Abstract
Background
Both primary lung adenocarcinoma and benign processes can have a ground-glass opacity (GGO) appearance on imaging. This study evaluated the incidence of and risk factors for malignancy in a diverse cohort of patients who underwent resection of a GGO suspicious for lung cancer.
Methods
All patients who underwent resection of a pulmonary nodule with a GGO component and suspected to be primary lung cancer at a single institution from 2001–2017 were retrospectively reviewed. Risk factors for malignancy were evaluated using multivariable logistic regression analysis that included nodule size, age, sex, and race as potential predictors.
Results
The incidence of pulmonary adenocarcinoma in the 243 patients who met inclusion criteria was 86% (n=208). The most common pathologic findings in 35 patients with a benign pathology was granulomatous inflammation (n=14, 40%). Risk factors for adenocarcinoma in multivariable logistic regression were age [odds ratio (OR) 1.06, P=0.003], GGO size (OR 2.76, P<0.001), female sex (OR 4.47, P=0.002), and Asian race (OR 8.35, P=0.002). In this cohort, adenocarcinoma was found in 100% (44/44) of Asian females, 86% (25/29) of Asian males, 84% (98/117) of non-Asian females, and 77% (41/53) of non-Asian males.
Conclusions
The likelihood of adenocarcinoma in lung nodules with a ground-glass component is influenced by sex and race. Asian females with a GGO have a much higher likelihood of having adenocarcinoma than men and non-Asians. This data can be used when deciding whether to pursue nodule resection or surveillance in a patient with a GGO.
Keywords: Lung cancer, pulmonary nodules, adenocarcinoma of lung, solitary pulmonary nodule, thoracic surgery
Introduction
The etiology of a lung nodule that has a ground glass opacity (GGO) on computerized tomography (CT) scan is often something on the lung adenocarcinoma spectrum, ranging from atypical adenomatous hyperplasia to in-situ disease to invasive carcinoma (1). A solid component generally reflects proliferation of invasive tumor cells, while the GGO is felt to be a lepidic, in-situ, non-invasive growth pattern of cells along preexisting alveolar structures (2-9). Lung adenocarcinomas with a GGO component may behave less aggressively than pure-solid adenocarcinomas and even display indolent behavior, and often occur in non-smokers, in particular in people of Asian descent. However, these lesions can still progress with growth and spread over time, with increasing solid density generally correlating with malignant behavior. Therefore, appropriate and timely diagnosis and therapy are key to optimizing outcomes (10-13).
However, not all lung nodules with a GGO component are secondary to lung cancer, as the differential diagnosis includes a variety of benign pathologies such as infection and pneumonia, granulomatous and other inflammatory processes, and pulmonary edema (14). The specific etiology of a GGO cannot be definitively identified based on the CT alone (15). The management decision process for patients when these are discovered must choose between observation, pursuing an invasive diagnostic procedure with biopsy, or even proceeding with therapy prior to a diagnostic procedure (13). Clinicians are increasingly having to make these decisions, because these nodules are often found secondary to lung cancer screening and increased CT scan use in general (16-21). This study was undertaken to provide data to support this clinical decision process using an experience with a diverse cohort of patients undergoing GGO resection, by testing the hypothesis that the likelihood of a GGO being secondary to lung adenocarcinoma can be determined based on pre-treatment clinical and radiologic factors. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-583/rc).
Methods
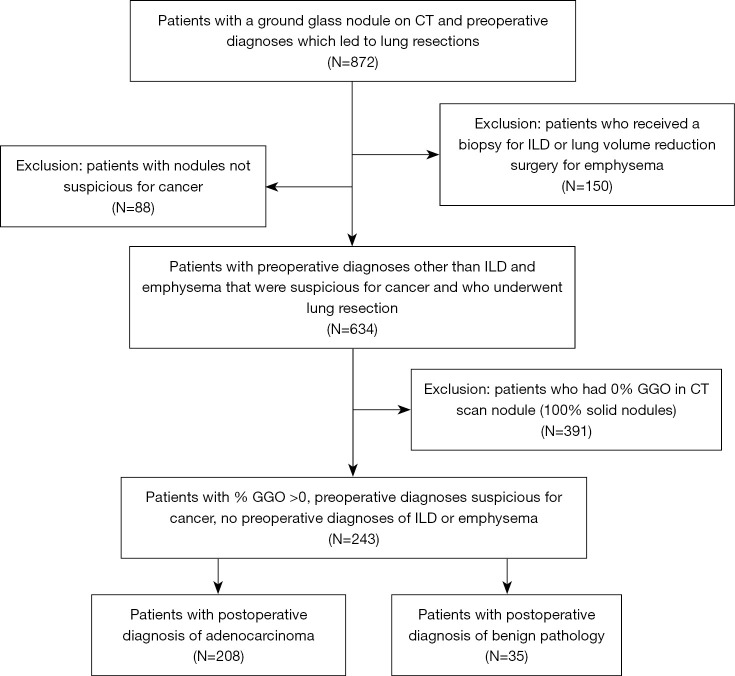
This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Stanford University institutional review board (protocol 21285) and individual consent for this retrospective analysis was waived. All patients who underwent surgical resection between 2001 and 2019 of a lung nodule that was clinically suspicious for lung cancer were reviewed using a prospectively collected clinical database. A nodule was generally deemed suspicious based on patient characteristic such as age, smoking history, history of other exposures known to be risk factors for lung cancer, history of previous cancer and other medical conditions, clinical symptoms, and radiologic appearance, including comparison to previous imaging studies when performed. Patients who were undergoing lung resection for a benign pre-operative diagnosis (diagnostic resection of interstitial lung disease, volume reduction surgery for emphysema, and nodules that were not suspicious for cancer) were excluded. Only patients who had a CT that had been performed within 3 months before surgery were included. Because the purpose of this study was to evaluate the risk of cancer in nodules that had any GGO component, patients whose nodule was pure solid (0% GGO) were excluded. If a CT report did not specify that nodule was part-solid or had a GGO component, the presence of a GGO component was determined by a repeat review of the CT images by a thoracic radiologist or an experienced thoracic surgeon (MB, JS) as previously described (1). GGO was defined by the presence of hazy, increased opacity of the lung with preservation of the bronchial and vascular margins, and the maximum diameter of the solid component was measured after excluding the areas of the GGO using lung windows, using high resolution imaging with 1 mm thick slices when available for each patient. This led to a final number of 243 patients in total, further subdivided into 208 adenocarcinoma patients and 35 benign pathology patients. Our consort diagram for the distribution of patients from each stage of the patient selection process is shown in Figure 1.
Figure 1.
Consort diagram for study populations. CT, computerized tomography; ILD, interstitial lung disease; GGO, ground-glass opacity.
Retrospective review was conducted to document demographics, preoperative characteristics, intraoperative details, and pathologic details. We were able to find complete data for all the patients in our two cohorts. The pre-operative predictions of the etiologies of the GGOs were compared to the post-operative surgical pathology report and discharge summaries for these patients, and the cohort was stratified in two groups according to whether their nodule was malignant versus benign. The use of positron emission tomography (PET) scans was documented. Results of PET scans were categorized as not performed if the patient did not have one, non-avid if there was no recorded PET activity in the suspicious nodule, mildly active if the maximum standardized uptake value (SUVmax) was 0.1 to 2.5, and hypermetabolic if the SUVmax was greater than 2.5.
Statistical analysis
Baseline demographic and clinical data between the two cohorts were compared via a chi square analysis for categorical variables and a T test analysis for continuous variables. Multivariable logistic regression analysis of the patients was performed to assess the impact of pre-operative characteristics on a cancer diagnosis. The model was constructed with a post-operative diagnosis of cancer as the outcome of interest. The risk factors chosen for analysis were those that have been previously shown or are clinically accepted to be strongly associated with a risk of lung cancer, and included total size of tumor (reported in cm, measured on CT), percentage of the nodule that was determined to be solid tumor (reported as percentile, measured by radiologist or physician based on CT), age (reported in years, measured as age on presentation), sex (reported as male or female, collected as demographic information from the patient’s chart), race (reported as Asian or non-Asian, collected as demographic information from the patient’s chart), and smoking history (reported as present with number of pack years noted, vs. not present).
Continuous data are presented as means ± standard deviations (SD) or medians with interquartile ranges, as noted. Categorical variables are presented as frequency and percentages. A nominal two-sided P value of less than 0.05 was considered significant. All statistical analysis was performed via the program SPSS V.26.
Results
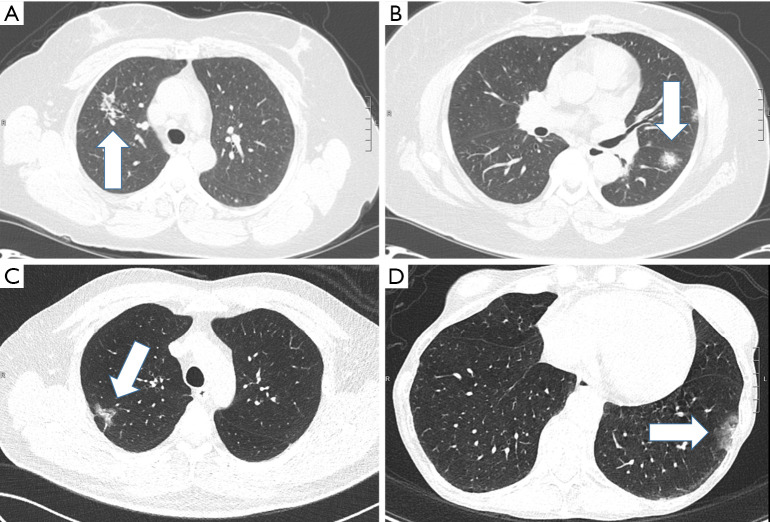
A total of 243 patients met all study criteria during the study period and included 208 patients (86%) whose post-operative pathology was primary adenocarcinoma and 35 patients (14%) who had a benign pathology. The majority of cases were in the years 2010 or later (n=202, 83.1%) while only 41 cases were in earlier years (16.9%). Figure 2 shows examples of the part-solid nodules observed in the cohort, for patients with both benign and malignant etiologies.
Figure 2.
Selected CT scans of patients with part-solid lung nodules (marked by arrows). Patients with the scans shown in (A) and (B) had benign histologies, while patients shown in (C) and (D) had lung adenocarcinoma. CT, computerized tomography.
Table 1 shows the baseline demographic characteristics of patients in both the adenocarcinoma and benign pathology cohorts. Age, race, and GGO size all had statistically significant associations with malignancy. In the cohort, 10.1% of the nodules found to be adenocarcinoma bore a composition of 100% ground glass opacity, whereas none of the patients with a benign histology had a pure ground glass opacity (P=0.05). The mean age for the adenocarcinoma cohort was 67.37 years (SD =10.04) and the mean age for the benign pathology cohort was 59.86 years (SD =13.30). Patients with a benign process were more likely to be of non-Asian race than Asian race. Non-Asians comprised 66.8% of patients with adenocarcinoma and 88.6% of the patients with benign pathology (P=0.009). Patients with adenocarcinoma also had larger ground glass opacity nodules than patients with a benign process (2.62 versus 1.35 cm, P<0.001). There was no statistically significant difference between the percent solid component of the lung nodule between the adenocarcinoma and the benign patients (59.6%±32.9% versus 65.7%±36.1%, P=0.32). There was also no statistically significant difference in PET scan results between benign and malignant patients (P=0.32). PET scan findings were either non-avid or only mild hypermetabolic in 38.9% (81/208) of the patients with adenocarcinoma, while the PET scan findings were hypermetabolic and suspicious for malignancy in 34.3% (12/35) of the patients with a benign histology.
Table 1. Demographic and clinical characteristics of patients who underwent resection of suspicious GGO nodules, stratified by benign versus malignant etiologies.
| Characteristics | Adenocarcinoma (N=208) | Benign pathology (N=35) | P value |
|---|---|---|---|
| Age (years), mean ± SD | 67.37±10.04 | 59.86±13.30 | <0.001* |
| Sex, n (%) | 0.106 | ||
| Male | 66 (31.7) | 16 (45.7) | |
| Female | 142 (68.3) | 19 (54.3) | |
| Race/Ethnicity, n (%) | 0.009* | ||
| Non-Asian | 139 (66.8) | 31 (88.6) | |
| Asian | 69 (33.2) | 4 (11.4) | |
| Smoking history, n (%) | 116 (55.8) | 20 (57.1) | 0.880 |
| Average smoking pack years, mean ± SD | 27.14±21.71 | 24.55±18.49 | 0.617 |
| Patients with no comorbidities, n (%) | 86 (41.3) | 14 (40.0) | 0.881 |
| Radiologic characteristics | |||
| Average total size of GGO, mean ± SD | 2.62±1.71 | 1.35±0.99 | <0.001* |
| Pure GGO (100%) nodules | 21 (10.1%) | 0 | 0.05 |
| Percent solid, mean ± SD | 59.6±32.9 | 65.7±36.1 | 0.32 |
| PET scan results, n (%) | 0.09 | ||
| Not done | 34 (16.3) | 10 (28.6) | |
| Non-avid | 20 (9.6) | 8 (22.9) | |
| Mild activity (0.1–2.5 SUV) | 61 (29.3) | 5 (14.3) | |
| Hypermetabolic (>2.5 SUV) | 93 (44.7) | 12 (34.3) |
*, statistically significant P value. GGO, ground glass opacity; SD, standard deviation; PET, positron emission tomography; SUV, maximum standardized uptake value.
Table 2 shows data regarding the approach, extent of resection, and perioperative course in the two groups. The majority of patients in each group had minimally invasive surgery. The majority (74%, 26/35) of patients in the benign group had a wedge resection, while the majority of patients (79%, 165/208) in the adenocarcinoma group had an anatomic resection (all other categories besides the wedge resection). For the nine patients in the benign group who had a lobectomy, the reasons a sublobar resection was not performed were: multiple abnormalities in one lobe (1 patient); nodule location in the central aspect of the middle lobe (3 patients); nodule presence location in the central aspect of the right or left upper lobe with suspicious appearance but previous non-diagnostic biopsy (4 patients); and nodule presence in a lobe that had previously been partially resected (1 patient). There were no perioperative deaths in either group, and the total rate of perioperative complications were 16% and 14% for the adenocarcinoma and benign groups respectively.
Table 2. Surgical procedure types and perioperative complications, stratified by benign versus malignant etiologies.
| Procedure types and complications | Adenocarcinoma (N=208) | Benign pathology (N=35) | P value |
|---|---|---|---|
| Procedure type, n (%) | |||
| Wedge resection | 43 (20.7) | 26 (74.3) | <0.001* |
| Segmentectomy | 14 (6.7) | 0 (0.0) | |
| Lobectomy | 147 (70.7) | 9 (25.7) | |
| Bilobectomy | 3 (1.4) | 0 (0.0) | |
| Pneumonectomy | 1 (0.5) | 0 (0.0) | |
| Approach, n (%) | |||
| Open | 76 (36.5) | 6 (17.1) | 0.025* |
| Video-assisted thoracic surgery | 132 (63.5) | 29 (82.9) | |
| Adenocarcinoma histology, n (%) | Not applicable | ||
| Not specified | 87 (41.8) | ||
| Acinar | 48 (23.1) | ||
| Bronchoalveolar carcinoma | 25 (12.5) | ||
| Lepidic | 18 (8.6) | ||
| Lepidic and acinar | 16 (7.7) | ||
| Papillary | 12 (5.8) | ||
| Papillary and acinar | 1 (0.5) | ||
| Postoperative mortality, n (%) | 0 (0.0) | 0 (0.0) | – |
| Perioperative complications, n (%) | 29 (13.9) | 4 (11.4) | 1.000a |
a, calculated using Fisher’s exact test; *, statistically significant P value. Perioperative complications are defined as including: air leak, atrial fibrillation, pneumothorax, hemothorax, empyema, bronchopulmonary fistula, pneumonia, other respiratory related complications, other complications.
The postoperative diagnoses for patients with GGOs that were initially suspicious for cancer but were found to be benign upon surgical resection of the nodule are shown in Table 3. The benign processes ranged from granulomas (n=14, 40%), inflammatory/infectious processes (n=10, 28.4%) such as necrosis, fibrosis, pneumonitis, abscesses, Mycobacterium-avium complex, and lymphoid hyperplasia (n=4, 11.4%), hamartomas (n=2, 5.7%), to other processes like bronchiolitis (n=5, 14.3%). In addition, 4 patients (11.4%) had atypical adenomatous hyperplasia. This process is technically benign but is a neoplastic process that could be a precursor to the development of adenocarcinoma in situ or ultimately true adenocarcinoma.
Table 3. Postoperative diagnoses for 35 patients with benign pathology after resection of a lung nodule with a ground-glass opacity component.
| Diagnosis | n (%) |
|---|---|
| Granuloma | 14 (40.0) |
| Inflammatory/infectious | 10 (28.5) |
| Necrosis, fibrosis, inflammation, pneumonitis | 6 |
| Abscess | 2 |
| Mycobacterium-avium complex | 1 |
| Lymphoid hyperplasia | 1 |
| Atypical adenomatous hyperplasia | 4 (11.4) |
| Hamartoma | 2 (5.71) |
| Other (bronchiolitis obliterans, no tumor seen) | 5 (14.3) |
Several factors were found to be independent predictors of adenocarcinoma in multivariate analysis (Table 4). Total size of the GGO nodule [odds ratio (OR) 2.76, 95% CI: 1.67–4.55, P<0.001], older age (OR 1.06, 95% CI: 1.02–1.11, P=0.003), female sex (OR 4.47, 95% CI: 1.72–11.57, P=0.002), and Asian race (OR 8.35, 95% CI: 2.24–31.05, P=0.002) were all significant predictors of malignancy in patients with a ground glass nodule on their computed tomography scan. Given that race and sex were both important independent risk factors, we also the examined the incidence of malignancy across these characteristics (Table 5). In the cohort, adenocarcinoma was found in 100% of Asian females, 86% of Asian males, 84% of non-Asian females, and 77% of non-Asian males.
Table 4. Probability of cancer in patients with suspicious GGO’s as a function of demographic and clinical covariates.
| Variable | Odds ratio | 95% confidence interval | P value |
|---|---|---|---|
| Age (per year) | 1.06 | 1.02–1.11 | 0.003* |
| Total size of GGO | 2.76 | 1.67–4.55 | <0.001* |
| Sex (female vs. male) | 4.47 | 1.72–11.57 | 0.002* |
| Race (Asian vs. non-Asian) | 8.35 | 2.24–31.05 | 0.002* |
| Smoking history (smoker vs. non-smoker) | 0.88 | 0.36–2.12 | 0.773 |
*, statistically significant P value. GGO, ground glass opacity.
Table 5. Incidence of malignancy compared by race and sex.
| Demographic | Incidence of adenocarcinoma, n [%] | Incidence of benign pathology, n [%] |
|---|---|---|
| Non-Asian males (n=53) | 41 [77] | 12 [23] |
| Non-Asian females (n=117) | 98 [84] | 19 [16] |
| Asian males (n=29) | 25 [86] | 4 [14] |
| Asian females (n=44) | 44 [100] | 0 [0] |
Discussion
Clinicians often must make management decisions for lung nodules that are discovered on imaging studies. The increased ease of obtaining as well as the use of CT scans in general as well as the implementation of lung cancer screening programs have made this decision process more common. Although guidelines that suggest management strategies based on various clinical factors exist, clinicians often make subjective decisions based on their interpretation of the clinical scenario. Though the Fleischner Society guidelines provide comprehensive criteria for managing pulmonary nodules, including nodule morphology and its change over time, more decisive size measurements, and understanding of patient risk factors, several studies have found a low guideline compliance and adherence to these recommendations. Much of the challenge in translating the guidelines to clinical practice is in its applicability to certain patient populations, some of which may have variability in risk factors and nodule characteristics (22). In this study, we sought to provide more objective data that could be used to guide management in the specific situation of patients found to have part-solid lung nodules.
Our study evaluated the incidence and risk factors for malignancy in a demographically diverse cohort of patients with ground-glass opacities on CT. In our binomial logistic regression analysis (Table 4), we demonstrate the incidence of malignancy as a function of the size of the GGO containing nodule, age, sex, and race, which we found to be statistically significant predictors of adenocarcinoma within a multivariate analysis. Our model shows that lung nodules are much more likely to be pulmonary adenocarcinoma in patients who are older, female, of Asian descent, and who have a larger ground glass opacity component to their nodule. Smoking history related inversely to development of adenocarcinoma but was not statistically significant within the analysis. In our findings of incidence of malignancy as compared among differences in sex and race, we saw that all of the Asian female patients in our study had nodules that were ultimately determined to be adenocarcinoma, whereas 86% of Asian male patients in our study had adenocarcinoma. In comparison, non-Asian female and male patients had a lower incidence of adenocarcinoma (84% and 77% respectively).
When evaluating patients with part solid lung nodules that have a GGO component, the radiologic factors most important in assessing the likelihood of malignancy are the total size and the total size of the solid component. In addition, we will take into account clinical factors such as the patient’s demographics, smoking history, personal history of lung cancer, family history of lung cancer, and history of possible either recent or remote inflammatory or infectious lung process. In general, a solid component of over 5 mm at presentation will prompt further evaluation with either PET, biopsy, or resection rather than observation. If a patient has previous imaging, we will evaluate for change over time, and change in the solid component will generally lead us to switch from observation to either biopsy or resection. Based on the lower incidence of adenocarcinoma in patients of non-Asian descent, the clinician may consider close observation of the nodule for these patients. Alternatively, even though the course of adenocarcinoma is often indolent, clinicians may want to pursue more aggressive investigation for patients of Asian descent, especially in older Asian females.
Of note, in Table 2, the types of surgery performed on our cohort are delineated. There are several factors that will guide a non-anatomic wedge resection versus an anatomic resection for a patient with a part-solid lung nodule in our practice. First, if a nodule is in a peripheral location amenable to wedge resection and a biopsy has not been obtained, we will generally initially perform a wedge resection and evaluate for malignancy with pathologic frozen section analysis. If that assessment is benign, then no further resection will be performed, though we will often sample lymph nodes so we have pathologic nodal data in the cases where the results of permanent processing differ from the frozen section results. When the nodule is known or shown to be malignant, the following factors are taken into account when deciding on wedge resection versus anatomic resection: tumor location and ability to obtain an adequate margin when the overall nodule size is less than 2 cm; the patient’s ability to tolerate parenchymal resection based on their overall functional status and their pulmonary function tests; the patient’s history of previous lung resection or lung diseases; and the presence of other part-solid nodules that may prove to require therapy in time if they grow or become more solid on follow-up.
Management of a suspicious GGO could involve biopsy or surgical resection depending on the other tumor characteristics, such as nodule size and percent of solid component. The diagnostic accuracy of needle biopsy is comparable for both solid and part-solid lesions, so the finding of a ground glass opacity on CT (therefore not entirely solid) should not necessarily preclude the clinician from recommending a biopsy prior to surgery. However, our results suggest that this step may be unnecessary in most patients and especially in Asian females, unless non-surgical management with stereotactic ablative body radiotherapy is considered and confirmation of a malignant process prior is needed. Based on our findings, a biopsy that does not show a malignant pathology may be most likely a false-negative in Asian patients. Of course, being able to perform safe surgery is a critical component to recommending resection for both diagnosis and therapy, and evidence shows that lung resection by specialized thoracic surgeons has been incrementally improving over time. In a study conducted by the Society of Thoracic Surgeons (STS) in 2019 on a cohort of 38,461 patients from their General Thoracic Surgery Database (GTSD) who underwent pulmonary resection for primary non-small cell lung cancer (NSCLC), overall operative mortality was 1.29% and major morbidity incidence was 7.92%. Morbidity and mortality rates have improved from prior analyses of the GTSD in 2008 yielding 2.2% and 8.6%, respectively- the study researchers suggest that this is due to increased use of minimally invasive techniques, sublobar resections, and better patient selection for surgery with more high-risk patients undergoing stereotactic radiotherapy or other nonsurgical therapies (23,24). In our series where most patients had minimally invasive surgery, morbidity and mortality rates were very low.
Because of how demographically diverse our patient cohort is, we believe that our own model has great potential for use in the diagnostic process of primary lung adenocarcinoma and can be applied to other patient populations, both institutions that have either a similar or different distribution of patient demographics. Our study and model are not without limitations, however. For one, there was inherent selection bias in the design of our study population, as we only included patients who have undergone surgery for their ground glass nodules and did not include other patients who deferred surgery. Secondly, we cannot generalize our model to simply any patient with a malignant or benign ground glass opacity on CT, as all of our patients had differential diagnoses which indicated that these GGOs were suspicious for malignancy- we excluded any patients with GGOs which were identified pre-operatively as a benign pathology such as interstitial lung disease or emphysema. We also acknowledge that with the advent of technological improvements in computed tomography over time, there may have been differences in the detection of ground glass opacities in more recent years compared to CT studies performed during the advent of the study in the early 2000s. Another limitation of the study is the relatively small cohort size. Finally, retrospective studies can have certain disadvantages, such as recall bias or errors in data collection. However, a major strength of our study compared to previously published studies in Asian-only cohorts is the demographic variability of our study population, and therefore potentially more generalizable to other patient populations in the world of thoracic surgery and oncology.
Conclusions
Our study concludes that the likelihood of adenocarcinoma in lung nodules with a ground-glass component is influenced by age, sex, and race, in addition to nodule size. Asian females with a GGO nodule have a much higher likelihood of having adenocarcinoma than men and non-Asians, both concurrently and respectively. These results can be used to assist the patient management process, and potentially optimize the therapeutic course while minimizing unnecessary diagnostic procedures or lengthy observation periods if management is ultimately inevitable.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Stanford University institutional review board (protocol 21285) and individual consent for this retrospective analysis was waived.
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-583/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-583/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-583/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-583/coif). NL reports grants from the Intuitive Foundation and Auspex Diagnostics. She serves as an unpaid editorial board member of Journal of Thoracic Disease from September 2021 to August 2023. LB reports grants from the Department of Veterans Affairs, National Institutes of Health, Chan Zuckerberg Institute, consulting fees from Bristol Squibb Myers, Genentech, and Johnson & Johnson, speaker honoraria from International Lung Cancer Congress, and support for attending meeting from national lung cancer roundtable. MFB serves as an unpaid editorial board member of Journal of Thoracic Disease from September 2020 to August 2022. The other authors have no conflicts of interest to declare.
References
- 1.Berry MF, Gao R, Kunder CA, et al. Presence of Even a Small Ground-Glass Component in Lung Adenocarcinoma Predicts Better Survival. Clin Lung Cancer 2018;19:e47-51. 10.1016/j.cllc.2017.06.020 [DOI] [PubMed] [Google Scholar]
- 2.Burt BM, Leung AN, Yanagawa M, et al. Diameter of Solid Tumor Component Alone Should be Used to Establish T Stage in Lung Adenocarcinoma. Ann Surg Oncol 2015;22 Suppl 3:S1318-23. 10.1245/s10434-015-4780-0 [DOI] [PubMed] [Google Scholar]
- 3.Takahashi M, Shigematsu Y, Ohta M, et al. Tumor invasiveness as defined by the newly proposed IASLC/ATS/ERS classification has prognostic significance for pathologic stage IA lung adenocarcinoma and can be predicted by radiologic parameters. J Thorac Cardiovasc Surg 2014;147:54-9. 10.1016/j.jtcvs.2013.08.058 [DOI] [PubMed] [Google Scholar]
- 4.Suzuki K, Koike T, Asakawa T, et al. A prospective radiological study of thin-section computed tomography to predict pathological noninvasiveness in peripheral clinical IA lung cancer (Japan Clinical Oncology Group 0201). J Thorac Oncol 2011;6:751-6. 10.1097/JTO.0b013e31821038ab [DOI] [PubMed] [Google Scholar]
- 5.Travis WD, Brambilla E, Noguchi M, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society: international multidisciplinary classification of lung adenocarcinoma: executive summary. Proc Am Thorac Soc 2011;8:381-5. 10.1513/pats.201107-042ST [DOI] [PubMed] [Google Scholar]
- 6.Austin JH, Garg K, Aberle D, et al. Radiologic implications of the 2011 classification of adenocarcinoma of the lung. Radiology 2013;266:62-71. 10.1148/radiol.12120240 [DOI] [PubMed] [Google Scholar]
- 7.Saito H, Kameda Y, Masui K, et al. Correlations between thin-section CT findings, histopathological and clinical findings of small pulmonary adenocarcinomas. Lung Cancer 2011;71:137-43. 10.1016/j.lungcan.2010.04.018 [DOI] [PubMed] [Google Scholar]
- 8.Park CM, Goo JM, Lee HJ, et al. Nodular ground-glass opacity at thin-section CT: histologic correlation and evaluation of change at follow-up. Radiographics 2007;27:391-408. 10.1148/rg.272065061 [DOI] [PubMed] [Google Scholar]
- 9.Tsutani Y, Miyata Y, Nakayama H, et al. Prognostic significance of using solid versus whole tumor size on high-resolution computed tomography for predicting pathologic malignant grade of tumors in clinical stage IA lung adenocarcinoma: a multicenter study. J Thorac Cardiovasc Surg 2012;143:607-12. 10.1016/j.jtcvs.2011.10.037 [DOI] [PubMed] [Google Scholar]
- 10.Tsutani Y, Miyata Y, Yamanaka T, et al. Solid tumors versus mixed tumors with a ground-glass opacity component in patients with clinical stage IA lung adenocarcinoma: prognostic comparison using high-resolution computed tomography findings. J Thorac Cardiovasc Surg 2013;146:17-23. 10.1016/j.jtcvs.2012.11.019 [DOI] [PubMed] [Google Scholar]
- 11.Inoue M, Minami M, Sawabata N, et al. Clinical outcome of resected solid-type small-sized c-stage IA non-small cell lung cancer. Eur J Cardiothorac Surg 2010;37:1445-9. 10.1016/j.ejcts.2009.12.030 [DOI] [PubMed] [Google Scholar]
- 12.Gu B, Burt BM, Merritt RE, et al. A dominant adenocarcinoma with multifocal ground glass lesions does not behave as advanced disease. Ann Thorac Surg 2013;96:411-8. 10.1016/j.athoracsur.2013.04.048 [DOI] [PubMed] [Google Scholar]
- 13.Huang C, Wang C, Wang Y, et al. The Prognostic Significance of Pure Ground Glass Opacities in Lung Cancer Computed Tomographic Images. J Cancer 2019;10:6888-95. 10.7150/jca.33132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Engeler CE, Tashjian JH, Trenkner SW, et al. Ground-glass opacity of the lung parenchyma: a guide to analysis with high-resolution CT. AJR Am J Roentgenol 1993;160:249-51. 10.2214/ajr.160.2.8424326 [DOI] [PubMed] [Google Scholar]
- 15.Zheng B, Zhou X, Chen J, et al. A Modified Model for Preoperatively Predicting Malignancy of Solitary Pulmonary Nodules: An Asia Cohort Study. Ann Thorac Surg 2015;100:288-94. 10.1016/j.athoracsur.2015.03.071 [DOI] [PubMed] [Google Scholar]
- 16.National Lung Screening Trial Research Team ; Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. 10.1056/NEJMoa1102873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moyer VA, U.S. Preventive Services Task Force . Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2014;160:330-8. 10.7326/M13-2771 [DOI] [PubMed] [Google Scholar]
- 18.Pedersen JH, Saghir Z, Wille MM, et al. Ground-Glass Opacity Lung Nodules in the Era of Lung Cancer CT Screening: Radiology, Pathology, and Clinical Management. Oncology (Williston Park) 2016;30:266-74. [PubMed] [Google Scholar]
- 19.Bak SH, Lee HY, Kim JH, et al. Quantitative CT Scanning Analysis of Pure Ground-Glass Opacity Nodules Predicts Further CT Scanning Change. Chest 2016;149:180-91. 10.1378/chest.15-0034 [DOI] [PubMed] [Google Scholar]
- 20.Yu WS, Hong SR, Lee JG, et al. Three-Dimensional Ground Glass Opacity Ratio in CT Images Can Predict Tumor Invasiveness of Stage IA Lung Cancer. Yonsei Med J 2016;57:1131-8. 10.3349/ymj.2016.57.5.1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamamoto K, Andoh M, Uematsu K, et al. A case of adenocarcinoma of the lung presenting ground glass opacity detected by spiral CT in lung cancer screening. Nihon Ika Daigaku Zasshi 1998;65:481-3. 10.1272/jnms1923.65.481 [DOI] [PubMed] [Google Scholar]
- 22.MacMahon H, Naidich DP, Goo JM, et al. Guidelines for Management of Incidental Pulmonary Nodules Detected on CT Images: From the Fleischner Society 2017. Radiology 2017;284:228-43. 10.1148/radiol.2017161659 [DOI] [PubMed] [Google Scholar]
- 23.Broderick SR, Grau-Sepulveda M, Kosinski AS, et al. The Society of Thoracic Surgeons Composite Score Rating for Pulmonary Resection for Lung Cancer. Ann Thorac Surg 2020;109:848-55. 10.1016/j.athoracsur.2019.08.114 [DOI] [PubMed] [Google Scholar]
- 24.Shirvani SM, Jiang J, Chang JY, et al. Lobectomy, sublobar resection, and stereotactic ablative radiotherapy for early-stage non-small cell lung cancers in the elderly. JAMA Surg 2014;149:1244-53. 10.1001/jamasurg.2014.556 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as