Abstract
Background
Extracorporeal membrane oxygenation has a high risk of acute brain injury and resultant mortality. Transcranial Doppler characterizes cerebral hemodynamics in real time, but limited data exist on its interpretation in ECMO. Here, we report TCD mean flow velocity and pulsatility index in a large ECMO population.
Methods
This was a prospective cohort study at a tertiary care center. The patients were adults on venoarterial ECMO or venovenous ECMO undergoing TCD studies.
Results
A total of 135 patients underwent a total of 237 TCD studies while on VA-ECMO (n = 95, 70.3%) or VV-ECMO (n = 40, 29.6%). MFVs were captured reliably (approximately 90%) and were similar to a published healthy cohort in all vessels except the internal carotid artery. Presence of a recordable PI was strongly associated with ECMO mode (57% in VA vs. 95% in VV, p < 0.001). Absence of TCD pulsatility was associated with intraparenchymal hemorrhage (14.7 vs. 1.6%, p = 0.03) in VA-ECMO patients.
Conclusions
Transcranial Doppler analysis in a single-center cohort of VA-ECMO and VV-ECMO patients demonstrates similar MFVs and PIs. Absence of PIs was associated with a higher frequency of intraparenchymal hemorrhage and a composite bleeding event. However, cautious interpretation and external validation is necessary for these findings with a multicenter study with a larger sample size.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12028-022-01653-6.
Keywords: Extracorporeal membrane oxygenation, Transcranial doppler, Brain injury, Stroke
Introduction
Extracorporeal membrane oxygenation (ECMO) is a temporary mechanical circulatory support used for medically refractory pulmonary and/or cardiac failure. ECMO is used with exponentially increasing frequency in the United States, with 18,260 runs in 2020, up from 3,446 in 2010 [1]. Although often lifesaving, ECMO is commonly associated with thrombotic and hemorrhagic complications, which are significant risk factors for morbidity and mortality, and embolism propagated from a cardiac or circuit source is a potential source of acute brain injury (ABI) [2].
In-hospital mortality for ECMO patients remains high (56% in venoarterial [VA] and 36% in venovenous [VV] ECMO), but mortality risk approximately doubles in the presence of ABI such as ischemic stroke, intraparenchymal hemorrhage (IPH), and hypoxic-ischemic brain injury [3, 4]. These complications are common, ranging from 5–10% prevalence in Extracorporeal Life Support Organization registry studies [3–6]. These rates are likely underestimated, however, due to lack of standardized neuromonitoring and the challenges of neuroimaging ECMO patients, as is evidenced by the drastically higher prevalence (up to 85%) described in pathological [7–9] and prospective clinical studies [10–12].
Transcranial Doppler (TCD) is a noninvasive, low-risk, bedside technique that allows real-time characterization of regional cerebral hemodynamics. Because of the relative rarity of ECMO and the high operator proficiency required of TCD, data regarding cerebral hemodynamics and their clinical significance in this population remain limited. To date, five articles have recently described cerebral hemodynamics in ECMO patients, but with sample sizes ranging from 8–53 patients and limited clinical correlation with relevant hemodynamic parameters or clinical follow-up [13–17]. In this study, intracranial artery mean flow velocities (MFVs) and pulsatility indices (PIs) were captured in a large, clinically well-characterized cohort of VA-ECMO and VV-ECMO patients to describe cerebral hemodynamics, clinical correlates, and neurologic outcomes with TCD use in this population.
Materials and Methods
Study Design
This study derives from a multidisciplinary effort involving the cardiovascular intensive care unit, cardiac critical care unit, and neurocritical care unit to improve overall care and outcomes for patients treated with ECMO at a tertiary care medical center. All ECMO patients cannulated at the study site are included in a prospective cohort that undergoes a neurologic monitoring protocol [12]. This protocol, established in October 2017, includes neurocritical care consultation with baseline and serial neurologic examinations, serial TCDs as described below, and additional neurological studies as appropriate (electroencephalography, SSEPs, and computed tomography). This was a retrospective observational study reviewing prospectively collected TCD examinations from ECMO patients from November 2017 through November 2021 at a single tertiary care center. Although not a case–control study, for the purposes of comparison of TCD variables between ECMO and non-ECMO patients, a well-characterized previously published cohort was studied [18].
Study Participants
We included all adult patients (age > 18 years) who received ECMO. We excluded patients who underwent multiple runs to minimize potential bias resulting from severe illness, as we considered that morbidity during the inter-ECMO periods could introduce a significant confounder. Patients who did not receive TCD or had absent temporal windows were excluded. The reasons for not having TCD tests were early deaths or ECMO withdrawals and noncompliance with the standardized neuromonitoring protocol, largely due to logistical challenges in the early phase of the coronavirus disease of 2019 (COVID-19) pandemic. This study was approved by the Johns Hopkins University Institutional Review Board (IRB00216321), and all patients or their surrogates consented for participation.
Data Collection and Definitions
For all patients in the study, we collected pre-ECMO characteristics including demographics, past medical history, precannulation neurologic function (Glasgow Coma Scale), cardiac diagnoses, pulmonary diagnoses, and laboratory values. ECMO variables included the following: indication, cannulation method (central [right atrium-aorta] vs. peripheral [femoral-femoral, femoral-internal jugular, or internal jugular dual lumen]) [19], ECMO flow (L/min), and ECMO pump speed (revolutions per minute). A dedicated perfusion team assessed the ECMO circuit multiple times daily to identify the presence of fibrin and clot. Selected hemodynamic parameters (systolic blood pressure [SBP], diastolic blood pressure [DBP], mean arterial pressure, venous oxygen saturation, transthoracic echocardiographic ejection fraction, cardiac output, and cardiac index calculated by Fick’s formula) and laboratory findings (hemoglobin/hematocrit, arterial blood gas, aPTT, and INR) were collected at the closest available time to each TCD. Based on clinical availability, the closest available time was typically within several minutes for hemodynamic parameters and within hours for laboratory values. These parameters were selected based on physiological relevance to cerebral blood flow hemodynamics [20, 21].
TCD Protocol and Variables
Serial TCD (DWL; Compumedics DWL, El Paso, TX) studies were performed while patients were on ECMO. Standard TCD protocols were used, and all studies were performed by registered vascular technologists [22]. We assessed cerebral blood flow velocities (peak systolic velocity, end diastolic velocity, and MFV), measured bilaterally in anterior (internal carotid artery [ICA; C1 segment], middle cerebral [MCA; M1 segment], anterior cerebral artery [ACA; A1 segment]) and posterior (vertebral artery [VrA], basilar artery [BA]) intracranial circulation. As permitted by duration of ECMO support, three TCDs were scheduled routinely for all ECMO patients at ECMO day 1, days 3–5, and days 7–10 [12]. Additional studies were performed as clinically indicated, such as for positive microembolic signals, subarachnoid hemorrhage monitoring, or brain death evaluation. PIs were calculated for each vessel using Gosling’s formula: (peak systolic velocity − end diastolic velocity)/MFV.
Measures and Outcomes
Primary measures were MFV and PI on TCD, which were analyzed by ECMO mode. Secondary outcomes were clinical complications relevant to TCD including neurologic (ischemic stroke, ICH, subdural hemorrhage, subarachnoid hemorrhage, hypoxic-ischemic brain injury, seizure, brain death) and thromboembolic (intracardiac thrombus, pulmonary embolism, deep venous thrombosis, heparin-induced thrombocytopenia, disseminated intravascular coagulation) events. A composite bleeding event was defined as one of the following the bleeding events: surgical site, cannulation site, gastrointestinal, pulmonary, disseminated intravascular coagulation, or any intracranial hemorrhage. We investigated systemic thromboembolic and bleeding events with TCD measures as a surrogate marker for hypercoagulable state or low pulsatility leading to bleeding events. Neurologic outcomes were assessed by a study team of neurointensivists based on physical examination and imaging results as clinically indicated.
Statistical Analysis
Primary outcomes were described as proportions for categorical variables and as medians and interquartile ranges (IQRs) for continuous variables, applying χ2 test, Fisher’s exact test, or Mann–Whitney U-test as appropriate.
Using summary data from a published cohort of 364 healthy adults, mean TCD MFVs for each studied vessel were compared with our study results by using Welch’s t-test due to unequal variances; men and women were analyzed separately due to known differences in normal values of TCD MFV and PI [23]. MFVs and PIs were then compared between VA-ECMO and VV-ECMO as well as between VA-ECMO with and without intraaortic balloon pump (IABP). For the above analyses, each patient first TCD on ECMO was used.
Correlations between MFVs in bilateral paired vessels were assessed by Spearman’s coefficient. Due to high positive correlations (> 0.75 Spearman’s coefficient) between MFVs in left and right paired vessels, these values were averaged for further correlation with clinical and hemodynamic parameters at the time of TCD, using all available TCD studies. As previously described, the MCA M1 segment (the average of left and right MFV in each study) was used as a representative vessel when analyzing covariates of MFV and PI on binary logistic regression [21, 24].
p values below 0.05 were considered statistically significant. Analyses were conducted in STATA 16.0 (StataCorp LLC; College Station, TX).
Results
Study Participant Characteristics
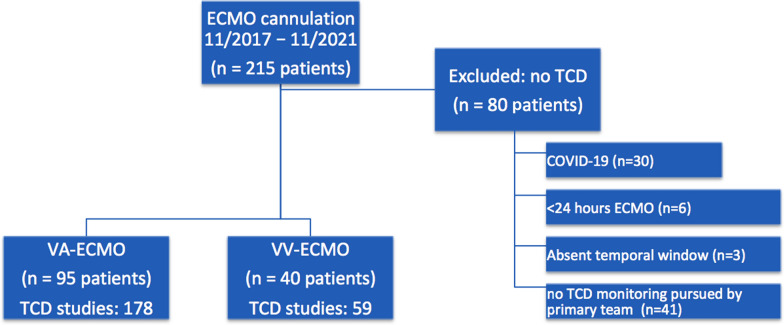
During the study period, 215 consecutive patients received ECMO support. Of these, 135 (62.7%) patients underwent at least one TCD study while on VA-ECMO (n = 95, 70.3%) or VV-ECMO (n = 40, 29.6%, Fig. 1). MFVs were reliably captured in 85% of VA-ECMO patients and 99% of VV-ECMO patients. Reasons for not completing a TCD study included COVID-19 infection (n = 30, 37.5%), less than 24 h of ECMO support (n = 6, 7.5%), absent temporal windows (n = 3, 3.8%), and no TCD monitoring pursued by primary team (n = 41, 51.2%). The study cohort was 60.7% men with a median age of 55 years (IQR 42–65); baseline characteristics of included patients are presented in Supplemental Table 1. The only significant difference in baseline characteristics between included patients and those excluded due to lack of a TCD study was a lower rate of COVID-19 in included VV-ECMO patients (50.0 vs. 76.9%, p = 0.02; Supplemental Table 2). Of 95 VA-ECMO patients, 50 (52.6%) were centrally cannulated whereas 45 (47.4%) were peripherally cannulated; 41 (30.4%) had an IABP and 7 (5.2%) had a left ventricular assist device. These 135 included patients underwent a total of 237 TCD studies, and median time to first study was 41 h (23–72) after ECMO cannulation (Fig. 2).
Fig. 1.
Study design and participant selection from a prospective cohort of venoarterial (VA) extracorporeal membrane oxygenation (ECMO) and venovenous (VV) ECMO at a single tertiary care center. COVID-19, coronavirus disease of 2019, TCD, transcranial Doppler
Fig. 2.
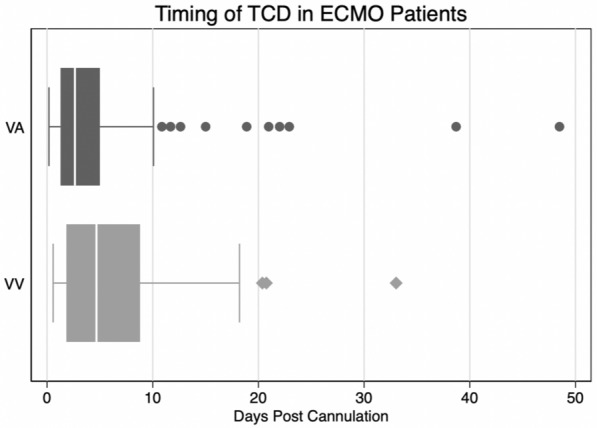
Number and timing of transcranial Doppler (TCD) studies in VA-ECMO and VV-ECMO patients. ECMO, extracorporeal membrane oxygenation, VA, venoarterial, VV, venovenous
TCD MFV
MFV values from the first TCD on ECMO for each insonated vessel by ECMO mode are described in Table 1. MFV was highest in the MCA M1 segment, followed by ICA C1 segment, ACA A1 segment, BA, and finally VrA. The BA, left VrA, and right VrA exhibited slightly lower MFVs in VA-ECMO as compared with VV-ECMO. This difference in MFV was preserved comparing peripherally cannulated VA-ECMO to VV-ECMO in all three recorded vessels (e.g., in the BA 31 [26–40] vs. 39 [33–45], p = 0.005), whereas there was no difference seen when comparing centrally cannulated VA-ECMO to VV-ECMO (BA 37 [24–45] cm/s vs. 39 [33–45] cm/s, p = 0.12). In VA-ECMO, no differences were observed in MFVs between central and peripheral cannulation, or between presence and absence of IABP (Supplemental Table 3).
Table 1.
Mean flow velocity from the first transcranial Doppler study on ECMO
| MFV | Normal, mean ± SD (cm/s) | VA-ECMO (n = 95 studies), n (%) or median (IQR) (cm/s) | VV-ECMO (n = 40 studies), n (%) or median (IQR) (cm/s) | p value (VA vs. VV) | ||
|---|---|---|---|---|---|---|
| Right | ||||||
| MCA M1 | 60.1 ± 12.1 | 84 (88.4%) | 52 (40–71) | 39 (97.5%) | 60 (45–65) | 0.28 |
| ACA A1 | 50.5 ± 10 | 78 (82.1%) | 42 (30–58) | 40 (100%) | 46 (38–59) | 0.13 |
| ICA C1 | 36.5 ± 9.4 | 81 (85.3%) | 50 (40–66) | 40 (100%) | 49 (33–64) | 0.50 |
| VrA | 34.3 ± 9.4 | 82 (86.3%) | 22 (18–28) | 40 (100%) | 26 (22–33) | 0.006 |
| Left | ||||||
| MCA M1 | 60.1 ± 12.1 | 82 (86.3%) | 51 (37–70) | 39 (97.5%) | 55 (47–68) | 0.52 |
| ACA A1 | 50.5 ± 10 | 74 (77.8%) | 44 (30–63) | 40 (100%) | 44 (38–62) | 0.96 |
| ICA C1 | 36.5 ± 9.4 | 79 (83.2%) | 50 (30–65) | 40 (100%) | 44 (34–70%) | 0.93 |
| VrA | 34.3 ± 9.4 | 80 (84.2%) | 33 (25–43) | 40 (100%) | 39 (32–45) | 0.04 |
| BA proximal | 39.7 ± 10.4 | 81 (85.3%) | 32 (25–43) | 40 (100%) | 39 (33–45) | 0.01 |
| Clinical parameters at time of TCD | ||||||
| Systolic blood pressure (mm Hg) | 87 (91.6%) | 92 (76–110) | 39 (97.5%) | 116 (103–130) | < 0.001 | |
| Diastolic blood pressure (mm Hg) | 87 (91.6%) | 62 (54–68) | 39 (97.5%) | 60 (54–68) | 0.60 | |
| Hematocrit (%) | 95 (100%) | 24.8 (22.8–28.2) | 40 (100%) | 25.5 (23.7–27.8) | 0.66 | |
| Arterial blood gas pCO2 (mm Hg) | 95 (100%) | 39 (34–45) | 39 (97.5%) | 45 (41–50) | < 0.001 | |
| Ejection fraction (%) | 67 (70.5%) | 30 (10–60) | 29 (72.5%) | 60 (50–65) | < 0.001 | |
Normal values from a healthy adult cohort are listed for reference [18]
Bold values are statistically significant (p < 0.05)
ACA, anterior cerebral artery, BA, basilar artery, ECMO, extracorporeal membrane oxygenation, ICA, internal carotid artery, IQR, interquartile range, MCA, middle cerebral artery, MFV, mean flow velocity, SD, standard deviation, VA, venoarterial, VrA, vertebral artery, VV, venovenous
MFVs were highly positively correlated between left and right paired vessels, as demonstrated by high Spearman’s coefficients: 0.72 for the MCA, 0.85 for the ACA, 0.78 for the ICA, and 0.78 for the VA. There were no statistically significant differences in median MFV by laterality, except for in the VrA (median [IQR] 35 [28–44] cm/s in the left vs. 23 [20–28] cm/s in the right, p < 0.001).
Given high concordance between left and right vessels, these values were averaged for further analysis. Compared by Welch’s t-test to normal values obtained from a cohort of 364 healthy adults, there was no significant difference in mean MFV of the MCA M1 segment, ACA A1 segment, BA, or VrA [23]. In the ICA C1 segment, however, MFVs for both men and women and for both VA-ECMO and VV-ECMO were significantly higher than published normal values (p values not shown, Table 2).
Table 2.
PI from the first transcranial Doppler study on ECMO
| PI | VA-ECMO (n = 95 studies), n (%) | VA-ECMO % abnormal (≥ 1.2), n (%) | PI, median (IQR) | VV-ECMO (n = 40 studies), n (%) | VV-ECMO % abnormal (≥ 1.2), n (%) | PI, median (IQR) | p value (PI) |
|---|---|---|---|---|---|---|---|
| Right | |||||||
| MCA M1 | 47 (49.5) | 22 (46.8) | 1.1 (1.0–1.6) | 38 (95.0) | 12 (31.6) | 1.1 (1.0–1.2) | 0.46 |
| ACA A1 | 44 (46.3) | 23 (52.2) | 1.2 (1.1–1.5) | 38 (95.0) | 18 (47.4) | 1.1 (1.1–1.3) | 0.23 |
| ICA C1 | 44 (46.3) | 19 (43.2) | 1.1 (1.0–1.4) | 38 (95.0) | 12 (31.6) | 1.1 (1.0–1.2) | 0.82 |
| VrA | 43 (45.3) | 28 (65.1) | 1.2 (1.1–1.6) | 37 (92.5) | 20 (54.1) | 1.2 (1.1–1.2) | 0.11 |
| Left | |||||||
| MCA M1 | 46 (48.4) | 25 (54.5) | 1.2 (1.0–1.6) | 38 (95.0) | 14 (36.8) | 1.1 (1.0–1.2) | 0.34 |
| ACA A1 | 42 (44.2) | 23 (54.8) | 1.2 (1.0–1.5) | 38 (95.0) | 15 (39.5) | 1.1 (1.1–1.2) | 0.55 |
| ICA C1 | 44 (46.3) | 23 (52.3) | 1.2 (1.0–1.6) | 38 (95.0) | 13 (34.2) | 1.1 (1.0–1.2) | 0.22 |
| VrA | 47 (49.5) | 30 (63.8) | 1.2 (1.1–1.6) | 36 (90.0) | 18 (50.0) | 1.2 (1.0–1.2) | 0.05 |
| BA proximal | 44 (46.3) | 28 (63.6) | 1.2 (1.1–1.5) | 37 (92.5) | 16 (43.2) | 1.1 (1.0–1.2) | 0.07 |
Pulsatility indices were calculated by Gosling’s formula: (peak systolic velocity − end diastolic velocity)/mean flow velocity
ACA, anterior cerebral artery, BA, basilar artery, ECMO, extracorporeal membrane oxygenation, IABP, intraaortic balloon pump, ICA, internal carotid artery, IQR, interquartile range, MCA, middle cerebral artery, PI, pulsatility index, VA, venoarterial, VrA, vertebral artery, VV, venovenous
No robust statistically significant associations were demonstrated between mean MFVs and any studied hemodynamic and clinical parameters (SBP, DBP, mean arterial pressure, Fick’s cardiac output, cardiac index, arterial blood gas pCO2 or pO2, hemoglobin/hematocrit, fibrinogen, or ECMO flow).
TCD PI
Presence of a recordable PI in any insonated vessel was strongly associated with ECMO mode: 54 (57%) in VA vs. 38 (95%) in VV (p < 0.001). PIs by ECMO mode for each insonated vessel from the first TCD on ECMO are described in Table 2. Compared with patients with only VA-ECMO, those who also had IABP exhibited similar PIs (Supplemental Table 4). Presence of a recordable PI in any insonated vessel on a patient’s first TCD examination was not significantly different between centrally and peripherally cannulated VA-ECMO (48.9 vs. 56.0%, p = 0.54).
Patients with recordable PI in any insonated vessel were younger than those without (53 [39–62] years in pulsatile TCD vs. 61 [49–69] years, p = 0.007). In VA-ECMO, hemodynamic covariates at the time of TCD study were strongly associated with presence versus absence of TCD pulsatility: pulse pressure (46 [34–60] mm Hg in pulsatile TCD vs. 18 [6–44] mm Hg in nonpulsatile TCD, p < 0.001), SBP (104 [90–118] mm Hg vs. 87 [72–100] mm Hg, p < 0.001), and DBP (60 [50–67] mm Hg vs. 62 [56–70] mm Hg, p = 0.02).
Clinical Outcomes
Clinical outcomes by ECMO type are presented in Supplemental Table 5. In VA-ECMO patients, absence of TCD pulsatility of any vessel in any study was more frequently associated with IPH (14.7% vs. 1.6%, p = 0.02) and was also more frequently associated with a composite event of any bleeding while on VA-ECMO (79.4% vs. 52.5%, p = 0.02).
Discussion
In this large cohort of ECMO patients with TCD studies, cerebral hemodynamics were characterized comprehensively and correlated with relevant physiologic parameters during TCD monitoring. MFVs were reliably captured and generally comparable to normal values. PIs were correlated with hemodynamic SBP and DBP and thus were often absent in VA-ECMO, an absence that was associated with a higher burden of IPH.
MFV
For both men and women on both VA-ECMO and VV-ECMO, MFVs were within published normal limits in all insonated vessels except the ICA C1 segment. This vessel exhibited slightly higher cerebral blood flow in both VA-ECMO and VV-ECMO, with MFVs approximately 5–10 cm/s above one standard deviation around the mean of a healthy cohort. This effect was preserved after subgrouping by ECMO type and cannulation method (central vs. peripheral, data not shown). Prior studies similarly exhibited MFVs largely within normal ranges in VA-ECMO [16].
Bilaterally paired vessels exhibited a high degree of correlation and similar MFVs, except in the VrA where the right side was approximately 10–13 cm/s lower than the left, an effect that was again conserved across analyzed subgroups, including cannulation method. Although too small to likely be clinically significant, this difference may be in part attributable to a higher prevalence of right VrA hypoplasia in the general population [25, 26].
When comparing VA-ECMO to VV-ECMO, MFVs were very similar in the anterior circulation, but studies in VA-ECMO exhibited 3–5 cm/s lower MFVs in both VrAs and the proximal BA. This difference was affected by cannulation method, as comparing peripherally cannulated VA-ECMO versus VV-ECMO preserved this effect, while no significant difference in MFV in these vessels was observed between centrally cannulated VA-ECMO and VV-ECMO.
PIs
Presence of pulsatile flow in at least one insonated vessel was more frequently observed in VV-ECMO as compared with VA-ECMO patients, and in VA patients with IABP as compared with those without IABP. These findings are expected given the underlying indications and mechanics of the two ECMO modes, but interestingly, no difference was observed in the presence of pulsatile flow between centrally and peripherally cannulated VA-ECMO patients. In contrast to a prior small study of TCD in VA-ECMO patients with severely reduced cardiac function that demonstrated invariably absent or very low PIs on VA-ECMO [13], here a majority of VA-ECMO TCD studies exhibited recordable PIs, with values in the normal or indeed slightly elevated range. TCD pulsatility was strongly associated with hemodynamic parameters at the time of TCD, namely SBP, DBP, pulse pressure, and ejection fraction, as previously described [13, 27]. Absence of pulsatile flow was associated with a higher frequency of IPH. Early low pulse pressure (< 20 mm Hg) has been associated with ABI in VA-ECMO patients, indicating low pulse pressure may serve as a marker of ABI risk, which is consistent with our TCD analysis [28]. However, this association needs to be interpreted carefully given the low number of patients with IPHs in our cohort.
Limitations
This study’s limitations include being conducted at a single center, which limits generalizability despite a sizable cohort. However, the high interobserver variability of TCD performance lends itself to a study design relying on a small group of experienced technologists [18]. The study was also subject to incomplete capture of eligible patients due to challenges obtaining TCD, such as clinical instability, short duration of ECMO support, staff availability, and COVID-19 restrictions. Most TCD studies were performed early during the ECMO support, limiting our analysis of the early phase of ECMO course and TCD measures. Lack of a suitable control population for comparison to normal TCD values is a further limitation, which was addressed by direct comparison to published literature but would benefit from paired case–control study in the future.
Conclusions
TCD analysis in a single-center cohort of VA-ECMO and VV-ECMO patients demonstrates similar MFVs and PIs. Absence of PIs was associated with a higher frequency of IPH and a composite bleeding event. However, cautious interpretation and external validation is necessary for these findings with a multicenter study with a larger sample size.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
HERALD (Hopkins Exploration, Research, and Advancement in Life support Devices) Investigators: Matthew Acton MD, Hannah Rando MD, Diane Alejo BA, Kate Calligy RN, R Scott Anderson BA, Benjamin Shou BS, Shrey Kapoor BA, Marc Sussman MD, Christopher Wilcox DO MS, Patricia Brown RD-AP CNSC, and Anna Peeler RN.
Author contributions
Concept/design: RGG, GJW, SMC, and WZ. Data collection: GC, LQZ, YM, AG, BE, and VP. Data analysis: GC. Data interpretation: GC, BSK, SK, RGG, GJW, SMC, and WZ. Drafting: GC, SMC, and WZ. Critical revision: LQZ, YM, AG, BE, VP, BSK, SK, RGG, GJW, SMC, and WZ. Approval: all authors.
Source of support
Sung-Min Cho is supported by the National Institutes of Health (NHLBI) 1K23HL157610. Dr. Ziai is supported by NIH R01 NS120557, R01AG069930, U24TR001609, and R01NS102583, and has received consultant fees from Integra and Bard.
Conflicts of interest
There are no conflicts of interest for the authors to disclose.
Ethical approval
All ethical guidelines were followed, and institutional review board approval was obtained.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Sung-Min Cho and Wendy Ziai have contributed equally as senior authors to this article.
Contributor Information
Giorgio Caturegli, Email: g.caturegli96@gmail.com.
HERALD (Hopkins Exploration, Research, and Advancement in Life support Devices) Investigators:
Matthew Acton, Hannah Rando, Diane Alejo, Kate Calligy, R Scott Anderson, Benjamin Shou, Shrey Kapoor, Marc Sussman, Christopher Wilcox, Patricia Brown, and Anna Peeler
References
- 1.Organization ELS, ECLS registry report international summary. Ann Arbor. 2020.
- 2.Abrams D, Combes A, Brodie D. Extracorporeal membrane oxygenation in cardiopulmonary disease in adults. J Am Coll Cardiol. 2014;63(25A):2769–2778. doi: 10.1016/j.jacc.2014.03.046. [DOI] [PubMed] [Google Scholar]
- 3.Cho SM, Canner J, Caturegli G, Choi CW, Etchill E, Giuliano K, et al. Risk factors of ischemic and hemorrhagic strokes during venovenous extracorporeal membrane oxygenation: analysis of data from the extracorporeal life support organization registry. Crit Care Med. 2021;49(1):91–101. doi: 10.1097/CCM.0000000000004707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cho SM, Canner J, Chiarini G, Calligy K, Caturegli G, Rycus P, et al. Modifiable risk factors and mortality from ischemic and hemorrhagic strokes in patients receiving venoarterial extracorporeal membrane oxygenation: results from the extracorporeal life support organization registry. Crit Care Med. 2020;48(10):e897–905. doi: 10.1097/CCM.0000000000004498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lorusso R, Barili F, Mauro MD, Gelsomino S, Parise O, Rycus PT, et al. In-hospital neurologic complications in adult patients undergoing venoarterial extracorporeal membrane oxygenation: results from the extracorporeal life support organization registry. Crit Care Med. 2016;44(10):e964–e972. doi: 10.1097/CCM.0000000000001865. [DOI] [PubMed] [Google Scholar]
- 6.Lorusso R, Gelsomino S, Parise O, Di Mauro M, Barili F, Geskes G, et al. Neurologic injury in adults supported with veno-venous extracorporeal membrane oxygenation for respiratory failure: findings from the extracorporeal life support organization database. Crit Care Med. 2017;45(8):1389–1397. doi: 10.1097/CCM.0000000000002502. [DOI] [PubMed] [Google Scholar]
- 7.Cho SM, Geocadin RG, Caturegli G, Chan V, White B, Dodd OJ, et al. Understanding characteristics of acute brain injury in adult extracorporeal membrane oxygenation: an autopsy study. Crit Care Med. 2020;48(6):e532–e536. doi: 10.1097/CCM.0000000000004289. [DOI] [PubMed] [Google Scholar]
- 8.Caturegli G, Cho SM, White B, Chen LL. Acute brain injury in infant venoarterial extracorporeal membrane oxygenation: an autopsy study. Pediatric Crit Care Med J Soc Crit Care Med World Fed Pediatr Intensive Crit Care Soc. 2021;22(3):297–302. doi: 10.1097/PCC.0000000000002573. [DOI] [PubMed] [Google Scholar]
- 9.Mateen FJ, Muralidharan R, Shinohara RT, Parisi JE, Schears GJ, Wijdicks EF. Neurological injury in adults treated with extracorporeal membrane oxygenation. Arch Neurol. 2011;68(12):1543–1549. doi: 10.1001/archneurol.2011.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sutter R, Tisljar K, Marsch S. Acute neurologic complications during extracorporeal membrane oxygenation: a systematic review. Crit Care Med. 2018;46(9):1506–1513. doi: 10.1097/CCM.0000000000003223. [DOI] [PubMed] [Google Scholar]
- 11.Cho SM, Deshpande A, Pasupuleti V, Hernandez AV, Uchino K. Radiographic and clinical brain infarcts in cardiac and diagnostic procedures: a systematic review and meta-analysis. Stroke. 2017;48(10):2753–2759. doi: 10.1161/STROKEAHA.117.017541. [DOI] [PubMed] [Google Scholar]
- 12.Cho SM, Ziai W, Mayasi Y, Gusdon AM, Creed J, Sharrock M, et al. Noninvasive neurological monitoring in extracorporeal membrane oxygenation. ASAIO J. 2020;66(4):388–393. doi: 10.1097/MAT.0000000000001013. [DOI] [PubMed] [Google Scholar]
- 13.Kavi T, Esch M, Rinsky B, Rosengart A, Lahiri S, Lyden PD. Transcranial doppler changes in patients treated with extracorporeal membrane oxygenation. J Stroke Cerebrovasc Dis Off J Natl Stroke Assoc. 2016;25(12):2882–2885. doi: 10.1016/j.jstrokecerebrovasdis.2016.07.050. [DOI] [PubMed] [Google Scholar]
- 14.Marinoni M, Migliaccio ML, Trapani S, Bonizzoli M, Gucci L, Cianchi G, et al. Cerebral microemboli detected by transcranial doppler in patients treated with extracorporeal membrane oxygenation. Acta Anaesthesiol Scand. 2016;60(7):934–944. doi: 10.1111/aas.12736. [DOI] [PubMed] [Google Scholar]
- 15.Rilinger JF, Smith CM, deRegnier RAO, Goldstein JL, Mills MG, Reynolds M, et al. Transcranial doppler identification of neurologic injury during pediatric extracorporeal membrane oxygenation therapy. J Stroke Cerebrovasc Dis Off J Natl Stroke Assoc. 2017;26(10):2336–2345. doi: 10.1016/j.jstrokecerebrovasdis.2017.05.022. [DOI] [PubMed] [Google Scholar]
- 16.Salna M, Ikegami H, Willey JZ, Garan AR, Cevasco M, Chan C, et al. Transcranial Doppler is an effective method in assessing cerebral blood flow patterns during peripheral venoarterial extracorporeal membrane oxygenation. J Card Surg. 2019;34(6):447–452. doi: 10.1111/jocs.14060. [DOI] [PubMed] [Google Scholar]
- 17.Melmed KR, Schlick KH, Rinsky B, Dumitrascu OM, Volod O, Nezhad M, et al. Assessing cerebrovascular hemodynamics using transcranial doppler in patients with mechanical circulatory support devices. J Neuroimaging Off J Am Soc Neuroimaging. 2020;30(3):297–302. doi: 10.1111/jon.12694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.White H, Venkatesh B. Applications of transcranial doppler in the ICU: a review. Intensive Care Med. 2006;32(7):981–994. doi: 10.1007/s00134-006-0173-y. [DOI] [PubMed] [Google Scholar]
- 19.Pavlushkov E, Berman M, Valchanov K. Cannulation techniques for extracorporeal life support. Ann Transl Med. 2017;5(4):70. doi: 10.21037/atm.2016.11.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schuurman PR, Albrecht KW. Intraoperative changes of transcranial doppler velocity: relation to arterial oxygen content and whole-blood viscosity. Ultrasound Med Biol. 1999;25(1):151–154. doi: 10.1016/S0301-5629(98)00135-5. [DOI] [PubMed] [Google Scholar]
- 21.D'Andrea A, Conte M, Scarafile R, Riegler L, Cocchia R, Pezzullo E, et al. Transcranial doppler ultrasound: physical principles and principal applications in neurocritical care unit. J Cardiovasc Echogr. 2016;26(2):28–41. doi: 10.4103/2211-4122.183746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alexandrov AV, Sloan MA, Wong LK, Douville C, Razumovsky AY, Koroshetz WJ, et al. Practice standards for transcranial doppler ultrasound: part I–test performance. J Neuroimaging Off J Am Soc Neuroimaging. 2007;17(1):11–18. doi: 10.1111/j.1552-6569.2006.00088.x. [DOI] [PubMed] [Google Scholar]
- 23.Tegeler CH, Crutchfield K, Katsnelson M, Kim J, Tang R, Passmore Griffin L, et al. Transcranial doppler velocities in a large, healthy population. J Neuroimaging Off J Am Soc Neuroimaging. 2013;23(3):466–472. doi: 10.1111/j.1552-6569.2012.00711.x. [DOI] [PubMed] [Google Scholar]
- 24.Sharma AK, Bathala L, Batra A, Mehndiratta MM, Sharma VK. Transcranial doppler: techniques and advanced applications: part 2. Ann Indian Acad Neurol. 2016;19(1):102–107. doi: 10.4103/0972-2327.173407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hong JM, Chung CS, Bang OY, Yong SW, Joo IS, Huh K. Vertebral artery dominance contributes to basilar artery curvature and peri-vertebrobasilar junctional infarcts. J Neurol Neurosurg Psychiatry. 2009;80(10):1087–1092. doi: 10.1136/jnnp.2008.169805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen X, Xu J, Zhang Y, Lin M, Wang H, Song Y. Evaluation of hemodynamic characteristics in posterior circulation infarction patients with vertebral artery dominance by color doppler flow imaging and transcranial doppler sonography. Int J Neurosci. 2021;131(11):1078–1086. doi: 10.1080/00207454.2020.1773820. [DOI] [PubMed] [Google Scholar]
- 27.Veraar CM, Rinösl H, Kühn K, Skhirtladze-Dworschak K, Felli A, Mouhieddine M, et al. Non-pulsatile blood flow is associated with enhanced cerebrovascular carbon dioxide reactivity and an attenuated relationship between cerebral blood flow and regional brain oxygenation. Crit Care. 2019;23(1):426. doi: 10.1186/s13054-019-2671-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shou BL, Wilcox C, Florissi I, Kalra A, Caturegli G, Zhang LQ, et al. Early low pulse pressure in VA-ECMO is associated with acute brain injury. Neurocrit Care. 2022 doi: 10.1007/s12028-022-01607-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.