Abstract
Whole genome sequencing for generating SNP data is increasingly used in population genetic studies. However, obtaining genomes for massive numbers of samples is still not within the budgets of many researchers. It is thus imperative to select an appropriate reference genome and sequencing depth to ensure the accuracy of the results for a specific research question, while balancing cost and feasibility. To evaluate the effect of the choice of the reference genome and sequencing depth on downstream analyses, we used five confamilial reference genomes of variable relatedness and three levels of sequencing depth (3.5×, 7.5× and 12×) in a population genomic study on two caddisfly species: Himalopsyche digitata and H. tibetana. Using these 30 datasets (five reference genomes × three depths × two target species), we estimated population genetic indices (inbreeding coefficient, nucleotide diversity, pairwise F ST, and genome‐wide distribution of F ST) based on variants and population structure (PCA and admixture) based on genotype likelihood estimates. The results showed that both distantly related reference genomes and lower sequencing depth lead to degradation of resolution. In addition, choosing a more closely related reference genome may significantly remedy the defects caused by low depth. Therefore, we conclude that population genetic studies would benefit from closely related reference genomes, especially as the costs of obtaining a high‐quality reference genome continue to decrease. However, to determine a cost‐efficient strategy for a specific population genomic study, a trade‐off between reference genome relatedness and sequencing depth can be considered.
Keywords: aquatic insects, de novo genomes, population genomics, reference genomes, sequencing depth, whole genome resequencing
To investigate the impacts of the reference genome and sequencing depth in population genomic studies, we compared results of population genetic indices and population structure derived from three levels of sequencing depth and five confamilial reference genomes of variable relatedness. Our study demonstrated that both distantly related reference genomes and lower sequencing depth lead to less accurate results. However, choosing a more closely related reference genome may significantly remedy the defects caused by low sequencing depth, which indicates that population genomic studies would benefit most from closely related reference genomes.
1. INTRODUCTION
As high‐throughput sequencing (HTS) technologies and bioinformatic tools are rapidly becoming more accurate and increasingly affordable, it is possible to generate whole genome resequencing (WGR) data for almost any species. High‐quality WGR data provide a remarkable amount of information, including a vast number of loci as well as a large number of genetic variants, thus enabling powerful population genomics analyses (Goodwin et al., 2016). Today, whole genome resequencing with low read depth, which indicates a low average number of reads that are aligned to a base in the reference genome, is widely applied in population studies (Lou et al., 2021; Nielsen et al., 2011; Sims et al., 2014). When initiating a project on population genetics using WGR, there are two prerequisites: (i) the availability of a reference genome representing the focal species (Ellegren, 2014) and (ii) estimating the necessary sequencing depth to support accurate results (Meisner & Albrechtsen, 2018).
The selection of reference genome and sequencing depth are therefore two important features in a population genetic study. However, with a fixed budget, it is important to find a balance between data quality and sequencing costs by compromising on the reference genome or sequencing depth. For every study, the reference genome needs to be carefully chosen to avoid bias in mapping and variant calling, considering the amount of sequence identity between the reference genome and the data resulting from resequencing (Nielsen et al., 2011). Ideally, such studies should include a high‐quality species‐specific reference genome, which is often not available for nonmodel organisms. Given the costs and time associated with generating a de novo reference genome, it can be more realistic to use an existing one, yet more distantly related, as is done most often in population genomic studies (Duchen & Salamin, 2021). Currently, several empirical studies have examined the impact of nonconspecific reference genomes in population genomics. For example, Gopalakrishnan et al. (2017) compared the use of dog and wolf genomes as reference genomes for either domestic dog or wolf populations. Their results showed that the selection of the reference genome only had a minor influence on downstream analyses, probably because of the close relatedness of these two taxa, which diverged approx. 30,000 years ago (Skoglund et al., 2015; Wang et al., 2013). By contrast, other studies have found that the use of distantly related reference genomes biases the results of resequencing analyses in bacteria (Valiente‐Mullor et al., 2021), fungi (Garcia‐Rubio et al., 2018), and mammals (Yang et al., 2019), but no studies are yet available for insects.
In comparison to choosing the reference genome based upon relatedness, sequencing depth needs more preliminary knowledge to determine. The sequencing depth needs to be tailored to each particular study based on many aspects, for instance genome size of the target species, the availability of funding, and of course the research question. Using the strategy of low sequencing depth in a population genetic study may result in data loss, thus causing statistical uncertainty during genotype and variant calling (Crawford & Lazzaro, 2012; Meisner & Albrechtsen, 2018; Nielsen et al., 2012). This statistical uncertainty is mainly due to the limited information provided by the low amount of reads, leading to poor discrimination between sequencing error and real variation, that is, SNPs (Meisner & Albrechtsen, 2018). To improve the accuracy of estimates in a cost‐limited population genetic study, especially using low sequencing depth, some researchers therefore demonstrated that employing a large sample size provides more accurate results, for instance, more than 50 individuals from a population with a sequencing depth of two (Buerkle & Gompert, 2013; Fumagalli, 2013; Han et al., 2014; Sims et al., 2014). However, unlike these studies that are based on simulated data, it is challenging to obtain a large number of samples for taxa such as the aquatic insect genus Himalopsyche. These species are apex predators in the benthic invertebrate community and naturally have small population sizes. Many other taxa are similarly rare. Thus, it is pragmatic to consider how we might improve population‐based inference through the optimization of reference genome choice or sequencing depth for these sample‐limited studies. Therefore, comprehensively exploring the influence of reference choice and sequencing depth on downstream analyses is urgently needed, particularly for insects. In this study, we will evaluate the effects of the reference genome and sequencing depth on a genus of caddisflies, for which we already have significant molecular and taxonomic data (Deng et al., 2021; Heckenhauer et al., 2022).
Himalopsyche is a genus of caddisflies (Insecta: Trichoptera) that is primarily distributed in the mountainous areas of central and east Asia (Hjalmarsson et al., 2018). Their larvae live as free‐roaming predators in cool fast‐flowing rivers and streams and are regarded as bioindicators of water quality (Hjalmarsson, 2019; Morse et al., 2019; Tsuruishi et al., 2006). There are currently 56 named species of Himalopsyche (Hjalmarsson, 2019), which are divided into five species groups: the tibetana group, the lepcha group, the kuldschensis group, the phryganea group, and the navasi group (Hjalmarsson et al., 2019). The species H. digitata and H. tibetana both belong to the tibetana group, which is distributed in the Himalayas. This area is characterized by a number of parallel north–south running river systems (such as the Karnali, the Narayani, and the Koshi) with sharp elevational gradients. Currently, climate change is causing a number of cascading effects on river flow via rapidly receding glaciers greatly affecting aquatic biodiversity (Xu et al., 2009). It is therefore crucial to investigate the patterns of genetic diversity of aquatic insects in the region to understand the current and past ecological processes, in order to promote the conservation of freshwater biodiversity (Geist, 2011). Genome‐wide analysis is an important conservation tool that can provide novel insights essential for identifying, for example, hotspots or reservoirs of genetic diversity, dispersal routes, ecological corridors, and stepping stone habitats (Barbosa et al., 2018; Brandies et al., 2019; Hohenlohe et al., 2021; Jasper et al., 2019). However, the quality and quantity of aquatic insect genomes are still relatively low compared with terrestrial insects (Hotaling et al., 2020, 2021). Despite Trichoptera covering ~275 million years of evolution (Thomas et al., 2020) and comprising ~16,300 known species (Morse et al., 2019), only 29 Trichoptera genome assemblies (26 species) have been published to date (Heckenhauer et al., 2019, 2022; Luo et al., 2018; Olsen et al., 2021; Ríos‐Touma et al., 2022). This represents <0.15% of all known species, which limits progress in genomics‐based research of this ecologically relevant group.
The number of studies using population genomics is rapidly increasing. With it, the need to test the effect of reference genome's selection and sequencing depth on the results. Indeed, such studies will allow to find a balance between data quality and sequencing costs by compromising on the reference genome or sequencing depth. Therefore, we conducted a case study using an empirical dataset to evaluate how reference genome selection, that is, degree of relatedness, and sequencing depth affect downstream population genetic analyses of the species H. digitata and H. tibetana. In addition, we tried to reveal the correlation between the inferences from population genetics and drainage network and provided insights for local biodiversity conservation of these enigmatic species.
2. MATERIALS AND METHODS
2.1. Study design
As illustrated in Figure 1, first, we generated three new de novo whole genome assemblies for H. tibetana (tibetana group), H. japonica (navasi group), and H. sp. (kuldschensis group) sensu Hjalmarsson et al., 2019, in addition to two previously published genomes (i.e., H. phryganea (phryganea group) and R. brunnea; Heckenhauer et al., 2022). Since the specimens of Himalopsyche sp. was collected as a larva that cannot be identified to species level by morphologic diagnosis, and the CO1 sequence of this specimen differed slightly from all hitherto known sequences of named species, we can only classify this specimen as a species belonging to the kuldschensis group. These five genomes represent a gradient of genetic relatedness with respect to our target species, which were used as reference genomes. We used populations of two Himalopsyche species (H. digitata and H. tibetana), each species including four populations with each population containing six individuals except for one population from H. digitata and H. tibetana, respectively, which contained only five individuals. The reads of the populations were subsampled into three separate datasets with an average depth of 12.5×, 7.5× and 3.5×, respectively. Reads from each dataset were mapped to the five different reference genomes separately. Afterward, variants were called from all datasets using two strategies: Genotype calling with GATK and direct estimation of the genotype likelihood using ANGSD. Variants identified with the first strategy were used to calculate the population genetic indices including inbreeding coefficient (F), nucleotide diversity (π), and pairwise fixation index (F ST); variants estimated from the second strategy were used in the principal component analysis (PCA) and admixture analysis. Finally, we compared population genetic indices and population structure with different references and sequencing depths.
FIGURE 1.
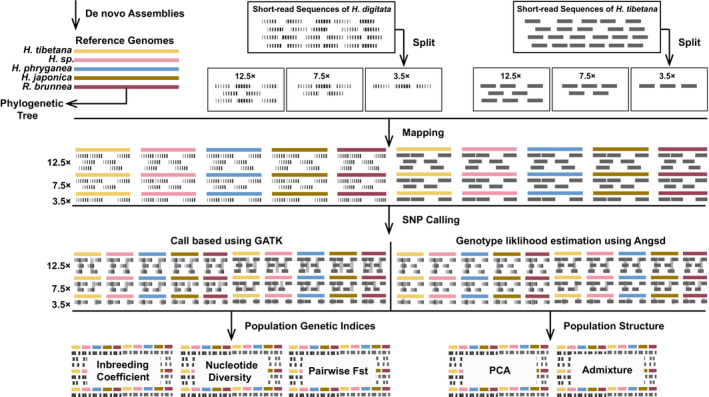
Workflow of data processing in this study showing the treatment of short‐read resequencing data from the two target species and subsequent mapping and variant calling with different reference genomes for assessing genetic diversity and population genetic structure (see details in Section 2.1).
2.2. De novo genomes of three reference species
We used the genome assemblies of four species of Himalopsyche (H. tibetana, H. sp., H. phryganea, and H. japonica) and of one species from the closely related genus Rhyacophila (R. brunneae, both family Rhyacophilidae) as reference genomes. The Himalopsyche species represent the four major taxonomic groups in the genus according to Hjalmarsson et al. (2018): the tibetana group (H. tibetana), the kuldschensis group (H. sp.), the phryganea group (H. phryganea), and the navasi group (H. japonica). Unfortunately, we could not obtain a sample of H. lepcha (the only species in the lepcha group). Rhyacophila is the most closely related genus to Himalopsyche (Thomas et al., 2020). The genomes of H. phryganea (JAGVSL000000000) and Rhyacophila brunnea (previous version of JAGYXB000000000, available at https://doi.org/10.6084/m9.figshare.c.6033011.v1) were previously sequenced and assembled (Heckenhauer et al., 2022). We generated new de novo assemblies of H. tibetana (collected from the Ê Ghunsa River, Nepal), H. sp. (kuldschensis group, collected from the Ê Ghunsa River, Nepal), and H. japonica (collected from the Nogami River, Kiso‐machi, Nagano Prefecture, Japan). Tissue of abdomen and thorax segments were used for DNA extraction after removal of the intestinal tract. We extracted high molecular weight genomic DNA using a salting‐out protocol adapted from Miller et al. (1988), as described in Heckenhauer et al. (2019). We quantified the DNA using a Qubit 4.0 fluorometer with the dsDNA Broad Range Kit (ThermoFisher Scientific) and checked its purity with a DS11 spectrophotometer (DeNovix). We used a low‐cost sequencing strategy that has been shown to produce contiguous genome assemblies, that is, employing a combination of short (Illumina) and long‐read (Oxford Nanopore) technologies to sequence the three new reference genomes, as described in Appendix S1 (Section 1.1).
We conducted a long‐read assembly of the Oxford Nanopore Technology sequencing reads with wtdbg2 v2.4 (Ruan & Li, 2020), followed by mapping and polishing with long reads with Minimap2 v14 (Li, 2018) and Racon v1.3.1 (Vaser et al., 2017). We then performed another round of long‐read polishing with nanopolish 0.11.1 (Loman et al., 2015) by first indexing the signal‐level data in the FAST5 files using nanopolish index, realigning the long reads to the Racon‐polished assembly with minimap2, and then sorting and indexing the bam file with samtools. We used nanopolish_makerange.py to split our draft genome assembly into 50‐kb segments and generated a consensus for each segment in parallel with nanopolish variants (−‐consensus ‐‐min‐candidate‐frequency 0.1). We generated the polished genome in FASTA format using nanopolish vcf2fasta. Because noisy long reads can suffer from indel errors, even with polishing, we further polished the assembly with high‐quality short‐read data with Pilon v1.22 (Walker et al., 2014). To do this, we mapped quality trimmed Illumina reads to the “nanopolished” assembly with bwa‐mem and sorted the read alignments by leftmost coordinates using the sort options in SAMtools v1.9 (Li et al., 2009). Finally, we used Pilon v1.22 (option ‐‐fix indels) to polish the assembly. Following polishing, we used purge_dups 1.2.3 (Roach et al., 2018) to purge haplotigs and overlaps in the assembly based on read depth.
For H. japonica, this pipeline did not meet the expected quality regarding contiguity and BUSCO completeness. Thus, we conducted a de novo hybrid assembly with the raw Illumina data together with the long reads using MaSuRCA v.3.1.1 (Zimin et al., 2013, 2017). In the config file, we specified the insert size and a standard deviation (10% of insert size) for the Illumina reads, as well as jellyfish hash size (estimated_genome_size*~long‐read coverage (equal to depth in this scenario)). All other parameters were left as defaults. We used purge_dups 1.2.3 to purge haplotigs and overlaps in the assembly based on read depth.
We calculated assembly statistics with QUAST v5.0.2 (Gurevich et al., 2013) and examined completeness with BUSCO v4.1.4 (Simão et al., 2015; Waterhouse et al., 2018) using the Endopterygota odb10 dataset with the options ‐‐long, −m = genome and ‐sp = fly. A summary of the assembly statistics and BUSCO completeness is given in Table 1. The final genome assemblies were screened for potential contaminations with taxon‐annotated GC‐coverage plots (TAGC plots) using BlobTools v1.0 (Laetsch & Blaxter, 2017). For this purpose, all preprocessed Illumina reads of the respective species were mapped against the final genome assemblies using BWA‐MEM v0.7.17‐r1188 (Li, 2013) and taxonomic assignment for BlobTools was done with blastn using ‐task megablast and ‐e‐value 1 e‐25. Details are given in Appendix S1 (Section 1.2).
TABLE 1.
Assembly statistics of reference genomes used in this study.
| Species | Accession number | Sequencing platform (depth) d | Assembly length bp | N50 (bp) | No of contigs | N's per 100 kb | BUSCOS % c | Number of proteins |
|---|---|---|---|---|---|---|---|---|
| Himalopsyche japonica a | JAHFWJ000000000 | Nanopore+Illumina (18× +170×) | 546,840,812 | 2,150,202 | 847 | 0 | C:97.2% [S:96.6%, D:0.6%], F:0.8%, M:2.0%, n:2124 | 9983 |
| Himalopsyche sp. a | JAHFWI000000000 | Nanopore+Illumina (26× +200×) | 592,402,457 | 7,599,818 | 528 | 0 | C:96.7% [S:96.3%, D:0.4%], F:1.0%, M:2.3%, n:2124 | 10,049 |
| Himalopsyche phryganea | JAGVSL000000000 | Nanopore+Illumina (36.8× +170×) | 633,785,554 | 4,634,010 | 710 | 0 | C:97.0% [S:96.5%, D:0.5%], F:1.0%, M:2.0%, n:2124 | 10,994 |
| Himalopsyche tibetana a | JAHFWH000000000 | Nanopore+Illumina (24× +150×) | 665,312,086 | 949,059 | 1853 | 0 | C:96.4% [S:95.6%, D:0.8%], F:1.0%, M:2.6%, n:2124 | 10,994 |
| Rhyacophila brunnea | JAGYXB000000000 b | Nanopore+Illumina (19× +154×) | 1,086,872,538 | 1,030,560 | 2125 | 0.36 | C:96.0% [S:93.3%, D:2.7%], F:1.1%, M:2.9%, n:2124 | 10,846 |
This study.
In this study, we used a previous version of this assembly for SNP calling.
Based on the Endopterygota odb10 dataset (2124 genes), C: complete, S: single, D: duplicated, F: fragmented, M: missing.
Based on Genomescope2 genome size estimation.
Genome size estimation and profiling was conducted from the short‐read sequence data with GenomeScope 2.0 (Ranallo‐Benavidez et al., 2020; Vurture et al., 2017) as described in Appendix S1 (Section 1.3).
2.3. Annotation
We identified and classified repetitive elements de novo and generated a library of consensus sequences for each genome using RepeatModeler 2.0 (Flynn et al., 2020). We then annotated and masked repeats in each assembly with RepeatMasker 4.1.0 (http://www.repeatmasker.org) using the custom repeat library for the species generated in the previous step. After masking repeats, genes were predicted using the homology‐based gene prediction tool GeMoMa v1.6.4 (Keilwagen et al., 2016, 2019) and the two previously published species (H. phryganea = RG 1 and R. brunnea = RG 2) as reference organisms as follows: GeMoMa ‐Xmx50G GeMoMaPipeline threads=$SLURM_NPROCS outdir=<out_dir > GeMoMa.Score=ReAlign AnnotationFinalizer.r=NO o=true t=<genome to be annotated> s=own i=<name of RG 1>a=<RG 1.gff>g=<RG 1 assembly.fasta>s=own i=i=<name of RG 2>a=a=<RG 2.gff>g=g=<RG 2 assembly.fasta>.
For functional annotation of predicted genes, we first split each amino acid FASTA file into multiple files with 50 sequences each using awk and then used blastp to search against the ncbi‐blast2.9.0+ nr database with an e‐value cutoff of 10–4, −max_target_seqs set to 10 and ‐out format 6. The resulting xml files were merged using cat and then functionally annotated using the command‐line version of Blast2GO (Götz et al., 2008).
2.4. Species tree reconstruction
To determine the phylogenetic relatedness among the five species, we estimated a species tree using single‐copy orthologs resulting from the BUSCO analyses of the five genome assemblies with an additional species, Glossosoma conforme, as an outgroup (Heckenhauer et al., 2022). For each single‐copy ortholog, we generated an unaligned FASTA file with sequences from each species. We then aligned each ortholog with the MAFFT L‐INS‐i algorithm (Katoh & Standley, 2013). We selected the best‐fit substitution model for each alignment using ModelFinder (option ‐m mfp, (Kalyaanamoorthy et al., 2017)) in IQtree v.2.0.6 (Minh et al., 2020) and estimated a maximum‐likelihood tree with 1000 ultrafast bootstrap replicates (Hoang et al., 2018) with the BNNI correction (options ‐bb 1000 ‐bnni). We then generated a multispecies coalescent species tree in ASTRAL‐III (Zhang et al., 2018) using the best maximum‐likelihood tree from each ortholog as input. We visualized the trees using FigTree v.1.4.4 (http://tree.bio.ed.ac.uk/software/figtree/).
2.5. Cactus alignment and Hal
To further characterize the variation among the five genomes, we computed a whole genome alignment using Cactus v1.0.0 (Armstrong et al., 2020) with a star tree of the five genomes as input: ((Rhyacophila_brunnea, Himalopsyche_kuldschensis, Himalopsyche_phryganea, Himalopsyche_japonica, Himalopsyche_tibetana)mr).
We used HALtools (Hickey et al., 2013) to obtain global alignment information with the halStats option. We then used “halSummarizeMutations” to generate mutation statistics, including the length of each mutation and the number of substitutions, transitions, transversions, gaps, insertions, deletions, inversions, duplications, and transpositions among genomes.
2.6. Population genomic analyses
2.6.1. Taxon sampling
We conducted whole genome resequencing on a total of 46 individuals from four H. tibetana populations and four H. digitata populations, with six individuals from each population except for pop 1 of H. digitata, which included five individuals. All 46 samples were collected as larvae in April 2018 and March 2019 in Nepal. All four H. digitata populations and two populations of H. tibetana were collected in the headwaters of the Gandaki basin with two additional populations of H. tibetana collected in the headwaters of Koshi basin (Figure 6). Since it is presently not possible to identify Himalopsyche larvae to species level solely based on morphological characters, we ensured correct identification of these samples based on two molecular markers: the mitochondrial COI and the nuclear CAD, using the methods outlined in Hjalmarsson et al. (2018).
FIGURE 6.

Location of populations, genetic diversity, and population structure of H. digitata and H. tibetana. (a) Map showing the eight population sites of the two species across two main drainage basins in Nepal. Population numbers are signed on the sample sites; (b) PCA plots and admixture proportions (k = 2, 3, 4) of H. digitata; (c) PCA plots and admixture proportions (k = 2, 3, 4) of H. tibetana; (d) nucleotide diversity (upper) and inbreeding coefficient (lower) of H. digitata, the mean values of each population were labeled with green dots and numbers; (e) nucleotide diversity (upper) and inbreeding coefficient (lower) of H. tibetana, the mean values of each population were labeled with green dots and numbers; (f) pairwise F ST of H. digitata (upper) and H. tibetana.
All the samples were preserved in 95% ethanol and archived in the collections of the Senckenberg Research Institute and Natural History Museum (SMF). Specimen and voucher information is shown in Appendix S2 and Figure 6.
2.6.2. DNA extraction, library preparation, and sequencing
Genomic DNA was extracted following a modified salting‐out protocol adapted from Miller et al. (1988), as described in Heckenhauer et al. (2019). In case of low purity DNA (A260/A230 purity ratio below 1.4 and DNA concentration higher than 100 μg/μl that was measured by DeNovix DS11 spectrophotometer), the template DNA was subject to an additional cleanup using magnetic beads as described in Appendix S1 (Section 2.1). Afterward, all the samples were sent to Novogene Co., Ltd. for DNA library preparation and sequencing. 150‐bp paired‐end reads were generated on an Illumina HiSeq 2000 platform.
2.6.3. Quality control, data processing of population sequence data, and variant calling
We assessed the quality of Illumina reads before and after each step using FastQC v0.11.8 (https://www.bioinformatics.babraham.ac.uk/projects/fastqc/) and MultiQC v1.7 (Ewels et al., 2016), details are described in Appendix S1 (Section 2.2). We trimmed overrepresented k‐mers using autotrim.pl v0.6.1 (Waldvogel et al., 2018) with Trimmomatic v0.38 (Bolger et al., 2014), and we removed adapters with Cutadapt v2.23 (Martin, 2011). After quality filtering, the average sequence depth was 18× and the minimal depth was 12.5× for all the samples. To maintain even depth of all the individuals and to simulate subsets with varying depth levels, we chose 12.5×, 7.5× and 3.5× as our test depth levels. We randomly subsampled the sequencing reads to a specified depth using rasusa 0.3.0. (Hall, 2022) with a random seed of 1 (−s 1), the target depth (−‐coverage 12.5, −‐coverage 7.5, −‐coverage 3.5) and the estimated genome size (−‐genome‐size 550 m) based on GenomeScope2 estimation (see below; Ranallo‐Benavidez et al., 2020; Vurture et al., 2017).
For population genomic analyses, FASTA files of the reference genomes were indexed with the function bowtie2‐build of bowtie2 2.3.5 (Langmead & Salzberg, 2012). We then mapped the reads of the three different depth datasets (12.5×, 7.5× and 3.5×) of the H. digitata and H. tibetana populations to each of the five different reference genomes separately using Bowtie2, which resulted in 30 datasets (2 species × 3 depth × 5 reference genome, Figure 1). After marking duplicate reads for each dataset using Picard v2.20.8 (Picard Tools – By Broad Institute), we conducted variant calling for downstream population genetic analysis using two strategies. In the first strategy, we called the haplotype of each individual separately, by running GATK v4.1.7.0 (GATK, broadinstitute.org) HaplotypeCaller on each bam file. Then, we called genotypes with GATK GenotypeGVCFs across all resulting vcf files. Ultimately, we selected the variants and filtered missing sites using VCFtools v0.1.17 (Danecek et al., 2011). With the second strategy, we estimated genotype likelihoods with appropriate filtering (including reads/sites/alleles/depth filtering, nonmissing individual, and SNP filtering) using ANGSD v0.931 (Korneliussen et al., 2014). For details and parameters used, see Appendix S1 (Sections 2.3 and 2.4).
2.6.4. Genetic diversity and population structure analysis
The variants called by GATK with genotype calling were used to estimate π, individual F, and pairwise F ST (Weir & Cockerham, 1984). These are commonly used measurements for population genetics. F is used to directly quantify the alleles inherited from common ancestors in an individual's lineage, π is an index for population‐level genetic diversity quantification, and F ST provides a primary description of population genetic differentiation. In this study, π and F ST were calculated using VCFtools with a 50‐kb window size. To better understand F ST within different datasets (species × reference genome × depth), we applied pairwise F ST estimates (pairwise populations among the four populations) and global F ST estimates (including all the four populations).
We used the genotype likelihoods estimated by ANGSD to generate a PCA with PCAngsd (Meisner & Albrechtsen, 2018) and individual admixture proportions estimating using NgsAdmix (Skotte et al., 2013) after Linkage pruning with ngsLD (Fox et al., 2019). Further details about tools, parameters, and commands were described in Appendix S1 (Section 2.4). Most plots were generated in Rstudio v3.6.1 (http://www.rstudio.org) (scripts for plotting were included in Appendix S1 (Section 3)).
3. RESULTS
3.1. New genomic resources
We combined long‐ and short‐read sequencing technologies to generate three new de novo genome assemblies for the genus Himalopsyche (Rhyacophilidae): H. japonica, H. sp. (kuldschensis group), and H. tibetana. For each species, we obtained ~150–200× Illumina and ∼18–26× Oxford Nanopore sequencing depth. All three assemblies are of high quality with respect to the number of contigs and contig N50 (Table 1). We identified >96% of the Endopterygota BUSCO gene set in the assemblies. BlobTools detected no contamination (Appendix S1: Figures 1–3). Remapping the Illumina reads back to the assemblies revealed more than 98% could be unambiguously placed (Appendix S1: Figures 1–3).
The estimated genome sizes resulting from the k‐mer‐based estimation with Genomescope2 were 481 Mb (H. japonica), 495 Mb (H. sp. (kuldschensis group)), and 568 Mb (H. tibetana; Appendix S1: Figures 4–6). Between 31% (H. japonica) and 44% (H. tibetana) of the genome assemblies were identified as repeats. A high percentage of the repeats were classified as interspersed repeats (approx. 28.5–40.3%). More than half of the interspersed repeats remain unclassified and therefore may be specific to Trichoptera. Details on repeat classes are given in Appendix S1 (Tables S1–S3). The annotation of the genomes resulted in the prediction of 9983 (H. japonica), 10,049 (H. sp. (kuldschensis group)), and 10,994 (H. tibetana) proteins. Most of the annotated proteins had functional Blast2GO annotations, were verified by BLAST, or were mapped to GO terms. GO Distributions were similar to previously annotated caddisfly genomes, that is, the major biological processes were cellular processes. Catalytic activity was the largest subcategory in molecular function, and the cell membrane subcategories were the largest cellular component (Appendix S1: Figures 7–12).
The Cactus alignment of the five reference genomes showed that Rhyacophila brunnea had the longest sequence length and largest number of contigs (Appendix S4). It also showed a higher level of mutations compared with the four Himalopsyche genomes, for instance gaps, insertions, inversions, and duplications. The number of mutations among the four Himalopsyche genomes was at similar levels in the Cactus alignment over all.
3.2. Phylogenetic relationships of the reference genomes—resolving the Himalopsyche backbone
We built a species tree for the five reference genomes to estimate their evolutionary history and to verify the phylogenetic relationship between the reference genomes and the two population‐level target species, H. digitata and H. tibetana. Phylogenetic relationships were strongly supported (Figure 2). Our phylogenetic tree showed two pairs of sister species (H. tibetana + H. sp. (kuldschensis group); H. phryganea + H. japonica) with R. brunnea forming the sister clade of all four Himalopsyche species. According to Hjalmarsson et al. (2018, 2019), H. digitata and H. tibetana both belong to the tibetana group, H. sp. to the kuldschensis group, H. phryganea to the phryganea group and H. japonica to the navasi group. In contrast to our results, Hjalmarsson et al., 2019 recovered the navasi group sister to all other Himalopsyche and a sister relationship between the phryganea and kuldschensis groups. Differences could be related to the different sampling strategies. Here, we present much more genome‐wide data per taxon, but reduced taxon sampling. For the purpose of assessing the impact of reference genome relatedness in SNP calling, H. tibetana and H. digitata (both belonging to tibetana group) are most closely related to H. tibetana, followed by H. sp. (kuldschensis group), H. phryganea and H. japonica, with R. brunnea being the most distantly related species.
FIGURE 2.

Astral tree of the five reference genomes generated from BUSCO genes. Numbers on the nodes indicate local posterior probabilities. G. conforme was used as an outgroup. Images taken from Hjalmarsson et al. (2019), (a) H. phryganea, (b) H. japonica, (c) H. gregoryi (identical with H. tibetana morphologically), (d) H. sylvicola (identical with H. sp. (kuldschensis group) morphologically).
3.3. The impacts of sequencing depth and phylogenetic relatedness of the reference genome on population genetic studies
To better understand the impacts of sequencing depth and reference genome on population genomic analyses, we compared the number of quality‐filtered variants, genetic diversity and population structure of the species H. digitata and H. tibetana among the datasets based on different reference genomes and varied sequencing depth. The results revealed that, perhaps unsurprisingly, the most distantly related reference genome, or the lowest sequencing depth, resulted in the least accurate downstream analysis.
We observed that reference genome selection has a measurable impact on the number of variants (Figure 3). With both strategies (variant calling using GATK and genotype likelihood estimation using Angsd), the number of variants sharply decreased when the reference genome was more distantly related, but remained similar with decreasing sequencing depth, especially when estimated by genotype likelihood. This was particularly striking for the H. tibetana populations when using ANGSD to call the variants, which dropped from millions to thousands when changing the reference genome from H. tibetana to the others (Figure 3). Consequently, the massive loss of information resulting from choosing a distant reference genome is likely to lead to poor performance in the downstream analyses.
FIGURE 3.

Number of variants called/estimated by two different strategies. Numbers of the Y‐axis were logarithmically scaled.
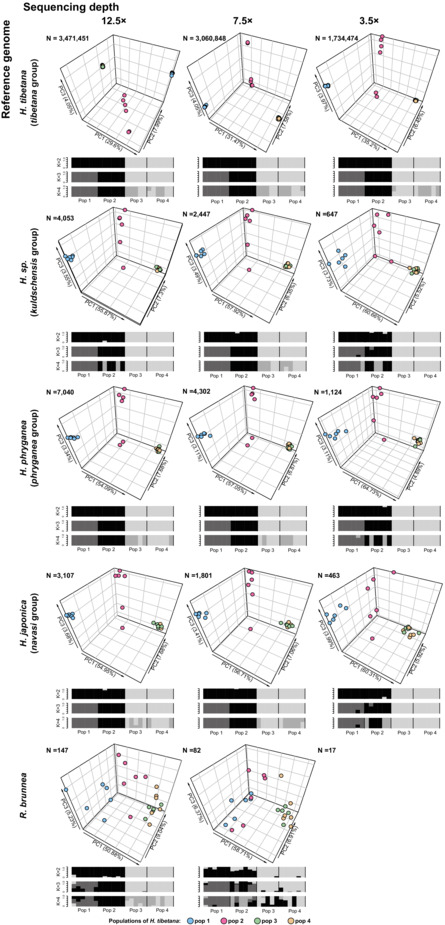
We observed that the choice of reference genome influenced population genetic values such as pairwise F ST (Appendix S3) and nucleotide diversity estimates (Appendix S1: Figure 14), the number of outliers in the inbreeding coefficient estimates (Appendix S1: Figure 13), as well as the resolution of the final outcome in genome‐wide F ST estimates (Figure 4), PCA, and admixture (Figure 5). For example, in the case of pairwise F ST estimates, when changing the reference genome from H. tibetana to R. brunnea (with the same sequencing depth, e.g., 12.5×), the F ST value between pop 1 and pop 3 of H. tibetana reduced from 0.17 to 0.06 (Appendix S3). The number of outliers (F < 0) in inbreeding coefficient estimates increased when using a reference genome that is distantly related to the target species, while the inbreeding coefficient values of individuals tended to fluctuate for both species, which might result in an ineffective or misleading conclusion (Appendix S1: Figure 13). The results of the PCA, admixture analysis, and the genome‐wide F ST clearly showed notable decreases in the resolution of the plots when selecting a more distantly related reference genome (Figure 5). For instance, for populations of H. digitata, regardless of the depth, the cluster of pop 1 was well defined when mapped to H. tibetana in the PCA plot and admixture analysis, but no distinguishable structure was discernible among all four populations when mapped to R. brunnea (Figure 5a). In addition, using a conspecific reference genome significantly improved the accuracy of genome‐wide F ST estimates, including both the global F ST value (the global F ST value of H. tibetana populations doubled when mapped to H. tibetana) and the distribution of F ST values from sliding windows. This effect was particularly pronounced with the abnormal value (highly qualified resolution, Figure 5b).
FIGURE 4.
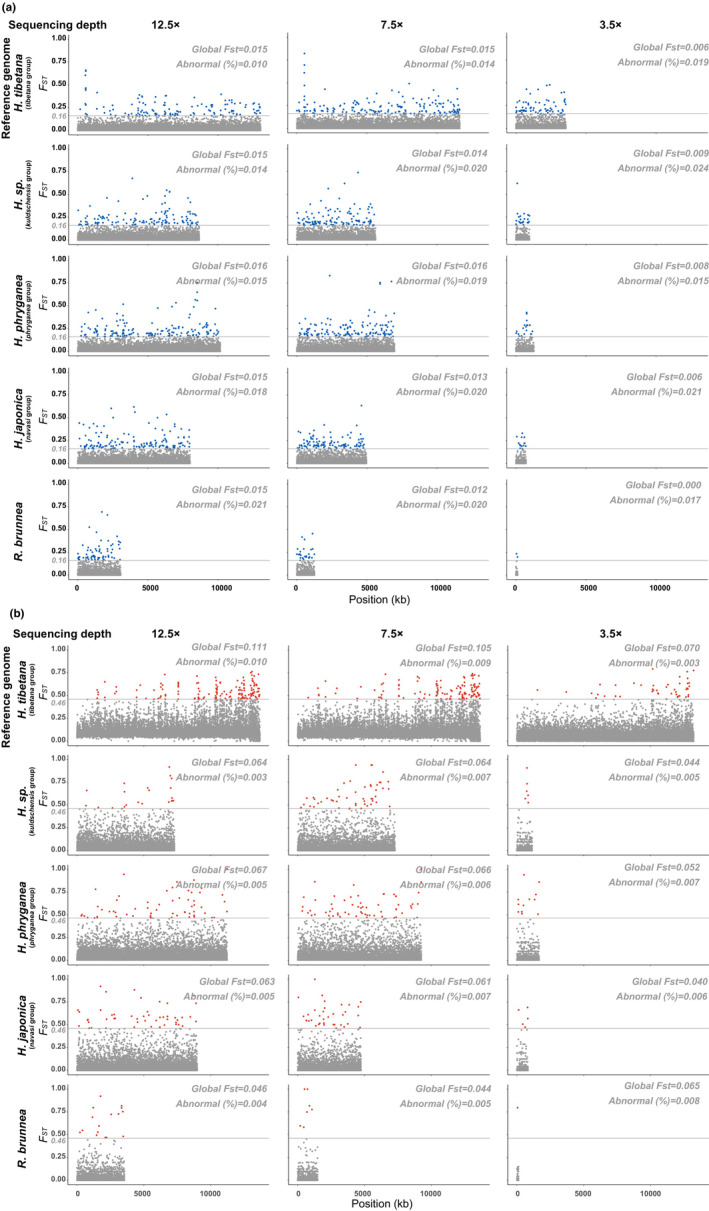
Genome‐wide distribution of F ST values (weighted) of (a) the H. digitata populations and (b) H. tibetana populations. F ST values were calculated in 50‐kb windows across contigs obtained from each reference genome; thus, the X‐axis (position) is not comparable among datasets. The horizontal gray lines indicate a threshold that is used for selecting the abnormal F ST windows for each species. The threshold is the minimum value of the top 1% F ST windows on the whole genome when using H. tibetana as reference genome and 12.5× as sequencing depth (0.16 for H. digitata and 0.28 for H. tibetana). The percentage of abnormal F ST windows is the number of F ST windows above the threshold over the total number of F ST windows. The global F ST is the genome‐wide weighted Fst value estimated based on Weir and Cockerham (1984).
FIGURE 5.

3D scatter plots of all individuals derived from PCA by using PCAngsd and population structure (k = 2, 3, 4, respectively) inferred from NgsAdmix depending on different reference genomes and different sequencing depth of (a) H. digitata and (b) H. tibetana populations. The explained variances are shown as percentages. Numbers on the top left of each plot show the number of SNPs used for the structure estimating. PCA and admixture were not able estimate when using R. brunnea as reference genome with 3.5× depth due to the limited number of variants. Colors in the plots represent the four populations of H. digitata and H. tibetana, respectively.
We also observed extensive influence of sequencing depth on the downstream analyses. In addition to decreasing number of variants, low sequencing depth also affected estimates of nucleotide diversity, the inbreeding coefficient, pairwise and global F ST, and population structure. In most cases, low sequencing depth reduced the accuracy of these inferences; however, the influence proved to be negligible or limited in some treatments. For example, the population structure of H. tibetana was highly differentiated regardless of depth when mapped to H. tibetana and was less differentiated when sequencing depth decreased while mapped to H. phryganea, but still sufficient to obtain a reliable result (Figure 5b). Likewise, the F value of all individuals changed when the sequencing depth decreased from 12.5× to 3.5×, but the differentiation among populations remained comparable (Appendix S1: Figure 13).
Furthermore, the results revealed that reference genome and sequencing depth had variable impacts between the populations of H. digitata and H. tibetana. More specifically, the same treatment (same reference genome and sequencing depth) might be sufficient to generate a reliable result for the populations of H. tibetana, but not for H. digitata. For instance, when mapped to H. japonica, the population structure of the H. tibetana populations was distinguishable regardless of sequencing depth, but it was barely detectable for the H. digitata populations even with 12.5× depth (Figure 5). Considering the inherent genetic variation of the two species, it is not surprising that more distantly related reference genome or lower sequencing depth were more tolerable for the populations with higher genetic diversity.
3.4. Genetic diversity and population structure of H. digitata and H. tibetana
Each population of H. digitata had a similar level of nucleotide diversity, but less consistent F values: pop 4 was slightly higher (0.31), whereas the other three were similar (0.22–0.27, Figure 6d). Population 1, which is located in the main river of Gandaki basin in central western Nepal, formed a distinct cluster in the PCA analyses (Figure 6a,b). Moreover, no admixture signal was detected in pop 1. As a population located in the middle of the basin, but more closely connected with pop3 and pop 4 by catchment, pop 2 formed a distinct cluster and adjoined pop3 and pop4 in the PCA analysis. Meanwhile, pop 2 was represented as genetic mixtures regardless of K values. Population 3 and 4 were mixed together in the PCA analyses, which was in line with the fact that they were located closely together in the most northeastern tributaries of the Gandaki basin. In addition, pop 3 and pop 4 were homogeneous when K = 2, but mixed when K = 3 or 4. This population structure was further supported by the pairwise F ST estimates using the base‐called variants (Figure 6f).
Following the pattern of H. digitata, the populations of H. tibetana had a very similar level of both nucleotide diversity and inbreeding coefficient (Figure 6e) and a more distinct population structure congruent with catchments (Figure 6c,f). Moreover, compared with H. digitata, H. tibetana showed a greater population diversity among the four populations, including higher nucleotide diversity (~ 12‐fold) and F ST (both pairwise and globalwise, Figures 6f and 4), lower inbreeding coefficient (~ 0.5‐fold), as well as a more distinct population structure (Figure 6b,c). This is in accordance with the fact that the geographic locality and catchment connection of the H. tibetana populations were further apart from each other compared with the ones of the H. digitata populations.
4. DISCUSSION
4.1. Reference genomes
In terms of BUSCO completeness and contiguity, the three de novo genomes provided in this study as references are of comparable quality than the other Trichoptera genomes published previously (Heckenhauer et al., 2019, 2022; Luo et al., 2018; Olsen et al., 2021; Ríos‐Touma et al., 2022). They contained 96%–97% of an Endopterygota core gene collection indicating an almost complete coverage of known single‐copy orthologs in the assembly. The backmapping rate of Illumina reads to the assemblies ranged between 96 and 98%, which also indicates the high quality of the assemblies.
Previous genomic studies have focused on sequencing a wide range of different families of Trichoptera and investigating variations across the order (Heckenhauer et al., 2022). These three new genome assemblies provide important new genomic resources for the scientific community, especially since Trichoptera and other aquatic insects are in general underrepresented in genomic research (Hotaling et al., 2020, 2021). Together with previously published ones (H. phryganea and R. brunnea), these newly available genomes adequately provide an initial perspective about the phylogenetic relationship of the four main taxonomic groups of Himalopsyche, as well as implying the genetic relatedness between the target species and the five different reference genomes, respectively, for this study.
4.2. Impacts of reference genome and sequencing depth on population genetic inferences
High‐throughput sequencing of individual specimens is poised to become the state‐of‐the‐art in population genetics studies. However, despite falling prices in sequencing, this approach can be prohibitively costly for large number of samples in species with large genomes. Many researchers may thus face the choice of either using an existing nonconspecific reference genome or lowering sequencing depth of individual samples. Previous studies have demonstrated that either the reference genome or the sequencing depth has a direct influence on population genetic estimates (Garcia‐Rubio et al., 2018; Gopalakrishnan et al., 2017; Valiente‐Mullor et al., 2021; Yang et al., 2019). Studies assessing the joint effects of the reference genome and sequencing depth are rare and hitherto lacking in insects. Here, we evaluated population diversity and structure based on a range of reference genomes and varied levels of low sequencing depth in the first case study on insects. The results revealed that the choice of the reference genome and sequencing depth both had an influence on estimating population genetic indices, including F, π and F ST, as well as the genetic structure inferred from genotype likelihoods. To some extent, the general trends are that (a) the more closely related the reference genome is, the more stable the estimates of population genetic indices are, and (b) the higher the sequencing depth, the better the resolution of the population structure analyses. However, the results also vary depending on the inherent genetic variation of the target species.
The choice of reference genome has a stronger influence on downstream analyses than resequencing depth, which is consistent with previous studies (Garcia‐Rubio et al., 2018; Günther & Nettelblad, 2019; Valiente‐Mullor et al., 2021). This is mainly due to the massive loss of reads while mapping to a distantly related reference genome. For example, in H. tibetana, mapping rates decrease from ~98% when using a conspecific reference genome, to 10% with a reference genome of a species of another genus (Rhyacophila brunnea; Appendix S5). This leads to a dramatic decline of variants (Figure 3). Moreover, increasing mismatches may also occur due to alternative alleles, thus increasing the so‐called “reference bias” (Günther & Nettelblad, 2019). Consequently, reference bias can impact variant calling by missing alternative alleles or by incorrectly calling heterozygous sites and therefore lead to an underestimation of variants, including rare/private variants (Günther & Nettelblad, 2019; Taub et al., 2010). Considering these two aspects, the effects related to the choice of reference genome may propagate to a certain degree to subsequent downstream analyses in a population genetic study, for example when investigating heterozygosity and genetic diversity, gene flow, as well as ancestry proportions (Brandt et al., 2015; Günther & Nettelblad, 2019). We have observed these in our results: the number of variants decreases logarithmically with genetic relatedness of the reference genome, especially for the genotype likelihood dataset called by ANGSD, which includes a strict filtering on base level, read level, sequencing depth, and other levels during the genotype calling. Moreover, when the genetic relatedness between reference genomes and target species decreases, the population genetic indices including F, π, and pairwise F ST tend to become less accurate, especially at a low depth (3.5×). In addition, estimates of population structure, including PCA and admixture, are strongly affected, resulting in unreliable, weakly supported findings.
Notably, even though population genetic analyses of both H. tibetana and H. digitata show the greatest accuracy when using H. tibetana as the reference genome, we have observed a remarkable improvement in the results when using a conspecific reference genome, for instance, in the high resolution of the population structure, especially with the low sequencing depth (Figure 5b). For example, when mapping H. tibetana population samples to the H. tibetana reference genome, we observed a mapping rate of 98%. However, mapping success decreases rapidly with decreased relatedness. When mapping H. digitata to H. tibetana, the mapping rate is only 35% and decreases to 20% when mapped to H. sp. kuldschensis and 18% when mapped to H. phryganea. As a consequence, even though the genome size of the two species is similar (Table 1), the number of variants called from the H. digitata population dataset is much lower than variants called from the H. tibetana population dataset, especially when estimated with genotype likelihood methods (Figure 3). Although H. digitata and H. tibetana are recovered in the same clade in previous phylogenetic analyses (Hjalmarsson et al., 2019), the poor mapping success suggests that genetic distance may still be high. Unfortunately, there are no established divergence times within the genus of himalopsyche at present. The most recent study (Thomas et al., 2020) shows that the divergence between Rhyacophila and Himalopsyche was approx. 90 Ma. Considering the distinct ecological niches of H. tibetana (inhabits high altitude) and H. digitata (inhabits a lower altitude) in the same distribution range (both endemic to the Himalayas), we hypothesize that the divergence between these two species may be associated with the uplift of the Qinghai–Tibet Plateau, which began ca. 45 Ma ago (Ding et al., 2022). In summary, considering the long evolutionary history of caddisflies in general (ca. 280 Ma), and the rapid radiation of caddisflies (Thomas et al., 2020), a high level of divergence between two closely related caddisflies species is not entirely unexpected. Therefore, we suggest that when selecting a closely related species as a reference genome in a population genomics study, it is important to consider genetic relatedness.
We also show that sequencing depth must be considered when designing population genomic analyses. Unlike de novo genome assembly which demands high sequencing depth, highly accurate results can be achieved with lower sequencing depth in population genomics by combining information from a large number of individuals either during SNP calling or other processes (Buerkle & Gompert, 2013; Fumagalli, 2013; Han et al., 2014). Even though the accuracy of population genetic inferences can be improved by increasing the number of samples, the bias caused by low sequencing depth cannot be ignored, especially since it can often be difficult to obtain many samples for some populations of rare animals. As revealed by previous studies, low sequencing depth may cause erroneous SNP calls, due to the errors introduced and amplified during PCR during library prep (Sims et al., 2014). Moreover, it may also produce ambiguous reads during mapping to the reference genome (Taub et al., 2010). Such biases may lead to incorrect conclusions in population genetics inferences, including population genetic differentiation, population structure, and demography (Crawford & Lazzaro, 2012; Fumagalli, 2013; Han et al., 2014; Jiang et al., 2019; Korneliussen et al., 2013). Our results show that decreasing depth massively reduced the number of informative sites, which may largely result from the variant calling step, especially when applying a series of filtering approaches, thus leading to a less accurate result in downstream analyses. However, sequencing depth may have limited impact in some other cases: The results are not affected by depth when the reference genome is either very closely or very distantly related to the target species. For example, when using H. tibetana as reference genome with H. digitata (the most closely related reference), population structures are distinguishable regardless of sequencing depth (Figure 5a). However, when using H. japonica or R. brunnea as the reference genome (most distantly related), population structures are indistinguishable regardless of the sequencing depth. Therefore, increasing the sequencing depth may not improve the results in such cases where the reference genome is too distantly related.
To conclude, both reference genome and sequencing depth have various degrees of influence on downstream analyses, whereas their respective impact is different for each target species. Our results imply that populations with a higher genetic diversity are less affected by the relatedness of the reference genome and the sequencing depth in population structure analyses. In general, the results for H. tibetana appeared more robust despite the variation of reference genome and sequencing depth in comparison to those obtained for H. digitata. This may result from (1) different pairwise relatedness between reference genome and target species and (2) inherent population variation (or expected level of differentiation). Even though H. tibetana and H. digitata are very closely related and both belong to the tibetana group (Hjalmarsson et al., 2019), populations of H. tibetana show a higher level of population variation compared to those of H. digitata, as, for instance, shown by higher F ST value and nucleotide diversity of populations. This is likely caused by different distribution patterns with H. tibetana populations being more isolated at high elevations than H. digitata. A detailed investigation of this was not intended with this study, and our sampling is insufficient and thus inconclusive regarding the underlying biological reasons for the differences in species‐specific population structures.
Similar to the trade‐off between sequencing depth and sample size demonstrated by previous studies (Buerkle & Gompert, 2013; Fumagalli, 2013), we reveal that the roles of reference genome and sequencing depth in a study of population genetics could also be considered as a trade‐off. We suggest that population genetic study using genomic data may benefit from applying a more closely related reference genome. Undoubtedly, the optimal option would be a conspecific reference genome even with a low sequencing depth. Our results showed that a conspecific reference genome can significantly improve the accuracy and reliability of all kinds of analyses, in particular WGR‐based SNP imputation which will be promising in other genomic investigations like genome‐wide association studies. However, if resource limitations exist (in terms of funding, available biological material, time (i.e., wet‐ and dry lab efforts)), or the research is focused on populations with high interpopulation variation, a trade‐off between a more distant reference genome and a higher sequencing depth can be considered. In other words, in a population genetic study, the trade‐off between reference genome and sequencing depth is dictated by the focus of research. Although our case study is carried out on caddisflies and thus may not be universally applicable to other organisms, we believe that our results do provide a valuable example that enhances developing roadmaps involved in the choice of appropriate reference genome and sequencing depth in population genomic studies.
4.3. Concordance between genetic patterns and biogeography of H. digitata and H. tibetana
Irrespective of sequencing depth and reference genome, the results of the population genetic analyses for H. tibetana and H. tibetana are highly consistent with the geographic distribution of populations within the drainage and river networks. For example, in H. tibetana, the PCA plots show that pop1 and pop 2 form two distinct clusters, respectively, while pop 3 and pop 4 cluster together and are separated from pop1 and pop 2. This is also consistent with their geographical location: pop 3 and pop 4 are both located in the Kanchenjunga region in far eastern Nepal; pop 2 is located in the most northeastern tributaries of the Gandaki Basin, and pop 1 is located in the east of the Annapurna circuit, which is in the center of the Gandaki Basin. In the admixture plots, pop 1 and 2, as well as pop 3 and 4, share the same structure, respectively. These results are consistent with the basin structure of these populations (pop 1 and pop 2 in Gandaki basin; pop 3 and pop 4 in Koshi basin). Moreover, compared with H. digitata populations, which are all located in one basin, H. tibetana populations show greater genetic diversity and clearer population structure. Due to the dependency of larvae on the aquatic habitat (De Moor & Ivanov, 2007), the limited dispersal capabilities of the adults (Griffith et al., 1998; Petersen et al., 2004), and the unique geographic feature of the Himalayan region, it is not surprising that the population genetics of the two target species show a notable correlation with drainages and river network, like in other Trichoptera studied in the region (Hoppeler et al., 2016) and elsewhere (Altermatt et al., 2013; Engelhardt et al., 2011). The resequencing approach, even with low sequencing depth, appears to be a suitable methodological avenue to study population genomics of insect populations when reference genomes of at least moderate relatedness are available.
AUTHOR CONTRIBUTIONS
Xiling Deng: Data curation (equal); formal analysis (equal); methodology (equal); software (equal); validation (equal); visualization (equal); writing – original draft (equal); writing – review and editing (equal). Paul B. Frandsen: Conceptualization (equal); data curation (equal); formal analysis (equal); investigation (equal); methodology (equal); resources (equal); software (equal); supervision (equal); validation (equal); writing – review and editing (equal). Rebecca B Dikow: Data curation (equal); methodology (equal); resources (equal); software (equal); writing – review and editing (equal). Adrien Favre: Writing – review and editing (equal). Deep Narayan Shah: Investigation (equal); resources (equal); writing – review and editing (equal). Ram Devi Tachamo Shah: Data curation (equal); resources (equal); writing – review and editing (equal). Julio V. Schneider: Data curation (equal); methodology (equal); resources (equal); writing – review and editing (equal). Jacqueline Heckenhauer: Conceptualization (equal); data curation (equal); formal analysis (equal); investigation (equal); methodology (equal); resources (equal); software (equal); supervision (equal); validation (equal); visualization (equal); writing – original draft (equal); writing – review and editing (equal). Steffen Pauls: Conceptualization (equal); data curation (equal); funding acquisition (equal); investigation (equal); project administration (lead); resources (equal); supervision (equal); validation (equal); writing – review and editing (equal).
CONFLICT OF INTEREST
None declared.
Supporting information
Appendix S1
Appendix S2
Appendix S3
Appendix S4
Appendix S5
ACKNOWLEDGMENTS
We are thankful to Raphael Coimbra providing for valuable advice in population genetic analyses. We thank Koji Tojo and Robert Wisseman for providing specimens and numbers of Nepalese students for supporting our collection efforts in the field. We thank Subodh Sharma for obtaining the fieldwork permit from Kathmandu University. This research was funded by the Deutsche Forschungsgemeinschaft (DFG), grant number PA 1617/2‐1 and PA 1617/2‐2 awarded to Steffen Pauls, and AF 1117/1‐2 awarded to Adrien Favre. The sequencing of the reference genomes was funded through the LOEWE Center for Translational Biodiversity Genomics with a grant from the Hessen State Ministry of Higher Education, Research and the Arts (HMWK). Analyses were conducted on the BYU Fulton Supercomputer, the TBG Deepfry clusters, and the Smithsonian High‐Performance Cluster (SI/HPC), Smithsonian Institution (https://doi.org/10.25572/SIHPC). Open Access funding enabled and organized by Projekt DEAL.
Deng, X.‐L. , Frandsen, P. B. , Dikow, R. B. , Favre, A. , Shah, D. N. , Shah, R. D. T. , Schneider, J. V. , Heckenhauer, J. , & Pauls, S. U. (2022). The impact of sequencing depth and relatedness of the reference genome in population genomic studies: A case study with two caddisfly species (Trichoptera, Rhyacophilidae, Himalopsyche). Ecology and Evolution, 12, e9583. 10.1002/ece3.9583
Jacqueline Heckenhauer and Steffen U. Pauls equal contribution authors.
DATA AVAILABILITY STATEMENT
All the COI and CAD data for this research are available in the GenBank of National Center for Biotechnology Information (NCBI), and the accession codes of each individual are provided in Appendix S2. The raw data and genome assemblies of the three novel references have been deposited at NCBI under the Bioproject ID PRJNA728835, and the raw data of populations have been deposited at NCBI under the Bioproject ID PRJNA749154. All BUSCO genes, annotation gffs, and predicted proteins resulting from GEMOMA, blastp, and BLAST2GO results, as well as repeatmodeler and ‐masker results are available at: https://doi.org/10.6084/m9.figshare.c.6033011.v1 [dataset]. The two reference genome of H. phryganea and R. brunnea were previously published by Heckenhauer et al. (2022) and has been deposited at NCBI under the BioProject ID PRJNA558902.
REFERENCES
- Altermatt, F. , Seymour, M. , & Martinez, N. (2013). River network properties shape α‐diversity and community similarity patterns of aquatic insect communities across major drainage basins. Journal of Biogeography, 40(12), 2249–2260. [Google Scholar]
- Armstrong, J. , Hickey, G. , Diekhans, M. , Fiddes, I. T. , Novak, A. M. , Deran, A. , Fang, Q. , Xie, D. , Feng, S. , & Stiller, J. (2020). Progressive cactus is a multiple‐genome aligner for the thousand‐genome era. Nature, 587(7833), 246–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa, S. , Mestre, F. , White, T. A. , Paupério, J. , Alves, P. C. , & Searle, J. B. (2018). Integrative approaches to guide conservation decisions: Using genomics to define conservation units and functional corridors. Molecular Ecology, 27(17), 3452–3465. [DOI] [PubMed] [Google Scholar]
- Bolger, A. M. , Lohse, M. , & Usadel, B. (2014). Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics, 30(15), 2114–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandies, P. , Peel, E. , Hogg, C. J. , & Belov, K. (2019). The value of reference genomes in the conservation of threatened species. Genes, 10(11), 846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt, D. Y. , Aguiar, V. R. , Bitarello, B. D. , Nunes, K. , Goudet, J. , & Meyer, D. (2015). Mapping bias overestimates reference allele frequencies at the HLA genes in the 1000 genomes project phase I data. G3: Genes, Genomes, Genetics, 5(5), 931–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buerkle, C. A. , & Gompert, Z. (2013). Population genomics based on low coverage sequencing: How low should we go? Molecular Ecology, 22(11), 3028–3035. [DOI] [PubMed] [Google Scholar]
- Crawford, J. E. , & Lazzaro, B. P. (2012). Assessing the accuracy and power of population genetic inference from low‐pass next‐generation sequencing data. Frontiers in Genetics, 3, 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danecek, P. , Auton, A. , Abecasis, G. , Albers, C. A. , Banks, E. , DePristo, M. A. , Handsaker, R. E. , Lunter, G. , Marth, G. T. , & Sherry, S. T. (2011). The variant call format and VCFtools. Bioinformatics, 27(15), 2156–2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Moor, F. , & Ivanov, V. (2007). Global diversity of caddisflies (Trichoptera: Insecta) in freshwater. In Freshwater animal diversity assessment (pp. 393–407). Springer. [Google Scholar]
- Deng, X. L. , Favre, A. , Lemmon, E. M. , Lemmon, A. R. , & Pauls, S. U. (2021). Gene flow and diversification in Himalopsyche martynovi species complex (Trichoptera: Rhyacophilidae) in the Hengduan Mountains. Biology, 10(8), 816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding, L. , Kapp, P. , Cai, F. , Garzione, C. N. , Xiong, Z. , Wang, H. , & Wang, C. (2022). Timing and mechanisms of Tibetan plateau uplift. Nature Reviews Earth & Environment, 3, 1–16. [Google Scholar]
- Duchen, P. , & Salamin, N. (2021). A cautionary note on the use of genotype callers in Phylogenomics. Systematic Biology, 70(4), 844–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellegren, H. (2014). Genome sequencing and population genomics in non‐model organisms. Trends in Ecology & Evolution, 29(1), 51–63. [DOI] [PubMed] [Google Scholar]
- Engelhardt, C. H. , Haase, P. , & Pauls, S. U. (2011). From the Western Alps across Central Europe: Postglacial recolonisation of the tufa stream specialist Rhyacophila pubescens (Insecta, Trichoptera). Frontiers in Zoology, 8(1), 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewels, P. , Magnusson, M. , Lundin, S. , & Käller, M. (2016). MultiQC: Summarize analysis results for multiple tools and samples in a single report. Bioinformatics, 32(19), 3047–3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn, J. M. , Hubley, R. , Goubert, C. , Rosen, J. , Clark, A. G. , Feschotte, C. , & Smit, A. F. (2020). RepeatModeler2 for automated genomic discovery of transposable element families. Proceedings of the National Academy of Sciences USA, 117(17), 9451–9457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox, E. A. , Wright, A. E. , Fumagalli, M. , & Vieira, F. G. (2019). ngsLD: Evaluating linkage disequilibrium using genotype likelihoods. Bioinformatics, 35(19), 3855–3856. [DOI] [PubMed] [Google Scholar]
- Fumagalli, M. (2013). Assessing the effect of sequencing depth and sample size in population genetics inferences. PLoS One, 8(11), e79667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia‐Rubio, R. , Monzon, S. , Alcazar‐Fuoli, L. , Cuesta, I. , & Mellado, E. (2018). Genome‐wide comparative analysis of aspergillus fumigatus strains: The reference genome as a matter of concern. Genes, 9(7), 363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geist, J. (2011). Integrative freshwater ecology and biodiversity conservation. Ecological Indicators, 11(6), 1507–1516. [Google Scholar]
- Goodwin, S. , McPherson, J. D. , & McCombie, W. R. (2016). Coming of age: Ten years of next‐generation sequencing technologies. Nature Reviews Genetics, 17(6), 333–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopalakrishnan, S. , Castruita, J. A. S. , Sinding, M.‐H. S. , Kuderna, L. F. , Räikkönen, J. , Petersen, B. , Sicheritz‐Ponten, T. , Larson, G. , Orlando, L. , & Marques‐Bonet, T. (2017). The wolf reference genome sequence (Canis lupus lupus) and its implications for Canis spp. population genomics. BMC Genomics, 18(1), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Götz, S. , García‐Gómez, J. M. , Terol, J. , Williams, T. D. , Nagaraj, S. H. , Nueda, M. J. , Robles, M. , Talón, M. , Dopazo, J. , & Conesa, A. (2008). High‐throughput functional annotation and data mining with the Blast2GO suite. Nucleic Acids Research, 36(10), 3420–3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith, M. B. , Barrows, E. M. , & Perry, S. A. (1998). Lateral dispersal of adult aquatic insects (Plecoptera, Trichoptera) following emergence from headwater streams in forested Appalachian catchments. Annals of the Entomological Society of America, 91(2), 195–201. [Google Scholar]
- Günther, T. , & Nettelblad, C. (2019). The presence and impact of reference bias on population genomic studies of prehistoric human populations. PLoS Genetics, 15(7), e1008302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevich, A. , Saveliev, V. , Vyahhi, N. , & Tesler, G. (2013). QUAST: Quality assessment tool for genome assemblies. Bioinformatics, 29(8), 1072–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall, M. (2022). Rasusa: Randomly subsample sequencing reads to a specified coverage. Journal of Open Source Software, 7(69), 3941. 10.21105/joss.03941 [DOI] [Google Scholar]
- Han, E. , Sinsheimer, J. S. , & Novembre, J. (2014). Characterizing bias in population genetic inferences from low‐coverage sequencing data. Molecular Biology and Evolution, 31(3), 723–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckenhauer, J. , Frandsen, P. B. , Gupta, D. K. , Paule, J. , Prost, S. , Schell, T. , Schneider, J. V. , Stewart, R. J. , & Pauls, S. U. (2019). Annotated draft genomes of two caddisfly species Plectrocnemia conspersa CURTIS and Hydropsyche tenuis NAVAS (Insecta: Trichoptera). Genome Biology and Evolution, 11(12), 3445–3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckenhauer, J. , Frandsen, P. B. , Sproul, J. S. , Li, Z. , Paule, J. , Larracuente, A. M. , Maughan, P. J. , Barker, M. S. , Schneider, J. V. , Stewart, R. J. , & Pauls, S. U. (2022). Genome size evolution in the diverse insect order Trichoptera. GigaScience, 11, giac011. 10.1093/gigascience/giac011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey, G. , Paten, B. , Earl, D. , Zerbino, D. , & Haussler, D. (2013). HAL: A hierarchical format for storing and analyzing multiple genome alignments. Bioinformatics, 29(10), 1341–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjalmarsson, A. E. (2019). Delimitation and description of three new species of Himalopsyche (Trichoptera: Rhyacophilidae) from the Hengduan Mountains, China. Zootaxa, 4638(3), 419–441. [DOI] [PubMed] [Google Scholar]
- Hjalmarsson, A. E. , Graf, W. , Jähnig, S. C. , Vitecek, S. , & Pauls, S. U. (2018). Molecular association and morphological characterisation of Himalopsyche larval types (Trichoptera, Rhyacophilidae). ZooKeys, 773, 79–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjalmarsson, A. E. , Graf, W. , Vitecek, S. , Jähnig, S. C. , Cai, Q. , Sharma, S. , Tong, X. , Li, F. , Shah, D. N. , & Shah, R. D. T. (2019). Molecular phylogeny of Himalopsyche (Trichoptera, Rhyacophilidae). Systematic Entomology, 44(4), 973–984. [Google Scholar]
- Hoang, D. T. , Chernomor, O. , Von Haeseler, A. , Minh, B. Q. , & Vinh, L. S. (2018). UFBoot2: Improving the ultrafast bootstrap approximation. Molecular Biology and Evolution, 35(2), 518–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohenlohe, P. A. , Funk, W. C. , & Rajora, O. P. (2021). Population genomics for wildlife conservation and management. Molecular Ecology, 30(1), 62–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppeler, F. , Tachamo Shah, R. D. , Shah, D. N. , Jähnig, S. C. , Tonkin, J. D. , Sharma, S. , & Pauls, S. U. (2016). Environmental and spatial characterisation of an unknown fauna using DNA sequencing–an example with Himalayan Hydropsychidae (Insecta: Trichoptera). Freshwater Biology, 61(11), 1905–1920. [Google Scholar]
- Hotaling, S. , Kelley, J. L. , & Frandsen, P. B. (2020). Aquatic insects are dramatically underrepresented in genomic research. Insects, 11(9), 601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotaling, S. , Sproul, J. S. , Heckenhauer, J. , Powell, A. , Larracuente, A. M. , Pauls, S. U. , Kelley, J. L. , & Frandsen, P. B. (2021). Long‐reads are revolutionizing 20 years of insect genome sequencing. Genome Biology and Evolution, 13(8), evab138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasper, M. , Schmidt, T. L. , Ahmad, N. W. , Sinkins, S. P. , & Hoffmann, A. A. (2019). A genomic approach to inferring kinship reveals limited intergenerational dispersal in the yellow fever mosquito. Molecular Ecology Resources, 19(5), 1254–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, Y. , Jiang, Y. , Wang, S. , Zhang, Q. , & Ding, X. (2019). Optimal sequencing depth design for whole genome re‐sequencing in pigs. BMC Bioinformatics, 20(1), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyaanamoorthy, S. , Minh, B. Q. , Wong, T. K. , Von Haeseler, A. , & Jermiin, L. S. (2017). ModelFinder: Fast model selection for accurate phylogenetic estimates. Nature Methods, 14(6), 587–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh, K. , & Standley, D. M. (2013). MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Molecular Biology and Evolution, 30(4), 772–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keilwagen, J. , Hartung, F. , & Grau, J. (2019). GeMoMa: Homology‐based gene prediction utilizing intron position conservation and RNA‐seq data. In Gene prediction (pp. 161–177). Springer. http://www.jstacs.de/index.php/GeMoMa [DOI] [PubMed] [Google Scholar]
- Keilwagen, J. , Wenk, M. , Erickson, J. L. , Schattat, M. H. , Grau, J. , & Hartung, F. (2016). Using intron position conservation for homology‐based gene prediction. Nucleic Acids Research, 44(9), e89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korneliussen, T. S. , Albrechtsen, A. , & Nielsen, R. (2014). ANGSD: Analysis of next generation sequencing data. BMC Bioinformatics, 15(1), 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korneliussen, T. S. , Moltke, I. , Albrechtsen, A. , & Nielsen, R. (2013). Calculation of Tajima's D and other neutrality test statistics from low depth next‐generation sequencing data. BMC Bioinformatics, 14(1), 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laetsch, D. R. , & Blaxter, M. L. (2017). BlobTools: Interrogation of genome assemblies. F1000Research, 6(1287), 1287. [Google Scholar]
- Langmead, B. , & Salzberg, S. L. (2012). Fast gapped‐read alignment with bowtie 2. Nature Methods, 9(4), 357–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, H. (2013). Aligning sequence reads, clone sequences and assembly contigs with BWA‐MEM (Version 2). arXiv preprint arXiv: 1303.3997. 10.48550/ARXIV.1303.3997 [DOI] [Google Scholar]
- Li, H. (2018). Minimap2: Pairwise alignment for nucleotide sequences. Bioinformatics, 34(18), 3094–3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, H. , Handsaker, B. , Wysoker, A. , Fennell, T. , Ruan, J. , Homer, N. , Marth, G. , Abecasis, G. , & Durbin, R. (2009). The sequence alignment/map format and SAMtools. Bioinformatics, 25(16), 2078–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loman, N. J. , Quick, J. , & Simpson, J. T. (2015). A complete bacterial genome assembled de novo using only nanopore sequencing data. Nature Methods, 12(8), 733–735. [DOI] [PubMed] [Google Scholar]
- Lou, R. N. , Jacobs, A. , Wilder, A. P. , & Therkildsen, N. O. (2021). A beginner's guide to low‐coverage whole genome sequencing for population genomics. Molecular Ecology, 30(23), 5966–5993. [DOI] [PubMed] [Google Scholar]
- Luo, S. , Tang, M. , Frandsen, P. B. , Stewart, R. J. , & Zhou, X. (2018). The genome of an underwater architect, the caddisfly Stenopsyche tienmushanensis Hwang (Insecta: Trichoptera). Gigascience, 7(12), giy143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, M. (2011). Cutadapt removes adapter sequences from high‐throughput sequencing reads. EMBnet Journal, 17(1), 10–12. [Google Scholar]
- Meisner, J. , & Albrechtsen, A. (2018). Inferring population structure and admixture proportions in low‐depth NGS data. Genetics, 210(2), 719–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, S. , Dykes, D. , & Polesky, H. (1988). A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Research, 16(3), 1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minh, B. Q. , Schmidt, H. A. , Chernomor, O. , Schrempf, D. , Woodhams, M. D. , Von Haeseler, A. , & Lanfear, R. (2020). IQ‐TREE 2: New models and efficient methods for phylogenetic inference in the genomic era. Molecular Biology and Evolution, 37(5), 1530–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse, J. C. , Frandsen, P. B. , Graf, W. , & Thomas, J. A. (2019). Diversity and ecosystem services of Trichoptera. Insects, 10(5), 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen, R. , Korneliussen, T. , Albrechtsen, A. , Li, Y. , & Wang, J. (2012). SNP calling, genotype calling, and sample allele frequency estimation from new‐generation sequencing data. PLoS One, 7(7), e37558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen, R. , Paul, J. S. , Albrechtsen, A. , & Song, Y. S. (2011). Genotype and SNP calling from next‐generation sequencing data. Nature Reviews Genetics, 12(6), 443–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen, L. K. , Heckenhauer, J. , Sproul, J. S. , Dikow, R. B. , Gonzalez, V. L. , Kweskin, M. P. , Taylor, A. M. , Wilson, S. B. , Stewart, R. J. , & Zhou, X. (2021). Draft genome assemblies and annotations of Agrypnia vestita Walker, and Hesperophylax magnus Banks reveal substantial repetitive element expansion in tube case‐making caddisflies (Insecta: Trichoptera). Genome Biology and Evolution, 13(3), evab013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen, I. , Masters, Z. , Hildrew, A. , & Ormerod, S. J. (2004). Dispersal of adult aquatic insects in catchments of differing land use. Journal of Applied Ecology, 41(5), 934–950. [Google Scholar]
- Ranallo‐Benavidez, T. R. , Jaron, K. S. , & Schatz, M. C. (2020). GenomeScope 2.0 and Smudgeplot for reference‐free profiling of polyploid genomes. Nature Communications, 11(1), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ríos‐Touma, B. , Holzenthal, R. W. , Rázuri‐Gonzales, E. , Heckenhauer, J. , Pauls, S. U. , Storer, C. G. , & Frandsen, P. B. (2022). De novo genome assembly and annotation of an Andean caddisfly, Atopsyche davidsoni Sykora, 1991, a model for genome research of high elevation adaptations. Genome Biology and Evolution, 14(1), evab286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roach, M. J. , Schmidt, S. A. , & Borneman, A. R. (2018). Purge Haplotigs: Allelic contig reassignment for third‐gen diploid genome assemblies. BMC Bioinformatics, 19(1), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan, J. , & Li, H. (2020). Fast and accurate long‐read assembly with wtdbg2. Nature Methods, 17(2), 155–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simão, F. A. , Waterhouse, R. M. , Ioannidis, P. , Kriventseva, E. V. , & Zdobnov, E. M. (2015). BUSCO: Assessing genome assembly and annotation completeness with single‐copy orthologs. Bioinformatics, 31(19), 3210–3212. [DOI] [PubMed] [Google Scholar]
- Sims, D. , Sudbery, I. , Ilott, N. E. , Heger, A. , & Ponting, C. P. (2014). Sequencing depth and coverage: Key considerations in genomic analyses. Nature Reviews Genetics, 15(2), 121–132. [DOI] [PubMed] [Google Scholar]
- Skoglund, P. , Ersmark, E. , Palkopoulou, E. , & Dalén, L. (2015). Ancient wolf genome reveals an early divergence of domestic dog ancestors and admixture into high‐latitude breeds. Current Biology, 25(11), 1515–1519. [DOI] [PubMed] [Google Scholar]
- Skotte, L. , Korneliussen, T. S. , & Albrechtsen, A. (2013). Estimating individual admixture proportions from next generation sequencing data. Genetics, 195(3), 693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taub, M. A. , Bravo, H. C. , & Irizarry, R. A. (2010). Overcoming bias and systematic errors in next generation sequencing data. Genome Medicine, 2(12), 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas, J. A. , Frandsen, P. B. , Prendini, E. , Zhou, X. , & Holzenthal, R. W. (2020). A multigene phylogeny and timeline for Trichoptera (Insecta). Systematic Entomology, 45(3), 670–686. [Google Scholar]
- Tsuruishi, T. , Ketavan, C. , Suwan, K. , & Sirikajornjaru, W. (2006). Importance of water flow on larval growth and pupation of Himalopsyche acharai, (Malicky and Chantaramongkol, 1989) (Trichoptera: Rhyacophilidae). Hydrobiologia, 563(1), 537–540. [Google Scholar]
- Valiente‐Mullor, C. , Beamud, B. , Ansari, I. , Francés‐Cuesta, C. , García‐González, N. , Mejía, L. , Ruiz‐Hueso, P. , & González‐Candelas, F. (2021). One is not enough: On the effects of reference genome for the mapping and subsequent analyses of short‐reads. PLoS Computational Biology, 17(1), e1008678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaser, R. , Sović, I. , Nagarajan, N. , & Šikić, M. (2017). Fast and accurate de novo genome assembly from long uncorrected reads. Genome Research, 27(5), 737–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vurture, G. W. , Sedlazeck, F. J. , Nattestad, M. , Underwood, C. J. , Fang, H. , Gurtowski, J. , & Schatz, M. C. (2017). GenomeScope: Fast reference‐free genome profiling from short reads. Bioinformatics, 33(14), 2202–2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldvogel, A. M. , Wieser, A. , Schell, T. , Patel, S. , Schmidt, H. , Hankeln, T. , Feldmeyer, B. , & Pfenninger, M. (2018). The genomic footprint of climate adaptation in Chironomus riparius. Molecular Ecology, 27(6), 1439–1456. [DOI] [PubMed] [Google Scholar]
- Walker, B. J. , Abeel, T. , Shea, T. , Priest, M. , Abouelliel, A. , Sakthikumar, S. , Cuomo, C. A. , Zeng, Q. , Wortman, J. , & Young, S. K. (2014). Pilon: An integrated tool for comprehensive microbial variant detection and genome assembly improvement. PLoS One, 9(11), e112963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, G.‐D. , Zhai, W. , Yang, H.‐C. , Fan, R.‐X. , Cao, X. , Zhong, L. , Wang, L. , Liu, F. , Wu, H. , & Cheng, L.‐G. (2013). The genomics of selection in dogs and the parallel evolution between dogs and humans. Nature Communications, 4(1), 1–9. [DOI] [PubMed] [Google Scholar]
- Waterhouse, R. M. , Seppey, M. , Simão, F. A. , Manni, M. , Ioannidis, P. , Klioutchnikov, G. , Kriventseva, E. V. , & Zdobnov, E. M. (2018). BUSCO applications from quality assessments to gene prediction and phylogenomics. Molecular Biology and Evolution, 35(3), 543–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir, B. S. , & Cockerham, C. C. (1984). Estimating F‐statistics for the analysis of population structure. Evolution, 38(6), 1358–1370. [DOI] [PubMed] [Google Scholar]
- Xu, J. , Grumbine, R. E. , Shrestha, A. , Eriksson, M. , Yang, X. , Wang, Y. , & Wilkes, A. (2009). The melting Himalayas: Cascading effects of climate change on water, biodiversity, and livelihoods. Conservation Biology, 23(3), 520–530. [DOI] [PubMed] [Google Scholar]
- Yang, X. , Lee, W.‐P. , Ye, K. , & Lee, C. (2019). One reference genome is not enough. Genome Biology, 20(1), 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, C. , Rabiee, M. , Sayyari, E. , & Mirarab, S. (2018). ASTRAL‐III: Polynomial time species tree reconstruction from partially resolved gene trees. BMC Bioinformatics, 19(6), 15–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimin, A. V. , Marçais, G. , Puiu, D. , Roberts, M. , Salzberg, S. L. , & Yorke, J. A. (2013). The MaSuRCA genome assembler. Bioinformatics, 29(21), 2669–2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimin, A. V. , Puiu, D. , Luo, M.‐C. , Zhu, T. , Koren, S. , Marçais, G. , Yorke, J. A. , Dvořák, J. , & Salzberg, S. L. (2017). Hybrid assembly of the large and highly repetitive genome of Aegilops tauschii, a progenitor of bread wheat, with the MaSuRCA mega‐reads algorithm. Genome Research, 27(5), 787–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Appendix S2
Appendix S3
Appendix S4
Appendix S5
Data Availability Statement
All the COI and CAD data for this research are available in the GenBank of National Center for Biotechnology Information (NCBI), and the accession codes of each individual are provided in Appendix S2. The raw data and genome assemblies of the three novel references have been deposited at NCBI under the Bioproject ID PRJNA728835, and the raw data of populations have been deposited at NCBI under the Bioproject ID PRJNA749154. All BUSCO genes, annotation gffs, and predicted proteins resulting from GEMOMA, blastp, and BLAST2GO results, as well as repeatmodeler and ‐masker results are available at: https://doi.org/10.6084/m9.figshare.c.6033011.v1 [dataset]. The two reference genome of H. phryganea and R. brunnea were previously published by Heckenhauer et al. (2022) and has been deposited at NCBI under the BioProject ID PRJNA558902.


