Knowledge of portal vein (PV) anatomy is important for liver lesion segmentation, surgical methods selection, and intervention procedures. However, PV variants are quite frequent, with an incidence rate of 20–35% (1). Awareness and careful and accurate evaluation before surgery have significant impacts and may help avoid unnecessary complications, especially complex partial hepatectomy and liver transplantation. Herein, we report the case of a 34-year-old woman with a rare PV variant that, to our knowledge, has not been reported before.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this article. A copy of the written consent is available for review by the editorial office of this journal.
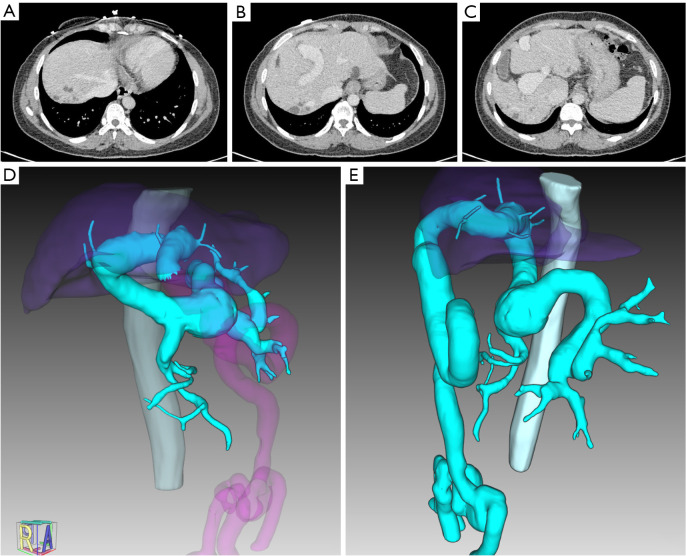
A 34-year-old woman presented to our hospital with liver fibrosis, severe anemia, and splenomegaly, without any previous history of surgery, viral hepatitis, or autoimmune hepatitis. Abdominal contrast-enhanced computed tomography (CT) revealed a single dilated intrahepatic PV coursing to the umbilical fissure without further branching (Figure 1). The intrahepatic PV remained single and maintained its median position throughout its course. Its diameter gradually decreased, but it did not divide into second- or third-order branches, with only some extremely small hypoplastic branches. Bifurcation was absent. The anatomy and course of the three hepatic veins (left, right, and middle hepatic veins) were normal. CT also showed features of portal hypertension, including splenomegaly, dilated umbilical and esophageal veins, and multiple splenic aneurysms. However, no dilation of the bile duct (BD) was observed on CT tomography and ultrasound.
Figure 1.
Imaging of abnormal intrahepatic portal vein without second-order branches. (A-C) Contrast-enhanced CT scans on portal venous phase showed a dilated portal trunk and absence of bifurcation. (D,E) Images of 3D reconstruction of portal vein and branches (vein in cyan). CT, computed tomography.
Currently, the Couinaud classification is the most acceptable and widely used liver segmentation scheme, which is based on the anatomy of the hepatic veins and bifurcation of the PV (2). The PV trunk is generally divided into two second-order branches in the liver hilum: the left portal vein (LPV) branch and the right portal vein (RPV) branch. The LPV supplies the left and caudate lobes of the liver, while the RPV further divides into the right anterior portal vein (RAPV) and right posterior portal vein (RPPV), supplying the right lobe of the liver. Any deviation from this anatomy should be considered an anatomical variant (3). Four main types of PV variants have been described before (1): Type 1, as PV trifurcation (the most common variants type); Type 2 or “Z” anomaly, as RPPV as the first branch originating from the PV trunk; Type 3, as segment VII branch as the separate branch of RPV; Type 4, as segment VI branch as the separate branch of the RPV. In this case, the PV trunk was dilated in the liver hilum and not divided into the LPV or RPV. Only extremely small hypoplastic branches originated from the PV, feeding the peripheral liver tissue. We speculate that this might be due to atresia failure of the umbilical vein causing blood shunting, leaving insufficient blood flow to power the PV to branch further into the liver tissue, which in turn causes liver tissue dysplasia and, thus, liver fibrosis. We deemed that the PV variant observed in this case was not classified into these four types and had not been previously reported.
Accurate liver segmentation and lesion localization are indispensable prerequisites for the operational evaluation and preparation. PV anatomy has an essential impact on liver segmentation because, in the Couinaud classification, each third-order branch of the PV defines the territory of a corresponding liver segment (4). Clinically, resecting the tumor completely and maximally while preserving liver function simultaneously has long become a difficult task in patients with hepatocellular carcinoma (HCC) (5). Third-order portal tributary segmentectomy and subsegmentectomy provide good solutions for balancing the oncologic advantage and remaining liver parenchyma, while PV variants increase the difficulty and complexity of liver surgery. Intraoperative clamping in patients with trifurcation variants is complex. In addition, surgeons without adequate preoperative evaluation may only ligate RAPV and forget about RPPV in patients with “Z” anomaly PV variants during right hepatectomy, which could lead to postoperative bleeding. PV variants are also related to portosystemic shunting (3), which greatly increases the complexity of interventional procedures, including PV embolization and transjugular intrahepatic portosystemic shunting.
PV variants are usually accompanied by biliary variants, with an incidence of 50–60% (6). Biliary-related complications are among the most severe complications of liver surgery. The gold standard method for BD variant assessment is invasive and depends on endoscopic retrograde cholangiopancreatography (ERCP). Magnetic resonance cholangiopancreatography (MRCP) is noninvasive but less accurate, and is more often used for the evaluation of patients with BD dilatation. PV variants can be evaluated noninvasively by CT or MRI, and many current imaging methods, such as 3-dimensional (3D) maximum intensity projection reconstruction, have greatly improved the detection rate of PV variants, reaching up to approximately 35% (7,8), for instance, three-dimensional maximum intensity projection reconstruction. In other words, PV variants may help predict and alert for BD variants to some extent. In this case, there were no clear signs of BD malformation or dilatation on CT or ultrasound. Of course, it is possible that the examinations were not sufficiently suggestive enough.
PV variants have a considerable effect on abnormal liver development. Agenesis of PV branches usually leads to insufficient nutrients and oxygen, resulting in agenesis or hypotrophy of the corresponding side of the liver parenchyma, fibrosis, and even cirrhosis (9). Several studies have demonstrated that hypoxia directly contributes to liver fibrosis through several mechanisms, including activation of hepatic stellate cells, mediating chronic inflammation, and upregulating the expression of hypoxia-inducible factor-1 (HIF-1) and nuclear factor kappa B (NF-κB) (10). The patient in this case had been diagnosed with liver fibrosis for two years, with a liver stiffness measurement of 11.9 Kpa.
In summary, we report a case of a rare PV variant and review the common types of PV variants. We further elaborated on the importance of accurate evaluation in terms of surgical method selection, BD variation indications, intraoperative injuries, and postoperative complication reduction. Current imaging equipment and 3D reconstruction techniques provide great assistance in this regard. With continuing advances in technology and algorithms, PV can be identified and extracted automatically, which offers great assistance to liver surgeons and radiologists.
Supplementary
The article’s supplementary files as
Acknowledgments
The authors thank IQQA-3D, EDDA Technology, Princeton, NJ, USA for providing 3D reconstruction to this research.
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this article. A copy of the written consent is available for review by the editorial office of this journal.
Footnotes
Provenance and Peer Review: This article was a standard submission to the journal. The article did not undergo external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-22-516/coif). The authors have no conflicts of interest to declare.
References
- 1.Cheng YF, Huang TL, Lee TY, et al. Variation of the intrahepatic portal vein; angiographic demonstration and application in living-related hepatic transplantation. Transplant Proc 1996;28:1667-8. [PubMed] [Google Scholar]
- 2.Le foie CC. Etudes anatomiques et chirugicales [in French]. Paris, France: Paris, Masson & Cie, 1957. [Google Scholar]
- 3.Guerra A, De Gaetano AM, Infante A, et al. Imaging assessment of portal venous system: pictorial essay of normal anatomy, anatomic variants and congenital anomalies. Eur Rev Med Pharmacol Sci 2017;21:4477-86. [PubMed] [Google Scholar]
- 4.Huang SH, Wang BL, Cheng M, et al. A fast method to segment the liver according to Couinaud’s classification. International Conference on Medical Imaging and Informatics. Berlin, Heidelberg: Springer, 2007:270-6. [Google Scholar]
- 5.Takamoto T. Improvement and development in anatomical hepatectomy for hepatocellular carcinoma. Hepatobiliary Surg Nutr 2021;10:545-7. 10.21037/hbsn-21-247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takeishi K, Shirabe K, Yoshida Y, et al. Correlation between portal vein anatomy and bile duct variation in 407 living liver donors. Am J Transplant 2015;15:155-60. 10.1111/ajt.12965 [DOI] [PubMed] [Google Scholar]
- 7.Iqbal S, Iqbal R, Iqbal F. Surgical Implications of Portal Vein Variations and Liver Segmentations: A Recent Update. J Clin Diagn Res 2017;11:AE01-5. 10.7860/JCDR/2017/25028.9453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soler L, Nicolau S, Pessaux P, et al. Real-time 3D image reconstruction guidance in liver resection surgery. Hepatobiliary Surg Nutr 2014;3:73-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kouadio EK, Bessayah A, Valette PJ, et al. Anatomic variation: absence of portal vein bifurcation. Surg Radiol Anat 2011;33:459-63. 10.1007/s00276-010-0750-1 [DOI] [PubMed] [Google Scholar]
- 10.Cai J, Hu M, Chen Z, et al. The roles and mechanisms of hypoxia in liver fibrosis. J Transl Med 2021;19:186. 10.1186/s12967-021-02854-x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as