Abstract
This special issue highlights the unique role that social and behavioral science has to play at the forefront of genomics. Through the introduction of papers comprising this special issue, we outline priority research areas at the nexus of genomics and the social and behavioral sciences. These include: Discovery science; clinical and community translation, and equity, including engagement and inclusion of diverse populations in genomic science. We advocate for genomic discovery that considers social context, neural, cognitive, and behavioral endophenotypes, and that is grounded in social and behavioral science research and theory. Further, the social and behavioral sciences should play a leadership role in identifying best practices for effective clinical and community translation of genomic discoveries. Finally, inclusive research that engages diverse populations is necessary for genomic discovery and translation to benefit all. We also highlight ways that genomics can be a fruitful testbed for the development and refinement of social and behavioral science theory. Indeed, an expanded ecological lens that runs from genomes to society will be required to fully understand human behavior.
Keywords: Genomics, Genomic discovery, Clinical translation, Community translation, Genomic knowledge, Health equity
1. Introduction
In this special issue we consider how social and behavioral science disciplines can inform the discovery and translation of genomics into clinical and community contexts. Rapid advances in identifying genomic contributors to rare and common health conditions are outpacing our understanding of how best to use this information to improve individual and public health. In this context, we put forth this special issue of Social Science & Medicine that represents the state of the science and how social science theory can contribute to genomic discovery and optimal genomic translation, while promoting inclusion of diverse populations and reduced health inequities. The studies herein also illustrate how the context of genomics can provide a fruitful testbed to refine and expand social science theory.
The growth of precision medicine and the integration of genomic discoveries into health care and the public sphere has created the need to anticipate approaches for optimal translation of such discoveries. This optimal translation will require consideration of the broad swathe of pertinent psychosocial factors. These include, the complexities of integrating genomic, neurobiological, social, and personal data together; the perspectives and social determinants that patients and providers bring to the clinical encounter; the interpersonal mechanisms that underpin patient-provider interactions; and the challenges of broader information diffusion beyond the clinical setting into families and communities. With such complexity comes the need to take an organized approach, grounded in theoretical frameworks that describe how social and behavioral processes influence and are influenced by genomic information.
One goal of this special issue is to engage social and behavioral scientists in genomics-centered research and to put forth models for future work in this area. Genomics presents the opportunity to develop, extend, and test theories of health and social behavior that consider outcomes at many socio-ecological levels. That is, genomics is relevant to trans-individual domains, including families, communities, and health care systems that each represent spheres of influence on health. Relatedly, although social and behavioral science research frequently considers socio-ecological spheres, it is important and necessary to also include individual biology and genetic makeup to these frameworks. Doing so will advance consideration of how social and contextual factors influence individuals’ biological functioning and ultimately their health. As such, it is crucial to consider the ways that individuals’ genetic makeup interacts with a lifetime of social and environmental influences and exposures within the range of socio-ecological spheres. Influences on biological functioning may be particularly relevant as we consider the roots of inequities in health outcomes (Gibbons et al., 2007; Williams et al., 2010).
To investigate such complex interrelationships will require building and testing theories inclusive of social and behavioral science disciplines, health services research, and biomedical sciences. Only through transdisciplinary approaches can we understand the entirety of the multiple interlocking systems that drive human health. In this special issue, we bring forward innovative research grounded within the social sciences that span these other domains as well. We see three key themes driving this work: 1) Genomic discovery that considers social context and is informed by social and behavioral science research and theory; 2) investigation of social and behavioral factors relevant to translation of genomic discoveries; and 3) the interplay between social and structural factors, genomics, and health inequity (see Fig. 1). These first two themes focus on the discovery to translation pipeline, while the third theme underpins both discovery and translation efforts by highlighting the need to reach and engage diverse, often understudied populations in our science. Indeed, one size does not fit all – it is imperative that we build our understanding of those social and behavioral factors that underlie existing disparities as to who benefits from discovery and translation so that genomics can benefit all. To begin this undertaking, we introduce this special issue and the papers herein and, with an eye towards the future, consider gaps in genomic discovery and translation that the social and behavioral sciences are uniquely poised to address.
Fig. 1.
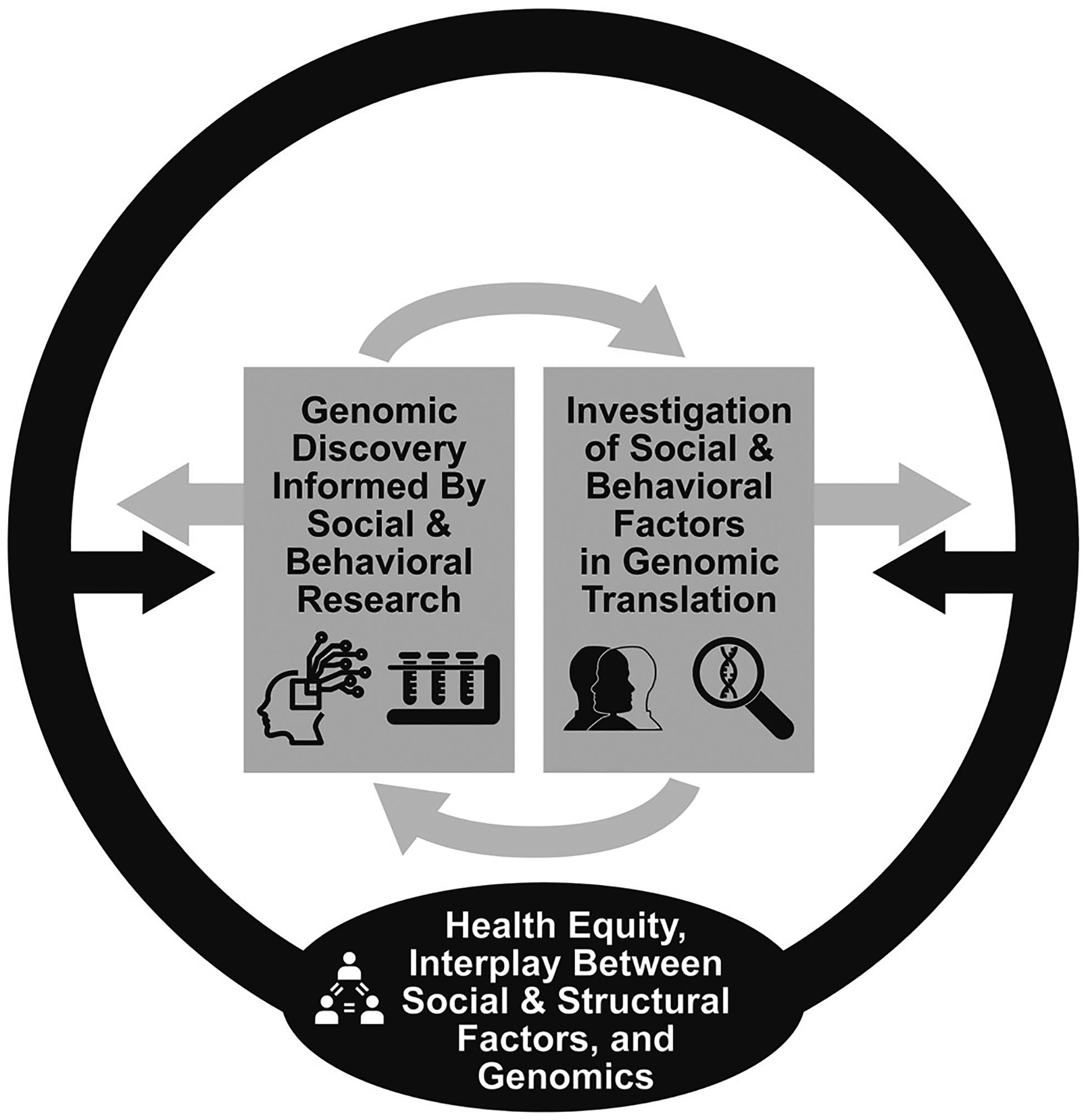
A framework for the interplay between social, behavioral and genomic science that promotes health equity.
2. Discovery science: integrating genomics, behavior and contextual factors
The social and behavioral sciences play a key, though sometimes underappreciated, role in discovery of the mechanisms that underpin the interplay of genomics, personal behavior, and social contexts in health and illness. We see this role as necessary for driving discovery in two areas. The first lies in the expertise of social and behavioral scientists to measure, in valid and reliable ways, important cognitive, social, and behavioral phenotypes that may impact health. For example, the fields of psychology and neuroscience have strong histories of developing measurement approaches that capture psychological phenomena and cognitions, social and lifestyle behaviors, and their underlying neural substrates, all important phenotypes to consider. Second, given the importance of environmental influences in modifying gene function, there is need to quantify the social, behavioral and environmental ‘exposome’ and its interactions with genotype in shaping health outcomes across the lifespan. Thus, specific consideration of social and behavioral phenotypes and their genetic underpinnings, along with epigenomic response to social processes or environmental insults is critical.
There are a multitude of potentially informative intermediate phenotypes for genetic study that sit firmly within the social and behavioral sciences’ purview. For example, obesity’s co-morbidities, such as heart disease, type 2 diabetes, and cancer, are leading causes of death worldwide (World Health Organization, 2018); as such, understanding how genomics contributes to obesity has important implications for public health and wellbeing. There has been progress in this domain, with identification of many gene variants associated with weight and obesity (Ghosh and Bouchard, 2017). Elucidating the role of genomic mechanisms underlying physical activity and dietary behaviors that contribute to or protect against obesity and common disease risk could move us closer to achieving personalized approaches to common disease prevention and management.
Progress in gene identification will be bolstered by using heritable intermediate phenotypes that lie on the pathways between genotypes and key health outcomes. In this issue, Lee and colleagues review evidence showing that an individual’s affective, rather than physiological, response to exercise is heritable and that some of this heritability is explained by common genetic variants (H. H. Lee et al., 2019, this issue). Genomics influences not only affective response to exercise, but also behavior-related phenotypes such as physical exertion capacity and eating behavior. For example, recent work has demonstrated genomic contributors to taste perception, eating in the absence of hunger, and satiety cognitions (Chamoun et al., 2018; Ghosh and Bouchard, 2017; Grimm and Steinle, 2011; Jacob et al., 2018). Moreover, recent research demonstrates that social processes, such as influence mechanisms, can shape physical activity and dietary behaviors (Bell et al., 2019; de Heer et al., 2016). While such social influences may represent social exposures that interact with genetic predispositions towards obesity, they may also represent intermediary neural phenotypes indicative of increased susceptibility to influence (Falk and Scholz, 2018).
Quantification and assessment of these experiential and cognitive variables are areas where social and behavioral research excels. Affective and social neuroscience can provide insights into intermediate phenotypes, characterized by brain structure and function, that might accelerate gene discovery in larger scale studies and help unravel the mechanisms of action. One advantage of such neural phenotypes is that they are quantitative traits that capture more information about inter-individual variability than categorical outcomes and may thus lend themselves more readily to genomic discovery. We recognize that uncovering the heritable basis of some of these intermediate phenotypes magnifies the complexities of genomic discovery. However, social and behavioral science often addresses such complexities, and thus can contribute in developing experimental or quasi-experimental designs to elucidate the underlying cognitive, social and behavioral mechanisms characterizing this complexity (D’Onofrio et al., 2013; Fletcher and Conley, 2013). And thus, we have an important role to play in designing studies that help to identify intermediate phenotypes critical to human health and wellbeing.
In addition to contributing toward gene discovery, the social and behavioral sciences also can contribute to understanding of the interplay between genetic risk and the social environment. The field perhaps has a broadened view of critical environmental influences, or the “exposome”, that can contribute to epigenomic modifications that impact health. For example, Cheon and Hong have demonstrated experimentally that the mere subjective experience of low social status relative to others, independent of actual economic status, can activate biological responses associated with food preferences, selection, and calorie intake. This work challenges assumptions about how low socioeconomic status increases obesity risk (Cheon and Hong, 2017). Another example demonstrates the important role that social networks play in health; here network composition and structure explain significant variation in systolic and diastolic blood pressure, above and beyond known genetic contributors, among African Americans (Fuller et al., 2018). Similarly, Song et al. (2018) demonstrated that the likelihood of binge drinking in a cohort of Mexican-heritage youth was best modeled as a function of affective traits, personal network composition, and specific genetic factors involved in serotonin pathways previously thought to impart susceptibility for risk-seeking behavior (Wilkinson et al., 2012). These works highlight how individual traits work in tandem with interpersonal social and behavioral processes to affect health and health-related behaviors. Indeed, recent meta-analyses show the importance of interpersonal relationships in health and well-being (Holt-Lunstad et al., 2010, 2015). These are just two examples of many constructs fundamentally grounded in the social and behavioral sciences pointing to important environmental exposures that can influence biological functioning. The social and behavioral sciences are at the vanguard of measuring such phenomena, whether implicitly, through ecological momentary assessments, or using multi-informant perspectives to capture social environment, and, as such, have a significant role in characterizing and quantifying the exposome.
As an example of the crucial influence that the social environment can have, Sharp and colleagues consider how place and family context impacts the clinical course of childhood attention deficit hyperactivity disorder (ADHD) (Sharp et al., 2019, this issue). These authors show that children who live in less affluent neighborhoods demonstrate a worsening of ADHD-related symptoms over their developmental course, compared to those in more affluent neighborhoods, though children share the same highly heritable disorder in both cases. However, family harmony and higher socio-economic standing, in terms of income and parental education, appear to modify the detrimental impact of living in less affluent neighborhoods on the clinical course of ADHD.
A central tenet in precision medicine is the aim to improve health outcomes by altering one’s environment in a manner that is mindful of how individual genotype might impact one’s response. Smith and colleagues, in this issue, demonstrate that one environmental factor - an individual’s level of education - moderates both baseline memory skill and the degree of age-related memory decline in carriers of well-established risk gene variants for Alzheimer’s disease (Smith et al., 2019, this issue). In brief, higher levels of education protect against the deleterious effects of risk-conferring genes. At one level, the findings are consistent with the diathesis-stress model of gene-environment interaction, in which genetic risk is accentuated in less-advantageous environments (such as a sub-optimal period of formal education) and attenuated in (educationally) enriched, optimized settings. The study by Smith and colleagues also illustrates the complexity of defining the two sides of the gene by environment equation. For instance, it is conceivable that the gene variants associated with Alzheimer’s disease overlap with genes involved in traits associated with success (and thus duration) in academic settings. Consequently, the environmental exposure of education is not entirely independent from an individual’s genotype - they might be correlated. Other contextual factors less proximal to the individual may be less likely to be determined by the individual’s genotype. To continue to use the example of Alzheimer’s disease, the association of disease onset with its best-established genetic risk factor—the apolipoprotein E e4 allele—has been found to be stronger in more psychosocially hazardous neighborhoods (Lee et al., 2011). That is, neighborhood level exposures and genotype may interact to determine cognitive decline. This is consistent with other work in the area of cardiovascular disease, where social and environmental factors have been found to influence the inflammatory molecular signature associated with increased risk of cardiovascular disease in African American populations, evidence of potential epigenomic mechanisms (Gaye et al., 2017). Importantly, neighborhood level exposures are largely independent of an individual’s genotype, per se, and may differentially impact under-resourced populations.
The study of gene-environment interplay has increased rapidly over the past few years, and future progress will benefit from the conceptual models developed to elucidate this interplay. Notably, social, behavioral, and environmental exposures accumulate over the life course and are multiplex, adding to the complexity of the gene-environment interplay that impacts health. As such, discovery research that identifies genomic underpinnings of social, behavioral, and neural phenotypes is potentially quite powerful as we imagine clinical and community translation of such discoveries through personalized intervention. As well, revealing aspects of the exposome that result in epigenomic modifications that impact health can inform translation efforts through improved, and perhaps more motivational, health communication efforts and intervention tailoring (McBride and Koehly, 2017).
3. Preparing for the clinical and community translations of genomic knowledge
It is undoubtedly important to understand the genetic influences of social and behavioral phenotypes, and to unravel the molecular mechanisms that allow socio-environmental exposures to ‘get under the skin’. However, if genomic knowledge is to be broadly useful to inform individual and family health decisions—and we argue that it is insofar as health risks and clinical outcomes are concerned—then it is equally important to consider the optimal translation of such knowledge into understanding. Genomic translation is necessarily social – whether occurring within the clinical encounter, via consumer channels, or through community engagement activities. For example, patients make health decisions based upon their genetic risk through discussions with their health care providers, family members, through direct-to-consumer genetic companies, and other routes. In turn, providers must consider their patients’ contexts to optimize the clinical interaction and the decisions that result. In communities, genomic translation can involve motivating behavior change within the population, including screening and lifestyle behaviors, to reduce risks conferred by genomic susceptibility. Interventions to promote these outcomes can be more efficacious when guided by behavioral and social sciences theory (Elder et al., 1999; Valente, 2012).
Much translational research aims to design optimal approaches for clinical and public health dissemination of complex genomics topics. Such translation should consider how individual and family characteristics (including genetic literacy, risk perception, culture, family norms, etc.) impact understanding of genomic information. Content development must occur alongside determination of best practices for educating various constituencies about genomics such that information is understood and motivating. Several papers in this issue focus on developing effective strategies for communicating genomic information to patients, clients, and the public to improve health outcomes. Strategies may differ, often requiring targeting and tailoring to recipients. Social and cultural norms are important to consider in genomic education programs and are best ascertained through the inclusion of diverse communities at all stages of translational research. Foundational research that helps form the bedrock of effective communication strategies also involves understanding individuals’ mental models of core concepts of genomics, which may or may not vary within diverse communities. Fiallos and colleagues bring to our attention potential variability in the mental models of disease inheritance that exist within genetic counseling clients (Fiallos et al., 2019, this issue). They highlight the increasing necessity to convey information about heritable conditions to immigrants to the United States, specifically in Latino populations, the largest minority group in the United States. In their study of Latina immigrants, Fiallos et al. found broadly similar mental models of inheritance between this Latina population and white, American populations. However, such similarities may not be observed in other communities. In addition, acknowledgement of existing gaps related to health care resources between communities (e.g., access to services) may generate additional challenges for translating scientific evidence into evidence-based clinical practice in diverse community settings.
Central to the clinical translation of genomic technologies is the need for effective and useable information systems and platforms such as electronic health records, online genomic information return approaches, and electronic decision aids. This important direction is illustrated in work by Paquin and colleagues (Paquin et al., 2019, this issue). This team developed aids to help parents make decisions about highly complex, often unfamiliar information related to whether-or-not to consent to genomic sequencing of a newborn child. Using an experimental design, informed by the reasoned action framework, this research showed that a parent’s active consideration of their values around newborn genomic sequencing decreased their negative attitudes towards sequencing, and increased uptake intentions. This type of work guides the design of decision aids, highlighting the strong influence of values clarification, and as such has implications for genomic translation in other contexts such as personal genome sequencing and secondary findings. According to Paquin and colleagues, decision aids for genomic testing need to consider the beliefs, preferences and goals of individual and family decision makers. Research in this area increasingly reveals the degree of uncertainty involved in potentially learning unanticipated results, and suggests it is imperative that patients and families are supported to make informed choices when deciding whether to undergo genomic testing.
The social and behavioral sciences have a central role to play in identifying effective approaches to facilitate diffusion of genomic information through families, communities, and healthcare teams. For example, one important question is - how can community-based health interventions be optimized to promote engagement in educational programs for those most at risk of common, heritable disorders? Guided by the health belief model, Prom-Wormley and colleagues identify which ‘cues for action’ – such as, family health history and perceived threat of disease - are most salient in motivating information seeking about common, heritable disorders. The study demonstrated how an individual’s perceived threat from a disease is a prime motivating factor for engagement in health education. The degree of perceived threat attenuates, but does not remove, the motivating properties of a having family history of these common disorders. Importantly, this work finds that the influence of these ‘cues’ did not differ between participants who were connected versus unconnected to traditional health care services. Thus, similar strategies may be effective in promoting engagement in health education about common heritable disorders in traditional clinical, as well as in non-medical community, settings. Building from this study, we can identify important related questions that require future research. For example, to what extent are individual-level and interpersonal-level factors associated with interest in community-based health education efforts? Recent work within Mexican-heritage families suggests that family-based education programs may be particularly effective in improving members’ family health history knowledge and activating interpersonal mechanisms aimed at increasing risk-reducing behaviors (de Heer et al., 2016; Lin et al., 2018). An open question is whether such approaches would be similarly successful in other cultural contexts or with a focus on different health conditions.
Looking toward the future of genomic translation in clinical and community settings, it is important to consider the changing landscape of translation, wherein direct-to-consumer and other industry applications are shortening the timeline from discovery to implementation and popular adoption. As such, social and behavioral science will need to quickly evaluate, and in many cases anticipate, genomic products and services to pave the way for effective translation. For example, genomic approaches related to prevention and treatment of common health conditions (e.g., type 2 diabetes, obesity) are forecasted and desired for implementation in clinical and community settings (Bray et al., 2016; Floyd and Psaty, 2016; Persky et al., 2018). However, the scientific evidence behind such products is often still emerging. With this future vision in mind, we must turn our attention to investigating communication about the inherent complexity of gene-environment interactions, gene-behavior interactions, and gene expression processes related to common conditions as these will be difficult to effectively convey to patients and community members. There are also unanswered questions about what information is useful, how much information should be provided, at what stage, and in what contexts. Communicating these concepts and related emerging tests and interventions to healthcare providers and health systems in ways that are useful and useable will prove a challenge as well. This is only one example of many communication and behavioral science issues that will emerge as genome sciences and medicine mature and shift toward whole genome sequencing and related technologies.
Let us also consider that the development of genomic tools for health and medicine is only one part of the implementation of personalized medicine. It will be important for the behavioral and social science community to consider ways of incorporating genomics alongside other growing and emerging health technologies (e.g., lifestyle trackers, home-based medical monitoring) in behavioral interventions of the future. Relatedly, the movement of many elements of health and medical assessment and continuity of care from the clinical to the online and home contexts will mark a change that needs evaluation. It is yet unknown how genomic information provided in this context will be applied and communicated in family and community settings. In short, there is much for social and behavioral science to do to get out front of approaching genomic advancements to optimize translation to clinical and community settings.
4. Driving research through diversity
In order for genomic translation to result in public health for all, there is a need to increase representation from racial, ethnic, socially disadvantaged, and other underrepresented groups in both discovery and translation research. To do so, one must consider novel mechanisms for fostering community engagement with such groups. Tailored recruitment strategies are needed to increase representation of understudied populations so that research is effectively powered. Creating and evaluating approaches to study the role of genomic technologies in addressing or unintentionally exacerbating health disparities is also needed.
We highlight papers in this issue that demonstrate inclusion of populations that are traditionally understudied, marginalized, or stigmatized in relation to genomics research. For example, the work of Prom-Wormley and colleagues focuses on a predominately African-American sample to identify factors that motivate an interest, through family history, in attaining health education on common, heritable disorders (Prom-Wormley et al., 2019, this issue). Fiallos and colleagues consider how Latina immigrant women think about core genomic concepts, asking whether there is diversity of mental models of genomics held by various groups - information that is important when engaging Latina immigrant populations in genomics research (Fiallos et al., 2019, this issue). Moreover, Milliken-Smith and colleagues consider how gender inequity may impact the conduct of basic molecular science – particularly in the area of epigenomics (Milliken-Smith and Potter, 2019, this issue). In their review, the authors highlight biases that arise by overemphasizing the role of the mother, and a converse neglect of the role of the father, in the epigenetic intergenerational transmission of obesity risk. While epidemiological evidence suggests maternal and paternal contributors to obesity risk are roughly equal, most molecular studies and pertinent public policies focus on the mother. The authors consider strategies for attaining an epigenomic research agenda that is gender equitable. This work is a model of how normative social biases may exacerbate or create inequities due to limited representation of specific populations in new fields of molecular inquiry.
These papers provide insight into how genomics and genetic risk can influence the health of subgroups that are understudied and underrepresented in this arena. More research is needed – including how adverse social exposure affects gene expression and the roles of admixture and exposome in disproportionately poor health outcomes among persons of color such as Latinos and African Americans. In particular, three topics related to inclusivity in the production of genomic science that require more attention include: 1) the conflation between attributes of identity (e.g., race/ancestry, sex/gender); 2) the misuse of genomic discovery for the advancement of agendas that create and exacerbate health inequities; and, 3) the need for greater representation from, and participation by, non-Western societies in the production of genomic evidence. These three topics cut across levels of analysis and relevance to highlight gaps in inclusivity from different perspectives: from individual, to group, to society.
A challenge for social scientists working in health disparities and genomics research is to communicate to the biomedical science communities what attributes of identity—for both individuals and groups—are socially constructed and how those social constructs may conflate or even challenge biological paradigms. Researchers often comingle and conjoin constructs such as ethnicity, ancestry, culture and race (Bonham et al., 2005; Duster, 2015; Fujimura and Rajagopalan, 2011). As evidence continues to accumulate that genetic diversity is at least as variable within racial and ethnic groups as it is between (Moreno-Estrada et al., 2014), more research is needed by social scientists to understand the relationship between these socially constructed attributes of identity and their health and social outcomes.
The consequences of confusion between the biological and social homologues of identity cut widely across domains of social and behavioral research. Research that conflates biological and social identities can lead to erroneous conclusions that exacerbate health disparities. For example, the diffusion of family health history information can be disrupted in pedigrees when social identity does not match its biological counterpart (for instance, a sister who is biologically a cousin). For personalized medicine, consideration of social identities could help ensure that clinical care supports both a patient’s biological and social identities.
As Fiallos and colleagues point out, ethnicity—which is based on cultural distinctions between groups primarily rooted in shared common-language—may shape mental models of disease inheritance (Fiallos et al., 2019, this issue). In turn these mental models should inform how genomic information is conveyed by genetic counsellors and other practitioners. Their qualitative work is exemplary of the type needed to fill gaps in within-group heterogeneity as they were careful to draw from the perspectives of Latina women from a variety of country origins.
Another area where social scientists are uniquely positioned to address issues that underlie health inequities is in challenging interpretations of genomic research that would serve to further marginalize certain social groups. Social science research is a primary means through which the research community demonstrates that health disparities are predominantly the result of social constructs. From a research perspective, the diffusion of genomic misinformation into the population, especially via social media, and how this misinformation may result in health disparities among targeted groups (i.e., by manufacturing the distrust of science or informing health policy that affects such groups) is an open area in great need of further investigation.
Most of the production of genomic knowledge is led by a few developed, Western nations. One way to foster genomics knowledge inclusivity is by building solidarity around science rather than around group membership. However, in their discussion of how large, state-sponsored genomic research efforts produce an “infrastructure of solidarity” around the science, van Hoyweghen and colleagues (van Hoyweghen and Aarden, 2019, this issue) propose that individual trust in science is insufficient to instantiate large health data studies involving genomics. Rather, institutional-state actors need to reconstitute the social contract: you (the citizens) provide your data and we (the state) will make medical and scientific discoveries. While this paper takes a broad perspective, the authors note that such social contracts may exclude those without access to, or who may distrust, the medical and research institutions steering the way.
The field of genomics has many incredibly exciting advances on the horizon. However, history has shown that scientific advances can generate and exacerbate health inequities. The genomic research community has come to recognize that the inclusion of diverse populations is essential to fully understand the role of human genetic variation has on health and disease (Hindorff et al., 2018; Landry et al., 2018; Popejoy and Fullerton, 2016). Historically, genomic research has not included diverse cultures, ancestries, and geographic communities that make up the rich global diversity. There has not been emphasis on understanding marginalized populations and how social inequalities interacts with genomics in health and disease. How do we make the research inclusive? Where do we go to from here?
International efforts are underway to increase global diversity in genomics research (H3Africa Consortium et al., 2014; Terry, 2014). These efforts would benefit from the integration of genomics-informed social science research to study the social context of health and disease in diverse global populations. This special issue highlights what can be gained from inclusion of more diverse populations (Smith et al., 2019, this issue). Without meaningful engagement and partnership with diverse communities, the discovery, translation and application of genomic and social science research for clinical and community benefit will be stymied and health inequities will be exacerbated (Bentley et al., 2017).
It is imperative for social and behavioral researchers to study questions of health equity. The first step is the rigorous integration of social science into genomics and vice versa. Scholars trained in interdisciplinary science are a prerequisite for this goal. Additionally, we should foster the growing awareness that the lack of inclusion of underrepresented communities in research is a barrier to good science and good health care. All benefit when all are included in a genomic research agenda driven by the full range and variation of human health and disease. To accomplish this, diverse study populations can and need to be engaged in a respectful, trustworthy manner by scientists to build partnerships for meaningful inclusion in research. Indeed, research agencies are beginning to hold scientists accountable for diversity in recruitment targets and are developing funding opportunities with clear goals regarding recruitment from underrepresented populations. Genomics-informed research conducted by social and behavioral scientists can be a leader in answering questions that are important for improving health equity and reducing health disparities.
5. Conclusion
Behavioral habits, social circumstances, environmental exposures, and access to care represent 70% of the proportional contributions to premature death (Schroeder, 2007). As such, the contributions of the social and behavioral sciences are pivotal in identifying solutions to the many challenges faced in improving public health and reducing illness and disease. All genomes lie within persons who lie within environments in space and time. Social and behavioral scientists are a primary source of expertise on the personal and environmental aspects of this interplay, and as such are a crucial ingredient to a comprehensive understanding of genomics. Conversely, all individuals are, in large part, a product of their genome, with behavior and response to environment driven in part by genetic makeup. As such, understanding of genomics is also an inescapable part of fully understanding human behavior and social systems. Yet, a full ecological lens that runs from genomes to society is rarely applied in research and intervention contexts. To access such scope and complexity will require teams that span the gamut through these ecological spheres. This work will also benefit from developing and testing social science theories that create a framework for investigations of this magnitude.
From a more immediately applied perspective, the science underlying the applications of genomics in precision medicine are in very early stages. As a result, there is still time for social and behavioral perspectives to contribute in formative ways. The papers included herein represent a sampling of the exciting science being conducted at this interface. There is, however, still much more work to be done. As discussed above, we see several areas as crucial ones for the social science community to begin to participate in more fully, in a transdisciplinary manner.
First, given the complexity of our social and behavioral worlds, and the potential impact of behavior and social exposures on health outcomes, there is a need for much more in-depth research on the influence of social and environmental factors on gene expression (and vice versa). In particular, the social and behavioral sciences can inform understanding of the range of potential exposures that may impact health through gene-environment interactions and epigenomic modifications. Such exposures may include interpersonal exposures, such as social support or social isolation, and behavioral exposures, such as physical activity or diet. In so doing, we can leverage our expertise in assessing cognitive, social, and behavioral constructs to develop well-measured phenotypes, endophenotypes, and phenotypic clusters for genomic discovery research.
Second, within the clinical setting, both providers and patients bring with them knowledge, experiences, preferences, attitudes, values and beliefs. New genomic discoveries, sometimes of unclear clinical significance, add a further layer to the clinical encounter. Patients are faced with integrating this complex, often probabilistic genomic information with their existing mental models and understanding of genetics and health. In turn, these individuals will often discuss and reinterpret this information with family members and with their communities. At each stage of this process, miscommunication and misunderstanding can occur that may impact health-related decisions and behaviors. Our research agenda should encompass how individual differences, as well as family and community characteristics, influence the process of translating genomic information into health promoting behaviors. This could inform the development of context-sensitive communication and training approaches to inform dissemination at every step in the process.
Finally, to improve public health through genomics, we must engage in inclusive science such that everyone is involved in discovery and translation research and dissemination efforts. Social and behavioral scientists bring with them an expertise in community engagement, the politics of identity, and theories of social action that can inform recruitment strategies of underserved, under-resourced, and understudied populations.
As we move this science forward, with consideration of how individual, family, community, and society-level factors impact discovery and translation, we anticipate that theories and models underpinning this work will be modified and updated as new data come available. We imagine that such new theories and frameworks are on the horizon that span genes to society such that research can more effectively interrogate the sprawling pathways that effect health and wellbeing. This process requires truly transdisciplinary contributions, allowing social science to inform genomics and vice versa. New initiatives in cross-training and funding that marry these disciplines by design are necessary to move the shared principles of discovery, translation, and health equity to the forefront of genomics.
Acknowledgements
This commentary was supported by the Intramural Research Program of the National Human Genome Research Institute at the National Institutes of Health, United States.
Footnotes
Ethical disclosures
This commentary is not an empirical paper and thus, did not require human subjects review. As such, human welfare was not compromised during the implementation of this work. No animals were involved in this research and as such the welfare of animals was not compromised during the implementation of this project.
References
- Bell Brooke M., Spruijt-Metz D, Vega Yon GG, Mondol AS, Alam R, Ma M, et al. , 2019. Sensing eating mimicry among family members. Transl. Behav. Med 9, 422–430. [DOI] [PubMed] [Google Scholar]
- Bentley AR, Callier S, Rotimi CN, 2017. Diversity and inclusion in genomic research: why the uneven progress? J. Commun. Genet 8, 255–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonham VL, Warshauer-Baker E, Collins FS, 2005. Race and ethnicity in the genome era: the complexity of the constructs. Am. Psychol 60, 9. [DOI] [PubMed] [Google Scholar]
- Bray MS, Loos RJ, McCaffery JM, Ling C, Franks PW, Weinstock GM, et al. , 2016. NIH Working Group Report - using genomic information to guide weight management: from universal to precision treatment. Obesity 24, 14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamoun E, Mutch DM, Allen-Vercoe E, Buchholz AC, Duncan AM, Spriet LL, et al. , 2018. A review of the associations between single nucleotide polymorphisms in taste receptors, eating behaviors, and health. Crit. Rev. Food Sci. Nutr 58. [DOI] [PubMed] [Google Scholar]
- Cheon BK, Hong Y-Y, 2017. Mere experience of low subjective socioeconomic status stimulates appetite and food intake. Proc. Natl. Acad. Sci. U. S. A 114, 72–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Onofrio BM, Lahey BB, Turkheimer E, Lichtenstein P, 2013. Critical need for family-based, quasi-experimental designs in integrating genetic and social science research. Am. J. Public Health 103, 46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Heer H, de la Haye K, Skapinsky K, Georgen AF, Wilkinson AV, Koehly LM, 2016. Let’s Move Together: impact of a family health history intervention on encouragement and co-engagement in physical activity of Mexican origin parents and children. Health Educ. Behav 44, 141–152.27198532 [Google Scholar]
- Duster T, 2015. A post-genomic surprise. The molecular reinscription of race in science, law and medicine. Br. J. Sociol 66, 1–27. [DOI] [PubMed] [Google Scholar]
- Elder JP, Ayala GX, Harris S, 1999. Theories and intervention approaches to health-behavior change in primary care. Am. J. Prev. Med 17, 275–284. [DOI] [PubMed] [Google Scholar]
- Falk E, Scholz C, 2018. Persuasion, influence, and value: perspectives from communication and social neuroscience. Annu. Rev. Psychol 69, 329–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiallos K, Owczarzak J, Bodurtha J, Margarit S, Erby L, 2019. Where culture meets genetics: exploring Latina immigrants’ lay beliefs of disease inheritance. Soc. Sci. Med this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher JM, Conley D, 2013. The challenge of causal inference in gene-environment interaction research: leveraging research designs from the social sciences. Am. J. Public Health 103, 42–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd JS, Psaty BM, 2016. The application of genomics in diabetes: barriers to discovery and implementation. Diabetes Care 39, 1858–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura JH, Rajagopalan R, 2011. Different differences: the use of ‘genetic ancestry’ versus race in biomedical human genetic research. Soc. Stud. Sci 41, 5–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller KC, McCarty C, Seaborn C, Gravlee CC, Mulligan CJ, 2018. ACE gene haplotypes and social networks: using a biocultural framework to investigate blood pressure variation in African Americans. PLoS One 13, e0204127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaye A, Gibbons GH, Barry C, Quarrells R, Davis SK, 2017. Influence of socioeconomic status on the whole blood transcriptome in African Americans. PLoS One 12, e0187290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Bouchard C, 2017. Convergence between biological, behavioural, and genetic determinants of obesity. Nat. Rev. Genet 18, 731–748. [DOI] [PubMed] [Google Scholar]
- Gibbons MC, Brock M, Alberg AJ, Glass T, LaVeist TA, Baylin S, et al. , 2007. The Sociobiologic Integrative Model (SBIM): enhancing the integration of socio-behavioral, environmental, and biomolecular knowledge in urban health and disparities research. J. Urban Health 84, 198–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm E, Steinle N, 2011. Genetics of eating behavior: established and emerging concepts. Nutr. Rev 69, 52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- H3Africa Consortium, Rotimi C, Abayomi A, Abimiku A, Adabayeri VM, Adebamowo C, et al. , 2014. Research capacity. Enabling the genomic revolution in Africa. Science 344, 1346–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindorff LA, Bonham VL, Ohno-Machado L, 2018. Enhancing diversity to reduce health information disparities and build an evidence base for genomic medicine. Pers. Med 15, 403–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt-Lunstad J, Smith TB, Baker M, Harris T, Stephenson D, 2015. Loneliness and social isolation as risk factors for mortality: a meta-analytic review. Perspect. Psychol. Sci 10, 227–237. [DOI] [PubMed] [Google Scholar]
- Holt-Lunstad J, Smith TB, Layton JB, 2010. Social relationships and mortality risk: a meta-analytic review. PLoS Med 7, e1000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob R, Drapeau V, Tremblay A, Provencher V, Bouchard C, Perusse L, 2018. The role of eating behavior traits in mediating genetic susceptibility to obesity. Am. J. Clin. Nutr 108, 445–452. [DOI] [PubMed] [Google Scholar]
- Landry LG, Ali N, Williams DR, Rehm HL, Bonham VL, 2018. Lack of diversity in genomic databases is a barrier to translating precision medicine research into practice. Health Aff. (Millwood) 37, 780–785. [DOI] [PubMed] [Google Scholar]
- Lee BK, Glass TA, James BD, Bandeen-Roche K, Schwartz BS, 2011. Neighborhood psychosocial environment, Apolipoprotein E Genotype, and cognitive function in older adults. Arch. Gen. Psychiatr 68, 314–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HH, Emerson JA, Connell L, Williams DM, 2019. Affective response to physical activity as an intermediate phenotype. Soc. Sci. Med this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Marcum CS, Wilkinson AV, Koehly LM, 2018. Developing shared appraisals of diabetes risk through family health history: the case of Mexican-heritage families. Ann. Behav. Med 52, 262–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride CM, Koehly LM, 2017. Imagining roles for epigenetics in health promotion research. J. Behav. Med 40, 229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milliken-Smith S, Potter CM, 2019. Paternal origins of obesity: emerging evidence for incorporating epigenetic pathways into the social determinants of health framework. Soc. Sci. Med this issue. [DOI] [PubMed] [Google Scholar]
- Moreno-Estrada A, Gignoux CR, Fernandez-Lopex JC, Zakharia F, Sikora M, Contreras AV, et al. , 2014. The genetics of Mexico recapitulates Native American substructure and affects biomedical traits. Science 344, 1280–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquin RS, Peinado S, Lewis MA, Biesecker BB, Rini C, Roche M, et al. , 2019. A behavior-theoretic evaluation of values clarification on parental beliefs and intentions toward genomic sequencing for newborns. Soc. Sci. Med this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persky S, Goldring MR, Cohen RW, 2018. Anticipated interest in genomics-informed clinical weight management. Pers. Med 15, 271–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popejoy AB, Fullerton SM, 2016. Genomics is failing on diversity. Nature 538, 161–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prom-Wormley E, Clifford JS, Bourdon J, Barr P, Blondino C, Ball KM, et al. , 2019. Developing community-based health education strategies with family history: assessing the association between community resident family history and interest in health education. Soc. Sci. Med this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder AA, 2007. We can do better - improving the health of American people. N. Engl. J. Med 357, 1221–1228. [DOI] [PubMed] [Google Scholar]
- Sharp W, Mangalmurti A, Hall C, Choudhury S, Shaw P, 2019. Associations between neighborhood and family factors on symptom change in childhood attention deficit hyperactivity disorder. Soc. Sci. Med this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JA, Kho M, Zhao W, Yu M, Mitchell C, Faul JD, 2019. Genetic effects and gene-by-education interactions on episodic memory performance and decline in an aging population. Soc. Sci. Med this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S, Marcum CS, Wilkinson AV, Shete S, Koehly LM, 2018. Genetic, psychological, and personal network factors associated with changes in binge drinking over 2 years among Mexican heritage adolescents in the USA. Ann. Behav. Med 53, 126–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry SF, 2014. The global alliance for genomics and health. Genet. Test. Mol. Biomark 18. [DOI] [PubMed] [Google Scholar]
- Valente T, 2012. Network interventions. Science 337, 49–53. [DOI] [PubMed] [Google Scholar]
- van Hoyweghen I, Aarden E, 2019. One for All, All for One! Achieving the promise of solidarity through precision medicine initiatives. Soc. Sci. Med this issue. [Google Scholar]
- Wilkinson AV, Bondy ML, Wu X, Wang J, Dong Q, D’Amelio AM, et al. , 2012. Cigarette experimentation in Mexican origin youth: psychosocial and genetic determinants. Cancer Epidemiol. Biomark. Prev 21, 228–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DR, Mohammed SA, Leavell J, Collins C, 2010. Race, socioeconomic status, and health: complexities, ongoing challenges, and research opportunities. Ann. N. Y. Acad. Sci 1186, 69–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization, 2018. The Top 10 Causes of Death.


