for 16S V4 region amplicon sequencing analysis. A TruSeq® DNA PCR‐Free Sample Preparation Kit was used to construct the library, which was then subjected to Qubit quantification. Hi‐Seq was used for online sequencing to perform a series of analyses, including cluster analysis, principal component analysis (PCA), LEfSe (LDA effect size) analysis and other statistical comparative analyses to compare the species composition of the different samples.
2.4. Statistical analysis
Statistical analysis was performed using SPSS v.22.0 software. Counted data are expressed as rates and percentages, while measurement data are expressed as ± SD. The means of two groups were compared using the independent sample t‐test and a p value of <0.05 was considered to indicate statistical significance. The level of IL‐6, TNF‐α, DA and IL‐1 Beta ELISA values were converted from OD to concentration using a standard curve. p Values were adjusted using a Benjamini–Hochberg correction. Pearson correlation analysis was conducted on data conformed to a normal distribution as determined using the normality test, otherwise the Spearman correlation test was used. The levels of intestinal microbiota, associated neurotransmitters and inflammatory factors were calculated and plotted in R Studio (R‐4.0.2, package corrplot_0.84). Correlations with an absolute coefficient value of >0.6 and an adjusted p value of <0.05 were considered to be statistically significant.
3. RESULTS
During the experiment, one rat in the control group developed diarrhea, and one in the CUMS intervention group died due to unknown causes. Thus, only nine animals were finally included in the analyses carried out on each group.
3.1. Behavioral tests
Based on the results of the SPT, the sucrose preference value of the control group was significantly higher compared with that of the CUMS group (t = 16.39, p <0.05). In addition, healthy control rats were immobile during the FST for a shorter period compared with the CUMS intervention group rats, which showed an obvious struggle during the initial period (t = 16.07, p <0.05). Specific important data are summarized in Figure 2, and clearly indicate that depression‐like symptoms were observed after the CUMS intervention.
FIGURE 2.
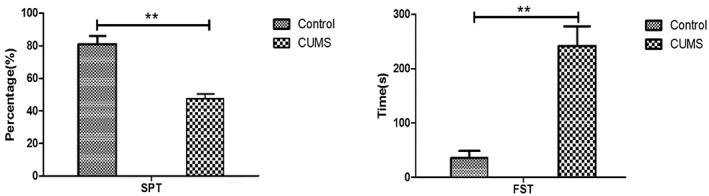
Results of the sucrose preference test and forced swimming test in the two groups
3.2. Effect of CUMS intervention on peripheral blood DA, 5‐HT, colon 5‐HT and metabolic factors
As shown in Figure 3, the levels of serum DA (t = 0.42, p = 0.79) and 5‐HT (t = 0.29, p = 0.29) were not significantly different between the groups. However, the 5‐HT content in the colon homogenate was significantly higher than that of the CUMS group (t = 6.36; p < 0.05), as shown in Figure 3.
FIGURE 3.
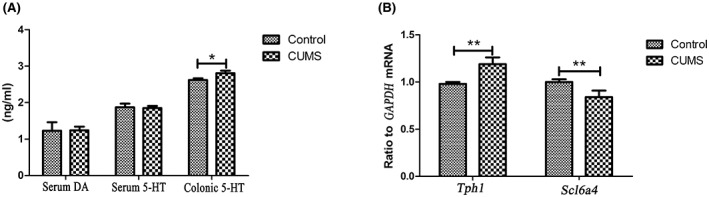
Comparison of serum DA, 5‐HT, colonic 5‐HT levels and the expression of Tph1 and Scl6a4 in both groups
To determine the source of colonic 5‐HT variability, we compared the expression levels of Tph1 mRNA/GAPDH mRNA and Scl6a4 mRNA/GAPDH mRNA. The relative expression level of Tph1 mRNA was significantly higher (t = 9.18, p < 0.01) in the colon tissue of the CUMS group compared with that of healthy controls (t = 6.90, p < 0.01), as shown in Figure 3.
3.3. Effect of CUMS intervention on the content of 5‐HT, Tph2 and IDO1 in the brain
To investigate the effect of 5‐HT in the brain, we obtained brain tissue from rats in both groups and isolated the prefrontal cortex and hippocampus. The 5‐HT content was significantly lower in the frontal lobes (t = 8.05, p < 0.01) and hippocampus (t = 7.57, p < 0.01) of the stressed rats compared with the controls. Furthermore, the prefrontal and hippocampal TH (t = 7.57, p < 0.01; t = 7.44, p < 0.01) and Tph2 (t = 7.79, p < 0.01; t = 4.87, p < 0.01) levels were also lower in the CUMS, compared with the control group. In contrast, the prefrontal and hippocampal IDO1 levels were significantly higher in the CUMS group (t = 3.93, p < 0.01; t = 5.71, p < 0.01). The results are shown in Figure 4.
FIGURE 4.
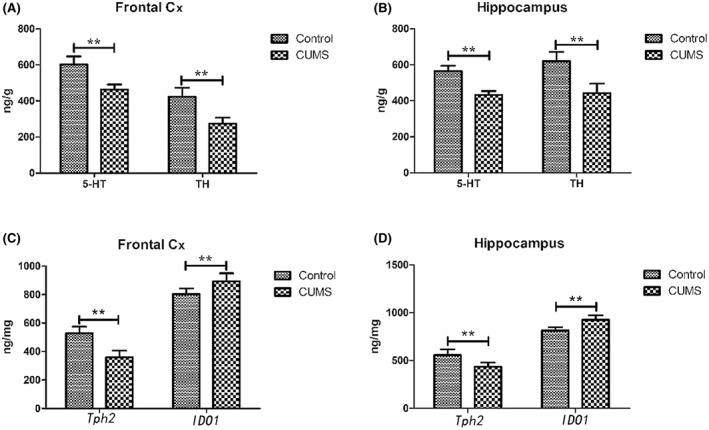
5‐HT, TH, Tph2 and IDO1 levels in the prefrontal cortex and hippocampus of rats in both groups
3.4. Effect of CUMS intervention on inflammatory factors and immune cells
The serum levels of inflammatory factors, including IL‐6 (t = 7.55, p < 0.01), IL‐1β (t = 2.76, p < 0.05) and TNF‐α (t = 3.50, p < 0.01) were significantly higher in the CUMS group compared with the control group (Figure 5).
FIGURE 5.
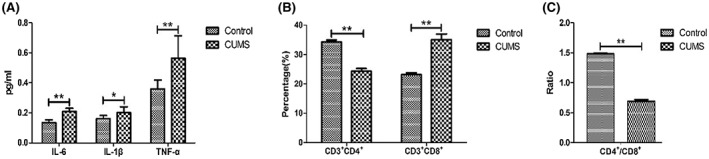
Levels of inflammatory factors and immune cells in the two groups
Furthermore, the percentage of CD3 + cells were similar between the groups (t = 1.58, p > 0.05). The proportion of CD3 +CD4 + double positive cells (t = 24.52, p < 0.01) and the CD4 +/CD8 + ratio (t = 55.31, p < 0.01) were lower in the CUMS group, while that of the CD3 +CD8 + double positive cells was significantly higher in the CUMS‐treated group, compared with the control group (t = 15.81, p < 0.01).
3.5. Analysis of intestinal microbiota
Based on species annotation, the top 10 abundant phyla, classes, orders, families and genera were determined, and their relative proportions were calculated (Figure 6A–E). PCA analysis based on OTU levels (Figure 6F) indicated significant differences between the intestinal microbiota of control and CUMS‐treated rats at each level of classification. In addition, the LDA value obtained from the LEfSe (Figure 6G) showed that each group showed a distinct composition of dominant species.
FIGURE 6.
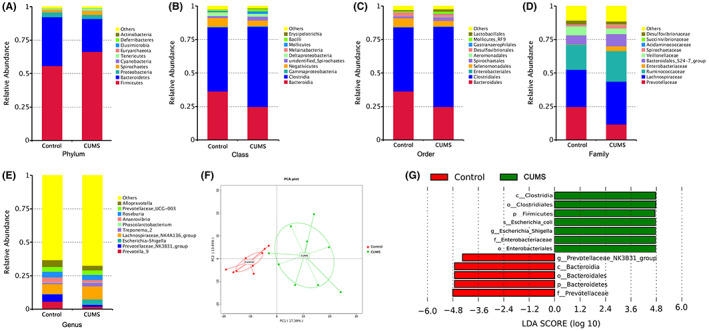
Analysis of the intestinal microbiota of the two groups. Note: Species with an LDA score greater than 4 were considered to be significantly different between the groups. The length of the histogram represents the magnitude of the impact of different species
The histogram of LDA distribution showed that the dominant species in the control group were Bacteroidetes, Bacteroidia, Bacteroidales, Prevotellaceae and Prevotellaceae_NK3B31_group. The dominant species in the CUMS group were Firmicutes, Clostridia, Clostridiales, Enterobacteriales, Enterobacteriaceae, Escherichia shigella and Escherichia coli.
3.6. Correlation analysis between significant taxonomical differences in the intestinal microbiota and the levels of 5‐HT and other biomarkers between the groups
We analyzed the correlation between the different types of microbiota and related biomarkers between the control group and the CUMS group. The significantly more abundant groups of intestinal microbiota in the CUMS group, such as Firmicutes, Clostridia, Clostridiales, Enterobacteriales and Escherichia Shigella, were negatively correlated with 5‐HT, Tph2 and TH levels in the prefrontal lobe and hippocampus, colonic Scl6a4/GAPDH mRNA, peripheral CD3+CD4+ double positive cells and CD4+/CD8+ ratio, but were positively correlated with IDO1 levels in the prefrontal lobe, colonic 5‐HT and Tph 1 /GAPDH mRNA levels, and blood inflammatory factors, IL‐6, IL‐1β and TNF‐α (p < 0.05; Figure 7). On the other hand, the dominant microbiota in the control group showed an opposite trend. At the genus level, the dominant microbiota in the control group, PrevotellaceaeNK3B31_group, was negatively correlated with 5‐HT and Tph2 levels in the prefrontal lobe, Tph2 level in hippocampus and colonic Scl6a4/GAPDH mRNA, and was positively correlated with colonic 5‐HT, Tph1/GAPDH mRNA, peripheral CD3+CD4+ double positive cells and CD4+/CD8+ ratio (p < 0.05; Figure 7).
FIGURE 7.
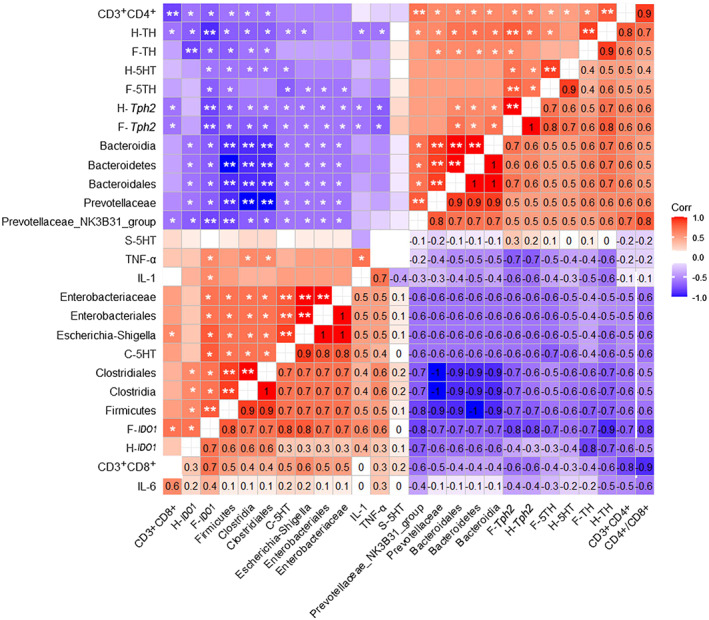
Pearson correlation analysis between the differential fecal metabolites, neurotransmitters in the prefrontal cortex and hippocampus, inflammatory factors and immune cells: F indicates the prefrontal cortex, H indicates hippocampus and S indicates serum. The numbers on the lower right panel show the correlation coefficient, while the symbols on the upper left panel show the results of the significance test. *, adjusted p <0.05; **, adjusted p <0.01
4. DISCUSSION
4.1. CUMS intervention can lead to changes in the structure and abundance of the intestinal microbiota and are associated with serotonin metabolism in the intestines and brain
In our study, the structure and abundance of the intestinal microbiota were analyzed using 16S rRNA amplification sequencing. PCA showed that CUMS intervention significantly changed the microbial composition of the colon, compared with the control group. The abundance of Firmicutes increased significantly, and the number of Bacteroidetes decreased significantly, leading to an increase in the F/B ratio. Consistent with the results of this study, Zu et al. showed that the relative abundance of Firmicutes was higher in a depressed rat model and depressed patients, while the relative abundance of Bacteroidetes was lower. 23 , 24 , 25 Tomova et al. also reported a significant increase in the intestinal F/B ratio in autistic children. 26 In addition, F/B ratios have also been reported to be elevated in patients with metabolic diseases, inflammatory bowel disease. 27 In addition, the F/B ratio at phylum level is closely associated with the occurrence of depression, and this study further analyzed the same at the class, order, family and genus levels. LEfSe analysis showed that the dominant bacteria in the control group were Bacteroidetes, Bacteroidia, Bacteroidales, Prevotellaceae and Prevotellaceae_NK3B31_group, and that the dominant bacteria in the CUMS group were Firmicutes, Clostridia, Clostridiales, Enterobacteriales, Enterobacteriaceae, Escherichia shigella and Escherichia coli. Consistent with the results of this study, Braun et al. found that an increase in the abundance of Clostridium in the intestinal tract of patients with mental diseases. 28 , 29 , 30 , 31 Although Clostridium forms part of the normal intestinal microbiota in humans and animals, most bacteria are opportunistic pathogens that can cause intestinal or neuro toxicity. Yano et al. showed that Clostridium has a direct regulatory effect on colon 5‐HT synthesis. 32 Lin et al. showed that Clostridium increased deoxycholic acid levels through 7‐dehydroxy activity, promoting 5‐HT synthesis in colonic epithelial cells. 24 , 33 Our study showed that Clostridiales was positively correlated with the colon 5‐HT level, positively correlated with IDO1 expression in the prefrontal lobe and hippocampus, and negatively correlated with Tph2 expression, TH and hippocampal 5‐HT levels in the prefrontal lobe and hippocampus. Dominant bacteria in the CUMS intervention group, such as Enterbacteriaceae (Order), Escherichia_shigella (Family) and Escherichia coli (Genus) also showed similar relevance. The dominant bacteria in the healthy control group, including Prevotellaceae (Family) and Prevotellaceae_NK3B31_group (Genus) were not only negatively correlated with intestinal 5‐HT expression, but were also positively correlated with TH expression in the prefrontal lobe and hippocampus. This suggests that intestinal microbiota may have follow a feedback regulation mechanism between 5‐HT and DA metabolism in brain tissue.
The neurotransmitter, 5‐HT, regulates immune responses and intestinal‐brain communication, 5 , 34 , 35 and a decrease in cerebral 5‐HT expression can lead to behavioral changes, including persistent grief and loss of interest. 36 In our study, CUMS intervention significantly decreased levels of 5‐HT in the colon and brain tissues. The role of circulating 5‐HT in depression is ambiguous. Some studies have reported of no change in the levels of 5‐HT in the peripheral blood of CUMS‐treated rats, 37 , 38 , 39 while other studies indicate that a lower blood 5‐HT level is a potential biomarker for depression. 40 , 41 , 42 We did not detect any significant differences in the serum 5‐HT level between the control and CUMS groups, suggesting that circulating 5‐HT may not directly reflect the state of depression. This may be attributed to the blood–brain barrier (BBB), which consists of tightly connected transmembrane proteins, such as claudins and tricellulin, which limit the diffusion of water‐soluble substances, such as 5‐HT, from the blood to the brain. 43 The elevation of 5‐HT expression in the colon tissues of CUMS‐exposed animals is indicative of the gut–brain axis. Although intestinal 5‐HT cannot directly affect the level of 5‐HT in the brain, tryptophan can cross the blood–brain barrier and promote 5‐HT synthesis in the brain. 4 Therefore, the increased synthesis of 5‐HT in the intestine may decrease the amount of tryptophan entering the brain, and eventually interfere with 5‐HT synthesis.
The rate‐limiting step of 5‐HT biosynthesis in intestinal chromaffin cells (ECs) is catalyzed by Tph1. 44 In addition, the rapid uptake of 5‐HT depends on SLC6A4, a transmembrane serotonin transporter (SERT) with a high affinity for 5‐HT that is mainly expressed on intestinal mucosal epithelial cells and chromaffin cells. 45 Colonic Tph1 expression was upregulated whereas SLC6A4 expression was downregulated after CUMS intervention. Decreased intestinal SLC6A4 levels can lead to intestinal 5‐HT accumulation, which in turn can increase intestinal mucosal stimuli, eventually leading to diarrhea. 46 Gastrointestinal diseases often occur in patients with depression, which may also be closely associated with the increase in the content of free 5‐HT in the intestines. 47 Further studies are needed to determine whether changes in intestinal microbiota and brain 5‐HT metabolism involve the vagus nerve and the overall mechanisms of action involved.
4.2. The structural and abundance changes of the intestinal microbiota are closely associated with the imbalance of immune cells and certain inflammatory factors
During an inflammatory state, immune cells produce high levels of pro‐inflammatory cytokines and metabolites, which enter systemic blood circulation 10 and can cross the blood–brain barrier, leading to severe behavioral changes. CUMS intervention significantly increased IL‐6, IL‐1β and TNF‐α levels, and decreased the CD4+/CD8+ ratio. The non‐neuronal cells in the brain, such as microglia and astrocytes, express TNF‐α and IL‐1β receptors. 48 , 49 , 50 Circulating IL‐1β and TNF‐α bind to these receptors after crossing the blood–brain barrier and induce the production of secondary cytokines that promote the development of depression. CD4+ T cells play a key role in the induction of T lymphocyte proliferation, while CD8+ T cells perform an immunosuppressive function. 51 , 52 A significant decrease in the CD4+/CD8+ ratio is suggestive of impaired immune surveillance and inflammation. Zdanowicz et al. conducted a study on 549 patients with severe depression and showed that depression and immunity are associated with each other. 53 In our study, the correlation analysis showed that the abundance of E. coli increased significantly in the CUMS intervention group. Porter et al. proved that the presence of E. coil was associated with intestinal inflammation in a mouse colitis model. 54 The correlation analysis showed that Enterbacteriales (Order), Enterbacteriaceae (Family), Escherichia_shigella (Genus) were significantly and positively correlated with TNF‐α and IL‐1 expression, and that Escherichia_shigella (Genus) was positively correlated with CD3+CD8+ expression. These results indicate that CUMS intervention could indeed lead to the proliferation of certain pathogenic bacteria in the intestinal tract, and which may then lead to the increase of inflammatory factors and immune cell disorders.
Kim et al. reported that intestinal microbiota can affect the maturation of immune cell subsets and innate immune response. 11 , 12 Prevotellaceae was found to be more abundant in individuals on a plant‐rich diet. 55 , 56 Simpson et al. showed that Prevotellaceae_NK3B31_group produces short‐chain fatty acids (SCFAs) through the fermentation of dietary fiber, which not only provides energy for the growth and metabolism of colon epithelial cells, but also improves intestinal barrier function and regulates the endocrine system. 57 Our study showed that Prevotellaceae and Prevotellaceae_NK3B31_group were significantly and positively correlated with the number of CD3+CD8+ cells and the CD4+/CD8+ ratio, indicating their roles in regulating immune monitoring and repairing inflammation damage.
5. CONCLUSIONS
Stress can lead to disturbances in the intestinal microbiota. Intestinal microbiota may indirectly regulate emotional and behavioral changes associated with depression by regulating 5‐HT metabolism and the expressions of immune cells and inflammatory cytokines. Therefore, regulation of the intestinal microbiota may be a promising intervention for depression that warrants further investigation.
CONFLICT OF INTEREST
The authors declare no conflict of interests.
ACKNOWLEDGMENTS
This work was supported by the Nation Natural Science Foundation of China (Nos. 81270448 and 81470890).
Li H, Wang P, Zhou Y, et al. Correlation between intestinal microbiotal imbalance and 5‐HT metabolism, immune inflammation in chronic unpredictable mild stress male rats. Genes, Brain and Behavior. 2022;21(6):e12806. doi: 10.1111/gbb.12806
Funding information National Natural Science Foundation of China, Grant/Award Numbers: 81270448, 81470890; Natural Science Foundation of China
DATA AVAILABILITY STATEMENT
Data availability statement The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Duan D, Tu Y, Yang X, Liu P. Electroacupuncture restores 5‐HT system deficit in chronic mild stress‐induced depressed rats. Evid Based Complement Alternat Med. 2016;2016:7950635. doi: 10.1155/2016/7950635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Anisman H, Merali Z, Hayley S. Neurotransmitter, peptide and cytokine processes in relation to depressive disorder: comorbidity of depression with neurodegenerative disorders. Prog Neurobiol. 2008;85(1):1‐74. doi: 10.1016/j.pneurobio.2008.01.004 [DOI] [PubMed] [Google Scholar]
- 3. O'Brien SM, Scott LV, Dinan TG. Cytokines: abnormalities in major depression and implications for harmacological treatment. Hum Psychopharmacol. 2004;19(6):397‐403. doi: 10.1002/hup.6090 [DOI] [PubMed] [Google Scholar]
- 4. Ruddick JP, Evans AK, Nutt DJ, et al. Tryptophan metabolism in the central nervous system: medical implications. Expert Rev Mol Med. 2006;8(20):1‐27. doi: 10.1017/S1462399406000068 [DOI] [PubMed] [Google Scholar]
- 5. O'Mahony S, Clarke G, Borre Y, Dinan T, Cryan J. Serotonin, tryptophan metabolism and the brain‐gut‐microbiome axis. Behav Brain Res. 2015;277:32‐48. doi: 10.1016/j.bbr.2014.07.027 [DOI] [PubMed] [Google Scholar]
- 6. Bruce‐Keller AJ, Salbaum JM, Berthoud H‐R. Harnessing gut microbes for mental health: getting from here to there. Biol Psychiatry. 2018;83(3):214‐223. doi: 10.1016/j.biopsych.2017.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Round JL, O'Connell RM, Mazmanian SK. Coordination of Tolerogenic immune responses by the commensal microbiota. J Autoimmun. 2010;34(3):J220‐J225. doi: 10.1016/j.jaut.2009.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. El Aidy S, van Baarlen P, Derrien M, et al. Temporal, spatial interplay of microbiota, and intestinal mucosa drive establishment of immune homeostasis in conventionalized mice. Mucosal Immunol. 2012;5(5):567‐579. doi: 10.1038/mi.2012.32 [DOI] [PubMed] [Google Scholar]
- 9. El Aidy S, Derrien M, Aardema R, et al. Transient inflammatory‐like state and microbial Dysbiosis are pivotal in establishment of mucosal homeostasis during colonisation of germ‐free mice. Benefic Microbes. 2014;5:67‐77. doi: 10.3920/BM2013.0018 [DOI] [PubMed] [Google Scholar]
- 10. Dantzer R. Cytokine, sickness behavior, and depression. Immunol Allergy Clin North Am. 2009;29:247‐264. doi: 10.1006/brbi.2000.0613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kim CH. Immune regulation by microbiome metabolites. Immunology. 2018;154(2):220‐229. doi: 10.1111/imm.12930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. RooksMG GWS. Gut microbiota, metabolites and host immunity. Nat Rev Immunol. 2016;16(6):341‐352. doi: 10.1038/nri.2016.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Willner P. Validity, reliability and utility of the chronic mild stress model of depression: a 10‐year review and evaluation. Psychopharmacology (Berl). 1997;134(4):319‐329. doi: 10.1007/s002130050456 [DOI] [PubMed] [Google Scholar]
- 14. Papp M, Moryl E, Willner P. Pharmacological validation of the chronic mild stress model of depression. Eur J Pharmacol. 1996;296(2):129‐136. doi: 10.1016/0014-2999(95)00697-4 [DOI] [PubMed] [Google Scholar]
- 15. Ossowska G, Danilczuk Z, Klenk‐Majewska B. Antidepressants in chronic unpredictable mild stress (cums) induced deficit of fighting behavior. Pol J Pharmacol. 2004;56(3):305‐311. [PubMed] [Google Scholar]
- 16. Li S, Wang C, Wang M, et al. Antidepressant like effects of piperine in chronic mild stress treated mice and its possible mechanisms. Life Sci. 2007;80:1373‐1381. doi: 10.1016/j.lfs.2006.12.027 [DOI] [PubMed] [Google Scholar]
- 17. Deng XY, Xue JS, Li HY, et al. Geraniol produces antidepressant‐like effects in a chronic unpredictable mild stress mice model. Physiol Behav. 2015;152 (Pt A):264‐271. doi: 10.1016/j.physbeh.2015.10.008 [DOI] [PubMed] [Google Scholar]
- 18. Cryan JF, Valentino RJ, Lucki I. Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neurosci Biobehav Rev. 2005;29(4–5):547‐569. doi: 10.1016/j.neubiorev.2005.03.008 [DOI] [PubMed] [Google Scholar]
- 19. Gao H, Zhu X, Xi Y, et al. Anti‐depressant‐like effect of atractylenolide I in a mouse model of depression induced by chronic unpredictable mild stress. Exp Ther Med. 2018;15(2):1574‐1579. doi: 10.3892/etm.2017.5517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cryan JF, Page ME, Lucki I. Differential behavioral effects of the antidepressants reboxetine, fluoxetine, and moclobemide in a modified forced swim test following chronic treatment. Psychopharmacology. 2005;182(3):335‐344. doi: 10.1007/s00213-005-0093-5 [DOI] [PubMed] [Google Scholar]
- 21. Gross M, Pinhasov A. Chronic mild stress in submissive mice: marked polydipsia and social avoidance without hedonic deficit in the sucrose preference test. Behav Brain Res. 2016;298 (Pt B):25‐34. doi: 10.1016/j.bbr.2015.10.049 [DOI] [PubMed] [Google Scholar]
- 22. Li Huawei, Wang P, Huang L, Li P, Zhang D. Effects of regulating gut microbiota on the serotonin metabolism in the chronic unpredictable mild stress rat model. Neurogastroenterol Motil. 2019. Oct; 31(10):e13677. doi: 10.1111/nmo.13677. Epub 2019 Jul 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xianpeng Z. Characterization of Metabolome and Intestinal Microbiota in Depressive Patients and Mechanism Study on the Antidepressive Effects of Bacopaside I [D]. Naval Military Medical University of the People's Liberation Army, Naval Military. Medical University; 2018. [Google Scholar]
- 24. Lin P, Ding B, Feng C, et al. Prevotella and Klebsiella proportions in fecal microbial communities are potential characteristic parameters for patients with major depressive disorder. J Affect Disord. 2017;207:300‐304. doi: 10.1016/j.jad.2016.09.051 [DOI] [PubMed] [Google Scholar]
- 25. Qu W, Liu S, Zhang W, et al. Impact of traditional Chinese medicine treatment on chronic unpredictable mild stress‐induced depression‐like behaviors: intestinal microbiota and gut microbiome function. Food Funct. 2019;10(9):5886‐5897. doi: 10.1039/c9fo00399a [DOI] [PubMed] [Google Scholar]
- 26. Tomova A, Husarova V, Lakatosova S, et al. Gastrointestinal microbiota in children with autism in Slovakia. Physiol Behav. 2015;138:179‐187. doi: 10.1016/j.physbeh.2014.10.033 [DOI] [PubMed] [Google Scholar]
- 27. Stojanov S, Berlec A, Štrukelj B. The influence of probiotics on the Firmicutes/Bacteroidetes ratio in the treatment of obesity and inflammatory bowel disease. Microorganisms. 2020;8(11):1715. doi: 10.3390/microorganisms8111715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Braun J. Tightening the case for gut microbiota in autism‐Spectrum disorder. Cell Mol Gastroenterol Hepatol. 2017;3(2):131‐132. doi: 10.1016/j.jcmgh.2017.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yu M, Jia H, Zhou C, et al. Variations in gut microbiota and fecal metabolic phenotype associated with depression by 16S rRNA gene sequencing and LC/MS‐based metabolomics. J Pharmaceut Biomed. 2017;138:231‐239. doi: 10.1016/j.jpba.2017.02.008 [DOI] [PubMed] [Google Scholar]
- 30. Grimaldi R, Cela D, Swann JR, Vulevic J, et al. In vitro fermentation of B‐GOS: impact on faecal bacterial populations and metabolic activity in autistic and non‐autistic children. FEMS Microbiol Ecol. 2017;93(2):pii: fiw233. doi: 10.1093/femsec/fiw233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rogers MA, Greene MT, Young VB, et al. Depression, antidepressant medications, and risk of Clostridium difficile infection. BMC Med. 2013;11:121. doi: 10.1186/1741-7015-11-121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yano JM, Yu K, Donaldson GP, et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161:264‐276. doi: 10.1016/j.cell.2015.02.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Larroya‐García A, Navas‐Carrillo D, Orenes‐Piñero E. Impact of gut microbiota on neurological diseases: Diet composition and novel treatments. Crit Rev Food Sci Nutr. 2019;59(19):3102‐3116. doi: 10.1080/10408398.2018.1484340 [DOI] [PubMed] [Google Scholar]
- 34. Le Floc'h N, Otten W, Merlot E. Tryptophan metabolism, from nutrition to potential therapeutic applications. Amino Acids. 2011;41(5):1195‐1205. doi: 10.1007/s00726-010-0752-7 [DOI] [PubMed] [Google Scholar]
- 35. Nguyen NT, Nakahama T, Le DH, et al. Aryl hydrocarbon receptor and kynurenine: recent advances in autoimmune disease research. Front Immunol. 2014;5:551. doi: 10.3389/fimmu.2014.00551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Catena‐Dell'Osso M, Rotella F, Dell'Osso A, et al. Inflammation, serotonin and major depression. Curr Drug Targets. 2013;14(5):571‐577. doi: 10.2174/13894501113149990154 [DOI] [PubMed] [Google Scholar]
- 37. Li X, Fan Y, Xiao S, et al. Decreased platelet 5‐hydroxytryptamin (5‐HT) levels: a response to antidepressants. J Affect Disord. 2015;187:84‐90. doi: 10.1016/j.jad.2015.08.025 [DOI] [PubMed] [Google Scholar]
- 38. Fidalgo S, Ivanov DK, Wood SH. Serotonin: from top to bottom. Biogerontology. 2013;14(1):21‐45. doi: 10.1007/s10522-012-9406-3 [DOI] [PubMed] [Google Scholar]
- 39. Lima L, Mata S, Urbina M. Allelic isoforms and decrease in serotonin transporter mRNA in lymphocytes of patients with major depression. Neuroimmunomodulation. 2005;12:299‐306. doi: 10.1159/000087108 [DOI] [PubMed] [Google Scholar]
- 40. Flores‐Ramos M, Moreno J, Heinze G, et al. Gonadal hormone levels and platelet tryptophan and serotonin concentrations in perimenopausal women with or without depressive symptoms. Gynecol Endocrinol. 2014;30:232‐235. doi: 10.3109/09513590.2013.875994 [DOI] [PubMed] [Google Scholar]
- 41. Sekiyama T, Nakatani Y, Yu X, et al. Increased blood serotonin concentrations are correlated with reduced tension/anxietyin healthy postpartum lactating women. Psychiatry Res. 2013;209(3):560‐565. doi: 10.1016/j.psychres.2013.03.009 [DOI] [PubMed] [Google Scholar]
- 42. Zahn D, Petrak F, Franke L, et al. Cortisol, platelet serotonin content and platelet activity in patients with major depression and type 2 diabetes: an exploratory investigation. Psychosom Med. 2015;77(2):145‐155. doi: 10.1097/PSY.0000000000000145 [DOI] [PubMed] [Google Scholar]
- 43. Waclawiková B, El Aidy S. Role of microbiota and tryptophan metabolites in the remote effect of intestinal inflammation on brain and depression. Pharmaceuticals (Basel). 2018;11(3):63. doi: 10.3390/ph11030063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gershon MD, Tack J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology. 2007;132(1):397‐414. doi: 10.1053/j.gastro.2006.11.002 [DOI] [PubMed] [Google Scholar]
- 45. Duman EA, Canli T. Influence of life stress, 5‐HTTLPR genotype, and SLC6A4 methylation on gene expression and stress response in healthy Caucasian males. Biol Mood Anxiety Disord. 2015;5:2. Published online 2015 May 14. doi: 10.1186/s13587-015-0017-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sjaarda CP, Hecht P, et al. Interplay between maternal Slc6a4 mutation and prenatal stress: a possible mechanism for autistic behavior development. Sci Rep. 2017;7:8735. Published online 2017 Aug 18. doi: 10.1038/s41598-017-07405-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lam D, Ancelin M‐L, Ritchie K, et al. Genotype‐dependent associations between serotonin transporter gene (SLC6A4) DNA methylation and late‐life depression. BMC Psychiatry. 2018;18:282. Published online 2018 Sep 4. doi: 10.1186/s12888-018-1850-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jensen CJ, Massie A, De Keyser J. Immune players in the CNS: the astrocyte. J Neuroimmune Pharmacol. 2013;8(4):824‐839. doi: 10.1007/s11481-013-9480-6 [DOI] [PubMed] [Google Scholar]
- 49. Yang I, Han SJ, Kaur G, et al. The role of microglia in central nervous system immunity and glioma immunology. J Clin Neurosci. 2010;17(1):6‐10. doi: 10.1016/j.jocn.2009.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nadeau S, Rivest S. Effects of circulating tumor necrosis factor on the neuronal activity and expression of the genes encoding the tumor necrosis factor receptors (P55 and P75) in the rat brain: a view from the blood–brain barrier. Neuroscience. 1999;93(4):1449‐1464. doi: 10.1016/s0306-4522(99)00225-0 [DOI] [PubMed] [Google Scholar]
- 51. Marini S, Vellante F, et al. Inflammatory markers and suicidal attempts in depressed patients: a review. Int J Immunopathol Pharmacol. 2016;29(4):583‐594. doi: 10.1177/0394632015623793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Manceaux P, Zdanowicz N. Immunity, coping and depression. Psychiatr Danub. 2016;28(Suppl‐1):165‐169. [PubMed] [Google Scholar]
- 53. Zdanowicz N, Reynaert C, Jacques D, Dubois T. Depression and immunity: a psychosomatic unit. Psychiatr Danub. 2017. Sep;29(Suppl 3):274‐278. [PubMed] [Google Scholar]
- 54. Porter NT, Martens EC. The critical roles of polysaccharides in gut microbial ecology and physiology. Annu Rev Microbiol. 2017;71:349‐369. doi: 10.1146/annurev-micro-102215-095316 [DOI] [PubMed] [Google Scholar]
- 55. Ley RE. Gut microbiota in 2015: Prevotella in the gut: choose carefully. Nat Rev Gastroenterol Hepatol. 2016;13(2):69‐70. doi: 10.1038/nrgastro.2016.4 [DOI] [PubMed] [Google Scholar]
- 56. Simpson HL, Campbell BJ. Review article:dietary fibre‐microbiota interactions. Aliment Pharmacol Ther. 2015;42(2):158‐179. doi: 10.1111/apt.13248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Elatrech I, Marzaioli V, Boukemara H, et al. Escherichia coli LF82 differentially regulates ROS production and mucin expression in intestinal epithelial T84 cells: implication of NOX1. Inflamm Bowel Dis. 2015;21(5):1018‐1026. doi: 10.1097/MIB.0000000000000365 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data availability statement The data that support the findings of this study are available from the corresponding author upon reasonable request.


