Abstract
TP53 mutation is the most frequent genetic event in head and neck squamous cell carcinoma (HNSCC), found in more than 80% of patients with human papillomavirus–negative disease. As mutations in the TP53 gene are associated with worse outcomes in HNSCC, novel therapeutic approaches are needed for patients with TP53-mutated tumors. The National Cancer Institute sponsored a Clinical Trials Planning Meeting to address the issues of identifying and developing clinical trials for patients with TP53 mutations. Subcommittees, or breakout groups, were tasked with developing clinical studies in both the locally advanced and recurrent and/or metastatic (R/M) disease settings as well as considering signal-seeking trial designs. A fourth breakout group was focused on identifying and standardizing biomarker integration into trial design; this information was provided to the other breakout groups prior to the meeting to aid in study development. A total of 4 concepts were prioritized to move forward for further development and implementation. This article summarizes the proceedings of the Clinical Trials Planning Meeting with the goal of developing clinical trials for patients with TP53-mutant HNSCC that can be conducted within the National Clinical Trials Network.
Worldwide, more than 550 000 people per year are diagnosed with head and neck squamous cell carcinoma (HNSCC). The principal risk factors include tobacco and alcohol use and human papillomavirus (HPV) infection (1). HPV-associated and HPV-unassociated (HPV-) HNSCC are clinically distinct entities that differ in pathophysiology and prognosis (2). Whereas it is rare in HPV-associated HNSCC, TP53 mutation is the most common genomic alteration in HPV- HNSCC, occurring in approximately 50%-85% of cases (3,4). As with many other types of cancer, TP53 mutation is an independent poor prognostic factor in HNSCC (5). If TP53 mutations are present, about 50% of patients with locally advanced disease and nearly all patients with metastatic disease will die of their illness.
In part, poor outcomes in patients with TP53-mutant HNSCC may be associated with suboptimal response to standard treatments, which include radiation and platinum-based chemotherapy. Typically, TP53-mutant tumors are associated with radioresistance, with one potential mechanism involving impaired senescence (6-8). Data on response to cisplatin in TP53-mutant HNSCC vary with evidence supporting both sensitization (9–11) and resistance (12–14) patterns. The effect of TP53 mutation on cisplatin sensitivity likely varies with the phenotype produced by a particular mutation or frequency of TP53 polymorphisms (eg, R72/R72 correlates with response to cisplatin) in the context of other coexisting genomic aberrations in the tumor, as well as other host factors.
Genomics and Preclinical Data
Unlike oncogenes, no hot spot mutations occur throughout the TP53 tumor suppressor gene, and various classifications have been explored to infer functional relevance from these genomic alterations. Poeta et al., in a seminal article (5), described disruptive vs nondisruptive alterations based on protein structure, wherein disruptive mutations occur in the L2 and L3 binding domains of the p53 protein and replace an amino acid of one polarity with an amino acid of another polarity. Nondisruptive mutations occur outside L2 and L3 or result in replacement of an amino acid of the same polarity. This classification scheme was compared with other computational methods in a double-blinded analysis, where investigators were given TP53 mutations and asked to classify them as disruptive or nondisruptive by methods of their choice (15). A total of 15 methods were used to divide patients into classes, including protein function as a predictive method, WAF 1 transactivation where less than 10% and less than 20% wild-type expression were each used as cutoffs to define the disruptive class (16), and in silico predictive methods rules devised by Poeta and including splice sites (Poeta rules + Splice). When Eastern Cooperative Oncology Group (ECOG) statisticians compared overall survival and progression-free survival (PFS) of the disruptive and nondisruptive groups, none of the methods were better than the Poeta rules + Splice for classifying HNSCC survival. Neskey et al. (17) developed an alternative TP53 computational approach termed Evolutionary Action Score of P53 to stratify patients with tumors harboring TP53 mutations as high or low risk and validated this system in in vivo and in vitro models. Patients with high-risk TP53 mutations had the poorest survival outcomes and the shortest time to the development of distant metastases in the The Cancer Genome Atlas HNSCC cohort.
Although advances have been made using targeted agents in cancers with a high prevalence of oncogene aberrations such as gain-of-function oncogenic mutations or gene rearrangements, there has been limited improvement in outcomes for malignancies defined by loss of function in tumor suppressor genes. The most promising preclinical and early clinical data have emerged for HNSCC through approaches involving synthetic lethality. TP53-mutated HNSCC cells rely on G2-M checkpoint for DNA-damage repair and survival (18). Wee-1 is one such regulatory nuclear kinase that prevents entry into mitosis in response to DNA damage. Preclinical models support the inhibition of such key regulatory genes such as wee-1, which leads to premature mitosis, by disabling the G2-M checkpoint and resulting in mitotic catastrophe in cells deficient of p53 signaling (19). Moser et al. (20) used functional RNA kinome drug screens on TP53-mutant murine and patient-derived HNSCC cell lines and identified AZD-1775 (adavosertib), which showed evidence of tumor reduction in xenograft-bearing mice, augmented by the concurrent administration of cisplatin chemotherapy. Similar observations were reported by Osman et al. in HNSCC cell lines (21).
Prospective Clinical Data
Early phase clinical data with the use of the wee-1 inhibitor adavosertib in HNSCC have been reported by Mendez and colleagues from a phase I study (22), wherein adavosertib was given orally, in combination with cisplatin and docetaxel in the neoadjuvant setting in patients with borderline resectable or unresectable disease. The maximum tolerated dose was established at 150 mg orally twice daily; the dose-limiting toxicity was grade 3 diarrhea observed in 3 patients. Among 10 evaluable patients, objective response by Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 were observed in 5, and among 7 patients who went on to surgical resection, 6 pathologic responses were noted; overall 9 patients had either a RECIST or a pathologic response after neoadjuvant therapy, and these were observed in all 3 dose levels explored. Next-generation sequencing revealed TP53 alterations in 5 of the 9 patients with either a RECIST or a pathologic response. The safety and preliminary efficacy results demonstrated in this study highlighted the potential for further investigation, particularly in the TP53-altered population, with specific attention to gastrointestinal toxicities. Adavosertib is being investigated in locally advanced HNSCC in combination with cisplatin and radiation (NCT03028766 and NCT02585973) and is under evaluation in combination with chemotherapy (carboplatin with paclitaxel and gemcitabine), immune checkpoint inhibitor (durvalumab), and poly (ADP-ribose) polymerase (PARP) inhibitor (olaparib). There is ongoing investigation of wee-1 inhibition in phase I clinical studies for advanced solid tumors (NCT04158336, NCT03968653, NCT04768868).
There is a paucity of TP53-directed studies in the HNSCC population. The ongoing ECOG study EA3132 (NCT02734537) is the first prospective trial testing the role of cisplatin with postoperative radiation by utilizing disruptive TP53 mutations as an integral biomarker. Using the Poeta rules + splice method, patients are stratified by TP53 mutational status (disruptive, nondisruptive, and wild type) and randomly assigned to postoperative radiation alone or with concurrent cisplatin 40 mg/m2 weekly. The rationale for this study is that sensitivity to the known radiosensitizer cisplatin is preserved or enhanced in the presence of disruptive TP53 mutation, providing a possible avenue to overcome radioresistance of TP53-mutant cells and thus mitigate the increased risk of relapse associated with TP53 mutation (5,9,23).
Description of Clinical Trials Planning Meeting (CTPM) and Goals
The objectives of the CTPM were to 1) identify promising synthetic lethal interaction partners and therapeutic combinations for randomized phase II trials in TP53-mutant HPV-negative HNSCC that are suitable to be conducted in a cooperative group setting, 2) design a phase II trial for stage III and IV HPV-negative HNSCC that employs genomic selection or stratification, 3) design a randomized phase II trial employing a novel combination in R/M HNSCC, and 4) advance the infrastructure for a generalizable approach to mutation calling (ie, process of identifying variants different in sequencing data from a reference sequence).
Each of 4 CTPM breakout groups comprised 2 chairs and 4-6 panel members. These groups met virtually from April to December 2020 to develop clinical trial designs based on the meeting goals and agenda. The CTPM meeting was held virtually on January 21-22, 2021, and gathered more than 60 experts in HNSCC from different disciplines (medical oncologists, radiation oncologists, surgical oncologists, biostatisticians, translational researchers, and industry partners) from across the United States and Canada to finalize the proposed study designs and prioritize development. The participants reviewed a series of scientific presentations including an extensive overview of the TP53 landscape, with key genomic and informatics updates, prior and ongoing studies involving synthetic lethality and various therapeutic agents leveraging such interactions, and algorithms to determine functionally relevant TP53 mutations. A discussion of TP53-mutated cancers and treatment resistance in minority populations was also highlighted. This article provides an overview of the trials proposed in the locally advanced and R/M settings as well as describes a focused effort on identifying and incorporating biomarkers relevant to TP53-mutated HNSCC in trials to improve survival and quality of life in these patients.
Breakout Group #1: Previously Untreated Locally Advanced HNSCC (PULA): Co-Chairs Drs Heath Skinner and Ranee Mehra
The goal of the locally advanced group was to evaluate clinical trial concepts relevant to this CPTM for treatment paradigms in the curative setting and benefit from the interaction with statistical support as well as a discussion with the biomarker committee. In the process of evaluating feasible trial designs, 2 of 4 proposed clinical trial concepts were deemed unsuitable for further development because of a paucity of safety data that preclude the use of these agents in previously untreated patients and absence of consensus over treatment selection based on TP53 status in the PULA setting. The 2 concepts put forward by this breakout group are described below and were presented at the CTPM.
PULA Concept #1
Phase II trial data have shown that neoadjuvant immunotherapy is feasible and effective in inducing a polyclonal T-cell antitumor response (24,25). Capitalizing on the importance of the G2-M cell cycle checkpoint among tumor cells harboring dysfunctional TP53 (20), this concept sought to combine adavosertib with the programmed death-ligand 1 (PD-L1) inhibitor durvalumab in the neoadjuvant setting for patients planned for surgical resections of their primary tumors. This rationale is further supported by data from Friedman et al. (26), which illustrated that wee-1 inhibition sensitizes TP53-mutant oral cavity tumor cells to granzyme B and tumor necrosis factor α–dependent killing by T cells. Although the scientific rationale was viewed favorably by committee members and the larger CPTM group, challenges were identified including adequate safety data and feasibility of obtaining a wee-1 inhibitor in the clinical setting, as well as the adequacy of tissue requirements for correlative studies. It was felt that the cooperative group mechanism was not the optimal forum for obtaining the needed preliminary data.
PULA Concept #2
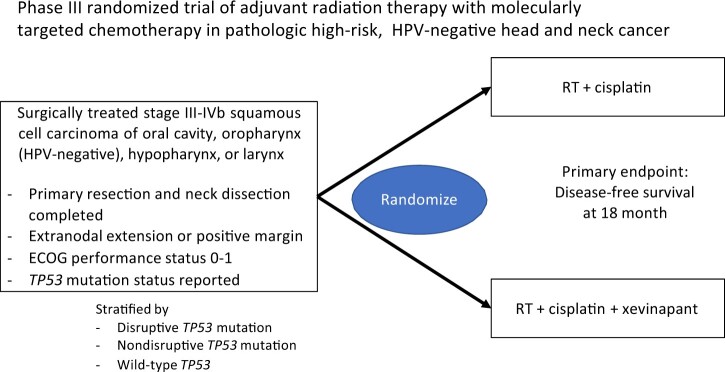
Another proposed clinical trial concept was based on the second mitochondrial-derived activator of caspases (SMAC) mimetic xevinapant (previously Debio1143). This class of agents acts to antagonize the inhibitor of apoptosis proteins XIAP, cIAP1, and cIAP2 (27), with a goal of restoring apoptosis in tumor cells resistant to this mode of cell death. SMAC mimetics have been extensively studied in HNSCC with demonstrated antitumor effects alone (28,29) or in combination with radiation (30–33). As tumors harboring TP53 mutations are generally more resistant to apoptosis (34) and additional genetic alterations commonly found in HNSCC have been shown to sensitize cells to the effects of SMAC mimetics (31,35), these agents seem to be an ideal avenue for clinical trial development. A recent randomized phase II clinical trial examining the addition of xevinapant to concurrent chemoradiation in HNSCC noted a greater than 20% increase in locoregional control compared with placebo, with an acceptable toxicity profile (36). A phase III trial of xevinapant with chemoradiation vs chemoradiation is ongoing (NCT04459715) and is currently enrolling patients treated in the nonsurgical, definitive radiation setting. Based on these data, a randomized trial in HPV-negative HNSCC patients treated with surgery and found to have high-risk features (extranodal extension or positive margins) in the resection specimen was proposed (Figure 1). Patients will be treated with postoperative radiation and concurrent cisplatin with or without xevinapant, with a primary endpoint of disease-free survival at 18 months and total goal accrual of 180 patients. Patients will be stratified based on stage, site, and performance status as well as TP53 status (disruptive vs nondisruptive vs wild type) (5,6,15). In addition to standard monitoring, circulating tumor DNA (Signatera) will be evaluated prior to and after treatment to potentially validate this assay as a biomarker of residual disease.
Figure 1.
Previously Untreated Locally Advanced Concept #2. ECOG = Eastern Cooperative Oncology Group; HPV = human papilloma virus; RT = radiation therapy.
Breakout Group #2: R/M HNSCC: Co-Chairs Drs Cristina Rodriguez and Hyunseok Kang
Breakout Group #2 was tasked with the design of a large clinical trial in the R/M setting based on biology of TP53-mutated HNSCC. First, the current treatment landscape of R/M HNSCC was surveyed and unmet clinical needs identified. After pembrolizumab was approved in first-line treatment of R/M HNSCC, avenues of scientific investigation included establishing treatment options in the second-line setting and improving the efficacy of single agent pembrolizumab in the first-line setting where overall response rates (ORRs) range from 10% to 19% in a biomarker-enriched population (37). The group also prioritized the need to explore immunotherapy combinations with better tolerability in the first-line setting. During the development of these clinical trial concepts, this group focused on designs in various treatment lines, but concepts in locally recurrent disease amenable to surgical salvage or re-irradiation were excluded. Strategies of patient selection, particularly focused on methods of identification of TP53 alterations and their impact on trial accrual, were discussed. Study design elements relating to the use of TP53 alterations as integrated or integral biomarkers were reviewed. The merit of each proposed trial design was evaluated based on its relevance for the current state of clinical trials in R/M HNSCC, potential changes in therapeutic standards that could be brought about by the study, feasibility of the trial conduct through the National Cancer Institute’s (NCI’s) Clinical Trials Network (NCTN), and competitive enrollment by other studies for a similar patient population. At the conclusion of the R/M breakout group meetings, 3 proposals were finalized: 1 in the first-line setting and 2 in the second-line population previously treated with immune checkpoint inhibitor.
R/M Concept #1
There was strong agreement within the group on proposing a first-line R/M concept wherein wee-1 inhibition would be combined with pembrolizumab, the current standard of care for combined positive score 1 or greater patients (Figure 2). Published preclinical evidence supports the synergy between wee-1 inhibition and immune checkpoint inhibitors through avenues involving cell cycle arrest and subsequent enhancement of cytotoxic T-cell and antibody-dependent cell cytotoxicity–mediated tumor cell death (26,38). Prospective clinical data have been reported in abstract form on the combination of adavosertib and the PD-L1 inhibitor durvalumab, and a recommended phase II dose has been determined (NCT02617277). This trial plans to enroll patients with R/M mucosal squamous cell carcinomas of the oral cavity, oropharynx, larynx, or hypopharynx who have not previously been treated with immune checkpoint inhibitors and have not received systemic therapy in the R/M setting. Additional eligibility criteria include a TP53 alteration, based on next-generation sequencing on archival tumor tissue. Patients must have a combined positive score of 1 or greater, and those with p16-positive oropharynx squamous cell carcinoma will be excluded. Patients will be randomly assigned 1:1 to either adavosertib (or alternative wee-1 inhibitors, several of which are in various earlier stages of clinical development and are detailed in Table 1) and a PD-1 inhibitor vs the PD-1 inhibitor with placebo. The primary endpoint would be PFS, with secondary endpoints of overall survival, ORR, and patient-reported quality of life. Because of limited clinical data for the wee-1 and PD-1 inhibitor combination, the first 6 patients enrolled in each arm would represent a safety lead in group. Correlative blood samples will be obtained for cell-free DNA testing at baseline and after 3 cycles of treatment. TP53 mutation subgroups (eg, disruptive vs nondisruptive) will not be used as a stratifying factor but can be explored in the post hoc analysis. Based on an expected PFS of 30% in the control arm and a 10% rate of patient loss, a sample size of 132 would yield an 81% power to detect a PFS of 45% in the experimental arm (corresponding to a hazard ratio = 0.66) using a 1-sided 0.10-level log-rank test. An estimate of 165 patients will be screened at an accrual rate of 6 eligible patients per month, for a total accrual period of 20 months. Trial completion is estimated in 2.7 years from activation.
Figure 2.
Recurrent/metastatic (R/M) Concept #1. CPS = combined proportion score; HNSCC = head and neck squamous cell carcinoma; HPV = humanpapilomavirus; PD-L = programmed death-ligand.
Table 1.
Wee-1 inhibitors in development
| Compound | Company | Current investigation |
|---|---|---|
| ZN-c3 | Zentalis | Phase I (NCT04158336, NCT04516447), I/II (NCT04833582) |
| Debio 0123 | Debiopharm | Phase I (NCT05100975) |
| IMP7068 | Impact Therapeutics | Phase I (NCT04768868) |
| NUV 569 | Nuvation Bio | Preclinical |
| SDR 7995, SDR 7778 | Schrodinger | Preclinical |
| PD0166285 | Pfizer | Preclinical |
R/M Concept #2
Encouraging clinical activity of the combination of adavosertib with cisplatin and docetaxel in locally advanced HNSCC by Mendez et al. (22) was the rationale for a proposed randomized phase II clinical trial for second-line treatment of R/M HNSCC. In this concept, R/M HNSCC patients who have progressed or are ineligible to receive standard first-line immune checkpoint inhibitor therapy will be eligible and will be randomly assigned to a wee-1 inhibitor or placebo in combination with weekly cisplatin 25 mg/m2 and docetaxel 35 mg/m2 (Figure 3). Substitution of cisplatin with carboplatin will not be allowed given the concern for overlapping toxicity of myelosuppression. Weekly chemotherapy will be continued up to 18 weeks, disease progression, or emergence of intolerable toxicity. There was group consensus that patients who have progressed on platinum chemotherapy within 6 months of study entry should be excluded. Enrollment will not be limited to TP53-mutated R/M HNSCC to explore the effects of wee-1 inhibition in TP53 wild-type patients with dysfunctional p53 (such as patients with HPV-positive HNSCC, where E6 oncoprotein impairs p53 function), but the random assignment will be stratified by TP53 mutation status (altered vs wild type). The trial assumes a 6-month PFS of 30% in the control arm and hypothesizes that the wee-1 inhibition combination would achieve a 6-month PFS of 45%, which corresponds to a hazard ratio of 0.66. The target enrollment is 114 TP53-mutant R/M HNSCC patients, which will yield an 81% power with a 1-sided alpha of 0.10. As 80% of R/M HNSCC patients are estimated to harbor TP53 mutation, the trial would enroll 156 patients accounting for a 10% dropout rate. One futility interim analysis was incorporated to occur after 54 PFS events for TP53-mutant patients. Exploratory endpoints include TP53-mutation analysis via the Poeta classification, as well as serum studies using circulating tumor DNA. Biomarker analysis for these correlative endpoints is expected to use existing infrastructure through the NCI, such as that currently in place for EA3132, as well as a potential partnership with an industry sponsor. The trial is expected to introduce a new standard of care for patients who fail first-line immune checkpoint inhibitor–based therapy or are ineligible for immune checkpoint inhibitor–based therapy.
Figure 3.
Recurrent/metastatic Concept #2.
R/M Concept #3
The group further explored clinical trial designs for heavily pretreated patients who had both immune checkpoint inhibitor- and platinum-based chemotherapy, a population without standard or investigational therapeutic options. Gemcitabine, which has been explored in R/M HNSCC both as a single agent (39) and in combination with cytotoxic therapies such as docetaxel (40) and pemetrexed (41), represented a potential alternative to guideline-recommended single agent treatment options such as methotrexate and cetuximab. Gemcitabine induces replication stress and thus is expected to be synergistic with wee-1 inhibitor and/or ATR/CHK1 inhibitors (42). Based on these preclinical and clinical data, the group proposed a randomized phase II study for HPV-positive or TP53-mutated HNSCC patients who progressed on immune checkpoint inhibitor and platinum-based chemotherapy (Figure 4). The group was in agreement with including HPV-related oropharynx cancers. Although this subset is generally characterized by wild-type TP53, the presence of HPV oncoproteins disrupts the function of p53 and Rb. The E2F activation triggered by Rb inactivation promotes G1-S transition leading to cellular vulnerability to replication stresses, making this therapeutic approach attractive. Patients will be stratified by TP53 mutation status and will be randomly assigned to a wee-1 inhibitor and gemcitabine, an ATR inhibitor and gemcitabine, or gemcitabine alone, in a 1:1:1 allocation. Potential candidates for the wee-1 inhibitor adavosertib, most advanced in clinical development, as well as others in clinical testing, are described in Table 1. Similarly, the ATR inhibitors in the latest stages of clinical study as of the time of this writing are celaracertib (AstraZeneca) and berzosertib (Merck); the use of these novel inhibitors would require partnerships with industry. Assuming a 10% ORRs in the gemcitabine-alone arm, it was hypothesized that an experimental arm would achieve a 30% ORR. The study will be powered to provide a comparison between gemcitabine and each experimental arm, and the result of this study would provide the foundation for the design of a larger phase III clinical trial comparing this novel combination with the current standard of care (either methotrexate, docetaxel, or cetuximab).
Figure 4.
Recurrent/metastatic Concept #3. HPV = human papilloma virus; IV = intravenous.
Breakout Group #3: Signal-Seeking Trials: Co-Chairs Drs Elsa Flores and Nabil Saba
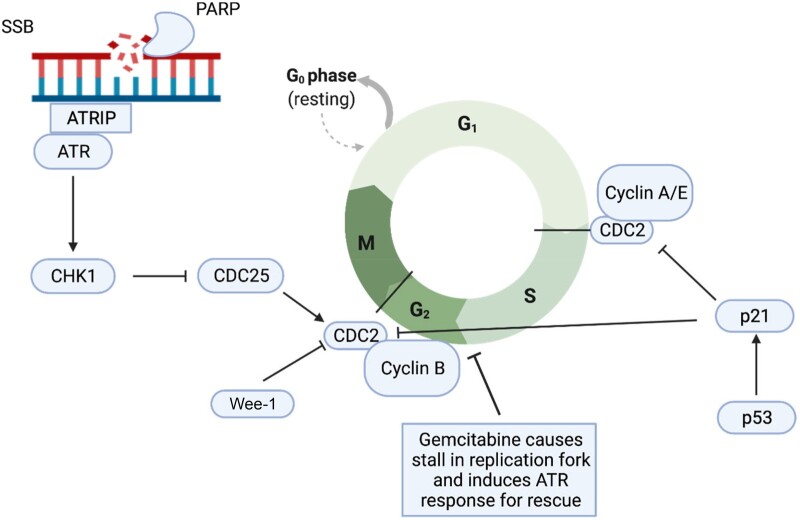
Breakout Group #3 focused on signal-seeking trial designs and early phase compounds. A close collaboration between clinicians and basic scientists focused on smaller studies mapping the terrain to inform the design of larger studies. One challenge that faced this group was the fact that NCTN does not support phase I trials. Tasks were assigned to different group members focusing on target interest, including wee-1 and PARP inhibitors, and targeting activation of wild-type TP53 (PRIMA1, MURA1, and APR256), as well as inhibiting mutant TP53 complexes with an emphasis on agents ready for application in an NCTN design setting. Combinatorial approaches with immunotherapy and radiation therapy were considered high priority given the role of these 2 modalities (radiation and immunotherapy) in the management of squamous cell carcinoma of head and neck. Prioritized agents included those agents targeting Aurora A, wee-1, PARP, ATR, ATM, PLK1, CHK-1, DNA-PK candidate agents (Figure 5).
Figure 5.
Targets of interest in TP53-altered head and neck cancer. PARP = poly (ADP-ribose) polymerase; SSB = single-strand break.
The group discussed a potential basket trial with multiple arms; with a wee-1 inhibitor as a possible backbone with the possible arms including wee-1 inhibitor monotherapy, wee-1 with chemotherapy, wee-1 and PD1 and PD-L1 inhibitor, and wee-1 and Aurora kinase inhibitors. Considerations were also given to having a DNA damage response or PARP inhibitor as a backbone in case wee-1 were not readily available. Data on novel combinations were reviewed, however, a number of them were noted to be poorly tolerated, such as wee-1 and ATR and wee-1 and PARP. As NCTN and the Cancer Therapy Evaluation Program tend to work with randomized trial designs focusing on agents with an established phase II dosing was necessary. In addition, it became more obvious to the group that relying on the molecular profiles of patients as a primary driver for signal seeking within new or existing randomized trials was most feasible. Various designs of phase II randomized trials in the first-line recurrent metastatic setting were entertained with the possibility of moving to a phase III trial depending on the phase II trial results. A randomized design of PARP inhibitor vs wee-1 inhibitor with immunotherapy in the first-line recurrent metastatic setting was deemed to be feasible and of high interest as it could potentially move to a phase III trial (Figure 6). There is increasing insight into the role of PARP inhibitors in enhancing tumor immunogenicity through accumulated DNA damage and increased tumor mutational burden, tumor-infiltrating lymphocyte recruitment, and changes in the tumor microenvironment (43–46). Various study designs in gynecologic (NCT04483544, NCT02657889) as well as gastrointestinal carcinomas (NCT05201612, NCT04592211) are incorporating immunotherapy and PARP inhibitor combinations and strengthen the rationale of this proposal.
Figure 6.
Signal-seeking concept. CPS = combined proportion score; HPV = human papilloma virus; PARP = poly (ADP-ribose) polymerase; PD-L = programmed death-ligand; R/M HNSCC = recurrent metastatic head and neck squamous cell carcinoma.
Breakout Group #4: Biomarkers Co-Chairs Drs James Ford and Jeffrey Meyers
The Biomarker Breakout Group (Breakout Group #4) met several times over the year prior to the CTPM to discuss multiple aspects of biomarker selection, technical methods of biomarker assessment including TP53 mutations, and computational methods for defining TP53 mutational risk stratification classification as well as the application of these classification schemes as prognostic and predictive markers to actual HNSCC clinical data sets. Dr Neil Hayes provided the genomic context with an overview of TP53 structure function relationships, and Dr Stanley Hamilton provided an overview on molecular pathology methodology of TP53-mutation assessment on tissue, blood, and oral rinses and described specific platforms and their strengths and limitations. Dr Christine Chung discussed the experience of the ECOG Head and Neck Cancer Group using the foundation medicine assay for TP53 mutations. Rachel Karchin provided the biostatistical rationale underlying the disruptive and nondisruptive TP53 mutational classification scheme, and Dr Jeff Myers spoke about the alternative Evolutionary Action Score of P53 scheme for classifying mutation on the basis of the principals of evolutionary action. Dr Curtis Pickering delineated the topics of integral vs integrated biomarker and stratified randomization vs stratified analysis, and Dr Ravi Uppaluri spoke on evaluating TP53 mutations as modifiers of HNSCC anti-PD-1 response. With this background in mind, at the CTPM, the group addressed the topics of the best way to sequence TP53, the best way to score TP53 mutations, how we should use TP53 mutational status (treatment selection vs stratification), and the best ways to incorporate these biomarker approaches into the clinical trials proposed by the 3 other breakout groups. Finally, the group addressed other biomarker data that should be considered in acquiring and/or analyzing in the context of these studies. There are a multitude of institutional molecular pathologic and commercial biotechnologic solutions for sequencing TP53 from human tumors and biofluids, as well as platforms for sequencing TP53 in ctDNA and quantifying tumor burden. The group was supportive of exploring partnerships with industry especially those that have established partnerships in NCTN trials.
With respect to molecular classification schemes, the Biomarker Breakout Group favored incorporation of the Poeta rules + Splice classification method of Dr Karchin and colleagues, as it has already been used in clinical trials of HNSCC from ECOG–American College of Radiology. The Poeta rules + Splice method is being evaluated prospectively in the study, EA3132 trial p16 negative patients; intermediate risk, margin negative, extranodal extension (ENE) negative randomized to radiotherapy or radiotherapy with cisplatin. Additional biomarkers considered for inclusion in the proposed clinical trials include tumor mutational burden, Casp-8 mutational status, 11 q amplification, POL-BPCR, and immune markers.
Summary of Priorities and Conclusions and Action Plan
At the conclusion of the CTPM, the various breakout group concepts were prioritized based on clinical need, feasibility, and interest. Five of the presented concepts were chosen for further development. Concept #3 from Breakout Group #2, exploring gemcitabine with ATR inhibitor, gemcitabine with wee-1 inhibitor vs gemcitabine alone in the second-line setting was prioritized. Concept #2 from Breakout Group #1 involving xevinapant plus cisplatin and postoperative radiation in high-risk resected non–HPV-related HNSCC similarly was considered high priority. The 2 other R/M studies, as well as the signal-seeking wee-1 and PARP inhibitor study, were judged worthy of further development. Discussions on drug provision for these trials are ongoing at the time of this manuscript’s writing.
The Executive Meeting Summary can be accessed at the following link: https://www.cancer.gov/about-nci/organization/ccct/steering-committees/nctn/head-neck/hnsc-ctpm-tp53-mutated-jan2021.
Funding
This meeting was supported by the NCI Coordinating Center for Clinical Trials and was developed under the leadership of the NCI Head & Neck Cancer Steering Committee.
Notes
Role of the funder: This meeting was supported by the NCI Coordinating Center for Clinical Trials and was developed under the leadership of the NCI Head & Neck Cancer Steering Committee. Both organizations approved the writing of this review and the decision to submit for publication.
Disclosures: CPR: BMS (research support paid to institution), Kura (research support paid to institution), Merck (research support paid to institution), Cue (advisory board, research support paid to institution), Coherus (advisory board). HK: Achilles (advisory board), MitoImmune (advisory board), Kura Oncology (research support paid to institution), PDS Biotechnology (research support paid to institution), NeoImmuneTech (research support paid to institution). JG: Regeneron (advisory board), Merck (advisory board), Exelexis (advisory board), Regeneron (research support paid to institution), Genentech (research support paid to institution), Debio (research support paid to institution), Alkermes (research support paid to institution). BB: Debio (consulting), Merck KgA(consulting), Vitrac Pharmaceuticals (consulting). CHC: Bristol-Myers Squibb (advisory board), CUE (advisory board), Sanofi (advisory board), Mirati (advisory board), Merck (advisory board), Brooklyn ImmunoTherapuetics (advisory board), and Exelixis (advisory board). QTL: Nanobiotix (consulting), Roche(consulting), Coherus (consulting). SSY: Merck (research support paid to institution), Bristol-Myers Squibb (research support paid to institution), Genentech (research support paid to institution), BioMimetix (research support paid to institution), EMD Serono (research support paid to institution). RM: Rakuten (consulting), AstraZeneca (research support paid to institution and uncompensated advisory board), Merck (research support paid to institution). NFS: Merck Coherus (advisory board), GSK Coherus (advisory board), Eisa (advisory board), Astra Zeneca (advisory board), Mirati (advisory board), Pfizer (advisory board), Aduro (advisory board), Vaccinex (advisory board). JMF: Pfizer (research support paid to institution), (research support paid to institution), AstraZeneca (research support paid to institution), Genentech (research support paid to institution), Merus (research support paid to institution), PUMA (research support paid to institution), and Incyte (research support paid to institution). RLF: Achilles Therapeutics (advisory board), Aduro Biotech (consultant), AstraZeneca/MedImmune (research support paid to institution), Bicara Therapeutics (consultant), BMS (advisory board, research support paid to institution), Brooklyn Immunotherapeutics (consultant), Catenion (consultant), EMD Serono (advisory board), Everest Clinical Research Corporation (consultant), F. Hoffman-La Roche (consultant), Genocea Biosciences (consultant), Hookipa Biotech (advisory board), Instil Bio (advisory board), Kowa Research Institute (consultant), Lifescience Dynamics Limited (advisory board), MacroGenics, Inc (advisory board), Merck (advisory board, research support paid to institution), Mirati Therapeutics (consultant), Nanobiotix (consultant), Novasenta (consultant, research support paid to institution), Numab Therapeutics (advisory board), Oncocyte (advisory board), Pfizer (advisory board), PPD Development (consultant), Rakuten Medical (advisory board), Sanofi (consultant), Seagen (advisory board), Tesaro (research support paid to institution), Vir Biotechnology (advisory board), Zymeworks (consultant). The remaining authors have no disclosures.
RLF, who is a JNCI Associate Editor and co-author on this review, was not involved in the editorial review or decision to publish this manuscript.
Author contributions: Conceptualization: BB, CHC, CPR Writing- Original Draft: CPR, HK, JLG, RM, HS, NS, ERF, JNM, CRP Writing—Review & Editing: CPR, HK, JLG, BB, CHC, CRP, CF, QTL, SSY, TJ, EG, AL, JS, SW, RM, HS, NFS, ERF, JNM, JMF, RK, RLF, CK, JML, SM.
Acknowledgements: We thank the meeting attendees for participating in the discussion and design of proposed clinical trials detailed in this review.
List of all invited participants to the TP53 Mutated Head and Neck Cancer Clinical Trials Planning Meeting: Doug Adkins, Clint Allen, Julie Bauman, Barbara Burtness, Zhong Chen, Christine Chung, Malgorzata Dominiewska, Deborah Doroshow, Anne Marie Egloff, Carole Fakhry, Robert Ferris, Elsa Flores, James Ford, Thomas Galloway, Jessica Geiger, Robert Godin, Erica Golemis, D. Neil Hayes, MD, Leah Hubbard, Zain Husain, Deborah Jaffe, Hyunseok Kang, Rachel Karchin, Tiffany Katz, Randy Kimple, Charles Kunos, Quynh Thu Le, Rom Leidner, Alice Li, Guillermina Lozano, Gregory Lubiniecki, Jean M. Lynn, Shakun Malik, Ranee Mehra, Luc Morris, Jeffrey Myers, Thomas Myers, Cheri-Ann Nathan, Giovanni Nitti, Brian O’Sullivan, Kym Pagel, Linda Paradiso, Curtis Pickering, Richard Piekarz, Mei Polley, Camille Ragin, Lovett Evan Reddick, Cristina Rodriguez, Angel Rodriguez, Dario Ruscica, Nabil Saba, Elena Schwartz, Geoffrey Shapiro, David Sher, Jeffery Shoop, Heath Skinner, Cheryl Solomon, Ramona Swaby, Paul Swiecicki, Aik Choon Tan, Ravi Uppaluri, Chiayeng Wang, Stuart Wong, Timothy Yap, Wendell Yarbrough, Sue Yom, Zhiwei Zhang.
Contributor Information
Cristina P Rodriguez, Department of Medicine, University of Washington, Seattle, WA, USA.
Hyunseok Kang, Department of Medicine, University of California San Francisco, San Francisco, CA, USA.
Jessica L Geiger, Department of Hematology and Medical Oncology, Cleveland Clinic Foundation, Cleveland, OH, USA.
Barbara Burtness, Department of Medicine, Yale University, New Haven, CT, USA.
Christine H Chung, Department of Head and Neck-Endocrine Oncology, Moffit Cancer Center, Tampa, FL, USA.
Curtis R Pickering, Department of Head and Neck Surgery, The University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Carole Fakhry, Division of Head and Neck Surgery, Department of Otolaryngology-Head and Neck Surgery, Johns Hopkins University, Baltimore, MD, USA.
Quynh Thu Le, Department of Radiation Oncology-Radiation Therapy, Stanford University, Palo Alto, CA, USA.
Sue S Yom, Department of Radiation Oncology, University of California San Francisco, San Francisco, CA, USA.
Thomas J Galloway, Department of Radiation Oncology, Fox Chase Cancer Center, Philadelphia, PA, USA.
Erica Golemis, Department of Radiation Oncology, University of California San Francisco, San Francisco, CA, USA.
Alice Li, Kaiser Permanente Oakland, Oakland, CA, USA.
Stuart Wong, Division of Neoplastic Diseases, Department of Medicine, Medical College of Wisconsin, Milwaukee, WI, USA.
Ranee Mehra, Division of Hematology/Oncology, Department of Medicine, University of Maryland, Baltimore, MD, USA.
Heath Skinner, Department of Radiation Oncology, University of Pittsburgh, Pittsburgh, PA, USA.
Nabil F Saba, Department of Hematology and Medical Oncology, Emory University, Atlanta, GA, USA.
Elsa R Flores, Department of Molecular Oncology, Moffit Cancer Center, Tampa, FL, USA.
Jeffrey N Myers, Department of Head and Neck Surgery, The University of Texas MD Anderson Cancer Center, Houston, TX, USA.
James M Ford, Department of Medicine, Stanford University, Palo Alto, CA, USA.
Rachel Karchin, Department of Oncology, Johns Hopkins University, Baltimore, MD, USA.
Robert L Ferris, Department of Otolaryngology, University of Pittsburgh, Pittsburgh, PA, USA.
Charles Kunos, National Institutes of Health, Bethesda, MD, USA.
Jean M Lynn, National Institutes of Health, Bethesda, MD, USA.
Shakun Malik, National Institutes of Health, Bethesda, MD, USA.
Data Availability
Data involving this clinical trials planning meeting are publicly available and accessible at https://www.cancer.gov/about-nci/organization/ccct/steering-committees/nctn/head-neck/hnsc-ctpm-tp53-mutated-jan2021
References
- 1. D’Souza G, Kreimer AR, Viscidi R, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356(19):1944-1956. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 2. Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24-35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Peltonen JK, Helppi HM, Pääkkö P, Turpeenniemi-Hujanen T, Vähäkangas KH.. p53 in head and neck cancer: functional consequences and environmental implications of TP53 mutations. Head Neck Oncol. 2010;2:36.doi: 10.1186/1758-3284-2-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stransky N, Egloff AM, Tward AD, et al. The mutational landscape of head and neck squamous cell carcinoma. Science. 2011;333(6046):1157-1160. doi: 10.1126/science.1208130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Poeta ML, Manola J, Goldwasser MA, et al. TP53 mutations and survival in squamous-cell carcinoma of the head and neck. N Engl J Med. 2007;357(25):2552-2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Skinner HD, Sandulache VC, Ow TJ, et al. TP53 disruptive mutations lead to head and neck cancer treatment failure through inhibition of radiation-induced senescence. Clin Cancer Res. 2012;18(1):290-300. doi: 10.1158/1078-0432.CCR-11-2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Alsner J, Sørensen SB, Overgaard J.. TP53 mutation is related to poor prognosis after radiotherapy, but not surgery, in squamous cell carcinoma of the head and neck. Radiother Oncol J Eur Soc Ther Radiol Oncol. 2001;59(2):179-185. doi: 10.1016/s0167-8140(01)00301-2. [DOI] [PubMed] [Google Scholar]
- 8. Koch WM, Brennan JA, Zahurak M, et al. p53 mutation and locoregional treatment failure in head and neck squamous cell carcinoma. J Natl Cancer Inst. 1996;88(21):1580-1586. doi: 10.1093/jnci/88.21.1580. [DOI] [PubMed] [Google Scholar]
- 9. Andrews GA, Xi S, Pomerantz RG, et al. Mutation of p53 in head and neck squamous cell carcinoma correlates with Bcl-2 expression and increased susceptibility to cisplatin-induced apoptosis. Head Neck. 2004;26(10):870-877. doi: 10.1002/hed.20029. [DOI] [PubMed] [Google Scholar]
- 10. Hoffmann TK, Sonkoly E, Hauser U, et al. Alterations in the p53 pathway and their association with radio- and chemosensitivity in head and neck squamous cell carcinoma. Oral Oncol. 2008;44(12):1100-1109. doi: 10.1016/j.oraloncology.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 11. Bradford CR, Zhu S, Ogawa H, et al. P53 mutation correlates with cisplatin sensitivity in head and neck squamous cell carcinoma lines. Head Neck. 2003;25(8):654-661. doi: 10.1002/hed.10274. [DOI] [PubMed] [Google Scholar]
- 12. Mandic R, Schamberger CJ, Müller JF, et al. Reduced cisplatin sensitivity of head and neck squamous cell carcinoma cell lines correlates with mutations affecting the COOH-terminal nuclear localization signal of p53. Clin Cancer Res. 2005;11(19 pt 1):6845-6852. doi: 10.1158/1078-0432.CCR-05-0378. [DOI] [PubMed] [Google Scholar]
- 13. Osman AA, Neskey DM, Katsonis P, et al. Evolutionary action score of TP53 coding variants is predictive of platinum response in head and neck cancer patients. Cancer Res. 2015;75(7):1205-1215. doi: 10.1158/0008-5472.CAN-14-2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gadhikar MA, Sciuto MR, Alves MVO, et al. CHK1/2 inhibition overcomes the cisplatin resistance of head and neck cancer cells secondary to the loss of functional p53. Mol Cancer Ther. 2013;12(9):1860-1873. doi: 10.1158/1535-7163.MCT-13-0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Masica DL, Li S, Douville C, et al. Predicting survival in head and neck squamous cell carcinoma from TP53 mutation. Hum Genet. 2015;134(5):497-507. doi: 10.1007/s00439-014-1470-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kato S, Han SY, Liu W, et al. Understanding the function-structure and function-mutation relationships of p53 tumor suppressor protein by high-resolution missense mutation analysis. Proc Natl Acad Sci USA. 2003;100(14):8424-8429. doi: 10.1073/pnas.1431692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Neskey DM, Osman AA, Ow TJ, et al. Evolutionary action score of TP53 identifies high-risk mutations associated with decreased survival and increased distant metastases in head and neck cancer. Cancer Res. 2015;75(7):1527-1536. doi: 10.1158/0008-5472.CAN-14-2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Geenen JJJ, Schellens JHM.. Molecular pathways: targeting the protein kinase Wee1 in cancer. Clin Cancer Res. 2017;23(16):4540-4544. doi: 10.1158/1078-0432.CCR-17-0520. [DOI] [PubMed] [Google Scholar]
- 19. De Witt Hamer PC, Mir SE, Noske D, Van Noorden CJF, Würdinger T.. WEE1 kinase targeting combined with DNA-damaging cancer therapy catalyzes mitotic catastrophe. Clin Cancer Res. 2011;17(13):4200-4207. doi: 10.1158/1078-0432.CCR-10-2537. [DOI] [PubMed] [Google Scholar]
- 20. Moser R, Xu C, Kao M, et al. Functional kinomics identifies candidate therapeutic targets in head and neck cancer. Clin Cancer Res. 2014;20(16):4274-4288. doi: 10.1158/1078-0432.CCR-13-2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Osman AA, Monroe MM, Ortega Alves MV, et al. Wee-1 kinase inhibition overcomes cisplatin resistance associated with high-risk TP53 mutations in head and neck cancer through mitotic arrest followed by senescence. Mol Cancer Ther. 2015;14(2):608-619. doi: 10.1158/1535-7163.MCT-14-0735-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mendez E, Rodriguez CP, Kao MC, et al. A phase I clinical trial of AZD1775 in combination with neoadjuvant weekly docetaxel and cisplatin before definitive therapy in head and neck squamous cell carcinoma. Clin Cancer Res. 2018;24(12):2740-2748. doi: 10.1158/1078-0432.CCR-17-3796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bernier J, Cooper JS, Pajak TF, et al. Defining risk levels in locally advanced head and neck cancers: a comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (# 9501). Head Neck. 2005;27(10):843-850. doi: 10.1002/hed.20279. [DOI] [PubMed] [Google Scholar]
- 24. Uppaluri R, Campbell KM, Egloff AM, et al. Neoadjuvant and adjuvant pembrolizumab in resectable locally advanced, human papillomavirus-unrelated head and neck cancer: a multicenter, phase II trial. Clin Cancer Res off J Am Assoc Cancer Res. 2020;26(19):5140-5152. doi: 10.1158/1078-0432.CCR-20-1695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Friedman J, Moore EC, Zolkind P, et al. Neoadjuvant PD-1 immune checkpoint blockade reverses functional immunodominance among tumor antigen-specific T cells. Clin Cancer Res. 2020;26(3):679-689. doi: 10.1158/1078-0432.CCR-19-2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Friedman J, Morisada M, Sun L, et al. Inhibition of WEE1 kinase and cell cycle checkpoint activation sensitizes head and neck cancers to natural killer cell therapies. J Immunother Cancer. 2018;6(1):59.doi: 10.1186/s40425-018-0374-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bai L, Smith DC, Wang S.. Small-molecule SMAC mimetics as new cancer therapeutics. Pharmacol Ther. 2014;144(1):82-95. doi: 10.1016/j.pharmthera.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Brands RC, Scheurer MJJ, Hartmann S, Seher A, Kübler AC, Müller-Richter UDA.. Apoptosis-sensitizing activity of birinapant in head and neck squamous cell carcinoma cell lines. Oncol Lett. 2018;15(3):4010-4016. doi: 10.3892/ol.2018.7783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brands RC, Herbst F, Hartmann S, et al. Cytotoxic effects of SMAC-mimetic compound LCL161 in head and neck cancer cell lines. Clin Oral Investig. 2016;20(9):2325-2332. doi: 10.1007/s00784-016-1741-3. [DOI] [PubMed] [Google Scholar]
- 30. Matzinger O, Viertl D, Tsoutsou P, et al. The radiosensitizing activity of the SMAC-mimetic, Debio 1143, is TNFα-mediated in head and neck squamous cell carcinoma. Radiother Oncol J Oncol. 2015;116(3):495-503. doi: 10.1016/j.radonc.2015.05.017. [DOI] [PubMed] [Google Scholar]
- 31. Uzunparmak B, Gao M, Lindemann A, et al. Caspase-8 loss radiosensitizes head and neck squamous cell carcinoma to SMAC mimetic-induced necroptosis. JCI Insight. 2020;5(23):139837.doi: 10.1172/jci.insight.139837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Xiao R, An Y, Ye W, et al. Dual antagonist of cIAP/XIAP ASTX660 sensitizes HPV- and HPV+ head and neck cancers to TNFα, TRAIL, and radiation therapy. Clin Cancer Res. 2019;25(21):6463-6474. doi: 10.1158/1078-0432.CCR-18-3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Xiao R, Allen CT, Tran L, et al. Antagonist of cIAP1/2 and XIAP enhances anti-tumor immunity when combined with radiation and PD-1 blockade in a syngeneic model of head and neck cancer. Oncoimmunology. 2018;7(9):e1471440.doi: 10.1080/2162402X.2018.1471440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Igney FH, Krammer PH.. Death and anti-death: tumour resistance to apoptosis. Nat Rev Cancer. 2002;2(4):277-288. doi: 10.1038/nrc776. [DOI] [PubMed] [Google Scholar]
- 35. Eytan DF, Snow GE, Carlson S, et al. SMAC mimetic birinapant plus radiation eradicates human head and neck cancers with genomic amplifications of cell death genes FADD and BIRC2. Cancer Res. 2016;76(18):5442-5454. doi: 10.1158/0008-5472.CAN-15-3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sun XS, Tao Y, Le Tourneau C, et al. Debio 1143 and high-dose cisplatin chemoradiotherapy in high-risk locoregionally advanced squamous cell carcinoma of the head and neck: a double-blind, multicentre, randomised, phase 2 study. Lancet Oncol. 2020;21(9):1173-1187. doi: 10.1016/S1470-2045(20)30327-2. [DOI] [PubMed] [Google Scholar]
- 37.Burtness B, Harrington KJ, Greil R, et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019;394(10212):1915-1928. doi: 10.1016/S0140-6736(19)32591-7 [DOI] [PubMed] [Google Scholar]
- 38. Patel P, Sun L, Robbins Y, et al. Enhancing direct cytotoxicity and response to immune checkpoint blockade following ionizing radiation with WEE1 kinase inhibition. Oncoimmunology. 2019;8(11):e1638207.doi: 10.1080/2162402X.2019.1638207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Raguse JD, Gath HJ, Bier J, Riess H, Oettle H.. Gemcitabine in the treatment of advanced head and neck cancer. Clin Oncol (R Coll Radiol). 2005;17(6):425-429. [DOI] [PubMed] [Google Scholar]
- 40. Sukari A, Al-Hajeili M, Salem M, et al. Biweekly gemcitabine and paclitaxel in patients with relapsed or metastatic squamous cell carcinoma of the head and neck. Avicenna J Med. 2015;5(2):36-41. doi: 10.4103/2231-0770.154195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fury MG, Haque S, Stambuk H, Shen R, Carlson D, Pfister D.. A phase 2 study of pemetrexed plus gemcitabine every 2 weeks for patients with recurrent or metastatic head and neck squamous cell cancer. Cancer. 2011;117(4):795-801. doi: 10.1002/cncr.25464. [DOI] [PubMed] [Google Scholar]
- 42. Koh SB, Wallez Y, Dunlop CR, et al. Mechanistic distinctions between CHK1 and WEE1 inhibition guide the scheduling of triple therapy with gemcitabine. Cancer Res. 2018;78(11):3054-3066. doi: 10.1158/0008-5472.CAN-17-3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wu Z, Cui P, Tao H, et al. The synergistic effect of PARP inhibitors and immune checkpoint inhibitors. Clin Med Insights Oncol. 2021;15:1179554921996288.doi: 10.1177/1179554921996288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jiao S, Xia W, Yamaguchi H, et al. PARP inhibitor upregulates PD-L1 expression and enhances cancer-associated immunosuppression. Clin Cancer Res. 2017;23(14):3711-3720. doi: 10.1158/1078-0432.CCR-16-3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Peyraud F, Italiano A.. Combined PARP inhibition and immune checkpoint therapy in solid tumors. Cancers. 2020;12(6):1502. doi: 10.3390/cancers12061502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Vikas P, Borcherding N, Chennamadhavuni A, Garje R.. Therapeutic potential of combining PARP inhibitor and immunotherapy in solid tumors. Front Oncol. 2020;10:570. doi: 10.3389/fonc.2020.00570 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data involving this clinical trials planning meeting are publicly available and accessible at https://www.cancer.gov/about-nci/organization/ccct/steering-committees/nctn/head-neck/hnsc-ctpm-tp53-mutated-jan2021