Abstract
The alveolo-capillary barrier is relatively impermeable, and facilitates gas exchange via the large alveolar surface in the lung. Disruption of alveolo-capillary barrier leads to accumulation of edema fluid in lung injury. Studies in animal models of various forms of lung injury provide evidence that TRPV4 channels play a critical role in disruption of the alveolo-capillary barrier and pathogenesis of lung injury. TRPV4 channels from capillary endothelial cells, alveolar epithelial cells, and immune cells have been implicated in the pathogenesis of lung injury. Recent studies in endothelium-specific TRPV4 knockout mice point to a central role for endothelial TRPV4 channels in lung injury. In this chapter, we review the findings on the pathological roles of endothelial TRPV4 channels in different forms of lung injury and future directions for further investigation.
1. Introduction
In 1969, Cosens and Manning reported a Drosophila mutant that was defective in light sensing and exhibited only transient light-induced receptor potentials (TRPs) instead of the normal sustained response (Cosens & Manning, 1969). This finding was explained by a defect in an ion channel, which later led to the discovery of the large family of TRP channels. The mammalian TRP channel superfamily consists of 28 members divided into six subfamilies based on their amino acid sequence homology: TRPC (Canonical), TRPM (Melastatin), TRPML (Mucolipins), TRPV (Vanilloid), TRPP (Polycystic), and TRPA (Ankyrin-rich protein). TRP channels exhibit diverse permeation and gating properties and regulate a plethora of signaling mechanisms that control physiological function in multiple cell types. TRP channels can be activated by polymodal stimuli, including physical (voltage, temperature, and mechanical stimuli) and chemical (neurohumoral mediators and second messengers) stimuli (Jara-Oseguera & Islas, 2013; Nieto-Posadas, Jara-Oseguera, & Rosenbaum, 2011). The TRPV subfamily can be further subdivided into six isoforms: TRPV1–TRPV6. Within the vasculature, TRPV channels are expressed in smooth muscle cells, endothelial cells, fibroblasts, and perivascular nerves (Filosa, Yao, & Rath, 2013; Ottolini & Sonkusare, 2021). Studies on TRPV channels at the plasma membrane demonstrate crucial roles for these channels in Ca2+ homeostasis in the vasculature. TRPV1 and TRPV4 are the most studied members of the TRPV family in the systemic and pulmonary vasculature.
In the lungs, TRPV4 channels are expressed in multiple cell types, including epithelial cells, immune cells, and cells in the vascular wall. TRPV4 channels have been associated with many physiological functions and pathological conditions in the lung. Studies in different models suggest a critical role for TRPV4 channels in alveolo-capillary barrier in the lung (Rajan et al., 2021), resulting in lung edema and injury. The alveolo–capillary barrier plays an essential role in gas exchange, permeability regulation, fluid clearance, and host defense. This barrier is formed by alveolar epithelium, capillary endothelium, and a basement membrane that separates alveolar epithelium and capillary endothelium. Disrupted alveolo-capillary barrier, which results in accumulation of edema fluid in alveolar spaces, is an important pathological feature of lung edema and injury. This chapter focuses on the role of TRPV4 channels in disrupting pulmonary alveolo-capillary barrier, thereby causing lung edema and injury. The role of TRPV4 channels in the airway and immune cells has been discussed in detail elsewhere (Belvisi & Birrell, 2017; Rajan et al., 2021).
2. Structure and properties
Recently elucidated single-particle cryo-electron microscopy (cryo-EM) structure of Xenopus tropicalis TRPV4 channel under near-atomic resolution revealed a symmetric tetramer formed by four subunits, each with six transmembrane segments (S1–S6) and linking loops (Deng et al., 2018). Among the six transmembrane segments, S5, S6, and the interconnecting loop form the central cation-permeable pore, and S3 and S4 appear to form an agonist-binding pocket that influences channel gating (recently reviewed in (Chen & Sonkusare, 2020; Rosenbaum et al., 2020; Toft-Bertelsen & MacAulay, 2021). Moreover, the inner helices formed an intracellular gate in the ion-conduction pore but lacked an extracellular gate in the selectivity filter that was seen in other TRPV family members, possibly explaining the less selective nature of the channel compared to other TRPV channels. Anomalous X-ray diffraction analyses also identified a single ion-binding site in an unusually wide selectivity filter (Deng et al., 2018). Thus, unique structural features of TRPV4 channels may underlie their distinct gating mechanisms and selectivity features when compared to other ion channels of TRP family.
The intracellular N- and C-termini of TRPV4 channels include multiple regions for protein–protein interactions that regulate the assembly and activity of the channel and its interaction with the cytoskeleton (Harteneck & Schultz, 2007; Nilius & Voets, 2013; White et al., 2016). The N-terminus contains ankyrin repeat domains, a feature found in all channels of TRPC and TRPV subfamilies, and proline-rich domain (Everaerts, Nilius, & Owsianik, 2010; Inada, Procko, Sotomayor, & Gaudet, 2012; Phelps, Huang, Lishko, Wang, & Gaudet, 2008). The C-terminal tail contains a TRP box that holds binding sites for calmodulin, actin, and tubulin and an amino acid stretch (Asp-Ala-Pro-Leu) similar to a PDZ-binding like motif.
TRPV4 is a cation channel characterized by a moderately high Ca2+ permeability ratio (PCa/PNa of ~6) and a single-channel conductance of ~90 pS (Clapham, Montell, Schultz, Julius, & International Union of Pharmacology, 2003). Residues Asp672 and Asp682 in the ionic pathway generated by the pore-forming loop between the S5 and S6 domains modulate the ion selectivity: alanine substitution of either or both asparagines causes a reduction in the relative permeability for divalent cations (Voets et al., 2002). Intracellular Ca2+ can inhibit or potentiate TRPV4 channels in a concentration-dependent manner (Sonkusare et al., 2012, 2014; Strotmann, Harteneck, Nunnenmacher, Schultz, & Plant, 2000), a mechanism that has been attributed to residue F707 (Watanabe et al., 2003). The biogenesis of TRPV4 channels involves N-glycosylation on residue N651 (Toft-Bertelsen & MacAulay, 2021) and oligomerization in the ER, followed by a transfer to the Golgi apparatus for subsequent maturation. The channel usually organizes as a homotetrameric structure (Arniges, Fernandez-Fernandez, Albrecht, Schaefer, & Valverde, 2006) requiring both the N- and the C-termini (Ryskamp et al., 2014), while heterotetramers can occur with TRPC1 (Ma et al., 2011), TRPP2 (Stewart, Smith, Sandford, & Edwardson, 2010), and TRPC1–TRPP2 channels (Du et al., 2014). Thus, studies of the biophysical properties of TRPV4 channels and TRPV4 heteromultimer may provide crucial insights into the mechanisms for altered channel function in lung injury.
3. TRPV4 channels in the lung
TRPV4 channels are expressed in numerous cells of the respiratory tract (airway smooth muscle cells, bronchial epithelial cells, alveolar type 1 (AT1) and type 2 (AT2) epithelial cells), pulmonary vascular cells (endothelial cells, smooth muscle cells and fibroblasts), and inflammatory cells (macrophages, neutrophils and T-cells) (Baxter et al., 2014; Daneva, Laubach, & Sonkusare, 2019; Groot-Kormelink, Fawcett, Wright, Gosling, & Kent, 2012; Hamanaka et al., 2010; Jia et al., 2004; Majhi et al., 2015; Marziano et al., 2017; Rahaman et al., 2014; Xia et al., 2013; Yang et al., 2012). While long and flat AT1 cells maintain the integrity of alveolar epithelial barrier, cubic AT2 cells produce surfactants to reduce surface tension and enhance gas exchange across the epithelium. Accordingly, epithelial barrier function was impaired and epithelial surfactant levels were lower in global TRPV4−/− mice (Weber et al., 2020). TRPV4 agonist GSK1016790A (GSK101) increased endothelial permeability in pulmonary capillaries (Rajan et al., 2021; Villalta & Townsley, 2013; Willette et al., 2008). Thus, TRPV4 channel activity appears to be essential for the regulation of epithelial and endothelial barrier function, and therefore, may play a role in defense against invading pathogens and toxicants in the lung.
TRPV4 channel dysfunction is observed in many diseases of the lung, including pulmonary hypertension, asthma, acute respiratory distress syndrome, chronic obstructive pulmonary disorder (COPD), cystic fibrosis (CF), lung fibrosis, pulmonary edema, and lung injury. TRPV4 channels in vascular smooth muscle and endothelial cells from small pulmonary arteries have distinct effects on pulmonary arterial pressure. Endothelium-specific TRPV4 knockout mice exhibited an elevated pulmonary arterial pressure, whereas smooth muscle-specific TRPV4 knockout mice showed unaltered pulmonary arterial pressure (Daneva, Marziano, et al., 2021; Daneva, Ottolini, et al., 2021). In pulmonary hypertension, the activity of smooth muscle TRPV4 channels is increased whereas the activity of endothelial TRPV4 channels is reduced (Daneva, Marziano, et al., 2021; Goldenberg et al., 2015). Airway smooth muscle TRPV4 channels increase the contractile response of the airway in asthma and promote transforming growth factor beta (TGFβ)-dependent remodeling of the airway (Bonvini et al., 2020). TRPV4 channels from multiple cell types in the lung contribute to the pathogenesis of acute lung injury (ALI) and acute respiratory distress syndrome (ARDS). In alveolar epithelial and endothelial cells, TRPV4 channel overactivation impairs epithelial and endothelial septal barrier, leading to edema and lung injury (Villalta & Townsley, 2013). TRPV4 channels from neutrophils increase ROS production (Scheraga, Southern, Grove, & Olman, 2017) and facilitate neutrophil activation in lung injury (Yin et al., 2016). In contrast, some studies show essential roles for TRPV4 channels in maintaining the integrity of alveolar barrier and protecting against edema formation (Weber et al., 2020). TRPV4 channels are potential therapeutic targets for lung disorders. Many specific TRPV4 channel activators and inhibitors have been developed in the past two decades (Chen & Sonkusare, 2020). Indeed, one TRPV4 inhibitor—GSK2798745—has been tested clinically and was found to be well-tolerated (Goyal et al., 2019). Identification of GSK2798745 as a clinically safe TRPV4 inhibitor may lead to additional clinical trials in individuals suffering from lung edema and injury.
4. TRPV4 channels in the pulmonary endothelium
Studies over the past two decades establish the functional expression of TRPV4 channels in arterial, capillary, and septal endothelium in the lung. Most studies have focused on the contribution of capillary endothelial TRPV4 channels to pulmonary pathologies, including lung edema and lung injury. However, the physiological roles of capillary endothelial TRPV4 channels have remained unclear. Recent studies show that the activation of endothelial TRPV4 channels dilates small, resistance-sized pulmonary arteries. In small pulmonary arteries, Ca2+ influx events through TRPV4 channels (TRPV4 sparklets) selectively activate endothelial nitric oxide synthase (eNOS) signaling and NO release (Daneva et al., 2019; Marziano et al., 2017; Ottolini, Daneva, et al., 2020). In resistance-sized systemic arteries, however, Ca2+ influx events through TRPV4 channels activate endothelial Ca2+-activated K+ (IK and SK) channels (Ottolini, Daneva, et al., 2020; Ottolini, Hong, et al., 2020; Ottolini, Hong, & Sonkusare, 2019; Ottolini & Sonkusare, 2021; Sonkusare et al., 2012, 2014). Studies in endothelium-specific TRPV4 channel knockout mice suggested that endothelial TRPV4 channels lower pulmonary artery constriction and resting pulmonary arterial pressure (Daneva, Marziano, et al., 2021; Daneva, Ottolini, et al., 2021). Moreover, in mouse models of pulmonary hypertension and pulmonary arterial hypertension patients, the activity of endothelial TRPV4 channels was decreased in small pulmonary arteries, contributing to reduced vasodilation and elevated pulmonary arterial pressure (Daneva, Marziano, et al., 2021). On the contrary, smooth muscle-specific TRPV4 channel knockout did not result in alteration of basal pulmonary arterial pressure (Daneva, Marziano, et al., 2021; Daneva, Ottolini, et al., 2021), indicating that smooth muscle TRPV4 channels may not play a role in the regulation of pulmonary arterial pressure under normal conditions. Other studies linked smooth muscle TRPV4 channels with hypoxia- and G-protein coupled receptor signaling-induced constriction of pulmonary arteries and elevation of pulmonary arterial pressure (Goldenberg et al., 2015; Xia et al., 2013; Yang et al., 2012). Thus, endothelial and smooth muscle TRPV4 channels appear to differentially regulate the pulmonary arterial pressure under normal conditions and in pulmonary hypertension. Recent studies also showed that the efflux of adenosine triphosphate (ATP) through endothelial Pannexin 1, an ATP efflux pathway at the cell membrane, activates endothelial TRPV4 channels via P2 purinergic receptor signaling and lowers pulmonary arterial pressure (Daneva, Ottolini, et al., 2021). Moreover, this pathway was shown to be activated by increases in flow/shear stress. Since pulmonary arteries are a high-flow, low-resistance circulation, shear stress-activation of endothelial pannexin 1–P2Y2 receptor–TRPV4 signaling pathway needs to be investigated as a new mechanism for flow-induced dilation of pulmonary arteries.
Pulmonary capillary endothelium is structurally and functionally different from arterial endothelium (Stevens, 2011) (Fig. 1). TRPV4 channel activity in capillary endothelial cells has mostly been associated with increased permeability and detrimental effects in pulmonary pathologies (Villalta & Townsley, 2013). Endothelial Ca2+ signaling mechanisms involving TRPV4 channels have distinct effects on capillary and arterial function (Fig. 1) (Alvarez et al., 2006; Marziano et al., 2017; Ottolini, Daneva, et al., 2020; Willette et al., 2008). TRPV4 channels couple to disparate targets and regulatory mechanisms in capillary and arterial endothelium (Harraz, Longden, Eubanks, & Nelson, 2018; Longden et al., 2017; Sonkusare et al., 2012; Sonkusare, Dalsgaard, Bonev, & Nelson, 2016). Capillary endothelium regulates capillary permeability but, unlike pulmonary arterial endothelium, does not play an active role in the regulation of pulmonary arterial pressure. Capillary endothelial TRPV4 channel activity has been associated with endothelial hyperpermeability, pulmonary edema, and lung injury. Current evidence in the literature suggests that pathological conditions affect capillary and arterial TRPV4 channels differently (Daneva, Marziano, et al., 2021; Suresh et al., 2018). Studies in primary microvascular endothelial cells, which likely represent capillary endothelium, suggest that the activity of endothelial TRPV4 channels is elevated in pulmonary hypertension, contributing to endothelial cell migration and proliferation (Suresh et al., 2018, 2015). This is in contrast with pulmonary artery endothelium, where the activity of TRPV4 channels is reduced in pulmonary hypertension (Daneva, Marziano, et al., 2021). Since capillary and alveolar septal endothelium is directly involved in lung edema and injury, the following sections will review the findings on TRPV4 channels in capillary or alveolar septal endothelium.
Fig. 1.
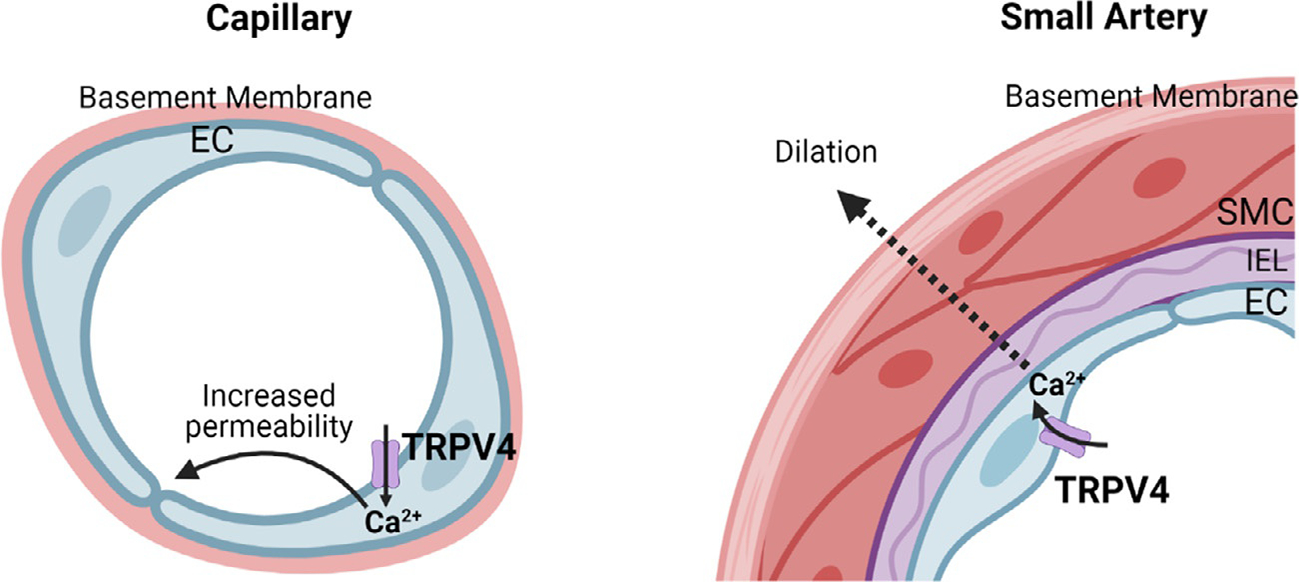
Distinct functions of endothelial TRPV4 channels in capillaries and arteries. Left, TRPV4 channels in capillary endothelial cells increase endothelial permeability; right, TRPV4 channels in arterial endothelial cells relax smooth muscle cells and cause vasodilation.
5. TRPV4 channels in lung edema and injury
The endothelium of the pulmonary circulation is an important barrier to protect the lung tissue from toxic substances and pathogens circulating in the blood. However, in response to bacterial infections of the lung tissue, endothelial cell permeability increases to facilitate the migration of immune cells from the blood to the alveoli. As a result, protein-rich fluid accumulates in the alveolar space, causing life-threatening pulmonary edema (Rajan et al., 2021). Pulmonary edema can be cardiogenic (heart failure) or non-cardiogenic (ALI or ARDS) (Komiya, Akaba, Kozaki, Kadota, & Rubin, 2017). Overwhelming evidence in the literature suggests that TRPV4 is a key ion channel for increasing pulmonary endothelial permeability in both cardiogenic and non-cardiogenic pulmonary edema. TRPV4 channel activation can induce lung endothelial/epithelial barrier disruption, a critical feature of lung injury, suggesting that TRPV4 channel inhibition could be of therapeutic value (Fig. 2). Interestingly, a recent study showed that macrophage TRPV4 channel protects the lung from injury upon intratracheal Pseudomonas aeruginosa exposure in mice (Scheraga, Abraham, et al., 2020). TRPV4 channels enhanced macrophage bacterial clearance and reduced the levels of proinflammatory cytokines. These studies indicate that TRPV4 channels in different pulmonary cell types can play distinct roles in lung injury, which can lead to different pathologies depending on whether infection-driven (e.g., Pseudomonas infection) or sterile-driven (e.g., ischemia-reperfusion) inflammatory processes are involved. Further studies using cell-specific knockout models will be necessary for understanding the cell type-specific roles of TRPV4 channels in lung injury.
Fig. 2.

Role of TRPV4 channels in different forms of pulmonary edema and lung injury. TRPV4 channels play an important role lung ischemia/reperfusion injury, heart failure-induced pulmonary edema, fluid-, acid-, ventilator-, and high pressure-induced lung injury. A potential role for TRPV4 channels has also been proposed in pulmonary edema and lung injury in COVID-19.
5.1. Heart failure-induced pulmonary edema
Heart failure-induced pulmonary edema resulting from high pulmonary venous pressure (PVP) is a major cause of morbidity and mortality in heart failure patients. High PVP results in increased capillary permeability, ultimately leading to pulmonary edema. Current treatment options do not address high PVP-induced pulmonary edema in heart failure. Recent evidence from rodent lungs suggests that heart failure-induced pulmonary edema is driven by activation of pulmonary capillary endothelial TRPV4 channels (Thorneloe et al., 2012). Furthermore, in both acute and chronic heart failure models, pretreatment with TRPV4 antagonist, GSK2193874, inhibited the formation of pulmonary edema and enhanced arterial oxygenation. GSK2193874 treatment also resolved pulmonary edema in a mouse model of myocardial infarction. These findings identified a crucial role for TRPV4 channels in the pathogenesis of heart failure-induced pulmonary edema and suggest that TRPV4 inhibition is a potential therapeutic strategy for pulmonary edema in heart failure patients (Thorneloe et al., 2012).
5.2. Lung ischemia-reperfusion injury
Lung ischemia-reperfusion injury (IRI), involving severe inflammation, edema, alveolar damage, and hypoxemia, is a major cause of primary graft failure after transplant (de Perrot, Liu, Waddell, & Keshavjee, 2003; Haywood et al., 2021). IRI is characterized by robust inflammation, alveolar damage, and increased vascular permeability. TRPV4 channel activation has been linked to lung injury through endothelial barrier disruption, edema, and inflammatory cell recruitment and activation. Therefore, TRPV4 channels may be a promising therapeutic target to mitigate lung IRI and decrease the incidence of primary graft dysfunction after transplant.
In this regard, Haywood and colleagues demonstrated that endothelial TRPV4 channel plays a key role in the development of inflammation and pulmonary edema after IR (Haywood et al., 2022). Using clinically relevant measurements of lung function (PaO2 and compliance), the authors found that endothelium-specific TRPV4 knockout or pharmacologic inhibition of TRPV4 channels preserves lung function and reduces inflammation and edema after IR. These findings supported previous reports demonstrating that TRPV4 channel activation can lead to increased endothelial permeability, edema, and immune cell infiltration and activation (Dutta et al., 2020; Rayees et al., 2019; Scheraga, Southern, Grove, & Olman, 2020; Villalta & Townsley, 2013). Studies in endothelium-specific TRPV4 knockout mice suggested that the primary protective effect of TRPV4 inhibition in lung IRI is localized to the pulmonary endothelium (Haywood et al., 2022). Therefore, pharmacological inhibition of TRPV4 channels may be an effective strategy to treat lung IRI and prevent primary graft dysfunction after transplantation.
Studies in pulmonary arteries have shown that ATP efflux through endothelial pannexin 1 can activate TRPV4 channels through P2 purinergic receptor signaling. In this regard, Sharma et al. used pannexin 1 endothelial knockout mice to demonstrate that endothelial pannexin 1 plays a key role in mediating vascular permeability, inflammation, edema, leukocyte infiltration, and lung dysfunction after IR (Sharma et al., 2018). Since endothelium-specific deletion of TRPV4 or pannexin 1 appears to be protective against lung IRI, it is plausible that an endothelial pannexin 1–TRPV4 interaction is involved in the pathogenies of lung IRI. A recent study by Daneva and colleagues showed an interaction between pannexin 1 and TRPV4 channels in the intact endothelium from small pulmonary arteries (Daneva, Ottolini, et al., 2021). Pannexin 1–TRPV4 interaction in capillary endothelial cells and its role in lung IRI has not yet been verified.
5.3. Fluid-induced acute lung injury
Rapid administration of fluids, or fluid resuscitation, is a common clinical practice. Notably, administration of bolus intravenous fluid has been associated with respiratory dysfunction and increased mortality (Maitland et al., 2011). Administration of intravenous saline solution resulted in pulmonary edema in rats and mice (Bihari et al., 2017). Global knockout of TRPV4 channels and administration of TRPV4 inhibitor prevented the development of intravenous fluid-induced pulmonary edema, suggesting that the activation of TRPV4 channels is a central mechanism in fluid-induced acute lung injury (Bihari et al., 2017). However, the exact cell-types contributing to the effect of TRPV4 channels in fluid-induced lung injury remain unknown.
5.4. Ventilator-induced lung injury
Mechanical ventilation is a crucial tool for supporting critically ill patients but can cause lung injury. Ventilator-induced lung injury is associated with disrupted pulmonary endothelial barrier and release of pro-inflammatory cytokines. TRPV4 channels play an essential role in the activation of macrophages in ventilator-induced lung injury (Hamanaka et al., 2010). Hamanaka et al. showed that high pressure ventilation induces pulmonary edema in mice, and this effect is absent in TRPV4−/− mice (Hamanaka et al., 2007). In a follow-up study, this group reported that alveolar macrophages from wild-type mice restored the susceptibility of TRPV4−/− mice to ventilator-induced lung injury, implying that TRPV4 channel activation in alveolar macrophages is critical to ventilator-induced lung injury. Michalik and colleagues demonstrated that pharmacological inhibition of TRPV4 channels and serum glucocorticoid-regulated kinase 1 (SGK1) reduces endothelial Ca2+ influx and vascular leakage in an isolated lung model of ventilation-induced lung injury (Michalick et al., 2017). Further studies demonstrated that lung ventilation promotes endothelial Ca2+ influx and barrier disruption through the activation of TRPV4 channels, presumably through phosphorylation at serine 824 residue by SGK1. Thus, TRPV4 and SGK1 were proposed as promising new targets for prevention or treatment of ventilator-induced lung injury (Michalick et al., 2017). A decrease in cytokine release and endothelial permeability following TRPV4 inhibition suggested that TRPV4 inhibitors may be utilized as a prophylactic treatment to improve the pathological response of lung cells during ventilation and potentially support patients receiving mechanical ventilation (Pairet et al., 2018). Intriguingly, exosomes derived from human adipocytes protected mice against ventilator-induced lung injury via inhibition of Ca2+ influx through TRPV4 channels (Yu et al., 2020). Thus, new approaches to lower TRPV4 channel activity may be effective in clinical treatment of lung edema and injury.
5.5. High intravascular pressure-induced lung injury
Abnormally high intravascular pressure can increase endothelial permeability at the alveolar-septal barrier. Disruption of the alveolar-septal barrier leads to acute lung injury, patchy alveolar flooding, and hypoxemia. Multiple studies implicate TRPV4 channels in disruption of the alveolar-septal barrier and pathogenesis of acute lung injury (Alvarez et al., 2006). Jian et al. showed that high intravascular pressure-induced disruption of alveolar-septal barrier was reduced in the lungs from global TRPV4−/− mice. Further, the authors suggested a critical role for epoxyeicosatrienoic acid-dependent activation of TRPV4 channels in disruption of alveolar-septal barrier following high intravascular pressure (Jian, King, Al-Mehdi, Liedtke, & Townsley, 2008). In a follow-up study, the group showed that increased lung endothelial permeability in response to 14,15-epoxyeicosatrienoic acid (14,15-EET) in rat lungs requires Ca2+ entry via TRPV4 channels and functional coupling of TRPV4 channels with Ca2+-sensitive, intermediate, and small conductance (IK and SK) K+ channels (Lin et al., 2015). Such “functional coupling” suggests that endothelial TRPV4 channels likely form signaling microdomains with IK and SK channels and dictate the extent of lung endothelial injury caused by 14,15-EET.
5.6. Acid exposure-induced lung injury
The treatment of acute lung injury caused by exposure to reactive chemicals remains challenging because of the lack of mechanism-based therapeutic approaches. In a study by Balakrishna and colleagues, the effects of two new TRPV4 inhibitors (GSK2220691, GSK2337429A) were examined in mice exposed to hydrochloric acid, mimicking acid exposure and acid aspiration injury, and to chlorine gas, a severe chemical threat with frequent exposures in domestic and occupational environments and transportation accidents. The authors demonstrated that TRPV4 inhibitors are potent and efficacious countermeasures against severe chemical exposures, acting against exaggerated inflammatory responses, and protecting tissue barriers and cardiovascular function (Balakrishna et al., 2014). These findings pointed to TRPV4 channel-dependent mechanisms of chemical-induced lung injury and provided proof-of-principle for TRPV4 channel inhibition as a treatment strategy.
6. Potential role of TRPV4 channels in lung edema and injury in COVID-19 patients
Disruption of the alveolo-capillary barrier as a direct consequence of severe acute respiratory syndrome coronavirus type 2 (SARS-CoV2) infection is a critical driver of lethality in the recent coronavirus disease 2019 (COVID-19) pandemic. Therefore, there is an urgent need for alveolo-capillary barrier-stabilizing drugs in the COVID-19 pandemic. TRPV4 channel inhibition is protective in various animal models of lung edema as described above, and overactivation of TRPV4 channels damages the alveolo-capillary barrier of the lungs (Rajan et al., 2021; Villalta & Townsley, 2013). Moreover, TRPV4 channels are expressed in all the cell types of the alveolo-capillary unit, including AT1 and AT2 cells and capillary endothelial cells. TRPV4 channels also regulate the activation of alveolar macrophages and neutrophils, which contribute to alveolo-capillary barrier disruption via the release of proteases, cytokines, and reactive oxygen species (Balakrishna et al., 2014; Dutta et al., 2020; Hamanaka et al., 2010; Scheraga, Southern, et al., 2020; Yin et al., 2016). Since SARS-CoV2 also infects endothelial cells (Liu et al., 2021), a recent article by Kuebler and colleagues (Kuebler, Jordt, & Liedtke, 2020) proposed that TRPV4 channel inhibition could be a promising approach to protect the alveolo-capillary barrier of the lungs in COVID-19 pandemic. The authors suggested that GSK2798745 (TRPV4 channel inhibitor) may be a powerful therapeutic option in COVID-19 patients. Indeed, in vitro screening has identified TRPV4 channel as a target for endothelial barrier stabilization in COVID-19 (Michalick et al., 2022). In the first clinical trial, GSK2798745 did not affect lipopolysaccharide (LPS)-induced elevation of total protein and neutrophils in the bronchoalveolar lavage despite blood and lung exposures that were predicted to be efficacious (Mole et al., 2020). However, the central role of TRPV4 channels in mediating vascular inflammation and endothelial barrier dysfunction provides rationale supporting investigation of TRPV4-targeting therapies against COVID-19 pandemic.
7. Conclusions and future directions
The current evidence in the literature points to a critical role for TRPV4 channels from endothelial cells, immune cells, and alveolar epithelial cells in the pathogenesis of lung injury. Modulating TRPV4 channel activity is a potentially useful therapeutic approach for lung edema and injury, but clinically successful drugs or genetic strategies need to be established. It should be noted that most studies in TRPV4 knockout mice have used global knockout mice, making it difficult to decipher the contribution of TRPV4 channels from individual cell types in the lung. Recent studies in endothelium-specific TRPV4 channel knockout mice indicate a central role for endothelial TRPV4 channels in lung ischemia-reperfusion injury. Similar studies using neutrophil/macrophage/alveolar epithelial cell-specific knockout mice are needed for confirming the contribution of TRPV4 channels from these cell types to lung injury. The interactions amongst immune cell, endothelial cells, and alveolar epithelial cells, and potential roles of TRPV4 channels in regulating these interactions will be an interesting area for future investigations on lung injury. For example, it is plausible that the release of cytokines from immune cells alters the activity of endothelial TRPV4 channels in lung injury. Previous studies in other vascular beds have shown that inflammatory cytokines can alter the activity of endothelial TRPV4 channels (Maier-Begandt et al., 2021).
TRPV4 channels are expressed and are functional in endothelial cells, immune cells, alveolar epithelial cells, and vascular and airway smooth muscle cells in the lung. Studies in cell-specific knockout mice will provide much needed information on the physiological roles of TRPV4 channels from different cell types in the lung. Although numerous studies in the literature point to a critical role of endothelial TRPV4 channels in lung injury, a direct recording of ionic currents through endothelial TRPV4 channels (Chen et al., 2022; Ottolini, Daneva, et al., 2020; Ottolini, Hong, et al., 2020) in a model of lung injury has not yet been reported. Furthermore, individual Ca2+ influx events through TRPV4 channels (TRPV4 sparklets) (Chen et al., 2021; Sonkusare et al., 2012) have not been recorded in endothelial cells in a lung injury model. Since intracellular Ca2+ is a major contributor to the effects of TRPV4 channels on endothelial permeability, studies of TRPV4 sparklets will provide a more in-depth understanding of alterations in TRPV4 channel activity in lung injury. Direct studies of TRPV4 channel activity– TRPV4 channel currents and TRPV4 sparklets–will enable a more thorough examination of TRPV4 channels as a therapeutic target in lung injury.
TRPV4 channels can interact with multiple other ion channels. Recent studies demonstrate direct or indirect interactions of TRPV4 channels with IK/SK channels, large conductance, Ca2+-activated BK channels and inward rectifier K+ channels (Chen et al., 2022; Earley, Heppner, Nelson, & Brayden, 2005; Sonkusare et al., 2012, 2016) in vascular endothelial and smooth muscle cells. Such interactions could contribute to the deleterious effect of TRPV4 channel activity in lung injury and may provide new targets to alter the harmful effects of TRPV4 channels. It should also be noted that multiple scaffolding proteins, including caveolin-1 (Cav-1) (Daneva, Marziano, et al., 2021; Daneva, Ottolini, et al., 2021) and A-kinase anchoring protein 150 (AKAP150) (Mercado et al., 2014; Ottolini, Hong, et al., 2020; Sonkusare et al., 2014) promote the activity of vascular TRPV4 channels. Whether alterations in these scaffolding proteins contribute to abnormal activity of TRPV4 channels in lung injury has not been investigated. Finally, nitric oxide is a major signaling molecule in the pulmonary circulation. Studies have shown that nitric oxide can limit the coupling amongst endothelial TRPV4 channels, thereby reducing their activity in pulmonary endothelial cells (Marziano et al., 2017). Thus, nitric oxide–TRPV4 channel interaction could be explored as a pathological mechanism for lung injury and potential therapeutic target for restoring TRPV4 channel activity. Future studies are expected to solidify our understanding of TRPV4 channel as a therapeutic target in lung injury and identify new proteins that can be targeted to lower the harmful effects of TRPV4 channels in lung injury.
Funding
This work was in part funded by awards HL146914, HL142808, and HL157407 from the National Institutes of Health.
References
- Alvarez DF, King JA, Weber D, Addison E, Liedtke W, & Townsley MI (2006). Transient receptor potential vanilloid 4-mediated disruption of the alveolar septal barrier: A novel mechanism of acute lung injury. Circulation Research, 99, 988–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arniges M, Fernandez-Fernandez JM, Albrecht N, Schaefer M, & Valverde MA (2006). Human TRPV4 channel splice variants revealed a key role of ankyrin domains in multimerization and trafficking. The Journal of Biological Chemistry, 281, 1580–1586. [DOI] [PubMed] [Google Scholar]
- Balakrishna S, Song W, Achanta S, Doran SF, Liu B, Kaelberer MM, et al. (2014). TRPV4 inhibition counteracts edema and inflammation and improves pulmonary function and oxygen saturation in chemically induced acute lung injury. American Journal of Physiology. Lung Cellular and Molecular Physiology, 307, L158–L172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter M, Eltom S, Dekkak B, Yew-Booth L, Dubuis ED, Maher SA, et al. (2014). Role of transient receptor potential and pannexin channels in cigarette smoke-triggered ATP release in the lung. Thorax, 69, 1080–1089. [DOI] [PubMed] [Google Scholar]
- Belvisi MG, & Birrell MA (2017). The emerging role of transient receptor potential channels in chronic lung disease. The European Respiratory Journal, 50, 1601357. [DOI] [PubMed] [Google Scholar]
- Bihari S, Dixon DL, Lawrence MD, De Bellis D, Bonder CS, Dimasi DP, et al. (2017). Fluid-induced lung injury-role of TRPV4 channels. Pflügers Archiv, 469, 1121–1134. [DOI] [PubMed] [Google Scholar]
- Bonvini SJ, Birrell MA, Dubuis E, Adcock JJ, Wortley MA, Flajolet P, et al. (2020). Novel airway smooth muscle-mast cell interactions and a role for the TRPV4-ATP axis in non-atopic asthma. The European Respiratory Journal, 56, 1901458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YL, Baker TM, Lee F, Shui B, Lee JC, Tvrdik P, et al. (2021). Calcium signal profiles in vascular endothelium from Cdh5-GCaMP8 and Cx40-GCaMP2 mice. Journal of Vascular Research, 58, 159–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YL, Daneva Z, Kuppusamy M, Ottolini M, Baker TM, Klimentova E, et al. (2022). Novel smooth muscle ca(2+)-signaling nanodomains in blood pressure regulation. Circulation. 101161circulationaha121058607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YL, & Sonkusare SK (2020). Endothelial TRPV4 channels and vasodilator reactivity. Current Topics in Membranes, 85, 89–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham DE, Montell C, Schultz G, Julius D, & International Union of Pharmacology. (2003). International union of pharmacology. XLIII. Compendium of voltage-gated ion channels: Transient receptor potential channels. Pharmacological Reviews, 55, 591–596. [DOI] [PubMed] [Google Scholar]
- Cosens DJ, & Manning A (1969). Abnormal electroretinogram from a drosophila mutant. Nature, 224, 285–287. [DOI] [PubMed] [Google Scholar]
- Daneva Z, Laubach VE, & Sonkusare SK (2019). Novel regulators and targets of redox signaling in pulmonary vasculature. Current Opinion in Physiology, 9, 87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneva Z, Marziano C, Ottolini M, Chen YL, Baker TM, Kuppusamy M, et al. (2021). Caveolar peroxynitrite formation impairs endothelial TRPV4 channels and elevates pulmonary arterial pressure in pulmonary hypertension. Proceedings of the National Academy of Sciences of the United States of America, 118, e2023130118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneva Z, Ottolini M, Chen YL, Klimentova E, Kuppusamy M, Shah SA, et al. (2021). Endothelial pannexin 1-TRPV4 channel signaling lowers pulmonary arterial pressure in mice. eLife, 10, e67777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Perrot M, Liu M, Waddell TK, & Keshavjee S (2003). Ischemia-reperfusion-induced lung injury. American Journal of Respiratory and Critical Care Medicine, 167, 490–511. [DOI] [PubMed] [Google Scholar]
- Deng Z, Paknejad N, Maksaev G, Sala-Rabanal M, Nichols CG, Hite RK, et al. (2018). Cryo-EM and X-ray structures of TRPV4 reveal insight into ion permeation and gating mechanisms. Nature Structural & Molecular Biology, 25, 252–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Ma X, Shen B, Huang Y, Birnbaumer L, & Yao X (2014). TRPV4, TRPC1, and TRPP2 assemble to form a flow-sensitive heteromeric channel. The FASEB Journal, 28, 4677–4685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta B, Arya RK, Goswami R, Alharbi MO, Sharma S, & Rahaman SO (2020). Role of macrophage TRPV4 in inflammation. Laboratory Investigation, 100, 178–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earley S, Heppner TJ, Nelson MT, & Brayden JE (2005). TRPV4 forms a novel Ca2+ signaling complex with ryanodine receptors and BKCa channels. Circulation Research, 97, 1270–1279. [DOI] [PubMed] [Google Scholar]
- Everaerts W, Nilius B, & Owsianik G (2010). The vanilloid transient receptor potential channel TRPV4: From structure to disease. Progress in Biophysics and Molecular Biology, 103, 2–17. [DOI] [PubMed] [Google Scholar]
- Filosa JA, Yao X, & Rath G (2013). TRPV4 and the regulation of vascular tone. Journal of Cardiovascular Pharmacology, 61, 113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg NM, Wang L, Ranke H, Liedtke W, Tabuchi A, & Kuebler WM (2015). TRPV4 is required for hypoxic pulmonary vasoconstriction. Anesthesiology, 122, 1338–1348. [DOI] [PubMed] [Google Scholar]
- Goyal N, Skrdla P, Schroyer R, Kumar S, Fernando D, Oughton A, et al. (2019). Clinical pharmacokinetics, safety, and tolerability of a novel, first-in-class TRPV4 Ion Channel inhibitor, GSK2798745, in healthy and heart failure subjects. American Journal of Cardiovascular Drugs, 19, 335–342. [DOI] [PubMed] [Google Scholar]
- Groot-Kormelink PJ, Fawcett L, Wright PD, Gosling M, & Kent TC (2012). Quantitative GPCR and ion channel transcriptomics in primary alveolar macrophages and macrophage surrogates. BMC Immunology, 13, 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamanaka K, Jian MY, Townsley MI, King JA, Liedtke W, Weber DS, et al. (2010). TRPV4 channels augment macrophage activation and ventilator-induced lung injury. American Journal of Physiology. Lung Cellular and Molecular Physiology, 299, L353–L362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamanaka K, Jian MY, Weber DS, Alvarez DF, Townsley MI, Al-Mehdi AB, et al. (2007). TRPV4 initiates the acute calcium-dependent permeability increase during ventilator-induced lung injury in isolated mouse lungs. American Journal of Physiology. Lung Cellular and Molecular Physiology, 293, L923–L932. [DOI] [PubMed] [Google Scholar]
- Harraz OF, Longden TA, Eubanks DH, & Nelson MT (2018). PIP2depletion promotes TRPV4 channel activity in mouse brain capillary endothelial cells. eLife, 7, 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harteneck C, & Schultz G (2007). TRPV4 and TRPM3 as volume-regulated cation channels. In Liedtke W, & Heller S (Eds.), TRP ion channel function in sensory transduction and cellular signaling cascades. Boca Raton, FL: CRC Press/Taylor & Francis. [Google Scholar]
- Haywood N, Ta HQ, Rotar E, Daneva Z, Sonkusare SK, & Laubach VE (2021). Role of the purinergic signaling network in lung ischemia-reperfusion injury. Current Opinion in Organ Transplantation, 26, 250–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haywood N, Ta HQ, Zhang A, Charles EJ, Rotar E, St N, et al. (2022). Endothelial transient receptor potential vanilloid 4 channels mediate lung ischemia-reperfusion injury. The Annals of Thoracic Surgery, 113, 1256–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inada H, Procko E, Sotomayor M, & Gaudet R (2012). Structural and biochemical consequences of disease-causing mutations in the ankyrin repeat domain of the human TRPV4 channel. Biochemistry, 51, 6195–6206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jara-Oseguera A, & Islas LD (2013). The role of allosteric coupling on thermal activation of thermo-TRP channels. Biophysical Journal, 104, 2160–2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Y, Wang X, Varty L, Rizzo CA, Yang R, Correll CC, et al. (2004). Functional TRPV4 channels are expressed in human airway smooth muscle cells. American Journal of Physiology. Lung Cellular and Molecular Physiology, 287, L272–L278. [DOI] [PubMed] [Google Scholar]
- Jian MY, King JA, Al-Mehdi AB, Liedtke W, & Townsley MI (2008). High vascular pressure-induced lung injury requires P450 epoxygenase-dependent activation of TRPV4. American Journal of Respiratory Cell and Molecular Biology, 38, 386–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komiya K, Akaba T, Kozaki Y, Kadota JI, & Rubin BK (2017). A systematic review of diagnostic methods to differentiate acute lung injury/acute respiratory distress syndrome from cardiogenic pulmonary edema. Critical Care, 21, 228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuebler WM, Jordt SE, & Liedtke WB (2020). Urgent reconsideration of lung edema as a preventable outcome in COVID-19: Inhibition of TRPV4 represents a promising and feasible approach. American Journal of Physiology. Lung Cellular and Molecular Physiology, 318, L1239–L1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin MT, Jian MY, Taylor MS, Cioffi DL, Yap FC, Liedtke W, et al. (2015). Functional coupling of TRPV4, IK, and SK channels contributes to ca(2+)-dependent endothelial injury in rodent lung. Pulmonary Circulation, 5, 279–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Han K, Blair R, Kenst K, Qin Z, Upcin B, et al. (2021). SARS-CoV-2 infects endothelial cells in vivo and in vitro. Frontiers in Cellular and Infection Microbiology, 11, 701278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longden TA, Dabertrand F, Koide M, Gonzales AL, Tykocki NR, Brayden JE, et al. (2017). Capillary K(+)-sensing initiates retrograde hyperpolarization to increase local cerebral blood flow. Nature Neuroscience, 20, 717–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Cheng KT, Wong CO, O’Neil RG, Birnbaumer L, Ambudkar IS, et al. (2011). Heteromeric TRPV4-C1 channels contribute to store-operated ca(2+) entry in vascular endothelial cells. Cell Calcium, 50, 502–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier-Begandt D, Comstra HS, Molina SA, Kruger N, Ruddiman CA, Chen YL, et al. (2021). A venous-specific purinergic signaling cascade initiated by pannexin 1 regulates TNFalpha-induced increases in endothelial permeability. Science Signaling, 14, eaba2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maitland K, Kiguli S, Opoka RO, Engoru C, Olupot-Olupot P, Akech SO, et al. (2011). Mortality after fluid bolus in African children with severe infection. The New England Journal of Medicine, 364, 2483–2495. [DOI] [PubMed] [Google Scholar]
- Majhi RK, Sahoo SS, Yadav M, Pratheek BM, Chattopadhyay S, & Goswami C (2015). Functional expression of TRPV channels in T cells and their implications in immune regulation. The FEBS Journal, 282, 2661–2681. [DOI] [PubMed] [Google Scholar]
- Marziano C, Hong K, Cope EL, Kotlikoff MI, Isakson BE, & Sonkusare SK (2017). Nitric oxide-dependent feedback loop regulates transient receptor potential vanilloid 4 (TRPV4) channel cooperativity and endothelial function in small pulmonary arteries. Journal of the American Heart Association, 6, e007157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercado J, Baylie R, Navedo MF, Yuan C, Scott JD, Nelson MT, et al. (2014). Local control of TRPV4 channels by AKAP150-targeted PKC in arterial smooth muscle. The Journal of General Physiology, 143, 559–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalick L, Erfinanda L, Weichelt U, van der Giet M, Liedtke W, & Kuebler WM (2017). Transient receptor potential vanilloid 4 and serum glucocorticoid-regulated kinase 1 are critical mediators of lung injury in overventilated mice in vivo. Anesthesiology, 126, 300–311. [DOI] [PubMed] [Google Scholar]
- Michalick L, Mandzimba-Maloko B, Hamedi S, Dohmen M, Brack MC, Schulz S, et al. (2022). In vitro screening identifies TRPV4 and PAR1 as targets for endothelial barrier stabilization in COVID-19. The FASEB Journal, 36. [Google Scholar]
- Mole S, Harry A, Fowler A, Hotee S, Warburton J, Waite S, et al. (2020). Investigating the effect of TRPV4 inhibition on pulmonary-vascular barrier permeability following segmental endotoxin challenge. Pulmonary Pharmacology & Therapeutics, 64, 101977. [DOI] [PubMed] [Google Scholar]
- Nieto-Posadas A, Jara-Oseguera A, & Rosenbaum T (2011). TRP channel gating physiology. Current Topics in Medicinal Chemistry, 11, 2131–2150. [DOI] [PubMed] [Google Scholar]
- Nilius B, & Voets T (2013). The puzzle of TRPV4 channelopathies. EMBO Reports, 14, 152–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottolini M, Daneva Z, Chen YL, Cope EL, Kasetti RB, Zode GS, et al. (2020). Mechanisms underlying selective coupling of endothelial Ca2+ signals with eNOS vs. IK/SK channels in systemic and pulmonary arteries. Journal of Physiology, 598, 3577–3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottolini M, Hong K, Cope EL, Daneva Z, DeLalio LJ, Sokolowski JD, et al. (2020). Local peroxynitrite impairs endothelial transient receptor potential vanilloid 4 channels and elevates blood pressure in obesity. Circulation, 141, 1318–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottolini M, Hong K, & Sonkusare SK (2019). Calcium signals that determine vascular resistance. Wiley Interdisciplinary Reviews. Systems Biology and Medicine, e1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottolini M, & Sonkusare SK (2021). The calcium signaling mechanisms in arterial smooth muscle and endothelial cells. Comprehensive Physiology, 11, 1831–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pairet N, Mang S, Fois G, Keck M, Kuhnbach M, Gindele J, et al. (2018). TRPV4 inhibition attenuates stretch-induced inflammatory cellular responses and lung barrier dysfunction during mechanical ventilation. PLoS One, 13, e0196055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps CB, Huang RJ, Lishko PV, Wang RR, & Gaudet R (2008). Structural analyses of the ankyrin repeat domain of TRPV6 and related TRPV ion channels. Biochemistry, 47, 2476–2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahaman SO, Grove LM, Paruchuri S, Southern BD, Abraham S, Niese KA, et al. (2014). TRPV4 mediates myofibroblast differentiation and pulmonary fibrosis in mice. The Journal of Clinical Investigation, 124, 5225–5238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan S, Schremmer C, Weber J, Alt P, Geiger F, & Dietrich A (2021). Ca(2 +) signaling by TRPV4 channels in respiratory function and disease. Cells, 10, 822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayees S, Joshi JC, Tauseef M, Anwar M, Baweja S, Rochford I, et al. (2019). PAR2-mediated cAMP generation suppresses TRPV4-dependent ca(2+) signaling in alveolar macrophages to resolve TLR4-induced inflammation. Cell Reports, 27, 793–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum T, Benitez-Angeles M, Sanchez-Hernandez R, Morales-Lazaro SL, Hiriart M, Morales-Buenrostro LE, et al. (2020). TRPV4: A physio and Pathophysiologically significant Ion Channel. International Journal of Molecular Sciences, 21, 3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryskamp DA, Jo AO, Frye AM, Vazquez-Chona F, MacAulay N, Thoreson WB, et al. (2014). Swelling and eicosanoid metabolites differentially gate TRPV4 channels in retinal neurons and glia. The Journal of Neuroscience, 34, 15689–15700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheraga RG, Abraham S, Grove LM, Southern BD, Crish JF, Perelas A, et al. (2020). TRPV4 protects the lung from bacterial pneumonia via MAPK molecular pathway switching. Journal of Immunology, 204, 1310–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheraga RG, Southern BD, Grove LM, & Olman MA (2017). The role of transient receptor potential vanilloid 4 in pulmonary inflammatory diseases. Frontiers in Immunology, 8, 503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheraga RG, Southern BD, Grove LM, & Olman MA (2020). The role of TRPV4 in regulating innate immune cell function in lung inflammation. Frontiers in Immunology, 11, 1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma AK, Charles EJ, Zhao Y, Narahari AK, Baderdinni PK, Good ME, et al. (2018). Pannexin-1 channels on endothelial cells mediate vascular inflammation during lung ischemia-reperfusion injury. American Journal of Physiology. Lung Cellular and Molecular Physiology, 315, L301–L312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonkusare SK, Bonev AD, Ledoux J, Liedtke W, Kotlikoff MI, Heppner TJ, et al. (2012). Elementary Ca2+ signals through endothelial TRPV4 channels regulate vascular function. Science, 336, 597–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonkusare SK, Dalsgaard T, Bonev AD, Hill-Eubanks DC, Kotlikoff MI, Scott JD, et al. (2014). AKAP150-dependent cooperative TRPV4 channel gating is central to endothelium-dependent vasodilation and is disrupted in hypertension. Science Signaling, 7, ra66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonkusare SK, Dalsgaard T, Bonev AD, & Nelson MT (2016). Inward rectifier potassium (Kir2.1) channels as end-stage boosters of endothelium-dependent vasodilators. The Journal of Physiology, 594, 3271–3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens T (2011). Functional and molecular heterogeneity of pulmonary endothelial cells. Proceedings of the American Thoracic Society, 8, 453–457. [DOI] [PubMed] [Google Scholar]
- Stewart AP, Smith GD, Sandford RN, & Edwardson JM (2010). Atomic force microscopy reveals the alternating subunit arrangement of the TRPP2-TRPV4 heterotetramer. Biophysical Journal, 99, 790–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strotmann R, Harteneck C, Nunnenmacher K, Schultz G, & Plant TD (2000). OTRPC4, a nonselective cation channel that confers sensitivity to extracellular osmolarity. Nature Cell Biology, 2, 695–702. [DOI] [PubMed] [Google Scholar]
- Suresh K, Servinsky L, Jiang H, Bigham Z, Yun X, Kliment C, et al. (2018). Reactive oxygen species induced ca(2+) influx via TRPV4 and microvascular endothelial dysfunction in the SU5416/hypoxia model of pulmonary arterial hypertension. American Journal of Physiology. Lung Cellular and Molecular Physiology, 314, L893–L907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suresh K, Servinsky L, Reyes J, Baksh S, Undem C, Caterina M, et al. (2015). Hydrogen peroxide-induced calcium influx in lung microvascular endothelial cells involves TRPV4. American Journal of Physiology. Lung Cellular and Molecular Physiology, 309, L1467–L1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorneloe KS, Cheung M, Bao W, Alsaid H, Lenhard S, Jian MY, et al. (2012). An orally active TRPV4 channel blocker prevents and resolves pulmonary edema induced by heart failure. Science Translational Medicine, 4 (159ra148). [DOI] [PubMed] [Google Scholar]
- Toft-Bertelsen TL, & MacAulay N (2021). TRPing to the point of clarity: Understanding the function of the complex TRPV4 Ion Channel. Cells, 10, 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalta PC, & Townsley MI (2013). Transient receptor potential channels and regulation of lung endothelial permeability. Pulmonary Circulation, 3, 802–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voets T, Prenen J, Vriens J, Watanabe H, Janssens A, Wissenbach U, et al. (2002). Molecular determinants of permeation through the cation channel TRPV4. The Journal of Biological Chemistry, 277, 33704–33710. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Vriens J, Janssens A, Wondergem R, Droogmans G, & Nilius B (2003). Modulation of TRPV4 gating by intra- and extracellular Ca2+. Cell Calcium, 33, 489–495. [DOI] [PubMed] [Google Scholar]
- Weber J, Rajan S, Schremmer C, Chao YK, Krasteva-Christ G, Kannler M, et al. (2020). TRPV4 channels are essential for alveolar epithelial barrier function as protection from lung edema. JCI Insight, 5, e134464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JP, Cibelli M, Urban L, Nilius B, McGeown JG, & Nagy I (2016). TRPV4: Molecular conductor of a diverse orchestra. Physiological Reviews, 96, 911–973. [DOI] [PubMed] [Google Scholar]
- Willette RN, Bao W, Nerurkar S, Yue TL, Doe CP, Stankus G, et al. (2008). Systemic activation of the transient receptor potential vanilloid subtype 4 channel causes endothelial failure and circulatory collapse: Part 2. The Journal of Pharmacology and Experimental Therapeutics, 326, 443–452. [DOI] [PubMed] [Google Scholar]
- Xia Y, Fu Z, Hu J, Huang C, Paudel O, Cai S, et al. (2013). TRPV4 channel contributes to serotonin-induced pulmonary vasoconstriction and the enhanced vascular reactivity in chronic hypoxic pulmonary hypertension. American Journal of Physiology. Cell Physiology, 305, C704–C715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XR, Lin AH, Hughes JM, Flavahan NA, Cao YN, Liedtke W, et al. (2012). Upregulation of osmo-mechanosensitive TRPV4 channel facilitates chronic hypoxia-induced myogenic tone and pulmonary hypertension. American Journal of Physiology. Lung Cellular and Molecular Physiology, 302, L555–L568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J, Michalick L, Tang C, Tabuchi A, Goldenberg N, Dan Q, et al. (2016). Role of transient receptor potential vanilloid 4 in neutrophil activation and acute lung injury. American Journal of Respiratory Cell and Molecular Biology, 54, 370–383. [DOI] [PubMed] [Google Scholar]
- Yu Q, Wang D, Wen X, Tang X, Qi D, He J, et al. (2020). Adipose-derived exosomes protect the pulmonary endothelial barrier in ventilator-induced lung injury by inhibiting the TRPV4/ca(2+) signaling pathway. American Journal of Physiology. Lung Cellular and Molecular Physiology, 318, L723–L741. [DOI] [PMC free article] [PubMed] [Google Scholar]


