Abstract
Background
Weight loss (WL) has been associated with shorter survival in patients with advanced cancer, while obesity has been associated with longer survival. Integrating body mass index (BMI) and WL provides a powerful prognostic tool but has not been well‐studied in lung cancer patients, particularly in the setting of clinical trials.
Methods
We analysed patient data (n = 10 128) from 63 National Cancer Institute sponsored advanced non‐small cell lung cancer (NSCLC) and small cell lung cancer (SCLC) trials. Risk matrices were created using BMI and WL percentage, which were divided into ‘grades’ based on median survival. Relationships between survival, BMI and WL percentage were examined using Kaplan–Meier estimators and Cox proportional hazards (PH) models with restricted cubic splines.
Results
For NSCLC, a twofold difference was noted in median survival between the BMI > 28 and WL ≤ 5% group (13.5 months) compared with the BMI < 20 and WL > 5% group (6.6 months). These associations were less pronounced in SCLC. Kaplan–Meier curves showed significant survival differences between grades for both NSCLC and SCLC (log‐rank, P < 0.0001). In Stage IV NSCLC, Cox PH analyses with restricted cubic splines demonstrated significant associations between BMI and survival in both WL ≤ 5% (P = 0.0004) and >5% (P = 0.0129) groups, as well as in WL > 5% in Stage III (P = 0.0306). In SCLC, these relationships were more complex.
Conclusions
BMI and WL have strong associations with overall survival in patients with advanced lung cancer, with a greater impact seen in NSCLC compared with SCLC. The integration of a BMI/WL grading scale may provide additional prognostic information and should be included in the evaluation of therapeutic interventions in future clinical trials in advanced lung cancer.
Keywords: lung cancer, body composition, BMI, weight loss
Introduction
Population trends for weight and body mass index (BMI) throughout the globe are increasing. Estimates show that nearly 1 in 2 adults in the United States will be obese by 2030. 1 Being overweight is associated with higher all‐cause mortality internationally. 2 In fact, studies show that mortality among obese individuals is increased in many chronic medical conditions, including cardiovascular disease, diabetes, liver disease and depression. 3 The risk of death increases by 20% to 40% in overweight individuals and by two to three times in obese individuals. 4 However, in individuals with acute illness, a BMI below the 15th percentile has also been shown to be an independent predictor of worse overall mortality. 5 Involuntary weight loss (WL) correlates with high morbidity and mortality, regardless of aetiology, 6 and is well described as a poor prognostic factor for survival in lung cancer. 7
The ‘obesity paradox’ refers to individuals with obesity and other comorbidities who actually have improved outcomes as a result of their body composition. 8 Although obesity is typically associated with increased all‐cause mortality at the population level, there is actually an associated survival advantage in some chronic conditions that are typically associated with WL and muscle wasting. 9 , 10 For example, in contrast to some chronic diseases like coronary artery disease and diabetes mellitus, where obesity has a negative impact on long‐term survival, obesity has been associated with longer overall survival (OS) in chronic diseases that are associated with wasting, like chronic heart failure, cancer, AIDS and rheumatoid arthritis. In these situations, the survival paradox is thought to be related to competition between the short‐term protective nature of the obesity in the setting of diseases where wasting carries a significant morbidity and the overall course of the disease itself. 11
This obesity paradox has been described in the oncologic population as well. Studies show that overweight and early‐obese states are associated with improved survival; however, some groups argue that this may be the result of confounding, bias and the imprecise nature of BMI as a measure. 12 In a study conducted by Dahlberg et al. in advanced non‐small cell lung cancer (NSCLC), obese patients were found to have significantly different OS compared with normal and overweight patients. 13 They described an inverse association between BMI and mortality, as well as an initial protective effect of obesity.
Martin et al. studied BMI and WL percentage prior to chemotherapy prospectively while following patients with a variety of malignancies until death. 14 They cited a history of involuntary WL as the primary diagnostic criterion for cancer cachexia, which is associated with a poorer prognosis. Individuals with stable weight and BMI > 25 had longer OS, whereas individuals in lower BMI groups and concurrent WL had shorter OS. Martin's study developed a novel way to combine BMI and WL trends by creating matrices for BMI and %WL, dividing the cohort into grades (0–4), and then recording median survival for each group. Grade 0 included individuals with the highest BMI and lowest %WL, while Grade 4 included individuals with the lowest BMI and greatest %WL. Martin's study included different malignancies with varied prognoses, with OS in the groups ranging anywhere from 4.2 to 77.9 months. Not only was there a wide overall range in OS, but there was also a wide range in OS between different malignancies.
Our study developed a similar grading system, with the purpose of applying it to a more homogeneous population of lung cancer patients. We were curious to determine new findings in individuals with advanced stage lung cancer and overall good performance status undergoing clinical trials receiving chemotherapy or combined modality treatments. Our ultimate goal was to improve our understanding of risk factors and outcomes within both NSCLC and SCLC, at the time of initial treatment.
Methods
Data were obtained from six US national cancer cooperative groups (American College of Surgeons Oncology Group, Cancer and Leukemia Group B, Eastern Cooperative Oncology Group [ECOG], North Central Cancer Treatment Group, Radiation Therapy Oncology Group and Southwest Oncology Group) with patient enrolment data spanning the years 1991 through 2008 and patient follow up through 2012. Individuals with NSCLC and SCLC were included in the study. The dataset initially included 27 007 individuals that were participants in a total of 135 clinical trials, and ECOG performance status was included. More specifics about the assembled data set can be found in the article by Pang et al. 15 Inclusion and exclusion criteria for trials and individual patients are included in Figure 1 .
Figure 1.
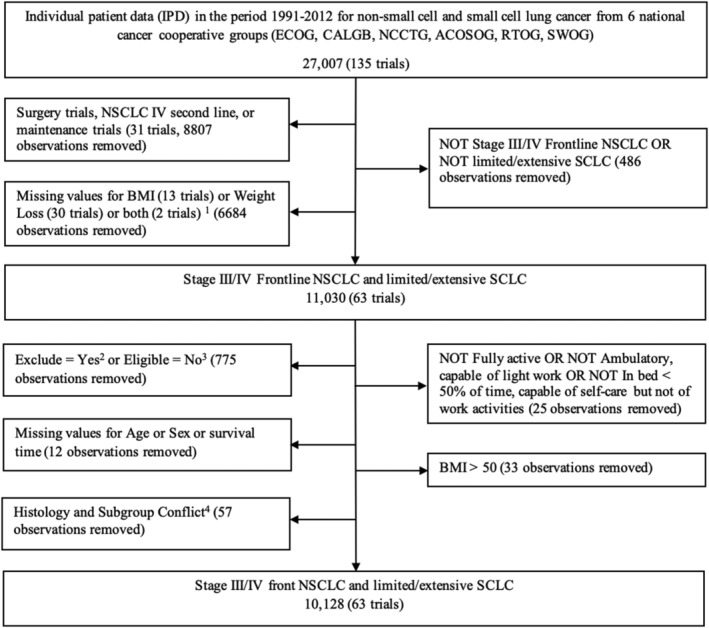
Inclusion and exclusion criteria for the validation cohort.
BMI was defined as weight in kilograms divided by the square of height in meters. Missing data for either weight or height rendered BMI missing. Missing data for race were imputed as ‘unknown’. WL was defined as a binary variable for whether or not greater than 5% WL occurred (over the prior 3 or 6 months, depending on the specific trial). Missing data for survival time were calculated by the date of the patient's status record and the date of the registration. Four subgroups, including Stages III and IV NSCLC, as well as limited and extensive stage SCLC, were selected by filtering the trials. Missing data for initial stage were imputed from the frequency of Stage III versus Stage IV NSCLC and limited versus extensive stage SCLC.
Trials were excluded if they were surgical trials, second line therapy trials, maintenance trials, included stage I or stage II malignancies, or had missing data for BMI, WL or survival time. After application of initial exclusion and inclusion criteria, the data set included 11 030 individuals in 63 clinical trials. This group included individuals with Stage III or IV NSCLC and individuals with limited or extensive stage SCLC. Trials and individual patients were further excluded if they had missing age, sex or survival time; if ECOG was 3 or higher; or if an individual's BMI was greater than 50, corresponding to the 99.67th percentile of the available data. Ultimately, our study population consisted of both NSCLC and SCLC patients who received first‐line chemotherapy with or without chest radiation. The final data set included 10 128 individuals spanning 63 clinical trials.
The primary outcome for the study was OS, which was defined as the time interval in months from registration or randomization to death from any cause. Individuals were followed from point of inception until death or last visit/contact. The primary variables of investigation were BMI and WL. OS was summarized using the Kaplan–Meier estimator and modelled using Cox proportional hazards (PH) models with and without restricted cubic splines (RCS), with BMI as a continuous variable and WL as a binary variable, respectively.
In order to determine the impacts of BMI and WL on survival, similar to the methods described by Martin et al., a 5 × 2 matrix was created that included the primary variables, BMI and WL. BMI was separated into 5 groups (>28, 25–28, 22–25, 20–22 and <20), and WL was a binary variable with 2 groups (>5% and ≤5%), creating 10 subgroups. A separate matrix with corresponding subgroups was created for both NSCLC and SCLC. After calculating median survival time (in months) and plotting the Kaplan–Meier curves for each of the 10 subgroups, the subgroups with adjacent Kaplan–Meier curves were then grouped into different ‘grades.’ Grade 1 represented matrix subgroups with the longest median survival. Higher grades (2–4) corresponded to decreased median survival, with Grade 4 representing the group with the lowest median survival. Grades 1–4 were used for NSCLC, and Grades 1–3 were used for SCLC. Survival distribution by grades was then estimated using the Kaplan–Meier method, and log‐rank tests were used to compare the survival distribution between grades.
RCS were utilized to examine the nonlinear effects of BMI on survival in different WL subgroups: Stage III NSCLC, Stage IV NSCLC, Limited Stage SCLC and Extensive Stage SCLC. 16 RCS transform the range of an independent variable (BMI) into four different ‘knots,’ which each represent a segment of data. In our sample, four knots were used at the 5th, 35th, 65th and 95th percentiles of BMI. Four knots were chosen to allow data flexibility and avoid loss of precision that can arise when overfitting a sample with too many knots. 17 , 18 Separate curves (or ‘splines’) were then applied to each segment to create a continuous and smooth curve. To adjust for the effects of other risk factors, including age, disease, performance status, race, gender and histology, multivariable Cox PH models were developed, and the PH assumptions were examined using Schoenfeld residuals. A nomogram based on the coefficients of the fitted Cox model was then used to visualize the impact of each covariate on OS. All P values were two‐sided without multiplicity adjustments. Unless specified otherwise, P values were from the Wald test on the regression coefficients of Cox PH models. All confidence intervals (CIs) were two‐sided 95% CIs. Statistical analyses were conducted using SAS (9.4) and R (4.0.4).
Results
As outlined in Figure 1 , the original data set was narrowed to 63 chemotherapy trials, encompassing 10 128 lung cancer patients.
Table 1 shows a breakdown of the sample population by age, race, gender, performance status, histology, disease subgroup, BMI and WL. Approximately 88% of the study population was composed of White individuals; African Americans represented 9% of the group. Additionally, the population had a very high functional status; 37% of the individuals had an ECOG score of 0, 55% had an ECOG score of 1, and only 8% had an ECOG score of 2. As noted earlier, patients with ECOG scores of 3 or higher (n = 25) were excluded. Of particular interest is the fact that 56% of the sample had a BMI greater than 25, corresponding with overweight or obese. It should be noted that 28% of the study population had WL greater than 5%.
Table 1.
Baseline characteristics of the cohort of lung cancer patients
| NSCLC (N = 7321) | SCLC (N = 2807) | Overall (N = 10 128) | |
|---|---|---|---|
| Subgroup | |||
| Stage III NSCLC | 3248, 44.37% | 0, 0.00% | 3248, 32.07% |
| Stage IV NSCLC | 4073, 55.63% | 0, 0.00% | 4073, 40.22% |
| Limited SCLC | 0, 0.00% | 1095, 39.01% | 1095, 10.81% |
| Extensive SCLC | 0, 0.00% | 1712, 60.99% | 1712, 16.90% |
| Weight loss | |||
| Weight loss ≤ 5% | 5399, 73.75% | 1940, 69.11% | 7339, 72.46% |
| Weight loss > 5% | 1922, 26.25% | 867, 30.89% | 2789, 27.54% |
| Race | |||
| Black | 704, 9.62% | 163, 5.81% | 867, 8.56% |
| Other | 332, 4.53% | 64, 2.28% | 396, 3.91% |
| White | 6285, 85.85% | 2580, 91.91% | 8865, 87.53% |
| Histology | |||
| Adenocarcinoma | 3005, 41.05% | 0, 0.00% | 3005, 29.67% |
| Other NSCLC | 2663, 36.37% | 0, 0.00% | 2663, 26.29% |
| SCLC | 0, 0.00% | 2807, 100.00% | 2807, 27.72% |
| Squamous | 1653, 22.58% | 0, 0.00% | 1653, 16.32% |
| Performance status | |||
| Ambulatory | 4203, 57.41% | 1415, 50.41% | 5618, 55.47% |
| Fully active | 2734, 37.34% | 987, 35.16% | 3721, 36.74% |
| In bed less than half of time | 384, 5.25% | 405, 14.43% | 789, 7.79% |
| Age | |||
| Age ≤ 60 | 2970, 40.57% | 1100, 39.19% | 4070, 40.19% |
| 60 < Age < 70 | 2558, 34.94% | 1091, 38.87% | 3649, 36.03% |
| Age ≥ 70 | 1793, 24.49% | 616, 21.95% | 2409, 23.79% |
| Gender | |||
| Female | 2702, 36.91% | 1188, 42.32% | 3890, 38.41% |
| Male | 4619, 63.09% | 1619, 57.68% | 6238, 61.59% |
| BMI | |||
| BMI < 20 | 703, 9.60% | 255, 9.08% | 958, 9.46% |
| 20 ≤ BMI < 22 | 847, 11.57% | 286, 10.19% | 1133, 11.19% |
| 22 ≤ BMI < 25 | 1755, 23.97% | 612, 21.80% | 2367, 23.37% |
| 25 ≤ BMI < 28 | 1789, 24.44% | 696, 24.80% | 2485, 24.54% |
| BMI ≥ 28 | 2227, 30.42% | 958, 34.13% | 3185, 31.45% |
| Disease | |||
| Advanced | 4073, 55.63% | 1712, 60.99% | 5785, 57.12% |
| Early | 3248, 44.37% | 1095, 39.01% | 4343, 42.88% |
Note: Categorical variables by N (%).
The 5 × 2 matrices with corresponding median survival times (in months) and subsequent grading schema for NSCLC and SCLC are shown in Figure 2 A–D . Sample sizes of each group are included in Figures S1 A,B. Based on OS outcomes, Grades 1–4 were assigned to the NSCLC group, and Grades 1–3 were assigned to the SCLC group. Within the NSCLC group, median survival in Grade 1 was nearly double that of Grade 4. Additionally, there was a clear demarcation between median survival in the WL ≤ 5% group (median survival ranging from 11.5 to 13.6 months) and the WL > 5% group (median survival ranging from 6.7 to 8.4 months). There was also a difference in median survival between BMI groups when comparing within specific WL categories, but this was not as pronounced. With respect to the SCLC group, the differences between median survivals were not as great. Median survival in the WL ≤ 5% group (range 11.3 to 12.9 months) was only slightly increased compared with the WL > 5% group (range 9.5 to 12 months). The most notable difference was in extreme values where WL ≤ 5% and BMI > 25 (median survival 12.7 to 12.9 months) compared with WL > 5% and BMI < 20 (median survival 9.5 months).
Figure 2.
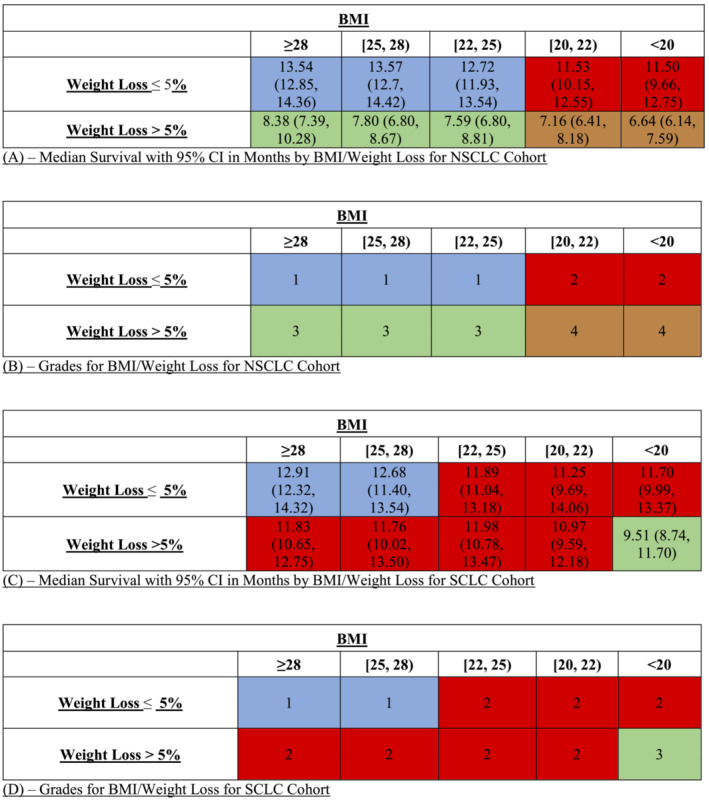
(A) Median survival with 95% confidence intervals (CIs) in months by body mass index (BMI)/weight loss for non‐small cell lung cancer (NSCLC) cohort. (B). Grades for BMI/weight loss for NSCLC cohort. (C) Median survival with 95% CI in months by BMI/weight loss for SCLC cohort. (D) Grades for BMI/weight loss for small cell lung cancer (SCLC) cohort.
Survival probability by grade was plotted using the Kaplan–Meier method (Figure 3 A for NSCLC, Figure 3 B for SCLC). Log‐rank tests were applied and showed a statistically significant difference (P < 0.0001) in median survival between grades for both NSCLC and SCLC. The curves suggested that survival decreases as BMI decreases and that survival also decreases as WL increases. As noted above, in the NSCLC group, the magnitude of impact on median survival in the WL groups was stronger than the magnitude of impact on median survival in the BMI groups. This trend did not hold true in the SCLC group, where BMI and WL appeared to have similar magnitudes of impact on median survival. More detailed information about survival in specific subgroups is included in the Supporting information figures. Figure S2 identifies 10 separate subgroups identified by combining different BMI values and WL above or below 5%. Figure S3A shows Kaplan–Meier curves for the 10 unique subgroups within the NSCLC cohort, while Figure S3B shows similar curves for the SCLC cohort.
Figure 3.
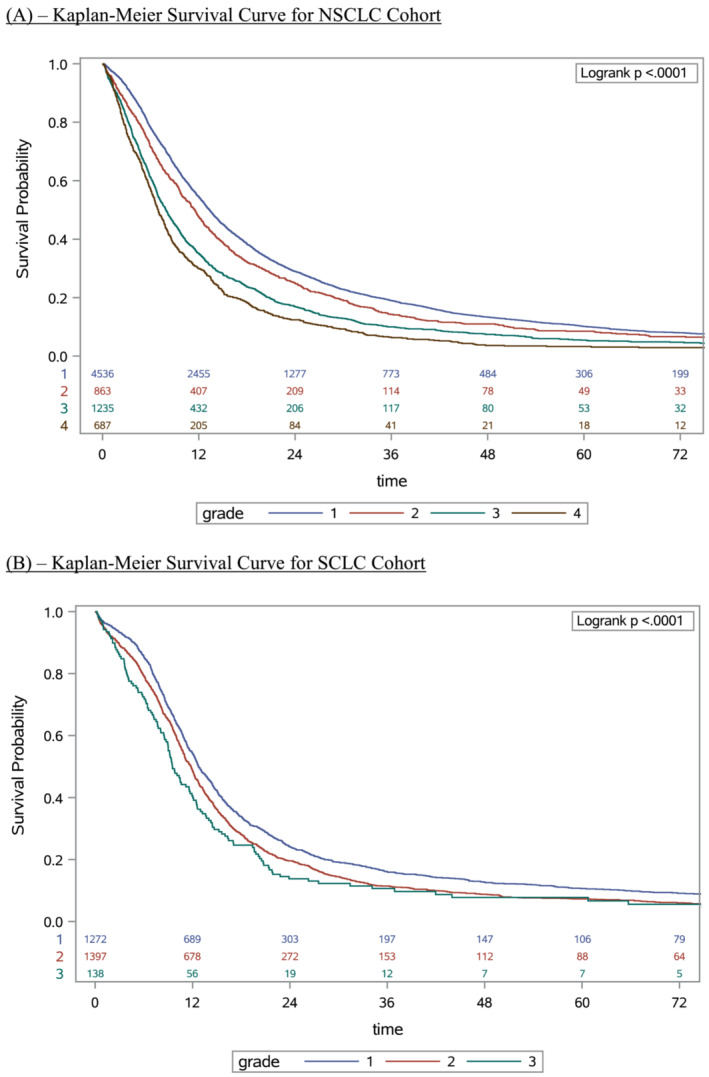
(A) Kaplan–Meier survival curve for non‐small cell lung cancer (NSCLC) cohort. (B) Kaplan–Meier survival curve for small cell lung cancer (SCLC) cohort.
Figure 4 A–D show RCS comparing the effects of BMI on survival in WL ≤ 5% versus WL > 5% subgroups manifested by the log (hazard ratio) on the y‐axis, without adjusting for other risk factors such as age.
Figure 4.
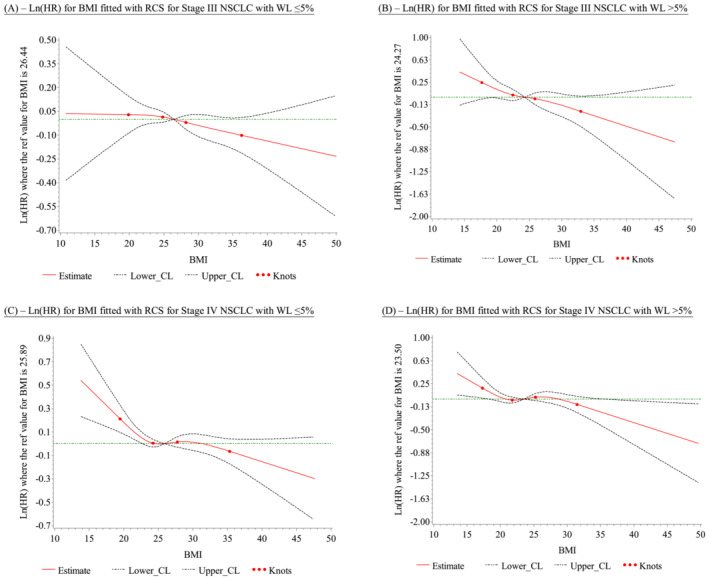
(A) Ln(HR) for body mass index (BMI) fitted with restricted cubic splines (RCS) for Stage III non‐small cell lung cancer (NSCLC) with WL ≤5%. (B) Ln(HR) for BMI fitted with RCS for Stage III NSCLC with weight loss (WL) >5%. (C) Ln(HR) for BMI fitted with RCS for Stage IV NSCLC with WL ≤ 5%. (D) Ln(HR) for BMI fitted with RCS for Stage IV NSCLC with WL >5%
Figure 4 A,B represents Stage III NSCLC, and Figure 4 C,D represents Stage IV NSCLC. A monotonic inverse relationship between BMI and survival can be observed in both Stage III and Stage IV NSCLC groups, regardless of WL. In Stage III NSCLC group, the only statistically significant overall association was between BMI and OS in patients with WL > 5% (P = 0.0306). However, in the Stage IV NSCLC group, the statistically significant overall association was seen both in patients with WL > 5% (P = 0.0129) and in patients with WL ≤ 5% (P = 0.0004). The nonlinear association between BMI and OS were statistically significant (P = 0.0455) in the WL ≤ 5% group and not statistically significant (P = 0.1035) in the WL > 5% group. Consequently, regardless of WL status, there was a statistically significant relationship between increasing BMI and decreasing log (HR), suggesting that increased BMI correlates with improved OS (Table S1 ).
Figure S4A ,B represents limited stage SCLC, and Figure S4C ,D represents extensive stage SCLC. From the trends in the estimation of log (HR) at different BMIs, no monotonic inverse associations between BMI and survival were seen in either the limited or extensive stage SCLC groups, regardless of WL, with no statistically significant nonlinear or overall associations observed (P > 0.05).
Table S2 shows overall sample size for each malignancy subgroup and percentage of individuals in each WL category. Additionally, for each malignancy subgroup, P values were calculated for both nonlinear association and for overall association and are listed in Table S2 .
A multivariable piecewise Cox PH model is shown in Table 2 . Histology was the only variable that did not show a statistically significant association with OS. It is important to note that many covariates had statistically significant hazard ratios (HRs). For instance, the overall mortality hazard was lower in female patients compared with that in male patients (HR = 0.81, CI [0.77, 0.84], P < 0.0001). Additionally, the overall mortality hazard difference was not significant in African Americans compared with that in White individuals (HR = 0.98, CI [0.91, 1.05], P = 0.57), but the overall mortality hazard was lower in ‘Other’ races compared with that in White individuals (HR = 0.87, CI [0.78, 0.97], P = 0.05). As expected, ECOG statuses of 1 and 2 were associated with increased HR of 1.32 (CI [1.26, 1.38], P < 0.0001) and 1.86 (CI [1.71, 2.03], P < 0.0001), respectively, suggesting decreased OS compared to ECOG status of 0. Additionally, age >70 years had a HR of 1.17 (CI [1.11, 1.23], P < 0.0001), corresponding to a decreased OS than younger individuals.
Table 2.
Multivariable piecewise cox proportional hazards model for lung cancer patient cohort
| Estimate | P value | Overall P value | Hazard ratio (95% CI) | ||
|---|---|---|---|---|---|
| Gender | Female | −0.22 | <0.0001 | <0.0001 | 0.81 [0.77, 0.84] |
| Male | |||||
| Race | Black or African American | −0.02 | 0.57 | 0.0377 | 0.98 [0.91, 1.05] |
| Other | −0.14 | 0.05 | 0.87 [0.78, 0.97] | ||
| White | |||||
| Performance status | Ambulatory | 0.28 | <0.0001 | <0.0001 | 1.32 [1.26, 1.38] |
| In bed < 50% of time | 0.62 | <0.0001 | 1.86 [1.71, 2.03] | ||
| Fully active | |||||
| Weight loss | Weight loss > 5% | 0.18 | <0.0001 | <0.0001 | 1.20 [1.14, 1.26] |
| Weight loss ≤ 5% | |||||
| Disease | Advanced | 0.77 | <0.0001 | <0.0001 | 2.16 [1.94, 2.42] |
| Early | |||||
| Histology | Small cell lung cancer | −0.02 | 0.80 | 0.1293 | 0.98 [0.87, 1.11] |
| Adenocarcinoma | 0.016 | 0.66 | 1.02 [0.95, 1.09] | ||
| Other NSCLC | 0.065 | 0.08 | 1.07 [0.99, 1.15] | ||
| Squamous | |||||
| Age | 60 < Age < 70 | 0.04 | 0.06 | <0.0001 | 1.05 [1.00, 1.10] |
| Age ≥ 70 | 0.15 | <0.0001 | 1.17 [1.11, 1.23] | ||
| Age ≤ 60 | |||||
| BMI | BMI ≥ 28 | −0.16 | <0.0001 | <0.0001 | 0.85 [0.79, 0.92] |
| 25 ≤ BMI < 28 | −0.14 | 0.00 | 0.87 [0.80, 0.94] | ||
| 22 ≤ BMI < 25 | −0.10 | 0.01 | 0.90 [0.84, 0.98] | ||
| 20 ≤ BMI < 22 | −0.04 | 0.41 | 0.96 [0.88, 1.05] | ||
| BMI < 20 |
Abbreviations: BMI, body mass index; CI, confidence interval.
The HRs of the BMI and WL groups were most notable with respect to our initial hypotheses. Compared with a BMI < 20, all groups except BMI 20–22 had statistically significantly decreased HRs with HR for BMI 22–25 of 0.90 (CI [0.84, 0.98], P = 0.01), for BMI 25–28 of 0.87 (CI [0.80, 0.94], P = 0.001), and BMI > 28 of 0.85 (CI [0.79, 0.92], P < 0.0001). As discussed above, this demonstrates that increased BMI was associated with a lower HR and consequently increased OS. Additionally, WL > 5% had a HR of 1.20 (CI [1.14, 1.26], P < 0.0001) when compared with WL ≤ 5%, suggesting that an increased amount of WL was associated with worse outcomes and decreased OS.
This information was used to plot a nomogram of the coefficients of the fitted Cox model to compare the corresponding impact that each covariate had on OS. 19 These results are shown in Figure S5 . Interestingly, disease severity and functional status had the largest magnitude of impact on OS. BMI and WL had similar magnitudes of impact on OS with low BMI and WL > 5% both corresponding to decreased OS rates.
Discussion
In this retrospective analysis of 10 128 patients with either advanced NSCLC or SCLC enrolled in 63 clinical trials spanning six cooperative oncology groups in the United States, of first line treatment with chemotherapy or chemotherapy and radiation treatment, we examined the association between BMI, WL and OS. Our results support the importance of both BMI and WL as composite measures on survival outcomes as initially proposed by Martin et al. and offer new findings in good performance status patients with advanced lung cancer.
Using BMI and %WL, individuals can be placed into different groups for prognosticating approximate OS. Each group had a statistically significant difference in median survival time, suggesting that these two variables, in combination, have utility in evaluating treatment outcomes for patients with advanced lung cancer. Differences were more pronounced in the NSCLC group compared with SCLC. It is also worth noting that %WL had a more substantial impact on survival than BMI did, especially in the NSCLC group, as median survival ranged from 6.7 to 8.4 months in the WL > 5% group but ranged from 11.5 to 13.6 months in the WL ≤ 5% group. Notably, groups with both low BMI and increased %WL had significantly decreased OS. This aligns with the ‘obesity paradox’, suggesting that individuals with low energy reserves who lose a significant amount of weight have a significant nutritional deficit and inability to sustain muscle mass. While this decrease in muscle mass is one of the mechanisms for shortened survival, there are multiple other factors that can explain this trend, including proportion of adipose tissue, overall nutritional stores and activity level. Both low energy reserves and changes in body composition, as well as some additional factors for which there is ongoing investigation, are the mechanisms by which low BMI and WL are associated with significantly shorter survival.
Multiple proposed mechanisms exist to explain improved outcomes in obese patients. The most obvious is the advantage of larger energy and protein reserves with relation to improved functional and mobility status, which correlate with lower complication rates, including venous thromboembolism and pneumonia. Other proposed explanations include synergy between peroxisome proliferator‐activated receptor (PPAR) ligands and platinum‐based chemotherapy agents, as well as induction of apoptosis in LKB1‐deficient NSCLC by metabolic drugs like metformin and phenformin. 20 , 21 The Dahlberg study emphasizes using serial weight measurements after randomization to better characterize survival trends, citing weight gain as an independent factor in outcomes in locally advanced NSCLC. 13 Based on our findings, we believe that both WL and BMI should be incorporated into baseline data for all clinical trials, as this information allows clinicians to incorporate objective evidence into treatment decisions, including additional therapies, palliative care, nutritional interventions and discussing prognosis.
Our study was unique in its use of RCS to create fluid statistical models for the impact of WL on outcomes in different BMI groups stratified by type and stage of malignancy. Although there were no statistically significant results in either limited stage or extensive stage SCLC, both Stage III and Stage IV NSCLC models showed statistically significant differences. There are several possible explanations for these trends, including the smaller sample size of the SCLC group, the role of treatment as a primary determinant in overall outcomes in SCLC, and the overall poorer outcome for patients with SCLC.
In our models, WL > 5% consistently showed a significant impact on overall outcomes in both Stage III and Stage IV NSCLC. This trend was similarly demonstrated in a study by Mytelka et al. evaluating WL during treatment. Their analysis was modelled after Martin's initial framework, but they limited their patient population to individuals with NSCLC. They found that cachexia was a stronger predictor of survival than even disease stage, performance status or age. Additionally, they noted that WL was the primary driver of poorer prognosis, independent of other variables and even more so than BMI. 22 Our study is unique in its complex statistical analysis and comparison between NSCLC and SCLC, with the advantage of using pretreatment baseline characteristics.
There was also a significant difference in nonlinear and overall association outcomes in Stage IV NSCLC in groups with WL ≤ 5%. What is most striking about this information is the fact that outcomes predicted by BMI and WL are most notable in Stage IV groups, suggesting that the role of initial BMI and %WL in determining clinical outcomes is of most utility in advanced stage disease in patients who have less energy stores. As BMI increased, HRs decreased across groups. On the other hand, HRs increased in the WL > 5% group. These findings support the direct relationship between BMI and OS, as well as the inverse relationship between WL and OS.
Patel et al. expanded on some of these findings by noting that individuals with NSCLC who gained weight during treatment with chemotherapy had improved outcomes, including statistically significant increases in OS, progression‐free survival (PFS), and tumour response. 23 A higher BMI was also associated with longer OS and PFS, particularly in the group with both BMI > 25 and a weight gain of >5%. They call for prospective studies to confirm these findings with the ultimate goal of developing therapeutic strategies to target mechanisms of cachexia and ultimately improve survival outcomes. Similar findings were noted in a pooled analysis of patients with NSCLC performed by Le‐Rademacher et al. 24 They found that a WL of 2% or more was associated with poorer OS compared to weight gain and even WL < 2%.
There are limitations to our study. The clinical trials used in this data set spanned the years 1991–2012, with treatment regimens of chemotherapy or chemotherapy and radiation. Given recent advances in treatment with targeted therapies and immunotherapy, the range of OS may be greater than what exists in our study, as these treatment options have radically changed the landscape of lung cancer treatment. How these therapies, BMI and weight changes interact is still somewhat unknown. A recent study of 2110 patients with advanced NSCLC receiving an immune checkpoint inhibitor demonstrated an association between BMI and OS. 25 This study used data only from patients with an ECOG score of 2 or better, which would be similar to the good performance status of the individuals in our study. It is unclear how the incorporation of patients with limited mobility would affect our results. Finally, BMI and %WL are general measurements that do not specifically define lean body or fat mass. They can be impacted by various factors, including oedema, ascites, organ volume and even tumour mass. These variables, however, should have been overcome given the large, diverse sample size of our study population.
It is worth noting that BMI and %WL are not perfect measures of true adiposity or muscle mass. There are many other methods that can be used to define degree of adiposity, including waist circumference and weight to height ratio. Additionally, more sophisticated mechanisms like computed tomography (CT) imaging to determine lean body mass and fat mass along with functional assessments (6‐min walk test, hand grip strength, etc.) can be used to more precisely define body composition. Given the frequency of CT imaging to assess tumour response, using software to characterize skeletal muscle and fat mass on staging scans is a promising area of development to further assess how nutritional deficiencies may impact OS. 26 By screening for early muscle mass loss, interventions, like resistance training, dietary supplementation and pharmacotherapy, could be used to slow the net negative energy balance and show promise in improving outcomes in patients with malignancy. 27
Cancer cachexia is a multifactorial syndrome defined by an ongoing loss of skeletal muscle mass that cannot be fully reversed by conventional nutritional support and leads to progressive functional impairment. 28 There are three major components of this syndrome: altered body composition via skeletal muscle mass loss, negative protein/energy balance due to poor intake and altered metabolism, and ultimate progressive functional impairment. Altered body composition clearly has clinical implications in ultimate functional status, survival, metabolism, immunity and even chemotherapy toxicities. 29 Specifically, it is the loss of muscle mass, or sarcopenia, that has been shown to impact these variables. The differences in outcomes in the two WL groups in our study could certainly be due to this sarcopenia. If we are able to detect sarcopenia early, treatment approaches like nutritional counselling, physical therapy and even pharmacologic interventions could be used to improve outcomes in these patients and continue to shape the future of lung cancer care.
Conclusion
In summary, BMI and WL are both important variables to consider when assessing prognosis or expected survival in individuals with both NSCLC and SCLC. In these specific populations, there appears to be a direct relationship between BMI and OS and an inverse relationship between WL percentage and OS. Using these two variables in combination with one another can provide additional information about OS and can be used to guide clinical decision making. These findings are more pronounced in NSCLC groups compared with SCLC groups, which is one of the new findings from our study. This information supports the ‘obesity paradox’. There is clearly a great need for additional investigation in these areas and for the development and implementation of methods to accurately assess body composition and muscle mass in patients with cancer and other wasting disorders. BMI and WL should be the baseline data that are incorporated into future clinical trials in individuals with lung cancer, as we evaluate the impact of newer treatment options on outcomes for our patients.
Conflict of interest
The authors declare that no conflict of interest relevant to this article exists.
Funding
The study is supported by grant no. R21‐AG042894 from the National Institutes of Health National Institute on Aging.
Supporting information
Figure S1. A Sample Size for BMI × Weight Loss Groups for NSCLC Cohort B Sample Size for BMI Weight Loss Groups for SCLC Cohort
Figure S2. Ten Subgroups Identified by BMI and WL
Figure S3A. Kaplan–Meier Survival Curve for Ten Subgroups within the NSCLC Cohort
Figure S3B. Kaplan–Meier Survival Curve for Ten Subgroups within the SCLC Cohort
Table S1. p‐values for RCS Non‐Linear and Overall Associations by Malignancy Type
* Represents a statistically significant p‐value
Figure S4A. Limited Stage SCLC RCS of BMI & Survival in WL ≤ 5% Group
Figure S4B. Limited Stage SCLC RCS of BMI & Survival in WL ≥ 5% Group
Figure S4A. Limited Stage SCLC RCS of BMI & Survival in WL ≤ 5% Group
Figure S4B. Limited Stage SCLC RCS of BMI & Survival in WL ≥ 5% Group
Figure S4C. Extensive Stage SCLC RCS of BMI & Survival in WL ≤ 5% Group
Figure S4D. Extensive Stage SCLC RCS of BMI & Survival in WL ≥ 5% Group
Table S2. Sample Sizes and Overall Percentage of Each Malignancy Subgroup by WL
Figure S5. Nomogram of Coefficients of Multivariable Piecewise Cox Proportional Hazards Model
Oswalt C., Liu Y., Pang H., Le‐Rademacher J., Wang X., and Crawford J., (2022) Associations between body mass index, weight loss and overall survival in patients with advanced lung cancer, Journal of Cachexia, Sarcopenia and Muscle, 13, 2650–2660, doi: 10.1002/jcsm.13095
Cameron Oswalt and Yingzhou Liu contributed equally to this paper.
REFERENCES
- 1. Ward ZJ, Bleich SN, Cradock AL, Barrett JL, Giles CM, Flax C, et al. Projected U.S. state‐level prevalence of adult obesity and severe obesity. N Engl J Med 2019;381:2440–2450. [DOI] [PubMed] [Google Scholar]
- 2. di Angelantonio E, Bhupathiraju SN, Wormser D, Gao P, Kaptoge S, de Gonzalez AB, et al. Body‐mass index and all‐cause mortality: individual‐participant‐data meta‐analysis of 239 prospective studies in four continents. Lancet 2016;388:776–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pi‐Sunyer X. The medical risks of obesity. Postgrad Med 2009;121:21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Adams KF, Schatzkin A, Harris TB, Kipnis V, Mouw T, Ballard‐Barbash R, et al. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med 2006;355:763–778. [DOI] [PubMed] [Google Scholar]
- 5. Galanos AN, Pieper CF, Kussin PS, Winchell MT, Fulkerson WJ, Harrell FE, et al. Relationship of body mass index to subsequent mortality among seriously ill hospitalized patients. Crit Care Med 1997;25:1962–1968. [DOI] [PubMed] [Google Scholar]
- 6. Wong CJ. Involuntary weight loss. Med Clin 2014;98:625–643. [DOI] [PubMed] [Google Scholar]
- 7. Yang R, Cheung MC, Pedroso FE, Byrne MM, Koniaris LG, Zimmers TA. Obesity and weight loss at presentation of lung cancer are associated with opposite effects on survival. J Surg Res 2011. Sept;170:e75–e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Spelta F, Fratta Pasini AM, Cazzoletti L, Ferrari M. Body weight and mortality in COPD: focus on the obesity paradox. Eat Weight Disord 2018;23:15–22. [DOI] [PubMed] [Google Scholar]
- 9. Obi Y, Qader H, Kovesdy CP, Kalantar‐Zadeh K. Latest consensus and update on protein‐energy wasting in chronic kidney disease. Curr Opin Clin Nutr Metab Care 2015;18:254–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vest AR, Chan M, Deswal A, Givertz MM, Lekavich C, Lennie T, et al. Nutrition, obesity, and cachexia in patients with heart failure: a consensus statement from the Heart Failure Society of America Scientific Statements Committee. J Card Fail 2019;25:380–400. [DOI] [PubMed] [Google Scholar]
- 11. Kalantar‐Zadeh K, Horwich TB, Oreopoulos A, Kovesdy CP, Younessi H, Anker SD, et al. Risk factor paradox in wasting diseases. Curr Opin Clin Nutr Metab Care 2007;10:433–442. [DOI] [PubMed] [Google Scholar]
- 12. Lennon H, Sperrin M, Badrick E, Renehan AG. The obesity paradox in cancer: a review. Curr Oncol Rep 2016;18:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dahlberg SE, Schiller JH, Bonomi PB, Sandler AB, Brahmer JR, Ramalingam SS, et al. Body mass index and its association with clinical outcomes for advanced non‐small‐cell lung cancer patients enrolled on Eastern Cooperative Oncology Group clinical trials. J Thorac Oncol 2013;8:1121–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Martin L, Senesse P, Gioulbasanis I, Antoun S, Bozzetti F, Deans C, et al. Diagnostic criteria for the classification of cancer‐associated weight loss. J Clin Oncol 2015;33:90–99. [DOI] [PubMed] [Google Scholar]
- 15. Pang HH, Wang X, Stinchcombe TE, Wong ML, Cheng P, Ganti AK, et al. Enrollment trends and disparity among patients with lung cancer in national clinical trials, 1990 to 2012. J Clin Oncol Off J Am Soc Clin Oncol 2016;34:3992–3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Desquilbet L, Mariotti F. Dose‐response analyses using restricted cubic spline functions in public health research. Stat Med 2010;29:1037–1057. [DOI] [PubMed] [Google Scholar]
- 17. Harrell FE Jr. Regression modeling strategies: with applications to linear models, logistic and ordinal regression, and survival analysis, 2nd ed. New York: Springer; 2015. [Google Scholar]
- 18. Croxford R. Restricted cubic spline regression: a brief introduction. London: Elsevier; 2016. [Google Scholar]
- 19. Zhang Z, Kattan MW. Drawing nomograms with R: applications to categorical outcome and survival data. Ann Transl Med 2017;5:211–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Girnun GD, Naseri E, Vafai SB, Qu L, Szwaya JD, Bronson R, et al. Synergy between PPARγ ligands and platinum‐based drugs in cancer. Cancer Cell 2007;11:395–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shackelford DB, Abt E, Gerken L, Vasquez DS, Seki A, Leblanc M, et al. LKB1 inactivation dictates therapeutic response of non‐small cell lung cancer to the metabolism drug phenformin. Cancer Cell 2013;23:143–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mytelka DS, Li L, Benoit K. Post‐diagnosis weight loss as a prognostic factor in non‐small cell lung cancer. J Cachexia Sarcopenia Muscle 2018;9:86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Patel JD, Pereira JR, Chen J, Liu J, Guba SC, John WJ, et al. Relationship between efficacy outcomes and weight gain during treatment of advanced, non‐squamous, non‐small‐cell lung cancer patients. Ann Oncol 2016;27:1612–1619. [DOI] [PubMed] [Google Scholar]
- 24. le‐Rademacher J, Lopez C, Wolfe E, Foster NR, Mandrekar SJ, Wang X, et al. Weight loss over time and survival: a landmark analysis of 1000+ prospectively treated and monitored lung cancer patients. J Cachexia Sarcopenia Muscle 2020;11:1501–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kichenadasse G, Miners JO, Mangoni AA, Rowland A, Hopkins AM, Sorich MJ. Association between body mass index and overall survival with immune checkpoint inhibitor therapy for non‐small cell lung cancer. JAMA Oncol 2020;6:512–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Crawford J. What are the criteria for response to cachexia treatment? Ann Palliat Med 2019;8:43–49. [DOI] [PubMed] [Google Scholar]
- 27. Brown JC, Cespedes Feliciano EM, Caan BJ. The evolution of body composition in oncology‐epidemiology, clinical trials, and the future of patient care: facts and numbers. J Cachexia Sarcopenia Muscle 2018;9:1200–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011;12:489–495. [DOI] [PubMed] [Google Scholar]
- 29. Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population‐based study. Lancet Oncol 2008;9:629–635. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. A Sample Size for BMI × Weight Loss Groups for NSCLC Cohort B Sample Size for BMI Weight Loss Groups for SCLC Cohort
Figure S2. Ten Subgroups Identified by BMI and WL
Figure S3A. Kaplan–Meier Survival Curve for Ten Subgroups within the NSCLC Cohort
Figure S3B. Kaplan–Meier Survival Curve for Ten Subgroups within the SCLC Cohort
Table S1. p‐values for RCS Non‐Linear and Overall Associations by Malignancy Type
* Represents a statistically significant p‐value
Figure S4A. Limited Stage SCLC RCS of BMI & Survival in WL ≤ 5% Group
Figure S4B. Limited Stage SCLC RCS of BMI & Survival in WL ≥ 5% Group
Figure S4A. Limited Stage SCLC RCS of BMI & Survival in WL ≤ 5% Group
Figure S4B. Limited Stage SCLC RCS of BMI & Survival in WL ≥ 5% Group
Figure S4C. Extensive Stage SCLC RCS of BMI & Survival in WL ≤ 5% Group
Figure S4D. Extensive Stage SCLC RCS of BMI & Survival in WL ≥ 5% Group
Table S2. Sample Sizes and Overall Percentage of Each Malignancy Subgroup by WL
Figure S5. Nomogram of Coefficients of Multivariable Piecewise Cox Proportional Hazards Model


