Abstract
Background
The extent to which vaccinated persons who become infected with SARS-CoV-2 contribute to transmission is unclear. During a SARS-CoV-2 Delta variant outbreak among incarcerated persons with high vaccination rates in a federal prison, we assessed markers of viral shedding in vaccinated and unvaccinated persons.
Methods
Consenting incarcerated persons with confirmed SARS-CoV-2 infection provided mid-turbinate nasal specimens daily for 10 consecutive days and reported symptom data via questionnaire. Real-time reverse transcription-polymerase chain reaction (RT-PCR), viral whole genome sequencing, and viral culture was performed on these nasal specimens. Duration of RT-PCR positivity and viral culture positivity was assessed using survival analysis.
Results
A total of 957 specimens were provided by 93 participants, of whom 78 (84 %) were vaccinated and 17 (16 %) were unvaccinated. No significant differences were detected in duration of RT-PCR positivity among vaccinated participants (median: 13 days) versus those unvaccinated (median: 13 days; p = 0.50), or in duration of culture positivity (medians: 5 days and 5 days; p = 0.29). Among vaccinated participants, overall duration of culture positivity was shorter among Moderna vaccine recipients versus Pfizer (p = 0.048) or Janssen (p = 0.003) vaccine recipients. In post-hoc analyses, Moderna vaccine recipients demonstrated significantly shorter duration of culture positivity compared to unvaccinated participants (p = 0.02). When restricted to participants without reported prior infection, the difference between Moderna vaccine recipients and unvaccinated participants was more pronounced (medians: 3 days and 6 days, p = 0.002).
Conclusions
Infectious periods for vaccinated and unvaccinated persons who become infected with SARS-CoV-2 are similar and can be highly variable, though some vaccinated persons are likely infectious for shorter durations. These findings are critically important, especially in congregate settings where viral transmission can lead to large outbreaks. In such settings, clinicians and public health practitioners should consider vaccinated, infected persons to be no less infectious than unvaccinated, infected persons.
Keywords: COVID-19 transmission, Vaccination, Virus shedding, Infectious disease outbreaks, Correctional facilities
Abbreviations: BOP, U.S. Bureau of Prisons; CDC, U.S. Centers for Disease Control and Prevention; CSTE, Council of State and Territorial Epidemiologists; Ct, Cycle Threshold; IQR, Interquartile Range; RT-PCR, Reverse Transcription-Polymerase Chain Reaction; VTM, Viral Transport Medium; WGS, Whole Genome Sequencing
1. Introduction
COVID-19 vaccines are highly effective in preventing severe illness and death from SARS-CoV-2 (the virus that causes COVID-19). However, because COVID-19 vaccines are not 100 % effective in preventing infection, some infections among vaccinated persons are expected to occur. As global vaccination coverage increases, the role of vaccinated persons in transmission will be a critical determinant of the pandemic’s future trajectory [1]. The extent to which vaccinated persons who become infected contribute to transmission of SARS-CoV-2 is not yet well understood. Some preprint manuscripts have reported comparable indicators of transmission potential regardless of vaccination status [2], while others have reported reduced viability of virus isolated from vaccinated persons [3].
The B.1.617.2 (Delta) variant was associated with a peak in COVID-19 cases in the United States beginning in July 2021 that included large outbreaks among vaccinated and unvaccinated persons in crowded settings [4], [5], [6]. These findings are of particular concern for congregate living environments such as correctional and detention facilities because of the potential for rapid transmission of SARS-CoV-2 and the high prevalence of underlying health conditions associated with severe COVID-19 [7], [8], [9].
In an outbreak involving the Delta variant in a federal prison in Texas, the cumulative incidence of infection in two affected housing units was 74 %; it was 93 % and 70 % among unvaccinated and vaccinated incarcerated persons, respectively [6]. Using serial mid-turbinate nasal specimens collected from a subset of incarcerated persons infected during this outbreak, this report assesses reverse transcription-polymerase chain reaction (RT-PCR) and viral culture characteristics as surrogate markers of transmission potential over time among vaccinated and unvaccinated persons. This report is one of the first longitudinal investigations of viral shedding from vaccinated persons infected with the Delta variant and contributes to the evidence base guiding infection prevention and control procedures across a variety of settings.
2. Methods
2.1. Investigational setting
On July 12, 2021, an outbreak of SARS-CoV-2 among vaccinated and unvaccinated persons was detected in a federal prison in Texas. Staff from the Centers for Disease Control and Prevention (CDC) and Federal Bureau of Prisons (BOP) deployed to the prison to investigate the outbreak as previously reported [6]. As part of this outbreak investigation, a subset of incarcerated persons provided serial mid-turbinate nasal specimens which were analyzed to evaluate the potential role of infected vaccinated and unvaccinated persons in transmission of SARS-CoV-2. This activity was reviewed and approved by the BOP Research Review Board and CDC and conducted consistent with applicable federal law and CDC policy.*.
2.2. Participant enrollment and serial specimen collection
Incarcerated persons living in four housing units where COVID-19 cases had been identified were invited to participate in serial swabbing. Persons were eligible to enroll if they had tested positive for SARS-CoV-2 between July 12 (the start of the outbreak) and August 4, 2021. Staff members who tested positive during the outbreak were not invited to participate in the investigation; per CDC guidelines, they were restricted from entering the facility during their home isolation period. CDC and BOP staff held information sessions to explain the purpose of the project and to answer questions, including privacy protections and how results of the study would be made available to participants. All persons who chose to participate signed consent forms, which were provided in English and Spanish.
Specimen collection occurred during July 19—August 9, 2021. CDC and BOP staff collected one nasal mid-turbinate specimen daily for 10 consecutive days from participants who had tested positive for SARS-CoV-2. Specimen collection began on July 19 for known cases; for cases identified after July 19 collection began on the date of participants’ first positive test. All incarcerated persons residing in housing units where cases were identified were placed under quarantine precautions. To assist in case-finding, consenting persons who were quarantined were tested every other day beginning on July 19 or on their first full day of quarantine; those who tested positive during quarantine were invited to participate in the 10 consecutive days of specimen collection for persons who had tested positive. All participants were asked to provide an eleventh specimen on August 6, which corresponded to a late timepoint in infection for most participants, to provide additional data on viral shedding (Fig. 1 ).
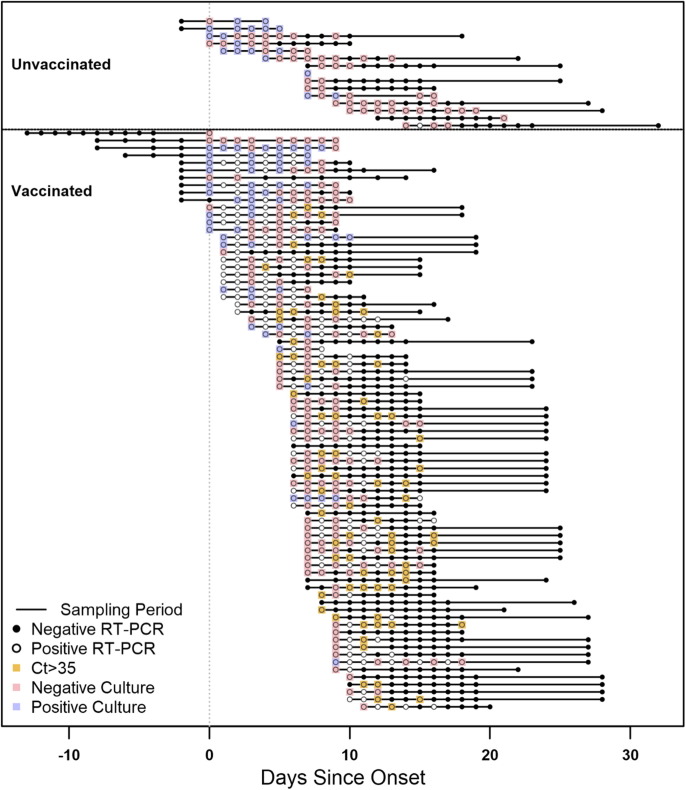
Fig. 1.
Timelines and results of nasal mid-turbinate specimens collected from enrolled participants, Federal prison, Texas, July 12—August 9, 2021. The timelines of specimen collection and laboratory results for 95 included participants are represented diagrammatically, indexed by the day of onset. Onset was determined to be either a) date of first onset of self-reported symptom(s) meeting the case definition of COVID-19 or b) date of first positive diagnostic SARS-CoV-2 test, whichever occurred first. Each participant is represented by a horizontal line corresponding to the investigation sampling period during their time-course of illness. Participants who were unvaccinated (including 2 participants who received only the first dose of a two-dose COVID-19 vaccine series) are depicted at the top of the figure, while vaccinated participants are depicted at the bottom. RT-PCR results are represented by solid circles (positive results) or open circles (negative results). For specimens with positive RT-PCR results for which viral culture was performed, culture results are indicated by overlaid blue boxes (positive culture results) or red boxes (negative culture results). Specimens with positive RT-PCR results with a cycle threshold (Ct) value>35 for which viral culture was not performed are indicated by overlaid orange boxes (indicated a presumptive negative viral culture result). Some participants provided specimens during case-finding testing while in quarantine and may have RT-PCR negative specimens collected prior to onset.
On the tenth day of specimen collection, participants were asked to complete a paper-based questionnaire to report COVID-19-like symptoms during the course of their illness, including date of symptom onset and symptom duration. Information on demographic characteristics, COVID-19 vaccination history, previous positive SARS-CoV-2 diagnostic tests, and underlying medical conditions was extracted from BOP electronic medical records for all participants.
2.3. Laboratory methods
Specimens were collected using nylon flocked minitip swabs, transferred into universal viral transport media (VTM) (Becton Dickinson, Franklin Lakes, NJ), immediately stored at 2–8 °C and frozen at −20 °C or below within 72 h, and sent to CDC for RT-PCR testing using the CDC Influenza SARS-CoV-2 Multiplex Assay. Remnant aliquots were stored at −70 °C or below for viral culture. Due to capacity limitations, viral culture was performed on a subset of specimens. Specimens were included for viral culture if they had been collected 0, 3, 5, 7, or 9 days since onset and had an accompanying positive RT-PCR test with cycle threshold (Ct) value < 35. For verification that this selected Ct cutoff did not exclude specimens containing culturable virus, viral culture was also performed on 25 of 102 specimens with Ct ≥ 35. (25/25 of these specimens were culture negative.) For more granular detail across the time-course of infection, viral culture was also performed on a subset of specimens collected on other days (see Supplemental Figs. 1-2 for details on specimens included for viral culture).
Specimens selected for culture were used to perform limiting-diluting inoculation of Vero CCL-81 cells expressing TMPRSS2, and cultures showing evidence of cytopathic effect were tested by RT-PCR for the presence of SARS-CoV-2 RNA. Viral recovery was conducted as previously described [10]. Whole genome sequencing (WGS) was performed for one RT-PCR-positive specimen per participant with Ct < 30 (per sequencing laboratory standard protocols).
2.4. Statistical Methods
Onset (used as time 0 in longitudinal analyses below) was defined to be either a) date of first onset of self-reported symptom(s) meeting the case definition of COVID-19 [11], or b) date of first positive diagnostic SARS-CoV-2 test, whichever occurred first. In two instances where a participant without symptoms had an initial positive test followed by at least 3 negative tests before subsequent positive tests, the date of second positive test was used.
Participants were considered vaccinated if ≥ 14 days had elapsed since they had completed all recommended doses of a COVID-19 primary vaccine series before the start of the outbreak. (No participant had completed a primary vaccine series < 14 days before the outbreak.) At the time of this investigation, additional/booster doses of COVID-19 vaccines were not recommended. Participants were considered unvaccinated if they had not received any doses; persons who had started but not completed a primary vaccine series were excluded from the analysis. Demographic characteristics of participants stratified by vaccination status were assessed using Fisher’s exact tests.
Three surrogate markers for assessing transmission potential were analyzed as primary outcomes: RT-PCR positivity (an indicator of current/recent infection), RT-PCR Ct value (a semi-quantitative indicator of relative level of viral nucleic acid), and viral culture positivity (an indicator of viable/infectious virus). Dichotomous laboratory results (RT-PCR positivity and viral culture positivity) were analyzed longitudinally with time 0 defined as the date of onset and the primary endpoints defined by a participant’s last positive test. Specimens for which viral culture was not performed were presumed to be culture negative if an accompanying RT-PCR test was negative or was positive with Ct ≥ 35. To account for variation in the interval between onset and enrollment, and intermittent participation in specimen collection by some participants (which can result in interval and right censoring), survival analyses were performed using Turnbull estimation using the “interval” package implementation in R [12]. Hypothesis testing of survival functions was performed using the generalized Wilcoxon-Mann-Whitney method for interval-censored data.
As a post-hoc evaluation of potential interactions between vaccination status, vaccine product, recency of vaccination, and known prior SARS-CoV-2 infections, stratified pairwise comparisons of survival functions were performed between vaccinated and unvaccinated participants in each subgroup (e.g., those vaccinated with known prior infection vs those unvaccinated with known prior infection) using the generalized Wilcoxon-Mann-Whitney method.
Non-dichotomous laboratory results (RT-PCR Ct values) were characterized by days since onset using medians and interquartile ranges (IQRs). Because Ct values are semi-parametric, distributions were compared non-parametrically using the Mann-Whitney U test with ties (for dichotomous variables) or the Kruskal-Wallis test (for categorical variables with>2 levels); negative RT-PCR results were assigned higher ranks than any Ct value from positive RT-PCR results. To account for multiple hypothesis testing across days, α thresholds were adjusted using Bonferroni correction. All hypothesis tests of Ct values are detailed in Supplementary Tables 1 and 2. All statistical analyses were performed in R version 4.0.2 (R Core Team, Vienna, Austria).
3. Results
3.1. Population characteristics
Among 189 persons with SARS-CoV-2 infection eligible to enroll, a total of 96 persons (51 %) consented to participate in serial specimen collection; one participant had a single positive diagnostic test (Ct = 36.2) followed by seven negative diagnostic tests and reported no symptoms and was excluded as a non-case, and two participants who had received single doses of a two-dose primary series were also excluded. Of the 93 included participants, 78 (84 %) were documented as being vaccinated against SARS-CoV-2 and 15 (16 %) were unvaccinated (Table 1 ). Among vaccinated participants, a majority (57/78, 73 %) received the Pfizer vaccine; smaller proportions received the Moderna vaccine (14/78, 18 %) or Janssen vaccine (7/78, 9 %). A majority (47/78, 60 %) of vaccinated participants completed their vaccination series>120 days prior to the start of the outbreak (IQR: 81–140 days prior to start). Recipients of Pfizer vaccines completed their series earlier (IQR: 131–131 days) than recipients of Moderna (IQR: 81–82 days prior to start) or Janssen (IQR: 46–70 days prior to start) vaccines (p < 0.001). A small number of participants (2/78 vaccinated, 3 %, and 2/15 unvaccinated, 13 %, p = 0.10) had a documented prior SARS-CoV-2 infection. Based on symptom self-report at the end of sampling, 68 % of participants reported at least one symptom in the COVID-19 case definition. The most commonly reported symptoms were runny or stuffy nose (59 %), loss of smell or taste (54 %), and cough (45 %). Of 93 specimens from 93 participants for which sequencing was attempted, 64 were successfully sequenced and passed quality metrics; all 64 (100 %) belonged to the B.1.617.2 (Delta) lineage and AY.3 sublineage.
Table 1.
Characteristics of enrolled participants who tested positive for SARS-CoV-2, Federal prison, Texas, July 12—August 9, 2021.
| All participants |
Vaccinated |
Unvaccinated* |
p-value† | ||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | N | % | ||
| Total | 93 | 100 % | 78 | 84 % | 15 | 16 % | |
| Sex | |||||||
| Male | 93 | 100 % | 78 | 84 % | 15 | 16 % | |
| Age | 0.5 | ||||||
| 18–29 | 5 | 5 % | 3 | 4 % | 2 | 13 % | |
| 30–39 | 22 | 23 % | 19 | 24 % | 3 | 20 % | |
| 40–49 | 26 | 29 % | 22 | 28 % | 4 | 27 % | |
| 50–59 | 25 | 26 % | 20 | 26 % | 5 | 33 % | |
| ≥ 60 | 15 | 16 % | 14 | 18 % | 1 | 7 % | |
| Race/Ethnicity | 0.01 | ||||||
| American Indian/Alaska Native | 2 | 2 % | 2 | 3 % | 0 | 0 % | |
| Asian | 1 | 1 % | 1 | 1 % | 0 | 0 % | |
| Black | 15 | 17 % | 8 | 10 % | 7 | 47 % | |
| Hispanic | 12 | 13 % | 10 | 13 % | 2 | 13 % | |
| White | 63 | 67 % | 57 | 73 % | 6 | 40 % | |
| Country of birth | 0.5 | ||||||
| Non US-born | 4 | 4 % | 3 | 4 % | 1 | 7 % | |
| US-born | 89 | 96 % | 75 | 96 % | 14 | 93 % | |
| Vaccination status | |||||||
| Vaccinated | 78 | 82 % | 78 | 100 % | 0 | 0 % | |
| Unvaccinated* | 15 | 16 % | 0 | 0 % | 15 | 100 % | |
| Vaccine product received | |||||||
| Janssen | 7 | 7 % | 7 | 9 % | 0 | 0 % | |
| Moderna | 14 | 15 % | 14 | 17 % | 0 | 0 % | |
| Pfizer | 57 | 60 % | 57 | 74 % | 0 | 0 % | |
| Time from full vaccination to outbreak (if vaccinated) | |||||||
| ≤120 days | 31 | 33 % | 31 | 33 % | 0 | 0 % | |
| >120 days | 47 | 49 % | 47 | 61 % | 0 | 0 % | |
| Medical comorbidities | |||||||
| Overweight‡ | 31 | 33 % | 24 | 31 % | 7 | 46 % | 0.2 |
| Obesity‡ | 46 | 49 % | 42 | 54 % | 4 | 26 % | |
| Severe obesity ‡ | 7 | 7 % | 6 | 8 % | 0 | 0 % | |
| History of smoking | 45 | 48 % | 42 | 54 % | 3 | 20 % | 0.02 |
| Hypertension | 42 | 45 % | 38 | 49 % | 4 | 26 % | 0.1 |
| Diabetes | 15 | 16 % | 14 | 18 % | 1 | 6 % | 0.3 |
| Moderate/severe asthma | 10 | 11 % | 8 | 10 % | 2 | 12 % | 0.7 |
| Chronic obstructive pulmonary disease | 6 | 6 % | 6 | 8 % | 0 | 0 % | 0.6 |
| Cancer | 1 | 1 % | 1 | 1 % | 0 | 0 % | 1.0 |
| Chronic kidney disease | 2 | 2 % | 2 | 3 % | 0 | 0 % | 1.0 |
| Immunocompromised state | 2 | 2 % | 2 | 3 % | 0 | 0 % | 1.0 |
| HIV | 0 | 0 % | 0 | 0 % | 0 | 0 % | |
| Serious cardiac conditions | 0 | 0 % | 0 | 0 % | 0 | 0 % | |
| Liver disease | 0 | 0 % | 0 | 0 % | 0 | 0 % | |
| Documented prior SARS-CoV-2 infection | 0.1 | ||||||
| No | 89 | 96 % | 76 | 97 % | 13 | 87 % | |
| Yes | 4 | 4 % | 2 | 3 % | 2 | 13 % | |
| COVID-19 disease outcomes | |||||||
| Hospitalization | 2 | 2 % | 1 | 1 % | 1 | 6 % | |
| Death | 0 | 0 % | 0 | 0 % | 0 | 0 % | |
| Reported Symptoms | |||||||
| Reported any symptoms in CSTE case definition§ | 64 | 68 % | 54 | 69 % | 10 | 67 % | 1.0 |
| Reported any symptoms | 70 | 75 % | 59 | 76 % | 11 | 73 % | 0.4 |
| Runny/Stuffy Nose | 54 | 59 % | 48 | 62 % | 6 | 47 % | 0.3 |
| Loss of Smell or Taste | 50 | 54 % | 43 | 55 % | 7 | 47 % | 1.0 |
| Cough | 43 | 45 % | 35 | 45 % | 7 | 47 % | 0.8 |
| Headache | 40 | 43 % | 33 | 42 % | 6 | 40 % | 1.0 |
| Muscle Aches | 38 | 41 % | 30 | 38 % | 8 | 53 % | 0.1 |
| Subjective Fever | 34 | 36 % | 27 | 35 % | 6 | 40 % | 0.5 |
| Measured Fever | 10 | 11 % | 6 | 8 % | 4 | 27 % | 0.04 |
| Chills | 28 | 30 % | 21 | 27 % | 7 | 47 % | 0.09 |
| Sore Throat | 22 | 24 % | 21 | 27 % | 1 | 7 % | 0.2 |
| Shortness of Breath | 19 | 20 % | 14 | 18 % | 5 | 33 % | 0.1 |
| Abdominal Pain, Nausea, Vomiting | 17 | 18 % | 12 | 15 % | 5 | 33 % | 0.1 |
| Diarrhea | 15 | 16 % | 11 | 14 % | 4 | 27 % | 0.2 |
| Other | 6 | 6 % | 6 | 8 % | 0 | 0 % | 1.0 |
| None Reported | 23 | 25 % | 19 | 24 % | 4 | 27 % | 1.0 |
*Unvaccinated participants include 15 who have not received any dose of a SARS-CoV-2 vaccine.
†P-values correspond to results of Fisher’s exact tests.
‡Overweight was defined as a body mass index (BMI) > 25 kg/m2 but < 30 kg/m2; obesity was defined as BMI ≥ 30 kg/m2 but < 40 kg/m2; severe obesity was defined as BMI ≥ 40 kg/m2.
§The COVID-19 case definition of the Council of State and Territorial Epidemiologists (CSTE) includes fever, chills, muscle aches, headache, sore throat, nausea/vomiting, diarrhea, fatigue, stuffy/runny nose, cough, shortness of breath, or loss of taste or smell.
¶ 8 participants (5 vaccinated and 3 unvaccinated) declined to report symptoms in addition to 15 (14 and 1, respectively) who reported that they had no symptoms.
3.2. RT-PCR positivity
From the 93 included participants, 957 specimens were collected for RT-PCR testing (825/957, 86 % from vaccinated participants). Specimens were collected ranging from 13 days prior to onset (among participants tested during quarantine prior to diagnosis) to 32 days following onset. See Fig. 1 for a diagrammatic representation of RT-PCR specimen collection from participants, and see Supplemental Fig. 1 for details of specimen collection by day since onset (stratified by vaccination status). A median of 6 days elapsed between onset and enrollment among vaccinated participants, compared with a median of 7 days among participants who were unvaccinated (p = 0.45).
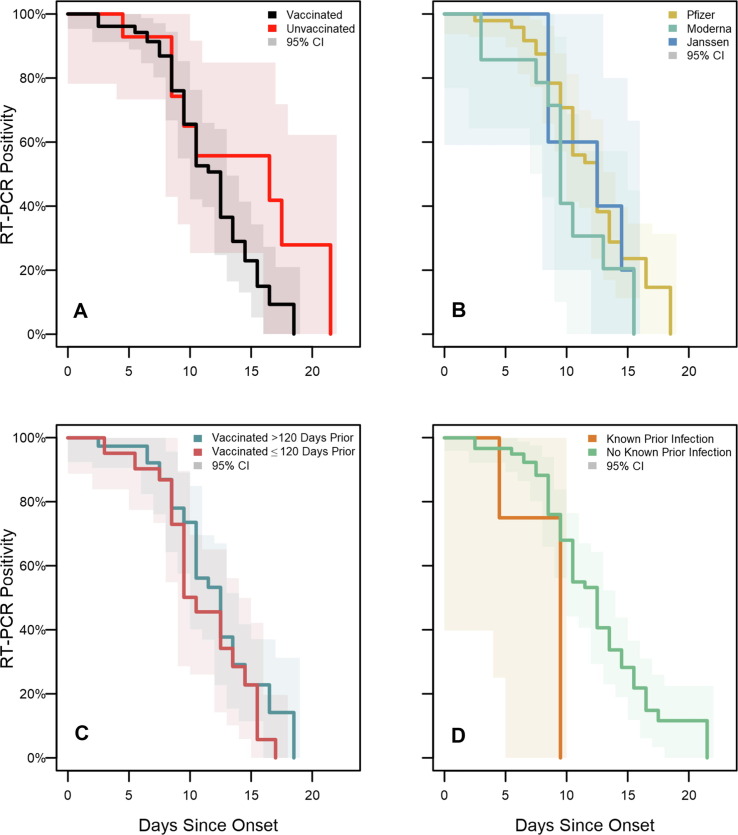
No significant differences in time to last RT-PCR positive test were found. Median duration of RT-PCR positivity was 13 days among vaccinated participants versus 17 days among participants who were unvaccinated (p = 0.32; Fig. 2); and 10 days among participants with known history of prior SARS-CoV-2 infection (regardless of vaccination) versus 13 days among participants without any known prior infection (p = 0.14). Among vaccinated participants, median duration of positivity was 10 days among Moderna vaccine recipients versus 13 days among Pfizer recipients and 13 days among Janssen recipients (p = 0.39); and 13 days among participants vaccinated >120 days prior to the outbreak versus 11 days among participants vaccinated 120 days or less prior to the outbreak (p = 0.32).
Fig. 2.
SARS-CoV-2 RT-PCR test positivity survival curves for enrolled participants, Federal prison, Texas, July 12—August 9, 2021. Panels illustrate the results of Turnbull estimation survival functions with a primary endpoint of last positive reverse transcription-polymerase chain reaction (RT-PCR) test result. Solid lines indicate nonparametric maximum likelihood estimates and shaded regions correspond to 95% confidence intervals estimated through modified bootstrap. Survival functions are plotted by Turnbull interval midpoints. Onset was determined to be either a) date of first onset of self-reported symptom(s) meeting the case definition of COVID-19 or b) date of first positive diagnostic SARS-CoV-2 test, whichever occurred first. Panel A depicts RT-PCR positivity by vaccination status. Panel B depicts positivity by vaccine product among vaccinated participants. Panel C depicts positivity according to the time from completion of a COVID-19 vaccine/series to onset. Panel D depicts positivity according to history of known prior SARS-CoV-2 infection.
3.3. Ct values
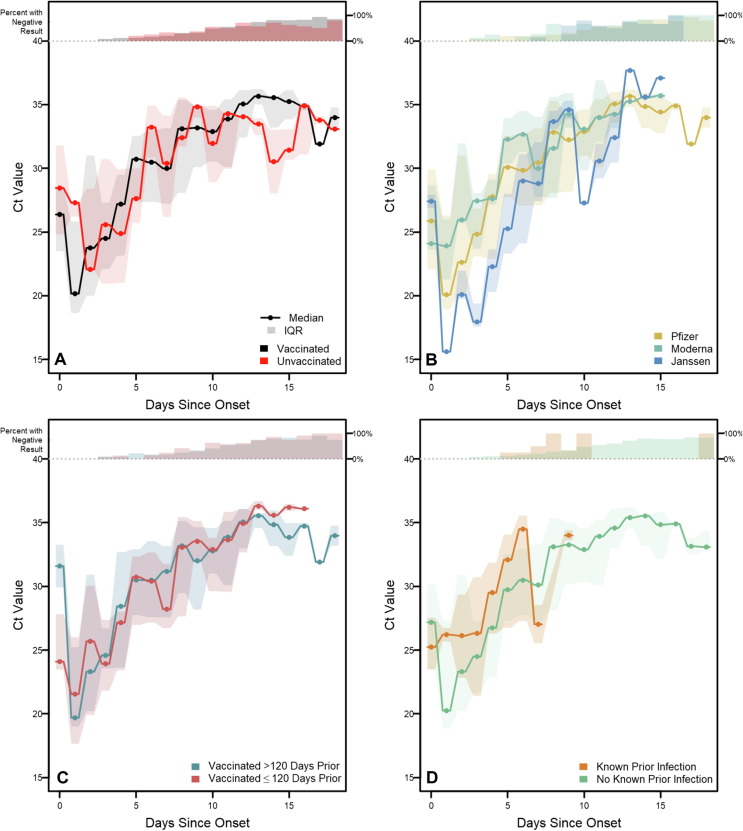
Ct values from specimens testing positive by RT-PCR increased with the number of days since onset (Fig. 3 ). Among specimens from vaccinated participants, Ct values increased from a median of 26.4 (IQR: 23.5–28.4) on the day of onset to a median of 32.9 on day 10 (IQR: 30.5–34.6), while Ct values from specimens from participants who were unvaccinated increased from a median of 28.5 (IQR:24.8–31.8) on the day of onset to a median of 31.9 on day 10 (IQR:28.9–34.9). Across the time-course of infection, no statistically significant difference was observed among Ct values by vaccination status on any day after Bonferroni correction (all p > 0.0026, the Bonferroni-corrected α threshold). Additionally, no significant differences were observed among Ct values when stratified by vaccine product, time since vaccination, or known prior SARS-CoV-2 infection. While not statistically significant, lower Ct values were observed early in the time-course of infection among Janssen vaccine recipients (day 3 median: 17.9; IQR: 17.6–19.4) than among Moderna (day 3 median: 27.4; IQR: 23.7–28.1) or Pfizer recipients (day 3 median: 24.8; IQR: 23.1–26.8; p = 0.016 while Bonferroni α = 0.0026).
Fig. 3.
RT-PCR Cycle Threshold distributions for enrolled participants with confirmed SARS-CoV-2 infection, Federal prison, Texas, July 12—August 9, 2021. Panels illustrate daily medians and interquartile ranges (IQRs) for reverse transcription-polymerase chain reaction (RT-PCR) cycle threshold (Ct) values among specimens with positive RT-PCR results. Solid lines indicate median Ct values and shaded regions indicate IQRs. Percentages at the top of each panel indicate the proportion of specimens with negative RT-PCR results each day. Onset was determined to be either a) date of first onset of self-reported symptom(s) meeting the case definition of COVID-19 or b) date of first positive diagnostic SARS-CoV-2 test, whichever occurred first. Panel A depicts RT-PCR positivity by vaccination status. Panel B depicts positivity by vaccine product among vaccinated participants. Panel C depicts positivity according to the time from completion of a COVID-19 vaccine/series to onset. Panel D depicts positivity according to history of known prior SARS-CoV-2 infection.
3.4. Viral culture positivity
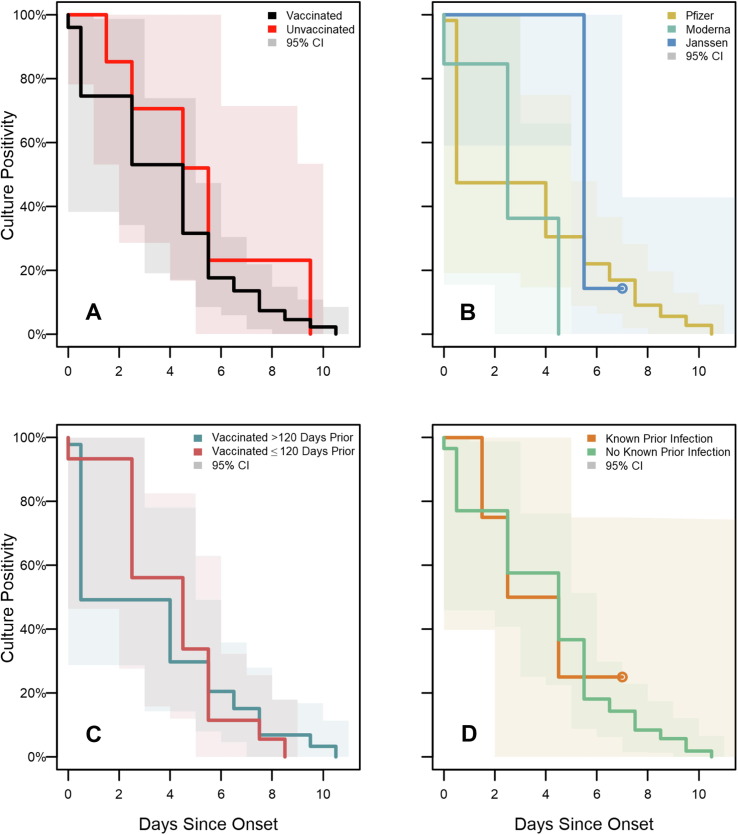
Of the 957 specimens collected, viral culture was performed on 283 (30 %); an additional 538 (56 %) were included as presumptive negative viral culture results due to an accompanying negative RT-PCR test (n = 461) or a positive RT-PCR test with a Ct value>35 (n = 77). Viral culture capture by day since onset stratified by vaccination status is detailed in Supplementary Fig. 2. Among the 821 specimens with a viral culture result, 75 (9 %) had a positive viral culture. Virus was recovered from 57/690 (8 %) of specimens from vaccinated participants, compared with 18/131 (14 %) of specimens from participants who were unvaccinated (p = 0.07).
No statistically significant difference was detected in the duration of viral culture positivity (Fig. 4 ) between participants who were vaccinated (median: 5 days) compared with those who were unvaccinated (median: 6 days; p = 0.192). (Viral culture results are illustrated as a function of days since onset and grouped by RT-PCR result in Supplementary Fig. 4). Cumulative hazard functions indicate overall shorter culture positivity for vaccinated participants who received the Moderna vaccine than those who received Pfizer (p = 0.048) or Janssen vaccines (p = 0.003), but there was no significant difference between recipients of Pfizer and Janssen vaccines (p = 0.12). No statistically significant differences in duration of culture positivity were detected when stratified according to time since vaccination (p = 0.79) or known prior infection (p = 0.99).
Fig. 4.
SARS-CoV-2 viral culture test positivity survival curves for enrolled participants, Federal prison, Texas, July 12—August 9, 2021. Panels illustrate the results of Turnbull estimation survival functions with a primary endpoint of last positive viral culture test result. Specimens were included as presumptive negative results if no culture was performed but were accompanied by negative RT-PCR results or positive RT-PCR results with Ct > 35. Solid lines indicate nonparametric maximum likelihood estimates and shaded regions correspond to 95 % confidence intervals estimated through modified bootstrap. Survival functions are plotted by Turnbull interval midpoints. When Turnbull intervals are bounded by positive infinity (resulting from right-censoring in subgroups), survival functions are truncated by open points at the rightmost non-infinite intervals. Onset was determined to be either a) date of first onset of self-reported symptom(s) meeting the case definition of COVID-19 or b) date of first positive diagnostic SARS-CoV-2 test, whichever occurred first. Panel A depicts viral culture positivity by vaccination status. Panel B depicts positivity by vaccine product among vaccinated participants. Panel C depicts positivity according to the time from completion of a COVID-19 vaccine/series to onset. Panel D depicts positivity according to history of known prior SARS-CoV-2 infection.
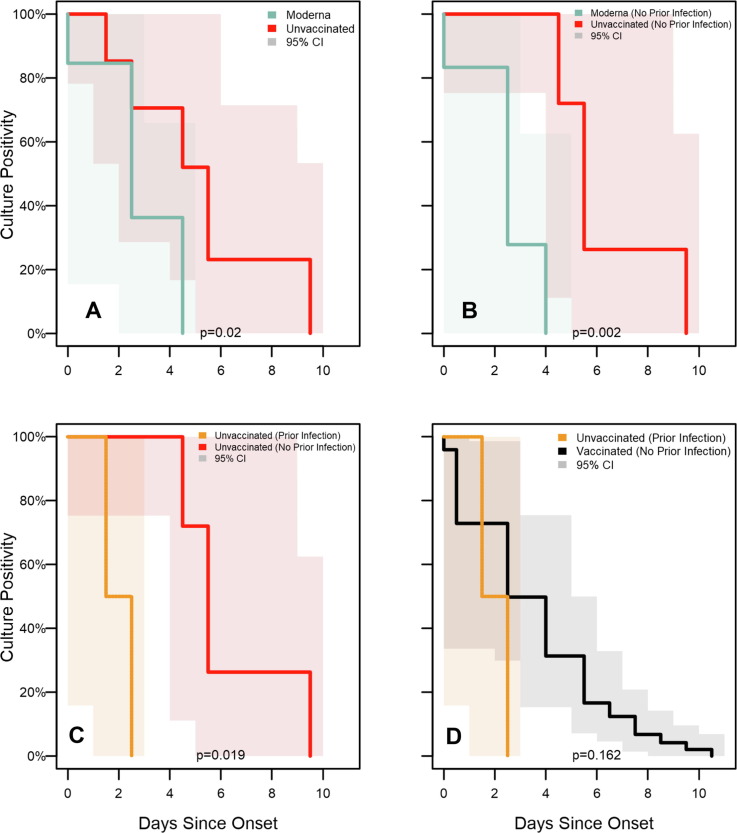
3.5. Post-Hoc pairwise testing
Due to the high degree of correlation between vaccine product, recency of vaccination, and history of known prior infection, subgroup identifiability limits the use of multivariate models to examine interactions between these factors. Instead, a series of post-hoc pairwise comparisons were performed across identifiable subgroups (Supplemental Table 3). In these comparisons (Fig. 5 ), duration of viral culture positivity was significantly shorter among Moderna vaccine recipients compared to unvaccinated participants (medians: 3 days vs 6 days; p = 0.02); this difference was more pronounced when restricted to participants with no known history of prior infection (medians: 3 days vs 6 days; p = 0.002). Additionally, among unvaccinated participants, persons with known prior infection were positive for significantly shorter than those without a known prior infection (medians: 2 days vs 6 days; p = 0.019). Duration of culture positivity was not significantly different between vaccinated participants with no known history of prior infection and unvaccinated participants with known prior infection (medians: 3 days vs 2 days; p = 0.162).
Fig. 5.
Subgroup analysis of SARS-CoV-2 viral culture test positivity survival curves for enrolled participants, Federal prison, Texas, July 12—August 9, 2021. Panels illustrate the results of Turnbull estimation survival functions with a primary endpoint of last positive viral culture test result. Specimens were included as presumptive negative results if no culture was performed but were accompanied by negative RT-PCR results or positive RT-PCR results with Ct > 35. Solid lines indicate nonparametric maximum likelihood estimates and shaded regions correspond to 95 % confidence intervals estimated through modified bootstrap. P-values of differences in survival functions (using the generalized Wilcoxon-Mann-Whitney method) are displayed at the bottom of each panel. Survival functions are plotted by Turnbull interval midpoints. When Turnbull intervals are bounded by positive infinity (resulting from right-censoring in subgroups), survival functions are truncated by open points at the rightmost non-infinite intervals. Onset was determined to be either a) date of first onset of self-reported symptom(s) meeting the case definition of COVID-19 or b) date of first positive diagnostic SARS-CoV-2 test, whichever occurred first. Panel A depicts viral culture positivity between Moderna vaccine recipients and unvaccinated participants. Panel B restricts this analysis to participants with no known prior infection, comparing positivity between Moderna vaccine recipients and unvaccinated participants Panel C restricts to unvaccinated participants and compares positivity between participants with and without known prior infection. Panel D compares positivity between vaccinated participants (any full primary series) without know prior infection versus unvaccinated participants with known prior infection.
4. Discussion
During a high-transmission outbreak of the SARS-CoV-2 Delta variant in a prison setting, we failed to find different durations of RT-PCR positivity, Ct values, or durations of viral culture positivity between persons who had received any primary series vaccine and persons who were unvaccinated. However, persons who had specifically received the Moderna vaccine demonstrated shorter duration of viral culture positivity than unvaccinated persons. In our data, outcomes varied by vaccine product as persons who received the Moderna vaccine had a shorter duration of culture positivity compared with Pfizer or Janssen vaccine recipients. Finally, among unvaccinated participants, the duration of viral culture positivity was shorter among those with prior infection than those without a known prior infection.
Collectively, our findings suggest that potential infectiousness may be decreased in some vaccinated persons with SARS-CoV-2 infection and in some unvaccinated persons with prior infection. However, vaccination with a primary series does not guarantee decreased infectiousness in those who become infected, and many vaccinated persons in our analysis demonstrated longer duration of viral culture positivity than some unvaccinated persons. As illustrated in this outbreak (in which 74 % of incarcerated persons in affected housing units became infected), introduction of infection into congregate and correctional settings can accelerate rapidly and result in hospitalizations and deaths [6]. In such settings, clinicians and public health practitioners should consider vaccinated persons who become infected as not significantly less infectious than unvaccinated persons for the purposes of public health action.
As viral infections in vaccinated persons can result from either a failure to mount a protective immune response following initial vaccination or a gradual waning of immunological protection following initially robust protection, the infectiousness of vaccinated persons may be variable. It is plausible that some participants in this investigation who became infected despite vaccination had weak or waning vaccine-induced protection and were therefore similar to unvaccinated persons in the markers of transmission potential that we evaluated.
This report adds to a limited body of scientific literature evaluating the transmission potential of SARS-CoV-2 infections in vaccinated persons. Reports of infections in vaccinated persons have found mixed results using markers of transmission potential, and no longitudinal studies of viral culture characteristics in vaccinated persons with Delta infections have been published. A multi-site serial testing investigation involving Alpha (B.1.1.7) and Gamma (P.1) infections found that duration of culture positivity was shorter among vaccinated persons compared with unvaccinated persons [13], [14]. One report using surveillance data found lower Ct values among unvaccinated persons, but this difference was only observed for two of three RT-PCR probes and only during one of three months [15]. One cross-sectional report found no difference in Ct value by vaccination status [2]. However, extrapolating from cross-sectional and surveillance data may be challenging without data to account for timing of specimen collection in the course of infection. Nevertheless, this finding is corroborated by analysis of a clinical convenience sample which found vaccination did not impact Ct values and reduced viral recovery of Alpha variant but did not reduce recovery of Delta variant virus [16]; similar findings were mirrored by two retrospective health-system cohorts [17], [18]. A report of health system workers found that viral culture positivity was reduced in vaccinated persons despite similar Ct values as those in unvaccinated persons [3]. A separate report found that early in the clinical course of infection, Ct values were comparable between vaccinated and unvaccinated persons, but among individuals who presented to care later in their course of illness, Ct values were higher in vaccinated persons [19]. A study of household transmission of Delta infections found similar peak viral loads regardless of vaccination status, but noted faster declines in vaccinated persons [20]. Cumulatively, available data have not clearly or consistently identified markers of reduced transmission potential in vaccinated persons with SARS-CoV-2 infection. This report, which to our awareness represents the first longitudinal investigation of viral culture characteristics of vaccinated persons with Delta variant infections, further demonstrates the potential of vaccinated persons to contribute to SARS-CoV-2 transmission.
While our investigation found mixed evidence of reduced transmission potential from vaccinated persons with infection, vaccination is known to reduce the risk of infection [6], [21], which prevents secondary transmission. In addition, vaccination remains a strongly protective factor against morbidity and mortality due to SARS-CoV-2 [22]. Protection against infection, morbidity, and mortality underscores the importance of maximizing vaccination coverage, particularly in settings where challenges to physical distancing can result in rapid, widespread transmission when infections do occur.
The evidence that vaccinated persons can transmit SARS-CoV-2 to others suggests that there is continued risk of widespread outbreaks when the virus is introduced into congregate settings, even when vaccination coverage is high. While quarantine is not recommended after exposure to someone with COVID-19 in general settings, some high-risk congregate settings may still choose to use quarantine protocols to limit transmission, especially when the population has a high prevalence of underlying health conditions associated with severe COVID-19 [7], [8]. Our findings suggest that when facilities use quarantine as a COVID-19 prevention strategy, they should implement it for anyone who is exposed, regardless of vaccination status.
This report is subject to several limitations. Due to the small proportion of participants who were unvaccinated (16 %), statistical comparisons on the basis of vaccination status were underpowered, and negative findings reported here warrant cautious interpretation. Only four participants had known prior infection, of which a higher proportion occurred in those unvaccinated; therefore, these participants may appear to have slightly greater immunological protection than those without prior infection. On average, unvaccinated participants enrolled earlier in the outbreak and later in their course of infection than vaccinated participants; we utilized Turnbull estimation in survival analyses to account for the possibility of interval censoring in this population. All symptom data was self-reported and collected at the end of the specimen collection period, which may have impacted the accuracy of participants’ recall related to the date of symptom onset. Ct values are semi-quantitative indicators of viral RNA levels and cannot be interpreted as quantitative markers of viral load or infectiousness. To avoid drawing quantitative conclusions around Ct values, we conservatively utilized non-parametric rank-based statistics (Mann-Whitney and Kruskal-Wallis) with Bonferroni correction to describe Ct values in this investigation. Information on prior SARS-CoV-2 infection was obtained from medical records; persons with earlier infections that were undiagnosed or diagnosed prior to incarceration and not documented in the BOP medical record may not have been correctly characterized. We did not attempt viral culture for 561 specimens with Ct > 35 and classified them as presumptively negative. This decision was based on negative viral culture results from 25/25 specimens with Ct > 35 for which viral culture was performed during this investigation, as well as previously published findings demonstrating an inability to recover viable virus from specimens that were RT-PCR negative [23]. Finally, our investigation took place in a setting and time in which prior infections were less common, vaccine boosters were not yet recommended, hybrid immunity (i.e., vaccination and prior infection) was uncommon, and the Delta variant was predominant, factors which may limit the generalizability of our findings to future outbreaks.
In this investigation, we found mixed evidence of reductions in transmission potential between vaccinated persons and persons who were unvaccinated. While recipients of a Moderna primary series demonstrated reduced viral culture positivity, recipients of other vaccines did not. Therefore, our findings indicate that prevention and mitigation measures should be applied without regard to vaccination status for persons in high-risk settings or those with significant exposures. Our data add to a growing body of evidence characterizing transmission potential from vaccinated persons. Future studies of transmission potential from vaccinated persons with infection, incorporating similar laboratory-based markers as well as evidence of transmission from secondary attack rates and network analysis, may help to further describe the contributions of vaccinated persons in chains of transmission as the pandemic evolves and new variants emerge.
Footnotes
* 45 C.F.R. part 46, 21C.F.R. part 56; 42 U.S.C. Sect. 241(d); 5 U.S.C. Sect. 552a; 44 U.S.C. Sect. 3501 et seq.
Disclaimer. The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of Centers for Disease Control and Prevention (CDC).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Mario Cordova, Torrey Haskins, Jennifer Jackson, Joshua Jett, Barbara Swopes, Tammy Winbush, Federal Bureau of Prisons.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2022.11.045.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
Data availability
Anonymized and de-identified data produced in this analysis may be made available upon reasonable request to the authors.
References
- 1.Mancuso M., Eikenberry S.E., Gumel A.B. Will vaccine-derived protective immunity curtail COVID-19 variants in the US? Infect Dis Model. 2021;6:1110–1134. doi: 10.1016/j.idm.2021.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Riemersma KK, Grogan BE, Kita-Yarbro A, Halfmann PJ, Segaloff HE, Kocharian A, et al. Shedding of Infectious SARS-CoV-2 Despite Vaccination. medRxiv. 2021: 2021.07.31.21261387. 10.1101/2021.07.31.21261387. [DOI] [PMC free article] [PubMed]
- 3.Shamier MC, Tostmann A, Bogers S, de Wilde J, IJpelaar J, van der Kleij WA, et al. Virological characteristics of SARS-CoV-2 vaccine breakthrough infections in health care workers. medRxiv. 2021: 2021.08.20.21262158. 10.1101/2021.08.20.21262158.
- 4.Brown CM, Vostok J, Johnson H, Burns M, Gharpure R, Sami S, et al. Outbreak of SARS-CoV-2 Infections, Including COVID-19 Vaccine Breakthrough Infections, Associated with Large Public Gatherings - Barnstable County, Massachusetts, July 2021. MMWR Morb Mortal Wkly Rep. 2021; 70(31): 1059-62. 10.15585/mmwr.mm7031e2. [DOI] [PMC free article] [PubMed]
- 5.Centers for Disease Control and Prevention. COVID Data Tracker. Atlanta, GA. US Department of Health and Human Services. Accessed October 16, 2021. [Available from: https://covid.cdc.gov/covid-data-tracker/#trends_dailycases.
- 6.Hagan L.M., McCormick D.W., Lee C., Sleweon S., Nicolae L., Dixon T., et al. Outbreak of SARS-CoV-2 B.1.617.2 (Delta) Variant Infections Among Incarcerated Persons in a Federal Prison - Texas, July-August 2021. MMWR Morb Mortal Wkly Rep. 2021;70(38):1349–1354. doi: 10.15585/mmwr.mm7038e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hagan L.M., Williams S.P., Spaulding A.C., Toblin R.L., Figlenski J., Ocampo J., et al. Mass Testing for SARS-CoV-2 in 16 Prisons and Jails - Six Jurisdictions, United States, April-May 2020. MMWR Morb Mortal Wkly Rep. 2020;69(33):1139–1143. doi: 10.15585/mmwr.mm6933a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maruschak L., Bronson J., Alper M. DC. US Department of Justice, Bureau of Justice Statistics; Washington: 2021. Medical problems reported by prisoners, survey of prison inmates, 2016. Available from: https://bjs.ojp.gov/sites/g/files/xyckuh236/files/media/document/mprpspi16st.pdf. [Google Scholar]
- 9.McMichael T.M., Clark S., Pogosjans S., Kay M., Lewis J., Baer A., et al. COVID-19 in a Long-Term Care Facility - King County, Washington, February 27-March 9, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(12):339–342. doi: 10.15585/mmwr.mm6912e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harcourt J., Tamin A., Lu X., Kamili S., Sakthivel S.K., Murray J., et al. Severe Acute Respiratory Syndrome Coronavirus 2 from Patient with Coronavirus Disease, United States. Emerg Infect Dis. 2020;26(6):1266–1273. doi: 10.3201/eid2606.200516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Council of State and Territorial Epidemiologists. Update to the standardized surveillance case definition and national notification for 2019 novel coronavirus disease (COVID-19). Accessed October 15, 2021. [Available from: https://cdn.ymaws.com/www.cste.org/resource/resmgr/21-ID-01_COVID-19_updated_Au.pdf.
- 12.Fay MP, Shaw PA. Exact and Asymptotic Weighted Logrank Tests for Interval Censored Data: The interval R package. J Stat Softw. 2010;36(2). 10.18637/jss.v036.i02. [DOI] [PMC free article] [PubMed]
- 13.Ke R., Martinez P.P., Smith R.L., Gibson L.L., Achenbach C.J., McFall S., et al. Longitudinal analysis of SARS-CoV-2 vaccine breakthrough infections reveal limited infectious virus shedding and restricted tissue distribution. medRxiv. 2021 doi: 10.1101/2021.08.30.21262701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ke R., Martinez P.P., Smith R.L., Gibson L.L., Mirza A., Conte M., et al. Daily sampling of early SARS-CoV-2 infection reveals substantial heterogeneity in infectiousness. medRxiv. 2021 doi: 10.1101/2021.07.12.21260208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Griffin JB, Haddix M, Danza P, Fisher R, Koo TH, Traub E, et al. SARS-CoV-2 Infections and Hospitalizations Among Persons Aged ≥16 Years, by Vaccination Status - Los Angeles County, California, May 1-July 25, 2021. MMWR Morb Mortal Wkly Rep. 2021; 70(34): 1170-6. 10.15585/mmwr.mm7034e5. [DOI] [PMC free article] [PubMed]
- 16.Luo C.H., Morris C.P., Sachithanandham J., Amadi A., Gaston D., Li M., et al. Infection with the SARS-CoV-2 delta variant is associated with higher infectious virus loads compared to the alpha variant in both unvaccinated and vaccinated individuals. medRxiv. 2021 doi: 10.1101/2021.08.15.21262077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Christensen PA, Olsen RJ, Long SW, Subedi S, Davis JJ, Hodjat P, et al. Delta variants of SARS-CoV-2 cause significantly increased vaccine breakthrough COVID-19 cases in Houston, Texas. medRxiv. 2021: 2021.07.19.21260808. 10.1101/2021.07.19.21260808. [DOI] [PMC free article] [PubMed]
- 18.Eyre DW, Taylor D, Purver M, Chapman D, Fowler T, Pouwels KB, et al. The impact of SARS-CoV-2 vaccination on Alpha & Delta variant transmission. medRxiv. 2021: 2021.09.28.21264260. 10.1101/2021.09.28.21264260.
- 19.Chia PY, Xiang Ong SW, Chiew CJ, Ang LW, Chavatte J-M, Mak T-M, et al. Virological and serological kinetics of SARS-CoV-2 Delta variant vaccine-breakthrough infections: a multi-center cohort study. medRxiv. 2021: 2021.07.28.21261295. 10.1101/2021.07.28.21261295. [DOI] [PMC free article] [PubMed]
- 20.Singanayagam A, Hakki S, Dunning J, Madon KJ, Crone M, Koycheva A, et al. Community transmission and viral load kinetics of SARS-CoV-2 Delta (B.1.617.2) variant in vaccinated and unvaccinated individuals. Preprint Available at SSRN: https://ssrncom/abstract=3918287 or http://dxdoiorg/102139/ssrn3918287. 2021. [DOI] [PMC free article] [PubMed]
- 21.Pouwels K.B., Pritchard E., Matthews P.C., Stoesser N., Eyre D.W., Vihta K.D., et al. Effect of Delta variant on viral burden and vaccine effectiveness against new SARS-CoV-2 infections in the UK. Nat Med. 2021 doi: 10.1038/s41591-021-01548-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tenforde M.W., Patel M.M., Ginde A.A., Douin D.J., Talbot H.K., Casey J.D., et al. Effectiveness of SARS-CoV-2 mRNA Vaccines for Preventing Covid-19 Hospitalizations in the United States. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab687. [DOI] [Google Scholar]
- 23.Ford L., Lee C., Pray I.W., Cole D., Bigouette J.P., Abedi G.R., et al. Epidemiologic characteristics associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antigen-based test results, real-time reverse transcription polymerase chain reaction (rRT-PCR) cycle threshold values, subgenomic RNA, and viral culture results from university testing. Clin Infect Dis. 2021;73(6):e1348–e1355. doi: 10.1093/cid/ciab303. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymized and de-identified data produced in this analysis may be made available upon reasonable request to the authors.