Abstract
In this study, the sigA gene situated on the she pathogenicity island of Shigella flexneri 2a was cloned and characterized. Sequence analysis showed that sigA encodes a 139.6-kDa protein which belongs to the SPATE (serine protease autotransporters of Enterobacteriaceae) subfamily of autotransporter proteins. The demonstration that SigA is autonomously secreted from the cell to yield a 103-kDa processed form and possesses a conserved C-terminal domain for export from the cell were consistent with the autotransporter pathway of secretion. Functional analysis showed that SigA is a secreted temperature-regulated serine protease capable of degrading casein. SigA was cytopathic for HEp-2 cells, suggesting that it may be a cell-altering toxin with a role in the pathogenesis of Shigella infections. SigA was at least partly responsible for the ability of S. flexneri to stimulate fluid accumulation in ligated rabbit ileal loops.
Shigella spp. are gram-negative bacteria that are the etiological agents of bacillary dysentery, a diarrheal disease responsible for the death of more than 500,000 people every year (37). Infections are transmitted via the fecal-oral route and are usually the result of direct person-to-person contact or the consumption of contaminated water or food (12). After ingestion, shigellae invade the colonic epithelium and then spread from cell to cell, resulting in cell destruction and inflammation (35). In the infected host, this leads to a watery diarrhea that often progresses to the bloody, mucoid diarrhea typical of bacillary dysentery (15).
A variety of virulence determinants play important roles in the pathogenesis of shigellosis. Several proteins required for epithelial cell invasion and intercellular spread, including the autotransporter VirG, are encoded by a large virulence plasmid found in all Shigella spp. (38). In addition, some Shigella spp. produce enterotoxins that probably play a role in the watery diarrhea phase of shigellosis. These include ShET1, an enterotoxin found predominantly in Shigella flexneri serotype 2a (15), and ShET2, which is found in more than 80% of Shigella strains of diverse serotypes (25). Other determinants include an aerobactin iron uptake system found in S. flexneri, S. sonnei, and S. boydii (45), SepA, which induces both mucosal atrophy and tissue inflammation, indicating that it is involved in tissue invasion by S. flexneri 5 (4), and the putative virulence protein Pic (originally ShMu), from S. flexneri 2a, which has mucinase and hemagglutinin activities (17; F. R. Noriega, unpublished data).
Both SepA and Pic belong to a rapidly expanding family of autotransporter proteins that share sequence similarity and a common pathway for autonomous secretion (18). This type of autosecretion is characterized by transport into the periplasm, usually via the Sec system, followed by insertion of the transported protein's C-terminal domain, termed the β domain, into the outer membrane. The β domain is thought to form a β-barrel pore through which the remainder of the protein, termed the passenger or α domain, is transported to the exterior of the cell. After transport across the outer membrane, exported proteins either remain tethered to the cell surface or are released from the cell by proteolytic cleavage (18). Neisseria gonorrhoeae immunoglobulin A (IgA) protease, the first member of the autotransporter family, is a serine protease that catalyzes its own release from the cell by autoproteolysis (28). However, in some cases autotransporters are released from the cell by the actions of distinct proteases located in the outer membrane (13, 40).
Several autotransporters have established or potential roles in bacterial virulence (18). Their various biological roles and activities include mediating adherence to cell surfaces, proteolysis, cytotoxicity, cell invasion, intracellular motility, and serum resistance (18). Recently, Pet, a member of this subfamily from enteroaggregative Escherichia coli, was shown to have cytopathic effects on HEp-2 cells as well as demonstrating enterotoxic activity.
The autotransporter family shows a considerable degree of divergence, and members have been classified into subfamilies based on amino acid sequence. Both SepA and Pic belong to the Tsh subfamily, otherwise known as the SPATE (serine protease autotransporters of Enterobacteriaceae) subfamily, and both possess a highly conserved serine protease motif shared by other members of the group (18). The serine protease activity of SepA and some other members of this subfamily has been demonstrated experimentally (5).
In S. flexneri 2a, the pic (originally she) gene, encoding Pic, is associated with a pathogenicity island (PAI) termed the she PAI (32). The she PAI is distinct from another unstable PAI-like element, encoding multiple antibiotic resistance in S. flexneri 2a (31), and the recently described Shi-2 PAI, encoding an aerobactin iron uptake system in a variety of Shigella strains (23, 45). Like several other PAIs, the she PAI is an unstable chromosomal locus and spontaneously deletes at a frequency of 10−5 to 10−6 per cell per generation (32). The extent of the PAI is not known, but she PAI deletants appear to have lost a 51-kb region that resides on NotI fragment A (32). Very little is known about the genes carried on the she PAI and their roles in virulence. Besides the pic gene, the she PAI carries set1A and set1B, the genes encoding the two subunits of the ShET1 enterotoxin (15). set1A and set1B overlap the pic gene and are transcribed in an opposite direction to pic. In a previous study, we discovered a sequence, lying 3.6 kb downstream of pic, which encoded a putative protein with similarity to the carboxy termini of several autotransporter proteins (32). This suggested that as well as Pic, the she PAI may encode a second autotransporter protein which was tentatively named SigA. In the present study, we have confirmed that sigA encodes a protein belonging to the autotransporter family of proteins. In addition, we report on the regulation of SigA expression in S. flexneri, its biological activities, and its role in virulence.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The bacterial strains and plasmids used in this study are described in Table 1. Escherichia coli DH5α and Shigella strains were grown in either Luria-Bertani or 2xYT medium at 37°C with aeration. When antibiotic selection was necessary, the growth medium was supplemented with ampicillin (100 μg/ml), kanamycin (50 μg/ml), tetracycline (10 μg/ml), streptomycin (25 μg/ml), or spectinomycin (25 μg/ml).
TABLE 1.
Bacterial strains and plasmids used
| Bacterial strain or plasmid | Description | Reference or source |
|---|---|---|
| E. coli DH5α | F−endA1 hsdr 17 (rK− mK+) thi-1 λ− recA1 gyrA96 relA1 φ80dlacZΔM15 | 16 |
| S. flexneri 2a | ||
| YSH6000 | Wild-type S. flexneri 2a Japanese isolate, Apr Strepr Tetr Cmr | 39 |
| YSH6200 | Derivative of YSH6000 cured of the 230-kb virulence plasmid | 33 |
| YSH6000T | Spontaneous Aps Streps Tets Cms variant of YSH6000 | 24 |
| SBA1336 | Derivative of YSH6000T harboring a Tcr cassette within she | 32 |
| SBA1351 | Derivative of YSH6000T harboring a Kmr cassette within sigA | This study |
| SBA1341 | Tcs derivative of SBA1336 exhibiting a 51-kb chromosomal deletion | 32 |
| SBA1356 | Derivative of SBA1336 harboring an Ω cassette within sepA | This study |
| SBA1361 | Derivative of SBA1341 harboring pSBA479 | This study |
| SBA1413 | Derivative of SBA1351 harboring pSBA572 | This study |
| Plasmids | ||
| pPBA1100 | pUC18-based high-copy-number vector, Kmr ΔlacZ′ | 20 |
| pWSK29 | pSC101-based low-copy-number vector, Apr ΔlacZ′ | 46 |
| pWSK30 | pSC101-based low-copy-number vector, Apr ΔlacZ′ | 46 |
| pWSK130 | pSC101-based low-copy-number vector, Kmr ΔlacZ′ | 46 |
| pCACTUS | pSC101-based low-copy-number vector with sacB, Cmr | 43 |
| pBz | Hybrid of pBluescriptSK+ and pZero 1.1, ccdB gene replaces ΔlacZ′ | S. Doughty, Monash University |
| pUC4K | Source of Kmr gene from Tn903, Apr Kmr | 44 |
| pPBA1180 | 2.0-kb Ω fragment consisting of the Strepr/Specr gene of the R100.1 plasmid cloned into pUC19 | 29; M. Hunt, Monash University |
| pSBA415 | 19.9-kb SalI fragment of SBA1336 bearing the 3′ truncated she gene with an inserted tetAR(B) cassette cloned into BamHI site of pWSK29, Apr Tcr | 32 |
| pSBA432 | 3.6-kb EcoRI fragment of pSBA415 cloned into EcoRI site of pWSK130, Kmr | N. Ngoc, Monash University |
| pSBA479 | 5.4-kb SalI/HindIII fragment of pSBA415 cloned into SalI/HindIII sites of pPBA1100, Kmr | This study |
| pSBA493 | 1.2-kb AccI fragment carrying a Kmr of pUC4K cloned in the unique ClaI site in pSBA479 | This study |
| pSBA501 | 6.7-kb Expand PCR product containing the insertionally disrupted sigA gene cloned in pCACTUS T-tailed SmaI site | This study |
| pSBA544 | 3.3-kb PCR product containing sepA cloned into BamHI site of pBz | This study |
| pSBA549 | 2.0-kb HindIII Ω fragment cloned into unique HindIII site in pSBA544 | This study |
| pSBA550 | 5.3-kb BamHI fragment (sepA-Ω) of pSBA549 cloned in the unique BamHI site in pCACTUS | This study |
| pSBA572 | 5.4-kb SalI/HindIII fragment of pSBA479 cloned into the SalI/HindIII site in pWSK30 | This study |
Recombinant DNA techniques.
The preparation of genomic DNA of S. flexneri 2a was described previously (2), as was the preparation of plasmid DNA (22). DNA ligations and restriction endonuclease digestions (2) were performed with enzymes supplied by Roche Molecular Biochemicals (Basel, Switzerland) or New England Biolabs (Beverly, Mass.). DNA was introduced into E. coli and S. flexneri 2a by electrotransformation (41) with a Bio-Rad (Hercules, Calif.) Gene Pulser. Standard methods (34) were used for cloning. PCR products for cloning experiments were amplified using a Expand high-fidelity PCR kit (Roche).
Nucleotide sequencing was performed with a PRISM Ready Reaction DyeDeoxy Terminator cycle sequencing kit (Perkin-Elmer Corp., Norwalk, Conn.), using universal vector primers or synthetic oligonucleotides designed on the basis of preceding sequences. The reactions were analyzed on an Applied Biosystems model 373A DNA sequencing system (Perkin-Elmer). The program Sequencher (GeneCodes Corporation, Ann Arbor, Mich.) was used for routine analysis of DNA sequence information. Similarities and identities between pairwise comparisons of protein sequences were determined with the BlastN, BlastP, or BlastX program (1). Comparisons of multiple sequence alignments were done using the program PileUp (Genetics Computer Group [Madison, Wis.] Wisconsin Package).
Construction of chromosomal mutants.
To construct a sigA mutant, plasmid pSBA479, which carries sigA on a SalI/HindIII fragment of pSBA415 (32), was cleaved at the unique ClaI site within sigA and ligated with an AccI fragment carrying the kanamycin resistance (Kmr) cassette from pUC4K to yield plasmid pSBA493. A 6.7-kb DNA fragment containing sigA-kan was then amplified by PCR from pSBA493, using vector primers. This PCR fragment was cloned into the SmaI-digested, T-tailed site of the vector pCACTUS (44). The resulting construct, pSBA501, was introduced into S. flexneri 2a YSH6000T by electroporation, and strains containing sigA-kan in the chromosome were selected by growth at 42°C on media containing 5% sucrose and kanamycin. Mutants resulting from double-crossover allelic exchange were identified by screening for chloramphenicol sensitivity. The presence of only an insertionally inactivated copy of sigA alone in the chromosome was confirmed by Southern blot analysis (data not shown). One sigA mutant strain, SBA1351, was selected for further experiments.
A sepA/pic double mutant was constructed by amplifying a truncated version of the sepA gene (3.3 kb) from S. flexneri 2a YSH6000T and cloning it into the BamHI site of pBz to yield pSBA544. A streptomycin-spectinomycin resistance (Strepr/Specr) Ω cassette was isolated from pPBA1180 by digestion with HindIII and ligated into an introduced HindIII site in pSBA544 present at the 5′ end of sepA to yield pSBA549. A 5.3-kb BamHI fragment (sepA-Ω) was subsequently excised from pSBA549 and cloned into the equivalent site of pCACTUS to produce pSBA550. Plasmid pSBA550 was then introduced into the pic strain SBA1336 (33), and the method described above was applied to select for double-crossover mutants resistant to tetracycline, streptomycin, and spectinomycin and sensitive to chloramphenicol. A putative mutant was analyzed by PCR and Southern blot hybridization to confirm allelic exchange and was named SBA1356 (data not shown).
Protein purification and analysis.
For the purification of SigA, culture supernatants were recovered by centrifugation at 8,000 × g for 10 min from bacteria grown to an optical density at 600 nm of 1.0 and then filter sterilized through 0.22-μm-pore-size filter units (Millipore, Bedford, Mass.). Supernatants were concentrated either 200-fold by ultrafiltration through an Amicon PM50 membrane or 2,000-fold by a combination of ammonium sulfate precipitation (50% saturation) and ultrafiltration using Centriplus-50 concentrators (Amicon, Lexington, Mass.). The retentates were dialyzed three times against phosphate-buffered saline, pH 7.2 (PBS). All manipulations were performed at 4°C. Concentrates were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (21) and assayed for proteolytic activity toward casein using an EnzChek protease assay kit (Molecular Probes, Leiden, The Netherlands). The intensity of fluorescence at 520 nm was measured at 37°C in samples excited at 485 nm with a Perkin-Elmer fluorescence spectrophotometer. A unit of activity was defined as an increase of one fluorescence unit over a period of 5 h.
Antisera specific for the 103-kDa secreted form of SigA were produced by immunization of New Zealand White rabbits with polyacrylamide gel slices that had been macerated and mixed with either Freund's incomplete adjuvant or water. The rabbits were boosted three times over the course of 6 months and exsanguinated; sera were collected, stored at −20°C, and used to detect SigA by immunoblotting (8).
Cell culture methods.
HEp-2 cells were grown in Dulbecco's modified Eagle's medium supplemented with 5% fetal calf serum (HyClone, Logan, Utah), 1% nonessential amino acids, 5 mM l-glutamine, penicillin (100 μg/ml), and streptomycin (100 μg/ml). Cells were maintained at 37°C in humidified 5% CO2–95% air. For experimental use, subconfluent HEp-2 cells were resuspended with EDTA-trypsin, plated in eight-well LabTek slides (VWR, Bridgeport, N.J.), and allowed to grow to 60% confluence. For all experiments, protein preparations were diluted directly in tissue culture medium on the cells to a final concentration of 25 μg/ml. To neutralize the serine protease activity of SigA, the SigA preparations were incubated for 15 min with 2 mM phenylmethylsulfonyl fluoride (PMSF), washed twice with 5 volumes of PBS in a centrifugal filter device with a 100-kDa cutoff to remove residual PMSF, and then added to HEp-2 cells in fresh medium. Equivalent quantities of control concentrates (from vector-containing bacteria) were added to additional wells as controls. After incubation for 6 h at 37°C in humidified 5% CO2–95% air, the medium was aspirated, and the cells were washed three times with PBS and stained with Giemsa stain as described previously (26).
Fluid accumulation assays.
Infection of rabbit ileal loops was performed as described previously (36). In brief, strains of S. flexneri were investigated for the ability to induce fluid accumulation in the ileum of New Zealand White rabbits. The ileum was ligated to construct loops approximately 5 cm long, after which 1 ml of PBS containing 109 bacteria was injected into the lumen of each loop, using a 25-gauge needle. After 18 h, the rabbits were euthanized, and the amount of fluid accumulation in the loop was measured. Results were expressed as the volume of fluid (milliliters) accumulated per centimeter of ligated intestinal segment. Data were analyzed by Student's t test.
Nucleotide sequence accession number.
The sequence data reported here have been assigned GenBank accession no. AF200692.
RESULTS
Cloning and sequence analysis of the sigA gene.
To obtain the entire sigA gene, we cloned a 5.4-kb SalI/HindIII fragment from pSBA415 (32) into pPBA1100 to construct pSBA479. Sequencing of the cloned insert in pSBA479 revealed a predicted open reading frame of 3,858 nucleotides which encodes a putative protein with a deduced molecular mass of 139.6 kDa and a predicted isoelectric point of 8.9. Comparison of the translated sequence of sigA with sequences in GenBank determined that SigA exhibited sequence similarity and was comparable in length to a large number of bacterial IgA1 protease-like autotransporter proteins, including Pet (72% similarity and 59% identity) (14), PssA (10), EspP (7), EspC (42), Pic (17; Noriega, unpublished data), SepA (4), and Tsh (30). Sequence identity to these proteins varied from 39 to 58%, while similarity varied from 59 to 72% (Table 2).
TABLE 2.
Homologies of SigA from the SPATE family of proteases
In common with this family of autotransporters, the S. flexneri 2a SigA protein appears to be divided into three domains comprising an N-terminal signal sequence typical of this subfamily of autotransporters, followed by a putative passenger or α domain and a carboxy-terminal β domain. The degree of similarity to other members of this family was much higher in the C-terminal domain of the protein, consistent with the putative role of this region in protein secretion (18). The polypeptide possessed additional features characteristic of other members of this family. A potential serine protease motif (G256DSGSGS) (18) was found, as well as a putative signal peptidase cleavage site at residues 54 and 55 (27). The conserved serine protease motif consisting of the sequence GDSGSPLF, where S is the active-site serine characteristic of serine proteases, has been identified as part of the catalytic site of the IgA1 proteases (3, 6). The cleavage site of the β domain was predicted to lie between residues N1007 and N1008 by comparison with other autotransporters.
Secretion of SigA from E. coli DH5α.
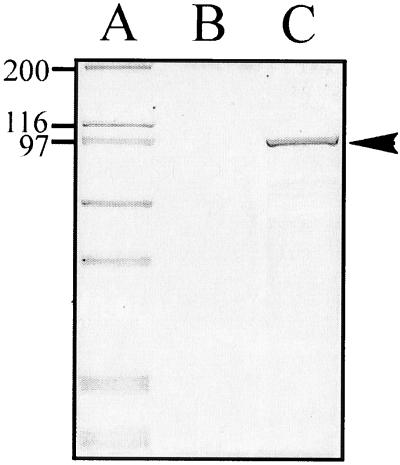
To establish whether the sigA open reading frame encoded a secreted protein, supernatants concentrated from logarithmic phase cultures of E. coli DH5α/pSBA479 (sigA) and the control strain DH5α/pPBA1100 (vector only) were analyzed by SDS-PAGE. Coomassie blue staining indicated that DH5α/pSBA479 expressed and secreted a protein of approximately 103 kDa, while no such protein was secreted from cells with the vector only (Fig. 1). The size of the protein coincided with the molecular weight of the predicted secreted product deduced from the sigA nucleotide sequence assuming cleavage of the N-terminal signal peptide and the C-terminal β-barrel pore.
FIG. 1.
Analysis of the expression and secretion of SigA in E. coli carrying the sigA gene. Concentrated culture supernatants were subjected to SDS-PAGE, and the gel was stained with Coomassie blue. Lanes: A, molecular mass markers (indicated in kilodaltons at the left); B, supernatant of DH5α/pPBA1100 (vector only); C, supernatant of DH5α/pSBA479 (sigA). The arrowhead indicates the 103-kDa secreted protein encoded by sigA.
The sequence comparison strongly suggested that the C-terminal domain was required for transport of the protein. Using the TopPredII computer program developed for the prediction of secondary structures adopted by membrane proteins (9), the putative β domain of the SigA polypeptide was found to consist of 14 amphiphilic β strands, strongly suggesting that these strands form a β-barrel pore for the translocation of SigA across the outer membrane. To test this hypothesis, a truncated form of sigA lacking the putative β-barrel domain was obtained by deleting the region of sigA downstream of nucleotide 2508, giving rise to plasmid pSBA432. A concentrated supernatant of E. coli harboring this truncated gene contained no secreted proteins when analyzed by SDS-PAGE (data not shown). These findings support the notion that the C terminus of SigA is indispensable for the secretion of the protein.
Expression of SigA is regulated by temperature in vitro.
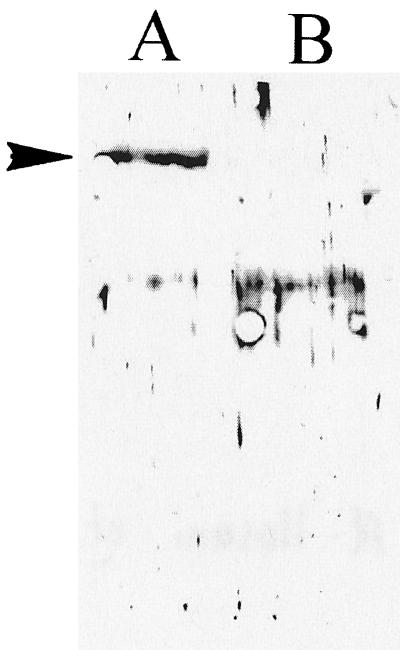
Since temperature affects the expression of a variety of virulence determinants, including the autotransporter proteins SepA (4), Tsh (30), EspP (7), and Pic (17), we sought to determine whether expression of SigA was also regulated by temperature. In this experiment, Shigella strain SBA1356, carrying mutations in sepA and pic but expressing SigA, was cultured at 37 or 21°C. Protein expression was evaluated by immunoblotting with antibodies specific for SigA. As shown in Fig. 2, SigA was expressed at 37 not 21°C.
FIG. 2.
Temperature regulation of SigA. Supernatants from S. flexneri SBA1356 grown at 37°C (lane A) or 21°C (lane B) were immunoblotted and probed with rabbit antiserum raised against SigA. The arrowhead indicates the 103-kDa SigA protein expressed at 37°C.
Proteolytic activity of SigA.
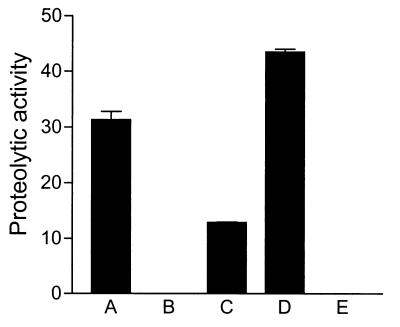
Based on the presence of a protease motif and homology with other proteins which have been demonstrated to act as proteases, we suspected that SigA also possess proteolytic activity. To determine whether SigA was a protease, concentrated supernatant from E. coli DH5α/pSBA479 was tested for its ability to degrade a casein-based fluorogenic substrate by using an EnzChek protein assay kit. Protease activity was detected in the supernatant derived from strain DH5α/pSBA479 (Fig. 3, column A), while no protease activity was detected in supernatants of the control E. coli DH5α/pPBA1100 (column B). As expected, supernatant from the wild-type S. flexneri 2a YSH6000T (column C) also showed proteolytic activity. To test whether this activity was attributable to SigA, we inserted a Kmr cassette into a unique ClaI site in the sigA gene and introduced it by allelic exchange to construct the Shigella sigA mutant SBA1351. No protease activity was observed with this strain (column E), indicating that SigA is the only secreted protease of S. flexneri 2a with activity for casein. Moreover, casein-specific proteolytic activity was fully restored in a she PAI deletant strain complemented with an intact sigA gene carried on pSBA479 (column D). The higher levels observed in the complemented strain SBA1361 appeared to be due to a gene dosage effect.
FIG. 3.
Protease activity of SigA. Proteolytic activity of concentrated supernatants derived from E. coli and S. flexneri 2a was measured as described in Materials and Methods. Columns: A, E. coli DH5α/pSBA479 (sigA); B, DH5α/pPBA1100 (vector only); C, S. flexneri 2a YSH6000T (parent); D, SBA1361 (sigA); E, SBA1351 (sigA::kan). Error bars indicate standard errors in assays performed at least in duplicate.
Biological role of SigA.
The properties of many autotransporter proteins suggest roles in bacterial virulence (18). Of the known autotransporters, the enterotoxin Pet from enteroaggregative E. coli has the greatest sequence similarity to SigA. To determine whether SigA had similar properties, we tested SigA for cytopathic activity toward HEp-2 cells.
Partially purified SigA protein was obtained by passage of culture supernatants from DH5α/pSBA479 through a 100-kDa-cutoff filter (Materials and Methods). After filtration, the protein was found in the retentate. The concentrated SigA protein preparation was applied to HEp-2 epithelial cells cultured in eight-well chamber slides.
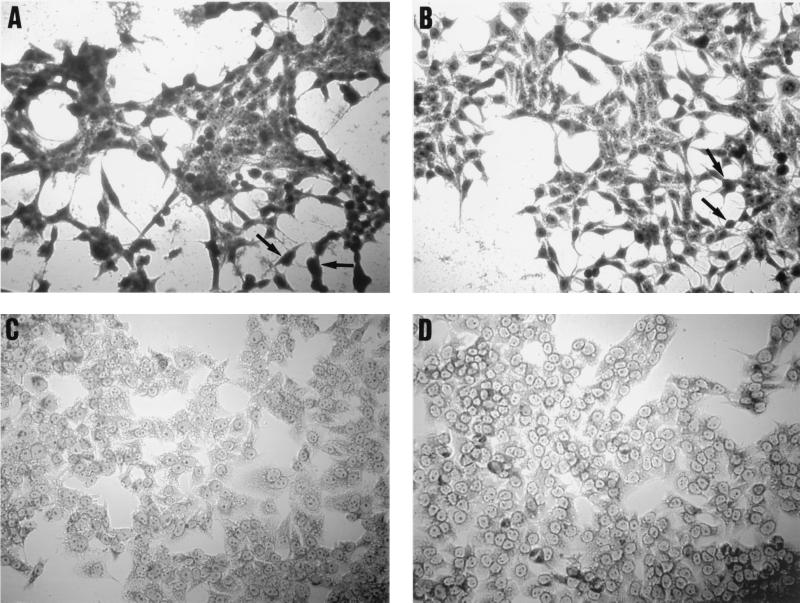
Supernatants containing the SigA protein caused extensive damage to the HEp-2 cells, as characterized by the release of cellular focal adhesion contacts from the glass substratum, rounding of cells, and detachment (Fig. 4B). Similar effects were observed with purified Pet toxin from enteroaggregative E. coli (Fig. 4A). However, the level of toxicity appeared to be more pronounced on treatment with Pet. The morphologies of the HEp-2 cells were unaltered by treatment of the cells with concentrated supernatants from DH5α/pPBA1100 (Fig. 4D). In addition, treatment of the SigA protein preparations with PMSF prior to addition of the preparations to the HEp-2 culture medium inhibited the cytopathic activity of the preparations on HEp-2 cells (Fig. 4C).
FIG. 4.
Effect of SigA protein on epithelial cells. SigA (B) and Pet (A) proteins were added to cell cultures at a final concentration of 25 μg/ml per well and incubated for 3 h. Release from the glass substratum of the cellular focal adhesion points and rounding of cells is indicated by arrows. (C) HEp-2 cells incubated with PMSF (25 μg/ml)-treated SigA preparations. No cell rounding or detachment of focal adhesion contacts is apparent. Appropriate vector control preparations were also added (D; see text).
To test whether SigA had enterotoxic activity, we compared the amount of fluid accumulation induced by a sigA mutant (SBA1351) and its parent strain (YSH6000T) in a rabbit ileal loop model of infection. Fluid accumulation induced by the sigA mutant was 30% lower than that of the wild-type YSH6000T (P = 0.004 by Student's t test, two tailed). Since sigA lies upstream of the genes encoding ShET1 enterotoxin (32), it was possible that the decreased fluid accumulation observed was due to polar effects on the expression of the enterotoxin genes. To test this, we compared the induction of fluid accumulation by SBA1351 (sigA) and by SBA1351 complemented with pSBA572 (sigA+). A mean fluid accumulation ratio of 0.79 was observed with the mutant as opposed to 1.05 with the complemented strain (P = 0.016), indicating that reduced fluid accumulation the phenotype of SBA1351 was due to disruption of sigA rather than a polar effect on downstream genes.
DISCUSSION
In this study, we have shown that in addition to Pic, the she PAI of S. flexneri 2a encodes another protein, SigA, belonging to the autotransporter family of proteins. The sigA gene encodes a protein with a predicted molecular mass of 139.6 kDa that has significant sequence similarity to several autotransporters from a variety of gram-negative bacteria. Like SepA and Pic, two other autotransporters found in Shigella, SigA is most closely related to the SPATE subfamily of autotransporters. SigA exhibits several highly conserved features of this family, including a long putative N-terminal signal sequence, a predicted C-terminal β domain, a putative β-domain cleavage site, a serine protease active-site motif in the putative α domain, and the C-terminal amino acid motif YSF, which is necessary for correct localization of autotransporters to the outer membrane (19). Secondary structure predictions also suggested that the putative β domain consists of 14 antiparallel, amphipathic β sheets, a common structural feature of autotransporters.
Many of its structural features suggested that SigA is autonomously secreted from the cell via an autosecretory pathway. This hypothesis was supported by our finding that SigA is secreted from E. coli carrying only the cloned sigA gene. This finding suggested that no Shigella-specific accessory genes are required for the secretion of SigA. Furthermore, the secreted form of SigA is a 103-kDa protein, indicating that the 139-kDa form was proteolytically processed. The molecular mass of the secreted form is consistent with the predicted proteolytic cleavage of the N-terminal signal sequence and the C-terminal β domain at the predicted residues 54-55 and 1007-1008, respectively. Further support for an autosecretory pathway for the secretion of SigA was obtained by deleting the putative β domain. The inability of bacteria to secrete a C-terminally truncated SigA protein showed that the putative β domain was essential for extracellular export.
The presence of a conserved serine protease motif suggested that, like other members of the SPATE subfamily, SigA is a serine protease. This hypothesis was confirmed by the demonstration that SigA had proteolytic activity for a casein-based substrate. By assaying isogenic sigA strains, we also demonstrated that SigA is the only secreted protease of S. flexneri 2a that has activity toward casein. Although SigA degrades casein in vitro, its natural substrate is not known. We predict that the casein hydrolytic activity of SigA from the cell is dependent on the conserved serine protease active-site motif in SigA.
The close linkage of sigA to the genes encoding the ShET1 enterotoxin and the putative virulence protein Pic suggested that SigA might also be involved in virulence. Since the expression of many bacterial virulence proteins, including the autotransporters SepA (4), Tsh (30), Pic (17), and EspP (7), is thermoregulated, we tested whether the expression of SigA was regulated in the same way. We found that the expression of SigA resembled that of many virulence proteins since high levels of SigA were detectable in the culture supernatant of Shigella grown at 37 but not 21°C.
Autotransporters have been implicated in a range of bacterial processes associated with virulence. Given that Pet, the most closely related homolog of SigA, is an enterotoxic and cytopathic protease from enteroaggregative E. coli (14), we examined SigA for similar properties. Our results indicate that SigA induces cytopathic effects in HEp-2 cells but not to the same magnitude as Pet.
To test SigA for enterotoxic properties, we examined the ability of S. flexneri 2a strains that were isogenic for sigA to induce fluid accumulation in a rabbit ileal loop model of infection. A 30% reduction in fluid accumulation was observed for the sigA mutant, suggesting that SigA is a putative enterotoxin. However, since the sigA mutant was still capable of inducing substantial fluid accumulation, SigA appears to be only one of a number of enterotoxins produced by S. flexneri 2a. This is consistent with the presence of the genes for the ShET1 enterotoxin in our test strain. In addition, it is likely that our test strain also produces ShET2, an enterotoxin that is encoded by a DNA sequence which is widely distributed in Shigella strains.
It appears likely that the cytopathic and enterotoxic activity of SigA are related to its proteolytic activity, although we have not established this as part of this study. Examples where such a relationship has been established include the Pet enterotoxin from enteroaggregative E. coli (26) and the enterotoxin of Bacteroides fragilis (11). Although their specific mechanisms of action are unknown, both proteases exert their toxic effects by affecting the eukaryotic cell cytoskeleton. SigA and its closest homolog, Pet, appear to share a number of similar functions, including proteolytic activity, cytopathic effects on cells, and possible enterotoxic activity. However, only the expression of sigA is temperature regulated.
In conclusion, we have characterized the novel autotransporter protein SigA, which is encoded by the she PAI of S. flexneri 2a, and established a possible role for it in virulence. sigA is the second autotransporter gene to be found on the she PAI and one of three such genes harbored by S. flexneri 2a strains which appear to contribute to virulence. Further investigation of the she PAI will reveal whether it carries additional virulence determinants and thus contribute to our understanding of the evolution of virulence in Shigella.
ACKNOWLEDGMENTS
This work was supported by project grants from the National Health and Medical Research Council, Canberra, Australia, and by Public Health Service grant AI 43615 to J.P.N.
The technical assistance of Vicki Vallance, Ian McPherson, Vicki Bennet-Wood, and Magdy Sourial is gratefully acknowledged.
REFERENCES
- 1.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 2.Ausubel F M, Brent R, Kingston R E, Moore D M, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: Greene Publishing Associates and Wiley-Interscience; 1991. [Google Scholar]
- 3.Bachovchin W W, Plaut A G, Flentke G R, Lynch M, Kettner C A. Inhibition of IgA1 proteinases from Neisseria gonorrhoeae and Haemophilus influenzae by peptide prolyl boronic acids. J Biol Chem. 1990;265:3738–3743. [PubMed] [Google Scholar]
- 4.Benjelloun-Touimi Z, Sansonetti P J, Parsot C. SepA, the major extracellular protein of Shigella flexneri: autonomous secretion and involvement in tissue invasion. Mol Microbiol. 1995;17:123–135. doi: 10.1111/j.1365-2958.1995.mmi_17010123.x. [DOI] [PubMed] [Google Scholar]
- 5.Benjelloun-Touimi Z, Tahar M S, Montecucco C, Sansonetti P J, Parsot C. SepA, the 110 kDa protein secreted by Shigella flexneri: two-domain structure and proteolytic activity. Microbiology. 1998;144:1815–1822. doi: 10.1099/00221287-144-7-1815. [DOI] [PubMed] [Google Scholar]
- 6.Brenner S. The molecular evolution of genes and proteins: a tale of two serines. Nature. 1988;334:528–530. doi: 10.1038/334528a0. [DOI] [PubMed] [Google Scholar]
- 7.Brunder W, Schmidt H, Karch H. EspP, a novel extracellular serine protease of enterohaemorrhagic Escherichia coli O157:H7, cleaves human coagulation factor V. Mol Microbiol. 1997;24:767–778. doi: 10.1046/j.1365-2958.1997.3871751.x. [DOI] [PubMed] [Google Scholar]
- 8.Chapman A J, Adler B, Faine S. Genus-specific antigens in Leptospira revealed by immunoblotting. Zentbl Bakteriol Mikrobiol Hyg Ser A. 1987;264:279–293. doi: 10.1016/s0176-6724(87)80046-9. [DOI] [PubMed] [Google Scholar]
- 9.Claros M G, Vonheijne G. Toppred II—an improved software for membrane protein structure predictions. Comput Appl Biosci. 1994;10:685–686. doi: 10.1093/bioinformatics/10.6.685. [DOI] [PubMed] [Google Scholar]
- 10.Djafari S, Ebel F, Deibel C, Kramer S, Hudel M, Chakraborty T. Characterization of an exported protease from Shiga toxin-producing Escherichia coli. Mol Microbiol. 1997;25:771–784. doi: 10.1046/j.1365-2958.1997.5141874.x. [DOI] [PubMed] [Google Scholar]
- 11.Donelli G, Fabbri A, Fiorentini C. Bacteroides fragilis enterotoxin induces cytoskeletal changes and surface blebbing in HT-29 cells. Infect Immun. 1996;64:113–119. doi: 10.1128/iai.64.1.113-119.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DuPont H L, Levine M M, Hornick R B, Formal S B. Inoculum size in shigellosis and implications for expected mode of transmission. J Infect Dis. 1989;159:1126–1128. doi: 10.1093/infdis/159.6.1126. [DOI] [PubMed] [Google Scholar]
- 13.Egile C, d'Hauteville H, Parsot C, Sansonetti P J. SopA, the outer membrane protease responsible for polar localization of IcsA in Shigella flexneri. Mol Microbiol. 1997;23:1063–1073. doi: 10.1046/j.1365-2958.1997.2871652.x. [DOI] [PubMed] [Google Scholar]
- 14.Eslava C, Navarro-Garcia F, Czeczulin J R, Henderson I R, Cravioto A, Nataro J P. Pet, an autotransporter enterotoxin from enteroaggregative Escherichia coli. Infect Immun. 1998;66:3155–3163. doi: 10.1128/iai.66.7.3155-3163.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fasano A, Noriega F R, Maneval D R, Jr, Chanasongcram S, Russell R, Guandalini S, Levine M M. Shigella enterotoxin 1: an enterotoxin of Shigella flexneri 2a active in rabbit small intestine in vivo and in vitro. J Clin Investig. 1995;95:2853–2861. doi: 10.1172/JCI117991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983;166:557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 17.Henderson I R, Czeczulin J, Eslava C, Noriega F, Nataro J P. Characterization of Pic, a secreted protease of Shigella flexneri and enteroaggregative Escherichia coli. Infect Immun. 1999;67:5587–5596. doi: 10.1128/iai.67.11.5587-5596.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henderson I R, Navarro-Garcia F, Nataro J P. The great escape: structure and function of the autotransporter proteins. Trends Microbiol. 1998;6:370–378. doi: 10.1016/s0966-842x(98)01318-3. [DOI] [PubMed] [Google Scholar]
- 19.Hendrixson D R, de la Morena M L, Stathopoulos C, St. Geme J W., III Structural determinants of processing and secretion of the Haemophilus influenzae hap protein. Mol Microbiol. 1997;26:505–518. doi: 10.1046/j.1365-2958.1997.5921965.x. [DOI] [PubMed] [Google Scholar]
- 20.Homchampa P, Strugnell R A, Adler B. Cross protective immunity conferred by a marker-free aroA mutant of Pasteurella multocida. Vaccine. 1997;15:203–208. doi: 10.1016/s0264-410x(96)00139-9. [DOI] [PubMed] [Google Scholar]
- 21.Laemmli U K, Molbert E, Showe M, Kellenberger E. Form-determining function of the genes required for the assembly of the head of bacteriophage T4. J Mol Biol. 1970;49:99–113. doi: 10.1016/0022-2836(70)90379-7. [DOI] [PubMed] [Google Scholar]
- 22.Le Gouill C, Parent J L, Rola-Pleszczynski M, Stankova J. Analysis of recombinant plasmids by a modified alkaline lysis method. Anal Biochem. 1994;219:164. doi: 10.1006/abio.1994.1250. [DOI] [PubMed] [Google Scholar]
- 23.Moss J E, Cardozo T J, Zychlinsky A, Groisman E A. The selC-associated SHI-2 pathogenicity island of Shigella flexneri. Mol Microbiol. 1999;33:74–83. doi: 10.1046/j.1365-2958.1999.01449.x. [DOI] [PubMed] [Google Scholar]
- 24.Nakata N, Sasakawa C, Okada N, Tobe T, Fukuda I, Suzuki T, Komatsu K, Yoshikawa M. Identification and characterization of virK, a virulence-associated large plasmid gene essential for intercellular spreading of Shigella flexneri. Mol Microbiol. 1992;6:2387–2395. doi: 10.1111/j.1365-2958.1992.tb01413.x. [DOI] [PubMed] [Google Scholar]
- 25.Nataro J P, Seriwatana J, Fasano A, Maneval D R, Guers L D, Noriega F, Dubovsky F, Levine M M, Morris J G. Identification and cloning of a novel plasmid-encoded enterotoxin of enteroinvasive Escherichia coli and Shigella strains. Infect Immun. 1995;63:4721–4728. doi: 10.1128/iai.63.12.4721-4728.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Navarro-Garcia F, Sears C, Eslava C, Cravioto A, Nataro J P. Cytoskeletal effects induced by Pet, the serine protease enterotoxin of enteroaggregative Escherichia coli. Infect Immun. 1999;67:2184–2192. doi: 10.1128/iai.67.5.2184-2192.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nielsen H, Engelbrecht J, Brunak S, von Heijne G. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 1997;10:1–6. doi: 10.1093/protein/10.1.1. [DOI] [PubMed] [Google Scholar]
- 28.Pohlner J, Halter R, Beyreuther K, Meyer T F. Gene structure and extracellular secretion of Neisseria gonorrhoeae IgA protease. Nature. 1987;325:458–462. doi: 10.1038/325458a0. [DOI] [PubMed] [Google Scholar]
- 29.Prentki P, Krisch H M. In vitro insertional mutagenesis with a selectable DNA fragment. Gene. 1984;29:303–313. doi: 10.1016/0378-1119(84)90059-3. [DOI] [PubMed] [Google Scholar]
- 30.Provence D L, Curtiss R., III Isolation and characterization of a gene involved in hemagglutination by an avian pathogenic Escherichia coli strain. Infect Immun. 1994;62:1369–1380. doi: 10.1128/iai.62.4.1369-1380.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rajakumar K, Sasakawa C, Adler B. A spontaneous 99-kb chromosomal deletion results in multi-antibiotic susceptibility and an attenuation of contact haemolysis in Shigella flexneri 2a. J Med Microbiol. 1996;45:64–75. doi: 10.1099/00222615-45-1-64. [DOI] [PubMed] [Google Scholar]
- 32.Rajakumar K, Sasakawa C, Adler B. Use of a novel approach, termed island probing, identifies the Shigella flexneri she pathogenicity island which encodes a homolog of the immunoglobulin A protease-like family of proteins. Infect Immun. 1997;65:4606–4614. doi: 10.1128/iai.65.11.4606-4614.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sakai T, Sasakawa C, Yoshikawa M. Expression of four virulence antigens of Shigella flexneri is positively regulated at the transcriptional level by the 30 kiloDalton virF protein. Mol Microbiol. 1988;2:589–597. doi: 10.1111/j.1365-2958.1988.tb00067.x. [DOI] [PubMed] [Google Scholar]
- 34.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 35.Sansonetti P J. Molecular mechanisms of cell and tissue invasion by Shigella flexneri. Infectious Agents Dis. 1993;2:201–206. . (Review.) [PubMed] [Google Scholar]
- 36.Sansonetti P J, Arondel J. Construction and evaluation of a double mutant of Shigella flexneri as a candidate for oral vaccination against shigellosis. Vaccine. 1989;7:443–450. doi: 10.1016/0264-410x(89)90160-6. [DOI] [PubMed] [Google Scholar]
- 37.Sasakawa C. Early stages of Shigella interaction with host cells. J Infect Chemother. 1997;3:63–72. [Google Scholar]
- 38.Sasakawa C, Buysse J M, Watanabe H. The large virulence plasmid of Shigella. Curr Top Microbiol Immunol. 1992;180:21–44. doi: 10.1007/978-3-642-77238-2_2. . (Review.) [DOI] [PubMed] [Google Scholar]
- 39.Sasakawa C, Kamata K, Sakai T, Murayama S Y, Makino S, Yoshikawa M. Molecular alteration of the 140-megadalton plasmid associated with loss of virulence and Congo red binding activity in Shigella flexneri. Infect Immun. 1986;51:470–475. doi: 10.1128/iai.51.2.470-475.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shere K D, Sallustio S, Manessis A, D'Aversa T G, Goldberg M B. Disruption of IcsP, the major Shigella protease that cleaves IcsA, accelerates actin-based motility. Mol Microbiol. 1997;25:451–462. doi: 10.1046/j.1365-2958.1997.4681827.x. [DOI] [PubMed] [Google Scholar]
- 41.Smith M, Jessee J, Landers T, Jordan J. High efficiency bacterial electroporation: 1 × 1010E. coli transformants/μg. Focus. 1990;12:38–40. [Google Scholar]
- 42.Stein M, Kenny B, Stein M A, Finlay B B. Characterization of EspC, a 110-kilodalton protein secreted by enteropathogenic Escherichia coli which is homologous to members of the immunoglobulin A protease-like family of secreted proteins. J Bacteriol. 1996;178:6546–6554. doi: 10.1128/jb.178.22.6546-6554.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van den Bosch L, Manning P A, Morona R. Regulation of O-antigen chain length is required for Shigella flexneri virulence. Mol Microbiol. 1997;23:765–775. doi: 10.1046/j.1365-2958.1997.2541625.x. [DOI] [PubMed] [Google Scholar]
- 44.Vieira J, Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982;19:259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- 45.Vokes S A, Reeves S A, Torres A G, Payne S M. The aerobactin iron transport system genes in Shigella flexneri are present within a pathogenicity island. Mol Microbiol. 1999;33:63–73. doi: 10.1046/j.1365-2958.1999.01448.x. [DOI] [PubMed] [Google Scholar]
- 46.Wang R F, Kushner S R. Construction of versatile low-copy-number vectors for cloning, sequencing and gene expression in Escherichia coli. Gene. 1991;100:195–199. [PubMed] [Google Scholar]