Abstract
BACKGROUND
The best treatment option for giant intracranial aneurysms (GIAs) is still debated. The authors report a case of a giant thrombosed cavernous carotid artery (CCA) aneurysm for which two sessions of flow diverter (FD) placement failed, leading to bilateral blindness.
OBSERVATIONS
A 66-year-old man presented to an outside center with a history of rapid-onset right-sided retro-orbital pain, visual deterioration, and restricted eye movements associated with headache, vomiting, and diminished sensations on the right side of the face. He was diagnosed with an unruptured thrombosed giant CCA aneurysm and was treated twice with unsuccessful FDs. At follow-up, he developed blindness in both eyes. After 1.5 years, he presented to the authors’ institution with headache, vomiting, and epistaxis, for which he underwent high-flow external carotid artery–M2 segment of the middle cerebral artery bypass grafting using the radial artery as a conduit and ligation of the internal carotid artery in the neck without any added neurological deficits.
LESSONS
This dreadful complication of bilateral blindness after being treated with repeated unsuccessful FDs has not been reported in the literature. It could have been avoided if microsurgery had been the primary modality of treatment.
Keywords: cavernous carotid artery aneurysm, extracranial-intracranial bypass, flow diverter, giant aneurysm, complication
ABBREVIATIONS : CCA = cavernous carotid artery, CT = computed tomography, DSA = digital subtraction angiography, FD = flow diverter, GIA = giant intracranial aneurysm, ICA = internal carotid artery, MRI = magnetic resonance imaging, PED = Pipeline embolization device
Giant intracranial aneurysms (GIAs) are those with a minimum diameter of 25 mm. GIAs are among the most difficult aneurysms, have an unfavorable prognosis, and result in 20%–30% combined morbidity and mortality from surgery.1 Recent refinements in both endovascular and microsurgical techniques show promise.2 GIAs of the cavernous carotid artery (CCA) are uncommon and account for 3%–5% of intracranial aneurysms. Because of their extradural location, the clinical features differ from those of other aneurysms in an intradural location. Mass effect is one of their common manifestations, causing cranial nerve deficits.3 Endovascular therapy (EVT) with a flow diverter (FD) is currently considered the preferred mode of treatment for symptomatic CCA aneurysm. The presented case demonstrates an example of GIA of the CCA that was treated with FDs, resulting in a yet unreported and unintended result.
Illustrative Case
A 66-year-old man presented to an outside facility with a history of retro-orbital pain; rapid deterioration of vision in the right eye; and associated severe headache, vomiting, and diminished sensations on the right side of the face. His right eye visual acuity was 1/6 finger counting; he had a sluggish pupillary reflex; and the right eyeball showed restricted abduction. The left eye was clinically normal. The fundus showed optic nerve atrophy in the right eye and disc pallor in both eyes. Magnetic resonance imaging (MRI) of the brain with orbit and four-vessel cerebral digital subtraction angiography (DSA) showed a partially thrombosed giant right CCA aneurysm approximately 40 × 28 mm in size (Fig. 1). The patient underwent pipeline FD placement (Fig. 2). He continued to have headaches and vomiting. After EVT, his right eye gradually lost vision, and he started complaining of diminution of vision in his left eye. Ophthalmological evaluation confirmed complete vision loss in his right eye and 3/6 finger counting in his left eye. A four-vessel cerebral DSA and computed tomography (CT) scan of the brain showed residual filling of the aneurysm with evidence of leak. He was advised to repeat FD placement. He then underwent balloon angioplasty and Surpass Evolve FD placement 4 months after the first procedure (Fig. 3). His vision in his left eye improved for 2 months after the procedure, but his vision again started deteriorating in his left eye, and in a span of 6 months, he became blind in both eyes. He also started complaining of recurrence of headache with vomiting episodes, and he became lethargic and started feeling dizzy in the morning. He was evaluated by an endocrinologist and was diagnosed with hypocortisolemia and started on replacement therapy.
FIG. 1.

Lateral four-vessel cerebral DSA showing a 40 × 28–mm partially thrombosed giant right CCA aneurysm.
FIG. 2.

Four-vessel cerebral DSA showing Pipeline FD placement for a CCA aneurysm.
FIG. 3.
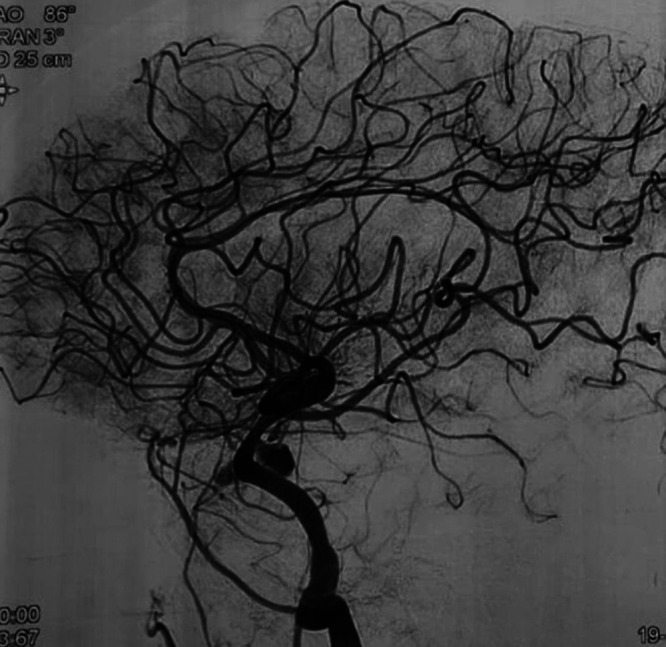
Lateral four-vessel cerebral DSA after Surpass Evolve FD placement for a CCA aneurysm.
Only 1.5 years after being diagnosed with a giant right CCA aneurysm, he was referred to us by an endovascular neurosurgeon. He had complaints of recurrent severe headache with multiple episodes of vomiting and episodic epistaxis of 2 weeks’ duration. He had been evaluated with DSA by the endovascular neurosurgeon. His collateral circulation was inadequate, and hence he was referred to us for extracranial-intracranial (EC-IC) arterial bypass. CT angiography of the head and neck vessels showed a 60 × 60 × 72–mm thrombosed right CCA aneurysm with extension into the nasal cavity and left maxillary sinus (Fig. 4). A microsurgical bypass was performed between the external carotid artery and M2 of the middle cerebral artery using a radial artery graft. After confirming anastomosis patency with indocyanine green angiography, ligation of the right internal carotid artery (ICA) was performed just after the bifurcation. The procedure was completed without any added neurological deficit. Postoperatively, he had significant improvement in headache, his vomiting subsided, and the epistaxis stopped. Postoperative three-dimensional reconstructed CT angiography of the head and neck vessels confirmed the patency of the graft (Fig. 5).
FIG. 4.
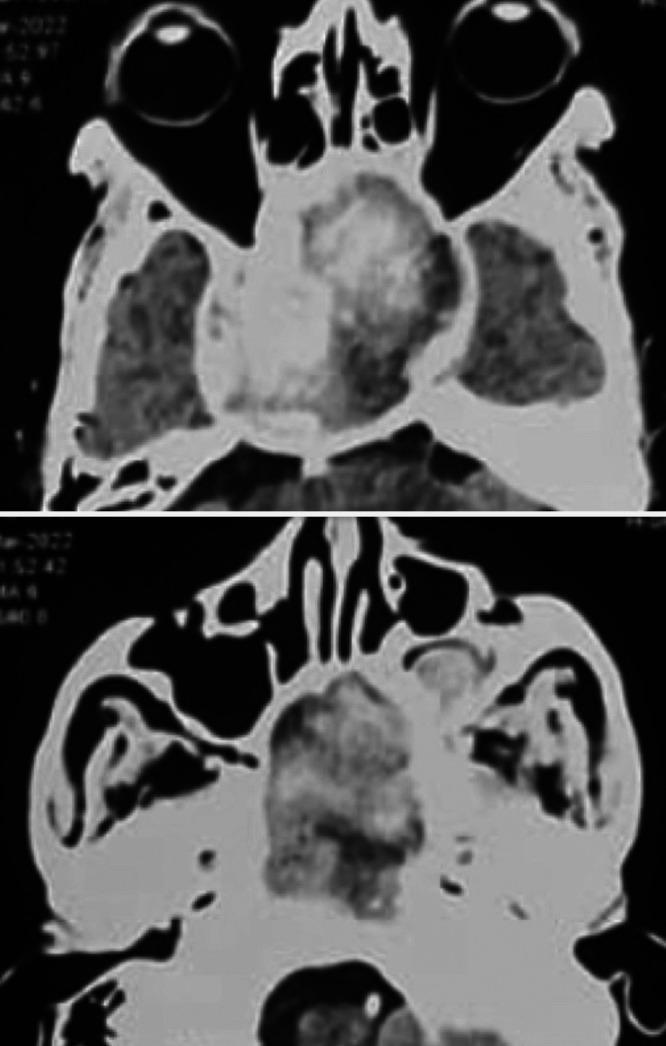
CT angiography of head and neck vessels showing 60 × 60 × 72–mm thrombosed right CCA aneurysm (upper) with extension into the nasal cavity and left maxillary sinus (lower).
FIG. 5.
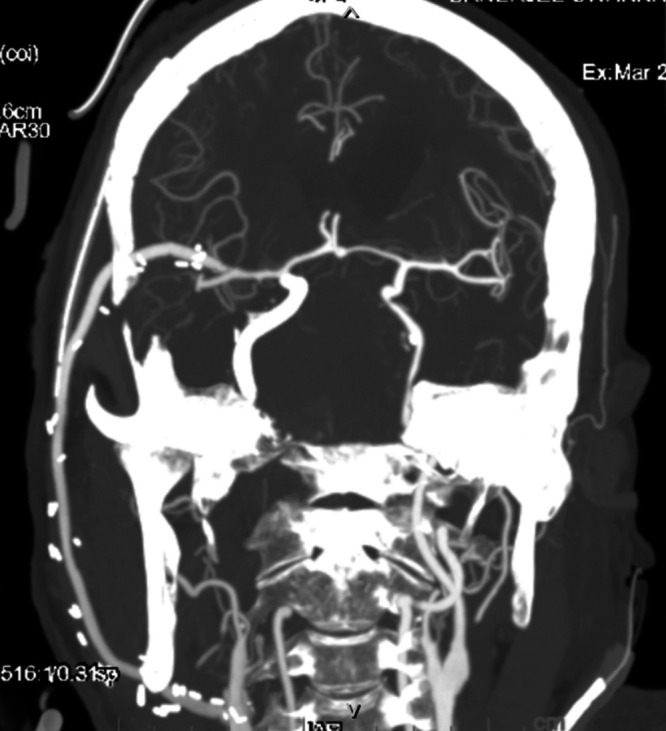
Postoperative three-dimensional reconstructed CT angiography of the head and neck vessels confirming patency of the external carotid artery–M2 segment of middle cerebral artery bypass graft.
Discussion
Observations
CCA aneurysm is not a common pathologic entity. Unruptured CCA aneurysms may cause compression of the cranial nerves, resulting in pain, double vision, ptosis, and/or visual failure.4 Direct clipping of such an aneurysm is rather challenging because of the unique anatomical neurovascular relationship. When such an aneurysm is approached via a direct surgical corridor, injury to the cranial nerves is inevitable, resulting in a significant postoperative neurological deficit. To avoid such neurological deficit postoperatively, alternative indirect strategies of flow diversion with or without EC-IC bypass have been developed. ICA occlusion proximally after a high-flow ECA-MCA radial bypass graft appears to be the most definitive treatment, resulting in symptomatic improvement and thrombosing the aneurysm with acceptable complications.3
FD devices are currently the preferred modality of treatment of GIAs of the CCA. In spite of the promise, several post-treatment complications, such as the risk of aneurysm rupture and mass effect, especially when treating giant ICA aneurysms, have been reported in the literature, which raises concern regarding the use of FD devices.5 Therefore, in the presence of the more conventional options, such as microsurgery and nonassisted endovascular coiling, the use of new neurovascular devices should be employed with caution until more definitive data are available. Results reported in some coiling and clipping series of GIAs are better than those achieved with FDs.6
Nurminen et al.7 reported a study of 76 ICA aneurysms (4 ruptured and 72 unruptured) in 62 patients. Flow diversion was employed in all cases and 6 of which underwent retreatment. The visual deficit improved in 60% cases, whereas the recovery rates for cranial nerves III and VI were 40%–50%. New post-procedural visual impairment was found to be a rare phenomenon that was seen in only 3% of the patients. The authors concluded that though results were acceptable after the use of FDs for cavernous and intradural ICA aneurysms, the probability of occlusion was uncertain, and improvement of cranial nerve dysfunction was incomplete in the presence of complex aneurysm features. A total of 316 cases treated with the Pipeline embolization device (PED) were reviewed by Daou et al.8 They observed no significant association between aneurysm size and aneurysm occlusion. Incomplete occlusion of the aneurysm was associated with age older than 65 years, aneurysm location in the distal anterior circulation, prior stent placement across the target aneurysm, and longer follow-up duration.
Moon et al.9 reported their experience with 127 cases of intracranial aneurysms treated with the PED, 22 of which had presented with ophthalmoparesis and facial hypoesthesia. In an average of 9.55 months of clinical follow-up, 15 patients (75%) had resolution or significant improvement of their cranial neuropathies, and the remaining patients had stable symptomatology. Symptomatic improvement at follow-up was most likely in patients with complete or near-complete occlusion of the aneurysm. Microsurgical bypass was needed for persistent symptoms in two patients. High rates of symptomatic relief in cranial nerve palsies were seen in patients with aneurysms treated with the PED. Novel modification of the device and new research may be necessary to treat the subset of patients who do not respond satisfactorily to the PED.
Worsening bilateral visual loss after treatment with the PED and coiling in a giant paraclinoid aneurysm was reported by Patel et al.10 Vision worsened after aneurysm thrombosis associated with an increase in aneurysm size and perilesional edema. Spontaneous improvement in vision at follow-up was seen associated with reduced mass effect, aneurysm size, and pulsation artifact on MRI. Puffer et al.11 did a prospective study in 44 patients with CCA aneurysms treated with FDs. The most frequent presenting symptoms included diplopia/cavernous cranial neuropathy followed by headache/retro-orbital pain and incidental diagnosis after an unrelated symptom with or without interval growth. There was significant improvement or complete resolution of their presenting symptoms in 90% of patients. Preexisting persistent problems included continued cranial neuropathy in two patients and minor headache in one patient.
Flow diversion by FD stents may be a safer and more effective method for treating the unruptured extradural CCA aneurysms than microsurgical flow diversion with or without bypass. Thus, EVT has become the first option of treatment for CCA aneurysms. However, a microsurgical approach of flow diversion with or without bypass may be better for selected GIAs of the CCA, especially those with significant mass effect as in the present case.
Lessons
Although EVT of symptomatic CCA aneurysms is an attractive and appropriate first choice, microsurgical flow diversion with or without bypass may be the treatment of choice for GIAs of the CCA, especially the ones presenting with significant mass effect, because of the high rate of complete thrombosis of the aneurysm with fewer complications and good symptomatic relief. A number of GIAs still get transformed into chronic disease requiring prolonged follow-up, frequent retreatments with additional risks, and frequent relapses after EVT.
Disclosures
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Author Contributions
Conception and design: Misra. Acquisition of data: Khandhar, Pradhan. Analysis and interpretation of data: Khandhar, Pradhan. Drafting the article: Khandhar, Pradhan. Critically revising the article: Misra. Reviewed submitted version of manuscript: all authors. Approved the final version of the manuscript on behalf of all authors: Misra. Administrative/technical/material support: Misra. Study supervision: Misra.
Supplemental Information
Previous Presentations
The case was presented (virtually) at the Annual Meeting of the World Academy of Neurological Surgeon (WANS) 2022, Salt Lake City, UT, April 27, 2022.
References
- 1. Sughrue ME, Saloner D, Rayz VL, Lawton MT. Giant intracranial aneurysms: evolution of management in a contemporary surgical series. Neurosurgery. 2011;69(6):1261–1271. doi: 10.1227/NEU.0b013e31822bb8a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Misra BK, Warade A. Microsurgery of giant intracranial aneurysms: current status. Neurol India. 2018;66(6):1614–1616. doi: 10.4103/0028-3886.246223. [DOI] [PubMed] [Google Scholar]
- 3. Sriamornrattanakul K, Sakarunchai I, Yamashiro K, et al. Surgical treatment of large and giant cavernous carotid aneurysms. Asian J Neurosurg. 2017;12(3):382–388. doi: 10.4103/1793-5482.180930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kupersmith MJ, Hurst R, Berenstein A, Choi IS, Jafar J, Ransohoff J. The benign course of cavernous carotid artery aneurysms. J Neurosurg. 1992;77(5):690–693. doi: 10.3171/jns.1992.77.5.0690. [DOI] [PubMed] [Google Scholar]
- 5. Peschillo S, Caporlingua A, Resta MC, et al. Endovascular treatment of large and giant carotid aneurysms with flow-diverter stents alone or in combination with coils: a multicenter experience and long-term follow-up. Oper Neurosurg (Hagerstown) 2017;13(4):492–502. doi: 10.1093/ons/opx032. [DOI] [PubMed] [Google Scholar]
- 6. Arrese I, Sarabia R, Pintado R, Delgado-Rodriguez M. Flow-diverter devices for intracranial aneurysms: systematic review and meta-analysis. Neurosurgery. 2013;73(2):193–200. doi: 10.1227/01.neu.0000430297.17961.f1. [DOI] [PubMed] [Google Scholar]
- 7. Nurminen V, Raj R, Numminen J, Kivisaari R, Niemelä M, Lehecka M. Flow diversion for internal carotid artery aneurysms: Impact of complex aneurysm features and overview of outcome. Clin Neurol Neurosurg. 2020;193:105782. doi: 10.1016/j.clineuro.2020.105782. [DOI] [PubMed] [Google Scholar]
- 8. Daou B, Atallah E, Chalouhi N, et al. Aneurysms with persistent filling after failed treatment with the Pipeline embolization device. J Neurosurg. 2019;130:1376–1382. doi: 10.3171/2017.12.JNS163090. [DOI] [PubMed] [Google Scholar]
- 9. Moon K, Albuquerque FC, Ducruet AF, Crowley RW, McDougall CG. Resolution of cranial neuropathies following treatment of intracranial aneurysms with the Pipeline Embolization Device. J Neurosurg. 2014;121(5):1085–1092. doi: 10.3171/2014.7.JNS132677. [DOI] [PubMed] [Google Scholar]
- 10. Patel S, Fargen KM, Peters K, Krall P, Samy H, Hoh BL. Return of visual function after bilateral visual loss following flow diversion embolization of a giant ophthalmic aneurysm due to both reduction in mass effect and reduction in aneurysm pulsation. J Neurointerv Surg. 2015;7(1):e1. doi: 10.1136/neurintsurg-2013-010960.rep. [DOI] [PubMed] [Google Scholar]
- 11. Puffer RC, Piano M, Lanzino G, et al. Treatment of cavernous sinus aneurysms with flow diversion: results in 44 patients. AJNR Am J Neuroradiol. 2014;35(5):948–951. doi: 10.3174/ajnr.A3826. [DOI] [PMC free article] [PubMed] [Google Scholar]


