Abstract
Background
Cardiovascular complications after Pfizer–BioNTech COVID-19 (BNT) vaccination are a concern, especially in adolescents. We analyzed the risk factors for myocarditis after BNT vaccination.
Methods
We used a special evaluation protocol for all patients aged 12–18 years who presented to our emergency department with cardiovascular symptoms after BNT vaccination.
Results
A total of 195 patients (109 boys and 86 girls) were enrolled. Eleven (5.6%) patients presented with arrhythmia (arrhythmia group), 14 (7.2%) had a diagnosis of pericarditis/myocarditis (the peri/myocarditis group), and the remaining 170 were controls (no cardiac involvement). Chest pain (77.6%) was the most common symptom. The median time from vaccination to symptom onset was 3 days. In the peri/myocarditis group (13 myocarditis and 1 pericarditis), the median time to the peak troponin T level was 5 days after vaccination. Abnormal electrocardiographic changes, including ST-T changes and conduction blocks, were more commonly detected in the peri/myocarditis group (85.7% vs. 12.4% in the control group, p < 0.01). Echocardiography revealed normal ventricular function in all patients. Symptoms were resolved before discharge in all, with the median duration of hospital stay being 4 days. The electrocardiography was the most appropriate screening tool for myocarditis, with a sensitivity and specificity of 85.7% and 87.6%, respectively.
Conclusion
Pericarditis or myocarditis was diagnosed in 7.2% of adolescents presenting to the emergency department with cardiovascular symptoms after BNT vaccination. In addition to the troponin T level, ECG change listed above can be used as a screening tool for vaccine-induced cardiac complications.
Keywords: COVID-19, BNT vaccine, Chest pain, Myocarditis, Electrocardiogram
Introduction
COVID-19 has emerged as a critical global health concern. Vaccination is a crucial tool for the prevention of COVID-19 infection. In adolescents, mRNA vaccines, such as the Pfizer–BioNTech COVID-19 (BNT) vaccine, have been approved by the United States Food and Drug Administration for COVID-19 prevention.1 Clinical trials have reported the safety and efficacy of the BNT vaccine in adolescents.1, 2, 3, 4 However, after nationwide vaccination programs in several countries, myocarditis has emerged as a crucial complication of the BNT vaccine in adolescents.5, 6, 7, 8 Vaccine-related myocarditis is frequently detected in boys and young adolescents,6, 7, 8, 9 with the most common symptoms being chest pain or chest tightness.10 Other complications, including arrhythmias, have also been reported.11 Studies have evaluated the benefits and risks of the vaccine8, 9 however, the differentiation of vaccine-related myocarditis from nonspecific chest pain remains challenging.
Taiwan has strict quarantine and mask policies, leading to a low incidence of COVID-19. As of January 31, 2022, the number of confirmed COVID-19 infections was 21,986, which is approximately 0.09% of the total population. Although the infection rate has been low in Taiwan, several outbreak episodes have occurred in the 2 years since the initial outbreak. Vaccination programs have become the main solution for COVID-19 control. A mass BNT vaccination program for high school students began on September 15, 2021, with the second dose being administered 12 weeks later (i.e., from December 15 to 29, 2021). The vaccines were administered using a school-based system supported by Taipei City Hospital. All students in the same high school received the BNT vaccine on the same day; both doses of the vaccine were administered within 15 days. Our center is the largest pediatric cardiology center in Taipei, and we evaluated the clinical characteristics, clinical course, and other factors associated with myocarditis in adolescents who presented with cardiovascular symptoms within 4 weeks after receiving either dose of the BNT vaccine through the vaccination program.
Methods
A total of 195 patients, aged 12–18 years, who presented to the emergency department of National Taiwan University Children's Hospital from September 2021 to January 2022 for cardiovascular symptoms within 28 days after BNT vaccination were included in this retrospective analysis. A COVID-19 test was performed using a polymerase chain reaction for all patients. The evaluation protocol for patients presenting with cardiovascular symptoms after BNT vaccination is displayed in Fig. 1 . An electrocardiogram (ECG) and tests measuring the levels of troponin T, white blood cells (WBCs), and c-reactive protein (CRP) levels were performed in all patients, and echocardiography was performed only in selected patients with equivocal findings after these tests. All patients with a diagnosis of myocarditis were admitted to the general ward or the intensive care unit for further management; the patients were discharged if the troponin T level declined. We reviewed the basic characteristics and clinical course of these patients, including initial symptoms, laboratory presentations, serial ECG changes, and echocardiography.
Figure 1.
Flow chart for patients with cardiovascular complaints after BNT vaccine in the pediatric emergency department. Abbreviation: SVT, supraventricular tachycardia; TnT, troponin-T; ER, emergency room; OPD, outpatient department; ECG, electrocardiogram; F/U, follow-up.
We divided the patients into pericarditis or myocarditis (peri/myocarditis), arrhythmia, and control groups. Myocarditis definition was based on a Centers for Disease Control and Prevention (CDC) working definition with some modification.12 According to this definition, the diagnosis of confirmed myocarditis requires clinical symptoms, elevated troponin T levels, and positive cardiac magnetic resonance image (MRI) findings. Because of a limited capacity for cardiac MRI, we made the diagnosis of myocarditis on the basis of clinical symptoms and elevated troponin T levels. The arrhythmia group comprised patients with arrhythmic change, such as paroxysmal supraventricular tachycardia (PSVT), atrial premature beats, ventricular premature beats, sinus bradycardia, and first-degree atrioventricular (AV) block, but with normal troponin T levels. Patients without a diagnosis of pericarditis, myocarditis, or arrhythmia were allocated to the control group.
Diagnostic ECG changes13 were ST-segment depression of at least 0.05 mV in at least two contiguous leads, ST-segment elevation of at least 0.2 mV in lead V2 or V3, ST-segment elevation of at least 0.1 mV in at least two contiguous leads, and T-wave inversion (TWI), defined as negative T-waves in at least two contiguous leads. Negative T-waves at precordial leads V1–V3 were considered a juvenile pattern and were not counted as TWI unless the serial ECG revealed upright T-waves after symptom resolution.13 Sinus bradycardia was defined as sinus rhythm and heart rate below 50 bpm, and incomplete right bundle branch block was defined as a QRS duration of less than 120 ms and the presence of right bundle branch block pattern.14
Statistical analysis
Data are expressed as medians and ranges. The nonparametric Wilcoxon rank sum test was used for numerical data analysis, and the chi-squared or Fisher's exact test was used for categorical data analysis. A p value < 0.05 indicated statistical significance.
Results
Clinical characteristics of the patients
During the study period, 195 adolescent patients (109 boys and 86 girls) with a median age of 15 years presented with cardiovascular symptoms after BNT vaccination. Among them, 11 (5.6%) had cardiac arrhythmias (arrhythmia group), 14 (7.2%) had pericarditis or myocarditis (peri/myocarditis group), and 170 (87.2%) had no cardiac involvement and were followed up at outpatient clinics (the control group). All patients tested negative for COVID-19. Cardiovascular symptoms occurred after the first dose of the BNT vaccine in 101 patients and the second dose of the BNT vaccine in 95 patients. Chest pain (77.6%) was the most common symptom, followed by palpitations (24.5%), dyspnea (14.8%), and dizziness or syncope (3.1%). The median duration from vaccination to symptom onset was 3 days (0–25 days) and that from vaccination to the emergency department visit was 4 days (0–90 days).
Clinical profiles of the arrhythmia group
Of the 11 patients who presented with arrhythmias, 4 (36.3%) required admission for monitoring, and 7 were treated at and discharged from the emergency department with outpatient follow-up. Most admissions occurred after the first dose, and the high admission rate may reflect our initial inexperience in BNT vaccine–related cardiac complications. The documented arrhythmias were PSVT in 2 patients, atrial premature beats in 2, ventricular premature beats in 4, sinus bradycardia in 1, sinus bradycardia with a long pause in 1, and first-degree AV block in 1. All but one had no history of arrhythmia, and the condition improved in all patients before discharge.
One of the 2 patients with PSVT required conversion by using adenosine. The troponin T level was 100.6 ng/L, which decreased to 12.5 ng/L (normal <14 ng/L) during the follow-up. Atenolol was prescribed for rhythm control after the discharge of the patient. The other patient with PSVT had a history of pulmonary atresia with an intact ventricular septum, which had been corrected using transcatheter intervention. This patient had had PSVT since September 2018; the condition was well controlled with regular atenolol use. However, it occurred twice within 2 weeks after the first BNT vaccine dose, and ultimately adenosine conversion was required at the emergency department. Propafenone was prescribed for arrhythmia control after discharge.
Of the 4 patients with ventricular premature beats, 1 presented with frequent ventricular bigeminy and was admitted to the general ward for further evaluation. A Holter test revealed frequent premature ventricular contractions (24%) that included quadruplets. Propranolol was prescribed for arrhythmia control, and the patient was discharged without incident to be followed up at the outpatient clinic.
No ST-segment changes were detected through ECG in any of the patients with arrhythmia after arrhythmia conversion. A total of 6 of the 11 patients with arrhythmias underwent echocardiography, which revealed a fair left ventricular systolic function without pericardial effusion. Other details of these 11 patients are listed in Table 1 .
Table 1.
Clinical characteristics of 195 patients according to diagnosis.
| Group 1 Arrythmia (n = 11) |
Group 2 Myocarditis (n = 14) | Group 3 Control (n = 170) |
P value | P value (compare group 2 & 3) | |
|---|---|---|---|---|---|
| Age (Median (Min-Max), years) | 16 (12–17) | 15 (13–17) | 15 (12–18) | 0.77 | 0.82 |
| Gender (M/F) | 9/2 | 11/3 | 89/81 | 0.03 | 0.09 |
| Vaccination (1stdose/2nddose) | 7/4 | 2/12 | 91/79 | 0.01 | <0.01 |
| Underlying heart disease | 1 (8.3%) | 0 | 2 (1.2%) | 0.13 | 0.20 |
| Onset of symptoms after vaccine (Median (Min-Max),days) | 4 (1–26) | 1.5 (0–8) | 3 (0–25) | 0.12 | 0.16 |
| ED visit after vaccine (Median (Min-Max),days) | 4 (2–26) | 3 (1–8) | 4 (0–90) | 0.57 | 0.51 |
| Symptoms | |||||
| Chest pain/chest tightness | 4 (36.4%) | 13 (92.9%) | 135 (79.4%) | <0.01 | 0.31 |
| Palpitation | 7 (63.6%) | 3 (21.4%) | 37 (21.8%) | <0.01 | 1.00 |
| Dyspnea | 0 | 1 (7.1%) | 28 (16.5%) | 0.21 | 0.70 |
| Dizziness/syncope | 0 | 0 | 6 (3.5%) | 0.62 | 1.00 |
| Initial laboratory survey | |||||
| Troponin-T (Median (Min-Max), ng/L) | 5.0 (3–100.6) | 191.2 (3.7–1728) | 3.4 (3–16.4) | <0.01 | <0.01 |
| WBC (Median (Min-Max), K/ L) | 7.4 (3.4–12.9) | 7.3 (4.3–11.4) | 7.0 (2.6–11.9) | 0.27 | 0.13 |
| CRP (Median (Min-Max), mg/dL) | 0.18 (0.02–3.01) | 0.96 (0.02–7.45) | 0.11 (0.02–4.38) | <0.01 | <0.01 |
| ECG | |||||
| ST segment elevation | 0 (0%) | 9 (64.3%) | 4 (2.98%) | <0.01 | <0.01 |
| Inferior lead | 4 | 0 | |||
| Lateral lead | 6 | 3 | |||
| Precordial lead | 1 | 0 | |||
| Diffused | 0 | 1 | |||
| ST segment depression | 1 (9.1%) | 3 (21.4%) | 1 (0.6%) | <0.01 | <0.01 |
| T wave inversion | 1 (9.1%) | 1 (7.1%) | 12 (7.1%) | 0.97 | 1.00 |
| Conduction block | 2 (18.2%) | 1 (7.1%) | 4 (2.4%) | 0.02 | 0.33 |
| Any of ECG change | 3 (27.3%) | 12 (85.7%) | 21 (12.4%) | <0.01 | <0.01 |
| Echocardiography (number) | 6 | 14 | 12 | ||
| LV systolic function55% | 0 | 0 | 0 | 1.00 | 1.00 |
| Pericardial effusion | 0 | 2 (14.3%) | 0% | 0.22 | 0.14 |
Abbreviation: CRP, C reactive protein; ECG, electrocardiography; ED, emergency department; LV, left ventricle; WBC, white blood cell count.
Clinical profile of the peri/myocarditis group
Of the 14 patients with a diagnosis of pericarditis or myocarditis, 13 had myocarditis, and 1 had pericarditis. A comparison of clinical characteristics between the peri/myocarditis and control groups is presented in Table 1.
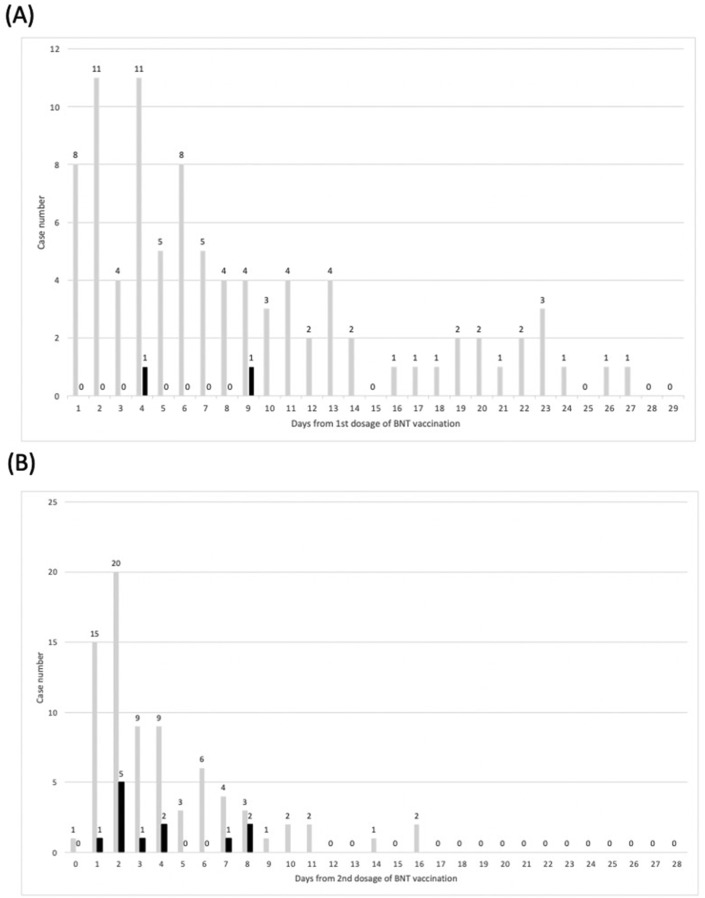
Of patients with pericarditis or myocarditis, 13 (92.9%) presented with chest pain or chest tightness, 3 (21.4%) with palpitations, and 1 (7.1%) with dyspnea. No statistically significant difference in the clinical presentation was observed between the peri/myocarditis and control groups. The median time from BNT vaccination to symptom onset in the peri/myocarditis group was 1.5 days versus 3 days in the control group (p = 0.16; Table 1 and Fig. 2 ).
Figure 2.
Time to emergency department visit (days) after (A) first dose and (B) second dose of BNT vacuine. Black bar, peri/myocarditis group; gray bar, control group.
WBC counts were similar between the peri/myocarditis and control groups; however, troponin T and CRP levels were significantly higher in the peri/myocarditis group than in the control group. The cardiac troponin T level was elevated in all 13 patients with myocarditis. The trend of troponin T levels is displayed in Fig. 3 . The median time to the peak troponin T level was 5 days after vaccination, ranging from 2 to 9 days. The patient who was diagnosed with pericarditis had a normal cardiac troponin T level with a small amount of pericardial effusion in echocardiography.
Figure 3.
Trend of cardiac troponin-T level in 14 patients diagnosed with peri/myocarditis.
Abnormal ECG changes, namely ST-segment changes, TWIs, and conduction blocks, were recorded in 85.7% of the patients with myocarditis and in 12.4% of the controls (p < 0.01). More detailed analysis indicated that ST-segment changes were more frequently detected in patients with myocarditis; however, TWIs and conduction blocks were not detected.
Echocardiography was performed to evaluate the basic systolic function and pericardial effusion of the patients (14 in the control and arrhythmia groups) who were discharged from the emergency department. All of them had a normal left ventricular systolic function, and 2 had minimal to small volumes of pericardial effusion.
Only one patient (the first in the peri/myocarditis group) underwent cardiac MRI, which revealed an elevated native T1 signal with late gadolinium enhancement at the midwall of the left ventricle, confirming the myocarditis diagnosis. Of the 14 patients, only 1 was admitted to the intensive care unit for vital sign monitoring because of inexperience in vaccine-related myocarditis. No inotropic agents or advanced cardiopulmonary support was required. Four out of the 14 patients required symptomatic control with nonsteroidal anti-inflammatory drugs or acetaminophen, and the median time from admission to symptom relief was 1 day (range, 0–3 days). The hospital stay ranged from 1 to 5 days, with a median of 3 days. All patients survived to discharge and did not have notable sequelae at the time of data collection.
Clinical risk factors for pericarditis and myocarditis
We compared the peri/myocarditis group with the control group to determine the clinical profile associated with the diagnosis of pericarditis or myocarditis. Multivariate logistic regression analysis of sex, vaccine dosage, chest pain, ECG change, and CRP level revealed that ECG changes (odds ratio [OR] 24.6, 95% confidence interval [CI] 4.7–130, p < 0.01) and CRP levels (OR 1.8, 95% CI 1.0–3.1, p = 0.047) were significant predictors of myocarditis. After the removal of laboratory data from the regression model, only ECG changes were significant (OR 42.5, 95% CI 8.9–203.6, p < 0.01). ECG as the first-line screening tool exhibited 85.7% sensitivity and 87.6% specificity for pericarditis and myocarditis, with positive and negative predictive values being 36.4% and 98.7%, respectively. The addition of chest pain in the screening model decreased the sensitivity to 78.6% and increased the specificity to 91.2% (see Table 2 ).
Table 2.
Predictive value for peri/myocarditis of ECG and chest pain.
| Sensitivity | Specificity | Positive predictive value | Negative predictive value | |
|---|---|---|---|---|
| Any ECG change | 85.7% | 87.6% | 36.4% | 98.7% |
| Any ECG change plus chest pain | 78.6% | 91.2% | 57.7% | 98.1% |
Discussion
The findings of this study can guide the diagnosis and management of cardiovascular symptoms after BNT vaccination in adolescents in the emergency department and outpatient settings. During our study period, the incidence of pericarditis or myocarditis after BNT vaccination in adolescent patients presenting to the emergency department with cardiovascular symptoms was 7.2%, with a predominance of boys. The clinical course was largely benign, and no patient required inotropic agents or other cardiovascular support. The median hospital stay was 3 days. An ECG change, especially an ST-segment change, is the most suitable tool for screening probable myocarditis with acceptable sensitivity and specificity.
Importance of pericarditis and myocarditis screening after BNT vaccination in the young population
An increasing risk of myocarditis after BNT vaccination has been reported.5 An Israel-based study7 reported that the overall incidence of vaccine-related myocarditis after the second dose of the BNT vaccine was 2.13 cases per 100,000 persons; the incidence of vaccine-related myocarditis was 10.69 cases per 100,000 persons, with the highest incidence being reported among men aged 16–29 years. The CDC in the United States reported that the incidence of myocarditis or pericarditis among individuals aged 12–39 years after the second dose of mRNA vaccines was 12.6 cases per million.15 These findings highlight the importance of rapid detection of vaccine-related pericarditis or myocarditis in the young population. Primary-care providers in emergency departments and outpatient clinics should have easy access to a quick screening method for pericarditis or myocarditis in young patients presenting with cardiovascular symptoms after BNT vaccination.
In Taipei, nearly 160,000 students received BNT vaccinations in a short time because of a school-based mass vaccination program.16 As the largest pediatric cardiology center in the country, our hospital is now the referral center for patients with cardiovascular complications after BNT vaccination. We collected data from a large number of patients with post-BNT vaccination cardiovascular symptoms, which can mitigate the influence of background myocarditis.
In other reports,11, 15, 17, 18 the clinical course of vaccine-related myocarditis was shorter than that of virus-related myocarditis. Vaccine-related myocarditis often occurs within 5 days after vaccination, most commonly within 2 days. In most cases, the clinical course has been benign, and aggressive treatment with an inotropic agent or advanced cardiopulmonary support has rarely been necessary. The most common symptom has been chest pain, with the troponin T levels peaking at 2–4 days after vaccination. Symptoms have been successfully managed using analgesics in most cases, with improvements in biomarker levels and imaging study results at discharge. In pediatric patients,19, 20 similar to young adolescents, most cases of BNT vaccine-related pericarditis or myocarditis have been reported after the second dose of the vaccine. Patients present at a median of 2 days after symptom onset, with the most common symptom being chest pain. The cardiac troponin T level generally peaks at 2–3 days after vaccination, and symptoms are commonly resolved within 7 days.
The present study also clarified the clinical course of BNT vaccine-related pericarditis or myocarditis in adolescents. The median time from vaccination to symptom onset was 1.5 days. The troponin T level peaked at a median of 5 days after vaccination and decreased rapidly thereafter. ECG changes were detected in 85.7% of the patients, with an improvement observed within 5 days in most cases. None of the patients had an abnormal ejection fraction in echocardiography, and none required inotropic support. All of the patients had their symptoms resolve before discharge, with the median length of hospital stay being 4 days.
Arrhythmia after BNT vaccination
A large-scale study in the United Kingdom explored the incidence of myocarditis, pericarditis, and arrhythmia among individuals who received three types of COVID-19 vaccines (ChAdOx1, BNT162b2, and mRNA-1273); the study discovered no association between the ChAdOx1 and BNT vaccines and cardiac arrhythmias.21 In the present study, the rate of post-BNT vaccine–related arrhythmia or conduction disturbance was 6.1% in pediatric patients presenting with cardiovascular symptoms to the emergency department. Unlike pericarditis and myocarditis, which had significantly higher incidences after the second dose of the vaccine, 67% of the arrhythmias occurred after the first dose. Most patients had no known history of arrhythmia. Because of the unavailability of baseline ECG or cardiac electrophysiology study results, we cannot conclude whether the arrhythmias were caused by vaccine-related injury or triggered by the vaccine in patients with underlying arrhythmia. However, this finding suggests that arrhythmia may occur in association with BNT vaccination in some susceptible patients. We reported 4 cases of ventricular tachyarrhythmia after vaccination with a COVID-19 mRNA vaccine. Two of these were in patients with underlying cardiomyopathy, and 1 occurred in a patient who had congenital heart disease with Eisenmenger syndrome. However, vaccine-induced arrhythmia may still be a critical concern deserving further survey.
Detection of pericarditis and myocarditis
Among adult patients presenting to the emergency department for cardiovascular adverse reactions after vaccination, more than half of the patients were women, most presented after the first dose of the vaccine, and myocarditis was diagnosed in 0.58% of the patients.22 The incidence of postvaccine pericarditis or myocarditis was higher in our study because we included adolescents and our hospital is the main referral center for children with cardiovascular complications after COVID-19 vaccination. We determined a protocol for the evaluation of these young patients at the emergency department, with the possibility of extending it to the outpatient clinic. In the emergency department, we performed ECG and evaluated the levels of troponin T and CRP in all patients with BNT-related cardiovascular symptoms; the results are readily available. However, the diagnosis of pericarditis or myocarditis by using this protocol might not be extendable to outpatient clinics because of the long wait for troponin T and CRP level results, which are usually available within 3 h at the emergency department. For patients presenting to outpatient clinics with cardiovascular symptoms after BNT vaccination, ECG can be applied because of the immediate availability of results in any setting. We discovered that ECG changes, either ST-T changes or conduction abnormalities, have high sensitivity and negative predictive value for the diagnosis of pericarditis or myocarditis in patients with cardiovascular complications after BNT vaccination. Therefore, ECG can be used as the first-line tool to detect vaccine-related pericarditis or myocarditis in the outpatient clinic.
Study limitations
Because this was a single-center study in the emergency department, the sample size and generalizability may be a problem. However, our hospital was assigned as the referral center for COVID-19 vaccine–related cardiovascular complications; therefore, the sample size was sufficiently large for analysis.
We did not analyze data on patients presenting to outpatient clinics because blood sampling and laboratory surveys were not performed in most such patients, leading to the underdetection of pericarditis or myocarditis. Not all patients with initial normal troponin T levels underwent echocardiography, which may have decreased the detection rate of pericarditis. Only one patient underwent cardiac MRI because of limited MRI capacities and because all patients had mild symptoms, which can also decrease the study power.
For simplicity, we categorized patients into arrhythmia and peri/myocarditis groups. Some patients with myocarditis may present with arrhythmia. Although only patients with normal troponin T levels can be categorized into the arrhythmia group, this categorization may result in underestimation of probable myocarditis as the cause of the arrhythmia.
Conclusion
Pericarditis or myocarditis was diagnosed in 7.2% of adolescents presenting to the emergency department with cardiovascular symptoms after BNT vaccination. In addition to the troponin T level, ECG change including ST-T change and conduction block can be used as a screening tool for vaccine-induced cardiac complications in the emergency department.
Declaration of competing interest
The authors have no conflicts of interest relevant to this article.
References
- 1.Barda N., Dagan N., Ben-Shlomo Y., Kepten E., Waxman J., Ohana R., et al. Safety of the BNT162b2 mRNA covid-19 vaccine in a nationwide setting. N Engl J Med. 2021;385(12):1078–1090. doi: 10.1056/NEJMoa2110475. [published Online First: Epub Date] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fowlkes A.L., Yoon S.K., Lutrick K., Gwynn L., Burns J., Grant L., et al. Effectiveness of 2-dose BNT162b2 (pfizer BioNTech) mRNA vaccine in preventing SARS-CoV-2 infection among children aged 5-11 Years and adolescents aged 12-15 Years - PROTECT cohort, july 2021-february 2022. MMWR Morb Mortal Wkly Rep. 2022;71(11):422–428. doi: 10.15585/mmwr.mm7111e1. [published Online First: Epub Date] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klein N.P., Stockwell M.S., Demarco M., Gaglani M., Kharbanda A.B., Irving S.A., et al. Effectiveness of COVID-19 pfizer-BioNTech BNT162b2 mRNA vaccination in preventing COVID-19-associated emergency department and urgent care encounters and hospitalizations among nonimmunocompromised children and adolescents aged 5-17 Years - VISION network, 10 States, april 2021-january 2022. MMWR Morb Mortal Wkly Rep. 2022;71(9):352–358. doi: 10.15585/mmwr.mm7109e3. [published Online First: Epub Date] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dooling K., Gargano J.W., Moulia D., Wallace M., Rosenblum H.G., Blain A.E., et al. Use of pfizer-BioNTech COVID-19 vaccine in persons aged >/=16 Years: recommendations of the advisory committee on immunization practices - United States, september 2021. MMWR Morb Mortal Wkly Rep. 2021;70(38):1344–1348. doi: 10.15585/mmwr.mm7038e2. [published Online First: Epub Date] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mevorach D., Anis E., Cedar N., Bromberg M., Haas E.J., Nadir E., et al. Myocarditis after BNT162b2 mRNA vaccine against covid-19 in Israel. N Engl J Med. 2021;385(23):2140–2149. doi: 10.1056/NEJMoa2109730. [published Online First: Epub Date] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Husby A., Hansen J.V., Fosbøl E., Thiesson E.M., Madsen M., Thomsen R.W., et al. SARS-CoV-2 vaccination and myocarditis or myopericarditis: population based cohort study. Br Med J. 2021;375 doi: 10.1136/bmj-2021-068665. [published Online First: Epub Date] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Witberg G., Barda N., Hoss S., Richter I., Wiessman M., Aviv Y., et al. Myocarditis after covid-19 vaccination in a large health care organization. N Engl J Med. 2021;385(23):2132–2139. doi: 10.1056/NEJMoa2110737. [published Online First: Epub Date] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lai F.T.T., Li X., Peng K., Huang L., Ip P., Tong X., et al. Carditis after COVID-19 vaccination with a messenger RNA vaccine and an inactivated virus vaccine : a case-control study. Ann Intern Med. 2022;175(3):362–370. doi: 10.7326/m21-3700. [published Online First: Epub Date] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krug A., Stevenson J., Hoeg T.B. BNT162b2 vaccine-associated Myo/pericarditis in adolescents: a stratified risk-Benefit analysis. Eur J Clin Invest. 2022 doi: 10.1111/eci.13759. [published Online First: Epub Date] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Law Y.M., Lal A.K., Chen S., Cihakova D., Cooper L.T., Deshpande S., et al. Diagnosis and management of myocarditis in children: a scientific statement from the American heart association. Circulation. 2021;144(6):e123–e135. doi: 10.1161/CIR.0000000000001001. [published Online First: Epub Date] [DOI] [PubMed] [Google Scholar]
- 11.Truong D.T., Dionne A., Muniz J.C., McHugh K.E., Portman M.A., Lambert L.M., et al. Clinically suspected myocarditis Temporally related to COVID-19 vaccination in adolescents and young adults: suspected myocarditis after COVID-19 vaccination. Circulation. 2022;145(5):345–356. doi: 10.1161/circulationaha.121.056583. [published Online First: Epub Date] [DOI] [PubMed] [Google Scholar]
- 12.Oster DM. Centers for disease control and prevention (CDC). Advisory committee on immunization practices (ACIP). Coronavirus disease 2019 (COVID-19) vaccines. . Secondary centers for disease control and prevention (CDC). Advisory committee on immunization practices (ACIP). Coronavirus disease 2019 (COVID-19) vaccines. Accessed July 6, 2021.
- 13.Regan W., O'Byrne L., Stewart K., Miller O., Pushparajah K., Theocharis P., et al. Electrocardiographic changes in children with Multisystem inflammation associated with COVID-19: associated with coronavirus disease 2019. J Pediatr. 2021;234:27–32 e2. doi: 10.1016/j.jpeds.2020.12.033. [published Online First: Epub Date] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Surawicz B., Childers R., Deal B.J., Gettes L.S., Bailey J.J., Gorgels A., et al. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part III: intraventricular conduction disturbances: a scientific statement from the American heart association electrocardiography and arrhythmias committee, council on clinical cardiology; the American college of cardiology foundation; and the heart rhythm society. Endorsed by the international society for computerized electrocardiology. J Am Coll Cardiol. 2009;53(11):976–981. doi: 10.1016/j.jacc.2008.12.013. [published Online First: Epub Date] [DOI] [PubMed] [Google Scholar]
- 15.Bozkurt B., Kamat I., Hotez P.J. Myocarditis with COVID-19 mRNA vaccines. Circulation. 2021;144(6):471–484. doi: 10.1161/CIRCULATIONAHA.121.056135. [published Online First: Epub Date] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.government DoeTc https://www.doe.gov.taipei/News_Content.aspx?n=0F560782595DACFC&s=561076331D4D9063
- 17.Woo W., Kim A.Y., Yon D.K. Clinical characteristics and prognostic factors of myocarditis associated with the mRNA COVID-19 vaccine. J Med Virol. 2022;94(4):1566–1580. doi: 10.1002/jmv.27501. [published Online First: Epub Date] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oster M.E., Shay D.K., Su J.R., Gee J., Creech C.B., Broder K.R., et al. Myocarditis cases reported after mRNA-based COVID-19 vaccination in the US from December 2020 to august 2021. JAMA. 2022;327(4):331–340. doi: 10.1001/jama.2021.24110. [published Online First: Epub Date] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Das B.B., Kohli U., Ramachandran P., Nguyen H.H., Greil G., Hussain T., et al. Myopericarditis after messenger RNA coronavirus disease 2019 vaccination in adolescents 12 to 18 Years of age. J Pediatr. 2021;238:26–32 e1. doi: 10.1016/j.jpeds.2021.07.044. [published Online First: Epub Date] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dionne A., Sperotto F., Chamberlain S., Baker A.L., Powell A.J., Prakash A., et al. Association of myocarditis with BNT162b2 messenger RNA COVID-19 vaccine in a case series of children. JAMA Cardiol. 2021;6(12):1446–1450. doi: 10.1001/jamacardio.2021.3471. [published Online First: Epub Date] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patone M., Mei X.W., Handunnetthi L., Dixon S., Zaccardi F., Shankar-Hari M., et al. Risks of myocarditis, pericarditis, and cardiac arrhythmias associated with COVID-19 vaccination or SARS-CoV-2 infection. Nat Med. 2022;28(2):410–422. doi: 10.1038/s41591-021-01630-0. [published Online First: Epub Date] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oh T.H., Woo S.H., Hong S., Lee C., Lee W.J., Jeong S.K. Clinical features of patients presenting to the emergency department with cardiovascular adverse reactions after COVID-19 mRNA vaccination. J Kor Med Sci. 2022;37(9):e73. doi: 10.3346/jkms.2022.37.e73. [published Online First: Epub Date] [DOI] [PMC free article] [PubMed] [Google Scholar]