Abstract
The study of protective immune mechanisms effective against filarial nematodes has been hampered by the inability of these important human pathogens to infect laboratory mice. Recently, Litomosoides sigmodontis, a natural parasite of rats, has been developed as a valuable model for the study of filarial infection. BALB/c mice are fully susceptible to infection with L. sigmodontis third-stage larvae and develop patent infection. In contrast, mice on the C57BL background are resistant, and parasites undergo only a single molt and do not mature to adulthood. We used interleukin-5 (IL-5)-deficient mice on the C57BL/6 background to address the role of IL-5 and eosinophils in the innate resistance of C57BL/6 mice. We found no differences in parasite survival between IL-5-deficient and C57BL/6 mice. However, when these mice were used for the analysis of vaccine-mediated immunity, a critical role for IL-5 was elucidated. Mice genetically deficient in IL-5 were unable to generate a protective immune response when vaccinated with irradiated larvae, whereas C57BL/6 mice were fully protected from challenge infection. These studies help to clarify the highly controversial role of eosinophils in filarial infection.
Filarial nematodes are the causative agents of lymphatic filariasis (elephantiasis) and onchocerciasis (river blindness). Together, these parasites (Wuchereria bancrofti, Brugia malayi, and Onchocerca volvulus) afflict more than 140 million people worldwide (37). A major stumbling block in the study of filarial disease is the inability of these human pathogens to establish infection in well-characterized laboratory animals. A recent advance in filariasis research has been the development of a murine model of infection, using the rodent filarial parasite Litomosoides sigmodontis (24). L. sigmodontis is the only filarial species able to complete its full development cycle in inbred laboratory mice.
One of the benefits of this new model is that susceptibility to infection is murine strain dependent, allowing genetic dissection of the mechanisms that determine innate resistance as has been done for other parasitic systems such as Trichuris muris and Leishmania major (11, 30). BALB/c mice are fully susceptible to L. sigmodontis infection, and parasites develop through to patency. In contrast, mice on the C57BL background are resistant and patent infections are never seen (27). Interestingly, this pattern of resistance and susceptibility is similar to that seen for the protozoan parasite L. major and opposite to that of the intestinal nematode T. muris.
How cells of the innate immune system (e.g., granulocytes and macrophages) might mediate protective immunity to nematode parasites is an unresolved and controversial issue. In particular, despite their distinctive association with nematode infection, the exact role of eosinophils is not established for either intestinal or tissue locales and remains an area of considerable scientific debate (3, 16, 35). Numerous studies have shown that the characteristic eosinophilia observed in nematode infection is dependent on host interleukin-5 (IL-5) (7, 15, 16). Not surprisingly, eosinophil recruitment to filarial infection is also IL-5 dependent (12, 22). However, without the ability to use the extensive array of murine reagents, the exact function of eosinophils in immunity to infection has been difficult to establish.
We have chosen the L. sigmodontis model to directly address the role of eosinophils in resistance to infection with vector-derived larvae. We hypothesized that if eosinophils were a key player in innate resistance to filarial parasites, then a genetic inability to recruit eosinophils might render resistant C57BL/6 mice more susceptible to infection. In this study, we demonstrate that parasite survival following primary infection of IL-5-deficient mice on the C57BL/6 background does not differ from that in infections in wild-type (WT) C57BL/6 mice. Parasite attrition in naive resistant mice normally occurs after larval migration and molting (23). In immunized mice, however, parasites die rapidly at the site of inoculation with eosinophils surrounding the dying larvae (20). We therefore chose to use these mice to additionally investigate vaccine-mediated protection. We found that in contrast to innate resistance, the rapid killing of larvae in vaccinated mice was highly dependent on IL-5.
MATERIALS AND METHODS
Parasites and mouse infection model.
L. sigmodontis is transmitted by the mite vector Ornithonyssus bacoti. Infective third-stage larvae (L3) migrate through the lymphatics to the thoracic cavity, where larvae mature to the adult stage. Adult parasites produce microfilariae that circulate in the bloodstream. Laboratory maintenance of L. sigmodontis and recovery of infective larvae from the mite vector were carried out as previously described (9, 27). IL-5-deficient mice on the C57BL/6 background were the kind gift of Manfred Kopf (Basel, Switzerland). All mice (C57BL/6 and IL-5 deficient) were bred on site, and 6- to 8-week-old males were used for all experiments.
(i) Primary infection.
In each experiment, age-matched groups of IL-5-deficient and WT C57BL/6 mice were infected subcutaneously with 25 infective larvae. Necropsies were performed 10, 20, or 40 days postinfection. For all experiments, the number of worms that had developed from infective larvae was counted as described by Bain et al. (4). Briefly, all dissections were performed in RPMI 1640 with 10% fetal calf serum. The internal organs (lungs, heart, and gut) were removed, and the rest of the body was cut transversely to separate the abdominal and thoracic regions. Parasites were enumerated for each cavity or organ separately.
(ii) Vaccination protocol.
Vaccinated groups of IL-5-deficient and WT mice were immunized three times with 25 irradiated (600 Gy, using a cesium source) infective larvae by subcutaneous inoculation into the lumbar area at weekly intervals and challenged with 25 infective larvae subcutaneously into the lumbar area 2 weeks after the last immunization. Control groups of IL-5-deficient and WT mice received only the challenge with 25 infective larvae subcutaneously into the lumbar area. Necropsies were performed 10 days after the challenge, at which point sera were collected and the spleens were removed for use in cellular assays.
Antigen preparation.
Somatic extract was prepared from adult female and male L. sigmodontis worms by homogenization followed by centrifugation at 12,000 × g for 10 min at 4°C. Protein content was determined by the Coomassie Plus protein assay (Pierce, Rockford, Ill.).
Cytokine assays.
Splenocytes were cultured in RPMI 1640 (Life Technologies) supplemented with 2 mM glutamine, penicillin (100 U/ml), streptomycin (100 μg/ml), and 10% fetal calf serum (complete medium). Spleens were teased apart, and then erythrocytes were lysed in red blood cell lysis buffer (Sigma Chemical Co.). Viable cells were counted by trypan blue exclusion. Whole splenocytes were incubated at 5 × 106 cells/ml with L. sigmodontis antigen or with concanavalin A (Sigma) at a final concentration of 10 μg/ml. After incubation for 72 h at 37°C, 100 μl of supernatant was taken for cytokine assays. IL-2 and IL-4 were measured by bioassay; gamma interferon (IFN-γ) and IL-5 were measured by enzyme-linked immunosorbent assay (ELISA). All assays were performed essentially as described by Lawrence et al. (18) except that streptavidin-alkaline phosphatase (Sigma) was used for detection with p-nitrophenyl phosphate (Sigma) as substrate.
Antibody isotype analysis.
Levels of parasite-specific immunoglobulin G1 (IgG1), IgG2a, IgG2b, and IgG3 in serum samples from individual mice were measured by ELISA. Plates were coated with L. sigmodontis antigen (1 μg/ml), diluted in carbonate buffer (pH 9.6) overnight at 4°C. The plates were blocked with 5% bovine serum albumin in phosphate-buffered saline–0.05% Tween 20 (PBST) for 90 min at 37°C. Serum samples were diluted 1:200 in PBST and incubated for 90 min at 37°C. Peroxidase-conjugated goat anti-IgG1 (1:6,000; Southern Biotechnology Associates Inc. [SBA] product no. 1070-05), anti-IgG2a (1:200; SBA 1080-05), anti-IgG2b (1:4,000; SBA 1090-05), anti-IgG3 (1:1,000; SBA 1100-05), or anti-IgM (1:1,000; SBA 1020-05) was diluted in PBST to the concentration indicated and incubated for 90 min at 37°C. After each incubation, the plate was washed five times in PBST. Finally 2,2′-azinodi(ethylbenzthiazoline-6-sulfonate) (ABTS) substrate (KPL Biotechnology) was added, and the enzyme reaction was determined photometrically in an ELISA reader at 405 nm. A capture antibody ELISA was used to measure the total serum IgE concentration as previously described (18).
Statistical analysis.
Significant differences between experimental groups were calculated using the two-tailed Mann-Whitney U test with P < 0.05 considered significant.
RESULTS
Innate resistance is not mediated by IL-5.
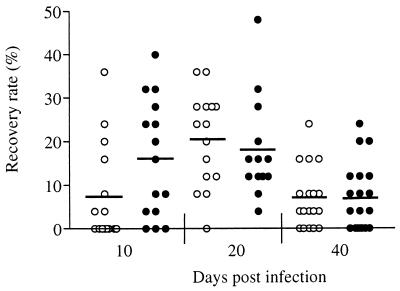
To evaluate whether innate resistance to infection was mediated by eosinophils in C57BL/6 mice, we inoculated WT and IL-5-deficient mice with 25 infective larvae and assessed parasite survival at days 10, 20, and 40. After the primary infection, no significant differences in recovery rate were observed between IL-5-deficient mice and WT mice at any time point (Fig. 1). It was notable (although not statistically significant) that at 10 days postinfection the IL-5-deficient mice had fewer parasites than the WT controls but numbers were equivalent by day 20. Since parasite numbers cannot increase following infection, this observation leads to the intriguing but highly speculative hypothesis that IL-5 influences early larval migration. In both groups, the recovery rate had declined significantly by 40 days after infection (P < 0.05). This is in contrast to BALB/c mice, in which no decline in parasite numbers is seen between days 20 and 40 (23).
FIG. 1.
Parasite recovery rate in a primary infection of IL-5-deficient (○) and WT (●) mice. Values are shown for individual mice. The mean of 15 to 20 mice per group for each time point is indicated by a horizontal bar.
We investigated the immune response of WT and IL-5-deficient mice during the course of primary infection to determine whether the absence of IL-5 had any significant effect on the immune response to L. sigmodontis infection. IL-2, IL-4, IL-5, and IFN-γ were measured using mitogenic and antigenic stimulation. No antigen-specific IL-2 or IFN-γ responses were seen in the spleen at any time postinfection, while antigen-specific IL-4 and IL-5 release was not detected until 40 days postinfection (data not shown). No significant differences in IL-4 production were observed between IL-5-deficient and WT animals. As expected, no IL-5 was produced in the gene-deficient animals.
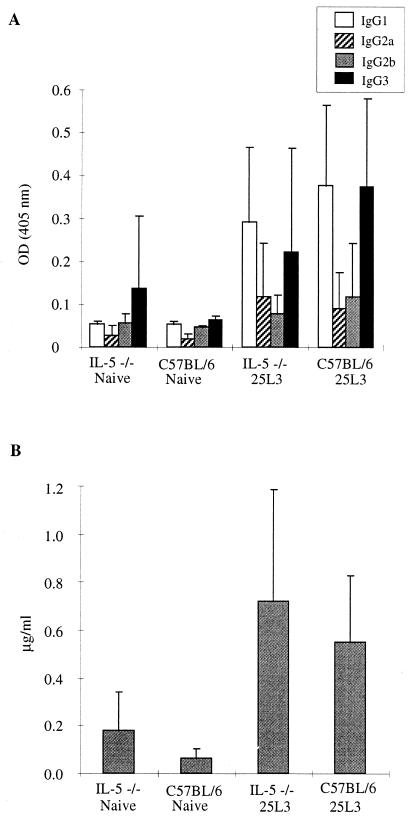
Parasite-specific antibody production was not observed until 40 days postinfection in both groups of mice. High levels of parasite-specific IgG1 were detected at day 40, in contrast to naive mice (P < 0.01), with no significant differences in isotype profile between the IL-5-deficient and WT mice (Fig. 2). Polyclonal IgE was observed at significant levels (P < 0.01) by day 20 (data not shown) and at day 40 (Fig. 2), but once again, no significant differences were observed in the absence of IL-5.
FIG. 2.
Antibody isotype production in IL-5-deficient and WT mice 40 days postinfection. (A) L. sigmondontis-specific IgG subclass responses; (B) total serum IgE. Values are expressed as the mean ± standard deviation of 11 to 15 mice per group. OD, optical density.
Vaccine-mediated protection is critically dependent on IL-5.
Numerous studies have demonstrated that immunization with irradiated filarial larvae can lead to significant levels of protection from challenge infection (1, 5, 21, 32, 34, 36, 38). Le Goff et al. (19) demonstrated that in the L. sigmodontis model this killing is very rapid, with larval destruction occurring within the first 2 days following challenge. We chose to use C57BL/6 mice to assess the role of eosinophils in this rapid protective immune response. Mice vaccinated three times with irradiated larvae demonstrated a 100% level of protection, with no parasites recovered from challenge, in contrast to unvaccinated C57BL/6 mice, in which parasites were recovered from all animals (Fig. 3; P < 0.01). Strikingly, this high level of protection was not observed in the IL-5-deficient mice, in which the parasite recovery rate following challenge did not differ significantly between vaccinated and unvaccinated animals (Fig. 3). Thus, the absence of IL-5 prevents the development of a protective immune response to challenge infection. This is in sharp contrast to the impact of IL-5 on primary exposure to L3.
FIG. 3.
Parasite recovery rate in challenged only and in vaccinated and challenged IL-5-deficient (○) and WT (●) mice at 10 days postchallenge. Values are shown for individual mice.
Consistent with our observations following primary infections, no measurable specific cytokine or antibody responses were seen at 10 days in the mice receiving only the challenge infection. In the vaccinated mice, high parasite-specific IgG1 was observed along with polyclonal IgE, but there were no significant differences in antibody isotype profile between IL-5-deficient and WT mice (Fig. 4). Similarly, with the exception of IL-5 (2.35 ± 1.16 ng/ml in the five WT mice; nil in the IL-5-deficient mice), there were no differences in cytokine responses (data not shown).
FIG. 4.
Antibody isotype production in vaccinated IL-5-deficient and WT mice 10 days following challenge (chal.) infection. (A) L. sigmondontis-specific IgG subclass responses; (B) total serum IgE. Values are expressed as the mean ± standard deviation of five mice per group. IgG1, IgG3, and IgE were significantly higher in vaccinated (Vac.) mice than naive mice (P < 0.05). OD, optical density.
DISCUSSION
The use of inbred mouse strains with differences in innate resistance has been a powerful tool in parasite biology to understand the fundamental requirements for innate resistance and susceptibility to infection with a variety of pathogens (6, 25). The identification of differential strain susceptibility in a mouse model of filarial infection may help us to understand the mechanisms by which some individuals appear never to become infected while others carry high worm burdens.
In this study, we wished to specifically address the role of IL-5 (and therefore eosinophils) in the innate resistance to primary infection that has been observed for many strains of laboratory mice (27), using the C57BL/6 strain as a prototypic resistant mouse. An important role for eosinophils has already been proposed for killing of Onchocerca microfilariae (10, 13), but this study was designed to address the nature of susceptibility and resistance to incoming larvae and not the later stages of infection.
We found that in the absence of eosinophils, parasite survival kinetics were identical to that seen in WT mice. Parasite numbers begin to decline significantly between days 20 and 40, suggesting that in the resistant host the nematode is unable to survive the L4-to-adult molt (23). Our results with IL-5-deficient mice demonstrate that parasite attrition at this stage is independent of eosinophils. Interestingly, Rajan et al. demonstrated that treatment of mice with nitric oxide (NO) synthase inhibitor abrogated resistance to infection with the human filarial parasite B. malayi (29). From this, we would hypothesize that classic inflammatory processes such as NO release by IFN-γ-activated macrophages mediate the parasite decline that we observe between days 20 and 40. To test this hypothesis, we will need to investigate the role of IFN-γ or NO at the critical point during infection when larval development is unable to proceed in the resistant host. Regardless of the mechanism, it is likely to be mediated via specific T cells, as Al-Qaoud et al. (2) have demonstrated a critical requirement for T cells in susceptibility to L. sigmodontis infection.
In striking contrast to primary infection, we found a critical role for IL-5 in the immune response against challenge infection consistent with the association of type 2 cytokines with vaccine-mediated protection (5). Evidence that eosinophils are important in vaccine-mediated immunity against filarial parasites has previously come from a study by Lange et al. with O. volvulus (17) in which neutralizing antibody to IL-5 prevents killing of the challenge in a micropore chamber model of infection. In a related study also using L3 in chambers, IL-4-deficient mice are not protected by vaccination (14). Consistent with our findings, lower numbers of eosinophils are present in the chambers of IL-4-deficient mice in contrast to IFN-γ-deficient mice that are fully protected. Surprisingly, the investigators find elevated levels of IL-5 in the chambers of all vaccinated groups, including the IL-4-deficient mice (14). A separation of IL-4 and IL-5 regulation has been described before (13, 31), and thus the interpretation that would be consistent with both our study and the Johnson study is that the failure of vaccine-mediated protection is due to insufficient numbers of eosinophils. Additionally, insufficient antibody responses in the IL-4-deficient mice may fail to trigger eosinophils.
Previous work in the L. sigmodontis vaccination model has shown that eosinophils are present and degranulate at the site of challenge infection (20). This observation in combination with our data here using mice genetically deficient in IL-5 and the work of others (14, 17) provides strong evidence that eosinophils are responsible for vaccine-mediated protection in filarial infection of mice. Killing of incoming larvae by eosinophils may be mediated via antibodies, as previous studies have shown reduced protection when heterologous larvae are used in the challenge (33) and eosinophils alone could not account for this specific immune recognition. However, we saw no significant differences in antibody levels or isotype distribution between WT and IL-5-deficient mice. These data suggest that the failure to achieve protection in the gene-deficient mice is likely due to the absence of eosinophils rather than an IL-5-mediated defect in B-cell function.
Nonetheless, it will be necessary to rule out other effects of IL-5 such as defects due to B-1 cells (15). In addition, IL-5-deficient mice infected with Toxoplasma gondii failed to make antigen-specific IgG1 in contrast to WT controls (28), consistent with previous in vitro studies (39). However, IgG1 levels were unimpaired in IL-5-deficient mice during L. sigmodontis infection, perhaps because very high levels of IL-4 are induced by nematode infection. IL-5 may be important only if levels of IL-4 are suboptimal. Although our data show no evidence for differences in antibody responsiveness between IL-5-deficient and WT mice, we cannot exclude the possibility that qualitative effects of IL-5 on antibody profiles are responsible for the protective immune response.
It is important to consider why eosinophils appear to contribute to the early response in vaccinated mice but are not important in the late primary response to the parasite. In both situations, parasite-specific antibody and eosinophils are present. Several factors may be responsible for this difference. First, the immune responses to important target antigens may be considerably greater in repeatedly vaccinated mice than in a late primary infection. Second, infective larvae may be intrinsically more susceptible to eosinophil-mediated killing than the later stages of development. Third, the location of the more mature parasites (outside the subcutaneous tissue) may facilitate their ability to mechanically avoid eosinophil attack. In addition, later in the infection process, immune suppressive mechanisms may counter important host effector mechanisms. Finally, the ability of IL-5-deficient mice to clear a primary infection does not rule out a role for eosinophils in a normal setting, as other mediators may be readily able to compensate for a lack of eosinophils in the gene-deficient mice.
The data on the role of IL-5 using both neutralizing antibodies and transgenic mice have not generated a consistent picture between different nematode species and even within parasite families (8). For example, our data contrast directly with those for Strongyloides ratti infection in mice, where IL-5 is important in the primary infection but is not a player in the protective response against a challenge infection (26). This is not surprising, as each parasite has evolved independently and has developed disparate survival strategies that may or may not involve direct avoidance of eosinophil-mediated killing. Further, the location and migration patterns of the parasite life cycle stages are certain to influence their susceptibility to a particular innate or adaptive immune mechanism. It is becoming apparent, therefore, that conclusions found within one system cannot be readily applied to another. However, we do hope that the data provided in this report will help to sharpen our image of the mechanisms involved in the destruction of filarial nematodes and help the development of effective strategies to prevent new infections.
ACKNOWLEDGMENTS
We thank Odile Bain for helping us initiate the L. sigmodontis life cycle and for subsequent advice, Kathryn Lamza for maintenance of the life cycle, and Rick Maizels for critical comments on the manuscript.
Judith Allen is an MRC Senior Fellow. P'ng Loke is a Wellcome Trust student. This work was supported by the Edna McConnell Clark Foundation and the Medical Research Council of the United Kingdom.
REFERENCES
- 1.Abraham D, Eberhard M L, Lange A M, Yutanawiboonchai W, Perler F B, Lok J B. Identification of surrogate rodent hosts for larval Onchocerca lienalis and induction of protective immunity in a model system. J Parasitol. 1992;78:447–453. [PubMed] [Google Scholar]
- 2.Al-Qaoud K M, Taubert A, Zahner H, Fleischer B, Hoerauf A. Infection of BALB/c mice with the filarial nematode Litomosoides sigmodontis: role of CD4+ T cells in controlling larval development. Infect Immun. 1997;65:2457–2461. doi: 10.1128/iai.65.6.2457-2461.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allen J E, Maizels R M. Th1-Th2: reliable paradigm or dangerous dogma? Immunol Today. 1997;18:387–392. doi: 10.1016/s0167-5699(97)01102-x. [DOI] [PubMed] [Google Scholar]
- 4.Bain O, Wanji S, Vuong P N, Maréchal P, Le Goff L, Petit G. Larval biology of six filariae of the sub-family Onchocercinae in a vertebrate host. Parasite. 1994;1:241–254. doi: 10.1051/parasite/1994013241. [DOI] [PubMed] [Google Scholar]
- 5.Bancroft A J, Grencis R K, Else K J, Devaney E. Cytokine production in BALB/c mice immunized with radiation attenuated third stage larvae of the filarial nematode, Brugia pahangi. J Immunol. 1993;150:1395–1402. [PubMed] [Google Scholar]
- 6.Behnke J M, Barnard C J, Wakelin D. Understanding chronic nematode infections—evolutionary considerations, current hypotheses and the way forward. Int J Parasitol. 1992;22:861–907. doi: 10.1016/0020-7519(92)90046-n. [DOI] [PubMed] [Google Scholar]
- 7.Coffman R L, Seymour B W P, Hudak S, Jackson J, Rennick D. Antibody to interleukin-5 inhibits helminth-induced eosinophilia in mice. Science. 1989;245:308–310. doi: 10.1126/science.2787531. [DOI] [PubMed] [Google Scholar]
- 8.Dent L A, Daly C M, Mayrhofer G, Zimmerman T, Hallett A, Bignold L P, Creaney J, Parsons J C. Interleukin-5 transgenic mice show enhanced resistance to primary infections with Nippostrongylus brasiliensis but not primary infections with Toxocara canis. Infect Immun. 1999;67:989–993. doi: 10.1128/iai.67.2.989-993.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diagne M, Petit G, Liot P, Cabaret J, Bain O. The filaria Litomosoides galizai in mites; microfilarial distribution in the host and regulation of the transmission. Ann Parasitol Hum Comp. 1990;65:193–199. doi: 10.1051/parasite/1990654193. [DOI] [PubMed] [Google Scholar]
- 10.Folkard S G, Hogarth P J, Taylor M J, Bianco A E. Eosinophils are the major effector cells of immunity to microfilariae in a mouse model of onchocerciasis. Parasitology. 1996;112:323–329. doi: 10.1017/s0031182000065847. [DOI] [PubMed] [Google Scholar]
- 11.Grencis R K. T cell and cytokine basis of host variability in response to intestinal nematode infections. Parasitology. 1996;112:S31–S37. [PubMed] [Google Scholar]
- 12.Hall L R, Mehlotra R K, Higgins A W, Haxhiu M A, Pearlman E. An essential role for interleukin-5 and eosinophils in helminth-induced airway hyperresponsiveness. Infect Immun. 1998;66:4425–4430. doi: 10.1128/iai.66.9.4425-4430.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hogarth P J, Taylor M J, Bianco A E. IL-5-dependent immunity to microfilariae is independent of IL-4 in a mouse model of onchocerciasis. J Immunol. 1998;160:5436–5440. [PubMed] [Google Scholar]
- 14.Johnson E H, Schynder-Candrian S, Rajan T V, Nelson F K, Lustigman S, Abraham D. Immune responses to third stage larvae of Onchocerca volvulus in interferon-γ and interleukin-4 knockout mice. Parasite Immunol. 1998;20:319–324. doi: 10.1046/j.1365-3024.1998.00148.x. [DOI] [PubMed] [Google Scholar]
- 15.Kopf M, Brombacher F, Hodgkin P D, Ramsay A J, Milbourne E A, Dai W J, Ovington K S, Behm C A, Kohler G, Young I G, Matthaei K I. IL-5-deficient mice have a developmental defect in CD5+ B-1 cells and lack eosinophilia but have normal antibody and cytotoxic T cell responses. Immunity. 1996;4:15–24. doi: 10.1016/s1074-7613(00)80294-0. [DOI] [PubMed] [Google Scholar]
- 16.Korenaga M, Tada I. The role of IL-5 in the immune responses to nematodes in rodents. Parasitol Today. 1994;10:234–236. doi: 10.1016/0169-4758(94)90125-2. [DOI] [PubMed] [Google Scholar]
- 17.Lange A M, Yutanawiboonchai W, Scott P, Abraham D. IL-4- and IL-5-dependent protective immunity to Onchocerca volvulus infective larvae in BALB/cBYJ mice. J Immunol. 1994;153:205–211. [PubMed] [Google Scholar]
- 18.Lawrence R A, Allen J E, Osborne J, Maizels R M. Adult and microfilarial stages of the filarial parasite Brugia malayi stimulate contrasting cytokine and Ig isotype responses in BALB/c mice. J Immunol. 1994;153:1216–1224. [PubMed] [Google Scholar]
- 19.Le Goff L, Maréchal P, Petit G, Taylor D W, Hoffman W, Bain O. Early reduction of the challenge recovery rate following immunization with irradiated infective larvae in a filaria mouse system. Trop Med Int Health. 1997;2:1170–1174. doi: 10.1046/j.1365-3156.1997.d01-218.x. [DOI] [PubMed] [Google Scholar]
- 20.Le Goff, L., C. Martin, I. P. Oswald, P. N. Vuong, G. Petit, M. N. Ungeheuer, and O. Bain. Parasitology and immunology of mice vaccinated with irradiated Litomosoides sigmodontis larvae. Parasitology, in press. [DOI] [PubMed]
- 21.Lucius R, Textor G, Kern A, Kirsten C. Acanthocheilonema viteae: vaccination of jirds with irradiation-attenuated stage-3 larvae and with exported larval antigens. Exp Parasitol. 1991;73:184–196. doi: 10.1016/0014-4894(91)90021-n. [DOI] [PubMed] [Google Scholar]
- 22.MacDonald, A. S., P. Loke, and J. E. Allen. Suppressive antigen-presenting cells in helminth infection. Pathobiology, in press. [DOI] [PubMed]
- 23.Maréchal P, Le Goff L, Petit G, Diagne M, Taylor D W, Bain O. The fate of the filaria Litomosoides sigmodontis in susceptible and naturally resistant mice. Parasite. 1996;3:25–31. doi: 10.1051/parasite/1996031025. [DOI] [PubMed] [Google Scholar]
- 24.Maréchal P, Petit G, Diagne M, Taylor D W, Bain O. Use of the Litomosoides sigmodontis-mouse model in development of an Onchocerca vaccine. II. L. sigmodontis in the BALB/c mouse: vaccination experiments; preliminary immunological studies. Parasite. 1994;1:31–32. [Google Scholar]
- 25.McLeod R, Buschman E, Arbuckle L D, Skamene E. Immunogenetics in the analysis of resistance to intracellular pathogens. Curr Opin Immunol. 1995;7:539–552. doi: 10.1016/0952-7915(95)80100-6. [DOI] [PubMed] [Google Scholar]
- 26.Ovington K S, McKie K, Matthaei K I, Young I G, Behm C A. Regulation of primary Strongyloides ratti infections in mice: a role for interleukin-5. Immunology. 1998;95:488–493. doi: 10.1046/j.1365-2567.1998.00620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petit G, Diagne M, Maréchal P, Owen D, Taylor D, Bain O. Maturation of the filaria Litomosoides sigmodontis in BALB/c mice; comparative susceptibility of nine other inbred strains. Ann Parasitol Hum Comp. 1992;67:144–150. doi: 10.1051/parasite/1992675144. [DOI] [PubMed] [Google Scholar]
- 28.Purkerson J M, Isakson P C. Interleukin 5 (IL-5) provides a signal that is required in addition to IL-4 for isotype switching to immunoglobulin (Ig) G1 and IgE. J Exp Med. 1992;175:973–982. doi: 10.1084/jem.175.4.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rajan T V, Porte P, Yates J A, Keefer L, Shultz L D. Role of nitric oxide in host defense against an extracellular, metazoan parasite, Brugia malayi. Infect Immun. 1996;64:3351–3353. doi: 10.1128/iai.64.8.3351-3353.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reiner S L, Locksley R M. The regulation of immunity to Leishmania major. Annu Rev Immunol. 1995;13:151–177. doi: 10.1146/annurev.iy.13.040195.001055. [DOI] [PubMed] [Google Scholar]
- 31.Sartono E, Kruize Y C M, Kurniawan-Atmadja A, Maizels R M, Yazdanbakhsh M. Depression of antigen-specific interleukin-5 and interferon-γ responses in human lymphatic filariasis as a function of clinical status and age. J Infect Dis. 1996;175:1276–1280. doi: 10.1086/593701. [DOI] [PubMed] [Google Scholar]
- 32.Schrempf-Eppstein B, Kern A, Textor G, Lucius R. Acanthocheilonema viteae: vaccination with irradiated L3 induces resistance in three species of rodents (Meriones unguiculatus, Mastomys coucha, Mesocricetus auratus) Trop Med Int Health. 1997;2:104–110. doi: 10.1046/j.1365-3156.1997.d01-129.x. [DOI] [PubMed] [Google Scholar]
- 33.Storey D M, Al-Mukhtar A S. Vaccination of jirds, Meriones unguiculatus, against Litomosoides carinii and Brugia pahangi using irradiated larvae of L. carinii. Tropenmed Parasitol. 1982;33:23–24. [PubMed] [Google Scholar]
- 34.Taylor M J, van Es R P, Shay K, Folkard S G, Townson S, Bianco A E. Protective immunity against Onchocerca volvulus and O. lienalis infective larvae in mice. Trop Med Parasitol. 1994;45:17–23. [PubMed] [Google Scholar]
- 35.Urban J F, Madden K B, Sveti'c A, Cheever A, Trotta P P, Gause W C, Katona I M, Finkelman F D. The importance of Th2 cytokines in protective immunity to nematodes. Immunol Rev. 1992;127:205–220. doi: 10.1111/j.1600-065x.1992.tb01415.x. [DOI] [PubMed] [Google Scholar]
- 36.Weil G J, Li B W, Liftis F, Chandrashekar R. Brugia malayi—antibody responses to larval antigens in infected and immunized jirds. Exp Parasitol. 1992;74:315–323. doi: 10.1016/0014-4894(92)90155-4. [DOI] [PubMed] [Google Scholar]
- 37.World Health Organization. Lymphatic filariasis: the disease and its control. Fifth report of the WHO Expert Committee on Filariasis. WHO Tech Rep Ser. 1992;821:1–71. [PubMed] [Google Scholar]
- 38.Yates J A, Higashi G I. Brugia malayi: vaccination of jirds with 60cobalt-attenuated infective stage larvae protects against homologous challenge. Am J Trop Med Hyg. 1985;34:1132–1137. doi: 10.4269/ajtmh.1985.34.1132. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Y, Denkers E Y. Protective role for interleukin-5 during chronic Toxoplasma gondii infection. Infect Immun. 1999;67:4383–4392. doi: 10.1128/iai.67.9.4383-4392.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]