Abstract
Background
Water T2 (T2H2O) mapping is increasingly being used in muscular dystrophies to assess active muscle damage. It has been suggested as a surrogate outcome measure for clinical trials. Here, we investigated the prognostic utility of T2H2O to identify changes in muscle function over time in limb girdle muscular dystrophies.
Methods
Patients with genetically confirmed dysferlinopathy were assessed as part of the Jain Foundation Clinical Outcomes Study in dysferlinopathy. The cohort included 18 patients from two sites, both equipped with 3‐tesla magnetic resonance imaging (MRI) systems from the same vendor. T2H2O value was defined as higher or lower than the median in each muscle bilaterally. The degree of deterioration on four functional tests over 3 years was assessed in a linear model against covariates of high or low T2H2O at baseline, age, disease duration, and baseline function.
Results
A higher T2H2O at baseline significantly correlated with a greater decline on functional tests in 21 out of 35 muscles and was never associated with slower decline. Higher baseline T2H2O in adductor magnus, vastus intermedius, vastus lateralis, and vastus medialis were the most sensitive, being associated bilaterally with greater decline in multiple timed tests. Patients with a higher than median baseline T2H2O (>40.6 ms) in the right vastus medialis deteriorated 11 points more on the North Star Ambulatory Assessment for Dysferlinopathy and lost an additional 86 m on the 6‐min walk than those with a lower T2H2O (<40.6 ms). Optimum sensitivity and specificity thresholds for predicting decline were 39.0 ms in adductor magnus and vastus intermedius, 40.0 ms in vastus medialis, and 40.5 ms in vastus lateralis from different sites equipped with different MRI systems.
Conclusions
In dysferlinopathy, T2H2O did not correlate with current functional ability. However, T2H2O at baseline was higher in patients who worsened more rapidly on functional tests. This suggests that inter‐patient differences in functional decline over time may be, in part, explained by different severities of the active muscle damage, assessed by T2H2O measure at baseline. Significant challenges remain in standardizing T2H2O values across sites to allow determining globally applicable thresholds. The results from the present work are encouraging and suggest that T2H2O could be used to improve prognostication, patient selection, and disease modelling for clinical trials.
Keywords: Magnetic resonance imaging, Water T2, Limb girdle muscular dystrophy, Limb girdle muscular dystrophy R2, Limb girdle muscular dystrophy 2B
Introduction
Predicting functional decline in muscular dystrophies is a difficult task. There are many paediatric and adult onset forms of muscular dystrophy and they display highly variable rates of disease progression, yet few clues to the cause of the variability have been identified. 1 In some diseases, particularly the more rapidly progressive Duchenne muscular dystrophy (DMD), identifying current functional ability may suggest the next function to be lost, leading to a predictable set of disease milestones. 2 , 3 Biomarkers that correlate with current function can therefore predict the next step in disease progression in DMD. 4 However, in the slowly progressive limb girdle muscular dystrophies (LGMD), predicting short‐term functional changes presents more of a challenge. Dysferlinopathy is a form of LGMD, most commonly described as LGMDR2 or Miyoshi myopathy (MMD1). 5 , 6 In this disease, from the same functional starting point, one patient may remain stable for 3 years, while another deteriorates significantly over only 1 year. 7 , 8
Being able to predict progression of muscular dystrophies has several advantages. Patients may benefit from clearer expectations about the future and more tailored care. Moreover, for clinical trials aiming to demonstrate an effective intervention, it is important to have a well‐matched cohort, or at least to understand the differences in anticipated disease progression without intervention. 9 , 10 As interventional therapies become a reality for the muscular dystrophies, the need to identify biomarkers able to predict upcoming functional decline has intensified. 11 , 12
Magnetic resonance imaging (MRI) has been proposed as one such biomarker. 4 , 11 , 12 , 13 The most used sequences in clinics are both qualitative and include T1‐weighted, which detects fatty replacement of the muscles, and fat‐suppressed T2‐weighted imaging, which signal is increased in several diseases being usually related to oedema and inflammation, although these can be masked by the presence of fatty infiltrations due to the fat suppression. 14 Although these sequences have been demonstrated to be useful for the diagnosis of patients with neuromuscular diseases, their interpretation can be biased and they lack reproducibility, which hampers their application in longitudinal follow‐up studies designed over short periods of time. 15 In contrast, quantitative MRI methods such as Dixon‐based fat‐fraction (FF) mapping, which provides an objective measurement of the amount of fat present in the skeletal muscles, have been demonstrated to correlate with muscle function and to capture changes in muscle structure over short periods of time. Such methods are being implemented in natural history studies and also clinical trials. 15 , 16 , 17 However, its role as a predictor of changes in muscle function has not been demonstrated so far.
T2 mapping sequences have also been used to study changes in muscle structure in several neuromuscular diseases (NMDs). Skeletal muscle T2 is elevated in the presence of oedema, inflammation, and necrosis, as a consequence of increased water mobility and disrupted tissue ultrastructure. However, fatty replacement is a common pathway in the disease progression of most NMDs, which also results in increased T2 values, because the T2 of fat is much longer than that of muscle. 15 , 18 In order to assess the current status of the residual skeletal muscle tissue, independently from the irreversible end‐stage fatty replacement, water T2 (T2H2O) mapping methods have been developed, allowing the detection of inflammatory, oedematous, and necrotic features in fatty‐infiltrated muscles. 19 , 20
T2H2O is increased in several NMDs and its capacity to predict changes in muscle structure, mainly the fat replacement that follows muscle fibre loss, has been reported in several diseases. 21 , 22 , 23 , 24 Recent data from the Clinical Outcomes Study (COS) baseline visit suggest that T2H2O correlates with fatty replacement over time in dysferlinopathy patients, confirming the capacity of T2H2O to identify active damage leading to muscle fibre loss and expansion of fat tissue. 25 However, the capacity of T2H2O to identify patients who will experience a quicker and more severe clinical progression over a short period of time remains to be demonstrated. If it could be shown to predict progression, T2H2O could be used both in clinics to identify patients at risk of progression and also in clinical trials to select patients who may deteriorate over a short period of time and whose results could be more informative about the effectivity of experimental therapies.
We hypothesized that inter‐patient variation in active muscle damage, assessed by T2H2O, may underlie and predict subsequent differences in disease progression from the same functional starting point in patients with dysferlinopathy. In this paper, we assess a series of quantitative MRI parameters, including FF, contractile cross‐sectional area (cCSA), and T2H2O value, in each of the lower limb muscles and how they relate to subsequent disease progression in a range of functional and timed tests in patients with dysferlinopathy, to determine whether any of these parameters can be effectively used to predict muscle function loss.
Methods
Patients
Patients were selected from the Jain Foundation COS in dysferlinopathy. 26 COS is an international, multicentric, prospective natural history study involving 15 centres in the USA, Europe, Asia, and Oceania. COS recruited and assessed 182 patients over a 3‐year period. Assessments performed included medical, physical therapist, biochemical, cardiovascular, and respiratory and MRI that were performed at baseline visit and then every year until Year 3. All patients had a diagnosis of dysferlinopathy, confirmed by genetic testing, with two pathogenic mutations or one pathogenic mutation and evidence of reduced dysferlin expression on a western blot of skeletal muscle and/or monocytes. The baseline, 1‐year data, and 3‐year functional progression and quantitative muscle MRI analysis have previously been published. 7 , 8 , 25
We selected a cohort of patients seen at Newcastle and Paris to limit the impact of inter‐site variability. These two sites had the largest number of patients with MRI results and have previously been shown to have high inter‐site reliability. In order to identify if quantitative MRI measurement could predict changes in muscle function, we only included ambulant patients who completed a set of four functional assessments including the North Star Assessment for limb girdle‐type muscular dystrophies [North Star Ambulatory Assessment for Dysferlinopathy (NSAD)], the 6‐min walk test (smwt), the timed up and go (TUG) test, and the 10‐m walk test (10MWT) at baseline, Year 1, and Year 3. The cohort included a total of 18 patients who had an NSAD score of more than 15 points (Figure 1).
Figure 1.
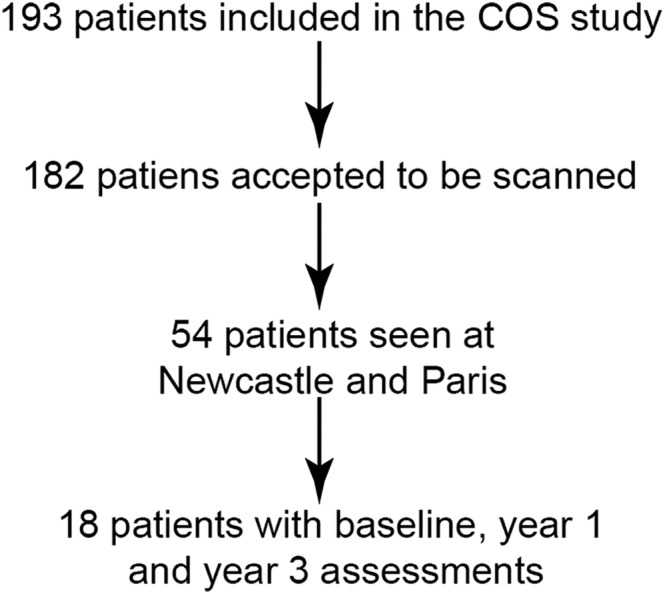
Patients included in this study from the original COS study. COS, Clinical Outcomes Study.
Quantitative muscle magnetic resonance imaging: acquisition and processing of Dixon and T2H2O imaging data
Patients were imaged using 3.0‐tesla MRI clinical scanners from two different vendors: Newcastle (Philips) and Paris (Siemens). MRI acquisition parameters were standardized across sites before the start of the study. Patients were positioned feet‐first supine, and all MRI sequences were centred at one‐third of the femur from the superior border of the patella and at the widest part of the calf. The acquisition protocol for FF and T2H2O mapping in the COS study has been recently reported. 25
Water and fat images were reconstructed using in‐house Matlab code (MathWorks, Natick, MA, USA), which incorporated hierarchical IDEAL (iterative decomposition of water and fat with echo asymmetry and least‐squares estimation) and the Tsao–Jiang algorithm for separating multiple chemical species by hierarchical decomposition and direct estimation of phase. 27 Using the mean‐square error (MSE) images, quantitative T2H2O maps were reconstructed based on a tri‐exponential fitting procedure. 19 , 21 Regions of interest (ROIs) were drawn manually using a free software tool (https://www.itksnap.org) in the same five central slices on the shortest echo time (TE) image of the MSE series images by a single investigator as has been already described. 25 ROIs were drawn on both the left and right sides for seven leg muscles (extensor digitorum, tibialis anterior, tibialis posterior, peroneus longus, soleus, gastrocnemius medialis, and gastrocnemius lateralis) and in nine thigh muscles (vastus lateralis, vastus intermedius, vastus medialis, gracilis, sartorius, adductor magnus, biceps femoris long head, semimembranosus, and semitendinosus). For the determination of FF and cross‐sectional area (CSA), the boundaries of the ROIs were drawn following individual muscle delineation, avoiding inclusion of other muscles, subcutaneous and intermuscular fat, tendons, and major blood vessels. cCSA was calculated for each muscle using cCSA = (1 − FFmean) * CSA. For the assessment of T2H2O, ROIs delineated the interior of the muscle, avoiding visible fasciae and blood vessels. ROIs that included <10 pixels were excluded for analysis.
After quality control, FF and cCSA calculation was not possible in all slices for all patients. This resulted in some slices for which there was a T2H2O value but not an FF and a cCSA value. Assessment of correlations between T2H2O and FF and cCSA used only those data sets where all variables were available (n numbers shown in Table 1).
Table 1.
Correlation between T2H2O and changes in muscle function, fat fraction, and contractile cross‐sectional area over 3 years
| Muscle [left (L)/right (R)] | Median T2H2O value (range) | ΔNSAD | Δ6MWT velocity in m/s | Δ6MWT distance in m | ΔTUG rate (event/s) | Δ10MWT velocity (m/s) | N with Dixon data | ΔFF (%) | ΔcCSA (mm2) |
|---|---|---|---|---|---|---|---|---|---|
| Vastus intermedius (L) | 40.0 (34.3–46.4 | −8 * | −0.18 * | −64.8 * | −0.04 ** | −0.06 * | 16 | 9.4 | −54.7 |
| Vastus intermedius (R) | 40.6 (35.8–47.2) | −11 ** | −0.14 | −50.4 | −0.04 ** | −0.06 ** | 16 | 7.8 | −181.1 |
| Vastus lateralis (L) | 41.4 (27.3–48.0) | −9 ** | −0.10 | −36.0 | −0.03 * | −0.05 * | 16 | 8.4 | −551.0 |
| Vastus lateralis (R) | 42.7 (35.2–51.4) | −6 | −0.08 | −28.8 | −0.03 * | −0.05 * | 16 | 10.3 * | −606.1 |
| Vastus medialis (L) | 39.5 (26.9–50.1) | −8 * | −0.20 * | −72.0 * | −0.02 | −0.05 * | 16 | 9.8 ** | −315.9 |
| Vastus medialis (R) | 42.0 (33.1–48.8) | −11 ** | −0.24 ** | −86.4 ** | −0.04 *** | −0.07 ** | 15 | 9.7 ** | −441.4 |
| Adductor magnus (L) | 39.3 (33.1–45.8) | −7 * | −0.14 | −50.4 | −0.03 ** | −0.04 | 16 | 15.5 | −40.7 |
| Adductor magnus (R) | 39.0 (33.6–47.2 | −10 ** | −0.18 * | −64.8 * | −0.04 *** | −0.07 ** | 16 | 13.3 | −156.9 |
| Gracilis (L) | 38.5 (33.4–43.0) | −4 | −0.15 | −54.0 | −0.03 ** | −0.03 | 16 | 9.1 | −123.6 |
| Gracilis (R) | 37.5 (33.0–44.3) | −6 | −0.17 * | −61.2 * | −0.03 ** | −0.03 | 16 | 3.5 | −141.9 |
| Sartorius (L) | 38.4 (32.0–43.9) | −4 | −0.16 * | −57.6 * | −0.03 ** | 0 | 16 | 12.0 | −142.6 |
| Sartorius (R) | 39.3 (34.5–44.8) | −8 ** | −0.11 | −39.6 | −0.03 ** | −0.03 | 16 | 7.9 | −88.1 |
| Biceps femoris (L) | 39.6 (26.4–51.5) | −2 | 0.05 | −18.0 | −0.02 | 0.02 | 15 | 5.9 | −162.9 |
| Biceps femoris (R) | 38.7 (27.7–50.4) | −9 ** | −0.16 | −57.6 | −0.03 * | −0.02 | 14 | 13.6 | −181.4 |
| Semimembranosus (L) | 38.3 (25.7–47.8) | −7 * | −0.13 | −46.8 | −0.03 | −0.02 | 13 | 13.1 | −113.1 |
| Semimembranosus (R) | 38.1 (20.3–46.2) | −3 | −0.08 | −28.8 | −0.01 | 0.02 | 13 | 10.0 * | −168.7 |
| Semitendinosus (L) | 36.7 (28.8–44.6) | −5 | −0.01 | −3.6 | −0.01 | −0.01 | 16 | 7.1 | −57.8 |
| Semitendinosus (R) | 34.8 (22.6–47) | −4 | −0.09 | −32.4 | −0.01 | 0.01 | 16 | 9.4 | −35.8 |
| Tibialis anterior (L) | 39.4 (23.9–49.4) | −7 * | −0.14 | −50.4 | −0.02 | 0 | 15 | 4.0 | −41.1 |
| Tibialis anterior (R) | 38.6 (31.1–53.3) | −2 | −0.04 | −14.4 | −0.01 | 0 | 15 | −2.4 | 21.0 |
| Extensor digitorum (L) | 40.5 (34.2–50) | −8 * | −0.11 | −39.6 | −0.03 ** | −0.01 | 15 | 7.7 * | −50.1 |
| Extensor digitorum (R) | 39.6 (34.8–48) | −2 | −0.03 | −10.8 | −0.02 | 0.01 | 15 | 11.3 | −95.3 |
| Peroneus (L) | 37.1 (23.9–50) | −2 | −0.12 | −43.2 | −0.01 | 0.01 | 15 | 14.5 | −159.6 |
| Peroneus (R) | 36.4 (25.2–48.2) | −3 | −0.11 | −39.6 | 0.02 | −0.02 | 14 | 13.2 | −184.5 |
| Tibialis posterior (L) | 40.2 (34.1–50.3) | −2 | −0.11 | −39.6 | −0.03 * | 0.01 | 15 | 6.9 | −133.8 |
| Tibialis posterior (R) | 39.9 (34.8–48.3) | −4 | −0.06 | −21.6 | −0.02 | −0.02 | 15 | 8.4 | −129.9 |
| Soleus (L) | 37.3 (23.6–50.4) | −1 | −0.09 | −32.4 | −0.02 | 0.01 | 14 | 6.1 | −14.9 |
| Soleus (R) | 38.1 (26.9–49.4) | −9 * | −0.26 ** | −93.6 ** | −0.04 ** | −0.03 | 15 | 10.9 | −40.5 |
| Gastrocnemius lateralis (L) | 36.9 (25.8–46.2) | 5 | 0.03 | 10.8 | 0.01 | 0.02 | 14 | 14.1 | −151.1 |
| Gastrocnemius lateralis (R) | 37.7 (23.1–47.6) | 1 | −0.06 | −21.6 | 0 | 0 | 13 | 15.8 | −34.0 |
| Gastrocnemius medialis (L) | 35.9 (26.2–57) | 3 | 0.07 | 25.2 | 0.02 | 0.05 * | 14 | 3.8 | −95.1 |
| Gastrocnemius medialis (R) | 38.5 (26.1–60.7) | −4 | −0.06 | −21.6 | −0.02 | 0 | 14 | 0.5 | −73.8 |
N, number; Δ6MWT, change in 6‐min walk test between Year 3 and baseline; Δ10MWT, change in 10‐m walk test between Year 3 and baseline; ΔcCSA, change in contractile cross‐sectional area between Year 3 and baseline; ΔFF, change in fat fraction between Year 3 and baseline; ΔNSAD, change in North Star Ambulatory Assessment for Dysferlinopathy between Year 3 and baseline; ΔTUG, change in timed up and go test between Year 3 and baseline.
The table shows mean changes in muscle function tests and their association with high T2H2O. Regression coefficients in bold were statistically significant at the 0.05 level.
P < 0.05.
P < 0.01.
P < 0.001.
Data analysis
Higher or lower than median T2H2O
The median T2H2O of each muscle in each patient was categorized as either (a) greater than or equal to the cohort median T2H2O value for that muscle (high T2H2O) or (b) less than the cohort median T2H2O value for that muscle (low T2H2O). The change in functional score (ΔNSAD) or change in velocity in the timed tests (Δsmwt, ΔTUG, and Δ10MWT) over 3 years was calculated using assessments from baseline and Year 3.
Demographic information was reviewed, including age, disease duration, and baseline functional assessment score. Demographic data and baseline functional assessment results were compared between high and low T2H2O values in each muscle. Median values were compared between groups using a Mann–Whitney test.
For each functional assessment, a linear model of the change in functional score over 3 years was performed with high or low baseline T2H2O as a predictor and disease duration, age, and baseline functional score as covariates. Timed tests were assessed as velocities to give normally distributed values. This model produces an estimate of the additional change in functional score seen in those with a high T2H2O compared with those with a low T2H2O value, when disease duration, age, and baseline functional score are held constant. The analysis was performed for each functional assessment using the T2H2O value from each of the 16 muscles on each side in turn (4 functional assessments × 32 muscles—left and right). This method was repeated with FF and cCSA data. For each muscle, the change in FF and cCSA over 3 years was modelled against the baseline T2H2O value, with disease duration, age, and baseline FF and cCSA as covariates.
Differences were considered significant if the P‐value was lower than 0.05. The P‐values were adjusted using the Holm–Bonferroni correction to assess statistical significance in the presence of multiple comparisons.
Identifying a T2H2O threshold that identifies higher changes in North Star Ambulatory Assessment for Dysferlinopathy than expected
Previous studies modelling the COS cohort of patients over time have demonstrated that NSAD score declines by 1.68 points per year, and therefore, over 3 years, a decline of more than 5.04 points identifies those with faster than average functional decline. 8 Each patient was classed as having a decline of more than 5 points in NSAD score (positive result) or <5 points (negative result). We calculated the cut‐off point in T2H2O with the higher sensitivity and specificity to distinguish between patients progressing more and less than 5 points in NSAD using receiver operating characteristic curves (ROC curves) using R software (https://www.r‐project.org). We included in this analysis only those muscles whose baseline T2H2O correlated with changes in muscle function tests from baseline to the last visit assessment.
Results
Demographics
The cohort consisted of 18 ambulant patients (7 male) assessed in Newcastle (12 patients) or Paris (6 patients). Patients had a median age of 32.5 years (range 19–71 years) and had had symptoms for a median of 11 years (range 3–22 years). Demographic information split by T2H2O value per muscle is listed in Supporting Information, Table S1 .
T2H2O values vary between muscles
Median T2H2O values varied from a minimum of 35.9 ms in gastrocnemius medialis (left) to a maximum of 42.7 ms in vastus lateralis (right) (Table 1). Within each muscle, T2H2O was not significantly different between left and right (Wilcoxon's test). T2H2O did not correlate with functional score, FF, or cCSA at baseline (Table S2 ).
High T2H2O value was significantly correlated with functional deterioration
Median T2H2O values at baseline significantly correlated with a worsening for at least one functional assessment over the next 3 years in 19 of the 32 muscles studied (Table 1). Muscles with high T2H2O were always associated with faster functional deterioration.
High T2H2O in adductor magnus and vastus intermedius/lateralis/medialis muscles stood out as being consistently and bilaterally significantly correlated with functional progression, demonstrating a greater deterioration in at least two functional assessments bilaterally (Table 1).
In vastus lateralis (bilateral), vastus medialis (right), semimembranosus (left), and extensor digitorum (left), median T2H2O values significantly correlated with an increase in FF over 3 years. Median T2H2O values did not correlate with a change in cCSA bilaterally in any muscle after 3 years of follow‐up (Table 1).
Determining a T2H2O value for prediction
We studied the T2H2O value thresholds that maximized sensitivity and specificity for predicting decline in function using ROC curves of the adductor magnus, vastus intermedius, vastus lateralis, and vastus medialis. The T2H2O value thresholds obtained were 39.0 ms [88% sensitive and 75% specific, area under the curve (AUC) 0.847 (0.634–1)] in adductor magnus, 39.4 ms [100% sensitive, 60% specific, AUC 0.917 (0.782–1)] in vastus intermedius, 40.5 ms [94% sensitive, 70% specific, AUC 0.889 (0.734–1)] in vastus lateralis, and 40.1 ms [94% sensitive, 40% specific, AUC 0.875 (0.699–1)] in vastus medialis (Figure 2).
Figure 2.
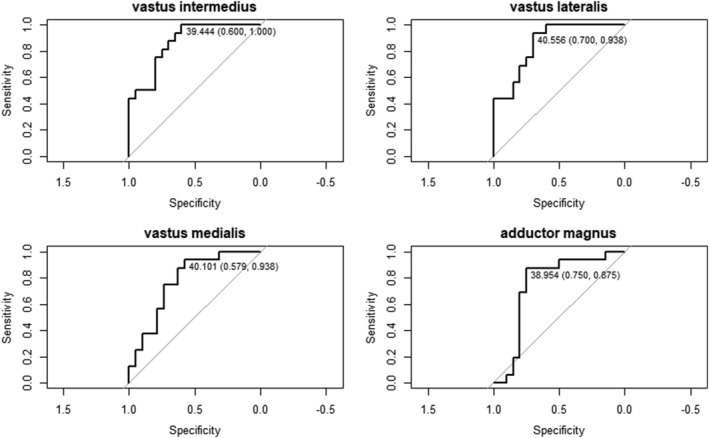
Receiver operating characteristic curve of the sensitivity and specificity achieved by all possible T2H2O relaxation times. The area under the curve is listed for each plot. In each curve, the threshold with the highest specificity (with zero false positives) and the threshold with optimum sensitivity and specificity are listed.
Validating T2H2O thresholds
Seventeen of the 18 patients had bilateral T2H2O results for adductor magnus, vastus intermedius, vastus lateralis, and vastus medialis and could be included in this analysis. The sensitivity of predicting decline on the NSAD score with the T2H2O thresholds over both 1 and 3 years is shown in Table 2 and Figure 3. As shown in Table 2, sensitivity and specificity of the measures suggested that those with high T2H2O values were generally rapid progressors. Sensitivity and specificity values weere not improved by requiring all four muscles to be over their indiviudal thresholds or requring only one of the four muscles to be over their individual theshold (Table 2).
Table 2.
Sensitivity and specificity of a T2H2O threshold in predicting decline in North Star Ambulatory Assessment for Dysferlinopathy (NSAD) score
| Muscle | Threshold | Analysis | Decline of >1 point on NSAD in 1 year | Decline of >5 points on NSAD in 3 years |
|---|---|---|---|---|
| Adductor magnus | 39.0 ms | Sensitivity | 57% | 63% |
| Specificity | 71% | 78% | ||
| Vastus intermedius | 39.4 ms | Sensitivity | 86% | 100% |
| Specificity | 57% | 67% | ||
| Vastus lateralis | 40.5 ms | Sensitivity | 71% | 88% |
| Specificity | 71% | 78% | ||
| Vastus medialis | 40.1 ms | Sensitivity | 86% | 88% |
| Specificity | 57% | 67% | ||
| Muscle grouping | ||||
| All four muscles over threshold bilaterally | Respective threshold for each muscle as above | Sensitivity | 43% | 63% |
| Specificity | 71% | 89% | ||
| At least one muscle over threshold bilaterally | Sensitivity | 86% | 100% | |
| Specificity | 57% | 56% | ||
Figure 3.
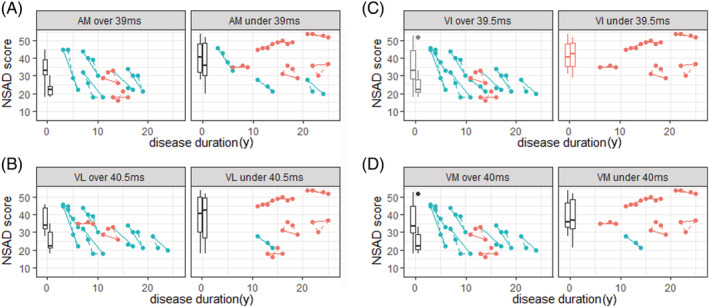
Disease progression in the cohort analysed, grouped by T2H2O threshold value identified for (A) adductor magnus (AM), (B) vastus intermedius (VI), (C) vastus lateralis (VL), and (D) vastus medialis (VM). Dots showing the North Star Ambulatory Assessment for Dysferlinopathy (NSAD) score at each time point are grouped by dashed lines to illustrate individual patient trajectories on the NSAD score over 3 years. Those deteriorating more than 5 points over 3 years are coloured blue, and those deteriorating 5 or less points are coloured red. Box plots represent the median, interquartile range, and range of NSAD score for patients at baseline (left) and Year 3 (right).
Discussion
We have shown that a higher T2H2O in some muscles of the lower limbs might be associated with greater functional deterioration in patients with dysferlinopathy. These findings could be particularly helpful in advising patients about prognosis, in selecting patients for clinical trials, and, potentially, as an outcome measure for interventional trials.
There is a large body of evidence already published demonstrating that the amount of fat present in the skeletal muscle, which can be quantified using quantitative MRI (Dixon method) or proton magnetic resonance spectroscopy (1H MRS), correlates with muscle function tests and is sensitive to change over short periods of time. 17 , 28 However, little is known about the relation of T2H2O, which may be an indicator of active muscle damage and muscle function. It has been observed that in dysferlinopathy, the T2H2O does not correlate with baseline functional scores. 25 T2H2O has been shown to be impacted by high levels of FF (i.e. >60%) as also described in dysferlinopathy. 25 The data described in the current study, however, were acquired in exclusively ambulant patients, where FF values were predominantly lower than 60%. This suggests that T2H2O could be compared across patients at different stages of muscle loss to offer a snapshot of the current level of disease activity.
Higher than threshold baseline T2H2O value predicted greater decline over both 1 and 3 years, being consistently more accurate over the 3‐year window. This suggests that change in function is a delayed downstream consequence of higher active muscle damage as quantified by the T2H2O. This also reflects the slowly progressive nature of the disease, which results generally in small changes in function over 1 year but more consistently over a longer period of 3 years of follow‐up. Despite this, even over a 1‐year period, T2H2O thresholds were able to predict functional decline, which could make T2H2O particularly useful for detecting patients that will probably progress significantly during a clinical trial with a shorter running time.
We found a correlation between T2H2O at baseline and changes in FF in some of the investigated muscles, including the vastus medialis and the vastus intermedius. Correlation between muscle histopathology and quantitative MRI in patients with muscular dystrophy demonstrates histopathological features of inflammation appearing in the muscle, detectable by T2H2O, before fat replacement begins. 29 In our population, it appears that active muscle damage, detected by higher T2H2O, correlated with a functional decline in some of the muscles affected. Given the progressive nature of dysferlinopathy, we may anticipate that with a longer period of follow‐up, this increased disease activity would ultimately translate into irreversibly increased FF and loss of cCSA. These findings suggest that T2H2O is a useful measure to demonstrate active muscle damage in remaining muscle in dysferlinopathy and to provide an accurate quantification of potentially salvageable tissue. This could be useful for monitoring response to future treatments, with T2H2O forming a potential early biomarker of treatment efficacy. 30
However, not all the muscles imaged showed an association between higher T2H2O value and risk of more severe disease progression. The muscles in which T2H2O did not correlate with functional decline were those of the posterior compartment of the thigh and muscles of the leg, apart from the soleus. There are several possible reasons for this:
Due to the functional outcome measures chosen: The distal muscle that did show involvement was the soleus, which is heavily involved in walking, while the gastrocnemius and peroneus are less involved when walking on an even surface. 31 Regarding the posterior compartment of the thigh, walking can be maintained, even in the presence of significant weakness, by using compensatory mechanisms, which may mean that active muscle damage in these muscles does not translate to impairment on these specific outcome measures. 32
Due to a lower degree of active muscle damage in these muscles at the time of assessment: The pattern of muscle involvement in dysferlinopathy includes an early and severe involvement of the soleus, both the gastrocnemius and the peroneus. 13 The first functional ability to be lost is usually the ability to stand on tiptoes, a function highly reliant on the gastrocnemius and soleus muscles. 33 The T2H2O values seen in these muscles were lower than those in the vasti muscles, and these muscles showed a higher fat replacement by the time of assessment as they are affected very early during the disease's progression. 13
Due to the higher FF in these muscles: FFs in the posterior compartment of the thigh and the gastrocnemius were among the highest of all the muscles assessed (Table S2 ). T2H2O is much more heterogeneous in more fatty‐replaced muscles, as has been shown. 34 In this sense, investigation in nine patients with more advanced dysferlinopathy and higher FFs found lower T2H2O in patients compared with healthy controls. 35 These data suggest that at those stages, T2H2O would probably not be useful to identify active muscle damage due to the massive replacement by fat. 34
The association of T2H2O with functional test outcomes in specific muscles demonstrates the importance of selecting an appropriate ‘reporter’ muscle that is related to the functional outcome of interest and stage of disease progression. In this analysis, the muscles most associated with the timed tests and NSAD score were the vasti and the adductor magnus muscles. However, we may also anticipate that gluteal muscles, hip extensors, and abductors, which are important in rising from the floor and walking, may constitute useful reporter muscles. 31 Unfortunately, the COS study did not include T2H2O data from the gluteal muscles and so we were unable to investigate this further. As disease progresses, useful reporter muscles may change to those with lower FFs. In non‐ambulant patients, where outcome measures focus more on the upper limbs, which are generally preserved for longer in dysferlinopathy, one of the arm muscles may be a better reporter muscle. 10 , 13 We hope to address this in future, after completion of the clinical outcome extension study, which includes upper limb MRI.
We felt that it would be clinically useful for prognostication or trial cohort selection if a specific threshold could be defined above which T2H2O is considered ‘high’ and patients may be expected to progress more rapidly. We tried to validate these thresholds in a second cohort of dysferlin patients participating in COS study that were scanned in 3‐ and 1.5‐tesla scanners at six other sites, but even though higher T2H2O levels were associated with faster progression, the accuracy, sensitivity, and specificity were lower as it is shown in the supporting information. There are many reasons that could explain these results including, among others, the different field strengths, which is known to affect the T2; the different vendor‐specific sequence details such as radio frequency pulses and crusher‐gradient schemes, which impact the measured magnetic resonance (MR) signal and hence the observed T2; and the transmitter and receiver coils that vary between vendors and even between system versions from the same vendor, which also impact the measured MR signal. While the scan protocols were standardized and the same post‐processing methodology was used for all T2H2O data and was conducted by the same team, normalization across sites by means of control data from the same volunteers in all sites was not practicable. 15 , 16 We also attempted to correct for the difference in field strength by applying a correction factor based on published data to the results acquired on 1.5‐tesla scanners. 36 However, this scaling factor is only an approximation that has been obtained in a few number of healthy controls and likely still leaves significant inter‐scanner variability reinforcing the need of further research in this specific topic. In addition, besides hardware‐related differences that affect T2, variations in patient status, such as recent exercise activity, are known to impact on the observed T2H2O. 15 In this regard, patients participating in the COS study were imaged before physio assessments were performed and were asked to avoid doing any exercise the week before the visit. Most of the patients used a taxi to go to the hospital or imaging centre, in order to reduce the impact that the exercise could have in the MRI values, and were also asked to lie down before being scanned. The data obtained in our extension cohort (supporting information results) unfortunately do not allow us to take any final conclusion about what could be the causes of the lack of validation of the thresholds obtained, indicating that more research is needed in this topic. In this sense, we think that clinical or clinical trial‐based applications attempting to use T2H2O for predicting disease progression across multiple sites would require additional research to identify the optimum standardization of scan protocol, T2H2O mapping, and normalization of data between sites before starting the trial. 37
This study was conducted in patients with dysferlinopathy. However, the findings may be applicable to other slowly progressive forms of muscular dystrophy, which show periods of both deterioration and stability. Muscular dystrophies share a common pathomechanism of repeated cycles of muscle damage and attempted repair, with release of pro‐inflammatory cytokines and immune cell influx. 38 , 39 , 40 , 41 Such patterns of inflammation and oedema are detected by T2H2O, suggesting that our finding may be extrapolated to other muscular dystrophies, although this needs to be confirmed in future studies. 29 , 37
The results presented in this paper suggest that faster functional disease progression in dysferlinopathy can be predicted by higher T2H2O values in muscles of the thigh, over 1 and 3 years. With further research, we predict that similar patterns would be demonstrated in other slowly progressive forms of muscular dystrophy. However, it is important to take into account that an effort in standardizing the measurements between centres is crucial if these observations are to be employed in clinical trial design and prognostication for patients with muscular dystrophy.
Conflict of interest
Ursula Moore, Ericky Caldas de Almeida Araujo, Harmen Reyngoudt, Heather Gordish‐Dressman, Fiona E. Smith, Ian Wilson, Meredith James, Anna Mayhew, Jown W. Day, Kristi J. Jones, Diana X. Bhraucha Goebel, Emmanuelle Salort Campana, Alan Pestronk, Maggie Walter, Carmen Paradas, Tanya Stojkovic, Madoka Mori‐Yoshimura, Elene Bravver, Elena Pegoraro, Jerry R. Mendel, Kate Bushby, Andrew M. Blamire, Volker Straub, Pierre Carlier, and Jordi Díaz‐Manera have received funding for research from the Jain Foundation during the conduct of the study.
Laura Rufibach is hired by the Jain Foundation.
Supporting information
Figure S1: Illustrative scheme describing the tri‐exponential fitting method for T2 H2O mapping applied in the present work.
In (A) are raw T2‐weighted MSE images at different TEs; The blue, red and black filled circles are placed in subcutaneous‐fat, spared muscle and highly fatty‐replaced muscle. Notice how the signal from spared muscle vanishes much faster than the signal from fat as TE evolves, due to its shorter T2 relaxation time. In (B) are the corresponding theoretically predicted signal evolutions; water signal is assumed to be mono‐exponential, while the fat signal, previously calibrated in the subcutaneous fat of healthy subjects, is described with a bi‐exponential model with known fixed T2 values and corresponding relative fractions. The mixed signal model is fitted to the actual data at each pixel by adjusting the relative water and fat signal fractions at TE = 0, and the T2 value characterizing the water signal.
Figure S2: Disease progression in the original and extension cohorts, grouped by T2H2O threshold value identified for A adductor magnus (AM), B vastus intermedius (VI), C vastus lateralis (VL) and D vastus medialis (VM). Dots showing the NSAD score at each time point are grouped by dashed lines to illustrate individual patient trajectories on the NSAD score over 3 years. Those deteriorating more than 5 points over 3 years are coloured blue, and those deteriorating 5 or less points are coloured red. Box plots represent the median, interquartile range (IQR) and range of NSAD score for patients in that panel at baseline (left) and year 3 (right).
Table S1: Median T2 water value of each muscle analysed in the cohort
Median T2 water value obtained on each muscle is shown as well as number of patients on each subgroup (higher or lower values than median), the median symptom duration and the median age of each group. L: left. R: Right
Table S2: Results of functional tests in patients with higher or lower than median T2 water value
The table shows the results of the muscle function tests in the patients that were included in the higher or lower than median T2 water value on each muscle. N: number, smwt: 6 minutes walking test, m/s: meters per second, TTRW: time to run/walk 10 meters, TTUG: time to up&go test,
Table S3: Sensitivity and specificity of a T2H2O threshold in predicting decline in NSAD score in the initial and extension cohort
Data S1. Supporting Information
Acknowledgements
This study has only been possible thanks to the international collaboration of several specialized centres promoted by the Jain Foundation. The Jain COS consortium would like to thank the study participants and their families for their invaluable contribution and would also like to acknowledge the ongoing support the Jain Foundation provides in the development, management, and analysis of this study. The Jain Foundation, based in Seattle, USA, is entirely focused on LGMD2B/dysferlinopathy/Miyoshi myopathy. The foundation does not solicit funding from patients but instead funds research and clinical studies worldwide with the goal of finding treatments for dysferlinopathy. Please visit www.jain‐foundation.org for more information about the foundation, and if you are a patient suffering from dysferlinopathy, please consider enrolling into their interactive dysferlinopathy registry that seeks to build a strong, engaged, and supportive community (patients@jain-foundation.org). We also acknowledge the help of Pierre‐Yves Baudin (NMR Laboratory, Institute of Myology, Paris, France) for assistance with MRI data processing.
Moore U., Caldas de Almeida Araújo E., Reyngoudt H., Gordish‐Dressman H., Smith F. E., Wilson I., James M., Mayhew A., Rufibach L., Day J. W., Jones K. J., Bharucha‐Goebel D. X., Salort‐Campana E., Pestronk A., Walter M. C., Paradas C., Stojkovic T., Mori‐Yoshimura M., Bravver E., Pegoraro E., Mendell J. R., The Jain COS Consortium , Bushby K., Blamire A. M., Straub V., Carlier P. G., and Diaz‐Manera J. (2022) Water T2 could predict functional decline in patients with dysferlinopathy, Journal of Cachexia, Sarcopenia and Muscle, 13, 2888–2897, doi: 10.1002/jcsm.13063
Ursula Moore, Ericky Caldas de Almeida Araujo, Pierre G. Carlier, and Jordi Diaz‐Manera equally contributed to the work.
References
- 1. Winckler PB, da Silva AMS, Coimbra‐Neto AR, Carvalho E, Cavalcanti EBU, Sobreira CFR, et al. Clinicogenetic lessons from 370 patients with autosomal recessive limb‐girdle muscular dystrophy. Clin Genet 2019;96:341–353. [DOI] [PubMed] [Google Scholar]
- 2. Brooke MH, Fenichel GM, Griggs RC, Mendell JR, Moxley R, Florence J, et al. Duchenne muscular dystrophy: patterns of clinical progression and effects of supportive therapy. Neurology 1989;39:475–481. [DOI] [PubMed] [Google Scholar]
- 3. Mayhew AG, Cano SJ, Scott E, Eagle M, Bushby K, Manzur A, et al. Detecting meaningful change using the North Star Ambulatory Assessment in Duchenne muscular dystrophy. Dev Med Child Neurol 2013;55:1046–1052. [DOI] [PubMed] [Google Scholar]
- 4. Barnard AM, Willcocks RJ, Triplett WT, Forbes SC, Daniels MJ, Chakraborty S, et al. MR biomarkers predict clinical function in Duchenne muscular dystrophy. Neurology 2020;94:e897–e909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bashlr R, Strachan T, Keers S, Stephenson A, Mahjneh I, Marconi G, et al. A gene for autosomal recessive limb‐girdle muscular dystrophy maps to chromosome 2p. Hum Mol Genet 1994;3:455–457. [DOI] [PubMed] [Google Scholar]
- 6. Miyoshi K, Kawai H, Iwasa M, Kusaka K, Nishino H. Autosomal recessive distal muscular dystrophy as a new type of progressive muscular dystrophy. Seventeen cases in eight families including an autopsied case. Brain 1986;109:31–54. [DOI] [PubMed] [Google Scholar]
- 7. Moore U, Jacobs M, James MK, Mayhew AG, Fernandez‐Torron R, Feng J, et al. Assessment of disease progression in dysferlinopathy: a 1‐year cohort study. Neurology 2019;92:e461–e474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jacobs MB, James MK, Lowes LP, Alfano LN, Eagle M, Muni Lofra R, et al. Assessing dysferlinopathy patients over three years with a new motor scale. Ann Neurol 2021;89:967–978. [DOI] [PubMed] [Google Scholar]
- 9. Hoffman EP. Causes of clinical variability in Duchenne and Becker muscular dystrophies and implications for exon skipping therapies. Acta Myol 2020;39:179–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Patino CM, Ferreira JC. Inclusion and exclusion criteria in research studies: definitions and why they matter. J Bras Pneumol 2018;44:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dahlqvist JR, Widholm P, Leinhard OD, Vissing J. MRI in neuromuscular diseases: an emerging diagnostic tool and biomarker for prognosis and efficacy. Ann Neurol 2020;88:669–681. [DOI] [PubMed] [Google Scholar]
- 12. Mul K, Vincenten SCC, Voermans NC, Lemmers RJLF, van der Vliet PJ, van der Maarel SM, et al. Adding quantitative muscle MRI to the FSHD clinical trial toolbox. Neurology 2017;89:2057–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Diaz‐Manera J, Fernandez‐Torron R, LLauger J, James MK, Mayhew A, Smith FE, et al. Muscle MRI in patients with dysferlinopathy: pattern recognition and implications for clinical trials. J Neurol Neurosurg Psychiatry 2018;89:1071–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nunez‐Peralta C, Alonso‐Perez J, Diaz‐Manera J. The increasing role of muscle MRI to monitor changes over time in untreated and treated muscle diseases. Curr Opin Neurol 2020;33:611–620. [DOI] [PubMed] [Google Scholar]
- 15. Carlier PG, Marty B, Scheidegger O, Loureiro de Sousa P, Baudin PY, Snezhko E, et al. Skeletal muscle quantitative nuclear magnetic resonance imaging and spectroscopy as an outcome measure for clinical trials. J Neuromuscul Dis 2016;3:1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Burakiewicz J, Sinclair CDJ, Fischer D, Walter GA, Kan HE, Hollingsworth KG. Quantifying fat replacement of muscle by quantitative MRI in muscular dystrophy. J Neurol 2017;264:2053–2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rooney WD, Berlow YA, Triplett WT, Forbes SC, Willcocks RJ, Wang DJ, et al. Modeling disease trajectory in Duchenne muscular dystrophy. Neurology 2020;94:e1622–e1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Carlier PG. Global T2 versus water T2 in NMR imaging of fatty infiltrated muscles: different methodology, different information and different implications. Neuromuscul Disord 2014;24:390–392. [DOI] [PubMed] [Google Scholar]
- 19. Azzabou N, Loureiro de Sousa P, Caldas E, Carlier PG. Validation of a generic approach to muscle water T2 determination at 3T in fat‐infiltrated skeletal muscle. J Magn Reson Imaging 2015;41:645–653. [DOI] [PubMed] [Google Scholar]
- 20. Keene KR, Beenakker JM, Hooijmans MT, Naarding KJ, Niks EH, Otto LAM, et al. T2 relaxation‐time mapping in healthy and diseased skeletal muscle using extended phase graph algorithms. Magn Reson Med 2020;84:2656–2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Carlier PG, Azzabou N, de Sousa PL, Hicks A, Boisserie JM, Amadon A, et al. Skeletal muscle quantitative nuclear magnetic resonance imaging follow‐up of adult Pompe patients. J Inherit Metab Dis 2015;38:565–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Morrow JM, Sinclair CD, Fischmann A, Machado PM, Reilly MM, Yousry TA, et al. MRI biomarker assessment of neuromuscular disease progression: a prospective observational cohort study. Lancet Neurol 2016;15:65–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. van de Velde NM, Hooijmans MT, Sardjoe Mishre ASD, Keene KR, Koeks Z, Veeger TTJ, et al. Selection approach to identify the optimal biomarker using quantitative muscle MRI and functional assessments in Becker muscular dystrophy. Neurology 2021;97:e513–e522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hooijmans MT, Niks EH, Burakiewicz J, Verschuuren JJ, Webb AG, Kan HE. Elevated phosphodiester and T2 levels can be measured in the absence of fat infiltration in Duchenne muscular dystrophy patients. NMR Biomed 2017;30:e3667. [DOI] [PubMed] [Google Scholar]
- 25. Reyngoudt H, Smith FE, Caldas de Almeida Araújo E, Wilson I, Fernández‐Torrón R, James MK, et al. Three‐year quantitative magnetic resonance imaging and phosphorus magnetic resonance spectroscopy study in lower limb muscle in dysferlinopathy. J Cachexia Sarcopenia Muscle 2022;13:1850–1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Harris E, Bladen CL, Mayhew A, James M, Bettinson K, Moore U, et al. The Clinical Outcome Study for dysferlinopathy: an international multicenter study. Neurol Genet 2016;2:e89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tsao J, Jiang Y. Hierarchical IDEAL: fast, robust, and multiresolution separation of multiple chemical species from multiple echo times. Magn Reson Med 2013;70:155–159. [DOI] [PubMed] [Google Scholar]
- 28. Spanish Pompe group , Figueroa‐Bonaparte S, Llauger J, Segovia S, Belmonte I, Pedrosa I, et al. Quantitative muscle MRI to follow up late onset Pompe patients: a prospective study. Sci Rep 2018;8:10898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lassche S, Küsters B, Heerschap A, Schyns MVP, Ottenheijm CAC, Voermans NC, et al. Correlation between quantitative MRI and muscle histopathology in muscle biopsies from healthy controls and patients with IBM, FSHD and OPMD. J Neuromuscul Dis 2020;7:495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Arpan I, Willcocks RJ, Forbes SC, Finkel RS, Lott DJ, Rooney WD, et al. Examination of effects of corticosteroids on skeletal muscles of boys with DMD using MRI and MRS. Neurology 2014;83:974–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bogey RA, Barnes LA. Estimates of individual muscle power production in normal adult walking. J Neuroeng Rehabil 2017;14:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Knarr BA, Reisman DS, Binder‐Macleod SA, Higginson JS. Understanding compensatory strategies for muscle weakness during gait by simulating activation deficits seen post‐stroke. Gait Posture 2013;38:270–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Moore U, Gordish H, Diaz‐Manera J, James MK, Mayhew AG, Guglieri M, et al. Miyoshi myopathy and limb girdle muscular dystrophy R2 are the same disease. Neuromuscul Disord 2021;31:265–280. [DOI] [PubMed] [Google Scholar]
- 34. Schlaeger S, Weidlich D, Klupp E, Montagnese F, Deschauer M, Schoser B, et al. Decreased water T2 in fatty infiltrated skeletal muscles of patients with neuromuscular diseases. NMR Biomed 2019;32:e4111. [DOI] [PubMed] [Google Scholar]
- 35. Arrigoni F, de Luca A, Velardo D, Magri F, Gandossini S, Russo A, et al. Multiparametric quantitative MRI assessment of thigh muscles in limb‐girdle muscular dystrophy 2A and 2B. Muscle Nerve 2018;58:550–558. [DOI] [PubMed] [Google Scholar]
- 36. Gold GE, Han E, Stainsby J, Wright G, Brittain J, Beaulieu C. Musculoskeletal MRI at 3.0 T: relaxation times and image contrast. AJR Am J Roentgenol 2004;183:343–351. [DOI] [PubMed] [Google Scholar]
- 37. Araujo ECA, Marty B, Carlier PG, Baudin PY, Reyngoudt H. Multiexponential analysis of the water T2‐relaxation in the skeletal muscle provides distinct markers of disease activity between inflammatory and dystrophic myopathies. J Magn Reson Imaging 2021;53:181–189. [DOI] [PubMed] [Google Scholar]
- 38. Fanin M, Angelini C. Muscle pathology in dysferlin deficiency. Neuropathol Appl Neurobiol 2002;28:461–470. [DOI] [PubMed] [Google Scholar]
- 39. Rosenberg AS, Puig M, Nagaraju K, Hoffman EP, Villalta SA, Rao VA, et al. Immune‐mediated pathology in Duchenne muscular dystrophy. Sci Transl Med 2015;7:299rv294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Angelini C, Nardetto L, Borsato C, Padoan R, Fanin M, Nascimbeni AC, et al. The clinical course of calpainopathy (LGMD2A) and dysferlinopathy (LGMD2B). Neurol Res 2010;32:41–46. [DOI] [PubMed] [Google Scholar]
- 41. Tasca G, Monforte M, Corbi M, Granata G, Lucchetti D, Sgambato A, et al. Muscle microdialysis to investigate inflammatory biomarkers in facioscapulohumeral muscular dystrophy. Mol Neurobiol 2018;55:2959–2966. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Illustrative scheme describing the tri‐exponential fitting method for T2 H2O mapping applied in the present work.
In (A) are raw T2‐weighted MSE images at different TEs; The blue, red and black filled circles are placed in subcutaneous‐fat, spared muscle and highly fatty‐replaced muscle. Notice how the signal from spared muscle vanishes much faster than the signal from fat as TE evolves, due to its shorter T2 relaxation time. In (B) are the corresponding theoretically predicted signal evolutions; water signal is assumed to be mono‐exponential, while the fat signal, previously calibrated in the subcutaneous fat of healthy subjects, is described with a bi‐exponential model with known fixed T2 values and corresponding relative fractions. The mixed signal model is fitted to the actual data at each pixel by adjusting the relative water and fat signal fractions at TE = 0, and the T2 value characterizing the water signal.
Figure S2: Disease progression in the original and extension cohorts, grouped by T2H2O threshold value identified for A adductor magnus (AM), B vastus intermedius (VI), C vastus lateralis (VL) and D vastus medialis (VM). Dots showing the NSAD score at each time point are grouped by dashed lines to illustrate individual patient trajectories on the NSAD score over 3 years. Those deteriorating more than 5 points over 3 years are coloured blue, and those deteriorating 5 or less points are coloured red. Box plots represent the median, interquartile range (IQR) and range of NSAD score for patients in that panel at baseline (left) and year 3 (right).
Table S1: Median T2 water value of each muscle analysed in the cohort
Median T2 water value obtained on each muscle is shown as well as number of patients on each subgroup (higher or lower values than median), the median symptom duration and the median age of each group. L: left. R: Right
Table S2: Results of functional tests in patients with higher or lower than median T2 water value
The table shows the results of the muscle function tests in the patients that were included in the higher or lower than median T2 water value on each muscle. N: number, smwt: 6 minutes walking test, m/s: meters per second, TTRW: time to run/walk 10 meters, TTUG: time to up&go test,
Table S3: Sensitivity and specificity of a T2H2O threshold in predicting decline in NSAD score in the initial and extension cohort
Data S1. Supporting Information


