Abstract
Lower limb muscle dysfunction is a key driver for impaired physical capacity and frailty status, both characteristics of sarcopenia. Sarcopenia is the key pathway between frailty and disability. Identifying biological markers for early diagnosis, treatment, and prevention may be key to early intervention and prevention of disability particularly mobility issues. To identify biological markers associated with lower limb muscle (dys)function in adults with sarcopenia, a systematic literature search was conducted in AMED, CINAHL, Cochrane Library, EMBASE, Medline, PubMed, Scopus, SPORTDiscus, and Web of Science databases from inception to 17 November 2021. Title, abstract, and full‐text screening, data extraction, and methodological quality assessment were performed by two reviewers independently and verified by a third reviewer. Depending on available data, associations are reported as either Pearson's correlations, regression R 2 or partial R 2, P value, and sample size (n). Twenty eligible studies including 3306 participants were included (females: 79%, males: 15%, unreported: 6%; mean age ranged from 53 to 92 years) with 36% in a distinct sarcopenic subgroup (females: 73%, males: 19%, unreported: 8%; mean age range 55–92 years). A total of 119 biomarkers were reported, categorized into: genetic and microRNAs (n = 64), oxidative stress (n = 10), energy metabolism (n = 18), inflammation (n = 7), enzyme (n = 4), hormone (n = 7), bone (n = 3), vitamin (n = 2), and cytokine (n = 4) markers) and seven lower limb muscle measures predominately focused on strength. Seven studies reported associations between lower limb muscle measures including (e.g. power, force, and torque) and biomarkers. In individuals with sarcopenia, muscle strength was positively associated with free testosterone (r = 0.40, P = 0.01; n = 46). In analysis with combined sarcopenic and non‐sarcopenic individuals, muscle strength was positively associated with combined genetic and methylation score (partial R 2 = 0.122, P = 0.03; n = 48) and negatively associated with sarcopenia‐driven methylation score (partial R 2 = 0.401, P < 0.01; n = 48). Biomarkers related to genetics (R 2 = 0.001–0.014, partial R 2 = 0.013–0.122, P > 0.05; n = 48), oxidative stress (r = 0.061, P > 0.05; n ≥ 77), hormone (r = 0.01, ρ = 0.052 p > 0.05, n ≥ 46) and combined protein, oxidative stress, muscle performance, and hormones (R 2 = 22.0, P > 0.05; n ≥ 82) did not report significant associations with lower limb muscle strength. Several biomarkers demonstrated associations with lower limb muscle dysfunction. The current literature remains difficult to draw clear conclusions on the relationship between biomarkers and lower limb muscle dysfunction in adults with sarcopenia. Heterogeneity of biomarkers and lower limb muscle function precluded direct comparison. Use of international classification of sarcopenia and a set of core standardized outcome measures should be adopted to aid future investigation and recommendations to be made.
Keywords: Lower limb, Cytokines, Muscle strength, Muscle mass, Inflammation
Introduction
Sarcopenia is an independent risk factor for poor health outcomes 1 , 2 , 3 , 4 , 5 including mortality, 6 defined as an age‐related loss of skeletal muscle strength, muscle mass, and physical performance. 7 Although poor health status is not a prerequisite, 4 , 8 , 9 the presence of multimorbidity nearly doubles the odds of sarcopenia prevalence, 10 which is likely to rise, in line with multimorbidity. 11 Remaining largely undiagnosed, 5 , 12 sarcopenia affects between 10 and 40% of community‐dwelling adults depending on the classification criteria used. 13 There are a variety of classification criteria used for the diagnosis of sarcopenia, including European Working Group on Sarcopenia in Older People (EWGSOP), 14 EWGSOP2, 15 Asian Working Group for sarcopenia criteria (AWG), 16 or International Working Group for Sarcopenia. 13 Sarcopenia classification is normally based on all or a combination of low muscle strength (e.g. knee extensor strength and handgrip strength), low lean mass [bioimpedance analysis, dual‐energy X‐ray absorptiometry (DEXA)], and low physical performance (gait speed and short physical performance battery score). The EWGSOP in 2019 recommended healthcare professionals treating patients at risk for sarcopenia to take actions to promote early detection and treatment, 15 with diagnosis, treatment and prevention of sarcopenia suggested to become part of routine clinical practice. 7
Sarcopenia constitutes one of the main components of frailty and plays a key etiological role in the frailty process. 17 Although sarcopenia is defined by low muscle mass, strength, and function, these do not always align with the functional aspect more relevant to frailty, physical disability including mobility issues, and falls. 17 Physical functional limitations including immobility have detrimental effects on an individual's independence and quality of life, often leading to falls, disability, and subsequent adverse health outcomes. 18 As such, the Society of Sarcopenia, Cachexia and Wasting Disorders suggested that sarcopenia with limited mobility should be considered as an important clinical entity. 19 Early diagnosis of sarcopenia may enable early targeted interventions to prevent or alleviate physical functional limitations, frailty, and disability. Therefore, identifying novel targets may be key to reducing frailty and disability and improving physical function associated with sarcopenia.
Sarcopenia is a complex geriatric syndrome of multifactorial pathogenesis, 1 including neuromuscular degeneration, changes in muscle protein turnover, hormone levels and sensitivity, chronic inflammation, oxidative stress, and behaviour/lifestyle factors. 20 Recently, there has been particular interest in identifying effective biomarkers to accurately diagnose 21 and treat sarcopenia through targeted, nutritional, pharmacological, and rehabilitation interventions. 7 This has resulted in the identification of numerous biomarkers associated with sarcopenic muscle function 21 and the establishment of a new disease code in ICD‐10‐CM. High levels of circulating inflammatory markers associated with lower skeletal muscle measures, 22 , 23 mammalian target of rapamycin, hormones including insulin‐like growth factor‐1 (IGF‐1), and insulin through muscle protein synthesis and breakdown 24 have been identified. As such, the identification of biomarkers associated with muscle function for the diagnosis, treatment, and prevention could aid in the development of targeted therapeutic therapies for treatment and prevention of sarcopenia and subsequent physical functional capacity and disability.
Despite the high prevalence of sarcopenia in older adults, 15 , 25 the potential for biological markers as tools to aid diagnosis, treatment, and prevention of sarcopenia is poorly understood. It is important to understand how any identified prognostic biomarkers are associated with skeletal muscle function, specifically lower limb skeletal muscle dysfunction as potential targets for early disease identification and diagnosis, and prevention of sarcopenia and subsequent disability through the development of new therapeutics. Although research is progressing in terms of the identification of prognostic biomarkers, previous studies provide variable results leading to inconsistent associations with muscle function and the use of biomarkers as indicators of change following treatment. Accordingly, the present review of literature aimed to identify associations between biological markers and lower limb muscle function in adults with sarcopenia.
Methods
Search strategy and selection criteria
The review was designed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses 26 with the protocol was predefined and registered on PROSPERO (CRD42020197544). Nine databases (AMED 1887–, CINAHL 1981–, Cochrane Library 1973–, EMBASE 1974–, Medline 1946–, PubMed 1801–, Scopus 1939‐ SPORTDiscus 1969–, Web of Science 1921–) were searched to identify original research articles published in peer‐reviewed journals published before 17 November 2021. Review articles, conference abstracts, and grey literature were excluded. Articles were limited to English language only. A systematic search strategy was developed in PubMed (Table S1) and was replicated as closely as possible in other bibliographic databases. The predefined search strategy was expanded to include all known subgroups of biomarkers. The search strategy inclusion and exclusion criteria were considered in line with Population, Intervention, Comparator, Outcomes, and Study design (Table 1). Studies included participants or a subgroup of participants with sarcopenia not related to other conditions, for example, renal cachexia to prevent diseases status influencing associations. A biomarker (defined as a characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention, 27 excluding imaging‐based biomarkers) was required to be reported. Studies were also required to report a lower limb measure, for example, muscle strength or mass. Following the removal of duplicates, a two‐phase screening strategy was undertaken by two independent reviewers (SLS and RLJ) based on (i) title and abstract, (ii) full‐text appraisal. Any discrepancies were resolved by consensus, where consensus could not be obtained a third reviewer (LP) was consulted. Additional studies were identified by manual searches of the references of included articles completed by SLS and RLJ. Reports were downloaded into Rayyan. 28
Table 1.
Population, intervention or exposure, comparator, outcomes, and study design (PICOS) criteria
| Population | Individuals were required to be human adults (aged >18 years) with all, or a distinct subgroup of participants diagnosis/classification of sarcopenia |
| Intervention or exposure | Individuals or a distinct subgroup of individuals were required to have a diagnosis/classification of sarcopenia. All definitions of sarcopenia were included within this review. Studies including at‐risk population without a diagnosis/classification of sarcopenia, and/or sarcopenia/cachexia related to co‐morbidities, for example, cancer, were excluded |
| Comparator | Examining the relationship between biological markers (biomarkers) and measurement of lower limb muscle function (e.g. strength, mass, and power) |
| Outcomes | Report on a biomarker (defined as a characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention, 27 excluding imaging‐based biomarkers) and measurement of lower limb muscle function (e.g. muscle strength, muscle mass, or power) regardless of measurement modality |
| Study design | Only original peer‐reviewed research articles in English language were included, with review articles, conference abstracts, and grey literature excluded. Any study design that included the information described above was considered for inclusion |
Data extraction
Data were extracted independently by two authors (SLS, RLJ) and verified by a third (LP). All data were extracted using a standardized piloted data extraction form. The information included the following for baseline only: first author; year of publication; study design; population; sex ratio; age; study origin; body mass index; physical activity levels; measure of biomarkers; and lower limb muscle measure. Associations between muscle dysfunction and biomarkers were extracted as adjusted and unadjusted correlations and regression coefficients. Additional data were requested from authors if they were not reported fully in the papers, and these studies were not included if the requested data were not provided.
Assessment of methodological quality (risk of bias)
The methodological quality of the papers was assessed independently by two reviewers (SLS, RLJ) using the Joanna Briggs Institute checklist for analytical cross‐sectional studies. 29 Regardless of study design, data were treated as cross‐sectional with only baseline data extracted. The primary measures extracted were cross‐sectional associations between biomarkers and lower limb muscle measures, rather than the likely cause of sarcopenia; therefore, prognosis criteria were deemed unsuitable. Each criterion was scored as ‘Yes’, ‘No’, ‘Unclear’, and ‘Not applicable’, and overall determined ‘include’, ‘exclude’, and ‘seek further information’ (Table 2).
Table 2.
Check list for study quality from Joanna Briggs Institute for cross‐sectional studies; scoring yes = +, no = −, unclear =?, not applicable = N/A
| Author | Were the criteria for inclusion in the study clearly defined? | Were the study subjects and the setting described in detail? | Was the exposure measured in a valid and reliable way? | Were objective; standard criteria used for measurement of the condition | Were confounding factors identified? | Were strategies to deal with confounding factors stated | Were the outcomes measured in a valid and reliable way? | Was appropriate statistical analysis used? | Overall appraisal |
|---|---|---|---|---|---|---|---|---|---|
| Chen et al. (2019) 30 | + | ? | + | + | + | − | + | + | Include |
| Chen et al. (2017) 31 | + | + | + | + | − | − | + | + | Include |
| He et al. (2020) 32 | + | + | + | + | + | − | + | + | Include |
| He et al. (2020) 33 | + | + | + | + | + | + | + | + | Include |
| Hinkley et al. (2020) 34 | + | ? | + | + | + | + | + | + | Include |
| Hofmann et al. (2015) 35 | + | + | + | + | + | + | + | + | Include |
| Khanal et al. (2021) 36 | + | + | + | + | + | + | + | + | Include |
| Kim et al. (2016) 37 | + | + | + | + | − | − | + | + | Include |
| Lim et al. (2019) 38 | + | + | + | + | + | + | + | + | Include |
| Lustosa et al. (2017) 39 | + | + | + | + | − | − | + | − | Include |
| Ma et al. (2021) 40 | + | + | + | + | − | − | ? | + | Include |
| Mafi et al. (2018) 41 | − | − | + | + | − | − | + | − | Exclude |
| Nabuco et al. (2019) 42 | + | + | + | + | − | − | + | + | Include |
| Negaresh et al. (2019) 43 | + | + | + | + | + | + | + | − | Include |
| Pietrangelo et al. (2009) 44 | ? | + | + | + | − | − | + | − | Exclude |
| Ratkevicius et al. (2011) 45 | + | + | + | + | − | − | + | + | Include |
| Rossi et al. (2020) 46 | + | + | + | + | + | + | + | + | Include |
| Sanada et al. (2010) 47 | + | + | + | + | + | + | + | − | Include |
| Sanada et al. (2012) 48 | + | + | ? | ? | + | + | + | − | Include |
| Seo et al. (2020) 49 | + | + | + | + | − | − | + | + | Include |
| Tay et al. (2018) 50 | + | + | + | + | + | + | + | + | Include |
| Vezzoli et al. (2019) 51 | + | + | + | + | − | − | + | + | Include |
Evidence synthesis
Summary statistics are presented as frequencies (%). Data were synthesized separately for (i) descriptive study data identifying biomarkers (all eligible studies) and (ii) associations between biomarkers and lower limb muscle measures (all studies reporting associations). Citations were categorized based on the biomarker's primary role and lower limb muscle measure extracted from the original source into Excel for tabulation. The included studies were heterogeneous with regard to associations between individual and categories of biomarkers and muscle dysfunction measures. Meta‐regression was considered inappropriate, and narrative data analysis was performed. 52 Depending on available data associations are reported as either correlation coefficients (Pearson or Spearman) or regression R 2 or partial R 2, P value, and sample size.
Results
In total, 7282 articles were identified through database and manual searches; following the removal of duplicates, 3859 articles were included for title and abstract screening, with 120 articles sought for full‐text screening (Figure 1). One article could not be sought; 97 were excluded with 22 studies meeting the inclusion criteria. Following risk of bias assessment, two studies 41 , 44 were excluded based on lack of clear inclusion criteria and inappropriate statistical analysis including identifying and dealing with confounders (Table 2). Of the remaining studies, the most common risk of bias was the lack of confounders being identified and dealt with and inappropriate statistical analysis. Overall agreement on risk of bias between reviewers was 94%.
Figure 1.
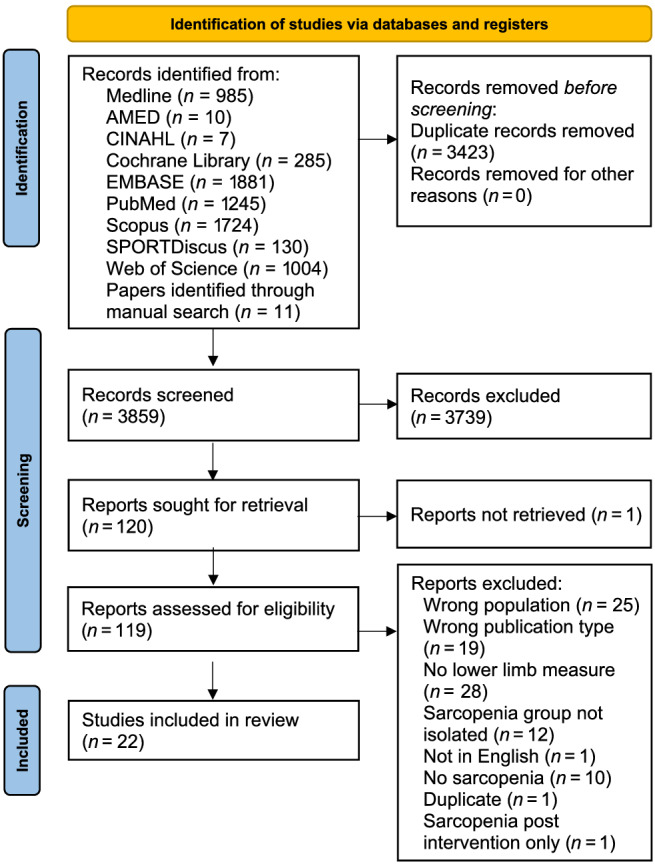
Flow diagram of the study selection process for eligible studies in the systematic review.
Study characteristics
Twenty published studies were included (Table 3), of which five were randomized controlled trials (RCT) and 13 were cross‐sectional in study design. All RCTs included an exercise intervention, of which two were combined with either nutrition or nutrition and health education. A total of 3306 participants were included [498 males; 2622 females; sex was not reported in one paper (n = 186)], with a mean age ranging from 53 to 92 years. Of these, 36% (1174) were in a distinct subgroup of participants diagnosed with sarcopenia mean age range 55–92 years, of which 73% were female; sex was not reported in 8%. All definitions of sarcopenia were included within this review, with sarcopenia defined using the EWGSOP (n = 4 34 , 35 , 39 , 50 ), AWG (n = 3 33 , 38 , 40 ), and combined EWSOP and International Working Group for Sarcopenia (n = 1 49 ). The remaining studies defined sarcopenia using body composition only (n = 7 30 , 31 , 42 , 43 , 46 , 47 , 51 ), body composition and handgrip strength (n = 4 32 , 36 , 37 , 48 ), or knee extensor strength (n = 1 45 ). Within these definitions, sarcopenia was further classified in five studies, 43 , 45 , 47 , 48 , 51 as 1–2 standard deviation (SD) below young group as mild 43 , 45 or Class I sarcopenia, 47 , 48 , 51 >2 SD below young group as moderate 43 or severe 45 or Class II sarcopenia. 47 , 51
Table 3.
Description of study papers
| Author/Country | Study design | Subgroups | Participants (M/F) | Age (years) | Diagnosis criteria | Lower limb muscle measures | Biomarkers |
|---|---|---|---|---|---|---|---|
|
Chen et al. (2017) 31 China |
RCT | Sarcopenic with aerobic training | 15 (1/14) | 69 ± 3 | ASMM (BIA) ≤ 32.5% men; ≤25.7% women | Knee extensor force | IGF‐1 |
| Sarcopenic with resistance training | 15 (3/12) | 69 ± 4 | |||||
| Sarcopenic with combination training | 15 (4/11) | 69 ± 3 | |||||
| Sarcopenic control | 15 (2/13) | 69 ± 3 | |||||
|
Chen et al. (2019) 30 USA |
Cross‐sectional | Sarcopenic | 1 (0/1) | 87 | BMD (DEXA) T‐score> − 1; SMMI (DEXA) ≤ 5.5 kg/m2 | Jump power | CTX‐1; CTX‐1/TRAP5b ratio; miR‐100‐5p; miR‐125b‐5p; miR‐133a‐3p; miR‐1‐3p; miR‐20; miR‐21–5p; miR‐23a‐3p; miR‐24‐3p; TRAP5b |
| Sarco‐osteopenic | 15 (0/15) | 69 ± 5 | BMD (DEXA) T‐score ≤ − 1; SMMI (DEXA) ≤ 5.5 kg/m2 | ||||
| Control | 13 (0/13) | 69 ± 7 | BMD (DEXA) T‐score ≤ − 1; SMMI (DEXA) > 5.5 kg/m2 | ||||
|
He et al. (2020) 32 China |
Cross‐sectional | Sarcopenic | 93 | 76 ± 1 | AWG (BIA) (sarcopenic) | Knee extension torque | Creatinine; HDLc; LDLc; miR‐1; miR‐126; miR‐133a; miR‐133b; miR‐146a; miR‐155; miR‐208b; miR‐20a; miR‐21; miR‐210; miR‐221; miR‐222; miR‐328; miR‐486; miR‐499; total cholesterol; triglycerides; HbA1c |
| Control | 93 | 76 ± 1 | |||||
|
He et al. (2020) 33 UK |
Cross‐sectional | Sarcopenic | 24 (0/24) | 73 ± 4 | Both SMMI<6.75 kg/m2 and HGS < 26 kg | Knee extension torque | Alpha‐actinin‐3 (ACTN3); angiotensin‐converting‐enzyme (ACE); ciliary neurotrophic factor (CNTF); fat mass and obesity‐associated (FTO); gene‐wise methylation score (MSSNP); hypoxia inducible factor 1 subunit alpha (HIF1A); methylation levels of sarcopenia‐driven CpG sites (MSSAR); myostatin (MSTN); vitamin D receptor |
| Control | 24 (0/24) | 70 ± 3 | |||||
|
Hinkley et al. (2020) 34 USA |
Cross‐sectional | Sarcopenic | 7 (2/5) | 71 ± 2 | EWGSOP (DEXA) (sarcopenic) | Knee extensor power; quadriceps muscle volume | Creatine; glycerol‐phosphoethanolamine (GPE); phosphatidylcholine, 108; phosphatidylethanolamine, 79; Phosphatidylglycerol, 157; Phosphocreatine (resting); phosphodiester peak |
| Control | 17 (10/7) | 71 ± 1 | |||||
|
Hofmann et al. (2015) 35 Austria |
Cross‐sectional | Sarcopenic | 9 (0/9) | 65–92 | EWGSOP (DEXA) (sarcopenic) | Knee extensor torque | Activin A; follistatin; GDF‐15; IGF‐1; Myostatin |
| Control | 70 (0/70) | 65–92 | |||||
|
Kim et al. (2016) 37 Japan |
RCT | Sarcopenic obese with exercise and nutrition interventions | 36 (0/36) | 81 ± 4 | Body fat (BIA) ≥ 32% and SMMI (BIA) < 5.67 kg/m2 or HGS < 17 kg or GS < 1 m/s. | Knee extensor force | Albumin; CRP; cystatin C; HDLc; IL‐6; leptin; total cholesterol; triglycerides; vitamin D (25OHD ‐ serum 25‐hydroxyvitamin D); HbA1c |
| Sarcopenic obese with exercise intervention | 35 (0/35) | 81 ± 4 | |||||
| Sarcopenic obese with nutrition intervention | 34 (0/34) | 81 ± 5 | |||||
| Sarcopenic obese with health education intervention | 34 (0/34) | 81 ± 5 | |||||
|
Khanal et al. (2021) 36 England/UK |
Cross‐sectional | Sarcopenic obese | 77 (0/77) | 73 | SMI (BIA) < 6.76 kg/m2, HGS < 28.5 kg, body fat >38% | Knee extensor torque; muscle‐specific strength | Activin A receptor Type 1B (ACVR1B) rs2854464, rs10783485; alpha‐actinin‐3 (ACTN3) rs1815739; angiotensin‐converting‐enzyme (ACE) rs4341 (I/D); ciliary neurotrophic factor (CNTF) rs1800169; ciliary neurotrophic factor (CNTF)R rs2070802; collagen Type I alpha 1 chain (COL1A1) rs1800012; erythrocyte sedimentation rate 1 rs4870044; fat mass and obesity‐associated (FTO) rs9939609; hypoxia inducible factor 1 subunit alpha (HIF1A) rs11549465; ID3 rs11574; IGF‐1 rs35767; IL‐6 rs1800795; methylenetetrahydrofolate reductase (MTHFR) rs1801131, rs1537516, rs17421511; NOS 3 rs1800000; protein tyrosine kinase 2 (PTK2) rs7843014, rs7460; the titin gene (TTN) rs10497520; thyrotropin‐releasing hormone receptor (TRHR) rs7832552; vitamin D receptor rs2228570 |
| Control obese | 176 (0/176) | 71 | SMI (BIA) ≥ 6.76 kg/m2, HGS ≥ 28.5 kg, body fat >38% | ||||
| Sarcopenic non obese | 7 (0/7) | 69 | SMI (BIA) < 6.76 kg/m2, HGS < 28.5 kg, body fat ≤38% | ||||
| Control (non‐Sarcopenic, non‐obese) | 47 (0/47) | 68 | SMI (BIA) ≥ 6.76 kg/m2, HGS ≥ 28.5 kg, body fat ≤38% | ||||
|
Lim et al. (2019) 38 Singapore |
Cross‐sectional | Sarcopenic | 26 (10/16) | 70 ± 7 | AWG (DEXA) (sarcopenic) | Knee extensor force | CRP; IL‐6; monocyte chemoattractant protein‐1; vitamin D (25OHD ‐ serum 25‐hydroxvitamin D) |
| Control non‐obese | 42 (19/23) | 67 ± 7 | |||||
| Sarcopenic obese | 17 (2/15) | 75 ± 10 | |||||
| Obese control | 102 (25/77) | 67 ± 7 | |||||
|
Lustosa et al. (2017) 39 Brazil |
Cross‐sectional | Sarcopenia | 31 (0/31) | 77 ± 5 | EWGSOP (DEXA) (sarcopenic) | Knee extensor power/torque | Soluble TNF‐alpha receptor‐1 (sTNFR1); IL‐6; TNF‐α |
| Control | 32 (0/32) | 77 ± 7 | |||||
|
Ma et al. (2021) 40 China |
Intervention | Waitlist control | 12 (6/6) | 70 ± 4 | AWG (sarcopenic) | Knee extensor force | Annexin A6 (ANXA6); bridging integrator 1 (BIN1); eukaryotic translation initiation factor 3 subunit E (EIF3E); histidine triad nucleotide‐binding protein 1 (HINT1); interleukin 7 (IL‐7)R; lactate dehydrogenase B (LDHB); leucine‐rich repeat protein 3 (LRRN3); lymphoid enhancer binding factor 1 (LEF1); musculoaponeurotic fibrosarcoma (MAF); protein kinase C theta (PRKCQ); RAS guanyl nucleotide‐releasing protein (RASGRP1); superoxide dismutase (SOD1); translocase of outer mitochondrial membrane 7 (TOMM7) |
| Exercise intervention | 11 (5/6) | 76 ± 7 | |||||
| Exercise and nutritional intervention | 23 (11/12) | 74 ± 6 | |||||
|
Nabuco et al. (2019) 42 Brazil |
RCT | Sarcopenic obese with exercise and protein intervention | 13 (0/13) | 68 ± 4 | Body fat (DEXA) ≥ 35% appendicular lean soft tissue (DEXA) ≤ 15.02 kg | Knee extensor force; lower soft tissue mass | Advanced oxidation protein products; albumin; CRP; creatine; glucose (fasted); HDLc; homeostasis model assessment insulin resistance (HOMA‐IR); insulin; IL‐6; LDLc; total cholesterol; total radical‐trapping antioxidant parameter; triglycerides; TNF‐α |
| Sarcopenic obese with exercise and placebo | 13 (0/13) | 70 ± 4 | |||||
|
Negaresh et al. (2019) 43 Iran |
RCT | Sarcopenic with resistance training | 16 (16/0) | 55–70 | 1–2 SD (mild) or >2 SD (severe) muscle mass below mean of healthy young group (skinfold) | Squat press force | Follistatin; IGF‐1; myostatin; testosterone |
| Control with resistance training | 15 (15/0) | 55–70 | |||||
|
Ratkevicius et al. (2011) 45 Scotland/UK |
Cross‐sectional | Mildly sarcopenic | 20 (20/0) | 69 ± 3 | Maximal knee extension torque 1–2 SD below young men | Knee extensor torque; knee extensor voluntary activation | Follistatin; follistatin‐related gene; GDF‐associated serum protein‐1; IGF‐1; IL‐6; myostatin; testosterone; TNF‐α |
| Severely sarcopenic | 26 (26/0) | 76 ± 6 | Maximal knee extension torque 2 SD below young below young men | ||||
|
Rossi et al. (2020) 46 Italy |
Longitudinal | Healthy | 116 (43/73) | 72 ± 2 | ASMM (DEXA) > 7 kg/m2 men, >5.18 kg.m2 women, body fat (DEXA) < 31.17% men, <44.01% women | Knee extensor torque | Albumin; fibrinogen; glucose; HDLc; total cholesterol; triglycerides; Vitamin D3 |
| Sarcopenia | 68 (24/44) | 72 ± 2 | ASMM (DEXA) < 7 kg/m2 men, <5.18 kg.m2 women, body fat (DEXA) < 31.17% men, <44.01% women | ||||
| Obese | 68 (24/44) | 72 ± 3 | ASMM (DEXA) > 7 kg/m2 men, >5.18 kg.m2 women, body fat (DEXA) > 31.17% men, >44.01% women | ||||
| Sarcopenic obese | 22 (8/14) | 71 ± 2 | ASMM (DEXA) < 7 kg/m2 men, <5.18 kg.m2 women, body fat (DEXA) > 31.17% men, >44.01% women | ||||
|
Sanada et al. (2010) 47 Japan |
Cross‐sectional | Mildly sarcopenic | 219 (63/156) | M: 65 ± 14 | Class 1: SMMI 1SD below sex‐specific reference value (DEXA) | Leg extension power; lean soft tissue mass | Glucose; HDLc; LDLc; total cholesterol; total cholesterol/HDLc ratio; triglyceride/HDL ratio; triglycerides; HbA1c |
| F: 61 ± 11 | |||||||
| Severely sarcopenic | 27 (5/22) | M: 67 ± 17 | Class 2: SMMI 2SD below sex‐specific reference value (DEXA) | ||||
| F: 63 ± 11 | |||||||
| Control | 713 (100/613) | M: 65 ± 8 | |||||
| F: 60 ± 9 | |||||||
|
Sanada et al. (2012) 48 Japan |
Cross‐sectional | Sarcopenic | 129 (0/129) | 62 ± 1 | Class 1: SMMI 6.7 kg/m2 and HGS 1SD below young mean (DEXA) | Leg extension power | Glucose (fasted); HDLc; triglyceride/HDLc ratio; triglycerides; HbA1c |
| Control | 404 (0/404) | 53 ± 1 | |||||
|
Seo et al. (2020) 49 Korea |
Cross‐sectional | Sarcopenic | 27 (0/27) | 72 ± 4 | Based on EWGSOP and IWGS (DEXA) ‐ GS < 1.0 m·s−1 and ASMI < 5.67 kg·m−2, or GS > 1.0 m·s−1, HGS < 20 kg and ASM I < 5.67 kg·m2 | Knee extension torque; relative knee extensor torque; thigh inter‐muscular adipose tissue volume; total thigh volume | Activin A; follistatin; GDF‐15; myostatin |
| Non‐Sarcopenic | 32 (0/32) | 71 ± 5 | Non‐sarcopenic (DEXA), body fat <35%, and lumbar or femur BMD T‐score <−2.5 | ||||
|
Tay et al. (2018) 50 Singapore |
Cross‐sectional | Pre‐sarcopenic | 14 (9/5) | 72 ± 8 | EWGSOP Asian gender‐specific cut‐off. Pre‐sarcopenia: Low muscle mass without impact on muscle strength or gait speed | Knee extensor force; muscle‐specific strength | APOE genotyping into APOEε2; 3; 4 isoforms; CRP; dehydroepiandrosterone sulphate (DHEAS); IGF‐1; IL‐6; soluble TNF‐alpha receptor‐1 (sTNFR1); TNF‐α; vitamin D (25OHD ‐ serum 25‐hydroxvitamin D) |
| Sarcopenic | 53 (18/35) | 78 ± 7 | EWGSOP Asian gender‐specific cut‐off (DEXA). Low muscle mass and weak HGS and/or slow GS | ||||
| Control | 108 (11/97) | 76 ± 6 | EWGSOP Asian gender‐specific cut‐off (DEXA). Normal muscle mass | ||||
|
Vezzoli et al. (2019) 51 Italy |
RCT | Sarcopenic with training | 15 (6/9) | 71 ± 3 | Class 1: SMMI 1SD below young mean (BIA) | Stair‐climbing power; leg press force | 8‐Isoprostane (8‐iso‐PGF2‐α); 8‐OH‐2‐deoxyguanosine (8‐OH0dG); creatinine; protein carbonyls; reactive oxygen species production rate; thiobarbituric acid‐reactive substances; total antioxidant capacity |
| Sarcopenic non‐training | 20 (10/10) | 73 ± 6 | Class 2: SMMI 2SD below young mean (BIA) |
ASMI, appendicular skeletal muscle index; ASMM, appendicular skeletal muscle mass; AWG, Asian Working Group Criteria; BIA, bioelectrical impedance analysis; BMD, bone mineral density; CRP, C‐reactive protein; CTX‐1 C‐terminal cross‐linking telopeptide of Type 1 collagen; DEXA, dual‐energy X‐ray absorptiometry; EWGSOP, European Working Group on Sarcopenia in Older People; GDF, growth/differentiation factor; GS, gait speed; HbA1c, whole‐blood glycated haemoglobin A1c; HDLc, high‐density lipoprotein cholesterol; HGS, handgrip strength; IGF‐1, insulin‐like growth factor‐1; IL‐6, interleukin 6; IWGS, International Working Group on Sarcopenia; LDLc, low‐density lipoprotein cholesterol; NOS, nitric oxide synthase; RCT, randomized controlled trial; SMMI, skeletal muscle mass index = (skeletal muscle mass/body mass) × 100; TNF‐α, tumour necrosis factor‐alpha; TRAP5b, tartrate‐resistant acid phosphatase 5b.
Data presented as mean ± SD.
A total of 119 biomarkers were reported across the 20 studies; biomarkers were grouped based on their primary characteristics into the following: genetic and microRNAs (n = 64), oxidative stress (n = 10), energy metabolism (n = 18), inflammation (n = 7), enzyme (n = 4), hormone (n = 7), bone (n = 3), vitamin (n = 2), and cytokine (n = 4) markers (Figure 2 and Table S2 ). Lower limb muscle function was assessed in a variety of measures, with seven differing outcomes (Table S3 ), the predominant outcomes focused on lower limb strength including power (n = 5), force (n = 3), muscle‐specific strength (n = 2), and torque (n = 2).
Figure 2.
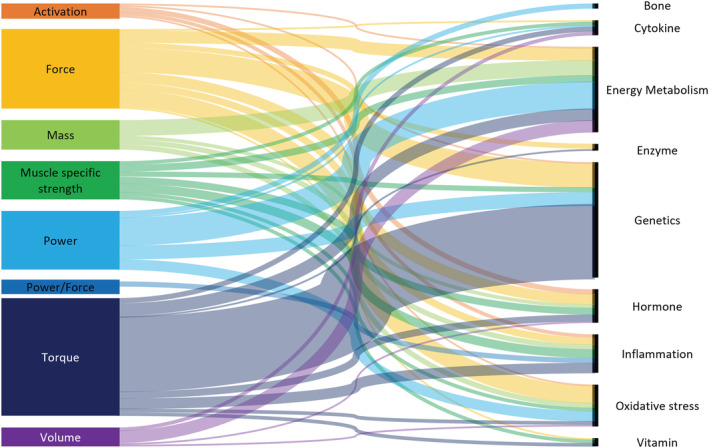
Sankey diagram of lower limb muscle measures and biomarkers (based on the primary role).
Associations between biomarkers and muscle
Of the 20 studies included, seven reported associations between biomarkers and lower limb muscle measures, of which two reported genetic biomarker associations, 30 , 33 one reported muscle performance biomarkers, 34 one reported oxidative stress biomarkers, 51 and three reported combined biomarkers 35 , 38 , 45 (Table 4). Two studies reported sarcopenia only associations, 38 , 45 whereas four studies combined sarcopenic and non‐sarcopenic populations 30 , 33 , 34 , 35 and another study combined pre and post 12‐week intervention in sarcopenia only population. 51
Table 4.
Association data between biomarkers and lower limb muscle measures
| Author | Analysis type | Outcome/associations |
|---|---|---|
| Chen et al. (2019) 30 | Spearman correlation (combined sarcopenic and non‐sarcopenic) |
|
| He et al. (2020) 33 | Multiple linear regression (combined sarcopenic and non‐sarcopenic) |
|
| Hinkley et al. (2020) 34 | Pearson's correlation (combined sarcopenic and non‐sarcopenic) |
|
| Hofmann et al. (2015) 35 | Spearman's Rho, multi‐linear regression (combined sarcopenic and non‐sarcopenic) |
|
| Lim et al. (2019) 38 | Pearson's correlation, Spearman's Rho, multiple linear regression (sarcopenic and sarcopenic obesity only) |
|
| Ratkevicius et al. (2011) 45 | Pearson's product–moment correlation coefficient (combined mildly and severely sarcopenic only) |
|
| Vezzoli et al. (2009) 51 | Pearson's product of moment (combined pre and post intervention in combined type 1 and type 2 sarcopenic only) |
|
BMI, body mass index; GDF, growth/differentiation factor; IGF‐1, insulin‐like growth factor‐1; IL‐6, interleukin 6; P, P‐value; n, sample size; r, Pearson's correlation; SNP, single nucleotide polymorphisms; ρ, spearman's rho.
Two studies examined the relationships between biomarkers and lower limb muscle strength in sarcopenia only individuals. Knee extensor muscle strength was positively associated with free testosterone (r = 0.40; P = 0.01; n = 46) 45 ; however, there was no association seen with IGF‐1, 45 follistatin, 45 myostatin, 45 follistatin‐related gene, and 45 GDF‐associated serum protein‐1. 45 Inter‐muscular adipose tissue ratio was not associated with inflammatory markers interleukin‐6 (IL‐6) and C‐reactive protein in the sarcopenic or sarcopenic obese groups. 38 Although monocyte chemoattractant protein‐1 was not associated with inter‐muscular adipose tissue ratio for sarcopenic individuals, it was positively associated with inter‐muscular adipose tissue ratio in the sarcopenic obese group (r = 0.556, P < 0.05; n = 17). 38 In individuals with sarcopenia, associations between biomarkers and lower limb muscle assessed via leg press were positively associated with total antioxidant capacity (R 2 = 0.33; n = 20) and negatively associated with protein carbonyls (R 2 = 0.31; n = 20), 51 when pre‐intervention and post‐intervention analysis was combined.
Four studies combined sarcopenic and non‐sarcopenic populations, of which two studies examined the relationship between biomarkers and lower limb muscle power and assessed via jump power 30 and knee extensor peak power. 34 Muscle power was positively associated with MicroRNA miR‐125b‐5p (r = 0.294, P = 0.028; n = 56) 30 and negatively associated with phosphatidylethanolamine (r = −0.433, P = 0.044; n = 23). 34 Yet, there were no associations between muscle power and miR‐1‐3p, miR‐133a‐3p, 30 phosphocreatine, 34 phosphodiester, 34 phosphatidylcholine, 34 or phosphatidylglycerol. 34
Two studies with combined sarcopenic and non‐sarcopenic populations assessed the association between knee extension torque and biomarkers. Knee extensor torque was negatively associated with sarcopenia‐driven methylation score (partial R 2 = 0.401, P = <0.01; n = 48) adjusted for age and BMI (R 2 = 0.406; n = 48) 33 and positively associated with combined genetic and methylation score (partial R 2 = 0.122, P = 0.03; n = 48) adjusted for age and BMI (R 2 = 0.112; n = 48). 33 That said, knee extensor torque was not associated with single nucleotide polymorphism‐driven methylation score, 33 muscle‐driven genetic predisposition score, 33 IGF‐1, 35 GDF‐15, 35 follistatin, 35 activin A, 35 or myostatin. 35
Associations between biomarkers and lower limb muscle volume was reported on only one occasion using combined sarcopenic and non‐sarcopenic populations. Negative associations were found between muscle volume and muscle performance biomarkers of phosphodiester (r = −0.625, P = 0.001; n = 21), phosphatidylcholine (r = −0.529, P = 0.011; n = 23), phosphatidylethanolamine (r = −0.522, P = 0.008; n = 23), and phosphatidylglycerol (r = −0.435, P = 0.043; n = 23) and no association with phosphocreatine was found in sarcopenia and non‐sarcopenic adults. 34
Discussion
The current study has summarized current literature focusing on biological markers and lower limb muscle dysfunction in adults with sarcopenia. The review's main finding was that although biomarkers and lower limb muscle measures were frequently reported, there was a lack of consistency in both biomarkers and lower limb muscle measures reported across the 20 studies included, with only seven studies including associations. Lower limb muscle strength (e.g. power, force, and torque) was significantly associated with several biomarkers whose primary role is related to genetics, muscle performance, hormones, and oxidative stress. However, numerous biomarkers also related to these roles did not report significant associations with lower limb muscle strength. Furthermore, current research is strongly weighted towards assessment of lower limb muscle strength, with research sparse in drawing associations between biomarkers and other contributors to sarcopenia. Based on the current literature, it remains difficult to understand the relationship between biomarkers and lower limb muscle dysfunction in adults with sarcopenia.
The research within this area is limited, with identification of biomarkers associated with muscle dysfunction vital given the potential to be novel therapeutic targets and aid with early diagnosis for the prevention and treatment of sarcopenia and disability. Within the current systematic review, 119 individual biomarkers were reported, ranging from genetic markers such as microRNAs to inflammatory markers such as IL‐6. This breadth of biomarkers indicates interest in this research area, yet most of the biomarkers included within this review were reported on a singular basis demonstrating a breadth of potential targets. It is vital that future researchers consider strengthening the depths of the current body of literature through validation and confirmation of biomarkers and their association with a range of muscle dysfunction measures, thus ensuring recommendation are based on robust data. Furthermore, to identify biomarkers as potential therapeutic targets, it is also important to determine if the biomarkers associated with lower limb muscle dysfunction are state (changeable) or trait (static) characteristics. Although there is limited evidence available, based on the current review, future research should consider examining the relationship between MicroRNA miR‐125b‐5p, phosphatidylethanolamine, sarcopenia‐driven methylation score, combined genetic and methylation score, free testosterone, total antioxidant capacity, protein carbonyls, monocyte chemoattractant protein‐1, and lower limb muscle measures associated with sarcopenia given the reported associations with measures of lower limb muscle strength. In support of the suggestion by the International Working Group on Sarcopenia, 53 , 54 it is vital to consider the multifactorial nature of sarcopenia, and although single markers are of interest, it may be the case that a composition of markers from a variety of mechanistic pathways provides a greater insight into the understanding of sarcopenia. However, a study reporting a composition of multiple with varying primary roles including protein, oxidative stress, muscle performance, and hormone found no association with lower limb muscle torque. 35 In the current systematic review, a variety of muscle strength measures (e.g. force, torque, and power; muscle‐specific strength) and other muscle function measures (e.g. volume and mass) were reported (Table S3). Whereas muscle strength plays a large role in mobility limitations, other muscle dysfunction measures such as muscle activation and tissue attenuation 55 warrant further investigation given their association with both mobility limitations and sarcopenia. Currently, the EWGSOP does not provide recommendations regarding methods of assessing muscle mass and other parameters of sarcopenia. That said, DEXA and bioelectrical impedance (BIA) are suggested in the evaluation of whole‐body muscle mass 14 including in the AWG criteria. 16 There needs to be consideration regarding the sensitivity of these methods and their clinical application when providing recommendations, for example, when assessing muscle mass by BIA, the prevalence of sarcopenia was higher compared with DEXA assessment in both males [BIA: 13% (95% CI: 7–19%); DEXA: 8% (95% CI 7–9%)] and females [BIA: 13% (95% CI 9–19%); DEXA: 8% (95%CI 6–11%)]. 56 Although prevalence is higher using BIA, it is less time consuming, quicker, and easier to perform in a clinical setting. Although it would be beneficial to provide recommendations regarding sarcopenia diagnostic assessment methods, real‐world considerations including access, ease of use, and cost effectiveness need to be considered. The same could be said for examination of biological biomarkers whereby certain markers, such as ESR and CRP, are routinely reported clinical practice settings such as rheumatology, whereas other markers, for example, microRNAs, are not routinely performed clinically. Additionally, although long‐term tracking of biological markers associated with sarcopenia could be a beneficial process, especially markers such as the inflammatory marker IL‐6, which has a pleiotropic nature, 57 the cost effectiveness of these recommendations would need to be evaluated. These recommendations do however support the increased efforts by researchers and clinical practitioners to define blood‐based biomarkers, providing a quick and cost‐effective practice that could aid in the facilitation of sarcopenia diagnosis, tracking changes over time, and aiding clinical and therapeutic decision processes. 53
The prevalence of sarcopenia is widely reported, 13 yet these data could be significantly impacted by the diagnostic criteria being implemented. 13 , 58 Of the eligible studies, four 34 , 35 , 39 , 50 defined sarcopenia based on the EWGSOP, 15 three studies 33 , 38 , 40 used the AWG criteria, 16 one study 49 combined the EWGSOP with the International Working Group on Sarcopenia, and the remaining studies aligned to population and sex‐specific cut‐offs for knee extensor strength 45 and body composition. 30 , 31 , 32 , 36 , 37 , 42 , 43 , 46 , 47 , 48 , 51 The inconsistencies in criteria make it difficult to accurately compare results across studies, even when similar outcomes are reported. This is even more difficult to evaluate with studies that have limited their sarcopenia diagnosis to one muscle assessment criterion, for example, body composition only, body composition and handgrip strength, or knee extensor strength. To add to the difficulty of evaluating data between studies, recently, the definition of sarcopenia according to the EWGSOP has been updated (EWGSOP2). The latest definition is based on low skeletal muscle mass and low muscle strength, whereas low physical performance is used to determine sarcopenia severity, rather than a diagnostic criterion. 7 Sarcopenia defined using EWGSOP2 is reportedly better than EWGSOP‐defined sarcopenia for predicting the 1‐year incidence of falls or hospitalization, especially when using the modified cut‐offs. 59 Yet, there has already been reported of discrepancies between the prevalence of sarcopenia when applying the EWGSOP and EWGSOP2 definitions 60 , 61 , 62 with the suggestion that cut‐off points for some of the measures might not be comparable and may lead to differing groups being identified as sarcopenic between different trials. 13 To aid with consistency, understanding, and impact of future research, it is proposed that sarcopenia should be diagnosed using a diagnostic criterion such as the EWGSOP2 consisting of all areas of sarcopenia (muscle strength, mass, and performance).
Sarcopenia is prevalent in both sexes, albeit to a greater degree in males compared with females. 63 , 64 It is therefore surprising that of the 3306 participants included within this systematic review, the majority were female (79%) and of the sarcopenic population included (n = 1220), 70% were female. Eleven of the eligible studies were single sex groups, with two males 43 , 45 and nine females only and two studies recruited mixed groups, then split data by sex. 32 , 40 , 51 The remaining five studies reported uneven sex ratio within groups. 31 , 38 , 46 , 47 , 50 Although there is limited evidence, one study reported differences between males and females in the associations between biological markers, muscle strength, and body composition. That said, individual factors contributing to sarcopenia have been suggested to differ between males and females, with the catabolic influence of myostatin in men potentially contributing to sarcopenia, whereas in women was due to anabolic decline represented by reduced IGF‐1. 64 Furthermore, there is an abundance of literature supporting differences in muscle function including, strength, wasting, 65 muscle morphology, 66 and mobility 67 measures between males and females. To support the understanding of this potential sex‐specific pathophysiological mechanism for sarcopenia, future work needs to look at sex‐specific associations and/or control for sex through normalization of data or the inclusion of sex as a cofounder factor in regression models. These processes should aid in more targeted clinical interventions, with potentially differing guidelines and recommendations based on sex‐specific data.
The current systematic review evaluated the quality of the research using Joanna Briggs Institute checklist for analytical cross‐sectional studies; several studies lacked the appropriate statistical information, thereby in some instances impacting the quality of analysis and data provided. Based on quality assessment, two papers were excluded from the review; these studies lacked in areas such as robust criteria for inclusion in the study and implementation and consideration of confounding factors and appropriate statistical measures (Table 2). Sarcopenia may be a composition of markers from a variety of mechanistic pathways; therefore, a strong statistical framework to examine these avenues is required. 53 , 54 Future research should consider the inclusion of robust reliability data, examination of cofounding variables, assessment of multiple relationships within set models, the inclusion of confidence intervals, and following reporting guidelines such as EQUATOR reporting guidelines. These recommendations would aid in improving the quality of published data within the field and thereby more robust recommendations for therapeutic interventions.
During the current review, studies were excluded if groups contained individuals with co‐morbidities, including hypertension, Type I and II diabetes, cancer, and osteoarthritis. This stringent criterion was designed to provide a greater understanding of impact of sarcopenia on the relationship between biological markers and skeletal muscle function, without the influence of diseases status, especially given the influence disease status can have on both biological markers and skeletal muscle function. That said, this approach does mean the exclusion of a wealth of data that may change the narrative of this relationship, especially given that sarcopenia is highly prevalent in individuals with cardiovascular disease, dementia, diabetes mellitus, and respiratory disease. 68 Additionally, given the requirement for real‐world knowledge and applicable recommendations to aid the early diagnosis, treatment, and prevention of sarcopenia, and given the potential worthwhile impact of this research area, and the demand for greater understanding regarding diagnosis, treatment, and prevention of sarcopenia, future research should confirm and validate the following biomarkers and their association with muscle dysfunction: microRNA 125b‐5p, sarcopenia‐driven methylation score, combined genetic and methylation score, total oxidant capacity, protein carbonyls, phosphodiester, phosphatidylcholine, phosphatidylethanolamine, phosphatidylglycerol, and free testosterone. Moreover, confirmation of associations of these biomarkers across different lower limb muscle measures, and in individuals with and without co‐morbidities especially given the large number of factors such as diagnosis time, severity, and treatment practices, which would significantly influence the assessment of sarcopenia‐related outcomes, important for identifying biomarkers to for diagnosis, treatment, and prevention of sarcopenia.
In conclusion, 119 different biomarkers and seven assessment methods of lower limb muscle function were identified, with association reported in seven out of the 20 studies included. Associations between biomarkers and lower limb muscle function are limited due to a lack of repetition of biomarkers and lower limb muscle measures. A lack of depth of biomarkers and heterogeneity of biomarkers and lower limb muscle measures make comparisons difficult. International classification of sarcopenia and a set of core standardized outcome measures should be adopted to aid future investigations. Future work needs to also include sex‐specific associations to understand the underlying sex‐specific pathophysiological mechanisms for sarcopenia, which may also confound associations when mixed‐sex groups are assessed.
Funding
The authors have received no financial support for the work presented in this article.
Conflict of interest
Rebecca Louise Jones, Lorna Paul, Martijn Steultjens, and Stephanie Louise Smith declare that they have no conflicts of interest.
Supporting information
Table S1. Study PubMed search strategy
Table S2. Biomarkers identified and groups based on primary role
Table S3. Lower limb muscle measures identified and grouped based on measurement type
Table S4. Preferred Reporting Items for Systematic Reviews and Meta‐Analysis (PRIMSA) checklist
Acknowledgements
The authors would like to acknowledge the support for this work from Glasgow Caledonian University, the University of Bedfordshire, and the University of Nottingham. The authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia, and Muscle 69 .
Jones R. L., Paul L., Steultjens M. P. M., and Smith S. L. (2022) Biomarkers associated with lower limb muscle function in individuals with sarcopenia: a systematic review, Journal of Cachexia, Sarcopenia and Muscle, 13, 2791–2806, doi: 10.1002/jcsm.13064
References
- 1. Santilli V, Bernetti A, Mangone M, Paoloni M. Clinical definition of sarcopenia. Clin Cases Miner Bone Metab 2014;11:177–180. [PMC free article] [PubMed] [Google Scholar]
- 2. Beaudart C, Zaaria M, Pasleau F, Reginster JY, Bruyère O. Health outcomes of sarcopenia: A systematic review and meta‐analysis. PLoS One 2017;12:e0169548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chang KV, Hsu TH, Wu WT, Huang KC, Han DS. Association Between Sarcopenia and Cognitive Impairment: A Systematic Review and Meta‐Analysis. J Am Med Dir Assoc 2016;17:1164.e15. [DOI] [PubMed] [Google Scholar]
- 4. Cruz‐Jentoft AJ, Landi F, Schneider SM, Zúñiga C, Arai H, Boirie Y, et al. Prevalence of and interventions for sarcopenia in ageing adults: A systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing 2014;43:48–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yeung SSY, Reijnierse EM, Pham VK, Trappenburg MC, Lim WK, Meskers CGM, et al. Sarcopenia and its association with falls and fractures in older adults: A systematic review and meta‐analysis. J Cachexia Sarcopenia Muscle Wiley Blackwell 2019;10:485–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chang S‐F, Lin P‐L. Systematic literature review and meta‐analysis of the association of sarcopenia with mortality. Worldviews Evid Based Nurs 2016;13:153–162. [DOI] [PubMed] [Google Scholar]
- 7. Cruz‐Jentoft AJ, Sayer AA. Sarcopenia. Lancet Publishing Group; 2019. p 2636–2646. [DOI] [PubMed] [Google Scholar]
- 8. Bianchi L, Abete P, Bellelli G, Bo M, Cherubini A, Corica F, et al. Prevalence and clinical correlates of sarcopenia, identified according to the EWGSOP definition and diagnostic algorithm, in hospitalized older people: The GLISTEN study. J Gerontol Series A 2017;72:1575–1581. [DOI] [PubMed] [Google Scholar]
- 9. Reijnierse EM, Buljan A, Tuttle CSL, van Ancum J, Verlaan S, Meskers CGM, et al. Prevalence of sarcopenia in inpatients 70 years and older using different diagnostic criteria. Nurs Open 2019;6:377–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dodds RM, Granic A, Robinson SM, Sayer AA. Sarcopenia, long‐term conditions, and multimorbidity: findings from UK Biobank participants. J Cachexia Sarcopenia Muscle 2020;11:62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kingston A, Robinson L, Booth H, Knapp M, Jagger C. Projections of multi‐morbidity in the older population in England to 2035: estimates from the Population Ageing and Care Simulation (PACSim) model. Age Ageing 2018;47:374–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Reijnierse EM, De Van Der Schueren MAE, Trappenburg MC, Doves M, Meskers CGM, Maier AB. Lack of knowledge and availability of diagnostic equipment could hinder the diagnosis of sarcopenia and its management. PLoS One 2017;12:e0185837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mayhew AJ, Amog K, Phillips S, Parise G, Mcnicholas PD, De Souza RJ, et al. The prevalence of sarcopenia in community‐dwelling older adults, an exploration of differences between studies and within definitions: a systematic review and meta‐analyses. Age Ageing 2019;48:48–56. [DOI] [PubMed] [Google Scholar]
- 14. Cruz‐Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010;39:412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cruz‐Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: Revised European consensus on definition and diagnosis. Oxford University Press; 2019. p 16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K, et al. Asian Working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc 2020;21:300–7.e2. [DOI] [PubMed] [Google Scholar]
- 17. Cruz‐Jentoft AJ, Michel JP. Sarcopenia: A useful paradigm for physical frailty. Eur Geriatr Med 2013;4:102–105. [Google Scholar]
- 18. Cunningham C, O'Sullivan R, Caserotti P, Tully MA. Consequences of physical inactivity in older adults: A systematic review of reviews and meta‐analyses. Scand J Med Sci Sports 2020;30:816–827. [DOI] [PubMed] [Google Scholar]
- 19. Morley JE, Abbatecola AM, Argiles JM, Baracos V, Bauer J, Bhasin S, et al. Sarcopenia with limited mobility: An international consensus. J Am Med Dir Assoc 2011;12:403–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Roubenoff R. Sarcopenia and its implications for the elderly. Eur J Clin Nutr 2000;54:S40–S47. [DOI] [PubMed] [Google Scholar]
- 21. Qaisar R, Karim A, Muhammad T, Shah I, Khan J. Prediction of sarcopenia using a battery of circulating biomarkers. Sci Rep 2021;11:8632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bano G, Trevisan C, Carraro S, Solmi M, Luchini C, Stubbs B, et al. Inflammation and sarcopenia: A systematic review and meta‐analysis. Maturitas 2017;96:10–15. [DOI] [PubMed] [Google Scholar]
- 23. Tuttle CSL, Thang LAN, Maier AB. Markers of inflammation and their association with muscle strength and mass: A systematic review and meta‐analysis. Ageing Res Rev 2020;64:101185. [DOI] [PubMed] [Google Scholar]
- 24. Ali S, Garcia JM. Sarcopenia, cachexia and aging: Diagnosis, mechanisms and therapeutic options ‐ A mini‐review. S Karger AG 2014;294–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kilgour AHM, Redmond P, Taylor A, Deary IJ, Starr JM, Shenkin SD. 70 prevalence of sarcopenia in a longitudinal UK cohort study using Ewgsop2 criteria varies widely depending on which measures of muscle strength and performance are used. Age Ageing 2020;49:i22–i23. [Google Scholar]
- 26. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: The PRISMA statement. BMJ 2009;339:b2535‐b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Biomarkers Definitions Working G . Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clin Pharmacol Ther 2001;69:89–95. [DOI] [PubMed] [Google Scholar]
- 28. Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan‐A web and mobile app for systematic reviews. Syst Rev 2016;5:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Moola S, Munn Z, Tufanaru C, Aromataris E, Sears K, Sfetc R, et al. Systematic reviews of etiology and risk. In Aromataris E, Munn Z, eds. JBI Manual for Evidence Synthesis. JBI; 2020. [Google Scholar]
- 30. Chen Z, Bemben MG, Bemben DA. Bone and muscle specific circulating microRNAs in postmenopausal women based on osteoporosis and sarcopenia status. Bone 2019;120:271–278. [DOI] [PubMed] [Google Scholar]
- 31. Chen HT, Chung YC, Chen YJ, Ho SY, Wu HJ. Effects of different types of exercise on body composition, muscle strength, and IGF‐1 in the elderly with sarcopenic obesity. J Am Geriatr Soc 2017;65:827–832. [DOI] [PubMed] [Google Scholar]
- 32. He N, Zhang YL, Zhang Y, Feng B, Zheng Z, Wang D, et al. Circulating microRNAs in plasma decrease in response to sarcopenia in the elderly. Front Genet 2020;11:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. He L, Khanal P, Morse CI, Williams A, Thomis M. Associations of combined genetic and epigenetic scores with muscle size and muscle strength: A pilot study in older women. J Cachexia Sarcopenia Muscle 2020;11:1548–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hinkley JM, Cornnell HH, Standley RA, Chen EY, Narain NR, Greenwood BP, et al. Older adults with sarcopenia have distinct skeletal muscle phosphodiester, phosphocreatine, and phospholipid profiles. Aging Cell 2020;19:e13135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hofmann M, Halper B, Oesen S, Franzke B, Stuparits P, Tschan H, et al. Serum concentrations of insulin‐like growth factor‐1, members of the TGF‐beta superfamily and follistatin do not reflect different stages of dynapenia and sarcopenia in elderly women. Exp Gerontol 2015;64:35–45. [DOI] [PubMed] [Google Scholar]
- 36. Khanal P, Williams AG, He L, Stebbings GK, Onambele‐Pearson GL, Thomis M, et al. Sarcopenia, obesity, and sarcopenic obesity: Relationship with skeletal muscle phenotypes and single nucleotide polymorphisms. J Clin Med 2021;10:4933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kim H, Kim M, Kojima N, Fujino K, Hosoi E, Kobayashi H, et al. Exercise and nutritional supplementation on community‐dwelling elderly Japanese women with sarcopenic obesity: A randomized controlled trial. J Am Med Dir Assoc 2016;17:1011–1019. [DOI] [PubMed] [Google Scholar]
- 38. Lim JP, Chong MS, Tay L, Yang YX, Leung BP, Yeo A, et al. Inter‐muscular adipose tissue is associated with adipose tissue inflammation and poorer functional performance in central adiposity. Arch Gerontol Geriatr 2019;81:1–7. [DOI] [PubMed] [Google Scholar]
- 39. Lustosa LP, Batista PP, Pereira DS, Pereira LSM, Scianni A, Ribeiro‐Samora GA. Comparison between parameters of muscle performance and inflammatory biomarkers of non‐sarcopenic and sarcopenic elderly women. Clin Interv Aging 2017;12:1183–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ma S‐L, Wu J, Zhu L, Chan RS‐M, Wang X, Huang D, et al. Peripheral blood T cell gene expression responses to exercise and HMB in sarcopenia. Nutrients 2021;13:2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mafi F, Biglari S, Ghardashi Afousi A, Gaeini AA. Improvement in skeletal muscle strength and plasma levels of follistatin and myostatin induced by an 8‐week resistance training and epicatechin supplementation in sarcopenic older adults. J Aging Phys Act 2019;27:384–391. [DOI] [PubMed] [Google Scholar]
- 42. Nabuco HCG, Tomeleri CM, Fernandes RR, Sugihara Junior P, Cavalcante EF, Cunha PM, et al. Effect of whey protein supplementation combined with resistance training on body composition, muscular strength, functional capacity, and plasma‐metabolism biomarkers in older women with sarcopenic obesity: A randomized, double‐blind, placebo‐controlled trial. Clin Nutr ESPEN 2019;32:88–95. [DOI] [PubMed] [Google Scholar]
- 43. Negaresh R, Ranjbar R, Baker JS, Habibi A, Mokhtarzade M, Gharibvand MM, et al. Skeletal muscle hypertrophy, insulin‐like growth factor 1, myostatin and follistatin in healthy and sarcopenic elderly men: The effect of whole‐body resistance training. Int J Prev Med 2019;10:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pietrangelo T, Mancinelli R, Toniolo L, Cancellara L, Paoli A, Puglielli C, et al. Effects of local vibrations on skeletal muscle trophism in elderly people: mechanical, cellular, and molecular events. Int J Mol Med 2009;24:503–512. [DOI] [PubMed] [Google Scholar]
- 45. Ratkevicius A, Joyson A, Selmer I, Dhanani T, Grierson C, Tommasi AM, et al. Serum concentrations of myostatin and myostatin‐interacting proteins do not differ between young and sarcopenic elderly men. J Gerontol A Biol Sci Med Sci 2011;66:620–626. [DOI] [PubMed] [Google Scholar]
- 46. Rossi AP, Urbani S, Fantin F, Nori N, Brandimarte P, Martini A, et al. Worsening disability and hospitalization risk in sarcopenic obese and dynapenic abdominal obese: A 5.5 years follow‐up study in elderly men and women. Front Endocrinol (Lausanne) 2020;11:314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sanada K, Miyachi M, Tanimoto M, Yamamoto K, Murakami H, Okumura S, et al. A cross‐sectional study of sarcopenia in Japanese men and women: Reference values and association with cardiovascular risk factors. Eur J Appl Physiol 2010;110:57–65. [DOI] [PubMed] [Google Scholar]
- 48. Sanada K, Iemitsu M, Murakami H, Gando Y, Kawano H, Kawakami R, et al. Adverse effects of coexistence of sarcopenia and metabolic syndrome in Japanese women. Eur J Clin Nutr 2012;66:1093–1098. [DOI] [PubMed] [Google Scholar]
- 49. Seo M‐W, Jung S‐W, Kim S‐W, Jung HC, Kim D‐Y, Song JK. Comparisons of muscle quality and muscle growth factor between sarcopenic and non‐sarcopenic older women. Int J Environ Res Public Health 2020;17:6581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tay L, Leung BP, Wee S, Tay KS, Ali N, Chan M, et al. Association of nutrition and immune‐endocrine dysfunction with muscle mass and performance in cognitively impaired older adults. Arch Gerontol Geriatr 2018;75:20–27. [DOI] [PubMed] [Google Scholar]
- 51. Vezzoli A, Mrakic‐Sposta S, Montorsi M, Porcelli S, Vago P, Cereda F, et al. Moderate intensity resistive training reduces oxidative stress and improves muscle mass and function in older individuals. Antioxidants (Basel) 2019;8. 10.3390/antiox8100431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. McKenzie JE, Brennan SE, Ryan RE, Thomson HJ, Johnston RV. Summarizing study characteristics and preparing for synthesis. In Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, eds. Cochrane Handbook for Systematic Reviews of Interventions. Cochrane; 2021. [Google Scholar]
- 53. Cesari M, Fielding RA, Pahor M, Goodpaster B, Hellerstein M, van Kan GA, et al. Biomarkers of sarcopenia in clinical trials‐recommendations from the International Working Group on Sarcopenia. J Cachexia Sarcopenia Muscle 2012;3:181–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Marzetti E, Calvani R, Lorenzi M, Marini F, D'Angelo E, Martone AM, et al. Serum levels of C‐terminal agrin fragment (CAF) are associated with sarcopenia in older hip fractured patients. Exp Gerontol 2014;60:79–82. [DOI] [PubMed] [Google Scholar]
- 55. Visser M, Goodpaster BH, Kritchevsky SB, Newman AB, Nevitt M, et al. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well‐functioning older persons. J Gerontol A Biol Sci Med Sci 2005;60:324–333. [DOI] [PubMed] [Google Scholar]
- 56. Shafiee G, Keshtkar A, Soltani A, Ahadi Z, Larijani B, Heshmat R. Prevalence of sarcopenia in the world: A systematic review and meta‐ analysis of general population studies. J Diabetes Metab Disord 2017;16:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Fontes JA, Rose NR, Čiháková D. The varying faces of IL‐6: From cardiac protection to cardiac failure. Cytokine 2015;74:62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Reijnierse EM, Trappenburg MC, Leter MJ, Blauw GJ, Sipilä S, Sillanpää E, et al. The impact of different diagnostic criteria on the prevalence of sarcopenia in healthy elderly participants and geriatric outpatients. Gerontology 2015;61:491–496. [DOI] [PubMed] [Google Scholar]
- 59. Yang M, Liu Y, Zuo Y, Tang H. Sarcopenia for predicting falls and hospitalization in community‐dwelling older adults: EWGSOP versus EWGSOP2. Sci Rep 2019;9:17636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Locquet M, Beaudart C, Petermans J, Reginster JY, Bruyère O. EWGSOP2 versus EWGSOP1: Impact on the prevalence of sarcopenia and its major health consequences. J Am Med Dir Assoc 2019;20:384–385. [DOI] [PubMed] [Google Scholar]
- 61. Phu S, Vogrin S, Zanker J, Bani Hassan E, Al Saedi A, Duque G. Agreement between initial and revised European working group on sarcopenia in older people definitions. J Am Med Dir Assoc 2019;20:382–3.e1. [DOI] [PubMed] [Google Scholar]
- 62. Reiss J, Iglseder B, Alzner R, Mayr‐Pirker B, Pirich C, Kässmann H, et al. Consequences of applying the new EWGSOP2 guideline instead of the former EWGSOP guideline for sarcopenia case finding in older patients. Age Ageing 2019;48:719–724. [DOI] [PubMed] [Google Scholar]
- 63. Castillo EM, Goodman‐Gruen D, Kritz‐Silverstein D, Morton DJ, Wingard DL, Barrett‐Connor E. Sarcopenia in elderly men and women: The Rancho Bernardo study. Am J Prev Med 2003;25:226–231. [DOI] [PubMed] [Google Scholar]
- 64. Tay L, Ding YY, Leung BP, Ismail NH, Yeo A, Yew S, et al. Sex‐specific differences in risk factors for sarcopenia amongst community‐dwelling older adults. Age 2015;37:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Anderson LJ, Liu H, Garcia JM. Sex differences in muscle wasting. In Mauvais‐Jarvis F, ed. Sex and Gender Factors Affecting Metabolic Homeostasis, Diabetes and Obesity. Cham: Springer International Publishing; 2017. p 153–197. [Google Scholar]
- 66. Behan FP, Maden‐Wilkinson TM, Pain MTG, Folland JP. Sex differences in muscle morphology of the knee flexors and knee extensors. PLoS One 2018;13:e0190903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Bloomberg M, Dugravot A, Landré B, Britton A, Steptoe A, Singh‐Manoux A, et al. Sex differences in functional limitations and the role of socioeconomic factors: A multi‐cohort analysis. Lancet Healthy Longevity 2021;2:e780–e790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Pacifico J, Geerlings MAJ, Reijnierse EM, Phassouliotis C, Lim WK, Maier AB. Prevalence of sarcopenia as a comorbid disease: A systematic review and meta‐analysis. Exp Gerontol 2020;131:110801. [DOI] [PubMed] [Google Scholar]
- 69. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: Update 2021. J Cachexia Sarcopenia Muscle 2021;12:2259–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Study PubMed search strategy
Table S2. Biomarkers identified and groups based on primary role
Table S3. Lower limb muscle measures identified and grouped based on measurement type
Table S4. Preferred Reporting Items for Systematic Reviews and Meta‐Analysis (PRIMSA) checklist


