Abstract
Purpose
To report a case of ocular involving monkeypox infection in the United States during the 2022 outbreak, and to review the literature regarding its clinical manifestations and management known to date.
Observations
A 36-year-old man with well controlled HIV presented to the emergency department with anal pain, diffuse rash, right eye pain, and right eye redness after he tested positive for monkeypox one week prior. Ocular examination showed bilateral periorbital vesicular lesions, right eye conjunctival injection, and a single white plaque on his right medial bulbar conjunctiva. Macular, vesicular, and pustular lesions were noted throughout his body, including the genital and perianal region. His ocular and systemic symptoms completely resolved after treatment with a ten-day course of 1% trifluridine and moxifloxacin drops in both eyes, as well as two weeks of oral tecovirimat.
Conclusion and Importance
In July of 2022, monkeypox virus was declared a global health emergency by the World Health Organization; however, there are no standard guidelines for monkeypox treatment. Data on its clinical presentation and course, especially pertaining to ocular manifestations, is limited. We highlight the importance of recognizing ophthalmic manifestations of monkeypox virus and a possible therapeutic approach to help guide the management of these patients.
Keywords: Monkeypox virus, Ocular manifestations, Conjunctivitis, Conjunctival lesion
Abbreviations: MPV, Monkeypox virus; PCP, Primary care physician; STI, Sexually transmitted infection; CDC, Centers for Disease Control and Prevention; OD, right eye; OS, left eye; OU, both eyes; VIG, Vaccinia immune globulin; ART, Antiretroviral therapy; OSSN, Ocular surface squamous neoplasia; OCT, Optical coherence tomography
1. Introduction
Monkeypox virus (MPV) is a zoonotic DNA virus from the Orthopoxvirus genus in the family of Poxviridae, to which smallpox also belongs.1 First identified in monkeys in the 1950s, MPV spread to humans in 1970 in the Democratic Republic of the Congo, where it is considered endemic.2 The current monkeypox outbreak was confirmed in May 2022 in the United Kingdom, and by June 13, 2022, the World Health Organization reported nearly 800 cases in 27 countries.3 On August 4th, 2022, the United States then declared the monkeypox outbreak a public health emergency as the number of confirmed cases approached 7000 in America.4
Most infections in the 2022 MPV outbreak have occurred in men who have sex with men, and transmission is thought to be caused by prolonged skin-to-skin contact, often during intimate exchanges.5,6 Patients with monkeypox generally experience systemic symptoms such as fever, myalgias, and lymphadenopathy, which often precede the development of genital, perianal, and/or diffuse mucocutaneous lesions.5,6
There is currently a paucity of data on the possible ophthalmological manifestations and treatment of MPV. Conjunctivitis, conjunctival ulcers, periorbital lesions, keratitis, corneal ulceration and even blindness were reported in two large case series in the 1980s in Africa; however, specific ocular examination findings and treatments were not reported.7,8 More recently, Benatti et al.9 described the case of a patient with MPV in Italy who developed a bulbar conjunctival ulcer which resolved in three weeks after treatment with co-formulated neomycin, polymyxin B, dexamethasone ointment, and dexamethasone drops.
In this report, we present the examination findings, clinical course, and treatment of a case of ocular MPV infection in the United States. We hope our findings and review of the literature will help guide clinical decision making as the monkeypox outbreak unfolds in real time.
Our report was conducted in accordance with the principles of the Declaration of Helsinki and was compliant with the Health Insurance Portability and Accountability Act. Institutional Review Board approval was not required for this case report.
2. Case presentation
2.1. Presentation
A 36-year-old man with a history of well controlled HIV on antiretroviral therapy (ART; CD4 444) presented to the emergency department with worsening skin lesions, anal pain, and right eye pain and redness one week after testing positive for MPV.
The patient was well until 2 weeks prior to presentation when he had unprotected receptive anal intercourse that resulted in a painful anal fissure. Five days later, he developed low grade fevers, sore throat, several new perianal lesions, and swollen inguinal lymph nodes. He presented to his primary care physician (PCP) and underwent sexually transmitted infection (STI) testing as well as an anal swab for MPV. Over the following three days, the patient developed an eruption of lesions over his entire body, starting with the right forearm. At the same time, he developed photophobia, right eye redness, vesicular eyelid lesions, and a single conjunctival lesion. He became febrile to 103° with chills, night sweats, and worsening anal pain. Four days later, he received a call from his PCP confirming infection with MPV. Due to his ocular symptoms, worsening rash, and intense anal pain, the patient was instructed to present to the emergency room for further management and for urgent ophthalmology evaluation.
In the emergency room, the patient was afebrile with normal vital signs. His physical exam was notable for diffuse, 2–5 mm macular, papular, vesicular, and pustular lesions over the entire body, half with central umbilication. Several lesions were seen on his head and face, inside both ears, and in the left posterior pharynx. He had tender submandibular and inguinal lymph nodes bilaterally, accompanied by lesions on the penile shaft, scrotum, torso, back, and right palm. There was a small anal fissure, and perianal white plaque-like areas with coalescing vesicular lesions.
A bedside ophthalmic examination showed 20/20 vision OD and 20/20 vision OS. Pupils, visual fields, color vision, and extraocular motility were all normal. His intraocular pressures were 12 OD and 13 OS. External examination was notable for two, 1 mm × 1 mm vesicular lesions on the right upper eyelid, one of which was eroded (Fig. 1). A 0.5 mm × 0.5 mm eroded lesion was noted on the right central upper eyelid margin (Fig. 2b). In addition, one fleshy lesion without central erosion, which had been present for years per the patient, was seen on the right medial lower eyelid margin (Fig. 4a). Diffuse 3+ conjunctival injection more pronounced nasally was seen in the right eye as well as a raised, well-defined, 6 mm (height) x 4 mm (width) white plaque-like lesion, which was located nasally on the bulbar conjunctiva and extended to the limbus from 3:30 to 4:30 o'clock (Fig. 2a). In the left eye, the conjunctiva was white and quiet, and a round, 0.5 mm × 0.5 mm vesicular lesion was observed on the palpebral conjunctiva on upper eyelid eversion (Fig. 3). There was no discharge from either eye. The corneas of both eyes were clear without epithelial defects or ulceration OU, and the anterior chamber was deep and appeared quiet without any evidence of layering, hypopyon, or keratic precipitates. Dilated fundus exam was also unremarkable OU.
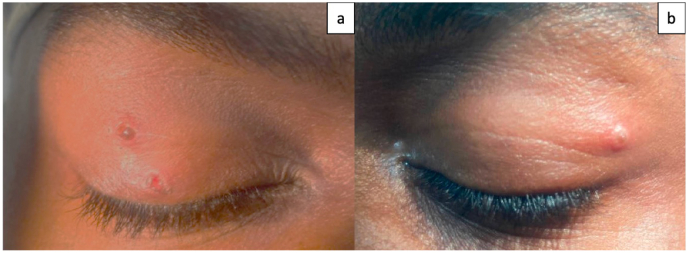
Fig. 1.
a. External photograph of the right eye showing one vesicular round lesion and one eroded lesion on the upper eyelid both measuring approximately 1 mm × 1 mm. b. External photograph of the left eye showing one vesicular round lesion on the lateral upper eyelid measuring approximately 1 mm × 1 mm.
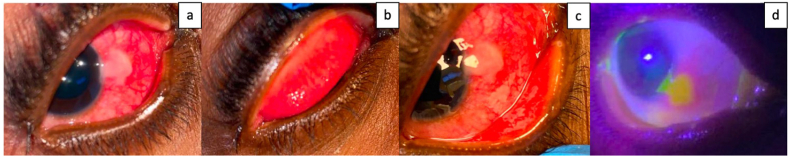
Fig. 2.
a. External photograph of the right eye showing diffuse 3+ conjunctival injection more pronounced nasally. An elevated white plaque like lesion measuring 6 mm (height) x 4 mm (width) is noted in the nasal bulbar conjunctiva extending to the limbus from 3:30 to 4:30 o'clock. b. A 0.5 mm × 0.5 mm eroded lesion on the right central upper eyelid margin was noted. Eversion of the right upper eyelid did not reveal any palpebral conjunctival lesions. Palpebral conjunctival injection is noted. c. Eversion of the right lower eyelid did not reveal any palpebral conjunctival lesions. Palpebral conjunctival injection is noted. d. The lesion stains with fluorescein stain under cobalt blue light. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
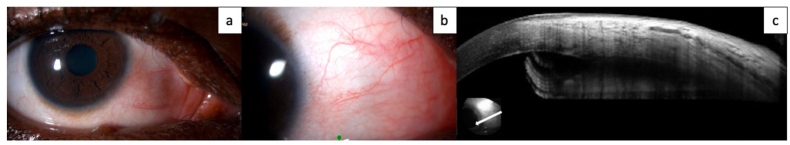
Fig. 4.
a. Slit lamp photograph of the right eye showing remaining trace nasal conjunctival injection. A fleshy round lesion is seen in the medial right lower eyelid which per patient has been present for years. b. Slit lamp photograph of the right eye focused on the nasal conjunctiva again showing nasal conjunctival injection and dilated conjunctival vessels. No corneal or conjunctival lesions are identified. c. Anterior segment optical coherence tomography (OCT) showing normal corneal and conjunctival architecture. Epithelium appears to be of normal thickness, without hyperreflectivity or abrupt transition.
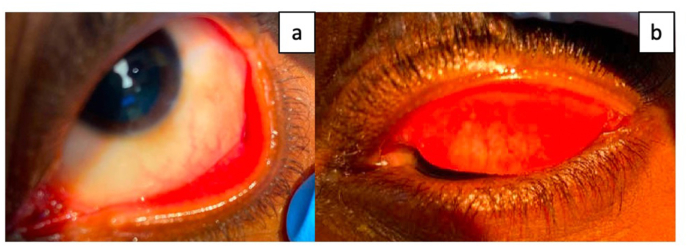
Fig. 3.
a. External photograph of the left eye showing relatively white bulbar conjunctiva. Eversion of the lower eyelid shows injected palpebral conjunctiva. Palpebral conjunctival injection is noted. b. Eversion of the left upper eyelid did not reveal any palpebral conjunctival lesions. A 0.5 mm × 0.5 mm vesicular conjunctival lesion was noted on the medial palpebral conjunctiva on examination however it is not well visible in this picture.
In coordination with the Centers for Disease Control and Prevention (CDC), health officials, emergency medical services, and hospital staff, the patient was admitted to an airborne isolation room for clinical observation and treatment, with providers following CDC recommendations for contact, droplet, and airborne precautions with eye protection.10
2.2. Treatment
On day 1 of hospitalization, the CDC and the Connecticut Department of Public Health were contacted to obtain tecovirimat for the patient's disseminated monkeypox infection. Tecovirimat was delivered the following day, and the patient was started on 600 mg twice daily orally.
For the patient's ocular manifestations, he was treated topically with one drop of trifluridine 1% every 2 hours while awake in both eyes for 3 days, followed by one drop 4 times per day in both eyes for 7 more days (total 10-day course). He was started on one drop of moxifloxacin in both eyes 4 times daily for ten days. We also recommended erythromycin ointment nightly on the eyelids for ten days, though bacitracin was used during hospitalization due to an erythromycin shortage in the hospital. Finally, one drop of artificial tears 4 times per day was recommended for comfort and lubrication. Oral morphine and acetaminophen controlled the patient's eye and anal pain.
2.3. Clinical course and follow up
Laboratory evaluation during the patient's hospital stay showed negative repeat STI testing for syphilis, gonorrhea, and chlamydia, in addition to negative anal HSV-1, and HSV-2 tests. Anal and skin lesions were swabbed and were positive for monkeypox. No eyelid or conjunctival swab was obtained. The patient's lesions began crusting over by hospital days 4–5, with 1/3 lesions crusting and desquamating. At this point, his photophobia and conjunctival injection in the right eye was significantly improved as well.
After five days in the hospital, the patient was discharged to home isolation until resolution of the rash. He was also provided tecovirimat to complete a 14-day course of treatment. Follow-up video visits with infectious disease were scheduled for continued monitoring. Three weeks later, the patient was seen in-person in the ophthalmology clinic, in accordance with CDC guidelines.10 His vision remained stable at 20/20 OU. Slit lamp examination showed complete resolution of his conjunctival lesions (Fig. 4a and b), which was confirmed with anterior segment OCT showing normal conjunctival architecture (Fig. 4c).
3. Discussion
After more than two years of confronting the global impact of COVID-19, the world now faces concerns of a new viral outbreak, monkeypox. The clinical syndrome of MPV is classically characterized by a prodromal phase of fever, rash, and lymphadenopathy. This is followed by diffuse body involvement of firm, deep-seated and well-circumscribed lesions, often with central umbilication that progress through sequential stages of macules, papules, vesicles, pustules, and scabs.6 Most cases associated with this MPV outbreak have initially presented as isolated involvement of the genital and anal areas. This can often be misdiagnosed as other sexually transmitted infections, including herpes, syphilis, lymphogranuloma venereum, chancroid, chlamydia or gonorrhea, as well as delay the start of isolation and treatment if needed. Transmission has been suspected to occur primarily through sexual activity6 as with our patient given his recent exposure; however, it is unknown whether the patient's sexual partner had symptoms at the time of exposure or developed symptoms subsequently.
Our case was notable for ocular involvement of MPV, including eye pain, eyelid lesions, conjunctivitis, and a single right conjunctival lesion. Most of the current literature on periocular and ocular manifestations of MPV was collected after outbreaks in western and central African countries.7, 8, 11, 12, 13 A case series of 282 patients in Zaire between 1980 and 19857 found eyelid margin lesions and focal conjunctival lesions in 13% of patients who received smallpox vaccination versus 17% of unvaccinated patients. Severe ocular complications such as corneal ulceration/keratitis were also more common in unvaccinated individuals (4.4% vs 3.1%, respectively). Unfortunately, corneal involvement led to visual changes, ranging from visual impairment (visual acuity was unspecified) to bilateral blindness in 11 children (10 unvaccinated, 1 vaccinated).
In another report from Hughes et al. in 2014, conjunctivitis was reported in 23.1% (n = 68) of MPV cases in the Democratic Republic of the Congo, with the majority of affected patients being children younger than 10 years old.12 Interestingly, the study showed that patients with conjunctivitis exhibited systemic symptoms in higher frequency when compared to patients without conjunctivitis, suggesting that ocular involvement may even be prognostic of disease course. In another cohort of 40 patients with MPV in Nigeria from 2017 to 2018, 22.5% (n = 9) of patients had photophobia and conjunctivitis, and 7.5% (n = 3) developed keratitis.13
Ocular manifestations of MPV can range in severity. The most common finding is conjunctivitis. Thus far, no intraocular manifestations have been fully documented in the literature. However, in a review of 11 cases of MPV in the Democratic Republic of the Congo in 2003, a child was noted to have a sustained conjunctivitis with “extensive damage”.14 Review of the photograph in that case showed inflamed sclera, corneal opacities and even possible small hypopyon, although it is difficult to gauge the extent of intraocular involvement from the report. In the Western Hemisphere, there is one case report of an adult with keratitis/corneal ulceration which required corneal transplantation.15 Given the wide range in severity of ophthalmic manifestations and the possibility for devastating ocular consequences, it is imperative to recognize and treat patients with MPV early. In the current monkeypox outbreak, ocular manifestations of MPV infection were noted in a 39-year-old male in Italy. In that case, 4 days after systemic symptom onset, the patient developed conjunctivitis of the left eye, a small vesicle on the left lower eyelid, and a whitish ulcer (10 mm) on the bulbar conjunctiva, similar to the findings we have described. This patient was treated with topical neomycin, polymyxin B and dexamethasone solution and ointment. Three weeks after symptom onset, his ocular and periocular lesions resolved with only remaining conjunctival injection.9 Another recent case report described a follicular conjunctivitis along with small white vesicles on the bulbar conjunctiva in a 39-year-old male in Switzerland. No eyelid or periocular skin lesions were reported. MPV was identified on conjunctival PCR swabs with a similar viral load to cutaneous lesions.16
Infection with MPV was first described in 1970, however, there are still no standard guidelines for management, and no currently licensed treatments in the U.S.17 In our case, the patient was treated with 1% trifluridine drops in both eyes for ten days, along with erythromycin or bacitracin ointment and moxifloxacin drops to prevent secondary bacterial superinfection. Topical trifluridine is a synthetic fluorinated pyrimidine nucleoside that inhibits viral DNA replication.18 It is predominately used and marketed for treatment of herpes simplex virus keratitis. However, trifluridine has also been used successfully for treatment of ocular vaccinia19,20; that is, conjunctivitis, conjunctival ulcers, keratitis, and/or intraocular inflammation secondary to the smallpox vaccine virus. Both MPV and the smallpox vaccinia virus belong to the same genus (Orthopoxvirus). In a 2004 case series in Ophthalmology, 10 patients with ocular vaccinia were treated with 1% trifluridine. Specifically, 7 patients were treated with topical trifluridine alone, 1 with trifluridine and ofloxacin drops, 1 with trifluridine and oral acyclovir, and 1 with trifluridine and vaccinia immune globulin (VIG). All but one patient's ocular vaccinia fully recovered after treatment; the remaining patient had a small stromal scar without decline in visual acuity.19 In another study of 56 rabbit eyes infected with the vaccinia virus, topical trifluridine alone (9 drops per day for ten days) was the most effective treatment for vaccinia induced corneal opacity, corneal discharge, and conjunctival chemosis compared to VIG alone or prednisolone acetate in combination with trifluridine or VIG20 based on the Modified MacDonald-Shadduck Scoring System.21 In fact, any combination of treatment with prednisolone impaired viral clearance and resulted in rebound disease.20
Trifluridine drops are typically well tolerated, but they have been associated with corneal toxicity, and therefore, should be used carefully. Adverse effects may include conjunctival cicatrization, punctate epithelial keratopathy, corneal epithelial dysplasia, corneal edema, filamentary keratitis, and even severe anterior segment ischemia.22, 23, 24 The general recommended dose and frequency of trifluridine is 1 drop every 2 h while awake for a maximum of nine drops per day. Once the cornea begins healing, one drop every 4 hours (maximum 5 drops per day) is continued for one week.23
Only two orally available antiviral drugs, tecovirimat and brincidofovir, are available for systemic treatment in the U.S. and have demonstrated efficacy against orthopoxviruses in animal models.25, 26 The patient in this case was successfully treated with tecovirimat, which has FDA-approval for treatment of human smallpox disease caused by variola virus, and is now used for treatment of non-variola orthopoxvirus infections through the CDC expanded access Investigational New Drug (EA-IND) protocol.27
In the setting of his HIV co-infection,28 the single conjunctival lesion the patient developed could represent ocular surface squamous neoplasia (OSSN) as it had a plaque like appearance and was adjacent to the limbus. However, the conjunctival lesion appeared around the same time his other lesions erupted and on follow up examination 3 weeks later, it had completely resolved (Fig. 4). This was confirmed with anterior segment OCT imaging, which has been shown to assist in the diagnosis of ocular surface masquerade lesions,29 and subclinical OSSN.30 Although spontaneous resolution has been reported after incisional biopsy of OSSN31 it would be unlikely for OSSN to completely spontaneously resolve after no intervention and in such a short timeframe.
4. Conclusions
We present a case of successfully treated systemic and ocular MPV infection in the United States during the 2022 outbreak using a collaborative and multidisciplinary approach between the ophthalmology and infectious disease teams. Given the rapid increase in cases, MPV infection should be considered when physicians encounter patients with ocular complaints, including eyelid lesions and conjunctivitis along with suspicious skin lesions. It is especially important that health care personnel utilize adequate personal protective equipment, including eye protection, when evaluating such patients that may first present to the emergency room or the clinic for evaluation. More data on the clinical presentation and management of monkeypox cases may help guide the management of future patients.
Patient consent
The patient verbally consented to publication of the case.
Funding
No Funding or Grant support to disclose.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Declaration of competing interest
The following authors have no financial disclosures: BP, DT, KL, EM, MM, MG, MK, JC, SG.
Acknowledgments
None.
References
- 1.Kumar N., Acharya A., Gendelman H.E., Byrareddy S.N. The 2022 outbreak and the pathobiology of the monkeypox virus. J Autoimmun. 2022;131 doi: 10.1016/j.jaut.2022.102855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marennikova S.S., Seluhina E.M., Mal’ceva N.N., Cimiskjan K.L., Macevic G.R. Isolation and properties of the causal agent of a new variola-like disease (monkeypox) in man. Bull World Health Organ. 1972;46(5):599–611. [PMC free article] [PubMed] [Google Scholar]
- 3.Anwar F., Waris A. Monkeypox virus outbreak: a brief timeline. New Microbes New Infect. 2022;48 doi: 10.1016/j.nmni.2022.101004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stolberg S.G., Mandavilli A. As Monkeypox Spreads, U.S. Declares a Health Emergency. New York Times. Date published August 4, 2022.
- 5.Patel A., Bilinska J., Tam J.C.H., et al. Clinical features and novel presentations of human monkeypox in a central London centre during the 2022 outbreak: descriptive case series. BMJ. 2022;378 doi: 10.1136/bmj-2022-072410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thornhill J.P., Barkati S., Walmsley S., et al. Monkeypox virus infection in humans across 16 countries - April-June 2022. N Engl J Med. 2022 doi: 10.1056/NEJMoa2207323. Published online July 21. [DOI] [PubMed] [Google Scholar]
- 7.Ježek Z., Szczeniowski M., Paluku K.M., Mutombo M. Human monkeypox: clinical features of 282 patients. J Infect Dis. 1987;156(2):293–298. doi: 10.1093/infdis/156.2.293. [DOI] [PubMed] [Google Scholar]
- 8.Ježek Z., Grab B., Szczeniowski M., Paluku K.M., Mutombo M. Clinico-epidemiological features of monkeypox patients with an animal or human source of infection. Bull World Health Organ. 1988;66(4):459–464. [PMC free article] [PubMed] [Google Scholar]
- 9.Benatti S.V., Venturelli S., Comi N., Borghi F., Paolucci S., Baldanti F. Ophthalmic manifestation of monkeypox infection. Lancet Infect Dis. 2022 doi: 10.1016/S1473-3099(22)00504-7. Published online July 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Infection Prevention and Control of Monkeypox in Healthcare Settings. Centers for Disease Control and Prevention; 2022. https://www.cdc.gov/poxvirus/monkeypox/clinicians/infection-control-healthcare.html Published August 11. [Google Scholar]
- 11.Abdelaal A., Serhan H.A., Mahmoud M.A., Rodriguez-Morales A.J., Sah R. Ophthalmic manifestations of monkeypox virus. Eye . Published online July. 2022;27 doi: 10.1038/s41433-022-02195-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hughes C., McCollum A., Pukuta E., et al. Ocular complications associated with acute monkeypox virus infection. DRC. Int J Infect Dis. 2014;21:276–277. [Google Scholar]
- 13.Ogoina D., Iroezindu M., James H.I., et al. Clinical course and outcome of human monkeypox in Nigeria. Clin Infect Dis. 2020;71(8):e210–e214. doi: 10.1093/cid/ciaa143. [DOI] [PubMed] [Google Scholar]
- 14.Learned L.A., Reynolds M.G., Wassa D.W., et al. Extended interhuman transmission of monkeypox in a hospital community in the Republic of the Congo, 2003. Am J Trop Med Hyg. 2005;73(2):428–434. [PubMed] [Google Scholar]
- 15.Huhn G.D., Bauer A.M., Yorita K., et al. Clinical characteristics of human monkeypox, and risk factors for severe disease. Clin Infect Dis. 2005;41(12):1742–1751. doi: 10.1086/498115. [DOI] [PubMed] [Google Scholar]
- 16.Meduri E., Malclès A., Kecik M. Conjunctivitis with monkeypox virus positive conjunctival swabs. Ophthalmology. 2022 doi: 10.1016/j.ophtha.2022.07.017. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adler H., Gould S., Hine P., et al. Clinical features and management of human monkeypox: a retrospective observational study in the UK. Lancet Infect Dis. 2022;22(8):1153–1162. doi: 10.1016/S1473-3099(22)00228-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guess S., Stone D.U., Chodosh J. Evidence-based treatment of herpes simplex virus keratitis: a systematic review. Ocul Surf. 2007;5(3):240–250. doi: 10.1016/s1542-0124(12)70614-6. [DOI] [PubMed] [Google Scholar]
- 19.Fillmore G.L., Ward T.P., Bower K.S., et al. Ocular complications in the department of defense smallpox vaccination program. Ophthalmology. 2004;111(11):2086–2093. doi: 10.1016/j.ophtha.2004.04.027. [DOI] [PubMed] [Google Scholar]
- 20.Altmann S., Brandt C.R., Murphy C.J., et al. Evaluation of therapeutic interventions for vaccinia virus keratitis. J Infect Dis. 2011;203(5):683–690. doi: 10.1093/infdis/jiq103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Altmann S., Emanuel A., Toomey M., et al. A quantitative rabbit model of vaccinia keratitis. Invest Ophthalmol Vis Sci. 2010;51(9):4531–4540. doi: 10.1167/iovs.09-5106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shearer D.R., Bourne W.M. Severe ocular anterior segment ischemia after long-term trifluridine treatment for presumed herpetic keratitis. Am J Ophthalmol. 1990;109(3):346–347. doi: 10.1016/s0002-9394(14)74564-7. [DOI] [PubMed] [Google Scholar]
- 23.Carmine A.A., Brogden R.N., Heel R.C., Speight T.M., Avery G.S. Trifluridine: a review of its antiviral activity and therapeutic use in the topical treatment of viral eye infections. Drugs. 1982;23(5):329–353. doi: 10.2165/00003495-198223050-00001. [DOI] [PubMed] [Google Scholar]
- 24.Udell I.J. Trifluridine-associated conjunctival cicatrization. Am J Ophthalmol. 1985;99(3):363–364. doi: 10.1016/0002-9394(85)90372-1. [DOI] [PubMed] [Google Scholar]
- 25.Grosenbach D.W., Honeychurch K., Rose E.A., et al. Oral tecovirimat for the treatment of smallpox. N Engl J Med. 2018;379(1):44–53. doi: 10.1056/NEJMoa1705688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chittick G., Morrison M., Brundage T., Nichols W.G. Short-term clinical safety profile of brincidofovir: a favorable benefit–risk proposition in the treatment of smallpox. Antivir Res. 2017;143:269–277. doi: 10.1016/j.antiviral.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 27.Information for healthcare providers on obtaining and using TPOXX (tecovirimat) for treatment of monkeypox. Centers for disease Control and prevention. 2022. https://www.cdc.gov/poxvirus/monkeypox/clinicians/obtaining-tecovirimat.html Published August 8.
- 28.Rathi S.G., Ganguly Kapoor A., Kaliki S. Ocular surface squamous neoplasia in HIV-infected patients: current perspectives. HIV AIDS. 2018;10:33–45. doi: 10.2147/HIV.S120517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Theotoka D., Wall S., Galor A., et al. The use of high resolution optical coherence tomography (HR-OCT) in the diagnosis of ocular surface masqueraders. Ocul Surf. 2022;24:74–82. doi: 10.1016/j.jtos.2022.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tran A.Q., Venkateswaran N., Galor A., Karp C.L. Utility of high-resolution anterior segment optical coherence tomography in the diagnosis and management of sub-clinical ocular surface squamous neoplasia. Eye Vis (Lond). 2019;6:27. doi: 10.1186/s40662-019-0152-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Theotoka D., Morkin M.I., Naranjo A., Dubovy S.R., Karp C.L. Spontaneous regression of ocular surface squamous neoplasia: possible etiologic mechanisms in cancer resolution. Ocul Surf. 2020;18(3):351–353. doi: 10.1016/j.jtos.2020.03.001. [DOI] [PubMed] [Google Scholar]