Short abstract
Content available: Audio Recording
Listen to an audio presentation of this article.
BACKGROUND
Tea consumption is an aspect of many cultures worldwide. Green tea, particularly made from the leaves of Camellia sinensis (L.), is part of daily tea rituals in the East. In addition to cultural considerations, green tea enjoys great popularity worldwide because of its potential preventive health effects.
Green tea contains a number of phytochemicals, which can be subdivided into three groups of substances: terpenes, alkaloids, and phenols. The medically most relevant group within the phenols are the flavanoids, of which the catechins account for up to 20% of the dry matter. The catechins consist of catechin (C), epicatechin (EC), gallocatechin, epigallocatechin (EGC), and epigallocatechin gallate (EGCG), of which the EGCG is the pharmacologically most active polyphenol. 1 Although the enjoyment of green tea as an undisputed health remedy is highly popular, more than 200 cases of liver failure, sometimes even requiring liver transplantation, associated with green tea have been published within the last 30 years. Although traditional green tea preparation rarely instigates major medical problems, extreme use of concentrated green tea extract (GTE), such as in mixed preparations taken in powder or tablet form for weight loss, have more frequently been described. 2 , 3
CASE DESCRIPTION
A 24‐year‐old woman with normal body mass index was transferred from a community hospital to the intensive care unit (ICU) of a university hospital with clinical and laboratory features of acute liver injury. The patient reported that she received a mixed preparation of GTE intravenously from a medical doctor specialized in alternative medicine 72 h and again 48 h before admission. The mixture contained high‐dose GTE, carnitine, riboflavin (vitamin B6), and other phytomedical components. She never received similar medication and did not experience any other organ‐specific side effects. She had already begun to feel unwell after the second infusion and suffered from nausea and abdominal pain. Complaints aggravated the day before hospital admission, with worsened upper abdominal pain, severe nausea, and repeated vomiting, which made eating and drinking impossible. Diarrhea did not occur. Jaundice had been noticed about 24 h before admission.
Five years ago, she went on a trip to Columbia, from where she returned with fever. Her general condition worsened since then, and she suffered from fatigue and from fructose and lactose intolerance; however, all diagnostics from tropical medicine were inconspicuous (Table 1). Because of concomitant depression, the patient took citalopram 20 mg/day. Alcohol and drug use were credibly denied; admittedly, the patient drank several cups of green tea per day for the last weeks and lost 3 kg within 14 days.
TABLE 1.
Laboratory results
| Parameter (Normal Value) | −6 Months | Admission | +8 Hours | +20 Hours | +32 Hours | +25 Days |
|---|---|---|---|---|---|---|
| Hemoglobin (14–17.5 g/dL) | 13.3 | 12.1 | 11.1 | 11.3 | 11.3 | 12.8 |
| White blood cells (3.8–11 x 109/L) | 6.1 | 9.0 | 7.9 | 16.2 | 7.2 | |
| Blood platelets (150–400 x 109/L) | 223 | 214 | 194 | 197 | 197 | 192 |
| Serum creatinine (0.7–1.2 mg/dL) | 0.51 | 0.44 | 0.5 | 0.46 | ||
| Serum urea (9–23 mg/dL) | 11 | 10 | 6 | 5 | ||
| Aspartate aminotransferase (<50 U/L) | 13 | 2647 | 1899 | 1313 | 885 | 21 |
| ALT (<50 U/L) | 20 | 3863 | 3254 | 3086 | 2678 | 50 |
| Gamma‐glutamyltransferase (<73 U/L) | 15 | 21 | 21 | 25 | 35 | 27 |
| Alkaline phosphatase (46–116 U/L) | 57 | 49 | 54 | 55 | 65 | |
| C‐reactive protein (<5 mg/L) | <5 | 29 | 20 | <5 | ||
| Total serum bilirubin (0.3–1.2 mg/dL) | 2.1 | 1.7 | 2.3 | 2.5 | 1.1 | |
| Conjugated serum bilirubin (<0.3 mg/dl) | 0.7 | 0.6 | 0.9 | 0.7 | ||
| Serum albumin (34–50 g/L) | 36.3 | 30.1 | 32.6 | 34.4 | 36.5 | |
| Glutamate dehydrogenase (<7 U/L) | 743 | 511 | 199 | 79 | ||
| Lactate dehydrogenase (120–246 U/L) | 1449 | 1098 | 512 | 289 | ||
| Quick (84%–129%) | 47 | 39 | 50 | 59 | ||
| International normalized ratio | 1.7 | 1.9 | 1.6 | 1.4 | ||
| Factor V (66%–149%) | 35.2 | 48 | 69.5 | 85.4 | ||
| Serum ammonia (16–53 μmol/L) | 46 | 44 | 45 |
At admission, she presented jaundice, severe nausea, and vomiting. Physical examination revealed a slightly enlarged liver and epigastric pain. The patient was exhausted and anguished, but there were no signs of hepatic encephalopathy. The initial laboratory examination showed significantly increased transaminases and hyperbilirubinemia and reduced coagulation performance. The ammonia level measured shortly after admission of 46 pmol/L was in the upper reference range. The other laboratory parameters were unremarkable (Table 1). The supplementary toxicological examination in urine did not bring up any additional information. The paracetamol level in the blood was below the reference range (<10 mg/L). Hepatitis serology (hepatitis A, B, C, D, or E virus) demonstrated no evidence of acute or chronic infection. The diagnosis of hepatotropic viruses, such as cytomegalovirus or herpes simplex virus, was unremarkable. Autoimmune diagnostics (anti‐nuclear antibody, anti‐mitochondrial antibody, smooth muscle actin, and anti‐liver‐kidney‐antibody) showed no evidence of underlying autoimmune hepatitis. The diagnostics for Wilson's disease and hemochromatosis also remained unremarkable. Besides minimal perihepatic ascites and a single lymph node of 6 mm within the liver hilus, ultrasound of the abdomen was unremarkable. The patient did not consent for a diagnostic laparoscopy.
Due to the clinical and laboratory picture of acute hepatopathy and the close temporal relationship with an intravenous administration of green tea, a probatory trial of N‐acetylcysteine (NAC) therapy following a standardized protocol was initiated (initially 150 mg/kg over 15 min, 50 mg/kg over 4 h, and 100 mg/kg initiated over 16 h). After this treatment, laboratory values improved (Table 1). After 3 days of ICU treatment, the patient was transferred to the gastroenterological normal ward.
During the following days, liver function steadily improved, but the patient developed Clostridium difficile–associated diarrhea. After oral treatment with vancomycin, the patient was clinically stabilized and discharged from the hospital. The first follow‐up indicated a complete clinical recovery of the patient; initially elevated transaminases and decreased markers of liver synthesis were almost normalized.
DISCUSSION
Although the consumption of green tea is generally considered to be harmless to health, several hundred case reports have been published in the past 30 years in which signs of liver damage and even liver failure requiring transplantation have been described in connection with green tea. Only a few of these cases involved green tea consumption as it is traditionally prepared. Most of them were concentrated GTE preparations taken in powder or tablet form for weight reduction. An overview of systematic reviews in which cases of green tea–associated liver damage were examined is summarized in Table 2. 2 , 4 , 5 , 6 , 7
TABLE 2.
Overview of systemic reviews
| Author | Study type | Data | Supplements | Intake type | Intake duration until liver damage | Authors’ conclusion |
|---|---|---|---|---|---|---|
| Sarma et al. (2008) 2 | Safety review | Case reports of experimental and clinical data (n = 216) | GTE (multi‐ingredient supplement | Oral application | 5–120 days | Expert Committee concluded that the safety information for green tea arising from diverse sources provides a signal for the possibility of liver damage caused by products that contain concentrated GTEs |
| Liver damage possible (n = 27) | ||||||
| Liver damage probable (n = 7) | ||||||
| (1966–2007) | ||||||
| Mazzanti et al. (2009) 5 | Review | Case reports (n = 34) | GTE (n = 15) | Oral application | 4–1460 days | Analysis of the published case reports suggests a causal association between green tea and liver damage |
| (1999–2008) | Multicomponent preparations (n = 19) | |||||
| Mazzanti et al. (2015) 4 | Review | Case reports (n = 19) | GTE (multi‐ingredient supplement) | Oral application | 14–365 days | Safety concern with GTE especially when green tea is associated with other ingredients |
| (2008–2015) | ||||||
| Oketch‐Rabah et al. (2019) 6 | Safety review | Experimental animal studies (n = 127) | GTE (different compositions) | Oral application | >14 days | Animal and human data indicate that repeated oral administration of bolus doses of GTE during fasting significantly increases bioavailability of catechins |
| Clinical data (n = 355) | Case reports associate hepatotoxicity with EGCG intake amounts from 140 mg to ∼1000 mg/day and substantial interindividual variability in susceptibility, possibly because of genetic factors | |||||
| Clinical trials (n = 4) | Toxicological studies show a hepatocellular pattern of liver injury | |||||
| Published case reports (n = 51) | ||||||
| Reviews and miscellaneous reports (n = 52 + 35) | ||||||
| (2008–2016) | ||||||
| Hoofnagle et al. (2021) 7 | Retrospective analysis | 40 cases of green tea–associated liver injury 3% of 1414 DILIN patients | GTE (multi‐ingredient supplement) | Oral application | 15–448 days (median 72 days) | The liver injury was typically hepatocellular (95%) with marked serum aminotransferase elevations and modest increases in alkaline phosphatase. Severe course in 14 patients (35%), liver transplantation in 3 (8%), and chronic injury 12 patients (3%). HLA typing indicated a high prevalence of HLA‐B*35:01, found in 72% (95% CI: 58%‐87%) of green tea cases, but only 15% (95% CI: 10%‐20%) caused by other supplements and 12% (95% CI: 10%‐14%) attributed to drugs, the latter rate being similar to population controls (95% CI: 10.5%‐11.5%), suggesting that it is idiosyncratic and immune mediated |
| (2004–2018) |
In 2003, the preparation Exolise (Arkopharma, Carros, France) was withdrawn from the market for the first time after 13 cases of documented hepatoxicity. 8 Subsequently, on the basis of a review in which more than 200 cases involving green tea products were examined, the affixing of a hazard label to green tea–containing preparations was considered by the US Pharmacopeia (USP) Dietary Supplement Information Expert Committee. Out of a total of 216 cases, 27 reports of liver damage were considered possible and likely caused by GTE‐induced toxicity. 2
The recommendation to add a warning label for powdered decaffeinated GTE was initially withdrawn in the course of the analysis. It was recommended to monitor the literature for a further period in anticipation of additional evidence. Shortly thereafter, a warning was issued by the US Food and Drug Administration for the green tea–containing preparation Hydroxycut (Iovate Health Sciences, Oakville, ON, Canada), and the preparation was withdrawn from the market. 9
A 2015 update of a review by Mazzanti et al. 4 , 5 described an additional 19 cases of hepatotoxic adverse events after green tea ingestion. Sixteen of the 19 patients (84%) were female and had taken larger amounts of green tea orally as an infusion, GTE, or mixed preparations containing GTE. The time to onset of hepatotoxic side effects varied from 14 to 365 days. All 19 patients had multifocal hepatocellular necrosis and apoptosis with inflammatory infiltrates. Four patients (three of four women) underwent liver transplantation. These patients had all previously taken mixed drugs orally with an unknown dose of GTE.
In 2016, the USP Dietary Supplements Admission Evaluations Joint Standard Setting Subcommittee (USP DSAE JS3) again reviewed the adverse effect data for GTE published since 2008 and recommended that the warning be reinserted into the USP Powdered Decaffeinated Green Tea Extract (PDGTE) monograph. The revised USP PDGTE monograph, which includes the warning, officially went into effect on March 1, 2019. 10
The European Food Safety Authority had also initiated a systemic review on the safety of catechins in animal and intervention studies in 2018. A systematic review identified transaminase and cholestasis parameter increases with daily intake of at least 800 mg EGCG in 9 of 38 intervention studies. Although traditional green tea infusion was considered harmless, a possible causal relationship for the observed liver damage was considered possible for food supplements with EGCG doses of cumulatively >800 mg/day. A safe dose could not be named. 3
A Green Tea Extract Hepatotoxicity Expert Panel was established by the USP, which conducted a further comprehensive analysis of the existing literature regarding potential interactions of GTE, preparation, pharmacokinetics, and dynamics. In a comprehensive review, Oketch‐Rabah et al. 6 suggested that repeated oral administration of GTE, particularly during periods of fasting, increased the bioavailability but also the toxicity of catechins. 11 Possible explanatory mechanisms for the reduced systemic availability of catechins when taken with food include interactions with dietary proteins, a pH >4, but also delayed absorption per se. 12 , 13
In this case of the 24‐year‐old patient, however, it was not a case of oral ingestion of GTE. The patient had received a GTE mixture intravenously 72 h and 48 h before admission to the ICU. Shortly after the first administration of the mixture, the patient already described clinical signs of poisoning, such as nausea and abdominal pain. Forty‐eight hours after the first infusion, the patient noticed jaundice. Admission to the ICU revealed markedly elevated aspartate aminotransaminase and alanine aminotransferase, hyperbilirubinemia, and hypalbuminemia. Coagulation parameters including factor V were decreased. The ammonia level was in the upper range (Table 1). Clinical signs of hepatic encephalopathy were not observed, so prognostic scores such as King's College criteria were not applicable. The calculation of the Model for End‐Stage Liver Disease score was 15 points.
The R ratio was 68, which corresponds to a hepatocellular pattern of damage already described in numerous case reports. The more accurate assessment of drug‐induced liver failure was presented by Danan and Benichou 14 in 1993. The Roussel Uclaf Causality Assessment Method (RUCAM), in which points in seven diagnostic criteria are summed up to a total value, allows the assessment of a possible causal relationship between a damaging drug and a liver injury that has occurred. 14 , 15
When using the RUCAM score, however, it must be taken into account that the signs of liver damage occurred within 48 h (category 1 = 1 point), which may be because of the unusual form of application. The immediate systemic availability bypassing the gastrointestinal tract might have potentiated the toxicity of GTE. In category 2 of the RUCAM, the course of alanine aminotransferase (ALT) is surveyed. In the present case, the ALT shows a rapid recovery. However, it has to be considered that this development could be caused by the application of acetylcysteine and may not reflect the natural course. Taking these limitations into account, a score of at least eight points can be calculated for GTE in the present case. Using the RUCAM currently revised by Hayashi, 16 the revised electronic causality assessment method (RECAM) domains, results in an even higher point value of 10 points (“highly probable” drug‐induced liver damage). The main reason for this is that in RECAM the hepatotoxic effect of green tea is already associated with three points in the likelihood score of the LiverTox likelihood categories.
In addition, the RECAM algorithm provides for a separate evaluation of concomitant medication. In this case, it was citalopram, a category C drug in the likelihood score. It usually causes only minor liver enzyme changes that are usually self‐limiting and do not require dose adjustment or discontinuation of therapy. The incidence of drug‐induced liver damage is 0.02% during treatment with citalopram. In the RECAM calculation, this results in five points, which corresponds to possible liver damage. Possible cumulative effects of citalopram and GTE can therefore not be excluded.
Even if the amount of GTE mixture administered to the patient is unknown, toxic effects have been described already from an amount of 140 mg of the catechin EGCG, whereby a considerable interindividual susceptibility is assumed. 6
In a recent analysis by Hoofnagle et al., 7 retrospective data from the US Drug‐Induced Liver Injury Network (DILIN) showed that the liver damage of 40 female patients (3% of the 1414 patients examined) was due to GTE. As in numerous previous studies, the patients were women around 40 years of age who had taken green tea products with several ingredients for weight loss. In the HLA typing that could be performed in 36 of the patients, the prevalence rate of HLA‐B*35:01 was 72% (95% confidence interval [CI]: 58%–87%). Comparison of cases with green tea–induced liver injury with and without HLA‐B*35:01 showed that those with the risk allele were younger (median age, 40 versus 47 years), had a shorter time to onset (median, 66 versus 139 days), higher median baseline ALT levels (2329 versus 696 U/L), and higher severity of liver injury. HLA‐B 35:01 has now also been linked to Polygonum multiflorum–associated liver injury and thus appears to be a risk factor for liver injury from herbal substances. 17 It is not known whether the patient in the present case has a corresponding allele constellation.
The potential induction of hepatic damage by GTE has meanwhile been documented in numerous case reports, clinical studies, and reviews. However, the pattern of damage induced by catechins is unclear. Clues to the metabolic pathways involved have emerged from experimental studies.
In animal experiments on mice and in vitro on isolated hepatocytes, EGCG showed a stronger cytotoxic effect than all other green tea catechins. A breakdown of the mitochondrial membrane potential and the formation of reactive oxygen species (ROS) are currently assumed to be the main mechanisms of damage. 18 , 19 For the various catechins, different pathways of metabolization have been described, such as via the cytochrome P450 enzyme series, uridine 5'‐diphospho‐ glucuronosyltransferase, ATP‐binding‐cassette transporter, and antioxidant enzymes. 20 , 21 James et al. 22 were also able to show that with increasing EGCG doses, histopathological signs of liver damage such as inflammation, necrosis, and bleeding were accompanied by oxidative stress (increase in hepatic lipid peroxidase and phosphorylated histones 2A.X) and a simultaneous decrease in reduced glutathione levels. At the same time, reduced hepatic mitochondrial density was observed with significantly reduced gene transcription for mitochondrial respiratory chain complexes I and III, glutathione peroxidase I, and superoxide dismutase. 22
Even though validated knowledge about the damage pattern of catechins is still lacking, and conclusions from this experimental study are based only on surrogate markers, they nevertheless offer a possible explanation for the rapid clinical and laboratory improvement under acetylcysteine therapy.
Acetylcysteine has traditionally been used as an antidote after paracetamol intoxication, as the provision of SH groups counteracts the depletion of the body's glutathione reserves and detoxifies the harmful N‐acetyl‐p‐benzoquinoneimine bound to glutathione. As a radical scavenger in reduced form, glutathione is a reaction partner of numerous metabolic pathways in which it can be substituted by halogen, sulfate, sulfonate, phosphate, and nitro groups and thus excreted via the kidneys. The timely use of acetylcysteine may have improved glutathione levels and enhanced the metabolic pathways of detoxification of GTEs. A highly simplified illustration of the possible metabolic pathways is shown in Figure 1.
FIGURE 1.
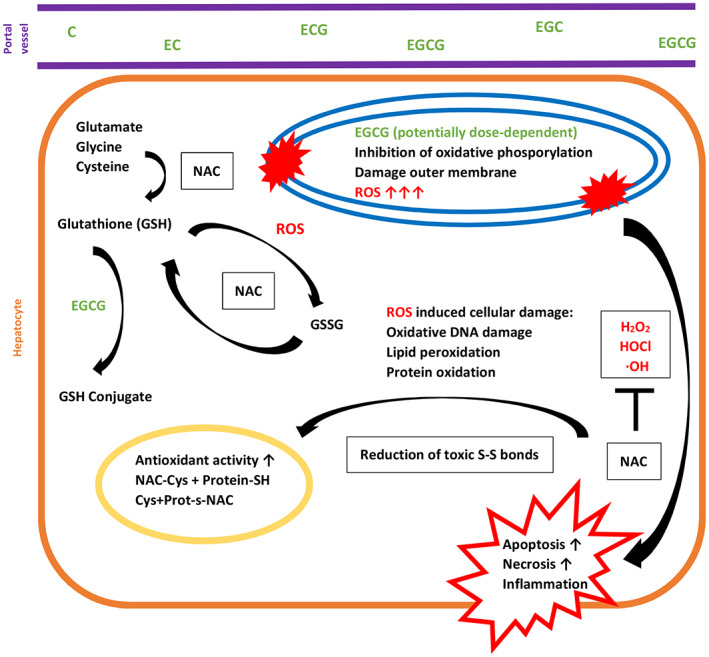
The catechins, especially EGCG, lead to inhibition of oxidative phosphorylation, damage to the mitochondrial outer membrane, and dose‐dependent formation of ROS. Glutathione deactivates ROS with formation of GSSG. Application of NAC and provision of cysteine can reduce glutathione dimer (GSSG) again. In addition, further glutathione can be replenished with ATP consumption by glutamate, glycine, and cysteine (also from NAC). Further, NAC inactivates ROS directly as a scavenger of ROS. By cleaving thiolated proteins, NAC also improves their antioxidant activity.
Using the search terms “green tea,” “GTE,” “liver,” “liver failure,” and “hepatoxicity,” only five reports could be identified that outlined the treatment of GTE‐associated liver injury with acetylcysteine. 8 , 23 , 24 , 25 , 26 In all of these cases, green tea or GTE was taken orally by the patients over a period of several weeks to months. Of seven patients who received acetylcysteine, five recovered completely within a few weeks, while the remaining two cases required liver transplantation.
However, the case presented in this report did not involve weeks of GTE ingestion. After intravenous administration of a GTE mixture, the clinical and laboratory signs of hepatocellular damage, as described in numerous studies, appeared after a short latency time. The direct application of NAC resulted in a rapid and complete recovery of the patient. This immediate antidote effect of NAC in GTE‐associated liver injury has not been described so far and requires further research.
The potential hepatotoxic effects of GTE have been outlined in numerous clinical and experimental studies for several decades and were ultimately decisive in the revised USP PDGTE monograph, which now includes the warning as of March 1, 2019. In Europe, comparable precautions are not yet required.
The present case illustrates that drug‐ or herb‐associated liver injury is an important differential diagnosis of acute liver damage and highlights the importance of NAC as a hepatic‐acting antidote.
CONFLICT OF INTEREST
Nothing to report.
Grajecki D, Ogica A, Boenisch O, Hübener P & Kluge S. Green tea extract–associated acute liver injury: Case report and review. Clinical Liver Disease. 2022;20:181–187. 10.1002/cld.1254
REFERENCES
- 1. Lambert JD, Elias RJ. The antioxidant and pro‐oxidant activities of green tea polyphenols: a role in cancer prevention. Arch Biochem Biophys. 2010;501:65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sarma DN, Barrett ML, Chavez ML, Gardiner P, Ko R, Mahady GB, et al. Safety of green tea extracts: a systematic review by the US Pharmacopeia. Drug Saf. 2008;31:469–84. [DOI] [PubMed] [Google Scholar]
- 3. Younes M, Aggett P, Aguilar F, Crebelli R, Dusemund B, Filipič M, et al. Scientific opinion on the safety of green tea catechins. EFSA J. 2018;16:5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mazzanti G, Di Sotto A, Vitalone A. Hepatotoxicity of green tea: an update. Arch Toxicol. 2015;89:1175–91. [DOI] [PubMed] [Google Scholar]
- 5. Mazzanti G, Menniti‐Ippolito F, Moro PA, Cassetti F, Raschetti R, Santuccio C, et al. Hepatotoxicity from green tea: a review of the literature and two unpublished cases. Eur J Clin Pharmacol. 2009;65:331–41. [DOI] [PubMed] [Google Scholar]
- 6. Oketch‐Rabah HA, Roe AL, Rider CV, Bonkovsky HL, Giancaspro GI, Navarro V, et al. United States Pharmacopeia (USP) comprehensive review of the hepatotoxicity of green tea extracts. Toxicol Rep. 2020;7:386–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hoofnagle JH, Bonkovsky HL, Phillips EJ, Li Y‐J, Ahmad J, Barnhart H, et al. HLA‐B*35:01 and Green Tea‐Induced Liver Injury. Hepatology. 2021;73:2484–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gloro R, Hourmand‐Ollivier I, Mosquet B, Mosquet L, Rousselot P, Salamé E, et al. Fulminant hepatitis during self‐medication with hydroalcoholic extract of green tea. Eur J Gastroenterol Hepatol. 2005;17:1135–7. [DOI] [PubMed] [Google Scholar]
- 9. Young S. Stop using Hydroxycut products, FDA says. CNN; 2009. [cited 2022 Aug 20]. Available from: https://www.cnn.com/2009/HEALTH/05/01/hydroxycut.fda.recall/index.html [Google Scholar]
- 10. USP‐NF . Update on the USP Green Tea Extract Monograph. 2009. April 10 [cited 2022 Sep 28]. Available from: https://www.uspnf.com/notices/retired‐compendial‐notices/update‐usp‐green‐tea‐extract‐monograph
- 11. Chow H‐HS, Hakim IA, Vining DR, Crowell JA, Ranger‐Moore J, Chew WM, et al. Effects of dosing condition on the oral bioavailability of green tea catechins after single‐dose administration of Polyphenon E in healthy individuals. Clin Cancer Res. 2005;11:4627–33. [DOI] [PubMed] [Google Scholar]
- 12. Naumovski N, Blades BL, Roach PD. Food inhibits the oral bioavailability of the major green tea antioxidant epigallocatechin gallate in humans. Antioxidants (Basel). 2015;4:373–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Egert S, Tereszczuk J, Wein S, Müller MJ, Frank J, Rimbach G, et al. Simultaneous ingestion of dietary proteins reduces the bioavailability of galloylated catechins from green tea in humans. Eur J Nutr. 2013;52:281–8. [DOI] [PubMed] [Google Scholar]
- 14. Danan G, Benichou C. Causality assessment of adverse reactions to drugs—I. A novel method based on the conclusions of international consensus meetings: application to drug‐induced liver injuries. J Clin Epidemiol. 1993;46:1323–30. [DOI] [PubMed] [Google Scholar]
- 15. Teschke R, Wolff A, Frenzel C, Schwarzenboeck A, Schulze J, Eickhoff A. Drug and herb induced liver injury: Council for International Organizations of Medical Sciences scale for causality assessment. World J Hepatol. 2014;6:17–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hayashi PH, Lucena MI, Fontana RJ, Bjornsson ES, Aithal GP, Barnhart H, et al. A revised electronic version of RUCAM for the diagnosis of DILI. Hepatology. 2022;76:18–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li C, Rao T, Chen X, Zou Z, Wei A, Tang J, et al. HLA‐B*35:01 allele is a potential biomarker for predicting polygonum multiflorum‐induced liver injury in humans. Hepatology. 2019;70:346–57. [DOI] [PubMed] [Google Scholar]
- 18. Galati G, Lin A, Sultan AM, O'Brien PJ. Cellular and in vivo hepatotoxicity caused by green tea phenolic acids and catechins. Free Radic Biol Med. 2006;40:570–80. [DOI] [PubMed] [Google Scholar]
- 19. Kucera O, Mezera V, Moravcova A, Endlicher R, Lotkova H, Drahota Z, et al. In vitro toxicity of epigallocatechin gallate in rat liver mitochondria and hepatocytes. Oxid Med Cell Longev. 2015;2015:476180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Feng WY. Metabolism of green tea catechins: an overview. Curr Drug Metab. 2006;7:755–809. [DOI] [PubMed] [Google Scholar]
- 21. Urban TJ, Daly AK, Aithal GP. Genetic basis of drug‐induced liver injury: present and future. Semin Liver Dis. 2014;34:123–33. [DOI] [PubMed] [Google Scholar]
- 22. James KD, Kennett MJ, Lambert JD. Potential role of the mitochondria as a target for the hepatotoxic effects of (−)‐epigallocatechin‐3‐gallate in mice. Food Chem Toxicol. 2018;111:302–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jimenez‐Saenz M, Martinez‐Sanchez C. Green tea extracts and acute liver failure: the need for caution in their use and diagnostic assessment. Liver Transpl. 2007;13:1067. [DOI] [PubMed] [Google Scholar]
- 24. Elinav E, Pinsker G, Safadi R, Pappo O, Bromberg M, Anis E, et al. Association between consumption of Herbalife nutritional supplements and acute hepatotoxicity. J Hepatol. 2007;47:514–20. [DOI] [PubMed] [Google Scholar]
- 25. Schoepfer AM, Engel A, Fattinger K, Marbet UA, Criblez D, Reichen J, et al. Herbal does not mean innocuous: ten cases of severe hepatotoxicity associated with dietary supplements from Herbalife products. J Hepatol. 2007;47:521–6. [DOI] [PubMed] [Google Scholar]
- 26. Lugg ST, Braganza Menezes D, Gompertz S. Chinese green tea and acute hepatitis: a rare yet recurring theme. BMJ Case Rep. 2015;2015:bcr2014208534. [DOI] [PMC free article] [PubMed] [Google Scholar]


