Keywords: myosin binding protein-C, slow-twitch skeletal muscle fiber, stretch activation, transient force overshoot, troponin I
Abstract
Stretch activation is defined as a delayed increase in force after rapid stretches. Although there is considerable evidence for stretch activation in isolated cardiac myofibrillar preparations, few studies have measured mechanisms of stretch activation in mammalian skeletal muscle fibers. We measured stretch activation following rapid step stretches [∼1%–4% sarcomere length (SL)] during submaximal Ca2+ activations of rat permeabilized slow-twitch skeletal muscle fibers before and after protein kinase A (PKA), which phosphorylates slow myosin binding protein-C. PKA significantly increased stretch activation during low (∼25%) Ca2+ activation and accelerated rates of delayed force development (kef) during both low and half-maximal Ca2+ activation. Following the step stretches and subsequent force development, fibers were rapidly shortened to original sarcomere length, which often elicited a shortening-induced transient force overshoot. After PKA, step shortening-induced transient force overshoot increased ∼10-fold following an ∼4% SL shortening during low Ca2+ activation levels. kdf following step shortening also increased after PKA during low and half-maximal Ca2+ activations. We next investigated thin filament regulation of stretch activation. We tested the interplay between cardiac troponin I (cTnI) phosphorylation at the canonical PKA and novel tyrosine kinase sites on stretch activation. Native slow-skeletal Tn complexes were exchanged with recombinant human cTn complex with different human cTnI N-terminal pseudo-phosphorylation molecules: 1) nonphosphorylated wild type (WT), 2) the canonical S22/23D PKA sites, 3) the tyrosine kinase Y26E site, and 4) the combinatorial S22/23D + Y26E cTnI. All three pseudo-phosphorylated cTnIs elicited greater stretch activation than WT. Following stretch activation, a new, elevated stretch-induced steady-state force was reached with pseudo-phosphorylated cTnI. Combinatorial S22/23D + Y26E pseudo-phosphorylated cTnI increased kdf. These results suggest that slow-skeletal myosin binding protein-C (sMyBP-C) phosphorylation modulates stretch activation by a combination of cross-bridge recruitment and faster cycling kinetics, whereas cTnI phosphorylation regulates stretch activation by both redundant and synergistic mechanisms; and, taken together, these sarcomere phosphoproteins offer precision targets for enhanced contractility.
INTRODUCTION
Stretch activation is defined as a delayed increase in force after rapid stretch of a Ca2+-activated striated muscle cell. Stretch activation was first observed in insect flight muscle (IFM) (1) and is widely accepted as a regulator of IFM contraction (2–6). During IFM contraction, two antagonistic muscle groups work synergistically to elicit stretch activation, i.e., contraction of an agonist muscle group stretches and consequently activates the antagonistic muscle group in an oscillatory manner (2, 5, 6). This mechanical feedback loop allows IFM to elicit 10–40 oscillatory wing beats per neural impulse (i.e., asynchronous contraction) (4).
Although mammalian cardiac muscle is controlled primarily by synchronous contractions (one contraction per electrical activation), its cyclical nature may use stretch activation processes to help match ventricular pressure with hemodynamic loads (7, 8). For instance, studies have shown that the mammalian ventricles utilize a bidirectional torsional contraction (9). The endocardium is activated first and contracts in a clockwise direction, after which the epicardium is activated and contracts in a counter-clockwise manner (viewed from apex) (9). It is postulated that timing of the different layers yields sequential stretch activation, eliciting a coordinated twisting of the ventricle for optimal wringing and ejection of blood. Consistent with this theory, the ventricle wall has a transmural gradient of 1) myosin heavy chain isoform (10), 2) myosin RLC phosphorylation patterns (11, 12), and 3) length-dependent activation properties (13), which may precisely time myocardial stretch to assist contraction/relaxation cycles (14–16).
Mammalian skeletal muscle tends to operate in a different physiological manner than cardiac and insect flight muscle whereby voluntary skeletal muscle contractions can be followed by long intervals of relaxation, whereas cardiac muscle functions as a pump that requires constant involuntary contraction/relaxation cycles. However, when a skeletal muscle group and its antagonist muscle group are taken into consideration, repetitive oscillatory contractions can arise during activity, i.e., a potential for stretch activation. While running, contraction of the quadriceps may stretch the hamstrings as they are beginning to be activated for the next cycle of knee flexion (17). There is also potential for dysfunctional stretch activation to elicit myogenic tremors associated with skeletal myopathies (18). Although there are limited studies of stretch activation in mammalian skeletal muscle fibers (19), there is considerable evidence for stretch activation in isolated cardiac myofibrillar preparations (9, 20, 21). Following a stretch of permeabilized cardiac myocyte preparations, there is a multiphasic mechanical response (12). First, there is an initial spike in force that coincides with stretch (phase 1), thought to be due to a strain on the attached cross bridges; followed by a quick decay of force (phase 2), thought to be the detachment of stained cross bridges from thin filaments; and finally, a slow, delayed increase in force (phase 3) from stretch activation. The mechanism of stretch activation in mammalian cardiac muscle remains uncertain although it likely involves myofilament cooperative activation (22). Myofilament cooperative activation is a mechanism by which the binding of one myosin head increases the likelihood of activation along the thin filament, thus, allowing greater force via recruitment of additional cross bridges from the noncycling pool to the cycling, force-generating pool. Stretch activation responses in mammalian cardiac muscle are modulated by 1) Ca2+ activation levels (21), 2) myosin heavy chain isoform (23), 3) phosphorylation state of myosin regulatory light chain (24), 4) sarcomere length (25), and 5) protein kinase A (PKA)-induced phosphorylation of cardiac myosin binding protein-C (cMyBP-C) (26). Thin filament regulation of stretch activation in mammalian striated muscle remains unclear.
We examined both thick and thin filament mechanisms that regulate stretch activation in mammalian striated muscle. To address these mechanisms and further illuminate stretch activation in skeletal muscle fibers, we implemented a rat slow-twitch skeletal muscle fiber experimental model. This experimental platform allowed us to first focus on PKA phosphorylation sites on MyBP-C since slow-twitch fibers lack PKA phosphorylation sites on troponin I (27). Next, using slow-twitch fibers, we tested thin filament regulation of stretch activation by exchange of endogenous slow skeletal troponin complex with cardiac troponin complex having site-specific cardiac troponin I (cTnI) PKA pseudo-phosphorylation (at serine sites 22/23). We also examined the effects of a novel phosphorylation site on cTnI [i.e., tyrosine 26 (Y26)], which is observed under basal conditions and decreased in failing human hearts (28). Tyrosine 26 resides in the same N-terminus cTnI region as serines 22/23; therefore, it stands to reason that phosphorylation of Y26 would have a similar physiological effect (29). However, tyrosine phosphorylation regulation remains unclear.
MATERIALS AND METHODS
Experimental Animals
All procedures involving animals were approved by the Animal Care and Use Committee of the University of Missouri. Male Sprague–Dawley rats (∼2–4 mo old), obtained from Envigo RMS, were housed in groups of two and provided food and water ad libitum.
Solutions
Relaxing solution for permeabilized skeletal muscle fibers contained 1 mM DTT, 100 mM KCl, 10 mM imidazole, 2.0 mM EGTA, 4.0 mM ATP, and 1 mM (free, 5 total) MgCl2. Minimal Ca2+ activating solution (pCa 9.0) for experimental protocol contained 7.00 mM EGTA, 20 mM imidazole, 5.42 mM MgCl2, 72.37 mM KCl, 0.016 mM CaCl2, 14.50 mM PCr, and 4.7 mM ATP. Maximal Ca2+ activating solution (pCa 4.5) for experimental protocol contained 7.00 mM EGTA, 20 mM imidazole, 5.26 mM MgCl2, 60.25 mM KCl, 7.01 mM CaCl2, 14.50 mM PCr, and 4.81 mM ATP. A range of Ca2+ concentrations for experiments was prepared by varying combinations of maximal and minimal Ca2+ solutions. Preactivating solution contained 0.5 mM EGTA, 20 mM imidazole, 5.42 mM MgCl2, 98.18 mM KCl, 0.016 mM CaCl2, 14.50 mM PCr, and 4.8 mM ATP. PKA solution was prepared by diluting 1 mg of DTT in 100 μL of ultrapure water; the DTT solution was then added to 400 U PKA (Sigma), and the 100 μL of PKA was then diluted in 700 μL pCa 9.0 solution to yield 0.5 U PKA/μL. Human cardiac troponin C (cTnC), cardiac troponin I (cTnI), and cardiac troponin T (cTnT) cDNA were isolated as previously described (30). Human cTnI cDNA was used to encode pseudo-phosphorylated human cTnI with serines 22/23 mutated to aspartic acid (S22/23D), tyrosine 26 to glutamic acid (Y26E), or all three sites by site-directed mutagenesis (S22/23D + Y26E) (Quick Change; Stratagene). Resultant constructs were verified by DNA sequencing. The individual recombinant human cTn subunits were expressed in Escherichia coli and purified to homogeneity as previously described for the human cTn subunits (31, 32). The varied cTn complexes were reconstituted by sequential dialysis and column purified as previously described (31, 33). Column fractions containing pure cTn were extensively dialyzed against exchange buffer (in mM: KCl, 200; MgCl2, 5; EGTA, 5; dithiothreitol, 1; MOPS, 20; pH 6.5). Aliquots were stored at −80°C until use. Troponin exchange was carried out in relaxing solution containing ∼0.5 mg/mL recombinant troponin overnight.
Skeletal Muscle Fiber Preparation
Slow-twitch skeletal muscle fibers were obtained from Sprague–Dawley rats anesthetized by inhalation of isoflurane (0.75 mL isoflurane:4.25 mL olive oil) and subsequently euthanized by excision of the heart. Slow-twitch skeletal muscle fibers were obtained from the soleus muscle. The muscles were isolated and placed in relaxing solution. Bundles of muscle fibers were separated and tied to capillary tubes. They were stored in the freezer in a 1:1 ratio of relaxing solution and glycerol for up to 1 mo. On the day of experimentation, single fibers were dissected from a bundle by gently pulling fibers from the end of the bundle (34).
Experimental Apparatus
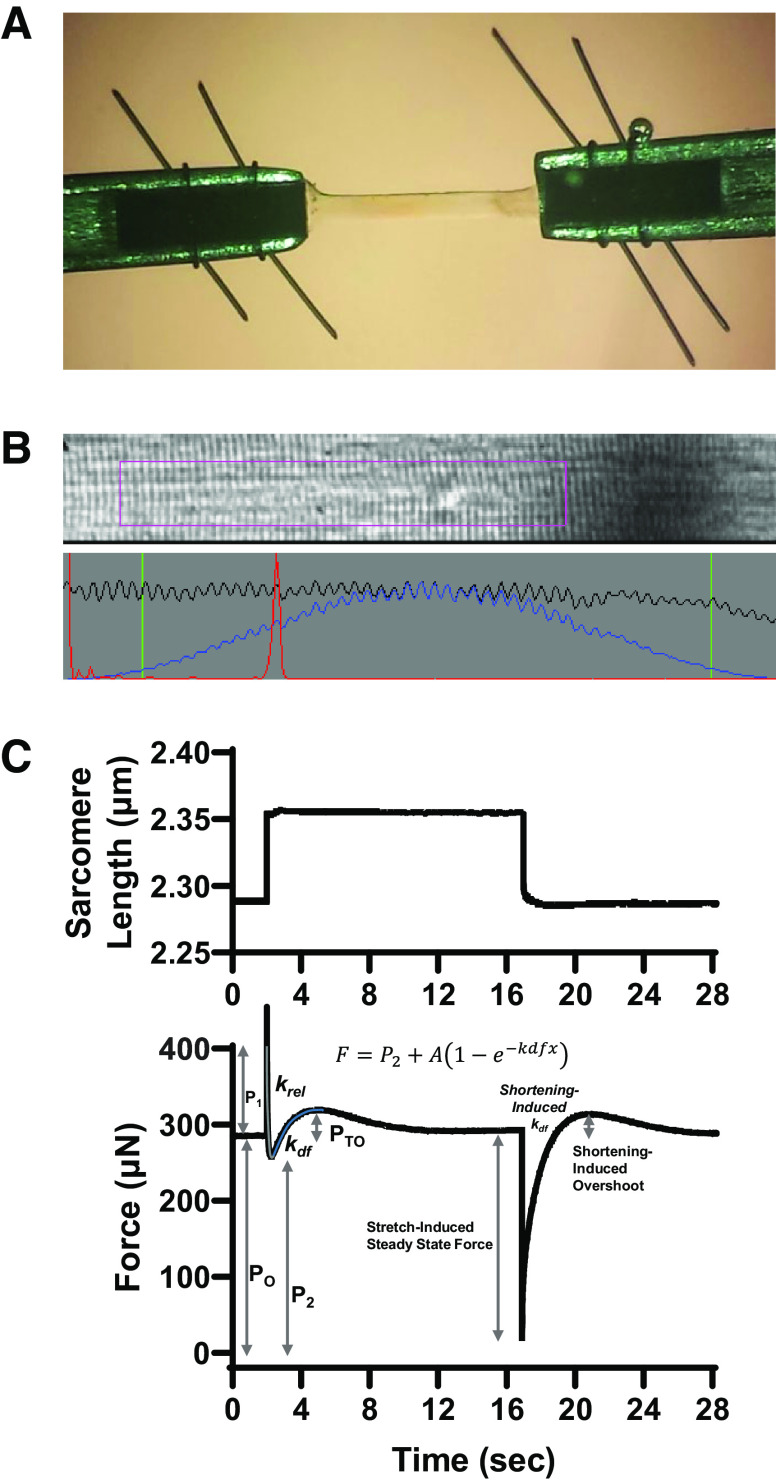
Prior to mechanical measurements, the experimental apparatus was mounted on the stage of an inverted microscope (model IX-70; Olympus Instrument Co.), which was placed on a pneumatic vibration isolation table. Mechanical measurements were performed using a capacitance gauge force transducer (Model 403, sensitivity of 20 mV/mg and resonant frequency of 600 Hz; Aurora Scientific). Length changes were presented to one end of the fiber via a DC torque motor (model 308c; Aurora Scientific) driven by voltage commands from a personal computer via a 16-bit D/A converter (AT-MIO-16E-1; National Instruments Corp.). Fibers were attached between the force transducer and length motor by placing the ends of the fiber into stainless steel troughs (25 gauge). The fiber ends were secured by overlaying a ∼0.5 mm length of 3-0 monofilament suture (Ethicon). The suture secured the fiber into the troughs by tightening two loops of 10-0 monofilament (Ethicon) at each end (Fig. 1A). The attachment procedure was performed under a stereomicroscope (×90 zoom). Force and length signals were digitized at 1 kHz and stored on a personal computer using Lab-View for Windows (National Instruments Corp.). Simultaneous sarcomere length measurements of force and length were obtained via IonOptix SarcLen system, which used a fast Fourier transform algorithm of the video image of the fiber (Fig. 1B). The region of interest is ∼220 × 30 μm; therefore, the sarcomere length is calculated from approximately one-tenth of the permeabilized skeletal muscle fiber preparation.
Figure 1.
Materials and methods of biomechanical techniques. A: permeabilized slow-twitch skeletal muscle fibers (∼1,000 µm long) were mounted between a motor and a force transducer. B: sarcomere length was monitored via IonOptix SarcLen system, which used a fast Fourier transform algorithm of the video image of the fiber. C: sarcomere length and force during a step-stretch and step-shortening protocol. Muscle fibers were allowed to develop steady-state tension at each pCa, then the fiber was rapidly stretched (∼1.5% and ∼4% original sarcomere length). After ∼12 s, the fiber was rapidly shortened to original muscle length. See materials and methods for further description of analysis.
Step-Stretch and Step-Shortening Protocol
All mechanical measurements of skeletal muscle fibers were performed at 15 ± 1°C. Rat permeabilized slow-twitch skeletal muscle fibers were placed in activating solution and adjusted to a sarcomere length (SL) of ∼2.40 ± 0.1 μm by manual manipulation of the length micrometer on fiber mount. The preparation was first transferred into pCa 4.5 solution for maximal Ca2+ activation then subsequently transferred into a series of submaximal Ca2+ activating solutions, ending back in pCa 4.5 maximal activating solution. At each pCa, steady-state tension was allowed to develop, and the fiber was stretched 1%–4% of the initial sarcomere length and held for ∼12 s, after which the preparation was shortened back to original sarcomere length (Fig. 1C). At each Ca2+ activation level, the fibers underwent approximately four step stretches and step shortening maneuvers, ranging from 1% to 4% sarcomere length in accordance with previous literature (21). These magnitudes of stretch also correlate to the stretch experienced by hamstrings muscles during the terminal swing phase of a stride cycle, i.e., early contraction (17). Forces in submaximal activating solutions were expressed as a fraction of force obtained during maximal Ca2+ activation. The maximal force value was calculated as an average of maximal force at the beginning and end of the experiment. To assess the effects of phosphorylation of slow-skeletal myosin binding protein-C (sMyBP-C) on stretch activation and rate of developed force, the step stretch protocol was performed before and after a 60-min incubation with PKA in relaxing solution. Step stretch protocol was also performed before and after human cTn exchange. Ca2+ activating solutions were adjusted to elicit desired relative forces (∼25%, 50%, and maximal Ca2+ activation) in accordance with previous literature. Physiologically, the small stretches of skeletal muscle occur during early activation and, thus, stretch activation occurs during low Ca2+ activation levels (17). The pCa solutions were adjusted following treatments to accommodate any changes in Ca2+ sensitivity and maintain similar relative forces in the respective fibers.
Western Blot
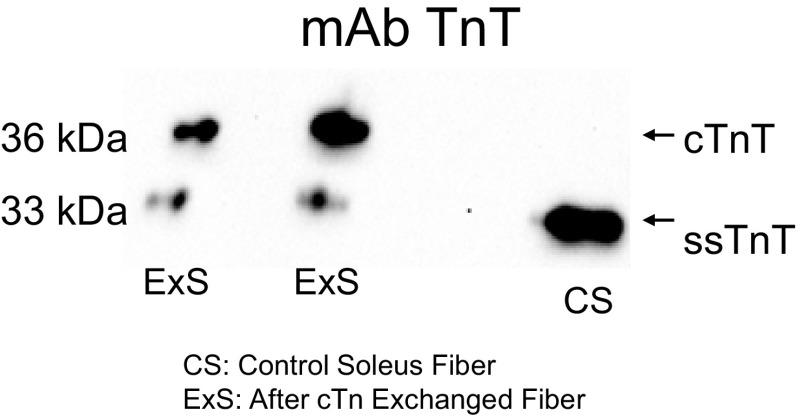
The level of residue-specific phosphorylation of sMyBP-C was assessed using SDS-PAGE followed by Western blotting. A glycerinated fiber bundle containing ∼20 fibers was isolated and cut into three equally sized sections, which were each placed into separate tubes containing 20 µL of relaxing solution and incubated with 5 µL of either 20 U PKA (Sigma), 2,000 U lambda phosphatase (New England Biolabs), or relaxing solution and incubated for 1 h at room temperature (∼20°C). The reaction was stopped by the addition of 25 µL of SDS-sample buffer. cTn exchange was confirmed by Western blots of single skeletal fibers after mechanical measurement using SDS-PAGE followed by Western blotting. The samples underwent SDS-PAGE using 12% polyacrylamide slab gels. After SDS-PAGE, the gels were placed on prewetted nitrocellulose membranes sandwiched between several sheets of 3MM chromatography paper, and the size-separated proteins were transferred to nitrocellulose using a semidry blot apparatus at constant current (120 mA) for 1.5 h. Immediately following transfer, the blots were stained with Ponceau S (Pierce) to verify equivalent protein loads and then placed in blocking buffer consisting of 5% dry milk, pH 7.4, and rocked for 1 h. To assess the level of MyBP-C phosphorylation, blots were then placed in small bags containing primary phospho-specific antibodies against Ser-59 sMyBP-C (35) diluted 1:500 in blocking buffer and incubated overnight at room temperature. To assess troponin exchange, blots were then incubated overnight with CT3 Troponin T antibodies (purchased from Developmental Studies Hybridoma Bank, Iowa City, IA) diluted 1:1,000 in blocking buffer. Blots were then washed in blocking buffer and incubated for 2 h with secondary peroxidase-conjugated goat anti-mouse or rabbit antibodies (Thermo Fisher Scientific) diluted 1:1,000 in blocking buffer. Blots were then washed with PBS and subsequently coated with Supersignal West Pico-chemiluminescent substrate (Pierce) and imaged using a Bio-Rad ChemDoc imaging system, and signal intensity was quantified using ImageJ software (National Institutes of Health). The difference in TnT isoform size (between endogenous slow skeletal troponin T (ssTnT) and the human exogenous cardiac TnT (cTnT)) was used to assess relative level of Tn exchange (Fig. 4).
Figure 4.
Confirmation of cardiac troponin (cTn) complex exchange into skeletal muscle. Native slow skeletal Tn complex was exchanged with recombinant human cTn complex in the same fibers that mechanics were measured. Western blots were performed using single skeletal fibers after mechanical measurement, and cTn exchange was quantified by the ratio of recombinant cTnT, endogenous slow-skeletal TnT. For all fibers, the Tn exchange averaged ∼60%.
Data Analysis
Step stretch and step shortening traces were fit with a single exponential rise to maximum equation as:
where F is force during force redevelopment, and P2 is the residual force before redevelopment, and A is the maximum force developed while kdf is the rate constant of developed force. Force redevelopment traces were fit from Fr to the force maximum.
Force redevelopment traces provided various force characteristic values (Fig. 1C). Permeabilized skeletal muscle fiber preparations first were submerged in Ca2+ activating solutions and allowed to rise to steady-state isometric force (PO). Following the mechanical perturbations, force often redeveloped transiently to an elevated maximal force (PO + PTO). Values for stretch activation were calculated by subtracting isometric force from the maximal redeveloped force [PTO = (PO + PTO) − PO) (Fig. 1C). Transient force overshoot was analyzed relative to isometric force and expressed as PTO/PO.
Rapid force decay traces were fit with a single exponential decay equation as:
where F is force during rapid force decay, and P2 is the force asymptote of decay, and krel is the rate constant of force decay while k is the curvature constant of force decay. Force decay traces were fit from P1 to P2.
Statistical Analysis
A Student–Newman–Keuls test was used for all pairwise comparisons among different groups. Paired t tests were used to compare the effects of PKA on contractile properties. One-way ANOVA was used to compare cTn exchange group differences in stretch magnitude, stretch activation, rates of developed force, and rate of rapid force decay. Values are expressed as means ± SE. P < 0.05 is accepted as statistically significant. n = number of fibers.
RESULTS
Effects of PKA-Induced sMyBP-C Phosphorylation on Stretch Activation
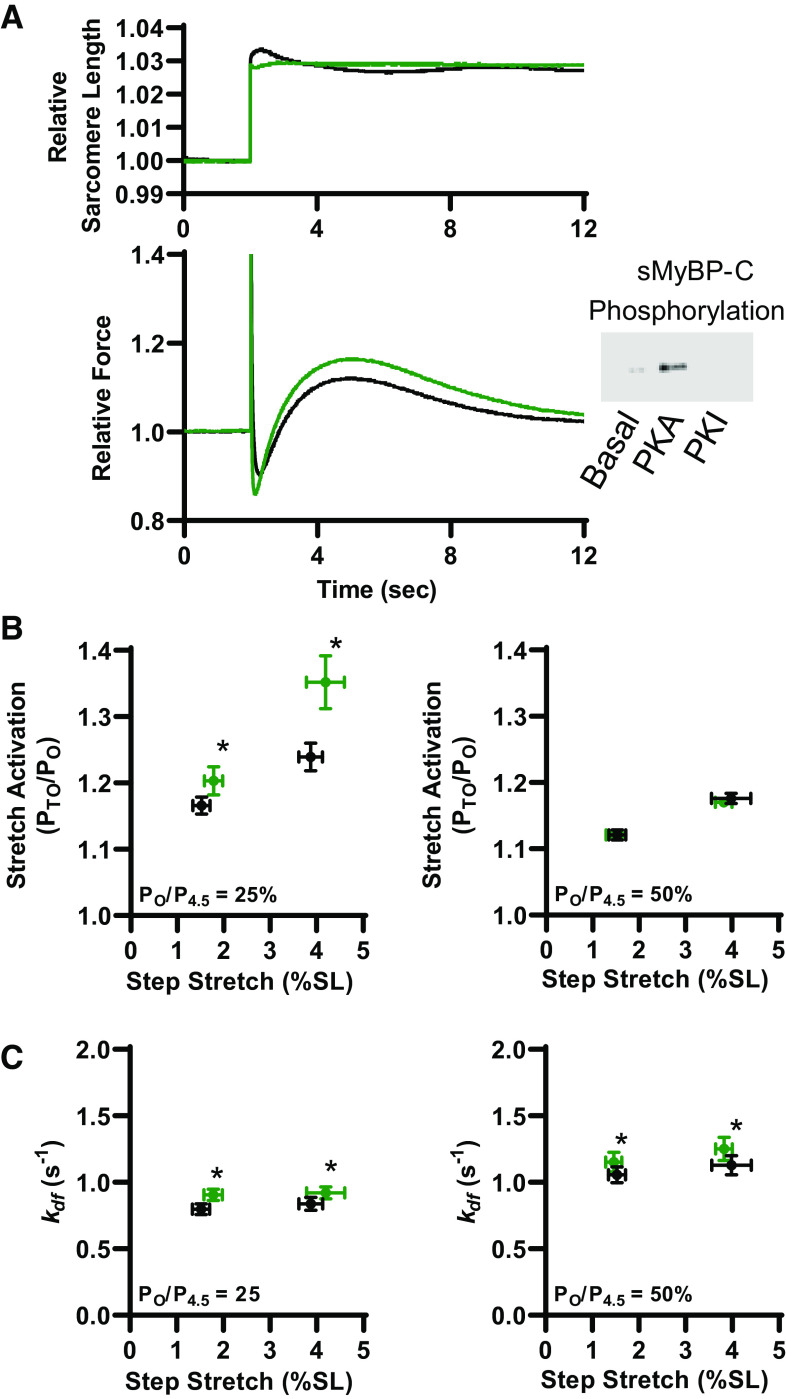
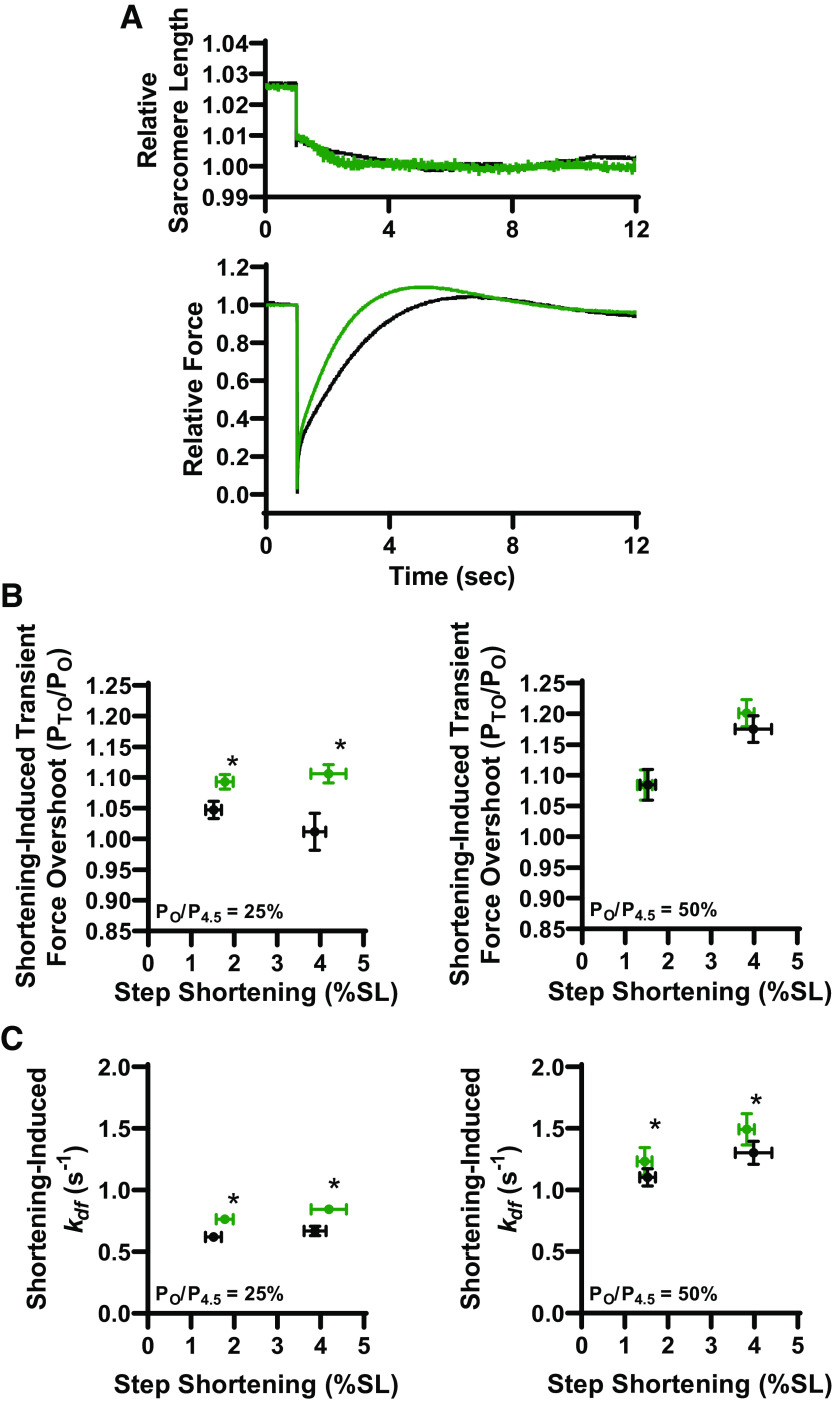
Stretch activation in rat slow-twitch skeletal muscle fibers was similar to previous reports using mammalian cardiac muscle preparations (21). There is an initial force spike that coincides with stretch (phase 1), followed by a quick decay of force that undershoots isometric force (phase 2), and over the next ∼5–8 s a delayed transient increase in force (phase 3) from stretch activation (Fig. 1C, Fig. 2A, and Fig. 5A). For these experiments, stretch activation was measured during low (∼25%) and half-maximal (∼50%) Ca2+ activation before and after PKA treatment of rat permeabilized slow-twitch skeletal muscle fibers that increased phosphorylation of sMyBP-C (Fig. 2). Before PKA, as stretch length increased [from ∼1.5% to ∼4% sarcomere length (SL)], stretch activation magnitude (PTO/PO) increased at both low and half-maximal Ca2+ activation (Fig. 2B). These results are consistent with previous reports in mammalian permeabilized striated muscle preparations (21). After PKA, the magnitude of stretch activation significantly increased during low Ca2+ activation levels. Quantitatively, after PKA, stretch activation was ∼20% and ∼50% greater (than before PKA) following ∼1.5% and ∼4% SL step stretches, respectively. PKA did not affect stretch activation magnitude during half-maximal Ca2+ activations (Fig. 2B). These results are similar to PKA effects on transient force overshoot magnitudes following a slack-restretch maneuver in rat permeabilized slow-twitch skeletal muscle fiber (36). Rates of delayed force development (kdf) following step stretches were faster after PKA during both low and half-maximal Ca2+ activation levels (Fig. 2C). Quantitatively, after PKA, kdf increased by ∼15% and ∼10% during low and half-maximal Ca2+ activations, respectively. Step stretch magnitude had no effect on kdf. These findings suggest that PKA phosphorylation of sMyBP-C accelerates processes that regulate myofilament stretch activation, which is consistent with previous cardiac myocardium studies (37–39). Additional parameters of stretch activation characterization, i.e., P1, P2, krel (see Fig. 1C) (29) were unchanged following PKA treatment (Table 1).
Figure 2.
Protein kinase A (PKA) effect on stretch activation in slow-twitch skeletal muscle fibers. A: representative sarcomere length (SL) and force trace during a step stretch maneuver (between 1% and 4% SL) before (black) and after PKA treatment (green). Inset shows a representative Western blot showing slow-skeletal myosin binding protein-C (sMyBP-C) phosphorylation under basal conditions, and after PKA or protein kinase I (PKI) treatment. B: larger stretches elicited greater stretch activation (PTO/PO) responses and the magnitude of stretch activation increased following PKA (green) under low Ca2+ conditions by ∼22% and ∼50% following a ∼1.5% and ∼4% sarcomere length step-stretch, respectively (left). PKA had no effect on the magnitude of stretch activation during half-maximal Ca2+ activations (right). C: step stretch-induced delayed force development rates during 25% (left) and 50% (right) Ca2+ activations before (black) and after (green) PKA treatment. Rate constant of delayed force development increased ∼15% and ∼10% after PKA at low activation levels and ∼10% during half-maximal Ca2+ activations. All data points are represented as means ± bidirectional SE. (n = 5) *P < 0.05 using paired t test analysis.
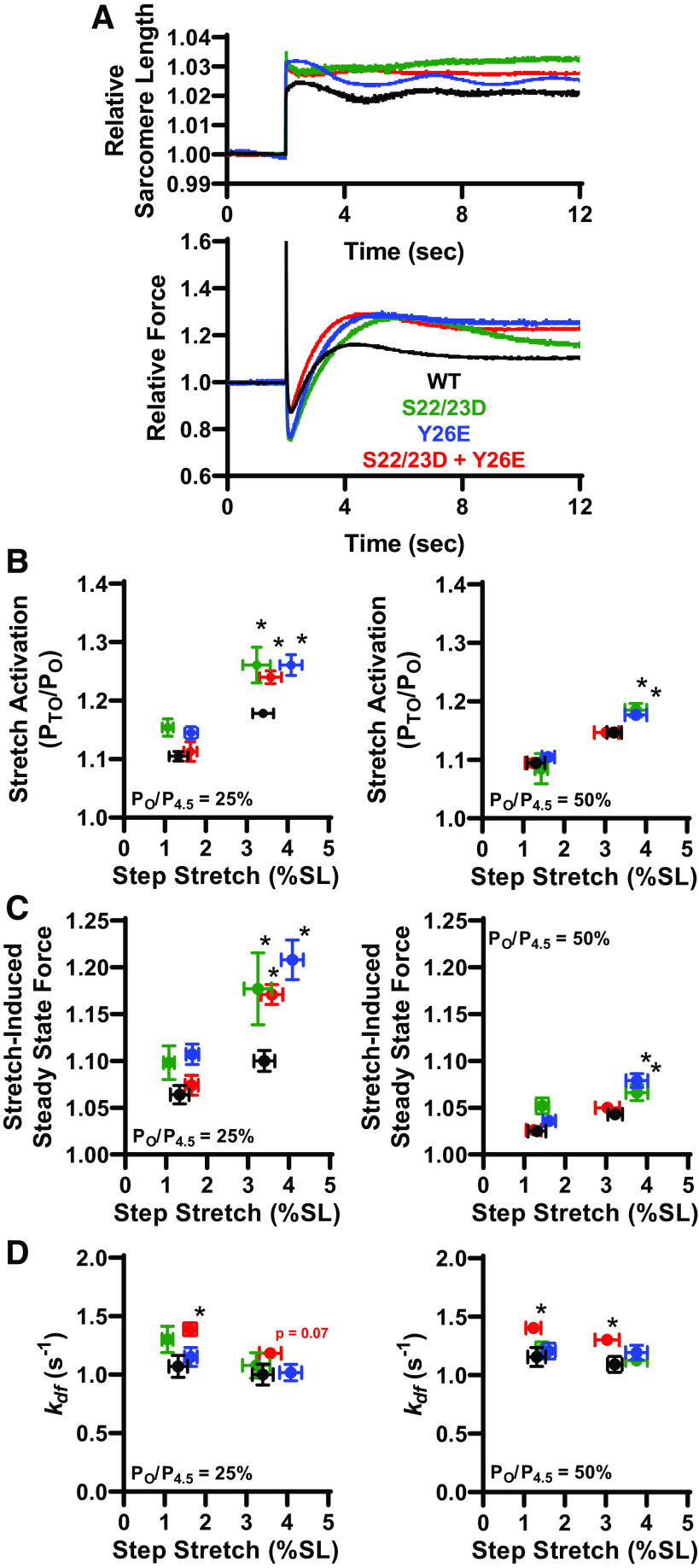
Figure 5.
Pseudo-phosphorylation of cardiac troponin I (cTnI) elevated stretch activation. A: representative sarcomere length (SL) and force trace during a step stretch maneuver (between 1% and 4% SL) after cTn exchange. B: at 25% Ca2+ activation (left), stretch activation increased with S22/23D (n = 4), Y26E (n = 7), and combinatorial S22/23D + Y26E (n = 4) pseudo-phosphorylated cTnI. At 50% Ca2+ activation (right), stretch activation increased with Y26E and S22/23D pseudo-phosphorylated cTnI. C: at 25% Ca2+ activation (left), stretch-induced steady-state force (relative to isometric force) increased with Y26E, S22/23D, and combinatorial S22/23D + Y26E pseudo-phosphorylated cTnI. At 50% Ca2+ activation (right), stretch activation increased with Y26E and S22/23D pseudo-phosphorylated cTnI. D: rate of delayed force development (kdf) increased with combinatorial Y26E + S22/23D cTnI exchange, which suggests that Y26E and S22/23D pseudo-phosphorylation of cTnI works synergistically to increase kdf. Pseudo-phosphorylated cTnI was compared with WT (n = 5) cTnI exchange via one-way ANOVA. *P < 0.05.
Table 1.
Characteristics of stretch activation in slow-twitch skeletal muscle fibers before versus after PKA treatment
| Treatment | Relative Force, PO/P4.5, % | Step Stretch Length, % | Stretch Activation, PTO/PO | Delayed Force Development Rate, kdf, s−1 | Maximum Force Peak, P1/PO | Rapid Force Decay Rate, krel, s−1 | Minimum Force Decay, P2/PO | Stretch-Induced Steady State Force | Shortening-Induced Overshoot (PTO/PO) | Shortening-Induced kdf, s−1 |
|---|---|---|---|---|---|---|---|---|---|---|
| Before PKA | 29.3 ± 2.3 | 1.5 ± 0.2 | 1.17 ± 0.01 | 0.80 ± 0.04 | 2.07 ± 0.06 | 121 ± 17 | 0.83 ± 0.01 | 1.08 ± 0.01 | 1.05 ± 0.01 | 0.62 ± 0.03 |
| 29.8 ± 2.7 | 3.9 ± 0.3 | 1.24 ± 0.02 | 0.84 ± 0.05 | 1.82 ± 0.26 | 122 ± 17 | 0.75 ± 0.02 | 1.14 ± 0.03 | 1.01 ± 0.03 | 0.67 ± 0.04 | |
| 45.8 ± 0.8 | 1.5 ± 0.2 | 1.121 ± 0.008 | 1.06 ± 0.06 | 2.17 ± 0.06 | 191 ± 17 | 0.89 ± 0.01 | 1.024 ± 0.003 | 1.09 ± 0.03 | 1.1 ± 0.07 | |
| 47.4 ± 0.9 | 4.0 ± 0.4 | 1.176 ± 0.008 | 1.13 ± 0.07 | 2.05 ± 0.11 | 190 ± 18 | 0.80 ± 0.04 | 1.045 ± 0.005 | 1.18 ± 0.02 | 1.3 ± 0.09 | |
| After PKA | 28.4 ± 2.5 | 1.8 ± 0.2 | 1.20 ± 0.02* | 0.91 ± 0.04* | 1.91 ± 0.04 | 107 ± 22 | 0.80 ± 0.02 | 1.10 ± 0.01 | 1.09 ± 0.01* | 0.76 ± 0.03* |
| 29.4 ± 2.5 | 4.2 ± 0.4 | 1.35 ± 0.04* | 0.92 ± 0.05* | 1.98 ± 0.05 | 100 ± 24 | 0.71 ± 0.02 | 1.18 ± 0.02 | 1.11 ± 0.02* | 0.85 ± 0.04* | |
| 48.4 ± 0.9 | 1.5 ± 0.2 | 1.121 ± 0.007 | 1.15 ± 0.07* | 2.02 ± 0.04 | 181 ± 18 | 0.87 ± 0.01 | 1.022 ± 0.002 | 1.08 ± 0.03 | 1.2 ± 0.11* | |
| 50.8 ± 0.9 | 3.8 ± 0.2 | 1.170 ± 0.003 | 1.25 ± 0.09* | 2.02 ± 0.03 | 190 ± 25 | 0.81 ± 0.01 | 1.033 ± 0.004 | 1.20 ± 0.02 | 1.5 ± 0.13* |
Stretch activation was tested before and after protein kinase A (PKA) treatment (n = 5) and compared via paired t test analysis. Values are represented as means ± SE.
P < 0.05.
Effects of PKA-Induced sMyBP-C Phosphorylation on Shortening-Induced Transient Force Overshoot
Following the step stretch and subsequent force development (results described earlier), the fiber was rapidly shortened to its original sarcomere length. This often elicited a shortening-induced transient force overshoot (PTO/PO) (Fig. 3A). Before PKA, step shortening-induced transient force overshoot magnitude was markedly greater during half-maximal Ca2+ activation compared with low Ca2+ activation (Fig. 3B). After PKA, step shortening-induced transient force overshoot doubled following ∼1.5% SL shortening and increased ∼10-fold following ∼4% SL shortening during low Ca2+ activation levels (Fig. 3B). PKA did not alter step shortening-induced transient force overshoot magnitude during half-maximal Ca2+ activations. Rate of force development (kdf) following step shortening increased by ∼25% and ∼15% after PKA during low and half-maximal Ca2+ activations (Fig. 3C). These results suggest that PKA phosphorylation of sMyBP-C markedly augments step shortening-induced recruitment of force-generating cross bridges, especially at low levels of Ca2+ activation.
Figure 3.
Step shortening-induced transient force overshoots increased following protein kinase A (PKA). A: following muscle stretch, the fiber was rapidly shortened back to original muscle length. Above is representative sarcomere length and force traces in response to a rapid step shortening (n = 5). B: during low Ca2+ activations (left) ∼1.5% sarcomere length (SL) step-shortening-induced transient force overshoot doubled after PKA (green) while ∼4% SL step-shortening-induced transient force overshoot significantly increased ∼10-fold. During half-maximal Ca2+ activations (right), step-shortening-induced transient force overshoot was unchanged following PKA treatment. C: rate of delayed force development, increased by ∼25% after PKA (green) during low Ca2+ activations in response to both ∼1.5% and ∼4% SL step shortenings (left). During half-maximal Ca2+ activations, kdf increased ∼15% after PKA in response to both ∼1.5% and ∼4% SL step shortenings (right). Data points are represented as means ± bidirectional SE. *P < 0.05 using paired t test analysis.
Site-Specific Pseudo-Phosphorylation of cTnI Effects on Stretch Activation
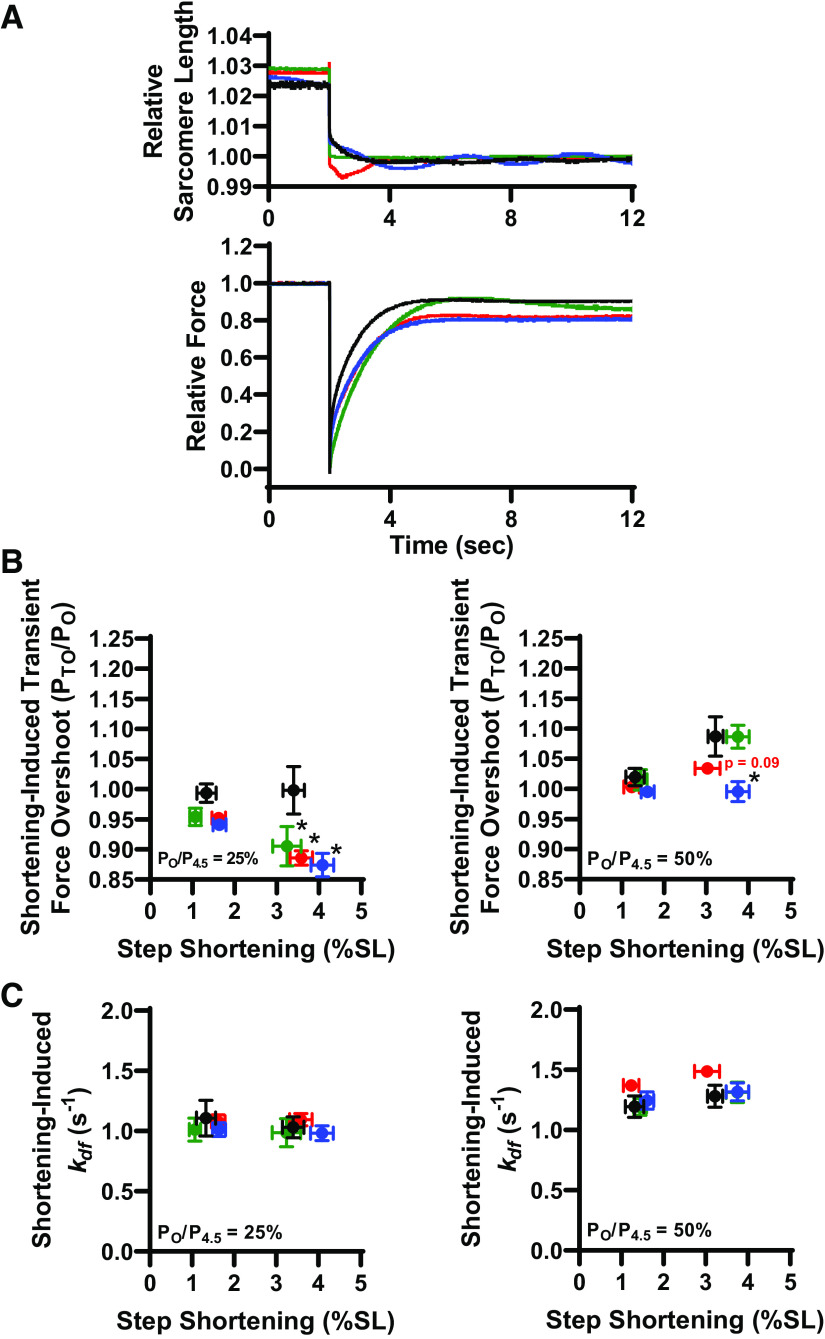
We next addressed the role of thin filament proteins on stretch activation. We continued to utilize our experimental platform since stretch activation was qualitatively similar between permeabilized slow-twitch skeletal muscle fibers and cardiac muscle preparations. Since PKA is known, in cardiac muscle, to phosphorylate the thin filament protein cardiac troponin I (cTnI), we tested the role of canonical cTnI PKA sites (i.e., serines 22/23) and a novel cTnI tyrosine phosphorylation site (i.e., tyrosine 26) on stretch activation. To assess these cTnI site-specific effects, we implemented our previously characterized Tn exchange protocol in single rat slow-twitch skeletal muscle fiber preparations (40). For these experiments after initial mechanical measurements, native slow-skeletal Tn complexes (which lack N-terminal cTnI phosphorylation sites) were exchanged in single fibers overnight with recombinant human cTn complex with different cTnI N-terminal pseudo-phosphorylation states (Fig. 4). We tested the hypothesis that pseudo-phosphorylation of TnI will increase stretch activation (PTO/PO) and rates of delayed force development (kdf). Four different human cTnI N-terminal pseudo-phosphorylation molecules were tested: 1) nonphosphorylated WT, 2) the canonical S22/23D PKA sites, 3) the tyrosine kinase Y26E site (29), and 4) the combinatorial S22/23D + Y26E cTnI to address functional effects of phosphorylation interplay between canonical PKA phosphorylation and novel tyrosine kinase sites (Table 2). The effect of cTnI phosphorylation on stretch activation was tested via rapid step stretches after cTn exchange (Fig. 5A). Following Tn exchange, fibers were Ca2+ activated at low (∼25%) and half-maximal (∼50%) levels and underwent rapid stretches of ∼1.5% and ∼4% SL. The canonical PKA phosphomimetic S22/23D cTnI elicited a ∼45% and ∼20% increase in stretch activation (PTO/PO) compared with WT cTnI at low and half-maximal Ca2+ activations, respectively, following a ∼4% SL stretch (Fig. 5B). Following Y26E cTnI exchange, stretch activation (PTO/PO) increased by ∼45% at low and ∼20% at half-maximal Ca2+ activations compared with WT cTnI, respectively, following a ∼4% SL stretch (Fig. 5B). Combinatorial S22/23D + Y26E pseudo-phosphorylated cTnI elicited at ∼35% increase in stretch activation compared with WT cTnI at low Ca2+ activation with a ∼4% SL step stretch (Fig. 5B). None of the cTnI modifications elicited an effect on stretch activation following a ∼1.5% SL step stretch (Fig. 5B). cTnI pseudo-phosphorylation elicited no difference in additional characteristics of stretch activation, i.e., P1, P2, krel (21), except for lower krel in the Y26E cTnI exchange group during ∼25% Ca2+ activated.
Table 2.
Characteristics of stretch activation in slow-twitch skeletal muscle fibers after cTn exchange with pseudo-phosphorylated cTnI
| Exchange | Relative Force, PO/P4.5, % | Step Stretch Length, % | Stretch Activation, PTO/PO | Delayed Force Development Rate, kdf, s−1 | Maximum Force Peak, P1/PO | Rapid Force Decay Rate, krel, s−1 | Minimum Force Decay, P2/PO | Stretch-Induced Steady-State Force | Shortening-Induced Overshoot (PTO/PO) | Shortening-Induced kdf, s−1 |
|---|---|---|---|---|---|---|---|---|---|---|
| WT | 25.8 ± 2.1 | 1.33 ± 0.23 | 1.11 ± 0.01 | 1.07 ± 0.09 | 1.94 ± 0.10 | 85 ± 8 | 0.90 ± 0.02 | 1.06 ± 0.01 | 0.99 ± 0.02 | 1.11 ± 0.15 |
| 27.9 ± 2.3 | 3.40 ± 0.25 | 1.18 ± 0.01 | 1.00 ± 0.09 | 2.28 ± 0.07 | 112 ± 6 | 0.84 ± 0.02 | 1.10 ± 0.01 | 1.00 ± 0.04 | 1.03 ± 0.09 | |
| 46.6 ± 1.8 | 1.31 ± 0.22 | 1.10 ± 0.01 | 1.16 ± 0.08 | 2.03 ± 0.10 | 164 ± 14 | 0.94 ± 0.01 | 1.03 ± 0.01 | 1.02 ± 0.01 | 1.19 ± 0.09 | |
| 47.5 ± 1.7 | 3.22 ± 0.18 | 1.15 ± 0.01 | 1.09 ± 0.07 | 2.45 ± 0.07 | 202 ± 15 | 0.90 ± 0.02 | 1.04 ± 0.01 | 1.09 ± 0.03 | 1.28 ± 0.09 | |
| S22/23D | 28.4 ± 2.8 | 1.07 ± 0.13 | 1.15 ± 0.01 | 1.30 ± 0.11 | 2.08 ± 0.09 | 82 ± 12 | 0.89 ± 0.02 | 1.10 ± 0.02 | 0.95 ± 0.01 | 1.01 ± 0.10 |
| 28.3 ± 3.2 | 3.24 ± 0.34 | 1.26 ± 0.03* | 1.08 ± 0.11 | 2.31 ± 0.06 | 98 ± 13 | 0.85 ± 0.02 | 1.18 ± 0.04* | 0.91 ± 0.03* | 0.99 ± 0.12 | |
| 42.7 ± 2.0 | 1.44 ± 0.16 | 1.08 ± 0.03 | 1.22 ± 0.07 | 2.16 ± 0.11 | 153 ± 15 | 0.93 ± 0.01 | 1.05 ± 0.01 | 1.02 ± 0.02 | 1.19 ± 0.07 | |
| 45.1 ± 2.2 | 3.75 ± 0.27 | 1.19 ± 0.01* | 1.13 ± 0.04 | 2.31 ± 0.07 | 165 ± 17 | 0.89 ± 0.01 | 1.07 ± 0.01* | 1.09 ± 0.02 | 1.31 ± 0.08 | |
| Y26E | 23.9 ± 1.8 | 1.64 ± 0.15 | 1.15 ± 0.01 | 1.15 ± 0.08 | 2.01 ± 0.06 | 50 ± 5* | 0.89 ± 0.01 | 1.11 ± 0.01 | 0.94 ± 0.01 | 1.01 ± 0.06 |
| 23.9 ± 1.9 | 4.08 ± 0.27 | 1.26 ± 0.02* | 1.02 ± 0.07 | 2.28 ± 0.03 | 64 ± 5* | 0.81 ± 0.01 | 1.21 ± 0.02* | 0.87 ± 0.02* | 0.98 ± 0.06 | |
| 47.4 ± 2.0 | 1.61 ± 0.15 | 1.11 ± 0.01 | 1.21 ± 0.07 | 2.02 ± 0.08 | 119 ± 14 | 0.94 ± 0.01 | 1.04 ± 0.01 | 1.00 ± 0.01 | 1.24 ± 0.07 | |
| 47.6 ± 2.0 | 3.75 ± 0.27 | 1.18 ± 0.01* | 1.19 ± 0.06 | 2.33 ± 0.03 | 153 ± 15 | 0.88 ± 0.02 | 1.08 ± 0.01* | 1.00 ± 0.02* | 1.32 ± 0.07 | |
| S22/23D + Y26E | 24.4 ± 1.9 | 1.62 ± 0.16 | 1.11 ± 0.02 | 1.39 ± 0.05* | 1.91 ± 0.10 | 78 ± 7 | 0.92 ± 0.01 | 1.07 ± 0.01 | 0.95 ± 0.01 | 1.08 ± 0.06 |
| 22.6 ± 1.0 | 3.58 ± 0.27 | 1.24 ± 0.01* | 1.18 ± 0.04 | 2.23 ± 0.06 | 94 ± 6 | 0.86 ± 0.02 | 1.17 ± 0.01* | 0.89 ± 0.01* | 1.10 ± 0.05 | |
| 49.0 ± 1.1 | 1.23 ± 0.18 | 1.10 ± 0.01 | 1.40 ± 0.05* | 2.03 ± 0.09 | 168 ± 19 | 0.947 ± 0.004 | 1.026 ± 0.002 | 1.003 ± 0.003 | 1.37 ± 0.03 | |
| 48.7 ± 1.5 | 3.03 ± 0.30 | 1.147 ± 0.004 | 1.30 ± 0.04* | 2.41 ± 0.04 | 209 ± 21 | 0.93 ± 0.01 | 1.050 ± 0.003 | 1.03 ± 0.01 | 1.49 ± 0.04 |
Stretch activation after cardiac troponin (cTn) exchange. Pseudo-phosphorylated cTnI (S22/23D (n = 4), Y26E (n = 7), or S22/23D + Y26E (n = 4)) was compared with WT cTnI (n = 5) via one-way ANOVA. Values are represented as means ± SE.
P < 0.05.
Further evidence of thin filament-mediated stretch activation was the consistent finding that stretch-induced steady-state force was increased by pseudo-phosphorylation of cTnI at low Ca2+ activation levels (Fig. 5C). At half-maximal Ca2+ activation, stretch-induced steady-state force increased with Y26E and S22/23D, but not combinatorial S22/23D + Y26E pseudo-phosphorylated cTnI (Fig. 5C). Rates of stretch activation development were unchanged following the canonical PKA pseudo-phosphorylation S22/23D and tyrosine kinase Y26E pseudo-phosphorylation. However, the combinatorial S22/23D + Y26E pseudo-phosphorylated cTnI elicited an increase in kdf at both low and half-maximal Ca2+ activation compared with WT cTnI (Fig. 5D), suggesting that Y26E and S22/23D pseudo-phosphorylation of cTnI works synergistically to increase kdf.
Site-Specific Pseudo-Phosphorylation of cTnI Effects on Shortening-Induced Transient Force Overshoot
Pseudo-phosphorylation of cTnI’s effect on step-shortening-induced transient force overshoot was tested by rapidly shortening the fiber to original sarcomere length following the rapid step stretch and subsequent force development. Shortening-induced transient force overshoot was unaltered by pseudo-phosphorylation of cTnI following ∼1.5% SL shortening (Fig. 6B). Following ∼4% SL shortening, shortening-induced PTO/PO was decreased with pseudo-phosphorylated cTnI during low (∼25%) Ca2+ activation (Fig. 6B). This is likely due to the elevated levels stretch-induced steady-state force with pseudo-phosphorylation of cTnI, i.e., elevated PO. Shortening-induced force development rates were similar in all groups (Fig. 6C).
Figure 6.
Cardiac troponin (cTn) exchange effect on step shortening-induced transient force overshoot and rates of delayed force development. A: representative sarcomere length and force traces during step shortening [between 1% and 4% sarcomere length (SL)] after cTn exchange with 1) nonphosphorylated WT (n = 5), 2) the canonical S22/23D protein kinase A (PKA) sites (n = 4), 3) the tyrosine kinase Y26E site (n = 7), and 4) the combinatorial S22/23D + Y26E cTnI (n = 4). B: relative step shortening-induced transient force overshoot was lower following modified cTn exchange compared with WT cTn. C: rate of delayed force development following step shortening was unaltered by pseudo-phosphorylated cTnI (B). *P < 0.05.
DISCUSSION
This study examined dynamic, subcellular regulation of stretch activation in rat-permeabilized slow-twitch skeletal muscle fibers. The main findings from these experiments were: 1) PKA phosphorylation of sMyBP-C increased stretch activation in slow-twitch skeletal muscle fibers but only at low (∼25%) Ca2+ activation levels (Fig. 2B), whereas rates of delayed force development (kdf) increased at all Ca2+ activation levels (Fig. 2C), which provides evidence that phosphorylation of MyBP-C increases the availability of force-generating cross bridges and accelerates myosin cross-bridge cycling kinetics; 2) step shortenings elicited similar transient force overshoot (as step stretches) and the shortening-induced transient force overshoot was markedly increased by PKA but again only at low (∼25%) Ca2+ activation levels, implicating phosphorylation of sMyBP-C relieves its constraint on a population of myosin cross bridges previously unavailable by step shortenings under basal, low stress conditions (Fig. 3B); 3) all cTnI pseudo-phosphorylation increased stretch activation (Fig. 5B), whereas only combinatorial S22/23D + Y26E cTnI synergistically increased rates of delayed force development (Fig. 5D); this suggests that cTnI phosphorylation regulates myofilament stretch activation by a combination of redundant and synergistic biophysical mechanisms, which may complement each other to tune beat-to-beat myocardial output to match hemodynamic demand.
Multiple lines of evidence have shown that small stretches of Ca2+-activated sarcomeres augment mammalian striated muscle force generation, i.e., stretch activation. Straight et al. (19) reported stretch activation in mouse slow-twitch and fast-twitch skeletal muscle fibers after small (1%) step stretches. A series of studies by Stelzer and Moss (21–26, 41) showed that step stretches of mouse myocardium yielded a delayed increase in force. The force redevelopment following a rapid step stretch was multiphasic. Force spiked immediately in response to a step stretch (P1), force then fell exponentially (P2), and finally over several seconds, a delayed force develops (PTO). This delayed force development is taken as evidence of stretch activation. Stelzer and Moss showed that the kinetics and magnitude of stretch activation response can be modulated by 1) Ca2+ activation levels (21), 2) myosin heavy chain isoform (23), 3) phosphorylation state of myosin regulatory light chain (24), 4) sarcomere length (25), and 5) PKA-induced phosphorylation of cardiac myosin binding protein-C (cMyBP-C) (26). In accordance with these studies and our previous studies on transient force overshoot following mechanical perturbation (i.e., slack-restretch maneuver) (36), we hypothesized that PKA phosphorylation of slow-skeletal MyBP-C (sMyBP-C) would augment stretch activation and accelerate delayed force development in rat slow-twitch skeletal muscle fibers. Indeed, PKA elevated stretch activation, but only at low (∼25%) Ca2+ activation levels (Fig. 2B). We propose that the increased stretch activation is likely due to phosphorylation of sMyBP-C increasing the likelihood of recruitment of myosin heads into the pool of cycling, force-generating cross bridges. In agreement with this theory, stretch in cardiac myocardium has been shown to influence disorder of the myosin backbone, i.e., less myosin heads in OFF state (42). In Lethocerus IFM, it is postulated that the myosin heads are capable of cycling from OFF to force generating at 30–40 Hz, similar to the oscillation frequency of the muscle; however, this sequence is still under investigation (8). PKA also elevated the rate of stretch activation force development (kdf) at all levels of Ca2+ activation in rat slow-twitch skeletal muscle fibers, suggesting that phosphorylation of sMyBP-C accelerates myosin cross-bridge cycling kinetics (Fig. 2C). Collectively, PKA-induced phosphorylation of sMyBP-C elicited qualitatively similar responses to both step-stretch and slack-restretch protocols (i.e., increased transient force overshoot and faster force development). In permeabilized myocardium, the slack-restretch protocol is the canonical test of force development/generation (43, 44). The step stretch protocol tests a different myocardial phenomena, i.e., stretch activation (2). Stretch activation involves stretching an active muscle to a new isometric length resulting in a new steady state. The two phenomena appear to be regulated, at least in part, through similar myofilament mechanisms.
To the best of our knowledge, shortening-induced transient force overshoot has not been experimentally measured, but it was described in a computational model of mammalian striated muscle stretch activation (16). Similar to transient force overshoot [with slack-restretch (36)] and stretch activation (following a step stretch), step-shortening-induced transient force overshoot increased following PKA. Interestingly, PKA caused an ∼10-fold increase following a ∼4% SL shortening albeit to lesser relative values than stretch-activated force response. There was minimal shortening-induced force overshoot before PKA treatment (∼1%), and following PKA phosphorylation of sMyBP-C, step shortening-induced force overshoot (PTO/PO) increased to ∼11% (Fig. 3B). Rates of shortening-induced transient force development were also elevated following PKA treatment at all Ca2+ activation levels (Fig. 3C). In a computational model, step shortening-induced transient force overshoot arose only in the presence of cross-bridge-mediated cooperative activation of thin filament regulatory units, and was interpreted as a myofilament memory behavior to mitigate shortening-induced force deactivation (16). Taken together, step-shortening-induced transient force overshoot further support the hypothesis that PKA phosphorylation of sMyBP-C increases the cooperative recruitment of force-generating myosin cross bridges and accelerate cross-bridge cycling kinetics.
This study also addressed the role of thin filaments on stretch activation, specifically the phosphorylation status of cardiac troponin I (cTnI). Canonical serine (Ser)-22/23 PKA sites are well-accepted regulators (e.g., effects Ca2+ sensitivity of force) of cardiac muscle contraction under physiological and pathophysiological stress (28, 40, 45). In addition, cTnI tyrosine (Tyr)-26 phosphorylation has recently been observed under basal conditions and also decreased in failing human hearts (28). Tyr-26 phosphorylation also decreases Ca2+ sensitivity of thin filaments and accelerates thin filament deactivation similar to Ser-22/23 phosphorylation (29). Tyr-26 phosphorylation is a novel, unexplored regulatory pathway of cardiac function. The serine kinase pathway has broad downstream targets (46), whereas, the tyrosine kinase signaling pathway is more specific (46) and thus better suited for potential therapeutic targets. We tested the phospho-specificity of these residues individually and in combination. Pseudo-phosphorylation of cTnI at either serine 22/23 or tyrosine-26 augmented stretch activation compared with WT cTnI following ∼4% SL step stretches (Fig. 5B). These results suggest that phosphorylation of cTnI has the capacity to contribute to a stress response (e.g., β-adrenergic stimulation) by amplified stretch activation. One possible mechanism of the augmented stretch activation of force is phosphorylation of cTnI increasing cooperativity of the thin filaments, i.e., increased myosin binding sites available on actin monomers due to increased thin filament coordination (40). There was no additive effect on stretch activation with combinatorial pseudo-phosphorylation of serine 22/23 and tyrosine 26. However, S22/23D and Y26E integration appears to synergistically accelerate rates of delayed force development (kdf), i.e., the combinatorial S22/23D + Y26E cTnI significantly increased kdf values following exchange (Fig. 5D). Combinatorial S22/23D + Y26E cTnI has also been shown to synergistically accelerate thin filament deactivation (29), which was theorized to aid myocardial lusitropy. Here, we provide evidence of cTnI ability to not only aid in cooperative deactivation of the thin filament, but also augment rates of stretch activation in the heart. Pseudo-phosphorylation of cTnI reduced shortening-induced transient force overshoot (Fig. 6B). Phosphorylation of TnI increases cooperative deactivation of the thin filaments (40). It stands to reason that the step-shortening-induced transient force overshoot was attenuated following exchange of pseudo-phosphorylated cTnI molecules due to increased cooperative deactivation of the thin filaments. It bears mentioning that cTnI PKA phosphorylation did not appear to augment stretch activation in mouse permeabilized myocardial preparations (26). However, the design of that study did not examine cTnI phosphorylation status in isolation from cMyBP-C effects. Another interpretation is that cTnI phosphorylation effects may depend on species variations in troponin molecules and/or manifest only in the presence of slow myosin heavy chain.
Taken together, these results suggest that MyBP-C and its phosphorylation state regulates stretch activation by a combination of modifying cross-bridge cycling kinetics and recruitment of cross bridges. TnI and its phosphorylation state appears to regulate stretch activation by a combination of redundant and synergistic biophysical mechanisms, which may complement each other to tune beat-to-beat myocardial output demand. This complementary mechanism could offer an advantage for targeting phosphorylation of cTnI tyrosine 26 for more precise cardiac contractility improvements, especially since PKA phosphorylates numerous cardiac proteins (47–51), many of which have a propensity for cardiac arrhythmias.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute (Grants R01-HL148785 to K.S.M and R01 HL114940 to B.J.B.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.C.R., L.M.H., and K.S.M. conceived and designed research; J.C.R., L.M.H., B.B., and K.S.M. performed experiments; J.C.R., L.M.H., and K.S.M. analyzed data; J.C.R., L.M.H., and K.S.M. interpreted results of experiments; J.C.R., L.M.H., and K.S.M. prepared figures; J.C.R., L.M.H., and K.S.M. drafted manuscript; J.C.R., L.M.H., B.B., and K.S.M. edited and revised manuscript; J.C.R., L.M.H., B.B., and K.S.M. approved final version of manuscript.
REFERENCES
- 1. Pringle JW. The excitation and contraction of the flight muscles of insects. J Physiol 108: 226–232, 1949. doi: 10.1113/jphysiol.1949.sp004326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pringle JW. The Croonian Lecture, 1977. Stretch activation of muscle: function and mechanism. Proc R Soc Lond B Biol Sci 201: 107–130, 1978. doi: 10.1098/rspb.1978.0035. [DOI] [PubMed] [Google Scholar]
- 3. Perz-Edwards RJ, Irving TC, Baumann BAJ, Gore D, Hutchinson DC, Kržič U, Porter RL, Ward AB, Reedy MK. X-ray diffraction evidence for myosin-troponin connections and tropomyosin movement during stretch activation of insect flight muscle. Proc Natl Acad Sci USA 108: 120–125, 2011. doi: 10.1073/pnas.1014599107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bullard B, Pastore A. Regulating the contraction of insect flight muscle. J Muscle Res Cell Motil 32: 303–313, 2011. doi: 10.1007/s10974-011-9278-1. [DOI] [PubMed] [Google Scholar]
- 5. Josephson RK, Malamud JG, Stokes DR. Asynchronous muscle: a primer. J Exp Biol 203: 2713–2722, 2000. doi: 10.1242/jeb.203.18.2713. [DOI] [PubMed] [Google Scholar]
- 6. Linari M, Reedy MK, Reedy MC, Lombardi V, Piazzesi G. Ca-activation and stretch-activation in insect flight muscle. Biophys J 87: 1101–1111, 2004. doi: 10.1529/biophysj.103.037374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vemuri R, Lankford EB, Poetter K, Hassanzadeh S, Takeda K, Yu ZX, Ferrans VJ, Epstein ND. The stretch-activation response may be critical to the proper functioning of the mammalian heart. Proc Natl Acad Sci USA 96: 1048–1053, 1999. doi: 10.1073/pnas.96.3.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bullard B, Pastore A. Through thick and thin: dual regulation of insect flight muscle and cardiac muscle compared. J Muscle Res Cell Motil 40: 99–110, 2019. doi: 10.1007/s10974-019-09536-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Epstein ND, Davis JS. When is a fly in the ointment a solution and not a problem? Circ Res 98: 1110–1112, 2006. doi: 10.1161/01.RES.0000223888.99864.3c. [DOI] [PubMed] [Google Scholar]
- 10. Stelzer JE, Norman HS, Chen PP, Patel JR, Moss RL. Transmural variation in myosin heavy chain isoform expression modulates the timing of myocardial force generation in porcine left ventricle. J Physiol 586: 5203–5214, 2008. doi: 10.1113/jphysiol.2008.160390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Davis JS, Hassanzadeh S, Winitsky S, Lin H, Satorius C, Vemuri R, Aletras AH, Wen H, Epstein ND. The overall pattern of cardiac contraction depends on a spatial gradient of myosin regulatory light chain phosphorylation. Cell 107: 631–641, 2001. doi: 10.1016/S0092-8674(01)00586-4. [DOI] [PubMed] [Google Scholar]
- 12. Sheikh F, Ouyang K, Campbell SG, Lyon RC, Chuang J, Fitzsimons D, Tangney J, Hidalgo CG, Chung CS, Cheng H, Dalton ND, Gu Y, Kasahara H, Ghassemian M, Omens JH, Peterson KL, Granzier HL, Moss RL, McCulloch AD, Chen J. Mouse and computational models link Mlc2v dephosphorylation to altered myosin kinetics in early cardiac disease. J Clin Invest 122: 1209–1221, 2012. doi: 10.1172/JCI61134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cazorla O, Le Guennec J-Y, White E. Length-tension relationships of subepicardial and sub-endocardial single ventricular myocytes from rat and ferret hearts. J Mol Cell Cardiol 32: 735–744, 2000. doi: 10.1006/jmcc.2000.1115. [DOI] [PubMed] [Google Scholar]
- 14. Chung CS, Hoopes CW, Campbell KS. Myocardial relaxation is accelerated by fast stretch, not reduced afterload. J Mol Cell Cardiol 103: 65–73, 2017. doi: 10.1016/j.yjmcc.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chung CS. How myofilament strain and strain rate lead the dance of the cardiac cycle. Arch Biochem Biophys 664: 62–67, 2019. doi: 10.1016/j.abb.2019.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Campbell KB, Chandra M. Functions of stretch activation in heart muscle. J Gen Physiol 127: 89–94, 2006. doi: 10.1085/jgp.200509483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schache AG, Dorn TW, Wrigley TV, Brown NA, Pandy MG. Stretch and activation of the human biarticular hamstrings across a range of running speeds. Eur J Appl Physiol 113: 2813–2828, 2013. doi: 10.1007/s00421-013-2713-9. [DOI] [PubMed] [Google Scholar]
- 18. Geist Hauserman J, Stavusis J, Joca HC, Robinett JC, Hanft L, Vandermeulen J, Zhao R, Stains JP, Konstantopoulos K, McDonald KS, Ward C, Kontrogianni-Konstantopoulos A. Sarcomeric deficits underlie MYBPC1-associated myopathy with myogenic tremor. JCI Insight 6: e147612, 2021. doi: 10.1172/jci.insight.147612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Straight CR, Bell KM, Slosberg JN, Miller MS, Swank DM. A myosin-based mechanism for stretch activation and its possible role revealed by varying phosphate concentration in fast and slow mouse skeletal muscle fibers. Am J Physiol Cell Physiol 317: C1143–C1152, 2019. doi: 10.1152/ajpcell.00206.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Steiger GJ. Stretch activation and myogenic oscillation of isolated contractile structures of heart muscle. Pflugers Arch 330: 347–361, 1971. doi: 10.1007/BF00588586. [DOI] [PubMed] [Google Scholar]
- 21. Stelzer JE, Larsson L, Fitzsimons DP, Moss RL. Activation dependence of stretch activation in mouse skinned myocardium: implications for ventricular function. J Gen Physiol 127: 95–107, 2006. doi: 10.1085/jgp.200509432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stelzer JE, Dunning SB, Moss RL. Ablation of cardiac myosin-binding protein-c accelerates stretch activation in murine skinned myocardium. Circ Res 98: 1212–1218, 2006. doi: 10.1161/01.RES.0000219863.94390.ce. [DOI] [PubMed] [Google Scholar]
- 23. Stelzer JE, Brickson SL, Locher MR, Moss RL. Role of myosin heavy chain composition in the stretch activation response of rat myocardium. J Physiol 579: 161–173, 2007. doi: 10.1113/jphysiol.2006.119719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stelzer JE, Patel JR, Moss RL. Acceleration of stretch activation in murine myocardium due to phosphorylation of myosin regulatory light chain. J Gen Physiol 128: 261–272, 2006. doi: 10.1085/jgp.200609547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stelzer JE, Moss RL. Contributions of stretch activation to length-dependent contraction in murine myocardium. J Gen Physiol 128: 461–471, 2006. doi: 10.1085/jgp.200609634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stelzer JE, Patel JR, Moss RL. Protein kinase a-mediated acceleration of the stretch activation response in murine skinned myocardium is eliminated by ablation of cMyBP-C. Circ Res 99: 884–890, 2006. doi: 10.1161/01.RES.0000245191.34690.66. [DOI] [PubMed] [Google Scholar]
- 27. Hanft LM, Cornell TD, McDonald CA, Rovetto MJ, Emter CA, McDonald KS. Molecule specific effects of PKA-mediated phosphorylation on rat isolated heart and cardiac myofibrillar function. Arch Biochem Biophys 601: 22–31, 2016. doi: 10.1016/j.abb.2016.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang P, Kirk JA, Ji W, dos Remedios CG, Kass DA, Van Eyk JE, Murphy AM. Multiple reaction monitoring to identify site-specific troponin I phosphorylated residues in the failing human heart. Circulation 126: 1828–1837, 2012. doi: 10.1161/CIRCULATIONAHA.112.096388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Salhi HE, Walton SD, Hassel NC, Brundage EA, de Tombe PP, Janssen PML, Davis JP, Biesiadecki BJ. Cardiac troponin I tyrosine 26 phosphorylation decreases myofilament Ca2+ sensitivity and accelerates deactivation. J Mol Cell Cardiol 76: 257–264, 2014. doi: 10.1016/j.yjmcc.2014.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kobayashi T, Solaro RJ. Increased Ca2 affinity of cardiac thin filaments reconstituted with cardiomyopathy-related mutant cardiac troponin I. J Biol Chem 281: 13471–13477, 2006. doi: 10.1074/jbc.M509561200. [DOI] [PubMed] [Google Scholar]
- 31. Nixon BR, Liu B, Scellini B, Tesi C, Piroddi N, Ogut O, John Solaro R, Ziolo MT, Janssen PML, Davis JP, Poggesi C, Biesiadecki BJ. Tropomyosin Ser-283 pseudo-phosphorylation slows myofibril relaxation. Arch Biochem Biophys 535: 30–38, 2013. doi: 10.1016/j.abb.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nixon BR, Thawornkaiwong A, Jin J, Brundage EA, Little SC, Davis JP, Solaro RJ, Biesiadecki BJ. AMP-activated protein kinase phosphorylates cardiac troponin I at Ser-150 to increase myofilament calcium sensitivity and blunt PKA-dependent function. J Biol Chem 287: 19136–19147, 2012. doi: 10.1074/jbc.M111.323048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Biesiadecki BJ, Kobayashi T, Walker JS, Solaro RJ, de Tombe PP. The troponin C G159D mutation blunts myofilament desensitization induced by troponin I Ser23/24 phosphorylation. Circ Res 100: 1486–1493, 2007. doi: 10.1161/01.RES.0000267744.92677.7f. [DOI] [PubMed] [Google Scholar]
- 34. McDonald KS. Ca2+ dependence of loaded shortening in rat skinned cardiac myocytes and skeletal muscle fibers. J Physiol 525: 169–181, 2000. doi: 10.1111/j.1469-7793.2000.00169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ackermann MA, Kontrogianni-Konstantopoulos A. Myosin binding protein-C slow is a novel substrate for protein kinase A (PKA) and C (PKC) in skeletal muscle. J Proteome Res 10: 4547–4555, 2011. doi: 10.1021/pr200355w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Robinett JC, Hanft LM, Geist J, Kontrogianni-Konstantopoulos A, McDonald KS. Regulation of myofilament force and loaded shortening by skeletal myosin binding protein C. J Gen Physiol 151: 645–659, 2019. doi: 10.1085/jgp.201812200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gresham KS, Mamidi R, Stelzer JE. The contribution of cardiac myosin binding protein-C ser282 phosphorylation to the rate of force generation and in vivo cardiac contractility. J Physiol 592: 3747–3765, 2014. doi: 10.1113/jphysiol.2014.276022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mamidi R, Gresham KS, Verma S, Stelzer JE. Cardiac myosin binding protein-C phosphorylation modulates myofilament length-dependent activation. Front Physiol 7: 38, 2016. doi: 10.3389/fphys.2016.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Patel JR, Fitzsimons DP, Buck SH, Muthuchamy M, Wieczorek DF, Moss RL. PKA accelerates rate of force development in murine skinned myocardium expressing α- or β-tropomyosin. Am J Physiol Heart Circ Physiol 280: H2732–H2739, 2001. doi: 10.1152/ajpheart.2001.280.6.H2732. [DOI] [PubMed] [Google Scholar]
- 40. Hanft LM, Biesiadecki BJ, McDonald KS. Length dependence of striated muscle force generation is controlled by phosphorylation of cTnI at serines 23/24. J Physiol 591: 4535–4547, 2013. doi: 10.1113/jphysiol.2013.258400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Stelzer JE, Patel JR, Walker JW, Moss RL. Differential roles of cardiac myosin-binding protein C and cardiac troponin I in the myofibrillar force responses to protein kinase A phosphorylation. Circ Res 101: 503–511, 2007. doi: 10.1161/CIRCRESAHA.107.153650. [DOI] [PubMed] [Google Scholar]
- 42. Hu Z, Taylor DW, Edwards RJ, Taylor KA. Coupling between myosin head conformation and the thick filament backbone structure. J Struct Biol 200: 334–342, 2017. doi: 10.1016/j.jsb.2017.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Brenner B. Effect of calcium on cross-bridge turnover kinetics in skinned single rabbit psoas fibers: implications for regulation of muscle contraction. Proc Natl Acad Sci USA 85: 3265–3269, 1988. doi: 10.1073/pnas.85.9.3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Metzger JM, Moss RL. Calcium-sensitive cross-bridge transitions in mammalian fast and slow skeletal muscle fibers. Science 247: 1088–1090, 1990. doi: 10.1126/science.2309121. [DOI] [PubMed] [Google Scholar]
- 45. Nixon BR, Walton SD, Zhang B, Brundage EA, Little SC, Ziolo MT, Davis JP, Biesiadecki BJ. Combined troponin I Ser-150 and Ser-23/24 phosphorylation sustains thin filament Ca(2+) sensitivity and accelerates deactivation in an acidic environment. J Mol Cell Cardiol 72: 177–185, 2014. doi: 10.1016/j.yjmcc.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lundby A, Lage K, Weinert BT, Bekker-Jensen DB, Secher A, Skovgaard T, Kelstrup CD, Dmytriyev A, Choudhary C, Lundby C, Olsen JV. Proteomic analysis of lysine acetylation sites in rat tissues reveals organ specificity and subcellular patterns. Cell Rep 2: 419–431, 2012. doi: 10.1016/j.celrep.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fukuda N, Wu Y, Nair P, Granzier HL. Phosphorylation of titin modulates passive stiffness of cardiac muscle in a titin isoform-dependent manner. J Gen Physiol 125: 257–271, 2005. doi: 10.1085/jgp.200409177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yamasaki R, Wu Y, McNabb M, Greaser ML, Labeit D, Granzier H. Protein kinase A phosphorylates titin's cardiac-specific N2B domain and reduces passive tension in rat cardiac myocytes. Circ Res 90: 1181–1188, 2002. doi: 10.1161/01.res.0000021115.24712.99. [DOI] [PubMed] [Google Scholar]
- 49. Metzger JM, Greaser ML, Moss RL. Variations in cross-bridge attachment rate and tension with phosphorylation of myosin in mammalian skinned skeletal muscle fibers: implications for twitch potentiation in intact muscle. J Gen Physiol 93: 855–883, 1989. doi: 10.1085/jgp.93.5.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sweeney HL, Stull JT. Phosphorylation of myosin in permeabilized mammalian cardiac and skeletal muscle cells. Am J Physiol Cell Physiol 250: C657–C660, 1986. doi: 10.1152/ajpcell.1986.250.4.C657. [DOI] [PubMed] [Google Scholar]
- 51. Wray HL, Gray RR, Olsson RA. Cyclic adenosine 3',5'-monophosphate-stimulated protein kinase and a substrate associated with cardiac sarcoplasmic reticulum. J Biol Chem 248: 1496–1498, 1973. doi: 10.1016/S0021-9258(19)44327-5. [DOI] [PubMed] [Google Scholar]