Abstract
Yersinia enterocolitica infection of epithelial cells results in interleukin-8 (IL-8) mRNA expression. Herein we demonstrate that besides IL-8, increased mRNA levels of five other cytokines, IL-1α, IL-1β, monocyte chemoattractant protein 1 (MCP-1), granulocyte-macrophage colony-stimulating factor (GM-CSF), and tumor necrosis factor alpha (TNF-α), can be detected upon infection of HeLa cells with Yersinia. Yersinia-triggered cytokine production was not affected by blocking phosphatidylinositol-3-phosphate kinase with wortmannin, which inhibited bacterial invasion. Comparable cytokine mRNA responses were triggered by Escherichia coli expressing Yersinia inv, while no response was triggered by an inv-deficient Yersinia mutant. Moreover, cytokine responses were independent from metabolic activity of the bacteria, as killed bacterial cells were sufficient for triggering cytokine responses in HeLa cells. Semiquantitative reverse transcription-PCR analysis was used to assess the kinetics of cytokine mRNA expression in infected HeLa cells. IL-8, IL-1α, IL-1β, MCP-1, GM-CSF, and TNF-α mRNA expression increased within 1 h postinfection, reached a maximum after 3 to 4 h, and then declined to preinfection levels within 3 h. IL-8, MCP-1, and GM-CSF were secreted by HeLa cells, whereas IL-1α and IL-1β were not secreted and thus were found exclusively intracellularly. TNF-α protein could not be detected in cell lysates or supernatants. Stimulation of HeLa cells with IL-1α was followed by increased IL-8 mRNA expression, whereas stimulation with IL-8 did not induce cytokine production. Likewise, MCP-1 and GM-CSF did not induce significant cytokine responses in HeLa cells. Our results implicate that the initial host response to Yersinia infection might be sustained by IL-8, MCP-1, and GM-CSF produced by epithelial cells.
Upon infection of the host, invasive and noninvasive enteric pathogens first encounter the host's mucosal surfaces lined by epithelial cells. The function of these cells in host defense goes far beyond the mere mechanical barrier that separates the host's internal milieu from the external environment. In fact, epithelial cells can be considered an integral component of the mucosal immune system, as they provide the underlying mucosa with the first signals of an infection (22, 36). Thus, invasion by enteropathogenic bacteria such as Salmonella enterica serovar Dublin, Shigella dysenteriae, Yersinia enterocolitica, Listeria monocytogenes, or enteroinvasive Escherichia coli prompts a rapid cytokine response in epithelial cells that orchestrates the early phase of immune reactions, including the initiation of the cellular host responses (20, 21, 24, 35).
On the other hand, cytokine responses by epithelial or phagocytic cells can be disrupted by some pathogenic bacteria, eventually enabling their escape of the host's immune system (for a review, see reference 62). For instance, inhibition of cytokine release in macrophages by bacterial products has been reported for Y. enterocolitica, Brucella suis, Vibrio cholerae, Bacillus anthracis, and Pseudomonas aeruginosa (9, 11, 30, 37, 56, 59).
Y. enterocolitica causes various clinical syndromes ranging from self-limited enterocolitis to potentially fatal systemic infection (16). The virulence of Y. enterocolitica is encoded by chromosomal (e.g., inv and yst) (18, 31, 42, 46) and Yersinia virulence plasmid (pYV)-encoded genes, including yadA and genes encoding Yersinia outer proteins (Yops) (for reviews, see references 13 to 15).
After orogastic infection of mice, Y. enterocolitica selectively invades M cells located in the follicle-associated epithelium overlying Peyer's patches (1, 26, 27). After transcytosis via M cells, Y. enterocolitica multiplies in Peyer's patch tissue, thereby triggering an enormous recruitment of polymorphonuclear and mononuclear phagocytes (3). This leads to the formation of microabscesses and destruction of the cytoarchitecture of Peyer's patches. Thereafter, yersiniae disseminate, and abscesses appear in the mesenteric lymph nodes (1). The massive influx of immune cells into the infected mucosal tissue and their simultaneous activation might be due to the activity of various cytokines released by epithelial cells. Interleukin-8 (IL-8), which is released by intestinal epithelial cells after exposure to Y. enterocolitica, is a potent chemoattractant from the family of CXC chemokines (21). Previous work from our laboratory suggested that Yersinia-triggered IL-8 secretion depends on cell adhesion rather than on bacterial invasion, suggesting that Yersinia adhesion to epithelial cells via the bacterial outer membrane protein invasin activates de novo synthesis and secretion of IL-8 (57). Other groups reported that secretion of IL-8 and other cytokines after infection of epithelial cells with Helicobacter pylori or S. enterica serovar Typhimurium was due to the activation of the transcription factor NF-κB in epithelial cells (29, 45).
To obtain a more detailed view of the early cytokine network in Yersinia-infected mucosa, we have now focused on other proinflammatory cytokines which have been reported to be activated via NF-κB (for reviews, see references 5, 8, and 39). By comparing the kinetics of cytokine mRNA expression, production, and secretion, we gained insight into their yet unclear role in Yersinia host defense. We further investigated the function of the Yersinia invasin protein in this process.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Plasmid-harboring (pYV+) and plasmid-cured (pYV−) Y. enterocolitica WA314 (28), inv mutant Y. enterocolitica (pYV−) WA314 (52), noninvasive E. coli HB101, and the E. coli HB101(pInv1914) strain expressing Y. enterocolitica inv (57) were routinely grown in Luria-Bertani broth (LB). For infection experiments, overnight bacterial cultures were diluted to an optical density at 600 nm (OD600) of 0.2 in LB and incubated for another 3 h at 27°C (Y. enterocolitica pYV−) or 37°C (E. coli and Y. enterocolitica pYV+). Bacteria were collected by centrifugation and washed twice with sterile phosphate-buffered saline (PBS), pH 7.4. After determination of the OD600, appropriate dilutions of the bacteria in PBS were performed to infect the cells with a multiplicity of infection (MOI) of 150 bacteria/cell. The actual number of bacteria administered was determined by plating serial dilutions on Mueller-Hinton agar and counting of CFU.
Killed bacterial cells for stimulation experiments were produced by the addition of gentamicin (100 μg/ml of medium) to bacterial cultures 1 h before infection. Alternatively, bacteria were heat killed by exposure to 60°C for 1 h. To control viability of killed bacteria, an aliquot of the suspensions was plated on Mueller-Hinton agar and incubated for 1 week.
Cell lines.
Human cervical epithelial cells (HeLa; ATCC CCL-2.1) and T84 epithelial colon carcinoma cell line were obtained from the American Type Culture Collection, Manassas, Va. Cells were grown in RPMI 1640 (Biochrom KG, Berlin, Germany)–10% heat-inactivated fetal bovine serum (Gibco BRL, Karlsruhe, Germany) supplemented with 2 mM l-glutamine (Gibco BRL), penicillin (100 U/ml), and streptomycin (100 μg/ml) (Biochrom KG) in a humidified 5% CO2 atmosphere at 37°C.
Stimulation of epithelial cells by infection or cytokines.
Confluent monolayers of cells, grown in six-well plates, were washed twice with PBS, and incubated in medium containing heat-inactivated fetal bovine serum without antibiotics. After 1 to 2 h of equilibration, bacterial samples were added. Monolayers and bacteria were incubated for 1 h to allow bacterial adherence and entry. After removal of the medium, cultures were washed three times with PBS to remove extracellular bacteria and further incubated for up to 4 h in the presence of 100 μg of gentamicin per ml of medium to kill remaining extracellular bacteria. Then culture supernatants were removed and centrifuged for 10 min at 15,000 × g to pellet residual bacteria and cells before cytokine determination. For the determination of intracellular cytokines, cells were lysed with double-distilled water in the presence of proteinase inhibitors (phenylmethylsulfonyl fluoride and Complete protease inhibitor cocktail tablets; Boehringer, Mannheim, Germany) and by freezing at −80°C and thawing; cells were then centrifuged for 20 min at 15,000 × g to pellet nonsoluble cell fragments. For reverse transcription-PCR (RT-PCR) analysis, cells were washed twice with PBS before total RNA extraction. We used bacterial lipopolysaccharide (LPS) derived from E. coli O55:B5 and Salmonella serovar Typhimurium (Bacto Lipopolysaccharides; Difco, Detroit, Mich.) in some stimulation experiments. Stock solutions of wortmannin (Sigma), 10 mM, were prepared in dimethyl sulfoxide. Tumor necrosis factor alpha (TNF-α) was a gift from Bender, Vienna, Austria; phorbol myristate acetate was obtained from Calbiochem. Human recombinant cytokines were obtained from R&D Systems, Wiesbaden-Nordenstadt, Germany (IL-1α, IL-1β, granulocyte-macrophage colony-stimulating factor [GM-CSF], and monocyte chemoattractant protein 1 [MCP-1]) and Pharmingen, San Diego, Calif. (IL-8). For cell stimulation, the cytokines were added to HeLa cell cultures at various concentrations. After 1 h, the medium was removed, the cell monolayers were washed extensively, and fresh medium without cytokines was added. After various intervals, the supernatants were harvested for enzyme-linked immunosorbent assay (ELISA), and the cells were harvested and RNA was prepared as described below.
Determination of cytokine production by ELISA.
The amounts of cytokines released into the culture supernatant or remaining in the cells were determined by ELISA. For IL-1α, IL-1β, GM-CSF, and MCP-1, assay kits from R&D Systems were used. An ELISA for IL-8 was established with optimal concentrations of a mouse anti-human IL-8 monoclonal antibody (MAb) and a biotinylated mouse anti-human IL-8 MAb as detecting antibody as described previously (57). ELISA microtiter plates (Nunc, Roskilde, Denmark) were coated overnight with anti-human IL-8 MAb (G265-5; Pharmingen). After nonspecific binding sites were blocked, supernatants were added to the wells and incubated overnight. After several washing steps, biotin-labeled anti-human IL-8 MAb (G265-8; Pharmingen) was added. Finally, an avidin-biotin-alkaline phosphatase complex (Strept ABC-AP kit; Dako, Glostrup, Denmark) was used. For signal development, the wells were incubated with p-nitrophenylphosphate disodium (Sigma), and the OD was determined at wavelengths of 405 and 490 nm. IL-8 concentrations were calculated from the straight-line portion of standard curves revealed by means of recombinant human IL-8 (Pharmingen).
RT-PCR analysis.
As previously described (57), total RNA of infected HeLa cells in six-well plates was extracted using 1 ml of TRIzol reagent (Gibco BRL). RNA (5 μg) was reverse transcribed as described by Bohn et al. (10). cDNA products were amplified by PCR in 50 μl of a mixture containing 10 mM Tris (pH 8.3), 50 mM KCl, and 2.5 mM MgCl2 plus 200 mM each dATP, dCTP, dGTP, and dTTP in the presence of 25 pmol each of 5′ and 3′ primer (35) and 2.0 U of AmpliTaq DNA polymerase or AmpliTaq Gold DNA polymerase (for IL-8 and TNF-α; Perkin-Elmer, Überlingen, Germany). Temperature profiles for the amplification were as described in reference 35; 1 min of denaturation at 95°C and 2.5 min of annealing and extension at 60°C (IL-1α, IL-1β, IL-8, and MCP-1), 65°C (GM-CSF), or 72°C (TNF-α and β-actin). The number of PCR cycles was adjusted as appropriate to maximize the differences between samples (β-actin, 22 cycles; IL-8, IL-1α, and MCP-1, 25 cycles; IL-1β and GM-CSF, 30 cycles; TNF-α, 35 cycles). Negative controls were performed by omitting RNA from the cDNA synthesis and specific PCR amplifications. PCR products were separated in 2% agarose gels and stained with ethidium bromide. Primers were obtained from Roth (Karlsruhe, Germany); primer sequences (35) are shown in Table 1.
TABLE 1.
Primers used
| Primer | Sequence |
|---|---|
| β-Actin | |
| 3′ | 5′-TAGAAGCATTGCGGTGGACGATGGAGGG-3′ |
| 5′ | 5′-TGACGGGGTCACCCACACTGTGCCCATCTA-3′ |
| IL-1α | |
| 3′ | 5′-CATGTCAAATTTCACTGCTTCATCC-3′ |
| 5′ | 5′-GTCTCTGAATCAGAAATCCTCTATC-3′ |
| IL-1β | |
| 3′ | 5′-TGGAGAACACCACTTGTTGCTCCA-3′ |
| 5′ | 5′-AAACAGATGAAGTGCTCCTTCCAGG-3′ |
| IL-8 | |
| 3′ | 5′-TCTCAGCCCTCTTCAAAAACTTCTC-3′ |
| 5′ | 5′-ATGACTTCCAAGCTGGCCGTGGCT-3′ |
| TNF-α | |
| 3′ | 5′-CACCAGCTGGTTATCTCTCAGCTC-3′ |
| 5′ | 5′-CGGGACGTGGAGCTGGCCGAGGAG-3′ |
| MCP-1 | |
| 3′ | 5′-GGGTAGAACTGTGGTTCAAGAGG-3′ |
| 5′ | 5′-TCTGTGCCTGCTGCTCATAGC-3′ |
| GM-CSF | |
| 3′ | 5′-TGGACTGGCTCCCAGCAGTCAAAGGGGATG-3′ |
| 5′ | 5′-ACACTGCTGAGATGAATGAAACAGTAG-3′ |
Semiquantitative mRNA levels were determined by digitally scanning the bands and calculating intensity of each cytokine band by FluroS MultiImager (Bio-Rad, Munich, Germany) and software (Multi-Analyst 1.1; Bio-Rad). Values were expressed in arbitrary units as ratio of the cytokine mRNA level to the corresponding β-actin mRNA level. The points of time of half-maximum cytokine expression were calculated to assess the kinetics of cytokine mRNA expression.
Statistical analysis.
Data were analyzed using Student's t test. P values of <0.05 were considered statistically significant. All experiments were repeated several times and yielded comparable results.
RESULTS
Y. enterocolitica-induced mRNA expression in HeLa cells.
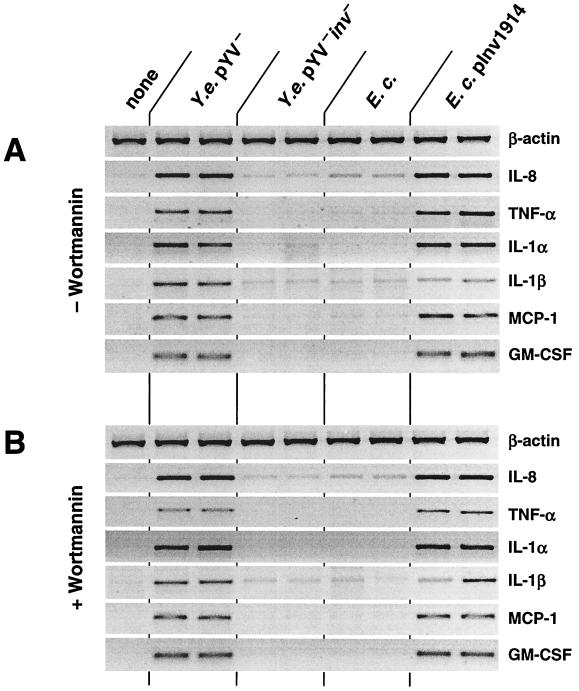
To assess the role of epithelial cells in generating signals for the underlying mucosa and circulating immune cells in Yersinia infections, we treated HeLa cells with Y. enterocolitica pYV− at an MOI of 150 bacteria/cell and analyzed mRNA extracted 3 h after infection. As shown in Fig. 1A, mRNA levels of the six proinflammatory cytokines, IL-8, TNF-α, IL-1α, IL-1β, MCP-1, and GM-CSF, were markedly increased upon Yersinia infection compared with noninfected cells. In keeping with previous results (55, 56), infection of HeLa cells with Y. enterocolitica pYV+ did not significantly induce expression of any of these cytokines, as secreted Yops suppress cytokine production. Furthermore, infection of other epithelial cell lines including T84 revealed similar results for IL-8 as infection with HeLa cells (data not shown). Cytokine mRNA induction could not be attributed to bacterial LPS since HeLa cells were completely unresponsive to LPS derived from E. coli or Salmonella serovar Typhimurium (not shown). This observation was made in both the presence and the absence of normal human serum.
FIG. 1.
Cytokine mRNA production of HeLa cells after Y. enterocolitica infection. (A) HeLa cells were infected with Y. enterocolitica (Y.e.) pYV− or inv mutant strain, E. coli (E.c.), or E. coli(pInv1914) expressing Y. enterocolitica inv (MOI of ∼150 bacteria/cell). After 1 h, gentamicin (100 μg/ml) was added to kill extracellular bacteria. Uninfected cells as controls received the same treatment without bacterial infection. After an additional 2 h, total cellular RNA was extracted from cells. PCR products were fractionated on a 3% agarose gel stained with ethidium bromide. (B) Bacterial invasion was inhibited by treating HeLa cells with wortmannin (100 ng/ml) 20 min prior to infection. Data shown are from a representative experiment with duplicate samples. Comparable results were obtained in additional experiments.
Adhesion of Yersinia to HeLa cells is sufficient to trigger proinflammatory cytokine responses.
Recently we showed that the phosphatidylinositol-3-phosphate kinase (PI3-K) inhibitor wortmannin blocks invasion of Y. enterocolitica into epithelial cells but does not affect Yersinia-induced IL-8 expression and secretion (57). In accordance with these results, inhibition of Yersinia invasion by wortmannin (not shown) did not alter the Yersinia-induced mRNA expression of IL-8, TNF-α, IL-1α, IL-1β, MCP-1, and GM-CSF (Fig. 1B). Wortmannin itself did not induce cytokine mRNA expression or modulate phorbol myristate acetate-induced cytokine mRNA expression in HeLa cells (not shown). From these data we conclude that bacterial adhesion, rather than invasion, triggers IL-8, TNF-α, IL-1α, IL-1β, MCP-1, and GM-CSF mRNA production in epithelial cells.
Yersinia invasin protein triggers cytokine mRNA expression.
In previous work we found that induction of IL-8 mRNA in HeLa cells after Yersinia infection was dependent on expression of the Y. enterocolitica outer membrane protein invasin (57). Invasin mediates bacterial invasion by binding to β1 integrins on the host cell surface (32). To evaluate the contribution of invasin to Yersinia-induced cytokine expression, we used a Yersinia mutant deficient in the inv gene (Y. enterocolitica pYV− inv), which is unable to invade epithelial cells (not shown). As shown in Fig. 1, the Yersinia inv mutant was unable to induce cytokine mRNA expression in HeLa cells. Likewise, a recombinant E. coli(pInv1914) strain expressing Y. enterocolitica inv, but not E. coli, was able to induce IL-8, TNF-α, IL-1α, IL-1β, MCP-1, and GM-CSF mRNA expression. Furthermore, although treatment with the PI3-K inhibitor wortmannin blocked E. coli pInv1914 invasion (not shown), this treatment did not affect cytokine mRNA production. These results suggest that invasin-mediated bacterial adhesion is sufficient to induce expression of proinflammatory cytokines in epithelial cells.
Killed Yersinia induce cytokine responses in HeLa cells.
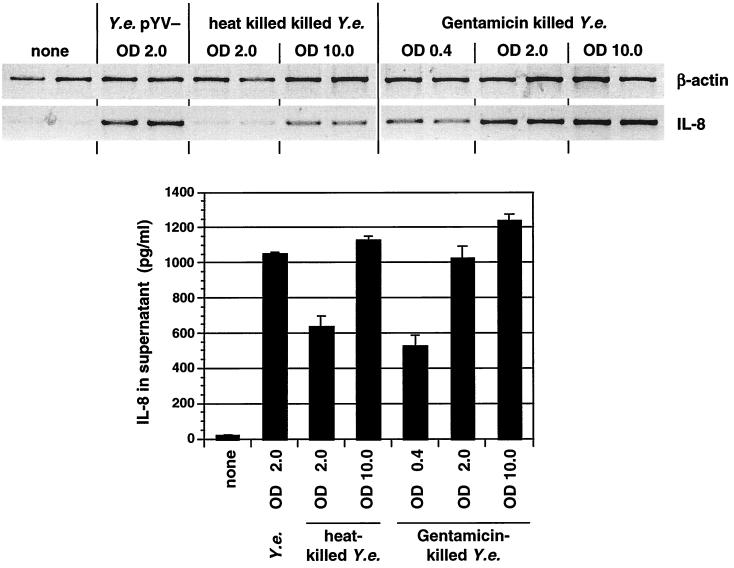
For several pathogens, including Salmonella spp., H. pylori, and Chlamydia spp., it has been demonstrated that metabolic activity or an active type III protein secretion system is necessary to induce cytokine responses in infected cells (40, 48, 58). As the Yersinia invasin protein appears to be essential for triggering cytokine responses in epithelial cells, we wanted to investigate the potential of metabolically inactive yersiniae expressing invasin to trigger cytokine production. For this purpose, Y. enterocolitica pYV− bacteria expressing invasin were killed by gentamicin or heat treatment. Killed bacterial cell suspensions were added to HeLa cells, and cytokine production was determined. As shown in Fig. 2, killed Y. enterocolitica pYV− cells induced IL-8 mRNA expression and IL-8 protein production to a similar degree as viable bacteria. Infection of cells with different numbers of bacteria showed a dose-dependent response in terms of IL-8 mRNA expression. Comparable results were achieved by infection with heat-killed E. coli(pInv1914) expressing Yersinia invasin (data not shown). These data show that metabolic activity of bacteria is not necessary for invasin-mediated cytokine production and secretion by HeLa cells.
FIG. 2.
Role of metabolic activity of Y. enterocolitica in IL-8 production of HeLa cells. Cells were exposed to viable, heat-killed, or gentamicin (100 μg/ml for 1 h)-killed Y. enterocolitica (Y.e.) pYV−; after 3 h IL-8 mRNA production and secretion in the supernatant were determined. ODs of the bacterial inoculum represent different MOIs; OD 2.0 is equivalent to an MOI of ∼150 bacteria/cell.
Kinetics of Yersinia-induced proinflammatory cytokine mRNA production in HeLa cells.
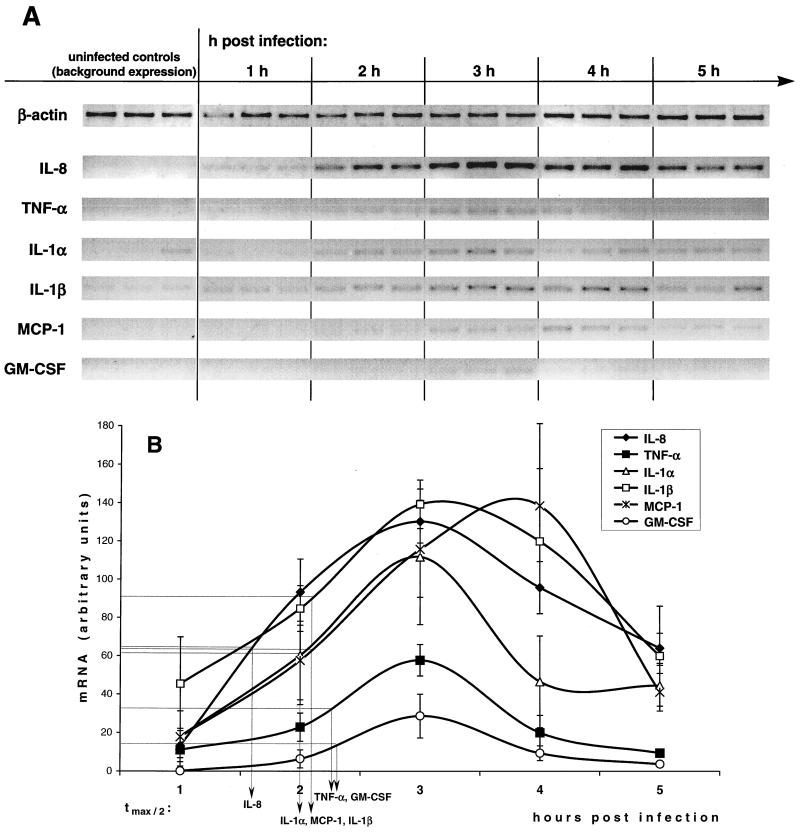
To obtain a more detailed view of the early Yersinia-induced cytokine network in epithelial cells, the kinetics of cytokine mRNA expression was assessed semiquantitatively by means of fluoroimager analysis. The data depicted in Fig. 3 show that IL-8, TNF-α, IL-1α, IL-1β, MCP-1, and GM-CSF mRNA expression was upregulated within 1 h postinfection to reach a maximum after 3 h. Only MCP-1 showed a slightly delayed peak between 3 and 4 h. mRNA levels then declined to levels before infection within the following 3 to 5 h. Points of time of half-maximum mRNA levels were calculated as indicators of the sequential order of cytokine mRNA expression. As indicated in Fig. 3, IL-8 was expressed first, about 20 to 30 min before IL-1α and MCP-1, which were closely followed by IL-1β, TNF-α, and GM-CSF.
FIG. 3.
Time course of cytokine mRNA expression in HeLa cells after Y. enterocolitica infection. HeLa cells were infected for 1 h with Y. enterocolitica pYV− at an MOI of ∼150 bacteria/cell. After 1 h of infection, bacteria were removed from the cultures by washing with PBS and further incubated in the presence of gentamicin for 5 h. Uninfected cells as controls received the same treatment without bacterial infection. Data shown are from a representative experiment with triplicate samples. Comparable results were obtained in additional experiments. (A) Qualitative RT-PCR analysis. (B) Semiquantitative mRNA levels determined by digitally scanning the bands and calculating intensity of each cytokine band by a fluoroimager. Values are expressed in arbitrary units as ratio of the cytokine mRNA level to the corresponding β-actin mRNA level (means of triplicate samples ± standard deviation, interpolated curves). The points of time of half-maximum cytokine expression (tmax/2) are indicated.
IL-8, MCP-1, and GM-CSF, but not IL-1α and IL-1β, are secreted by HeLa cells.
We next measured the amount of cytokine protein in cell culture supernatants and cell lysates of Yersinia-infected HeLa cells to gain additional information on whether secreted cytokines might augment the bacterium-stimulated cytokine responses of epithelial cells. The results shown in Table 2 demonstrate that 4 to 6 h postinfection considerable quantities of IL-8, MCP-1, and GM-CSF were secreted into culture supernatants by HeLa cells, whereas IL-1α and IL-1β could be detected intracellularly only in cell lysates. MCP-1 was detectable also in cell lysates, although in small quantities only. TNF-α was not detectable in culture supernatants or cell lysates by ELISA. At 24 h after infection, IL-1α and IL-1β still remained intracellular in gradually decreasing levels, and neither of the two cytokines was secreted. In contrast, MCP-1, like IL-8, was detectable only extracellularly in supernatants after 24 h. As observed after 4 to 6 h, TNF-α was not detectable by ELISA or Western blotting in all lysates or supernatants after 24 h (data not shown).
TABLE 2.
Cytokine production by HeLa cells after Y. enterocolitica infection
| Cytokine | Concn (pg/ml)a
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Controlb
|
Y. enterocolitica
|
|||||||
| SNc
|
Lysated
|
SN
|
Lysate
|
|||||
| 4–6 h | 24 h | 4 h | 24 h | 4–6 h | 24 h | 4 h | 24 h | |
| IL-8 | 29 ± 2.4 | 218 ± 0.0 | <5 | <5 | 890 ± 10.3* | 897 ± 83.8* | <5 | <5 |
| TNF-α | <15 | <15 | <15 | <15 | <15 | <15 | <15 | <15 |
| IL-1α | <0.5 | <0.5 | <0.5 | <0.5 | <0.5 | <0.5 | 107 ± 12.5* | 52 ± 27.5* |
| IL-1β | <1 | <1 | <1 | <1 | <1 | <1 | 26 ± 8.6* | 4 ± 5.4* |
| MCP-1 | <10 | 141 ± 56.3 | <10 | <10 | 304 ± 18.3* | 817 ± 32.5* | 26 ± 12.8* | <10 |
| GM-CSF | <2.8 | ND | <2.8 | ND | 137 ± 8.7* | ND | <2.8 | ND |
Mean ± standard deviation of triplicate samples from a representative experiment. *, statistically significant difference at P < 0.05. ND, not determined.
HeLa cells were exposed to medium (control) or Y. enterocolitica pYV− (MOI of 150) for 1 h. Cells were then washed with PBS, and medium containing gentamicin was added to kill extracellular bacteria.
Cytokine concentration in culture supernatant (SN) was measured by ELISA at the indicated times postinfection.
Cells were lysed as described in Materials and Methods after the indicated time points to determine intracellular cytokine concentrations by ELISA.
IL-1α, but not IL-8, stimulates cytokine responses in HeLa cells.
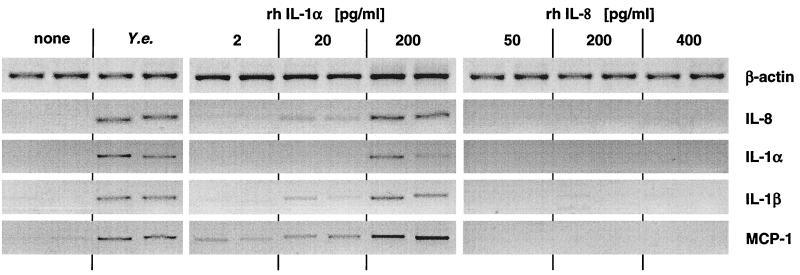
To investigate whether IL-8, which was produced and secreted by Yersinia-stimulated HeLa cells, might augment epithelial cytokine production, HeLa cells were stimulated with various quantities of IL-8, and at various intervals culture supernatants as well as cell lysates were analyzed for the presence of cytokines by RT-PCR (Fig. 4) and ELISA (not shown). As controls, Yersinia- or IL-1-stimulated HeLa cells were used. The results in Fig. 4 show that IL-8 did not stimulate cytokine production in HeLa cells, whereas Yersinia and IL-1 stimulated all of the cytokines investigated (IL-8, IL-1α, IL-1β, and MCP-1) in a dose-dependent manner. Moreover, exposure of HeLa cells to various concentrations of MCP-1 or GM-CSF did not induce significant cytokine responses (data not shown).
FIG. 4.
Cytokine mRNA production of HeLa cells after Y. enterocolitica pYV− infection or exposure to IL-1 or IL-8. Cells were treated with various concentrations of recombinant human IL-1α or IL-8 for 1 h. Then the cells were washed and incubated with medium for another 2 h, when total RNA was extracted for RT-PCR analysis (see Materials and Methods). Data shown are from a representative experiment with duplicate samples. Comparable results were obtained in additional experiments.
DISCUSSION
Although several studies with detailed histologic approaches analyzed the inflammatory response of the infected mucosa to infection by Y. enterocolitica (2, 4), it is still unclear which pathogen and host signals may trigger and promote this reaction. Epithelial cells, the first cells to encounter yersiniae upon orogastric infection, are attributed a key role in generating signals to the underlying mucosa, thereby initiating the host's immune response. To evaluate the role of epithelial cells in intestinal inflammation upon Yersinia infection, we used an epithelial cell monolayer infection model and analyzed Yersinia-triggered cytokine production in these cells.
Infection of HeLa cells with Y. enterocolitica resulted in mRNA expression of several proinflammatory cytokines, including IL-8, TNF-α, IL-1α, IL-1β, MCP-1, and GM-CSF. However, only IL-8, MCP-1, and GM-CSF were secreted into the extracellular environment, while IL-1 was produced but not secreted. TNF-α protein was not detectable at all. Although we cannot exclude that the ELISA and Western blot assays used for detection of TNF-α protein production were not sensitive enough, TNF-α mRNA expression was detected only after 35 PCR cycles, suggesting that very low quantities of TNF-α mRNA (and possibly protein) were produced.
The CXC chemokine IL-8, which could be detected in our model immediately after Yersinia infection, attracts and activates predominantly neutrophils but also monocytes and T lymphocytes (6). MCP-1, a CC chemokine, has a more specific chemotactic activity for monocytes and basophils (38; see reference 7 for a review). GM-CSF, which was also found after Yersinia infection of HeLa cells, is a strong chemoattractant for neutrophils and eosinophils (60). Moreover, GM-CSF stimulates the proliferation and differentiation of neutrophilic, eosinophilic, and monocytic cell lineages and functionally activates the corresponding mature forms of these cells by, e.g., enhancing phagocytosis of bacteria by neutrophils (17, 25, 41, 61). All of the aforementioned effects of IL-8, MCP-1, and GM-CSF might be involved in triggering cellular immune responses against Yersinia as observed in a mouse infection model. In Yersinia-infected mice, polymorphonuclear leukocytes are recruited into infected Peyer's patches 24 h after infection and give rise to microabscesses (1, 3). Several days thereafter, further inflammatory cells including mononuclear phagocytes are recruited into Yersinia-induced Peyer's patch lesions and promote tissue destruction.
While IL-8, MCP-1, and GM-CSF were secreted, both IL-1α and IL-1β were produced but not secreted by HeLa cells upon Yersinia infection and thus remained intracellular for more than 24 h. This observation argues for a special role of IL-1 in Y. enterocolitica infection. Similar to infections with other pathogens such as Entamoeba histolytica (23), it might well be that a second challenge to the epithelial cells (e.g., cytotoxicity) may be required for IL-1 release during Yersinia infection. Trophozoites of the protozoan parasite Entamoeba histolytica induce increased mRNA expression of several proinflammatory cytokines in HeLa cells similar to that observed after Y. enterocolitica infection (23). However, cytokine production and secretion are predominantly due to the paracrine action of preformed, constitutively expressed IL-1α which is released after Entamoeba-induced cytolysis (23). After Chlamydia infection of epithelial cells, mRNA expression and secretion of IL-8, GM-CSF, and other proinflammatory cytokines is upregulated (48). Similar to Entamoeba histolytica infection, cytokine mRNA upregulation could be attributed mainly to the paracrine action of IL-1α, which was passively released by epithelial cells damaged by Chlamydia infection (48). However, an additional signaling pathway for direct induction of IL-8 production and secretion was postulated. In Shigella flexneri infection, presynthesized IL-1α is released from macrophages as a stress signal paralleled by apoptosis. However, Shigella itself is incapable of inducing de novo synthesis of cytokines in macrophages (53). Taken together, in infections with Shigella, Entamoeba, or Chlamydia, IL-1α is the actual inducer of proinflammatory cytokine production and secretion leading to tissue inflammation.
Unlike infection with Entamoeba histolytica, Chlamydia, or S. flexneri, production and secretion of IL-8 in Yersinia infection was not linked to the release of intracellular IL-1. In fact, in our experiments Y. enterocolitica pYV− infected HeLa cells were not damaged and did not undergo apoptosis consistent with other publications (43, 44, 51). Therefore, both IL-1α and IL-1β remained intracellular for more than 24 h after infection. However, pathogenic yersiniae harbor a virulence plasmid which encodes Yop effector proteins, e.g., YopE mediating cytotoxicity (50). Therefore, it is conceivable that such events might lead to the release of IL-1 from epithelial cells during Yersinia infection in vivo.
In contrast to IL-1, both IL-8 and MCP-1 as well as GM-CSF did not augment the inflammatory response of epithelial cells. In keeping with these observations, recent investigations (19, 34) demonstrated a distinct pattern of CC/CXC receptor expression on human colon epithelial cells such as HT29, Caco-2, and T84 cells. Hence, CCR1-8 and CXCR4-5 are all constitutively expressed on these cells, whereas CXCR1 and CXCR2 are little, if at all, expressed. As IL-8 binds to CXCR1 and CXCR2, and GM-CSF and MCP-1 bind to CCR2 or CCR10, it is evident that these cytokines do not act on epithelial cells. Although HeLa cells have not been included in the aforementioned studies, our results may suggest that HeLa cells do not express CXCR1, CXCR2, CCR2, and CCR10. In keeping with these data, we found that IL-1α, but not IL-8, MCP-1, or GM-CSF, is a potent stimulus for transcription and secretion in HeLa cells of proinflammatory cytokines such as IL-8, MCP-1, and GM-CSF. Furthermore, IL-1α itself stimulates IL-1α and IL-1β production in HeLa cells (this work) and other epithelial cells (23, 35). Nevertheless, further work is required on cytokine receptor expression on the various epithelial cell lines as well as on intestinal epithelial cells in situ.
Yersinia-induced cytokine production could be attributed to the activity of a virulence factor, the Yersinia outer membrane protein invasin, which is known to bind to β1 integrins of mammalian cells, thereby mediating, e.g., bacterial internalization into host cells (32). As a further pathogenic effect of invasin, we found that binding of bacteria via invasin to β1 integrins on epithelial cells leads directly to the expression of proinflammatory cytokines including IL-8. For several other bacteria infecting epithelial surfaces (e.g., Salmonella spp. [29, 40], H. pylori [45, 58], or Chlamydia [48]), it was shown that bacterial protein synthesis or a type III or IV protein secretion system was necessary to induce a cytokine responses. In Yersinia infection, however, the type III protein secretion system might counteract invasin-triggered cytokine production (56). Moreover, in contrast to other enteropathogenic bacteria like Salmonella serovar Dublin or enteroinvasive E. coli (21), we could show that Yersinia invasin-mediated cytokine induction did not require bacterial invasion, as inhibition of bacterial entry with the PI3-K inhibitor wortmannin did not alter the Yersinia-induced cytokine pattern. Furthermore, killed invasin-expressing bacterial cells trigger cytokine production comparable to that induced by viable bacteria.
Based on these and previous results, we propose the following scenario for Yersinia infection in vivo. Invasin-expressing yersiniae are translocated efficiently through M cells, and invasin plays an important role in the early phase of Peyer's patch infection (1, 12, 47). Via interaction with β1 integrins on host cells, invasin might trigger production and release of proinflammatory cytokines and chemokines. Thereafter, yersiniae express plasmid-encoded pathogenicity factors (1, 33) in order to evade the innate host immune response. Yersiniae might translocate effector proteins such as YopE, YopH, YopM, and YopJ into the cytosol of host cells (for a review, see reference 15). YopE may disrupt host cells by destroying the actin cytoskeleton (49), and YopJ/YopP may induce apoptosis of macrophages (43, 44). It is tempting to speculate that these events might lead to release of both preformed as well as invasin-induced de novo-produced IL-1. In turn, this process might augment the inflammatory response initially triggered by invasin-induced IL-8, MCP-1, and GM-CSF release.
Whether the inflammatory response induced by Yersinia invasin has protective (anti-Yersinia) or deleterious (destruction of Peyer's patches by the inflammatory response) effects is a matter of ongoing research in our laboratory. Current investigations in our laboratory focus on the chemokine production including production of IL-8-like chemokines in the early phase of the Yersinia infection in mice. Moreover, we have to take into account that in vivo cells other than epithelial cells (e.g., dendritic cells) might also contribute to chemokine production (54).
In summary, our observations may have implications for understanding the early cytokine network operating in mucosal Yersinia infections and further define the role of Y. enterocolitica invasin as an important outer membrane protein with a high contribution to Yersinia pathogenicity in the early phase of mucosal infection.
ACKNOWLEDGMENTS
This work was supported by a grant from the Deutsche Forschungsgemeinschaft.
We thank Nicole Bücheler and Sonja Preger for expert technical assistance.
REFERENCES
- 1.Autenrieth I B, Firsching R. Penetration of M cells and destruction of Peyer's patches by Yersinia enterocolitica: an ultrastructural and histological study. J Med Microbiol. 1996;44:285–294. doi: 10.1099/00222615-44-4-285. [DOI] [PubMed] [Google Scholar]
- 2.Autenrieth I B, Hantschmann P, Heymer B, Heesemann J. Immunohistological characterization of the cellular immune response against Yersinia enterocolitica in mice: evidence for the involvement of T lymphocytes. Immunobiology. 1993;187:1–16. doi: 10.1016/S0171-2985(11)80241-X. [DOI] [PubMed] [Google Scholar]
- 3.Autenrieth I B, Kempf V, Sprinz T, Preger S, Schnell A. Defense mechanisms in Peyer's patches and mesenteric lymph nodes against Yersinia enterocolitica involve integrins and cytokines. Infect Immun. 1996;64:1357–1368. doi: 10.1128/iai.64.4.1357-1368.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Autenrieth I B, Vogel U, Preger S, Heymer B, Heesemann J. Experimental Yersinia enterocolitica infection in euthymic and T-cell-deficient athymic nude C57BL/6 mice: comparison of time course, histomorphology, and immune response. Infect Immun. 1993;61:2585–2595. doi: 10.1128/iai.61.6.2585-2595.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baeuerle P A, Henkel T. Function and activation of NF-kappa B in the immune system. Annu Rev Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 6.Baggiolini M, Dewald B, Moser B. Interleukin-8 and related chemotactic cytokines—CXC and CC chemokines. Adv Immunol. 1994;55:97–179. [PubMed] [Google Scholar]
- 7.Baggiolini M, Dewald B, Moser B. Human chemokines: an update. Annu Rev Immunol. 1997;15:675–705. doi: 10.1146/annurev.immunol.15.1.675. [DOI] [PubMed] [Google Scholar]
- 8.Baldwin A S J. The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 9.Beuscher H U, Rodel F, Forsberg A, Rollinghoff M. Bacterial evasion of host immune defense: Yersinia enterocolitica encodes a suppressor for tumor necrosis factor alpha expression. Infect Immun. 1995;63:1270–1277. doi: 10.1128/iai.63.4.1270-1277.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bohn E, Heesemann J, Ehlers S, Autenrieth I B. Early gamma interferon mRNA expression is associated with resistance of mice against Yersinia enterocolitica. Infect Immun. 1994;62:3027–3032. doi: 10.1128/iai.62.7.3027-3032.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caron E, Gross A, Liautard J P, Dornand J. Brucella species release a specific, protease-sensitive, inhibitor of TNF-alpha expression, active on human macrophage-like cells. J Immunol. 1996;156:2885–2893. [PubMed] [Google Scholar]
- 12.Clark M A, Hirst B H, Jepson M A. M-cell surface beta1 integrin expression and invasin-mediated targeting of Yersinia pseudotuberculosis to mouse Peyer's patch M cells. Infect Immun. 1998;66:1237–1243. doi: 10.1128/iai.66.3.1237-1243.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cornelis G R. Yersinia, finely tuned pathogens. Symp Soc Gen Microbiol. 1992;49:231–265. [Google Scholar]
- 14.Cornelis G R. Yersinia pathogenicity factors. Curr Top Microbiol Immunol. 1994;192:243–263. doi: 10.1007/978-3-642-78624-2_11. [DOI] [PubMed] [Google Scholar]
- 15.Cornelis G R, Boland A, Boyd A P, Geuijen C, Iriarte M, Neyt C, Sory M P, Stainier I. The virulence plasmid of Yersinia, an antihost genome. Microbiol Mol Biol Rev. 1998;62:1315–1352. doi: 10.1128/mmbr.62.4.1315-1352.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cover T L, Aber R C. Yersinia enterocolitica. N Engl J Med. 1989;321:16–24. doi: 10.1056/NEJM198907063210104. [DOI] [PubMed] [Google Scholar]
- 17.Danis V A, Franic G M, Rathjen D A, Brooks P M. Effects of granulocyte-macrophage colony-stimulating factor (GM-CSF), IL-2, interferon-gamma (IFN-gamma), tumour necrosis factor-alpha (TNF-alpha) and IL-6 on the production of immunoreactive IL-1 and TNF-alpha by human monocytes. Clin Exp Immunol. 1991;85:143–150. doi: 10.1111/j.1365-2249.1991.tb05695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Delor I, Cornelis G R. Role of Yersinia enterocolitica Yst toxin in experimental infection of young rabbits. Infect Immun. 1992;60:4269–4277. doi: 10.1128/iai.60.10.4269-4277.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dwinell M B, Eckmann L, Leopard J D, Varki N M, Kagnoff M F. Chemokine receptor expression by human intestinal epithelial cells. Gastroenterology. 1999;117:359–367. doi: 10.1053/gast.1999.0029900359. [DOI] [PubMed] [Google Scholar]
- 20.Eckmann L, Jung H C, Schurer M C, Panja A, Morzycka W E, Kagnoff M F. Differential cytokine expression by human intestinal epithelial cell lines: regulated expression of interleukin 8. Gastroenterology. 1993;105:1689–1697. doi: 10.1016/0016-5085(93)91064-o. [DOI] [PubMed] [Google Scholar]
- 21.Eckmann L, Kagnoff M F, Fierer J. Epithelial cells secrete the chemokine interleukin-8 in response to bacterial entry. Infect Immun. 1993;61:4569–4574. doi: 10.1128/iai.61.11.4569-4574.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eckmann L, Kagnoff M F, Fierer J. Intestinal epithelial cells as watchdogs for the natural immune system. Trends Microbiol. 1995;3:118–120. doi: 10.1016/s0966-842x(00)88894-0. [DOI] [PubMed] [Google Scholar]
- 23.Eckmann L, Reed S L, Smith J R, Kagnoff M F. Entamoeba histolytica trophozoites induce an inflammatory cytokine response by cultured human cells through the paracrine action of cytolytically released interleukin-1 alpha. J Clin Investig. 1995;96:1269–1279. doi: 10.1172/JCI118161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fierer J, Eckmann L, Kagnoff M. IL-8 secreted by epithelial cells invaded by bacteria. Infect Agents Dis. 1993;2:255–258. [PubMed] [Google Scholar]
- 25.Fleischmann J, Golde D W, Weisbart R H, Gasson J C. Granulocyte-macrophage colony-stimulating factor enhances phagocytosis of bacteria by human neutrophils. Blood. 1986;68:708–711. [PubMed] [Google Scholar]
- 26.Grützkau A, Hanski C, Hahn H, Riecken E O. Involvement of M cells in the bacterial invasion of Peyer's patches: a common mechanism shared by Yersinia enterocolitica and other enteroinvasive bacteria. Gut. 1990;31:1011–1015. doi: 10.1136/gut.31.9.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hanski C, Kutschka U, Schmoranzer H P, Naumann M, Stallmach A, Hahn H, Menge H, Riecken E O. Immunohistochemical and electron microscopic study of interaction of Yersinia enterocolitica serotype O:8 with intestinal mucosa during experimental enteritis. Infect Immun. 1989;57:673–678. doi: 10.1128/iai.57.3.673-678.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heesemann J, Laufs R. Construction of a mobilizable Yersinia enterocolitica virulence plasmid. J Bacteriol. 1983;155:761–767. doi: 10.1128/jb.155.2.761-767.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hobbie S, Chen L M, Davis R J, Galan J E. Involvement of mitogen-activated protein kinase pathways in the nuclear responses and cytokine production induced by Salmonella typhimurium in cultured intestinal epithelial cells. J Immunol. 1997;159:5550–5559. [PubMed] [Google Scholar]
- 30.Hoover D L, Friedlander A M, Rogers L C, Yoon I K, Warren R L, Cross A S. Anthrax edema toxin differentially regulates lipopolysaccharide-induced monocyte production of tumor necrosis factor alpha and interleukin-6 by increasing intracellular cyclic AMP. Infect Immun. 1994;62:4432–4439. doi: 10.1128/iai.62.10.4432-4439.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Isberg R R. Mammalian cell adhesion functions and cellular penetration of enteropathogenic Yersinia species. Mol Microbiol. 1989;3:1449–1453. doi: 10.1111/j.1365-2958.1989.tb00128.x. [DOI] [PubMed] [Google Scholar]
- 32.Isberg R R, Leong J M. Multiple beta 1 chain integrins are receptors for invasin, a protein that promotes bacterial penetration into mammalian cells. Cell. 1990;60:861–871. doi: 10.1016/0092-8674(90)90099-z. [DOI] [PubMed] [Google Scholar]
- 33.Jacobi C A, Roggenkamp A, Rakin A, Zumbihl R, Leitritz L, Heesemann J. In vitro and in vivo expression studies of yopE from Yersinia enterocolitica using the gfp reporter gene. Mol Microbiol. 1998;30:865–882. doi: 10.1046/j.1365-2958.1998.01128.x. [DOI] [PubMed] [Google Scholar]
- 34.Jordan N J, Kolios G, Abbot S E, Sinai M A, Thompson D A, Petraki K, Westwick J. Expression of functional CXCR4 chemokine receptors on human colonic epithelial cells. J Clin Investig. 1999;104:1061–1069. doi: 10.1172/JCI6685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jung H C, Eckmann L, Yang S K, Panja A, Fierer J, Morzycka W E, Kagnoff M F. A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J Clin Investig. 1995;95:55–65. doi: 10.1172/JCI117676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kagnoff M F, Eckmann L. Epithelial cells as sensors for microbial infection. J Clin Investig. 1997;100:6–10. doi: 10.1172/JCI119522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leal-Berumen I, Snider D P, Barajas-Lopez C, Marshall J S. Cholera toxin increases IL-6 synthesis and decreases TNF-alpha production by rat peritoneal mast cells. J Immunol. 1996;156:316–321. [PubMed] [Google Scholar]
- 38.Leonard E J, Yoshimura T. Human monocyte chemoattractant protein-1 (MCP-1) Immunol Today. 1990;11:97–101. doi: 10.1016/0167-5699(90)90035-8. [DOI] [PubMed] [Google Scholar]
- 39.May M J, Ghosh S. Signal transduction through NF-kappa B. Immunol Today. 1998;19:80–88. doi: 10.1016/s0167-5699(97)01197-3. [DOI] [PubMed] [Google Scholar]
- 40.McCormick B A, Colgan S P, Delp A C, Miller S I, Madara J L. Salmonella typhimurium attachment to human intestinal epithelial monolayers: transcellular signalling to subepithelial neutrophils. J Cell Biol. 1993;123:895–907. doi: 10.1083/jcb.123.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Metcalf D. The molecular biology and functions of the granulocyte-macrophage colony-stimulating factors. Blood. 1986;67:257–267. [PubMed] [Google Scholar]
- 42.Miller V L, Falkow S. Evidence for two genetic loci in Yersinia enterocolitica that can promote invasion of epithelial cells. Infect Immun. 1988;56:1242–1248. doi: 10.1128/iai.56.5.1242-1248.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mills S D, Boland A, Sory M P, van der Smissen P, Kerbourch C, Finlay B B, Cornelis G R. Yersinia enterocolitica induces apoptosis in macrophages by a process requiring functional type III secretion and translocation mechanisms and involving YopP, presumably acting as an effector protein. Proc Natl Acad Sci USA. 1997;94:12638–12643. doi: 10.1073/pnas.94.23.12638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Monack D M, Mecsas J, Ghori N, Falkow S. Yersinia signals macrophages to undergo apoptosis and YopJ is necessary for this cell death. Proc Natl Acad Sci USA. 1997;94:10385–10390. doi: 10.1073/pnas.94.19.10385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Munzenmaier A, Lange C, Glocker E, Covacci A, Moran A, Bereswill S, Baeuerle P A, Kist M, Pahl H L. A secreted/shed product of Helicobacter pylori activates transcription factor nuclear factor-kappa B. J Immunol. 1997;159:6140–6147. [PubMed] [Google Scholar]
- 46.Pai C H, Mors V. Production of enterotoxin by Yersinia enterocolitica. Infect Immun. 1978;19:908–911. doi: 10.1128/iai.19.3.908-911.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pepe J C, Miller V L. Yersinia enterocolitica invasin: a primary role in the initiation of infection. Proc Natl Acad Sci USA. 1993;90:6473–6477. doi: 10.1073/pnas.90.14.6473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rasmussen S J, Eckmann L, Quayle A J, Shen L, Zhang Y X, Anderson D J, Fierer J, Stephens R S, Kagnoff M F. Secretion of proinflammatory cytokines by epithelial cells in response to Chlamydia infection suggests a central role for epithelial cells in chlamydial pathogenesis. J Clin Investig. 1997;99:77–87. doi: 10.1172/JCI119136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rosqvist R, Forsberg A, Wolf-Watz H. Intracellular targeting of the Yersinia YopE cytotoxin in mammalian cells induces actin microfilament disruption. Infect Immun. 1991;59:4562–4569. doi: 10.1128/iai.59.12.4562-4569.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rosqvist R, Wolf-Watz H. Virulence plasmid-associated HeLa cell induced cytotoxicity of Yersinia pseudotuberculosis. Microb Pathog. 1986;1:229–240. doi: 10.1016/0882-4010(86)90047-1. [DOI] [PubMed] [Google Scholar]
- 51.Ruckdeschel K, Roggenkamp A, Lafont V, Mangeat P, Heesemann J, Rouot B. Interaction of Yersinia enterocolitica with macrophages leads to macrophage cell death through apoptosis. Infect Immun. 1997;65:4813–4821. doi: 10.1128/iai.65.11.4813-4821.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ruckdeschel K, Roggenkamp A, Schubert S, Heesemann J. Differential contribution of Yersinia enterocolitica virulence factors to evasion of microbicidal action of neutrophils. Infect Immun. 1996;64:724–733. doi: 10.1128/iai.64.3.724-733.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sansonetti P J, Arondel J, Cavaillon J M, Huerre M. Role of interleukin-1 in the pathogenesis of experimental shigellosis. J Clin Investig. 1995;96:884–892. doi: 10.1172/JCI118135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sato K, Kawasaki H, Nagayama H, Serizawa R, Ikeda J, Morimoto C, Yasunaga K, Yamaji N, Tadokoro K, Juji T, Takahashi T A. CC chemokine receptors, CCR-1 and CCR-3, are potentially involved in antigen-presenting cell function of human peripheral blood monocyte-derived dendritic cells. Blood. 1999;93:34–42. [PubMed] [Google Scholar]
- 55.Schulte R, Autenrieth I B. Yersinia enterocolitica-induced interleukin-8 secretion by human intestinal epithelial cells depends on cell differentiation. Infect Immun. 1998;66:1216–1224. doi: 10.1128/iai.66.3.1216-1224.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schulte R, Wattiau P, Hartland E L, Robins B R, Cornelis G R. Differential secretion of interleukin-8 by human epithelial cell lines upon entry of virulent or nonvirulent Yersinia enterocolitica. Infect Immun. 1996;64:2106–2113. doi: 10.1128/iai.64.6.2106-2113.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schulte R, Zumbihl R, Kampik D, Fauconnier A, Autenrieth I B. Wortmannin blocks Yersinia invasin-triggered internalization, but not interleukin-8 production by epithelial cells. Med Microbiol Immunol (Berl) 1998;187:53–60. doi: 10.1007/s004300050074. [DOI] [PubMed] [Google Scholar]
- 58.Sharma S A, Tummuru M K, Miller G G, Blaser M J. Interleukin-8 response of gastric epithelial cell lines to Helicobacter pylori stimulation in vitro. Infect Immun. 1995;63:1681–1687. doi: 10.1128/iai.63.5.1681-1687.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Staugas R E, Harvey D P, Ferrante A, Nandoskar M, Allison A C. Induction of tumor necrosis factor (TNF) and interleukin-1 (IL-1) by Pseudomonas aeruginosa and exotoxin A-induced suppression of lymphoproliferation and TNF, lymphotoxin, gamma interferon, and IL-1 production in human leukocytes. Infect Immun. 1992;60:3162–3168. doi: 10.1128/iai.60.8.3162-3168.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Warringa R A, Koenderman L, Kok P T, Kreukniet J, Bruijnzeel P L. Modulation and induction of eosinophil chemotaxis by granulocyte-macrophage colony-stimulating factor and interleukin-3. Blood. 1991;77:2694–2700. [PubMed] [Google Scholar]
- 61.Weisbart R H, Golde D W, Clark S C, Wong G G, Gasson J C. Human granulocyte-macrophage colony-stimulating factor is a neutrophil activator. Nature. 1985;314:361–363. doi: 10.1038/314361a0. [DOI] [PubMed] [Google Scholar]
- 62.Wilson M, Seymour R, Henderson B. Bacterial perturbation of cytokine networks. Infect Immun. 1998;66:2401–2409. doi: 10.1128/iai.66.6.2401-2409.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]