Abstract
Background:
In Indonesia, around 400 health workers died due to Covid-19 between June–July 2021, therefore the health workers need to be given further immunity. Health workers were among the first to get a booster shoot. However, they may experience side effects after vaccination. We aimed to describe side effects of Moderna vaccine as a booster in health workers.
Methods:
A cross sectional study was conducted on health workers who received mRNA Covid-19 vaccine booster (Moderna) at Sulianti Saroso Infectious Disease Hospital, Indonesia and had filled the questionnaire assessing side effects form. We associated the form of the questionnaire assessing side effects from the originating source of hospital immunization unit in September 2021.
Results:
A total of 101 health workers who received mRNA Covid-19 vaccine booster in Jul–Aug 2021 were included. Most of health worker experienced more than 3 side effects. The side effects were sore arm (100%); chills (72%); fatigue (57%); headache (53%) and fever (51%), other symptoms (28%). The side effects mostly happened a day of receiving a booster shot (61.4%). There was no association between age, gender, comorbid to amount of side effects (P>0.05).
Conclusion:
Since the public must fulfil the immunization program during pandemic, it is the responsibility of the healthcare provider to inform about the potential side effects and benefits of a new Covid-19 vaccine.
Keywords: Moderna, Booster vaccine, Side effects, Indonesia
Introduction
Second Covid-19 pandemic wave in June–July 2021 and new variants of the virus kept emerging. It was said mutation of new variant keep advancing, more infectious and causing more severe stage of disease (1–3). Vaccination program for health worker has been done with non mRNA vaccines as a first and second shot. In fact, 1569 health workers died due to Covid-19, including nearly 400 between June and July 2021, therefore the health workers need to be given further immunity with vaccination strategy (4).
Ministry of health of Indonesia recommended the booster shot by mRNA Covid-19 vaccine (Moderna) to health workers in July 2021. Moderna as mRNA vaccine was determined as its effectivity to prevent infection reach 94.1% (5). It was reported in earlier study, adverse event of Moderna such as pain, oedema, redness, or rash around injection site or systemic symptom like fever and chills (6). These consequences could disturb the workers and caused missed work (6).
We aimed to describe side effects of Moderna vaccine as a booster in health workers and finding out its correlation to various variables.
Methods
We conducted a cross sectional study. The inclusion criteria were health workers of Sulianti Saroso Infectious Disease Hospital, Indonesia who received mRNA Covid-19 vaccine booster (Moderna) and had filled the questionnaire assessing the side effects form. Moderna vaccine was administered as a booster Covid-19 vaccine in July–September 2021. We associated the form of the questionnaire assessing the side effects from the originating source of hospital immunization unit.
Distribution of demography data and side effects were presented in percentages. Fisher's exact test was performed to analyse whether there was a significant relationship between characteristics of subjects (gender, age, comorbidities) and amount of side effects of Moderna vaccine. All statistical analyses were conducted with SPSS version 20 (IBM Corp., Armonk, NY, USA).
Ethics approval
All patients’ data were pseudonymised through coding of personal identifiers. This study was approved by the ethical committee (Ethics no. 29/XXXVIII.10/IX/2021).
Result
A total of 101 health workers received mRNA Covid-19 vaccine booster. They were 17–65 years old, included doctor, nurse/midwifes, and other health workers. Less than half of health workers had history of Covid-19 infection and comorbid disease. Table 1 shows demography data and amount of side effects.
Table 1:
Demography data and amount of side effects
| Variable | Frequency (n=101) | Percentage (%) |
|---|---|---|
| Age (yr) | ||
| 17–25 | 21 | 20.8 |
| 26–35 | 25 | 24.8 |
| 36–45 | 35 | 34.7 |
| 46–55 | 19 | 18.8 |
| 56–65 | 1 | 1.0 |
| Gender | ||
| Male | 33 | 32.7 |
| Female | 68 | 67.3 |
| Profession | ||
| Doctor | 20 | 19.8 |
| Nurse/Midwife | 36 | 35.6 |
| Other Health Worker | 45 | 44.6 |
| History of Covid-19 Infection | 29 | 28.7 |
| Comorbidities | 11 | 10.9 |
| Amount of side effects | ||
| < 3 side effects | 11 | 10.9 |
| ≥ 3 side effects | 90 | 89.1 |
| Onset of side effects | ||
| Day 0 * | 33 | 32.7 |
| Day 1 | 62 | 61.4 |
| Day 2 | 6 | 5.9 |
Notes:
Day 0: the day of receiving a booster shot
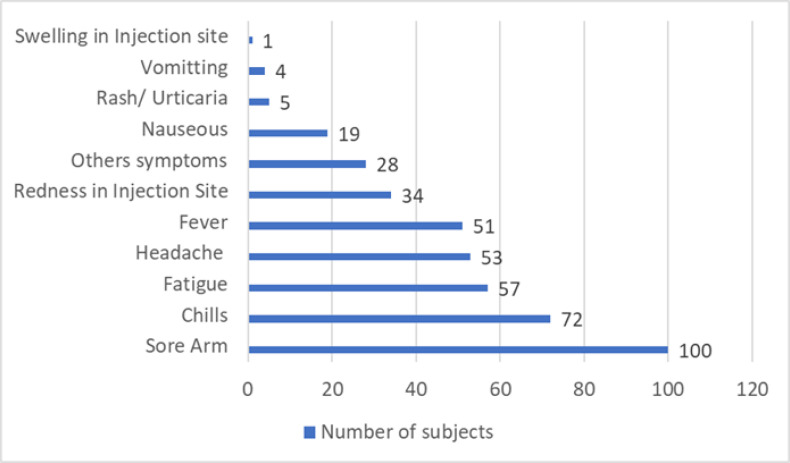
All participant (100%) experienced a sore arm after being injected with Moderna as booster vaccine. Most of participant (89.1%) had more than three side effects, including a sore arm. Meanwhile, 34.7% of participants experienced 3 side effects in the same time such as arm pain, rash and swelling at the injection site. Side effects mostly happened one day after the day of injection (61.4%). Fig. 1 describes the distributions of side effects among health workers who received mRNA Covid-19 vaccine booster (Moderna).
Fig 1:
Distributions of Symptoms among health workers who received mRNA Covid-19 vaccine booster (Moderna). Other symptoms: sleep disruption, less appetite and diarrhea
We analysed association between age, gender, comorbid disease to amount of side effects. We found no significance among the variables. Table 2 shows the association between age, gender, and comorbid disease to amount of side effects.
Table 2:
Association between ages, gender, comorbidities to amount of side effects
| Variable | Amount of Side effects | p | |||
|---|---|---|---|---|---|
|
| |||||
| < 3 side effects | ≥ 3 side effects | ||||
| n | % | n | % | ||
| Age in years | |||||
| 18 - < 37 | 4 | 8.2 | 45 | 91.6 | 0.593 |
| ≥ 37 | 7 | 13.5 | 45 | 86.5 | |
| Gender | |||||
| Male | 4 | 12.1 | 29 | 87.9 | 0.746 |
| Female | 7 | 10.3 | 61 | 89.7 | |
| Comorbidities | |||||
| Yes | 2 | 18.2 | 9 | 81.8 | 0.342 |
| No | 9 | 10.0 | 81 | 90.0 | |
Discussion
Moderna was recommended by our government as a vaccine booster since its effectivity to prevent Covid-19 infection. Moderna had been granted emergency use authorization for individual more than 18 years old. In many clinical trials, the efficacy of this vaccine concerning the preventing of Covid-19 was more than 90% (7–9). Participants who received a booster at least 5 months after a second dose of BNT162b2 had 90% lower mortality due to Covid-19 than participants who did not receive a booster (7). In a randomized control trial of 30,420 volunteers who received either mRNA-1273 vaccine or placebo, vaccine efficacy was 94.1% (95% CI, 89.3 to 96.8%; P<0.001) after two doses (9). Health workers were among the first to get a booster shoot. As vaccine booster, it was needed once 0.5 ml (5–7). In this study, most of health workers were 36–45 years old (34.7%), followed by 25–35 years old (24.8%) and mostly women (67.3%).
Adverse Event Following Immunization (AEFI) is a medical incident correlated to vaccination including adverse reaction (side effects), unrelated health problems following vaccination and health problems with an unknown cause (10). Adverse Event Following Immunization can be divided to two categories; local and systemic. Local side effects include pain, tenderness, hyperaemic and oedema around injection site. Systemic symptoms can be fever, myalgia, fatigue, syncope and anaphylactic shock. Side effects can occur in 1 month after getting a shot. Observation at least 30 minutes after vaccination must be done to ascertain deadly effect such anaphylactic shock. (7,9,11,12).
In this study, all participant (100%) experienced a sore arm after being administered Moderna (11). Injection site reactions are common with most vaccines and attributed to injection technique or immune or inflammatory responses to the antigens or excipients injected. Especially, mRNA vaccines require mRNA translation to produce endogenously produced viral spike proteins by which an immune response is generated. Meanwhile, conventional vaccines (non mRNA) where the immunizing agent is a killed or attenuated component of the infectious agent. Injection site reactions typically occur within a day or two of vaccination and resolve within a day or two although some can be more prolonged (13,14). Meanwhile, the delayed onset reactions after mRNA (Moderna) vaccine assumed that the specific polyethylene glycol (1,2-dimyristoyl-racglycero-3-methoxypolyethylene glycol-2000 may cause more inflammation or be more immunogenic than the compound in other mRNA vaccine (Pfizer/BioNTech) (15).
In this study, most of our health workers experienced more than three side effects after a day after getting a shot. There were 34.7% of participants that experienced 3 side effects in the same time such as arm pain, rash and swelling at the injection site. In contrast to our study, some studies found delayed onset AEFI were more common in their respondents (15–17). Delayed-onset, injection site pruritic rashes after mRNA-1273 (Moderna) SARS-CoV-2 vaccine administration, lasting up to 1 week, occur commonly in females, did not lead to serious sequela (15). Two other studies discovered similar finding in their studies (16,17). This difference was based on that our study was a booster vaccine while their observation was hold after primary and secondary vaccination.
Our study stated that 89.1% of health workers had no comorbidities. Subjects with uncontrolled comorbid disease could not receive vaccine as its inability to form antibody efficiently. In Indonesia, these subjects should have their condition checked to get letter of permission to receive vaccine.
Some studies in Japan and United States explained younger age and women was the risk factor to AEFI, though in our study we found no association between age and gender to amounts of side effects (15,18). A limitation of study was the data collected from the questionnaire assessing of side effects that might impact on subjectivity.
Conclusion
Since the public must fulfil the immunization program during pandemic, we highlighted that it is the responsibility of the healthcare provider to inform about the potential side effects and benefits of a new Covid-19 vaccine.
Journalism Ethics considerations
Ethical issues (Including plagiarism, informed consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc.) have been completely observed by the authors.
Acknowledgements
We would like to thank Sulianti Saroso Infectious Disease Hospital to facilitate the study. We would also like to acknowledge all data collectors, supervisor and participants without whom this research would not have been realized. The authors received no funding for the research, authorship and/or publication of this article.
Footnotes
Conflict of Interest
The authors declare that there is no conflict of interests.
References
- 1.Ershkov SV, Rachinskaya A. (2021). A new approximation of mean-time trends for the second wave of COVID-19 pandemic evolving in key six countries. Nonlinear Dyn, 106(2): 1433–52. doi: 10.1007/s11071-021-06244-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hendra Permana Y, Sahadewo GA. (2020). A Tale of Two Peaks: Predicting the Peak of Covid-19 Infections in Indonesia. Preprint, doi: 10.13140/RG.2.2.29574.80966 [DOI]
- 3.Nugraha DNS, Fridiana SW, Listiani N, et al. (2021). A Critical Discourse Analysis to Prevent the Second Wave of COVID-19 Pandemic in Indonesia. Rev Int Geogr Educ Online, 11(5):906–12. [Google Scholar]
- 4.Widianto S. (2021). Indonesia considers COVID-19 booster shots for wider use -govt official. Reuters. Available from: https://www.reuters.com/business/healthcare-pharmaceuticals [Google Scholar]
- 5.Barda N, Dagan N, Cohen C, et al. (2021). Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: an observational study. Lancet, 398(10316):2093–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen DA, Greenberg P, Formanowski B, Parikh PD. (2022). Are COVID-19 mRNA vaccine side effects severe enough to cause missed work? Cross-sectional study of health care-associated workers. Medicine (Baltimore), 101(7):e28839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arbel R, Hammerman A, Sergienko R, et al. (2021). BNT162b2 Vaccine Booster and Mortality Due to Covid-19. N Engl J Med, 385(26):2413–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Riad A, Schünemann H, Attia S, et al. (2021). Covid-19 vaccines safety tracking (Covast): Protocol of a multi-center prospective cohort study for active surveillance of covid-19 vaccines’ side effects. Int J Environ Res Public Health, 18(15): 7859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baden LR, el Sahly HM, Essink B, et al. (2021). Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med, 384(5):403–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for diseases control and prevention (2021). Vaccine safety. Available from: https://www.cdc.gov/vaccinesafety/ensuringsafety/sideeffects/index.html
- 11.Rutkowski K, Mirakian R, Till S, Rutkowski R, Wagner A. (2021). Adverse reactions to COVID-19 vaccines: A practical approach. Clin Exp Allergy, 51(6):770–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Idayanti N, Sutiningsih D, Hadi M, Abrori I. (2021). Adverse effects Associated with Third-Booster COVID-19 Vaccine (Heterologous Vaccines by Sinovac-Moderna) among Health Care Workers. Int J Engl Lang Lit Stud, 6(6): 250–2. [Google Scholar]
- 13.Ramos CL, Kelso JM. (2021). Clinical Communications “COVID Arm”: Very delayed large injection site reactions to mRNA COVID-19 vaccines. J Allergy Clin Immunol Pract, 9:2480–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Annisa D. (2021). Current situation of Corona-virus Disease (COVID-19) 5 Aug. 2021. Ministry of health of Indonesia. Available from: https://infeksiemerging.kemkes.go.id/laporan-harian-covid-19 [Google Scholar]
- 15.Jacobson MA, Zakaria A, Maung Z, et al. (2022). Incidence and Characteristics of Delayed Injection Site Reaction to the mRNA-1273 Severe Acute Respiratory Syndrome Corona-virus 2 (SARS-CoV-2) Vaccine (Moderna) in a Cohort of Hospital Employees. Clin Infect Dis, 74(4):591–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Papadimitriou I, Bakirtzi K, Sotiriou E, Vakirlis E, Hatzibougias D, Ioannides D. (2022). Delayed localized hypersensitivity reactions to COVID-19 mRNA vaccines: a 6-month retrospective study. Clin Exp Dermatol, 47(1): 157–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wei N, Fishman M, Wattenberg D, Gordon M, Lebwohl M. (2021). “COVID arm”: A reaction to the Moderna vaccine. JAAD Case Rep, 10:92–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suehiro M, Okubo S, Nakajima K, et al. (2022). Adverse events following COVID-19 virus vaccination in Japanese young population: The first cross-sectional study conducted by a questionnaire survey after the first-time-injection. Arch Clin Biomed Res, 6(1): 9–29. [Google Scholar]