Abstract
BACKGROUND
Both abdominal obesity, defined as waist circumference (WC) ≥102 cm for men and WC ≥88 cm for women and increased body mass index (BMI; kg/m2) are known to be associated with hypertension. The aim of this study was to examine the independent and the combined relationship between abdominal obesity and increased BMI and hypertension by age, race, and gender in a national sample.
METHODS
This report is based on national level cross-sectional data for adults aged 18 years and older (11,145 participants) from the US National Health and Nutrition Examination Survey (NHANES) 2007–2010.
RESULTS
Abdominal obesity, after adjusting for BMI categories and other covariables, was independently associated with hypertension. That is, survey participants classified as abdominally obese had almost 50% increased odds of being hypertensive (odds ratio (OR) 1.51, 95% confidence interval (CI) 1.27–1.81) after controlling for BMI. After adjusting for covariables, the groups of individuals classified as abdominally obese and normal BMI; as abdominally obese and overweight; and abdominally obese and obese each had a progressive increase in the odds of hypertension when compared with individuals who had a normal BMI and no abdominal obesity (OR 1.81, 95% CI 1.28–2.57, OR 1.87, 95% CI 1.55–2.25, and OR 3.23, 95% CI 2.63–3.96, respectively)
CONCLUSIONS
Abdominal obesity is independently associated with hypertension after adjusting for BMI. After adjusting for covariables and parameterizing BMI categories and abdominal obesity the new variable showed a progressive increase in the odds of hypertension. Both BMI and WC should be included in models assessing hypertension risks.
Keywords: abdominal obesity, blood pressure, BMI, hypertension, NHANES
Cardiovascular disease is the leading cause of death in the United States.1 Increased body mass index (BMI) (calculated as (weight (kg)/height2 (m))) is a surrogate for total body fat and abdominal obesity (estimated by abdominal waist circumference (WC)) cutoff criteria (WC ≥102 cm for men and WC ≥88 cm for women) is a surrogate for abdominal subcutaneous and visceral fat stores. Both increased BMI and abdominal obesity are associated with diabetes, cardiovascular disease, and mortality.2–8 A number of studies suggest that abdominal obesity is independently associated with cardiovascular diseases after adjusting for BMI.9,10 Specifically, increased accumulation of visceral fat is thought to be associated with increased insulin resistance, which may contribute to the development of atherosclerosis and hypertension.11
Recent trends in obesity (BMI ≥30) and abdominal obesity show that between 1999 and 2008, abdominal obesity increased both in men and women, whereas obesity increased only in women.12 Comparing the trends in BMI and WC suggests that WC has increased independently from BMI (average 0.9 cm) between the years 1988–1994 and 2005–2006.13 Aside from one study using the third National Health and Nutrition Examination Survey (NHANES 1988–1994) data, no other study using more recent NHANES data has assessed the specific association between abdominal obesity and hypertension.14 Another motivation for the present study is the fact that a number of current studies suggested that at the population level, BMI and WC all predicted cardiovascular risk factors equally well; suggesting that WC is interchangeable with BMI as a risk predictor.15,16
The objectives of our study are twofold. The first objective is to examine the independent association between abdominal obesity and hypertension, adjusting for BMI, demographic and other covariates shown to be associated with hypertension. The second objective is to examine the combined effect of BMI and abdominal obesity on hypertension risk adjusting for demographic and other covariates shown to be associated with hypertension.17,18
METHODS
Survey description.
The NHANES 2007–2008 and 2009–2010 surveys were fielded by the US National Center for Health Statistics, a part of the Centers for Disease Control and Prevention. The procedures to select the sample and conduct the interview and examination have been previously described.19 This study is based on the 2007–2010 NHANES data.
Sample.
A total of 17,170 individuals 18 years of age and older were sampled. Of these, 12,755 (74%) were interviewed and 12,355 (72%) were examined. Of those examined, 1,210 individuals were excluded as follows: 125 due to pregnancy; 717 due to missing data on WC; 22 due to missing data on BMI; and 346 due to missing blood pressure (BP) data. These exclusions resulted in a final analytic sample of 11,145 participants aged 18 years and older.
Outcome variables.
A maximum of three brachial systolic and diastolic BP readings were collected for each participant: the mean of these recorded values was used to represent the participants’ systolic and diastolic BP. All BP readings were obtained during a single examination visit. Trained physicians following a standard protocol measured BP at the MEC using a Baumanometer true gravity wall model and standard mercury Baumanometer cuffs (small adult (17–22 cm), adult (22–32 cm), large adult (32–42 cm), and X-large adult (42–50 cm)).20 Appropriate BP cuff sizes were based on the measurement of the mid-arm circumference. The study BP measurements were obtained after the participant had been seated and resting for a minimum of 5 min; three individual BP determinations were taken 30 s apart.20 A participant was defined as having hypertension if at least one of the following conditions applied: a systolic BP of 140 mm Hg or greater; a diastolic BP of 90 mm Hg or greater; or currently taking prescribed medications for high BP.
Abdominal obesity was obtained from measured WC. A standard protocol was followed, in which the measurement was taken at the uppermost lateral border of the right ilium, to the nearest 0.1 cm, and at the end of the examinee’s normal expiration of breath. Abdominal obesity was defined as a WC of ≥102 cm for males and ≥88 cm for females.2
Demographic covariates.
Age was categorized into the following groups: 18–39, 40–59, 60–79, and 80 years or older. Race/ethnicity, based on self-reported information, was classified as non-Hispanic white, non-Hispanic black, and Hispanic. Participants not fitting the above self-classification were classified as “other.” Data for the “other” group, including persons who reported multiple races, were included in the total sample results, but because of small sample sizes are not reported separately in the data tables. Education attainment was categorized as: less than high school, high school, and more than high school education. Income status was based on the family income to poverty ratio (IPR). Families that have IPR values below 1.00 have incomes that are below the official poverty threshold; and IPR values of ≥1.00 are above the poverty level (US Census Bureau 2003).
Other covariates.
BMI was calculated as weight in kilograms over height in meters squared (kg/m²), and was categorized using criteria established by the National Institutes of Health (NIH) as underweight (<18.5 kg/m²), normal (18.5–24.9 kg/m²), overweight (25.0–29.9 kg/m2), and obese (≥30 kg/m2). Due to the relatively small number of respondents in the underweight category, the underweight category was joined with the normal category after a sensitivity analysis showed little difference in the results between excluding the underweight category and including them in the normal weight category. Responses to the medical conditions section of the NHANES household interview were used to establish a history of risk factors.20 A participant was defined as “diabetic” if they reported they had ever been told by a doctor that they had diabetes. History of cardiovascular disease was ascertained by a positive response to any of the following conditions “Has a doctor or other health care professionals ever told you had: congestive heart failure, coronary heart disease, angina, or heart attack.”20 Smoking status was ascertained by responses to the smoking section of the household questionnaire and participants were classified as never, former, and current smokers. Smokers were defined as persons who had smoked at least 100 cigarettes during their lifetime and were currently smoking.20 Leisure–time physical activity (LTPA) was measured using the World Health Organization’s Global Physical Activity Questionnaire.21 Respondents were asked about the usual amount of time they engaged in vigorous and moderately intense LTPA during a typical week. Total LTPA time was categorized as: none; 0 to <300 min; and 300 or more min/week.
Statistical analyses.
Analyses were conducted using SAS (version 9.2; SAS Institute, Cary, NC), SUDAAN (version 10.0; Research Triangle Institute, Research Triangle Park, NC), and R (version 2.13; The R Foundation for Statistical Computing). MEC examination sample weights and the appropriate sample design variables were used in the analysis. The MEC examination sample weights account for the complex survey design (including oversampling), survey nonresponse, and are also post-stratified to obtain nationally representative estimates of the US civilian non-institutionalized population.
Except for age-specific estimates, all prevalence estimates for hypertension were adjusted for age. Age-adjustment was performed, by the direct method, using the year 2000 projected US population with the aforementioned age groups. The SUDAAN Taylor series linearization method was used to calculate 95% confidence intervals (CIs) for the estimated prevalences. Statistical testing was performed using t-tests with an α-level of < 0.05 denoting statistical significance. Adjusted odds ratios (OR) and 95% CIs were calculated using four different logistic regression models. The first model included all the covariates and abdominal obesity; the second included the covariates and BMI; the third included the covariates, BMI and abdominal obesity. We also examined a third model that included an interaction between BMI and abdominal obesity; the interaction was near significant (P = 0.051). Therefore, the fourth model was added that included all the covariates and a cross-classification of BMI and abdominal obesity as a single variable (see Table 3 for the parameterized variable). Here, the odds of hypertension were calculated for the major different combinations of BMI and WC subgroups, using the subgroup with normal body weight and no abdominal obesity as a referent. Adjusted ORs having a 95% CI, not including unity, were considered statistically significant. In order to graphically display the distributions of WC by gender, BMI category, and hypertension status, the WC distribution was smoothed using svysmooth in the R survey package.22
Table 3 |.
Adjusted odds ratios for hypertension: NHANES 2007–2010
| Odds ratio (95% CI) | ||||
|---|---|---|---|---|
| Age group (years) | ||||
| 18–39 | — | — | — | — |
| 40–59 | 4.43 (3.51–5.61) | 4.78 (3.81–6.00) | 4.66 (3.70–5.86) | 4.63 (3.69–5.82) |
| 60–79 | 14.6 (11.6–18.4) | 16.7 (13.3–21.0) | 16.0 (12.7–20.1) | 16.0 (12.7–20.1) |
| 80 or more | 33.7 (24.2–46.9) | 42.6 (30.5–59.6) | 40.2 (28.8–56.1) | 40.1 (28.7–56.1) |
| Gender | ||||
| Male | — | — | — | — |
| Female | 0.75 (0.66–0.86) | 0.90 (0.81–1.01) | 0.82 (0.73–0.93) | 0.82 (0.73–0.93) |
| Race/ethnicity | ||||
| Hispanic | 0.69 (0.60–0.80) | 0.65 (0.56–0.76) | 0.67 (0.57–0.78) | 0.67 (0.58–0.78) |
| Non-Hispanic White | — | — | — | — |
| Non-Hispanic Black | 1.90 (1.55–2.34) | 1.76 (1.44–2.14) | 1.80 (1.47–2.21) | 1.82 (1.49–2.23) |
| Education | ||||
| Less than high school | — | — | — | — |
| High school | 1.00 (0.83–1.21) | 1.00 (0.82–1.21) | 0.99 (0.82–1.20) | 0.99 (0.82–1.20) |
| More than high school | 0.83 (0.69–0.99) | 0.83 (0.70–0.99) | 0.83 (0.69–0.98) | 0.83 (0.69–0.98) |
| Diabetes | ||||
| Yes | 2.67 (2.17–3.29) | 2.53 (2.06–3.12) | 2.49 (2.03–3.05) | 2.47 (2.01–3.04) |
| No | — | — | — | — |
| Cardiovascular disease | ||||
| Yes | 1.83 (1.42–2.34) | 1.84 (1.44–2.36) | 1.81 (1.41–2.31) | 1.82 (1.42–2.32) |
| No | — | — | — | — |
| Cigarette smoking | ||||
| Never | — | — | — | — |
| Former | 0.96 (0.83–1.12) | 0.99 (0.86–1.15) | 0.98 (0.84–1.13) | 0.98 (0.84–1.13) |
| current | 0.87 (0.72–1.05) | 0.93 (0.77–1.13) | 0.92 (0.76–1.11) | 0.92 (0.76–1.12) |
| Leisure–time physical activity | ||||
| None | 1.42 (1.16–1.74) | 1.41 (1.14–1.74) | 1.38 (1.12–1.70) | 1.38 (1.12–1.70) |
| Less than 300 min/week | 1.29 (1.04–1.61) | 1.30 (1.06–1.61) | 1.29 (1.04–1.59) | 1.29 (1.04–1.59) |
| 300 or more min/week | — | — | — | — |
| Abdominal obesity a | ||||
| Yes | 2.32 (1.98–2.71) | 1.51 (1.27–1.81) | ||
| No | — | na | — | na |
| Body mass index (BMI) | ||||
| Normal/underweight (<25.0) | na | — | — | na |
| Overweight (25.0–29.9) | 1.55 (1.32–1.84) | 1.26 (1.03–1.54) | ||
| Obese (≥30.0) | 2.96 (2.43–3.60) | 2.05 (1.63–2.59) | ||
| Abdominal obesity, body mass index | ||||
| Yes, normal | 1.81 (1.28–2.57) | |||
| Yes, overweight | 1.87 (1.55–2.25) | |||
| Yes, obese | 3.23 (2.63–3.96) | |||
| No, normal | na | na | na | — |
| No, overweight | 1.38 (1.08–1.77) | |||
| No, obese | 1.17 (0.62–2.20) | |||
Adjusted model includes age group, gender, race/ethnicity, education, diabetes, cardiovascular disease, cigarette smoking, and leisure–time physical activity.
Abdominal obesity is defined as a waist circumference of 102 or more centimeters for males and 88 or more centimeters for females.
CI, confidence interval; NHANES, US National Health and Nutrition Examination Survey; —, denotes reference group; na, not included in model.
RESULTS
Table 1 shows the prevalence of abdominal obesity by demographic and other covariates. Overall, the prevalence of abdominal obesity was 52.8% and it was significantly associated with all of the covariates except family IPR. It was higher in females (62.6%) then males (42.7%), higher in self-reported diabetics (82.0%) than nondiabetics, and higher in those reporting no LTPA (60.9%) than in those reporting 300 min or more of LTPA per week (38.4%).
Table 1 |.
Prevalence of abdominal obesity by selected characteristics: NHANES 2007–2010
| Number | Abdominal obesity | P value* | ||
|---|---|---|---|---|
| Yes | No | |||
| Row percent (95% CI) | ||||
| Total | 11,145 | 52.8 (50.9–54.7) | 47.2 (45.3–49.1) | |
| Age group (years) | <0.001 | |||
| 18–39 | 3,958 | 38.7 (35.8–41.7) | 61.3 (58.3–64.2) | |
| 40–59 | 3,569 | 57.9 (55.2–60.4) | 42.1 (39.6–44.8) | |
| 60–79 | 2,964 | 69.3 (66.9–71.6) | 30.7 (28.4–33.1) | |
| 80 or more | 654 | 61.8 (58.4–65.1) | 38.2 (34.9–41.6) | |
| Gender | <0.001 | |||
| Male | 5,554 | 42.7 (40.3–45.1) | 57.3 (54.9–59.7) | |
| Female | 5,591 | 62.6 (60.5–64.6) | 37.4 (35.4–39.5) | |
| Race/ethnicity | <0.001 | |||
| Hispanic | 3,230 | 51.8 (48.0–55.6) | 48.2 (44.4–52.0) | |
| Non-Hispanic white | 5,267 | 54.3 (51.9–56.6) | 45.7 (43.4–48.1) | |
| Non-Hispanic black | 2,132 | 56.8 (53.5–60.0) | 43.2 (40.0–46.5) | |
| Education | <0.001 | |||
| Less than high school | 3,116 | 57.4 (54.2–60.4) | 42.6 (39.6–45.8) | |
| High school | 2,532 | 57.0 (54.5–59.5) | 43.0 (40.5–45.5) | |
| More than high school | 4,966 | 51.4 (48.8–54.0) | 48.6 (46.0–51.2) | |
| Family income to poverty ratio | >0.05 | |||
| <1.0 | 2,214 | 51.7 (47.6–55.8) | 48.3 (44.2–52.4) | |
| 1.0–1.9 | 2,763 | 56.1 (52.7–59.4) | 43.9 (40.6–47.3) | |
| 2.0–3.9 | 2,641 | 53.3 (50.7–55.9) | 46.7 (44.1–49.3) | |
| ≥4.0 | 2,518 | 51.5 (48.2–54.8) | 48.5 (45.2–51.8) | |
| Body mass index (kg/m2) | <0.001 | |||
| Normal/underweight (<25.0) | 3,337 | 8.0 (6.8–9.5) | 92.0 (90.5–93.2) | |
| Overweight (25.0–29.9) | 3,764 | 52.1 (49.7–54.6) | 47.9 (45.4–50.3) | |
| Obese (≥30.0) | 4,044 | 96.5 (95.6–97.1) | 3.5 (2.9–4.4) | |
| Diabetes | <0.001 | |||
| Yes | 1,276 | 82.0 (78.6–85.0) | 18.0 (15.0–21.4) | |
| No | 9,860 | 50.2 (48.2–52.2) | 49.8 (47.8–51.8) | |
| Cardiovascular disease | <0.001 | |||
| Yes | 849 | 72.6 (68.4–76.5) | 27.4 (23.5–31.6) | |
| No | 9,666 | 52.6 (50.5–54.7) | 47.4 (45.3–49.5) | |
| Cigarette smoking | <0.001 | |||
| Never | 5,613 | 53.3 (50.4–56.2) | 46.7 (43.8–49.6) | |
| Former | 2,637 | 61.4 (59.0–63.8) | 38.6 (36.2–41.0) | |
| Current | 2,358 | 46.7 (44.0–49.4) | 53.3 (50.6–56.0) | |
| Leisure–time physical activity | <0.001 | |||
| None | 6,042 | 60.9 (58.8–63.0) | 39.1 (37.0–41.2) | |
| Less than 300 min/week | 2,792 | 51.6 (48.6–54.7) | 48.4 (45.3–51.4) | |
| 300 or more min/week | 2,294 | 38.4 (35.1–41.7) | 61.6 (58.3–64.9) | |
Abdominal obesity is defined as a waist circumference of 102 or more centimeters for males and 88 or more centimeters for females.
CI, confidence interval; NHANES, US National Health and Nutrition Examination Survey.
P value for χ2-test of independence.
Age-adjusted and age-specific prevalences of hypertension, according to abdominal obesity category, are shown in Table 2. Overall, there was a statistically significant difference in the age-adjusted prevalence of hypertension between the abdominal obesity group (35.0%) and the group without abdominal obesity (21.0%). Consistently across all levels of the demographic and other covariates, participants categorized as abdominally obese had a higher prevalence of hypertension when compared with participants categorized as not abdominally obese. These differences, with the exception of the 80 or more years of age category, were all statistically significant. Also of particular interest was the finding of a higher prevalence of hypertension in the abdominal obesity subgroup within all three levels of BMI.
Table 2 |.
Age-adjusted and age-specific prevalence of hypertension by abdominal obesity: NHANES 2007–2010
| Number | Abdominal obesity | P value | ||
|---|---|---|---|---|
| Yes | No | |||
| % Hypertension (95% CI) | ||||
| Total* | 11,145 | 35.0 (33.6–36.4) | 21.0 (19.5–22.7) | <0.001 |
| Age group (years) | ||||
| 18–39 | 3,958 | 11.4 (9.3–13.8) | 4.3 (3.4–5.5) | <0.001 |
| 40–59 | 3,569 | 39.7 (37.1–42.3) | 19.8 (16.7–23.3) | <0.001 |
| 60–79 | 2,964 | 69.8 (67.2–72.4) | 49.3 (44.6–54.1) | <0.001 |
| 80 or more | 654 | 81.2 (76.5–85.2) | 75.4 (69.4–80.5) | >0.05 |
| Gender * | ||||
| Male | 5,554 | 37.9 (35.4–40.4) | 23.1 (21.2–25.0) | <0.001 |
| Female | 5,591 | 33.1 (31.8–34.5) | 17.9 (15.7–20.2) | <0.001 |
| Race/ethnicity * | ||||
| Hispanic | 3,230 | 30.9 (29.3–32.5) | 19.6 (17.9–21.4) | <0.001 |
| Non-Hispanic white | 5,267 | 34.1 (31.9–36.2) | 20.2 (18.1–22.4) | <0.001 |
| Non-Hispanic black | 2,132 | 47.4 (43.9–51.0) | 29.2 (26.5–32.0) | <0.001 |
| Education* | ||||
| Less than high school | 3,116 | 37.3 (34.8–39.9) | 24.2 (21.8–26.7) | <0.001 |
| High school | 2,532 | 37.8 (34.9–40.7) | 23.0 (20.3–26.0) | <0.001 |
| More than high school | 4,966 | 33.2 (31.2–35.3) | 19.5 (17.4–21.8) | <0.001 |
| Family income to poverty ratio * | ||||
| <1.0 | 2,214 | 37.9 (34.6–41.3) | 21.2 (18.5–24.3) | <0.001 |
| 1.0–1.9 | 2,763 | 36.2 (34.1–38.3) | 23.7 (20.9–26.6) | <0.001 |
| 2.0–3.9 | 2,641 | 35.5 (32.3–38.9) | 23.8 (20.8–26.9) | <0.001 |
| ≥4.0 | 2,518 | 33.8 (31.2–36.6) | 19.5 (16.8–22.5) | <0.001 |
| Body mass index (kg/m2) * | ||||
| Normal/underweight (< 25.0) | 3,337 | 24.2 (20.1–28.7) | 19.4 (17.6–21.3) | <0.05 |
| Overweight (25.0–29.9) | 3,764 | 27.0 (25.4–28.7) | 23.5 (21.0–26.2) | <0.05 |
| Obese (≥ 30.0) | 4,044 | 39.7 (38.1–41.2) | 23.0 (17.7–29.3) | <0.001 |
| Diabetes * | ||||
| Yes | 1,276 | 59.7 (53.1–65.9) | 40.0 (29.8–51.2) | <0.01 |
| No | 9,860 | 32.6 (31.2–34.0) | 20.4 (18.8–22.1) | <0.001 |
| Cardiovascular disease | ||||
| Yes | 849 | 59.1 (47.3–69.9) | 41.7 (27.8–57.0) | >0.05 |
| No | 9,666 | 34.1 (32.5–35.7) | 20.5 (19.0–22.1) | <0.001 |
| Cigarette smoking * | ||||
| Never | 5,613 | 34.6 (32.8–36.6) | 21.0 (19.2–23.1) | <0.001 |
| Former | 2,637 | 36.9 (33.7–40.1) | 19.9 (16.9–23.2) | <0.001 |
| Current | 2,358 | 34.6 (32.0–37.2) | 22.6 (19.9–25.5) | <0.001 |
| Leisure–time physical activity * | ||||
| None | 6,042 | 37.3 (35.5–39.2) | 22.3 (20.8–23.8) | <0.001 |
| Less than 300 min/week | 2,792 | 33.9 (31.8–36.0) | 21.1 (18.7–23.8) | <0.001 |
| 300 or more min/week | 2,294 | 29.5 (26.0–33.2) | 18.7 (15.8–22.1) | <0.001 |
Abdominal obesity is defined as a waist circumference of 102 or more centimeters for males and 88 or more centimeters for females.
CI, confidence interval; NHANES, US National Health and Nutrition Examination Survey.
Age-adjusted.
Table 3 shows the results of the four logistic regression models. The first model, which did not include BMI, shows the main effects of abdominal obesity on hypertension risk, after adjusting for the effects of age, gender, race/ethnicity, education, IPR, BMI, diabetes, cardiovascular disease, smoking, and LTPA. The odds of hypertension here were about 2.3 times higher among those with abdominal obesity than in those without abdominal obesity. The second model, which did not include WC, examined the main effect of BMI on hypertension risk. This model showed that, after adjusting for the covariables, the odds of hypertension increase with an increasing BMI. In model two, the odds of hypertension were about 1.6 times higher for overweight persons as compared to those with a normal BMI, and 3.0 times higher for obese persons as compared to those with normal weight.
Both WC and BMI are included as terms in the third model, which shows that after adjusting for the covariables, the odds of hypertension were about 1.5 times higher among those with abdominal obesity as compared to those without abdominal obesity. The fourth model shows the results of an analysis using a combined, cross-classified BMI and abdominal obesity variable. This allows estimation of ORs for hypertension risk in the major different combinations of BMI and WC subgroups. After adjusting for the covariables and using the BMI/WC subgroup with normal body weight and no abdominal obesity as a referent, the highest OR for hypertension was seen in the subgroup of individuals who were classified as both obese and abdominally obese (OR 3.23; 95% CI 2.63–3.96). The lowest OR for hypertension was seen in the subgroup of individuals who were obese but without abdominal obesity (OR 1.17; 95% CI 0.62–2.20).
Figure 1 shows smoothed density plots of the distribution of WC measurements for males by their BMI category and Figure 2 shows the same analysis for females. While we were unable to test differences between the density plots, in each of the three BMI subgroups (normal weight, overweight, and obese) there is a clear and consistent pattern of an overall rightward shift in WC for those with hypertension as compared to those without hypertension. Also evident in Figures 1 and 2 is an overall rightward shift in the distribution of WC with increasing BMI category.
Figure 1 |.
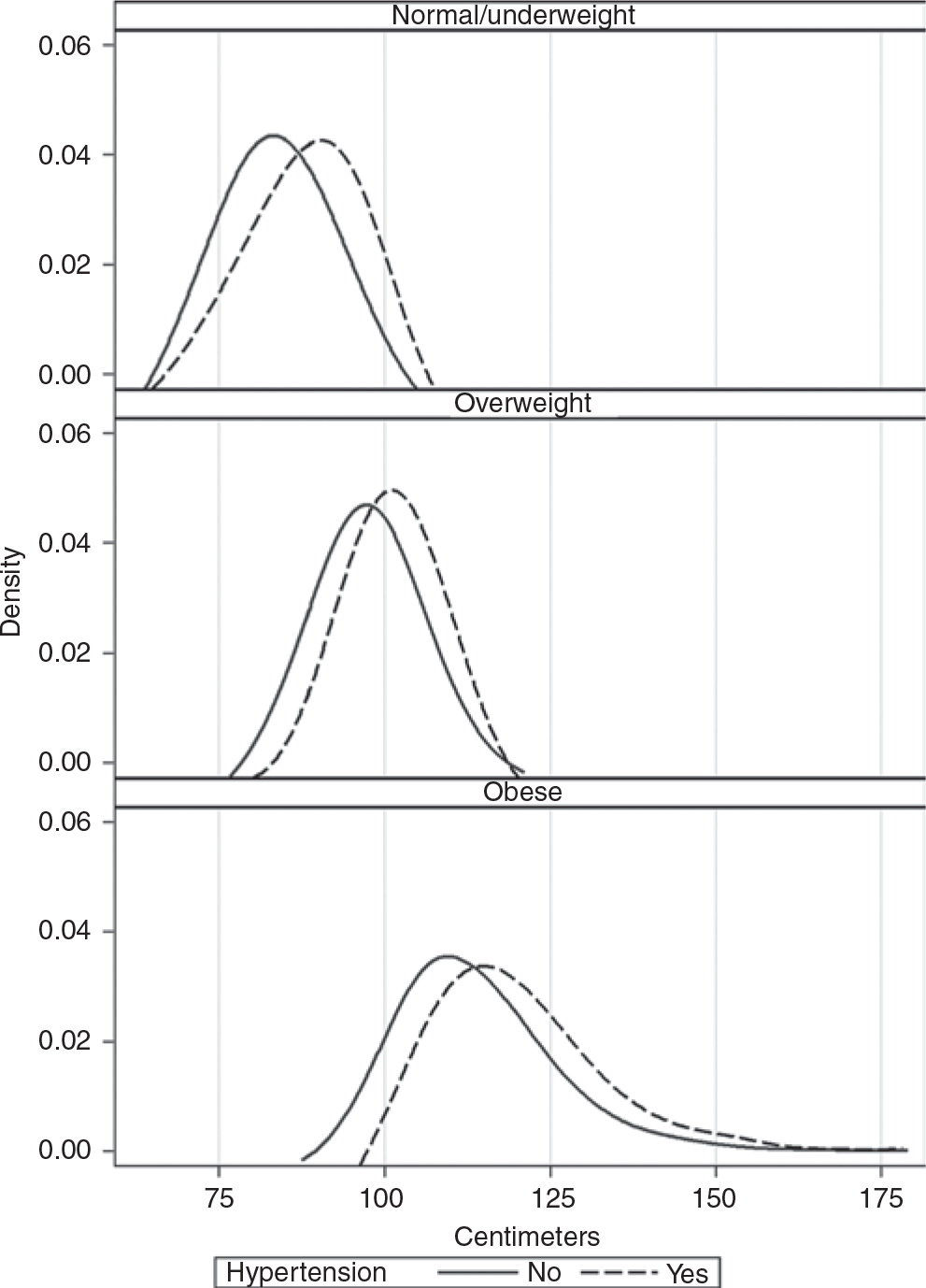
Distribution of waist circumference for males by body mass index category and hypertension status: US adults aged 18+ years, NHANES 2007–2010. NHANES, US National Health and Nutrition Examination Survey.
Figure 2 |.
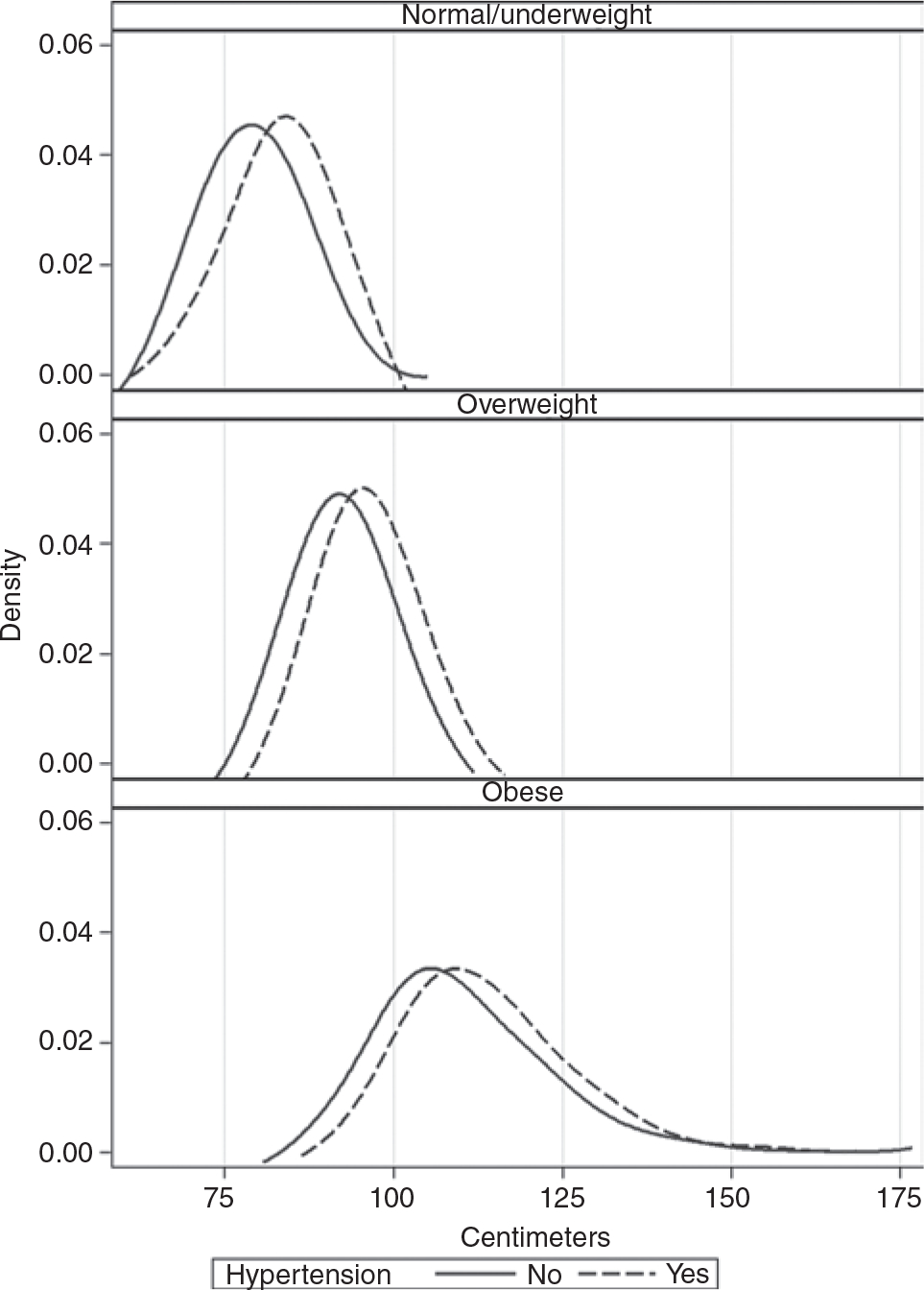
Distribution of waist circumference for females by body mass index category and hypertension status: US adults aged 18+ years, NHANES 2007–2010. NHANES 2007–2010. NHANES, US National Health and Nutrition Examination Survey.
DISCUSSION
Our results suggest that abdominal obesity, as defined by the NIH’s WC cutpoints, was independently associated with increased odds of hypertension even after adjusting for all other covariates. Adjusting for BMI participants classified as abdominally obese had almost 50% increased odds of being hypertensive when compared with individuals not classified as being abdominally obese. After adjusting for covariates, the groups of individuals classified as abdominally obese and normal BMI; as abdominally obese and overweight; and abdominally obese and obese each had a progressive increase in the odds of hypertension when compared with individuals who had a normal BMI and no abdominal obesity. In the present study, some 271 participants were classified as abdominally obese, yet had a normal BMI and yet, were at higher odds of being hypertensive when compared to reference (OR 1.81, 95% CI 1.27–1.81). The overwhelming majority of these were females (n = 258), non-Hispanic white (n = 175), with a mean age of 57 years and median age of 60 years. It may be possible to explain these findings by pointing to a number of studies suggesting that older women undergo physiological changes resulting in an increase in fat mass and a redistribution of fat to the abdominal area.22–24 It is not clear if this physiological phenomenon, whether it is a function of aging or due to a decline in estrogen levels (i.e., menopause).25 Similar results were previously reported by Ghandehari et al. using NHANES 2003–2004 data, survey participants with normal BMI levels and abdominal obesity had higher odds of being hypertensive when compared to reference (OR 1.86, 95% CI 1.33–2.61).26
It should be noted that while WC is a proxy for abdominal subcutaneous and visceral fat and BMI is a proxy for total body fat, the two measures are correlated to some extent, and relatively few participants with abdominal obesity were not classified as overweight or obese. Conceptually, if two variables are too highly correlated it is difficult to assess their independent effect on the response variable. Therefore, following the Allison method, we estimated the logistic regression model using an equivalent weighted linear regression model with the collinearity diagnostic option (Proc REG/VIF, SAS 9.2).23 Commonly, VIF value >10 is an indicator of multicollinearity, however VIF as low as 4 have been used in other studies to indicate serious multicollinearity.27,28 None of the independent variables in the third model had a VIF value close to 10; the highest VIF value was 3.5 for the independent variable obesity.
In a recent commentary Bouchard stated “it is hard to believe that much new information can be added by WAIST (WC; italic mine) once BMI is known.”29 Our results suggest that BMI and abdominal obesity are independent predictors of hypertension risk, abdominal obesity as measured by increased WC is a proxy for abdominal subcutaneous and visceral fat and BMI is a proxy for total body fat. So while there is some degree of overlap, the variables are not interchangeable but appear to have additive effects. Therefore abdominal obesity as measured by WC may independently add to our knowledge about risk factors related to hypertension.
That the independent effect of abdominal obesity is a surrogate for the independent effect of abdominal subcutaneous and visceral fat on hypertension and it is supported by recent studies. Specifically, these studies suggest that excess visceral fat is causally related to metabolic abnormalities resulting in increased insulin resistance. The studies propose three explanatory models to explain such a causal association: the portal vein free-fatty acid model; the endocrine model; and the ectopic fat deposition model.3,30–33 Insulin may exert its effect on the vascular tone through metabolic actions applied on endothelial cells stimulating nitric oxide production. Therefore, insulin resistance could result in a decreased ability of insulin to mediate vasodilatation in vascular tissue resulting in increased BP.11,34,35
Currently, there is no universal agreement on the cutpoints to define abdominal obesity, despite the fact that WC is one of the components of the Adult Treatment Panel III’s (ATP III) and the International Diabetes Federation’s (IDF) definitions of metabolic syndrome.36,37 Flegal et al., using NHANES III data, pointed out that ATP III/ criteria corresponds very closely to the 95% distribution of WC in healthy men and women.38
Further complicating the issue is the fact that there is no universally accepted anatomical site to measure WC (Mason and Katzmarzyk suggest four anatomical locations: superior border of the iliac crest, midpoint between the iliac crest and lower rib, umbilicus, and minimal waist.39) and the choice of site significantly influences abdominal obesity classification especially in women.39 The measurement of WC at the level of the iliac crest is recommended by NIH and the National Heart and Lung Institute (NHLBI)2 and has been the NHANES method of measuring WC since 1988 (start of NHANES III). It is felt that this landmark is easily identifiable and reproducible both in men and women (ICC, r = 0.998 and r = 0.999, respectively).4
Considering the above definitional issues, the review of the literature will be limited to studies using the NHANES method of measuring WC.
A number of studies using NHANES data examined the relationship between WC and cardiovascular risk factors. Janssen et al., using NHANES III data, examined whether nesting BMI categories within WC (normal and abdominal obesity) would improve the prediction power of BMI when assessing obesity related comorbidities, including hypertension.14 Their findings showed that after adjusting for BMI, WC as a continuous variable significantly predicted hypertension, whereas BMI levels nested within WC categories were not a significant predictor of hypertension.14 Ghandehari et al., using NHANES 2003–2004 data, examined the relationship between WC, BMI, and cardiometabolic risk factors. They reported that abdominal obesity was independently and significantly associated with more than three cardiometabolic risk factors, and among these was hypertension.26 Okosun et al., using NHANES III data, reported that abdominal obesity was independently and significantly associated with twofold and threefold increased risks of hypertension in men and women, respectively.39 Their reported ORs were higher than those reported in our current analysis. In our report, participants classified as abdominally obese had almost 60% increased odds of being hypertensive after controlling for BMI. This discrepancy may reflect the fact that Okosun et al. did not include BMI in their logistics models, whereas our model included BMI, which is highly correlated with WC, and therefore the observed magnitude of the WC effect is less.
The findings in this report are subject to several limitations. First, the cross-sectional study design provides only a one-time assessment of WC; therefore, in a strict sense, no causality of the currently observed association between hypertension and WC can be determined. Second, the analysis used NHLBI cutpoints which do not account for an age and race/ethnicity effect on WC.
The study results suggest that the NHLBI definition of abdominal obesity measured at the iliac crest level was independently associated with hypertension. WC is an easy and effective way to measure abdominal obesity. This study’s results also suggest that it may useful to measure across BMI categories (normal, overweight, and obese) when assessing hypertension risks.
Acknowledgments:
This paper is dedicated to the memory of Dr Lester (Randy) Curtin a friend and a mentor; you are going to be missed. Also, we thank Ms Michele Chiappa for all her editorial help.
Footnotes
Disclosure: The authors declared no conflict of interest.
References
- 1.Kochanek KD, Jiaguqan X, Murphy SL, Minino AM, Ching KH. Deaths: Preliminary data for 2009. National Vital Statistics 59:2011. [PubMed] [Google Scholar]
- 2.Clinical guidelines on the identification evaluation, and treatment of overweight and obesity in adults. <http://www.nhlbi.nih.gov/guidelines/obesity/ob_gdlns.pdf> Accessed 4 June 2012. [PubMed]
- 3.Canoy D Coronary heart disease and body fat distribution. Curr Atheroscler Rep 2010; 12:125–133. [DOI] [PubMed] [Google Scholar]
- 4.Klein S, Allison DB, Heymsfield SB, Kelley DE, Leibel RL, Nonas C, Kahn R. Waist Circumference and Cardiometabolic Risk: a Consensus Statement from Shaping America’s Health: Association for Weight Management and Obesity Prevention; NAASO, the Obesity Society; the American Society for Nutrition; and the American Diabetes Association. Obesity (Silver Spring) 2007; 15:1061–1067. [DOI] [PubMed] [Google Scholar]
- 5.de Koning L, Merchant AT, Pogue J, Anand SS. Waist circumference and waist-to-hip ratio as predictors of cardiovascular events: meta-regression analysis of prospective studies. Eur Heart J 2007; 28:850–856. [DOI] [PubMed] [Google Scholar]
- 6.Grundy SM, Cleeman JI, Merz CN, Brewer HB Jr, Clark LT, Hunninghake DB, Pasternak RC, Smith SC Jr, Stone NJ; Coordinating Committee of the National Cholesterol Education Program. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Arterioscler Thromb Vasc Biol 2004; 24:e149–e161. [DOI] [PubMed] [Google Scholar]
- 7.Cornier MA, Després JP, Davis N, Grossniklaus DA, Klein S, Lamarche B, Lopez-Jimenez F, Rao G, St-Onge MP, Towfighi A, Poirier P; American Heart Association Obesity Committee of the Council on Nutrition; Physical Activity and Metabolism; Council on Arteriosclerosis; Thrombosis and Vascular Biology; Council on Cardiovascular Disease in the Young; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing, Council on Epidemiology and Prevention; Council on the Kidney in Cardiovascular Disease, and Stroke Council. Assessing adiposity: a scientific statement from the american heart association. Circulation 2011; 124:1996–2019. [DOI] [PubMed] [Google Scholar]
- 8.Jacobs EJ, Newton CC, Wang Y, Patel AV, McCullough ML, Campbell PT, Thun MJ, Gapstur SM. Waist circumference and all-cause mortality in a large US cohort. Arch Intern Med 2010; 170:1293–1301. [DOI] [PubMed] [Google Scholar]
- 9.Casanueva FF, Moreno B, Rodríguez-Azeredo R, Massien C, Conthe P, Formiguera X, Barrios V, Balkau B. Relationship of abdominal obesity with cardiovascular disease, diabetes and hyperlipidaemia in Spain. Clin Endocrinol (Oxf) 2010; 73:35–40. [DOI] [PubMed] [Google Scholar]
- 10.Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature 2006; 444:875–880. [DOI] [PubMed] [Google Scholar]
- 11.Kotchen TA. Obesity-related hypertension: epidemiology, pathophysiology, and clinical management. Am J Hypertens 2010; 23:1170–1178. [DOI] [PubMed] [Google Scholar]
- 12.Ford ES, Li C, Zhao G, Tsai J. Trends in obesity and abdominal obesity among adults in the United States from 1999–2008. Int J Obes (Lond) 2011; 35:736–743. [DOI] [PubMed] [Google Scholar]
- 13.Walls HL, Stevenson CE, Mannan HR, Abdullah A, Reid CM, McNeil JJ, Peeters A. Comparing trends in BMI and waist circumference. Obesity (Silver Spring) 2011; 19:216–219. [DOI] [PubMed] [Google Scholar]
- 14.Janssen I, Katzmarzyk PT, Ross R. Waist circumference and not body mass index explains obesity-related health risk. Am J Clin Nutr 2004; 79:379–384. [DOI] [PubMed] [Google Scholar]
- 15.Bosy-Westphal A, Geisler C, Onur S, Korth O, Selberg O, Schrezenmeir J, Müller MJ. Value of body fat mass vs anthropometric obesity indices in the assessment of metabolic risk factors. Int J Obes (Lond) 2006; 30:475–483. [DOI] [PubMed] [Google Scholar]
- 16.Dalton M, Cameron AJ, Zimmet PZ, Shaw JE, Jolley D, Dunstan DW, Welborn TA; AusDiab Steering Committee. Waist circumference, waist-hip ratio and body mass index and their correlation with cardiovascular disease risk factors in Australian adults. J Intern Med 2003; 254:555–563. [DOI] [PubMed] [Google Scholar]
- 17.Ostchega Y, Hughes JP, Wright JD, McDowell MA, Louis T. Are demographic characteristics, health care access and utilization, and comorbid conditions associated with hypertension among US adults? Am J Hypertens 2008; 21:159–165. [DOI] [PubMed] [Google Scholar]
- 18.Sattelmair J, Pertman J, Ding EL, Kohl HW 3rd, Haskell W, Lee IM. Dose response between physical activity and risk of coronary heart disease: a meta-analysis. Circulation 2011; 124:789–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Curtin LR. Mohadjer L. Dohrmann S. The National Health and Nutrition Examination Survey (NHANES) Survey: Sample design, 1999–2006. National Center for Health Statistics. Vital Health Stat 2012; 2. [PubMed] [Google Scholar]
- 20.National Centre for Health Statistics, Centres for Disease Control and Prevention. National Health and Nutrition Examination Survey (NHANES) Questionnaire and Exam Protocol. <http://www.cdc.gov/nchs/about/major/nhanes/questexam>. Accessed 4 June 2012.
- 21.U.S. Department of Health and Human Services. 2008. Physical Activity Guidelines for Americans. <http://www.health.gov/PAGuidelines/>. Accessed 4 June 2012.
- 22.Lumley T (2011). Survey: Analysis of Complex Survey Samples. R package version 3.26. [Google Scholar]
- 23.Toth MJ, Tchernof A, Sites CK, Poehlman ET. Menopause-related changes in body fat distribution. Ann N Y Acad Sci 2000; 904:502–506. [DOI] [PubMed] [Google Scholar]
- 24.Poehlman ET, Tchernof A. Traversing the menopause: changes in energy expenditure and body composition. Coron Artery Dis 1998; 9:799–803. [PubMed] [Google Scholar]
- 25.Stevens J, Katz EG, Huxley RR. Associations between gender, age and waist circumference. Eur J Clin Nutr 2010; 64:6–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghandehari H, Le V, Kamal-Bahl S, Bassin SL, Wong ND. Abdominal obesity and the spectrum of global cardiometabolic risks in US adults. Int J Obes (Lond) 2009; 33:239–248. [DOI] [PubMed] [Google Scholar]
- 27.Allison P Logistic Regression Using the SAS System Theory and Application. SAS Institute: Cary, North Carolina. [Google Scholar]
- 28.O’Brien RM. A caution regarding rules of thumb for variance inflation factors. Quality & Quantity 2007; 41:673–690. [Google Scholar]
- 29.Bouchard C BMI, fat mass, abdominal adiposity and visceral fat: where is the ‘beef’? Int J Obes (Lond) 2007; 31:1552–1553. [DOI] [PubMed] [Google Scholar]
- 30.Ronti T, Lupattelli G, Mannarino E. The endocrine function of adipose tissue: an update. Clin Endocrinol (Oxf) 2006; 64:355–365. [DOI] [PubMed] [Google Scholar]
- 31.Windham BG, Griswold ME, Farasat SM, Ling SM, Carlson O, Egan JM, Ferrucci L, Najjar SS. Influence of leptin, adiponectin, and resistin on the association between abdominal adiposity and arterial stiffness. Am J Hypertens 2010; 23:501–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Whaley-Connell A, Sowers JR. Aldosterone and risk for insulin resistance. Hypertension 2011; 58:998–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cornier MA, Després JP, Davis N, Grossniklaus DA, Klein S, Lamarche B, Lopez-Jimenez F, Rao G, St-Onge MP, Towfighi A, Poirier P; On behalf of the American Heart Association Obesity Committee of the Council on Nutrition. Physical Activity and Metabolism, Council on Arteriosclerosis, Thrombosis and Vascular Biology, Council on Cardiovascular Disease in the Young, Council on Cardiovas. Assessing Adiposity: A Scientific Statement from the American Heart Association. Circulation 2011. [DOI] [PubMed] [Google Scholar]
- 34.Sowers JR. Insulin resistance and hypertension. Am J Physiol Heart Circ Physiol 2004; 286:H1597–H1602. [DOI] [PubMed] [Google Scholar]
- 35.Manrique C, Lastra G, Gardner M, Sowers JR. The renin angiotensin aldosterone system in hypertension: roles of insulin resistance and oxidative stress. Med Clin North Am 2009; 93:569–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adult Treatment Panel III’s (ATP III). <http://www.nhlbi.nih.gov/guidelines/cholesterol/atglance.pdf>. Accessed 4 June 2012.
- 37.International Diabetes Federation (IDF) definitions of metabolic syndrome (online). <http://www.idf.org/webdata/docs/IDF_Meta_def_final.pdf>. Accessed 4 June 2012.
- 38.Flegal KM. Waist circumference of healthy men and women in the United States. Int J Obes (Lond) 2007; 31:1134–1139. [DOI] [PubMed] [Google Scholar]
- 39.Mason C, Katzmarzyk PT. Variability in waist circumference measurements according to anatomic measurement site. Obesity (Silver Spring) 2009; 17:1789–1795. [DOI] [PubMed] [Google Scholar]


