Keywords: cardioprotection, ischemia-reperfusion, myocardial infarction, Ossabaw minipig, remote ischemic conditioning
Abstract
Ischemic preconditioning (IPC; brief cycles of coronary occlusion/reperfusion) is operative in all species tested so far and reduces infarct size through the release of trigger molecules and activation of signal transducer and activator of transcription (STAT)3 in pigs. We have recently demonstrated that IPC failed to protect Ossabaw minipigs, which had a genetic predisposition to, but not yet established a metabolic syndrome, from infarction and did not activate STAT3. We now subjected Ossabaw minipigs to remote ischemic conditioning (RIC; 4 × 5 min/5 min bilateral hindlimb ischemia-reperfusion) and analyzed the release of cardioprotective triggers into the circulation with the aim to distinguish whether IPC failed to stimulate trigger release or to activate intracellular signaling cascades upstream of STAT3. RIC or a placebo protocol, respectively, was induced in anesthetized pigs before 60 min/180 min coronary occlusion/reperfusion. Plasma, prepared from Ossabaw minipigs after RIC or placebo, was infused into isolated rat hearts subjected to 30 min/120 min global ischemia-reperfusion. In the Ossabaw minipigs, RIC did not reduce infarct size (49.5 ± 12.1 vs. 56.0 ± 11.8% of area at risk with placebo), and STAT3 was not activated. In isolated rat hearts, infusion of RIC plasma reduced infarct size (19.7 ± 6.7 vs. 33.2 ± 5.5% of ventricular mass with placebo) and activated STAT3. Pretreatment of rat hearts with the STAT3 inhibitor stattic abrogated such infarct size reduction and STAT3 activation. In conclusion, Ossabaw minipigs release cardioprotective triggers in response to RIC into the circulation, and lack of cardioprotection is attributed to myocardial nonresponsiveness.
NEW & NOTEWORTHY Ischemic conditioning reduces myocardial infarct size in all species tested so far. In the present study, we used Ossabaw minipigs that had a genetic predisposition to, but not yet established a metabolic syndrome. In these pigs, remote ischemic conditioning (RIC) induced the release of cardioprotective triggers but did not reduce infarct size. Transfer of their plasma, however, reduced infarct size in isolated recipient rat hearts, along with signal transducer and activator of transcription (STAT)3 activation.
INTRODUCTION
There is still a medical need for adjunct cardioprotection beyond that by timely reperfusion, since one-year mortality after reperfused acute myocardial infarction is still at 14% (1) and the rate of postinfarct heart failure development remains high (2). However, the translation of mechanical and pharmacological cardioprotective interventions from preclinical animal studies to the clinical benefit of patients with myocardial infarction has been difficult. With one exception (3), phase III studies on cardioprotection in patients with reperfused acute myocardial infarction (4, 5) have failed to reduce infarct size and improve clinical outcomes. However, post hoc and non-prespecified analyses of two studies reported better clinical outcomes of remote ischemic conditioning or ischemic postconditioning, respectively (6, 7). Preclinical studies often use healthy, young animals with a homogenous genetic background (8). Such studies, however, neglect both the advanced age and the genetic heterogeneity of patients and their typical comorbidities and comedications, which may interfere with cardioprotective interventions (9–11). In the Ossabaw minipig, a particular strain with a genetic predisposition for the development of a diet-inducible metabolic syndrome (12), we have recently demonstrated that ischemic preconditioning (IPC), repetitive nonlethal cycles of coronary occlusion-reperfusion before a more severe episode of myocardial ischemia, failed to reduce infarct size (13, 14). Of note, IPC is unequivocally the strongest and most robust cardioprotective intervention and has reduced infarct size in all species tested so far (5). IPC has been efficacious in all published studies using pigs of different strains, sex, and age, using different models with different anesthesia and different IPC protocols (15–28). Thus, the lack of protection in the Ossabaw minipig was indeed surprising (13, 14).
The absence of cardioprotection in the Ossabaw minipig was accompanied by a lack of increased phosphorylation in signal transducer and activator of transcription (STAT)3 in the myocardium, which has been previously demonstrated to be causally involved in IPC’s cardioprotection in rodents (29–31) and pigs (28, 32). Whether the lack of cardioprotection resulted from a failure of IPC to act as a stimulus for the release of triggering molecules or from an intracellular blockade of signaling cascades upstream to STAT3 activation remained unclear in our previous study. IPC in pigs stimulates the release of local triggers such as adenosine and bradykinin (19, 33) that subsequently activate intracellular signaling cascades (34). The analysis of locally released cardioprotective triggers during IPC, however, requires complex invasive techniques to collect trigger-containing medium, i.e., coronary venous cannulation to withdraw blood samples or microdialysis to drain interstitial myocardial fluid samples (19, 33). The release of cardioprotective triggers is much easier to study during remote ischemic conditioning (RIC; brief cycles of ischemia and reperfusion in organs/tissues remote from the heart). With RIC, the cardioprotective triggers are released into the circulating blood that then initiates cardioprotection through activation of intracellular signaling cascades within the myocardium (35, 36). In patients with manifest diabetes and neuropathy, however, this release of circulating cardioprotective triggers was impaired in response to RIC (37). Whereas the nature of these circulating cardioprotective factors is still enigmatic, their action can be analyzed by the transfer of circulating blood cells, blood plasma, or its derivatives to isolated perfused recipient hearts where they mediate cardioprotection (30, 37–47). Of note, in isolated perfused recipient rodent hearts, RIC’s cardioprotective factors activate intracellular signaling cascades, i.e., in mouse and rat hearts STAT3 (30, 42), as also seen in pig hearts in response to various ischemic conditioning strategies (28, 30, 32, 43, 44, 48).
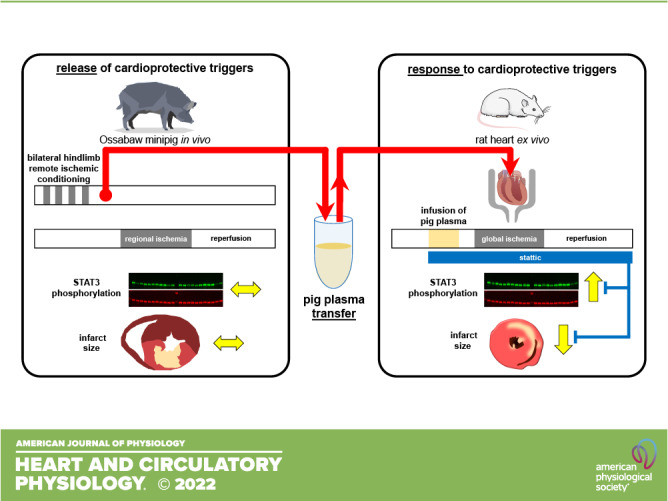
Therefore, we have now subjected Ossabaw minipigs to RIC with subsequent transfer experiments in isolated rat hearts with the aim to distinguish between the release of cardioprotective factors and/or their efficacy to serve as cardioprotection-mediating triggers in the heart. We found that RIC as IPC before (13) did not reduce infarct size in pigs and failed to activate STAT3. In contrast, the transfer of pig plasma to isolated rat hearts reduced infarct size and activated STAT3, indicating that myocardial nonresponsiveness was the culprit of failure of cardioprotection in Ossabaw minipigs but not impaired cardioprotective trigger release.
METHODS
The authors declare that all supporting data of the present exploratory study are available in the article. The experimental protocols were approved by the Bioethical Committee of the District of Düsseldorf (G1610/17; G1777/20) and conform to the guidelines from Directive 2010/63/EU of the European Parliament on the protection of animals used for scientific purposes. We followed the ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines 2.0 (49, 50). Experiments were performed between September 2017 and September 2022. Unless otherwise specified, materials were obtained from Sigma-Aldrich (Deisenhofen, Germany).
Experimental Preparation and Protocols in Pigs
Anesthesia and ventilation.
Ossabaw minipigs (females, n = 12; age, 16 ± 2 mo; and 56.7 ± 5.0 kg body wt; and castrated males, n = 12; aged 14 ± 1 mo; and 57.0 ± 7.5 kg body wt) were purchased from CorVus Biomedical (Crawfordsville, IN). Male piglet castration was performed at around 4 wk of age under local anesthesia with lidocaine and/or bupivacaine. Here, we only used Ossabaw minipigs with homozygous single nucleotide polymorphisms encoding for isoleucine rather than valine in the γ-subunit of adenosine monophosphate-activated protein kinase (12, 51) in which we have seen a trend toward infarct size reduction with IPC (13), with the idea to maximize a potential cardioprotective effect with RIC in this subgroup of pigs. Pigs were housed in tiled rooms (∼2 m2/pig) with straw bedding at 12-h:12-h light/dark cycles, fed with standard chow (500 g twice/day, No. V4133. Ssniff, Soest, Germany) and had ad libitum access to water. Pigs were sedated with flunitrazepam (0.8 mg/kg im). Anesthesia was induced with etomidate (0.3 mg/kg iv, Piramal Critical Care, Hypnomidat; Voorschoten, The Netherlands) and sufentanil (1 µg/kg iv, Sufentanil-hameln, Hameln Pharma, Hameln, Germany). Anesthesia was maintained with isoflurane (2%, TEVA, Eastbourne, UK) during artificial ventilation with room air. Muscle relaxation during electrosurgery was induced with a single bolus of rocuronium (1.2 mg/kg iv, B. Braun, Melsungen, Germany). This anesthetic regimen is identical to that used in our institution for patients undergoing surgical coronary revascularization (52). The pigs were placed on a heated table and covered with blankets to keep esophageal temperature between 37.0°C and 39.0°C. ECG lead II was continuously recorded using a single-channel, calibrated amplifier. A midline cervical incision was performed. After tracheotomy for mechanical ventilation, the left jugular vein was cannulated for volume replacement and intravenous drug administration, and the right common carotid artery was cannulated to measure arterial pressure.
RIC protocol.
Twelve pigs (females/castrated males, n = 6 each) were subjected to RIC. We explicitly performed RIC on both hindlimbs to maximize the RIC stimulus. A tourniquet was placed around each hindlimb, respectively, and both were tightened for 5 min. Pale skin was taken to indicate leg ischemia. The tourniquets were quickly released after 5 min ischemia, and the limbs were reperfused for 5 min. Skin blush indicated reperfusion. The ischemia-reperfusion cycles in the hindlimbs were performed four times. Arterial blood (200 mL) was sampled 60 min after completion of the last ischemia-reperfusion cycle in the hindlimbs. The heart was exposed by a left lateral thoracotomy and instrumented with a micromanometer (DPT-6000, Codan-PVB, Forsting, Germany) in the left ventricle to measure left ventricular pressure (LVP) and a Teflon catheter in the left atrium for the injection of colored microspheres. The distal aortic arch was cannulated to withdraw a reference sample for regional blood flow measurement using colored microspheres (53). The left anterior descending coronary artery (LAD) was dissected and prepared distal to its second diagonal branch for later coronary occlusion. When baseline heart rate was below 95 beats/min, left atrial pacing at 100 beats/min was performed with bipolar rectangular pulses of 2-ms duration and 2- to 4-V amplitude using an analog stimulus isolator (model 2200; A-M Systems/ADInstruments, Dunedin, New Zealand). At baseline, systemic hemodynamics and regional myocardial blood flow were measured. Myocardial drill biopsies (2–10 mg) were taken from the designated area at risk, immediately snap frozen in liquid nitrogen, and stored at −80°C for later Western blot analysis. Arterial blood (200 mL) was sampled 60 min after completion of the last ischemia-reperfusion cycle in the hindlimbs, and immediately before coronary occlusion since any protective substances would need to be present then to exert their protective function. Blood was sampled into vials containing lithium-heparin (B. Braun, Melsungen, Germany) and immediately centrifuged at 4°C with 800 g for 10 min. The separated plasma was again centrifuged at 4°C with 4,500 g for 10 min and then stored at −80°C for later use. Unfractionated heparin (intravenous, 500 I.E. LEO Pharma, Neu-Isenburg, Germany) was administered and the LAD occluded distal to its second diagonal branch using a microvascular clamp (TKL-1, Biover, Hergiswill, Switzerland). The heparin administration was repeated at 30 and 55 min coronary occlusion. After 5 and 55 min coronary occlusion, systemic hemodynamics and regional myocardial blood flow were measured again. Biopsies were again taken at 55 min coronary occlusion. Reperfusion was induced after 60 min coronary occlusion by quick removal of the vascular clamp and visually confirmed by the reappearance of red color on the surface of the reperfused myocardium. Systemic hemodynamics were again measured at 10, 60, 120, and 180 min reperfusion and regional myocardial blood flow at 10 and 180 min reperfusion. Additional myocardial biopsies were taken at 10 min reperfusion. Ventricular fibrillation during ischemia or reperfusion, as identified from the continuous lead II ECG recording, was immediately terminated by intrathoracic defibrillation (up to 50 W; 6/4 ms biphasic pulse; Zoll R Series Monitor & Defibrillator, Zoll Medical Cooperation, Chelmsford, MA). We did not use any antiarrhythmic or inotropic agents since they might interfere with the infarction process and/or cardioprotection. At the end of the experiment, pigs were euthanized by intracardiac injection of 20 mL potassium chloride (1 mol/L).
Placebo protocol.
Twelve pigs (females/castrated males, n = 6 each) were subjected to placebo. The placebo protocol was identical to that of RIC, except that the conditioning maneuver on the hindlimbs was omitted.
Regional myocardial blood flow.
Colored microspheres were recovered from transmural myocardial samples taken from the central area at risk by digestion with 4 mol/L KOH and subsequent filtration (8-µm pore size, Pieper Filter, Bad Zwischenahn, Germany). Fluorescent dye was resolved from the microspheres and quantified in a spectrophotometer (F-7100, Hitachi High-Tech, Krefeld, Germany). Blood flow was calculated as blood flow per tissue mass (53).
Area at risk and infarct size.
The LAD was reoccluded at the same location as for the index ischemia at the end of 180 min of reperfusion, and 5 mL blue dye (Patentblau V, Guerbet, Sulzbach, Germany) was quickly injected into the left atrium to delineate the area at risk as remaining unstained. The heart was quickly removed from the chest, rinsed with cold saline, and cut into five slices perpendicular to the ventricular long axis. After we documented the slices using a digital camera, the slice shape and the demarcated area at risk were transferred manually to a transparent film. Thereafter, infarcted tissue was demarcated by triphenyl-tetrazolium chloride (TTC) staining (1% dissolved in 90 mmol/L sodium phosphate buffer containing 8% dextran, Roth, Karlsruhe, Germany). The TTC-stained slices were again photographed and together with the tissue areas that remained unstained by TTC transferred to the same transparent film, which was used to document the area at risk. The transparent films were scanned and analyzed using digital planimetry. Total area of the left ventricle, the area at risk, and the area of TTC-negative (infarcted) tissue were calculated and averaged for both sides of each slice using the slice weight for normalization. The tissue masses for the area at risk and the infarcted area were calculated. In addition, the area at risk was calculated as a fraction of the left ventricle, and the infarct size was calculated as a fraction of the area at risk (54).
Transfer Experiments in Isolated Rat Hearts
Male Lewis rats (200–350 g, 2.0–3.5 mo, Central Animal Laboratory, University of Duisburg-Essen, Medical School, Essen, Germany) were euthanized with pentobarbital sodium (800 mg/kg ip, narcoderm, CP-Pharma, Burgdorf, Germany). Hearts were excised and arrested in cold saline (8°C, supplemented with unfractionated heparin 300 IU/mL). The aorta was immediately cannulated, and hearts were mounted on a Langendorff apparatus and perfused with modified Krebs–Henseleit buffer, consisting of (in mmol/L) 118.0 NaCl, 4.7 KCl, 16.0 MgSO4, 1.2 KH2PO4, 5.6 glucose, 24.9 NaHCO3, 2.0 sodium pyruvate, and 2.0 CaCl2, gassed with 95% O2-5% CO2 in a prewarmed reservoir (pH 7.40) at a constant pressure of 65–70 mmHg. Coronary flow (CF) was measured with an inline ultrasonic flow probe (TS410, Transsonic Systems, Ithaca, NY) above the aortic cannula. A fluid-filled latex balloon was inserted into the left ventricular cavity and connected to a pressure transducer to measure LVP. End-diastolic LVP was set to 5–15 mmHg at baseline by graded balloon inflation during the initial 5 min perfusion. Left ventricular developed pressure (LVDP) was calculated as the difference between peak and end-diastolic LVP. CF and end-diastolic and peak LVP were continuously recorded. Hearts were allowed to stabilize for 10–20 min. Preparations with CF <10.0 mL/min or >18.0 min or LVDP < 60 mmHg after the stabilization period were excluded. Nine isolated heart preparations in total did not meet baseline criteria and were thus excluded from further analysis. Heart rate was kept constant at 360 beats/min by right atrial pacing. Hearts were immersed in prewarmed oxygenated modified Krebs–Henseleit buffer. The temperatures of the perfusion and immersion buffers were monitored with probes in the aortic cannula and in the immersion buffer chamber throughout the experiment and kept between 37.5°C and 37.8°C by heat exchangers.
Pig plasma samples (placebo, RIC) were filtered (5 µm pore size, Machery-Nagel, Düren, Germany) and added with a syringe pump to the perfusate (dilution 1:10 volume ratio) before passing the heat exchanger for 8 min, followed by 2-min washout to avoid adherence of proteins from stagnant plasma in the Langendorff apparatus. Global ischemia-reperfusion (GI/R) was then induced by a 30-min full stop of perfusion and subsequent 120 min reperfusion (placebo plasma + GI/R, n = 12; RIC plasma + GI/R, n = 12). Saline infusion served as control, respectively (saline + GI/R, n = 10). In an additional set of experiments, pig plasma samples were infused into isolated hearts that were perfused with modified Krebs–Henseleit buffer supplemented with the STAT3 blocker stattic (4 µmol/L; placebo plasma + GI/R + stattic, n = 11; RIC plasma + GI/R + stattic, n = 12) throughout the entire experiment. The volume of plasma sampled from one female Ossabaw minipig that had been subjected to placebo protocol was very low; thus, plasma from this particular pig was only infused into hearts without stattic blockade, and plasma infusion under blockade with stattic was omitted. The stattic concentration did not impact on infarct size per se in preliminary experiments (saline + GI/R + stattic, n = 9). CF and LVDP were calculated as mean values during the last minute of each of the stabilization period (baseline), at the last minute of plasma/saline infusion, and at the last minute of the washout phase at 5 and 25 min ischemia and at 10, 30, and 60 min reperfusion, respectively. After completion of reperfusion, the apex of each heart was cut off (∼50–100 mg) and quickly frozen in liquid nitrogen for later protein analysis by Western blot analysis. The hearts were frozen in Cryomatrix (Thermo Fisher Scientific, Schwerte, Germany) at −20°C and cut into transverse 2-mm-thick slices. Infarcted tissue was demarcated by staining with 0.09 mol/L sodium phosphate buffer containing 1.5% triphenyl tetrazolium chloride (TTC) at 37°C for 5 min. Stained slices were photographed from both sides. The total slice area and the infarcted areas were quantified by computer-assisted planimetry (ImageJ 1.48v, National Institutes of Health, Bethesda, MD), and infarct size was calculated as percentage of the sum of left and right ventricular mass (percentage of ventricular mass).
STAT3 and Phosphorylation of STAT3
Snap-frozen biopsies from pig and rat hearts were homogenized in 100.0 mmol/L tris(hydroxymethyl)aminomethane with 2% sodium dodecyl sulfate (wt/vol; SERVA Electrophoresis, Heidelberg, Germany), heated to 70°C for 5 min and centrifuged at 16,000 g for 10 min. The protein lysate-containing supernatant was stored at −80°C in aliquots to prevent freeze and thaw cycles (55). In preliminary experiments, the combined linear range had been determined for STAT3 protein and its phosphorylated form for both pig and rat myocardium, according to the manufacturer’s recommendation (56), using series of 8, 12, 16, 20, 24, 28, and 32 µg protein lysate. The determined protein quantity used for Western blotting of STAT3 was 32 µg for pig and 16 µg for rat myocardium. Protein lysates from 22 of the 24 Ossabaw minipigs were loaded randomly in blocks per pig on gels. One Ossabaw minipig (castrated male, placebo) was excluded from analysis because the baseline biopsy was missing and another Ossabaw minipig (female, RIC) was excluded since no biopsy was available due to technical failure of the biopsy device. Myocardial lysates from rat hearts treated with pig plasma without stattic (19 of 24) and treated with pig plasma under blockade with stattic (22 of 23) were randomly loaded on gels. Myocardial lysates from four rat hearts with infusion of placebo plasma and from two rat hearts with infusion of RIC plasma were excluded because of visible protein degradation.
Protein lysate aliquots were electrophoretically separated on precast stain-free 7.5% sodium dodecyl sulfate polyacrylamide electrophoresis gels (Bio-Rad, Hercules, CA). Total protein was visualized by an ultraviolet light-induced fluorescence reaction of protein-tryptophan with tri-halo compounds within the stain-free gels and imaged using the Gel Doc EZ system (Bio-Rad). Proteins were transferred to 0.45-µm low-fluorescence polyvinylidene difluoride membranes (Merck, Chemicals, Darmstadt, Germany) using the Trans-Blot Turbo transfer system (Bio-Rad). Fluorescence signals of the protein-tryptophan tri-halo compounds on the membranes were imaged (Gel Doc EZ system), and the membranes were dried. After reactivation with 100% methanol, membranes were incubated with Revert (1:15; LI-COR Biosciences, Lincoln, NE) for total protein staining and imaged using the LI-COR Odyssey FC system (LI-COR Biosciences). Membranes were destained, cut horizontally, blocked (1:10; EveryBlot blocking buffer, Bio-Rad) for 5 min at room temperature, and rinsed with Tris-buffered saline. Membranes were then incubated with the primary antibody directed against STAT3 phosphorylated at tyrosine-705 (Cell Signaling, No. 9138, 1:250). Membranes were washed four times for 5 min with Tris-buffered saline containing polyoxyethylene-20-sorbitan monolaurate plus Tween 20 (TBST) before being incubated with antibodies directed against total STAT3 (Cell signaling, No. 9139, 1:500). Membranes were again washed four times for 5 min using Tris-buffered saline with TBST [before incubation with the secondary antibodies (LI-COR Biosciences): IRDye 800CW goat anti-mouse IgG1, 1:5,000, for phosphorylated STAT3; IRDye 680LT goat anti-mouse IgG2a, 1:20,000, for total STAT3]. The primary antibody was diluted in TBST containing 5% bovine serum albumin. Secondary antibodies were diluted in EveryBlot blocking buffer (1:10; Bio-Rad) supplemented with 0.02% sodium dodecyl sulfate. Signals were detected by fluorescence, and the signal intensities of phosphorylated STAT3 and total STAT3 were imaged using the LI-COR Odyssey FC system. Detected signals were analyzed with the LI-COR Biosciences Empiria studio software (v. 2.1.0.134). All signal intensities were normalized to that of the reference sample on the respective membrane. The sum of all fluorescent signal intensities (100–38 kDa per lane) was used as a reference to normalize the signal intensity of the STAT3 total protein signal. The signal intensity of phosphorylated STAT3 protein was normalized to that of STAT3 total protein.
Statistics
Investigators who quantitatively analyzed hemodynamics, regional myocardial blood flow, infarct size, and Western blots were blinded to protocols in pigs. Investigators who analyzed infarct size, time courses of CF and LVDP, and Western blots from isolated perfused rat hearts were blinded with respect to the infused pig plasma sample. Data were tested for normal distribution using the Shapiro–Wilk test. Data are presented as means ± SD; individual data on infarct size are also presented as scatterplots. Data of female and castrated male Ossabaw minipigs were analyzed together, but are presented by different symbols in the figures, according to Lindsey et al. (57). Data on area at risk and infarct size from Ossabaw minipigs and Western blots from transfer experiments in rat hearts were analyzed by one-way analysis of variance (ANOVA). Infarct size in isolated perfused rat hearts with RIC-plasma or placebo-plasma infusion without or with stattic blockade were analyzed by two-way ANOVA. Hemodynamics in Ossabaw minipigs and time courses of CF and LVDP in isolated perfused rat hearts, regional myocardial blood flow in pigs, and Western blot data from pigs were analyzed by two-way ANOVA for repeated measures (time course vs. protocol). When ANOVA indicated a significant main effect or interaction, Fisher’s least significant difference post hoc tests were used to compare individual mean values. Differences were considered significant at the level of P < 0.05 (SigmaStat 3.5, Erkrath, Germany).
RESULTS
Systemic Hemodynamics, Regional Myocardial Blood Flow, Area at Risk, and Infarct Size in Ossabaw Minipigs
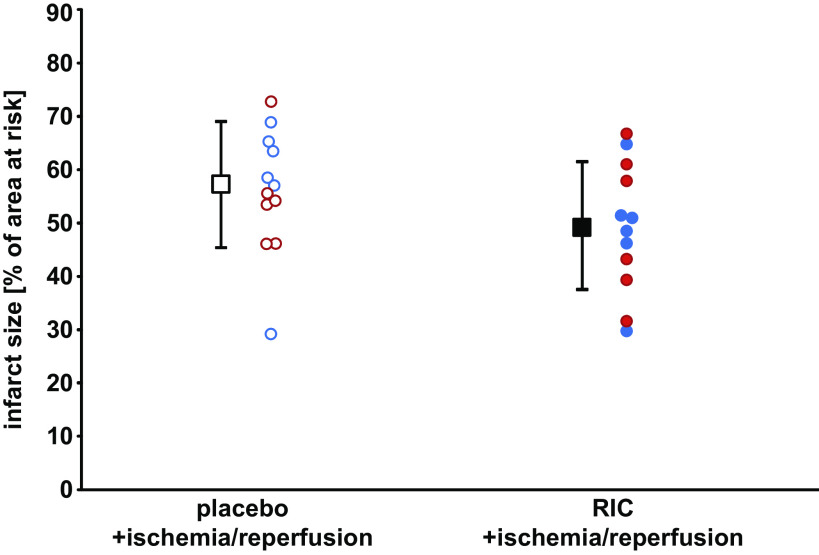
Heart rate and LVP at baseline were not different between both groups of Ossabaw minipigs. During ischemia, heart rate remained constant and LVP decreased, without differences between the placebo and RIC groups. During reperfusion, LVP did not fully recover in both groups (Table 1). Regional myocardial blood flow at baseline was not different between groups but decreased markedly at 5 and 55 min ischemia and recovered with a wide range of intraindividual variation over 180 min reperfusion. Area at risk was 20%–22% of left ventricular myocardium and not different between the placebo and RIC groups. With RIC, infarct size was 49.5 ± 12.1% of area at risk (females, 48.8 ± 11.3%; and castrated males, 50.3 ± 13.9%; Fig. 1) and not different from that with placebo, where infarct size was 56.0 ± 11.8% (females, 57.2 ± 14.3%; and castrated males, 54.9 ± 9.8%; Fig. 1).
Table 1.
AAR, RMBF in AAR, HR, and LVP in Ossabaw minipigs subjected to either a placebo protocol or RIC before I/R
| Placebo + I/R |
RIC + I/R |
|||
|---|---|---|---|---|
| P value (vs. baseline) | P value (vs. baseline) | |||
| AAR, %LV | 20.5 ± 5.1 | 22.4 ± 4.8 | ||
| RMBF, mL/min/g | ||||
| Baseline | 1.14 ± 0.23 | 1.04 ± 0.27 | ||
| I5 | 0.04 ± 0.02 | 1.2E-11 | 0.03 ± 0.02 | 6.6E-11 |
| I55 | 0.03 ± 0.02 | 1.0E-11 | 0.03 ± 0.01 | 2.4E-10 |
| R10 | 1.16 ± 0.64 | 1.18 ± 0.63 | ||
| R180 | 0.73 ± 0.43 | 5.1E-03 | 0.75 ± 0.31 | 3.5E-02 |
| HR, beats/min | ||||
| Baseline | 100 ± 0 | 99 ± 10 | ||
| I5 | 100 ± 0 | 100 ± 11 | ||
| I55 | 100 ± 4 | 100 ± 10 | ||
| R10 | 102 ± 12 | 101 ± 11 | ||
| R180 | 95 ± 12 | 104 ± 18 | ||
| LVP, mmHg | ||||
| Baseline | 97 ± 10 | 97 ± 17 | ||
| I5 | 87 ± 12 | 5.7E-05 | 90 ± 19 | 2.1E-02 |
| I55 | 85 ± 11 | 5.7E-06 | 89 ± 17 | 8.7E-03 |
| R10 | 83 ± 10 | 3.3E-07 | 83 ± 18 | 4.8E-06 |
| R180 | 77 ± 8 | 6.1E-10 | 83 ± 17 | 1.1E-05 |
Values are means ± SD. Baseline, before I/R; I5 and I55, 5 and 55 min ischemia, respectively; R10 and R180, 10 and 180 min reperfusion, respectively; I/R, ischemia-reperfusion. Area at risk (AAR) was analyzed by Student’s t test, whereas regional myocardial blood flow (RMBF), heart rate (HR), and left ventricular pressure (LVP) were analyzed by two-way analysis of variance for repeated measures [time course, placebo vs. remote ischemic conditioning (RIC)] and Fisher’s least significant difference post hoc tests. Exact P values given for P < 0.05 vs. baseline.
Figure 1.
Lack of cardioprotection in Ossabaw minipigs. Ossabaw minipigs were subjected to remote ischemic conditioning (RIC) or placebo before 60 min coronary occlusion and 180 min reperfusion. Data are presented as means ± SD. Red circles, female; blue circles, castrated male Ossabaw minipigs; open symbols, placebo; closed symbols, RIC protocol.
LVDP, CF, and Infarct Size in Isolated Perfused Rat Hearts
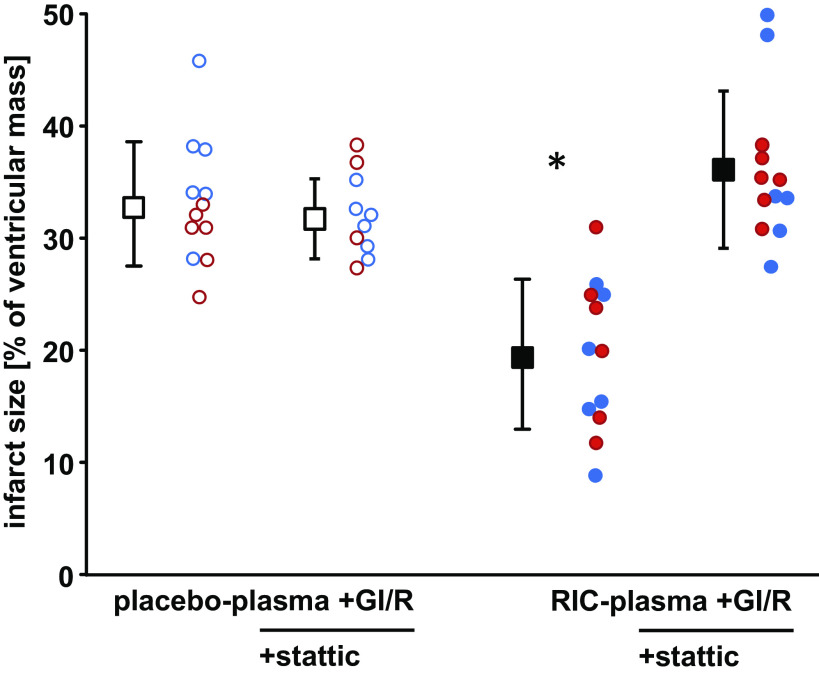
LVDP and CF were not different between groups with infusion of placebo plasma and RIC plasma, respectively, at baseline. Infusion of pig plasma slightly decreased LVDP and CF, with full recovery during washout. In all groups, LVDP and CF decreased to zero during global ischemia, and the recovery of LVDP and CF during reperfusion was not different between groups. Stattic impacted neither on LVDP and CF at baseline nor on their recovery during reperfusion (Table 2). With infusion of saline before GI/R, infarct size was 34.8 ± 4.0% of ventricular mass in isolated perfused rat hearts (Supplemental Fig. S4; https://doi.org/10.6084/m9.figshare.21277953.v1). In saline controls, stattic had no impact on infarct size per se (36.1 ± 4.0%, Supplemental Fig. S4). Infarct size with infusion of plasma from Ossabaw minipigs subjected to RIC was 19.7 ± 6.7% and less than that with infusion of plasma from pigs with placebo (33.2 ± 5.5%, Fig. 2). Pretreatment of isolated perfused hearts with stattic abolished the infarct size reduction by infusion of RIC (Fig. 3A) pig plasma but had no impact on infarct size per se (placebo plasma + GI/R + stattic, 31.8 ± 3.6%; and RIC plasma + GI/R + stattic, 36.1 ± 7.0%; Fig. 2).
Table 2.
CF and LVDP in isolated rat hearts perfused without or with the inhibitor of STAT3 stattic and infusion of saline, plasma from Ossabaw minipigs subjected to a placebo protocol, or to RIC
| Placebo Plasma + GI/R |
RIC Plasma + GI/R |
Placebo Plasma + GI/R + Stattic |
RIC Plasma + GI/R + Stattic |
Saline + GI/R |
Saline + GI/R + Stattic |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P value (vs. baseline) |
P value (vs. baseline) |
P value (vs. baseline) |
P value (vs. baseline) |
P value (vs. baseline) |
P value (vs. baseline) |
|||||||
| n | 12 | 12 | 11 | 12 | 10 | 9 | ||||||
| CF, mL/min | ||||||||||||
| Baseline | 15.2 ± 2.0 | 14.9 ± 2.0 | 13.9 ± 1.2 | 13.2 ± 1.5 | 15.0 ± 1.9 | 13.3 ± 2.3 | ||||||
| Infusion | 11.4 ± 2.2 | 4.3E-08 | 11.2 ± 3.0 | 2.7E-08 | 10.9 ± 2.5 | 2.1E-06 | 10.1 ± 1.6 | 8.9E-07 | 15.1 ± 1.7 | 13.4 ± 2.0 | ||
| Washout | 14.2 ± 1.8 | 14.2 ± 3.0 | 13.3 ± 1.7 | 12.4 ± 1.9 | 15.1 ± 1.8 | 13.5 ± 1.9 | ||||||
| I5 | 0.0 ± 0.0 | <1.0E-15 | 0.0 ± 0.0 | <1.0E-15 | 0.0 ± 0.0 | <1.0E-15 | 0.0 ± 0.0 | <1.0E-15 | 0.0 ± 0.0 | <1.0E-15 | 0.0 ± 0.0 | <1.0E-15 |
| I25 | 0.0 ± 0.0 | <1.0E-15 | 0.0 ± 0.0 | <1.0E-15 | 0.0 ± 0.0 | <1.0E-15 | 0.0 ± 0.0 | <1.0E-15 | 0.0 ± 0.0 | <1.0E-15 | 0.0 ± 0.0 | <1.0E-15 |
| R10 | 8.6 ± 2.1 | <1.0E-15 | 9.4 ± 3.1* | <1.0E-15 | 8.2 ± 2.4 | <1.0E-15 | 7.1 ± 1.8 | <1.0E-15 | 8.5 ± 1.8 | <1.0E-15 | 9.9 ± 2.0 | <1.0E-15 |
| R30 | 8.9 ± 1.6 | <1.0E-15 | 9.3 ± 2.2 | 1.0E-15 | 8.9 ± 2.6 | <1.0E-15 | 8.5 ± 2.0 | <1.0E-15 | 8.7 ± 1.9 | <1.0E-15 | 10.1 ± 1.5 | <1.0E-15 |
| R60 | 8.8 ± 1.4 | <1.0E-15 | 9.1 ± 2.3 | <1.0E-15 | 8.4 ± 2.0 | <1.0E-15 | 8.0 ± 1.7 | <1.0E-15 | 8.8 ± 1.0 | <1.0E-15 | 9.6 ± 1.5 | <1.0E-15 |
| LVDP, mmHg | ||||||||||||
| Baseline | 103 ± 12 | 104 ± 19 | 108 ± 12 | 103 ± 14 | 100 ± 10 | 109 ± 15 | ||||||
| Infusion | 84 ± 21 | 2.2E-03 | 78 ± 30 | 3.1E-05 | 82 ± 22 | 3.1E-05 | 87 ± 19 | 1.5-05 | 100 ± 9 | 108 ± 14 | ||
| Washout | 97 ± 15 | 94 ± 25 | 99 ± 18 | 101 ± 20 | 100 ± 9 | 108 ± 14 | ||||||
| I5 | 1 ± 0 | <1.0E-15 | 1 ± 1 | <1.0E-15 | 1 ± 0 | <1.0E-15 | 1 ± 0 | <1.0E-15 | 0 ± 0 | <1.0E-15 | 0 ± 0 | <1.0E-15 |
| I25 | 1 ± 1 | <1.0E-15 | 1 ± 2 | <1.0E-15 | 2 ± 4 | <1.0E-15 | 1 ± 1 | <1.0E-15 | 0 ± 0 | <1.0E-15 | 0 ± 0 | <1.0E-15 |
| R10 | 10 ± 8 | <1.0E-15 | 17 ± 16 | <1.0E-15 | 19 ± 13 | <1.0E-15 | 22 ± 17 | <1.0E-15 | 11 ± 9 | <1.0E-15 | 7 ± 5 | <1.0E-15 |
| R30 | 28 ± 14 | <1.0E-15 | 33 ± 20 | <1.0E-15 | 39 ± 21 | <1.0E-15 | 33 ± 19 | <1.0E-15 | 22 ± 29 | <1.0E-15 | 11 ± 9 | <1.0E-15 |
| R60 | 37 ± 13 | <1.0E-15 | 37 ± 18 | <1.0E-15 | 37 ± 17 | <1.0E-15 | 35 ± 15 | <1.0E-15 | 26 ± 25 | <1.0E-15 | 10 ± 10 | <1.0E-15 |
Values are means ± SD. Baseline, before plasma/saline infusion; infusion, 1st min during plasma/saline infusion; washout, last minute during washout after plasma/saline infusion; I5 and I25, 5 and 25 min ischemia, respectively; R10, R30, and R60, 10, 30, and 60 min reperfusion, respectively. GI/R, global ischemia-reperfusion; RIC, remote ischemic conditioning; STAT3, signal transducer and activator of transcription 3. Baseline, coronary flow (CF), and left ventricular developed pressure (LVDP) at baseline were compared between all hearts by one-way ANOVA. Time courses of CF and LVDP of hearts infused with plasma were analyzed by two-way ANOVA for repeated measures (time course, groups) and Fisher’s least significant difference post hoc tests; exact P values given for P < 0.05 vs. baseline; *P = 0.0033 vs. RIC plasma + GI/R + stattic.
Figure 2.
Humoral transfer of remote ischemic conditioning´s (RIC) cardioprotection through plasma from Ossabaw minipigs with RIC to isolated perfused recipient rat hearts despite nonresponsiveness of the pig’s myocardium to cardioprotection. Isolated perfused rat hearts were subjected to 30 min global ischemia and 120 min reperfusion (GI/R) without or with blockade of signal transducer and activator of transcription (STAT)3 by stattic and infusion of plasma taken from Ossabaw minipigs subjected to a placebo or RIC protocol. Red circles, data obtained with plasma from female; blue circles, data obtained with plasma from castrated male Ossabaw minipigs; open symbols, placebo; closed symbols, RIC protocol. Data are presented as means ± SD. *P < 0.001 vs. all other groups, respectively; two-way analysis of variance with Fisher’s least significant differences post hoc tests.
STAT3 in Pig and Rat Myocardium
Myocardial STAT3 expression decreased slightly over time in pigs with placebo and RIC (Fig. 3A). In rat hearts, there was no difference of STAT3 expression after infusion of plasma from Ossabaw minipigs with placebo or RIC (Fig. 3B). In the Ossabaw minipigs, there was a decrease in STAT3 phosphorylation during ischemia and an increase at 10 min reperfusion, which was not different between placebo and RIC (Fig. 3A). In isolated perfused rat hearts, STAT3 phosphorylation was increased with infusion of plasma from Ossabaw minipigs with RIC in comparison to hearts which had been infused with plasma from Ossabaw minipigs subjected to placebo (Fig. 3B). Pretreatment of isolated perfused rat hearts with the STAT3 blocker stattic abrogated the increase of rat myocardial STAT3 phosphorylation by infusion of plasma from Ossabaw pigs with RIC (Fig. 3C). All original Western blots are displayed in Supplemental Fig. S1 (pigs) and Supplemental Fig. S3 (rats).
Figure 3.
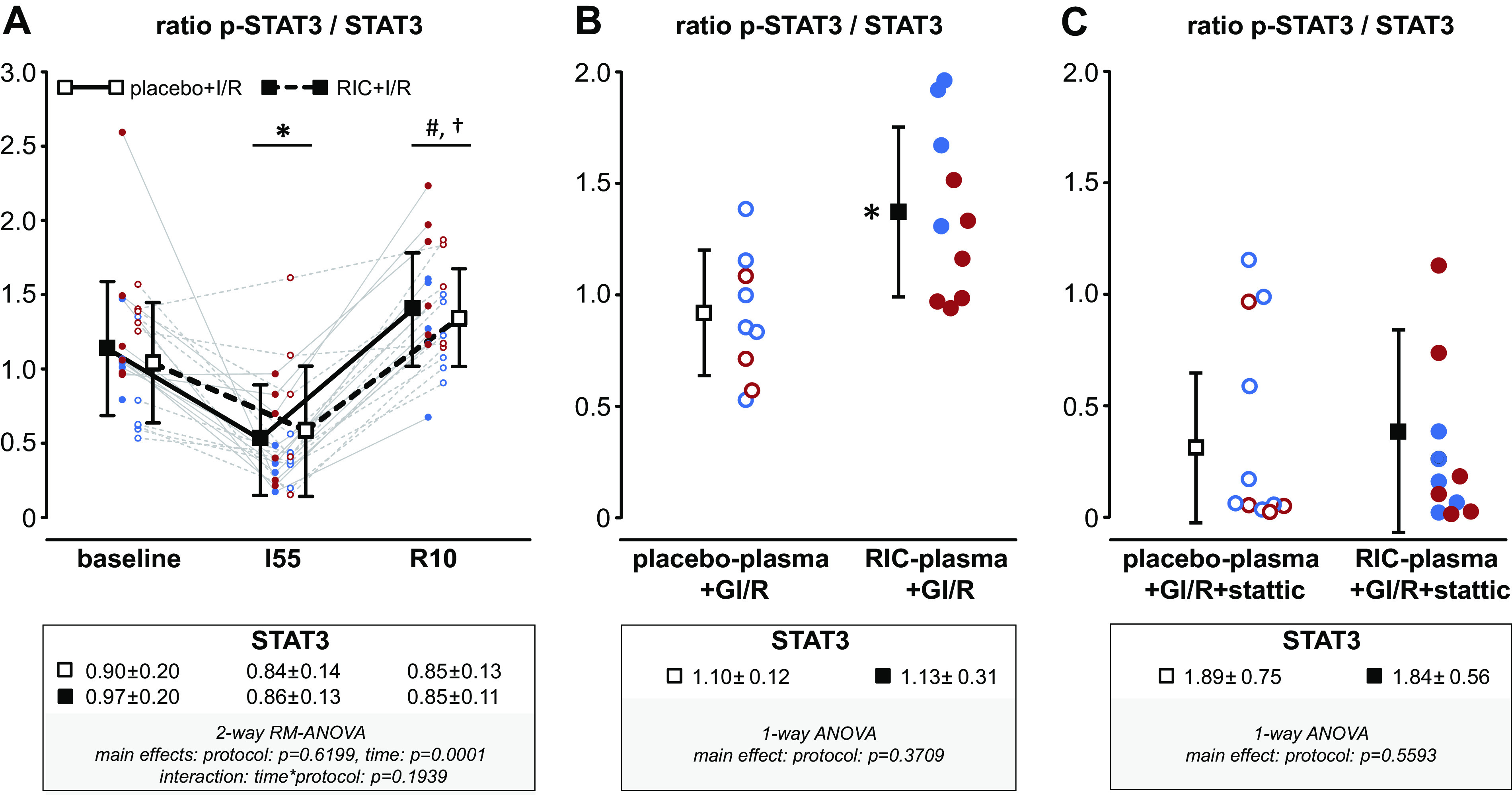
A: remote ischemic conditioning (RIC) does not activate the signal transducer and activator of transcription (STAT)3tyr705 in the Ossabaw minipig’s myocardium. STAT3tyr705 phosphorylation (p-STAT3) was analyzed in myocardial biopsies taken at baseline, during ischemia and at early reperfusion, respectively, from Ossabaw minipigs which had been subjected to a RIC (females/castrated males, n = 5/6) or to a placebo (females/castrated males, n = 6/5) protocol before ischemia-reperfusion by 60 min coronary occlusion and 180 min. Continuous lines indicate samples taken from one individual Ossabaw minipig with RIC protocol, respectively. Dashed lines indicate samples taken from one individual Ossabaw minipig with placebo protocol. Red circles, female; blue circles, castrated male Ossabaw minipigs. Data are presented as means ± SD. I55, 55 min ischemia; R10, 10 min reperfusion. *P < 0.0001 vs. baseline; #P < 0.001 vs. I55; †P = 0.0183 vs. baseline. B: infusion of plasma from Ossabaw minipigs subjected to RIC increases the phosphorylation of STAT3tyr705 in isolated perfused rat hearts in comparison to infusion of plasma from pigs with placebo protocol. *P < 0.01 vs. placebo plasma+global ischemia-reperfusion (GI/R), one-way analysis of variance with Fisher’s least significant post hoc tests. C: pretreatment of isolated perfused rat hearts with the STAT3 inhibitor stattic abrogates the increase in STAT3tyr705 phosphorylation induced by infusion of plasma from Ossabaw minipigs with RIC. Total STAT3 signals are normalized to the aggregate fluorescence signal on the membrane and displayed in the boxes below the x-axes of A, B, and C, respectively.
DISCUSSION
The translation of mechanical and pharmacological strategies to protect the heart from ischemia-reperfusion injury, which were highly successful in preclinical animal studies to the benefit of patients with reperfused acute myocardial infarction, has been largely disappointing (5, 58). The lack of translation is also seen in studies using RIC (11), with only one exception that reported better clinical outcomes in terms of reduced mortality and hospitalization for heart failure but still no infarct size reduction in terms of biomarker release (3). However, post hoc and non-prespecified analyses of two studies reported better clinical outcomes: in the CONDI-1 trial (6), RIC before primary percutaneous coronary intervention improved long-term clinical outcomes in patients with ST segment-elevation myocardial infarction (STEMI). Also, in the DANAMI-3-iPOST trial, ischemic postconditioning in addition to primary percutaneous coronary intervention reported reduced all-cause mortality and hospitalization for heart failure in patients with STEMI when not treated with thrombectomy (7). The difficulties in translation have been attributed to the advanced age and to comorbidities and comedications that patients with acute myocardial infarction typically have but the animals in preclinical studies mostly did not (9, 10). We have recently proposed that not only established comorbidities interfere with cardioprotection but that there may also be a genetically determined primordial obstacle to cardioprotection. Using Ossabaw minipigs, a strain that prone to develop a full metabolic syndrome, including obesity, insulin resistance up to type 2 diabetes, hyperlipidemia, hypertension with subsequent coronary atherosclerosis, and occasional spontaneous myocardial infarction with a high-fat diet (12, 59, 60), we observed lack of infarct size reduction by ischemic preconditioning(13), the strongest and most robust cardioprotective intervention (5). Notably, this lack of cardioprotection was observed when the Ossabaw minipigs were lean and before they had received the high-fat diet and developed a metabolic syndrome (12, 51). The lack of infarct size reduction was associated with a lack of STAT3 activation in Western blot analysis and in bioinformatics analysis with a difference in several clusters of protein-encoding genes, notably in mitochondrial and Janus kinase-STAT protein-encoding genes between the Ossabaw minipigs and our established model of Göttingen minipigs that have marked infarct size reduction with ischemic preconditioning (28, 32) and also Sus scrofa. In that study, however, we could not determine whether the failure to induce cardioprotection was secondary to a failure of the stimulus to release the cardioprotective trigger molecules or a nonresponsiveness of the myocardial signal transduction cascade. We therefore now extended our studies in Ossabaw minipigs from local to remote ischemic conditioning. With RIC, the potential cardioprotective triggering molecules are released into the circulating blood, where they can more easily be measured.
Confirming our previous study (13), we noted that the Ossabaw minipig was again resistant to ischemic conditioning’s cardioprotection: RIC, as IPC in our previous study, did not reduce infarct size in the Ossabaw minipig and again, the absence of infarct size reduction was accompanied by a lack of activation of myocardial STAT3. With respect to the above clinical trial that reported better clinical outcomes in patients with reperfused acute myocardial infarction when subjecting a leg (3) rather than an arm, as in a neutral larger phase III trial (61), to repeated brief ischemia and reperfusion, we used bilateral limb occlusion/reperfusion to induce RIC as in our previous study in rats (62), thus subjecting a larger tissue mass to ischemia and reperfusion and creating a stronger stimulus for cardioprotection. Infarct size in the rat hearts was reduced by plasma from RIC pigs, although the concentration of cardioprotective factor(s) was diluted 1:10 as compared with the pigs where infarct size was not reduced, reflecting a conservative mismatch of doses between rat and pig hearts. Indeed, RIC released cardioprotective triggers as evidenced by the transfer of Ossabaw minipig plasma to isolated perfused recipient rat hearts: plasma taken after RIC reduced infarct size in isolated rat hearts through rat myocardial STAT3 activation. The observed protection in our humoral transfer experiments was comparable to that seen in our previous studies in Göttingen minipigs (30, 43–45), but different from the impaired release of cardioprotective factor(s) in patients with diabetes and neuropathy when undergoing RIC (37).
In pigs, as in rats, RIC involves vagal activation of the spleen to induce the release of cardioprotective factors from the spleen into the circulating blood (43), rendering the spleen an important relay organ between neuronal and humoral cardioprotective signal transduction (63), a signal transduction which is apparently intact in the Ossabaw minipig.
Although the exact nature of the released triggers that induce cardioprotection during RIC is still unknown and these triggers probably differ from those released by IPC, i.e., adenosine and bradykinin (33), our observation of a humoral transfer of cardioprotection through plasma from pigs with RIC to recipient rat hearts strongly suggests that myocardial nonresponsiveness of the Ossabaw minipig to cardioprotection is located upstream of STAT3 activation. Mitochondria are considered as end effectors of ischemic conditioning’s cardioprotection (34), and a causal role of mitochondrial STAT3 activation was demonstrated for ischemic postconditioning’s cardioprotection(48).
Further experiments are required to clarify in more detail where exactly the block of ischemic conditioning’s signal transduction is located. Treatment of Ossabaw minipigs with substances that mediate cardioprotection via STAT3 activation, such as gp130 receptor agonists (64), may help to distinguish whether the block is upstream of STAT3 and attributable to lack of its activation or due to impaired function of STAT3 itself. In addition, substances that bypass STAT3 activation to induce cardioprotection directly in mitochondria, e.g., diazoxide (65, 66) or malonate (67, 68), could be used.
Conclusion and Outlook
Our present study may help to identify novel risk factors for acute myocardial infarction, i.e., a primordial resistance to adjunct cardioprotective interventions. The search for such risk factors would require analysis of genetic databases to associate potential genetic variants and an apparent resistance of patients to cardioprotective interventions in past, ongoing, but also future studies on ischemic conditioning’s cardioprotection. One useful approach may be to specifically analyze in bioassay approaches (46) blood samples from patients in whom RIC may induce the release of circulating cardioprotective factors, but who have no infarct size reduction, as seen in the present study using the Ossabaw minipig model.
SUPPLEMENTAL DATA
Supplemental Figs. S1–S3: https://doi.org/10.6084/m9.figshare.21277953.v1.
GRANTS
This work was supported by German Research Foundation Grant SFB 1116 B08 (to G.H. and P.K.); European-CARDIOPROTECTION COST-Action in Grant CA16225 (to G.H.); and National Institute of Diabetes and Digestive and Kidney Diseases Grant DK097512 (to M.S.).
DISCLOSURES
Michael Sturek is cofounder and Chief Scientific Officer of CorVus Biomedical, LLC, which produces Ossabaw minipigs. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
Petra Kleinbongard is an editor of American Journal of Physiology-Heart and Circulatory Physiology and was not involved and did not have access to information regarding the peer-review process or final disposition of this article. An alternate editor oversaw the peer-review and decision-making process for this article.
AUTHOR CONTRIBUTIONS
G.H. and P.K. conceived and designed research; H.R.L. and P.K. performed experiments; H.R.L. and A.S. analyzed data; H.R.L., M.S., G.H., and P.K. interpreted results of experiments; H.R.L. and A.S. prepared figures; H.R.L. drafted manuscript; A.S., M.S., G.H., and P.K. edited and revised manuscript; H.R.L., A.S., M.S., G.H., and P.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Kerstin Abu Hamed, Julia Husmann, Sandra Krüger, Marion Pesch, and Anita van de Sand for excellent technical assistance.
REFERENCES
- 1. Szummer K, Wallentin L, Lindhagen L, Alfredsson J, Erlinge D, Held C, James S, Kellerth T, Lindahl B, Ravn-Fischer A, Rydberg E, Yndigegn T, Jernberg T. Improved outcomes in patients with ST-elevation myocardial infarction during the last 20 years are related to implementation of evidence-based treatments: experiences from the SWEDEHEART registry 1995-2014. Eur Heart J 38: 3056–3065, 2017. doi: 10.1093/eurheartj/ehx515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jenča D, Melenovský V, Stehlik J, Staněk V, Kettner J, Kautzner J, Adámková V, Wohlfahrt P. Heart failure after myocardial infarction: incidence and predictors. ESC Heart Fail 8: 222–237, 2021. doi: 10.1002/ehf2.13144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gaspar A, Lourenço AP, Álvares Pereira M, Azevedo P, Roncon-Albuquerque R Jr, Marques J, Leite-Moreira AF. Randomized controlled trial of remote ischaemic conditioning in ST-elevation myocardial infarction as adjuvant to primary angioplasty (RIC-STEMI). Basic Res Cardiol 113: 14, 2018. doi: 10.1007/s00395-018-0672-3. [DOI] [PubMed] [Google Scholar]
- 4. Heusch G, Gersh BJ. Is cardioprotection salvageable? Circulation 141: 415–417, 2020. doi: 10.1161/CIRCULATIONAHA.119.044176. [DOI] [PubMed] [Google Scholar]
- 5. Heusch G. Myocardial ischaemia-reperfusion injury and cardioprotection in perspective. Nat Rev Cardiol 17: 773–789, 2020. doi: 10.1038/s41569-020-0403-y. [DOI] [PubMed] [Google Scholar]
- 6. Sloth AD, Schmidt MR, Munk K, Kharbanda RK, Redington AN, Schmidt M, Pedersen L, Sørensen HT, Bøtker HE; CONDI Investigators. Improved long-term clinical outcomes in patients with ST-elevation myocardial infarction undergoing remote ischaemic conditioning as an adjunct to primary percutaneous coronary intervention. Eur Heart J 35: 168–175, 2014. doi: 10.1093/eurheartj/eht369. [DOI] [PubMed] [Google Scholar]
- 7. Nepper-Christensen L, Høfsten DE, Helqvist S, Lassen JF, Tilsted HH, Holmvang L, Pedersen F, Joshi F, Sørensen R, Bang L, Bøtker HE, Terkelsen CJ, Maeng M, Okkels Jensen L, Aarøe J, Kelbæk H, Køber L, Engstrøm T, Lønborg J. Interaction of ischaemic postconditioning and thrombectomy in patients with ST-elevation myocardial infarction. Heart 106: 24–32, 2020. doi: 10.1136/heartjnl-2019-314952. [DOI] [PubMed] [Google Scholar]
- 8. Lecour S, Andreadou I, Bøtker HE, Davidson SM, Heusch G, Ruiz-Meana M, Schulz R, Zuurbier CJ, Ferdinandy P, Hausenloy DJ; on behalf of the European Union-CARDIOPROTECTION COST ACTION CA16225. IMproving Preclinical Assessment of Cardioprotective Therapies (IMPACT) criteria: guidelines of the EU-CARDIOPROTECTION COST action. Basic Res Cardiol 116: 52, 2021. doi: 10.1007/s00395-021-00893-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ferdinandy P, Hausenloy DJ, Heusch G, Baxter GF, Schulz R. Interaction of risk factors, comorbidities and comedications with ischemia/reperfusion injury and cardioprotection by preconditioning, postconditioning, and remote conditioning. Pharmacol Rev 66: 1142–1174, 2014. doi: 10.1124/pr.113.008300. [DOI] [PubMed] [Google Scholar]
- 10. Kleinbongard P, Bøtker HE, Ovize M, Hausenloy DJ, Heusch G. Co-morbidities and co-medications as confounders of cardioprotection - does it matter in the clinical setting? Br J Pharmacol 177: 5252–5269, 2020. doi: 10.1111/bph.14839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bell RM, Basalay M, Bøtker HE, Beikoghli Kalkhoran S, Carr RD, Cunningham J, Davidson SM, England TJ, Giesz S, Ghosh AK, Golforoush P, Gourine AV, Hausenloy DJ, Heusch G, Ibanez B, Kleinbongard P, Lecour S, Lukhna K, Ntsekhe M, Ovize M, Salama AD, Vilahur G, Walker JM, Yellon DM. Remote ischaemic conditioning: defining critical criteria for success-report from the 11th Hatter Cardiovascular Workshop. Basic Res Cardiol 117: 39, 2022. doi: 10.1007/s00395-022-00947-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sturek M, Alloosh M, Sellke FW. Swine disease models for optimal vascular engineering. Annu Rev Biomed Eng 22: 25–49, 2020. doi: 10.1146/annurev-bioeng-082919-053009. [DOI] [PubMed] [Google Scholar]
- 13. Kleinbongard P, Lieder HR, Skyschally A, Alloosh M, Gödecke A, Rahmann S, Sturek M, Heusch G. Non-responsiveness to cardioprotection by ischaemic preconditioning in Ossabaw minipigs with genetic predisposition to, but without the phenotype of the metabolic syndrome. Basic Res Cardiol 117: 58, 2022. doi: 10.1007/s00395-022-00965-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bolli R, Tang XL. New insights into cardioprotection, gained by adopting the CAESAR standards of rigor. Basic Res Cardiol 117: 57, 2022. doi: 10.1007/s00395-022-00964-1. [DOI] [PubMed] [Google Scholar]
- 15. Schott RJ, Rohmann S, Braun ER, Schaper W. Ischemic preconditioning reduces infarct size in swine myocardium. Circ Res 66: 1133–1142, 1990. doi: 10.1161/01.res.66.4.1133. [DOI] [PubMed] [Google Scholar]
- 16. Koning MM, Gho BC, van Klaarwater E, Duncker DJ, Verdouw PD. Endocardial and epicardial infarct size after preconditioning by a partial coronary artery occlusion without intervening reperfusion. Importance of the degree and duration of flow reduction. Cardiovasc Res 30: 1017–1027, 1995. doi: 10.1016/S0008-6363(95)00178-6. [DOI] [PubMed] [Google Scholar]
- 17. Ovize M, Aupetit J-F, Rioufol G, Loufoua J, André-Fouët X, Minaire Y, Faucon G. Preconditioning reduces infarct size but accelerates time to ventricular fibrillation in ischemic pig heart. Am J Physiol Heart Circ Physiol 269: H72–H79, 1995. doi: 10.1152/ajpheart.1995.269.1.H72. [DOI] [PubMed] [Google Scholar]
- 18. Sanz E, García Dorado D, Oliveras J, Barrabés JA, Gonzalez MA, Ruiz-Meana M, Solares J, Carreras MJ, García-Lafuente A, Desco M, Soler-Soler J. Dissociation between anti-infarct effect and anti-edema effect of ischemic preconditioning. Am J Physiol Heart Circ Physiol 37: H233–H241, 1995. doi: 10.1152/ajpheart.1995.268.1.H233. [DOI] [PubMed] [Google Scholar]
- 19. Schulz R, Rose J, Post H, Heusch G. Involvement of endogenous adenosine in ischaemic preconditioning in swine. Pflugers Arch 430: 273–282, 1995. doi: 10.1007/BF00374659. [DOI] [PubMed] [Google Scholar]
- 20. Shattock MJ, Lawson CS, Hearse DJ, Downey JM. Electrophysiological characteristics of repetitive ischemic preconditioning in the pig heart. J Mol Cell Cardiol 28: 1339–1347, 1996. doi: 10.1006/jmcc.1996.0124. [DOI] [PubMed] [Google Scholar]
- 21. Grund F, Sommerschild HT, Kirkebøen KA, Ilebekk A. Proarrhythmic effects of ischemic preconditioning in anesthetized pigs. Basic Res Cardiol 92: 417–425, 1997. [Erratum in Basic Res Cardiol 93: 497, 1998]. doi: 10.1007/BF00796216. [DOI] [PubMed] [Google Scholar]
- 22. Martin BJ, McClanahan TB, Van Wylen DGL, Gallagher KP. Effects of ischemia, preconditioning and adenosine deaminase inhibition on interstitial adenosine levels and infarct size. Basic Res Cardiol 92: 240–251, 1997. [Erratum in Basic Res Cardiol 92: 435, 1997].doi: 10.1007/BF00788519. [DOI] [PubMed] [Google Scholar]
- 23. Kudej RK, Shen YT, Peppas AP, Huang CH, Chen W, Yan L, Vatner DE, Vatner SF. Obligatory role of cardiac nerves and α1-adrenergic receptors for the second window of ischemic preconditioning in conscious pigs. Circ Res 99: 1270–1276, 2006. doi: 10.1161/01.RES.0000251282.79411.44. [DOI] [PubMed] [Google Scholar]
- 24. Schwartz LM, Lagranha CJ. Ischemic postconditioning during reperfusion activates AKT and ERK without protecting against lethal myocardial ischemia-reperfusion injury in pigs. Am J Physiol Heart Circ Physiol 290: H1011–H1018, 2006. doi: 10.1152/ajpheart.00864.2005. [DOI] [PubMed] [Google Scholar]
- 25. Depre C, Park JY, Shen YT, Zhao X, Qiu H, Yan L, Tian B, Vatner SF, Vatner DE. Molecular mechanisms mediating preconditioning following chronic ischemia differ from those in classical second window. Am J Physiol Heart Circ Physiol 299: H752–H762, 2010. doi: 10.1152/ajpheart.00147.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Poulsen RH, Rasmussen JT, Bøtker HE, Waehrens LS, Falborg L, Heegaard CW, Rehling M. Imaging the myocardium at risk with (9)(9)mTc-lactadherin administered after reperfusion in a porcine model. Nucl Med Biol 41: 114–119, 2014. doi: 10.1016/j.nucmedbio.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 27. Jones SP, Tang XL, Guo Y, Steenbergen C, Lefer DJ, Kukreja RC, Kong M, Li Q, Bhushan S, Zhu X, Du J, Nong Y, Stowers HL, Kondo K, Hunt GN, Goodchild TT, Orr A, Chang CC, Ockaili R, Salloum FN, Bolli R. The NHLBI-sponsored consortium for preclinicAL assESsment of cARdioprotective therapies (CAESAR): a new paradigm for rigorous, accurate, and reproducible evaluation of putative infarct-sparing interventions in mice, rabbits, and pigs. Circ Res 116: 572–586, 2015. doi: 10.1161/CIRCRESAHA.116.305462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kleinbongard P, Lieder H, Skyschally A, Heusch G. No sex-related differences in infarct size, no-reflow and protection by ischaemic preconditioning in Göttingen minipigs. Cardiovasc Res cvac062, 2022. doi: 10.1093/cvr/cvac062. [DOI] [PubMed] [Google Scholar]
- 29. Goodman MD, Koch SE, Afzal MR, Butler KL. STAT subtype specificity and ischemic preconditioning in mice: is STAT-3 enough? Am J Physiol Heart Circ Physiol 300: H522–H526, 2011. doi: 10.1152/ajpheart.00231.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Skyschally A, Gent S, Amanakis G, Schulte C, Kleinbongard P, Heusch G. Across-species transfer of protection by remote ischemic preconditioning with species-specific myocardial signal transduction by reperfusion injury salvage kinase and survival activating factor enhancement pathways. Circ Res 117: 279–288, 2015. doi: 10.1161/CIRCRESAHA.117.306878. [DOI] [PubMed] [Google Scholar]
- 31. Sawashita Y, Hirata N, Yoshikawa Y, Terada H, Tokinaga Y, Yamakage M. Remote ischemic preconditioning reduces myocardial ischemia-reperfusion injury through unacylated ghrelin-induced activation of the JAK/STAT pathway. Basic Res Cardiol 115: 50, 2020. doi: 10.1007/s00395-020-0809-z. [DOI] [PubMed] [Google Scholar]
- 32. Gent S, Skyschally A, Kleinbongard P, Heusch G. lschemic preconditioning in pigs: a causal role for signal transducer and activator of transcription 3. Am J Physiol Heart Circ Physiol 312: H478–H484, 2017. doi: 10.1152/ajpheart.00749.2016. [DOI] [PubMed] [Google Scholar]
- 33. Schulz R, Post H, Vahlhaus C, Heusch G. Ischemic preconditioning in pigs: a graded phenomenon. Its relation to adenosine and bradykinin. Circulation 98: 1022–1029, 1998. doi: 10.1161/01.CIR.98.10.1022. [DOI] [PubMed] [Google Scholar]
- 34. Heusch G. Molecular basis of cardioprotection: signal transduction in ischemic pre-, post-, and remote conditioning. Circ Res 116: 674–699, 2015. doi: 10.1161/CIRCRESAHA.116.305348. [DOI] [PubMed] [Google Scholar]
- 35. Heusch G, Bøtker HE, Przyklenk K, Redington A, Yellon DM. Remote ischemic conditioning. J Am Coll Cardiol 65: 177–195, 2015. doi: 10.1016/j.jacc.2014.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kleinbongard P, Skyschally A, Heusch G. Cardioprotection by remote ischemic conditioning and its signal transduction. Pflugers Arch 469: 159–181, 2017. [Erratum in Pflugers Arch 469: 843, 2017].doi: 10.1007/s00424-016-1922-6. [DOI] [PubMed] [Google Scholar]
- 37. Jensen RV, Støttrup NB, Kristiansen SB, Bøtker HE. Release of a humoral circulating cardioprotective factor by remote ischemic preconditioning is dependent on preserved neural pathways in diabetic patients. Basic Res Cardiol 107: 285, 2012. doi: 10.1007/s00395-012-0285-1. [DOI] [PubMed] [Google Scholar]
- 38. Shimizu M, Tropak M, Diaz RJ, Suto F, Surendra H, Kuzmin E, Li J, Gross G, Wilson GJ, Callahan J, Redington AN. Transient limb ischaemia remotely preconditions through a humoral mechanism acting directly on the myocardium: evidence suggesting cross-species protection. Clin Sci (Lond) 117: 191–200, 2009. doi: 10.1042/CS20080523. [DOI] [PubMed] [Google Scholar]
- 39. Merlocco AC, Redington KL, Disenhouse T, Strantzas SC, Gladstone R, Wei C, Tropak MB, Manlhiot C, Li J, Redington AN. Transcutaneous electrical nerve stimulation as a novel method of remote preconditioning: in vitro validation in an animal model and first human observations. Basic Res Cardiol 109: 406, 2014. doi: 10.1007/s00395-014-0406-0. [DOI] [PubMed] [Google Scholar]
- 40. Schmidt MR, Støttrup NB, Michelsen MM, Contractor H, Sørensen KE, Kharbanda RK, Redington AN, Bøtker HE. Remote ischemic preconditioning impairs ventricular function and increases infarct size after prolonged ischemia in the isolated neonatal rabbit heart. J Thorac Cardiovasc Surg 147: 1049–1055, 2014. doi: 10.1016/j.jtcvs.2013.05.022. [DOI] [PubMed] [Google Scholar]
- 41. Contractor H, Lie RH, Cunnington C, Li J, Støttrup NB, Manlhiot C, Bøtker HE, Schmidt MR, Forfar JC, Ashrafian H, Redington A, Kharbanda RK. Adenosine receptor activation in the “trigger” limb of remote pre-conditioning mediates human endothelial conditioning and release of circulating cardioprotective factor(s). JACC Basic Transl Sci 1: 461–471, 2016. doi: 10.1016/j.jacbts.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hildebrandt HA, Kreienkamp V, Gent S, Kahlert P, Heusch G, Kleinbongard P. Kinetics and signal activation properties of circulating factor(s) from healthy volunteers undergoing remote ischemic pre-conditioning. JACC Basic Transl Sci 1: 3–13, 2016. doi: 10.1016/j.jacbts.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lieder HR, Kleinbongard P, Skyschally A, Hagelschuer H, Chilian WM, Heusch G. Vago-splenic axis in signal transduction of remote ischemic preconditioning in pigs and rats. Circ Res 123: 1152–1163, 2018. doi: 10.1161/CIRCRESAHA.118.313859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Skyschally A, Kleinbongard P, Lieder HR, Gedik N, Stoian L, Amanakis G, Elbers E, Heusch G. Humoral transfer and intramyocardial signal transduction of protection by remote ischemic perconditioning in pigs, rats, and mice. Am J Physiol Heart Circ Physiol 315: H159–H172, 2018. [Erratum in Am J Physiol Heart Circ Physiol 322: H1086, 2022]. doi: 10.1152/ajpheart.00152.2018. [DOI] [PubMed] [Google Scholar]
- 45. Lieder HR, Skyschally A, Heusch G, Kleinbongard P. Plasma from remotely conditioned pigs reduces infarct size when given before or after ischemia to isolated perfused rat hearts. Pflugers Arch 471: 1371–1379, 2019. doi: 10.1007/s00424-019-02314-y. [DOI] [PubMed] [Google Scholar]
- 46. Lieder HR, Tüller P, Braczko F, Zandi A, Kamler M, Thielmann M, Heusch G, Kleinbongard P. Bioassays of humoral cardioprotective factors released by remote ischemic conditioning in patients undergoing coronary artery bypass surgery. J Cardiovasc Pharmacol Ther 27: 10742484221097273, 2022. doi: 10.1177/10742484221097273. [DOI] [PubMed] [Google Scholar]
- 47. Lieder HR, Tsoumani M, Andreadou I, Schrör K, Heusch G, Kleinbongard P. Platelet-mediated transfer of cardioprotection by remote ischemic conditioning and its abrogation by aspirin, but not by ticagrelor. Cardiovasc Drugs Ther 2022. doi: 10.1007/s10557-022-07345-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Heusch G, Musiolik J, Gedik N, Skyschally A. Mitochondrial STAT3 activation and cardioprotection by ischemic postconditioning in pigs with regional myocardial ischemia/reperfusion. Circ Res 109: 1302–1308, 2011. doi: 10.1161/CIRCRESAHA.111.255604. [DOI] [PubMed] [Google Scholar]
- 49. Percie Du Sert N, Ahluwalia A, Alam S, Avey MT, Baker M, Browne WJ, Clark A, Cuthill IC, Dirnagl U, Emerson M, Garner P, Holgate ST, Howells DW, Hurst V, Karp NA, Lazic SE, Lidster K, MacCallum CJ, Macleod M, Pearl EJ, Petersen OH, Rawle F, Reynolds P, Rooney K, Sena ES, Silberberg SD, Steckler T, Würbel H. Reporting animal research: explanation and elaboration for the ARRIVE guidelines 2.0. PLoS Biol 18: e3000411, 2020. doi: 10.1371/journal.pbio.3000411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Percie Du Sert N, Hurst V, Ahluwalia A, Alam S, Avey MT, Baker M, Browne WJ, Clark A, Cuthill IC, Dirnagl U, Emerson M, Garner P, Holgate ST, Howells DW, Karp NA, Lazic SE, Lidster K, MacCallum CJ, Macleod M, Pearl EJ, Petersen OH, Rawle F, Reynolds P, Rooney K, Sena ES, Silberberg SD, Steckler T, Würbel H. The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. PLoS Biol 18: e3000410, 2020. doi: 10.1371/journal.pbio.3000410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lloyd PG, Fang M, Brisbin ILJ, Andersson L, Sturek M. AMP kinase gene mutation is consistent with a thrifty phenotype (metabolic syndrome) in a population of feral swine. FASEB J 20, A299–A299, 2006. doi: 10.1096/fasebj.20.4.A299-d. [DOI] [Google Scholar]
- 52. Thielmann M, Kottenberg E, Kleinbongard P, Wendt D, Gedik N, Pasa S, Price V, Tsagakis K, Neuhäuser M, Peters J, Jakob H, Heusch G. Cardioprotective and prognostic effects of remote ischaemic preconditioning in patients undergoing coronary artery bypass surgery: a single-centre randomised, double-blind, controlled trial. Lancet 382: 597–604, 2013. [Erratum in Lancet 382: 940, 2013]. doi: 10.1016/S0140-6736(13)61450-6. [DOI] [PubMed] [Google Scholar]
- 53. Kowallik P, Schulz R, Guth BD, Schade A, Paffhausen W, Gross R, Heusch G. Measurement of regional myocardial blood flow with multiple colored microspheres. Circulation 83: 974–982, 1991. doi: 10.1161/01.CIR.83.3.974. [DOI] [PubMed] [Google Scholar]
- 54. Bøtker HE, Hausenloy D, Andreadou I, Antonucci S, Boengler K, Davidson SM, Deshwal S, Devaux Y, Di Lisa F, Di Sante M, Efentakis P, Femminò S, Garcia-Dorado D, Girícz Z, Ibanez B, Iliodromitis E, Kaludercic N, Kleinbongard P, Neuhäuser M, Ovize M, Pagliaro P, Rahbek-Schmidt M, Ruiz-Meana M, Schlüter KD, Schulz R, Skyschally A, Wilder C, Yellon DM, Ferdinandy P, Heusch G. Practical guidelines for rigor and reproducibility in preclinical and clinical studies on cardioprotection. Basic Res Cardiol 113: 39, 2018. doi: 10.1007/s00395-018-0696-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kleinbongard P, Skyschally A, Gent S, Pesch M, Heusch G. STAT3 as a common signal of ischemic conditioning: a lesson on “rigor and reproducibility” in preclinical studies on cardioprotection. Basic Res Cardiol 113: 3, 2018. doi: 10.1007/s00395-017-0660-z. [DOI] [PubMed] [Google Scholar]
- 56. Pillai-Kastoori L, Schutz-Geschwender AR, Harford JA. A systematic approach to quantitative Western blot analysis. Anal Biochem 593: 113608, 2020. doi: 10.1016/j.ab.2020.113608. [DOI] [PubMed] [Google Scholar]
- 57. Lindsey ML, LeBlanc AJ, Ripplinger CM, Carter JR, Kirk JA, Hansell Keehan K, Brunt KR, Kleinbongard P, Kassiri Z. Reinforcing rigor and reproducibility expectations for use of sex and gender in cardiovascular research. Am J Physiol Heart Circ Physiol 321: H819–H824, 2021. doi: 10.1152/ajpheart.00418.2021. [DOI] [PubMed] [Google Scholar]
- 58. Heusch G. Critical issues for the translation of cardioprotection. Circ Res 120: 1477–1486, 2017. doi: 10.1161/CIRCRESAHA.117.310820. [DOI] [PubMed] [Google Scholar]
- 59. Trask AJ, Katz PS, Kelly AP, Galantowicz ML, Cismowski MJ, West TA, Neeb ZP, Berwick ZC, Goodwill AG, Alloosh M, Tune JD, Sturek M, Lucchesi PA. Dynamic micro- and macrovascular remodeling in coronary circulation of obese Ossabaw pigs with metabolic syndrome. J Appl Physiol (1985) 113: 1128–1140, 2012. doi: 10.1152/japplphysiol.00604.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zhang Y, Fan G, Liu X, Skovgaard K, Sturek M, Heegaard PMH. The genome of the naturally evolved obesity-prone Ossabaw miniature pig. iScience 24: 103081, 2021. doi: 10.1016/j.isci.2021.103081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hausenloy DJ, Kharbanda RK, Møller UK, Ramlall M, Aarøe J, Butler R, et al. Effect of remote ischaemic conditioning on clinical outcomes in patients with acute myocardial infarction (CONDI-2/ERIC-PPCI): a single-blind randomised controlled trial. Lancet 394: 1415–1424, 2019. doi: 10.1016/S0140-6736(19)32039-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lieder HR, Irmert A, Kamler M, Heusch G, Kleinbongard P. Sex is no determinant of cardioprotection by ischemic preconditioning in rats, but ischemic/reperfused tissue mass is for remote ischemic preconditioning. Physiol Rep 7: e14146, 2019. doi: 10.14814/phy2.14146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Heusch G. The spleen in myocardial infarction. Circ Res 124: 26–28, 2019. doi: 10.1161/CIRCRESAHA.118.314331. [DOI] [PubMed] [Google Scholar]
- 64. Boengler K, Hilfiker-Kleiner D, Drexler H, Heusch G, Schulz R. The myocardial JAK/STAT pathway: from protection to failure. Pharmacol Ther 120: 172–185, 2008. doi: 10.1016/j.pharmthera.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 65. Pain T, Yang XM, Critz SD, Yue Y, Nakano A, Liu GS, Heusch G, Cohen MV, Downey JM. Opening of mitochondrial K(ATP) channels triggers the preconditioned state by generating free radicals. Circ Res 87: 460–466, 2000. doi: 10.1161/01.res.87.6.460. [DOI] [PubMed] [Google Scholar]
- 66. Heinzel FR, Luo Y, Li X, Boengler K, Buechert A, García-Dorado D, Di Lisa F, Schulz R, Heusch G. Impairment of diazoxide-induced formation of reactive oxygen species and loss of cardioprotection in connexin 43 deficient mice. Circ Res 97: 583–586, 2005. doi: 10.1161/01.RES.0000181171.65293.65. [DOI] [PubMed] [Google Scholar]
- 67. Prag HA, Aksentijevic D, Dannhorn A, Giles AV, Mulvey JF, Sauchanka O, Du L, Bates G, Reinhold J, Kula-Alwar D, Xu Z, Pellerin L, Goodwin RJA, Murphy MP, Krieg T. Ischemia-selective cardioprotection by malonate for ischemia/reperfusion injury. Circ Res 131: 528–541, 2022. doi: 10.1161/CIRCRESAHA.121.320717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Schulz R, Heusch G. Targeted mito- and cardioprotection by malonate. Circ Res 131: 542–544, 2022. doi: 10.1161/CIRCRESAHA.122.321582. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figs. S1–S3: https://doi.org/10.6084/m9.figshare.21277953.v1.