Abstract
The hippocampus is one of the most widely investigated brain regions with its massive contributions to multiple behaviours. Especially, the hippocampus is subdivided into the dorsal and ventral parts playing distinct roles. In this review, we will focus on the ventral hippocampus, especially the ventral CA1 (vCA1), whose role is being actively discovered. vCA1 is well known to be associated with emotion‐like behaviour, in both positive (reward) and negative (aversive) stimuli. How can this small region in volume mediate such variety of responses? This question will be answered with technologies up to date that have allowed us to study in‐depth the specific neural circuit and to map the complex connectivity.
Keywords: anxiety, autism, fear conditioning, hippocampus, learning and memory, reward, ventral CA1
1. INTRODUCTION
Hippocampus is known to be the most critical brain region in learning and memory. The substantial hippocampus, which physically takes up a large portion of the brain, was primarily subdivided into septal and temporal poles simply to categorize domains. However, studies revealed differentiating roles within the hippocampus allowing for categorization according to the function – dorsal and ventral hippocampus (vHip). Anatomical and electrophysiological studies have demonstrated long‐axis gradients, whilst gene expression studies showed that demarcated functional domains are superimposed. 1
Early studies have led to a strong belief in the critical role of the dorsal hippocampus (dHip) in learning and memory, resulting in concentrated research in this region. 2 , 3 As for the vHip, the study of this specific region began in an attempt to better understand emotion‐related behaviour, especially those that are associated with anxiety. 4 , 5 , 6 , 7 Especially, understanding how emotions are generated from the vCA1 can further provide information related to various diseases such as addiction, autism and anxiety. Neuroscience began with the observation of human brain anatomy which permitted defining its structure; thus, it was limited to defining the function. Since hippocampus is a relatively conserved brain structure among mammals, studying hippocampus in animal models like rodents can indirectly provide information about human phenotypes.
This review is focused on studies primarily performed in rodents to define the role of vCA1 participating in different behaviours with multiple neural projections‐starting from the earliest studies of the vHip to more in‐depth studies focused on social memory, fear, anxiety and reward‐related behaviours.
2. HIPPOCAMPUS, THE PREMISE OF MEMORY
Before understanding the role of the hippocampus, it is necessary to mention the most relatable H.M case – over hundreds of hippocampal studies were originated from this patient. The subject was treated with bilateral medial temporal lobectomy to cure epilepsy. The treatment resolved epileptic symptoms, however, the subject suffered from severe side effects that led to the inability to form new memories. This severity of H.M's memory impairment has inspired scientists to further investigate the role of the hippocampus in learning and memory. 8 H.M's case has intrigued many scientists to work on the hippocampus from its anatomical structure to its function. Similar to the study of other brain sites, research of the hippocampus was initially focused on lesion studies. However, the advancement of molecular biology, chemogenetics and optogenetics has allowed the experiment to proceed in more detail – in a cell‐type and a circuit‐specific manner.
Since the beginning of the hippocampal research, the subdivision into the dorsal and vHip was clear, whereas there has been an active debate on the individual role and function of the two regions 9 (Figure 1). Discovering the roles of each subdivision has been a critical step for further understanding how the hippocampus affects our memory system. The clarification of each subdivision with each specific role and its mechanism will be the first clue to provide a breakthrough for various clinical treatments on mental disorders (Figure 2).
FIGURE 1.
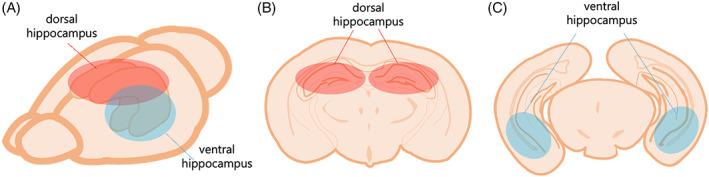
Illustration of the hippocampal section (A) Mouse brain with the hippocampus along its longitudinal axis. (B) Coronal section of the dHip. (C) Coronal section of the ventral hippocampus
FIGURE 2.
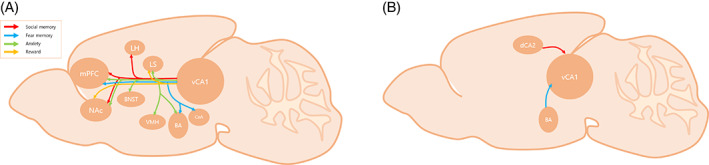
Input and output pathways of the vCA1 (A) efferent pathway and (B) afferent pathway
3. THE EARLIEST STUDIES OF THE vHip
Studies on the two subdivisions have allowed for a clear and further in‐depth understanding of the hippocampus. The spatial information is processed by the dHip with its connection to the cortical regions, whilst the vHip is more related to emotion due to its projection towards the amygdala and hypothalamus. 3 , 10 , 11 , 12 , 13 , 14 Lesion studies have historically been a fundamental technique in neuroscience to investigate the necessity of the brain region under behavioural observation.
Preliminary neuroscientific studies were less focused on the vHip, but rather, mostly focused on the dorsal regions as considered important for spatial learning. 3 , 10 , 11 Male Long‐Evans rats were tested for a double dissociation between dHip and vHip lesions, and the only dHip‐lesioned mice showed impairment of spatial memory. 11 Subsequent studies have shown controversial results 10 , 15 , 16 as vHip N‐methyl‐d‐aspartate (NMDA) lesions affected the ability to rapidly acquire a new context during the Morris water maze in male Long–Evans rats. 10
vCA1 and vCA3 are subregions in the vHip that play distinct roles in learned approach‐avoidance conflict processing. 17 Both are also well‐known regions that encode spatial information. In CA3, place cells are particularly distinct, especially along the longitudinal axis, wherein the ventral axis has a larger spatial map than the dorsal axis. 18
Also, the vCA1 participating in spatial navigation further led to the basic study of fear memory. Ventral hippocampus lesions disrupt exquisite surroundings, which impacts the impairment of contextual or spatial navigation (i.e. defence‐like behaviour). 12 , 13 , 14 Furthermore, a decrease in the rate of habituation of exploratory responses, retarded retention and extinction of fear conditioning were discovered in the vHip lesioned mice. 13 , 19
Lastly, there are many studies related to the cause of the anxiolytic effect. It has been reported that the selective temporal hippocampal lesions decrease anxiety, exhibiting an anxiolytic effect by the failure of avoidance in the open arm task. Also, a decreased neuroendocrine stress response was observed during confinement to a brightly lit chamber, 12 which was not found in the lesion of the septal pole. As for the dHip, its lesion failed to drastically change any defensive behavioural measure. Opposing results were obtained in the vHip, which was conducted to fade defensive behaviour. 14 , 20
4. LIMITATIONS OF THE LESION STUDY
Lesion studies have provided the first idea that these subdivisions play a different role, in an independent manner. Yet, as there cannot be a fine line in lesion specification, studies that claimed to be conducted under the same conditions showed conflicting results. 6 , 21 , 22 Controversies on the distinction of the hippocampal subregions were raised from the results of lesion studies. 10 , 15 , 16 One explanation could be that these differences were derived from the ambiguity of the ‘dorsal’ and the ‘ventral’ hippocampus. 6 Bannerman et al. 23 defined the dorsal part as 50% of the whole hippocampus from the septal pole, and the remaining half as the ventral part. 7 Whilst studies from the Moser group fixated on 20% of each pole, which was more restricting but did not cover the lesion of the whole hippocampus. 3 , 12
Furthermore, recently another subdivision of the hippocampus was defined – the intermediate hippocampus. 24 This is evidence that partition of lesion is a limited method as it lacks accuracy. This imprecision could possibly affect other regions than the region of interest. For more intricate hippocampal research, recent technology has allowed for more accuracy. The hippocampus has been categorized beyond the dorsal and ventral – the region has been further subdivided within. The ventral and the dHip both are independently subdivided into CA1, CA2 and CA3. This review will be mainly focusing on the vCA1 subregion, which is actively being studied among other vHip subdivisions.
5. vCA1 AND THE EFFECT ON SOCIAL MEMORY
The term ‘social memory’ was first implicated by Thor and Holloway in 1982, by observing rodents that possess the ability to discriminate conspecifics proving the capability of social recognition. 25 , 26 This is important for rodents as it gives the ability to forage, mate and create a social hierarchy. The absence of sociability may even lead to negative emotions, as it was reported that once mice were isolated, they showed impaired social memory 27 and displayed depressive‐like behaviour. 28 , 29 Preceding studies, which were simply focused on observations of lesions, proved that the hippocampus plays a critical role in sociability, but there was controversy on which specific part of the hippocampus needs to be explored. 21 , 22 , 26 , 30
It has been reported that the sociability of rodents can be reproduced in the laboratory by observing social interaction or social memory. 30 , 31 Under this kind of setting, researchers could elaborate neural pathways related to social behaving in rodents. Rodents show curiosity towards a never‐before‐met mouse, thus showing higher investigating time to the “novel” mouse compared to the ‘familiar’ mouse. By artificially inhibiting and stimulating activated vCA1 neurons through optogenetics, Okuyama et al. 32 proposed that the ‘engram cells’ among the Nucleus Accumbens (NAc)‐projecting vCA1 pyramidal cells are the key to storing social memory. Moreover, Tonegawa's group showed social memory recall via optogenetically reactivating the ‘engram cells’, vCA1 neurons responding to the familiar mouse, after a certain amount of time from the first exposure. Rao's study extends to monitoring regions of the brain and concluded that the vHip specifically responds to the presence of conspecifics with elevated sharp‐wave ripple activities, 33 indicating that the vCA1 is the core for memory and recognition, thus, discriminating familiar and novel mice.
Additionally, recent research has connected social memory to the dorsal CA2 (dCA2)‐vCA1‐NAc circuit. 32 , 34 , 35 , 36 , 37 The input of dCA2 towards the vCA1 is required for memory processing including memory encoding, consolidation and recall. Then, social memory is stored in the vCA1. 35 , 37 , 38 The experimental mice tended to show less interest towards familiar mice and interacted more with the novel mouse. Such behaviour is mediated by the Nac that receives inputs from the vCA1. 32
In parallel, another pivotal pathway is rising from the vCA1 to medial prefrontal cortex (mPFC). Chemogenetic activation and inactivation were performed chronically with continuous clozapine‐N‐oxide via water delivery to observe the participation of the vCA1‐mPFC circuit in social memory‐ in both cases, social memory was impaired. Philips et al. have further demonstrated that this vCA1‐mPFC pathway mediates hyperactivation in Mecp2‐mutant mice, with overrepresented vHip fibres in the mPFC. The phenotype of this Rett syndrome mouse model was similar to autistic‐like behaviour, resulting in the loss of ability to discriminate against familiar mice. 39
6. AUTISM AND SOCIAL MEMORY
A key characteristic of autism is the deterioration of social function; therefore, observing mice with gene mutations associated with autism is a textbook condition for observing sociability. 40 , 41 , 42 , 43 Affected regions of the brain can be identified through gene knockout (KO), and this technique has been adopted by many studies to explore the influence of autism on the brain. 27 , 39 , 41 , 43 Once the importance of vCA1 in social memory was discovered, studies can precisely define which phenotype is the result of social memory in mouse model of autism.
The first target phenotype that creates autistic conditions is X‐linked heterozygous mutations in methyl CpG binding protein 2 (MeCP2). This has been proven by human studies that show patients suffering from autism between 6 and 18 months specifically carry phenotypes with deteriorated cognitive and social functions. 44 The deletion of MeCP2 made differences between the MeCP2 KO and wildtype mice evident, as basal Fos activity was higher in the vHip of the MeCP2 KO mouse. 39 The three‐chamber test and unrestricted paradigms have proven that MeCP2 KO mice are incapable of storing social memory. Owing to the superiority in number of activated mPFC projecting neurons compared to that of the lateral hypothalamus (LH) projecting‐vHip neurons, Phillips et al. 39 have focused on the identification of the vHip‐mFPC neurons. The long‐term potentiation was not induced and chemogenetic inhibition via hM4Di has rescued the social deficit of MeCP2 KO mice.
Various ex vivo and in vivo recordings have shown the connection between pyramidal neurons of vCA1 to the mPFC mainly located in layers 2/3 and 5. 39 , 45 , 46 Additionally, recent studies revealed that the vCA1 pyramidal neurons also innervate PV+ neurons in the mPFC. 47 Phillips et al. 39 performed wheat germ agglutinin (or WGA) transsynaptic labelling with immunohistochemistry to determine the pattern of innervating vHip axons in the mPFC. In WT mice, WGA‐labelled neurons were mainly excitatory pyramidal neurons whereas MeCP2 KO mice showed an articulated fraction of PV+ neurons ‐expressing inhibitory γ‐aminobutyric acid (GABAergic) interneurons. 39 It had been previously reported that the PV+ neurons are required for social discrimination, 27 , 47 but in excess, these neurons may cause an imbalance between excitation and inhibition in the mPFC, which can affect the social memory formation. 39
Shank3, a gene known to cause neurodevelopmental and neurobehavioural deficits in 22q13 deletion syndrome, is another clear feature associated with autism. 48 Shank3 KO in mice resulted in reduced levels of proteins, which are believed to function as scaffolds in the postsynaptic density of glutamatergic synapses. 48 , 49 Moreover, it is reported that by deleting Shank3 gene in mice resulted in higher levels of self‐grooming alongside social behaviour and novel object recognition impairment. 50 , 51 Impairment in long‐term social memory (24 h) was observed in Shank3 KO mice, whilst the social memory was maintained in the short term (30 min). The connection of mPFC with the hippocampus was observed earlier in Shank3 mice by Harony‐Nicolcas et al. 52 describing that the dorsal CA1 (dCA1)‐prelimbic mPFC circuitry displayed attenuated synaptic plasticity. In Shank3 KO mice, reduced sociability has led to observed disruption of sharp‐wave ripples. Neurons activated during social behaviour underlying in the vCA1 were minor in Shank3 KO mice, which resulted in an inability to socially discriminate. 53
Defining the synaptic bases related to social behaviour would provide an insight to understand psychiatric disorders such as schizophrenia or depression, as this phenotype is articulated in a mouse model of autism. 53 Among the hippocampal subregions, dysfunction in CA1 is specific to schizophrenia, whereas dysfunction in CA2/CA3 is attributed to schizophrenia and bipolar disorder. Given that the vCA1 has a strong projection to the mPFC and ventral subiculum (vSUB) which are both involved in schizophrenia it is a suitable region to study psychiatric disorder. 39 , 54
7. vCA1 AND THE EFFECT ON FEAR MEMORY
Fear conditioning being simple yet compelling acts as the premise of a behavioural paradigm allowing in‐depth study of circuit mechanisms. For a whilst, research was mainly focused on the dorsal hippocampal CA1 as a key region of contextual fear memory. 2 , 56 , 57 Early studies based on the lesion studies demonstrated that the vHip was not involved in contextual fear memory. Beginning with ibotenate lesion studies in the 1990s, the role in fear memory of the vCA1 was first mentioned. 3 , 10 , 11 , 12 , 13 , 14 However, these lesion studies showed some discrepancies over the functions and even were not enough to study in circuit levels. New approaches not only allowed to discover the role of vCA1, but also its circuit and its underlying mechanism.
To elucidate the importance of vCA1 in encoding contextual information, various manipulations were performed both in vivo and ex vivo. Recent studies remarkably empowered the role of vCA1 by using miniscope calcium imaging, electrophysiology and optogenetics. Memory retrieval was the primarily well‐known role of the vCA1 during contextual fear memory with projections towards both mPFC and basal amygdala (BA) pathways. 13 , 19 , 57 , 58 , 59 , 60 , 61 , 62 It has been reported that a subset of vCA1 neurons send dual projections to mPFC and BA. 57 , 59 , 60 , 61 , 63 Furthermore, as vCA1 neurons are important for context acquisition, it is also important to mention its role in the memory encoding process. 62 Jimenez et al. 58 recently revealed that activated vCA1 neurons during fear conditioning were necessary for memory encoding, and another subset of neurons activated during memory retrieval are correlated to the shock‐encoding neurons. However, there still are discrepancies to defining vCA1 neurons between the shock‐encoding neurons and contextual neurons. 58 , 62 , 64
As the amygdala is the core region of fear responses, vCA1 neurons also project into the central amygdala (CeA), inspite of having a weaker response. 65 , 66 A further studies by Xu et al. 67 added that the central amygdala is required for context‐dependent retrieval of cued fear memory.
Furthermore, the connection between the vHip and mPFC in fear memory is also being actively investigated. Both PL and IL receive input from the vHip that are uniquely involved in fear renewal rather than the suppression of fear extinction. Maren et al. 56 have several works on this subject, 56 , 59 , 60 , 68 , 69 from which they have asserted that fear renewal is driven by vHip neurons by fostering fear expression with PL activation and limiting fear suppression by inhibiting IL. 70 Also, inhibition of the vHip‐PL during memory acquisition disabled memory recall, suggesting that the vHip updates any current spatial information to the PL. 71 In a behavioural paradigm in which mice learn to discriminate between a safety cue and foot shock cue, vCA1 neurons projecting to the prelimbic cortex showed higher population activity when exposed to safety cues. This suggests a possible role for this circuit in inhibiting fear expression. 72 In addition, Val 66 Met, a human variant of the brain‐derived neurotrophic factor (BDNF) gene, is known to be associated with impaired fear extinction. Giza et al. 73 demonstrated that the BDNF Met prodomain reduces dendritic spines and eliminates synapses in the hippocampus. Their study was prolonged to an in vivo study, where they confirmed similar morphological changes (reduction of dendritic spine density and prelimbic projections) were observed in the vCA1 of periadolecent mice, coupled with impaired fear extinction. In adolescent BdnfMet/Met mice, the similar spine and prelimbic innervation deficits were found. Also, fibre photometry experiment has proven that vCA1 neurons projecting to the prelimbic encode extinction. As with relapse of fear memory, vCA1 neurons projecting to the CeA is known to mediate this process as inhibiting this pathway abolishes fear renewal response. 67 vCA1 neurons projecting to the infralimbic cortex was shown to recruit PV+ interneurons thereby applying feed‐forward inhibition to mediate fear relapse. 74
8. VCA1 AND THE EFFECT ON ANXIETY
A strong connectivity between the mPFC and vCA1 has been reported in many previous studies, yet it was unknown whether vCA1‐mPFC neurons are related to anxiety. 39 , 57 , 59 , 60 , 61 , 63 , 72 , 75 Neurons residing in the mPFC are able to differentiate safe and aversive locations during elevated plus maze (EPM). 75 Also, the high correlation between theta‐frequency activity of the mPFC and vCA1 in an anxiogenic environment strengthens the importance of this circuit. 75 , 76 Anxiety‐related behaviour can be examined by inhibiting vCA1‐mPFC circuit by hyperpolarising ion pumps, known as Archaerhodopsin (ArchT). Moreover, observation of live cell recording of vCA1 neurons projecting to various regions shows inputs towards the mPFC exhibit higher neuronal activity than other brain regions during EPM. 77
However, a different claim also exists – the work by Kheirbek's lab proposed a conflicting result concluding that showed a different result, that the activation of vCA1 axon terminals projected to the LH increases anxiety‐like state. 78 Both electrophysiology and miniature miniscope recordings have revealed that the vCA1 neurons were selectively activated in the open‐arm session during the EPM, in which the neurons exhibited a significantly higher rate of Ca2+, and especially neurons projecting from the LH are responsible for inducing anxiety‐like behaviour and avoidance. 78 The authors suggest that this inconsistency was found in line with the theory of LeDoux, who proclaimed that thalamic ‘low’ and cortical ‘high’ roads are both critical to auditory fear processing. 79
Accordingly, a similar paradigm was performed in the vCA1‐NAc, vCA1‐lateral septum (LS) circuit demonstrating that the input of the vCA1 towards NAc and LS also impacts anxiogenic behaviour. 80 , 81 Glangetas et al. 82 added that this anxiolytic behaviour is driven by NMDA receptors dependent long‐term potentiation of vCA1/vSUB to BNST (bed nucleus of the stria terminalis) pathway. This was proven as the inhibition with NMDA receptors antagonist in BNST blocked (disrupted) anxiolytic effect mediated by high frequency stimulation of vSUB and vCA1. 82
The last downstream target is the ventromedial hypothalamus (VMH), and the social interaction was observed to see the influence on aggressive behaviour. Unlike the previously reviewed studies, this report focuses on maximized aggressive behaviour manipulated by post‐weaning social isolation, which is followed by foot shock‐induced stress then exposure to a stranger mouse. This setting increased attacking behaviour which was the mainly observed response. By suppressing the activity of vCA1‐VMH neurons with hM4Di the attacking behaviour was decreased, contrary to when the activated attacking behaviour was restored. These findings show connectivity between attacking behaviour and vCA1‐VMH. Open field test (OFT) testing was also conducted by artificially activating VMH‐vCA1 projecting neurons resulting in anxiogenic behaviour in rats depict by the preferred periphery positioning compared to centred positioning. 84
Projections from the posterior BLA are well‐known for the afferent pathway involved in anxiogenic behaviour. 78 , 84 , 85 , 86 , 87 , 88 Pi et al. 86 have subdivided BLA in anterior–posterior, and vCA1 with deep layer (calbindin1‐negative), and superficial layer (calbindin1‐positive). These subregions are specifically connected to the corresponding subregion: anterior BLA (aBLA) innervating deep layer whereas posterior BLA (pBLA) is innervated in the outer layer of vCA1. Photostimulation of the pBLA‐vCA1 has led the mice to exhibit anxiolytic behaviour, whilst aBLA‐vCA1 mediated anxiety‐like behaviour. 86 Anxiety can also cause such symptoms when a subject experiences unpredictable chronic mild stress. 85 , 87 Decreased phosphorylation of GluA1 affecting AMPAR (α‐amino‐3‐hydroxy‐5‐methyl‐4‐isoxazole propionic acid receptor) function due to chronic stress can weaken the connectivity of pBLA‐vCA1, inducing depressive‐like state and anxiety. 85
Chronic restraint stress (CRS), which mediates social avoidance and anxiety‐like behaviours also has an effect on the increase in acetylcholine muscarinic receptor (mAChR) in vHip. This social avoidance behaviour was further rescued by mAChRs antagonist, by suppressing excitatory synaptic transmission. 89 Furthermore, unlike male subjects, anxiety behaviour was unnoticed in females. Fos immunohistochemistry shows stronger enrolment of vHip in innate anxiety in male mice. 90 This anxiety‐like behaviour was shown to be specifically associated with the activation of vCA1 among other subregions in the vHip. 17
9. REWARD‐RELATED BEHAVIOUR, A GUIDE TOWARDS ADDICTION
Motivational behaviour can lead to two opposing consequences – either extremely strong (addiction) or practically absent (depression). This is due to how the subject inputs the surrounding environment and interprets them. The subjects' interpretation of surrounding input is then behaviourally shown as the subjects' deportment. Studying motivational behaviour can be replicated in two ways; either exhibiting approached seeking behaviour or oppositely avoiding punishment.
Between the two different motivational behaviours, an avoidance mediated paradigm requires only simple and straightforward tasks, whilst rewarding rodents take a lot longer for training due to complex behavioural tasks. Seeking behaviour is the most common behaviour of rodents to translate their motivation – thus, most of the experimental animals undergo food or water deprivation before the experiment. Nucleus accumbens (NAc) has always been the core region mediating social behaviour, commonly known to generate goal‐directed behaviour. 91 , 92 , 93 In reward‐related behaviour, the NAc is the most observed region of the brain as it is considered as the neural interface between motivation and action with its dynamic dopaminergic effect. 92 , 94 Especially, the connectivity between ventral tegmental areas, another source of dopamine, is well studied. 95 , 96
Recent studies have yielded strong evidence that the role of the hippocampus is important, as much as NAc, which is necessary and sufficient to modulate seeking behaviour during reward. 97 , 98 The subregions of the hippocampus are known to encode differently during reward; dCA1 is more likely to encode reward proximity, whilst vCA1 neurons are more likely to predict reward. 99 To understand the connectivity of the vCA1 to the NAc, Zhou et al. 100 , 101 investigated the labelling of engram cells in each region during cocaine‐conditioned place preference (CPP). Their chemogenetic inactivation data revealed that the non‐cocaine control engram cells in the vCA1 affect the retrieval of cocaine CPP memory, suggesting that the vCA1 engram cells are involved in only contextual information for a specific reward memory. Activation of the NAc core was sufficient for CPP, whilst vCA1 neurons were silenced, suggesting that the NAc core engram cells projecting from the vCA1 are important for memory recall. 100 , 101 Moreover, by using D1‐cre driver line, Zhou et al. 100 , 101 reported that the main cell type of vCA1 to NAc core engram cells were D1 cells, rather than D2 cells.
Due to the diverse connectivity of vCA1, not only the NAc but other regions are also connected to the vCA1. A symbolic region is a lateral septum and this region plays an essential role in feeding behaviour. The LS connection to the vHip (mainly targeted ventral CA3 [vCA3] and dentate gyrus) was previously discovered and showed that optogenetic activation of the vHip‐LS reduces food intake. 103 As for the vCA1, during the goal‐directed food‐seeking lever‐press behaviour task, vCA1‐LS neurons exhibited a higher rate of calcium activity, especially during the food intake. 81 Reward omission reduced the activity of LS‐projecting vCA1 neurons, suggesting that vCA1‐LS neurons are specific to the food reward. 103
Interestingly, another study by Yoshida et al. 104 demonstrated that decreased calcium activity of vCA1 pyramidal neurons promotes the sustainment of goal‐directed lever pressing. Here, goal‐directed behaviours not only include the obtention of a reward but also the avoidance of punishment, wherein such behaviour was enhanced when vCA1 neuron activity decreases due to suppressed aversive emotional states as mediated by serotonergic neurons from the median raphe region. 104
Motivated behaviour can be observed simply by providing direct ‘reward’ to the subject, but it can also be observed indirectly by inducing ‘hopefulness (HF)’ or ‘helplessness (HL)’‐modulated behaviour. The two notions were respectively introduced to the subject by anticipated avoidance training and foot shocks. HF animals appeared to carry potentiation of spatial learning with strengthened posterior BLA‐vCA1 connectivity since this connection was faded in HL mice accompanied with memory deficit. Optogenetic modulation has allowed to artificially potentiate pBLA‐vCA1 connections in the HL mice, and this was sufficient to mimic the phenotype of HF mice. 105 Another very interesting study was recently carried out by Lin's group showing that the inputs to dCA3 from vCA1 are crucial in spatial memory. 106 This was proven as silencing of vCA1‐dCA3 circuit impaired spatial learning and memory during the object location memory test and the object recognition memory test. 106
10. COMPLEX CONNECTIVITY
Various studies have been conducted to show the diversity of vCA1 neurons; studies focusing on behavioural, circuit specificity, cell type and its relying molecular mechanisms. 64 , 78 , 79 , 82 Recent techniques have allowed accurate investigation of neural projections in a cell‐, circuit‐ and time‐specific manner. A subset of vCA1 neurons can be activated in multiple behavioural tasks, and several projections play multiple roles.
In recent years, active interest of interneurons has risen supporting the idea that these neurons are as critical as excitatory neurons. Furthermore, through mapping technology, we are now able to visualise the connectivity of vCA1 neurons into different regions. The question of whether the vCA1 neurons are necessary or sufficient to trigger such phenotype will be the next confronted question. Similar to the importance of pyramidal neurons in social memory, among interneurons, PV+ neurons residing in the vCA1 region have been the focus of research as a key cell type that mediates social memory. 27 , 32 , 35 It has been reported that hippocampal GABAergic interneurons exhibit greater cellular diversity compared to pyramidal neurons. This firing action potential of the theta cycle, which are interacted with glutamatergic inputs that are necessary for synaptic temporal dynamics and network oscillations. 106 , 107 Moreover, among different types of interneurons, PV+ neurons have been shown to control the activity of their target cells. 107 , 108
Pyramidal neurons and PV+ neurons both play an important role in social memory retrieval and recognition of conspecifics, yet it is still unclear whether and how these neurons are interrelated. Along with the importance of PV+ neurons in social memory, the plasticity of PV+ neurons critically mediates long‐term consolidation of spatial memory. It is partially influenced by D1/5 dopamine receptor signals, which causes DARPP‐32 and ERK phosphorylation in the PV+ interneurons. 110 Studies that define cell types remain lacking, hence, molecular approaches will be an upcoming challenge.
In addition to the studies that were previously mentioned above, mapping axonal projections enabled visualizing neural projections deriving from the vCA1. It has been studied that vCA1 are connected to various distinct brain regions – projecting to the NAc, mPFC, LS, LH, BNST, VMH and amygdala. These findings were first performed by observing neural alterations in a specific behaviour task and adding to this recently, reconstruction and mapping of long‐range projections consolidated the information regarding the heterogeneity of vCA1 neurons. The manoeuvre of juxtacellular labelling permitted tagging single neurons with neurobiotin. By determining soma location and dendritic arborization, the author identified three major distinct routes of vCA1 pyramidal neurons. 110 To analyse how various types of information are controlled in these diverse pathways, Ciocchi et al. manipulated tetrode recordings and antidromic spiking analysis with an optic stimulus in vCA1, whilst performing different behavioural tasks – spatial memory, reward and anxiety. 77 vCA1 neurons seemed to be selective to each task with its selective pathways, but triple projecting neurons which are responsive to the overall task were exigent exhibiting a high firing pattern. More recently, the assessment of high‐throughput sequencing of genetically barcoded neurons (MAPseq) enabled axonal tracing of thousands of vCA1 neurons. Distinct pathways selectively receiving the vCA1 inputs and outputs were identified via molecular profiling in support with the viral tracing of vCA1. 63
The transcriptional profiles of vCA1 and dCA1 are distinct from each other. 112 Although most of the inputs of the former come from the intrahippocampal regions, which are stronger along the rostral‐caudal axis and are distinct from those of dCA1, both have regions that still receive similar inputs from the amygdala nuclei and olfactory areas. 113
To understand the heterogeneity of vCA1, researchers focused on tracing vCA1 neurons in distinct pathways over time and achieved to define multi‐projecting neurons. Nonetheless, several neural projections participate in multiple behavioural features, such as the vCA1‐mPFC pathway engaged in the social memory and fear memory. In addition, between vCA1 and BLA, the projection is not unilateral but rather bidirectional. Understanding how neurons interplay within the same pathway whilst the subject is exposed to different behaviours seems to be prominent in the future.
11. CONCLUSION
The hippocampal complex is one of the most important fields of neuroscience. Hippocampal research has been mostly centred on the dorsal region, thus, only recently ventral region studies focusing on the role of the vCA1 as a pre‐region has become a topic of interest. The recent study driven by Tao et al. 113 has proposed that unlike the dCA1, vCA1 receives inputs from the subregions of olfactory areas and amygdala nuclei. Even though several afferent regions are known, studies focusing on the inputs towards the vCA1 with its specific role would be prominent.
Also, unlike the dHip, subregions other than the vCA1 in the vHip require further investigation. vCA3 and the vSUB are progressively being investigated, but there are still many questions to be answered. Recent papers still use the term ‘vHip’ rather than specifying the subregion. 88 , 114 It has been suggested that the inner layer and the outer layer within the vCA1 play different roles. The rise of the technology will provide an insight of heterogeneity in the vHip.
The connections of vCA1 are complex, most of the neurons are projected towards two distinct regions, and some neurons exhibit even triple connectivity. It has been proved that vCA1‐BA and vCA1‐mPFC have individual projections during fear‐related memory, whilst some mPFC‐projecting vCA1 neurons were indirectly projecting to the BA. 62 The interpretation of these overall task‐activated cells would be the next challenge.
To enclose, the hippocampus has remained largely unfamiliar to neuroscientists, and through the exploration of its role in memory and spatial processing and navigation, we can understand how memory is formed and stored. Rodents were used in this study as they have genetic similarities with humans. Human episodic memories have been proven to be analogous to those of rodents and both also have similar heterogeneity functions along the anteroposterior axis. 73 , 115 However, there are still discrepancies in the frequency of theta between the two species 116 ; hence, it is necessary to perform further investigation to graft our findings to human behaviour.
CONFLICT OF INTEREST
The author declares that there is no conflict of interest to disclose.
Hong I, Kaang B‐K. The complexity of ventral CA1 and its multiple functionalities. Genes, Brain and Behavior. 2022;21(7):e12826. doi: 10.1111/gbb.12826
Funding information National Research Foundation, Grant/Award Number: NRF‐2012R1A3A1050385
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Strange B, Witter M, Lein E, et al. Functional organization of the hippocampal longitudinal axis. Nat Rev Neurosci. 2014;15:655‐669. doi: 10.1038/nrn3785 [DOI] [PubMed] [Google Scholar]
- 2. Bast T, Zhang WN, Feldon J. The ventral hippocampus and fear conditioning in rats. Different anterograde amnesias of fear after tetrodotoxin inactivation and infusion of the GABA(A) agonist muscimol. Exp Brain Res. 2001;139(1):39‐52. doi: 10.1007/s002210100746 [DOI] [PubMed] [Google Scholar]
- 3. Moser MB, Moser EI, Forrest E, Andersen P, Morris RG. Spatial learning with a minislab in the dorsal hippocampus. Proc Natl Acad Sci USA. 1995;92(21):9697‐9701. doi: 10.1073/pnas.92.21.9697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bannerman DM, Rawlins JN, McHugh SB, et al. Regional dissociations within the hippocampus‐‐memory and anxiety. Neurosci Biobehav Rev. 2004;28(3):273‐283. doi: 10.1016/j.neubiorev.2004.03.004 [DOI] [PubMed] [Google Scholar]
- 5. Bannerman DM, Sprengel R, Sanderson DJ, et al. Hippocampal synaptic plasticity, spatial memory and anxiety. Nat Rev Neurosci. 2014;15(3):181‐192. doi: 10.1038/nrn3677 [DOI] [PubMed] [Google Scholar]
- 6. Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65(1):7‐19. doi: 10.1016/j.neuron.2009.11.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bannerman DM, Deacon RM, Offen S, Friswell J, Grubb M, Rawlins JN. Double dissociation of function within the hippocampus: spatial memory and hyponeophagia. Behav Neurosci. 2002;116(5):884‐901. doi: 10.1037//0735-7044.116.5.884 [DOI] [PubMed] [Google Scholar]
- 8. Andersen P, Morris R, Amaral D. In: Bliss T, O'Keefe J, eds. The Hippocampus Book. Oxford University Press; 2007:551‐554. [Google Scholar]
- 9. Amaral DG, Witter MP. Hippocampal formation. In: Paxinos G, ed. The Rat Nervous System. 2nd ed. Academic Press; 1995:443‐493. doi: 10.1016/B978-012547638-6/50022-5 [DOI] [Google Scholar]
- 10. Ferbinteanu J, McDonald RJ. Dorsal and ventral hippocampus: same or different? Psychobiology. 2000;28:314‐324. doi: 10.3758/BF03331990 [DOI] [Google Scholar]
- 11. Hock BJ Jr, Bunsey MD. Differential effects of dorsal and ventral hippocampal lesions. J Neurosci. 1998;18(17):7027‐7032. doi: 10.1523/JNEUROSCI.18-17-07027.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kjelstrup KG, Tuvnes FA, Steffenach HA, Murison R, Moser EI, Moser MB. Reduced fear expression after lesions of the ventral hippocampus. Proc Natl Acad Sci U S A. 2002;99(16):10825‐10830. doi: 10.1073/pnas.152112399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rogers JL, Hunsaker MR, Kesner RP. Effects of ventral and dorsal CA1 subregional lesions on trace fear conditioning. Neurobiol Learn Mem. 2006;86(1):72‐81. doi: 10.1016/j.nlm.2006.01.002 [DOI] [PubMed] [Google Scholar]
- 14. Trivedi MA, Coover GD. Lesions of the ventral hippocampus, but not the dorsal hippocampus, impair conditioned fear expression and inhibitory avoidance on the elevated T‐maze. Neurobiol Learn Mem. 2004;81(3):172‐184. doi: 10.1016/j.nlm.2004.02.005 [DOI] [PubMed] [Google Scholar]
- 15. Loureiro M, Lecourtier L, Engeln M, et al. The ventral hippocampus is necessary for expressing a spatial memory. Brain Struct Funct. 2012;217(1):93‐106. doi: 10.1007/s00429-011-0332-y [DOI] [PubMed] [Google Scholar]
- 16. Pothuizen HH, Zhang WN, Jongen‐Rêlo AL, Feldon J, Yee BK. Dissociation of function between the dorsal and the ventral hippocampus in spatial learning abilities of the rat: a within‐subject, within‐task comparison of reference and working spatial memory. Eur J Neurosci. 2004;19(3):705‐712. doi: 10.1111/j.0953-816x.2004.03170.x [DOI] [PubMed] [Google Scholar]
- 17. Schumacher A, Villaruel FR, Ussling A, Riaz S, Lee A, Ito R. Ventral hippocampal CA1 and CA3 differentially mediate learned approach‐avoidance conflict processing. Curr Biol. 2018;28(8):1318‐1324.e4. doi: 10.1016/j.cub.2018.03.012 [DOI] [PubMed] [Google Scholar]
- 18. Kjelstrup KB, Solstad T, Brun VH, et al. Finite scale of spatial representation in the hippocampus. Science (New York, N.Y.). 2008;321(5885):140‐143. doi: 10.1126/science.1157086 [DOI] [PubMed] [Google Scholar]
- 19. Nadel L. Dorsal and ventral hippocampal lesions and behavior. Physiol Behav. 1968;3(6):891‐900. doi: 10.1016/0031-9384(68)90174-1 [DOI] [Google Scholar]
- 20. Pentkowski NS, Blanchard DC, Lever C, Litvin Y, Blanchard RJ. Effects of lesions to the dorsal and ventral hippocampus on defensive behaviors in rats. Eur J Neurosci. 2006;23(8):2185‐2196. doi: 10.1111/j.1460-9568.2006.04754.x [DOI] [PubMed] [Google Scholar]
- 21. Bannerman DM, Lemaire M, Beggs S, Rawlins JNP, Iversen SD. Cytotoxic lesions of the hippocampus increase social investigation but do not impair social‐recognition memory. Exp Brain Res. 2001;138(1):100‐109. doi: 10.1007/s002210100687 [DOI] [PubMed] [Google Scholar]
- 22. Squires AS, Peddle R, Milway SJ, Harley CW. Cytotoxic lesions of the hippocampus do not impair social recognition memory in socially housed rats. Neurobiol Learn Mem. 2006;85(1):95‐101. doi: 10.1016/j.nlm.2005.08.012 [DOI] [PubMed] [Google Scholar]
- 23.Bannerman DM, Yee BK, Good MA, Heupel MJ, Iversen SD, Rawlins JN. Double dissociation of function within the hippocampus: a comparison of dorsal, ventral, and complete hippocampal cytotoxic lesions. Behav Neurosci. 1999;113(6):1170‐1188. doi: 10.1037//0735-7044.113.6.1170 [DOI] [PubMed] [Google Scholar]
- 24. Kenney J, Manahan‐Vaughan D. Learning‐facilitated synaptic plasticity occurs in the intermediate hippocampus in association with spatial learning. Front Synap Neurosci. 2013;5:10. doi: 10.3389/fnsyn.2013.00010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Thor DH, Holloway WR. Social memory of the male laboratory rat. J Comp Physiol Psychol. 1982;96(6):1000‐1006. doi: 10.1037/0735-7036.96.6.1000 [DOI] [Google Scholar]
- 26. von Heimendahl M, Rao RP, Brecht M. Weak and nondiscriminative responses to conspecifics in the rat hippocampus. J Neurosci. 2012;32(6):2129‐2141. doi: 10.1523/JNEUROSCI.3812-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Deng X, Gu L, Sui N, Guo J, Liang J. Parvalbumin interneuron in the ventral hippocampus functions as a discriminator in social memory. Proc Natl Acad Sci U S A. 2019;116(33):16583‐16592. doi: 10.1073/pnas.1819133116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ieraci A, Mallei A, Popoli M. Social isolation stress induces anxious‐depressive‐like behavior and alterations of neuroplasticity‐related genes in adult male mice. Neural Plast. 2016;2016:6212983‐6212913. doi: 10.1155/2016/6212983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Patel D, Kas MJ, Chattarji S, Buwalda B. Rodent models of social stress and neuronal plasticity: relevance to depressive‐like disorders. Behav Brain Res. 2019;369:111900. doi: 10.1016/j.bbr.2019.111900 [DOI] [PubMed] [Google Scholar]
- 30. Kogan JH, Frankland PW, Silva AJ. Long‐term memory underlying hippocampus‐dependent social recognition in mice. Hippocampus. 2000;10(1):47‐56. doi:10.1002/(SICI)1098‐1063(2000)10:1<47::AID‐HIPO5>3.0.CO;2‐6 [DOI] [PubMed] [Google Scholar]
- 31. Grant EC, Mackintosh JH. A comparison of the social postures of some common laboratory rodents. Behaviour. 1963;21(3–4):246‐259. doi: 10.1163/156853963X00185 [DOI] [Google Scholar]
- 32. Okuyama T, Kitamura T, Roy DS, Itohara S, Tonegawa S. Ventral CA1 neurons store social memory. Science (New York, NY). 2016;353(6307):1536‐1541. doi: 10.1126/science.aaf7003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rao RP, von Heimendahl M, Bahr V, Brecht M. Neuronal responses to conspecifics in the ventral CA1. Cell Rep. 2019;27(12):3460‐3472.e3. doi: 10.1016/j.celrep.2019.05.081 [DOI] [PubMed] [Google Scholar]
- 34. Elise C, Wang S, Waters RC, Vasquez B, Gould E. Activation of the CA2‐vCA1 pathway reverses social discrimination dysfunction in Shank3B knockout mice. bioRxiv. 2022. doi: 10.1101/2022.03.28.486130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Meira T, Leroy F, Buss EW, Oliva A, Park J, Siegelbaum SA. A hippocampal circuit linking dorsal CA2 to ventral CA1 critical for social memory dynamics. Nat Commun. 2018;9(1):4163. doi: 10.1038/s41467-018-06501-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Raam T, McAvoy KM, Besnard A, Veenema AH, Sahay A. Hippocampal oxytocin receptors are necessary for discrimination of social stimuli. Nat Commun. 2017;8(1):2001. doi: 10.1038/s41467-017-02173-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Harony‐Nicolas H, Kay M, du Hoffmann J, et al. The hippocampal CA2 region is essential for social memory. Nature. 2014;508(1):88‐92. doi: 10.1038/nature13028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Watarai A, Tao K, Wang MY, Okuyama T. Distinct functions of ventral CA1 and dorsal CA2 in social memory. Curr Opin Neurobiol. 2021;68:29‐35. doi: 10.1016/j.conb.2020.12.008 [DOI] [PubMed] [Google Scholar]
- 39. Phillips ML, Robinson HA, Pozzo‐Miller L. Ventral hippocampal projections to the medial prefrontal cortex regulate social memory. eLife. 2019;8:e44182. doi: 10.7554/eLife.44182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. DeLorey TM, Sahbaie P, Hashemi E, Homanics GE, Clark JD. Gabrb3 gene deficient mice exhibit impaired social and exploratory behaviors, deficits in non‐selective attention and hypoplasia of cerebellar vermal lobules: A potential model of autism spectrum disorder. Behav Brain Res. 2008;187(2):207‐220. doi: 10.1016/j.bbr.2007.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hamilton SM, Spencer CM, Harrison WR, et al. Multiple autism‐like behaviors in a novel transgenic mouse model. Behav Brain Res. 2011;218(1):29‐41. doi: 10.1016/j.bbr.2010.11.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nadler JJ, Moy SS, Dold G, et al. Automated apparatus for quantitation of social approach behaviors in mice. Genes Brain Behav. 2004;3(5):303‐314. doi: 10.1111/j.1601-183X.2004.00071.x [DOI] [PubMed] [Google Scholar]
- 43. Silverman JL, Turner SM, Barkan CL, et al. Sociability and motor functions in Shank1 mutant mice. Brain Res. 2011;1380:120‐137. doi: 10.1016/j.brainres.2010.09.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Guy J, Hendrich B, Holmes M, Martin JE, Bird A. A mouse Mecp2‐null mutation causes neurological symptoms that mimic Rett syndrome. Nat Genet. 2001;27(3):322‐326. doi: 10.1038/85899 [DOI] [PubMed] [Google Scholar]
- 45. Dégenètais E, Thierry AM, Glowinski J, Gioanni Y. Synaptic influence of hippocampus on pyramidal cells of the rat prefrontal cortex: an in vivo intracellular recording study. Cereb Cortex (New York, N.Y.: 1991). 2003;13(7):782‐792. doi: 10.1093/cercor/13.7.782 [DOI] [PubMed] [Google Scholar]
- 46. Liu X, Carter AG. Ventral hippocampal inputs preferentially drive Corticocortical neurons in the Infralimbic prefrontal cortex. J Neurosci. 2018;38(33):7351‐7363. doi: 10.1523/JNEUROSCI.0378-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sun Q, Li X, Li A, et al. Ventral hippocampal‐prefrontal interaction affects social behavior via Parvalbumin positive neurons in the medial prefrontal cortex. iScience. 2020;23(3), 100894. doi: 10.1016/j.isci.2020.100894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Peça J, Feliciano C, Ting JT, et al. Shank3 mutant mice display autistic‐like behaviours and striatal dysfunction. Nature. 2011;472(7344):437‐442. doi: 10.1038/nature09965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Moessner R, Marshall CR, Sutcliffe JS, et al. Contribution of SHANK3 mutations to autism spectrum disorder. Am J Hum Genet. 2007;81(6):1289‐1297. doi: 10.1086/522590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bozdagi O, Sakurai T, Papapetrou D, et al. Haploinsufficiency of the autism‐associated Shank3 gene leads to deficits in synaptic function, social interaction, and social communication. Mol Autism. 2010;1(1):15. doi: 10.1186/2040-2392-1-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yang M, Bozdagi O, Scattoni ML, et al. Reduced excitatory neurotransmission and mild autism‐relevant phenotypes in adolescent Shank3 null mutant mice. J Neurosci. 2012;32(19):6525‐6541. doi: 10.1523/JNEUROSCI.6107-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Harony‐Nicolas H, Kay M, du Hoffmann J, Klein ME, Bozdagi‐Gunal O, Riad M, Daskalakis NP, Sonar S, Castillo PE, Hof PR, Shapiro ML, Baxter MG, Wagner S, Buxbaum JD.. Oxytocin improves behavioral and electrophysiological deficits in a novel Shank3‐deficient rat. eLife. 2017;6:e18904. doi: 10.7554/eLife.18904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tao K, Chung M, Watarai A, Huang Z, Wang MY, Okuyama T. Disrupted social memory ensembles in the ventral hippocampus underlie social amnesia in autism‐associated Shank3 mutant mice. Mol Psychiatry. 2022;27:2095‐2105. doi: 10.1038/s41380-021-01430-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zhou Y, Kaiser T, Monteiro P, et al. Mice with Shank3 mutations associated with ASD and schizophrenia display both shared and distinct defects. Neuron. 2016;89(1):147‐162. doi: 10.1016/j.neuron.2015.11.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ewing SG, Winter C. The ventral portion of the CA1 region of the hippocampus and the prefrontal cortex as candidate regions for neuromodulation in schizophrenia. Med Hypotheses. 2013;80(6):827‐832. doi: 10.1016/j.mehy.2013.03.026 [DOI] [PubMed] [Google Scholar]
- 56. Maren S, Holt W. The hippocampus and contextual memory retrieval in Pavlovian conditioning. Behav Brain Res. 2000;110(1–2):97‐108. doi: 10.1016/s0166-4328(99)00188-6 [DOI] [PubMed] [Google Scholar]
- 57. Richmond MA, Yee BK, Pouzet B, et al. Dissociating context and space within the hippocampus: effects of complete, dorsal, and ventral excitotoxic hippocampal lesions on conditioned freezing and spatial learning. Behav Neurosci. 1999;113(6):1189‐1203. doi: 10.1037/0735-7044.113.6.1189 [DOI] [PubMed] [Google Scholar]
- 58. Ishikawa A, Nakamura S. Ventral hippocampal neurons project axons simultaneously to the medial prefrontal cortex and amygdala in the rat. J Neurophysiol. 2006;96(4):2134‐2138. doi: 10.1152/jn.00069.2006 [DOI] [PubMed] [Google Scholar]
- 59. Jimenez JC, Berry JE, Lim SC, Ong SK, Kheirbek MA, Hen R. Contextual fear memory retrieval by correlated ensembles of ventral CA1 neurons. Nat Commun. 2020;11(1):3492. doi: 10.1038/s41467-020-17270-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Jin J, Maren S. Fear renewal preferentially activates ventral hippocampal neurons projecting to both amygdala and prefrontal cortex in rats. Sci Rep. 2015a;5:8388. doi: 10.1038/srep08388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Jin J, Maren S. Prefrontal‐hippocampal interactions in memory and emotion. Front Syst Neurosci. 2015b;9:170. doi: 10.3389/fnsys.2015.00170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kim WB, Cho JH. Synaptic targeting of double‐projecting ventral CA1 hippocampal neurons to the medial prefrontal cortex and basal amygdala. J Neurosci. 2017;37(19):4868‐4882. doi: 10.1523/JNEUROSCI.3579-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kim WB, Cho JH. Encoding of contextual fear memory in hippocampal‐amygdala circuit. Nat Commun. 2020;11(1):1382. doi: 10.1038/s41467-020-15121-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Gergues MM, Han KJ, Choi HS, et al. Circuit and molecular architecture of a ventral hippocampal network. Nat Neurosci. 2020;23(11):1444‐1452. doi: 10.1038/s41593-020-0705-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Silva BA, Burns AM, Gräff J. A cFos activation map of remote fear memory attenuation. Psychopharmacology (Berl). 2019;236(1):369‐381. doi: 10.1007/s00213-018-5000-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Cenquizca LA, Swanson LW. Spatial organization of direct hippocampal field CA1 axonal projections to the rest of the cerebral cortex. Brain Res Rev. 2007;56(1):1‐26. doi: 10.1016/j.brainresrev.2007.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kishi T, Tsumori T, Yokota S, Yasui Y. Topographical projection from the hippocampal formation to the amygdala: a combined anterograde and retrograde tracing study in the rat. J Comp Neurol. 2006;496(3):349‐368. doi: 10.1002/cne.20919 [DOI] [PubMed] [Google Scholar]
- 68. Xu C, Krabbe S, Gründemann J, et al. Distinct hippocampal pathways mediate dissociable roles of context in memory retrieval. Cell. 2016;167(4):961‐972.e16. doi: 10.1016/j.cell.2016.09.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Giustino TF, Maren S. The role of the medial prefrontal cortex in the conditioning and extinction of fear. Front Behav Neurosci. 2015;9:298. doi: 10.3389/fnbeh.2015.00298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Orsini CA, Kim JH, Knapska E, Maren S. Hippocampal and prefrontal projections to the basal amygdala mediate contextual regulation of fear after extinction. J Neurosci. 2011;31(47):17269‐17277. doi: 10.1523/JNEUROSCI.4095-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wang Q, Jin J, Maren S. Renewal of extinguished fear activates ventral hippocampal neurons projecting to the prelimbic and infralimbic cortices in rats. Neurobiol Learn Memory. 2016;134 Pt A:38‐43. doi: 10.1016/j.nlm.2016.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Twining RC, Lepak K, Kirry AJ, Gilmartin MR. Ventral hippocampal input to the Prelimbic cortex dissociates the context from the cue Association in Trace Fear Memory. J Neurosci. 2020;40(16):3217‐3230. doi: 10.1523/JNEUROSCI.1453-19.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Meyer HC, Odriozola P, Cohodes EM, et al. Ventral hippocampus interacts with prelimbic cortex during inhibition of threat response via learned safety in both mice and humans. Proc Natl Acad Sci. 2019;116(52):26970‐26979. doi: 10.1073/PNAS.1910481116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Giza JI, Kim J, Meyer HC, et al. The BDNF Val66Met Prodomain disassembles dendritic spines altering fear extinction circuitry and behavior. Neuron. 2018;99(1):163‐178.e6. doi: 10.1016/j.neuron.2018.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Marek R, Jin J, Goode TD, et al. Hippocampus‐driven feed‐forward inhibition of the prefrontal cortex mediates relapse of extinguished fear. Nat Neurosci. 2018;21(3):384‐392. doi: 10.1038/s41593-018-0073-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Adhikari A, Topiwala MA, Gordon JA. Synchronized activity between the ventral hippocampus and the medial prefrontal cortex during anxiety. Neuron. 2010;65(2):257‐269. doi: 10.1016/j.neuron.2009.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Padilla‐Coreano N, Bolkan SS, Pierce GM, et al. Direct ventral hippocampal‐prefrontal input is required for anxiety‐related neural activity and behavior. Neuron. 2016;89(4):857‐866. doi: 10.1016/j.neuron.2016.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Ciocchi S, Passecker J, Malagon‐Vina H, Mikus N, Klausberger T. Brain computation. Selective information routing by ventral hippocampal CA1 projection neurons. Science (New York, N.Y.). 2015;348(6234):560‐563. doi: 10.1126/science.aaa3245 [DOI] [PubMed] [Google Scholar]
- 79. Jimenez JC, Su K, Goldberg AR, et al. Anxiety cells in a hippocampal‐hypothalamic circuit. Neuron. 2018;97(3):670‐683.e6. doi: 10.1016/j.neuron.2018.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155‐184. doi: 10.1146/annurev.neuro.23.1.155 [DOI] [PubMed] [Google Scholar]
- 81. Kosugi K, Yoshida K, Suzuki T, et al. Activation of ventral CA1 hippocampal neurons projecting to the lateral septum during feeding. Hippocampus. 2021;31(3):294‐304. doi: 10.1002/hipo.23289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Muir J, Tse YC, Iyer ES, et al. Ventral hippocampal afferents to nucleus Accumbens encode both latent vulnerability and stress‐induced susceptibility. Biol Psychiatry. 2020;88(11):843‐854. doi: 10.1016/j.biopsych.2020.05.021 [DOI] [PubMed] [Google Scholar]
- 83. Glangetas C, Massi L, Fois GR, et al. NMDA‐receptor‐dependent plasticity in the bed nucleus of the stria terminalis triggers long‐term anxiolysis. Nat Commun. 2017;8:14456. doi: 10.1038/ncomms14456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Chang CH, Gean PW. The ventral hippocampus controls stress‐provoked impulsive aggression through the ventromedial hypothalamus in post‐weaning social isolation mice. Cell Rep. 2019;28(5):1195‐1205.e3. doi: 10.1016/j.celrep.2019.07.005 [DOI] [PubMed] [Google Scholar]
- 85. Felix‐Ortiz AC, Beyeler A, Seo C, Leppla CA, Wildes CP, Tye KM. BLA to vHPC inputs modulate anxiety‐related behaviors. Neuron. 2013;79(4):658‐664. doi: 10.1016/j.neuron.2013.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Ma H, Li C, Wang J, et al. Amygdala‐hippocampal innervation modulates stress‐induced depressive‐like behaviors through AMPAR receptors. Proc Nat Acad Sci USA. 2021;118(6):e2019409118. doi: 10.1073/pnas.2019409118/-/DCSupplemental [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Pi G, Gao D, Wu D, et al. Posterior basolateral amygdala to ventral hippocampal CA1 drives approach behaviour to exert an anxiolytic effect. Nat Commun. 2020;11(1):183. doi: 10.1038/s41467-019-13919-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Sherafat Y, Bautista M, Fowler JP, Chen E, Ahmed A, Fowler CD. The interpeduncular‐ventral hippocampus pathway mediates active stress coping and natural reward. ENeuro. 2020;7(6):1‐17. doi: 10.1523/ENEURO.0191-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Zhang WH, Zhang JY, Holmes A, Pan BX. Amygdala circuit substrates for stress adaptation and adversity. Biol Psychiatry. 2021;89(9):847‐856. doi: 10.1016/j.biopsych.2020.12.026 [DOI] [PubMed] [Google Scholar]
- 90. Mei L, Zhou Y, Sun Y, et al. Acetylcholine muscarinic receptors in ventral hippocampus modulate stress‐induced anxiety‐like behaviors in mice. Front Molec Neurosci. 2020;13:598811. doi: 10.3389/fnmol.2020.598811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Wang C, Zhang Y, Shao S, Cui S, Wan Y, Yi M. Ventral hippocampus modulates anxiety‐like behavior in male but not female C57BL/6 J mice. Neuroscience. 2019;418:50‐58. doi: 10.1016/j.neuroscience.2019.08.032 [DOI] [PubMed] [Google Scholar]
- 92. Gale JT, Shields DC, Ishizawa Y, Eskandar EN. Reward and reinforcement activity in the nucleus accumbens during learning. Front Behav Neurosci. 2014;8:114. doi: 10.3389/fnbeh.2014.00114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Gallo EF, Meszaros J, Sherman JD, et al. Accumbens dopamine D2 receptors increase motivation by decreasing inhibitory transmission to the ventral pallidum. Nature. Communications. 2018;9(1):1086. doi: 10.1038/s41467-018-03272-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. LeGates TA, Kvarta MD, Tooley JR, et al. Reward behaviour is regulated by the strength of hippocampus–nucleus accumbens synapses. Nature. 2018;564(7735):258‐262. doi: 10.1038/s41586-018-0740-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Ikemoto S, Panksepp J. The role of nucleus accumbens dopamine in motivated behavior: a unifying interpretation with special reference to reward‐seeking. Brain Res Brain Res Rev. 1999;31(1):6‐41. doi: 10.1016/s0165-0173(99)00023-5 [DOI] [PubMed] [Google Scholar]
- 96. Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35(1):4‐26. doi: 10.1038/npp.2009.129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Pignatelli M, Bonci A. Spiraling connectivity of NAc‐VTA circuitry. Neuron. 2018;97(2):261‐262. doi: 10.1016/j.neuron.2017.12.046 [DOI] [PubMed] [Google Scholar]
- 98. Davidson TL, Chan K, Jarrard LE, Kanoski SE, Clegg DJ, Benoit SC. Contributions of the hippocampus and medial prefrontal cortex to energy and body weight regulation. Hippocampus. 2009;19(3):235‐252. doi: 10.1002/hipo.20499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Ruediger S, Spirig D, Donato F, Caroni P. Goal‐oriented searching mediated by ventral hippocampus early in trial‐and‐error learning. Nat Neurosci. 2012;15(11):1563‐1571. doi: 10.1038/nn.3224 [DOI] [PubMed] [Google Scholar]
- 100. Jarzebowski P, Hay YA, Grewe BF, Paulsen O. Different encoding of reward location in dorsal and intermediate hippocampus. Curr Biol. 2022;32(4):834‐841.e5. doi: 10.1016/j.cub.2021.12.024 [DOI] [PubMed] [Google Scholar]
- 101. Zhou Y, Zhu H, Liu Z, et al. A ventral CA1 to nucleus accumbens core engram circuit mediates conditioned place preference for cocaine. Nat Neurosci. 2019;22(12):1986‐1999. doi: 10.1038/s41593-019-0524-y [DOI] [PubMed] [Google Scholar]
- 102. Zhou Y, Yan E, Cheng D, et al. The projection from ventral CA1, not prefrontal cortex, to nucleus Accumbens Core mediates recent memory retrieval of cocaine‐conditioned place preference. Front Behav Neurosci. 2020;14:558074. doi: 10.3389/fnbeh.2020.558074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Sweeney P, Yang Y. An excitatory ventral hippocampus to lateral septum circuit that suppresses feeding. Nat Commun. 2015;6:10188. doi: 10.1038/ncomms10188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Yoshida K, Drew MR, Mimura M, Tanaka KF. Serotonin‐mediated inhibition of ventral hippocampus is required for sustained goal‐directed behavior. Nat Neurosci. 2019;22(5):770‐777. doi: 10.1038/s41593-019-0376-5 [DOI] [PubMed] [Google Scholar]
- 105. Yang Y, Wang ZH, Jin S, et al. Opposite monosynaptic scaling of BLP‐vCA1 inputs governs hopefulness‐ and helplessness‐modulated spatial learning and memory. Nat Commun. 2016;7:11935. doi: 10.1038/ncomms11935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Lin X, Amalraj M, Blanton C, et al. Noncanonical projections to the hippocampal CA3 regulate spatial learning and memory by modulating the feedforward hippocampal trisynaptic pathway. PLoS Biol. 2021;19(12):e3001127. doi: 10.1371/journal.pbio.3001127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Klausberger T, Somogyi P. Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science (New York, N.Y.). 2008;321(5885):53‐57. doi: 10.1126/science.1149381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Hu H, Gan J, Jonas P. Interneurons. Fast‐spiking, parvalbumin+ GABAergic interneurons: from cellular design to microcircuit function. Science (New York, N.Y.). 2014;345(6196):1255263. doi: 10.1126/science.1255263 [DOI] [PubMed] [Google Scholar]
- 109. Pelkey KA, Chittajallu R, Craig MT, Tricoire L, Wester JC, McBain CJ. Hippocampal GABAergic inhibitory interneurons. Physiol Rev. 2017;97(4):1619‐1747. doi: 10.1152/physrev.00007.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Karunakaran S, Chowdhury A, Donato F, Quairiaux C, Michel CM, Caroni P. PV plasticity sustained through D1/5 dopamine signaling required for long‐term memory consolidation. Nat Neurosci. 2016;19(3):454‐464. doi: 10.1038/nn.4231 [DOI] [PubMed] [Google Scholar]
- 111. Arszovszki A, Borhegyi Z, Klausberger T. Three axonal projection routes of individual pyramidal cells in the ventral CA1 hippocampus. Front Neuroanat. 2014;8:53. doi: 10.3389/fnana.2014.00053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Cembrowski MS, Bachman JL, Wang L, Sugino K, Shields BC, Spruston N. Spatial gene‐expression gradients underlie prominent heterogeneity of CA1 pyramidal neurons. Neuron. 2016;89(2):351‐368. doi: 10.1016/j.neuron.2015.12.013 [DOI] [PubMed] [Google Scholar]
- 113. Tao S, Wang Y, Peng J, et al. Whole‐brain mapping the direct inputs of dorsal and ventral CA1 projection neurons. Front Neural Circuits. 2021;15:643230. doi: 10.3389/fncir.2021.643230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Park AJ, Harris AZ, Martyniuk KM, et al. Reset of hippocampal–prefrontal circuitry facilitates learning. Nature. 2021;591(7851):615‐619. doi: 10.1038/s41586-021-03272-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Collin SH, Milivojevic B, Doeller CF. Memory hierarchies map onto the hippocampal long axis in humans. Nat Neurosci. 2015;18(11):1562‐1564. doi: 10.1038/nn.4138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Goyal A, Miller J, Qasim SE, et al. Functionally distinct high and low theta oscillations in the human hippocampus. Nat Commun. 2020;11(1):2469. doi: 10.1038/s41467-020-15670-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


