Abstract
From population estimates to social evolution, much of our understanding of the family Hyaenidae is drawn from studies of known individuals. The extant species in this family (spotted hyenas, Crocuta crocuta, brown hyenas, Parahyaena brunnea, striped hyenas, Hyaena hyaena, and aardwolves, Proteles cristata) are behaviorally diverse, presenting an equally diverse set of logistical constraints on capturing and marking individuals. All these species are individually identifiable by their coat patterns, providing a useful alternative to man-made markings. Many studies have demonstrated the utility of this method in answering a wide range of research questions across all four species, with some employing a creative fusion of techniques. Despite its pervasiveness in basic research on hyenas and aardwolves, individual identification has rarely been applied to the conservation and management of these species. We argue that individual identification using naturally occurring markings in applied research could prove immensely helpful, as this could further improve accuracy of density estimates, reveal characteristics of suitable habitat, identify threats to population persistence, and help to identify individual problem animals.
Supplementary Information
The online version contains supplementary material available at 10.1007/s42991-022-00309-4.
Keywords: Aardwolf, Camera traps, Carnivores, Hyena, Individual identification, Mark-recapture
Introduction
Our understanding of the biology of many mammals, including mammalian carnivores, has been greatly enhanced by studies of individually recognizable animals (Clutton-Brock and Sheldon 2010; Schneider et al. 2019; Karczmarski et al. 2022a, b). Such studies have shed light on demography, natural selection, life histories, ontogeny, social behavior, and intrapopulation variation in diverse species and populations (Clutton-Brock and Sheldon 2010). In free-living populations, however, capturing and marking individuals (Mills 1982a, 1983a, b) may be impractical due to limited funds, difficulty obtaining permits, rarity or elusiveness of subjects, or—in some group-living species—a large number of subjects, making it impractical to capture every individual in the study population. Individual identification using naturally occurring markings is a practical, cheap, and noninvasive alternative to capturing and marking wild animals (Powell and Proulx 2003; Mendoza et al. 2011; Kelly et al. 2012; Schneider et al. 2019). This method has been applied to various mammalian carnivores with unique pelage patterns (Karanth and Nichols 1998; Heilbrun et al. 2003; Harihar et al. 2010). Even in more subtly marked species, individuals can be consistently differentiated using whisker patterns (Anderson et al. 2007; Osterrieder et al. 2015; Elliot and Gopalaswamy 2017), coloration, facial markings, leg and tail markings, body and tail structure or carriage, kinks in tails, ear nicks, or scars (Trolle et al. 2006; Kelly et al. 2008; Sarmento et al. 2009; Zheng et al. 2016). Natural markings are used to identify individual animals both in direct observations (Smith et al. 2017) and during use of camera traps (Dheer et al. 2022).
Studies of individually known members of the family Hyaenidae have proven particularly fruitful. This family contains only four extant species, but these species exhibit impressive diversity in morphology, diet, and social organization. The three bone-cracking hyena species include spotted (Crocuta crocuta), brown (Parahyaena brunnea), and striped (Hyaena hyaena) hyenas. Although spotted hyenas kill most of their own prey, the other two bone-cracking forms live mainly on carrion. In contrast, aardwolves (Proteles cristata) feed almost exclusively on termites. These species span a wide spectrum of social behavior, from aardwolves, which are solitary except when breeding, to spotted hyenas, which sometimes live in the largest groups of any terrestrial carnivore (Green et al. 2018). The diversity within this family makes these species excellent models for basic research. Furthermore, human–hyena conflict and the Near Threatened status of brown and striped hyenas (AbiSaid and Dloniak 2015; Wiesel 2015) suggest that further study of these animals should facilitate their conservation and management.
In this review, we briefly describe the morphology and ecology of each extant species in the family Hyaenidae and describe how best to distinguish individuals based on their unique markings. We then highlight studies of demographic and socioecological processes that illustrate the utility of individual identification in studies of free-living hyaenids. Finally, we discuss previous applied work using individual identification, and identify important gaps in our knowledge of hyaenids that could be filled using the identification methods we describe.
The four extant hyaenid species
Spotted hyenas are large, gregarious carnivores that occur throughout much of sub-Saharan Africa. Adults typically weigh roughly 55.00 kg and stand 0.77–0.80 m tall at the shoulder, with females being about 10% larger than males (Swanson et al. 2013), making spotted hyenas the largest of the extant hyaenids. Their fur ranges in color from sandy to grey or brown, with dark spots on their flanks, backs, rumps, and legs (Holekamp and Kolowski 2009). The spotted hyena is the only extant hyaenid whose post-cranial anatomy is adapted for cursorial hunting of medium- and large-sized herbivores, and 65–95% of their diet consists of prey they kill themselves (Holekamp and Dloniak 2010). Spotted hyenas exhibit social behavior convergent with that of cercopithecine primates. They live in stable, fission–fusion social groups, called “clans,” that may contain up to 126 individuals (Green et al. 2018). Each clan is structured by a linear dominance hierarchy in which adult females outrank most breeding males. Although they are experiencing global decline, spotted hyenas are still abundant throughout sub-Saharan Africa, and are listed as a species of Least Concern by the International Union for Conservation of Nature (IUCN; Bohm and Höner 2015).
Brown hyenas occur in southern Africa, where they are widespread throughout Botswana (Winterbach et al. 2017) and most of Namibia (Wiesel 2015). They also occur in Angola, Zimbabwe, and South Africa. Body size varies regionally, with adults weighing 28.00–47.50 kg, and typically standing around 0.74 (females) or 0.79 (males) m tall at the shoulder (Holekamp and Kolowski 2009). Their long, shaggy fur is dark or reddish brown on their torsos and has a lighter tawny color on their necks and shoulders. The face is covered with short, dark hair, and their front and hind legs are striped. They are opportunistic foragers, feeding mainly on vertebrate remains. Hunting plays a minor role in this species, although brown hyenas living along the southern Namib Desert coast regularly kill Cape fur seal (Arctocephalus pusillus pusillus) pups at mainland seal colonies (Wiesel 2010). Brown hyenas live in small clans of 4 to 14 individuals, within which females sometimes breed cooperatively (Mills 1982b, 1990). Males either remain in their natal clan, disperse to a new clan to breed, or become nomadic breeders. The IUCN lists this species as Near Threatened, with a population estimate of fewer than 10,000 mature individuals (Wiesel 2015).
Striped hyenas have the largest geographic range of the extant hyaenids, stretching from the northwestern coast of Africa and as far south as Tanzania, through the Middle East and the southern Caucasus, and eastward through much of India (AbiSaid and Dloniak 2015) and into Nepal (Bhandari et al. 2020). They are smaller than spotted or brown hyenas, typically weighing 26.00–41.00 kg and standing 0.66–0.75 m tall at the shoulder. Striped hyenas have a somewhat shaggy appearance, a bushy tail, and the most prominent mane of any hyaenid. They have black muzzles, black throat patches, and black or brown stripes on lighter colored fur (Holekamp and Kolowski 2009). These hyenas are omnivorous scavengers that hunt infrequently and opportunistically (Kruuk 1976; Holekamp and Kolowski 2009). Despite their expansive range and ecological importance (Beasley et al. 2015), they remain poorly understood. This void in information is likely due to their low density, nocturnality, elusiveness, the rough terrain they sometimes inhabit, and confusion with spotted hyenas where the two species co-occur (Kruuk 1976; Holekamp and Kolowski 2009). Traditionally, striped hyenas have been considered solitary (Kruuk 1976), but recent research suggests that this is not the case for all populations (Wagner et al. 2008; Califf et al. 2020; Tichon et al. 2020). The striped hyena has been extirpated from many parts of its range, and populations continue to decline globally. Conservation of this Near Threatened species requires further research on its behavior and demography (Mills and Hofer 1998; Holekamp and Kolowski 2009; AbiSaid and Dloniak 2015).
Aardwolves occur in East and southern Africa (Green 2015). As the smallest member of the hyena family, they typically weigh 8.00–12.00 kg and stand 0.45–0.50 m tall at the shoulder (Holekamp and Kolowski 2009). Aardwolves feed predominantly on termites (Trinervitermes spp.; Kruuk and Sands 1972; Koehler and Richardson 1990; Anderson 2013; Green 2015). They are primarily nocturnal and forage alone (Smithers 1983; Koehler and Richardson 1990; Anderson 2013). During the breeding season, mated pairs of aardwolves occupy a territory with their youngest offspring (Koehler and Richardson 1990; Richardson 1991). The conservation status of aardwolves is listed as Least Concern, and their global population is considered stable (Green 2015).
Individual identification by naturally occurring markings
All four hyena species have unique coat patterns that are consistent throughout the animals’ lives. Individuals can be distinguished using these markings and any other unique characteristics, such as scars or ear damage. To avoid misidentification, reference photographs must be maintained for each study population, preferably including high-quality reference photos of the left and right sides of each animal, as the markings on the two sides differ. Such images can be obtained easily by taking video footage of both sides of an individual animal as it moves around, freezing the frames in which left- and right-side markings are clearest, and printing those images. If photographs of each individual’s left and right side cannot be matched for any reason, two separate sets of records should be kept, one for the right-side images and one for left-side images (Karanth 1995), and they should be analyzed separately (Gupta et al. 2009; Harihar et al. 2010; Kent and Hill 2013; Dheer et al. 2022). Identification relies on distinctive patterning of the coat in all four species, so images need not be in color; in fact, converting photographs to black and white and increasing the contrast may be helpful for maximizing clarity of coat patterns. Images may be annotated with other useful, unique physical characteristics. These databases require upkeep as new individuals are born or immigrate and existing hyenas develop new scars or injuries, emigrate, or die. Digital reference photographs of known individuals may be most useful for identifying animals in camera trap images, as they allow the observer to zoom in on individual body parts (Henschel and Ray 2003). Although future technological advances may lead to digital devices that are practical for field identification, in general, hard copies of reference photos are currently necessary for identification during direct observations. Hard copies of reference photos also serve as invaluable backup records. If time allows, photographs can be taken of individuals in the field and immediately compared to the reference photos to confirm identities. It is important that the observer uses spots or stripes from multiple body parts whenever possible to confirm an identity. Before they can identify individuals without their work being checked by an expert, observers need a substantial period of training. An expert should observe their work and decide when new observers are ready to work independently. Ideally, two or more independent observers will confirm each identity.
Spotted hyenas
Long-term (1979 to present, Holekamp and Strauss 2020; 1987 to present, Hofer and East 1993) and shorter term (Henschel and Skinner 1990) studies of spotted hyenas demand that observers be able to recognize each hyena individually based on its unique spot patterns, ear damage, and other permanent markings (e.g., missing tail, significant scars). Fortunately, except after mud-bathing, individuals are recognizable, as they have unique, permanent spot patterns that vary among individuals (Fig. 1). The Mara Hyena Project (Kenya) maintains a photo album for each of its study clans showing left- and right-side spot patterns of each clan member (Fig. 2a). Photo albums for all nearby study clans are also carried in each research vehicle during data collection. Position and shape of ear damage (e.g., cut, notch, missing ear; Fig. 2b), when present, also aids in individual identification, but examining spot patterns in reference photos is critical to confirm identities, even for highly experienced observers. Different age-sex classes (i.e., cub, subadult, adult female, adult male) have different body-shape profiles (Johnson-Ulrich et al. 2018), so organizing photos by age-sex class reduces the number of spot patterns one must check for confirmation. Further dividing adult hyenas into residents and “aliens” can further facilitate the process of individual identification. Hyenas that are seen passing through the territory of a study hyena clan that are not members of that particular clan are considered “aliens”; these are often dispersing males. Thus, we recommend organizing each photo album into five sections: cubs, subadults, resident adult females, resident adult males, and “aliens”.
Fig. 1.
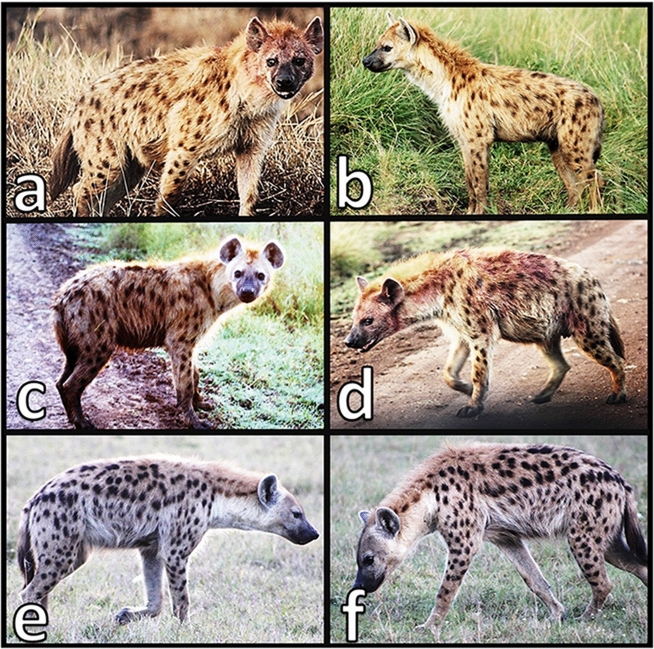
Photographs of two different hyenas in the Maasai Mara National Reserve, captured on different occasions. These photographs were all taken manually by observers in the field, using digital cameras. Contrast, brightness, and sharpness were edited to enhance visibility of spots. Shown here are reference photographs of the right (a, c) and left (b, d) sides of a female hyena named Pike. Spotted hyenas’ spots are not bilaterally symmetrical, so reference photographs should ideally show both sides. Her identity may be confirmed by comparison of her spot patterns on her right and/or left shoulder(s), flank(s), hip(s), and legs. Spots from multiple body parts should be used, but the specific parts used may depend on visibility (e.g., whether grass is in the way, whether the hyena is muddy, or depending on the animal’s posture or the angle from which the animal is seen). The right (e) and left (f) sides of a different hyena, a male named Kaikura, are quite distinct from Pike’s
Fig. 2.
a A photo album of one of the Mara Hyena Project’s study clans in the Mara Triangle, Kenya. The photo album is organized by age–sex class. For each subject, photos of the left and right sides are shown together and oriented in the same fashion to facilitate comparison across subjects. These photos are updated throughout the hyenas’ lifetimes, and each photo is marked with the date taken. Photos are edited to increase contrast, so that spots are clearly visible. The position and shape of each hyena’s ear damage, if present, are noted on reference photos. Whereas a hyena’s spots never change, ear damage often varies over a hyena’s lifetime. Therefore, ear damage should be used to narrow the list of likely candidates, but final confirmation should always rely on examination of spot patterns. b Ears of eight different spotted hyenas in the Mara Triangle, Kenya. Ear damage is acquired while fighting with conspecifics or other large carnivores. Clearly, ear damage among individuals can be quite variable and distinctive, from a small nick to a missing ear. Photo credit for (a) to Erin Person
Spotted hyena cubs are seldom seen aboveground before they are a few weeks old. They are born with solid dark brown or black natal coats, and, upon first seeing them, their age can be estimated to within 7 days based on their pelage, size, and other features (Pournelle 1965; Holekamp et al. 1996). Although cubs are often difficult to tell apart before replacement of the natal coat, this can sometimes be done based on slight differences in fur color, size differences, patterns of abrasion on the skin covering the carpal bones, small nicks in the ears (often inflicted during the early fighting between litter-mates; Frank et al. 1991; Fig. 3), and differential patterns of scarring on cubs’ backs, necks, and shoulders. Although female spotted hyenas have male-like genitalia, one can determine the sex of each cub older than a few weeks by using the dimorphic morphology of the glans of the erect phallus (Frank et al. 1990; Drea et al. 1999; Cunha et al. 2003; McCormick et al. 2021; Dheer et al. 2022). Cubs change somewhat in appearance as they grow, mainly due to having fur of variable length and texture between consecutive molts, but, once the spot pattern appears, it never changes. The spots typically begin to fade by the time a hyena reaches its mid-teens and continue fading as the hyena ages. The oldest hyenas recorded by the Mara Hyena Project were 26 years old at the time of their deaths.
Fig. 3.

Distinct ear damage on a spotted hyena cub with its natal coat. This cub, Black Bear, was the only cub at this communal den with a slit on this part of its ear (indicated by white arrow), so he could be clearly distinguished from the other cubs, including his littermate. This allowed researchers to identify Black Bear before he shed his black natal coat and developed spots
There is a lack of consistent criteria to define age-sex classes of spotted hyenas, and definitions of the age classes used are project-specific (e.g., Trinkel et al. 2004; Höner et al. 2005; Belton et al. 2018). For example, Holekamp et al. (2012) consider hyenas to be subadults once they are no longer dependent on the communal den, which typically happens at 9–12 months of age, whereas Kruuk (1972) defines subadults based on a specific range of tooth-wear values. Similarly, females may be considered adults when they are first known to conceive litters, when their teeth are worn down to a certain degree, or when they reach a specific age.
Brown hyenas
Before the advances of digital photography and camera trap technologies, triangular ear notches were cut into brown hyenas’ ears for quick individual identification (Mills 1982a, 1983a, b). However, because man-made and natural ear notches may change over time (Fig. 4), they are not a reliable stand-alone method of identification. Instead, the Brown Hyena Research Project (Namibia) distinguishes individuals by the unique stripe patterns on their fore- and hindlegs. Brown hyena cubs have no solid natal coat, and their leg stripes are faintly visible from birth, and become clearer as the hyenas grow. These stripes are permanent over the course of a hyena’s lifetime (Fig. 5), but their appearance is greatly influenced by the viewing angle (Fig. 6). The stripes are often indistinct, due to the small and round surface area of the legs, and the sometimes-changing directional position of the hair. Therefore, the combined use of stripes on the anterior, lateral, and medial surfaces of the forelegs, stripes on the lateral surfaces of the hindlegs, and any ear notches result in the highest likelihood of correct identification. Photographs of the anterior surfaces of the forelegs as well as the left and right sides of the body should be maintained for identification purposes. Unlike spotted hyenas, female brown hyenas do not have masculinized genitalia, but external morphology is otherwise similar between sexes and they are generally considered to be sexually monomorphic (Mills 1982a; Butler-Valverde et al. 2015; Dheer et al. 2022). As a result, it is generally not possible to distinguish between males and females in the field without capture.
Fig. 4.
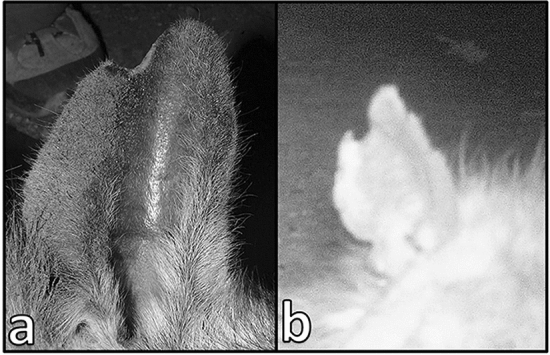
A natural ear notch on the right ear of the same brown hyena at the ages of five (a) and 15 years (b). The shape of the original notch has changed, and new smaller notches have appeared over time
Fig. 5.
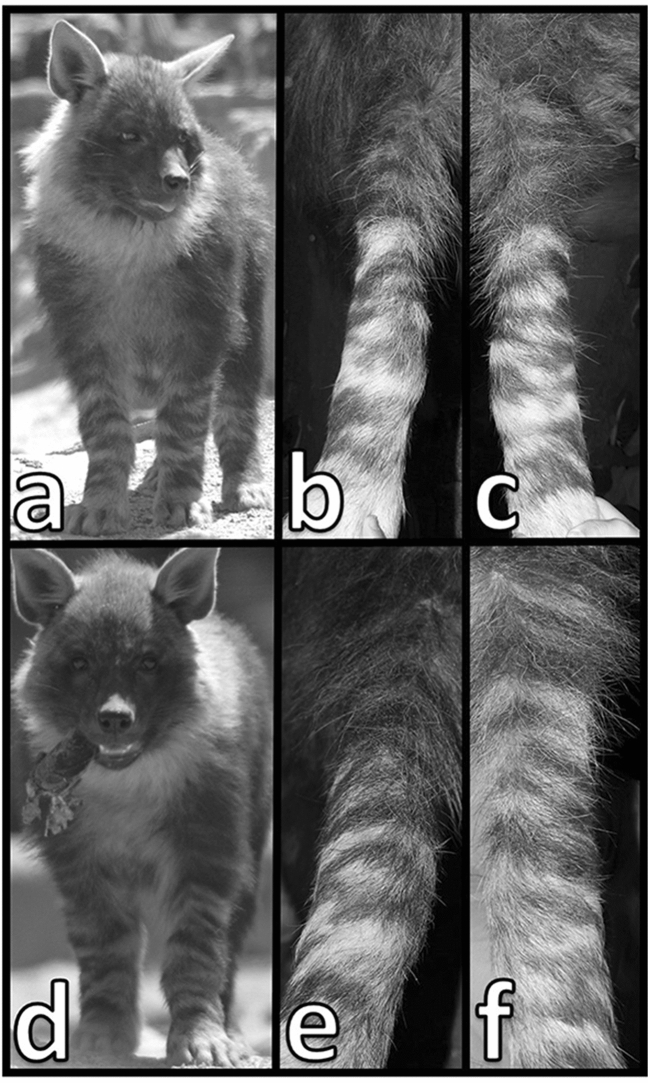
The top row (a–c) shows the anterior surfaces of the forelegs of a male brown hyena named Kai-Alex, and the bottom row (d–f) shows those of a second male, Lloyd. Kai-Alex’s leg stripes are the same at six months (a) and 2.5 years of age (right foreleg, b; left foreleg, c). Similarly, Lloyd’s foreleg stripes are consistent from cubhood (d) to adulthood (right foreleg, e; left foreleg, f). Photos of leg stripes at 2.5 years of age were taken while hyenas were anesthetized
Fig. 6.
A single female brown hyena, Alaika, is shown at the ages of five, eight, and 12 years. The first frame (a) shows the lateral view of Alaika’s right foreleg at five (left), eight (center), and 12 (right) years of age. We can see that the stripes are consistent over her lifetime. In the second frame (b), we see that the anterior view of the right foreleg looks quite different from the lateral view of the same leg, but, again, the stripes are consistent throughout her lifetime (5 years of age, left; 8 years, center; 12 years, right). Photographs in a and b were taken while Alaika was anesthetized for handling on three different occasions. c Remote camera trap images of Alaika with sufficient clarity to identify her using lateral stripes of right foreleg (left, center), and insufficient clarity for confident identification (right)
Striped hyenas
Striped hyenas have vertical stripes on their flanks, and diagonal and horizontal stripes on all four limbs (Holekamp and Kolowski 2009). They have no natal coat and, instead, are born sporting clear, conspicuous stripes (Rieger 1979; Bothma and Walker 1999). The position and shape of these stripes are consistent throughout a hyena’s lifetime, although they may become distorted with seasonal variation in coat length or fade slightly with age (Rieger 1979; Jhala 2013). Striped hyenas can be individually identified by the patterns of stripes and dots on their shoulders, flanks, hips, forelimbs, and hindlimbs, as well as by any other conspicuous features (e.g., ear notches, scars; Fig. 7). Stripes on the hind- (hip and upper hindleg) and forequarters (shoulder and foreleg) are the most useful, as they are prominent and vary between individuals (Singh 2008; Harihar et al. 2010), while stripes on the flank may be distorted by shaggy fur. One should use stripe patterns from multiple body parts to confirm individual identification (Gupta et al. 2009). Determination of sex is difficult without capture (Dheer et al. 2022) but may be possible for females that have dependent cubs, for example, if they are visibly lactating or being followed by their cubs (Alam 2011; Tichon et al. 2020).
Fig. 7.
Left sides of two known striped hyenas in a population near Shompole, Kenya. a, b Two images of the same individual, male M113, captured on different occasions. M113’s mane is erect in a, revealing some stripes on his left flank, but is not erect in b, thus partially covering and distorting these stripes. The stripes and spots on the fore- and hindquarters are more useful than the flanks or ears for identification of striped hyenas. c, d Two images of a second individual, male M114, captured on different occasions. In (c), M114’s legs are partially obscured by grass. Leg stripes may certainly be useful when visible, but they are more likely to be out of view than the shoulders and hips, which also have similarly variable and prominent markings. In this population, many hyenas have a single solid stripe over a double stripe on their left hip (white boxes; a, c). As an observer becomes more experienced identifying subjects in a given population, they should recognize interindividual similarities such as this and focus on more variable features (e.g., left thigh stripes, shoulder stripes). Therefore, slight similarities among hyenas should not necessarily lead to a reduction in accuracy, particularly if observers use patterns from multiple body parts to confirm each identification. Images captured by handheld digital camera (a) and remote digital camera traps (b–d) by Aaron Wagner
Aardwolves
The coats of aardwolves are yellowish in color and the face and throat are grayer than the rest of the body. They have three or more vertical black stripes on each flank, and one or two diagonal stripes across their fore- and hindquarters. Irregular horizontal stripes run across the legs, and are darkest near their feet. Stripes and spots are sometimes present on the neck (Smithers 1983; Koehler and Richardson 1990). Individuals can be distinguished using their stripe and spot patterns (Richardson 1991; Silwa 1996; O’Brien and Kinnaird 2011; Rich et al. 2019; Fig. 8). Coat patterns can also be paired with natural scars and ear nicks, if present (Richardson 1991; Silwa 1996). Natural features have also been supplemented with reflective collars and man-made ear notches to aid in recognition (Richardson 1987a, b, 1990, 1991; Silwa 1996). Visual determination of sex is unlikely to be feasible without capture (Dheer et al. 2022).
Fig. 8.

Incidental photographic captures of aardwolves by remote camera traps used by the Brown Hyena Research Project in Namibia. a, b Two captures of the same individual, viewed from different angles. c A second individual. These two individuals can be easily distinguished by the stripes on their shoulders. While individuals can often be recognized even when viewed from different angles, applying bait or taking advantage of natural or man-made trails can help position animals relative to the camera lens for optimal visibility
Past and present use in basic research
Our knowledge of the ecology of the four hyaenid species has been acquired, in large part, from studies taking advantage of natural markings. Here we describe findings from selected studies that have used this method to address a wide array of research questions about hyaenids. This body of literature is vast, particularly for spotted hyenas, and this is by no means intended to represent a comprehensive overview of the literature. Rather, the studies highlighted here are meant to serve as examples of the diversity of past and present applications of this method and to demonstrate its value in basic research.
Individual recognition of wild hyaenids has greatly improved the accuracy with which we can assess their demography. Most large carnivores, including hyaenids, are rare, elusive, primarily nocturnal, or maintain large home ranges, making them difficult to detect (Cozzi et al. 2013; Chutipong et al. 2014; Green et al. 2020). Low detection probability renders common methods for estimating density of large terrestrial mammals, such as aerial transects and line surveys, inappropriate for these carnivores (Cozzi et al. 2013). Instead, density estimates for some large carnivores were historically based on call-in (also known as call-up) station surveys and sign surveys (most commonly track counts), which are demonstrably unreliable (Karanth and Nichols 1998; Kelly et al. 2012; Vissia et al. 2021). Photographic capture–recapture analyses of individually identifiable animals in camera trap images represents a substantial methodological advance over call-in station and sign surveys. Since its first application to estimating tiger (Panthera tigris) abundance and density in India (Karanth 1995; Karanth and Nichols 1998), photographic capture–recapture has emerged as a powerful method for quantifying population characteristics of elusive carnivores (Treves et al. 2010; Kelly et al. 2012; Johansson et al. 2020). Camera traps were deployed in the field by 2007 for brown hyenas (Thorn et al. 2009), 2007–2008 for striped hyenas (Gupta et al. 2009; Harihar et al. 2010; Singh et al. 2010; Athreya et al. 2013), and 2008 for aardwolves (O’Brien and Kinnaird 2011). Photographic capture–recapture has been used to estimate the density of spotted hyenas in many countries throughout their geographic range, such as Congo (Henschel et al. 2014; Bohm 2015), Uganda (Braczkowski et al. 2022), Kenya (O’Brien and Kinnaird 2011), Botswana (Rich et al. 2019; Vitale et al. 2020), Namibia (Stratford et al. 2020), and South Africa (de Blocq 2014). In rare cases in which all study animals were known from extensive long-term direct observations, researchers derived precise counts of spotted hyenas within their study area (rather than using capture–recapture methods to estimate abundance; Watts and Holekamp 2009; Green et al. 2018), but extensive direct observations yield a better return-on-investment for behavioral studies than for population estimation (de Blocq 2014). In addition to population size, studies of known individuals have yielded estimates of other demographic parameters, such as population growth rate (Benhaiem et al. 2018; Green et al. 2018; Mandal 2018), mortality rate (White 2005; Watts and Holekamp 2009; Höner et al. 2012; Mandal 2018), birth rate (Holekamp and Smale 1995; Watts and Holekamp 2009), and sex ratio (Holekamp and Smale 1995).
Incorporation of environmental data into models of spatial or temporal variation in demographic parameters can reveal processes underlying population ecology. For example, the past decade has revealed several ecological correlates of striped hyena population characteristics. Singh et al. (2010, 2014) found that, unlike in other parts of their geographic range, striped hyenas in Rajasthan, India occurred at higher densities closer to human settlements than farther away. They posited that this high density was supported by the availability of unexploited livestock carcasses near settlements. They also found rugged terrain and forest cover to be important components of suitable habitats for striped hyenas in India, likely because they provide undisturbed den sites and daytime refugia where hyenas can rest undetected by humans and domestic dogs (Singh et al. 2010, 2014). In another Indian population of striped hyenas, vehicular traffic regulation had tremendous effects on rates of survival and population growth (Mandal 2018).
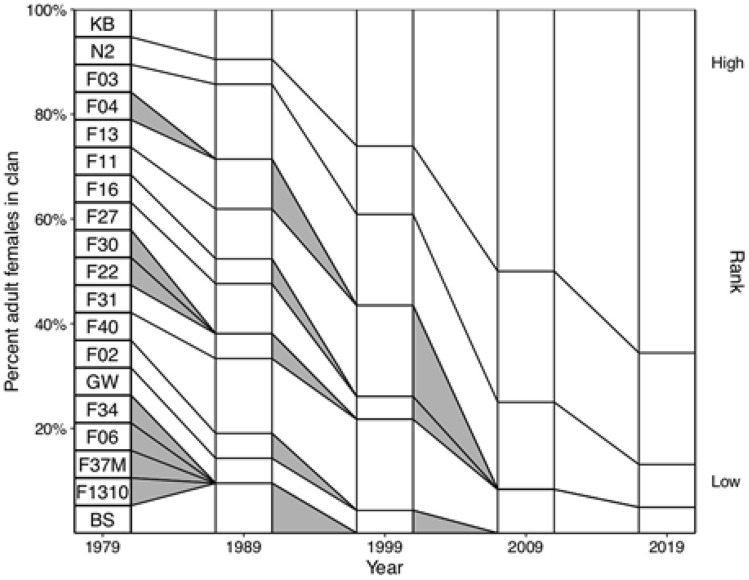
Studies of spatial and temporal variation in population size have aimed to illuminate ecological drivers of demography in spotted hyenas as well. In the Maasai Mara National Reserve, Kenya, the Talek hyena clan, which became known as the Talek West clan after a permanent clan fission event in 2000, has been studied from 1988 to present. Observers know each hyena by its unique natural markings and opportunistically collect biological samples (for genetic, dietary, hormonal, and other analyses) and fit hyenas with collars (VHF and/or GPS). The size of the Talek clan remained remarkably stable between 1989 and 1995 (Fig. 9; Green et al. 2018), given that outbreaks of rabies (1989–1991) and canine distemper (1994–1995) decimated sympatric populations of African wild dogs (Lycaon pictus; Macdonald 1992; Alexander and Appel 1994; Kat et al. 1995) and lions (Panthera leo; Roelke-Parker et al. 1996; Kock et al. 1998), respectively. The slight reduction in hyena clan size in 1989 (Fig. 9; Green et al. 2018) was due to emigration rather than increased mortality. This is consistent with previous findings documenting strong resistance of spotted hyenas to diseases that substantially increase mortality among sympatric carnivore species (Alexander et al., 2015; East et al. 2001; 2004; Watts and Holekamp 2009). However, disease-induced mortality has certainly been recorded for spotted hyenas (Roelke-Parker et al. 1996), including a Streptococcus outbreak that correlated with a 78% increase in mortality and slight population decline for 2 years in a spotted hyena population in the Ngorongoro Crater, Tanzania (Höner et al. 2006; 2012). Trends in the size of the Talek West clan (Kenya) between 2008 and 2013 reflected the top-down effects of human disturbance on spotted hyenas. During this time period (2008–2013), livestock grazing inside the Reserve increased more than sixfold, resulting in significantly fewer sightings of lions, which are the top competitor and mortality source of spotted hyenas, after humans. Meanwhile, the Talek West clan increased in size by 95%, eventually reaching 126 individuals and making it the largest spotted hyena clan ever documented (Fig. 9; Green et al. 2018).
Fig. 9.
Variation over time in the sizes of six study clans in the Maasai Mara National Reserve, Kenya. For each time point, the annual mean clan size is shown (with standard error bars when multiple population counts were performed within the given year). Data for each of six study clans, represented by symbols indicated in the key at the top left, were included from the first year each was studied through 2013. The Talek West clan split into two daughter clans in both 1989 and 2000, indicated by horizontal black bars over the data points. Each of these two clan fissions resulted in a reduction in size of the parent clan, as a subset of this clan’s members left to form a new clan in each case.
Reproduced from Green et al. (2018) with permission from Biodiversity and Conservation
Several studies have investigated ecological correlates of brown hyena density. The highest published densities to date were found in enclosed reserves (Welch and Parker 2016; Edwards et al. 2019), but a substantial proportion of the global population may live outside of protected areas (Kent and Hill 2013). Some studies have documented suppression of brown hyena density by sympatric apex carnivores (e.g., spotted hyenas) through competition (Williams et al. 2021), while others have found brown hyenas and apex carnivores to co-occur at high densities. Co-occurrence could be due to high prey density and divergent foraging strategies (hunting vs. scavenging; Vissia et al. 2021), or because the hunting activity of apex predators creates additional scavenging opportunities for brown hyenas (Yarnell et al. 2013). More work is needed to disentangle the environmental drivers of brown hyena density.
Behavioral studies of individually recognizable hyaenas have allowed researchers to investigate dispersal as well as its potential functions. Spotted hyenas exhibit male-biased dispersal (Höner et al. 2007), but dispersal processes and patterns may vary in different environments. In the Maasai Mara National Reserve, Kenya, most males disperse to new clans from their natal ones, and dispersers seem to experience greater mortality than non-dispersers (Smale et al. 1997), but also more mating opportunities. The reproductive success was compared between philopatric males (i.e., adult natal males who had not yet dispersed) and immigrant males by pairing observational data with genetic samples of known individuals from this study population (Engh et al. 2002; Van Horn et al. 2004; Watts et al. 2011). Engh et al. (2002) reported that, although 20% of the adult males in their study clan were philopatric males, they sired only 3% of cubs born. Thus, the immigrant males had much higher reproductive success than philopatric males who had not dispersed. Engh et al. (2002) also found that immigrant males’ reproductive success increased with their length of tenure within the new clan. However, spotted hyenas in the Ngorongoro Crater, Tanzania, exhibited quite a different pattern of dispersal. While the majority of males there dispersed, dispersers and philopatric adult males distributed themselves similarly across clans, electing to join clans with the highest number of potential mates, and attained similar fitness (Davidian et al. 2016). The difference in patterns between these two study sites may reflect either variation in constraints on dispersal or variation in the underlying processes of males’ decisions to disperse or not, but further work on known individuals using consistent methodology across study sites is needed to address these hypotheses (Davidian et al. 2016). Collectively, studies of individually recognizable spotted hyenas have revealed that female mate choice drives male-biased dispersal (Smale et al. 1997; Engh et al. 2002; East et al. 2003; Höner et al. 2007; Davidian et al. 2016).
Striped hyenas also seem to be plastic in their dispersal behavior. Califf et al. (2020) compared space use of known individuals between populations of striped hyenas in central (Laikipia) and southern Kenya (Shompole). Whereas food resources in Laikipia were relatively scarce, they were plentiful in Shompole. Striped hyenas in these two populations showed very different dispersal patterns, with Laikipia females dispersing (Califf et al. 2020), while Shompole females were philopatric (Califf et al. 2020). Females in central Laikipia may have dispersed to avoid competing with close kin for food, whereas the high density of prey in Shompole allowed females to remain near their natal home range (Califf et al. 2020).
Individual identification is particularly critical in studies of social behavior (spotted hyenas, Frank 1986; brown hyenas, Mills 1983a; striped hyenas, Califf et al. 2020; aardwolves, Richardson 1987b, 1991). Repeated behavioral observations of individually recognizable spotted hyenas allow researchers to determine each individual’s precise social rank in its clan, and to track ontogenetic changes in individuals’ social ranks (Smale et al. 1993; Strauss and Holekamp 2019a). Like many juvenile primates, young spotted hyenas learn their ranks in the clan’s dominance hierarchy early in life through a process known as “maternal rank inheritance,” where they acquire ranks immediately below those of their mothers, and above those of their older siblings (Holekamp and Smale 1991; Engh et al. 2000). Despite variability in the timing of rank acquisition among juveniles, most young hyenas come to attain the precise rank predicted by the rules of maternal rank inheritance. Nevertheless, transient variation in early-life rank acquisition is associated with long-term fitness consequences; juveniles that “underperformed” at acquiring their expected ranks show reduced survival and lower lifetime reproductive success than better performing peers, and this relationship was independent of both maternal rank and rank achieved in adulthood (Strauss et al. 2020). In adulthood, rank changes often occur due to passive processes (e.g., births, emigration), but, on rare occasions, they arise from active processes (e.g., rank reversals; Strauss and Holekamp 2019b). That is, individual adult females who repeatedly form coalitions with their top allies may improve their position in the clan’s hierarchy, suggesting that social alliances facilitate revolutionary social change. Using lifetime reproductive success as a fitness measure, Strauss and Holekamp (2019b) demonstrated that these status changes can have major fitness consequences. Furthermore, these fitness consequences may increase over multiple generations (Fig. 10), as small differences in social rank become amplified over time. Thus, knowing each hyena individually allows investigation of rank changes within individuals’ lifetimes, as well as the fitness consequences that unfold over many generations.
Fig. 10.
Representation among adult female spotted hyenas of the Talek clan at decade-long intervals (Holekamp and Strauss 2020) of descendants of the original 19 adult females studied by Frank (1983).
Reproduced from Holekamp and Strauss (2020) with permission from Integrative and Comparative Biology
Camera trapping has scarcely been used to study spotted hyena sociality, but a recent pioneering study (Stratford et al. 2020) suggests that camera traps can be used to shed light on the diversity in group composition and dynamics within this species. Clan size of spotted hyenas is highly variable, ranging from six to over a hundred individuals (Kruuk 1972; Green et al. 2018), depending on environmental factors (Stratford et al. 2020). Extensive direct observations have been used to study the social structures of several large clans of spotted hyenas in grassland ecosystems (Hofer and East 1993; Holekamp and Strauss 2020), where relatively high visibility makes such observations possible. However, direct observations are challenging in dense forests and rugged terrain, so little is known about the social structure of spotted hyenas living in smaller clans in these environments (Stratford et al. 2020). Stratford et al. (2020) demonstrated the utility of camera traps to fill this gap in knowledge using individual identification. They deployed camera traps at waterholes in a Namibian game reserve to capture images of resident hyenas. They used the photographic captures to estimate clan size, assign clan membership, and estimate individuals’ connectedness within their clan (Stratford et al. 2020).
Studies of known individual striped hyenas have recently revealed that these animals are not strictly solitary, as previously thought, and that their social behavior varies greatly among populations under different ecological conditions. For example, Califf et al. (2020) found that females in the resource-rich Shompole region exhibited high home range overlap, particularly between kin, whereas those in the resource-poor Laikipia region exhibited no home range overlap. In the Laikipia population, most females’ territories were occupied by at least one male (Wagner et al. 2008). In fact, although striped hyenas typically forage solitarily, many studies have observed social aggregations (Wagner 2006; Tichon et al. 2020), particularly at active dens. Alam (2011) observed groups of three to eight striped hyenas gathering at a single den, and cubs from previous litters often helping to rear their younger siblings, sometimes even provisioning them with food (Alam 2011; Mandal 2018). Recently, den-sharing by a pair of closely related female striped hyenas was documented in Shompole (Spagnuolo 2016; Califf et al. 2020).
Future directions in basic research
There are countless potential future directions for the role of individual identification in basic research on wild hyenas and aardwolves. Camera traps could be used to expand our knowledge of brown hyenas, striped hyenas, and aardwolves, as well as spotted hyenas inhabiting dense forests and rugged terrain; these are currently poorly understood, especially compared to our knowledge base about the spotted hyena populations inhabiting grassland ecosystems (Dheer et al. 2022). For example, camera traps can be effective at collecting data on the two most elusive hyaenids, striped hyenas and aardwolves, the basic biology of which remain poorly understood. Striped hyenas and aardwolves are nocturnal, persist at low densities, and inhabit rugged terrain, making them difficult to detect. In areas thought to be occupied by residents of these species—based on reported sightings, presence of spoor or scat, or by-catch data from camera trap studies of other species—camera traps can be strategically placed to capture images of striped hyenas or aardwolves (Schuette et al. 2013). That is, camera traps can be systematically distributed on a grid (optionally baited with an attractant) or placed at points of interest, such as waterholes, artificial or natural trails, known dens, or locations where spoor, scat, or direct sightings have been reported recently. If sufficient data can be obtained, then density, space use, movement, and activity patterns could be assessed. For striped hyenas, if the goal is to investigate social group size and composition, fission–fusion dynamics, or social networks, camera traps will likely need to be stationed at active dens, because this species typically forages solitarily but sometimes convenes at dens. These camera traps should be equipped to capture short videos, because higher quality behavioral data can be extracted from videos than from still images alone.
For all four hyaenid species, more work is needed to delineate ecological drivers of demography and behavior. Studies should collectively cover the diversity of habitats and geographic range of each species. We recommend camera traps for data collection, as these can be used even for elusive populations in dense vegetative cover or rough terrain (Treves et al. 2010; Kelly et al. 2012; Johansson et al. 2020). Similar methods must be used across different studies, so that the findings can be compared or, ideally, eventually incorporated into meta-analyses. These methods can be adapted as needed for the given species or study area, or to meet additional objectives of the research project.
This review presents examples of studies of known individual hyaenids across diverse disciplines, and the possible future directions are equally diverse. We presented several examples of interesting avenues for future research, but the possibilities certainly are not limited to those presented here. Hyena biologists with research foci other than those touched on herein should certainly seek applications that fit their own area of research in the literature, or consider new applications to their interests.
Past and present use in conservation and management
Individual identification of hyaenids facilitates research that informs conservation and management of free-living populations. First, individual identification has been employed to develop methods for density estimation that are far superior to traditional methods (Karanth and Nichols 1998; Treves et al. 2010; Kelly et al. 2012; Green et al. 2020; Vissia et al. 2021). Population density is critical information needed for wildlife conservation (Rich et al. 2019), and the IUCN has identified estimation of population size as a top research priority for conservation of all four hyaenid species (AbiSaid and Dloniak 2015; Bohm and Höner 2015; Green 2015; Wiesel 2015). Photographic capture–recapture methods have been used to estimate density of hyaenid populations, even for rare or elusive hyaenids (O’Brien and Kinnaird 2011; Alam et al. 2015; Edwards et al. 2019; Vitale et al. 2020; Braczkowski et al. 2022) and those occupying areas only accessible to researchers on foot (Henschel et al. 2014). Accurate estimates of density are important as this allows for better estimation of global population size and the designation of appropriate conservation status (Akçakaya et al. 2006).
Second, information on hyaenids’ social systems gleaned from studies of known individuals may also be useful in conservation planning. Camera trap (Mandal 2018; Tichon et al. 2020) and observational studies (Frank 1986) form the basis of much of our understanding of social grouping, which may inform density estimation. This body of research has also provided evidence of cooperative breeding in brown hyenas (Mills 1990) and, more recently, in striped hyenas (Alam 2011; Spagnuolo 2016; Mandal 2018; Califf et al. 2020). This knowledge may help to predict how reduced social group sizes (resulting from declines in population density) may affect reproductive success in cooperatively breeding populations (van der Meer et al. 2013; Tichon et al. 2020).
Our knowledge of population connectivity has benefited from direct observations of spotted hyenas bearing unique natural markings, sometimes paired with complementary data from radio collars, GPS collars, or genetic samples. The resultant findings have illuminated patterns of space use and movement, including natal dispersal (Smale et al., 1997; Boydston et al. 2005), and reproductive success in spotted hyenas (Engh et al. 2002; Watts et al. 2011). Gene flow among spotted hyena clans may have important implications for metapopulation persistence (McCullough 1996; Hanski and Simberloff 1997; Dolrenry et al. 2014).
Finally, individual identification of hyaenids can be used to investigate their role within the ecosystem, to the benefit of multiple species. Ongoing research into predictors of demographic parameters identifies components of suitable habitat and threats to hyaenid population persistence (Singh et al. 2010, 2014; Mandal 2018), which may be directly targeted by conservation efforts. Camera traps can be used to monitor multiple species within the same study area (Kelly et al. 2012; Green et al. 2020). Illumination of the dynamics within large carnivore guilds, as well as the ecological relationships among carnivores and their wild and domestic prey (Green et al. 2018), may enable scientists and practitioners to foresee the consequences of changes within one species or trophic level for other sympatric species. Spotted hyena movement may also shed light on impending shifts in the sympatric large carnivore community; Green et al. (2019) found that, within spotted hyena territories, the areas frequented by low-ranking (rather than high-ranking) hyenas and in which hyenas moved at the highest speeds exhibited declines in carnivore species richness in the following months. The spatial resolution of this analysis was small (200 × 200 m cells), much smaller than a large carnivore’s home range, so this may reflect changes in space use by sympatric carnivores rather than their density in the study area. More research is needed to explore this relationship, to unravel the underlying mechanisms, and to determine appropriate applications to conservation and management.
Future directions in conservation and management
Many practical applications of individual identification of hyaenids remain unexplored. One potential application is the identification of population sinks. Source–sink theory posits that average fitness varies across subpopulations within a metapopulation due to variable habitat quality. Subpopulations inhabiting patches of high-quality habitat (source populations) are expected to experience high fitness, thus producing a surplus of individuals, while subpopulations inhabiting poor-quality habitats (sink populations) experience low fitness, with mortality exceeding local recruitment, resulting in a population deficit (Pulliam 1988; Kristan 2003; van der Meer et al. 2015; Kelt et al. 2019). Population sinks are maintained by immigration from source populations (Pulliam 1988). A common example of source-sink dynamics in large carnivores is edge effects on populations in protected areas; that is, conflict with humans along the boundaries of protected areas often turns border areas into population sinks, with populations in core areas acting as sources (Woodroffe and Ginsberg 1998; Balme et al. 2010). Knowledge of edge effects have been used to improve conservation and management of leopards in South Africa, potentially to the benefit of other carnivores, including spotted hyenas (Balme et al. 2010). Several studies have found evidence of edge effects on probability of population persistence (Woodroffe and Ginsberg 1998) and mortality (Newmark 2008; Pangle and Holekamp 2010) of spotted hyenas, but there is still much work to do in this area.
More urgently, we encourage researchers to take advantage of hyaenids’ unique natural markings to identify ecological traps. An ecological trap represents an extreme case of a population sink, in which the animals actually prefer the sink to the source area (Gates and Gysel 1978; Delibes et al. 2001; Donovan and Thompson 2001). Under classical source-sink theory, it is assumed that animals can accurately assess habitat quality and therefore prefer source habitat, only immigrating to sink habitat when the source habitat is already occupied. When vacancies become available in the source area, individuals should emigrate from the sink to the source (Kristan 2003; Kelt et al. 2019). Through continuous density-dependent immigration, sources and sinks can persist. In fact, sink patches may contribute to the persistence of the metapopulation as a whole (Pulliam 1988; Howe et al. 1991; Kelt et al. 2019). However, when environmental cues are uncoupled from true habitat quality—most often through anthropogenic disturbance—a population sink may become more attractive to animals than nearby source areas, representing an ecological trap (Gates and Gysel 1978; Delibes et al. 2001; Donovan and Thompson 2001). Elevated mortality rates in ecological traps create vacancies that invite immigration from nearby source populations, creating a “vacuum effect” that can lead to extirpation of the entire metapopulation over time (Balme et al. 2010). Occupancy and density data alone are insufficient to identify population sinks and ecological traps (Kelt et al. 2019). Rather, the relationship between habitat preference and habitat quality must be delineated, for example, using data on fecundity, mortality, and dispersal over time (Pulliam 1988; Kelt et al. 2019). Empirical evidence of ecological traps has been found for other large carnivores (van der Meer et al. 2013, 2015; Lamb et al. 2017), but we are unaware of any research on ecological traps for hyaenids specifically. In the face of continuing anthropogenic change, ecological traps may become increasingly widespread threats to the persistence of large carnivore populations (Balme et al. 2010). This could certainly be the case for hyenas, which could potentially be attracted to human settlements (e.g., by livestock, crops, or refuse; Kruuk 1976; Kolowski and Holekamp 2008; Kissui et al. 2019). Conservation planning should incorporate identification of and differentiation between conventional population sinks and ecological traps (van der Meer et al. 2015).
Increasing anthropogenic disturbance presents many natural opportunities to assess the responses of hyaenids to human activity. Monitoring efforts should be initiated in areas subject to imminent increases in anthropogenic activity, such as livestock grazing or tourist visitation, to provide baseline data. These efforts—whether direct behavioral observations or camera trap studies—can run continuously or be repeated at future time points using the same methods each time to quantify demographic or behavioral changes that occur alongside changing levels of human disturbance. This same process could also be applied to sites targeted for restoration or at which prohibitions against human activities are newly enforced to assess resilience of hyaenid populations. The COVID-19 pandemic presented another natural “experiment,” because travel restrictions interrupted ecotourism. In many countries, pre-pandemic data could be compared to data collected after the imposition of travel restrictions to investigate effects of tourism on hyaenids. Anthropogenic removal of sympatric carnivores or natural prey could allow researchers to delineate demographic and behavioral responses to reduction of apex carnivores (Green et al. 2018), mesocarnivores, or prey.
Identification of problem animals (Linnell et al. 1999) represents another potential future direction for applied research on individually identifiable spotted hyenas. Livestock depredation imposes a financial burden on affected households and can prompt retaliatory killings of hyenas and sympatric carnivores (Kissui 2008). Although we are unaware of any studies that sought to determine whether livestock depredation is attributable to a subset of problem animals, previous research on intrapopulation variation in hyaenid behavior suggests that this is likely. First, socioecological conditions affect space use in spotted hyenas, putting some individuals at higher risk of conflict with humans. In the Maasai Mara National Reserve, Kenya, low-ranking females without den-dependent cubs maintain the largest home ranges of any adult females, particularly in times of prey scarcity (Boydston et al. 2003; Green et al. 2019). Upon reaching puberty, males begin making exploratory forays beyond the boundaries of their territory, and adult males maintain larger home ranges than their female clanmates, venturing three to four times as far from the center of their territory as females (Boydston et al. 2005). Thus, males and low-ranking females may be more likely to venture into human-disturbed landscapes than high-ranking females, and thus experience an elevated risk of human-caused mortality.
Second, hyena personalities may affect individuals’ likelihoods of engaging in conflict with humans. Animal personality refers to individual variation in behavioral traits such as boldness, neophobia, and exploration that is consistent across time and context (Yoshida et al. 2016; Greenberg and Holekamp 2017; Turner et al. 2020). Individual personalities converge to affect population responses to novel environmental conditions, such as human disturbance (Merrick and Koprowski 2017). Greenberg and Holekamp (2017) and Turner et al. (2020) found that spotted hyenas from highly human-disturbed areas were less bold, less neophobic, and more exploratory than those in areas of low human disturbance. Boldness was negatively correlated with survivorship to adulthood in populations exposed to both low and high human disturbance. The relationships among intrapopulation variation in behavioral traits (personality) and space use have yet to be empirically studied in the context of human–hyena conflict.
If livestock depredation varies among individual spotted hyenas, one would also expect to observe intrapopulation variation in selection of native prey. Currently, the strength of individual diet specialization is poorly understood in hyaenids. This could be investigated through behavioral observations of hunting and feeding of known individuals or by analyzing tissue samples or repeat scat samples from known individuals. However, most published studies of scat analyses in hyenas used scat from unknown individuals (Henschel and Skinner 1990; Abay et al. 2011; Yirga et al. 2013), hindering the study of dietary specialization in hyaenids.
Intrapopulation variation in encounter rates with livestock affects livestock depredation risk and represents another exciting frontier in human–hyena conflict mitigation. For example, a camera trap grid could be placed to cover a mosaic of areas of livestock use (e.g., livestock corrals) and non-use, large enough to encapsulate at least one entire spotted hyena clan territory. Network analyses can be used to identify clans (Vitale et al. 2020), and clan membership can be paired with locations of photographic capture events to delineate territory boundaries. The camera trap data could be analyzed in a spatially explicit capture–recapture framework to estimate the size of a clan whose entire territory is included within the camera trap array and encompasses livestock corrals. Observers could then determine the number of visits to livestock corrals by each individual hyena. Variation in corral visit frequency would likely reveal differences in individuals’ tendencies to pass through these areas, representing individual-level differences in encounter rate with livestock. We are unaware of any camera trap studies at livestock corrals or grazing areas that differentiate among individual hyenas. In fact, we are only aware of one study in which camera traps were deployed at livestock corrals (Hoffmann et al. 2022). In this study, Hoffmann et al. (2022) used camera trap data to quantify encounter and attack rates at the species level but did not distinguish among individuals of each species. Innovative research methods are worthy of exploration, as are analytical applications to investigate human–hyena interactions and dynamics in shared landscapes.
Resources for conflict mitigation are limited, so identification of high-conflict areas is essential. Hyenas who frequent human settlements are likely at a higher risk of human-induced mortality (e.g., spearing, poison, snares) than others, regardless of their interactions with livestock. Individual-level space use and mortality data from known hyenas could be useful in identifying age-sex class and rank of hyenas at the highest risk of human-caused mortality. Furthermore, by identifying which high-use areas are correlated with the highest hyena mortality rates, efforts to reduce human–hyena conflict could be concentrated where they are most needed.
Current limitations
Despite its advantages, there are some drawbacks to individual identification using natural markings. To demonstrate these points, we draw from examples of hyaenids. There are certainly limitations of other methods discussed herein, such as camera trapping (Green et al. 2020; Dheer et al. 2022), but here we specifically focus on the limitations of individual identification of hyaenids in both camera trap studies and direct observations.
Using naturally occurring markings to identify individuals can be time-consuming and is not always reliable. For example, one study tested agreement among observers in individual identification of striped hyenas from camera trap images through a double-blind experiment in which three independent observers identified hyenas in 26 photographic captures (Harihar et al. 2010), following methods of Kelly et al. (2008). All three observers only agreed on the identity of the hyena for 76.80% of the capture events (Harihar et al. 2010).
According to Johansson et al. (2020), each time an attempt is made to identify an individual, there are five possible outcomes: correct identification, a splitting error, a combination error, a shifting error, or exclusion of the datum. A splitting error occurs when the focal individual is already present in the dataset but is mistakenly identified as a new individual. A combination error occurs when the focal individual has not yet been observed but is mistaken for a different animal that has already been observed. A shifting error occurs when the focal individual has already been observed and is mistaken for a different individual, who has also already been observed. Finally, a datum may be deemed unusable and excluded from the dataset either correctly (true exclusion) or erroneously (exclusion error). A true exclusion occurs when identification is truly infeasible, for example, due to poor lighting or blockage of the observer's view by features of the environment (e.g., tall grass) or another animal (Kelly et al. 2008; Harihar et al. 2010). An exclusion error occurs when sufficient information is available to make the identification, but the observer fails to identify the individual (Johansson et al. 2020).
Identification errors can skew the results of the studies in which they occur. In estimation of population size or density, splitting errors lead to overestimation, whereas combination errors lead to underestimation (Johansson et al. 2020). Shifting and exclusion errors do not necessarily bias the results of traditional capture–recapture analyses but shifting errors are problematic in spatially explicit capture–recapture analyses (Johansson et al. 2020). In a camera trap study of 16 snow leopards, photographic captures had an 8.70% probability of being excluded from the dataset. Of the remaining photographic captures, the probability of splitting errors (9.10%) was far higher than that of combination or shifting errors, leading to an overall misidentification rate of 12.50% (Johansson et al. 2020). The prevalence of splitting errors ultimately inflated the estimated population size by about one third (Johansson et al. 2020). Population estimates are central to conservation and management planning, and overestimation of population size may potentially undermine conservation efforts (Choo et al. 2020; Johansson et al. 2020). Splitting errors may be the most pervasive error type and are especially problematic in studies of threatened and endangered species, but more studies of identification error are needed to better understand the prevalence of error types across species with distinct individual natural markings. The other types of error (combination, shifting, and exclusion) also have the potential to bias estimates of demographic parameters, specifically if they are not randomly distributed across the population (Johansson et al. 2020), such as if some age-sex classes are more difficult to identify than others. For example, subadult spotted hyenas go through a phase in which they become very fluffy, making their spot patterns difficult to see. Additionally, ear damage and scars accumulate over a hyena’s lifetime, and are therefore relatively uncommon in young hyenas. On extremely old hyenas, on the other hand, spots may fade. For these reasons, it may be easier to identify middle-aged hyenas than those that are very young or very old. In addition to yielding erroneous population estimates (Johansson et al. 2020), misidentification can obscure the results of behavioral or genomic studies.
Standards for reporting methods and accuracy of individual identification are severely lacking. Most studies do not describe the methods they used to avoid misidentification, provide photographic evidence that individuals can be differentiated, or quantify the error rates of identification, thus precluding reviewers and readers from critically assessing the studies’ reliability and robustness (Choo et al. 2020). In summary, the methods by which data on known individuals are collected, analyzed, and reported certainly need further development. This is not to say that we should not use individual identification, but rather to stress the importance of quantifying, rectifying, and reporting errors, as well as refining methods for managing interobserver discrepancies and unclassifiable capture events (Choo et al. 2020; Johansson et al. 2020).
Future directions in methodology
Many of the current limitations of individual identification represent potential targets for methodological improvements. These include observer training, testing for errors, error prevention, transparency and accountability in reporting, and software to aid observers in identification. We also suggest methods by which the resultant data could be used to improve parameter estimation and answer novel questions.
More information is needed to determine what specific training or experience decreases observers’ misidentification rates. Virtual training tools, such as that recently produced for photographic identification of snow leopards (Johansson et al. 2020), may be helpful in improving training as well as in testing identification error. By testing rates of specific error types, targeted training approaches and identification methods can be identified to avoid the common error types (Choo et al. 2020; Johansson et al. 2020). Johansson et al. (2020) suggested prioritization of verification of new individuals, based on the prevalence of splitting errors. When feasible, multiple independent observers should identify individual animals, and methods for resolving disagreement among observers and management of images deemed unclassifiable should be carefully chosen (Choo et al. 2020; Johansson et al. 2020). Using multiple independent observers not only helps to detect and rectify identification errors but allows authors to report rates of interobserver agreement. Even when errors cannot be effectively rectified, quantification of error rates can allow for selection or development of modeling approaches that minimize the impacts of these errors on the results (Yoshizaki et al. 2009; Mendoza et al. 2011; Johansson et al., 2020). To achieve higher transparency and accountability in reporting, we recommend following the Individual Identification Reporting Checklist presented by Choo et al. (2020).
Software programs to automate individual identification have been successfully applied to multiple mammal species (Bolger et al. 2012; Crall et al. 2013; Schneider et al. 2019; Choo et al. 2020), and could potentially be applicable to hyaenids in the future. Computer vision has been used to assist manual identification by human observers in other carnivores with distinct coat patterns, such as cheetahs (Acinonyx jubatus; Kelly 2001), tigers (Hiby et al. 2009), and bobcats (Lynx rufus; Mendoza et al. 2011). A computer-aided approach relies on a human observer to confirm the final classification, but can reduce the man-hours needed to process large datasets (Kelly 2001) and reduce rates of misidentification (Hiby et al. 2009; Mendoza et al. 2011). Automated identification methods are currently underdeveloped and face many of the same challenges that human observers do (e.g., poor image quality, camera angle; Johansson et al. 2020). However, with further development, these methods could become highly effective and widely used in the future (Schneider et al. 2019). We are unaware of any studies that have used automatic methods to identify individual hyaenids, but suspect that this would be difficult, especially in species whose patterns are especially prone to distortion, such as by shifting position of long fur (spotted and striped hyenas) or due to camera angle (brown hyenas).
Data from individually identifiable animals can be applied to improve model parameter estimation. Counterintuitively, although studies of occupancy do not require differentiation of individuals, they could still benefit from examination of unique markings. Individual identity may be useful in determining whether or not the assumptions of spatial independence and population closure have been met (Edwards et al. 2018). Additionally, further research on personality may better inform estimates of detection probability for hyaenids. This research by definition requires individual hyaenids to be identified and studied across time and contexts.
New research questions may be addressable by combining multiple data types. Individually recognizable animals can be studied by pairing visual observations (direct or through photos) with data collected with either noninvasive (Table 1) or invasive (radio collars, Stratford et al. 2020; Edwards et al. 2020; biosamples, Engh et al. 2002; Van Horn et al. 2004; Watts et al. 2011) methods. Additionally, experts could visually identify individuals in geotagged photos of sufficient quality submitted by local people or tourists. There are also many large datasets of existing images produced by camera trap studies that could be used to answer new research questions. Thus, advances in methodology of individual hyaenid identification could facilitate citizen science and new collaborations.
Table 1.
Creative combinations of methods used with spotted hyenas have allowed researchers to collect biological samples noninvasively from known individuals to pair with direct observations. Here, we report methods paired with direct behavioral observations, the type of complementary data yielded by this method, and citations of researchers who have successfully employed this fusion
| Method(s) | Data type | Citation(s) |
|---|---|---|
| Opportunistically pluck cubs’ hair | Genetic | Höner et al. (2007) |
| Necropsies of dead hyenas | Genetic | Höner et al. (2007) |
| Feces collection | Genetic | Watts et al. (2011) |
| Feces collection | Hormones | Dloniak et al. (2006) |
| Van Meter et al. (2008, 2009) | ||
| Sampling by saliva stick | Hormones | Montgomery et al. (2022) |
| Feces collection | Microbiome | Rojas et al. (2020) |
The most vocal hyaenids, spotted hyenas, may be individually distinguishable not only through visual identification, but possibly through acoustic identification as well (Lehmann et al. 2022). Spotted hyenas’ loudest vocalizations, called “whoops,” are emitted in bouts and can be heard from up to five kilometres away (Kruuk 1972; East and Hofer 1991a). Whooping serves important functions, such as recruiting clanmates to cooperate in defense of a shared resource, and are used in various contexts to transmit information about the callers’ location, age, sex, affective state, and, importantly, identity (East and Hofer 1991a, b; Theis et al. 2007; Benson-Amram et al. 2011; Gersick et al. 2015; Lehmann 2020). Acoustic variation among different individuals (signal strength) and consistency of individuals’ acoustic signatures over time (signal stability) are necessary for acoustic identification of individuals (AIID; Linhart et al. 2022). If the acoustic variation quantified by Lehmann et al. (2022) proves to be consistent over time, as suggested by East and Hofer (1991a), then AIID could become a powerful tool for studying spotted hyenas.
Vocalizations may be collected through focal or passive recording. In focal recording, an observer typically uses a handheld microphone and digital recorder. In passive recording, vocalizations are captured by autonomous recording units (ARUs) placed in the animal’s environment. Focal recording has many benefits, such as yielding high-quality samples and allowing the observer to record the emitter’s identity, the emitter’s posture and orientation in relation to the microphone, the distance between the emitter and the microphone, and contextual information. However, focal recording is much more time consuming than passive recording. Using focal recordings for AIID in a capture–recapture framework is important for feature selection and external validation, and can be treated as a pilot study, with the ultimate goal of developing methods for AIID using passive recording (Linhart et al. 2022). We are unaware of any work that has identified individual hyenas based on passive recording, but this could be an interesting area for future exploration. Next steps should include developing software to perform AIID through machine learning and to externally validate the results. Additionally, the maximum distance at which a whoop can be correctly assigned to the emitter should be determined. These steps all require pilot data from focal recordings. If whoops can be recorded from kilometres away and the emitter accurately identified, then an ARU could potentially obtain much more data than a camera trap placed at the same location.
Conclusion
The unique markings of hyaenids are indispensable in ongoing research and have greatly enhanced our understanding of these species. We have highlighted several interesting studies that exemplify this, but this is by no means a comprehensive review of the literature built on individual identification of hyaenids. In addition to the fields discussed here, this method has facilitated studies of hyena cognition (Johnson-Ulrich et al. 2020), disease ecology (Höner et al. 2012; Gering et al. 2020), mate choice (Engh et al. 2002; Szykman et al. 2001), behavioral endocrinology (Dloniak et al. 2006; Montgomery et al. 2022), and microbiota (Theis et al. 2013; Rojas et al. 2020), among many others. Comparative work within the family Hyaenidae is especially useful, as the socioecological diversity within this family allows investigation of the evolution of various traits in closely related species and has been particularly useful in studies of social evolution and intelligence (Holekamp 2007; Holekamp et al. 2007; Holekamp and Benson-Amram 2017; Johnson-Ulrich 2017). Applied work with hyenas in situ is timely and important for protecting human livelihoods from crop raiding and livestock depredation, and for conserving rare striped and brown hyenas. Furthermore, results of such studies may help to inform conservation and management of other large mammalian carnivores or even whole ecosystems (Green et al. 2019).
Despite the wealth of knowledge about spotted hyenas, many unanswered questions remain, and relatively little is known about the other hyaenid species, especially striped hyenas. We have identified several of the many gaps in our current understanding that can be answered using studies of free-living, individually recognizable hyenas or aardwolves. Furthermore, identification by natural markings has been combined with other methods to generate novel datasets. For example, some studies have fitted subjects with collars to aid in visual identification or location of subjects for repeated behavioral observations, or to supplement data from direct observations with spatial data from GPS collars, while also using coat patterns to differentiate individuals (Richardson 1987a, b, 1991; Silwa 1996; Boydston et al. 2003; Califf et al. 2020). Individually known subjects may also be captured to obtain biosamples to complement behavioral data (Höner et al. 2007; Califf et al. 2020). Identification by natural markings has also been combined with various noninvasive methods (Table 1) to produce complementary datasets. Many possible combinations of methods have yet to be used for hyenas, such as pairing camera traps with hair snares or identifying prey hair or DNA in scat from known individuals to study individual diet variation and specialization (Larson et al. 2020). Noninvasive methods are unlikely to replace invasive methods completely, but they can be beneficial for minimizing stress and risk of injury to the animals, circumventing logistical limitations (including obtaining permits) and trap shyness, maximizing sample size, and detecting elusive species (Kelly et al. 2012).
Noninvasive individual identification has proven critical to building our understanding of the demography, social behavior, reproduction, and ecology of wild hyenas and aardwolves. The literature reviewed herein was selected to showcase the value of this method and to showcase the diversity of its applications, but we merely scratch the surface of this vast body of work. We encourage researchers and conservation practitioners to seek out papers that describe the use of this method in their own areas of interest and to consider how their current or future projects may benefit from incorporation of individual identification of study animals. Researchers already using individual identification should strive to meet the criteria specified in the Individual Identification Reporting Checklist (Choo et al. 2020). Besides the large body of published work to date, there are certainly many unexplored uses of individual identification. Researchers should consider new applications of this method to address basic research questions and methodological advances to address the limitations discussed above. Importantly, there are many avenues of applied research that have gone largely unpursued to date in hyenas, including identification of problem animals in livestock and crop damage. We encourage creative fusions of methods and the application of individual identification to basic and applied research questions. The methods we have discussed should be useful in future studies of wild hyaenids, as well as other mammalian carnivores, by facilitating new research, improving reliability and transparency of published work, and informing conservation and management strategies.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank all past and present members of the Mara Hyena Project for their work collecting and curating the data presented here. We thank the National Commission for Science, Technology and Innovation, the Kenya Wildlife Service, the Wildlife Research and Training Institute, the Narok County Government, the Government of Namibia, the Naboisho Conservancy, and the Mara Conservancy for permission to conduct research on spotted hyenas. We thank the Namdeb Diamond Corporation for allowing access to the study area for brown hyenas, and the Namibian Ministry of Environment, Forestry and Tourism for granting annual research permits to study brown hyenas. We thank Kenna Lehmann, Tracy Montgomery, Julie Jarvey, Lily Johnson-Ulrich, Eli Strauss, Jackie Spagnuolo, Darren Incorvaia, and both editors Scott Y.S. Chui and Leszek Karczmarski for their valuable feedback on this manuscript. We thank Sabrina Salome and Malit Ole Pioon for their help obtaining photographs of spotted hyenas.
Authors' contribution
O.S.B.S. and K.E.H. conceptualized this paper. O.S.B.S. conducted background research on each of the four hyaenid species. K.E.H. assisted in background research on spotted hyenas, I.W. did so for brown hyenas, and M.A.L. did so for aardwolves. Each author also found photos to illustrate points made about her species. Each author then submitted her text to O.S.B.S., who organized the manuscript and added figures and tables. O.S.B.S. and K.E.H. made manuscript revisions.
Funding
This work was supported by National Science Foundation (NSF) Grants OISE1853934 and IOS1755089 to KEH and the Namdeb Diamond Corporation funds granted to IW.
Declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Footnotes
Handling editors: Scott Y.S. Chui and Leszek Karczmarski.
This article is a contribution to the special issue on “Individual Identification and Photographic Techniques in Mammalian Ecological and Behavioural Research – Part 2: Field Studies and Applications” — Editors: Leszek Karczmarski, Stephen C.Y. Chan, Scott Y.S. Chui and Elissa Z. Cameron.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Olivia S. B. Spagnuolo, Email: spagnu39@msu.edu
Marie A. Lemerle, Email: marie.lemerle157@gmail.com
Kay E. Holekamp, Email: holekamp@msu.edu
Ingrid Wiesel, Email: ingrid.wiesel@strandwolf.org.
References
- Abay GY, Bauer H, Gebrihiwot K, Deckers J. Peri-urban spotted hyena (Crocuta crocuta) in northern Ethiopia: diet, economic impact, and abundance. Eur J Wildl Res. 2011;57:759–765. doi: 10.1007/s10344-010-0484-8. [DOI] [Google Scholar]
- AbiSaid M, Dloniak SMD (2015) Hyaena hyaena. The IUCN Red List of Threatened Species. 10.2305/IUCN.UK.2015-2.RLTS.T10274A45195080.en. Accessed 10 Dec 2020
- Akçakaya HR, Butchart SHM, Mace GM, Stuart SN, Hilton-Taylor C. Use and misuse of the IUCN Red List Criteria in projecting climate change impacts on biodiversity. Glob Change Biol. 2006;12:2037–2043. doi: 10.1111/j.1365-2486.2006.01253.x. [DOI] [Google Scholar]
- Alam MS, Khan JA, Pathak BJ. Striped hyena (Hyaena hyaena) status and factors affecting its distribution in the Gir National Park and Sanctuary, India. J Vertebr Biol. 2015;64:32–39. doi: 10.25225/fozo.v64.i1.a4.2015. [DOI] [Google Scholar]
- Alam MS (2011) Status ecology and conservation of striped hyena (Hyaena hyaena) in Gir National Park and Sanctuary, Gujarat. Dissertation, Aligarh Muslim University
- Alexander KA, Appel MJ. African wild dogs (Lycaon pictus) endangered by a canine distemper epizootic among domestic dogs near the Masai Mara National Reserve, Kenya. J Wildlife Dis. 1994;30:481–485. doi: 10.7589/0090-3558-30.4.481. [DOI] [PubMed] [Google Scholar]
- Alexander JS, Gopalaswamy AM, Shi K, Riordan P. Face value: towards robust estimates of snow leopard densities. PLoS ONE. 2015;10:e0134815. doi: 10.1371/journal.pone.0134815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MD. Proteles cristatus. In: Kingdon J, Hoffmann M, editors. The mammals of Africa. Volume V: carnivores, pangolins, equids, rhinoceroes. London: Bloomsbury Publishing; 2013. p. 560. [Google Scholar]
- Anderson CJR, Roth JD, Waterman JM. Can whisker spot patterns be used to identify individual polar bears? J Zool. 2007;273:333–339. doi: 10.1111/j.1469-7998.2007.00340.x. [DOI] [Google Scholar]
- Athreya V, Odden M, Linnell JD, Krishnaswamy J, Karanth U. Big cats in our backyards: persistence of large carnivores in a human dominated landscape in India. PLoS ONE. 2013;8:e57872. doi: 10.1371/journal.pone.0057872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balme GA, Slotow R, Hunter LTB. Edge effects and the impact of non-protected areas in carnivore conservation: leopards in the Phinda-Mkhuze Complex, South Africa. Anim Conserv. 2010;13:315–323. doi: 10.1111/j.1469-1795.2009.00342.x. [DOI] [Google Scholar]
- Beasley JC, Olson ZH, DeVault TL. Ecological role of vertebrate scavengers. In: Benbow ME, Tomberlin JK, Tarone AM, editors. Carrion ecology, evolution and their applications. Boca Raton: CRC Press; 2015. pp. 107–127. [Google Scholar]
- Belton LE, Cameron EZ, Dalerum F. Spotted hyaena visitation at anthropogenic sites in the Kruger National Park, South Africa. Afr Zool. 2018;53:113–118. doi: 10.1080/15627020.2018.1518728. [DOI] [Google Scholar]
- Benhaiem S, Marescot L, East ML, Kramer-Schadt S, Gimenez O, Lebreton JD, Hofer H. Slow recovery from a disease epidemic in the spotted hyena, a keystone social carnivore. Commun Biol. 2018;1:1–12. doi: 10.1038/s42003-018-0197-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson-Amram S, Heinen VK, Dryer SL, Holekamp KE. Numerical assessment and individual call discrimination by wild spotted hyaenas, Crocuta crocuta. Anim Behav. 2011;82:743–752. doi: 10.1016/j.anbehav.2011.07.004. [DOI] [Google Scholar]
- Bhandari S, Morley C, Aryal A, Shrestha UB. The diet of the striped hyena in Nepal's lowland regions. Ecol Evol. 2020;10:7953–7962. doi: 10.1002/ece3.6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohm T, Höner OR (2015) Crocuta crocuta. The IUCN Red List of Threatened Species. 10.2305/IUCN.UK.2015-2.RLTS.T5674A45194782.en. Accessed 10 Dec 2020
- Bohm T (2015) Population ecology, conservation status and genetics of the spotted hyena (Crocuta crocuta) in the Odzala-Kokoua National Park, Republic of Congo, including an assessment of the status of spotted hyenas in southeast Gabon. Dissertation, Free University of Berlin
- Bolger DT, Morrison TA, Vance B, Lee D, Farid H. A computer-assisted system for photographic mark–recapture analysis. Methods Ecol Evol. 2012;3:813–822. doi: 10.1111/j.2041-210X.2012.00212.x. [DOI] [Google Scholar]
- Bothma JP, Walker C. The striped hyaena. In: Bothma JP, Walker C, editors. Larger carnivores of the African savannas. Berlin, New York: Springer; 1999. pp. 201–211. [Google Scholar]
- Boydston EE, Kapheim KM, Szykman M, Holekamp KE. Individual variation in space use by female spotted hyenas. J Mammal. 2003;84:1006–1018. doi: 10.1644/BOS-038. [DOI] [Google Scholar]
- Boydston EE, Kapheim KM, Van Horn RC, Smale L, Holekamp KE. Sexually dimorphic patterns of space use throughout ontogeny in the spotted hyena (Crocuta crocuta) J Zool. 2005;267:271–281. doi: 10.1017/S0952836905007478. [DOI] [Google Scholar]
- Braczkowski A, Schenk R, Samarasinghe D, Biggs D, Richardson A, Swanson M, Dheer A, Fattebert J. Leopard and spotted hyena densities in the Lake Mburo National Park, southwestern Uganda. PeerJ. 2022;10:e12307. doi: 10.7717/peerj.12307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Califf KJ, Green DS, Wagner AP, Scribner KT, Beatty K, Wagner ME, Holekamp KE. Genetic relatedness and space use in two populations of striped hyenas (Hyaena hyaena) J Mammal. 2020;101:361–372. doi: 10.1093/jmammal/gyz165. [DOI] [Google Scholar]
- Choo YR, Kudavidanage EP, Amarasinghe TR, Nimalrathna T, Chua MA, Webb EL. Best practices for reporting individual identification using camera trap photographs. Glob Ecol Conserv. 2020;24:e01294. doi: 10.1016/j.gecco.2020.e01294. [DOI] [Google Scholar]
- Chutipong W, Lynam AJ, Steinmetz R, Savini T, Gale GA. Sampling mammalian carnivores in western Thailand: issues of rarity and detectability. Raffles Bull Zool. 2014;62:521–535. [Google Scholar]
- Clutton-Brock T, Sheldon BC. Individuals and populations: the role of long-term, individual-based studies of animals in ecology and evolutionary biology. Trends Ecol Evol. 2010;25:562–573. doi: 10.1016/j.tree.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Cozzi G, Broekhuis F, McNutt JW, Schmid B. Density and habitat use of lions and spotted hyenas in northern Botswana and the influence of survey and ecological variables on call-in survey estimation. Biodivers Conserv. 2013;22:2937–2956. doi: 10.1007/s10531-013-0564-7. [DOI] [Google Scholar]
- Crall JP, Stewart CV, Berger-Wolf TY, Rubenstein DI, Sundaresan SR (2013) HotSpotter-Patterned species instance recognition. In: 2013 IEEE Workshop Applications of Computer Vision WACV 2013, Proceedings of IEEE Workshop on Applications of Computer Vision, pp 230–237. 10.1109/WACV.2013.6475023
- Cunha GR, Wang Y, Place NJ, Liu W, Baskin L, Glickman SE. Urogenital system of the spotted hyena (Crocuta crocuta Erxleben): a functional histological study. J Morphol. 2003;256:205–218. doi: 10.1002/jmor.10085. [DOI] [PubMed] [Google Scholar]
- Davidian E, Courtiol A, Wachter B, Hofer H, Höner OP. Why do some males choose to breed at home when most other males disperse? Sci Adv. 2016;2:e1501236. doi: 10.1126/sciadv.1501236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Blocq AD (2014) Estimating spotted hyaena (Crocuta crocuta) population density using camera trap data in a spatially-explicit capture-recapture framework. Bachelor’s thesis, University of Cape Town
- Delibes M, Gaona P, Ferreras P. Effects of an attractive sink leading into maladaptive habitat selection. Am Nat. 2001;158:277–285. doi: 10.1086/321319. [DOI] [PubMed] [Google Scholar]
- Dheer A, Samarasinghe D, Dloniak SM, Braczkowski A. Using camera traps to study hyenas: challenges, opportunities, and outlook. Mammal Biol (special Issue) 2022 doi: 10.1007/s42991-021-00188-1. [DOI] [Google Scholar]
- Dloniak SM, French JA, Holekamp KE. Rank-related maternal effects of androgens on behaviour in wild spotted hyaenas. Nature. 2006;440:1190–1193. doi: 10.1038/nature04540. [DOI] [PubMed] [Google Scholar]
- Dolrenry S, Stenglein J, Hazzah L, Lutz RS, Frank L. A metapopulation approach to African lion (Panthera leo) conservation. PLoS ONE. 2014;9:e88081. doi: 10.1371/journal.pone.0088081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan TM, Thompson FR., III Modeling the ecological trap hypothesis: a habitat and demographic analysis for migrant songbirds. Ecol Appl. 2001;11(3):871–882. doi: 10.1890/1051-0761(2001)011[0871:MTETHA]2.0.CO;2. [DOI] [Google Scholar]
- Drea CM, Coscia EM, Glickman SE. Hyenas. In: Knobil E, Neill J, Licht P, editors. Encyclopaedia of reproduction. San Diego: Academic Press; 1999. pp. 718–725. [Google Scholar]
- East ML, Hofer H. Loud calling in a female-dominated mammalian society: I. Structure and composition of whooping bouts of spotted hyaenas, Crocuta crocuta. Anim Behav. 1991;42:637–649. doi: 10.1016/S0003-3472(05)80246-5. [DOI] [Google Scholar]
- East ML, Hofer H. Loud calling in a female-dominated mammalian society: II. Behavioural contexts and functions of whooping of spotted hyaenas, Crocuta crocuta. Anim Behav. 1991;42:651–669. doi: 10.1016/S0003-3472(05)80247-7. [DOI] [Google Scholar]
- East ML, Hofer H, Cox JH, Wulle U, Wiik H, Pitra C. Regular exposure to rabies virus and lack of symptomatic disease in Serengeti spotted hyenas. Proc Natl Acad Sci USA. 2001;98:15026–15031. doi: 10.1073/pnas.261411898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- East ML, Burke T, Wilhelm K, Greig C, Hofer H. Sexual conflicts in spotted hyenas: male and female mating tactics and their reproductive outcome with respect to age, social status and tenure. Proc R Soc B. 2003;270:1247–1254. doi: 10.1098/rspb.2003.2363f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- East ML, Moestl K, Benetka V, Pitra C, Höner OP, Wachter B, Hofer H. Coronavirus infection of spotted hyenas in the Serengeti ecosystem. Vet Microbiol. 2004;102:1–9. doi: 10.1016/j.vetmic.2004.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S, Cooper S, Uiseb K, Hayward M, Wachter B, Melzheimer J. Making the most of by-catch data: assessing the feasibility of utilising non-target camera trap data for occupancy modelling of a large felid. Afr J Ecol. 2018;56:885–894. doi: 10.1111/aje.12511. [DOI] [Google Scholar]
- Edwards S, Noack J, Heyns L, Rodenwoldt D. Evidence of a high-density brown hyena population within an enclosed reserve: the role of fenced systems in conservation. Mammal Res. 2019;64:519–527. doi: 10.1007/s13364-019-00432-7. [DOI] [Google Scholar]
- Edwards S, Noack J, Heyns L, Rodenwoldt D, Tarjan LM. Socioecology of a high-density brown hyaena population within an enclosed reserve. Mammal Res. 2020;65:223–233. doi: 10.1007/s13364-020-00477-z. [DOI] [Google Scholar]
- Elliot NB, Gopalaswamy AM. Toward accurate and precise estimates of lion density. Conserv Biol. 2017;31:934–943. doi: 10.1111/cobi.12878. [DOI] [PubMed] [Google Scholar]
- Engh AL, Esch K, Smale L, Holekamp KE. Mechanisms of maternal rank ‘inheritance’ in the spotted hyaena, Crocuta crocuta. Anim Behav. 2000;60:323–332. doi: 10.1006/anbe.2000.1502. [DOI] [PubMed] [Google Scholar]
- Engh AL, Funk SM, Van Horn RC, Scribner KT, Bruford MW, Libants S, Szykman M, Smale L, Holekamp KE. Reproductive skew among males in a female-dominated society. Behav Ecol. 2002;13:193–200. doi: 10.1093/beheco/13.2.193. [DOI] [Google Scholar]
- Frank LG. Social organization of the spotted hyaena Crocuta crocuta. II. Dominance and reproduction. Anim Behav. 1986;34:1510–1527. doi: 10.1016/S0003-3472(86)80221-4. [DOI] [Google Scholar]
- Frank LG, Glickman SE, Powch I. Sexual dimorphism in the spotted hyaena (Crocuta crocuta) J Zool. 1990;221:308–313. doi: 10.1111/j.1469-7998.1990.tb04001.x. [DOI] [Google Scholar]
- Frank LG, Glickman SE, Licht P. Fatal sibling aggression, precocial development, and androgens in neonatal spotted hyaenas. Science. 1991;252:702–704. doi: 10.1126/science.2024122. [DOI] [PubMed] [Google Scholar]
- Frank LG (1983) Reproduction and intra-sexual dominance in the spotted hyena (Crocuta crocuta). Dissertation, University of California, Berkeley
- Gates JE, Gysel LW. Avian nest dispersion and fledging success in field-forest ecotones. Ecology. 1978;59:871–883. doi: 10.2307/1938540. [DOI] [Google Scholar]
- Gering E, Laubach Z, Weber P, Hussey G, Turner J, Lehmann K, Montgomery T, Holekamp K, Getty T. Time makes you older, parasites make you bolder—Toxoplasma gondii infections predict hyena boldness toward definitive lion hosts. In: Banzhaf W, Cheng BHC, Deb K, Holekamp KE, Lenski RE, Ofria C, Pennock RT, Punch WF, Whittaker DJ, editors. Evolution in action: past, present and future. Genetic and evolutionary computation. Cham: Springer; 2020. pp. 205–224. [Google Scholar]
- Gersick AS, Cheney DL, Schneider JM, Seyfarth RM, Holekamp KE. Long-distance communication facilitates cooperation among wild spotted hyaenas, Crocuta crocuta. Anim Behav. 2015;103:107–116. doi: 10.1016/j.anbehav.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DS, Johnson-Ulrich L, Couraud HE, Holekamp KE. Anthropogenic disturbance induces opposing population trends in spotted hyenas and African lions. Biodivers Conserv. 2018;27:871–889. doi: 10.1007/s10531-017-1469-7. [DOI] [Google Scholar]
- Green DS, Farr MT, Holekamp KE, Strauss ED, Zipkin EF. Can hyena behaviour provide information on population trends of sympatric carnivores? Philos Trans R Soc B. 2019;374:20180052. doi: 10.1098/rstb.2018.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green AM, Chynoweth MW, Şekercioğlu ÇH. Spatially explicit capture-recapture through camera trapping: a review of benchmark analyses for wildlife density estimation. Front Ecol Evol. 2020;8:563477. doi: 10.3389/fevo.2020.563477. [DOI] [Google Scholar]
- Green DS (2015) Proteles cristata. The IUCN Red List of Threatened Species. 10.2305/IUCN.UK.2015-2.RLTS.T18372A45195681.en. Accessed 10 Dec 2020
- Greenberg JR, Holekamp KE. Human disturbance affects personality development in a wild carnivore. Anim Behav. 2017;132:303–312. doi: 10.1016/j.anbehav.2017.08.023. [DOI] [Google Scholar]
- Gupta S, Mondal K, Sankar K, Qureshi Q. Estimation of striped hyena Hyaena hyaena population using camera traps in Sariska tiger reserve, Rajasthan, India. J Bombay Nat Hist Soc. 2009;106:284–288. [Google Scholar]
- Hanski I, Simberloff D. The metapopulation approach, its history, conceptual domain, and application to conservation. In: Hanski I, Gilpin ME, McCauley DE, editors. Metapopulation biology. San Diego: Academic Press; 1997. pp. 5–26. [Google Scholar]
- Harihar A, Ghosh M, Fernandes M, Pandav B, Goyal SP. Use of photographic capture-recapture sampling to estimate density of Striped Hyena (Hyaena hyaena): implications for conservation. Mammalia. 2010;74:83–87. doi: 10.1515/mamm.2009.072. [DOI] [Google Scholar]
- Heilbrun RD, Silvy NJ, Tewes ME, Peterson MJ. Using automatically triggered cameras to individually identify bobcats. Wildlife Soc B. 2003;31:748–755. [Google Scholar]
- Henschel P, Ray J. Leopards in African rainforests: survey and monitoring techniques. Bronx: Wildlife Conservation Society; 2003. [Google Scholar]
- Henschel JR, Skinner JD. The diet of the spotted hyaenas Crocuta crocuta in Kruger National Park. Afr J Ecol. 1990;28:69–82. doi: 10.1111/j.1365-2028.1990.tb01138.x. [DOI] [Google Scholar]
- Henschel P, Malanda GA, Hunter L. The status of savanna carnivores in the Odzala-Kokoua National Park, northern Republic of Congo. J Mammal. 2014;95:882–892. doi: 10.1644/13-MAMM-A-306. [DOI] [Google Scholar]
- Hiby L, Lovell P, Patil N, Kumar NS, Gopalaswamy AM, Karanth KU. A tiger cannot change its stripes: using a three-dimensional model to match images of living tigers and tiger skins. Biol Lett. 2009;5:383–386. doi: 10.1098/rsbl.2009.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer H, East ML. The commuting system of Serengeti spotted hyaenas: how a predator copes with migratory prey: I. Social organization. Anim Behav. 1993;46:547–557. doi: 10.1006/anbe.1993.1222. [DOI] [Google Scholar]
- Hoffmann CF, Pilfold NW, Ruppert K, Letoluai A, Lenguya L, Limo I, Montgomery RA. The integral nature of encounter rate in predicting carnivore depredation of livestock risk. Glob Ecol Conserv. 2022 doi: 10.3389/fcosc.2022.808043. [DOI] [Google Scholar]
- Holekamp KE. Questioning the social intelligence hypothesis. Trends Cogn Sci. 2007;11:65–69. doi: 10.1016/j.tics.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Holekamp KE, Benson-Amram S. The evolution of intelligence in mammalian carnivores. Interface Focus. 2017;7:20160108. doi: 10.1098/rsfs.2016.0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holekamp KE, Dloniak SM. Intra-specific variation in the behavioral ecology of a tropical carnivore, the spotted hyena. Adv Stud Behav. 2010;42:189–229. doi: 10.1016/S0065-3454(10)42006-9. [DOI] [Google Scholar]
- Holekamp KE, Kolowski JM. Family Hyaenidae (Hyenas) In: Wilson DE, Mittermeier RA, editors. Handbook of the mammals of the world 1 carnivores. Barcelona: Lynx Edicions; 2009. pp. 234–261. [Google Scholar]
- Holekamp KE, Smale L. Dominance acquisition during mammalian social development: the "inheritance" of maternal rank. Am Zool. 1991;31:306–317. doi: 10.1093/icb/31.2.306. [DOI] [Google Scholar]
- Holekamp KE, Smale L. Rapid change in offspring sex ratios after clan fission in the spotted hyena. Am Nat. 1995;145:261–278. doi: 10.1086/285739. [DOI] [Google Scholar]
- Holekamp KE, Strauss ED. Reproduction within a hierarchical society from a female’s perspective. Integr Comp Biol. 2020;60:753–764. doi: 10.1093/icb/icaa068. [DOI] [PubMed] [Google Scholar]
- Holekamp KE, Smale L, Szykman M. Rank and reproduction in the female spotted hyaena. Reproduction. 1996;108:229–237. doi: 10.1530/jrf.0.1080229. [DOI] [PubMed] [Google Scholar]
- Holekamp KE, Sakai ST, Lundrigan BL. The spotted hyena (Crocuta crocuta) as a model system for study of the evolution of intelligence. J Mammal. 2007;88:545–554. doi: 10.1644/06-MAMM-S-361R1.1. [DOI] [Google Scholar]
- Holekamp KE, Smith JE, Strelioff CC, Van Horn RC, Watts HE. Society, demography and genetic structure in the spotted hyena. Mol Ecol. 2012;21:613–632. doi: 10.1111/j.1365-294X.2011.05240.x. [DOI] [PubMed] [Google Scholar]
- Höner OP, Wachter B, East ML, Runyoro VA, Hofer H. The effect of prey abundance and foraging tactics on the population dynamics of a social, territorial carnivore, the spotted hyena. Oikos. 2005;108:544–554. doi: 10.1111/j.0030-1299.2005.13533.x. [DOI] [Google Scholar]
- Höner OP, Wachter B, Speck S, Wibbelt G, Ludwig A, Fyumagwa RD, Wohlsein P, Lieckfeldt D, Hifer H, East ML. Severe Streptococcus infection in spotted hyenas in the Ngorongoro Crater, Tanzania. Vet Microbiol. 2006;115:223–228. doi: 10.1016/j.vetmic.2005.12.018. [DOI] [PubMed] [Google Scholar]
- Höner OP, Wachter B, East ML, Streich WJ, Wilhelm K, Burke T, Hofer H. Female mate-choice drives the evolution of male-biased dispersal in a social mammal. Nature. 2007;448:798–801. doi: 10.1038/nature06040. [DOI] [PubMed] [Google Scholar]
- Höner OP, Wachter B, Goller KV, Hofer H, Runyoro V, Thierer D, Fyumagwa RD, Müller T, East ML. The impact of a pathogenic bacterium on a social carnivore population. J Anim Ecol. 2012;81:36–46. doi: 10.1111/j.1365-2656.2011.01873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe RW, Davis GJ, Mosca V. The demographic significance of ‘sink’populations. Biol Conserv. 1991;57:239–255. doi: 10.1016/0006-3207(91)90071-G. [DOI] [Google Scholar]
- Jhala YV. Striped hyena (Hyaena hyaena) In: Johnsingh AJT, Manjrekar N, editors. Mammals of South Asia. Hyderabad: Universities Press; 2013. pp. 522–530. [Google Scholar]
- Johansson Ö, Samelius G, Wikberg E, Chapron G, Mishra C, Low M. Identification errors in camera-trap studies result in systematic population overestimation. Sci Rep. 2020;10:1–10. doi: 10.1038/s41598-020-63367-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson-Ulrich L. The social intelligence hypothesis. In: Shackelford T, Weekes-Shackelford V, editors. Encyclopedia of evolutionary psychological science. Cham: Springer; 2017. pp. 1–7. [Google Scholar]
- Johnson-Ulrich L, Lehman KDS, Turner JW, Holekamp KE. Hyenas – Testing cognition in the umwelt of the spotted hyena. In: Bueno-Guerra N, Amici F, editors. Field and laboratory methods in animal cognition: a comparative guide. Cambridge: Cambridge University Press; 2018. pp. 244–265. [Google Scholar]
- Johnson-Ulrich L, Holekamp KE, Hambrick DZ. Innovative problem-solving in wild hyenas is reliable across time and contexts. Sci Rep-UK. 2020;10:1–12. doi: 10.1038/s41598-020-69953-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karanth KU. Estimating tiger Panthera tigris populations from camera-trap data using capture—recapture models. Biol Conserv. 1995;71:333–338. doi: 10.1016/0006-3207(94)00057-W. [DOI] [Google Scholar]
- Karanth KU, Nichols JD. Estimation of tiger densities in India using photographic captures and recaptures. Ecology. 1998;79:2852–2862. doi: 10.1890/0012-9658(1998)079[2852:EOTDII]2.0.CO;2. [DOI] [Google Scholar]
- Karczmarski L, Chan SCY, Rubenstein DI, Chui SYS, Cameron EZ (2022a) Individual identification and photographic techniques in mammalian ecological and behavioural research — Part 1: methods and concepts. Mamm Biol (Special Issue) 102(3). https://link.springer.com/journal/42991/volumes-and-issues/102-3
- Karczmarski L, Chan SCY, Chui SYS, Cameron EZ (2022b) Individual identification and photographic techniques in mammalian ecological and behavioural research — Part 2: Field studies and applications. Mamm Biol (Special Issue) 102(4). https://link.springer.com/journal/42991/volumes-and-issues/102-4
- Kat PW, Alexander KA, Smith JS, Munson L. Rabies and African wild dogs in Kenya. Proc R Soc B. 1995;262:229–233. doi: 10.1098/rspb.1995.0200. [DOI] [PubMed] [Google Scholar]
- Kelly MJ. Computer-aided photograph matching in studies using individual identification: an example from Serengeti cheetahs. J Mammal. 2001;82:440–449. doi: 10.1644/1545-1542(2001)082<0440:CAPMIS>2.0.CO;2. [DOI] [Google Scholar]
- Kelly MJ, Noss AJ, Di Bitetti MS, Maffei L, Arispe RL, Paviolo A, De Angelo CD, Di Blanco YE. Estimating puma densities from camera trapping across three study sites: Bolivia, Argentina, and Belize. J Mammal. 2008;89:408–418. doi: 10.1644/06-MAMM-A-424R.1. [DOI] [Google Scholar]
- Kelly MJ, Betsch J, Wultsch C, Mesa B, Mills LS. Noninvasive sampling for carnivores. In: Boitani L, Powell RA, editors. Carnivore ecology and conservation: a handbook of techniques. Oxford: Oxford University Press; 2012. pp. 47–69. [Google Scholar]
- Kelt DA, Heske EJ, Lambin X, Oli MK, Orrock JL, Ozgul A, Pauli JN, Prugh LR, Sollmann R, Sommer S. Advances in population ecology and species interactions in mammals. J Mammal. 2019;100:965–1007. doi: 10.1093/jmammal/gyz017. [DOI] [Google Scholar]
- Kent VT, Hill RA. The importance of farmland for the conservation of the brown hyaena Parahyaena brunnea. Oryx. 2013;47:431–440. doi: 10.1017/S0030605312001007. [DOI] [Google Scholar]
- Kissui BM. Livestock predation by lions, leopards, spotted hyenas, and their vulnerability to retaliatory killing in the Maasai steppe, Tanzania. Anim Conserv. 2008;11:422–432. doi: 10.1111/j.1469-1795.2008.00199.x. [DOI] [Google Scholar]
- Kissui BM, Kiffner C, König HJ, Montgomery RA. Patterns of livestock depredation and cost-effectiveness of fortified livestock enclosures in northern Tanzania. Ecol Evol. 2019;9:11420–11433. doi: 10.1002/ece3.5644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kock R, Chalmers WSK, Mwanzia J, Chillingworth C, Wambua J, Coleman PG, Baxendale W. Canine distemper antibodies in lions of the Masai Mara. Vet Rec. 1998;142:662–665. doi: 10.1136/vr.142.24.662. [DOI] [PubMed] [Google Scholar]
- Koehler CE, Richardson PRK. Proteles cristatus. Mamm Species. 1990 doi: 10.2307/3504197. [DOI] [Google Scholar]
- Kolowski JM, Holekamp KE. Effects of an open refuse pit on space use patterns of spotted hyenas. Afr J Ecol. 2008;46:341–349. doi: 10.1111/j.1365-2028.2007.00846.x. [DOI] [Google Scholar]
- Kristan WB., III The role of habitat selection behavior in population dynamics: source–sink systems and ecological traps. Oikos. 2003;103:457–468. doi: 10.1034/j.1600-0706.2003.12192.x. [DOI] [Google Scholar]
- Kruuk H. The spotted hyena: a study of predation and social behavior. Chicago: University of Chicago Press; 1972. [Google Scholar]
- Kruuk H. Feeding and social behaviour of the striped hyaena (Hyaena vulgaris Desmarest) Afr J Ecol. 1976;14:91–111. doi: 10.1111/j.1365-2028.1976.tb00155.x. [DOI] [Google Scholar]
- Kruuk H, Sands WA. The aardwolf (Proteles cristatus Sparrman) 1783 as predator of termites. Afr J Ecol. 1972;10:211–227. doi: 10.1111/j.1365-2028.1972.tb00728.x. [DOI] [Google Scholar]
- Lamb CT, Mowat G, McLellan BN, Nielsen SE, Boutin S. Forbidden fruit: human settlement and abundant fruit create an ecological trap for an apex omnivore. J Anim Ecol. 2017;86:55–65. doi: 10.1111/1365-2656.12589. [DOI] [PubMed] [Google Scholar]
- Larson RN, Brown JL, Karels T, Riley SPD. Effects of urbanization on resource use and individual specialization in coyotes (Canis latrans) in southern California. PLoS ONE. 2020;15:e0228881. doi: 10.1371/journal.pone.0228881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann KDS, Jensen FH, Gersick AS, Strandburg-Peshkin A, Holekamp KE. Long-distance vocalizations of spotted hyenas contain individual, but not group, signatures. Proc R Soc B. 2022 doi: 10.1098/rspb.2022.0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann KD (2020) Communication and cooperation in silico and nature. Dissertation, Michigan State University
- Linhart P, Mahamoud-Issa M, Stowell D, Blumstein DT. The potential for acoustic individual identification in mammals. Mamm Biol. 2022;102:1–17. doi: 10.1007/s42991-021-00222-2. [DOI] [Google Scholar]
- Linnell JDC, Odden J, Smith ME, Aanes R, Swenson JE. Large carnivores that kill livestock: do" problem individuals" really exist? Wildlife Soc B. 1999;27:698–705. [Google Scholar]
- Macdonald DW. Cause of wild dog deaths. Nature. 1992;360:633–634. doi: 10.1038/360633b0. [DOI] [PubMed] [Google Scholar]
- Mandal DK (2018) Ecology of striped hyena in Sariska Tiger reserve Rajasthan. Dissertation, Saurashtra University
- McCormick SK, Holekamp KE, Smale L, Weldele ML, Glickman SE, Place NJ. Sex differences in spotted hyenas. Cold Spring Harb Perspect Biol. 2021 doi: 10.1101/cshperspect.a039180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough DR. Metapopulations and wildlife conservation. Washington: Island Press; 1996. [Google Scholar]
- Mendoza E, Martineau PR, Brenner E, Dirzo R. A novel method to improve individual animal identification based on camera-trapping data. J Wildlife Manag. 2011;75:973–979. doi: 10.1002/jwmg.120. [DOI] [Google Scholar]
- Merrick MJ, Koprowski JL. Should we consider individual behavior differences in applied wildlife conservation studies? Biol Conserv. 2017;209:34–44. doi: 10.1016/j.biocon.2017.01.021. [DOI] [Google Scholar]
- Mills MGL. The mating system of the brown hyaena, Hyaena brunnea in the southern Kalahari. Behav Ecol Sociobiol. 1982;10:131–136. doi: 10.1007/BF00300173. [DOI] [Google Scholar]
- Mills MGL. Factors affecting group size and territory size of the brown hyaena, Hyaena brunnea in the southern Kalahari. J Zool. 1982;198:39–51. doi: 10.1111/j.1469-7998.1982.tb02059.x. [DOI] [Google Scholar]
- Mills MGL. Behavioural mechanisms in territory and group maintenance of the brown hyaena, Hyaena brunnea, in the southern Kalahari. Anim Behav. 1983;31:503–510. doi: 10.1016/S0003-3472(83)80072-4. [DOI] [Google Scholar]
- Mills MGL. Mating and denning behaviour of the brown hyaena Hyaena brunnea and comparisons with other Hyaenidae. Z Tierpsychol. 1983;63:331–342. doi: 10.1111/j.1439-0310.1983.tb00748.x. [DOI] [Google Scholar]
- Mills MGL. Kalahari hyaenas. London: Unwin Hyman; 1990. [Google Scholar]
- Mills G, Hofer H. Hyaenas: status survey and conservation action plan. Gland: IUCN SSC Hyaena Specialist Group; 1998. [Google Scholar]
- Montgomery TM, Greenberg JR, Gunson JL, John K, Laubach ZM, Nonnamaker E, Person ES, Rogers H, Ronis E, Smale L, Steinfield K, Strong R, Holekamp KE, Beehner JC. Measuring salivary cortisol in wild carnivores. Horm Behav. 2022;137:105082. doi: 10.1016/j.yhbeh.2021.105082. [DOI] [PubMed] [Google Scholar]
- Newmark WD. Isolation of African protected areas. Front Ecol Environ. 2008;6:321–328. doi: 10.1890/070003. [DOI] [Google Scholar]
- O’Brien TG, Kinnaird MF. Density estimation of sympatric carnivores using spatially explicit capture–recapture methods and standard trapping grid. Ecol Appl. 2011;21:2908–2916. doi: 10.1890/10-2284.1. [DOI] [Google Scholar]
- Osterrieder SK, Kent CS, Anderson CJ, Parnum IM, Robinson RW. Whisker spot patterns: a noninvasive method of individual identification of Australian sea lions (Neophoca cinerea) J Mammal. 2015;96:988–997. doi: 10.1093/jmammal/gyv102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangle WM, Holekamp KE. Lethal and nonlethal anthropogenic effects on spotted hyenas in the Masai Mara National Reserve. J Mammal. 2010;91:154–164. doi: 10.1644/08-MAMM-A-359R.1. [DOI] [Google Scholar]
- Pournelle GH. Observations on birth and early development of the spotted hyena. J Mammal. 1965;46:503. doi: 10.2307/1377649. [DOI] [Google Scholar]
- Powell RA, Proulx G. Trapping and marking terrestrial mammals for research: integrating ethics, performance criteria, techniques, and common sense. ILAR J. 2003;44:259–276. doi: 10.1093/ilar.44.4.259. [DOI] [PubMed] [Google Scholar]
- Pulliam HR. Sources, sinks, and population regulation. Am Nat. 1988;132(5):652–661. doi: 10.1086/284880. [DOI] [Google Scholar]
- Rich LN, Miller DAW, Muñoz DJ, Robinson HS, McNutt JW, Kelly MJ. Sampling design and analytical advances allow for simultaneous density estimation of seven sympatric carnivore species from camera trap data. Biol Conserv. 2019;233:12–20. doi: 10.1016/j.biocon.2019.02.018. [DOI] [Google Scholar]
- Richardson PRK. Food consumption and seasonal variation in the diet of the aardwolf Proteles cristatus in southern Africa. Z Säugetierkd. 1987;52:307–325. [Google Scholar]
- Richardson PRK. Aardwolf mating system: overt cuckoldry in an apparently monogamous mammal. S Afr J Sci. 1987;83:405–410. [Google Scholar]
- Richardson PRK. Scent marking and territoriality in the aardwolf. Chem Signal. 1990;5:379–387. [Google Scholar]
- Richardson PRK. Territorial significance of scent marking during the non-mating season in the aardwolf Proteles cristatus (Carnivora: Protelidae) Ethology. 1991;87:9–27. doi: 10.1111/j.1439-0310.1991.tb01184.x. [DOI] [Google Scholar]
- Rieger I. A review of the biology of striped hyaenas, Hyaena hyaena (Linné, 1758) Saüge Mitt. 1979;27:81–95. [Google Scholar]
- Roelke-Parker ME, Munson L, Packer C, Kock R, Cleaveland S, Carpenter M, O’Brien SJ, Pospischil A, Hofmann-Lehmann R, Lutz H, Mwamengele GLM, Mgasa MN, Machange GA, Summers BA, Appel MJG. A canine distemper virus epidemic in Serengeti lions (Panthera leo) Nature. 1996;379:441–445. doi: 10.1038/379441a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas CA, Holekamp KE, Winters AD, Theis KR. Body site-specific microbiota reflect sex and age-class among wild spotted hyenas. FEMS Microbiol Ecol. 2020 doi: 10.1093/femsec/fiaa007. [DOI] [PubMed] [Google Scholar]
- Sarmento P, Cruz J, Eira C, Fonseca C. Evaluation of camera trapping for estimating red fox abundance. J Wildlife Manage. 2009;73:1207–1212. doi: 10.2193/2008-288. [DOI] [Google Scholar]
- Schneider S, Taylor GW, Linquist S, Kremer SC. Past, present and future approaches using computer vision for animal re-identification from camera trap data. Methods Ecol Evol. 2019;10:461–470. doi: 10.1111/2041-210X.13133. [DOI] [Google Scholar]
- Schuette P, Wagner AP, Wagner ME, Creel S. Occupancy patterns and niche partitioning within a diverse carnivore community exposed to anthropogenic pressures. Biol Conserv. 2013;158:301–312. doi: 10.1016/j.biocon.2012.08.008. [DOI] [Google Scholar]
- Silwa A (1996) A functional analysis of scent marking and mating behaviour in the aardwolf, Proteles cristatus (Sparrman, 1783). Dissertation, University of Pretoria
- Singh P, Gopalaswamy AM, Karanth KU. Factors influencing densities of striped hyenas (Hyaena hyaena) in arid regions of India. J Mammal. 2010;91:1152–1159. doi: 10.1644/09-MAMM-A-159.1. [DOI] [Google Scholar]
- Singh R, Qureshi Q, Sankar K, Krausman PR, Goyal SP, Nicholson KL. Population density of striped hyenas in relation to habitat in a semi-arid landscape, western India. Acta Theriol. 2014;59:521–527. doi: 10.1007/s13364-014-0187-8. [DOI] [Google Scholar]
- Singh P (2008) Population density and feeding ecology of the striped hyena (Hyaena hyaena) in relation to land use patterns in an arid region of Rajasthan. Dissertation, MS thesis, The Manipal University, Bangalore, India
- Smale L, Frank LG, Holekamp KE. Ontogeny of dominance in free-living spotted hyaenas: juvenile rank relations with adult females and immigrant males. Anim Behav. 1993;46:467–477. doi: 10.1006/anbe.1993.1215. [DOI] [Google Scholar]
- Smale L, Nunes S, Holekamp KE. Sexually dimorphic dispersal in mammals: patterns, causes, and consequences. Adv Stud Behav. 1997;26:181–250. doi: 10.1016/S0065-3454(08)60380-0. [DOI] [Google Scholar]
- Smith JE, Lehmann KDS, Montgomery TM, Strauss ED, Holekamp KE. Insights from long-term field studies of mammalian carnivores. J Mammal. 2017;98:631–641. doi: 10.1093/jmammal/gyw194. [DOI] [Google Scholar]
- Smithers RHN. The mammals of the southern African subregion. Pretoria: University of Pretoria Press; 1983. [Google Scholar]
- Spagnuolo O (2016) Pasting behaviour suggests cryptic sociality in the striped hyaena, Hyaena hyaena. MS Thesis, University of Michigan
- Stratford K, Stratford S, Périquet S. Dyadic associations reveal clan size and social network structure in the fission–fusion society of spotted hyaenas. Afr J Ecol. 2020;58:182–192. doi: 10.1111/aje.12641. [DOI] [Google Scholar]
- Strauss ED, Holekamp KE. Inferring longitudinal hierarchies: framework and methods for studying the dynamics of dominance. J Anim Ecol. 2019;88:521–536. doi: 10.1111/1365-2656.12951. [DOI] [PubMed] [Google Scholar]
- Strauss ED, Holekamp KE. Social alliances improve rank and fitness in convention-based societies. PNAS. 2019;116:8919–8924. doi: 10.1073/pnas.1810384116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss ED, Shizuka D, Holekamp KE. Juvenile rank acquisition is associated with fitness independent of adult rank. P R Soc B. 2020;287:20192969. doi: 10.1098/rspb.2019.2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson EM, McElhinny TL, Dworkin I, Weldele ML, Glickman SE, Holekamp KE. Ontogeny of sexual size dimorphism in the spotted hyena (Crocuta crocuta) J Mammal. 2013;94:1298–1310. doi: 10.1644/12-MAMM-A-277.1. [DOI] [Google Scholar]
- Szykman M, Engh AL, Van Horn RC, Funk SM, Scribner KT, Holekamp KE. Association patterns among male and female spotted hyenas (Crocuta crocuta) reflect male mate choice. Behav Ecol Sociobiol. 2001;50:231–238. doi: 10.1007/s002650100356. [DOI] [Google Scholar]
- Theis KR, Greene KM, Benson-Amram SR, Holekamp KE. Sources of variation in the long-distance vocalizations of spotted hyenas. Behaviour. 2007;144:557–584. doi: 10.1163/156853907780713046. [DOI] [Google Scholar]
- Theis KR, Venkataraman A, Dycus JA, Koonter KD, Schmitt-Matzen EN, Wagner AP, Holekamp KE, Schmidt TM. Symbiotic bacteria appear to mediate hyena social odors. Proc Natl Acad Sci U S A. 2013;110:19832–19837. doi: 10.1073/pnas.1306477110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorn M, Scott DM, Green M, Bateman PW, Cameron EZ. Estimating brown hyaena occupancy using baited camera traps. Afr J Wildl Res. 2009;39:1–10. doi: 10.3957/056.039.0101. [DOI] [Google Scholar]
- Tichon J, Gilchrist JS, Rotem G, Ward P, Spiegel O. Social interactions in striped hyena inferred from camera trap data: is it more social than previously thought? Curr Zool. 2020;66:345–353. doi: 10.1093/cz/zoaa003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treves A, Mwima P, Plumptre AJ, Isoke S. Camera-trapping forest-woodland wildlife of western Uganda reveals how gregariousness biases estimates of relative abundance and distribution. Biol Conserv. 2010;143:521–528. doi: 10.1016/j.biocon.2009.11.025. [DOI] [Google Scholar]
- Trinkel M, Fleischmann PH, Steindorfer AF, Kastberger G. Spotted hyenas (Crocuta crocuta) follow migratory prey. Seasonal expansion of a clan territory in Etosha. Namibia J Zool. 2004;264:125–133. doi: 10.1017/S0952836904005588. [DOI] [Google Scholar]
- Trolle M, Noss AJ, De Lima ES, Dalponte JC. Camera-trap studies of maned wolf density in the Cerrado and the Pantanal of Brazil. In: Hawksworth DL, Bull AT, editors. Vertebrate conservation and biodiversity. Dordrecht: Springer; 2006. pp. 371–378. [Google Scholar]
- Turner JW, LaFleur RM, Richardson AT, Holekamp KE. Risk-taking in free-living spotted hyenas is associated with anthropogenic disturbance, predicts survivorship, and is consistent across experimental contexts. Ethology. 2020;126:97–110. doi: 10.1111/eth.12964. [DOI] [Google Scholar]
- van der Meer E, Fritz H, Blinston P, Rasmussen GSA. Ecological trap in the buffer zone of a protected area: effects of indirect anthropogenic mortality on the African wild dog Lycaon pictus. Oryx. 2013 doi: 10.1017/S0030605312001366. [DOI] [Google Scholar]
- van der Meer E, Rasmussen GSA, Fritz H. Using an energetic cost–benefit approach to identify ecological traps: the case of the African wild dog. Anim Conserv. 2015;18:359–366. doi: 10.1111/acv.12182. [DOI] [Google Scholar]
- Van Horn RC, Engh AL, Scribner KT, Funk SM, Holekamp KE. Behavioural structuring of relatedness in the spotted hyena (Crocuta crocuta) suggests direct fitness benefits of clan-level cooperation. Mol Ecol. 2004;13:449–458. doi: 10.1046/j.1365-294X.2003.02071.x. [DOI] [PubMed] [Google Scholar]
- Van Meter PE, French JA, Bidali K, Weldele ML, Brown JL, Holekamp KE. Non-invasive measurement of fecal estrogens in the spotted hyena (Crocuta crocuta) Gen Comp Endocr. 2008;155:464–471. doi: 10.1016/j.ygcen.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Meter PE, French JA, Dloniak SM, Watts HE, Kolowski JM, Holekamp KE. Fecal glucocorticoids reflect socio-ecological and anthropogenic stressors in the lives of wild spotted hyenas. Horm Behav. 2009;55:329–337. doi: 10.1016/j.yhbeh.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vissia S, Wadhwa R, van Langevelde F. Co-occurrence of high densities of brown hyena and spotted hyena in central Tuli, Botswana. J Zool. 2021;314:143–150. doi: 10.1111/jzo.12873. [DOI] [Google Scholar]
- Vitale JD, Jordan NR, Gilfillan GD, McNutt JW, Reader T. Spatial and seasonal patterns of communal latrine use by spotted hyenas (Crocuta crocuta) reflect a seasonal resource defense strategy. Behav Ecol Sociobiol. 2020;74:120. doi: 10.1007/s00265-020-02895-0. [DOI] [Google Scholar]
- Wagner AP, Frank LG, Creel S. Spatial grouping in behaviourally solitary striped hyaenas, Hyaena hyaena. Anim Behav. 2008;75:1131–1142. doi: 10.1016/j.anbehav.2007.08.025. [DOI] [Google Scholar]
- Wagner AP (2006) Behavioral ecology of the striped hyena (Hyaena hyaena). Dissertation, Montana State University-Bozeman
- Watts HE, Holekamp KE. Ecological determinants of survival and reproduction in the spotted hyena. J Mammal. 2009;90:461–471. doi: 10.1644/08-MAMM-A-136.1. [DOI] [Google Scholar]
- Watts HE, Scribner KT, Garcia HA, Holekamp KE. Genetic diversity and structure in two spotted hyena populations reflects social organization and male dispersal. J Zool. 2011;285:281–291. doi: 10.1111/j.1469-7998.2011.00842.x. [DOI] [Google Scholar]
- Welch RJ, Parker DM. Brown hyaena population explosion: rapid population growth in a small, fenced system. Wildlife Res. 2016;43:178–187. doi: 10.1071/WR15123. [DOI] [Google Scholar]
- White PA. Maternal rank is not correlated with cub survival in the spotted hyena, Crocuta crocuta. Behav Ecol. 2005;16:606–613. doi: 10.1093/beheco/ari033. [DOI] [Google Scholar]
- Wiesel I. Killing of Cape fur seal (Arctocephalus pusillus pusillus) pups by brown hyenas (Parahyaena brunnea) at mainland breeding colonies along the coastal Namib Desert. Acta Ethol. 2010;13:93–100. doi: 10.1007/s10211-010-0078-1. [DOI] [Google Scholar]
- Wiesel I (2015) Parahyaena brunnea. The IUCN Red List of Threatened Species. 10.2305/IUCN.UK.2015-4.RLTS.T10276A82344448.en. Accessed 10 Dec 2020
- Williams KS, Pitman RT, Mann GK, Whittington-Jones G, Comley J, Williams ST, Hill RA, Balme GA, Parker DM. Utilizing bycatch camera-trap data for broad-scale occupancy and conservation: a case study of the brown hyaena Parahyaena brunnea. Oryx. 2021;55:216–226. doi: 10.1017/S0030605319000747. [DOI] [Google Scholar]
- Winterbach CW, Maude G, Neo-Mahupeleng G, Klein R, Boast L, Rich LN, Somers MJ. Conservation implications of brown hyaena (Parahyaena brunnea) population densities and distribution across landscapes in Botswana. Koedoe. 2017;59:1–16. doi: 10.4102/koedoe.v59i2.1441. [DOI] [Google Scholar]
- Woodroffe R, Ginsberg JR. Edge effects and the extinction of populations inside protected areas. Science. 1998;280:2126–2128. doi: 10.1126/science.280.5372.2126. [DOI] [PubMed] [Google Scholar]
- Yarnell RW, Phipps WL, Burgess LP, Ellis JA, Harrison SWR, Dell S, MacTavish D, MacTavish LM, Scott DM. The influence of large predators on the feeding ecology of two African mesocarnivores: the black-backed jackal and the brown hyaena. S Afr J Wildl Res. 2013;43:155–216. doi: 10.3957/056.043.0206. [DOI] [Google Scholar]
- Yirga G, Ersino W, De Iongh HH, Leirs H, Gebrehiwot K, Deckers J, Bauer H. Spotted hyena (Crocuta crocuta) coexisting at high density with people in Wukro district, northern Ethiopia. Mamm Biol. 2013;78:193–197. doi: 10.1016/j.mambio.2012.09.001. [DOI] [Google Scholar]
- Yoshida KCS, Van Meter PE, Holekamp KE. Variation among free-living spotted hyenas in three personality traits. Behaviour. 2016;153:1665–1722. doi: 10.1163/1568539X-00003367. [DOI] [Google Scholar]
- Yoshizaki J, Pollock KH, Brownie C, Webster RA. Modeling misidentification errors in capture–recapture studies using photographic identification of evolving marks. Ecology. 2009;90:3–9. doi: 10.1890/08-0304.1. [DOI] [PubMed] [Google Scholar]
- Zheng X, Owen MA, Nie Y, Hu Y, Swaisgood RR, Yan L, Wei F. Individual identification of wild giant pandas from camera trap photos–a systematic and hierarchical approach. J Zool. 2016;300:247–256. doi: 10.1111/jzo.12377. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.