Abstract
Background:
In Thailand, data on colorectal cancer (CRC) patient characteristics and overall survival (OS) rates are limited. We aimed to describe the overall 5-year, 10-year survival and to examine factors effecting the survival outcome among patients who were diagnoses of colorectal cancer.
Methods:
We reviewed medical records of patients diagnosed with invasive CRC from 2007 through 2016. Demographic and clinical data were collected upon diagnosis. Kaplan-Meier method and Cox proportional hazards model to evaluate the association of overall (OS) with risk factors.
Results:
A total of 3,402 CRC patients (colon 59.4%, rectum 34. 5%, and rectosigmoid 6.1%) were identified. Mean (SD) and median age were 62.9 (12.7) and 63 years old (rang 14–98 years). Stages at diagnosis were I (10.1%), II (23.3), III (35.9%) and IV (30.7%). Five-year and 10-year OS of the entire cohort were 52.7% and 41.5%, respectively. Over the part 10 years, there was a trend toward improved 5-year OS in stages I, II and III. However, 3-year OS in stage IV patients remained unchanged. Confirmed poor prognostic factors included patient age ≥65 years, high grade, and advanced stage at diagnosis.
Conclusion:
Advanced disease was a significant prognostic factor for shorter survival. A trend toward improvement in 5-year OS in early stages over the past decade might be related to better surgical quality, improved radiation technique, and adjuvant chemotherapy. Given that patients received better systemic treatment in stage IV disease, the reason their OS was not improved should be examined.
Keywords: Colorectal cancer, Right-sided, Left-sided, Survival, Thailand
Introduction
Colorectal cancer (CRC) ranks as the third most common cancer globally. Worldwide, CRC is the third most common cancer in males (1,026,215 cases (10.9%)) and the second most common cancer in females (823,303 cases (9.5%)) (1–4). The age-standardized incidence rates (World) are 11.1 per 100,000 males and 8.6 per 100,000 females (2). Approximately 9,555, 027 CRC-related deaths have occurred in 2018, and the age-standardized mortality rate (World) is 8.9 per 100,000 people (1). In Thailand, CRC is the third and fourth most common cancer in males and females, respectively, with incidence rates higher in males than females. CRC occurs in 16.2 and 11.1 per 100,000 males and females, respectively (5). The survival rate is one of the main indicators for evaluating the effect of healthcare and disease control, and for measuring the effect of treatment. Survival rates of CRC patients are comparable among Asian and non-Asian countries. The 5-year survival rates for people with CRC in the United States (USA), Korea, Taiwan and Thailand are 65.0%, 75.0%, 64.2% and 44.0%, respectively (1,2,7). Unfortunately, many colorectal cancer patients in Thailand presented with distant metastatic disease. The treatment aim was palliative not curative.
Treatment of colorectal cancer required cancer required multidisciplinary approaches such as medical oncologist, surgeon, radiologist and palliative care team. Many resources are being used for treatment care due to increasing incidence of new patients according to the aging community. Data of incidence and survival will guide planning, evaluation of cancer control programs and set priorities all allocating health resources. To date, data on CRC patient characteristics and survival rate remain low in Thailand.
We therefore conducted a 10-year registry study of patients with CRC who diagnosed and treated at Ramathibodi Hospital to determine the survival outcomes and factors affecting survival outcomes among patients with CRC.
Material and Methods
Study design and population
All patients diagnosed with CRC and registered in the Ramathibodi Cancer Registry (RCR) were identified through the database. The RCR, established in 1985, contains registered data on patients who were newly diagnosed with invasive CRC between 2007 and 2016. A hospital-based cancer registry collects incidence and survival data on all cancer patients who are hospitalized, diagnosed, and/or treated for cancer at the Ramathibodi Hospital. Medical records, operation related notes, and pathological data were retrospective reviewed.
Patient demographics and clinical characteristics were collected form the time of initial diagnosis. All patients received the standard treatment given in accordance with the treatment protocol. We identified all new cases of CRC from the RCR database. All patient follow-up was completed by March 31, 2021. All patient statuses were verified as cancers by the Bureau of Registration Administration (BORA). The healthcare service schemes are divided into three groups: 1) Civil Servant Medical Benefit Scheme (CSMBS), 2) Social Security Scheme (SSS), and Universal Health Coverage (UC). Tumor location was identified using the international Classification of Disease, Oncology, Third Revision, diagnostic codes (ICD-O-3) C18.0–C18.9, which included the rectosigmoid junction (C19.9) and rectum (C20.9) (8). Only subjects with their first malignant primary tumor being an adenocarcinoma of the colon were included. For comparison of left-versus right-sided CRC outcomes, tumors occurring from the cecum to the transverse colon were considered as right-sided colon cancer (RCC), and those occurring from the descending colon, splenic flexure, sigmoid colon, rectosigmoid, and rectum, as left-sided colon cancer (LCC). Mortality status of all patients was validated and confirmed through the Thai Social Security Death Index database.
The ethic committee of Ramathibodi Hospital, Mahidol University approved this study (ID number: MURA202/1287)
Statistical analyses
Patient's baseline characteristics were summarized using descriptive statistics, and patients’ clinical characteristics were defined as categorical variables. Continuous variables were categorized according to clinical characteristics. Age at diagnosis was divided by 2 group; <65 years and ≥65 years. Overall survival (OS) was calculated from the date of diagnosis to the date of death from any cause. Cumulative survival percentages were estimated using the Kaplan-Meier method. Statistical significance of the difference in cumulative survival was tested using the log-rank statistic for homogeneity. A Cox proportional hazards model was used to evaluate the significance of the associations between other factors and death, which are represented as hazard ratios (HRs) and the 95% confidence interval (95%CI). P-value were considered significant when less than 0.05, and 95% CI were computed for survival proportions and survival rates. Statistical analysis was performed using StataCorp STATA 16.0 (College Station, Texas 77845 USA).
Result
Patient characteristics and histopathological characteristics
From Jan 2007 to Dec 2016, 3,402 patients were diagnosed with colorectal cancer. The topography of CRC were as follows: colon 59.4%, rectum 34.5%, and rectosigmoid 6.1%. The most common healthcare system was the civil servant medical benefit scheme (CSMBS). The majority of patients were male (52.8%), with mean (SD) and median (range) ages at first diagnosis were 62.9 (SD=12.7) years and 63 (14–98) years, respectively (Table 1).
Table 1:
Clinical characteristics of patients diagnosed with colorectal cancer between January 2007 and December 2016
| Variables | Number (3,402) | 100 % |
|---|---|---|
| Gender | ||
| Male | 1,796 | 52.8 |
| Female | 1,606 | 47.2 |
| Age group | ||
| <65 | 1,811 | 53.2 |
| ≥65 | 1,591 | 46.8 |
| Mean (SD) | 62.9 (12.7) | |
| Median(min:max) | 63 (14:98) | |
| Sided | ||
| RCC | 638 | 20.0 |
| LCC | 2,549 | 79.0 |
| Tumor Grade | ||
| Well diff. | 799 | 59.4 |
| Moderately diff | 1,759 | 64.5 |
| Poorly diff. | 170 | 6.2 |
| Topography | ||
| Colon | 2,022 | 88.9 |
| Rectosigmoid | 206 | 6.1 |
| Rectum | 1,174 | 34.5 |
| Type of patients | ||
| New cases | 3,024 | 88.9 |
| Referred | 378 | 11.1 |
| Staging | ||
| Stage I | 319 | 10.1 |
| Stage II | 737 | 23.3 |
| Stage III | 1,135 | 35.9 |
| Stage IV | 970 | 30.7 |
| Healthcare scheme | ||
| CSMBS | 835 | 65.8 |
| SSS | 59 | 34.6 |
| UHC | 375 | 29.6 |
Survival and factor associations
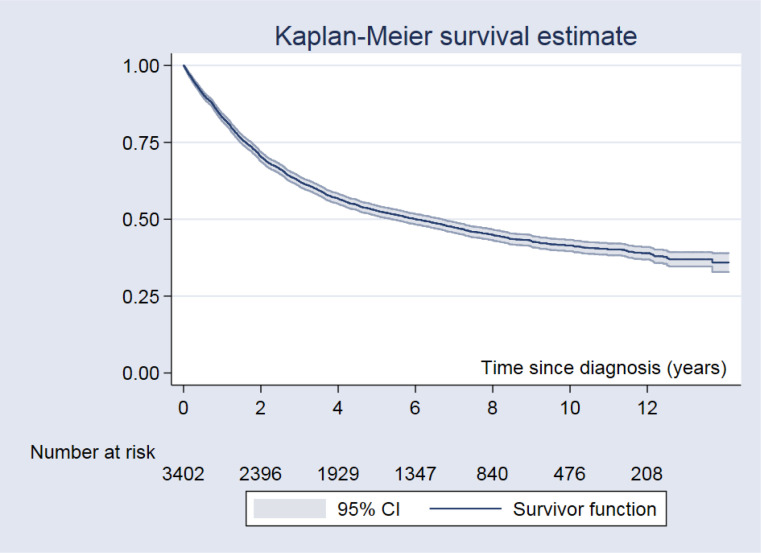
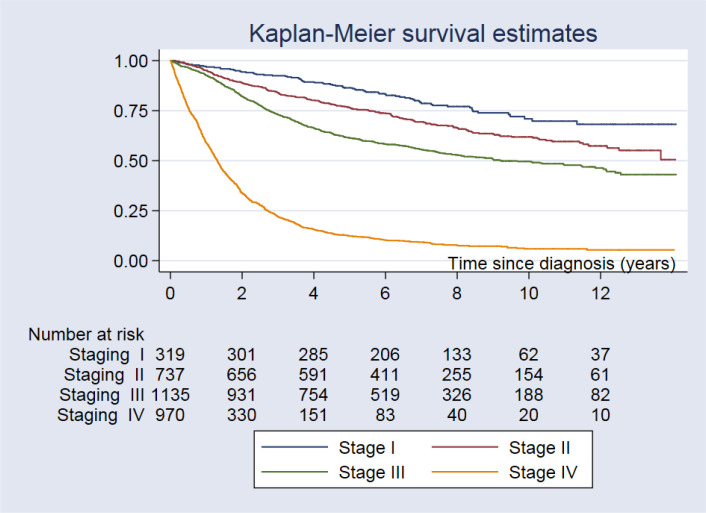
The median follow-up period was 5.17 years (0.01–14.1 years). There were 1,886 deaths, 922 (48.9%) and 964 (51.1%) in the <65 and ≥65-year age groups, respectively. Regarding follow-up of 17,607 person-years, the mortality rate was 10.7 per 100 person-years, and median survival time was 6.0 years (95% CI: 5.4 to 6.7). The 5-year and 10-year OS rates were 52.7% and 41.5%, respectively. The 5-year and 10-year OS rates are shown in Table 2, Fig.1 and 2. OS rates differed according to tumor type and stage at diagnosis. The best outcomes with the longest OS were detected in patients with well and moderately differentiated CRCs, while the worst outcomes were found in those with poorly differentiated CRCs.
Table 2:
The 5-year and 10-year survival rate according of different prognostic factor using Kaplan-Meier analysis
| Variables | Median survival (years) (95%CI) | 5-yr OS (95%CI) | 10-yr OS (95%CI) | P-value |
|---|---|---|---|---|
| Overall | 6.0 (5.4–6.7) | 52.7 (51.0–54.4) | 41.5 (39.6–43.3) | |
| Gender | 0.156 | |||
| Male | 5.7 (4.9–6.4) | 51.9 (49.6–54.3) | 39.2 (36.7–41.7) | |
| Female | 6.6 (5.6–7.8) | 53.6 (51.1–56.0) | 44.2 (41.3–46.7) | |
| Age group | <0.001 | |||
| <65 | 7.3 (6.2–9.2) | 54.6 (52.2–56.8) | 47.1 (44.6–49.6) | |
| ≥65 | 5.3 (4.5–5.9) | 50.6 (48.1–53.1) | 34.9 (32.2–37.7) | |
| Sided | 0.804 | |||
| RCC | 5.7 (4.5–7.9) | 52.4 (48.4–56.2) | 44.9 (40.7–49.0) | |
| LCC | 6.2 (5.4–6.8) | 53.1 (51.2–55.1) | 40.8 (38.7–43.0) | |
| Tumor Grade | <0.001 | |||
| Well diff. | 7.8 (6.5–9.0) | 57.3 (53.8–60.6) | 44.5 (40.7–48.1) | |
| Moderately diff. | 6.8 (6.0–7.7) | 55.7 (53.3–58.0) | 44.2 (41.4–46.9) | |
| Poorly diff. | 2.1 (1.7–2.7) | 35.2 (28.1–42.4) | 27.5 (20.4–35.1) | |
| Staging | <0.001 | |||
| Stage I | - | 86.4 (82.1–89.7) | 70.9 (64.1–76.7) | |
| Stage II | - | 76.6 (73.3–79.4) | 61.8 (57.6–65.8) | |
| Stage III | 9.2 (7.8–2.1) | 61.4 (58.4–64.1) | 49.6 (46.3–52.8) | |
| Stage IV | 1.3 (1.2–1.4) | 12.3 (10.3–14.5) | 5.6 (4.3–7.9) | |
| Topography | 0.114 | |||
| Colon | 6.2 (5.5–7.0) | 53.5 (51.3–55.6) | 42.2 (39.8–44.6) | |
| Rectosigmoid | 4.3 (3.3–6.7) | 47.1 (40.1–53.7) | 32.7 (25.7–39.9) | |
| Rectum | 6.0 (4.8–7.0) | 52.4 (49.5–55.3) | 41.8 (38.6–45.0) | |
| Type of patients | <0.001 | |||
| New cases | 6.4 (5.7–7.0) | 53.5 (51.7–55.3) | 42.2 (40.2–44.2) | |
| Referred | 4.0 (3.4–5.1) | 46.2 (41.1–51.2) | 35.6 (30.6–40.6) | |
| Healthcare scheme | 0.003 | |||
| CSMBS | 8.1 (7.0–11.0) | 59.9 (56.5–63.2) | 48.2 (43.4–52.8) | |
| SSS | 4.9 (2.2–8.7) | 48.9 (35.7–60.9) | 46.8 (33.6–59.0) | |
| UHC | 5.5 (4.3–6.9) | 52.0 (46.8–56.9) | 40.2 (33.7–46.7) |
Fig. 1:
The Kaplan-Meier survival curve for colorectal cancer patients
Fig. 2:
The Kaplan-Meier survival curve for colorectal cancer patients by stage
A confirmed poor prognostic factor was advanced stage at diagnosis (P<0.001), age at diagnosis (P<0.001), tumor grade (P<0.001), type of patient (P<0.001) and healthcare scheme (P=0.003) (Table 2).
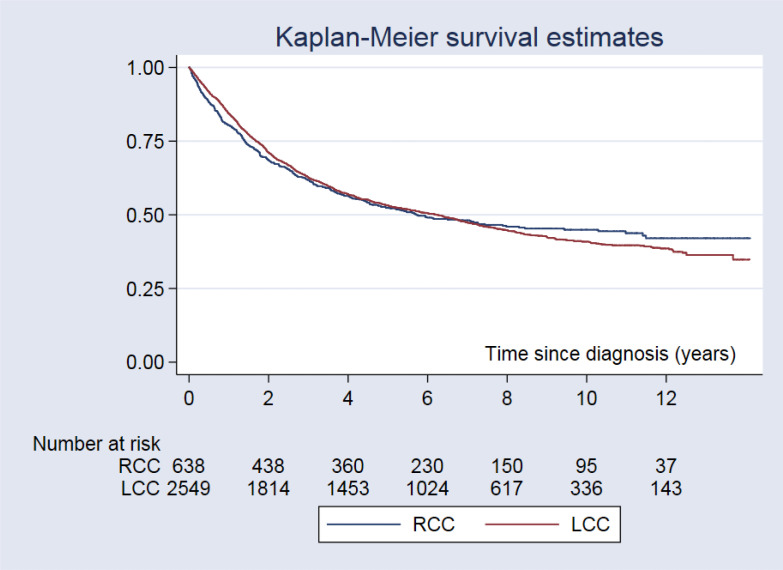
There was no statistical difference detected between RCC and LCC regarding the depth of tumor infiltration (P=0.854). The 5 and 10-year survival rates among CRC patients with RCC were 52.4% and 44.9%, respectively, while those of CRC patients with LCC were 53.1% and 40.9%, respectively (Fig.3).
Fig. 3:
The Kaplan-Meier survival curve for colorectal cancer patients by sided of colorectal cancer patients
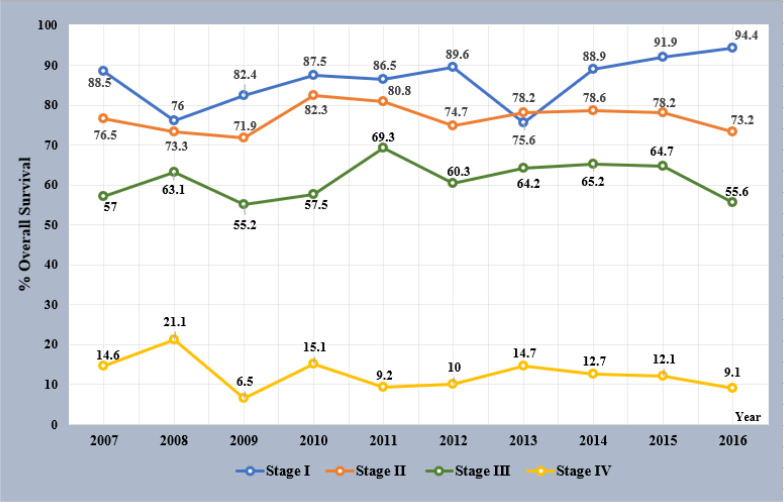
Between 2007 and 2016, the 5-year survival trends for each stage were comparatively better in patients with stage I, II, and III CRCs initially diagnosed in later than in earlier years. Especially in patients with stage I CRC, 5-year OS increased each year from 75.6% to 88.9% to 91.9% to 94.4% in patients diagnosed in 2013, 2014, 2015 and 2016, respectively (Fig.4).
Fig. 4:
Five years OS by staging and year of diagnosed
Using Cox's proportional adjusted hazard ratio, age at diagnosis ≥65 (P=0.001), histological tumor grade (P<0.001), stage at diagnosis (P<0.001), referred patients (P=0.035), and patient insurance coverage (P=0.001) were significant poor predictive factors for OS. Colorectal cancer patient who age at diagnosis ≥65 years showed adjusted hazard ratio (AHR) = 1.49 (95% CI: 1.23–1.80) time to death compared with those patients with age under 65 years. A significant factor for poor prognosis was advanced disease. Thus, CRC patient stage III and stage IV had 2.14 (95% CI: 1.35–3.40) and 11.67 (95% CI: 7.38–18.45) higher risks of death, respectively, compared that of stage I disease. For tumor location, LCC did not confer a significantly higher risk of death (AHR=0.92 times; 95% CI: 0.73–1.16, P=0.485). We found that factors predicting favorable prognosis was coverage under the CSMBS. Patient covered under the SSS and UC experienced AHR were 1.57 (95% CI: 1.03–2.39) and 1.44 (95% CI: 1.18–1.76), respectively, while compared with those covered by the CSMBS. Table 3: shows the estimated hazard ratios for all-cause mortality.
Table 3:
Crude and Adjusted hazard ratios with 95% confidence intervals for death from colorectal cancer using cox regression
| Variables | Simple Cox regression | Multiple Cox regression | ||
|---|---|---|---|---|
|
| ||||
| Crude HR (95%CI) | P-value | Adjusted HR (95%CI) | P-value | |
| Gender | 0.042 | 0.352 | ||
| Male | Ref | Ref | ||
| Female | 0.91 (0.83–0.99) | 10.9 (0.91–1.32) | ||
| Age group | <0.001 | <0.001 | ||
| <65 | Ref | Ref | ||
| ≥65 | 1.28 (1.17–1.40) | 1.49 (1.23–1.80) | ||
| Sided | 0.855 | 0.485 | ||
| RCC | Ref | Ref | ||
| LCC | 1.01 (0.90–1.14) | 0.92 (0.73–1.16) | ||
| Tumor Grade | <0.001 | <0.001 | ||
| Well diff. | Ref | Ref | ||
| Moderately diff. | 1.01 (0.90–1.13) | 1.17 (0.91–1.52) | ||
| Poorly diff. | 1.83 (1.49–2.24) | 2.15 (1.40–3.29) | ||
| Staging | <0.001 | <0.001 | ||
| Stage I | Ref | Ref | ||
| Stage II | 1.57 (1.22–2.04) | 1.68 (1.04–2.7) | ||
| Stage III | 2.45 (1.92–3.11) | 2.14 (2.14–3.40) | ||
| Stage IV | 11.20 (8.83–14.20) | 11.67 (7.38–18.45) | ||
| Type of patients | 0.002 | 0.035 | ||
| New cases | Ref | Ref | ||
| Referred | 1.23 (1.08–1.42) | 1.46 (1.03–2.07) | ||
| Healthcare scheme | 0.006 | <0.001 | ||
| CSMBS | Ref | Ref | ||
| SSS | 1.36 (0.95–1.97) | 1.57 (1.03–2.39) | ||
| UC | 1.31 (1.10–1.55) | 1.44 (1.18–1.76) | ||
Discussion
In our study, the mean 5-year OS rate for all CRC patients was 52.7%. though the total number of patients in this study was quite large (3,402 cases), the 5-year OS rate was lower than in Korea 75.0%, Japan 73.0%, Taiwan 68.7%, USA 63.7% and Norway 59.9% (9–12). However, our 5-year OS rate was comparable with that of Spain (55.5%, Iran (54.0%), United Kingdom (50.0%), and China 43.0% (10,12–14). The reason for this difference might be that our hospital is a medical school and tertiary referral health care center that receives many advanced cancer case referrals from the entire country. In this study, 378 of 3,402 CRC patients (11.1%) were referred cases from other hospitals. In addition, this difference might also be because of the higher number of advanced stage CRC patients in our study. patients with stage IV disease at diagnosis in our study was 31%, which was higher in comparison with other countries such as Taiwan (15.9%), Korea (15.6%) and USA (19.3%), as well as stage III–IV in Spain (8.0%) (6,9,15). Cancer stage was a strong prognostic factor associated with patient survival in our study, which corresponded well with previous studies (1,3,4,9,13,15,16). Advanced disease stage was a poor prognostic factor. In this study, the 5-year OS rate was 12.1% in patients with stage IV CRC, which was comparable with other countries. Several previous studies showed various OS rates in CRC patients with stage IV disease. The 5-year OS rates for stage IV disease were 21.7%, 18.6%, 14.2% and 5.5% in Taiwan, Korea, USA, and Spain, respectively (6,11,14,15)
However, in this study, only 10.1% of patients were stage I, whereas 30.7% of patients presented with stage IV disease at the time of diagnosis. OS rates for patients with stage II and stage III CRC were similar. Thus, this might result in an overestimation of survival outcome.
Over the past 10 years, there has been a trend toward improved 3-year OS in patients with stage I, II, and III CRC in our study. However, 3-year OS in stage IV patients remained unchanged (Fig. 3). Good survival outcome for patients with early stage disease has been improving. CRC screening has been launched in other countries including the USA (1960), Japan (1992), Korea (2004), Taiwan (2004), and Singapore (2009) (17–20). In Thailand, a policy for national CRC screening was launched in 2018, but this has not yet been implemented nationwide. If we can diagnose a high proportion of CRC patients with early stage disease, then we can expect OS rates to increase in the future.
However, the OS rate in our study was higher when compared with some studies in cancer centers in Thailand (21,22). One important reason might be that patients in this study could receive CSMBS healthcare service (47.6%). Patients who were with SSS and UC healthcare services had 1.57 and 1.44 times the death rate, respectively, compared with those who had CSMBS. Patients who had CSMBS might receive more chemotherapeutic agents and targeted therapies such as oxaliplatin, irinotecan, bevacizumab, and cetuximab, as these have been reimbursed by the CSMBS program (12,14,15)
We found that LCC significantly outnumbered RCC cases, with 80.0% of patients having LCC and 20.0% having RCC (P<0.001). This was because we included patients with rectal cancer (C20.0) in the LCC group. It remains unclear whether the prognosis of patients with RCC is different from those with LCC. Although several previous studies showed lower survival in patients with RCC, some studies showed little difference (14,25–29). In this study, we did not find any significant difference. By the end of follow-up, the 5-year OS rate for patients with RCC was comparable to those with LCC. Interestingly, the average age of patients with RCC was higher than for those with LCC (65.5 vs 62.3 years; P<0.001). This age difference might contribute to the non-significant difference in the overall 5-year OS between the two groups in our study. Further research on the prognosis of CRC will improve oncological outcomes for patients with either RCC or LCC. Our study had a number of strengths and limitations. Major strengths included the large number of CRC cases, the long follow-up period, and the low percentage of cases with and unknown stage at diagnosis. Limitations included that this was a retrospective and single-center study. The period of data collection was during 2007 and 2016, which needed to be updated. A multicenter study will need to be conducted in the future.
Conclusion
Longer follow-up data on this specific group of patients in Thailand will provide a clearer picture of the nature and course of CRC and benefit patients seeking successful treatments in the future. Poor prognostic factors were older age at diagnosis and advanced stages of the disease. A favorable prognostic factor was being under the CSMBS health scheme. Access to screening colonoscopies and inclusion in our database should be explored further. These results suggest that comprehensive cancer control strategies and efforts should be based on a continuing examination of cancer statistics.
Acknowledgements
The authors acknowledge the staff members of Ramathibodi Comprehensive Cancer Center, Faculty of Medicine, Ramathibodi Hospital, Mahidol University for their assistance and providing data; Mark Abramovitz, PhD for assistance with preparing the manuscript.
Footnotes
Conflict of interest
The authors declare that they have no conflicts of interest.
References
- 1.Moghimi-Dehkordi B, Safaee A. (2012). An overview of colorectal cancer survival rates and prognosis in Asia. World J Gastrointeat Oncol, 4(4):71–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bray F, Ferlay J, Soerjomataram I, et al. (2018). Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin, 68(6):394–424. [DOI] [PubMed] [Google Scholar]
- 3.Arnold M, Sierra MS, Laversanne M, et al. (2017). Global patterns and trends in colorectal cancer incidence and mortality. Gut, 66(4):683–691. [DOI] [PubMed] [Google Scholar]
- 4.Rawla P, Sunkara T, Barsouk A. (2019). Epidemiology of colorectal cancer: incidence, mortality, survival, and risk factors. Prz Gastroenterol, 14(2):89–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Imsamran W, Pattatang A, Supaattagron P, et al. (2018). Cancer in Thailand Volume IX:2013–2015. [cited 2020 December 15] Available from: www.nci.go.th/th/File_download/Nci%20Cancer%20in%20Thailand%20IX%20OK.pdf
- 6.Hong S, Won YJ, Park YR, et al. (2020). Cancer Statistics in Korea: Incidence, Mortality, Survival, and Prevalence in 2017. Cancer Res Treat, 52(2):335–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kittrongsiri K, Wanitsuwan W, Prechawittayakul P, et al. (2020). Survival analysis of colorectal cancer patients in a Thai hospital-based cancer registry. Expert Rev Gastroenterol Hepatol: 14(4):291–300. [DOI] [PubMed] [Google Scholar]
- 8.Fritz A, Percy C, Jack A, et al. International Classification of Diseases for Oncology: Third Edition. [cited 2020 December 20] Available from: http://www.who.int/standards/classifications/other-classifications/international-classification-of-diseases-for-oncology.
- 9.Tamakoshi A, Nakamura K, Ukawa S, et al. (2017). Characteristics and prognosis of Japanese colorectal cancer patients: The Biobank Japan Project. J Epidemiol, 27(3S):S36–S42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiang CJ, Lo WC, Yang YW, et al. (2016). Incidence and survival of adult cancer patients in Taiwan, 2002–2012. J Formos Med Assoc, 115(12):1076–1088. [DOI] [PubMed] [Google Scholar]
- 11.White A, Joseph D, Rim SH, et al. (2017). Colon cancer survival in the United States by race and stage (2001–2009): Findings from the CONCORD-2 study. Cancer, 123(Supl 24):5014–5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brenner H, Bouvier AM, Foschi R, et al. (2012). Progress in colorectal cancer survival in Europe from the late 1980s to the early 21st century: The EUROCARE study. Int J Cancer, 131(7):1649–58. [DOI] [PubMed] [Google Scholar]
- 13.Stor Z, Blagus R, Tropea A, Biondi A. (2019). Net survival of patients with colorectal cancer: a comparison of two periods. Updates Surg, 71(4): 687–694. [DOI] [PubMed] [Google Scholar]
- 14.AGuero F, Murta-Nascimento C, Gallen M, et al. (2012). Colorectal cancer survival: Result from a hospital-based cancer registry. Rev esp Enferm Dig, 104(11):572–577 [DOI] [PubMed] [Google Scholar]
- 15.Lee CH, Cheng SC, Tung HY, et al. (2018). The Risk Factors Affecting Survival in Colorectal Cancer in Taiwan. Iran J Public Health, 47(4):519–530. [PMC free article] [PubMed] [Google Scholar]
- 16.Hippisley-Cox J, Coupland C. (2017). Development and validation of risk prediction equations to estimate survival in patients with colorectal cancer: cohort study. BMJ, 357:j2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiu HM, Hsu WF, Chang LC, Wu MH. (2017). Colorectal Cancer Screening in Asia. Curr Gastroenterol Rep, 19(10):47. [DOI] [PubMed] [Google Scholar]
- 18.Doubeni CA. (2014). The impact of colorectal cancer screening on the US population: Is it time to celebrate?: CRC Screening and Incidence. Cancer, 120(8):2810–2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zavoral M, Suchanek S, Zavad F, et al. (2009). Colorectal cancer screening in Europe. World J Gastroenterol, 15(47):5907–5915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang YW, Chen HH, Wu MS, Chiu HM. (2018). Current status and future challenge of population-based organized colorectal cancer screening: Lesson from the first decade of Taiwanese program. J Formos Med Assoc, 117(5):358–364. [DOI] [PubMed] [Google Scholar]
- 21.Phimha S, Promthet S, Suwanrungruang K, et al. (2019). Health Insurance and Colorectal Cancer Survival in Khon Kaen, Thailand. Asian Pac J Cancer Prev, 20(6):1797–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Phiphatpatthamaamphan K, Vilaichone RK. (2016). Colorectal Cancer in the Central Region of Thailand. Asian Pac J Cancer Prev, 17(7):3647–3650. [PubMed] [Google Scholar]
- 23.Chindaprasirt J, Sookprasert A, Wirasorn K, et al. (2012). Cost of Colorectal Cancer Care in Hospitalized Patients of Thailand. J Med Assoc Thai, 95(Suppl 7):S196–200. [PubMed] [Google Scholar]
- 24.Tangcharoensathien V, Patcharanarumol W, Suwanwela W, et al. (2020). Defining the Benefit Package of Thailand Universal Coverage Scheme: From Pragmatism to Sophistication. Int J Health Policy Manag, 9(4), 133–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meguid RA, Slidell MB, Wolfgang CL, et al. (2008). Is There a Difference in Survival Between Right-Versus Left-Sided Colon Cancers? Ann Surg Oncol, 15(9):2388–2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yahagi M, Okabayashi K, Hasegawa H, et al. (2016). The Worse Prognosis of Right-Sided Compared with Left-Silded Colon Cancers: a Systematic Review and Mete-analysis. J Gastrointest Surg, 20(3):648–655. [DOI] [PubMed] [Google Scholar]
- 27.Nitsche U, Stogbauer F, Spath C, et al. (2016). Right-Sided Colon Cancer as a Distinct Histopathological Subtype with Reduced Prognosis. Dig Surg, 33(2):157–163. [DOI] [PubMed] [Google Scholar]
- 28.Warschkow R, Sulz MC, Marti L, et al. (2016). Better survival in right-sided versus left-sided stage I–III colon cancer patients. BMC Cancer, 16:554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Narayanan S, Gabriel E, Attwood K, et al. (2018). Association of Clinicopathologic and Molecular Markers on Stage-specific Survival of Right Versus Left Colon Cancer. Clin Colorectal Cancer, 17(4):e671–e678. [DOI] [PMC free article] [PubMed] [Google Scholar]