Abstract
Background
Guidelines recommend that hepatocellular carcinoma (HCC) patients with portal vein tumor thrombosis (PVTT) and/or hepatic vein tumor thrombosis (HVTT) should undergo systemic therapy. However, recent data suggest that surgical resection may be beneficial in selected cases, but outcomes are heterogenous. We aimed to estimate pooled overall survival (OS), recurrence free survival (RFS) and complication rates in HCC patients with macrovascular invasion (MVI) following surgical resection.
Methods
In this systematic review and meta-analysis, two investigators independently searched PubMed, Embase, and Cochrane databases from inception to Nov 10, 2020, without language restrictions, for studies reporting outcomes of adult HCC patients with MVI who underwent liver resection with curative intent.
Results
We screened 8,598 articles and included 40 studies involving 8,218 patients. Among all patients with MVI, the pooled median OS was 14.39 months [95% confidence interval (CI): 10.99–18.84], 1-year OS was 54.47% (95% CI: 46.12–62.58%) and 3-year OS was 23.20% (95% CI: 16.61–31.42%). Overall, 1- and 3-year RFS were 27.70% (95% CI: 21.00–35.57%) and 10.06% (95% CI: 6.62–15.01%), respectively. Among patients with PVTT, median OS was 20.41 months in those with segmental/2nd order involvement compared to 12.91 months if 1st order branch was involved and 6.41 months if the main trunk was involved. The pooled rate of major complications was 6.17% (95% CI: 3.53–10.56%).
Conclusions
Overall median survival was 14.39 months for HCC patients with MVI following resection. Median survival was higher in PVTT with segmental/2nd order involvement at 20.41 versus 6.41 months if the main trunk was involved.
Keywords: Hepatocellular carcinoma (HCC), resection, portal vein, macrovascular invasion (MVI), recurrence
Introduction
Hepatocellular carcinoma (HCC) is the third leading cause of cancer death worldwide (1), with an overall 5-year survival of less than 20% (2). Surgical resection, along with liver transplantation and radiofrequency ablation, are the only curative therapies for HCC (3). However, portal vein tumor thrombosis (PVTT) is present in more than a quarter of cases at the point of diagnosis, while hepatic vein tumor thrombosis (HVTT) is present in around 13% (4-6). These are often considered a contraindication to surgical treatment. As a result, patients with tumor macrovascular invasion (MVI) are classified as Barcelona Clinic Liver Cancer (BCLC) stage C, with treatment options limited to mostly palliative systemic therapy. Prognosis is poor, with untreated patients having a median overall survival (OS) of around 6–8 months and a 1-year survival of 25% (2,7-9).
More recently, data from Asia and the US suggest a survival benefit with surgical resection in well selected HCC patients with MVI (10-12). However, available data are heterogenous likely due in part to the inclusion of patients with tumor thrombus that involved different anatomic levels of the vasculature in different studies. Therefore, the primary purpose of this study was to conduct a systematic review and meta-analysis to evaluate OS, recurrence free survival (RFS) and perioperative complications of HCC patients with MVI who underwent liver resection with curative intent. Our secondary aims were to evaluate the effect of different anatomic sub-classes of MVI on clinical outcomes, as well as to identify factors associated with survival and recurrence. We present the following article in accordance with the PRISMA reporting checklist (available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-21-419/rc).
Methods
In accordance with the PRISMA statement, we conducted and reported the meta-analysis as recommended for meta-analyses of observational studies (Appendix 1) (13).
Search strategy and selection criteria
We searched PubMed, Embase, and the Cochrane Library databases from inception to Nov 10, 2020 for original full-text research articles, using search terms based on “HCC”, “resection”, and “survival” as developed in collaboration with a medical librarian (CW) from the Lane Medical Library at Stanford University, CA, USA. Details of the search terms and study selection criteria are available in the Appendix 1. Briefly, we included original research studies published as full-text articles that provided data on adults aged ≥18 years with HCC and portal vein and/or hepatic vein invasion who had undergone primary surgical resection with curative intent and reported OS and/or RFS outcomes. In order to discern the impact of liver resection on survival outcomes of patients with MVI, we excluded studies with patients who received neo-adjuvant therapy for HCC.
Two authors independently searched the databases for relevant articles, screened through them by title and abstract review, followed by a full-text review of potentially eligible articles. Discordance was resolved by consensus or consultation with a third and senior author. Data was extracted from eligible studies using a case report form developed for this study. Quality assessment of included studies was performed using scales developed for this review based on the Newcastle-Ottawa scale (NOS) for retrospective studies (14).
Statistical analysis
We used a random-effects model to determine pooled estimates of demographic and clinical characteristics of HCC patients with MVI. We also used a random-effects model to estimate pooled percentages and 95% confidence intervals (CI) of median, 1-, 3-, and 5-year OS and RFS. We performed pre-planned analyses if there were sufficient data available for the following subgroups: studies that included PVTT only versus PVTT and/or HVTT, sub-classification of PVTT (as recommended by the Liver Cancer Study Group of Japan and the Cheng’s classification) (15,16), country/region, alpha-fetoprotein (AFP) levels, number of tumor nodules, tumor histology, status of hepatic function, presence of cirrhosis and etiology of the underlying liver disease. We performed meta-regression to evaluate factors associated with 3- and 5-year OS and RFS for variables with available data such as age, etiology of liver disease, presence of cirrhosis, tumor number, tumor size, sub-classification of MVI, AFP levels and platelet levels.
We assessed for inter-study heterogeneity with the Higgins’ and Thompson’s I2 statistics derived from the Cochran’s Q test, with heterogeneity considered significant if I2>50% (17). We utilized the Egger’s test and the funnel plot to assess for publication bias. All statistical analyses were carried out with the meta-packages in R statistical software (version 3.6.1).
Results
Study selection and study characteristics
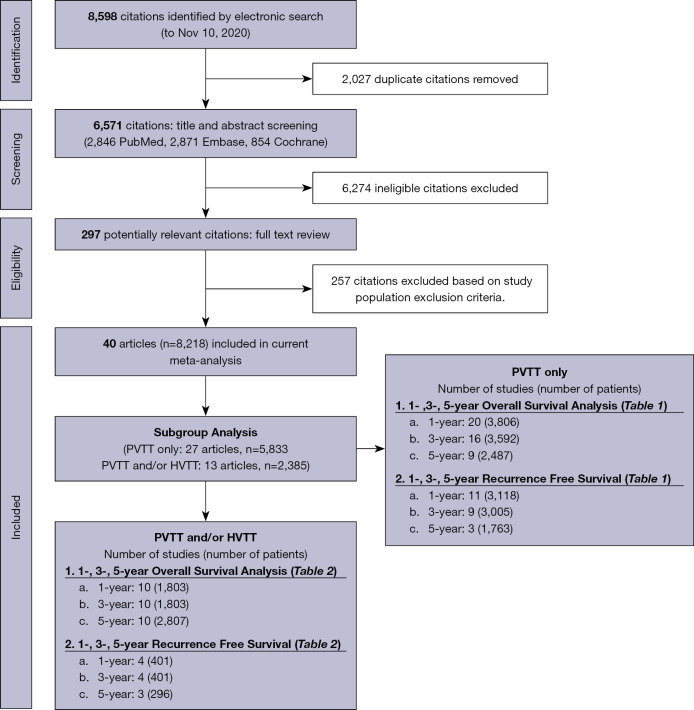
We screened 8,598 articles, removed 2,027 duplicates, reviewed titles and abstracts of 4,646 articles, identified and reviewed the full text of 297 potentially eligible articles and finally selected 40 studies involving 8,218 patients from 8 countries/regions that met our study inclusion/exclusion criteria (Figure 1, Tables 1,2). Of the included studies, 34 were from Asia, 4 from Europe, 1 from North America and 1 from multiple regions. The study sample size ranged from 12 to 1,517. Details of individual study characteristics are reported in Appendix 1, while each study’s patient and tumor characteristics are summarized in Appendix 1. The quality assessment for each study is shown in Appendix 1. Overall, 38 studies were of high quality, 2 studies were of moderate quality, and none were of low quality.
Figure 1.
Flow chart of systematic literature search and screening for analysis of HCC resection outcomes in patients with MVI. PVTT, portal vein tumor thrombosis; HVTT, hepatic vein tumor thrombosis; HCC, hepatocellular carcinoma; MVI, macrovascular invasion.
Table 1. OS and RFS after liver resection in patients with HCC and only PVTT (not inclusive of patients with HVTT).
| Country/region | n/n | 1-year, % (95% CI) | P | n/n | 3-year, % (95% CI) | P | n/n | 5-year, % (95% CI) | P |
|---|---|---|---|---|---|---|---|---|---|
| OS* | |||||||||
| Global | 20/3,806 | 51.77 (40.71–62.65) | – | 16/3,592 | 20.16 (11.52–32.87) | – | 9/2,487 | 21.19 (11.16–36.54) | – |
| By country/region* | |||||||||
| China | 15/3,411 | 46.68 (34.93–58.81) | <0.0001 | 12/3,228 | 15.65 (7.69 – 29.23) | <0.0001 | 6/2,166 | 18.25 (8.15 – 35.95) | <0.0001 |
| Japan | 1/29 | 62.10 (43.62–77.63) | 1/29 | 24.10 (11.94–42.65) | 1/29 | 17.20 (7.34–35.27) | |||
| Korea | 2/74 | 72.43* (61.21–81.39) | 1/43 | 42.00 (28.33–57.02) | 0/0 | – | |||
| Taiwan | 1/247 | 85.00 (79.98–88.93) | 1/247 | 68.00 (61.93–73.52) | 1/247 | 61.00 (54.78–66.89) | |||
| France | 1/45 | 30.80 (19.12–45.60) | 1/45 | 20.50 (11.11–34.71) | 1/45 | 15.40 (7.49–29.03) | |||
| RFS* | |||||||||
| Global | 11/3,118 | 22.67 (16.97–29.60) | – | 9/3,005 | 7.05 (4.99–9.87) | – | 3/1,763 | 0.65 (0.01–26.72) | – |
| By country* | |||||||||
| China | 8/2,999 | 20.29 (14.27–28.02) | 0.15 | 7/2,917 | 6.22 (4.39–8.75) | 0.04 | 2/1,718 | 0.10** (0.00–31.83) | 0.12 |
| Korea | 2/74 | 31.17 (19.73–45.48) | 1/43 | 16.00 (7.77–30.09) | 0/0 | – | |||
| France | 1/45 | 32.50 (20.51–47.33) | 1/45 | 11.60 (5.01–24.63) | 1/45 | 11.60 (5.01–24.63) | |||
n/n, studies/patients; *, some studies encompassed multiple regions, so they were included in the global analysis but not in the regional/country analysis; **, all I2>65.2 with P value <0.05, except for values marked. OS, overall survival; RFS, recurrence free survival; HCC, hepatocellular carcinoma; PVTT, portal vein tumor thrombosis; HVTT, hepatic vein tumor thrombosis.
Table 2. OS and RFS after liver resection in patients with HCC from studies that included PVTT and/or HVTT.
| Country/region | n/n | 1-year, % (95% CI) | P | n/n | 3-year, % (95% CI) | P | n/n | 5-year, % (95% CI) | P |
|---|---|---|---|---|---|---|---|---|---|
| OS* | |||||||||
| Global | 10/1,803 | 60.07 (49.22–70.00) | – | 10/1,803 | 27.34 (19.36–37.10) | – | 10/2,807 | 19.78 (13.85–27.44) | – |
| By country/region* | |||||||||
| China | 5/830 | 54.83 (41.60–67.41) | <0.0001 | 5/830 | 17.62** (15.17–20.36) | <0.0001 | 2/607 | 11.47* (9.16–14.25) | <0.0001 |
| Japan | 1/651 | 80.00 (76.75–82.90) | 1/651 | 56.60 (52.76–60.36) | 2/917 | 32.42 (19.01–49.51) | |||
| Taiwan | 0/0 | – | 0/0 | – | 1/76 | 15.70 (9.12–25.68) | |||
| France | 1/26 | 38.00 (21.73–57.50) | 1/26 | 20.00 (8.73–39.52) | 1/26 | 13.00 (4.55–31.91) | |||
| Italy | 1/62 | 53.30 (49.93–65.27) | 1/62 | 30.10 (20.02–42.56) | 1/62 | 20.00 (11.83–31.78) | |||
| Spain | 1/12 | 66.70 (37.62–86.93) | 1/12 | 33.30 (13.07–62.38) | 1/12 | 22.20 (6.81–52.68) | |||
| United States | 0/0 | – | 0/0 | – | 1/165 | 14.00 (9.49–20.17) | |||
| RFS* | |||||||||
| Global | 4/401 | 45.30 (38.00–52.79) | – | 4/401 | 20.88 (11.36–35.22) | – | 3/296 | 17.66 (13.72–22.42) | – |
| By country* | |||||||||
| China | 1/105 | 51.90 (42.39–61.27) | 0.03 | 1/105 | 7.90 (4.05–14.84) | 0.003 | 0/0 | – | 0.59 |
| Italy | 1/62 | 31.70 (21.37–44.21) | 1/62 | 20.80 (12.45–32.66) | 1/62 | 15.60 (8.52–26.85) | |||
| Spain | 1/12 | 58.30 (30.74–81.50) | 1/12 | 43.70 (19.88–70.83) | 1/12 | 21.90 (6.66–52.41) | |||
n/n, studies/patients; *, some studies encompassed multiple regions, so they were included in the global analysis but not in the regional/country analysis; **, all I2>89.2, all P value for available I2 were <0.05. OS, overall survival; RFS, recurrence free survival; HCC, hepatocellular carcinoma; PVTT, portal vein tumor thrombosis; HVTT, hepatic vein tumor thrombosis.
Study patient characteristics, overall and by presence of PVTT only or PVTT and/or HVTT
Study and patient characteristics are shown in Table 3, and the studies that provided data for these analyses are listed in Appendix 1. Overall, the majority of patients were male (86.80%, 95% CI: 83.39–89.59%), and the pooled mean age was 52.93 years (95% CI: 51.15–54.70) (Table 3). More than three-quarter of patients (79.49%, 95% CI: 65.30–88.87%) had cirrhosis, and the pooled mean Model for End-Stage Liver Disease (MELD) score was 7.34 (95% CI: 6.23–8.45). The most common underlying liver disease was hepatitis B virus (HBV) infection (74.37%, 95% CI: 61.77–83.90%), followed by hepatitis C virus (HCV) infection (16.16%, 95% CI: 7.63–31.04%) and alcohol (4.92%, 95% CI: 3.00–7.98%). The pooled mean AFP level was 892.91 ng/mL (496.50–1,289.32) (Appendix 1). With regards to tumor characteristics, the pooled mean tumor size was 7.43 cm (95% CI: 5.44–9.42), the proportion of patients with poorly differentiated HCC was 36.99% (95% CI: 13.08–69.61%), and the proportion with lymphatic invasion was 11.97% (95% CI: 8.48–16.65%) (Appendix 1). The proportion of patients that underwent anatomical resection (6 studies, 517 patients) and non-anatomical resection (5 studies, 493 patients) were 73.60% (95% CI: 44.61–90.61%) and 36.92% (95% CI: 19.14–59.15%) respectively. The pooled median follow-up was 16.60 months (95% CI: 12.35–20.85).
Table 3. Study and patient characteristics.
| Characteristics | Overall | PVTT only | PVTT and/or HVTT | P* | |||||
|---|---|---|---|---|---|---|---|---|---|
| n/n | Mean/median/% (95% CI) | n/n | Mean/median/% (95% CI) | n/n | Mean/median/% (95% CI) | ||||
| Study characteristics | |||||||||
| Median study year | 40/8,218 | 2007 | 27/5,833 | 2007 | 13/2,385 | 2004 | 0.21 | ||
| Median follow-up (months) | 9/1,161 | 16.60 (12.35–20.85) | 8/1,133 | 17.14 (12.66–21.62) | 1/28 | 11.00 (7.70–14.30) | 0.06 | ||
| Patient characteristics | |||||||||
| Male (%) | 35/6,128 | 86.80 (83.39–89.59) | 26/4,316 | 86.99 (82.56–90.42) | 9/1,812 | 85.28 (81.11–88.66) | 0.54 | ||
| Age (years) | 31/4,389 | 52.93 (51.15–54.70) | 22/2,953 | 50.93 (49.62–52.24) | 9/1,436 | 57.86 (52.46–63.27) | 0.01 | ||
| Platelet (109/L) | 12/2,221 | 196.60 (174.45–218.75) | 8/1,080 | 195.68 (163.72–227.65) | 4/1,141 | 195.68 (163.72–227.65) | 0.94 | ||
| MELD score | 5/928 | 7.34 (6.23–8.45) | 5/928 | 7.34 (5.93–8.76) | 0/0 | – | – | ||
| Cirrhosis (%) | 24/3,930 | 79.49 (65.30–88.87) | 20/3,531 | 78.30 (60.73–89.39) | 4/399 | 84.51 (64.74–94.19) | 0.56 | ||
| Alcohol (%) | 6/890 | 4.92 (3.00–7.98) | 4/428 | 4.44** (2.85–6.85) | 2/462 | 5.78 (2.65–12.15) | 0.56 | ||
| HBV (%) | 29/5,290 | 74.37 (61.77–83.90) | 21/3,454 | 80.92 (71.73–87.64) | 8/1,836 | 49.75 (19.75–79.93) | 0.055 | ||
| HCV (%) | 16/2,622 | 16.16 (7.63–31.04) | 12/1,447 | 13.84 (5.63–30.19) | 4/1,175 | 41.23 (35.30–47.43) | 0.42 | ||
| Child-Pugh class A (%) | 28/5,051 | 96.21 (93.23–97.91) | 21/3,924 | 93.76 (89.99–96.17) | 7/1,127 | 99.87 (93.85–100.00) | 0.051 | ||
| Child-Pugh class B (%) | 27/4,886 | 4.25 (2.43–7.34) | 21/3,924 | 6.24 (3.83–10.01) | 6/962 | 0.26 (0.01–7.62) | 0.07 | ||
n/n, studies/patients; *, between PVTT only and PVTT and/or HVTT; **, all I2>54.6 with P value <0.05, except for values marked; PVTT, portal vein tumor thrombosis; HVTT, hepatic vein tumor thrombosis; MELD, Model for End-Stage Liver Disease; HBV, hepatitis B virus; HCV, hepatitis C virus.
When studies including only PVTT (without HVTT) were compared against studies including PVTT and/or HVTT, the two groups were distinct in terms of age with the former being significantly younger (50.93 years, 95% CI: 49.62–52.24 versus 57.86 years, 95% CI: 52.46–63.27, P=0.01) but similar in terms of gender (P=0.54) and presence of cirrhosis (P=0.56).
OS
Overall analysis (OS)
Overall, 21 studies (3,909 patients) provided data for median OS (Asia 17 studies, 3,611 patients; Europe 3 studies, 133 patients; North America 1 study, 165 patients). The pooled median OS was 14.39 months (95% CI: 10.99–18.84) (Table 4). The 1-year (30 studies, 5,609 patients), 3-year (26 studies, 5,395 patients) and 5-year OS (19 studies, 4,574 patients) were 54.47% (95% CI: 46.12–62.58%), 23.20% (95% CI: 16.61–31.42%) and 20.29% (95% CI: 14.23–28.08%), respectively (Table 5 and Appendix 1).
Table 4. Median survival, complication rates, operation time and blood loss of liver resection for HCC with MVI.
| Outcomes and complications | Number of studies | Number of patients | Refer to sub-header |
|---|---|---|---|
| Median survival (months) | |||
| Overall | 21 | 3,909 | 14.39 (10.99–18.84) |
| PVTT only | 13 | 2,437 | 12.97 (10.48–16.06) |
| PVTT and/or HVTT | 8 | 1,472 | 16.83 (10.12–27.98) |
| All complications (%) | |||
| Overall | 13 | 1,698 | 30.52 (23.60–38.44) |
| PVTT only | 8 | 1,039 | 27.37 (19.72–36.63) |
| PVTT and/or HVTT | 5 | 659 | 36.59 (24.44–50.72) |
| Minor complications (%) | |||
| Overall | 9 | 669 | 24.87 (20.09–30.36) |
| PVTT only | 6 | 474 | 21.44 (17.15–26.45)* |
| PVTT and/or HVTT | 3 | 195 | 31.79 (25.64–38.66)* |
| Major complications (%) | |||
| Overall | 16 | 1,687 | 6.17 (3.53–10.56) |
| PVTT only | 12 | 1,327 | 4.86 (2.10–10.82) |
| PVTT and/or HVTT | 4 | 360 | 9.17 (6.59–12.61)* |
| Operation time (min) | |||
| Overall | 9 | 1,253 | 219.42 (182.77–256.07) |
| PVTT only | 5 | 749 | 185.89 (181.06–190.73) |
| PVTT and/or HVTT | 4 | 504 | 146.96 (137.32–156.60) |
| Blood loss (mL) | |||
| Overall | 8 | 1,290 | 655.76 (434.94–876.58) |
| PVTT only | 6 | 824 | 618.28 (342.81–893.75) |
*, all I2>57.4 with P value <0.05, except for values marked. HCC, hepatocellular carcinoma; MVI, macrovascular invasion; PVTT, portal vein tumor thrombosis; HVTT, hepatic vein tumor thrombosis.
Table 5. OS and RFS after liver resection in patients with HCC and MVI with only PVTT and in those with PVTT and/or HVTT.
| Country/region | n/n | 1-year, % (95% CI) | P | n/n | 3-year, % (95% CI) | P | n/n | 5-year, % (95% CI) | P |
|---|---|---|---|---|---|---|---|---|---|
| OS* | |||||||||
| Global | 30/5,609 | 54.47 (46.12–62.58) | – | 26/5,395 | 23.20 (16.61–31.42) | – | 19/4,574 | 20.29 (14.23–28.08) | – |
| By country/region* | |||||||||
| China | 20/4,241 | 48.77 (39.14–58.49) | <0.0001 | 17/4,058 | 17.40 (11.26–25.93) | <0.0001 | 8/2,773 | 16.13 (8.61–28.16) | <0.0001 |
| Japan | 2/680 | 79.24 (76.02–82.12) | 2/680 | 41.82 (20.97–66.05) | 3/946 | 28.21 (16.64–43.60) | |||
| Korea | 2/74 | 72.43** (61.21–81.39) | 1/43 | 42.00 (28.33–57.02) | 0/0 | – | |||
| Taiwan | 1/247 | 85.00 (79.98–88.93) | 1/247 | 68.00 (61.93–73.52) | 2/323 | 35.59 (11.01–71.15) | |||
| France | 2/71 | 33.44* (23.48–45.13) | 2/71 | 20.32 (12.51–31.25) | 2/71 | 14.52 (8.07–24.74) | |||
| Italy | 1/62 | 53.30 (40.93–65.27) | 1/62 | 30.10 (20.02–42.56) | 1/62 | 20.00 (11.83–31.78) | |||
| Spain | 1/12 | 66.70 (37.62–86.93) | 1/12 | 33.30 (13.07–62.38) | 1/12 | 22.20 (6.81–52.68) | |||
| United States | 0/0 | – | 0/0 | – | 1/165 | 14.00 (9.49–20.17) | |||
| RFS* | |||||||||
| Global | 15/3,519 | 27.70 (21.00–35.57) | – | 13/3,406 | 10.06 (6.62–15.01) | – | 6/2,059 | 4.31 (0.61–24.76) | – |
| By country* | |||||||||
| China | 9/3,104 | 22.81 (15.45–32.33) | 0.002 | 8/3,022 | 6.35 (4.63–8.65) | <0.0001 | 2/1,718 | 0.10* (0.00–31.83) | 0.37 |
| Korea | 2/74 | 31.17 (19.73–45.48) | 1/43 | 16.00 (7.77–30.09) | 0/0 | – | |||
| France | 1/45 | 32.50 (20.51–47.33) | 1/45 | 11.60 (5.01–24.63) | 1/45 | 11.60 (5.01–24.63) | |||
| Italy | 1/62 | 31.70 (21.37–44.21) | 1/62 | 20.80 (12.45–32.66) | 1/62 | 15.60 (8.52–26.85) | |||
| Spain | 1/12 | 58.30 (30.74–81.50) | 1/12 | 43.70 (19.88–70.83) | 1/12 | 21.90 (6.66–52.41) | |||
n/n, studies/patients; *, some studies encompassed multiple regions, so they were included in the global analysis but not in the regional/country analysis; **, all I2>66.0 with P value <0.05, except for values marked. OS, overall survival; RFS, recurrence free survival; HCC, hepatocellular carcinoma; MVI, macrovascular invasion; PVTT, portal vein tumor thrombosis; HVTT, hepatic vein tumor thrombosis.
Subgroup analyses by presence of PVTT only or PVTT and/or HVTT and by country/region (OS)
Among studies that provided data for PVTT only (without HVTT), median OS was 12.97 months (95% CI: 10.48–16.06) (Table 4). The 1-year (20 studies, 3,806 patients), 3-year (16 studies, 3,592 patients) and 5-year OS (9 studies, 2,487 patients) were 51.77% (95% CI: 40.71–62.65%), 20.16% (95% CI: 11.52–32.87%) and 21.19% (95% CI: 11.16–36.54%) (Table 1, Appendix 1), respectively.
Among studies that provided data for PVTT and/or HVTT, median OS was 16.83 (95% CI: 10.12–27.98) (Table 4). The 1-year (10 studies, 1,803 patients), 3-year (10 studies, 1,803 patients) and 5-year OS (10 studies, 2,807 patients) were 60.07% (95% CI: 49.22–70.00%), 27.34% (95% CI: 19.36–37.10%) and 19.78% (95% CI: 13.85–27.44%), respectively (Table 2, Appendix 1). There were no significant differences in 1-, 3- and 5-year OS between the PVTT only group versus the PVTT and/or HVTT only group (all P>0.32).
Country/region level data for OS, where available, are shown in Tables 1,2,5. For OS, most of the studies (n=20) came from China, with other countries contributing 1–2 studies each. The studies included in the analyses of OS are listed in Appendix 1.
Subgroup analyses by sub-classification of PVTT
There were significant differences in the median OS among patients with different levels of vascular invasion. The median OS among patients with segmental/2nd order portal vein branch involvement was 20.41 months (95% CI: 15.16–27.48; 3 studies, 612 patients), versus 12.91 months (95% CI: 9.97–16.72; 3 studies, 466 patients) among patients with 1st order branch involvement and 6.41 months (95% CI: 5.07–8.10; 2 studies, 214 patients) among those with main portal vein trunk/superior mesenteric vein (SMV) involvement, P<0.0001.
The pooled 1-year OS for segmental and second-order branch involvement, first-order branch involvement and main trunk/SMV involvement were 57.04% (95% CI: 38.92–73.45%), 42.16% (95% CI: 22.71–64.38%) and 19.59% (95% CI: 8.75–38.23%), respectively (Table 6).
Table 6. OS and RFS after liver resection in patients with HCC patients by sub-classification of PVTT.
| PVTT sub-classification | n/n | 1-year, % (95% CI) | P | n/n | 3-year, % (95% CI) | P | n/n | 5-year, % (95% CI) | P | n/n | Median survival (months) (95% CI) | P |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OS | ||||||||||||
| Segmental & second-order brancha | 3/396 | 57.04 (38.92–73.45) | 0.02 | 3/396 | 28.55 (21.47–36.86) | 0.72 | 1, 20 | 21.75 (8.77–44.57) | 0.98 | 3, 612 | 20.41 (15.16–27.48) | <0.0001 |
| First-order branchb | 4/223 | 42.16 (22.71–64.38) | 3/172 | 17.85 (4.94–47.60) | 1, 21 | 19.00 (7.31–41.10) | 3, 466 | 12.91 (9.97–16.72) | ||||
| Main trunk & SMVc | 3/101 | 19.59 (8.75–38.23) | 2/70 | 0.00** (0.00–100.00) | 1, 50 | 0.00 (0.00–100.00) | 2, 214 | 6.41** (5.07–8.10) | ||||
| RFS | ||||||||||||
| Segmental & second-order brancha | 1/308 | 16.98 (13.19–21.59) | <0.0001 | 1/308 | 5.50 (3.44–8.67) | 1.00 | 0 0 |
– | – | – | – | – |
| First-order branchb | 2/129 | 3.67 (2.32–5.76) | 1/78 | 0.00 (0.00–100.00) | 0, 0 | – | – | – | ||||
| Main trunk & SMVc | 2/51 | 0.24** (0.01–8.70) | 1/20 | 0.00 (0.00–100.00) | 0, 0 | – | – | – | ||||
n/n, studies/patients; a, segmental & second-order branch corresponds to Cheng’s classification I and Japan’s VP classification VP1 and VP2; b, first-order branch corresponds to Cheng’s classification II and Japan’s VP classification VP3; c, main trunk & SMV corresponds to Cheng’s classification III and Japan’s VP classification VP4; **, all available I2>72.9 and all P value for available I2 were <0.05, except for values marked. OS, overall survival; RFS, recurrence free survival; HCC, hepatocellular carcinoma; PVTT, portal vein tumor thrombosis; SMV, superior mesenteric vein.
The pooled 3-year OS for segmental and second-order branch involvement, first-order branch involvement and main trunk/SMV involvement were 28.55% (95% CI: 21.47–36.86%), 17.85% (95% CI: 4.94–47.60%) and 0.00% (95% CI: 0.00–100.00%), respectively (Table 6 and Appendix 1).
There were insufficient studies reporting 5-year OS for meta-analysis.
RFS
Overall analysis (RFS)
Overall, the 1-year (15 studies, 3,519 patients), 3-year (13 studies, 3,406 patients) and 5-year RFS (6 studies, 2,059 patients) were 27.70% (95% CI: 21.00–35.57%), 10.06% (95% CI: 6.62–15.01%) and 4.31% (95% CI: 0.61–24.76%), respectively (Table 5, Appendix 1). The pooled proportion of recurrences that were intrahepatic (10 studies, 1,701 patients) and extrahepatic (7 studies, 494 patients) were 56.61% (95% CI: 43.65–68.73%) and 38.75% (95% CI: 16.42–67.07%) respectively.
Among studies that provided data for PVTT only (without HVTT), the 1-year (11 studies, 3,118 patients), 3-year (9 studies, 3,005 patients) and 5-year RFS (3 studies, 1,763 patients) were 22.67% (95% CI: 16.97–29.60%), 7.05% (95% CI: 4.99–9.87%) and 0.65% (95% CI: 0.01–26.72%), respectively (Table 1).
Subgroup analyses by presence of PVTT only or PVTT and/or HVTT and by country/region (RFS)
Among studies that provided data for PVTT and/or HVTT, the 1-year (4 studies, 401 patients), 3-year (4 studies, 401 patients) and 5-year RFS (3 studies, 296 patients) were 45.30% (95% CI: 38.00–52.79%), 20.88% (95% CI: 11.36–35.22%) and 17.66% (95% CI: 13.72–22.42%), respectively (Table 2).
There were differences in 1-, 3- and 5-year RFS between the PVTT only (without HVTT) group versus the PVTT and/or HVTT only group (P=0.02 for 1-year, P=0.03 for 3-year, P=0.05 for 5-year).
RFS data, by subclassification of PVTT are shown in Table 6.
Country/region level data for RFS, where available, are shown in Tables 1,2,5. The studies included in the analyses of RFS are listed in Appendix 1.
Meta-regression of factors associated with survival
Meta-regression of study-level demographic, clinical, and biochemical characteristics for potentially relevant factors with sufficient data did not show any significant association with 5-year OS including age (15 studies, 2,242 patients), cirrhosis (10 studies, 1,237 patients), platelets (7 studies, 1,531 patients), HBV infection (13 studies, 2,441 patients), HCV infection (11 studies, 1,869 patients) or tumor size (8 studies, 1,335 patients) (Appendix 1).
Complications, blood loss and operative time
Overall, pooled complication rates were 30.52% (95% CI: 23.60–38.44%; 13 studies, 1,698 patients) for overall complications and 6.17% (95% CI: 3.53–10.56%; 16 studies, 1,687 patients) for major complications (defined as Clavien-Dindo classification III/IV) (Table 4). Subgroup analysis for complication rates in studies reporting outcomes in PVTT only versus PVTT and/or HVTT were similar to the overall analysis (Table 4). The pooled operative time and blood loss in the overall analysis was 219.42 (95% CI: 182.77–256.07) min and 655.76 (95% CI: 434.94–876.58) mL, respectively.
Additional subgroup analyses
We collected survival data when available for various subgroups (AFP <400 ng/mL, AFP ≥400 ng/mL, presence and absence of cirrhosis, presence of HBV, viral versus non-viral etiology of liver disease, isolated hepatic vein involvement and open versus minimally invasive approaches for surgery). As there were insufficient data to perform meta-analysis, we reported the data in the form of a systematic review in Appendix 1.
Heterogeneity and publication bias
There was moderate heterogeneity among most of the studies (I2 statistic >54.6%, except for the analysis for alcohol as an etiology of liver disease where heterogeneity was low). The funnel plot (Appendix 1) and Egger’s test did not suggest potential publication bias (P=0.13) for 5-year OS.
Discussion
In this systematic review and meta-analysis of 40 studies and 8,218 patients from 8 countries/regions, we determined that HCC patients with MVI who underwent surgical resection with curative intent had a 1- and 3-year OS of 54.5% and 23.2%, respectively. Among studies that reported outcomes for PVTT only (without HVTT), the 1- and 3-year OS were 51.8% and 20.2%, while among studies that reported outcomes for PVTT and/or HVTT, the 1- and 3-year OS were 60.1% and 19.8% respectively. Overall, 1- and 3-year RFS were 27.8% and 10.1%, with similar outcomes in the PVTT only (without HVTT) group (22.7% and 7.1% respectively) and better outcomes in the PVTT and/or HVTT group (45.3% and 20.9%), however, there were limited studies (≤4) that provided RFS data for the PVTT and/or HVTT subgroup and these findings require cautious interpretation. By contrast, a meta-analysis of phase III trials for the treatment of advanced HCC reported a median OS of 10 months for sorafenib; a recent randomized trial demonstrated a median OS of 13 months for lenvatinib and the Imbrave150 trial reported a median OS of 19.2 months with atezolizumab and bevacizumab (18-22). These data suggest that the median survival of resected patients with MVI in general (14 months) may be comparable to that of systemic therapy (2,8), although caution is required in interpreting these data as the current study was not designed to compare outcomes between liver resection and systemic therapy.
Furthermore, in patients with segmental or 2nd order PVTT, median OS was 20.4 months, 1-year OS was 57% and 3-year OS approached 30%, suggesting that surgical resection in this situation may be superior to the multi-kinase inhibitors and comparable to atezolizumab plus bevacizumab. In addition, many patients from countries of lower socioeconomic status do not have access to the newer systemic therapies such as lenvatinib or atezolizumab plus bevacizumab or are unable to afford such costly treatment. Ongoing systemic therapy is often associated with multiple systemic side effects including increased bleeding risk, severe immune associated hepatitis or dermatitis, further complicating the management of these complex patients who are already struggling with poor hepatic reserve and impaired quality of life (23,24). Therefore, surgery may be a viable alternative option in the setting of segmental or 2nd order PVTT (25). Our data is in line with a recent study by Govalan et al. (10) which was published after our search was performed. The authors evaluated 11,259 HCC patients with American Joint Commission on Cancer (AJCC) 7th clinical stage TNM (26) T3BN0M0 and demonstrated that those who underwent resection had a median OS of 21 versus 8 months in those that received systemic therapy. Of note, only 3% of the cohort in the Govalan study received surgery, suggesting that these were highly selected patients, compared with 38% that received systemic therapy. Taken together, these data suggest that a personalized approach should be adopted for HCC patients with MVI, especially those with segmental or 2nd order PVTT, contrary to the latest AJCC 8th edition staging system where any MVI or invasion into adjacent organs are both considered as T4 (27). However, caution must be exercised when interpreting the results, as patients with MVI who underwent liver resection were likely to be highly selected and potentially had fewer comorbidities then those undergoing systemic therapy. In addition, the mean age of patients who underwent resection was only 53 years, younger that the patients included in most clinical trials for systemic therapy (28).
Despite survival being fair in segmental or 2nd order PVTT, 1-year RFS is poor (17%), emphasizing the need for early detection of tumor recurrence in these patients. A study of 734 HCC patients that underwent resection found that lack of tumor surveillance was an independent predictor of mortality (29). We suggest imaging be obtained every 4 months for the first 2 years after surgery, then twice a year thereafter, in line with the National Comprehensive Cancer Network guidelines (30). More data are required regarding the use of neo-adjuvant and adjuvant therapy among patients with MVI undergoing resection, given the extremely high recurrence rate.
However, in should be noted that in the setting of tumor involvement of the first order branch or main trunk PV/SMV, the median survival rates were much poorer (12.9 and 6.4 months respectively). Such patients should be considered for systemic therapy rather than invasive surgery.
Regardless of the vascular extent of tumor thrombus and types of therapy, the outcome of patients with advanced HCC is dismal. This highlights the importance of increasing compliance to primary HCC surveillance among patients at risk for HCC, to increase the likelihood of such patients being diagnosed before the development of MVI. Unfortunately, compliance to HCC surveillance has been reported to be very poor in the real world, with most HCC patients diagnosed at a late stage (31-35). A recent nationwide USA study of 82,427 patients with cirrhosis reported that HCC surveillance took place in barely 10% of patients (32). Therefore, there is an urgent need to improve compliance to HCC surveillance and linkage to care (33,36).
In the current study, majority of the included patients (74%) had HBV as the underlying etiology for liver disease, followed by HCV (16%). Although treatment with antivirals has been shown to improve survival and reduce recurrence after HCC treatment (37,38), there is gross under-utilization of anti-viral treatment (37-40). In a multi-center study involving 2,518 patients with HBV-related HCC from the USA and Asia, only 17% of patients were on anti-viral therapy at the time of HCC diagnosis, and only half received HBV anti-viral therapy at any time, highlighting a substantial care gap (38,41) and a significant opportunity for intervention.
We acknowledge the following limitations. There were a lack of data for major underlying liver disease etiologies such as non-alcoholic fatty liver disease (NAFLD) (42) and alcohol-associated liver disease (43), and further studies are needed to examine the outcomes for these populations, especially since the burden of NAFLD and alcohol-associated liver disease are rising. Due to poorer surveillance rates among patients with alcohol-associated liver disease and NAFLD compared with viral-associated liver disease, the proportion of patients with advanced HCC stage and MVI at presentation may increase, therefore, more outcome data for these subgroups are required (35,44). There was marked variation in outcomes among countries/regions, with studies from Taiwan and Japan reporting 1-year OS of around 80% of more, while studies from other countries such as China and France reported 1-year OS of 49% and 33% respectively. However, there were few studies from countries outside of China, and a complete lack of data from South America, Africa and the Middle East. More data are required to accurately determine survival outcomes in these countries. In addition, the outcomes from the sub-classification of PVTT should be interpreted with caution as some of the subgroup analyses only contained a limited number of studies. There were insufficient data among the included studies regarding cases where tumor thrombus crossed the portal vein bifurcation from left to right, or vice versa without entering the main portal vein. More studies are required to evaluate outcomes in this subgroup. The observational nature of the included studies, moderate heterogeneity and the lack of certain data points are further limitations to this study. In addition, this study was not designed to compare the outcomes of surgical resection with systemic therapy, and more data in this area are required.
Conclusions
HCC patients with PVTT and HVTT generally have poor survival after surgical resection, though the median survival is comparable to patients who receive systemic therapy. However, survival is particularly poor if the main portal vein trunk/SMV or 1st order portal vein is involved, suggesting that invasive surgical treatment should be avoided in this setting. On the other hand, more favorable median survival is observed in patients with segmental or 2nd order branch portal vein invasion, suggesting that surgical resection may be a reasonable option in select patients.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-21-419/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-21-419/coif). MHN has received research grants from Glycotest, Gilead, B. K. Kee Foundation, and National Cancer Institute; MHN serves on the Advisory Board of Intercept, Laboratory of Advanced Medicine, Bayer, Eisai, Gilead, Novartis, Janssen, Eli Lilly, and Exact Sciences. DQH has received a research grant from the National Medical Research Council [Singapore (MOH-000595-01)] and the Exxon-Mobil NUS Scholarship for Clinicians (MOH-000595-01); DQH serves on the Advisory Board of Eisai. The other authors have no conflicts of interest to declare.
References
- 1.GLOBOCAN 2020: Global Cancer Observatory. International Agency for Research on Cancer. 2020.
- 2.European Association for the Study of the Liver . Electronic address: easloffice@easloffice.eu; European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol 2018;69:182-236. 10.1016/j.jhep.2018.03.019 [DOI] [PubMed] [Google Scholar]
- 3.Koh JH, Tan DJH, Ong Y, et al. Liver resection versus liver transplantation for hepatocellular carcinoma within Milan criteria: a meta-analysis of 18,421 patients. Hepatobiliary Surg Nutr 2022;11:78-93. 10.21037/hbsn-21-350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheung TK, Lai CL, Wong BC, et al. Clinical features, biochemical parameters, and virological profiles of patients with hepatocellular carcinoma in Hong Kong. Aliment Pharmacol Ther 2006;24:573-83. 10.1111/j.1365-2036.2006.03029.x [DOI] [PubMed] [Google Scholar]
- 5.Costentin CE, Ferrone CR, Arellano RS, et al. Hepatocellular Carcinoma with Macrovascular Invasion: Defining the Optimal Treatment Strategy. Liver Cancer 2017;6:360-74. 10.1159/000481315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mähringer-Kunz A, Meyer FI, Hahn F, et al. Hepatic vein tumor thrombosis in patients with hepatocellular carcinoma: Prevalence and clinical significance. United European Gastroenterol J 2021;9:590-7. 10.1002/ueg2.12098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cabibbo G, Enea M, Attanasio M, et al. A meta-analysis of survival rates of untreated patients in randomized clinical trials of hepatocellular carcinoma. Hepatology 2010;51:1274-83. 10.1002/hep.23485 [DOI] [PubMed] [Google Scholar]
- 8.Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018;67:358-80. 10.1002/hep.29086 [DOI] [PubMed] [Google Scholar]
- 9.Omata M, Cheng AL, Kokudo N, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int 2017;11:317-70. 10.1007/s12072-017-9799-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Govalan R, Lauzon M, Luu M, et al. Comparison of Surgical Resection and Systemic Treatment for Hepatocellular Carcinoma with Vascular Invasion: National Cancer Database Analysis. Liver Cancer 2021;10:407-18. 10.1159/000515554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kokudo T, Hasegawa K, Matsuyama Y, et al. Survival benefit of liver resection for hepatocellular carcinoma associated with portal vein invasion. J Hepatol 2016;65:938-43. 10.1016/j.jhep.2016.05.044 [DOI] [PubMed] [Google Scholar]
- 12.Kokudo T, Hasegawa K, Yamamoto S, et al. Surgical treatment of hepatocellular carcinoma associated with hepatic vein tumor thrombosis. J Hepatol 2014;61:583-8. 10.1016/j.jhep.2014.04.032 [DOI] [PubMed] [Google Scholar]
- 13.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PloS Med 2009;6:e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.GA Wells, B Shea, D O’Connell, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of andomized ed studies in meta-analyses. 2014.
- 15.Ikai I, Kudo M, Arii S, et al. Report of the 18th follow-up survey of primary liver cancer in Japan. Hepatol Res 2010;40:1043-59. 10.1111/j.1872-034X.2010.00731.x [DOI] [PubMed] [Google Scholar]
- 16.Shi J, Lai EC, Li N, et al. A new classification for hepatocellular carcinoma with portal vein tumor thrombus. J Hepatobiliary Pancreat Sci 2011;18:74-80. 10.1007/s00534-010-0314-0 [DOI] [PubMed] [Google Scholar]
- 17.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539-58. 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 18.Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a andomized phase 3 non-inferiority trial. Lancet 2018;391:1163-73. 10.1016/S0140-6736(18)30207-1 [DOI] [PubMed] [Google Scholar]
- 19.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378-90. 10.1056/NEJMoa0708857 [DOI] [PubMed] [Google Scholar]
- 20.Finn RS, Qin S, Ikeda M, et al. Imbrave150: Updated overall survival (OS) data from a global, randomized, open-label phase III study of atezolizumab (atezo) + bevacizumab (bev) versus sorafenib (sor) in patients (pts) with unresectable hepatocellular carcinoma (HCC). J Clin Oncol 2021;39:267. 10.1200/JCO.2021.39.3_suppl.267 [DOI] [Google Scholar]
- 21.Cabibbo G, Cucchetti A, Cammà C, et al. Outcomes of hepatocellular carcinoma patients treated with sorafenib: a meta-analysis of Phase III trials. Future Oncol 2019;15:3411-22. 10.2217/fon-2019-0287 [DOI] [PubMed] [Google Scholar]
- 22.Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med 2020;382:1894-905. 10.1056/NEJMoa1915745 [DOI] [PubMed] [Google Scholar]
- 23.Llovet JM, Kelley RK, Villanueva A, et al. Hepatocellular carcinoma. Nat Rev Dis Primers 2021;7:6. 10.1038/s41572-020-00240-3 [DOI] [PubMed] [Google Scholar]
- 24.Sangro B, Chan SL, Meyer T, et al. Diagnosis and management of toxicities of immune checkpoint inhibitors in hepatocellular carcinoma. J Hepatol 2020;72:320-41. 10.1016/j.jhep.2019.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang X, Wang J, Shi J, et al. Cost-effectiveness of Atezolizumab Plus Bevacizumab vs Sorafenib for Patients With Unresectable or Metastatic Hepatocellular Carcinoma. JAMA Netw Open 2021;4:e214846. 10.1001/jamanetworkopen.2021.4846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010;17:1471-4. [DOI] [PubMed] [Google Scholar]
- 27.Kamarajah SK, Frankel TL, Sonnenday C, et al. Critical evaluation of the American Joint Commission on Cancer (AJCC) 8th edition staging system for patients with Hepatocellular Carcinoma (HCC): A Surveillance, Epidemiology, End Results (SEER) analysis. J Surg Oncol 2018;117:644-50. [DOI] [PubMed] [Google Scholar]
- 28.Han Y, Zhi WH, Xu F, et al. Selection of first-line systemic therapies for advanced hepatocellular carcinoma: A network meta-analysis of randomized controlled trials. World J Gastroenterol 2021;27:2415-33. 10.3748/wjg.v27.i19.2415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu XF, Xing H, Han J, et al. Risk Factors, Patterns, and Outcomes of Late Recurrence After Liver Resection for Hepatocellular Carcinoma: A Multicenter Study From China. JAMA Surg 2019;154:209-17. 10.1001/jamasurg.2018.4334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.NCCN. NCCN Clinical Practice Guidelines in Oncology: Hepatobiliary Cancers. 2021. [DOI] [PMC free article] [PubMed]
- 31.Wolf E, Rich NE, Marrero JA, et al. Use of Hepatocellular Carcinoma Surveillance in Patients With Cirrhosis: A Systematic Review and Meta-Analysis. Hepatology 2021;73:713-25. 10.1002/hep.31309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yeo YH, Hwang J, Jeong D, et al. Surveillance of patients with cirrhosis remains suboptimal in the United States. J Hepatol 2021;75:856-64. 10.1016/j.jhep.2021.04.042 [DOI] [PubMed] [Google Scholar]
- 33.Huang DQ, Yeo YH, Nguyen MH. Letter to the Editor: Hepatocellular Carcinoma Surveillance in Cirrhosis Patients: Is the Real-World Situation Even Worse Than Reported? Hepatology 2021;74:1714-5. [DOI] [PubMed] [Google Scholar]
- 34.Huang DQ, Hoang JK, Leong J, et al. Differential characteristics and outcomes of Asian and non-Asian patients with HBV-related hepatocellular carcinoma. Liver Int 2021;41:1922-32. 10.1111/liv.14877 [DOI] [PubMed] [Google Scholar]
- 35.Chen VL, Yeh ML, Yang JD, et al. Effects of Cirrhosis and Diagnosis Scenario in Metabolic-Associated Fatty Liver Disease-Related Hepatocellular Carcinoma. Hepatol Commun 2020;5:122-32. 10.1002/hep4.1606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang DQ, Hoang JK, Nguyen MH. HBV-related hepatocellular carcinoma: A call to improve surveillance and linkage to care. Liver Int 2021;41:2238-9. 10.1111/liv.15023 [DOI] [PubMed] [Google Scholar]
- 37.Dang H, Yeo YH, Yasuda S, et al. Cure With Interferon-Free Direct-Acting Antiviral Is Associated With Increased Survival in Patients With Hepatitis C Virus-Related Hepatocellular Carcinoma From Both East and West. Hepatology 2020;71:1910-22. 10.1002/hep.30988 [DOI] [PubMed] [Google Scholar]
- 38.Chen VL, Yeh ML, Le AK, et al. Anti-viral therapy is associated with improved survival but is underutilised in patients with hepatitis B virus-related hepatocellular carcinoma: real-world east and west experience. Aliment Pharmacol Ther 2018;48:44-54. 10.1111/apt.14801 [DOI] [PubMed] [Google Scholar]
- 39.Ogawa E, Yeo YH, Dang N, et al. Diagnosis Rates of Chronic Hepatitis B in Privately Insured Patients in the United States. JAMA Netw Open 2020;3:e201844. 10.1001/jamanetworkopen.2020.1844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guarino M, Di Costanzo GG, Bruzzese D, et al. Incidence of HCC recurrence after DAA treatment for HCV in a multicentre Italian cohort study. Liver Cancer Int 2020;1:12-24. 10.1002/lci2.13 [DOI] [Google Scholar]
- 41.Huang DQ, Nguyen MH. Treatment eligibility in hepatitis B: a call for better linkage to optimal care. Lancet Gastroenterol Hepatol 2021;6:160. 10.1016/S2468-1253(20)30391-5 [DOI] [PubMed] [Google Scholar]
- 42.Huang DQ, El-Serag HB, Loomba R. Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol 2021;18:223-38. 10.1038/s41575-020-00381-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Global Burden of Disease Liver Cancer Collaboration ; Akinyemiju T, Abera S, et al. The Burden of Primary Liver Cancer and Underlying Etiologies From 1990 to 2015 at the Global, Regional, and National Level: Results From the Global Burden of Disease Study 2015. JAMA Oncol 2017;3:1683-91. 10.1001/jamaoncol.2017.3055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bucci L, Garuti F, Camelli V, et al. Comparison between alcohol- and hepatitis C virus-related hepatocellular carcinoma: clinical presentation, treatment and outcome. Aliment Pharmacol Ther 2016;43:385-99. 10.1111/apt.13485 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as