Many antivirals have been shown effective at preventing COVID-19 infection from turning severe, especially if used in the early stages of infection, often in combination with antibiotics and supplements (c19early.org/). Fluvoxamine, which is not an antiviral, nor an antibiotic but an antidepressant of the selective serotonin reuptake inhibitor class, was first shown effective in preventing serious consequences of COVID-19 infection in hospitalized patients during a placebo-controlled, randomized, trial reported in November 2020. According to (Reis et al., 2022), Fluvoxamine reduced both the risk of death and the need for intensive care in acutely symptomatic COVID-19 patients. An animal model (Rosen et al., 2019) first noticed as Fluvoxamine reduced inflammation in mice with sepsis. The rationale behind the use of Fluvoxamine for COVID-19 infection was then the opportunity to benefit from the anti-inflammatory action of this drug when inflammation in COVID-19-infected patients was excessive.
Fluvoxamine mechanism of action on COVID-19 infection is everything but intuitive. While its use is aimed at conditions such as depression and obsessive-compulsive disorder, it also reduces immune responses and alleviates tissue damage. Especially these two effects are acknowledged for the achievement in the trial (Reis et al., 2022). Other trials have been performed with Fluvoxamine, unfortunately not that many, small in size, and everything but free from criticism, same as (Reis et al., 2022). At present, the database of all Fluvoxamine COVID-19 studies (c19early.org/ 2022) includes 13 clinical trials which are used to summarize the benefits as shown in Fig. 1 .
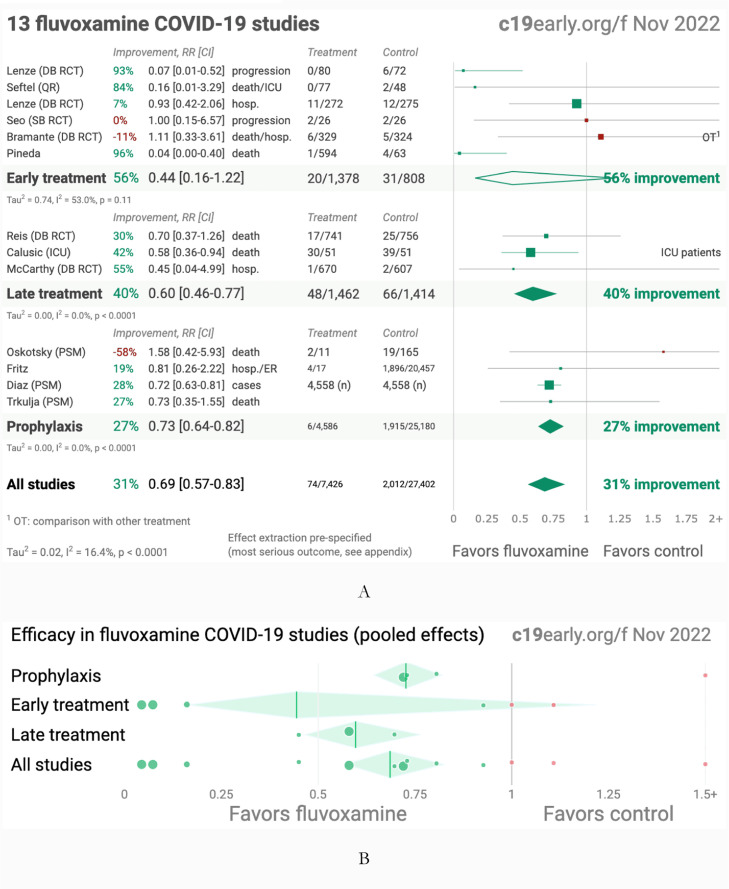
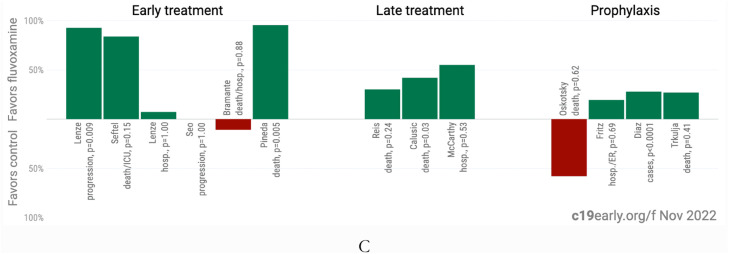
Fig. 1.
A. Random effects meta-analysis. B. Scatter plot showing the most serious outcome in all studies, along with the result of random effects meta-analysis. C. History of all reported effects (chronological within treatment stages). Images from (c19early.org/ 2022). Credit c19early.org. Supporting works are Reis doi.org/10.1016/S2214-109X(21)00,448-4, Lenze doi.org/10.1001/jama.2020.22760, Seftel doi.org/10.1093/ofid/ofab050, Lenze dcricollab.dcri.duke.edu/sites/NIHKR/KR/GR-Slides-08–20–21.pdf, Seo doi.org/10.3947/ic.2021.0142, Bramante doi.org/10.1056/NEJMoa2201662, Pineda doi.org/10.1101/2022.09.27.22280428, Calusic doi.org/10.1111/bcp.15126, McCarthy doi.org/10.1101/2022.10.17.22281178, Oskotsky doi.org/10.1001/jamanetworkopen.2021.33090, Fritz doi.org/10.1038/s41398-022-02,109-3, Diaz doi.org/10.4088/PCC.22br03337 and Trkulja doi.org/10.21203/rs.3.rs-2,239,187/v1.
All the proposed mechanisms of action for fluvoxamine against COVID-19 infection are also described in (c19early.org/ 2022). It is a functional inhibitor of acid sphingomyelinase (FIASMA). Inhibition of the ASM/ceramide system activated by SARS-CoV02 to facilitate the entry of the virus may prevent viral entry. It may influence σ−1 (S1R) receptor activation reducing excessive cytokine production. It may inhibit platelet activation contributing to COVID-19 severity. It may have lysosomotropic properties interfering with endolysosomal viral trafficking used by SARS-CoV-2 to leave infected cells. It may increase intracellular heme oxygenase (HO-1) thus reducing the COVID-19 risk which is correlated to low HO-1. It is a cytoprotective and anti-inflammatory agent. Fluvoxamine may decrease mast cell degranulation reducing the cytokine storm. It may inhibit CYP1A2 and CYP2C19 thus increasing the level of melatonin which has beneficial effects on COVID-19 infection.
All the COVID-19 treatments have been working better in the early stages of infection, as SARS-CoV-2 elimination is a race against viral build-up. While the best results have been achieved by using drugs with antiviral action, Fluvoxamine, which has been mostly working as an anti-inflammatory agent, still had its merit. The few trials conducted so far have been positive. By using the relative risk ratio RR (risk of an event in the exposed group versus the risk of the event in the other nonexposed group (Ranganathan et al., 2015)), in the list of substances that had a positive impact on COVID-19 infection patients of (c19early.org/ 2022), Fluvoxamine is ranked 31st, based on an improvement of 31% (RR = 0.69, CI = 0.57–0.83) compared to control, from 13 studies and 34,828 patients. Given the many different mechanisms of action, fluvoxamine may still have use in the few cases of patients infected by the current variants of SARS-CoV-2 and facing more serious effects mostly because of excessive cytokine production.
The above conclusions are consistent with the very recent findings of the meta-analysis (Fico et al., 2022) which investigated the role of several psychotropic drugs, including antidepressants (AD), mood stabilizers, and antipsychotics (AP), which have all been suggested to have positive effects in the treatment of COVID-19. Based on the review of the relevant articles published up to December 13, 2021, 29 studies total, of whom 15 were clinical, 9 of them quantitative, 9 preclinical, and 5 computational, the relative risk ratio RR as well as the odds ratio OR (odds of an event in the exposed group versus the odds of the event in the nonexposed group (Ranganathan et al., 2015)) was used to assess the efficacy. AD did not increase the risk of severe infection, RR = 1.71, CI = 0.65–4.51, or mortality, as RR = 0.94, CI = 0.81–1.09. Specifically, Fluvoxamine was associated with a reduced risk of mortality, OR = 0.15, CI = 0.02–0.95. AP increased the risk of severe infection, RR = 3.66, CI = 2.76–4.85, and mortality OR = 1.53, CI = 1.15–2.03. Ref. (Fico et al., 2022) concluded as Fluvoxamine could have been beneficial for COVID-19 infection due to its anti-inflammatory and antiviral potential, while evidence on other AD is controversial, which is consistent with the conclusion of (c19early.org/ 2022) and this work.
Funding
No funding.
Ethics approval statement
Not applicable.
Contributorship statement
Single author.
Declaration of Competing Interest
No competing interests.
Acknowledgements
Nothing to acknowledge.
Editor: Dr Jose Sanchez
References
- c19early.org/.
- Fico G., Isayeva U., De Prisco M., Oliva V., Solè B., Montejo L., Grande I., Arbelo N., Gomez-Ramiro M., Pintor L., Carpiniello B. Psychotropic drug repurposing for COVID-19: a systematic review and meta-analysis. Eur. Neuropsychopharmacol. 2022 doi: 10.1016/j.euroneuro.2022.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganathan P., Aggarwal R., Pramesh C.S. Common pitfalls in statistical analysis: odds versus risk. Perspect. Clin. Res. 2015;6(4):222. doi: 10.4103/2229-3485.167092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis G., dos Santos Moreira-Silva E.A., Silva D.C.M., Thabane L., Milagres A.C., Ferreira T.S., Dos Santos C.V.Q., de Souza Campos V.H., Nogueira A.M.R., de Almeida A.P.F.G., Callegari E.D. Effect of early treatment with fluvoxamine on risk of emergency care and hospitalisation among patients with COVID-19: the TOGETHER randomised, platform clinical trial. The Lancet Global Health. 2022;10(1):e42–e51. doi: 10.1016/S2214-109X(21)00448-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen D.A., Seki S.M., Fernández-Castañeda A., Beiter R.M., Eccles J.D., Woodfolk J.A., Gaultier A. Modulation of the sigma-1 receptor–IRE1 pathway is beneficial in preclinical models of inflammation and sepsis. Sci. Transl. Med. 2019;11(478):eaau5266. doi: 10.1126/scitranslmed.aau5266. [DOI] [PMC free article] [PubMed] [Google Scholar]