Abstract
Toxin B from Clostridium difficile is a monoglucosylating toxin that targets substrates within the cytosol of mammalian cells. In this study, we investigated the impact of acidic pH on cytosolic entry and structural changes within toxin B. Bafilomycin A1 was used to block endosomal acidification and subsequent toxin B translocation. Cytopathic effects could be completely blocked by addition of bafilomycin A1 up to 20 min following toxin treatment. Furthermore, providing a low extracellular pH could circumvent the effect of bafilomycin A1 and other lysosomotropic agents. Acid pH-induced structural changes were monitored by using the fluorescent probe 2-(p-toluidinyl) naphthalene-6-sulfonic acid, sodium salt (TNS), inherent tryptophan fluorescence, and relative susceptibility to a specific protease. As the toxin was exposed to lower pH there was an increase in TNS fluorescence, suggesting the exposure of hydrophobic domains by toxin B. The change in hydrophobicity appeared to be reversible, since returning the pH to neutrality abrogated TNS fluorescence. Furthermore, tryptophan fluorescence was quenched at the acidic pH, indicating that domains may have been moving into more aqueous environments. Toxin B also demonstrated variable susceptibility to Staphylococcus aureus V8 protease at neutral and acidic pH, further suggesting pH-induced structural changes in this protein.
The large clostridial toxins are a unique class of virulence factors produced by at least three pathogenic clostridial species. Clostridium difficile produces toxins A and B, Clostridium novyi produces alpha-toxin, and Clostridium sordellii produces lethal toxin and hemorrhagic toxin. These toxins not only are unique because of their exceptionally large size (ranging from 260 to 308 kDa) but also demonstrate a novel enzymatic activity (1, 3, 4). Each of these toxins targets members of the Ras superantigen of GTPases by acting as a glycosyltransferase. Toxin A, toxin B, lethal toxin, and hemorrhagic toxin all use UDP-glucose as a cosubstrate, whereas alpha-toxin uses UDP-GlcNAc to modify targets.
The mechanism of action for C. difficile toxins A and B is of particular interest since this organism causes pseudomembranous colitis, a serious human disease usually occurring in hospitalized patients undergoing antibiotic therapy (2). C. difficile toxin A acts as an enterotoxin and is considered to be the major contributor to the intestinal damage caused by C. difficile. Toxin B is an effective cytotoxin that demonstrates less cell tropism than toxin A and is responsible for systemic intoxication (1).
While there has been significant progress in identifying the enzymatic action of these toxins, little is known about how these proteins translocate to the cytosol of target cells. By definition, intracellular bacterial toxins must cross the target cell membrane in order to enter the interior of the target cell. The most common means of accomplishing this appears to be via a three-step process: (i) receptor binding, (ii) receptor-triggered endocytosis, and (iii) membrane insertion and translocation following endosomal acidification. In this pathway, the cell is triggered to endocytose the toxin following its binding to a target cell receptor. Subsequent acidification, proteolysis, and reduction may contribute to stimulating the toxin to insert into and cross the vesicle membrane. Several bacterial toxins appear to enter the cell by using this process, although subsequent steps in translocation are varied. For example, anthrax toxin uses a binary combination of proteins to provide membrane translocation whereas diphtheria toxin acts as a single polypeptide that is reduced, proteolyzed, and translocated following endosomal acidification (5, 10). In the case of both toxins, acidification of the endosomal vesicle triggers structural changes and membrane insertion by the toxin.
Previous reports indicate that toxin B also requires an acidified endosome for toxic activity (7, 8). These earlier reports involved a protein reported to be 440 kDa in size, much larger than toxin B. Herein, we briefly readdress this issue and clarify these experiments using a protein clearly shown to be toxin B. Additionally, we report the use of fluorescent approaches and proteolytic digest to provide the first insight into pH-induced structural changes in toxin B. The results from this study suggest that toxin B shows a significant increase in hydrophobicity and change in structure at acidic pH, all perhaps as a prelude to membrane insertion and translocation.
MATERIALS AND METHODS
Cell culture.
Chinese hamster ovary-K1 (CHO) cells were used in these studies. This line was maintained in Ham's F-12 medium (Gibco BRL, Rockville, Md.) supplemented with 10% fetal bovine serum. Cultures were grown at 37°C in the presence of 6% CO2.
Purification of toxin B.
A modified protocol derived from two previously reported methods was used to isolate toxin B (11, 13). In this protocol, C. difficile VPI 10463 (American Type Culture Collection, Manassas, Va.) was grown in 10,000- to 12,000-molecular-weight-cutoff dialysis tubing suspended in 10 liters of brain heart infusion broth. The culture was grown at 37°C for 72 h, at which point the culture was centrifuged and the supernatant was collected. Toxin B was subsequently purified by consecutive steps of ammonium sulfate precipitation, anion-exchange (Q-Sepharose) chromatography, gel filtration, and high-resolution anion-exchange (Mono-Q) chromatography. The final product was passed once over a benzamidine-Sepharose column (Amersham Pharmacia, Piscataway, N.J.) to remove trace amounts of contaminating proteases. A cocktail of protease inhibitors, TLCK (Nα-p-tosyl-l-lysine chloromethyl ketone), TPCK (l-1-tosylamide-2-phenylmethyl chloromethyl ketone), and phenylmethylsulfonyl fluoride (Sigma Chemical, St. Louis, Mo.), was included during each step of the isolation protocol. Purification steps were followed by cytotoxicity on CHO cells, Western blot analysis using toxin B polyclonal antiserum (a generous gift from Rodney Tweten), and visualization by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Following the final step of purification, the protein concentration was determined by a Bradford assay (Bio-Rad Laboratories, Hercules, Calif.), and the sample was frozen at −80°C in 100-μl aliquots. Prior to assays, samples were thawed on ice and used immediately. Due to increased degradation, toxin B was not refrozen after thawing.
Bafilomycin A1 assays.
CHO cells were plated at 5 × 104 cells/well in a 96-well plate and incubated overnight. The following day, toxin B was added to cells at a final concentration of 0.5 μg/ml. At the indicated time points, the cells were washed to remove unbound toxin, and bafilomycin A1 (Sigma) was added to the cells at a final concentration of 5 × 10−7 M. Each sample was monitored for 8 and 16 h, and cytopathic effects were determined by visualization.
Acid pulse experiments.
CHO cells were plated at 5 × 104 cells/well in a 96-well plate and incubated overnight. The following day, cells were incubated with either 100 mM ammonium chloride or 5 × 10−7 M bafilomycin A1 for 30 min. Toxin B was added to cells at concentrations ranging from 10 pmol to 1 fmol. Cells were incubated with toxin B for 1 h and then washed to remove unbound toxin. A pH pulse was performed by lowering the pH to 4.0 with buffered medium for 10 min and then returning it to pH 7.8 using neutralized medium. The cells were then monitored for 8 h, and cytopathic effect was determined by visualization.
TNS fluorescence analysis of toxin B.
2-(p-Toluidinyl) naphthalene-6-sulfonic acid, sodium salt (TNS; Molecular Probes, Eugene, Oreg.) solutions were prepared in the appropriate buffers for each pH to be analyzed: for pH 4.0, 4.5, 5.0, and 5.5, 100 mM NaCl–100 mM ammonium acetate–1 mM EDTA; for pH 6.0 and 6.5, 100 mM NaCl–100 mM morpholineethanesulfonic acid–1 mM EDTA; for pH 7.0 and 7.5, 100 mM sodium chloride–100 mM HEPES–1 mM EDTA. TNS was added to each buffer to a final concentration of 150 μM; 20 pmol of toxin B was added to each buffer in a final volume of 2 ml. These mixtures were allowed to incubate at 37°C for 20 min. Each sample was analyzed on an SLM 8100 photon-counting fluorometer (Spectronic Instruments, Rochester, N.Y.) with an excitation of 366 nm and an emission scan of 380 to 500 nm with a slit width of 2.0. For the pH shift experiments, 40 pmol of toxin B was dialyzed into 50 mM ammonium acetate–1 mM EDTA–100 mM NaCl (pH 4.0), and 20 pmol of this sample was saved. The remaining 20 pmol of toxin B was incubated for 5 min at 37°C. The pH was then adjusted to pH 7.5 by the gradual addition of 1 N NaOH. The volume for both the pH 4.0 and pH 7.5 samples was adjusted to 1.5 ml, and 0.5 ml of 20 mM TNS was added (this resulted in a final TNS concentration approximately fivefold less than that used in the previous experiment). The samples were then analyzed as described above. Since the pH 7.5 sample was not within the buffering range of ammonium acetate, the pH of the sample was checked following the analysis to confirm that the neutral pH condition was maintained.
Tryptophan analysis.
Tryptophan fluorescence was analyzed at pH 7.0 and 4.0. Twenty picomoles of toxin B was incubated at pH 7.0 (100 mM HEPES) or pH 4.0 (100 mM ammonium acetate). Samples were analyzed using an excitation of 270 nm and an emission scan of 300 nm to 400 nm. Slit widths were set at 4.0.
V8 protease analysis.
Toxin B was dialyzed overnight against 1 liter of 50 mM ammonium acetate (pH 4.0) or 50 mM ammonium bicarbonate (pH 7.8). Dialysis was carried out at 4°C in a microdialyzer using 12,000- to 14,000-molecular-weight-cutoff membranes. V8 protease (Boehringer Mannheim, Indianapolis, Ind.) was made as a stock at 1 mg/ml in either 50 mM ammonium acetate buffer (pH 4.0) or 50 mM ammonium bicarbonate buffer (pH 7.8). Toxin B (75 pmol) at both pHs was incubated with increasing dilutions of V8 protease (100 ng to 100 pg) at 37°C for 90 min. The reactions were stopped by the addition of SDS-PAGE tracking dye and analyzed immediately by SDS-PAGE.
RESULTS
Bafilomycin A1 inhibition of toxin B cytopathic effects.
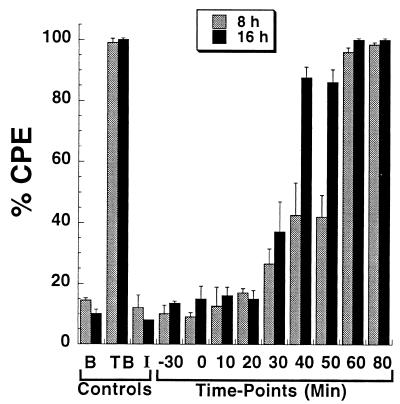
Bafilomycin A1 is a potent inhibitor of the endosomal vacuolar ATPase pump and blocks the acidification of early and late endosomes as well as lysosomes (12). In this study, we used bafilomycin A1 to confirm the role of endosomal acidification on toxin B entry. Furthermore, we were able to gain insight into the time course of toxin B translocation. As shown in Fig. 1, treatment of CHO cells with bafilomycin A1 prevents cytopathic effects by toxin B. A complete protective effect can be achieved by adding bafilomycin A1 up to 20 min following toxin B treatment. Approximately 20% of the killing can also be prevented by addition of bafilomycin A1 up to 50 min following treatment. Only after almost 60 min does addition of the inhibitor have no effect on toxin activity. Samples observed at 18 h start to show cytopathic effects at the earlier time points, suggesting about a 20-min time period for entry. These results suggested that toxin B may remain within the vacuolar compartment for a significant period of time following entry. For this reason, we also investigated the effect that brefeldin A (disrupts the Golgi and trans-Golgi network) had on toxin entry. Treatment of cells with brefeldin A had no effect on toxin B activity (data not shown), indicating that toxic activity is not dependent on trafficking to the Golgi.
FIG. 1.
Bafilomycin A1 inhibition of toxin B-mediated cytopathic effects. CHO cells were incubated with toxin B (TB; 50 ng) in a 96-well plate, and bafilomycin A1 (I; 5 × 10−7 M) was added at 10-min intervals from 0 to 80 min. Each sample was performed in triplicate, and cytopathic effects were determined at 8 and 16 h. The error bars mark the standard deviation from the mean. Similar levels of inhibition were found in two subsequent repetitions of this experiment. B, phosphate-buffered saline control.
Cellular intoxication following an acid pulse.
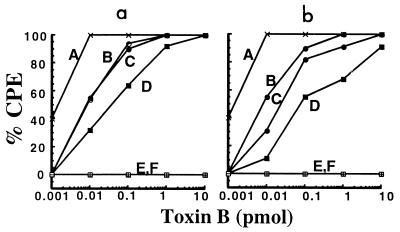
To determine if the effects of bafilomycin A1 could be bypassed, cells were incubated with toxin B in the presence of the inhibitor, and a brief acid pulse was provided. Following a 10-min acid pulse at pH 4.0, the cells were washed to remove unbound toxin B and the pH was returned to neutrality. Subsequent cytopathic effects were monitored for 8 h. As shown in Fig. 2a, the lysosomotropic effects of bafilomycin A1 could be overcome by the acid pulse. This effect could be found in samples that were predialyzed to pH 4.0 or in samples at neutral pH.
FIG. 2.
Acid pulse-induced entry of toxin B. Endosomal acidification was inhibited with either ammonium chloride or bafilomycin A1. Cells were then subjected to a brief acid pulse at pH 4.0 followed by a return to neutral pH. Samples were followed for 8 h, and cytopathic effects (CPE) were determined by visualization. (a) Bafilomycin inhibitor and pH pulse; (b) ammonium chloride inhibitor and pH pulse. Curves: A, toxin B (pH 7.8); B, toxin B (pH 7.8) plus inhibitor plus acid pulse; C, toxin B predialyzed to pH 4.0; D, toxin B (pH 4.0) plus inhibitor plus acid pulse; E, toxin B (pH 7.8) plus inhibitor; F, toxin B (pH 4.0) plus inhibitor.
Previous work had indicated toxin B could be delivered following an acid pulse, but only if the toxin was reduced with dithiothreitol (14). Since this previous work also involved using ammonium chloride instead of bafilomycin A1 as the inhibitor, we carried out this experiment in the presence of ammonium chloride. As can be seen in Fig. 2b, the effects of ammonium chloride could be bypassed by providing an acid pulse. Furthermore, this bypass could be accomplished in the absence of any reducing agent.
TNS fluorescence analysis of pH-induced changes in toxin B hydrophobicity.
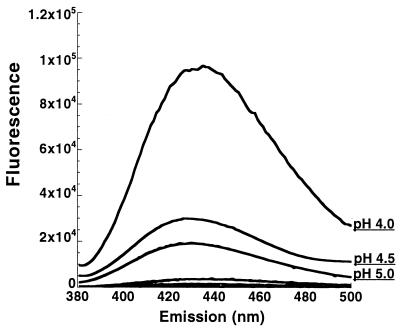
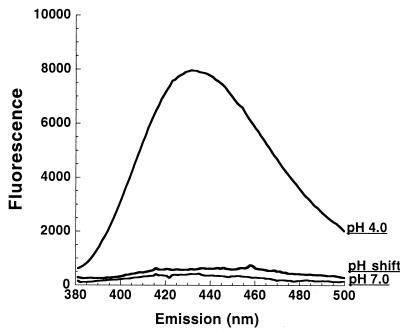
TNS is a convenient probe for determining the exposure or sequestering of hydrophobic domains under various conditions. To identify pH-induced changes in toxin B hydrophobicity, the protein was preincubated with 150 μM TNS for 20 min at pH 7.0, 6.5, 6.0, 5.5, 5.0, 4.5, or 4.0. The samples were then analyzed for changes in TNS fluorescence. As shown in Fig. 3, toxin B exhibits a significant increase in hydrophobicity as pH declines. While there is little difference in the range of pH 5.5 to 7.0, at pH 5.0 there is a significant increase in fluorescence and the intensity continues to increase at pH 4.0. At the lowest pH, the fluorescent intensity has increased almost 100-fold over that of the neutral pH. This pH-induced transition in hydrophobicity seems to be reversible since samples shifted from pH 4.0 to pH 7.0 demonstrate TNS fluorescence at background levels (Fig. 4).
FIG. 3.
TNS analysis of pH-induced hydrophobic transitions in toxin B. Twenty picomoles of toxin B was incubated with 150 μM TNS for 20 min at 37°C. Samples were analyzed for changes in TNS fluorescence. The fluorescent spectrum of each pH is shown and labeled; each spectrum represents the experimental sample with background (TNS and buffer alone) subtracted. TNS fluorescence for samples above pH 5.0 were not above background levels. Similar relative fluorescence was obtained in two consecutive repetitions of this experiment.
FIG. 4.
TNS analysis of toxin B hydrophobicity following pH shift. TNS was added to toxin B at pH 4.0 and incubated for 20 min at 37°C, and the emission profile was determined. The pH was then raised to pH 7.0, and the TNS emission spectrum was generated. The spectra represent TNS-toxin B fluorescence after subtraction of background (TNS plus buffer alone). Similar relative fluorescence was obtained in two consecutive repetitions of this experiment.
Tryptophan fluorescence.
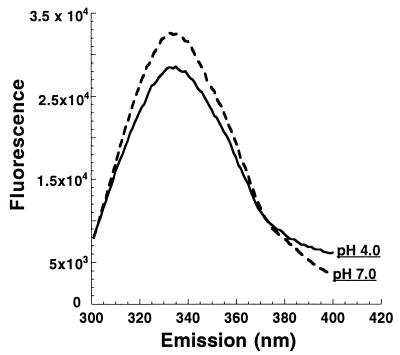
Presumably the changes in hydrophobicity involve the movement of buried or less accessible hydrophobic regions into more aqueous environments. Since tryptophan fluorescence should be quenched under these conditions, changes in the inherent fluorescence of the protein at neutral and acidic pHs should be revealed. In these experiments, toxin B was incubated at 37°C at pH 4.0 or 7.5. Environmental changes surrounding tryptophans were then monitored by fluorescence using an excitation of 270 nm and an emission scan from 300 to 400 nm. As shown in Fig. 5, there is a subtle shift in the fluorescent profile between pH 4.0 and 7.5. Tryptophan fluorescence is decreased at the lower pH, indicating that these residues are in a more solvent accessible environment.
FIG. 5.
Tryptophan fluorescence of toxin B at acidic and neutral pH. Toxin B (20 pmol) was dialyzed to pH 4.0 or 7.0, and the tryptophan fluorescence was determined. The fluorescent spectrum of each sample is shown and labeled; each spectrum represents the experimental sample minus background. Similar relative fluorescence was obtained in two consecutive repetitions of this experiment.
V8 protease analysis.
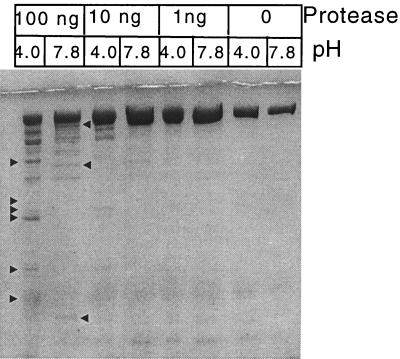
To further track the pH-induced conformational changes in toxin B, we subjected the protein to protease digestion at pH 4.0 or 7.8. The Staphylococcus aureus-derived V8 protease was selected since it maintains the same activity and specificity (peptide bond hydrolysis on the carboxylic side of glutamines) at both pHs (6, 9). In this experiment, toxin B was incubated with V8 protease and the resulting digest was resolved by SDS-PAGE. As shown in Fig. 6, there is a difference in the proteolytic digest profile between the two pHs. We also made direct comparisons with different ratios of toxin to protease, to confirm that the effect was not due to various degrees of activity at pH 4.0 and 7.8. Regardless of the ratio, we did not find a condition where the peptide profile was similar.
FIG. 6.
Relative susceptibility of toxin B to V8 protease at pH 4.0 and 7.8. Toxin B (75 pmol) was incubated with increasing dilutions of V8 protease at pH 4.0 or 7.8 for 1.5 h at 37°C. Controls of toxin B alone were included and incubated at the same pH and temperature. The samples were resolved by SDS-PAGE (10% gel) and stained with Coomassie blue. The pH, amounts of V8 protease, and controls are shown at the top. Arrowheads mark bands unique to each condition.
DISCUSSION
Since intracellular bacterial toxins need to be relatively soluble following release from their host bacterium, they are not able to expose significant apolar domains. This presents a conundrum since the toxin must insert into a very hydrophobic environment, namely, the phospholipid bilayer of the target cell membrane. For this reason, intracellular bacterial toxins have had to evolve elegant mechanisms to alter their structure immediately prior to membrane insertion. By using pH as a signal, these proteins can exploit the natural processes of endocytosis and vacuolar acidification as a means to trigger the exposure of membrane-insertable regions. Thus, the hydrophilic molecule can remain soluble while outside the target cell and expose its membrane-inserting domains when assured of having an accessible lipid bilayer. The purpose of this work was to determine if toxin B uses this type of mechanism for altering its structure and exposing hydrophobic regions.
Earlier work by Florin and Thelestam had addressed the issue of lysosomal involvement in intoxication by toxin B (7, 8). While this work focused on a cytotoxin from C. difficile, the reports indicated a protein significantly different in size from our isolated form of toxin B (440 instead of 270 kDa). In a later paper, these investigators report the amino-terminal sequence, which matched the predicted sequence within toxin B. Interestingly, their protein seems to migrate as a larger form than ours and requires treatment with dithiothreitol for optimal toxic activity (14). Our purified form of toxin B shares some similar characteristics with this larger form. Like the larger form, ours has an amino-terminal region matching predicted sequences; however, reducing agents do not appear to enhance the activity of our purified toxin B. These differences suggested that we should confirm endosomal involvement in toxin B intoxication with our form before looking at the impact of acidification on toxin B structure. Using bafilomycin A1, an inhibitor of endosomal acidification, we found that acidification of the endosome is important for toxin B entry (Fig. 1). Additionally, we analyzed the temporal nature of entry and found that most of toxin B toxicity could be prevented up to 20 min following treatment. We were also able to circumvent this block by providing a brief acid pulse (Fig. 2). These results were in agreement with what was found by Florin and Thelestam with the larger, reductant-dependent form of toxin B. However, we were able to get pH pulse-induced entry of toxin B from the cell surface in the absence of reducing agents. We do not know what the actual differences, structurally, are between these two forms of the toxin. Nonetheless, the data indicate that acid conditions have an impact on toxin B entry and that low pH alone seems to be sufficient for triggering translocation.
To identify the impact that acidification has on toxin B structure, we monitored pH-induced structural changes by TNS fluorescence, tryptophan fluorescence, and susceptibility to V8 protease. While toxin hydrophobicity at pH 5.5 and above is not higher than background, beginning at pH 5.0 TNS fluorescence increases in the presence of toxin B (Fig. 3). TNS fluorescence continues to increase at pH 4.5 and 4.0. This increase in hydrophobicity appears to be reversible, since returning the pH to neutral conditions results in decreased fluorescence (Fig. 4). Whether the shift back to neutral pH causes refolding to the original conformation is currently being investigated. The toxin does maintain toxic activity following acidification, since toxin dialyzed to pH 4.0 still causes cell rounding (Fig. 2). This toxic activity may be due to direct action of the pH 4.0 form or could be the result of the toxin refolding under the neutral conditions of the culture medium. Earlier reports suggested toxin B was inactivated by acid pH (15), which conflicts with our findings. The difference may be due to the fact that we slowly dialyzed toxin B into pH 4.0 buffer at 4°C, while the earlier work used a rapid dialysis method and incubation at room temperature.
The decrease in tryptophan fluorescence (Fig. 5) suggests that tryptophan-containing domains move into more aqueous environments or that hydrophobic pockets move away from tryptophans. In either case, the results suggest that toxin B alters its structure in response to acidification. Finally, results from the V8 protease analysis further support our hypothesis that toxin B undergoes pH-induced structural changes (Fig. 6). We are now investigating whether these structural changes and increases in hydrophobicity are in preparation for oligomerization and/or membrane insertion.
ACKNOWLEDGMENTS
This work was supported in part by an Oklahoma Center for the Advancement of Science and Technology grant to J.D.B.
The advice from and helpful discussions with Laura Shepard and R. K. Tweten are greatly appreciated.
REFERENCES
- 1.Aktories K. Bacterial toxins that target Rho proteins. J Clin Investig. 1997;99:827–829. doi: 10.1172/JCI119245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ayyagari A, Sharma P, Venkateswarlu T, Mehta S, Agarwal K C. Prevalence of Clostridium difficile in pseudomembranous and antibiotic-associated colitis in north India. J Diarrhoeal Dis Res. 1986;4:157–160. [PubMed] [Google Scholar]
- 3.Ball D W, Van Tassell R L, Roberts M D, Hahn P E, Lyerly D M, Wilkins T D. Purification and characterization of alpha-toxin produced by Clostridium novyi type A. Infect Immun. 1993;61:2912–2918. doi: 10.1128/iai.61.7.2912-2918.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boquet P, Munro P, Fiorentini C, Just I. Toxins from anaerobic bacteria: specificity and molecular mechanisms of action. Curr Opin Microbiol. 1998;1:66–74. doi: 10.1016/s1369-5274(98)80144-6. [DOI] [PubMed] [Google Scholar]
- 5.Collier R J. Mechanism of membrane translocation by anthrax toxin: insertion and pore formation by protective antigen. J Appl Microbiol. 1999;87:283. doi: 10.1046/j.1365-2672.1999.00889.x. [DOI] [PubMed] [Google Scholar]
- 6.Drapeau G R, Boily Y, Houmard J. Purification and properties of an extracellular protease of Staphylococcus aureus. J Biol Chem. 1972;247:6720–6726. [PubMed] [Google Scholar]
- 7.Florin I, Thelestam M. Internalization of Clostridium difficile cytotoxin into cultured human lung fibroblasts. Biochim Biophys Acta. 1983;763:383–392. doi: 10.1016/0167-4889(83)90100-3. [DOI] [PubMed] [Google Scholar]
- 8.Florin I, Thelestam M. Lysosomal involvement in cellular intoxication with Clostridium difficile toxin B. Microb Pathog. 1986;1:373–385. doi: 10.1016/0882-4010(86)90069-0. [DOI] [PubMed] [Google Scholar]
- 9.Houmard J, Drapeau G R. Staphylococcal protease: a proteolytic enzyme specific for glutamoyl bonds. Proc Natl Acad Sci USA. 1972;69:3506–3509. doi: 10.1073/pnas.69.12.3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.London E. Diphtheria toxin:membrane interaction and membrane translocation. Biochim Biophys Acta. 1992;1113:25–51. doi: 10.1016/0304-4157(92)90033-7. [DOI] [PubMed] [Google Scholar]
- 11.Meador J D, Tweten R K. Purification and characterization of toxin B from Clostridium difficile. Infect Immun. 1988;56:1708–1714. doi: 10.1128/iai.56.7.1708-1714.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Menard A, Altendorf K, Breves D, Mock M, Montecucco C. The vacuolar ATPase proton pump is required for the cytotoxicity of Bacillus anthracis lethal toxin. FEBS Lett. 1996;386:161–164. doi: 10.1016/0014-5793(96)00422-x. [DOI] [PubMed] [Google Scholar]
- 13.Pothoulakis C, Barone L M, Ely R, Faris B, Clark M E, Franzblau C, LaMont J T. Purification and properties of Clostridium difficile cytotoxin B. J Biol Chem. 1986;261:1316–1321. [PubMed] [Google Scholar]
- 14.Shoshan M C, Bergman T, Thelestam M, Florin I. Dithiothreitol generates an activated 250,000 mol. wt form of Clostridium difficile toxin B. Toxicon. 1993;31:845–852. doi: 10.1016/0041-0101(93)90219-9. [DOI] [PubMed] [Google Scholar]
- 15.Sullivan N M, Pellett S, Wilkins T D. Purification and characterization of toxins A and B of Clostridium difficile. Infect Immun. 1982;35:1032–1040. doi: 10.1128/iai.35.3.1032-1040.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]