Abstract
Introduction
To evaluate the clinical validity of free water (FW), a diffusion tensor imaging–based biomarker kit proposed by the MarkVCID consortium, by investigating the association between mean FW (mFW) and executive function.
Methods
Baseline mFW was related to a baseline composite measure of executive function (EFC), adjusting for relevant covariates, in three MarkVCID sub‐cohorts, and replicated in five, large, independent legacy cohorts. In addition, we tested whether baseline mFW predicted accelerated EFC score decline (mean follow‐up time: 1.29 years).
Results
Higher mFW was found to be associated with lower EFC scores in MarkVCID legacy and sub‐cohorts (p‐values < 0.05). In addition, higher baseline mFW was associated significantly with accelerated decline in EFC scores (p = 0.0026).
Discussion
mFW is a sensitive biomarker of cognitive decline, providing a strong clinical rational for its use as a marker of white matter (WM) injury in multi‐site observational studies and clinical trials of vascular cognitive impairment and dementia (VCID).
Keywords: biomarker, diffusion tensor imaging, free water, small vessel disease, vascular contributions to cognitive impairment and dementia, VCID, white matter injury
1. BACKGROUND
In recent efforts to improve early identification, staging, and prediction of risk of persons at risk for vascular contributions to vascular cognitive impairment and dementia (VCID) in relation with small vessel disease, the US National Institutes of Health (NIH) National Institute of Neurological Disorders and Stroke (NINDS) funded the MarkVCID consortium to identify and validate fluid‐ and imaging‐based biomarkers for the small vessel diseases associated with VCID. 1 Free water (FW) measured from a diffusion magnetic resonance imaging (MRI) is one of the 11 biomarkers selected for validation for VCID. The FW measure is derived from diffusion tensor imaging (DTI) and reflects the amount of water molecules that are relatively unrestricted by their local microenvironment in the white matter (WM) tissue. FW has received recent attention as a sensitive measure of cerebral injury in association with cognition. 2 , 3 In addition, FW has been found to be associated with a network of inflammatory biomarkers in older individuals, 4 with vascular risk factors (VRFs) in relatively healthy adults (including systolic blood pressure and arterial stiffness), 5 and with white matter hyperintensities (WMHs), 6 supporting the role of FW as a marker of vascular disease.
The biomarker validation consisted of two parts following recommendations proposed by the US Food and Drug Administration (FDA‐NIH) Biomarker Working Group 7 : (1) instrument validation and (2) clinical validation. The instrument validation consisted of demonstrating that the measurement was reproducible with respect to: (1) test‐retest measurements, (2) measurements across different scanner types, and (3) data processing done by different users. The FW kit instrumental validation phase was recently completed 8 and revealed that the mean FW (mFW) biomarker kit demonstrated very high inter‐rater reliability (intraclass correlation coefficient [ICC] = 0.997), test‐retest repeatability (ICC = 0.995), and inter‐scanner reproducibility (ICC = 0.96). Because of the high reproducibility shown in the instrument validation study no further data harmonization is required within the MarkVCID data sets.
The clinical validation hypothesis of the FW kit, pre‐specified before the start of the study (https://markvcid.partners.org/consortium‐protocols‐resources) is that mFW will be cross‐sectionally associated with a composite score of executive functions (EFCs) from the MarkVCID cohorts. In order to show that these results are also valid in other cohorts, we replicated the analysis in five independent data sources for which clinical and DTI data were available. Finally, to further provide supporting evidence of the FW kit's clinical relevance, we explored whether baseline mFW measures predicted future decline in EFC scores using individuals from the MarkVCID cohorts for whom longitudinal data were available.
2. METHOD
2.1. Cohorts
2.1.1. MarkVCID cohorts
MarkVCID is a consortium of seven sites 1 : Johns Hopkins University School of Medicine (JHU); Rush University Medical Center/Illinois Institute of Technology (RUSH); Universities of California San Francisco, Davis, and Los Angeles (UCSF/UCD/UCLA); University of Kentucky (UKY); University of New Mexico Health Sciences Center (UNM); University of Southern California (USC), and the University of Texas Health Science Center at San Antonio (UTHSCSA, operating as part of the Cohorts for Heart and Aging Research in Genomic Epidemiology [CHARGE] consortium site); and a central coordinating center (Massachusetts General Hospital) working with the NINDS and National Institute on Aging (NIA) under cooperative agreements. Six MarkVCID sites that participated in the clinical validation of the FW kit included UNM, UCSF, UKY, USC, JHU, and UTHSCSA. The data used in this study excluded participants with unstable major medical illness, major primary psychiatric disorder, prevalent stroke at the MRI assessment, or other neurological disorders that might confound the assessment of brain volumes, resulting in a total sample of 523 participants. Recruitment sources and inclusion and exclusion criteria for each site cohort are summarized in Table S1. A detailed description of the MarkVCID approach for participant enrollment, clinical and cognitive testing, and sample collection can be found elsewhere. 1 Among these individuals, 180 underwent a second cognitive exam ∼1 year (mean ± SD: 1.29 ± 0.39 years) after their initial exam. Table 1 summarizes subjects’ demographic and clinical characteristics by site for the MarkVCID cohorts used in this study.
TABLE 1.
Participants' characteristics for all MarkVCID participants and each sub‐cohort
| Cohort | N | Age | Sex (F) | Education | Hypertension (Y) | Diabetes (Y) | Smoking Status (Y) | mFW | EFC | WMH | TCV |
|---|---|---|---|---|---|---|---|---|---|---|---|
| All | 523 | 71.6 ± 8.1 [32.2; 90] | 321; 61.4 |
15.4 ± 3.1 [4; 24] |
265; 50.7 | 103; 19.7 | 195; 37.3 | 0.23 ± 0.05 [0.13; 0.47] |
−0.51 ± 0.89 [−2.9; 1.75] |
7.45 ± 11.32 [0; 87.17] |
1349.0 ± 152.8 [997.2; 1876.6] |
| JHU | 86 | 69.3 ± 6.9 [53.2; 88.9] | 55; 64 |
16.2 ± 2.5 [12; 24] |
43; 50 | 21; 24.4 | 35; 40.7 | 0.23 ± 0.04 [0.16; 0.32] |
−0.18 ± 0.79 [−2.2; 1.63] |
9.68 ± 13.55 [0.07; 70.91] |
1312.5 ± 129.5 [1022.6; 1642.8] |
| UCSF | 54 | 77.4 ± 7.4 [55.1; 90] | 28; 51.9 | 17.0 ± 2.1 [12; 20] | 26; 48.1 | 6; 11.1 | 21; 38.9 | 0.25 ± 0.04 [0.17; 0.37] |
−0.19 ± 0.86 [−2.52; 1.23] |
9.16 ± 12.16 [0.13; 59.57] |
1407.9 ± 168.8 [1134.1; 1785.7] |
| UKY | 125 | 74.2 ± 7.7 [55.8; 90] | 75; 60 | 16.1 ± 2.6 [12; 22] | 71; 56.8 | 22; 17.6 | 47; 37.6 | 0.24 ± 0.04 [0.17; 0.35] |
−0.41 ± 0.86 [−2.9; 1.38] |
7.06 ± 8.69 [0; 57.98] |
1386.6 ± 140.4 [1068.4; 1794.4] |
| UNM | 79 | 70.8 ± 8.8 [47.5; 90] | 38; 48.1 | 16.4 ± 2.7 [10; 20] | 31; 39.2 | 4; 5.1 | 35; 44.3 | 0.26 ± 0.06 [0.18; 0.47] |
−0.54 ± 1.01 [−2.86; 1.75] |
13.26 ± 17.4 [0; 87.17] |
1416.2 ± 155.4 [1028.3; 1876.6] |
| USC | 58 | 68.7 ± 9.0 [32.2; 90] | 38; 65.5 |
11.0 ± 3.3 [4; 17] |
35; 60.3 | 22; 37.9 | 24; 41.4 | 0.21 ± 0.03 [0.16; 0.37] |
−1.09 ± 0.78 [−2.35; 0.72] |
4.23 ± 5.08 [0.19; 24.45] |
1267.7 ± 142.7 [997.2; 1629.8] |
| UTHSCSA | 121 | 69.9 ± 6.9 [55.9; 89.3] | 87; 71.9 |
14.5 ± 2.7 [6; 20] |
59; 48.8 | 28; 23.1 | 33; 27.3 | 0.19 ± 0.03 [0.13; 0.27] |
−0.68 ± 0.76 [−2.36; 1.34] |
3.26 ± 5.04 [0.18; 42.91] |
1304.4 ± 138.4 [1045.1; 1634.9] |
Note: Continuous variable: mean ± SD [min; max]; categorical variable: N; %. Cohort 1 includes UKY and UCSF, Cohort 2 UNM and JHU, and Cohort 3 UTHSCSA and USC.
Abbreviations: EFC, executive function composite score; mFW, mean free water; TCV, total cranial volume (cc); WMH, white matter hyperintensity (cc). JHU, Johns Hopkins University School of Medicine; RUSH, Rush University Medical Center/Illinois Institute of Technoly; UCSF, Universities of California San Francisco, Davis, and Los Angeles; UKY, University of Kentucky; UNM, University of New Mexico Health Sciences Center; USC, University of Southern California; UTHSCSA. University of Texas Health Science Center at San Antonio.
2.1.2. Legacy cohorts
Five independent data sources (legacy cohorts) from MarkVCID collaborators were used for replication: Data from UCSF and RUSH, previously acquired data from UCD Alzheimer Disease Research Center, Framingham Heart Study (FHS), including Offspring and Third Generation (Gen3) cohorts, and Atherosclerosis Risk in Communities (ARIC) study from the CHARGE consortium. Briefly, the UCSF cohort includes community‐dwelling older adults with normal cognition or mild cognitive impairment (MCI) recruited from the University of California, San Francisco Memory and Aging Center during the 2‐year discovery phase of the MarkVCID consortium. 4 RUSH data includes data from the Rush Alzheimer Disease Center collected for the MarkVCID project on 302 Rush Memory and Aging Project (MAP), Religious Orders Study (ROS), Minority Aging Research Study (MARS), Clinical Core, Latino Core non‐demented participants. 9 The UCD ADRC cohort includes 173 individuals recruited at the University of California Davis Alzheimer Disease Research Center with ∼74% of the participants recruited through community‐based recruitment protocols designed to enhance racial and ethnic diversity and the spectrum of cognitive dysfunction with an emphasis on normal cognition and MCI. 10 FHS is a three‐generation, single‐site, community‐based, ongoing cohort study initiated in 1948 to investigate the risk factors for cardiovascular disease. The present study includes individuals from the Offspring and Gen3 cohorst. 11 , 12 The ARIC study is a population‐based cohort study of atherosclerosis and clinical atherosclerotic diseases. 13 Participants were between age 45 and 64 years at their baseline examination in 1987–1989. Between 2011 and 2013, ARIC conducted a fifth examination, during which MRI scan was also conducted. Table 2 summarizes subjects’ demographic and clinical characteristics for each of the five legacy cohorts.
TABLE 2.
Participants' characteristics for each legacy cohorts
| Cohort | N | Age | Sex (F) | Education | Hypertension (Y) | Diabetes (Y) | Smoking Status (Y) | mFW | EFC |
|---|---|---|---|---|---|---|---|---|---|
| UCSF | 182 |
70.0 ± 10.8 [45.5; 91] |
96; 52.7 |
17.8 ± 2.8 [5;26] |
46; 25.3 | 8; 4.4 | 46; 25.3 |
0.23 ± 0.038 [0.11; 0.36] |
−1.09 ± 0.74 [−2.34; 0.44] |
| ADRC UCD | 173 |
76.1 ± 6.9 [65; 97] |
11; 63.6 |
14.9 ± 3.9 [0; 20] |
105; 60.7 | 51; 29.5 | Not Available |
0.22 ± 0.05 [0.14; 0.58] |
−0.66 ± 0.79 [−2.62; 1.15] |
| RUSH | 302 |
77.5 ± 6.5 [62.1; 97.8] |
249; 82.5 |
16.0 ± 3.7 [5; 30] |
205; 68.1 | 73; 25.3 | 123; 40.7 |
0.24 ± 0.037 [0.15; 0.37] |
−0.41.3 ± 0.72 [−2.43; 1.55] |
| ARIC | 1837 |
76.3 ± 5.3 [67.5; 90] |
1105; 60.15 |
15.3 ± 4.2 [1; 21] |
1370; 75.2 | 600; 33.1 | 97; 5.5 |
0.27 ± 0.062 [0.17; 0.70] |
−0.94.3 ± 0.74 [−2.71; 1.42] |
| FHS | 2902 |
55 ± 14 [25; 92] |
1569; 54.1 |
15.1 ± 3.3 [4; 22] |
932; 32.1 | 212; 7.3 | 213; 7.3 |
0.19 ± 0.034 [0.12; 0.38] |
−0.23 ± 0.74 [−2.76; 2.09] |
| Offspring | 889 |
71 ± 8 [48; 92] |
501; 54.1 | 506; 56.9 | 118; 13.3 | 40; 4.5 | 0.23 ± 0.04 [0.15; 0.38] |
−0.58 ± 0.73 [−2.63; 1.36] |
|
| Gen3 | 1937 |
48 ± 9 [ 25; 85] |
1068; 55.1 | 418; 21.6 | 92; 4.8 | 166; 8.6 | 0.18 ± 0.02 [0.12; 0.30] |
−0.07 ± 0.69 [−2.76; 2.09] |
Note: Continuous variable: mean ± SD [min; max]; categorical variable: N; %.
Abbreviations: EFC, executive function composite score; mFW, mean free water.
RESEARCH IN CONTEXT
Systematic review: This article describes the clinical validation of free water (FW) content, one of the imaging‐based biomarkers selected by the MarkVCID consortium. To assess the clinical relevance of the biomarker, the group reviewed existing publicly available methodological papers. References to these sources are appropriately cited.
Interpretation: Our results indicate that baseline FW is a sensitive biomarker of cognitive decline, providing a strong clinical rational for its use as a marker of white matter (WM) injury in multi‐site observational studies and clinical trials of vascular cognitive impairment and dementia (VCID).
Future directions: The next step consists of evaluating the longitudinal association between change in FW and change in cognitive performance.
Institutional review boards approved all participating studies, and study participants provided written informed consent.
2.2. MRI acquisition protocol
2.2.1. MarkVCID protocol
Protocols for MarkVCID DTI sequence have been described previously. 1 Briefly, to balance accuracy and scan time, the MarkVCID DTI protocol uses a single‐shell (b = 1000 s/mm2), 40‐direction diffusion sequence with a voxel size of 2.0 × 2.0 × 2.0 mm3 and six b = 0 s/mm2. A separate scan using a reverse‐polarity phase encoding gradient was acquired and used to estimate and correct image distortions in the DTI data.
2.2.2. Legacy cohorts
MRI scans were performed in each study separately following a standard site‐specific procedure. Briefly, magnetic field strength of the scanners used in the different studies ranged from 1.5 to 3.0 Tesla. All cohorts used a single‐shell DTI acquisition, with non‐b‐zero values equal to 1000 s/mm2. Studies used a 2 × 2 × 2 mm3 resolution (UCSF, UCD ADRC, and RUSH), except FHS and ARIC (2.7 × 2.7 × 3 and 1.8 × 1.8 × 5 mm3, respectively).
DTI acquisition parameters characteristics for the different cohorts are reported in Table S2.
2.3. Free water kit
Methodology for the FW kit has been described previously. 8 Briefly, the kit requires, as inputs, a four‐dimensional (4D) diffusion‐weighted (DW) volume (corrected for eddy current distortion), a brain mask, and files containing the b‐vector and b‐values values for each volume. The model considers two coexisting compartments per voxel: one compartment is a FW compartment, which models isotropic diffusion with a diffusion coefficient of water at body temperature (37°C) fixed to 3 × 10−3 mm2/s, 14 and a second compartment accounts for all other molecules, that is, all intra‐ and extra‐cellular molecules that are hindered or restricted by physical barriers such as axonal membranes and myelin. 15 The kit software generates a measure of mean FW (or mFW) within the WM tissue.
WMH and total intracranial volume were also computed using automated procedures as described previously 16 (see Supplemental Method). WMH burden was defined as WMH volume log‐transformed and normalized by total intracranial.
2.4. Clinical outcome
Because executive function has been consistently reported to be associated vascular disease, 17 including FW, 18 , 19 , 20 the clinical outcome measure used by the FW kit is a composite measure of executive function (EFC) derived using item‐response theory (IRT) generated score. 21 Composite scores of executive functions offer advantages such as better reliability, fewer statistical comparisons, and improved power to detect longitudinal change with smaller sample sizes. 22 , 23 The EFC incorporates scores from the following NACC Uniform Data Set (UDS) Version 3 neuropsychological tests 24 : Trail Making Test, Part B (number of correct lines per minute), Number Span Backward (total score), Phonemic fluency (number of correct F‐words in 1 min), and Category fluency (number of correct animal responses in 1 min).
2.5. Biomarker clinical evaluation
The imaging‐based biomarker kit clinical validation procedure was pre‐defined by the MarkVCID Coordinating Center and requires the kit's pre‐specified primary biological hypothesis (see below) to be tested in at least three MarkVCID cohorts. 1 It also encourages the application of the kit procedure to other independent data sources to provide further evidence of kit validity.
2.5.1. Primary pre‐specified biological hypothesis
The FW kit's primary pre‐specified biological hypothesis is that baseline mFW will be negatively associated with baseline EFC scores with a minimal sample size of 123 individuals (https://markvcid.partners.org/consortium‐protocols‐resources). To reach this minimum sample size and because the kit previously demonstrated excellent inter‐rater reliability, test‐retest repeatability, and inter‐scanner reproducibility, 8 cross‐sectional data from the six MarkVCID participating sites were pooled into three sub‐cohorts based solely on recruitment similarity (see Figure 1). Briefly, Euclidian distance was computed between each pair of MarkVCID sites based on average age; education; proportion of female individuals; and proportion of individuals with hypertension, diabetes, and smoking history. At each step, the lowest pairwise distance was used for grouping corresponding sites, resulting in three groups: Cohort 1 (UNM and JHU, N = 169), Cohort 2 (UKY and UCSF, N = 179), and Cohort 3 (UTHSCSA and USC, N = 179). Table S3 summarizes subjects’ demographic and clinical characteristics of each sub‐cohort.
FIGURE 1.
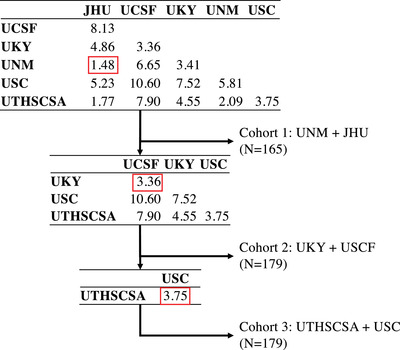
Flowchart describing stepwise sub‐cohort grouping. Tables indicate Euclidian distance between each pair of MarkVCID sites based on average age, education, proportion of female, and proportion of individuals with hypertension, diabetes, and smoking history. At each step (top to bottom), the lowest pairwise distance was used for grouping corresponding sites, which were in turn removed from the table
2.5.2. Secondary pre‐specified biological hypothesis
The FW kit protocol also includes an optional secondary biological hypothesis: higher baseline mFW will predict EFC decline (ΔEFC) over time with a minimal sample size of 175 individuals (https://markvcid.partners.org/consortium‐protocols‐resources). ΔEFC is defined as the difference between follow‐up and baseline EFC score normalized by the time between the two cognitive assessments. To reach the minimum sample size required by the kit, available longitudinal data from all participating MarkVCID sites were pooled together (N = 180).
2.5.3. Testing of primary hypothesis in multiple independent data sources
The FW kit's primary pre‐specified clinical hypothesis was tested on each legacy cohort for replication and generalizability of the findings in the MarkVCID sub‐cohort.
2.6. Statistical analyses
2.6.1. Primary pre‐specified biological hypothesis
To test this hypothesis, a linear regression was used with baseline EFC score as the dependent variable and baseline mFW as the independent variable, adjusting for age, sex, and education (model M1). In a second model, model M1 was additionally adjusted for VRF including presence of diabetes, smoking, and hypertension (model M1 + VRF). This model aimed to estimate the added contribution of mFW above VRF on executive functions.
2.6.2. Secondary pre‐specified biological hypothesis
To evaluate whether baseline mFW correlates with the trajectory of executive functions, we used a linear regression with ΔEFC as the dependent variable and baseline mFW as the independent variable, adjusting for age, sex, education, and baseline EFC score (model M2). In a second analysis, we adjusted model M2 for VRF (Model M2 + VRF).
2.6.3. Contribution of mFW and WMH burden to EFC scores
To assess the contribution of mFW to explain baseline and longitudinal trajectory of EFC in addition to a classic small vessel disease (SVD) marker such as WMH, we added WMH burden as an independent variable to models M1 + VRF and M2 + VRF. Finally, because mFW is generated within the entire WM mask, including WMH, and to disentangle the contribution of FW and WMH, we replicated these analyses using mFW measures computed within the normal appearing WM voxels only, that is, WM mask excluding WMH voxels.
3. RESULTS
3.1. Subjects’ characteristics
As described in the preceding text, enrolled subjects in MarkVCID were pooled into three sub‐cohorts to achieve targeted sample sizes. Table S3 summarizes subjects’ demographic and clinical characteristics for the three MarkVCID sub‐cohorts. Individuals were 72 ± 8 years of age on average, with older individuals in Cohort 2 (see Table S4). Significant differences appear between Cohort 3 and the two other cohorts: individuals in Cohort 3 were less educated, more likely to have diabetes history, and to be a female, and they had lower mFW, EFC scores, and WMH burden (see Table S4).
Individuals from the legacy cohorts were found to be ≈75 years of age on average, except in the FHS cohort (mean ± SD: 55 ± 14 years old) due to the large number of adult individuals in the Gen3 sub‐cohort (mean ± SD: 48 ± 9 years old). Participants were predominantly female (54.1% to 82.5%). The cohorts were found to be very diverse in terms of VRF including the proportion of individuals with hypertension (21.6% to 75.2%), with diabetes (4.4% to 33.1%), and with smoking history (4.5% to 40.7%).
3.2. Association between baseline EFC scores and baseline mFW in MarkVCID sub‐cohorts
In model M1, analyses performed in each MarkVCID sub‐cohort revealed that higher baseline mFW was significantly associated with decreased baseline EFC score (Cohort 1: ß = −7.417, p < 0.0001; Cohort 2: ß = −8.927, p < 0.0001; and Cohort 3: ß = −4.027, p = 0.026—see Table 3 and Figure 2). In model M1 + VRF, this association remained significant for Cohorts 1 and 2 (ß = −7.325, p < 0.0001 and ß = −8.585, p < 0.0001, respectively), but was not significant for Cohort 3 (ß = , p = 0.068; see Table 3).
TABLE 3.
Effect of mFW on EFC scores in the different cohorts
| Cohort | Model M1 | Model M1 + VRF | ||||
|---|---|---|---|---|---|---|
| Beta | SE | p‐value | Beta | SE | p‐value | |
| Cross‐sectional MarkVCID cohorts | ||||||
| Cohort 1 | −7.417 | 1.279 | <0.001 | −7.325 | 1.315 | <0.001 |
| Cohort 2 | −8.927 | 1.807 | <0.001 | −8.585 | 1.891 | <0.001 |
| Cohort 3 | −4.027 | 1.789 | 0.026 | −3.425 | 1.864 | 0.068 |
| Cross‐sectional legacy cohorts | ||||||
| UCSF | −9.87 | 1.76 | <0.0001 | −10.63 | 2.55 | <0.0001 |
| UCD ADRC | −6.18 | 0.603 | <0.0001 | −6.16 | 0.605 | <0.0001 |
| RUSH | −1.905 | 0.69 | 0.00597 | −2.07 | 0.66 | 0.0018 |
| ARIC | −1.24 | 0.304 | <0.0001 | −1.08 | 0.32 | 0.0007 |
| FHS (Offspring and Gen3 combined) | −4.06 | 0.51 | <0.0001 | −3.80 | 0.51 | <0.0001 |
| Offspring | −5.17 | 0.74 | <0.0001 | −5.03 | 0.75 | <0.0001 |
| Gen3 | −1.62 | 0.77 | 0.036 | −1.09 | 0.79 | 0.16 |
Note: Model M1 included baseline EFC as the dependent variable and mFW as the independent variable, adjusting for age, sex, and education. Results for FHS sub‐cohorts (Offspring and Gen3) are reported with italic font. RUSH used log10‐transformed FW measures to normalize distribution.
Abbreviations: EFC, executive function composite score; mFW, mean free water; SE, standard error; VRF, vascular risk factors including diabetes, smoking, and hypertension status. Beta: regression coefficient.
FIGURE 2.
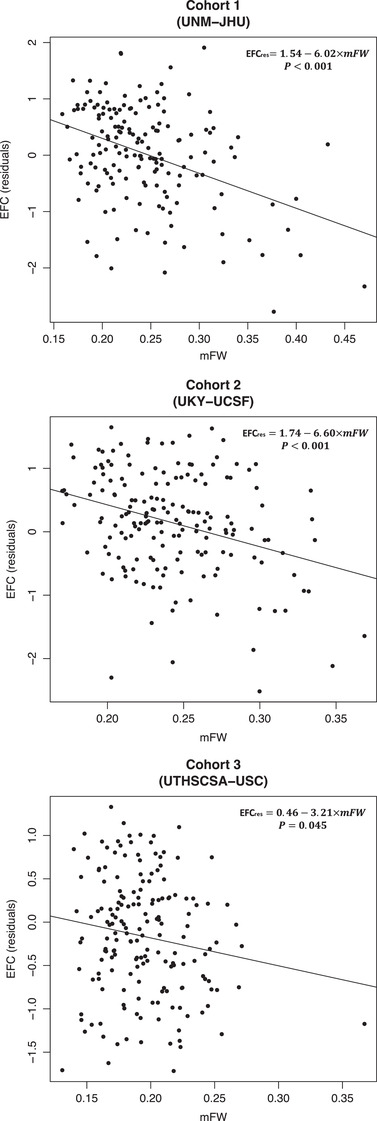
Cross‐sectional association between mean Free Water (mFW) and executive function composite (EFC) scores (residualized against age, sex, and education, [EFCres]) in MarkVCID sub‐cohorts. Johns Hopkins University School of Medicine; UCSF, Universities of California San Francisco, Davis, and Los Angeles; UKY, University of Kentucky; UNM, University of New Mexico Health Sciences Center; USC, University of Southern California; UTHSCSA. University of Texas Health Science Center at San Antonio
3.3. Association between EFC scores and mFW in legacy cohorts
The five legacy cohorts all reported significant association between higher baseline mFW and lower baseline EFC score in both models M1 and M1 + VRF (p < 0.001, see Table 3 and Figure 3). In addition, FHS investigators were asked to analyze separately the two sub‐cohorts of FHS, that is, Offspring and Gen3. The effect of mFW on EFC score was found significant in both Offspring and Gen3 for model M1 (ß = −5.17, p < 0.0001 and ß = −1.62, p = 0.036, respectively) indicating that FW is a sensitive biomarker of concomitant cognition even in a younger adult population. When adding VRF as covariates, the association remained in the expected direction but significant in Offspring only (Offspring: ß = −5.03, p < 0.0001 and Gen3: ß = −1.09, p = 0.16, respectively; see Table 3).
FIGURE 3.
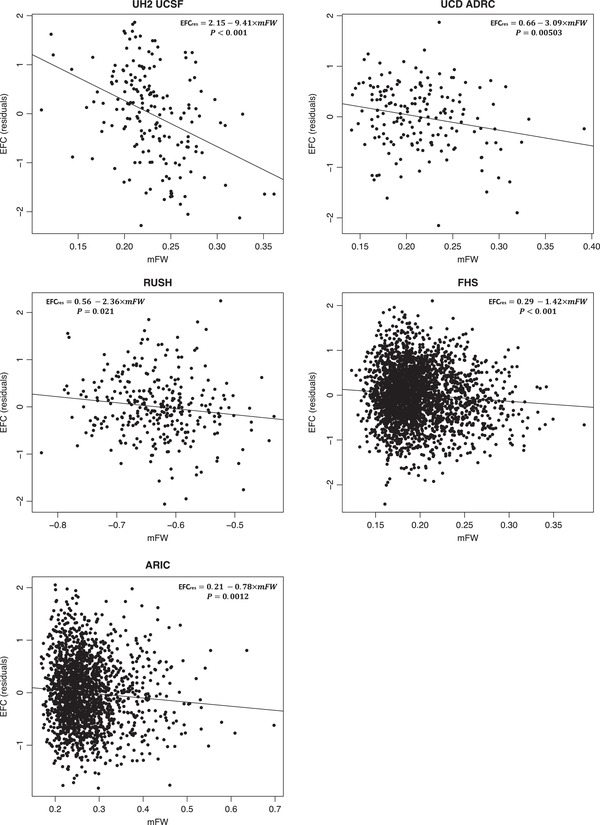
Cross‐sectional association between mFW and EFC scores (residualized against age, sex, and education, [EFCres]) in legacy cohorts. RUSH used log10‐transformed FW measures to normalize distribution
3.4. Association between changes in EFC scores and baseline mFW in MarkVCID cohorts
Mean (± SD) ΔEFC was found to be −0.032/year (± 0.46). Effect of baseline mFW on ΔEFC was found to be significant in models M2 and M2 + VRF (ß = −2.75, p = 0.0026 and ß = −2.20, p = 0.0206, respectively), indicating that higher baseline FW is associated with an accelerated decrease in EFC over time (see Figure 4).
FIGURE 4.
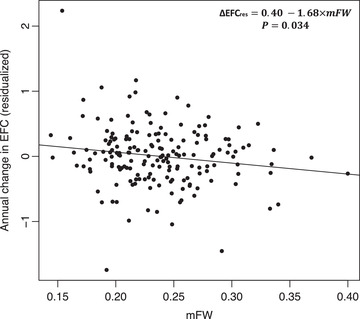
Longitudinal association between mFW and annual change in EFC scores (residualized against age, sex, and education, [∆EFCres]) in the MarkVCID longitudinal cohort
3.5. Contribution of mFW and WMH burden in MarkVCID cohorts
Adding WMH burden to model M1 + VRF did not change the relationships reported in Table 2 between baseline mFW and baseline EFC scores (see Table 4). Of interest, baseline WMH burden was found to be associated with baseline EFC in Cohort 1 (ß = −0.159, p = 0.034) but not in Cohorts 2 and 3 (p‐values > 0.05, see Table 4). Similarly, including WMH with model M2 + VRF did not change the relationships between baseline mFW and annual change in EFC scores (ß = −2.80, p = 0.0096). Baseline WMH burden was not found to be associated with annual change in EFC scores (p = 0.23).
TABLE 4.
Effect of baseline mFW and WMH burden on baseline and change in EFC scores
| mFW | WMH burden | |||||
|---|---|---|---|---|---|---|
| Beta | SE | p‐value | Beta | SE | p‐value | |
| Model M1 + VRF + WMH | ||||||
| Cohort 1 | −7.38 | 1.46 | <0.001 | 0.007 | 0.076 | 0.93 |
| Cohort 2 | −10.737 | 2.234 | <0.001 | 0.176 | 0.094 | 0.062 |
| Cohort 3 | −3.75 | 2.091 | 0.075 | 0.029 | 0.083 | 0.73 |
| Model M2 + VRF + WMH | ||||||
| Longitudinal MarkVCID cohort | −2.80 | 1.07 | 0.0096 | 0.49 | 0.41 | 0.23 |
Note: Model M1 and M2 included baseline executive function composite score (EFC) and annual change in EFC as the dependent variable, respectively, and mFW as the independent variable, adjusting for age, sex, and education.
Abbreviations: mFW, mean free water; VRF, vascular risk factors including presence of diabetes, smoking, and hypertension; WMH burden corresponds to log‐transformed WMH volume normalized by total cranial volume and scaled; WMH, white matter hyperintensity.
It is important to note that excluding WMH voxels from the WM mask when computing mean FW measures did not change the significance of the associations reported above (see Table S5). These findings suggest that independently of WMH, mFW is a sensitive biomarker of WM integrity in association with baseline EFC and EFC decline over a year.
4. DISCUSSION
FW is one of the seven imaging‐based biomarker kits classified by the MarkVCID consortium as a susceptibility/risk biomarker for the early identification of VCID subjects at risk for cognitive decline, as these participants would be ideal for early intervention. The goal of MarkVCID is to evaluate biomarkers through a formal process with instrumental and clinical validation phases, which, to date, has been only partially addressed in the literature. 25 Expanding upon the FW kit's recently published instrumental validation, 8 the most novel finding of this work is that mFW content is cross‐sectionally negatively associated with a composite measure of executive function (EFC) in three MarkVCID cohorts, and, importantly, across independent subject groups from multiple legacy cohorts, offering robust support for the biomarker's biological validity for detecting clinically meaningful changes in WM microstructure. We discuss the results of this formal process and describe the potential benefits for the use of mFW in a clinical trial context.
4.1. Biological evaluation
Initially introduced to correct conventional metrics derived from single‐shell DWI, including fractional anisotropy or mean diffusivity, for extracellular water partial volume contamination, FW has received increasing attention for the past decade as a metric of interest by itself and has been reported consistently as a measure that is sensitive to cognitive decline in recent clinical studies. 2 , 3 , 20 , 27 , 28 , 29 , 30 , 31 , 32 In two studies on cognitively normal individuals with limited sample size (N = 47 and 68), increased FW measures were found to be associated with accelerated changes in verbal fluency and with poorer fluid cognitive function. 33 , 34 The underlying biological mechanism being measured by mFW, however, and how mFW is related to cognition, is unclear. Previous studies reported that mFW is associated with VRF 5 and with inflammatory biomarkers. 4 One explanation for these associations is that abnormal hemodynamics, including high blood pressure and arterial stiffness, may result in endothelial injury and subtle blood‐brain barrier dysfunction, shifting the equilibrium point between arterial and osmotic pressure, and resulting in excess FW. Such alteration may contribute to subtle demyelination and axonal destruction over time, 35 leading to the development of WMH. It is notable that abnormal FW levels can be detected years before pathophysiological manifestations of SVD injury become evident (WMH, lacunes, small subcortical infarcts), thus positioning mFW as a promising early biomarker of vascular injury. 36 Because anisotropic water in perivascular space (PVS), another SVD marker, has been found to influence conventional DTI‐derived measures, 37 including fraction anisotropy and mean diffusivity, it would be of interest to investigate whether the effects of PVS and WMH on mFW can be distinguished. The primary biological hypothesis, pre‐specified in the FW kit's protocol, was evaluated and demonstrated in three sub‐cohorts of the MarkVCID consortium and successfully replicated in five, large, independent data sources, strongly supporting that mean FW content in the WM tissue, as proposed by the FW kit, is a strong predictor of executive function, as expressed by a composite score based on NACC‐derived tests. Although not the primary focus of our study, our results also suggest that higher baseline mFW can predict accelerated EFC decline over a period as short as 1.3 years on average, extending findings from a recent longitudinal study that discovered associations between baseline mFW and cognitive decline in cognitively normal, MCI, and Alzheimer's disease (AD) individuals followed annually for years. 20 These findings support the utility of the FW kit measure as a reliable and reproducible biomarker sensitive to cognition.
4.2. Benefits of mFW in a clinical trial context
There is critical need for simple imaging biomarkers capable of measuring SVD severity for selecting individuals with cognitive complaints or early cognitive impairment potentially attributable to SVD for trials of targeted therapeutics. Using measures of late‐stage pathophysiological manifestations of vascular injury, including WMH, previous clinical trials that aimed to evaluate drug treatment benefits on SVD‐related injury have had mixed results. 38 , 39 The lack of current biomarkers to capture subtle and early cerebral changes in association with SVD and the heterogeneity in available methodologies to quantify these biomarkers limit the ability of clinical trials to efficiently stratify patients. Our work suggests that mFW is sensitive to cognition even as early as the fifth decade (Gen3 cohort, see Table 3), extending findings from a previous study showing that effect of WMH on concomitant cognitive performances were fully mediated by mFW and supporting mFW as an early and sensitive biomarker of cognitive abilities. Correcting for VRF reduced the association between mFW and EFC scores in the MarkVCID Cohort 3 but not in Cohort 1 and Cohort 2 or in the legacy cohorts. It is notable that among all the cohorts, MarkVCID Cohort 3, despite the absence of evident recruitment strategy disparity, was also found to have the lowest average mFW content and WMH burden (see Tables S3 and S4), suggesting that SVD may be under‐represented in this cohort. This finding emphasizes the importance of individual stratification during the recruitment stage of clinical trials that aim to assess cognitive decline in association with cerebrovascular disease and the promising role of mFW in this attempt. Indeed, our finding suggests that mFW may be used as a prognostic biomarker to select individuals with certain levels of cognitive decline and SVD and at high risk of experiencing accelerated cognitive decline and aggravated SVD overtime, thereby enhancing trial efficiency.
4.3. Strengths and limitations
A strength of the FW kit is that it demonstrates remarkable analytical performance. First, mFW requires DWI data, which is a non‐invasive technology widely available. Therefore, as a new biomarker, it can be quickly operationalized into practice for drug development trials. Second, the FW kit includes protocol, script, and instructions that are publicly available online (https://markvcid.partners.org/consortium‐protocols‐resources). Third, mFW is extremely robust and precise in terms of inter‐rater reliability, test‐retest repeatability, and inter‐scanner reproducibility. 8 Third, DWI sequence parameters for major MRI scanner manufacturers, including Philips, Siemens Trio, Siemens Prisma, and GE, and optimized for mFW kit's performances are publicly available, 1 enabling multi‐site MRI data research and joint and integrated data analyses. As a limitation, our statistical models considered a set of covariates, including age, sex, education, hypertension, diabetes, and smoking status. Other demographic variables, not included in this study, may have an impact on the association between FW and cognition, and further studies are needed to investigate whether other demographic variables may impact the association between FW and cognition. In the present study we focused on a composite measure of executive function. mFW is also reported to be associated with the trajectory of episodic memory and functional performances, including clinical dementia rating. 20 However, further studies are needed to assess the sensitivity of mFW as a potential imaging biomarker of other cognitive domains or more global measures of cognition, including the Montreal Clinical Assessment (MoCA).
In summary, the present study gives clinical validation of the FW kit, an imaging‐based biomarker of SVD selected by the MarkVCID consortium. Combined results from the MarkVCID cohort, and from five independent data sources, give further evidence that mean FW is a sensitive biomarker of WM injury and is significantly associated with executive function, making it a suitable candidate for future multi‐site observational studies and clinical trials in the context of VCID.
CONFLICTS OF INTEREST
Dr. Pauline Maillard has no conflict of interest to report. Laura J. Hillmer has no conflict of interest to report. Dr. Hanzhang Lu has no conflict of interest to report. Dr. Konstantinos Arfanakis is on the advisory board for External Advisory Committee, NIH‐funded clinical research study titled “Determinants of Incident Stroke Cognitive Outcomes and Vascular Effects on RecoverY Network (DISCOVERY).” Dr. Brian T. Gold has no conflict of interest to report. Dr. Christopher E. Bauer has no conflict of interest to report. Dr. Lara Stables has no conflict of interest to report. Dr. Danny JJ Wang has no conflict of interest to report. Dr. Sudha Seshadri is a consultant for Biogen. Dr. Claudia L. Satizabal has received support for attending meetings and/or travel from the NIH (payment to Dr. Satizabal). Dr. Alexa Beiser has no conflict of interest to report. Dr. Myriam Fornage has no conflict of interest to report. Dr. Thomas H. Mosley has no conflict of interest to report. Dr. Gary A. Rosenberg has no conflict of interest to report. Dr. Mohamad Habes is a consultant for Biogen on ARIA. Baljeet Singh has no conflict of interest to report. Dr. Joel H. Kramer has received royalties from Pearson, Inc. Dr. Adam M. Staffaroni is a consultant to Passage Bio and Takeda. Herpreet Singh has no conflict of interest to report. Kristin Schwab has no conflict of interest to report. Dr. Karl G. Helmer has no conflict of interest to report. Dr. Steven M. Greenberg is a consultant for Roche (payment to Dr. Greenberg) and Washington University/IQVIA (payment to Dr. Greenberg), Bayer (payment to Dr. Greenberg), and Biogen (payment to Dr. Greenberg), and receives royalties or licenses from Up‐To‐Date (payment to Dr. Greenberg). Dr. Charles DeCarli is a consultant to Novartis on a safety trial for heart failure. Dr. Arvind Caprihan has no conflict of interest to report. Author disclosures are available in the supporting information.
Supporting information
SUPPORTING INFORMATION
SUPPORTING INFORMATION
ACKNOWLEDGMENTS
The authors thank: Bruce Fischl, Department of Radiology, Massachusetts General Hospital; Arnold M. Evia, Rush Alzheimer's Disease Center, Rush University Medical Center; Nazanin Makkinejad, Department of Biomedical Engineering, Illinois Institute of Technology; Xin Li and Yang Li, Department of Radiology, Johns Hopkins University School of Medicine; Samantha Ma, Departments of Neurology and Radiology, Keck School of Medicine, University of Southern California; Elyas Fadaee, Glenn Biggs Institute for Alzheimer's and Neurodegenerative Diseases, University of Texas Health San Antonio; Andrew Warren, Mitchell Horn, and Vanessa Gonzalez, Department of Neurology, Massachusetts General Hospital; and Linda McGavern from National Institute of Health (NIH) andNational Institute of Neurological Disorders and Stroke (NINDS). The Atherosclerosis Risk in Communities (AStudy is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute (NHLBI) contracts (HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700005I, HHSN268201700004I). Neurocognitive data are collected by U01 2U01HL096812, 2U01HL096814, 2U01HL096899, 2U01HL096902, 2U01HL096917 from the NIH (NHLBI, NINDS, National Institute of Aging (NIA), and National Institute on Deafness and Communication Disorders (NIDCD), and with previous brain MRI examinations funded by R01‐HL70825 from the NHLBI. The authors thank the staff and participants of the ARIC study for their important contributions. U24NS100591; UH3NS100599; UH3NS100588; UH3NS100608; UH3NS100605; UH3NS100606; UH3NS100598; UH3NS100614; P30 AG 010129; 5UH2NS100614‐02; K23AG061253; P30 GM 122734. The PRISMA 3T upgrade at The Mind Research Network was supported by the NIH award S10OD025313. Dr. Konstantinos Arfanakis: R01AG064233 (support to institution); R01AG052200 (support to institution); Dr. Danny Wang: R01EB028297 (support to institution); R01NS114382 (support to institution); Dr. Joel Kramer: NIH funding (support to institution); Dr. Adam Staffaroni: NIH (support to institution), Bluefield Project to Cure Frontotemporal Dementia (support to institution); Dr. Karl Helmer: NIH (support to institution), Boston Scientific (support to institution), Minoryx (support to institution); Dr. Steven Greenberg: NIH (support to Dr. Greenberg); Dr. Charles DeCarli: NIH funding (support to institution); Dr. Claudia Satizabal: Texas Alzheimer's Research and Care Consortiium/State of Texas 2020‐58‐81‐CR (support to Dr. Satizabal and to institution).
Maillard P, Hillmer LJ, Lu H, et al. MRI free water as a biomarker for cognitive performance: Validation in the MarkVCID consortium. Alzheimer's Dement. 2022;14:e12362. 10.1002/dad2.12362
REFERENCES
- 1. Lu H, Kashani A, Arfanakis K, et al. MarkVCID cerebral small vessel consortium: II. Neuroimaging protocols. Alzheimers Dement. 2021;17(4):71‐6725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ji F, Pasternak O, Liu S, et al. Distinct white matter microstructural abnormalities and extracellular water increases relate to cognitive impairment in Alzheimer's disease with and without cerebrovascular disease. Alzheimers Res Ther. 2017;9:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Duering M, Finsterwalder S, Baykara E, et al. Free water determines diffusion alterations and clinical status in cerebral small vessel disease. Alzheimers Dement. 2018;14:764‐774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Altendahl M, Maillard P, Harvey D, et al. An IL‐18‐centered inflammatory network as a biomarker for cerebral white matter injury. PLoS One. 2020;15:e0227835. 10.1371/journal.pone.eCollection_2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Maillard P, Mitchell GF, Himali JJ, et al. Aortic stiffness, increased white matter free water, and altered microstructural integrity: a continuum of injury. Stroke. 2017;48:1567‐1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mayer C, Nagele FL, Petersen M, et al. Free‐water diffusion MRI detects structural alterations surrounding white matter hyperintensities in the early stage of cerebral small vessel disease. J Cereb Blood Flow Metab. 2022:42(9):1707‐1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. BEST (Biomarkers, EndpointS, and other Tools) Resource. Silver Spring (MD): https://www.ncbi.nlm.nih.gov/books/NBK326791/; 2016. [PubMed]
- 8. Maillard P, Lu H, Arfanakis K, et al. Instrumental validation of free water, peak‐width of skeletonized mean diffusivity, and white matter hyperintensities: MarkVCID neuroimaging kits. Alzheimers Dement (Amst). 2022;14:e12261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Makkinejad N, Evia AM, Tamhane AA, et al. ARTS: a novel in‐vivo classifier of arteriolosclerosis for the older adult brain. Neuroimage Clin. 2021;31:102768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Carmichael O, Mungas D, Beckett L, et al. MRI predictors of cognitive change in a diverse and carefully characterized elderly population. Neurobiol Aging. 2012;33(1):83‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Feinleib M, Kannel WB, Garrison RJ, McNamara PM, Castelli WP. The framingham offspring study. Design and preliminary data. Prev Med. 1975;4:518‐525. [DOI] [PubMed] [Google Scholar]
- 12. Splansky GL, Corey D, Yang Q, et al. The Third Generation Cohort of the National Heart, Lung, and Blood Institute's Framingham Heart Study: design, recruitment, and initial examination. Am J Epidemiol. 2007;165:1328‐1335. [DOI] [PubMed] [Google Scholar]
- 13. Wright JD, Folsom AR, Coresh J, et al. The ARIC (Atherosclerosis Risk In Communities) Study: JACC Focus Seminar 3/8. J Am Coll Cardiol. 2021;77:2939‐2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pierpaoli C, Basser PJ. Toward a quantitative assessment of diffusion anisotropy. Magn Reson Med. 1996;36:893‐906. [DOI] [PubMed] [Google Scholar]
- 15. Pasternak O, Sochen N, Gur Y, Intrator N, Assaf Y. Free water elimination and mapping from diffusion MRI. Magn Reson Med. 2009;62:717‐730. [DOI] [PubMed] [Google Scholar]
- 16. DeCarli C, Fletcher E, Ramey V, Harvey D, Jagust WJ. Anatomical mapping of white matter hyperintensities (WMH): exploring the relationships between periventricular WMH, deep WMH, and total WMH burden. Stroke. 2005;36:50‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hamilton OKL, Backhouse EV, Janssen E, et al. Cognitive impairment in sporadic cerebral small vessel disease: a systematic review and meta‐analysis. Alzheimers Dement. 2021;17:665‐685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Berger M, Pirpamer L, Hofer E, et al. Free water diffusion MRI and executive function with a speed component in healthy aging. NeuroImage. 2022;257:119303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Maillard P, Fletcher E, Singh B, et al. Cerebral white matter free water: a sensitive biomarker of cognition and function. Neurology. 2019;92:e2221‐e2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Archer DB, Moore EE, Shashikumar N, et al. Free‐water metrics in medial temporal lobe white matter tract projections relate to longitudinal cognitive decline. Neurobiol Aging. 2020;94:15‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Staffaroni AM, Asken BM, Casaletto KB, et al. Development and validation of the Uniform Data Set (v3.0) executive function composite score (UDS3‐EF). Alzheimers Dement. 2021;17:574‐583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gibbons LE, Carle AC, Mackin RS, et al. A composite score for executive functioning, validated in Alzheimer's Disease Neuroimaging Initiative (ADNI) participants with baseline mild cognitive impairment. Brain Imaging Behav. 2012;6:517‐527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Staffaroni AM, Bajorek L, Casaletto KB, et al. Assessment of executive function declines in presymptomatic and mildly symptomatic familial frontotemporal dementia: NIH‐EXAMINER as a potential clinical trial endpoint. Alzheimers Dement. 2020;16:11‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Besser L, Kukull W, Knopman DS, et al. Version 3 of the National Alzheimer's Coordinating Center's Uniform Data Set. Alzheimer Dis Assoc Disord. 2018;32:351‐358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Konieczny MJ, Dewenter A, Ter Telgte A, et al. Multi‐shell diffusion MRI models for white matter characterization in cerebral small vessel disease. Neurology. 2021;96:e698‐e708. [DOI] [PubMed] [Google Scholar]
- 26. Huang P, Zhang R, Jiaerken Y, et al. White matter free water is a composite marker of cerebral small vessel degeneration. Transl Stroke Res. 2022;13(1):56‐64. [DOI] [PubMed] [Google Scholar]
- 27. Reas ET, Hagler DJ, Kuperman JM, et al. Associations between microstructure, amyloid, and cognition in amnestic mild cognitive impairment and dementia. J Alzheimers Dis. 2020;73:347‐357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vipin A, Ng KK, Ji F, et al. Amyloid burden accelerates white matter degradation in cognitively normal elderly individuals. Hum Brain Mapp. 2019;40:2065‐2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dumont M, Roy M, Jodoin PM, et al. Free water in white matter differentiates MCI and AD from control subjects. Front Aging Neurosci. 2019;11:270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bergamino M, Walsh RR, Stokes AM. Free‐water diffusion tensor imaging improves the accuracy and sensitivity of white matter analysis in Alzheimer's disease. Sci Rep. 2021;11:6990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hoy AR, Ly M, Carlsson CM, et al. Microstructural white matter alterations in preclinical Alzheimer's disease detected using free water elimination diffusion tensor imaging. PloS one. 2017;12:e0173982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Edde M, Theaud G, Rheault F, et al. Free water: a marker of age‐related modifications of the cingulum white matter and its association with cognitive decline. PLoS One. 2020;15:e0242696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gullett JM, O'Shea A, Lamb DG, et al. The association of white matter free water with cognition in older adults. NeuroImage. 2020;219:117040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li X, Li TQ, Andreasen N, Wiberg MK, Westman E, Wahlund LO. The association between biomarkers in cerebrospinal fluid and structural changes in the brain in patients with Alzheimer's disease. J Intern Med. 2014;275:418‐427. [DOI] [PubMed] [Google Scholar]
- 35. Maillard P, Himali J, Beiser A, et al. IC‐P‐087: association between cognition and cerebral white matter free water in adults from the framingham heart study: a diffusion tensor imaging voxel‐based study. Alzheimer Dement. 2019;15:P77‐P78. [Google Scholar]
- 36. Sepehrband F, Cabeen RP, Choupan J, et al. Perivascular space fluid contributes to diffusion tensor imaging changes in white matter. NeuroImage. 2019;197:243‐254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jennings GL. Recent clinical trials of hypertension management. Hypertension. 2013;62:3‐7. [DOI] [PubMed] [Google Scholar]
- 38. Dufouil C, Chalmers J, Coskun O, et al. Effects of blood pressure lowering on cerebral white matter hyperintensities in patients with stroke: the PROGRESS (Perindopril Protection Against Recurrent Stroke Study) Magnetic Resonance Imaging Substudy. Circulation. 2005;112:1644‐1650. [DOI] [PubMed] [Google Scholar]
- 39. SMIftSR Group, Nasrallah IM, Pajewski NM, et al. Association of intensive vs standard blood pressure control with cerebral white matter lesions. JAMA. 2019;322:524‐534. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPORTING INFORMATION
SUPPORTING INFORMATION


