Abstract
Background
Cancer cachexia negatively impacts patient outcomes, quality of life and survival. Identification and management of cancer cachexia remains challenging to healthcare professionals (HCPs). The aim of this assessment was to identify current gaps in HCPs' knowledge and practice for identifying and managing adults with cancer‐related cachexia. Results may guide development of new educational programmes to close identified gaps and improve outcomes of cancer patients.
Methods
An international assessment was conducted using a mixed‐methods approach including focus group interviews with subject matter experts and an electronic survey of practising HCP. The assessment was led by the Society on Sarcopenia, Cachexia and Wasting Disorders (SCWD) and was supported by in‐country collaborating organizations.
Results
A quantitative survey of 58 multiple‐choice questions was completed by physicians, nurses dietitians and other oncology HCP (N = 2375). Of all respondents, 23.7% lacked confidence in their ability to provide care for patients with cancer cachexia. Patients with gastrointestinal, head and neck, pulmonary cancers and leukaemia/lymphoma were reported as those at highest risk for cachexia. Only 29.1% of respondents recognized a key criterion of cancer cachexia as >5% weight loss from baseline, but many (14.4%) did not utilize a standardized definition of cancer cachexia. Despite this, most clinicians (>84%) were able to identify causes of weight loss—reduced oral intake, progressive disease, side effects of therapy and disease‐related inflammation. Of all respondents, 52.7% indicated newly diagnosed patients with cancer should be screened for weight loss. In practice, 61.9% reported that patient weight was systematically tracked over time, but only 1125 (47.4%) reported they weigh their cancer patients at each visit. Treatment of cachexia focused on increasing the patient's nutritional intake by oral nutritional supplements (64.2%), energy and protein fortified foods (60.3%) and counselling by a dietitian (57.1%). Whereas many respondents (37.3%) considered cachexia inevitable, most (79.2%) believed that an interprofessional team approach could improve care and that use of standardized tools is critical.
Conclusions
Findings from this international assessment highlight the challenges associated with the care of patients with cancer cachexia, opportunities for interventions to improve patient outcomes and areas of variance in care that would benefit from further analysis.
Keywords: Cancer, cachexia, needs assessment, guidelines, nutrition, education
Background
Cancer cachexia is frequently observed in patients with malignant disease. It is defined as a multifactorial syndrome characterized by skeletal muscle mass loss (with or without loss of fat mass) due to negative protein and energy balance that is driven by a variable combination of reduced food intake and abnormal metabolism. 1 Cancer cachexia is associated with a decrease in patients' quality of life and increased mortality. 2 , 3 , 4 Furthermore, the condition may be responsible for up to 50% of patients suffering and more than 20% of patient deaths. 5 , 6 , 7 , 8 , 9
Identifying and managing cancer cachexia presents a challenge to healthcare professionals (HCPs) and may be complicated by the lack of globally accepted criteria for cancer cachexia, limited availability of effective treatments and lack of knowledge among clinicians.
Although, there is growing data on basic science level of therapeutic targets including antibodies, muscle stem cells and mesenchymal stromal cell transplantation, 10 there are still few evidence‐based interventions for the treatment of cachexia, and a clear definition of cachexia and effective screening tools are urgently needed. 10 , 11 , 12 Leading organizations have published guidelines for the management of cachexia including the European Society of Medical Oncology, 13 the American Society of Clinical Oncology 14 and the Japanese Society for Palliative Medicine. 15 Organizations have also published oncology nutrition guidelines, for example, the European Society for Clinical Nutrition and Metabolism (ESPEN). 16 Although guidelines differ in their recommendations on components of care/interventions, all highlight the role of reduced food intake and abnormal metabolism. It is known that it is important to have the appropriate quality and quantity of nutrients to maintain muscle mass. 17 , 18 , 19 , 20 , 21 This can be challenging as a recent study shows that cancer patients with advanced stages of cachexia were associated with having lack of appetite and reduced dietary intakes. 22 The lack of effective pharmaceutical or nutritional interventions for management of cancer cachexia may contribute to the clinical perception that cachexia is an irreversible end‐stage condition. As a possible result of this perception, few intervention studies for cachexia have been conducted in some regions to date. 23 Nevertheless, there are positive developments with the approval of anamorelin for treatment of cachexia in patients with non‐small cell lung cancer, gastric, pancreatic and colorectal cancer in Japan. 24 The approval of anamorelin will lead to the development of consensus papers and clinical practice guideline in Japan and Asia and therefore to a change in clinical practice of cancer cachexia in Japan. 24
New advances in understanding the pathophysiology of cancer cachexia require an understanding of how HCPs currently identify, assess and manage patients with cachexia. A recent questionnaire for clinicians from Japan investigated the use of sarcopenia and cachexia (not limited to cancer cachexia) evaluations in the workplace. It revealed that only 17.3% of the respondents actually assessed patients for cachexia. 25
Another study conducted among dietitians from different settings in four European countries investigated whether dieticians have sufficient knowledge regarding malnutrition, starvation, cachexia and sarcopenia and whether they use the terms in their daily clinical practice. The study determined that the percentage of dieticians with sufficient knowledge regarding these terms was unsatisfactory (13%). 26 In a survey of medical oncologists and haematologists worldwide, respondents most commonly defined cancer cachexia as weight loss (86%) or loss of appetite (46%). 27 Of those surveyed, 48% reported waiting to treat patients with cachexia until weight loss was greater than 15%, and 61–77% did not prescribe pharmaceutical agents to manage cachexia until Stage IV disease, regardless of tumour type. 27 It is notable that a review of the published literature mostly reflects a single‐profession approach with a limited number of studies reporting assessment across professions or an interprofessional approach to the care of patients with cancer with cachexia. These studies showed that multidisciplinary, multimodel cancer cachexia treatment has a positive effect on patient outcomes. 28 , 29 This difficulty to identify and manage cachexia by clinicians worldwide has an obvious impact on patient outcomes and survival.
The aim of this international, interprofessional, mixed‐methods, educational needs assessment was to identify gaps in HCPs' knowledge and practice for identifying and managing adults with cancer cachexia with a view to developing educational programmes. Ultimately, such programmes are expected to help fill knowledge gaps, update care for patients with cancer at risk of cachexia and improve patient outcomes. This project was led by the Society on Sarcopenia, Cachexia and Wasting Disorders (SCWD) in collaboration with the European Cancer Organization, the Multinational Association of Supportive Care in Cancer (MASCC), the Japanese Association of Supportive Care in Cancer (JASCC), the American Society for Parenteral and Enteral Nutrition (ASPEN) and the European Society for Parenteral and Enteral Nutrition (ESPEN).
Methods
This international assessment followed a proven mixed‐methods approach that included (1) a literature review of classic and recent articles on the definition, causes and management of cancer cachexia, (2) focus group interviews and (3) a survey of practising HCPs, who are members of the partner societies, to collect quantitative data. The assessment was led by the SCWD and was supported by collaborating organizations in the surveyed countries. An international advisory board (IAB) was recruited to analyse the current state of care for patients with cancer at risk for or with cachexia and to identify professional practice gaps among clinicians and healthcare teams in their respective countries. The information gathered from the IAB was used to develop the survey that was subsequently reviewed and refined by regional advisory boards (RABs) from Japan, Europe and North America to ensure in‐country applicability and subsequent validation of the survey.
Survey
The quantitative survey consisted of 58 multiple‐choice Likert scale or free‐response items. The estimated online completion time was 20 min. The survey was organized into respondent demographics (10 items), knowledge domain/defining cachexia (five items), knowledge domain/assessing risk (two items), practice domain/screening (five items), practice domain/diagnosing (five items), practice domain/treating (nine items), perceptions/attitudes domain (seven items), interprofessional practice (five items), facilitators and barriers (four items) and education (six items). The survey was available in English, French, German, Italian, Spanish and Japanese to increase in‐country clinician response rates. The finalized survey was disseminated electronically using SurveyMonkey® from 18 January 2021 to 10 March 2021 in Japan and from 18 January 2021 to 14 May 2021 throughout Europe and North America. Completion of the survey by clinicians was encouraged by the participating organizations, IAB and RAB members. All data were collected confidentially and rendered anonymous with removal of all IP addresses.
Following the close of the survey, data were downloaded from SurveyMonkey® and analysed. Respondents who did not pursue the survey (Japan 12.7% and Europe/North America 11.6%) beyond the self‐reported confidence items were removed from the original file and not included in the analysis.
Data analyses
Analyses included descriptive statistics (mean, standard deviation, %) and one‐way analysis of variance (ANOVA) with Welch statistic and Games–Howell post hoc using profession as the independent variable to assess differences in total years of experience, years of experience treating patients with cancer cachexia and confidence in ability to care for patients at risk or diagnosed with cachexia by profession. Professions with less than 35 respondents were removed (physician assistant, occupational therapist, social worker, psychologist, other and multiple) to conduct one‐way ANOVA as the sample sizes were sharply unequal to the other professions.
For the purposes of evaluating percent response, single response items were divided by the total number of respondents per item. For multiple response items, percent response was calculated by the number of respondents who proceeded after the self‐confidence item (N = 2375) regardless of the respondent drop off over time.
Results
Survey participation and respondent characteristics
Data were collected from 2705 survey responders. Of these, 330 were excluded from analysis because they did not continue the survey following the survey item assessing self‐reported confidence, leaving a total of 2375 for analysis. Respondents were 59.7% female, with a mean age of 44.4 (10.9 SD). One‐third (32.8%) of the respondents were physicians, followed by dietitians/nutritionists (27.7%), nurses/advanced practice nurses (13.6%), pharmacists (12.4%) and physical therapists (7.1%). Respondents averaged 18.7 (10.5 SD) years of experience as a healthcare provider and 11.9 (9.8 SD) years of experience treating patients with cancer cachexia. Most respondents (73.8%) provided patient care in a hospital or cancer centre. Only about one‐third (32%) of the respondents were confident in their ability to provide care for patients at risk for or diagnosed with cancer cachexia. When confidence level was assessed by profession, dietitians/nutritionists (65.8%) had the highest confidence score, followed by nurses/advanced practice nurses (64.8%) and physicians (60%).
Defining cachexia
Respondents reported not utilizing a standardized definition of cancer cachexia to diagnose cancer cachexia (Table 1). In the domain of knowledge in defining cachexia in patients with cancer and identifying risk for developing cachexia, responses demonstrated variation across domain items.
Table 1.
Domain (Knowledge): defining cachexia in cancer patients (N = 2375)
| N | Statistic | ||
|---|---|---|---|
| What point in the continuum of weight loss defines the presence of cachexia? | |||
| >5% | 691 | 29.1% | |
| >10% | 597 | 25.1% | |
| >15% | 193 | 8.1% | |
| >20% | 133 | 5.6% | |
| There is no consistent definition of cachexia | 419 | 17.6% | |
| I do not know | 342 | 14.4% | |
| Which threshold of BMI (kg/m2) do you consider representative of cachexia? | |||
| 25 | 24 | 1.0% | |
| 22 | 44 | 1.9% | |
| 20 | 521 | 21.9% | |
| 18.5 | 642 | 27.0% | |
| 17 | 459 | 19.3% | |
| I do not know | 685 | 28.5% | |
| A cancer patient with a BMI of 30 kg/m2 has experienced weight loss of 15% over 6 months, would you define them as having (elect all that apply) (Japan ONLY; N = 1228): | |||
| Malnutrition | 633 | 51.6% | |
| Cachexia | 696 | 56.7% | |
| Non‐detrimental weight loss | 321 | 26.1% | |
| I do not know | 186 | 15.2% | |
| A cancer patient with a BMI of 30 kg/m2 has experienced weight loss of 15% over 6 months, would you define them as having (select 1) (All other countries; N = 1147): | |||
| Malnutrition | 482 | 42.0% | |
| Cachexia | 483 | 42.1% | |
| Non‐detrimental weight loss | 92 | 8.0% | |
| I do not know | 90 | 7.8% | |
| Cachexia and cachectic state are the same thing | |||
| Strongly agree | 84 | 3.5% | |
| Agree | 589 | 24.8% | |
| Neither agree nor disagree | 953 | 40.1% | |
| Disagree | 699 | 29.4% | |
| Strongly disagree | 50 | 2.1% | |
| In your opinion, causes of weight loss in cancer patients may include (select all that apply): | |||
| Inflammation | 2012 | 84.7% | |
| Reduced oral intake | 2291 | 96.5% | |
| Progressive disease | 2127 | 89.6% | |
| Toxicity or side effects of chemotherapy | 2067 | 87.0% | |
| Other | 370 | 15.6% | |
Definition of cancer cachexia severity is guided by international consensus. 1 A robust grading system incorporating the independent prognostic significance of both BMI and %WL has been developed 30 (Figure 1) and has been prospectively validated 30 ; it is included in ESPEN and ASCO guidelines. Respondents to the survey were aware of a spectrum of cachexia severity, but 14% of respondents did not know which level of weight loss, and 28.5% of respondents did not know which level of BMI depletion defined cachexia. Many respondents' understanding of threshold values to define cachexia was limited to values that essentially define weight loss of the most severe Grade 4 (13.7% of respondents identified >15% weight loss, and 67.9% identified BMI < 20 kg/m2 as cut‐offs defining cachexia). There were diverse perspectives on the importance of weight loss in patients of obese body habitus (BMI > 30 20 kg/m2) with up to 26% identifying high weight loss as non‐detrimental and up to 15% unsure as to whether it was detrimental or not.
Figure 1.
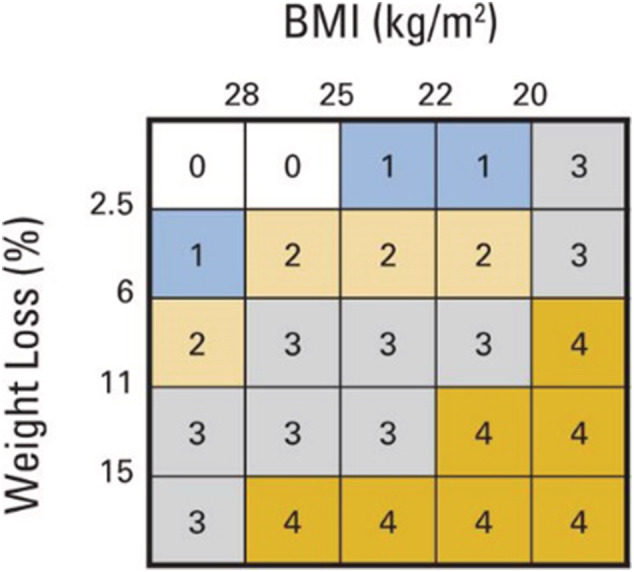
Risk of reduced survival is a function of body mass index (BMI) and per cent weight loss (%WL). Panel represent a 5 × 5 matrix analysis of five categories of BMI and five categories of %WL. Reference categories are BMI ≥ 28.0 kg/m2 and weight stable ±2.4%. Different colours represent significant differences (P < .05) in median overall survival. BMI‐adjusted WL grading system (Grades 0–4) are shown.
Most respondents were able to identify the causes of weight loss in patients with cancer as reduced oral intake (96.5%), progressive disease (89.6%), toxicity/side effects of chemotherapy (87.0%) and inflammation (84.7%).
Risk of developing cachexia in patients with cancer
Respondents accurately identified patients with cancer at highest risk for developing cachexia by primary tumour site—gastrointestinal (80.4%), head and neck (57.3%) pulmonary (55.9%), leukaemia/lymphoma (32.5%) and included breast (9.1%) and prostate (4.8%). Once a patient had been identified as ‘at risk’ of developing cachexia, respondents focused on ways to increase the patients' dietary intake by consultation with a dietitian/nutritionist (70%), informing the patient/family of risk factors and ways to increase oral intake (64.2%), increasing the frequency of nutritional screening (51.6%), providing patient education materials on increasing oral intake (48.8%) increasing frequency for checking patient's weight (44.3%) and consulting a rehabilitation doctor/physical therapist (41.4%).
Screening patients with cancer for cachexia
Screening for cachexia was not done routinely by respondents (Table 2). About half of respondents (52.7%) indicated that patients should be screened for weight loss upon receiving a cancer diagnosis. Most respondents (91.5%) indicated that screening for weight loss should be conducted with follow‐up frequency as all the time (59.5%) or most of the time (32.0%) during treatment. Slightly less than half of the respondents (47.4%) indicated that patients with cancer should be weighed at each visit with weight systematically tracked over time (61.9%). In most cases (39.8%), respondents reported the nurse as being responsible for screening the patient for weight loss. The main reasons given by respondents for not routinely screening patients for cachexia was that they do not know how to effectively screen patients (43.6%), there are no standardized tools/instruments for screening (38.8%), the belief that weight loss is an expected side effect of treatment (35.1%), and that screening is not a priority (32.0%). Respondents also identified healthcare system impacts on the screening of patients for cachexia. The system impacts identified by respondents included not enough personnel or fiscal resources available to screen patients (25.0%), screening was not a regulatory priority (23.5%), and there was no electronic medical record documentation requirement (21.8%). Finally, respondents identified that they did not have the resources available to treat patients if cachexia was diagnosed (17.2%) and that there were no curative treatment options (15.3%).
Table 2.
Domain (Practice): screening cancer patients for cachexia
| N | Statistic | ||
|---|---|---|---|
| When should cancer patients be screened for weight loss (select all that apply)? | |||
| At the time of diagnosis | 1252 | 52.7% | |
| All the time throughout treatment | 1413 | 59.5% | |
| Most of the time during treatment | 759 | 32.0% | |
| When the patient has lost >10% body weight in the last 3 months | 435 | 18.3% | |
| I do not know | 30 | 1.3% | |
| I do not screen cancer patients therefore not applicable for my professional role/responsibility | 82 | 3.5% | |
| In your practice setting, are cancer patients weighed at each visit? | |||
| Yes | 1125 | 47.4% | |
| No | 678 | 28.5% | |
| Unsure | 140 | 5.9% | |
| Not applicable for my practice setting | 308 | 13.0% | |
| Missing data | 124 | 5.2% | |
| In your practice setting, is the weight of cancer patients systematically tracked over time? | |||
| Yes | 1470 | 61.9% | |
| No | 400 | 16.8% | |
| Unsure | 167 | 7.0% | |
| Not applicable for my practice setting | 214 | 9.0% | |
| Missing data | 124 | 5.2% | |
| In your practice setting, who is responsible for screening cancer patients for weight loss? | |||
| The primary treating physician | 554 | 23.3% | |
| The nurse (nurse or advanced practice nurse) | 945 | 39.8% | |
| The dietitian/registered dietitian/nutritionist (non‐physician) | 324 | 13.6% | |
| I do not know | 125 | 5.3% | |
| Other (free text) | 233 | 9.8% | |
| Not applicable for my practice setting | 70 | 2.9% | |
| Missing data | 124 | 5.2% | |
| In my opinion, clinicians do not regularly screen patients for cachexia because (select all that apply): | |||
| They do not know how to effectively screen patients | 1036 | 43.6% | |
| There are no standardized tools or instruments to screen patients for cachexia | 922 | 38.8% | |
| They believe that weight loss is an expected side effect of treatment | 834 | 35.1% | |
| They do not have sufficient resources to screen patients for cachexia (e.g. fiscal or personnel) | 595 | 25.0% | |
| They do not have sufficient resources to treat patients if cachexia is diagnosed | 409 | 17.2% | |
| There are no curative treatment options | 364 | 15.3% | |
| There is no regulatory requirement to do so | 557 | 23.5% | |
| It is not a priority | 760 | 32.0% | |
| There is no cue to do so (e.g. required field in the medical record that must be completed) | 518 | 21.8% | |
| Other: free text | 138 | 5.8% | |
Diagnosing patients with cancer with cachexia
Respondents reported that there is a low level (43.5%) or a moderate level (33.9%) of attention given to the diagnosis of cachexia in patients with cancer, whereas only 9% believe that the level of attention is high and 3.8% reported that no attention is given to the diagnosis of cachexia (Table 3). Respondents reported that the diagnosis of cancer cachexia includes the identification of anorexia and decreased oral intake (82.1%), evaluation of physical function (68.5%), evaluation of inflammatory markers (67.4%), determination of calorie and protein requirements (60%), collection of clinical and collection of laboratory data to validate the diagnosis of cachexia (54.9%) and measurement of body composition (50.8%). One‐fifth (21.5%) of respondents reported they use their own clinical judgement to identify and diagnose cancer cachexia, whereas 22% reported that the diagnosis of cancer cachexia was not within their scope of responsibility. Almost 17% of the respondents utilized national and regional clinical practice guidelines to diagnosis patients, and utilization of international guidelines remained below 18% across the individual societies. Terms used by respondents to diagnose cancer‐related weight loss included malnutrition (73.9%), anorexia (67%), cachexia (67%) and unintentional weight loss (57.9%). Respondents reported that malnutrition may be used as a surrogate diagnosis for cachexia since it is more easily understood (46.7%) and is often conflated with cachexia (41.7%) and because there are standardized tools to screen and diagnosis malnutrition (22.5%).
Table 3.
Domain (Practice): diagnosing cancer patients with cachexia
| N | Statistic | ||
|---|---|---|---|
| In your opinion, what is the level of attention that is given to the diagnosis of cachexia? | |||
| High attention | 214 | 9.0% | |
| Moderate attention | 804 | 33.9% | |
| Low attention | 1033 | 43.5% | |
| No attention | 91 | 3.8% | |
| Missing data | 233 | 9.8% | |
| Diagnosing cancer patients with cachexia should include the following actions (select all that apply): | |||
| Identifying the presence of anorexia and decreased oral intake | 1949 | 82.1% | |
| Determining calorie and protein requirements | 1426 | 60.0% | |
| Measuring body composition | 1206 | 50.8% | |
| Evaluating inflammatory markers (i.e. acute phase proteins, pro‐inflammatory cytokines and WBC) | 1601 | 67.4% | |
| Evaluating physical function | 1628 | 68.5% | |
| Collecting clinical and laboratory data to validate the diagnosis of cachexia | 1304 | 54.9% | |
| Other: free text | 48 | 2.0% | |
| I do not know | 50 | 2.1% | |
| I do not conduct these types of assessments therefore not applicable for my professional | 401 | 16.9% | |
| I use the definition and/or diagnostic criteria from professional organizations to support and confirm my identification of or diagnosis of cancer cachexia (select all that apply): | |||
| American Society of Clinical Oncology (ASCO) | 328 | 13.8% | |
| Spanish Society of Medical Oncology (SEOM) Clinical Guidelines on Nutrition in Cancer Patients | 35 | 1.5% | |
| American Society for Parenteral and Enteral Nutrition (ASPEN) | 365 | 15.4% | |
| European Society for Clinical Nutrition and Metabolism (ESPEN) | 418 | 17.6% | |
| European Palliative Care Research Collaborative (EPCRC) | 149 | 6.3% | |
| Designated national/regional guidelines | 398 | 16.8% | |
| My institutional guidelines | 186 | 7.8% | |
| My own clinical judgement | 510 | 21.5% | |
| Other: free text | 83 | 3.5% | |
| I do not diagnose cachexia in cancer patients therefore not applicable for my professional role/responsibility | 535 | 22.5% | |
| Terms used for diagnosing cancer‐related weight loss in my practice setting include (select all that apply): | |||
| Unintentional weight loss | 1374 | 57.9% | |
| Anorexia | 1592 | 67.0% | |
| Malnutrition | 1755 | 73.9% | |
| Cachexia | 1592 | 67.0% | |
| Other (free text) | 78 | 3.3% | |
| Malnutrition may be used as a diagnosis for cachexia because (select all that apply): | |||
| There are standardized tools to screen for and diagnose malnutrition | 535 | 22.5% | |
| Insurance companies reimburse for interventions related to a diagnosis of malnutrition | 258 | 10.9% | |
| Malnutrition is more easily understood | 1108 | 46.7% | |
| Clinicians confuse malnutrition with cachexia | 991 | 41.7% | |
| Other: free text | 91 | 3.8% | |
Treating patients with cancer cachexia
Respondents reported utilizing nutrition support, physical exercise, pharmaceutical agents and psychosocial interventions when treating patients with cancer cachexia (Table 4). Once a diagnosis of cachexia is made, respondents initiated nutritional interventions that included oral nutritional supplements (64.2%), energy and protein‐fortified foods (60.3%) and prescribed nutritional counselling (57.1%). Respondents (40.7%) acknowledged that making nutritional recommendations for patients with cachexia is challenging. Other interventions for patients with cachexia included physical exercise, pharmaceutical agents and psychosocial support. About one‐third (34.4%) of respondents prescribed physical exercise. Pharmaceutical interventions prescribed included the use of corticosteroids (26.8%), anti‐inflammatory agents (15.8%), progestational agents (12.0%) and cannabinoids (9.9%). Approximately, a quarter (23.0%) of respondents referred patients for psychosocial support. Respondents (34.8%) utilized evidence‐based guidelines to guide care for their patients with cancer cachexia. Recommendations from ESPEN (19.2%) and ASPEN (16.6%) were the most widely used guidelines followed by the use of regional cancer centre guidelines (17.1%). Most referrals for patients with cachexia were made by physicians (58.7%), nurses/advanced practice nurses (51.2%) and dietitians/nutritionists (32.3%). Responsibility for referral follow‐up was most often delegated to nurses/advanced practice nurses (63.7%), dietitian/nutritionists (54.9%) and physicians (52.3%). However, respondents (24.4%) reported that specialists have limited availability for consultation. Most often, the specialists who were not available for consultation were the physician (22.1%), nurse/advanced practice (13.1%) and dietitians/nutritionists (12.7%).
Table 4.
Domain (Practice): treating cancer patients with cachexia
| N | Statistic | ||
|---|---|---|---|
| When a diagnosis of cachexia is made, treatments may include (select all that apply): | |||
| Incorporate energy and protein‐fortified foods in the diet | 1433 | 60.3% | |
| Recommend oral nutritional supplements | 1524 | 64.2% | |
| Prescribe corticosteroids | 636 | 26.8% | |
| Prescribe progestational agents | 284 | 12.0% | |
| Prescribe cannabinoids | 234 | 9.9% | |
| Prescribe anti‐inflammatory agents | 375 | 15.8% | |
| Prescribe physical exercise | 817 | 34.4% | |
| Prescribe nutritional counselling | 1357 | 57.1% | |
| Refer to specialist for psychosocial support | 547 | 23.0% | |
| Other: free text | 72 | 3.0% | |
| I do not know | 55 | 2.3% | |
| I do not diagnose cachexia in cancer patients therefore not applicable for my professional role/responsibility | 199 | 8.4% | |
| I diagnose cachexia in cancer patients but I cannot prescribe or order treatments, so not applicable for my professional role/responsibility | 108 | 4.5% | |
| Providing nutrition recommendations for patients with cachexia is challenging for me | |||
| Strongly agree | 257 | 10.8% | |
| Agree | 710 | 29.9% | |
| Neither agree or disagree | 403 | 17.0% | |
| Disagree | 399 | 16.8% | |
| Strongly disagree | 163 | 6.9% | |
| Not applicable for my professional role/responsibility | 70 | 2.9% | |
| Missing data | 373 | 15.7% | |
| I use tools and resources such as evidence‐based guidelines developed by experts to care for my cancer patients with cachexia | |||
| Yes, all the time | 240 | 10.1% | |
| Yes, most of the time | 587 | 24.7% | |
| Rarely | 415 | 17.5% | |
| No, I prefer to use my own clinical judgement | 202 | 8.5% | |
| No, I am not aware of tools and resources to care for my cancer patients with cachexia | 280 | 11.8% | |
| No, I do not use tools and resources because I do not have access to them | 81 | 3.4% | |
| Not applicable for my professional role/responsibility | 197 | 8.3% | |
| Missing data | 373 | 15.7% | |
| If yes, to above, I use evidence‐ based guidelines from the following organization(s) to care for my cancer patients with cachexia (select all that apply): | |||
| American Society of Clinical Oncology (ASCO) | 302 | 12.7% | |
| Spanish Society of Medical Oncology (SEOM) Clinical Guidelines on Nutrition in Cancer Patients | 27 | 1.1% | |
| American Society for Parenteral and Enteral Nutrition (ASPEN) | 367 | 16.6% | |
| European Society for Parenteral and Enteral Nutrition (ESPEN) | 457 | 19.2% | |
| Designated regional cancer centre guidelines | 406 | 17.1% | |
| Other: free text | 106 | 4.5% | |
| Not applicable for my professional role/responsibility | 165 | 6.9% | |
| In my practice setting, referrals for cachexia are most often initiated by the following health care team member – ALL THAT APPLY (Europe, NA and ROW only): | |||
| Specialist physician/medical doctor | 673 | 58.7% | |
| Physician assistant | 146 | 12.7% | |
| Advanced practice nurse (e.g. nurse practitioner) | 293 | 25.5% | |
| Registered nurse/nurse | 295 | 25.7% | |
| Pharmacist | 30 | 2.6% | |
| Dietitian/registered dietitian/nutritionist (non‐physician) | 370 | 32.3% | |
| Occupational therapist | 19 | 1.7% | |
| Physical therapist | 47 | 4.1% | |
| Social worker | 32 | 2.8% | |
| Psychologist | 31 | 2.7% | |
| Other (free text) | 0 | 0% | |
| Not applicable for my professional role/responsibility | 80 | 7.0% | |
| In my practice setting, referrals for cachexia are most often initiated by the following health care team member – SINGLE RESPONSE ONLY (Japan only data): | |||
| Specialist physician/medical doctor | 300 | 24.4% | |
| Physician assistant | 1 | 0.1% | |
| Advanced practice nurse (e.g. nurse practitioner) | 125 | 10.2% | |
| Registered nurse/nurse | 195 | 15.9% | |
| Pharmacist | 14 | 1.1% | |
| Dietitian/registered dietitian/nutritionist (non‐physician) | 229 | 18.7% | |
| Occupational therapist | 2 | 0.2% | |
| Physical therapist | 19 | 1.6% | |
| Social worker | 1 | 0.1% | |
| Psychologist | 2 | 0.2% | |
| Other (free text) | 35 | 2.9% | |
| Not applicable for my professional role/responsibility | 86 | 7.0% | |
| Missing data | 219 | 17.8% | |
| Once a referral is initiated, it is up to the following individual(s) to ensure that it is executed (all that apply): | |||
| Specialist physician/medical doctor | 1241 | 52.3% | |
| Physician assistant | 150 | 6.3% | |
| Advanced practice nurse (e.g. nurse practitioner) | 661 | 27.8% | |
| Registered nurse/nurse | 853 | 35.9% | |
| Pharmacist | 428 | 18.0% | |
| Dietitian/registered dietitian/nutritionist (non‐physician) | 1303 | 54.9% | |
| Occupational therapist | 214 | 9.0% | |
| Physical therapist | 397 | 16.7% | |
| Social worker | 159 | 6.7% | |
| Psychologist | 137 | 5.8% | |
| Other (free text) | 83 | 3.5% | |
| I do not refer my patients to other healthcare professionals; I manage their care myself | 33 | 1.4% | |
| I do not know | 79 | 3.3% | |
| Not applicable for my professional role/responsibility | 85 | 3.6% | |
| There are sufficient specialists available for me to refer my cancer patients with cachexia | |||
| Yes, all the time | 145 | 6.1% | |
| Yes, most of the time | 435 | 18.3% | |
| Rarely | 478 | 20.1% | |
| No | 710 | 29.9% | |
| Not applicable for my professional role/responsibility | 234 | 9.9% | |
| Missing data | 373 | 15.7% | |
| The following specialists are often not available for my patients even if I refer them (select all that apply): | |||
| Specialist physician/medical doctor | 526 | 22.1% | |
| Physician assistant | 180 | 7.6% | |
| Advanced practice nurse (e.g. nurse practitioner) | 152 | 6.4% | |
| Registered nurse/nurse | 159 | 6.7% | |
| Pharmacist | 166 | 7.0% | |
| Dietitian/registered dietitian/nutritionist (non‐physician) | 301 | 12.7% | |
| Occupational therapist | 206 | 8.7% | |
| Physical therapist | 231 | 9.7% | |
| Social worker | 204 | 8.6% | |
| Psychologist | 249 | 10.5% | |
| Other (free text) | 41 | 17.2% | |
| Not applicable for my professional role/responsibility | 551 | 23.2% | |
Perceptions and attitudes in the care of patients with cancer cachexia
Commonly held perceptions and attitudes often impacted the care that is given to patients with cancer cachexia (Table 5). Respondents (37.3%) believed that cachexia is unavoidable. However, half of respondents (49.5%) recognized that weight loss in obese patients with cancer is not a positive finding. Most respondents (79.2%) believed that an interprofessional team approach improves the care of patients with cancer cachexia and that the use of standardized tools (77%) to evaluate weight loss is critical. Slightly more than half (53.7%) of respondents believed that the lack of high‐quality evidence to guide the care and treatment of patients with cachexia makes it difficult to initiate treatment. Respondents also reported that the lack of safe and effective medications (54.5%) and sufficient time to provide psychosocial support for the patient and their families (59.8%) results in poor outcomes for patients suffering from cachexia.
Table 5.
Domain (Perceptions and Attitudes): perceptions and attitudes in the care of cancer patients with cachexia
| N | Statistic | ||
|---|---|---|---|
| Cachexia is unavoidable in cancer patients | |||
| Strongly agree | 203 | 8.5% | |
| Agree | 685 | 28.8% | |
| Neither agree nor disagree | 550 | 23.2% | |
| Disagree | 464 | 19.5% | |
| Strongly disagree | 58 | 2.4% | |
| Missing data | 415 | 17.5% | |
| When an obese cancer patient starts to lose weight during the treatment phase, it is a good thing | |||
| Strongly agree | 15 | 0.6% | |
| Agree | 98 | 4.1% | |
| Neither agree nor disagree | 672 | 28.3% | |
| Disagree | 762 | 32.1% | |
| Strongly disagree | 413 | 17.4% | |
| Missing data | 415 | 17.5% | |
| An interprofessional team approach in the care of cancer patients with cachexia improves patient outcomes | |||
| Strongly agree | 1193 | 50.2% | |
| Agree | 689 | 29.0% | |
| Neither agree nor disagree | 70 | 2.9% | |
| Disagree | 5 | 0.2% | |
| Strongly disagree | 3 | 0.1% | |
| Missing data | 415 | 17.5% | |
| The regular use of standardized tools to evaluate cancer patients for weight loss is critical | |||
| Strongly agree | 1036 | 43.6% | |
| Agree | 793 | 33.4% | |
| Neither agree nor disagree | 104 | 4.4% | |
| Disagree | 21 | 0.9% | |
| Strongly disagree | 6 | 0.3% | |
| Missing data | 415 | 17.5% | |
| Lack of high‐quality evidence to guide the care and treatment of cancer patients with cachexia makes it challenging for me as a clinician when starting treatment | |||
| Strongly agree | 366 | 15.4% | |
| Agree | 909 | 38.3% | |
| Neither agree nor disagree | 368 | 15.5% | |
| Disagree | 175 | 7.4% | |
| Strongly disagree | 51 | 2.1% | |
| Not applicable for my professional role/responsibility | 91 | 3.8% | |
| Missing data | 415 | 17.5% | |
| Lack of safe and consistently effective medications that are available to treat cancer patients with cachexia make it challenging for me as a clinician | |||
| Strongly agree | 419 | 17.6% | |
| Agree | 877 | 36.9% | |
| Neither agree nor disagree | 315 | 13.3% | |
| Disagree | 111 | 4.7% | |
| Strongly disagree | 15 | 0.6% | |
| I do not prescribe medications | 223 | 9.4% | |
| Missing data | 415 | 17.5% | |
| Clinicians lack sufficient time to provide psychosocial support for patients who have been diagnosed with cachexia and their families | |||
| Strongly agree | 462 | 19.5% | |
| Agree | 957 | 40.3% | |
| Neither agree nor disagree | 335 | 14.1% | |
| Disagree | 187 | 7.9% | |
| Strongly disagree | 19 | 0.8% | |
| Missing data | 415 | 17.5% | |
Interprofessional care of patients with cancer cachexia
Just over 45% of respondents reported that they practise as members of interprofessional teams in the care of patients with cancer cachexia all or most of the time (45.2%); and just over 30% reported that they rarely or never practiced as members of interprofessional teams (30.5%). Similar distributions were found in the clarity of roles and responsibilities of healthcare team members, with 41.7% of respondents reporting roles/responsibilities in relation to cachexia were clear all or most of the time, and rarely or not clear in 28.3% of respondents. Respondents did report that they were able to easily collaborate with other HCPs all or most of the time almost half the time (55.6%) and there was a high level of respect for each profession among the members of the healthcare team all or most of the time (55.4%). Over 40% of respondents disagreed or strongly disagreed that care by a team of multiple professionals could result in no one being responsible for overall care.
Care facilitators and barriers for patients with cachexia
Over half of respondents (54.3%) indicated that the care prescribed to patients is impacted by reimbursement. Over half of respondents (55.4%) believed that patients comply with the cachexia treatment regime prescribed by their oncologist. However, respondents (67.9%) believed patients' personal choices impacted their compliance with the cachexia treatment protocol and may affect the overall effectiveness of the treatment. In addition, respondents (71.6%) believed that compliance with the cachexia treatment regime may be affected by challenges related to the disease process such as fatigue.
Education for care of patients with cancer cachexia
It is critical to ensure that clinicians receive education to optimize the care and outcomes for patients with cancer and cancer cachexia (Table 6). According to respondents, there was a lack of general nutrition information incorporated into curricula at all levels of education with even less information on the management of patients with cancer cachexia. More respondents reported that they engaged in continuing education (CE) or continuing professional development (CPD) in nutrition for patients with cancer (30.7%) and all patients (29.3%) as compared with those who reported that they participated in CE/CPD for patients with cancer cachexia (19.1%). More than 25% of respondents reported that they do not engage in any CE/CPD on nutrition (27.3%). For those who do participate in CE/CPD, 30.3% participated in less than five educational activities annually. When accessing CE/CPD, respondents preferred online formats [video lectures (52.2%) or case‐based learning (41.3%)]; attending live regional activities (42.9%); attending national or international conferences or symposia (36.0%); reading journals or other printed materials (35.5%); and answering questions at the end of e‐learning activities (23.4%). Respondents were less likely to prefer authoring professional papers and books, serving as a supervisory physician, hands‐on learning or listening to podcasts.
Table 6.
Nutrition education within formal educational programmes
| General nutrition education | Nutrition education specific to cancer patients | Nutrition education specific to cancer patients with cachexia | |
|---|---|---|---|
| Undergraduate | 32.3% | 15.4% | 11.2% |
| Residency or fellowship | 13.3% | 11.7% | 10.5% |
| Graduate | 17.1% | 14.8% | 12.6% |
| No nutrition education | 24.2% | 36.7% | 41.0% |
Discussion
This international, interprofessional, mixed‐methods educational needs assessment is among the first to explore professional practice gaps across multiple HCPs involved in the care of patients with cancer cachexia, both within and across professions. It found gaps in use of a standardized definition of cachexia and in identifying and treating patients with cancer cachexia (Box 1 ). Almost half of the respondents were very or somewhat confident in their ability to care for patients at risk or diagnosed with cachexia. These numbers were primarily driven by the confidence reported by physicians/medical doctors and registered dieticians/nutritionists. There was a considerable variation among respondents when asked to identify the percentage of weight loss and the BMI threshold that define cachexia. This variation may reflect a number of root causes that include but are not limited to the evolving understanding of cachexia; lack of access to or outdated evidence‐based resources; differences among professions; and lack of engagement in education in this area. More than half of respondents used both terms ‘malnutrition’ and ‘cachexia’ to describe weight loss in patients with cancer. This mirrors the overlapping definitions of cancer cachexia 1 and disease‐associated malnutrition 31 and that both have weight loss as their cardinal diagnostic criterion.
The vast majority was able to identify that causes of weight loss in patients with cancer included inflammation in addition to other pathophysiologic causes of the disease process and/or side effects of chemotherapy. There was a lack of consistency among respondents when asked about when patients with cancer should be screened for weight loss, how frequently they should be weighed and how weight loss should be tracked over time. This variation may reflect the perspective of different professions, the setting where respondents practice and whether respondents have control over that decision. This variation may also indicate an opportunity to standardize and/or widely communicate guidelines for HCPs. Respondents reported using their own clinical judgement and referring to guidelines from ASCO, ASPEN and ESPEN to care for patients with cancer with cachexia. Additionally, 16.8% used national or regional guidelines. It will be important to ensure that national/regional guidelines reflect the most up‐to‐date evidence.
Treatment recommendations remain challenging for respondents and most interventions involved increasing oral intake and incorporating energy and protein‐fortified foods. More than 25% of respondents rarely or never used evidence‐based tools or resources or preferred to use his/her own clinical judgement to care for patients with cancer cachexia, which may reflect a low level of attention, but could also reflect lack of access at point of care. An interprofessional team approach to the care of patients with cancer was strongly supported by respondents as was the use of standardized tools and resources. Most respondents also reported a lack of high‐quality evidence to guide the care and treatment of patients with cancer cachexia may reflect a lack of availability of evidence‐based tools and resources at the point of acre rather than a lack of desire to use those resources.
Box 1. Cancer cachexia education: insights and gaps
Almost half of respondents were very or somewhat confident in their ability to provide care for patients diagnosed with cancer cachexia. These numbers were primarily driven by the confidence reported by physicians/medical doctors and registered dieticians/nutritionists.
Many respondents did not recognize or utilize a standardized definition of cancer cachexia; however, most could identify weight loss as a ‘red flag’ and correctly identify potential causes of weight loss as inflammation, reduced oral intake, disease progression and side effects of chemotherapy.
About half of respondents conducted nutrition screening for weight loss on newly diagnosed patients. Reasons for not routinely screening patients were lack of knowledge on how to effectively screen patients, lack of standardized tools and belief that weight loss is an expected side effect of treatment.
Respondents confirmed the diagnosis of cachexia through the presence of anorexia, decreased oral intake, determination of calorie and protein requirements, evaluation of physical function and laboratory findings such as elevated inflammatory markers.
Treatment of cancer cachexia focused on increasing the patient's nutritional intake, via energy and protein‐fortified foods, oral nutritional supplements and counselling by a dietitian/nutritionist.
Whereas many respondents considered cachexia an unavoidable consequence of cancer, most believed that the interprofessional team approach could improve the care of patients with cancer cachexia and that the use of standardized tools is critical. However, more than half of respondents believed that there is a lack of high‐quality evidence to guide the care and treatment of patients suffering from cancer cachexia.
Strengths and limitations
The strengths of this international study included the interdisciplinary approach and the large number of survey respondents (N = 2375) from North America (19.3%), Europe (20.0%), Japan (51.7%) and the rest of world (9.0%). Respondents in this survey had clinical experience and expertise in the management of patients with cancer cachexia. Physicians and dietitians/nutritionists had the highest levels of response to the survey; such responses likely correlate with their direct roles in identification and management of patients with cachexia. The combination of clinical expertise and interest in optimizing the management of patients with cancer cachexia makes the results of this survey extremely relevant to the development of highly effective educational programmes for practising clinicians and for preparing new practitioners. In addition, respondents provided specific information on their preferred learning methods, which will further enhance participation and ensure the success of the educational programs.
Participation to this assessment was voluntary and may reflect bias towards HCPs who are most actively involved and interested in the care of patients with cachexia. There were few respondents from certain professions (occupational therapists, social workers, psychologists, dentists, speech pathologists, palliative care specialists). There was an abrupt decrease in the number of respondents after the confidence survey item. The results thus most likely reflect the opinions of those HCPs who were the most confident in treating patients with cancer cachexia.
Conclusions
The findings from this international analysis of a mixed‐methods educational needs assessment present a picture of challenges associated with the care of patients with cancer cachexia, as well as opportunities for interventions that may improve the care and outcomes of patients and their families. HCPs need to better understand the pathophysiology, identification of and treatment of cancer cachexia. There is a need for clear, concise and pragmatic guidelines that are widely available at point of care. HCPs need to participate as members of collaborative, interprofessional care teams. Finally, HCPs need affordable CE/CPD that is easily accessible and can be delivered in multimodal forms including at the point of care where HCPs are practising.
Conflict of interest
VB reports receiving consultancy fees from Pfizer and Nestle and has received research grant funding from Baxter healthcare. SDA reports receiving fees from Abbott, Actimed, Bayer, Boehringer Ingelheim, Cardiac Dimension, Cordio, Impulse Dynamics, Novartis, Occlutech, Servier and Vifor Pharma, and grant support from Abbott and Vifor Pharma. AC declares having received honoraria and/or lecture fees from AstraZeneca, Boehringer Ingelheim, Menarini, Novartis, Servier, Vifor, Abbott, Actimed, Arena, Cardiac Dimensions, Corvia, CVRx, Enopace, ESN Cleer, Faraday, Impulse Dynamics, Respicardia and Viatris. All other authors have no conflict of interest.
Funding
Funding for this project was provided by Pfizer as an independent educational grant.
Acknowledgements
International Advisory Board
Vickie Baracos, PhD, Department of Oncology Division of Palliative Care Medicine, University of Alberta, Alberta, Canada; Stefan Anker, MD, Charité‐Universitätsmedizin, Berlin, Germany; Andrew Coats, MD, Joint Academic Vice‐President of Monash University, Australia and the University of Warwick, UK; Matti Aapro, MD, Dean, Multidisciplinary Oncology Institute, Genolier, Switzerland; Andreas Charalambous, PhD, Cyprus University of Technology; Egidio del Fabbro, MD, Virginia Commonwealth University Massey Cancer Center, USA; Stefan Gijssels, Chief Executive Officer, Member of the Board, Digestive Cancers Europe, Brussels, Belgium; Alessandro Laviano, MD, PhD, Director, Clinical Nutrition, Department of Clinical Medicine, Sapienza University, Rome, Italy; Mary Marian, RDN, MS, Dept Nutritional Science, University of Arizona, USA; Maurizio Muscaritoli, MD, Director, Clinical Nutrition Management, Sapienza University, Rome, Italy; Tateaki Naito, MD, Thoracic Cancer Medicine, Shizuoka Cancer Center, Japan; Hidetaka Wakabayashi, MD, Dept Rehabilitation Medicine, Tokyo Women's Medical University, Japan.
North American Advisory Board
Aminah Jatoi, MD, Mayo Clinic, Rochester, Minnesota; Jeffrey Crawford, MD, Duke University School of Medicine, Cancer institute, North Caroline; Puneeth Lyengar, MD, PhD, UT Southwestern Medical Center, Dallas, Texas; Kelay Trentham, MS, RDN, CSO, FAND, MultiCare Health System, Tacoma, Washington; Todd Mattox, PharmD, BCNSP, FASPEN, Moffitt Cancer Center Tampa, Florida; Valaree Williams, MS, RD, CSO, CNSC, LDN, FAND, Penn Medicine, University of Pennsylvania Health System, Pennsylvania; Kim Gorsuch, BSN, RN, CNSC, Cancer Treatment Centers of America, Illinois; Richard Dunne, MD, University of Rochester Medical Center, Minnesota.
European Advisory Board
Josep Argiles, PhD, Cancer Research Group, Department of Biochemistry and Molecular Biomedicine, Faculty of Biology, University of Barcelona, Barcelona, Spain; Sarah Liptrott, MS, RN, CNS, European Institute of Oncology, Italy; Florian Strasser, MD, Cantonal Hospital Section Oncology, Dept Internal Medicine, St Gallen, Switzerland; Olivier Mir, MD, PhD, MPH, Gustave Roussy Cancer Institute, University of Paris‐Sud, Villejuif, France; Annemie Schols, PhD, Dean, Faculty of Health, Medicine and Life Sciences at Maastricht University, Maastricht, Netherlands; Pierre Senesse, MD, PhD, Institut du Cancer de Montpellier Val d'Aurelle, and Laboratoire Epsylon, Montpellier, France; Richard Skipworth, MD, Edinburgh University, Edinburgh, Scotland.
Japanese Advisory Board
Hidenori Arai, MD, PhD, National Center for Geriatrics and Gerontology; Sakiko Aso, RN, Division of Nursing, Shizuoka Cancer Center; Akio Inui, MD, PhD, Department of Pharmacological Sciences of Herbal Medicine, Graduate School of Medical and Dental Sciences; Masaaki Konishi, MD, PhD, Department of Medical Science and Cardiorenal Medicine, Yokohama City University School of Medicine; Tateaki Naito, MD, PhD, Division of Thoracic Oncology, Shizuoka Cancer Center; Masakazu Saitoh, RPT, PhD, Department of Physical Therapy, Faculty of Health Science, Juntendo University; Noriyasu Shirotani, MD, PhD, Shin‐Yokohama Home Care Clinic; Hidetaka Wakabayashi, MD, PhD, Department of Rehabilitation Medicine, Tokyo Women's Medical University Hospital.
Editorial support as provided by Daniel Beard (MedEd Global Solutions, Paris, France), and statistical analysis was performed by Kathy Chappell, PhD, RN, FNAP, FAAN.
The authors certify that they comply with the ethical guidelines for authorship and publishing of the Journal of Cachexia, Sarcopenia and Muscle. 32
Baracos V. E., Coats A. J., Anker S. D., Sherman L., Klompenhouwer T., and on behalf of the International Advisory Board, and Regional Advisory Boards for North America, Europe, and Japan (2022) Identification and management of cancer cachexia in patients: Assessment of healthcare providers' knowledge and practice gaps, Journal of Cachexia, Sarcopenia and Muscle, 13, 2683–2696, doi: 10.1002/jcsm.13105
References
- 1. Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol 2011;12:489–495. [DOI] [PubMed] [Google Scholar]
- 2. Ferraro CD, Grant M, Koczywas M, Dorr‐Uyemura LA. Management of anorexia‐cachexia in late stage lung cancer patients. J Hosp Palliat Nurs 2012;14:397–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kalantar‐Zadeh K, Rhee C, Sim JJ, Stenvinkel P, Anker SD, Kovesdy CP. Why cachexia kills: Examining the causality of poor outcomes in wasting conditions. J Cachexia Sarcopenia Muscle 2013;4:89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Poisson J, Martinez‐Tapia C, Heitz D, Geiss R, Albrand G, Falandry C, et al. Prevalence and prognostic impact of cachexia among older patients with cancer: A nationwide cross‐sectional survey (NutriAgeCancer). J Cachexia Sarcopenia Muscle 2021;12:1477–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. von Haehling S, Anker MS, Anker SD. Prevalence and clinical impact of cachexia in chronic illness in Europe, USA, and Japan: facts and numbers update 2016. J Cachexia Sarcopenia Muscle 2016;7:507–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Arends J, Baracos V, Bertz H, Bozzetti F, Calder PC, Deutz NEP, et al. ESPEN expert group recommendations for action against cancer‐related malnutrition. Clin Nutr 2017;36:1187–1196. [DOI] [PubMed] [Google Scholar]
- 7. The PreMiO Study Group , Muscaritoli M, Lucia S, Farcomeni A, Lorusso V, Saracino V, et al. Prevalence of malnutrition in patients at first medical oncology visit: the PreMiO study. Oncotarget 2017;8:79884–79896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tisdale MJ. Mechanisms of cancer cachexia. Physiol Rev 2009;89:381–410. [DOI] [PubMed] [Google Scholar]
- 9. Argiles JM, Lopez‐Soriano FJ, Busquets S. Mechanisms and treatment of cancer cachexia. Nutr Metab Cardiovasc Dis 2013;23:S19–S24. [DOI] [PubMed] [Google Scholar]
- 10. Ebner N, Anker SD, Von Haehling S. Recent developments in the field of cachexia, sarcopenia and muscle wasting: Highlights from the 12th Cachexia Conference. J Cachexia Sarcopenia Muscle 2020;11:274–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Roeland EJ. Cancer cachexia: The elephant in the room? J Cachexia Sarcopenia Muscle 2022;13:3–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Garcia JM, Dunne RF, Santiago K, Martin L, Birnbaum MJ, Crawford J, et al. Addressing unmet neeeds for people with cancer cachexia: Recommendations from a multistakeholder workshop. J Cachexia Sarcopenia Muscle 2022;13:1418–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Arends J, Strasser F, Gonella S, Solheim TS, Madeddu C, Ravasco P, et al. Cancer cachexia in adult patients: ESMO clinical practice guidelines. ESMO Open 2021;6:100092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Roeland EJ, Bohlke K, Baracos VE, Bruera E, del Fabbro E, Dixon S, et al. Management of cancer cachexia: ASCO guideline. J Clin Oncol 2020;38:2438–2453. [DOI] [PubMed] [Google Scholar]
- 15. Higashiguchi T, Ikegaki J, Sobue K, Tamura Y, Nakajima N, Futamura A, et al. Guidelines for parenteral fluid management for terminal cancer patients. Jpn J Clin Oncol 2016;46:986–992. [DOI] [PubMed] [Google Scholar]
- 16. Muscaritoli M, Arends J, Bachmann P, Baracos V, Barthelemy N, Bertz H, et al. ESPEN practical guideline: Clinical nutrition in cancer. Clin Nutr 2021;40:2898–2913. [DOI] [PubMed] [Google Scholar]
- 17. Prado CM, Purcell SA, Laviano A. Nutrition interventions to treat low muscle mass in cancer. J Cachexia Sarcopenia Muscle 2020;11:366–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chapek MA, Martindale RG. Nutrition in cancer therapy: overview for the cancer patient. J Parenter Enteral Nutr 2021;45:1–25. [DOI] [PubMed] [Google Scholar]
- 19. Prado CM, Anker SD, Coats AJS, Laviano A, von Haehling S. Nutrition in the spotlight in cachexia, sarcopenia and muscle: Avoiding the wildfire. J Cachexia Sarcopenia Muscle 2021;12:3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tan S, Meng Q, Jiang Y, Zhuang Q, Xi Q, Xu J, et al. Impact of oral nutritional supplements in post‐discharge patients at nutritional risk following colorectal cancer surgery: a randomised clinical trial. Clin Nutr 2021;40:47–53. [DOI] [PubMed] [Google Scholar]
- 21. Meng Q, Tan S, Jiang Y, Han J, Xi Q, Zhuang Q, et al. Post‐discharge oral nutritional supplements with dietary advice in patients at nutritional risk after surgery for gastric cancer: A randomized clinical trial. Clin Nutr 2021;40:40–46. [DOI] [PubMed] [Google Scholar]
- 22. Amano K, Baracos V, Morita T, Miura T, Mori N, Tatara R, et al. The impact of cachexia on dietary intakes, symptoms and quality of life in advanced cancer. JCSM Rapid Commun 2022;5:162–170. [Google Scholar]
- 23. Aoyagi T, Terracina KP, Raza A, Matsubara H, Takabe K. Cancer cachexia, mechanism and treatment. World J Gastrointest Oncol 2015;7:17–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wakabayashi H, Arai H, Inui A. The regulatory approval of anamorelin for treatment of cachexia in patients with non‐small call lung cancer, gastric cancer, pancreatic cancer, and colorectal cancer in Japan: facts and numbers. J Cachexia Sarcopenia Muscle 2021;12:14–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nakahara S, Wakabayashi H, Maeda K, Nishioka S, Kokura Y. Sarcopenia and cachexia evaluation in different healthcare settings: A questionnaire survey of health professionals. Asia Pac J Clin Nutr 2018;27:167–175. [DOI] [PubMed] [Google Scholar]
- 26. ter Beek L, Vanhauwaert E, Slinde F, Orrevall Y, Henriksen C, Johansson M, et al. Unsatisfactory knowledge and use of terminology regarding malnutrition, starvation, cachexia and sarcopenia among dietitians. Clin Nutr 2016;35:1450–1456. [DOI] [PubMed] [Google Scholar]
- 27. Muscaritoli M, Rossi Fanelli F, Molfino A. Perspectives of health care professionals on cancer cachexia: Results from three global surveys. Ann Oncol 2016;27:2230–2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Parmar MP, Vanderbyl BL, Kanbalian M, Windholz TY, Tran AT, Jagoe RT. A multidisciplinary rehabilitation programme for cancer cachexia improves quality of life. BMJ Support Palliat Care 2017;7:441–449. [DOI] [PubMed] [Google Scholar]
- 29. Bland KA, Harrison M, Zopf EM, Sousa MS, Currow DC, Ely M, et al. Quality of life and symptom burden improve in patients attending a multidisciplinary clinical service for cancer cachexia: A retrospective observational review. J Pain Symptom Manage 2021;62:e164–e176. [DOI] [PubMed] [Google Scholar]
- 30. Martin L, Senesse P, Gioulbasanis I, Antoun S, Bozzetti F, Deans C, et al. Diagnostic criteria for the classification of cancer‐associated weight loss. J Clin Oncol 2015;33:90–99. [DOI] [PubMed] [Google Scholar]
- 31. Jensen GL, Mirtallo J, Compher C, Dhaliwal R, Forbes A, Grijalba RF, et al. Adult starvation and disease‐related malnutrition: A proposal for etiology‐based diagnosis in the clinical practice setting from the International Consensus Guideline Committee. JPEN J Parenter Enteral Nutr 2010;34:156–159. [DOI] [PubMed] [Google Scholar]
- 32. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: Update 2021. J Cachexia Sarcopenia Muscle 2021;12:2259–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]


