Abstract
This report of the European Food Safety Authority and the European Centre for Disease Prevention and Control presents the results of zoonoses monitoring and surveillance activities carried out in 2021 in 27 MSs, the United Kingdom (Northern Ireland) and nine non‐MSs. Key statistics on zoonoses and zoonotic agents in humans, food, animals and feed are provided and interpreted historically. In 2021, the first and second most reported zoonoses in humans were campylobacteriosis and salmonellosis, respectively. Cases of campylobacteriosis and salmonellosis increased in comparison with 2020, but decreased compared with previous years. In 2021, data collection and analysis at the EU level were still impacted by the COVID‐19 pandemic and the control measures adopted in the MSs, including partial or total lockdowns. Sixteen MSs and the United Kingdom (Northern Ireland) achieved all the established targets in poultry populations for reduction in Salmonella prevalence for the relevant serovars. Salmonella samples from carcases of various animal species and samples for Campylobacter quantification from broiler carcases were more frequently positive when performed by the competent authorities than when own‐checks were conducted. Yersiniosis was the third most reported zoonosis in humans, followed by Shiga toxin‐producing Escherichia coli (STEC) and Listeria monocytogenes infections. L. monocytogenes and West Nile virus infections were the most severe zoonotic diseases, with the most hospitalisations and highest case fatality rates. Overall, MSs reported more foodborne outbreaks and cases in 2021 than in 2020. S. Enteritidis remained the most frequently reported causative agent for foodborne outbreaks. Salmonella in ‘eggs and egg products’ and in ‘mixed foods’ were the agent/food pairs of most concern. Outbreaks linked to ‘vegetables and juices and products thereof’ rose considerably compared with previous years. This report also provides updates on brucellosis, Coxiella burnetii (Q fever), echinococcosis, rabies, toxoplasmosis, trichinellosis, tuberculosis due to Mycobacterium bovis or M. caprae, and tularaemia.
Keywords: Campylobacter, foodborne outbreaks, Listeria, monitoring, parasites, Salmonella, zoonoses
Introduction
Legal basis of European Union‐coordinated zoonoses monitoring
The European Union (EU) system for the monitoring and collection of information on zoonoses is based on Zoonoses Directive 2003/99/EC 1 , which obliges EU Member States (MSs) to collect relevant and, when applicable, comparable data on zoonoses, zoonotic agents, antimicrobial resistance and foodborne outbreaks. In addition, MSs shall assess the trends and sources of these agents, as well as outbreaks in their territory, submitting an annual report each year by the end of May to the European Commission covering the data collected. The European Commission should subsequently forward these reports to the European Food Safety Authority (EFSA). EFSA is assigned the tasks of examining these data and publishing the EU Annual Summary Reports. In 2004, the European Commission entrusted EFSA with the task of setting up an electronic reporting system and database for monitoring zoonoses (EFSA Mandate No 2004‐0178, continued by M‐2015‐0231 2 ).
Data collection on human diseases from MSs is conducted in accordance with Decision 1082/2013/EU 3 on serious cross‐border threats to health. In October 2013, this decision replaced Decision 2119/98/EC on setting up a network for the epidemiological surveillance and control of communicable diseases in the EU. The case definitions to be followed when reporting data on infectious diseases to the European Centre for Disease Prevention and Control (ECDC) are described in Decision 2018/945/EU 4 . ECDC has provided data on zoonotic infections in humans, as well as their analyses, for the EU Summary Reports since 2005. Since 2008, data on human cases have been received via The European Surveillance System (TESSy), maintained by ECDC.
Reporting requirements
In accordance with List A of Annex I of Zoonoses Directive 2003/99/EC, data on animals, food and feed must be reported on a mandatory basis for the following eight zoonotic agents: Salmonella, Campylobacter, Listeria monocytogenes, Shiga toxin‐producing Escherichia coli (STEC), Mycobacterium bovis, Brucella, Trichinella and Echinococcus. In addition, and based on the epidemiological situations in the MSs, data must be reported on the following agents and zoonoses (List B of Annex I of the Zoonoses Directive): (i) viral zoonoses: calicivirus, hepatitis A virus, influenza virus, rabies, viruses transmitted by arthropods; (ii) bacterial zoonoses: borreliosis and agents thereof, botulism and agents thereof, leptospirosis and agents thereof, psittacosis and agents thereof, tuberculosis due to agents other than M. bovis, vibriosis and agents thereof, yersiniosis and agents thereof; (iii) parasitic zoonoses: anisakiasis and agents thereof, cryptosporidiosis and agents thereof, cysticercosis and agents thereof, toxoplasmosis and agents thereof; and (iv) other zoonoses and zoonotic agents such as Francisella and Sarcocystis. Furthermore, MSs provided data on certain other microbiological contaminants in foods: histamine, staphylococcal enterotoxins and Cronobacter sakazakii, for which food safety criteria are set down in the EU legislation.
In accordance with Article 9 of the Directive, MSs shall assess the trends and sources of zoonoses, zoonotic agents and antimicrobial resistance in their territory and each MS shall send to the European Commission every year by the end of May a report on the trends and sources of zoonoses, zoonotic agents and antimicrobial resistance. Reports and any summaries of these shall be made publicly available.
Terms of Reference
In accordance with Article 9 of Directive 2003/99/EC, EFSA shall examine the national reports and data submitted by the EU MSs regarding their 2021 zoonoses monitoring activities as described above and publish an EU Summary Report on the trends and sources of zoonoses, zoonotic agents and antimicrobial resistance in the EU.
The 2021 data of MSs on antimicrobial resistance in zoonotic agents are published in a separate EU Summary Report.
Data sources and report production
Since 2019, the annual EU Summary Reports on zoonoses, zoonotic agents and foodborne outbreaks have been renamed the ‘EU One Health Zoonoses Summary Report’ (EUOHZ), which is co‐authored by EFSA and ECDC.
Since 2020, the production of the annual EUOHZ report has been supported by the ZOE (Zoonoses under a One health perspective in the EU) Consortium's Work‐package 1 composed of the Istituto Superiore di Sanità (Rome, Italy), the Istituto Zooprofilattico Sperimentale delle Venezie (Padova, Italy), the French Agency for Food, Environmental and Occupational Health & Safety (Maisons‐Alfort, France), the Istituto Zooprofilattico Sperimentale dell'Abruzzo e del Molise (Teramo, Italy) and the Istituto Zooprofilattico Sperimentale della Lombardia e dell'Emilia Romagna (Brescia, Italy), under the coordination of the Istituto Zooprofilattico Sperimentale dell'Abruzzo e del Molise (Teramo, Italy).
The efforts made by the MSs, the reporting non‐MSs and the European Commission in the reporting of zoonoses data and in the preparation of this report are gratefully acknowledged.
The MSs, other reporting countries, the European Commission, members of EFSA's Scientific Panels on Biological Hazards (BIOHAZ) and Animal Health and Welfare (AHAW) and the relevant European Union Reference Laboratories (EURLs) were consulted while preparing the EUOHZ 2021 report.
The EUOHZ 2021 report focuses on the most relevant information on zoonoses and foodborne outbreaks within the EU in 2021. Where substantial differences when compared to the previous years were observed, they have been reported.
In 2020, data collection was affected by the reduction in the number of EU MSs from 28 to 27, due to the withdrawal of the United Kingdom from the EU. 5 On 1 February 2020, the United Kingdom became a third country. In descriptive tables, data from the United Kingdom were included in the EU statistics for 2019 and previous years, whereas the 2020 statistical data from the United Kingdom, when available (for EFSA data), were assigned to the non‐MSs group. Human data from the United Kingdom were not collected by ECDC as of 2020. With regard to trend analyses of human data, only countries having contributed data for all the years of the considered period were taken into account. For trend analyses of the estimated prevalence at EU level of Salmonella in poultry populations covered by National Control Programs, any data provided by the reporting MSs were taken into account in the model. The United Kingdom data were only included when available for 2019 and previous years.
In 2021, the only United Kingdom data that were reported to EFSA were from Northern Ireland. In accordance with the Agreement on the withdrawal of the United Kingdom from the European Union, and in particular with the Protocol on Ireland/Northern Ireland, the European Union requirements on data sampling are also applicable to and in the United Kingdom with respect to Northern Ireland. Therefore, pursuant to Article 5(4) and Section 24 of Annex 2 of the Protocol on Ireland/Northern Ireland, which is an integral part of the Agreement on the withdrawal of the United Kingdom of Great Britain and Northern Ireland from the European Union and the European Atomic Energy Community, for the purpose of this report, references to MSs should be read as including Northern Ireland, despite it being part of the United Kingdom. Hence, the European Union requirements on data sampling were also applicable to Northern Ireland and data transmitted by the United Kingdom (Northern Ireland) have been assigned to the MSs group. For the collection of data, EFSA aligned with the guidelines of the Commission concerning customs registration, 6 which lay down the following abbreviations and terminology:
GB, which stands for ‘the United Kingdom’ and refers to: Great Britain, Northern Ireland, the Channel Islands and the Isle of Man.
XI, which stands for ‘the United Kingdom (Northern Ireland)’, and is used when the United Kingdom is identified with respect to Northern Ireland.
XU, which stands for ‘the United Kingdom (excluding Northern Ireland)’, and is used when the United Kingdom excluding Northern Ireland is identified.
Human data collection for 2021
In the 2021 EUOHZ report, the analyses of data from infections in humans were prepared by the Food‐ and Waterborne Diseases and Zoonoses (FWD) domain (brucellosis, campylobacteriosis, congenital toxoplasmosis, echinococcosis, listeriosis, salmonellosis, Shiga toxin‐producing E. coli infection, trichinellosis and yersiniosis), the Emerging and Vectorborne Diseases (EVD) domain (Q fever, rabies, tularaemia and West Nile virus (WNV) infection) and the tuberculosis (TB) domain (TB due to Mycobacterium bovis and M. caprae) at ECDC. Please note, as explained above, that the numbers presented in the report may differ from those in national reports due to differences in the case definitions used at the EU and at national levels, or due to differing dates of data submission and extraction. The latter may also result in some divergence in the case numbers presented in the different ECDC reports.
TESSy is a software platform in which data on 56 diseases and special health issues are collected. Both aggregated and case‐based data are reported to TESSy by MSs and other European countries. Although aggregated data did not include individual case‐based information, both reporting formats were included, when possible, to calculate the number of cases and country‐specific case notification rates. Human data used in the report were extracted from TESSy as of 20 July 2022 for EVD, as of 25 July 2022 for FWD and as of 30 September 2022 for TB due to M. bovis and M. caprae . The denominators used for calculating notification rates were based on the human population data from Eurostat on 01 January 2022.
Data on human cases were received from 27 MSs and from three non‐MSs (Iceland, Liechtenstein and Norway). Switzerland reported its data on human cases directly to EFSA. For 2021, Liechtenstein reported distinct data on human cases for the first time since 2008, whereas in the past years, these data were reported together with Switzerland.
Since the United Kingdom became a third country on 1 February 2020, human data from the United Kingdom were not collected by ECDC for 2020 and afterwards.
When interpreting data, data quality issues should be considered, as well as the differences between MS surveillance systems; comparisons between countries should therefore be undertaken with caution.
Data collection on food, animals, feed and foodborne outbreaks
In 2021, 27 MSs and the United Kingdom (Northern Ireland) submitted data and national zoonoses reports on monitoring results in food, animals, feed and foodborne outbreaks. In addition, data and reports were submitted by four non‐MSs and the European Free Trade Association (EFTA) countries: Iceland, Norway, Switzerland and Liechtenstein. 7 For some food, animal and feed matrices, and for foodborne outbreaks, EFSA received data and reports from the following pre‐accession countries: Albania, Bosnia and Herzegovina, the Republic of North Macedonia, Montenegro and Serbia.
Data were submitted electronically to the EFSA zoonoses database, through EFSA's Data Collection Framework (DCF). MSs could also update their data from previous years.
The deadline for data submission was 31 May 2022. Two data validation procedures were carried out, from 2 June to 14 June 2022 and from 7 July to 15 July 2022, respectively. Validated data on food, animals, feed and foodborne outbreaks used in the report were extracted from the EFSA zoonoses database on 26 July 2022.
The draft EUOHZ report was sent to the MSs for consultation on 12 October 2022 and comments were collected by 26 October 2022. The utmost effort was made to incorporate comments within the available time frame. Data amended after the data validation period that ended on 26 July 2022 have been not considered in summary calculations or other analyses, and footnotes to tables and figures have been added to inform on these late data corrections. The report was finalised by 11 November 2022 and published online by EFSA and ECDC on 8 December 2022.
A detailed description of the terms used in the report is available in EFSA's manuals for reporting on zoonoses (EFSA, 2022, 2022b,c).
The national zoonoses reports submitted in accordance with Directive 2003/99/EC are published on the EFSA website together with the EU One Health Zoonoses Report. They are available online at http://www.efsa.europa.eu/en/biological-hazards-data/reports.
Data analyses and presentation
Comparability and quality of data
Humans
For data on human infections, please note that the numbers presented in this report may differ from national zoonoses reports due to differences in case definitions used at the EU and national levels or because of differing dates of data submission and extraction. Results are not directly comparable among the MSs.
Food–animals–feed and foodborne outbreaks
For data on food, animals and feed, please note that the numbers presented in this report may differ from national zoonoses reports due to differing dates of data submission and extraction.
The data obtained by the EFSA DCF can vary according to the level of data quality and harmonisation. Therefore, the type of data analyses suggested by EFSA for each zoonosis and matrix (food, animals, feed or foodborne outbreaks) strongly depended on this level of harmonisation and can either be a descriptive summary of submitted data, the follow‐up of trends (trend watching) or the (quantitative) analyses of trends. Data analyses were carried out according to Table 1, as adapted from Boelaert et al. (2016). Food, animals, feed and foodborne outbreak data can be classified into three categories according to the zoonotic agent monitored and the design of the monitoring or surveillance carried out. It follows that the type of data analyses that can be implemented is conditioned by these three distinct categories.
Table 1.
Categorisation of the data used in the EU One Health Zoonoses 2021 Summary Report (adapted from Boelaert et al., 2016)
| Category | Type of analysis | Type/comparability between MSs | Examples | |
|---|---|---|---|---|
| I |
Descriptive summaries at the national level and EU level EU trend watching (trend monitoring) Spatial and temporal trend analyses at the EU level |
o |
Programmed harmonised monitoring or surveillance Comparable between MSs Results at the EU level are interpretable |
Salmonella national control programmes in poultry, bovine tuberculosis, bovine and small ruminant brucellosis, Trichinella in pigs at slaughterhouse |
| II |
Descriptive summaries at national level and EU level EU trend watching (trend monitoring) No EU trend analysis |
|
Monitoring or surveillance not fully harmonised Not fully comparable between MSs Caution needed when interpreting results at the EU level |
Foodborne outbreak data; official samplings related to process hygiene criteria for carcases at the slaughterhouse for Salmonella and Campylobacter and to food safety criteria for Campylobacter, L. monocytogenes, Salmonella and STEC in the context of Regulation (EC) No. 2073/2005; Rabies monitoring, West Nile virus |
| III |
Descriptive summaries at national level and EU level No EU trend watching (trend monitoring) No EU trend analysis |
|
Non‐harmonised monitoring or surveillance data with no (harmonised) reporting requirements Not comparable between MSs; extreme caution needed when interpreting results at the EU level |
Campylobacter, Yersinia, Q fever, Francisella tularensis, Taenia spp., Toxoplasma and other zoonoses |
Rationale of the table of contents
In keeping with the rationale of the zoonoses listing in Annex I of Directive 2003/99/EC, for the mandatory reporting of foodborne outbreaks and of the above‐mentioned categorisation of food, animal and feed data (Table 1), the following table of contents has been adopted for the 2021 EUOHZ report.
Zoonoses and zoonotic agents included in compulsory annual monitoring (Directive 2003/99/EC List A):
Campylobacter
Salmonella
Listeria
Shiga toxin‐producing Escherichia coli
Tuberculosis due to Mycobacterium bovis and Mycobacterium caprae
Brucella
Trichinella
Echinococcus
Foodborne and waterborne outbreaks (according to Directive 2003/99/EC).
Zoonoses and zoonotic agents monitored according to the epidemiological situation (Directive 2003/99/EC List B):
Yersinia
Toxoplasma gondii
Rabies
Q fever
West Nile virus
Tularaemia
Other zoonoses and zoonotic agents
Microbiological contaminants subject to food safety criteria (Regulation (EC) No 2073/2005).
After the section on ‘Microbiological contaminants subject to food safety criteria (Regulation (EC) No 2073/2005)’, a note was added in the 2021 EUOHZ report on outbreak data of SARS‐CoV‐2 in minks, mustelids and raccoon dogs, in EU.
Chapter sections
The 2021 EUOHZ Report presents a harmonised structure for each chapter:
‘Key facts’,
‘Monitoring and surveillance' in the EU for the specific disease,
‘Results’, summarising the major findings of 2021 as regards trends and sources, starting with a table displaying summary statistics for the last 5 years (2017–2021) for human cases, food matrices and major animal species,
A ‘Discussion’ section. For foodborne and waterborne outbreaks, the main findings are presented and discussed in a joint ‘Results and discussion’ section and key messages are summarised in the ‘Conclusions’ section.
For each chapter, overview tables present the data reported by each country. However, for the tables summarising MS‐specific results and providing EU‐level results for food, animals and feed, unless stated otherwise, data from industry own‐check programmes, hazard analysis and critical control point (HACCP) sampling, as well as data from suspect sampling, selective sampling and outbreak or clinical investigations are excluded. Moreover, regional data reported by countries for food, animals and feed, without statistics at the national level were also excluded from these summary tables.
Data analyses
Statistical trend analyses in humans were carried out to evaluate the significance of temporal variations in the EU (further details in each chapter) in years 2017–2021. The number of confirmed cases for the EU by month is presented as a trend figure for years 2012–2021. All countries that consistently reported cases – or reported zero cases over the whole reporting period – were included. The trend figure also shows a centred 12‐month moving average over the last 5 years, illustrating the overall trend by smoothing seasonal and random variations. Moreover, the same trend analysis was carried out separately for each country (MS and non‐MS countries). Analyses were carried out considering confirmed cases only, except for WNV infection, for which total locally acquired cases (i.e. probable and confirmed cases) were considered.
The notification rates were calculated taking into account the coverage of the human population under surveillance (percentage of national coverage). For countries where surveillance did not cover the whole population, the estimated coverage – if provided – was used to calculate the country‐specific rate. Cases and populations of those countries not providing information on national coverage or reporting incomplete data were excluded from the EU notification rate.
ESRI ArcMap 10.5.1 was used to map the data. Choropleth maps with graduated colours over five class scales of values, according to the natural breaks function proposed by the ArcGIS software, were used to map the proportion of positive sample units across the EU and other reporting countries. In the maps included in the present report, EU MSs and the United Kingdom (Northern Ireland) were represented with a blue label, whereas all the non‐EU MSs (including the EFTA countries: Iceland, Norway, Switzerland and Liechtenstein; and the pre‐accession countries: Albania, Bosnia and Herzegovina, the Republic of North Macedonia, Montenegro and Serbia) were represented with an orange label.
Statistical trend analysis of foodborne outbreaks was performed to evaluate the significance of temporal variations at the single MS level over the 2012–2021 period.
Summary data and figures on food, animals, feed and foodborne outbreaks used to produce this report, as well as additional information on related projects and internet sources are published for this report on the EFSA Knowledge Junction on the Zenodo general‐purpose open‐access repository here. All country‐specific data on food, animals, feed and foodborne outbreaks updated until 30 November 2022 are also available at this URL.
With the present report, EFSA has also published interactive communication tools:
the EFSA story maps on Campylobacter (here), Salmonella (here), Listeria monocytogenes (here) and foodborne outbreaks (here).
the EFSA dashboards on Campylobacter (here), Salmonella (here), Listeria monocytogenes (here) and foodborne outbreaks (here).
Data used in these communication tools were extracted from the EFSA zoonoses database on 26 July 2022.
Summary of human zoonoses data for 2021
The numbers of confirmed human cases of the zoonoses presented in this report are summarised in Figure 1. In 2021, campylobacteriosis was confirmed as the most commonly reported zoonosis (as it has been since 2005). It accounted for more than 62% of all the reported confirmed human cases in 2021. After campylobacteriosis, salmonellosis, yersiniosis, STEC infections and listeriosis were the most frequently reported. The severity of the diseases was descriptively analysed based on hospitalisations and the outcomes of reported cases (Table 2). Based on severity data, listeriosis and West Nile virus infection were the two most severe diseases with the highest case fatality and hospitalisation rates. Almost all confirmed cases with available hospitalisation data for these two diseases were hospitalised (96.5% of cases for listeriosis and 84.3% of cases for West Nile virus infection, respectively). The highest number of deaths was associated with listeriosis (N = 196; 13.7%), followed by salmonellosis (N = 71; 0.18%) and STEC infections (N = 18; 0.41%). For West Nile infections, the number of deaths was lower (N = 11), but the fatality rate was higher (7.2%) than salmonellosis and STEC infections.
Figure 1.
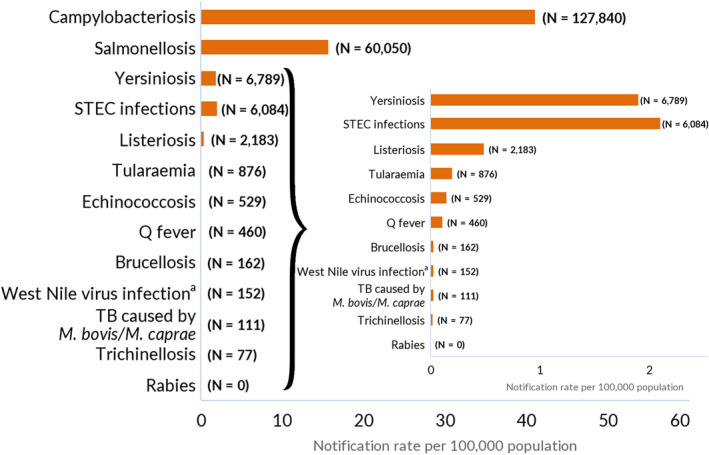
Reported numbers of cases and notification rates for confirmed human zoonoses in the EU, 2021
-
Data on congenital toxoplasmosis are not shown since 2021 data are not available yet.Note: The total number of confirmed cases is indicated in parentheses at the end of each bar.(a) Regarding West Nile virus infection, the total number of locally acquired cases was used (includes probable and confirmed cases).
Table 2.
Reported hospitalisations and deaths due to zoonoses in confirmed human cases and among foodborne outbreak cases in the EU, 2021
| Disease | Surveillance data on human cases (source: ECDC) | Foodborne outbreaks (FBO) (source: EFSA) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Confirmed human cases | Hospitalisation | Deaths | Outbreaks | Cases | Hospitalisations and proportion of hospitalised cases | Deaths and case fatality | |||||||||||
| Status available | Reporting MSs (b) | Cases and proportion of hospitalised cases | Outcome available | Reporting MSs (b) | Deaths and case fatality | ||||||||||||
| N | N | % | N | N | % | N | % | N | N | % | N | N | N | % | N | % | |
| Campylobacteriosis | 127,840 | 45,121 | 35.3 | 15 | 10,469 | 23.2 | 91,177 | 71.3 | 16 | 26 | 0.03 | 249 | 1,051 | 134 | 12.7 | 6 | 0.6 |
| Salmonellosis | 60,050 | 30,951 | 51.5 | 16 | 11,785 | 38.1 | 38,658 | 64.4 | 16 | 71 | 0.18 | 773 | 6,755 | 1,123 | 16.6 | 1 | 0.1 |
| Yersiniosis | 6,789 | 1,564 | 23.0 | 13 | 508 | 32.5 | 3,596 | 53.0 | 21 | 0 | 0 | 21 | 125 | 14 | 11.2 | 0 | 0 |
| STEC infections | 6,084 | 2,133 | 35.1 | 17 | 901 | 42.2 | 4,366 | 71.8 | 20 | 18 | 0.41 | 31 | 275 | 47 | 13.5 | 0 | 0 |
| Listeriosis | 2,183 | 956 | 43.8 | 16 | 923 | 96.5 | 1,427 | 65.4 | 14 | 196 | 13.7 | 23 | 104 | 48 | 46.2 | 12 | 11.5 |
| Tularaemia | 876 | 221 | 25.2 | 10 | 112 | 50.7 | 341 | 38.9 | 11 | 2 | 0.59 | 0 | 0 | 0 | – | 0 | – |
| Echinococcosis | 529 | 121 | 22.9 | 13 | 51 | 42.1 | 270 | 51.0 | 16 | 0 | 0 | 0 | 0 | 0 | – | 0 | – |
| Q fever | 460 | NA | NA | NA | NA | NA | 270 | 58.7 | 11 | 4 | 1.5 | 0 | 0 | 0 | – | 0 | – |
| Brucellosis | 162 | 60 | 37.0 | 10 | 36 | 60.0 | 59 | 36.4 | 11 | 0 | 0 | 1 | 2 | 2 | 100 | 0 | – |
| West Nile virus infection (a) | 152 | 83 | 54.6 | 6 | 70 | 84.3 | 152 | 100 | 8 | 11 | 7.2 | NA | NA | NA | NA | NA | NA |
| Trichinellosis | 77 | 26 | 33.8 | 6 | 10 | 38.5 | 28 | 36.4 | 6 | 0 | 0 | 1 | 2 | 0 | 100 | 0 | – |
| Rabies | 0 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
Data on congenital toxoplasmosis are not shown since 2021 data are not available yet.
MSs: Member States.
NA: Not applicable as the information is not collected for this dsease.
: Total number of locally acquired infections (probable and confirmed cases).
: Not all countries observed cases for all diseases.
With regard to foodborne outbreaks (FBOs), Salmonella, Norovirus and Campylobacter accounted for the highest number of outbreaks and cases.
Comparison of human zoonoses data for 2021, 2020 and previous years
In order to estimate the trends of human infections, especially in relation to the COVID‐19 pandemic, the 2021 data (number of cases and notification rates) were compared with those from 2020 as well as with the mean annual number of cases and mean notification rates from the prepandemic years (2017–2019). Moreover, some statistics were also collected to obtain indications concerning the effects of the withdrawal of the United Kingdom from the EU. The absolute and relative difference between the number of cases and the notification rates reported in the EU for 2021 compared with 2020, and for 2021 compared with the annual average for each disease in the 2017–2019 period, were estimated (Table 3).
Table 3.
Number of confirmed human cases and notification rates (per 100,000 population) in 2021 by zoonosis, including the absolute and relative (%) difference with regard to 2020, and the mean annual cases and mean notification rates (per 100,000 population) for the 2017–2019 period, EU
| Zoonosis | EU level (a) | Cases (N) | Notification rates (N confirmed cases *100,000) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 2021 | 2020 difference | 2017–2019 (mean annual) | 2021 | 2020 | 2017–2019 (mean annual) | ||||
| Absolute difference | Relative difference (%) | Absolute difference | Relative difference (%) | ||||||
| Campylobacteriosis | EU | 127,840 | +7,296 | −110,076 | 41.1 | +0.86 | +2.1 | −23.2 | −36.1 |
| EU‐27 | −47,665 | −16.1 | −28.1 | ||||||
| Salmonellosis | EU | 60,050 | +7,360 | −30,401 | 15.7 | +1.96 | +14.3 | −3.8 | −19.6 |
| EU‐27 | −20,638 | −4.7 | −23.1 | ||||||
| Yersiniosis | EU | 6,789 | +1,128 | −153 | 1.9 | +0.20 | +11.8 | +0.19 | +11.3 |
| EU‐27 | +15 | −0.19 | −8.9 | ||||||
| STEC infections | EU | 6,084 | +1,595 | −1,262 | 2.1 | +0.55 | +36.9 | +0.19 | +9.9 |
| EU‐27 | +211 | +0.26 | +14.2 | ||||||
| Listeriosis | EU | 2,183 | +296 | −363 | 0.49 | +0.06 | +14.0 | +0.02 | +4.3 |
| EU‐27 | −203 | −0.01 | −2.0 | ||||||
| Tularaemia | EU | 876 | +235 | +252 | 0.20 | +0.05 | +33.3 | +0.08 | +66.7 |
| EU‐27 | +252 | +0.06 | +42.9 | ||||||
| Echinococcosis | EU | 529 | −15 | −283 | 0.15 | −0.01 | −7.5 | −0.04 | −23.0 |
| EU‐27 | −280 | −0.06 | −30.4 | ||||||
| Q‐fever | EU | 460 | −63 | −415 | 0.11 | −0.01 | −12.0 | −0.07 | −38.8 |
| EU‐27 | −399 | −0.09 | −45.8 | ||||||
| West Nile virus (b) | EU | 152 | −178 | −573 | 0.03 | −0.04 | −53.9 | −0.11 | −76.4 |
| EU‐27 | −573 | −0.13 | −79.5 | ||||||
| Brucellosis | EU | 162 | +30 | −178 | 0.03 | < 0.01 | +12.3 | −0.04 | −54.8 |
| EU‐27 | −170 | −0.04 | −55.5 | ||||||
| Tuberculosis caused by M. bovis, M caprae | EU | 111 | +12 | −71 | 0.03 | < 0.01 | +12.2 | −0.01 | −28.3 |
| EU‐27 | −38 | −0.01 | −25.3 | ||||||
| Trichinellosis | EU | 77 | −40 | −33 | 0.02 | −0.01 | −32.5 | <−0.01 | −17.6 |
| EU‐27 | −33 | −0.01 | −28.6 | ||||||
| Rabies | EU | 0 | 0 | −2 | 0 | 0 | – | 0 | – |
| EU‐27 | ‐2 | 0 | – | ||||||
: Data from the United Kingdom are taken into account for 2017–2019 because the United Kingdom was an EU MS, but it became a third country on 1 February 2020. To calculate the difference between 2021 and 2017–2020 (mean annual statistics), data from the United Kingdom for 2017–2019 were included in the ‘EU Total’ difference, whereas human data from the United Kingdom were not collected by ECDC for 2020 and 2021 (‘EU‐27’).
: For West Nile virus infection, the total number of locally acquired cases was used (including probable and confirmed cases).
For all zoonoses except echinococcosis, Q fever, WNV infection and trichinellosis, there was an increase in the number of confirmed cases for 2021 when compared to 2020, whereas the comparison in terms of the number of confirmed cases for 2021 vs. the annual mean for the 2017–2019 period showed a decrease in 2021 for all zoonoses except tularaemia.
For STEC infections (+36.9%), tularaemia (+33.3%), salmonellosis (+14.3%), listeriosis (+14.0%), brucellosis (+12.3%), yersiniosis (+11.8%) and campylobacteriosis (+2.1%), there was an increase in the relative difference in notification rates (*100,000 population) in 2021 as compared with 2020, whereas for all the other zoonoses, the opposite trend was observed. The same comparison (relative difference) between the 2021 notification rates and the annual averages of the 2017–2019 notification rates, conducted to evaluate the effect of the pandemic, revealed a sharp decrease in WNV infections (−76.4%), brucellosis (−54.8%), Q fever (−38.8%), campylobacteriosis (−36.1%), trichinellosis (−28.6%), echinococcosis (−23.0%) and salmonellosis (−19.6%), whereas an opposite trend was seen for tularaemia (+66.7%), yersiniosis (+11.3%), STEC infections (+9.9%) and listeriosis (+4.3%). For each disease, the relative difference in EU notification rates was also calculated based on EU‐27 data only (i.e. excluding data reported by the United Kingdom for 2017, 2018 and 2019) (Table 3) in order to provide evidence of the effect of the withdrawal of the United Kingdom from the EU. The comparison of the relative difference in notification rates based on EU and EU‐27 data showed the same trend for almost all of the zoonoses with very few exceptions (e.g. yersiniosis).
Zoonoses included in compulsory annual monitoring (in accordance with Directive 2003/99 List A)
1. Campylobacter
Summary data substantiating this chapter, as well as additional information on related projects and internet sources are published for this report on the EFSA Knowledge Junction on Zenodo here. Summary statistics on Campylobacter food monitoring data and on campylobacteriosis foodborne outbreaks reported in the framework of Directive 2003/99/EC, with downloadable files, are retrievable using the EFSA Campylobacter dashboard and the EFSA foodborne outbreaks dashboard, respectively available here and here. Summary statistics on human surveillance data with downloadable files are retrievable using the ECDC Surveillance Atlas of Infectious Diseases here.
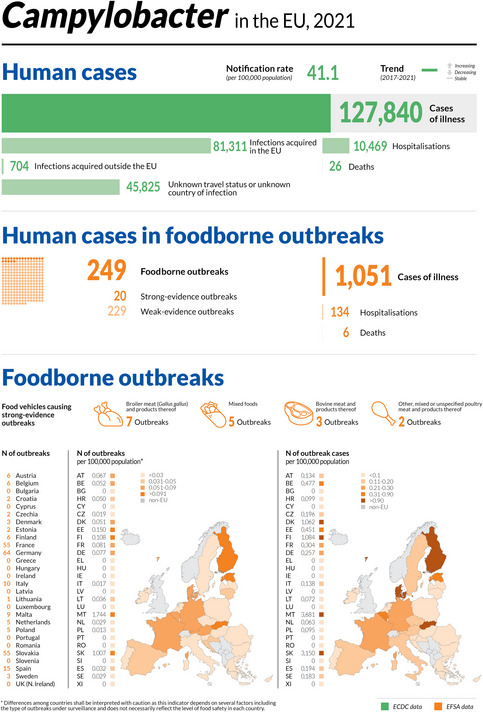
1.1. Key facts
Campylobacteriosis is the most commonly reported foodborne gastrointestinal infection in humans in the EU and has been so since 2007.
In 2021, the number of confirmed cases of human campylobacteriosis was 127,840, corresponding to an EU notification rate of 41.1 per 100,000 population. This was an increase of 2.1% compared with 2020 (40.2 per 100,000 population).
In 2020, ECDC recorded the lowest number of human cases of campylobacteriosis in any year since surveillance began in 2007, owing to the impact of the COVID‐19 pandemic and the withdrawal of the United Kingdom from the EU.
Compared with the rate before the COVID‐19 pandemic (2017–2019 annual mean), the 2021 EU notification rate decreased by 36.1% and 28.1%, with and without the data from the United Kingdom, respectively. The overall trend for the campylobacteriosis in the period 2017–2021 in the EU showed no statistically significant increase or decrease.
In most of the cases (99.1%), where the origin was known, the infection was acquired in the EU.
Twenty‐four MSs reported data in 2021 in the context of the Campylobacter process hygiene criterion, set out in Regulation (EC) No 2073/2005. Fifteen MSs reported 8,063 test results from official controls, with 42.1% Campylobacter‐positive samples and 18.4% exceeding the limit of 1,000 CFU/g. Twenty MSs reported 53,351 test results from the monitoring of food business operators, with 27.2% positive samples and 16.4% exceeding the limit of 1,000 CFU/g. Eleven MSs reported results from both samplers, showing that the number of samples exceeding the limit was significantly higher in official samples (19.4%) than in own‐checks (7.3%).
In 2021, 3,220 ‘ready‐to‐eat’ and 14,158 ‘non‐ready‐to‐eat’ results from food sampling units were reported by 12 and 16 MSs, respectively. In the former category, 0.31% of the units were positive to Campylobacter as compared to 10.9% in the latter. The highest level of contamination in the ‘non‐ready‐to‐eat’ food category was in ‘meat and meat products’ with 11.9% of positive units. Overall, Campylobacter was isolated from all fresh meat categories, with meat from turkeys and broilers showing the highest percentage of Campylobacter‐positive sampling units at 12.9% and 11.5%, respectively.
Campylobacter spp. was detected by 16 MSs and three non‐MSs in more than 30 different animal categories in 2021. The vast majority of units tested in the EU (N = 10,162) were from broilers with a proportion of positives of 10.5%. The proportion of positive sampling units for pigs, cattle and ‘cats and dogs’ was 41.3%, 13.5% and 12.3%, respectively.
Alongside the present report, EFSA has also published two new interactive communication tools on Campylobacter: the EFSA story map (available here) and the dashboard (available here).
1.2. Surveillance and monitoring of Campylobacter in the EU
1.2.1. Humans
In 2021, 27 EU MSs reported information on campylobacteriosis in humans. Surveillance of campylobacteriosis is mandatory in 22 EU MSs. In five MSs (Belgium, France, Greece, Italy and the Netherlands), notification is based on a voluntary system. In 2020–2021, Spain did not receive data from all the regions that normally report, so the case number might not be complete. The EU case definition was used by 21 MSs. Four MSs used a different case definition for reporting (Denmark, France, Germany and Italy), Greece did not use any case definition and the Netherlands did not specify which case definition it used. All MSs, except four (Belgium, Greece, Italy and the Netherlands) had a comprehensive surveillance system.
The campylobacteriosis surveillance systems cover the whole population in all MSs except four (France, Italy, the Netherlands and Spain). The estimated coverage of the surveillance system is 20% in France and 64% in the Netherlands. These estimated proportions of population coverage were used in the calculation of notification rates for these two MSs. No estimates of population coverage in Italy and Spain were provided, so no notification rates were calculated for these two MSs. All countries reported case‐based data except Belgium, Bulgaria and Greece, which reported aggregated data.
1.2.2. Food and animals
Campylobacter is monitored along the food chain during the primary production stage (farm animals), during harvest/slaughter, manufacturing and at the distribution stage.
Campylobacter data in the context of Regulation (EC) No 2073/2005
A regulatory limit (microbiological process hygiene criterion (PHC)) of 1,000 CFU/g of Campylobacter on the neck skins of chilled broiler carcases was set by Regulation (EC) N. 2073/2005 8 (point 2.1.9 of Chapter 2 of Annex I). This limit applies to a set of 50 pooled samples from 10 consecutive sampling sessions. As of 2021, a maximum number of 15 samples with values exceeding this limit are considered as acceptable. Food business operators (FBOp) failing to comply with this limit are required to carry out corrective actions involving validation and verification of their food safety management procedures based on HACCP principles and good manufacturing practices (GMP). The PHC has been in force since 1 January 2018. On 14 December 2019, Commission Implementing Regulation (EU) 2019/627 9 came into force, harmonising sampling procedures for official controls. This legislation requires the Competent Authority (CA) to verify whether the FBOp is correctly implementing and checking the PHC on broiler carcases by choosing one of two approaches: implementing ad hoc official sampling 10 or collecting all the information from the samples taken by the FBOp. The results obtained in official controls enable improved trend watching and trend analyses (Table 1).
Other monitoring data for food and animals
Campylobacter monitoring data from food and animals submitted to EFSA in compliance with Chapter II ‘Monitoring of zoonoses and zoonotic agents’ of the Zoonoses Directive 2003/99/EC 11 are collected without a harmonised procedure. These data allow descriptive summaries at EU level, but they do not support EU‐level trend analyses and trend watching (Table 1).
In 2021, general data on food and animals reported to EFSA by MSs and non‐MSs were obtained mainly from official sampling, industry sampling HACCP and own‐checks, as part of national monitoring and surveillance, and/or organised surveys. In addition, for animal data, other reported samples were obtained from clinical investigations by private veterinarians and industry (e.g. artificial insemination centres).
The occurrence of Campylobacter reported in the main food categories for the year 2021 and for the 4‐year period of 2017–2020 was descriptively summarised, making a distinction between RTE and non‐RTE food. Data sets were extracted using the strategy of ‘objective sampling’, meaning that the reporting MS collected the samples as part of a planned strategy based on the selection of random samples that are statistically representative of the population to be analysed.
Other Campylobacter monitoring data, intended solely for monitoring antimicrobial resistance, are obtained from selected animal species and their carcases/meat, using the harmonised sampling scheme set out in the Commission Implementing Decision (EU) 2020/1729. These antimicrobial resistance results are published in a separate EU Summary Report.
The detection and confirmation of Campylobacter in food and animals is generally based on culture, with the use of international standards or equivalent validated methods. Species identification is carried out using biochemical and molecular methods (PCR based), as well as matrix‐assisted laser desorption/ionisation time‐of flight mass spectrometry (MALDI‐TOF MS).
EFSA story map on Campylobacter
The EFSA story map on Campylobacter is a new interactive communication tool developed by EFSA in 2022, available online (here) and geared to the general public. This story map provides general information on the pathogen and its epidemiology, including information on where the pathogen can be found, how people and animals get infected, the occurrence of this pathogen in different sources, the disease it causes and how to prevent infection. In addition, this story map also illustrates the monitoring activities implemented in the EU and the role of EFSA with respect to these activities. Users can easily display and explore the content of the different sections in the story map, browsing the dynamic maps, images, text and multimedia features.
1.3. Data analyses
A comparison was made of Campylobacter results exceeding 1,000 CFU/g from the neck skins of broiler carcases after chilling, as obtained by the CA and FBOp as part of the Campylobacter PHC in compliance with Regulation (EC) No 2073/2005. The significance of any differences was verified by the one‐tailed Fisher's exact probability test in cases where the expected values of any of the cells in a contingency table were below 5; otherwise, the one‐tailed z test was used. The official control sampling results by the CA and the own‐check results by the FBOp were expressed as prevalence ratios with an exact binomial confidence interval of 95%. A p‐value < 0.10 (Clayton and Hills, 2013) was considered as significant, in order to highlight every possible indication of differences between the data collected by the FBOp and the CA. R software (www.r-project.org, version 4.0.5) was used to conduct the above analyses.
EFSA dashboard on Campylobacter
The EFSA dashboard on Campylobacter (available online here) is a graphical user interface for searching and querying the large amount of data collected each year by EFSA from EU MSs and other reporting countries based on Zoonoses Directive 2003/99/EC. The Campylobacter dashboard shows summary statistics for the monitoring results of the pathogen with regard to major food categories, Campylobacter‐positive official samples exceeding the Process Hygiene Criterion limit of 1,000 CFU/g for chilled broiler carcases and the occurrence of Campylobacter in major food categories. The Campylobacter data and related statistics can be displayed interactively using charts, graphs and maps in the online EFSA dashboard. In this tool, the main statistics can also be viewed and downloaded in tabular format. Detailed information on the use and features of the Campylobacter dashboard can be found in the user guide available on Zenodo here and can also be downloaded from the online tool. Links to the dashboard are available in the relevant sections of this chapter.
1.4. Results
1.4.1. Overview of key statistics, EU, 2017–2021
Table 4 summarises EU statistics on human campylobacteriosis and on the occurrence and prevalence of Campylobacter in food and animals, respectively, during 2017–2021. In 2021, a slight increase was observed in notified human cases and the EU notification rate, despite the ongoing COVID‐19 pandemic. However, the notification rate remained low compared with the pre‐pandemic period of 2017–2019. The food data of interest in this report were classified into two major categories: ‘meat and meat products’ and ‘milk and milk products’, aggregated by year to obtain an annual overview of the volume of data submitted. Since 2019, the number of sampling units reported for ‘meat and meat products’ has increased sharply, probably owing to the Commission Implementing Regulation (EU) 2019/627 establishing compulsory reporting of Campylobacter PHC monitoring data (see above). Animal data were collected principally from broilers, cattle and pigs, showing comparable and consistent test numbers over the considered period. More detailed descriptions of these statistics are provided in the below subsections and in the chapter on foodborne outbreaks.
Table 4.
Summary of Campylobacter statistics related to humans, major food categories and the main animal species, EU, 2017–2021
| 2021 (a) | 2020 | 2019 (b) | 2018 (b) | 2017 (b) | Data source | |
|---|---|---|---|---|---|---|
| Humans | ||||||
| Total number of confirmed cases | 127,840 | 120,544 | 220,639 | 246,570 | 246,538 | ECDC |
| Total number of confirmed cases/100,000 population (notification rates) | 41.1 | 40.2 | 60.6 | 66.0 | 66.1 | ECDC |
| Number of reporting MSs | 27 | 27 | 28 | 28 | 28 | ECDC |
| Infection acquired in the EU | 81,311 | 70,772 | 109,937 | 116,246 | 122,280 | ECDC |
| Infection acquired outside the EU | 704 | 1,586 | 6,514 | 7,685 | 6,583 | ECDC |
| Unknown travel status or unknown country of infection | 45,825 | 48,186 | 104,188 | 122,639 | 117,675 | ECDC |
| Number of foodborne outbreak‐related cases | 1,051 | 1,319 | 1,770 | 2,365 | 3,608 | EFSA |
| Total number of foodborne outbreaks | 249 | 317 | 542 | 537 | 395 | EFSA |
| Food (c) | ||||||
| Meat and meat products (d) | ||||||
| Number of sampling units | 87,808 | 66,099 | 57,027 | 26,514 | 21,521 | EFSA |
| Number of reporting MSs | 25 | 25 | 25 | 26 | 22 | EFSA |
| Milk and milk products (e) | ||||||
| Number of sampling units | 2,125 | 2,145 | 2,749 | 3,227 | 2,317 | EFSA |
| Number of reporting MSs | 11 | 11 | 11 | 13 | 13 | EFSA |
| Animals | ||||||
| Cattle (bovine animals) | ||||||
| Number of sampling units (f) | 7,529 | 4,387 | 6,850 | 4,220 | 7,312 | EFSA |
| Number of reporting MS | 11 | 7 | 10 | 8 | 10 | EFSA |
| Gallus gallus (chicken) | ||||||
| Number of sampling units (f) | 10,162 | 13,628 | 10,472 | 14,093 | 10,133 | EFSA |
| Number of reporting MSs | 6 | 15 | 8 | 16 | 7 | EFSA |
| Pigs | ||||||
| Number of sampling units(f) | 4,502 | 2,110 | 4,308 | 2,481 | 3,824 | EFSA |
| Number of reporting MSs | 14 | 4 | 11 | 5 | 10 | EFSA |
ECDC: European Centre for Disease Prevention and Control; EFSA: European Food Safety Authority; MSs: Member States.
: Data on food and animal samples from the United Kingdom (Northern Ireland) are taken into account for 2021. In accordance with the agreement on the withdrawal of the United Kingdom from the EU, and in particular with the Protocol on Ireland/Northern Ireland, the EU requirements on data sampling are also applicable to Northern Ireland.
: Data from the United Kingdom are taken into account for 2017–2019, because the United Kingdom was an EU MS, but it became a third country on 1 February 2020.
: Summary statistics referring to MSs were obtained by totalling all sampling units (single samples, samples from batches and samples from slaughter batches), sampling stages (farm, packing centre, processing plant, cutting plant, slaughterhouse, catering, hospital or medical care facility, restaurant or cafe or pub or bar or hotel or catering service, retail, wholesale, border control posts, school or kindergarten, unspecified), sampling strategies (census, convenience sampling, selective sampling, objective sampling and unspecified) and samplers (official sampling, official and industry sampling, private sampling, unspecified).
: ‘Meat and meat products’ refer to carcases and fresh meat/ready‐to‐eat (RTE), cooked and fermented products.
: ‘Milk and milk products’ refer to raw and pasteurised milk and all dairy products including cheeses.
: Summary statistics referring to MSs were obtained by totalling all sampling units (single animals, slaughter animal batches and herds or flocks).
For a further interactive look at Campylobacter monitoring results, dashboards have been implemented (different filters can be applied to query the data) ( link ).
1.4.2. Human campylobacteriosis
In 2021, 127,840 confirmed cases of human campylobacteriosis were reported by the 27 EU MSs, corresponding to an EU notification rate of 41.1 cases per 100,000 population (Table 4). This is an increase of 2.1% compared with 2020 (40.2 per 100,000 population) and a decrease of 36.1% and 28.1% compared with the average annual notification rate from 2017 to 2019 (pre‐pandemic period), with and without United Kingdom data, respectively (Table 3). France, Ireland, Latvia, Malta and Portugal had an increased rate in 2021 compared with the pre‐pandemic period. The highest country‐specific notification rates in 2021 were observed in Czechia (152.4 cases per 100,000), Slovakia (111.7), Luxembourg (92.8) and Malta (73.2). The lowest rates in 2021 were observed in Poland, Romania, Bulgaria, Greece and Cyprus (≤ 2.7 per 100,000) (Table 5).
Table 5.
Reported confirmed human cases of campylobacteriosis and notification rates per 100,000 population in EU MSs and non‐MS countries, by country and year, 2017–2021
| Country | 2021 | 2020 | 2019 | 2018 | 2017 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| National coverage (a) | Data format (a) | Confirmed cases and rates | Confirmed cases and rates | Confirmed cases and rates | Confirmed cases and rates | Confirmed cases and rates | ||||||
| Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | |||
| Austria | Y | C | 6,019 | 67.4 | 5,406 | 60.7 | 6,572 | 74.2 | 7,999 | 90.7 | 7,204 | 82.1 |
| Belgium | Y | A | 3,273 | 28.3 | 5,693 | 49.4 | 7,337 | 64.0 | 8,086 | 70.9 | 8,649 | 76.2 |
| Bulgaria | Y | A | 130 | 1.9 | 127 | 1.8 | 229 | 3.3 | 191 | 2.7 | 195 | 2.7 |
| Croatia | Y | C | 1,173 | 29.1 | 1,054 | 26.0 | 1,722 | 42.2 | 1,965 | 47.9 | 1,686 | 40.6 |
| Cyprus | Y | C | 24 | 2.7 | 18 | 2.0 | 21 | 2.4 | 26 | 3.0 | 20 | 2.3 |
| Czechia | Y | C | 16,305 | 152.4 | 17,517 | 163.8 | 22,894 | 215.0 | 22,895 | 215.8 | 24,326 | 230.0 |
| Denmark | Y | C | 3,740 | 64.0 | 3,742 | 64.3 | 5,402 | 93.0 | 4,559 | 78.9 | 4,255 | 74.0 |
| Estonia | Y | C | 185 | 13.9 | 265 | 19.9 | 347 | 26.2 | 411 | 31.2 | 285 | 21.7 |
| Finland | Y | C | 1,798 | 32.5 | 2,074 | 37.5 | 4,382 | 79.4 | 5,099 | 92.5 | 4,289 | 77.9 |
| France (b) | N | C | 8,875 | 65.6 | 7,920 | 58.8 | 7,712 | 57.4 | 7,491 | 55.9 | 6,579 | 49.2 |
| Germany | Y | C | 47,912 | 57.6 | 46,378 | 55.8 | 61,277 | 73.8 | 67,585 | 81.6 | 69,251 | 83.9 |
| Greece | Y | A | 260 | 2.4 | 218 | 2.0 | 366 | 3.4 | 357 | 3.3 | 344 | 3.2 |
| Hungary | Y | C | 5,088 | 52.3 | 4,461 | 45.7 | 6,400 | 65.5 | 7,117 | 72.8 | 7,807 | 79.7 |
| Ireland | Y | C | 3,147 | 62.9 | 2,419 | 48.7 | 2,776 | 56.6 | 3,044 | 63.0 | 2,779 | 58.1 |
| Italy (c) | N | C | 1,542 | – | 1,418 | – | 1,633 | – | 1,356 | – | 1,060 | – |
| Latvia | Y | C | 158 | 8.3 | 104 | 5.5 | 133 | 6.9 | 87 | 4.5 | 59 | 3.0 |
| Lithuania | Y | C | 357 | 12.8 | 684 | 24.5 | 1,221 | 43.7 | 919 | 32.7 | 990 | 34.8 |
| Luxembourg | Y | C | 589 | 92.8 | 729 | 116.4 | 271 | 44.1 | 625 | 103.8 | 613 | 103.8 |
| Malta | Y | C | 378 | 73.2 | 206 | 40.0 | 278 | 56.3 | 333 | 70.0 | 231 | 50.2 |
| Netherlands (d) | N | C | 2,692 | 24.1 | 2,549 | 25.2 | 3,415 | 34.1 | 3,091 | 34.6 | 2,890 | 32.5 |
| Poland | Y | C | 616 | 1.6 | 414 | 1.1 | 715 | 1.9 | 719 | 1.9 | 874 | 2.3 |
| Portugal | Y | C | 973 | 9.4 | 790 | 7.7 | 887 | 8.6 | 610 | 5.9 | 596 | 5.8 |
| Romania | Y | C | 348 | 1.8 | 300 | 1.6 | 805 | 4.1 | 573 | 2.9 | 467 | 2.4 |
| Slovakia | Y | C | 6,099 | 111.7 | 4,921 | 90.2 | 7,690 | 141.1 | 8,339 | 153.2 | 6,946 | 127.8 |
| Slovenia | Y | C | 856 | 40.6 | 811 | 38.7 | 1,085 | 52.1 | 1,305 | 63.1 | 1,408 | 68.2 |
| Spain (c) , (e) | N | C | 11,244 | – | 6,891 | – | 9,658 | – | 18,410 | – | 18,860 | – |
| Sweden | Y | C | 4,059 | 39.1 | 3,435 | 33.3 | 6,693 | 65.4 | 8,132 | 80.4 | 10,608 | 106.1 |
| EU Total 27 | – | – | 127,840 | 41.1 | 120,544 | 40.2 | 161,921 | 54.0 | 181,324 | 58.3 | 183,271 | 59.0 |
| United Kingdom | – | – | – | – | – | – | 58,718 | 88.1 | 65,246 | 98.4 | 63,267 | 96.1 |
| EU Total (f) | – | – | 127,840 | 41.1 | 120,544 | 40.2 | 220,639 | 60.6 | 246,570 | 66.0 | 246,538 | 66.1 |
| Iceland | Y | C | 58 | 15.7 | 95 | 26.1 | 136 | 38.1 | 145 | 41.6 | 119 | 35.2 |
| Norway | Y | C | 2,049 | 38.0 | 2,422 | 45.1 | 4,154 | 78.0 | 3,668 | 69.3 | 3,883 | 73.8 |
| Liechtenstein | Y | C | 38 | 97.3 | 6,196 | 71.7 | 7,200 | 83.9 | 7,696 | 90.3 | 7,217 | 85.3 |
| Switzerland (g) | Y | C | 6,755 | 77.9 | ||||||||
–: Data not reported.
: Y: yes; N: no; A: aggregated data; C: case‐based data.
: Sentinel surveillance; notification rates calculated with an estimated coverage of 20%.
: The notification rate was not calculated since information on estimated coverage was not available.
: Sentinel surveillance; notification rates calculated with an estimated coverage of 64% in 2021, 58% in 2019–2020, 52% in 2017–2018.
: Data not complete in 2020–2021, rate not estimated.
: Cases reported by the United Kingdom for the period 2017–2019 were also considered for this estimation (EU‐28). When the United Kingdom data were collected for the period 2017–2019, the United Kingdom was an EU MS, but it became a third country on 1 February 2020.
: Switzerland provided data directly to EFSA. The human data for Switzerland include data from Liechtenstein for the years 2017–2020.
For most (99.1%) of the reported campylobacteriosis cases of known origin, the infection was contracted in the EU (Table 4) as compared with 98.5% in 2020. Twenty countries reported data on the importation of cases. The proportion of domestic cases with known data was over 95% in all reporting countries except five MSs, which reported the highest proportion of travel‐associated cases: Finland (34.2%), Sweden (19.1%), Denmark (12.7%), Portugal (6%) and Germany (5.1%). The proportion of travel‐associated cases observed in 2021 (3.8%) compared with 2020 (3.7%) were very much at the same level, differing from the higher proportion of travel‐associated cases in 2019 (10.8%), before the COVID‐19 pandemic. However, the number of cases acquired outside the EU decreased considerably in 2021 compared with 2020 (Table 4). Of the 3,138 travel‐associated cases reported by MSs with a known country of origin, 2,063 cases (65.7%) were linked to travel within the EU, with most of the infections being acquired in Spain, Croatia, Greece and Italy (28.1%, 12.6%, 10.5% and 9.5%, respectively). Turkey, Serbia, Bosnia and Herzegovina, Pakistan and Ukraine were the most frequently reported probable countries of infection outside the EU (29.8%, 7.7%, 7%, 3.1% and 3.1%, respectively). Campylobacteriosis cases were reported in all age groups, with the highest proportion of reported cases belonging to the youngest age group from 0 to 4 years (20,884 cases: 16.8%).
Between 2012 and 2021, the number of confirmed campylobacteriosis cases reported in the EU showed a clear seasonal trend, peaking in the summer months. Annual winter peaks were also observed in January from 2012 to 2021, although peak numbers were lower than those observed during the summer. As in 2020, a decrease in the number of cases was observed in 2021, probably due to the COVID‐19 pandemic. However, the overall campylobacteriosis trend in 2017–2021 showed no statistically significant increase or decrease (Figure 2). Belgium, Finland, Hungary, Poland, Slovenia and Sweden reported significantly decreasing trends (p < 0.05) during the period 2017–2021. Latvia and Portugal reported a significantly increasing trend over the same period.
Figure 2.
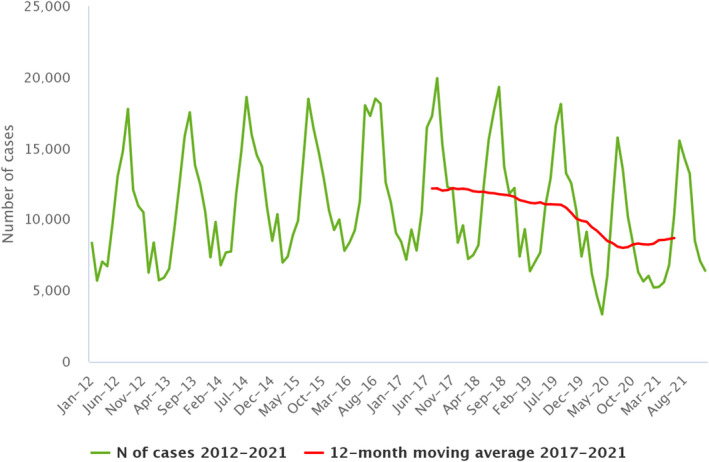
- Source: Austria, Cyprus, Czechia, Denmark, Estonia, Finland, France, Germany, Hungary, Ireland, Italy, Latvia, Luxembourg, Malta, the Netherlands, Poland, Romania, Slovenia and Sweden.
Information on hospitalisation status was provided for 35.3% of all campylobacteriosis cases by 15 MSs. Of the cases with known hospitalisation status, 10,469 (23.2%) were hospitalised. The highest hospitalisation rates were reported in Latvia (95.6%), Cyprus (91.7%) and Poland (80%), where most of the reported cases were hospitalised. Outcomes were reported for 71.3% of all cases by 16 MSs. Twenty‐six deaths from campylobacteriosis were reported in 2021, resulting in an EU case fatality rate of 0.03%. The average percentage of fatal outcomes observed has remained unchanged over the past 5 years (range 0.03–0.05%). Information on gender was provided for 123,990 confirmed cases in the EU, 54.7% were male and 45.3% female.
Campylobacter species information was provided by 22 MSs for 65.1% of confirmed cases reported in the EU, representing a slight increase compared with 2020 (64.7%). Of these cases, 88.4% were Campylobacter jejuni, 10.1% C. coli, 0.18% C. fetus, 0.12% C. upsaliensis and 0.09% Campylobacter lari. Other Campylobacter species accounted for 1.1% of cases, but most of those cases were reported at the national level as ‘C. jejuni/C. coli/C. lari not differentiated’. Belgium, Bulgaria, Denmark, Greece and Sweden provided no information on species.
1.4.3. Campylobacter in food
Campylobacter data in the context of Regulation (EC) No 2073/2005
The Campylobacter PHC monitoring data reported in Table 6 show test results obtained using a culture‐based enumeration method (ISO 10272‐2:2017), from the neck skins of chilled broiler carcases sampled at slaughterhouses within the EU. Fifteen MSs reported ad hoc official sampling results, 20 MSs reported monitoring results from FBOp and 11 MSs reported data from both samplers. In total, MSs reported 8,936 (31%) Campylobacter‐positive units out of 28,823 neck skin units tested.
Table 6.
Comparison of proportions (%) of Campylobacter‐positive single samples and samples exceeding the Campylobacter PHC limit collected from the neck skins of chilled broiler carcases sampled at the slaughterhouses in accordance with Regulation (EC) No. 2073/2005, by sampler and reporting MS, EU, 2021
| Country | Competent Authority (CA) | Food business operator (FBOp) | p‐value (b) , (c) | Interpretation (c) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N samples Tested | N (%) samples positive | N (%) samples above 1,000 CFU/g | CI95 samples above 1,000 CFU/g | N samples Tested | N (%) samples positive | N (%) samples above 1,000 CFU/g | CI95 samples above 1,000 CFU/g | |||
| Austria | – | – | – | – | 957 | NA | 61 (6.4) | [4.9; 8.1] | – | – |
| Belgium | 633 | NA | 89 (14.1) | [11.4; 17.0] | 2,421 | NA | 172 (7.1) | [6.1; 8.2] | < 0.001 | CA > FBOp |
| Bulgaria | 1,048 | 218 (20.8) | 16 (1.5) | [0.88; 2.5] | – | – | – | – | – | – |
| Croatia | 832 | 417 (50.1) | 253 (30.4) | [27.3; 33.7] | – | – | – | – | – | – |
| Cyprus | 220 | 180 (81.8) | 113 (51.4) | [44.6; 58.1] | – | – | – | – | – | – |
| Czechia | – | – | – | – | 4,110 | 2,573 (62.6) | 1,620 (39.4) | [37.9; 40.9] | – | – |
| Denmark | – | – | – | – | 1,150 | 164 (14.3) | 86 (7.5) | [6,0; 9.2] | – | – |
| Estonia | 12 | 1 (8.3) | 1 (8.3) | [0.21; 38.5] | 260 | 0 | 0 | [0; 1.4] (a) | 0.04 | CA > FBOp |
| Finland | – | – | – | – | 585 | 1 (0.17) (f) | 1 (0.17) | [0; 0.95] | – | – |
| France | – | – | – | – | 16,357 | NA | 4,389 (26.8) | [26.2; 27.5] | – | – |
| Germany | 28 | NA | 9 (32.1) | [15.9; 52.4] | 6,604 | NA | 510 (7.7) | [7.1; 8.4] | < 0.001 | CA > FBOp |
| Greece | 75 | 52 (69.3) | 33 (44.0) | [32.5; 55.9] | 612 | 31 (5.1) | 31 (5.1) | [3.5; 7.1] | < 0.001 | CA > FBOp |
| Hungary | 344 | 41 (11.9) | 14 (4.1) | [2.2; 6.7] | – | – | – | – | – | – |
| Ireland | 164 | 96 (58.5) | 10 (6.1) | [3; 10.9] | 1,031 | 379 (36.8) | 75 (7.3) | [5.8; 9.0] | NS | |
| Italy | 1,233 | 639 (51.8) | 310 (25.1) | [22.7; 27.7] | 5,591 | NA | 466 (8.3) | [7.6; 9.1] | < 0.001 | CA > FBOp |
| Latvia | 100 | 4 (4.0) | 0 (0) | [0; 3.6] (a) | 434 | 90 (20.7) | 24 (5.5) | [3.6; 8.1] | 0.01 | CA < FBOp |
| Netherlands | 333 | 79 (23.7) | 10 (3.0) | [1.4; 5.5] | 3,336 | 201 (6.0) | 201 (6.0) | [5.2; 6.9] | 0.0239 | CA < FBOp |
| Poland | 885 | 287 (32.4) | 174 (19.7) | [17.1; 22.4] | 1,365 | 112 (8.2) | 109 (8.0) | [6.6; 9.6] | < 0.001 | CA > FBOp |
| Portugal | – | – | – | – | 3,528 | 1,006 (28.5) | 521 (14.8) | [13.6; 16.0] | – | – |
| Romania | 1,399 | 521 (37.2) | 84 (6.0) | [4.8; 7.4] | 1,450 | 491 (33.9) | 6 (0.41) | [0.15; 0.90] | < 0.001 | CA > FBOp |
| Slovakia | – | – | – | – | 1,075 | 20 (1.9) | 0 | [0; 0.34] (a) | – | – |
| Slovenia | – | – | – | – | 804 | 595 (74.0) | 333 (41.4) | [38; 44.9] | – | – |
| Spain | 757 | 584 (77.1) | 370 (48.9) | [45.3; 52.5] | 635 | 139 (21.9) | 139 (21.9) | [18.7; 25.3] | < 0.001 | CA > FBOp |
| Sweden | – | – | – | – | 1,046 | 15 (1.4) | 15 (1.4) | [0.8; 2.4] | – | – |
| EU Total (27 + XI) | 8,063 | 3,119 (42.1) (d) | 1,486 (18.4) | [17.6; 19.3] | 53,351 | 5,817 (27.2) (e) | 8,759 (16.4) | [16.1; 16.7] | < 0.001 | CA > FBOp |
| EU Total (27 + XI) providing CA and FBOp data | 5,619 | 2,263 (45.6) (d) | 1,090 (19.4) | [18.4; 20.5] | 23,739 | 1,443 (15.8) (e) | 1,733 (7.3) | [7.0; 7.6] | < 0.001 | CA > FBOp |
NA: not available.
: One‐sided, 97.5% confidence interval.
: p‐value: NS, not significant.
: Related to the percentage of positive samples above 1,000 CFU/g.
: Belgium and Germany did not report Campylobacter‐positive samples below 1,000 CFU/g from test results of the Competent Authority.
: Austria, Belgium, France, Germany and Italy did not report Campylobacter‐positive samples below 1,000 CFU/g from test results of food business operators.
: Reporting error. Finland indicated, during the last phase of the production of this report, that the number of Campylobacter‐positive samples below 1,000 CFU/g from the FBOp was unknown.
From ad hoc official sampling of 8,063 units, 1,486 (18.4%) exceeded the limit of 1,000 CFU/g. Moderate variability was observed in percentage test results exceeding the limit. In particular, Latvia reported none, six MSs (Bulgaria, Estonia, Hungary, Ireland, Netherlands, Romania) reported fewer than 8.3% of units exceeding the limit, while eight MSs (Belgium, Croatia, Cyprus, Germany, Greece, Italy, Poland, Spain) reported a higher number of units above the limit, ranging from 14.1% to 51.4%. The number of Campylobacter‐positive units, reported by 13 MSs totalled 3,119 (42.1%).
FBOp reported test results for 53,351 neck skin samples from own‐check sampling activities, of which 8,759 (16.4%) exceeded the limit of 1,000 CFU/g. For four MSs (Estonia, Finland, Romania, Slovakia), the levels of positives exceeding the limit ranged from 0 to 0.41%. In Switzerland and Montenegro, 77 of 805 (9.5%) and 2 of 50 (4%) units exceeded the limit, respectively. The number of Campylobacter‐positive units reported by 15 MSs totalled 5,817 (27.2%).
Eleven MSs reported results from both samplers and the overall total number of units exceeding the limit was significantly higher in official samples (19.4%, N = 1,090) than in own‐checks (7.3%, N = 1,773). A far higher percentage of units exceeding the limit was also observed in official samples compared with FBOp in eight MSs (Belgium, Estonia, Germany, Greece, Italy, Poland, Romania, Spain). A substantial difference in percentage Campylobacter‐positive units was also observed, with results for official controls (N = 2,263; 45.6%) being higher than own‐checks (N = 1,443; 15.8%).
Overall, for the Campylobacter PHC monitoring data provided by 24 MSs, the percentage of units exceeding the limit was significantly higher in official samples (18.4%) than in FBOp samples (16.4%).
For a further interactive look at Campylobacter monitoring results, dashboards have been implemented (different filters can be applied to query the data) (link).
Other food monitoring data
Table 7 summarises the reported occurrence of Campylobacter in the main food categories in 2021 and over the 4‐year period of 2017–2020 within the EU. A distinction is made between RTE and non‐RTE food, and fresh meat.
Table 7.
Occurrence of Campylobacter in the main food categories, EU, 2021 and 2017–2020
| Food | 2021 | 2017–2020 (a) | ||||
|---|---|---|---|---|---|---|
| N reporting MSs | N sampled units | Positive N (%) | N reporting MSs | N sampled units | Positive N (%) | |
| RTE food | ||||||
| All | 12 | 3,220 | 10 (0.31) | 15 | 11,429 | 19 (0.17) |
| Meat and meat products | 7 | 421 | 1 (0.24) | 10 | 1,301 | 3 (0.23) |
| Meat and meat products from broilers | 2 | 6 | 1 (16.7) | 3 | 39 | 0 |
| Meat and meat products from turkeys | 0 | 0 | 0 | 2 | 15 | 1 (6.7) |
| Other meat and meat products | 5 | 415 | 0 | 9 | 1,247 | 2 (0.16) |
| Milk and milk products | 7 | 909 | 1 (0.11) | 6 | 3,029 | 10 (0.33) |
| Milk | 3 | 361 | 1 (0.28) | 6 | 1,055 | 10 (0.95) |
| Raw milk (b) | 2 | 212 | 1 (0.47) | 6 | 1,036 | 10 (0.97) |
| Cheese | 5 | 546 | 0 | 7 | 1,959 | 0 |
| Dairy products excluding cheeses, butter, cream, ice cream, whey, yoghurt and fermented dairy products | 1 | 2 | 0 | 3 | 15 | 0 |
| Fruits, vegetables and juices | 5 | 1,215 | 0 | 5 | 2,530 | 1 (0.04) |
| Ready‐to‐eat salads | 2 | 367 | 1 (0.27) | 5 | 640 | 1 (0.16) |
| Other processed food products and prepared dishes | 4 | 239 | 3 (1.3) | 6 | 3,275 | 4 (0.12) |
| Other foods | 5 | 69 | 4 (5.8) | 6 | 654 | 0 |
| Non‐RTE food | ||||||
| All | 16 | 14,158 | 1,543 (10.9) | 22 | 73,990 | 17,431 (23.6) |
| Meat and meat products | 15 | 12,704 | 1,511 (11.9) | 22 | 66,947 | 17,353 (25.9) |
| Meat and meat products from broilers | 14 | 10,287 | 1,219 (11.8) | 20 | 38,220 | 12,666 (33.1) |
| Meat and meat products from turkeys | 9 | 610 | 85 (13.9) | 10 | 3,876 | 1,058 (27.3) |
| Other meat and meat products | 11 | 1,807 | 207 (11.5) | 18 | 24,851 | 3,629 (14.6) |
| Milk and milk products | 5 | 394 | 1 (0.25) | 8 | 2,556 | 51 (2) |
| Fruits, vegetables and juices | 4 | 337 | 0 | 6 | 1,500 | 2 (0.13) |
| Other food | 6 | 723 | 31 (4.3) | 8 | 2,987 | 25 (0.84) |
| Fresh meat (c) | ||||||
| All | 13 | 11,783 | 1,381 (11.7) | 22 | 61,805 | 16,061 (26.0) |
| Fresh meat from broilers | 13 | 9,845 | 1,135 (11.5) | 20 | 35,858 | 11,857 (33.1) |
| Fresh meat from turkeys | 8 | 583 | 75 (12.9) | 10 | 3,419 | 957 (28.0) |
| Fresh meat from pigs | 2 | 239 | 6 (2.5) | 10 | 2,571 | 110 (4.3) |
| Fresh meat from bovine animals | 3 | 192 | 1 (0.52) | 12 | 4,174 | 40 (0.96) |
| Other fresh meat | 8 | 924 | 164 (17.7) | 13 | 15,783 | 3,097 (19.6) |
RTE: ready‐to‐eat.
: Data from the United Kingdom are taken into account for 2017–2019, because the United Kingdom was an EU MS, but it became a third country on 1 February 2020.
: Raw RTE milk sampling units are a subset of RTE milk.
: Fresh meat sampling units are a subset of the two main categories above.
The proportion of Campylobacter‐positive samples in the RTE and non‐RTE categories was 0.31% and 10.9%, respectively. In fresh meat, 11.7% of sampling units were positive.
In 2021, most of the results from the 3,220 RTE food sampling units reported by 12 MSs came from ‘fruit, vegetables and juices’ (37.7%), followed by ‘milk and milk products’ (28.2%) and ‘meat and meat products’ (13.1%). In total, Campylobacter was detected in 10 RTE food samples: one from ‘raw milk’, one from ‘meat products’, one from ‘ready‐to‐eat salads’, three from meat‐based dishes reported as ‘other processed food products and prepared dishes’ and four from oysters reported as ‘other foods’. During the period 2017–2020, the percentage of Campylobacter‐positive sampling units in RTE food was low, at below 1% for all categories. Over the entire period for the main RTE categories, the highest percentage of Campylobacter‐positive units was for ‘raw milk’: 10 positives out of 1,036 (0.97%) sample units tested.
The results reported in 2021 by 16 MSs for non‐RTE food show that ‘meat and meat products’ was the most contaminated food category, followed by ‘other food’ and ‘milk and milk products’. During 2017–2020, ‘meat and meat products’ was the most contaminated food category, followed by ‘milk and milk products’.
Thirteen MSs reported results for fresh meat categories. The percentage of Campylobacter‐positive units was similar for meat from turkey (12.9%) and broilers (11.5%), and higher for ‘other fresh meat’ (17.7%). The percentage for fresh meat from pigs and bovine animals remained relatively low; 2.5% and 0.52%, respectively.
In 2021, a substantial decrease in the number of positive units was observed for non‐RTE food and fresh meat, compared with the period 2017–2020, except for ‘other fresh meat’, for which the positive percentage was comparable.
For a further interactive look at Campylobacter monitoring results, dashboards have been implemented (different filters can be applied to query the data) (link).
1.4.4. Campylobacter in animals
Table 8 shows the number of positive Campylobacter spp. samples detected during 2021 in the five main animal species, as well as in the ‘other animals’ category containing more than 30 different animal groups. In total, 16 MSs and three non‐MSs reported data, primarily relating to broilers (39.6%, N = 12,733), followed by cattle (24.6%, N = 7,923) and pigs (15.2%, N = 4,877). The overall proportion of positive units in EU was highest in pigs (41.3%) followed by cattle (13.5%), cats and dogs (12.3%) and finally broilers (10.5%). Although fewer samples were tested for ‘other animals’, a considerable proportion of positive units were detected in samples from birds collected by MS (25.1%, N = 131).
Table 8.
Summary of Campylobacter statistics related to major animal species, reporting EU MSs and non‐MS countries, 2021
| Animals | EU MSs | Non‐MS countries | ||||||
|---|---|---|---|---|---|---|---|---|
| N reporting countries | N tested (a) sampling units | Positive sampling units | N reporting countries | N tested (a) sampling units | Positive sampling units | |||
| N | % | N | % | |||||
| Gallus gallus (broilers) | 6 | 10,162 | 1,065 | 10.5 | 2 | 2,571 | 117 | 4.6 |
| Bovine animals (b) | 11 | 7,529 | 1,015 | 13.5 | 2 | 394 | 168 | 42.6 |
| Pigs | 13 | 4,428 | 1,827 | 41.3 | 2 | 449 | 338 | 75.3 |
| Small ruminants | 6 | 2,934 | 109 | 3.7 | 1 | 21 | 5 | 23.8 |
| Cats and dogs | 3 | 913 | 112 | 12.3 | 2 | 1,581 | 95 | 6.0 |
| Other animals (c) | 4 | 876 | 143 | 16.3 | 2 | 284 | 12 | 4.2 |
| Total | 16 | 26,842 | 4,271 | 15.9 | 3 | 5,300 | 735 | 13.9 |
MS: Member State.
: Summary statistics were obtained by totalling all sampling units (single samples, batch samples, animals, slaughter animal batches and herds or flocks).
: Animals from the sampling stage ‘Artificial insemination stations’ were not included.
: Other animals include Alpacas, Antelopes, Badgers, Barbary sheep, Bears, Birds, Budgerigars, Camels, Canary, Cantabrian chamois, Chinchillas, Deer, Elephants, Falcons, Ferrets, Foxes, Geese, Guinea pigs, Hedgehogs, Kangaroos, Land game mammals, Leporidae, Lions, Llamas, Martens, Monkeys, Oscine birds, Other animals, Other carnivores, Parrots, Pigeons, Raccoons, Rats, Reindeer, Reptiles, Rodents, Sea lions, Snakes, Solipeds, domestic Steinbock, Turkeys, Turtles, Water buffalos, Wild boars, Wild cats (Felis silvestris), Wild ducks, Zoo animals, all.
1.5. Discussion
Campylobacteriosis has been the most frequently reported zoonosis in humans across the EU since 2007. Despite comprehensive surveillance and national coverage in most MSs, the number of reported cases is underestimated in the EU (Teunis et al., 2013). In 2019, in two‐thirds of EU MSs, the number of confirmed campylobacteriosis cases decreased. A decrease in the number of cases was also observed in 2020, probably due to the withdrawal of the United Kingdom from the EU and the COVID‐19 pandemic, which had an impact on surveillance, including diagnosis and reporting. In 2021, although the COVID‐19 pandemic was still ongoing, a slight increase was observed in the number of confirmed campylobacteriosis cases compared with 2020. The gradual scaling down of national public response measures to COVID‐19 in the EU in 2021, 12 along with the return of mass gatherings, sporting and recreational/social events and the reopening of bars, restaurants and catering facilities (i.e. schools, workplaces) may explain this slight increase. However, the overall campylobacteriosis trend in 2017–2021 showed no statistically significant increase or decrease.
Compared with 2020, no difference in travel‐associated campylobacteriosis cases was observed. The number of travel‐associated cases remained below pre‐pandemic level. European and Mediterranean countries were the main places of origin for travel‐associated campylobacteriosis. Campylobacter has a characteristic seasonality with cases increasing sharply in the summer. Campylobacteriosis cases have been positively associated with temperature and, to a lesser degree, precipitation (Lake et al., 2019). Recent studies have even evidenced a possible association between campylobacteriosis and global climate change (Kuhn et al., 2020).
A smaller but distinct winter peak in the EU has become apparent in the past 10 years, including in 2021. Disease onsets concerning cases that were notified during the winter peaks occurred predominantly in January. This points to an exposure around the Christmas/New Year period. Reports indicate that meat fondues or table‐top grilling, which are popular during the festive season, could promote the transmission of Campylobacter in some countries, causing the winter peak (Bless et al., 2017; Rosner et al., 2021).
In 2021, more than 10,000 cases of campylobacteriosis were hospitalised, making it the second most common foodborne agent associated with the number of hospitalisations, after salmonellosis. The proportion of hospitalised campylobacteriosis cases was higher than expected in some MSs, where all or most of the confirmed cases were hospitalised. These MSs also reported the lowest notification rates, indicating that surveillance focuses primarily on hospitalised (i.e. severe) cases. This can lead to an overestimation of the proportion of hospitalised cases in some countries. As in previous years, C. jejuni and C. coli were the main species notified by MSs in 2021. Unfortunately, there was still a high percentage (34.9%) of campylobacteriosis cases in which the Campylobacter species was not determined. Although the proportion of characterised isolates at species level has increased over the last 5 years, further improvement is still necessary. A One Health approach, using whole genome sequencing (WGS) for typing Campylobacter isolates in humans, food and animals, will allow for better species characterisation and will enhance the monitoring of zoonotic transmission, improving public health surveillance.
In 2021, as part of a food control strategy, 24 MSs submitted Campylobacter PHC data, with 11 reporting both official and own‐check results, four only official results and nine only own‐check results. Official control and FBOp monitoring data from 2021 showed that about one in five and one in six samples exceeded the limit of 1,000 CFU/g, respectively. For the MSs that submitted data from both samplers, the results exceeding the limit concerned one in five units for the CA and one in 14 for the FBOp, respectively. The results reported by the CA were always significantly higher than those reported by FBOp. Moreover, the CA also reported considerably higher percentages of Campylobacter positive units. This discrepancy, observed for the second year, deserves more thorough investigation in order to identify the critical parameters and factors explaining these differences. Monitoring Campylobacter for the purposes of improving biosecurity measures on farms is of paramount importance (Newell et al., 2011). The aim here is to stimulate action to lower Campylobacter counts on broiler carcases and to reduce the number of human campylobacteriosis cases caused by the consumption or handling of contaminated chicken/broiler meat. It was recently reported that a 3‐log10 reduction in broiler caecal concentrations would reduce the relative risk within the EU of human campylobacteriosis attributable to broiler meat by a substantial 58% (EFSA BIOHAZ Panel et al., 2020a). Overall, the comparison with 2020 percentages of Campylobacter samples exceeding the limit revealed a slight increase and a slight decrease, respectively, for CA and FBOp. The number of samples reported and the number of reporting MSs increased compared to previous years, which can be explained by the need for MSs to comply with the EU regulation.
Monitoring of other food in the EU showed overall percentages of Campylobacter‐positive units in RTE and non‐RTE foods of 0.31% and 10.9%, respectively. Although the presence of Campylobacter in RTE was low and has remained stable over the years, these findings are of concern given that contaminated RTE products directly expose consumers to infection. The RTE foods found to be contaminated with Campylobacter were ‘raw milk’, ‘meat and meat products’, ‘fruit, vegetables and juices’ along with ‘other food’, including meat‐based dishes. This confirms the importance of those categories over the period 2017–2020. Moreover, positive results were observed for four ‘other foods’, including oysters. Given the risk of contracting campylobacteriosis from raw shellfish (Teunis et al., 1997), efforts must be encouraged to increase the sampling frequency of these food products, eaten raw in some regions. Overall, a small number of sampling units could have resulted in an imprecise estimation of the prevalence of RTE food samples contaminated by Campylobacter.
Monitoring data for non‐RTE food showed positive results for 1 in 10 ‘meat and meat products’, and 1 in 400 ‘milk and milk products’. Although the Campylobacter contamination observed in fresh meat categories was moderate in 2021, a decrease in the number of positive units was observed compared with the period 2017–2020. Nevertheless, the data continue to underline the key role of these products in campylobacteriosis epidemiology, either through direct handling or through cross‐contamination of other foods.
In 2021, 16 MSs and three non‐MSs reported data from several animal groups. Campylobacter spp. was detected in all the major animal categories: broilers, pigs, cattle, small ruminants, cats and dogs. Broilers were tested most frequently and accounted for 24.5% of test results, followed by cattle and pigs. The highest percentage of positive units, however, was observed for pigs. Despite an increase in the number of tested units, only about half of MSs reported broiler data in 2021, thereby hampering a fair comparison of data and probably indicating the fluctuation in positive results as being associated with the testing of a different epidemiological scenario.
2. Salmonella
Summary data substantiating this chapter, as well as additional information on related projects and Internet sources are published for this report on the EFSA Knowledge Junction on Zenodo here. Summary statistics on Salmonella food and animal monitoring data and on salmonellosis foodborne outbreaks reported in the framework of Directive 2003/99/EC, with downloadable files, are retrievable using the EFSA Salmonella dashboard and the EFSA foodborne outbreaks dashboard, respectively available here and here. Summary statistics on human surveillance data with downloadable files are retrievable using the ECDC Surveillance Atlas of Infectious Diseases here.
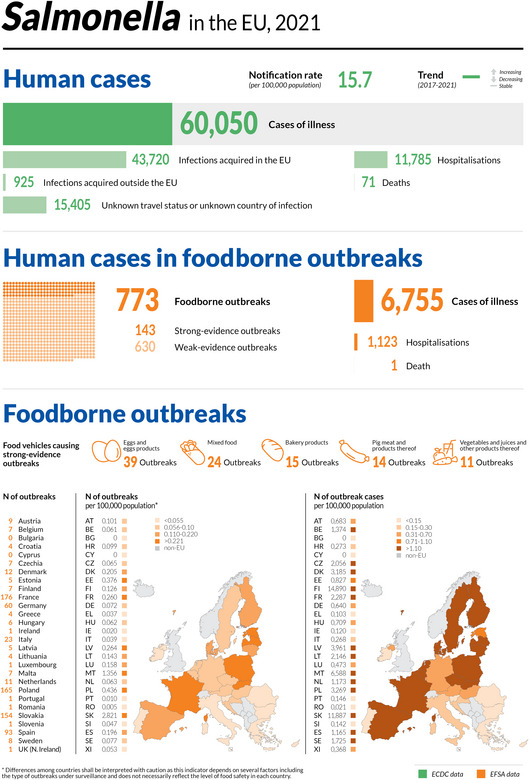
2.1. Key facts
Salmonellosis was the second most commonly reported foodborne gastrointestinal infection in humans after campylobacteriosis and was a major cause of foodborne outbreaks in EU MSs and non‐MS countries.
In 2021, the number of confirmed cases of human salmonellosis was 60,050, corresponding to an EU notification rate of 15.7 per 100,000 population. This was an increase of 14.3% compared with the rate in 2020.
In 2020, ECDC recorded the lowest number of human Salmonella cases since 2007, when salmonellosis surveillance started. The number of cases was impacted by the COVID‐19 pandemic and the United Kingdom's withdrawal from the EU.
Compared with the rate before the COVID‐19 pandemic (2017–2019 annual mean), the 2021 EU notification rate decreased by 19.6% and 23.1%, with and without the data from the United Kingdom, respectively.
Notwithstanding, the overall trend for salmonellosis in 2017–2021 did not show any statistically significant increase or decrease.
The proportion of hospitalised cases was 38.1%, which was higher than in 2020, with an EU case fatality rate of 0.18%, which is similar to 2020.
The top five Salmonella serovars involved in human infections overall were distributed as follows: S. Enteritidis (54.6%), S. Typhimurium (11.4%), monophasic S. Typhimurium (1,4,[5],12:i:‐) (8.8%), S. Infantis (2.0%) and S. Derby (0.93%).
Sampling to verify compliance with process hygiene criteria on carcases at the slaughterhouse found the highest proportions of positive samples among those collected by the competent authorities for broilers (14%), turkeys (7.4%), pigs (1.7%) and sheep (1.2%). Conversely, for goats, the highest proportion of positive samples was for those collected by food business operators (2.1%).
In 2021, 73,238 ‘ready‐to‐eat’ food sampling units were collected with a very low proportion of Salmonella‐positive units (0.23%), overall. The highest proportions of positives were found for ‘meat and meat products from pigs’ (0.82%) and ‘spices and herbs’ (0.72%). For ‘non‐ready‐to‐eat’ food, 466,290 sampling units were collected and the proportion of positive samples was low (2.1%). ‘Meat and meat products’ (2.2%) and especially those from broilers (4.4%) and turkeys (3.6%) were the food categories with the highest proportions of positive units.
Sixteen MSs and the United Kingdom (Northern Ireland) reporting on Salmonella control programmes met the reduction targets for all poultry populations. The number of MSs that did not meet the reduction targets was seven for laying hens, five for breeding Gallus gallus, three for broilers, one for breeding turkeys and one for fattening turkeys.
For flocks of broilers and of breeding and fattening turkeys, for which results of Salmonella control programmes are required to be reported separately by sampler, the prevalence reported by food business operators was significantly lower than that reported by competent authorities.
A significant increase in the estimated breeding turkey flock prevalence of Salmonella was noted in 2021 compared with 2016, when the estimated prevalence reached the lowest value seen in the entire study period (2010–2021). Flock prevalence trends for target Salmonella serovars have, in contrast, been fairly stable over the last few years for all poultry populations.
Considering the top 20 EU ranking of Salmonella serovars by food‐animal source, focusing on those most frequently isolated from humans, S. Enteritidis was the most commonly reported serovar in laying hens and the second most commonly reported one in broilers. Amongst the top three for pig and bovine isolates were S. Typhimurium and its monophasic variant. Some other very common serovars were S. Infantis for broilers (one out of two isolates from this source belonged to this serovar) and S. Derby for pigs.
With the present report, EFSA has also published two new interactive communication tools on Salmonella: the EFSA story map (available here) and the dashboard (available here).
2.2. Surveillance and monitoring of Salmonella in the EU
EFSA story map on Salmonella
The EFSA story map on Salmonella is a new interactive communication tool developed by EFSA in 2022, available online (here) and geared to the general public. This story map provides general information on the pathogen and its epidemiology, including information on where the pathogen can be found, how people and animals get infected, the occurrence of this pathogen in different sources, the disease it causes and how to prevent infection. In addition, this story map also illustrates the monitoring activities implemented in the EU and the role of EFSA with respect to these activities. Users can easily display and explore the content of the different sections in the story map, browsing the dynamic maps, images, text and multimedia features.
2.2.1. Humans
In 2021, 27 EU MSs reported information on non‐typhoidal salmonellosis infections in humans. The notification of salmonellosis is mandatory in 24 EU MSs, whereas it is voluntary in three MSs (Belgium, France and the Netherlands). The EU case definition was used by 23 MSs, while four MSs (Denmark, France, Germany and Italy) reported using other case definitions. All countries except the Netherlands had a comprehensive surveillance system. The surveillance systems for salmonellosis covered the whole population in all MSs except three: France, the Netherlands and Spain. The estimated coverage of the surveillance system was 48% in France and 64% in the Netherlands. These estimated proportions of population coverage were used in the calculation of notification rates for these two MSs. No estimated population coverage in Spain was provided, so notification rates were not calculated. In 2020–2021, Spain did not receive data from all the regions that normally report, due to COVID‐19, so the case numbers may therefore not be complete. All countries reported case‐based data except Bulgaria, which reported aggregated data.
2.2.2. Food, animals and feed
Salmonella data in the context of Regulation (EC) No 2073/2005
Regulation (EC) No 2073/2005 lays down microbiological criteria, intended as food safety criteria (FSC) and process hygiene criteria (PHC), for Salmonella in specific food categories. Compliance with these criteria must be legally verified by the individual food business operator (FBOp) as part of their own HACCP programme. 13 In addition, the competent authority (CA), through official sampling or oversight of data, should ensure that the FBOp complies with these regulatory requirements. The Salmonella FSC require that the pathogen not be detected in different products when they are on the market, during their shelf‐life. Moreover, in fresh poultry meat (from species covered by national control programmes), the FSC require the absence of target serovars (S. Enteritidis and S. Typhimurium including monophasic S. Typhimurium (1,4,[5],12:i:‐) 14 ). The Salmonella PHC are regulated for carcases of pigs, cattle, sheep, goats, horses, broilers and turkeys, as sampled by the FBOp. Moreover, in accordance with Regulation (EU) No 2019/627 15 , the CA has to verify whether the FBOp correctly implements and checks these PHC for carcases by choosing between different approaches: (i) implementing official sampling, (ii) collecting all information on the total number and the number of Salmonella‐positive samples from own checks by the FBOp and/or (iii) collecting information on the total number and the number of Salmonella‐positive samples as part of national control programmes in the MSs with special guarantees (Regulation (EC) No 853/2004 16 ).
Data for compliance with national control programmes for Salmonella in poultry populations
In accordance with Regulation (EC) No 2160/2003 17 and its subsequent amendments, MSs have to set up national control programmes (NCPs) for Salmonella aimed at reducing the prevalence of Salmonella serovars that are considered relevant for public health (from this point forward, termed ‘target serovars’). Currently, prevalence targets have been defined for breeding flocks of Gallus gallus, laying hens, broilers and breeding and fattening turkeys and correspond to the maximum annual percentage of flocks positive for S. Enteritidis and S. Typhimurium, including its monophasic variant, except for breeding flocks of Gallus gallus, where S. Infantis, S. Virchow and S. Hadar are considered to be relevant as well. The prevalence target is equal to 1% or less for breeding flocks of Gallus gallus, 18 broilers 19 and breeding and fattening turkeys 20 ; it is 2% for laying hens. 21 Every year, MSs must report results for their Salmonella NCP and, for broiler flocks and breeding and fattening turkey flocks, results for sampling conducted by the CA and FBOp must also be reported separately.
Other monitoring data for food, animals and feed including serovars
Food, animal and feed data other than those described above are not collected in a harmonised way, because there are no specific legal requirements. The reported occurrence of Salmonella in the main food categories was descriptively summarised, with a distinction being made between ‘ready‐to‐eat (RTE) and non‐RTE food’. Data sets were extracted with ‘objective sampling’ being specified as the sampling strategy, which means that samples were representative of the population being analysed and were collected according to a planned strategy.
The occurrence of Salmonella in animal populations was descriptively summarised considering all data collected in different sampling contexts and reported as different sampling units (e.g. ‘holding’, ‘herd/flock’, ‘animal’ and ‘slaughter animal batch’), with the exception of data related to poultry populations covered by NCPs, which have been discussed separately.
The reported data on Salmonella serovars were also descriptively summarised. MSs are required to report the target serovars as part of their NCPs for poultry, whereas for the samples collected in different contexts, serotyping is not mandatory. Also, for the food sector, the FSC is the absence of Salmonella, except for fresh poultry meat, for which the criterion is the absence of target serovars. The compulsory reporting of target serovars in some contexts (NCPs for poultry and FSC for fresh poultry meat) guarantees the consistency of such data over the years and among MSs but could result in these target serovars being overestimated compared with the other serovars.
2.3. Data analyses
2.3.1. Comparison between competent authority and food business operator sampling results
CA and FBOp Salmonella results in the context of NCPs for those poultry populations requiring separate reporting (i.e. broilers and fattening and breeding turkeys) were compared, as were Salmonella PHC monitoring data from carcases (of pigs, cattle, goats, sheep, horses, broilers and turkeys). The significance of differences was verified by the one‐tailed Fisher's exact probability test, in cases where the expected values in any of the cells of a contingency table were below five; otherwise, the z‐statistic one‐tailed test was performed. CA control sampling results and the own‐check results of the FBOp were expressed as prevalence and exact binomial confidence interval (95% level). A p‐value < 0.10 (Clayton and Hills, 2013) was considered significant to include all possible evidence of differences between data collected by the FBOp and CA.
R software (www.r-project.org, version 4.2.1) was used to conduct the above‐mentioned analyses.
2.3.2. Statistical trend analyses for poultry monitoring data
Statistical trend analyses were carried out with the objective of evaluating the significance of temporal variations in the EU‐level flock prevalence of Salmonella and target Salmonella serovars in poultry since the start of NCP implementation.
The tested flocks were either positive or negative for target serovars and Salmonella, and so the status of the flocks is a dichotomous outcome variable. Therefore, the binomial probability distribution for the response variable was assumed and the logit link function was computed in the model for the trend analysis. The logit is defined as the logarithm of p/(1 − p), where p/(1 − p) is the odds of being positive for Salmonella.
According to temporal flock prevalence trends in the MSs, B‐spline basic models for the logit of the probability of flocks being positive were fitted for the different poultry populations over the entire period of NCP implementation. Moreover, attention was paid to the period after achievement of the minimum prevalence reported to date, to capture any evidence of a significant increase in Salmonella prevalence. Marginal and conditional generalised linear models for repeated measures were used to perform these trend analyses (EFSA, 2009a, 2011). Details about the estimated parameters of the models, odds ratios, prevalence rates and graphical analyses (conditional and marginal) are published in the file ‘Salmonella poultry outcome trend analyses’ on the EFSA Knowledge Junction on Zenodo here.
To investigate EU‐level prevalence, considering the relevant heterogeneity among MSs for the flock prevalence of Salmonella and target serovars over time, the results obtained using the conditional generalised mixed model for longitudinal binary data were summarised and discussed in the report, for all poultry populations covered by NCPs. To take into account the different levels (baselines) of probability of MSs having positive flocks, yet with similar patterns over time, a random MS‐specific intercept effect was included in the model. To evaluate the trend over time, the ‘time' variable was included in the model as a fixed effect. The correlation between repeated observations in the same MS in subsequent years was considered using a first autoregressive or exchangeable structure of the correlation matrix for the residuals. To evaluate the significance of the overall effect of fixed factors specified in the model, Type III F‐tests were applied, whereas the receiver operating characteristic (ROC) curve was used to assess the goodness of fit of the model. A p‐value < 0.10 was considered to be significant for both random and fixed effects.
GLIMMIX and SGPLOT procedures in SAS 9.4 software were used to fit the models and to produce the graphical outputs, respectively.
2.3.3. Descriptive analyses of Salmonella serovars
With the aims of evaluating the distribution of Salmonella serovars across the food chain and identifying potential sources of human infections, a Sankey diagram was provided to link the food and food‐producing animal sources to the five most commonly reported Salmonella serovars from human cases acquired within the EU (domestically or during travel within the EU). For animal categories covered by NCPs, only serovar data reported in the context of these programmes were presented. For cattle, meat‐producing animals were considered, whereas for pigs, data from fattening animals were used. In addition to possible reporting biases as regards serovars, reporting for animal or food categories may also have been unbalanced and specific sources (e.g. cattle) may have been underrepresented.
EFSA dashboard on Salmonella
The EFSA dashboard on Salmonella (available online here) is a graphical user interface for searching and querying the large amount of data collected each year by EFSA from EU MSs and other reporting countries based on Zoonoses Directive 2003/99/EC. The Salmonella dashboard shows summary statistics for the monitoring results of the pathogen with regard to major food categories and animal species, Salmonella‐positive official samples in the context of FSC and HPC, the occurrence of Salmonella in major food categories and the achievement of Salmonella reduction targets in poultry populations. The Salmonella data and related statistics can be displayed interactively using charts, graphs and maps in the online EFSA dashboard. In this tool, the main statistics can also be viewed and downloaded in tabular format. Detailed information on the use and features of the Salmonella dashboard can be found in the user guide available on Zenodo here and can also be downloaded from the online tool. Links to the dashboard are available in the relevant sections of this chapter.
2.4. Results
2.4.1. Overview of key statistics, EU, 2017–2021
Humans
In total, the number of reported human salmonellosis cases and the notification rate in 2021 were higher than in 2020 (Table 9). The number of reported human salmonellosis cases acquired in the EU (i.e. by domestic infection and through travel within the EU), the number of outbreak‐related cases and the total number of foodborne salmonellosis outbreaks were higher in 2021 than in 2020, but lower than in previous years.
Table 9.
Summary of Salmonella statistics related to humans, major food categories and the main animal species, EU, 2017–2021
| 2021 (a) | 2020 | 2019 (b) | 2018 (b) | 2017 (b) | Data source | |
|---|---|---|---|---|---|---|
| Humans | ||||||
| Total number of confirmed cases | 60,050 | 52,690 | 87,908 | 91,858 | 91,587 | ECDC |
| Total number of confirmed cases/100,000 population (notification rate) | 15.7 | 13.7 | 19.5 | 19.6 | 19.4 | ECDC |
| Number of reporting MSs | 27 | 27 | 28 | 28 | 28 | ECDC |
| Infection acquired in the EU | 43,720 | 38,247 | 58,157 | 59,763 | 59,642 | ECDC |
| Infection acquired outside the EU | 925 | 973 | 6,343 | 6,376 | 6,001 | ECDC |
| Unknown travel status or unknown country of infection | 15,405 | 13,470 | 23,408 | 25,719 | 25,944 | ECDC |
| Number of outbreak‐related cases | 6,755 | 3,686 | 10,240 | 11,631 | 9,607 | EFSA |
| Total number of outbreaks | 773 | 694 | 1,284 | 1,588 | 1,241 | EFSA |
| Food | ||||||
| Meat and meat products | ||||||
| Number of sampling units | 977,434 | 557,341 | 552,590 | 433,197 | 380,000 | EFSA |
| Number of reporting MSs | 28 | 26 | 28 | 28 | 28 | EFSA |
| Milk and milk products | ||||||
| Number of sampling units | 43,902 | 38,492 | 46,797 | 44,078 | 30,796 | EFSA |
| Number of reporting MSs | 25 | 24 | 25 | 24 | 24 | EFSA |
| Fish and fishery products | ||||||
| Number of sampling units | 14,882 | 16,486 | 13,974 | 17,075 | 13,486 | EFSA |
| Number of reporting MSs | 25 | 23 | 24 | 22 | 22 | EFSA |
| Eggs and egg products | ||||||
| Number of sampling units | 14,696 | 11,579 | 12,093 | 10,611 | 15,435 | EFSA |
| Number of reporting MSs | 22 | 18 | 21 | 21 | 23 | EFSA |
| Fruit and vegetables (including juice) | ||||||
| Number of sampling units | 12,483 | 17,222 | 17,068 | 10,888 | 7,579 | EFSA |
| Number of reporting MSs | 23 | 23 | 22 | 22 | 25 | EFSA |
| Animals | ||||||
| Gallus gallus (chickens) | ||||||
| Number of sampling units (c) | 812,163 | 620,141 | 752,172 | 720,717 | 736,534 | EFSA |
| Number of reporting MSs | 28 | 26 | 27 | 27 | 28 | EFSA |
| Turkeys | ||||||
| Number of sampling units (c) | 70,869 | 63,473 | 65,950 | 68,009 | 74,739 | EFSA |
| Number of reporting MSs | 25 | 22 | 23 | 24 | 26 | EFSA |
| Ducks and geese | ||||||
| Number of sampling units (c) | 3,751 | 412 | 8,700 | 9,846 | 5,743 | EFSA |
| Number of reporting MSs | 10 | 6 | 9 | 6 | 8 | EFSA |
| Pigs | ||||||
| Number of sampling units (c) | 16,689 | 17,234 | 18,619 | 17,868 | 19,239 | EFSA |
| Number of reporting MSs | 15 | 10 | 14 | 14 | 17 | EFSA |
| Cattle (bovine animals) | ||||||
| Number of sampling units (c) | 26,061 | 28,363 | 86,871 | 30,302 | 654,593 | EFSA |
| Number of reporting MSs | 14 | 11 | 14 | 14 | 15 | EFSA |
ECDC: European Centre for Disease Prevention and Control; EFSA: European Food Safety Authority; MSs: Member States.
: Data on food and animal samples from the United Kingdom (Northern Ireland) are taken into account for 2021. In accordance with the agreement on the withdrawal of the United Kingdom from the EU, and in particular with the Protocol on Ireland/Northern Ireland, the EU requirements on data sampling are also applicable to Northern Ireland.
: Data from the United Kingdom are taken into account for 2017–2019, because the United Kingdom was an EU MS, but it became a third country on 1 February 2020.
: Summary statistics referring to MSs were obtained by totalling all sampling units (single animals, slaughter animal batches and herds or flocks).
Food categories
The number of sampling units reported in 2021 for three of the five food categories was higher than in 2020, and for all food categories, there was an increase in the number of reporting MSs except for ‘fruit and vegetables (including juice)’. The largest increase in the number of reported sampling units was for ‘meat and meat products’. Conversely, for ‘fruit and vegetables (including juice)’ and ‘fish and fishery products’, there was a decrease in the number of sampled units reported in 2021 compared to 2020.
Animal categories
For all animal categories, there was an increase in the number of reporting MSs. In general, there was an increase in the number of sampling units between 2020 and 2021, except for ‘cattle' and ‘pigs’, for which the number was fairly stable between the 2 years.
More detailed descriptions of the above statistics are provided in the below subsections and in the chapter on foodborne outbreaks.
For a further interactive look at Salmonella monitoring results, dashboards have been created (different filters can be applied to query the data) (link).
2.4.2. Human salmonellosis
In total, 60,050 human salmonellosis cases were reported by 27 MSs in 2021, corresponding to an EU notification rate of 15.7 cases per 100,000 population (Table 10). This was an increase of 14.3% compared with the rate in 2020 and a decrease of 19.6% and 23.1% compared with the rates in pre‐pandemic years (2021 vs. 2017–2019) with and without the data from the United Kingdom, respectively.
Table 10.
Reported confirmed human cases of salmonellosis and notification rates per 100,000 population in EU MSs and non‐MS countries, by country and year, 2017–2021
| Country | 2021 | 2020 | 2019 | 2018 | 2017 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| National coverage (a) | Data format (a) | Confirmed cases and rates | Confirmed cases and rates | Confirmed cases and rates | Confirmed cases and rates | Confirmed cases and rates | ||||||
| Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | |||
| Austria | Y | C | 993 | 11.1 | 817 | 9.2 | 1,866 | 21.1 | 1,538 | 17.4 | 1,667 | 19.0 |
| Belgium | Y | C | 2,084 | 18.0 | 1,595 | 13.8 | 2,527 | 22.1 | 2,958 | 26.0 | 2,298 | 20.2 |
| Bulgaria | Y | A | 241 | 3.5 | 187 | 2.7 | 594 | 8.5 | 586 | 8.3 | 796 | 11.2 |
| Croatia | Y | C | 605 | 15.0 | 786 | 19.4 | 1,308 | 32.1 | 1,323 | 32.2 | 1,242 | 29.9 |
| Cyprus | Y | C | 41 | 4.6 | 70 | 7.9 | 62 | 7.1 | 44 | 5.1 | 59 | 6.9 |
| Czechia | Y | C | 10,032 | 93.7 | 10,516 | 98.3 | 13,009 | 122.2 | 10,901 | 102.7 | 11,473 | 108.5 |
| Denmark | Y | C | 692 | 11.8 | 614 | 10.5 | 1119 | 19.3 | 1168 | 20.2 | 1067 | 18.6 |
| Estonia | Y | C | 112 | 8.4 | 91 | 6.8 | 150 | 11.3 | 314 | 23.8 | 265 | 20.1 |
| Finland | Y | C | 474 | 8.6 | 516 | 9.3 | 1,175 | 21.3 | 1,431 | 26.0 | 1,535 | 27.9 |
| France (b) | N | C | 9,315 | 28.7 | 7,071 | 21.9 | 8,935 | 27.7 | 8,936 | 27.8 | 7,993 | 24.9 |
| Germany | Y | C | 8,144 | 9.8 | 8,664 | 10.4 | 13,495 | 16.3 | 13,293 | 16.1 | 14,051 | 17.0 |
| Greece | Y | C | 284 | 2.7 | 381 | 3.6 | 643 | 6.0 | 640 | 6.0 | 672 | 6.2 |
| Hungary | Y | C | 3,298 | 33.9 | 2,964 | 30.3 | 4,452 | 45.6 | 4,161 | 42.6 | 3,922 | 40.0 |
| Ireland | Y | C | 173 | 3.5 | 214 | 4.3 | 347 | 7.1 | 352 | 7.3 | 379 | 7.9 |
| Italy | Y | C | 3768 | 6.4 | 2713 | 4.5 | 3256 | 5.4 | 3635 | 6.0 | 3347 | 5.5 |
| Latvia | Y | C | 218 | 11.5 | 296 | 15.5 | 438 | 22.8 | 409 | 21.1 | 225 | 11.5 |
| Lithuania | Y | C | 281 | 10.1 | 419 | 15.0 | 736 | 26.3 | 779 | 27.7 | 1,005 | 35.3 |
| Luxembourg | Y | C | 133 | 21.0 | 93 | 14.9 | 131 | 21.3 | 135 | 22.4 | 118 | 20.0 |
| Malta | Y | C | 249 | 48.2 | 176 | 34.2 | 131 | 26.5 | 116 | 24.4 | 107 | 23.2 |
| Netherlands (c) | N | C | 862 | 7.7 | 695 | 6.2 | 1,197 | 10.8 | 1,061 | 9.6 | 954 | 8.7 |
| Poland | Y | C | 7,702 | 20.4 | 5,192 | 13.7 | 8,373 | 22.0 | 9,064 | 23.9 | 8,921 | 23.5 |
| Portugal | Y | C | 361 | 3.5 | 262 | 2.5 | 432 | 4.2 | 302 | 2.9 | 462 | 4.5 |
| Romania | Y | C | 518 | 2.7 | 408 | 2.1 | 1,383 | 7.1 | 1,410 | 7.2 | 1,154 | 5.9 |
| Slovakia | Y | C | 4,439 | 81.3 | 3,385 | 62.0 | 4,992 | 91.6 | 6,791 | 124.8 | 5,789 | 106.5 |
| Slovenia | Y | C | 185 | 8.8 | 214 | 10.2 | 362 | 17.4 | 274 | 13.3 | 275 | 13.3 |
| Spain (d) , (e) | N | C | 3,913 | – | 3,526 | – | 5,087 | – | 8,730 | – | 9,426 | – |
| Sweden | Y | C | 933 | 9.0 | 825 | 8.0 | 1,990 | 19.5 | 2,041 | 20.2 | 2,280 | 22.8 |
| EU Total 27 | 60,050 | 15.7 | 52,690 | 13.7 | 78,190 | 20.4 | 82,392 | 20.5 | 81,482 | 20.1 | ||
| United Kingdom | – | – | – | – | – | – | 9,718 | 14.6 | 9466 | 14.3 | 10,105 | 15.3 |
| EU Total (f) | 60,050 | 15.7 | 52,690 | 13.7 | 87,908 | 19.5 | 91,858 | 19.6 | 91,587 | 19.4 | ||
| Iceland | Y | C | 54 | 14.6 | 32 | 8.8 | 50 | 14.0 | 63 | 18.1 | 64 | 18.9 |
| Norway | Y | C | 389 | 7.2 | 441 | 8.2 | 1,092 | 20.5 | 961 | 18.1 | 992 | 18.9 |
| Liechtenstein | Y | C | 7 | 17.9 | 1,260 | 14.6 | 1,538 | 17.9 | 1,468 | 17.2 | 1,835 | 21.7 |
| Switzerland (g) | Y | C | 1,480 | 17.1 | ||||||||
–: Data not reported.
: Y: yes; N: no; A: aggregated data; C: case‐based data.
: Sentinel system; notification rates calculated with an estimated population coverage of 48%.
: Sentinel system; notification rates calculated with an estimated population coverage of 64%.
: The notification rate was not calculated since information on estimated coverage was not available.
: Data not complete in 2020 and 2021: rate not estimated.
: Cases reported by the United Kingdom for the period 2017–2019 were also considered for this estimation (EU‐28). When the United Kingdom data were collected for the period 2017–2019, the United Kingdom was an EU MS, but it became a third country on 1 February 2020.
: Switzerland provided data directly to EFSA. The human data for Switzerland include data from Liechtenstein for the years 2017–2020.
As in the previous year, the highest notification rates in 2021 were reported by Czechia (93.7 cases per 100,000 population) and Slovakia (81.3 cases per 100,000 population), while the lowest rates were reported by Bulgaria, Cyprus, Greece, Ireland, Romania and Portugal (≤ 4.6 cases per 100,000 population).
The proportion of domestic vs. travel‐associated cases varied markedly between countries, but most of the confirmed salmonellosis cases were acquired in the EU (72.8%), whereas 1.5% reported travel outside the EU and 25.7% of infections were of unknown origin (Table 9). Considering all cases in EU MSs, the highest proportions of domestic cases (100%) were reported by Croatia, Hungary, Malta, Poland, Romania, Slovakia and Spain. The highest proportions of travel‐associated cases were reported by four countries: France (21%), Sweden (20%), Slovenia (19%) and Ireland (16%). Of 1,443 travel‐associated cases with known information on the probable country of infection, 64.1% involved travel outside the EU. Turkey, Egypt, Morocco and Kosovo were the most frequently reported travel destinations outside the EU (21.2%, 7.1%, 6.9% and 3.7%, respectively). For travel‐associated cases in the EU, the most common countries of infection were Spain and Italy.
A seasonal trend was observed for confirmed salmonellosis cases in the EU in 2012–2021, with more cases reported during summer months (Figure 3). In 2021, a slight increase in notified human cases was registered compared with 2020. Notwithstanding, the overall trend for salmonellosis in 2017–2021 did not show any statistically significant increase or decrease.
Figure 3.
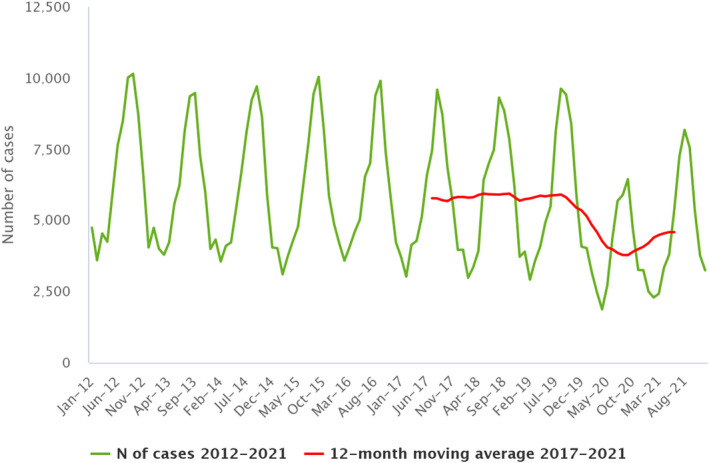
- Source: Austria, Belgium, Cyprus, Czechia, Denmark, Estonia, Germany, Greece, Finland, France, Hungary, Ireland, Italy, Luxembourg, Latvia, Malta, the Netherlands, Poland, Portugal, Romania, Sweden, Slovenia and Slovakia.
Denmark, Estonia, Finland, Ireland, Romania and Sweden reported a significantly decreasing trend (p < 0.05) in the last 5 years (2017–2021). Only Malta reported a significantly increasing trend (p < 0.05) in 2017–2021.
The most affected age groups were 0–4 years (27.7%), 5–9 years (13.8%) and over 65 years (16.5%). It is important to underline that for 74.4% of the samples, information about the type of specimen was present; 69.9% of specimens were faeces and the rest consisted of 1.8% other, 1.6% blood, 1.1% urine and 0.03% cerebrospinal fluid and pus.
Sixteen MSs provided information on hospitalisation for 30,951 cases (51.5%) at the EU level. Among these, the proportion of hospitalised cases was 38.1%, which was higher than in 2020. The highest proportions of hospitalised cases were reported, as in previous years, in Cyprus, Greece and Lithuania. Two of these countries also reported the lowest notification rates for salmonellosis, which may suggest that the surveillance systems in these countries primarily capture the most severe cases. Within different classes of specimens, considering cases with information on hospitalisation, higher rates of cases were reported from blood (82.4%), pus (62.5%), urine (39.1%) and faeces (36.5%).
Sixteen EU MSs provided data on the outcome of salmonellosis, accounting for 64.4% of confirmed cases. Among these, 10 reported 71 fatal cases, resulting in an EU case fatality rate of 0.18%.
2.4.3. Salmonella in food
Data collected in the context of Regulation (EC) No 2073/2005
Food safety criteria
The number of official single samples collected at manufacturing (N = 32,212 samples, notified by 14 MSs) was higher than that at distribution (N = 22,614 samples, notified by 14 MSs), whereas the proportions of positive samples were similar at the two stages (2.81% at manufacturing and 2.53% at distribution) (Table 11).
Table 11.
Proportion (%) of Salmonella‐positive samples from official sampling as part of the verification of Salmonella FSC in accordance with Regulation (EC) No 2073/2005, by stage in the food chain, EU, 2021
| Food matrices | Manufacturing stage (including processing) | Distribution stage (including retail) | ||||
|---|---|---|---|---|---|---|
| N MSs | N tested samples | N (%) testing positive | N MSs | N tested samples | N (%) testing positive | |
| Cheeses, butter and cream made from raw milk or milk that has undergone a lower heat treatment than pasteurisation | 9 | 1,718 | 4 (0.23) | 7 | 2,614 | 6 (0.23) |
| Cooked crustaceans and molluscan shellfish | 5 | 390 | 0 | 7 | 846 | 6 (0.71) |
| Dried follow‐on formulae | 1 | 90 | 1 (1.1) | 4 | 259 | 0 |
| Dried infant formulae and dried dietary foods for special medical purposes intended for infants below 6 months of age | – | – | – | 5 | 449 | 1 (0.22) |
| Egg products, excluding products where the manufacturing process or the composition of the product will eliminate the Salmonella risk | 5 | 203 | 0 | 4 | 292 | 9 (3.1) |
| Fresh poultry meat | 9 | 16,322 | 501 (3.1) | 10 | 4,889 | 319 (6.5) |
| Gelatine and collagen | 2 | 45 | 0 | 7 | 114 | 0 |
| Ice cream, excluding products where the manufacturing process or the composition of the product will eliminate the Salmonella risk | 5 | 352 | 1 (0.28) | 7 | 483 | 0 |
| Live bivalve molluscs and live echinoderms, tunicates and gastropods | 2 | 535 | 0 | 4 | 371 | 2 (0.54) |
| Meat products intended to be eaten raw, excluding products where the manufacturing process or the composition of the product will eliminate the Salmonella risk | 9 | 1,130 | 3 (0.27) | 7 | 968 | 4 (0.41) |
| Meat products made from poultry meat intended to be eaten cooked | 2 | 30 | 3 (10.0) | 1 | 40 | 0 |
| Mechanically separated meat (MSM) | 7 | 188 | 25 (13.3) | 2 | 30 | 0 |
| Milk powder and whey powder | 8 | 184 | 0 | 4 | 143 | 0 |
| Minced meat and meat preparations intended to be eaten raw | 4 | 118 | 0 | 4 | 194 | 0 |
| Minced meat and meat preparations made from other species than poultry intended to be eaten cooked | 11 | 3,879 | 41 (1.1) | 12 | 5,935 | 80 (1.3) |
| Minced meat and meat preparations made from poultry meat intended to be eaten cooked | 9 | 6,236 | 325 (5.2) | 9 | 1,654 | 144 (8.7) |
| Precut fruit and vegetables (ready‐to‐eat) | 5 | 185 | 0 | 9 | 1,915 | 2 (0.1) |
| Ready‐to‐eat foods containing raw egg, excluding products where the manufacturing process or the composition of the product will eliminate the Salmonella risk | 1 | 285 | 0 | 2 | 50 | 0 |
| Sprouted seeds (ready‐to‐eat) | 4 | 67 | 0 | 6 | 383 | 0 |
| Unpasteurised fruit and vegetable juices (ready‐ to‐eat) | 4 | 255 | 0 | 7 | 985 | 0 |
| EU Total (a) | 14 | 32,212 | 904 (2.8) | 14 | 22,614 | 573 (2.5) |
MSs: Member States; FSC: food safety criteria.
: Data from the United Kingdom (Northern Ireland) are taken into account for 2021. In accordance with the agreement on the withdrawal of the United Kingdom from the EU, and in particular with the Protocol on Ireland/Northern Ireland, the EU requirements on data sampling are also applicable to Northern Ireland.
At manufacturing level, the highest percentages of Salmonella‐positive samples were reported from ‘mechanically separated meat (MSM)’ (13.3%), ‘meat products made from poultry meat intended to be eaten cooked’ (10%), ‘minced meat and meat preparations made from poultry meat intended to be eaten cooked’ (5.2%) and ‘fresh poultry meat’ (3.1%). Next, there were ‘dried follow‐on formulae' (1.1%, one positive out of 90 tested samples) and ‘minced meat and meat preparations made from other species than poultry intended to be eaten cooked’ (1.1%). For the other food matrices, the percentage of positive samples was lower than 0.30%, or no Salmonella‐positive samples were reported.
At distribution level, the highest proportions of Salmonella‐positive samples were reported in ‘minced meat and meat preparations made from poultry meat intended to be eaten cooked’ (8.7%), ‘fresh poultry meat’ (6.5%), ‘egg products, excluding products where the manufacturing process or the composition of the product will eliminate the Salmonella risk’ (3.1%) and ‘minced meat and meat preparations made from other species than poultry intended to be eaten cooked’ (1.3%). For the other food matrices covered by Regulation (EC) No 2073/2005, the percentage of positive samples was consistently lower than 0.70% and for the majority of them, no Salmonella‐positive samples were reported.
For a further interactive look at Salmonella monitoring results, dashboards have been created (different filters can be applied to query the data) ( link ).
Process hygiene criteria
Considering data on Salmonella from carcases of different species, irrespective of the sampler, the prevalence values found for neck skin samples from broilers and turkeys were much higher than those reported for carcase surfaces of ruminants (cattle, sheep and goats) and horses.
Carcases of pigs
Considering all PHC monitoring data from pig carcases collected at the slaughterhouse after dressing but before chilling sent by a total of 23 MSs, the overall proportion of Salmonella‐positive samples based on official controls was 1.7% (N = 24,802) and was significantly higher than that based on own checks (1.4%, N = 103,270) (Table 12). The same finding was true considering overall data from those MSs (nine) that reported data collected by both the CA (2.2%, N = 15,617) and the FBOp (0.85%, N = 55,699) and specifically for six MSs (Belgium, Estonia, Germany, Ireland, Italy and Spain).
Table 12.
Comparisons of proportions (%) of Salmonella‐positive single samples from pig carcases after dressing, but before chilling, by sampler and reporting MS, EU, 2021
| Country | Competent authority (CA) | Food business operator (FBOp) | p‐value (b) | Interpretation | ||||
|---|---|---|---|---|---|---|---|---|
| N tested samples | N (%) positive samples | CI95 | N tested samples | N (%) positive samples | CI95 | |||
| Austria | – | – | – | 4,056 | 2 (0.05) | [0.01; 0.18] | – | – |
| Belgium | 955 | 59 (6.2) | [4.7; 7.9] | 4,108 | 62 (1.5) | [1.2; 1.9] | < 0.001 | CA > FBOp |
| Bulgaria | 5,049 | 0 | [0; 0.07] (a) | 305 | 0 | [0; 1.2] (a) | NS | |
| Croatia | 1,630 | 4 (0.24) | [0.07; 0.63] | – | – | – | – | – |
| Cyprus | 2 | 0 | [−] | – | – | – | – | – |
| Czechia | 4,508 | 35 (0.78) | [0.54; 1.1] | – | – | – | – | – |
| Denmark | – | – | – | 10,773 | 80 (0.74) | [0.59; 0.92] | – | – |
| Estonia | 357 | 6 (1.7) | [0.62; 3.6] | 1,581 | 12 (0.76) | [0.39; 1.3] | 0.096 | CA > FBOp |
| France | – | – | – | 13,662 | 628 (4.6) | [4.3; 5.0] | – | – |
| Germany | 200 | 4 (2.0) | [0.55; 5.0] | 24,885 | 156 (0.63) | [0.53; 0.73] | 0.039 | CA > FBOp |
| Hungary | 3,045 | 44 (1.4) | [1.1; 1.9] | – | – | – | – | – |
| Ireland | 391 | 19 (4.9) | [3.0; 7.5] | 2,064 | 25 (1.2) | [0.78; 1.8] | < 0.001 | CA > FBOp |
| Italy | 5,147 | 174 (3.4) | [2.9; 3.9] | 11,494 | 107 (0.93) | [0.76; 1.1] | < 0.001 | CA > FBOp |
| Latvia | – | – | – | 579 | 3 (0.52) | [0.11; 1.5] | – | – |
| Luxembourg | – | – | – | 345 | 2 (0.58) | [0.07; 2.1] | – | – |
| Malta | – | – | – | 120 | 13 (10.8) | [5.9; 17.8] | – | – |
| Netherlands | – | – | – | 5,635 | 120 (2.1) | [1.8; 2.5] | – | – |
| Poland | 273 | 0 | [0; 1.3] (a) | 5,576 | 3 (0.05) | [0.01; 0.16] | NS | |
| Portugal | – | – | – | 8,222 | 66 (0.8) | [0.62; 1.0] | – | – |
| Romania | 2,540 | 0 | [0; 0.14] (a) | 2,881 | 0 | [0; 0.13] (a) | NS | |
| Slovakia | – | – | – | 3,209 | 0 | [0; 0.12] (a) | – | – |
| Slovenia | – | – | – | 970 | 13 (1.3) | [0.72; 2.3] | – | – |
| Spain | 705 | 82 (11.6) | [9.4; 14.2] | 2,805 | 109 (3.9) | [3.2; 4.7] | < 0.001 | CA > FBOp |
| EU Total (27 + XI) | 24,802 | 427 (1.7) | [1.6; 1.9] | 103,270 | 1401 (1.4) | [1.3; 1.4] | < 0.001 | CA > FBOp |
| EU Total (27 + XI) providing CA and FBOp data | 15,617 | 344 (2.2) | [2.0; 2.4] | 55,699 | 474 (0.85) | [0.78; 0.93] | < 0.001 | CA > FBOp |
–: Data not reported.
[−]: The confidence interval is not provided because of the small sample size.
: One‐sided, 97.5% confidence interval.
: p‐value: NS, not significant.
Finland, Sweden and Norway, which are countries with special guarantees in relation to Salmonella on pig carcases (in accordance with Regulation (EU) No 853/2004), reported the following monitoring results: one positive out of 2,369 own‐check samples taken by the FBOp (0.04%) in Finland, one positive out of 2,833 official samples (0.03%) in Norway and one positive out of 6,426 official samples (0.01%) in Sweden. Moreover, Switzerland reported zero positive out of 1,117 and Montenegro zero positive out of nine own‐check samples collected by the FBOp.
Carcases of broilers
The overall proportion of Salmonella‐positive PHC neck skin samples collected at the slaughterhouse from broiler carcases after chilling based on official controls was 14.0% (N = 6,544), which was significantly higher than that based on own checks (3.2%, N = 56,532) (Table 13). Similarly, for the five MSs that reported data collected by both samplers, the overall proportion detected in samples collected by the CA (12.8%, N = 3,285) was significantly higher than that reported by the FBOp (4.1%, N = 10,955), and this finding was specifically confirmed for four MSs (Belgium, Italy, Romania and Spain).
Table 13.
Comparisons of proportions (%) of Salmonella‐positive single samples from broiler carcases (neck skin samples) after chilling, by sampler and reporting MS, EU, 2021
| Country | Competent authority (CA) | Food business operator (FBOp) | p‐value (b) | Interpretation | ||||
|---|---|---|---|---|---|---|---|---|
| N tested samples | N (%) positive samples | CI95 | N tested samples | N (%) positive samples | CI95 | |||
| Austria | – | – | – | 1,029 | 147 (14.3) | [12.2; 16.6] | – | – |
| Belgium | 631 | 62 (9.8) | [7.6; 12.4] | 2,634 | 128 (4.9) | [4.1; 5.8] | < 0.001 | CA > FBOp |
| Croatia | 1,290 | 270 (20.9) | [18.7; 23.3] | – | – | – | – | – |
| Cyprus | 220 | 65 (29.5) | [23.6; 36] | – | – | – | – | – |
| Czechia | 1,040 | 65 (6.2) | [4.9; 7.9] | – | – | – | – | – |
| Denmark | – | – | – | 263 | 0 | [0; 1.4] (a) | – | – |
| Estonia | – | – | – | 260 | 0 | [0; 1.4] (a) | – | – |
| France | – | – | – | 13,739 | 319 (2.3) | [2.1; 2.6] | – | – |
| Germany | – | – | – | 19,509 | 504 (2.6) | [2.4; 2.8] | – | – |
| Greece | – | – | – | 1,991 | 0 | [0; 0.18] (a) | – | – |
| Hungary | 709 | 94 (13.3) | [10.8; 16.0] | – | – | – | – | – |
| Ireland | 279 | 3 (1.1) | [0.22; 3.1] | 1,190 | 11 (0.92) | [0.46; 1.6] | NS | |
| Italy | 1,125 | 277 (24.6) | [22.1; 27.2] | 4,605 | 300 (6.5) | [5.8; 7.3] | < 0.001 | CA > FBOp |
| Latvia | – | – | – | 599 | 23 (3.8) | [2.4; 5.7] | – | – |
| Netherlands | – | – | – | 3,536 | 273 (7.7) | [6.9; 8.7] | – | – |
| Portugal | – | – | – | 2,862 | 5 (0.17) | [0.06; 0.41] | – | – |
| Romania | 800 | 59 (7.4) | [5.7; 9.4] | 1,891 | 9 (0.48) | [0.22; 0.90] | < 0.001 | CA > FBOp |
| Slovakia | – | – | – | 945 | 0 | [0; 0.39] (a) | – | – |
| Slovenia | – | – | – | 844 | 59 (7.0) | [5.4; 8.9] | – | – |
| Spain | 450 | 19 (4.2) | [2.6; 6.5] | 635 | 4 (0.63) | [0.17; 1.6] | < 0.001 | CA > FBOp |
| EU Total (27 + XI) | 6,544 | 914 (14.0) | [13.1; 14.8] | 56,532 | 1782 (3.2) | [3.0; 3.3] | < 0.001 | CA > FBOp |
| EU Total (27 + XI) providing CA and FBOp data | 3,285 | 420 (12.8) | [11.7; 14.0] | 10,955 | 452 (4.1) | [3.8; 4.5] | < 0.001 | CA > FBOp |
–: Data not reported.
: One‐sided, 97.5% confidence interval.
: p‐value: NS, not significant.
Finland, Sweden and Norway, which are countries with special guarantees in relation to Salmonella on broiler carcases (in accordance with Regulation (EU) No 853/2004), reported the following monitoring results: Finland (N = 1,271 collected by the FBOp) and Sweden (N = 1,846 collected by the CA) did not report any positive samples, whereas Norway did not report any data for broiler carcases. Moreover, Switzerland reported 13 positive out of 845 (1.5%) and Montenegro two positive out of 50 tested broiler carcase samples collected by the FBOp.
Carcases of turkeys
The overall percentage of Salmonella‐positive PHC neck skin samples collected at the slaughterhouse from turkey carcases after chilling based on official controls was 7.4% (N = 1,321) and was significantly higher than the percentage based on own‐check samples collected by the FBOp (3.2%, N = 7,941) (Table 14). The same finding was true considering the overall proportion of positive samples for the four MSs that reported data from both samplers, whereas looking at the data of the single MSs, this finding was confirmed only for Italy.
Table 14.
Comparisons of proportions (%) of Salmonella‐positive single samples from turkey carcases (neck skin samples) after chilling, by sampler and reporting MS, EU, 2021
| Country | Competent authority (CA) | Food business operator (FBOp) | p‐value (b) | Interpretation | ||||
|---|---|---|---|---|---|---|---|---|
| N tested samples | N (%) positive samples | CI95 | N tested samples | N (%) positive samples | CI95 | |||
| Austria | – | – | – | 135 | 5 (3.7) | [1.2; 8.4] | – | – |
| Belgium | 49 | 0 | [0; 7.3] (a) | 135 | 0 | [0; 2.7] (a) | NS | |
| Czechia | 300 | 3 (1.0) | [0.21; 2.9] | – | – | – | – | – |
| France | – | – | – | 2,948 | 35 (1.2) | [0.83; 1.6] | – | – |
| Germany | – | – | – | 1,611 | 102 (6.3) | [5.2; 7.6] | – | – |
| Greece | – | – | – | 116 | 0 | [0; 3.1] (a) | – | – |
| Hungary | 651 | 32 (4.9) | [3.4; 6.9] | – | – | – | – | – |
| Ireland | 47 | 0 | [0; 7.5] (a) | 538 | 0 | [0; 0.68] (a) | NS | |
| Italy | 144 | 29 (20.1) | [13.9; 27.6] | 1,079 | 91 (8.4) | [6.8; 10.3] | < 0.001 | CA > FBOp |
| Latvia | – | – | – | 5 | 0 | [−] | – | – |
| Poland | – | – | – | 371 | 7 (1.9) | [0.76; 3.8] | – | – |
| Portugal | – | – | – | 752 | 6 (0.80) | [0.29; 1.7] | – | – |
| Romania | 30 | 0 | [0; 11.6] (a) | 25 | 0 | [0; 13.7] (a) | NS | |
| Slovakia | – | – | – | 60 | 0 | [0; 6.0] (a) | – | – |
| Slovenia | – | – | – | 166 | 5 (3.0) | [0.98; 6.9] | – | – |
| Spain | 100 | 34 (34.0) | [24.8; 44.2] | – | – | – | – | – |
| EU Total (27 + XI) | 1,321 | 98 (7.4) | [6.1; 9.0] | 7,941 | 251 (3.2) | [2.8; 3.6] | < 0.001 | CA > FBOp |
| EU Total (27 + XI) providing CA and FBOp data | 270 | 29 (10.7) | [7.3; 15.1] | 1,777 | 91 (5.1) | [4.1; 6.2] | < 0.001 | CA > FBOp |
–: Data not reported.
: One‐sided, 97.5% confidence interval.
: p‐value: NS, not significant.
Finland, Sweden and Norway are countries with special guarantees in relation to Salmonella on turkey carcases (in accordance with Regulation (EU) No 853/2004). Finland (N = 271 collected by the FBOp) and Sweden (N = 124 collected by the CA) did not report any positive samples, whereas Norway did not report any data on turkey carcases. Moreover, Switzerland reported zero positive out of 135 tested turkey samples collected by the FBOp.
Carcases of cattle
The overall percentage of Salmonella‐positive PHC samples from bovine carcases collected at the slaughterhouse after dressing but before chilling based on official controls was 0.81% (N = 13,017) and was significantly higher than that based on own checks conducted by the FBOp (0.24%, N = 69,412) (Table 15). The same finding was true considering the overall proportion of positive samples for the five MSs that reported data from both samplers (1.1%, N = 5,784 collected by the CA and 0.42%, N = 15,828 collected by the FBOp) and specifically for Italy and Spain. Only Belgium reported a higher percentage of Salmonella‐positive samples based on own checks (0.34%, N = 2,667) than for samples based on official controls (0%, N = 1,164).
Table 15.
Comparisons of proportions (%) of Salmonella‐positive single samples from bovine carcases after dressing but before chilling, by sampler and reporting MS, EU, 2021
| Country | Competent authority (CA) | Food business operator (FBOp) | p‐value (b) | Interpretation | ||||
|---|---|---|---|---|---|---|---|---|
| N tested samples | N (%) positive samples | CI95 | N tested samples | N (%) positive samples | CI95 | |||
| Austria | – | – | – | 2,607 | 0 | [0; 0.14] (a) | – | – |
| Belgium | 1,164 | 0 | [0; 0.32] (a) | 2,667 | 9 (0.34) | [0.15; 0.64] | 0.038 | CA < FBOp |
| Bulgaria | 44 | 0 | [0; 8.0] (a) | – | – | – | – | – |
| Croatia | 2,819 | 32 (1.1) | [0.78; 1.6] | – | – | – | – | – |
| Czechia | 3,951 | 9 (0.23) | [0.1; 0.43] | – | – | – | – | – |
| Denmark | – | – | – | 4,971 | 3 (0.06) | [0.01; 0.18] | – | – |
| Estonia | 222 | 3 (1.4) | [0.28; 3.9] | – | – | – | – | – |
| France | – | – | – | 18,088 | 38 (0.21) | [0.15; 0.29] | – | – |
| Germany | – | – | – | 11,283 | 7 (0.06) | [0.03; 0.13] | – | – |
| Greece | – | – | – | 167 | 0 | [0; 2.2] (a) | – | – |
| Hungary | 197 | 1 (0.51) | [0.01; 2.8] | – | – | – | – | – |
| Ireland | – | – | – | 6,203 | 1 (0.02) | [0; 0.09] | – | – |
| Italy | 2,409 | 38 (1.6) | [1.1; 2.2] | 10,009 | 58 (0.58) | [0.44; 0.75] | < 0.001 | CA > FBOp |
| Latvia | – | – | – | 539 | 0 | [0; 0.68] (a) | – | – |
| Luxembourg | – | – | – | 255 | 0 | [0; 1.4] (a) | – | – |
| Malta | – | – | – | 120 | 26 (21.7) | [14.7; 30.1] | – | – |
| Netherlands | – | – | – | 2,572 | 9 (0.35) | [0.16; 0.66] | – | – |
| Poland | 105 | 0 | [0; 3.5] (a) | 108 | 0 | [0; 3.4] (a) | NS | |
| Portugal | – | – | – | 3,120 | 14 (0.45) | [0.25; 0.75] | – | – |
| Romania | 1,471 | 0 | [0; 0.25] (a) | 2,194 | 0 | [0; 0.17] (a) | NS | |
| Slovakia | – | – | – | 2,107 | 0 | [0; 0.17] (a) | – | – |
| Slovenia | – | – | – | 1,552 | 0 | [0; 0.24] (a) | – | – |
| Spain | 635 | 23 (3.6) | [2.3; 5.4] | 850 | 0 | [0; 0.43] (a) | < 0.001 | CA > FBOp |
| EU Total (27 + XI) | 13,017 | 106 (0.81) | [0.67; 0.98] | 69,412 | 165 (0.24) | [0.2; 0.28] | < 0.001 | CA > FBOp |
| EU Total (27 + XI) providing CA and FBOp data | 5,784 | 61 (1.1) | [0.81; 1.4] | 15,828 | 67 (0.42) | [0.33; 0.54] | < 0.001 | CA > FBOp |
–: Data not reported.
: One‐sided, 97.5% confidence interval.
: p‐value: NS, not significant.
Finland, Sweden and Norway are countries with special guarantees in relation to Salmonella on bovine carcases (in accordance with Regulation (EU) No 853/2004). Finland (N = 2,177 collected by the FBOp) and Norway (N = 3,172 collected by the CA) did not report any positive samples, whereas Sweden reported two positive out of 3,831 tested samples collected by the CA (0.05%). Moreover, Switzerland reported two positive samples out of 1,017 (0.2%), Iceland zero positive out of 300 and Montenegro zero positive out of 66 tested samples collected by the FBOp.
Carcases of sheep
The overall percentage of Salmonella‐positive PHC samples from sheep carcases collected at the slaughterhouse after dressing but before chilling based on official controls was 1.2% (N = 3,693) and was significantly higher than that based on own checks (0.49%, N = 16,683) (Table 16). The same finding was true considering overall data sent by the four MSs providing both CA (0.81%, N = 1,238) and FBOp (0.11%, N = 4,673) data and specifically for Belgium and Italy. Moreover, Switzerland reported zero positive out of 367, Iceland zero positive out of 132 and Montenegro zero positive out of 12 tested samples collected by the FBOp. There are no special guarantees in relation to Salmonella in sheep carcases.
Table 16.
Comparisons of proportions (%) of Salmonella‐positive single samples from sheep carcases after dressing but before chilling, by sampler and reporting MS, EU, 2021
| Country | Competent authority (CA) | Food business operator (FBOp) | p‐value (b) | Interpretation | ||||
|---|---|---|---|---|---|---|---|---|
| N tested samples | N (%) positive samples | CI95 | N tested samples | N (%) positive samples | CI95 | |||
| Austria | – | – | – | 229 | 0 | [0; 1.6] (a) | – | – |
| Belgium | 338 | 4 (1.2) | [0.32; 3.0] | 641 | 1 (0.16) | [0; 0.87] | 0.051 | CA > FBOp |
| Bulgaria | 120 | 0 | [0; 3.0] (a) | – | – | – | – | – |
| Croatia | 2,034 | 33 (1.6) | [1.1; 2.3] | – | – | – | – | – |
| Cyprus | 10 | 0 | [0; 30.9] (a) | – | – | – | – | – |
| Czechia | 291 | 0 | [0; 1.3] (a) | – | – | – | – | – |
| Finland | – | – | – | 57 | 0 | [0; 6.3] (a) | – | – |
| France | – | – | – | 6,265 | 51 (0.81) | [0.61; 1.1] | – | – |
| Germany | – | – | – | 907 | 3 (0.33) | [0.07; 0.96] | – | – |
| Ireland | – | – | – | 1,402 | 2 (0.14) | [0.02; 0.51] | – | – |
| Italy | 346 | 6 (1.7) | [0.64; 3.7] | 3,135 | 4 (0.13) | [0.04; 0.33] | < 0.001 | CA > FBOp |
| Latvia | – | – | – | 143 | 0 | [0; 2.5] (a) | – | – |
| Luxembourg | – | – | – | 78 | 0 | [0; 4.6] (a) | – | – |
| Malta | – | – | – | 96 | 4 (4.2) | [1.1; 10.3] | – | – |
| Netherlands | – | – | – | 288 | 0 | [0; 1.3] (a) | – | – |
| Portugal | – | – | – | 2,101 | 17 (0.81) | [0.47; 1.3] | – | – |
| Romania | 464 | 0 | [0; 0.79] (a) | 652 | 0 | [0; 0.56] (a) | NS | |
| Slovakia | – | – | – | 352 | 0 | [0; 1.0] (a) | – | – |
| Slovenia | – | – | – | 92 | 0 | [0; 3.9] (a) | – | – |
| Spain | 90 | 0 | [0; 4.0] (a) | 245 | 0 | [0; 1.5] (a) | NS | |
| EU Total (27 + XI) | 3,693 | 43 (1.2) | [0.84; 1.6] | 16,683 | 82 (0.49) | [0.39; 0.61] | < 0.001 | CA > FBOp |
| EU Total (27 + XI) providing CA and FBOp data | 1,238 | 10 (0.81) | [0.39; 1.5] | 4,673 | 5 (0.11) | [0.04; 0.25] | < 0.001 | CA > FBOp |
–: Data not reported.
: One‐sided, 97.5% confidence interval.
: p‐value: NS, not significant.
Carcases of goats
The overall percentage of Salmonella‐positive PHC samples from goat carcases collected at the slaughterhouse after dressing but before chilling based on own checks was 2.1% (N = 1,211) and was significantly higher than that based on official controls (0.51%, N = 585). This finding was markedly influenced by the results of two MSs (France and Portugal) that reported the large majority of the positive samples from goat carcases notified by the FBOp (Table 17). There are no special guarantees in relation to Salmonella in goat carcases.
Table 17.
Comparisons of proportions (%) of Salmonella‐positive single samples from goat carcases after dressing but before chilling, by sampler and reporting MS, EU, 2021
| Country | Competent authority (CA) | Food business operator (FBOp) | p‐value (b) | Interpretation | ||||
|---|---|---|---|---|---|---|---|---|
| N tested samples | N (%) positive samples | CI95 | N tested samples | N (%) positive samples | CI95 | |||
| Austria | – | – | – | 13 | 0 | [0; 24.7] (a) | – | – |
| Belgium | 113 | 2 (1.8) | [0.22; 6.2] | 55 | 0 | [0; 6.5] (a) | NS | |
| Bulgaria | 5 | 0 | [−] | – | – | – | – | – |
| Croatia | 314 | 1 (0.32) | [0.01; 1.8] | – | – | – | – | – |
| Czechia | 70 | 0 | [0; 5.1] (a) | – | – | – | – | – |
| France | – | – | – | 171 | 15 (8.8) | [5.0; 14.1] | – | – |
| Germany | – | – | – | 36 | 0 | [0; 9.7] (a) | – | – |
| Greece | – | – | – | 203 | 0 | [0; 1.8] (a) | – | – |
| Italy | 53 | 0 | [0; 6.7] (a) | 114 | 1 (0.88) | [0.02; 4.8] | NS | |
| Malta | – | – | – | 24 | 1 (4.2) | [0.10; 21.1] | – | – |
| Netherlands | – | – | – | 42 | 0 | [0; 8.4] (a) | – | – |
| Portugal | – | – | – | 531 | 9 (1.7) | [0.78; 3.2] | – | – |
| Slovakia | – | – | – | 7 | 0 | [−] | – | – |
| Slovenia | – | – | – | 15 | 0 | [0; 21.8] (a) | – | – |
| Spain | 30 | 0 | [0; 11.6] (a) | – | – | – | – | – |
| EU Total (27 + XI) | 585 | 3 (0.51) | [0.11; 1.5] | 1,211 | 26 (2.1) | [1.4; 3.1] | 0.005 | CA < FBOp |
| EU Total (27 + XI) providing CA and FBOp data | 166 | 2 (1.2) | [0.15; 4.3] | 169 | 1 (0.59) | [0.01; 3.3] | NS | |
–: Data not reported.
: One‐sided, 97.5% confidence interval.
: p‐value: NS, not significant.
Carcases of horses
The overall percentage of Salmonella‐positive PHC samples from horse carcases collected at the slaughterhouse after dressing but before chilling based on official controls was 0.19% (N = 517) and was not significantly higher than that based on FBOp own checks (0.15%, N = 1,360) (Table 18). There are no special guarantees in relation to Salmonella in horse carcases.
Table 18.
Comparisons of proportions (%) of Salmonella‐positive single samples from horse carcases after dressing but before chilling, by sampler and reporting MS, EU, 2021
| Country | Competent authority (CA) | Food business operator (FBOp) | p‐value (b) | Interpretation | ||||
|---|---|---|---|---|---|---|---|---|
| N tested samples | N (%) positive samples | CI95 | N tested samples | N (%) positive samples | CI95 | |||
| Austria | – | – | – | 6 | 0 | [−] | – | – |
| Belgium | 69 | 0 | [0; 5.2] (a) | 114 | 0 | [0; 3.2] (a) | NS | |
| Croatia | 49 | 0 | [0; 7.3] (a) | – | – | – | – | – |
| Czechia | 7 | 0 | [−] | – | – | – | – | – |
| Finland | – | – | – | 1 | 0 | – | – | – |
| France | – | – | – | 70 | 0 | [0; 5.1] (a) | – | – |
| Germany | – | – | – | 21 | 0 | [0; 16.1] (a) | – | – |
| Ireland | – | – | – | 62 | 0 | [0; 5.8] (a) | – | – |
| Italy | 177 | 1 (0.56) | [0.01; 3.1] | 820 | 1 (0.12) | [0; 0.68] | NS | |
| Latvia | – | – | – | 3 | 0 | [−] | – | – |
| Poland | 5 | 0 | [−] | – | – | – | – | – |
| Portugal | – | – | – | 52 | 1 (1.9) | [0.05; 10.3] | – | – |
| Romania | 145 | 0 | [0; 2.5] (a) | 186 | 0 | [0; 2.0] (a) | NS | |
| Slovenia | – | – | – | 25 | 0 | [0; 13.7] (a) | – | – |
| Spain | 65 | 0 | [0; 5.5] (a) | – | – | – | – | – |
| EU Total (27 + XI) | 517 | 1 (0.19) | [0; 1.1] | 1,360 | 2 (0.15) | [0.02; 0.53] | NS | |
| EU Total (27 + XI) providing CA and FBOp data | 391 | 1 (0.26) | [0.01; 1.4] | 1,120 | 1 (0.09) | [0; 0.5] | NS | |
–: Data not reported.
: One‐sided, 97.5% confidence interval.
: p‐value: NS, not significant.
For a further interactive look at Salmonella monitoring results, dashboards have been created (different filters can be applied to query the data) (link).
Occurrence in food
Monitoring data reported for food samples, which do not fit with the criteria described in the previous paragraphs, were described by merging investigations from all sampling stages (primary production, manufacturing, distribution, other and unspecified), all samplers except ‘HACCP and own checks’ and ‘private sampling’ and all sampling units (single, batch and slaughter animal batch). Only samples collected through ‘objective sampling’ were considered in this context.
RTE food and non‐RTE food
In 2021, 73,238 RTE and 466,290 non‐RTE food sampling units were reported from 23 and 26 MSs with very low (0.23%) and low (2.1%) proportions of positive samples, respectively (Table 19).
Table 19.
Occurrence of Salmonella in the main food categories, EU, 2021 and 2017–2020
| Food | 2021 (a) | 2017–2020 (b) | ||||
|---|---|---|---|---|---|---|
| N reporting MSs | N sampled units | N positive (%) | N reporting MSs | N sampled units | N positive (%) | |
| RTE food | ||||||
| All | 23 | 73,238 | 169 (0.23) | 25 | 279,268 | 746 (0.27) |
| Meat and meat products | 21 | 22,589 | 99 (0.44) | 21 | 66,284 | 320 (0.48) |
| Meat and meat products from broilers | 12 | 1,706 | 0 | 16 | 5,128 | 25 (0.49) |
| Meat and meat products from turkeys | 9 | 213 | 0 | 14 | 1,593 | 7 (0.44) |
| Meat and meat products from pigs | 18 | 8,014 | 66 (0.82) | 19 | 27,874 | 110 (0.39) |
| Meat and meat products from bovine animals | 16 | 1,219 | 2 (0.16) | 19 | 4,243 | 14 (0.33) |
| Mixed | 10 | 2,956 | 4 (0.14) | 17 | 5,289 | 18 (0.34) |
| Other meat and meat products | 13 | 8,481 | 27 (0.32) | 17 | 22,157 | 146 (0.66) |
| Milk and milk products | 21 | 21,583 | 22 (0.1) | 24 | 93,802 | 111 (0.12) |
| Milk | 12 | 634 | 1 (0.16) | 15 | 2,828 | 1 (0.04) |
| Raw milk (c) | 5 | 211 | 0 | 7 | 1,812 | 0 |
| Cheese | 21 | 15,422 | 19 (0.12) | 23 | 48,587 | 74 (0.15) |
| Dairy products excluding cheeses (butter, cream, ice cream, whey, yoghurt and fermented dairy products) | 20 | 5,527 | 2 (0.04) | 21 | 42,387 | 36 (0.08) |
| Fruits and vegetables and juices | 18 | 6,261 | 3 (0.05) | 20 | 20,591 | 22 (0.11) |
| Fish and fishery products | 22 | 2,809 | 11 (0.39) | 22 | 12,909 | 10 (0.08) |
| Spices and herbs | 16 | 1,529 | 11 (0.72) | 19 | 5,636 | 41 (0.73) |
| Bakery products | 17 | 2,940 | 0 (0) | 18 | 16,110 | 34 (0.21) |
| Salads | 9 | 2,194 | 1 (0.05) | 15 | 12,866 | 47 (0.37) |
| Other processed food products and prepared dishes | 17 | 8,891 | 16 (0.18) | 16 | 30,362 | 122 (0.4) |
| Eggs and egg products | 3 | 46 | 0 | 8 | 244 | 0 |
| Sprouts (sprouted seeds) | 9 | 512 | 0 | 11 | 1,234 | 2 (0.16) |
| Cereals and nuts | 9 | 859 | 1 (0.12) | 15 | 3,008 | 3 (0.1) |
| Confections | 4 | 73 | 0 | 7 | 3,740 | 3 (0.08) |
| Infant formulae and follow‐on formulae–RTE | 12 | 1,127 | 1 (0.09) | 16 | 4,933 | 22 (0.45) |
| Foodstuffs intended for special nutritional uses | 10 | 588 | 1 (0.17) | 14 | 1,905 | 1 (0.05) |
| Non‐RTE food | ||||||
| All | 26 | 466,290 | 9,764 (2.1) | 28 | 1,034,606 | 22,086 (2.1) |
| Meat and meat products | 26 | 443,056 | 9,593 (2.2) | 28 | 945,084 | 21,747 (2.3) |
| Meat and meat products from broilers | 25 | 109,342 | 4,763 (4.4) | 26 | 148,380 | 9,521 (6.4) |
| Meat and meat products from turkeys | 22 | 13,049 | 472 (3.6) | 25 | 21,513 | 1,097 (5.1) |
| Meat and meat products from pigs | 26 | 136,975 | 2,050 (1.5) | 28 | 390,797 | 6,789 (1.7) |
| Meat and meat products from bovine animals | 25 | 86,507 | 237 (0.27) | 27 | 135,711 | 532 (0.39) |
| Mixed | 14 | 6,102 | 66 (1.1) | 19 | 25,656 | 218 (0.85) |
| Other meat and meat products | 25 | 91,081 | 2,005 (2.2) | 25 | 223,027 | 3,590 (1.6) |
| Milk and milk products | 11 | 996 | 0 (0) | 13 | 4,902 | 1 (0.02) |
| Fruits, vegetables and juices | 13 | 1,795 | 1 (0.06) | 21 | 9,078 | 54 (0.59) |
| Fish and fishery products | 18 | 7,093 | 46 (0.65) | 21 | 27,074 | 147 (0.54) |
| Eggs and egg products | 18 | 6,501 | 53 (0.82) | 20 | 27,281 | 92 (0.34) |
| Sprouts (sprouted seeds) | 5 | 85 | 0 | 8 | 1,337 | 5 (0.37) |
| Infant formulae | 4 | 175 | 0 | 4 | 244 | 0 |
| Foodstuffs intended for special nutritional uses | 5 | 317 | 0 | 7 | 789 | 2 (0.25) |
| Cereals, dried seeds | 9 | 409 | 1 (0.24) | 18 | 2,700 | 21 (0.78) |
| Other processed food products and prepared dishes | 10 | 3,779 | 38 (1) | 18 | 10,240 | 12 (0.12) |
| Fresh meat (d) | ||||||
| All | 26 | 387,152 | 8,219 (2.1) | 26 | 122,337 | 18,812 (2.4) |
| Fresh meat from broilers | 25 | 99,370 | 4,382 (4.4) | 25 | 17,843 | 8,586 (7.0) |
| Fresh meat from turkeys | 22 | 12,600 | 441 (3.5) | 28 | 348,156 | 989 (5.5) |
| Fresh meat from pigs | 26 | 124,467 | 1,902 (1.5) | 27 | 129,721 | 6,205 (1.8) |
| Fresh meat from bovine animals | 25 | 83,703 | 214 (0.26) | 24 | 154,371 | 481 (0.37) |
| Other fresh meat | 25 | 67,012 | 1,280 (1.9) | 26 | 122,337 | 2,551 (1.7) |
MSs: Member States; RTE: ready‐to‐eat.
: Data from the United Kingdom (Northern Ireland) are taken into account for 2021. In accordance with the agreement on the withdrawal of the United Kingdom from the EU, and in particular with the Protocol on Ireland/Northern Ireland, the EU requirements on data sampling are also applicable to Northern Ireland.
: Data from the United Kingdom are taken into account for 2017–2019, because the United Kingdom was an EU MS, but it became a third country on 1 February 2020.
: Raw RTE milk sampling units are a subset of RTE milk.
: Fresh meat sampling units are a subset of the two main categories above.
Within the category of RTE food, all matrices with Salmonella isolates had very low proportions of positive samples and the highest percentages were from ‘meat and meat products from pigs’ (0.82%), ‘spices and herbs’ (0.72%), ‘fish and fishery products’ (0.39%) and ‘other meat and meat products’ (0.32%).
Within the category of non‐RTE food, the highest percentages of positive samples were reported for ‘meat and meat products from broilers’ (4.4%), ‘meat and meat products from turkeys’ (3.6%), ‘other meat and meat products’ (2.2%), ‘meat and meat products from pigs’ (1.5%), ‘mixed meat and meat products’ (1.1%) and ‘other processed food products and prepared dishes’ (1.0%). Some Salmonella isolates were also reported for ‘eggs and egg products’ (0.82%) and ‘fish and fishery products’ (0.65%).
Comparing the results for the year 2021 and the 4‐year period of 2017–2020, the overall percentage of Salmonella‐positive RTE food samples remained rather stable. The greatest percentage reductions in Salmonella‐positive samples were found for ‘meat and meat products from broilers’, ‘meat and meat products from turkeys’, ‘infant formulae and follow‐on formulae–RTE', ‘other meat and meat products’ and ‘salads’. In contrast, a small increase was reported for ‘meat and meat products from pigs’ and for ‘fish and fishery products’.
Similarly, also for non‐RTE food, the overall percentage of Salmonella‐positive samples reported in 2021 was very close to the average reported over the period 2017–2020. The greatest reductions in proportions of Salmonella‐positive samples were found for ‘meat and meat products from broilers’, ‘meat and meat products from turkeys’, ‘fruits, vegetables and juices’ and ‘cereals and dried seeds’. Conversely, an increase was reported in the last year for ‘other processed food products and prepared dishes’, ‘other meat and meat products’ and ‘eggs and egg products’.
Fresh meat
For fresh meat, in 2021, 2.1% of sampling units (N = 387,152) were positive for Salmonella. Within this category, the highest percentages of positive samples were reported for ‘fresh meat from broilers’ (4.4%) and for ‘fresh meat from turkeys’ (3.5%), with a general reduction vs. the proportions of positive samples recorded in the previous years.
For a further interactive look at Salmonella monitoring results, dashboards have been created (different filters can be applied to query the data) ( link ).
2.4.4. Salmonella in animals
For a further interactive look at Salmonella monitoring results, dashboards have been created (different filters can be applied to query the data) ( link ).
Poultry monitoring data according to the national control programmes for Salmonella
Achievement of Salmonella reduction targets
Breeding flocks of Gallus gallus
In total, 25 MSs, the United Kingdom (Northern Ireland) and three non‐MSs reported Salmonella NCP data for breeding flocks of Gallus gallus. Luxembourg and Malta do not have such flocks. In the EU in 2021, considering merged data from the CA and FBOp, Salmonella was found in 348 (2.5%) of the 13,983 flocks tested, compared with 2.0% and 2.3% for 2020 and 2019, respectively. The prevalence of flocks that were positive for any of the five target serovars was 0.58% in 2021, while it was 0.52% in 2020 and 0.62% in 2019. In 2020, Poland did not report any data for Gallus gallus breeders. All reporting countries, except Austria, Belgium, Greece, Ireland and Poland, met the flock prevalence target of 1% maximum (Figure 4). The most frequently reported target serovar was S. Enteritidis (EU flock prevalence of 0.39%, 55 positive flocks), with 44 flocks (80.0%) reported by Poland. The total number of S. Enteritidis‐positive breeding flocks (55) increased compared to 2020 (29 positive flocks) and 2019 (53 positive flocks). S. Typhimurium (including the monophasic variant) was the second most commonly reported target serovar (with 15 positive flocks). The third most commonly reported serovar was S. Infantis (with six positive flocks). With regard to the other target serovars, three flocks tested positive for S. Virchow (0.02%) and two flocks tested positive for S. Hadar (0.01%) (Table 20).
Figure 4.
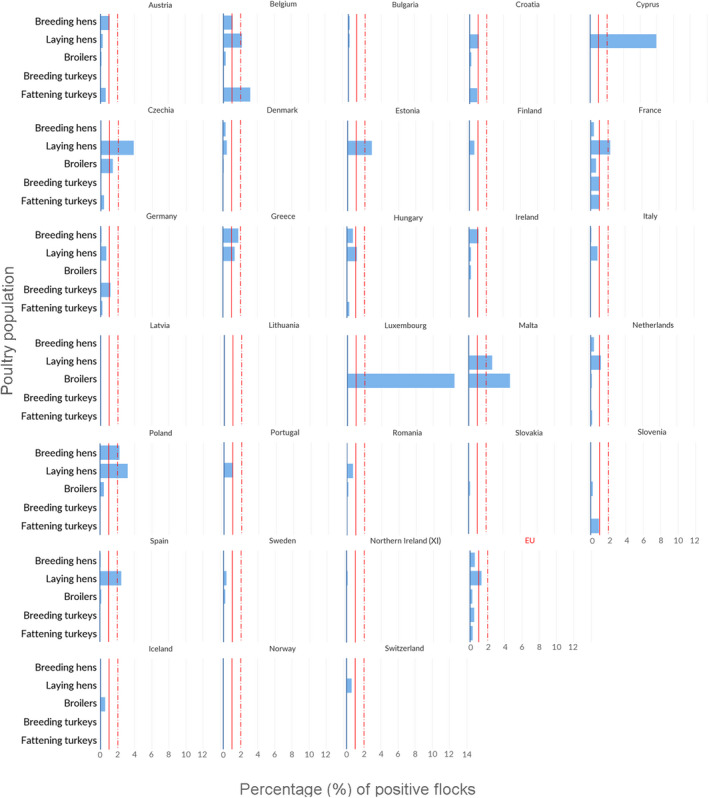
- Vertical bars indicate the target to be reached, which was set at 1% for all poultry populations with the exception of laying hens, for which it was 2%.
Table 20.
Salmonella in breeding flocks of Gallus gallus during the production period (all types of breeding flocks, flock‐based data) in countries running control programmes in accordance with Regulation (EC) No 2160/2003, 2021
| Country | N tested flocks | N (%) positive for Salmonella spp. | N (%) positive for target serovars | N (%) positive samples for | ||||
|---|---|---|---|---|---|---|---|---|
| S. Enteritidis | S. Typhimurium | S. Hadar | S. Infantis | S. Virchow | ||||
| Austria | 184 | 6 (3.3) | 2 (1.1) | 1 (0.54) | 1 (0.54) | 0 | 0 | 0 |
| Belgium | 556 | 12 (2.2) | 6 (1.1) | 1 (0.18) | 4 (0.72) | 0 | 1 (0.18) | 0 |
| Bulgaria | 608 | 3 (0.49) | 1 (0.16) | 0 | 0 | 0 | 1 (0.16) | 0 |
| Croatia | 95 | 0 | 0 | – | – | – | – | – |
| Cyprus | 34 | 0 | 0 | – | – | – | – | – |
| Czechia | 641 | 0 | 0 | – | – | – | – | – |
| Denmark | 308 | 1 (0.32) | 1 (0.32) | 0 | 1 (0.32) | 0 | 0 | 0 |
| Estonia | 7 | 0 | 0 | – | – | – | – | – |
| Finland | 145 | 0 | 0 | – | – | – | – | – |
| France | 1,555 | 22 (1.4) | 6 (0.39) | 2 (0.13) | 3 (0.19) | 0 | 1 (0.06) | 0 |
| Germany | 882 | 42 (4.8) | 1 (0.11) | 1 (0.11) | 0 | 0 | 0 | 0 |
| Greece | 224 | 30 (13.4) | 4 (1.8) | 1 (0.45) | 0 | 0 | 0 | 3 (1.3) |
| Hungary | 554 | 4 (0.72) | 4 (0.72) | 1 (0.18) | 1 (0.18) | 1 (0.18) | 1 (0.18) | 0 |
| Ireland | 175 | 3 (1.7) | 2 (1.1) | 2 (1.1) | 0 | 0 | 0 | 0 |
| Italy | 1,176 | 60 (5.1) | 1 (0.09) | 0 | 1 (0.09) | 0 | 0 | 0 |
| Latvia | 38 | 0 | 0 | – | – | – | – | – |
| Lithuania | 47 | 0 | 0 | – | – | – | – | – |
| Netherlands | 1,540 | 12 (0.78) | 6 (0.39) | 2 (0.13) | 3 (0.20) | 0 | 1 (0.06) | 0 |
| Poland | 1,991 | 53 (2.7) | 45 (2.3) | 44 (2.2) | 0 | 0 | 1 (0.05) | 0 |
| Portugal | 476 | 3 (0.63) | 0 | – | – | – | – | – |
| Romania | 457 | 7 (1.5) | 0 | – | – | – | – | – |
| Slovakia | 115 | 0 | 0 | – | – | – | – | – |
| Slovenia | 131 | 0 | 0 | – | – | – | – | – |
| Spain | 1,620 | 90 (5.6) | 2 (0.12) | 0 | 1 (0.06) | 1 (0.06) | 0 | 0 |
| Sweden | 156 | 0 | 0 | – | – | – | – | – |
| United Kingdom (Northern Ireland) | 268 | 0 | 0 | – | – | – | – | – |
| EU Total (27 + XI) | 13,983 | 348 (2.5) | 81 (0.58) | 55 (0.39) | 15 (0.11) | 2 (0.01) | 6 (0.04) | 3 (0.02) |
| Iceland | 43 | 0 | 0 | – | – | – | – | – |
| Norway | 230 | 0 | 0 | – | – | – | – | – |
| Switzerland | 122 | 0 | 0 | – | – | – | – | – |
Flocks of laying hens
All MSs, the United Kingdom (Northern Ireland) and three non‐MSs reported Salmonella NCP data for laying hen flocks. Considering merged data from the CA and FBOp, Salmonella was found in 1,323 flocks (3.3%), compared with 1,389 (4.0%) in 2020. The EU prevalence of laying hen flocks that were positive for either of the two target serovars was 1.3%, which was stable compared with 2020, when 1.3% of tested flocks were positive for target serovars.
Seven MSs (Belgium, Cyprus, Czechia, France, Malta, Poland and Spain) did not meet the reduction target of 2% (Figure 4). The most frequently reported target serovar was S. Enteritidis (EU flock prevalence of 1.0%), with 81.3% of 407 S. Enteritidis‐positive flocks reported by six MSs. France alone accounted for 28.0% (114 positive flocks) of the S. Enteritidis notified; this situation was similar to that of the previous year (29.2%). For S. Typhimurium (including the monophasic variant), 126 positive flocks were reported (EU flock prevalence of 0.32%) and the majority (36.5%; 46 positive flocks) were reported by France (Table 21).
Table 21.
Salmonella in laying hen flocks of Gallus gallus during the production period (flock‐based data) in countries running control programmes in accordance with Regulation (EC) No 2160/2003, 2021
| Country | N tested flocks | N (%) positive for Salmonella spp. | N (%) positive for target serovars | N (%) positive samples for | |
|---|---|---|---|---|---|
| S. Enteritidis | S. Typhimurium | ||||
| Austria | 3,318 | 25 (0.75) | 10 (0.3) | 10 (0.3) | 0 |
| Belgium | 642 | 35 (5.5) | 14 (2.2) | 11 (1.7) | 3 (0.47) |
| Bulgaria | 1,194 | 6 (0.50) | 2 (0.17) | 2 (0.17) | 0 |
| Croatia | 364 | 18 (4.9) | 4 (1.1) | 2 (0.55) | 2 (0.55) |
| Cyprus | 126 | 18 (14.3) | 10 (7.9) | 7 (5.6) | 3 (2.4) |
| Czechia | 491 | 22 (4.5) | 19 (3.9) | 13 (2.6) | 6 (1.2) |
| Denmark | 429 | 4 (0.93) | 2 (0.47) | 2 (0.47) | 0 |
| Estonia | 35 | 1 (2.9) | 1 (2.9) | 1 (2.9) | 0 |
| Finland | 651 | 4 (0.61) | 4 (0.61) | 2 (0.31) | 2 (0.31) |
| France | 6,996 | 300 (4.3) | 160 (2.3) | 114 (1.6) | 46 (0.66) |
| Germany | 7,070 | 73 (1.0) | 48 (0.68) | 37 (0.52) | 11 (0.16) |
| Greece | 728 | 45 (6.2) | 10 (1.4) | 6 (0.82) | 4 (0.55) |
| Hungary | 958 | 11 (1.1) | 11 (1.1) | 7 (0.73) | 4 (0.42) |
| Ireland | 383 | 1 (0.26) | 1 (0.26) | 0 | 1 (0.26) |
| Italy | 4,300 | 243 (5.7) | 35 (0.81) | 26 (0.6) | 9 (0.21) |
| Latvia | 71 | 0 | 0 | – | – |
| Lithuania | 47 | 3 (6.4) | 0 | – | – |
| Luxembourg | 46 | 0 | 0 | – | – |
| Malta | 109 | 25 (22.9) | 3 (2.8) | 2 (1.8) | 1 (0.92) |
| Netherlands | 2,744 | 51 (1.9) | 33 (1.2) | 32 (1.2) | 1 (0.04) |
| Poland | 2,242 | 89 (4.0) | 72 (3.2) | 69 (3.1) | 3 (0.13) |
| Portugal | 375 | 18 (4.8) | 4 (1.1) | 4 (1.1) | 0 |
| Romania | 873 | 49 (5.6) | 6 (0.69) | 6 (0.69) | 0 |
| Slovakia | 288 | 0 | 0 | – | – |
| Slovenia | 263 | 3 (1.1) | 0 | – | – |
| Spain | 3,201 | 268 (8.4) | 80 (2.5) | 53 (1.7) | 27 (0.84) |
| Sweden | 891 | 3 (0.34) | 3 (0.34) | 1 (0.11) | 2 (0.22) |
| United Kingdom (Northern Ireland) | 711 | 8 (1.1) | 1 (0.14) | 0 | 1 (0.14) |
| EU Total (27 + XI) | 39,546 | 1,323 (3.3) | 533 (1.3) | 407 (1.0) | 126 (0.32) |
| Iceland | 55 | 0 | 0 | – | – |
| Norway | 910 | 1 (0.11) | 0 | – | – |
| Switzerland | 677 | 4 (0.59) | 4 (0.59) | 4 (0.59) | 0 |
Broiler flocks
All MSs, the United Kingdom (Northern Ireland) and three non‐MSs reported Salmonella NCP data for broiler flocks. Considering merged data from the CA and FBOp, Salmonella was found in 3.8% (N = 12,040) of the tested flocks, compared with 3.9% in 2020 and 3.6% in 2019. The EU prevalence of broiler flocks positive for either of the two target Salmonella serovars was 0.28% (corresponding to 901 flocks), which was similar to the prevalence in previous years (0.25% in 2020 and 0.20% in 2019). Three MSs (Czechia, Luxembourg and Malta) did not meet the target of 1% or less of broiler flocks positive for S. Enteritidis and/or S. Typhimurium, like in the previous year, when the same three countries did not meet the target. As already reported in 2019 and 2020, the EU prevalence was very similar for the two target serovars: in 2021, S. Enteritidis accounted for 51.4% of flocks positive for target serovars, whereas S. Typhimurium accounted for 48.6%. France and Poland accounted for 65.0% of all the EU flocks positive for S. Enteritidis, and France alone accounted for 64.6% of all the EU broiler flocks positive for S. Typhimurium (Table 22).
Table 22.
Salmonella in broiler flocks of Gallus gallus before slaughter (flock‐based data) in countries running control programmes in accordance with Regulation (EC) No 2160/2003, 2021
| Country | N tested flocks | N (%) positive for Salmonella spp. | N (%) positive for target serovars | N (%) positive samples for | |
|---|---|---|---|---|---|
| S. Enteritidis | S. Typhimurium | ||||
| Austria | 6,153 | 167 (2.7) | 9 (0.15) | 3 (0.05) | 6 (0.10) |
| Belgium | 11,194 | 413 (3.7) | 30 (0.27) | 9 (0.08) | 21 (0.19) |
| Bulgaria | 8,482 | 2 (0.02) | 0 | – | – |
| Croatia | 3,120 | 134 (4.3) | 7 (0.22) | 3 (0.10) | 4 (0.13) |
| Cyprus | 1,033 | 5 (0.48) | 0 | 0 | 0 |
| Czechia | 4,945 | 97 (2.0) | 70 (1.4) | 65 (1.3) | 5 (0.10) |
| Denmark | 3,758 | 6 (0.16) | 4 (0.11) | 0 | 4 (0.11) |
| Estonia | 669 | 0 | 0 | – | – |
| Finland | 4,081 | 0 | 0 | – | – |
| France | 65,469 | 1,955 (3.0) | 414 (0.63) | 131 (0.20) | 283 (0.43) |
| Germany | 25,929 | 254 (0.98) | 6 (0.02) | 1 (< 0.01) | 5 (0.02) |
| Greece | 7,743 | 17 (0.22) | 3 (0.04) | 0 | 3 (0.04) |
| Hungary | 8,864 | 3 (0.03) | 3 (0.03) | 2 (0.02) | 1 (0.01) |
| Ireland | 3,416 | 29 (0.85) | 8 (0.23) | 7 (0.20) | 1 (0.03) |
| Italy | 29,281 | 6,341 (21.7) | 7 (0.02) | 2 (0.01) | 5 (0.02) |
| Latvia | 794 | 13 (1.6) | 0 | – | – |
| Lithuania | 143 | 3 (2.1) | 0 | – | – |
| Luxembourg | 8 | 1 (12.5) | 1 (12.5) | 0 | 1 (12.5) |
| Malta | 413 | 43 (10.4) | 20 (4.8) | 1 (0.24) | 19 (4.6) |
| Netherlands | 15,587 | 567 (3.6) | 22 (0.14) | 6 (0.04) | 16 (0.10) |
| Poland | 37,701 | 373 (0.99) | 175 (0.46) | 170 (0.45) | 5 (0.01) |
| Portugal | 11,074 | 10 (0.09) | 6 (0.05) | 2 (0.02) | 4 (0.04) |
| Romania | 13,520 | 338 (2.5) | 26 (0.19) | 24 (0.18) | 2 (0.01) |
| Slovakia | 2,824 | 13 (0.46) | 5 (0.18) | 5 (0.18) | 0 |
| Slovenia | 2,480 | 363 (14.6) | 6 (0.24) | 2 (0.08) | 4 (0.16) |
| Spain | 38,465 | 876 (2.3) | 70 (0.18) | 21 (0.06) | 49 (0.13) |
| Sweden | 4,077 | 11 (0.27) | 9 (0.22) | 9 (0.22) | 0 |
| United Kingdom (Northern Ireland) | 7,571 | 6 (0.08) | 0 | – | – |
| EU Total (27 + XI) | 318,794 | 12,040 (3.8) | 901 (0.28) | 463 (0.14) | 438 (0.14) |
| Iceland | 687 | 9 (1.3) | 4 (0.58) | 0 | 4 (0.58) |
| Norway | 4,674 | 0 | 0 | – | – |
| Switzerland | 612 | 0 | 0 | – | – |
Regulation (EU) No 200/2012 requires that MSs separately report the results obtained by the FBOp and by the CA for broiler flocks. Most MSs (23) and the United Kingdom (Northern Ireland) reported both the overall merged results collected as part of the NCP and separate results from the CA and FBOp investigations, for their broiler flocks. One MS (Hungary) reported only data collected by the CA. Three MSs (Croatia, Lithuania and the Netherlands) did not comply. Considering all the data sent by the MSs providing data from both the CA and the FBOp, the EU flock prevalence of target Salmonella serovars based on CA sampling was 0.74% (N = 49,950), which was significantly higher than that based on FBOp sampling (0.24%, N = 286,741). The flock prevalence of target Salmonella serovars in broilers obtained by the CA was also significantly higher for Czechia, France, Germany, Greece, Italy, Poland and Spain. For the remaining reporting MSs, the differences between the results of the two types of samplers were not significant, or the sample sizes for one or both samplers were too small to be analysed (Table 23).
Table 23.
Comparisons of the prevalence of target Salmonella serovar‐positive broiler flocks, by sampler and by reporting MS, EU, 2021
| Country | Competent authority (CA) | Food business operator (FBOp) | p‐value (b) | Interpretation | ||||
|---|---|---|---|---|---|---|---|---|
| N tested flocks | N (%) flocks positive for target serovars | CI95 | N tested flocks | N (%) flocks positive for target serovars | CI95 | |||
| Austria | 115 | 0 | [0; 3.2] (a) | 6,041 | 9 (0.15) | [0.07; 0.28] | NS | |
| Belgium | 89 | 1 (1.1) | [0.03; 6.1] | 11,190 | 30 (0.27) | [0.18; 0.38] | NS | |
| Bulgaria | 260 | 0 | [0; 1.4] (a) | 8,222 | 0 | [0; 0.04] (a) | NS | |
| Cyprus | 9 | 0 | – | 1,033 | 0 | [0; 0.36] (a) | – | – |
| Czechia | 41 | 8 (19.5) | [8.8; 34.9] | 4,906 | 62 (1.3) | [0.97; 1.6] | < 0.001 | CA > FBOp |
| Denmark | 276 | 0 | [0; 1.3] (a) | 3,758 | 4 (0.11) | [0.03; 0.27] | NS | |
| Estonia | 642 | 0 | [0; 0.57] (a) | 254 | 0 | [0; 1.4] (a) | NS | |
| Finland | 498 | 0 | [0; 0.74] (a) | 3,583 | 0 | [0; 0.10] (a) | NS | |
| France | 559 | 14 (2.5) | [1.4; 4.2] | 65,212 | 400 (0.61) | [0.56; 0.68] | < 0.001 | CA > FBOp |
| Germany | 282 | 5 (1.8) | [0.58; 4.1] | 25,929 | 3 (0.01) | [0; 0.03] | < 0.001 | CA > FBOp |
| Greece | 87 | 3 (3.4) | [0.72; 9.7] | 7,743 | 0 | [0; 0.05] (a) | < 0.001 | CA > FBOp |
| Hungary (c) | 8,864 | 0 | [0; 0.04] (a) | – | – | – | – | – |
| Ireland | 82 | 0 | [0; 4.4] (a) | 3,416 | 8 (0.23) | [0.10; 0.46] | NS | |
| Italy | 419 | 2 (0.48) | [0.06; 1.7] | 29,228 | 5 (0.02) | [0.01; 0.04] | 0.004 | CA > FBOp |
| Latvia | 3 | 0 | [−] | 791 | 0 | [0; 0.47] (a) | – | – |
| Luxembourg | 3 | 1 (33.3) | [−] | 8 | 0 | [−] | – | – |
| Malta | 6 | 1 (16.7) | [−] | 407 | 19 (4.7) | [2.8; 7.2] | – | – |
| Poland | 41,409 | 296 (0.72) | [0.64; 0.80] | 35,535 | 34 (0.10) | [0.07; 0.13] | < 0.001 | CA > FBOp |
| Portugal | 111 | 0 | [0; 3.3] (a) | 11,060 | 6 (0.05) | [0.02; 0.12] | NS | |
| Romania | 355 | 1 (0.28) | [0.01; 1.6] | 13,165 | 25 (0.19) | [0.12; 0.28] | NS | |
| Slovakia | 36 | 0 | [0; 9.7] (a) | 2,807 | 5 (0.18) | [0.06; 0.42] | NS | |
| Slovenia | 41 | 0 | [0; 8.6] (a) | 2,439 | 6 (0.25) | [0.09; 0.54] | NS | |
| Spain | 449 | 8 (1.8) | [0.77; 3.5] | 38,405 | 64 (0.17) | [0.13; 0.21] | < 0.001 | CA > FBOp |
| Sweden | 139 | 0 | [0; 2.6] (a) | 4,077 | 9 (0.22) | [0.10; 0.42] | NS | |
| United Kingdom (Northern Ireland) | 39 | 0 | [0; 9.0] (a) | 7,532 | 0 | [0; 0.05] (a) | NS | |
| EU Total (27 + XI) | 54,814 | 340 (0.62) | [0.56; 0.69] | 286,741 | 689 (0.24) | [0.22; 0.26] | < 0.001 | CA > FBOp |
| EU Total (27 + XI) providing CA and FBOp data | 49,950 | 340 (0.74) | [0.66; 0.82] | 286,741 | 689 (0.24) | [0.22; 0.26] | < 0.001 | CA > FBOp |
–: Data not reported.
[−]: The confidence interval is not provided because of the small sample size.
: One‐sided, 97.5% confidence interval.
: p‐value: NS, not significant.
: Considering merged CA and FBOp data, Hungary notified three flocks positive for target serovars (Table 22), but in the context of separate reporting, data from the FBOp were not provided.
Breeding flocks of turkeys
For breeding turkeys, 12 MSs and two non‐MSs reported Salmonella NCP data. Hungary did not provide data to assess achievement of the reduction target. Considering merged data from the CA and FBOp, Salmonella was found in 48 of the 1,219 flocks tested (3.9%), compared with 5.1% in 2020 and 5.2% in 2019. In 2021, the prevalence of flocks positive for either of the two target Salmonella serovars was 0.49% (six positive flocks), compared with 0.48% and 0.30% in 2020 and 2019, respectively. Five of these positive flocks were notified by France and one by Germany (Table 24). France did not meet the reduction target of 1% or less of breeding flocks of turkeys positive for S. Enteritidis and/or S. Typhimurium (Table 24).
Table 24.
Salmonella in breeding flocks of turkeys during the production period (flock‐based data) in countries running control programmes in accordance with Regulation (EC) No 2160/2003, 2021
| Country | N tested flocks | N (%) positive for Salmonella spp. | N (%) positive for target serovars | N (%) positive samples for | |
|---|---|---|---|---|---|
| S. Enteritidis | S. Typhimurium | ||||
| Bulgaria | 2 | 0 | 0 | – | – |
| Croatia | 1 | 0 | 0 | – | – |
| Finland | 7 | 0 | 0 | – | – |
| France | 481 | 18 (3.7) | 5 (1.0) | 2 (0.42) | 3 (0.62) |
| Germany | 86 | 1 (1.2) | 1 (1.2) | 0 | 1 (1.2) |
| Greece | 9 | 1 (11.1) | 0 | – | – |
| Ireland | 4 | 2 (50.0) | 0 | – | – |
| Italy | 293 | 23 (7.8) | 0 | – | – |
| Poland | 178 | 0 | 0 | – | – |
| Slovakia | 68 | 0 | 0 | – | – |
| Spain | 86 | 3 (3.5) | 0 | – | – |
| Sweden | 4 | 0 | 0 | – | – |
| EU Total (27 + XI) | 1,219 | 48 (3.9) | 6 (0.49) | 2 (0.16) | 4 (0.33) |
| Iceland | 5 | 0 | 0 | – | – |
| Norway | 15 | 0 | 0 | – | – |
In accordance with Regulation (EC) No 1190/2012, Salmonella NCP monitoring data for breeding turkey flocks must be reported separately for sampling performed by the CA and the FBOp, in addition to the overall merged data. Eleven MSs complied with this requirement, whereas Croatia did not report separate results from the CA and the FBOp and Hungary only reported results from the CA. Considering all the data sent by those MSs that provided data from both samplers (CA and FBOp), the EU prevalence of target Salmonella serovar‐positive flocks based on CA sampling was 0.70% (N = 711), which was significantly higher than that based on FBOp sampling (0.08%, N = 1,210). This finding was markedly influenced by the data reported by one single MS, France (Table 25), which was the only MS with a reported prevalence of target Salmonella serovar‐positive flocks by CA significantly higher than that reported by FBOp.
Table 25.
Comparisons of the prevalence of target Salmonella serovar‐positive flocks of breeding turkeys, by sampler and by reporting MS, EU, 2021
| Country | Competent authority (CA) | Food business operator (FBOp) | p‐value (b) | Interpretation | ||||
|---|---|---|---|---|---|---|---|---|
| N tested flocks | N (%) flocks positive for target serovars | CI95 | N tested flocks | N (%) flocks positive for target serovars | CI95 | |||
| Bulgaria | 1 | 0 | [−] | 1 | 0 | [−] | – | – |
| Finland | 7 | 0 | [−] | 7 | 0 | [−] | – | – |
| France | 188 | 4 (2.1) | [0.58; 5.4] | 481 | 1 (0.21) | [0; 1.2] | 0.023 | CA > FBOp |
| Germany | 74 | 1 (1.4) | [0.03; 7.3] | 83 | 0 | [0; 4.3] (a) | NS | |
| Greece | 5 | 0 | [−] | 9 | 0 | [−] | – | – |
| Hungary | 105 | 0 | [0; 3.5] (a) | – | – | – | – | – |
| Ireland | 4 | 0 | [−] | 4 | 0 | [−] | – | – |
| Italy | 163 | 0 | [0; 2.2] (a) | 293 | 0 | [0; 1.3] (a) | NS | |
| Poland | 154 | 0 | [0; 2.4] (a) | 178 | 0 | [0; 2.1] (a) | NS | |
| Slovakia | 61 | 0 | [0; 5.9] (a) | 68 | 0 | [0; 5.3] (a) | NS | |
| Spain | 50 | 0 | [0; 7.1] (a) | 82 | 0 | [0; 4.4] (a) | NS | |
| Sweden | 4 | 0 | [−] | 4 | 0 | [−] | – | – |
| EU Total (27 + XI) | 816 | 5 (0.61) | [0.20; 1.4] | 1,210 | 1 (0.08) | [0; 0.46] | 0.042 | CA > FBOp |
| EU Total (27 + XI) providing CA and FBOp data | 711 | 5 (0.70) | [0.23; 1.6] | 1,210 | 1 (0.08) | [0; 0.46] | 0.029 | CA > FBOp |
–: Data not reported.
[−]: The confidence interval is not provided because of the small sample size.
: One‐sided, 97.5% confidence interval.
: p‐value: NS, not significant.
Flocks of fattening turkeys
For fattening turkey flocks, 22 MSs, the United Kingdom (Northern Ireland) and three non‐MSs provided data. In the EU in 2021, considering merged data from the CA and FBOp, Salmonella was found in 3,012 (9.1%) fattening turkey flocks, compared with 8.8% and 5.8% in 2020 and 2019, respectively. The EU prevalence of flocks positive for either of the two target Salmonella serovars was 0.31%, compared with 0.38% in 2020 and 0.24% in 2019. Belgium did not meet the reduction target of 1% (Figure 4), as in previous years. The EU flock prevalence was higher for S. Typhimurium (0.22%, 73 flocks) than for S. Enteritidis (0.09%, 28 flocks), with 63.0% and 53.6% of the flocks positive for S. Typhimurium and S. Enteritidis being reported by France, which was similar to the situation in previous years (Table 26).
Table 26.
Salmonella in fattening flocks of turkeys before slaughter (flock‐based data) in countries running control programmes, 2021
| Country | N tested flocks | N (%) positive for Salmonella spp. | N (%) positive for target serovars | N (%) positive samples for | |
|---|---|---|---|---|---|
| S. Enteritidis | S. Typhimurium | ||||
| Austria | 470 | 14 (3.0) | 3 (0.64) | 3 (0.64) | 0 |
| Belgium | 188 | 8 (4.3) | 6 (3.2) | 1 (0.53) | 5 (2.7) |
| Bulgaria | 4 | 0 | 0 | – | – |
| Croatia | 439 | 53 (12.1) | 4 (0.91) | 2 (0.46) | 2 (0.46) |
| Cyprus | 7 | 3 (42.9) | 0 | – | – |
| Czechia | 260 | 2 (0.77) | 1 (0.38) | 1 (0.38) | 0 |
| Denmark | 115 | 0 | 0 | – | – |
| Finland | 302 | 0 | 0 | – | – |
| France | 6,219 | 262 (4.2) | 61 (0.98) | 15 (0.24) | 46 (0.74) |
| Germany | 4,538 | 16 (0.35) | 10 (0.22) | 2 (0.04) | 8 (0.18) |
| Greece | 50 | 2 (4.0) | 0 | – | – |
| Hungary | 1,874 | 5 (0.27) | 5 (0.27) | 1 (0.05) | 4 (0.21) |
| Ireland | 553 | 1 (0.18) | 0 | – | – |
| Italy | 5,467 | 1,881 (34.4) | 1 (0.02) | 0 | 1 (0.02) |
| Netherlands | 567 | 11 (1.9) | 1 (0.18) | 1 (0.18) | 0 |
| Poland | 5,980 | 14 (0.23) | 4 (0.07) | 1 (0.02) | 3 (0.05) |
| Portugal | 1,191 | 5 (0.42) | 1 (0.08) | 0 | 1 (0.08) |
| Romania | 274 | 1 (0.36) | 0 | – | – |
| Slovakia | 98 | 1 (1.0) | 0 | – | – |
| Slovenia | 104 | 9 (8.7) | 1 (0.96) | 0 | 1 (0.96) |
| Spain | 4,041 | 724 (17.9) | 3 (0.07) | 1 (0.03) | 2 (0.05) |
| Sweden | 175 | 0 | 0 | – | – |
| United Kingdom (Northern Ireland) | 55 | 0 | 0 | – | – |
| EU Total (27 + XI) | 32,971 | 3,012 (9.1) | 101 (0.31) | 28 (0.09) | 73 (0.22) |
| Iceland | 31 | 0 | 0 | – | – |
| Norway | 235 | 0 | 0 | – | – |
| Switzerland | 38 | 0 | 0 | – | – |
Salmonella NCP monitoring data for fattening turkey flocks must be reported separately for sampling performed by the CA and the FBOp, in addition to the overall merged results, as defined in Regulation (EU) No 1190/2012. Nineteen MSs and the United Kingdom (Northern Ireland) complied with the requirement, whereas the two MSs Croatia and the Netherlands did not report separate results from the CA and the FBOp and the MS Hungary only reported results from the CA. Considering all the data sent by those MSs that provided data from both samplers (CA and FBOp), the EU prevalence of target Salmonella serovar‐positive flocks based on CA sampling was 1.3% (N = 1,161), which was significantly higher than that based on FBOp sampling (0.26%, N = 29,840). The same finding was also seen for data provided by Germany, Italy, Poland and Spain (Table 27).
Table 27.
Comparisons of the prevalence of target Salmonella serovar‐positive flocks of fattening turkeys, by sampler and by reporting MS, EU, 2021
| Country | Competent authority (CA) | Food business operator (FBOp) | p‐value (b) | Interpretation | ||||
|---|---|---|---|---|---|---|---|---|
| N tested flocks | N (%) flocks positive for target serovars | CI95 | N tested flocks | N (%) flocks positive for target serovars | CI95 | |||
| Austria | 22 | 0 | [0; 15.4] (a) | 462 | 3 (0.65) | [0.13; 1.9] | NS | |
| Belgium | 4 | 1 (25.0) | – | 188 | 5 (2.7) | [0.87; 6.1] | – | – |
| Bulgaria | 1 | 0 | [−] | 3 | 0 | [−] | – | – |
| Cyprus | 3 | 0 | [−] | 6 | 0 | [−] | – | – |
| Czechia | 16 | 0 | [0; 20.6] (a) | 250 | 1 (0.40) | [0.01; 2.2] | NS | |
| Denmark | 106 | 0 | [0; 3.4] (a) | 115 | 0 | [0; 3.2] (a) | NS | |
| Finland | 40 | 0 | [0; 8.8] (a) | 262 | 0 | [0; 1.4] (a) | NS | |
| France | 150 | 2 (1.3) | [0.16; 4.7] | 6,171 | 59 (0.96) | [0.73; 1.2] | NS | |
| Germany | 160 | 6 (3.8) | [1.4; 8.0] | 4,538 | 5 (0.11) | [0.04; 0.26] | < 0.001 | CA > FBOp |
| Greece | 7 | 0 | [−] | 49 | 0 | [0; 7.3] (a) | – | – |
| Hungary (c) | 1,874 | 0 | [0; 0.20] (a) | – | – | – | – | – |
| Ireland | 16 | 0 | [0; 20.6] (a) | 553 | 0 | [0; 0.66] (a) | NS | |
| Italy | 111 | 1 (0.90) | [0.02; 4.9] | 5,462 | 0 | [0; 0.07] (a) | 0.020 | CA > FBOp |
| Poland | 350 | 3 (0.86) | [0.18; 2.5] | 5,891 | 1 (0.02) | [0; 0.10] | < 0.001 | CA > FBOp |
| Portugal | 14 | 0 | [0; 23.2] (a) | 1,191 | 1 (0.08) | [0; 0.47] | NS | |
| Romania | 28 | 0 | [0; 12.3] (a) | 246 | 0 | [0; 1.5] (a) | NS | |
| Slovakia | 7 | 0 | [−] | 98 | 0 | [0; 3.7] (a) | – | – |
| Slovenia | 6 | 0 | [−] | 98 | 1 (1.0) | [0.03; 5.6] | – | – |
| Spain | 77 | 2 (2.6) | [0.32; 9.1] | 4,031 | 2 (0.05) | [0.01; 0.18] | 0.002 | CA > FBOp |
| Sweden | 39 | 0 | [0; 9.0] (a) | 175 | 0 | [0; 2.1] (a) | NS | |
| United Kingdom (Northern Ireland) | 4 | 0 | [−] | 51 | 0 | [0; 7.0] (a) | – | – |
| EU Total (27 + XI) | 3,035 | 35 (0.49) | [0.28; 0.81] | 29,840 | 78 (0.26) | [0.21; 0.33] | 0.011 | CA > FBOp |
| EU Total (27 + XI) providing CA and FBOp data | 1,161 | 15 (1.3) | [0.72; 2.1] | 29,840 | 78 (0.26) | [0.21; 0.33] | < 0.001 | CA > FBOp |
–: Data not reported.
[−]: The confidence interval is not provided because of the small sample size.
: One‐sided, 97.5% confidence interval.
: p‐value: NS, not significant.
: Considering merged CA and FBOp results, Hungary notified five flocks positive for target serovars (Table 26), but in the context of separate reporting, data from the FBOp were not provided.
Salmonella prevalence trends in poultry flocks
Trends in the estimated EU prevalence of poultry flocks positive for Salmonella spp. and target Salmonella serovars, for different poultry populations, since the implementation of the EU‐wide 2007–2021 NCP, are displayed in (Figure 5). In 2020, data provided by the United Kingdom were not considered, whereas in 2021, data provided by the United Kingdom (Northern Ireland) were taken into account.
Figure 5.
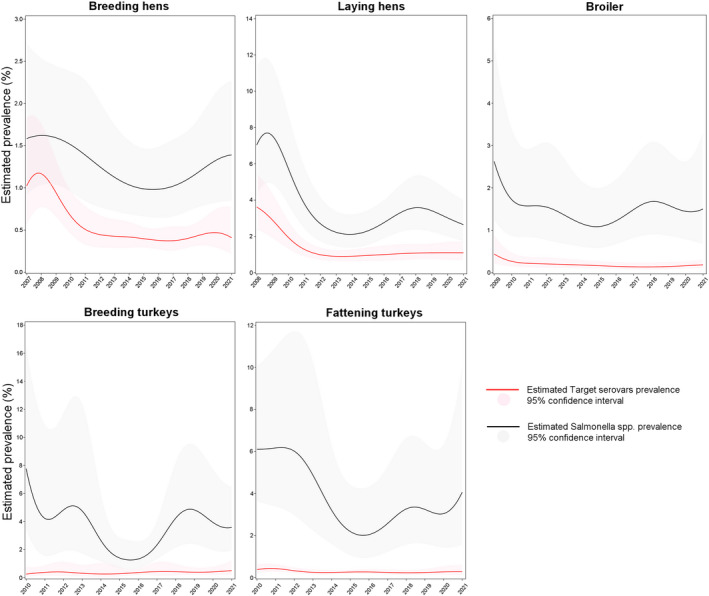
Trend in the estimated prevalence of poultry flocks positive for Salmonella spp. and target Salmonella serovars, at EU level for different poultry populations, 2007–2021
In the file ‘Salmonella poultry outcome trend analyses’ published on the EFSA Knowledge Junction on Zenodo here, the EU percentages of positive flocks for Salmonella, target and non‐target Salmonella serovars and S. Enteritidis over time are shown and compared for each poultry population covered by the NCP. Moreover, figures show the modelling of prevalence trends for Salmonella and target Salmonella serovars in poultry flocks. Detailed outputs of trend analyses (at subject level and population level) are reported.
The apparent discrepancy between the proportion of positive flocks (both for target Salmonella serovars and for Salmonella, as described in the previous paragraphs) and the estimated prevalence shown below is due to the fact that the first value is the ratio of all positive to all tested flocks, whereas the estimated prevalence is obtained by modelling the ratio of positive to all tested flocks in each reporting country, taking into account inter‐country variability and the correlation between years.
Breeding flocks of Gallus gallus: 2007–2021
Since the beginning of the NCP, there has been an overall decreasing trend for the prevalence of breeding Gallus gallus flocks positive for target serovars (Figure 5). The prevalence estimated by modelling decreased from 1% CI95 [0.57; 1.8] in 2007 to 0.38% CI95 [0.27; 0.52] in 2016, when the estimated prevalence reached its lowest value. Over the next 5 years, the estimated prevalence slightly increased, reaching 0.41% CI95 [0.22; 0.76] in 2021, but this increase was not statistically significant.
The estimated EU prevalence of Salmonella‐positive breeding flocks was 1.6% CI95 [0.92; 2.7] in 2007 and then decreased, reaching the minimum value of 0.98% CI95 [0.65; 1.5] in 2016. During the following years, it increased slightly to reach 1.4% CI95 [0.85; 2.3] in 2021, but this increase was not statistically significant.
Flocks of laying hens: 2008–2021
Since the beginning of the NCP, there has been an overall decreasing trend for the prevalence of flocks positive for target serovars (Figure 5). The prevalence estimated by modelling was 3.6% CI95 [2.4; 5.4] in 2008 and decreased to reach the lowest value of 0.90% CI95 [0.65; 1.2] in 2013, with a steep downturn. From 2014 onwards, it increased slightly and stabilised at 1.1% CI95 [0.71; 1.7] in 2021. This prevalence was not significantly different from that of the previous 2 years or compared with the lowest prevalence estimated in 2013.
The estimated EU prevalence of Salmonella spp. in laying hen flocks was 7.0% CI95 [4.3; 11.3] in 2008 and decreased to 2.1% CI95 [1.4; 3.2] in 2014, with a steep downturn. During the following years, it increased and reached 2.6% CI95 [1.7; 4.0] in 2021. In 2021, the estimated Salmonella prevalence in laying hen flocks was not significantly different from that in the previous 2 years or compared with the lowest prevalence estimated in 2014.
Broiler flocks: 2009–2021
From the beginning of the NCP, the flock prevalence of target serovars estimated by the model steeply decreased in the first time interval (until 2011) and then further decreased (Figure 5). The estimated prevalence was 0.44% CI95 [0.22; 0.87] in 2009 and decreased to 0.18% CI95 [0.11; 0.30] in 2021. This prevalence was not significantly different from that during the previous 2 years.
The EU prevalence of Salmonella spp.‐positive broiler flocks estimated by modelling decreased from 2.6% CI95 [1.3; 5.3] in 2009 to 1.1% CI95 [0.59; 2.0] in 2015 and then increased to 1.5% CI95 [0.68; 3.3] in 2021. Nevertheless, the estimated EU prevalence of Salmonella‐positive broiler flocks in 2021 was not significantly different to that of the previous 2 years or that of 2015, when the estimated prevalence reached its lowest value.
Breeding turkey flocks: 2010–2021
From the beginning of the NCP, the prevalence of target Salmonella serovar‐positive breeding turkey flocks fluctuated between 0.26% CI95 [0.08; 0.78] in 2010 and 0.51% CI95 [0.22; 1.2] (Figure 5) in 2021. This trend may have been affected by the low number of MSs with breeding turkey flocks positive for target Salmonella serovars.
With regard to EU‐level Salmonella spp.‐positive breeding turkey flocks, after an initial fluctuation in the EU prevalence from 7.8% CI95 [3.5; 16.4] in 2010 to 1.3% CI95 [0.69; 2.6] in 2016, when this estimated prevalence reached the lowest value seen in the entire study period, the estimated prevalence increased over time to reach 3.6% CI95 [2.0; 6.4] in 2021. This estimated prevalence in 2021 was not significantly different from that of the previous 2 years, but it was significantly higher than the estimated prevalence in 2016 (p‐value = 0.0261).
Fattening turkey flocks: 2010–2021
The estimated flock prevalence of target serovars was 0.38% CI95 [0.23; 0.62] in 2010; it decreased to 0.25% CI95 [0.17; 0.35] in 2014 and then increased to 0.28% CI95 [0.13; 0.59] in 2021, after some small temporal fluctuations (Figure 5). Nevertheless, there were no significant differences in the estimated prevalence of the target Salmonella serovars in EU fattening turkey flocks in the last 2 years or compared with the lowest prevalence estimated in 2014.
For this poultry category, after an initial fluctuation in the EU prevalence of Salmonella spp.‐positive flocks from 6.1% CI95 [3.6; 10.0] in 2010 to 2.0% CI95 [0.96; 4.3] in 2016, when the estimated prevalence reached its lowest value, the prevalence increased to 4.1% CI95 [1.6; 10.0] in 2021. Nevertheless, the prevalence in 2021 was not significantly different from that in the previous 2 years or from the lowest estimated prevalence in 2016.
Salmonella data for other animals
Considering all the collected data on the presence of Salmonella in different categories of animal species in the EU with the exception of data collected in the framework of NCPs for poultry, 70,326 samples collected from animals of various species were reported by 20 MSs (Table 28). The overall prevalence of Salmonella‐positive samples was 4.0% (N = 2,843). The highest number of samples was from cattle (bovine animals) (N = 26,412 notified by 14 MSs) and 3.5% were reported as being positive for Salmonella. The highest prevalence of positive samples were notified for small ruminants (9.0% for nine MSs) and wild boar (6.6% for three MSs). For pigs, based on data reported by 17 MSs, the prevalence of positive samples was 2.9% (520 positive samples, N = 17,927). For solipeds, 2.9% of samples were positive for Salmonella (18 positive samples) and were notified by seven MSs.
Table 28.
Summary of Salmonella statistics related to major animal species, reporting MSs and non‐MS countries, 2021
| Animals | EU (a) MSs | Non‐MS countries | ||||||
|---|---|---|---|---|---|---|---|---|
| N reporting countries | N tested animals | Positive animals | N reporting countries | N tested animals | Positive animals | |||
| N | % | N | % | |||||
| Birds (b) | 12 | 13,150 | 480 | 3.7 | 3 | 336 | 21 | 6.3 |
| Cats | 5 | 1,072 | 50 | 4.7 | 2 | 559 | 20 | 3.6 |
| Cattle (bovine animals) | 14 | 26,412 | 920 | 3.5 | 4 | 5,390 | 242 | 4.5 |
| Dogs | 8 | 1,995 | 53 | 2.7 | 2 | 1,082 | 46 | 4.3 |
| Pigs | 17 | 17,927 | 520 | 2.9 | 3 | 4,517 | 151 | 3.3 |
| Small ruminants (c) | 9 | 5,622 | 505 | 9.0 | 3 | 262 | 28 | 10.7 |
| Solipeds | 7 | 616 | 18 | 2.9 | 2 | 364 | 11 | 3.0 |
| Wild boar | 3 | 1,175 | 78 | 6.6 | 1 | 287 | 13 | 4.5 |
| Wild ungulates (d) | 3 | 388 | 2 | 0.52 | 1 | 1 | 0 | 0 |
| Others/Not specified (e) | 13 | 1,969 | 217 | 11.0 | 2 | 422 | 68 | 16.1 |
| Total | 20 | 70,326 | 2,843 | 4.0 | 5 | 13,220 | 600 | 4.5 |
MSs: Member States.
: Data from the United Kingdom (Northern Ireland) are taken into account for 2021. In accordance with the agreement on the withdrawal of the United Kingdom from the EU, and in particular with the Protocol on Ireland/Northern Ireland, the EU requirements on data sampling are also applicable to Northern Ireland.
: Included in this category: geese, canaries, ducks, parrots, pheasants, Gallus gallus (fowl), owls, partridges, turkeys, doves, falcons, gulls, magpies, night herons, ostriches, peafowl, starlings, swans, poultry (unspecified), guinea fowl, budgerigars, songbirds.
: Included in this category: sheep and goats.
: Included in this category: deer and Cantabrian chamois.
: Included in this category: shellfish, quails, fish, monkeys, pigeons, badgers, foxes, other animals, Leporidae, alpacas, antelopes, Barbary sheep, bats, bears, bison, camels, capybaras, chinchillas, dolphins, dromedaries, ferrets, guinea pigs, hedgehogs, jays, kangaroos, leopards, lions, lynxes, marine mammals, martens, mice, minks, mouflon, other carnivores, otter, passerines, unspecified, Psittacidae, raccoon dogs, rats, reptiles, rodents, seals, snakes, squirrels, Alpine ibex, turtles, water buffalo, wildcats (Felis silvestris), wolves, zoo animals, all, beavers, raccoons, ratites (ostriches, emus, nandus), other ruminants, penguins, moose, reindeer.
For a further interactive look at Salmonella monitoring results, dashboards have been created (different filters can be applied to query the data) ( link ).
2.4.5. Salmonella in feed
In 2021, the overall EU‐level occurrence of Salmonella‐positive samples in any ‘animal and vegetable‐derived feed’ was 0.55% (N = 71,965). In compound feed (finished feed for animals), the prevalence of Salmonella‐positive units was 0.40% (N = 15,463) for samples from poultry, 0.58% for samples from cattle (N = 2,909) and 0.36% for samples from pigs (N = 4,123). There were no noticeable isolates in 2021, except for fish, where in over 134 tested units, the prevalence of Salmonella‐positive samples was 1.5%, and for rabbits, where in 55 tested units, the prevalence was 1.8%. Regarding compound feedingstuffs for fur animals, Salmonella was reported in 76 out of 176 tested units (43.2%) with all the positive samples having been detected in Poland. Lastly, the prevalence of Salmonella‐positive sampling units for pet food was 1.0% (N = 3,196). For non‐specified compound feedingstuffs, Salmonella was reported in 0.23% of over 8,222 tested units, mainly by Sweden.
2.4.6. Salmonella serovars in humans, food and animals
Humans
Serovars among all confirmed salmonellosis cases
For humans, information on Salmonella serovars was available for 84.6% of the total number of confirmed cases (50,817 cases out of 60,050) for 25 MSs (Bulgaria, Luxembourg and Spain did not report serovar data). Data included all cases reported with serovar information regardless of travel status. The proportion of cases of Salmonella with serovar data available decreased compared to 2020 (87.9%). As in previous years, the three most commonly reported Salmonella serovars in 2021 were S. Enteritidis (54.6%), S. Typhimurium (11.4%) and monophasic S. Typhimurium (1,4,[5],12:i:‐) (8.8%), representing 74.8% of the 50,817 confirmed human cases. S. Enteritidis increased by 15.5%, when considering the absolute number of cases of this serovar, but it increased by only 2.8% compared with 2020 with respect to the total number of isolates. Monophasic S. Typhimurium (1,4,[5],12:i:‐) apparently decreased by 4.3% compared with 2020, when considering the absolute number of cases of this serovar in these years, but the proportion decreased by 1.3% compared with 2020 with respect to the total number of isolates in the relative years.
The proportions of these three serovars, mainly driven by S. Enteritidis, increased during the last 3 years: from 70.5% in 2019 to 72.2% in 2020 and to 74.8% in 2021. The fourth and fifth serovars, S. Infantis and S. Derby, were at the same levels as in 2020 and 2019 (Table 29).
Table 29.
Distribution of reported confirmed cases of human salmonellosis in the EU, 2019–2021, for the 20 most frequent Salmonella serovars in 2021
| Serovar | 2021 | 2020 | 2019 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Cases | MSs | % | Cases | MSs | % | Cases | MSs | % | |
| Enteritidis | 27,734 | 24 | 54.6 | 24,008 | 25 | 51.8 | 39,451 | 27 | 50.4 |
| Typhimurium | 5,781 | 24 | 11.4 | 5,337 | 25 | 11.5 | 9,288 | 27 | 11.9 |
| Monophasic Typhimurium 1.4.[5]0.12:i:‐ | 4,495 | 14 | 8.8 | 4,697 | 16 | 10.1 | 6,432 | 18 | 8.2 |
| Infantis | 1,019 | 24 | 2.0 | 1,064 | 23 | 2.30 | 1,912 | 26 | 2.4 |
| Derby | 474 | 17 | 0.93 | 525 | 20 | 1.13 | 719 | 23 | 0.92 |
| Coeln | 463 | 15 | 0.91 | 324 | 19 | 0.70 | 441 | 18 | 0.56 |
| Braenderup | 373 | 15 | 0.73 | 93 | 12 | 0.20 | 292 | 18 | 0.37 |
| Napoli | 352 | 12 | 0.69 | 412 | 12 | 0.89 | 493 | 18 | 0.63 |
| Chester | 316 | 12 | 0.62 | 129 | 13 | 0.28 | 340 | 17 | 0.43 |
| Newport | 311 | 20 | 0.61 | 336 | 21 | 0.73 | 846 | 24 | 1.08 |
| Montevideo | 219 | 11 | 0.43 | 102 | 14 | 0.22 | 244 | 19 | 0.31 |
| Brandenburg | 213 | 15 | 0.42 | 309 | 16 | 0.67 | 288 | 17 | 0.37 |
| Bovismorbificans | 205 | 14 | 0.40 | 337 | 15 | 0.73 | 452 | 19 | 0.58 |
| Oranienburg | 193 | 14 | 0.38 | 90 | 14 | 0.19 | 236 | 19 | 0.30 |
| Stanley | 190 | 16 | 0.37 | 208 | 21 | 0.45 | 509 | 19 | 0.65 |
| Virchow | 170 | 16 | 0.33 | 121 | 16 | 0.26 | 469 | 21 | 0.60 |
| Dublin | 148 | 10 | 0.29 | 196 | 9 | 0.42 | 207 | 13 | 0.26 |
| Anatum | 143 | 11 | 0.28 | 57 | 7 | 0.12 | 134 | 15 | 0.17 |
| Rissen | 139 | 14 | 0.27 | 114 | 13 | 0.25 | 252 | 19 | 0.32 |
| Agona | 137 | 16 | 0.27 | 157 | 18 | 0.34 | 490 | 20 | 0.63 |
| Other | 7,742 | 15.2 | 7,725 | 16.7 | 14,787 | 18.9 | |||
| Total (a) , (b) | 50,817 | 24 | 100 | 46,341 | 25 | 100 | 78,282 | 27 | 100 |
MSs: Member States.
: Source(s): 2021 – 25 MSs: Austria, Belgium, Cyprus, Czechia, Germany, Denmark, Estonia, Greece, Finland, France, Croatia, Hungary, Ireland, Italy, Lithuania, Latvia, Malta, Netherlands, Poland, Portugal, Romania, Sweden, Slovenia, Slovakia. 2020 – 25 MSs: Austria, Belgium, Cyprus, Czechia, Germany, Denmark, Estonia, Greece, Finland, France, Croatia, Hungary, Ireland, Italy, Latvia, Luxembourg, Malta, Netherlands, Poland, Portugal, Romania, Spain, Sweden, Slovenia, Slovakia. 2019 – 27 MSs: Austria, Belgium, Cyprus, Czechia, Germany, Denmark, Estonia, Greece, Finland, France, Croatia, Hungary, Ireland, Italy, Lithuania, Latvia, Luxembourg, Malta, Netherlands, Poland, Portugal, Romania, Spain, Sweden, Slovenia, Slovakia the United Kingdom.The total number of confirmed cases with information on Salmonella serovars includes cases classified as S. enterica, S. SubspI, S. SubspIIIb, S. SubspIIIa, S. SubspIV, S. SubspII, S. SubspV, S. SubspVI and other.
: Data from the United Kingdom are taken into account for the year 2019, because the United Kingdom was an EU MS, but it became a third country on 1 February 2020.
In 2021, the proportion of cases of the S. Coeln serovar increased by 0.21% and 0.35% compared with 2020 and 2019, respectively; it increased by 42.9% compared with 2020 while it decreased by 4.9% compared with 2019, when considering the absolute number of cases of this serovar in these years. For the first time since 2019, four serovars, S. Braenderup, S. Montevideo, S. Oranienburg and S. Rissen, entered the top 20 list of the most frequent serovars in 2021. In particular, the proportion of cases of S. Braenderup increased by 0.53% and 0.36% compared with 2020 and 2019, respectively; the absolute number of cases increased by 301% and 27.7% compared with 2020 and 2019, respectively. The proportion of S. Montevideo cases increased by 0.21% and 0.12% compared with 2020 and 2019, respectively, but the absolute number of cases increased by 114.7% compared with 2020 and decreased by 10.2% compared with 2019. Furthermore, S. Virchow entered the top 20 list of the most frequent serovars in 2019.
Serovars acquired in the EU
To estimate the impact of Salmonella infections acquired at the EU level, serovar data were analysed for domestic and travel‐associated cases in which the probable country of infection was an EU MS. Information on Salmonella serovars with travel data was available from 23 MSs, representing 72.0% of cases with known serovar data in 2021. Most cases (97.7%) with a known serovar and with travel data were infected within the EU. For the travel‐associated cases, the most frequently reported travel destinations in the EU were Spain (16.6%), Italy (15%), Greece (11.4%) and Poland (10.2%). For the reported cases of human salmonellosis acquired in the EU, S. Enteritidis dominated and 64.6% of these reported cases were infected with this serovar. S. Enteritidis, S. Typhimurium and monophasic S. Typhimurium (1,4,[5],12:i:‐) together represented 79.1% of the confirmed human cases acquired in the EU in 2021 (Table 30). S. Enteritidis cases were predominantly (85.2%) infected within the EU. The proportions of cases of S. Enteritidis, S. Typhimurium and its monophasic variant (1,4,[5],12:i:‐) were at the same level as in 2019–2020, when considering the number of these serovars vs. the total number of reported serovars. Also, S. Infantis and S. Derby remained at the same level as in 2020.
Table 30.
Distribution of reported confirmed cases of human salmonellosis acquired in the EU, 2019–2021, for the six most frequent Salmonella serovars in 2021
| Serovar | 2021 | 2020 | 2019 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Cases | MSs | % | Cases | MSs | % | Cases | MSs | % | |
| Enteritidis | 23,634 | 23 | 64.6 | 21,203 | 23 | 63.1 | 32,010 | 24 | 61.6 |
| Typhimurium | 4,027 | 23 | 11.0 | 3,702 | 22 | 11.0 | 6,044 | 24 | 11.6 |
| Monophasic Typhimurium 1.4.[5]0.12:I:‐ | 1,269 | 14 | 3.5 | 1,530 | 16 | 4.6 | 2,688 | 17 | 5.2 |
| Infantis | 633 | 23 | 1.7 | 716 | 21 | 2.1 | 1,215 | 24 | 2.3 |
| Derby | 239 | 16 | 0.65 | 260 | 17 | 0.77 | 396 | 20 | 0.76 |
| Coeln | 315 | 14 | 0.86 | 201 | 17 | 0.60 | 270 | 15 | 0.52 |
| Other | 6,462 | – | 17.7 | 6,009 | – | 17.9 | 9,378 | – | 18.0 |
| Total (a) | 36,579 | 23 | 100 | 33,621 | 23 | 100 | 52,001 | 24 | 100 |
MSs: Member States.
: Data from the United Kingdom are taken into account for the year 2019, because the United Kingdom was an EU MS, but it became a third country on 1 February 2020.
A seasonal trend was observed for confirmed S. Enteritidis infections acquired in the EU in 2017–2021, with more cases reported during summer months. A decrease in cases was observed in 2020, probably due to the COVID‐19 pandemic. Notwithstanding, the overall trend for S. Enteritidis in 2017–2021 did not show any statistically significant increase or decrease (Figure 6). Belgium, Estonia and Greece showed a significantly decreasing (p < 0.05) trend in S. Enteritidis infections within the EU over the last 5 years (2017–2021). A significant increasing trend (p < 0.05) was observed in France and Malta.
Figure 6.
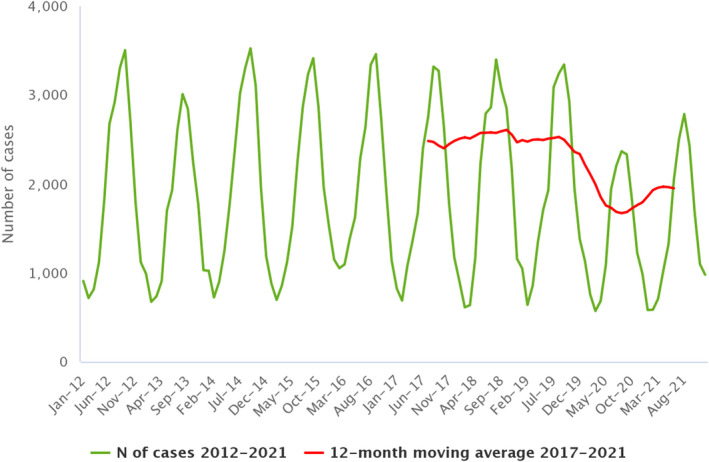
- Source: Austria, Belgium, Czechia, Germany, Denmark, Estonia, Greece, Finland, Hungary, Ireland, Italy, Latvia, Malta, the Netherlands, Portugal, Sweden and Slovakia.
Food and animals
Descriptive analyses were undertaken using serotyped isolates from food and animals. In this context, only isolates related to the most common food‐producing animal species and food matrices thereof were considered and were aggregated into the following categories for further analysis: ‘broiler flocks – broiler meat’, ‘laying hen flocks – eggs’, ‘fattening turkey flocks – turkey meat’, ‘fattening pigs – pig meat’ and ‘cattle – bovine meat’. Overall, a selection of 20,020 serotyped Salmonella isolates meeting the mentioned inclusion criteria were obtained (Table 31).
Table 31.
Distribution of Salmonella isolates (number and percentage of positive sampling units) with and without serotype identification among the different selected sources (food and animals), EU, 2021
| Source | Salmonella positive‐sampling units without serotyped isolates | Salmonella positive‐sampling units with serotyped isolates | ||
|---|---|---|---|---|
| N | % | N | % | |
| Broilers | 973 | 14.0 | 11,143 | 55.7 |
| Broiler meat | 2,905 | 41.7 | 3,206 | 16 |
| Cattle | 30 | 0.43 | 124 | 0.62 |
| Cattle meat | 138 | 2.0 | 261 | 1.3 |
| Pigs | 71 | 1.0 | 394 | 2.0 |
| Pig meat | 1,545 | 22.2 | 1,114 | 5.6 |
| Turkeys | 663 | 9.5 | 2,373 | 11.9 |
| Turkey meat | 395 | 5.7 | 203 | 1.0 |
| Layers | 198 | 2.8 | 1,166 | 5.8 |
| Eggs and egg products | 43 | 0.62 | 36 | 0.18 |
| Total | 6,961 | 100 | 20,020 | 100 |
The large majority of the serotyped isolates were from ‘broilers’ (both animals (55.7%) and food (16.0%)). ‘Turkey’ sources (animals and food) accounted for 12.9% of the serotyped isolates, while ‘pig’ and ‘laying hen’ sources represented 7.6% and 6.0% of the serotyped isolates, respectively. Serotyped isolates from ‘cattle' sources made up about 1.9% of the total.
Isolates belonging to the five most frequently reported Salmonella serovars involved in cases of human salmonellosis acquired in the EU in 2021 were considered for further analysis, which were S. Enteritidis, S. Typhimurium, monophasic S. Typhimurium (1,4,[5],12:i:‐), S. Infantis and S. Derby. From the above‐mentioned food‐animal sources a total of 20,020 serotyped isolates were reported, from which S. Infantis accounted for 33.9%, S. Enteritidis for 8.2%, S. Typhimurium for 3.8%, monophasic S. Typhimurium (1,4,[5],12:i:‐) for 3.2% and S. Derby for 2.2%.
A Sankey diagram (Figure 7) illustrates how these top five EU‐level Salmonella serovars involved in human salmonellosis cases acquired in the EU were linked with the major animal species.
Figure 7.
- The left side of the diagram shows the five most commonly reported Salmonella serovars involved in human salmonellosis cases acquired in the EU: S. Enteritidis (blue), S. Infantis (green), S. Typhimurium (orange), monophasic S. Typhimurium (1,4,[5],12:i:‐) (violet) and S. Derby (magenta). Animal and food data from the same source were merged: ‘broiler’ includes isolates from broiler flocks and broiler meat, ‘bovine' includes isolates from bovines for meat production and from bovine meat, ‘pig’ includes isolates from fattening pigs and pig meat, ‘turkey’ includes isolates from fattening turkey flocks and turkey meat and ‘layers’ includes isolates from laying hen flocks and eggs. The right side shows the five sources considered (broilers, bovines, pigs, turkeys and layers). The width of the coloured bands linking the sources and serovars is proportional to the percentage of isolates of each serovar from each source.
S. Enteritidis was primarily related to ‘broiler’ sources (70.0% of the S. Enteritidis isolates were from broiler flocks and meat) and also to ‘layers and eggs’ (26.0%). S. Typhimurium isolates were distributed among the different sources, although they were mainly related to ‘broiler’ and ‘pig’ sources (43.2% and 29.7% of the isolates were from these sources respectively), followed by ‘laying hen’ and ‘turkey’ sources (15.0% and 9.1%). Monophasic S. Typhimurium (1,4,[5],12:i:‐) was related mainly to ‘pig’ (65.4%) and secondly to ‘broiler’ (21.2%) sources. S. Infantis was strictly related to ‘broiler’ sources (95.2%). S. Derby was primarily related to ‘pig’ (75.3%) and secondly to ‘turkey’ (13.5%) sources. To interpret these data, it is important to be aware that the distribution of the serotyped isolates among the different sources is very unbalanced in terms of the number of isolates per source, and the large majority of the serotyped isolates for the subsets considered were from poultry populations covered by NCPs, especially broilers.
Table 32 shows the top 20 serovars notified, considering all serotyped isolates (including those from both food and animals) from the following species: laying hens, broilers, turkeys, pigs and bovines. For laying hens, 44.9% of the isolates (N = 1,198) belonged to two target serovars (S. Enteritidis (35.5%) and S. Typhimurium (9.4%)). The other most common serovars from this source were S. Kentucky (10.3%) and S. Infantis (7.1%). For broilers (N = 14,348), four serovars (S. Infantis, S. Enteritidis, S. Livingstone and S. Mbandaka) represented 65.9% of the serotyped isolates, with S. Infantis including more than 45% of all strains. S. Typhimurium and its monophasic variant accounted for 2.9% of the isolates from this species. Also, for turkey sources (N = 2,639), two serovars (S. Anatum (29.8%) and S. Agona (23.5%)) were by far the most common ones, followed by S. Infantis (7.2%). Overall, S. Typhimurium, S. Enteritidis and the monophasic variant of S. Typhimurium accounted for 5.6% of the isolates from this species. For pigs, the monophasic variant of S. Typhimurium (28.2%) as well as S. Derby (22.3%) represented half of the serotyped strains (N = 1,559) from this source, with S. Typhimurium (15.3%) and S. Rissen (6.6%) being two other common serovars. Considering serotyped isolates from bovine sources (N = 891), two serovars were by far the most common ones: S. Dublin and S. Typhimurium represented 31.5% and 29.7% of the serotyped isolates from this source respectively.
Table 32.
Distribution of the top 20 Salmonella serovars by food–animal source (laying hens, broilers, turkeys, pigs and bovines), EU, 2021
| Poultry population | |||||||||||
| Laying hens | Serovar | N (%) positive samples |
N MSs |
Broilers | Serovar | N (%) positive samples |
N MSs |
Turkeys | Serovar | N (%) positive samples |
N MSs |
| Enteritidis | 425 (35.5) | 22 | Infantis | 6,468 (45.1) | 23 | Anatum | 785 (29.8) | 7 | |||
| Kentucky | 124 (10.3) | 5 | Enteritidis | 1,147 (8.0) | 21 | Agona | 620 (23.5) | 9 | |||
| Typhimurium | 113 (9.4) | 15 | Livingstone | 998 (7.0) | 7 | Infantis | 189 (7.2) | 9 | |||
| Enterica, subspecies enterica | 97 (8.1) | 3 | Mbandaka | 838 (5.8) | 10 | Newport | 94 (3.6) | 9 | |||
| Infantis | 85 (7.1) | 12 | Java | 439 (3.1) | 5 | Senftenberg | 90 (3.4) | 4 | |||
| Braenderup | 24 (2.0) | 8 | Enterica, subspecies enterica | 436 (3.0) | 4 | Enterica, subspecies enterica | 81 (3.1) | 1 | |||
| Mbandaka | 20 (1.7) | 8 | Thompson | 374 (2.6) | 7 | Typhimurium | 71 (2.7) | 9 | |||
| Anatum | 19 (1.6) | 5 | Typhimurium | 330 (2.3) | 17 | Derby | 61 (2.3) | 6 | |||
| Corvallis | 17 (1.4) | 6 | Montevideo | 301 (2.1) | 5 | Bredeney | 55 (2.1) | 5 | |||
| Montevideo | 16 (1.3) | 6 | Group O:7 | 281 (2.0) | 3 | Saintpaul | 55 (2.1) | 4 | |||
| Ohio | 16 (1.3) | 4 | Senftenberg | 273 (1.9) | 8 | Hadar | 52 (2.0) | 4 | |||
| Thompson | 15 (1.2) | 6 | Newport | 171 (1.2) | 15 | Lagos | 40 (1.5) | 1 | |||
| Newport | 13 (1.1) | 5 | Agona | 170 (1.2) | 13 | Monophasic Typhimurium | 40 (1.5) | 2 | |||
| Senftenberg | 13 (1.1) | 6 | Kedougou | 156 (1.1) | 7 | Kedougou | 38 (1.4) | 3 | |||
| Livingstone | 11 (0.92) | 5 | enterica | 150 (1.0) | 3 | Enteritidis | 37 (1.4) | 11 | |||
| Mikawasima | 11 (0.92) | 2 | Virchow | 99 (0.69) | 5 | Coeln | 32 (1.2) | 5 | |||
| Agona | 10 (0.83) | 4 | Kentucky | 96 (0.67) | 9 | Napoli | 27 (1.0) | 3 | |||
| Hadar | 10 (0.83) | 3 | Mkamba | 92 (0.64) | 1 | Kentucky | 24 (0.91) | 7 | |||
| Mkamba | 10 (0.83) | 1 | Napoli | 81 (0.56) | 2 | Thompson | 18 (0.68) | 1 | |||
| Monophasic Typhimurium | 9 (0.75) | 1 | Monophasic Typhimurium | 81 (0.56) | 2 | Haifa | 15 (0.57) | 1 | |||
| Other | 140 (11.7) | 26 | Other | 1,367 (9.5) | 27 | Other | 215 (8.2) | 19 | |||
| Total | 1,198 | Total | 14,348 | Total | 2,639 | ||||||
| Pigs and cattle (bovine animals) | |||||||||||
| Pigs | Serovar | N (%) positive samples |
N MSs |
Bovines | Serovar | N (%) positive samples |
N MSs |
||||
| Monophasic Typhimurium | 440 (28.2) | 16 | Dublin | 281 (31.5) | 9 | ||||||
| Derby | 347 (22.3) | 21 | Typhimurium | 265 (29.7) | 15 | ||||||
| Typhimurium | 239 (15.3) | 19 | Monophasic Typhimurium | 80 (9.0) | 7 | ||||||
| Rissen | 103 (6.6) | 8 | Havana | 57 (6.4) | 2 | ||||||
| enterica | 81 (5.2) | 3 | enterica | 28 (3.1) | 3 | ||||||
| Brandenburg | 44 (2.8) | 8 | Group B | 19 (2.1) | 3 | ||||||
| Infantis | 39 (2.5) | 10 | Derby | 18 (2.0) | 8 | ||||||
| Group B | 24 (1.5) | 4 | Enteritidis | 18 (2.0) | 6 | ||||||
| Group O:4 | 22 (1.4) | 1 | Infantis | 14 (1.6) | 7 | ||||||
| Enterica, subspecies enterica | 19 (1.2) | 4 | Altona | 9 (1.0) | 1 | ||||||
| London | 19 (1.2) | 7 | Agona | 6 (0.67) | 2 | ||||||
| 4,12:a:‐ | 14 (0.9) | 1 | Group C | 6 (0.67) | 1 | ||||||
| Goldcoast | 12 (0.77) | 3 | Montevideo | 6 (0.67) | 1 | ||||||
| Afula | 10 (0.64) | 1 | Group C1 | 5 (0.56) | 1 | ||||||
| Enteritidis | 10 (0.64) | 5 | Mbandaka | 5 (0.56) | 2 | ||||||
| Livingstone | 10 (0.64) | 3 | Kentucky | 4 (0.45) | 2 | ||||||
| Agona | 9 (0.58) | 2 | Muenster | 4 (0.45) | 1 | ||||||
| Bovismorbificans | 6 (0.38) | 2 | Newport | 4 (0.45) | 2 | ||||||
| Choleraesuis | 6 (0.38) | 2 | Give | 3 (0.34) | 1 | ||||||
| Choleraesuis var. Kunzendorf | 6 (0.38) | 1 | Rissen | 3 (0.34) | 3 | ||||||
| Other | 99 (6.3) | 26 | Other | 56 (6.3) | 21 | ||||||
| Total | 1,559 | Total | 891 | ||||||||
MSs: Member States.
2.5. Discussion
Salmonellosis remained the second most common foodborne zoonosis in humans in the EU in 2021 after campylobacteriosis. The previous decreasing trend for confirmed cases has stabilised since 2014. In 2020, the number of reported confirmed human cases and the EU notification rate reached the lowest levels observed since the beginning of Salmonella surveillance at the EU level in 2007. This was probably due to the COVID‐19 pandemic and the withdrawal of the United Kingdom from the EU. In 2021, a slight increase in notified human cases was registered compared with 2020, caused in part by the lower impact of the COVID‐19 pandemic compared with the previous year. The slight increase in human cases of salmonellosis in 2021 could be associated with the reduction of COVID‐19 pandemic restriction measures and with the slow recovery of daily activities (social events, doctor's visits, travel). The numbers of human salmonellosis cases acquired in the EU, outbreak‐related cases and foodborne salmonellosis outbreaks were higher in 2021 than in 2020 but lower than in previous years, and may have been consequences of reduced COVID‐19 restrictions. Notwithstanding, the overall EU trend for salmonellosis in 2017–2021 did not show any statistically significant increase or decrease. Conversely, over the period 2017–2021, Denmark, Estonia, Finland, Ireland, Romania and Sweden reported a decreasing trend.
In addition, notification rates for salmonellosis in humans varied between MSs, reflecting potential variations, for example, in the quality, coverage and disease‐severity focus of the surveillance systems, sampling and testing practices, disease prevalence in the food‐producing animal population and food and animal trade between MSs.
The hospitalisation rate varied from 23.9% to 91.9%. Countries reporting the lowest notification rates for salmonellosis had the highest proportions of hospitalisation, suggesting that the surveillance systems in these countries are focused on the most severe cases and underlining the variability of national surveillance systems. It is important to note that a higher hospitalisation rate was reported for patients with specimens from blood (82.4%), suggesting that people with systemic infection are more likely to be hospitalised.
In 2021, a decrease in the proportion of reported data for Salmonella serovars was also observed compared with 2020. This was probably due to a lack of serovar data from Spain and Luxembourg. For Spain, serovar information was not available when Salmonella serovar data were analysed for this report (this information will be shown in the ECDC Surveillance Atlas). In 2021, the proportion of monophasic S. Typhimurium (1,4,[5],12:i‐) decreased and the proportion of S. Enteritidis increased compared with previous years. Concerning the increased proportion of S. Enteritidis cases, it is worth noting that Poland provided serovar data in 2021, whereas it had not provided such data in 2020; it is to be remembered that Poland had been involved, during previous years, in a long‐lasting multi‐country outbreak associated with contaminated eggs from Polish farms.
Regarding the cases acquired in the EU, the ranking of the five most common serovars was stable, but the proportion of S. Enteritidis was much higher than in relation to total cases. Overall, the three most commonly reported human serovars, S. Enteritidis, S. Typhimurium and its monophasic variant (1,4,[5],12:i:‐) continued to account for over 70% of human cases acquired in the EU, as has been observed since 2014, reaching 79.1% in 2021. S. Infantis has consistently been the fourth most frequently reported serovar involved in domestically acquired and travel‐associated human infections. After S. Infantis, S. Derby was the fifth most frequently reported serovar in 2021, while S. Napoli slid to eighth position, from sixth in 2020, preceded by S. Braenderup, probably due to a multi‐country outbreak of S. Braenderup ST22 that was presumed to be linked to imported melons (ECDC and EFSA, 2021a).
S. Enteritidis continued to be the serovar most frequently reported in human cases and was associated with different sources, especially broilers and laying hens. Eggs and egg products were confirmed as the primary source of salmonellosis outbreaks in 2021, as had been the case during previous years (Chanamé Pinedo et al., 2022). The importance of eggs as a source of human salmonellosis was underlined by the recurrent high number (seven) of MSs that did not meet the Salmonella reduction target in laying hens under their NCPs, even though that target was set at 2% instead of 1% as for other poultry populations. It is also of note that for breeding flocks of Gallus gallus, a number (five) of MSs did not meet the reduction target, which had also been the case in the last few years. Moreover, trend analyses confirmed that during the last few years, there was no significant reduction in target Salmonella serovar flock prevalence in any of the poultry populations covered by NCPs. This indicates that Salmonella elimination and eradication, also in relation to those serovars that are considered relevant for public health, are not in sight. These consolidated findings suggest that some revisions of programmes such as changes to the frequency of sampling on farms and the vaccination policy, especially for groups with long production cycles, could be considered in order to reduce the risk of Salmonella spreading. Broiler meat is another source of Salmonella and the contamination of broiler farms has been increasingly associated with persistent serovars, such as S. Infantis (Mughini‐Gras et al., 2021). These data confirm that it is vital to identify efficient and cost‐effective measures to control contamination, primarily on farms, which may even include evaluating measures aimed at non‐regulated Salmonella serovars (Newton et al., 2021) in relation to the epidemiological situation in each MS. Pigs are another species that deserves attention as a putative source of salmonellosis (Chanamé Pinedo et al., 2022). S. Typhimurium and its monophasic variant, which are among the top serovars causing human infections, also appear as some of the serovars most commonly associated with this animal species. In pigs, the Salmonella holding prevalence varies widely across MSs, as was ascertained with the EU baseline survey conducted in 2008 (EFSA, 2009b). Over the last few years, these differences may also have become larger due to the fact that some MSs (e.g. Norway, Finland, Sweden, Denmark, Germany and Estonia) have implemented control programmes for Salmonella in their pig populations (Bonardi et al., 2021) in an attempt to reduce the risk of this pathogen reaching humans (Ferrari et al., 2019). For bovine animals, similar to pigs, no harmonised control programmes are implemented at EU level, although some MSs have their own national control programmes in place. For this animal species, the prevalence situation is even more unclear than for pigs, because no baseline surveys have been carried out so far at EU level. Moreover, also looking at the 2021 food data and Salmonella PHC data on carcases at the slaughterhouse, it is evident how meat has continued to play a central role in the dissemination of the pathogen (one in seven broiler carcases and one in 13 turkey carcases were positive for Salmonella at the slaughterhouse). The safety of carcases and derived meat products is dependent on both farm and slaughterhouse performance (Cegar et al., 2022). Regarding poultry meat, although NCPs for poultry populations have been implemented for many years, the EU trends for different species have stabilised both for Salmonella spp. and for ‘target serovars’ without any significant decrease in the prevalence notified recently. Control at farm level is undeniably important for reducing the prevalence of Salmonella in the next phases of the process; however, further implementation aiming to reduce the contamination of final products could also address the slaughter process. The risk categorisation of slaughterhouses based on process hygiene criteria has been suggested as one of the essential components of the risk‐based meat safety assurance system (EFSA BIOHAZ Panel, 2012). Therefore, efforts to control Salmonella at the primary production level should be combined with the categorisation of slaughterhouses in terms of their capacity to reduce carcase contamination (Cegar et al., 2022). Food chain information, accompanying animals to be admitted to the slaughterhouse, serves as the bridge between these two phases (Bonardi et al., 2021). Flocks from high‐risk farms can be directed to low‐risk slaughterhouses, thanks to their capacity to reduce carcase contamination. High‐risk slaughterhouses could accept flocks from low‐risk farms or could apply additional final carcase interventions (EFSA BIOHAZ Panel, 2012).
Regulation (EU) 2019/627 has defined the obligation, for competent authorities, to verify the correct implementation by the FBOp of PHC for Salmonella in carcases at the slaughterhouse. Data collected in this context confirmed that, especially for poultry (broiler and turkey) carcases, the proportion of positive samples, identified by sampling conducted by the CA, was significantly higher than that notified for samples taken by the FBOp. This discrepancy was also observed for national control programmes for poultry, where separate reporting on controls carried out by the CA and FBOp is mandatory. The prevalence of target Salmonella serovars in samples from controls conducted by competent authorities was consistently higher than for controls carried out by the FBOp, for both broilers and fattening turkeys. Investigations to define the reasons for discrepancies between the results of sampling conducted by the CA and FBOp should be encouraged as an essential prerequisite to ensure the trustworthiness of the data collected in both contexts.
Although animal‐based foods are the most common sources of Salmonella transmission to humans (Ferrari et al., 2019), and while prevalence data collected in 2021 from food samples confirmed that the pathogen is seldom isolated from non‐animal products, large multi‐country outbreaks related to non‐animal products (e.g. melons, sesame‐based products and chocolate products) (ECDC and EFSA, 2021b,a, 2022a) recently occurred in the EU. Therefore, for such products, efforts to control Salmonella contamination must also be maintained at a high level.
3. Listeria monocytogenes
Summary data substantiating this chapter, as well as additional information on related projects and internet sources are published for this report on the EFSA Knowledge Junction on Zenodo here. Summary statistics on Listeria monocytogenes food monitoring data and on listeriosis foodborne outbreaks reported in the framework of Directive 2003/99/EC, with downloadable files, are retrievable using the EFSA Listeria monocytogenes dashboard and the EFSA foodborne outbreaks dashboard, respectively available here and here. Summary statistics on human surveillance data with downloadable files are retrievable using the ECDC Surveillance Atlas of Infectious Diseases here.
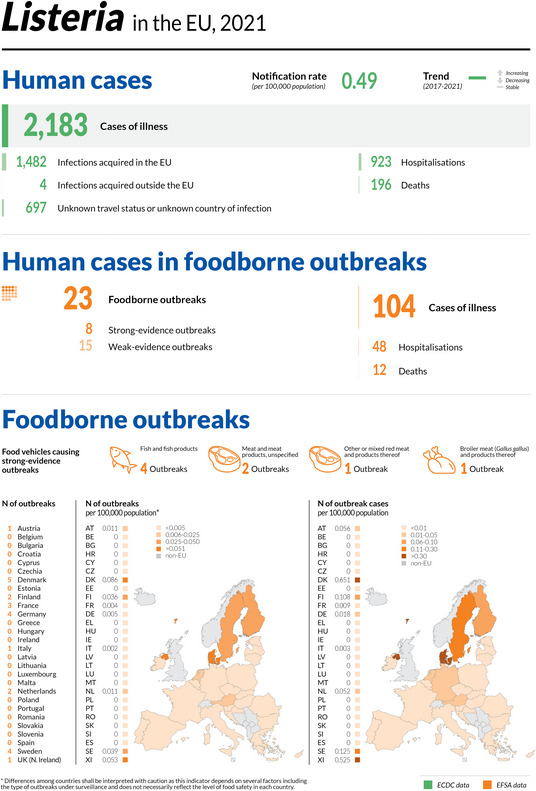
3.1. Key facts
In 2021, 27 MSs reported 2,183 confirmed invasive human cases of Listeria monocytogenes infection to ECDC. These cases resulted in 923 hospitalisations and 196 deaths in the EU. Listeriosis was the fifth most commonly reported zoonosis in humans in the EU, and is one of the most serious foodborne diseases under EU surveillance.
The EU notification rate was 0.49 per 100,000 population, 14.0% higher compared with the rate in 2020 (0.43 per 100,000 population). Reporting in 2020 – which showed the lowest number of human cases/lowest rate since the start of listeriosis surveillance in 2007 − was impacted by the COVID‐19 pandemic and the withdrawal of the United Kingdom from the EU.
Compared with the rate before the COVID‐19 pandemic (2017–2019 annual mean), the 2021 EU notification rate increased by 4.3% and decreased by 2.0% with and without data from the United Kingdom, respectively.
The overall EU trend for listeriosis in the period 2017–2021 did not show any statistically significant increase or decrease.
The overall EU case fatality rate was high (13.7%), similar to 2020 and slightly lower than 2019 (13.0% and 17.6%, respectively).
L. monocytogenes infections were most commonly reported in the age group ‘over 64 years’ and particularly in the age group ‘over 84 years’.
24 MSs reported a total of 244,357 samples from different ‘ready‐to‐eat’ food categories, from the distribution or manufacturing stages.
The occurrence of L. monocytogenes gives an indication of the reasonably foreseeable contamination rate in these ‘ready‐to‐eat’ food categories. Results varied according to the ‘ready‐to‐eat’ food category, the sampling stage, the number of tested samples and the number of reporting countries. In the framework of objective sampling, all samplers and sampling units included, occurrences remained generally rare (< 0.1%) to low (> 1% to 10%) in these categories. The highest values (from 2% to 5%) were observed for fish and fishery products, meat products from bovines or pigs, fruits and vegetables and cheeses from sheep milk.
At distribution, the proportions of positive results for single sample enumeration tests for L. monocytogenes carried out by the Competent Authorities (CAs) as part of verification of L. monocytogenes food safety criteria listed in Regulation (EC) No 2073/2005 remained very low to low (< 1.0%) in 9 out of 11 RTE food categories. The highest proportions at distribution were observed in ‘ready‐to‐eat’ ‘meat products, fermented sausage' (3.1%) and ‘fish’ (1.5%).
At manufacturing, the proportions of single samples positive for L. monocytogenes based on the detection test were systematically higher compared with those at the distribution level, for all categories of ‘ready‐to‐eat’ food. The highest proportions at manufacturing were observed for fishery products (3.1%), products of meat origin other than fermented sausages (2.5%) and fish (1.8%).
In primary production, the percentage of positive units was very low in cattle (1.1%), which is the most sampled animal species in the EU. The low number of data reported by MSs reflects the absence of minimum legal requirements for harmonised sampling and reporting at primary production.
With the present report, EFSA has also published two new interactive communication tools on: the EFSA story map (available here) and the dashboard (available here).
3.2. Surveillance and monitoring of Listeria monocytogenes in the EU
EFSA story map on Listeria monocytogenes
The EFSA story map on Listeria monocytogenes is a new interactive communication tool developed by EFSA in 2022, available online (here) and geared to the general public. This story map provides general information on the pathogen and its epidemiology, including information on where the pathogen can be found, how people and animals get infected, the occurrence of this pathogen in different sources, the disease it causes and how to prevent infection. In addition, this story map also illustrates the monitoring activities implemented in the EU and the role of EFSA with respect to these activities. Users can easily display and explore the content of the different sections in the story map, browsing the dynamic maps, images, text and multimedia features.
3.2.1. Humans
Surveillance of listeriosis in humans in the EU focuses on invasive forms of L. monocytogenes infection, mostly manifesting as septicaemia, influenza‐like symptoms, meningitis, or spontaneous abortion. Diagnosis of Listeria infections in humans is generally carried out by culture, from blood, cerebrospinal fluid and vaginal swabs, or by nucleic acid detection. Since 2018, MSs have been able to submit whole genome sequencing (WGS) data for L. monocytogenes to TESSy to be used for EU‐wide surveillance and cross‐sectoral comparison.
Surveillance of listeriosis is mandatory in all MSs, except in one country (Belgium). The EU case definition was used by 22 countries. Four countries (Denmark, Germany, Hungary and Italy) reported using different case definitions, and one (France) did not specify which case definition was used.
All countries had comprehensive surveillance systems. The surveillance systems for listeriosis cover the whole population in all MSs, except in Belgium, where the estimated coverage of the surveillance system is 80%. This estimated coverage was used in the calculation of notification rates for Belgium. In 2020–2021, Spain did not receive data from all regions that normally report, so the case numbers may therefore not be complete. Bulgaria reported aggregated data, while the other 26 countries reported case‐based data.
3.2.2. Food, animals and feed
Monitoring of L. monocytogenes is carried out along the food chain, at primary production, manufacturing and distribution.
The public health risk associated with RTE foods depends mainly on the effectiveness of control measures implemented by food business operators (FBOps), including:
-
–
Good Agricultural Practices (GAP) at primary production.
-
–
Good Manufacturing Practices (GMP) and Hazard Analysis and Critical Control Point (HACCP) programmes at manufacturing and distribution.
-
–
Microbiological criteria for RTE foods as defined by Regulation (EC) No 2073/2005.
Official sampling is scheduled by national Competent Authorities (CAs) to verify whether FBOps correctly implement the legal framework of their own‐check programmes. Official control samples are thus part of the verification of L. monocytogenes food safety criteria (FSC). Data provided to EFSA within this context enable a descriptive summary of contamination levels, especially of RTE foods, at the EU level.
The rationale for surveillance and monitoring of L. monocytogenes in animals, feed and food at the different stages along the food chain and the number of samples provided to EFSA are shown in Figure 8.
Figure 8.
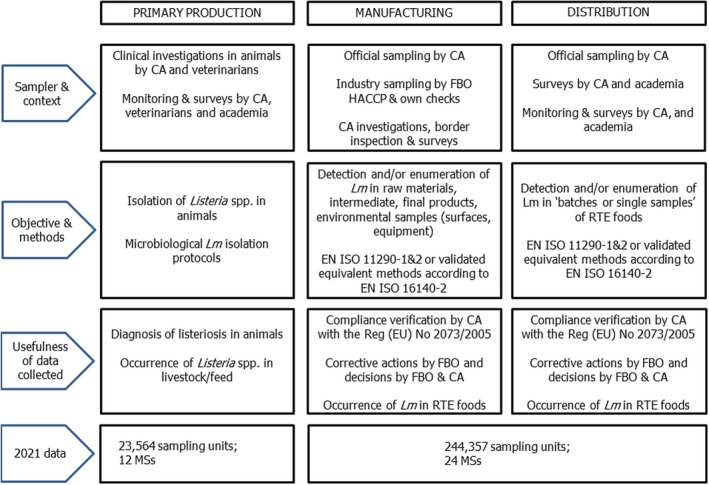
Overview of Listeria monocytogenes testing along the food chain according to the sampling stage, the sampler and the objective of the sampling, EU
At the manufacturing and distribution stages, in 2021, 24 MSs reported 301,195 samples tested for L. monocytogenes involving different RTE food categories. Due to the impact of the COVID‐19 pandemic on food surveillance activities, 2021 results were not comparable with 2020 results. Compared with 2019, the number of reporting MSs was stable (25 MSs in 2019), and the number of tested samples at these stages increased by 11.9% (222,659 samples tested in 2019). In 2020, 24 MSs reported 184,570 sampling units.
At primary production, data are far more comparable with previous years, with 13 MSs reporting 23,564 samples in 2021, compared with 22,567 samples in 2020 (13 MSs) and 22,135 samples in 2019 (13 MSs). Most of the reported data in animals and feed are generated by non‐harmonised monitoring schemes across MSs, and for which mandatory reporting requirements do not exist. Data on the occurrence of L. monocytogenes in feed are only collected as part of clinical investigations in farm animals. Hence, monitoring data on L. monocytogenes in animal feed are rarely available.
3.3. Data analyses
3.3.1. Data on RTE food in the context of Regulation (EC) No 2073/2005 on microbiological criteria
These data are based on single samples collected by CAs as part of verification of L. monocytogenes FSC listed in Regulation (EC) No 2073/2005, which are to be complied with by FBOps. These FSC are specified by RTE food category and by sampling stage, and are underpinned by the results of either detection or enumeration analytical methods (Table 33). Data were extracted from the database using the values ‘official sampling’ for the sampler, ‘single units’ for the sampling unit and ‘objective sampling’ for the sampling strategy (Table 36).
Table 33.
Listeria monocytogenes FSC as described in Regulation (EC) No 2073/2005 for the different RTE categories across the food chain, 2021
| Sampling stage | RTE foods intended for infants and RTE foods for special medical purposes | Other RTE foods | |
|---|---|---|---|
| Able to support growth of Lm | Unable to support growth of Lm | ||
| Manufacturing (a) | NA |
Based on detection method: Lm not detected in 25 g of sample (n = 5, c = 0) (c) |
NA |
| Distribution (b) |
Based on detection method: Lm not detected in 25 g of sample (n = 10, c = 0) |
Based on enumeration method: limit of 100 CFU/g (n = 5, c = 0) (d) |
Based on enumeration method: limit of 100 CFU/g (n = 5, c = 0) |
Lm: Listeria monocytogenes; NA: not applicable; RTE: ready‐to‐eat.
: Before the food has left the immediate control of the food business operator that has produced it.
: Products placed on the market during their shelf‐life.
: n = number of units comprising the sample (number of sample units per food batch that are required for testing); c = the maximum allowable number of sample units yielding unsatisfactory test results. In a two‐class attributes sampling plan defined by n = 10, c = 0 and a microbiological limit of ‘not detected in 25 g’, in order for the food batch to be considered acceptable, L. monocytogenes must not be detected in qualitative (detection) analyses of 25 g food test portions obtained from each one of 10 sample units taken from the batch. If even one of the sample units from the batch is found to contain L. monocytogenes (detected in 25 g), then the entire batch is deemed unacceptable. This criterion applies to products before they have left the immediate control of the producing food business operator, when the operator is not able to demonstrate, to the satisfaction of the CA, that the product will not exceed the limit of 100 CFU/g throughout the shelf‐life.
: This criterion applies if the manufacturer is able to demonstrate, to the satisfaction of the CA, that the product will not exceed the limit of 100 CFU/g throughout the shelf‐life. The operator may fix intermediate limits during the process that should be low enough to guarantee that the limit of 100 CFU/g is not exceeded at the end of the shelf‐life.
Table 36.
Proportions (%) of positive single samples at the manufacturing and distribution stage from official sampling by CAs in the context of verification of implementation by FBOps of the Listeria monocytogenes FSC in accordance with Regulation (EC) No 2073/2005, EU, 2021
| RTE food category (a) | Manufacturing stage (b) | Distribution (c) | |||
|---|---|---|---|---|---|
| Analytical method | |||||
| Detection (EN ISO 11290‐1) | Enumeration (EN ISO 11290‐2) | Detection (EN ISO 11290‐1) | Enumeration (EN ISO 11290‐2) | ||
| % positive samples (N samples tested, N reporting MS) (d) | % positive samples (N samples tested, N reporting MS) (d) | % positive samples (N samples tested, N reporting MS) (d) | % of samples exceeding 100 CFU/g (e) | ||
| Foods intended for infants and for medical purposes: data reported from BE, BG, CY, ES, HU, IT, RO, SI, SK 22 | 0 (N = 1,358; 9 MSs) | ||||
| Fish: data reported from BE, BG, CY, CZ, DK, EE, ES, FR, GR, HR, HU, IT, LV, SK | 1.8 (N = 501; 8 MSs) | 1.5 (N = 1,516; 14 MSs) | 0.66 | ||
| Fishery products: data reported from BE, BG, CY, DK, EE, ES, FR, HR, IT, LV, RO, SI, SK | 3.1 (N = 716; 8 MSs) | 0.62 (N = 1,285; 11 MSs) | 0.08 | ||
| Cheeses, soft and semi‐soft: data reported from BE, BG, CZ, EE, ES, GR, HR, HU, IT, LV, RO, SK | 0.95 (N = 4,922; 10 MSs) | 0.05 (N = 1,995; 10 MSs) | 0 | ||
| Cheeses, hard: data reported from BG, ES, IT, SK | 0 (N = 244; 4 MSs) | 0 | |||
| Cheeses, unspecified: data reported from BE, ES HU, IT, RO, SI | 0.77 (N = 1,686; 5 MSs) | 0.19 (N = 530; 4 MSs) | 0 | ||
| Other dairy products (excluding cheeses) – entire category: data reported from BE, BG, CZ, EE, ES, GR, HR, HU, IT, LV, RO, SI, SK | 0.27 (N = 1,485; 10 MSs) | 0 (N = 1,231; 10 MSs) | 0 | ||
| Milk: data reported from BG, CZ, EE, ES, GR, HR, IT, RO, SK | 0.26 (N = 388; 7 MSs) | 0 (N = 285; 6 MSs) | 0 | ||
| Products of meat origin, fermented sausages: data reported from BE, DK, ES, HR, HU, SI, SK | 3.1 (N = 447; 7 MSs) | 0.45 | |||
| Products of meat origin, other than fermented sausages: data reported from BE, BG, CZ, DK, EE, ES, GR, HR, HU, IT, LV, PL, RO, SI, SK | 2.5 (N = 14,136; 12 MSs) | 0.19 (N = 4,271; 14 MSs) | 0.02 | ||
| Other products: data reported from BE, BG, CY, CZ, EE, ES, GR, HU, HR, IT, LV, RO, SI, SK | 0.72 (N = 1,658; 8 MSs) | 0.65 (N = 3,849; 14 MSs) | 0.03 | ||
MSs: Member States; N: number of single samples tested.
Grey boxes are not applicable in relation to the analytical method for the specific food category and sampling stage in the context of Regulation (EC) No 2073/2005.
: In the absence of relevant physicochemical data (pH, aw), EFSA assumes that foods listed under ‘fish and fishery products’, ‘soft and semi‐soft cheeses’, ‘unspecified cheeses’, ‘milk’, ‘products of meat origin other than fermented sausages’, ‘other dairy products’ and ‘other products’ belong to the category of foods that are able to support the growth of L. monocytogenes. EFSA assumes that ‘fermented sausages’ and ‘hard cheeses’ belong to the category of foods that are unable to support the growth of L. monocytogenes, because foods classified under these two categories of RTE products undergo ripening/fermentation and are expected to have low pH and moderate aw values. For ‘other dairy products’, EFSA presents the results in a conservative way, by considering all foods included in this category as capable of supporting the growth of L. monocytogenes.
: Includes sampling units that were collected from ‘cutting plants’ and ‘processing plants’.
: Includes sampling units that were obtained from ‘catering’, ‘hospital or medical care facility’, ‘retail’, ‘wholesale', ‘not available', ‘unspecified’, ‘restaurant or cafe or pub or bar or hotel or catering service', ‘automatic distribution system for raw milk’, ‘border inspection’ and ‘packing centre'.
: Proportion (%) of positive samples (detection of L. monocytogenes in 25 g of sample for qualitative analyses, or number of L. monocytogenes > or < 100 CFU/g for enumeration analyses); in parentheses the number of tested samples and the number of reporting MSs.
: Proportion of samples exceeding 100 CFU/g for enumeration analyses.
Data reported by MSs were classified into the different categories of RTE food/sampling stages based on the assumptions described in the EU Summary zoonoses and foodborne outbreaks report of 2016 (EFSA and ECDC, 2017). Briefly, these assumptions are: all sampling units that were collected from ‘cutting plants’ and ‘processing plants’ were considered units collected at the manufacturing stage, while sampling units that were obtained from ‘catering’, ‘hospital or medical care facility’, ‘retail’, ‘wholesale', ‘restaurant or cafe or pub or bar or hotel or catering service', ‘border inspection activities’, ‘packing centre' and ‘automatic distribution system for raw milk’ were considered units collected at distribution. When the stage was ‘not available' or ‘unspecified’, data were also considered part of the distribution stage. Other assumptions concerning the RTE categories are presented in the footnotes of Table 36.
3.3.2. Other monitoring data for Listeria monocytogenes in RTE food
The overall occurrence of L. monocytogenes in different RTE food categories, regardless of the stage, was assessed from detection results reported by MSs (Appendix B). All levels of sampling unit (single and batches), sampling stages, sampler and sampling contexts (surveillance, monitoring and surveillance – based on Regulation (EC) No 2073/2005) were considered. Only data obtained from the sampling strategies ‘objective sampling’ and ‘census sampling’ were used, excluding data reported from ‘convenient sampling’, ‘suspect sampling’, ‘selective sampling’ and ‘other’ contexts.
Since data were mostly reported by a limited number of MSs and are of a heterogeneous nature, results may not be representative of the EU level or directly comparable across years.
3.3.3. Monitoring data for Listeria monocytogenes in animals and feed
For animals and feed, all sampling strategies were included, even data reported for ‘suspect sampling’ and ‘selective sampling’.
EFSA dashboard on Listeria monocytogenes
The EFSA dashboard on Listeria monocytogenes (available online here) is a graphical user interface for searching and querying the large amount of data collected each year by EFSA from EU MSs and other reporting countries based on Zoonoses Directive 2003/99/EC. The Listeria monocytogenes dashboard shows summary statistics for the monitoring results of the pathogen with regard to major RTE food categories, Listeria monocytogenes‐positive official samples in the context of FSC in accordance with Regulation (EC) No 2073/2005 and official samples exceeding the FSC limit of 100 CFU/g for specified food matrices. Other monitoring statistics for Listeria monocytogenes in RTE foods are also displayed. The Listeria monocytogenes data and related statistics can be displayed interactively using charts, graphs and maps in the online EFSA dashboard. In this tool, the main statistics can also be viewed (and downloaded) in tabular format. Detailed information on the use and features of the Listeria monocytogenes dashboard can be found in the user guide available on Zenodo here and can also be downloaded from the online tool. Links to the dashboard are available in the relevant sections of this chapter.
3.4. Results
3.4.1. Overview of key statistics, EU, 2017–2021
Table 34 summarises EU‐level statistics on human listeriosis and on samples from RTE food tested for L. monocytogenes during the period 2017–2021. Food data of interest reported were classified into the major categories and aggregated by year to obtain an annual overview of the volume of data submitted. The sampling efforts of the MSs in 2021 for L. monocytogenes in some major RTE food categories can be found In Appendix A. More detailed descriptions of these statistics are provided in the below subsections and in the chapter on foodborne outbreaks.
Table 34.
Summary of Listeria monocytogenes statistics related to invasive human infections and on sampled major RTE food categories in the EU, 2017–2021
| 2021 (a) | 2020 | 2019 (b) | 2018 (b) | 2017 (b) | Data source | |
|---|---|---|---|---|---|---|
| Humans | ||||||
| Total number of confirmed cases | 2,183 | 1,887 | 2,621 | 2,544 | 2,474 | ECDC |
| Total number of confirmed cases/100,000 population (notification rates) | 0.49 | 0.43 | 0.46 | 0.47 | 0.47 | ECDC |
| Number of reporting MSs | 27 | 27 | 28 | 28 | 28 | ECDC |
| Infection acquired in the EU | 1,482 | 1,285 | 1,816 | 1,640 | 1,639 | ECDC |
| Infection acquired outside the EU | 4 | 5 | 14 | 8 | 4 | ECDC |
| Unknown travel status or unknown country of infection | 697 | 597 | 791 | 896 | 831 | ECDC |
| Number of outbreak‐related cases | 104 | 120 | 349 | 159 | 39 | EFSA |
| Total number of outbreaks | 23 | 16 | 21 | 14 | 10 | EFSA |
| Sampled major RTE food categories (c) | ||||||
| Meat and meat products | ||||||
| Number of sampling units | 107,198 | 40,291 | 64,971 | 58,060 | 45,322 | EFSA |
| Number of reporting MSs | 23 | 22 | 22 | 22 | 24 | EFSA |
| Fish and fishery products | ||||||
| Number of sampling units | 29,273 | 11,212 | 13,366 | 14,031 | 12,603 | EFSA |
| Number of reporting MSs | 24 | 23 | 22 | 22 | 24 | EFSA |
| Milk and milk products | ||||||
| Number of sampling units | 66,633 | 49,132 | 61,866 | 59,313 | 56,428 | EFSA |
| Number of reporting MSs | 23 | 23 | 23 | 23 | 23 | EFSA |
| Products intended for infants or special medical purposes | ||||||
| Number of sampling units | 2,764 | 2,394 | 2,346 | 2,433 | 1,943 | EFSA |
| Number of reporting MSs | 19 | 19 | 19 | 18 | 21 | EFSA |
| Other products | ||||||
| Number of sampling units | 94,817 | 81,541 | 80,110 | 28,143 | 27,946 | EFSA |
| Number of reporting MSs | 23 | 24 | 24 | 23 | 23 | EFSA |
ECDC: European Centre for Disease Prevention and Control; EFSA: European Food Safety Authority; MSs: Member States; RTE: ready‐to‐eat.
: Data on food and animal samples from the United Kingdom (Northern Ireland) are taken into account for 2021. In accordance with the agreement on the withdrawal of the United Kingdom from the EU, and in particular with the Protocol on Ireland/Northern Ireland, EU requirements on data sampling are also applicable to Northern Ireland.
: Data from the United Kingdom are taken into account for 2017–2019, because the United Kingdom was an EU MS, but it became a third country on 1 February 2020.
: Number of sampling units tested by detection or enumeration method; number of reporting MSs. More details on the number of samples per MS and for non‐MSs can be found in Appendix A.
For a further interactive look at Listeria monocytogenes monitoring results, dashboards have been created (different filters can be applied to query the data) ( link ).
3.4.2. Human listeriosis
In 2021, 27 MSs reported 2,183 confirmed cases of invasive listeriosis in humans (Tables 34 and 35). The EU notification rate was 0.49 cases per 100,000 population, 14.0% higher than 2020 (0.43 per 100,000 population), a year for which the COVID‐19 pandemic affected the comparability of data. Comparing the notification rate in 2021 with the rate before the COVID‐19 pandemic (2017–2019 annual mean), this rate increased by 4.3% and decreased by 2.0% with and without the data from the United Kingdom, respectively. In 2021, the highest notification rates were observed for Finland, Denmark, Sweden and Slovenia, with 1.3, 1.1, 1.0 and 0.9 cases per 100,000 population, respectively. The lowest notification rates were reported by Bulgaria, Croatia, Cyprus, Greece and Romania (≤ 0.20 per 100,000).
Table 35.
Reported confirmed human cases of invasive listeriosis and notification rates per 100,000 population in EU MSs and non‐MS countries, by country and year, 2017–2021
| Country | 2021 | 2020 | 2019 | 2018 | 2017 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| National coverage (a) | Data format (a) | Confirmed cases and rates | Confirmed cases and rates | Confirmed cases and rates | Confirmed cases and rates | Confirmed cases and rates | ||||||
| Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | |||
| Austria | Y | C | 38 | 0.43 | 41 | 0.46 | 38 | 0.43 | 27 | 0.31 | 32 | 0.36 |
| Belgium (b) | N | C | 65 | 0.70 | 54 | 0.59 | 66 | 0.72 | 74 | 0.81 | 73 | 0.80 |
| Bulgaria | Y | A | 3 | 0.04 | 4 | 0.06 | 13 | 0.19 | 9 | 0.13 | 13 | 0.18 |
| Croatia | Y | C | 8 | 0.20 | 5 | 0.12 | 6 | 0.15 | 4 | 0.10 | 8 | 0.19 |
| Cyprus | Y | C | 1 | 0.11 | 2 | 0.23 | 1 | 0.11 | 1 | 0.12 | 0 | 0 |
| Czechia | Y | C | 24 | 0.22 | 16 | 0.15 | 27 | 0.25 | 31 | 0.29 | 30 | 0.28 |
| Denmark | Y | C | 62 | 1.1 | 43 | 0.7 | 61 | 1.0 | 49 | 0.85 | 57 | 0.99 |
| Estonia | Y | C | 5 | 0.38 | 3 | 0.23 | 21 | 1.6 | 27 | 2.0 | 4 | 0.30 |
| Finland | Y | C | 70 | 1.3 | 94 | 1.7 | 50 | 0.91 | 80 | 1.5 | 89 | 1.6 |
| France | Y | C | 435 | 0.64 | 334 | 0.50 | 373 | 0.56 | 338 | 0.50 | 370 | 0.55 |
| Germany | Y | C | 560 | 0.67 | 546 | 0.66 | 571 | 0.69 | 678 | 0.82 | 721 | 0.87 |
| Greece | Y | C | 21 | 0.20 | 20 | 0.19 | 10 | 0.09 | 19 | 0.18 | 20 | 0.19 |
| Hungary | Y | C | 35 | 0.36 | 32 | 0.33 | 39 | 0.40 | 24 | 0.25 | 36 | 0.37 |
| Ireland | Y | C | 14 | 0.28 | 6 | 0.12 | 17 | 0.35 | 21 | 0.43 | 14 | 0.29 |
| Italy | Y | C | 241 | 0.41 | 155 | 0.26 | 202 | 0.34 | 178 | 0.29 | 164 | 0.27 |
| Latvia | Y | C | 10 | 0.53 | 8 | 0.42 | 6 | 0.31 | 15 | 0.78 | 3 | 0.15 |
| Lithuania | Y | C | 7 | 0.25 | 7 | 0.25 | 6 | 0.21 | 20 | 0.71 | 9 | 0.32 |
| Luxembourg | Y | C | 4 | 0.63 | 4 | 0.64 | 3 | 0.49 | 5 | 0.83 | 5 | 0.85 |
| Malta | Y | C | 0 | 0 | 5 | 0.97 | 5 | 1.0 | 1 | 0.21 | 0 | 0 |
| Netherlands | Y | C | 86 | 0.49 | 90 | 0.52 | 103 | 0.60 | 69 | 0.40 | 108 | 0.63 |
| Poland | Y | C | 120 | 0.32 | 57 | 0.15 | 121 | 0.32 | 128 | 0.34 | 116 | 0.31 |
| Portugal | Y | C | 0 | 0 | 47 | 0.46 | 56 | 0.54 | 64 | 0.62 | 42 | 0.41 |
| Romania | Y | C | 11 | 0.06 | 2 | 0.01 | 17 | 0.09 | 28 | 0.14 | 10 | 0.05 |
| Slovakia | Y | C | 13 | 0.24 | 7 | 0.13 | 18 | 0.33 | 17 | 0.31 | 12 | 0.22 |
| Slovenia | Y | C | 19 | 0.90 | 26 | 1.2 | 20 | 0.96 | 10 | 0.48 | 13 | 0.63 |
| Spain (c) , (d) | N | C | 224 | – | 191 | – | 504 | – | 370 | – | 284 | – |
| Sweden | Y | C | 107 | 1.0 | 88 | 0.85 | 113 | 1.1 | 89 | 0.88 | 81 | 0.81 |
| EU Total 27 | – | – | 2,183 | 0.49 | 1,887 | 0.43 | 2,467 | 0.49 | 2,376 | 0.50 | 2,314 | 0.51 |
| United Kingdom | – | – | – | – | – | – | 154 | 0.23 | 168 | 0.25 | 160 | 0.24 |
| EU Total (e) | – | – | 2,183 | 0.49 | 1,887 | 0.43 | 2,621 | 0.46 | 2,544 | 0.47 | 2,474 | 0.47 |
| Iceland | Y | C | 5 | 1.4 | 4 | 1.1 | 4 | 1.1 | 2 | 0.57 | 6 | 1.8 |
| Norway | Y | C | 20 | 0.37 | 37 | 0.69 | 27 | 0.51 | 24 | 0.45 | 16 | 0.30 |
| Liechtenstein | Y | C | 0 | 0 | 58 | 0.67 | 36 | 0.42 | 53 | 0.62 | 45 | 0.53 |
| Switzerland (f) | Y | C | 33 | 0.38 | ||||||||
–: Data not reported.
: Y: yes; N: no; A: aggregated data; C: case‐based data.
: Sentinel system; notification rates calculated with an estimated population coverage of 80% for Belgium.
: The notification rate was not calculated since information on estimated coverage was not available.
: Data not complete in 2020–2021, rate not calculated.
: Cases reported by the United Kingdom for the period 2017–2019 were also considered for this estimation (EU‐28). When the United Kingdom data were collected for the period 2017–2019, the United Kingdom was an EU MS, but it became a third country on 1 February 2020.
: Switzerland provided data directly to EFSA. The human data for Switzerland include data from Liechtenstein for the years 2017–2020.
Most (67.9%; 1,482) listeriosis cases with known origin of infection were reported to have been acquired in the EU in 2021 (Table 34). Only four travel‐associated listeriosis cases were reported from outside the EU (the United Kingdom, Syria, India and the Dominican Republic) in 2021 vs. five outside the EU in 2020. No data on travel status or country of infection were reported for 697 cases (31.9%).
The distribution by month appears to be relatively stable. Over the last 5 years (2017–2021), there has been slightly greater reporting of cases in the second half of the year (Figure 9). Although a slight increase in cases was observed in 2021, the overall EU trend for listeriosis cases in the period 2017–2021 did not show any statistically significant increase or decrease. A significantly (p < 0.05) decreasing trend was reported by Romania over the period 2017–2021.
Figure 9.
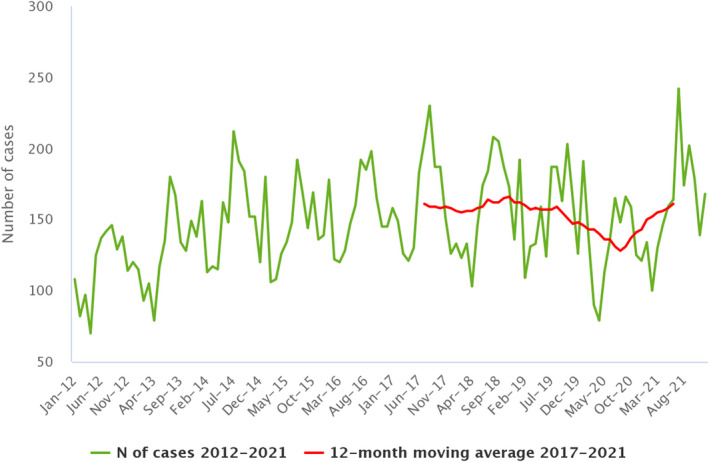
- Source: Austria, Belgium, Czechia, Cyprus, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Ireland, Italy, Latvia, Lithuania, Malta, Netherlands, Poland, Romania, Slovakia, Slovenia and Sweden.
Information on hospitalisation was provided by 16 MSs for 43.8% of all confirmed cases in 2021, similar to the previous year. Among the cases with information on hospitalisation status, 96.5% were hospitalised totalling 923 hospitalised cases. Listeriosis had the highest proportion of hospitalised cases of all zoonoses under EU surveillance. Outcome was reported for 1,427 confirmed cases (65.4%). Fourteen MSs reported a total of 196 deaths from listeriosis in 2021. This represented a 17.4% increase compared with 2020 (167 deaths). There has been a steady increase in the annual number of deaths between 2010 and 2019 (annual average: 217) which dropped in 2020. However, the overall EU case fatality rate among cases with known outcome was 13.7%, similar to previous years (13.0%) and lower than in 2019 (17.6%).
France reported the highest number of fatal cases (75), followed by Spain (34), Poland (25) and Germany (20). L. monocytogenes infections were most commonly reported in the age group over 64. At the EU level, the proportion of listeriosis cases in this age group has steadily increased from 56.1% in 2008 to 64.5% in 2019, and 72.5% in 2020. In the age group over 84, there was an increase from 7.3% to 17.1% in 2019 and 2020, respectively. Within fatal cases of listeriosis, 58.1% of cases were in the age group 64–84 years, while 22.8% were in the age group over 84 years.
3.4.3. Listeria monocytogenes in RTE food
In the food sector 24 MSs reported 244,357 samples in different RTE categories at the distribution or manufacturing stages. This sampling effort increased by 80% compared with 2020 (136,346 sampling units) and was comparable with 2019 (218,438 sampling units).
Data collected in the context of Regulation (EC) No 2073/2005
In total, 17 MSs reported data in the context of the FSC according to the specifications mentioned above (Section 3.3.1) for 11 RTE food categories (Table 36).
Overall L. monocytogenes occurrences reported from official sampling were low, both at distribution and manufacturing (from 0% to 3.1%). Food categories for which data were reported both at manufacturing and distribution systematically showed lower occurrences at distribution. The highest occurrence was found at distribution for ‘Product of meat origin, fermented sausages’ (3.1%) and at manufacturing for ‘Fishery products’ (3.1%) and ‘Products of meat origin, other than fermented sausages’ (2.5%).
For all food categories, the proportion of samples exceeding the limit of the criteria at distribution (100 CFU/g) was very low (0% to 0.66%). 22
For a further interactive look at Listeria monocytogenes monitoring results, dashboards have been created (different filters can be applied to query the data) ( link ).
Other monitoring data for Listeria monocytogenes in RTE food
Details on the occurrence of L. monocytogenes (detection results) in the main RTE food matrices in 2021, together with 2020 and 2019 results, are presented in Appendix B. The below text summarises these results for the major food categories.
Fish and fishery products, RTE
In 2021, 22 MSs and four non‐MSs (Albania, Iceland, North Macedonia and Serbia) reported such data for fish or fishery products.
In the EU, as for previous years, the occurrence of L. monocytogenes remained one of the highest of all RTE food categories from 3.5% to 5.4%, with an overall mean of 4.7% (N = 9,967). The number of tested samples from fish batches was particularly high for Poland (4,893 tested samples reported in 2021). Excluding Poland, four MSs (France, Italy, the Netherlands and Spain) contributed 67% of the remaining reported data for these categories.
Meat and meat products, RTE
In 2021, 20 MSs and three non‐MSs (Albania, North Macedonia and Serbia) reported detection data for meat and meat products.
In the EU, the overall occurrence of L. monocytogenes, all matrices included, was 2.3% (N = 40,710 tested units for detection). In all, 68.4% of tested units were assigned to the four main animal species (pigs, bovine animals, broilers and turkeys). The remaining 31.6% of tested samples were reported from other animals, unspecified or mixed meats. RTE meat and meat products from pigs were by far the most frequently tested (60.8%). RTE meat and meat products from bovine animals, broilers and turkeys represented 5.4%, 1.9% and 0.31% of all tested samples, respectively.
Pork meat products
Eighteen MSs and three non‐MSs (Albania, North Macedonia and Serbia) reported data for pork meat products. In the EU, the overall occurrence of L. monocytogenes remained comparable with previous years: 2.7% in 2021 (N = 24,751), 3.0% in 2020 (N = 6,585) and 4.2% in 2019 (N = 14,035). In 2021, four MSs provided 91% of data on pork meat products (Czechia, Poland, Romania and Spain).
Poultry meat products (broilers and turkeys)
Fourteen MSs and one non‐MS (Albania) reported data for RTE poultry meat products (broiler and turkey meat products). The overall occurrence of L. monocytogenes in sampled RTE poultry meat products (N = 895) in EU was 1.3%. This is comparable with 2020 (0.65%, N = 464) and 2019 results (1.9%, N = 931). All positive samples in 2021 (N = 12) were from RTE broiler meat as all 126 samples from RTE meats from turkeys were negative. Six MSs provided 90% of data on poultry meat products (Bulgaria, Czechia, Hungary, Poland, Romania and Spain).
Bovine meat products
Fourteen MSs and two non‐MSs (Albania and Serbia) reported data for RTE bovine meat products. In the EU, the overall occurrence of L. monocytogenes reported in RTE bovine meat products was 3.9% (N = 2,217). Four MSs provided 89% of data on bovine meat products (Czechia, Luxembourg, the Netherlands and Poland). For comparison, occurrences were 7.4% in 2020 (N = 856) and 4.2% in 2019 (N = 1,248).
Milk and milk products, RTE
In 2021, 20 MSs and four non‐MSs (Albania, Montenegro, North Macedonia and Serbia) reported data for RTE milk and milk products, all categories included.
In the EU, the overall occurrence of L. monocytogenes in RTE milk products, all matrices included, was 0.51% (N = 26,154 tested units for detection). This occurrence was 0.69% for cheeses (N = 14,985) and 0.30% for milk (N = 1,642). Seven MSs (Czechia, Hungary, Italy, the Netherlands, Poland, Romania and Spain) provided 81% of data. Additionally, 36.5% of data were assigned to milk or specified cheeses from bovine, goat or sheep origin (Appendix B). The remaining data were reported from other dairy products and cheeses made from mixed milk or unspecified animals.
Among assigned tested samples, 79.1% of cheese samples and 80.5% of milk samples were of bovine origin. Compared with previous years, occurrences observed in all categories of cheeses were rare to very low (< 1%), regardless of the animal sector, except for cheeses from unpasteurised sheep milk where higher occurrences were observed (ranging from 1.7% to 4.6%).
Fruits, vegetables and juices, RTE
Fifteen MSs reported data for RTE fruits, vegetables and juices. The overall occurrence was 3.0% (N = 1,407). A total of 80.9% of data were reported by four MSs (Germany, Hungary, Italy and Spain). The results are comparable with previous years. The higher occurrence reported for batches in this category in 2021 compared with previous years may not be representative as a result of a low number of tested samples (N = 24).
3.4.4. Listeria spp. in animals
In 2021, 12 MSs and two non‐MSs (North Macedonia and Switzerland) reported data on several animal categories (food‐producing, wild‐, zoo‐ and pet animals, including birds) from different species (Table 37).
Table 37.
Summary of Listeria species statistics related to major animal species, MSs, 2021
| Animal species | N reporting MSs | N tested units | % positive units | N positive units for L. monocytogenes | N positive units for L. ivanovii | N positive units for L. innocua | N positive units for other Listeria species |
|---|---|---|---|---|---|---|---|
| Cattle | 12 | 16,687 | 1.1 | 157 | 0 | 0 | 31 |
| Sheep | 10 | 1,154 | 5.8 | 14 | 2 | 0 | 51 |
| Pigs | 7 | 2,137 | < 0.01 | 1 | 0 | 0 | 0 |
| Others | 12 | 3,586 | 3.3 | 45 | 10 | 3 | 59 |
| Total EU | 12 | 23,564 | 1.6 | 217 | 12 | 3 | 141 |
In the EU, reported data were mainly results from ‘animals’ (98.7%, N = 23,257) compared with other sampling unit levels (‘herd/flock’ and ‘holding’). Considering all sampling unit levels, cattle were the major animal species concerned by Listeria testing (70.8% of total units tested, N = 16,687), followed by sheep (15.2%, N = 3,586) and pigs (9.1%, N = 2,137). The sample size, as well as the sampling strategy and the proportion of positive samples, varied considerably among the reporting countries and animal species. Most EU data were reported by 2 MSs: the Netherlands (62.4%) and Ireland (26.0%).
Among the positive units, only limited positive findings were reported as L. ivanovii (3.2%) and L. innocua (0.80%). As in previous years, many positive findings for Listeria (37.8%) were reported as other or unspecified species.
In 2021, a few data (all negative) were reported by Romania in silage (N = 101 units tested) and Greece in feed (N = 65 units tested).
For a further interactive look at Listeria monocytogenes monitoring results, dashboards have been created (different filters can be applied to query the data) ( link ).
3.5. Discussion
In 2021, the number of confirmed cases of human listeriosis was 2,183, corresponding to an EU notification rate of 0.49 per 100,000 population and resulting in an increase of 14% in the notification rate compared with the rate in 2020. Fewer cases were reported in 2020, which could partially be explained by the impact of the COVID‐19 pandemic on national healthcare systems and the withdrawal of the United Kingdom from the EU. Listeriosis remains one of the most serious foodborne diseases under EU surveillance due to the high rate of hospitalisations, and high morbidity and mortality, particularly among elderly people.
The general trend for listeriosis in 2017–2021 showed no statistically significant increase or decrease, except for Romania with a statistically significant decrease. Listeriosis had a fairly stable notification rate even during the COVID‐19 pandemic, when compared with other foodborne diseases. Clinical manifestations in susceptible categories such as fever, meningitis and septicaemia require hospitalisation or medical treatment and subsequent notification to the national health system (Radoshevich and Cossart, 2018; Quereda et al., 2021). Furthermore, outbreaks, mainly related to the consumption of ready‐to‐eat foods, meat and meat products, smoked fish and frozen vegetables are not necessarily linked to social and community events (ECDC and EFSA, 2018a,b, 2019). Most infections and outbreaks are domestic.
Listeriosis continues to be one of the foodborne infections with the highest number of fatal cases in the EU, particularly among elderly people. With the ageing of the European population (20.8% of the European population is over 65) (Eurostat, 2022) and the increase in chronic age‐related diseases (EFSA BIOHAZ Panel, 2020c), more groups are entering the high‐risk categories for severe forms of Listeria infections. It is important to continue raising awareness of listeriosis and its risks associated with certain consumption habits and types of food (e.g. RTE fish products and frozen vegetables) (ECDC and EFSA, 2018b; EFSA BIOHAZ Panel, 2018; ECDC and EFSA, 2019).
The molecular characterisation of clinical isolates of Listeria is now based on WGS, combined with core genome multi‐locus sequence typing (cgMLST) for strain typing. Almost all MSs have moved from pulsed‐field gel electrophoresis (PFGE) (Bergis et al., 2021) to WGS (ECDC, 2019), ensuring greater accuracy in typing L. monocytogenes. Prior to the use of WGS, a system of rapid and accurate identification of clusters was lacking in sensitivity and, at the European level, many outbreaks were not detected early (Van Walle et al., 2018).
In the food sector, the sampling effort at manufacturing and distribution remained focused on RTE products of animal origin. The occurrence of L. monocytogenes as assessed from detection results reported by MSs (covering objective sampling of single samples and batches) varied according to the food category and sample stage. Occurrences remained generally rare to low in RTE foods. The highest values were observed for fish and fishery products (3.5–5.4%), meat products from bovine or pig origin (2.7–3.9%), fruits and vegetables (2.5%) and hard cheeses from raw or low heat‐treated sheep milk (4.6%).
As in previous years, the highest proportions of positive official control samples as part of verification of L. monocytogenes FSC were observed at manufacturing compared with distribution. One exception concerned fermented sausages, for which occurrence at distribution was 3.1%. The results from official sampling showed that the proportion (%) of samples at distribution exceeding the limit of 100 CFU/g was very low (< 1%).
In primary production, cattle were the most frequently sampled animal species and presented a low proportion of positive units (1.1%).
Interpreting national data and trends for the occurrence of L. monocytogenes in food or animals over time must be carried out with caution. Currently, surveillance data come from systems that are not fully harmonised and that vary in nature and effectiveness depending on the MS. Moreover, data may be reported by a limited number of MSs depending on each food/animal category.
Combining human, animal and food epidemiological data with molecular and genotyping data provides an efficient methodology to better understand the ecology of this pathogen at different stages of the food chain, and will improve the investigation of listeriosis outbreaks affecting one or more MSs (ECDC et al., 2021). L. monocytogenes surveillance in the EU currently uses tools based on genotyping to characterise food and animal contamination and listeriosis outbreaks. ECDC and EFSA have jointly created interoperable databases in order to quickly identify outbreaks. In 2022, the EFSA One Health WGS System that interoperates with the ECDC Molecular Typing system was opened to MSs for the exchange of cgMLST profiles and minimum metadata (EFSA, 2022d). The aim is to collect typing information to detect clusters of foodborne disease and to generate hypotheses on the possible food vehicles involved, supporting risk managers. At the international level, the FAO and WHO (2022) also issued an open call for experts to obtain more representative data on L. monocytogenes in foods and to develop a new, full farm‐to‐table risk assessment.
4. Shiga toxin‐producing Escherichia coli (STEC)
Summary data substantiating this chapter, as well as additional information on related projects and internet sources are published for this report on the EFSA Knowledge Junction on Zenodo here. Summary statistics on STEC infections in humans associated with foodborne outbreaks reported in the framework of Directive 2003/99/EC, with downloadable files, are retrievable using the EFSA foodborne outbreaks dashboard available here. Summary statistics on human surveillance data with downloadable files are retrievable using the ECDC Surveillance Atlas of Infectious Diseases here.
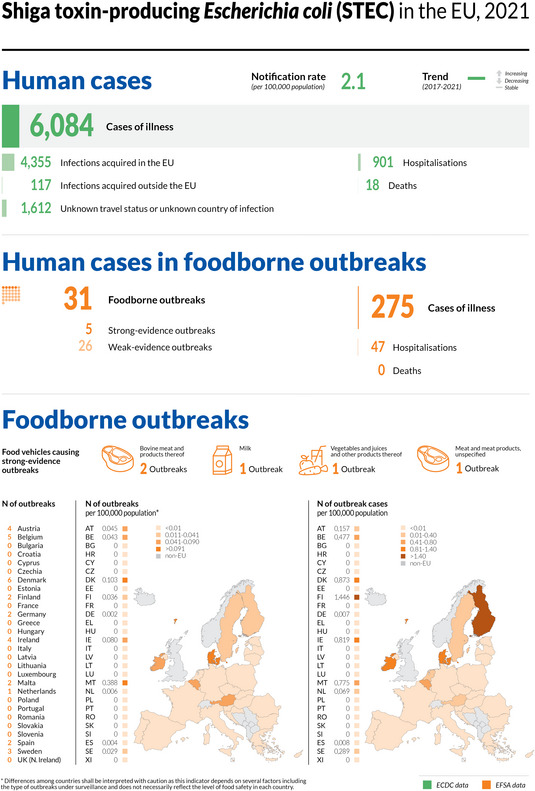
4.1. Key facts
In 2021, the number of confirmed cases of human STEC infection was 6,084. This made STEC infection the fourth most commonly reported foodborne gastrointestinal infection in humans in the EU.
The EU notification rate in 2021 was 2.1 per 100,000 population, representing an increase of 36.9% compared with the rate in 2020 (1.5 per 100,000 population).
Compared with the rate before the COVID‐19 pandemic (2017–2019 annual mean), the 2021 EU notification rate increased by 9.9% and 14.2% with and without the data from the United Kingdom, respectively.
In 2020, STEC cases reported to ECDC showed the lowest rate since 2007, when the disease surveillance started. The reporting was most likely impacted by the COVID‐19 pandemic and withdrawal of the United Kingdom from the EU.
The overall trend for STEC infections did not show any statistically significant increase or decrease in 2017–2021.
In 2021, 22 MSs reported the presence of STEC in 3.6% of 17,516 food samples taken according to an ‘objective sampling’ strategy, compared with 2.4% and 2.8% in 2020 and 2019, respectively.
‘Sprouted seeds’ were tested by 10 MSs in the context of Regulation (EC) No 2073/2005 and one batch was positive for STEC out of 617 sampling units.
Overall, STEC was most commonly found in ‘fresh meat derived from different animal species’ (7% STEC‐positive), followed by bakery products (6.3%) and ‘non‐ready‐to‐eat’ (RTE) ‘milk and milk products’ (2%), whereas ‘fruits and vegetables’ was the least contaminated category (0.5%).
Sixteen MSs tested 7,444 RTE food samples for STEC with 112 (1.5%) positives, including 20 (1.6%) from ‘meat and meat product samples’, 39 (1.7%) from ‘milk and milk product samples’, 1 (0.3%) from ‘spices and herbs’ and 10 STEC‐positive samples from ‘fruits, vegetables and juices’ (0.5%).
Of the food isolates, 27.4% were provided with information on the serogroup and many of these belonged to the top 20 STEC serogroups reported in human infections to ECDC in 2021.
Most of the virulotypes of STEC isolates from food and animals were also identified in severe STEC infections in humans. Only 32.5% (N = 284) of the STEC isolated from food in 2021 were reported with information on virulence gene typing (stx1 or stx2 and eae) and 9% (N = 79) were provided with the stx gene subtypes.
Testing of animal samples increased compared with previous years, but still was not widely carried out in the EU. In fact, 3,746 animal samples were reported by seven MSs in 2021 with 6.1% of positives. Most of the sample units (88.5%) were taken from a single animal species, cattle.
4.2. Surveillance and monitoring of STEC infections in the EU
4.2.1. Humans
In 2021, all 27 MSs reported information on STEC infections in humans.
STEC surveillance is mandatory in 24 MSs, voluntary in two MSs (Belgium and France) and one country (Italy) adopted another, non‐specified system. The surveillance systems for STEC infections cover the whole population in all EU MSs except for three MSs (France, Italy and Spain). The notification rates were not calculated in these three countries for the following reasons: (a) in France, STEC surveillance in humans is based on paediatric haemolytic‐uraemic syndrome (HUS) cases; (b) in Italy, STEC surveillance is sentinel and primarily based on the HUS cases reported through the national registry of HUS; (c) no estimation for population coverage of STEC cases was provided by Spain. In 2020 and 2021, Spain did not receive data from all regions; therefore, the number of reported cases might not be complete. Twenty‐six MSs reported case‐based data, whereas Bulgaria reported aggregated data.
The EU case definition was used by 26 countries. Four countries reported using a different case definition, and in two instances, the case definition used was not indicated.
4.2.2. Food and animals
Data collected in the context of Regulation (EC) No 2073/2005
The food safety criterion (Regulation (EC) No 2073/2005) specifies that sprouts placed on the market during their shelf‐life shall not contain detectable STEC O157, O26, O111, O103, O145 and O104:H4 in 25 g of product. The ISO TS 13136:2012 method with the adaptation developed by the EURL for E. coli for detecting O104:H4 or alternative methods validated according to the requirements of the ISO standard 16140 are indicated as reference methodologies for testing.
Nevertheless, the sampling objectives and the sampling frequency vary or are interpreted differently among MSs, resulting in non‐harmonised data.
Other STEC monitoring data from food and animals
All the other food and animal testing data originate from the reporting obligations of MSs under Directive 2003/99/EC (i.e. the Zoonoses Directive). Due to the absence of explicitly indicated sampling strategies in this directive, the data generated by MSs are based on investigations with non‐harmonised sampling programmes. Therefore, STEC monitoring data in accordance with Directive 2003/99/EC are not comparable between MSs and preclude assessing temporal and spatial trends at the EU level.
In certain food categories, different sampling designs and inaccuracies due to limited numbers of samples also preclude an accurate estimation of prevalence.
Nevertheless, descriptive summaries of sample statistics at the EU level can be compiled and used to indicate the circulation of certain STEC types in food and animals, provided that the above‐mentioned relevant limitations of the data set are taken into consideration.
To improve the quality of EU data on STEC monitoring of food and animals, EFSA issued technical specifications for harmonised monitoring and reporting of STEC in animals and foodstuffs in 2009 (EFSA, 2009c). With an additional Scientific Opinion, EFSA encouraged MSs to extend the monitoring and reporting of data on STEC serogroups (EFSA BIOHAZ Panel, 2013c). More recently, it has been recommended that the presence of the main virulence genes be reported, considering the most recent developments in STEC testing and risk assessment (FAO and WHO, 2018; NACMCF, 2019; EFSA BIOHAZ Panel, 2020b). Finally, the latest published EFSA Scientific Opinion on the pathogenicity assessment of STEC presents important considerations related to the virulence of the different STEC types and underlines the significance of determining the virulence gene combinations (virulotypes) of the isolated STEC strains, with an emphasis on stx gene subtyping, which would facilitate a more precise assessment of the risk connected with different STEC isolates (EFSA BIOHAZ Panel, 2020b).
4.3. Data analyses
4.3.1. Occurrence in food and animals
The monitoring data on sprouts as part of Regulation (EC) No 2073/2005 were aggregated and summarised for trend watching according to the following ‘filters’: Sampling context: ‘surveillance, based on Regulation No 2073/2005’; Sampling unit type: ‘single'; Sampling stage: as appropriate; Sampling strategy: ‘objective sampling’ and Sampler: ‘official sampling’.
For the description of the occurrence of STEC‐positive samples in the different food categories, the subset of the monitoring data with ‘objective sampling’ specified as sampling strategy was used (N = 17,516), meaning that the reporting MSs collected the samples according to a planned strategy based on the selection of random samples statistically representative of the population to be analysed.
4.3.2. Serogroups and virulence features in food and animals
The full data set was used for the descriptive analyses of STEC findings in food (N = 23,659) and animals (N = 3,746), primarily those on virulence gene types and on their frequency distribution.
To analyse the STEC serogroups, the data were grouped according to the test method used, because 3.8% of food samples and 41.5% of animal samples were still assayed using methods detecting only E. coli O157. This distinction between methods was necessary to minimise the impact of E. coli O157‐specific methods on the analysis of the serogroup distribution given that the latter do not identify other STEC possibly present in the samples.
In 2021, the methods targeting any STEC included the ISO TS 13136:2012 method (ISO, 2012), stx gene PCR‐based methods and the DIN10118:2004 (DIN, 2004a) standard based on the immunochemical detection of Stx by colony blot. The methods designed to detect only E. coli O157 were the ISO 16654:2001 method (ISO, 2001), the NMKL 164:2005 method (NMKL, 2005) and the DIN 10167:2004–03 (DIN, 2004b) method. The method indicated as the OIE method for E. coli O157 is an adaptation of the ISO 16654:2001 method used for animal samples (WOAH, 2018b).
4.4. Results
4.4.1. Overview of key statistics, EU, 2017–2021
Table 38 summarises EU‐level statistics on human STEC infections and on samples from food and animals tested for STEC during 2017–2021. Food and animal data were classified into major categories and aggregated by year to obtain an annual overview of the volume of data submitted. More detailed descriptions of these statistics are provided in the below subsections and in the chapter on foodborne outbreaks.
Table 38.
Summary of STEC statistics related to humans, major food categories and the main animal species, EU, 2017–2021
| 2021 | 2020 | 2019 (a) | 2018 (a) | 2017 (a) | Data source | |
|---|---|---|---|---|---|---|
| Humans | ||||||
| Total number of confirmed cases | 6,084 | 4,489 | 7,801 | 8,167 | 6,071 | ECDC |
| Total number of confirmed cases/100,000 population (notification rates) | 2.1 | 1.5 | 1.9 | 2.1 | 1.7 | ECDC |
| Number of reporting MSs | 27 | 27 | 28 | 28 | 28 | ECDC |
| Infection acquired in the EU | 4,355 | 3,370 | 4,836 | 5,783 | 4,747 | ECDC |
| Infection acquired outside the EU | 117 | 148 | 751 | 693 | 525 | ECDC |
| Unknown travel status or unknown country of infection | 1,612 | 971 | 2,214 | 1,691 | 799 | ECDC |
| Number of foodborne outbreak‐related cases | 275 | 208 | 273 | 390 | 260 | EFSA |
| Total number of foodborne outbreaks | 31 | 34 | 42 | 50 | 48 | EFSA |
| Food | ||||||
| All | ||||||
| Number of sampling units | 23,659 | 22,119 | 25,030 | 20,498 | 19,351 | EFSA |
| Number of reporting MSs | 22 | 22 | 22 | 20 | 22 | EFSA |
| Meat and meat products | ||||||
| Number of sampling units | 12,160 | 10,866 | 14,110 | 9,250 | 10,706 | EFSA |
| Number of reporting MSs | 19 | 17 | 20 | 17 | 18 | EFSA |
| Milk and milk products | ||||||
| Number of sampling units | 4,094 | 4,665 | 5,479 | 5,339 | 3,485 | EFSA |
| Number of reporting MSs | 11 | 10 | 13 | 14 | 10 | EFSA |
| Fruits and vegetables | ||||||
| Number of sampling units | 3,976 | 3,398 | 2,696 | 3,371 | 2,323 | EFSA |
| Number of reporting MSs | 14 | 15 | 13 | 13 | 15 | EFSA |
| Animals (b) | ||||||
| All | ||||||
| Number of sampling units | 3,746 | 2,112 | 2,588 | 1,631 | 2,217 | EFSA |
| Number of reporting MSs | 7 | 6 | 9 | 5 | 7 | EFSA |
| Cattle (bovine animals) | ||||||
| Number of sampling units (b) | 3,316 | 868 | 1,615 | 1,112 | 1,681 | EFSA |
| Number of reporting MSs | 5 | 3 | 7 | 5 | 6 | EFSA |
| Small ruminants: sheep, goats, deer | ||||||
| Number of sampling units (b) | 151 | 227 | 320 | 188 | 210 | EFSA |
| Number of reporting MSs | 2 | 2 | 6 | 4 | 2 | EFSA |
ECDC: European Centre for Disease Prevention and Control; EFSA: European Food Safety Authority; MSs: Member States; STEC: Shiga toxin‐producing Escherichia coli.
: Data from the United Kingdom are taken into account for 2017–2019, because the United Kingdom was an EU MS, but it became a third country on 1 February 2020.
: Individual animals.
4.4.2. STEC infections in humans
In 2021, 6,084 confirmed cases of STEC infections were reported in the EU (Table 39). Twenty‐four MSs reported at least one confirmed STEC case and three MSs reported zero cases. In 2021, the EU notification rate was 2.1 per 100,000 population. In 2021, there was an increase of 9.9% and 14.2% compared with the average annual notification rate calculated for years 2017–2019 with and without the data from the United Kingdom, respectively. If 2020 data are considered, the increase in the notification rate was higher: 36.9%. However, 2020 data collection was likely affected by the COVID‐19 pandemic.
Table 39.
Reported human cases of STEC and notification rates per 100,000 population in EU MSs and non‐MS countries, by country and year, 2017–2021
| Country | 2021 | 2020 | 2019 | 2018 | 2017 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| National coverage (a) | Data format (a) | Confirmed cases and rates | Confirmed cases and rates | Confirmed cases and rates | Confirmed cases and rates | Confirmed cases and rates | ||||||
| Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | |||
| Austria | Y | C | 383 | 4.3 | 288 | 3.2 | 284 | 3.2 | 305 | 3.5 | 250 | 2.8 |
| Belgium | Y | C | 124 | 1.1 | 84 | 0.7 | 131 | 1.1 | 112 | 0.98 | 123 | 1.1 |
| Bulgaria | Y | A | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Croatia | Y | C | 12 | 0.30 | 8 | 0.20 | 22 | 0.54 | 10 | 0.24 | 7 | 0.17 |
| Cyprus | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Czechia | Y | C | 36 | 0.34 | 32 | 0.30 | 33 | 0.31 | 26 | 0.25 | 37 | 0.35 |
| Denmark | Y | C | 927 | 15.9 | 445 | 7.6 | 623 | 10.7 | 493 | 8.5 | 263 | 4.6 |
| Estonia | Y | C | 7 | 0.53 | 10 | 0.75 | 6 | 0.45 | 7 | 0.53 | 3 | 0.23 |
| Finland | Y | C | 288 | 5.20 | 175 | 3.2 | 311 | 5.6 | 210 | 3.8 | 123 | 2.2 |
| France (b) | N | C | 298 | – | 262 | – | 335 | – | 259 | – | 260 | – |
| Germany | Y | C | 1,635 | 2.0 | 1,409 | 1.7 | 1,907 | 2.3 | 2,226 | 2.7 | 2,065 | 2.5 |
| Greece | Y | C | 10 | 0.09 | 3 | 0.03 | 5 | 0.05 | 1 | 0.01 | 3 | 0.03 |
| Hungary | Y | C | 24 | 0.25 | 8 | 0.08 | 23 | 0.24 | 14 | 0.14 | 12 | 0.12 |
| Ireland | Y | C | 878 | 17.5 | 734 | 14.8 | 798 | 16.3 | 966 | 20.0 | 795 | 16.6 |
| Italy (b) | N | C | 65 | – | 45 | – | 62 | – | 73 | – | 92 | – |
| Latvia | Y | C | 13 | 0.69 | 2 | 0.10 | 48 | 2.5 | 3 | 0.16 | 1 | 0.05 |
| Lithuania | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Luxembourg | Y | C | 10 | 1.6 | 0 | 0 | 4 | 0.65 | 3 | 0.50 | 1 | 0.17 |
| Malta | Y | C | 68 | 13.2 | 43 | 8.4 | 53 | 10.7 | 41 | 8.6 | 9 | 2.0 |
| Netherlands | Y | C | 484 | 2.8 | 323 | 1.9 | 459 | 2.7 | 488 | 2.8 | 392 | 2.3 |
| Poland | Y | C | 7 | 0.02 | 3 | 0.01 | 14 | 0.04 | 6 | 0.02 | 4 | 0.01 |
| Portugal | Y | C | 2 | 0.02 | 5 | 0.05 | 1 | 0.01 | 2 | 0.02 | 1 | 0.01 |
| Romania | Y | C | 6 | 0.03 | 14 | 0.07 | 36 | 0.19 | 20 | 0.10 | 11 | 0.06 |
| Slovakia | Y | C | 5 | 0.09 | 1 | 0.02 | 3 | 0.06 | 12 | 0.22 | 3 | 0.06 |
| Slovenia | Y | C | 48 | 2.3 | 30 | 1.4 | 31 | 1.5 | 32 | 1.5 | 33 | 1.6 |
| Spain (c) , (d) | N | C | 101 | – | 74 | – | 269 | 0.57 | 126 | 0.27 | 86 | – |
| Sweden | Y | C | 653 | 6.3 | 491 | 4.7 | 756 | 7.4 | 892 | 8.8 | 504 | 5.0 |
| EU Total 27 | – | – | 6,084 | 2.0 | 4,489 | 1.5 | 6,214 | 1.8 | 6,327 | 1.9 | 5,078 | 1.7 |
| United Kingdom | – | – | – | – | – | – | 1,587 | 2.4 | 1,840 | 2.8 | 993 | 1.5 |
| EU Total (e) | – | – | 6,084 | 2.1 | 4,489 | 1.5 | 7,801 | 1.9 | 8,167 | 2.1 | 6,071 | 1.7 |
| Iceland | Y | C | 7 | 1.9 | 4 | 1.1 | 27 | 7.6 | 3 | 0.86 | 3 | 0.89 |
| Norway | Y | C | 437 | 8.1 | 331 | 6.2 | 511 | 9.6 | 494 | 9.3 | 381 | 7.2 |
| Liechtenstein | Y | C | 6 | 15.4 | 715 | 8.3 | 966 | 11.3 | 887 | 10.4 | 746 | 8.8 |
| Switzerland (f) | Y | C | 915 | 10.6 | ||||||||
–: Data not reported.
: Y: yes; N: no; A: aggregated data; C: case‐based data.
: Sentinel surveillance; mainly haemolytic‐uraemic syndrome (HUS) cases are notified.
: Notification rate was not calculated because information on estimated coverage was not available.
: Data incomplete for 2021 and 2020, rate not estimated.
: Cases reported by the United Kingdom for the 2017–2019 period were also considered for this estimation (EU‐28). When the United Kingdom data were collected for the period 2017–2019, the United Kingdom was an EU MS, but it became a third country on 1 February 2020.
: Switzerland provided the data directly to EFSA. The human data for Switzerland include data from Liechtenstein for years 2017–2020.
The highest country‐specific notification rates among the EU MSs were observed in Ireland, Denmark and Malta (17.54, 15.87 and 13.18 cases per 100,000 population, respectively). Five countries (Romania, Portugal, Greece, Slovakia and Poland) reported ≤ 0.1 cases per 100,000 population.
Most STEC cases reported were infected in the EU (71.6% domestic cases or travel in the EU, 1.9% travel outside EU and 26.5% of unknown travel history or unknown country of infection) (Table 38). Overall, for the year 2021, 98.6% of the 4,355 reported STEC cases in humans who acquired the infection in the EU (Table 38) were domestic (acquired within the home country) and 1.4% were acquired through travel in the EU. The proportion of human STEC cases infected within the EU remained stable during 2017–2021, excluding the decrease observed in 2020 during the pandemic.
In 2021, the number of cases infected through travel outside the EU continued to be considerably lower than that reported in 2019, as already observed for the 2020 data. In fact, the proportion decreased from 9.7% in 2019 to 3.1% in 2020 (EFSA and ECDC, 2021) and reached 2.6% in 2021, probably reflecting the disruption in travel caused by the COVID‐19 pandemic still in the first half of 2021.
Germany, Sweden, the Netherlands, Austria and Finland reported the highest number of travel‐associated cases (107, 97, 33, 18 and 16, respectively), altogether representing 92.2% of all the imported cases (EU and non‐EU).
Turkey was most frequently reported as the probable country of infection, followed by Egypt and Kosovo among the non‐EU countries (36.8%, 11.1% and 5.1% of the cases with a known probable country of infection, respectively).
The seasonal trend in confirmed STEC cases observed in the EU between 2012 and 2020 was maintained in 2021, with more cases reported during the summer months (Figure 10). The observed STEC infection seasonality is in line with that reported in the literature (Sapountzis et al., 2020). The overall trend for STEC in 2017–2021 did not show any statistically significant increase or decrease, even considering the drop observed in 2020, likely due to the COVID‐19 pandemic. At the MS level, a statistically significant increasing trend (p < 0.05) was observed over years 2017–2021 in Denmark and Malta.
Figure 10.
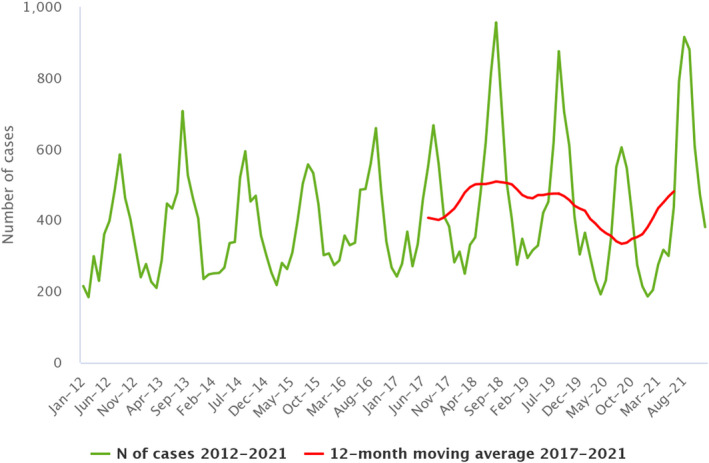
- Source: Austria, Cyprus, Denmark, Estonia, Finland, France, Germany, Greece, Ireland, Italy, Lithuania, Luxembourg, Malta, Netherlands, Poland, Romania, Slovenia and Sweden.
Seventeen MSs provided information on hospitalisation for 35.1% of all confirmed STEC cases in the EU in 2021. Out of the 2,133 cases with known hospitalisation status, 42.2% were hospitalised. The highest proportions of hospitalised cases were reported by Greece, Poland and Slovakia (100% each). However, these MSs only reported a few cases of infection (10, 7 and 5, respectively). Other MSs also reporting high proportions of hospitalised cases were Italy (83.1%), Latvia (76.9%), Estonia (71.4%) and Romania (66.7%). Noteworthily, the high proportion of hospitalised cases observed in Italy is due to the sentinel surveillance system adopted, which is mainly based on HUS notification. A total of 362 HUS cases were reported in 2021 in almost all age groups, with the highest proportion of patients in the youngest age groups from 0–4 years (233 cases; 64.4%) to 5–14 years (71 cases; 19.6%). The most common serogroups among HUS cases were O26 (34% of all cases with serogroup reported), O157 (19.6%), O80 (11%) and O145 (7.6%).
In 2021, 18 fatalities in patients with a STEC infection were reported in the EU, compared with 13 deaths in 2020. Seven MSs reported one to five fatal cases each and 13 MSs reported no fatal cases. This resulted in an EU case fatality of 0.4% among the 4,366 confirmed cases with known outcome (71.8% of all reported confirmed cases). Deaths were mostly reported in the 0–4 years age group (27.8%), followed by the 85+ age group (16.7%).
4.4.3. STEC in food
For the year 2021, 22 MSs provided results from analyses of 23,659 food units (batches or single samples).
The most recent source attribution analysis available for STEC underlined that ‘bovine meat and products thereof’, ‘milk and dairy products’ and ‘vegetables, fruits and products thereof’ were the vehicles most frequently implicated in STEC infections in the EU during the 2012–2017 period (inclusive) (EFSA BIOHAZ Panel, 2020b), confirming the results of previous reports (FAO and WHO, 2018). These categories are indeed the most tested in the EU and in 2021 represented 74.5% of the food sample units tested, considering the subset of the monitoring data with ‘objective sampling’ specified as sampling strategy (N = 17,516) (Table 40).
Table 40.
Occurrence of Shiga toxin‐producing E. coli (STEC) in the main food categories, EU, 2021 and 2017–2020
| Food | 2021 | 2017–2020 (a) | ||||
|---|---|---|---|---|---|---|
| N reporting MSs | N sampling units | Positive N (%) | N reporting MSs | N sampling units | Positive N (%) | |
| RTE food | ||||||
| All | 16 | 7,444 | 112 (1.5) | 19 | 24,429 | 226 (0.93) |
| Meat and meat products | 8 | 1,276 | 20 (1.6) | 9 | 5,116 | 72 (1.4) |
| Meat and meat products from bovine animals | 5 | 643 | 11 (1.7) | 7 | 2,517 | 45 (1.8) |
| Meat and meat products from pigs | 3 | 155 | 1 (0.65) | 6 | 494 | 5 (1.0) |
| Other meat and meat products | 6 | 478 | 8 (1.7) | 6 | 2,105 | 22 (1.0) |
| Milk and milk products | 8 | 2,271 | 39 (1.7) | 11 | 7,507 | 98 (1.3) |
| Milk | 2 | 199 | 4 (2.0) | 5 | 727 | 33 (4.5) |
| Raw milk (b) | 1 | 107 | 2 (1.9) | 4 | 714 | 33 (4.6) |
| Cheese | 8 | 1,930 | 32 (1.7) | 11 | 6,363 | 63 (1.0) |
| Dairy products excluding cheeses (butter, cream, ice cream, whey, yoghurt and fermented dairy products) | 3 | 142 | 3 (2.1) | 5 | 417 | 2 (0.48) |
| Bakery products | 2 | 587 | 37 (6.3) | 3 | 106 | 2 (1.9) |
| Fruits, vegetables and juices | 11 | 1,922 | 10 (0.52) | 10 | 6,350 | 9 (0.14) |
| Spices and herbs | 3 | 296 | 1 (0.34) | 7 | 1,953 | 8 (0.41) |
| Ready‐to‐eat salads | 2 | 301 | 0 (0) | 4 | 531 | 1 (0.19) |
| Seeds, sprouted | 10 | 617 | 1 (0.16) | 11 | 1,730 | 0 |
| Non‐RTE food | ||||||
| All | 17 | 10,072 | 518 (5.1) | 22 | 40,921 | 1,329 (3.2) |
| Meat and meat products | 16 | 7,848 | 503 (6.4) | 21 | 32,172 | 1,217 (3.8) |
| Milk and milk products | 6 | 714 | 14 (2.0) | 9 | 2,916 | 103 (3.5) |
| Fruits, vegetables and juices | 9 | 1,188 | 0 (0) | 10 | 2,716 | 1 (0.04) |
| Fresh meat (c) | ||||||
| All | 16 | 6,700 | 469 (7.0) | 21 | 27,219 | 1,022 (3.8) |
| Fresh meat from bovine animals | 16 | 5,095 | 288 (5.7) | 21 | 21,017 | 514 (2.4) |
| Fresh meat from pigs | 7 | 604 | 100 (16.6) | 9 | 1,226 | 63 (5.1) |
| Fresh meat from goats | 2 | 2 | 0 (0) | 3 | 26 | 1 (3.8) |
| Fresh meat from sheep | 4 | 505 | 49 (9.7) | 6 | 2,943 | 309 (10.5) |
| Other fresh meat | 7 | 494 | 32 (6.5) | 10 | 2,007 | 135 (6.7) |
STEC: Shiga toxin‐producing Escherichia coli; N: Number; MSs: Member States; RTE: ready‐to‐eat.
: Data from the United Kingdom are taken into account for 2017–2019, because the United Kingdom was an EU MS, but it became a third country on 1 February 2020.
: Raw RTE milk sampling units are a subset of RTE milk.
: Fresh meat sampling units are a subset of the two main categories (RTE food and non‐RTE food).
Data collected in the context of Regulation (EC) No 2073/2005
As regards 2021 data for STEC on sprouted seeds from the at the distribution level in the context of Regulation (EC) No 2073/2005, 617 samples were tested by 10 MSs with one positive batch (Table 40). This included 388 single official control samples, taken by CAs to verify the compliance of FBOp with the STEC food safety criterion, with none positive. As noted in previous years, testing sprouted seeds is still not widely applied at the EU level, although a microbiological criterion for this food commodity has been laid down in Regulation (EC) No 2073/2005 since 2013.
Other STEC monitoring data from food
Overall, 3.6% of the 17,516 food sample units tested by 21 MSs, and collected using an objective sampling strategy, were positive for STEC. For years 2020, 2019, 2018 and 2017, the reported percentages of STEC‐positive food samples were lower, namely 2.4%, 2.8%, 2.8% and 1.5%, respectively (EFSA and ECDC, 2021). In Table 40, these monitoring results are summarised, and a distinction is made between RTE food, non‐RTE food and ‘fresh meat’.
RTE food
As regards RTE food, testing of 7,444 samples was reported. The food category with the highest number of sample units tested was ‘milk and milk products’ with 2,271 samples assayed, notably cheeses (25.9%), followed by ‘fruits, vegetables and juices’ (25.8%), ‘meat and meat products’ (17.1%), ‘seeds, sprouted’ (8.3%) and bakery products (6.3%). In total, 112 RTE food samples were found to be positive for STEC: 1.6% in ‘meat and meat products’ (notably of bovine origin), 1.7% in ‘milk and milk products’ particularly milk, 0.5% in the ‘fruits, vegetables and juices’ category and 0.3% in ‘spices and herbs’ (Table 40).
RTE and non‐RTE food
To provide overall statistics, the following descriptive analyses are based on merged data from RTE food and non‐RTE food.
Meat and meat products
Bovine meat
In 2021, 5,095 units of fresh and unprocessed bovine meat were tested. At the distribution stage, 2,815 units were sampled by 12 MSs with 4.1% of positives. Samples (N = 2,280) taken at the manufacturing level were the most contaminated (7.5%, 9 MSs), particularly at the slaughterhouse with 9.1% of the samples testing positive for STEC.
Sheep and goat meat
Small ruminants are an important reservoir of STEC as reported in the literature (Persad and LeJeune, 2014). In 2021, four MSs reported the results of an investigation on 505 sample units of fresh sheep meat with 9.7% of these being STEC‐positive, whereas two MSs reported on fresh goat meat with no STEC‐positive sampling units out of the two tested (Table 40). This proportion of contaminated samples is consistent with that from the analysis of 2017–2020 data (10.5%). However, this food commodity is generally tested in surveys by very few MSs.
The sampling stage that yielded the highest proportion of positive sheep meat samples was the distribution level, with 14.3% of samples producing a STEC isolate. Conversely, the samples taken at the slaughterhouse showed a much lower STEC contamination rate (4.6%).
Meat from other ruminants
Only three MSs provided information on the presence of STEC in fresh deer meat samples. In total, 101 samples were taken and 17 were found to be contaminated with STEC (16.8%). The sampling stage with highest percentage of samples contaminated by STEC was the manufacturing level (29.4%). Deer hunting may have contributed to this result because the use of firearms and the slaughtering procedures may favour gut perforation and the contamination of meat, respectively.
Meat from other animal species
Seven MSs tested fresh pig meat in 2021 and reported data on 604 samples, with 100 of these being positive for STEC (16.6%) (Table 40).
Fresh meat from animal species other than bovine, ovine, goat, pig and deer species was tested in 2021 by seven MSs that reported on the analyses carried out on 494 sample units (Table 40). These included samples taken from poultry, ducks, wild and farmed game, geese, horses, rabbits, wild boars and unspecified meat. Thirty‐two samples were reported as STEC‐positive (6.5%) (Table 40).
Meat products and meat preparations
Meat products and meat preparations other than fresh meat were sampled in 2021 by 11 MSs that tested 2,485 samples, resulting in 58 isolated STEC strains (2.3%).
Milk and milk products
Overall, STEC was found in 53 (1.8%) out of 2,985 samples of RTE and non‐RTE milk and milk products including cheese reported by eight MSs (Table 40).
In 2021, seven MSs reported on the testing of 503 sample units of raw cows' milk with seven positive units (1.4%). One MS reported monitoring results on 13 sample units of raw goats' milk, and two MS reported only 8 samples of raw sheep milk. Both categories recorded one positive sample each.
The presence of STEC in RTE dairy products other than milk and cheeses was reported by three MSs, which tested 142 sample units of butter, cream, ice cream, yoghurt and fermented dairy products. Three positive samples were detected (Table 40).
For the cheese samples, 1,930 samples were tested for the presence of STEC, with 32 (1.7%) positive units from eight MSs in 2021.
Vegetables and fruits
STEC were found in 10 (0.3%) out of 3,110 samples of fruits and vegetables (Table 40). In total, 1,214 sample units of RTE spice and herbs, salads and sprouted seeds were tested and 2 STEC‐positive units were reported (0.2%) (Table 40).
Other foodstuffs
This category contains miscellaneous food commodities not comprised in the previously mentioned categories, and included cereals and meals, bakery products, fish and fishery products, infant formula and others. For the whole category, 865 samples were analysed by five MSs with 39 (4.5%) positive samples reported from the cereals and meals (2) and bakery products (37) food categories.
4.4.4. STEC in animals
For the year 2021, results from 3,746 sampling units (single heads or herds or flocks) from animals were reported by seven MSs, (Table 41), and no data from non‐MSs have been reported. This number has increased compared with the number of animals tested during 2017–2020.
Table 41.
Summary of STEC statistics related to major animal species, reporting EU MSs, 2021
| Animals | N reporting MSs | N tested sampling units | Positive sampling units | |
|---|---|---|---|---|
| N | % | |||
| Cattle | 5 | 3,316 | 195 | 5.9 |
| Sheep and goats | 1 | 48 | 7 | 14.6 |
| Other ruminants (a) | 2 | 121 | 1 | 0.83 |
| Pigs | 1 | 51 | 6 | 11.8 |
| Other animals/not specified | 2 | 210 | 20 | 9.6 |
| Total | 7 | 3,746 | 229 | 6.1 |
MSs: Member States; STEC: Shiga toxin‐producing Escherichia coli.
: Other ruminants include Cantabrian chamois, deer and water buffalos.
The most tested animal category in 2021 was cattle, with 3,316 sample units tested (88.5% of all animal samples tested) by five MSs and 6.1% of positives (Table 41). The number of samples tested for this animal category showed an approximately four‐fold increase with respect to the previous year and a two‐fold increase considering the 2017–2019 period (EFSA and ECDC, 2021). The proportion of positive samples varied significantly with sampling stage. The 322 sample units taken at the farm level yielded 17.4% of positives, and the testing of the 2,994 samples taken at the slaughterhouse returned 139 STEC positive samples (4.6%).
The other animal species tested included pigs, Cantabrian chamois, deer and sheep among others, with only few samples assayed per category by three MSs.
4.4.5. Focus on STEC strain features: virulence genes and serogroups
Humans
Data on STEC serogroups were reported in 2021 by 22 MSs for 56.5% of the confirmed human cases. The most frequently reported serogroups were O157 (15.2% of the human cases reported with information on the serogroup) and O26 (14.8%). These two serogroups together represented 30% of the total number of confirmed human cases with known serogroups in 2021. The proportion of non‐typable STEC isolates rose to 25.9% in 2021 (18.3% in 2020).
Data on virulotypes (based on Shiga toxin genes stx1, stx2 and the intimin‐coding gene eae) were reported for 35% (N = 2,130) of confirmed STEC infections (N = 6,084) in 2021. The most frequently reported virulence gene combination in strains isolated from severe cases (HUS, bloody diarrhoea and hospitalised cases) was stx2+/eae+, accounting for 34.1% of the strains with a known virulotype (Table 42), half of which were from HUS cases (84 strains). The proportion of the second most common virulotype stx1+/stx2+/eae + accounted for 26.2% of these cases (Table 42). The most common stx gene subtypes in strains from severe human infections were stx2a (38.3% of isolates with reported stx gene subtyping data), stx1a (27.7%), stx2c (8.8%) and stx2d (9.0%) (Table 43).
Table 42.
Virulotypes (stx type and presence of eae) in food, animal and human STEC isolates causing severe infection (haemolytic‐uraemic syndrome (HUS), hospitalisation and bloody diarrhoea) in 2021 and comparison with those associated with severe disease in humans during 2012–2017, EU
| Virulence genes profile | N of animal isolates in 2021 (a) | N of food isolates in 2021 (a) | N of human isolates in 2021 (%) | Relative frequency of the virulotype in (b) | ||
|---|---|---|---|---|---|---|
| HUS | Hospitalisation | Bloody diarrhoea | ||||
| stx2; eae+ | 53 | 23 | 164 (34.1) | 17.7 | 42.0 | 40.2 |
| stx1; stx2; eae+ | 17 | 10 | 126 (26.2) | 5.9 | 35.7 | 64.8 |
| stx1; eae+ | 43 | 34 | 58 (12.1) | 1.2 | 27.4 | 27.3 |
| stx2; eae‐ | – | 139 | 74 (15.4) | 2.7 | 24.3 | 14.8 |
| stx1; eae‐ | – | 61 | 34 (7.1) | 0.3 | 20.3 | 14.1 |
| stx1; stx2, eae‐ | – | 17 | 25 (5.2) | 1.4 | 15.3 | 19.4 |
| Total | 77 | 284 | 481 (100) | – | – | – |
STEC: Shiga toxin‐producing Escherichia coli; HUS: haemolytic‐uraemic syndrome. The stx genes were characterised at the type level (stx1 and stx2).
: Due to the low number of isolates virulotyped for food and animals, only the number of isolates is shown.
: Relative frequencies (%) of the different combinations of stx gene types with or without the eae gene in STEC isolated from severe disease (TESSy data, 2012–2017) (EFSA BIOHAZ Panel, 2020b).
Table 43.
Virulotypes (stx subtype) of food and human STEC isolates causing severe infection (haemolytic‐uraemic syndrome, hospitalisation and bloody diarrhoea) in 2021 and comparison with those associated with severe disease in humans during 2012–2017, EU
| stx genes subtypes combinations | N of food isolates in 2021 (a) | N of human isolates in 2021 (%) | Relative frequency of the stx gene subtype combinations in (b) | |||||
|---|---|---|---|---|---|---|---|---|
| HUS | Hospitalisation | Bloody diarrhoea | ||||||
| eae+ | eae− | eae+ | eae− | eae+ | eae− | |||
| stx2a | 20 | 184 (38.3) | 27.4 | 10.4 | 56.4 | 32 | 58.4 | 26.3 |
| stx1a | 15 | 133 (27.7) | 1.2 | 0 | 27.6 | 20.7 | 27.3 | 8 |
| stx2d | 7 | 43 (9.0) | NR | 10.3 | NR | 33.3 | NR | 16 |
| stx2c | 7 | 42 (8.8) | 4.3 | 5 | 19.8 | NR | 23.9 | NR |
| stx2b | 1 | 29 (6.0) | NR | 0.5 | NR | 21.3 | NR | 10.5 |
| stx1c | 2 | 18 (3.8) | NR | 0.6 | NR | 18.9 | NR | 19.5 |
| stx2f | ND | 18 (3.8) | 3.8 | NR | 21 | NR | 8.7 | NR |
| stx2c;stx2a | ND | 5 (1.0) | 29 | NR | 57.1 | NR | 65.5 | NR |
| stx2g | 3 | 3 (0.6) | NR | – | – | NR | NR | NR |
| stx2c;stx2d | ND | 3 (0.6) | – | – | – | – | – | – |
| stx2e | 8 | 1 (0.2) | – | NR | NR | NR | NR | 31.8 |
| stx2b;stx2c | ND | 1 (0.2) | – | – | – | – | – | – |
| stx2d;stx1a | 5 | – | – | – | – | – | – | |
| stx1d | 2 | – | – | – | – | – | – | |
| stx2a;stx1a | 7 | 20.8 | 4.5 | 59.3 | NR | 56.6 | NR | |
| stx2d;stx2c;stx2a | 1 | – | – | – | – | – | – | |
| stx2c;stx1a | 1 | – | – | – | – | – | – | |
| Total | 79 | 480 | – | – | – | – | – | – |
Note: Two STEC isolates with subtypes stx2a and stx2c/stx2a, respectively, were reported in animals in 2021.
STEC: Shiga toxin‐producing Escherichia coli; HUS: haemolytic‐uraemic syndrome. The stx genes were characterised at the subtype level.
NR: Data present in the TESSy data set used with fewer than 20 isolates.
ND: Not detected.
–: not present in the TESSy database during the 2012–2017 period.
: Due to the low number of isolates virulotyped for food, only the number of isolates is given.
: Relative frequencies (%) of the different combinations of stx gene subtypes in STEC isolated from severe disease (TESSy data. 2012–2017). Human data from: EFSA Journal 2020;18(1):5967.
Food
Most of the 20 most common STEC serogroups isolated from human infections were also found in the STEC isolated from food in 2021, except for serogroups O55, O63, O80, O125 and O177. The information on the serogroup was available for 239 (27.4%) out of 872 strains isolated from the overall 23,659 sample units tested in 2021.
Information on stx1 and/or stx2 and eae genes was provided for 284 (32.6%) of the STEC strains (Table 42). Only 79 STEC isolates were subtyped for the stx gene (Table 43). This was 27.8% of the isolates with stx types and eae genes reported, and was 9.1% of the total number of STEC isolated from food in 2021. Tables 42 and 43 show the combinations of the virulence genes determined in the food, animal and human STEC isolates in 2021 and the numbers and frequencies of these combinations in the STEC isolated from severe human disease in the EU in 2012–2017, as analysed in the latest pathogenicity assessment of STEC (EFSA BIOHAZ Panel, 2020b). Given the scarce amount of data on the virulence genes characterised in food and animal isolates in 2021, the number of isolates are displayed instead of the relative frequency for each virulotype.
Animals
This section includes the analysis of the data related to the 3,746 sampling units from animals tested, of which 6.1% (N = 229) were positive for the presence of STEC.
For the analysis of the distribution of STEC serogroups and virulotypes, all STEC strains isolated from the animal sampling units were considered and from some units multiple isolates were obtained. In total there were 245 STEC strains and of 201 strains (82%) were provided with information on the serogroups. However, 36 isolates were obtained using the analytical methods detecting the O157 serogroup only. The isolates with serogroup information belonged to 22 different O‐groups, with 8 of these represented in the top 20 serogroups isolated from human disease in 2021.
For the analysis of the virulence genes of STEC strains, 113 STEC animal isolates (46.1%) were provided with the virulotype based on the identification of the stx1, stx2 and eae genes (Table 42). Two MSs also carried out stx gene subtyping and reported this information for only two STEC strains (stx2a/stx2c; stx2a).
All data provided by the reporting countries were used to generate atlases of the STEC serogroups identified in the different food and animal categories compared with those determined in human isolates for years 2017–2021 (Appendix C) and for 2021 for food and animal isolates only (Appendices D and E).
4.5. Discussion
In 2020, there was a decline in the notification rates of STEC infections, probably due to the COVID‐19 pandemic. In 2021, the number of reported cases was comparable to those reported during the pre‐pandemic period (2017–2019).
In 2021, more than half of the confirmed human cases reported by EU MSs had information on the serogroup with the most frequently reported being O157, followed by O26, although the two proportions diverged by only 0.4%.
It has been proposed that the highest predictive power in terms of pathogenicity potential of STEC strains resides in the characterisation of the Shiga toxin‐coding genes (stx) and, to a lesser extent, the intimin‐coding eae gene (FAO and WHO, 2018; NACMCF, 2019; EFSA BIOHAZ Panel, 2020b). Moreover, stx gene subtyping helps to identify the strains that have a higher frequency of association with severe disease (EFSA BIOHAZ Panel, 2020b). Regarding subtyping capacity, more than half of the MSs' national public health laboratories reported the ability to perform whole‐genome sequencing for STEC isolates (EFSA BIOHAZ Panel, 2020b). This is a promising perspective, possibly fostering increased reporting of typing and subtyping data for STEC isolates in the coming years.
In 2021, more than one third of the STEC cases were reported with information on the stx (stx1 or stx2) and the eae genes and only 22.5% of the isolates with a reported stx1, stx2 and eae virulotype were provided with information on stx gene subtypes. Based on the analysis of the stx subtypes reported in TESSy from 2012 to 2017 (EFSA BIOHAZ Panel, 2020b), all STEC virulence gene combinations and most of the stx gene subtypes identified in 2021 can be associated with severe illness, albeit at different frequencies.
More than 40% of the cases with information on hospitalisation status were hospitalised. The highest proportions of hospitalised cases were reported by Greece, Poland and Slovakia, countries that accounted for 100% of the hospitalised cases; however, these countries reported low numbers of cases, below or equal to 10. A total of 362 HUS cases were reported in 2021 in almost all age groups, with the highest proportion of patients in the youngest age groups, from 0 to 4 years. Most cases of deaths were reported in this age group followed by the 85+ age class.
In 2021, 22 EU MSs reported monitoring results of STEC in 23,659 food samples. Not all reporting MSs have tested all food categories equally. After aggregating the food samples into macro‐categories in 2021, the number of MSs testing and reporting data on the presence of STEC in food ranged from 19 MSs reporting the testing for STEC in meat and vegetables (including seeds) to 11 MSs testing milk and dairy products. Sprouted seeds were tested by 10 MSs, considering the samples taken in the context of Regulation (EC) No 2073/2005. As noted in previous years, although the microbiological criterion for the presence of STEC in seeds and sprouts has a normative value, the sampling of this food categories in the EU appears to be infrequent.
The analytical procedures for testing food in the EU have been substantially harmonised. In 2021, all the reporting countries used the ISO TS 13136:2012 or equivalent method to test 22,760 samples (96.2%) out of the 23,659 total samples tested. The remaining data reported by some MSs (four) for specific surveys were obtained with the ISO 16654:2001 or equivalent method. This method detects serogroup O157 only and does not give information on any other STEC serogroups possibly present in the sample.
The general extent of observed STEC contamination in food, assessed using food samples taken according to an ‘objective sampling’ strategy, was 3.6%. As observed in previous years, the frequencies of STEC contamination varied among the different major food categories, RTE and non‐RTE. The most contaminated food categories included commodities of animal origin, whereas vegetables and fruits were the least contaminated or did not yield positive results at all. The finding of 6.3% of bakery products samples contaminated with STEC is interesting. However, most of the contaminated samples were reported by one single MS, which tested the majority of the samples. Such a high level of contamination might be related to the reported contamination of the flour, which has been identified as the cause of some outbreaks of STEC infections in the US and Canada (Gill et al., 2019). Additionally, behaviours such as tasting and manipulating raw dough should also be considered in relation to the risk of STEC infections from contaminated flour (Crowe et al., 2017).
STEC‐positive units were detected in all types of RTE food. Importantly, only a few MSs reported data for certain food categories or with a limited sampling effort for certain foods (e.g. one MS reported 107 raw milk sample results). The testing of RTE food commodities for STEC is important because these foods are consumed without any treatment to reduce or eliminate the possible presence of the pathogen, posing a direct risk to the consumer.
In 2021, 27.4% of the food isolates were provided with information on the serogroup, compared with 28.2% and 34.4% observed in 2020 and 2019, respectively, and 41.8% in 2018 (EFSA and ECDC, 2021). Serogroup determination clearly shows a decreasing trend, which probably reflects the awareness that this character is of limited importance in assessing the virulence of STEC strains. Most of the top 20 STEC serogroups isolated from human infections in 2021 were also found in the STEC isolated from food in the same year.
As regards the animal monitoring results for 2021, overall, 6.1% of the 3,746 samples taken were STEC‐positive. However, in 2021, the samples tested from cattle represented 88.5% of the total animal sample units assayed by five MSs and 5.9% of these were contaminated with STEC. In any case, the number of animal sampling units tested, although considerably increased with respect to 2020, continued to be very low, possibly biasing the estimates.
The analysis of the presence and subtypes of virulence genes is important for pathogenicity assessment (EFSA BIOHAZ Panel, 2020b). Unfortunately, this level of characterisation is still far from being routinely carried out for food and animal isolates, and only 32.6% of the STEC isolated from food in 2021 were reported together with the information on the stx gene types (stx1 or stx2) and the intimin‐coding gene eae. The number of strains with information on stx gene subtyping was even lower (N = 79), representing 27.8% of the isolates with stx types and eae genes reported, and 9.1% of the total number of STEC isolated from food in 2021. Given that this characterisation strategy represents the basis for a molecular risk assessment of STEC circulating in the vehicles of infection, MSs should be encouraged to continue to adopt this approach.
The virulotyping of STEC isolated from food in 2021, although conducted on a small subset of isolates, confirmed that all the virulotypes identified, based on the stx types and the eae gene, matched those associated with the STEC strains isolated from severe disease (EFSA BIOHAZ Panel, 2020b) (Table 42). As far as the stx gene subtyping is concerned, 9 out of the 13 combinations identified in food isolates were also represented among those associated with severe disease (Table 43).
On the other hand, 46.1% of animal isolates (N = 113) were reported with data on the characterisation of the virulence genes, but only two animal isolates had undergone stx gene subtyping by two MSs. Also in this case, all the stx1/stx2/eae profiles and the two subtype combinations identified – both eae positive – were represented in STEC isolated from human severe disease during the 2012–2017 period (EFSA BIOHAZ Panel, 2020b) (Tables 42 and 43).
Given the predictive power of the stx gene subtyping, a wider adoption of this approach is recommended. Raising awareness on the need to enhance the characterisation of STEC strains will lead to an improved risk assessment of STEC, ultimately supporting actions to mitigate the impact of STEC on public health.
5. Tuberculosis due to Mycobacterium bovis or Mycobacterium caprae
Summary data substantiating this chapter, as well as additional information on related projects and internet sources are published for this report on the EFSA Knowledge Junction on Zenodo here. Summary statistics on human surveillance data with downloadable files are retrievable using the ECDC Surveillance Atlas of Infectious Diseases here.

5.1. Key facts
In 2021, the number of confirmed cases of human tuberculosis due to Mycobacterium bovis or Mycobacterium caprae was 111, corresponding to an EU notification rate of 0.03 cases per 100,000 population. This is an increase of 12.4% compared with the rate in 2020 (0.026 per 100,000 population).
Compared with the EU notification rate before the COVID‐19 pandemic (2017–2019 annual mean), there was a decrease of 28.3% and 25.3% with and without the data from the United Kingdom, respectively.
In both 2021 and 2020, the M. bovis or M. caprae reporting recorded the lowest number of human cases and rates since 2017, because it was impacted by the COVID‐19 pandemic and withdrawal of the United Kingdom from the EU.
In 2021, the M. bovis and M. caprae case notification rate was similar (0.03 cases per 100,000) among EU MSs with disease‐free status and EU MSs with non‐disease‐free status in the bovine population.
Almost half (49.5%) of M. bovis and M. caprae cases in humans were of EU origin (native cases and/or cases originating from other MSs).
No foodborne outbreaks due to M. bovis or M. caprae have ever been reported to EFSA since the start of the data collection on foodborne outbreaks in 2004; 2021 was no exception.
In 2021, the overall prevalence of bovine tuberculosis due to M. bovis or M. caprae increased slightly (0.6%) compared with the previous year, and the number of infected cattle herds in the EU increased from 7,372 to 9,690 herds. This increase was mainly due to the United Kingdom (Northern Ireland) data, which were not included in the EU 2020 statistics. Infection of cattle with M. tuberculosis was not reported to EFSA.
Fourteen MSs and the United Kingdom (Northern Ireland) detected the presence of bovine tuberculosis in 2021. Similar to previous years, the distribution of infected herds was heterogeneous and spatially clustered, with a national herd‐level prevalence ranging from < 0.01% (Poland) to 11.3% (the United Kingdom (Northern Ireland)).
Seventeen MSs had disease‐free status during 2021. Ten MSs and the United Kingdom (Northern Ireland) were non‐disease‐free, of which three MSs had disease‐free zones.
Overall, 139 cattle herds proved infected with M. bovis or M. caprae in the disease‐free zones (0.014%), confirming that infection occurs rarely in these zones.
In the non‐disease‐free zones of 10 MSs and the United Kingdom (Northern Ireland) 9,551 cattle herds (1.3% of total herds) showed infection with M. bovis or M. caprae in 2021. The United Kingdom (Northern Ireland) (11.3%), Ireland (4.6%) and Spain (1.3%) were the only countries that reported prevalence higher than 1%. No infected herds were reported by Malta or Cyprus.
In the last 10 years (2012–2021), the annual number of infected cattle herds and the prevalence of bovine tuberculosis in non‐disease‐free zones has decreased by 47.5% and 4.2%, respectively. This decrease is mainly attributable to the withdrawal of the United Kingdom from the EU in 2020. In fact, the annual prevalence of herds infected with M. bovis or M. caprae in all the zones of the United Kingdom was higher than 10% between 2012 and 2019. For the same reason, the inclusion of the United Kingdom (Northern Ireland) among the EU zones explains the increase in both the number of infected herds and the prevalence recorded in 2021.
5.2. Surveillance and monitoring of tuberculosis due to Mycobacterium bovis or Mycobacterium caprae in the EU
5.2.1. Humans
The notification of tuberculosis in humans is mandatory in all 27 EU MSs and covers the whole population. All countries report case‐based data. The proportion of tuberculosis cases caused by M. bovis and M. caprae was calculated using the preliminary estimate of the total number of confirmed tuberculosis cases in 2021 among reporting MSs' species‐specific data. No human data on M. bovis and M. caprae cases are available for France because this MS does not report species‐specific data within the Mycobacterium tuberculosis complex (MTBC) for human tuberculosis cases. In addition, Latvia did not report any MTBC data during 2018–2020 and Iceland in 2021.
Given that tuberculosis is a chronic disease with a long incubation period, it is not possible to assess travel‐associated cases in the same way as for other, acute‐onset zoonoses detailed in this report. Instead, a distinction is made between individuals with the disease originating from an EU MS (cases of EU origin) and those originating from outside the EU (case originating outside of the EU). In the analysis, origin is mainly based on the reported birthplace, except for cases from Austria, Belgium, Greece, Hungary and Poland, whose origin is based on their reported citizenship. The treatment outcome for tuberculosis due to M. bovis and M. caprae is assessed 1 year (12 months) after case notification, because the shortest duration for treatment completion is 6 months, according to the international treatment guidelines for tuberculosis.
5.2.2. Animals
Bovine tuberculosis surveillance data
On 21 April 2021, Commission Implementing Regulation (CIR) (EU) 2020/2002 23 under the Animal Health Law (AHL) entered into force, with Union notification and reporting covering infection with Mycobacterium tuberculosis complex (M. bovis, M. caprae and M. tuberculosis) (MTBC), and bovine tuberculosis is also covered by Directive 2003/99/EC. Article 2 of the AHL states that its scope applies to transmissible diseases, including zoonoses, without prejudice to the rules laid down in Directive 2003/99/EC. Therefore, the annual zoonosis data reporting requirements for MSs in accordance with Directive 2003/99/EC, implemented by EFSA using specific tools, manuals and guidance, are not affected by the entry into force of CIR (EU) 2020/2002. The EU MSs need to report to the EU ADIS 24 on outbreaks of infection with MTBC in bovine (cattle, buffalo and bison), caprine and ovine animal populations, in other even‐toed ungulates (Artiodactyla) and in terrestrial mammals. Annual summary reports are published online. CIR (EU) 2020/2002 details compulsory notification and annual reporting requirements from MSs to ADIS. MSs or zones thereof with disease‐free status must also notify ADIS of MTBC outbreaks in terrestrial animals.
In accordance with the Zoonoses Directive 2003/99/EC, MSs must report annual surveillance data for bovine tuberculosis. These data originate from the compulsory national eradication and surveillance programmes that the MSs implement in accordance with EU legislation (Animal Health Law). The reports submitted by the MSs are harmonised and can be used for the assessment of the epidemiological situation and analysis of trends in MSs and MS zones.
In accordance with the EU legislation in force, disease‐free status can be assigned by the European Commission to an MS or a zone thereof if during the previous 3 years at least 99.8% of the bovine herds have maintained their disease‐free status from infection with MTBC, and the herd incidence rate during the last year has not exceeded 0.1%. Because the risk of infection with MTBC in disease‐free zones is different from that of non‐disease‐free zones, these zones have been considered separately in this report.
Differently to the procedure for the above‐mentioned statistics for humans, for which infections with M. bovis and M. caprae are treated separately, all cases of tuberculosis in cattle caused by the infectious MTBC members (M. bovis, M. caprae) are considered cases of bovine tuberculosis and were taken into account to summarise the EU situation on bovine tuberculosis. Whenever possible, reporting MSs distinguish descriptively between Mycobacterium tuberculosis complex, M. bovis and M. caprae. M. tuberculosis in cattle was not reported to EFSA during 2016–2020, whereas rare cases were reported earlier.
Mycobacterium surveillance data from food and from animals other than bovine animals
Mycobacterium spp. monitoring data from food and from animals other than bovine animals are submitted to EFSA in accordance with Directive 2003/99/EC. Data collected allow for descriptive summaries to be compiled at the EU level, but do not allow trend watching or trend analyses (Table 1).
In accordance with CIR (EU) 2020/2002, notification to ADIS and surveillance rules apply to other Artiodactyla (such as camelids, caprine animals and cervids) as species susceptible to infection with MTBC.
5.3. Results
5.3.1. Overview of key statistics, EU, 2017–2021
Table 44 summarises the EU‐level statistics on human tuberculosis due to M. bovis or M. caprae and on bovine tuberculosis during 2017–2021. More detailed descriptions of these statistics are provided in the below subsections.
Table 44.
Summary statistics related to tuberculosis due to Mycobacterium bovis and Mycobacterium caprae in humans and bovine animals (stratified by disease‐free (a) and non‐disease‐free status zones), EU, 2017–2021
| 2021 (b) | 2020 | 2019 (c) | 2018 (c) | 2017 (c) | Data source | |
|---|---|---|---|---|---|---|
| Humans | ||||||
| Number of confirmed M. bovis cases | 103 | 96 | 141 | 168 | 204 | ECDC |
| Number of confirmed M. caprae cases | 8 | 3 | 11 | 13 | 9 | ECDC |
| Total number of confirmed cases | 111 | 99 | 152 | 181 | 213 | ECDC |
| Total number of confirmed cases/100,000 population (notification rates) | 0.03 | 0.03 | 0.03 | 0.04 | 0.05 | ECDC |
| Number of EU MSs that reported data on M. bovis or M. caprae cases | 26 | 25 | 26 | 26 | 27 | ECDC |
| M. bovis or M. caprae cases in individuals of EU origin | 55 | 53 | 107 | 105 | 143 | ECDC |
| M. bovis or M. caprae cases in individuals originating from outside the EU | 47 | 35 | 40 | 68 | 62 | ECDC |
| M. bovis or M. caprae cases in individuals of unknown origin | 9 | 11 | 5 | 8 | 8 | ECDC |
| Total number of foodborne outbreaks | 0 | 0 | 0 | 0 | 0 | EFSA |
| Number of outbreak‐related cases | 0 | 0 | 0 | 0 | 0 | EFSA |
| Animals | ||||||
| Bovine animals | ||||||
| Number of infected herds in disease‐free zones | 139 | 139 | 143 | 172 | 134 | EFSA |
| Number of reporting disease‐free MSs | 17 | 17 | 17 | 17 | 18 | EFSA |
| Number of infected herds in non‐disease‐free zones | 9,551 | 7,233 | 16,277 | 18,801 | 18,857 | EFSA |
| Number of reporting non‐disease‐free MSs | 11 | 9 (d) | 11 | 11 | 10 | EFSA |
ECDC: European Centre for Disease Prevention and Control; EFSA: European Food Safety Authority; MSs: Member States.
: The Member States or zones thereof with disease‐free status from infection with the Mycobacterium tuberculosis complex (M. bovis, M. caprae and M. tuberculosis).
: Data on animals from the United Kingdom (Northern Ireland) are taken into account for 2021. In accordance with the agreement on the withdrawal of the United Kingdom from the EU, and in particular with the Protocol on Ireland/Northern Ireland, the EU requirements on data sampling are also applicable to Northern Ireland.
: Data from the United Kingdom are taken into account for 2017–2019, because the United Kingdom was an EU MS, but it became a third country on 1 February 2020.
: No data were reported from Bulgaria.
5.3.2. Tuberculosis due to Mycobacterium bovis and Mycobacterium caprae in humans
In 2021, 111 confirmed human cases of tuberculosis due to M. bovis or M. caprae were reported by eight MSs (Austria, Belgium, Germany, Ireland, Italy, the Netherlands, Spain and Sweden) (Table 45). Tuberculosis cases due to M. bovis (103 cases) were reported in all these countries, and only three MSs (Austria, Germany and Spain) also notified cases caused by M. caprae (8 cases). Overall, tuberculosis cases due to M. bovis or M. caprae accounted for a small proportion (0.3%) of total tuberculosis cases reported in the EU by 26 MSs with species‐specific data on MTBC available in 2021 (see Section 5.2.1). Eighteen MSs did not report any cases.
Table 45.
Reported cases of human tuberculosis due to Mycobacterium bovis and Mycobacterium caprae and notification rates per 100,000 population in EU MS and non‐MS countries by country and year, 2017–2021
| Country | 2021 | 2020 | 2019 | 2018 | 2017 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Status | National coverage | Data format (a) | Confirmed cases and rates | Confirmed cases and rates | Confirmed cases and rates | Confirmed cases and rates | Confirmed cases and rates | ||||||
| Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | ||||
| Austria | DFS | Y | C | 4 | 0.04 | 0 | 0 | 3 | 0.03 | 2 | 0.02 | 2 | 0.02 |
| Belgium | DFS | Y | C | 10 | 0.09 | 6 | 0.05 | 0 | 0 | 5 | 0.04 | 6 | 0.05 |
| Bulgaria | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Croatia | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Cyprus | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Czechia | DFS | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.01 | 0 | 0 |
| Denmark | DFS | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.02 |
| Estonia | DFS | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Finland | DFS | Y | C | 0 | 0 | 1 | 0.02 | 0 | 0 | 0 | 0 | 0 | 0 |
| France (b) | DFS | – | – | – | – | – | – | – | – | – | – | – | – |
| Germany | DFS | Y | C | 42 | 0.05 | 37 | 0.04 | 51 | 0.06 | 64 | 0.08 | 48 | 0.06 |
| Greece | Y | C | 0 | 0 | 2 | 0.02 | 1 | 0.01 | 0 | 0 | 1 | 0.01 | |
| Hungary | DFS | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ireland | Y | C | 2 | 0.04 | 4 | 0.08 | 7 | 0.14 | 7 | 0.14 | 4 | 0.08 | |
| Italy | Y | C | 12 | 0.02 | 6 | 0.01 | 11 | 0.02 | 17 | 0.03 | 21 | 0.03 | |
| Latvia | DFS | Y | C | 0 | 0 | – | – | – | – | – | – | 0 | 0 |
| Lithuania | DFS | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Luxembourg | DFS | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Malta | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Netherlands | DFS | Y | C | 5 | 0.03 | 6 | 0.03 | 5 | 0.03 | 11 | 0.06 | 11 | 0.06 |
| Poland | DFS | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Portugal | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Romania | Y | C | 0 | 0 | 1 | 0.01 | 1 | 0.01 | 0 | 0 | 2 | 0.01 | |
| Slovakia | DFS | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Slovenia | DFS | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Spain | Y | C | 32 | 0.07 | 30 | 0.06 | 35 | 0.07 | 46 | 0.10 | 73 | 0.16 | |
| Sweden | DFS | Y | C | 4 | 0.04 | 6 | 0.06 | 3 | 0.03 | 4 | 0.04 | 3 | 0.03 |
| EU Total 27 | – | – | 111 | 0.03 | 99 | 0.03 | 117 | 0.03 | 157 | 0.04 | 172 | 0.05 | |
| United Kingdom (c) | – | – | – | – | – | – | 35 | 0.05 | 24 | 0.04 | 41 | 0.06 | |
| EU Total | – | – | 111 | 0.03 | 99 | 0.03 | 152 | 0.03 | 181 | 0.04 | 213 | 0.05 | |
| Iceland (d) | Y | C | – | – | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Norway | DFS | Y | C | 0 | 0 | 0 | 0 | 1 | 0.02 | 0 | 0 | 3 | 0.06 |
| Liechtenstein | DFS | Y | C | 0 | 0 | 3 | 0.03 | 4 | 0.05 | 3 | 0.04 | 3 | 0.04 |
| Switzerland (e) | DFS | Y | C | 3 | 0.03 | ||||||||
EU: European Union.
–: Data not reported.
DFS: Disease‐free status, i.e. free of infection with M. bovis and M. caprae in bovine animal population.
: Y: yes; N: no; A: aggregated data; C: case‐based data.
: Not reporting species of the M. tuberculosis complex.
: Cases reported by the United Kingdom for the period 2017–2019 were also considered for this estimation (EU‐28). When the United Kingdom data were collected for the period 2017–2019, the United Kingdom was an EU MS, but it became a third country on 1 February 2020.
: In Iceland, which has no special agreement regarding animal health (status) with the EU, the last outbreak of bovine tuberculosis was in 1959.
: Switzerland provided data directly to EFSA. The human data for Switzerland include data from Liechtenstein for the years 2017–2020.

All Zones of the MS have disease‐free status.

Not all Zones of the MS have disease‐free status.

No Zones of the MS have disease‐free status.
The EU notification rate in 2021 was 0.03 cases per 100,000 population, which is similar to the rate observed in 2020 (Table 45). The EU notification rate decreased by 28.3% and 25.3% compared with the mean notification rate observed over the pre‐pandemic years 2017–2019 with and without the data from the United Kingdom, respectively.
In 2021, the highest notification rate was reported by Belgium (0.09 per 100,000), followed by Spain (0.07 per 100,000).
Among the 17 MSs with disease‐free status in 2021, 16 MSs reported on MTBC species. M. bovis and M. caprae human cases were reported in five MSs. The notification rate in these 16 MSs reporting on MTBC species was 0.026 cases per 100,000 population. The same notification rate for M. bovis and M. caprae human cases (0.026 cases per 100,000 population) was also observed in the 10 MSs with non‐disease‐free status in 2021.
Approximately half of the cases reported in 2021 (55/111; 49.5%) were of EU origin (native cases and/or cases originating from other MSs). The other cases originated from outside the EU (N = 47; 42.3%) or had unknown origin (N = 9; 8.1%) (Table 44). Notification rates of M. bovis and M. caprae human cases of EU origin were similar in disease‐free MSs (N = 27; 48.2%) and non‐disease‐free MSs (N = 29; 51.8%).
Information on treatment outcome after 12 months was reported for 91.9% (91/99) of the human M. bovis and M. caprae cases notified in 2020. The treatment was reported to be successful for 58 cases (63.7%). Three cases (3.3%) were still being treated at 12 months. Deaths were reported in 10 cases (11.0%). Twenty cases (22.0%) were reported as lost to follow‐up.
Regarding drug resistance, only one case was reported to be resistant to isoniazid treatment out of 77 M. bovis or M. caprae cases with test results reported in 2021 for rifampicin and isoniazid. This finding confirms that drug resistance is relatively rare among M. bovis or M. caprae, considering that neither rifampicin‐resistant nor multidrug‐resistant cases were reported in 2021.
Figure 11 shows, for the year 2021, the number of confirmed tuberculosis cases due to M. bovis and to M. caprae in individuals of EU origin overlaid with the national aggregated herd prevalence of bovine tuberculosis.
Figure 11.
Map of the number of confirmed tuberculosis cases due to Mycobacterium bovis and Mycobacterium caprae in individuals of EU origin, and national herd prevalence of tuberculosis in the bovine population in EU MS and non‐MS countries, 2021
5.3.3. Mycobacterium in food
No Mycobacterium spp. monitoring data from food were submitted for the year 2021.
5.3.4. Tuberculosis in bovine animals
Bovine tuberculosis surveillance data
Seventeen MSs had DFS from MTBC during 2021 (Figure 12). Of the remaining 10 MSs and the United Kingdom (Northern Ireland), three MSs had disease‐free zones or provinces:
-
–
Italy: 11 regions and 12 provinces;
-
–
Portugal: 1 region (Algarve) and all of the Azores islands, except São Miguel;
-
–
Spain: 3 autonomous communities (Canary Islands, Galicia and Asturias).
Figure 12.
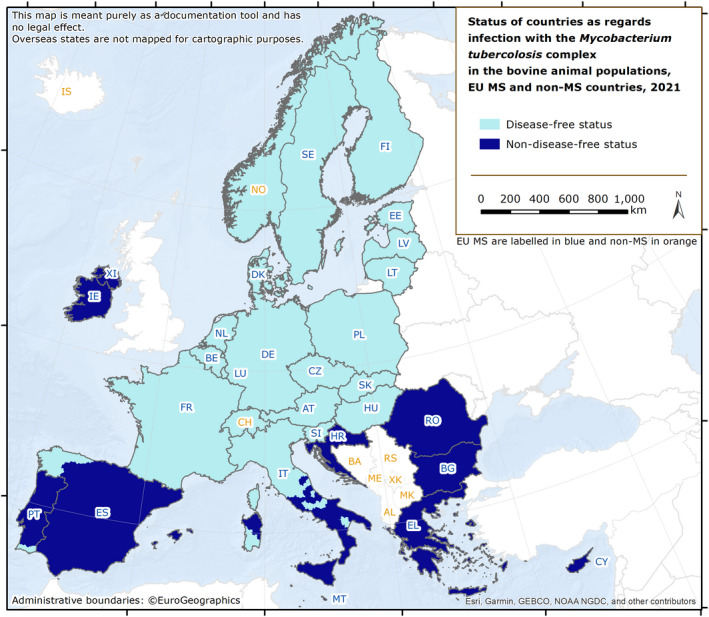
Status of countries for infection with the Mycobacterium tuberculosis complex (M. bovis, M. caprae and M. tuberculosis) in the bovine animal population, EU MSs and non‐MSs, 2021
Seven MSs had no DFS from MTBC zones. The United Kingdom (Northern Ireland) has no disease‐free region either.
Norway, Switzerland and Liechtenstein had disease‐free status, in accordance with the EU legislation. In Iceland, which has no special agreement with the EU on animal health status, the last outbreak of bovine tuberculosis was reported in 1959.
During 2021, in the EU, the overall prevalence of cattle herds infected with MTBC was very low (9,690 out of 1,726,451 herds; 0.6%), slightly higher than 2020 (0.4%). Compared with the previous year, the number of bovine herds infected with MTBC in the EU increased from 7,372 to 9,690 herds in 2021. This increase was mainly due to 2021 data from the United Kingdom (Northern Ireland), which has a high prevalence, whereas its 2020 data were not included in the overall EU statistics. Thirteen MSs (11 disease‐free and 2 non‐disease‐free) reported no cases of bovine tuberculosis in cattle (Table 46). The remaining MSs reported bovine tuberculosis with a wide range of prevalence at the national level. MTBC infection in cattle herds primarily affected the EU zones with non‐disease‐free status: the overall occurrence of bovine tuberculosis in non‐disease‐free zones (1.3%) was 92.8 times higher than in disease‐free zones (0.014%).
Table 46.
Status of countries as regards bovine tuberculosis and related prevalence, EU, 2021
| Member state (MS) | Status | N of infected herds in disease‐free zones | Prevalence (%) of infected herds in DFS (a) zones | N of infected herds in non‐DFS (a) zones | Prevalence (%) of infected herds in non‐DFS (a) zones |
|---|---|---|---|---|---|
| Austria | DFS | 6 (b) | 0.01 | – | – |
| Belgium | DFS | 5 (c) | 0.02 | – | – |
| Bulgaria | – | – | 6 | 0.01 | |
| Croatia | – | – | 2 | 0.01 | |
| Cyprus | – | – | 0 | 0 | |
| Czechia | DFS | 0 | 0 | – | – |
| Denmark | DFS | 0 | 0 | – | – |
| Estonia | DFS | 0 | 0 | – | – |
| Finland | DFS | 0 | 0 | – | – |
| France | DFS | 99 (c) | 0.06 | – | – |
| Germany | DFS | 8 | 0.01 | – | – |
| Greece | – | – | 140 | 0.76 | |
| Hungary | DFS | 4 (c) | 0.02 | – | – |
| Ireland | – | – | 5,071 | 4.6 | |
| Italy | 9 (c) | 0.02 | 179 | 0.35 | |
| Latvia | DFS | 0 | 0 | – | – |
| Lithuania | DFS | 0 | 0 | – | – |
| Luxembourg | DFS | 0 | 0 | – | – |
| Malta | – | – | 0 | 0 | |
| Netherlands | DFS | 0 | 0 | – | – |
| Poland | DFS | 8 (c) | < 0.01 | – | – |
| Portugal | 0 | 0 | 99 | 0.35 | |
| Romania | – | – | 31 | 0.01 | |
| Slovakia | DFS | 0 | 0 | – | – |
| Slovenia | DFS | 0 | 0 | – | – |
| Spain | 0 | 0 | 1,424 | 1.33 | |
| Sweden | DFS | 0 | 0 | – | – |
| United Kingdom (Northern Ireland) | – | – | 2,599 | 11.3 | |
| EU Total (27 + XI) | 139 | 0.014 | 9,551 | 1.3 |
MS: Member State; DFS: Disease‐free status.
: Data from the United Kingdom (Northern Ireland) are taken into account for 2021. In accordance with the agreement on the withdrawal of the United Kingdom from the EU, and in particular with the Protocol on Ireland/Northern Ireland, the EU requirements on data sampling also apply to Northern Ireland, which is non‐disease‐free.
: Only Mycobacterium caprae identified.
: Only Mycobacterium bovis identified.

All Zones of the MS have disease‐free status.

Not all Zones of the MS have disease‐free status.

No Zones of the MS have disease‐free status.
MSs and MSs' zones with disease‐free status from Mycobacterium tuberculosis complex
The majority of the whole EU cattle herd population (56.1%) is located in the disease‐free zones of the 20 MSs having such zones. However, the number of cattle herds has been steadily decreasing (−29.4% in 2021 compared with 2012), impacting on the calculation of the prevalence of herds infected with MTBC. From 2012 to 2021, there was a marked decrease in the annual number of infected cattle herds (−38.8%), whereas the prevalence has remained stable. Infected herds were rare events (Figure 13).
Figure 13.
- (*): In contrast to years 2012–2019, the year 2020 does not include United Kingdom data. Since 1 February 2020, the United Kingdom has withdrawn from the EU and has become a third country. (**): In accordance with the agreement on the withdrawal of the United Kingdom from the EU, and in particular with the Protocol on Ireland/Northern Ireland, the European Union requirements on data sampling also apply to Northern Ireland.
Seven MSs with disease‐free zones reported a total of 139 MTBC‐infected bovine herds (Table 46), confirming that the detection of bovine tuberculosis in disease‐free zones is sporadic. When comparing data from 2021 and 2020, the number of MTBC‐infected cattle herds remained the same, with an almost unchanged prevalence (0.013% vs. 0.014%), whereas the total number of cattle herds has decreased by 6.5%.
MSs and MSs' zones with non‐disease‐free status from Mycobacterium tuberculosis complex
The zones with non‐disease‐free status, belonging to 10 MSs and the United Kingdom (Northern Ireland), account for 43.9% of the whole EU cattle herd population, which has been steadily decreasing over time (−45.2% in 2021 compared with 2012; Figure 14). The significant decrease in 2020 is due to the withdrawal of the United Kingdom from the EU. Moreover, Bulgaria did not report data in that year. The increase in the number of 2021 cattle herds can be attributed to the data provided by Bulgaria after its 2020 hiatus and by the United Kingdom (Northern Ireland).
Figure 14.
- (*): In contrast to years 2012–2019, the year 2020 does not include the United Kingdom data. Since 1 February 2020, the United Kingdom has withdrawn from the EU and has become a third country. No data were reported from Bulgaria. (**): In accordance with the agreement on the withdrawal of the United Kingdom from the EU, and in particular with the Protocol on Ireland/Northern Ireland, the European Union requirements on data sampling also apply to Northern Ireland.
Eight MSs and the United Kingdom (Northern Ireland) reported a total of 9,551 cattle herds infected with MTBC (Table 46). Over the last 10 years, the annual number of infected cattle herds in non‐disease‐free zones has decreased (−47.5% in 2021 compared with 2012). During the same period, the overall prevalence of bovine tuberculosis decreased from 1.32% to 1.26% (−4.2%). This decrease can be attributed to the withdrawal of the United Kingdom from the EU in 2020.
In 2021, prevalence varied widely among MSs with non‐disease‐free status: Ireland (4.6%) and Spain (1.3%) were the only MSs that reported prevalence higher than 1%; no infected herds were reported by Malta or Cyprus (Table 46). The United Kingdom (Northern Ireland), which has no disease‐free zones, reported prevalence of 11.3%. Compared with 2020, the prevalence of infected herds increased in 2021, mainly due to the inclusion of United Kingdom (Northern Ireland) data, which were not included in the 2020 EU‐level statistics.
Most MSs reported MTBC infections without specifying the species involved. Infection with M. bovis was specifically detected in Bulgaria, France, Germany, Hungary, Ireland, Italy, Poland, Romania and the United Kingdom (Northern Ireland), whereas infection with M. caprae was specifically reported by Austria, Germany and Romania. M. tuberculosis in cattle was not reported.
Figure 15 shows the prevalence trends in the five MSs (Greece, Ireland, Italy, Spain, Portugal) and the United Kingdom (Northern Ireland) presenting the highest prevalence between 2005 and 2021. In most of the MSs (Greece, Ireland, Spain) and the United Kingdom (Northern Ireland), no significant improvements were observed over time given that these data refer to zones where activities related to eradication programmes are more difficult due to unfavourable local environmental conditions (free‐range animals, common grazing land, transhumance, etc.) (Ciaravino et al., 2021; Byrne et al., 2022). However, in Italy, Spain and Portugal, several zones obtained disease‐free status during this period.
Figure 15.
Prevalence of bovine tuberculosis‐infected herds in non‐disease‐free zones of five MSs and the United Kingdom (Northern Ireland), 2005–2021
Non‐Member States and pre‐accession countries
Bovine tuberculosis was not detected in 2021 in Iceland, Liechtenstein, Norway or Switzerland. Among the pre‐accession countries, Montenegro, as in 2020, reported no infected herds, whereas the Republic of North Macedonia reported a prevalence of 0.13% MTBC‐infected herds (20/14,898), a value slightly lower than that reported in 2020 (0.15%).
Mycobacterium surveillance data from animals other than bovine animals, and complementary reporting from cattle
In 2021, three MSs (Finland, Slovenia, Sweden) and one non‐MS (Norway) reported surveillance data on infection in farmed deer, with no cases of MTBC infection detected. For the first time in 2021, Greece reported cases of infection with MTBC in goats via ADIS.
Complementary to their 2021 reports from cattle, MSs also reported cases of MTBC infection in other mammal species. In particular, M. bovis was detected in farmed alpacas, small ruminants (sheep and goats), pigs, cats, wild deer, wild boars and badgers, whereas M. caprae was reported in wild red deer in Austria and in wild boars in Hungary.
5.4. Discussion
In 2021, the reporting of human cases of tuberculosis due to M. bovis and M caprae at the EU level was the lowest in the last 5 years, with the exception of 2020 and taking into account the withdrawal of the United Kingdom from the EU. This finding should be interpreted with caution. It may be an important indication of the successful application of the eradication programmes for tuberculosis in bovine animals in EU MSs. On the other hand, it may be a consequence of the disruption in healthcare services due to COVID‐19. Like for other zoonoses, the reporting of fewer M. bovis and M. caprae cases in 2020, i.e. the first pandemic year, may reflect the impact of the COVID‐19 pandemic on healthcare systems, even for a long‐standing condition such as tuberculosis. A WHO document released in early in the COVID‐19 pandemic in Europe called attention to the need to maintain the continuity of essential services for people affected with tuberculosis during the COVID‐19 pandemic (WHO, 2020). Tuberculosis‐related services and programmes were perturbed by the COVID‐19 pandemic and the impact of the COVID‐19 pandemic on tuberculosis services — leading to hindered access to care, delayed diagnosis and delayed reporting — was particularly severe in 2020 (Migliori et al., 2021; Nikolayevskyy et al., 2021). The increased reporting of cases in 2021 at the EU level and in many MSs can be considered a sign of the healthcare systems' resilience during the second pandemic year. The significant reduction in the proportion of M. bovis and M. caprae cases lost to follow‐up for the evaluation of treatment outcome after 12 months is another important indication of a progressive recovery of tuberculosis programmes to their pre‐pandemic capacity. In 2020, this proportion accounted for almost half (49.3%) of the cases with reported treatment outcome, whereas in 2021 it was only 22.0%, although this proportion is still much higher compared with pre‐pandemic years (lost‐to‐follow‐up cases were 1.2% in 2019, 2.2% in 2018 and 2.4% in 2017). However, compared to M. tuberculosis, M. bovis and M. caprae represent a minor proportion of all MTBC cases in the human population and the limited number of human M. bovis and M. caprae cases involves some degree of uncertainty, which hampers coming to definitive conclusions.
The characteristics of M. bovis and M. caprae cases reported in 2021 and indicators of treatment outcome among cases occurred in 2020 did not substantially differ from those reported in pre‐pandemic years. Successful treatment and deaths were reported in 63.7% and 11% of cases, respectively. This finding suggests that there was no direct effect of COVID‐19 on patients with M. bovis and M caprae, albeit the high proportion of lost‐to‐follow‐up cases challenges this conclusion.
The notification rate of M. bovis and M. caprae cases in 2021 was the lowest among all zoonoses analysed in this report, apart from rabies, meaning that exposure of humans to M. bovis and M. caprae and the development of clinical disease is apparently a rare event, although the consequences are severe for patients. It is possible that the annual number of cases of zoonotic tuberculosis in the EU and the overall burden may be much higher, because not all MSs implement routine surveillance able to discriminate M. bovis and M. caprae cases from M. tuberculosis cases. Moreover, lower access to healthcare services by the non‐EU native population, which account for 42.3% of the total number of tuberculosis cases, may also contribute to the underdiagnosis or underreporting of M. bovis and M. caprae cases.
The high proportion of deaths among patients evaluated for treatment outcome that are reported every year at the 12‐month follow‐up argues for the need to continue eradication programmes in the bovine population to reduce the risk of exposure to M. bovis and M. caprae in the human population. A call for action launched in 2017 acknowledged the importance of a cross‐sectorial and multidisciplinary approach linking animal, human and environmental health, particularly to protect the most vulnerable and marginalised communities (Olea‐Popelka et al., 2017).
M. bovis and M. caprae notification rates did not differ significantly between EU countries with disease‐free status in the bovine population and those with non‐disease‐free status. This finding appears to suggest that the health status of the bovine population does not directly influence the risk of human infection by M. bovis and M. caprae, which remains a rare event and therefore highly subject to peculiar individual habits and behaviours. On the other hand, notification rates are strongly influenced by the sensitivity of surveillance at the country level and differences in case reporting among MSs are considerable. For example, Germany which is a disease‐free country regarding its bovine population, reported more than one third of the total M. bovis and M. caprae human cases notified in the EU (42 cases, 37.8%), revealing highly sensitive surveillance. Data reported to ECDC show that the proportion of MSs not reporting any M. bovis and M. caprae cases in the last 5 years were higher among MSs with non‐disease‐free status in the bovine population (5/10) than among MSs with disease‐free status (7/16; 2021). This comparison suggests lower surveillance sensitivity in some MSs in the non‐disease‐free status group.
M. bovis and M. caprae cases originating from outside the EU were reported in 2021 (42.3%) and 2020 (35.4%) in higher proportions compared with MTBC cases originating from outside the EU and EEA in 2020 (33.0%) (ECDC and WHO, 2022). Although these statistics are not fully comparable among the two groups because the 2021 data do not include cases originating from EEA countries, this difference raises the question of exposure to M. bovis and M. caprae being higher in people originating from outside the EU. For example, employees in slaughterhouses and cattle farms are frequently non‐EU natives, which may lead to differential occupational exposure to aerosols from infected animals and their carcases in this population.
The regulatory framework for bovine tuberculosis changed substantially in the EU during 2021. Tuberculosis caused by MTBC infection is currently considered a disease to be controlled in all MSs with the goal of eradicating the disease in bovine animals throughout the EU, while being kept under surveillance in other mammals. All MSs must have a surveillance or a control‐and‐eradication programme approved by the European Commission. This should lead, in the coming years, to progressive improvement in the already satisfactory epidemiological situation. In 2021, the overall EU prevalence of cattle herds infected with MTBC was 0.6%, slightly higher than in 2020. Bovine tuberculosis was reported by 14 MSs and by the United Kingdom (Northern Ireland). Its distribution was highly heterogeneous and spatially clustered in the EU, with herd prevalence ranging from 0% to 11.3%, confirming the results of a previous EFSA report (EFSA AHAW Panel, 2014).
Seventeen MSs had disease‐free status and three non‐disease‐free MSs also had such zones. The number of disease‐free zones increased during 2021. Thirteen of these MSs reported no cases of bovine tuberculosis in cattle. In the disease‐free zones, the detection of bovine tuberculosis remained a rare event, as in the previous years. From 2012 to 2021, the overall annual number of infected cattle herds, the prevalence and the total number of cattle herds decreased.
Eight non‐disease‐free MSs and the United Kingdom (Northern Ireland) detected bovine tuberculosis during 2021, with an overall infected herd prevalence of 1.3%. When comparing 2021 with 2020 data, the overall annual number of infected cattle herds, the prevalence and the total number of cattle herds increased in these non‐disease‐free zones. This increase is mainly due to the withdrawal of the United Kingdom from the EU in 2020 and to the subsequent (2021) inclusion of data from the United Kingdom (Northern Ireland), a region with high bovine tuberculosis prevalence. In the last 10 years (2012–2021), the overall annual number of infected cattle herds reported in the non‐disease‐free zones decreased by 47.5%, whereas prevalence decreased only by 4.2%. These different trends can partly be attributed to (1) the withdrawal of the United Kingdom from the EU; (2) the decreased number of herds; (3) the gradual obtention of disease‐free status in zones within non‐disease‐free MSs and (4) unfavourable environmental conditions hindering the eradication process in several non‐disease‐free zones (Byrne et al., 2022).
There is a major obstacle to bovine tuberculosis eradication in cattle in areas where infection is endemic in wildlife animals. Successfully tackling bovine tuberculosis also involves addressing the wildlife reservoir of the disease. Ireland introduced a vaccination policy in the Eurasian badger (Meles meles), a species known as a maintenance host of M. bovis, in 2018 and is also, among other control measures, reducing the badger population (Gormley and Corner, 2013). On the other hand, in the United Kingdom (Northern Ireland), no active badger intervention or vaccination took place in 2021. Stagnating or increasing trends in the prevalence of bovine tuberculosis‐infected cattle herds demonstrate that eradication of this disease is a challenge, owing to the complex interactions between the pathogen, hosts and the local environments (EFSA AHAW Panel, 2014). MS‐specific evaluations of status, trends and of the relevance of bovine tuberculosis as a source of disease for humans can be found in the 2021 Annual National Zoonoses Country Reports referenced in Section 5.5.
In 2021, M. bovis was reported to be isolated – apart from bovine animals – from a wide range of mammal species, both domestic and wild, reflecting that this pathogen has a broad host range. M. caprae, acknowledged to cause bovine tuberculosis, was reported in cattle, and also in wild boar and red deer.
6. Brucella
Summary data substantiating this chapter, as well as additional information on related projects and internet sources are published for this report on the EFSA Knowledge Junction on Zenodo here. Summary statistics on brucellosis foodborne outbreaks reported in the framework of Directive 2003/99/EC, with downloadable files, are retrievable using the EFSA foodborne outbreaks dashboard available here. Summary statistics on human surveillance data with downloadable files are retrievable using the ECDC Surveillance Atlas of Infectious Diseases here.
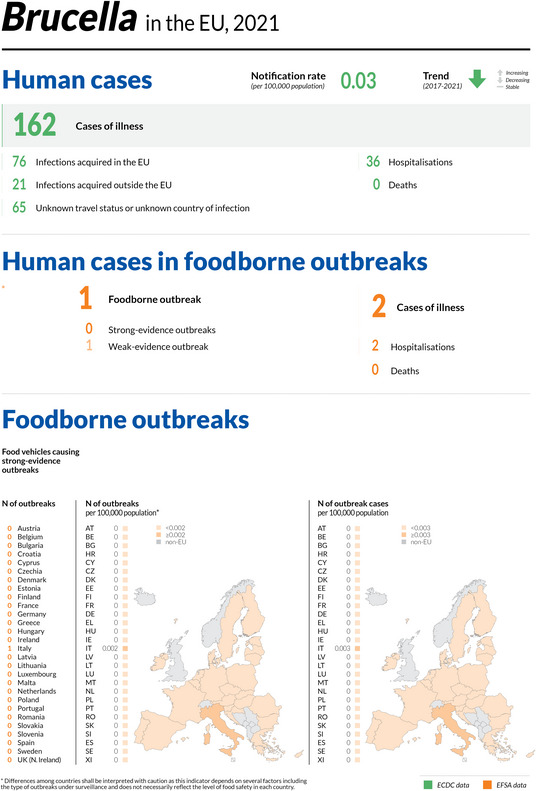
6.1. Key facts
In 2021, the number of confirmed cases of human brucellosis was 162 in the EU. The EU notification rate of 0.03 per 100,000 population was the same as that reported in 2020, which was the lowest since surveillance began in the EU in 2007.
Compared with the rate before the COVID‐19 pandemic (2017–2019 annual mean), there was a decrease of 52.2% and 51.1% with and without the data from the United Kingdom, respectively.
From 2017 to 2021 there has been a significantly declining trend of confirmed human cases of brucellosis in the EU.
Three MSs (Germany, Greece and Italy) have had significantly decreasing 5‐year trends from 2017 to 2021.
Brucella melitensis was reported as the aetiological agent in 51 (87.9%) out of 58 human cases of brucellosis with information reported on the Brucella species.
Croatia became disease‐free from infection with B. abortus, B. melitensis and B. suis 25 in 2021 in bovine animal populations. In total, 21 MSs and the United Kingdom (Northern Ireland) were disease‐free while 6 MSs (Bulgaria, Greece, Hungary, Italy, Portugal and Spain) were non‐disease‐free. Overall, in the disease‐free zones of the EU, there were eight infected herds in 2021, demonstrating a rare occurrence (prevalence < 0.001%). In the non‐disease‐free zones, bovine brucellosis remained very low, with 546 herds reported to be infected (0.43%). The number of infected herds stabilised at between 648 and 485 during 2017–2021.
Spain became disease‐free from infection with B. abortus, B. melitensis and B. suis in ovine and caprine populations in 2021. In total, 20 MSs and the United Kingdom (Northern Ireland) were disease‐free while seven MSs (Bulgaria, Croatia, overseas French regions, Greece, Italy, Malta and Portugal) were non‐disease‐free. Overall, in the disease‐free zones of the EU, there were 15 infected herds in 2021, demonstrating an extremely low prevalence (< 0.01%). In the non‐disease‐free zones, brucellosis in sheep and goats remained very low, with 331 herds reported to be infected (0.18%). The number of infected herds in these zones decreased from 815 in 2017 to 331 in 2021.
Brucellosis is still an animal health concern with public health relevance in southern European countries that are not brucellosis disease‐free.
6.2. Surveillance and monitoring of Brucella in the EU
6.2.1. Humans
In 2021, 26 MSs reported information on brucellosis in humans. Surveillance is mandatory in 25 MSs. Belgium has another (unspecified) system. The surveillance systems cover the whole population in all MSs reporting brucellosis data. Denmark has no surveillance system in place for brucellosis, and the disease is not notifiable or reported at the EU level. All countries reported case‐based data except Belgium and Bulgaria, which reported aggregated data. The EU case definition was used by 23 of these 26 countries, all of which had a comprehensive surveillance system. France reported using a different case definition, and Germany and Italy did not specify which case definition was used.
6.2.2. Food and animals
Monitoring data for Brucella from bovine animals, and from sheep and goats, originating from the National Control and Eradication Programmes and/or from countries or zones with disease‐free status
On 21 April 2021, Commission Implementing Regulation (CIR) (EU) 2020/2002 26 under the Animal Health Law (AHL) entered into force, with Union notification and reporting covering infection with Brucella abortus, B. melitensis and B. suis. Reporting on brucellosis (any Brucella species) is also covered by the Directive 2003/99/EC, Annex I list A ‘brucellosis and agents thereof’. Article 2 of the AHL states that its scope applies to transmissible diseases, including zoonoses, without prejudice to the rules laid down in Directive 2003/99/EC. Therefore, the zoonoses annual data reporting requirements for MSs in accordance with Directive 2003/99/EC, implemented by the European Food Safety Authority (EFSA) using specific tools, manuals and guidance, are not affected by the entry into force of the CIR (EU) 2020/2002. The EU MSs need to report outbreaks of infection with Brucella abortus, B. melitensis and B. suis in bovine, caprine and ovine animal populations, and other terrestrial animals to the EU ADIS, 27 and annual summary reports are published online. CIR (EU) 2020/2002 details compulsory notification and annual reporting requirements from MSs to ADIS.
In accordance with the EU legislation in force, disease‐free status can be assigned by the European Commission to an MS or a zone thereof if during the previous 3 years at least 99.8% of bovine herds have maintained their disease‐free status from infection with brucellosis, and the herd incidence rate during the last year has not exceeded 0.1%. Because the risk of infection with brucellosis in disease‐free zones is different from that of non‐disease‐free zones, these zones have been considered separately in this report.
Regulation (EU) 2016/429 of the European Parliament and of the Council concerns approval of the disease‐free and non‐vaccination status of MSs or zones or compartments. CIR (EU) 2018/1882 includes Bos ssp., Bubalus ssp., Bison ssp., Ovis ssp., Capra ssp. as species for notification, surveillance, prevention, certification and compulsory eradication. Commission Delegated Regulation 2020/689 (Annex IV) describes the conditions required to grant, maintain or suspend the status of free from infection with B. abortus, B. melitensis and B. suis for an establishment keeping bovine animals, and sheep and goats.
The reports submitted by the MSs are based on Regulation (EU) 2016/429 and subsequent legislation and are essential for assessment of the epidemiological situation in MSs and MS zones, whether declared disease‐free (DF) in cattle and/or in sheep and goats.
Annual surveillance programmes are carried out in DFS zones to confirm the absence of infection with Brucella abortus, B. melitensis and B. suis in cattle, and in sheep and goats. In non‐DF zones, control and eradication programmes for brucellosis in cattle, and sheep and goats are in place. These data are comparable across MSs because the monitoring schemes are harmonised, and the data collected and reported to EFSA originate from the census‐as‐sampling framework or a randomised design. These data can be used to carry out trend analyses both at the EU and MS levels, to perform trend watching and produce descriptive summaries and to assess the impact of control and eradication programmes (Table 1).
Monitoring data for Brucella from food, and animals other than bovine animals, and sheep and goats
Monitoring data for Brucella from food, and animals other than bovine animals, and sheep and goats are submitted to EFSA in accordance with Directive 2003/99/EC. They preclude trend analyses and trend watching at the EU level (Table 1). In accordance with CIR (EU) 2020/2002, notification to ADIS and surveillance rules apply to other Artiodactyla species (such as camelids or cervids) as susceptible species for brucellosis. Data collected allow for descriptive summaries to be compiled at the EU level.
6.3. Results
6.3.1. Overview of key statistics, EU, 2017–2021
Table 47 displays statistics at the EU level on human and animal brucellosis, along with data on detection of Brucella in food, between 2017 and 2021. Results are described in detail in this chapter.
Table 47.
Summary of Brucella statistics related to humans, major food categories and main animal species (stratified by disease‐free status (a) and non‐disease‐free status zones), EU, 2017–2021
| 2021 (b) | 2020 | 2019 (c) | 2018 (c) | 2017 (c) | Data source | |
|---|---|---|---|---|---|---|
| Humans | ||||||
| Total number of confirmed cases | 162 | 132 | 309 | 332 | 378 | ECDC |
| Total number of confirmed cases/100,000 population (notification rates) | 0.03 | 0.03 | 0.06 | 0.08 | 0.09 | ECDC |
| Number of reporting MSs | 26 | 26 | 27 | 26 | 26 | ECDC |
| Infection acquired in the EU | 76 | 68 | 126 | 133 | 148 | ECDC |
| Infection acquired outside the EU | 21 | 14 | 50 | 51 | 46 | ECDC |
| Unknown travel status or unknown country of infection | 65 | 50 | 133 | 148 | 184 | ECDC |
| Number of outbreak‐related cases | 2 | 2 | 2 | 0 | 2 | EFSA |
| Total number of outbreaks | 1 | 1 | 1 | 0 | 1 | EFSA |
| Food | ||||||
| Milk and milk products | ||||||
| Number of sampling units | 320 | 275 | 586 | 1,005 | 1,338 | EFSA |
| Number of reporting MSs | 3 | 3 | 2 | 3 | 3 | EFSA |
| Animals | ||||||
| Cattle (Bovine animals) | ||||||
| Number of infected herds in disease‐free zones | 8 | 6 | 4 | 3 | 0 | EFSA |
| Number of reporting disease‐free MSs | 22 | 19 | 20 | 20 | 20 | EFSA |
| Number of infected herds in non‐disease‐free zones | 546 | 603 | 485 | 563 | 648 | EFSA |
| Number of reporting non‐disease‐free MSs | 6 | 7 | 8 | 8 | 8 | EFSA |
| Sheep and goats | ||||||
| Number of infected herds in disease‐free zones | 15 | 3 | 1 | 0 | 7 | EFSA |
| Number of reporting disease‐free MSs | 22 | 19 | 20 | 20 | 20 | EFSA |
| Number of infected herds in non‐disease‐free zones | 331 | 349 | 451 | 620 | 815 | EFSA |
| Number of reporting non‐disease‐free MSs | 6 | 7 (d) | 8 | 8 | 8 | EFSA |
ECDC: European Centre for Disease Prevention and Control; EFSA: European Food Safety Authority; MSs: Member States.
: Disease‐free status from infection with B. abortus, B. melitensis and B. suis in bovine animal populations, and/or ovine and caprine animal populations.
: Data on food and animal samples from the United Kingdom (Northern Ireland) are taken into account for 2021. In accordance with the agreement on the withdrawal of the United Kingdom from the EU, and in particular with the Protocol on Ireland/Northern Ireland, EU requirements on data sampling are also applicable to Northern Ireland.
: Data from the United Kingdom are taken into account for 2017–2019, because the United Kingdom was an EU MS over this period but it became a third country on 1 February 2020.
: No data were reported from Bulgaria.
6.3.2. Human brucellosis
In 2021, 162 confirmed cases were reported in the EU, which was a slight increase compared to 2020. The notification rate was 0.03 cases per 100,000 population, which is equal to the notification rate in 2020. In 2021, 26 MSs provided data and information on brucellosis in humans (Table 47). Compared with the rate before the COVID‐19 pandemic (2017–2019 mean annual), there was a decrease of 52.2% and 51.1%, with and without the data from the United Kingdom, respectively.
In 2021, 10 MSs (Bulgaria, Estonia, Finland, Hungary, Ireland, Latvia, Lithuania, Malta, Romania and Slovenia) reported zero cases (Table 48).
Table 48.
Reported confirmed human cases of brucellosis and notification rates per 100,000 population in EU MSs and non‐MSs countries, by country and year, 2017–2021
| Country | 2021 | 2020 | 2019 | 2018 | 2017 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Status | National coverage (c) | Data format (c) | Confirmed cases and rates | Confirmed cases and rates | Confirmed cases and rates | Confirmed cases and rates | Confirmed cases and rates | |||||||
| Bv (a) | Ov,Cp (b) | Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | |||
| Austria | DFS | DFS | Y | C | 6 | 0.07 | 8 | 0.09 | 6 | 0.07 | 7 | 0.08 | 6 | 0.07 |
| Belgium | DFS | DFS | Y | A | 7 | 0.06 | 4 | 0.03 | 3 | 0.03 | 9 | 0.08 | 8 | 0.07 |
| Bulgaria | Y | A | 0 | 0 | 1 | 0.01 | 0 | 0 | 1 | 0.01 | 2 | 0.03 | ||
| Croatia | DFS | Y | C | 2 | 0.05 | 1 | 0.02 | 3 | 0.07 | 3 | 0.07 | 1 | 0.02 | |
| Cyprus | DFS | DFS | Y | C | 1 | 0.11 | 0 | 0.00 | 0 | 0 | 0 | 0 | 0 | 0 |
| Czechia | DFS | DFS | Y | C | 1 | 0.01 | 0 | 0.00 | 4 | 0.04 | 4 | 0.04 | 1 | 0.01 |
| Denmark (d) | DFS | DFS | – | – | – | – | – | – | – | – | – | – | – | – |
| Estonia | DFS | DFS | Y | C | 0 | 0 | 0 | 0.00 | 1 | 0.08 | 1 | 0.08 | 0 | 0 |
| Finland | DFS | DFS | Y | C | 0 | 0 | 0 | 0.00 | 0 | 0 | 0 | 0 | 1 | 0.02 |
| France | DFS | Y | C | 21 | 0.03 | 19 | 0.03 | 34 | 0.05 | 0 | 0 | 21 | 0.03 | |
| Germany | DFS | DFS | Y | C | 13 | 0.02 | 19 | 0.02 | 36 | 0.04 | 37 | 0.04 | 41 | – |
| Greece | Y | C | 24 | 0.22 | 30 | 0.28 | 65 | 0.61 | 97 | 0.90 | 94 | 0.87 | ||
| Hungary | DFS | Y | C | 0 | 0 | 0 | 0.00 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Ireland | DFS | DFS | Y | C | 0 | 0 | 0 | 0.00 | 0 | 0 | 0 | 0 | 2 | 0.04 |
| Italy | Y | C | 32 | 0.05 | 18 | 0.03 | 49 | 0.08 | 94 | 0.16 | 99 | 0.16 | ||
| Latvia | DFS | DFS | Y | C | 0 | 0 | 1 | 0.05 | 0 | 0 | 0 | 0 | 0 | 0 |
| Lithuania | DFS | DFS | Y | C | 0 | 0 | 0 | 0.00 | 0 | 0 | 0 | 0 | 0 | 0 |
| Luxembourg | DFS | DFS | Y | C | 1 | 0.16 | 0 | 0.00 | 0 | 0 | 0 | 0 | 0 | 0 |
| Malta | DFS | Y | C | 0 | 0 | 0 | 0.00 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Netherlands | DFS | DFS | Y | C | 2 | 0.01 | 2 | 0.01 | 7 | 0.04 | 5 | 0.03 | 2 | 0.01 |
| Poland | DFS | DFS | Y | C | 1 | < 0.01 | 0 | 0.00 | 2 | 0.01 | 0 | 0 | 2 | 0.01 |
| Portugal | Y | C | 10 | 0.10 | 9 | 0.09 | 33 | 0.32 | 19 | 0.18 | 16 | 0.16 | ||
| Romania | DFS | DFS | Y | C | 0 | 0.00 | 0 | 0.00 | 1 | 0.01 | 1 | 0.01 | 3 | 0.02 |
| Slovakia | DFS | DFS | Y | C | 6 | 0.11 | 2 | 0.04 | 1 | 0.02 | 0 | 0 | 1 | 0.02 |
| Slovenia | DFS | DFS | Y | C | 0 | 0 | 1 | 0.05 | 6 | 0.29 | 3 | 0.15 | 1 | 0.05 |
| Spain (e) | DFS | Y | C | 25 | – | 10 | – | 20 | 0.04 | 40 | 0.09 | 63 | 0.14 | |
| Sweden | DFS | DFS | Y | C | 10 | 0.10 | 7 | 0.07 | 14 | 0.14 | 11 | 0.11 | 14 | 0.14 |
| EU Total 27 | – | – | – | 162 | 0.03 | 132 | 0.03 | 285 | 0.06 | 332 | 0.08 | 378 | 0.09 | |
| United Kingdom (f) | – | – | – | – | – | – | – | – | 24 | 0.04 | – | – | – | – |
| EU Total | – | – | – | 162 | 0.03 | 132 | 0.03 | 309 | 0.06 | 332 | 0.08 | 378 | 0.09 | |
| Iceland | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Norway | DFS | DFS | Y | C | 3 | 0.06 | 2 | 0.04 | 4 | 0.08 | 3 | 0.06 | 3 | 0.06 |
| Liechtenstein | DFS | DFS | Y | C | 0 | 0 | 3 | 0.03 | 7 | 0.08 | 5 | 0.06 | 9 | 0.11 |
| Switzerland (g) | DFS | DFS | Y | C | 6 | 0.07 | ||||||||
–: Data not reported.
DFS: Disease‐free status from infection with B. abortus, B. melitensis and B. suis in bovine animal populations, and/or in ovine and caprine animal populations.
: Bovine animal population.
: Ovine and caprine animal populations.
: Y: yes; N: no; A: aggregated data; C: case‐based data.
: No surveillance system.
: Data not complete for 2020–2021, rate not estimated.
: Cases reported by the United Kingdom for the period 2017–2019 were also considered for this estimation (EU‐28). When the United Kingdom data were collected for the period 2017–2019, the United Kingdom was an EU MS, but it became a third country on 1 February 2020.
: Switzerland provided data directly to EFSA. The human data for Switzerland include data from Liechtenstein for the years 2017–2020.

All Zones of the MS have disease‐free status.

Not all Zones of the MS have disease‐free status.

No Zones of the MS have disease‐free status.
Clear seasonality was observed in the number of confirmed brucellosis cases in the EU, with more cases reported from April to August. There was a significantly (p < 0.01) declining EU trend from 2017 to 2021 (Figure 16). Three MSs (Germany, Greece and Italy) had significantly decreasing 5‐year trends from 2017 to 2021.
Figure 16.
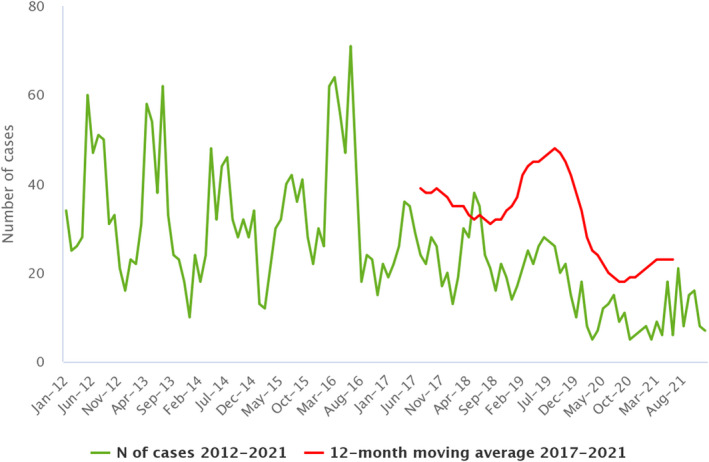
- Source: Austria, Cyprus, Czechia, Estonia, Finland, France, Germany, Greece, Hungary, Ireland, Italy, Lithuania, Latvia, Malta, Netherlands, Poland, Portugal, Romania, Slovakia, Slovenia and Sweden.
Ten MSs provided information on hospitalisation. Out of 162 human cases, 60 (37.0%) were reported with information on hospitalisation. Among these, 36 (60.0%) were hospitalised. This is a decrease of 6.7% compared to the data for 2020 (64.3%). Eleven MSs provided information on the outcome. Out of the 162 cases of human brucellosis, 59 (36.4%) were reported with information on disease outcomes. No fatalities out of the 59 cases were notified.
Out of 162 human cases, 58 (35.8%) were reported with information on the Brucella species by 12 MSs. B. melitensis was reported as the aetiological agent in 51 cases (87.9%); B. suis was reported in three cases (5.2%); B. abortus in two cases (3.4%); and other (not specified) Brucella species in two cases (3.4%).
The proportion of B. melitensis infections was the same compared to the data in 2020 (87.8%). Only 11 (30.6%) out of 36 hospitalised cases were reported with information on the Brucella species. B. melitensis was reported as the aetiological agent in 10 hospitalised cases and B. abortus in 1 hospitalised case.
The number of confirmed, domestically acquired brucellosis cases in humans (patients not having been outside the country of notification during the disease incubation period) is overlaid with the national prevalence data on Brucella‐positive cattle herds, and sheep and goat herds in the EU in 2021 in Figure 17. Greece, Italy and Spain have the highest number of confirmed brucellosis cases in humans, while Greece, Italy and Portugal have the highest prevalence of Brucella‐positive ruminant herds. Italy, which has reported a high number of human brucellosis cases over the years, did not report the origin of infection for 2021.
Figure 17.
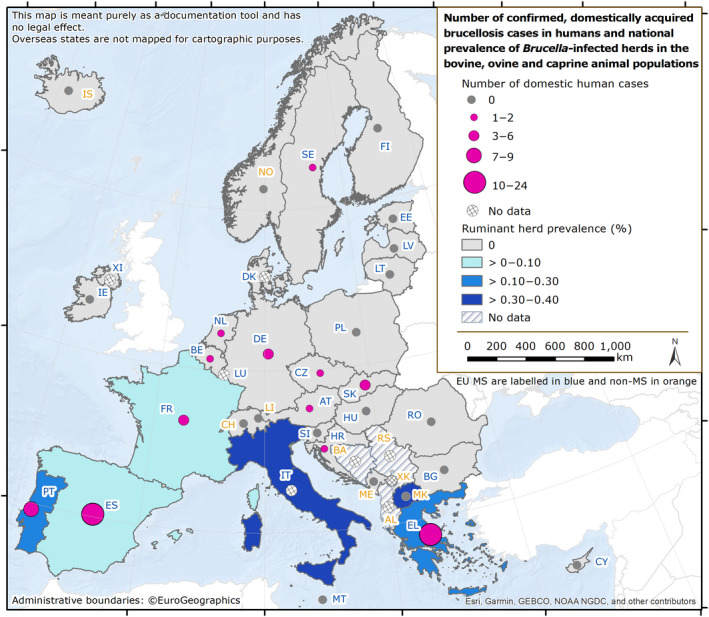
Number of confirmed, domestically acquired brucellosis cases in humans and national prevalence of Brucella‐positive cattle herds, and sheep and goat herds, in EU MSs and non‐MS countries, 2021
6.3.3. Brucella in food
Very few monitoring data for Brucella were submitted in 2021, as was the case in previous years. In total, 307 samples of ‘milk’, ‘cheese' and ‘other dairy products’ were collected from processing plants by three MSs (Italy, Portugal and Spain). Two Italian samples from ‘raw sheep's milk’ taken at the processing plant level identified as positive for an unspecified Brucella species.
6.3.4. Brucella in animals
Monitoring data for Brucella from bovine animals, and from sheep and goats, originating from the National Control and Eradication Programmes and/or from countries or zones with disease‐free status
Cattle
The status of countries for brucellosis in cattle, as of 31 December 2021, showed a favourable situation, with 21 MSs and the United Kingdom (Northern Ireland) being disease‐free in 2021 (Figure 18). Croatia obtained disease‐free status (DFS) in 2021. Out of the six non‐disease‐free status MSs, Italy, Portugal and Spain have increasing numbers of disease‐free zones. Since 2019, Spain has reported no positive herds, indicating an extremely low level of prevalence, and absence of infection. Bulgaria, Greece and Hungary have no disease‐free zones.
Figure 18.
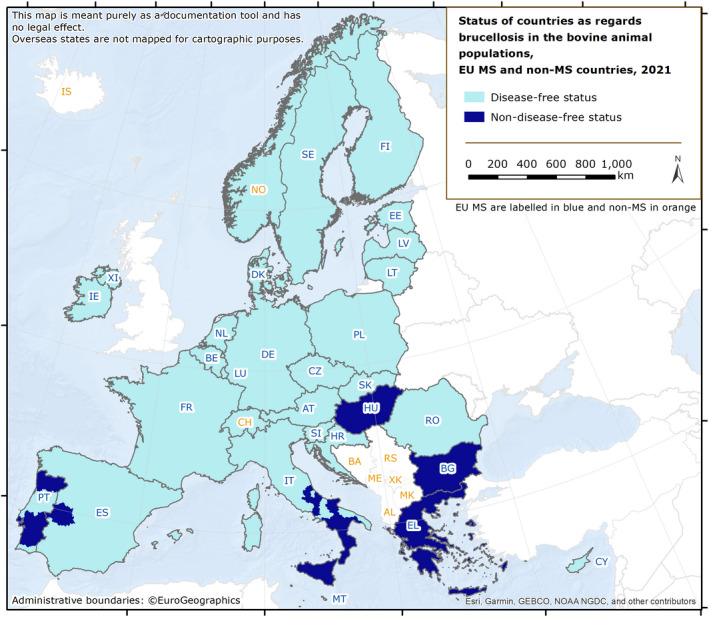
Status of countries as regards brucellosis in bovine animal population (Bison ssp., Bos ssp., Bubalus ssp.), MSs and non‐MSs, 2021
Three countries had disease‐free zones or provinces in cattle:
-
–
Italy: 13 regions and 9 provinces.
-
–
Portugal: 1 region (Algarve) and 14 districts (from the Centre region and from the Azores islands).
-
–
Spain: 16 autonomous communities and 1 province (Badajoz).
Lichtenstein, Norway, Switzerland and the United Kingdom (Northern Ireland) are disease‐free in accordance with the EU legislation. Iceland, which has no special agreement on animal health (status) with the EU, has never reported any brucellosis cases caused by B. abortus, B. melitensis or B. suis (Figure 18). 28
In 2021, the overall proportion of cattle herds infected with B. abortus, B. melitensis or B. suis in the EU remained very low (0.04%; 554 out of 1,719,963 herds), with 22 MSs and the United Kingdom (Northern Ireland) reporting no cases of brucellosis in cattle. Greece, Italy and Portugal reported most infected herds.
In DFS MSs or in the DFS zones of non‐DFS MSs, the overall prevalence was extremely low (< 0.001) (Table 49). Only eight infected cattle herds were reported in these DFS zones of the EU (six in 2020), with Italy reporting seven positive herds and France one.
Table 49.
Status of countries as regards brucellosis in bovine animals (Bos ssp., Bubalus ssp. and Bison ssp.) and related prevalence, EU, 2021
| Member State (MS) | Disease‐free status from brucellosis in bovine animals (a) | N of infected herds in DFS zones | Prevalence (%) of infected herds in DFS (a) zones | N of infected herds in non‐DFS (a) zones | Prevalence (%) of infected herds in non‐DFS (a) zones |
|---|---|---|---|---|---|
| Austria | DFS | 0 | 0 | – | – |
| Belgium | DFS | 0 | 0 | – | – |
| Bulgaria | – | – | 0 | 0 | |
| Croatia | DFS | 0 | 0 | – | – |
| Cyprus | DFS | 0 | 0 | – | – |
| Czechia | DFS | 0 | 0 | – | – |
| Denmark | DFS | 0 | 0 | – | – |
| Estonia | DFS | 0 | 0 | – | – |
| Finland | DFS | 0 | 0 | – | – |
| France | DFS | 1 | < 0.01 | – | – |
| Germany | DFS | 0 | 0 | – | – |
| Greece | – | – | 69 | 0.63 | |
| Hungary | – | – | 0 | 0 | |
| Ireland | DFS | 0 | 0 | – | – |
| Italy | 7 | 0.01 | 453 | 1.3 | |
| Latvia | DFS | 0 | 0 | – | – |
| Lithuania | DFS | 0 | 0 | – | – |
| Luxembourg | DFS | 0 | 0 | – | – |
| Malta | DFS | 0 | 0 | – | – |
| Netherlands | DFS | 0 | 0 | – | – |
| Poland | DFS | 0 | 0 | – | – |
| Portugal | 0 | 0 | 24 | 0.11 | |
| Romania | DFS | 0 | 0 | – | – |
| Slovakia | DFS | 0 | 0 | – | – |
| Slovenia | DFS | 0 | 0 | – | – |
| Spain | 0 | 0 | 0 | 0 | |
| Sweden | DFS | 0 | 0 | – | – |
| United Kingdom (Northern Ireland) (b) | DFS | 0 | 0 | – | – |
| EU Total (27 + XI) | 8 | < 0.001 | 546 | 0.43 |
–: not applicable (no such zones).
DFS: Disease‐free status.
: Disease‐free status from infection with B. abortus, B. melitensis and B. suis, in bovine animal populations.
: Data from the United Kingdom (Northern Ireland) are taken into account for 2021. In accordance with the agreement on the withdrawal of the United Kingdom from the EU, and in particular with the Protocol on Ireland/Northern Ireland, EU requirements on data sampling are also applicable to Northern Ireland, which is disease‐free.

All Zones of the MS have disease‐free status.

Not all Zones of the MS have disease‐free status.

No Zones of the MS have disease‐free status.
In 2021, the prevalence of brucellosis‐infected cattle herds remained very low in the non‐DFS zones of the six non‐disease‐free MSs, with 546 positive herds (0.43%; Table 49), compared with 603 (0.38%) positive herds in 2020. The number of positive herds out of all herds reported by Italy in its non‐DFS zones was 453 (504 in 2020), while this number was 24 in Portugal (27 in 2020). Greece reported 69 positive herds (72 in 2020).
Comparing data for 2012 with data for 2021, the overall annual number of reported infected cattle herds in non‐DFS zones decreased by 53.7%, from 1,181 to 546, while the prevalence of infected cattle herds increased from 0.10% to 0.43% (Figure 19). This is due to the reduction in the number of non‐DFS zones and the resulting drop in the total number of cattle herds of interest from 1,162,978 to 127,000 during the same period. No speciation of Brucella isolates was reported for cattle.
Figure 19.
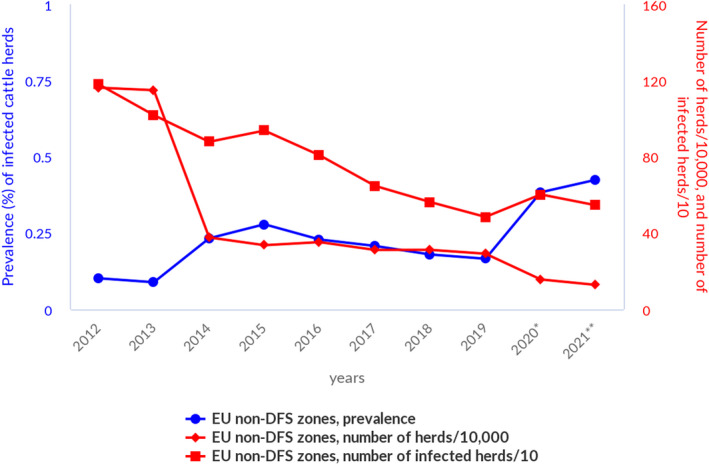
Prevalence of Brucella‐positive cattle herds, in non‐DF zones, EU, 2012–2021
Figure 20 displays trends between 2005 and 2021 in the reported prevalence of brucellosis test‐positive cattle herds in Greece, Italy and Portugal. The prevalence in Greece showed considerable annual variation from a minimum of 2% in 2008 to a maximum of 12% in 2012. The prevalence in Italy has remained under 2% since 2012, with a minimum value of 1.3% in 2019. The prevalence in Portugal has decreased consistently from about 1% in 2005 to 0.11% in 2021.
Figure 20.
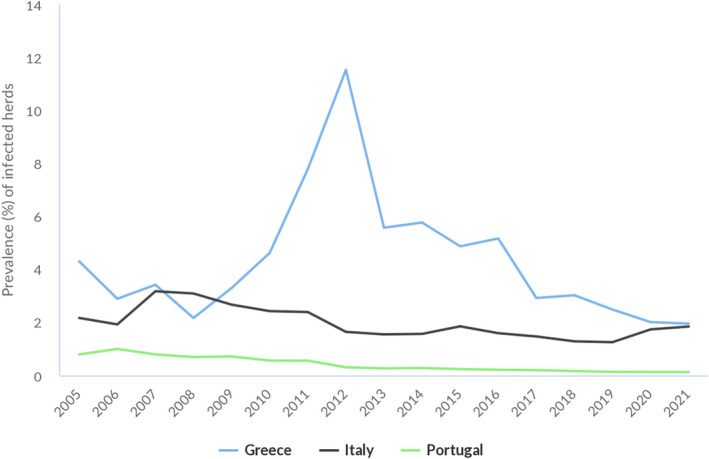
Prevalence of Brucella‐positive cattle herds, in two MSs with non‐DFS zones (Italy and Portugal) and in one non‐DFS MS (Greece), 2005–2021
In 2021, bovine brucellosis was not detected in the following non‐MS countries: Iceland, Montenegro, Norway and Switzerland. In the Balkans area, the disease is still present in cattle, with 25 infected (0.17%) herds out of 14,898 herds reported by North Macedonia, along with positive results reported by Bosnia and Herzegovina, and Serbia from national monitoring data. Bosnia and Herzegovina, and Serbia reported 108 positive animals out of 111,785 (0.10%) and 13 positive animals out of 488,587 (0.003%), respectively.
Sheep and goats
The status of countries as regards ovine and caprine brucellosis, reflecting the situation on 31 December 2021, is presented in Figure 21 and in Table 50. In 2021, 20 MSs and the United Kingdom (Northern Ireland) had disease‐free status, with Spain obtaining the disease‐free status in 2021. Out of the seven other MSs, three had DFS zones (France, Italy and Portugal) and four MSs had no zones with a disease‐free status (Bulgaria, Croatia, Greece and Malta). In France, no cases of brucellosis have been reported in small ruminants since 2003, and the whole of metropolitan France obtained disease‐free status in 2021.
Figure 21.
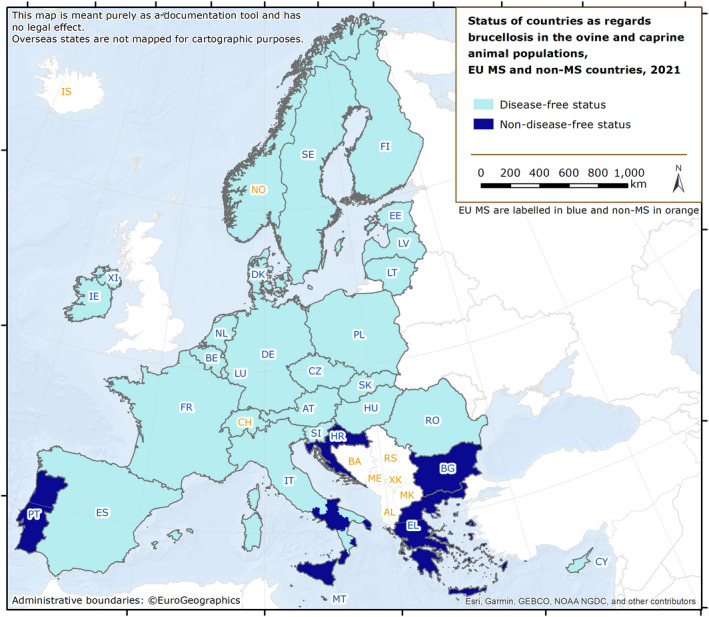
Status of countries as regards ovine and caprine brucellosis, MSs 29 and non‐MS countries, 2021
Table 50.
Status of countries for brucellosis in sheep (Ovis ssp.) and goat (Capra ssp.) populations and related prevalence, EU, 2021
| Member State | Disease‐free status from brucellosis in sheep and goats (a) | N of infected herds in DFS zones | Prevalence (%) of infected herds in DFS (a) zones | N of infected herds in non DFS (a) zones | Prevalence (%) of infected herds in non‐DFS (a) zones |
|---|---|---|---|---|---|
| Austria | DFS | 0 | 0 | – | – |
| Belgium | DFS | 0 | 0 | – | – |
| Bulgaria | – | – | 0 | 0 | |
| Croatia | – | – | 0 | 0 | |
| Cyprus | DFS | 0 | 0 | – | – |
| Czechia | DFS | 0 | 0 | – | – |
| Denmark | DFS | 0 | 0 | – | – |
| Estonia | DFS | 0 | 0 | – | – |
| Finland | DFS | 0 | 0 | – | – |
| France (b) | 0 | 0 | – | – | |
| Germany | DFS | 0 | 0 | – | – |
| Greece | – | – | 26 | 0.10 | |
| Hungary | DFS | 0 | 0 | – | – |
| Ireland | DFS | 0 | 0 | – | – |
| Italy | 6 | < 0.01 | 107 | 0.40 | |
| Latvia | DFS | 0 | 0 | – | – |
| Lithuania | DFS | 0 | 0 | – | – |
| Luxembourg | DFS | 0 | 0 | – | – |
| Malta | – | – | 0 | 0 | |
| Netherlands | DFS | 0 | 0 | – | – |
| Poland | DFS | 0 | 0 | – | – |
| Portugal | 0 | 0 | 198 | 0.38 | |
| Romania | DFS | 0 | 0 | – | – |
| Slovakia | DFS | 0 | 0 | – | – |
| Slovenia | DFS | 0 | 0 | – | – |
| Spain | DFS | 9 | < 0.01 | – | – |
| Sweden | DFS | 0 | 0 | – | – |
| United Kingdom (Northern Ireland) (c) | DFS | 0 | 0 | – | – |
| EU Total (27 + XI) | 15 | < 0.01 | 331 | 0.18 |
–: not applicable (no such zones).
DFS: Disease‐free status.
: Disease‐free status from infection with B. abortus, B. melitensis and B. suis in ovine and caprine animal populations
: The whole of metropolitan France has disease‐free status.
: Data from the United Kingdom (Northern Ireland) are taken into account for 2021. In accordance with the agreement on the withdrawal of the United Kingdom from the EU, and in particular with the Protocol on Ireland/Northern Ireland, EU requirements on data sampling are also applicable to Northern Ireland, which is disease‐free.

All Zones of the MS have disease‐free status.

Not all Zones of the MS have disease‐free status.

No Zones of the MS have disease‐free status.
Three countries had disease‐free zones or provinces for sheep and goats:
-
–
France: all 13 metropolitan regions.
-
–
Italy: 15 regions and 5 provinces.
-
–
Portugal: 1 region (the autonomous region of the Azores).
In 2021, the overall proportion of sheep and goat herds infected with B. abortus, B. melitensis or B. suis in the EU remained very low (0.03%; 346 out of 1,069,048 herds). In 2021, 23 MSs and the United Kingdom (Northern Ireland) reported no cases of infection with B. abortus, B. melitensis or B. suis in sheep and goat herds (Table 50). Infected or positive herds were reported by Greece, Italy, Portugal and Spain.
In particular, in the DFS zones the prevalence was extremely low, with 21 MSs reporting no cases of brucellosis, and Italy and Spain together reporting 15 infected herds (3 in 2020 reported by Italy), resulting in an overall prevalence in the DFS zones lower than 0.01% (0.0004% in 2020). 29
In 2021, the seven MSs with non‐DFS zones reported 331 infected herds (0.18%), compared to 349 (0.22%) in 2020 (Table 50). The number of infected herds reported by these MSs was 26 in Greece (33 in 2020), 107 in Italy (120 in 2020) and 198 in Portugal (196 in 2020). No infected herds were reported by Bulgaria, Croatia, France and Malta. For the last 2 years, Croatia has reported zero infected herds, indicating that in the coming years, eradication of sheep and goat brucellosis is an achievable goal.
Spain reported that nine sheep and goat herds in its DFS zones were infected with Brucella melitensis. No other speciation of isolates was reported for sheep and goats.
From 2012 to 2021, the overall annual number of reported infected sheep and goat herds in the non‐disease‐free zones decreased by 80.4%, from 1,693 to 331, while the prevalence of positive herds decreased by 60%, from 0.45% to 0.18% (Figure 22).
Figure 22.
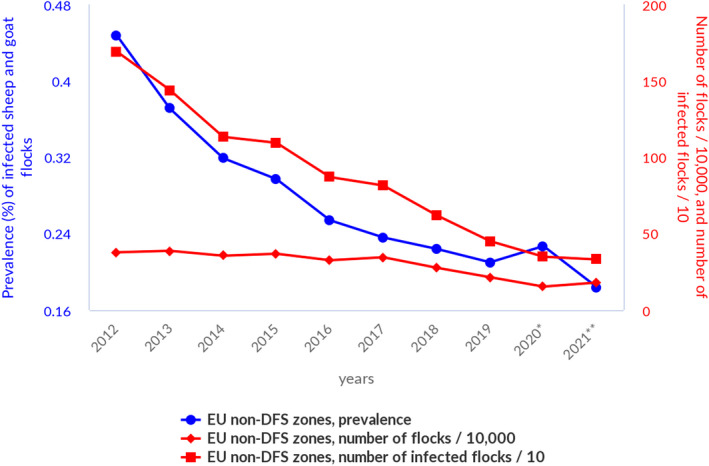
Prevalence of Brucella‐positive sheep and goat herds, in non‐DFS zones, EU, 2012–2021
Figure 23 displays trends from 2004 to 2020 in the reported prevalence of brucellosis test‐positive sheep and goat herds in non‐DFS zones of the three MSs reporting the majority of infected herds in the EU. The prevalence in Greece showed high annual variation from a minimum of 0.4% in 2015 to a maximum of 8.6% in 2012. This may be related to the low proportion of tested herds, which leads to a lack of precision in estimates. Italy and Portugal reported low (> 1–10%) to very low (0.1–1%) prevalence during this period, decreasing for both MSs.
Figure 23.
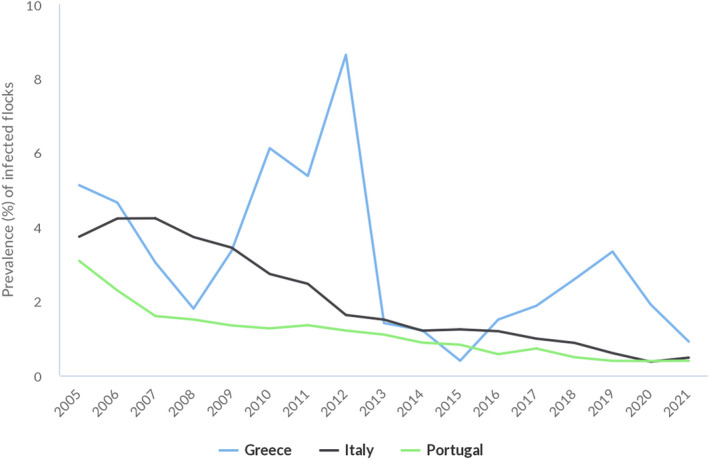
Prevalence of Brucella‐positive sheep and goat herds, in two MSs with non‐DFS zones (Italy and Portugal) and in one non‐DFS MS (Greece), 2005–2021
Brucellosis was not detected in sheep and goat herds in 2021 in the following non‐MS countries: Iceland, Montenegro, Norway and Switzerland. North Macedonia reported 44 infected herds (0.79%) out of 5,574 and submitted national monitoring data on ovine and caprine brucellosis. From national monitoring data, North Macedonia and Serbia reported 1,406 positive animals out of 502,864 (0.28%) and 8 positive animals out of 1,406,330 (0.001%), respectively.
Monitoring data for Brucella from animals other than bovine animals, and sheep and goats
Similarly to 2021 reports on Brucella from cattle, and from sheep and goats, Brucella species were reported from a wide range of animal species. B. canis was isolated in dogs from six MSs (Finland, France, Italy, the Netherlands, Sweden and Romania) and from one non‐MS (Norway). Five MSs reported 44 positive dogs out of 414 tested animals (10.6%). B. microti was isolated from ‘farmed’ frogs in France; B. suis biovar 2 or unspecified Brucella species were reported in pigs, wild boars and wild hares in seven MSs (Finland, France, Germany, Italy, Romania, Slovakia and Spain); B. melitensis was isolated from Alpine ibex in France; unspecified Brucella species were found in wild bears, wild deer and wild boars; and B. ceti was found in dolphins in Italy and marine mammals in Sweden.
6.4. Discussion
The 5‐year EU trend of confirmed cases of brucellosis in humans declined significantly from 2017 to 2021. The EU notification rate was 0.03 per 100,000 population, which was the same as that reported in 2020, the lowest since surveillance began in the EU in 2007. According to Kefaloudi et al. (2022), human brucellosis remains a persistent public health problem with an annual notification rate averaging 0.9/100,000 population (standard deviation [SD]: 0.35).
Because international travel was significantly reduced during the first part of the COVID‐19 pandemic, it has been postulated that the risk of human infection of travellers to endemic areas traditionally suited to tourism was likely smaller. This could have had an impact on the trend for human brucellosis. More evidence‐based data should be acquired to provide insights into the true efficacy of the eradication plans in animals on the incidence of brucellosis in humans.
Like in 2020, human cases in non‐DFS countries, such as Greece and Portugal, can be inferred to be mainly of domestic origin, while human cases in DFS countries, such as Austria, Germany, France and Sweden are of non‐domestic origin. Italy, which also reported several human cases, did not provide information on the origin of infection. Interestingly, both Bulgaria (non‐disease‐free) and Hungary (non‐disease‐free for cattle) did not report any cases of human brucellosis. Where such information was available, it was clear that human cases were commonly associated with hospitalisation.
In 2021, human cases of brucellosis were mainly caused by infection with B. melitensis. Furthermore, B. melitensis was the major species involved in cases of hospitalisation, where speciation information was provided. This information is very important when optimising risk management to further reduce disease in humans, considering that B. melitensis is mainly, if not completely, associated with brucellosis in sheep and goats. Three human cases were attributed to B. suis. This finding should be monitored in the next few years to detect a possible trend for a potentially emerging condition.
Bovine brucellosis, and ovine and caprine brucellosis have been eradicated by most EU MSs. In MSs and zones with disease‐free status, very few infected herds were reported for 2021: 8 infected cattle herds, and 15 infected sheep and goat herds.
Eradication of brucellosis in cattle has been achieved in Croatia and Spain. Metropolitan France has eradicated brucellosis in sheep and goats. Some MSs were not disease‐free free from bovine brucellosis and/or from brucellosis in sheep and goats, and both infections were mostly reported in Greece, Italy and Portugal. In Greece, the proportion of tested herds remained limited, which may affect the precision of surveillance. The overall number of infected herds should be interpreted differently between countries, as the proportion of tested herds was highly variable between MSs.
Nevertheless, from 2012 to 2021, the overall annual number of reported positive ruminants in the non‐DFS zones decreased, with a decrease in prevalence. In Italy, problems were concentrated in the southern part of the country and Sicily. Moreover, the infection was present in water buffalo from the provinces of Salerno and Caserta, where in the latter province a drastic increase in the prevalence and incidence of infection in buffalo has been observed in the last years. About 80% of Campania's buffalo population is found in Caserta. The infection is concentrated in the geographic areas with the highest density of animals and farms per km2 (Ottaiano et al., 2022). In non‐DFS zones, non‐food‐borne transmission of brucellosis to humans may still occur through direct contact with infected animals. People working with farm animals, including farmers, livestock breeders, butchers, abattoir workers and veterinarians, are known to be at increased risk of brucellosis in endemic countries.
In 2021, the regulatory framework for brucellosis in animals changed substantially in the EU. Infection with B. abortus, B. melitensis and B. suis in bovine animals, and sheep and goats is currently considered a disease to be controlled in all MSs with the goal of eradicating the disease in these animals throughout the EU, while being kept under surveillance in other even‐toed ungulates (Artiodactyla). All MSs must have a surveillance, or a control and eradication programme in place, approved by the EC. This should lead, in the coming years, to further progressive improvement in the already satisfactory epidemiological situation.
Attention should also be paid to canine brucellosis cases due to B. canis, which is also considered zoonotic and which is covered by the Directive 2003/99/EC, Annex I list A ‘brucellosis and agents thereof’. In the last 2 years, an increased number of cases was described in Italy (De Massis et al., 2021), France, the Netherlands (van Dijk et al., 2021) and the United Kingdom (England; source APHA). Moreover, despite the lack of bacterial speciation, it is important to highlight that many different zoonotic species of Brucella are isolated from many hosts, including marine mammals and amphibians. Rouzic et al. (2021) reported the first human case of brucellosis caused by an isolate whose genome is identical to that of a frog isolate from Texas, demonstrating the zoonotic potential of atypical amphibian‐type Brucella.
In conclusion, although cases of brucellosis in humans and ruminants are declining in the EU, brucellosis is still an animal health concern with public health relevance, especially in southern European countries that are not brucellosis disease‐free.
7. Trichinella
Summary data substantiating this chapter, as well as additional information on related projects and internet sources are published for this report on the EFSA Knowledge Junction on Zenodo here. Summary statistics on trichinellosis foodborne outbreaks reported in the framework of Directive 2003/99/EC, with downloadable files, are retrievable using the EFSA foodborne outbreaks dashboard available here. Summary statistics on human surveillance data with downloadable files are retrievable using the ECDC Surveillance Atlas of Infectious Diseases here.

7.1. Key facts
In 2021, the number of confirmed cases of human trichinellosis was 77, corresponding to an EU notification rate of 0.02 per 100,000 population. This was a decrease of 32.5% compared with 2020 (0.03 per 100,000 population).
Compared with the rate before the COVID‐19 pandemic (2017–2019 annual mean), the 2021 EU notification rate decreased by 17.5% and 28.6% with and without the data from the United Kingdom, respectively.
The overall trend for trichinellosis during the period 2017–2021 in the EU did not show any statistically significant increase or decrease.
In 2021, no infections with Trichinella were reported in tested fattening pigs (54 million) or breeding pigs (0.9 million) kept under controlled housing conditions, confirming that farming conditions are a key factor in preventing infection with this zoonosis.
In pigs not kept under controlled housing conditions, 0.0001% (120 out of 161 million) were positive for Trichinella. Romania accounted for most of the positive pigs (81), followed by Poland (19), Spain (13), Croatia (five) and Finland and France (one each).
No Trichinella infections were detected in domestic solipeds in the EU in 2021, as during the 2017–2020 period.
In 2021, the proportion of hunted wild boar that tested positive was 0.07%.
The proportion of Trichinella‐positive foxes (indicator animals) was 1.6% in 2021, while for 2020, a rate of 0.9% was reported.
7.2. Surveillance and monitoring of Trichinella in the EU
7.2.1. Humans
In 2021, 26 EU MSs reported information on trichinellosis in humans. The surveillance of Trichinella infections is mandatory in all reporting MSs, except in Belgium and France, where surveillance systems are voluntary. There is no surveillance system in place for trichinellosis in Denmark and the disease is not notifiable or reported at the EU level. The surveillance systems cover the whole population in all MSs reporting trichinellosis data. All countries reported case‐based data except Bulgaria, which reported aggregated data. The EU case definition was used by 22 MSs, other case definitions were used by three MSs (France, Germany and Italy) and Belgium did not specify the case definition used.
7.2.2. Animals
Trichinella monitoring data for domestic pigs (both fattening and breeding animals), farmed wild boar and solipeds
In accordance with Commission Implementing Regulation (EU) 2015/1375 30 , all Trichinella‐susceptible animals intended for human consumption in the EU, i.e. domestic pigs (both fattening and breeding animals), farmed wild boar and solipeds, should be tested for the presence of Trichinella larvae in the muscles unless carcases have undergone a freezing treatment (freezing inactivates the parasite). ISO 18743/2015 or an equivalent method should be used (Commission Implementing Regulation (EU) 2015/1375). Therefore, data on Trichinella infections in these animals are comparable across MSs because the monitoring schemes are harmonised and the data collected and reported to EFSA originate from census sampling (EFSA BIOHAZ Panel, 2013a,b) (Table 51).
Table 51.
Summary of Trichinella statistics related to humans and main animal species, EU, 2017–2021
| 2021 (a) | 2020 | 2019 (b) | 2018 (b) | 2017 (b) | Data source | |
|---|---|---|---|---|---|---|
| Humans | ||||||
| Total number of confirmed cases | 77 | 117 | 97 | 66 | 168 | ECDC |
| Total number of confirmed cases/100,000 population (notification rates) | 0.02 | 0.03 | 0.02 | 0.01 | 0.03 | ECDC |
| Number of reporting MSs | 26 | 26 | 27 | 27 | 27 | ECDC |
| Infections acquired in the EU | 29 | 99 | 26 | 18 | 81 | ECDC |
| Infections acquired outside the EU | 2 | 2 | 2 | 1 | 2 | ECDC |
| Unknown travel status or unknown country of infection | 46 | 16 | 69 | 47 | 85 | ECDC |
| Number of outbreak‐related cases | 2 | 119 | 44 | 114 | 199 | EFSA |
| Total number of outbreaks | 1 | 6 | 5 | 10 | 11 | EFSA |
| Animals | ||||||
| Domestic pigs RCHC | ||||||
| Number of units tested (c) | 55,177,802 | 55,989,292 | 73,633,900 | 77,794,786 | 72,227,074 | EFSA |
| % of positive units | 0 | 0 | 0 | 0 | 0 | EFSA |
| Number of reporting MSs | 17 | 16 | 16 | 15 | 14 | EFSA |
| Domestic pigs NRCHC | ||||||
| Number of units tested (c) | 161,129,635 | 139,637,631 | 145,213,445 | 152,922,322 | 124,689,434 | EFSA |
| % of positive units | 0.0001 | 0.0001 | 0.0002 | 0.0003 | 0.0002 | EFSA |
| Number of reporting MSs | 22 | 22 | 25 | 25 | 25 | EFSA |
| Farmed wild boar | ||||||
| Number of units tested (c) | 5,755 | 3,922 | 7,570 | 6,343 | 17,799 | EFSA |
| % of positive units | 0 | 0 | 0 | 0 | 0.74 | EFSA |
| Number of reporting MSs | 8 | 6 | 7 | 7 | 8 | EFSA |
| Hunted wild boar | ||||||
| Number of units tested (c) | 1,786,892 | 1,470,830 | 1,757,383 | 1,465,788 | 1,398,905 | EFSA |
| % of positive units | 0.0664 | 0.0484 | 0.0778 | 0.0891 | 0.0878 | EFSA |
| Number of reporting MSs | 20 | 21 | 23 | 23 | 22 | EFSA |
| Foxes | ||||||
| Number of units tested (c) | 6,776 | 5,764 | 6,696 | 6,612 | 6,486 | EFSA |
| % of positive units | 1.5939 | 0.8501 | 1.3292 | 1.6334 | 1.2180 | EFSA |
| Number of reporting MSs | 11 | 9 | 10 | 10 | 11 | EFSA |
ECDC: European Centre for Disease Prevention and Control; EFSA: European Food Safety Authority; MSs: Member States.
RCHC: raised under controlled housing conditions; NRCHC: not raised under controlled housing conditions.
: Data on animals from the United Kingdom (Northern Ireland) are taken into account for 2021. In accordance with the agreement on the withdrawal of the United Kingdom from the EU, and in particular with the Protocol on Ireland/Northern Ireland, the EU requirements on data sampling are also applicable to Northern Ireland.
: Data from the United Kingdom are taken into account for 2017–2019, because the United Kingdom was an EU MS, but it became a third country on 1 February 2020.
: Units: animals and/or slaughter animal batches.
Domestic pigs, farmed and hunted wild boar and other wild animals (e.g. bears) that are not processed to be placed on the EU market (e.g. those intended for own consumption) are exempted from Commission Implementing Regulation (EU) 2015/1375 and their control falls under national legislation. Commission Implementing Regulation (EU) 2015/1375 states that the reporting of data for domestic pigs shall, at least, provide specific information related to the number of animals tested that were raised under controlled housing conditions (RCHC) as well as the number of breeding sows, boar and fattening pigs tested. Furthermore, the regulation states that a negligible risk status for a country or region is no longer recognised.
Trichinella monitoring data for animals other than domestic pigs, farmed wild boar and solipeds
MSs should monitor the circulation of these nematodes in the main natural reservoir hosts (carnivorous and omnivorous animals) to acquire information on the risk of transmission to domestic animals (and from these to humans) and on the introduction of new Trichinella species from non‐EU countries. However, monitoring data provided by the MSs to EFSA are generated by non‐harmonised monitoring schemes across MSs without mandatory reporting requirements. Wild animals are the main reservoir hosts of Trichinella, and their biology and ecology vary from one MS to another and from one region or habitat in the same MS to another due to the human and environmental impact on ecosystems, resulting in different transmission patterns and prevalence rates of infection. Therefore, data on Trichinella in wild animals are not fully comparable among MSs, as neither harmonised monitoring schemes nor mandatory reporting requirements are in place, and the reported findings must be interpreted with caution. These data allow descriptive summaries to be produced at the EU level but preclude any subsequent data analysis such as an assessment of temporal and spatial trends (Table 51).
7.3. Results
7.3.1. Overview of key statistics, EU, 2017–2021
Table 51 summarises EU‐level statistics on human trichinellosis and on Trichinella in animals, for the 2017–2021 period. More detailed descriptions of these statistics are provided in the below subsections and in the chapter on foodborne outbreaks.
7.3.2. Human trichinellosis
In 2021, 77 confirmed cases of trichinellosis were reported by 26 MSs, which was a decrease compared with 2020 (Table 51). The EU notification rate decreased by 32.5% from 0.03 per 100,000 in 2020 to 0.02 per 100,000 in 2021. This decrease was mainly due to the decrease in the number of outbreak‐related cases reported by Italy, who reported for 2020 a large outbreak. Fifteen countries, including Italy, reported zero cases in 2021. Compared with the rate before the COVID‐19 pandemic (2017–2019 annual mean), there were decreases of 17.5% and 28.6% with and without the data from the United Kingdom, respectively.
In 2021, Bulgaria and Croatia had the highest notification rates in the EU (0.42 cases per 100,000 in both countries), followed by Latvia (0.37 cases per 100,000) and Austria (0.11 cases per 100,000) (Table 52). Together, these four countries (Austria, Bulgaria, Croatia and Latvia) accounted for 80% of all confirmed trichinellosis cases reported at the EU level in 2021.
Table 52.
Reported confirmed human cases of trichinellosis and notification rates per 100,000 population in EU MSs and non‐MS countries, by country and year, 2017–2021
| Country | 2021 | 2020 | 2019 | 2018 | 2017 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| National coverage (a) | Data format (a) | Confirmed cases and rates | Confirmed cases and rates | Confirmed cases and rates | Confirmed cases and rates | Confirmed cases and rates | ||||||
| Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | |||
| Austria | Y | C | 10 | 0.11 | 6 | 0.07 | 1 | 0.01 | 2 | 0.02 | 3 | 0.03 |
| Belgium (b) | Y | A | 0 | – | – | – | – | – | 0 | – | 0 | – |
| Bulgaria | Y | A | 29 | 0.42 | 13 | 0.19 | 55 | 0.79 | 45 | 0.64 | 55 | 0.77 |
| Croatia | Y | C | 17 | 0.42 | 0 | 0 | 3 | 0.07 | 0 | 0 | 21 | 0.51 |
| Cyprus | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Czechia | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Denmark (c) | – | – | – | – | – | – | – | – | – | – | – | – |
| Estonia | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Finland | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| France | Y | C | 2 | < 0.01 | 1 | < 0.01 | 2 | < 0.01 | 0 | 0 | 8 | 0.01 |
| Germany | Y | C | 2 | < 0.01 | 1 | < 0.01 | 3 | < 0.01 | 0 | 0 | 2 | < 0.01 |
| Greece | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.01 |
| Hungary | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0.02 | 0 | 0 |
| Ireland | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Italy | Y | C | 0 | 0 | 79 | 0.13 | 10 | 0.02 | 2 | < 0.01 | 4 | 0.01 |
| Latvia | Y | C | 7 | 0.37 | 1 | 0.05 | 1 | 0.05 | 1 | 0.05 | 1 | 0.05 |
| Lithuania | Y | C | 1 | 0.04 | 0 | 0 | 0 | 0 | 0 | 0 | 9 | 0.32 |
| Luxembourg | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Malta | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Netherlands | Y | A | 0 | 0 | 0 | 0 | 1 | 0.01 | 0 | 0 | 0 | 0 |
| Poland | Y | C | 2 | 0.01 | 11 | 0.03 | 2 | 0.01 | 2 | 0.01 | 9 | 0.02 |
| Portugal | Y | C | 0 | 0 | 0 | 0 | 1 | 0.01 | 0 | 0 | 1 | 0.01 |
| Romania | Y | C | 6 | 0.03 | 4 | 0.02 | 6 | 0.03 | 10 | 0.05 | 48 | 0.24 |
| Slovakia | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.02 |
| Slovenia | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Spain | Y | C | 1 | < 0.01 | 1 | < 0.01 | 12 | 0.03 | 2 | < 0.01 | 5 | 0.01 |
| Sweden | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| EU Total 27 | 77 | 0.02 | 117 | 0.03 | 97 | 0.02 | 66 | 0.02 | 168 | 0.04 | ||
| United Kingdom | Y | C | – | – | – | – | 0 | 0 | 0 | 0 | 0 | 0 |
| EU Total (d) | 77 | 0.02 | 117 | 0.03 | 97 | 0.02 | 66 | 0.01 | 168 | 0.03 | ||
| Iceland | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Norway | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Liechtenstein | Y | C | 0 | 0 | 4 | 0.05 | 3 | 0.03 | 0 | 0 | 1 | 0.01 |
| Switzerland (e) | Y | C | 0 | 0 | ||||||||
–: Data not reported.
: Y: yes; N: no; A: aggregated data; C: case‐based data.
: Sentinel surveillance, disease not under formal surveillance. Notification rate not calculated.
: No surveillance system.
: Cases reported by the United Kingdom for the period 2017–2019 were also considered for this estimation (EU‐28). When the United Kingdom data were collected for the period 2017–2019, the United Kingdom was an EU MS, but it became a third country on 1 February 2020.
: Switzerland provided data directly to EFSA. The human data for Switzerland include data from Liechtenstein for the years 2017–2020.
In 2021, 29 cases (36.7%) of trichinellosis with known travel status and with known country of infection were reported to be acquired in the EU. One MS (Austria) reported two travel‐associated trichinellosis cases infected outside the EU. For 46 cases (59.7%), travel information was not reported (Table 51).
The EU trend in confirmed cases of trichinellosis did not show any statistically significant decrease or increase over the period 2017–2021 (Figure 24). During the same period, only Romania reported a significant decreasing trend and none of the MSs observed a significant increasing trend. Bulgaria, which had reported most of the cases until 2019 and had the highest notification rates in the EU in 2017–2021, was not included in the EU trend calculations since monthly data were not available.
Figure 24.
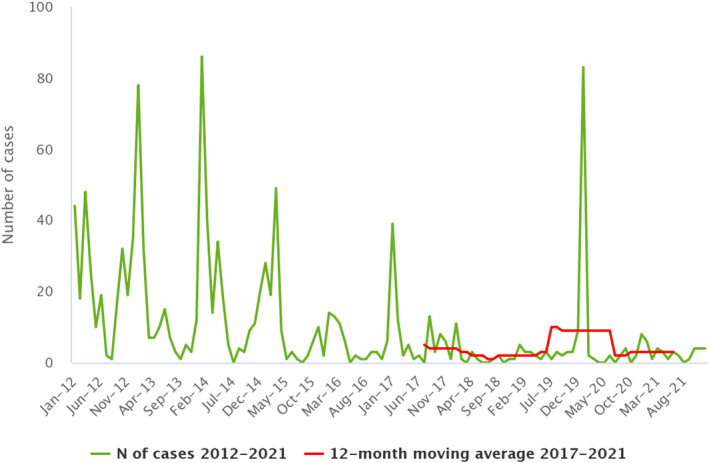
- Source: Austria, Cyprus, Czechia, Estonia, Finland, France, Germany, Greece, Hungary, Ireland, Italy, Latvia, Lithuania, Luxembourg, Malta, Poland, Portugal, Romania, Slovakia, Slovenia, Spain and Sweden.
Of the 10 MSs reporting confirmed cases for 2021, six provided information on hospitalisation (26 cases, 33.8% of all confirmed cases reported in the EU). Of these cases, 10 (38.5%) were hospitalised, which was a decrease compared with 2020 (72.7%). Six MSs provided information on the outcome of the cases (28 cases, 35.4% of all confirmed cases); no deaths were reported. Trichinella spiralis was identified as the causative agent of all the 27 (34.2%) confirmed human cases for which species identification was reported by five MSs.
7.3.3. Trichinella infection in food and animals
In 2021, one MS (Croatia) reported monitoring data for four meat products (fermented sausages made with pork) samples taken at the farm level and all tested positive. T. spiralis was identified in three samples.
In 2021, 31 countries (27 MSs, the United Kingdom (Northern Ireland) and three non‐MSs) provided information on Trichinella in domestic animals (pigs and/or farmed wild boar). Sixteen MSs, the United Kingdom (Northern Ireland) and one non‐MS (Iceland) reported data on breeding and/or fattening pigs RCHC; no positive findings were reported in these domestic pigs RCHC. No positive findings were found in farmed wild boar (Table 53).
Table 53.
Trichinella monitoring results for domestic pigs and farmed wild boar in reporting EU MSs and non‐MS countries, by housing conditions (a) , 2021
| Country | N positive/tested (% positive) | ||||
|---|---|---|---|---|---|
| Not controlled housing conditions (NCHC) | Controlled housing conditions | ||||
| Farmed wild boar | Fattening pigs | Breeding pigs | Fattening pigs | Breeding pigs | |
| Austria | 0/80 (0) | 0/5,024,563 (0) | 0/90,865 (0) | – | – |
| Belgium | – | 0/498,359 (0) | 0/1,598,668 (0) | 0/8,213,983 (0) | – |
| Bulgaria | – | – | – | 0/98,945 (0) | – |
| Croatia | – | 5/152,512 (< 0.001) | – | 0/1,045,327 (0) | 0/70,710 (0)(j) |
| Cyprus | – | 0/572,556 (0) | 0/13,059 (0) | – | – |
| Czechia | – | – | – | 0/2,385,961 (0) | – |
| Denmark | 0/394 (0) | 0/771,963 (0) | 0/271,526 (0) | 0/17,164,861 (0) | 0/289,868 (0) |
| Estonia | – | 0/149,163 (0) | 0/224,415 (0) | – | |
| Finland | 0/198 (0) | 1/1,904,914 (< 0.001) | 0/34,690 (0) | 0/2,165 (0) | 0/108 (0) |
| France | 0/455 (0) | 1/524,576 (< 0.001) (b) | 0/14,380 (0) | 0/25,734 (0) | 0/308,567 (0) |
| Germany | 0/1,157 (0) | 0/51,923,389 (0) | – | – | – |
| Greece | 0/1,323 (0) | 0/1,014,741 (0) (c) | 0/20,981 (0) | 0/7,899 (0) | – |
| Hungary | – | 0/4,973,495 (0) | 0/363,435 (0) | – | – |
| Ireland | – | – | – | 0/3,603,287 (0) | 0/90,444 (0) |
| Italy | 0/1,773 (0) | 0/9,776,660 (0) (d) | – | 0/37,931 (0) | 0/176,313 (0) |
| Latvia | – | 0/473,831 (0) | – | – | – |
| Lithuania | – | – | – | 0/901,388 (0) | – |
| Luxembourg | – | 0/144,880 (0) | – | – | – |
| Malta | – | 0/52,797 (0) | 0/1,244 (0) | – | – |
| Netherlands | – | – | – | 0/14,955,097 (0) | – |
| Poland | – | 19/21,438,846 (< 0.001) | – | – | – |
| Portugal | – | 0/4,072,097 (0) | 0/29,033 (0) | – | – |
| Romania | – | 81/211,260 (< 0.001) | – | 0/3,731,300 (0) | 0/9,323 (0) |
| Slovakia | – | 0/662,881 (0) (e) | 0/13,373 (0) | – | – |
| Slovenia | – | 0/242,584 (0) (f) | – | – | – |
| Spain | 0/375 (0) | 10/52,803,353 (< 0.001) (g) | 3/818,668 (< 0.001) (h) | 0/50,377 (0) | |
| Sweden | – | 0/451,423 (0) | 0/18,870 (0) | 0/1,746,698 (0) | 0/35,819 (0) |
| United Kingdom (Northern Ireland) | – | – | – | 0/1,282 (0) (i) | – |
| EU Total (27 + XI) | 0/5,755 (0) | 117/157,840,843 (< 0.001) | 3/3,288,792 (< 0.001) | 0/54,196,650 (0) | 0/981,152 (0) |
| Iceland | – | – | – | 0/78,267 (0) | – |
| Norway | – | 0/1,568,000 (0) | – | – | – |
| Switzerland | – | 0/2,265,215 (0) | 0/29,836 (0) | – | – |
| Total non‐EU Countries | – | 0/3,833,215 (0) | 0/29,836 (0) | 0/78,267 (0) | – |
| Total EU (27+ XI) + non‐EU countries | 0/5,755 (0) | 117/161,674,058 (< 0.001) | 3/3,318,628 (< 0.001) | 0/54,274,917 (0) | 0/981,152 (0) |
MSs: Member States.
: No pigs reported in 2021 with unspecified housing conditions.
: Comprising 21,832 pigs in mixed herds including one positive pig.
: Comprising 3,104 pigs for own consumption.
: Comprising 60,509 pigs for own consumption.
: Comprising 1,020 pigs for own consumption.
: Pigs in mixed herds.
: Comprising 9,972 pigs for own consumption and 37,066 piglets.
: Comprising 35,220 piglets.
: Pigs in mixed herds.
: Comprising 62,393 piglets and 747 boars.
Six MSs (Croatia, Finland, France, Poland, Romania and Spain) reported positive findings for domestic pigs NRCHC for 2021. For fattening pigs NRCHC, 117 (< 0.01%) (including one pig from a mixed herd) were positive, whereas only three breeding pigs were positive (< 0.01%). Romania accounted for more than half of the positive pigs (81, 67%), followed by Poland (19, 16%), Spain (13, 11%), Croatia (five, 4.2%), Finland (one, 0.8%) and France (one, 0.8%). Species identification was reported for 60 (50%) out of 120 pigs. T. spiralis was detected in 50 pigs (83%), T. britovi in eight pigs (13%), T. pseudospiralis in one pig (1.6%) and T. nativa in one pig (1.6%). As in previous years, these Trichinella infections were found in free‐range and backyard pigs reared in rural EU regions.
As shown in Figure 25, from 2012 to 2018 (7‐year period), Trichinella spp. were not reported in domestic pigs (RCHC or NRCHC) or farmed wild boar in 16 MSs, unlike in the other 12 MSs. In 2019, 2020 and 2021, Trichinella spp. in pigs were reported by six (Bulgaria, Croatia, France, Poland, Romania and Spain), seven (Bulgaria, Croatia, France, Greece, Italy, Romania and Spain) and six (Croatia, Finland, France, Poland, Romania and Spain) MSs, respectively.
Figure 25.
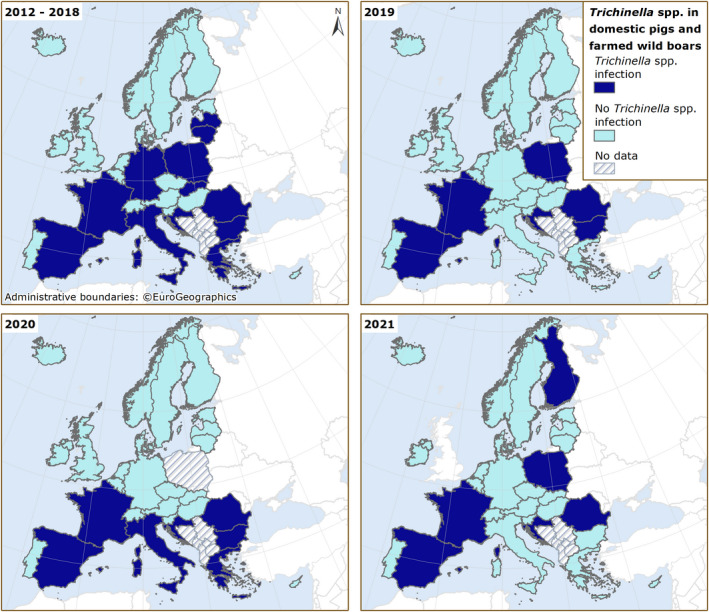
In 2021, as in the previous 5‐year period (2016–2020), positive findings were not reported either in 103,745 domestic solipeds tested in 20 MSs or in 8,276 domestic solipeds tested in two non‐MSs (Iceland and Switzerland) (Table 54).
Table 54.
Trichinella monitoring results for hunted wild boar and wild boar with unspecified habitat, bears, foxes and domestic solipeds, in reporting EU MSs and non‐MS countries, 2021
| Country | N positive/tested (% positive) | |||
|---|---|---|---|---|
| Hunted or unspecified wild boar | Bears | Foxes | Domestic solipeds | |
| Austria | 0/26,524 (0) | – | 0/3 (0) | 0/440 (0) |
| Belgium | 0/22,305 (0) | – | – | 0/7,937 (0) |
| Croatia | 74/56,661 (0.13) | 1/108 (0.93) | – | 0/579 (0) |
| Cyprus | – | – | 0/75 (0) | – |
| Czechia | 0/232,355 (0) | – | 3/2,758 (0.11) | 0/77 (0) |
| Denmark | – | – | – | 0/761 (0) |
| Estonia | 3/1,705 (0.18) | 9/68 (13.2) | 0/8 (0) | |
| Finland | 1/931 (0.11) | 11/279 (3.9) | 77/161 (47.8) | 0/757 (0) |
| France | 2/51,511 (0) | – | – | 0/3,815 (0) |
| Germany | 40/590,369 (0.01) | – | 0/732 (0) | 0/3,744 (0) |
| Hungary | 3/48,791 (0.01) | – | – | 0/223 (0) |
| Ireland | – | – | – | 0/1,662 (0) |
| Italy | 14/208,148 (0.01) | 0/1 (0) | 8/2,316 (0.35) | 0/28,800 (0) |
| Latvia | 16/7,641 (0.21) | – | 0/34 (0) | |
| Luxembourg | 0/6,747 (0) | – | 0/116 (0) | 0/10 (0) |
| Netherlands | 0/5,726 (0) | – | 0/1,777 (0) | |
| Poland | 453/201,263 (0.23) | – | 16/85 (18.8) | |
| Portugal | 0/121 (0) | – | – | 0/649 (0) |
| Romania | 46/9,207 (0.50) | 1/11 (9.1) | – | 0/29,946 (0) |
| Slovakia | 0/13,072 (0) | 0/3 (0) | 3/93 (3.2) | |
| Slovenia | 0/2,274 (0) | 0/86 (0) | – | 0/1,057 (0) |
| Spain | 531/170,715 (0.31) | – | – | 0/20,361 (0) |
| Sweden | 3/130,826 (0) | 0/325 (0) | 1/41 (2.4) | 0/1,108 (0) |
| United Kingdom (Northern Ireland) | – | – | 0/396 (0) | – |
| EU Total (27 + XI) | 1,186/1,786,892 (0.07) | 22/881 (2.5) | 108/6,776 (1.6) | 0/103,745 (0) |
| Iceland | – | – | – | 0/7,158 (0) |
| Norway | 0/292 (0) | – | – | – |
| Republic of North Macedonia | 14/1,218 (1.1) | – | – | – |
| Switzerland | 1/10,741 (0.01) | 0/1 (0) | – | 0/1,118 (0) |
| Total non‐EU Countries | 15/12,251 (0.12) | 0/1 (0) | – | 0/8,276 (0) |
| Total EU (27+ XI) + non‐EU countries | 1,201/1,799,143 (0.07) | 22/882 (2.5) | – | 0/112,021 (0) |
Twelve MSs reported positive findings for hunted wild boar (1,186 positive findings out of 1,786,892 animals tested (0.07%)). Species identification was provided for 865 wild boar (73%), of which 722 (83.4%) were infected with T. spiralis, 140 (16.2%) with T. britovi, two (0.23%) with T. pseudospiralis and one (0.11%) with T. nativa and T. britovi (mixed infection). For 321 (27%) animals, species identification was not reported.
Six MSs (Czechia, Finland, Italy, Poland, Slovakia and Sweden) reported positive findings for Trichinella in foxes (Vulpes vulpes) with, in total, 108 (1.6%) positive out of 6,776 tested animals in 11 MSs. T. britovi was identified in 26 (76%) animals, T. spiralis in four (12%), T. nativa in three (8.8%) and T. pseudospiralis in one (2.9%), out of 34 positive foxes with species identification provided. Four MSs (Croatia, Estonia, Finland and Romania) reported positive findings for Trichinella in brown bears (Ursus arctos) with 22 (2.5%) positive units out of 881 tested in eight MSs (Table 54). T. nativa, T. britovi and T. pseudospiralis were identified in 13 (59%), eight (36%) and one (4.5%) brown bear(s), respectively; one of them presented with a mixed T. nativa and T. britovi infection. Nine MSs and one non‐MS reported data on Trichinella in wild animals other than foxes, brown bears and wild boar. Positive findings were detected for raccoon dogs (32.9%), lynxes (17.2%), wolves (16.8%), birds (0.72%), martens (0.44%), badgers (0.15%) and other animals (0.4%), as shown in Table 55. In wild animals, T. spiralis was reported in Poland (one beaver); T. britovi was identified in three MSs (Italy (18 wolves), Poland (one wolf) and Sweden (two lynxes)) and in one non‐MS (Switzerland (one jackal)). T. nativa was documented in Finland (12 racoon dogs, one beaver and four lynxes) and Sweden (four lynxes and one wolf). T. pseudospiralis was documented in Finland (one goshawk and one owl) and Italy (one marsh harrier).
Table 55.
Trichinella monitoring results in other wild animals in reporting EU MSs and non‐MS countries, 2021
| Country | N positive/tested (% positive) | ||||||
|---|---|---|---|---|---|---|---|
| Badgers | Wolves | Raccoon dogs | Birds | Lynxes | Martens | Other animals | |
| Austria | 0/64 (0) | 0/4 (0) (j) | |||||
| Croatia | 0/2 (0) | 0/4 (0) (j) | |||||
| Cyprus | 0/1 (0) (k) | ||||||
| Finland | 1/11 (9.1) (a) | 19/41 (46.3) (b) | 91/231 (39.4) (e) | 2/71 (2.8) (f) | 20/40 (50.0) (h) | 1/2 (50.0) (b) | 1/51 (1.9) (l) |
| Germany | 0/56 (0) | 0/46 (0) | 0/331 (0) (m) | ||||
| Hungary | 0/3 (0) (n) | ||||||
| Italy | 0/521 (0) | 40/279 (14.3) (c) | 0/46 (0) | 1/344 (0.29) (g) | 0/179 (0) | 0/89 (0) (o) | |
| Poland | 1/1 (100) (d) | 1/1 (100) (p) | |||||
| Sweden | 0/9 (0) | 1/42 (2.4) (a) | 6/111 (5.4) (i) | 0/15 (0) (q) | |||
| EU Total (27 + XI) | 1/663 (0.15) | 61/363 (16.8) | 91/277 (32.9) | 3/415 (0.72) | 26/151 (17.2) | 1/227 (0.44) | 2/499 (0.40) |
| Switzerland | 0/9 (0) | 2/22 (9.1) (d) | 1/34 (2.9) (d) | 1/4 (25.0) (r) | |||
| Total EU (27+ XI) + non‐EU countries | 1/672 (0.15) | 63/385 (16.4) | 91/277 (32.9) | 3/415 (0.72) | 27/185 (14.6) | 1/227 (0.44) | 3/503 (0.59) |
: T. nativa.
: Unknown species.
: Eighteen infected with T. britovi.
: T. britovi.
: Twelve infected with T. nativa.
: Comprising three crows, seven eagles, 21 white‐tailed eagles, one falcon, 30 goshawks (one positive for T. pseudospiralis) and nine owls (one positive for T. pseudospiralis).
: Comprising one marsh harrier (positive for T. pseudospiralis) and 343 unspecified birds.
: Four positive for T. nativa.
: Four positive for T. nativa and two for T. britovi.
: All deer.
: Bats.
: Comprising four minks, 45 otters and two wolverines (one positive for unknown species).
: Comprising 12 coypus, 181 racoons and 138 unspecified animals.
: Rats.
: Comprising five roe deer, 19 hedgehogs, 33 jackals, three polecats, five weasels and 24 wildcats (Felis silvestris).
: One T. spiralis‐infected beaver.
: Comprising six beavers, two lions, four seals and three wolverines.
: Comprising one coypu, one pet dog, one jackal (positive for T. britovi) and one wildcat (Felis silvestris).
7.4. Discussion
Trichinellosis is a rare but serious human disease that is still present in some EU MSs. Fifteen out of 26 MSs reported zero cases including three MSs (Cyprus, Luxembourg and Malta) that have never reported any trichinellosis cases since the beginning of EU‐level surveillance in 2007.
The COVID‐19 pandemic did not appear to impact the reporting of human cases of trichinellosis in 2020 and 2021, whereas the number of reported FBOs and outbreak‐related human cases dropped in 2021. One MS (Portugal) reported a decrease in the number of wild boar hunted in 2020 and 2021 compared with that in the previous years (2017–2010), due to lockdowns associated with this pandemic.
In general, Trichinella infections in humans are often linked to FBOs; therefore, the EU trend for trichinellosis has been affected by the number and size of FBOs. The EU notification rate was not higher than 0.03 per 100,000 population in the last 5 years, from 2017 to 2021, with the highest rate (0.03) reported in 2017 and 2020, and the lowest rate (0.01) reported in 2018; this was the lowest rate ever reported since the beginning of EU‐level trichinellosis surveillance in 2007. In 2021, Bulgaria and Croatia accounted for more than half (58%) of all confirmed cases in the EU and did not report any trichinellosis FBOs. Romania, which had experienced the most Trichinella outbreaks in the previous years, showed a significant decrease in the 5‐year trend from 2017 to 2021. In 2021, only one weak‐evidence outbreak was reported in the EU, by France.
Pigs represent the largest livestock category in the EU. More than 216 million pigs were tested for Trichinella in MSs and non‐MSs in 2021 out of more than 246 million pigs reared in the EU (Marquer et al., 2014; European Parliament, 2020), with only 120 positive animals, i.e. about 0.49 per million reared pigs. Only six out of 22 MSs reported Trichinella in pigs in 2021, with an overall prevalence rate of 0.00005%. All positive findings were for pigs NRCHC. Most pigs at risk for this infection are backyard or free‐range pigs, which are usually slaughtered at home, where veterinary control or recording can be easily evaded. In the EU, infected pigs are usually clustered in five MSs (Bulgaria, Croatia, Poland, Romania and Spain) and sporadic infections are documented in other MSs (Pozio, 2014). Bulgaria did not report any 2021 data for pigs NRCHC and reported zero positive out of all the tested pigs RCHC, confirming that RCHC is a key condition to prevent infection with this zoonosis. Finland reported one outdoor‐reared T. nativa‐positive Mangalica pig; this MS had not reported any positive findings in domestic pigs since 2012, although Trichinella spp. circulate among wild animals in this country. EFSA has identified that not raising domestic pigs under controlled housing conditions is a major risk factor for Trichinella infections (EFSA and ECDC, 2011; EFSA BIOHAZ Panel et al., 2011). Identification of Trichinella larvae at species level in 2021 confirmed that T. spiralis was more prevalent than T. britovi in pigs (83% vs. 13%) (Pozio et al., 2009). However, T. spiralis was patchily distributed. T. pseudospiralis was reported in only one out of 60 pigs (1.8%) for which the Trichinella species was available; these data confirmed the low prevalence of this species in target animals (Pozio, 2016).
Hunted wild boar are a major source of trichinellosis infections in humans. Human behaviour can strongly influence sylvatic cycles, both favouring and reducing the transmission of Trichinella spp. For example, carcases of Trichinella‐infected animals left by hunters, or of such animals that have died in road accidents, may be scavenged by other wild animals, thereby contributing to transmission.
No positive findings were reported for solipeds in 2021. Over the last 12 years, only four horses tested positive out of more than one million tested, in 2008, 2010 and 2012 (EFSA and ECDC, 2009, 2010, 2011, 2012, 2013, 2014). This extremely low (< 0.001%) prevalence may have been related to effective control which, according to the EFSA BIOHAZ Panel (2013b), should be maintained if there is no full and reliable traceability system in place, especially since meat from solipeds can be eaten raw in some EU countries.
Trichinella spp. circulate among wild animals in large parts of Europe. The reporting of negative findings in MSs could be explained by an insufficient number of surveys, inadequate sample sizes or investigations in regions whose environmental conditions do not favour the transmission of these zoonotic nematodes among wildlife. Some MSs (Austria, Croatia and Italy) tested non‐susceptible wild animals (deer) for Trichinella infection. Apart from horses, other herbivores are considered accidental hosts of Trichinella spp. (Kärssin et al., 2021). High endemicity for Trichinella in the sylvatic cycles of these countries and/or convenience sampling might justify surveillance in these animals. Foxes (indicator animals), having a large and widespread population, can be considered as the main natural reservoir of Trichinella in Europe. In 2021, the prevalence of Trichinella infection in this animal species was 1.59%, which was the highest level observed in the last 3 years. This increase was mainly due to the positive findings reported by Poland, which had not reported data on this animal species in 2020, and Finland. In 2021, this MS reported 77 positive animals out of 161 tested (47.8%), whereas in 2020, it had documented 35 positive findings out of 210 (16.7%). In this case, the prevalence of Trichinella infection in animals may vary among the different sampling areas. In 2021, as in 2020, the proportion of positive samples from wildlife was higher in raccoon dogs (32.9%), wolves (16.8%) and lynxes (17.2%) than in other animals sampled but their population sizes and distributions in Europe are generally limited to a few countries.
In some MSs (Bulgaria, Croatia, etc.), there has been an increasing number of pigs RCHC and increased control at slaughter of pigs NRCHC during the last few years. These measures, in combination with trichinellosis awareness‐raising and farmers' education activities, may have contributed to a reduction in the parasite biomass in domestic habitats and in the probability of acquiring an infection for humans.
Farming practices at risk of transmitting Trichinella spp. (rearing backyard or free‐range pigs) occur, in general, in disadvantaged and poor areas where veterinary services do not exist or are unable to control many small pig units, or where veterinary supervision can be circumvented (Pozio, 2014). There are examples from the past, where countries had suitable controls in place for parasite management in domestic pigs, but where changes in pork production affected by socioeconomic conflicts resulted in the re‐emergence of trichinellosis as a serious public health problem (Djordjevic et al., 2003; Cuperlovic et al., 2005). The increasing number of wild boar and foxes and the spread of the raccoon dog population from eastern to western Europe and of the jackal population from southern–eastern to northern–western Europe may increase the prevalence of Trichinella circulating among wild animals (Alban et al., 2011; Széll et al., 2013).
8. Echinococcus
Summary data substantiating this chapter, as well as additional information on related projects and internet sources are published for this report on the EFSA Knowledge Junction on Zenodo here. Summary statistics on human surveillance data with downloadable files are retrievable using the ECDC Surveillance Atlas of Infectious Diseases here.

8.1. Key facts
In 2021, the number of confirmed cases of human echinococcosis was 529, corresponding to an EU notification rate of 0.15 per 100,000 population. This is a decrease of 7.5% compared with 2020 (0.16 per 100,000 population). The notification rate in 2021 was the lowest since EU surveillance of echinococcosis began in 2007.
Compared with the rate before the COVID‐19 pandemic (2017–2019 annual mean), the notification rate in humans decreased by 23.0% and 30.4% with and without the data from the United Kingdom, respectively.
Echinococcus granulosus sensu lato (s.l.) accounted for 67.0% (N = 278) of human cases reported with species information for 2021 while Echinococcus multilocularis accounted for 33.0% (N = 137).
In total, 22 Member States and two non‐Member States provided monitoring data for Echinococcus spp. in animals in 2021.
For E. multilocularis, 14 Member States and two non‐Member States reported data on 6,318 and 513 tested foxes, respectively, with nine Member States and one non‐Member State reporting positive findings and an overall proportion of test‐positives of 15.7%.
Data for 2020 from Finland, Ireland, Malta, the United Kingdom and mainland Norway confirmed the free status of these countries for E. multilocularis in accordance with Commission Delegated Regulation (EU) No 2018/772 (EFSA and Zancanaro, 2021).
For E. granulosus s.l., 19 Member States and two non‐Member States reported data from around 96 million animals, primarily domestic livestock (> 99%), with an overall proportion of test‐positives of 0.06%. Positive samples came mainly from small ruminants (sheep and goats; 57.2%, mostly from Spain, Greece, Italy and Slovakia), with cattle (bovine animals) accounting for 20.5% of total positives (mostly from Spain, Italy and Greece) and pigs for 22.1% (mostly from Poland and Spain).
8.2. Surveillance and monitoring of cystic and alveolar echinococcosis in humans and animals in the EU
8.2.1. Humans
In 2021, 25 MSs reported information on echinococcosis infections in humans. Surveillance is mandatory in 22 MSs and voluntary in three MSs (Belgium, France and the Netherlands), and two MSs (Denmark and Italy) do not have surveillance systems for echinococcosis. The surveillance systems for echinococcosis cover the whole population in all MSs where surveillance systems are in place. All countries reported case‐based data, except Bulgaria and the Netherlands, which reported aggregated data. The EU case definition was used by 23 countries; Germany reported using a different case definition, and France did not specify which case definition was used.
Alveolar echinococcosis (AE) caused by the tapeworm Echinococcus multilocularis and cystic echinococcosis (CE) caused by E. granulosus sensu lato (s.l.) are listed under the common disease name ‘echinococcosis’ in the EU case definition, thus making no distinction between these two diseases. AE and CE can be reported by species, and as of 2019 (2018 data), by clinical presentation of the disease in the ECDC TESSy database.
8.2.2. Animals
Surveillance of E. multilocularis in Europe is usually carried out on a voluntary basis, except in the reporting countries claiming to be free of this parasite in accordance with the Commission Delegated Regulation (EU) 2018/772 supplementing Regulation (EU) No 576/2013 31 . Surveillance is mainly carried out in red fox, which is Europe's main definitive host. In 2020, Finland, Ireland, mainland Norway (Svalbard archipelago excluded) and the United Kingdom demonstrated the absence of E. multilocularis through the implementation of an annual surveillance programme in compliance with Regulation (EU) 2018/772 (EFSA and Zancanaro, 2021). In accordance with said Regulation, Malta is not required to implement a surveillance programme, due to the absence of the definitive red fox host on its territory. In all other MSs, data on E. multilocularis depend on whether findings are notifiable, monitoring is in place or studies on E. multilocularis are performed. Given that data on E. multilocularis in animals vary geographically (and also within countries) and over time, depending on the sampling effort, it is difficult to compare reported cases of E. multilocularis within and between countries. According to a meta‐analysis based on studies published between 1900 and 2015, E. multilocularis has been documented in red foxes from 21 countries (Oksanen et al., 2016) (Figure 26). Since 2018, this parasite has also been found in golden jackals from Croatia, Hungary, Serbia and Slovenia (Dušek et al., 2020; Balog et al., 2021).
Figure 26.
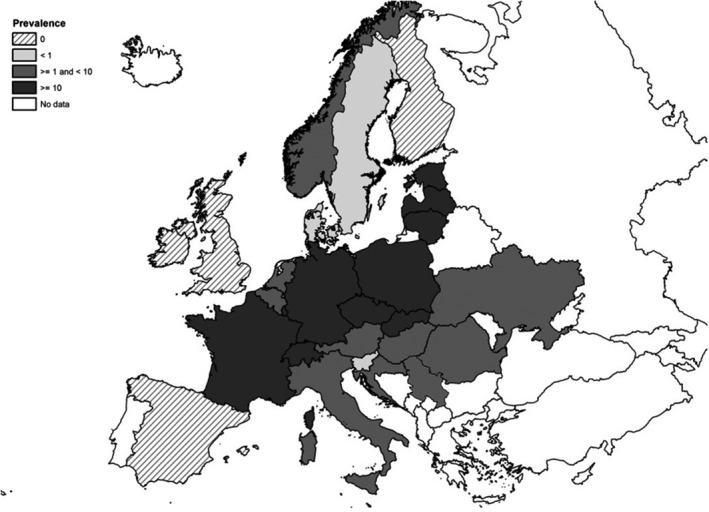
Pooled prevalence of Echinococcus multilocularis in red and Arctic foxes within the EU and adjacent countries, depicting the current epidemiological situation in Europe (Oksanen et al., 2016)
In accordance with CIR (EU) 2020/2002, disease‐free EU MSs must notify outbreaks of infection with E. multilocularis in Canidae to the EU ADIS. 32
Surveillance of E. granulosus s.l. is carried out in livestock intermediate hosts during slaughterhouse inspections. In particular, necropsy on sheep liver and lungs is used to detect the presence of parasitic cysts, while molecular PCR‐based methods are used to confirm and identify genotype/species belonging to the Echinococcus genus (Siles‐Lucas et al., 2017). Although Regulation (EU) 2018/772 is in force for E. multilocularis, no specific EU regulation is in place for detecting E. granulosus s.l. in animals or humans. Surveillance of the latter parasite therefore depends on national regulations. Approximate geographical distribution of E. granulosus sensu stricto, E. canadensis and E. ortleppi species causing human cystic echinococcosis in Europe are reported in Figure 27 (Casulli et al., 2022).
Figure 27.
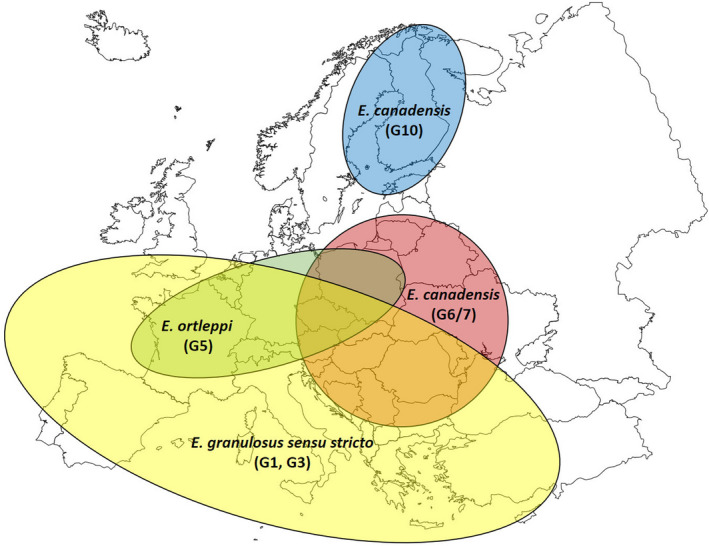
Approximate geographical distribution of the Echinococcus granulosus sensu lato species causing human cystic echinococcosis in Europe (2000–2021) (Casulli et al., 2022)
8.3. Results
8.3.1. Overview of key statistics, EU, 2017–2021
Table 56 summarises EU‐level statistics aggregated by year for CE and AE in humans for E. granulosus s.l. and E. multilocularis in their most relevant definitive and intermediate animal hosts in 2017–2021. More detailed descriptions of these statistics are provided in the below subsections.
Table 56.
Summary of echinococcosis in humans, caused by Echinococcus multilocularis or Echinococcus granulosus sensu lato (s.l.) in the most relevant definitive and intermediate animal hosts in the EU, 2017–2021
| 2021 (a) | 2020 | 2019 (b) | 2018 (b) | 2017 (b) | Data source | |
|---|---|---|---|---|---|---|
| Humans | ||||||
| Total number of confirmed cases | 529 | 544 | 769 | 815 | 851 | ECDC |
| Total number of confirmed cases/100,000 population (notification rates) | 0.15 | 0.16 | 0.17 | 0.21 | 0.19 | ECDC |
| Number of reporting MSs | 25 | 25 | 26 | 25 | 26 | ECDC |
| Infection acquired in the EU | 128 | 62 | 176 | 149 | 169 | ECDC |
| Infection acquired outside the EU | 81 | 77 | 96 | 83 | 77 | ECDC |
| Unknown travel status or unknown country of infection | 320 | 405 | 497 | 583 | 605 | ECDC |
| Animals (c) | ||||||
| Echinococcus multilocularis in foxes | ||||||
| Number of animals tested | 6,318 | 5,506 | 6,326 | 6,566 | 7,148 | EFSA |
| % positive animals | 17.0 | 16.1 | 13.7 | 18.4 | 16.9 | EFSA |
| Number of reporting MSs | 14 | 10 | 13 | 13 | 11 | EFSA |
| Echinococcus spp. in dogs | ||||||
| Number of animals tested | 2,942 | 2,515 | 2,113 | 2,605 | 2,538 | EFSA |
| % positive animals | 0.07 | 0.08 | 0.24 | 0.08 | 0 | EFSA |
| Number of reporting MSs | 5 | 5 | 6 | 6 | 7 | EFSA |
| Echinococcus granulosus s.l. in cattle (bovine animals) | ||||||
| Number of animals tested | 7,065,934 | 7,035,066 | 10,956,688 | 9,920,327 | 9,833,614 | EFSA |
| % positive animals | 0.21 | 0.21 | 0.17 | 0.23 | 0.22 | EFSA |
| Number of reporting MSs | 16 | 15 | 16 | 17 | 15 | EFSA |
| Echinococcus granulosus s.l. in sheep and goats | ||||||
| Number of animals tested | 10,817,922 | 11,089,043 | 36,890,847 | 38,870,491 | 38,278,556 | EFSA |
| % positive animals | 0.38 | 0.96 | 0.38 | 0.37 | 0.40 | EFSA |
| Number of reporting MSs | 14 | 12 | 15 | 15 | 14 | EFSA |
ECDC: European Centre for Disease Prevention and Control; EFSA: European Food Safety Authority; MSs: Member States.
: Data on animals from the United Kingdom (Northern Ireland) are taken into account for 2021. In accordance with the agreement on the withdrawal of the United Kingdom from the EU, and in particular with the Protocol on Ireland/Northern Ireland, the EU requirements on data sampling are also applicable to Northern Ireland.
: Data from the United Kingdom are taken into account for 2017–2019, because the United Kingdom was an EU MS, but it became a third country on 1 February 2020.
: Single tested animals.
8.3.2. Human echinococcosis
In 2021, 529 confirmed echinococcosis cases were reported by 25 MSs, corresponding to an EU notification rate of 0.15 cases per 100,000 population (Table 57). This was a decrease of 7.5% compared with 2020 (0.16 per 100,000 population). A mean notification rate of 0.19 was reported over 2017–2019. Compared with the rate before the COVID‐19 pandemic, the notification rate in humans decreased by 23.0% and 30.4% with and without the data from the United Kingdom, respectively. In 2021, 22 MSs reported at least one confirmed case, while three MSs reported zero cases (Cyprus, Malta and the Netherlands). In 2021, the highest notification rate was observed in Bulgaria, with 1.3 cases per 100,000 population, followed by Lithuania, Slovenia and Austria with 0.72, 0.52 and 0.47 cases per 100,000 population, respectively. Germany, Bulgaria and France reported the highest numbers of cases, with 152 (28.7%), 89 (16.8%) and 75 (14.2%) cases out of 529, respectively.
Table 57.
Reported human cases of cystic and alveolar echinococcosis and notification rates per 100,000 population in EU MSs and non‐MS countries, by country and year, 2017–2021
| Country | 2021 | 2020 | 2019 | 2018 | 2017 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| National coverage (a) | Data format (a) | Confirmed cases and rates | Confirmed cases and rates | Confirmed cases and rates | Confirmed cases and rates | Confirmed cases and rates | ||||||
| Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | |||
| Austria | Y | C | 42 | 0.47 | 34 | 0.38 | 36 | 0.41 | 46 | 0.52 | 50 | 0.57 |
| Belgium | Y | C | 18 | 0.16 | 19 | 0.16 | 22 | 0.19 | 15 | 0.13 | 13 | 0.11 |
| Bulgaria | Y | A | 89 | 1.29 | 95 | 1.37 | 193 | 2.76 | 206 | 2.92 | 218 | 3.07 |
| Croatia | Y | C | 3 | 0.07 | 3 | 0.07 | 3 | 0.07 | 4 | 0.10 | 15 | 0.36 |
| Cyprus | Y | C | 0 | 0 | 1 | 0.11 | 0 | 0 | 0 | 0 | 0 | 0 |
| Czechia | Y | C | 1 | 0.01 | 4 | 0.04 | 1 | 0.01 | 4 | 0.04 | 1 | 0.01 |
| Denmark (b) | – | – | – | – | – | – | – | – | – | – | – | – |
| Estonia | Y | C | 4 | 0.30 | 1 | 0.08 | 2 | 0.15 | 0 | 0 | 1 | 0.08 |
| Finland (c) | Y | C | 6 | 0.11 | 4 | 0.07 | 8 | 0.14 | 1 | 0.02 | 5 | 0.09 |
| France | Y | C | 75 | 0.11 | 53 | 0.08 | 45 | 0.07 | 62 | 0.09 | 53 | 0.08 |
| Germany | Y | C | 152 | 0.18 | 170 | 0.20 | 150 | 0.18 | 176 | 0.21 | 141 | 0.17 |
| Greece | Y | C | 4 | 0.04 | 7 | 0.07 | 7 | 0.07 | 11 | 0.10 | 15 | 0.14 |
| Hungary | Y | C | 7 | 0.07 | 4 | 0.04 | 10 | 0.10 | 9 | 0.09 | 14 | 0.14 |
| Ireland (c) | Y | C | 1 | 0.02 | 0 | 0 | 0 | 0 | 2 | 0.04 | 0 | 0 |
| Italy (b) | – | – | – | – | – | – | – | – | – | – | – | – |
| Latvia | Y | C | 6 | 0.32 | 5 | 0.26 | 6 | 0.31 | 10 | 0.52 | 6 | 0.31 |
| Lithuania | Y | C | 20 | 0.72 | 37 | 1.32 | 81 | 2.90 | 50 | 1.78 | 53 | 1.86 |
| Luxembourg | Y | C | 1 | 0.16 | 3 | 0.48 | 1 | 0.16 | 0 | 0 | 2 | 0.34 |
| Malta (c) | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Netherlands | Y | A | 0 | 0 | 48 | 0.28 | 48 | 0.28 | 42 | 0.24 | 38 | 0.22 |
| Poland | Y | C | 26 | 0.07 | 18 | 0.05 | 70 | 0.18 | 51 | 0.13 | 75 | 0.20 |
| Portugal | Y | C | 2 | 0.02 | 1 | 0.01 | 5 | 0.05 | 9 | 0.09 | 2 | 0.02 |
| Romania | Y | C | 1 | 0.01 | 0 | 0 | 1 | 0.01 | 4 | 0.02 | 14 | 0.07 |
| Slovakia | Y | C | 2 | 0.04 | 3 | 0.05 | 11 | 0.20 | 10 | 0.18 | 7 | 0.13 |
| Slovenia | Y | C | 11 | 0.52 | 3 | 0.14 | 6 | 0.29 | 6 | 0.29 | 7 | 0.34 |
| Spain (d) | Y | C | 33 | – | 8 | – | 34 | 0.07 | 68 | 0.15 | 83 | 0.18 |
| Sweden | Y | C | 25 | 0.24 | 23 | 0.22 | 26 | 0.25 | 29 | 0.29 | 34 | 0.34 |
| EU Total 27 | 529 | 0.15 | 544 | 0.16 | 766 | 0.20 | 815 | 0.21 | 847 | 0.22 | ||
| United Kingdom (c) | Y | C | – | – | – | – | 3 | 0 | – | – | 4 | 0.01 |
| EU Total (e) | 529 | 0.15 | 544 | 0.16 | 769 | 0.17 | 815 | 0.21 | 851 | 0.19 | ||
| Iceland | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Norway (c) | Y | C | 11 | 0.20 | 6 | 0.11 | 7 | 0.13 | 7 | 0.13 | 6 | 0.11 |
| Liechtenstein (b) | – | – | – | – | – | – | – | – | – | – | – | – |
| Switzerland (b) | – | – | – | – | ||||||||
–: Data not reported.
: Y: yes; N: no; A: aggregated data; C: case‐based data.
: No surveillance system.
: Finland, Ireland, Malta, the United Kingdom and mainland Norway have been declared free of Echinococcus multilocularis.
: Data not complete for 2020–2021, rate not estimated.
: Cases reported by the United Kingdom for the period 2017–2019 were also considered for this estimation (EU‐28). When the United Kingdom data were collected for the period 2017–2019, the United Kingdom was an EU MS, but it became a third country on 1 February 2020.
Most echinococcosis cases (60.5%; 320/529) were reported without data on their importation and probable country of infection; 61.2% (128/209) of cases reported with such information were domestic or related to travel within the EU, while 38.8% (81/209) were associated with travel outside the EU. In 2021, 14 MSs out of the 25 reporting MSs notified that all their Echinococcus spp. infections were domestically acquired. Considering data with information on travel‐related status, the highest proportions of cases (N = 107) were reported by Germany (100%; 59 vs. 0 non‐travel‐related cases), Sweden (100%; 18 vs. 0 cases), Belgium (72%; 13 vs. 5 cases) and Austria (48%; 11 vs. 12 cases). At the species level, human E. granulosus s.l. infections were more often reported as travel‐associated than human E. multilocularis infections, accounting for 95.2% (N = 79) and 4.8% (N = 4) of cases reported with this information, respectively. Of the 81 travel‐associated cases of Echinococcus spp. for which the origin of infection is known, most (83.5%) were reported as originating from outside the EU, mainly from Syria (37%), followed by Turkey (11.9%), Iraq (9.9%) and Morocco (7.4%). Within the EU, Bulgaria (45%), Romania (40%), Luxembourg (10%) and Austria (5%) were reported as the probable countries of infection in 20 cases.
In 2021, species information was provided for 415 confirmed echinococcosis cases (78.4%) out of 529 confirmed cases reported by 19 MSs (Table 58). In 2021, human infections caused by E. multilocularis accounted for 137 cases (33.0% of cases with known species information), more than in 2020 and slightly fewer than in 2017–2019. In 2021, Germany and France reported the highest number of human cases caused by E. multilocularis, accounting for 38.7% and 29.2% of all reported E. multilocularis cases, respectively. Human infections caused by E. granulosus s.l. accounted for 67.0% (278 cases) of the cases with species information available. In 2021, Bulgaria and Germany reported the highest number of human cases caused by E. granulosus s.l., accounting in both cases for 32.0% of all reported E. granulosus s.l. cases.
Table 58.
Reported human cases of cystic and alveolar echinococcosis in EU MSs and non‐MS countries, by country, year and Echinococcus species, 2017–2021
| Country | 2021 | 2020 | 2019 | 2018 | 2017 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total Cases | Eg | Em | Total Cases | Eg | Em | Total Cases | Eg | Em | Total Cases | Eg | Em | Total Cases | Eg | Em | |
| Austria | 42 | 24 | 7 | 34 | 18 | 4 | 36 | 16 | 13 | 46 | 29 | 12 | 50 | 37 | 8 |
| Belgium | 18 | 11 | 7 | 19 | 10 | 8 | 22 | 12 | 10 | 15 | 10 | 5 | 13 | 9 | 4 |
| Bulgaria | 89 | 89 | 0 | 95 | 95 | 0 | 193 | 193 | 0 | 206 | 206 | 0 | 218 | 218 | 0 |
| Croatia | 3 | – | – | 3 | – | – | 3 | – | – | 4 | – | – | 15 | – | – |
| Cyprus | 0 | 0 | 0 | 1 | – | – | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Czechia | 1 | 0 | 1 | 4 | 1 | 2 | 1 | – | – | 4 | 1 | 2 | 1 | – | – |
| Denmark (a) | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Estonia | 4 | – | – | 1 | 1 | 0 | 2 | – | – | 0 | 0 | 0 | 1 | 0 | 1 |
| Finland (b) | 6 | 5 | – | 4 | 3 | – | 8 | 8 | 0 | 1 | 1 | 0 | 5 | 5 | 0 |
| France | 75 | 22 | 53 | 53 | 11 | 42 | 45 | 10 | 35 | 62 | 21 | 41 | 53 | 5 | 48 |
| Germany | 152 | 89 | 40 | 170 | 80 | 54 | 150 | 87 | 41 | 176 | 93 | 59 | 141 | 86 | 35 |
| Greece | 4 | – | – | 7 | 7 | 0 | 7 | – | – | 11 | – | – | 15 | – | – |
| Hungary | 7 | – | – | 4 | 1 | – | 10 | – | – | 9 | – | – | 14 | 1 | 1 |
| Ireland (b) | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | – | – | 0 | 0 | 0 |
| Italy (a) | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Latvia | 6 | 6 | 0 | 5 | 5 | 0 | 6 | 4 | – | 10 | 5 | 1 | 6 | 4 | – |
| Lithuania | 20 | 2 | 14 | 37 | – | – | 81 | 30 | 21 | 50 | 11 | 17 | 53 | 19 | 20 |
| Luxembourg | 1 | – | – | 3 | 3 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 2 | 2 | 0 |
| Malta (b) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Netherlands | 0 | 0 | 0 | 48 | – | – | 48 | – | – | 42 | – | – | 38 | – | – |
| Poland | 26 | 12 | 11 | 18 | 8 | 6 | 70 | 21 | 25 | 51 | 17 | 19 | 75 | 27 | 31 |
| Portugal | 2 | 2 | 0 | 1 | 1 | 0 | 5 | 5 | 0 | 9 | 9 | 0 | 2 | – | – |
| Romania | 1 | – | – | 0 | 0 | 0 | 1 | – | – | 4 | – | – | 14 | – | – |
| Slovakia | 2 | 0 | 2 | 3 | 1 | 2 | 11 | 3 | 8 | 10 | 3 | 3 | 7 | 2 | 3 |
| Slovenia | 11 | 2 | 2 | 3 | 1 | – | 6 | 1 | – | 6 | 3 | – | 7 | – | – |
| Spain (c) | 33 | 1 | – | 8 | 1 | – | 34 | 6 | – | 68 | 12 | – | 83 | 4 | – |
| Sweden | 25 | 12 | 0 | 23 | 8 | 3 | 26 | 17 | 2 | 29 | 5 | 2 | 34 | 11 | 4 |
| EU Total 27 | 529 | 278 | 137 | 544 | 255 | 121 | 766 | 414 | 155 | 815 | 426 | 161 | 847 | 430 | 155 |
| United Kingdom (b) | – | – | – | – | – | – | 3 | 3 | 0 | – | – | – | 4 | 4 | 0 |
| EU Total (d) | 529 | 278 | 137 | 544 | 255 | 121 | 769 | 417 | 155 | 815 | 426 | 161 | 851 | 434 | 155 |
| Iceland | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Norway (b) | 11 | 5 | – | 6 | 1 | 1 | 7 | 2 | – | 7 | 5 | – | 6 | 3 | 1 |
| Liechtenstein (a) | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Switzerland (a) | – | – | – | – | |||||||||||
Eg: Echinococcus granulosus sensu lato; Em: Echinococcus multilocularis.
–: Data not reported.
: No surveillance system.
: Finland, Ireland, Malta, the United Kingdom and mainland Norway have been declared free of E. multilocularis.
: Data not complete for 2020–2021.
: Cases reported by the United Kingdom for the period 2017–2019 were also considered for this estimation (EU‐28). When the United Kingdom data were collected for the period 2017–2019, the United Kingdom was an EU MS, but it became a third country on 1 February 2020.
In 2021, 13 MSs provided information on hospitalisations, covering 22.9% (121/529) of all confirmed cases of echinococcosis in the EU. The overall hospitalisation rate was 42.1%. Information on the outcome of the cases was provided by 15 MSs for 51.0% of confirmed cases with no deaths reported. Information on age was reported by 19 MSs with the highest proportion of cases (83.1%) occurring in over 30‐year‐olds.
8.3.3. Echinococcus spp. in animals and food
Monitoring data for Echinococcus multilocularis
Table 59 summarises the most relevant definitive and intermediate host species tested for E. multilocularis. In 2021, results were reported by 18 MSs and two non‐MSs (Norway and Switzerland). Austria, Bulgaria, Croatia, Lithuania and Portugal reported no animal monitoring data for E. multilocularis or E. granulosus s.l.
Table 59.
Echinococcus multilocularis monitoring results in wild and domestic animals in reporting EU MSs and non‐MSs, 2021
| Country | Presence of Em/Eg(a) | N Positive/N tested (% positive) | ||||||
|---|---|---|---|---|---|---|---|---|
| Beavers | Cats | Coypu | Dogs | Foxes | Jackals | Pigs | ||
| Czechia | Em/Eg | – | – | – | – | 645/2,758 (23.4) | – | – |
| Denmark | Em | – | – | – | – | – | – | 0/18,592,853 (0) |
| Estonia | Em/Eg | – | – | – | – | – | – | 0/548,849 (0)(e) |
| Finland | Eg | – | – | – | – | 0/244 (0) | – | – |
| France | Em/Eg | 1/1 (100) | 0/1 (0)(e) | – | 1/52 (1.9)(e) | 32/233 (13.7) | – | – |
| Germany | Em | – | 0/2 (0) | – | 0/322 (0) | 179/607 (29.5) | – | 0/1 (0) |
| Hungary | Em/Eg | – | – | – | – | 0/1 (0) | – | 0/61 (0)(e) |
| Ireland(b) | Eg | – | – | – | – | 0/398 (0) | – | – |
| Italy | Em/Eg | – | – | – | 0/58 (0)(e) | 0/139 (0) | – | 0/5,087,079 (0)(e) |
| Latvia | Em/Eg | – | – | – | – | – | – | 0/473,831 (0)(e) |
| Luxembourg | Em | – | – | – | – | 22/107 (20.6) | – | 0/122,548 (0) |
| Netherlands | Em | – | – | – | – | 3/176 (1.7) | – | – |
| Poland | Em/Eg | – | – | – | 1/90 (1.1)(e) | 120/340 (35.3)(e) | – | 13,086/21,439,046 (0.06)(e) |
| Romania | Em/Eg | – | – | – | – | – | – | 0/53 (0)(e) |
| Slovakia | Em/Eg | – | 0/768 (0)(e) | – | 0/2,420 (0)(e) | 5/96 (5.2)(e) | – | 43/675,234 (0.01)(e) |
| Slovenia | Em/Eg | – | – | – | – | 49/184 (26.6) | – | 0/242,584 (0)(e) |
| Sweden | Em/Eg | – | – | – | – | 16/343 (4.7) | – | 0/2,651,110 (0)(e) |
| United Kingdom (Northern Ireland)(c) | Eg | – | – | – | – | 0/692 (0) | – | – |
| EU Total (27 + XI) | 1/1 (100) | 0/771 (0) | – | 2/2,942 (0.07) | 1071/6,318 (17) | – | 13,129/49,833,249 (0.03) | |
| Norway(d) | Eg | – | – | – | – | 0/511 (0) | – | |
| Switzerland | Em | 0/2 (0) | 1/7 (14.3) | 1/1 (100) | 14/42 (33.3) | 1/2 (50) | 1/1 (1) | 5/5 (100) |
| Total non‐EU countries | 0/2 (0) | 1/7 (14.3) | 1/1 (100) | 14/42 (33.3) | 1/513 (0.2) | 1/1 (1) | 5/5 (100) | |
| Total EU (27+ XI) + non‐EU countries | 1/3 (33.3) | 1/778 (0.13) | 1/1 (100) | 16/2,984 (0.54) | 1,072/6,831 (15.7) | 1/1 (1) | 13,134/49,833,254 (0.03) | |
| Country | Presence of Em/Eg (a) | N Positive/N tested (% positive) | ||||||
|---|---|---|---|---|---|---|---|---|
| Raccoon dogs | Raccoons | Wild boars | Wild cats (Felis silvestris) | Wolves | Voles | Lynx | ||
| Czechia | Em/Eg | – | – | – | – | – | – | – |
| Denmark | Em | – | – | – | – | – | – | – |
| Estonia | Em/Eg | – | – | – | – | – | – | – |
| Finland | Eg | 0/409 (0) | – | – | – | – | 0/618 (0) | – |
| France | Em/Eg | – | – | – | – | – | – | – |
| Germany | Em | – | 0/3 (0) | 4/4 (100) | 0/1 (0) | – | – | |
| Hungary | Em/Eg | – | – | – | – | – | – | – |
| Ireland (b) | Eg | – | – | – | – | – | – | – |
| Italy | Em/Eg | – | – | 0/55,673 (0) | 0/55 (0) (e) | – | – | |
| Latvia | Em/Eg | – | – | – | – | – | – | – |
| Luxembourg | Em | – | – | – | – | – | – | – |
| Netherlands | Em | – | – | – | – | – | – | – |
| Poland | Em/Eg | – | – | – | – | – | – | – |
| Romania | Em/Eg | – | – | – | – | – | – | – |
| Slovakia | Em/Eg | – | – | – | 0/1 (0) | 0/9 (0) (e) | – | – |
| Slovenia | Em/Eg | – | – | – | – | – | – | |
| Sweden | Em/Eg | – | – | 0/16,569 (0) | – | 0/51 (0) (e) | – | – |
| United Kingdom (Northern Ireland) (c) | Eg | – | – | – | – | – | – | – |
| EU Total (27 + XI) | 0/409 (0) | 0/3 (0) | 4/72,246 (0.01) | 0/1 (0) | 0/116 (0) | 0/618 (0) | – | |
| Norway (d) | Eg | – | – | – | – | – | – | – |
| Switzerland | Em | – | – | 1/1 (100) | 0/1 (0) | 3/5 (60) | – | 1/1 (100) |
| Total non‐EU countries | – | – | 1/1 (100) | 0/1 (0) | 3/5 (60) | – | 1/1 (100) | |
| Total EU (27+ XI) + non‐EU countries | 0/409 (0) | 0/3 (0) | 5/72,247 (0.01) | 0/2 (0) | 3/121 (2.5) | 1/1 (100) | ||
–: Data not reported.
Em: Echinococcus multilocularis; Eg: Echinococcus granulosus sensu lato.
: Presence in the country of Echinococcus multilocularis (Em) and/or Echinococcus granulosus sensu lato (Eg).
: Member States listed in the Annex to Commission Implementing Regulation (EU) 2018/878 on the application of preventive health measures for the control of E. multilocularis infection in dogs.
: Data on animals from the United Kingdom (Northern Ireland) are taken into account for 2021. In accordance with the agreement on the withdrawal of the United Kingdom from the EU, and in particular with the/Northern Ireland protocol, the EU requirements on data sampling are also applicable to Northern Ireland.
: Mainland Norway (excluding the Svalbard archipelago where E. multilocularis was documented).
: Positive samples reported from dogs, cats, wolves and pigs without Echinococcus species information were mentioned in the table only for countries with known circulation of both E. multilocularis and E. granulosus sensu lato.
In 2021, 13 MSs and the United Kingdom (Northern Ireland) reported monitoring data from 6,318 foxes examined for E. multilocularis. Also, two non‐MSs (Norway and Switzerland) reported on monitoring data from a total of 513 foxes examined for E. multilocularis. Nine MSs and one non‐MS (Switzerland) reported a total of 15.7% positive samples: Czechia (23.4% positive samples), France (13.7%), Germany (29.5%), Luxembourg (20.6%), the Netherlands (1.7%), Poland (35.3%), Slovakia (5.2%), Sweden (4.7%) and Switzerland (50%). Czechia (N = 645) reported the largest number of infected foxes in Europe, accounting for 60.2% of positive findings.
In addition to its presence in foxes (as definitive hosts), Echinococcus spp. have been reported in one beaver (France), one cat (Switzerland), 16 dogs (France, Poland and Switzerland), one golden jackal (Switzerland), five wild boars (Germany and Switzerland), three wolves (Switzerland) and one lynx (Switzerland). Moreover, 13,086 and 34 positive pigs were reported by two MSs (Poland and Slovakia), co‐endemic for both Echinococcus species and five pigs from one non‐MS, endemic only for E. multilocularis (Switzerland). Poland reported 10 cases as E. multilocularis and 13,076 as Echinococcus unspecified; Slovakia reported 43 cases as Echinococcus unspecified, while Switzerland reported five cases as E. multilocularis. It should also be emphasised that pigs are good hosts for E. granulosus s.l. while E. multilocularis metacestodes in pigs are abortive, and their presence is often used as a sentinel for the circulation of this parasite in animal hosts. For this reason, the presence of both E. multilocularis and E. granulosus s.l. may be overestimated in co‐endemic countries with unknown species identification. In this context, it should also be noted that positive samples from pigs, dogs and wolves without species identification were mentioned in Tables 59 and/or 60 only for countries with known circulation of both E. granulosus s.l. and E. multilocularis. E. multilocularis was also detected in one sample batch of fresh leaf vegetables in France.
Table 60.
Echinococcus granulosus sensu lato monitoring results in wild and domestic animals in reporting EU MSs and non‐MSs, 2021
| Country | Presence of Em/Eg(a) | N Positive/N tested (% positive) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Cantabrian chamois | Cats | Cattle (bovine animals) | Deer | Dogs | Moose | Mouflons | Pigs | ||
| Belgium(b) | Em | – | – | 0/770,235 (0) | – | – | – | – | – |
| Cyprus | Eg | – | – | – | – | – | – | 2/43 (4.7) | – |
| Denmark(b) | Em | – | – | 0/452,600 (0) | – | – | – | – | – |
| Estonia | Em/Eg | – | – | 0/34,011 (0) | – | – | – | – | 0/548,849 (0)(c) |
| Finland | Eg | – | – | 0/257,680 (0) | 0/2,765 (0) | – | 1/212 (0.47) | – | 0/1,942,376 (0) |
| France | Em/Eg | – | 0/1 (0)(c) | 0/1 (0) | – | 0/51 (0)(c) | – | – | |
| Germany | Em | – | – | 0/12 (0) | 0/2 (0) | – | – | – | |
| Greece | Eg | – | – | 930/45,462 (2.0) | – | – | – | – | 29/250,835 (0.01) |
| Hungary | Em/Eg | – | – | 4/8 (50.0) | – | – | – | – | 1/62 (1.6)(c) |
| Italy | Em/Eg | 0/1 (0) | – | 2,515/2,493,943 (0.10) | 0/629 (0) | 0/58 (0)(c) | – | 0/12 (0) | 10/5,087,089 (< 0.001)(c) |
| Latvia | Em/Eg | – | – | 0/73,531 (0) | – | – | – | – | 0/473,831 (0)(c) |
| Luxembourg(b) | Em | – | – | 0/27,326 (0) | – | – | – | – | |
| Malta | Eg | – | – | – | – | – | – | – | |
| Poland | Em/Eg | – | – | – | – | 0/89 (0)(c) | – | – | 13,076/21,439,036 (0.06)(c) |
| Romania | Em/Eg | – | – | 18/21 (85.7) | – | – | – | – | 0/53 (0)(c) |
| Slovakia | Em/Eg | – | 0/768 (0)(c) | 0/34,771 (0) | – | 0/2,420 (0)(c) | – | – | 43/675,234 (0.01)(c) |
| Slovenia | Em/Eg | – | – | 0/123,961 (0) | – | – | – | – | 0/242,584 (0)(c) |
| Spain | Eg | – | – | 11,302/2,340,722 (0.48) | 2/185,894 (0) | – | – | 0/7,412 (0) | 2,714/41,148,859 (0.01) |
| Sweden | Em/Eg | – | – | 0/411,650 (0) | 0/6,631 (0) | – | – | – | 0/2,651,110 (0)(c) |
| EU Total (27 + XI) | 0/1 (0) | 0/769 (0) | 14,769/7,065,934 (0.21) | 2/195,921 (0) | 0/2,618 (0) | 1/212 (0.47) | 2/7,467 (0.03) | 15,873/74,459,918 (0.02) | |
| Norway | Eg | – | – | 0/301,000 (0) | – | – | – | – | 0/1,568,000 (0) |
| Switzerland(b) | Em | – | – | 0/2 (0) | 0/1 (0) | – | – | – | |
| Total non‐EU countries | – | – | 0/301,002 (0) | 0/1 (0) | – | – | – | 0/1,568,000 (0) | |
| Total EU (27+ XI) + non‐EU countries | 0/1 (0) | 0/769 (0) | 14,769/7,366,936 (0.20) | 2/195,922 (0) | 0/2,618 (0) | 1/212 (0.47) | 2/7,467 (0.03) | 15,873/76,027,918 (0.02) | |
| Country | Presence of Em/Eg (a) | N Positive/N tested (% positive) | ||||||
|---|---|---|---|---|---|---|---|---|
| Reindeers | Goats | Sheep | Solipeds, domestic | Water buffalos | Wild boars | Wolves | ||
| Belgium (b) | Em | – | – | – | – | – | – | – |
| Cyprus | Eg | – | – | – | – | – | – | – |
| Denmark (b) | Em | – | – | – | – | – | – | – |
| Estonia | Em/Eg | – | 0/171 (0) | 0/8,378 (0) | 0/8 (0) | – | ||
| Finland* | Eg | 7/56,079 (0.01) | 0/748 (0) | 0/62,724* (0) | 0/757 (0) | – | 0/183 (0) | 7/41 (17.1) |
| France | Em/Eg | – | – | 0/2 (0) | – | – | – | – |
| Germany | Em | – | – | 0/2 (0) | – | – | – | – |
| Greece | Eg | – | 1,477/259,488 (0.57) | 4,490/621,068 (0.72) | – | – | – | – |
| Hungary | Em/Eg | – | – | 3/3 (100) | – | – | – | – |
| Italy | Em/Eg | – | 272/48,039 (0.57) | 1,292/107,2250 (0.12) | 0/537 (0) | 114/33,912 (0.34) | 7/55,680 (0.01) | 0/55 (0) (c) |
| Latvia | Em/Eg | – | 0/510 (0) | 0/26,943 (0) | 0/34 (0) | – | – | – |
| Luxembourg (b) | Em | – | – | – | – | – | – | – |
| Malta | Eg | – | – | 0/7,373 (0) | – | – | – | – |
| Poland | Em/Eg | – | – | – | – | – | – | |
| Romania | Em/Eg | – | 0/3 (0) | 0/30 (0) | – | – | – | – |
| Slovakia | Em/Eg | – | 0/91 (0) | 775/6,448 (12.0) | – | – | – | 0/9 (0) (c) |
| Slovenia | Em/Eg | – | 0/1,172 (0) | 0/10860 (0) | 0/1,057 (0) | – | – | – |
| Spain | Eg | – | 3,803/783,974 (0.49) | 28,969/7,679,758 (0.38) | 0/6,637 (0) | – | 5/138,599 (< 0.001) | – |
| Sweden | Em/Eg | 0/40,528 (0) | 0/817 (0) | 0/227,070 (0) | 1/1,200 (0.08) | – | 0/16,569 (0) | 0/51 (0) (c) |
| EU Total (27 + XI) | 7/96,607 (0.01) | 5,552/1,095,013 (0.51) | 35,529/9,722,909 (0.37) | 1/10,230 (0.01) | 114/33,912 (0.34) | 12/211,031 (0.01) | 7/156 (4.5) | |
| Norway | Eg | – | 0/28,600 (0) | 0/1,204,600 (0) | – | – | – | |
| Switzerland (b) | Em | – | – | – | – | – | – | |
| Total non‐EU countries | – | 0/28,600 (0) | 0/1,204,600 (0) | – | – | – | – | |
| Total EU (27+ XI) + non‐EU countries | 7/96,607 (0.01) | 5,552/1,123,613 (0.49) | 35,529/10,927,509 (0.33) | 1/10,230 (0.01) | 114/33,912 (0.34) | 12/211,031 (0.01) | 7/156 (4.5) | |
–: Data not reported
Em: Echinococcus multilocularis; Eg: Echinococcus granulosus sensu lato.
: During the finalisation of this report, Finland indicated a reporting error: only 51,221 sheep were tested.
: Presence in the country of E. multilocularis (Em) and/or E. granulosus sensu lato (Eg).
: Reporting countries with known circulation of E. multilocularis only, and which tested suitable hosts for E. granulosus sensu lato.
: Positive samples from dogs, cats, wolves and pigs without Echinococcus species information reported, were mentioned in the table only for MSs with known circulation of both E. multilocularis and E. granulosus sensu lato.
Monitoring data for Echinococcus granulosus sensu lato
Table 60 summarises the most relevant definitive and intermediate host species tested for E. granulosus s.l. In 2021, 19 MSs and two non‐MSs (Norway and Switzerland) reported monitoring data for E. granulosus s.l. The data reported were from around 93 million domestic and wild animals tested for E. granulosus s.l., of which more than 99% were domestic animals (sheep, cattle, goats, pigs, horses, water buffalos, dogs and cats). A large proportion of these data was obtained from domestic livestock during meat inspections at the slaughterhouse. Wild animals tested included Cantabrian chamois, deer, moose, mouflons, reindeer, wild boars and wolves. A total of 71,869 (0.07%) positive samples were reported by 11 MSs, mainly from domestic animals. These positive samples came mainly from small ruminants (sheep and goats; N = 41,081), accounting for 57.2% of positive results. Positive tests in small ruminants were mainly reported by Spain (79.8%), followed by Greece, Italy and Slovakia. Results showed 14,769 positive cattle (20.5% of animals positive for E. granulosus s.l.), mainly reported by Spain (76.52%), followed by Italy and Greece, and 15,874 positive pigs (22.1% of animals positive for E. granulosus s.l.), of which 82.4% were reported by Poland, followed by Spain.
Belgium, Denmark, Estonia, Ireland, Latvia, Malta and Slovenia among MSs and Norway among non‐MSs, reported no positive findings for E. multilocularis or E. granulosus s.l. Austria, Bulgaria, Croatia, Lithuania and Portugal reported no animal monitoring data for E. multilocularis or E. granulosus s.l.
Figures 28 and 29 show the cumulative proportion of positive samples from different intermediate hosts of E. granulosus s.l. and their geographical distribution in EU MSs and other European countries for the period between 2017 and 2021. Small ruminants (sheep and goats) accounted for 75.5% (2017–2021) of all positive samples and were reported by a few countries with large animal populations (Greece, Italy and Spain and the United Kingdom). Positive results in cattle (11.8%; 2017–2021) were also mainly reported by Greece, Italy and Spain. Positive results in pigs (12.6%; 2017–2021) were mainly reported by Poland, followed by Spain.
Figure 28.
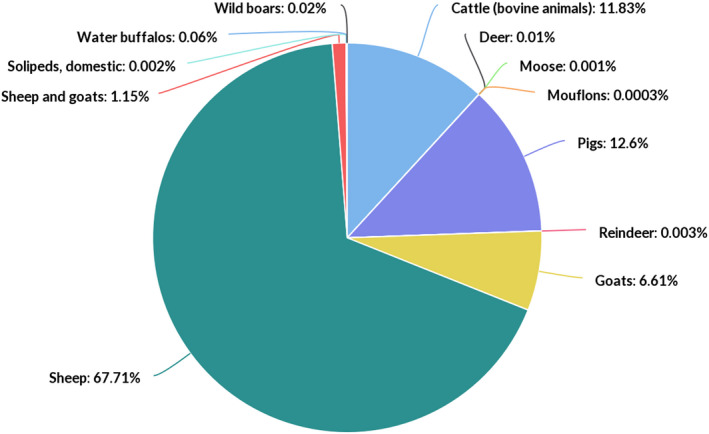
Cumulative proportion (%) of test‐positive animals for Echinococcus granulosus sensu lato, by intermediate host species, in EU MSs and non‐MSs, 2017–2021
Figure 29.
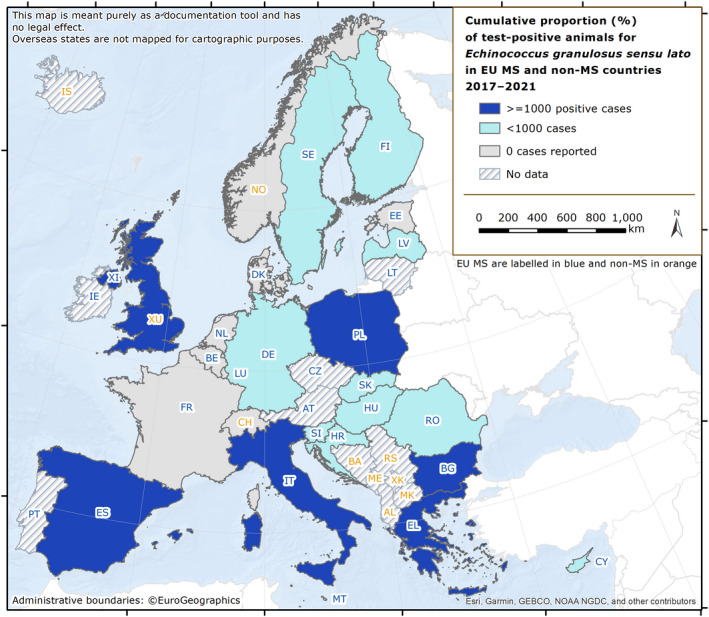
- Intermediate hosts included on the map are cattle, deer, goats, horses, moose, mouflons, pigs, reindeer, sheep, water buffalos and wild boars. It should be stressed that pigs are intermediate hosts for both E. multilocularis and E. granulosus s.l. For this reason, data from pigs were excluded from co‐endemic countries – Poland, Romania, Slovakia and Slovenia – where Echinococcus species information was not reported.Spain (N = 302,433), Italy, (N = 248,530), United Kingdom 33 (N = 71,861), Greece (N = 53,140), Bulgaria (N = 12,214), Poland (N = 2,630) and Slovakia (N = 995) were the countries with the highest endemicity for E. granulosus s.l. in the EU in 2017–2021.
8.4. Discussion
The EU notification rate of confirmed human echinococcosis cases decreased slightly from 2017 to 2019. In 2020 and 2021, the EU notification rates for infections caused by Echinococcus species fell sharply compared with the previous 3 years, probably due to the COVID‐19 pandemic and partly due to the withdrawal of the United Kingdom from the EU. In 2021, although the COVID‐19 pandemic was still ongoing, a slight decrease was observed in the number of confirmed echinococcosis cases with respect to 2020.
In a few countries, the increase in case numbers over the last few years may be explained by increased human surveillance activities and improved notification systems for these diseases. Increased awareness of the diseases among clinicians, as well as immigration from endemic countries, may also have influenced the number of diagnosed cases in some countries (Richter et al., 2019). It should be emphasised that the true prevalence of these diseases is extremely difficult to estimate due to the long incubation period, high proportion of asymptomatic or paucisymptomatic carriers who never seek medical attention, non‐specific symptoms and under‐reporting/misdiagnosed cases. The above‐mentioned factors contribute to the neglected status of these diseases (Casulli, 2020). For these reasons, the patchy data reported by MSs on the number of people with echinococcosis, currently represent the ‘tip of the iceberg’ for infections, with asymptomatic carriers and misdiagnosed cases of CE/AE making up the invisible portion. It has been estimated that the official figures from hospital records should be far higher, with true values 10 and 700 times greater for Bulgaria and Romania, respectively (Tamarozzi et al., 2018). The prospective, observational, multicentre and online ‘European Register of Cystic Echinococcosis’ (ERCE) (Rossi et al., 2020) is currently seeking to establish a voluntary system to collect harmonised clinical data in the EU. The European (Alveolar) Echinococcosis Registry (Kern et al., 2003) organised a similar initiative in the past.
In 2021, 22 MSs reported monitoring data on E. granulosus s.l. and/or E. multilocularis in animals. The highest numbers of animals infected with E. granulosus s.l. were reported in Spain, Greece, Italy and Poland, and were mainly observed in sheep intermediate hosts, and secondarily in cattle and pigs. Most of the animals (mainly red foxes) infected with E. multilocularis were reported by Czechia, followed by France, Germany, Poland and Slovenia. The surveillance of E. multilocularis in foxes is important to assess the prevalence of AE in Europe, given that its geographical distribution seems to have widened over the last decades. It is difficult to establish whether the increased geographical distribution of E. multilocularis is due to a growing fox population in Europe (Oksanen et al., 2016) and the expansion of their habitat to urban areas (Deplazes et al., 2004), or whether it reflects greater efforts in surveillance, as there is a general lack of baseline data and standardised detection methods. One growing source of concern is the detection in recent years of E. multilocularis in golden jackals, which are increasing their geographical range from southern–eastern to northern–western Europe, and therefore contributing to the dispersion of this parasite over long distances (Dušek et al., 2020; Balog et al., 2021). Moreover, in animals, notification is necessary to obtain reliable data, and information on parasite speciation is essential for risk management efforts, since E. granulosus s.l. and E. multilocularis have different epidemiology and pose different health risks for humans (Possenti et al., 2016; Conraths et al., 2017; Casulli, 2020). For E. granulosus s.l., a notification requirement would ensure that comparable data between MSs are obtained from meat inspections of food‐producing animals. For E. multilocularis, while the need for general notification by all MSs may raise questions, it is nevertheless required in countries that are free from this parasite, in accordance with Regulation (EU) No 2018/772.
In general terms, it should be emphasised that animal findings from most endemic countries fluctuated from year to year over the period 2017–2021, although positive findings in animals and humans were reported in most years. The fluctuations over the last 5 years probably reflect the investigational efforts performed during a particular year, rather than a change in the true prevalence of the disease. For instance, Italy reported 1,292 (0.12%) test‐positive sheep in 2021, but documented 60,608 (6.5%) in 2020. Moreover, these fluctuations in animals can be partially explained by the United Kingdom leaving the EU: in 2019 the United Kingdom accounted for 68.8% and 37.2% of all EU‐tested sheep and goats, and cattle, respectively.
Foodborne outbreaks (in accordance with Directive 2003/99/EC)
Summary data and figures substantiating this chapter, as well as additional information on related projects and internet sources are published for this report on the EFSA Knowledge Junction on Zenodo here. Summary statistics on foodborne outbreaks reported in the framework of Directive 2003/99/EC are retrievable using the EFSA dashboard on foodborne outbreaks available here. Summary statistics on human surveillance data with downloadable files are retrievable using the ECDC Surveillance Atlas of Infectious Diseases here.
1. Key facts
In 2021, 27 EU MSs and the United Kingdom (Northern Ireland) reported 4,005 foodborne outbreaks, 32,543 cases of illness, 2,495 hospitalisations and 31 deaths. At the same time, 83 outbreaks, 1,270 cases of illness, 65 hospitalisations and 2 deaths were reported by seven non‐MSs.
In 2021, foodborne outbreaks in the EU increased by 29.8% compared with the previous year (3,086 in 2020). Human cases and hospitalisations also increased, by 62.6% (20,017 cases in 2020) and 49.0% (1,675 hospitalisations in 2020), respectively. However, the total number of outbreaks, cases and hospitalisations reported in 2021 was lower on average than in the most recent pre‐pandemic years (2017–2019), resulting in a relative decrease of 28.5% for outbreaks (5,601 outbreaks in 2017–2019, on average), 34.2% for cases (49,444 cases reported in 2017–2019, on average) and 44.3% for hospitalisations (4,482 hospitalisations in 2017–2019, on average). The number of reported deaths decreased compared with both 2020 and the period 2017–2019, by 8.8% (34 deaths in 2020) and 30.1% (44 deaths/year in 2017–2019, on average), respectively.
In 2021, the foodborne outbreak reporting rate in the EU was 0.89% per 100,000 population. This represents an increase of 29.0% compared with 2020 (0.69 per 100.000 population) and a decrease of 18.3% compared with the pre‐pandemic years (1.09 per 100.000 population in 2017–2019).
These findings suggest that the impact of the COVID‐19 pandemic was still considerable in 2021 in the EU, in relation to both the occurrence of foodborne outbreaks and their detection, investigation and reporting. However, the rise in the number of foodborne outbreaks, compared with 2020, might indicate a progressive return of foodborne outbreak surveillance to pre‐pandemic stability for most MSs, although higher consumer exposure to contaminated foods may have contributed to this rise in 2021.
The fall in foodborne outbreaks in the second year of the COVID‐19 pandemic compared with pre‐pandemic levels, did not affect all causative agents equally. For example, the number of outbreaks caused by Listeria monocytogenes was the highest since EFSA started collecting data.
Salmonella was identified as causative agent in most foodborne outbreaks in the EU (N = 773), making up 19.3% of total outbreaks. This pathogen was also associated with the highest number of cases (20.8% of outbreak‐associated cases) and hospitalisations (45.0% of outbreak‐associated hospitalisations). S. Enteritidis was the predominant serovar (N = 350; 79.7% of all Salmonella outbreaks).
A total of 31 deaths among outbreak cases were notified to EFSA by six MSs and the United Kingdom (Northern Ireland). L. monocytogenes was associated with 12 of these deaths (36.4% of all deaths), while Campylobacter was associated with six (18.2% of all deaths).
A total of 355 strong‐evidence outbreaks were reported in 2021 (8.9% of all outbreaks). Among these, food vehicles of animal origin (i.e. ‘meat and meat products’, ‘fish and fishery products’, ‘eggs and egg products’ and ‘milk and milk products’) were implicated in most FBOs (56.9%). Salmonella was the most frequent agent paired with various food items (e.g. eggs and egg products, mixed foods, vegetables and juices), in terms of the number of outbreaks, human cases and hospitalisations.
‘Composite foods or multi‐ingredient foods’ caused the highest number of cases in strong‐evidence outbreaks (39.3% of all cases), with ‘mixed foods’ being the most frequently reported cause. Outbreaks implicating these foods were associated with a wide range of causative agents.
Outbreaks associated with the consumption of ‘vegetables and juices and other products thereof’ rose considerably compared with both 2020 and the pre‐pandemic years. This foodstuff was second only to ‘mixed food’ in the overall number of cases reported in FBOs in 2021.
As in recent years, most outbreaks identified in 2021 took place on domestic premises (121 outbreaks; 34.1%. of all strong‐evidence outbreaks). ‘Restaurant or cafe or pub or bar or hotel or catering service' were the most frequently reported places of exposure for outbreaks occurring in other types of setting (77 outbreaks; 21.7%). ‘School or kindergarten’ was the place of exposure associated with the highest number of FBO cases (2,104 cases; 30.0% of all cases in strong‐evidence outbreaks). These findings highlight the importance of proper implementation of HACCP in public catering as well as the need to improve the awareness of both consumers and food business operators on correct procedures for handling and consuming food.
Alongside the present report, data on foodborne outbreaks may be explored through the EFSA interactive communication tools on foodborne outbreaks: the story map (available here), addressed to the general public, and the dashboard (available here) for an interactive data visualisation.
2. Surveillance and monitoring of foodborne outbreaks in the EU
Every year, EU Member States (MSs) and non‐MSs report to EFSA data concerning the foodborne outbreaks (FBOs) occurring in their country, in compliance with Directive 2003/99/EC. The reporting of data is based on the standard set out in the guidance documents, published annually by EFSA (EFSA, 2021a,b).
EFSA is assigned the task of describing the causative agents and foodstuffs implicated in FBOs, along with their time trends. The aim is to assess the health impact of FBOs in Europe and to characterise the food production and distribution chains most frequently implicated. Outbreaks are categorised as having ‘strong evidence' or ‘weak evidence' based on the strength of evidence implicating a suspected food vehicle as the cause of the outbreak (EFSA, 2014). Data on the circumstances and risk factors contributing to consumer exposure to contaminated food are also analysed. The analysis takes account of any uncertainty around the evidence implicating a given food as the vehicle of the outbreak, by looking only at strong‐evidence outbreaks for some analyses.
The current data reporting system is known as the European Union Foodborne Reporting System (EU‐FORS). It applies to outbreaks caused by bacteria, viruses, parasites, algae, fungi and their products, such as toxins and biological amines (e.g. histamine), either typical foodborne agents or agents for which foodborne transmission is usually accidental. Outbreaks caused by the ingestion of drinking water are also considered in FBO reporting, since drinking water is defined as a food in Regulation (EC) No. 178/2002.
FBO data reporting is mandatory for EU MSs and the key findings are summarised in this report.
A description of the national systems in place for foodborne outbreak surveillance and reporting can be found in the national zoonoses reports submitted in accordance with Directive 2003/99/EC, which are published on the EFSA website and available here.
More details on the surveillance and monitoring of foodborne outbreaks in the EU are available on the EFSA story map (available here; see sections on ‘What foodborne outbreaks are and how they are classified’, ‘Who investigates foodborne outbreaks’ and ‘EU regulatory framework and the role of EFSA’).
3. Data analyses
Basic indicators used to describe the impact of FBOs on human health include the total number of outbreaks and cases, the number and proportion of cases (%) leading to hospitalisations or deaths, the mean outbreak size (cases per outbreak) and the range of cases per outbreak (minimum and maximum). Outbreak and case reporting rates (per 100,000 population) are used as relative measures of occurrence in the population, allowing a direct comparison among MSs, independently of the size of the population and any variations over time. However, due to the lack of full harmonisation of FBO surveillance among MSs, any direct comparison of findings between countries should be interpreted with caution. The case reporting rate is a new measure of the impact of FBOs, introduced in this report for the first time. Counts of hospitalisations and deaths and the proportion (%) of hospitalisations and deaths among outbreak cases are used as indicators for outbreak severity, However, since the total number of hospitalisations and deaths can be reported as ‘unknown’ by MSs, these values might be underestimated and should be analysed with caution.
The causative agents in FBOs in 2021 have been grouped in accordance with the following criteria:
-
–
‘E. coli other than STEC’ includes any pathogenic E. coli other than ‘Shiga Toxin‐producing E. coli (STEC)’. In 2021, this group included ‘Enteroinvasive E. coli’ (EIEC), ‘Enterotoxigenic E. coli’ (ETEC) and ‘E. coli, unspecified’.
-
–
‘Bacillus cereus toxins’ includes ‘B. cereus’ and ‘B. cereus enterotoxins’.
-
–
‘Staphylococcus aureus toxins'’ includes ‘S. aureus', ‘Staphylococcus unspecified’ and ‘Staphylococcal enterotoxins'.
-
–
‘Clostridium perfringens toxins’ includes ‘Clostridium, unspecified’ and ‘C. perfringens’.
-
–
‘Norovirus (and other caliciviruses)’ includes ‘calicivirus, unspecified’, ‘norovirus’ and ‘sapovirus’.
To optimise the description of the findings and avoid data sparsity, information on ‘food vehicle' and ‘place of exposure' have been grouped, where necessary. Details concerning single entities in the group are described in the footnotes to the graphs or tables. Food vehicles have been grouped according to the general criteria set out in the EFSA data catalogues (EFSA, 2022). Places of exposure have been grouped according to the general characteristics and level of risk associated with the setting, as well as the process behind food preparation. For more information concerning the grouping criteria for causative agents, food vehicles and places of exposure, see ‘Addendum foodborne outbreaks, 2021′ from Section 1 to Section 3, published on the EFSA Knowledge Junction on Zenodo here.
Key statistics at EU level included the United Kingdom for 2019 and previous years since it was a MS. For 2021, according to the Protocol on Ireland/Northern Ireland, FBOs reported by the United Kingdom (Northern Ireland) were included in EU statistics.
Eurostat data on the resident population (updated on 1 January 2022) were used to calculate reporting rates at national and EU level, while data from the NISRA 34 Census 2021 were used for the United Kingdom (Northern Ireland).
Short‐term variations over time were described using three main time frames to emphasise the impact of the COVID‐19 pandemic on data reporting. In the main tables displaying key statistics at EU level (Tables 61, 62, 63 and 68), FBO and case reporting rates were described separately for 2021, 2020 and 2017–2019 (annual mean). The purpose was to enable an easier comparison between the trends of 2021, the first year of the pandemic and the pre‐pandemic period.
Table 61.
Number of foodborne outbreaks, human cases, hospitalisations and deaths, in reporting EU MSs and non‐MS countries, 2021
| Country | Outbreaks | Cases | Hospitalised | Deaths | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total (Strong‐evidence) | % of total | Outbreaks per 100,000 | N | % of total | Mean outbreak size (N) and range (min–max) | Cases per 100,000 | N | % of total | N | % of total | |||||
| N | 2021 | 2020 | 2017–2019 (a) (mean) | 2021 (a) | 2020 | 2017–2019 (b) (mean) | |||||||||
| Austria | 20 (4) | 0.50 | 0.22 | 0.24 | 0.64 | 92 | 0.30 | 4.6 (2–31) | 1.0 | 0.75 | 4.7 | 27 | 1.1 | 2 | 6.5 |
| Belgium | 547 (3) | 13.7 | 4.7 | 2.9 | 3.7 | 2,070 | 6.4 | 3.8 (2–60) | 17.9 | 10.9 | 17.8 | 78 | 3.1 | 0 | – |
| Bulgaria | 0 (−) | – | 0 | 0.09 | 0.26 | 0 | – | – | 0 | 2.1 | 3.0 | 0 | – | 0 | – |
| Croatia | 9 (1) | 0.20 | 0.22 | 0.32 | 1.0 | 102 | 0.30 | 11.3 (2–71) | 2.5 | 2.3 | 11.4 | 4 | 0.20 | 0 | – |
| Cyprus | 0 (−) | – | 0 | 0.11 | 0.15 | 0 | – | – | 0 | 1.4 | 2.3 | 0 | – | 0 | – |
| Czechia | 42 (7) | 1.0 | 0.39 | 0.20 | 0.25 | 1,976 | 6.1 | 47.0 (6–230) | 18.5 | 7.9 | 8.4 | 260 | 10.4 | 0 | – |
| Denmark | 63 (12) | 1.6 | 1.1 | 0.60 | 1.0 | 1,257 | 3.9 | 20.0 (2–85) | 21.5 | 25.1 | 27.2 | 155 | 6.2 | 0 | – |
| Estonia | 8 (0) | 0.20 | 0.60 | 1.1 | 0.93 | 20 | 0.10 | 2.5 (2–4) | 1.5 | 2.2 | 10.0 | 8 | 0.30 | 0 | – |
| Finland | 48 (20) | 1.2 | 0.87 | 0.65 | 1.0 | 1,385 | 4.3 | 28.9 (2–728) | 25.0 | 10.8 | 20.9 | 28 | 1.1 | 0 | – |
| France | 1,286 (93) | 32.1 | 1.9 | 1.5 | 2.4 | 10,836 | 33.3 | 8.4 (2–329) | 16.0 | 10.1 | 22.0 | 561 | 22.5 | 17 | 54.8 |
| Germany (b) | 168 (21) | 4.2 | 0.20 | 0.23 | 0.48 | 1,179 | 3.6 | 7.1 (2–98) | 1.4 | 1.4 | 2.7 | 196 | 7.9 | 2 | 6.5 |
| Greece | 6 (1) | 0.10 | 0.06 | 0.04 | 0.06 | 55 | 0.20 | 9.2 (2–30) | 0.52 | 1.7 | 3.1 | 5 | 0.20 | 0 | – |
| Hungary | 19 (6) | 0.50 | 0.20 | 0.11 | 0.40 | 564 | 1.7 | 29.7 (2–104) | 5.8 | 2.8 | 17.0 | 43 | 1.7 | 0 | – |
| Ireland | 6 (0) | 0.10 | 0.12 | 0.46 | 0.47 | 73 | 0.20 | 12.2 (2–35) | 1.5 | 0.97 | 2.4 | 1 | 0.04 | 0 | – |
| Italy | 94 (21) | 2.3 | 0.16 | 0.12 | 0.20 | 1,142 | 3.5 | 12.1 (2–150) | 1.9 | 0.92 | 1.8 | 115 | 4.6 | 0 | – |
| Latvia | 12 (0) | 0.30 | 0.63 | 0.89 | 1.5 | 454 | 1.4 | 37.8 (10–102) | 24.0 | 6.0 | 19.8 | 24 | 1.0 | 0 | – |
| Lithuania | 7 (5) | 0.20 | 0.25 | 0.18 | 1.7 | 71 | 0.20 | 10.1 (2–43) | 2.5 | 1.1 | 9.4 | 39 | 1.6 | 0 | – |
| Luxembourg | 1 (1) | 0.02 | 0.16 | 0.16 | 0.11 | 3 | 0.01 | 3.0 (−) | 0.47 | 0.32 | 0.40 | 0 | – | 0 | – |
| Malta | 26 (3) | 0.60 | 5.0 | 4.9 | 9.0 | 77 | 0.20 | 3.0 (2–19) | 14.9 | 33.8 | 50.0 | 8 | 0.30 | 0 | – |
| Netherlands | 838 (7) | 20.9 | 4.8 | 3.2 | 4.2 | 3,517 | 10.8 | 4.2 (2–402) | 20.1 | 11.0 | 17.1 | 21 | 0.80 | 4 | 12.9 |
| Poland | 299 (39) | 7.5 | 0.79 | 0.40 | 1.3 | 3,513 | 10.8 | 11.7 (2–152) | 9.3 | 3.2 | 14.9 | 509 | 20.4 | 0 | – |
| Portugal | 17 (4) | 0.40 | 0.17 | 0.04 | 0.14 | 495 | 1.5 | 29.1 (2–68) | 4.8 | 0.55 | 4.9 | 80 | 3.2 | 0 | – |
| Romania | 2 (2) | 0.05 | 0.01 | 0.02 | 0.08 | 48 | 0.10 | 24 (4–44) | 0.25 | 0.21 | 2.4 | 6 | 0.20 | 0 | – |
| Slovakia | 214 (17) | 5.3 | 3.9 | 5.9 | 11.7 | 844 | 2.6 | 3.9 (2–41) | 15.5 | 13.0 | 43.3 | 172 | 6.9 | 0 | – |
| Slovenia | 1 (0) | 0.02 | 0.05 | 0 | 0.05 | 3 | 0.01 | 3.0 (−) | 0.14 | 0 | 2.2 | 0 | – | 0 | – |
| Spain | 221 (64) | 5.5 | 0.47 | 0.34 | 1.1 | 2,052 | 6.3 | 9.3 (2–114) | 4.3 | 2.8 | 11.7 | 129 | 5.2 | 2 | 6.5 |
| Sweden | 49 (24) | 1.2 | 0.47 | 0.48 | 1.6 | 698 | 2.1 | 14.2 (2–90) | 6.7 | 9.0 | 25.0 | 12 | 0.50 | 1 | 3.2 |
| United Kingdom (Northern Ireland) (c) | 2 (0) | 0.05 | 0.11 | – | – | 17 | 0.10 | 8.5 (7–10) | 0.89 | – | – | 14 | 0.60 | 3 | 9.7 |
| EU Total (27 + XI) (b) | 4,005 (355) | 100 | 0.89 | 0.69 | 1.1 | 32,543 | 100 | 8.1 (2–728) | 7.3 | 4.5 | 9.7 | 2,495 | 100 | 31 | 100 |
| Bosnia and Herzegovina | 2 (0) | – | 0.06 | 0.03 | 0.10 | 66 | – | 33.0 (10–56) | 2.0 | 1.9 | 1.4 | 1 | – | 0 | – |
| Iceland | 6 (0) | – | 1.6 | 0.27 | 1.6 | 47 | – | 7.8 (3–13) | 12.7 | 12.4 | 50.5 | 4 | – | 0 | – |
| Montenegro | 6 (1) | – | 0.97 | 0.32 | 1.1 | 43 | – | 7.2 (2–20) | 6.9 | 2.6 | 21.8 | 1 | – | 0 | – |
| Norway | 25 (9) | – | 0.46 | 0.43 | 0.79 | 327 | – | 13.1 (3–30) | 6.1 | 9.3 | 26.6 | 4 | – | 0 | – |
| Republic of North Macedonia | 3 (2) | – | 0.15 | 0.05 | 0.40 | 195 | – | 65.0 (12–93) | 9.4 | 0.48 | 24.0 | 12 | – | 0 | – |
| Serbia | 4 (2) | – | 0.06 | 0.13 | 0.74 | 47 | – | 11.8 (5–24) | 0.68 | 0.77 | 7.9 | 3 | – | 0 | – |
| Switzerland (b) | 37 (12) | – | 0.43 | 0.15 | 0.21 | 545 | – | 15.1 (2–126) | 6.3 | 1.9 | 3.4 | 40 | – | 2 | – |
: Data on FBOs from the United Kingdom are taken into account for 2017–2019, because the United Kingdom was an EU MS, but it became a third country on 1 February 2020.
: For one outbreak, information on cases was not available.
: Data on FBOs from the United Kingdom (Northern Ireland) are taken into account for 2021. In accordance with the agreement on the withdrawal of the United Kingdom from the EU, and in particular with the Protocol on Ireland/Northern Ireland, the EU requirements on data sampling are also applicable to Northern Ireland.
Table 62.
Number of foodborne outbreaks, human cases, hospitalisations and deaths, by causative agents, in reporting EU MSs, 2021
| Type of agent | Outbreaks | Cases of illness | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total (Strong‐evidence) | % of total (a) | Reporting rate per 100,000 | Human cases | Mean outbreak size (cases) and range (min–max) | Hospitalisations | Deaths | |||||||
| N | 2021 (b) | 2020 | 2017–2019 (c) (Mean) | N | % of total (a) | N | % of cases (d) | N | % of cases (d) | ||||
| Bacteria | Aeromonas | 1 (1) | 0.02 | < 0.01 | 0 | < 0.01 | 19 | 0.10 | 19.0 (−) | 0 | 0 | 0 | 0 |
| Brucella | 1 (0) | 0.02 | < 0.01 | < 0.01 | < 0.01 | 2 | 0.01 | 2.0 (−) | 2 | 100 | 0 | 0 | |
| Campylobacter (e) | 249 (20) | 6.2 | 0.06 | 0.07 | 0.10 | 1,051 | 3.2 | 4.2 (2–39) | 134 | 12.7 | 6 | 0.57 | |
| Cronobacter sakazakii | 1 (1) | 0.02 | < 0.01 | 0 | < 0.01 | 4 | 0.01 | 4.0 (−) | 4 | 100 | 1 | 25.0 | |
| Escherichia coli other than STEC | 27 (4) | 0.70 | 0.01 | < 0.01 | < 0.01 | 327 | 1.0 | 12.1 (2–85) | 44 | 13.5 | 0 | 0 | |
| Listeria monocytogenes | 23 (8) | 0.60 | 0.01 | < 0.01 | < 0.01 | 104 | 0.30 | 4.5 (2–11) | 48 | 46.2 | 12 | 11.5 | |
| Salmonella | 773 (143) | 19.3 | 0.17 | 0.16 | 0.27 | 6,755 | 20.8 | 8.7 (2–728) | 1,123 | 16.6 | 1 | 0.01 | |
| Shiga toxin‐producing E. coli (STEC) | 31 (5) | 0.80 | 0.01 | 0.01 | 0.01 | 275 | 0.80 | 8.9 (2–76) | 47 | 17.1 | 0 | 0 | |
| Shigella | 11 (1) | 0.30 | < 0.01 | < 0.01 | 0.01 | 63 | 0.20 | 5.7 (2–21) | 4 | 6.3 | 0 | 0 | |
| Vibrio cholera (non‐toxigenic) | 1 (1) | 0.02 | < 0.01 | < 0.01 | 47 | 0.10 | 47.0 (−) | 1 | 2.1 | 0 | 0 | ||
| Vibrio parahaemolyticus | 3 (1) | 0.10 | < 0.01 | < 0.01 | < 0.01 | 10 | 0.03 | 3.3 (2–6) | 0 | 0 | 0 | 0 | |
| Yersinia | 21 (4) | 0.50 | < 0.01 | < 0.01 | < 0.01 | 125 | 0.40 | 6.0 (2–26) | 14 | 11.2 | 0 | 0 | |
| Other bacteria/unspecified | 1 (0) | 0.02 | < 0.01 | < 0.01 | < 0.01 | 16 | 0.05 | 16.0 (−) | 0 | 0 | 0 | 0 | |
| Subtotal (e) | 1,143 (189) | 28.5 | 0.25 | 0.24 | 0.39 | 8,798 | 27.0 | 7.7 (2–728) | 1,421 | 16.2 | 20 | 0.23 | |
| Bacterial toxins | Bacillus cereus toxins | 87 (15) | 2.2 | 0.02 | 0.02 | 0.04 | 679 | 2.1 | 7.8 (2–93) | 9 | 1.3 | 1 | 0.15 |
| Clostridium botulinum toxins | 7 (4) | 0.20 | < 0.01 | < 0.01 | < 0.01 | 24 | 0.10 | 3.4 (2–8) | 15 | 62.5 | 0 | 0 | |
| Clostridium perfringens toxins | 40 (20) | 1.0 | 0.01 | 0.01 | 0.02 | 778 | 2.4 | 19.5 (2–69) | 25 | 3.2 | 4 | 0.51 | |
| Staphylococcus aureus toxins | 61 (20) | 1.5 | 0.01 | 0.01 | 0.04 | 640 | 2.0 | 10.5 (2–62) | 51 | 8.0 | 0 | 0 | |
| Bacterial toxins, unspecified | 484 (13) | 12.1 | 0.11 | 0.08 | 0.13 | 4,257 | 13.1 | 8.8 (2–329) | 210 | 4.9 | 2 | 0.05 | |
| Subtotal | 679 (72) | 17.0 | 0.15 | 0.12 | 0.18 | 6,378 | 19.6 | 9.4 (2–329) | 310 | 4.9 | 7 | 0.11 | |
| Viruses | Adenovirus | 1 (0) | 0.02 | < 0.01 | 0 | < 0.01 | 2 | 0.01 | 2.0 (−) | 0 | 0 | 0 | 0 |
| Flavivirus (including tick‐borne Encephalitis virus) | 1 (1) | 0.02 | < 0.01 | < 0.01 | < 0.01 | 5 | 0.02 | 5.0 (−) | 5 | 100 | 0 | 0 | |
| Hepatitis A | 13 (0) | 0.30 | < 0.01 | < 0.01 | 0.01 | 264 | 0.80 | 20.3 (2–199) | 209 | 79.2 | 0 | 0 | |
| Hepatitis E | 1 (0) | 0.02 | < 0.01 | < 0.01 | < 0.01 | 3 | 0.01 | 3.0 (−) | 1 | 33.3 | 0 | 0 | |
| Norovirus (and other Calicivirus) | 251 (41) | 6.3 | 0.06 | 0.03 | 0.07 | 6,545 | 20.1 | 26.1 (2–230) | 156 | 2.4 | 1 | 0.02 | |
| Other viruses, unspecified | 4 (0) | 0.10 | < 0.01 | < 0.01 | 0.01 | 23 | 0.10 | 5.8 (2–12) | 2 | 8.7 | 0 | 0 | |
| Subtotal | 271 (42) | 6.8 | 0.06 | 0.03 | 0.10 | 6,842 | 21.0 | 25.2 (2–230) | 373 | 5.5 | 1 | 0.01 | |
| Parasites | Cryptosporidium | 2 (1) | 0.05 | < 0.01 | < 0.01 | < 0.01 | 25 | 0.10 | 12.5 (2–23) | 0 | 0 | 0 | 0 |
| Giardia | 5 (0) | 0.10 | < 0.01 | < 0.01 | < 0.01 | 11 | 0.03 | 2.2 (2–3) | 0 | 0 | 0 | 0 | |
| Trichinella | 1 (0) | 0.02 | < 0.01 | < 0.01 | < 0.01 | 2 | 0.01 | 2.0 (−) | 0 | 0 | 0 | 0 | |
| Subtotal | 8 (1) | 0.20 | < 0.01 | < 0.01 | 0.01 | 38 | 0.10 | 4.8 (2–23) | 0 | 0 | 0 | 0 | |
| Other causative agents | Histamine and Scombrotoxin | 47 (15) | 1.2 | 0.01 | 0.01 | 0.02 | 209 | 0.60 | 4.4 (2–14) | 16 | 7.7 | 0 | 0 |
| Marine biotoxins | 17 (5) | 0.40 | < 0.01 | 0.01 | 0.01 | 83 | 0.30 | 4.9 (2–38) | 3 | 3.6 | 0 | 0 | |
| Mushroom toxins | 6 (4) | 0.10 | < 0.01 | 0 | < 0.01 | 23 | 0.10 | 3.8 (2–6) | 21 | 91.3 | 0 | 0 | |
| Other agents (incl. unspecified) | 3 (1) | 0.10 | < 0.01 | < 0.01 | < 0.01 | 10 | 0.03 | 3.3 (2–6) | 2 | 20.0 | 0 | 0 | |
| Subtotal | 73 (25) | 1.8 | 0.02 | 0.02 | 0.03 | 325 | 1.0 | 4.5 (2–38) | 42 | 12.9 | 0 | 0 | |
| Unknown | Unknown/Unspecified | 1,831 (26) | 45.7 | 0.41 | 0.27 | 0.39 | 10,162 | 31.2 | 5.5 (2–402) | 349 | 3.4 | 3 | 0.03 |
| EU Total (e) | 4,005 (355) | 100 | 0.89 | 0.69 | 1.1 | 32,543 | 100 | 8.1 (2–728) | 2,495 | 7.7 | 31 | 0.10 | |
‘Escherichia coli’ other than STEC’ includes Enteroinvasive Escherichia coli (EIEC) (5), Enteropathogenic Escherichia coli (EPEC) (2) and other unspecified Escherichia coli (20).
‘Marine biotoxins’ includes ciguatoxin (8) and other unspecified toxins (9).
‘Other causative agents’ includes atropine (1) lectin (2).
: Percentage out of the total number of cases reported in the EU.
: Data on FBOs from the United Kingdom (Northern Ireland) are taken into account for 2021. In accordance with the agreement on the withdrawal of the United Kingdom from the EU, and in particular with the Protocol on Ireland/Northern Ireland, the EU requirements on data sampling are also applicable to Northern Ireland.
: Data on FBOs from the United Kingdom are taken into account for 2017–2019, because the United Kingdom was an EU MS, but it became a third country on 1 February 2020.
: Percentage out of the total number of cases caused by the causative agent.
: For one outbreak, information on cases was not available. This outbreak was excluded from the calculation of the mean outbreak size.
Table 63.
Frequency distribution of strong‐evidence foodborne outbreaks, by food vehicle, in reporting EU MSs, 2021
| Type of vehicle | Strong‐evidence outbreaks | Reporting rate per 100,000 | Rank | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Outbreaks | Cases | Hospitalisations | Deaths | 2021 (a) | 2020 | 2017–2019 (b) (mean) | 2021 (a) | 2017–2020 (b) | |||||
| N | % of total | N | % of total | N | % of total | N | % of total | ||||||
| Composite foods, multi‐ingredients foods and other foods | |||||||||||||
| Mixed foods | 76 | 21.4 | 2,156 | 30.8 | 181 | 24.4 | 2 | 15.4 | 0.017 | 0.006 | 0.017 | 1 | 3 |
| Bakery products | 18 | 5.1 | 258 | 3.7 | 52 | 7.0 | 0 | 0 | 0.004 | 0.002 | 0.008 | 8 | 5 |
| Buffet meals | 5 | 1.4 | 205 | 2.9 | 33 | 4.5 | 0 | 0 | 0.001 | 0.001 | 0.003 | 14 | 13 |
| Sweets and chocolate | 2 | 0.60 | 34 | 0.50 | 8 | 1.1 | 0 | 0 | < 0.001 | 0.001 | 0.001 | 17 | 21 |
| Other foods | 5 | 1.4 | 103 | 1.5 | 2 | 0.30 | 0 | 0 | 0.001 | 0.002 | 0.007 | 14 | 7 |
| Subtotal | 106 | 29.9 | 2,756 | 39.3 | 276 | 37.2 | 2 | 15.4 | 0.024 | 0.013 | 0.037 | – | – |
| Meat and meat products | |||||||||||||
| Pig meat and products thereof | 22 | 6.2 | 347 | 5.0 | 69 | 9.3 | 3 | 23.1 | 0.005 | 0.004 | 0.007 | 6 | 6 |
| Broiler meat (Gallus gallus) and products thereof | 21 | 5.9 | 202 | 2.9 | 42 | 5.7 | 0 | 0 | 0.005 | 0.002 | 0.005 | 7 | 10 |
| Meat and meat products, unspecified | 17 | 4.8 | 237 | 3.4 | 18 | 2.4 | 2 | 15.4 | 0.004 | 0.002 | 0.007 | 10 | 8 |
| Bovine meat and products thereof | 13 | 3.7 | 201 | 2.9 | 12 | 1.6 | 0 | 0 | 0.003 | 0.001 | 0.002 | 11 | 17 |
| Other or mixed red meat and products thereof | 2 | 0.60 | 11 | 0.20 | 0 | 0 | 0 | 0 | < 0.001 | 0.001 | 0.002 | 17 | 14 |
| Other, mixed or unspecified poultry meat and products thereof | 2 | 0.60 | 14 | 0.20 | 0 | 0 | 0 | 0 | < 0.001 | < 0.001 | 0.002 | 17 | 19 |
| Subtotal | 77 | 21.7 | 1,012 | 14.4 | 141 | 19 | 5 | 38.5 | 0.017 | 0.010 | 0.026 | – | – |
| Fish and fishery products | |||||||||||||
| Fish and fish products | 30 | 8.5 | 190 | 2.7 | 41 | 5.5 | 4 | 30.8 | 0.007 | 0.006 | 0.01 | 4 | 4 |
| Crustaceans, shellfish, molluscs and products thereof | 25 | 7.0 | 171 | 2.4 | 13 | 1.8 | 0 | 0 | 0.006 | 0.008 | 0.017 | 5 | 2 |
| Subtotal | 55 | 15.5 | 361 | 5.2 | 54 | 7.3 | 4 | 30.8 | 0.012 | 0.015 | 0.027 | – | – |
| Food of non‐animal origin | |||||||||||||
| Vegetables and juices and products thereof | 34 | 9.6 | 1,700 | 24.3 | 131 | 17.7 | 0 | 0 | 0.008 | 0.003 | 0.006 | 3 | 9 |
| Cereal products including rice and seeds/pulses | 9 | 2.5 | 194 | 2.8 | 17 | 2.3 | 0 | 0 | 0.002 | 0.001 | 0.002 | 12 | 18 |
| Fruit, berries and juices and products thereof | 2 | 0.60 | 15 | 0.20 | 0 | 0 | 0 | 0 | < 0.001 | 0.001 | 0.002 | 17 | 20 |
| Subtotal | 45 | 12.7 | 1,909 | 27.3 | 148 | 20.0 | 0 | 0 | 0.010 | 0.005 | 0.01 | – | – |
| Eggs and egg products | 42 | 11.8 | 439 | 6.3 | 90 | 12.1 | 1 | 7.7 | 0.009 | 0.009 | 0.024 | 2 | 1 |
| Milk and milk products | |||||||||||||
| Cheese | 18 | 5.1 | 235 | 3.4 | 11 | 1.5 | 0 | 0 | 0.004 | 0.001 | 0.002 | 8 | 15 |
| Dairy products (other than cheeses) | 6 | 1.7 | 119 | 1.7 | 7 | 0.90 | 1 | 7.7 | 0.001 | 0.001 | 0.001 | 13 | 22 |
| Milk | 4 | 1.1 | 55 | 0.80 | 13 | 1.8 | 0 | 0 | 0.001 | 0.002 | 0.004 | 16 | 11 |
| Subtotal | 28 | 7.9 | 409 | 5.8 | 31 | 4.2 | 1 | 7.7 | 0.006 | 0.004 | 0.007 | – | – |
| Water (and other beverages) | |||||||||||||
| Water | 2 | 0.60 | 119 | 1.7 | 1 | 0.10 | 0 | 0 | < 0.001 | 0.001 | 0.002 | 17 | 16 |
| Subtotal | 2 | 0.60 | 119 | 1.7 | 1 | 0.10 | 0 | 0 | < 0.001 | 0.001 | 0.002 | – | – |
| EU Total (b) | 355 | 100 | 7,005 | 100 | 741 | 100 | 13 | 100 | 0.079 | 0.055 | 0.266 | – | – |
Note: Single food items are consolidated into major groups according to their origin. The ‘Outbreak Reporting Rate' columns include the mean outbreak reporting rate per 100,000 for 2021 and for the previous years (2017–2020) for trend watching. The ranking of each food item provides a visual demonstration of the relative importance of the item, among all food vehicles implicated in foodborne outbreaks, for the same year and period.
: Data on FBOs from the United Kingdom (Northern Ireland) are taken into account for 2021. In accordance with the agreement on the withdrawal of the United Kingdom from the EU, and in particular with the Protocol on Ireland/Northern Ireland, the EU requirements on data sampling are also applicable to Northern Ireland.
: Data on FBOs from the United Kingdom are taken into account for 2017–2019, because the United Kingdom was an EU MS, but it became a third country on 1 February 2020.
‘Bakery products’ includes ‘Bakery products’, ‘Bakery products – cakes’, ‘Bakery products – cakes – containing heat‐treated cream’, ‘Bakery products – desserts – containing raw cream’, ‘Bakery products – desserts – containing raw eggs’, ‘Bakery products – pastry’.
‘Bovine meat and products thereof’ include ‘Bovine meat and products thereof’, ‘Cooked cured (or seasoned) bovine meat’, ‘Meat from bovine animals – meat products’, ‘Meat from bovine animals – meat products – raw and intended to be eaten raw’, ‘Meat from bovine animals – minced meat’.
‘Broiler meat (Gallus gallus) and products thereof’ include ‘Broiler meat (Gallus gallus) and products thereof’, ‘Meat from broilers (Gallus gallus)’, ‘Meat from broilers (Gallus gallus) – meat products’.
‘Cheese' includes ‘Cheese', ‘Cheeses made from sheep's milk – fresh’.
‘Crustaceans, shellfish, molluscs and products thereof’ includes ‘Crustaceans, shellfish, molluscs and products thereof’, ‘Molluscan shellfish’.
‘Eggs and egg products’ include ‘Eggs’, ‘Eggs and egg products’.
‘Fish and fish products’ include ‘Fish’, ‘Fish – cooked’, ‘Fish – Fishery products from fish species associated with a high amount of histidine – not enzyme maturated’, ‘Fish – raw’, ‘Fish – smoked’, ‘Fish and fish products’, ‘Roe – chilled’.
‘Milk’ includes ‘Milk’, ‘Milk, cows’ – raw milk’, ‘Milk, goats' – raw milk’.
‘Mixed food’ includes ‘Foodstuffs intended for special nutritional uses – dietary foods for special medical purposes’, ‘Foodstuffs intended for special nutritional uses – other food for infants and children’, ‘Mixed food’, ‘Other processed food products and prepared dishes’, ‘Other processed food products and prepared dishes – fish and seafood based dishes’, ‘Other processed food products and prepared dishes – meat based dishes’, ‘Other processed food products and prepared dishes – mushroom based dishes’, ‘Other processed food products and prepared dishes – pasta’, ‘Other processed food products and prepared dishes – pasta based dishes’, ‘Other processed food products and prepared dishes – sandwiches – non‐meat’, ‘Other processed food products and prepared dishes – sushi’, ‘Other processed food products and prepared dishes – unspecified’, ‘Ready‐to‐eat salads’, ‘Sauce and dressings’, ‘Sauce and dressings – mayonnaise', ‘Soups’.
Other or mixed red meat and products thereof includes ‘Meat, mixed meat – meat products – ready‐to‐eat’, ‘Other or mixed red meat and products thereof’.
Other, mixed or unspecified poultry meat and products thereof includes ‘Meat from duck – meat products – ready‐to‐eat’, ‘Meat from poultry, unspecified’.
‘Pig meat and products thereof’ includes ‘Cooked cured (or seasoned) pork meat’, ‘Meat from pig – meat products’, ‘Meat from pig – meat products – meat specialities’, ‘Meat from pig – meat products – ready‐to‐eat’, ‘Meat from pig – offal’, ‘Pig meat and products thereof’.
‘Sweets and chocolate' include ‘Confectionery products and pastes – hard candy’, ‘Sweets and chocolate'.
‘Vegetables and juices and other products thereof’ include ‘Alfalfa sprouts’, ‘Lettuce', ‘Melons (except watermelon)’, ‘Mushrooms’, ‘Spring onion’, ‘Vegetables – pre‐cut’, ‘Vegetables – products – cooked’, ‘Vegetables and juices and other products thereof’.
‘Water’ includes ‘Tap water, including well water’.
Table 68.
Frequency distribution of strong‐evidence foodborne outbreaks by place of exposure, in reporting EU, MSs, 2021
| Type of setting | Strong‐evidence outbreaks | Outbreak Reporting Rate per 100,000 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Outbreaks | Cases | Hospitalisations | Deaths | 2021 (a) | 2020 | 2017–2019 (mean) (b) | |||||
| N | % of total | N | % of total | N | % of total | N | % of total | ||||
| Domestic setting | 121 | 34.1 | 803 | 11.5 | 161 | 21.7 | 0 | 0 | 0.027 | 0.022 | 0.054 |
| Restaurant, pub, street vendors, take away, etc. | |||||||||||
| Restaurant or Cafe or Pub or Bar or Hotel or Catering service | 77 | 21.7 | 1,134 | 16.2 | 72 | 9.7 | 0 | 0 | 0.017 | 0.012 | 0.037 |
| Take‐away or fast‐food outlet | 4 | 1.1 | 26 | 0.37 | 0 | 0 | 0 | 0 | 0.001 | 0.002 | 0.001 |
| Mobile retailer or market/street vendor | 1 | 0.28 | 13 | 0.19 | 0 | 0 | 0 | 0 | < 0.01 | < 0.01 | 0.001 |
| Canteen or catering at workplace, school, etc. | |||||||||||
| School or kindergarten | 42 | 11.8 | 2,104 | 30.0 | 146 | 19.7 | 0 | 0 | 0.009 | 0.003 | 0.007 |
| Canteen or workplace catering | 3 | 0.85 | 48 | 0.69 | 0 | 0 | 0 | 0 | 0.001 | 0.002 | 0.005 |
| Health care and residential facilities | |||||||||||
| Residential institution (nursing home or prison or boarding school) | 21 | 5.9 | 449 | 6.4 | 20 | 2.7 | 6 | 46.2 | 0.005 | 0.004 | 0.005 |
| Hospital and medical care facility | 11 | 3.1 | 228 | 3.3 | 14 | 1.9 | 1 | 7.7 | 0.002 | < 0.01 | 0.002 |
| Multiple places of exposure | |||||||||||
| Multiple places of exposure in one country | 20 | 5.6 | 715 | 10.2 | 111 | 15.0 | 6 | 46.2 | 0.004 | 0.003 | 0.005 |
| Multiple places of exposure in more than one country | 6 | 1.7 | 306 | 4.4 | 90 | 12.1 | 0 | 0 | 0.001 | 0.001 | 0.001 |
| Other place of exposure | |||||||||||
| Others | 14 | 3.9 | 361 | 5.2 | 29 | 3.9 | 0 | 0 | 0.003 | 0.004 | 0.008 |
| Camp or picnic | 8 | 2.3 | 220 | 3.1 | 39 | 5.3 | 0 | 0 | 0.002 | < 0.01 | 0.002 |
| Farm | 1 | 0.28 | 39 | 0.56 | 0 | 0 | 0 | 0 | < 0.01 | < 0.01 | 0.001 |
| Temporary mass catering (fairs or festivals) | 1 | 0.28 | 19 | 0.27 | 1 | 0.13 | 0 | 0 | < 0.01 | < 0.01 | < 0.01 |
| Primary production | 1 | 0.28 | 3 | 0.04 | 0 | 0 | 0 | 0 | < 0.01 | – | – |
| Unknown | 24 | 6.8 | 537 | 7.7 | 58 | 7.8 | 0 | 0 | 0.005 | 0.003 | 0.008 |
| EU Total (b) | 355 | 100 | 7,005 | 100 | 741 | 100 | 13 | 100 | 0.079 | 0.055 | 0.137 |
: Data on FBOs from the United Kingdom (Northern Ireland) are taken into account for 2021. In accordance with the agreement on the withdrawal of the United Kingdom from the EU, and in particular with the Protocol on Ireland/Northern Ireland, the EU requirements on data sampling are also applicable to Northern Ireland.
: Data on FBOs from the United Kingdom are taken into account for 2017–2019, because the United Kingdom was an EU MS, but it became a third country on 1 February 2020.
Long‐term variations were also described, taking 2012–2021 as the reference time period. Frequency distributions and trends are shown at EU level. Trends were analysed only at MS level, according to the rationale described in Boelaert et al. (2016) for data quality (Table 1).
Key statistics on FBOs for 2020 and previous years may differ from those published in the European Union One Health 2020 Zoonoses Report, as MSs may have updated their data from previous years.
Time trends were tested for statistical significance over the period 2012–2021 using the Cox‐Stuart sign test, a nonparametric test appropriate for limited numbers of observations. A p‐value of < 0.05 was considered to identify a statistically significant trend, beyond chance. The detection of significant trends at national level should be interpreted with caution, following changes in the reporting specifications for FBO, introduced in 2014 (EFSA, 2014). MSs with incomplete datasets for the 2012–2021 period were excluded from the trend analysis.
4. Results and discussion
4.1. Overview of countries reporting foodborne outbreak data in 2021
In 2021, 27 MSs and the United Kingdom (Northern Ireland) reported a total of 4,005 FBOs with 32,543 human cases, 2,495 hospitalisations and 31 deaths. At the same time, seven non‐MSs (Bosnia and Herzegovina, Iceland, Montenegro, Norway, Republic of North Macedonia, Serbia, Switzerland) reported 83 outbreaks, 1,270 human cases, 65 hospitalisations and two deaths (Table 61).
The total number of FBOs reported by each MS in 2021 varied substantially, with most outbreaks concerning a small number of MSs. Belgium, France, the Netherlands and Poland accounted for most of the total number of FBOs (2,970 outbreaks or 74.2% of the total number) and for the majority of human cases reported in the EU in 2021 (19,936 cases or 61.3% of the total number).
Table 61 shows the substantial variations among MSs with respect to the numbers of outbreaks and cases reported in 2021. Outbreak reporting rates (per 100,000 population) ranged from 0.01 (Romania) to 5.0 (Malta) outbreaks per 100,000 population, corresponding to a 504‐fold variation. The variation for case reporting rates (per 100,000 population) was lower but still considerable, ranging from 0.14 (Slovenia) to 25.0 (Finland).
The ‘mean outbreak size' (i.e. the mean number of cases per outbreak) and the ‘range of cases per outbreak’ complete the characterisation of the FBOs occurring in 2021 in MSs and non‐MSs. Taken together, the indicators in Table 61 describe the considerable differences among countries in terms of the occurrence of FBOs and their impact on health. These differences are largely influenced by the characteristics of the surveillance put in place by the MSs, and in particular the type of FBOs and causative agents under surveillance (Figure 32).
Figure 32.
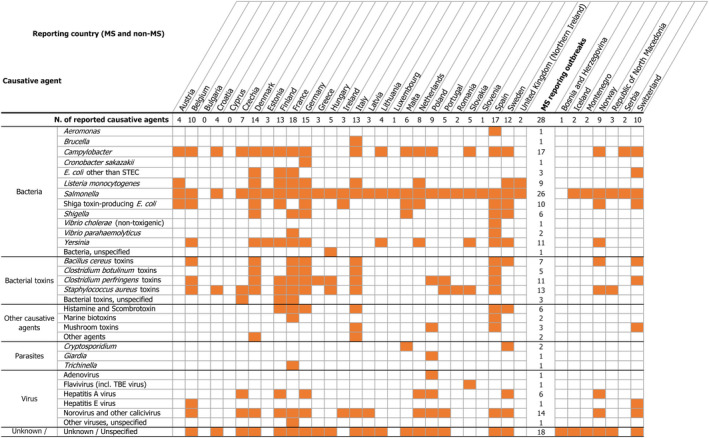
- The table may be read by column (country) or by row (causative agent). The number at the end of each row is the number of countries reporting a given causative agent for outbreaks in 2021, while the number at the top of each column indicates the number of causative agents identified in outbreaks by a given country in 2021. ‘Escherichia coli’ other than STEC includes Enteroinvasive E. coli (EIEC), Enterotoxigenic E. coli (ETEC) and E. coli, unspecified. ‘Bacillus toxins’ include Bacillus cereus, Bacillus cereus enterotoxins. ‘Staphylococcus aureus toxins’ include staphylococcal enterotoxins. ‘Norovirus (and other calicivirus)’ include norovirus (Norwalk‐like virus) and calicivirus unspecified. ‘Marine biotoxins’ include ciguatoxin and other unspecified marine toxins. ‘Other agents’ include atropine and lectin.
In 2021, FBOs classified as ‘general outbreaks’ (N = 1,550; 38.7%) outnumbered ‘household outbreaks’ (N = 794; 19.8%) even though not all MSs reported ‘household outbreaks’. Overall, 17 MSs (Austria, Belgium, Croatia, Estonia, France, Germany, Greece, Ireland, Italy, Malta, the Netherlands, Poland, Romania, Slovakia, Slovenia, Spain, Sweden) provided data on ‘household outbreaks’ (i.e. FBOs involving single households) in 2021. The reporting of ‘household’ outbreaks, most of which are small and occur in domestic settings (N = 745; 93.8% of household outbreaks), has a direct consequence on the total count of FBOs occurring in a country and on the relative measures of FBO occurrence. This explains why the MSs reporting ‘household’ outbreaks generally have higher FBO reporting rates than MSs that do not notify this type of FBO to EFSA.
The case reporting rate is a sensitive measure of the health impact of FBOs as it is largely influenced by the size of the outbreaks. In 2021, the MSs with the highest case reporting rates (i.e. Finland, Latvia and Denmark) did not report ‘household’ outbreaks but provided information on several large (i.e. ≥ 50 cases) or very large (i.e. ≥ 100 cases) outbreaks. Finland reported a single FBO caused by S. Typhimurium in pre‐cut vegetables involving 728 cases, and four other large FBOs caused by norovirus, STEC and S. Typhimurium. In Latvia, norovirus was responsible for two large FBOs. Denmark also reported four large FBOs caused by norovirus, and two other large FBOs caused by Enteroinvasive E. coli (EIEC) and S. Typhimurium, respectively, implicating food of non‐animal origin.
Information regarding the number of FBOs and cases, hospitalisations and deaths at EU and single MS level during 2016–2021, can be viewed dynamically on the dashboard (available here, see page ‘reporting countries’ for information on FBOs by reporting country). In 2021, the severity of outbreaks did not significantly change compared with 2020. The proportion of hospitalisations and deaths at EU‐level was 7.7% and 0.1%, respectively, based on the total number of outbreak cases. Six MSs (Austria, France, Germany, the Netherlands, Spain, Sweden) and the United Kingdom (Northern Ireland) and one non‐MS (Switzerland) reported a number of deaths among FBO cases. The number of deaths in the EU did not substantially change compared with 2020 (N = 34), although the distribution among MSs showed a considerable imbalance, with France (17 deaths) accounting for over half of the total deaths reported in the EU. This is the highest number of deaths among FBO cases reported by a single MS in 1 year, since 2012. At EU level, 15 deaths – including 13 in France – were reported as general outbreaks in ‘health care and residential facilities’, thus emphasising the risks of foodborne hazards for vulnerable populations.
Strong‐evidence outbreaks accounted for 355 FBOs (8.9% of all FBOs) and were reported by 21 MSs (all MSs except Bulgaria, Cyprus, Estonia, Ireland, Latvia, Slovenia, United Kingdom (Northern Ireland)). For five MSs (Finland, Lithuania, Luxembourg, Romania, Sweden) strong‐evidence outbreaks accounted for more than a third of total reported FBOs, while for four MSs (Belgium, France, the Netherlands, Slovakia), this proportion did not exceed 10%.
In 2021, although the number of FBOs reported in the EU was higher than in 2020, it did not generally return to the level of pre‐pandemic years (Figure 30). EU MSs reported overall 919 FBOs more than in 2020 (a 29.8% relative increase) but 1,596 fewer than the mean annual total for the period 2017–2019 (a 28.5% relative decrease). A similar pattern can be seen for cases of illness and hospitalisation, with 12,526 cases and 820 hospitalisations more than in 2020 (a relative increase of 62.6% and 49.0%, respectively) and 16,901 cases and 1,987 hospitalisations fewer, on average, than in 2017–2019 (a relative decrease of 34.2% and 44.3%). The number of deaths decreased both compared with 2020 (three fewer deaths; a relative decrease of 8.8%) and the pre‐pandemic years (13 fewer deaths; a 30.1% relative decrease compared with 2017–2019).
Figure 30.
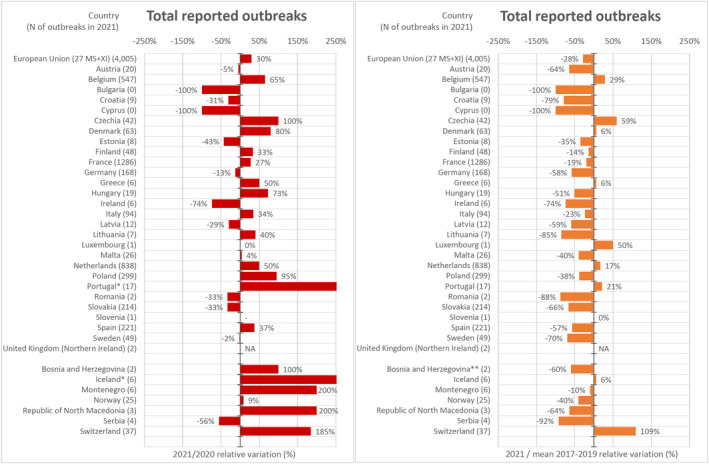
- * 2021/2020 relative variation for Portugal was 325% and for Iceland was 500%. ** The mean annual total of FBOs reported by Bosnia and Herzegovina was calculated considering only the years 2017–2018, since no FBO data were reported in 2019.
Details of the annual variations in FBOs reported for 2021 as compared with 2020 and the pre‐pandemic years (mean total of FBOs per year for the period 2017–2019) are shown in Figure 30, by country. Details on relative variations by causative agent and country are available in the ‘Addendum foodborne outbreaks, 2021’, published on the EFSA Knowledge Junction on Zenodo here, in sections 4 and 5.
Eight MSs (Finland, France, Hungary, Italy, Lithuania, Malta, Poland, Spain) and four non‐MSs (Bosnia and Herzegovina, Montenegro, Norway, Republic of North Macedonia) reported more FBOs in 2021 than in 2020 but fewer than in the pre‐pandemic years 2017–2019, on average. Eleven MSs (Austria, Bulgaria, Croatia, Cyprus, Estonia, Germany, Ireland, Latvia, Romania, Slovakia, Sweden) and one non‐MS (Serbia) reported fewer FBOs in 2021 on average than in both 2020 and the period 2017–2019. Taken together, these results suggest that the COVID‐19 pandemic and the associated control measures continued to have a major impact in 2021 on the occurrence of FBOs and their reporting in European countries. Six MSs (Belgium, Czechia, Denmark, Greece, the Netherlands, Portugal) and two non‐MSs (Iceland and Switzerland) reported more FBOs in 2021 than in 2020 and 2017–2019, on average. Both an improvement in surveillance sensitivity and an increase in FBOs taking place in a domestic setting were mentioned by some countries as a possible reason for this increase.
Over the long term (2012–2021), the number of FBOs reported annually to EFSA increased significantly for the Netherlands and Switzerland, while decreased significantly for Austria, Slovenia and Norway (Figure 31).
Figure 31.
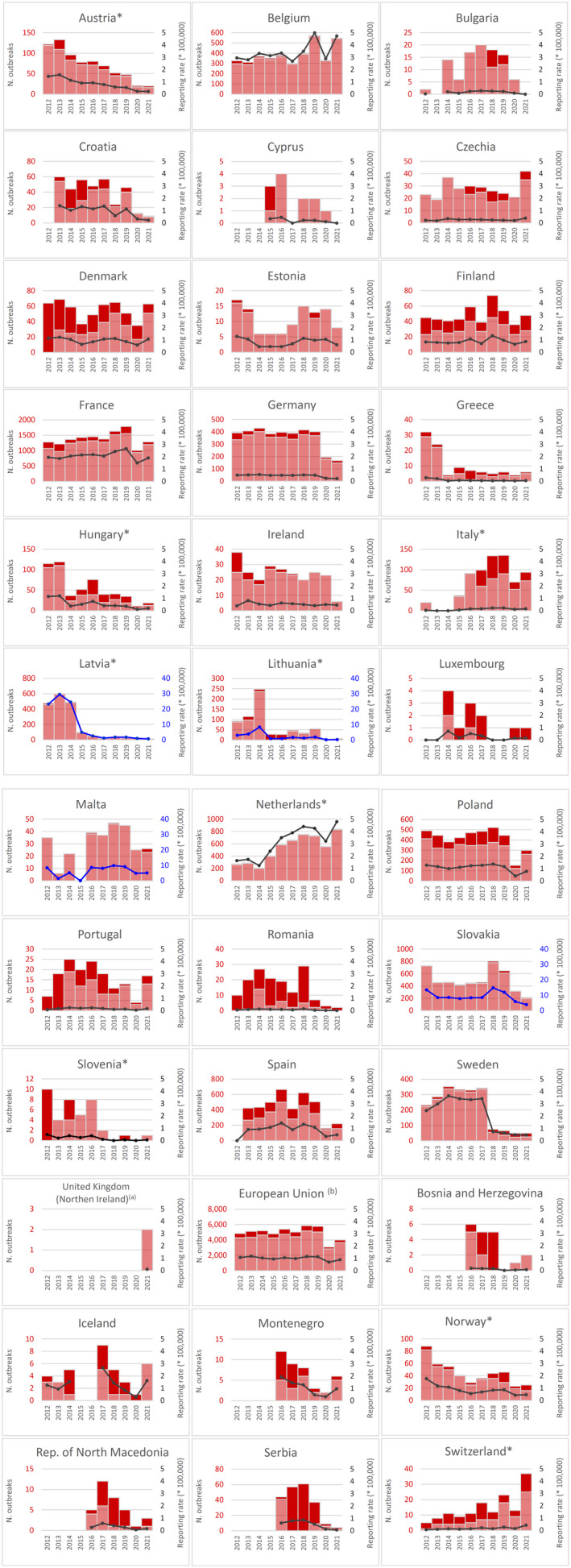
- Note: * indicates countries with a statistically significant trend (p < 0.05) over the period. Dark red and light red show strong‐ and weak‐evidence outbreaks, respectively. Black dots and lines show FBO reporting rates. The dots, lines and secondary Y‐axis in blue showing the outbreak reporting rates have been used for Latvia, Lithuania, Malta and Slovakia, in order to draw attention to a scale that is different to that of the other countries. (a): Data on FBOs from the United Kingdom (Northern Ireland) are taken into account for 2021. In accordance with the agreement on the withdrawal of the United Kingdom from the EU, and in particular with the Protocol on Ireland/Northern Ireland, the EU requirements on data sampling are also applicable to Northern Ireland. (b): Data on FBOs from the United Kingdom are taken into account for 2017–2019, because the United Kingdom was an EU MS, but it became a third country on 1 February 2020.
4.2. Overview of causative agents in foodborne outbreaks in 2021
In 2021, a causative agent was identified in 2,174 FBOs (54.3% of the total) reported in the EU, causing 22,381 cases (68.8% of the total), 2,146 hospitalisations (86.0% of the total) and 28 deaths (90.3% of the total).
Table 62 shows the key statistics for FBOs reported in the EU, by causative agent. More information on the agents most frequently implicated in FBOs are shown on the dashboard (available here, see the page on ‘causative agents’ for more information on FBOs per causative agent).
Most outbreaks implicated bacteria, which were also responsible for the highest number of cases, hospitalisations and deaths. Salmonella was responsible for the majority of FBOs (about one in five), human cases and hospitalisations, by far. Listeria monocytogenes was associated with the highest number of deaths. After bacteria, the most frequently reported causative agents were bacterial toxins and viruses, in particular norovirus. Parasites and other causative agents including histamine were much less frequently reported. It is noteworthy that in 2021, reports listed one severe FBO caused by Cronobacter sakazakii and one by non‐toxigenic Vibrio cholerae, a first since EFSA began collecting data on FBOs.
The breakdown of causative agents by country is shown in Figure 32. The MSs of Belgium, Denmark, Finland, France, Germany, Italy and Spain and one non‐MS (Switzerland) were the countries with the highest number (more than 10) and variety of causative agents reported in FBOs in 2021. Salmonella, Campylobacter, norovirus (and other calicivirus), Staphylococcus aureus toxins and Clostridium perfringens toxins were the pathogens reported by most countries.
For a further interactive look at FBO data: see the dashboard here (different filters can be applied; outbreaks by causative agent are shown on different pages of the dashboard).
See the EFSA story map (available here) on FBOs, section on ‘what organisms and symptoms’.
4.2.1. Bacteria
Salmonella
In 2021, 26 MSs and six non‐MSs (Table 61 and Figure 32) reported FBOs caused by Salmonella. France (N = 176), Poland (N = 165), Slovakia (N = 154) and Spain (N = 93) were the main contributors. In 15 MSs (Austria, Belgium, Croatia, Estonia, Greece, Hungary, Italy, Lithuania, Luxembourg, the Netherlands, Poland, Romania, Slovakia, Slovenia, Spain) and the United Kingdom (Northern Ireland), and in five non‐MSs (Iceland, Montenegro, Republic of North Macedonia, Serbia, Switzerland), Salmonella was either the leading cause or the only cause of FBOs.
Information on Salmonella serovars was available for 439 FBOs (56.8% of all Salmonella FBOs). S. Enteritidis was the predominant serovar (N = 350; 79.7%), followed by S. Typhimurium (N = 50; 11.4%), S. Braenderup (N = 9; 2.1%) and S. Typhimurium monophasic (N = 6; 1.4%).
The number of Salmonella FBOs in the EU fell considerably in 2021 (N = 773) and 2020 (N = 694) compared with the pre‐pandemic years (N = 1,371, on average), after several years of relative stagnation. This decline can be primarily attributed to the impact of the COVID‐19 pandemic and was mainly driven by a decrease in the number of FBOs caused by S. Enteritidis, S. Typhimurium and S. Typhimurium monophasic, which together were responsible for 92.5% of all Salmonella FBOs in 2021, with information on the serovar provided. The number of FBOs caused by S. Enteritidis gradually fell in 2020 and 2021, accounting for 452 and 504 fewer FBOs than over the period 2017–2019 (N = 854 reported FBOs on average), respectively. Slovakia (N = 146) and Poland (N = 125) reported most of the FBOs caused by S. Enteritidis in 2021, accounting for 77.4% of the total FBOs linked to this serovar in the EU. The results of a source attribution and trend analysis exercise recently carried out in Europe as part of the DiSCoVeR project showed an increased number of Salmonella outbreaks in the pre‐pandemic years, which was mostly driven by S. Enteritidis outbreaks identified in Eastern Europe (Chanamé Pinedo et al., 2022). In 2020 and 2021, a relative drop could also be seen for FBOs caused by S. Typhimurium and S. Typhimurium monophasic, with 60 and 55 fewer reported FBOs in 2020 and 2021 than for the period 2017–2019 (N = 111 reported FBOs on average), respectively.
Over the same period, a similar relative decrease was observed for the number of cases and hospitalisations arising from FBOs caused by the main Salmonella serovars except S. Typhimurium. In 2021, this serovar caused 1,222 cases (1,062 cases more than in 2020; a 663.8% increase) mainly due to a single FBO in Finland with 728 cases. The outbreak (a strong‐evidence FBO) was associated with the consumption of pre‐cut vegetables and was not only the largest FBO reported in 2021 in the EU, but also the largest FBO caused by S. Typhimurium ever reported since data collection on FBOs began in 2004.
In 2021, a major outbreak caused by S. Braenderup leading to hundreds of cases was reported by multiple European countries (Austria, Belgium, Denmark, France, Luxembourg, the Netherlands and Switzerland). This FBO is noteworthy since this is a serovar rarely reported in outbreaks. According to the ECDC and EFSA rapid outbreak assessment (ECDC and EFSA, 2021a), this multi‐country outbreak was probably associated with the consumption of contaminated ‘Galia melons’ imported from Honduras. More information on this outbreak is available in the ‘ECDC and EFSA rapid outbreak assessment of multi‐country foodborne outbreaks’ section (see Section 4.8. ECDC and EFSA rapid outbreak assessment of multi‐country foodborne outbreaks).
Altogether, the other 17 Salmonella serovars were responsible for 24 outbreaks (5.5% of the total with known serovars). They included S. Agona, S. Bareilly, S. Bovismorbificans, S. Chester, S. Coeln, S. Corvallis, S. Haifa, S. Infantis, S. Kedougou, S. Litchfield, S. Mikawasima, S. Montevideo, S. Muenchen, S. Oranienburg, S. Stanley, S. Strathcona and S. Virchow. Cases of infection caused by S. Chester and S. Montevideo were reported as being ‘Part of a multi‐country outbreak’.
Campylobacter
In 2021, Campylobacter was the fourth most frequently reported causative agent in FBOs in the EU (Table 62). It was reported by 17 MSs (Austria, Belgium, Croatia, Czechia, Denmark, Estonia, Finland, France, Germany, Italy, Lithuania, Malta, the Netherlands, Poland, Slovakia, Spain and Sweden) and three non‐MSs (Norway, Serbia, Switzerland) (Figure 32). Germany (N = 64), France (N = 55) and Slovakia (N = 55) reported most of these FBOs, making up 69.9% of the total. Campylobacter was the main causative agent in two MSs (Germany, Malta) and in Norway. Of the 112 FBOs reported by MSs with known Campylobacter species, C. jejuni was the causative agent in 106 FBOs (94.6%) and C. coli in six (5.4%). For 137 FBOs, species information was missing. Campylobacter FBOs are generally medium or small size outbreaks characterised by relatively mild illnesses. However, six severe outbreaks leading to deaths or a high proportion of hospitalisations were reported in 2021 by four MSs (Denmark, France, Spain, Sweden). The number of deaths observed in 2021 was the highest ever reported involving Campylobacter FBOs since 2007. Nevertheless, the number of FBOs and cases fell gradually in both 2020 and 2021. In 2021, the number of FBOs and cases reported in the EU decreased by 21.5% and 20.3% respectively, compared with 2020 (68 fewer outbreaks and 268 fewer cases). Campylobacter FBOs and cases halved compared with the pre‐pandemic period of 2017–2019 on average (242 and 1,530 fewer FBOs and cases respectively).
Listeria monocytogenes
Key statistics on FBOs caused by Listeria monocytogenes are available in Table 62. In 2021, this agent was reported in FBOs by eight MSs (Austria, Denmark, Finland, France, Germany, Italy, the Netherlands, Sweden) and the United Kingdom (Northern Ireland) (Figure 32). As in recent years, L. monocytogenes was associated with one of the highest numbers of deaths among outbreak cases and case fatality rates. The number of FBOs reported for 2021 was the highest since EFSA first started collecting data even though the United Kingdom is no longer included in the analysis since it left the EU in 2020. However at EU level, fewer cases, hospitalisations and deaths were reported in 2021 compared with both 2020 and 2017–2019, on average. One possible reason for this could be an increased detection of small listeriosis outbreaks in the population. This could be related to more sensitive laboratory‐based surveillance of listeriosis with the widespread implementation of fine‐tuning characterisation methods, in particular Whole Genome Sequencing (WGS), for L. monocytogenes (EFSA BIOHAZ Panel, 2019). This is a promising perspective since WGS data collection in humans and food, along with interoperable systems to support surveillance of L. monocytogenes outbreaks, have been recently implemented at EU level under the coordination of ECDC and EFSA (EFSA et al., 2022d).
Shiga toxin‐producing E. coli (STEC)
In 2021, Shiga toxin‐producing E. coli (STEC) was the third most frequently reported bacterial agent detected in FBOs in the EU (Table 62). STEC were reported by 10 MSs (Austria, Belgium, Denmark, Finland, Germany, Ireland, Malta, the Netherlands, Spain, Sweden) and two non‐MSs (Norway, Switzerland) (Figure 32). STEC was confirmed as the leading causative agent of FBOs in Ireland. The number of FBOs and cases of illness caused by STEC has fallen gradually and steadily over the past 4 years. FBOs fell from 50 outbreaks reported in 2018 to 31 in 2021 and in parallel cases decreased from 390 in 2018 to 275 in 2021. Conversely, the number of hospitalisations and deaths did not show a similar variation over the same period. Information on the STEC serogroup was available for 22 FBOs reported by MSs (all countries except Germany, Malta and Spain) and two FBOs reported by one non‐MS (Norway). Overall, the identified serogroups among STEC FBOs were O157 (nine outbreaks), O26 (six outbreaks), O103 (five outbreaks) and O12, O145, O146, O91 (one outbreak each).
Other bacteria
Among the FBOs caused by other bacteria (Table 62), the number of outbreaks caused by E. coli other than STEC rose considerably in 2021 compared with recent years. Enteroinvasive E. coli (EIEC) were reported in five FBOs in France and Denmark, leading to 159 cases and 27 hospitalisations. The largest outbreak was associated with two different EIEC strains (O136 and O96) and was identified in Denmark. It involved 85 cases with infections taking place around the country. The implicated food was spring onion. An Enterotoxigenic E. coli (ETEC) strain was reported in 2021 in two single FBOs reported by Finland (29 cases) and Denmark (16 cases), linked to the consumption of vegetables and multi‐ingredient foods, respectively. Both ETEC and EIEC were previously reported as causative agents of FBOs only in 2016, 2017 and 2018. Unfortunately, for most of these FBOs no information was available on the characteristics of the E. coli (20 outbreaks).
Summary statistics on the FBOs caused by Yersinia enterocolitica are shown in Table 62. In 2021, this pathogen was detected in FBOs reported by 11 MSs (Belgium, Denmark, Estonia, Finland, France, Germany, Lithuania, the Netherlands, Slovakia, Spain, Sweden) and one non‐MS (Norway) (Figure 32). For three FBOs, the serotype involved was O:3. The number of FBOs reported in 2021 was relatively stable compared with 2020 and the preceding pre‐pandemic years.
Six MSs (Denmark, France, Germany, Malta, Spain, Sweden) reported 11 FBOs caused by Shigella . S. sonnei was identified in six FBOs, while the information on species was missing for the remaining FBOs.
In 2021, for the first time since EFSA began collecting data in 2004, one FBO caused by Cronobacter sakazakii was reported. The outbreak notified by Germany involved four newborns (premature) and caused one death. The implicated food vehicle was a hospital‐mixed probiotic formula for infants. This report is not surprising since one of the main routes of transmission of this pathogen is powdered infant formula (PIF), which is not a sterile product (Forsythe, 2015). Food safety criteria for Cronobacter spp. are set out in Reg. (EC) No 2073/2005 for infant formulae and dried dietary foods intended for infants below 6 months of age. This report highlights the importance of good hygiene practices in reconstituting the product (Forsythe, 2015).
V. cholerae (non‐toxigenic) was also reported for the first time in 2021 as the causative agent of an FBO. The FBO was detected in Spain, in a ‘residential institution (nursing home or prison or boarding school)’ and was linked to the consumption of mixed food. Usually, non‐toxigenic serogroups cause self‐limiting gastroenteritis and have a limited enteroinvasive potential (Sack et al., 2004). In literature, human infection is mainly associated with the intake of contaminated water or food, such as bivalve molluscs (Sack et al., 2004).
Other bacterial agents causing FBOs in the EU in 2021 included Brucella , Aeromonas and Vibrio parahaemoloyticus .
4.2.2. Bacterial toxins
In 2021, 16 MSs (Belgium, Croatia, Czechia, Denmark, Finland, France, Germany, Greece, Hungary, Italy, Poland, Portugal, Romania, Slovakia, Spain, Sweden) and three non‐MSs (Norway, Republic of North Macedonia, Switzerland) reported FBOs caused by bacterial toxins (Figure 32). Bacterial toxins were the leading cause of FBOs in France and, as in recent years, France was also by far the country reporting the most FBOs of this type in the EU (611 outbreaks; 90.0% of all FBOs caused by bacterial toxins). In France, three different major FBOs caused by bacterial toxins (unspecified) led to 708 cases and 97 hospitalisations following the consumption of food in ‘Canteen or catering at workplace, school, etc.’. All the seven deaths in cases of FBOs caused by bacterial toxins took place in France and involved people living in ‘Health care and residential facilities’.
Although the number of FBOs involving bacterial toxins increased in 2021 (152 FBOs more than in 2020), it was still lower on average than for the period 2017–2019 (242 fewer FBOs; a 26.3% relative fall compared with 2017–2019).
In 2021, information on the toxigenic bacteria involved in FBOs was available only for 195 outbreaks (28.0% of FBOs caused by bacterial toxins). Although FBOs implicating Bacillus cereus toxins were associated with the highest number of FBOs among bacterial toxins, Clostridium perfringens toxins caused the highest number of cases and deaths and Staphylococcus aureus toxins the highest number of hospitalisations (Table 62). S. aureus toxins were the leading causative agent of FBOs in Portugal, Romania and Republic of North Macedonia. The number of FBOs caused by Clostridium botulinum toxins did not substantially change, compared with recent years, with two fewer outbreaks being reported in 2021, compared with 2020.
4.2.3. Viruses
Norovirus (and other calicivirus)
In 2021, norovirus (and other calicivirus) were the third most frequently reported agent causing FBOs in the EU (Table 62), and were reported by 14 MSs (Belgium, Czechia, Denmark, Finland, France, Germany, Ireland, Italy, Latvia, the Netherlands, Poland, Portugal, Spain, Sweden) and two non‐MSs (Norway, Switzerland) (Figure 32). Norovirus (and other calicivirus) were the leading causative agent in six MSs (Belgium, Czechia, Denmark, Finland, Latvia, Sweden). France contributed the most to the total number of FBOs with 112 outbreaks (43.6% of all FBOs caused by norovirus (and other calicivirus)). In 2021, the FBOs caused by norovirus had the highest mean outbreak size (cases per outbreak) with MSs reporting 24 large (≥ 50 cases) and 13 very large (≥ 100 cases) outbreaks.
At EU level, although the reporting of FBOs caused by norovirus (and other calicivirus) increased last year compared with 2020 (121 FBOs more than 2020), the number of FBOs was still below that of the pre‐pandemic years 2017–2019, on average (107 fewer outbreaks; a 29.9% relative decrease). The number of cases of illness, hospitalisations and deaths followed a similar downward trend.
Hepatitis A
Overall, six MSs (Czechia, Finland, Germany, the Netherlands, Poland, Sweden) notified EFSA of FBOs caused by hepatitis A in 2021 (Figure 32). None of the FBOs were strong‐evidence outbreaks (Table 62). One strong‐evidence outbreak was reported by Norway, involving 20 cases. This outbreak was associated with the consumption of ‘fruit, berries and juices and other products thereof’. Czechia reported a major weak‐evidence outbreak of hepatitis A, involving 199 cases, almost all of which required hospitalisation (N = 195). Mixed food was the suspected food vehicle. Other statistics on hepatitis A FBOs are shown in Table 62.
Other viruses
Within the EU, FBOs caused by hepatitis E virus have been reported continuously since the first report in 2017, albeit in small numbers. In 2021, hepatitis E FBOs were reported from Belgium and Switzerland (Figure 32). The Swiss FBO was the largest ever reported to EFSA (105 cases) and was particularly severe in terms of the number of hospitalisations (N = 29) and deaths (N = 2). The outbreak was declared to be linked to the hepatitis E virus subtype 3 h, which is highly prevalent in the Swiss pig population (Office fédérel de la santé publique, 2022). The FBO was classified as a weak‐evidence outbreak and no information on the suspect food vehicle was provided.
Of the other viruses reported in FBOs in 2021 (Table 62), Slovakia reported a single strong‐evidence FBO caused by the tick‐borne encephalitis virus, associated with the consumption of raw goat's milk, while Poland reported one FBO caused by adenovirus.
4.2.4. Parasites
Trichinella
The number of countries reporting FBOs caused by Trichinella, as well as the total number of outbreaks and cases of trichinellosis has fallen steadily across the EU in the last 10 years, reaching the lowest value ever reported in 2021, with only one FBO and two cases (Table 62) reported by France. The outbreak was a weak‐evidence FBO and neither information on the Trichinella species nor details on the implicated ‘meat and meat product’ was available.
Cryptosporidium
FBOs caused by Cryptosporidium were reported by two MSs (Malta and Sweden) in 2021, and accounted for two outbreaks (Table 62 and Figure 32). The outbreak notified by Sweden was the largest (23 cases) and was a strong‐evidence FBO associated with the consumption of kale, a food that has been recurrently reported in various outbreaks in Sweden over the past year. The species involved was C. parvum . Kale is a cruciferous vegetable belonging to the Brassicaceae family (Šamec et al., 2019), which has gained great popularity in Europe due its nutritional properties, cheapness and high availability on the market (Kapusta‐Duch et al., 2011; Šamec et al., 2019).
Other parasites
All FBOs caused by other parasites in 2021 (Table 62) were associated with Giardia intestinalis (lamblia) and were reported by Poland. No detail on the suspect food vehicles was available.
4.2.5. Other causative agents
This group of outbreaks includes mainly ‘histamine and scombrotoxin’, ‘marine biotoxins’, ‘mushroom toxins’ and a few other chemical agents of biological origin that may accidentally contaminate food or its ingredients (Table 62). The data collected on FBOs caused by ‘other causative agents’ are the least harmonised among MSs, since these agents are not regularly covered by national outbreak surveillance programmes. This type of food poisoning is therefore likely to be underestimated at EU level.
In 2021, FBOs caused by ‘histamine and scombrotoxin’ were reported by six EU MSs (Finland, France, Germany, Italy, Spain, Sweden) (Figure 32). Most of the cases concerned France (33 outbreaks; 70.2%). No substantial variations in reporting were observed, compared with recent years. ‘Fish and fish products’ were the food implicated in all strong‐evidence FBOs caused by ‘histamine and scombrotoxin’, except one, which was linked to the consumption of ‘cheese'. Fish and fish products and ripened cheeses are considered the most common sources of histamine intoxication (EFSA BIOHAZ Panel, 2011).
Two MSs (France and Spain) reported FBOs caused by marine biotoxins (Figure 32), which are mainly produced by algae or phytoplankton accumulating in fish and filter‐feeding molluscan shellfish. Ciguatoxin was responsible for eight FBOs (nine in 2020 and 26 over the period 2017–2019, on average). The increasing threat of ciguatoxin has been highlighted by a recent publication under the EuroCigua Project, as findings confirmed the presence of toxin‐producing microalgae (e.g. Gambierdiscus) and fish with ciguatoxins in Europe (Canals et al., 2021).
In 2021, mushroom toxin poisoning was reported as the cause of FBOs by three MSs (Italy, Poland, Spain) and by Switzerland (Table 62 and Figure 32).
Two weak‐evidence FBOs caused by lectin poisoning were reported in 2021, by Denmark, while one strong‐evidence FBO associated with atropine was notified by Italy. This last outbreak was linked to the consumption of vegetables. Atropine poisoning can occur following incidental ingestion of contaminated food if toxic plants enter the food chain during harvest or processing (Adamse et al., 2014).
4.2.6. Outbreaks caused by unknown/unspecified agents
In 2021, FBOs of unknown aetiology (Table 62) accounted for 45.7% of all outbreaks in the EU, and were reported by 18 MSs and six non‐MSs (Figure 32). In six MSs, unknown agents were the most frequent category of causative agents of FBOs. These countries were: the Netherlands (N = 810; 96.7% of all FBOs reported by this country), Belgium (N = 512; 93.6%), Portugal (N = 10; 58.8%), Hungary (N = 9; 47.4%), Italy (N = 35; 37.2%) and Sweden (N = 12; 24.5%).
FBOs of unknown aetiology tend to be small outbreaks, supporting the hypothesis that these outbreaks occur mainly in confined environments such as domestic settings or small groups, where it is relatively easy to identify a link between cases. However, 22 large (≥ 50 cases) FBOs were reported by 11 MSs (Croatia, Czechia, Denmark, France, Hungary, Italy, the Netherlands, Poland, Portugal, Spain, Sweden). Several reasons may explain the reporting of unknown/unspecified agents, including the late reporting of illness, failure to detect the causative agents in patients or food, the unavailability of clinical or food samples (e.g. leftovers), etc.
Short‐term relative variations (%) in the number of FBOs reported in 2021 compared with 2020 and the pre‐pandemic period (2017–2019), by causative agents and by countries, are shown in the ‘Addendum foodborne outbreaks, 2021’, published on the EFSA Knowledge Junction on Zenodo here, in sections 4 and 5.
4.3. Overview of food vehicles implicated in foodborne outbreaks
The description of the food vehicles most frequently implicated in FBOs provides useful indications on the sources to be targeted by control policies, either at primary production level or in the various food preparation sectors, in order to reduce the public health impact of FBOs.
4.3.1. Food vehicles implicated in strong‐evidence outbreaks
The summary statistics described in this section refer only to strong‐evidence FBOs, in order to minimise uncertainty around the reported findings. In 2021, 355 strong‐evidence FBOs (8.9% of total FBOs) were reported by 21 MSs (all MSs except Bulgaria, Cyprus, Estonia, Ireland, Latvia, Slovenia). In addition, 26 strong‐evidence FBOs were reported by five non‐MSs (Montenegro, Norway, Republic of North Macedonia, Serbia, Switzerland). Details of the food vehicles implicated in strong‐evidence FBOs in MSs and non‐MSs can be interactively searched using the EFSA dashboard (available here, see page ‘food vehicles’ for information on the FBOs per implicated food vehicle).
Table 63 describes the food vehicles implicated in the FBOs reported by MSs in 2021. Overall, three MSs (France, Spain, Poland) accounted for 55.2% of all strong‐evidence FBOs (N = 196) reported in the EU. The remaining outbreaks (N = 159) were notified by 18 MSs.
Food of animal origin
In 2021, FBOs in the EU were mainly associated with the consumption of foods of animal origin (i.e. ‘meat and meat products’, ‘fish and fishery products’, ‘eggs and egg products’ and ‘milk and milk products’). Foods of animal origin were implicated in 202 outbreaks (56.9% of all strong‐evidence FBOs), 2,221 cases (31.7%), 316 hospitalisations (42.6%) and 11 deaths (84.6%).
‘Meat and meat products’ were the most frequently reported food group in 2021 (Table 63). Sixteen MSs (Austria, Czechia, Denmark, Finland, France, Germany, Greece, Hungary, Italy, Lithuania, Malta, the Netherlands, Poland, Slovakia, Spain, Sweden) and four non‐MSs (Norway, Republic of North Macedonia, Serbia, Switzerland) notified EFSA of FBOs associated with this food. ‘Pig meat and products thereof’ ranked first among all the items included in this group. The causative agents associated with this vehicle were Salmonella (14 FBOs caused by S. Typhimurium monophasic, S. Enteritidis, S. Bovismorbificans, Salmonella unspecified), Clostridium perfringens toxins (three FBOs), Bacillus cereus toxins, Staphylococcus aureus toxins, Yersinia and unspecified bacterial toxins (one FBO each). In one FBO, the agent was unknown. ‘Broiler meat (Gallus gallus) and products thereof’ were implicated in more FBOs than in 2020 (14 FBOs more than in 2020) but fewer than the mean total of FBOs reported for the period 2017–2019 (5 FBOs fewer). Salmonella (10 FBOs including S. Enteritidis, S. Oranienburg, S. Typhimurium and Salmonella unspecified), Campylobacter (seven FBOs), Staphylococcus aureus toxins (two FBOs), Clostridium perfringens toxins and Listeria monocytogenes (one FBO each) were the agents identified in these FBOs. In 2021, ‘broiler meat (Gallus gallus) and products thereof’ ranked first among the food vehicles implicated in FBOs caused by Campylobacter. The number of FBOs associated with ‘bovine meat and products thereof’ was the highest for the last 5 years (seven FBOs more than in 2020 and two more than in the period 2017–2019, on average). The causative agents of these FBOs included Clostridium perfringens toxins (five FBOs), Campylobacter (three FBOs), Salmonella (three FBOs including S. Enteritidis and Salmonella unspecified) and STEC O157 (two FBOs). In 2021, ‘bovine meat and products thereof’ ranked first among the food vehicles implicated in FBOs caused by STEC. ‘Other, mixed and/or unspecified poultry meat and products thereof’ and ‘other or mixed red meat and products thereof’ were each identified as the implicated vehicle in two FBOs, caused by Campylobacter (two FBOs), Listeria monocytogenes and Staphylococcus aureus toxins.
In 2021, ‘fish and fishery products’ were reported by 10 MSs (Croatia, Denmark, Finland, France, Germany, Italy, the Netherlands, Poland, Spain, Sweden) (Table 63). The number of FBOs associated with this foodstuff was lower than in 2020 (10 FBOs fewer) and also lower than the mean total of FBOs for the period 2017–2019 (83 FBOs fewer). Among the foods included in this group, ‘fish and fish products’ were the fourth most frequently reported vehicles in strong‐evidence FBOs in the EU, with notifications by nine MSs (Denmark, Finland, France, Germany, Italy, the Netherlands, Poland, Spain, Sweden). Note that ‘fish and fish products’ had a considerable health impact in 2021, leading to 190 cases, 41 hospitalisations and four deaths, the highest number of deaths in strong‐evidence outbreaks. The agents implicated in these FBOs were histamine and scombrotoxin (14 FBOs), marine biotoxins (five FBOs), Listeria monocytogenes (four FBOs), unspecified bacterial toxins (three FBOs), Bacillus cereus toxins, Clostridium botulinum toxins, Salmonella and Staphylococcus aureus toxins (one FBO each). All the deaths concerned two listeriosis FBOs identified in the Netherlands and associated with the consumption of different smoked fishes (smoked salmon, eel and mackerel). In 2021, ‘fish and fish products’ ranked first among the food vehicles implicated in FBOs caused by Listeria monocytogens, histamine and scombrotoxin and marine biotoxins. ‘Crustaceans, shellfish, molluscs and products thereof’, were implicated in FBOs reported by five MSs (Croatia, France, Italy, Spain, Sweden). Norovirus (and other calicivirus) were the agents most frequently reported in these FBOs (19 FBOs), followed by Bacillus cereus toxins, Staphylococcus aureus toxins and Vibrio parahaemolyticus (one outbreak each). In three FBOs, the agent was unknown. In 2021, ‘crustaceans, shellfish, molluscs and products thereof’ ranked first among the food vehicles implicated in FBOs caused by norovirus (and other caliciviruses).
‘Eggs and egg products’ were the second food vehicle group most frequently implicated in FBOs in the EU in 2021 (Table 63), reported by eight MSs (Denmark, France, Hungary, Lithuania, Poland, Slovakia, Spain, Sweden) and one non‐MS (Switzerland). Almost all the FBOs linked to ‘eggs and egg products’, were associated with Salmonella (39 outbreaks, including S. Enteritidis, S. Typhimurium, S. Oranienburg, S. Infantis and Salmonella unspecified). One FBO was caused by norovirus and involved patients in a ‘residential institution (nursing home or prison or boarding school)’, causing one death. For two FBOs the causative agent was unknown. In 2021, ‘eggs and egg products’ ranked first among the food vehicle implicated in FBOs caused by Salmonella spp., S. Typhimurium including the monophasic variant.
‘Milk and milk products’ were reported by seven MSs (Finland, France, Germany, Lithuania, Poland, Slovakia, Spain) and one non‐MS (Norway). The number of FBOs notified to EFSA by MSs concerning the consumption of ‘milk and milk products’ was higher than in 2020 (12 FBOs more) but lower on average than during the period 2017–2019, (8 FBOs fewer). This increase can be attributed to the reporting of more FBOs associated with the consumption of ‘cheese' in 2021 than in 2020 (14 more FBOs). The number of FBOs associated with the consumption of ‘cheese' reported in 2021 was even higher than in the pre‐pandemic years on average (5 more FBOs). The causative agents of FBOs linked to cheese included Salmonella (eight FBOs, including S. Enteritidis and Salmonella unspecified), Staphylococcus aureus toxins (four FBOs), histamine and unspecified bacterial toxins (one FBO each). For four FBOs, the information was not available. FBOs caused by ‘dairy products’ were also associated with Salmonella (S. Enteritidis) and Staphylococcus aureus toxins. In addition, one severe FBO caused by Cronobacter sakazakii in contaminated probiotic formula caused 119 human cases, seven hospitalisations and one death in Germany. Among the FBOs linked to the consumption of ‘milk’, three FBOs were associated with raw milk from cows (two FBOs) and from goats (one FBO), contaminated with either Campylobacter, STEC or tick‐borne encephalitis virus (one outbreak each), consumed on the farm or in a domestic setting.
Food of non‐animal origin
In 2021, FBOs associated with the consumption of foods of non‐animal origin were reported by 14 MSs (Austria, Belgium, Denmark, Finland, France, Germany, Hungary, Italy, Lithuania, Luxembourg, the Netherlands, Poland, Spain, Sweden) and two non‐MSs (Switzerland, Norway). In 2021, the number of FBOs associated with foods of non‐animal origin doubled compared with 2020 (22 FBOs more), almost reaching the mean annual total reported during the period 2017–2019 (5 FBOs fewer, on average) (Table 63). This increase was mainly driven by FBOs implicating ‘vegetables and juices and other products thereof’. This food group was associated with the largest variety of causative agents. Among the bacteria: Salmonella (11 FBOs, including S. Braenderup, S. Typhimurium, S. Enteritidis, S. Coeln, S. Kedougou and Salmonella unspecified), Enteroinvasive E. coli (EIEC) (two FBOs), Shiga toxin‐producing E. coli (one FBO) and Yersinia enterocolitica (two FBOs), Enterotoxigenic E. coli (ETEC) (one FBO); bacterial toxins including Bacillus cereus (two FBOs), Clostridium botulinum (two FBOs), Staphylococcus aureus (one FBO) and bacterial toxins, unspecified (two FBOs); mushroom toxins (four FBOs); viruses including Norovirus (and other calicivirus) (three FBOs): parasite: Cryptosporidium parvum (one FBO). Nine large (≥ 50 cases) or very large (≥ 100 cases) FBOs associated with ‘vegetables and juices and other products thereof’ were reported in 2021 in the EU. This explains why the mean size of the FBOs linked to this foodstuff (50 cases/outbreak) was one of the highest, outnumbering the FBOs associated with foods of animal origin (11 cases/outbreak). Multiple mechanisms leading to the primary contamination of crops and the cross‐contamination of vegetables during processing have been described in literature. Pre‐cut vegetables were implicated in five FBOs, all reported by Finland. Of this total, one was associated with S. Typhimurium, causing 728 cases. Of the single outbreaks linked to this product in 2021, we should mention the large FBO associated with ‘Alfalfa sprouts' and caused by S. Coeln in Sweden. ‘Galia melons' imported from Honduras were responsible for a large multi‐country outbreak of infections by S. Braenderup (see Section 4.8. ECDC and EFSA rapid outbreak assessment of multi‐country foodborne outbreaks). Other foods of non‐animal origin implicated in FBOs in 2021 were ‘cereal products including rice and seeds/pulses (nuts, almonds)’, reported in outbreaks caused mainly by Bacillus cereus (four FBOs), Salmonella, unspecified (three FBOs), Clostridium botulinum (one FBO) and unspecified bacterial toxins (one FBO, each) and ‘fruit, berries and juices and other products thereof’, reported in outbreaks caused by Salmonella unspecified and Yersinia enterocolitica. In 2021, among non‐MSs, Switzerland reported one large general outbreak caused by norovirus and associated with the consumption of berries and small fruits.
Composite foods, multi‐ingredient foods and other foods
This food group includes foods resulting from the assembly of multiple ingredients, or highly processed or manipulated foods. A range of foodstuffs belong to this category, namely ‘bakery products’, ‘buffet meals’, ‘mixed foods’, ‘sweets and chocolate' and ‘other foods’. Eighteen MSs and four non‐MSs (Switzerland, Republic of North Macedonia, Montenegro, Norway) reported FBOs associated with ‘composite foods, multi‐ingredient foods and other’. The number of FBOs reported was higher than in 2020 (50 FBOs more) but still lower than the total mean reported annually during the period 2017–2019 (85 fewer on average). As was the case for foods of non‐animal origin, the mean size of FBOs implicating this food group was 26 cases/outbreak, larger than those associated with foods of animal origin.
In 2021, ‘mixed foods’ were the foodstuffs most frequently reported in FBOs. Food items in this group are highly heterogeneous. Details are provided in (Table 63). Many different causative agents were linked to ‘mixed foods’, mainly bacteria: Salmonella (24 FBOs, including S. Enteritidis, S. Typhimurium, S. Typhimurium monophasic, S. Corvallis, S. Bareilly, Salmonella, unspecified), Campylobacter (five FBOs), Aeromonas, E. coli unspecified, Enterotoxigenic E. coli (ETEC), Shigella, non‐toxigenic Vibrio cholerae (one FBO, each); viruses: norovirus (13 FBOs); bacterial toxins: Bacillus cereus (six FBOs), Clostridium perfringens (seven FBOs), Staphylococcus aureus (seven FBOs) and unspecified (five FBOs). For five FBOs, the causative agent was unknown. Interestingly, the majority of FBOs were characterised as general outbreaks, accounting for 80.3% of all FBOs caused by ‘mixed foods’. In 2021, mixed foods' ranked first among the food vehicles implicated in FBOs caused by S. Enteritidis, Bacillus cereus toxins, Staphylococcus aureus toxins and Clostridium perfringens toxins.
‘Bakery products’ were reported in FBOs mainly caused by Salmonella (15 FBOs, including S. Enteritidis and Salmonella, unknown). Norovirus (and other calicivirus) and unknown agents were also reported (one and two FBOs, respectively). ‘Buffet meals’ were associated with Salmonella (two FBOs, including S. Typhimurium monophasic, S. Enteritidis), norovirus (two FBOs) and Campylobacter (one FBO). Finally, ‘sweets and chocolate' led to two FBOs caused by S. Enteritidis, while ‘other foods’ were linked to FBOs caused by S. Enteritidis (one FBO) and Clostridium perfringens toxins (two FBOs) and an unknown agent (three FBOs).
For more information concerning the causative agent associated with the consumption of different types of food implicated in strong‐evidence FBOs, see the dashboard (available here, see page ‘Food vehicles and causative agents’ for information on FBOs by food vehicle and by causative agent).
4.3.2. Food vehicles implicated in weak‐evidence outbreaks
In 2021, a total of 1,873 weak‐evidence FBOs with a suspected food vehicle (46.8% of all FBOs) were reported by 20 MSs. No major discrepancies were observed in the ranking of food types for weak‐evidence outbreaks, compared with strong‐evidence outbreaks, in terms of causative agents, with a few exceptions. One of the main discrepancies observed in 2021 concerned STEC FBOs. Although ‘bovine meat and products thereof’ was the main food category reported in strong‐evidence outbreaks (two strong‐evidence outbreaks, one weak‐evidence outbreak), ‘water’ was actually the most frequently suspected source, even though the evidence is weak (four weak‐evidence outbreaks). This situation is consistent with the observations of previous years within the EU, and once again draws attention to environmental pathways in the transmission of STEC infections to humans.
Information regarding the foodstuffs implicated in strong‐ and weak‐evidence foodborne outbreaks by reporting MSs in 2021, for each causative agent, are available on the dashboard (available here, see page ‘Food vehicles and causative agents’ for information on FBOs by food vehicle and by causative agent).
4.3.3. Top‐10 agent/food pairs in strong‐evidence outbreaks associated with the highest impact on health in the EU, 2021
Tables 64, 65, 66, 67–64, 65, 66, 67 show the top‐10 pairs of causative agents and food vehicles for the strong‐evidence outbreaks with the highest health impact in 2021 in the EU, in terms of total outbreaks, cases, hospitalisations and deaths, respectively. The number of MSs that reported FBOs implicating each food/agent pair is also included in the tables, indicating how common these types of outbreaks were in EU MSs. When looking at the tables, it is important to remember that the ranking of the pairs may be influenced by the scale of the contribution made by each MS to data collection. The same information is provided for the 2017–2019 period, for trend watching purposes.
Table 64.
Top 10 pathogen/food vehicle pairs causing the highest number of strong‐evidence outbreaks in reporting EU MSs, 2021
| 2021 (a) | 2017–2020 (b) | Evaluation | ||||||
|---|---|---|---|---|---|---|---|---|
| Rank (c) | Causative agent | Food vehicle | Outbreaks (N) | Reporting MSs (N outbreaks) | Rank (c) | Outbreaks (N/year) (range) (d) | Reporting MSs (N/year) (d) | 2021 vs. 2017‐2020 (e) |
| 1 | Salmonella | Eggs and egg products (f) | 39 | Poland (13), Spain (12), France (8), Sweden (2), Slovakia (1), Hungary (1), Denmark (1), Lithuania (1) | 1 | 97.2 (37–135) | 10.0 | ↓↓ |
| 2 | Salmonella | Mixed foods (g) | 24 | Slovakia (10), Poland (3), Finland (3), Spain (3), Belgium (1), Denmark (1), Malta (1), Germany (1), Italy (1) | 5 | 22.2 (3–34) | 7.7 | – |
| 3 | Norovirus (and other calicivirus) | Crustaceans, shellfish, molluscs and products thereof (h) | 19 | France (15), Sweden (2), Spain (1), Italy (1) | 2 | 55.7 (8–144) | 5.75 | ↓↓ |
| 4 | Salmonella | Bakery products (i) | 15 | Poland (7), Spain (3), Romania (1), Slovakia (1), Malta (1), Italy (1), Czechia (1) | 3 | 28.7 (9–45) | 2.7 | ↓ |
| 5 | Histamine and scombrotoxin | Fish and fish products (j) | 14 | Sweden (5), France (4), Italy (3), Finland (1), Germany (1) | 4 | 28.2 (14–55) | 7.2 | ↓↓ |
| 5 | Salmonella | Pig meat and products thereof (k) | 14 | France (9), Germany (3), Italy (2) | 6 | 16.5 (11–26) | 7.7 | ‐ |
| 6 | Norovirus (and other calicivirus) | Mixed foods (l) | 13 | Finland (3), France (3), Spain (2), Sweden (2), Portugal (1), Belgium (1), Italy (1) | 11 | 8.5 (5–11) | 4.5 | ↑↑ |
| 7 | Salmonella | Vegetables and juices and other products thereof (m) | 11 | Finland (3), Belgium (1), Netherlands (1), Luxembourg (1), Denmark (1), Spain (1), Sweden (1), Austria (1), Germany (1) | 29 | 4.0 (1–6) | 3.0 | ↑↑ |
| 8 | Salmonella | Broiler meat (Gallus gallus) and products thereof | 10 | Spain (3), Poland (2), France (2), Sweden (1), Hungary (1), Malta (1) | 16 | 7.0 (2–13) | 3.7 | ↑ |
| 9 | Salmonella | Cheese | 8 | France (7), Poland (1) | 26 | 4.2 (1–8) | 1.5 | ↑↑ |
: Data on FBOs from the United Kingdom (Northern Ireland) are taken into account for 2021. In accordance with the agreement on the withdrawal of the United Kingdom from the EU, and in particular with the Protocol on Ireland/Northern Ireland, the EU requirements on data sampling are also applicable to Northern Ireland.
: Data on FBOs from the United Kingdom are taken into account for 2017–2019, because the United Kingdom was an EU MS, but it became a third country on 1 February 2020.
: Ranking of the food vehicle based on the number of strong‐evidence FBOs in which the combination (causative agent/food vehicle) was implicated (rank 1 is the highest rank, meaning the most commonly implicated). Strong‐evidence outbreaks with unknown causative agents are not included.
: Outbreaks reported by the United Kingdom are also included.
: A single arrow indicates variations of 25% and 50% in the number of outbreaks; double arrows indicate variations > 50%; a ‘stable' value indicates variations of between −25% and +25%.
: ‘Eggs and egg products’ include ‘Eggs’, ‘Eggs and egg products’.
: ‘Mixed food’ includes ‘Foodstuffs intended for special nutritional uses – dietary foods for special medical purposes’, ‘Mixed food’, ‘Other processed food products and prepared dishes’, ‘Other processed food products and prepared dishes – fish and seafood‐based dishes’, ‘Other processed food products and prepared dishes – pasta’, ‘Other processed food products and prepared dishes – sushi’, ‘Sauce and dressings – mayonnaise'.
: ‘Crustaceans, shellfish, molluscs and products thereof’ includes ‘Crustaceans, shellfish, molluscs and products thereof’, ‘Molluscan shellfish’.
: ‘Bakery products’ include ‘Bakery products’, ‘Bakery products – cakes’, ‘Bakery products – cakes – containing heat‐treated cream’, ‘Bakery products – desserts – containing raw cream’, ‘Bakery products – desserts – containing raw eggs’.
: ‘Fish and fish products’ include ‘Fish’, ‘Fish – cooked’, ‘Fish – Fishery products from fish species associated with a high amount of histidine – not enzyme maturated’, ‘Fish – raw’, ‘Fish and fish products’.
: ‘Pig meat and products thereof’ includes ‘Meat from pig – meat products’, ‘Meat from pig – meat products – ready‐to‐eat’, ‘Meat from pig – offal’, ‘Pig meat and products thereof’.
: ‘Mixed food’ includes ‘Mixed food’, ‘Other processed food products and prepared dishes’, ‘Other processed food products and prepared dishes – sushi’, ‘Ready‐to‐eat salads’.
: ‘Vegetables and juices and other products thereof’ include ‘Alfalfa sprouts’, ‘Melons (except watermelon)’, ‘Vegetables – pre‐cut’, ‘Vegetables and juices and other products thereof.
Table 65.
Top‐10 pathogen/food vehicle pairs causing the highest number of cases in strong‐evidence outbreaks in reporting EU MSs, 2021
| 2021 (a) | 2017–2020 (b) | Evaluation | ||||||
|---|---|---|---|---|---|---|---|---|
| Rank (c) | Causative agent | Food vehicle | Cases (N) | Reporting MSs (N cases) | Rank (c) | Cases (N/year) (range) (d) | Reporting MSs (N/year) (d) | 2021 vs. 2017–2020 (e) |
| 1 | Salmonella | Vegetables and juices and other products thereof (f) | 1,103 | Finland (797), Germany (82), Belgium (55), Sweden (53), Denmark (41), Netherlands (37), Spain (24), Austria (11), Luxembourg (3) | 24 | 108.2 (7–244) | 3.0 | ↑↑ |
| 2 | Norovirus (and other calicivirus) | Mixed foods (g) | 487 | France (227), Portugal (68), Italy (62), Finland (49), Sweden (44), Spain (31), Belgium (6) | 7 | 377.7 (223–735) | 4.5 | ↑ |
| 3 | Salmonella | Mixed foods (h) | 486 | Slovakia (187), Spain (134), Denmark (52), Italy (37), Finland (25), Germany (19), Malta (19), Poland (9), Belgium (4) | 2 | 777.8 (91–1,595) | 8.0 | ↓ |
| 4 | Salmonella | Eggs and egg products (i) | 403 | France (228), Poland (60), Spain (59), Denmark (26), Sweden (14), Hungary (9), Slovakia (5), Lithuania (2) | 1 | 1,140.8 (303–1,989) | 10.0 | ↓↓ |
| 5 | Norovirus (and other calicivirus) | Vegetables and juices and other products thereof (j) | 263 | France (118), Germany (98), Poland (47) | 14 | 227.0 (151–332) | 3.0 | – |
| 6 | Salmonella | Pig meat and products thereof (k) | 236 | France (104), Italy (67), Germany (65) | 12 | 235.3 (69–341) | 7.8 | – |
| 7 | Salmonella | Bakery products (l) | 178 | Poland (110), Czechia (29), Spain (14), Slovakia (14), Malta (4), Romania (4), Italy (3) | 8 | 352.5 (56–621) | 3.3 | ↓ |
| 8 | Staphylococcus aureus toxins | Mixed foods (m) | 166 | Portugal (93), Romania (44), Spain (15), Denmark (11), Italy (3) | 25 | 107.0 (3–212) | 4.5 | ↑↑ |
| 9 | Clostridium perfringens toxins | Mixed foods (n) | 161 | Italy (69), France (39), Portugal (20), Germany (15), Finland (12), Denmark (6) | 6 | 380.8 (292–507) | 5.3 | ↓↓ |
| 10 | Norovirus (and other calicivirus) | Crustaceans, shellfish, molluscs and products thereof (o) | 147 | France (53), Italy (49), Sweden (37), Spain (8) | 4 | 658.0 (104–1,152) | 5.8 | ↓↓ |
: Data on FBOs from the United Kingdom (Northern Ireland) are taken into account for 2021. In accordance with the agreement on the withdrawal of the United Kingdom from the EU, and in particular with the Protocol on Ireland/Northern Ireland, the EU requirements on data sampling are also applicable to Northern Ireland.
: Data on FBOs from the United Kingdom are taken into account for 2017–2019, because the United Kingdom was an EU MS, but it became a third country on 1 February 2020.
: Ranking of the food vehicle based on the number of strong‐evidence FBOs in which the combination (causative agent/food vehicle) was implicated (rank 1 is the highest rank meaning the most commonly implicated). Strong‐evidence outbreaks with unknown causative agents are not included.
: Outbreaks reported by the United Kingdom are also included.
: A single arrow indicates variations of 25% and 50% in the number of outbreaks; double arrows indicate variations > 50%; a ‘stable' value indicates variations of between −25% and +25%.
: ‘Vegetables and juices and other products thereof’ include ‘Alfalfa sprouts’, ‘Melons (except watermelon)’, ‘Vegetables – pre‐cut’, ‘Vegetables and juices and other products thereof’.
: ‘Mixed food’ includes ‘Mixed food’, ‘Other processed food products and prepared dishes’, ‘Other processed food products and prepared dishes – sushi’, ‘Ready‐to‐eat salads’.
: ‘Mixed food’ includes ‘Foodstuffs intended for special nutritional uses – dietary foods for special medical purposes’, ‘Mixed food’, ‘Other processed food products and prepared dishes’, ‘Other processed food products and prepared dishes – fish and seafood‐based dishes’, ‘Other processed food products and prepared dishes – pasta’, ‘Other processed food products and prepared dishes – sushi’, ‘Sauce and dressings – mayonnaise'.
: ‘Eggs and egg products’ include ‘Eggs’, ‘Eggs and egg products’.
: ‘Vegetables and juices and other products thereof’ include ‘Lettuce', ‘Vegetables and juices and other products thereof’.
: ‘Pig meat and products thereof’ includes ‘Meat from pig – meat products’, ‘Meat from pig – meat products – ready‐to‐eat’, ‘Meat from pig – offal’, ‘Pig meat and products thereof’.
: ‘Bakery products’ include ‘Bakery products’, ‘Bakery products – cakes’, ‘Bakery products – cakes – containing heat‐treated cream’, ‘Bakery products – desserts – containing raw cream’, ‘Bakery products – desserts – containing raw eggs’.
: ‘Mixed food’ includes ‘Mixed food’, ‘Other processed food products and prepared dishes’, ‘Other processed food products and prepared dishes – meat‐based dishes’, ‘Other processed food products and prepared dishes – mushroom‐based dishes’.
: ‘Mixed food’ includes ‘Mixed food’, ‘Other processed food products and prepared dishes’, ‘Other processed food products and prepared dishes – unspecified’, ‘Sauce and dressings’, ‘Soups’.
: ‘Crustaceans, shellfish, molluscs and products thereof’ includes ‘Crustaceans, shellfish, molluscs and products thereof’, ‘Molluscan shellfish’.
Table 66.
Top‐10 pathogen/food vehicle pairs causing the highest number of hospitalisations in strong‐evidence outbreaks in reporting EU MSs, 2021
| 2021 (a) | 2017–2020 (b) | Evaluation | ||||||
|---|---|---|---|---|---|---|---|---|
| Rank (c) | Causative agent | Food vehicle | Hospitalisations (N) | Reporting MSs (N hospitalisations) | Rank (c) | Hospitalisations (N/year) (range) (d) | Reporting MSs (N/year) (d) | 2021 vs 2017–2020 (e) |
| 1 | Salmonella | Mixed foods (f) | 82 | Denmark (30), Slovakia (25), Spain (10), Germany (4), Finland (4), Poland (3), Italy (3), Belgium (2), Malta (1) | 2 | 100.5 (11–198) | 7.8 | – |
| 2 | Salmonella | Eggs and egg products (g) | 79 | France (24), Spain (22), Poland (18), Denmark (12), Hungary (2), Lithuania (1) | 1 | 251.5 (46–382) | 10.0 | ↓↓ |
| 3 | Salmonella | Vegetables and juices and other products thereof (h) | 75 | Germany (30), Denmark (19), Belgium (17), Austria (7), Finland (2) | 35 | 7.0 (2–12) | 3.0 | ↑↑ |
| 4 | Salmonella | Bakery products (i) | 48 | Poland (35), Slovakia (4), Romania (3), Italy (3), Czechia (2), Spain (1) | 3 | 90.5 (21–148) | 3.3 | ↓ |
| 5 | Salmonella | Pig meat and products thereof (j) | 44 | Germany (22), France (18), Italy (4) | 6 | 54.5 (22–94) | 7.8 | – |
| 6 | E. coli other than STEC | Vegetables and juices and other products thereof (k) | 35 | Denmark (24), France (11) | – | – | – | NA |
| 7 | Salmonella | Buffet meals | 33 | Lithuania (23), Austria (10) | 4 | 63.8 (3–168) | 2.0 | ↓ |
| 8 | Norovirus (and other calicivirus) | Mixed foods (l) | 32 | Portugal (24), Italy (7), Belgium (1) | 65 | 2.3 (0–6) | 1.0 | ↑↑ |
| 8 | Campylobacter | Broiler meat (Gallus gallus) and products thereof (m) | 32 | Denmark (29), Spain (3) | 61 | 2.8 (1–8) | 1.3 | ↑↑ |
| 9 | Campylobacter | Mixed foods (n) | 25 | Spain (17), Slovakia (4), Finland (2), Poland (2) | 67 | 2.0 (0–3) | 1.5 | ↑↑ |
NA: Not available.
: Data on FBOs from the United Kingdom (Northern Ireland) are taken into account for 2021. In accordance with the agreement on the withdrawal of the United Kingdom from the EU, and in particular with the Protocol on Ireland/Northern Ireland, the EU requirements on data sampling are also applicable to Northern Ireland.
: Data on FBOs from the United Kingdom are taken into account for 2017–2019, because the United Kingdom was an EU MS, but it became a third country on 1 February 2020.
: Ranking of the food vehicle based on the number of strong‐evidence FBOs in which the combination (causative agent/food vehicle) was implicated (rank 1 is the highest rank meaning the most commonly implicated). Strong‐evidence outbreaks with unknown causative agents are not included.
: Outbreaks reported by the United Kingdom are also included.
: A single arrow indicates variations of 25% and 50% in the number of outbreaks; double arrows indicate variations > 50%; a ‘stable' value indicates variations of between −25% and +25%.
: ‘Mixed food’ includes ‘Foodstuffs intended for special nutritional uses – dietary foods for special medical purposes’, ‘Mixed food’, ‘Other processed food products and prepared dishes’, ‘Other processed food products and prepared dishes – fish and seafood‐based dishes’, ‘Other processed food products and prepared dishes – pasta’, ‘Other processed food products and prepared dishes – sushi’, ‘Sauce and dressings – mayonnaise'.
: ‘Eggs and egg products’ include ‘Eggs’, ‘Eggs and egg products’.
: ‘Vegetables and juices and other products thereof’ include ‘Alfalfa sprouts’, ‘Melons (except watermelon)’, ‘Vegetables – pre‐cut’, ‘Vegetables and juices and other products thereof’.
: ‘Bakery products’ include ‘Bakery products’, ‘Bakery products – cakes’, ‘Bakery products – cakes – containing heat‐treated cream’, ‘Bakery products – desserts – containing raw cream’, ‘Bakery products – desserts – containing raw eggs’.
: ‘Pig meat and products thereof’ includes ‘Meat from pig – meat products’, ‘Meat from pig – meat products – ready‐to‐eat’, ‘Meat from pig – offal’, ‘Pig meat and products thereof’.
: ‘Vegetables and juices and other products thereof’ include ‘Spring onion’, ‘Vegetables – pre‐cut’, ‘Vegetables and juices and other products thereof’.
: ‘Mixed food’ includes ‘Mixed food’, ‘Other processed food products and prepared dishes’, ‘Other processed food products and prepared dishes – sushi’, ‘Ready‐to‐eat salads’
: ‘Broiler meat (Gallus gallus) and products thereof’ include ‘Broiler meat (Gallus gallus) and products thereof’, ‘Meat from broilers (Gallus gallus)’.
: ‘Mixed food’ includes ‘Foodstuffs intended for special nutritional uses – other food for infants and children’, ‘Mixed food’.
Table 67.
Top seven pathogen/food vehicle pairs causing the highest number of deaths in strong‐evidence outbreaks in reporting EU MSs, 2021
| 2021 (a) | 2017–2020 (b) | Evaluation | ||||||
|---|---|---|---|---|---|---|---|---|
| Rank (c) | Causative agent | Food vehicle | Deaths (N) | Reporting MSs (N deaths) | Rank (c) | Deaths (N/year) (range) (d) | Reporting MSs (N/year) (d) | 2021 vs. 2017–2020 (e) |
| 1 | Listeria monocytogenes | Fish and fish products (f) | 4 | Netherlands (4) | 2 | 2.0 (0–8) | 0.5 | ↑↑ |
| 2 | Clostridium perfringens toxins | Pig meat and products thereof | 3 | France (3) | 22 | 0.3 (0–1) | 0.3 | ↑↑ |
| 3 | Listeria monocytogenes | Meat and meat products, unspecified | 2 | Austria (2) | 1 | 2.5 (0–9) | 0.8 | – |
| 4 | Cronobacter sakazakii | Dairy products (other than cheese) | 1 | Germany (1) | – | – | – | NA |
| 4 | Norovirus (and other calicivirus) | Eggs and egg products | 1 | France (1) | – | – | – | NA |
| 4 | Salmonella | Mixed foods | 1 | Spain (1) | 12 | 0.5 (0–1) | 0.5 | ↑↑ |
| 4 | Bacterial toxins, unspecified |
Mixed foods (g) |
1 | France (1) | – | – | – | NA |
NA: Not available.
: Data on FBOs from the United Kingdom (Northern Ireland) are taken into account for 2021. In accordance with the agreement on the withdrawal of the United Kingdom from the EU, and in particular with the Protocol on Ireland/Northern Ireland, the EU requirements on data sampling are also applicable to Northern Ireland.
: Data on FBOs from the United Kingdom are taken into account for 2017–2019, because the United Kingdom was an EU MS, but it became a third country on 1 February 2020.
: Ranking of the food vehicle based on the number of strong‐evidence FBOs in which the combination (causative agent/food vehicle) was implicated (rank 1 is the highest rank meaning the most commonly implicated). Strong‐evidence outbreaks with unknown causative agents are not included.
: Outbreaks reported by the United Kingdom are also included.
: A single arrow indicates variations of 25% and 50% in the number of outbreaks; double arrows indicate variations > 50%; a ‘stable' value indicates variations of between −25% and +25%.
: ‘Fish and fish products’ include ‘Fish – smoked’.
: ‘Mixed food’ includes ‘Other processed food products and prepared dishes’.
4.4. Overview of the places of exposure in strong‐evidence outbreaks
Table 68 describes the characteristics of strong‐evidence FBOs by place of exposure. More information regarding the places of exposures implicated in strong‐ and weak‐evidence FBOs in 2021, with data per causative agent, are available on the dashboard (available here, see page ‘Places of exposures’).
In 2021, most reported FBOs occurred on ‘domestic premises’ similarly to what has been observed in recent years. In 2021, 103 FBOs taking place in a ‘domestic setting’ were described as household FBOs (85.1% of FBOs in domestic settings). However, since not all MSs communicate data on household FBOs, which frequently occur in a domestic setting, this value is likely to be underestimated. 10 MSs (Czechia, Finland, France, Germany, Italy, the Netherlands, Poland, Romania, Slovakia, Spain) reported strong‐evidence FBOs on ‘domestic premises’. In 2021, the proportion of strong‐evidence FBOs occurring in this setting (34.1%) was the lowest since 2012. In 2020 it was 37.1% and over the period 2017–2019 this proportion stood at an average of 39.1%. Bacteria, in particular Salmonella were the causative agent most frequently reported in this context in 2021 (68 FBOs; 56.2%).
After domestic settings, ‘restaurants, pubs, street vendors, takeaway, etc.’ were the most frequently mentioned places of exposure in FBOs, with almost one in four FBOs (23.1%) in the EU occurring in these settings. The proportion was similar in 2020 (25.0%). In the pre‐pandemic years (2017–2019), FBOs in ‘restaurants, pubs, street vendors, takeaway, etc.’ accounted for 28.5% of total strong‐evidence FBOs. The main causative agents included Salmonella (24 FBOs; 29.3%), bacterial toxins (Staphylococcus aureus, Clostridium perfringens, Bacillus cereus, bacterial toxins unspecified; 17 FBOs; 20.7%), norovirus (and other calicivirus) (14 FBOs; 17.1%) and histamine (10 FBOs; 12.2%).
Most human cases arising from FBOs in 2021 were exposed to contaminated food in ‘canteen or catering at workplace, school, etc.‘(N = 2,152), especially in ‘school or kindergarten’ (97.8% of FBOs in ‘canteen or catering at workplace, school, etc.’). FBOs in ‘school or kindergarten’ saw a considerable relative increase, accounting for 11.8% of total strong‐evidence FBOs in 2021, 4.8% in 2020 and 5.5% during the period 2017–2019. It is worth remembering that many catering activities in schools and workplaces were suspended in 2020, owing to COVID‐19. Salmonella was the causative agent mostly reported in FBOs occurring in ‘school or kindergarten’ (10 FBOs, 23.8%).
A considerable increase was also observed in the proportion of FBOs in ‘Health care and residential facilities’, which accounted for 5.1% of strong‐evidence FBOs during the period 2017–2019, 7.7% in 2020 and 9.0% in 2021. Outbreaks in ‘Health care and residential facilities’ caused the highest number of deaths (N = 7; 53.8% of all deaths), mainly as a result of FBOs in a ‘residential institution (nursing home or prison or boarding school)’ (N = 6). C. perfringens, Salmonella and Norovirus (and other calicivirus) were the main causative agents.
‘Restaurant or cafe or pub or bar or hotel or catering service' was the most frequent place of exposure described for ‘general outbreaks’ (76 outbreaks, 32.2% of strong‐evidence general outbreaks).
FBOs in ‘school or kindergarten’ were the largest in terms of the mean number of cases per FBO (50.1 cases). The mean size of FBOs in ‘restaurant or cafe or pub or bar or hotel or catering service' was much smaller (14.7 mean cases).
4.5. Contributing factors in strong‐evidence foodborne outbreaks
Information on the factors contributing to food contamination or improper food preparation and storage were available for 126 FBOs reported by MSs in 2021 (35.5% of strong‐evidence FBOs). ‘Cross‐contamination’ was reported in 34 FBOs caused by Salmonella, Campylobacter, bacterial toxins, norovirus, Listeria monocytogenes, Aeromonas or non‐toxigenic Vibrio cholera. ‘Cross‐contamination’ was the most frequently reported factor in settings such as ‘canteen or catering at workplace, school, etc.’ and ‘health care and residential facilities’. The use of an ‘unprocessed contaminated ingredient’ was reported in 39 FBOs, mainly involving Salmonella, and was the main contributory factor in domestic settings. An ‘infected food handler’ is a major source of virus transmission (Teunis et al., 2015) and was identified in 26 FBOs implicating mainly norovirus and Salmonella. This was the most important factor in ‘restaurants, pubs, street vendors, takeaway, etc.’ ‘Time/temperature storage abuse' was reported in 14 FBOs caused by bacterial toxins, histamine, Salmonella and Cronobacter sakazakii. ‘Inadequate heat treatment’ and ‘Inadequate chilling’ contributed to 11 and 10 FBOs, respectively, involving bacterial toxins, Salmonella and histamine. ‘Untreated drinking water’ was linked to one Norovirus FBO.
4.6. Temporal trends by causative agent
4.6.1. Temporal trend at EU level
Figure 33 describes the number of FBOs reported in the EU over the period 2012–2021, by causative agent. Given that the collection of FBO data is not fully harmonised across the EU, and that changes in the surveillance of FBOs by MSs may have taken place over time, annual variations in the frequency distribution of the causative agents shown in Figure 33 may not necessarily reflect the true epidemiological pattern at EU level.
Figure 33.
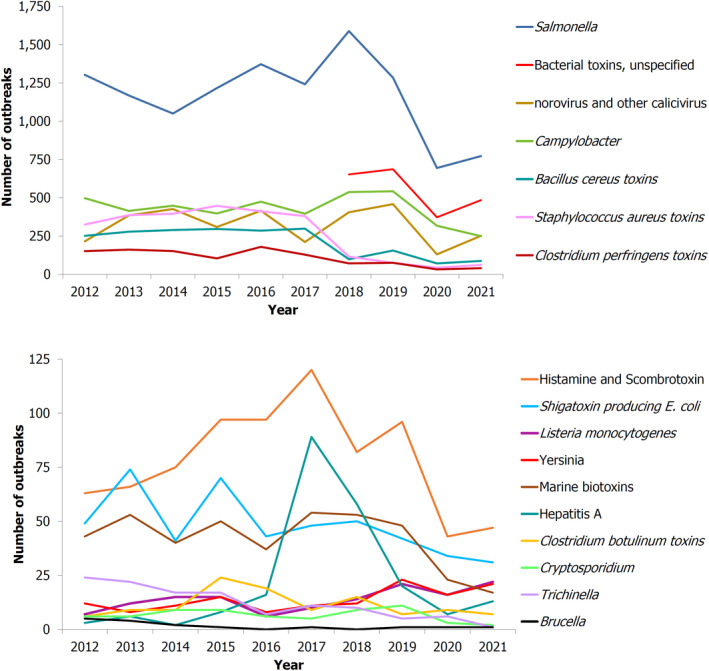
Number of foodborne outbreaks by causative agent, reported to the EU by MSs, 2012–2021
-
Note: Outbreaks reported by the United Kingdom are included for the years 2012–2019. However, data from United Kingdom (Northern Ireland) are taken into account for 2021. In accordance with the agreement on the withdrawal of the United Kingdom from the EU, and in particular with the Protocol on Ireland/Northern Ireland, the EU requirements on data sampling are also applicable to Northern Ireland.‘Marine biotoxins’ include ciguatoxin, muscle‐paralysing toxin, okadaic acid.‘Norovirus (and other calicivirus)’ include norovirus (Norwalk‐like virus), sapovirus (Sapporo‐like virus) and calicivirus unspecified.‘Other bacterial agents’ include Aeromonas, Arcobacter, Cronobacter sakazakii, E. coli other than STEC, Enterococcus, Francisella, Leptospira, Shigella, Streptococcus, non‐toxigenic Vibrio cholerae, Vibrio parahaemolyticus and other unspecified bacterial agents.
For a further interactive look into data, see the dashboard on FBOs (available here), where there is a dedicate page on time trends.
4.6.2. Country‐specific temporal trends by causative agent over the period 2012–2021
Figures on statistically significant country‐specific temporal trends by causative agent, for the period 2012–2021, are available in ‘Addendum foodborne outbreaks, 2021’ Section 6, published on the EFSA Knowledge Junction on Zenodo here.
A trend analysis of FBOs caused by Salmonella showed that three MSs (Germany, Latvia, Lithuania) reported a statistically significant decreasing number of FBOs over the period 2012–2021. For Germany and Lithuania, this trend was mainly driven by a significant decrease of S. Enteritidis FBOs over the same period. FBOs caused by S. Enteritidis also followed a statistically significant decreasing trend in three other MSs (Austria, Belgium and Hungary). A progressive fall in the number of FBOs caused by this serovar was also observed for Croatia, Czechia and Spain, even though it was not statistically significant. Findings from a study carried out in Europe as part of the DiSCoVeR project emphasised that outbreaks caused by S. Enteritidis significantly decreased in Northern and Southern Europe between 2015 and 2019, and significantly increased in Eastern Europe (Chanamé Pinedo et al., 2022). Austria reported a significant negative trend for S. Typhimurium and S. Typhimurium monophasic. For Germany and Slovakia a similar trend was also observed, although it was not statistically significant. These trends could point to the successful implementation of National Control Programmes (NCP) for Salmonella in poultry. Information on the results achieved with the NCPs in poultry by EU MSs is available in Section 2.4.4. (Salmonella in animals) of the Salmonella chapter.
In Austria, FBOs caused by Campylobacter fell significantly, while in France, figures followed an increasing trend over the same period, although the number of Campylobacter FBOs reported last year was lower than in 2020.
In France, FBOs caused by Clostridium perfringens toxins followed a statistically decreasing trend, even though annual reporting of FBOs caused by this agent has been relatively variable over the years.
In Germany, norovirus (and other calicivirus) saw a considerable decrease over the period 2012–2021.
Except in the case of Salmonella, hypotheses explaining these trends cannot be easily formulated based on the information reported to EFSA. Most of the FBOs were actually weak‐evidence outbreaks characterised by a high degree of uncertainty concerning the implicated food vehicles and the environment in which the outbreak occurred.
In the Netherlands and Switzerland, the number of FBOs of unknown origin increased significantly during the study period, while Norway reported a substantial decrease. In 2021, the FBOs reported by the Netherlands (N = 810) accounted for 20.2% of all FBOs reported in the EU, totalling more than 3.5 times the number of FBOs notified to EFSA in 2012 (N = 216). The reasons underlying increasing or decreasing trends in FBOs caused by unknown agents may reflect changes in the sensitivity of outbreak surveillance, as well as changes in laboratory sample testing and subtyping. As a result, the diversion of both human and technical resources in recent years, to fight the COVID‐19 pandemic, should be considered as a possible reason for the increase.
Information on the number of strong‐evidence outbreaks, by causative agent, over the years 2016–2021 can be viewed on the dashboard (available here).
4.6.3. Temporal trends by implicated food vehicle
Figures on statistically significant country‐specific temporal trends by food vehicle, for the period 2012–2020, are available in the ‘Addendum foodborne outbreaks, 2021’ Section 7, published on the EFSA Knowledge Junction on Zenodo here.
Few statistically significant increasing and decreasing trends were detected for food vehicles over the 2012–2021 period. Germany reported an increasing trend in FBOs associated with the consumption of ‘cereal products including rice and seeds/pulses (nuts, almonds)’. The number of FBOs involving this foodstuff in annual reports was very low (< 10 FBOs/year), making it difficult to investigate possible reasons underlying this finding.
In France, FBOs caused by the consumption of ‘fish and fish products’ saw a statistically significant decrease over the period 2012–2021. This fall was mainly linked to a reduction in the number of FBOs caused by ‘histamine and scombrotoxin’ and ‘marine biotoxins’.
Information on the number of strong‐evidence outbreaks, by food vehicle, over the years 2016–2021 can be viewed on the dashboard (available here).
4.7. Waterborne outbreaks
In 2021, seven MSs (Austria, Finland, Greece, Ireland, Italy, Spain and Sweden) reported a total of 12 waterborne outbreaks caused by the intake of ‘tap water, including well water’ (11 outbreaks) and ‘potable water’ (one outbreak). Moreover, three outbreaks were notified by two non‐MSs (Switzerland and Republic of North Macedonia) caused by ‘tap water, including well water’ (two outbreaks) and ‘water, unspecified’.
At EU level, the number of waterborne outbreaks and cases reported in 2021 decreased by 55.6% and 28.1% compared with 2020 (27 outbreaks and 278 human cases in 2020), respectively. Compared with the pre‐pandemic years, the number of outbreaks and human cases reported in 2021 were considerably lower than during the 2017–2019 period, with a relative decrease of 69.5% (39 mean outbreaks reported annually during 2017–2019) and 85.9% (1,422 cases on average, during 2017–2019) respectively. In 2021, the mean outbreak size in EU MSs was 16.7.
It is worth mentioning a large general outbreak reported in 2021 by the Republic of North Macedonia, which involved 93 human cases. No information was available on the causative agent and the outbreak was associated with ‘water, unspecified’.
Four strong‐evidence outbreaks were reported in 2021 by two MSs (Finland and Spain, one FBO each) and by Switzerland (N = 2), involving 146 human cases. All outbreaks were associated with the intake of ‘tap water including well water’ and were caused by ‘norovirus’ in MSs and by ‘E. coli other than STEC’ in Switzerland. Remarkably, the outbreak reported by Spain was described as a general outbreak and involved a total of 114 human cases.
Eleven weak‐evidence outbreaks were reported by six MSs (Austria, Finland, Greece, Ireland, Italy and Sweden) and one non‐MS (Republic of North Macedonia). In the EU, ‘tap water, including well water’ was the main source of waterborne outbreaks (N = 9), followed by ‘potable water’ (one outbreak). The weak‐evidence outbreak reported by the Republic of North Macedonia is described above. All together weak‐evidence outbreaks reported in 2021 were caused by STEC (N = 4) and Campylobacter (N = 2). For five weak‐evidence waterborne outbreaks, the agent was ‘unknown’.
4.8. ECDC and EFSA rapid outbreak assessment (ROA) of multi‐country foodborne outbreaks
The ECDC and EFSA jointly assessed the public health risk posed by various FBOs occurring in 2021, publishing their Joint Rapid Outbreak Assessments (ROA). The first ROA concerned an FBO attributable to Salmonella Braenderup sequence type 22, which was responsible for 348 cases, and 68 hospitalisations, in 12 European countries and the United Kingdom. The cases occurred between March and July 2021. Evidence from epidemiological, microbiological and trace‐back investigations identified imported melons as the suspected source of the outbreak. In particular, following case interviews and an analytical epidemiological study, Galia melons were identified as the probable vehicle of infection. Although S. Braenderup matching the outbreak strain was isolated in melons, including Galia melons imported from Honduras, at the time of the publication of the ROA it was not possible to establish the exact point of contamination in the food production chain. Withdrawals and recalls of contaminated products were implemented as control measures (ECDC and EFSA, 2021a).
Another ROA concerned an FBO caused by multiple serovars of Salmonella enterica, namely S. Amsterdam, S. Havana, S. Kintambo, S. Mbandaka, S. Orion and S. Senftenberg, which has been ongoing since 2019. It involved 121 confirmed cases, and 22 hospitalisations from five EU/EEA countries (Denmark, Germany, the Netherlands, Norway and Sweden), between 2019 and 2021. Noticeably, almost half of the cases and most hospitalisations (12/22) involved children under 10 years of age. Epidemiological, microbiological and food tracing investigations identified sesame‐based products imported from Syria as the probable vehicles of infection. Although control measures (withdrawals, recalls and destructions) have been implemented since 2020, these were not sufficient to prevent the occurrence of human cases in 2021. Moreover, sesame‐based products have a long shelf life and could be stored in people's homes over a long period (ECDC and EFSA, 2021b), thereby highlighting the risk of a prolonged pattern of human exposure to Salmonella caused by these foodstuffs.
Another ROA concerned an FBO caused by Salmonella Enteritidis ST11 in 2021. In total, 272 confirmed human cases, 25 hospitalisations and two deaths were notified by five European countries and the United Kingdom. France reported the highest number of cases (N = 216), followed by Spain (N = 22), the Netherlands (N = 12), the United Kingdom (N = 12), Norway (N = 7) and Denmark (N = 3). Following epidemiological, microbiological and traceability investigations, eggs from farms in Spain were identified as the potential source of the outbreak. Table eggs from the farms implicated in the outbreak were withdrawn and redirected for use in heat‐treated egg products. Interestingly, this outbreak was microbiologically linked to a historical outbreak reported by the Netherlands in 2019. However, no epidemiological link between the two events was identified (ECDC and EFSA, 2022b).
5. Conclusions
5.1. Health impact, causative agents and trends
In 2021, an increase was observed in the reporting of FBOs by most MSs and non‐MSs, compared with 2020. Across the EU and the United Kingdom (Northern Ireland) a total of 4,005 FBOs were reported, an increase of approximately one‐third (29.8%) compared with 2020. However, this number was still well below the mean number of FBOs reported to EFSA in the pre‐pandemic years (5,601 FBOs/year reported on average during the period 2017–2019). A similar pattern could be observed for other indicators relating to the impact of FBOs on public health (primarily cases and hospitalisations). To properly interpret these data, since FBO surveillance is not fully harmonised among MSs, it is important to remember that statistics at EU level reflect mostly the pattern of FBO occurrence of those MSs that contributed the most to data collection in 2021. The findings described above suggest that the consequences of the COVID‐19 pandemic were still visible in 2021, in both the occurrence of FBOs in the EU and the activities of detection, investigation and reporting. This is also supported by the fact that the drop in FBO reporting observed in a few countries in 2020 following the arrival of the COVID‐19 pandemic, was not reversed in 2021.
The year 2021 was the second year of the COVID‐19 pandemic, with several new waves being reported across Europe. Also in this second year, a range of control measures were adopted by National Authorities in the EU to limit the spread of the virus, with content varying from one country to another. These measures included actions to reduce contact (e.g. stay‐at‐home orders, banning of private gatherings, etc.), promote hygiene and safety measures (e.g. the use of protective equipment, disinfection procedures, etc.) and other preventive measures. 35 The control measures implemented in 2021 were less stringent than in 2020, but could still explain the downturn in FBOs for the second year of the pandemic. These control measures could have had an impact in decreasing the foodborne exposure of the population to zoonotic agents (e.g. better hygiene, closure of restaurants, etc.), improving hygiene measures by consumers (e.g. gloves, hand sanitiser, cleaning of surfaces and equipment, etc.) or even in reducing travel‐related FBOs, as observed in the literature (Ray et al., 2021; van Deursen et al., 2022). The diversion of technical, financial and human resources towards activities relating to the COVID‐19 pandemic may also have had an important role. In many countries, the COVID‐19 pandemic placed healthcare services under severe strain and negatively impacted doctor‐patient interaction, leading to healthcare difficulties, with problems of under‐diagnosis and under‐reporting of non‐COVID diseases (Kastritis et al., 2020; Verhoeven et al., 2020; Lim et al., 2021; WHO, 2022). Nonetheless, the increase in the number of FBOs compared with 2020 might indicate a gradual return to pre‐pandemic stability in FBO surveillance for most MSs.
Overall, the causative agents associated with FBOs in 2021 were similar to those observed in previous years. In the EU, Salmonella remained the most frequently reported pathogen, for the number of FBOs (i.e. one in five), as well as for human cases and hospitalisations. S. Enteritidis and S. Typhimurium were the predominant serovars. At national level, Salmonella was the main cause of FBOs in most EU MSs (17 MSs) and the United Kingdom (Northern Ireland), as well as in six non‐MSs. However, in a few countries, the number of FBOs caused by Salmonella was surpassed by other agents including either Campylobacter, STEC, Staphylococcus aureus toxins or norovirus. This finding highlights considerable differences in the epidemiology of FBOs among MSs, which is probably attributable to different risks of exposure to pathogens through food consumption, and differences in the structure of FBO surveillance.
One critical finding emerging from the 2021 data analysis concerns the number of listeriosis FBOs reported to EFSA, which was higher than in both 2020 and the pre‐pandemic years. However, these outbreaks were apparently less severe compared with recent years, as fewer cases, hospitalisations and deaths were reported in 2021. Possible reasons for this increase are discussed in the section dedicated to Listeria (4.2.1 Bacteria). The rise in the reporting of FBOs caused by Listeria monocytogenes is a matter of concern since high rates of hospitalisation and death are associated with this pathogen (Buchanan et al., 2017), especially in vulnerable and fragile populations, whose numbers are increasing in the EU. It is possible that FBO cases involving severe conditions, such as sepsis and meningitis associated with invasive listeriosis, are less likely to be under‐diagnosed and under‐reported.
In 2021, two strong‐evidence FBOs caused by Cronobacter sakazakii and Vibrio cholerae (non‐toxigenic) were reported for the first time to EFSA since the start of data collection. Both outbreaks occurred in ‘Health care and residential facilities’. One new‐born child infected by C. sakazakii died after the intake of a contaminated probiotic formula. These events draw attention to the high susceptibility of vulnerable population groups to FBOs.
5.2. Food vehicles and places of exposure
In 2021, the range of foodstuffs implicated in FBOs closely reflected the established epidemiology of the implicated causative agents. Overall, considering only single food items, ‘mixed food’ was the foodstuff implicated in most outbreaks, leading to the highest number of cases and hospitalisations. ‘Mixed foods’ are complex foods by definition since they include a variety of ingredients, making it difficult to identify the primary source of contamination. Many factors may contribute to the contamination of these foodstuffs, including unsafe food mixing, processing and handling of ingredients by infected food handlers, cross‐contamination or even the development of favourable growing conditions for pathogens during food preparation. ‘Eggs and egg products’ were the second most frequently reported foodstuff in FBOs, followed by ‘vegetables and juices and products thereof’ and ‘fish and fish products’. In 2021, FBOs caused by Salmonella coupled with a variety of food vehicles (i.e. ‘eggs and egg products’, ‘mixed foods’, ‘vegetables and juices and other products thereof’ and ‘bakery products’) led to some of the most severe health impacts in terms of total outbreaks, cases and hospitalisations in the EU.
Interestingly, the implication of ‘vegetables and juices and other products thereof’ in FBOs increased considerably compared with both 2020 and the pre‐pandemic years. In addition, these foods caused the second highest number of human cases. For extensively handled vegetables and fruit, such as pre‐cut or frozen items, the food chain leading to the shop or place of consumption may be complex and long, as indicated by the Salmonella Braenderup found in melons. Contamination may take place at various levels of the food chain, from the primary production level with the growers, up to the processing and retail chain (Olaimat and Holley, 2012; Alegbeleye et al., 2018). This is the reason why FBOs implicating this foodstuff may have a critical impact on public health and require strong public health and food safety preparedness, with intersectoral coordination for effective control.
As observed in the past, domestic settings remain the most frequently reported place of exposure to foodborne agents. FBOs occurring in this setting were mostly described as ‘household’ outbreaks. However, it is highly probable that these outbreaks have been under‐reported, since household FBOs could have remained undetected or been poorly investigated, especially in the pandemic years. In 2021, these outbreaks were reported only by seven MSs across the EU. This finding highlights the importance of communicating with the public in order to improve awareness of the risk posed by handling food in domestic kitchens, and the need to adopt the correct practices for food preparation and storage.
One remarkable aspect highlighted in 2021 is the increase of outbreaks in ‘canteen or catering at workplace, school, etc.’, mainly due to the contribution of FBOs occurring in ‘school or kindergarten’. Outbreaks occurring in ‘school or kindergarten’ are relatively easy to detect because they are often point‐source outbreaks and/or involve a large number of people. In 2021, outbreaks in ‘school or kindergarten’ led to the highest number of cases and had a high mean outbreak size (50 cases per FBO). The highest number of deaths was observed in outbreaks occurring in ‘health care and residential facilities', drawing attention to the high risks of foodborne outbreaks for vulnerable population groups. The causative agents frequently identified in these places of exposure were mostly unspecified bacterial toxins, Salmonella and norovirus. Interestingly, an infected food handler was the main factor contributing to FBOs. This finding underlines the importance of strengthening hygiene standards and HACCP protocols in the food production chain, including training food handlers in food safety principles and procedures.
In conclusion, it is important to remember that trends in the occurrence of FBOs over the years are highly influenced not only by the level of food contamination at consumer level, but also by changes in food consumption habits. Globalisation has revolutionised the food supply system, resulting in an extensive and complex food production chain that may also contribute to an increasing number of foodborne outbreaks (Adinolfi et al., 2016). In addition, the EU is in the throes of demographic change. The ageing of the population will probably affect all EU countries, resulting in the considerably increased susceptibility of vulnerable population groups (Eurostat, 2020).
Zoonoses monitored in accordance with the epidemiological situation (Directive 2003/99 List B)
1. Yersinia
Summary data substantiating this chapter, as well as additional information on related projects and internet sources are published for this report on the EFSA Knowledge Junction on Zenodo here. Summary statistics on yersiniosis foodborne outbreaks reported in the framework of Directive 2003/99/EC, with downloadable files, are retrievable using the EFSA foodborne outbreaks dashboard available here. Summary statistics on human surveillance data with downloadable files are retrievable using the ECDC Surveillance Atlas of Infectious Diseases here.
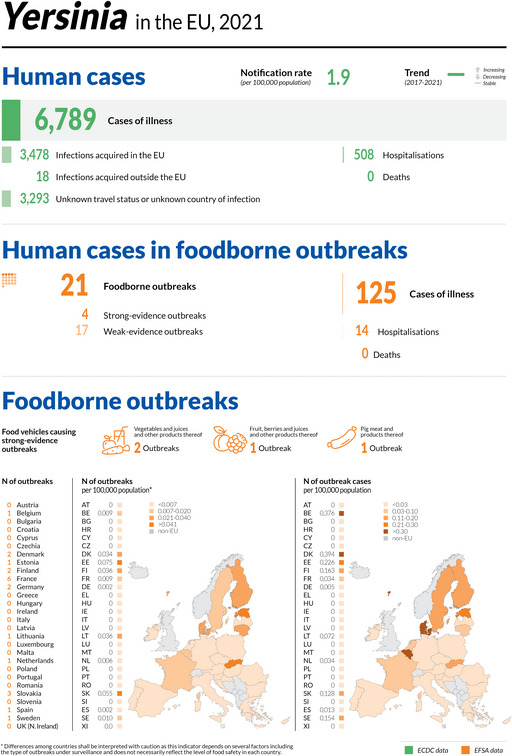
1.1. Key facts
Yersiniosis is the third most commonly reported zoonosis in humans in the EU.
In 2021, the number of confirmed cases of human yersiniosis was 6,789, corresponding to an EU notification rate of 1.9 per 100,000 population. This was an increase of 11.8% compared with the rate in 2020 (1.7 per 100,000 population).
In 2020, yersiniosis reporting recorded the lowest number of human cases since 2007, when Yersinia surveillance began. This low number was related to the impact of the COVID‐19 pandemic and the withdrawal of the United Kingdom from the EU.
Compared with the rate before the COVID‐19 pandemic (2017–2019 annual mean), the 2021 EU notification rate increased by 11.3% and decreased 8.9% with and without data from the United Kingdom, respectively.
The overall yersiniosis trend in 2017–2021 showed no statistically significant increase or decrease.
In 2021, six MSs reported information on 355 ‘ready‐to‐eat’ food sampling units tested for the presence of Yersinia. There were three positive units, and all were from the ‘ready‐to‐eat’ meat and meat products category, in particular ‘meat and meat products from pigs’ (2.1% positive samples).
In ‘non‐ready‐to‐eat’ food, six MSs provided results on 736 sampling units. There were 38 positive units and all originated from ‘meat and meat products’ with 5.4% positives in this category. In ‘fresh meat’, five MSs provided information from 418 sampling units, reporting 29 positive units (6.9%). In particular, Yersinia was found in 26 (7.1%) out of 366 samples tested in the ‘fresh meat of pigs’ food category.
In animals, seven MSs and one non‐MS reported results of sampling activities in 2021; the highest overall proportion of Yersinia‐positive sampling units in farmed animals was observed in ‘small ruminants’ (N = 96, 2.7%).
1.2. Surveillance and monitoring of Yersinia in the EU
1.2.1. Humans
In 2021, 26 MSs reported information on yersiniosis in humans. Surveillance of yersiniosis is mandatory in 22 MSs. In four MSs (Belgium, France, Greece and Italy) notification is based on a voluntary system. No yersiniosis surveillance system is in place in the Netherlands. The surveillance systems for yersiniosis have national coverage in all reporting countries, except for three: France, Italy and Spain. For these three countries, no information on population coverage was provided, and notification rates were not calculated. In 2020–2021, Spain did not received data from all regions that normally report, so the case number might not be complete. The EU case definition was used by 21 MSs, four MSs used a different case definition for reporting (Denmark, France, Germany and Italy), and Greece did not use any case definition. All countries reporting data on yersiniosis in 2021 provided case‐based data, except Belgium, Bulgaria and Greece, which reported aggregated data.
1.2.2. Food and animals
Although it is not mandatory to report the presence of Yersinia in food and animals, MSs can report monitoring data on Yersinia to EFSA, in accordance with the Zoonoses Directive (Directive 2003/99/EC). The directive specifies that, in addition to the zoonoses and zoonotic agents for which monitoring is mandatory, zoonoses such as yersiniosis and agents thereof must also be monitored if warranted by the epidemiological situation in an MS (Annex I, B. 2., in compliance with Article 4.1 of the same Directive). At present, no harmonised Yersinia monitoring plan is in place for food or animals in the EU. Therefore, data on Yersinia in food and animals submitted to EFSA by the MSs are not harmonised. Data allow for descriptive summary statistics at the EU level only, and do not support trend analyses and trend watching (Table 1). Harmonised monitoring and reporting criteria for Y. enterocolitica in slaughter pigs were recommended by EFSA in a scientific report (EFSA, 2009). The reported occurrence of Yersinia in major food categories for the year 2021 and for the four‐year period 2017–2020 was summarised descriptively, making a distinction between ‘ready‐to‐eat’ (RTE) and non‐RTE foods.
For the purpose of the 2021 data analysis, only results obtained from samples collected and tested for Yersinia under an ‘objective sampling’ strategy were considered, in order to limit selection bias. Objective sampling means that MSs collected and tested the samples according to a planned strategy based on a random sampling design representative of the population under study.
1.3. Results
1.3.1. Overview of key statistics, EU, 2017–2021
Tables 69 and 70 summarise EU‐level statistics on human yersiniosis, and on the occurrence and prevalence of Yersinia in food and animals, for the period 2017–2021. Although yersiniosis was the third most frequently reported zoonosis in the EU in 2021, data on Yersinia in food and animals continue to be reported by few MSs, like in previous years. More detailed descriptions of these statistics are provided in the below subsections and in the chapter on foodborne outbreaks.
Table 69.
Summary of Yersinia statistics related to humans, major food categories and main animal species, EU, 2017–2021
| 2021 (a) | 2020 | 2019 (b) | 2018 (b) | 2017 (b) | Data source | |
|---|---|---|---|---|---|---|
| Humans | ||||||
| Total number of confirmed cases | 6,789 | 5,661 | 6,967 | 7,015 | 6,844 | ECDC |
| Total number of confirmed cases/100,000 population (notification rate) | 1.9 | 1.7 | 1.7 | 1.7 | 1.7 | ECDC |
| Number of reporting MSs | 26 | 26 | 27 | 27 | 27 | ECDC |
| Infection acquired in the EU | 3,478 | 2,686 | 3,468 | 3,446 | 3,410 | ECDC |
| Infection acquired outside the EU | 18 | 61 | 96 | 106 | 88 | ECDC |
| Unknown travel status or unknown country of infection | 3,293 | 2,914 | 3,403 | 3,463 | 3,346 | ECDC |
| Number of foodborne outbreak‐related cases | 125 | 236 | 160 | 58 | 130 | EFSA |
| Total number of foodborne outbreaks | 21 | 16 | 23 | 12 | 11 | EFSA |
| Food | ||||||
| Meat and meat products | ||||||
| Number of sampling units | 1,789 | 1,597 | 2,304 | 1,470 | 1,211 | EFSA |
| Number of reporting MSs | 6 | 6 | 6 | 6 | 7 | EFSA |
| Fruits and vegetable products | ||||||
| Number of sampling units | 142 | 256 | 17 | 7 | 116 | EFSA |
| Number of reporting MSs | 3 | 4 | 2 | 2 | 4 | EFSA |
| Animals (c) | ||||||
| Bovine animals | ||||||
| Number of sampling units | 19,218 | 14,796 | 16,885 | 13,101 | 17,404 | EFSA |
| Number of reporting MSs | 5 | 5 | 5 | 6 | 8 | EFSA |
| Pigs | ||||||
| Number of sampling units | 2,164 | 2,368 | 2,591 | 2,347 | 2,781 | EFSA |
| Number of reporting MSs | 5 | 4 | 6 | 7 | 7 | EFSA |
ECDC: European Centre for Disease Prevention and Control; EFSA: European Food Safety Authority; MSs: Member States.
: Data on food and animals from the United Kingdom (Northern Ireland) are taken into account for 2021. In accordance with the agreement on the withdrawal of the United Kingdom from the EU, and in particular with the Protocol on Ireland/Northern Ireland, EU requirements on data sampling are also applicable to Northern Ireland.
: Data from the United Kingdom are taken into account for 2017–2019, because the United Kingdom was an EU MS, but it became a third country on 1 February 2020.
: Samples as single animals.
Table 70.
Reported confirmed human cases of yersiniosis and notification rates per 100,000 population in EU MSs and non‐MS countries, by country and year, 2017–2021
| Country | 2021 | 2020 | 2019 | 2018 | 2017 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| National coverage (a) | Data format (a) | Confirmed cases and rates | Confirmed cases and rates | Confirmed cases and rates | Confirmed cases and rates | Confirmed cases and rates | ||||||
| Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | |||
| Austria | Y | C | 138 | 1.5 | 128 | 1.4 | 112 | 1.3 | 136 | 1.5 | 95 | 1.1 |
| Belgium | Y | A | 418 | 3.6 | 260 | 2.3 | 406 | 3.5 | 392 | 3.4 | 317 | 2.8 |
| Bulgaria | Y | A | 5 | 0.07 | 4 | 0.06 | 11 | 0.16 | 9 | 0.13 | 17 | 0.24 |
| Croatia | Y | C | 14 | 0.35 | 11 | 0.27 | 12 | 0.29 | 20 | 0.49 | 29 | 0.7 |
| Cyprus | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Czechia | Y | C | 456 | 4.3 | 437 | 4.1 | 618 | 5.8 | 622 | 5.9 | 611 | 5.8 |
| Denmark | Y | C | 453 | 7.8 | 413 | 7.1 | 221 | 3.8 | 282 | 4.9 | 206 | 3.6 |
| Estonia | Y | C | 45 | 3.4 | 44 | 3.3 | 42 | 3.2 | 63 | 4.8 | 43 | 3.3 |
| Finland | Y | C | 331 | 6.0 | 386 | 7.0 | 406 | 7.4 | 529 | 9.6 | 423 | 7.7 |
| France (b) | N | C | 1,451 | – | 988 | – | 1,135 | – | 929 | – | 738 | – |
| Germany | Y | C | 1,912 | 2.3 | 1,860 | 2.2 | 2,164 | 2.6 | 2,193 | 2.6 | 2,581 | 3.1 |
| Greece | Y | A | 7 | 0.07 | 3 | 0.0 | 13 | 0.12 | 21 | 0.20 | 19 | 0.18 |
| Hungary | Y | C | 50 | 0.51 | 25 | 0.26 | 38 | 0.39 | 36 | 0.37 | 30 | 0.31 |
| Ireland | Y | C | 19 | 0.38 | 13 | 0.26 | 9 | 0.18 | 8 | 0.17 | 6 | 0.13 |
| Italy (b) | N | C | 35 | – | 21 | – | 12 | – | 14 | – | 8 | – |
| Latvia | Y | C | 83 | 4.4 | 88 | 4.6 | 60 | 3.1 | 68 | 3.5 | 47 | 2.4 |
| Lithuania | Y | C | 153 | 5.5 | 123 | 4.4 | 181 | 6.5 | 139 | 4.9 | 174 | 6.1 |
| Luxembourg | Y | C | 12 | 1.9 | 26 | 4.2 | 18 | 2.9 | 16 | 2.7 | 15 | 2.5 |
| Malta | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Netherlands (c) | – | – | – | – | – | – | – | – | – | – | – | – |
| Poland | Y | C | 142 | 0.38 | 90 | 0.24 | 196 | 0.52 | 170 | 0.45 | 191 | 0.50 |
| Portugal | Y | C | 34 | 0.33 | 25 | 0.24 | 29 | 0.28 | 30 | 0.29 | 35 | 0.34 |
| Romania | Y | C | 15 | 0.08 | 6 | 0.03 | 36 | 0.19 | 22 | 0.11 | 36 | 0.2 |
| Slovakia | Y | C | 213 | 3.9 | 168 | 3.1 | 255 | 4.7 | 259 | 4.8 | 242 | 4.5 |
| Slovenia | Y | C | 49 | 2.3 | 26 | 1.2 | 28 | 1.3 | 32 | 1.5 | 18 | 0.87 |
| Spain (b) , (d) | N | C | 444 | – | 296 | – | 409 | – | 549 | – | 585 | – |
| Sweden | Y | C | 310 | 3.0 | 220 | 2.1 | 393 | 3.8 | 278 | 2.7 | 236 | 2.4 |
| EU Total 27 | – | – | 6,789 | 1.9 | 5,661 | 1.7 | 6,804 | 2.1 | 6,817 | 2.1 | 6,702 | 2.1 |
| United Kingdom | – | – | – | – | – | – | 163 | 0.24 | 198 | 0.30 | 142 | 0.22 |
| EU Total (e) | – | – | 6,789 | 1.9 | 5,661 | 1.7 | 6,967 | 1.7 | 7,015 | 1.7 | 6,844 | 1.7 |
| Iceland | Y | C | 4 | 1.1 | 3 | 0.82 | 2 | 0.56 | 2 | 0.57 | 0 | 0 |
| Norway | Y | C | 85 | 1.6 | 83 | 1.5 | 85 | 1.6 | 105 | 2.0 | 67 | 1.3 |
| Liechtenstein | – | – | – | – | – | – | – | – | – | – | – | – |
| Switzerland | – | – | – | – | ||||||||
–: Data not reported.
: Y: yes; N: no; A: aggregated data; C: case‐based data.
: Sentinel surveillance; no information on estimated coverage. Notification rate not estimated.
: No surveillance system.
: Data not complete in 2020–2021, rate not estimated.
: Cases reported by the United Kingdom for the period 2017–2019 were also considered for this estimation (EU‐28). When the United Kingdom data were collected for the period 2017–2019, the United Kingdom was an EU MS, but it became a third country on 1 February 2020.
1.3.2. Human yersiniosis
In 2021, 6,789 confirmed cases of yersiniosis were reported by 26 MSs. As in recent years, Germany accounted for the highest number of cases, followed by France (Table 70). Cases reported by these countries together accounted for 49.5% of all confirmed yersiniosis cases in the EU. In particular, the number of cases increased in France and Denmark from 2017 to 2021. The highest notification rates (per 100,000 population) were observed for Denmark (7.8), Finland (6.0), Lithuania (5.5) and Latvia (4.4).
The notification rate of confirmed yersiniosis cases in the EU was 1.9 cases per 100,000 population. This corresponds to an increase of 11.8% compared with the rate in 2020 (1.7 per 100,000 population), and an increase of 11.3% and decrease of 8.9% compared with the average annual notification rate from 2017 to 2019 (pre‐pandemic period) with and without United Kingdom data, respectively.
The majority of yersiniosis cases, i.e. 3,478 (99.5%) reported with known origin of infection were from the EU (Table 70). Germany, Sweden, Denmark, Austria and Finland reported the highest number of travel‐associated cases (33, 16, 15, 6 and 6 cases, respectively) out of a total of 84 cases. Overall, the proportion of travel‐associated cases of yersiniosis was only 2.4%, including 60 travel‐associated cases in the EU (1.7%), and 18 cases (0.5%) outside the EU reported by 18 MSs and 6 with origin unknown (0.2%).
The trend of human yersiniosis cases in the EU for 2017–2021 showed no statistically significant increase or decrease (Figure 34). At the MS level, a statistically significant decrease (p < 0.05) was observed for Germany and Romania. For the same time period, there was a statistically significant increase (p < 0.05) for four MSs (Denmark, France, Italy and Latvia).
Figure 34.
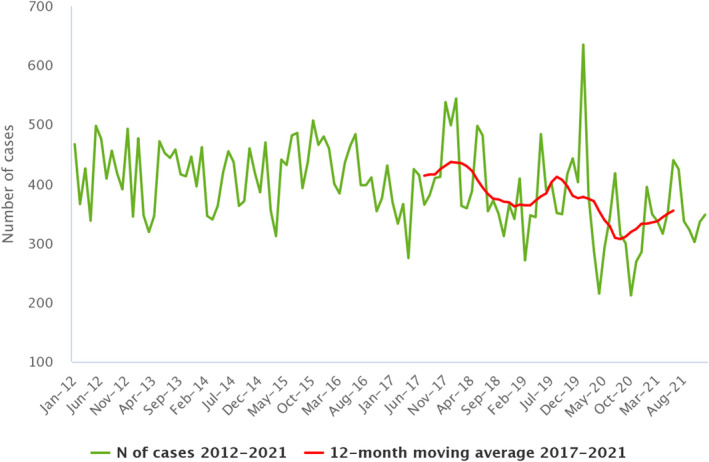
Trends in reported confirmed human cases of yersiniosis in the EU, by month, 2012–2021
- Source: Austria, Czechia, Denmark, Estonia, Finland, Germany, Hungary, Ireland, Italy, Latvia, Luxembourg, Malta, Poland, Romania, Slovakia, Slovenia and Sweden.
Of the 1,564 (23.0%) yersiniosis cases with available hospitalisation information reported by 13 MSs, 508 cases (32.5%) were hospitalised. No deaths were reported among the 3,596 confirmed cases with available clinical outcome information. The outcome was reported for 53.0% of all confirmed cases of yersiniosis by 21 MSs.
Information on the Yersinia species detected in confirmed cases was reported by 21 MSs for 6,063 cases (95.3%) in 2021. Y. enterocolitica was the most commonly reported species in all countries, among 5,950 human cases (98.1% of all cases with information on species available). Serotype information on Y. enterocolitica was provided for 2,984 (50.2%) confirmed cases. The most commonly reported serotype was O3 (83.1% of cases with information on serotype available), followed by O9 (12.3%). Overall, the other serotypes (O1, O5, O5, 27, O8 and others) accounted for 4.6% of cases with known serotype. Biotype information was provided for 1,727 (29.0%) confirmed cases. The most commonly reported biotype of Y. enterocolitica was biotype 4 (82.3% of all cases with this information available), followed by biotype 2 (16.7%), biotype 1B (0.6%) and biotype 3 (0.4%). Information on Y. enterocolitica bioserotypes was provided for 1,682 (28.3%) confirmed cases. The most common bioserotypes were 4/O3 (83.2%) and 2/O9 (15.3%). Comparing these data with those for 2020, there was a slight decrease in the reporting of biotype 4/O3, from 87.7% in 2020 to 83.2% in 2021, while reporting of biotype 2/O9 increased from 10.9% in 2020 to 15.3% in 2021.
Eleven MSs reported a total of 113 Y. pseudotuberculosis cases in 2021.
1.3.3. Yersinia in food
Statistics on the presence of Yersinia in the major food categories in 2021 and for 2017–2020 are summarised in Table 71 according to food type (i.e. RTE and non‐RTE foods).
Table 71.
Occurrence of Yersinia in the main food categories, EU, 2021 and 2017–2020
| Food | 2021 (a) | 2017–2020 (b) | ||||
|---|---|---|---|---|---|---|
| N reporting MSs | N sampling units | N positive (%) | N reporting MSs | N sampling units | N positive (%) | |
| RTE food | ||||||
| All | 6 | 355 | 3 (0.85) | 6 | 1,768 | 122 (6.9) |
| Meat and meat products | 4 | 232 | 3 (1.3) | 4 | 1,707 | 121 (7.1) |
| Meat and meat products from pigs | 2 | 144 | 3 (2.1) | 2 | 36 | 0 (0) |
| Mixed meat and meat products from bovine animals and pigs | 0 | 0 | 0 (0) | 2 | 1,553 | 111 (7.1) |
| Mixed meat | 1 | 37 | 0 (0) | 2 | 100 | 10 (10) |
| Meat from other animals and raw cured (or seasoned) meat | 2 | 51 | 0 (0) | 3 | 18 | 0 (0) |
| Milk and milk products | 1 | 43 | 0 (0) | 3 | 31 | 0 (0) |
| Fruits and vegetables | 1 | 55 | 0 (0) | 4 | 10 | 0 (0) |
| RTE salads | 1 | 4 | 0 (0) | 0 | 0 | 0 (0) |
| Other processed food products and prepared dishes | 1 | 21 | 0 (0) | 2 | 4 | 1 (25.0) |
| Other food | 0 | 0 | 0 (0) | 2 | 16 | 0 (0) |
| Non‐RTE food | ||||||
| All | 6 | 736 | 38 (5.2) | 8 | 4,092 | 283 (6.9) |
| Meat and meat products | 5 | 698 | 38 (5.4) | 7 | 3,722 | 254 (6.8) |
| Milk and milk products | 1 | 23 | 0 (0) | 3 | 238 | 29 (12.2) |
| Other food | 2 | 15 | 0 (0) | 5 | 132 | 0 (0) |
| Fresh meat (c) | ||||||
| All fresh meat | 5 | 418 | 29 (6.9) | 7 | 4,026 | 310 (7.7) |
| Fresh meat from pigs | 5 | 366 | 26 (7.1) | 5 | 2,400 | 148 (6.2) |
| Fresh meat from bovine animals | 1 | 10 | 0 (0) | 3 | 34 | 1 (2.9) |
| Fresh meat from bovine animals and pigs | 0 | 0 | 0 (0) | 2 | 1,401 | 129 (9.2) |
| Other fresh meat | 2 | 42 | 3 (7.1) | 3 | 191 | 32 (16.8) |
MSs: Member States; RTE: ‘ready‐to‐eat’.
Fresh meat includes RTE and non‐RTE food.
Other RTE food includes: Bakery products, Cereals and nuts – RTE, Chocolate, Other food – RTE
Other non‐RTE food includes: Cereals, dried seeds – non‐RTE, Egg and egg products – non‐RTE, Fish and fishery products – non‐RTE, Fruits, vegetables and juices – non‐RTE, Foodstuffs intended for special nutritional uses – non‐RTE, Other processed food products and prepared dishes – non‐RTE and Seeds, sprouted – non‐RTE.
: Data from the United Kingdom (Northern Ireland) are taken into account for 2021. In accordance with the agreement on the withdrawal of the United Kingdom from the EU, and in particular with the Protocol on Ireland/Northern Ireland, the EU requirements on data sampling are also applicable to Northern Ireland.
: Data from the United Kingdom are taken into account for 2017–2019, because the United Kingdom was an EU MS, but it became a third country on 1 February 2020.
: Fresh meat sampling units are a subset of the two main categories above.
As in recent years, most RTE food samples analysed for the presence of Yersinia (N = 355) in 2021 belonged to ‘meat and meat products’ (232 samples; 65.3%) and were reported by four MSs. However, compared with 2020, the number of RTE samples decreased by 54%. A total of 3 (2.1%) sampling units of ‘meat and meat products from pigs’ were found to be positive for Yersinia in 2021. In the period 2017–2020, 121 (7.1%) sampling units positive for the presence of Yersinia were found in RTE food from ‘meat and meat products’, of which 111 (7.1%) sampling units in ‘meat and mixed meat products from bovine animals and pigs’ and 10 (10%) in ‘mixed meat’ samples. Compared with 2017–2020, no sampling was conducted in 2021 for the category ‘mixed meat and meat products from bovine animals and pigs’.
Despite evidence supporting the need to sample RTE foods, few MSs reported monitoring this type of food for Yersinia to EFSA, even though they represent a direct risk to consumers.
Regarding non‐RTE foods, sampling and testing results were reported in 2021 by six MSs. As in recent years, most non‐RTE food samples analysed for the presence of Yersinia (N = 736) belonged to ‘meat and meat products’ (698 samples; 94.8%). A total of 38 (5.4%) sampling units of ‘meat and meat products’ were positive for Yersinia. Yersinia was detected in 26 (7.1%) sampling units of ‘fresh meat from pigs’ (N = 366) belonging to the food category ‘all fresh meat’ (N = 418).
1.3.4. Yersinia in animals
Table 72 summarises the reported occurrence of Yersinia in animals for the year 2021 in MSs and non‐MS countries. In 2021, seven MSs and one non‐MS country reported monitoring data on the presence of Yersinia in animals. A total of 27,756 animals were analysed in EU for Yersinia. The MSs sampled different animal species, mainly cattle, small ruminants, pigs and pet animals with 223 (0.8%) positive animals. The non‐MS country sampled mainly pet animals (N = 1,341) with only one positive sample (0.07%).
Table 72.
Summary of Yersinia enterocolitica statistics related to major animal species, reported by EU MSs and non‐MS countries, 2021
| Animals | EU MSs (a) | Non‐MS countries | ||||||
|---|---|---|---|---|---|---|---|---|
| N reporting countries | N sampling units tested (b) | Positive sampling units | N reporting countries | N sampling units tested (b) | Positive sampling units | |||
| N | % | N | % | |||||
| Cattle (bovine animals) | 5 | 19,206 | 106 | 0.55 | 1 | 39 | 0 | 0 |
| Small ruminants (sheep and goats) | 4 | 3,531 | 96 | 2.7 | 1 | 18 | 0 | 0 |
| Pigs | 5 | 2,164 | 2 | 0.09 | 1 | 11 | 0 | 0 |
| Other domestic/farmed animals | 3 | 266 | 0 | 0 | 1 | 90 | 0 | 0 |
| Pet animals | 4 | 159 | 6 | 3.8 | 1 | 1,341 | 1 | 0.07 |
| Wild animals (e.g. birds, roe and fallow deer, wild boar, foxes) | 3 | 448 | 9 | 2.0 | 0 | 0 | 0 | 0 |
| Zoo animals | 5 | 80 | 4 | 5.0 | 0 | 0 | 0 | 0 |
| Other (unspecified habitat) | 4 | 1,902 | 0 | 0 | 1 | 6 | 0 | 0 |
| Total | 7 | 27,756 | 223 | 0.80 | 1 | 1,494 | 1 | 0.07 |
Other domestic\farmed animals: Alpacas – farmed, Poultry unspecified, Solipeds domestic – horses, Wild ducks – farmed, Camels – farmed, rabbits – farmed, Reindeers – farmed.
Pet animals: Cats – pet animals, Dogs – pet animals, Other animals – exotic pet animals, Rabbits – pet animals, Budgerigars – pet animals, Canaries – pet animals, Chinchillas – pet animal, Ferrets – pet animals, Guinea pigs – pet animals, Parrots – pet animals, Rats – pet animal, Reptiles – pet animals, Turtles – pet animals.
Wild: Badgers – wild, Birds – wild, Cantabrian chamois – wild, Deer – wild, Deer – wild – fallow deer, Deer – wild – roe deer, Falcons – wild, Ferrets – wild, Foxes – wild, Martens – wild, Otters – wild, Rats – wild, Squirrels – wild, Steinbocks – wild, Turtles – wild, Wild boars – wild, Wolves – wild.
Zoo animals: Fish, Kangaroos – zoo animal, Monkeys – zoo animal, Penguins – zoo animals, Snakes – zoo animal, Wild cats (Felis silvestris) – zoo animals, Zoo animals, all.
Other (unspecified habitat): Fish, Cats, Deer, Hares, Rabbits, Geese, Kangaroos, Oscine birds and Pigeons.
: Data from the United Kingdom (Northern Ireland) are taken into account for 2021. In accordance with the agreement on the withdrawal of the United Kingdom from the EU, and in particular with the Protocol on Ireland/Northern Ireland, EU requirements on data sampling are also applicable to Northern Ireland.
: The summary statistics were obtained summing all sampling units (single and batch samples).
1.4. Discussion
In 2021, yersiniosis was the third most commonly reported foodborne zoonotic disease in the EU. The EU notification rate for yersiniosis remained stable even during the COVID‐19 pandemic when compared with other foodborne diseases and the overall trend for yersiniosis in 2017–2021 showed no statistically significant increase or decrease. The lowest number of yersiniosis cases were reported in 2020, which could be partially explained by the impact of the COVID‐19 pandemic on national healthcare systems, and less importantly by the withdrawal of the United Kingdom from the EU. The EU notification rate in 2020, remained comparable with the previous years owing to the withdrawal of the United Kingdom from the EU. In 2021, the number of reported cases was comparable to those reported before the pandemic and the EU notification rate of yersiniosis increased compared to both 2020 and to the pre‐pandemic period (2017–2019).
Among the two pathogenic species that are notified, Y. enterocolitica and Y. pseudotuberculosis, the first caused the majority (98.1%) of human infections. Information on biotypes would allow for better characterisation of the epidemiology of Y. enterocolitica infection in humans and a better investigation of relevant animal sources (Espenhain et al., 2019; Gruber et al., 2021). In 2021 as in previous years, the most common biotypes in humans were 4/O3 and 2/O9. In 2021, few MSs reported data on Yersinia sampling activities in food and animals. This is probably due to the lack of mandatory monitoring plans for non‐human sources, resulting in significant differences between MSs in their approach to monitoring Yersinia in food and animals. The lack of homogeneity makes statistical analysis and monitoring of trends impossible. An EFSA scientific report suggested technical specifications for the harmonised monitoring and reporting of Y. enterocolitica in slaughter pigs in the EU (EFSA, 2009).
In 2021, only six MSs provided data on RTE food for Yersinia and the number of samples analysed was rather low (N = 355) compared with 2020 (N = 766), with a proportion of positive samples of 0.85% compared with 5.2% in 2020. Instead, it would be beneficial to sample more RTE foods that are consumed without any processing to reduce or eliminate Yersinia contamination.
Yersinia enterocolitica was isolated in all foods that were found to be positive. Information on Y. enterocolitica biotypes was provided for a small fraction of data, although documenting trends and sources of Yersinia along the food chain, including reporting of information on Y. enterocolitica biotypes (EFSA BIOHAZ Panel, 2007), is essential to the overall goal of reducing yersiniosis at both the primary production level and at other critical points along the food production chain.
In this context, it would be useful to adopt real‐time PCR‐based methods that are able to rapidly provide information on the pathogenicity of Y. enterocolitica that may be present, even though they do not provide an indication of the specific biotype. Such methods can also easily be used for the detection of strains isolated from faeces, food, feed and environmental samples (Hallanvuo et al., 2019). For this purpose, the ISO method (ISO, 2015) describes horizontal methods based on real‐time PCR for detection of the pathogenic bioserotypes of Y. enterocolitica and one for detection of Y. pseudotuberculosis in products for human consumption, animal feeding stuffs and environmental samples (Rivas et al., 2021).
2. Toxoplasma gondii
Summary data substantiating this chapter, as well as additional information on related projects and internet sources are published for this report on the EFSA Knowledge Junction on Zenodo here. Summary statistics on human surveillance data with downloadable files are retrievable using the ECDC Surveillance Atlas of Infectious Diseases here.
2.1. Key facts
Only confirmed cases of congenital toxoplasmosis are reported to ECDC, with a two‐year delay in human data analyses at the EU level as data from France are reported to TESSy with a two‐year delay.
In 2020, the number of confirmed cases of congenital toxoplasmosis was 133, corresponding to an EU notification rate of 5.1 cases per 100,000 live births. Compared with the rates in 2019 with and without data from the United Kingdom (5.1 and 6.2 per 100,000 live births), this is unchanged or decreased by 17.7%, respectively.
In 2020, France accounted for 82.7% of reported cases of congenital toxoplasmosis due to compulsory screening of pregnant women.
Overall, the number of human cases of congenital toxoplasmosis in the EU has shown a constant decrease in the 2016–2020 period, mainly due to the reduction in cases reported by a single MS (France), but also due to the COVID‐19 pandemic and withdrawal of the United Kingdom from the EU.
In total, 14 MSs and three non‐MSs reported 2021 monitoring data on Toxoplasma gondii infections in animals. Most animals tested were sheep and goats, which also showed the highest overall prevalence of T. gondii infections in animals (16.8%), as reported by 12 MSs. Most samples were obtained from clinical investigations. It is not possible to accurately estimate the prevalence of T. gondii infections in animals due to the use of different diagnostic methods, the different sampling schemes in the MSs and the lack of information on the animals' ages and rearing conditions.
2.2. Surveillance and monitoring of Toxoplasma in the EU
2.2.1. Humans
In 2020, surveillance of toxoplasmosis was mandatory in 18 MSs and in one (France) it was voluntary. Data from France are reported to TESSy with a 2‐year delay. Eight MSs (Austria, Belgium, Denmark, Greece, Italy, the Netherlands, Portugal and Sweden) do not have a surveillance system for toxoplasmosis.
All reporting MSs except one (Spain) had a comprehensive surveillance system with full national coverage. Spain did not have national surveillance and could not provide any estimate for population coverage, so no notification rate was calculated. In 2020, Spain has not received data from all regions that normally report, so the case number might not be complete. Case‐based data were reported by all MSs except Bulgaria, which reported aggregated data. The EU case definition was used by 17 MSs and two MSs (France and Germany) reported use of different case definitions.
Six MSs (Austria, Belgium, France, Greece, Slovakia and Slovenia) have active surveillance of congenital cases, with compulsory screening of pregnant women. Austria and Belgium, however, do not report to TESSy. In the case of Austria, the disease is not notifiable and official data are therefore lacking. In Belgium, there are no clear recommendations on the follow‐up of seroconversion cases during pregnancy. In 2020, Greece did not report to TESSy. Four MSs (Bulgaria, Czechia, Germany and Hungary) have voluntary screening (ECDC, 2022).
On 1 February 2020, the United Kingdom became a third country, whereas before it was a MS. Human data were not collected from the United Kingdom for 2020 by ECDC.
2.2.2. Animals and food
There are no EU regulations concerning the surveillance and monitoring of T. gondii in animals and food. Therefore, the available and reported information relies on national legislation and whether the countries have a mandatory reporting system following the detection of T. gondii. As a result, data allow only for descriptive summaries at the EU level (Table 73). The main animal species tested are those intended for human consumption (small ruminants (goats and sheep), cattle, pigs) as well as pet animals (cats and dogs), using samples from aborted animals (ruminants) or clinically suspect animals. Mainly blood samples, but also samples from tissues and organs, are analysed either by direct methods, such as PCR, histology and immunohistochemistry (IHC), or by indirect antibody detection methods, including enzyme‐linked immunosorbent assay (ELISA), latex agglutination test (LAT), direct agglutination (DA) and immunofluorescence assay (IFA). For food testing, meat is the main matrix to be analysed either by PCR or by direct agglutination (DA).
Table 73.
Reported confirmed human cases of congenital toxoplasmosis and notification rates per 100,000 live births in EU MSs and non‐MS countries, by country and year, 2016–2020
| Country | 2020 | 2019 | 2018 | 2017 | 2016 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| National coverage (a) | Data format (a) | Confirmed cases and rates | Confirmed cases and rates | Confirmed cases and rates | Confirmed cases and rates | Confirmed cases and rates | ||||||
| Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | |||
| Austria | – | – | – | – | – | – | – | – | – | – | – | – |
| Belgium | – | – | – | – | – | – | – | – | – | – | – | – |
| Bulgaria | Y | A | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 3.1 | 0 | 0 |
| Croatia | Y | C | 0 | 0 | 0 | 0 | 1 | 2.7 | 0 | 0 | 0 | 0 |
| Cyprus | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Czechia | Y | C | 0 | 0 | 1 | 0.9 | 0 | 0 | 2 | 1.8 | 0 | 0 |
| Denmark | – | – | – | – | – | – | – | – | – | – | – | – |
| Estonia | Y | C | 0 | 0 | 0 | 0 | 1 | 7.2 | 0 | 0 | 0 | 0 |
| Finland | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1.8 |
| France | Y | C | 110 | 15.5 | 134 | 18.8 | 151 | 20.8 | 153 | 20.7 | 195 | 25.9 |
| Germany | Y | C | 14 | 1.8 | 17 | 2.2 | 18 | 2.3 | 8 | 1.0 | 10 | 1.3 |
| Greece | – | – | – | – | 0 | 0 | 0 | 0 | 0 | 0 | – | – |
| Hungary | Y | C | 0 | 0 | 1 | 1.1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ireland | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Italy | – | – | – | – | – | – | – | – | – | – | – | – |
| Latvia | Y | C | 0 | 0 | 1 | 5.2 | 0 | 0 | 0 | 0 | 0 | 0 |
| Lithuania | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Luxembourg | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Malta | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Netherlands | – | – | – | – | – | – | – | – | – | – | – | – |
| Poland | Y | C | 9 | 2.5 | 14 | 3.7 | 25 | 6.3 | 18 | 4.8 | 20 | 5.5 |
| Portugal | – | – | – | – | – | – | – | – | – | – | – | – |
| Romania | Y | C | 0 | 0 | 0 | 0 | 1 | 0.48 | 0 | 0 | 0 | 0 |
| Slovakia | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 3.6 |
| Slovenia | Y | C | 0 | 0 | 1 | 5.1 | 2 | 9.9 | 2 | 9.8 | 1 | 4.8 |
| Spain (b) , (c) | N | C | 0 | – | 0 | – | 2 | – | 3 | – | 5 | – |
| Sweden | – | – | – | – | – | – | – | – | – | – | – | – |
| EU Total 27 | – | – | 133 | 5.1 | 169 | 6.2 | 201 | 7.1 | 188 | 6.6 | 234 | 8.7 |
| United Kingdom | – | – | – | – | 7 | 1.0 | 7 | 0.9 | 7 | 0.91 | 8 | 1.0 |
| EU Total (d) | – | – | 133 | 5.1 | 176 | 5.1 | 208 | 5.8 | 195 | 5.4 | 242 | 6.9 |
| Iceland | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Norway | – | – | – | – | – | – | – | – | – | – | – | – |
| Liechtenstein | – | – | – | – | – | – | – | – | – | – | – | – |
| Switzerland | – | – | – | – | ||||||||
–: Data not reported.
: Y: yes; N: no; A: aggregated data; C: case‐based data.
: Notification rate was not calculated since information on estimated coverage was not available.
: Data not complete for 2020–2021, rate not estimated.
: Cases reported by the United Kingdom for the period 2016–2019 were also considered for this estimate (EU‐28). When the United Kingdom data were collected for the period 2017–2019, the United Kingdom was an EU MS, but it became a third country on 1 February 2020.
2.3. Results
2.3.1. Human congenital toxoplasmosis
In 2020, 19 MSs reported congenital toxoplasmosis data (Table 73) with a total of 133 confirmed cases. Sixteen MSs reported zero cases, whereas eight MSs (Austria, Belgium, Denmark, Greece, Italy, the Netherlands, Portugal and Sweden) did not report congenital toxoplasmosis at the EU level (Table 73). The notification rate was 5.1 per 100,000 live births in the EU, with the highest rate in France (15.5), followed by Poland (2.5) and Germany (1.8) (Table 73).
France accounted for 82.7% of all reported cases in the EU, followed by Germany and Poland. France regularly reports the highest number of congenital toxoplasmosis cases in the EU, whereas the other two MSs accounted for 17.3% of the cases reported in the EU.
Between 2016 and 2020, a gradual decrease in the number of reported cases was observed from France (43.6%) and Poland (55%, excluding data from 2018). A similar decrease was not observed for Germany.
As a result of fewer cases reported by France (and Poland), in 2020 the notification rate in the EU decreased by 17.7% compared with 2019, without data from the United Kingdom (Table 73), whereas no decrease was observed compared with 2019, including data from the United Kingdom.
In 2020, information on hospitalisation was provided by one MS (Poland) on 9 cases, representing 100% of cases reported by the MS. Cases with known fatal outcomes were 5 out of 109 (case fatality of 4.6%), with all fatal cases reported by France. Germany reported one case as imported, with Turkey being the probable country of infection.
2.3.2. Toxoplasma gondii in food and animals
Toxoplasma gondii in food
One MS (Poland) submitted monitoring results for T. gondii in non‐RTE food in 2021. In total, 694 samples were reported from meat products from pigs (641) and bovine animals (53). Seventy‐three (11.4%) and five (9.4%) samples were positive from pigs and from bovine animals, respectively.
Toxoplasma gondii in animals
Monitoring data on T. gondii in livestock, pet or zoo animals and wildlife were provided by 14 MSs, and by three non‐MSs (North Macedonia, Norway and Switzerland).
In small ruminants (sheep and goats), 12 MSs and three non‐MSs (North Macedonia, Norway and Switzerland) reported data. In total, 4,552 animals were tested and 762 were found to be positive (16.7%). In cattle, seven MSs (Austria, Germany, Ireland, Italy, Latvia, Slovakia and the United Kingdom (Northern Ireland)) and one non‐MS (Switzerland) reported data on Toxoplasma. In total, 728 animals were tested and 18 were found to be positive (2.5%). In pigs, five MSs (Austria, Germany, Ireland, Italy and the United Kingdom (Northern Ireland)) reported monitoring data that are found in Table 74. For other livestock (solipeds, alpacas, fowl and water buffaloes), four MSs (Austria, Germany, Italy and the United Kingdom (Northern Ireland)) reported monitoring data: in total, 371 animals were tested and four (1.1%) were found to be positive. In pet animals (cats, dogs, birds and rodents), eight MSs (Austria, Finland, Germany, Italy, Latvia, the Netherlands, Slovakia and Slovenia) and one non‐MS (Switzerland) tested in total 5,463 animals (3,640 cats, 1,776 dogs, 33 birds and 14 rodents). There were 778 (14.2%) positive samples, 428 (11.7%) samples from cats, 349 (19.6%) samples from dogs and 1 (3%) sample from birds obtained mainly from clinical investigations. As regards zoo animals, five MSs (Austria, Finland, Germany, Italy and Slovakia) and two non‐MSs (North Macedonia and Switzerland) tested in total 810 animals and 14 (1.7%) were positive. Three MSs (Germany, Italy and Slovakia) and one non‐MS (Switzerland) reported on testing for T. gondii in wildlife. In total, 1,299 animals (mainly from Italy and Slovakia) were tested and 150 were positive (11.5%).
Table 74.
Summary of Toxoplasma gondii statistics in the main animal species, EU, 2017–2021
| Animals (a) | 2021 (a) | 2020 | 2019 (b) | 2018 (b) | 2017 (b) | Data source |
|---|---|---|---|---|---|---|
| Small ruminants | ||||||
| Number of tested animals | 4,525 | 6,113 | 12,120 | 6,756 | 6,410 | EFSA |
| % of positive animals | 16.8 | 21.3 | 13.5 | 18.3 | 18.3 | EFSA |
| Number of reporting MSs | 12 | 10 | 11 | 12 | 13 | EFSA |
| Bovine animals | ||||||
| Number of tested animals | 726 | 254 | 664 | 158 | 2,163 | EFSA |
| % of positive animals | 2.5 | 9.8 | 9.2 | 27.8 | 10.6 | EFSA |
| Number of reporting MSs | 7 | 4 | 6 | 6 | 7 | EFSA |
| Pigs | ||||||
| Number of tested animals | 599 | 948 | 1,108 | 263 | 689 | EFSA |
| % of positive animals | 5.0 | 9.7 | 11.7 | 22.1 | 15.2 | EFSA |
| Number of reporting MSs | 5 | 3 | 4 | 4 | 4 | EFSA |
| Cats | ||||||
| Number of tested animals | 3,275 | 1,880 | 1,525 | 1,382 | 690 | EFSA |
| % of positive animals | 9.8 | 6.5 | 5.2 | 4.7 | 7.5 | EFSA |
| Number of reporting MSs | 8 | 6 | 8 | 9 | 8 | EFSA |
Summary statistics referring to MSs were obtained by totalling all sampling units (single animals, slaughter animal batches, and herds or flocks).
For the summary statistics, indirect and direct diagnostic methods were taken together to calculate the proportion of positive units.
MS: Member State.
: Data from the United Kingdom (Northern Ireland) are taken into account for 2021. In accordance with the agreement on the withdrawal of the United Kingdom from the EU, and in particular with the Protocol on Ireland/Northern Ireland, the EU requirements on data sampling are also applicable to Northern Ireland.
: Data from the United Kingdom are taken into account for 2017–2019, because the United Kingdom was an EU MS, but it became a third country on 1 February 2020.
2.4. Discussion
Cases of congenital toxoplasmosis in the EU are strongly biased by the high reporting rate of France, which has accounted for most reported cases since 2009 (ECDC Surveillance Atlas of Infectious Diseases), representing 83.3% to 75.1% of overall EU cases in 2016–2020 (excluding data from the United Kingdom). The high reporting rate for France reflects systematic screening for toxoplasmosis in pregnant women first established in 1978 and mandatory since 1992. Seronegative women are followed up during pregnancy to detect seroconversion early, and congenital toxoplasmosis cases are laboratory confirmed. The reported constant decrease in cases in the EU clearly mirrors the lower number of cases reported by France in the 2016–2020 period. In this 5‐year period, the most remarkable decrease in reported cases occurred in 2016 and 2017 (20.7% and 21.5, respectively) and continued in subsequent years, with the lowest rate in 2020. Decreased seroprevalence in pregnant women in France (from 54% in 1995, to 31% in 2016) and a decreased number of seroconversions during pregnancy (from 5.4 per 1,000 at‐risk pregnancies in 1995, to 2.1 in 2010, and expected to be 1.6 by 2020) have been reported, and may explain the decreasing number of reported cases of congenital toxoplasmosis in France (Robinson et al., 2021). Interestingly, the number of reported cases in France decreased by 11.2% and 17.9% between 2018 and 2019 and between 2019 and 2020, respectively, suggesting an additional effect of the COVID‐19 pandemic in the case reporting decrease in 2020. An educational campaign for pregnant women and reduced exposure to contaminated raw/undercooked meat (e.g. changes in food habits and improved hygiene practices in meat production) or other raw foods at risk of contamination (e.g. fresh produce, molluscs and raw milk) have likely contributed to the reduced incidence of T. gondii infection during pregnancy in France, reflecting the lower number of congenital toxoplasmosis cases (Opsteegh et al., 2015; Robinson et al., 2021). By contrast, surveillance for congenital toxoplasmosis among the other MSs is highly variable, with countries reporting zero cases or simply not reporting to ECDC, or countries lacking any surveillance. This may reflect discussions about the cost‐effectiveness of screening pregnant women in preventing or reducing the impact of congenital toxoplasmosis.
Due to imbalanced data, it is not possible to estimate the prevalence of congenital toxoplasmosis in the EU, limiting assessment of the burden of this form of the disease. In addition to prenatal screening, sensitive and effective postnatal diagnostic screening should be implemented and improved in the EU in order to detect cases of congenital toxoplasmosis. This is particularly important as most prenatal infections are sub‐clinical at birth or when maternal anti‐toxoplasma treatment is performed, which may decrease diagnostic sensitivity, thus generating false negatives (Guegan et al., 2021). Primary infection in pregnancy, particularly if early in gestation, and even in the asymptomatic form, is of particular concern. Even though the risk of congenital infection increases if the mother becomes infected late in pregnancy (third trimester), outcomes of congenital disease are much more severe if the mother became infected in the first trimester. This can result in abortion, still‐birth, perinatal death or congenital diseases with immediate or late (up to adolescence) manifestations, including ocular diseases, seizures and learning disabilities. All possible strategies for the prevention of congenital toxoplasmosis, including appropriate information to pregnant woman and active screening, should be reinforced.
The 2021 monitoring data from animals reported by MSs show that T. gondii is present in most livestock species across the EU, as well as in pet/zoo animals and wildlife. The limitations of these surveillance data preclude any trend watching or prevalence trend analysis in animals.
The current European surveillance system of T. gondii in animals is strongly affected by several important limitations: (i) the small number of tested animals, (ii) the use of different indirect and direct detection methods, which have not been validated by an independent body in most cases; (iii) unknown age of the tested animals; and (iv) no information on the type of husbandry system (housing). Furthermore, there is no relationship between the presence of anti‐T. gondii antibodies and infecting parasites in cattle and horses (Aroussi et al., 2015; Blaga et al., 2019; Opsteegh et al., 2019). For pigs, poultry and small ruminants, serological methods could be useful for the detection of high‐risk animals or herds, but not as an indicator of infection in individual animals, as the agreement between direct and indirect methods was estimated to be low to moderate.
The above‐mentioned limitations associated with toxoplasmosis detection and diagnosis, and surveillance rules, do not allow for direct comparison of the reported data across MSs.
3. Rabies
Summary data substantiating this chapter, as well as additional information on related projects and internet sources are published for this report on the EFSA Knowledge Junction on Zenodo here. Summary statistics on human surveillance data with downloadable files are retrievable using the ECDC Surveillance Atlas of Infectious Diseases here.
3.1. Key facts
In 2021, EU MSs and non‐MS countries reported no human lyssavirus infections, as in 2020. However, travel‐associated human rabies cases occurred regularly in Europe, as reported in recent years (N = 4 in 2019, N = 1 per year in the 2017–2018 period).
In animals excluding bats, a total of 118 cases of rabies of autochthonous origin were reported by two MSs: 113 cases in Poland (96 foxes, 2 wild roe deer, 2 martens, 2 raccoon dogs, 7 cats, one stray cat, 2 dogs and one stray dog) and five cases in Romania (four cows and one fox). The total number of reported indigenous rabies cases in non‐flying animals in the EU increased in 2021 compared with previous years (N = 12 in 2020, N = 5 in 2019; N = 8 in 2018; N = 6 in 2017). This is due to a rabies outbreak since 2021 in a Polish region that had been rabies‐free for more than 16 years.
Surveillance data on lyssavirus in bats were reported by 16 EU MSs. Four MSs reported 29 positive results for lyssavirus, mainly European bat 1 lyssavirus (EBLV‐1).
A case of rabies was reported by Germany in an illegally imported dog infected with a rabies virus (RABV) strain.
3.2. Surveillance and monitoring of rabies in the EU
3.2.1. Humans
Reporting of rabies is mandatory in all 27 MSs. In 2021, all countries except Denmark reported case‐based data. Twenty‐three MSs used the EU case definition, three countries (Denmark, Germany and Italy) used another case definition and France did not specify the case definition used. Disease surveillance is comprehensive in all reporting countries.
3.2.2. Animals
The objective of passive rabies surveillance is to detect the presence and the geographic distribution of the virus over time, to allow timely dissemination of information for immediate integrated control actions among different sectors, such as the public health and veterinary sectors. For rabies‐free countries, surveillance aims to confirm the absence of the disease. In accordance with Regulation (EU) No 652/2014 36 and Commission Delegated Regulation (EU) No 2020/689 37 , 37 multiannual programmes for eradication of rabies may be co‐financed by the EU. In 2021, 12 MSs had approved elimination, control and surveillance programmes for rabies and oral rabies vaccination (ORV) campaigns were conducted in 8 MSs (Croatia, Estonia, Finland, Hungary, Latvia, Lithuania, Poland and Slovakia), as well as in some of the EU‐bordering countries. Surveillance of rabies is carried out by sampling and testing ‘indicator animals’; these are wild or domestic animals (foxes, raccoon dogs, jackals, badgers, dogs, cattle, cats, sheep, equines, goats, etc.) that are found dead (including road‐killed) and/or suspect animals, i.e. animals showing neurological clinical signs or abnormal behaviour compatible with rabies like biting, licking a wound or scratching a human being in the absence of clear neurological signs.
To monitor the efficacy of the ORV campaigns, healthy animals of the wild species targeted by oral vaccination, which are foxes, raccoon dogs and golden jackals, are hunted. These animals' carcases are used to determine immunity and oral vaccine bait uptake. This specific active rabies surveillance is traditionally designated as ‘ORV monitoring’ or ‘monitoring’. These hunted animals can also be tested for rabies and very few of them (below 5%) are usually found to be positive for the disease.
Imported or travel‐related companion animals (mainly dogs and cats) from territories and non‐EU countries not included in Annex II of Commission Implementing Regulation (EU) No 577/2013 38 are currently tested for rabies virus neutralising antibodies.
In accordance with CIR (EU) 2020/2002, EU MSs must notify outbreaks of infection with rabies virus in non‐flying animals to the EU ADIS 3 ; these are the animal species and groups of species Carnivora, Bovidae, Suidae, Equidae, Cervidae and Camelidae, but not Chiroptera (bats).
The data reported here include all animals tested for rabies, collected for disease surveillance and for ORV monitoring (active surveillance) purposes.
3.3. Results
3.3.1. Overview of key statistics, EU, 2017–2021
A summary of EU‐level rabies statistics in humans and in wild and domestic animals is shown in Table 75. For animals, the total number of samples analysed for passive surveillance from foxes, raccoon dogs, golden jackals, dogs, cats and bats, as well as the number of MSs from which these samples originated, are shown. An increase was observed in the number of tested samples of foxes, which are the main reservoir of the virus, compared with 2020. The number of tested raccoon dogs remained stable, while the number of golden jackals more than doubled in 2021 compared with 2020. In 2021, the number of tested bats remained stable as compared with 2020, and the substantial decrease observed in 2020 was confirmed for 2021 as compared with the 2017–2019 period. For cats, dogs and farmed animals, the number of tested samples remained stable in 2021 as compared with 2020.
Table 75.
Summary of rabies lyssavirus statistics related to humans and the main animal reservoirs, EU, 2017–2021
| 2021 (a) | 2020 | 2019 (b) | 2018 (b) | 2017 (b) | Data Source | |
|---|---|---|---|---|---|---|
| Humans | ||||||
| Total number of confirmed cases | 0 | 0 | 4 | 1 | 1 | ECDC |
| Total number of confirmed cases/100,000 population (notification rates) | 0 | 0 | 0 | 0 | 0 | ECDC |
| Number of reporting countries | 26 | 26 | 28 | 28 | 28 | ECDC |
| Infection acquired in the EU | 0 | 0 | 1 | 0 | 0 | ECDC |
| Infection acquired outside the EU | 0 | 0 | 3 | 1 | 1 | ECDC |
| Unknown travel status or unknown country of infection | 0 | 0 | 0 | 0 | 0 | ECDC |
| Animals under passive surveillance | ||||||
| Dogs (Canis lupus familiaris) | ||||||
| Number of tested animals | 1,838 | 1,732 | 1,901 | 2,097 | 2,334 | EFSA |
| Number of positive animals | 4 | 4 | – | 1 | 1 | EFSA |
| Number of reporting MSs | 21 | 22 | 22 | 23 | 22 | EFSA |
| Cats (Felis catus) | ||||||
| Number of tested animals | 2,335 | 2,440 | 2,389 | 2,661 | 2,722 | EFSA |
| Number of positive animals | 8 | 2 | – | – | 1 | EFSA |
| Number of reporting MSs | 20 | 21 | 22 | 21 | 23 | EFSA |
| Farmed mammals (c) | ||||||
| Number of tested animals | 406 | 392 | 394 | 570 | 796 | EFSA |
| Number of positive animals | 4 | 3 | 1 | 1 | 3 | EFSA |
| Number of reporting MSs | 17 | 17 | 15 | 17 | 17 | EFSA |
| Wild animals – Red foxes (Vulpes vulpes) | ||||||
| Number of tested animals | 12,907 | 9,805 | 5,338 | 5,833 | 10,808 | EFSA |
| Number of positive animals | 97 | 6 | 2 | 6 | 2 | EFSA |
| Number of reporting MSs | 17 | 18 | 16 | 16 | 17 | EFSA |
| Wild animals – Raccoon dogs (Nyctereutes procyonoides) | ||||||
| Number of tested animals | 1,339 | 1,214 | 1,241 | 1,335 | 712 | EFSA |
| Number of positive animals | 2 | – | – | – | – | EFSA |
| Number of reporting MSs | 6 | 6 | 7 | 7 | 6 | EFSA |
| Wild animals – Jackals (Canis aureus) | ||||||
| Number of tested animals | 230 | 102 | 42 | 44 | 1,000 | EFSA |
| Number of positive animals | – | – | – | – | – | EFSA |
| Number of reporting MSs | 5 | 6 | 5 | 4 | 5 | EFSA |
| Wild animals – Bats (order Chiroptera) | ||||||
| Number of tested animals | 1,316 | 1,308 | 2,069 | 2,278 | 2,079 | EFSA |
| Number of positive animals | 29 | 31 | 39 | 45 | 39 | EFSA |
| Number of reporting MSs | 16 | 15 | 19 | 18 | 19 | EFSA |
| Animals under active surveillance (ORV monitoring) (d) | ||||||
| Red foxes (Vulpes vulpes) | ||||||
| Number of tested animals | 10,581 | 14,416 | 17,805 | 15,737 | 19,677 | EFSA |
| Number of positive animals | – | – | 1 | – | – | EFSA |
| Number of reporting MSs | 9 | 10 | 9 | 9 | 9 | EFSA |
| Raccoon dogs (Nyctereutes procyonoides) | ||||||
| Number of tested animals | 369 | 324 | 301 | 23 | 280 | EFSA |
| Number of positive animals | – | – | – | – | – | EFSA |
| Number of reporting MSs | 4 | 4 | 3 | 2 | 3 | EFSA |
| Jackals (Canis aureus) | ||||||
| Number of tested animals | 1,499 | 1,319 | 1,045 | 1,304 | 870 | EFSA |
| Number of positive animals | – | – | – | – | – | EFSA |
| Number of reporting MSs | 3 | 3 | 2 | 3 | 2 | EFSA |
ECDC: European Centre for Disease Prevention and Control; EFSA: European Food Safety Authority; MSs: Member States. ORV oral rabies vaccination.
: Data on animal samples from the United Kingdom (Northern Ireland) are taken into account for 2021. In accordance with the agreement on the withdrawal of the United Kingdom from the EU, and in particular with the Protocol on Ireland/Northern Ireland, the EU requirements on data sampling are also applicable to Northern Ireland.
: Data from the United Kingdom are taken into account for the 2017–2019 period because the United Kingdom was an EU MS, but it became a third country on 1 February 2020.
: Animals that are commonly raised or kept for breeding purposes or animal by‐products.
: Samples collected in the framework of oral rabies vaccination monitoring (active surveillance).
Table 75 also displays the active surveillance (ORV monitoring) statistics for ORV target species (foxes, raccoon dogs and golden jackals). The number of tested foxes decreased considerably, while the number of tested raccoon dogs and golden jackals increased in 2021, as compared with the previous years.
3.3.2. Humans
EU MSs and non‐MS countries reported no human lyssavirus infections for 2021, as was observed in 2020. In the 2017–2019 period, travel‐associated cases were reported each year from EU MSs. In 2017, France reported one travel‐associated case, with exposure occurring in Sri Lanka. In 2018, the United Kingdom reported one human case following exposure in Morocco. In 2019, four human lyssavirus infections were reported by EU MSs. Three of these were travel‐associated, reported by Italy, Latvia and Spain, and acquired in Tanzania, India and Morocco, respectively. Most of the human cases were linked to dog exposure. The fourth human infection in 2019 was reported in France and was due to European bat lyssavirus 1 (EBLV‐1).
3.3.3. Animals
Rabies cases in wildlife
In 2021, 12,907 foxes (Vulpes vulpes) were tested using passive surveillance by 17 MSs. The majority of the tested samples (60.5%) were analysed by two MSs: Poland and Romania. In total, 97 cases of rabies in foxes were detected in the EU: 96 cases in Poland and one in Romania. The geographical distribution and number of cases in foxes, as well as a choropleth map of the total number of foxes sampled per MS for passive surveillance, are shown in Figure 35. Three non‐EU countries (Norway, North Macedonia, and Switzerland) reported a total of 21 foxes tested under passive surveillance schemes and found none positive.
Figure 35.
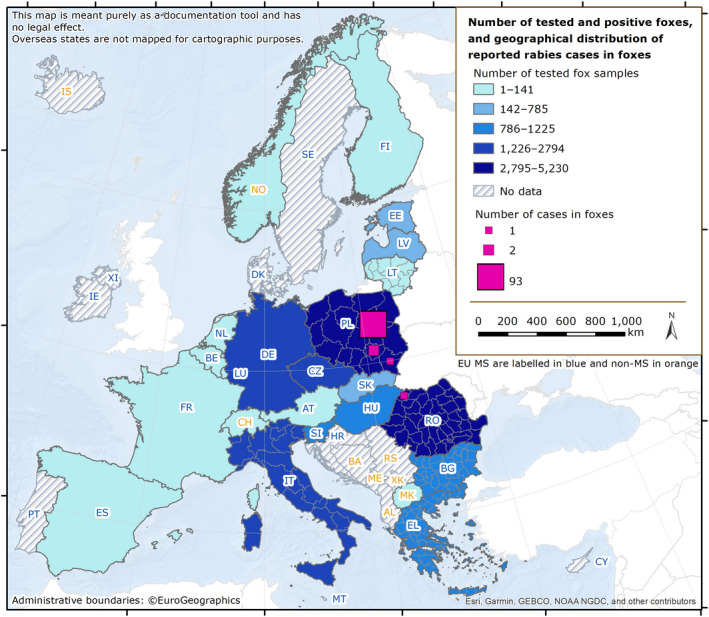
Choropleth map of the number of tested and positive foxes, and the geographical distribution of the rabies cases reported in foxes in EU MSs and non‐EU countries, 2021
In 2021, 1,339 raccoon dogs (Nyctereutes procyonoides) were tested for rabies by six MSs (Estonia, Finland, Latvia, Lithuania, Poland and Slovakia). Most (93.4%) of these samples came from raccoon dogs originating from two MSs (Estonia and Latvia). Two tested samples (out of a total of 38) originating from Poland were positive for rabies. Regarding golden jackals, 89.5% of the samples tested came from two MSs (Bulgaria and Romania).
Seventeen MSs reported data of passive surveillance for 714 non‐flying wild animals other than foxes, raccoon dogs or golden jackals. The other most widely tested wild animal species were deer and roe deer (152), badgers (148), martens (108) and raccoons (98). In Poland, two roe deer and two martens were tested positive for rabies. Other species tested included wolf, lynx, bear, ferret, hedgehog, mouse, mink, otter, polecat, rat, squirrel, wild boar, wolverine, coypus, moose, beaver, weasel and mole. All the animals tested negative for rabies.
In 2021, 16 MSs and two non‐MSs reported surveillance data on bats. In total, 1,316 bats were investigated in the EU (Figure 36). Of these, 29 samples tested positive in four MSs: Germany (16 EBLV‐1), France (5 EBLV‐1), Poland (5 unspecified lyssavirus) and Spain (3 EBLV‐1). Two non‐MSs, Norway and Switzerland, analysed 4 and 18 bats, respectively and all samples tested negative.
Figure 36.
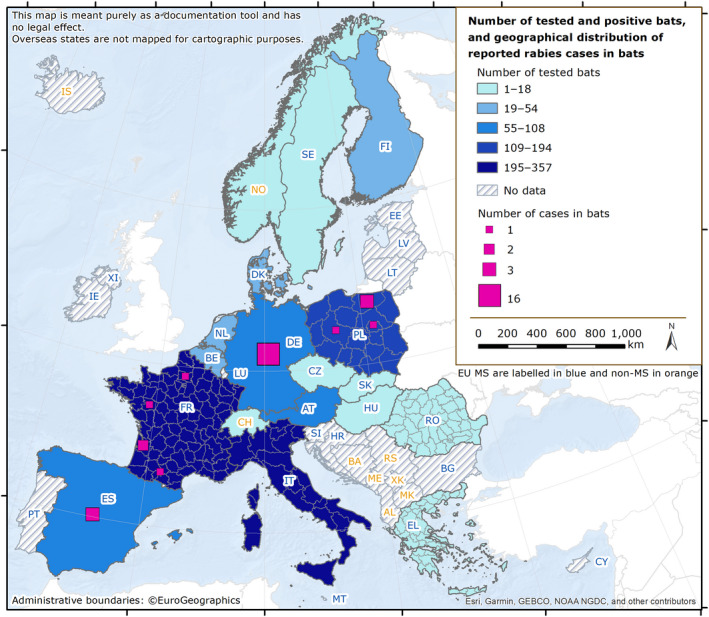
- For Germany and Spain, the geographical distribution of reported cases was not provided.
Rabies cases in domestic animals
In 2021, a total of 15 autochthonous domestic animals were tested positive for rabies. Romania reported four cases of rabies (unspecified lyssavirus) in cows. Poland recorded two cases in pet dogs, one case in a stray dog, seven cases in pet cats and one case in a stray cat. Except for the stray cat for which a rabies virus (RABV) strain was identified, all the other cases reported the strains as unspecified lyssavirus.
In Germany, one dog was reported positive for rabies. This unvaccinated 8‐week‐old puppy had been imported illegally from Turkey via Bulgaria. Genetic analysis revealed that it was an RABV strain showing a high sequence identity with a Turkish RABV fox isolate.
The geographical distribution and number of tested and reported cases in pets (dogs and cats) are shown in Figure 37
Figure 37.
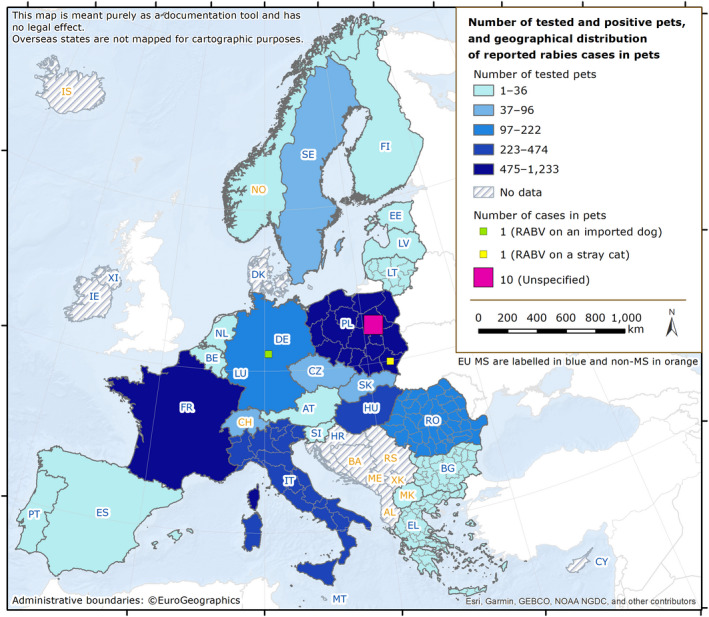
- For Germany, the geographical distribution of the reported case was not provided.
In 2021, 21 MSs tested and reported 4,173 samples for dogs (1,838) and cats (2,335 from 20 MSs). The number of samples reported for both species remained similar to 2019 (1,732 dogs and 2,440 cats were reported in 2019). Three non‐EU countries (Norway, North Macedonia and Switzerland) reported in total 72 tested dogs and 24 tested cats. None of them reported positive cases for rabies.
A total of 406 samples from farmed mammals (Figure 38) were tested by 17 MSs (reports included mainly cattle, small ruminants and domestic solipeds). The number of samples tested from domestic farmed mammals in 2021 was slightly higher than in 2020 (392 samples tested) but lower than in 2017 or and 2018.
Figure 38.
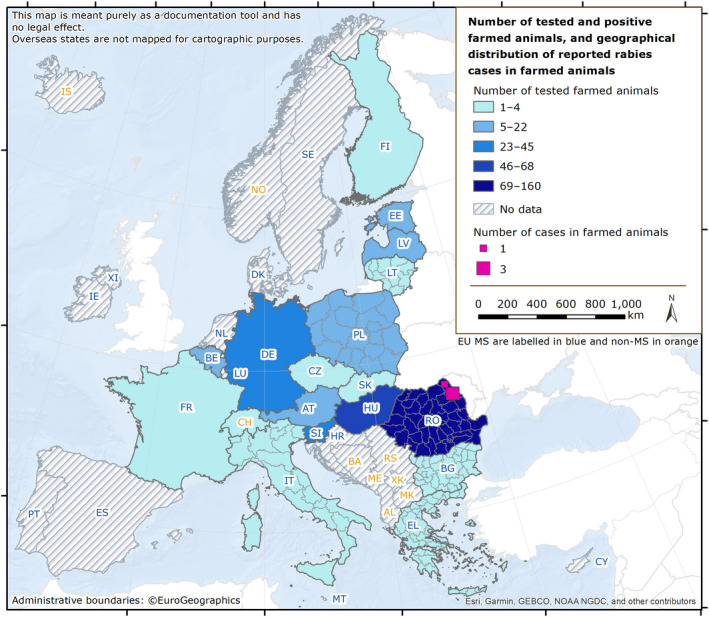
Choropleth map of the number of tested and positive farmed animals, and the geographical distribution of the rabies cases reported in farmed animals in EU MSs and non‐EU countries, 2021
3.4. Discussion
In Europe, human rabies is a rare disease, with the last autochthonous EU case of RABV human infection dating back to 2012 (Romania). Nowadays, the infection is mainly acquired abroad in countries where dog rabies is endemic and the development of the disease is due to the absence of pre‐exposure prophylaxis or late/inappropriate/incomplete administration of post‐exposure treatment. The illegal importation of pets also poses a constant risk of rabies introduction (Klevar et al., 2015). Another rare source of infection is through organ transplantation (Maier et al., 2010). The absence of human rabies deaths in 2021, and this for the second consecutive year, might be attributable to reduced travel due to the COVID‐19 mitigation measures applied in many MSs.
Among infections caused by lyssaviruses other than RABV, five human deaths have been reported so far in Europe, more specifically in Ukraine (1977: species not characterised), Russia (1985: EBLV‐1), Finland (1985: EBLV‐2), the United Kingdom (2002: EBLV‐2) and France (2019: EBLV‐1) (Fooks et al., 2003; Regnault et al., 2022). All these infections were linked to direct exposure to infected bats; however, indirect exposure to lyssaviruses by contact with infected domestic animals (mainly free‐roaming cats, occasionally infected by a bat lyssavirus) must not be overlooked. In this context, the absence of tools to prevent divergent lyssaviruses from circulating in European bats must be underlined (Echevarría et al., 2019).
The EU programmes for rabies eradication include disease surveillance, oral vaccination campaigns, the monitoring of ORV and awareness activities. Results show spread of the infection in 2021 in the European wildlife reservoir, with a total of 118 autochthonous rabies cases reported in foxes and in domestic animals in Poland (113 cases) and Romania (5 cases), as compared with 12 cases reported in the same countries in 2020. The epidemiological data suggest stability in rabies incidence, with no more than five cases reported annually since 2017 in non‐flying animals in most of the MSs reporting infections, except in Poland, which has been experiencing a rabies outbreak with domestic animal infections since 2021 in a Polish region that had been rabies‐free for more than 16 years.
Data relating to rabies passive surveillance in wildlife, mainly in foxes and raccoon dogs, show relative stability in the number of samples tested, and even a slight increase in the fox sampling effort, due to the surveillance efforts maintained by two MSs for controlling the last foci. Data related to active surveillance (ORV monitoring) are reported for foxes, raccoon dogs and jackals. This surveillance is conducted by hunting apparently healthy animals and using the animals' carcases to assess the efficacy of vaccination campaigns in infected and rabies‐free countries involved in eradication programmes. A sample size linked to the area covered by vaccination is recommended. The data show a substantial decrease over the years in the number of analysed foxes, reflecting the decrease in the size of areas vaccinated in the EU, hence the success of ORV programmes. The cases still being reported for several years in the few remaining MSs with infections, or a re‐emergence of rabies, highlight the importance of a sustainable surveillance programme and awareness campaigns for the general public and professionals to ensure the early detection of any potential rabies cases.
Regarding rabies surveillance in bats, the number of tests has decreased since 2020. This is partly due to the withdrawal of the United Kingdom from the EU, since the UK analysed 347 bats in 2020 and 488 in 2019. The decrease observed is also related to a slightly lower number of tested bats in most reporting countries (for example, 660 and 308 samples analysed in France in 2019 and 2021, respectively and 275 and 194 bats tested in Poland in 2019 and 2021, respectively), as well as a slight decrease in the number of MSs reporting. Positive results obtained in the framework of bat surveillance (N = 29 cases) are in line with the findings of the previous years and confirm that European bats act as reservoirs for lyssaviruses other than rabies virus, reaffirming the public recommendation to handle bats with utmost caution, if at all. The public health hazard of bat lyssaviruses in Europe should not be underestimated.
In 2021, one case of imported rabies in a pet (a dog located in Lower Saxony in Germany) was reported. Such imported cases are recurrent in the EU (at a frequency of approximately one imported case per year (EFSA et al., 2022a)) and might pose a threat of rabies reintroduction into rabies‐free areas.
This also underlines the need to improve public awareness, particularly among travellers, with regard to rabies risks and legislation involving pet movements. As rabies is still endemic in countries bordering the EU, in areas not far from the borders, several MSs are involved in collaborations with these countries for the implementation of vaccination and testing schemes in buffer zones. The Global Framework for the Progressive Control of Transboundary Animal Diseases (GF‐TADs) created a new Standing Group of Experts on Rabies (SGE RAB) in 2019, and the third meeting was organised in 2021 with the goal of coordinating rabies control and improving surveillance activities, primarily in the Balkan sub‐region, where a case was detected in a dog in 2020.
Maintaining appropriate surveillance is of paramount importance for all MSs, due to resurgence of the disease in Poland and the persistence of active foci in Romania. Apparent disappearance of the virus has been achieved in most EU territories, and maintaining appropriate surveillance efforts remains the most challenging issue to attaining rabies elimination in the EU.
4. Q fever
Summary data substantiating this chapter, as well as additional information on related projects and internet sources are published for this report on the EFSA Knowledge Junction on Zenodo here. Summary statistics on human surveillance data with downloadable files are retrievable using the ECDC Surveillance Atlas of Infectious Diseases here.
4.1. Key facts
In 2021, the number of confirmed cases of human Q fever was 460 corresponding to an EU notification rate of 0.11 per 100,000 population. This is a decrease of 12.0% compared with the rate in 2020 (0.12 per 100,000 population).
Compared with the rate before the COVID‐19 pandemic (2017–2019 annual mean), there was a decrease of 38.8% and 45.8% with or without data from the United Kingdom.
Over the past 5 years (2017–2021), a significant decreasing trend (p < 0.05) in the number of Q fever cases was observed.
In 2021, Q fever cases occurred from April to September, in line with the spring/summer seasonal pattern. Cases were highest for the 50–55 years age group.
In animals, cattle and small ruminants were mostly sampled during clinical investigations and passive monitoring of animals suspected to be infected with Coxiella burnetii. However, in the absence of harmonised reporting data in animals in the EU, the data reported to EFSA cannot be used to analyse spatial representativeness and trends over the years for Q fever at the EU level or to compare differences among reporting countries.
In total, 17 MSs (15 in 2020) and five non‐MSs (six in 2020) reported 2021 data for C. burnetii. The proportion of positive animals with direct tests was 5.9% in sheep (8.7% in 2020), 16.5% in goats (11.3% in 2020) and 5.2% in cattle (3.8% in 2020). The proportion of positive herds with direct tests was 4.1% in sheep (1.4% in 2020), 2.0% in goats (1.2% in 2020) and 4.8% in cattle (6.7% in 2020). The proportion of seropositive animals was 10.3% in sheep (11.4% in 2020), 24.6% in goats (25.0% in 2020) and 12.2% in cattle (9.6% in 2020). The proportion of seropositive herds was 18.9% in sheep (5.9% in 2020), 50.0% in goats (78.7% in 2020) and 15.1% in cattle (14.4% in 2020). Results from various other domestic and wild animal species were reported and only Italy reported positive results, mainly from dogs (73.2% out of 541) and water buffalos (4.7% out of 43).
4.2. Surveillance and monitoring of Coxiella burnetii in the EU
4.2.1. Humans
In 2021, 25 EU MSs provided data on Q fever in humans. No surveillance system is in place in Austria. Denmark did not report data for 2020 or 2021. Surveillance is mandatory in 24 MSs and voluntary in France. In 2020–2021, Spain did not receive data from all its regions; case numbers may therefore be incomplete. The EU case definition was used by 22 countries; three MSs (France, Germany and Italy) reported use of another case definition. All reporting countries had a comprehensive surveillance system. All countries reported case‐based data except Belgium and Bulgaria, which provided aggregated data.
4.2.2. Animals
Commission Implementing Regulation CIR (EU) 2018/1882 39 lists Q fever as a category E disease, meaning that, in accordance with CIR (EU) 2020/2002 40 , MSs shall report to the ADIS by 30 April of every year, covering the previous calendar year, on the detection of disease that has been confirmed on their territory in Bison ssp., Bos ssp., Bubalus ssp., Ovis ssp. and Capra ssp. MSs also submit data from annual surveillance and monitoring activities to EFSA in accordance with Directive 2003/99/EC. Q fever falls under Annex I, B. 4. ‘Other zoonoses and zoonotic agents’ as an agent to be monitored if warranted by the epidemiological situation in an MS, in compliance with Article 4.1 of the same Directive.
As there is no harmonised monitoring system in place for Q fever in animals in the EU, data can only be used for descriptive summaries and preclude analyses such as tracking or assessing EU‐level temporal and spatial trends.
The main animal species tested are sheep, goats and cattle. Samples are mostly blood samples, samples from fetuses and stillborn animals, placentas, vaginal swabs from animals suspected of being infected with C. burnetii, as well as bulk milk samples for screening. Samples are tested either by serological methods (proving past or recent exposure to C. burnetii) or direct detection (showing carriage). In most MSs, reporting was based on clinical investigation and passive monitoring. A few countries (Belgium, Bulgaria, the Netherlands, Poland) implemented planned surveillance in cattle and small ruminants, using mainly direct detection in herds in Poland (N = 4,940) and Belgium (N = 237), and mainly ELISA serological tests at the individual animal level. Belgium and the Netherlands carried out regular PCR tests on bulk tank milk from dairy sheep and goats. Some countries (Denmark, Finland, Slovenia and Spain) reported very low numbers of tests (< 100 units), corresponding to local surveys or selective tests. In addition, samples were taken from other domestic animals (dogs in particular for Italy in 2021) and wild animals on farms, in zoos or from natural habitats.
4.3. Results
4.3.1. Overview of key statistics, EU, 2017–2021
Table 76 summarises EU‐level statistics on Q fever in humans and in major animal species, respectively, during 2017–2021. More detailed descriptions of these statistics are provided in the below subsections.
Table 76.
Summary of Coxiella burnetii statistics regarding humans and the main animal species, EU, 2017–2021
| 2021 | 2020 | 2019 (a) | 2018 (a) | 2017 (a) | Data source | |
|---|---|---|---|---|---|---|
| Humans | ||||||
| Total number of confirmed cases | 460 | 523 | 951 | 790 | 884 | ECDC |
| Total number of confirmed cases/100,000 population (notification rates) | 0.11 | 0.12 | 0.19 | 0.16 | 0.18 | ECDC |
| Number of reporting EU MSs | 25 | 25 | 27 | 27 | 27 | ECDC |
| Infection acquired in the EU | 359 | 347 | 810 | 629 | 720 | ECDC |
| Infection acquired outside the EU | 3 | 6 | 14 | 12 | 9 | ECDC |
| Unknown travel status or unknown country of infection | 98 | 170 | 127 | 149 | 155 | ECDC |
| Animals | ||||||
| Sheep (b) | ||||||
| Animals | ||||||
| Serology (c) | ||||||
| Number of tested animals | 750 | 911 | 666 | 2,986 | 867 | EFSA |
| % positive animals | 10.3 | 11.4 | 9.9 | 14.4 | 6.0 | EFSA |
| Number of reporting MSs | 8 | 8 | 9 | 10 | 7 | EFSA |
| Direct detection (d) | ||||||
| Number of tested animals | 85 | 358 | 224 | 358 | 359 | EFSA |
| % positive animals | 5.9 | 8.7 | 18.3 | 12.8 | 8.9 | EFSA |
| Number of reporting MSs | 5 | 4 | 3 | 4 | 3 | EFSA |
| Other methods/unknown | ||||||
| Number of tested animals | 521 | 1,555 | 2,244 | 802 | 1,166 | EFSA |
| % positive animals | < 0.01 | 21.7 | 13.3 | 0.1 | 15.2 | EFSA |
| Number of reporting MSs | 1 | 2 | 2 | 3 | 2 | EFSA |
| Herds | ||||||
| Serology (c) | ||||||
| Number of tested herds | 2,238 | 17 | 25 | 197 | 3,654 | EFSA |
| % positive herds | 18.9 | 5.9 | 28.0 | 8.6 | 2.7 | EFSA |
| Number of reporting MSs | 1 | 2 | 1 | 3 | 4 | EFSA |
| Direct detection (d) | ||||||
| Number of tested herds | 3,130 | 2,890 | 2,902 | 3,480 | 896 | EFSA |
| % positive herds | 4.1 | 1.4 | 1.2 | 0.9 | 9.6 | EFSA |
| Number of reporting MSs | 4 | 4 | 4 | 4 | 3 | EFSA |
| Other methods/unknown | ||||||
| Number of tested herds | 0 | 39 | 53 | 1 | 342 | EFSA |
| % positive herds | 0 | 15.4 | 30.2 | < 0.01 | 20.2 | EFSA |
| Number of reporting MSs | 0 | 1 | 1 | 1 | 1 | EFSA |
| Goats | ||||||
| Animals | ||||||
| Serology (c) | ||||||
| Number of tested animals | 540 | 651 | 656 | 947 | 464 | EFSA |
| % positive animals | 24.6 | 25.0 | 18.0 | 20.6 | 3.7 | EFSA |
| Number of reporting MSs | 6 | 7 | 8 | 9 | 8 | EFSA |
| Direct detection (d) | ||||||
| Number of tested animals | 200 | 248 | 189 | 217 | 148 | EFSA |
| % positive animals | 16.5 | 11.3 | 9.5 | 12.4 | 9.5 | EFSA |
| Number of reporting MSs | 4 | 3 | 4 | 5 | 2 | EFSA |
| Other methods/unknown | ||||||
| Number of tested animals | 777 | 831 | 845 | 1,076 | 1,241 | EFSA |
| % positive animals | 0.13 | 0 | 0 | 0 | 8.0 | EFSA |
| Number of reporting MSs | 1 | 2 | 2 | 2 | 2 | EFSA |
| Herds | ||||||
| Serology (c) | ||||||
| Number of tested herds | 4 | 141 | 30 | 212 | 216 | EFSA |
| % positive herds | 50.0 | 78.7 | 73.3 | 25.5 | 25.9 | EFSA |
| Number of reporting MS | 1 | 1 | 1 | 3 | 3 | EFSA |
| Direct detection (d) | ||||||
| Number of tested herds | 1,283 | 1,175 | 1,167 | 1,318 | 1,251 | EFSA |
| % positive herds | 2.0 | 1.2 | 2.8 | 3.0 | 2.6 | EFSA |
| Number of reporting MSs | 5 | 4 | 4 | 4 | 4 | EFSA |
| Other methods/unknown | ||||||
| Number of tested herds | 0 | 12 | 207 | 11 | 0 | EFSA |
| % positive herds | 0 | 0.0 | 84.1 | 36.4 | 0 | EFSA |
| Number of reporting MSs | 0 | 1 | 1 | 1 | 0 | EFSA |
| Cattle (bovine animals) | ||||||
| Animals | ||||||
| Serology (c) | ||||||
| Number of tested animals | 3,405 | 4,664 | 8,722 | 14,795 | 8,760 | EFSA |
| % positive animals | 12.2 | 9.6 | 10.5 | 9.8 | 12.4 | EFSA |
| Number of reporting MSs | 11 | 9 | 11 | 11 | 10 | EFSA |
| Direct detection (d) | ||||||
| Number of tested animals | 458 | 842 | 739 | 4,703 | 4,963 | EFSA |
| % positive animals | 5.2 | 3.8 | 3.0 | 5.1 | 6.0 | EFSA |
| Number of reporting MSs | 458 | 842 | 739 | 4,703 | 4,963 | EFSA |
| Other methods/unknown | ||||||
| Number of tested animals | 343 | 3,860 | 4,240 | 3,963 | 2,549 | EFSA |
| % positive animals | 0.29 | 0.08 | 0.66 | 2.8 | 0.43 | EFSA |
| Number of reporting MSs | 1 | 2 | 2 | 3 | 4 | EFSA |
| Herds | ||||||
| Serology (c) | ||||||
| Number of tested herds | 1,201 | 312 | 551 | 2,283 | 246 | EFSA |
| % positive herds | 15.1 | 14.4 | 30.7 | 10.2 | 21.1 | EFSA |
| Number of reporting MSs | 1 | 2 | 2 | 4 | 2 | EFSA |
| Direct detection (d) | ||||||
| Number of tested herds | 4,311 | 3,571 | 3,673 | 1,262 | 1,244 | EFSA |
| % positive herds | 4.8 | 6.7 | 6.3 | 2.6 | 14.4 | EFSA |
| Number of reporting MSs | 8 | 5 | 5 | 5 | 4 | EFSA |
| Other methods/unknown | ||||||
| Number of tested herds | 0 | 0 | 43 | 132 | 395 | EFSA |
| % positive herds | 0 | 0 | 95.3 | 3.8 | 3.8 | EFSA |
| Number of reporting MSs | 0 | 0 | 1 | 1 | 1 | EFSA |
ECDC: European Centre for Disease Prevention and Control; EFSA: European Food Safety Authority; MSs: Member States.
: Data from the United Kingdom are taken into account for 2017–2019, because the United Kingdom was an EU MS, but it became a third country on 1 February 2020.
: ‘Sheep’ includes the ‘sheep and goats’ category (NB, some countries report both livestock types together).
: Serology includes the complement fixation test (CFT), ELISA, competitive ELISA (C‐ELISA), indirect ELISA (I‐ELISA), IgG ELISA and the immunofluorescence antibody test (IFAT).
: Direct detection methods include fluorescent in situ hybridisation (FISH), immunohistochemistry (IHC), microbiological tests, multiplex PCR, PCR, real‐time PCR, real‐time PCR (CEN TC 275/WG 6).
Animal categories
Animal data of interest were classified into three major species categories (sheep, goats and cattle) and aggregated by year to obtain an annual overview of the volume of data submitted. The total number and the proportions of positive animals and herds were categorised according to the type of analytical testing method used (serological or direct detection).
In 2021, compared with the year 2020, the total number of samples from animals submitted by EU MSs decreased slightly for goats and more drastically for sheep and cattle. Concomitantly, the number of serological results from herds increased for sheep and for cattle (only reported this year by Italy), and slightly decreased for goats; however, the number of direct tests increased slightly for all three species.
4.3.2. Coxiella burnetii in humans
In 2021, 25 EU MSs reported a total of 460 confirmed cases of Q fever, with a notification rate of 0.11 cases per 100,000 population. This is a decrease of 12.0% compared with the rate in 2020 (0.12 per 100,000 population) and a decrease of 38.8% and 45.8% compared with the average annual notification rate from 2017 to 2019 (pre‐pandemic period), with or without data from the United Kingdom. In 2021, Bulgaria had the highest notification rate with 0.45 cases per 100,000 population, followed by Hungary and Spain with 0.42 and 0.31 cases per 100,000 population, respectively (Table 77).
Table 77.
Reported confirmed human cases of Q fever and notification rates per 100,000 population in EU MSs and non‐MS countries, by country and year, 2017–2021
| Country | 2021 | 2020 | 2019 | 2018 | 2017 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| National coverage (a) | Data format (a) | Confirmed cases and rates | Confirmed cases and rates | Confirmed cases and rates | Confirmed cases and rates | Confirmed cases and rates | ||||||
| Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | |||
| Austria (b) | – | – | – | – | – | – | – | – | – | – | – | – |
| Belgium | Y | A | 6 | 0.05 | 4 | 0.03 | 10 | 0.09 | 6 | 0.05 | 7 | 0.06 |
| Bulgaria | Y | A | 31 | 0.45 | 103 | 1.48 | 36 | 0.51 | 45 | 0.64 | 28 | 0.39 |
| Croatia | Y | C | 0 | 0 | 2 | 0.05 | 8 | 0.20 | 11 | 0.27 | 23 | 0.55 |
| Cyprus | Y | C | 2 | 0.22 | 1 | 0.11 | 1 | 0.11 | 0 | 0 | 3 | 0.35 |
| Czechia | Y | C | 1 | 0.01 | 1 | 0.01 | 1 | 0.01 | 1 | 0.01 | 0 | 0 |
| Denmark | Y | C | – | – | – | – | 0 | 0 | 0 | 0 | 0 | 0 |
| Estonia | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Finland | Y | C | 0 | 0 | 0 | 0 | 2 | 0.04 | 2 | 0.04 | 4 | 0.07 |
| France | Y | C | 92 | 0.14 | 96 | 0.14 | 156 | 0.23 | 172 | 0.26 | 194 | 0.29 |
| Germany | Y | C | 99 | 0.12 | 55 | 0.07 | 148 | 0.18 | 91 | 0.11 | 107 | 0.13 |
| Greece | Y | C | 4 | 0.04 | 4 | 0.04 | 14 | 0.13 | 13 | 0.12 | 4 | 0.04 |
| Hungary | Y | C | 41 | 0.42 | 34 | 0.35 | 47 | 0.48 | 28 | 0.29 | 29 | 0.30 |
| Ireland | Y | C | 0 | 0 | 2 | 0.04 | 2 | 0.04 | 0 | 0 | 2 | 0.04 |
| Italy | Y | C | 0 | 0 | 0 | 0 | 6 | 0.01 | 1 | < 0.01 | 7 | 0.01 |
| Latvia | Y | C | 0 | 0 | 1 | 0.05 | 0 | 0 | 0 | 0 | 0 | 0 |
| Lithuania | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Luxembourg | Y | C | 0 | 0 | 2 | 0.32 | 0 | 0 | 0 | 0 | 0 | 0 |
| Malta | Y | C | 1 | 0.19 | 0 | 0 | 1 | 0.20 | 2 | 0.42 | 0 | 0 |
| Netherlands | Y | C | 6 | 0.03 | 7 | 0.04 | 16 | 0.09 | 18 | 0.10 | 22 | 0.13 |
| Poland | Y | C | 0 | 0 | 0 | 0 | 4 | 0.01 | 0 | 0 | 0 | 0 |
| Portugal | Y | C | 18 | 0.17 | 22 | 0.21 | 32 | 0.31 | 36 | 0.35 | 48 | 0.47 |
| Romania | Y | C | 5 | 0.03 | 12 | 0.06 | 109 | 0.56 | 22 | 0.11 | 46 | 0.23 |
| Slovakia | Y | C | 2 | 0.04 | 5 | 0.09 | 1 | 0.02 | 2 | 0.04 | 0 | 0 |
| Slovenia | Y | C | 0 | 0 | 1 | 0.05 | 6 | 0.29 | 1 | 0.05 | 3 | 0.15 |
| Spain | Y | C | 149 | 0.31 | 170 | 0.36 | 332 | 0.71 | 313 | 0.67 | 333 | 0.72 |
| Sweden | Y | C | 3 | 0.03 | 1 | 0.01 | 10 | 0.10 | 7 | 0.07 | 3 | 0.03 |
| EU Total 27 | 460 | 0.11 | 523 | 0.12 | 942 | 0.22 | 771 | 0.18 | 863 | 0.20 | ||
| United Kingdom | – | – | – | – | 9 | 0.01 | 19 | 0.03 | 21 | 0.03 | ||
| EU Total (c) | 460 | 0.11 | 523 | 0.12 | 951 | 0.19 | 790 | 0.16 | 884 | 0.18 | ||
| Iceland | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Norway | Y | C | 3 | 0.06 | 5 | 0.09 | 8 | 0.15 | 5 | 0.09 | 4 | 0.08 |
| Liechtenstein | Y | C | 0 | 0 | 55 | 0.64 | 103 | 1.20 | 52 | 0.61 | 41 | 0.48 |
| Switzerland (d) | Y | C | 111 | 1.3 | ||||||||
–: Data not reported.
: Y: yes; N: no; A: aggregated data; C: case‐based data.
: Not notifiable, no surveillance system exists.
: Cases reported by the United Kingdom for the period 2017–2019 were also considered in this estimation (EU‐28). When the United Kingdom data were collected for the period 2017–2019, the United Kingdom was an EU MS, but it became a third country on 1 February 2020.
: Switzerland provided data directly to EFSA. The human data for Switzerland include data from Liechtenstein for years 2017–2020.
In 2021, 359 cases (78%) were acquired in the EU. Germany and Sweden reported travel‐associated cases. Three cases were imported from Ethiopia, Iran and Sudan (0.7%), whereas for 98 cases (21.3%), there were no data on travel or the country of infection (Table 76).
In 2021, 11 MSs reported outcomes for 270 cases (58.7%) with four deaths (in Germany, Hungary, Spain and Portugal) recorded in patients over 50 years old, resulting in an EU case fatality of 1.5%.
In 2021, cases occurred year‐round. Most Q fever cases occurred from April to September, in line with the spring/summer seasonal pattern (Figure 39).
Figure 39.
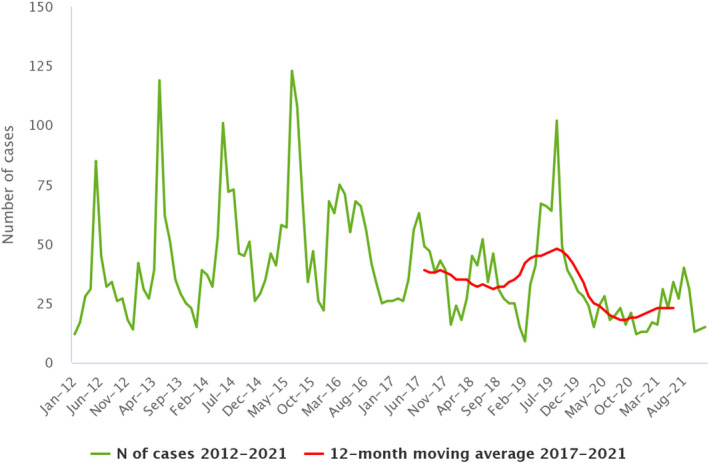
Trend in reported confirmed human cases of Q fever in the EU by month, 2017–2021
- Source: Data from Cyprus, Czechia, Estonia, Finland, France, Germany, Greece, Hungary, Ireland, Latvia, Lithuania, Malta, the Netherlands, Poland, Portugal, Romania, Slovakia, Slovenia and Sweden. Austria, Belgium, Bulgaria, Croatia, Denmark, Italy, Luxembourg and Spain did not report data at the level of detail required for the analysis.
In 2021, the number of Q fever cases decreased compared with 2020 and the 2017–2019 period. In particular, a significant (p < 0.01) decreasing trend was noted for France, the Netherlands and Portugal. However, over the past 5 years, a significant decrease (p < 0.05) in Q fever cases has been observed in the EU. The rate of confirmed human Q fever cases was higher among men than women with a male‐to‐female ratio of 2.1:1. The highest notification rate occurred in the 50–55 years age group (12.5%).
4.3.3. Coxiella burnetii in animals
Sheep (including the ‘sheep and goats’ category)
Seven MSs and three non‐MSs (North Macedonia, Serbia and Switzerland) provided data in 2021. In total, 5,368 herds and 1,356 animals were tested. At the animal level, serological tests were more often used (N = 750, 10.3% positive) than direct detection tests (N = 85, 5.9% positive). Most individual serological tests were reported by Greece (N = 288, 17.7% positive), Bulgaria (N = 185, 8.1% positive), Ireland (N = 123, 0.8% positive) and Sweden (N = 98, 0.0% positive). At the herd level, Italy accounted for all the reported serological tests (N = 2,238, 18.9% positive), and most direct tests were reported by Poland (N = 2,452, 0.1% positive), Italy (N = 508, 20.9% positive) and Belgium (N = 162, 8.6% positive).
Goats
Eleven MSs and one non‐MS (Switzerland) provided data in 2021. In total, 1,287 herds and 1,517 animals were tested. At the animal level, serological tests were more often used (N = 540, 24.6% positive) than direct detection tests (N = 200, 16.5% positive). Most serological tests were reported by Italy (N = 215, 27.9% positive) and Bulgaria (N = 158, 36.7% positive). Italy and Switzerland reported the most direct detection tests (N = 162, 17.9% positive; N = 159, 11.3% positive, respectively). At the herd level, almost all tests were direct detection tests, mostly carried out in Poland (N = 926, 0.0% positive), Belgium (N = 241, 5.4% positive) and Spain (N = 81, 12.3% positive).
Cattle
Sixteen MSs and five non‐MSs (Iceland, Norway, North Macedonia, Serbia and Switzerland) provided data in 2021. In total, 5,512 herds and 4,206 animals were tested. At the animal level, serological tests were more often employed (N = 3,405, 12.2% positive) than direct detection tests (N = 458, 5.2%). Slovakia (N = 1,137, 3.2% positive), Austria (N = 965, 13.7% positive) and Hungary (N = 451, 37.7% positive) provided 75% of the serological tests, whereas Switzerland tested 3,277 animals using direct detection tests, resulting in 1.7% positivity. For herds, most tests were direct detection methods and mainly reported from Belgium (N = 2,199, 7.6% positive) and Poland (N = 1,864, 1.0% positive), whereas Italy reported data mostly obtained using serological methods (N = 1,201, 15.1% positive).
Other animal species
Four MSs (Austria, Cyprus, Italy and Sweden) and two non‐MSs (Norway and Switzerland) reported data in 2021 on animals other than sheep, goats and cattle. In total, 38 herds and 706 animals were tested from various domestic and wild animal species, such as other ruminant species (water buffalo, llama, etc.), pets (cats and dogs) or diverse other species. Positive results were only reported by Italy, mainly from dogs (N = 541, 73.2% positive) and water buffalos (N = 43, 4.7% positive).
4.4. Discussion
C. burnetii is the aetiological agent of the Q fever disease and, if aerosolised, is also considered a potential biological weapon. Humans can acquire the infection mainly through environmental contamination due to bacterial shedding of infected animals, but also through tick‐borne or foodborne transmissions. In Europe, the majority of clinical cases are sporadic. However, several outbreaks among humans have been reported. Up to 2016, France and Germany reported most of the confirmed cases. In 2017, two outbreaks were reported from Bulgaria in the Gabrovo and Blagoevgrad regions (Genova‐Kalou et al., 2019). Since 2017, Spain has reported the highest number of cases annually. The increase in the number of human cases reported by Spain is most likely explained by a change in their reporting system from voluntary to mandatory.
In 2021, the number of human Q fever cases in the EU was the lowest recorded over the past 5 years. Cases decreased compared with the COVID‐19 pandemic year 2020, but also compared with the pre‐pandemic 2017–2019 period. Compared with 2020, a decrease was observed in Bulgaria and Spain. However, Spain accounted for about one third of the overall number of cases, whereas Germany reported an increase in Q fever disease in humans. Several Q fever cases recorded in Germany and Spain were linked to occupational (waste treatment plant or laboratory staff) or recreational activities, and the exposure was from animal and environmental contamination (ProMED‐mail, 2021a,b,c). Overall, a significant decreasing trend was observed over the last 5 years (2017–2021). Year 2021 showed a reduction in case fatality (1.48%) compared with 2020 (2.13%). Moreover, no data were available for 2021 regarding the impact of the pandemic on Q fever surveillance and reporting.
The results obtained from animals – mainly from small ruminants and cattle – are insufficient for analysing trends for Q fever at the EU level. The start of annual Union reporting in 2021 (Q fever is a ‘Category E' disease under the new EU Animal Health Law) has not drastically increased the total number of countries reporting to EFSA under Directive 2003/99/EC. The results submitted by different MSs and non‐MSs are not directly comparable, mainly due to differences in sampling strategy (sample types, testing methods, coverage of the monitoring), data completeness and sensitivity of the surveillance method. Importantly, EU MSs should first agree on case definitions in domestic ruminants at the animal and herd levels and also improve data reporting harmonisation among countries. Case definitions could be based on EFSA harmonisation guidelines (Sidi‐Boumedine et al., 2010) and the WOAH case definition draft (WOAH, 2022). Furthermore, monitoring could be extended to include species other than domestic ruminants, such as pets, which may be reservoirs. In 2021, Italy reported a high number (394) and proportion of positive dogs (76.8%), compared with published 2020 data indicating 8.2% positivity in Italian dogs (Ebani et al., 2020). Pets have already been suspected to be reservoirs for C. burnetii in Australia (Tozer et al., 2014; Orr et al., 2022), Asia (Lyoo et al., 2017) and Africa (Kelly et al., 1993; Boni et al., 1998). They may also be considered as sentinels for humans and farm animals. In coming years, it is of utmost importance to collect more data on the persistence of environmental contamination (animal waste, dust) to better assess risk factors (Carrié et al., 2019). The major challenge is to reduce human exposure to this zoonosis through a preventive ‘One Health’ approach.
5. West Nile virus
Summary data substantiating this chapter, as well as additional information on related projects and internet sources are published for this report on the EFSA Knowledge Junction on Zenodo here. Summary statistics on human surveillance data with downloadable files are retrievable using the ECDC Surveillance Atlas of Infectious Diseases here.
5.1. Key facts
In 2021, the number of locally acquired probable and confirmed human cases of West Nile virus (WNV) infection was 152, corresponding to an EU notification rate of 0.03 per 100,000 population. This is a decrease of 57% compared with 2020 (0.07 per 100,000 population) and the lowest notification rate reported in the period 2017–2021.
Most locally acquired infections were reported by Italy and Greece, accounting respectively for 43% and 39% of the total number of probable/confirmed human cases in the EU.
Since the epidemic year of 2018, which was characterised by an unusually intense transmission season, the trend in the number of reported human cases of infection seems to be slowly decreasing. However, no significant decrease (p = 0.89) has been observed over the past 5 years (2017–2021). Nevertheless, over this same period, 2021 was the year in which countries reported the lowest number of cases in humans and the lowest number of outbreaks in animals.
In Spain, a country that experienced intense transmission in 2020, a significant decrease in the number of human cases was documented over the same period.
In 2021, 15 EU MSs and Switzerland submitted WNV monitoring and surveillance data from birds and/or equids to EFSA. Italy and Spain submitted respectively 62.3% and 13.7% of these data on birds, while Spain, Greece and Germany submitted most of the data for equids, at 42.8%, 17.6% and 15.1% respectively.
Italy, Germany, Slovenia and Spain reported infections in birds (only 2 MSs reported outbreaks in ADIS) with Italy and Germany reporting the highest number of avian cases (73.3% and 23.3% respectively). Germany, Spain, Italy, Portugal, France, Hungary and Greece reported equid outbreaks to ADIS, with the highest number of cases being reported by Germany, Spain and Italy, accounting for 40.4%, 23.4% and 14.9% of the total number of cases respectively.
The data reported to ECDC, ADIS and EFSA for 2021 indicated WNV circulation in central and eastern Europe (Germany, Hungary, Austria, Romania and Slovenia) as well as in southern and western Europe (Greece, Italy, France, Spain and Portugal). WNV infections of humans, equids and birds now regularly occur in these countries.
5.2. WNV ecology and epidemiology
West Nile fever, also known as West Nile virus disease, is an arboviral disease caused by an arbovirus called the West Nile virus (WNV). WNV genetic lineages one and two are associated with both human and animal diseases.
Mosquitoes serve as vectors and birds are the main amplifying hosts. This virus is transmitted to humans and equids through the bite of infected mosquitoes, mainly of the Culex genus (Hubálek and Halouzka, 1999). Occasionally, infection can occur through the transfusion/transplantation of substances of human origin (SoHO) (i.e. blood, organs or cells), percutaneous or conjunctival exposure in laboratories or transplacental passage from mother to fetus. The number of EU countries reporting locally acquired WNV infections in humans and cases of infection in birds/equids has increased in recent years.
WNV circulation is influenced by environmental conditions, and is helped by low precipitation in winter and high spring temperatures (Marini et al., 2021; Farooq et al., 2022). These conditions are beneficial to the Culex mosquito population, and enhance virus replication in the vector (Reisen et al., 2006; Fornasiero et al., 2020).
5.3. Surveillance and monitoring of West Nile virus in the EU
5.3.1. Humans
In 2021, 25 MSs reported information on WNV infections in humans. Surveillance is mandatory in 23 countries, in France it is voluntary, and Germany did not specify their surveillance system. There is no surveillance system in place in Denmark and the disease is not notifiable or reported at EU level. The EU case definition was used by 23 MSs, France reported another case definition and Germany did not specify which case definition was used. All countries conducting surveillance had a comprehensive surveillance system with full national coverage, except Germany, which did not specify the surveillance system but had full national coverage. All countries reported case‐based data.
5.3.2. Animals
From a veterinary standpoint, WNV is the causative agent of West Nile Fever (WNF), a disease that develops into asymptomatic forms, benign forms (flu‐like syndrome) and neuro‐invasive forms (WOAH, 2018a). WNV surveillance in animals involves mostly passive surveillance, including surveillance based on the diagnosis of neuro‐invasive cases in equids, but some countries implement active surveillance of equids and/or captive birds and/or wild birds. Alongside EU MSs, Switzerland submits reports to EFSA on animal surveillance and monitoring activities in animals. Two sources of information are used to complete this report. Firstly, data are submitted to EFSA by EU MSs and Switzerland from annual surveillance and monitoring activities in accordance with Directive 2003/99/EC. WNV is listed in Annex I, Part B (viruses transmitted by arthropods) as a virus to be monitored, if warranted by the epidemiological situation in an MS, in compliance with Article 4.1 of the same Directive. Secondly, it is mandatory for MSs to notify outbreaks of equine and avian WNV to ADIS, 41 in accordance with CIR (EU) 2020/2002.
5.4. Results
5.4.1. Overview of key statistics, EU, 2017–2021
In 2021, WNV cases were reported in humans, equids and birds. Over the past 5 years, there has been an increase in the number of countries reporting surveillance data in animals (Table 78). While the number of tested equids appears to correlate with WNV circulation, increasing in years when a greater number of cases are reported and decreasing when WNV circulation is observed to fall, there has been an overall increase in the number of tested birds over time, reflecting an intensification of the surveillance system in place.
Table 78.
Summary of WNV infection statistics related to humans, birds and equids EU, 2017–2021
| 2021 (a) | 2020 | 2019 (b) | 2018 (b) | 2017 (b) | Data source | |
|---|---|---|---|---|---|---|
| Humans | ||||||
| Total number of confirmed and probable cases | 158 | 333 | 443 | 1,615 | 208 | ECDC |
| Total number of confirmed and probable cases/100,000 population (notification rates) | 0.04 | 0.08 | 0.09 | 0.32 | 0.05 | ECDC |
| Number of reporting MSs | 26 | 26 | 27 | 27 | 26 | ECDC |
| Infection acquired in the EU | 153 (c) | 331 | 435 | 1,573 | 205 | ECDC |
| Infection acquired outside the EU | 5 | 2 | 5 | 29 | 2 | ECDC |
| Unknown travel status or unknown country of infection | 0 | 0 | 3 | 13 | 1 | ECDC |
| Animals (d) | ||||||
| Birds | ||||||
| Number of animals tested | 19,596 | 11,141 | 14,932 | 13,970 | 11,173 | EFSA |
| Number of positive animals by PCR (e) ‐based methods | 146 | 165 | 104 | 425 | 93 | EFSA |
| Number of MSs reporting surveillance/monitoring data to EFSA | 13 | 11 | 13 | 11 | 7 | EFSA |
| Number of outbreaks notified to ADIS | 8 | 2 | 53 | 22 | 0 | ADIS |
| Number of MSs notifying outbreaks to ADIS | 2 | 1 | 2 | 6 | 0 | ADIS |
| Equids | ||||||
| Number of animals tested | 5,985 | 6,749 | 5,563 | 13,785 | 11,668 | EFSA |
| Number of positive animals by PCR (e) ‐based methods | 1 | 1 | 4 | 7 | 1 | EFSA |
| Number of animals positive for IgM by ELISA | 47 | 209 | 74 | 393 | 110 | EFSA |
| Number of MSs reporting surveillance/monitoring data to EFSA | 14 | 14 | 14 | 12 | 12 | EFSA |
| Number of outbreaks notified to ADIS | 45 | 189 | 100 | 292 | 84 | ADIS |
| Number of MSs notifying outbreaks to ADIS | 7 | 9 | 8 | 10 | 7 | ADIS |
ADIS: Animal Disease Information System; ECDC: European Centre for Disease Prevention and Control; EFSA: European Food Safety Authority; ELISA: enzyme‐linked immunosorbent assay; MSs: Member States; PCR: polymerase chain reaction.
: Data on animals from the United Kingdom (Northern Ireland) are taken into account for 2021. In accordance with the agreement on the withdrawal of the United Kingdom from the EU, and in particular with the Protocol on Ireland/Northern Ireland, EU requirements on data sampling are also applicable to Northern Ireland.
: Data from the United Kingdom are taken into account for 2017–2019, because the United Kingdom was an EU MS, but it became a third country on 1 February 2020.
: One case reported from the EU was later found to have been infected in Mayotte.
: Samples tested using an unspecified analytical method are not included.
: PCR: polymerase chain reaction.
The number of probable and confirmed human cases of West Nile virus (WNV) infection reported in 2021 was 158, corresponding to an EU notification rate of 0.04 per 100,000 population. This is a decrease of 50% compared with 2020 (0.08 per 100,000 population) and the lowest notification rate reported in the period 2017–2021.
More detailed descriptions of these statistics are provided in the below subsections.
5.4.2. West Nile virus infections in humans
In 2021, 158 confirmed and probable WNV infections in humans were reported. Of these, 153 were acquired in the EU. Of the 153 cases acquired in the EU, 152 were locally acquired (Table 79) and one was imported from another EU country. Of the 152 cases of probable/confirmed infection that were locally acquired in 2021, 93 concerned males, (61.2%), and over 80% of cases occurred in people aged 50 or older. Five cases of travel‐associated WNV infection acquired outside the EU (Dominican Republic, Turkey and the United States) were also reported.
Table 79.
Locally acquired human WNV cases (confirmed and probable) and notification rates per 100,000 population in EU MSs and non‐MS countries, by country and year, 2017–2021
| Country | 2021 | 2020 | 2019 | 2018 | 2017 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| National coverage (a) | Data format (a) | Confirmed cases | Total cases* and rates | Total cases* and rates | Total cases* and rates | Total cases* and rates | Total cases* and rates | ||||||
| Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | ||||
| Austria | Y | C | 3 | 3 | 0.03 | 0 | 0 | 4 | 0.05 | 21 | 0.24 | 6 | 0.07 |
| Belgium | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Bulgaria | Y | C | 0 | 0 | 0 | 1 | 0.01 | 5 | 0.07 | 15 | 0.21 | 1 | 0.01 |
| Croatia | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 58 | 1.4 | 5 | 0.12 |
| Cyprus | Y | C | 0 | 0 | 0 | 0 | 0 | 23 | 2.6 | 1 | 0.12 | 0 | 0 |
| Czechia | Y | C | 0 | 0 | 0 | 0 | 0 | 1 | 0.01 | 5 | 0.05 | 0 | 0 |
| Denmark (b) | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Estonia | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Finland | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| France | Y | C | 1 (c) | 1(c) | < 0.01 | 0 | 0 | 2 | < 0.01 | 27 | 0.04 | 2 | < 0.01 |
| Germany | Y | C | 4 | 4 | < 0.01 | 22 | 0.03 | 5 | 0.01 | 1 | < 0.01 | – | – |
| Greece (d) | Y | C | 27 | 59 | 0.55 | 144 | 1.3 | 227 | 2.1 | 315 | 2.9 | 48 | 0.45 |
| Hungary | Y | C | 6 | 7 | 0.07 | 3 | 0.03 | 36 | 0.37 | 215 | 2.2 | 20 | 0.20 |
| Ireland | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Italy | Y | C | 65 | 65 | 0.11 | 69 | 0.12 | 54 | 0.09 | 610 | 1.0 | 53 | 0.09 |
| Latvia | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Lithuania | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Luxembourg | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Malta | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Netherlands | Y | C | 0 | 0 | 0 | 8 | 0.05 | 0 | 0 | 0 | 0 | 0 | 0 |
| Poland | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Portugal | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Romania | Y | C | 6 | 7 | 0.04 | 6 | 0.03 | 67 | 0.35 | 277 | 1.4 | 66 | 0.34 |
| Slovakia | Y | C | 0 | 0 | 0 | 0 | 0 | 1 | 0.02 | 0 | 0 | 0 | 0 |
| Slovenia | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0.19 | 0 | 0 |
| Spain | Y | C | 6 | 6 | 0.01 | 77 | 0.16 | 0 | 0 | 0 | 0 | 0 | 0 |
| Sweden | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| EU Total 27 | – | – | 118 | 152 | 0.03 | 330 | 0.07 | 425 | 0.10 | 1.55 | 0.35 | 201 | 0.06 |
| United Kingdom | – | – | – | – | – | – | – | 0 | 0 | 0 | 0 | 0 | 0 |
| EU Total (e) | – | – | 118 | 152 | 0.03 | 330 | 0.07 | 425 | 0.08 | 1.55 | 0.31 | 201 | 0.05 |
| Iceland | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Norway | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Liechtenstein | Y | C | 0 | 0 | 0 | 1 | 0.01 | 1 | 0.01 | 0 | 0 | 0 | 0 |
| Switzerland (f) | Y | C | 0 | 0 | 0 | ||||||||
–: Data not reported.
: Y: yes; N: no; A: aggregated data; C: case‐based data.
: No surveillance system.
: This case reported by France acquired the WNV infection in Mayotte.
: Only locally acquired cases diagnosed in the country of exposure are included in the table.
: Cases reported by the United Kingdom for the years 2017–2019 were also considered for this estimation (EU‐28). When United Kingdom data were collected for the period 2017–2019, the United Kingdom was an EU MS, but it became a third country on 1 February 2020.
: Switzerland provided data directly to EFSA. The human data for Switzerland include data from Liechtenstein for the years 2017–2020.
Six EU MSs reporting locally acquired infections in humans provided data on the hospitalisation status of their cases. Of the cases with known hospitalisation status (83 cases, 55% of total infections) in 2021, 84% (N = 70) were hospitalised.
Clinical manifestations were reported for all of the 152 locally acquired cases. Among those, 63% (N = 96) of infections were neuro‐invasive (Table 80) and 16% (N = 25) were asymptomatic. This compares with 79% (N = 252) neuro‐invasive and 6% (N = 19) asymptomatic infections in 2020. In 2021, the remaining 31 cases (20%) were symptomatic cases with non‐neurological symptoms. This compares with 46 cases (15%) in 2020.
Table 80.
Locally acquired human WNND cases (confirmed and probable) and notification rates per 100,000 population in the EU MSs and non‐MS countries, by country and year, 2017–2021
| Country | 2021 | 2020 | 2019 | 2018 | 2017 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| National coverage (a) | Data format (a) | Confirmed cases | Total cases* and rates | Total cases* and rates | Total cases* and rates | Total cases* and rates | Total cases* and rates | ||||||
| Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | ||||
| Austria | Y | C | 1 | 1 | 0.01 | 0 | 0 | 1 | 0.01 | 4 | 0.05 | 2 | 0.02 |
| Belgium | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Bulgaria | Y | C | 0 | 0 | 0 | 1 | 0.01 | 4 | 0.06 | 13 | 0.18 | 1 | 0.01 |
| Croatia | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 47 | 1.1 | 5 | 0.12 |
| Cyprus | Y | C | 0 | 0 | 0 | 0 | 0 | 20 | 2.3 | 1 | 0.12 | 0 | 0 |
| Czechia | Y | C | 0 | 0 | 0 | 0 | 0 | 1 | 0.01 | 3 | 0.03 | 0 | 0 |
| Denmark (b) | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Estonia | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Finland | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| France | Y | C | 0 | 0 | 0 | 0 | 0 | 1 | < 0.01 | 7 | 0.01 | 0 | 0 |
| Germany | Y | C | 2 | 2 | < 0.01 | 7 | 0.01 | 3 | < 0.01 | 0 | 0 | – | – |
| Greece (c) | Y | C | 22 | 38 | 0.36 | 116 | 1.1 | 140 | 1.3 | 241 | 2.2 | 28 | 0.26 |
| Hungary | Y | C | 6 | 7 | 0.07 | 1 | 0.01 | 23 | 0.24 | 152 | 1.6 | 17 | 0.17 |
| Ireland | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Italy | Y | C | 35 | 35 | 0.06 | 46 | 0.08 | 24 | 0.04 | 243 | 0.40 | 26 | 0.04 |
| Latvia | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Lithuania | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Luxembourg | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Malta | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Netherlands | Y | C | 0 | 0 | 0 | 6 | 0.03 | 0 | 0 | 0 | 0 | 0 | 0 |
| Poland | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Portugal | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Romania | Y | C | 6 | 7 | 0.04 | 6 | 0.03 | 65 | 0.33 | 277 | 1.4 | 66 | 0.34 |
| Slovakia | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Slovenia | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0.19 | 0 | 0 |
| Spain | Y | C | 6 | 6 | 0.01 | 72 | 0.15 | 0 | 0 | 0 | 0 | 0 | 0 |
| Sweden | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| EU Total 27 | – | – | 78 | 96 | 0.02 | 255 | 0.06 | 282 | 0.06 | 992 | 0.23 | 145 | 0.04 |
| United Kingdom | – | – | – | – | – | – | – | 0 | 0 | 0 | 0 | 0 | 0 |
| EU Total (d) | – | – | 78 | 96 | 0.02 | 255 | 0.06 | 282 | 0.06 | 992 | 0.20 | 145 | 0.03 |
| Iceland | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Norway | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Liechtenstein | Y | C | 0 | 0 | 0 | – | – | – | – | – | – | – | – |
| Switzerland (e) | Y | C | – | – | – | ||||||||
WNND: West Nile Neuro‐invasive Disease.
–: Data not reported.
: Y: yes; N: no; A: aggregated data; C: case‐based data.
: No surveillance system.
: Only locally acquired cases diagnosed in the country of exposure are included in the table.
: Cases reported by the United Kingdom for the period 2017–2020 were also considered for this estimation (EU‐28). When the United Kingdom data were collected for the period 2017–2020, the United Kingdom was an EU MS, but it became a third country on 1 February 2020.
: Switzerland provided data directly to EFSA. The human data for Switzerland include data from Liechtenstein for the years 2017–2020.
Data on the outcome of infections were reported for all of the 152 locally acquired cases. In 2021, 11 deaths among cases with WNV infections were reported, compared with 39 in 2020. The case fatality rate in 2021 was 7% (12% in 2020) among all locally acquired WNV infections and 11% (15% in 2020) among locally acquired WNV infections with West Nile neuro‐invasive disease (WNND).
Eight EU MSs (Austria, France, Germany, Greece, Hungary, Italy, Romania and Spain) reported at least one locally acquired human case of WNV infection in 2021. Most of the locally acquired infections were reported by Italy and Greece, accounting respectively for 43% and 39% of the total number of probable/confirmed cases in the EU.
5.4.3. West Nile virus infections in animals
In 2021, 15 EU MSs and Switzerland submitted WNV monitoring and surveillance data from birds and/or equids to EFSA. None of the analyses undertaken on other species (sheep and goats at farm, wild martens and one crocodile in a zoo) were positive. Summaries by country of reported cases, and of outbreaks, in birds and equids are provided in Table 81, which displays data from both data sources. In some cases, the statistics from both sources may show discrepancies, so the following points should be taken into consideration for interpreting the data:
-
–
The reported number of animals tested based on surveillance activities submitted to EFSA includes the following registered methods: detection of IgG and IgM antibodies, seroneutralisation and the detection of flavivirus or WNV genomes using a PCR‐based method. Samples tested using an unspecified analytical method are not included.
-
–
The notification of outbreaks in equids and birds to ADIS is mandatory. However, some countries did not report confirmed outbreaks among birds to ADIS, while nevertheless including them in their national reports. An outbreak can refer to more than one infected animal if they constitute a single epidemiological unit and/or are from the same location.
-
–
The number of positive animals includes cases for which the disease was confirmed clinically and/or in a laboratory setting either by the detection of IgM‐specific antibodies indicating a recent infection by WNV (available for equids only) or by the detection of the WNV genome using a PCR‐based method indicating an ongoing infection (for both birds and equids). The results of serum neutralisation testing and IgG ELISA, including observation of seroconversion in avian or equid sentinel animals by detecting specific neutralising antibodies, are not included, because it is not possible to determine when the infection occurred.
-
–
Some countries did not submit data to either ADIS or EFSA.
Table 81.
Summary of WNV surveillance/monitoring results in animals reported to EFSA, and WNV outbreaks notified to ADIS, by EU MSs and non‐MS countries, 2021
| Country (EU MSs, non‐EU country) | Birds (a) | Equids (a) | |||||
|---|---|---|---|---|---|---|---|
| Data on surveillance activities submitted to EFSA | N (%) outbreaks notified in ADIS | Data on surveillance activities submitted to EFSA | N (%) outbreaks notified in ADIS | ||||
| N (%) animals tested | N (%) animals positive using PCR‐based methods (b) | N (%) animals tested | N (%) animals positive using ELISA‐IgM (c) | N (%) animals positive using PCR‐based methods (b) | |||
| Austria | 170 (0.87) | 0 (0) | NR | 16 (0.27) | – | 0 (0) | NR |
| Cyprus | 498 (2.5) | – | NR | 103 (1.7) | 0 (0) | – | NR |
| Czechia | NR | – | NR | 783 (13.1) | – | – | NR |
| Denmark | 891 (4.5) | – | NR | NR | – | – | NR |
| Finland | 25 (0.13) | 0 (0) | NR | 144 (2.4) | 0 (0) | – | NR |
| France | 85 (0.43) | 0 (0) | NR | 22 (0.37) | 3 (6.4) | 0 (0) | 2 (4.4) |
| Germany | 1,909 (9.7) | 34 (23.3) | NR | 906 (15.1) | 19 (40.4) | – | 18 (40.0) |
| Greece | 38 (0.19) | 0 (0) | NR | 1,052 (17.6) | 0 (0) | 1 (100) | 1 (2.2) |
| Hungary | 794 (4.1) | 0 (0) | NR | 109 (1.8) | 3 (6.4) | 0 (0) | 3 (6.7) |
| Italy | 12,204 (62.3) | 107 (73.3) | NR | 195 (3.3) | 7 (14.9) | – | 6 (13.3) |
| Portugal | NR | – | NR | 6 (0.10) | 4 (8.5) | – | 4 (8.9) |
| Romania | 6 (0.03) | – | NR | 84 (1.4) | 0 (0) | – | NR |
| Slovenia | 38 (0.19) | 1 (0.68) | 1 (12.5) | 2 (0.03) | – | 0 (0) | NR |
| Spain | 2,684 (13.7) | 4 (2.7) | 7 (87.5) | 2,562 (42.8) | 11 (23.4) | – | 11 (24.4) |
| Sweden | 254 (1.3) | 0 (0) | NR | 1 (0.02) | – | 0 (0) | NR |
| EU Total (27 + XI) | 19,596 (100) | 146 (100) | 8 (100) | 5,985 (100) | 47 (100) | 1 (100) | 45 (100) |
| Switzerland | 0 (0) | – | NR | 9 (0) | – | 0 (0) | NR |
MSs: Member States; ADIS: Animal Disease Information System.
NR: Not reported to EFSA or to ADIS. These countries did not submit data for WNF surveillance activities to EFSA or did not notify outbreaks to ADIS.
–: Analytical method not used.
0: Analytical method used with negative results.
: Samples tested with an unspecified analytical method are not included.
: PCR: polymerase chain reaction (for identification of the virus genome).
: ELISA: enzyme‐linked immunosorbent assay.
The geographical distribution of outbreaks in animal is shown in Figure 40.
Figure 40.
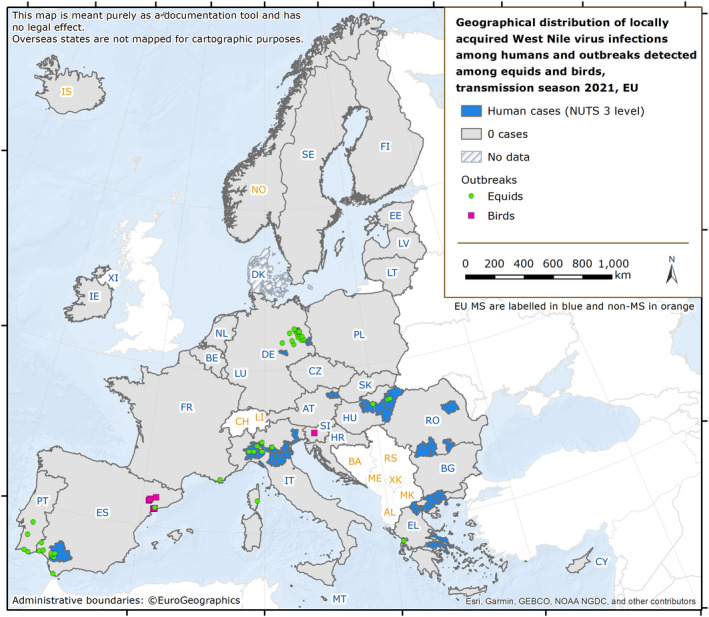
Geographical distribution of locally acquired West Nile virus infections among humans (NUTS 3 level) and outbreaks notified to ADIS among equids and birds (XY coordinates) across the EU, 2021 transmission season
In birds, 13 MSs reported a total of 19,596 analysed samples as part of their surveillance and monitoring activities (Tables 78 and 81). Italy, Spain and Germany submitted 62.3%, 13.7% and 9.7% of these data respectively (Table 81). Of the total number, 16,247 samples from Hungary, Greece, France, Slovenia, Italy, Finland, Spain, Germany, Sweden, Austria were tested using a WNV‐specific PCR method. A total of 146 infected birds were reported to EFSA by Italy, Germany, Slovenia and Spain (Table 81). WNV infection occurred in crows, doves, goshawks, jays, magpies, owls, Passeriformes, pheasants, pigeons and starlings. Only Slovenia and Spain reported avian outbreaks to ADIS.
In equids, 14 MSs reported a total of 5,985 analysed samples as part of their surveillance and monitoring activities (Tables 78 and 81). Spain, Greece and Germany submitted 42.8%, 17.6% and 15.1% of these data respectively. Seven MSs reported a total of 48 equid cases that were confirmed either through IgM by ELISA (47 animals) or PCR methods (1 animal) (Table 81). As a result, 45 outbreaks were notified to ADIS by France, Germany, Greece, Hungary, Italy, Portugal and Spain. Together, Germany, Spain and Italy reported a total of 77.7% of the outbreaks in equids (Table 81). One non‐EU country, Switzerland, reported 9 analyses with no outbreaks.
5.4.4. Joint analysis of trends and seasonality
WNV is endemic in several EU countries and both animal and human cases of WNV infections are reported every year (Figure 41). Infections in Europe occur seasonally, with most cases being reported between June and October (Figures 42 and 43). However, since the epidemic year of 2018, which was characterised by an unusually intense transmission season, no significant decrease (p = 0.89) has been observed over the past 5 years (2017–2021). Notwithstanding, the trend in the number of reported human cases of infection seems to be slowly decreasing (Figure 41), and 2021 is the year, within that period, in which reports indicated the lowest number of cases among humans (total = 158; Table 78 and Figure 42) and the lowest number of outbreaks in animals (total = 53; Table 78 and Figure 43). In Spain, a country that experienced an intense transmission season in 2020, a significant decrease in the number of cases was documented over the same period (García San Miguel Rodríguez‐Alarcón et al., 2021).
Figure 41.
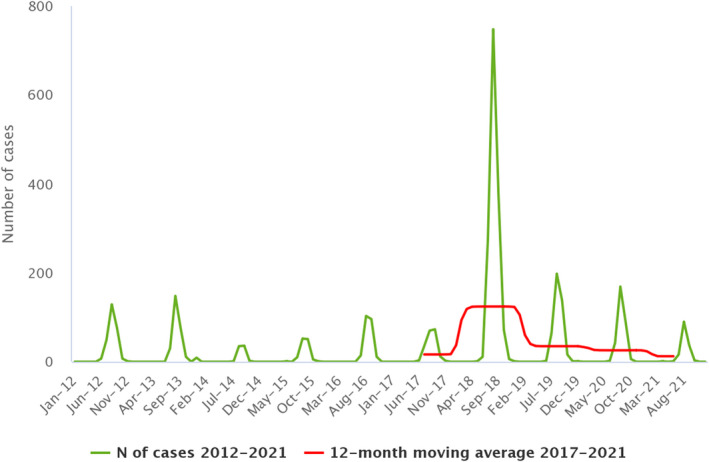
Trend in locally acquired human WNV infections reported in EU MSs, by month, 2017–2022
- Source: Austria, Belgium, Bulgaria, Cyprus, Czechia, Estonia, Greece, Spain, Finland, France, Hungary, Ireland, Italy, Lithuania, Luxembourg, Latvia, Malta, Netherlands, Poland, Portugal, Romania, Sweden, Slovenia, Slovakia.
Figure 42.
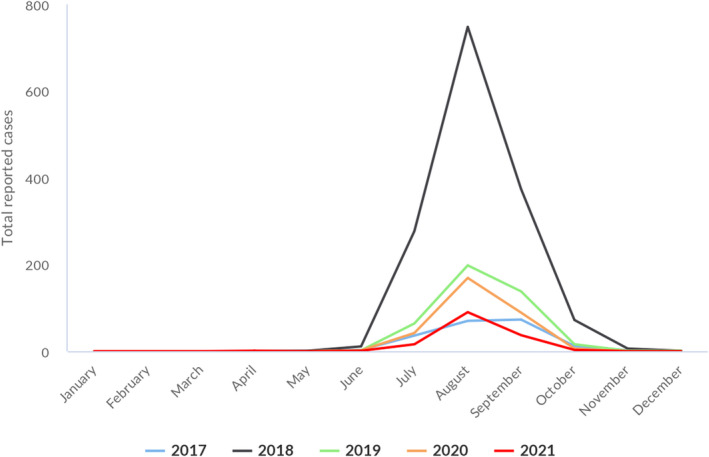
Reported human cases of West Nile virus infection in the EU MSs, by month, 2017–2021
- The data set includes only locally acquired WNF cases and only countries that consistently reported cases (or reported zero cases) over the whole reporting period (last 5 years) and to the level of detail required for trend analysis (not aggregated).
Figure 43.
Outbreaks of West Nile infection in animals in the EU MSs, by month, 2017–2021
-
Data source: ADIS for animal outbreaks. Outbreaks in birds or equids that were not notified to ADIS are not included.The dataset includes only locally acquired WNF cases and only countries that consistently reported cases (or reported zero cases) over the whole reporting period (last 5 years) and to the level of detail required for trend analysis (not aggregated).
The transmission season of 2021 showed a similar pattern to pre‐pandemic years, with an increase of human cases in the month of July and a peak in the month of August, albeit with lower intensity.
The season started early in 2021 with the first locally acquired human case reported in June (the earlier case reported in April was in the Island of Mayotte, France and does not really reflect the EU season) and the first animal outbreak also reported in June, with cases peaking, as in previous years, in August for humans (90 reported cases, Trend Statistical Analysis (TSA), Figure 42) and in September for animals (30 outbreaks, TSA, Figure 43).
5.5. Discussion
Human cases of WNV infection are reported every year by some EU MSs while in others WNV is only diagnosed in people who have travelled in endemic areas.
The number of countries reporting local WNV transmission has increased in EU MSs in recent years (Hubálek and Halouzka, 1999; Young et al., 2021). In 2020, the Netherlands reported its first cases in birds and humans (Sikkema et al., 2020). Moreover, since detecting its first epizoonosis in 2018, Germany has reported locally‐acquired human and animal cases every year, suggesting the establishment of local transmission (Ziegler et al., 2020). In 2021, all the countries that reported locally acquired cases of WNV in humans had also reported locally acquired cases in the past.
The intensity of WNV transmission varies from year to year depending on environmental conditions. In 2021, the number of reported human cases and reported animal outbreaks was lower than in previous years. The number of avian cases was higher in 2021 than in 2017 and 2019, but this could be the result of more intense surveillance. That year was characterised by higher‐than‐average precipitation over much of Europe in winter, followed by a dry, temperature‐neutral spring and a warm, rainy summer. 42 In contrast, past epidemic years, such as 2018, with case counts up to 10 times higher than expected, were marked by a dry winter and warm spring, favouring WNV replication and transmission by mosquitoes (ECDC, 2018; Haussig et al., 2018; Riccardo et al., 2018; Beck et al., 2020; Pervanidou et al., 2020; Riccardo et al., 2020; Young et al., 2021). Even though fewer human cases and animal outbreaks have been reported in Europe over the past 3 years, we can expect to see changes in the epidemiological pattern of WNV circulation in Europe during the coming years due to an overall increase in spring temperatures.
The two major lineages of WNV (lineage 1 and lineage 2) are considered endemic in Europe. They are derived from a limited number of independent introductions from Africa, followed by local spread and evolution. Lineage 2 has become the dominant lineage over the past few years and has been associated with significant human epidemics (Papa et al., 2011; Hernández‐Triana et al., 2014; Beck et al., 2020; Pacenti et al., 2020). Yet lineage 1 is occasionally detected, indicating that it is still circulating. During the 2021 transmission season, and 8 years after the last human case of WNV lineage 1 in Italy, the co‐circulation of both lineage 1 and lineage 2 was confirmed (Veneto region) (Barzon et al., 2022), revealing the introduction/re‐introduction of WNV lineage 1.
The impact of WNV on human health in EU MSs is relevant in terms of hospitalisation and case fatality. As in prior years, neuro‐invasive infections continued to be the most frequently reported clinical presentation in 2021. Notwithstanding, substantial under‐detection/under‐reporting of clinically asymptomatic and/or mildly symptomatic WNV infections exists and should be considered when reading the data presented. Similarly, in animals, most EU countries limit screening of WNV to suspected symptomatic cases.
Integrated One Health active surveillance provides a concrete advantage in the rapid detection and characterisation of WNV circulation across the human‐animal interface and can effectively guide action to ensure the safety of substances of human origin (e.g. blood and organs) and enhance risk communication (Riccardo et al., 2020). Active surveillance, with planned sampling in sentinels or wild animals, and sometimes mosquito screening, allows earlier detection of cases and is useful to indicate when and where to start the screening of blood/organ donors.
6. Tularaemia
Summary data substantiating this chapter, as well as additional information on related projects and internet sources are published for this report on the EFSA Knowledge Junction on Zenodo here. Summary statistics on human surveillance data with downloadable files are retrievable using the ECDC Surveillance Atlas of Infectious Diseases here.
6.1. Key facts
In 2021, the number of confirmed cases of human tularaemia was 876, corresponding to an EU notification rate of 0.20 per 100,000 population. This is an increase of 33.3% compared with the rate in 2020 (0.15 per 100,000 population), and a decrease compared with the rate in 2019 (0.25 per 100,000 population).
Compared with the rate before the COVID‐19 pandemic (2017–2019 annual mean), there was an increase of 66.7% and 42.9%, with or without the data from the United Kingdom, respectively.
Over the past 5 years (2017–2021), a significant increasing trend (p = 0.0005) in the number of tularaemia cases has been observed.
In 2021, the seasonal pattern was similar to that in previous years with infections peaking in summer months. Cases increased with age and were highest in the 55‐ to 60‐year age group.
Tularaemia in animals is rarely reported in the EU as data are submitted to EFSA on a voluntary basis. In 2021, four MSs (Austria, Finland, the Netherlands and Sweden) reported data on the occurrence of Francisella tularensis, mainly in hares and dogs. One non‐MS (Switzerland) reported samples from wild species, zoo animals and pets.
Austria, Finland, the Netherlands and Sweden together reported that 75 out of 335 animals tested positive, 70 of which were hares. Among pets, four dogs tested serologically positive. In Switzerland, seven (41.2%) out of 17 tested hares were positive for Francisella tularensis.
6.2. Surveillance and monitoring of tularaemia in the EU
6.2.1. Humans
In 2021, 26 EU MSs provided data on tularaemia in humans. Surveillance is mandatory and surveillance systems are comprehensive with full national coverage in all reporting countries. There is no surveillance system in place for tularaemia in Denmark and the disease is not notifiable or reported at the EU level. In 2020–2021, Spain did not receive data from all regions that normally report, so the case numbers may therefore not be complete. The EU case definition was used by 25 MSs, Germany and Italy reported using other case definitions. All countries reported case‐based data except Belgium, which reported aggregated data.
6.2.2. Animals
Tularaemia in animals is an internationally reportable disease (with reporting to WOAH); therefore, at European level, each country receives communications from its veterinary services. In some countries, notification is mandatory under national law. Animal monitoring data on F. tularensis are submitted to EFSA on a voluntary basis by EU MSs and non‐MS countries. Notably, for 2021, four EU MSs (Austria, Finland, the Netherlands and Sweden) and one non‐MS (Switzerland) reported these data to EFSA. Surveillance was mostly passive.
6.3. Results
6.3.1. Overview of key statistics, EU, 2017–2021
Table 82 summarises EU‐level statistics on tularaemia in humans and in major animal species, respectively, for 2017–2021. More detailed descriptions of these statistics are provided in the below subsections.
Table 82.
Summary of Francisella tularensis statistics related to humans and the main animal species, EU, 2017–2021
| 2021 (a) | 2020 | 2019 (b) | 2018 (b) | 2017 (b) | Data source | |
|---|---|---|---|---|---|---|
| Humans | ||||||
| Total number of confirmed cases | 876 | 641 | 1,279 | 270 | 323 | ECDC |
| Total number of confirmed cases/100,000 population (notification rates) | 0.20 | 0.15 | 0.25 | 0.05 | 0.06 | ECDC |
| Number of reporting MSs | 26 | 26 | 27 | 27 | 27 | ECDC |
| Infection acquired in the EU | 715 | 466 | 1,118 | 233 | 236 | ECDC |
| Infection acquired outside the EU | 1 | 2 | 3 | 3 | 2 | ECDC |
| Unknown travel status or unknown country of infection | 160 | 173 | 158 | 34 | 85 | ECDC |
| Number of foodborne outbreak‐related cases | 0 | 0 | 0 | 0 | 0 | EFSA |
| Total number of foodborne outbreaks | 0 | 0 | 0 | 0 | 0 | EFSA |
| Animals | ||||||
| Hares | ||||||
| Number of sampled animals | 317 | 222 | 211 | 112 | 39 | EFSA |
| Number of positive animals | 70 | 81 | 67 | 20 | 7 | EFSA |
| % of positive animals | 22.1 | 36.5 | 31.8 | 17.9 | 17.9 | EFSA |
| Number of reporting MSs | 3 | 3 | 2 | 2 | 1 | EFSA |
| Animals other than hares | ||||||
| Number of sampled animals | 18 | 5 | 152 | 0 | 0 | EFSA |
| Number of positive animals | 5 | 1 | 8 | 0 | 0 | EFSA |
| % of positive animals | 27.8 | 20.0 | 5.3 | – | – | EFSA |
| Number of reporting MSs | 3 | 1 | 1 | 0 | 0 | EFSA |
ECDC: European Centre for Disease Prevention and Control; EFSA: European Food Safety Authority; MSs: Member States.
: Data on animals from the United Kingdom (Northern Ireland) are taken into account for 2021. In accordance with the agreement on the withdrawal of the United Kingdom from the EU, and in particular with the Protocol on Ireland/Northern Ireland, the EU requirements on data sampling are also applicable to Northern Ireland.
: Data from the United Kingdom are taken into account for 2017–2019, because the United Kingdom was an EU MS, but it became a third country on 1 February 2020.
6.3.2. Tularaemia in humans
In 2021, 26 EU MSs reported a total of 876 confirmed cases of tularaemia with a notification rate of 0.20 cases per 100,000 population. This was an increase of 33.3% compared with the rate in 2020 (0.15 per 100,000 population) and an increase of 66.7% and 42.9% compared with the average annual notification rate from 2017 to 2019 (pre‐pandemic period), with and without the data from the United Kingdom, respectively. In 2021, Sweden had the highest notification rate with 2.8 per 100,000 population, followed by Slovenia and Finland, with 2.6 and 1.6 per 100,000 population, respectively (Table 83).
Table 83.
Reported confirmed human cases of tularaemia and notification rates per 100,000 population in EU MSs and non‐MS countries, by country and by year, 2017–2021
| Country | 2021 | 2020 | 2019 | 2018 | 2017 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| National coverage (a) | Data format (a) | Confirmed cases and rates | Confirmed cases and rates | Confirmed cases and rates | Confirmed cases and rates | Confirmed cases and rates | ||||||
| Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | |||
| Austria | Y | C | 58 | 0.65 | 33 | 0.37 | 20 | 0.23 | 7 | 0.08 | 13 | 0.15 |
| Belgium | Y | A | 7 | 0.06 | 1 | 0.01 | 4 | 0.03 | 0 | 0 | 5 | 0.04 |
| Bulgaria | Y | A | 0 | 0 | 2 | 0.03 | 1 | 0.01 | 1 | 0.01 | 1 | 0.01 |
| Croatia | Y | C | 0 | 0 | 0 | 0 | 1 | 0.02 | 0 | 0 | 3 | 0.07 |
| Cyprus | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Czechia | Y | C | 50 | 0.47 | 67 | 0.63 | 102 | 0.96 | 32 | 0.30 | 51 | 0.48 |
| Denmark (b) | – | – | – | – | – | – | – | – | – | – | – | – |
| Estonia | Y | C | 1 | 0.08 | 1 | 0.08 | 2 | 0.15 | 1 | 0.08 | 0 | 0 |
| Finland | Y | C | 86 | 1.6 | 143 | 2.6 | 48 | 0.87 | 7 | 0.13 | 32 | 0.58 |
| France | Y | C | 143 | 0.21 | 45 | 0.07 | 45 | 0.07 | 11 | 0.02 | 19 | 0.03 |
| Germany | Y | C | 113 | 0.14 | 59 | 0.07 | 70 | 0.08 | 52 | 0.06 | 52 | 0.06 |
| Greece | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hungary | Y | C | 7 | 0.07 | 20 | 0.20 | 22 | 0.23 | 17 | 0.17 | 11 | 0.11 |
| Ireland | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Italy | Y | C | 3 | 0.01 | 0 | 0 | 1 | < 0.01 | 0 | 0 | 2 | < 0.01 |
| Latvia | Y | C | 0 | 0 | 0 | 0 | 2 | 0.10 | 0 | 0 | 0 | 0 |
| Lithuania | Y | C | 7 | 0.25 | 2 | 0.07 | 4 | 0.14 | 5 | 0.18 | 5 | 0.18 |
| Luxembourg | Y | C | 3 | 0.47 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Malta | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Netherlands | Y | C | 5 | 0.03 | 1 | 0.01 | 3 | 0.02 | 2 | 0.01 | 1 | 0.01 |
| Poland | Y | C | 43 | 0.11 | 5 | 0.01 | 21 | 0.06 | 16 | 0.04 | 30 | 0.08 |
| Portugal | Y | C | 0 | 0 | 1 | 0.01 | 1 | 0.01 | 2 | 0.02 | 0 | 0 |
| Romania | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Slovakia | Y | C | 0 | 0 | 12 | 0.22 | 20 | 0.37 | 6 | 0.11 | 2 | 0.04 |
| Slovenia | Y | C | 54 | 2.6 | 1 | 0.05 | 7 | 0.34 | 4 | 0.19 | 1 | 0.05 |
| Spain | Y | C | 4 | 0.01 | 1 | < 0.01 | 88 | 0.19 | 4 | 0.01 | 11 | 0.02 |
| Sweden | Y | C | 292 | 2.8 | 247 | 2.4 | 817 | 8.0 | 102 | 1.0 | 84 | 0.84 |
| EU Total 27 | – | – | 876 | 0.20 | 641 | 0.15 | 1,279 | 0.29 | 269 | 0.06 | 323 | 0.07 |
| United Kingdom | – | – | – | – | – | – | 0 | 0 | 1 | < 0.01 | 0 | 0 |
| EU Total (c) | – | – | 876 | 0.20 | 641 | 0.15 | 1,279 | 0.25 | 270 | 0.05 | 323 | 0.06 |
| Iceland | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Norway | Y | C | 95 | 1.8 | 99 | 1.8 | 183 | 3.4 | 58 | 1.1 | 92 | 1.7 |
| Liechtenstein | Y | C | 1 | 2.6 | 133 | 1.5 | 154 | 1.8 | 117 | 1.4 | 152 | 1.8 |
| Switzerland (d) | Y | C | 212 | 2.4 | ||||||||
–: Data not reported.
: Y: yes; N: no; A: aggregated data; C: case‐based data.
: No surveillance system.
: Cases reported by the United Kingdom for the period 2017–2019 were also considered for this estimation (EU‐28). When the United Kingdom data were collected for the period 2017–2019, the United Kingdom was an EU MS, but it became a third country on 1 February 2020.
: Switzerland provided data directly to EFSA. The human data for Switzerland include data from Liechtenstein for the years 2017–2020.
In 2021, 715 cases (81.6%) were acquired in the EU. Austria, France, Germany and the Netherlands reported travel‐associated cases. Of those, only one case was imported from outside the EU, namely Nigeria, and for 160 cases (18.3%), there were no data on travel or the country of infection (Table 82).
Tularaemia shows a seasonal pattern, with most cases occurring from July to November, but some cases are also observed in winter. In 2021, infections peaked in summer months, in line with the mean for the 2017–2020 period (Figure 44).
Figure 44.
Trends in reported confirmed human cases of tularaemia in the EU, by month and year, 2017–2021
- Source: Austria, Cyprus, Czechia, Estonia, Finland, France, Germany, Greece, Hungary, Ireland, Latvia, Luxembourg, Malta, Poland, Romania, Slovakia, Slovenia, Spain and Sweden. Belgium, Bulgaria, Croatia, Denmark, Italy, Lithuania, the Netherlands and Portugal did not report data to the level of detail required for the analysis.
In 2021, the number of tularaemia cases increased compared with 2020 and vs. the 2017–2019 pre‐pandemic period. Over the past 5 years, from 2017 to 2021, a significant increasing trend in the number of tularaemia cases was observed in the EU (Figure 44). At the country level, a statistically significant (p < 0.01) increasing trend was observed in Austria, Czechia, France, Germany and Sweden.
Data on hospitalisation status were provided by 10 MSs for 221 confirmed cases. A total of 112 hospitalisations were reported by eight MSs (Austria, Czechia, Hungary, Lithuania, the Netherlands, Poland, Slovenia and Spain), corresponding to 50.7% of confirmed cases with reported hospitalisation status; more than 35% were counted in the 45‐ to 60‐year age group. The highest hospitalisation rates were reported in Lithuania (100%), Austria (64%) and Poland (55.8%). In 2021, 11 MSs reported outcomes for 341 cases (38.9%), with two deaths recorded (> 70 years) and an EU case fatality rate of 0.59%.
The proportion of male cases was higher, with a male‐to‐female ratio of 1.8:1. Children under 14 years of age accounted for 44 cases (5%). The number of cases increased with age up to 60 years and slowly decreased after 60 years. The highest notification rate was for the 55‐ to 60‐year age group, followed by the 60‐ to 65‐year age group.
6.3.3. Tularaemia in animals
In 2021, overall, four EU MSs reported 70 hares, four dogs and one muskrat positive for tularaemia, while Switzerland (not an EU MS) reported 10 positive animals (Table 84).
Table 84.
Francisella tularensis monitoring results for animals, by species in reporting EU MSs and non‐MS countries, 2021
| Country | N positive/N tested (% positive) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Domestic animals | Wild animals | Zoo animals | |||||||||
| Cats | Dogs | Guinea pigs | Rabbits | Hares | Beavers | Foxes | Muskrats | Shrews | Hares | Other zoo animals | |
| Austria | – | – | – | – | 20/52 (38.5) | – | – | – | – | – | – |
| Finland | – | – | – | – | 30/199 (15.1) | – | – | 1/1 (100) | – | – | – |
| Netherlands | – | – | – | – | – | – | – | 0/2 (0) | – | – | – |
| Sweden | – | 4/11 (36.4) | 0/1 (0) | 0/2 (0) | 20/66 (30.3) | – | – | – | 0/1 (0) | – | – |
| EU Total (27 + XI) | – | 4/11 (36.4) | 0/1 (0) | 0/2 (0) | 70/317 (22.1) | – | – | 1/3 (33.3) | 0/1 (0) | – | – |
| Switzerland | 1/5 (20.0) | 0/1 (0) | – | 0/3 (0) | 7/17 (41.2) | 0/2 (0) | 0/1 (0) | – | – | 1/3 (33.3) | 1/7 (14.3) |
| Total EU (27+ XI) + non‐EU countries | 1/5 (20.0) | 4/12 (33.3) | 0/1 (0) | 0/5 (0) | 77/334 (23.1) | 0/2 (0) | 0/1 (0) | 1/3 (33.3) | 0/1 (0) | 1/3 (33.3) | 1/7 (14.3) |
The number of samples and number of animal species tested were greater than in 2020. Moreover, one additional MS country (the Netherlands) reported data.
6.4. Discussion
Francisella tularensis is the causative agent of tularaemia and is also considered a potential biological weapon. Humans can acquire the disease through several routes. The disease shows a seasonal pattern in humans (Hestvik et al., 2015), consistent with a higher likelihood of exposure in summer and autumn months due to recreational outdoor activities (notably hunting), exposure to contaminated water (Hennebique et al., 2019) and vector bites. Tularaemia is widely distributed throughout most of Europe, and in endemic regions within Scandinavian countries, it is typically transmitted by mosquito bites (Kenney et al., 2017). Notification rates for tularaemia vary among MSs and over time. Sweden has reported cases of tularaemia since 1931 and recorded two major outbreaks in 2015 and 2019 (Dryselius et al., 2019), whereas in 2016, Finland had the highest notification rate. In 2018, an outbreak occurred in France, whereas in 2019, Norway and Sweden reported highest number of cases. In 2020, the number of human cases in the EU was about half that observed in 2019.
In 2021, the number of human cases increased in the EU compared with the 2020 pandemic year but also vs. the 2017–2019 pre‐pandemic period. More than 70% of cases were reported from Finland, France, Germany and Sweden. In particular, France recorded an increasing number of cases over a 5‐year period, mainly due to the change of the case definition used in 2021 (EU case definition), being less restrictive for confirmed cases. However, from 2018, a significant increase in tularaemia cases was noted in France. In particular, the Brittany region reported more pulmonary forms than other regions, but the reasons for this deviating pattern are unknown (Figoni et al., 2022). Overall, from 2017 to 2021, there was a statistically significant increase in the trend for confirmed cases in the EU. Moreover, no data were available for 2021 regarding the impact of the COVID‐19 pandemic on tularaemia surveillance and reporting.
Wildlife continues to play a role in maintaining F. tularensis in the ecological cycle, and also in the occurrence of human cases. Francisella spp. are present in wild animals (such as hares) and in vectors (e.g. ticks and mosquitos), which can be sources of infections for humans (WHO, 2007; Maurin and Gyuranecz, 2016).
Lately, the circulation of F. tularensis among wild animals has been reported in numerous countries in north‐central Europe, where it is considered endemic (Hestvik et al., 2015; Faber et al., 2018; Seiwald et al., 2020), as well as in Spain (Mínguez‐González et al., 2021).
In the last 5 years, among the reporting MSs, the number of hares tested has increased (from 39 to 317) and the rate of positivity has ranged from 17.9% to 36.5%.
The reporting of positive dogs (4/11) is interesting because dogs live in close contact with humans and may therefore share exposure to F. tularensis (Kwit et al., 2020).
As tularaemia surveys are very often passive, they do not reflect the status of the entire population, so it is difficult to paint an accurate picture of the spread of the disease among animals. For example, the positivity rate reported in dogs in 2021 is not indicative of the spread of the disease in this population.
It should be highlighted that risks of exposure and/or new outbreaks in humans are often preceded by the appearance of the disease in animals, which means that wildlife monitoring (hares are good indicators) is crucial.
Tularaemia is a disease with a multifaceted epidemiology; it is therefore difficult to control. All these aspects underline the importance of collaborative work between public health and veterinary units for the control of this zoonotic disease.
7. Other zoonoses and zoonotic agents
In 2021, data on Bacillus, Chlamydia, Clostridium, Cronobacter sakazakii, Klebsiella, non‐pathogenic Escherichia coli, Proteus, Shigella, Staphylococcus, Streptococcus, Vibrio, caliciviruses, flaviviruses other than West Nile virus, hepatitis virus, Cysticercus, Leishmania, Leptospira and Sarcocystis were reported to EFSA.
7.1. Bacillus spp.
Luxembourg and Portugal submitted 2021 data on Bacillus spp. in various foods collected at hospitals or medical care facilities, retail establishments, collective catering establishments (restaurant or cafe or pub or bar, or hotel or catering service) and wholesale establishments. Out of 165 batches and 1,130 single samples, 29 single samples (2.6%) tested positive. The positive food categories involved ‘other processed food products and prepared dishes’ or ‘sauces and dressings’.
Greece reported one positive bovine and one positive sheep sample (15.4%) for Bacillus anthracis in 13 samples collected at the farm level during clinical investigations.
7.2. Caliciviruses (including norovirus)
Five MSs (Bulgaria, Croatia, France, Portugal and Romania) reported data on caliciviruses for a total of six batch samples and 937 single samples. Only one sample (0.1%) of non‐pre‐cut fruit collected at the retail level in France was positive.
7.3. Chlamydia spp.
Three MSs (Austria, Denmark and Greece) and one non‐MS (North Macedonia) reported data on Chlamydia spp. in various animal species. Austria reported 106 (4.6%) positives out of 2,297 samples, Denmark reported 26 (24.5%) positives out of 106 samples and North Macedonia reported 12 (29.3%) positives out of 41 samples. Greece reported no positives from 12 tested samples.
7.4. Clostridium spp.
Greece and non‐MS North Macedonia reported data on Clostridium spp. from various ruminant species for a total of 54 samples. Greece detected 30 (56.6%) positives out of 53 animal samples collected during clinical investigations and North Macedonia detected one (100%) positive sample from the one sample collected during monitoring.
Three MSs (Lithuania, Romania and Slovenia) and non‐MS North Macedonia provided data on Clostridium spp. in foods for a total of 302 tested sampling units. In an investigation at the manufacturing level on five samples, four (80.0%) positives were found in Lithuania, and the identified species was Clostridium perfringens.
7.5. Hepatitis virus
Three MSs (Bulgaria, France and Romania) provided data on hepatitis virus in non‐pre‐cut fruit and leaf vegetables. None of the 153 tested samples were positive.
7.6. Proteus spp.
Greece tested for the presence of Proteus spp. in 179 samples of milk and organs or tissues collected from various animals during clinical investigations, resulting in 11 (6.1%) positives.
7.7. Staphylococcus spp. and staphylococcal enterotoxins
Four MSs (Bulgaria, Germany, Greece and Italy) provided data on Staphylococcus spp. (reported as Staphylococcus, Staphylococcus spp. unspecified or S. aureus) in various food matrices (N = 7,734) and animals (N = 2,491). Overall, 16.5% of food and 66.4% of animal sampling units were reported positive. ‘Milk from other animal species or unspecified – pasteurised milk’, ‘cheeses, made from unspecified milk or other animal milk – unspecified’, ‘bakery products – pastry’ and ‘other processed food products and prepared dishes – pasta’ were the food categories with the highest numbers of positive results.
Thirteen MSs reported data on staphylococcal enterotoxins collected in contexts other than those stipulated in Regulation (CE) No 2073/2005. None of the 118 tested batches were positive, whereas 12 out of 3,897 (0.3%) single samples were positive. ‘Cheeses, made from unspecified milk or other animal milk’ collected in Italy at the processing plant was the food category positive for staphylococcal enterotoxins.
7.8. Cysticercus spp.
Eight MSs (Belgium, Finland, Luxembourg, Malta, Slovakia, Slovenia, Spain and Sweden) submitted data on Cysticercus spp. in various animal species. Belgium collected 770,235 bovine carcases from slaughterhouses, revealing 857 positive samples (0.111%). None of the 2,200,672 carcases from cattle, pigs and wild boars collected in Finland were positive. Luxembourg found 74 positive bovine carcases out of the 27,326 collected samples (0.271%). None of the 65,334 cattle, pig, sheep or goat carcases collected in Malta were positive. Slovakia found three positive cattle carcases out of 34,771 (0.009%) and seven pig carcases out of 675,234 (0.001%) samples collected at the slaughterhouse. Slovenia provided results on 123,961 cattle and 242,584 pig carcases collected at the slaughterhouse, detecting 11 positive samples in cattle carcases (0.009%). Spain provided data on Cysticercus spp. in various animal species: 125 out of 2,332,666 cattle (0.005%), 17,332 out of 799,767 goats (2.18%), 2,902 out of 41,059,466 pigs (0.007%), 200,810 out of 7,077,050 sheep (2.84%), 110 out of 4,544 solipeds (2.42%) and 33 of 111,100 wild boars (0.03%) tested positive. No positives were detected upon testing 7,415 mouflons and 118,899 deer. Sweden detected one positive out of 411,650 cattle carcases and no positives from 2,651,110 pig carcases collected at the slaughterhouse.
7.9. Leishmania
Greece and non‐MS North Macedonia provided data on Leishmania collected from 6,006 (22.0%) dog blood samples, detecting 1,324 positive samples.
7.10. Sarcocystis spp.
Belgium reported data from 770,235 cattle carcases collected at the slaughterhouse, whereof 81 (0.01%) were positive for Sarcocystis spp.
7.11. Other
Twelve MSs reported data on Cronobacter sakazakii collected in contexts other those stipulated in Regulation (EC) No 2073/2005. ‘Infant formulae', ‘dried dietary foods for special medical purposes intended for infants below 6 months of age', ‘dairy products intended for infants and young children’, ‘processed cereal‐based food for infants and young children’, ‘milk and whey powder’, ‘ice cream’, ‘follow‐on formulae' and ‘ready‐to‐eat infant formulae' were collected as single samples and batches at the processing plant, retail, wholesale, hospital or medical care facility and farm and road transport levels. Three (0.25%) of the 1,205 single samples and three (0.74%) of the 404 batches were positive.
Two MSs (Greece and Latvia) provided data on Escherichia coli and non‐pathogenic E. coli collected at the farm and slaughterhouse levels, detecting 293 (74.5%) positives out of 393 tested samples.
Slovenia tested 41 milk and milk products collected at the retail level to verify the presence of flaviviruses other than West Nile virus, obtaining no positive samples. Four MSs (Ireland, Italy, the Netherlands and Slovakia) and the non‐MS Switzerland reported data collected from various animal categories. None of 12,474 collected samples tested positive.
Greece reported data on Klebsiella spp. in milk and organs or tissues collected from various ruminants (dairy cows, goats, sheep), obtaining four (2.2%) positives out of 179 tested samples.
Greece provided data on Streptococcus spp. in milk and organs or tissues collected from cattle and small ruminants, obtaining 37 (18.1%) positives out of 204 tested samples.
The Netherlands tested 750 single samples or batches of crustaceans, fishes, live bivalve molluscs and leaf vegetables to verify the presence of Vibrio spp. Vibrio cholerae was detected in 21 samples, whereas Vibrio parahaemolyticus was detected in 45 samples, for a total of 66 (8.8%) positives.
Slovenia provided data on Leptospira in various animal species, with no positives detected from 146 collected samples.
Microbiological contaminants subject to food safety criteria (Regulation (EC) No 2073/2005)
This chapter summarises the 2021 information and data provided by reporting countries on microbiological contaminants in food for which food safety criteria (FSC) have been set down in EU legislation (Regulation (EC) No 2073/2005), histamine, staphylococcal enterotoxins and Cronobacter sakazakii.
1. Histamine
1.1. Histamine data in the context of Regulation (EC) No 2073/2005
Histamine is a thermostable biogenic amine occurring naturally in the human body. However, its ingestion at high concentrations through food is associated with the onset of health disorders such as scombroid poisoning.
Regulation (EC) No 2073/2005 on microbiological criteria for foodstuffs defines three categories concerning FSC for histamine in food at the retail level: ‘fishery products from fish species associated with a high amount of histidine' (food category 1.26), ‘fishery products which have undergone enzyme maturation treatment in brine, manufactured from fish species associated with a high amount of histidine' (food category 1.27) and ‘fish sauce produced by fermentation of fishery products’ (food category 1.27a). Information was also obtained at the manufacturing level, indicating the correct application of GMP (good manufacturing practices) and the proper maintenance of the cold chain, which is essential to avoid the development or increase of histamine in fish and fish products.
Data on histamine were reported by 15 MSs (Belgium, Cyprus, Czechia, Denmark, Estonia, France, Germany, Greece, Latvia, Luxembourg, Portugal, Romania, Slovakia, Slovenia and Spain) and two non‐MSs (Iceland and Serbia).
In official control samples (n = 3,154) for histamine in food category 1.26 at the distribution level (wholesale establishments, retail establishments, border control posts and restaurants), 0.89% had a histamine content higher than 200 mg/kg, 0.98% a histamine content of between 100 and 20 mg/kg and 35.76% a histamine content above the limit of detection, but less than or equal to 100 mg/kg. Of the total number of samples, 13.9% were of EU origin (Latvia, Romania, European Union), 21.7% were of non‐EU origin (Cape Verde, Colombia, Ecuador, El Salvador, Indonesia, Mauritius, Morocco, Papua New Guinea, Philippines, Senegal, Seychelles, Taiwan, Thailand, Vietnam, non‐EU origin), while for 64.2% no information was available. Of the total number of official control samples, 15.6% came from canned fish and 3.9% from raw fish, while for 80% no information was provided.
At the manufacturing level (processing plants, packaging centres), 2,178 official control sampling units were collected and the results were as follows: 0.14% had a histamine content higher than 200 mg/kg, 0.18% a histamine content of between 100 and 200 mg/kg and 60.2% a histamine content higher than the limit of detection, but less than or equal to 100 mg/kg. Of the total number of samples, 30% were of EU origin (Estonia, European Union, Latvia, Portugal, Spain), while for 67.2% no information was reported. Of the total number of control sample units, 24.8% came from canned fish and 17.9% from raw fish, while for 57.1% no information was reported.
For food category 1.27, 1,223 and 162 official control sample units were collected at the distribution and manufacturing levels, respectively. At the distribution level, 63.2% of the samples had a histamine concentration less than or equal to 200 mg/kg and 0.33% a histamine content between 200 and 400 mg/kg. At the manufacturing level, the percentage of samples with a histamine concentration less than or equal to 200 mg/kg was 44.4%. At the distribution level, 7.35% of samples were of EU origin, 8.1% were of non‐EU origin, while for 84% no information was reported.
For food category 1.27a at the distribution level, 6 official control samples were reported: 67% of samples had a histamine content of less than 400 mg/kg but above the limit of detection; at the manufacturing level, 1 sample was collected and had a histamine content below 400 mg/kg.
1.2. Other monitoring or surveillance data for histamine
MSs also collected and analysed fishery products in contexts other than Regulation (EC) 2073/2005 on microbiological criteria for foodstuffs, such as Surveillance and Monitoring.
A total of 2,162 samples and 20 batches were collected as part of Surveillance and Monitoring activities, respectively.
Monitoring
A total 20 batches were collected at the distribution level. Of this number, 60% of batches were of EU origin and 40% of non‐EU origin. All the batches fell into category 1.26 and had a histamine content of less than 100 mg/kg.
Surveillance
A total of 1,803 and 359 sampling units were collected at the distribution and manufacturing levels, respectively.
At the distribution level, 99% of samples were taken at border control posts; 5% and 95% of the collected samples were classified in categories 1.26 and 1.27, respectively. At the manufacturing level, all samples were taken at processing plants; 87% and 13% of the samples were classified in categories 1.26 and 1.27, respectively.
All samples taken (n = 2,162) were negative.
2. Staphylococcal enterotoxins
Data on staphylococcal enterotoxins were reported by seven MSs (Bulgaria, Croatia, Cyprus, Estonia, Romania, Slovakia and Spain). No positives were reported out of 1,391 collected official control samples.
3. Cronobacter sakazakii
Data on Cronobacter sakazakii in ‘infant formulae' and ‘foodstuffs intended for special nutritional uses – dried dietary foods for special medical purposes intended for infants under 6 months of age' collected at the distribution level (retail and wholesale) were reported by four MSs (Croatia, Hungary, Slovenia, Slovakia). No positives were detected out of the 364 collected official control samples.
Estonia and Spain collected samples at the processing plant, detecting one (3.1%) positive out of 32 tested official control samples.
Note on outbreak data of SARS‐CoV‐2 in minks, mustelids and raccoon dogs, in EU
In accordance with Commission Implementing Decision (CID) (EU) 2021/788 43 Member States monitor and report infections with SARS‐CoV‐2 in minks, mustelids and raccoon dogs to the European Commission. The European Commission forwards these reports to EFSA, who, in accordance with Article 9(2) of Directive 2003/99/EC is required to summarise these outbreak data in the EU One Health Zoonoses report. EFSA and the European Commission agreed that the summary of these data will be made available in the EFSA scientific opinion ‘SARS‐CoV‐2 in animals: susceptibility of animal species, monitoring, prevention and control’ to be released by EFSA in January 2023.
Abbreviations
- ADIS
Animal Disease Information System
- AE
Alveolar echinococcosis
- AHAW
EFSA Panel on Animal Health and Welfare
- BIOHAZ
EFSA Panel on Biological Hazards
- CA
Competent authority
- CE
Cystic echinococcosis
- CFT
Complement fixation test
- CFU
Colony‐forming unit
- CHC
Controlled housing conditions
- CONTAM
EFSA Panel on Contaminants in the Food Chain
- COVID‐19
Coronavirus disease 2019
- DA
Direct agglutination
- DCF
Data Collection Framework
- DFS
Disease‐free‐status
- DHs
Definitive hosts
- EBLV
European bat lyssavirus
- ECDC
European Centre for Disease Prevention and Control
- EEA
European Economic Area
- EFTA
European Free Trade Association
- Eg
Echinococcus granulosus
- ELISA
Enzyme‐linked immunosorbent assay
- Em
Echinococcus multilocularis
- ERCE
European Register of Cystic Echinococcosis
- EU‐FORS
European Union Foodborne Reporting System
- EUOHZ
European Union One Health Zoonoses Report
- EURL
European Union Reference Laboratory
- EVD
Emerging and vector‐borne disease
- FAO
Food and Agriculture Organization
- FBO
Foodborne outbreak
- FBOp
Food business operator
- FISH
Fluorescent in situ hybridisation
- FNAO
Food of non‐animal origin
- FSC
Food safety criteria
- FWD
Foodborne and waterborne disease
- g
gram
- GAPs
Good Agricultural Practices
- GMP
Good Manufacturing Practices
- GP
Good Practices
- HACCP
Hazard analysis and critical control point
- HUS
Haemolytic–uraemic syndrome
- i‐ELISA
Indirect enzyme‐linked immunosorbent assay
- IFA
Immunofluorescence assay
- IFAT
Immunofluorescence antibody test
- IHC
Immunohistochemistry
- IHs
Intermediate hosts
- ISO
International Organization for Standardization
- JEMRA
Joint FAO/WHO Expert Meeting on Risk Assessment
- LAT
Latex agglutination test
- LHT
Low heat‐treated
- MS
Member State
- MSM
Mechanically separated meat
- MTBC
Mycobacterium tuberculosis complex
- N
Number
- NCP
National control programmes
- NMKL
Nordic Committee on Food Analysis
- NRCHC
Not raised under controlled housing conditions
- NT
Not typable
- OIE
World Organisation for Animal Health
- ORV
Oral rabies vaccination
- PCR
Polymerase chain reaction
- PHC
Process hygiene criteria
- RABV
Rabies virus
- RCHC
Raised under controlled housing conditions
- ROA
Rapid Outbreak Assessments
- RTE
Ready‐to‐eat
- s.l.
sensu lato
- STEC
Shiga toxin‐producing Escherichia coli
- SoHO
Substances of human origin
- TB
Tuberculosis
- TBE
Tick‐borne encephalitis
- TESSy
The European Surveillance System
- vs.
Versus
- WGS
Whole‐genome sequencing
- WHO
World Health Organization
- WNF
West Nile fever
- WNV
West Nile virus
- WNND
West Nile neuroinvasive disease
- WOAH
World Organisation for Animal Health
Country codes
- Albania
AL
- Austria
AT
- Belgium
BE
- Bosnia and Herzegovina
BA
- Bulgaria
BG
- Croatia
HR
- Cyprus
CY
- Czechia
CZ
- Denmark
DK
- Estonia
EE
- Finland
FI
- France
FR
- Germany
DE
- Greece
GR
- Hungary
HU
- Iceland
IS
- Ireland
IE
- Italy
IT
- Latvia
LV
- Liechtenstein
LI
- Lithuania
LT
- Luxembourg
LU
- Malta
MT
- Montenegro
ME
- Netherlands
NL
- Norway
NO
- North Macedonia
MK
- Poland
PL
- Portugal
PT
- Romania
RO
- Serbia
RS
- Slovakia
SK
- Slovenia
SI
- Spain
ES
- Switzerland
CH
- Sweden
SE
- United Kingdom
GB
- United Kingdom (Northern Ireland)
XI
Appendix A – Number of tested samples for Listeria monocytogenes for the main ready‐to‐eat (RTE) food categories, by reporting MS and non‐MS countries, 2021
| Country | RTE milk and milk products | RTE fish and fishery products | RTE meat and meat products | Other RTE products | RTE food intended for infants and for medical purposes |
|---|---|---|---|---|---|
| Austria | 905 | 176 | 742 | 688 | 1 |
| Belgium | 2,501 | 777 | 2,459 | 984 | 881 |
| Bulgaria | 4,518 | 421 | 932 | 4,309 | 1 |
| Croatia | 502 | 142 | 994 | 230 | 1 |
| Cyprus | 388 | 19 | 126 | 479 | 21 |
| Czechia | 2,196 | 156 | 6,128 | 728 | 2 |
| Denmark | – | 424 | 388 | – | – |
| Estonia | 84 | 227 | 174 | 183 | – |
| France | 1,427 | 912 | 2,367 | 1,937 | 18 |
| Germany | 8,764 | 3,991 | 11,111 | 9,493 | 316 |
| Greece | 512 | 102 | 166 | 737 | 6 |
| Hungary | 1,266 | 70 | 462 | 2,127 | 79 |
| Ireland | 931 | 198 | 1,329 | 1,678 | 200 |
| Italy | 11,324 | 967 | 300 | 1,629 | 101 |
| Latvia | 60 | 140 | 55 | 20 | – |
| Lithuania | 80 | 6 | – | 30 | – |
| Luxembourg | 53 | 2 | 304 | 621 | 10 |
| Netherlands | 3,520 | 1,218 | 633 | 1,264 | 107 |
| Poland | 8,251 | 14,972 | 54,532 | 15 | – |
| Portugal | 402 | 30 | 324 | 1,079 | 6 |
| Romania | 13,960 | 2,176 | 16,922 | 56,491 | 26 |
| Slovakia | 1,749 | 625 | 1,997 | 1,699 | 460 |
| Slovenia | 71 | 29 | 90 | 234 | 5 |
| Spain | 3,169 | 2,003 | 4,663 | 8,162 | 523 |
| United Kingdom (Northern Ireland) | – | – | – | – | – |
| EU Total (27 + XI) | 66,633 | 29,783 | 107,198 | 94,817 | 2,764 |
| Albania | 75 | 4 | 28 | 14 | – |
| Iceland | – | 30 | – | – | – |
| Montenegro | 1,381 | 3 | 261 | – | – |
| North Macedonia | 45 | 5 | 60 | – | – |
| Serbia | 779 | 39 | 424 | 121 | – |
| Switzerland | 3,410 | – | – | – | – |
| Total non‐EU countries | 5,690 | 81 | 773 | 135 | – |
| Total EU (27+ XI) + non‐EU countries | 73,323 | 29,864 | 107,971 | 94,952 | 2,764 |
–: no data available; RTE: ready‐to‐eat.
For each food category, the number of samples reported in the table was obtained without exclusion criteria.
Appendix B – Occurrence of Listeria monocytogenes at all sampling stages combined in ready‐to eat (RTE) food categories using a detection method, EU, 2019–2021
| RTE food category | Food subcategories | Sampling unit | 2021 (a) | 2020 | 2019 (b) | |||
|---|---|---|---|---|---|---|---|---|
| N tested samples | Positive samples (%) | N tested samples | Positive samples (%) | N tested samples | Positive samples (%) | |||
| Fish and fishery products | Fish | Batch | 5,089 | 5.4 | 124 | 1.6 | 22 | 0 |
| Single | 2,417 | 4.4 | 2,521 | 4.4 | 3,775 | 4.3 | ||
| Fishery products | Batch | 196 | 4.1 | 113 | 2.7 | 25 | 0 | |
| Single | 2,265 | 3.5 | 1,625 | 4.2 | 2,472 | 3.7 | ||
| Milk | Pasteurised | Batch | 156 | 0 | 153 | 0 | 144 | 0 |
| Single | 1,215 | 0.08 | 297 | 0 | 813 | 0.12 | ||
| UHT | Batch | – | – | 5 | 0 | – | – | |
| Single | 122 | 0 | 64 | 0 | 99 | 0 | ||
| Raw, intended for direct human consumption | Batch | 138 | 1.4 | 132 | 0 | 144 | 0.69 | |
| Single | 10 | 20 | 336 | 1.5 | 44 | 0 | ||
| Hard cheeses from pasteurised milk | From cow milk | Batch | 1,836 | 0.33 | 2,031 | 0.3 | 1,912 | 0.1 |
| Single | 1,123 | 0 | 805 | 0 | 1,273 | 0 | ||
| From goat milk | Batch | 3 | 0 | 8 | 0 | 107 | 0 | |
| Single | 37 | 0 | 42 | 0 | 105 | 0 | ||
| From sheep milk | Batch | 12 | 0 | 20 | 0 | 4 | 0 | |
| Single | 29 | 0 | 45 | 6.7 | 30 | 0 | ||
| Hard cheeses from raw or low heat‐treated milk | From cow milk | Batch | 450 | 0.67 | 542 | 1.1 | 541 | 0.55 |
| Single | 259 | 0.39 | 516 | 0 | 727 | 1.1 | ||
| From goat milk | Batch | – | – | – | ||||
| Single | 32 | 0 | 34 | 0 | 29 | 3.4 | ||
| From sheep milk | Batch | – | – | 5 | 0 | – | – | |
| Single | 259 | 4.6 | 240 | 5.4 | 170 | 3.5 | ||
| Soft and semi‐soft cheeses from pasteurised milk (including fresh cheese) | From cow milk | Batch | 40 | 0 | 71 | 0 | 172 | 0 |
| Single | 2,673 | 0.86 | 844 | 1.1 | 1,465 | 0.55 | ||
| From goat milk | Batch | 18 | 0 | 32 | 0 | 17 | 0 | |
| Single | 316 | 0.95 | 61 | 0 | 25 | 0 | ||
| From sheep milk | Batch | 53 | 0 | 23 | 0 | 19 | 0 | |
| Single | 76 | 0 | 40 | 0 | 45 | 0 | ||
| Soft and semi‐soft cheeses from raw or low heat‐treated milk (including fresh cheese) | From cow milk | Batch | 66 | 0 | 82 | 0 | 130 | 0 |
| Single | 427 | 0.70 | 878 | 0.46 | 651 | 1.4 | ||
| From goat milk | Batch | – | – | – | – | – | – | |
| Single | 26 | 0 | 15 | 0 | 42 | 0 | ||
| From sheep milk | Batch | – | – | – | – | – | – | |
| Single | 178 | 1.7 | 127 | 3.1 | 164 | 0 | ||
| Meat products | From bovine animals | Batch | 2 | 0 | 12 | 0 | 3 | 0 |
| Single | 2,215 | 3.9 | 844 | 7.5 | 1,245 | 4.3 | ||
| From broilers | Batch | 2 | 100 | 11 | 0 | – | – | |
| Single | 767 | 1.3 | 291 | 0.69 | 803 | 2 | ||
| From turkeys | Batch | 3 | 0 | 5 | 0 | 3 | 0 | |
| Single | 123 | 0 | 157 | 0.64 | 125 | 1.6 | ||
| From pigs | Batch | 649 | 0.77 | 115 | 0 | 127 | 4.7 | |
| Single | 24,102 | 2.7 | 6,470 | 3 | 13,908 | 4.2 | ||
| Other RTE products | Salads (c) | Batch | 126 | 0.79 | 99 | 1 | 47 | 0 |
| Single | 844 | 0.95 | 2,664 | 2.3 | 2,993 | 3.6 | ||
| Bakery products (d) | Batch | 4 | 0 | 3 | 0 | 22 | 0 | |
| Single | 1,481 | 0.2 | 2,563 | 0.55 | 3,202 | 0.34 | ||
| Fruits, vegetables and juices (e) | Batch | 24 | 4.2 | 17 | 0 | 66 | 0 | |
| Single | 1,383 | 3 | 1,857 | 2.9 | 1,717 | 2.3 | ||
| Sauces & dressings (f) | Batch | 4 | 0 | 4 | 0 | – | – | |
| Single | 182 | 0 | 315 | 0.32 | 83 | 1.2 | ||
| Egg products | Batch | 2 | 0 | 6 | 0 | 3 | 0 | |
| Single | 16 | 0 | 1 | 0 | 23 | 0 | ||
| Confectionery products & pastes (g) | Batch | – | – | 2 | 0 | 3 | 0 | |
| Single | 26 | 0 | 28 | 0 | 37 | 0 | ||
| Spices & herbs (h) | Batch | – | – | 1 | 0 | 2 | 0 | |
| Single | 115 | 0 | 113 | 0 | 139 | 1.4 | ||
| Other processed food products & prepared dishes | Batch | 96 | 2.1 | 123 | 0 | 252 | 0 | |
| Single | 21,001 | 0.34 | 10,365 | 0.39 | 10,585 | 1.1 | ||
: Data from the United Kingdom (Northern Ireland) are taken into account for 2021. In accordance with the agreement on the withdrawal of the United Kingdom from the EU, and in particular with the Protocol on Ireland/Northern Ireland, EU requirements on data sampling are also applicable to Northern Ireland.
: doi:Data from the United Kingdom are taken into account for 2019, because the United Kingdom was an EU MS, but it became a third country on 1 February 2020.
: doi:Includes RTE salads containing mayonnaise.
: doi:Includes bread, cakes, desserts and pastry.
: doi:Includes fruits and vegetables pre‐cut, products; fruit or vegetable juice, mixed juice.
: doi:Includes sauce and dressings containing mayonnaise.
: Includes confectionery products and pastes such as chocolate‐based product and soft and hard candy.
: Includes spices and herbs, either dried, fresh or frozen.
Appendix C – Atlas of STEC serogroups in food, animals and humans in reporting MSs, EU, 2017–2021
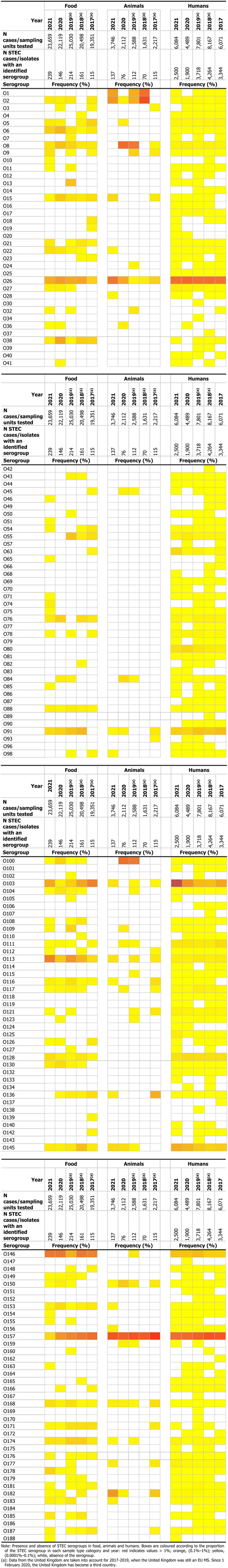
Appendix D – STEC serogroups in food and animals, by reporting MS, EU, 2021
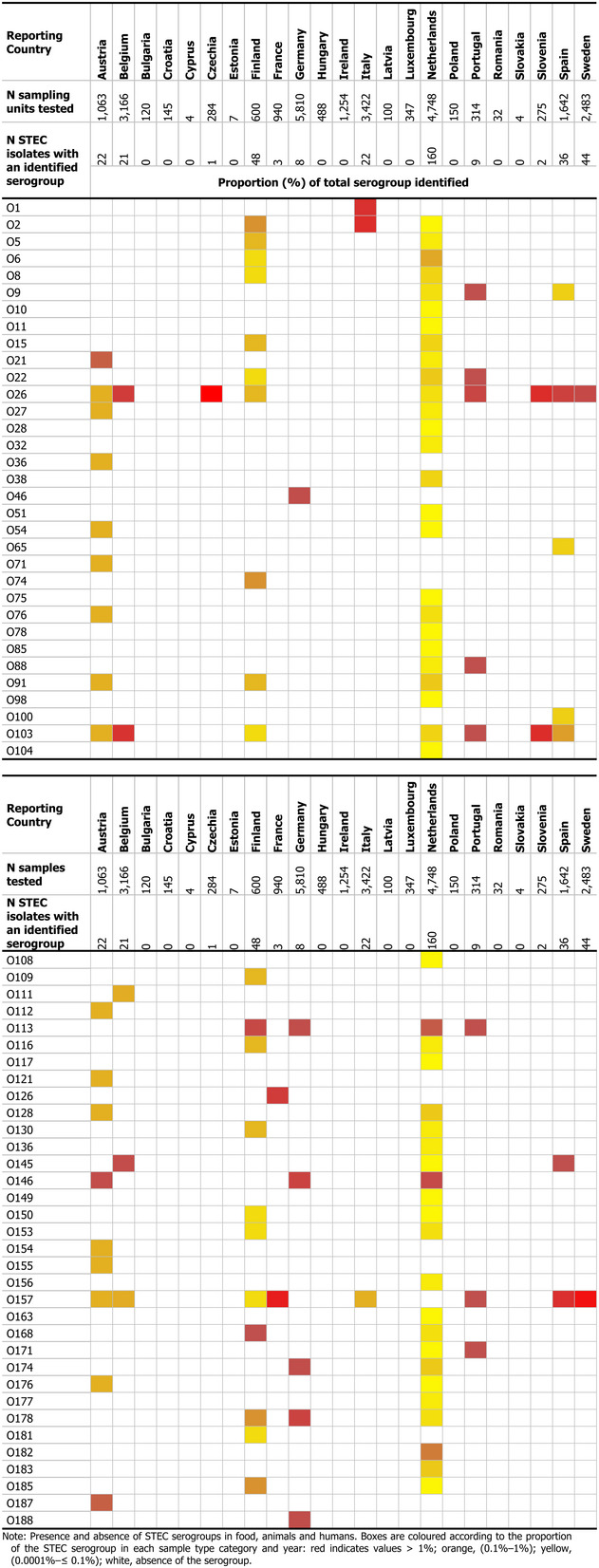
Appendix E – Atlas of STEC serogroups in food and animals in reporting MSs, EU, 2021
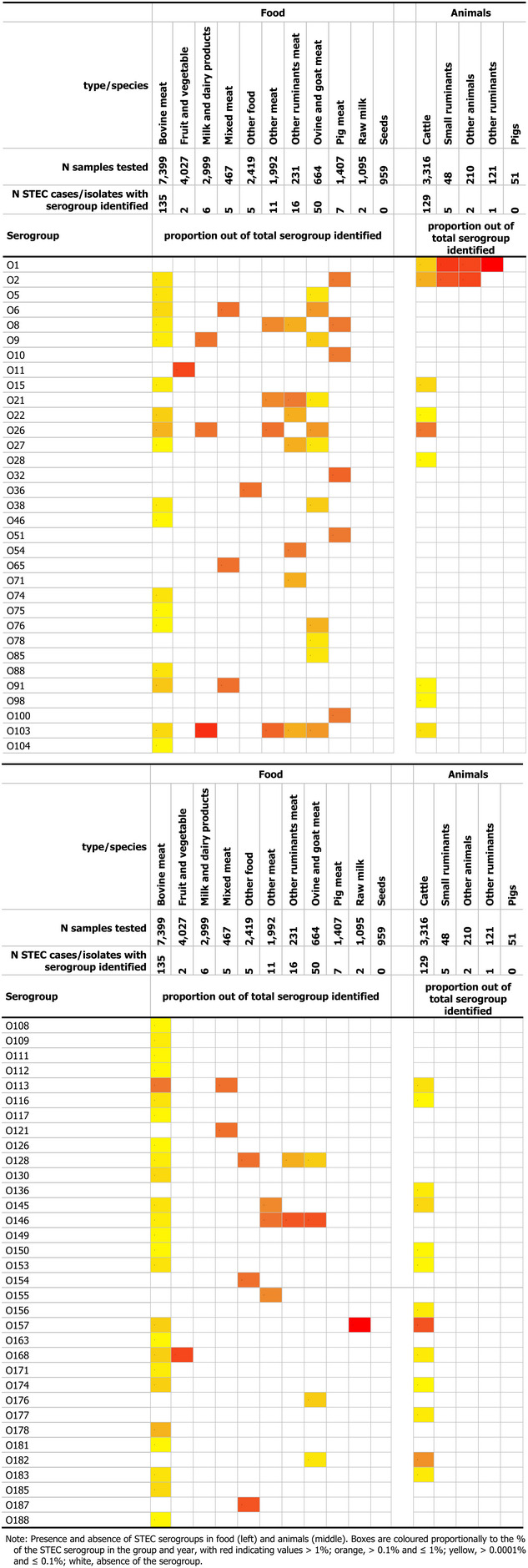
Supporting information
The European Union One Health 2021 Zoonoses Report
Suggested citation: EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control) , 2022. The European Union One Health 2021 Zoonoses Report. EFSA Journal 2022;20(12):7666, 273 pp. 10.2903/j.efsa.2022.7666
Requestor European Commission
Question number EFSA‐Q‐2021‐00762
Declarations of interest The declarations of interest of all scientific experts active in EFSA's work are available at https://ess.efsa.europa.eu/doi/doiweb/doisearch.
Acknowledgements EFSA and the ECDC wish to thank the members of the EFSA Scientific Network for Zoonoses Monitoring Data and of the ECDC Food and Waterborne Diseases and Zoonoses Network, the ECDC Emerging and Vector‐borne Diseases Network and the ECDC Tuberculosis Network, who provided the data and reviewed the report; the members of the Scientific Network for Zoonoses Monitoring Data for their endorsement of this scientific report; the EFSA staff members (Frank Boelaert, Giusi Amore, Valentina Rizzi, Anca Stoicescu, Maria Teresa Da Silva Felício, Sandra Luisa De Almeida Florentino Correia, Michaela Hempen, Winy Messens, Sotiria‐Eleni Antoniou, Francesca Baldinelli, Alessandro Broglia, Sofie Dhollander, Lisa Kohnle and Lina Mur), the ECDC staff members (Taina Niskanen, Joana Haussig and Veronica Cristea) and the EFSA and ECDC contractor: Istituto Superiore di Sanità (Gaia Scavia, Ilaria Altieri, Fabrizio Anniballi, Antonino Bella, Simone Cacciò, Adriano Casulli, Alessandra Ciervo, Elisabetta Delibato, Michele Luca D'Errico, Aurora Garcia Fernandez, Maria Angeles Gomez Morales, Francesca Iacoponi, Arnold Knijn, Marco Lalle, Claudia Lucarelli, Stefano Morabito, Marco Ortoffi, Paolo Pasquali, Pierfrancesco Barbariol, Flavia Riccardo, Elisabetta Suffredini, Rosangela Tozzoli, Eleonora Ventola and Laura Villa); Istituto Zooprofilattico delle Venezie (Giorgia Angeloni, Lisa Barco, Grazia Manca, Katia Capello, Giulia Cento, Laura Ciot, Paola De Benedictis, Laura Gagliazzo, Annie Ishebabi, Elena Lazzaro, Monica Lorenzetto, Marzia Mancin, Claudio Mantovani, Martina Simonato and Marica Toson); Istituto Zooprofilattico Sperimentale dell'Abruzzo e del Molise ‘G. Caporale' (Paolo Calistri, Enrico De Albentiis, Giuliano Garofolo, Diana Palma, Carla Ippoliti, Americo Bonanni, Susanna Tora, Alessio Di Lorenzo, Mauro Ferella, Andrea De Ruvo and Annamaria Conte); Istituto Zooprofilattico Sperimentale della Lombardia ed Emilia Romagna B. Ubertini – IZSLER (Maria Beatrice Boniotti, Nadia Vicari, Maria Lodovica Pacciarini, Silvia Bellini and Gabriele Casadei) for the support provided to this scientific report; the French Agency for Food, Environmental and Occupational Health & Safety (Adrien Asseré, Christophe Cordevant, Aurélie Couesnon, Radu Blaga, Florence Cliquet, Corinne Danan, Nolwenn Dheilly, Claire Ponsart, Dana Pottratz, Emmanuelle Robardet and Elodie Rousset).
Plain language summary A plain language summary of this opinion is available under Supporting Information. It aims to enhance transparency and inform interested parties on the topic using simplified language. Those interested in the more technical analysis should consult the full report.
Approved: 11 November 2022
Notes
Directive 2003/99/EC of the European Parliament and of the Council of 17 November 2003 on the monitoring of zoonoses and zoonotic agents, amending Council Decision 90/424/EEC and repealing Council Directive 92/117/EEC. OJ L 325, 12.12.2003 pp. 31–40.
See mandate M‐2015‐0231 in OpenEFSA Question: https://open.efsa.europa.eu/questions/EFSA-Q-2020-00787
Decision No. 1082/2013/EU of the European Parliament and of the Council of 22 October 2013 on serious cross‐border threats to health and repealing Decision No 2119/98/EC. OJ L 293, 5.11.2013, pp. 1–15.
Commission Implementing Decision 2018/945/EU on the communicable diseases and related special health issues to be covered by epidemiological surveillance as well as relevant case definitions. OJ L 170, 6.7.2018, pp. 1–74.
Agreement on the withdrawal of the United Kingdom of Great Britain and Northern Ireland from the European Union and the European Atomic Energy Community. OJ L 29, 31.1.2020, p. 7 (‘Withdrawal Agreement’).
Based on the customs union treaty of the Principality of Liechtenstein with Switzerland, Liechtenstein is part of the Swiss customs territory. Due to the strong connection between the veterinary authorities of Liechtenstein and Switzerland, and Liechtenstein's integration into the Swiss system in the veterinary field, in principal, all legislation, rules and data on contagious diseases are identical for both Switzerland and Liechtenstein. If not mentioned otherwise, the Swiss data also include the data from Liechtenstein.
Commission Regulation (EC) No 2073/2005 of 15 November 2005 on microbiological criteria for foodstuffs OJ L 338, 22.12.2005, pp. 1–26.
Commission Implementing Regulation (EU) 2019/627 of 15 March 2019, laying down uniform practical arrangements for the performance of official controls on products of animal origin intended for human consumption in accordance with Regulation (EU) 2017/625 of the European Parliament and of the Council and amending Commission Regulation (EC) No 2074/2005 regarding official controls. OJ L 131, 17.5.2019, pp. 51–100.
This means official sampling using the same method and sampling area as food business operators. At least 49 random samples shall be taken in each slaughterhouse each year. The number of samples may be reduced in small slaughterhouses and based on a risk evaluation.
Directive 2003/99/EC of the European Parliament and of the Council of 17 November 2003 on the monitoring of zoonoses and zoonotic agents, amending Council Decision 90/424/EEC and repealing Council Directive 92/117/EEC. OJ L 325, 12.12.2003, pp. 31–40.
More information is available at: https://www.ecdc.europa.eu/en/publications-data/download-data-response-measures-covid-19
Regulation (EC) No 852/2004 of the European Parliament and of the Council of 29 April 2004 on the hygiene of foodstuffs. OJ L 139, 30.4.2004, pp. 1–54.
Commission Regulation (EU) No 1086/2011 of 27 October 2011 amending Annex II to Regulation (EC) No 2160/2003 of the European Parliament and of the Council and Annex I to Commission Regulation (EC) No 2073/2005 as regards salmonella in fresh poultry meat Text with EEA relevance. OJ L 281, 28.10.2011, pp. 7–11.
Commission Implementing Regulation (EU) 2019/627 of 15 March 2019 laying down uniform practical arrangements for the performance of official controls on products of animal origin intended for human consumption in accordance with Regulation (EU) 2017/625 of the European Parliament and of the Council and amending Commission Regulation (EC) No 2074/2005 as regards official controls. OJ L 131, 17.5.2019, pp. 51–100.
Regulation (EC) No 853/2004 of the European Parliament and of the Council of 29 April 2004 laying down specific hygiene rules for food of animal origin. OJ L 139, 30.4.2004, pp. 55–205.
Regulation (EC) No 2160/2003 of the European Parliament and of the Council of 17 November 2003 on the control of salmonella and other specified foodborne zoonotic agents. OJ L 325, 12.12.2003, pp. 1–15.
Commission Regulation (EU) No 200/2010 of 10 March 2010 implementing Regulation (EC) No 2160/2003 of the European Parliament and of the Council as regards a Union target for the reduction of the prevalence of Salmonella serotypes in adult breeding flocks of Gallus (Text with EEA relevance). OJ L 61, 11.3.2010, pp. 1–9.
Commission Regulation (EU) No 200/2012 of 8 March 2012 concerning a Union target for the reduction of Salmonella Enteritidis and Salmonella Typhimurium in flocks of broilers, as provided for in Regulation (EC) No 2160/2003 of the European Parliament and of the Council Text with EEA relevance. OJ L 71, 9.3.2012, pp. 31–36.
Commission Regulation (EU) No 1190/2012 of 12 December 2012 concerning a Union target for the reduction of Salmonella Enteritidis and Salmonella Typhimurium in flocks of turkeys, as provided for in Regulation (EC) No 2160/2003 of the European Parliament and of the Council Text with EEA relevance. OJ L 340, 13.12.2012, p. 29–34.
Commission Regulation (EU) No 517/2011 of 25 May 2011 implementing Regulation (EC) No 2160/2003 of the European Parliament and of the Council as regards a Union target for the reduction of the prevalence of certain Salmonella serotypes in laying hens of Gallus and amending Regulation (EC) No 2160/2003 and Commission Regulation (EU) No 200/2010. OJ L 138, 26.5.2011, pp. 45–51.
GR reported enumeration data (N = 6, 0 positive samples).
Commission Implementing Regulation (EU) 2020/2002 of 7 December 2020 laying down rules for the application of Regulation (EU) 2016/429 of the European Parliament and of the Council with regard to Union notification and Union reporting of listed diseases, to formats and procedures for submission and reporting of Union surveillance programmes and of eradication programmes and for application for recognition of disease‐free status, and to the computerised information system, OJ L 412, 8.12.2020, pp. 1–28.
EU Animal Diseases Information System (ADIS). More information is available at https://food.ec.europa.eu/animals/animal-diseases/animal-disease-information-system-adis_en
In 2021, the regulatory framework for brucellosis in animals changed substantially in the EU. Infection with B. abortus, B. melitensis and B. suis in bovine animals, and sheep and goats became a disease to be controlled in all MSs with the goal of eradicating the disease in these animals throughout the EU, while being kept under surveillance in other even‐toed ungulates (Artiodactyla). Previous, repealed legislation established requirements relating to Officially brucellosis‐free status in cattle (OBF) and/or officially B. melitensis‐free status in sheep and goats (ObmF), in certain Member States and regions of Member States.
Commission Implementing Regulation (EU) 2020/2002 of 7 December 2020 laying down the rules for the application of Regulation (EU) 2016/429 of the European Parliament and of the Council with regard to Union notification and Union reporting of listed diseases, to formats and procedures for submission and reporting of Union surveillance programmes and of eradication programmes, and for application for recognition of disease‐free status, and to the computerised information system, OJ L 412, 8.12.2020, pp. 1–28.
EU Animal Diseases Information System (ADIS). More information is available at: https://food.ec.europa.eu/animals/animal-diseases/animal-disease-information-system-adis_en
French, Spanish overseas territories are defined as DFS zones; Portuguese overseas territories are defined as non‐DFS zones.
French, Portuguese and Spanish overseas territories are defined as non‐DFS zones.
Commission Implementing Regulation (EU) 2015/1375 of 10 August 2015 laying down specific rules on official controls for Trichinella in meat (text with EEA relevance). OJ L 212, 11.8.2015, pp. 7–34.
Commission Delegated Regulation (EU) 2018/772 of 21 November 2017 supplementing Regulation (EU) No 576/2013 of the European Parliament and of the Council with regard to preventive health measures for the control of Echinococcus multilocularis infection in dogs and repealing Delegated Regulation (EU) No 1152/2011 C/2017/7619 OJ L 130, 28.5.2018, pp. 1–10.
EU Animal Diseases Information System (ADIS). More information is available at: https://food.ec.europa.eu/animals/animal-diseases/animal-disease-information-system-adis_en
Data from the United Kingdom are taken into account for 2017–2019, because the United Kingdom was an EU MS over this period but it became a third country on 1 February 2020. United Kingdom (Northern Ireland) did not report monitoring data for 2021 from intermediate hosts for Echinococcus granulosus sensu lato.
Northern Ireland Statistics and Research Agency (NISRA) https://www.nisra.gov.uk/
More information is available at: https://covid-statistics.jrc.ec.europa.eu/RMeasures
Regulation (EU) No 652/2014 of the European Parliament and of the Council of 15 May 2014 laying down provisions for the management of expenditure relating to the food chain, animal health and animal welfare, and relating to plant health and plant reproductive material, amending Council Directives 98/56/EC, 2000/29/EC and 2008/90/EC, Regulations (EC) No 178/2002, (EC) No 882/2004 and (EC) No 396/2005 of the European Parliament and of the Council, Directive 2009/128/EC of the European Parliament and of the Council and Regulation (EC) No 1107/2009 of the European Parliament and of the Council and repealing Council Decisions 66/399/EEC, 76/894/EEC and 2009/470/EC. OJ L 189, 27.6.2014, pp. 1–32.
Commission Delegated Regulation (EU) 2020/689 of 17 December 2019 supplementing Regulation (EU) 2016/429 of the European Parliament and of the Council as regards rules for surveillance, eradication programmes, and disease‐free status for certain listed and emerging diseases.
Commission Implementing Regulation (EU) No 577/2013 of 28 June 2013 on the model identification documents for the non‐commercial movement of dogs, cats and ferrets, the establishment of lists of territories and third countries and the format, layout and language requirements of the declarations attesting compliance with certain conditions provided for in Regulation (EU) No. 576/2013 of the European Parliament and of the Council. OJ L 178, 28.6.2013, pp. 109–148.
Commission Implementing Regulation (EU) 2018/1882 of 3 December 2018 on the application of certain disease prevention and control rules to categories of listed diseases and establishing a list of species and groups of species posing a considerable risk for the spread of those listed diseases.
Commission Implementing Regulation (EU) 2020/2002 of 7 December 2020 laying down rules for the application of Regulation (EU) 2016/429 of the European Parliament and of the Council with regard to Union notification and Union reporting of listed diseases, to formats and procedures for submission and reporting of Union surveillance programmes and of eradication programmes and for application for recognition of disease‐free status, and to the computerised information system.
Animal Disease Information System.
More information is available at: https://climate.copernicus.eu/
Commission Implementing Decision (EU) 2021/788 laying down rules for the monitoring and reporting of infections with SARS‐CoV‐2 in certain animal species. OJ L 173, 17.5.2021, pp. 6–14.
References
- Adamse P, Van Egmond H, Noordam M, Mulder P and De Nijs M, 2014. Tropane alkaloids in food: poisoning incidents. Quality Assurance and Safety of Crops & Foods, 6, 15–24. 10.3920/QAS2013.0314 [DOI] [Google Scholar]
- Adinolfi F, Di Pasquale J and Capitanio F, 2016. Economic issues on food safety. Ital J Food Saf, 5, 5580. 10.4081/ijfs.2016.5580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alban L, Pozio E, Boes J, Boireau P, Boué F, Claes M, Cook AJ, Dorny P, Enemark HL, van der Giessen J, Hunt KR, Howell M, Kirjusina M, Nöckler K, Rossi P, Smith GC, Snow L, Taylor MA, Theodoropoulos G, Vallée I, Viera‐Pinto MM and Zimmer IA, 2011. Towards a standardised surveillance for Trichinella in the European Union. Prev Vet Med, 99, 148–160. 10.1016/j.prevetmed.2011.02.008 [DOI] [PubMed] [Google Scholar]
- Alegbeleye OO, Singleton I and Sant'Ana AS, 2018. Sources and contamination routes of microbial pathogens to fresh produce during field cultivation: a review. Food Microbiol, 73, 177–208. 10.1016/j.fm.2018.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aroussi A, Vignoles P, Dalmay F, Wimel L, Dardé ML, Mercier A and Ajzenberg D, 2015. Detection of Toxoplasma gondii DNA in horse meat from supermarkets in France and performance evaluation of two serological tests. Parasite, 22, 14. 10.1051/parasite/2015014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balog T, Nagy G, Halász T, Csányi E, Zomborszky Z and Csivincsik Á, 2021. The occurrence of Echinococcus spp. in golden jackal (Canis aureus) in southwestern Hungary: should we need to rethink its expansion? Parasitology International, 80, 102214. 10.1016/j.parint.2020.102214 [DOI] [PubMed] [Google Scholar]
- Barzon L, Montarsi F, Quaranta E, Monne I, Pacenti M, Michelutti A, Toniolo F, Danesi P, Marchetti G, Gobbo F, Sinigaglia A, Riccetti S, Dal Molin E, Favero L, Russo F and Capelli G, 2022. Early start of seasonal transmission and co‐circulation of West Nile virus lineage 2 and a newly introduced lineage 1 strain, northern Italy, June 2022. Euro Surveill, 27. 10.2807/1560-7917.Es.2022.27.29.2200548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck C, Leparc Goffart I, Franke F, Gonzalez G, Dumarest M, Lowenski S, Blanchard Y, Lucas P, Lamballerie X, Grard G, Durand GA, Zientara S, Tapprest J, L'Ambert G, Durand B, Desvaux S and Lecollinet S, 2020. Contrasted epidemiological patterns of west nile virus lineages 1 and 2 infections in France from 2015 to 2019. Pathogens, 9, 908. 10.3390/pathogens9110908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergis H, Bonanno L, Asséré A and Lombard B, 2021. EURL Lm technical guidance document on challenge tests and durability studies for assessing shelf‐life of ready‐to‐eat foods related to Listeria monocytogenes; c2021 [cited 4 Aug 2021]. Available online: https://food.ec.europa.eu/system/files/2021-07/biosafety_fh_mc_tech-guide-doc_listeria-in-rte-foods_en_0.pdf
- Blaga R, Aubert D, Thébault A, Perret C, Geers R, Thomas M, Alliot A, Djokic V, Ortis N, Halos L, Durand B, Mercier A, Villena I and Boireau P, 2019. Toxoplasma gondii in beef consumed in France: regional variation in seroprevalence and parasite isolation. Parasite, 26, 77. 10.1051/parasite/2019076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bless PJ, Schmutz C and Mäusezahl D, 2017. The recurrent campylobacteriosis epidemic over Christmas and New Year in European countries, 2006‐2014. BMC Res Notes, 10, 266. 10.1186/s13104-017-2587-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boelaert F, Amore G, Van der Stede Y and Hugas M, 2016. EU‐wide monitoring of biological hazards along the foodchain: achievements, challenges and EFSA vision for the future. Current Opinion in Food Science, 12, 52–62. 10.1016/j.cofs.2016.08.004 [DOI] [Google Scholar]
- Bonardi S, Blagojevic B, Belluco S, Roasto M, Gomes‐Neves E and Vågsholm I, 2021. Food chain information in the European pork industry: where are we? Trends in Food Science & Technology, 118, 833–839. 10.1016/j.tifs.2021.10.030 [DOI] [Google Scholar]
- Boni M, Davoust B, Tissot‐Dupont H and Raoult D, 1998. Survey of seroprevalence of Q fever in dogs in the southeast of France, French Guyana, Martinique, Senegal and the Ivory Coast. Vet Microbiol, 64, 1–5. 10.1016/s0378-1135(98)00247-8 [DOI] [PubMed] [Google Scholar]
- Buchanan RL, Gorris LG, Hayman MM, Jackson TC and Whiting RC, 2017. A review of Listeria monocytogenes: an update on outbreaks, virulence, dose‐response, ecology, and risk assessments. Food control, 75, 1–13. 10.1016/j.foodcont.2016.12.016 [DOI] [Google Scholar]
- Byrne AW, Barrett D, Breslin P, O'Keeffe J, Murphy KJ, Conteddu K, Morera‐Pujol V, Ryan E and Ciuti S, 2022. Disturbance ecology meets bovine tuberculosis (bTB) Epidemiology: a before‐and‐after study on the association between forest clearfelling and bTB herd risk in cattle herds. Pathogens, 11, 807. 10.3390/pathogens11070807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canals A, Martínez CV, Diogène J, Gago‐Martínez A, Cebadera‐Miranda L, de Vasconcelos FM, Gómez IL, Sánchez EVM, Alférez RC and Núñez D, 2021. Risk characterisation of ciguatera poisoning in Europe. EFSA Supporting Publications, 18, 6647E. 10.2903/sp.efsa.2021.EN-6647 [DOI] [Google Scholar]
- Carrié P, Barry S, Rousset E, de Crémoux R, Sala C, Calavas D, Perrin JB, Bronner A, Gasqui P, Gilot‐Fromont E, Becker CAM, Gache K and Jourdain E, 2019. Swab cloths as a tool for revealing environmental contamination by Q fever in ruminant farms. Transbound Emerg Dis, 66, 1202–1209. 10.1111/tbed.13137 [DOI] [PubMed] [Google Scholar]
- Casulli A, 2020. Recognising the substantial burden of neglected pandemics cystic and alveolar echinococcosis. The Lancet Global Health, 8, e470–e471. 10.1016/S2214-109X(20)30066-8 [DOI] [PubMed] [Google Scholar]
- Casulli A, Massolo A, Saarma U, Umhang G, Santolamazza F and Santoro A, 2022. Species and genotypes belonging to Echinococcus granulosus sensu lato complex causing human cystic echinococcosis in Europe (2000‐2021): a systematic review. Parasit Vectors, 15, 109. 10.1186/s13071-022-05197-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cegar S, Kuruca L, Vidovic B, Antic D, Hauge SJ, Alvseike O and Blagojevic B, 2022. Risk categorisation of poultry abattoirs on the basis of the current process hygiene criteria and indicator microorganisms. Food control, 132, 108530. 10.1016/j.foodcont.2021.108530 [DOI] [Google Scholar]
- Chanamé Pinedo L, Mughini‐Gras L, Franz E, Hald T and Pires SM, 2022. Sources and trends of human salmonellosis in Europe, 2015–2019: an analysis of outbreak data. International Journal of Food Microbiology, 379, 109850. 10.1016/j.ijfoodmicro.2022.109850 [DOI] [PubMed] [Google Scholar]
- Ciaravino G, Laranjo‐González M, Casal J, Sáez‐Llorente JL and Allepuz A, 2021. Most likely causes of infection and risk factors for tuberculosis in Spanish cattle herds. Vet Rec, 189, e140. 10.1002/vetr.140 [DOI] [PubMed] [Google Scholar]
- Clayton D and Hills M, 2013. Statistical models in epidemiology. OUP Oxford, Chapter 11.2 pp.
- Conraths FJ, Probst C, Possenti A, Boufana B, Saulle R, La Torre G, Busani L and Casulli A, 2017. Potential risk factors associated with human alveolar echinococcosis: systematic review and meta‐analysis. PLoS Neglected Tropical Diseases, 11, e0005801. 10.1371/journal.pntd.0005801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe SJ, Bottichio L, Shade LN, Whitney BM, Corral N, Melius B, Arends KD, Donovan D, Stone J, Allen K, Rosner J, Beal J, Whitlock L, Blackstock A, Wetherington J, Newberry LA, Schroeder MN, Wagner D, Trees E, Viazis S, Wise ME and Neil KP, 2017. Shiga toxin‐producing E. coli infections associated with flour. N Engl J Med, 377, 2036–2043. 10.1056/NEJMoa1615910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuperlovic K, Djordjevic M and Pavlovic S, 2005. Re‐emergence of trichinellosis in southeastern Europe due to political and economic changes. Vet Parasitol, 132, 159–166. 10.1016/j.vetpar.2005.05.047 [DOI] [PubMed] [Google Scholar]
- De Massis F, Sacchini F, Averaimo D, Garofolo G, Lecchini P, Ruocco L, Lomolino R, Santucci U, Sgariglia E, Crotti S, Petrini A, Migliorati G, D'Alterio N, Gavaudan S and Tittarelli M, 2021. First isolation of Brucella canis from a breeding kennel in Italy. Vet Ital, 57, 3. 10.12834/VetIt.2497.15848.1 [DOI] [PubMed] [Google Scholar]
- Deplazes P, Hegglin D, Gloor S and Romig T, 2004. Wilderness in the city: the urbanization of Echinococcus multilocularis . Trends Parasitol, 20, 77–84. 10.1016/j.pt.2003.11.011 [DOI] [PubMed] [Google Scholar]
- van Deursen B, Hagenaars M, Meima A, van Asten L, Richardus JH, Fanoy E and Voeten H, 2022. A sharp decrease in reported non‐COVID‐19 notifiable infectious diseases during the first wave of the COVID‐19 epidemic in the Rotterdam region, the Netherlands: a descriptive study. BMC Infect Dis, 22, 208. 10.1186/s12879-022-07209-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk MAM, Engelsma MY, Visser VXN, Keur I, Holtslag ME, Willems N, Meij BP, Willemsen PTJ, Wagenaar JA, Roest HIJ and Broens EM, 2021. Transboundary spread of Brucella canis through import of infected dogs, the Netherlands, November 2016–December 2018. Emerg Infect Dis, 27, 1783–1788. 10.3201/eid2707.201238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIN (Deutsches Institut Fur Normung E.V. ‐ German National Standard‐) , 2004a. DIN 10118:2004‐06. Analysis of foodstuffs ‐ detection of verotoxine‐forming Escherichia (E.) coli‐strains (VTEC) in food derived from animals.
- DIN (Deutsches Institut Fur Normung E.V. ‐ German National Standard‐) , 2004b. DIN 10167:2004 DE. Nachweis von Escherichia coli O157 in Fleisch und Fleischerzeugnissen. Detection of Escherichia coli O157 in meat and meat products.
- Djordjevic M, Bacic M, Petricevic M, Cuperlovic K, Malakauskas A, Kapel CM and Murrell KD, 2003. Social, political, and economic factors responsible for the reemergence of trichinellosis in Serbia: a case study. J Parasitol, 89, 226–231. 10.1645/0022-3395(2003)089[0226:Spaefr]2.0.Co;2 [DOI] [PubMed] [Google Scholar]
- Dryselius R, Hjertqvist M, Mäkitalo S, Lindblom A, Lilja T, Eklöf D and Lindström A, 2019. Large outbreak of tularaemia, central Sweden, July to September 2019. Euro Surveill, 24. 10.2807/1560-7917.Es.2019.24.42.1900603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dušek D, Vince A, Kurelac I, Papić N, Višković K, Deplazes P and Beck R, 2020. Human alveolar Echinococcosis, Croatia. Emerging Infectious Diseases, 26, 364–366. 10.3201/eid2602.181826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebani VV, Bertelloni F, Najar B, Nardoni S, Pistelli L and Mancianti F, 2020. Antimicrobial activity of essential oils against staphylococcus and malassezia strains isolated from canine dermatitis. Microorganisms, 8, 252. 10.3390/microorganisms8020252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECDC (European Centre for Disease Prevention and Control) , 2018. Early large increase in West Nile virus infections in the EU/EEA and EU neighbouring countries. Available online: https://www.ecdc.europa.eu/en/publications-data/rapid-risk-assessment-early-large-increase-west-nile-virus-infections-reported
- ECDC (European Centre for Disease Prevention and Control) , 2019. ECDC strategic framework for the integration of molecular and genomic typing into European surveillance and multi‐country outbreak investigations – 2019–2021. 10.2900/805317 Available online: https://www.ecdc.europa.eu/en/publications-data/ecdc-strategic-framework-integration-molecular-and-genomic-typing-european [DOI]
- ECDC and EFSA (European Centre for Disease Prevention and Control and European Food Safety Authority) , 2018a. Multi‐country outbreak of Listeria monocytogenes serogroup IVb, multi‐locus sequence type 6, infections probably linked to frozen corn. EFSA Supporting Publications. 2397‐8325. 10.2903/sp.efsa.2018.EN-1402 [DOI] [Google Scholar]
- ECDC and EFSA (European Centre for Disease Prevention and Control and European Food Safety Authority) , 2018b. Multi‐country outbreak of Listeria monocytogenes sequence type 8 infections linked to consumption of salmon products. EFSA Supporting Publications . 2397‐8325. 10.2903/sp.efsa.2018.EN-1496 [DOI] [Google Scholar]
- ECDC (European Centre for Disease Prevention and Control) and EFSA (European Food Safety Authority) , 2019. Multi‐country outbreak of Listeria monocytogenes sequence type 6 infections linked to ready‐to‐eat meat products. 10.2903/sp.efsa.2019.EN-1745 [DOI]
- ECDC (European Centre for Disease Prevention and Control) and EFSA (European Food Safety Authority) , 2021a. Multi‐country outbreak of Salmonella Braenderup ST22, presumed to be linked to imported melons. EFSA Supporting Publications. Available online: https://www.efsa.europa.eu/en/efsajournal/pub/en-6807 [Google Scholar]
- ECDC (European Centre for Disease Prevention and Control) and EFSA (European Food Safety Authority) , 2021b. Multi‐country outbreak of multiple Salmonella enterica serotypes linked to imported sesame‐based products. EFSA Supporting Publications, 18, 6922E. 10.2903/sp.efsa.2021.EN-6922 [DOI] [Google Scholar]
- ECDC (European Centre for Disease Prevention and Control) and EFSA (European Food Safety Authority) , 2022a. Multi‐country outbreak of monophasic Salmonella Typhimurium sequence type 34 linked to chocolate products–first update–18 May 2022a. EFSA Supporting Publications, 19, 7352E. 10.2903/sp.efsa.2022.EN-7352 [DOI] [Google Scholar]
- ECDC (European Centre for Disease Prevention and Control) and EFSA (European Food Safety Authority) , 2022b. Multi‐country outbreak of Salmonella Enteritidis sequence type (ST) 11 infections linked to eggs and egg products–8 February 2022b. EFSA Supporting Publications, 19, 7180E. 10.2903/sp.efsa.2022.EN-7180 [DOI] [Google Scholar]
- ECDC (European Centre for Disease Prevention and Control) and WHO (WHO Regional Office for Europe) , 2022. Tuberculosis surveillance and monitoring in Europe 2022 – 2020 data. Copenhagen: WHO Regional Office for Europe and Stockholm, Licence: CC BY 3.0 IGO. Available online: https://www.ecdc.europa.eu/en/publications-data/tuberculosis-surveillance-and-monitoring-europe-2022-2020-data
- ECDC (European Centre for Disease Prevention and Control) , EFSA (European Food Safety Authority) and ANSES (French Agency for Food, Environmental and Occupational Health and Safety) , 2021. European Listeria typing exercise (ELiTE). 10.2900/314391 Available online: https://www.ecdc.europa.eu/en/publications-data/joint-ecdc-efsa-and-eurl-lm-report-european-listeria-typing-exercise-elite [DOI]
- ECDC (European Centre for Disease Prevention and Control) , 2022. Congenital toxoplasmosis ‐ Annual epidemiological report for 2019. Available online: https://www.ecdc.europa.eu/en/publications-data/congenital-toxoplasmosis-annual-epidemiological-report-2019
- Echevarría JE, Banyard AC, McElhinney LM and Fooks AR, 2019. Current rabies vaccines do not confer protective immunity against divergent Lyssaviruses circulating in Europe. Viruses, 11, 892. 10.3390/v11100892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) , 2009. Technical specifications for harmonised national surveys on Yersinia enterocolitica in slaughter pigs. EFSA Journal 2009;7(11):1374, 23 pp. 10.2903/j.efsa.2009.1374. Available online: www.efsa.europa.eu/efsajournal [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , 2009a. Statistical analysis of temporal and spatial trends of zoonotic agents in animals and food Part I: Critical review of the statistical analysis carried out on the Community Summary Report 2006 data. EFSA Journal 2009;7(5):253r, 77 pp. 10.2903/j.efsa.2009.253r [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , 2009b. Analysis of the baseline survey on the prevalence of Salmonella in holdings with breeding pigs in the EU, 2008‐Part A: Salmonella prevalence estimates. EFSA Journal 2009;7(12):1377, 93 pp. 10.2903/j.efsa.2009.1377 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , 2009c. Technical specifications for the monitoring and reporting of verotoxigenic Escherichia coli (VTEC) on animals and food (VTEC surveys on animals and food). EFSA Journal 2009;7(11):1366, 43 pp. 10.2903/j.efsa.2009.1366 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , 2011. Statistical analysis of temporal and spatial trends of zoonotic agents in animals and food Part II: Applications of spatial analysis and further developments of temporal analysis. EFSA Journal 2011;9(8):2331, 72 pp. 10.2903/j.efsa.2011.2331 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , 2014. Update of the technical specifications for harmonised reporting of foodborne outbreaks through the European Union reporting system in accordance with Directive 2003/99/EC. EFSA Journal 2014;12(3):3598, 25 pp. 10.2903/j.efsa.2014.3598 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , 2022. Catalogues for 2021 zoonoses data reporting. Zenodo. 10.5281/zenodo.6395940 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) and ECDC (European Centre for Disease Prevention and Control) , 2009. The community summary report on trends and sources of zoonoses and zoonotic agents in the European Union in 2007. EFSA Journal 2009;7(1):223r, 350 pp. 10.2903/j.efsa.2009.223r [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) and ECDC (European Centre for Disease Prevention and Control) , 2010. The community summary report on trends and sources of zoonoses, zoonotic agents and foodborne outbreaks in the European Union in 2008. EFSA Journal 2010;8:1496, 410 pp. 10.2903/j.efsa.2010.1496 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) and ECDC (European Centre for Disease Prevention and Control) , 2011. The European Union summary report on trends and sources of zoonoses, zoonotic agents and foodborne outbreaks in 2009. EFSA Journal 2011;9(3):2090, 378 pp. 10.2903/j.efsa.2011.2090 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) and ECDC (European Centre for Disease Prevention and Control) , 2012. The European Union summary report on trends and sources of zoonoses, zoonotic agents and foodborne outbreaks in 2010. EFSA Journal 2012;10(3):2597, 442 pp. 10.2903/j.efsa.2012.2597 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) and ECDC (European Centre for Disease Prevention and Control) , 2013. The European Union summary report on trends and sources of zoonoses, zoonotic agents and foodborne outbreaks in 2011. EFSA Journal 2013;11(4):3129, 250 pp. 10.2903/j.efsa.2013.3129 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) and ECDC (European Centre for Disease Prevention and Control) , 2014. The European Union summary report on trends and sources of zoonoses, zoonotic agents and foodborne outbreaks in 2012. EFSA Journal 2014;12(2):3547, 312 pp. 10.2903/j.efsa.2014.3547 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) and ECDC (European Centre for Disease Prevention and Control) , 2015a. The European Union summary report on trends and sources of zoonoses, zoonotic agents and foodborne outbreaks in 2014. EFSA Journal 2015;13(12):4329, 190 pp. 10.2903/j.efsa.2015.4329 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) and ECDC (European Centre for Disease Prevention and Control) , 2015b. The European Union summary report on trends and sources of zoonoses, zoonotic agents and foodborne outbreaks in 2013. EFSA Journal 2015;13(1):3991, 165 pp. 10.2903/j.efsa.2015.3991 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) and ECDC (European Centre for Disease Prevention and Control) , 2016. The European Union summary report on trends and sources of zoonoses, zoonotic agents and foodborne outbreaks in 2015. EFSA Journal 2016;14(12):4634, 231 pp. 10.2903/j.efsa.2016.4634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) and ECDC (European Centre for Disease Prevention and Control) , 2017. The European Union summary report on trends and sources of zoonoses, zoonotic agents and foodborne outbreaks in 2016. EFSA Journal 2017;15(12):5077, 228 pp. 10.2903/j.efsa.2017.5077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) and ECDC (European Centre for Disease Prevention and Control) , 2021. The European Union one health 2020 zoonoses report. EFSA Journal 2021;19(12):6971, 324 pp. 10.2903/j.efsa.2021.6971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) and Zancanaro G, 2021. Annual assessment of Echinococcus multilocularis surveillance reports submitted in 2020 in the context of Commission Delegated Regulation (EU) 2018/772. EFSA Journal 2021;19(1):6382, 63 pp. 10.2903/j.efsa.2021.6382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) , Amore G, Beloeil P‐A, Bocca V, Boelaert F, Gibin D, Papanikolaou A, Rizz V and Stoicescu A‐V, 2021a. Zoonoses, antimicrobial resistance and foodborne outbreaks guidance for reporting 2020 data. EFSA Supporting Publication; 2021:EN‐6438, 112 pp. 10.2903/sp.efsa.2021. Available online: www.efsa.europa.eu/efsajournal [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , Amore G, Boelaert F, Papanikolaou A, Rizzi V and Stoicescu A‐V, 2021b. Manual for reporting on zoonoses and zoonotic agents,within the framework of Directive 2003/99/EC, and on some other pathogenic microbiological agents for information derived from the year 2020. EFSA Supporting Publication 2021:EN‐6440, 67 pp. 10.2903/sp.efsa.2021.EN-6440 Available online: www.efsa.europa.eu/efsajournal [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , Amore G, Boelaert F, Gibin D, Papanikolaou A, Rizzi V and Stoicescu A‐V, 2022. Zoonoses and foodborne outbreaks guidance for reporting 2021 data. EFSA Supporting Publications. EN‐7131. 10.2903/sp.efsa.2022.EN-7131 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , Alvarez J, Nielsen SS, Robardet E, Stegeman A, Van Gucht S, Vuta V, Antoniou SE, Aznar I and Papanikolaou A, 2022a. Risks related to a possible reduction of the waiting period for dogs after rabies antibody titration to 30 days compared with 90 days of the current EU legislative regime. EFSA Journal 2022;20(6):7350, 78 pp. 10.2903/j.efsa.2022.7350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) , Amore G, Boelaert F, Papanikolaou A, Rizzi V and Stoicescu A‐V, 2022b. Manual for reporting on zoonoses and zoonotic agents, within the framework of Directive 2003/99/EC, and on some other pathogenic microbiological agents for information derived from the year 2021. EFSA Supporting Publication 2022:EN‐7130, 78 pp. 10.2903/sp.efsa.2022.EN-7130 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , Boelaert F, Gibin D, Papanikolaou A, Rizzi V and Stoicescu A‐V, 2022c. Prevalence sample‐based guidance for reporting 2021 data. EFSA Supporting Publications; 2022:EN‐7129. 10.2903/sp.efsa.2022.EN-7129 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , Costa G, Di Piazza G, Koevoets P, Iacono G, Liebana E, Pasinato L, Rizzi V and Rossi M, 2022d. Guidelines for reporting Whole Genome Sequencing‐based typing data through the EFSA One Health WGS System. EFSA Supporting Publications; 2022:EN‐7413. 10.2903/sp.efsa.2022.EN-7413 [DOI] [Google Scholar]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare) , 2014. Statement on a conceptual framework for bovine tuberculosis. EFSA Journal 2014;12(5):3711, 59 pp. 10.2903/j.efsa.2014.3711 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards,) , EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain) and EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare) , 2012. Scientific Opinion on the public health hazards to be covered by inspection of meat (poultry). EFSA Journal 2012;10(6):2741, 179 pp. 10.2903/j.efsa.2012.2741 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards) , 2007. Scientific Opinion of the Panel on BIOHAZ on a request from EFSA on monitoring and identification of human enteropathogenic Yersinia spp. EFSA Journal 2007;595:1–30, 30 pp. 10.2903/j.efsa.2007.595 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards) , 2011. Scientific opinion on risk based control of biogenic amine formation in fermented foods. EFSA Journal 2011;9(10):2393, 93 pp. 10.2903/j.efsa.2011.2393 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards) , 2013a. Scientific Opinion on the public health hazards to be covered by inspection of meat from farmed game. EFSA Journal 2013;11(6):3264, 181 pp. 10.2903/j.efsa.2013.3264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards) , 2013b. Scientific Opinion on the public health hazards to be covered by inspection of meat (solipeds). EFSA Journal 2013;11(6):3263, 161 pp. 10.2903/j.efsa.2013.3263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards) , 2013c. Scientific Opinion on VTEC‐seropathotype and scientific criteria regarding pathogenicity assessment. EFSA Journal 2013;11(4):3138, 106 pp. 10.2903/j.efsa.2013.3138 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards) , EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain) and EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare) , 2011. Scientific opinion on the public health hazards to be covered by inspection of meat (swine). EFSA Journal 2011;9(10):2351, 198 pp. 10.2903/j.efsa.2011.2351 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards) , Ricci A, Allende A, Bolton D, Chemaly M, Davies R, Fernández Escámez PS, Girones R, Herman L, Koutsoumanis K, Nørrung B, Robertson L, Ru G, Sanaa M, Simmons M, Skandamis P, Snary E, Speybroeck N, Ter Kuile B, Threlfall J, Wahlström H, Takkinen J, Wagner M, Arcella D, Da Silva Felicio MT, Georgiadis M, Messens W and Lindqvist R, 2018. Scientific Opinion on the Listeria monocytogenes contamination of ready‐to‐eat foods and the risk for human health in the EU. EFSA Journal 2018;16(1):5134, 173 pp. 10.2903/j.efsa.2018.5134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards) , Koutsoumanis K, Allende A, Alvarez‐Ordóñez A, Bolton D, Bover‐Cid S, Chemaly M, Davies R, De Cesare A, Hilbert F, Lindqvist R, Nauta M, Peixe L, Ru G, Simmons M, Skandamis P, Suffredini E, Jenkins C, Malorny B, Ribeiro Duarte AS, Torpdahl M, da Silva Felício MT, Guerra B, Rossi M and Herman L, 2019. Whole genome sequencing and metagenomics for outbreak investigation, source attribution and risk assessment of foodborne microorganisms. EFSA Journal 2019;17(12):5898, 78 pp. 10.2903/j.efsa.2019.5898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards) , Koutsoumanis K, Allende A, Alvarez‐Ordóñez A, Bolton D, Bover‐Cid S, Davies R, De Cesare A, Herman L, Hilbert F, Lindqvist R, Nauta M, Peixe L, Ru G, Simmons M, Skandamis P, Suffredini E, Alter T, Crotta M, Ellis‐Iversen J, Hempen M, Messens W and Chemaly M, 2020a. Update and review of control options for Campylobacter in broilers at primary production. EFSA Journal 2020;18(4):6090, 89 pp. 10.2903/j.efsa.2020.6090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards) , Koutsoumanis K, Allende A, Alvarez‐Ordóñez A, Bover‐Cid S, Chemaly M, Davies R, De Cesare A, Herman L, Hilbert F, Lindqvist R, Nauta M, Peixe L, Ru G, Simmons M, Skandamis P, Suffredini E, Jenkins C, Monteiro Pires S, Morabito S, Niskanen T, Scheutz F, da Silva Felício MT, Messens W and Bolton D, 2020b. Pathogenicity assessment of Shiga toxin‐producing Escherichia coli (STEC) and the public health risk posed by contamination of food with STEC. EFSA Journal 2020;18(1):5967, 105 pp. 10.2903/j.efsa.2020.5967 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards) , Koutsoumanis K, Alvarez‐Ordóñez A, Bolton D, Bover‐Cid S, Chemaly M, Davies R, De Cesare A, Herman L, Hilbert F, Lindqvist R, Nauta M, Peixe L, Ru G, Simmons M, Skandamis P, Suffredini E, Jordan K, Sampers I, Wagner M, Da Silva Felicio MT, Georgiadis M, Messens W, Mosbach‐Schulz O and Allende A, 2020c. The public health risk posed by Listeria monocytogenes in frozen fruit and vegetables including herbs, blanched during processing. EFSA Journal 2020;18(4):6092, 102 pp. 10.2903/j.efsa.2020.6092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espenhain L, Riess M, Müller L, Colombe S, Ethelberg S, Litrup E, Jernberg C, Kühlmann‐Berenzon S, Lindblad M, Hove NK, Torpdahl M and Mörk MJ, 2019. Cross‐border outbreak of Yersinia enterocolitica O3 associated with imported fresh spinach, Sweden and Denmark, March 2019. Euro Surveill, 24, pii=1900368. 10.2807/1560-7917.Es.2019.24.24.1900368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Parliament , 2020. The EU pig meat sector. Available online: https://www.europarl.europa.eu/RegData/etudes/BRIE/2020/652044/EPRS_BRI(2020)652044_EN.pdf
- Eurostat (European Commission) , 2020. Ageing Europe — looking at the lives of older people in the EU. Statistical Books. 10.2785/628105 [DOI]
- Eurostat (European Commission) 2022. Population structure and ageing. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Population_structure_and_ageing [Accessed: 03/11/2022].
- Faber M, Heuner K, Jacob D and Grunow R, 2018. Tularemia in Germany‐A Re‐emerging Zoonosis. Front Cell Infect Microbiol, 8, 40. 10.3389/fcimb.2018.00040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO (Food and Agriculture Organization of the United Nations) and WHO (World Health Organization) , 2018. Shiga Toxin‐producing Escherichia Coli (STEC) and Food: Attribution, Characterization and Monitoring. World Health Organization; Available online: https://www.fao.org/documents/card/en/c/CA0032EN [Google Scholar]
- FAO (Food and Agriculture Organization of the United Nations) and WHO (World Health Organization) , 2022. Listeria monocytogenes in ready‐to‐eat (RTE) foods: attribution, characterization and monitoring ‐ Meeting report. Microbiological Risk Assessment Series No. 38. Rome. 10.4060/cc2400en [DOI]
- Farooq Z, Rocklöv J, Wallin J, Abiri N, Sewe MO, Sjödin H and Semenza JC, 2022. Artificial intelligence to predict West Nile virus outbreaks with eco‐climatic drivers. Lancet Reg Health Eur, 17, 100370. 10.1016/j.lanepe.2022.100370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari RG, Rosario DKA, Cunha‐Neto A, Mano SB, Figueiredo EES and Conte‐Junior CA, 2019. Worldwide epidemiology of Salmonella serovars in animal‐based foods: a meta‐analysis. Appl Environ Microbiol, 85. 10.1128/aem.00591-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figoni J, Gagnière B, Pouey J, Mailles A, Decors A, Madani N, Revest M, Boisset S and Caspar Y, 2022. Augmentation de l'incidence de la tularémie en France: données de la déclaration obligatoire 2012–2021. Médecine et Maladies Infectieuses Formation, 1, S18–S19. 10.1016/j.mmifmc.2022.03.063 [DOI] [Google Scholar]
- Fooks AR, Johnson N, Brookes SM, Parsons G and McElhinney LM, 2003. Risk factors associated with travel to rabies endemic countries. J Appl Microbiol, 94(Suppl), 31s–36s. 10.1046/j.1365-2672.94.s1.4.x [DOI] [PubMed] [Google Scholar]
- Fornasiero D, Mazzucato M, Barbujani M, Montarsi F, Capelli G and Mulatti P, 2020. Inter‐annual variability of the effects of intrinsic and extrinsic drivers affecting West Nile virus vector Culex pipiens population dynamics in northeastern Italy. Parasit Vectors, 13, 271. 10.1186/s13071-020-04143-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsythe SJ, 2015. Chapter 13 ‐ New Insights into the Emergent Bacterial Pathogen Cronobacter. In: Ricke SC, Donaldson JR and Phillips CA (eds). Food Safety. Academic Press, San Diego. pp. 265–308 Available online: https://www.sciencedirect.com/science/article/pii/B9780128002452000137 [Google Scholar]
- García San Miguel Rodríguez‐Alarcón L, Fernández‐Martínez B, Sierra Moros MJ, Vázquez A, Julián Pachés P, García Villacieros E, Gómez Martín MB, Figuerola Borras J, Lorusso N, Ramos Aceitero JM, Moro E, de Celis A, Oyonarte S, Mahillo B, Romero González LJ, Sánchez‐Seco MP, Suárez Rodríguez B, Ameyugo Catalán U, Ruiz Contreras S, Pérez‐Olmeda M and Simón Soria F, 2021. Unprecedented increase of West Nile virus neuroinvasive disease, Spain, summer 2020. Euro Surveill, 26. 10.2807/1560-7917.Es.2021.26.19.2002010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genova‐Kalou P, Vladimirova N, Stoitsova S, Krumova S, Kurchatova A and Kantardjiev T, 2019. Q fever in Bulgaria: laboratory and epidemiological findings on human cases and outbreaks, 2011 to 2017. Euro Surveill, 24. 10.2807/1560-7917.Es.2019.24.37.1900119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill A, Carrillo C, Hadley M, Kenwell R and Chui L, 2019. Bacteriological analysis of wheat flour associated with an outbreak of Shiga toxin‐producing Escherichia coli O121. Food Microbiology, 82, 474–481. 10.1016/j.fm.2019.03.023 [DOI] [PubMed] [Google Scholar]
- Gormley E and Corner LA, 2013. Control strategies for wildlife tuberculosis in Ireland. Transbound Emerg Dis, 60(Suppl 1), 128–135. 10.1111/tbed.12095 [DOI] [PubMed] [Google Scholar]
- Gruber JF, Morris S, Warren KA, Kline KE, Schroeder B, Dettinger L, Husband B, Pollard K, Davis C, Miller J, Weltman A, Mattioli M, Ray L, Tarr C and Longenberger AH, 2021. Yersinia enterocolitica outbreak associated with pasteurized milk. Foodborne Pathog Dis, 18, 448–454. 10.1089/fpd.2020.2924 [DOI] [PubMed] [Google Scholar]
- Guegan H, Stajner T, Bobic B, Press C, Olariu RT, Olson K, Srbljanovic J, Montoya JG, Djurković‐Djaković O and Robert‐Gangneux F, 2021. Maternal anti‐toxoplasma treatment during pregnancy is associated with reduced sensitivity of diagnostic tests for congenital infection in the neonate. J Clin Microbiol, 59. 10.1128/jcm.01368-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallanvuo S, Herranen M, Jaakkonen A, Nummela M, Ranta J, Botteldoorn N, De Zutter L, Fredriksson‐Ahomaa M, Hertwig S, Johannessen GS, Ludewig M, Messelhäußer U, Sigvart‐Mattila P, Thisted‐Lambertz S, Thure T and Vatunen E, 2019. Validation of EN ISO method 10273 ‐ detection of pathogenic Yersinia enterocolitica in foods. Int J Food Microbiol, 288, 66–74. 10.1016/j.ijfoodmicro.2018.01.009 [DOI] [PubMed] [Google Scholar]
- Haussig JM, Young JJ, Gossner CM, Mezei E, Bella A, Sirbu A, Pervanidou D, Drakulovic MB and Sudre B, 2018. Early start of the West Nile fever transmission season 2018 in Europe. Euro Surveill, 23, 1800428. 10.2807/1560-7917.Es.2018.23.32.1800428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennebique A, Boisset S and Maurin M, 2019. Tularemia as a waterborne disease: a review. Emerg Microbes Infect, 8, 1027–1042. 10.1080/22221751.2019.1638734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández‐Triana LM, Jeffries CL, Mansfield KL, Carnell G, Fooks AR and Johnson N, 2014. Emergence of west Nile virus lineage 2 in Europe: a review on the introduction and spread of a mosquito‐borne disease. Front Public Health, 2, 271. 10.3389/fpubh.2014.00271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hestvik G, Warns‐Petit E, Smith LA, Fox NJ, Uhlhorn H, Artois M, Hannant D, Hutchings MR, Mattsson R, Yon L and Gavier‐Widen D, 2015. The status of tularemia in Europe in a one‐health context: a review. Epidemiol Infect, 143, 2137–2160. 10.1017/s0950268814002398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubálek Z and Halouzka J, 1999. West Nile fever‐‐a reemerging mosquito‐borne viral disease in Europe. Emerg Infect Dis, 5, 643–650. 10.3201/eid0505.990505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISO (International Organization for Standardization) , 2001. ISO 16654:2001. Microbiology of food and animal feeding stuffs – Horizontal method for the detection of Escherichia coli O157. [DOI] [PubMed]
- ISO (International Organization for Standardization) , 2012. ISO TS 13136:2012. . Microbiology of food and animal feed – Real‐time polymerase chain reaction (PCR)‐based method for the detection of foodborne pathogens – Horizontal method for the detection of Shiga toxin‐producing Escherichia coli (STEC) and the determination of O157, O111, O26, O103 and O145 serogroups.
- ISO (International Organization for Standardization) , 2015. ISO/TS 18867. Microbiology of the Food Chain‐Polymerase Chain Reaction (PCR) for the Detection of Food‐Borne Pathogens‐ Detection of Pathogenic Yersinia enterocolitica and Yersinia pseudotubculosis .
- Kapusta‐Duch J, Leszczyńska T, Florkiewicz A and Filipiak‐Florkiewicz A, 2011. Comparison of calcium and magnesium contents in cruciferous vegetables grown in areas around steelworks, on organic farms, and those available in retail. Ecol Food Nutr, 50, 155–167. 10.1080/03670244.2011.552373 [DOI] [PubMed] [Google Scholar]
- Kärssin A, Remes N, Korge K, Viigipuu M, Stensvold CR, Gómez‐Morales MA, Ludovisi A, Jokelainen P and Lassen B, 2021. Herbivores as accidental hosts for Trichinella: search for evidence of trichinella infection and exposure in free‐ranging moose (alces alces) in a highly endemic setting. J Wildl Dis, 57, 116–124. 10.7589/jwd-d-19-00011 [DOI] [PubMed] [Google Scholar]
- Kastritis E, Tsitsimpis K, Anninos E, Stamatelopoulos K, Kanakakis I, Lampropoulos C, Chatzidou S, Michopoulos S, Papamichail C, Kostis E, Manios E, Kontogiannis S, Paraskevaidis I, Terpos E, Mitrakou A and Dimopoulos MA, 2020. Significant reduction in the visits to the emergency room department during the COVID‐19 pandemic in a tertiary hospital in Greece: Indirect victims of the pandemic? Medicine (Baltimore), 99, e23845. 10.1097/md.0000000000023845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kefaloudi C, Mellou K, Dougas G, Vorou R, Mitrou K and Kontopidou F, 2022. Human Brucellosis in Greece, 2005–2020: a persistent public health problem. Vector Borne Zoonotic Dis, 22, 163–169. 10.1089/vbz.2021.0050 [DOI] [PubMed] [Google Scholar]
- Kelly PJ, Matthewman LA, Mason PR and Raoult D, 1993. Q fever in Zimbabwe. A review of the disease and the results of a serosurvey of humans, cattle, goats and dogs. S Afr Med J, 83, 21–25. [PubMed] [Google Scholar]
- Kenney A, Cusick A, Payne J, Gaughenbaugh A, Renshaw A, Wright J, Seeber R, Barnes R, Florjanczyk A and Horzempa J, 2017. The potential for flower nectar to allow mosquito to mosquito transmission of Francisella tularensis. PLoS One, 12, e0175157. 10.1371/journal.pone.0175157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern P, Bardonnet K, Renner E, Auer H, Pawlowski Z, Ammann RW, Vuitton DA and Kern P, 2003. European echinococcosis registry: human alveolar echinococcosis, Europe, 1982–2000. Emerg Infect Dis, 9, 343–349. 10.3201/eid0903.020341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klevar S, Høgåsen HR, Davidson RK, Hamnes IS, Treiberg Berndtsson L and Lund A, 2015. Cross‐border transport of rescue dogs may spread rabies in Europe. Vet Rec, 176, 672. 10.1136/vr.102909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn KG, Nygård KM, Guzman‐Herrador B, Sunde LS, Rimhanen‐Finne R, Trönnberg L, Jepsen MR, Ruuhela R, Wong WK and Ethelberg S, 2020. Campylobacter infections expected to increase due to climate change in Northern Europe. Scientific Reports, 10, 13874. 10.1038/s41598-020-70593-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwit NA, Middaugh NA, VinHatton ES, Melman SD, Onischuk L, Aragon AS, Nelson CA, Mead PS and Ettestad PJ, 2020. Francisella tularensis infection in dogs: 88 cases (2014‐2016). J Am Vet Med Assoc, 256, 220–225. 10.2460/javma.256.2.220 [DOI] [PubMed] [Google Scholar]
- Lake IR, Colón‐González FJ, Takkinen J, Rossi M, Sudre B, Dias JG, Tavoschi L, Joshi A, Semenza JC and Nichols G, 2019. Exploring Campylobacter seasonality across Europe using the European Surveillance System (TESSy), 2008 to 2016. Euro Surveill, 24. 10.2807/1560-7917.Es.2019.24.13.180028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J, Broughan J, Crowley D, O'Kelly B, Fawsitt R, Burke MC, McCombe G, Lambert JS and Cullen W, 2021. COVID‐19's impact on primary care and related mitigation strategies: A scoping review. Eur J Gen Pract, 27, 166–175. 10.1080/13814788.2021.1946681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyoo KS, Kim D, Jang HG, Lee SJ, Park MY and Hahn TW, 2017. Prevalence of antibodies against Coxiella burnetii in Korean native cattle, dairy cattle, and dogs in South Korea. Vector Borne Zoonotic Dis, 17, 213–216. 10.1089/vbz.2016.1977 [DOI] [PubMed] [Google Scholar]
- Maier T, Schwarting A, Mauer D, Ross RS, Martens A, Kliem V, Wahl J, Panning M, Baumgarte S, Müller T, Pfefferle S, Ebel H, Schmidt J, Tenner‐Racz K, Racz P, Schmid M, Strüber M, Wolters B, Gotthardt D, Bitz F, Frisch L, Pfeiffer N, Fickenscher H, Sauer P, Rupprecht CE, Roggendorf M, Haverich A, Galle P, Hoyer J and Drosten C, 2010. Management and outcomes after multiple corneal and solid organ transplantations from a donor infected with rabies virus. Clin Infect Dis, 50, 1112–1119. 10.1086/651267 [DOI] [PubMed] [Google Scholar]
- Marini G, Manica M, Delucchi L, Pugliese A and Rosà R, 2021. Spring temperature shapes West Nile virus transmission in Europe. Acta Trop, 215, 105796. 10.1016/j.actatropica.2020.105796 [DOI] [PubMed] [Google Scholar]
- Marquer P, Rabade T and Forti R, 2014. Pig farming in the European Union: considerable variations from one Member State to another. Statistics in focus 15/2014.
- Maurin M and Gyuranecz M, 2016. Tularaemia: clinical aspects in Europe. Lancet Infect Dis, 16, 113–124. 10.1016/s1473-3099(15)00355-2 [DOI] [PubMed] [Google Scholar]
- Migliori GB, Thong PM, Alffenaar JW, Denholm J, Tadolini M, Alyaquobi F, Blanc FX, Buonsenso D, Cho JG, Codecasa LR, Danila E, Duarte R, García‐García JM, Gualano G, Rendon A, Silva DR, Souleymane MB, Tham SM, Thomas TA, Tiberi S, Udwadia ZF, Goletti D, Centis R, D'Ambrosio L, Sotgiu G and Ong CWM, 2021. Gauging the impact of the COVID‐19 pandemic on tuberculosis services: a global study. Eur Respir J, 58, 2101786. 10.1183/13993003.01786-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mínguez‐González O, Gutiérrez‐Martín CB, Martínez‐Nistal MDC, Esquivel‐García MDR, Gómez‐Campillo JI, Collazos‐Martínez J, Fernández‐Calle LM, Ruiz‐Sopeña C, Tamames‐Gómez S, Martínez‐Martínez S, Caminero‐Saldaña C, Hernández M, Rodríguez‐Lázaro D and Rodríguez‐Ferri EF, 2021. Tularemia outbreaks in Spain from 2007 to 2020 in humans and domestic and wild animals. Pathogens, 10, 892. 10.3390/pathogens10070892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mughini‐Gras L, van Hoek A, Cuperus T, Dam‐Deisz C, van Overbeek W, van den Beld M, Wit B, Rapallini M, Wullings B, Franz E, van der Giessen J, Dierikx C and Opsteegh M, 2021. Prevalence, risk factors and genetic traits of Salmonella Infantis in Dutch broiler flocks. Vet Microbiol, 258, 109120. 10.1016/j.vetmic.2021.109120 [DOI] [PubMed] [Google Scholar]
- NACMCF (National Advisory Committee on Microbiological Criteria for Foods) , 2019. Response to Questions Posed by the Food and Drug Administration Regarding Virulence Factors and Attributes that Define Foodborne Shiga Toxin‐Producing Escherichia coli (STEC) as Severe Human Pathogens. J Food Prot, 82, 724–767. 10.4315/0362-028x.Jfp-18-479 [DOI] [PubMed] [Google Scholar]
- Newell DG, Elvers KT, Dopfer D, Hansson I, Jones P, James S, Gittins J, Stern NJ, Davies R, Connerton I, Pearson D, Salvat G and Allen VM, 2011. Biosecurity‐based interventions and strategies to reduce Campylobacter spp. on poultry farms. Appl Environ Microbiol, 77, 8605–8614. 10.1128/aem.01090-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton K, Withenshaw SM, Cawthraw SA and Davies R, 2021. In‐depth farm investigations and an exploratory risk factor analysis for the presence of Salmonella on broiler farms in Great Britain. Prev Vet Med, 197, 105498. 10.1016/j.prevetmed.2021.105498 [DOI] [PubMed] [Google Scholar]
- Nikolayevskyy V, Holicka Y, van Soolingen D, van der Werf MJ, Ködmön C, Surkova E, Hillemann D, Groenheit R and Cirillo D, 2021. Impact of the COVID‐19 pandemic on tuberculosis laboratory services in Europe. Eur Respir J, 57, 2003890. 10.1183/13993003.03890-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- NMKL (Nordisk Metodikkomité for Næringsmidler ‐ Nordic Committee on Food Analysis) , 2005. Escherichia coli O157. Detection in food and feeding stuffs. NMKL No. 164, 2. Available online: http://www.nmkl.org/index.php?option=com_zoo&task=item&item_id=337&Itemid=319&lang=en
- Office fédérel de la santé publique , 2022. Bulletin semaine 4/2022; magazine d'information pour professionnels de la santé et pour les médias. Available online: https://www.bag.admin.ch/dam/bag/fr/dokumente/cc/Kampagnen/Bulletin/2022/bu-4-22.pdf.download.pdf/BU_4_22_FR.pdf
- Oksanen A, Siles‐Lucas M, Karamon J, Possenti A, Conraths FJ, Romig T, Wysocki P, Mannocci A, Mipatrini D, La Torre G, Boufana B and Casulli A, 2016. The geographical distribution and prevalence of Echinococcus multilocularis in animals in the European Union and adjacent countries: a systematic review and meta‐analysis. Parasit Vectors, 9, 519. 10.1186/s13071-016-1746-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olaimat AN and Holley RA, 2012. Factors influencing the microbial safety of fresh produce: a review. Food Microbiol, 32, 1–19. 10.1016/j.fm.2012.04.016 [DOI] [PubMed] [Google Scholar]
- Olea‐Popelka F, Muwonge A, Perera A, Dean AS, Mumford E, Erlacher‐Vindel E, Forcella S, Silk BJ, Ditiu L and El Idrissi A, 2017. Zoonotic tuberculosis in human beings caused by Mycobacterium bovis ‐ a call for action. The Lancet Infectious Diseases, 17, e21–e25. 10.1016/S1473-3099(16)30139-6 [DOI] [PubMed] [Google Scholar]
- Opsteegh M, Kortbeek TM, Havelaar AH and van der Giessen JW, 2015. Intervention strategies to reduce human Toxoplasma gondii disease burden. Clin Infect Dis, 60, 101–107. 10.1093/cid/ciu721 [DOI] [PubMed] [Google Scholar]
- Opsteegh M, Spano F, Aubert D, Balea A, Burrells A, Cherchi S, Cornelissen JBWJ, Dam‐Deisz C, Guitian J, Györke A, Innes EA, Katzer F, Limon G, Possenti A, Pozio E, Schares G, Villena I, Wisselink HJ and van der Giessen JWB, 2019. The relationship between the presence of antibodies and direct detection of Toxoplasma gondii in slaughtered calves and cattle in four European countries. International Journal for Parasitology, 49, 515–522. 10.1016/j.ijpara.2019.01.005 [DOI] [PubMed] [Google Scholar]
- Orr B, Malik R, Westman ME and Norris JM, 2022. Seroprevalence of Coxiella burnetii in pig‐hunting dogs from north Queensland, Australia. Aust Vet J, 100, 230–235. 10.1111/avj.13151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottaiano M, Brunetti R, Calistri P, Di Lorenzo A, Di Sabatino D, Cito F, Chiodini P, Baldi L and De Carlo E, 2022. Risk factors for the persistence and spread of Brucellosis in buffaloes in the province of Caserta (2015–2020). Available online: https://brucellosis2022.izs.it/wp-content/uploads/2022/09/Conference_Proceedings_Brucellosis2022.pdf
- Pacenti M, Sinigaglia A, Franchin E, Pagni S, Lavezzo E, Montarsi F, Capelli G and Barzon L, 2020. Human West Nile Virus Lineage 2 infection: epidemiological, clinical, and virological findings. Viruses, 12. 10.3390/v12040458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papa A, Xanthopoulou K, Gewehr S and Mourelatos S, 2011. Detection of West Nile virus lineage 2 in mosquitoes during a human outbreak in Greece. Clin Microbiol Infect, 17, 1176–1180. 10.1111/j.1469-0691.2010.03438.x [DOI] [PubMed] [Google Scholar]
- Persad AK and LeJeune JT, 2014. Animal reservoirs of Shiga toxin‐producing Escherichia coli. Microbiol Spectr, 2, Ehec‐0027‐2014. 10.1128/microbiolspec.EHEC-0027-2014 [DOI] [PubMed] [Google Scholar]
- Pervanidou D, Vakali A, Georgakopoulou T, Panagiotopoulos T, Patsoula E, Koliopoulos G, Politis C, Stamoulis K, Gavana E, Pappa S, Mavrouli M, Emmanouil M, Sourvinos G, Mentis A, Tsakris A, Hadjichristodoulou C, Tsiodras S and Papa A, 2020. West Nile virus in humans, Greece, 2018: the largest seasonal number of cases, 9 years after its emergence in the country. Euro Surveill, 25. 10.2807/1560-7917.Es.2020.25.32.1900543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Possenti A, Manzano‐Román R, Sánchez‐Ovejero C, Boufana B, La Torre G, Siles‐Lucas M and Casulli A, 2016. Potential risk factors associated with human cystic echinococcosis: systematic review and meta‐analysis. PLoS Neglected Tropical Diseases, 10, e0005114. 10.1371/journal.pntd.0005114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozio E, 2014. Searching for Trichinella: not all pigs are created equal. Trends Parasitol, 30, 4–11. 10.1016/j.pt.2013.11.001 [DOI] [PubMed] [Google Scholar]
- Pozio E, 2016. Trichinella pseudospiralis an elusive nematode. Veterinary Parasitology, 231, 97–101. 10.1016/j.vetpar.2016.03.021 [DOI] [PubMed] [Google Scholar]
- Pozio E, Rinaldi L, Marucci G, Musella V, Galati F, Cringoli G, Boireau P and La Rosa G, 2009. Hosts and habitats of Trichinella spiralis and Trichinella britovi in Europe. Int J Parasitol, 39, 71–79. 10.1016/j.ijpara.2008.06.006 [DOI] [PubMed] [Google Scholar]
- ProMED‐mail , 2021a. Q fever‐Spain (02): (PV) bouldering, Baltzola caves, RFI. 04 May: 20210504.8343274. Available online: https://www.promedmail.org
- ProMED‐mail , 2021b. Q fever‐Spain: (PV) recurrent, waste treatment facility, RFI. 27 March: 20210327.8273195. Available online: https://www.promedmail.org
- ProMED‐mail , 2021c. Q fever‐Germany: (Berlin) sheep, human, laboratory staff, RFI. 26 September: 20210926.8698730 Available online: https://www.promedmail.org
- Quereda JJ, Morón‐García A, Palacios‐Gorba C, Dessaux C, García‐Del Portillo F, Pucciarelli MG and Ortega AD, 2021. Pathogenicity and virulence of Listeria monocytogenes: A trip from environmental to medical microbiology. Virulence, 12, 2509–2545. 10.1080/21505594.2021.1975526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radoshevich L and Cossart P, 2018. Listeria monocytogenes: towards a complete picture of its physiology and pathogenesis. Nat Rev Microbiol, 16, 32–46. 10.1038/nrmicro.2017.126 [DOI] [PubMed] [Google Scholar]
- Ray LC, Collins JP, Griffin PM, Shah HJ, Boyle MM, Cieslak PR, Dunn J, Lathrop S, McGuire S, Rissman T, Scallan Walter EJ, Smith K, Tobin‐D'Angelo M, Wymore K, Kufel JZ, Wolpert BJ, Tauxe R and Payne DC, 2021. Decreased incidence of infections caused by pathogens transmitted commonly through food during the COVID‐19 pandemic ‐ foodborne diseases active Surveillance Network, 10 U.S. Sites, 2017‐2020. MMWR Morb Mortal Wkly Rep, 70, 1332–1336. 10.15585/mmwr.mm7038a4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regnault B, Evrard B, Plu I, Dacheux L, Troadec E, Cozette P, Chrétien D, Duchesne M, Vallat JM, Jamet A, Leruez M, Pérot P, Bourhy H, Eloit M and Seilhean D, 2022. First case of lethal encephalitis in Western Europe due to European Bat Lyssavirus Type 1. Clin Infect Dis, 74, 461–466. 10.1093/cid/ciab443 [DOI] [PubMed] [Google Scholar]
- Reisen WK, Fang Y and Martinez VM, 2006. Effects of temperature on the transmission of west Nile virus by Culex tarsalis (Diptera: Culicidae). J Med Entomol, 43, 309–317. 10.1603/0022-2585(2006)043[0309:Eotott]2.0.Co;2 [DOI] [PubMed] [Google Scholar]
- Riccardo F, Monaco F, Bella A, Savini G, Russo F, Cagarelli R, Dottori M, Rizzo C, Venturi G, Di Luca M, Pupella S, Lombardini L, Pezzotti P, Parodi P, Maraglino F, Costa AN, Liumbruno GM and Rezza G, 2018. An early start of West Nile virus seasonal transmission: the added value of One Heath surveillance in detecting early circulation and triggering timely response in Italy, June to July 2018. Euro Surveill, 23. 10.2807/1560-7917.Es.2018.23.32.1800427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccardo F, Bolici F, Fafangel M, Jovanovic V, Socan M, Klepac P, Plavsa D, Vasic M, Bella A, Diana G, Rosi L, Pezzotti P, Andrianou XD, Di Luca M, Venturi G, Maraglino F, Pervanidou D, Cenciarelli O, Baka A, Young J, Bakonyi T, Rezza G and Suk JE, 2020. West Nile virus in Europe: after action reviews of preparedness and response to the 2018 transmission season in Italy, Slovenia. Serbia and Greece. Global Health, 16, 47. 10.1186/s12992-020-00568-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter J, Esmann L, Lindner AK, Trebesch I, Equihua‐Martinez G, Niebank M, Georgi S, Schürmann D, Pfäfflin F, Pasić M and Gertler M, 2019. Cystic echinococcosis in unaccompanied minor refugees from Afghanistan and the Middle East to Germany, July 2016 through June 2017. Eur J Epidemiol, 34, 611–612. 10.1007/s10654-019-00492-8 [DOI] [PubMed] [Google Scholar]
- Rivas L, Strydom H, Paine S, Wang J and Wright J, 2021. Yersiniosis in New Zealand. Pathogens, 10. 10.3390/pathogens10020191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson E, de Valk H, Villena I, Le Strat Y and Tourdjman M, 2021. National perinatal survey demonstrates a decreasing seroprevalence of Toxoplasma gondii infection among pregnant women in France, 1995 to 2016: impact for screening policy. Euro Surveill, 26. 10.2807/1560-7917.Es.2021.26.5.1900710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosner BM, Gassowski M, Albrecht S and Stark K, 2021. Investigating the Campylobacter enteritis winter peak in Germany, 2018/2019. Scientific Reports, 11, 22902. 10.1038/s41598-021-02423-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi P, Tamarozzi F, Galati F, Akhan O, Cretu CM, Vutova K, Siles‐Lucas M, Brunetti E and Casulli A, 2020. The European Register of Cystic Echinococcosis, ERCE: state‐of‐the‐art five years after its launch. Parasit Vectors, 13, 236. 10.1186/s13071-020-04101-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouzic N, Desmier L, Cariou ME, Gay E, Foster JT, Williamson CHD, Schmitt F, Le Henaff M, Le Coz A, Lorléac'h A, Lavigne JP, O'Callaghan D and Keriel A, 2021. First case of Brucellosis caused by an amphibian‐type Brucella . Clin Infect Dis, 72, e404–e407. 10.1093/cid/ciaa1082 [DOI] [PubMed] [Google Scholar]
- Sack DA, Sack RB, Nair GB and Siddique AK, 2004. Cholera. Lancet, 363, 223–233. 10.1016/s0140-6736(03)15328-7 [DOI] [PubMed] [Google Scholar]
- Šamec D, Urlić B and Salopek‐Sondi B, 2019. Kale (Brassica oleracea var. acephala) as a superfood: review of the scientific evidence behind the statement. Critical Reviews in Food Science and Nutrition, 59, 2411–2422. 10.1080/10408398.2018.1454400 [DOI] [PubMed] [Google Scholar]
- Sapountzis P, Segura A, Desvaux M and Forano E, 2020. An overview of the elusive passenger in the gastrointestinal tract of cattle: the Shiga toxin producing Escherichia coli. Microorganisms, 8, 877. 10.3390/microorganisms8060877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiwald S, Simeon A, Hofer E, Weiss G and Bellmann‐Weiler R, 2020. Tularemia goes west: epidemiology of an emerging infection in Austria. Microorganisms, 8, 1597. 10.3390/microorganisms8101597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidi‐Boumedine K, Rousset E, Henning K, Ziller M, Niemczuck K, Roest H and Thiéry R, 2010. Development of harmonised schemes for the monitoring and reporting of Q‐fever in animals in the European Union. EFSA Supporting Publications, 7, 2397–8325, 48E pp. Available online: https://efsa.onlinelibrary.wiley.com/doi/epdf/10.2903/sp.efsa.2010.EN-48 [Google Scholar]
- Sikkema RS, Schrama M, van den Berg T, Morren J, Munger E, Krol L, van der Beek JG, Blom R, Chestakova I, van der Linden A, Boter M, van Mastrigt T, Molenkamp R, Koenraadt CJ, van den Brand JM, Oude Munnink BB, Koopmans MP and van der Jeugd H, 2020. Detection of West Nile virus in a common whitethroat (Curruca communis) and Culex mosquitoes in the Netherlands, 2020. Euro Surveill, 25. 10.2807/1560-7917.Es.2020.25.40.2001704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siles‐Lucas M, Casulli A, Conraths FJ and Müller N, 2017. Laboratory diagnosis of Echinococcus spp. in human patients and infected animals. Adv Parasitol, 96, 159–257. 10.1016/bs.apar.2016.09.003 [DOI] [PubMed] [Google Scholar]
- Széll Z, Marucci G, Pozio E and Sréter T, 2013. Echinococcus multilocularis and Trichinella spiralis in golden jackals (Canis aureus) of Hungary. Veterinary Parasitology, 197, 393–396. 10.1016/j.vetpar.2013.04.032 [DOI] [PubMed] [Google Scholar]
- Tamarozzi F, Akhan O, Cretu CM, Vutova K, Akinci D, Chipeva R, Ciftci T, Constantin CM, Fabiani M, Golemanov B, Janta D, Mihailescu P, Muhtarov M, Orsten S, Petrutescu M, Pezzotti P, Popa AC, Popa LG, Popa MI, Velev V, Siles‐Lucas M, Brunetti E and Casulli A, 2018. Prevalence of abdominal cystic echinococcosis in rural Bulgaria, Romania, and Turkey: a cross‐sectional, ultrasound‐based, population study from the HERACLES project. Lancet Infect Dis, 18, 769–778. 10.1016/s1473-3099(18)30221-4 [DOI] [PubMed] [Google Scholar]
- Teunis P, Havelaar A, Vliegenthart J and Roessink G, 1997. Risk assessment of Campylobacter species in shellfish: identifying the unknown. Water Science and Technology, 35, 29–34. 10.1016/S0273-1223(97)00230-8 [DOI] [Google Scholar]
- Teunis PF, Falkenhorst G, Ang CW, Strid MA, De Valk H, Sadkowska‐Todys M, Zota L, Kuusi M, Rota MC, Simonsen JB, Mølbak K, Van Duynhoven YT and Van Pelt W, 2013. Campylobacter seroconversion rates in selected countries in the European Union. Epidemiol Infect, 141, 2051–2057. 10.1017/s0950268812002774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teunis PF, Sukhrie FH, Vennema H, Bogerman J, Beersma MF and Koopmans MP, 2015. Shedding of norovirus in symptomatic and asymptomatic infections. Epidemiol Infect, 143, 1710–1717. 10.1017/s095026881400274x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozer SJ, Lambert SB, Strong CL, Field HE, Sloots TP and Nissen MD, 2014. Potential animal and environmental sources of Q fever infection for humans in Queensland. Zoonoses Public Health, 61, 105–112. 10.1111/zph.12051 [DOI] [PubMed] [Google Scholar]
- Van Walle I, Björkman JT, Cormican M, Dallman T, Mossong J, Moura A, Pietzka A, Ruppitsch W and Takkinen J, 2018. Retrospective validation of whole genome sequencing‐enhanced surveillance of listeriosis in Europe, 2010 to 2015. Euro Surveill, 23. 10.2807/1560-7917.Es.2018.23.33.1700798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoeven V, Tsakitzidis G, Philips H and Van Royen P, 2020. Impact of the COVID‐19 pandemic on the core functions of primary care: will the cure be worse than the disease? A qualitative interview study in Flemish GPs. BMJ Open, 10, e039674. 10.1136/bmjopen-2020-039674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (World Health Organization) , 2007. WHO Guidelines on tularaemia. Available online: https://apps.who.int/iris/handle/10665/43793
- WHO (World Health Organization) , 2020. COVID‐19: Considerations for tuberculosis (TB) care. 12, Available online: https://www.who.int/docs/default-source/documents/tuberculosis/infonote-tb-covid-19.pdf
- WHO (World Health Organization) , 2022. Third round of the global pulse survey on continuity of essential health services during the COVID‐19 pandemic: November–December 2021: Interim Report, 7 February 2022. Available online: file:///C:/Users/ISS/Downloads/WHO-2019-nCoV-EHS-continuity-survey-2022.1-eng.pdf
- WOAH (World Organisation for Animal Health) , 2018a. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. 8th Edition, 2018 Edition. Available online: https://www.woah.org/en/produit/manual-of-diagnostic-tests-and-vaccines-for-terrestrial-animals-2018/
- WOAH (World Organisation for Animal Health) , 2018b. Verocytotoxigenic Escherichia coli. In. OIE Terrestrial Manual. Available online: https://www.woah.org/fileadmin/Home/eng/Health_standards/tahm/3.09.10_VERO_E_COLI.pdf
- WOAH (World Organisation for Animal Health) , 2022. Report of the meeting of the scientific commission for animal diseases. Annex 10: report of the development of the case definition for infection with Coxiella burnetii (Q fever). Available online: https://www.woah.org/en/document/report-of-the-meeting-of-the-oie-scientific-commission-for-animal-diseases-scad/
- Young JJ, Haussig JM, Aberle SW, Pervanidou D, Riccardo F, Sekulić N, Bakonyi T and Gossner CM, 2021. Epidemiology of human West Nile virus infections in the European Union and European Union enlargement countries, 2010 to 2018. Euro Surveill, 26, 2001095. 10.2807/1560-7917.Es.2021.26.19.2001095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler U, Santos PD, Groschup MH, Hattendorf C, Eiden M, Höper D, Eisermann P, Keller M, Michel F, Klopfleisch R, Müller K, Werner D, Kampen H, Beer M, Frank C, Lachmann R, Tews BA, Wylezich C, Rinder M, Lachmann L, Grünewald T, Szentiks CA, Sieg M, Schmidt‐Chanasit J, Cadar D and Lühken R, 2020. West Nile virus epidemic in Germany triggered by epizootic emergence, 2019. Viruses, 12, 448. 10.3390/v12040448 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The European Union One Health 2021 Zoonoses Report