Abstract
Tetanus toxoid has been used widely as an adjuvant. The atoxic fragment C from tetanus toxin (TetC) is potently immunogenic when expressed in Salmonella vaccine strains and has been used as a fusion partner for antigens (Ag). However, there has been no formal comparison of the immunomodulatory impact of TetC on its fusion partners. In this study, we have addressed this important issue. The protective 28-kDa glutathione S-transferase (GST) from Schistosoma haematobium (Sh28GST) was expressed either as a fusion to TetC or as the full-length Sh28GST alone in a nonvirulent aroA-attenuated strain of Salmonella enterica serovar Typhimurium. The Sh28GST proteins were soluble and stably expressed in Salmonella, as evaluated by Western blotting with TetC and/or Sh28GST antisera. Mice were immunized orally with a single dose of the live recombinant Salmonella. The constructs were stable in mice but, dramatically, only the strain expressing the TetC-Sh28GST fusion elicited significant antibody (Ab) responses directed against Sh28GST as determined by enzyme-linked immunosorbent assay. An analysis of the isotype profiles showed that these mice also produced anti-Sh28GST immunoglobulin A and GST-neutralizing assays revealed high levels of neutralizing Abs in sera. These are important correlates of protection in schistosomiasis. In addition, stimulation of spleen cells from immunized mice with Sh28GST Ag showed that both strains, expressing Sh28GST alone or the TetC-Sh28GST fusion, were able to stimulate the secretion of Th1-related cytokines (gamma interferon and interleukin 2) to comparable levels. Thus, TetC has modulated the immune responses generated against its fusion partner, Sh28GST, by markedly enhancing the Ab responses elicited. These results have important implications in the rational development of live vaccines.
Human schistosomiasis remains a major global health problem with 200 million individuals currently infected (41). Chemotherapy can be used to control schistosomiasis, but rapid reinfection demands frequent retreatment, which makes this approach expensive. Moreover, the number of cases in the world has not been reduced over the last 20 years, and the recent observations of strains with decreased drug susceptibility (15, 23) emphasize the need for the development of vaccines for a more long-term approach.
A prime candidate in the development of such a vaccine is the 28-kDa glutathione S-transferase (GST) (3, 9, 31). This antigen is present in the adult, egg, and larval stages of the parasite and is thought to detoxify electrophilic compounds generated by the host (38). Purified and recombinant GST from different schistosome species has been successfully used to elicit protective immunity in animal models (2, 4, 5, 8, 37). For a vaccine to be successful, it does not necessarily need to induce sterile immunity. A vaccine inducing partial resistance and/or affecting fecundity and egg viability would be of value in controlling infection intensity and disease severity (12). A significant body of research on the development of schistosomiasis vaccines has dealt with the 28-kDa GST from Schistosoma mansoni (Sm28GST); however, phase I clinical trials have recently been initiated with the Schistosoma haematobium 28-kDa GST (Sh28GST) (9). The Sh28GST has been selected because resistance to S. haematobium is correlated with immune system-mediated inhibition of worm fecundity in humans and also because a noninvasive assessment of pathology to evaluate the vaccine efficacy can be easily applied to urinary schistosomiasis (9).
In humans and in animal models an important correlate in protection is the presence of antibodies which neutralize the enzymatic activity of the GST (42). Intriguingly, this effect appears to be mediated by immunoglobulin A (IgA) (17, 18). This observation has important implications in the rational development of effective vaccines. Thus, a vaccine that can be delivered to a mucosal surface may be capable of eliciting the desired immune responses.
Live salmonella vaccines have been used as carriers for the mucosal delivery of heterologous antigens from viruses, bacteria, and parasites to the immune system. These recombinant Salmonella strains were able to induce cellular, humoral, and secretory immune responses to the recombinant antigens (1, 29, 32, 34). Tetanus toxoid has been used widely as an adjuvant. The atoxic fragment C from tetanus toxin (TetC) is potently immunogenic when expressed in Salmonella vaccine strains. We have previously described the expression of antigens from worms and viruses as a fusion to TetC. This has resulted in the development of multivalent Salmonella vaccines which have elicited protective immunity (10).
This expression system has been used with great success. This system has also served to allow the expression of antigens from worms and bacteria which had proved otherwise difficult to express in Salmonella spp., a process which has been referred to as “expression rescue” (16, 24). In a previous study (24), we have described the expression and immunogenicity of Sm28GST as a fusion to TetC. However, there has been no formal comparison of the immunomodulatory impact of TetC on its fusion partners. In this study, we have addressed this important issue and have selected Sh28GST, which is undergoing clinical vaccine trials, rather than Sm28GST. The Sh28GST was expressed either as a fusion to TetC or as the full-length Sh28GST alone. Mice were immunized orally with a single dose of the live Aro-attenuated Salmonella vaccine strains harboring each construct, and the ensuing immune responses are described.
MATERIALS AND METHODS
Bacterial strains, plasmids, oligonucleotides, and DNA sequencing.
Bacterial strains and plasmids used in this study are summarized in Table 1. Bacteria were cultured in Luria-Bertani (LB) broth and on LB agar with ampicillin (50 μg/ml), when appropriate.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Characteristics | Source or reference |
|---|---|---|
| Strains | ||
| E. coli TG2 | recA | 33 |
| S. enterica serovar Typhimurium | ||
| SL5338 | galE r− m+ | 7 |
| JJ502 | SL5338 harboring pTECH2 plasmid | This study |
| JJ503 | SL5338 harboring pTECH2-Sh28 plasmid | This study |
| JJ504 | SL5338 harboring pTECH10-Sh28 plasmid | This study |
| SL3261 | SL1344 aroA | 20 |
| JJ702 | SL3261 harboring pTECH2 plasmid | This study |
| JJ703 | SL3261 harboring pTECH2-Sh28 plasmid | This study |
| JJ704 | SL3261 harboring pTECH10-Sh28 plasmid | This study |
| Plasmids | ||
| pTECH2 | pnirB TetC fusion vector | 25 |
| pTECH2-Sh28 | pTECH2 carrying Sh28GST ORFa | This study |
| pTECH10-Sh28 | pnirB Sh28GST expression plasmid | This study |
ORF, open reading frame.
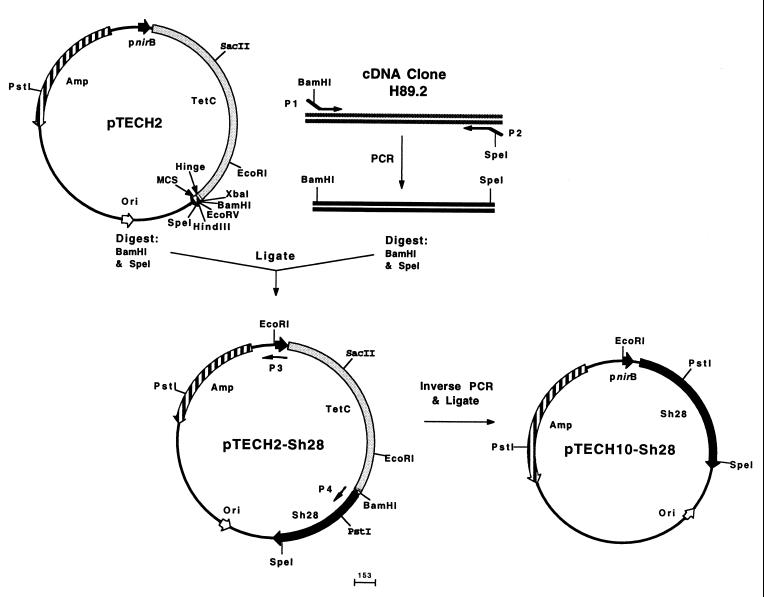
Plasmid pTECH2 has been described previously (25). The full-length gene coding for Sh28GST was amplified by PCR from cDNA clone H89.2 (39) using primers P1 (forward primer; 5′-TGAGGATCCGTCGACATGACTGGTGATCATATC-3′) and P2 (reverse primer; 5′-GTCACTAGTCTCGAGTTAGAAGGGAGTTGCAGC-3′), which allowed the directional cloning of the Sh28GST gene into pTECH2. The resulting plasmid was designated pTECH2-Sh28. PCR was performed using the high-fidelity thermostable DNA polymerase from Pyrococcus furiosus (Stratagene, Cambridge, United Kingdom). The modified vector pTECH10-Sh28 expressing Sh28GST alone was constructed by inverse PCR with pTECH2-Sh28 template DNA, using primers P3 (forward primer; 5′-ATGGCTGGCGAGCATATC-3′) and P4 (reverse primer; 5′-CAGAAAGTCTCCTGTGGA-3′) (Fig. 1).
FIG. 1.
Construction of pTECH2-Sh28 and pTECH10-Sh28. See Results for details.
DNA sequencing was performed on an automatic sequencer (Perkin-Elmer Biosystems; model 877) by the university facility for molecular biology, using the dideoxynucleotide chain termination method modified with fluorescent tags. All sequences were confirmed in both directions using sequencing primers and plasmids isolated from transformants.
SDS-PAGE and Western blotting.
Expression of the TetC-Sh28GST fusion and Sh28GST alone was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting as described previously (10). Cells growing in mid-log phase, with antibiotic selection, were harvested by centrifugation, resuspended in phosphate-buffered saline (PBS) containing 1% Triton X-100, and disrupted on ice for 10 s at 10 μ with a sonicator (Soniprep 150; MSE). The lysates were fractionated by SDS–10% PAGE, as described by Laemmli (28). The proteins were transferred to a nitrocellulose membrane by electroblotting and reacted with either a polyclonal rabbit antiserum directed against recombinant TetC (rTetC; Boehringer Mannheim, Sussex, United Kingdom) or a polyclonal mouse antiserum directed against recombinant Sh28GST (rSh28GST). The blots were then probed with horseradish peroxidase (HRP) conjugated to either goat anti-rabbit Ig or goat anti-mouse Ig antibodies (Dako, Bucks, United Kingdom) and developed with 4-chloro-1-naphthol (Sigma, Dorset, United Kingdom).
Animals, immunizations, and viable counting on organ homogenates.
Female BALB/c mice were purchased from Harlan Olac (Blackthorn, Bicester, United Kingdom) and used when at least 8 weeks of age. Bacteria were grown in LB broth supplemented with 50 μg of ampicillin/ml as required. For oral immunization, 4 × 1010 to 5 × 1010 CFU of each construct in 200 μl of PBS was given once intragastrically by gavage tube to anesthetized mice. The immunization doses were checked by viable counts on LB agar plates. The mice were bled at 3, 5, 7, 9, and 11 weeks after immunization, and sera were stored individually at −20°C.
For viable counts on organ homogenates, groups of three mice were killed at day 14, the livers and spleens were homogenized separately in 10 ml of distilled water in a Colworth stomacher (21), and viable counts were conducted on LB agar supplemented with 50 μg of ampicillin/ml as required.
Measurement of antibody responses.
Antibody responses were measured by enzyme-linked immunosorbent assay (ELISA) as described previously (11). Briefly, 96-well microtiter plates (Nunc, Paisley, United Kingdom) were coated overnight at 4°C with either 0.1 μg of rTetC (produced in Escherichia coli; Boehringer Mannheim) or 0.1 μg of rSh28GST (produced in yeast; F. Trottein, Institut Pasteur de Lille) diluted in 0.1 M carbonate buffer (pH 9.6) per well. Twofold serial dilutions of antisera in PBS–1% bovine serum albumin were added, and the plates were incubated for 90 min at 37°C. HRP-conjugated antibodies specific to mouse Ig (Dako) and each isotype (Zymed, Cambridge Bioscience, Cambridge, United Kingdom) were added according to the manufacturer's instructions. For IgA determination, biotinylated goat anti-mouse IgA antibodies diluted 1:1,000 and streptavidin-HRP diluted 1:500 (Sigma) were used. The plates were developed with o-phenylene diamine (Sigma) prepared according to the manufacturer's instructions. After 20 min at 37°C, the reaction was stopped with 3 M H2SO4 and plates were read at 490 nm.
For antibody titration, serial twofold dilutions of pooled sera were assessed by ELISA as described above. Titers were defined as the highest dilution of the antisera that gave an absorbancy three times higher than background (26).
Purification of Sh28GST from recombinant salmonellae.
Recombinant salmonellae were grown overnight in LB broth supplemented with 50 μg of ampicillin/ml, harvested, washed once with PBS, resuspended in PBS containing 1% Triton X-100, and disrupted on ice for 2 min. After sonication, the lysates were clarified by centrifugation at 25,000 × g for 20 min at 4°C. The soluble fraction was recovered and diluted in equilibration buffer (PBS containing 1 mM EDTA and 0.5 mM phenylmethylsulfonyl fluoride). Glutathione (GSH)-agarose beads (Sigma) were suspended in equilibration buffer for 2 h before use, packed into a column (10 by 50 mm), and equilibrated with the same buffer. The protein sample was applied at a flow rate of 1 ml/min. After extensive washing with equilibration buffer, Sh28GST was eluted with 5 mM GSH (Sigma) in elution buffer (50 mM Tris-HCl, pH 8.0). Fractions of 1 ml were collected and analyzed for the presence of Sh28GST by SDS-PAGE and Coomassie blue staining. Fractions containing the protein were pooled, concentrated by ultrafiltration on a Centricon-10 concentrator (Amicon Ltd., Gloucestershire, United Kingdom), and dialyzed against PBS. The protein concentration was determined using the bicinchoninic acid protein assay reagent kit (Pierce, Chester, United Kingdom), according to the manufacturer's instructions.
Enzymatic activity of Sh28GST.
The enzyme activity of the Sh28GST purified from recombinant Salmonella was measured as described by Habig et al. (19), with the following modifications. In 820 μl of reaction buffer (100 mM potassium phosphate [pH 6.5] containing 1 mM 1-chloro-2,4-dinitrobenzene [Sigma] and 5 mM GSH), 10 μl of enzyme (10 μg/ml) was added. The enzymatic reaction was monitored spectrophotometrically at 340 nm for 60 min.
The neutralizing activity of the anti-Sh28GST antisera was analyzed as described by Kremer et al. (26). Briefly, in a total reaction volume of 430 μl containing 400 μl of reaction buffer, 30 μl of enzyme mixture was added. This enzyme mixture contained 10 μl of enzyme (10 μg/ml) incubated with 20 μl of potassium phosphate (pH 6.5) or of antisera for 1 h at 37°C, followed by 1 h at 4°C. The reaction was started by the addition of the enzyme mixture and was monitored continuously for 20 min at 340 nm.
Cytokine assays.
Cytokine levels in spleen cell culture supernatant were measured after antigenic stimulation. Fifteen weeks after immunization, two mice from each of the immunized groups were killed, and the spleens were aseptically removed and placed in RPMI 1640 (Sigma; HEPES modification). Single-cell suspensions were prepared by mashing the spleens through a sieve with a syringe plunger and washing them in RPMI 1640 and complete RPMI 1640 (CRPMI; RPMI 1640 supplemented with 10% heat-inactivated fetal calf serum, 50 μM 2-mercaptoethanol, 2 mM glutamine, 100 μg of streptomycin/ml, and 100 U of penicillin/ml). Erythrocytes were lysed by the addition of 5 ml of Gey's solution per spleen and incubated on ice for 4 min. The cells were then washed twice before being resuspended in CRPMI and counted. Cell cultures were set up in round-bottom 96-well plates (Corning Glass Works, Corning, N.Y.) at 5 × 105 cells/well in 200 μl and incubated at 37°C in 95% humidity with 5% CO2. Antigens were added in triplicate. At intervals, supernatants were collected in flat-bottom 96-well plates and frozen at −80°C until use in cytokine ELISAs. rTetC and rSh28GST were the same as those used for antibody determinations and were diluted in CRPMI at concentrations of 5 and 10 μg per well, respectively. For Salmonella antigens, a whole-cell extract designated C5 lysate was prepared as described previously (40). The protein concentration of C5 lysate was determined by the bicinchoninic acid method.
Interleukin 2 (IL-2), gamma interferon (IFN-γ), and IL-4 were assayed by ELISA as described previously (11), using a pair of specific monoclonal antibodies (capture and detection) against each cytokine and dilutions of a recombinant cytokine for the construction of standard curves (Pharmingen, San Diego, Calif.).
RESULTS
Construction of pTECH2-Sh28 and pTECH10-Sh28.
A Sh28GST expression cassette was synthesized by PCR using the Sh28GST cDNA clone H89.2 (39) as a template. Oligonucleotide primers P1 and P2 were designed to amplify the gene, beginning with the start codon and terminating with the stop codon. In addition, forward and reverse primers were tailored with BamHI and SpeI, respectively, to allow directional cloning into pTECH2. The PCR product was gel purified and digested with BamHI and SpeI and then cloned into pTECH2, which had been digested with the same enzymes and subsequently gel purified. The construct pTECH2-Sh28 directed the expression of a TetC-Sh28GST fusion protein. The vector pTECH10-Sh28, expressing Sh28GST alone without the TetC fusion partner, was constructed by inverse PCR using pTECH2-Sh28 as the template DNA. The inverse PCR was conducted with high-fidelity thermostable Pfu DNA polymerase and primers P3 and P4. The PCR product was gel purified and ligated, resulting in the complete removal of the TetC gene (Fig. 1).
Expression of Sh28GST in salmonella.
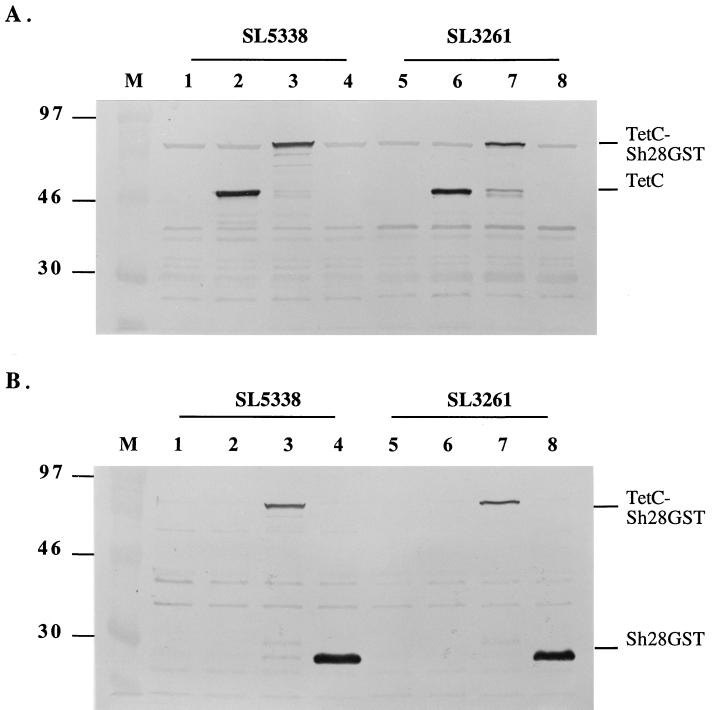
The recombinant plasmids were first transformed into E. coli TG2, and expression on cells harboring the constructs was evaluated by SDS-PAGE and Western blotting. The TetC-Sh28GST fusion protein and Sh28GST remained soluble and reacted with antisera to TetC and Sh28GST, respectively. Single bands of the expected molecular masses for the TetC-Sh28GST fusion protein (80 kDa) and Sh28GST alone (28 kDa) were recognized in several independent transformants, and DNA restriction analysis of the plasmid DNA confirmed their identity (data not shown). The constructs were initially transformed into the intermediate Salmonella strain SL5338 (r− m+) to increase the efficiency of electroporation into the vaccine strain SL3261 (r+ m+) and finally transformed into the vaccine strain SL3261. The resulting recombinant strains were designated as in Table 1. The identity of the expression plasmids pTECH2-Sh28, isolated from JJ503 and JJ703, and pTECH10-Sh28, isolated from JJ504 and JJ704, was further verified by DNA sequencing using sequencing primers. The expression of the recombinant proteins in SL5338 and SL3261 genetic backgrounds was judged by SDS-PAGE and Western blotting. Western blot analysis showed that the TetC-Sh28GST fusion and Sh28GST alone were similarly expressed in both background strains (Fig. 2). However, it was estimated by densitometer that the expression level of Sh28GST alone in JJ504 and JJ704 was approximately fivefold higher than that of the TetC-Sh28GST fusion in JJ503 and JJ703 (Fig. 2B).
FIG. 2.
Expression of TetC-Sh28GST fusion and Sh28GST as determined by SDS-PAGE and Western blotting. Shown are the results of probing with a rabbit anti-TetC polyclonal antiserum (A) and a mouse anti-Sh28GST polyclonal antiserum (B). The constructs were in S. enterica serovar Typhimurium strains SL5338 and SL3261, as indicated. Lane M, low-molecular-mass marker proteins (kilodaltons). Lanes 1 to 8, SL5338, JJ502, JJ503, JJ504, SL3261, JJ702, JJ703, and JJ704 cell lysate, respectively.
Stability of the plasmid constructs in vivo and immunization of mice.
BALB/c mice were immunized orally with approximately 4 × 1010 to 5 × 1010 CFU of Salmonella enterica serovar Typhimurium strains SL3261, JJ702, JJ703, and JJ704. Viable counts of homogenates of liver and spleen were conducted at day 14 after immunization. The recombinant constructs grew but persisted in the tissues at a level 1 log unit lower than that of parental strain, SL3261. Viable counts of JJ703 and JJ704 for media with and without ampicillin were very similar, indicating that the plasmid was not being lost in vivo. In addition, when colonies recovered from livers and spleens were analyzed, all of them retained the constructs and Western analysis showed that recombinant proteins were still well expressed (data not shown).
Antibody response induced after immunization with recombinant salmonellae in mice.
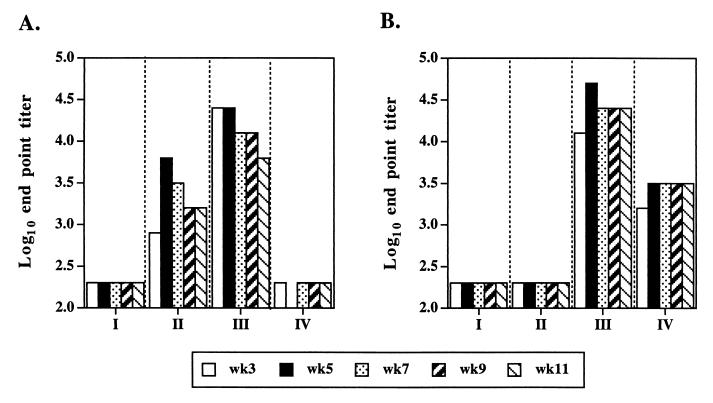
Tail bleeds were taken at weeks 3, 5, 7, 9, and 11 from all eight mice per group, and all antisera were assessed simultaneously by ELISA against TetC (Fig. 3A) and Sh28GST (Fig. 3B). A single oral dose of 4 × 1010 CFU of recombinant Salmonella was sufficient to generate specific immune responses against TetC and Sh28GST. The antibody responses against TetC and Sh28GST were detected as early as week 3 and peaked at week 5 after immunization, with no significant decrease over the time of the study. Although all mice immunized with JJ702 and JJ703 expressing TetC alone or the TetC-Sh28GST fusion invoked a strong antibody response to TetC, the antibody titer in sera from mice immunized with JJ703 was much higher than that in sera from mice immunized with JJ702. As expected no anti-TetC antibodies were detected in sera from mice immunized with SL3261 or JJ704 (Fig. 3A). Mice immunized with JJ703 developed a significant anti-Sh28GST response. In contrast, mice immunized with JJ704 developed a weaker response (Fig. 3B). While a peak anti-Sh28GST antibody titer of 1/51,200 was elicited in mice immunized with JJ703, a peak titer of 1/3,200 was elicited in mice immunized with JJ704.
FIG. 3.
Antibody responses against rTetC (A) and rSh28GST (B), as detected by ELISA, from mice immunized orally with S. enterica serovar Typhimurium strains SL3261 (I), JJ702 harboring pTECH2 (II), JJ703 harboring pTECH2-Sh28 (III), and JJ704 harboring pTECH10-Sh28 (IV). Pooled sera from a group of eight mice bled at weeks 3, 5, 7, 9, and 11 were analyzed, and each value is expressed as the log10 end point titer as described in Materials and Methods.
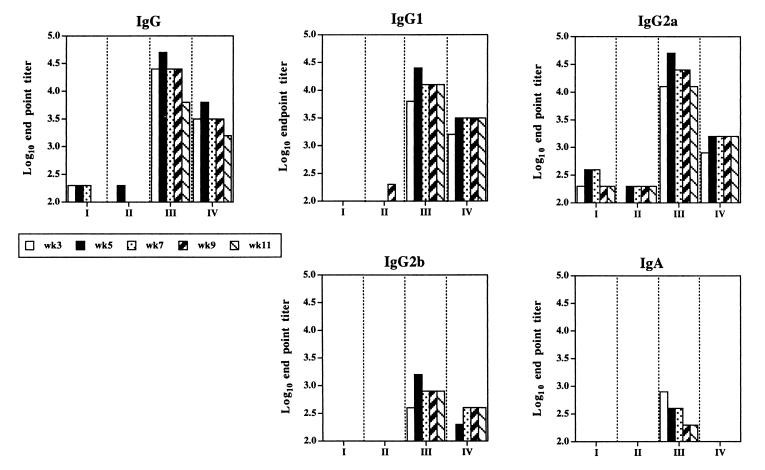
The isotype profiles of antibodies directed against Sh28GST are shown in Fig. 4. Mice immunized with JJ703 elicited clear IgG1, IgG2a, IgG2b, and IgA responses, while mice immunized with JJ704 showed IgG1, IgG2a, and IgG2b responses. Interestingly, significant levels of anti-Sh28GST IgA antibodies were detected in mice immunized with JJ703. The peak IgG1, IgG2a, and IgG2b responses in mice immunized with JJ703 were at week 5 after immunization, whereas the peak IgA response was at week 3. However, no IgG3 or IgM was detected in any group of mice (data not shown).
FIG. 4.
Antibody isotype profiles against rSh28GST as detected by ELISA from mice immunized orally with S. enterica serovar Typhimurium strains SL3261 (I), JJ702 harboring pTECH2 (II), JJ703 harboring pTECH2-Sh28 (III), and JJ704 harboring pTECH10-Sh28 (IV). Pooled sera from a group of eight mice bled at weeks 3, 5, 7, 9, and 11 were analyzed, and each value is expressed as the log10 end point titer as described in Materials and Methods.
Purification and enzymatic activity of Sh28GST expressed in salmonella.
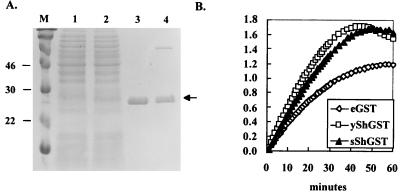
As the strain expressing Sh28GST alone, JJ704, elicited only a weak antibody response against Sh28GST, it is possible that the recombinant protein may have misfolded significantly and lost its immunogenicity. Furthermore, it has been reported that protective immunity is associated with antibodies directed against the active site of Sm28GST (42). Therefore, it may be important that the active site of the recombinant Sh28GST expressed in Salmonella also remain intact. These possibilities were investigated by purification of Sh28GST using affinity chromatography and by determining the enzymatic activity of the purified recombinant Sh28GST expressed in JJ704. As shown in Fig. 5A, the Sh28GST produced in S. enterica serovar Typhimurium strain JJ704 harboring the pTECH10-Sh28 construct was purified in a single step by affinity chromatography on a GSH-agarose column. Eluted fractions were monitored by measuring the optical density at 280 nm, and the fractions containing protein were pooled and then analyzed by SDS-PAGE. The protein purified from S. enterica serovar Typhimurium strain JJ704 comigrated exactly with the protein purified from yeast, which was used as a positive control. These results suggest that the Sh28GST expressed in Salmonella folds properly and binds to GSH in a reversible manner, since Sh28GST can be competitively eluted with free GSH.
FIG. 5.
Purification and enzymatic activity of the Sh28GST expressed by JJ704 harboring pTECH10-Sh28. (A) Sh28GST was purified by single-step affinity chromatography on a GSH-agarose column. Purified proteins were subjected to SDS-PAGE and Coomassie blue staining. Lane 1, whole-cell lysate of SL3261; lane 2, whole-cell lysate of JJ704 harboring pTECH10-Sh28; lane 3, purified Sh28GST produced in JJ704; lane 4, purified Sh28GST produced in yeast (∼2 μg). Arrow, position of Sh28GST. The molecular mass markers are shown in lane M, and their sizes in kilodaltons are at the left. (B) GST activity catalyzed by Sh28GST purified from yeast (open squares), S. enterica serovar Typhimurium strain typhimurium JJ704 (solid triangles), and commercially purified GST of equine origin (Sigma; open diamonds) in a time-dependent fashion.
To test whether the recombinant protein was enzymatically active, the enzyme activity of the purified protein was determined (Fig. 5B). The enzymatic activity of the purified protein from S. enterica serovar Typhimurium strain JJ704 was found to be very similar to that of the purified recombinant Sh28GST protein obtained from yeast, which was used as a positive control, and was compared with that of purified GST from equine liver (Sigma). This result demonstrates that the Sh28GST produced in JJ704 retained full enzymatic activity, implying that rSh28GST has conserved the three-dimensional structure of its active site. Therefore, it appears that the protein has not misfolded so as to lose its immunogenicity when expressed alone without TetC in JJ704.
Neutralization of the Sh28GST enzymatic activity by sera from immunized mice.
Since the induction of neutralizing antibodies has been related to protection against schistosomiasis in humans (31), the ability of the anti-Sh28GST antisera to neutralize the enzymatic activity of Sh28GST was also investigated using an enzymatic inhibition assay. As shown in Table 2, only the group of mice immunized with JJ703 expressing the TetC-Sh28GST fusion produced significant levels of neutralizing antibodies. The inhibition value was as high as 85.4% 3 weeks after a single oral immunization and gradually diminished with time. In contrast, no significant inhibition was observed with sera obtained from mice immunized with SL3261, JJ702, and JJ704 expressing Sh28GST alone.
TABLE 2.
Inhibition of Sh28GST enzymatic activity by antisera
| Wk after oral immunization | % Inhibition of enzymatic activitya in serab from mice immunized with:
|
|||
|---|---|---|---|---|
| SL3261 | JJ702 | JJ703 | JJ704 | |
| 3 | 11.0 | 23.0 | 85.4 | 32.7 |
| 7 | 9.0 | 17.7 | 64.2 | 27.7 |
| 11 | 0.0 | 28.1 | 53.8 | 31.6 |
The percentages of inhibition were calculated by comparison to a negative control where Sh28GST was incubated in the presence of the same amount of normal mouse serum.
Ten microliters of serum from individual mice in each group was pooled, and 10 μl of pooled sera was used for the inhibition assay.
Cellular response induced in mice immunized with recombinant salmonella.
To investigate the cellular immune responses induced in mice immunized with the recombinant Salmonella, spleen cells obtained 15 weeks after immunization were stimulated in vitro with C5 lysate, rTetC, and rSh28GST antigens. Cytokine levels in cell culture supernatants were measured at various time points after antigenic stimulation.
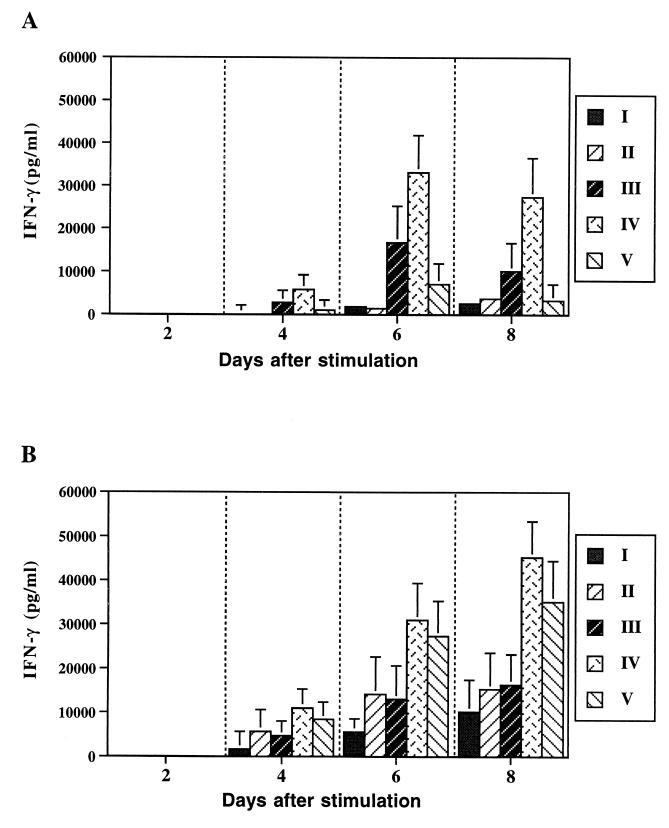
The IFN-γ responses upon stimulation with the different antigens are shown in Fig. 6. Stimulation with S. enterica serovar Typhimurium C5 lysate induced similar responses in all immunized groups and the responses remained stable with no significant decrease over the time of the study (data not shown). The IFN-γ response upon stimulation with rTetC and rSh28GST varied with the strains used for immunization. rTetC stimulation of cells from mice immunized with JJ702 and JJ703 expressing TetC or the TetC-Sh28GST fusion resulted in IFN-γ responses that were most apparent at 6 days after stimulation, although IFN-γ responses at weeks 4 and 8 were also statistically significant (P < 0.01). Furthermore, the response to rTetC obtained with JJ703-immunized mice was much greater than that obtained with JJ702-immunized mice (Fig. 6A). Stimulation with rSh28GST elicited significantly (P < 0.01) greater production of IFN-γ in splenocytes from mice immunized with JJ703 and JJ704 expressing TetC-Sh28GST and Sh28GST alone than in those from mice immunized with JJ702 expressing TetC alone, 8 days poststimulation (Fig. 6B).
FIG. 6.
IFN-γ production by whole spleen cells after stimulation with rTetC (A) and rSh28GST (B). Results are expressed as the concentration of cytokine in supernatants of cell cultures collected up to 8 days after stimulation, as detected by ELISA. Each value is the average of the results for three different cultures plus the standard deviation. Statistical significances were analyzed by Student's t test. P values <0.05 were considered significant. Groups: I, naive mice; II, mice immunized with SL3261; III, mice immunized with JJ702; IV, mice immunized with JJ703; V, mice immunized with JJ704.
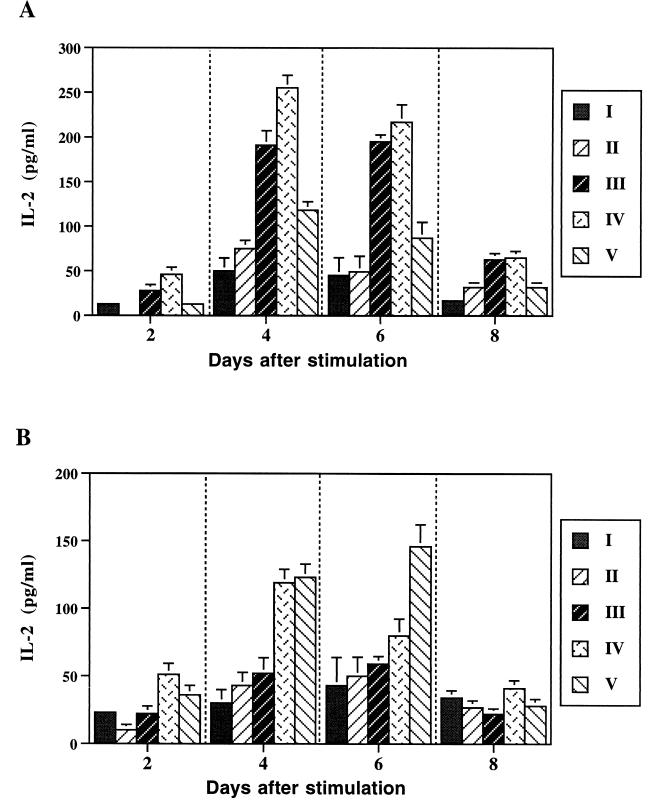
The IL-2 production from splenocytes of immunized animals upon stimulation with rTetC and rSh28GST was determined (Fig. 7). Upon stimulation with C5 lysate, IL-2 production was induced in cells from all groups of mice at day 2 (data not shown), which is consistent with previous observation (11). IL-2 responses were obtained in mice immunized with JJ702 and JJ703 in supernatants collected after rTetC stimulation (Fig. 7A). IL-2 production from splenocytes of mice immunized with JJ702 and JJ703 peaked at 4 days after stimulation, although it was significant throughout the time of the study (P < 0.01). rSh28GST-specific IL-2 responses were detectable in the culture supernatants of spleen cells from mice immunized with JJ703 and JJ704. IL-2 production in cell culture supernatant peaked earlier in JJ703-immunized mice, 4 days after stimulation, and later in JJ704-immunized mice, 6 days after stimulation (Fig. 7B). Low levels of IL-4 were obtained in experimental groups stimulated with rTetC (155 to 177 pg/ml) or rSh28GST (251 to 360 pg/ml). However, these levels were not significantly different from those for the control groups (P > 0.05).
FIG. 7.
IL-2 production by whole spleen cells after stimulation with rTetC (A) and rSh28GST (B). Results are expressed as the concentration of cytokine in supernatants of cell cultures collected up to 8 days after stimulation, as detected by ELISA. Each value is the average of the results for three different cultures plus the standard deviation. Groups: I, naive mice; II, mice immunized with SL3261; III, mice immunized with JJ702; IV, mice immunized with JJ703; V, mice immunized with JJ704.
DISCUSSION
Tetanus toxoid has been universally exploited as a potent adjuvant for chemically coupled antigens. Fragment C from tetanus toxin is highly immunogenic when expressed in Salmonella. In order to enhance the immune response, precise genetic fusions of recombinant antigens with TetC have been constructed in live Salmonella vaccines (25). This strategy has received considerable attention; however, a formal proof of the immunomodulatory nature of TetC is urgently required. A number of carrier proteins have been used in live vaccines for the presentation of heterologous epitopes from antigens including flagellin, E. coli heat-labile toxin B, outer membrane protein A (OmpA), phage lambda receptor (LamB), maltose binding protein E (MalE), and hepatitis B core antigen (13, 14, 29, 30, 35, 36). In a number of cases, these carrier molecules have been shown to enhance the immune responses to otherwise nonimmunogenic heterologous epitopes when expressed in Salmonella. However, little is known of the immunological impact these carrier molecules on full-length antigens. The results presented in this study for the first time provide strong evidence to suggest that TetC can enhance the immune responses to a fusion partner, in this case Sh28GST. This molecule has been expressed either as the native protein or as a fusion to TetC. Both TetC-Sh28GST and Sh28GST are stably expressed in S. enterica serovar Typhimurium strain SL3261. However, Sh28GST alone in JJ704 was expressed to approximately fivefold greater levels than the TetC-Sh28GST fusion in JJ703. The expression plasmids in both strains JJ703 and JJ704 were stably retained in vitro in the absence of drug selection. Furthermore, viable counts performed on organ homogenates of livers and spleens in groups of mice immunized orally with JJ703 and JJ704 indicated that the recombinant strains persist in tissues equally well. In addition, after in vivo passage the strains retained the ability to express the respective recombinant proteins, implying that the constructs were stable in vivo and not prone to genetic rearrangements.
However, after a single oral inoculation the immune responses generated by these constructs were very different. The antibody responses to Sh28GST that were generated were much greater in mice inoculated with JJ703 expressing the TetC-Sh28GST fusion than in mice inoculated with JJ704 expressing Sh28GST alone. Thus, despite the significantly lower in vitro expression of the fusion TetC-Sh28GST than of Sh28GST alone, JJ703 was even more effective at invoking antibody responses against Sh28GST. The low antibody responses generated by JJ704 expressing Sh28GST alone cannot be attributed to misfolding of the molecule, as Sh28GST retains full enzymatic activity. In addition, JJ703 was capable of eliciting antibody responses to TetC greater than those obtained with JJ702. This observation is consistent with the anti-TetC responses to TetC-antigen fusions observed previously (10, 11, 24, 25).
Adjuvants are capable of modulating antibody subclasses against specific epitopes of antigen (22). The analysis of anti-Sh28GST antibody subclasses in mice immunized with recombinant salmonellae showed the presence of IgG1, IgG2a, and IgG2b antibodies. However, there were no apparent qualitative differences in the antibody subclasses elicited by JJ703 and JJ704. Thus, it appears that the greater antibody responses generated by TetC to its fusion partner Sh28GST cannot be attributable to a particular antibody subclass. It has been shown that different routes of immunization elicit different antibody isotype profiles directed against the S. mansoni Sm28GST (26) and the S. haematobium Sh28GST (27) in mice immunized with rBCG. The intranasal and intravenous immunizations led to an initial production of high titers of IgG2a and low titers of IgG1 and IgG2b. Immediately after boosting, high titers of IgG2b also emerged. The results obtained by using the pTECH system have consistently shown that high levels of IgG1 antibodies can be elicited against several recombinant antigens, delivered by different Salmonella vaccine strains and by both oral and intravenous immunizations (10, 11). The results obtained in this study with a single oral immunization of recombinant Salmonella show that the main antibody subclasses directed against Sh28GST were IgG1 and IgG2a, with lower levels of IgG2b antibodies. Interestingly, notable levels of specific IgA antibodies were also detected in the sera from mice immunized with JJ703. Thus TetC enhanced the IgA response to Sh28GST. This is of particular interest since it has been suggested that IgA antibodies might participate in the effector immune responses, which protect against schistosomiasis in experimental models (17) and humans (18). The lack of detection of IgM was puzzling; either it is not induced or more likely appeared and disappeared below detectable level by week 3.
Furthermore, the antibodies elicited by JJ703 expressing TetC-Sh28GST, but not JJ704 expressing Sh28GST alone, have significant GST-neutralizing activity. In humans and animal models, an important correlate of protection from schistosomiasis is the presence of GST-neutralizing antibodies. It has been reported that antisera with a GST-neutralizing activity of 60 to 70% result in a significant reduction in worm fecundity (G. J. Riveau, unpublished observations). In this study, we have reported inhibition values as high as 85.4% 21 days after a single immunization, which gradually fell with time. It may be that boosting helps to maintain such high levels of neutralizing antibodies. Hence not only can TetC enhance the antibody response to its fusion partner Sh28GST but also it can stimulate the induction of IgA and antibodies with significant GST neutralization activity.
Stimulation of splenocytes from immunized animals with rSh28GST and rTetC resulted in clear IFN-γ and IL-2 responses. In contrast to the significantly different antibody responses elicited by the strains, splenocytes from mice immunized with either JJ703 or JJ704 produced comparable amounts of both IFN-γ and IL-2 when stimulated with Sh28GST. However, no specific IL-4 response could be detected, possibly because whole spleen cells rather than purified CD4+ T cells were assayed. This is consistent with cytokine patterns we have observed previously (10, 11). Upon stimulation with rTetC, splenocytes from mice immunized with JJ702 and JJ703 also secreted similar levels of IFN-γ and IL-2. Thus, TetC does not appear to have altered the secretion of the cytokines investigated after stimulation of splenocytes from JJ703-immunized mice with Sh28GST antigen. Not only can a vaccine adjuvant increase the potency of an antigen, it can also modulate the humoral or cell-mediated immune responses generated. The TetC fusion partner may increase the antibody responses to Sh28GST by providing T-cell help, but the precise molecular mechanisms need further investigation. It is also possible that the augmented antibody responses have been influenced by the levels of other cytokines such as IL-13 and IL-12. For example, cholera toxin, a potent mucosal adjuvant, has recently been demonstrated to elicit T-helper-cell type 2 responses by inhibiting the production of IL-12 (6).
In summary, the results presented in this study demonstrate the immunomodulatory effects of TetC in raising the antibody responses to fused antigens. Thus, a single dose of JJ703 expressing TetC-Sh28GST is able to potently elicit antibodies against Sh28GST, in contrast to JJ704 expressing Sh28GST alone. Furthermore, IgA antibodies are produced and the sera have significant GST neutralization activity, all important correlates of protection against schistosomiasis in the mouse model. The ability of these constructs to confer protection against S. haematobium is currently under investigation. The approach presented in this investigation may represent a general strategy for eliciting antibody responses to recombinant antigens expressed in live vaccines. Thus TetC may be exploited as a potent tool to immunostimulate the production of antibodies to antigens in live vaccines when these are the protective responses required.
ACKNOWLEDGMENTS
This work was supported by grants from the European Union.
We thank David Dunne, University of Cambridge, for helpful comments.
REFERENCES
- 1.Aggarwal A, Kumar S, Jaffe R, Hone D, Gross M, Sadoff J. Oral Salmonella malaria circumsporozoite recombinants induce specific CD8+ cytotoxic cells. J Exp Med. 1990;172:1083–1090. doi: 10.1084/jem.172.4.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balloul J M, Grxych J M, Pierce R J, Capron A. A purified 28,000 dalton protein from Schistosoma mansoni adult worms protects rats and mice against experimental schistosomiasis. J Immunol. 1987;138:3448–3453. [PubMed] [Google Scholar]
- 3.Bergquist N R, Colley D G. Schistosomiasis vaccines: research to development. Parasitol Today. 1998;14:99–104. doi: 10.1016/s0169-4758(97)01207-6. [DOI] [PubMed] [Google Scholar]
- 4.Boulanger D, Reid G D, Sturrock R F, Wolowczuk I, Balloul J-M, Grezel D, Pierce R J, Otieno M F, Guerret S, Grimaud J A, Butterworth A E, Capron A. Immunization of mice and baboons with the recombinant Sm28GST affects both worm viability and fecundity after experimental infection with Schistosoma mansoni. Parasite Immunol. 1991;13:473–490. doi: 10.1111/j.1365-3024.1991.tb00545.x. [DOI] [PubMed] [Google Scholar]
- 5.Boulanger D, Warter A, Sellin B, Lindner V, Pierce R J, Chippaux J-P, Capron A. Vaccine potential of a recombinant glutathione S-transferase cloned from Schistosoma haematobium in primates experimentally infected with an homologous challenge. Vaccine. 1999;17:319–326. doi: 10.1016/s0264-410x(98)00202-3. [DOI] [PubMed] [Google Scholar]
- 6.Braun M C, He J, Wu C-Y, Kelsall B L. Cholera toxin suppresses interleukin (IL)-12 production and IL-12 receptor b1 and b2 chain expression. J Exp Med. 1999;189:541–552. doi: 10.1084/jem.189.3.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown A, Hormaeche C E, Demarco de Hormaeche R, Dougan G, Winther M, Maskell D, Stocker B A D. An attenuated aroA S. typhimurium vaccine elicits humoral and cellular immunity to cloned β-galactosidase in mice. J Infect Dis. 1987;155:86–92. doi: 10.1093/infdis/155.1.86. [DOI] [PubMed] [Google Scholar]
- 8.Capron A, Riveau G, Grzych J-M, Boulanger D, Capron M, Pierce R J. Development of a vaccine strategy against human and bovine schistosomiasis: background and update. Trop Geogr Med. 1994;46:242–246. [PubMed] [Google Scholar]
- 9.Capron A. Schistosomiasis: forty years' war on the worm. Parasitol Today. 1998;14:379–384. doi: 10.1016/s0169-4758(98)01322-2. [DOI] [PubMed] [Google Scholar]
- 10.Chabalgoity J A, Khan C M A, Nash A A, Hormaeche C E. A Salmonella typhimurium htrA live vaccine expressing multiple copies of a peptide comprising amino acids 8-23 of herpes simplex virus glycoprotein D as a genetic fusion to tetanus toxin fragment C protects mice from herpes simplex virus infection. Mol Microbiol. 1996;19:791–801. doi: 10.1046/j.1365-2958.1996.426965.x. [DOI] [PubMed] [Google Scholar]
- 11.Chabalgoity J A, Harrison J A, Esterves A, Demarco de Hormaeche R, Ehrlich R, Khan C M A, Hormaeche C E. Expression and immunogenicity of an Echinococcus granulosus fatty acid-binding protein in live attenuated Salmonella vaccine strains. Infect Immun. 1997;65:2402–2412. doi: 10.1128/iai.65.6.2402-2412.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chan M S, Woolhouse M E S, Bundy D A P. Human schistosomiasis: potential long-term consequences of vaccination programmes. Vaccine. 1997;15:1545–1550. doi: 10.1016/s0264-410x(97)00071-6. [DOI] [PubMed] [Google Scholar]
- 13.Charbit A, Sobczak E, Michel M-L, Molla A, Tiollais P, Hofnung M. Presentation of two epitopes of the preS2 region of hepatitis B virus on live recombinant bacteria. J Immunol. 1987;139:1658–1664. [PubMed] [Google Scholar]
- 14.Charbit A, Martineau P, Ronco J, Leclerc C, Lo-Man R, Michel V, O'Callaghan D, Hofnung M. Expression and immunogenicity of the V3 loop from the envelope of human immunodeficiency virus type 1 in an attenuated aroA strain of Salmonella typhimurium upon genetic coupling to 2 Escherichia coli carrier proteins. Vaccine. 1993;11:1221–1228. doi: 10.1016/0264-410x(93)90046-z. [DOI] [PubMed] [Google Scholar]
- 15.Fallon P G, Mubarak J S, Fookes R E, Niang M, Butterworth A E, Sturrock R F, Doenhoff M J. Schistosoma mansoni: maturation rate and drug susceptibility of different geographic isolates. Exp Parasitol. 1997;86:29–36. doi: 10.1006/expr.1997.4149. [DOI] [PubMed] [Google Scholar]
- 16.Gomez-Duarte O G, Galen J, Chatfield S N, Rappuoli R, Eidels L, Levine M. Expression of fragment C of tetanus toxin fused to a carboxyl-terminal fragment of diphtheria toxin in Salmonella typhi CVD 908 vaccine strain. Vaccine. 1995;13:1596–1602. doi: 10.1016/0264-410x(95)00094-h. [DOI] [PubMed] [Google Scholar]
- 17.Grezel D, Capron M, Grzych J-M, Fontaine J, Lecocq J-P, Capron A. Protective immunity induced in rat schistosomiasis by a single dose of the Sm28GST recombinant antigen: effector mechanisms involving IgE and IgA antibodies. Eur J Immunol. 1993;23:454–460. doi: 10.1002/eji.1830230223. [DOI] [PubMed] [Google Scholar]
- 18.Grzych J-M, Grezel D, Xu C B, Neyrinck J-L, Capron M, Ouma J H, Butterworth A E, Capron A. IgA antibodies to a protective antigen in human schistosomiasis mansoni. J Immunol. 1993;150:527–535. [PubMed] [Google Scholar]
- 19.Habig W H, Pabst M J, Jakoby W B. Glutathione S-transferases; the first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- 20.Hoiseth S K, Stocker B A D. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature. 1981;291:238–239. doi: 10.1038/291238a0. [DOI] [PubMed] [Google Scholar]
- 21.Hormaeche C E. Natural resistance to Salmonella typhimurium in different inbred mouse strains. Immunology. 1979;37:311–318. [PMC free article] [PubMed] [Google Scholar]
- 22.Hunter R L, Lal A A. Copolymer adjuvants in malaria vaccine development. Am J Trop Med Hyg. 1994;50:52–58. doi: 10.4269/ajtmh.1994.50.52. [DOI] [PubMed] [Google Scholar]
- 23.Ismail M, Metwally A, Farghaly A, Bruce J, Tao L-F, Bennett J L. Characterization of isolates of Schistosoma mansoni from Egyptian villagers that tolerate high doses of praziquantel. Am J Trop Med Hyg. 1996;55:214–218. doi: 10.4269/ajtmh.1996.55.214. [DOI] [PubMed] [Google Scholar]
- 24.Khan C M A, Villarreal-Ramos B, Pierce R J, Demarco de Hormaeche R, McNeil H, Ali T, Chatfield S, Capron A, Dougan G, Hormaeche C E. Construction, expression, and immunogenicity of the Schistosoma mansoni glutathione S-transferase as a genetic fusion to tetanus toxin fragment C in a live Aro attenuated vaccine strain of Salmonella. Proc Natl Acad Sci USA. 1994;91:11261–11265. doi: 10.1073/pnas.91.23.11261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khan C M A, Villarreal-Ramos B, Pierce R J, Demarco de Hormaeche R, McHeil H, Ali T, Chatfield S, Capron A, Dougan G, Hormaeche C E. Construction, expression, and immunogenicity of multiple tandem copies of the Schistosoma mansoni peptide 115-131 of the glutathione S-transferase expressed as C-terminal fusions to tetanus toxin fragment C in a live aro-attenuated vaccine strain of Salmonella. J Immunol. 1994;153:5634–5642. [PubMed] [Google Scholar]
- 26.Kremer L, Riveau G, Baulard A, Capron A, Locht C. Neutralizing antibody responses elicited in mice immunized with recombinant bacillus Calmette-Guerin producing the Schistosoma mansoni glutathione S-transferase. J Immunol. 1996;156:4309–4317. [PubMed] [Google Scholar]
- 27.Kremer L, Dupre L, Riveau G, Capron A, Locht C. Systemic and mucosal immune response after intranasal administration of recombinant Mycobacterium bovis bacillus Calmette-Guérin expressing glutathione S-transferase from Schistosoma haematobium. Infect Immun. 1998;66:5669–5676. doi: 10.1128/iai.66.12.5669-5676.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 29.Newton S M C, Jacob C O, Stocker B A D. Immune response to cholera toxin epitope inserted in Salmonella fragellin. Science. 1989;244:70–72. doi: 10.1126/science.2468182. [DOI] [PubMed] [Google Scholar]
- 30.Pistor S, Hobom G. Expression of viral hemagglutinin on the surface of E. coli. Klin Wochenschr. 1988;66:110–116. doi: 10.1007/BF01774224. [DOI] [PubMed] [Google Scholar]
- 31.Riveau G J, Capron A. Vaccination against schistosomiasis: concepts and strategies. In: Kaufmann S H E, editor. Concepts in vaccine design. Berlin, Germany: Walter de Gruyter; 1996. pp. 509–532. [Google Scholar]
- 32.Sadoff J C, Ballou W R, Baron L S, Majarian W R, Brey R N, Hockmeyer W T, Young J F, Cryz J J, Ou J, Lowell G H, Chulay J D. Oral Salmonella typhimurium vaccine expressing the circumsporozoite protein protects against malaria. Science. 1988;240:336–338. doi: 10.1126/science.3281260. [DOI] [PubMed] [Google Scholar]
- 33.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Vol. 3. Cold Spring Harbor, N.Y.: Cold Spring Harbor Laboratory Press; 1989. p. A.12. [Google Scholar]
- 34.Schodel F, Moriarty A M, Peterson D L, Zheng J, Hughes J L, Will H, Leturq D J, McGee J S, Milich D R. The position of heterologous epitopes inserted in hepatitis B virus core particles determines their immunogenicity. J Virol. 1992;66:106–114. doi: 10.1128/jvi.66.1.106-114.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schodel F, Milich D R, Will H. Hepatitis B virus nucleocapsid/pre-S2 fusion proteins expressed in attenuated Salmonella for oral vaccination. J Immunol. 1990;145:4317–4321. [PubMed] [Google Scholar]
- 36.Schodel F, Kelly S M, Peterson D L, Milich D R, Curtiss R., III Hybrid hepatitis B virus core-pre-S proteins synthesized in avirulent Salmonella typhimurium and Salmonella typhi for oral vaccination. Infect Immun. 1994;62:1669–1676. doi: 10.1128/iai.62.5.1669-1676.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shuxian L, Guangchen S, Yuxian X, Yang W, McManus D P. Immunization of mice with recombinant Sjc26GST induces a pronounced anti-fecundity effect after experimental infection with Chinese Schistosoma japonicum. Vaccine. 1995;13:603–607. doi: 10.1016/0264-410x(94)00045-o. [DOI] [PubMed] [Google Scholar]
- 38.Taylor J B, Vidal A, Torpier G, Meyer D J, Roitsch C, Balloul J-M, Southan C, Sondermeyer P, Pemble S, Lecocq J-P, Capron A, Ketterer B. The glutathione transferase activity and tissue distribution of a cloned Mr28K protective antigen of Schistosoma mansoni. EMBO J. 1988;7:465–472. doi: 10.1002/j.1460-2075.1988.tb02834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trottein F, Godin C, Pierce R J, Sellin B, Taylor M G, Gorillot I, Silva M S, Lecocq J-P, Capron A. Inter-species variation of schistosome 28-kDa glutathione S-transferases. Mol Biochem Parasitol. 1992;54:63–72. doi: 10.1016/0166-6851(92)90095-2. [DOI] [PubMed] [Google Scholar]
- 40.Villarreal B, Mastroeni P, Demarco de Hormaeche R, Hormaeche C E. Proliferative and T-cell specific interleukin (IL-2/IL-4) production responses in spleen cells from mice vaccinated with aroA live attenuated Salmonella vaccines. Microb Pathog. 1992;13:305–315. doi: 10.1016/0882-4010(92)90040-u. [DOI] [PubMed] [Google Scholar]
- 41.World Health Organization. WHO fact sheet 115. New York, N.Y.: World Health Organization, W.H.O. Publications Center; 1996. [Google Scholar]
- 42.Xu C B, Verwaerde C, Grzych J-M, Fontaine J, Capron A. A monoclonal antibody blocking the Schistosoma mansoni 28 kDa glutathione S-transferase activity reduces female worm fecundity and egg viability. Eur J Immunol. 1991;21:1801–1807. doi: 10.1002/eji.1830210804. [DOI] [PubMed] [Google Scholar]