Abstract
Background
Catheter ablation for atrial fibrillation (AF) is a proven alternative to pharmacologic rhythm control in patients with heart failure with reduced ejection fraction (HFrEF). Whether outcomes differ in patients with heart failure with preserved ejection fraction (HFpEF) is of interest.
Methods
Medline, Scopus, and Cochrane Central Register of Controlled Trials were systematically searched to identify relevant studies. Primary efficacy outcomes of interest include atrial arrythmia recurrence and repeat ablation. Harm outcomes of interest include all‐cause mortality, all‐cause hospitalizations, cardiovascular hospitalizations, stroke/transient ischemic attack, and cardiac tamponade.
Results
We included 7 observational studies comprising 2554 patients with HFpEF who underwent catheter ablation for AF. When comparing patients with HFpEF versus without HF, there was no significant difference in atrial arrhythmia recurrence (risk ratio [RR] 1.39; 95% confidence interval [CI] 0.91–2.13), stroke or transient ischemic attack (TIA) (RR 0.47; 95% CI 0.03–6.54), or cardiac tamponade (RR 1.20; 95% CI 0.12–12.20). When comparing patients with HFpEF versus HFrEF, there was no significant difference in atrial arrhythmia recurrence (RR 1.12; 95% CI 0.92–1.37), repeat ablation (RR 1.19; 95% CI 0.74–1.93), all‐cause mortality (RR 0.87; 95% CI 0.67–1.13), all‐cause hospitalizations (RR 1.10; 95% CI 0.94–1.30), cardiovascular hospitalizations (RR 0.83; 95% CI 0.69–1.01), stroke or TIA (RR 0.81; 95% CI 0.29–2.25), or cardiac tamponade (RR 0.98; 95% CI 0.19–5.16).
Conclusions
Non‐randomized studies suggest that catheter ablation for AF in patients with HFpEF is associated with similar arrythmia‐free survival and safety profile when compared to patients with HFrEF or without heart failure.
Keywords: atrial fibrillation, catheter ablation, heart failure with preserved ejection fraction
Non‐randomized studies suggest that catheter ablation for AF in patients with HFpEF is associated with similar arrythmia free survival and safety profile when compared to patients with HFrEF and without heart failure. Ultimately, this suggests that patients with AF and HFpEF may benefit equally as those with AF and HFrEF.

1. INTRODUCTION
Atrial fibrillation (AF) is the most common sustained arrhythmia in clinical practice and is associated with an increased risk of stroke, heart failure (HF), and mortality. 1 , 2 , 3 Due to its global disease burden and projected increase in prevalence, it poses a significant healthcare issue. 4 Patients with concurrent AF and HF have particularly poor outcomes. 5 Effective management of AF can mitigate this. In recent years, there exists a growing interest in shifting from rate control approaches to rhythm control strategies relatively early in the disease course. Landmark trials have demonstrated the safety and efficacy of catheter ablation (CA) for AF patients. 6 , 7 , 8 , 9
In select patients with AF and HF with reduced ejection fraction (HFrEF), studies have demonstrated reduced AF recurrence, as well as improvements in mortality and hospitalization rates with CA. 6 , 10 , 11 Guidelines have provided Class IIb recommendation for CA in symptomatic AF patients who have HFrEF. 4 , 12 However, evidence for outcomes of CA in patients with HF with preserved ejection (HFpEF) is limited to a few retrospective studies. 13 , 14 , 15 The objective of this meta‐analysis is to assess the efficacy and safety of CA in patients with AF and HFpEF.
2. METHODS
2.1. Data sources and search strategy
This systematic review and meta‐analysis was reported according to the Preferred Reporting Items for Systematic Review and Meta‐Analyses (PRISMA) guidelines. 16 Medline, Scopus, and Cochrane Central Register of Controlled Trials were searched from database inception through March 2021 using the following combination of keywords: heart failure OR heart failure with preserved ejection fraction OR congestive heart failure OR HFpEF AND atrial fibrillation AND catheter ablation. Only articles with available abstracts and free full text were included. Language was restricted to English. We also searched trial registries, www.clinicaltrialresults.org, www.clinicaltrials.gov, abstracts, and presentations from major cardiovascular proceedings. All citations retrieved from the search were transferred to EndNote X7.5 (Thompson ISI ResearchSoft) Reference Manager and duplicates were removed.
2.2. Study selection
All citations were screened by two reviewers (MUS, JJ). Eligible studies reported on AF recurrence, CA procedure characteristics, and CA procedure complications in patients with HFpEF and AF. We included randomized and non‐randomized studies. Exclusion criteria included: studies with data on only patients with HFrEF or studies comparing CA to antiarrhythmic drug (AAD) therapy in patients with AF and HF.
Patients were identified using AF ablation registries or through International Classification of Disease, Ninth Revision, Clinical Modification (ICD 9 CM) codes for AF and CA. Classification of HFpEF was based on the individual ejection fraction (EF) cutoffs within each included study. When possible, HFpEF was classified as patients with HF and ejection fraction (EF) ≥50% measured by echocardiogram, whereas HFrEF was defined as HF with EF <50%. If a study used a different EF cutoff for HFpEF, the study's classification of HFpEF was used.
Main efficacy outcomes of interest were atrial arrythmia recurrence and repeat CA. Harm outcomes included all‐cause mortality, all‐cause hospitalizations, cardiovascular hospitalizations, stroke/transient ischemic attacks (TIA), or cardiac tamponade.
2.3. Data extraction and risk of bias
Two independent reviewers (MUS, JJ) extracted the data on year of publication, study design, inclusion criteria, primary endpoints, and follow‐up time using a standardized data extraction form. Risk of bias was assessed using the Modified Newcastle‐Ottawa scale for observational studies, which assesses three domains: patient selection, comparability, and outcome assessment. 17 The methodological quality of a study was graded as high or low based on whether the study had adequate adjustment for confounders, which we judged to be the most critical domain affecting the outcome of atrial arrythmia recurrence. 18
2.4. Statistical analysis and certainty in the estimates
We extracted or calculated a risk ratio (RR) and 95% confidence intervals (CI) from each study. RR's were pooled using a random effect model to account for between study variance, independent of estimated heterogeneity. 19 The I 2‐statistic was quantified to measure heterogeneity with values <25%, 50%, and 75% consistent with low, moderate, and high degrees of heterogeneity, respectively. 20 Review Manager Software v5.4 was used for analysis. p‐values <.05 were considered statistically significant. Certainty in the evidence (i.e., confidence in the final estimates) was assessed using the GRADE approach (Grades of Recommendation, Assessment, Development, and Evaluation) based on the risk of bias, imprecision, indirectness, inconsistency, and publication bias. 21
3. RESULTS
3.1. Study selection
Of 548 potential articles screened, 7 studies comprising 6692 patients were included (Figure S1). 13 , 14 , 15 , 22 , 23 , 24 , 25 Of these, 2554 patients had HFpEF, 3582 patients had HFrEF, and 556 patients had no HF. Eitel et al. divided patients into three groups based on left ventricular EF (LVEF): HFpEF (LVEF ≥50%), HF with mid‐range LVEF (LVEF 40%–49%) (HFmrEF), and HFrEF (LVEF <40%). 14 For the purposes of this meta‐analysis, the HFmrEF group in the study by Eitel et al. was classified as HFrEF. Additionally, Ichijo et al. categorized HFpEF as LVEF >45% and HFrEF as LVEF ≤45%. 15 The LVEF cutoff of 45% in Ichijo et al. was used to stratify patients into HFrEF or HFpEF. Otherwise, an LVEF cut‐off of 50% was used for all the remaining studies to stratify patients. Arora et al. did not mention the EF cutoff for HFpEF and HFrEF. 22
Table 1 summarizes the characteristics of the included studies. All studies included were observational (non‐randomized). Table 2 summarizes the baseline characteristics of included patients. Out of 6692 patients included in this analysis, 2881 were female (43%). The mean age of the patients included in this analysis was 64.2 years. Patient follow‐up ranged between 1–5 years with a mean follow‐up duration of 2.6 years.
TABLE 1.
Characteristics of included studies
| Study | Design | Experimental arm | Control arm | Endpoints | Follow‐up duration | Type of atrial fibrillation |
|---|---|---|---|---|---|---|
| Cha et al., 2011 | Prospective cohort study | Radiofrequency ablation on LV diastolic dysfunction group (abnormal diastolic function and LVEF ≥50%) and LV systolic dysfunction group (abnormal diastolic function and LVEF≤40%) | Radiofrequency ablation on normal LV function group (normal diastolic function, LVEF ≥50%) |
Primary: AAD free AF elimination 1 year after ablation Secondary: ablation times and complications |
5 years | Paroxysmal, persistent, or permanent |
| Black‐Maier et al., 2018 | Retrospective cohort study | Radiofrequency ablation on HFpEF (LVEF ≥50%) | Radiofrequency ablation on HFrEF (LVEF <50%) | Primary: freedom from recurrent atrial arrhythmia at 1 year, in‐hospital adverse events | 1 year | Paroxysmal, persistent |
| Ichijo et al., 2018 | Retrospective cohort study | Radiofrequency ablation on HFpEF (LVEF >45%) | Radiofrequency ablation on HFrEF (LVEF ≤45%) |
Primary: freedom from AF Secondary: all‐cause mortality, stroke, HF‐related unplanned hospitalizations |
4 years | Paroxysmal, persistent |
| Eitel et al., 2019 | Prospective cohort study |
Ablation (radiofrequency, cryoballoon, or AV‐node ablation) on HFpEF (LVEF ≥50%) Ablation (radiofrequency, cryoballoon, or AV‐node ablation) on HFmrEF (LVEF 40–49%) |
Ablation (radiofrequency, cryoballoon, or AV‐node ablation) on HFrEF (LVEF <40%) | Primary: AF recurrence, all‐cause mortality, peri‐procedural complications | 1 year | Paroxysmal, persistent, or permanent |
| Aldaas et al., 2020 | Retrospective cohort study |
Radiofrequency ablation on HFpEF (LVEF ≥50%) Radiofrequency ablation on HFrEF (LVEF <50%) |
Radiofrequency ablation on patients without HF | Primary: in‐hospital adverse events, recurrence of atrial arrhythmia, all‐cause hospitalization and mortality | 5 years | Paroxysmal, persistent |
| Arora et al., 2020 | Retrospective cohort study | Radiofrequency ablation on HFpEF (no mention of EF cutoff) | Radiofrequency ablation on HFrEF (no mention of EF cutoff) |
Primary: composite of HF readmission and mortality Secondary: HF readmission, mortality, AF readmission |
1 year | Paroxysmal, persistent |
| Vecchio et al., 2019 | Prospective cohort study | Radiofrequency ablation on HFpEF (LVEF ≥50%) | Radiofrequency ablation on HFrEF (LVEF <45%) |
Primary: freedom from AF Secondary: peri‐procedural |
1 year | Paroxysmal, persistent |
Abbreviations: AAD, anti‐arrhythmic drug; AF, atrial fibrillation; AV, atrioventricular; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; LV, left ventricular; LVEF, left ventricular ejection fraction.
TABLE 2.
Patient baseline characteristics
| Study | Cha et al., 2011 | Black‐Maier et al., 2018 | Ichijo et al., 2018 | Eitel et al., 2019 | Aldaas et al., 2020 | Arora et al., 2020 | Vecchio et al., 2019 * | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HFpEF | HFrEF | No HF | HFpEF | HFrEF | HFpEF | HFrEF | HFpEF | HFrEF | HFpEF | HFrEF | No HF | HFpEF | HFrEF | All patients | |
| Patients | 157 | 111 | 100 | 133 | 97 | 55 | 51 | 333 | 395 | 51 | 40 | 456 | 1790 | 2841 | 82 |
| Age, mean (SD) or median (IQR) | 62.2 (54.4–70.5) | 54.7 (49.3–61.2) | 52.8 (43.9–59.7) | 68.0 (60.0–74.0) | 67.0 (58.0–73.0) | 64 (10) | 60 (11) | 65 (10) | 66 (10) | 67.6 (56.6–74.7) | 68.2 (58.4–73.8) | 64.3 (57.6–70.5) | 73 (10) | 70 (12) | 62 (10) |
| Female, no. (%) | 50 (32%) | 6 (5%) | 25 (25%) | 56 (42%) | 16 (17%) | 11 (20%) | 10 (20%) | 113 (34%) | 111 (28%) | 20 (39%) | 8 (20%) | 149 (33%) | 610 (34%) | 1673 (41%) | 23 (28%) |
| Paroxysmal atrial fibrillation, no. (%) | 78 (50%) | 31 (28%) | 61 (61%) | 45 (37%) | 35 (38%) | 23 (42%) | 12 (22%) | 153 (46%) | 143 (36%) | 25 (49%) | 15 (39%) | 331 (74%) | 628 (35%) | 1364 (41%) | 35 (45%) |
| Hypertension, no. (%) | 75 (48%) | 42 (38%) | 29 (29%) | 113 (85%) | 78 (80%) | 33 (60%) | 23 (45%) | 255 (77%) | 282 (71%) | 38 (75%) | 27 (69%) | 243 (53%) | 1500 (84%) | 2438 (86%) | 55 (67%) |
| Diabetes mellitus, no. (%) | 15 (10%) | 7 (6%) | 5 (5%) | 38 (29%) | 19 (20%) | 13 (24%) | 8 (16%) | 36 (11%) | 81 (21%) | 8 (16%) | 3 (8%) | 44 (10%) | 614 (34%) | 1034 (36%) | 7 (9%) |
| Coronary artery disease, no. (%) | 27 (17%) | 14 (13%) | 15 (15%) | NA | NA | 10 (18%) | 8 (44%) | 151 (45%) | 192 (49%) | 19 (37%) | 15 (39%) | 51 (11%) | 992 (55%) | 1168 (41%) | 17 (21%) |
| Prior cerebrovascular accident, no. (%) | 8 (5%) | 7 (6%) | 4 (4%) | 20 (15%) | 13 (13%) | 5 (9%) | 2 (4%) | 24 (7%) | 10 (3%) | 5 (10%) | 4 (10%) | 38 (8%) | 202 (11%) | 358 (13%) | 4 (5%) |
| CHA2DS2‐VASc score, mean (SD) or median (IQR) | NA | NA | NA | 5 (4–6) | 5 (3–6) | NA | NA | 2.5 (1.2) | 3 (1.7) | 3 (2–4) | 3 (1–3) | 2 (1–3) | NA | NA | NA |
| Anti‐arrhythmic drug use, no. (%) | 85 (54%) | 74 (67%) | 61 (61%) | 83 (62%) | 64 (66%) | 24 (44%) | 24 (47%) | 177 (53%) | 145 (37%) | 38 (75%) | 23 (58%) | 322 (71%) | NA | NA | 65 (79%) |
| Beta‐blocker use, no. (%) | 102 (65%) | 89 (80%) | 70 (70%) | 97 (73%) | 83 (86%) | 33 (60%) | 28 (55%) | 240 (72%) | 315 (80%) | 33 (65%) | 22 (55%) | 217 (48%) | NA | NA | 45 (55%) |
| Calcium channel blocker use, no. (%) | 31 (20%) | 25 (23%) | 20 (20%) | NA | NA | 15 (27%) | 10 (20%) | NA | NA | 8 (16%) | 13 (33%) | 124 (27%) | NA | NA | NA |
| LVEF %, mean (SD) or median (IQR) | 62 (60–65) | 35 (30–40) | 63 (60–65) | 55 (55–55) | 35 (30–45) | 57 (8) | 38 (6) | NA | NA | 58 (52–65) | 40 (35–45) | 64 (60–68) | NA | NA | 49 (13) |
Abbreviations: HF, heart failure; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; IQR, inter‐quartile range; LVEF, left ventricular ejection fraction; NA, not applicable as unreported; no., number; SD, standard deviation.
Baseline characteristics of all included patients (n = 82). Baseline characteristics stratified by HFpEF, HFrEF, or no HF were not provided.
Age, prior cerebrovascular accident/transient ischemic attack, hypertension, CHA2DS2VaSc Score, and calcium channel blocker use were similar between the HFpEF and HFrEF groups. Patients with HFpEF that underwent ablation were more likely than to be male (65.9% vs. 48.4%, p < .01), have comorbid coronary artery disease (50.3% vs. 40.6%, p < .01), and use anti‐arrhythmic agents (55.8% vs. 47.6%, p < .01) as compared to those with HFrEF. Those with HFpEF were also less likely to have paroxysmal AF (37.8% vs. 45.3%, p < .01), comorbid diabetes (28.7% vs. 32.6%, p < .01), or use beta blocker therapy (69.3% vs. 77.4%, p < .01).
Table S1 shows the risk of bias assessment. Two studies did not adjust for confounders and therefore had high risk for confounding bias. 14 , 15 There was high risk of selection bias in all the seven studies given the lack of randomization and blinding. We were unable to statistically evaluate publication bias due to the small number of included studies.
3.2. Efficacy and harm outcomes
Tables S2 and S3 summarizes the number and risk ratios of outcome events. When comparing CA of AF in patients with HFpEF versus patients without HF, pooled results of the two studies did not identify any statistical difference in atrial arrythmia recurrence (RR 1.39; 95% CI 0.91–2.13; Figure 1).
FIGURE 1.
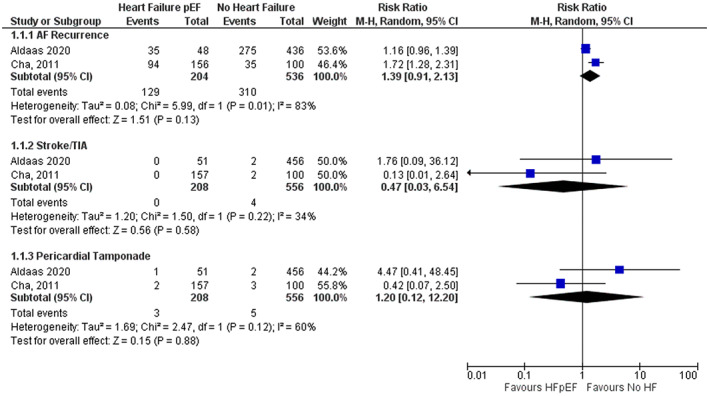
Forest plot for primary and harm outcomes comparing HFpEF versus no HF. The pooled risk ratio with 95% confidence interval were calculated using a random effects model. Weight refers to the contribution of each study to the pooled estimate. Squares and horizontal lines denote the point estimate and 95% confidence interval for each study's risk ratio. The diamond signifies the pooled risk ratio; the diamond center denotes the point estimate, and the width denotes the 95% confidence interval.
When comparing CA of AF in patients with HFpEF versus patients with HFrEF, pooled results of the 7 studies did not identify any statistical difference in atrial arrythmia recurrence 2.6 years after catheter ablation (RR 1.12; 95% CI 0.92–1.37; Figure 2). Similarly, pooled results of the 4 studies did not identify any statistical difference in repeat ablation (RR 1.19; 95% CI 0.74–1.93; Figure 2).
FIGURE 2.
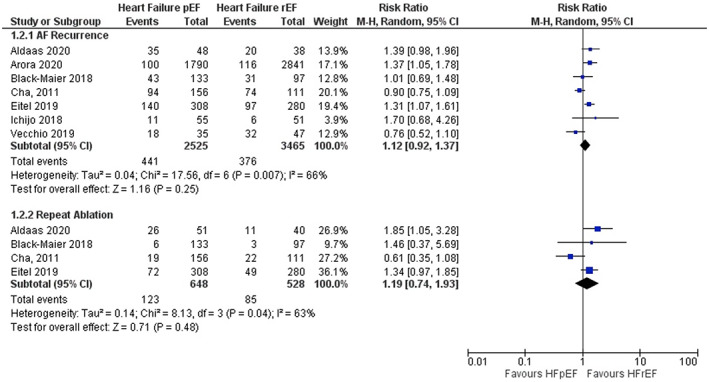
Forest plot for primary outcomes comparing HFpEF and HFrEF. The pooled risk ratio with 95% confidence interval were calculated using a random effects model. Weight refers to the contribution of each study to the pooled estimate. Squares and horizontal lines denote the point estimate and 95% confidence interval for each study's risk ratio. The diamond signifies the pooled risk ratio; the diamond center denotes the point estimate, and the width denotes the 95% confidence interval.
When comparing CA of AF in patients with HFpEF versus patients without HF, pooled results of the two studies did not identify any statistical difference in stroke/TIA (RR 0.47; 95% CI 0.03–6.54; Figure 1) or cardiac tamponade (RR 1.20; 95% CI 0.12–12.20; Figure 1).
When comparing CA of AF in patients with HFpEF versus those with HFrEF, pooled results did not identify any statistical difference in all‐cause mortality (RR 0.87; 95% CI 0.67–1.13), all‐cause hospitalizations (RR 1.10; 95% CI 0.94–1.30), cardiovascular hospitalizations (RR 0.83; 95% CI 0.69–1.01), stroke/TIA (RR 0.81; 95% CI 0.29–2.25), or cardiac tamponade (RR 0.98; 95% CI 0.19–5.16) (Figure 3).
FIGURE 3.
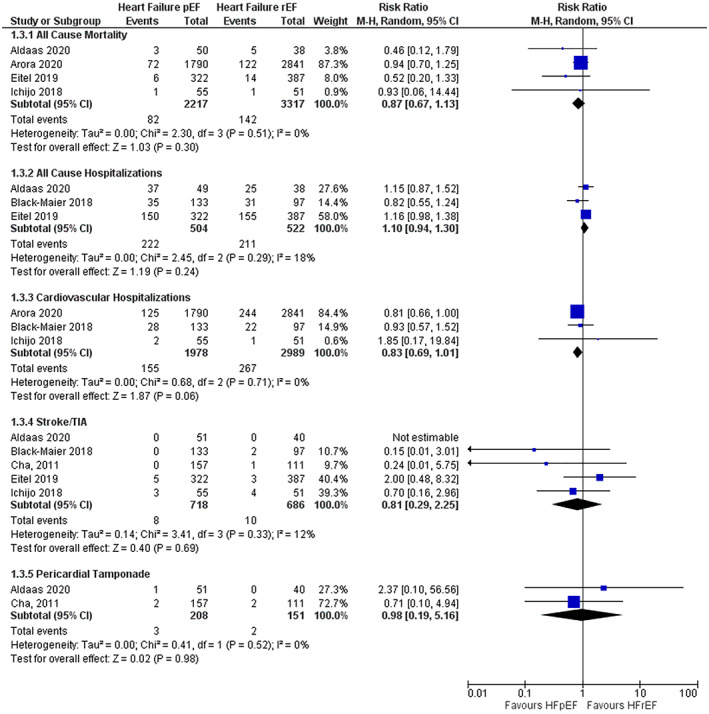
Forest plot for harm outcomes comparing HFpEF and HFrEF. The pooled risk ratio with 95% confidence interval were calculated using a random effects model. Weight refers to the contribution of each study to the pooled estimate. Squares and horizontal lines denote the point estimate and 95% confidence interval for each study's risk ratio. The diamond signifies the pooled risk ratio; the diamond center denotes the point estimate, and the width denotes the 95% confidence interval.
3.3. Sensitivity analysis
The sensitivity analysis of the pooled findings after the exclusion of the unadjusted data from the studies by Ichijo et al. and Eitel et al. when comparing HFpEF with HFrEF showed results consistent with the overall RR of atrial arrythmia recurrence (RR 1.06; 95% CI 0.84–1.34) (Figure S2). 14 , 15 The Chi‐squared test for sub‐group differences was also not significant (p = .15).
3.4. Subgroup analysis
Subgroup analysis based on whether studies were prospective or retrospective was performed to evaluate any difference in the risk of atrial fibrillation recurrence. The pooled results of prospective studies demonstrated no difference in risk of atrial fibrillation recurrence when comparing catheter ablation in HFpEF with HFrEF (RR 0.99; 95% CI 0.72–1.35). In contrast, the result was statistically significant in favor of HFrEF when the data was pooled for retrospective studies (RR 1.29; 95% CI 1.08–1.55) (Figure 4). The Chi‐squared test for sub‐group differences was not significant (p = .14).
FIGURE 4.
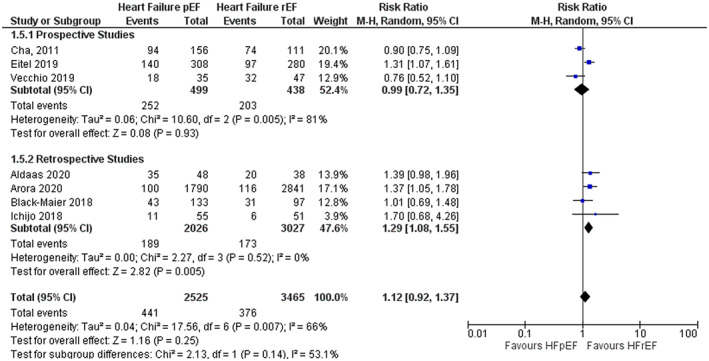
Subgroup analysis based on study type for risk of atrial fibrillation recurrence. The pooled risk ratio with 95% confidence interval were calculated using a random effects model. Weight refers to the contribution of each study to the pooled estimate. Squares and horizontal lines denote the point estimate and 95% confidence interval for each study's risk ratio. The diamond signifies the pooled risk ratio; the diamond center denotes the point estimate, and the width denotes the 95% confidence interval.
3.5. Certainty in the estimates
The included studies were observational with variable methodological quality with increased risk of selection and confounding bias. The estimates were precise for atrial arrythmia recurrence, repeat ablation, all‐cause mortality, all‐cause hospitalizations, and cardiovascular hospitalizations (large number of events). However, stroke/TIA and cardiac tamponade analyses had less than 100 events. There was no indirectness or evidence of publication bias. Heterogeneity was noted among the included studies. The quantified I2 value for each individual outcome investigated for HFpEF versus HFrEF are as follows: atrial arrythmia recurrence 66% (moderate), repeat ablation 63% (moderate), all‐cause mortality 0% (none), all‐cause hospitalizations 18% (low), cardiovascular hospitalizations 0% (none), stroke/TIA 12% (low) and tamponade 0% (none). Overall, the certainty in the estimates in all the outcomes was judged to be low.
4. DISCUSSION
This meta‐analysis demonstrated no difference in atrial arrhythmia recurrence, repeat ablation, stroke/TIA, cardiac tamponade, cardiovascular hospitalizations, all‐cause hospitalizations, or all‐cause mortality in patients with AF and HFpEF undergoing CA versus those with AF and HFrEF. Additionally, when comparing AF and HFpEF to those without HF, there was no difference in atrial arrhythmia recurrence, stroke/TIA, or cardiac tamponade.
Improvements in CA for AF is occurring at a rapid pace, with the introduction of contact force measurements, automated lesion assessment, and next‐generation catheters seen in recent years. Evidence for CA of AF in HFrEF is more available, with updated guidelines supporting it in selected patients. 4 , 12 Until recently, data on HFpEF patients have been limited to few retrospective and prospective studies. A separate meta‐analysis comparing the utility of CA in patients with AF and HFpEF versus HFrEF found no significant differences in the recurrence of AF after 1 year, procedure time, peri‐procedural adverse events, or hospitalizations. 26 However, the study found that HFpEF patients had significantly less mortality over follow‐up. In contrast, we included the work by Arora et al. 22 in our analysis of mortality, and pooled findings detected no difference when comparing HFpEF versus HFrEF. We also performed a sensitivity analysis by excluding unadjusted data, calculated certainty in the estimates, and classified peri‐procedural complications. Regardless, both meta‐analyses demonstrate that patients with AF and HFpEF undergoing CA have similar outcomes as those with AF and HFrEF. Ultimately, this should encourage randomized controlled trials (RCTs) to confirm the benefit of CA in this patient population, as they have not been included in the most current guidelines.
The benefit derived from CA depends on multiple factors, most notably New York Heart Association Functional Classification (NYHA), ventricular scar burden, degree of atrial fibrosis, duration of AF, age, and comorbid conditions. 27 CASTLE‐AF was an RCT comparing CA versus medical therapy for AF in addition to guideline‐based therapy for HFrEF. 6 Importantly, sub‐group analysis showed that patients with NYHA functional class II were more likely to have benefit from CA compared to NYHA class III. Similarly, AMICA and CAMERA‐MRI trials identified greater benefit of CA in patient with mild HFrEF compared to those with severe HFrEF. In contrast, data on whether CA in HFpEF is more beneficial in a subset of patients is lacking. 28 , 29 In the study by Ichijo et al., NYHA functional class improved immediately post‐ablation. 15 However, there was no difference in NYHA functional class improvement after 1 year in the study by Black‐Maier et al. 23 It remains to be elucidated whether patients with HFpEF who are the most symptomatic would benefit from CA at all. Future trials should differentiate the utility of CA based on differing NYHA class, comorbidities, and degree of atrial and ventricular remodeling in patients with HFpEF.
The interaction between AF and HFpEF is important. Prevalent and incident AF are associated with increased mortality in HFpEF. 30 Conversely, the presence of HF worsens the prognosis in those with AF. 31 However, the interaction between HFpEF and AF is complex, and not all studies are able to delineate causation between HFpEF or AF. These conditions often co‐exist and perpetuate the other. Patients with AF and HFpEF share common risk factors and co‐morbidities. 32 , 33 , 34 Those with HFpEF have impaired contractile reserve and left atrial (LA) enlargement, which is a well‐established pro‐arrhythmic substrate associated with atrial fibrosis. 35 The most commonly recognized mechanism by which HFpEF leads to AF is through the structural and functional remodeling of the LA. Nonetheless, because AF itself leads to LA dilation and atrial cardiomyopathy, it can be a direct cause of HFpEF. 36 Additionally, systemic inflammation may link HFpEF and AF. It has been proposed that HFpEF may be an inflammatory disorder where its co‐morbidities trigger endothelial dysfunction and oxidative stress, leading to end‐organ damage, which includes diastolic dysfunction. 37 Indeed, histological findings in atrial biopsies have demonstrated pro‐inflammatory changes of HFpEF as a major contributor to AF occurrence and maintenance. 38
4.1. Limitations
This meta‐analysis has limitations primarily due to limitations in the studies that were included. There was heterogeneity in the CA techniques used in the studies included. Arora et al. did not specify the specific CA technique utilized, 22 Eitel et al. included cryoablation, radiofrequency, and atrioventricular nodal ablation, 14 while the remaining studies all utilized radiofrequency ablation. Different CA techniques may not be completely comparable with each other. The EF cut‐off used to stratify patients into HFpEF or HFrEF categories also differed between studies. Ichijo et al. used a cut‐off of 45%, while Arora et al. did not specify an EF cut‐off. 15 , 22 The rest of the studies used a cut‐off of 50%. Methods utilized to detect arrythmia recurrence differed among studies as well, but all followed consensus guidelines. 39 Additionally, all studies included were observational in design and lacked randomization, which increases the possibility of selection bias and confounding.
5. CONCLUSION
Current guidelines recommend that CA may be reasonable in symptomatic patients with AF and HFrEF. The evidence in patients with HFpEF is less clear. This meta‐analysis demonstrates no difference in atrial arrhythmia recurrence, repeat ablation, or harm outcomes in patients with AF and HFpEF undergoing CA versus those with HFrEF. Ultimately, this suggests that patients with AF and HFpEF may benefit equally as those with AF and HFrEF. Future large RCTs can confirm the utility of CA in this patient population.
AUTHOR CONTRIBUTIONS
Design: MS, JJ. Data collection: MS, JJ, JR, AA, AP, and KL. Manuscript: MS. Supervision: MS. Analysis: JJ, JR, AA, and AP. Manuscript: JJ, JR, AA, AP, KL, RA, and DF. Supervision: RA and DF.
FUNDING INFORMATION
The authors have no sources of funding for this research to declare.
CONFLICT OF INTEREST
The authors declare that they have no competing interests. The results presented in this paper have not been published previously in whole or part, except in abstract form.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was a meta‐analysis that did not require approval from our institutional review board. This article does not contain any studies with animals performed by any of the authors.
CONSENT FOR PUBLICATION
Not applicable.
CODE AVAILABILITY
Not applicable.
Supporting information
Figure S1
Figure S2
Table S1–S3
ACKNOWLEDGMENTS
Not applicable.
Siddiqui MU, Junarta J, Riley JM, Ahmed A, Pasha AK, Limbrick K, et al. Catheter ablation in patients with atrial fibrillation and heart failure with preserved ejection fraction: A systematic review and meta‐analysis. J Arrhythmia. 2022;38:981–990. 10.1002/joa3.12794
DATA AVAILABILITY STATEMENT
Data are safely kept in a password protected security system at Thomas Jefferson University Hospital. The datasets used and/or analysed during the current study are de‐identified and available from the corresponding author on reasonable request.
REFERENCES
- 1. Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, et al. Worldwide epidemiology of atrial fibrillation: a global burden of disease 2010 study. Circulation. 2014;129(8):837–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kannel WB, Wolf PA, Benjamin EJ, Levy D. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population‐based estimates. Am J Cardiol. 1998;82(8A):2 N–9 N. [DOI] [PubMed] [Google Scholar]
- 3. Wang TJ, Larson MG, Levy D, Vasan RS, Leip EP, Wolf PA, et al. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham heart study. Circulation. 2003;107(23):2920–5. [DOI] [PubMed] [Google Scholar]
- 4. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomstrom‐Lundqvist C, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio‐Thoracic Surgery (EACTS). Eur Heart J. 2021;42(5):373–498. [DOI] [PubMed] [Google Scholar]
- 5. Mamas MA, Caldwell JC, Chacko S, Garratt CJ, Fath‐Ordoubadi F, Neyses L. A meta‐analysis of the prognostic significance of atrial fibrillation in chronic heart failure. Eur J Heart Fail. 2009;11(7):676–83. [DOI] [PubMed] [Google Scholar]
- 6. Marrouche NF, Brachmann J, Andresen D, Siebels J, Boersma L, Jordaens L, et al. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med. 2018;378(5):417–27. [DOI] [PubMed] [Google Scholar]
- 7. Wazni OM, Dandamudi G, Sood N, Hoyt R, Tyler J, Durrani S, et al. Cryoballoon ablation as initial therapy for atrial fibrillation. N Engl J Med. 2021;384(4):316–24. [DOI] [PubMed] [Google Scholar]
- 8. Andrade JG, Wells GA, Deyell MW, Bennett M, Essebag V, Champagne J, et al. Cryoablation or drug therapy for initial treatment of atrial fibrillation. N Engl J Med. 2021;384(4):305–15. [DOI] [PubMed] [Google Scholar]
- 9. Kuniss M, Pavlovic N, Velagic V, Hermida JS, Healey S, Arena G, et al. Cryoballoon ablation vs. antiarrhythmic drugs: first‐line therapy for patients with paroxysmal atrial fibrillation. Europace. 2021;23:1033–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Di Biase L, Mohanty P, Mohanty S, Santangeli P, Trivedi C, Lakkireddy D, et al. Ablation versus amiodarone for treatment of persistent atrial fibrillation in patients with congestive heart failure and an implanted device: results from the AATAC multicenter randomized trial. Circulation. 2016;133(17):1637–44. [DOI] [PubMed] [Google Scholar]
- 11. Packer DL, Piccini JP, Monahan KH, Al‐Khalidi HR, Silverstein AP, Noseworthy PA, et al. Ablation versus drug therapy for atrial fibrillation in heart failure: results from the CABANA trial. Circulation. 2021;143(14):1377–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC Jr, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the Management of Patients with Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the Heart Rhythm Society in collaboration with the Society of Thoracic Surgeons. Circulation. 2019;140(2):e125–e51. [DOI] [PubMed] [Google Scholar]
- 13. Aldaas OM, Malladi CL, Mylavarapu PS, Lupercio F, Darden D, Han FT, et al. Comparison of outcomes after ablation of atrial fibrillation in patients with heart failure with preserved versus reduced ejection fraction. Am J Cardiol. 2020;136:62–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Eitel C, Ince H, Brachmann J, Kuck KH, Willems S, Gerds‐Li JH, et al. Atrial fibrillation ablation strategies and outcome in patients with heart failure: insights from the German ablation registry. Clin Res Cardiol. 2019;108(7):815–23. [DOI] [PubMed] [Google Scholar]
- 15. Ichijo S, Miyazaki S, Kusa S, Nakamura H, Hachiya H, Kajiyama T, et al. Impact of catheter ablation of atrial fibrillation on long‐term clinical outcomes in patients with heart failure. J Cardiol. 2018;72(3):240–6. [DOI] [PubMed] [Google Scholar]
- 16. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–9, w64. [DOI] [PubMed] [Google Scholar]
- 17. Stang A. Critical evaluation of the Newcastle‐Ottawa scale for the assessment of the quality of nonrandomized studies in meta‐analyses. Eur J Epidemiol. 2010;25(9):603–5. [DOI] [PubMed] [Google Scholar]
- 18. Viswanathan M, Patnode CD, Berkman ND, Bass EB, Chang S, Hartling L, et al. Recommendations for assessing the risk of bias in systematic reviews of health‐care interventions. J Clin Epidemiol. 2018;97:26–34. [DOI] [PubMed] [Google Scholar]
- 19. Murad MHMV, Ioannidis JPA, Prasad K, Cook DJ, Guyatt G. Advanced topics in systematic reviews: fixed‐effects and random‐effects models. McGraw Hill: The JAMA Network; 2014. [Google Scholar]
- 20. Turner RM, Davey J, Clarke MJ, Thompson SG, Higgins JP. Predicting the extent of heterogeneity in meta‐analysis, using empirical data from the Cochrane database of systematic reviews. Int J Epidemiol. 2012;41(3):818–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Murad MH, Mustafa RA, Schünemann HJ, Sultan S, Santesso N. Rating the certainty in evidence in the absence of a single estimate of effect. Evid Based Med. 2017;22(3):85–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Arora S, Jaswaney R, Jani C, Zuzek Z, Thakkar S, Patel HP, et al. Catheter ablation for atrial fibrillation in patients with concurrent heart failure. Am J Cardiol. 2020;137:45–54. [DOI] [PubMed] [Google Scholar]
- 23. Black‐Maier E, Ren X, Steinberg BA, Green CL, Barnett AS, Rosa NS, et al. Catheter ablation of atrial fibrillation in patients with heart failure and preserved ejection fraction. Heart Rhythm. 2018;15(5):651–7. [DOI] [PubMed] [Google Scholar]
- 24. Cha YM, Wokhlu A, Asirvatham SJ, Shen WK, Friedman PA, Munger TM, et al. Success of ablation for atrial fibrillation in isolated left ventricular diastolic dysfunction: a comparison to systolic dysfunction and normal ventricular function. Circ Arrhythm Electrophysiol. 2011;4(5):724–32. [DOI] [PubMed] [Google Scholar]
- 25. Vecchio N, Ripa L, Orosco A, Tomas L, Mondragón I, Acosta A, et al. Atrial fibrillation in heart failure patients with preserved or reduced ejection fraction. Prognostic significance of rhythm control strategy with catheter ablation. J Atr Fibrillation. 2019;11(5):2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Aldaas OM, Lupercio F, Darden D, Mylavarapu PS, Malladi CL, Han FT, et al. Meta‐analysis of the usefulness of catheter ablation of atrial fibrillation in patients with heart failure with preserved ejection fraction. Am J Cardiol. 2021;142:66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gopinathannair R, Chen LY, Chung MK, Cornwell WK, Furie KL, Lakkireddy DR, et al. Managing atrial fibrillation in patients with heart failure and reduced ejection fraction: a scientific statement from the American Heart Association. Circ Arrhythm Electrophysiol. 2021;14(6):Hae0000000000000078. [DOI] [PubMed] [Google Scholar]
- 28. Kuck KH, Merkely B, Zahn R, Arentz T, Seidl K, Schlüter M, et al. Catheter ablation versus best medical therapy in patients with persistent atrial fibrillation and congestive heart failure: the randomized AMICA trial. Circ Arrhythm Electrophysiol. 2019;12(12):e007731. [DOI] [PubMed] [Google Scholar]
- 29. Prabhu S, Taylor AJ, Costello BT, Kaye DM, McLellan AJA, Voskoboinik A, et al. Catheter ablation versus medical rate control in atrial fibrillation and systolic dysfunction: the CAMERA‐MRI study. J Am Coll Cardiol. 2017;70(16):1949–61. [DOI] [PubMed] [Google Scholar]
- 30. Zakeri R, Chamberlain AM, Roger VL, Redfield MM. Temporal relationship and prognostic significance of atrial fibrillation in heart failure patients with preserved ejection fraction: a community‐based study. Circulation. 2013;128(10):1085–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Santhanakrishnan R, Wang N, Larson MG, Magnani JW, McManus DD, Lubitz SA, et al. Atrial fibrillation begets heart failure and vice versa: temporal associations and differences in preserved versus reduced ejection fraction. Circulation. 2016;133(5):484–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vermond RA, Geelhoed B, Verweij N, Tieleman RG, Van der Harst P, Hillege HL, et al. Incidence of atrial fibrillation and relationship with cardiovascular events, heart failure, and mortality: a community‐based study from the Netherlands. J Am Coll Cardiol. 2015;66(9):1000–7. [DOI] [PubMed] [Google Scholar]
- 33. Mentz RJ, Kelly JP, von Lueder TG, Voors AA, Lam CS, Cowie MR, et al. Noncardiac comorbidities in heart failure with reduced versus preserved ejection fraction. J Am Coll Cardiol. 2014;64(21):2281–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37(38):2893–962. [DOI] [PubMed] [Google Scholar]
- 35. Knackstedt C, Gramley F, Schimpf T, Mischke K, Zarse M, Plisiene J, et al. Association of echocardiographic atrial size and atrial fibrosis in a sequential model of congestive heart failure and atrial fibrillation. Cardiovasc Pathol. 2008;17(5):318–24. [DOI] [PubMed] [Google Scholar]
- 36. Manning WJ, Silverman DI, Katz SE, Riley MF, Come PC, Doherty RM, et al. Impaired left atrial mechanical function after cardioversion: relation to the duration of atrial fibrillation. J Am Coll Cardiol. 1994;23(7):1535–40. [DOI] [PubMed] [Google Scholar]
- 37. Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62(4):263–71. [DOI] [PubMed] [Google Scholar]
- 38. Frustaci A, Chimenti C, Bellocci F, Morgante E, Russo MA, Maseri A. Histological substrate of atrial biopsies in patients with lone atrial fibrillation. Circulation. 1997;96(4):1180–4. [DOI] [PubMed] [Google Scholar]
- 39. January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC Jr, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the Heart Rhythm Society. Heart Rhythm. 2019;16(8):e66–93. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Figure S2
Table S1–S3
Data Availability Statement
Data are safely kept in a password protected security system at Thomas Jefferson University Hospital. The datasets used and/or analysed during the current study are de‐identified and available from the corresponding author on reasonable request.


