Abstract
Protection against Candida infection involves both innate and acquired immune responses, and cytokines produced by monocytes during the innate response may modify the acquired immune response by T cells. We hypothesized that Candida species which differ in pathogenicity can differentially induce production of immunoregulatory cytokines by human monocytes, which in turn modify T cells for immune responses to Candida. To test this hypothesis, we examined the effects of Candida albicans and Candida krusei on immunoregulatory cytokine production by human monocytes and gamma interferon (IFN-γ) production by peripheral blood mononuclear cells (PBMC). Purified monocytes were incubated with live or heat-killed strains of C. albicans and C. krusei at the optimal Candida/monocyte ratio of 0.5. Cytokines in the supernatants were measured by enzyme-linked immunosorbent assay. Our data demonstrated that live C. albicans and C. krusei significantly induced interleukin-10 (IL-10), monocyte chemotactic factor 1, IL-1β, and tumor necrosis factor alpha production by monocytes relative to unstimulated monocytes. In contrast, unlike C. krusei, pathogenic live strains of C. albicans induced no or only a minimal level of IL-12. The expression of IL-12 p40 mRNA levels by reverse transcription-PCR corroborated the IL-12 protein (p70) findings. In human PBMC, human blood monocytes were the major source of both IL-10 and IL-12 production in response to C. albicans and C. krusei. Upon activation of T cells in the presence of Candida-modified monocytes and antigen-presenting cells, IL-12 production by PBMC treated with Candida organisms correlated strongly with the level of IFN-γ production by T cells. These results indicate that the virulence of C. albicans may be related to its ability to induce the monocytic type II cytokine IL-10, with a selective inhibition of IL-12 production, which may be responsible for the observed lack of T-cell IFN-γ and may restrain an effective type I immune response to Candida.
Candida albicans is a major opportunistic fungal pathogen which may be present in humans as a commensal microbial flora; most importantly, it causes candidiasis in immunocompromised hosts due to malignant tumors, major surgery, organ transplantation, or treatment with cytotoxic or immunosuppressive drugs (13). In addition to C. albicans, other Candida species, even much less virulent non-albicans Candida species such as C. krusei (1, 13, 36, 42, 43), have been reported as pathogens causing systemic candidiasis.
The importance of polymorphonuclear leukocytes has been extensively studied in the pathogenesis of candidiasis (4, 5, 33). However, systemic candidiasis has also occurred in hosts with normal neutrophil function, suggesting that cells other than neutrophils also play an important role in host defense. When hosts are neutropenic, mononuclear cells, especially monocytes/macrophages, contribute to the defense against the infection (17); nonetheless, their functions in pathogenesis of candidiasis in humans have not been fully explored.
Monocytes have the capacity to produce chemokines (23), proinflammatory cytokines (6), and particularly the immunoregulatory cytokines interleukin-10 (IL-10) and IL-12 (9, 41). Immunoregulatory cytokines released as a result of the initial contact of Candida with host monocytes/macrophages can also be a major factor, which can potentially regulate the acquired immune response through T-cell development, in host defense. IL-12 is essential for inducing type I immune responses, and the development of gamma interferon (IFN-γ)-producing T cells (25, 41), which in turn are associated with resistance to candidal infection (34, 35). Its reciprocal immunoregulatory cytokine, IL-10, inhibits IL-12 and IFN-γ production (2, 8, 24), favoring type II immune responses (8, 9, 11), which are associated with susceptibility to C. albicans infection (7, 40).
We hypothesized that Candida species which differ in pathogenicity can differentially induce production of immunoregulatory cytokines by human monocytes, which in turn modify T cells for immune responses to Candida. In this study, we show that C. albicans and C. krusei differentially induce IL-12 production; i.e., C. krusei, but not C. albicans, clearly induced IL-12 by monocytes. These results indicate that pathogenic Candida species have the ability to create an environment rich in IL-10 and poor in IL-12 and IFN-γ, which would generate a more susceptible state of the host to candidiasis.
MATERIALS AND METHODS
PBMC, monocytes, and nonadherent mononuclear cells (NAC).
Peripheral blood mononuclear cells (PBMC) were isolated from heparinized blood of healthy volunteer by Histopaque-1077 (Sigma Chemical Company, St. Louis, Mo.) gradient centrifugation. Monocytes were isolated by incubation of PBMC in tissue culture dishes for 1 h at 37°C followed by harvesting adherent cells using 0.5 mM EDTA in Hanks balanced salt solution (Life Technologies, Grand Island, N.Y.). The T, B, and natural killer cells and erythrocytes following treatment with antibody mixture (anti-CD2, -3, -19, -56 and -glycophorin A) and dextran-iron (as instructed by the manufacturer [Stem Cell Technologies, Vancouver, British Columbia, Canada]) were removed by adherence to a MACS separation column against a MidiMACS magnet (Miltenyi Biotec, Auburn, Calif.) (44).
NAC were collected after PBMC adherence to plastic dishes at 37°C for 1 h in a 5% CO2 incubator. The cells were adjusted to 2 × 106/ml in RPMI 1640 plus penicillin, streptomycin, and 10% fetal bovine serum. Endotoxin was determined in the supernatants by using E-Toxate reagent (Sigma).
Fungal organisms.
The C. albicans strains (SC5314, 1442, 2307, and 2183) and C. krusei isolates (6258 and A-L) used in this study have been described previously (15, 16, 21). Candida strains were stored in a mixed medium composed of glycerol and Sabouraud's dextrose broth (1:1) at −70°C. The organisms were streaked onto Sabouraud's dextrose agar, and the plates were incubated at 37°C overnight. One colony was transferred to 10 ml of Sabouraud's dextrose broth, and the cells were incubated overnight at 37°C in a shaking water bath. The organisms were centrifuged at 1,500 rpm for 8 min, washed twice with phosphate-buffered saline (PBS), then resuspended in RPMI 1640 plus penicillin, streptomycin, and 10% FBS, and adjusted to a final concentration of 107 cells/ml. In some experiments, organisms were heat killed by suspension in RPMI 1640 by incubation in a 60°C water bath for 30 min.
Coculture of fungal cells with PBMC, monocytes, and NAC.
To prevent stimulation of blood cells with plastic, six-well plates were coated with 0.05% bovine serum albumin in PBS for 1 h, followed by three washes with PBS. PBMC, monocytes, or NAC (1 ml of 2 × 106 cells/ml) were added to each well and incubated for 45 min. Then yeast cells were added to the blood cells at different ratios (see Results) and incubated for 20 h at 37°C in a 5% CO2 incubator. Following incubation, supernatants were collected and stored at −70°C until use. The viability of monocytes in coculture with live C. albicans and C. krusei was monitored using a lactate dehydrogenase (LDH) assay kit (Boehringer Mannheim Corporation, Indianapolis, Ind.).
Cytokine ELISA.
Cytokine proteins in cell supernatants were quantitated by enzyme-linked immunosorbent assay (ELISA) with antibody pairs for either IL-12, tumor necrosis factor alpha (TNF-α; R&D Systems Inc., Minneapolis, Minn.), IL-1β (Endogen, Woburn, Mass.), IFN-γ, monocyte chemotactic protein 1 (MCP-1), IL-4, or IL-10 (PharMingen International, San Diego, Calif.). The sensitivity of all ELISAs was ≥10 pg/ml.
RNA extraction and RT-PCR.
Reverse transcription-PCR (RT-PCR) was performed as previously described (19). Briefly, total RNA of monocytes was extracted by an RNeasy Total RNA kit (Qiagen, Chatsworth, Calif.) and quantified by spectrophotometric measurement. cDNA was synthesized from 200 ng of total RNA. The primers used for PCR were as follows: IL-12 p40 (nucleotides 806 to 822 of sense strand [5′-CCACATTCCTACTTCTC-3′] and nucleotides 1061 to 1077 of antisense strand [5′-GTCTATTCCGTTGTGTC-3′]; 272 bp) and β-actin (447 bp). Thirty-two cycles were conducted in Quarther Bath Thermal Cycler (Inotech, Lansing, Mich.) with denaturation at 94°C for 1 min, annealing at 55°C (60°C for β-actin) for 1 min, and 72°C for 2 min. PCR products were electrophoresed with 2% agarose gel with ethidium bromide.
Statistics.
Results were expressed as mean ± standard error for n number of repeat experiments. Statistical significance was determined by Student's t test. The correlation coefficient of two variables was evaluated by using linear regression, and statistical significance was determined by t test. A P value of <0.05 was considered significant.
RESULTS
C. albicans as well as C. krusei induced MCP-1, IL-1β, and TNF-α by human blood monocytes.
To determine whether live C. albicans and C. krusei can differentially induce cytokine production by human blood monocytes, we first tried to determine the optimal ratio of Candida organisms to monocytes for use in subsequent experiments. The yeast/monocyte ratios examined were 0.001, 0.01, 0.05, 0.1, 0.5, 1, 10, and 50 (data not shown). These preliminary experiments showed that a yeast/monocyte ratio of 0.5 caused significant stimulation of IL-1β and TNF-α production by monocytes. A lower yeast/monocyte ratio (0.01) was needed to cause significant induction of MCP-1. At the ratio of 0.5, Candida organisms caused no statistically significant increase of LDH release by monocytes relative to unstimulated controls. Therefore, the ratio of 0.5 was chosen to determine the immunoregulatory cytokine production by monocytes following stimulation by Candida cells in all subsequent experiments. As shown in Table 1, both C. albicans SC5314 and C. krusei 6258 induced high and significant levels of MCP-1, IL-1β, and TNF-α production by monocytes relative to unstimulated controls (P < 0.05). The endotoxin level in the supernatants was determined and found to be below the detectable level (<0.06 endotoxin unit/ml), indicating that our preparations were not contaminated by endotoxin.
TABLE 1.
Live C. albicans- and C. krusei-induced IL-1β, TNF-α, and MCP-1 production by monocytesa
| Monocyte treatment | Mean concn (pg/ml) ± SD (n)
|
||
|---|---|---|---|
| IL-1β | TNF-α | MCP-1 | |
| Control | <10 (15) | <10 (15) | 2,011 ± 619 (16) |
| C. albicans SC5314 | 1,823 ± 241* (11) | 5,736 ± 329* (11) | 7,561 ± 449* (16) |
| C. krusei 6258 | 1,885 ± 298* (5) | 6,546 ± 617* (5) | 8,581 ± 967* (16) |
Human peripheral blood monocytes were treated with live C. albicans SC5314 or C. krusei 6258. Supernatants were collected after incubation for 20 h. Cytokines were measured by ELISA. ∗, P < 0.05 versus control.
Unlike C. krusei, which significantly induced the production of IL-10 and IL-12 by human monocytes, C. albicans induced IL-10 production only.
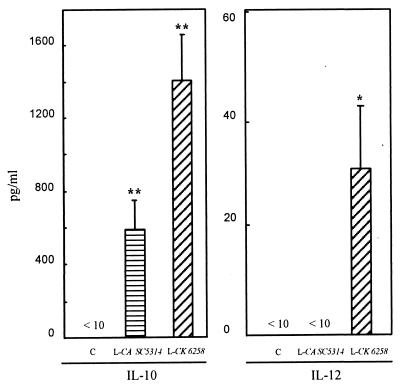
Although monocytes have the capacity to produce IL-10 and IL-12 (9, 20, 41), the ability of C. albicans to induce IL-10 and IL-12 by monocytes has not been investigated. Thus, in this study we examined the production of these two cytokines by monocytes following stimulation with C. albicans and C. krusei. Our results showed that both live C. krusei 6258 and live C. albicans SC5314 stimulated IL-10 production; however, C. krusei 6258 but not C. albicans SC5314 induced significant IL-12 production by monocytes (32 ± 12 pg/ml for C. krusei versus 0 pg/ml for both C. albicans and controls; P < 0.05) (Fig. 1). These results indicate that production of the immunoregulatory cytokine IL-12 by monocytes is differentially induced by different Candida species and differs from its reciprocal counterpart IL-10.
FIG. 1.
IL-10 and IL-12 production by monocytes treated with live C. albicans SC5341 or live C. krusei 6258. Human PBMC were treated with live C. albicans SC5314 (L-CA SC5314) or C. krusei 6258 (L-CK 6258). Supernatants were collected after incubation for 20 h. Cytokines were measured by ELISA. Monocytes without fungal cells were used as controls (C). n = 18 for IL-10; n = 16 for IL-12; ∗, P < 0.05 versus control; ∗∗, P, <0.01 versus control.
To determine whether induction of IL-12 production by monocytes with C. krusei but not C. albicans is species or strain specific, we repeated the above experiments using additional strains of these candidal species. Three C. albicans strains (1442, 2307, and 2183) have been previously characterized for their virulence (16). The first strain is highly virulent, while the other two are of low virulence as determined by animal survival studies (16). A second C. krusei strain (A-L) was also included in these experiments. In general, C. albicans, unlike C. krusei, failed to induce IL-12 production by monocytes (levels of <10 pg/ml). However, one strain of C. albicans that was characterized by Graybill's group (16) to be of low virulence (strain 2183) stimulated the production of minimal but insignificant (P > 0.05) amount of IL-12 p70 protein (10 ± 5 pg/ml) by monocytes. C. krusei 6258 and A-L stimulated IL-12 production to levels of 31 ± 12 (n = 16) and 283 ± 167 pg/ml (n = 4).
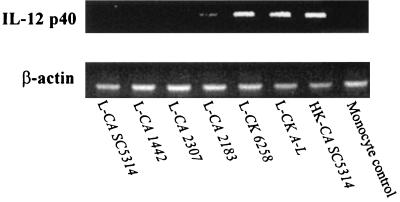
Next we performed RT-PCR to determine whether IL-12 p70 protein production is mirrored at the mRNA level. As shown in Fig. 2, the pattern of IL-12 p40 mRNA expression paralleled IL-12 p70 protein production following stimulation with C. krusei (strains 6258 and A-L both caused remarkable expression of IL-12 p40 mRNA). In contrast, C. albicans did not stimulate or induced a minimal level (strain 2183) of mRNA expression (Fig. 2). As expected, monocytes incubated in the absence of fungal cells as a negative control did not express IL-12 p40 mRNA (Fig. 2).
FIG. 2.
Expression of IL-12 p40 mRNA in human peripheral blood monocytes was inhibited by C. albicans species. Human peripheral blood monocytes were cocultured with live C. albicans SC5314, 1442, 2307, and 2183 (L-CA SC5314, L-CA 1442, L-CA 2183, and L-CA 2307, respectively), C. krusei 6258 and A-L (L-CK 6258 and L-CK A-L, respectively), or monocytes alone as control for 20 h. Total RNA was extracted, and RT-PCR was conducted with primers for IL-12 p40 and β-actin. Results are representative of two separate experiments that yielded similar results.
IL-12 is markedly induced by human blood monocytes treated with heat-killed C. albicans and C. krusei.
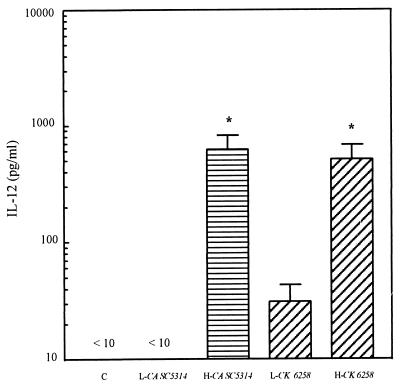
To determine whether viability is critical for the inability of C. albicans to induce IL-12, C. albicans SC5314 and C. krusei 6258 were heat killed and cocultured with monocytes. IL-12 production by monocytes treated with heat-killed and live C. albicans SC5314 or C. krusei 6258 was monitored. As expected, live C. albicans failed to induce IL-12 whereas live C. krusei did. In contrast, heat-killed C. albicans and C. krusei induced high levels of IL-12 production by monocytes (P < 0.05) (Fig. 3), indicating that the inhibition of IL-12 production by C. albicans is an active process requiring viable Candida cells.
FIG. 3.
IL-12 production by monocytes treated with live C. albicans SC5314, live C. krusei 6258, heat-killed C. albicans SC5314, and heat-killed C. krusei 6258. Human peripheral blood monocytes were treated with live C. albicans SC5314 (L-CA SC5314), live C. krusei 6258 (L-CK 6258), heat-killed C. albicans SC5314 (H-CA SC5314), and heat-killed C. krusei 6258 (H-CK 6258). Supernatants were collected after incubation for 20 h. Cytokine was measured by ELISA. Monocytes without fungal cells were used as control (C). n = 16 for L-CA and L-CK; n = 6 for H-CA SC5314 and H-CK 6258; ∗, P < 0.05 versus L-CA SC5314 or L-CK 6258.
Human blood monocytes represent the major source of IL-10 and IL-12 production by PBMC in response to C. albicans and C. krusei stimulation.
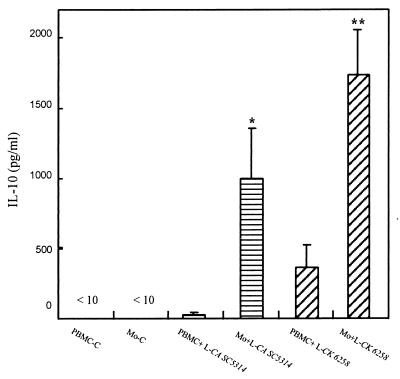
Although purified monocytes in the above experiments were clearly capable of responding to Candida directly, it is possible that in vivo, where complex mixtures of immunocytes interact, other cell types might participate in or regulate monocyte immunoregulatory cytokine production. To determine if monocytes are the major producer of IL-10 in the mix of T cells, B cells, monocytes, dendritic cells, basophils and NK cells contained in PBMC, whole PBMC were cocultured with either live C. albicans SC5314 or live C. krusei 6258. IL-10 protein in the supernatants was determined and compared between purified monocytes and PBMC treated with the same candidal strain. Monocytes/PBMC at the concentration of 2 × 106/ml with the yeast-to-monocyte/PBMC ratio of 0.5 were cultured as described above. The percentage of monocytes in the purified monocyte preparations was >90%, whereas in PBMC monocytes represented only 7.4% ± 2.1% (n = 2; as determined by flow cytometry for CD14); this represented an approximately 12-fold enrichment. IL-10 was significantly higher in the supernatants of cultured monocytes stimulated with either live C. albicans SC5314 (P < 0.05) or C. krusei 6258 (P < 0.01) relative to that in the PBMC supernatants (Fig. 4). Next, to determine if the monocytes are the major producer of IL-12 in stimulated PBMC, monocytes, PBMC, and NAC were cocultured with live C. krusei 6258, and IL-12 protein levels in the supernatants were determined. Since live C. albicans SC5314 was unable to stimulate monocytes and PBMC to produce IL-12, it was not included in these experiments. After adherence, the monocytes, as determined by flow cytometry, in NAC dropped to 2.7% ± 0.8%, compared to 7.4% ± 2.1% in PBMC. Our data show that IL-12 was not detected in NAC cocultured with C. krusei, while live C. krusei induced significantly higher IL-12 production by PBMC (80 ± 32 pg/ml; n = 6 [Table 2]) than that of NAC (P < 0.05). Because different cell types within PBMC regulate IL-12, we were unable to show significance in the production of this cytokine between PBMC and enriched monocytes. Taken together, these results indicate that monocytes are the major producer of both IL-10 and IL-12 in PBMC after Candida stimulation.
FIG. 4.
Comparison of IL-10 production by human blood monocytes treated with live C. albicans SC5314 and C. krusei 6258 to that by PBMC. Monocytes (Mo) and PBMC were treated with live C. albicans SC5314 (L-CA SC5314) or C. krusei 6258 (L-CK 6258). Supernatants were collected after incubation for 20 h. Cytokine was measured by ELISA. Monocytes and PBMC without fungal cells were used as controls. PBMC-C, PBMC control; Mo-C, monocyte control; n = 6; ∗, P < 0.05 versus PBMC+L-CA SC5314; ∗∗, P < 0.01 versus PBMC+L-CK 6258.
TABLE 2.
Correlation of IL-12 and IFN-γ production by PBMC treated with Candida organismsa
| Treatment | Mean concn (pg/ml) ± SD (n = 6)
|
r2 | P | |
|---|---|---|---|---|
| IL-12 | IFN-γ | |||
| C. albicans SC5314 | ||||
| Live | <10 | <10 | ||
| Heat killed | 639 ± 158 | 1,281 ± 586 | 0.81 | <0.01 |
| C. krusei 6258 | ||||
| Live | 80 ± 32 | 459 ± 620 | 0.84 | <0.01 |
| Heat killed | 440 ± 106 | 1,201 ± 570 | 0.82 | <0.01 |
PBMC were treated with live or heat-killed C. albicans SC5314 and C. krusei 6258. Supernatants were collected after incubation for 20 h. Cytokines were measured by ELISA.
IFN-γ induction correlates strongly with the level of IL-12 in the supernatants of PBMC treated with Candida organisms.
Production of IFN-γ by T cells is dependent on IL-12 and is critical for immune responses associated with cell-mediated immunity against microorganisms, including Candida. To determine if a correlation exists between the inability of C. albicans to induce IL-12 and IFN-γ production, PBMC were incubated with live or heat-killed C. albicans SC5314 or C. krusei 6258, and the supernatants were assayed for the simultaneous presence of IL-12 and IFN-γ. As can be seen in Table 2, live C. albicans SC5314 failed to produce either IL-12 or IFN-γ. In contrast, stimulation of PBMC with either C. krusei 6258 or heat-killed C. albicans led to the production of significant amounts of both IL-12 and IFN-γ. IL-4 was not detectable in the supernatants of PBMC treated with live C. albicans SC5314 and C. krusei 6258 (data not shown). Therefore, these data indicated that IFN-γ production by PBMC strongly correlated with IL-12 production (r2 = 0.81, P < 0.01 for heat-killed C. albicans SC5314; r2 = 0.84, P < 0.01 for live C. krusei 6258; r2 = 0.82, P < 0.01 for heat-killed C. krusei 6258) (Table 2).
DISCUSSION
In this study, we showed that C. albicans-stimulated monocytes produced high levels of MCP-1 and proinflammatory cytokines IL-1β and TNF-α (Table 1). These findings agree with earlier reports (3, 6, 10, 18). Production of these cytokines under the influence of C. albicans may enhance acute inflammatory cell influx into infected tissues, activate leukocytes, and promote early noncognate elimination of the organism prior to the development and/or mobilization of an adaptive specific immune response (30, 38). Furthermore, we found that clinical isolates of C. albicans and C. krusei which differ in pathogenicity can also induce similar levels of the immunoregulatory cytokine IL-10. Interestingly, these yeast species differentially induce IL-12 production by monocytes.
It has been well documented that IL-10 plays an inhibitory role in monocytes and neutrophils against Candida (7, 29, 39). In the murine models of candidiasis, neutralization of IL-10 upregulates nitric oxide production and protects susceptible mice from challenge with C. albicans (28, 33). This indicates that IL-10 suppresses protective type I responses in mice with C. albicans infection. The ultimate net response in vivo may depend on host immunogenetics and immune status and the virulence of the fungal strain.
Our data showed that live C. albicans SC5314 failed to induce IL-12 production whereas C. krusei 6258 induced the production of significant levels of IL-12 by monocytes (P < 0.05). To determine whether this observation is strain specific or species specific, we extended our studies to include three additional strains of C. albicans and one strain of C. krusei. The relative virulence of the three strains of C. albicans was shown to be 1442 > 2307 > 2183 (16). Similar to C. albicans SC5314, the virulent strain 1442 failed to induce IL-12 production by monocytes. Although C. albicans 2307 and 2183 (low-virulence strains) induced a trace amount of IL-12, this level was not statistically significant. In contrast, A-L, the second C. krusei strain tested, like C. krusei 6258, induced high levels of IL-12 production by monocytes (see Results).
Failure to detect IL-12 in the culture of monocytes treated with C. albicans was also shown by mRNA level. The expression of IL-12 p40 mRNA by monocytes stimulated with C. albicans and C. krusei was in line with the IL-12 p70 protein levels (Fig. 2). Thus, the possibility of extrinsic factors such as absorption or degradation by C. albicans in the medium which might affect detection of IL-12 is excluded.
The mechanism(s) responsible for the inhibition of IL-12 induction by monocytes needs to be explored. We asked whether C. albicans essentially failed to produce IL-12 by monocytes is due to the viability of C. albicans. Our data showed that both heat-killed C. albicans and heat-killed C. krusei induce high levels of IL-12 production by monocytes (Fig. 3). Overall, it is quite clear that live virulent and avirulent strains of C. albicans are all substantially incapable of stimulating IL-12 production, the true distinction being that between live and inactivated cells. These results indicate that inhibition of IL-12 production by monocytes is an active process on the part of C. albicans and may be associated with virulence factors such as germination, production of enzymes (such as phospholipase), and complement activation. It is likely that phagocytosis of yeasts by monocytes is involved in the production of IL-12, because C. krusei does not make true, uningestible hyphae and heat-killed C. albicans does not make hyphae either. Fulton et al. (14) reported that inhibition of phagocytosis by cytochalasin D reduced the IL-12 p40 mRNA expression by monocytes treated with Mycobacterium tuberculosis. C. albicans appears as both yeast and hyphae in RPMI 1640; therefore, whether hyphae and phagocytosis of yeasts play a role in regulation of IL-12 production by monocytes is currently being investigated.
We further determined whether monocytes are the main source of the immunoregulatory cytokines IL-10 and IL-12 in PBMC treated with Candida species. Levitz and North (22) reported that PBMC treated with heat-killed C. albicans could produce IL-10 and postulated that IL-10 could be dependent on the presence of peripheral blood monocytes. We compared the IL-10 production by monocytes and PBMC treated with both live C. albicans and live C. krusei. We also compared IL-12 protein production by PBMC and NAC treated with live C. krusei, in which monocytes were depleted by adherence. As shown in Results, monocytes are the main source of IL-10 and IL-12 in PBMC treated with these Candida organisms. The importance of monocytes as the main source of IL-10 and IL-12 in PBMC is that they exert a central functional effect on differentiation of immune responses. To determine whether the production of IL-12 by monocytes stimulated with Candida organisms affected pivotal T-cell function, IFN-γ was monitored along with IL-12 in the supernatants of PBMC cocultured with the Candida organism. Our results show that IFN-γ production was absent upon stimulation with C. albicans but clearly induced by the low-virulence C. krusei and heat-killed C. albicans. IFN-γ production correlated strongly with IL-12 production in PBMC challenged with Candida species (Table 2). It is well documented that a type I immune response is characterized by increased IFN-γ, which can enhance the antifungal activity of neutrophils (31, 32, 37) in vitro and can protect endothelial cells from organism-induced damage (12, 35), thereby leading to host resistance and onset of protective immunity (27, 28, 38). In contrast, IL-10 inhibits type I cell development and favors susceptibility to C. albicans (26, 33). Therefore, the ability of live C. albicans to induce IL-10 while failing to induce IL-12 production by monocytes may create a circumstance in which type I response is suppressed, thus increasing the susceptibility of the host to candidiasis. In the case of C. krusei, however, IL-12 was clearly induced by monocytes, which leads to type I response and favors cell-mediated immunity against C. krusei infection. These results may partly explain the high and low incidences of infections due to C. albicans and C. krusei, respectively. IFN-γ was induced, while IL-4 was not detectable, in PBMC treated with live C. krusei, indicating that a type II immune response was not induced in place of a type I response, at least upon a single round of stimulation. Taken together, these results indicate that the virulence of C. albicans may be related to its ability to selectively induce IL-10, with simultaneous inhibition of monocytic IL-12 and T-cell IFN-γ.
ACKNOWLEDGMENTS
This work was supported in part by grants from Pfizer Pharmaceutical Group, New York (M.A.G. and K.K.), National Institutes of Health grants AI-35097-04 (M.A.G.) and AI-41766 (K.D.C.), and the University Hospitals of Cleveland Research and Education Fund.
We thank John R. Graybill (University of Texas San Antonio) for providing C. albicans virulence strains 1442, 2307, and 2183, Steven D. Leidich and Chad Jessup for preparing the other Candida isolates, and Guofen Chen for technical assistance.
REFERENCES
- 1.Anaissie E, Hachem R, Tin-U C K-, Stephens L C, Bodey G P. Experimental hematogenous candidiasis caused by Candida krusei and Candida albicans: species differences in pathogenicity. Infect Immun. 1993;61:1268–1271. doi: 10.1128/iai.61.4.1268-1271.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aste-Amezaga M, Ma X, Sartori A, Trinchieri G. Molecular mechanisms of the induction of IL-12 and its inhibition by IL-10. J Immunol. 1998;160:5936–5944. [PubMed] [Google Scholar]
- 3.Ayray C, Imir T. Tumor necrosis factor (TNF) induction from monocyte/macrophages by Candida species. Immunobiology. 1996;196:363–374. doi: 10.1016/S0171-2985(96)80059-3. [DOI] [PubMed] [Google Scholar]
- 4.Calderone R, Diamond R, Senet J-M, Warmington J, Filler S, Edwards J E. Host cell-fungal cell interactions. J Med Vet Mycol. 1994;32:151–168. doi: 10.1080/02681219480000801. [DOI] [PubMed] [Google Scholar]
- 5.Calderone R, Sturtevant J. Macrophage interactions with Candida. In: Zwilling B W, Eisenstein T K, editors. Macrophage-pathogen interaction. New York, N.Y: Marcel Dekker, Inc.; 1994. pp. 505–515. [PubMed] [Google Scholar]
- 6.Castro M, Bjoraker J A, Rohrbach M S, Limper A H. Candida albicans induces the release of inflammatory mediators from human peripheral blood monocytes. Inflammation. 1996;20:107–122. doi: 10.1007/BF01487749. [DOI] [PubMed] [Google Scholar]
- 7.Cenci E, Romani L, Mencacci A, Spaccapelo R, Schiaffella E, Puccetti P, Bistoni F. Interleukin-4 and interleukin-10 inhibit nitric oxide-dependent macrophage killing of Candida albicans. Eur J Immunol. 1993;23:1034–1038. doi: 10.1002/eji.1830230508. [DOI] [PubMed] [Google Scholar]
- 8.D'Andrea A, Aste-Amezaga M, Valiante N M, Ma X, Kubin M, Trinchieri G. Interleukin 10 (IL-10) inhibits human lymphocyte interferon gamma-production by suppressing natural killer cell stimulatory factor/IL-12 synthesis in accessory cells. J Exp Med. 1993;178:1041–1048. doi: 10.1084/jem.178.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Waal Malefyt R, Abrams J, Bennett B, Figdor C G, De Vries J E. Interleukin 10 (IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991;174:1209–1220. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Djeu J Y, Blanchard D K, Richards A L, Friedman H. Tumor necrosis factor induction by Candida albicans from human natural killer cells and monocytes. J Immunol. 1988;141:4047–4052. [PubMed] [Google Scholar]
- 11.Fiorentino D F, Zlotnik A, Vieira P, Mosmann T R, Howard M, Moore K W, O'Garra A. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J Immunol. 1991;146:3444–3451. [PubMed] [Google Scholar]
- 12.Fratti R A, Ghannoum M A, Edwards J E, Jr, Filler S G. Gamma interferon protects endothelial cells from damage by Candida albicans by inhibiting endothelial cell phagocytosis. Infect Immun. 1996;64:4714–4718. doi: 10.1128/iai.64.11.4714-4718.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fridkin S K, Jarvis W R. Epidemiology of nosocomial fungal infections. Clin Microbiol Rev. 1996;9:499–511. doi: 10.1128/cmr.9.4.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fulton S A, Johnsen J M, Wolf S F, Sieburth D S, Boom W H. Interleukin-12 production by human monocytes infected with Mycobacterium tuberculosis: role of phagocytosis. Infect Immun. 1996;64:2523–2531. doi: 10.1128/iai.64.7.2523-2531.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghannoum M A, Okogbule-Wonodi I, Bhat N, Sanati H. Antifungal activity of voriconazole (UK-109,496), fluconazole and amphotericin B against hematogenous Candida krusei infection in neutropenic guinea pig model. J Chemother. 1999;11:34–39. doi: 10.1179/joc.1999.11.1.34. [DOI] [PubMed] [Google Scholar]
- 16.Graybill J R, Montalbo E, Kirkpatrick W R, Luther M F, Revankar S G, Patterson T F. Fluconazole versus Candida albicans: a complex relationship. Antimicrob Agents Chemother. 1999;42:2938–2942. doi: 10.1128/aac.42.11.2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jensen J, Warner T, Balish E. The role of phagocytic cells in resistance to disseminated candidiasis in granulocytopenic mice. J Infect Dis. 1994;170:900–905. doi: 10.1093/infdis/170.4.900. [DOI] [PubMed] [Google Scholar]
- 18.Jiang Y, Russell T R, Graves D T, Cheng H, Nong S-H, Levitz S M. Monocyte chemoattractant protein 1 and interleukin-8 production in mononuclear cells stimulated by oral microorganisms. Infect Immun. 1996;64:4450–4455. doi: 10.1128/iai.64.11.4450-4455.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kang K, Hammerberg C, Meunier L, Cooper K D. CD11b+ macrophages that infiltrate human epidermis after in vivo ultraviolet exposure potently produce IL-10 and represent the major secretory source of epidermal IL-10 protein. J Immunol. 1994;153:5256–5264. [PubMed] [Google Scholar]
- 20.Larsson S, Linden M. Effects of a corticosteroid, budesonide, on production of bioactive IL-12 by human monocytes. Cytokine. 1998;10:786–789. doi: 10.1006/cyto.1998.0362. [DOI] [PubMed] [Google Scholar]
- 21.Leidich S D, Ibrahim A S, Fu Y, Koul A, Jessup C, Vitullo J, Fonzi W, Mirbod F, Shigeru N, Nozawa Y, Ghannoum M A. Cloning and disruption of caPLB1, a phospholipase B gene involved in the pathogenicity of Candida albicans. J Biol Chem. 1998;273:26078–26086. doi: 10.1074/jbc.273.40.26078. [DOI] [PubMed] [Google Scholar]
- 22.Levitz S M, North E A. Lymphoproliferation and cytokine profiles in human peripheral blood mononuclear cells stimulated by Cryptococcus neoformans. J Med Vet Mycol. 1997;35:229–236. doi: 10.1080/02681219780001201. [DOI] [PubMed] [Google Scholar]
- 23.Levitz S M, North E A, Jiang Y, Nong S H, Kornfeld H, Harrison T S. Variables affecting production of monocyte chemotactic factor 1 from human leukocytes stimulated with Cryptococcus neoformans. Infect Immun. 1997;65:903–908. doi: 10.1128/iai.65.3.903-908.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Macatonia S E, Doherty T M, Knight S C, O'Garra A. Differential effect of IL-10 on dendritic cell-induced T cell proliferation and IFN-gamma production. J Immunol. 1993;150:3755–3765. [PubMed] [Google Scholar]
- 25.McKnight A J, Zimmer G J, Fogelman I, Wolf S F, Abbas A K. Effects of IL-12 on helper T cell-dependent immune responses in vivo. J Immunol. 1994;152:2172–2179. [PubMed] [Google Scholar]
- 26.Moore K W, O'Garra A, de Waal Malefyt R, Vieira P, Mosmann T R. Interleukin-10. Annu Rev Immunol. 1993;11:165–190. doi: 10.1146/annurev.iy.11.040193.001121. [DOI] [PubMed] [Google Scholar]
- 27.Murphy J W, Bistoni F, Deepe G S, Blackstock R A, Buchanan K, Ashman R B, Romani L, Mencacci A, Cenci C, Fe D'Ostiani C, Del Sero G, Calich V L G, Kashino S S. Type I and type 2 cytokines: from basic science to fungal infections. Med Mycol. 1998;36:109–118. [PubMed] [Google Scholar]
- 28.Puccetti P, Romani L, Bistoni F. A TH1-TH2-like switch in candidiasis: new perspectives for therapy. Trends Microbiol. 1995;3:237–240. doi: 10.1016/s0966-842x(00)88931-3. [DOI] [PubMed] [Google Scholar]
- 29.Roilides E, Anastasiou-Katsiardani A, Dimitriadou-Georgiadou A, Kadiltsoglou I, Tsaparidou S, Panteliadis C, Walsh T J. Suppressive effects of interleukin-10 on human mononuclear phagocyte function against Candida albicans and Staphylococcus aureus. J Infect Dis. 1998;178:1734–1742. doi: 10.1086/314479. [DOI] [PubMed] [Google Scholar]
- 30.Roilides E, Dignani M C, Anaissie E J, Rex J H. The role of immunoreconstitution in the management of refractory opportunistic fungal infections. Med Mycol. 1998;36:12–25. [PubMed] [Google Scholar]
- 31.Roilides E, Holmes A, Blake C, Pizzo P A, Walsh T J. Effects of granulocyte colony-stimulating factor and interferon-gamma on antifungal activity of human polymorphonuclear neutrophils against pseudohyphae of different medically important Candida species. J Leukoc Biol. 1995;57:651–656. doi: 10.1002/jlb.57.4.651. [DOI] [PubMed] [Google Scholar]
- 32.Roilides E, Uhlig K, Venzon D, Pizzo P A, Walsh T J. Neutrophil oxidative burst in response to blastoconidia and pseudohyphae of Candida albicans: augmentation by granulocyte colony-stimulating factor and interferon-gamma. J Infect Dis. 1992;166:668–673. doi: 10.1093/infdis/166.3.668. [DOI] [PubMed] [Google Scholar]
- 33.Romani L, Mencacci A, Cenci E, Puccetti P, Bistoni F. Neutrophils and the adaptive immune response to Candida albicans. Res Immunol. 1996;147:512–518. doi: 10.1016/s0923-2494(97)85216-9. [DOI] [PubMed] [Google Scholar]
- 34.Romani L, Mencacci A, Cenci E, Spaccapelok R, Mosci P, Puccetti P, Bistoni F. CD4+ subset expression in murine candidiasis. Th responses correlate directly with genetically determined susceptibility or vaccine-induced resistance. J Immunol. 1993;150:925–931. [PubMed] [Google Scholar]
- 35.Romani L, Puccetti P, Bistoni F. Biological role of Th cell subsets in candidiasis. Chem Immunol. 1996;63:115–137. [PubMed] [Google Scholar]
- 36.Samaranayake Y H, Samaranayake L P. Candida krusei: biology, epidemiology, pathogenicity and clinical manifestations of an emerging pathogen. Med Microbiol. 1994;41:295–310. doi: 10.1099/00222615-41-5-295. [DOI] [PubMed] [Google Scholar]
- 37.Stevenhagen A, van Furth R. Interferon-gamma activates the oxidative killing of Candida albicans by human granulocytes. Clin Exp Immunol. 1993;91:170–175. doi: 10.1111/j.1365-2249.1993.tb03374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stevens D A, Walsh T J, Bistoni F, Cenci E, Clemons K V, Del Sero G, Fe D'Ostiani C, Kullberg B J, Mencacci A, Roilides E, Romani L. Cytokines and mycoses. Med Mycol. 1998;36:174–182. [PubMed] [Google Scholar]
- 39.Tascini C, Baldelli F, Monari C, Retini C, Pietrella D, Francisci D, Bistoni F, Vecchiarelli A. Inhibition of fungicidal activity of polymorphonuclear leukocytes from HIV-infected patients by interleukin (IL)-4 and IL-10. AIDS. 1996;10:477–483. doi: 10.1097/00002030-199605000-00005. [DOI] [PubMed] [Google Scholar]
- 40.Tonnetti L, Spaccapelo R, Cenci E, Mencacci A, Puccetti P, Coffman R L, Bistoni F, Romani L. Interleukin-4 and -10 exacerbate candidiasis in mice. Eur J Immunol. 1995;25:1559–1565. doi: 10.1002/eji.1830250614. [DOI] [PubMed] [Google Scholar]
- 41.Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251–276. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 42.Wingard J R. Importance of Candida species other than C. albicans as pathogens in oncology patients. Clin Infect Dis. 1995;20:115–125. doi: 10.1093/clinids/20.1.115. [DOI] [PubMed] [Google Scholar]
- 43.Wingard J R, Merz W G, Rinaldi M G, Johnson T R, Karp J E, Saral R. Increase in Candida krusei infection among patients with bone marrow transplantation and neutropenia treated prophylactically with flucanazole. N Engl J Med. 1991;325:1274–1277. doi: 10.1056/NEJM199110313251803. [DOI] [PubMed] [Google Scholar]
- 44.Yoshida Y, Kang K, Berger M, Chen G, Gilliam A C, Moser A, Wu L, Hammerberg C, Cooper K D. Monocyte induction of IL-10 and down-regulation of IL-12 by iC3b deposited in ultraviolet-exposed human skin. J Immunol. 1998;161:5873–5879. [PubMed] [Google Scholar]