Abstract
Background
The association between sarcopenia and mild cognitive impairment (MCI) among elderly adults in China remains unclear. The present study aimed to examine the association based on a nationally representative large‐scale survey.
Methods
The study used two waves of data from China Health and Retirement Longitudinal Study (CHARLS) in 2015 and 2018. All subjects met the inclusion criteria were classified based on Asia Working Group for Sarcopenia 2019 criteria. Aging‐associated cognitive decline is used to define MCI, and cognitive function is measured based on four dimensions: orientation, computation, memory, and drawing. OLS and logistic regression model were conducted to analyse the cross‐sectional association between sarcopenia and different cognitive functions. Logistic regression model was conducted to analyse the longitudinal association between sarcopenia and MCI.
Results
Totally, 5715 participants aged over 60 years (43.8% women; mean age 67.3 ± 6.0 years) were enrolled in a cross‐sectional association study in 2015, and further 2982 elderly adults were followed up in 2018. During the period, sarcopenia and possible sarcopenia increased from 8.5% to 29.6%. Scores of cognitive and four dimensions (orientation, computation, memory, and drawing) exhibited a decreasing trend from non‐sarcopenia to sarcopenia (P < 0.001). In the fully adjusted OLS regression model, scores of four dimensions were lower in possible sarcopenia and sarcopenia groups when compared with the non‐sarcopenia group (P < 0.05) respectively. The incidence of MCI was 10.1%, 16.5%, and 24.2% for non‐sarcopenia, possible sarcopenia, and sarcopenia groups from 2015 to 2018, with a significantly statistical difference (P < 0.001). Logistic regression model revealed an odds ratio of 1.43 [95% confidence interval (CI): 1.06–1.91, P = 0.017] for the possible sarcopenia group and 1.72 (95% CI: 1.04–2.85, P = 0.035) for sarcopenia group when compared with the non‐sarcopenia group.
Conclusions
Sarcopenia is associated with worse cognitive impairment, which provided new evidence for a strong association that warrants further research into mechanistic insights.
Keywords: CHARLS, mild cognitive impairment, sarcopenia
1. Introduction
With the rapid development of China's society and economy, the aging of China's population is further deepened. According to the latest seventh population census in 2020, the population aged 60 and above in China is 264 018 766, accounting for 18.70% of the total population, among which the population aged 65 and above is 190 635 280, accounting for 13.50% of the total population. 1 A large‐scale elderly population will greatly increase the burden of disease and medical care needs of the whole society. It is estimated the percentage of gross domestic product (GDP) spent on pensions, healthcare, welfare, and facilities will increase from 7.33% in 2015 to 26.24% in 2050. 2 Among many health‐related factors leading to disability in the elderly, sarcopenia and cognitive impairment have received widespread attention in academic circles and clinics.
Sarcopenia is mainly defined by progressive and widespread skeletal muscle disease, including accelerated loss of muscle mass and function. However, there are challenges in clinical diagnostic approaches. 3 The occurrence of sarcopenia is related to age, nutritional intake, physical inactivity, disease, and iatrogenic factors. 4 Sarcopenia increases the risk of a variety of adverse events, including falls, reduced physical function, vulnerability, and death. 5 So far, many academic organizations including the European Working Group on Sarcopenia in Older People (EWGSOP), 4 the Asian Working Group for Sarcopenia (AWGS), 6 and the Foundation for the National Institutes of Health (FNIH) 7 have put forward the diagnostic criteria about sarcopenia. The prevalence of sarcopenia was estimated in different countries and regions based on the diagnostic criteria. A meta‐analysis 8 suggested that the prevalence of sarcopenia among the elderly in Chinese communities was 12.9% in males and 11.2% in females and varied by diagnostic criteria and regions.
The fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM‐5) classified mild cognitive impairment (MCI) as mild neurocognitive impairment. 9 Specifically, it refers to the subjective and objective decline in the functional level of one or more cognitive dimensions compared with the past, but it does not seriously affect the daily instrumental activities and does not occur mental or other psychological diseases. 9 Studies have shown that MCI is associated with age, gender, apolipoprotein E allele, family history, and the presence of cardiovascular risk factors such as hypertension, hyperlipidaemia, coronary heart disease, and stroke. 10 , 11 , 12 Currently, the diagnostic criteria of MCI are not uniform. A meta‐analysis 13 found that the prevalence of cognitive impairment among the Chinese elderly aged over 55 years was 15.4% and varied by the diagnostic criteria. Cognitive impairment was more likely to be experienced by women, the elderly, illiterates, people living in rural areas, and those with unhealthy lifestyle and co‐morbidities.
Given the common influencing factors between sarcopenia and cognitive impairment, studies have suggested a possible link between sarcopenia and cognitive impairment. 14 Currently available evidence 15 , 16 , 17 , 18 on the association between sarcopenia and cognitive impairment is based on cross‐sectional data and needs to be verified by large‐scale longitudinal studies. To fill the research gap, in this study, we used the nationally representative data from the China Health and Retirement Longitudinal Study (CHARLS), in conducting a cross‐sectional analysis in 2015 at wave 3 to investigate the association between sarcopenia and cognitive function in Chinese elderly adults aged 60 years and above, and further analysing the longitudinal association of sarcopenia with MCI based on data in 2018 at wave 4, aiming to provide objective scientific evidence on aetiology, early intervention, and prevention strategies of MCI.
2. Methods
2.1. Study population
CHARLS project aims to collect a set of high‐quality microdata representing households and individuals aged 45 and above in China to analyse the aging of China's population and promote interdisciplinary research on aging. CHARLS national baseline survey was conducted in 2011 using the multi‐stage probability to proportional to size (PPS) sampling method. The samples covered 450 villages, 150 counties, and 28 provinces, involving more than 17 000 people from about 10 000 households. The CHARLS is an ongoing survey with exams performed every 2 to 3 years. The participants were interviewed face‐to‐face in their homes through computer‐assisted personal interviewing (CAPI) technology. The survey included basic demographic information of the respondents and their families, transfer payments between family members, health status of the respondents, medical care and insurance, employment, income, expenditure and assets, and so on. Besides, CHARLS included 13 physical measurements and blood sample collection. To date, CHARLS has released four waves data of national baseline survey (wave 1, 2011y), the first follow‐up survey (wave 2, 2013y), second follow‐up survey (wave 3, 2015y), and third follow‐up survey (wave 4, 2018y). The detailed information about CHARLS had been published in previous literature. 19 The CHARLS datasets can be downloaded at the CHARLS home page at http://charls.pku.edu.cn/en. The CHARLS survey project was approved by the Biomedical Ethics Committee of Peking University, and all participants were required to sign informed consent.
This study used data from two waves collected in 2015 (wave 3) and 2018 (wave 4), respectively. The sample size in wave 3 was 21 097. We excluded 15 382 individuals due to (1) no information on cognition, (2) no information on sarcopenia, and (3) age <60 years. The cross‐sectional analysis included 5715 participants. In the longitudinal analysis, we excluded the participants with a cognitive score less than the threshold in wave 3 20 , 21 and missing information on cognitive in wave 4, which resulted in 2982 eligible individuals. The detailed flowchart of the sample selection process is shown in Figure 1.
Figure 1.
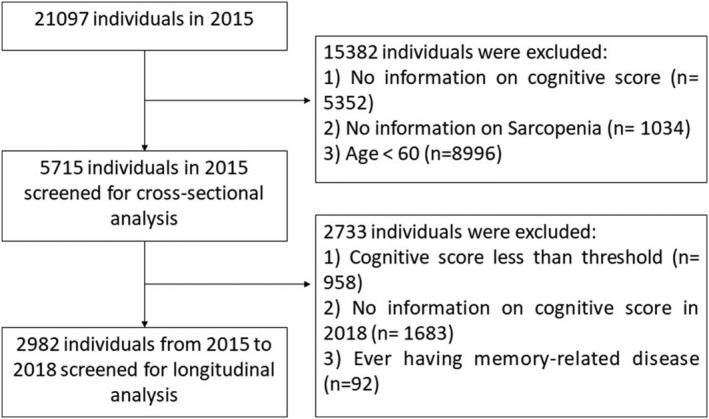
Flowchart of the sample selection process.
2.2. Measurement of cognitive function
We measured the cognitive function based on the method used in the American Health and Retirement Study (HRS). 22 The participants received a face‐to‐face assessment from four cognitive function dimensions: orientation, memory, computation, and drawing. Orientation and computation were determined using the Telephone Interview for Cognitive Status (TICS). Items for orientation included year, month, day, the day of the week, and the current season. The total score of the orientation dimension is five points, with one point for each item. For computation, the participants were asked to successively subtract 7 from 100 for five times, with one point awarded for each successful operation. Ten words were randomly read to each participant, and the immediate word recall was assessed by counting how many words could be recalled immediately. Delayed word recall was evaluated after the participant completed the survey of depression scale, computation, and drawing tests. The total score of memory, defined as the sum scores of immediate and delayed word recall, was 20 points, with one point for each word. The interviewer showed a picture of two pentacle stars overlapping each other to check whether the participant could draw them appropriately to test their drawing ability. If correct, the participant scored one point. The total score of cognitive was defined as the sum scores of orientation (5 points), computation (5 points), memory (20 points), and drawing (1 point), resulting in 31 points. 23
There is no consensus on the diagnostic criteria of MCI. In our study, we used aging‐associated cognitive decline (AACD) to define MCI, namely, at least 1 standard deviation (SD) below the age standard. 20 , 21 All participants over 60 years old were grouped for every 5 years of age. The participant in each age group who met the AACD criteria would be classified as MCI. In longitudinal analysis, participants with MCI at baseline (wave 3, n = 958) were excluded from longitudinal analysis. Three hundred seventy‐three new MCI cases were diagnosed during the follow‐up.
2.3. Assessment of sarcopenia
Sarcopenia was assessed based on the criteria recommended by the AWGS2019, 24 including muscle strength, appendicular skeletal muscle mass (ASM), and physical performance. Handgrip strength (unit: kg) was measured in the dominant hand and non‐dominant hand, with the participant squeezing a YuejianTM WL‐1000 dynamometer as hard as possible. Each participant was tested in duplicate for both hands by holding the dynamometer at a right angle (90°). The average of the available maximum strength data was used. If one of the participant's hands could not be measured for some reason, the maximum value with the other hand was recorded. According to AWGS 2019, the cut‐off point for low handgrip strength was defined as less than 28 kg for men and less than 18 kg for women. ASM for the Chinese population was estimated using a physical measurement formula reported by a previous study 25 :
where the weight was measured by OmronTM HN‐286 scale and the height was measured by SecaTM213 height meter. If male, gender was set to 1, otherwise to 0. Several studies have shown that ASM calculated by this formula is in good agreement with dual‐energy X‐ray absorptiometry (DXA). 25 , 26 Similar to previous studies, 27 the cut‐off for low muscle mass was based on the sex‐specific lowest 20% of the height‐adjusted muscle mass (ASM/height2) among the study population, with <4.89 kg/m2 in women and <6.79 kg/m2 in men.
Physical performance included the gait speed, the five‐time chair stand test, and the short physical performance battery (SPPB). For gait speed, each participant was asked to walk a 2.5‐m distance at a normal pace two times (there and back). The time to complete was recorded. The five‐time chair stand test measures the amount of time needed for the participants to rise continuously five times keeping their arms folded across their chest from the height of the 47‐cm chair. In addition to the previous gait speed and the five‐time chair stand test, the assessment of SPPB also included three‐position tests for 10 s each: (1) a side‐by‐side position; (2) semi‐tandem position (the heel of one foot beside the big toe of the other foot); and (3) tandem position (the heel of one foot in front of and touching the toes of the other foot). The total score of SPPB was 12 points with 4 points for each test. According to the AWGS 2019 recommendations, low physical performance was defined as a gait speed on <1.0 m/s, 5‐time chair stand test ≥12 s, or SPPB score <9.
Possible sarcopenia is defined as low muscle strength or low physical performance. Sarcopenia is defined as low muscle mass plus low muscle strength or combined low physical performance. Therefore, all participants were divided into three groups: non‐sarcopenia, possible sarcopenia, and sarcopenia.
2.4. Potential covariates
According to prior knowledge, we also considered sociodemographic characteristics and health‐related factors in our study. Sociodemographic characteristics included age, gender, residential area (urban and rural), education (illiterate, primary school, middle school, high school/vocational high school, and junior college or above), average household income (<1000, 1000, 5000, 10 000, and 20 000 Chinese Yuan), marital status (married, separated, and unmarried/divorced/widowed). Health‐related factors included ever/current smoke, ever/current alcohol, daily sleep time, and 14 common co‐morbidities (cancer, chronic lung diseases, heart disease, stroke, emotional and mental disorders, arthritis, dyslipidaemia, hepatic disease, kidney disease, digestive system disease, asthma, memory‐related disease, hypertension, and hyperglycaemia). Body mass index (BMI) was defined as the weight (unit: kg) divided by the square of height (unit: m). Depression was assessed by the 10‐item Center for Epidemiologic Studies Depression Scale (CESD‐10), with 30 of the total scores.
2.5. Statistical analysis
Quantitative data with normal distribution were described as mean and SD, and differences among three groups (non‐sarcopenia, possible sarcopenia, and sarcopenia) were compared using one‐way ANOVA. Qualitative data were reported using percentages, and differences among groups were compared using the χ 2 test.
OLS regression model was adopted to investigate the cross‐sectional association between scores of total cognitive function and three dimensions (orientation, memory, and computation) and sarcopenia in 2015, expressed in regression coefficients (β) and 95% confidence intervals (CIs), while the association between drawing dimension and sarcopenia was analysed using a logistic regression model, expressed in odds ratios (ORs) and 95% CIs. We also used a logistic regression model to analyse the relationship between sarcopenia and the occurrence of MCI based on longitudinal data from 2015 and 2018. We used different combinations of covariates in five models. More specifically, model 1 included only cognitive function; model 2 included age, gender, residential area, education, average household income, and marital status; model 3 additionally included ever/current smoke, ever/current alcohol, and daily sleep time; model 4 additionally included co‐morbidities and CESD score; and model 5 included BMI. All statistical analyses were conducted using STATA 16.0 software, and the significance level of statistical tests was 0.05.
3. Results
Table 1 presented the baseline characteristics of the study population in wave 3. The average age of the 5715 participants was 67.3 years (SD: 6.0 years), and 56.2% of the participants were male. Based on AWGS criteria, 486 (8.5%) and 1689 (29.6%) participants were diagnosed with sarcopenia and possible sarcopenia, respectively. Sarcopenia individuals were more likely to be more advanced age, rural residents, lower education, lower average household income, and more unmarried/divorced/widowed. The distribution of co‐morbidities among non‐sarcopenia, possible sarcopenia, and sarcopenia groups was a statistical difference (P < 0.05), except for cancer and hepatic disease. Individuals with sarcopenia had higher CESD scores and lower BMI.
Table 1.
Baseline characteristics of study population in wave 3
| Variables | Total | Non‐ sarcopenia | Possible sarcopenia | Sarcopenia | P‐value |
|---|---|---|---|---|---|
| Age (years, M ± SD) | 67.3 ± 6.0 | 66.0 ± 5.1 | 68.7 ± 6.3 | 72.5 ± 6.6 | <0.001 |
| Gender | |||||
| Male | 56.2 | 58.4 | 50.9 | 58.9 | <0.001 |
| Female | 43.8 | 41.6 | 49.1 | 41.1 | |
| Residential area | |||||
| Urban | 41.6 | 44.6 | 40.6 | 23.5 | <0.001 |
| Rural | 58.4 | 55.4 | 59.4 | 76.5 | |
| Education | |||||
| Illiterate | 44.5 | 40.4 | 49.9 | 55.4 | <0.001 |
| Primary school | 30.2 | 31.0 | 28.5 | 30.0 | |
| Middle school | 16.9 | 18.5 | 15.6 | 9.7 | |
| High school/vocational high school | 6.7 | 8.2 | 4.5 | 3.9 | |
| Junior college or above | 1.7 | 1.9 | 1.4 | 1.0 | |
| Average household income (CNY) | |||||
| <1000 | 24.1 | 22.7 | 24.4 | 33.5 | <0.001 |
| 1000 | 28.8 | 26.1 | 32.8 | 34.8 | |
| 5000 | 12.0 | 12.3 | 11.7 | 11.1 | |
| 10 000 | 15.0 | 16.1 | 13.3 | 12.7 | |
| 20 000 | 20.1 | 22.8 | 17.8 | 7.9 | |
| Marital status | |||||
| Married | 80.2 | 84.0 | 75.5 | 69.3 | <0.001 |
| separated | 2.9 | 3.1 | 2.9 | 1.7 | |
| Unmarried/divorced/widowed | 16.9 | 13.0 | 21.6 | 29.0 | |
| Ever/current smoke | |||||
| No | 48.3 | 48.0 | 51.0 | 40.1 | <0.001 |
| Yes | 51.7 | 52.0 | 49.0 | 59.9 | |
| Ever/current alcohol | |||||
| No | 49.1 | 46.8 | 53.7 | 49.8 | <0.001 |
| Yes | 50.9 | 53.2 | 46.3 | 50.2 | |
| Daily sleep time (h) | |||||
| <6 | 24.6 | 22.0 | 29.1 | 28.8 | <0.001 |
| 6 | 16.2 | 17.3 | 14.6 | 13.7 | |
| 7 | 17.2 | 18.5 | 15.1 | 15.2 | |
| 8 | 18.4 | 19.0 | 16.5 | 21.1 | |
| 9 | 23.6 | 23.4 | 24.6 | 21.1 | |
| Co‐morbidities | |||||
| Cancer | 1.68 | 1.42 | 2.04 | 2.38 | 0.134 |
| Chronic lung disease | 17.5 | 14.9 | 19.9 | 28.0 | <0.001 |
| Heart disease | 22.6 | 20.5 | 27.7 | 20.8 | <0.001 |
| Stroke | 4.5 | 3.0 | 7.7 | 4.3 | <0.001 |
| Emotional and mental disorders | 2.0 | 1.6 | 2.9 | 2.4 | <0.001 |
| Arthritis | 46.6 | 43.2 | 53.5 | 46.9 | <0.001 |
| Dyslipidaemia | 21.8 | 20.4 | 27.9 | 11.6 | <0.001 |
| Hepatic disease | 6.8 | 6.8 | 7.0 | 6.3 | 0.892 |
| Kidney disease | 11.5 | 10.6 | 13.3 | 11.8 | 0.023 |
| Digestive system disease | 31.7 | 30.6 | 31.4 | 41.0 | <0.001 |
| Asthma | 8.0 | 6.4 | 9.4 | 14.1 | <0.001 |
| Memory‐related disease | 3.5 | 2.5 | 5.2 | 4.4 | <0.001 |
| Hypertension | 40.1 | 36.9 | 50.9 | 30.3 | <0.001 |
| Hyperglycaemia | 12.0 | 10.8 | 16.4 | 5.5 | <0.001 |
| CESD score, M ± SD | 8.0 ± 6.2 | 7.14 ± 5.8 | 9.2 ± 6.7 | 9.6 ± 6.8 | <0.001 |
| BMI (kg/m2, M ± SD) | 23.5 ± 3.7 | 23.6 ± 3.5 | 24.7 ± 3.3 | 18.7 ± 1.5 | <0.001 |
Abbreviations: BMI, body mass index; CESD, Center for Epidemiologic Studies Depression; CNY, Chinese Yuan; M ± SD, mean ± standard deviation.
The mean score of total cognitive function, orientation, memory, computation, and drawing in the overall population was 14.4 ± 4.9, 3.9 ± 1.2, 6.2 ± 3.4, 3.6 ± 1.5, and 0.6 ± 0.5, respectively, which exhibited a decreasing trend from non‐sarcopenia to sarcopenia group (P < 0.001) (Table 2).
Table 2.
Cross‐sectional association between sarcopenia and cognitive score in wave 3 (M ± SD)
| Cognitive score | Total (n = 5715) | Non‐sarcopenia (n = 3540) | Possible sarcopenia (n = 1689) | Sarcopenia (n = 486) | P‐value |
|---|---|---|---|---|---|
| Total score | 14.4 ± 4.9 | 15.3 ± 4.7 | 13.5 ± 4.9 | 11.8 ± 5.0 | <0.001 |
| Orientation | 3.9 ± 1.2 | 4.0 ± 1.1 | 3.8 ± 1.3 | 3.5 ± 1.4 | <0.001 |
| Memory | 6.2 ± 3.4 | 6.7 ± 3.4 | 5.7 ± 3.3 | 4.6 ± 3.3 | <0.001 |
| Computation | 3.6 ± 1.5 | 3.8 ± 1.4 | 3.4 ± 1.6 | 3.2 ± 1.7 | <0.001 |
| Drawing | 0.6 ± 0.5 | 0.7 ± 0.5 | 0.6 ± 0.5 | 0.5 ± 0.5 | <0.001 |
Abbreviations: M ± SD, mean ± standard deviation.
Table 3 showed the cross‐sectional association between sarcopenia and cognitive score at wave 2015. In the crude model, the total cognitive score of possible sarcopenia (β = −1.78, 95% CI: −2.06, −1.50) and sarcopenia (β = −3.44, 95% CI: −3.90, −2.98) was lower than that of non‐sarcopenia. Compared with the non‐sarcopenia group, possible sarcopenia showed low scores for orientation, memory, computation, and drawing dimensions, and even lower scores were observed in the sarcopenia group. In the fully adjusted model by age, gender, residential area, education, average household income, marital status, ever/current smoke, ever/current alcohol, daily sleep time, co‐morbidities, CESD score, and BMI, similar patterns were also observed with statistical significance (all P < 0.05).
Table 3.
OLS or logistic regression model on sarcopenia and cognitive score
| Cognitive score | Models | Non‐sarcopenia | Possible sarcopenia | Sarcopenia | ||
|---|---|---|---|---|---|---|
| β (95% CI) | P‐value | β (95% CI) | P‐value | |||
| Total score | Model 1 | Reference | −1.78 (−2.06, −1.50) | <0.001 | −3.44 (−3.90, −2.98) | <0.001 |
| Model 2 | Reference | −0.78 (−1.04, −0.53) | <0.001 | −1.30 (−1.73, −0.88) | <0.001 | |
| Model 3 | Reference | −0.74 (−1.00, −0.49) | <0.001 | −1.27 (−1.70, −0.85) | <0.001 | |
| Model 4 | Reference | −0.62 (−0.90, −0.34) | <0.001 | −1.20 (−1.67, −0.74) | <0.001 | |
| Model 5 | Reference | −0.64 (−0.92, −0.36) | <0.001 | −0.98 (−1.47, −0.50) | <0.001 | |
| Orientation | Model 1 | Reference | −0.26 (−0.33, −0.19) | <0.001 | −0.52 (−0.63, −0.40) | <0.001 |
| Model 2 | Reference | −0.11 (−0.18, −0.04) | 0.001 | −0.22 (−0.33, −0.10) | <0.001 | |
| Model 3 | Reference | −0.10 (−0.17, −0.03) | 0.003 | −0.20 (−0.32, −0.09) | <0.001 | |
| Model 4 | Reference | −0.08 (−0.16, −0.01) | 0.030 | −0.19 (−0.32, −0.07) | 0.002 | |
| Model 5 | Reference | −0.09 (−0.17, −0.02) | 0.017 | −0.14 (−0.27, −0.01) | 0.038 | |
| Memory | Model 1 | Reference | −1.05 (−1.25, −0.86) | <0.001 | −2.10 (−2.42, −1.78) | <0.001 |
| Model 2 | Reference | −0.47 (−0.66, −0.29) | <0.001 | −0.72 (−1.03, −0.40) | <0.001 | |
| Model 3 | Reference | −0.45 (−0.64, −0.27) | <0.001 | −0.70 (−1.02, −0.39) | <0.001 | |
| Model 4 | Reference | −0.38 (−0.58, −0.17) | <0.001 | −0.71 (−1.05, −0.36) | <0.001 | |
| Model 5 | Reference | −0.39 (−0.60, −0.18) | <0.001 | −0.60 (−0.97, −0.24) | 0.001 | |
| Computation | Model 1 | Reference | −0.36 (−0.45, −0.27) | <0.001 | −0.61 (−0.76, −0.47) | <0.001 |
| Model 2 | Reference | −0.16 (−0.24, −0.08) | <0.001 | −0.28 (−0.42, −0.14) | <0.001 | |
| Model 3 | Reference | −0.15 (−0.23, −0.07) | <0.001 | −0.28 (−0.42, −0.14) | <0.001 | |
| Model 4 | Reference | −0.13 (−0.22, −0.04) | 0.006 | −0.22 (−0.38, −0.07) | 0.005 | |
| Model 5 | Reference | −0.13 (−0.22, −0.04) | 0.006 | −0.17 (−0.33, −0.01) | 0.040 | |
| Drawing* | Model 1 | Reference | −0.47 (−0.59, −0.35) | <0.001 | −0.88 (−1.07, −0.68) | <0.001 |
| Model 2 | Reference | −0.20 (−0.34, −0.07) | 0.003 | −0.40 (−0.63, −0.18) | <0.001 | |
| Model 3 | Reference | −0.19 (−0.33, −0.06) | 0.005 | −0.40 (−0.62, −0.18) | <0.001 | |
| Model 4 | Reference | −0.15 (−0.30, −0.00) | 0.044 | −0.38 (−0.62, −0.13) | 0.003 | |
| Model 5 | Reference | −0.16 (−0.31, −0.01) | 0.041 | −0.33 (−0.59, −0.07) | 0.014 | |
Notes: Model 1: No adjustment; Model 2: Adjusted for age, gender, residential area, education, average household income, and marital status; Model 3: model 2 + ever/current smoke, ever/current alcohol, and daily sleep time; Model 4: model 3 + co‐morbidities and CESD score; Model 5: model 4 + BMI.
Logistic regression model.
Out of 2982 longitudinal analytic samples, 373 participants (12.5%) developed into new‐onset MCI at wave 4. The incidence of MCI in non‐sarcopenia, possible sarcopenia, and sarcopenia groups was 10.1%, 16.5%, and 24.2%, respectively, with statistical significance (P < 0.001) (Figure 2). Table 4 showed the longitudinal association between sarcopenia and MCI based on logistic regression models. In the crude model, compared with the non‐sarcopenia group, the risk of the occurrence of MCI for individuals with possible sarcopenia was higher (OR = 1.75, 95% CI: 1.38–2.23), and even higher in the sarcopenia group (OR = 2.85, 95% CI: 1.94–4.18). In the fully adjusted model by age, gender, residential area, education, average household income, marital status, ever/current smoke, ever/current alcohol, daily sleep time, co‐morbidities, CESD score, and BMI, similar patterns were also observed with statistical significance (all P < 0.05).
Figure 2.
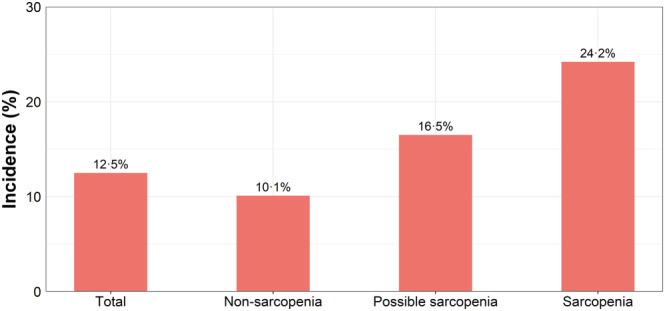
Prevalence of MCI in different groups.
Table 4.
Longitudinal analysis on sarcopenia and MCI based on wave 3 and wave 4
| Models | Non‐sarcopenia | Possible sarcopenia | Sarcopenia | ||
|---|---|---|---|---|---|
| OR (95% CI) | P‐value | OR (95% CI) | P‐value | ||
| Model 1 | Reference | 1.75 (1.38, 2.23) | <0.001 | 2.85 (1.94, 4.18) | <0.001 |
| Model 2 | Reference | 1.54 (1.19, 1.99) | 0.001 | 2.16 (1.42, 3.30) | <0.001 |
| Model 3 | Reference | 1.52 (1.18, 1.97) | 0.001 | 2.18 (1.42, 3.34) | <0.001 |
| Model 4 | Reference | 1.46 (1.09, 1.94) | 0.010 | 1.89 (1.18, 3.05) | 0.009 |
| Model 5 | Reference | 1.43 (1.06, 1.91) | 0.017 | 1.72 (1.04, 2.86) | 0.035 |
Notes: Model 1: No adjustment; Model 2: Adjusted for age, gender, residential area, education, average household income, and marital status; Model 3: model 2 + ever/current smoke, ever/current alcohol, and daily sleep time; Model 4: model 3 + co‐morbidities and CESD score; Model 5: model 4 + BMI.
4. Discussion
To the best of our knowledge, this is the first study to examine the longitudinal association between sarcopenia and cognitive impairment among the elderly population aged over 60 years in Chinese communities using the nationally representative data. In a cross‐sectional analysis, we found a negative association between sarcopenia and cognitive scores. Further, the elderly with possible sarcopenia or sarcopenia were more likely to develop new‐onset MCI in a longitudinal analysis.
The cross‐sectional analysis found a negative association between sarcopenia and cognitive scores. The elderly with sarcopenia had lower cognitive scores than those with non‐sarcopenia. Our results were consistent with several cross‐sectional studies 16 , 17 , 18 and meta‐analyses. 14 , 15 However, the etiological association between sarcopenia and cognitive impairment requires further longitudinal data. The longitudinal analysis based on nationally representative data suggested that sarcopenia was an independent influence factor associated with MCI. Individuals with sarcopenia were 1.72 times more likely to develop MCI than those without sarcopenia in the fully adjusted model. The exact mechanisms of sarcopenia involved in MCI remained unclear. It was suspected that both of them shared common risk factors, including physical inactivity and malnutrition. Meta‐analysis indicated that physical activity could not only reduce the onset of sarcopenia 28 but also delay cognitive decline. 29 One possible explanation was that cytokines and peptides secreted by skeletal muscle could enhance the function of the brain, including cognitive ability, suggesting a muscle‐brain dialogue. 30 Poor nutrition might be due to inadequate dietary intake of nutrients that were common influence factors to sarcopenia and cognitive impairment. 31 , 32 In addition, physical activity and poor nutrition contributed to various chronic diseases, such as metabolic syndrome, which also increased the co‐morbidities of sarcopenia and cognitive impairment. 33 , 34
Based on AWGS 2019 criteria, we also analyse the influence of possible sarcopenia on cognitive function to facilitate early prevention interventions. A cross‐sectional association suggested that the elderly with possible sarcopenia had lower cognitive scores when compared with non‐sarcopenia groups, which was consistent with one study in Korea. 35 Longitudinal analysis found that individuals with possible sarcopenia were 1.43 times more likely to develop MCI when compared with non‐sarcopenia ones in the fully adjusted model, which implied a causality correlation between sarcopenia and MCI.
The results derived from the cross‐sectional analysis in wave 3 found that possible sarcopenia and sarcopenia were both associated with the scores of total cognitive function and four sub‐dimensions, namely, orientation, memory, computation, and drawing. The evaluation and measurement methods of cognitive function varied in different studies. Several studies suggested that sarcopenia was only associated with some specific dimensions. For example, the findings from a survey in Mexican adults showed that sarcopenia was associated with immediate verbal recall, delayed verbal recall, and semantic verbal fluency. 36 A cross‐sectional study from the Brazil study suggested a correlation between sarcopenia and verbal fluency test. 37
There are several strengths of this study. First, a nationally representative longitudinal survey for the Chinese elderly was employed, and the extrapolation of results was relatively high quality. Second, this is the first study to examine the association of sarcopenia with cognitive impairment in China based on cross‐sectional and longitudinal analysis adjusting multiple confounders. Third, this study provided new evidence for the utility of AWGS 2019 criteria on the screening of sarcopenia, which was important for the prevention and early intervention of cognitive impairment.
Nevertheless, the limitations of this study must be acknowledged. First, although we have adjusted a set of potential confounders based on prior knowledge, some extra confounders were not considered in our study, such as physical activity and dietary intake. The results were based on an observational study, and recall bias was inevitable in a questionnaire survey. Second, gait speed was measured at a distance of 2.5‐m instead of the 6‐m standard one. However, one study 38 suggested that the measurement of gait speed by CHARLS in 2015 was consistent with the previous studies in China, indicating that distance did not influence the gait speed. Third, although the correlation between sarcopenia and cognitive impairment derived from longitudinal study achieved stronger than that of the cross‐sectional study, we could not interpret the potential biological mechanisms. Therefore, further experimental studies are necessary to confirm this association.
In conclusion, this study suggested a correlation between sarcopenia and the occurrence of MCI in the Chinese elderly population aged 60 and above, providing new evidence for a causal relation. In the context of China's population aging rapidly, enhancing physical activity and nutrition intervention is a benefit for the prevention of sarcopenia, which further contributes to decreasing and delaying the development of MCI and dementia among the elderly. As a result, it is critical to reduce the burden of chronic disease and improve the quality of life for the elderly.
Conflicts of interest
We declare no competing interests.
Acknowledgements
We thank the China Health and Retirement Longitudinal Study team for providing data and training in using the datasets. We thank the students who participated in the survey for their cooperation. We thank all volunteers and staff involved in this research. The authors certify that they comply with the ethical guidelines for authorship and publishing of the Journal of Cachexia, Sarcopenia and Muscle. 39
Hu Y., Peng W., Ren R., Wang Y., and Wang G. (2022) Sarcopenia and mild cognitive impairment among elderly adults: The first longitudinal evidence from CHARLS, Journal of Cachexia, Sarcopenia and Muscle, 13, 2944–2952, doi: 10.1002/jcsm.13081
Yisong Hu and Wenjia Peng contributed equally.
Funding information: This analysis uses data or information from the Harmonized CHARLS dataset and Codebook, Version D as of June 2021 developed by the Gateway to Global Aging Data. This work was supported by the National Natural Science Foundation of China (71673055).
Contributor Information
Ying Wang, Email: wangying1013@fudan.edu.cn.
Gang Wang, Email: wg11424@rjh.com.cn.
References
- 1. Ren R, Qi J, Lin S, Liu X, Yin P, Wang Z, et al. The China Alzheimer Report 2022. General Psychiatry 2022;35:e100751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Liu Y, Zheng Z, Rao K, Wang S. Blue Book of Elderly Health: Annual Report on Elderly Health in China (2018). China: Social Science Academic Press, Beijing; 2019. [Google Scholar]
- 3. Cesari M, Kuchel GA. Role of Sarcopenia Definition and Diagnosis in Clinical Care: Moving from Risk Assessment to Mechanism‐Guided Interventions. J Am Geriatr Soc 2020;68:1406–1409. [DOI] [PubMed] [Google Scholar]
- 4. Cruz‐Jentoft AJ, Sayer AA. Sarcopenia. Lancet 2019;393:2636–2646. [DOI] [PubMed] [Google Scholar]
- 5. Cruz‐Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019;48:16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen LK, Liu LK, Woo J, Assantachai P, Auyeung TW, Bahyah KS, et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc 2014;15:95–101. [DOI] [PubMed] [Google Scholar]
- 7. Studenski SA, Peters KW, Alley DE, Cawthon PM, McLean RR, Harris TB, et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci 2014;69:547–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen Z, Li WY, Ho M, Chau PH. The Prevalence of Sarcopenia in Chinese Older Adults: Meta‐Analysis and Meta‐Regression. Nutrients 2021;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. American Psychiatric A . Diagnostic and statistical manual of mental disorders. Arlington: American Psychiatric Publishing; 2013. [Google Scholar]
- 10. Au B, Dale‐McGrath S, Tierney MC. Sex differences in the prevalence and incidence of mild cognitive impairment: A meta‐analysis. Ageing Res Rev 2017;35:176–199. [DOI] [PubMed] [Google Scholar]
- 11. Caselli RJ, Dueck AC, Osborne D, Sabbagh MN, Connor DJ, Ahern GL, et al. Longitudinal modeling of age‐related memory decline and the APOE epsilon4 effect. N Engl J Med 2009;361:255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pal K, Mukadam N, Petersen I, Cooper C. Mild cognitive impairment and progression to dementia in people with diabetes, prediabetes and metabolic syndrome: a systematic review and meta‐analysis. Soc Psychiatry Psychiatr Epidemiol 2018;53:1149–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Deng Y, Zhao S, Cheng G, Yang J, Li B, Xu K, et al. The Prevalence of Mild Cognitive Impairment among Chinese People: A Meta‐Analysis. Neuroepidemiology 2021;55:79–91. [DOI] [PubMed] [Google Scholar]
- 14. Peng TC, Chen WL, Wu LW, Chang YW, Kao TW. Sarcopenia and cognitive impairment: A systematic review and meta‐analysis. Clin Nutr 2020;39:2695–2701. [DOI] [PubMed] [Google Scholar]
- 15. Chang KV, Hsu TH, Wu WT, Huang KC, Han DS. Association Between Sarcopenia and Cognitive Impairment: A Systematic Review and Meta‐Analysis. J Am Med Dir Assoc 2016;17:e7–e15. [DOI] [PubMed] [Google Scholar]
- 16. Chen X, Han P, Yu X, Zhang Y, Song P, Liu Y, et al. Relationships between sarcopenia, depressive symptoms, and mild cognitive impairment in Chinese community‐dwelling older adults. J Affect Disord 2021;286:71–77. [DOI] [PubMed] [Google Scholar]
- 17. Wu B, Lyu YB, Cao ZJ, Wei Y, Shi WY, Gao X, et al. Associations of Sarcopenia, Handgrip Strength and Calf Circumference with Cognitive Impairment among Chinese Older Adults. Biomed Environ Sci 2021;34:859–870. [DOI] [PubMed] [Google Scholar]
- 18. Bai A, Xu W, Sun J, Liu J, Deng X, Wu L, et al. Associations of sarcopenia and its defining components with cognitive function in community‐dwelling oldest old. BMC Geriatr 2021;21:292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhao Y, Hu Y, Smith JP, Strauss J, Yang G. Cohort profile: the China Health and Retirement Longitudinal Study (CHARLS). Int J Epidemiol 2014;43:61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Levy R. Aging‐associated cognitive decline. Working Party of the International Psychogeriatric Association in collaboration with the World Health Organization. Int Psychogeriatr 1994;6:63–68. [PubMed] [Google Scholar]
- 21. Richards M, Touchon J, Ledesert B, Richie K. Cognitive decline in ageing: are AAMI and AACD distinct entities? Int J Geriatr Psychiatry 1999;14:534–540. [DOI] [PubMed] [Google Scholar]
- 22. Crimmins EM, Kim JK, Langa KM, Weir DR. Assessment of cognition using surveys and neuropsychological assessment: the Health and Retirement Study and the Aging, Demographics, and Memory Study. J Gerontol B Psychol Sci Soc Sci 2011;66:i162–i171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cao L, Zhao Z, Ji C, Xia Y. Association between solid fuel use and cognitive impairment: A cross‐sectional and follow‐up study in a middle‐aged and older Chinese population. Environ Int 2021;146:106251. [DOI] [PubMed] [Google Scholar]
- 24. Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K, et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J Am Med Dir Assoc 2020;21:e2. [DOI] [PubMed] [Google Scholar]
- 25. Wen X, Wang M, Jiang CM, Zhang YM. Anthropometric equation for estimation of appendicular skeletal muscle mass in Chinese adults. Asia Pac J Clin Nutr 2011;20:551–556. [PubMed] [Google Scholar]
- 26. Yang M, Hu X, Wang H, Zhang L, Hao Q, Dong B. Sarcopenia predicts readmission and mortality in elderly patients in acute care wards: a prospective study. J Cachexia Sarcopenia Muscle 2017;8:251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Delmonico MJ, Harris TB, Lee JS, Visser M, Nevitt M, Kritchevsky SB, et al. Alternative definitions of sarcopenia, lower extremity performance, and functional impairment with aging in older men and women. J Am Geriatr Soc 2007;55:769–774. [DOI] [PubMed] [Google Scholar]
- 28. Steffl M, Bohannon RW, Sontakova L, Tufano JJ, Shiells K, Holmerova I. Relationship between sarcopenia and physical activity in older people: a systematic review and meta‐analysis. Clin Interv Aging 2017;12:835–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dauwan M, Begemann MJH, Slot MIE, Lee EHM, Scheltens P, Sommer IEC. Physical exercise improves quality of life, depressive symptoms, and cognition across chronic brain disorders: a transdiagnostic systematic review and meta‐analysis of randomized controlled trials. J Neurol 2021;268:1222–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Scisciola L, Fontanella RA, Surina CV, Paolisso G, Barbieri M. Sarcopenia and Cognitive Function: Role of Myokines in Muscle Brain Cross‐Talk. Life (Basel) 2021;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Beaudart C, Sanchez‐Rodriguez D, Locquet M, Reginster JY, Lengele L, Bruyere O. Malnutrition as a Strong Predictor of the Onset of Sarcopenia. Nutrients 2019;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gomez‐Pinilla F. Brain foods: the effects of nutrients on brain function. Nat Rev Neurosci 2008;9:568–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liu M, He Y, Jiang B, Wu L, Wang J, Yang S, et al. Association between metabolic syndrome and mild cognitive impairment and its age difference in a Chinese community elderly population. Clin Endocrinol (Oxf) 2015;82:844–853. [DOI] [PubMed] [Google Scholar]
- 34. Zhang H, Lin S, Gao T, Zhong F, Cai J, Sun Y, et al. Association between Sarcopenia and Metabolic Syndrome in Middle‐Aged and Older Non‐Obese Adults: A Systematic Review and Meta‐Analysis. Nutrients 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lee I, Cho J, Hong H, Jin Y, Kim D, Kang H. Sarcopenia Is Associated with Cognitive Impairment and Depression in Elderly Korean Women. Iran J Public Health 2018;47:327–334. [PMC free article] [PubMed] [Google Scholar]
- 36. Salinas‐Rodriguez A, Palazuelos‐Gonzalez R, Rivera‐Almaraz A, Manrique‐Espinoza B. Longitudinal association of sarcopenia and mild cognitive impairment among older Mexican adults. J Cachexia Sarcopenia Muscle 2021;12:1848–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Szlejf C, Suemoto CK, Lotufo PA, Bensenor IM. Association of Sarcopenia With Performance on Multiple Cognitive Domains: Results From the ELSA‐Brasil Study. J Gerontol A Biol Sci Med Sci 2019;74:1805–1811. [DOI] [PubMed] [Google Scholar]
- 38. Wu X, Li X, Xu M, Zhang Z, He L, Li Y. Sarcopenia prevalence and associated factors among older Chinese population: Findings from the China Health and Retirement Longitudinal Study. PLoS One 2021;16:e0247617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2021. J Cachexia Sarcopenia Muscle 2021;12:2259–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]


