Keywords: epidermis, keratinocytes, melanocytes, neuropeptides, neurohormones
Abstract
The skin, which is comprised of the epidermis, dermis, and subcutaneous tissue, is the largest organ in the human body and it plays a crucial role in the regulation of the body’s homeostasis. These functions are regulated by local neuroendocrine and immune systems with a plethora of signaling molecules produced by resident and immune cells. In addition, neurotransmitters, endocrine factors, neuropeptides, and cytokines released from nerve endings play a central role in the skin's responses to stress. These molecules act on the corresponding receptors in an intra-, juxta-, para-, or autocrine fashion. The epidermis as the outer most component of skin forms a barrier directly protecting against environmental stressors. This protection is assured by an intrinsic keratinocyte differentiation program, pigmentary system, and local nervous, immune, endocrine, and microbiome elements. These constituents communicate cross-functionally among themselves and with corresponding systems in the dermis and hypodermis to secure the basic epidermal functions to maintain local (skin) and global (systemic) homeostasis. The neurohormonal mediators and cytokines used in these communications regulate physiological skin functions separately or in concert. Disturbances in the functions in these systems lead to cutaneous pathology that includes inflammatory (i.e., psoriasis, allergic, or atopic dermatitis, etc.) and keratinocytic hyperproliferative disorders (i.e., seborrheic and solar keratoses), dysfunction of adnexal structure (i.e., hair follicles, eccrine, and sebaceous glands), hypersensitivity reactions, pigmentary disorders (vitiligo, melasma, and hypo- or hyperpigmentary responses), premature aging, and malignancies (melanoma and nonmelanoma skin cancers). These cellular, molecular, and neural components preserve skin integrity and protect against skin pathologies and can act as “messengers of the skin” to the central organs, all to preserve organismal survival.
THE SKIN IN A NUTSHELL
The skin is the largest organ in the body and is strategically located as the interface between external and internal environments. As such, it plays a crucial role in the regulation and preservation of the body’s homeostasis (1–5). It is composed of the epidermis, dermis, and hypodermis, which are, respectively, of ectodermal and mesodermal origin (6, 7). It is a multifunctional self-regulating organ the functional integrity of which is sustained by the anatomical connections between the three compartments and the mutual signaling between them and other skin cohabitants (1, 3, 4). The skin protects against physical and chemical insults, invasion by microorganisms, injury caused by mechanical stress, retention of body fluids, regulation of body temperature, storage and production of energy, insulation against temperature variation and mechanical insults, sensation, and production of vitamin D, folate, and other factors. In addition, it has immune functions, is important for temperature regulation in the body, and is a source of social communication and camouflage (1, 4, 7–15).
The epidermis represents the outermost layer of the skin, which is predominantly formed by proliferating and differentiating keratinocytes forming, accordingly, stratum basale, suprabasale, spinosum, granulosum, and, most superficially, the acellular stratum corneum and stratum lucidum (1, 3). The latter is present only on acral skin. The epidermis contains melanocytes and Merkel cells of neural crest origin, and Langerhans cells of mesodermal lineage (6, 7). Having no blood supply, the epidermis is nourished through the diffusion of nutrients from the dermis through the basement membrane, which separates it from the dermis. The epidermis is oxygenated by diffusion from the air. It also contains sensory nerves network that can even penetrate to the stratum corneum (16–19). This is in addition to classical sensory functions including the detection of pain, temperature, touch, and vibration (20–22).
The dermis is divided into papillary (adjacent to the epidermis) and reticular components. It comprises fibroblasts/fibrocytes, myofibroblasts, resident and circulating immune cells including mast cells. It contains hair follicles, sebaceous and sweat glands, smooth muscle, nerve bundles and ending, and superficial vascular plexuses and lymphatics. Adjacent to the dermis is the lowermost component of the skin, the hypodermis, which is predominantly composed of adipocytes with other components including fibroblasts, histiocytes, nerve bundles, and deep vascular plexuses and lymphatics. It also contains distal aspect of anagen hair follicles (23).
AN OVERVIEW OF THE NEUROENDOCRINE FUNCTIONS OF THE SKIN
Neuro-Immuno-Endocrinology of the Skin
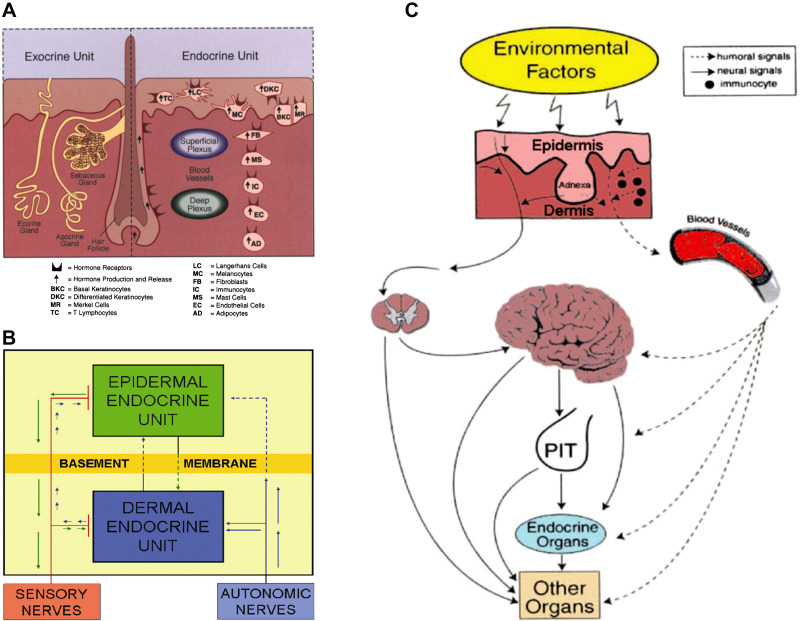
It has been over two decades since the formulation of the concept that the skin acts as a neuroendocrine organ (24) equipped with corticotropin-releasing hormone (CRH) and proopiomelanocortin (POMC) signaling systems to regulate skin responses to stress (25). A myriad of papers has confirmed the original hypotheses as reviewed in Refs. 2, 4, 26–44. This concept is consistent with other theories on neuroendocrine self-regulating pathways in peripheral organs, including the immune system (28, 45–51). The skin cells produce classic hormones and neurotransmitters including neuropeptides that act on their receptors through para-, auto-, and intracrine mechanisms, which in turn regulate local homeostasis (Fig. 1A). These neuroendocrine mediators with their receptors are organized into epidermal and dermal (including hypodermis) units, which communicate with each other (Fig. 1B) and systemically through humoral and neural pathways (Fig. 1C) to induce local homeostatic self-regulating activities, to preserve and maintain the skin structural and functional integrity under environmental pressure and, by inference, participate in systemic homeostasis (24, 25). Examples of such neuropeptides expressed in the skin are CRH (52–60), urocortins (58, 61), and POMC-derived peptides (44, 62–68). In addition, immune cells primed in the skin by neuroendocrine mediators released by skin cells or neurotransmitters released locally from nerve endings serve as additional cutaneous neuroendocrine messengers regulating systemic homeostasis (24, 28). Finally, neuromediators can be produced by the skin microbiome (69).
Figure 1.
Neuroendocrine organization of the skin. A: components of the exocrine and endocrine units of the skin produce hormones, neural mediators, and cytokines that act upon the corresponding receptors expressed on skin cells. B: the communication between epidermal and dermal neuroendocrine units and the systemic level. C: the cutaneous neuroendocrine system communicates with the central nervous, endocrine, and immune system or other organs through humoral or neural pathways. Reprinted from Ref. 24 with the permission of the publisher.
The elements of the nervous, immune, and endocrine systems present in the skin can be organized into different regulatory axes, circuits, and pathways, which not only regulate skin’s homeostasis but due to its rich innervation and vasculature can regulate other body organs, including the brain (12, 24). Examples include the cutaneous equivalent of the hypothalamus-pituitary-adrenal axis (HPA) (70–75), which is organized in a similar manner as the central HPA (76–82), with some local specificity (78) to be discussed later. The skin also contains its own cholinergic (83, 84) and catecholaminergic (84–88) systems with l-tyrosine and l-DOPA (L-3,4-dihydroxyphenylalanine) also functioning as bioregulators (89–91). It contains local and autonomous serotoninergic (92–98), melatoninergic (92, 99–106), and cannabinoid (33, 107–109) systems.
Small molecules synthesized in skin play important roles not only for epidermal homeostasis, but also for cutaneous inflammation, sensory perception, and vascular permeability. For example, nitric oxide (NO) synthesized by epidermal keratinocytes play a crucial role in endothelial cells by regulating vascular permeability in the skin, and ATP and other chemical factors released from keratinocytes contribute to cutaneous sensory and inflammatory responses (22, 110–113). The skin also expresses functional elements of the hypothalamic-pituitary-thyroid axis (114–119) and is a target for prolactin and growth hormone (GH) (120–122). It also produces and responds to leptin (32, 123). Importantly, skin is a recognized steroidogenic organ (34, 124) and can synthesize estrogens, androgens, and corticosteroids including corticosterone and cortisol in a highly organized fashion (27, 28, 34, 51, 73–75, 125–142), which has significant implications for skin immune functions. This can be started de novo by CYP11A1 expressed in skin cells (70, 143) with implications for the production of Δ7-steroids (144, 145) and generation of novel secosteroidal metabolites (15, 146, 147). Finally, it also produces calcitonin gene-related peptide (CGRP), corticosteroids, gonadotrophins, hemokinin-A (HKA), leptin, neurotensin (NT), neurotrophins, parathyroid hormone (PTH), PTH-related peptide (PTHRP), pituitary adenylate cyclase-activating peptide (PACAP), calcitonin gene-related peptide (CGRP), substance P (SP) neuropeptide Y (NPY), and vasoactive intestinal peptide (VIP) among other neuropeptides (24, 29, 32, 40, 148–166).
Cutaneous HPA Axis
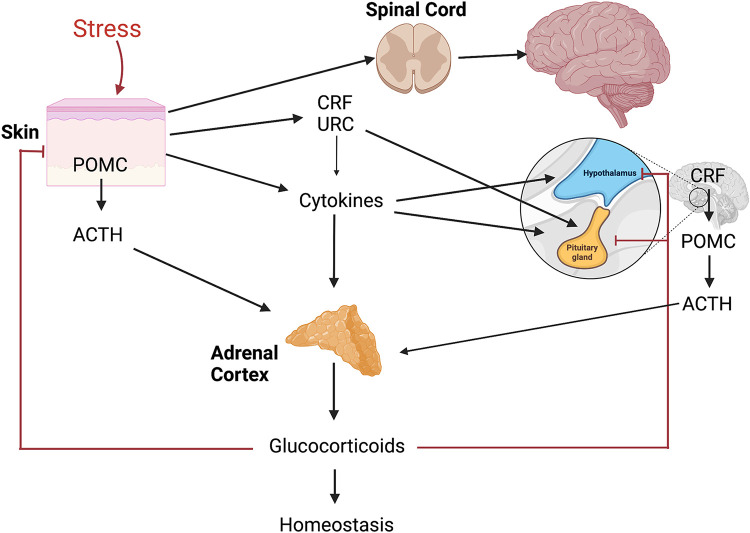
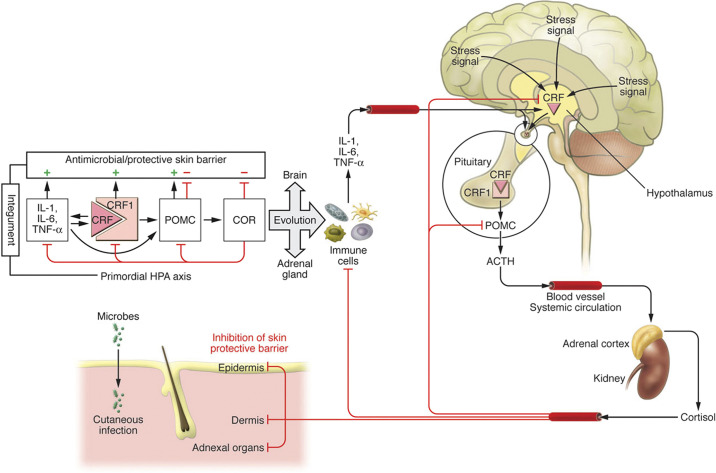
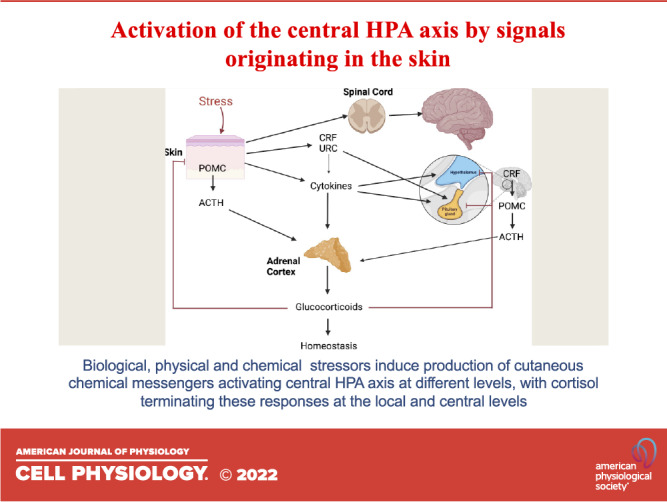
The HPA is one of the most important neuroendocrine axes regulating responses to stress (Fig. 2) (79, 82) and its homologue is also expressed locally to coordinate skin responses to stress (71, 72). However, there are differences and similarities between the central and cutaneous HPA (cHPA). Although the central HPA axis involves interactions between different organs (brain, pituitary, and adrenals) organized along the structure CRH→CRHR1→POMC→ACTH→COR (cortisol/corticosterone) with input from cytokines (IL-1, IL-6, TNF), the cutaneous HPA (cHPA) involves the interaction between the epidermis, dermis, hypodermis, or the cells that are in close contact. This close contact and lack of significant spatial separation lead to several scenarios in which elements of the cutaneous HPA can also operate in a coordinated or independent manner in para-, auto-, and intracrine fashions, leading to various phenotypic effects and possible pathology when feedback mechanisms, characteristic for the central HPA, are disconnected (5, 78, 125, 167). The key element is the activation of the CRHR1 by either CRH or urocortin with follow-up signaling cascade leading to production and release of POMC peptides including ACTH with their downstream effects that include corticosteroidogenesis. Different biological and physicochemical stressors such as ultraviolet radiation (UVR) and stress-induced cytokines can activate the entire axis, CRH→CRHR1→POMC→ACTH→COR, or its proximal, intermediate, and distal elements which are cell type or compartment dependent and may lead to opposing biological effects. For example, the direct CRH effect in the periphery (168–170) including skin will be proinflammatory (32, 39, 78, 171–173), which is exactly the opposite as that which occurs in the central HPA, unless attenuated by downstream POMC-derived peptides or glucocorticosteroids (72, 125, 174). In skin, the number of the elements and regulators of cHPA axis includes endorphins and MSH peptides (25, 44, 175–178) as wells as ACTH, CRHR2, and alternatively spliced isoforms of CRHR1 (59, 179–184). These differences with central organization could be secondary to the origin of the primordial HPA axis, which would have developed first in the integument for organismal defense against environmental stressors and pathogens (Fig. 3) (185).
Figure 2.
The organization of central hypothalamus-pituitary-adrenal (HPA) axis that can be activated at different levels by stress originating in the skin. CRH, corticotropin releasing hormone; POMC, proopiomelanocortin; URC, urocortin. Figure was created using BioRender.
Figure 3.
The evolutionary origin of the hypothalamus-pituitary-adrenal (HPA) axis. Right: organization of the central HPA axis; left: primordial integumental HPA axis. CRH, corticotropin releasing hormone; POMC, proopiomelanocortin. Reprinted from Ref. 185 with the permission of the publisher.
The cHPA plays an important role in the regulation of biological functions of the skin (26, 75, 78, 139, 186, 187). Its deregulation or pathological overexpression or downregulation of its functional elements can lead to skin pathology including inflammatory disorders (32, 36, 130, 132, 188–196), wound healing (134, 197–199), skin aging (31, 35), or skin cancers (200–204). Importantly, skin factors can activate the central HPA after cutaneous stressors such as ultraviolet B (UVB) (205). UVB can also trigger other neuroendocrine mechanisms affecting the body’s homeostasis (206–209), which has recently been discussed (12, 210, 211).
Nerve Fibers
An important element of the skin neuroendocrine system is its innervation composed of networks of somatosensory and autonomic nerve fibers (20, 111) terminating in each skin layer, which contributes to the physiological skin functions or development of disease (2, 16, 24, 26, 29, 32, 37, 40, 78, 155, 156, 188, 212, 213). This is in addition to detecting sensory inputs such as pain, touch, temperature, or vibration (20, 21). Cutaneous nerve fibers derived from neurons localized either in the dorsal root ganglia (DRG), the trigeminal ganglion (face and neck), or autonomic nerve centers (19, 20, 37). Depending on their nature they can release neurotransmitters including amines, neuropeptides, neurotrophins, bioactive lipids, and even gases to facilitate neural transmission through the synapses and to act on corresponding receptors present on effector skin cells. Thus, cutaneous nerve fibers can release classical neurotransmitters such as epinephrine and nonepinephrine, dopamine, acetylcholine (ACh), γ-aminobutyric acid (GABA), glutamate, serotonin, and perhaps melatonin; neuropeptides including CRH, urocortins, substance P (SP), PACAP, calcitonin gene-related peptide (CGRP), galanin (GAL), somatostatin (SOM), vasoactive intestinal peptide (VIP), neuropeptide Y (NPY), α-, β-, and γ-MSH, β-endorphin, dynorphins (DYN), enkephalins; bioactive lipids like cannabinoids; gases: NO, CO; and neurotrophins: like nerve growth factor (NGF). The same molecules released not only by cutaneous nerves but depending on the context, are also produced by resident skin cells and circulating immune cells to act as neurotransmitters, hormones, cytokines, or biological modulators (2, 16, 24, 25, 30, 37, 78, 97, 162, 188, 214, 215). A good example of such a molecule is CRH which in the skin is released from cutaneous nerve fibers as well as produced by keratinocytes, melanocytes, fibroblasts, mast cells, and other skin immune cells (53, 56, 58, 59, 216). The important feature of sensory nerve fibers is the ability to transmit signals through orthodromic (afferently to the spinal cord and brain), and antidromic (efferently, releasing neurotransmitters into effector skin cells), which is important for homeostatic communication between different skin components (24) (Fig. 1B and Fig. 4). Examples of mediators used by sensory nerves in the skin including epidermis include CRH, NPY, SP, VIP, GAL, CGRP, NGF, serotonin, β-endorphin, and other opioids and MSH peptides (37, 155, 217, 218). Cutaneous sensory receptors can be activated by physicochemical and biological factors such as UVR, visible light, pollutants, changes in proton concentrations, reactive oxygen species, toxins, allergens, and mechanical stimuli such as trauma, touch, or vibration (12, 16, 20, 37, 219, 220), This can lead to orthodromic and antidromic signals to regulate skin and systemic homeostasis (12, 24, 221).
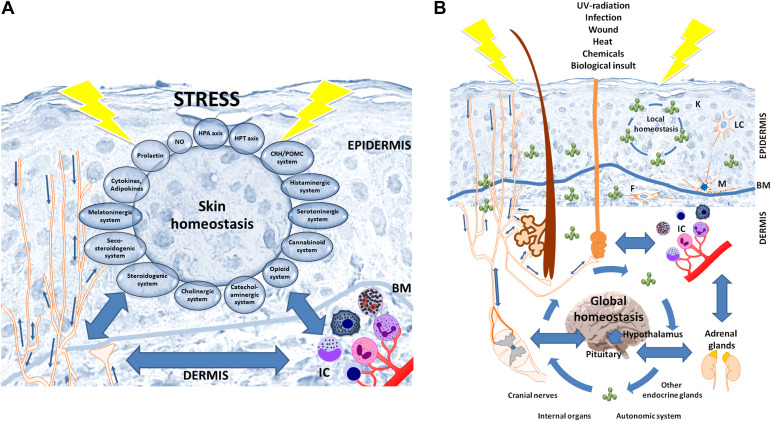
Figure 4.
Skin neuroimmunoendocrine system encompassing the epidermal neuroendocrine system (A), and interactions with the central systems and organs (B) in a coordinated stress response mode. A: epidermal cells are not only are sensitive to neurohormonal regulation but also produce elements of hypothalamus-pituitary-adrenal (HPA) or hypothalamus-pituitary-thyroid (HPT) axes, other neuropeptides, biogenic amines, serotonin, melatonin, nitric oxide, opioids, cannabinoids, catecholamines, acetylcholine, steroids, secosteroids, neurotrophins, and cytokines. B: the skin neuroimmunoendocrine system can activate the central responses with direct homeostatic, metabolic, and phenotypic consequences. The constant exchange of neuroendocrine mediators between skin and other organs is responsible for the maintenance of local and global homeostasis. BM, basement membrane; F, fibrocytes/fibroblasts; IC, immune cells; K, keratinocytes; LC, Langerhans cells; M, melanocytes. Reprinted from Ref. 12 with the permission of the publisher.
NEUROENDOCRINOLOGY OF THE EPIDERMIS
Epidermis as a Unique Neuroendocrine Unit
Being directly exposed to the environment, the epidermis not only serves as a physical barrier against environmental stressors, but it also senses and responds to the environmental signals through a sophisticated stress response system with a computing capability to regulate local and systemic homeostasis involving communication with the central nervous and endocrine systems (Fig. 4) (2, 4, 12, 24, 26, 78). Neurohormonal mediators and cytokines produced in the epidermis are predominantly used by the neuroendocrine system in that location since epidermal soluble factors would need to travel through the basement membrane and diffuse efficiently without degradation through an acellular dermal matrix component to reach dermal, hypodermal, or systemic components unless there was a specific transport system for these neuroendocrine mediators (24). Therefore, their concerted activity is predominantly to regulate epidermal functions, and forming an autonomous epidermal neuroendocrine system, which can communicate with dermal or hypodermal systems via nerve endings, unless there is sufficiently intense stress allowing the release of large amounts of mediators that could diffuse to other components of the skin (Fig. 4) (24, 25). In addition, the epidermal surface serves as an ecosystem for a variety of microorganisms forming the microbiome (69, 222, 223) that can affect skin physiology in a positive or negative manner. This microbiome keeps the skin immune system in equilibrium with its metabolic activity involving the various functions of the skin (69, 223). It can also regulate the neuroendocrine activity of the skin (69).
Epidermal cholinergic (83, 84, 224, 225), catecholaminergic that include dopamine and adrenergic receptors (84–86, 88, 135, 226–230), serotoninergic (97), melatoninergic (101), canabinoidogenic (107, 109), steroidogenic (27, 34, 124, 126, 231–233), vitamin D (15, 234, 235), nitric oxide (NO) (110), mitochondrial ATP (111), purinergic P2X (236–239), γ-aminobutyric acid (GABA) (240, 241), glutamatergic (242–245), parathyroid hormone, and calcitonin and related peptides (157, 160, 161, 214), and other local neuropeptide signaling systems (26, 29, 156, 157, 214, 218, 241, 246); all have also been cited in several reviews listed earlier (Fig. 4A). All of these neuromodulators are produced by keratinocytes and melanocytes and to some degree by Langerhans cells or they are released by sensory nerve endings entering the epidermis. They act on the corresponding membrane-bound receptors, which depending on the chemical structure and target receptor, stimulate downstream signaling systems such as cyclic adenosine monophosphate (cAMP), cyclic guanosine monophosphate (cGMP), inositol trisphosphate (IP3), diacylglycerol (DAG), or calcium (Ca+2) or are coupled with ion channels. Different locally produced steroids and secosteroids act on corresponding receptors to regulate epidermal functions (8, 34, 126, 198, 234, 247). They act in a para-, juxta-, endo-, or autocrine fashions depending on the distance from the target cell. With the exception of Langerhans cells, human epidermis does not have other immune cells under physiological conditions. However, keratinocytes and melanocytes act as epidermal immunocompetent cells producing and releasing cytokines in response to different stimuli including allergens (11, 14, 78, 133, 177, 248–251). In the epidermis, melanocytes act as sensory cells, detecting environmental changes directly or indirectly through extensive dendrite processes and then after computation sending regulatory signals to multiple target to counteract the environmental damage through mechanisms described previously(12, 252, 253).
The epidermal neuroendocrine system can be affected by chemical, biological, and physical factors. As relates to the different wave length of solar radiation including visible spectrum and ultraviolet B (UVB) and A (UVA), each will have different mechanisms of action with UVB acting through chromophores and UVA or visible light predominantly through reactive oxygen species (ROS) or other mechanisms (11, 12, 235, 254). This raises the question whether there are specific photoreceptors in the skin. This concept was first formulated in relation to regulation of melanin pigmentation and was further advanced by Pawelek group (220, 255). Previous reports demonstrated that rhodopsin, opsin, and the biochemical cascade for the signal transduction were expressed in the epidermal keratinocytes (256, 257). Recent data also indicate that opsins can act as receptors for blue light (258, 259) or UVA (260, 261). The aforementioned advancements represent confirmation of original studies on light detection by melanophores (262, 263).
CRH SIGNALING
CRH and related peptides are produced by human keratinocytes and melanocytes under physiological and pathological conditions or after stimulation by UVB or microbial antigens (52, 54, 58, 59, 61, 139, 195, 201, 264–267) and by active forms of vitamin D (268). Also, their receptors including CRHR1 and CRHR2 are expressed on human keratinocytes and melanocytes affected by their level of differentiation (59, 171, 179, 181, 216, 264, 269–274). Both CRH and related peptides including urocortin 1, 2, and 3 as well as CRHR1 and CRHR2 are also expressed on follicular keratinocytes and melanocytes (57, 58, 61, 75, 264, 266, 270, 274), which are mentioned here because of their direct connection between the epidermis and outer rout sheath of the hair follicle. It must be noted that CRH and urocortin 1 have a high affinity for CRHR1 with urocortin also having a high affinity for CRHR2 and urocortin 2 having a high affinity only for CRHR2.
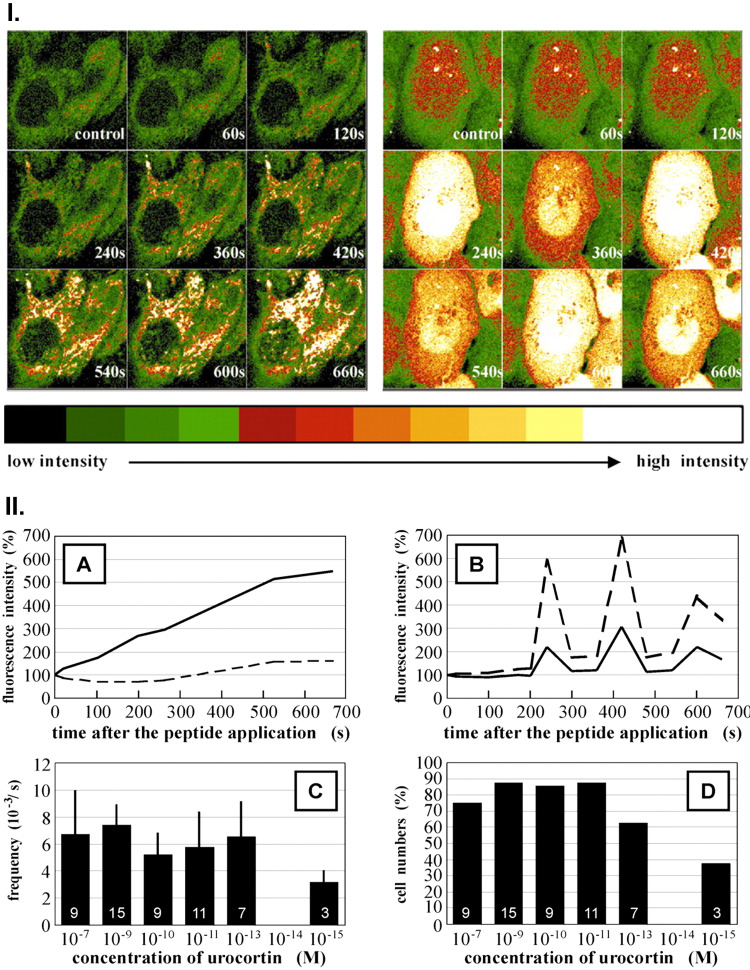
Since the predominant CRH receptor in the human epidermis is CRHR1, with CRHR2 expressed predominantly in the dermal compartment or adnexal structures (56, 58, 78, 171, 266), the discussion will focus on epidermal keratinocytes and melanocytes. Activation of CRHR1 on epidermal cells is coupled to cAMP, IP3, DAG, or Ca+2 signaling (171, 216, 269, 270, 273) with downstream activation of protein kinases A (PKA) and C (PKC) dependent pathways and activator protein-1 including Jun D or cAMP responsive element binding protein (CREB) (171, 272). Ca+2 signaling is caused by Ca influx from the extracellular space, including through voltage-activated Ca+2 ion channels, which are cyclic nucleotide-gated ion channels, and/or through its mobilization from the intracellular stores (216, 269, 271, 273). Interestingly, confocal laser scanning microscopy has shown that in HaCaT keratinocytes CRH induces Ca+2 flux into the cytoplasm, while urocortin induces Ca+2 flux into the nucleus with a remarkable oscillatory effect (273) (Fig. 5).
Figure 5.
Time course of the changes of intracellular Ca2+ after corticotropin-releasing hormone (CRH) or urocortin (URC) stimulation. I): time galleries of fluorescence images of the Ca2+ indicators in the HaCaT cells after stimulation with CRF (10−7 M, left) and urocortin (URC) (10−7 M, right). The intensity range (8 bit) is indicated below the image galleries. II), A and B: time course of the relative fluorescence intensities (unbroken line, cytosol; broken line, nucleus) of the Ca2+ indicators in HaCaT cells after stimulation with CRF (A, 10−7 M) and URC (B, 10−7 M). C: dose-dependent frequency of the oscillations in HaCaT-cells after stimulation with URC (means ± SD). Number of cells recorded (n) is shown in the respective columns. D: dose-dependent numbers of HaCaT cells with oscillations after stimulation with URC (percentage of total number of cells recorded). Number of cells recorded with oscillations (n) is shown in the respective columns. Reprinted from Ref. 273 with the permission of the publisher.
CRHR1 and CRHR2 undergo alternative splicing in epidermal keratinocytes and melanocytes with the production of multiple functionally active isoforms (59, 179–181, 183, 184, 275), consistent with the original studies of Hillhouse and Grammatopoulos in other tissues (276–280). In the skin, the alternative splicing and processing of the CRHR1 are subject to environmental regulation, affected by UVB, phorbol esters, factors raising intracellular cAMP, and cell culture conditions and pathology (58, 179–181, 184). The predominant splice form coupled to PKA and PKC signaling is CRHR1α. Other forms, including β, c, d, e, f, g, and h, were detected with the ones missing transmembrane domain serving as soluble forms potentially sequestering CRH or urocortin while others having part of the transmembrane domain with a predominant intracellular location. These could dimerize with CRHR1α affecting its presentation on the cell surface and signal transduction. Co-expression of CRHR1 isoforms, therefore, modulates the sensitivity of cells to the ligands and influences downstream coupling to G-proteins; it may affect fast mRNA and/or protein turnover or have a decoy receptor function of CRHR1 isoforms (179, 180, 184). In the epidermis, a gradient of CRHR1 is also present with the strongest expression at the basal layer (179).
CRH and CRH-related peptides can regulate proliferation and differentiation programs as well as immune and endocrine activities of keratinocytes and melanocytes through interaction with CRHR1 or CRHR2 receptors (57, 74, 75, 171–174, 186, 265, 266, 270–272, 274, 281–286). Specifically, CRH and related peptides can inhibit normal keratinocytes proliferation with the shape of the inhibition curve determined by the media calcium concentration and cellular context (171, 270, 272, 281, 284). It included inhibition of the G0/1 to the S phase transition of the cell cycle, which was accompanied by an increased expression of cdk inhibitor p16 (Ink4a) protein. The antiproliferative effect was attenuated by inhibition of PKC but not when inhibitors of PKA and MAP kinases were used (272). The cell cycle withdrawal was associated with the induction of keratinocyte differentiation with increased expression of CK1, involucrin, decreased expression of CK14, and increased granularity (272, 284). Therefore, it was proposed that the program, triggered by CRH interaction with CRH-R1, includes induction of a transduction pathway involving the sequential activation of phospholipase C, protein kinase C, activator protein-1 (including Jun D), and p16 (272). As relates to regulation of immune functions CRH enhanced the interferon-γ stimulated expression of the homing cell adhesion molecule (HCAM) and intercellular adhesion molecules and of the human leukocyte antigen DR (HLA-DR) antigen (281), rapidly stimulated IL-1β, IL-6, and TNFα genes expression (172) and stimulated nuclear factor-kappa B (NF-κβ) activity (283). In immortalized HaCaT keratinocytes, the effects were dependent on culture conditions, e.g., in serum-free media CRH stimulated NF-κβ activity and IL-1β (171), whereas in serum-containing media CRH inhibited NF-κβ activity and IL-1β but stimulated IL-6 and IL-11 (282, 286). Thus, the local CRH effects on keratinocytes are dependent on coupling to the type of receptor (CRHR1 vs. CRHR2), receptor signaling (coupling to PKA or PKC), or related to stimulation of POMC and corticosteroidogenesis (78, 134). For example, in closely related human follicular keratinocytes CRHR1 agonists inhibit keratinocytes proliferation and hair shaft elongation, whereas CRHR2 agonists maintain follicular keratinocytes in an anagen-like state, which indicates their stimulation of keratinocyte proliferation and retardation of follicular keratinocyte differentiation in undifferentiated hair bulb matrix keratinocytes (78, 171, 186).
CRH and related urocortins also regulate proliferation, viability, differentiation program, and endocrine activities of human melanocytes (74, 78, 171, 266, 267, 271). In normal and immortalized epidermal melanocytes, CRH inhibited melanocyte proliferation in growth factors containing medium through G2 arrest, whereas in growth factor-depleted media it enhanced DNA synthesis (through increased transition from G1/G0 to S phase and decreased subG1 component, indicating DNA degradation) and inhibited early and late apoptosis in the same cells, indicating that on epidermal melanocytes CRH acts as a survival factor under the stress of starvation and as an inhibitor of growth factors induced cell proliferation (271). Activation of CRHR1 induced generation of both cAMP and IP3 in epidermal melanocytes (271). In follicular melanocytes, CRH and specific CRHR1 agonists stimulated dendricity and melanogenesis, whereas CRHR2 agonists inhibited melanocyte differentiation program (266). In addition, CRH stimulated POMC expression with production of POMC peptides in normal and malignant melanocytes (74, 174, 267). In addition, UVB stimulated POMC expression and production of ACTH was dependent on CRH production and stimulation of CRHR1 (265). Furthermore, CRH stimulated cortisol production through action on CRHR1 coupled to cAMP signaling and production of POMC and its product ACTH (74). It also inhibited NF-κβ activity through similar mechanisms secondary to POMC expression and processing to final peptides (174).
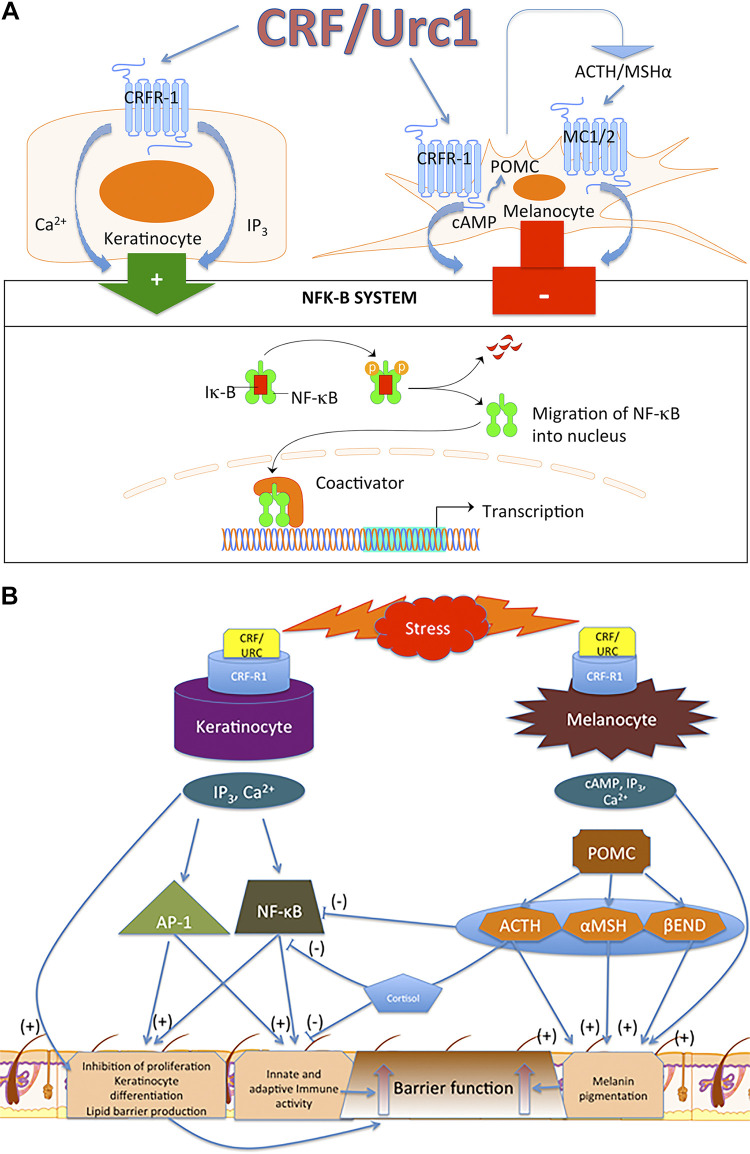
In summary, CRH and related urocortin can regulate functions of the epidermis through receptor and context-dependent mechanisms. Induction of differentiation programs in epidermal keratinocytes and melanocytes as well as of immune and endocrine activity would strengthen its barrier function as discussed previously and the responsiveness to stress with a feedback mechanism to terminate such responses (5, 78, 167, 185). The differences and similarities in CRH-mediated regulation of keratinocytes and melanocytes functions are presented in Fig. 6.
Figure 6.
Differential and overlapping phenotypic effects secondary to activation of corticotropin-releasing hormone receptor 1 (CRHR1) in human keratinocytes and melanocytes. A: activation of CRHR1 in normal epidermal keratinocytes and melanocytes, respectively stimulates and inhibits NF-κB activity. In immortalized HaCaT keratinocytes, CRF both inhibits and stimulates NF-κB activity, depending on the environmental context (171). B: in keratinocytes, activation of CRHR1 directly inhibits proliferation and stimulates differentiation and immune activity via stimulation of NF-κB. This enhances protective epidermal barrier function. In melanocytes, CRHR1 directly and indirectly [through proopiomelanocortin (POMC) peptides] stimulates differentiation and melanin production leading to enhancement of protective barrier function. In contrast to keratinocytes, there is an indirect (through POMC peptides) inhibition of NF-κB with subsequent suppression of immune activity, which can be amplified by production of cortisol by melanocytes. Reprinted from Ref. 78 with the permission of the publisher.
POMC SIGNALING
Since their initial detection in epidermal keratinocytes and melanocytes (65, 200, 287–289), there have been a number of reports that have addressed this issue. Their activities in the epidermal response system to the environmental stressors will therefore be discussed only briefly. For further information, the reader is referred to in-depth reviews on this topic reported elsewhere (2, 12, 25, 26, 41, 44, 68, 78, 177, 178, 188, 290, 291). POMC is expressed in human epidermal and follicular keratinocytes and melanocytes and is processed to ACTH, ACTH1-13, α-MSH, β-MSH, γ1–3MSH, β-LPH, and β-endorphin. These products together with POMC precursor are being released into extracellular space (44, 55, 65, 67, 75, 287, 292). This process follows the molecular principles operating at the central level (brain and pituitary) (76) and involves the action of prohormone convertases 1, -2 and regulatory protein 7B2 (44, 292–294). A similar process occurs in murine skin (294), dermal human skin cells (295–297), and human retinal pigment epithelium (298). Local expression of the POMC gene and protein and production of POMC peptides can be triggered by external stressors including UVB (139, 265, 289, 299–301), or other bioregulatory factors such as cytokines (25, 68, 134), active forms of vitamin D (268), and CRH (73–75, 174, 265, 267). The induction of POMC expression by UVB in epidermal melanocytes involves the activation of CRH and the PKA signaling pathway (265).
The phenotypic effects of POMC peptides on keratinocytes and melanocytes are mediated through their interaction with corresponding melanocortin or opioid receptors (4, 25, 26, 41, 67, 177, 178, 255, 291, 292, 302–310). The best characterized is the regulation of melanin skin pigmentation and melanocytic activity by MSH through an interaction with the melanocortin type 1 (MC1) receptors coupled to the cAMP signaling cascade, reviewed in Refs. 177, 255, 290, 291, 306, and 309. MC1 can also be activated by ACTH and to lesser degree by other MSH peptides. In normal human melanocytes, these molecules stimulate melanogenesis, dendrite formation, cell proliferation as well as melanosomes transfer to epidermal keratinocytes. An additional function of ligand-induced MC1 activity is stimulation of antiapoptotic, cytoprotective, and antioxidative cellular mechanisms that include DNA damage repair, making this axis important in protection against UVR-induced damage and carcinogenesis (254, 304, 305, 309–312). In addition, this pathway inhibits the immune activities of melanocytes including inhibition of the NF-κβ signaling (313–315). Additional MC receptors that are targets for MSH and ACTH peptides are expressed on melanocytes and keratinocytes include MC2, MC3, and MC4 (70, 177, 178, 268). MC2 is also expressed in murine skin (316). However, their functional activity in epidermal skin cells remains to be established. The role of γ1–3-MSH in the regulation of melanin pigmentation also remains to be defined (317, 318). POMC-derived and locally produced β-endorphin also regulates the melanocyte differentiation program through the μ-opioid receptor system with potent melanogenic, mitogenic, and dendritogenic effects (44, 67).
Human keratinocytes also express receptors for MSH, ACTH peptides, and β-endorphin (41, 68, 178, 270, 274, 300, 303, 307, 319–323). Through their actions on these membrane-bound receptors, the POMC-derived peptides can regulate keratinocytes proliferation and differentiation, as well as immune activities, wound healing, and cytoprotection (41, 176, 178, 303, 321–323). It should be noted that β-endorphin and MSH can be released by sensory nerve endings in the skin or be produced by circulating immune cells (24, 25, 37, 68, 176). In conclusion, the local POMC system operates to preserve barrier function of the epidermis with POMC peptides acting directly on keratinocytes or indirectly through stimulation of epidermal melanocytes to enhance the protective barrier through production of melanin pigment. Melanin and melanogenesis due to its diverse bioregulatory activity (324), in addition to its light scattering property, can strengthen the barrier function (252, 325). The anti-inflammatory activities of β-endorphin, ACTH, and MSH peptides and their indirect effects through stimulation of cortisol production are also important for epidermal physiology. The local HPA axis can operate at the cellular level in a para- and autocrine fashion as has been shown for melanocytes (74).
OTHER NEUROPEPTIDES AND NEUROTROPHINS
Enkephalins
Enkephalins can either be produced locally in the epidermis, released from sensory nerve endings, or produced by circulating immune cells (211, 218, 326–328). Epidermal melanocytes and keratinocytes express proenkephalin (PENK) with production of the corresponding Met- and Leu-enkephalin peptides (218). UVB, Toll-like receptor (TLR)4, and TLR2 agonists stimulated PENK gene expression in melanocytes and keratinocytes in a time- and dose-dependent manner and Met/Leu-ENK peptides are expressed in differentiating keratinocytes of the epidermis in the outer root sheath of the hair follicle, in myoepithelial cells of the eccrine gland, and in the basement membrane/basal lamina separating epithelial and mesenchymal components (218). These enkephalins are altered in a variety of hyperproliferative and inflammatory cutaneous diseases, especially psoriasis (218, 326, 328, 329). Its expression was also noted in epidermal skin tumors including squamous and basal cell carcinoma (218, 327). Through the interaction of the corresponding receptors, they can regulate the proliferation and differentiation of keratinocytes and inflammatory skin responses (326, 328, 330). Thus, enkephalins together with other opioid peptides can affect epidermal homeostasis and improper signaling can lead to hyperproliferative or inflammatory disorders (41, 175, 176, 302, 303, 326, 328–330).
Thyroid Stimulating Hormone and Thyroid Releasing Hormone
Since initial detection of functional thyroid stimulating hormone (TSH) receptors and genes expressing TSH and thyroid releasing hormone (TRH) in human skin (114), significant progress was made to define their function in that location (115–119, 331–333). It is thought that they regulate epidermal and follicular functions through corresponding receptors in a direct or indirect manner. TRH can also be involved in progression of melanocytic tumors (334) and its role in regulation of hair pigmentation was proposed (335). Furthermore, skin-derived TRH can stimulate anterior pituitary to release TSH with a downstream stimulation of thyroid gland to release thyroid hormones.
Pituitary and Hypothalamic Hormones
An important role for prolactin in skin physiology and pathology was initially proposed by Paus (121). This is supported by detection of prolactin in human scalp and discovery of prolactin receptors in skin cells, identification of its regulators, and defining its phenotypic effects (117, 120, 336).
The epidermis also responds to the action of growth hormone (GH) directly through GH receptor expressed on keratinocytes or indirectly via induction of IGF-1 production in dermis (IGF-1 receptor is expressed by epidermal cells) (337, 338). However, the GH is not expressed in the human epidermis (122).
Somatostatin is also expressed in the epidermis and hair follicle. It is thought to have a regulatory effect on keratinocyte proliferation and differentiation (339, 340). However, the mechanisms regulating its cutaneous expression and definitive functions remain to be investigated.
Oxytocin, a hypothalamic hormone released from the posterior pituitary, is also produced by human keratinocytes (341). It regulates proliferation of keratinocytes as well as having immunomodulatory and antioxidative activities in these cells (342). Its exact role in epidermal homeostasis remains to be investigated.
Other Neuropeptides
A variety of neuropeptides are produced in the human epidermis locally, in addition to their release from nerve endings (2, 24, 26, 37, 39, 157–162, 188, 215, 339, 343, 344). Examples include dynorphins, CGRP, NT, PTH, PTHRP, PACAP, CGRP, SP, NPY, PPY, and VIP. Their functions in skin physiology and pathology have been extensively reviewed previously (2, 24, 26, 37, 39, 156–166, 188, 215, 339, 343, 344). Briefly, they can affect keratinocytes proliferation, production of cytokines, and epidermal immune activities and they play a role in inflammatory skin diseases. However, their exact role in regulation of epidermal barrier or regulation of melanocyte pigmentary activity remains to be investigated.
Neurotrophins
Neurotrophins including NGF are recognized as neurotrophic factors supporting the synthesis and development of sensory neurons in the central and peripheral nervous system. They are also produced by keratinocytes (148, 149, 345–349). Neurotrophins including NGF act on the corresponding membrane-bound receptors present on epidermal and follicular keratinocytes and melanocytes to regulate their proliferation, survival, and other functions (148, 149, 325, 349–355). They also regulate the innervation of skin by sensory and sympathetic neurons (148, 348, 351). NGF also protects keratinocytes and melanocytes from UVR-induced damage and in the regulation of epidermal barrier functions.
CONCLUDING REMARKS
Because of its strategic location at the interface with the external environment, the skin, including its epidermal compartment, is crucial for organismal survival. These functions are regulated by local neuroendocrine, immune, pigmentary, and adnexal systems that detect, integrate, and respond to a diverse range of stressors to protect skin integrity and functions. This is achieved through precise calibration of stress responses with a high degree of local autonomy by sophisticated sensory, computing, and signaling systems that differentially respond to environmental changes. The skin constituents communicate cross-functionally through local nervous, immune, and endocrine systems to maintain essential epidermal functions. In fact the skin has been recognized as a brain on the outside (24, 43) and stress organ (25).
The epidermis also has its own autonomous self-regulating neuroendocrine system composed of hormonal and neuropeptide networks with corresponding receptors produced and expressed by keratinocytes, melanocytes, Langerhans cells, and Merkel cells that strengthen its barrier function and set the homeostatic responses in the most optimal mode. This system includes epidermal sensory nerves acting in a coordinated manner to orchestrate homeostatic responses and to interact with the local microbiome. Melanocytes, because of their secretory and pigmentary functions, through communication via an extensive dendrite network, represent an additional coordinator of the local stress responses. The skin is also tightly networked to central stress axes, which contribute to the maintenance of body homeostasis (Fig. 1C and Fig. 4B).
In addition, recent advances in genomics have provided big data analysis opportunities for skin research. For example, genomic landscapes of melanocytes from human skin were characterized (356). A large amount of data collected should allow to ask important questions on the gene signatures that may serve as disease biomarkers for screening, diagnostic, therapeutic, and prognosis solutions (357). The database PAGER, which is continuously developing, contains large number of gens related to the skin (358, 359), which forms the background for computational approaches using systems biology or artificial intelligence (AI). Thus, the computational biology represents a future for modeling of neuroendocrine connections in the skin with implications for cutaneous physiology and pathology that would also increase the precision of the diagnosis and therapy.
GRANTS
We acknowledge the support of NIH Grants 1R01AR073004-01A1, R01AR071189-01A1, and a VA Merit Grant No. 1I01BX004293-01A1 (to A.T.S.); R21 AI149267-01A1 (to C.R. and A.T.S.); R21ES034595 and RO1CA1933885 (to C.E.); and U54TR001005, U01CA223976 Grants (to J.Y.C.), U01 AR 078544 (to M.A.). The cited work was also supported by NSF Grants IOS-0918934, IBN-9604364, 9896030, and 049087 and NIH Grants RO1AR052190, 1R01AR056666, R21AR0665051, and AR-047079 (to A.T.S.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.T.S. conceived and designed research; A.T.S. and R.M.S. performed experiments; A.T.S., R.M.S., C.R., J.Y.C., M.A., and C.E. analyzed data; A.T.S., R.M.S., C.R., J.Y.C., M.A., and C.E. interpreted results of experiments; A.T.S. prepared figures; A.T.S., R.M.S., C.R., J.Y.C., M.A., and C.E. drafted manuscript; A.T.S., R.M.S., C.R., J.Y.C., M.A., and C.E. edited and revised manuscript; A.T.S., R.M.S., C.R., J.Y.C., M.A., and C.E. approved final version of manuscript.
ACKNOWLEDGMENTS
This article is part of the special collection "Skin Homeostasis: Peptides, Hormones, Proteases and More." Alexander Nyström, PhD, served as Guest Editor of this collection.
REFERENCES
- 1. Fuchs E. Epithelial skin biology: three decades of developmental biology, a hundred questions answered and a thousand new ones to address. Curr Top Dev Biol 116: 357–374, 2016. doi: 10.1016/bs.ctdb.2015.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ramot Y, Bohm M, Paus R. Translational neuroendocrinology of human skin: concepts and perspectives. Trends Mol Med 27: 60–74, 2021. doi: 10.1016/j.molmed.2020.09.002. [DOI] [PubMed] [Google Scholar]
- 3. Elias PM. Structure and function of the stratum corneum extracellular matrix. J Invest Dermatol 132: 2131–2133, 2012. doi: 10.1038/jid.2012.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Slominski AT, Zmijewski MA, Skobowiat C, Zbytek B, Slominski RM, Steketee JD. Sensing the environment: regulation of local and global homeostasis by the skin’s neuroendocrine system. Adv Anat Embryol Cell Biol 212: v, vii, 1–115, 2012. doi: 10.1007/978-3-642-19683-6_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Slominski A, Wortsman J, Paus R, Elias PM, Tobin DJ, Feingold KR. Skin as an endocrine organ: implications for its function. Drug Discov Today Dis Mech 5: 137–144, 2008. doi: 10.1016/j.ddmec.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fitzpatrick TE, Wolff K, Freedberg IM, Austen KF. Dermatology in General Medicine. New York: McGraw Hill, 1993. [Google Scholar]
- 7. Griffiths CEM, Barker J, Bleiker T, Chalmers R, Creamer D. Rook’s Textbook of Dermatology. Oxford: Wiley-Blackwell, 2016, p. 4992. [Google Scholar]
- 8. Bikle DD. Vitamin D and the skin: physiology and pathophysiology. Rev Endocr Metab Disord 13: 3–19, 2012. doi: 10.1007/s11154-011-9194-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Holick MF. Vitamin D: a millenium perspective. J Cell Biochem 88: 296–307, 2003. doi: 10.1002/jcb.10338. [DOI] [PubMed] [Google Scholar]
- 10. Zouboulis CC, Ganceviciene R, Liakou AI, Theodoridis A, Elewa R, Makrantonaki E. Aesthetic aspects of skin aging, prevention, and local treatment. Clin Dermatol 37: 365–372, 2019. doi: 10.1016/j.clindermatol.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 11. Bernard JJ, Gallo RL, Krutmann J. Photoimmunology: how ultraviolet radiation affects the immune system. Nat Rev Immunol 19: 688–701, 2019. [DOI] [PubMed] [Google Scholar]
- 12. Slominski AT, Zmijewski MA, Plonka PM, Szaflarski JP, Paus R. How UV light touches the brain and endocrine system through skin, and why. Endocrinology 159: 1992–2007, 2018. doi: 10.1210/en.2017-03230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Misery L. [Sensitive skin, reactive skin]. Ann Dermatol Venereol 146: 585–591, 2019. doi: 10.1016/j.annder.2019.05.007. [DOI] [PubMed] [Google Scholar]
- 14. Kobayashi T, Naik S, Nagao K. Choreographing immunity in the skin epithelial barrier. Immunity 50: 552–565, 2019. doi: 10.1016/j.immuni.2019.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Slominski AT, Chaiprasongsuk A, Janjetovic Z, Kim TK, Stefan J, Slominski RM, Hanumanthu VS, Raman C, Qayyum S, Song Y, Song Y, Panich U, Crossman DK, Athar M, Holick MF, Jetten AM, Zmijewski MA, Zmijewski J, Tuckey RC. Photoprotective properties of vitamin D and lumisterol hydroxyderivatives. Cell Biochem Biophys 78: 165–180, 2020. doi: 10.1007/s12013-020-00913-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cevikbas F, Lerner EA. Physiology and pathophysiology of itch. Physiol Rev 100: 945–982, 2020. doi: 10.1152/physrev.00017.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Besné I, Descombes C, Breton L. Effect of age and anatomical site on density of sensory innervation in human epidermis. Arch Dermatol 138: 1445–1450, 2002. doi: 10.1001/archderm.138.11.1445. [DOI] [PubMed] [Google Scholar]
- 18. Arthur RP, Shelley WB. The innervation of human epidermis. J Invest Dermatol 32: 397–411, 1959. doi: 10.1038/jid.1959.69. [DOI] [PubMed] [Google Scholar]
- 19. Lauria G. Innervation of the human epidermis. A historical review. Ital J Neurol Sci 20: 63–70, 1999. doi: 10.1007/s100720050013. [DOI] [PubMed] [Google Scholar]
- 20. Kandel ER. Principles of Neural Science (5th ed.). New York: McGraw-Hill Education, 2013. [Google Scholar]
- 21. Zimmerman A, Bai L, Ginty DD. The gentle touch receptors of mammalian skin. Science 346: 950–954, 2014. doi: 10.1126/science.1254229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Baumbauer KM, DeBerry JJ, Adelman PC, Miller RH, Hachisuka J, Lee KH, Ross SE, Koerber HR, Davis BM, Albers KM. Keratinocytes can modulate and directly initiate nociceptive responses. eLife 4: e09674, 2015. doi: 10.7554/eLife.09674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. O'Sullivan JDB, Nicu C, Picard M, Cheret J, Bedogni B, Tobin DJ, Paus R. The biology of human hair greying. Biol Rev Camb Philos Soc 96: 107–128, 2021. doi: 10.1111/brv.12648. [DOI] [PubMed] [Google Scholar]
- 24. Slominski A, Wortsman J. Neuroendocrinology of the skin. Endocr Rev 21: 457–487, 2000. [Erratum in Endocr Rev 23: 364, 2002]. doi: 10.1210/edrv.21.5.0410. [DOI] [PubMed] [Google Scholar]
- 25. Slominski A, Wortsman J, Luger T, Paus R, Solomon S. Corticotropin releasing hormone and proopiomelanocortin involvement in the cutaneous response to stress. Physiol Rev 80: 979–1020, 2000. doi: 10.1152/physrev.2000.80.3.979. [DOI] [PubMed] [Google Scholar]
- 26. Cirillo N. The local neuropeptide system of keratinocytes. Biomedicines 9: 1854, 2021. doi: 10.3390/biomedicines9121854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Slominski RM, Raman C, Elmets C, Jetten AM, Slominski AT, Tuckey RC. The significance of CYP11A1 expression in skin physiology and pathology. Mol Cell Endocrinol 530: 111238, 2021. doi: 10.1016/j.mce.2021.111238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Slominski RM, Tuckey RC, Manna PR, Jetten AM, Postlethwaite A, Raman C, Slominski AT. Extra-adrenal glucocorticoid biosynthesis: implications for autoimmune and inflammatory disorders. Genes Immun 21: 150–168, 2020. doi: 10.1038/s41435-020-0096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pondeljak N, Lugovic-Mihic L. Stress-induced interaction of skin immune cells, hormones, and neurotransmitters. Clin Ther 42: 757–770, 2020. doi: 10.1016/j.clinthera.2020.03.008. [DOI] [PubMed] [Google Scholar]
- 30. Clayton RW, Langan EA, Ansell DM, de Vos I, Gobel K, Schneider MR, Picardo M, Lim X, van Steensel MAM, Paus R. Neuroendocrinology and neurobiology of sebaceous glands. Biol Rev Camb Philos Soc 95: 592–624, 2020. doi: 10.1111/brv.12579. [DOI] [PubMed] [Google Scholar]
- 31. Bocheva G, Slominski RM, Slominski AT. Neuroendocrine aspects of skin aging. Int J Mol Sci 20: 2798, 2019. doi: 10.3390/ijms20112798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Theoharides TC. Neuroendocrinology of mast cells: challenges and controversies. Exp Dermatol 26: 751–759, 2017. doi: 10.1111/exd.13288. [DOI] [PubMed] [Google Scholar]
- 33. Szöllősi AG, Oláh A, Bíró T, Tóth BI. Recent advances in the endocrinology of the sebaceous gland. Dermatoendocrinol 9: e1361576, 2017. doi: 10.1080/19381980.2017.1361576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nikolakis G, Stratakis CA, Kanaki T, Slominski A, Zouboulis CC. Skin steroidogenesis in health and disease. Rev Endocr Metab Disord 17: 247–258, 2016. doi: 10.1007/s11154-016-9390-z. [DOI] [PubMed] [Google Scholar]
- 35. Chen Y, Lyga J. Brain-skin connection: stress, inflammation and skin aging. Inflamm Allergy Drug Targets 13: 177–190, 2014. doi: 10.2174/1871528113666140522104422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kim JE, Cho BK, Cho DH, Park HJ. Expression of hypothalamic-pituitary-adrenal axis in common skin diseases: evidence of its association with stress-related disease activity. Acta Derm Venereol 93: 387–393, 2013. doi: 10.2340/00015555-1557. [DOI] [PubMed] [Google Scholar]
- 37. Roosterman D, Goerge T, Schneider SW, Bunnett NW, Steinhoff M. Neuronal control of skin function: the skin as a neuroimmunoendocrine organ. Physiol Rev 86: 1309–1379, 2006. doi: 10.1152/physrev.00026.2005. [DOI] [PubMed] [Google Scholar]
- 38. O'Kane M, Murphy EP, Kirby B. The role of corticotropin-releasing hormone in immune-mediated cutaneous inflammatory disease. Exp Dermatol 15: 143–153, 2006. doi: 10.1111/j.1600-0625.2006.00382.x. [DOI] [PubMed] [Google Scholar]
- 39. Theoharides TC, Stewart JM, Taracanova A, Conti P, Zouboulis CC. Neuroendocrinology of the skin. Rev Endocr Metab Disord 17: 287–294, 2016. doi: 10.1007/s11154-016-9369-9. [DOI] [PubMed] [Google Scholar]
- 40. Paus R. Exploring the “brain-skin connection”: leads and lessons from the hair follicle. Curr Res Transl Med 64: 207–214, 2016. doi: 10.1016/j.retram.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 41. Bigliardi PL, Dancik Y, Neumann C, Bigliardi-Qi M. Opioids and skin homeostasis, regeneration and ageing—what’s the evidence? Exp Dermatol 25: 586–591, 2016. doi: 10.1111/exd.13021. [DOI] [PubMed] [Google Scholar]
- 42. Harvima IT, Nilsson G. Stress, the neuroendocrine system and mast cells: current understanding of their role in psoriasis. Expert Rev Clin Immunol 8: 235–241, 2012. doi: 10.1586/eci.12.1. [DOI] [PubMed] [Google Scholar]
- 43. Tobin DJ. Biochemistry of human skin—our brain on the outside. Chem Soc Rev 35: 52–67, 2006. doi: 10.1039/b505793k. [DOI] [PubMed] [Google Scholar]
- 44. Tobin DJ, Kauser S. β-Endorphin: the forgotten hair follicle melanotropin. J Investig Dermatol Symp Proc 10: 212–216, 2005. doi: 10.1111/j.1087-0024.2005.10108.x. [DOI] [PubMed] [Google Scholar]
- 45. Blalock JE. The immune system as the sixth sense. J Intern Med 257: 126–138, 2005. doi: 10.1111/j.1365-2796.2004.01441.x. [DOI] [PubMed] [Google Scholar]
- 46. Blalock JE. The syntax of immune-neuroendocrine communication. Immunol Today 15: 504–511, 1994. doi: 10.1016/0167-5699(94)90205-4. [DOI] [PubMed] [Google Scholar]
- 47. Besedovsky HO, del Rey A. Immune-neuro-endocrine interactions: facts and hypotheses. Endocr Rev 17: 64–102, 1996. doi: 10.1210/edrv-17-1-64. [DOI] [PubMed] [Google Scholar]
- 48. Bohm M, Grassel S. Role of proopiomelanocortin-derived peptides and their receptors in the osteoarticular system: from basic to translational research. Endocr Rev 33: 623–651, 2012. doi: 10.1210/er.2011-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Taves MD, Gomez-Sanchez CE, Soma KK. Extra-adrenal glucocorticoids and mineralocorticoids: evidence for local synthesis, regulation, and function. Am J Physiol Endocrinol Metab 301: E11–E24, 2011. doi: 10.1152/ajpendo.00100.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ahmed A, Schmidt C, Brunner T. Extra-adrenal glucocorticoid synthesis in the intestinal mucosa: between immune homeostasis and immune escape. Front Immunol 10: 1438, 2019. doi: 10.3389/fimmu.2019.01438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Merk VM, Phan TS, Brunner T. Regulation of tissue immune responses by local glucocorticoids at epithelial barriers and their impact on interorgan crosstalk. Front Immunol 12: 672808, 2021. doi: 10.3389/fimmu.2021.672808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Slominski A, Baker J, Ermak G, Chakraborty A, Pawelek J. Ultraviolet B stimulates production of corticotropin releasing factor (CRF) by human melanocytes. FEBS Lett 399: 175–176, 1996. doi: 10.1016/s0014-5793(96)01315-4. [DOI] [PubMed] [Google Scholar]
- 53. Roloff B, Fechner K, Slominski A, Furkert J, Botchkarev VA, Bulfone-Paus S, Zipper J, Krause E, Paus R. Hair cycle-dependent expression of corticotropin-releasing factor (CRF) and CRF receptors in murine skin. FASEB J 12: 287–297, 1998. doi: 10.1096/fasebj.12.3.287. [DOI] [PubMed] [Google Scholar]
- 54. Slominski A, Ermak G, Mazurkiewicz JE, Baker J, Wortsman J. Characterization of corticotropin-releasing hormone (CRH) in human skin. J Clin Endocrinol Metab 83: 1020–1024, 1998. doi: 10.1210/jcem.83.3.4650. [DOI] [PubMed] [Google Scholar]
- 55. Slominski A, Szczesniewski A, Wortsman J. Liquid chromatography-mass spectrometry detection of corticotropin-releasing hormone and proopiomelanocortin-derived peptides in human skin. J Clin Endocrinol Metab 85: 3582–3588, 2000. doi: 10.1210/jcem.85.10.6863. [DOI] [PubMed] [Google Scholar]
- 56. Zouboulis CC, Seltmann H, Hiroi N, Chen W, Young M, Oeff M, Scherbaum WA, Orfanos CE, McCann SM, Bornstein SR. Corticotropin-releasing hormone: an autocrine hormone that promotes lipogenesis in human sebocytes. Proc Natl Acad Sci USA 99: 7148–7153, 2002. doi: 10.1073/pnas.102180999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ito N, Ito T, Betterman A, Paus R. The human hair bulb is a source and target of CRH. J Invest Dermatol 122: 235–237, 2004. doi: 10.1046/j.1523-1747.2003.22145.x. [DOI] [PubMed] [Google Scholar]
- 58. Slominski A, Pisarchik A, Tobin DJ, Mazurkiewicz JE, Wortsman J. Differential expression of a cutaneous corticotropin-releasing hormone system. Endocrinology 145: 941–950, 2004. doi: 10.1210/en.2003-0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Slominski A, Wortsman J, Pisarchik A, Zbytek B, Linton EA, Mazurkiewicz JE, Wei ET. Cutaneous expression of corticotropin-releasing hormone (CRH), urocortin, and CRH receptors. FASEB J 15: 1678–1693, 2001. doi: 10.1096/fj.00-0850rev. [DOI] [PubMed] [Google Scholar]
- 60. Slominski A, Ermak G, Hwang J, Mazurkiewicz J, Corliss D, Eastman A. The expression of proopiomelanocortin (POMC) and of corticotropin releasing hormone receptor (CRH-R) genes in mouse skin. Biochim Biophys Acta 1289: 247–251, 1996. doi: 10.1016/0304-4165(95)00159-x. [DOI] [PubMed] [Google Scholar]
- 61. Slominski A, Roloff B, Curry J, Dahiya M, Szczesniewski A, Wortsman J. The skin produces urocortin. J Clin Endocrinol Metab 85: 815–823, 2000. doi: 10.1210/jcem.85.2.6381. [DOI] [PubMed] [Google Scholar]
- 62. Slominski A, Paus R, Mazurkiewicz J. Proopiomelanocortin expression in the skin during induced hair growth in mice. Experientia 48: 50–54, 1992. doi: 10.1007/BF01923606. [DOI] [PubMed] [Google Scholar]
- 63. Luger TA, Scholzen T, Brzoska T, Becher E, Slominski A, Paus R. Cutaneous immunomodulation and coordination of skin stress responses by α-melanocyte-stimulating hormone. Ann N Y Acad Sci 840: 381–394, 1998. doi: 10.1111/j.1749-6632.1998.tb09577.x. [DOI] [PubMed] [Google Scholar]
- 64. Slominski A, Botchkareva NV, Botchkarev VA, Chakraborty A, Luger T, Uenalan M, Paus R. Hair cycle-dependent production of ACTH in mouse skin. Biochim Biophys Acta 1448: 147–152, 1998. doi: 10.1016/s0167-4889(98)00124-4. [DOI] [PubMed] [Google Scholar]
- 65. Slominski A. Identification of beta-endorphin, alpha-MSH and ACTH peptides in cultured human melanocytes, melanoma and squamous cell carcinoma cells by RP-HPLC. Exp Dermatol 7: 213–216, 1998. doi: 10.1111/j.1600-0625.1998.tb00326.x. [DOI] [PubMed] [Google Scholar]
- 66. Furkert J, Klug U, Slominski A, Eichmuller S, Mehlis B, Kertscher U, Paus R. Identification and measurement of β-endorphin levels in the skin during induced hair growth in mice. Biochim Biophys Acta 1336: 315–322, 1997. doi: 10.1016/s0304-4165(97)00046-9. [DOI] [PubMed] [Google Scholar]
- 67. Kauser S, Schallreuter KU, Thody AJ, Gummer C, Tobin DJ. Regulation of human epidermal melanocyte biology by β-endorphin. J Invest Dermatol 120: 1073–1080, 2003. doi: 10.1046/j.1523-1747.2003.12242.x. [DOI] [PubMed] [Google Scholar]
- 68. Luger T, Paus R, Slominski A, Lipton J. Cutaneous neuromodulation: the proopiomelanocortin system. Ann NY Acad Sci 885: 1–479, 1999. doi: 10.1159/000087012. [DOI] [PubMed] [Google Scholar]
- 69. Racine PJ, Janvier X, Clabaut M, Catovic C, Souak D, Boukerb AM, Groboillot A, Konto-Ghiorghi Y, Duclairoir-Poc C, Lesouhaitier O, Orange N, Chevalier S, Feuilloley MGJ. Dialog between skin and its microbiota: emergence of “Cutaneous Bacterial Endocrinology”. Exp Dermatol 29: 790–800, 2020. doi: 10.1111/exd.14158. [DOI] [PubMed] [Google Scholar]
- 70. Slominski A, Ermak G, Mihm M. ACTH receptor, CYP11A1, CYP17 and CYP21A2 genes are expressed in skin. J Clin Endocrinol Metab 81: 2746–2749, 1996. doi: 10.1210/jcem.81.7.8675607. [DOI] [PubMed] [Google Scholar]
- 71. Slominski A, Mihm MC. Potential mechanism of skin response to stress. Int J Dermatol 35: 849–851, 1996. doi: 10.1111/j.1365-4362.1996.tb05049.x. [DOI] [PubMed] [Google Scholar]
- 72. Slominski A, Wortsman J, Tuckey RC, Paus R. Differential expression of HPA axis homolog in the skin. Mol Cell Endocrinol 265-266: 143–149, 2007. doi: 10.1016/j.mce.2006.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Slominski A, Zbytek B, Semak I, Sweatman T, Wortsman J. CRH stimulates POMC activity and corticosterone production in dermal fibroblasts. J Neuroimmunol 162: 97–102, 2005. doi: 10.1016/j.jneuroim.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 74. Slominski A, Zbytek B, Szczesniewski A, Semak I, Kaminski J, Sweatman T, Wortsman J. CRH stimulation of corticosteroids production in melanocytes is mediated by ACTH. Am J Physiol Endocrinol Metab 288: E701–E706, 2005. [Erratum in Am J Physiol Endocrinol Metab 290: E204, 2006]. doi: 10.1152/ajpendo.00519.2004. [DOI] [PubMed] [Google Scholar]
- 75. Ito N, Ito T, Kromminga A, Bettermann A, Takigawa M, Kees F, Straub RH, Paus R. Human hair follicles display a functional equivalent of the hypothalamic-pituitary-adrenal axis and synthesize cortisol. FASEB J 19: 1332–1334, 2005. doi: 10.1096/fj.04-1968fje. [DOI] [PubMed] [Google Scholar]
- 76. Cawley NX, Li Z, Loh YP. 60 YEARS OF POMC: biosynthesis, trafficking, and secretion of pro-opiomelanocortin-derived peptides. J Mol Endocrinol 56: T77–T97, 2016. doi: 10.1530/JME-15-0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Turnbull AV, Rivier CL. Regulation of the hypothalamic-pituitary-adrenal axis by cytokines: actions and mechanisms of action. Physiol Rev 79: 1–71, 1999. doi: 10.1152/physrev.1999.79.1.1. [DOI] [PubMed] [Google Scholar]
- 78. Slominski AT, Zmijewski MA, Zbytek B, Tobin DJ, Theoharides TC, Rivier J. Key role of CRF in the skin stress response system. Endocr Rev 34: 827–884, 2013. doi: 10.1210/er.2012-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Chrousos GP. Stress and disorders of the stress system. Nat Rev Endocrinol 5: 374–381, 2009. doi: 10.1038/nrendo.2009.106. [DOI] [PubMed] [Google Scholar]
- 80. Miller WL, Auchus RJ. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr Rev 32: 81–151, 2011. [Erratum in Endocr Rev 32: 579, 2011]. doi: 10.1210/er.2010-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Dallman MF, Akana SF, Levin N, Walker CD, Bradbury MJ, Suemaru S, Scribner KS. Corticosteroids and the control of function in the hypothalamo-pituitary-adrenal (HPA) axis. Ann N Y Acad Sci 746: 22–31, 1994. discussion 31–22, 64–27. doi: 10.1111/j.1749-6632.1994.tb39206.x. [DOI] [PubMed] [Google Scholar]
- 82. Seyle H. The Stress of Life. New York: McGraw-Hill Book Company, 1976, p. 515. [Google Scholar]
- 83. Grando SA. Cholinergic control of epidermal cohesion. Exp Dermatol 15: 265–282, 2006. doi: 10.1111/j.0906-6705.2006.00410.x. [DOI] [PubMed] [Google Scholar]
- 84. Grando SA, Pittelkow MR, Schallreuter KU. Adrenergic and cholinergic control in the biology of epidermis: physiological and clinical significance. J Invest Dermatol 126: 1948–1965, 2006. doi: 10.1038/sj.jid.5700151. [DOI] [PubMed] [Google Scholar]
- 85. Schallreuter KU, Lemke KR, Pittelkow MR, Wood JM, Korner C, Malik R. Catecholamines in human keratinocyte differentiation. J Invest Dermatol 104: 953–957, 1995. doi: 10.1111/1523-1747.ep12606218. [DOI] [PubMed] [Google Scholar]
- 86. Schallreuter KU. Epidermal adrenergic signal transduction as part of the neuronal network in the human epidermis. J Investig Dermatol Symp Proc 2: 37–40, 1997. doi: 10.1038/jidsymp.1997.9. [DOI] [PubMed] [Google Scholar]
- 87. Chavan B, Gillbro JM, Rokos H, Schallreuter KU. GTP cyclohydrolase feedback regulatory protein controls cofactor 6-tetrahydrobiopterin synthesis in the cytosol and in the nucleus of epidermal keratinocytes and melanocytes. J Invest Dermatol 126: 2481–2489, 2006. doi: 10.1038/sj.jid.5700425. [DOI] [PubMed] [Google Scholar]
- 88. Gillbro JM, Marles LK, Hibberts NA, Schallreuter KU. Autocrine catecholamine biosynthesis and the beta-adrenoceptor signal promote pigmentation in human epidermal melanocytes. J Invest Dermatol 123: 346–353, 2004. doi: 10.1111/j.0022-202X.2004.23210.x. [DOI] [PubMed] [Google Scholar]
- 89. Slominski A, Paus R. Are l-tyrosine and l-DOPA hormone-like bioregulators. J Theor Biol 143: 123–138, 1990. doi: 10.1016/s0022-5193(05)80292-9. [DOI] [PubMed] [Google Scholar]
- 90. Slominski A, Zmijewski MA, Pawelek J. l-tyrosine and l-dihydroxyphenylalanine as hormone-like regulators of melanocyte functions. Pigment Cell Melanoma Res 25: 14–27, 2012. doi: 10.1111/j.1755-148X.2011.00898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Slominski A, Paus R. Towards defining receptors for l-tyrosine and l-DOPA. Mol Cell Endocrinol 99: C7–C11, 1994. doi: 10.1016/0303-7207(94)90001-9. [DOI] [PubMed] [Google Scholar]
- 92. Slominski A, Pisarchik A, Semak I, Sweatman T, Wortsman J, Szczesniewski A, Slugocki G, McNulty J, Kauser S, Tobin DJ, Jing C, Johansson O. Serotoninergic and melatoninergic systems are fully expressed in human skin. FASEB J 16: 896–898, 2002. doi: 10.1096/fj.01-0952fje. [DOI] [PubMed] [Google Scholar]
- 93. Slominski A, Pisarchik A, Semak I, Sweatman T, Szczesniewski A, Wortsman J. Serotoninergic system in hamster skin. J Invest Dermatol 119: 934–942, 2002. doi: 10.1046/j.1523-1747.2002.00156.x. [DOI] [PubMed] [Google Scholar]
- 94. Slominski A, Pisarchik A, Zbytek B, Tobin DJ, Kauser S, Wortsman J. Functional activity of serotoninergic and melatoninergic systems expressed in the skin. J Cell Physiol 196: 144–153, 2003. doi: 10.1002/jcp.10287. [DOI] [PubMed] [Google Scholar]
- 95. Slominski A, Pisarchik A, Semak I, Sweatman T, Wortsman J. Characterization of the serotoninergic system in the C57BL/6 mouse skin. Eur J Biochem 270: 3335–3344, 2003. doi: 10.1046/j.1432-1033.2003.03708.x. [DOI] [PubMed] [Google Scholar]
- 96. Slominski A, Pisarchik A, Johansson O, Jing C, Semak I, Slugocki G, Wortsman J. Tryptophan hydroxylase expression in human skin cells. Biochim Biophys Acta 1639: 80–86, 2003. doi: 10.1016/s0925-4439(03)00124-8. [DOI] [PubMed] [Google Scholar]
- 97. Slominski A, Wortsman J, Tobin DJ. The cutaneous serotoninergic/melatoninergic system: securing a place under the sun. FASEB J 19: 176–194, 2005. doi: 10.1096/fj.04-2079rev. [DOI] [PubMed] [Google Scholar]
- 98. Slominski AT, Kim T-K, Kleszczyński K, Semak I, Janjetovic Z, Sweatman T, Skobowiat C, Steketee JD, Lin Z, Postlethwaite A, Li W, Reiter RJ, Tobin DJ. Characterization of serotonin and N-acetylserotonin systems in the human epidermis and skin cells. J Pineal Res 68: e12626, 2020. doi: 10.1111/jpi.12626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Slominski A, Baker J, Rosano TG, Guisti LW, Ermak G, Grande M, Gaudet SJ. Metabolism of serotonin to N-acetylserotonin, melatonin, and 5-methoxytryptamine in hamster skin culture. J Biol Chem 271: 12281–12286, 1996. doi: 10.1074/jbc.271.21.12281. [DOI] [PubMed] [Google Scholar]
- 100. Slominski A, Semak I, Pisarchik A, Sweatman T, Szczesniewski A, Wortsman J. Conversion of l-tryptophan to serotonin and melatonin in human melanoma cells. FEBS Lett 511: 102–106, 2002. doi: 10.1016/s0014-5793(01)03319-1. [DOI] [PubMed] [Google Scholar]
- 101. Slominski AT, Hardeland R, Zmijewski MA, Slominski RM, Reiter RJ, Paus R. Melatonin: a cutaneous perspective on its production, metabolism, and functions. J Invest Dermatol 138: 490–499, 2018. doi: 10.1016/j.jid.2017.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Slominski AT, Zmijewski MA, Semak I, Kim TK, Janjetovic Z, Slominski RM, Zmijewski JW. Melatonin, mitochondria, and the skin. Cell Mol Life Sci 74: 3913–3925, 2017. doi: 10.1007/s00018-017-2617-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Kim TK, Lin Z, Tidwell WJ, Li W, Slominski AT. Melatonin and its metabolites accumulate in the human epidermis in vivo and inhibit proliferation and tyrosinase activity in epidermal melanocytes in vitro. Mol Cell Endocrinol 404: 1–8, 2015. doi: 10.1016/j.mce.2014.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Kim TK, Kleszczynski K, Janjetovic Z, Sweatman T, Lin Z, Li W, Reiter RJ, Fischer TW, Slominski AT. Metabolism of melatonin and biological activity of intermediates of melatoninergic pathway in human skin cells. FASEB J 27: 2742–2755, 2013. doi: 10.1096/fj.12-224691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Kobayashi H, Kromminga A, Dunlop TW, Tychsen B, Conrad F, Suzuki N, Memezawa A, Bettermann A, Aiba S, Carlberg C, Paus R. A role of melatonin in neuroectodermal-mesodermal interactions: the hair follicle synthesizes melatonin and expresses functional melatonin receptors. FASEB J 19: 1710–1712, 2005. doi: 10.1096/fj.04-2293fje. [DOI] [PubMed] [Google Scholar]
- 106. Fischer TW, Sweatman TW, Semak I, Sayre RM, Wortsman J, Slominski A. Constitutive and UV-induced metabolism of melatonin in keratinocytes and cell-free systems. FASEB J 20: 1564–1566, 2006. [Erratum in FASEB J 21: 630, 2007]. doi: 10.1096/fj.05-5227fje. [DOI] [PubMed] [Google Scholar]
- 107. Biro T, Toth BI, Hasko G, Paus R, Pacher P. The endocannabinoid system of the skin in health and disease: novel perspectives and therapeutic opportunities. Trends Pharmacol Sci 30: 411–420, 2009. doi: 10.1016/j.tips.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Olah A, Toth BI, Borbiro I, Sugawara K, Szollosi AG, Czifra G, Pal B, Ambrus L, Kloepper J, Camera E, Ludovici M, Picardo M, Voets T, Zouboulis CC, Paus R, Biro T. Cannabidiol exerts sebostatic and antiinflammatory effects on human sebocytes. J Clin Invest 124: 3713–3724, 2014. doi: 10.1172/JCI64628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Toth KF, Adam D, Biro T, Olah A. Cannabinoid signaling in the skin: therapeutic potential of the “c(ut)annabinoid” system . Molecules 24: 918, 2019. doi: 10.3390/molecules24050918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Man MQ, Wakefield JS, Mauro TM, Elias PM. Regulatory role of nitric oxide in cutaneous inflammation. Inflammation 45: 949–964, 2022. doi: 10.1007/s10753-021-01615-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Sreedhar A, Aguilera-Aguirre L, Singh KK. Mitochondria in skin health, aging, and disease. Cell Death Dis 11: 444, 2020. doi: 10.1038/s41419-020-2649-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Moehring F, Cowie AM, Menzel AD, Weyer AD, Grzybowski M, Arzua T, Geurts AM, Palygin O, Stucky CL. Keratinocytes mediate innocuous and noxious touch via ATP-P2X4 signaling. eLife 7: e31684, 2018. doi: 10.7554/eLife.31684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Sadler KE, Moehring F, Stucky CL. Keratinocytes contribute to normal cold and heat sensation. eLife 9: e58625, 2020. doi: 10.7554/eLife.58625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Slominski A, Wortsman J, Kohn L, Ain KB, Venkataraman GM, Pisarchik A, Chung JH, Giuliani C, Thornton M, Slugocki G, Tobin DJ. Expression of hypothalamic-pituitary-thyroid axis related genes in the human skin. J Invest Dermatol 119: 1449–1455, 2002. doi: 10.1046/j.1523-1747.2002.19617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. van Beek N, Bodo E, Kromminga A, Gaspar E, Meyer K, Zmijewski MA, Slominski A, Wenzel BE, Paus R. Thyroid hormones directly alter human hair follicle functions: anagen prolongation and stimulation of both hair matrix keratinocyte proliferation and hair pigmentation. J Clin Endocrinol Metab 93: 4381–4388, 2008. doi: 10.1210/jc.2008-0283. [DOI] [PubMed] [Google Scholar]
- 116. Bodo E, Kromminga A, Biro T, Borbiro I, Gaspar E, Zmijewski MA, van Beek N, Langbein L, Slominski AT, Paus R. Human female hair follicles are a direct, nonclassical target for thyroid-stimulating hormone. J Invest Dermatol 129: 1126–1139, 2009. doi: 10.1038/jid.2008.361. [DOI] [PubMed] [Google Scholar]
- 117. Langan EA, Ramot Y, Hanning A, Poeggeler B, Biro T, Gaspar E, Funk W, Griffiths CE, Paus R. Thyrotropin-releasing hormone and oestrogen differentially regulate prolactin and prolactin receptor expression in female human skin and hair follicles in vitro. Br J Dermatol 162: 1127–1131, 2010. doi: 10.1111/j.1365-2133.2010.09676.x. [DOI] [PubMed] [Google Scholar]
- 118. Paus R. Exploring the “thyroid-skin connection”: concepts, questions, and clinical relevance. J Invest Dermatol 130: 7–10, 2010. doi: 10.1038/jid.2009.359. [DOI] [PubMed] [Google Scholar]
- 119. Cianfarani F, Baldini E, Cavalli A, Marchioni E, Lembo L, Teson M, Persechino S, Zambruno G, Ulisse S, Odorisio T, D'Armiento M. TSH receptor and thyroid-specific gene expression in human skin. J Invest Dermatol 130: 93–101, 2010. doi: 10.1038/jid.2009.180. [DOI] [PubMed] [Google Scholar]
- 120. Foitzik K, Langan EA, Paus R. Prolactin and the skin: a dermatological perspective on an ancient pleiotropic peptide hormone. J Invest Dermatol 129: 1071–1087, 2009. doi: 10.1038/jid.2008.348. [DOI] [PubMed] [Google Scholar]
- 121. Paus R. Does prolactin play a role in skin biology and pathology? Med Hypotheses 36: 33–42, 1991. doi: 10.1016/0306-9877(91)90161-q. [DOI] [PubMed] [Google Scholar]
- 122. Slominski A, Malarkey WB, Wortsman J, Asa SL, Carlson A. Human skin expresses growth hormone but not the prolactin gene. J Lab Clin Med 136: 476–481, 2000. doi: 10.1067/mlc.2000.110605. [DOI] [PubMed] [Google Scholar]
- 123. Poeggeler B, Schulz C, Pappolla MA, Bodó E, Tiede S, Lehnert H, Paus R. Leptin and the skin: a new frontier. Exp Dermatol 19: 12–18, 2010. doi: 10.1111/j.1600-0625.2009.00930.x. [DOI] [PubMed] [Google Scholar]
- 124. Slominski A, Zbytek B, Nikolakis G, Manna PR, Skobowiat C, Zmijewski M, Li W, Janjetovic Z, Postlethwaite A, Zouboulis CC, Tuckey RC. Steroidogenesis in the skin: implications for local immune functions. J Steroid Biochem Mol Biol 137: 107–123, 2013. doi: 10.1016/j.jsbmb.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Slominski AT, Manna PR, Tuckey RC. On the role of skin in the regulation of local and systemic steroidogenic activities. Steroids 103: 72–88, 2015. doi: 10.1016/j.steroids.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Slominski AT, Manna PR, Tuckey RC. Cutaneous glucocorticosteroidogenesis: securing local homeostasis and the skin integrity. Exp Dermatol 23: 369–374, 2014. doi: 10.1111/exd.12376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Slominski A, Zbytek B, Szczesniewski A, Wortsman J. Cultured human dermal fibroblasts do produce cortisol. J Invest Dermatol 126: 1177–1178, 2006. doi: 10.1038/sj.jid.5700204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Slominski A, Gomez-Sanchez CE, Foecking MF, Wortsman J. Active steroidogenesis in the normal rat skin. Biochim Biophys Acta 1474: 1–4, 2000. doi: 10.1016/s0304-4165(99)00215-9. [DOI] [PubMed] [Google Scholar]
- 129. Slominski A, Gomez-Sanchez CE, Foecking MF, Wortsman J. Metabolism of progesterone to DOC, corticosterone and 18OHDOC in cultured human melanoma cells. FEBS Lett 455: 364–366, 1999. doi: 10.1016/s0014-5793(99)00889-3. [DOI] [PubMed] [Google Scholar]
- 130. Hannen RF, Michael AE, Jaulim A, Bhogal R, Burrin JM, Philpott MP. Steroid synthesis by primary human keratinocytes; implications for skin disease. Biochem Biophys Res Commun 404: 62–67, 2011. doi: 10.1016/j.bbrc.2010.11.059. [DOI] [PubMed] [Google Scholar]
- 131. Cirillo N, Prime SS. Keratinocytes synthesize and activate cortisol. J Cell Biochem 112: 1499–1505, 2011. doi: 10.1002/jcb.23081. [DOI] [PubMed] [Google Scholar]
- 132. Hannen R, Udeh-Momoh C, Upton J, Wright M, Michael A, Gulati A, Rajpopat S, Clayton N, Halsall D, Burrin J, Flower R, Sevilla L, Latorre V, Frame J, Lightman S, Perez P, Philpott M. Dysfunctional skin-derived glucocorticoid synthesis is a pathogenic mechanism of psoriasis. J Invest Dermatol 137: 1630–1637, 2017. doi: 10.1016/j.jid.2017.02.984. [DOI] [PubMed] [Google Scholar]
- 133. Phan TS, Schink L, Mann J, Merk VM, Zwicky P, Mundt S, Simon D, Kulms D, Abraham S, Legler DF, Noti M, Brunner T. Keratinocytes control skin immune homeostasis through de novo-synthesized glucocorticoids. Sci Adv 7: eabe0337, 2021. doi: 10.1126/sciadv.abe0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Vukelic S, Stojadinovic O, Pastar I, Rabach M, Krzyzanowska A, Lebrun E, Davis SC, Resnik S, Brem H, Tomic-Canic M. Cortisol synthesis in epidermis is induced by IL-1 and tissue injury. J Biol Chem 286: 10265–10275, 2011. doi: 10.1074/jbc.M110.188268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Stojadinovic O, Gordon KA, Lebrun E, Tomic-Canic M. Stress-induced hormones cortisol and epinephrine impair wound epithelization. Adv Wound Care (New Rochelle) 1: 29–35, 2012. doi: 10.1089/wound.2011.0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Terao M, Katayama I. Local cortisol/corticosterone activation in skin physiology and pathology. J Dermatol Sci 84: 11–16, 2016. doi: 10.1016/j.jdermsci.2016.06.014. [DOI] [PubMed] [Google Scholar]
- 137. Tiganescu A, Hupe M, Uchida Y, Mauro T, Elias PM, Holleran WM. Increased glucocorticoid activation during mouse skin wound healing. J Endocrinol 221: 51–61, 2014. doi: 10.1530/JOE-13-0420. [DOI] [PubMed] [Google Scholar]
- 138. Tiganescu A, Hupe M, Uchida Y, Mauro T, Elias PM, Holleran WM. Topical 11β-hydroxysteroid dehydrogenase type 1 inhibition corrects cutaneous features of systemic glucocorticoid excess in female mice. Endocrinology 159: 547–556, 2018. doi: 10.1210/en.2017-00607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Skobowiat C, Dowdy JC, Sayre RM, Tuckey RC, Slominski A. Cutaneous hypothalamic-pituitary-adrenal axis homolog: regulation by ultraviolet radiation. Am J Physiol Endocrinol Metab 301: E484–E493, 2011. doi: 10.1152/ajpendo.00217.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Skobowiat C, Sayre RM, Dowdy JC, Slominski AT. Ultraviolet radiation regulates cortisol activity in a waveband-dependent manner in human skin ex vivo. Br J Dermatol 168: 595–601, 2013. doi: 10.1111/bjd.12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Inoue T, Miki Y, Kakuo S, Hachiya A, Kitahara T, Aiba S, Zouboulis CC, Sasano H. Expression of steroidogenic enzymes in human sebaceous glands. J Endocrinol 222: 301–312, 2014. doi: 10.1530/JOE-14-0323. [DOI] [PubMed] [Google Scholar]
- 142. Samson M, Labrie F, Zouboulis CC, Luu-The V. Biosynthesis of dihydrotestosterone by a pathway that does not require testosterone as an intermediate in the SZ95 sebaceous gland cell line. J Invest Dermatol 130: 602–604, 2010. doi: 10.1038/jid.2009.225. [DOI] [PubMed] [Google Scholar]
- 143. Slominski A, Zjawiony J, Wortsman J, Semak I, Stewart J, Pisarchik A, Sweatman T, Marcos J, Dunbar C, Tuckey RC. A novel pathway for sequential transformation of 7-dehydrocholesterol and expression of the P450scc system in mammalian skin. Eur J Biochem 271: 4178–4188, 2004. doi: 10.1111/j.1432-1033.2004.04356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Slominski AT, Zmijewski MA, Semak I, Sweatman T, Janjetovic Z, Li W, Zjawiony JK, Tuckey RC. Sequential metabolism of 7-dehydrocholesterol to steroidal 5,7-dienes in adrenal glands and its biological implication in the skin. PLoS One 4: e4309, 2009. doi: 10.1371/journal.pone.0004309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Slominski AT, Kim TK, Chen J, Nguyen MN, Li W, Yates CR, Sweatman T, Janjetovic Z, Tuckey RC. Cytochrome P450scc-dependent metabolism of 7-dehydrocholesterol in placenta and epidermal keratinocytes. Int J Biochem Cell Biol 44: 2003–2018, 2012. doi: 10.1016/j.biocel.2012.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Slominski AT, Kim TK, Shehabi HZ, Semak I, Tang EK, Nguyen MN, Benson HA, Korik E, Janjetovic Z, Chen J, Yates CR, Postlethwaite A, Li W, Tuckey RC. In vivo evidence for a novel pathway of vitamin D(3) metabolism initiated by P450scc and modified by CYP27B1. Faseb j 26: 3901–3915, 2012. doi: 10.1096/fj.12-208975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Slominski AT, Kim TK, Li W, Postlethwaite A, Tieu EW, Tang EKY, Tuckey RC. Detection of novel CYP11A1-derived secosteroids in the human epidermis and serum and pig adrenal gland. Sci Rep 5: 14875, 2015. doi: 10.1038/srep14875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Truzzi F, Marconi A, Pincelli C. Neurotrophins in healthy and diseased skin. Dermatoendocrinol 3: 32–36, 2011. doi: 10.4161/derm.3.1.14661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Botchkarev VA, Yaar M, Peters EM, Raychaudhuri SP, Botchkareva NV, Marconi A, Raychaudhuri SK, Paus R, Pincelli C. Neurotrophins in skin biology and pathology. J Invest Dermatol 126: 1719–1727, 2006. doi: 10.1038/sj.jid.5700270. [DOI] [PubMed] [Google Scholar]
- 150. Donadio V, Incensi A, Vacchiano V, Infante R, Magnani M, Liguori R. The autonomic innervation of hairy skin in humans: an in vivo confocal study. Sci Rep 9: 16982, 2019. doi: 10.1038/s41598-019-53684-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Kos K, Harte AL, James S, Snead DR, O'Hare JP, McTernan PG, Kumar S. Secretion of neuropeptide Y in human adipose tissue and its role in maintenance of adipose tissue mass. Am J Physiol Endocrinol Metab 293: E1335–E1340, 2007. doi: 10.1152/ajpendo.00333.2007. [DOI] [PubMed] [Google Scholar]
- 152. Pincelli C, Fantini F, Massimi P, Giannetti A. Neuropeptide Y-like immunoreactivity in Langerhans cells from patients with atopic dermatitis. Int J Neurosci 51: 219–220, 1990. doi: 10.3109/00207459008999700. [DOI] [PubMed] [Google Scholar]
- 153. Johansson O. A detailed account of NPY-immunoreactive nerves and cells of the human skin. Comparison with VIP-, substance P- and PHI-containing structures. Acta Physiol Scand 128: 147–153, 1986. doi: 10.1111/j.1748-1716.1986.tb07961.x. [DOI] [PubMed] [Google Scholar]
- 154. Alysandratos KD, Asadi S, Angelidou A, Zhang B, Sismanopoulos N, Yang H, Critchfield A, Theoharides TC. Neurotensin and CRH interactions augment human mast cell activation. PLoS One 7: e48934, 2012. doi: 10.1371/journal.pone.0048934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Peters EM, Ericson ME, Hosoi J, Seiffert K, Hordinsky MK, Ansel JC, Paus R, Scholzen TE. Neuropeptide control mechanisms in cutaneous biology: physiological and clinical significance. J Invest Dermatol 126: 1937–1947, 2006. doi: 10.1038/sj.jid.5700429. [DOI] [PubMed] [Google Scholar]
- 156. Choi JE, Di Nardo A. Skin neurogenic inflammation. Semin Immunopathol 40: 249–259, 2018. doi: 10.1007/s00281-018-0675-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. N'Diaye A, Gannesen A, Borrel V, Maillot O, Enault J, Racine PJ, Plakunov V, Chevalier S, Lesouhaitier O, Feuilloley MG. Substance P and calcitonin gene-related peptide: key regulators of cutaneous microbiota homeostasis. Front Endocrinol (Lausanne) 8: 15, 2017. doi: 10.3389/fendo.2017.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Dallos A, Kiss M, Polyanka H, Dobozy A, Kemeny L, Husz S. Effects of the neuropeptides substance P, calcitonin gene-related peptide, vasoactive intestinal polypeptide and galanin on the production of nerve growth factor and inflammatory cytokines in cultured human keratinocytes. Neuropeptides 40: 251–263, 2006. doi: 10.1016/j.npep.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 159. Kobayashi T, Kronenberg HM, Foley J. Reduced expression of the PTH/PTHrP receptor during development of the mammary gland influences the function of the nipple during lactation. Dev Dyn 233: 794–803, 2005. doi: 10.1002/dvdy.20406. [DOI] [PubMed] [Google Scholar]
- 160. Cho YM, Lewis DA, Koltz PF, Richard V, Gocken TA, Rosol TJ, Konger RL, Spandau DF, Foley J. Regulation of parathyroid hormone-related protein gene expression by epidermal growth factor-family ligands in primary human keratinocytes. J Endocrinol 181: 179–190, 2004. doi: 10.1677/joe.0.1810179. [DOI] [PubMed] [Google Scholar]
- 161. Thomson M, McCarroll J, Bond J, Gordon-Thomson C, E DW, Moore GP. Parathyroid hormone-related peptide modulates signal pathways in skin and hair follicle cells. Exp Dermatol 12: 389–395, 2003. doi: 10.1034/j.1600-0625.2003.00068.x. [DOI] [PubMed] [Google Scholar]
- 162. Anderson ZT, Dawson AD, Slominski AT, Harris ML. Current insights into the role of neuropeptide Y in skin physiology and pathology. Front Endocrinol (Lausanne) 13: 838434, 2022. doi: 10.3389/fendo.2022.838434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Helyes Z, Kun J, Dobrosi N, Sándor K, Németh J, Perkecz A, Pintér E, Szabadfi K, Gaszner B, Tékus V, Szolcsányi J, Steinhoff M, Hashimoto H, Reglődi D, Bíró T. Pituitary adenylate cyclase-activating polypeptide is upregulated in murine skin inflammation and mediates transient receptor potential vanilloid-1-induced neurogenic edema. J Invest Dermatol 135: 2209–2218, 2015. doi: 10.1038/jid.2015.156. [DOI] [PubMed] [Google Scholar]
- 164. Banki E, Pakai E, Gaszner B, Zsiboras C, Czett A, Bhuddi PR, Hashimoto H, Toth G, Tamas A, Reglodi D, Garami A. Characterization of the thermoregulatory response to pituitary adenylate cyclase-activating polypeptide in rodents. J Mol Neurosci 54: 543–554, 2014. doi: 10.1007/s12031-014-0361-0. [DOI] [PubMed] [Google Scholar]
- 165. Botz B, Imreh A, Sándor K, Elekes K, Szolcsányi J, Reglődi D, Quinn JP, Stewart J, Zimmer A, Hashimoto H, Helyes Z. Role of pituitary adenylate-cyclase activating polypeptide and Tac1 gene derived tachykinins in sensory, motor and vascular functions under normal and neuropathic conditions. Peptides 43: 105–112, 2013. doi: 10.1016/j.peptides.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 166. Mikami N, Fukada S, Yamamoto H, Tsujikawa K. Neuronal derivative mediators that regulate cutaneous inflammations. Crit Rev Immunol 32: 307–320, 2012. doi: 10.1615/critrevimmunol.v32.i4.20. [DOI] [PubMed] [Google Scholar]
- 167. Slominski A. On the role of the corticotropin-releasing hormone signalling system in the aetiology of inflammatory skin disorders. Br J Dermatol 160: 229–232, 2009. doi: 10.1111/j.1365-2133.2008.08958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Karalis K, Sano H, Redwine J, Listwak S, Wilder RL, Chrousos GP. Autocrine or paracrine inflammatory actions of corticotropin-releasing hormone in vivo. Science 254: 421–423, 1991. doi: 10.1126/science.1925600. [DOI] [PubMed] [Google Scholar]
- 169. Crofford LJ, Sano H, Karalis K, Friedman TC, Epps HR, Remmers EF, Mathern P, Chrousos GP, Wilder RL. Corticotropin-releasing hormone in synovial fluids and tissues of patients with rheumatoid arthritis and osteoarthritis. J Immunol 151: 1587–1596, 1993. [PubMed] [Google Scholar]
- 170. Crofford LJ, Sano H, Karalis K, Webster EL, Goldmuntz EA, Chrousos GP, Wilder RL. Local secretion of corticotropin-releasing hormone in the joints of Lewis rats with inflammatory arthritis. J Clin Invest 90: 2555–2564, 1992. doi: 10.1172/JCI116150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Slominski A, Zbytek B, Zmijewski M, Slominski RM, Kauser S, Wortsman J, Tobin DJ. Corticotropin releasing hormone and the skin. Front Biosci 11: 2230–2248, 2006. doi: 10.2741/1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Zbytek B, Slominski AT. CRH mediates inflammation induced by lipopolysaccharide in human adult epidermal keratinocytes. J Invest Dermatol 127: 730–732, 2007. doi: 10.1038/sj.jid.5700607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Isard O, Knol AC, Castex-Rizzi N, Khammari A, Charveron M, Dreno B. Cutaneous induction of corticotropin releasing hormone by Propionibacterium acnes extracts. Dermatoendocrinol 1: 96–99, 2009. doi: 10.4161/derm.1.2.8102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Zbytek B, Pfeffer LM, Slominski AT. CRH inhibits NF-κB signaling in human melanocytes. Peptides 27: 3276–3283, 2006. doi: 10.1016/j.peptides.2006.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Slominski AT. On the role of the endogenous opioid system in regulating epidermal homeostasis. J Invest Dermatol 135: 333–334, 2015. doi: 10.1038/jid.2014.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Bigliardi PL, Tobin DJ, Gaveriaux-Ruff C, Bigliardi-Qi M. Opioids and the skin—where do we stand? Exp Dermatol 18: 424–430, 2009. doi: 10.1111/j.1600-0625.2009.00844.x. [DOI] [PubMed] [Google Scholar]
- 177. Slominski A, Tobin DJ, Shibahara S, Wortsman J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev 84: 1155–1228, 2004. doi: 10.1152/physrev.00044.2003. [DOI] [PubMed] [Google Scholar]
- 178. Bohm M, Luger TA, Tobin DJ, Garcia-Borron JC. Melanocortin receptor ligands: new horizons for skin biology and clinical dermatology. J Invest Dermatol 126: 1966–1975, 2006. doi: 10.1038/sj.jid.5700421. [DOI] [PubMed] [Google Scholar]
- 179. Zmijewski MA, Slominski AT. CRF1 receptor splicing in epidermal keratinocytes: potential biological role and environmental regulations. J Cell Physiol 218: 593–602, 2009. doi: 10.1002/jcp.21632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Zmijewski MA, Slominski AT. Modulation of corticotropin releasing factor (CRF) signaling through receptor splicing in mouse pituitary cell line AtT-20—emerging role of soluble isoforms. J Physiol Pharmacol 60, Suppl 4: 39–46, 2009. [PMC free article] [PubMed] [Google Scholar]
- 181. Pisarchik A, Slominski AT. Alternative splicing of CRH-R1 receptors in human and mouse skin: identification of new variants and their differential expression. FASEB J 15: 2754–2756, 2001. doi: 10.1096/fj.01-0487fje. [DOI] [PubMed] [Google Scholar]
- 182. Pisarchik A, Slominski A. Corticotropin releasing factor receptor type 1: molecular cloning and investigation of alternative splicing in the hamster skin. J Invest Dermatol 118: 1065–1072, 2002. doi: 10.1046/j.1523-1747.2002.01770.x. [DOI] [PubMed] [Google Scholar]
- 183. Pisarchik A, Slominski A. Molecular and functional characterization of novel CRFR1 isoforms from the skin. Eur J Biochem 271: 2821–2830, 2004. doi: 10.1111/j.1432-1033.2004.04216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Zmijewski MA, Slominski AT. Emerging role of alternative splicing of CRF1 receptor in CRF signaling. Acta Biochim Pol 57: 1–13, 2010. [PMC free article] [PubMed] [Google Scholar]
- 185. Slominski A. A nervous breakdown in the skin: stress and the epidermal barrier. J Clin Invest 117: 3166–3169, 2007. doi: 10.1172/JCI33508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Fischer TW, Bergmann A, Kruse N, Kleszczynski K, Skobowiat C, Slominski AT, Paus R. New effects of caffeine on corticotropin-releasing hormone (CRH)-induced stress along the intrafollicular classical hypothalamic-pituitary-adrenal (HPA) axis (CRH-R1/2, IP3 -R, ACTH, MC-R2) and the neurogenic non-HPA axis (substance P, p75(NTR) and TrkA) in ex vivo human male androgenetic scalp hair follicles. Br J Dermatol 184: 96–110, 2021. doi: 10.1111/bjd.19115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Skobowiat C, Nejati R, Lu L, Williams RW, Slominski AT. Genetic variation of the cutaneous HPA axis: an analysis of UVB-induced differential responses. Gene 530: 1–7, 2013. doi: 10.1016/j.gene.2013.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Marek-Jozefowicz L, Czajkowski R, Borkowska A, Nedoszytko B, Zmijewski MA, Cubala WJ, Slominski AT. The brain-skin axis in psoriasis-psychological, psychiatric, hormonal, and dermatological aspects. Int J Mol Sci 23: 669, 2022. doi: 10.3390/ijms23020669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Tagen M, Stiles L, Kalogeromitros D, Gregoriou S, Kempuraj D, Makris M, Donelan J, Vasiadi M, Staurianeas NG, Theoharides TC. Skin corticotropin-releasing hormone receptor expression in psoriasis. J Invest Dermatol 127: 1789–1791, 2007. doi: 10.1038/sj.jid.5700757. [DOI] [PubMed] [Google Scholar]
- 190. Zhou C, Yu X, Cai D, Liu C, Li C. Role of corticotropin-releasing hormone and receptor in the pathogenesis of psoriasis. Med Hypotheses 73: 513–515, 2009. doi: 10.1016/j.mehy.2009.02.051. [DOI] [PubMed] [Google Scholar]
- 191. Ito N, Sugawara K, Bodo E, Takigawa M, van Beek N, Ito T, Paus R. Corticotropin-releasing hormone stimulates the in situ generation of mast cells from precursors in the human hair follicle mesenchyme. J Invest Dermatol 130: 995–1004, 2010. doi: 10.1038/jid.2009.387. [DOI] [PubMed] [Google Scholar]
- 192. Cemil BC, Canpolat F, Yilmazer D, Eskioğlu F, Alper M. The association of PASI scores with CRH-R1 expression in patients with psoriasis. Arch Dermatol Res 304: 127–132, 2012. doi: 10.1007/s00403-012-1218-4. [DOI] [PubMed] [Google Scholar]
- 193. Vasiadi M, Therianou A, Sideri K, Smyrnioti M, Sismanopoulos N, Delivanis DA, Asadi S, Katsarou-Katsari A, Petrakopoulou T, Theoharides A, Antoniou C, Papadavid E, Stavrianeas N, Kalogeromitros D, Theoharides TC. Increased serum CRH levels with decreased skin CRHR-1 gene expression in psoriasis and atopic dermatitis. J Allergy Clin Immunol 129: 1410–1413, 2012. doi: 10.1016/j.jaci.2012.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Loite U, Kingo K, Reimann E, Reemann P, Vasar E, Silm H, Koks S. Gene expression analysis of the corticotrophin-releasing hormone-proopiomelanocortin system in psoriasis skin biopsies. Acta Derm Venereol 93: 400–405, 2013. doi: 10.2340/00015555-1524. [DOI] [PubMed] [Google Scholar]
- 195. Kim JE, Cho DH, Kim HS, Kim HJ, Lee JY, Cho BK, Park HJ. Expression of the corticotropin-releasing hormone-proopiomelanocortin axis in the various clinical types of psoriasis. Exp Dermatol 16: 104–109, 2007. doi: 10.1111/j.1600-0625.2006.00509.x. [DOI] [PubMed] [Google Scholar]
- 196. Bohm M. Neuroendocrine regulators: novel trends in sebaceous gland research with future perspectives for the treatment of acne and related disorders. Dermatoendocrinol 1: 136–140, 2009. doi: 10.4161/derm.1.3.9666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Slominski AT, Zmijewski MA. Glucocorticoids inhibit wound healing: novel mechanism of action. J Invest Dermatol 137: 1012–1014, 2017. doi: 10.1016/j.jid.2017.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Jozic I, Vukelic S, Stojadinovic O, Liang L, Ramirez HA, Pastar I, Tomic Canic M. Stress signals, mediated by membranous glucocorticoid receptor, activate PLC/PKC/GSK-3β/β-catenin pathway to inhibit wound closure. J Invest Dermatol 137: 1144–1154, 2017. doi: 10.1016/j.jid.2016.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Slominski AT, Brożyna AA, Tuckey RC. Cutaneous glucocorticoidogenesis and cortisol signaling are defective in psoriasis. J Invest Dermatol 137: 1609–1611, 2017. doi: 10.1016/j.jid.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Slominski A, Wortsman J, Mazurkiewicz JE, Matsuoka L, Dietrich J, Lawrence K, Gorbani A, Paus R. Detection of the proopiomelanocortin-derived antigens in normal and pathologic human skin. J Lab Clin Med 122: 658–666, 1993. [PubMed] [Google Scholar]
- 201. Funasaka Y, Sato H, Chakraborty AK, Ohashi A, Chrousos GP, Ichihashi M. Expression of proopiomelanocortin, corticotropin-releasing hormone (CRH), and CRH receptor in melanoma cells, nevus cells, and normal human melanocytes. J Investig Dermatol Symp Proc 4: 105–109, 1999. doi: 10.1038/sj.jidsp.5640192. [DOI] [PubMed] [Google Scholar]
- 202. Slominski A, Heasley D, Mazurkiewicz JE, Ermak G, Baker J, Carlson JA. Expression of proopiomelanocortin (POMC)-derived melanocyte-stimulating hormone (MSH) and adrenocorticotropic hormone (ACTH) peptides in skin of basal cell carcinoma patients. Hum Pathol 30: 208–215, 1999. doi: 10.1016/s0046-8177(99)90278-2. [DOI] [PubMed] [Google Scholar]
- 203. Nagahama M, Funasaka Y, Fernandez-Frez ML, Ohashi A, Chakraborty A, Ueda M, Ichihashi M. Immunoreactivity of a-melanocyte-stimulating hormone, adrenocorticotrophic hormone, and b-endorphin in cutaneous malignant melanoma and benign melanocytic naevi. Br J Dermatol 138: 981–985, 1998. doi: 10.1046/j.1365-2133.1998.02263.x. [DOI] [PubMed] [Google Scholar]
- 204. Slominski AT, Carlson JA. Melanoma resistance: a bright future for academicians and a challenge for patient advocates. Mayo Clin Proc 89: 429–433, 2014. doi: 10.1016/j.mayocp.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Skobowiat C, Slominski AT. UVB activates hypothalamic-pituitary-adrenal axis in C57BL/6 mice. J Invest Dermatol 135: 1638–1648, 2015. doi: 10.1038/jid.2014.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Skobowiat C, Postlethwaite AE, Slominski AT. Skin exposure to ultraviolet B rapidly activates systemic neuroendocrine and immunosuppressive responses. Photochem Photobiol 93: 1008–1015, 2017. doi: 10.1111/php.12642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Skobowiat C, Slominski AT. Ultraviolet B stimulates proopiomelanocortin signalling in the arcuate nucleus of the hypothalamus in mice. Exp Dermatol 25: 120–123, 2016. doi: 10.1111/exd.12890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Hiramoto K, Yanagihara N, Sato EF, Inoue M. Ultraviolet B irradiation of the eye activates a nitric oxide-dependent hypothalamopituitary proopiomelanocortin pathway and modulates functions of α-melanocyte-stimulating hormone-responsive cells. J Invest Dermatol 120: 123–127, 2003. doi: 10.1046/j.1523-1747.2003.12004.x. [DOI] [PubMed] [Google Scholar]
- 209. Han M, Ban JJ, Bae JS, Shin CY, Lee DH, Chung JH. UV irradiation to mouse skin decreases hippocampal neurogenesis and synaptic protein expression via HPA axis activation. Sci Rep 7: 15574, 2017. doi: 10.1038/s41598-017-15773-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Slominski AT. Ultraviolet radiation (UVR) activates central neuro-endocrine-immune system. Photodermatol Photoimmunol Photomed 31: 121–123, 2015. doi: 10.1111/phpp.12165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Slominski AT, Slominski RM, Raman C. UVB stimulates production of enkephalins and other neuropeptides by skin-resident cells. Proc Natl Acad Sci USA 118: e2020425118, 2021. doi: 10.1073/pnas.2020425118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Misery L, Loser K, Stander S. Sensitive skin. J Eur Acad Dermatol Venereol 30, Suppl 1: 2–8, 2016. doi: 10.1111/jdv.13532. [DOI] [PubMed] [Google Scholar]
- 213. Riol-Blanco L, Ordovas-Montanes J, Perro M, Naval E, Thiriot A, Alvarez D, Paust S, Wood JN, von Andrian UH. Nociceptive sensory neurons drive interleukin-23-mediated psoriasiform skin inflammation. Nature 510: 157–161, 2014. doi: 10.1038/nature13199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Ding W, Stohl LL, Saab J, Azizi S, Zhou XK, Mehta D, Granstein RD. Regulation of cutaneous immunity in vivo by calcitonin gene-related peptide signaling through endothelial cells. J Immunol 208: 633–641, 2022. doi: 10.4049/jimmunol.2100139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Lambert RW, Campton K, Ding W, Ozawa H, Granstein RD. Langerhans cell expression of neuropeptide Y and peptide YY. Neuropeptides 36: 246–251, 2002. doi: 10.1016/s0143-4179(02)00020-3. [DOI] [PubMed] [Google Scholar]
- 216. Slominski AT, Botchkarev V, Choudhry M, Fazal N, Fechner K, Furkert J, Krause E, Roloff B, Sayeed M, Wei E, Zbytek B, Zipper J, Wortsman J, Paus R. Cutaneous expression of CRH and CRH-R. Is there a “skin stress response system?”. Ann N Y Acad Sci 885: 287–311, 1999. doi: 10.1111/j.1749-6632.1999.tb08686.x. [DOI] [PubMed] [Google Scholar]
- 217. McGlone F, Reilly D. The cutaneous sensory system. Neurosci Biobehav Rev 34: 148–159, 2010. doi: 10.1016/j.neubiorev.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 218. Slominski AT, Zmijewski MA, Zbytek B, Brozyna AA, Granese J, Pisarchik A, Szczesniewski A, Tobin DJ. Regulated proenkephalin expression in human skin and cultured skin cells. J Invest Dermatol 131: 613–622, 2011. doi: 10.1038/jid.2010.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Arenas OM, Zaharieva EE, Para A, Vásquez-Doorman C, Petersen CP, Gallio M. Activation of planarian TRPA1 by reactive oxygen species reveals a conserved mechanism for animal nociception. Nat Neurosci 20: 1686–1693, 2017. doi: 10.1038/s41593-017-0005-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Slominski A, Pawelek J. Animals under the sun: effects of ultraviolet radiation on mammalian skin. Clin Dermatol 16: 503–515, 1998. doi: 10.1016/s0738-081x(98)00023-6. [DOI] [PubMed] [Google Scholar]
- 221. Slominski A, Wortsman J. Self-regulated endocrine systems in the skin. Minerva Endocrinol 28: 135–143, 2003. [PubMed] [Google Scholar]
- 222. Gallo RL, Hooper LV. Epithelial antimicrobial defence of the skin and intestine. Nat Rev Immunol 12: 503–516, 2012. doi: 10.1038/nri3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Liu CF, Li XL, Zhang ZL, Qiu L, Ding SX, Xue JX, Zhao GP, Li J. Antiaging effects of urolithin A on replicative senescent human skin fibroblasts. Rejuvenation Res 22: 191–200, 2019. doi: 10.1089/rej.2018.2066. [DOI] [PubMed] [Google Scholar]
- 224. Grando SA, Kawashima K, Wessler I. Introduction: the non-neuronal cholinergic system in humans. Life Sci 72: 2009–2012, 2003. doi: 10.1016/s0024-3205(03)00063-8. [DOI] [PubMed] [Google Scholar]
- 225. Kurzen H, Wessler I, Kirkpatrick CJ, Kawashima K, Grando SA. The non-neuronal cholinergic system of human skin. Horm Metab Res 39: 125–135, 2007. doi: 10.1055/s-2007-961816. [DOI] [PubMed] [Google Scholar]
- 226. Pullar CE, Rizzo A, Isseroff RR. β-Adrenergic receptor antagonists accelerate skin wound healing: evidence for a catecholamine synthesis network in the epidermis. J Biol Chem 281: 21225–21235, 2006. doi: 10.1074/jbc.M601007200. [DOI] [PubMed] [Google Scholar]
- 227. Pullar CE, Le Provost GS, O'Leary AP, Evans SE, Baier BS, Isseroff RR. beta2AR antagonists and beta2AR gene deletion both promote skin wound repair processes. J Invest Dermatol 132: 2076–2084, 2012. doi: 10.1038/jid.2012.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Sivamani RK, Porter SM, Isseroff RR. An epinephrine-dependent mechanism for the control of UV-induced pigmentation. J Invest Dermatol 129: 784–787, 2009. doi: 10.1038/jid.2008.262. [DOI] [PubMed] [Google Scholar]
- 229. Sivamani RK, Lam ST, Isseroff RR. β Adrenergic receptors in keratinocytes. Dermatol Clin 25: 643–653, x, 2007. doi: 10.1016/j.det.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Yang HY, Steenhuis P, Glucksman AM, Gurenko Z, La TD, Isseroff RR. α and β Adrenergic receptors modulate keratinocyte migration. PLoS One 16: e0253139, 2021. doi: 10.1371/journal.pone.0253139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Farman N, Maubec E, Poeggeler B, Klatte JE, Jaisser F, Paus R. The mineralocorticoid receptor as a novel player in skin biology: beyond the renal horizon? Exp Dermatol 19: 100–107, 2010. doi: 10.1111/j.1600-0625.2009.01011.x. [DOI] [PubMed] [Google Scholar]
- 232. Lili LN, Klopot A, Readhead B, Baida G, Dudley JT, Budunova I. Transcriptomic network interactions in human skin treated with topical glucocorticoid clobetasol propionate. J Invest Dermatol 139: 2281–2291, 2019. doi: 10.1016/j.jid.2019.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Nguyen VT, Farman N, Maubec E, Nassar D, Desposito D, Waeckel L, Aractingi S, Jaisser F. Re-epithelialization of pathological cutaneous wounds is improved by local mineralocorticoid receptor antagonism. J Invest Dermatol 136: 2080–2089, 2016. doi: 10.1016/j.jid.2016.05.101. [DOI] [PubMed] [Google Scholar]
- 234. Bikle DD. Vitamin D: newer concepts of its metabolism and function at the basic and clinical level. J Endocr Soc 4: bvz038, 2020. doi: 10.1210/jendso/bvz038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Reichrath J, Reichrath S, Vogt T, Romer K. Crosstalk between vitamin D and p53 signaling in cancer: an update. Adv Exp Med Biol 1268: 307–318, 2020. doi: 10.1007/978-3-030-46227-7_15. [DOI] [PubMed] [Google Scholar]
- 236. Pastore S, Mascia F, Gulinelli S, Forchap S, Dattilo C, Adinolfi E, Girolomoni G, Di Virgilio F, Ferrari D. Stimulation of purinergic receptors modulates chemokine expression in human keratinocytes. J Invest Dermatol 127: 660–667, 2007. doi: 10.1038/sj.jid.5700591. [DOI] [PubMed] [Google Scholar]
- 237. Granstein RD, Ding W, Huang J, Holzer A, Gallo RL, Di Nardo A, Wagner JA. Augmentation of cutaneous immune responses by ATP γ S: purinergic agonists define a novel class of immunologic adjuvants. J Immunol 174: 7725–7731, 2005. [Erratum in J Immunol 181: 2933, 2008]. doi: 10.4049/jimmunol.174.12.7725. [DOI] [PubMed] [Google Scholar]
- 238. Slater M, Scolyer RA, Gidley-Baird A, Thompson JF, Barden JA. Increased expression of apoptotic markers in melanoma. Melanoma Res 13: 137–145, 2003. doi: 10.1097/00008390-200304000-00005. [DOI] [PubMed] [Google Scholar]
- 239. Denda M, Inoue K, Fuziwara S, Denda S. P2X purinergic receptor antagonist accelerates skin barrier repair and prevents epidermal hyperplasia induced by skin barrier disruption. J Invest Dermatol 119: 1034–1040, 2002. doi: 10.1046/j.1523-1747.2002.19505.x. [DOI] [PubMed] [Google Scholar]
- 240. Denda M, Inoue K, Inomata S, Denda S. γ-Aminobutyric acid (A) receptor agonists accelerate cutaneous barrier recovery and prevent epidermal hyperplasia induced by barrier disruption. J Invest Dermatol 119: 1041–1047, 2002. doi: 10.1046/j.1523-1747.2002.19504.x. [DOI] [PubMed] [Google Scholar]
- 241. Xu X, Yu C, Xu L, Xu J. Emerging roles of keratinocytes in nociceptive transduction and regulation. Front Mol Neurosci 15: 982202, 2022. doi: 10.3389/fnmol.2022.982202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Hoogduijn MJ, Hitchcock IS, Smit NP, Gillbro JM, Schallreuter KU, Genever PG. Glutamate receptors on human melanocytes regulate the expression of MiTF. Pigment Cell Res 19: 58–67, 2006. doi: 10.1111/j.1600-0749.2005.00284.x. [DOI] [PubMed] [Google Scholar]
- 243. Morhenn VB, Murakami M, O'Grady T, Nordberg J, Gallo RL. Characterization of the expression and function of N-methyl-d-aspartate receptor in keratinocytes. Exp Dermatol 13: 505–511, 2004. doi: 10.1111/j.0906-6705.2004.00186.x. [DOI] [PubMed] [Google Scholar]
- 244. Fischer M, Glanz D, William T, Klapperstuck T, Wohlrab J, Marsch W. N-methyl-d-aspartate receptors influence the intracellular calcium concentration of keratinocytes. Exp Dermatol 13: 512–519, 2004. doi: 10.1111/j.0906-6705.2004.00200.x. [DOI] [PubMed] [Google Scholar]
- 245. Fuziwara S, Inoue K, Denda M. NMDA-type glutamate receptor is associated with cutaneous barrier homeostasis. J Invest Dermatol 120: 1023–1029, 2003. doi: 10.1046/j.1523-1747.2003.12238.x. [DOI] [PubMed] [Google Scholar]
- 246. Ständer S, Steinhoff M, Schmelz M, Weisshaar E, Metze D, Luger T. Neurophysiology of pruritus: cutaneous elicitation of itch. Arch Dermatol 139: 1463–1470, 2003. doi: 10.1001/archderm.139.11.1463. [DOI] [PubMed] [Google Scholar]
- 247. Zmijewski MA, Carlberg C. Vitamin D receptor(s): in the nucleus but also at membranes? Exp Dermatol 29: 876–884, 2020. doi: 10.1111/exd.14147. [DOI] [PubMed] [Google Scholar]
- 248. Nestle FO, Di Meglio P, Qin JZ, Nickoloff BJ. Skin immune sentinels in health and disease. Nat Rev Immunol 9: 679–691, 2009. doi: 10.1038/nri2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Nguyen AV, Soulika AM. The dynamics of the skin's immune system. Int J Mol Sci 20: 1811, 2019. doi: 10.3390/ijms20081811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Bocheva GS, Slominski Rm, Slominski AT. Immunological aspects of skin aging in atopic dermatitis. Int J Mol Sci 22: 5729, 2021. doi: 10.3390/ijms22115729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Wood LC, Jackson SM, Elias PM, Grunfeld C, Feingold KR. Cutaneous barrier perturbation stimulates cytokine production in the epidermis of mice. J Clin Invest 90: 482–487, 1992. doi: 10.1172/JCI115884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Slominski A. Neuroendocrine activity of the melanocyte. Exp Dermatol 18: 760–763, 2009. doi: 10.1111/j.1600-0625.2009.00892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Slominski A, Paus R, Schadendorf D. Melanocytes as “sensory” and regulatory cells in the epidermis. J Theor Biol 164: 103–120, 1993. doi: 10.1006/jtbi.1993.1142. [DOI] [PubMed] [Google Scholar]
- 254. D'Orazio J, Jarrett S, Amaro-Ortiz A, Scott T. UV radiation and the skin. Int J Mol Sci 14: 12222–12248, 2013. doi: 10.3390/ijms140612222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Pawelek JM, Chakraborty AK, Osber MP, Orlow SJ, Min KK, Rosenzweig KE, Bolognia JL. Molecular cascades in UV-induced melanogenesis: a central role for melanotropins? Pigment Cell Res 5: 348–356, 1992. doi: 10.1111/j.1600-0749.1992.tb00561.x. [DOI] [PubMed] [Google Scholar]
- 256. Tsutsumi M, Ikeyama K, Denda S, Nakanishi J, Fuziwara S, Aoki H, Denda M. Expressions of rod and cone photoreceptor-like proteins in human epidermis. Exp Dermatol 18: 567–570, 2009. doi: 10.1111/j.1600-0625.2009.00851.x. [DOI] [PubMed] [Google Scholar]
- 257. Goto M, Ikeyama K, Tsutsumi M, Denda S, Denda M. Phosphodiesterase inhibitors block the acceleration of skin permeability barrier repair by red light. Exp Dermatol 20: 568–571, 2011. doi: 10.1111/j.1600-0625.2011.01255.x. [DOI] [PubMed] [Google Scholar]
- 258. Regazzetti C, Sormani L, Debayle D, Bernerd F, Tulic MK, De Donatis GM, Chignon-Sicard B, Rocchi S, Passeron T. Melanocytes sense blue light and regulate pigmentation through Opsin-3. J Invest Dermatol 138: 171–178, 2018. doi: 10.1016/j.jid.2017.07.833. [DOI] [PubMed] [Google Scholar]
- 259. Castellano-Pellicena I, Uzunbajakava NE, Mignon C, Raafs B, Botchkarev VA, Thornton MJ. Does blue light restore human epidermal barrier function via activation of Opsin during cutaneous wound healing? Lasers Surg Med 51: 370–382, 2019. doi: 10.1002/lsm.23015. [DOI] [PubMed] [Google Scholar]
- 260. de Assis LVM, Moraes MN, Magalhães-Marques KK, Castrucci AML. Melanopsin and rhodopsin mediate UVA-induced immediate pigment darkening: unravelling the photosensitive system of the skin. Eur J Cell Biol 97: 150–162, 2018. doi: 10.1016/j.ejcb.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 261. de Assis LVM, Tonolli PN, Moraes MN, Baptista MS, de Lauro Castrucci AM. How does the skin sense sun light? An integrative view of light sensing molecules. J Photochem Photobiol C: Photochem Rev 47: 100403, 2021. doi: 10.1016/j.jphotochemrev.2021.100403. [DOI] [Google Scholar]
- 262. Potenza MN, Lerner MR. A rapid quantitative bioassay for evaluating the effects of ligands upon receptors that modulate cAMP levels in a melanophore cell line. Pigment Cell Res 5: 372–378, 1992. doi: 10.1111/j.1600-0749.1992.tb00565.x. [DOI] [PubMed] [Google Scholar]
- 263. Daniolos A, Lerner AB, Lerner MR. Action of light on frog pigment cells in culture. Pigment Cell Res 3: 38–43, 1990. doi: 10.1111/j.1600-0749.1990.tb00260.x. [DOI] [PubMed] [Google Scholar]
- 264. Kono M, Nagata H, Umemura S, Kawana S, Osamura RY. In situ expression of corticotropin-releasing hormone (CRH) and proopiomelanocortin (POMC) genes in human skin. FASEB J 15: 2297–2299, 2001. doi: 10.1096/fj.01-0254fje. [DOI] [PubMed] [Google Scholar]
- 265. Zbytek B, Wortsman J, Slominski A. Characterization of a ultraviolet B-induced corticotropin-releasing hormone-proopiomelanocortin system in human melanocytes. Mol Endocrinol 20: 2539–2547, 2006. doi: 10.1210/me.2006-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Kauser S, Slominski A, Wei ET, Tobin DJ. Modulation of the human hair follicle pigmentary unit by corticotropin-releasing hormone and urocortin peptides. FASEB J 20: 882–895, 2006. doi: 10.1096/fj.05-5257com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Sato H, Nagashima Y, Chrousos GP, Ichihashi M, Funasak Y. The expression of corticotropin-releasing hormone in melanoma. Pigment Cell Res 15: 98–103, 2002. doi: 10.1034/j.1600-0749.2002.1o063.x. [DOI] [PubMed] [Google Scholar]
- 268. Wierzbicka JM, Żmijewski MA, Piotrowska A, Nedoszytko B, Lange M, Tuckey RC, Slominski AT. Bioactive forms of vitamin D selectively stimulate the skin analog of the hypothalamus-pituitary-adrenal axis in human epidermal keratinocytes. Mol Cell Endocrinol 437: 312–322, 2016. doi: 10.1016/j.mce.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Fazal N, Slominski A, Choudhry MA, Wei ET, Sayeed MM. Effect of CRF and related peptides on calcium signaling in human and rodent melanoma cells. FEBS Lett 435: 187–190, 1998. doi: 10.1016/s0014-5793(98)01067-9. [DOI] [PubMed] [Google Scholar]
- 270. Slominski AT, Roloff B, Zbytek B, Wei ET, Fechner K, Curry J, Wortsman J. Corticotropin releasing hormone and related peptides can act as bioregulatory factors in human keratinocytes. In Vitro Cell Dev Biol Anim 36: 211–216, 2000. doi:. [DOI] [PubMed] [Google Scholar]
- 271. Slominski A, Zbytek B, Pisarchik A, Slominski RM, Zmijewski MA, Wortsman J. CRH functions as a growth factor/cytokine in the skin. J Cell Physiol 206: 780–791, 2006. doi: 10.1002/jcp.20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Zbytek B, Slominski AT. Corticotropin-releasing hormone induces keratinocyte differentiation in the adult human epidermis. J Cell Physiol 203: 118–126, 2005. doi: 10.1002/jcp.20209. [DOI] [PubMed] [Google Scholar]
- 273. Wiesner B, Roloff B, Fechner K, Slominski A. Intracellular calcium measurements of single human skin cells after stimulation with corticotropin-releasing factor and urocortin using confocal laser scanning microscopy. J Cell Sci 116: 1261–1268, 2003. doi: 10.1242/jcs.00301. [DOI] [PubMed] [Google Scholar]
- 274. Wierzbicka JM, Żmijewski MA, Antoniewicz J, Sobjanek M, Slominski AT. Differentiation of keratinocytes modulates skin HPA analog. J Cell Physiol 232: 154–166, 2017. doi: 10.1002/jcp.25400. [DOI] [PubMed] [Google Scholar]
- 275. Pisarchik A, Wortsman J, Slominski A. A novel microarray to evaluate stress-related genes in skin: effect of ultraviolet light radiation. Gene 341: 199–207, 2004. doi: 10.1016/j.gene.2004.06.046. [DOI] [PubMed] [Google Scholar]
- 276. Hillhouse EW, Grammatopoulos DK. The molecular mechanisms underlying the regulation of the biological activity of corticotropin-releasing hormone receptors: implications for physiology and pathophysiology. Endocr Rev 27: 260–286, 2006. doi: 10.1210/er.2005-0034. [DOI] [PubMed] [Google Scholar]
- 277. Grammatopoulos DK. The role of CRH receptors and their agonists in myometrial contractility and quiescence during pregnancy and labour. Front Biosci 12: 561–571, 2007. doi: 10.2741/2082. [DOI] [PubMed] [Google Scholar]
- 278. Grammatopoulos DK. CRH-R splicing in estrogen-sensitive breast cancer. Cell Cycle 13: 687–688, 2014. doi: 10.4161/cc.27858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Grammatopoulos DK, Chrousos GP. Functional characteristics of CRH receptors and potential clinical applications of CRH-receptor antagonists. Trends Endocrinol Metab 13: 436–444, 2002. doi: 10.1016/s1043-2760(02)00670-7. [DOI] [PubMed] [Google Scholar]
- 280. Grammatopoulos DK, Ourailidou S. CRH receptor signalling: potential roles in pathophysiology. Curr Mol Pharmacol 10: 296–310, 2017. doi: 10.2174/1874467210666170110125747. [DOI] [PubMed] [Google Scholar]
- 281. Quevedo M-E, Slominski A, Pinto W, Wei E, Wortsman J. Pleiotropic effects of corticotropin releasing hormone (CRH) on normal human keratinocytes. In Vitro Cell Dev Biol Anim 37: 50–54, 2001. doi:. [DOI] [PubMed] [Google Scholar]
- 282. Zbytek B, Mysliwski A, Slominski A, Wortsman J, Wei ET, Mysliwska J. Corticotropin-releasing hormone affects cytokine production in human HaCaT keratinocytes. Life Sci 70: 1013–1021, 2002. doi: 10.1016/s0024-3205(01)01476-x. [DOI] [PubMed] [Google Scholar]
- 283. Zbytek B, Pfeffer LM, Slominski AT. Corticotropin-releasing hormone stimulates NF-kappaB in human epidermal keratinocytes. J Endocrinol 181: R1–R7, 2004. doi: 10.1677/joe.0.181r001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284. Zbytek B, Pikula M, Slominski RM, Mysliwski A, Wei E, Wortsman J, Slominski AT. Corticotropin-releasing hormone triggers differentiation in HaCaT keratinocytes. Br J Dermatol 152: 474–480, 2005. doi: 10.1111/j.1365-2133.2005.06217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285. Park HJ, Kim HJ, Lee JH, Lee JY, Cho BK, Kang JS, Kang H, Yang Y, Cho DH. Corticotropin-releasing hormone (CRH) downregulates interleukin-18 expression in human HaCaT keratinocytes by activation of p38 mitogen-activated protein kinase (MAPK) pathway. J Invest Dermatol 124: 751–755, 2005. doi: 10.1111/j.0022-202X.2005.23656.x. [DOI] [PubMed] [Google Scholar]
- 286. Zbytek B, Pfeffer LM, Slominski AT. Corticotropin-releasing hormone inhibits nuclear factor-kappaB pathway in human HaCaT keratinocytes. J Invest Dermatol 121: 1496–1499, 2003. doi: 10.1111/j.1523-1747.2003.12612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. Schauer E, Trautinger F, Kock A, Schwarz A, Bhardwaj R, Simon M, Ansel JC, Schwarz T, Luger TA. Proopiomelanocortin-derived peptides are synthesized and released by human keratinocytes. J Clin Invest 93: 2258–2262, 1994. doi: 10.1172/JCI117224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288. Slominski A, Ermak G, Hwang J, Chakraborty A, Mazurkiewicz JE, Mihm M. Proopiomelanocortin, corticotropin releasing hormone and corticotropin releasing hormone receptor genes are expressed in human skin. FEBS Lett 374: 113–116, 1995. doi: 10.1016/0014-5793(95)01090-2. [DOI] [PubMed] [Google Scholar]
- 289. Chakraborty AK, Funasaka Y, Slominski A, Bolognia J, Sodi S, Ichihashi M, Pawelek JM. UV light and MSH receptors. Ann NY Acad Sci 885: 100–116, 1999. doi: 10.1111/j.1749-6632.1999.tb08668.x. [DOI] [PubMed] [Google Scholar]
- 290. Upadhyay PR, Ho T, Abdel-Malek ZA. Participation of keratinocyte- and fibroblast-derived factors in melanocyte homeostasis, the response to UV, and pigmentary disorders. Pigment Cell Melanoma Res 34: 762–776, 2021. doi: 10.1111/pcmr.12985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291. Yardman-Frank JM, Fisher DE. Skin pigmentation and its control: from ultraviolet radiation to stem cells. Exp Dermatol 30: 560–571, 2021. doi: 10.1111/exd.14260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292. Rousseau K, Kauser S, Pritchard LE, Warhurst A, Oliver RL, Slominski A, Wei ET, Thody AJ, Tobin DJ, White A. Proopiomelanocortin (POMC), the ACTH/melanocortin precursor, is secreted by human epidermal keratinocytes and melanocytes and stimulates melanogenesis. FASEB J 21: 1844–1856, 2007. doi: 10.1096/fj.06-7398com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Peters EM, Tobin DJ, Seidah NG, Schallreuter KU. Pro-opiomelanocortin-related peptides, prohormone convertases 1 and 2 and the regulatory peptide 7B2 are present in melanosomes of human melanocytes. J Invest Dermatol 114: 430–437, 2000. doi: 10.1046/j.1523-1747.2000.00913.x. [DOI] [PubMed] [Google Scholar]
- 294. Mazurkiewicz JE, Corliss D, Slominski A. Spatiotemporal expression, distribution, and processing of POMC and POMC-derived peptides in murine skin. J Histochem Cytochem 48: 905–914, 2000. doi: 10.1177/002215540004800703. [DOI] [PubMed] [Google Scholar]
- 295. Scholzen TE, Brzoska T, Kalden DH, Hartmeyer M, Fastrich M, Luger TA, Armstrong CA, Ansel JC. Expression of functional melanocortin receptors and proopiomelanocortin peptides by human dermal microvascular endothelial cells. Ann N Y Acad Sci 885: 239–253, 1999. doi: 10.1111/j.1749-6632.1999.tb08681.x. [DOI] [PubMed] [Google Scholar]
- 296. Scholzen TE, Kalden DH, Brzoska T, Fisbeck T, Fastrich M, Schiller M, Bohm M, Schwarz T, Armstrong CA, Ansel JC, Luger TA. Expression of proopiomelanocortin peptides in human dermal microvascular endothelial cells: evidence for a regulation by ultraviolet light and interleukin-1. J Invest Dermatol 115: 1021–1028, 2000. [Erratum in J Invest Dermatol 116: 829, 2001]. doi: 10.1046/j.1523-1747.2000.00174.x. [DOI] [PubMed] [Google Scholar]
- 297. Schiller M, Raghunath M, Kubitscheck U, Scholzen TE, Fisbeck T, Metze D, Luger TA, Bohm M. Human dermal fibroblasts express prohormone convertases 1 and 2 and produce proopiomelanocortin-derived peptides. J Invest Dermatol 117: 227–235, 2001. doi: 10.1046/j.0022-202x.2001.01412.x. [DOI] [PubMed] [Google Scholar]
- 298. Zmijewski MA, Sharma RK, Slominski AT. Expression of molecular equivalent of hypothalamic-pituitary-adrenal axis in adult retinal pigment epithelium. J Endocrinol 193: 157–169, 2007. doi: 10.1677/joe.1.06927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299. Jussila A, Huotari-Orava R, Ylianttila L, Partonen T, Snellman E. Narrow-band ultraviolet B radiation induces the expression of beta-endorphin in human skin in vivo. J Photochem Photobiol B 155: 104–108, 2016. doi: 10.1016/j.jphotobiol.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 300. Schiller M, Brzoska T, Bohm M, Metze D, Scholzen TE, Rougier A, Luger TA. Solar-simulated ultraviolet radiation-induced upregulation of the melanocortin-1 receptor, proopiomelanocortin, and alpha-melanocyte-stimulating hormone in human epidermis in vivo. J Invest Dermatol 122: 468–476, 2004. doi: 10.1046/j.0022-202X.2004.22239.x. [DOI] [PubMed] [Google Scholar]
- 301. Fell GL, Robinson KC, Mao J, Woolf CJ, Fisher DE. Skin β-endorphin mediates addiction to UV light. Cell 157: 1527–1534, 2014. doi: 10.1016/j.cell.2014.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302. Slominski A. Beta-endorphin/mu-opiate receptor system in the skin. J Invest Dermatol 120: xii–xiii, 2003. doi: 10.1046/j.1523-1747.2003.12258.x. [DOI] [PubMed] [Google Scholar]
- 303. Leong C, Neumann C, Ramasamy S, Rout B, Yi Wee L, Bigliardi-Qi M, Bigliardi PL. Investigating endogenous µ-opioid receptors in human keratinocytes as pharmacological targets using novel fluorescent ligand. PLoS One 12: e0188607, 2017. doi: 10.1371/journal.pone.0188607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304. Abdel-Malek ZA, Kadekaro AL, Swope VB. Stepping up melanocytes to the challenge of UV exposure. Pigment Cell Melanoma Res 23: 171–186, 2010. doi: 10.1111/j.1755-148X.2010.00679.x. [DOI] [PubMed] [Google Scholar]
- 305. Kadekaro AL, Chen J, Yang J, Chen S, Jameson J, Swope VB, Cheng T, Kadakia M, Abdel-Malek Z. α-Melanocyte-stimulating hormone suppresses oxidative stress through a p53-mediated signaling pathway in human melanocytes. Mol Cancer Res 10: 778–786, 2012. doi: 10.1158/1541-7786.MCR-11-0436. [DOI] [PubMed] [Google Scholar]
- 306. Tsatmali M, Ancans J, Thody AJ. Melanocyte function and its control by melanocortin peptides. J Histochem Cytochem 50: 125–133, 2002. doi: 10.1177/002215540205000201. [DOI] [PubMed] [Google Scholar]
- 307. Luger TA, Brzoska T, Scholzen TE, Kalden DH, Sunderkotter C, Armstrong C, Ansel J. The role of alpha-MSH as a modulator of cutaneous inflammation. Ann NY Acad Sci 917: 232–238, 2000. doi: 10.1111/j.1749-6632.2000.tb05388.x. [DOI] [PubMed] [Google Scholar]
- 308. Brzoska T, Luger TA, Maaser C, Abels C, Bohm M. Alpha-melanocyte-stimulating hormone and related tripeptides: biochemistry, antiinflammatory and protective effects in vitro and in vivo, and future perspectives for the treatment of immune-mediated inflammatory diseases. Endocr Rev 29: 581–602, 2008. doi: 10.1210/er.2007-0027. [DOI] [PubMed] [Google Scholar]
- 309. Swope VB, Abdel-Malek ZA. MC1R: front and center in the bright side of dark eumelanin and DNA repair. Int J Mol Sci 19: 2667, 2018. doi: 10.3390/ijms19092667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310. Herraiz C, Martinez-Vicente I, Maresca V. The alpha-melanocyte-stimulating hormone/melanocortin-1 receptor interaction: a driver of pleiotropic effects beyond pigmentation. Pigment Cell Melanoma Res 34: 748–761, 2021. doi: 10.1111/pcmr.12980. [DOI] [PubMed] [Google Scholar]
- 311. Bohm M, Wolff I, Scholzen TE, Robinson SJ, Healy E, Luger TA, Schwarz T, Schwarz A. alpha-Melanocyte-stimulating hormone protects from ultraviolet radiation-induced apoptosis and DNA damage. J Biol Chem 280: 5795–5802, 2005. doi: 10.1074/jbc.M406334200. [DOI] [PubMed] [Google Scholar]
- 312. Cao J, Wan L, Hacker E, Dai X, Lenna S, Jimenez-Cervantes C, Wang Y, Leslie NR, Xu GX, Widlund HR, Ryu B, Alani RM, Dutton-Regester K, Goding CR, Hayward NK, Wei W, Cui R. MC1R is a potent regulator of PTEN after UV exposure in melanocytes. Mol Cell 51: 409–422, 2013. doi: 10.1016/j.molcel.2013.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313. Haycock JW, Wagner M, Morandini R, Ghanem G, Rennie IG, Mac Neil S. α-Melanocyte-stimulating hormone inhibits NF-κB activation in human melanocytes and melanoma cells. J Invest Dermatol 113: 560–566, 1999. doi: 10.1046/j.1523-1747.1999.00739.x. [DOI] [PubMed] [Google Scholar]
- 314. Eves PC, MacNeil S, Haycock JW. α-Melanocyte stimulating hormone, inflammation and human melanoma. Peptides 27: 444–452, 2006. doi: 10.1016/j.peptides.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 315. Haycock JW, Wagner M, Morandini R, Ghanem G, Rennie IG, MacNeil S. α-MSH immunomodulation acts via rel/NF-kappa B in cutaneous and ocular melanocytes and in melanoma cells. Ann NY Acad Sci 885: 396–399, 1999. doi: 10.1111/j.1749-6632.1999.tb08698.x. [DOI] [PubMed] [Google Scholar]
- 316. Ermak G, Slominski A. Production of POMC, CRH-R1, MC1, and MC2 receptor mRNA and expression of tyrosinase gene in relation to hair cycle and dexamethasone treatment in the C57BL/6 mouse skin. J Invest Dermatol 108: 160–165, 1997. doi: 10.1111/1523-1747.ep12332925. [DOI] [PubMed] [Google Scholar]
- 317. Slominski A, Costantino R, Wortsman J, Paus R, Ling N. Melanotropic activity of γ MSH peptides in melanoma cells. Life Sci 50: 1103–1108, 1992. doi: 10.1016/0024-3205(92)90347-r. [DOI] [PubMed] [Google Scholar]
- 318. Slominski AT, Slominski RM, Zmijewski MA. Targeting melanocortin receptor type 1 with small peptides. Br J Dermatol 181: 17–18, 2019. doi: 10.1111/bjd.18022. [DOI] [PubMed] [Google Scholar]
- 319. Elliott RJ, Szabo M, Wagner MJ, Kemp EH, MacNeil S, Haycock JW. alpha-Melanocyte-stimulating hormone, MSH 11-13 KPV and adrenocorticotropic hormone signalling in human keratinocyte cells. J Invest Dermatol 122: 1010–1019, 2004. doi: 10.1111/j.0022-202X.2004.22404.x. [DOI] [PubMed] [Google Scholar]
- 320. Cuellar-Barboza A, Cardenas-de la Garza JA, Cruz-Gomez LG, Barboza-Quintana O, Flores-Gutierrez JP, Gomez-Flores M, Welsh O, Ocampo-Candiani J, Herz-Ruelas ME. Local secretion of stress hormones increases in alopecia areata lesions after treatment with UVA-1 phototherapy. Exp Dermatol 29: 259–264, 2020. doi: 10.1111/exd.14077. [DOI] [PubMed] [Google Scholar]
- 321. Haycock JW, Rowe SJ, Cartledge S, Wyatt A, Ghanem G, Morandini R, Rennie IG, MacNeil S. α-Melanocyte-stimulating hormone reduces impact of proinflammatory cytokine and peroxide-generated oxidative stress on keratinocyte and melanoma cell lines. J Biol Chem 275: 15629–15636, 2000. doi: 10.1074/jbc.275.21.15629. [DOI] [PubMed] [Google Scholar]
- 322. Moustafa M, Szabo M, Ghanem GE, Morandini R, Kemp EH, MacNeil S, Haycock JW. Inhibition of tumor necrosis factor-α stimulated NFκB/p65 in human keratinocytes by α-melanocyte stimulating hormone and adrenocorticotropic hormone peptides. J Invest Dermatol 119: 1244–1253, 2002. doi: 10.1046/j.1523-1747.2002.19602.x. [DOI] [PubMed] [Google Scholar]
- 323. Curry JL, Pinto W, Nickoloff BJ, Slominski AT. Human keratinocytes express functional alpha-MSH (MC1-R) receptors. In Vitro Cell Dev Biol Anim 37: 234–236, 2001. doi: 10.1007/BF02577535. [DOI] [PubMed] [Google Scholar]
- 324. Slominski RM, Sarna T, Płonka PM, Raman C, Brożyna AA, Slominski AT. Melanoma, melanin, and melanogenesis: the Yin and Yang relationship. Front Oncol 12: 842496, 2022. doi: 10.3389/fonc.2022.842496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325. Elias PM, Menon G, Wetzel BK, Williams J. Barrier requirements as the evolutionary “driver” of epidermal pigmentation in humans. Am J Hum Biol 22: 526–537, 2010. doi: 10.1002/ajhb.21043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326. Nissen JB, Avrach WW, Hansen ES, Stengaard-Pedersen K, Kragballe K. Decrease in enkephalin levels in psoriatic lesions after calcipotriol and mometasone furoate treatment. Dermatology 198: 11–17, 1999. doi: 10.1159/000018057. [DOI] [PubMed] [Google Scholar]
- 327. Yazdani Abyaneh MA, Engel P, Slominski A, Ragsdale B, Agag R, Cramer D, Carlson JA. Giant basal cell carcinomas express neuroactive mediators and show a high growth rate: a case-control study and meta-analysis of etiopathogenic and prognostic factors. Am J Dermatopathol 39: 189–194, 2017. doi: 10.1097/DAD.0000000000000640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328. Yang DJ, Lee KS, Ko CM, Moh SH, Song J, Hur LC, Cheon YW, Yang SH, Choi YH, Kim KW. Leucine-enkephalin promotes wound repair through the regulation of hemidesmosome dynamics and matrix metalloprotease. Peptides 76: 57–64, 2016. doi: 10.1016/j.peptides.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 329. Nagui NA, Ezzat MA, Abdel Raheem HM, Rashed LA, Abozaid NA. Possible role of proenkephalin in psoriasis. Clin Exp Dermatol 41: 124–128, 2016. doi: 10.1111/ced.12679. [DOI] [PubMed] [Google Scholar]
- 330. Nissen JB, Egekvist H, Bjerring P, Kragballe K. Effect of intradermal injection of methionine-enkephalin on human skin. Acta Derm Venereol 79: 23–26, 1999. doi: 10.1080/000155599750011642. [DOI] [PubMed] [Google Scholar]
- 331. Poeggeler B, Knuever J, Gaspar E, Biro T, Klinger M, Bodo E, Wiesner RJ, Wenzel BE, Paus R. Thyrotropin powers human mitochondria. FASEB J 24: 1525–1531, 2010. doi: 10.1096/fj.09-147728. [DOI] [PubMed] [Google Scholar]
- 332. Ramot Y, Zhang G, Biro T, Lisztes E, Funk W, Ingber A, Langbein L, Paus R. TSH is a novel neuroendocrine regulator of selected keratins in the human hair follicle. J Dermatol Sci 64: 67–70, 2011. doi: 10.1016/j.jdermsci.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 333. Vidali S, Knuever J, Lerchner J, Giesen M, Biro T, Klinger M, Kofler B, Funk W, Poeggeler B, Paus R. Hypothalamic-pituitary-thyroid axis hormones stimulate mitochondrial function and biogenesis in human hair follicles. J Invest Dermatol 134: 33–42, 2014. doi: 10.1038/jid.2013.286. [DOI] [PubMed] [Google Scholar]
- 334. Ellerhorst JA, Naderi AA, Johnson MK, Pelletier P, Prieto VG, Diwan AH, Johnson MM, Gunn DC, Yekell S, Grimm EA. Expression of thyrotropin-releasing hormone by human melanoma and nevi. Clin Cancer Res 10: 5531–5536, 2004. doi: 10.1158/1078-0432.CCR-03-0368. [DOI] [PubMed] [Google Scholar]
- 335. Slominski A, Plonka PM, Pisarchik A, Smart JL, Tolle V, Wortsman J, Low MJ. Preservation of eumelanin hair pigmentation in proopiomelanocortin-deficient mice on a nonagouti (a/a) genetic background. Endocrinology 146: 1245–1253, 2005. doi: 10.1210/en.2004-0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336. Langan EA, Vidali S, Pigat N, Funk W, Lisztes E, Biro T, Goffin V, Griffiths CE, Paus R. Tumour necrosis factor alpha, interferon gamma and substance P are novel modulators of extrapituitary prolactin expression in human skin. PLoS One 8: e60819, 2013. doi: 10.1371/journal.pone.0060819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337. Sandru F, Popa A, Paduraru DN, Filipescu A, Carsote M, Ghemigian A. Skin anomalies in acromegalic patients (Review of the practical aspects). Exp Ther Med 22: 1330, 2021. doi: 10.3892/etm.2021.10765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338. Kanaka-Gantenbein C, Kogia C, Abdel-Naser MB, Chrousos GP. Skin manifestations of growth hormone-induced diseases. Rev Endocr Metab Disord 17: 259–267, 2016. doi: 10.1007/s11154-016-9378-8. [DOI] [PubMed] [Google Scholar]
- 339. Scholzen T, Armstrong CA, Bunnett NW, Luger TA, Olerud JE, Ansel JC. Neuropeptides in the skin: interactions between the neuroendocrine and the skin immune systems. Exp Dermatol 7: 81–96, 1998. doi: 10.1111/j.1600-0625.1998.tb00307.x. [DOI] [PubMed] [Google Scholar]
- 340. Breitkopf T, Lo BK, Leung G, Wang E, Yu M, Carr N, Zloty D, Cowan B, Shapiro J, McElwee KJ. Somatostatin expression in human hair follicles and its potential role in immune privilege. J Invest Dermatol 133: 1722–1730, 2013. doi: 10.1038/jid.2013.53. [DOI] [PubMed] [Google Scholar]
- 341. Denda S, Takei K, Kumamoto J, Goto M, Tsutsumi M, Denda M. Oxytocin is expressed in epidermal keratinocytes and released upon stimulation with adenosine 5'-[γ-thio]triphosphate in vitro. Exp Dermatol 21: 535–537, 2012. doi: 10.1111/j.1600-0625.2012.01507.x. [DOI] [PubMed] [Google Scholar]
- 342. Deing V, Roggenkamp D, Kuhnl J, Gruschka A, Stab F, Wenck H, Burkle A, Neufang G. Oxytocin modulates proliferation and stress responses of human skin cells: implications for atopic dermatitis. Exp Dermatol 22: 399–405, 2013. doi: 10.1111/exd.12155. [DOI] [PubMed] [Google Scholar]
- 343. Luger TA. Neuromediators—a crucial component of the skin immune system. J Dermatol Sci 30: 87–93, 2002. doi: 10.1016/s0923-1811(02)00103-2. [DOI] [PubMed] [Google Scholar]
- 344. Dumont Y, Bastianetto S, Duranton A, Breton L, Quirion R. Immunohistochemical distribution of neuropeptide Y, peptide YY, pancreatic polypeptide-like immunoreactivity and their receptors in the epidermal skin of healthy women. Peptides 70: 7–16, 2015. doi: 10.1016/j.peptides.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 345. Stefanato CM, Yaar M, Bhawan J, Phillips TJ, Kosmadaki MG, Botchkarev V, Gilchrest BA. Modulations of nerve growth factor and Bcl-2 in ultraviolet-irradiated human epidermis. J Cutan Pathol 30: 351–357, 2003. doi: 10.1034/j.1600-0560.2003.00065.x. [DOI] [PubMed] [Google Scholar]
- 346. Fukuoka M, Sakurai K, Ohta T, Kiyoki M, Katayama I. Tacalcitol, an active vitamin D3, induces nerve growth factor production in human epidermal keratinocytes. Skin Pharmacol Appl Skin Physiol 14: 226–233, 2001. doi: 10.1159/000056351. [DOI] [PubMed] [Google Scholar]
- 347. Pincelli C, Marconi A. Autocrine nerve growth factor in human keratinocytes. J Dermatol Sci 22: 71–79, 2000. doi: 10.1016/s0923-1811(99)00065-1. [DOI] [PubMed] [Google Scholar]
- 348. Pincelli C. Nerve growth factor and keratinocytes: a role in psoriasis. Eur J Dermatol 10: 85–90, 2000. [PubMed] [Google Scholar]
- 349. Yaar M, Grossman K, Eller M, Gilchrest BA. Evidence for nerve growth factor-mediated paracrine effects in human epidermis. J Cell Biol 115: 821–828, 1991. doi: 10.1083/jcb.115.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350. Truzzi F, Marconi A, Lotti R, Dallaglio K, French LE, Hempstead BL, Pincelli C. Neurotrophins and their receptors stimulate melanoma cell proliferation and migration. J Invest Dermatol 128: 2031–2040, 2008. doi: 10.1038/jid.2008.21. [DOI] [PubMed] [Google Scholar]
- 351. Bothwell M. Neurotrophin function in skin. J Investig Dermatol Symp Proc 2: 27–30, 1997. doi: 10.1038/jidsymp.1997.7. [DOI] [PubMed] [Google Scholar]
- 352. Krasagakis K, Garbe C, Zouboulis CC, Orfanos CE. Growth control of melanoma cells and melanocytes by cytokines. Recent Results Cancer Res 139: 169–182, 1995. doi: 10.1007/978-3-642-78771-3_12. [DOI] [PubMed] [Google Scholar]
- 353. Yaar M, Eller MS, DiBenedetto P, Reenstra WR, Zhai S, McQuaid T, Archambault M, Gilchrest BA. The trk family of receptors mediates nerve growth factor and neurotrophin-3 effects in melanocytes. J Clin Invest 94: 1550–1562, 1994. doi: 10.1172/JCI117496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354. Peacocke M, Yaar M, Mansur CP, Chao MV, Gilchrest BA. Induction of nerve growth factor receptors on cultured human melanocytes. Proc Natl Acad Sci USA 85: 5282–5286, 1988. doi: 10.1073/pnas.85.14.5282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355. Zhai S, Yaar M, Doyle SM, Gilchrest BA. Nerve growth factor rescues pigment cells from ultraviolet-induced apoptosis by upregulating BCL-2 levels. Exp Cell Res 224: 335–343, 1996. doi: 10.1006/excr.1996.0143. [DOI] [PubMed] [Google Scholar]
- 356. Tang J, Fewings E, Chang D, Zeng H, Liu S, Jorapur A, Belote RL, McNeal AS, Tan TM, Yeh I, Arron ST, Judson-Torres RL, Bastian BC, Shain AH. The genomic landscapes of individual melanocytes from human skin. Nature 586: 600–605, 2020. doi: 10.1038/s41586-020-2785-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357. Naylor S, Chen JY. Unraveling human complexity and disease with systems biology and personalized medicine. Per Med 7: 275–289, 2010. doi: 10.2217/pme.10.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358. Yue Z, Kshirsagar MM, Nguyen T, Suphavilai C, Neylon MT, Zhu L, Ratliff T, Chen JY. PAGER: constructing PAGs and new PAG-PAG relationships for network biology. Bioinformatics 31: i250–i257, 2015. doi: 10.1093/bioinformatics/btv265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359. Yue Z, Zheng Q, Neylon MT, Yoo M, Shin J, Zhao Z, Tan AC, Chen JY. PAGER 2.0: an update to the pathway, annotated-list and gene-signature electronic repository for Human Network Biology. Nucleic Acids Res 46: D668–D676, 2018. doi: 10.1093/nar/gkx1040. [DOI] [PMC free article] [PubMed] [Google Scholar]