Abstract
Introduction
Even after the enforcement of the lockdown, the government was unable to control the spread of the COVID-19 infection. Vaccination is the only remaining hope for preventing and controlling COVID-19 infections. The knowledge and attitude of the recipients can influence vaccine acceptance. In this study, we aim to assess the knowledge and attitude toward the COVID-19 vaccine among the general rural population of India.
Methodology
A community-based, prospective, cross-sectional study was conducted from May 2021 to October 2021 in the rural part of the Mandya district of Karnataka, India. Individuals over the age of 18 who met the Ministry of Health and Family Welfare's vaccination eligibility criteria were included in the study. Demographic details of participants and assessment of knowledge and attitude towards the COVID-19 vaccine were done in a designed and validated data collection form.
Results
The study included 596 participants, with females dominating males by 54.9 % (327). The average age of the participants was 31 years. Among them, 81.71% (487) had adequate knowledge, and 81.5% (486) had a positive attitude towards the COVID-19 vaccine. Females (85.3%, 279) tend to have a more positive attitude than males (77%, 207). Positive attitude participants (86.86 %, 423) have a higher level of knowledge about the COVID-19 vaccine than negative attitude participants (57.79 %, 63).
Conclusion
In the study, we found that 81.71% had adequate knowledge and 81.5% had a positive attitude toward the COVID-19 vaccine.
Keywords: COVID-19 vaccine, Knowledge, Attitude
Abstract
Introducción
Incluso tras la obligatoriedad del confinamiento el gobierno fue incapaz de controlar la propagación de la infección por COVID-19. La vacuna es la única esperanza que queda para prevenir y controlar las infecciones por COVID-19. El conocimiento y la actitud de los receptores pueden influir en la aceptación de la vacuna. En este estudio, nuestro objetivo fue evaluar el conocimiento y la actitud hacia la vacuna contra la COVID-19 entre la población rural general de India.
Metodología
Se realizó un estudio transversal, prospectivo y con base comunitaria de mayo a octubre de 2021 en la zona rural del distrito Mandya de Karnataka, India. Se incluyó en el estudio a los individuos mayores de 18 años que cumplieron los criterios de elegibilidad del Ministerio de Sanidad y Bienestar Familiar. Los datos demográficos de los participantes y la evaluación del conocimiento y la actitud hacia la vacuna contra la COVID-19 se incluyeron en un formulario de recopilación de datos diseñado y validado.
Resultados
El estudio incluyó a 596 participantes, siendo más numerosas las mujeres que los hombres en un 54,9 % (327). La edad media de los participantes fue de 31 años. Entre ellos, el 81,71% (487) tenía un conocimiento adecuado, y el 81,5% (486) una actitud positiva hacia la vacuna contra la COVID-19. Las mujeres (85,3%, 279) tendieron a tener una actitud más positiva que los hombres (77%, 207). Los participantes con actitud positiva (86,86 %, 423) tuvieron un mayor nivel de conocimiento sobre la vacuna contra la COVID-19 que los participantes con actitud negativa (57,79 %, 63).
Conclusión
En el estudio, encontramos que el 81,71% tuvo un conocimiento adecuado, y el 81,5% una actitud positiva hacia la vacuna contra la COVID-19.
Palabras clave: Vacuna contra la COVID-19, Conocimiento, Actitud
Introduction
After being discovered in Wuhan, China, in December 2019, the Severe Acute Respiratory Syndrome Corona Virus-2 (SARS-CoV-2) spread rapidly throughout the world.1 , 2 The SARS-CoV-2 virus infected almost all countries and had a significant negative impact on health, society, and the economy.3 A wide range of medications have been used off-label to treat the disease.4., 5., 6., 7. Drugs repurposed for treatment do not show promising results in curing the disease. On the other hand, excessive medication use increased the risk of developing drug-related adverse effects and microbial resistance.8 , 9 The government strictly implemented preventive measures such as wearing masks, keeping a safe distance, regular hand washing, avoiding crowds, and locking down to prevent the spread of the disease. Even so, it was unable to stop spreading. Hence, there is no other option than vaccination to prevent the spread of disease and decrease morbidities and motility associated with disease and economic crisis.
COVID-19 vaccines are developed to guide the production of SARS-CoV-2 neutralising antibodies by primarily targeting the surface spike protein which prevents the disease invasion.10 , 11 Its top priorities are to combat infection, prevent disease spread, and control the disease.12 Even after the manufacturer claimed that the vaccine was effective against SARS-CoV-2,13., 14., [15], [16], 17., 18. people doubted the vaccine due to its rushed development by skipping steps and spreading rumours about the adverse reaction. It is important to evaluate the general public's knowledge and attitude to vaccination to tackle all potential barriers to vaccination.
The COVID-19 immunisation campaign in India began on January 16, 2021, with the restricted use authorization in the emergency of Covishield®, Covaxin®, and Sputnik V®.2 , 19 India's COVID-19 vaccine drive will be the largest and most challenging because of its large population size and diverse socioeconomic background. Before developing a vaccination strategy to achieve the goal of COVID-19 vaccination, authorities must first understand the target population's knowledge and attitude. It aids in determining the lack and cause of hesitancy as well as encouraging participation in the vaccination campaign. The goal of this study is to assess the general rural Indian population's knowledge and attitude toward the COVID-19 vaccine.
Methods and methodology
Study design
A community-based, prospective, cross-sectional study was conducted from May 2021 to October 2021 in the rural part of the Mandya district of Karnataka, India after obtaining ethical clearance from the Institutional Ethics Committee of Adichunchanagiri Hospital and Research Centre (AH & RC), B.G. Nagara (Approval No. IEC/AH&RC/AC/012/2021).
Study population
We obtained 385 as the sample size for the study by using the total population of the Mandya district as the population size, a 5% margin of error, a response distribution of 50%, and a 95% confidence level using Raosoft® sample size calculation software.
Participants
Individuals over the age of 18 who met the Ministry of Health and Family Welfare's vaccination eligibility criteria (https://www.mohfw.gov.in/covid_vaccination/vaccination/index.html) and were willing to consent to participate in the study were included. Individuals with psychological disorders and those under the age of 18 were excluded from the study.
Study instrument
The questionnaire was created using a thorough literature review and expert opinions. The preliminary draught of the questionnaire design was discussed with two academic experts in the field and modified based on their suggestions. The questionnaire was split into two sections: Section A and Section B. Section A was used to collect demographic information, and Section B was used to assess vaccine knowledge and attitudes. The total of seven questions, four for knowledge and three for attitude, shows consistency and was acceptable with a Cronbach’s alpha coefficient of 0.758. Each correct answer received one point, while incorrect answers received zero points. The maximum score for knowledge is 4 and 3 for attitude. Those who scored more than 80% on the knowledge and attitude questionnaire were considered to have adequate knowledge and attitude toward the COVID vaccine.
Study procedure and data collection
The study team was a part of the COVID warriors belonging to AH & RC, Adichunchanagiri University, and were fully vaccinated with available COVID vaccines. Following all the preventive measures, the study team spread into the rural part of the Mandya district, explained the study in detail, and requested to take participants. Data was collected from the general rural population in a data collection form after screening for inclusion criteria and signing a consent form. After the collection of data, participants were explained in detail about the vaccines, their efficacy, side effects, benefits of vaccination, and the requirement for continuation of the preventive measure to prevent the SARS-CoV-2 infection after vaccination.
Statistical analysis
The Statistical Package for the Social Sciences (SPSS) version 26 was used to analyse the data. Gender, habits, occupation, socioeconomic status, source of vaccine information, and vaccination status were represented in frequency and percentage. Age, knowledge, and attitude were represented in the mean, standard deviation (S.D), and interquartile range (IQR). The Chi-Square test was used to determine the relationship between variables.
Results
Demographic details of the participants
The study included 596 participants, with females dominating males by 54.9 % (327). The average age of the participants was 31.24 ± 15.87, with an IQR of 10.75 (34.75, 24). Among them, 20% (119) were inoculated with the available COVID-19 vaccine in their area. News, radio, television, and social media were the primary sources of COVID vaccine information, accounting for 60.3 % (359). Since the study was conducted in rural Mandya, 45.13 % (269) of the participants earn less than one lakh rupees per year, indicating a low socioeconomic status (Table 1, Table 2 ).
Table 1.
Demographic details of the participants.
| Characters | Frequency | Percent | |
|---|---|---|---|
| Gender | Female | 327 | 54.9 |
| Male | 269 | 45.1 | |
| Age in years | 18-45 | 505 | 84.7 |
| 45-60 | 77 | 12.9 | |
| Above 60 | 14 | 2.3 | |
| Habits | Alcohol | 33 | 5.5 |
| Smoking | 12 | 2 | |
| Occupation | Self-employed | 173 | 29 |
| Student | 325 | 54.5 | |
| Employed | 98 | 16.5 | |
| Socio-economic status | 1–5 lakhs | 218 | 36.6 |
| 5–10 lakhs | 109 | 18.3 | |
| Less than 1 lakhs | 269 | 45.1 | |
| Source of information for COVID vaccine | Friends and family | 50 | 8.4 |
| Covid warrior | 144 | 24.2 | |
| Social media, radio, T V and news | 359 | 60.2 | |
| Government agency | 43 | 7.2 | |
| Vaccination status | Yes | 119 | 20 |
| No | 477 | 80 | |
Table 2.
Descriptive analysis of age, knowledge, and attitude of the participants.
| Characters | Mean | SD | IQR (Q3, Q1) |
|---|---|---|---|
| Age in years | 31.24 | 15.87 | 10.75 (34.75, 24) |
| Knowledge | 3.72 | 0.72 | 0 (4, 4) |
| Attitude | 2.72 | 0.66 | 0 (3, 3) |
Knowledge about COVID-19 vaccines
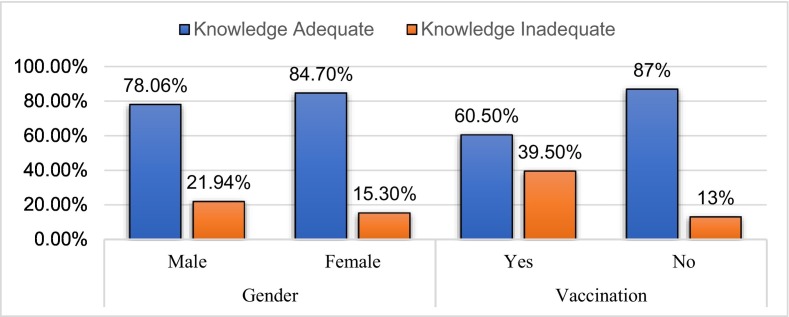
In the assessment of knowledge about COVID-19 vaccines, the mean score was 3.72 ± 0.72 (Table 2). Of 596, 81.71% (487) had adequate knowledge about the COVID-19 vaccine. 84.7% (277) of the total female participants reported having adequate knowledge and 78.06% (210) of the total male participants reported having adequate knowledge about the COVID-19 vaccine. There was no statistically significant difference in adequate knowledge among males and females (p-value, 0.37). We observed that 60.5% (72) of the vaccinated participants had adequate knowledge and 39.5% (47) had a lack of adequate knowledge. Similarly, 87% (415) of the not-vaccinated participants reported having adequate knowledge and 13% (62) of the total not-vaccinated reported a lack of adequate knowledge. There was a statistically significant difference in adequate knowledge among the vaccinated and not-vaccinated (p-value < 0.001) (Table 3, Table 4 , Fig. 1 ).
Table 3.
Correct response to the questionnaire about knowledge and attitudes.
| Questionnaires | Frequency | Percentage | |
|---|---|---|---|
| Knowledge | Single dose of the COVID vaccine is enough to prevent infection. (Q1) | 557 | 93.5% |
| The reason of inoculating the Covid-19 vaccine is to develop the neutralizing antibodies against COVID-19. (Q2) | 568 | 95.3% | |
| The common side effects of Covid-19 vaccines are Injection site pain, myalgia, fever and chills. (Q3) | 523 | 87.8% | |
| All the age group can be vaccinated. (Q4) | 569 | 95.5% | |
| Participants scored above 80% | 487 | 81.7% | |
| Attitude | I think Covid-19 vaccines are safe. (Q5) | 542 | 90.9% |
| I should not follow the preventive measures after vaccination. (Q6) | 517 | 86.7% | |
| I will recommend my family and friends to get vaccinated (Q7) | 563 | 94.5% | |
| Participants scored above 80% | 486 | 81.5% | |
Table 4.
Association between variables.
| Adequate Knowledge toward COVID vaccine |
P value | Positive Attitude toward COVID vaccine |
P value | ||||
|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | ||||
| Vaccination | Yes | 72 (60.5%) | 47 (39.5%) | < 0.001 | 61 (51.26%) | 58 (48.74%) | < 0.001 |
| No | 415 (87%) | 62 (13%) | 425 (89.1%) | 52 (10.9%) | |||
| Gender | Male | 210 (78.06%) | 59 (21.94%) | 0.37 | 207 (77%) | 62 (23%) | 0.009 |
| Female | 277 (84.7%) | 50 (15.3%) | 279 (85.3%) | 48 (14.7%) | |||
| Positive Attitude toward vaccine | Yes | 423 (86.86%) | 64 (13.14%) | < 0.001 | |||
| No | 63 (57.79%) | 46 (42.21%) | |||||
Level of significant 0.05.
Fig. 1.
Distribution of knowledge about COVID vaccine based on gender and vaccination groups.
Attitude towards COVID-19 vaccines
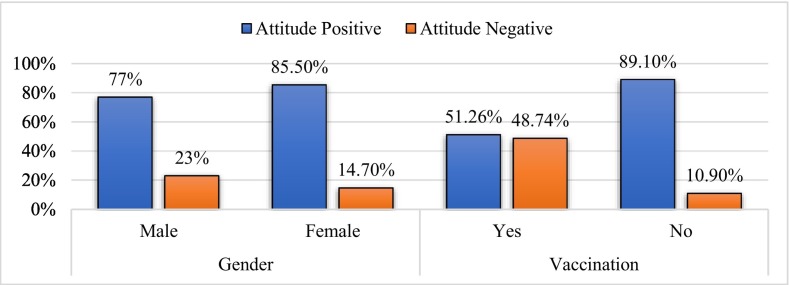
In the assessment of attitudes towards COVID-19 vaccines, the mean score was 2.72 ± 0.66 (Table 2). Out of 596, 81.5% (486) had a positive attitude towards the COVID-19 vaccine. 85.3% (279) of the total female participants reported having a positive attitude and 77% (207) of the total male participants reported having a positive attitude towards the COVID-19 vaccine. There was no statistically significant difference in positive attitudes among males and females (p-value < 0.01). We observed that 51.26% (61) of the vaccinated participants had a positive attitude and 48.74% (58) had a negative attitude. Similarly, 89.1% (425) of the not-vaccinated participants reported having a positive attitude and 10.9% (52) of the total not-vaccinated reported having a negative attitude. There was a statistically significant difference in positive attitude among the vaccinated and not-vaccinated (p-value < 0.001) (Table 3, Table 4, Fig. 2 ).
Fig. 2.
Distribution of attitude towards COVID vaccine based on gender and vaccination groups.
Association between knowledge and attitude
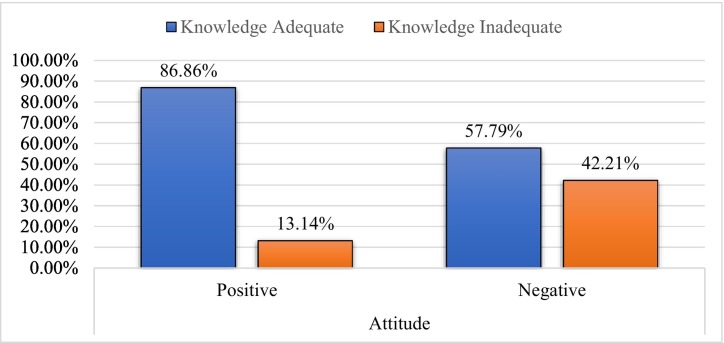
Among the participants with a positive attitude towards the COVID-19 vaccine, 86.86% (423) had adequate knowledge, and the remaining 13.14% (64) had a lack of adequate knowledge. Of those who had a negative attitude toward a vaccine, 57.79% (63) had adequate knowledge, and the remaining 42.2% (46) lacked adequate knowledge. There was a statistically significant difference in adequate knowledge among positive and negative attitudes (p-value < 0.001) (Table 3, Table 4, Fig. 3 ).
Fig. 3.
Distribution of knowledge about COVID vaccine based on attitude towards COVID vaccine.
Discussion
The study assessed the general rural Indian population's knowledge and attitude toward the COVID-19 vaccine. We observed that 81.71% (487) had adequate knowledge and 81.5% (486) had a positive attitude toward COVID-19 vaccines. The studies conducted by Popa GL et al. in Romania and Shekhar R et al. in Greece show greater levels of knowledge than our study.20 , 21 While Obarisiagbon OE et al. in Nigeria and Asmara Adella G et al. in Ethiopia show lower levels of knowledge than our study.22 , 23 A greater positive attitude towards the COVID-19 vaccine was found in our study than in Obarisiagbon OE et al., in Nigeria; Alle YF et al., in Ethiopia; and Khan ZA et al., in Pakistan.22 , 24 , 25 A similar positive attitude was reported in the study conducted by Jankowska-Polaska B et al. in Poland.26
We observed no statistically significant difference between the knowledge of males and females (p-value, 0.37). However, females (84.7%, 277) were found to have slightly more knowledge about the COVID-19 vaccine than males (78.06%, 210). The majority of study participants (93.5%) were aware that the full dose of vaccination required two doses of available Covid-19 vaccine, which was higher than Asmare Adella G et al..23 Among genders, females (85.3%, 279) tend to have a higher positive attitude than males (77%, 207) (p-value < 0.01). The trust in the safety of the COVID-19 vaccine (90.9%) was the same as reported by Bălan A et al. and higher than Bagic D et al..27 , 28 The majority of study participants (95.4%), which was higher than the percentage reported by Shekhar R et al., Moskova M et al., and Islam MS et al.,21 , 29 , 30 wanted to recommend vaccination to their friends and family.
The study shows that there was a statistically significant difference in the level of knowledge between the non-vaccinated and vaccinated (p-value < 0.001). Non-vaccinated participants (87%, 415) appeared to have a higher level of knowledge than vaccinated participants (60.5%, 72) (Fig. 1, Fig. 2). It could be influenced by the government vaccination strategy, where vaccination campaign phase 1 was to protect health care facilities by vaccinating front-line workers, then reduce motility by vaccinating vulnerable individuals (vaccination campaign phase 2), and finally control the disease by vaccinating adults and children (vaccination campaign phase 3).31 There were a high number of geriatrics vaccinated and a shortage of vaccine during the study duration, which might have affected the level of knowledge among the vaccinated. The literacy level of Mandya's rural population may have an impact on vaccine knowledge, as the majority relied on news, social media, and radio for information. Moskova M et al also reported radio and television as major sources of information.29
In the study, there is a statistically significant difference in knowledge about the COVID-19 vaccine between participants with positive attitudes and those with negative attitudes (p-value < 0.001). Positive attitude participants (86.86 %, 423) have a higher level of knowledge about the COVID-19 vaccine than negative attitude participants (57.79 %, 63) (Fig. 3). This emphasized the significance of maintaining a positive attitude in an attempt to acquire adequate knowledge.
Limitations
The study was carried out during a government-enforced lockdown, which limited the number of participants. Vaccination campaigns phases 1 and 2 ended during the study period, and vaccination campaign phase 3 began, with the maximum number of geriatrics vaccinated and adults waiting in line. These were the limitations of the study.
Conclusion
The study concluded that 81.71% (487) of the total participants had adequate knowledge and 81.5% (486) had a positive attitude toward COVID-19 vaccines. There is no gender difference in knowledge of the COVID-19 vaccine. When compared to the male, the female has a more positive attitude toward the COVID-19 vaccine. Further study with a diverse study population is necessary to corroborate the findings of the study.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Funding
This study did not receive any particular funding.
Declaration of Competing Interest
The authors declare that they have no potential conflict of interest in the publication of this research output.
Acknowledgement
Firstly, the authors would like to express the most profound gratitude to all of the respondents who participated in this study. Also we would like to thank Adichunchanagiri Hospital and Research Centre for the approval of ethical clearance.
References
- 1.Dai L., Gao G.F. Viral targets for vaccines against COVID-19. Nat Rev Immunol. 2020 Dec;18:1–10. doi: 10.1038/s41577-020-00480-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shrestha Y., Venkataraman R., Moktan J.B., Chitti R., Yadav S.K. 2021 Apr 29. COVID-19 vaccine authorized in India-a mini review. Available at SSRN 3836545. [Google Scholar]
- 3.Islam M.S., Siddique A.B., Akter R., Tasnim R., Sujan M.S., Ward P.R., Sikder M.T. Knowledge, attitudes and perceptions towards COVID-19 vaccinations: a cross-sectional community survey in Bangladesh. BMC Public Health. 2021 Dec;21(1) doi: 10.1186/s12889-021-11880-9. 1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gautret P., Lagier J.C., Parola P., Meddeb L., Mailhe M., Doudier B., et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020 Jul 1;56(1):105949. doi: 10.1016/j.ijantimicag.2020.105949. pmid:32205204. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5.Mahmud R., Rahman M.M., Alam I., Ahmed K.G.U., Kabir A.K.M.H., Sayeed S.K.J.B., et al. Ivermectin in combination with doxycycline for treating COVID-19 symptoms: a randomized trial. J Int Med Res. 2021;49(5) doi: 10.1177/03000605211013550. pmid:33983065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-kuraishy H.M., Al-Gareeb A.I., Qusty N., Cruz-Martins N., El-Saber Batiha G. Sequential doxycycline and colchicine combination therapy in Covid-19: The salutary effects. Pulm Pharmacol Ther [Internet] 2021 Apr;67(January):102008. doi: 10.1016/j.pupt.2021.102008. pmid:33727066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arshad S., Kilgore P., Chaudhry Z.S., Jacobsen G., Wang D.D., Huitsing K., et al. Treatment with hydroxychloroquine, azithromycin, and combination in patients hospitalized with COVID-19. Int J Infect Dis. 2020 Aug 1;(97):396–403. doi: 10.1016/j.ijid.2020.06.099. pmid:32623082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shrestha Y., Venkataraman R., Moktan J.B., Mallikarjuna S., Narayan S.S., Madappa M.H., Satya P.A. Impact of medication complexity assessment on admission to pharmacotherapy evaluation in COVID patients. J Young Pharm. 2022;14(3):322–326. [Google Scholar]
- 9.Shrestha Y., Shivalingegowda R.K., Avinash M.J., Kenchegowda S.B., Moktan J.B., Doddasamiah S.M., Tambat R.M., Golshetty D.G., Ganesh V.S., Venkataraman R. The rise in antimicrobial resistance: an obscure issue in COVID-19 treatment. PLOS Global Public Health. 2022;2(7) doi: 10.1371/journal.pgph.0000641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Piccoli L., Park Y.J., Tortorici M.A., Czudnochowski N., Walls A.C., Beltramello M., et al. Mapping neutralizing and immunodominant sites on the SARS-CoV-2 spike receptor-binding domain by structure-guided high-resolution serology. Cell. 2020;183(4):1024–1042.e21. doi: 10.1016/j.cell.2020.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shrestha Y., Venkataraman R. The prevalence of inverse health consequences of COVID-19 vaccines: a post-vaccination study. Vacunas. 2022 Mar 24 doi: 10.1016/j.vacun.2022.03.002. Epub ahead of print. PMID: 35345826; PMCID: PMC8942717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hodgson S.H., Mansatta K., Mallett G., Harris V., Emary K.R., Pollard A.J. What defines an efficacious COVID-19 vaccine? A review of the challenges assessing the clinical efficacy of vaccines against SARS-CoV-2. Lancet Infect Dis. 2021;21(2):e26–e35. doi: 10.1016/S1473-3099(20)30773-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Voysey M., Clemens S.A., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., Angus B., Baillie V.L., Barnabas S.L., Bhorat Q.E., Bibi S. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021 Jan 9;397(10269):99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramasamy M.N., Minassian A.M., Ewer K.J., Flaxman A.L., Folegatti P.M., Owens D.R., et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2020 Dec 19;396(10267):1979–1993. doi: 10.1016/S0140-6736(20)32466-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ella R., Vadrevu K.M., Jogdand H., Prasad S., Reddy S., Sarangi V., Ganneru B., Sapkal G., Yadav P., Abraham P., Panda S. Safety and immunogenicity of an inactivated SARSCoV-2 vaccine, BBV152: a double-blind, randomised, phase 1 trial. Lancet Infect Dis. 2021;21(5):637–646. doi: 10.1016/S1473-3099(20)30942-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ella R., Reddy S., Jogdand H., Sarangi V., Ganneru B., Prasad S., et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: interim results from a double-blind, randomised, multicentre, phase 2 trial, and 3-month follow-up of a double-blind, randomised phase 1 trial. Lancet Infect Dis. 2021;21(7):950–961. doi: 10.1016/S1473-3099(21)00070-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Logunov D.Y., Dolzhikova I.V., Zubkova O.V., Tukhvatullin A.I., Shcheblyakov D.V., Dzharullaeva A.S., et al. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: two open, non-randomised phase 1/2 studies from Russia. Lancet. 2020 Sep 26;396(10255):887–897. doi: 10.1016/S0140-6736(20)31866-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Logunov D.Y., Dolzhikova I.V., Shcheblyakov D.V., Tukhvatulin A.I., Zubkova O.V., Dzharullaeva A.S., et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021 Feb 20;397(10275):671–681. doi: 10.1016/S0140-6736(21)00234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar V.M., Pandi-Perumal S.R., Trakht I., Thyagarajan S.P. Strategy for COVID19 vaccination in India: the country with the second highest population and number of cases. NPJ Vaccines. 2021;6(1):1–7. doi: 10.1038/s41541-021-00327-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Popa G.L., Muntean A.A., Muntean M.M., Popa M.I. Knowledge and attitudes on vaccination in southern romanians: a cross-sectional questionnaire. Vaccines. 2020;8(4):1–7. doi: 10.3390/vaccines8040774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shekhar R., Sheikh A.B., Upadhyay S., Singh M., Kottewar S. COVID19 vaccine acceptance among health care workers in the United States. Vaccines. 2021;9:1–15. doi: 10.3390/vaccines9020119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Obarisiagbon O.E., Mokogwu N. Assessment of knowledge, attitude and factors influencing uptake of COVID-19 vaccine among traders at Edaiken Market, Uselu, Benin City, Edo State, Nigeria. West Afr J Med. 2022;39(4):327–335. [PubMed] [Google Scholar]
- 23.Asmare Adella G. Knowledge and attitude toward the second round of COVID-19 vaccines among teachers working at southern public universities in Ethiopia. Hum Vaccin Immunother. 2022 Dec 31;18(1):2018895. doi: 10.1080/21645515.2021.2018895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alle Y.F., Oumer K.E. Attitude and associated factors of COVID-19 vaccine acceptance among health professionals in Debre Tabor Comprehensive Specialized Hospital, North Central Ethiopia; 2021: cross-sectional study. Virusdisease. 2021 Jun;32(2):272–278. doi: 10.1007/s13337-021-00708-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khan Z.A., Allana R., Afzal I., Ali A.S., Mariam O., Aslam R., Shah I.A., Allana A., Haider M.M., Jandani R., Khan Z., Siddiqui A.M., Shah J.A., Butt U. Assessment of attitude and hesitancy toward vaccine against COVID-19 in a Pakistani population: A mix methods survey. Vacunas. 2022 May;23:S26–S32. doi: 10.1016/j.vacun.2021.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jankowska-Polańska B., Sarzyńska K., Czwojdziński E., Świątoniowska-Lonc N., Dudek K., Piwowar A. Attitude of Health Care Workers and Medical Students towards Vaccination against COVID-19. Vaccines (Basel) 2022;10(4):535. doi: 10.3390/vaccines10040535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bălan A., Bejan I., Bonciu S., Eni C.E., Ruță S. Romanian medical students' attitude towards and perceived knowledge on COVID-19 vaccination. Vaccines (Basel) 2021 Aug 4;9(8):854. doi: 10.3390/vaccines9080854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bagic D., Suljok A., Ancic B. Determinants and reasons for coronavirus disease 2019 vaccine hesitancy in Croatia. Croat Med J. 2022;63:89–97. doi: 10.3325/cmj.2022.63.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moskova M., Zasheva A., Kunchev M., Popivanov I., Dimov D., Vaseva V., Kundurzhiev T., Tsachev I., Baymakova M. Students' attitudes toward COVID-19 vaccination: an inter-university study from Bulgaria. Int J Environ Res Public Health. 2022;19(16):9779. doi: 10.3390/ijerph19169779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Islam M.S., Siddique A.B., Akter R., Tasnim R., Sujan M.S.H., Ward P.R., Sikder M.T. Knowledge, attitudes and perceptions towards COVID-19 vaccinations: a cross-sectional community survey in Bangladesh. BMC Public Health. 2021 Oct 13;21(1):1851. doi: 10.1186/s12889-021-11880-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Government of India, Ministry of Health and Family Welfare Liberalised Pricing and Accelerated National Covid-19 Vaccination Strategy. https://www.mohfw.gov.in/pdf/LiberalisedPricingandAcceleratedNationalCovid19VaccinationStrategy2042021.pdf Available at.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.