Abstract
Background
Fibro‐adipogenic progenitors (FAPs) in the muscles have been found to interact closely with muscle progenitor/stem cells (MPCs) and facilitate muscle regeneration at normal conditions. However, it is not clear how FAPs may interact with MPCs in aged muscles. Senolytics have been demonstrated to selectively eliminate senescent cells and generate therapeutic benefits on ageing and multiple age‐related disease models.
Methods
By studying the muscles and primary cells of age matched WT mice and Zmpste24−/− (Z24−/−) mice, an accelerated ageing model for Hutchinson–Gilford progeria syndrome (HGPS), we examined the interaction between FAPs and MPCs in progeria‐aged muscle, and the potential effect of senolytic drug fisetin in removing senescent FAPs and improving the function of MPCs.
Results
We observed that, compared with muscles of WT mice, muscles of Z24−/− mice contained a significantly increased number of FAPs (2.4‐fold; n > =6, P < 0.05) and decreased number of MPCs (2.8‐fold; n > =6, P < 0.05). FAPs isolated from Z24−/− muscle contained about 44% SA‐β‐gal+ senescent cells, in contrast to about 3.5% senescent cells in FAPs isolated from WT muscle (n > =6, P < 0.001). The co‐culture of Z24−/− FAPs with WT MPCs resulted in impaired proliferation and myogenesis potential of WT MPCs, with the number of BrdU positive proliferative cells being reduced for 3.3 times (n > =6, P < 0.001) and the number of myosin heavy chain (MHC)‐positive myotubes being reduced for 4.5 times (n > =6, P < 0.001). The treatment of the in vitro co‐culture system of Z24−/− FAPs and WT MPCs with the senolytic drug fisetin led to increased apoptosis of Z24−/− FAPs (14.5‐fold; n > =6, P < 0.001) and rescued the impaired function of MPCs by increasing the number of MHC‐positive myotubes for 3.1 times (n > =6, P < 0.001). Treatment of Z24−/− mice with fisetin in vivo was effective in reducing the number of senescent FAPs (2.2‐fold, n > =6, P < 0.05) and restoring the number of muscle stem cells (2.6‐fold, n > =6, P < 0.05), leading to improved muscle pathology in Z24−/− mice.
Conclusions
These results indicate that the application of senolytics in the progeria‐aged muscles can be an efficient strategy to remove senescent cells, including senescent FAPs, which results in improved function of muscle progenitor/stem cells. The senescent FAPs can be a potential novel target for therapeutic treatment of progeria ageing related muscle diseases.
Keywords: Senolytics, Progeria, Cellular senescence, Muscle stem cells, Muscle atrophy
Introduction
Muscle progenitor/stem cells (MPCs) progressively lose their capacity for proliferation and myogenic differentiation during the ageing process, 1 , 2 likely through both autonomous and non‐autonomous mechanisms. 3 , 4 The cell autonomous mechanism mainly involves increased DNA damage, telomere shortening and activation of chronic inflammatory signalling (i.e. NF‐κB), whereas the cell non‐autonomous mechanism involves the regulatory roles of other types of neighbouring cells in the tissues on the function of local stem cells. 3 , 4 , 5 However, the mechanism of MPCs being regulated by neighbouring cells during the ageing process of muscles remains largely unknown.
Adjacent to MPCs in the skeletal muscle are myofibres, immune cells, blood vessel endothelial cells and fibro‐adipogenic progenitors (FAPs). 6 , 7 , 8 FAPs are tissue‐resident mesenchymal stromal cells (MSCs) characterized by the high expression of PDGFR‐α that play important roles in the homeostasis and repair of multiple tissues. 9 , 10 In the skeletal muscle, FAPs interact closely with MPCs and facilitate muscle regeneration in normal conditions. 10 , 11 However, in diseased or dystrophic muscle, FAPs can play a negative role for proper muscle regeneration by promoting fibrosis and fatty infiltration. 12 , 13 , 14 Our recent study in a muscular dystrophy mouse model demonstrated that partial ablation of FAPs in dystrophic muscle was able to mitigate muscle damage and delay the loss of muscle function. 15
Senescent cells (SnCs) accumulate with age in multiple tissues where they are considered to be the driving factor for numerous age‐related disorders. 16 , 17 Senescence is a cell fate elicited in response to external and internal cellular stress signals, which causes extensive changes in gene expression, histone modifications, organelle function, elevated protein production and profound morphologic and metabolic shifts. 18 A significant fraction of SnCs release inflammatory factors, chemokines, growth factors, proteases, bioactive lipids, prostenoids, extracellular vesicles and pro‐coagulant factors, termed the senescence‐associated secretory phenotype or SASP. 19 , 20 , 21 The genetic or pharmacologic elimination of senescent cells in mouse models has shown therapeutic benefits on ageing and multiple age‐related disease models. 21 , 22 , 23 , 24 Compounds able to induce apoptosis of SnCs specifically, termed senolytics, include the combination of dasatinib and quercetin (D + Q), navitoclax (ABT263), 17‐DMAG and fisetin. 21 , 22 , 23 , 25 , 26 , 27 , 28 , 29 Senolytics selectively eliminate senescent cells by targeting senescent cell anti‐apoptotic (SCAP) signalling pathways that are overtly upregulated in senescent cells, such as BCL2 and BCL‐xL. 21 , 22 , 23 , 25
Hutchinson–Gilford progeria syndrome (HGPS) is an autosomal dominant disease associated with premature ageing (progeria). 30 , 31 Among various ageing‐related symptoms, severe muscle atrophy is also developed in HGPS patients. 30 , 31 Cells in HGPS patients is defective in generating normal Lamin A, a nuclear envelope protein. 30 , 32 In normal cells, prelamin A needs to undergo a final processing step mediated by the zinc metalloprotease Zmpste24 that catalyses the cleavage of farnesylated cysteine to produce mature, unfarnesylated lamin A; however, genetic mutations in HGPS cells diminish this final proteolytic cleavage step, resulting in the accumulation of permanently farnesylated forms of prelamin A. 30 , 31 , 32 Farnesyl‐prelamin A is targeted to the nuclear envelope, where it interferes with the integrity of the nuclear envelope and causes misshapen cell nuclei. 30 , 31 , 32 Zmpste24 is Lamin A‐processing zinc metalloproteinase required for generating normal Lamin A, and knocking out Zmpste24 (Z24−/−) in mice leads to accelerated ageing and ageing‐related pathologies common to HGPS. Z24−/− mice have been studied as an important murine model for HGPS and progeria ageing. 33 , 34 , 35 Z24−/− mice are short lived (~6 months) and develop severe musculoskeletal abnormalities including muscle atrophy. 33 , 34 , 35
In this study, we have utilized the Z24−/− mouse model to investigate the potential correlation between FAPs and MPCs in aged muscle tissue. Our recent study of skeletal muscle of Z24−/− mice revealed the increased dominance of PDGFR‐α+ FAPs and exhaustion of MPCs in skeletal muscle, leading to a higher ratio of FAPs/MPCs. 36 Here, we demonstrate that Z24−/− FAPs undergo cellular senescence and impair the function of MPCs through a paracrine effect. Depletion of senescent FAPs with the senolytic drug fisetin improved the function of MPCs (i.e. proliferation and myogenesis potentials), suggesting a clinically relevant approach for improving MPC function in aged skeletal muscle and potential treatment of HGPS.
Methods
Animal models
Zmpste24−/− (Z24−/−) mice are deficient in Zmpste24, a metalloproteinase involved in the formation of mature lamin A, and are an established model for HGPS disease and premature ageing. Age‐matched C57/BL6J mice were used as wild‐type (WT) controls. All mice were housed and maintained in the Center for Laboratory Animal Medicine and Care (CLAMC) at UTHealth (University of Texas Health Science Center at Houston) in accordance with established guidelines and protocols approved by the UTHealth Animal Welfare Committee. Both male and female mice were used for this study.
Isolation of FAPs and MPCs
Gastrocnemius muscles of Z24−/− mice and WT mice (~5 months old) were surgically harvested and incubated in minimal essential media containing 0.1% Collagenase A at 37°C for 1.5 h, as previously described. 36 , 37 MSCs adhere to the culture surface quickly in hours, and MPCs maintain floating in medium and only attach and start to grow 3 or 4 days later. 36 , 37 MPCs were then cultured in the proliferation medium (DMEM with 20% FBS and 1% CEE/chicken embryo extracts). 36 , 37 FAPs (PDGFR‐α + MSCs) were isolated and purified from MSCs with flow cytometry sorting after incubation with anti‐PDGFRα antibody (Cell Signaling). FAPs were known to specifically express PDGFR‐α, but not PDGFR‐β (a marker for perivascular cells such as pericytes), and PDGFR‐β was applied as a negative marker in cell sorting here to avoid potential collection of cells positive with both PDGFR‐α and PDGFR‐β. FAPs were then cultured in growth medium (DMEM supplemented with 10% FBS). Muscle stem/progenitor cells (MPCs) were isolated fusing the modified preplate technique, 36 , 37 based on their slow adhering capacity to collagen‐coated surface/substrate. MPCs were then cultured in growth medium (DMEM supplemented with 20% FBS and 1% chicken embryo essentials/CEE).
mRNA analysis via reverse transcriptase‐PCR
Total RNA was obtained from MPCs using the RNeasy Mini Kit (Qiagen, Inc., Valencia, CA) according to the manufacturer's instructions. Reverse transcription was performed using an iScript cDNA Synthesis Kit (Bio‐Rad Laboratories, Inc., Hercules, CA). Quantitative real‐time PCR was performed using an iCycler thermal cycler (Bio‐Rad Laboratories). The gene‐specific primer sequences used are listed in Table 1 for TNF‐α, IL‐1α, IL‐1β, CXCL1, MCP1, IFN‐γ, TGF‐β1, p16 and IL‐10 and Gapdh, which was used as an internal control to normalize gene expression. The cycling parameters used for all primers were as follows: 95°C for 10 min; PCR, 40 cycles of 30 s at 95°C for denaturation, 1 min at 54°C for annealing and 30 s at 72°C for extension. All data were normalized to the expression of GAPDH.
Table 1.
RT‐PCR primer sequences
| Gene | Primer sequence |
|---|---|
| GAPDH |
Forward: TCCATGACAACTTTGGCATTG Reverse: TCACGCCACAGCTTTCCA |
| TNF‐α |
Forward: CCTGTAGCCCACGTCGTAG Reverse: GGGAGTAGACAAGGTACAACCC |
| TGF‐β1 |
Forward: CTCCCGTGGCTTCTAGTGC Reverse: GCCTTAGTTTGGACAGGATCTG |
| IL‐1α |
Forward: TCTCAGATTCACAACTGTTCGTG Reverse: AGAAAATGAGGTCGGTCTCACTA |
| IL‐1β |
Forward: GCAACTGTTCCTGAACTCAACT Reverse: ATCTTTTGGGGTCCGTCAACT |
| CXCL1 |
Forward: CTGGGATTCACCTCAAGAACATC Reverse: CAGGGTCAAGGCAAGCCTC |
| MCP1 |
Forward: TAAAAACCTGGATCGGAACCAAA Reverse: GCATTAGCTTCAGATTTACGGGT |
| IFN‐γ |
Forward: CAGCAACAGCAAGGCGAAAAAGG Reverse: TTTCCGCTTCCTGAGGCTGGAT |
| p16 |
Forward: CGCAGGTTCTTGGTCACTGT Reverse: TGTTCACGAAAGCCAGAGCG |
| IL‐10 |
Forward: ATTTGAATTCCCTGGGTGAGAAG Reverse: CACAGGGGAGAAATCGATGACA |
SA‐β‐gal staining for senescent cells
The percent of senescent cells cultured in vitro and in skeletal muscle tissue was measured using the SA‐β‐gal Staining Kit (Cell Signaling Technology) following the manufacturer's protocol. The number of cells positive for β‐gal activity at pH 6, a known characteristic of senescent cells, was determined. The quantification of SA‐β‐gal+ cells in muscle tissues was performed by calculating the ratio of the number of SA‐β‐gal+ cells to the number of myofibres (number of SA‐β‐gal+ cells/number of myofibres).
Cell co‐culture system of FAPs and MPCs
MPCs from WT muscle were seeded in the lower chamber of a transwell, with WT FAPs or Z24−/− FAPs being seeded in the upper chamber. The pore size of the transwell membrane was 0.4 μm and coated with a layer of Matrigel (20% of Matrigel in water, 0.2 cm of thickness).
Fisetin treatment of cell co‐culture system
For in vitro cell treatment, fisetin (Selleckchem, Houston TX) was applied to treat the cell co‐culture system of Z24−/− FAPs and WT MPCs at 20 μM of concentration, and the proliferation assay and myogenesis assay of WT MPCs were performed and compared with non‐treated controls.
Proliferation assay of WT‐MPCs in co‐culture system
For proliferation assay, WT MPCs revived from frozen vial were seeded as ~70% confluence in the lower chamber of transwell, with WT or Z24−/− FAPs being co‐cultured in the upper chamber (8000 cells/cm2). Cells were then co‐cultured in the proliferation medium (DMEM medium containing 20% FBS and 1% CEE) for 24 h (with or without fisetin) and continued to be cultured with BrdU added in the medium for another 12 h. Thus, MPCs were fixed before cells reaching confluency to make sure the cells were examined during their proliferation state. MPCs were then imaged to compare the number of BrdU+ WT MPCs for proliferation potential in different cell groups (WT FAPs+ WT FAPs, WT FAPs+ Z24−/− FAPs, WT FAPs+ WT FAPs+ fisetin and WT FAPs+ Z24−/− FAPs+ fisetin).
Myogenesis assay of WT‐MPCs in co‐culture system
For myogenesis assay, WT MPCs were seeded as ~90% confluence in the lower chamber of transwell, with WT or Z24−/− FAPs being co‐cultured in the upper chamber (8000 cells/cm2); cells were co‐cultured in the myogenic differentiation medium (DMEM medium containing 2% horse serum/HS), and allowed for differentiation for 96 h with or without fisetin, before being fixed and imaged to compare the number of myotubes formed by WT MPCs in different cell groups (WT FAPs+ WT FAPs, WT FAPs+ Z24−/− FAPs, WT FAPs+ WT FAPs+ fisetin and WT FAPs+ Z24−/− FAPs+ fisetin). Multinucleated cells with two or more nuclei in the cell were classified as myotubes. Immunofluorescent staining of fMHC (fast‐type myosin heavy chain, a marker of myofibres/myotubes) was performed to mark myotubes and indicate the progression of myogenic differentiation.
Fisetin treatment of mice
For in vivo treatment of Z24−/− mice, fisetin was solved in 10% EtOH and 90% PEG‐400 in 10 mg/ml stock, and 4‐month‐old mice were weighed and given fisetin (100 mg/kg) weekly via oral gavage for 4 weeks. A control group of Z24−/− mice were given the same volume of 10% EtOH and 90% PEG‐400 vehicle solution as the group with fisetin treatment. Muscle tissues (gastrocnemius) were then harvested and frozen for histology assays.
Immunofluorescent staining and imaging
Cultured FAPs and MPCs were fixed with 4% paraformaldehyde, and frozen tissue sections were fixed with 10% formalin. The primary antibodies used ‐ PDGFR‐α (Cell Signaling), CD68 (Abcam), p‐4E‐BP1 (Cell Signaling), Lamin A/C (Cell Signaling), fMHC (fast‐type myosin heavy chain, R&D Systems), dystrophin (Abcam), collagen type IV (Cell Signaling) and Pax7 (DSHB) were all applied at a 1:100 to 1:300 dilution. Cell nucleus was stained with DNA binding reagent 4′,6‐diamidino‐2‐phenylindole (DAPI). Immunofluorescent images of cells were imaged and photographed with a Nikon high‐resolution microscope.
Trichrome staining
Fibrosis formation in muscle tissues was visualized by Masson trichrome staining with the Trichrome Stain (Masson) Kit (Sigma‐Aldrich). Sections were incubated in Weigert's iron haematoxylin working solution for 10 min and rinsed under running water for 10 min. Slides were transferred to Biebrich scarlet‐acid fuchsin solution for 15 min before incubation in aniline blue solution for another 5 min. Slides were then rinsed, dehydrated and mounted as earlier. The ratio of the area of fibrotic collagen (blue) to the area of normal muscle (red) was quantified to measure fibrosis formation.
Muscle strength test
Four‐limb hanging test was performed to measure and compare muscle strength of Z24−/− mice with or without fisetin treatment. Four‐limb hanging test was done as described in TREAT‐NMD standard operating procedure DMD_M.2.1.005. 38 , 39 Briefly, the mouse was placed on a wire grid and allowed to accommodate to this environment for 3–5 s before the wire grid being turned upside down above a cage filled with bedding. The wire grid holding time (or ‘hang time’ in seconds) is defined as the amount of time that it takes the mouse to fall from the inverted screen, and is measured from the time the wire grid is inverted to the time that the mouse falls off the wire grid. The holding impulse associated with the hang test was calculated as the longest hanging time multiplied by the body weight (given as Newton‐second; conversion factor is 9.806 Newton/kg).
Measurements of results and statistical analysis
Image analysis was performed using Nikon NIS‐Elements (Nikon Instruments, Inc.) and ImageJ software (version 1.32j; National Institutes of Health, Bethesda, MD). Data from at least six samples from each subject were pooled for statistical analysis. Prism software (GraphPad) was used to plot graphs as mean ± standard deviation (SD). The statistical significance of any difference was calculated using Student's t‐test or one‐way ANOVA test. P values < 0.05 were considered statistically significant.
Results
Z24−/− muscle displays increased number of FAPs and decreased number of muscle stem cells
To interrogate the relative number of FAPs and MPCs in the Z24−/− model, immunofluorescent staining of PDGFR‐α (a marker for FAPs) and Pax7 (a marker for muscle progenitor/stem cells, MPCs) was performed with cryosections of gastrocnemius muscle from aged‐matched 5‐month‐old WT and Z24−/− mice. Co‐staining of dystrophin was performed to verify the interstitial location of PDGFR‐α + FAPs or Pax7 + MPCs among myofibres. Results showed that, compared with WT muscle, there was a significant increase in the number of PDGFR‐α + cells and a decrease in the number of Pax7 + MPCs in Z24−/− muscle (Figure 1A), demonstrating an increased ratio of FAPs/MPCs in the muscle of Z24−/− mice.
Figure 1.
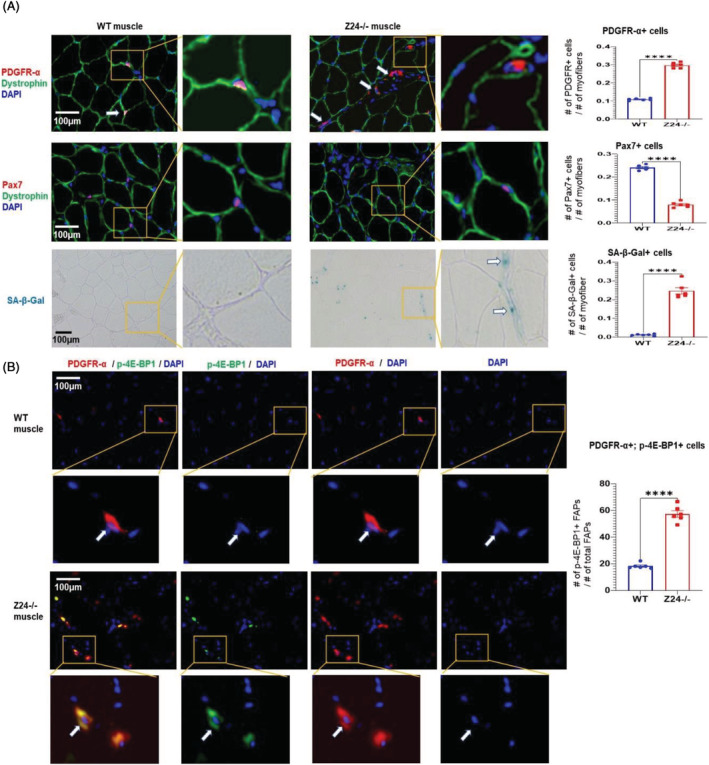
Increased number of senescent FAPs in the Zmpste24 −/− muscle. (A) Immunofluorescent staining were performed with WT and Z24−/− muscle tissues for PDGFR‐α + FAPs, and Pax7 + MPCs, with co‐staining of dystrophin being applied to verify the interstitial location of PDGFR‐α + FAPs or Pax7 + MPCs among myofibres. SA‐β‐Gal staining was performed to identify senescent cells. The number of FAPs and senescent cells was increased in Z24−/− muscle, whereas the number of MPCs was decreased. Data analysis was performed with random pictures from each group of muscle sections, and the sample number here N = 6 (biological replicates). (B) Co‐immunofluorescent staining of WT and Z24−/− muscle tissues for PDGFR‐α+/p‐4E‐BP1+ cells, confirming that a higher fraction of FAPs is positive with p‐4E‐BP1 in Z24−/− muscle. Data analysis was performed with random pictures from each group of muscle sections, and the sample number here N = 6 (biological replicates).
FAPs in Z24−/− muscle develop a senescence‐like phenotype
To explore the presence of senescent cells in skeletal muscle from Z24−/− mice, cryosections of skeletal muscle of WT and Z24−/− mice stained for SA‐β‐gal showed that there is increase in SA‐β‐Gal+ cells in Z24−/− muscle (Figure 1A). mTORC1 activation is a hallmark of senescent cells, which is independent of growth factor signalling and is relevant to higher metabolic stress and cellular dysfunction. 40 , 41 Immunofluorescence staining of PDGFR‐α and p‐4E‐BP1 (a substrate of mTORC1) indicated that a large fraction of PDGFR‐α + FAPs were also positive with p‐4E‐BP1 (Figure 1B), indicating higher mTORC1 activation in these FAPs. In order to further characterize potential cellular senescence in Z24−/− FAPs, PDGFR‐α + FAPs were isolated from WT and Z24−/− gastrocnemius muscles via flow cytometry‐based cell sorting (Figure 2A) and cultured for 6 h before being fixed for p‐4E‐BP1 immunostaining and SA‐β‐gal staining (~2 × 104 FAPs/one well of 24‐well plate). In contrast to WT FAPs, it showed that there were much more Z24−/− FAPs being positive with p‐4E‐BP1 and SA‐β‐gal ( a marker of cellular senescence) (Figure 2B), suggesting increased cellular senescence in Z24−/− FAPs. Thus, in Z24−/− muscle, there was increased cellular senescent in FAPs and decreased number of MPCs, highlighting a potential interactive correlation between the two types of cells, which is to be elucidated in the following experiments.
Figure 2.
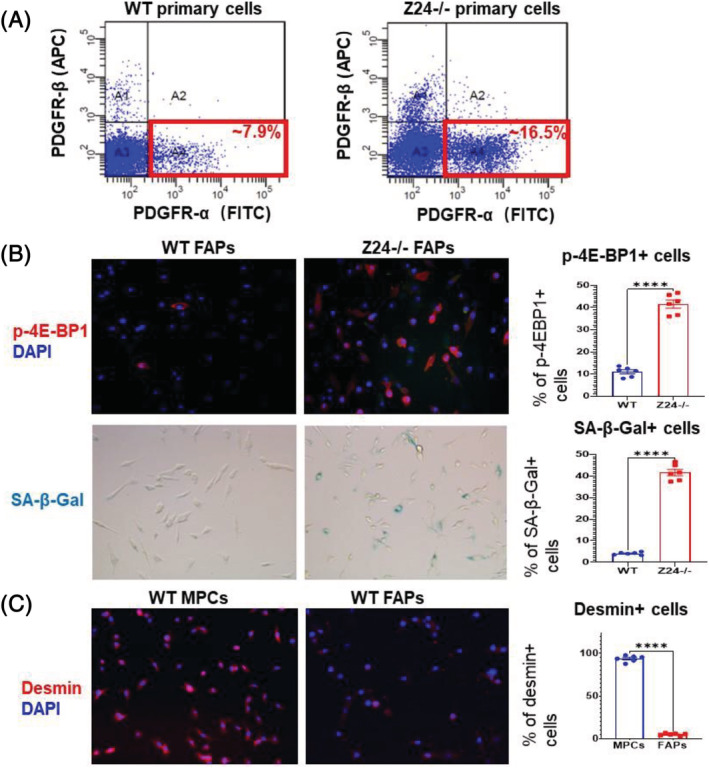
Isolated primary FAPs from aged muscle contain higher ratio of senescent cells. (A) PDGFR‐α + FAPs were isolated from WT and Z24−/− skeletal muscle by FACS based on PDGFR‐α expression. (B) Immunofluorescent staining of WT and Z24−/− FAPs for p‐4E‐BP1 show that the ratio of p‐4E‐BP1+ cells are higher in Z24−/− FAPs, compared with WT FAPs; SA‐β‐gal staining for senescent cells further confirm that there is increased ratio of senescent cells in Z24−/− FAPs. Data analysis was performed with random pictures from each group of cells, and the sample number here N = 6 (three biological replicates with two technical replicates for each). (C) Immunofluorescent staining of WT MPCs and WT FAPs for desmin to verify the myogenic nature of MPCs, in contrast to FAPs. Data analysis was performed with random pictures from each group of cells, and the sample number here N = 6 (three biological replicates with two technical replicates for each).
Co‐culture of Z24−/− FAPs with WT MPCs results in impaired function of WT MPCs
Given the increased number of FAPs with senescent signatures in Z24−/− muscle, we next set up cell co‐culture system to examine the potential impact of FAPs from Z24−/− muscle on healthy muscle stem cells. MPCs were isolated from the muscle of WT mice, and the myogenic purity of the cell population was verified by immunofluorescent staining of desmin (a marker for myogenic cells), in contrast to FAPs from muscle of WT mice (Figure 2C). FAPs from muscles of WT or Z24−/− mice were co‐cultured with WT MPCs in a transwell system, with FAPs in the upper chamber and WT MPCs in the lower chamber. Cell proliferation and myogenesis assays of WT MPCs showed that Z24−/− FAPs in the cell co‐culture system led to the impaired proliferation and myogenesis potential of WT MPCs (Figure 3A–C).
Figure 3.
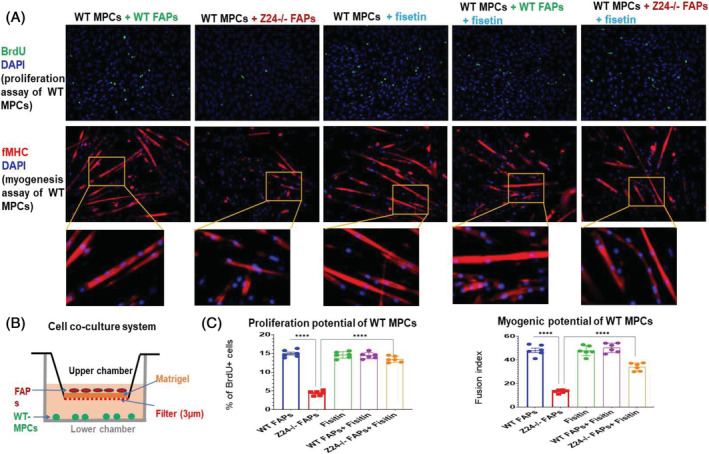
Fisetin treatment of cell co‐culture system of Z24−/− FAPs and WT MPCs. (A) BrdU proliferation assay and myogenesis assay showed that the proliferation and myogenesis potentials of WT MPCs were decreased when being co‐cultured with Z24−/− FAPs; while the treatment of the co‐culture system of WT MPCs and Z24−/− with fisetin effectively rescued the proliferation potential of the WT MPCs; also, fisetin treatment of WT MPCs or co‐culture system of WT MPCs and WT FAPs did not affect the proliferation and myogenesis potentials of WT MPCs. Immunofluorescent staining of f‐MHC (fast‐type myosin heavy chain) in WT MPCs was performed to compare myogenesis potential (myotube formation) in the co‐culture system. (B) Schematic presentation of the transwell co‐culture system with WT FAPs or Z24−/− FAPs being cultured in the upper chamber and WT MPCs being cultured in the lower chamber. (C) Quantitation of proliferation and myogenesis of the different groups shown in (A). The quantification of myotube formation was presented by the fusion index of muscle cells, which is the number of nucleus incorporated into multinucleate myotubes (f‐MHC+), compared with total number of nuclei. Data analysis was performed with random pictures from each group of cells, and the sample number here N = 6 (three biological replicates with two technical replicates for each).
Treatment of Z24−/− FAPs with fisetin rescues the impaired function of co‐cultured WT MPCs
In order to directly implicate the potential effect of senescent FAPs from Z24−/− skeletal muscle in impacting the function of healthy WT MPC, we examined whether the removal of senescent cells in FAPs with senolytic drug fisetin might rescue the function of MPCs from healthy WT donors. When fisetin (20 μM) was applied to the cell co‐culture system of Z24−/− FAPs and WT MPCs for 48 h, there was improved proliferation and myogenesis capacities of WT MPCs compared with non‐treated controls (Figure 3A–C ). Fisetin treatment of WT MPCs was also performed as a control group here to verify the specific effect of fisetin on senescent cells (Figure 3A–C ).
Fisetin treatment of Z24−/− FAPs causes the apoptosis of senescent cells
In order to verify that fisetin can cause the apoptosis of senescent cells in Z24−/− FAPs, WT FAPs and Z24−/− FAPs were treated with or without fisetin for 36 h before performing SA‐β‐Gal staining. It showed that the percentage of SA‐β‐Gal+ cells in Z24−/− FAPs was obviously reduced with fisetin treatment (Figure 4A). Also, TUNEL assay was performed with WT FAPs and Z24−/− FAPs treated with or without fisetin for 24 h, and it showed that there was increased apoptotic cells in Z24−/− FAPs treated with fisetin, but not in WT FAPs (Figure 4B). Further, qRT‐PCR analysis of mRNA from WT FAPs, Z24−/− FAPs and fisetin‐treated Z24−/− FAPs demonstrated up‐regulated expression of SASP factors and p16 in Z24−/− FAPs, which however was down‐regulated by fisetin treatment of Z24−/− FAPs (Figure 4C).
Figure 4.
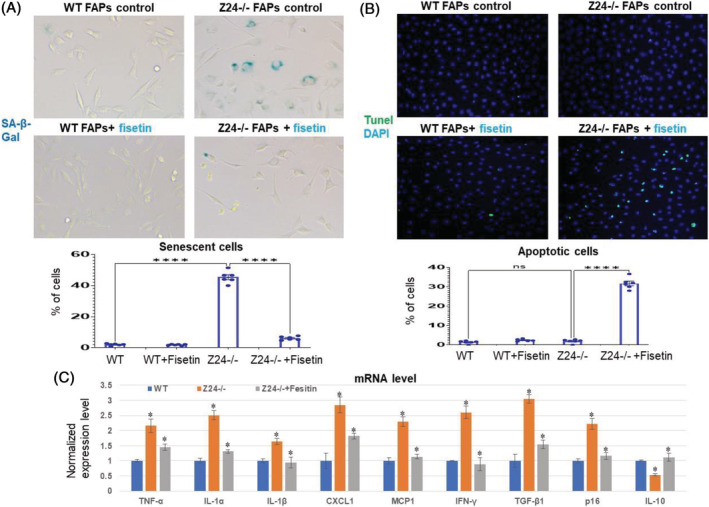
Fisetin treatment of WT and Z24−/− FAPs. (A) WT FAPs and Z24−/− FAPs were treated with or without fisetin for 36 h before performing SA‐β‐gal staining. It showed that the percentage of SA‐β‐gal+ cells in Z24−/− FAPs was obviously reduced with fisetin treatment. Data analysis was performed with random pictures from each group of cells, and the sample number here N = 6 (three biological replicates with two technical replicates for each). (B) TUNEL assay was performed with WT FAPs and Z24−/− FAPs treated with or without fisetin for 24 h, and it showed that there was obviously increased number of apoptotic cells in Z24−/− FAPs treated with fisetin, but not in WT FAPs. Data analysis was performed with random pictures from each group of cells, and the sample number here N = 6 (three biological replicates with two technical replicates for each). (C) Quantitative RT‐PCR results for gene expression level of SASP factors, p16 and IL‐10 in WT FAPs, Z24−/− FAPs and Z24−/− FAPs treated with fisetin.
Treatment of Z24−/− mice with fisetin leads to increased number of muscle stem cells and improved muscle phenotypes
In order to investigate the efficacy of senolytics on muscle health in vivo, adult Z24−/− mice were treated with fisetin (100 mg/kg bw) once per week via oral gavage for 4 weeks. Immunostaining of muscle cryosections demonstrated fisetin treatment decreased the number of PDGFR‐α + cells and increased the number of Pax7 + MPCs (Figure 5 ). In addition, muscle pathology was also improved as evidenced by reduced fibrosis formation (decreased collagen deposition), reduced muscle atrophy (increased myofibre size) and increased muscle strength (Figure 5). Taken together, these data suggest that increased abundance of senescent PDGFR‐α + FAPs in Z24−/− skeletal muscle may be driving MPC loss and dysfunction leading to age‐associated muscle pathology, which effect however can be rescued by senolytic removal of senescent PDGFR‐α + FAPs.
Figure 5.
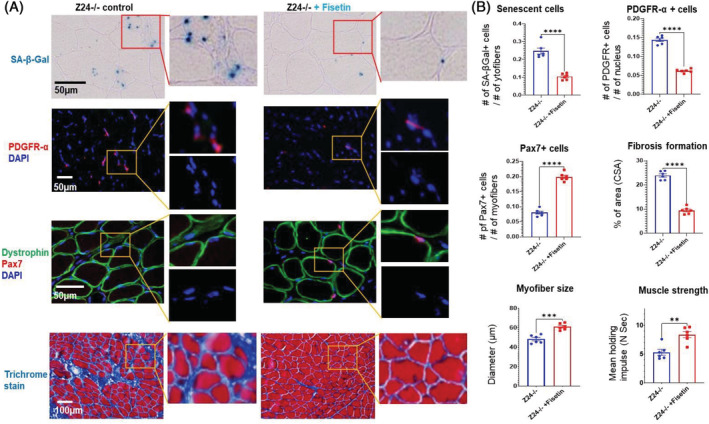
Intermittent treatment of Z24−/− mice with fisetin by oral gavage effectively reduced the number of senescent FAPs and increased the number of MPCs in muscles. (A) SA‐β‐gal staining of Z24−/− skeletal muscle with or without fisetin treatment was performed to compare the number of senescent cells; immunofluorescent staining of PDGFR‐α + FAPs in Z24−/− skeletal muscle with or without fisetin treatment was performed to compare the number of FAPs; immunofluorescent staining of Pax7 + MPCs in Z24−/− muscles with or without fisetin treatment was also performed to compare the number of muscle stem cells, with dystrophin staining to show the location of myofibres; the trichrome staining of Z24−/− muscles with or without fisetin treatment was performed to show the differential deposition of collagen and fibrosis formation. (B) Quantification of the amount of senescent cells (number of cells per myofibre), PDGFR‐α + FAPs (number of cells per nucleus), Pax7 + MPCs (number of cells per myofibre), fibrotic tissues (% of cross‐sectional area/CSA), myofibre size (diameter, μm) and muscle strength (mean holding impulse, N s). Data analysis was performed with random pictures from each group of muscle section samples, and the sample number here N = 6 (six biological replicates).
Discussion
Cellular senescence is a defined hallmark of ageing, contributing to the ageing process and age‐related diseases and disorders. Senotherapeutics, including senolytics and senomorphics, have emerged as a promising strategy to extend human healthspan, given the ability of senotherapeutics to extent healthspan and, in some cases, lifespan of rodent models of ageing. 21 , 22 , 23 In recent years, senolytics have been widely studied in various types of tissues and disease models and well have been well‐proven for their beneficial effect in delaying ageing or treating age‐related diseases. 21 , 22 , 23 , 24 , 25 However, the potential effect and mechanism of senolytics in regulating the function of MPCs in aged skeletal muscle remains unclear. Here we demonstrate that a significantly higher percentage of FAPs in the muscle from the Z24−/− mouse model of HGPS develop senescence. Furthermore, the SASP, including extracellular vesicles, inhibit the proper function of MPCs, and treatment of senescent FAPs with the senolytic fisetin rescued the function of MPCs in culture. Finally, intermittent treatment of Z24−/− mice with fisetin decreased the number of PDGFR+ cells and increased the number of Pax7 + MPCs as well as improved muscle pathology of progeria‐aged mice.
FAPs are tissue‐resident mesenchymal stromal cells (MSCs) characterized by the high expression of PDGFR‐α and are heavily involved in tissue homoeostasis and repair processes. 9 , 10 , 11 Similarly, FAPs in the skeletal muscle can be activated by injury to promote muscle regeneration. 10 , 11 However, studies with severely diseased skeletal muscle demonstrate that FAPs can become overtly activated, leading to increased fatty infiltration and fibrosis formation, while also potently repressing the proliferation and function of resident muscle stem cells. 12 , 13 , 14 Our recent study in muscular dystrophic mice also revealed that partial ablation of FAPs in dystrophic muscle can be achieved with a peptide targeting adipose stromal cells (ASCs) that was able to mitigate muscle damage. 15 The results described here in Z24−/− mice show a similar interaction between FAPs and MPCs, with the cell ratio of FAPs/MPCs being elevated and the function of MPCs being repressed like by senescent FAPs.
As senescent cells accumulate in the aged tissues, they can exert profound effects on the growth and function of normal cells by releasing SASP factors including exosomes. 18 , 20 , 42 Senescent cells play a critical role in inducing or mediating various types of ageing‐related diseases. 16 , 17 , 18 , 19 , 20 A reduction in the senescent cell burden can be achieved with several different classes of senolytics including the combination of dasatinib and quercetin (D + Q), navitoclax (ABT263) and related Bcl‐2 family member inhibitors, HSP90 inhibitors such as 17‐DMAG and the flavonoid fisetin. 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29
Fisetin is a member of the flavonoid family, a family of naturally occurring polyphenolic compounds, present in many fruits and vegetables such as apples, persimmon, grapes, onions and cucumbers and, in particular, in strawberries. Fisetin has been reported to have multiple functions including blocking the PI3K/AKT/mTOR pathway, inhibiting topoisomerase inhibitor, increasing the catalytic activity of SIRT1 and inhibiting the activity of several pro‐inflammatory cytokines, including TNF‐α, IL‐6 and the transcription factor NF‐κB. Fisetin has direct activity as a reducing agent, chemically reacting with and neutralizing reactive oxygen species. Fisetin is anti‐oxidant that scavenges free radicals as well as up‐regulates synthesis of glutathione. It also has been reported to inhibit BCL‐XL, 25 which may also be important for its senolytic activity. Because senescent cell usually takes weeks to re‐accumulate, intermit administration of senolytics is efficient to achieve therapeutic effects. 21 , 22 , 23 Here, we demonstrated that weekly administration of fisetin by oral gavage is effective in eliminating many of senescent FAPs and rescuing the growth and function of MPCs.
In summary, our results demonstrate that senescent FAPs play an important role in negatively regulating MPC function in muscle from Z24−/− mice and that the elimination of senescent FAPs with senolytics is effective in rescuing MPC number and function. Importantly, these results suggest that senescent FAPs represent a new therapeutic target for delaying muscle pathology associated with HGPS and possibly also with natural ageing.
Conflict of interest
Drs Lei Liu, Zhihui Wang, William S. Hambright, Polina Matre, Yan Cui, George Rodney, Paul D. Robbins and Xiaodong Mu and Mr/Mrs Xianlin Yue, Zewei Sun, Jianming Wei, and Ying Li declare that they have no conflict of interest. Dr. Johnny Huard discloses the fact that he receives royalties from Cook Myosite, Inc. for muscle stem cell technologies.
Acknowledgements
We greatly appreciate the funding or supports from Shandong First Medical University and Shandong Academy of Medical Science, University of Texas Health Science Center at Houston (UThealth Houston) and Steadman Philippon Research Institute (SPRI). This work was also supported by grant P01AG062412 from the National Institutes of Health to Paul D. Robbins., and 1UG3AR077748‐01 to Johnny Huard. We also greatly appreciate Dr. Yanling Mu's group at Shandong First Medical University for their great supports. The authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. 43
Liu L., Yue X., Sun Z., Hambright W. S., Wei J., Li Y., Matre P., Cui Y., Wang Z., Rodney G., Huard J., Robbins P. D., and Mu X. (2022) Reduction of senescent fibro‐adipogenic progenitors in progeria‐aged muscle by senolytics rescues the function of muscle stem cells, Journal of Cachexia, Sarcopenia and Muscle, 13, 3137–3148, doi: 10.1002/jcsm.13101
References
- 1. Sousa‐Victor P, García‐Prat L, Serrano AL, Perdiguero E, Muñoz‐Cánoves P. Muscle stem cell aging: Regulation and rejuvenation. Trends Endocrinol Metab 2015;26:287–296. [DOI] [PubMed] [Google Scholar]
- 2. García‐Prat L, Sousa‐Victor P, Muñoz‐Cánoves P. Functional dysregulation of stem cells during aging: A focus on skeletal muscle stem cells. FEBS J 2013;280:4051–4062. [DOI] [PubMed] [Google Scholar]
- 3. Cho JS, Kook SH, Robinson AR, Niedernhofer LJ, Lee BC. Cell autonomous and nonautonomous mechanisms drive hematopoietic stem/progenitor cell loss in the absence of DNA repair. Stem Cells 2013;31:511–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Good SC, Dewison KM, Radford SE, van Oosten‐Hawle P. Global proteotoxicity caused by human β2 microglobulin variants impairs the unfolded protein response in C. elegans . Int J Mol Sci 2021;22:10752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Miller HA, Dean ES, Pletcher SD, Leiser SF. Cell non‐autonomous regulation of health and longevity. Elife 2020;10:e62659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Boonen KJ, Post MJ. The muscle stem cell niche: regulation of satellite cells during regeneration. Tissue Eng Part B Rev 2008;14:419–431. [DOI] [PubMed] [Google Scholar]
- 7. Kann AP, Hung M, Krauss RS. Cell‐cell contact and signaling in the muscle stem cell niche. Curr Opin Cell Biol 2021;73:78–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Joe AW, Yi L, Natarajan A, Le Grand F, So L, Wang J, et al. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol 2010;12:153–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Low M, Eisner C, Rossi F. Fibro/adipogenic progenitors (FAPs): Isolation by FACS and culture. Methods Mol Biol 2017;1556:179–189. [DOI] [PubMed] [Google Scholar]
- 10. Wosczyna MN, Konishi CT, Perez Carbajal EE, Wang TT, Walsh RA, Gan Q, et al. Mesenchymal stromal cells are required for regeneration and homeostatic maintenance of skeletal muscle. Cell Rep 2019;27:2029–2035.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Biferali B, Proietti D, Mozzetta C, Madaro L. Fibro‐adipogenic progenitors cross‐talk in skeletal muscle: The social network. Front Physiol 2019;10:1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lukjanenko L, Karaz S, Stuelsatz P, Gurriaran‐Rodriguez U, Michaud J, Dammone G, et al. Aging disrupts muscle stem cell function by impairing matricellular WISP1 secretion from fibro‐adipogenic progenitors. Cell Stem Cell 2019;24:433–446.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Contreras O, Rebolledo DL, Oyarzún JE, Olguín HC, Brandan E. Connective tissue cells expressing fibro/adipogenic progenitor markers increase under chronic damage: Relevance in fibroblast‐myofibroblast differentiation and skeletal muscle fibrosis. Cell Tissue Res 2016;364:647–660. [DOI] [PubMed] [Google Scholar]
- 14. Starling S. FAPs linked with muscle degeneration in T2DM. Nat Rev Endocrinol 2021;18:3. [DOI] [PubMed] [Google Scholar]
- 15. Gao Z, Lu A, Daquinag AC, Yu Y, Huard M, Tseng C, et al. Partial ablation of non‐myogenic progenitor cells as a therapeutic approach to Duchenne muscular dystrophy. Biomolecules 2021;11:1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Childs BG, Durik M, Baker DJ, van Deursen JM. Cellular senescence in aging and age‐related disease: From mechanisms to therapy. Nat Med 2015;21:1424–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yousefzadeh MJ, Flores RR, Zhu Y, Schmiechen ZC, Brooks RW, Trussoni CE, et al. An aged immune system drives senescence and ageing of solid organs. Nature 2021;594:100–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Robbins PD, Jurk D, Khosla S, Kirkland JL, LeBrasseur NK, Miller JD, et al. Senolytic drugs: Reducing senescent cell viability to extend health span. Annu Rev Pharmacol Toxicol 2021;61:779–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Coppé JP, Desprez PY, Krtolica A, Campisi J. The senescence‐associated secretory phenotype: The dark side of tumor suppression. Annu Rev Pathol 2010;5:99–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Birch J, Gil J. Senescence and the SASP: Many therapeutic avenues. Genes Dev 2020;34:1565–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hickson LJ, Langhi Prata LGP, Bobart SA, Evans TK, Giorgadze N, Hashmi SK, et al. Senolytics decrease senescent cells in humans: Preliminary report from a clinical trial of dasatinib plus quercetin in individuals with diabetic kidney disease. EBioMedicine 2019;47:446–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kirkland JL, Tchkonia T. Senolytic drugs: from discovery to translation. J Intern Med 2020;288:518–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kirkland JL, Tchkonia T, Zhu Y, Niedernhofer LJ, Robbins PD. The clinical potential of senolytic drugs. J Am Geriatr Soc 2017;65:2297–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Camell CD, Yousefzadeh MJ, Zhu Y, Prata LGPL, Huggins MA, Pierson M, et al. Senolytics reduce coronavirus‐related mortality in old mice. Science 2021;373:eabe4832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yousefzadeh MJ, Zhu Y, McGowan SJ, Angelini L, Fuhrmann‐Stroissnigg H, Xu M, et al. Fisetin is a senotherapeutic that extends health and lifespan. EBioMedicine 2018;36:18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhu Y, Tchkonia T, Pirtskhalava T, Gower AC, Ding H, Giorgadze N, et al. The Achilles' heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell 2015;14:644–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fuhrmann‐Stroissnigg H, Ling YY, Zhao J, McGowan SJ, Zhu Y, Brooks RW, et al. Identification of HSP90 inhibitors as a novel class of senolytics. Nat Commun 2017;8:422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhu Y, Doornebal EJ, Pirtskhalava T, Giorgadze N, Wentworth M, Fuhrmann‐Stroissnigg H, et al. New agents that target senescent cells: the flavone, fisetin, and the BCL‐XL inhibitors, A1331852 and A1155463. Aging (Albany NY) 2017;9:955–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xu M, Pirtskhalava T, Farr JN, Weigand BM, Palmer AK, Weivoda MM, et al. Senolytics improve physical function and increase lifespan in old age. Nat Med 2018;24:1246–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dahl KN, Scaffidi P, Islam MF, Yodh AG, Wilson KL, Misteli T. Distinct structural and mechanical properties of the nuclear lamina in Hutchinson‐Gilford progeria syndrome. Proc Natl Acad Sci U S A 2006;103:10271–10276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Verstraeten VL, Ji JY, Cummings KS, Lee RT, Lammerding J. Increased mechanosensitivity and nuclear stiffness in Hutchinson‐Gilford progeria cells: Effects of farnesyltransferase inhibitors. Aging Cell 2008;7:383–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schreiber KH, Kennedy BK. When lamins go bad: nuclear structure and disease. Cell 2013;152:1365–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Young SG, Fong LG, Michaelis S. Thematic review series: Lipid Posttranslational Modifications. Prelamin A, Zmpste24, misshapen cell nuclei, and progeria—new evidence suggesting that protein farnesylation could be important for disease pathogenesis. J Lipid Res 2005;46:2531–2558. [DOI] [PubMed] [Google Scholar]
- 34. Fong LG, Ng JK, Meta M, Coté N, Yang SH, Stewart CL, et al. Heterozygosity for Lmna deficiency eliminates the progeria‐like phenotypes in Zmpste24‐deficient mice. Proc Natl Acad Sci U S A 2004;101:18111–18116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bergo MO, Gavino B, Ross J, Schmidt WK, Hong C, Kendall LV, et al. Zmpste24 deficiency in mice causes spontaneous bone fractures, muscle weakness, and a prelamin A processing defect. Proc Natl Acad Sci U S A 2002;99:13049–13054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mu X, Tseng C, Hambright WS, Matre P, Lin CY, Chanda P, et al. Cytoskeleton stiffness regulates cellular senescence and innate immune response in Hutchinson‐Gilford progeria syndrome. Aging Cell 2020;19:e13152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gharaibeh B, Lu A, Tebbets J, Zheng B, Feduska J, Crisan M, et al. Isolation of a slowly adhering cell fraction containing stem cells from murine skeletal muscle by the preplate technique. Nat Protoc 2008;3:1501–1509. [DOI] [PubMed] [Google Scholar]
- 38. Aartsma‐Rus A, van Putten M. Assessing functional performance in the mdx mouse model. J Vis Exp 2014;e51303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Carlson G. DMD M.2.1.005: The use of four limb hanging tests to monitor muscle strength and condition over time. TREAT‐NMD Neuromuscular Network; 2011 [updated May 27, 2019]. Available from: https://treat‐nmd.org/wp‐content/uploads/2016/08/MDX‐DMD_M.2.1.005.pdf
- 40. Yang Z, Ming XF. mTOR signalling: the molecular interface connecting metabolic stress, aging and cardiovascular diseases. Obes Rev 2012;13:58–68. [DOI] [PubMed] [Google Scholar]
- 41. Nacarelli T, Azar A, Sell C. Aberrant mTOR activation in senescence and aging: A mitochondrial stress response? Exp Gerontol 2015;68:66–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Takasugi M, Okada R, Takahashi A, Virya Chen D, Watanabe S, Hara E. Small extracellular vesicles secreted from senescent cells promote cancer cell proliferation through EphA2. Nat Commun 2017;8:15729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: Update 2017. J Cachexia Sarcopenia Muscle 2017;8:1081–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]


