Abstract
Background
Immune thrombocytopenia (ITP) has been reported following COVID-19 vaccination. After index case fatalities, there was concern among patients both with and without a prior history of ITP in Australia.
Objectives
To describe treatment outcomes of ITP after COVID-19 vaccination and compare relapsed vs historical pre-COVID-19 ITP cohorts.
Methods
We collected ITP cases in Australia within 6 weeks of receiving any COVID-19 vaccination as part of primary vaccination (up to October 17, 2021). Second, we reviewed platelet charts in a historical ITP cohort to determine whether platelet variability was distinct from relapsed ITP after vaccination.
Results
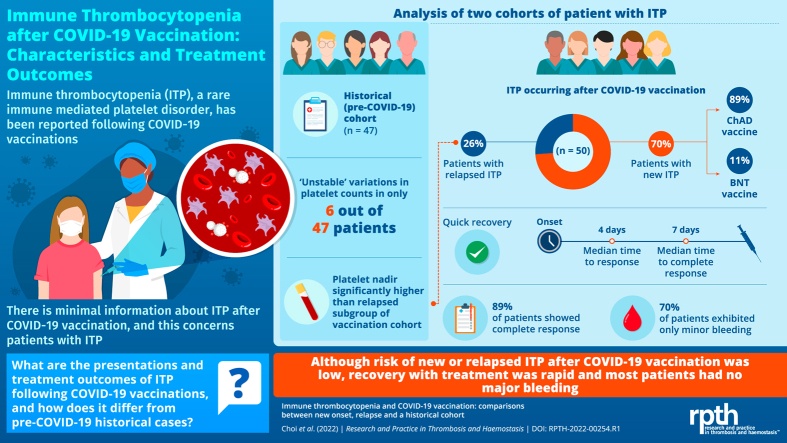
We report on 50 patients (37 de novo, 13 relapsed ITP) vaccinated from March 22, 2021, to October 17, 2021. Although there was 1 fatality, bleeding was otherwise mostly minor: (70% WHO bleeding grade <2). De novo ITP was more likely after AstraZeneca ChAdOx1 nCoV-19 (89%) than Pfizer BNT162b2 (11%). Most patients responded quickly (median, 4 days; complete response, 40 of 45 [89%]). In the historical cohort, only 6 of 47 patients exhibited platelet variability (>50% decrease and platelets <100 × 109/L), but median platelet nadir was significantly higher than vaccination relapse (27 vs 6 × 109/L, P =.005).
Conclusion
ITP was more frequently reported after AstraZeneca ChAdOx1 nCoV-19 than Pfizer BNT162b2 vaccination. Standard ITP treatments remain highly effective for de novo and relapsed ITP (96%). Although thrombocytopenia can be severe after vaccination, bleeding is usually mild. Despite some sampling bias, our data do not support a change in treatment strategies for patients with ITP after vaccination.
KeyWords: BNT162 vaccine, ChAdOx1 nCov-19, COVID-19 vaccines, immune thrombocytopenia, treatment outcome, vaccination
Graphical abstract
Essentials
-
•
Immune thrombocytopenia (ITP) has been reported after COVID-19 vaccinations.
-
•
Fifty cases of ITP after vaccination were reviewed for distinguishing features and treatment outcomes.
-
•
Most cases (36 of 50) in our study presented after first-dose ChAdOx1 (AstraZeneca) vaccination.
-
•
ITP after vaccination presents with infrequent bleeding and responds well to conventional treatments.
1. Introduction
Rare immune-mediated complications have been reported following COVID-19 vaccination [[1], [2], [3], [4]]. Immune thrombocytopenia (ITP) has also been described following COVID-19 and other vaccinations [[5], [6], [7], [8]]. A Scottish National Registry study examined hospital-coded and general practice data to identify a small increased incidence of ITP diagnosis within 4 weeks of AstraZeneca ChAdOx1 nCoV-19 (ChAd) vaccination but published information on treatment outcomes is limited [9,10].
Rare thrombotic complications with thrombocytopenia after adenovirus-based COVID-19 vaccinations were well publicized and have led to a heightened scrutiny of platelet counts and cautious observation for symptoms of thrombosis and bleeding [11,12]. Against this backdrop, segments of the population expressed concern and anxiety with vaccination, particularly those with pre-existing conditions related to autoimmunity and thrombocytopenia.
To better understand the apparent emerging phenomenon and guide future management considerations, we established an online registry to collaboratively collect clinically relevant characteristics and treatment outcomes of patients diagnosed with ITP following COVID-19 vaccinations in Australia. The aim of this study was to contrast the presentations of ITP in patients after vaccination (de novo vs relapse from prior diagnosis) with those in a pre-COVID-19 historical cohort, while also considering possible differences in outcomes between ChAd vs Pfizer BNT162b2 (BNT) vaccination.
2. Methods
2.1. Post–COVID-19 cohort
We established a post–COVID-19 ITP registry and advertised it to all hematologists in Australia from May 12, 2021, through society networks targeting hemostasis experts via medical society websites, emails, social media, and word-of-mouth at conference meetings.
We aimed to collect data on any case of adult ITP diagnosed within 6 weeks of any COVID-19 vaccination received from the beginning of the national rollout to October 17, 2021. We elected to perform an interim analysis of available data by January 31, 2022, as the general Australian population had begun receiving COVID-19 vaccination third dose boosters.
Participating hematologists accessed a secure web-based software platform (REDCap electronic data capture tools hosted at ACT Health) and uploaded deidentified clinical data on adult patients aged 18 years and above, including patient demographics, platelet counts, bleeding assessments, and treatment outcomes for up to 3 months after presentation, from May 12, 2021, to January 31, 2022.
Hematologists did not register these cases as ITP if the clinical presentation suggested an alternative diagnosis, such as vaccine-induced immune-mediated thrombosis and thrombocytopenia (VITT) or thrombotic thrombocytopenia syndrome [13].
2.2. Historical cohort
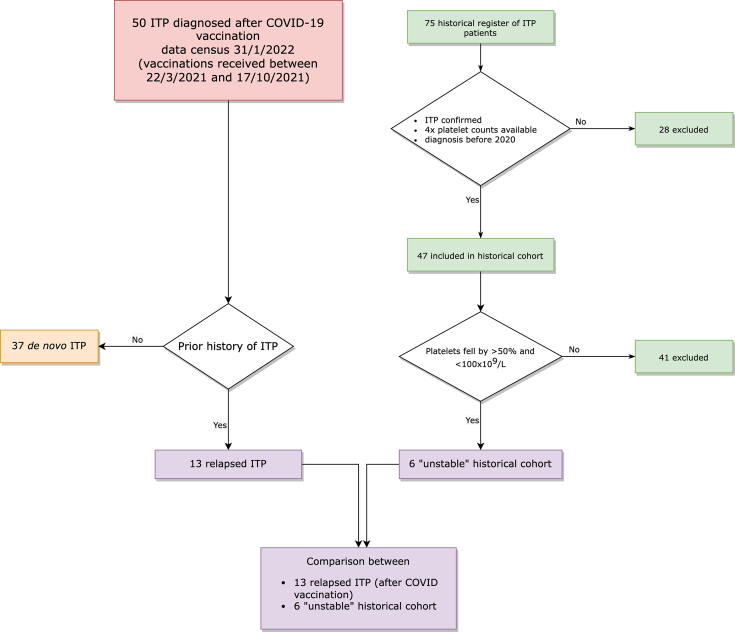
A cohort drawn from a historical platelet diseases registry of patients diagnosed with ITP at The Canberra Hospital from August 16, 2017, to December 31, 2019, was used as a prepandemic comparator. From a total registry of 75 patients, 28 cases were excluded because of alternative diagnoses, ITP diagnosis from 2020 onward, or insufficient platelet count results (4 values) available for comparison. The remaining 47 patients were all managed at an Australian tertiary referral hospital experienced at treating patients consistently with local and international guidelines [[14], [15], [16]].
2.3. ITP definition and severity
All ITP cases were diagnosed and verified by their treating hematologist with responses recorded using standard international consensus definitions (response [R], ≥30 × 109/L and at least double baseline without bleeding; complete response [CR], ≥100 × 109/L without bleeding; failure: loss of response and/or need for additional ITP-therapy), ITP-BAT (skin/mucosa/organ involvement with severity graded 0–4), and WHO criteria for bleeding (graded 0–4) [[17], [18], [19]]. Only patients presenting within 6 weeks of any COVID-19 vaccination were included in this analysis in line with other studies of postvaccination ITP [20].
2.4. Population vaccination statistics
Population vaccination statistics (number of vaccines administered; doses: first or second) for the period (March 22 to October 17, 2021) during which ITP cases were collected for the present study were obtained from the Therapeutic Goods Administration (TGA) [21], along with general population statistics from the Australian Bureau of Statistics for September 2021 (released March 17, 2022) [22]. From a population of 20.0 million eligible vaccine recipients in Australia aged 18 years and above, 32.7 million COVID-19 vaccination doses had been administered by October 17, 2021: 19.6 million BNT, 12.6 million ChAd, and 397,000 Moderna mRNA-1273 [21]. At that time, 18.3 million of these were first doses (56.0%) and 14.4 million were second doses (44.0%). The Australian public health authority (TGA) acknowledged receipt of 85 cases of suspected ITP in relation to COVID-19 vaccination by this same date. However, their clinical features, treatment outcomes, and confirmation of diagnosis were unknown.
2.5. Case number estimation
Total number of estimated cases of ITP was calculated as follows:
As a temporal relationship to COVID-19 vaccination was defined by presentation with thrombocytopenia within 6 weeks of either dose, the total exposure to risk varied from a maximum of 84 days after 2 consecutive ChAd vaccinations to only 63 days after 2 BNT vaccinations, as the recommended dosing interval was only 3 weeks for BNT (overlapping risk period of 3 weeks after second BNT vaccination—see Supplementary Figure 1). For our calculations, we assumed patients completed their primary vaccination sequence with no switching from BNT to ChAd vaccinations as available evidence suggests that this occurred rarely in this phase of the pandemic. Although there was also an accelerated program to shorten the primary ChAd vaccination sequence to 4 weeks (70 days exposure risk) during the Delta outbreak in certain jurisdictions with the highest risk, there were no data available to estimate how many individuals this affected [23]. Based on the annualized incidence estimates from French insurance-based literature (2.9 per 100,000) [24], the prepandemic estimated case number of ITP among ChAd recipients was as follows:
Following BNT vaccination,
Thus, we estimated a total of 91 de novo cases of ITP among vaccination recipients based on prepandemic estimates.
2.6. Statistical analyses
Analyses were conducted in GraphPad Prism version 9.3.1 for macOS (GraphPad Software). Missing data were censored from analysis. Group differences were assessed using Mann–Whitney U-test for continuous variables and Fisher’s exact for categorical variables. Differences in paired platelet counts over time were assessed with Repeated Measures anova. Relative risk estimations between groups were computed with logistic regression. Significance was set at P ≤.05 (2-tailed).
We probed for differences in outcomes between vaccination types (ChAd vs BNT) and de novo vs relapse in patients with a prior history of ITP. Subanalyses were also performed to identify differences in outcomes based on number of vaccines received, severity of thrombocytopenia at presentation, antecedent influenza vaccination (within 1 month), and onset in days between vaccination and presentation.
In addition, possible similarities between patients with relapsed ITP after COVID-19 vaccination and “unstable” ITP patients with prepandemic data were explored. To provide a basis for comparison with patients who relapsed after COVID-19 vaccination, we defined “unstable” ITP patients in the historical cohort as those with evidence of a decrease in their platelet by >50% and to a count <100 × 109/L within their 4 most recent consecutive historical values. A schematic of cohort and group selection is presented in Figure 1.
Figure 1.
Flow diagram of recruitment, classification, and comparison with historical cohort. ITP, immune thrombocytopenia.
2.7. Ethical approval
Approval to collect deidentified information from medical records in the post–COVID-19 cohort was obtained from the ACT Health Human Research Ethics committee (ACT-HREC; 2021/ETH00723). Approval for the historical cohort data registry had been previously obtained from the ACT-HREC; 2017/ETH.2.17.029.
3. Objectives
To describe treatment outcomes of ITP diagnosed after COVID-19 vaccination and compare presenting features with a historical ITP cohort before COVID-19.
4. Results
During the period of our data collection (April 1, 2021, to January 31, 2022), we received and analyzed 50 ITP cases following COVID-19 vaccinations meeting criteria for inclusion (37 de novo and 13 relapses of a prior history of ITP) vaccinated between March 22, 2021, and October 17 (Figure 1). The TGA acknowledged the receipt of 85 case reports of ITP following COVID-19 vaccination during this same period of time [21], and thus, our cohort represents 59% of all suspected (but not confirmed by consensus clinical criteria) cases in Australia at that time.
The patients were 56% female with a median age of 63.5 years. The median time from vaccination to presentation with thrombocytopenia was 15 days, and the average time between presentation with thrombocytopenia and enrolment onto the study was 110 days. Demographics are presented in Table 1. Forty patients were diagnosed after ChAd and 10 after BNT. This equates to incident reporting rates of 3.2 cases/million ChAd doses and 0.5 cases/million BNT doses.
Table 1.
Demographics and presenting features of patients diagnosed with ITP within 6 weeks of COVID-19 vaccination.
| Characteristics | No. (%) | Range [IQR] | |
|---|---|---|---|
| Female | 28 (56) | ||
| Age in median, y | 63.5 | 20-97 [51.8-77.0] | |
| ChAd | 40 (80) | ||
| Prior history of ITP | 13 (26) | ||
| Time from vaccination to presentation in median, d | 15 | 1-41 [8.3-23.8] | |
| Platelet count at presentation (×109/L) | 7 | 0-87 [4.0-13.0] | |
| Platelet nadir (×109/L) | 5 | 0-50 [1.0-12.8] | |
| WHO bleeding grade | |||
| 0 | 8 (16) | ||
| 1 | 27 (54) | ||
| 2 | 9 (18) | ||
| 3 | 3 (6) | ||
| 4 | 2 (4) | ||
| 5 | 1 (2) | ||
| ITP-BAT skin (n = 35) | |||
| 0 | 8 (23) | ||
| 1 | 15 (43) | ||
| 2 | 6 (17) | ||
| 3 | 6 (17) | ||
| 4 | 0 | ||
| 5 | 0 | ||
| ITP-BAT mucosa (n = 35) | |||
| 0 | 21 (60) | ||
| 1 | 8 (23) | ||
| 2 | 3 (9) | ||
| 3 | 1 (3) | ||
| 4 | 3 (9) | ||
| 5 | 0 | ||
| ITP-BAT organ (n = 35) | |||
| 0 | 25 (71) | ||
| 1 | 5 (14) | ||
| 2 | 1 (3) | ||
| 3 | 3 (9) | ||
| 4 | 1 (3) | ||
| 5 | 1 (3) |
BAT, Bleeding Assessment Tool; ITP, immune thrombocytopenia.
Most patients responded quickly and completely (median time to response [TTR], 4 days [IQR, 2-7]; median time to complete response [TTCR], 7 days [IQR, 4-19]; overall R, 45 of 47 [96%]; and CR of 40 of 45 [89%]). First-line treatment used included 25 prednisolone/IVIg (2 with methylprednisolone loading), 7 prednisolone only, 7 dexamethasone/IVIg, 5 dexamethasone only, 1 IVIg only, and 6 observation only. One patient was started with prednisolone/mycophenolate combination upfront. Sex, age, antecedent influenza vaccination, and severity of thrombocytopenia had no significant impact on bleeding at presentation, response rates, relapse rates, time to response, or need for ongoing treatments at day 90 (Table 2). Bleeding was mostly minor, with 35 of 50 (70%) having WHO bleeding grade <2, but there was 1 notable fatality (see below). Platelet counts were not significantly lower in patients with WHO bleeding grade ≥2 compared with those with only WHO bleeding grade 0 to 1 (5 vs 8 × 109/L, P =.08).
Table 2.
Outcomes and subgroup comparisons; significant results in red.
| Outcomes | n | R (%) | TTR, median [IQR] | CR (%) | TTCR, median [IQR] | Second line needed (%) | Treatment at day 90 (%) | Platelets, 10 × 109/L [IQR] | ||
|---|---|---|---|---|---|---|---|---|---|---|
| At diagnosis | Day 30 | Day 90 | ||||||||
| Overall | 50 | 45/47 (96) | 4 [2-6] | 40/45 (89) | 7 [4-15.8] | 17/50 (34) | 14/30 (47) | 7 [4-13] | 111 [44-175] | 175 [62-218] |
| ChAd | 40 | 35/37 (95) | 3.5 [2-6.8] | 31/36 (86) | 6.5 [4-22.8] | 16/40 (40) | 11/25 (44) | 7.5 [3.3-14.5] | 108 [42.5-177] | 131.5 [60.3-215] |
| BNT | 10 | 10/10 (100) | 4 [2-5.8] | 9/9 (100) | 7 [5-14] | 1/10 (10) | 3/5 (60) | 5 [3.3-9] | 122.5 [41-157] | 202 [115-246.5] |
| De novo | 37 | 32/34 | 4 [2-11] | 30/33 | 7.5 [4-28.3] | 14/37 | 12/24 | 7 [3-15] | 116 [40-171.3] | 175 [67-214] |
| Relapsed | 13 | 13/13 | 4 [2-4] | 10/12 | 5 [4-6.3] | 3/13 | 2/6 | 6 [4.5-11.5] | 77 [44.5-224.5] | 82 [47.5-205.5] |
| First dose | 42 | 37/39 | 4 [2-8] | 32/37 | 6.5 [4-19] | 13/42 | 11/26 | 7.5 [3-16] | 123 [63-176] | 175 [67-214] |
| Second dose | 8 | 8/8 | 3 [2.3-4.8] | 8/8 | 7 [4.5-20] | 4/8 | 3/4 | 5 [4-8] | 40 [22-170] | 164 [51-288] |
| Flu vaccine | 5 | 5/5 | 18 [2.5-59] | 5/5 | 23 [6.5-59] | 3/5 | 1/3 | 5 [1.5-38] | 144 [46-168] | 163 [90-282] |
| No flu vaccine | 45 | 40/42 | 4 [2-5.5] | 35/40 | 6 [4-15] | 14/45 | 13/27 | 7 [4-13] | 110 [43-175] | 175 [55-218] |
| Plt <10x109/L at presentation | 35 | 31/33 | 4 [2-6] | 27/32 | 6.5 [4-15] | 15/35 | 12/21 | 3 [1-5] | 121 [32-175] | 180 [55-220] |
| Plt ≥10x109/L | 15 | 14/14 | 4 [2-12] | 13/13 | 7 [4.8-30] | 2/15 | 2/9 | 15 [12-27] | 108 [74-170] | 110 [71-204] |
| Presentation from vaccination | ||||||||||
| <7 d | 12 | 12/12 | 3 [2-4.8] | 10/12 | 6 [3.8-9.3] | 5/12 | 6/7 | 7 [5-8] | 53 [34-138] | 82 [49-181] |
| ≥7 d | 38 | 33/35 | 4 [2-7.3] | 30/33 | 7 [4-22] | 12/38 | 8/23 | 7 [3-14] | 125 [73-178] | 178 [74-232] |
| <14 d | 23 | 23/23 | 4 [3-8] | 18/22 | 7 [5-37] | 8/23 | 8/14 | 5 [3-8] | 73 [26-151] | 146 [54-207] |
| ≥14 d | 37 | 22/24 | 3 [2-5] | 22/23 | 6 [4-18] | 9/27 | 6/16 | 10 [4-17] | 138 [104-178] | 175 [65-227] |
| Treatment | ||||||||||
| Prednisone | 31 | 28/29 | 3.5 [2-5.8] | 27/28 | 7 [5-15] | 11/31 | 10/17 | 5 [3-13] | 149 [93-206] | 180 [82-220] |
| Dexamethasone | 12 | 11/12 | 2.5 [2-4] | 10/12 | 3 [3-41] | 6/12 | 4/9 | 5 [3.3-10] | 36 [19-91] | 85 [33-211] |
| IVIg used | 33 | 31/32 | 3 [2-5] | 30/32 | 7 [4-12] | 14/33 | 11/20 | 5 [3-11] | 135 [48-178] | 178 [60-219] |
| No IVIg | 17 | 14/15 | 4.5 [4-27] | 10/13 | 18 [4.5-45] | 3/17 | 3/10 | 12 [5-27] | 75 [20-110] | 112 [71-230] |
CR, complete response; IVIg, intravenous immunoglobulin; TTCR, time to complete response; TTR, time to response.
A 61-year-old woman presented on day 17 after her first ChAd vaccination with WHO bleeding grade 4, ITP-BAT S3M4O4. Her platelet count at presentation was 5 × 109/L, and despite treatment with pulse dexamethasone 40 mg and IVIg 1 g/kg on days 1 and 2, she developed catastrophic posterior fossa and cerebellar intracranial hemorrhage resulting in tentorial herniation within 24 hours of admission. Platelet transfusions and neurosurgical evacuation were attempted, but brainstem reflexes were lost and she died on day 4 of presentation.
Despite high response rates, 18 patients (39%) needed additional therapy. The most commonly used second-line therapy was 7 eltrombopag. Other options used included 5 rituximab, 3 romiplostim, 3 mycophenolate mofetil, 2 azathioprine, 1 vincristine, and 1 IVIg. A noticeable minority of patients (13%) experienced refractory disease requiring >2 lines of therapy. Overall, 10 patients (20%) required thrombopoietin receptor agonist therapy, and such immune-sparing approaches were preferred over rituximab or splenectomy.
Routine anti-platelet factor 4 (PF4) ELISA was not recommended by local guidelines for isolated thrombocytopenia. No patients presented with thrombosis. PF4 ELISA was positive in only 1 of 18 cases after ChAd (functional testing in this case was negative).
Community transmission of COVID-19 was very low in Australia at the time of the COVID-19 vaccine roll out, and none of the cases in this analysis was complicated by a prior history of COVID-19 infection.
4.1. Predictors of response
The following predictors of response were explored: time from vaccination to presentation, severity of thrombocytopenia at presentation, use of prednisolone vs dexamethasone, IVIg vs no IVIg, first-dose vs second-dose vaccination, sex, severity of bleeding, and concomitant or antecedent influenza vaccinations.
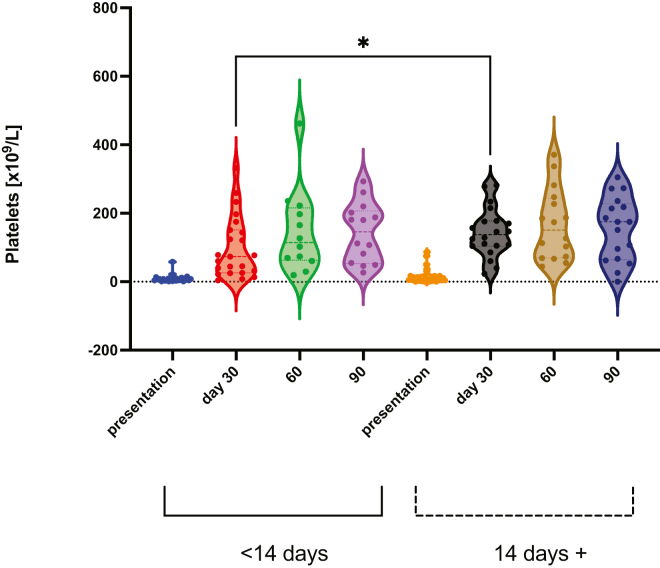
A shorter time from vaccination to presentation (<14 days vs ≥14 days) was associated with lower platelet count by day 30 (73 vs 138 × 109/L, P =.03), but this difference was not sustained (Figure 2).
Figure 2.
Platelet responses were higher in patients who presented with ITP later after vaccination (14 days or more), than those presenting earlier (<14 days) (median 73 vs 138 ×109/L, P =.03∗). ITP, immune thrombocytopenia.
Patients treated initially with dexamethasone (with or without IVIg) had lower platelet counts at day 30 than those treated with prednisolone (with or without IVIg) (median, 36 vs 149 × 109/L, P <.001), but 24 of 25 patients were still on prednisolone at that time compared with only 5 of 11 dexamethasone patients on any ongoing therapy. TTCR was shorter for dexamethasone recipients at 3 vs 7 days for prednisolone (P =.03), but not TTR at 2.5 vs 3.5 days (P =.21).
Upfront IVIg recipients had lower platelet counts at presentation as expected (5 vs 12 × 109/L, P =.02), but by day 30, their platelet counts were higher 135 vs 75 × 109/L, P =.02). Even though TTR was shorter (3 vs 4.5 days, P =.007), there was no reduction in the need for second-line therapy, which was still required in 14 of 33 (42%) IVIg recipients compared with 3 of 17 (18%) patients who did not receive IVIg. In addition, 12 of 15 (80%) patients with WHO bleeding grade ≥2 received IVIg upfront compared with 21 of 35 (60%) with lesser grades of bleeding.
4.2. Subgroup analyses
Key summary differences in presentation and outcomes based on vaccination received and de novo vs relapsed ITP are presented in Tables 3 and 4, respectively.
Table 3.
Subgroup analysis of ChAd vs BNT demographics and outcomes. BNT patients were younger, but this was expected because the vaccination policy median age was 35 vs 68 (P <.001).
| ChAd | BNT | OR (95% CI) | P | |
|---|---|---|---|---|
| Demographics | ||||
| Male | 20 | 2 | 4.0 (0.74-20) | .15 |
| Female | 20 | 8 | ||
| De novo | 33 | 4 | 7.1 (1.7-26) | .01 |
| Prior ITP | 7 | 6 | ||
| First dose | 36 | 6 | 6.0 (1.4-26) | .04 |
| Second dose | 4 | 4 | ||
| Time from vaccination to presentation, median, d [IQR] | 17.0 [10.0–25.25] | 7.5 [2.0–19.25] | (Mann–Whitney U-test) | .11 |
| Outcomes | ||||
| WHO bleeding 0–1 | 26 | 9 | 0.21 (0.018 to 1.3) | .25 |
| WHO bleeding ≥2 | 14 | 1 | ||
| Need for second-line therapy | 16 | 1 | 6.0 (0.95 to 69) | .13 |
| No need for second-line therapy | 24 | 9 | ||
| On treatment at day 90 | 12 | 4 | 0.56 (0.13 to 2.5) | .68 |
| Off treatment at day 90 | 16 | 3 |
ITP, immune thrombocytopenia.
Table 4.
Subgroup analysis of ChAd vs BNT demographics and outcomes between de novo vs relapsed ITP.
| De novo | Relapsed | OR (95% CI) | P | |
|---|---|---|---|---|
| Demographics | ||||
| Male | 19 | 4 | 2.4 (0.66-7.8) | .33 |
| Female | 18 | 9 | ||
| ChAd | 4 | 9 | 19 (3.6-83) | <.001 |
| BNT | 33 | 4 | ||
| First dose | 33 | 9 | 3.7 (0.89-14) | .18 |
| Second dose | 4 | 4 | ||
| Time from vaccination to presentation, median, d [IQR] | 17 [10–24.5] | 4 [2–25] | (Mann–Whitney U-test) | .03 |
| Outcomes | ||||
| WHO bleeding 0–1 | 23 | 12 | 0.14 (0.012-1.0) | .08 |
| WHO bleeding ≥2 | 14 | 1 | ||
| Need for second-line therapy | 23 | 3 | 5.5 (1.4-20) | .02 |
| No need for second-line therapy | 14 | 10 | ||
| On treatment at day 90 | 14 | 2 | 1.9 (0.36-11) | .67 |
| Off treatment at day 90 | 15 | 4 |
4.2.1. ChAd vs BNT
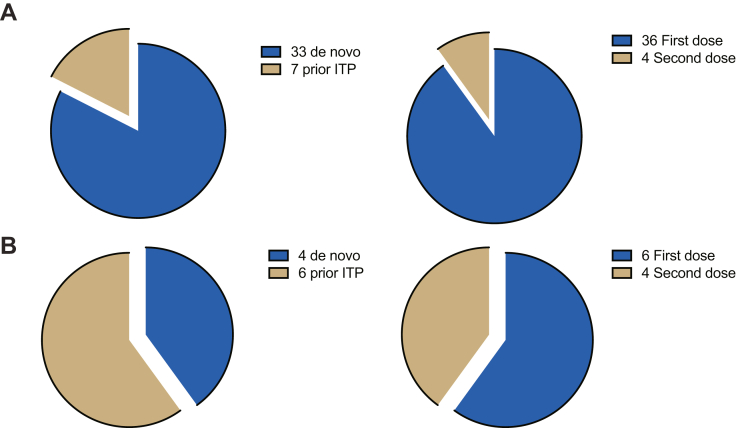
Forty cases presented after ChAd vaccination (36 first dose and 4 second dose) and 10 after BNT (6 first dose and 4 second dose). Compared with ITP diagnosed after BNT, ChAd-associated ITP presented (40 of 50) more frequently after the first dose than after the second dose (36 of 40 vs 4 of 40; OR, 6.0; 95% CI, 1.4-26; P =.04), with de novo cases more often than relapsed ITP (33 of 40 vs 7 of 40; OR, 7.1; 95% CI, 1.7-26; P =.01) (see Figure 3). However, there was no difference between ChAd and BNT in time from vaccination to ITP presentation, thrombocytopenia severity at presentation, ITP-BAT bleeding, TTR or TTCR, or ongoing platelet responses at days 30, 60, and 90 (see Supplementary Figure S2).
Figure 3.
(A) ITP after ChAd vaccinations were more commonly associated with de novo presentations (OR 7.1, 95% CI: 1.7 to 26, p = 0.01∗) and first dose vaccinations (OR 6.0, 95% CI: 1.4-26, P =.04∗) compared with (B) ITP after BNT vaccinations. ITP, immune thrombocytopenia.
However, patients with ITP after BNT seemed to have disease that was easier to treat: only 1 of 10 (10%) patients needed second-line treatments compared with 16 of 40 (40%) after ChAd (OR, 0.17; 95% CI, 0.014-1.1; P =.13), and only 1 of 10 (10%) presented with WHO bleeding grade >1 compared with 14 of 40 (35%) after ChAd (OR, 0.21; 95% CI, 0.018-1.3; ns) (see Supplemental Figures S3–5).
4.2.2. De novo vs relapsed ITP
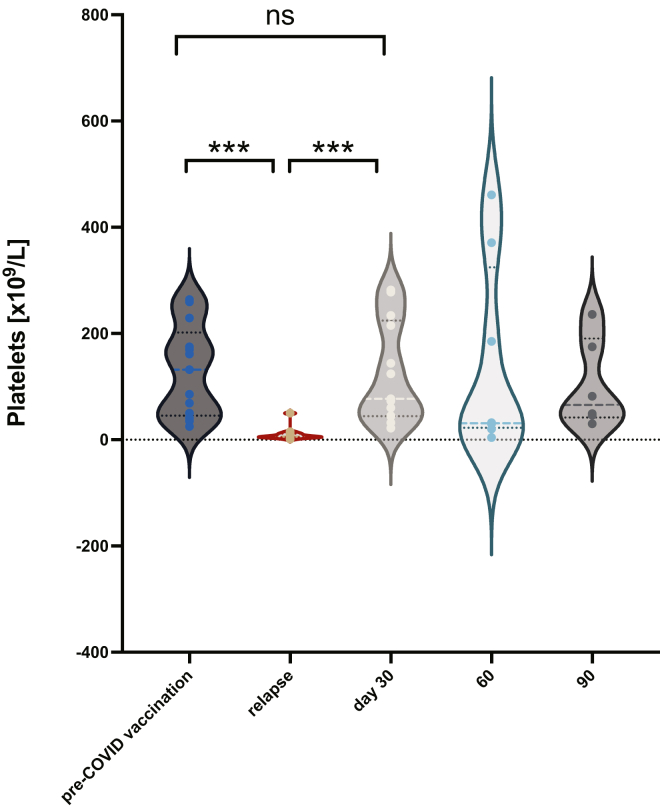
Compared with relapsed ITP (13 of 50), de novo cases (37 of 50) were more likely to follow ChAd (33 of 37) vaccination than BNT (4 of 37) (OR, 19; 95% CI, 3.6-83; P <.001), were more likely to require second-line therapy (14 of 17 vs 3 of 17; OR, 5.5; 95% CI, 1.4-20; P =.02), and presented after a longer time from vaccination (median, 17 vs 4 days; P =.03), but there was no difference in age, thrombocytopenia severity at presentation, ITP-BAT bleeding scores, TTR or TTCR, or ongoing platelet responses at days 30, 60, and 90 (see Supplementary Figure S6). Almost all cases (93%) with WHO bleeding scores of ≥2 had de novo ITP. Platelet counts in relapsed ITP after vaccination returned to pre-COVID-19 vaccination levels as soon as day 30 (Figure 4), with median platelet counts at day 30 of 77 × 109/L (IQR, 44.5-224.5) vs baseline platelets prevaccination of 94 × 109/L (IQR, 65.5-208.5) (Repeated Measures One-Way anova of platelet counts from baseline, to presentation, then day 30, P <.0001).
Figure 4.
Platelet counts for patients whose ITP relapsed after vaccination: platelets returned to pre-COVID-19 vaccination levels. ITP, immune thrombocytopenia.
4.2.3. De novo case reporting compared with case number estimation
There was no observed increase in the number of ITP cases reported after COVID-19 vaccination compared with prepandemic estimates of incident ITP. There were 37 new cases on our database compared with an estimated case number of 91. Although the reported number of cases after ChAd was 33 and was closer to the estimated number of 42, the reported number after BNT was only 4 in contrast to the estimated 49 cases.
Compared with a prepandemic estimate of annualized incident ITP of 2.9 per 100,000, the reporting incidence ITP was 1.13 (based on adult population statistics) and 1.41 per 100,000 (based on vaccination receipts). Specifically, after ChAd vaccination, the incidence was 2.72 per 100,000, but it was only 0.28 per 100,000 after BNT vaccination.
4.2.4. Background variability vs relapse of ITP after vaccination—cohort study
The historical prepandemic cohort (n = 47) comprised 25 women (53%) with a median age of 58.6 years (IQR, 35.0-74.2), with 13 receiving ongoing TPO-RA therapy (28%), 4 splenectomized (8.5%), with a median prior lines of therapy of 2 (IQR 1-3.5), and with 22 of them being treatment-free (47%) for the preceding 3 months.
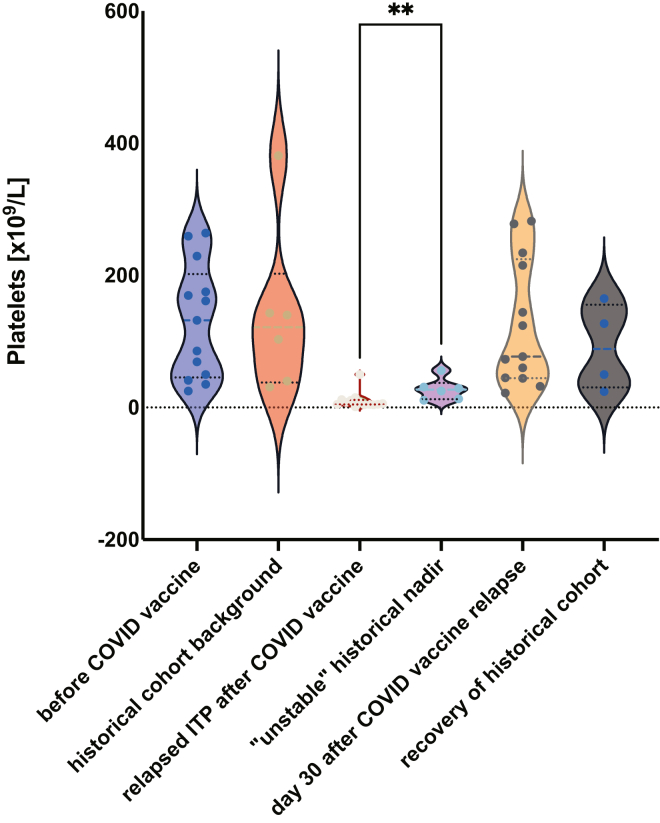
Only 6 of 47 patients (13%) had platelet counts that fluctuated sufficiently to be classified as “unstable.” These historically “unstable” patients with ITP had nadir platelet counts that were significantly higher than the presenting thrombocytopenia of relapsed ITP after COVID-19 vaccination (median, 27 vs 6 × 109/L; P =.005), even though platelet counts before decrease and after recovery were similar between the 2 cohorts (Figure 5). Demographics of the “unstable” historical cohort are compared with the relapsed ITP subset in Table 5.
Figure 5.
Platelet nadirs in ITP relapse after COVID-19 vaccination were significantly lower than nadirs in “unstable” chronic ITP patients in the historical cohort (median 27 vs 6 × 109/L, P =.005∗∗). ITP, immune thrombocytopenia.
Table 5.
Demographic comparison of “unstable” ITP drawn from historical cohort vs relapsed ITP after COVID-19 vaccination.
| “Unstable” historical cohort (n = 6) | Relapsed ITP after COVID-19 vaccination (n = 13) | P | |
|---|---|---|---|
| Sex (female, %) | 3 (50) | 9 (69) | |
| Age (median, y) | 71 | 64 | |
| Splenectomy (%) | 2 (33) | 1 (8) | |
| Stable off treatment (%) | – | 11 (85) | .001 |
| TPO-RA (%) | 5 (83) | 2 (15) | .01 |
| Baseline platelets (median, ×109/L) | 122 [IQR 38-203] | 132 [IQR 46-202] | |
| Nadir platelets (median, ×109/L) | 27 [IQR 13-37] | 6 [IQR 4.5-12] | .005 |
| Postnadir platelets (median, ×109/L) | 77 [IQR 45-225] | 89 [IQR 31-156] |
ITP, immune thrombocytopenia; TPO-RA, thrombopoietin receptor agonist.
5. Discussion
We report a higher number of ITP cases diagnosed within 6 weeks of ChAd vs BNT vaccination (3.2 cases/million doses vs 0.5 cases/million doses). Although there was a longer time window in which exposure to risk after ChAd could be assessed (12 weeks vs 9 weeks for BNT because of a shorter primary vaccination sequence of only 3 weeks—see Supplementary Figure 1), only 4 patients were diagnosed with ITP after second-dose ChAd (at days 6, 10, 19, and 29). Thus, despite the wider period of recruitment for ChAd compared with BNT, only relatively few cases of ITP were diagnosed late after second-dose ChAd.
Most de novo cases presented after the first dose of vaccination (33 of 37). However, 12 of 29 patients diagnosed with ITP after first dose ChAd presented between days 22 and 38 after vaccination (median, 26.5 days). Seven of these patients might have presented within 1 week of second-dose vaccination if ChAd was administered on the same 3-week schedule as BNT. Therefore, the dosing schedule of BNT may have underestimated the number of ITP cases after the first dose and inflated that after the second dose, which may explain the greater proportion of ITP cases observed after the second dose of vaccination.
With public concern surrounding VITT after ChAd, ascertainment bias cannot be excluded because of increased blood testing and scrutiny after ChAd. Patients with VITT and pre-VITT [25] were unlikely to be mistaken for patients with ITP in our case series as no patients presented with thrombosis or headaches, none had gross elevations of D-dimer levels, and ELISA for anti-PF4 antibodies was negative in nearly all cases tested (17 of 18).
Despite the heightened awareness of platelet disorders after vaccination, the total number reported in our database of Australian cases was lower than predicted after extrapolating from annualized French incidence data (37 new cases vs 91 predicted) [24,26]. Public health measures to ameliorate the pandemic may have inadvertently impacted the presentation of ITP. Social distancing, working from home, and mask wearing may have reduced the seasonal effect of environmental triggers, such as pollen, and the transmission of viruses previously implicated in observed cyclical fluctuations of ITP incidence throughout the calendar year [[27], [28], [29]]. In Australia, flu activity was at historically low levels in 2021, adding further uncertainty to comparisons with prepandemic epidemiology on the incidence of ITP [30]. These prepandemic seasonal variations in the presentation of ITP may somewhat cloud the interpretation of surveys that compare data in epochs of <12 continuous months such as the self-controlled case series analysis of the Scottish registry [9,24]. Additionally, patients may have been discouraged from visiting their doctor or presenting for routine blood testing during COVID-19 lockdowns, further diminishing opportunities for diagnosing milder, more transient forms of ITP.
We received 59% of the total number of suspected ITP cases notified to the TGA. Although the diagnosis of cases reported to public health authorities may not have been confirmed by a hematologist, selection bias by our participating clinicians may have enriched our report with more difficult memorable cases requiring lengthier treatment interactions and hospitalization. Our collaborators work across the major tertiary referral centers in the country, and we believe that most serious cases requiring hospitalization during the collection period have been included in this registry, whereas many milder cases of ITP (platelets 50-100 × 109/L) otherwise meeting diagnostic criteria for ITP are less likely to have been included in our analysis even though they may have been notified to the TGA. Only 3 of 50 (6%) cases in our dataset had a platelet count of >50 × 109/L. Despite this risk of bias toward more severe disease, standard first-line treatments for ITP were highly effective (RR, 96%), even though as many as 34% eventually required second-line therapies.
Our study also has other important limitations, including selection bias by participating clinicians, incomplete data collection because of the observational nature of the study including data on ethnicity or race, and the small number of “unstable” patients with ITP from the historical cohort. Moreover, a greater scrutiny of platelet counts after vaccination due to initial clinical reports from the United States, heightened population concerns, and extensive media interest may have led to some ascertainment bias. However, this effect is likely to have been minimized by the design of this registry, which appealed to specialist clinicians who diagnosed these cases as ITP at the exclusion of other causes of thrombocytopenia.
Despite these limitations, our data reaffirm the safety of vaccinating patients with pre-existing ITP, as even in the rare instance of relapse, bleeding is mild (92% WHO bleeding grade <2) and platelets respond quickly (TTCR, 5 days). In contrast to poor outcomes described in our previous brief analysis [31], concomitant influenza vaccination had no significant impact on key outcomes such as bleeding at presentation, response rates, relapse rates, time to response, and need for ongoing treatments at day 90. Likewise, age, sex, and severity of thrombocytopenia at presentation had no impact on outcomes. Patients with severe disease appear to be receiving IVIg in first-line: lower platelet count at presentation (5 vs 12 × 109/L; P =.02), 80% of patients with WHO bleeding grade ≥2, and 14 of 17 (82%) patients who will eventually require second-line therapies.
Although median platelet counts at day 30 seem substantially higher for prednisolone recipients vs dexamethasone recipients (36 vs 149 × 109/L; P <.001), this is probably a reflection of ongoing corticosteroid exposure, with nearly all patients still receiving prednisone at this time (96%) compared with dexamethasone recipients, of whom 6 of 11 (55%) are treatment-free.
Uncertainty remains as to whether these cases represent coincident ITP unrelated to vaccination, susceptibility to ITP unmasked by vaccination (as occurs after viral illness), or whether there is a cohort of secondary ITP truly induced by COVID-19 vaccination with novel culprits already described, such as spike-dependent platelet-activating antibodies [32]. Assuming underreporting of ITP cases onto our database because of our study design (only 4 cases after BNT vs 41 estimated by prepandemic incidence), the large difference between the annualized incidence of new ITP after ChAd vs BNT (2.72 vs 0.28 per 100,000 vaccination recipients) strongly suggests either a unique reporting bias based on patient or medical fears surrounding ChAd, a protective effect of BNT vaccination against ITP, or a pathologic impact of ChAd vaccination not observed with BNT. There is a strong relationship between increasing age and incidence of ITP [24], and the vaccination program in Australia encouraged older patients onto ChAd over BNT, possibly accounting for some of the observed differences in incidence.
The Scottish-linked database analysis identified a small increased risk of ITP after ChAd (but not BNT) corresponding to an estimated incidence of 11.3/million doses after identifying 22 cases from a study population of 2.53 million [9]. However, this study had numerous limitations, including reliance on accurate hospital coding in lieu of confirmation by a specialist diagnosis, inability to discriminate between de novo and relapsed ITP cases, 48% of identified patients had concurrent prescriptions of medications associated with thrombocytopenia or drug-induced thrombocytopenia, and no follow-up data were available on the clinical response to immunomodulatory therapies such as corticosteroids and intravenous immunoglobulins, which often help confirm suspicion of immune-mediated thrombocytopenia.
Smaller clinician-guided studies consistently demonstrate a small subset of patients with chronic ITP (10%-15%) who experience a significant decrease in platelet counts after any COVID-19 vaccination (mRNA or adenovirus vector based), with similarly swift platelet recovery and infrequent bleeding events (<3%) [8,10,33,34]. However, the incidence of the reported platelet variability is within the limits of our own observed “unstable” historical cohort, where 6 of 47 (12.7%) also experienced significant random fluctuations in their platelet counts (pre-2020). Our subsequent comparison with relapsed ITP cases suggests that this fluctuation may be deeper after vaccination (lower platelet nadir media, 6 vs 27 × 109/L; P =.005) but with a similarly early platelet response and minimal bleeding in a potentially overlapping predisposed subset of “unstable” chronic ITP.
Compelling evidence linking other rare outcomes from COVID-19 vaccination, such as VITT, has only been established by the sensitivity of anti-PF4 antibody immunoassays and PF4-enhanced functional tests [1,35]. Direct evidence of ITP causation by COVID-19 vaccines (either de novo or relapse) remains beyond the scope of this paper. Without analogous tools to confirm the diagnosis of ITP or elucidate a mechanism behind vaccine-associated ITP, the rarity of these events could be lost within seasonal variance and the impact of COVID-19 pandemic measures on health outcomes generally.
However, viral infections induce interferon responses that may exacerbate thrombocytopenia through well-described inhibitory effects on terminal megakaryocyte development and platelet production [36,37]. Vaccinations that mimic viral infection likely contribute to transient interferon-mediated thrombocytopenia often seen in clinical practice, but many other mechanisms of enhanced platelet destruction have also been proposed [38,39]. Ultimately, any reduction in the pool of circulating platelets may diminish platelet-derived TGF-β immunoregulation of previously balanced autoimmunity against platelet glycoproteins in susceptible individuals leading to the development or relapse of ITP [40].
New challenges will emerge as we advise patients on updated vaccinations for rapidly evolving variants and the safety of booster doses. Many patients with ITP after COVID-19 vaccination received some form of immunosuppression which may impair their vaccination efficiency.
Ongoing surveillance is critical as we begin to vaccinate younger populations, and collaborative networks need to be developed to better identify rare, emergent safety signals before future iterations of population-wide vaccination campaigns.
Acknowledgments
We would like to thank in-kind support from the Thrombosis and Haemostasis Society of Australia and New Zealand and all the clinicians who have collaborated with us to pool this national expertise—Agnes Yuen, Angeline Josiah, Beng Chong, Cecily Forsyth, Chris Ward, Kris Ma, David Bishop, Daniel Giles, Emily Blyth, Fernando Roncolato, Gianna Pastore, Jessie Zhao, Julianne Falconer, Kate Melville, Kathleen Crozier, Kim Cartwright, Lisa Clarke, Maple Huang, Michelle Spanevello, Richar Blennerhassett, Sharon Avery, Susan Maccallum, Tracey Batt, Vanessa Manitta, and Zoe Loh.
Funding
We have no funding to declare.
Author contributions
P.C. and R.B. conceived the study, collected data, and prepared the manuscript. N.C. provided expert epidemiologic advice and prepared the manuscript. All other authors (D.H., H.A.T., C.W.T., A.E., V.M.Y.C., E.M., A.Y., J.S., J.C., and D.P.) contributed to the design of the study, data collection, and manuscript preparation. P.C. prepared the first draft, data analysis, and final submission.
Relationship Disclosure
P.C. has received speaking fees from Novartis, is a consultant with Sobi and Sanofi, and is on the advisory board for Janssen. R.B. has received speaking fees from Amgen and Novartis, is on the advisory board for Amgen and Novartis, and is a consultant with Sobi. D.H. has received speaking fees from Amgen and Novartis and is a consultant with Sobi. D.P. has provided consultancy with Sanofi. All other authors—H.A.T., C.W.T., A.E., V.M.Y.C., A.Y., E.M., J.S., E.G., N.C., and J.C.—do not have any conflicts of interest to declare.
Informed patient consent
Human Rights Ethics Committee approval waived the need for informed patient consent to collect, analyse and disseminate this data.
Footnotes
Funding information There is no funding to declare.
Handling Editor: P Angchaisuksiri
The online version contains supplementary material available at https://doi.org/10.1016/j.rpth.2022.100009
Supplementary material
References
- 1.Greinacher A., Thiele T., Warkentin T.E., Weisser K., Kyrle P.A., Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 2021;384:2092–2101. doi: 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gerber G.F., Yuan X., Yu J., Cher B.A.Y., Braunstein E.M., Chaturvedi S., Brodsky R.A. COVID-19 vaccines induce severe hemolysis in paroxysmal nocturnal hemoglobinuria. Blood. 2021;137:3670–3673. doi: 10.1182/blood.2021011548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patel S.U., Khurram R., Lakhani A., Quirk B. Guillain-Barre syndrome following the first dose of the chimpanzee adenovirus-vectored COVID-19 vaccine, ChAdOx1. BMJ Case Rep. 2021;14 doi: 10.1136/bcr-2021-242956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Torjesen I. Covid-19: First UK vaccine safety data are “reassuring,” says regulator. BMJ. 2021;372:n363. doi: 10.1136/bmj.n363. [DOI] [PubMed] [Google Scholar]
- 5.Miller E., Waight P., Farrington C.P., Andrews N., Stowe J., Taylor B. Idiopathic thrombocytopenic purpura and MMR vaccine. Arch Dis Child. 2001;84:227–229. doi: 10.1136/adc.84.3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee E.-J., Cines D.B., Gernsheimer T., Kessler C., Michel M., Tarantino M.D., et al. Thrombocytopenia following Pfizer and Moderna SARS-CoV-2 vaccination. Am J Hematol. 2021;96:534–537. doi: 10.1002/ajh.26132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cines D.B., Bussel J.B., Liebman H.A., Prak ET Luning. The ITP syndrome: pathogenic and clinical diversity. Blood. 2009;113:6511–6521. doi: 10.1182/blood-2009-01-129155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuter D.J. Exacerbation of immune thrombocytopenia following Covid-19 vaccination. Br J Haematol. 2021;195:365–370. doi: 10.1111/bjh.17645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simpson C.R., Shi T., Vasileiou E., Katikireddi S.V., Kerr S., Moore E., et al. First-dose ChAdOx1 and BNT162b2 COVID-19 vaccines and thrombocytopenic, thromboembolic and hemorrhagic events in Scotland. Nat Med. 2021;27:1290–1297. doi: 10.1038/s41591-021-01408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee E.-J., Beltrami-Moreira M., Al-Samkari H., Cuker A., DiRaimo J., Gernsheimer T., et al. SARS-CoV-2 vaccination and ITP in patients with de novo or preexisting ITP. Blood. 2022;139:1564–1574. doi: 10.1182/blood.2021013411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen V.M., Curnow J.L., Tran H.A., Choi P.Y. Australian and New Zealand approach to diagnosis and management of vaccine-induced immune thrombosis and thrombocytopenia. Med J Aust. 2021;215:245–249.e1. doi: 10.5694/mja2.51229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nazy I., Sachs U.J., Arnold D.M., McKenzie S.E., Choi P., Althaus K., et al. Recommendations for the clinical and laboratory diagnosis of VITT against COVID-19: communication from the ISTH SSC subcommittee on platelet immunology. J Thromb Haemost. 2021;19:1585–1588. doi: 10.1111/jth.15341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi P.Y., Grace R.F., Therese Ahlen M., Nazy I., Sachs U.J., Arnold D.M., et al. The SSC platelet immunology register of VITT and VIITP: Toward standardization of laboratory and clinical parameters. J Thromb Haemost. 2021;19:2094–2095. doi: 10.1111/jth.15402. [DOI] [PubMed] [Google Scholar]
- 14.Choi P.Y., Merriman E., Bennett A., Enjeti A.K., Tan C.W., Goncalves I., et al. Consensus guidelines for the management of adult immune thrombocytopenia in Australia and New Zealand. Med J Aust. 2022;216:43–52. doi: 10.5694/mja2.51284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neunert C., Terrell D.R., Arnold D.M., Buchanan G., Cines D.B., Cooper N., et al. American Society of Hematology 2019 guidelines for immune thrombocytopenia. Blood Adv. 2019;3:3829–3866. doi: 10.1182/bloodadvances.2019000966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neunert C., Lim W., Crowther M., Cohen A., Solberg L., Crowther M.A., et al. The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood. 2011;117:4190–4207. doi: 10.1182/blood-2010-08-302984. [DOI] [PubMed] [Google Scholar]
- 17.Rodeghiero F., Stasi R., Gernsheimer T., Michel M., Provan D., Arnold D.M., et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009;113:2386–2393. doi: 10.1182/blood-2008-07-162503. [DOI] [PubMed] [Google Scholar]
- 18.Miller A., Hoogstraten B., Staquet M., Winkler A. Reporting results of cancer treatment. Cancer. 1981;47:207–214. doi: 10.1002/1097-0142(19810101)47:1<207::aid-cncr2820470134>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 19.Rodeghiero F., Michel M., Gernsheimer T., Ruggeri M., Blanchette V., Bussel J.B., et al. Standardization of bleeding assessment in immune thrombocytopenia: report from the International Working Group. Blood. 2013;121:2596–2606. doi: 10.1182/blood-2012-07-442392. [DOI] [PubMed] [Google Scholar]
- 20.Cecinati V., Principi N., Brescia L., Giordano P., Esposito S. Vaccine administration and the development of immune thrombocytopenic purpura in children. Hum Vaccin Immunother. 2013;9:1158–1162. doi: 10.4161/hv.23601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Therapeutic Goods Administration . In: COVID-19 vaccine safety monitoring and reporting. Care DoHaA, editor. Australian Government; 2021. COVID-19 vaccine weekly safety report - 21-10-2021. [Google Scholar]
- 22.Australian Bureau of Statistics. National, state and territory population. September 2021. ABS; https://www.abs.gov.au/statistics/people/population/national-state-and-territory-population/sep-2021; 2021 [accessed September 27, 2002].
- 23.Australian Technical Advisory Group on Immunisation. ATAGI statement regarding COVID-19 vaccines in the setting of transmission of the Delta variant of concern. https://www.health.gov.au/news/atagi-statement-regarding-covid-19-vaccines-in-the-setting-of-transmission-of-the-delta-variant-of-concern. [accessed September 27, 2022].
- 24.Moulis G., Palmaro A., Montastruc J.L., Godeau B., Lapeyre-Mestre M., Sailler L. Epidemiology of incident immune thrombocytopenia: a nationwide population-based study in France. Blood. 2014;124:3308–3315. doi: 10.1182/blood-2014-05-578336. [DOI] [PubMed] [Google Scholar]
- 25.Salih F., Schönborn L., Kohler S., Franke C., Möckel M., Dörner T., et al. Vaccine-induced thrombocytopenia with severe headache. N Engl J Med. 2021;385:2103–2105. doi: 10.1056/NEJMc2112974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Australian Bureau of Statistics. National, state and territory population (September 2021 reference period) 2022 [updated 17 March 2022]. https://www.abs.gov.au/statistics/people/population/national-state-and-territory-population/sep-2021. [accessed September 27, 2022].
- 27.Lim J.H., Kim Y.K., Min S.H., Kim S.W., Lee Y.H., Lee J.M. Epidemiology and viral etiology of pediatric immune thrombocytopenia through Korean public health data analysis. J Clin Med. 2021;10:1356. doi: 10.3390/jcm10071356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tombak A., Boztepe B., Tiftik N., Cömert M., Salim O., Aydın K., et al. Seasonal association of immune thrombocytopenia in adults. Balkan Med J. 2015;32:347–351. doi: 10.5152/balkanmedj.2015.151223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moulis G., Guénin S., Limal N., Michel M., Bierling P., Godeau B., et al. Seasonal variations of incident primary immune thrombocytopenia in adults: an ecological study. Eur J Intern Med. 2017;37:e26–e28. doi: 10.1016/j.ejim.2016.09.025. [DOI] [PubMed] [Google Scholar]
- 30.Government Australia. Australian Influenza Surveillance Report No. 14, 2021, reporting fortnight 27 September to 10 October. 2021. https://www1.health.gov.au/internet/main/publishing.nsf/Content/3ED373A81D484A6DCA25876F000A1FF2/$File/flu-14-2021.pdf. [accessed June 8, 2022].
- 31.Choi P.Y.-I., Hsu D., Tran H.A., Tan C.W., Enjeti A., Chen V.M.Y., et al. Immune thrombocytopenia following vaccination during the COVID-19 pandemic. Haematologica. 2021;107:1193–1196. doi: 10.3324/haematol.2021.279442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Appelbaum J., Arnold D.M., Kelton J.G., Gernsheimer T., Jevtic S.D., Ivetic N., et al. SARS-CoV-2 spike-dependent platelet activation in COVID-19 vaccine-induced thrombocytopenia. Blood Adv. 2022;6:2250–2253. doi: 10.1182/bloodadvances.2021005050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Visser C., Swinkels M., van Werkhoven E.D., Croles F.N., Noordzij-Nooteboom H.S., Eefting M., et al. COVID-19 vaccination in patients with immune thrombocytopenia. Blood Adv. 2022;6:1637–1644. doi: 10.1182/bloodadvances.2021006379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aharoni M., Leader A., Shochat T., Raanani P., Spectre G. Exacerbation of immune thrombocytopenia following initial and booster vaccination with Pfizer-BioNTech COVID-19 vaccine. Platelets. 2022;33:781–786. doi: 10.1080/09537104.2022.2071856. [DOI] [PubMed] [Google Scholar]
- 35.Scully M., Singh D., Lown R., Poles A., Solomon T., Levi M., et al. Pathologic antibodies to platelet factor 4 after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384:2202–2211. doi: 10.1056/NEJMoa2105385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamane A., Nakamura T., Suzuki H., Ito M., Ohnishi Y., Ikeda Y., et al. Interferon-α2b–induced thrombocytopenia is caused by inhibition of platelet production but not proliferation and endomitosis in human megakaryocytes. Blood. 2008;112:542–550. doi: 10.1182/blood-2007-12-125906. [DOI] [PubMed] [Google Scholar]
- 37.Katze M.G., He Y., Gale M. Viruses and interferon: a fight for supremacy. Nat Rev Immunol. 2002;2:675–687. doi: 10.1038/nri888. [DOI] [PubMed] [Google Scholar]
- 38.Teijaro J.R., Farber D.L. COVID-19 vaccines: modes of immune activation and future challenges. Nat Rev Immunol. 2021;21:195–197. doi: 10.1038/s41577-021-00526-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Assinger A. Platelets and infection – an emerging role of platelets in viral infection. Front Immunol. 2014;5:649. doi: 10.3389/fimmu.2014.00649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aoki C.A., Borchers A.T., Li M., Flavell R.A., Bowlus C.L., Ansari A.A., Gershwin M.E. Transforming growth factor beta (TGF-beta) and autoimmunity. Autoimmun Rev. 2005;4:450–459. doi: 10.1016/j.autrev.2005.03.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.