Abstract
Ophthalmoparesis and ptosis can be caused by a wide range of rare or more prevalent diseases, several of which can be successfully treated. In this review, we provide clues to aid in the diagnosis of these diseases, based on the clinical symptoms, the involvement pattern and imaging features of extra‐ocular muscles (EOM). Dysfunction of EOM including the levator palpebrae can be due to muscle weakness, anatomical restrictions or pathology affecting the innervation. A comprehensive literature review was performed to find clinical and imaging clues for the diagnosis and follow‐up of ptosis and ophthalmoparesis. We used five patterns as a framework for differential diagnostic reasoning and for pattern recognition in symptomatology, EOM involvement and imaging results of individual patients. The five patterns were characterized by the presence of combination of ptosis, ophthalmoparesis, diplopia, pain, proptosis, nystagmus, extra‐orbital symptoms, symmetry or fluctuations in symptoms. Each pattern was linked to anatomical locations and either hereditary or acquired diseases. Hereditary muscle diseases often lead to ophthalmoparesis without diplopia as a predominant feature, while in acquired eye muscle diseases ophthalmoparesis is often asymmetrical and can be accompanied by proptosis and pain. Fluctuation is a hallmark of an acquired synaptic disease like myasthenia gravis. Nystagmus is indicative of a central nervous system lesion. Second, specific EOM involvement patterns can also provide valuable diagnostic clues. In hereditary muscle diseases like chronic progressive external ophthalmoplegia (CPEO) and oculo‐pharyngeal muscular dystrophy (OPMD) the superior rectus is often involved. In neuropathic disease, the pattern of involvement of the EOM can be linked to specific cranial nerves. In myasthenia gravis this pattern is variable within patients over time. Lastly, orbital imaging can aid in the diagnosis. Fat replacement of the EOM is commonly observed in hereditary myopathic diseases, such as CPEO. In contrast, inflammation and volume increases are often observed in acquired muscle diseases such as Graves' orbitopathy. In diseases with ophthalmoparesis and ptosis specific patterns of clinical symptoms, the EOM involvement pattern and orbital imaging provide valuable information for diagnosis and could prove valuable in the follow‐up of disease progression and the understanding of disease pathophysiology.
Keywords: Ophthalmoparesis, Ptosis, Extra‐ocular muscles, Imaging, Neuromuscular disease, Involvement pattern
1. Introduction
Ophthalmoparesis is dysfunction of the extra‐ocular muscles (EOM), usually caused by muscle weakness, anatomical restrictions or pathology affecting their innervation. Three pairs of antagonizing EOMs move the eye in all directions: horizontally (medial rectus [MR] and lateral rectus [LR]) and vertically (superior rectus [SR], inferior rectus [IR], superior oblique [SO] and inferior oblique [IO]). Ptosis refers to the drooping of the upper eyelid and can be a disabling or disfiguring symptom. Ptosis is often caused by weakness of the levator palpebrae superioris (LPS) muscle. which is responsible for elevating the upper eyelid, with help from the superior tarsal muscle (Muller's muscle). Eye lid retraction, often accompanied by bulging of the eye called proptosis, is generally caused by an increased orbital volume. In this review, we aimed to explore whether the symptomatology, the involvement pattern of EOM, and imaging can aid in diagnosis, follow‐up and understanding of diseases with ophthalmoparesis and ptosis.
Causes of ophthalmoparesis and ptosis can be broadly divided into diseases affecting four anatomical locations: brain, nerve, synapse or muscle. These diseases can be either acquired or hereditary. Examples of brain diseases that present with ophthalmoparesis are Wernicke's encephalopathy and progressive supranuclear palsy. Nerve dysfunction, as seen in congenital fibrosis of the extra‐ocular muscles (CFEOM) 1 or acquired nerve disorders, like Miller–Fisher syndrome 2 or Tolosa–Hunt syndrome, 3 can cause ophthalmoparesis and ptosis following the innervation pattern of the affected cranial nerves. The group of synaptic diseases include congenital myasthenic syndromes 4 and acquired synaptic disease such as myasthenia gravis, which is caused by auto‐antibodies against neuromuscular junction‐proteins. 5 Finally, the group of muscle diseases comprises disorders that directly affect the EOM. In acquired muscle disease, ophthalmoparesis and ptosis are often due to inflammation or enlargement of the EOM, orbital fat or other orbital structures. An example of acquired muscle disease is Graves' orbitopathy, in which thyroid stimulating hormone receptor‐antibodies cause orbital inflammation. 6 Examples of hereditary muscle diseases that present with ophthalmoparesis are chronic progressive external ophthalmoplegia (CPEO), 7 caused by mitochondrial dysfunction and oculo‐pharyngeal muscular dystrophy (OPMD), with pharyngeal and ocular muscle weakness caused by a mutation in the PABPN1 gene. 8
To facilitate diagnostic reasoning, we used five main clinical patterns of symptoms that correspond to specific anatomical locations and disease characteristics pointing towards a hereditary or acquired cause of the disease (Figure 1 ). We used these five patterns as a starting point for pattern recognition in symptomatology, EOM involvement and imaging results of individual patients. They do not provide a stringent classification because the patterns are not completely mutually exclusive and exceptions do occur. Per pattern, we describe the severity of ptosis and ophthalmoparesis, the presence of diplopia, the symmetry, the presence of fluctuations and accompanying symptoms like pain and CNS symptoms. Per disease, we describe the involvement pattern of individual EOM. Lastly, for imaging, we describe identification of causes of ophthalmoparesis and ptosis by identifying primary tumours, metastasis, infection or inflammation and changes due to dysinnervation. In addition, we describe how imaging can identify the involved EOM in specific diseases and help understand the pathophysiology of ophthalmoparesis and ptosis in specific diseases. Disorders of brain and nerve and orbital diseases causing secondary muscle dysfunction (e.g., tumours and infections) are beyond the scope of this paper, but are for a large part reviewed in a recent paper. 9
Figure 1.
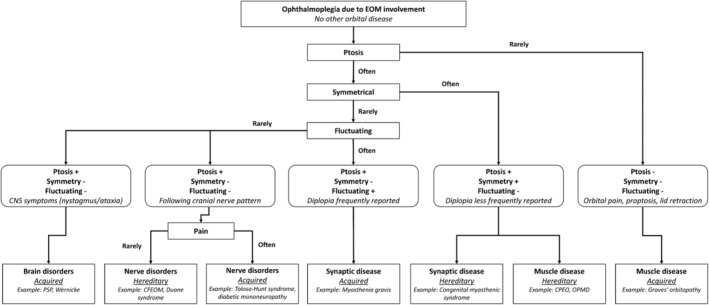
Five patterns characterized by the presence of combination of ptosis, ophthalmoparesis, diplopia, pain, proptosis, nystagmus, extra‐orbital symptoms, symmetry or fluctuations in symptoms. Each pattern was linked to anatomical locations and either hereditary or acquired diseases, as a starting point for clinical evaluation rather than providing a stringent, mutually exclusive classification.
2. Five patterns of clinical presentation of ophthalmoparesis and ptosis
Diseases with ophthalmoparesis or ptosis can be classified using different patterns of signs and symptoms (Figure 1 and Table 1 ). Diplopia is a patient‐reported symptom, while ophthalmoparesis can be assessed with physical examination and quantified using orthoptic measures like ductions as measured with a synoptophore, Hess chart or with the Goldmann perimeter. 53 We defined ptosis as a decreased distance between the borders of the eyelids due to drooping of the upper eyelid. Several reports provide criteria for ptosis using defined physical landmarks like the border of the eyelids and express their distance in millimetres. 9 This implies that the upper eyelid is lower than its normal anatomical position, typically 1–2 mm below the superior corneoscleral limbus. 54 Similarly, proptosis on magnetic resonance imaging (MRI) or computed tomography (CT) is defined as a distance >23 mm from the anterior surface of the globe to the interzygomatic line 55 or an asymmetry >2 mm as measured with an exopthalmometer. 56 These strict definitions are not consistently used in the literature that we collected on the many different diseases. Therefore, we accepted the definition that was used in the selected papers.
Table 1.
Differential diagnosis ophthalmoparesis and/or ptosis.
| Hereditary disorders | Ptosis | Ophthalmoparesis | Diplopia | Asymmetry | Pain | Fluctuating | Most frequently involved EOM* | Other symptoms |
|---|---|---|---|---|---|---|---|---|
| Nerve | ||||||||
| CFEOM 1 | Yes | Yes | No | No | No | No | LPS, SR (depends on affected nerves) 1 , 10 , 11 , 12 | No |
| Duane syndrome 9 , 13 | Yes | Yes | No | No | No | No | LPS, LR, MR (aberrant innervation) | No |
| Blepharophimosis syndrome (BPES) 9 , 13 | Yes | No | No | No | No | No | LPS | Horizontal narrowing of the eyelids, epicanthus inversus, lacrimal duct abnormalities |
| Marcus‐Gunn syndrome 9 , 13 | Yes | No | No | No | No | No | LPS | Upper eye lid retraction when chewing or laughing. |
| Synapse | ||||||||
|
Presynaptic congenital myasthenic syndromes 14 : Congenital Lambert–Eaton‐like, choline acetyltransferase deficiency, reduced quantal release, paucity of synaptic vesicles and reduced quantal release |
Yes | Rare | No | No | No | No | Other muscles | |
| Synaptic congenital myasthenic syndromes 14 : Endplate AChE deficiency, CMS with LAMB2 mutation | Yes | Yes | No | No | No | No | LPS, LR, SR, IO 4 | Other muscles |
| Post‐synaptic congenital myasthenic syndromes 14 : Slow channel syndrome, AChR deficiency, fast channel syndrome, Rapsyn deficiency, plectin deficiency, Dok‐7 myasthenia | Yes | Yes | Rare | No | No | No | LPS, LR, SR, IO 4 | Other muscles |
| Muscle | ||||||||
| Progressive external ophthalmoplegia 7 | Yes | Yes | Half of patients 15 | No | No | No | LPS, SR 7 | Other muscles and organs (heart) |
| Pompe disease 16 | Yes | No | No | Yes | No | No | LPS 16 | Other muscles |
| OPMD 8 | Yes | Yes | Rare 17 | No | No | No | LPS, SR, LR 8 | Pharyngeal and leg muscles |
| Myotonic dystrophy Type 1 18 , 19 | Yes | Rare 20 , 21 | No | No | No | No | LPS (cases of LR and MR). 21 | Other muscles |
| Centronuclear myopathy 22 , 23 | Yes | Yes | No | No | No | No | LPS, SR, LR 22 , 23 | Other muscles |
| Acquired disorders | Ptosis | Ophthalmoparesis | Diplopia | Asymmetry | Pain | Fluctuating | Most frequently involved EOM* | Other symptoms |
|---|---|---|---|---|---|---|---|---|
| Brain | ||||||||
| Progressive supranuclear palsy | No | Yes | No | No | No | No | SR, IO, IR, SO 24 | Parkinsonism, balance, dementia, bulbar symptoms |
| Internuclear ophthalmoparesis (MS/stroke) | No | Yes | Yes | No | No | No | MR | Other CNS symptoms and nystagmus |
| Wernicke encephalopathy | Rare | Yes | Yes | No | No | No | LR 25 | Encephalopathy and ataxia. Predominantly nystagmus. |
| Brain stem tumour 26 | Yes | Yes | Yes | Yes | No | No | Location dependent. | Other cranial nerves and lateralized CNS symptoms |
| Nerve | ||||||||
| Miller–Fisher syndrome 27 | Yes | Yes | Yes | No | No | No | LR > LPS, SR, IR, MR 27 | Vestibular and facial |
| Recurrent painful ophthalmoplegic neuropathy | Yes | Yes | Yes | Yes | Yes | No | LPS, SR, IR, MR, IO (N. III) 28 | Attacks of headache |
| Horner syndrome | Yes | No | No | Yes | No | No | No EOM (superior tarsal muscle) 29 | Anhidrosis and myosis |
| Tolosa–Hunt syndrome 30 | Yes | Yes | Yes | Yes | Yes | No | LPS, SR, IR, MR, IO (N. III) orLR (N. VI) 3 | No |
| Diabetic mononeuropathy 31 | Rare | Yes | Yes | Yes | No | No |
LPS, SR, IR, MR, IO (N. III) or LR (N. VI) 31 |
Transient palsy. Presence of diabetes. |
| Synapse | ||||||||
| Autoimmune LEMS 14 , 32 , 33 | Yes | Rare 34 | Yes | Yes | No | Yes | LPS 32 | Other muscles and autonomic |
| Autoimmune myasthenia gravis 14 | Yes | Yes | Yes | Yes | No | Yes | LPS, IO, SR > LR, MR 35 , 36 , 37 | Other muscles (bulbar, neck) |
| Botulism 38 , 39 | Yes | Yes | Yes | No | No | No | LPS 39 | Other muscles |
| Acetylcholinesterase intoxication 40 | Yes | Yes | Yes | No | No | No | Unknown | Other muscles |
| Muscle | ||||||||
| Orbital lymphoma | Rare | Yes | Yes | Yes | Rare | No | Location dependent. 41 | Depends on localization |
| Idiopathic Orbital myositis 42 | Rare | Yes | Yes | Yes | Yes | No | LR, SR, MR, IR 43 , 44 | Chemosis, Proptosis. Involvement of lacrimal gland and orbital fat. |
| IgG4‐related disease of the orbit 45 , 46 | Rare | Yes | Yes | Yes | Yes | No | LR 47 > IR, SR 43 , 48 | Proptosis. Involvement of lacrimal gland, orbital fat and nerves. |
| Thyroid orbitopathy/Graves' disease 49 , 50 | No | Yes | Yes | Yes | Yes | No | IR, MR, SR 43 , 44 | Eyelid retraction, proptosis and thyroid involvement. |
| Systemic auto‐inflammatory diseases 45 , 51 | No | Rare | Rare | Yes | Yes | No | ‐ | Involvement of other organs |
| Rare presentation of amyloidosis 52 | No | Rare | Rare | Yes | Rare | No | LR, MR 52 | Other organs |
Note: Symptomatology is described as the presence of ptosis, ophthalmoparesis, diplopia, asymmetrical symptoms, pain, fluctuating symptoms and the presence of other non‐ocular symptoms. The frequently involved extra‐ocular muscle (EOM) are mentioned for each disease (* a more specific description of the EOM involvement pattern can be found in Table S1 ). The neuromuscular diseases are categorized in acquired and hereditary, and then clustered by the localization of the pathology.
Abbreviations: IO, inferior oblique muscle; IR, inferior rectus muscle; LPS, levator palpebrae superior; LR, lateral rectus muscle; MR, medical rectus muscle; OPMD, oculo‐pharyngeal muscular dystrophy; SO, superior oblique muscle; SR, superior rectus muscle.
Diplopia is less frequently seen in hereditary neuromuscular disease with ophthalmoparesis (e.g. in at least half of CPEO patients 15 ), probably due to its slow progression, symmetrical involvement and facultative suppression. 7 Ptosis generally occurs symmetrically in these disorders. The co‐occurrence of ptosis and ophthalmoparesis is commonly seen in hereditary neuromuscular disease and in synaptic disease, whereas ptosis is rarely present in acquired myopathic disease, commonly associated with pain, proptosis and/or swelling of eyelids and conjunctiva due to EOM enlargement and inflammation.
In the first pattern, constant ophthalmoparesis with diplopia is present with or without ptosis, and accompanied by central nervous system (CNS)‐abnormalities such as upper gaze paralysis, nystagmus or other neurological extra‐ocular manifestations. This pattern is characteristic of acquired brain disease, in which diplopia and ophthalmoparesis co‐occur with evident CNS abnormalities and the presence of nystagmus. The presence of nystagmus is evident in internuclear ophthalmoparesis caused by multiple sclerosis (MS) or stroke: when one eye adducts, nystagmus of the contralateral eye is observed. Examples of CNS abnormalities accompanying the ocular symptoms are parkinsonism, dementia and swallowing problems (or dysphagia) in progressive supranuclear palsy and the triad of ataxia, ocular symptoms and an altered mental state in Wernicke encephalopathy. Moreover, nystagmus is also commonly present in Wernicke encephalopathy.
The second pattern consists of constant ophthalmoplegia or ptosis, and is often asymmetrical or painful. This pattern points towards nerve disorders, either acquired or hereditary. A patient with this pattern typically has constant painless ophthalmoplegia or ptosis that can be attributed to the innervation pattern of a specific cranial nerve. For example in CFEOM, the muscles that lack cranial nerve innervation become fibrotic. In CFEOM type I there is agenesis of the superior division of N. III 1 , 10 and in CFEOM type II, there is agenesis of the entire N III and N IV. 1 , 11 In other congenital ptosis syndromes, atrophy of the EOM are often secondary to abnormal innervation and development: in Duane syndrome the N. VI is absent and the LR is innervated by a branch of the N. III, Marcus‐Gunn syndrome is caused by an anomalous connection of motor fibres from the N. V to the n III and blepharophimosis syndrome (BPES) with narrowing of the eyelids. 9 , 13 In acquired nerve diseases, the symptomatology is often asymmetrical because the nerve is affected unilaterally, for example, in Horner syndrome, where only the superior tarsal muscle is affected, or diabetic mononeuropathy (often N. III 31 ). If the cause is within the cavernous sinus (e.g. Tolosa–Hunt syndrome), multiple ipsilateral cranial nerves can be affected (N. III in 80% of cases, N. VI in 70% of cases and N. IV in 30% of cases 3 ). An exception to this pattern is Miller–Fisher syndrome, which often has a symmetrical bilateral involvement of several cranial nerves.
In the third pattern, patients present with fluctuating ophthalmoparesis with diplopia and asymmetrical ptosis, which is indicative of an acquired synaptic disease like myasthenia gravis. 57 In this pattern, muscle weakness fluctuates over larger periods of time and within one day, with symptoms becoming worse during the day and improving after a period of rest. In myasthenia gravis, the EOM are involved in about 80% of patients, but other muscles are often involved like the bulbar, neck and limb muscles. 58
The fourth pattern is characterized by constant ophthalmoparesis with diplopia, but ptosis is not usual. These symptoms are most commonly asymmetrical and painful, but again without clear fluctuation. This is typical for acquired muscle disease. This pattern most often points to inflammatory disease: Graves' orbitopathy, IgG‐4 related orbital disease (IgG4‐ROD), idiopathic orbital inflammation and idiopathic orbital myositis. 42 , 45 , 46 , 59 , 60 Patients experience asymmetrical retrobulbar pain with accompanying ophthalmoparesis and sometimes proptosis. The differential diagnosis of painful ophthalmoparesis included the inflammatory diseases mentioned, but is very wide. It also includes vascular diseases such as aneurysms and dissections, neoplastic diseases such as lymphoma and metastases, infectious diseases including tuberculosis and extended bacterial infections and other inflammatory diseases like Tolosa–Hunt syndrome (for a wider differential see Montagnese et al and Gladstone et al.). 42 , 61 Rarely, systemic auto‐inflammatory diseases may present with a painful ophthalmoplegia, including amyloidosis, 52 systemic lupus erythematosus, 62 , 63 sarcoidosis 64 and Crohn's disease. 51 , 65 , 66 Therefore, a systemic diagnostic evaluation in inflammatory orbital diseases is important, as a systemic disease should be thoroughly excluded before labelling the disease idiopathic. 67 In these diseases ptosis is rarely observed or masked by proptosis; however, due to bulging of one eye and contralateral lid retraction, the presence of ptosis is sometimes reported. This is referred to as pseudoptosis.
Lastly, the fifth pattern consists of ophthalmoparesis and ptosis, but without diplopia as a predominant feature. The pattern of symptoms indicates a hereditary muscle disease. OPMD and CPEO are hereditary muscle diseases that are directly associated with ophthalmoparesis and ptosis. 7 , 8 , 11 , 14 , 68 In general, diplopia is less frequently reported in these diseases. 7 In addition, there is no clear asymmetry, pain or fluctuation. The lack of diplopia in the presence of clear ophthalmoparesis points towards a hereditary muscle disease, because the brain can adapt to the slowly developing ophthalmoparesis, a phenomenon called facultative suppression. However, throughout their disease at least half of for example CPEO patients do experience diplopia at some time in the disease course. 15 In centronuclear myopathy 22 and myotonic dystrophy Type 1, 18 , 19 , 20 ptosis and ophthalmoparesis occur regularly. In later stages of Pompe's disease, ptosis 16 may occur. In many other hereditary muscle diseases, including Duchenne muscular dystrophy, 69 the eye muscles are remarkably spared. Congenital myasthenic syndrome, although a synaptic disease, also presents with ophthalmoplegia, often without diplopia, and symmetrical ptosis. The postsynaptic types of congenital myasthenic syndrome (slow channel syndrome, AChR deficiency, fast channel syndrome, Rapsyn deficiency, and Plectin deficiency) present more frequently with ocular symptoms than synaptic and presynaptic congenital myasthenic syndromes. 14 For clinical reasoning, it is important to note that some congenital myasthenic syndromes can have an onset in early or even late adulthood, especially congenital myasthenic syndrome associated with rapsyn, 70 agrin, plectin, ALG14 and GMPPB gene mutations. 71
3. Imaging of extra‐ocular muscles
Magnetic Resonance Imaging is the primary tool for the assessment of ophthalmoparesis. It provides detailed information on orbital soft tissues, cranial nerves and the posterior cranial fossa. Besides anatomic sequences (T1 weighted [T1w] and T2 weighted [T2w] images) functional sequences such as diffusion weighted imaging (DWI), reflecting the Brownian motion of water molecules and thereby sensitivity to tissue architecture. Also contrast‐enhanced perfusion may be added to characterize lesions. 43 , 72 CT scans also demonstrate orbital pathology, in particular of bony origin. In MRI, there is a difference between quantitative and qualitative scans. 73 Quantitative scans allow for the numeric measurements of T1 and T2 (multi‐echo spin‐echo) relaxation times and for example chemical shift‐based water–fat separation scans allow for the quantification of fat fractions in tissue. Qualitative anatomical sequences can be used to identify a focal mass or changes in volume and cross‐sectional area of the EOM, reflecting EOM enlargement due to hypertrophy or inflammation or atrophy. Changes in signal intensities on qualitative T1w, T2w or DWI images, and enhancement and contrast scans can all indicate different types of tissue alterations. For instance, oedema and/or inflammation will cause an increase in the T2 relaxation time of water, and thereby signal intensity increase on T2w images, which can be observed on fat‐suppressed images. Fat replacement, that is, the replacement of muscle tissue with fat, will be hyperintense on both T1w and T2w images 74 due to the shorter T1 relaxation and longer T2 relaxation time of fat. On fat‐suppressed images, this will be shown as signal loss. 73 Fibrotic tissue has a very short T1 and T2 relaxation time, and hence is hardly visible on T1w and T2w images. The location and number of involved EOM may be suggestive of a specific disease (e.g., in IgG4 related orbitopathy usually bilateral involvement with a predilection for the lateral rectus muscle 47 is seen, while IOM is typically unilateral and mostly affects the medial rectus muscle). Supportive findings such as involvement of the lacrimal glands, increased orbital fat and vascular engorgement aid in the differential diagnosis but fall outside the scope of this paper.
For all diseases, we included MRI and CT studies in this review in which the EOM were studied or mentioned. Such studies were not available for all mentioned diseases; No MRI or CT studies describing the EOM were reported in Pompe's disease, in OPMD, Lambert–Eaton myasthenic syndrome (LEMS) and congenital myasthenic syndromes. For some diseases, we found only a small number of case reports; one case report describing a patient with myotonic dystrophy, in which no changes in the EOM were reported 20 and a small number of case reports of EOM involvement for systemic auto‐inflammatory disease. 51 , 62 , 63 , 65 , 66 (Table 2 ).
Table 2.
Reported imaging findings in the extra‐ocular muscles for orbital disease with ophthalmoparesis and ptosis interpreted as histopathological changes: fat increases, inflammation, enlargement and atrophy.
| Disease | Volume changes on anatomical sequences | T2 weighted (T2w) imaging with fat suppression | T1 weighted imaging (T1w)/T2 weighted (T2w) imaging without fat suppression/chemical shift‐based water–fat separation (Dixon) | Other findings |
|---|---|---|---|---|
| Atrophy/swelling | Inflammation | Fat increase | ||
| Acquired disease | ||||
| Idiopathic orbital myositis | EOM enlargement with tendon involvement. 42 , 43 | Increased signal on T2w imaging of the affected EOM. 59 , 75 , 76 | Surrounding T2 increases due to cellulitis. 75 | |
| IgG4‐related disease of the orbit | EOM enlargement with tendon sparing (fusiform enlargement), mostly LR. 47 | Decreased signal on T2w imaging of the affected EOM. 75 |
Lacrimal gland and infra‐orbital nerve enlargement. 47 Optic nerve enlargement. 42 |
|
| Thyroid orbitopathy/Graves' orbitopathy |
Fusiform enlargement of all EOM, often with tendon sparing, most pronounced in IR, MR and SR. 6 , 77 Increases in volume most pronounced in active stage; volume decreases in chronic but may still be enlarged. 78 |
Increased signal in the acute inflammatory phase 6 , 79 | T1 hyperintense signal, mainly in chronic patients. 6 , 44 , 80 , 81 |
Increased orbital fat volume. Enlarged lacrimal glands. Vascular engorgement. |
| Systemic auto‐inflammatory diseases |
Sarcoidosis: Bilateral EOM enlargement. 72 , 82 Crohn's disease: Enlargement of the SR and LPS. 65 Enlargement of the LR. 51 Enlargement of the IR. 66 Often with tendon involvement. |
T2w hypointensity or hyperintensity of affected EOM. 51 , 65 , 66 | Sarcoidosis: enlarged lacrimal gland, thickening and enhancement optic nerve | |
| Amyloidosis | Enlargement of the LR muscle with tendon sparing in amyloidosis. 52 | No hyperintensity on T2w‐STIR. 52 | An area without contrast enhancement, as compared with the rest of the EOM, is observed. 52 | |
| Orbital lymphoma | Muscle enlargement or focal mass. 83 | Focal hyperintense or isointense (as compared with the EOM) mass. 83 , 84 | Focal hypointense or isointense (as compared with the EOM) mass. 83 | Diffusion restriction: low ADC value. 11 , 49 |
| Synaptic disease | ||||
| Autoimmune LEMS | Not reported. | Not reported. | Not reported. | |
| Autoimmune myasthenia gravis |
Decreases in volume in chronically untreated/treatment resistant patients 85 , 86 , 87 and in Muscle‐specific tyrosine kinase (MUSK) positive patients. 88 No changes or small increases in volume in chronically treated and recent myasthenia gravis patients. 81 , 89 |
No hyperintensity on T2w scan in 20 myasthenia gravis patients. 90 |
Increases in fat fraction in the EOM in myasthenia gravis on Dixon scans. 89 Central hypo‐intensity on T2w scans with fat suppression. 90 |
|
| Congenital myasthenic syndrome | Not reported. | Not reported. | Not reported. | |
| Hereditary disease | ||||
| Chronic progressive external ophthalmoplegia (CPEO) |
Decrease in volume in all EOM, most pronounced in SR/LPS. 81 Decrease in cross‐sectional area in all EOMS (43%). 80 |
Two patients with STIR hyperintensities. 80 |
T1 hyperintense signal that is hypointense on STIR. 80 , 81 Prolonged (global) T2 relaxation time. 80 |
|
| Pompe disease | Not reported. | Not reported. | Not reported. | Not reported. |
| OPMD | Not reported. | Not reported. | Not reported. | Not reported. |
| Myotonic dystrophy | Case report: No changes in EOM. 20 | Case report: No changes in EOM. 20 | Case report: No changes in EOM. 20 | |
| CFEOM |
Severe atrophy of LPS and SR in CFEOM type 1. 91 Atrophy of all EOM, especially also SO and LR is relatively spared in CFEOM type 2. 11 , 12 |
Bright signal regions and longitudinal fissures in LR and MR in CFEOM type 2 on T1w imaging. 91 |
Absence of superior division of N. III in CFEOM type 1. 10 , 91 Absence of N. III and N. IV in CFEOM type 2. 11 |
|
| Congenital ptosis syndromes (e.g., Duane syndrome and BPES) | Atrophy/hypoplasia of the SO in Duane syndrome. 92 Atrophy of the LPS in BPES 93 | Not reported | Increases in T2 showing fatty patches in the LPS. 93 | Aberrant innervation of LR by branch of N. III and absence of N. VI in Duane syndrome. 94 |
Note: The neuromuscular diseases are categorized in acquired, synaptic and hereditary.
Abbreviation: CFEOM, congenital fibrosis of the extra‐ocular muscles; EOM, extra‐ocular muscles; LPS, levator palpebrae superioris; OPMD, oculo‐pharyngeal muscular dystrophy
4. MRI and volume of the extra‐ocular muscles
In acquired muscle disease of the orbit, EOM enlargement in different extend with or without tendon involvement is a frequent observation. In idiopathic orbital myositis, EOM volume is increased with involvement of the tendons, presenting as tendon thickening. In contrast, Graves' orbitopathy 6 , 77 and IgG4‐related orbital disease 47 (in 96% of patients) cause EOM enlargement of on average twice the size 42 , 78 with tendon sparing, known as fusiform enlargement. This specific pattern of enlargement can therefore be useful in differential diagnostics. An example of EOM enlargement in Graves' orbitopathy can be found in Figure 2 . In systemic diseases with EOM involvement, like Crohn's disease, sarcoidosis, systemic lupus erythematosus and amyloidosis, EOM volumes are increased as qualitatively described in several case reports. 52 , 63 , 64 , 65 , 66 , 95 In Graves' orbitopathy, EOM volume usually decreases in the inactive stage of the disease (e.g., for the SR: 1.1 cm3 in chronic inactive compared with 1.3 cm3 in chronic active), but is still more than double compared with healthy EOM (0.6 cm3). 78 EOM atrophy is a common finding in hereditary neuromuscular disease. In CPEO, the mean cross‐sectional area of all EOM was 43% lower than for controls. 80 An example of atrophy in CPEO can be seen in Figure 2 . The most pronounced reduction is in the SR and the LPS muscle, which is consistent with the clinical presentation with ptosis and gaze limitations in elevation. 81 One case report reported normal volumes of the EOM in a patient with ophthalmoparesis associated with myotonic dystrophy. 21 In CFEOM, many imaging studies have shown that the denervated EOM are atrophic. In CFEOM Type 1, caused by agenesis of the upper branch of the oculomotor nerve, there is atrophy of the LPS and the SR in all subjects, with an average volume reduction of 60% in the SR. 91 In CFEOM Type 2, with more global dysgenesis of the oculomotor nerve, the SO and LR are relatively spared, and the other EOM are severely atrophic. 11 , 12 In myasthenia gravis, an acquired synaptic disease, case reports describe qualitatively decreases in volumes in chronically untreated or treatment‐resistant patients 85 , 86 and in muscle‐specific tyrosine kinase (MUSK) positive myasthenia gravis patients. 88 However, normal volumes or slight increased volumes (0.8 ± 0.2 cm3 in MG and 0.6 ± 0.2 cm3 in healthy controls, all recti EOM averaged) were observed in recently diagnosed and chronic myasthenia gravis patients. 81 , 89
Figure 2.
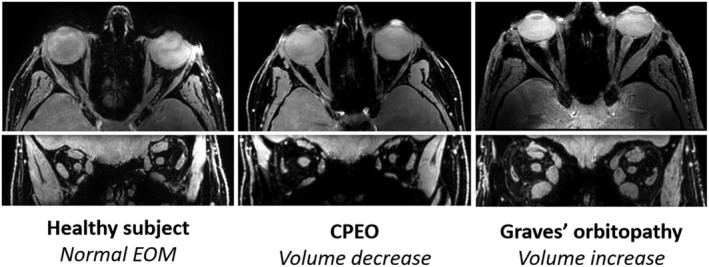
Magnetic resonance imaging scans of the orbit. Chemical shift‐based water–fat separation (using the Dixon technique) was used and the water image is shown; on top the transverse image and on bottom the coronal image. Volume decrease, indicative of atrophy, of the extra‐ocular muscle (EOM) is clearly demonstrated in chronic progressive external ophthalmoplegia and enlargement of the EOM in Graves' orbitopathy.
5. MRI and inflammation
When inflammation of orbital fat or muscle is the cause of ophthalmoparesis and ptosis, the inflammatory process is often shown as hyperintense on T2w MRI images due to the presence of oedema. The EOMs have an increased signal on T2w imaging with fat suppression in idiopathic orbital myositis, 59 , 75 , 76 in active Graves' orbitopathy 6 , 79 and in systemic auto‐inflammatory diseases like Crohn's disease, sarcoidosis and systemic lupus erythematosus. 51 , 65 , 66 , 96 IgG4‐ROD 75 demonstrates relatively low signal intensity on T2w ‐MR images because of its increased cellularity and amount of fibrosis. In hereditary muscle and synaptic disease, however, evidence of inflammation is sparse. In two patients with CPEO, hyperintensities were observed on a T2w scan with fat suppression. 80 No hyperintensities were present on T2w scans in 20 myasthenia gravis patients. 90
6. MRI and fat replacement
In the chronic stages of Graves' orbitopathy, an increase in fat in the EOM is observed on MRI, which is the result of adipogenesis by fibroblasts and fibrocytes. 44 , 77 , 97 In CPEO, an increase in signal intensity on a T1w scan has been described within EOM, 81 as well as a quantitative increase in T2 relaxation time, 80 both indicative of fat replacement in the EOM. Signal loss on fat‐suppressed images confirmed the presence of fat in this study. An example of fat replacement of the EOM as quantitatively measured with chemical shift‐based water–fat separation imaging (using the Dixon technique) in an OPMD patient can be seen in Figure 3 . The fat fraction of the EOM increases to up to 10% in myasthenia gravis patients on chemical shift‐based water–fat separation scans. 89 In addition, central hypo‐intensities were observed on T2w scans with fat suppression in myasthenia gravis. 90 In myasthenia gravis, fat replacement appears to be less frequently reported in literature than in CPEO. In CFEOM Type II, T1w imaging shows bright signal regions and longitudinal fissures in the LR and the MR, also indicative of fat replacement. 91
Figure 3.
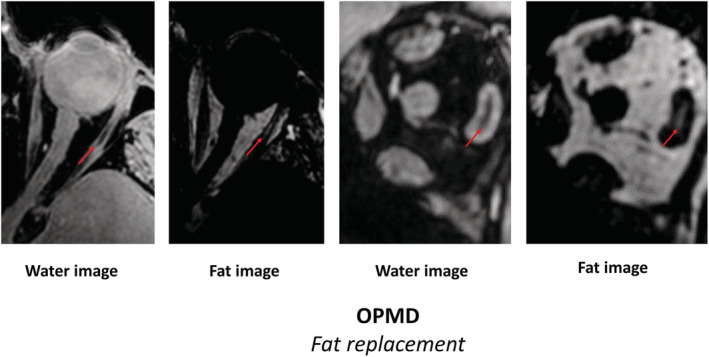
Magnetic resonance imaging scans of the orbit. Chemical shift‐based water–fat separation (using the Dixon technique) was used and the transverse water image (first), transverse fat image (second), the coronal water image (third) and the coronal fat image (fourth) are shown. This MRI scan demonstrates fat replacement of the extra‐ocular muscle in a patient with oculo‐pharyngeal muscular dystrophy. The fat replacement is predominantly observed in the lateral rectus muscles (red arrows).
7. Correlating quantitative MRI parameters with disease activity and disease severity
Only in a small number of the reviewed imaging studies, quantitative MRI parameters were correlated with disease activity or disease stage. In CPEO the range of eye movement was correlated with the global T2 relaxation time of the EOM, a parameter mainly reflecting fat replacement: patients with a smaller range of motion had more fat replacement. 80 In Graves' orbitopathy, EOM volume as measured with MRI and ultrasound is strongly correlated with disease activity. Mild and pronounced active stages of disease (stages G1 and G2) show progressively larger EOM volumes (e.g., for the SR: 1.1 cm3 in chronic inactive compared with 1.3 cm3 in chronic active) than normal subjects. Hereafter, volumes decreases in later stages with longstanding active disease (Stage G3) and chronic stages without active disease (Stage G4) but remain about 10% higher than in normal subjects. 78 In another study into the natural course of Graves' orbitopathy with a follow‐up of at least 4 years, also a slight increase of orbital fat volume from 15 to 16.8 ml, a decrease in muscle volume of 1 ml (decrease of ~20% on a muscle of 4.5 ml), and visible intramuscular fat was observed. 98 In case reports of the EOM in myasthenia gravis patients, long untreated and chronic myasthenia gravis patients appear to have EOM atrophy and fat replacement as qualitatively described. 85 , 86 On the contrary, a slight increase in EOM volume of 0.2 cm3 was observed in chronically treated and recent myasthenia gravis patients, also suggesting differences between different disease stages. 81 , 89
8. Clinical and radiological clues from the different combinations of extra‐ocular muscle weakness
To accurately assess the involvement pattern of individual EOM in orbital disease, both imaging studies and orthoptic studies were reviewed (Table S1 and Figure 4 ). For the three most commonly acquired orbital diseases the involvement pattern is well described in literature. In Graves' orbitopathy, the IR, MR and SR are most predominantly asymmetrically involved with remarkable sparing of the LR and the oblique muscles. In idiopathic orbital myositis, involvement is unilateral and the LR is not spared. 43 , 44 In IgG4‐ROD the LR is most often involved bilaterally, 47 with the IR and SR affected subsequently. 48 Auto‐inflammatory diseases with orbital involvement have a variety of involvement patterns, for example, as extensively described in case reports for Crohn's disease with MR and oblique muscles relatively spared. 51 , 65 , 66 In a case report of systemic lupus erythematosus, the MR and IR were involved, 62 and in another case report, the LR was solely involved. 63 In amyloidosis, the horizontal rectus muscles seem most predominantly affected. 52
Figure 4.
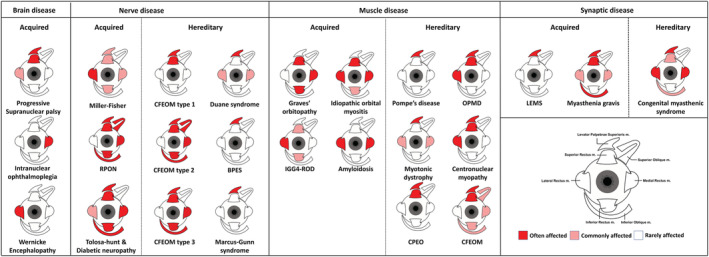
Involvement pattern of the six extra‐ocular muscles and the levator palpebrae superioris muscle for brain diseases, nerve diseases, muscle diseases and synaptic disease split into either acquired or hereditary. An example of the right eye is shown.
In hereditary muscle disease with orbital muscle involvement the LPS is most frequently affected. In CPEO, elevation limitation due to weakness of the SR is the second most present ocular symptom. 7 In OPMD, the SR is the most affected muscle, followed by the LR. 8 In Pompe's disease, 16 centronuclear myopathy 22 and myotonic dystrophy, 18 , 19 the LPS is often the only affected ocular muscle, but case reports have described horizontal ophthalmoparesis with weakness of the MR and LR in myotonic dystrophy 20 , 21 and elevation and abduction limitations in centronuclear myopathy. 23
In synaptic disorders, the individual variation of EOM involvement is high, with ptosis being more common than ophthalmoparesis. In literature, the ocular involvement pattern in myasthenia gravis differs; however, elevation is most often found to be limited (IO > SR), followed by horizontal limitations (LR > MR). 35 , 36 , 37 In LEMS, the LPS is frequently weak, causing ptosis in up to 54% of patients. EOM weakness does not occur frequently in LEMS, but 30% of patients have ocular motility abnormalities. 32 The pattern of congenital myasthenia gravis is similar to autoimmune myasthenia gravis with almost no limitations in depression; however, horizontal eye movements (LR > MR) are more frequently limited than elevation. 4
In disorders where nerve pathology and lack of innervation cause muscle dysfunction, the involvement pattern is confined to the innervation area of one or more cranial nerves. In CFEOM the agenesis of (a branch) of the oculomotor nerve, or less common the abducens nerve, causes atrophy of the innervated muscles. Similarly, diabetic mononeuropathy also affects the oculomotor nerve and the abducens nerve. 31 In Tolosa–Hunt syndrome, the oculomotor nerve is affected in 80% of individuals, followed by the abducens nerve in 70% and the trochlear nerve in 30%. 3 In Miller–Fisher syndrome, dysfunction of the LR, due to involvement of the abducens nerve, is most commonly involved and the last to recover. 27 Interestingly in Miller–Fisher syndrome, the ophthalmoparesis is mostly symmetrical. 27
In brain disease with ophthalmoparesis, ptosis is unusual except for brain stem pathology such as brain stem tumours. Brain stem tumours are known to mimic other diseases, for example, myasthenia gravis, and symptoms are dependent on tumour localization. In Wernicke's encephalopathy, limitations in abduction due to palsies of the LR are most common. 25 Progressive supranuclear palsy is a disease that can mimic dementia and Parkinson's, and ophthalmoparesis is always in the vertical direction. Internuclear ophthalmoparesis is a rare gaze abnormality characterized by impaired adduction of the affected eye, with nystagmus of the abducted contralateral eye.
9. Discussion
This review combines clinical symptoms and EOM involvement pattern on imaging to provide clues for diagnosis of diseases with ophthalmoparesis and ptosis, including acquired and hereditary diseases with pathology located in the brain, nerve, synapse or muscle.
Some general conclusions can be made, based on the combination of clinical symptoms, and the involvement pattern and imaging features of the different EOMs. First, in brain disease, some specific EOM weakness patterns, often accompanied by nystagmus, point towards a specific disease, with for example vertical gaze limitation in progressive supranuclear palsy and abduction limitation in Wernicke's encephalopathy. Second, nerve diseases follow the specific cranial nerve innervation pattern and in hereditary muscle diseases, the LPS and the SR are more frequently involved than the other EOM, and the IR is usually spared. Third, in synaptic diseases in general, the involvement pattern is highly variable per patient and over time, but the LPS and SR are most often involved, followed by the LR and MR; the IR is often spared. LEMS is an exception: Ophthalmoparesis is rare, and ptosis is more frequently observed. In acquired muscle diseases, the presence of pain and proptosis is often evident. The involvement pattern can be very specific per disease, for example, in Graves' orbitopathy the progressive involvement of the EOM generally follows a specific order: IR > MR > SR > LR > SO. In general, the LPS and the oblique muscles are spared and the IR is involved, in contrast with hereditary diseases.
Imaging studies of the EOM in hereditary and acquired neural and synaptic diseases describe atrophy and an increase in fat in the EOM, indicating that denervated EOM have a tendency towards atrophy accompanied by fat replacement of muscle fibres. In hereditary muscle diseases such as CPEO, fat replacement is also observed, which is also indicative of progressive muscle wasting. 81 Consequently, in myasthenia gravis, a synaptic disease, small increases in fat fraction are observed in patients with longstanding chronic or untreated disease and they show a small decrease in EOM volume, 87 but more recent findings rather point to a small and variable increase in muscle volume. 89 In acquired orbital diseases, inflammation seen as hyperintensity on T2w scans with fat suppression is observed in active stages of disease, accompanied by increases in muscle volume. In the chronic stages of Graves' orbitopathy, an increase in fat in the EOM is observed due to adipogenesis by orbital fibroblasts and fibrocytes. 97
Not in all neuromuscular diseases the EOMs are affected. This could provide valuable clues in the pathophysiology of EOM involvement and in disease pathophysiology in general, since the EOM differ anatomically and physiologically from skeletal muscles. They have distinct fibre type composition, 99 multiple innervation, 100 smaller motor units, 101 higher levels of utrophin expression, 102 a distinct contraction–excitation coupling, 103 have an increased capability of regeneration and preferentially use glucose‐based aerobic metabolic pathways. 69 The latter means that EOM are packed with mitochondria, explaining the predominant ocular phenotype in mitochondrial diseases such as CPEO. In other primary muscle diseases, such as Duchenne muscular dystrophy, the EOMs are remarkably spared, which has been hypothesized to be mainly due to the increased regenerative capacity of the EOM and higher levels of utrophin expression. 104 The LPS is relatively spared in acquired orbital disease with an inflammatory origin and is frequently affected in hereditary neuromuscular disease. This may be explained by differences between the EOM and the LPS. The LPS carries thicker muscle fibers 105 and has a higher arteriole–nerve distance. 106
Fat increase in the EOM does occur in OPMD, indicative that muscle damage of the EOM also causes the replacement of muscle tissue by fat in dystrophic disease with EOM involvement. Fat increase in the EOM also occurs in synaptic neuromuscular disorders. This may indicate that (relative) muscle denervation of the EOM causes the replacement of muscle tissue with fat. This phenomenon has been described previously in denervating disease with skeletal muscle involvement. 107 In addition, there is evidence from histological studies of the EOM in myasthenia gravis that atrophy and fat replacement occur. 108 In chronic stages of acquired orbital muscle disease with an inflammatory origin, such as Graves' orbitopathy, there is evidence of adipogenesis by fibroblasts in a later stage of the disease. 6 , 44 , 80 , 81 , 97 Therefore, because intramuscular fat increase seems to correlate with the disease stage to some extent, it may be a valuable biomarker in the follow‐up of disease progression.
In neuromuscular disease with known EOM involvement, MRI of the EOM is rarely performed compared with MRI of skeletal muscles. For OPMD, Pompe disease and congenital myasthenic syndromes imaging of the EOM has not been described, and in other diseases, like myotonic dystrophy, only a few case reports have been published. There are challenges when performing orbital MRI because the eyes are prone to motion and close to air‐bone‐tissue interfaces causing artefacts. However, scans can be optimized for these challenges. 109 Scan time can be reduced, and cued blinking could be used 89 to prevent movement artefacts and for example spin‐echo sequences are less sensitive to field inhomogeneities as compared with gradient‐echo sequences. 110 Currently, it is recommended to include T1w sequences, T2w sequences with and without fat suppression, T1w scans with contrast and DWI. 43 Given the small size of the orbital structures, sequences with an in plane resolution of at least 0.8 mm and slices of 3 mm are generally sufficient to detect clinically relevant EOM swelling and atrophy in our experience, although reports show that an isotropic resolution below 0.6 mm are also clinically feasible. 111 These can be obtained with a brain MRI setup on 3 T. To more directly assess the EOM pathophysiology in the context of research, we recommend including scans with water–fat separation to study fat fraction increases of the EOM. T2w imaging with fat suppression is recommended to differentiate intra‐muscular fat from inflammation/oedema, as is commonly performed in skeletal muscle imaging studies. 112 Many orbital inflammatory diseases are now labelled as idiopathic, and a systemic diagnostic evaluation might be needed to identify underlying and associated disease as proposed by McNab. 67 We believe that imaging can play an important role in this respect.
Additionally, quantitative MRI could be a valuable addition in the follow‐up of disease progression and in the correlation of MRI to disease activity and disease progression, as is known in the field of neuromuscular diseases like Duchenne and Becker muscular dystrophy. In general, for these muscle diseases, the recommended technique to quantify fat fraction is chemical shift‐based water–fat separation. 74 The feasibility of performing such scans of the EOM has been previously shown. 89 To quantify inflammation, T2 relaxation time maps of the water component could be acquired as is done in skeletal muscle. 73 Applying these quantitative techniques to study fat replacement and T2 relaxation time changes in the EOM could prove valuable in disease with EOM involvement. Finally, EOM volume can be quantified using anatomical scans such as T1w, T2 or Dixon. Clinical diagnostic evaluation of scans is conventionally performed by comparing the cross‐sectional area of the EOM, which is generally sufficient to detect EOM swelling or atrophy. In the context of research however, we believe volume to be a more robust measure than cross‐sectional area because the entire EOM is included and the measurement is more independent of variations in EOM shape or position. Also, we observe a high variation in EOM volume in healthy controls (e.g., for the medial rectus 569 ± 129 mm3), 113 therefore including a healthy control group for reference in studies is recommended. To determine the source of this variation, future studies should focus on and the influence of orbital volume, age and race on EOM volume. In conclusion, in diseases with ophthalmoparesis and ptosis specific patterns of clinical symptoms, the EOM involvement pattern and orbital imaging provide valuable information for diagnosis. Additionally, orbital imaging could prove valuable in the follow‐up of disease progression and the understanding of disease pathophysiology.
Conflicts of interest
K. R. Keene reports involvement in myasthenia gravis research sponsored by Argenx, Alexion Pharmaceuticals and the CHDR, with all reimbursements received by Leiden University Medical Center and research support from Philips Healthcare. H. E. Kan reports research support from Philips Healthcare, trial support from ImagingDMD; no personal fees are received, and all revenues go to the LUMC S. van Meeren reports no disclosures. B. M. Verbist reports no disclosures. M. R. Tannemaat reports trial support from Argen‐X and Alexion. J. W. M. Beenakker reports research support from Philips Healthcare. J. J. G. M. V. has been involved in MG research sponsored by the Princes Beatrix Fonds, Health Holland and consultancies for Argen‐X, Alexion, and NMD Pharma. Reimbursements were received by the LUMC. He is a co‐inventor on patent applications based on MuSK‐related research. The LUMC receives royalties for MuSK antibody assays.
Supporting information
Table S1. Involved EOM in different orbital disease with ophthalmoparesis and ptosis. The neuromuscular diseases are categorized in acquired, synaptic and hereditary.
Acknowledgements
Authors of this paper are members of the EURO‐NMD European Reference Network. The authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. 114
Keene K. R., Kan H. E., van der Meeren S., Verbist B. M., Tannemaat M. R., Beenakker J.‐W. M., and Verschuuren J. J. G. M. (2022) Clinical and imaging clues to the diagnosis and follow‐up of ptosis and ophthalmoparesis, Journal of Cachexia, Sarcopenia and Muscle, 13, 2820–2834, doi: 10.1002/jcsm.13089
References
- 1. Heidary G, Engle EC, Hunter DG. Congenital fibrosis of the extraocular muscles. Semin Ophthalmol 2008;23:3–8. [DOI] [PubMed] [Google Scholar]
- 2. Al Othman B, Raabe J, Kini A, Lee AG. Update: the Miller–Fisher variants of Guillain–Barré syndrome. Curr Opin Ophthalmol 2019;30:462–466. [DOI] [PubMed] [Google Scholar]
- 3. Filho ARG, Faccenda PG, Estacia CT, Correa BS, Curi I. Tolosa–Hunt syndrome. Rev Bras Oftalmol 2018;77:289–291. [Google Scholar]
- 4. Mansukhani SA, Bothun ED, Diehl NN, Mohney BG. Incidence and ocular features of pediatric myasthenias. Am J Ophthalmol 2019;200:242–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gilhus NE, Tzartos S, Evoli A, Palace J, Burns TM, Verschuuren JJGM. Myasthenia gravis. Nat Rev Dis Primers 2019;5:30. [DOI] [PubMed] [Google Scholar]
- 6. Parmar H, Ibrahim M. Extrathyroidal manifestations of thyroid disease: thyroid ophthalmopathy. Neuroimaging Clin N Am 2008;18:527–536. [DOI] [PubMed] [Google Scholar]
- 7. McClelland C, Manousakis G, Lee MS. Progressive external ophthalmoplegia. Curr Neurol Neurosci Rep 2016;16:1–10. [DOI] [PubMed] [Google Scholar]
- 8. Renard D, Ferraro A, Lorenzini MC, Jeanjean L, Portal MC, Llinares E, et al. Orthoptic and video‐oculographic analyses in oculopharyngeal muscular dystrophy. Muscle Nerve 2015;52:554–558. [DOI] [PubMed] [Google Scholar]
- 9. Díaz‐Manera J, Luna S, Roig C. Ocular ptosis: differential diagnosis and treatment. Curr Opin Neurol 2018;31:618–627. [DOI] [PubMed] [Google Scholar]
- 10. Engle EC, Goumnerov BC, McKeown CA, Schatz M, Johns DR, Porter JD, et al. Oculomotor nerve and muscle abnormalities in congenital fibrosis of the extraocular muscles. Ann Neurol 1997;41:314–325. [DOI] [PubMed] [Google Scholar]
- 11. Bosley TM, Oystreck DT, Robertson RL, Al Awad A, Abu‐Amero K, Engle EC. Neurological features of congenital fibrosis of the extraocular muscles type 2 with mutations in PHOX2A. Brain 2006;129:2363–2374. [DOI] [PubMed] [Google Scholar]
- 12. Razek AAKA, Maher H, Kasem MA, Helmy E. Imaging of congenital cranial dysinnervation disorders: what radiologist wants to know? Clin Imaging 2021;71:106–116. [DOI] [PubMed] [Google Scholar]
- 13. Marenco M, Macchi I, Macchi I, Galassi E, Massaro‐Giordano M, Lambiase A. Clinical presentation and management of congenital ptosis. Clin Ophthalmol 2017;11:453–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Verschuuren JJGM, Palace J, Erik Gukgys N. Clinical aspects of myasthenia explained. Autoimmunity 2010;43:344–352. [DOI] [PubMed] [Google Scholar]
- 15. Bau V, Zierz S. Update on chronic progressive external ophthalmoplegia. Strabismus 2005;13:133–142. [DOI] [PubMed] [Google Scholar]
- 16. Van Der Beek NAME, De Vries JM, Hagemans MLC, Hop WCJ, Kroos MA, Wokke JHJ, et al. Clinical features and predictors for disease natural progression in adults with Pompe disease: a nationwide prospective observational study. Orphanet J Rare Dis 2012;7:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nadaj‐Pakleza A, Richard P, Łusakowska A, Gajewska J, Jamrozik Z, Kostera‐Pruszczyk A, et al. Oculopharyngeal muscular dystrophy: phenotypic and genotypic characteristics of 9 Polish patients. Neurol Neurochir Pol 2009;43:113–120. [PubMed] [Google Scholar]
- 18. Thornton CA. Myotonic dystrophy. Neurol Clin 2014;32:705–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Choi SH, Yang HK, Hwang JM, Park KS. Ocular findings of myotonic dystrophy type 1 in the Korean population. Graefes Arch Clin Exp Ophthalmol 2016;254:1189–1193. [DOI] [PubMed] [Google Scholar]
- 20. Thiriez C, Vignal C, Papeix C, Yaici S, Vidailhet M, Roze E. Ophthalmoplegia as the presenting muscle‐related manifestation of myotonic dystrophy. Rev Neurol (Paris) 2010;166:538–541. [DOI] [PubMed] [Google Scholar]
- 21. Lessell S, Coppeto J, Samet S. Ophthalmoplegia in myotonic dystrophy. Am J Ophthalmol 1971;71:1231–1235. [DOI] [PubMed] [Google Scholar]
- 22. Jeannet PY, Bassez G, Eymard B, Laforêt P, Urtizberea JA, Rouche A et al. Clinical and histologic findings in autosomal centronuclear myopathy. 2004. [DOI] [PubMed] [Google Scholar]
- 23. Fischer D, Herasse M, Bitoun M, Barragán‐Campos HM, Chiras J, Laforêt P, et al. Characterization of the muscle involvement in dynamin 2‐related centronuclear myopathy. Brain 2006;129:1463–1469. [DOI] [PubMed] [Google Scholar]
- 24. Troost BT, Daroff RB. The ocular motor defects in progressive supranuclear palsy. Ann Neurol 1977;2:397–403. [DOI] [PubMed] [Google Scholar]
- 25. Isen DR, Kline LB. Neuro‐ophthalmic manifestations of wernicke encephalopathy. Eye Brain 2020;12:49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Straube A, Witt TN. Oculo‐bulbar myasthenic symptoms as the sole sign of tumour involving or compressing the brain stem. 1990. [DOI] [PubMed] [Google Scholar]
- 27. Ryu WY, Kim YH, Yoon BA, Park HT, Bae JS, Kim JK. Pattern of extraocular muscle involvements in Miller–Fisher syndrome. J Clin Neurol 2019;15:308–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liu Y, Wang M, Bian X, Qiu E, Han X, Dong Z, et al. Proposed modified diagnostic criteria for recurrent painful ophthalmoplegic neuropathy: five case reports and literature review. Cephalalgia 2020;40:1657–1670. [DOI] [PubMed] [Google Scholar]
- 29. Martin TJ. Horner syndrome: a clinical review. ACS Chem Nerosci 2018;9:177–186. [DOI] [PubMed] [Google Scholar]
- 30. Jain R, Sawhney S, Koul RL, Chand P. Tolosa–Hunt syndrome: MRI appearances. J Med Imaging Radiat Oncol 2008;52:447–451. [DOI] [PubMed] [Google Scholar]
- 31. Watanabe K, Hagura R, Akanuma Y, Takasu T, Kajinuma H, Kuzuya N, et al. Characteristics of cranial nerve palsies in diabetic patients. Diabetes Res Clin Pract 1990;10:19–27. [DOI] [PubMed] [Google Scholar]
- 32. Young JD, Leavitt JA. Lambert–Eaton myasthenic syndrome: ocular signs and symptoms. J Neuro‐Ophthalmology 2016;36:20–22. [DOI] [PubMed] [Google Scholar]
- 33. Schoser B, Eymard B, Datt J, Mantegazza R. Lambert–Eaton myasthenic syndrome (LEMS): a rare autoimmune presynaptic disorder often associated with cancer. J Neurol 2017;264:1854–1863. [DOI] [PubMed] [Google Scholar]
- 34. Wirtz PW, Sotodeh M, Nijnuis M, Van Doorn PA, Van Engelen BGM, Hintzen RQ, et al. Difference in distribution of muscle weakness between myasthenia gravis and the Lambert–Eaton myasthenic syndrome. J Neurol Neurosurg Psychiatry 2002;73:766–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. De Meel RHP, Raadsheer WF, Van Zwet EW, Tannemaat MR, Verschuuren JJGM. Ocular weakness in myasthenia gravis: changes in affected muscles are a distinct clinical feature. J Neuromuscul Dis 2019;6:369–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Almog Y, Ben‐David M, Nemet AY. Inferior oblique muscle paresis as a sign of myasthenia gravis. J Clin Neurosci 2016;50–53. [DOI] [PubMed] [Google Scholar]
- 37. Cleary M, Williams GJ, Metcalfe RA. The pattern of extra‐ocular muscle involvement in ocular myasthenia. Strabismus 2008;16:11–18. [DOI] [PubMed] [Google Scholar]
- 38. Luigetti M, Sabatelli M. Cranial botulism. Neuromuscul Disord 2012;22:995–996. [DOI] [PubMed] [Google Scholar]
- 39. Chatham‐Stephens K, Fleck‐Derderian S, Johnson SD, Sobel J, Rao AK, Meaney‐Delman D. Clinical features of foodborne and wound botulism: a systematic review of the literature, 1932–2015. Clin Infect Dis 2017;66:S11–S16. [DOI] [PubMed] [Google Scholar]
- 40. Azam L, McIntosh JM. Alpha‐conotoxins as pharmacological probes of nicotinic acetylcholine receptors. Acta Pharmacol Sin 2009;30:771–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Olsen TG, Heegaard S. Orbital lymphoma. Surv Ophthalmol 2019;64:45–66. [DOI] [PubMed] [Google Scholar]
- 42. Montagnese F, Wenninger S, Schoser B. “Orbiting around” the orbital myositis: clinical features, differential diagnosis and therapy. J Neurol 2016;263:631–640. [DOI] [PubMed] [Google Scholar]
- 43. Ferreira TA, Saraiva P, Genders SW, Buchem MV, Luyten GPM, Beenakker JW. CT and MR imaging of orbital inflammation. Neuroradiology 2018;60:1253–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kirsch E, Hammer B, Von Arx G. Graves' orbitopathy: current imaging procedures. Swiss Med Wkly 2009;139:618–623. [DOI] [PubMed] [Google Scholar]
- 45. Fraser CL, Skalicky SE, Gurbaxani A, McCluskey P. Ocular myositis. Curr Allergy Asthma Rep 2013;13:315–321. [DOI] [PubMed] [Google Scholar]
- 46. Goto H, Takahira M, Azumi A. Diagnostic criteria for IgG4‐related ophthalmic disease. Jpn J Ophthalmol 2015;59. [DOI] [PubMed] [Google Scholar]
- 47. Tiegs‐Heiden CA, Eckel LJ, Hunt CH, Diehn FE, Schwartz KM, Kallmes DF, et al. Immunoglobulin G4‐related disease of the orbit: imaging features in 27 patients. Am J Neuroradiol 2014;35:1393–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sogabe Y, Ohshima KI, Azumi A, Takahira M, Kase S, Tsuji H, et al. Location and frequency of lesions in patients with IgG4‐related ophthalmic diseases. Graefes Arch Clin Exp Ophthalmol 2014;252:531–538. [DOI] [PubMed] [Google Scholar]
- 49. Claytor B, Li Y. Challenges in diagnosing coexisting ocular myasthenia gravis and thyroid eye disease. Muscle Nerve 2021;63:631–639. [DOI] [PubMed] [Google Scholar]
- 50. Melcescu E, Horton WB, Kim D, Vijayakumar V, Corbett JJ, Crowder KW, et al. Graves orbitopathy: update on diagnosis and therapy. South Med J 2014;107:34–43. [DOI] [PubMed] [Google Scholar]
- 51. Bourikas LA, Roussomoustakaki M, Papadaki E, Valatas V, Koutroubakis IE, Papadakis KA, et al. A case of orbital myositis preceding the intestinal symptoms of Crohn's disease. J Crohns Colitis 2010;4:349–350. [DOI] [PubMed] [Google Scholar]
- 52. Nishikawa N, Kawaguchi Y, Konno A, Kitani Y, Takei H, Yanagi Y. Primary isolated amyloidosis in the extraocular muscle as a rare cause of ophthalmoplegia: a case report and literature review. Am J Ophthalmol Case Reports 2021;22:101052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Holmes JM, Leske DA, Kupersmith MJ. New methods for quantifying diplopia. Ophthalmology 2005;112:2035–2039. [DOI] [PubMed] [Google Scholar]
- 54. Latting MW, Huggins AB, Marx DP, Giacometti JN. Clinical evaluation of blepharoptosis: distinguishing age‐related ptosis from masquerade conditions. Semin Plast Surg 2017;31:5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ozgen A, Ariyurek M. Normative measurements of orbital structures using CT. Am J Roentgenol 1998;170:1093–1096. [DOI] [PubMed] [Google Scholar]
- 56. Park NR, Moon JH, Lee JK. Hertel exophthalmometer versus computed tomography scan in proptosis estimation in thyroid‐associated orbitopathy. Clin Ophthalmol 2019;13:1461–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Smith SV, Lee AG. Update on ocular myasthenia gravis. Neurol Clin 2017;35:115–123. [DOI] [PubMed] [Google Scholar]
- 58. Grob D, Brunner N, Namba T, Pagala M. Lifetime course of myasthenia gravis. Muscle Nerve 2008;37:141–149. [DOI] [PubMed] [Google Scholar]
- 59. Lacey B, Chang W, Rootman J. Nonthyroid causes of extraocular muscle disease. Surv Ophthalmol 1999;44:187–213. [DOI] [PubMed] [Google Scholar]
- 60. Nagy EV, Toth J, Kaldi I, Damjanovich J, Mezosi E, Lenkey A, et al. Graves' ophthalmopathy: eye muscle involvement in patients with diplopia. Eur J Endocrinol 2000;142:591–597. [DOI] [PubMed] [Google Scholar]
- 61. Gladstone JP, Dodick DW. Painful ophthalmoplegia: overview with a focus on Tolosa–Hunt syndrome. Curr Pain Headache Rep 2004;8:321–329. [DOI] [PubMed] [Google Scholar]
- 62. Jenkins PO, Soper C, Mackinnon AD, O'Sullivan E, Nitkunan A. Systemic lupus erythematosus presenting as orbital myositis. Neuro‐Ophthalmology 2014;38:264–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Chan AJ, Rai AS, Lake S. Orbital myositis in systemic lupus erythematosus: a case report and literature review. Eur J Rheumatol 2020;7:135–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kim JS, Scawn RL, Lee BW, Lin JH, Korn BS, Kikkawa DO. Masquerading orbital sarcoidosis with isolated extraocular muscle involvement. Open Ophthalmol J 2016;10:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Pimentel R, Lago P, Pedroto I. Recurrent orbital myositis as an extra‐intestinal manifestation of Crohn's disease. J Crohns Colitis 2012;6:958–959. [DOI] [PubMed] [Google Scholar]
- 66. Ramalho J, Castillo M. Imaging of orbital myositis in Crohn's disease. Clin Imaging 2008;32:227–229. [DOI] [PubMed] [Google Scholar]
- 67. McNab AA. Orbital myositis: a comprehensive review and reclassification. Ophthal Plast Reconstr Surg 2020;109–117. [DOI] [PubMed] [Google Scholar]
- 68. Nair AG, Patil‐Chhablani P, Venkatramani DV, Gandhi RA. Ocular myasthenia gravis: a review. Indian J Ophthalmol 2014;62:985–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Fischer MD, Budak MT, Bakay M, Gorospe JR, Kjellgren D, Pedrosa‐Domellöf F, et al. Definition of the unique human extraocular muscle allotype by expression profiling. Physiol Genomics 2005;22:283–291. [DOI] [PubMed] [Google Scholar]
- 70. Burke G, Cossins J, Maxwell S, Owens G, Vincent A, Robb S, et al. Rapsyn mutations in hereditary myasthenia: distinct early‐ and late‐onset phenotypes. Neurology 2003;61:826–828. [DOI] [PubMed] [Google Scholar]
- 71. Finsterer J. Congenital myasthenic syndromes. Orphanet J Rare Dis 2019;14:1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Lakerveld M, van der Gijp A. Orbital muscle enlargement: what if it's not Graves' disease? Curr Radiol Rep 2022;10:9–19. [Google Scholar]
- 73. Strijkers GJ, Araujo ECA, Azzabou N, Bendahan D, Blamire A, Burakiewicz J, et al. Exploration of new contrasts, targets, and MR imaging and spectroscopy techniques for neuromuscular disease—a workshop report of working group 3 of the biomedicine and molecular biosciences COST action BM1304 MYO‐MRI. J Neuromuscul Dis 2019;6:1–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Burakiewicz J, Sinclair CDJ, Fischer D, Walter GA, Kan HE, Hollingsworth KG. Quantifying fat replacement of muscle by quantitative MRI in muscular dystrophy. J Neurol 2017;264:2053–2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Pakdaman MN. Orbital inflammatory disease: pictorial review and differential diagnosis. World J Radiol 2014;6:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ding ZX, Lip G, Chong V. Idiopathic orbital pseudotumour. Clin Radiol 2011;66:886–892. [DOI] [PubMed] [Google Scholar]
- 77. Garau LM, Guerrieri D, De Cristofaro F, Bruscolini A, Panzironi G. Extraocular muscle sampled volume in Graves' orbitopathy using 3‐T fast spin‐echo MRI with iterative decomposition of water and fat sequences. Acta Radiol Open 2018;7:205846011878089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Lennerstrand G, Tian S, Isberg B, Högbeck IL, Bolzani R, Tallstedt L, et al. Magnetic resonance imaging and ultrasound measurements of extraocular muscles in thyroid‐associated ophthalmopathy at different stages of the disease. Acta Ophthalmol Scand 2007;85:192–201. [DOI] [PubMed] [Google Scholar]
- 79. Tortora F, Cirillo M, Ferrara M, Belfiore MP, Carella C, Caranci F et al. Disease activity in graves' ophthalmopathy: diagnosis with orbital MR imaging and correlation with clinical score. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Pitceathly RDS, Morrow JM, Sinclair CDJ, Woodward C, Sweeney MG, Rahman S, et al. Extra‐ocular muscle MRI in genetically‐defined mitochondrial disease. Eur Radiol 2016;26:130–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Ortube MC, Bhola R, Demer JL. Orbital magnetic resonance imaging of extraocular muscles in chronic progressive external ophthalmoplegia: specific diagnostic findings. J AAPOS 2006;10:414–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Marinò M, Ionni I, Lanzolla G, Sframeli A, Latrofa F, Rocchi R, et al. Orbital diseases mimicking Graves' orbitopathy: a long‐standing challenge in differential diagnosis. J Endocrinol Invest 2020;43:401–411. [DOI] [PubMed] [Google Scholar]
- 83. Xian J, Zhang Z, Wang Z, Li J, Yang B, Man F, et al. Value of MR imaging in the differentiation of benign and malignant orbital tumors in adults. Eur Radiol 2010;20:1692–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Fatima Z, Ichikawa T, Ishigame K, Motosugi U, Waqar AB, Hori M, et al. Orbital masses: the usefulness of diffusion‐weighted imaging in lesion categorization. Clin Neuroradiol 2014;24:129–134. [DOI] [PubMed] [Google Scholar]
- 85. Chan JW, Orrison WW. Ocular myasthenia: a rare presentation with MuSK antibody and bilateral extraocular muscle atrophy [6]. Br J Ophthalmol 2007;91:842–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Gratton SM, Herro A, Bermudez‐Magner JA, Guy J. Atrophy and fibrosis of extra‐ocular muscles in anti‐acetylcholine receptor antibody myasthenia Gravis. Open J Ophthalmol 2014;04:117–119. [Google Scholar]
- 87. Velonakis G, Papadopoulos VE, Karavasilis E, Filippiadis DK, Zouvelou V. MRI evidence of extraocular muscle atrophy and fatty replacement in myasthenia gravis. Neuroradiology 2021;63:1531–1538. [DOI] [PubMed] [Google Scholar]
- 88. Ricciardi D, Todisco V, Tedeschi G, Cirillo G. Anti‐MuSK ocular myasthenia with extrinsic ocular muscle atrophy: a new clinical phenotype? Neurol Sci 2020;41:221–223. [DOI] [PubMed] [Google Scholar]
- 89. Keene KR, van Vught L, van de Velde NM, Ciggaar IA, Notting IC, Genders SW, et al. The feasibility of quantitative MRI of extra‐ocular muscles in myasthenia gravis and Graves' orbitopathy. NMR Biomed 2021;34:e4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Lueangaram S, Tritanon O, Siriyotha S, Vanikieti K, Padungkiatsagul T, Preechawat P, et al. Radiological characteristics of extraocular muscles in myasthenia gravis patients with ocular manifestations: a case‐control study. Clin Ophthalmol 2021;15:2279–2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Demer JL, Clark RA, Engle EC. Magnetic resonance imaging evidence for widespread orbital dysinnervation in congenital fibrosis of extraocular muscles due to mutations in KIF21A. Invest Ophthalmol Vis Sci 2005;46:530–539. [DOI] [PubMed] [Google Scholar]
- 92. El‐Sayed Mojahed M, Thabet EM, El‐Khateeb MG, Elsayed MA. Ocular vestibular evoked myogenic potential in patients with myasthenia gravis: a prospective clinical study. Auris Nasus Larynx 2018;45:407–411. [DOI] [PubMed] [Google Scholar]
- 93. Decock CE, De Baere EE, Bauters W, Shah AD, Delaey C, Forsyth R, et al. Insights into levator muscle dysfunction in a cohort of patients with molecularly confirmed blepharophimosis‐ptosis‐epicanthus inversus syndrome using high‐resolution imaging, anatomic examination, and histopathologic examination. Arch Ophthalmol 2011;129:1564–1569. [DOI] [PubMed] [Google Scholar]
- 94. Xia S, Li RL, Li YP, Qian XH, Chong V, Qi J. MRI findings in Duane's ocular retraction syndrome. Clin Radiol 2014;69. [DOI] [PubMed] [Google Scholar]
- 95. Culver EL, Salmon JF, Frith P, Travis SPL. Recurrent posterior scleritis and orbital myositis as extra‐intestinal manifestations of Crohn's disease: case report and systematic literature review. J Crohns Colitis 2008;2:337–342. [DOI] [PubMed] [Google Scholar]
- 96. Mombaerts I, Rose GE, Garrity JA. Orbital inflammation: biopsy first. Surv Ophthalmol 2016;61:664–669. [DOI] [PubMed] [Google Scholar]
- 97. Dik WA, Virakul S, van Steensel L. Current perspectives on the role of orbital fibroblasts in the pathogenesis of Graves' ophthalmopathy. Exp Eye Res 2016;142:83–91. [DOI] [PubMed] [Google Scholar]
- 98. Potgieser PW, de Win MMML, Wiersinga WM, Mourits MP. Natural course of mild graves orbitopathy: increase of orbital fat but decrease of muscle volume with increased muscle fatty degeneration during a 4‐year follow‐up. Ophthal Plast Reconstr Surg 2019;35:456–460. [DOI] [PubMed] [Google Scholar]
- 99. Yellin H. Unique intrafusal and extraocular muscle fibers exhibiting dual actomyosin ATPase activity. Exp Neurol 1969;25:153–163. [DOI] [PubMed] [Google Scholar]
- 100. Liu JX, Pedrosa DF. A novel type of multiterminal motor endplate in human extraocular muscles. Invest Ophthalmol Vis Sci 2018;59:539–548. [DOI] [PubMed] [Google Scholar]
- 101. Bishop KN, McClung JR, Goldberg SJ, Shall MS. Anatomic and physiological characteristics of the ferret lateral rectus muscle and abducens nucleus. J Appl Physiol 2007;103:1706–1714. [DOI] [PubMed] [Google Scholar]
- 102. Sekulic‐Jablanovic M, Ullrich ND, Goldblum D, Palmowski‐Wolfe A, Zorzato F, Treves S. Functional characterization of orbicularis oculi and extraocular muscles. J Gen Physiol 2016;147:395–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Sekulic‐Jablanovic M, Palmowski‐Wolfe A, Zorzato F, Treves S. Characterization of excitation‐contraction coupling components in human extraocular muscles. Biochem J 2015;466:29–36. [DOI] [PubMed] [Google Scholar]
- 104. McLoon LK, Rowe J, Wirtschafter J, McCormick KM. Continuous myofiber remodeling in uninjured extraocular myofibers: myonuclear turnover and evidence for apoptosis. Muscle Nerve 2004;29:707–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Kjellgren D, Thornell LE, Andersen J, Pedrosa‐Domellöf F. Myosin heavy chain isoforms in human extraocular muscles. Invest Ophthalmol Vis Sci 2003;44:1419–1425. [DOI] [PubMed] [Google Scholar]
- 106. Kitamura K, Cho KH, Jang HS, Murakami G, Yamamoto M, Ichi AS. Distance between intramuscular nerve and artery in the extraocular muscles: a preliminary immunohistochemical study using elderly human cadavers. Surg Radiol Anat 2017;39:3–9. [DOI] [PubMed] [Google Scholar]
- 107. Morrow JM, Sinclair CDJ, Fischmann A, Machado PM, Reilly MM, Yousry TA, et al. MRI biomarker assessment of neuromuscular disease progression: a prospective observational cohort study. Lancet Neurol 2016;15:65–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Europa TA, Nel M, Heckmann JM. A review of the histopathological findings in myasthenia gravis: clues to the pathogenesis of treatment‐resistance in extraocular muscles. Neuromuscul Disord 2019;29:381–387. [DOI] [PubMed] [Google Scholar]
- 109. Niendorf T, Beenakker JWM, Langner S, Erb‐Eigner K, Bach Cuadra M, Beller E, et al. Ophthalmic magnetic resonance imaging: where are we (heading to)? Curr Eye Res 2021;46:1251–1270. [DOI] [PubMed] [Google Scholar]
- 110. Ferreira TA, Fonk LG, Jaarsma‐Coes MG, van Haren GGR, Marinkovic M, Beenakker JWM. MRI of uveal melanoma. Cancers (Basel) 2019;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Jaarsma‐Coes MG, Marinkovic M, Astreinidou E, Schuurmans MS, Peters FP, Luyten GPM, et al. Measuring eye deformation between planning and proton beam therapy position using magnetic resonance imaging. Phys Imaging Radiat Oncol 2020;16:33–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Chardon JW, Díaz‐Manera J, Tasca G, Bönnemann CG, Gómez‐Andrés D, Heerschap A, et al. MYO‐MRI diagnostic protocols in genetic myopathies. Neuromuscul Disord 2019;29:827–841. [DOI] [PubMed] [Google Scholar]
- 113. Howard JF, Vu T, Mantegazza R, Kushlaf H, Suzuki S, Wiendl H, et al. MUS MGFA abstracts: MRI of the extra‐ocular muscles in myasthenia gravis show small volume and fat fraction increases. Muscle Nerve 2022;65:S1–S47. [Google Scholar]
- 114. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2021. J Cachexia Sarcopenia Muscle 2021;8:1081–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Involved EOM in different orbital disease with ophthalmoparesis and ptosis. The neuromuscular diseases are categorized in acquired, synaptic and hereditary.


