Abstract
Objective
Hypercoagulability is common in severe acute respiratory syndrome coronavirus 2 and has been associated with arterial thrombosis leading to acute limb ischemia (ALI). Our objective was to determine the outcomes of concurrent coronavirus disease 2019 (COVID-19) infection and ALI, particularly during the Delta variant surge and the impact of vaccination status.
Methods
A retrospective review was performed of patients treated at a single health care system between March 2020 and December 2021 for ALI and recent (<14 days) COVID-19 infection or who developed ALI during hospitalization for the same disease. Patients were grouped by year as well as by pre and post Delta variant emergence in 2021 based on the World Health Organization timeline (January to May vs June to December). Baseline demographics, imaging, interventions, and outcomes were evaluated. A control cohort of all patients with ALI requiring surgical intervention for a 2-year period prior to the pandemic was used for comparison. Primary outcomes were in-hospital mortality and amputation-free survival. Kaplan-Meier survival and Cox proportional hazards analysis were performed.
Results
Forty acutely ischemic limbs were identified in 36 patients with COVID-19, the majority during the Delta surge (52.8%) and after the wide availability of vaccines. The rate of COVID-19-associated ALI, although low overall, nearly doubled during the Delta surge (0.37% vs 0.20%; P = .09). Intervention (open or endovascular revascularization vs primary amputation) was performed on 31 limbs in 28 individuals, with the remaining eight treated with systemic anti-coagulation. Postoperative mortality was 48%, and overall mortality was 50%. Major amputation following revascularization was significantly higher with COVID-19 ALI (25% vs 3%; P = .006) compared with the pre-pandemic group. Thirty-day amputation-free survival was significantly lower (log-rank P < .001). COVID-19 infection (adjusted hazard ratio, 6.2; P < .001) and age (hazard ratio, 1.1; P = .006) were associated with 30-day amputation in multivariate analysis. Severity of COVID-19 infection, defined as vasopressor usage, was not associated with post-revascularization amputation. There was a higher incidence of re-thrombosis in the latter half of 2021 with the Delta surge, as reintervention for recurrent ischemia of the same limb was more common than our previous experience (21% vs 0%; P = .55). COVID-19-associated limb ischemia occurred almost exclusively in non-vaccinated patients (92%).
Conclusions
ALI observed with Delta appears more resistant to standard therapy. Unvaccinated status correlated highly with ALI occurrence in the setting of COVID-19 infection. Information of limb loss as a COVID-19 complication among non-vaccinated patients may help to increase compliance.
Keywords: Acute limb ischemia (ALI), COVID-19
Article Highlights.
-
•
Type of Research: Retrospective, cohort study of data collected from a single-institute, multi-hospital network
-
•
Key Findings: The rate of coronavirus disease 2019 (COVID-19) associated acute limb ischemia (ALI), though low overall, nearly doubled during the Delta surge (0.37% vs 0.20%; P = .09). Major amputation following revascularization was significantly higher with COVID-19 ALI (25% vs 3%; P = .006) compared with the pre-pandemic group. COVID-19-associated limb ischemia occurred almost exclusively in non-vaccinated patients (92%).
-
•
Take Home Message: The overall post-revascularization mortality and major amputation rates were significantly increased in the setting of concurrent COVID-19 infection. Unvaccinated status correlated highly with ALI occurrence in the setting of COVID-19 infection.
Since the beginning of 2020, the severe acute respiratory syndrome coronavirus 2 (coronavirus disease 2019 [COVID-19]) pandemic has had a devastating impact on the United States, with over 82 million cases and over 1 million deaths.1 , 2 Although the primary manifestation of COVID-19 infection involves the pulmonary system, there has been documented involvement of various other organ systems, including cardiovascular, neurologic, renal, and hematologic.3
Hypercoagulable states have been shown to be associated with COVID-19 infection primarily in the venous system, with several studies documenting the presence of venous thromboembolisms and pulmonary embolisms in patients with COVID-19.4 , 5 It has been postulated that the prothrombotic state arises from underlying endothelial dysfunction and endotheliitis due to direct infection of the vascular endothelium by the COVID-19 virus, leading to thrombus formation.6, 7, 8, 9 Several case reports and small multi-center studies have shown an elevated incidence of arterial thrombosis leading to acute limb ischemia (ALI) in COVID-19 infections with increased mortality, amputation rates, and intervention failure.10, 11, 12, 13, 14, 15 However, most of these series were prior to the advent of vaccines.
The COVID-19 vaccines were approved by the United States Food and Drug Administration for emergency use in December of 2020.16 Shortly after the wide-availability of vaccines, in May of 2021, the World Health Organization identified the emergence of the Delta COVID-19 variant, putting an even larger strain on the health care system, with high numbers of breakthrough infection, increased virulence, and transmissibility.17 Currently, there is no study evaluating the association of the Delta variant COVID-19 infection with the incidence of ALI.
This study serves to determine the outcomes of concurrent COVID-19 infection and ALI, particularly during the Delta variant surge and the impact of vaccination status. We hypothesize that the hypercoagulable state induced by active COVID-19 infection leads to worsened outcome in instances of ALI, particularly in unvaccinated patients.
Methods
This was a retrospective study evaluating the incidence of COVID-19-associated ALI across at a single health care, multi-hospital network between January of 2020 and March of 2022. The University of Pittsburgh Institutional Review Board approved this study (STUDY21100134). Informed consent was obtained for all patients undergoing intervention.
Data source and patient cohort
The electronic medical record (EMR) was queried for patients aged >18 years with ALI and a recent (<14 days) COVID-19 positive test or for those who developed limb ischemia during hospitalization for COVID-19.18 COVID-19 infection was confirmed by polymerase chain reaction testing or positive testing documentation from outside facilities. Patients presenting with ALI and negative COVID-19 testing or no COVID-19 testing were excluded. A control cohort of unmatched patients with ALI requiring surgical intervention for a 2-year period prior to the COVID-19 pandemic (2018-2019) was used for comparison of outcomes.
Baseline demographic information, imaging, interventions, and outcomes were obtained from the EMR. Clinical indicators of ALI were evaluated using the Rutherford scale of ALI based on the presence of limb pain, motor, or sensory deficit and pulse/doppler exam. Preoperative laboratory results and imaging were collected with the location of the occlusion documented. Vasculature patency was assessed using duplex ultrasound, computed tomography angiography (CTA), or digital subtraction angiography. Interventions were defined as either open surgical, endovascular, hybrid, primary amputation, or nonoperative based on operative reports and documentation. Postoperative outcomes and follow-up information were documented. Major adverse limb events include the need for subsequent major amputation and open or endovascular reintervention due to vessel re-thrombosis. Additional complications included initiation of vasopressor support or renal replacement therapy, cardiac failure defined by myocardial infarction, or pulmonary failure as represented by mechanical ventilation and mortality either during the index hospitalization or on subsequent readmission/follow-up. The initiation of postoperative vasopressor support was used as a surrogate for severity of COVID-19 infection in our study.
Outcomes
Primary outcomes were all-cause in-hospital mortality and 30-day amputation-free survival. The EMR, which is linked to the Social Security death index, was used to identify the death date as well as the amputation date. The cause of death was determined by documented death summaries and classified as COVID-19-related (respiratory failure, multisystem organ failure, or septic shock secondary to initial COVID-19 infection), non-COVID-19-related (any other cause of death), or unknown. Thirty-day amputation-free survival was defined as not having a major amputation or death within 30 days of the index admission date. Major amputation was defined as either above-ankle amputation, or upper limb above-wrist amputation.
Secondary outcomes included hospital length of stay and major adverse limb events (rate of reintervention, and 30-day major amputation post-revascularization). Reintervention was defined as a return to the operating room due to recurrent ischemia or loss of patency after the primary intervention and was categorized as open surgical, endovascular, hybrid, or amputation.
Statistical analysis
Baseline characteristics for pre-COVID and COVID-19 cohorts were compared using the Pearson χ2 and Fisher exact tests for categorical variables and presented as number (frequency %). For continuous variables, the Mann-Whitney and the Student t test were used and presented as mean (± standard deviation). Amputation-free survival at 30 days between the two groups was compared by Kaplan-Meyer survival analysis with associated log-rank testing. Cox proportional hazard analysis was also utilized to assess for factors associated with amputation-free survival. The models generated adjusted hazard ratios (aHRs) with 95% confidence intervals (95% CIs). A P-value ≤ .05 was determined to be statistically significant. All analyses were performed with Stata 17 (StataCorp, College Station, TX). Informed consent was obtained with consent to be treated, and all patients were de-identified.
Subgroup analysis
To assess the effect of the emergence of the Delta variant and vaccination status on COVID-19-associated ALI, patients were grouped as pre-Delta (all cases from 2020 until May 2021) and post-Delta (June 2021 until December 2021) based on the World Health Organization timeline. For this subgroup analysis, we compared baseline characteristics using the same statistical methods described above. We also described characteristics and outcomes of individuals who presented with COVID-19-associated ALI after the emergence of the Omicron variant in 2022.
Results
A total of 40 COVID-19-associated ischemic limbs in 36 patients were identified throughout the study period in comparison with 74 ischemic limbs in 68 patients in the pre-COVID-19 cohort. 18 of 40 limbs (45%) were initially admitted for COVID-19 infection, whereas the remaining 22 (55%) were admitted for ALI and found to be COVID-19-positive after testing or tested positive at home but did not meet hospitalization criteria based on their COVID-19 symptoms. The majority of subjects presented during the Delta surge (52.8%) and after the wide availability of vaccines. COVID-19 variant information, however, was not available at the time of the study. A total of 13,522 cases of COVID-19 hospitalization were identified in the health care network between 2020 and 2021. The rate of COVID-19-associated ALI, though low overall, nearly doubled during the Delta surge (0.37% vs 0.20%; P = .09). A total of 212 cases of ALI were identified in the health care network between 2020 and 2021. The incidence of COVID-19-associated ALI during this study period was 17%. The average age of the COVID-19-associated ALI cohort was significantly older than the pre-COVID-19 group (69.1 ± 11.4 years vs 63.3 ± 14.4 years; P = .03). In both groups, the majority were male and non-Hispanic Whites, matching the demography of the western Pennsylvania patients (Table I ). Within the COVID-19-associated ALI group, there was a 32.5% incidence of peripheral arterial disease, although the symptoms were not obtainable, as well as a significantly larger proportion who have had no prior history of vascular interventions (72% vs 59%; P < .001) as compared with the control. There was a higher proportion of patients with a history of stroke or transient ischemic attack within the COVID-19-associated ALI group (30% vs 12%; P = .02). There were no significant differences in the proportion of patients with a history of venous thromboembolism or hypercoagulable disorders between the two groups. The COVID-19-associated ALI group more often presented with Rutherford Class I ischemia on initial evaluation when compared with the unmatched control group (28% vs 8%; P = .03).
Table I.
Baseline demographics of the pre-coronavirus 2019 (COVID-19) acute limb ischemia (ALI) cohort vs the COVID-19-associated ALI cohort
| Pre-COVID-19 ALI n = 74 (64.9%) | COVID-19 ALI n = 40 (35.1%) | P-value | |
|---|---|---|---|
| Age, years | 63.3 (14.4) | 69.1 (11.4) | .029 |
| Gender | |||
| Male | 45 (61) | 29 (72) | .23 |
| Female | 29 (39) | 11 (28) | |
| Ethnicity | |||
| White | 65 (88) | 36 (90) | .032 |
| Black | 7 (9) | 0 (0) | |
| Hispanic | 0 (0) | 1 (2) | |
| Other/unknown | 2 (3) | 3 (8) | |
| Smoking | |||
| Non-smoker | 26 (35) | 18 (46) | .38 |
| Current smoker | 19 (26) | 6 (15) | |
| Previous smoker | 29 (39) | 15 (38) | |
| Intravenous drug use | 4 (5) | 0 (0) | .30 |
| History of VTE | 6 (8) | 3 (8) | 1.00 |
| Hypertension | 48 (65) | 32 (80) | .13 |
| CAD | 28 (38) | 13 (32) | .68 |
| CHF | 4 (5) | 5 (12) | .27 |
| Diabetes | 25 (34) | 16 (40) | .54 |
| CKD | 8 (11) | 6 (15) | .56 |
| ESRD | 3 (4) | 2 (5) | 1.00 |
| History of stroke/TIA | 9 (12) | 12 (30) | .024 |
| HLD | 29 (39) | 14 (35) | .69 |
| History of hypercoagulable disease | 6 (8) | 3 (8) | 1.00 |
| Malignancy | 10 (14) | 7 (18) | .59 |
| COPD | 15 (20) | 11 (28) | .48 |
| History of vascular intervention | |||
| None | 44 (59) | 29 (72) | <.001 |
| Endoscopic | 30 (41) | 6 (15) | |
| Open | 0 (0) | 5 (12) | |
| History of amputation | |||
| None | 65 (88) | 35 (88) | .67 |
| Minor amputation | 6 (8) | 2 (5) | |
| Major amputation | 3 (4) | 3 (8) | |
| Anticoagulation | 17 (23) | 8 (20) | .81 |
| Aspirin | 34 (46) | 17 (42) | .84 |
| Antiplatelets | 26 (35) | 8 (20) | .13 |
| Rutherford classification | |||
| Class I | 6 (8) | 11 (28) | .025 |
| Class IIa | 30 (42) | 13 (32) | |
| Class IIb | 34 (47) | 13 (32) | |
| Class III | 2 (3) | 3 (8) |
CAD, Coronary artery disease; CHF, congestive heart failure; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; ESRD, end-stage renal disease; HLD, hyperlipidemia; TIA, transient ischemic attack; VTE, venous thromboembolism.
Data are presented as number (%) or mean (standard deviation).
In the COVID-19-associated ALI group, intervention (open or endovascular revascularization vs primary amputation) was performed on 32 limbs in 28 individuals, with the remaining eight patients treated with systemic anti-coagulation. Of the 32 limbs undergoing interventions, 12 required adjunctive treatment during the index operation that was presumed to address underlying disease present within the native vasculature (stenting, balloon angioplasty or endarterectomy with and without patch angioplasty). Eleven of the 40 ischemic limbs were on pressors prior to any intervention or at the time of vascular intervention, with 45% that were treated with medical management. Only one patient was on veno-veno extracorporeal membrane oxygenation and was managed with anticoagulation alone. In the 10 ischemic limbs treated solely with endovascular intervention, all underwent catheter-directed intervention with the use of thrombolytic agents, and six interventions employed the use of catheter-directed aspiration as an adjunct. Of the combined hybrid and open interventions listed in Table II , there were four bypasses, with the remainder of interventions being thromboendarterectomies. All but one of the hybrid interventions involved concomitant stenting proximal to site of thrombectomy/bypass, with the remaining concluding with only a diagnostic angiogram. There was a significantly higher proportion of subjects who received either nonsurgical management or primary amputation (30% vs 0%; P < .001) in the COVID-19-associated ALI group as compared with the control, with the majority of the nonoperative and primary amputation patients intubated at the time of ALI diagnosis (70%). There were 37 ischemic lower extremities and three ischemic upper extremities in our cohort. The majority of the thrombus were located proximally (17/40) (ie, iliac/femoral or subclavian/axillary/brachial) as opposed to distally (12/40) (ie, popliteal/tibial or radial/ulnar), with the remaining being multi-level in nature (9/40), occurring mainly during the Delta surge (6/9). There were, however, no differences in the management or outcomes when stratifying by occlusion location (Supplementary Table I, online only). There was also a significantly longer length of stay (19.3 ± 14.1 days vs 10.4 ± 9.3 days; P < .001), as well as a higher rate of 30-day major amputation (25% vs 3%; P < .001) within the COVID-19-associated ALI group as compared with the control (Table II).
Table II.
Treatment and postintervention outcomes of pre-coronavirus disease 2019 (COVID-19) acute limb ischemia (ALI) vs COVID-19-associated ALI
| Pre-COVID-19 ALI n = 74 (64.9%) | COVID-19 ALI n = 40 (35.1%) | P-value | |
|---|---|---|---|
| Treatment type | |||
| Open | 21 (28) | 11 (28) | <.001 |
| Endovascular | 19 (26) | 10 (25) | |
| Hybrid | 34 (46) | 7 (17.5) | |
| Medical only | 0 (0) | 9 (22) | |
| Amputation | 0 (0) | 3 (7.5) | |
| Reintervention | 7 (16) | 7 (17.5) | .88 |
| Total length of stay, days | 10.4 (9.3) | 19.3 (14.1) | <.001 |
| In-hospital mortality | 6 (8) | 19 (48) | <.001 |
| Postintervention amputation | 11 (14.9) | 7 (17.5) | .54 |
| 30-day amputation | 2 (3) | 11 (28) | .001 |
| 30-day mortality | 8 (11) | 17 (42) | <.001 |
Data are presented as number (%) or mean (standard deviation).
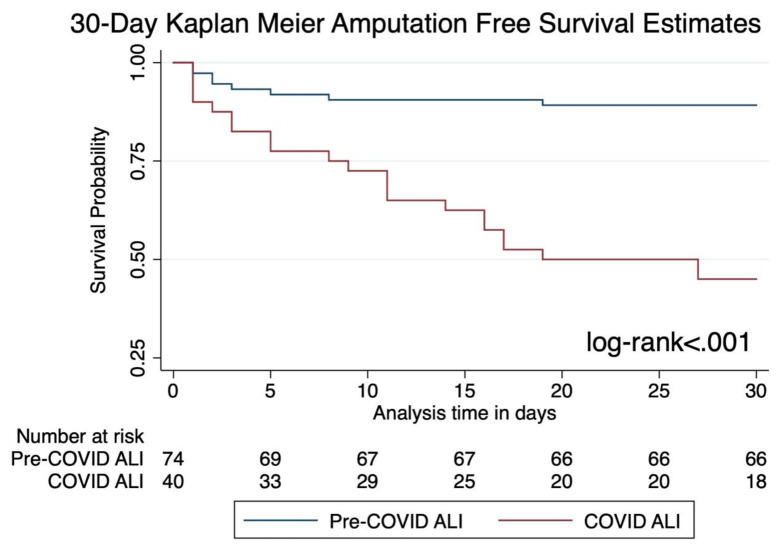
Postoperative in-hospital mortality was 48%, and overall mortality was 50% in the COVID-19-associated ALI cohort. Almost all deaths in the COVID-19-associated ALI cohort were COVID-19-related, with only one patient having a non-COVID-19-related mortality. This individual survived their index COVID-19 hospitalization, with the cause of death being hemorrhagic shock secondary to gastrointestinal bleeding. Patients who were treated nonoperatively had a higher mortality rate when compared with others who received some form of intervention (63%). Among the eight nonsurgically managed patients, five were deceased secondary to COVID-19 pneumonia, and the remaining three did not require any major amputations. The 30-day amputation-free survival in the COVID-19-associated ALI group was 45%, which was significantly lower than the pre-COVID-19 cohort (89%) by Kaplan-Meier analysis (Fig ). On multivariate analysis, COVID-19 infection was associated with increased 30-day amputation (aHR, 6.2; 95% CI, 2.3-16.8; P < .001) and in-hospital mortality (aHR, 10.1; 95% CI, 2.7-37.4; P = .001). Increased age was also a determinant of 30-day amputation and in-hospital mortality (Supplementary Tables II and III, online only). Severity of COVID infection, defined by vasopressor usage in our cohort, was not associated with post-revascularization amputation (P = .68) or nonoperative management (P = .5).
Fig.
Thirty-day Kaplan-Meier amputation-free survival curve. ALI, Acute limb ischemia; COVID-19, coronavirus disease 2019.
Subgroup analysis
Since the beginning of 2021 with implementation of COVID-19 vaccines, there have been 26 ischemic limbs, with 19 (73%) presenting during the Delta surge. There was a significantly lower proportion of subjects who exclusively underwent endovascular revascularization as compared with the months prior to the surge (0% vs 41%; P < .001). The instances of COVID-19-associated ALI occurred almost exclusively in nonvaccinated patients, with only two vaccinated individuals presenting during the Delta surge with concurrent infection and limb ischemia, both of whom received two doses of the Pfizer vaccine without any clinical evidence of vaccine-induced thrombotic thrombocytopenia (Table III ). Furthermore, there was also a higher incidence of re-thrombosis during the Delta surge, as reintervention for recurrent ischemia of the same limb was more common when compared with the first half of 2021 (Table IV ), although not statistically significant (21% vs 0%; P = .55).
Table III.
Baseline demographics of pre-Delta coronavirus disease 2019 (COVID-19) acute limb ischemia (ALI) vs Delta COVID-19 ALI cohorts
| Pre-Delta COVID-19 ALI n = 17 (47.2%) | Delta COVID-19 ALI n = 19 (52.8%) | P-value | |
|---|---|---|---|
| Age, years | 71.6 (12.1) | 66.4 (11.2) | .19 |
| Gender | |||
| Male | 14 (82) | 12 (63) | .27 |
| Female | 3 (18) | 7 (37) | |
| Ethnicity | |||
| White | 15 (88) | 17 (89) | .79 |
| Hispanic | 1 (6) | 0 (0) | |
| Other/unknown | 1 (6) | 2 (11) | |
| Vaccination status | 0 (0) | 2 (11) | .49 |
| Smoking | |||
| Non-smoker | 8 (47) | 9 (50) | 1.00 |
| Current smoker | 2 (12) | 3 (17) | |
| Previous smoker | 7 (41) | 6 (33) | |
| History of PAD | 6 (35) | 6 (32) | 1.00 |
| History of VTE | 3 (18) | 0 (0) | .09 |
| Hypertension | 15 (88) | 14 (74) | .41 |
| CAD | 7 (41) | 5 (26) | .48 |
| CHF | 4 (24) | 1 (5) | .17 |
| Diabetes | 8 (47) | 6 (32) | .50 |
| CKD | 6 (35) | 0 (0) | .006 |
| ESRD | 1 (6) | 1 (5) | 1.00 |
| History of stroke/TIA | 6 (35) | 6 (32) | 1.00 |
| HLD | 9 (53) | 3 (16) | .03 |
| History of hypercoagulable disease | 2 (12) | 1 (5) | .59 |
| Malignancy | 4 (24) | 2 (11) | .39 |
| COPD | 5 (29) | 5 (26) | 1.00 |
| History of vascular intervention | |||
| None | 12 (71) | 14 (74) | .88 |
| Endoscopic | 2 (12) | 3 (16) | |
| Open | 3 (18) | 2 (11) | |
| History of amputation | |||
| None | 14 (82) | 17 (89) | .79 |
| Minor amputation | 1 (6) | 1 (5) | |
| Major amputation | 2 (12) | 1 (5) | |
| Anticoagulation | 4 (24) | 4 (21) | 1.00 |
| Aspirin | 9 (53) | 7 (37) | .50 |
| Antiplatelets | 6 (35) | 1 (5) | .03 |
| Multiple limbs affected | 1 (6) | 7 (37) | .04 |
| Rutherford classification | |||
| Class I | 5 (29) | 6 (32) | .59 |
| Class IIa | 6 (35) | 4 (21) | |
| Class IIb | 4 (24) | 8 (42) | |
| Class III | 2 (12) | 1 (5) |
CAD, Coronary artery disease; CHF, congestive heart failure; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; ESRD, end-stage renal disease; HLD, hyperlipidemia; PAD, peripheral arterial disease; TIA, transient ischemic attack; VTE, venous thromboembolism.
Data are presented as number (%) or mean (standard deviation).
Table IV.
Treatment and postintervention outcomes of pre-Delta coronavirus 2019 (COVID-19) acute limb ischemia (ALI) vs Delta COVID-19 ALI cohorts
| Pre-Delta COVID-19 ALI n = 17 (47.2%) | Delta COVID-19 ALI n = 19 (52.8%) | P-value | |
|---|---|---|---|
| Treatment type | |||
| Open | 4 (24%) | 6 (32%) | .01 |
| Endovascular | 7 (41%) | 0 (0%) | |
| Hybrid | 1 (6%) | 6 (32%) | |
| Medical only | 4 (24%) | 5 (26%) | |
| Amputation | 1 (6%) | 2 (11%) | |
| Reintervention | 3 (17.6%) | 4 (21%) | .79 |
| Reintervention type | |||
| Open | 3 (100%) | 1 (25%) | .05 |
| Hybrid | 0 (0%) | 3 (75%) | |
| Postintervention amputation | 2 (12%) | 4 (22%) | .66 |
| Total length of stay, days | 19.6 (13.9) | 18.6 (12.4) | .82 |
| In-hospital mortality | 6 (35%) | 12 (63%) | .18 |
| 30-day amputation | 3 (18%) | 8 (42%) | .13 |
| 30-day mortality | 7 (41%) | 9 (47%) | .75 |
Data are presented as number (%) or mean (standard deviation).
A total of four ALIs in four individuals were identified during the Omicron surge (January 2022-March 2022), which was a drastic, though nonsignificant, drop-off compared with the Delta surge (0.19% vs 0.37%; P = 1.00). Only one subject had been vaccinated with two doses of the Pfizer vaccine. One patient underwent open revascularization, and three patients underwent endovascular revascularization. There were no major amputations or mortalities at 30 days within this subgroup.
Discussion
The results of this study demonstrated that limb ischemia in the setting of acute COVID-19 infection is associated with significant mortality as well as an increased risk of limb loss despite revascularization. Thirty-six ischemic limbs were identified between 2020 and 2021 during the COVID-19 pandemic, with a near two-fold increase in incidence during the more virulent and severe Delta variant surge compared with previous variants (0.37% vs 0.21%). The overall incidence of ALI in the setting of COVID-19 infection for our study was similar at 0.27%, compared that seen at various New York City hospital systems early in the pandemic at approximately 0.4%.19 , 20 Our study demonstrated a drastic change in the outcomes of revascularization in ALI when associated with concurrent COVID-19 infection. The majority of ischemic events in the COVID-19-associated ALI cohort were de novo, in patients without a history of vascular disease or prior vascular interventions when compared with the control ALI cohort (72% vs 59%), highlighting the viremia-induced hypercoagulability that has been documented in the literature.21, 22, 23 Although there have been multiple studies reporting the instances and outcomes of COVID-19-associated thrombotic events, most of these studies were published at the beginning of the pandemic and in small patient cohorts.15 , 19 , 23, 24, 25 To the authors’ knowledge, this is a unique institutional series evaluating the outcomes of COVID-associated limb ischemia with a particular focus on the Delta variant surge.
It is important to highlight the potential preventive effects of appropriate vaccination in our patient cohort, as COVID-associated ALI occurred almost exclusively among the unvaccinated subjects. The overall effects of the Delta wave during the COVID-19 pandemic have been highlighted by Manzur-Pineda et al, with an increase in the overall rate of thrombotic events, the majority of which were venous in nature.26 Furthermore, similar to the vaccination status of our cohort (92% unvaccinated), COVID-related thrombotic complications occurred almost exclusively within the unvaccinated population (94%) in their study. This could potentially serve as an important basis for the promotion of public health initiatives and improvement in vaccination rates in local communities.
The results of our study fall in line with the previously documented findings with a 30-day major amputation rate of 28% as well as a 30-day mortality rate of 42%, both of which were significantly higher than the control ALI group from 2 years prior. Etkins et al20 reported an overall in-hospital mortality of 46% and Faries et al19 noted an in-hospital mortality of 33%. This was further reflected in the significant decrease in 30-day amputation-free survival when compared with the control ALI group (45% vs 89%). The results are even more striking when separated based on the emergence of the Delta variant, with no incidents of post-revascularization reintervention or amputation in the post-vaccination era (January 2021-May 2021) prior to the emergence of the Delta variant (0% vs 21%). This may highlight the increased virulence and lethality of the Delta variant, which has been demonstrated in the mortality data by the Centers for Disease Control and Prevention.17 Among our cohort of COVID-19-associated ALI, there were six limbs (15%) where acute ischemia was the sole initial manifestation of their underlying COVID-19 infection, as has been previously reported.14 , 27 It was interesting to note that the 30-day major amputation rates for our study population varied dramatically when comparing between patients with COVID-19 and non-COVID-19 ALI at 28% vs 3% (P = .001). Although Kahlberg et al reported a secondary major limb amputation rate of 5.4% in their cohort out of Milan during their 2-month study period,28 Faries et al noted a more comparable major amputation rate of 28% at 6 months in their patients with COVID-19-associated ALI.19 Furthermore, Goldman et al also noted a similar amputation rate of 25% among their cohort of patients with COVID-19 in their 3-month study period.25 This could potentially be caused by the change in the virus itself, as a large portion of major 30-day amputations originated since the emergence of the Delta variant (73%).
The results of our multivariate Cox regression models showed that the only significant predictors for in-hospital mortality and 30-day amputation when controlling for other variables (ie, age and gender) were COVID-19 infection and age. There was also a notable change in the treatment algorithm for ALI that has emerged since the pandemic, with a significant increase in nonoperative management and primary amputation within our COVID-19 cohort (30% vs 0%), and a shift away from endovascular revascularization during the Delta surge (0% vs 41%) (Table II). This highlights the tenuous presentation status of patients with COVID-19 and a shift towards a more palliative/conservative treatment model in severe cases of this particular variant. Furthermore, the elevated mortality rate seen with COVID-19-associated ALI occurred almost exclusively due to complications from their index COVID-19 infection (ie, multi-system organ failure or respiratory decompensation) and could also be largely preventable with appropriate vaccination. As new variants emerge, their impact on the incidence and outcomes of COVID-19-associated ALI remains to be seen. Preliminary data from our institution during the Omicron emergence demonstrates a decreased rate of reintervention after index revascularization as well as a decreased rate of major amputation and mortality when compared with the Delta variant.
There are several limitations to our study. First, given the retrospective nature, the total number of COVID-19-associated ischemic limbs were likely larger than those reported in our study. This could in part be due to the substantial rate of false negatives among polymerase chain reaction tests leading to exclusion of ischemic limbs with negative COVID-19 tests during our study period.29 Although it had become standard protocol among the vascular division to test all patients presenting with ALI for COVID-19 during the peak of the pandemic as well as the Delta surge, additional patients could still have been missed because of subclinical undiagnosed COVID-19 infection with concurrent ALI, and also based on the search criteria in our EMRs, as those whom both COVID-19 and limb ischemia were not documented were excluded. Particularly at the beginning of the pandemic, critically ill patients with acute arterial thrombosis may have had their ischemic events attributed to increasing vasopressor support or systemic illness without exploration or documentation defined limb ischemia. Although hypercoagulable testing was not routinely performed on the COVID-19-associated ALI cohort as active COVID-19 infection, once discovered, was suspected to be the most likely cause of their underlying limb ischemia, there were no difference in the history of previously documented hypercoagulability disorder between our cohorts. Given the unequal distribution of COVID-19 cases within the United States, our small cohort size may be a result of stricter public health restrictions and the lower population density of western Pennsylvania. The rarity of the events also prevents adequate matching from our control group, although despite the lower rate of vascular disease comorbidities, there was still a significantly lower rate of amputation-free survival within our COVID-19-associated ALI group. Finally, given our temporal restriction of 14 days between ALI and COVID-19 symptoms/testing, there may be a significant number of delayed ALI after COVID-19 infection that may have been excluded and will need to be further investigated in the future.
Conclusions
In our evaluation of COVID-19-associated ALI in the University of Pittburgh Medical Center health system, ALI observed with the Delta variant appeared more frequently and more resistant to standard therapy, as demonstrated by the higher rate of reintervention. The overall post-revascularization mortality and major amputation rates were significantly increased in the setting of concurrent COVID-19 infection. In our clinical experience, unvaccinated status correlated highly with ALI, suggesting a potential protective effect of vaccination against thrombotic limb-threatening ischemia. Whether the protection is due to the vaccination status or other correlative factors needs to be clarified. Increased awareness of limb loss as a COVID-19 complication among nonvaccinated individuals may help to increase compliance and vaccination rates in the community.
Author Contributions
Conception and design: BX, NS
Analysis and interpretation: BX, DS, MB, NA, RK, EA, US, RC, ME, MM, NS
Data collection: BX, DS, MB, NA, RK, EA
Writing the article: BX, DS, US, RC, ME, MM, NS
Critical revision of the article: BX, DS, MB, NA, RK, EA, US, RC, ME, MM, NS
Final approval of the article: BX, DS, MB, NA, RK, EA, US, RC, ME, MM, NS
Statistical analysis: BX, DS
Obtained funding: Not applicable
Overall responsibility: BX
From the Society for Vascular Surgery
Footnotes
Research reported in this publication was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number T32HL098036 (E.A.A., B.X.). The University of Pittsburgh holds a Physician-Scientist Institutional Award from the Burroughs Wellcome Fund (E.A.A.).
Author conflict of interest: none.
The editors and reviewers of this article have no relevant financial relationships to disclose per the JVS policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
Additional material for this article may be found online at www.jvascsurg.org.
Additional material for this article may be found online at www.jvascsurg.org.
Appendix (online only).
Supplementary Table I (online only).
Management and outcomes of coronavirus 2019 (COVID-19)-associated acute limb ischemia (ALI) by thrombus location
| Proximal n = 17 (44.7%) | Distal n = 12 (31.6%) | Multi-level n = 9 (23.7%) | P-value | |
|---|---|---|---|---|
| Limb | ||||
| Lower extremity | 15 (88) | 11 (92) | 9 (100) | .78 |
| Upper extremity | 2 (12) | 1 (8) | 0 (0) | |
| Treatment type | ||||
| Open | 7 (41) | 1 (8) | 3 (33) | .09 |
| Endovascular | 1 (6) | 4 (33) | 5 (56) | |
| Hybrid | 3 (18) | 3 (25) | 1 (11) | |
| Medical only | 5 (28) | 3 (25) | 0 (0) | |
| Amputation | 2 (12) | 1 (8) | 0 (0) | |
| Reintervention | 3 (18) | 2 (17) | 2 (22) | 1.00 |
| 30-day amputation | 4 (24) | 4 (33) | 3 (33) | .81 |
Data are presented as number (%).
Supplementary Table II (online only).
Multivariate Cox proportional hazard model for 30-day amputation
| Hazard ratio | 95% CI | P-value | |
|---|---|---|---|
| COVID-19 infection | 6.2 | 2.3-16.8 | <.001 |
| Age | 1.1 | 1.0-1.1 | .006 |
| Gendera | 0.6 | 0.2-1.5 | .268 |
| History of vascular interventionb | |||
| Endoscopic | 0.4 | 0.11-1.5 | .168 |
| Open | 1.4 | 0.4-5.4 | .63 |
| Rutherford classificationc | |||
| Class IIa | 2.7 | 0.7-10.8 | .156 |
| Class IIb | 2.8 | 0.7-11.7 | .158 |
| Class III | 3.6 | 0.5-23.9 | .186 |
| Treatment typed | |||
| Endovascular | 0.3 | 0.1-1.7 | .179 |
| Hybrid | 1.3 | 0.4-4.1 | .591 |
| Medical only | 0.9 | 0.2-4.1 | .984 |
| Amputation | 4.5 | 0.5-42.9 | .196 |
CI, Confidence interval; COVID-19, coronavirus 2019.
Male gender is the reference group.
No history of vascular interventions is the reference group.
Rutherford Class I is the reference group.
Open surgical treatment is the reference group.
Supplementary Table III (online only).
Multivariate Cox proportional hazard model for in-hospital mortality
| Hazard ratio | 95% CI | P-value | |
|---|---|---|---|
| COVID-19 infection | 10.1 | 2.7-37.4 | .001 |
| Age | 1.1 | 1.0-1.1 | .047 |
| Gendera | 0.7 | 0.2-2.4 | .581 |
| History of vascular interventionb | |||
| Endoscopic | 0.1 | 0.1-0.6 | .019 |
| Open | 0.7 | 0.1-3.7 | .684 |
| Rutherford classificationc | |||
| Class IIa | 3.2 | 0.7-14.1 | .126 |
| Class IIb | 7.5 | 1.4-40.3 | .019 |
| Class III | 7.9 | 1.1-60.1 | .047 |
| Treatment typed | |||
| Endovascular | 2.2 | 0.3-14.7 | .415 |
| Hybrid | 3.6 | 0.9-14.9 | .074 |
| Medical only | 3.2 | 0.6-16.1 | .16 |
| Amputation | 0.6 | 0.1-6.6 | .662 |
CI, Confidence interval; COVID-19, coronavirus 2019.
Male gender is the reference group.
No history of vascular interventions is the reference group.
Rutherford Class I is the reference group.
Open surgical treatment is the reference group.
References
- 1.Centers for Disease C COVID Data Tracker. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html Available at:
- 2.COVID Data Tracker. 2022. https://covid.cdc.gov/covid-data-tracker/#cases-deaths-testing-trends Available at:
- 3.Polak S.B., Van Gool I.C., Cohen D., von der Thusen J.H., van Paassen J. A systematic review of pathological findings in COVID-19: a pathophysiological timeline and possible mechanisms of disease progression. Mod Pathol. 2020;33:2128–2138. doi: 10.1038/s41379-020-0603-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klok F.A., Kruip M., van der Meer N.J.M., Arbous M.S., Gommers D., Kant K.M., et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nopp S., Moik F., Jilma B., Pabinger I., Ay C. Risk of venous thromboembolism in patients with COVID-19: a systematic review and meta-analysis. Res Pract Thromb Haemost. 2020;4:1178–1191. doi: 10.1002/rth2.12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mosleh W., Chen K., Pfau S.E., Vashist A. Endotheliitis and endothelial dysfunction in patients with COVID-19: its role in thrombosis and adverse outcomes. J Clin Med. 2020;9:1862. doi: 10.3390/jcm9061862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lei Y., Zhang J., Schiavon C.R., He M., Chen L., Shen H., et al. SARS-CoV-2 spike protein impairs endothelial function via downregulation of ACE 2. Circ Res. 2021;128:1323–1326. doi: 10.1161/CIRCRESAHA.121.318902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ilonzo N., Judelson D., Al-Jundi W., Etkin Y., O'Banion L.A., Rivera A., et al. A review of acute limb ischemia in COVID-positive patients. Semin Vasc Surg. 2021;34:8–12. doi: 10.1053/j.semvascsurg.2021.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shalhub S. The mystery of COVID-19-associated arterial thrombosis. J Vasc Surg. 2021;73:390–391. doi: 10.1016/j.jvs.2020.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fournier M., Faille D., Dossier A., Mageau A., Nicaise Roland P., Ajzenberg N., et al. Arterial thrombotic events in adult inpatients with COVID-19. Mayo Clin Proc. 2021;96:295–303. doi: 10.1016/j.mayocp.2020.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bilaloglu S., Aphinyanaphongs Y., Jones S., Iturrate E., Hochman J., Berger J.S. Thrombosis in hospitalized patients with COVID-19 in a New York City health system. JAMA. 2020;324:799–801. doi: 10.1001/jama.2020.13372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Attisani L., Pucci A., Luoni G., Luzzani L., Pegorer M.A., Settembrini A.M., et al. COVID-19 and acute limb ischemia: a systematic review. J Cardiovasc Surg (Torino) 2021;62:542–547. doi: 10.23736/S0021-9509.21.12017-8. [DOI] [PubMed] [Google Scholar]
- 13.Sanchez J.B., Cuipal Alcalde J.D., Ramos Isidro R., Luna C.Z., Cubas W.S., Coaguila Charres A., et al. Acute limb ischemia in a Peruvian cohort infected by COVID-19. Ann Vasc Surg. 2021;72:196–204. doi: 10.1016/j.avsg.2020.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thompson O., Pierce D., Whang D., O'Malley M., Geise B., Malhotra U. Acute limb ischemia as sole initial manifestation of SARS-CoV-2 infection. J Vasc Surg Cases Innov Tech. 2020;6:511–513. doi: 10.1016/j.jvscit.2020.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Indes J.E., Koleilat I., Hatch A.N., Choinski K., Jones D.B., Aldailami H., et al. Early experience with arterial thromboembolic complications in patients with COVID-19. J Vasc Surg. 2021;73:381–389.e1. doi: 10.1016/j.jvs.2020.07.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kadlec R. Services DoHaH; 2020. Emergency Use Authorization Declaration; pp. 18250–18251. [Google Scholar]
- 17.Centers for Disease C. Johnson A.G., Amin A.B., Ali A.R. COVID-19 Incidence and death rates among unvaccinated and fully vaccinated adults with and without booster doses during periods of delta and omicron variant emergence — 25 U.S. Jurisdictions, April 4–December 25, 2021. MMWR Morb Mortal Wkly Rep. 2022;71:132–138. doi: 10.15585/mmwr.mm7104e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lauer S.A., Grantz K.H., Bi Q., Jones F.K., Zheng Q., Meredith H.R., et al. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann Intern Med. 2020;172:577–582. doi: 10.7326/M20-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Faries C.M., Rao A., Ilonzo N., Hwong S., Krishnan P., Farhan S., et al. Follow-up after acute thrombotic events following COVID-19 infection. J Vasc Surg. 2022;75:408–415.e1. doi: 10.1016/j.jvs.2021.08.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Etkin Y., Conway A.M., Silpe J., Qato K., Carroccio A., Manvar-Singh P., et al. Acute arterial thromboembolism in patients with COVID-19 in the New York City area. Ann Vasc Surg. 2021;70:290–294. doi: 10.1016/j.avsg.2020.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ilonzo N., Rao A., Safir S., Vouyouka A., Phair J., Baldwin M., et al. Acute thrombotic manifestations of coronavirus disease 2019 infection: experience at a large New York City health care system. J Vasc Surg. 2021;73:789–796. doi: 10.1016/j.jvs.2020.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li T., Lu H., Zhang W. Clinical observation and management of COVID-19 patients. Emerg Microbes Infect. 2020;9:687–690. doi: 10.1080/22221751.2020.1741327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bellosta R., Luzzani L., Natalini G., Pegorer M.A., Attisani L., Cossu L.G., et al. Acute limb ischemia in patients with COVID-19 pneumonia. J Vasc Surg. 2020;72:1864–1872. doi: 10.1016/j.jvs.2020.04.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schweblin C., Hachulla A.L., Roffi M., Glauser F. Delayed manifestation of COVID-19 presenting as lower extremity multilevel arterial thrombosis: a case report. Eur Heart J Case Rep. 2020;4:1–4. doi: 10.1093/ehjcr/ytaa371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goldman I.A., Ye K., Scheinfeld M.H. Lower-extremity arterial thrombosis associated with COVID-19 is Characterized by greater thrombus burden and increased rate of amputation and death. Radiology. 2020;297:E263–E269. doi: 10.1148/radiol.2020202348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manzur-Pineda K., O’Neil C.F., Bornak A., Lalama M.J., Shao T., Kang N., et al. COVID-19 related thrombotic complications experience before and during delta wave. J Vasc Surg. 2022;76:1374–1382.e1. doi: 10.1016/j.jvs.2022.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gonzalez-Urquijo M., Gonzalez-Rayas J.M., Castro-Varela A., Hinojosa-Gonzalez D.E., Ramos-Cazares R.E., Vazquez-Garza E., et al. Unexpected arterial thrombosis and acute limb ischemia in COVID-19 patients. Results from the Ibero-Latin American acute arterial thrombosis registry in COVID-19: (ARTICO-19) Vascular. 2022;30:1107–1114. doi: 10.1177/17085381211052033. [DOI] [PubMed] [Google Scholar]
- 28.Kahlberg A., Mascia D., Bellosta R., Attisani L., Pegorer M., Socrate A.M., et al. Vascular surgery during COVID-19 emergency in hub hospitals of lombardy: experience on 305 patients. Eur J Vasc Endovasc Surg. 2021;61:306–315. doi: 10.1016/j.ejvs.2020.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pecoraro V., Negro A., Pirotti T., Trenti T. Estimate false-negative RT-PCR rates for SARS-CoV-2. A systematic review and meta-analysis. Eur J Clin Invest. 2022;52:e13706. doi: 10.1111/eci.13706. [DOI] [PMC free article] [PubMed] [Google Scholar]