Abstract
Bone and joint infections are a rare but serious problem worldwide. Lactoferrin’s antimicrobial and antibiofilm activity coupled with its bone-regenerating effects may make it suitable for improving bone and joint infection treatment. However, free lactoferrin (LF) has highly variable oral bioavailability in humans due to potential for degradation in the stomach and small intestine. It also has a short half-life in blood plasma. Therefore, encapsulating LF in nanocarriers may slow degradation in the gastrointestinal tract and enhance LF absorption, stability, permeability and oral bioavailability. This review will summarize the literature on the encapsulation of LF into liposomes, solid lipid nanoparticles, nanostructured lipid carriers, polymeric micro and nanoparticles and hydroxyapatite nanocrystals. The fabrication, characterization, advantages, disadvantages and applications of each system will be discussed and compared.
Keywords: Lactoferrin, Bone, Nanoparticles, Microparticles, Hydroxyapatite
Introduction
Bone and joint infections are difficult to treat, require high healthcare costs and are highly debilitating conditions (Pereira Rosa et al. 2015). Reports of osteomyelitis are as high as 1 in 675 hospital admissions in the United States annually (Momodu and Savaliya 2022). Around 6–12 weeks of antibiotics are needed to treat osteomyelitis (Baldwin et al. 2018), including spending about 2 weeks in hospital to receive intravenous antibiotics (Webb et al. 2022). Osteomyelitis can lead to severe complications including sinus tract formation, contiguous soft tissue infection, abscess, septic arthritis, systemic infection, bony deformity and fracture (Lalani and Schmitt 2022).
Lactoferrin (LF) is a single-chain globular glycoprotein of the transferrin family with ~ 700 amino acids with a molecular weight of ~ 80 kDa (González-Chávez et al. 2009). Its molecular weight varies with the amount of glycosylation (Avery et al. 2021). It has an isoelectric point (pI) around 8–9 (Roohinejad et al. 2018). This means that LF is positively charged at the physiological pH of 7.4 and at a pH below its isoelectric point (Abad et al. 2021). The melting temperature is 60–85 °C (Roohinejad et al. 2018). The protein is folded into two globular lobes called the N and C lobes, which each bind one Fe3+ ion (Ammons and Copié 2013). Each iron binding requires synergistic binding of one bicarbonate (Prieels et al. 1978) or carbonate anion (Adlerova et al. 2008). LF is present in mammalian secretions and has a high homology between mammalian species (Icriverzi et al. 2019).
Several mechanisms for LF’s direct antimicrobial and antibiofilm activity have been found. LF chelates iron, an essential nutrient for many bacteria including S. aureus (Hammer and Skaar 2011). S. aureus is a common causative pathogen of osteomyelitis and prosthetic joint infection (Brady et al. 2007; Berbari et al. 2021; Krogstad 2021). Iron chelation also helps prevent biofilm formation (Vogel 2012). Furthermore, the N lobe of LF can interact with bacterial membranes resulting in membrane permeabilization (van Veen et al. 2002), opsonization (Jenssen and Hancock 2009) and release of bacterial lipopolysaccharide from the cell wall leading to lysis of bacteria (Wang et al. 2017).
In vitro and in vivo studies have shown that bovine and human LF have bacteriostatic effects against gram positive and gram negative bacteria (Bhimani et al. 1999; González-Chávez et al. 2009; Wang et al. 2017; Avery et al. 2021). Clinical trials have shown mixed reports that bovine LF-fortified formula given to neonates and infants reduces the incidence of diarrhoeal illness and respiratory disease (King et al. 2007; Chen et al. 2016). To explain these findings, it is proposed that orally delivered bovine LF (bLF) alters the gut microbiota and gut mucosal immune system, modulating the immunity of other mucous membranes such as the respiratory tract (Chen et al. 2016; Kowalczyk et al. 2022).
Bone-regenerating properties of LF have also been documented. Subcutaneous injections of bLF into rat calvariae increases bone growth compared to control (Cornish et al. 2004; Görmez et al. 2015; Gul Koca et al. 2022). In vitro, bLF produces a dose-related increase in the proliferation of rat osteoblast-like cells (Cornish et al. 2004). Many mechanisms for this osteoblast mitogenesis have been described: bLF increases COX2 and NFATc1 activity (Cornish and Naot 2010; Naot et al. 2011); bLF also binds to LRP1, a protein found on the osteoblast cell membrane, activating p42/44 MAPK signalling (Naot et al. 2004); other mechanisms include activation of PI3 kinase, Akt and upregulation of IGF-R1 (Cornish and Naot 2010; Icriverzi et al. 2019).
In considering its antimicrobial and bone-regenerating effects, LF could be delivered intravenously or intraosseously, however, the most convenient mode is oral delivery. Analysis of the literature shows that the bioavailability of orally delivered LF depends on multiple factors. Longer gastric emptying times as well as low pH of 1.5–2—the optimum for pepsin digestion—leads to greater gastric digestion of LF (Wang et al. 2017). Under fasting conditions, the intragastric pH of adults is ~ 5–6 and it takes up to 100 min to generate enough gastric acid to reach the optimum pH (Wang et al. 2017). These findings correlate well with one clinical trial, in which bovine LF (bLF) administered before meals, in contrast to during meals, was found to survive gastric degradation and improve the blood profile of pregnant women with hereditary thrombophilia and anaemia of inflammation (Rosa et al. 2020). Meanwhile, gastric pH higher than 4 and gastric emptying rate of 30 min have shown to be partially ineffective at digesting bLF (Troost et al. 2001).
Intact bLF that survives gastric degradation can then be degraded by the intestinal enzymes trypsin and chymotrypsin, based on in vitro studies (Yao et al. 2013, 2014a). However, bLF can also be absorbed in intact form by intestinal epithelial cells by binding to surface receptors and undergoing transcytosis; then, according to findings from rat studies, bLF enters the lymphatic system, travels through the thoracic duct lymph and enters the systemic circulation (Takeuchi et al. 2004; Nojima et al. 2008; Kilic et al. 2017). Here, free LF has a short half life of 12–60 min in blood plasma (van Snick et al. 1974; Beauchamp et al. 1983; Nojima et al. 2009; Shiga et al. 2015), due to rapid removal by the reticuloendothelial system, the liver and spleen (van Snick et al. 1974; Beauchamp et al. 1983; Onishi 2011).
Given its short half-life, it is not surprising that oral formulations of LF tend to produce low levels of LF in human serum, regardless of the formulation (Dix and Wright 2018). Prof. Harada could detect bLF in human blood after oral delivery of 900 mg of enteric-coated bLF to a 60 kg adult (Shimizu 2004). The concentration of bLF was only ~ 150 ng/ml 4 h after administration (Shimizu 2004). It should also be noted that the endogenous LF concentration in blood of healthy humans is 0.02 to 2 μg/ml rising to 200 μg/ml during inflammation and infection (Sienkiewicz et al. 2021). Why then, do some oral formulations of lactoferrin seem to produce therapeutic effects? Two models have been proposed. The first is that LF and its degradation products could exert distal effects even if it remains in the wall of the gut (Kowalczyk et al. 2022). This could occur by interaction of LF with gut associated lymphoid tissue (Kilic et al. 2017). The second is that LF is absorbed, as previously described, and accumulates in target organs exerting direct effects (Shimizu 2004). Little information exists on the oral bioavailability of bLF and the relationship between bLF’s effects and its concentration in the blood (Nojima et al. 2009). Future studies could address this issue by using fluorescent-labelled LF and calculating the concentration of absorbed LF based on fluorescence intensity (Kilic et al. 2017).
LF has immunomodulatory effects. An immune response normally begins with the deposition of pathogens in host tissue. In osteomyelitis, bacteria can colonize the bone marrow, soft tissue surrounding bone or the osteocyte-lacuno canalicular network (Masters et al. 2019). Microbial surface components recognizing adhesive matrix molecules (MSCRAMMs) allow bacteria to adhere to host polysaccharides like fibronectin, fibrinogen and collagen (Schmitt 2017). LF can prevent adherence of bacteria to epithelial cells (Ammons and Copié 2013). Neutrophils can recognize bacterial lipopolysaccharide (LPS). LF can bind LPS, reducing the activation of pro-inflammatory pathways (Fischer et al. 2006; Siqueiros-Cendón et al. 2014). Bacterial LPS can also stimulate osteoclastogenesis (Yamano et al. 2010; Janani et al. 2021). The extent of immune stimulation during sepsis, which can be a sequela or precursor to osteomyelitis, is also reduced by LF due to LF attenuating the LPS/CD14/TLR-4 pathway (Vogel 2012; Siqueiros-Cendón et al. 2014).
LF may have a role in osteoimmunology. Importantly, receptor activator of NF-κB ligand (RANKL) is expressed on osteoblasts and activated T cells, while RANK is expressed on osteoclasts and dendritic cells (Fan et al. 2018). RANKL-RANK binding results in bone resorption by osteoclasts. bLF orally administered to an osteoporosis mouse model decreased serum RANKL and increased serum OPG—these effects favour bone preservation (Fan et al. 2018). bLF was found to increase serum IFN-γ, IL-5 and IL-10. IFN-γ is known to inhibit RANKL/RANK signalling; IL-5 and IL-10 are known to increase OPG expression (Fan et al. 2018). RANKL and tumour necrosis factor (TNF) play an important role in bone destruction in rheumatoid arthritis (RA) (Firestein and Guma 2022). Oral liposomal bLF reduces osteoclastic bone destruction in a RA mouse model (Yanagisawa et al. 2022). This effect could be due to bLF-induced increase in Treg cells relative to Th17 cells and bLF-induced suppression of TNF-α production (Antoshin et al. 2021; Yanagisawa et al. 2022).
LF may have a role in coronavirus disease 2019 (COVID-19) treatment. LF’s antiviral activities are well known. It can bind to intelectin-1 receptor on host cells triggering the intracellular production of interferon which inhibits viral replication (Sienkiewicz et al. 2021). LF also down-regulates IL-6 which helps prevent intracellular iron overload, a situation which favours viral replication (Campione et al. 2021a). In particular for the SARS-CoV-2 virus that causes COVID-19, moieties of LF can attach to heparan sulfate proteoglycans, limiting the binding of the virus to ACE2, a protein expressed on the surface of multiple human epithelial cells that facilitates viral fusion with host epithelial cells (Sienkiewicz et al. 2021). In vivo studies have demonstrated that oral or intranasal liposomal bLF enables faster SARS-CoV-2 RNA negativization for patients with asymptomatic or mild-to-moderate infection compared to standard of care-treated or untreated patients (Rosa et al. 2021; Campione et al. 2021b). Negativization refers to the negative conversion of naso-oropharyngeal swab results for COVID-19 patients.
Considering its antimicrobial role in infections such as osteomyelitis, endogenous LF is secreted in high concentrations at the infection site. It binds to neutrophil extracellular traps (NETs) that help contain bacterial pathogens. These NETs help expose bacteria to high local concentrations of LF and other antimicrobial peptides (Vogel 2012). If exogenous LF is to be used as part of local therapy for osteomyelitis and other infections, it would need to be delivered to bacteria at high concentrations for a prolonged period in order to effectively eliminate the pathogen. High concentrations of LF would also help regenerate injured bone tissue. Therefore, a review of drug delivery carriers of LF would be useful to introduce effective formulation approaches that can enhance LF stability for parenteral use. The review will also discuss oral formulations of LF, to explore its possible role as an adjuvant for systemic infection (Vincent et al. 2015; Sherman et al. 2016). Oral formulations of LF may be able to enhance oral bioavailability and increase the permeability of LF through mucosal tissue and uptake by target cells. Therefore, we will discuss the applications of liposomes, solid lipid nanoparticles, nanostructured lipid carriers, polymeric micro and nanoparticles and hydroxyapatite nanocrystals and microspheres as potential methods of delivering LF orally and/or parenterally.
Liposomes
Liposomes are vesicles made of bilayer(s) of phospholipid enclosing an aqueous environment. They can be fabricated by four methods—thin film hydration, microfluidization also known as high pressure homogenization, reverse phase evaporation and ether injection (Guan et al. 2012). Liposomal LF (L-LF) has been administered intra-articularly, topically or orally (Table 1, Fig. 1).
Table 1.
Details of studies of LF-loaded micro/nano carriers
| Carrier type | Study authors | Mode of delivery | Species of LF used | Components of vehicle | Entrapment efficiency and particle size | Half life of delivery vehicle | Advantages of the carriers | Limitations of the carriers | |
|---|---|---|---|---|---|---|---|---|---|
| Liposomes | Trif et al. 2016; Icriverzi et al. 2019 | Intra-articular | Human | Negatively or positively charged liposomes | Not stated | Not stated | Fast action |
Requires specialist training Invasive/painful Risk of contamination Costly |
|
| Liposomes | López-Machado et al. 2021b | Topical—eye | Bovine | Hyaluronic acid-coated liposomes made from fat-free soybean phospholipids with 70% phosphatidylcholine (lipoid S75), cholesterol and polysorbate 80 | ~ 50%; ~ 90.5 nm | > 3 min | Prolonged/sustained release | Time taken to diffuse into cells, poor penetration | |
| Liposomes | Ishikado et al. 2005; Yamano et al. 2010 | Oral | Bovine |
Egg yolk phosphatidylcholine Phytosterol |
42%; 580 nm | Not stated | Safe, convenient and no pain for administration, Able to self-administer |
Slow action, Gut enzymes may degrade |
|
| Liposomes | Kawazoe et al. 2013 | Oral | Bovine | Soy phosphatidylcholine | EE not available; 70 nm | Not stated | Safe, convenient and no pain for administration, Able to self-administer |
Slow action, Gut enzymes may degrade |
|
| Liposomes | Vergara and Shene 2019; Vergara et al. 2020 | Oral | Bovine |
Rapeseed phospholipid Stigmasterol Hydrogenated phosphatidylcholine LF |
~ 90%; ~ 200 nm | Not stated | Safe, convenient and no pain for administration, Able to self-administer |
Slow action, Gut enzymes may degrade |
|
| Liposomes | Yao et al. 2014b; Yao 2015 | Oral | Bovine | Pectin or chitosan | 58.14%; 301.7 nm | ~ 5 h | Safe, convenient and no pain for administration, Able to self-administer |
Slow action, Gut enzymes may degrade |
|
| Liposomes | Campione et al. 2021b |
Oral or Intra-nasal |
Bovine | Not stated | Not stated; not stated | Not stated |
Oral delivery: Safe, convenient and no pain for administration, Able to self-administer |
Oral delivery: Slow action, Gut enzymes may degrade |
|
|
Intra-nasal delivery: Easy to administer Rapid onset of action Avoids first-pass metabolism |
Intra-nasal delivery: Only small volumes of drug can be administered |
||||||||
| Liposomes | Rosa et al. 2021 | Oral | Bovine | Not stated | Not stated; not stated | Not stated | Safe, convenient and no pain for administration, Able to self-administer |
Slow action, Gut enzymes may degrade |
|
| Solid lipid nanoparticles | Yao et al. 2015 | Oral | Bovine | Pectin or chitosan | ~ 92.02%; 283.1 nm | ~ 5 h | Safe, convenient and no pain for administration, Able to self-administer |
Slow action, Gut enzymes may degrade |
|
| Nanostructured lipid carriers | Varela-Fernández et al. 2022 | Topical – eye | Not stated |
Glycerol monostearate Soy lecithin Cholesterol Compritol 888 ATO Capryol® 90 Miglyol® 812 N Poloxamer 407 Poloxamer 188 D-α-Tocopherol Polyethylene Glycol 1000 Succinate |
~ 75%; ~ 119.45 nm | ~ 75 min | Prolonged/sustained release | Time taken to diffuse into cells, poor penetration | |
| Polymeric nanoparticles | Duarte et al. 2022 | In vitro | Bovine | Gellan gum | Not applicable; 92.03 nm | Not stated | Not applicable due to in vitro delivery | Not applicable due to in vitro delivery | |
| Polymeric nanoparticles | López-Machado et al. 2021a | Topical – eye | Bovine |
PLGA Resomer® 50:50 503H Ethyl acetate Kolliphor ® P188 |
32–56%; 130–146 nm | Not stated | Prolonged/sustained release | Time taken to diffuse into cells, poor penetration | |
| Polymeric nano-to-micro particles | Yang et al. 2020 | Oral or parenteral | Bovine | Oat β-glucan | Not applicable; Increase with rise in oat β-glucan concentration from nano to microscale | Not stated |
Oral delivery: Safe, convenient and no pain for administration, Able to self-administer Parenteral delivery: Fast action |
Oral delivery: Slow action, Gut enzymes may degrade Parenteral delivery: Painful, costly, risk of contamination |
|
| Polymeric microparticles | Kumar 2010; Kumar et al. 2013 | Oral | Bovine | Barley β-glucan | 63.2–91.5%; 5–50 μm | Not stated | Safe, convenient and no pain for administration, Able to self-administer |
Slow action, Gut enzymes may degrade |
|
| Polymeric microspheres | Kim et al. 2014 | In vitro | Not stated |
Poly-D,L-lactide-co-glycolide (PLGA) Poly vinyl alcohol (PVA) Dichloromethane Gelatin Dopamine Heparin |
9.31–54.4%; 400 μm | Months (Scholz 2009) | Not applicable due to in vitro delivery | Not applicable due to in vitro delivery | |
| Polymeric microspheres | Görmez et al. 2015 | Intraosseous | Bovine | Gelatin | Not stated; not stated | Not stated | Fast action |
Requires specialist training Invasive Costly Risk of contamination |
|
| Hydroxyapatite nanocrystals | Nocerino et al. 2014 | In vitro | Bovine | Hydroxyapatite | Not stated; 110 nm | Not stated | Not applicable due to in vitro delivery | Not applicable due to in vitro delivery | |
| Hydroxyapatite nanorods | Shi et al. 2017 | In vitro | Not stated | Hydroxyapatite | Not applicable; 150 nm | Not stated | Not applicable due to in vitro delivery | Not applicable due to in vitro delivery | |
| Hydroxyapatite microspheres | Shi et al. 2017 | In vitro | Not stated | Hydroxyapatite | Not applicable; 2–10 μm | Not stated | Not applicable due to in vitro delivery | Not applicable due to in vitro delivery | |
| Hydroxyapatite nanocrystals | Montesi et al. 2015a, b | In vitro | Bovine | Hydroxyapatite | 6.6–15.1%; 115 nm | Not stated | Not applicable due to in vitro delivery | Not applicable due to in vitro delivery | |
| Hydroxyapatite nanoparticles | Kim et al. 2016 | In vitro | Not stated |
Hydroxyapatite Dopamine Heparin 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide |
88.7%-90.03%; 107.5–119.1 nm | Not stated | Not applicable due to in vitro delivery | Not applicable due to in vitro delivery | |
Fig. 1.
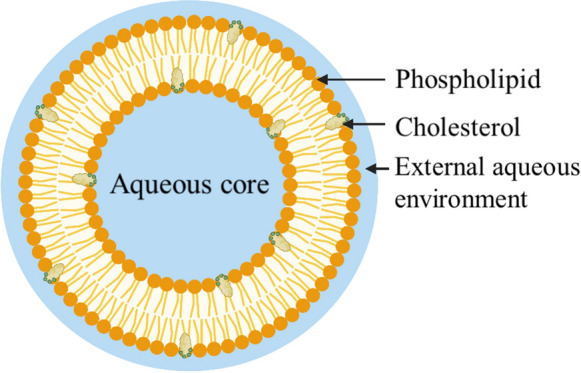
Liposome in cross-section (Buya et al. 2021). Created with BioRender.com
Liposomes can be characterized by: particle size; particle size distribution which is also known as polydispersity index (PDI); zeta potential; entrapment efficiency (EE); in vitro drug release; morphology by scanning electron microscopy (SEM); fourier transform infrared spectroscopy (FTIR) and differential scanning calorimetry (DSC).
Particle size and PDI can be measured by laser light scattering (Liu 2019). Zeta potential indicates the amount of surface charge of the liposome. The greater the surface charge, the greater the electrostatic repulsion between two liposomes. Small particle size and high zeta potential—particularly above 30 mV in modulus (Chen et al. 2019; Anali Bazán Henostroza et al. 2022)—increase the stability of liposomes. A positive zeta potential can be achieved by adding cationic compounds to liposomes, such as dioleoylphosphatidylethanolamine (DOPE) (Ding et al. 2009) and 1,2-dioleoyl-3-trimethylammonium propane (DOTAP) (Tonguc-Altin et al. 2015). A negative zeta potential can be achieved by adding 1,2-dioleoyl-sn-glycero-3-phospho-l-serine (DOPS) (Smith et al. 2017). Entrapment efficiency (EE) refers to the proportion of the drug trapped within the liposome. Several studies show that the EE for liposomal LF can range from 42 to 90% (Table 1).
In vitro drug release can be measured by dialysis tubing or Franz diffusion cell analysis. Both methods involve the release of the drug from the liposome followed by the permeation of free drug through a dialysis membrane. Samples are taken at specified time intervals and the amount of released drug is often measured (Chen et al., 2019) by high performance liquid chromatography (HPLC). FTIR and DSC can detect whether LF is loaded within the aqueous compartment or within the bilayer of the liposome.
Liposomes have many advantages as drug delivery vehicles. They are able to contain hydrophilic and hydrophobic drugs (Icriverzi et al. 2019); as their components are found endogenously (Anabousi et al. 2006), they are biocompatible (Icriverzi et al. 2019), biodegradable (dos Santos Ramos et al. 2020) and have low toxicity (Icriverzi et al. 2019; dos Santos Ramos et al. 2020); they have low immunogenicity (Icriverzi et al. 2019), their surface can be modified to target delivery of the drug (Icriverzi et al., 2019). They are able to prolong the release of drugs (Al‐amin et al. 2020).
Liposomes have several challenges to their widespread use. The main issue is poor stability compared to other drug carriers (Roohinejad et al. 2018; Thorn et al. 2021). Traditional liposomes greater than 100 nm are rapidly cleared from blood circulation by circulating macrophages or dendritic cells as part of the reticuloendothelial system (Buya et al. 2021; Anali Bazán Henostroza et al. 2022). Hydrophilic polymers such as polyethylene glycol, pectin or chitosan can protectively coat the surface of liposomes, increasing their residence time in the blood circulation (Anabousi et al. 2006; Icriverzi et al. 2019; Buya et al. 2021). It has also been shown that liposomes prepared from milk derived phospholipids or rapeseed oil can slow the digestion of LF in simulated gastric and intestinal conditions (Liu et al. 2013; Vergara et al. 2020).
Liposomes are difficult and costly to make on an industrial scale (Al‐amin et al. 2020). Microfluidization may achieve scalability with low batch-to-batch differences, however, this high energy process may damage proteins (Al‐amin et al. 2020). Supercritical carbon dioxide technique is a recently developed technique used to prepare liposomes and niosomes (Hallan et al. 2022). It is an inexpensive, inert, harmless, fire-resistant and environmentally friendly approach that avoids the use of organic solvent. The method involves atomized water droplets used to coat phospholipid vesicles under high diffusion of carbon dioxide. Several studies have demonstrated encapsulation efficiencies above 66% for various drugs using this method (Hallan et al. 2022).
Locally delivered L-LF can greatly prolong LF residence time at the administration site. Human LF (hLF) entrapped in positively charged liposomes delivered intra-articularly to mice with collagen-induced arthritis was retained longer in the injected joint compared to free protein or neutral or anionic liposome formulations (Trif et al. 2001; Icriverzi et al. 2019). However, negatively charged liposomes containing hLF had enhanced accumulation in human synovial fibroblasts from rheumatoid arthritis patients (Trif et al. 2001). Additionally coating liposomes with hyaluronic acid has increased the residence time of LF on the corneal surface (Table 1) (López-Machado et al. 2021b).
Liposomes, especially when coated with hydrophilic polymers such as chitosan, can increase the oral bioavailability of LF by protecting it from gastrointestinal degradation and delaying its removal from the systemic circulation by the reticuloendothelial system (Yao et al. 2015; Gorantla et al. 2021; Mohammadi et al. 2023). Two studies have found that orally administered L-LF inhibits bacterial LPS-induced bone resorption of alveolar bone in a rat periodontitis model (Table 1) (Yamano et al. 2010; Kawazoe et al. 2013). Yamano et al. (2010) administered L-bLF to rats for 7 days, then stimulated periodontitis by administering LPS. Therefore, it was concluded that L-LF can reduce alveolar bone destruction in periodontitis patients. This effect is probably due partly to the gastrointestinal ingestion and absorption of L-LF because of the pre-administration of bLF before stimulating periodontitis.
Vergara Shene (2019); Vergara et al. (2020) used combinations of rapeseed phospholipid, stigmasterol and hydrogenated phosphatidylcholine to make L-bLF with an entrapment efficiency of ~ 90%. A high entrapment efficiency is beneficial as it means relatively less amount of excipient can encapsulate a large amount of drug, increasing the cost-effectiveness and safety of the formulation. The liposomes of Vergara Shene (2019); Vergara et al. (2020) also had improved stability, delaying hydrolysis in gastric and intestinal environments. Further study needs to be done to investigate the therapeutic effects of this oral formulation of bLF. Another study by Yao et al. (2014b) reported that liposomes and solid lipid nanoparticles modified with chitosan or pectin increased the oral bioavailability of bLf 1.95–2.69 times in vivo compared to free bLF.
Solid lipid nanoparticles (SLNs)
SLNs are made of a core of biodegradable lipids that are solid at room and body temperature (Buya et al. 2021) surrounded by a layer of surfactant. The term “lipids” is used broadly here, and includes long chain triglycerides, partial triglycerides, fatty acids, phospholipids, waxes, cetyl palmitate and alkanoic acids (Pignatello et al. 2018; Buya et al. 2021). These are highly biocompatible. The surfactants used may have a concentration ranging between 1 and 5% (w/v) and can include polysorbate 80, poloxamer 188 and/or lecithin (Buya et al. 2021). Bioactive compounds, both hydrophilic and lipophilic (Moutinho et al. 2012), are encapsulated into the solid lipid matrix and released in a controlled manner (Buya et al. 2021). SLNs are generally spherical, with particle sizes of 10–1000 nm (Buya et al. 2021) (Fig. 2).
Fig. 2.
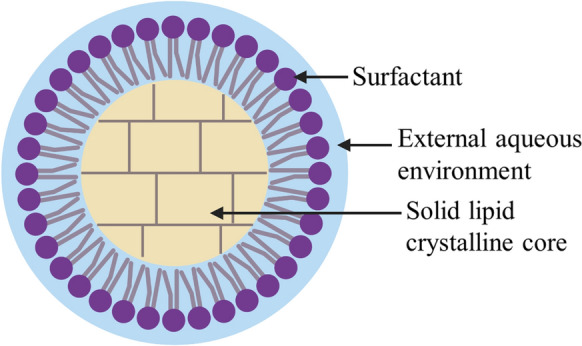
Solid lipid nanoparticle (Roohinejad et al. 2018; Buya et al. 2021). Created with BioRender.com
Only one group investigated the encapsulation of LF into solid lipid nanoparticles and compared this with liposomal-LF. SLN-LF showed higher heat resistance and greater electrolyte tolerance than L-LF (Yao et al. 2015). Furthermore, SLN-LF was physically more stable, demonstrated by pH and thermal treatment, ionic strength and storage at room and body temperature. This suggests that SLN-LF is, in general, more resistant to degradation in the gastrointestinal tract than L-LF. The rank order of oral bioavailability was chitosan-modified SLNs > pectin-modified liposomes > pectin-modified SLNs > chitosan-modified liposomes, with chitosan-modified SLNs showing 2.69-fold increase in oral bioavailability compared with free bLF (Yao et al. 2014b).
SLNs have the potential to be implanted into bone defects via embedding in hydrogels. One study investigated resveratrol loaded SLNs (Res-SLNs) embedded in a gelatin methacrylate (GelMA) hydrogel scaffold (Wei et al. 2021). Resveratrol is known to promote osteogenic differentiation and bone formation. Res-SLNs-GelMA was implanted into rat calvarial critical-size defects. Micro-CT results showed that the Res-SLNs-GelMA group showed the highest bone regeneration rate compared to GelMA only or SLNs-GelMA without Res. The study also found that SLNs significantly prolonged the release of Res from GelMA: 14% of the total drug was released at 0.5 days and 75% was released at 28 days. One limitation of this study is that the micro-CT results of Res-GelMA without SLNs weren’t obtained. This would shed more light on the synergistic effect of SLNs and GelMA hydrogel on bone regeneration.
SLNs can be characterized similarly to liposomes, namely particle size, zeta potential and entrapment efficiency (Wei et al. 2021). Their surface morphology can be determined by transmission electron microscopy (Wei et al. 2021). SLNs can be freeze-dried and their crystalline structure determined using an X-ray diffractometer (Wei et al. 2021), in order to ascertain whether LF has been successfully incorporated into the SLN.
Similar to liposomes, SLNs demonstrate sustained drug delivery, low toxicity, increased bioavailability compared to free drug and biodegradability (Naseri et al. 2015; Sayed 2017). However, unlike liposomes, SLNs and nanostructured lipid carriers (NLCs) have improved shelf-life stability (Thorn et al. 2021). SLNs can protect the drug from degradation (from light or oxygen) (Patel and San Martin-Gonzalez 2012; Pignatello et al. 2018; Thorn et al. 2021). Storage stability can be further increased by lyophilization and spray-drying (Hallan et al. 2022). Interestingly, SLNs can be designed to have prolonged circulation in the blood and may be able to accumulate in the bone marrow. This study (Göppert and Müller 2003) showed that poloxamer-188-stabilized SLNs (P188-SLNs) had prolonged circulation time, possibly due to the adsorption of albumin, a dysopsonic protein, on the P188-SLNs. The P188-SLNs also adsorbed apolipoprotein C-II and C-III in sufficient amounts that the researchers postulated that P188-SLNs could accumulate in the bone marrow, similar to poloxamer 407 polystyrene particles (Göppert and Müller 2003). Compared to liposomes and polymeric nanocarriers, SLNs are also easier and cheaper to mass-produce (Naseri et al. 2015; Sayed 2017; Hallan et al. 2022) and sterilize (Pignatello et al. 2018).
The main disadvantage of SLNs is that loading highly polar compounds often results in very low encapsulation (Furneri et al. 2017). However, there are ways to circumvent this, including: loading a non-polar basic form of the drug, coating the drug with a surfactant capsule before loading into SLN, utilizing lipophilic prodrugs or using hydrophobic ion-pairing (Thorn et al. 2021).
Nanostructured lipid carriers (NLCs)
NLCs are similar to SLNs but have a less structured lipid matrix composed of a mix of solid and liquid lipids (Buya et al. 2021). This allows NLCs to increase the encapsulation of active drug compared to SLNs, demonstrate higher loading capacity, reduce expulsion of drug during storage and prolong stability of the drug (Ali 2015; Roohinejad et al. 2018; Buya et al. 2021). NLCs have a greater capacity to store hydrophilic and lipophilic drugs compared to SLNs (Buya et al. 2021) and are more able to penetrate cell membranes (Buya et al. 2021). NLCs are also biodegradable, exhibit low toxicity and are easy and cost-effective to mass-manufacture (Roohinejad et al. 2018) (Fig. 3).
Fig. 3.
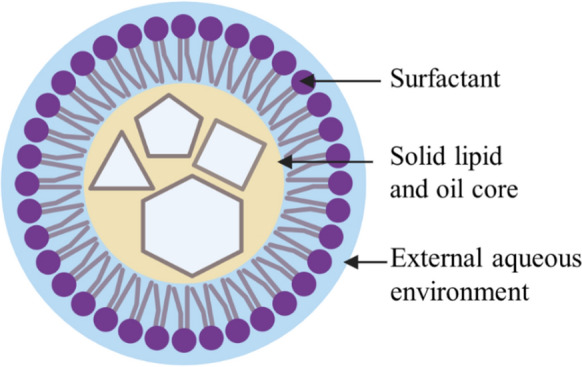
Nanostructured lipid carrier (NLC). The cores of NLCs are composed of liquid and solid lipids resulting in the formation of imperfect crystals. This allows more space to incorporate bioactive compounds (Roohinejad et al. 2018; Buya et al. 2021). Created with BioRender.com
The main methods for fabricating NLCs are hot homogenization, cold homogenization and solvent emulsification-evaporation (Roohinejad et al. 2018). Solvent emulsification-evaporation is typically employed to encapsulate hydrophilic drugs like the protein LF (Varela-Fernández et al. 2022).
NLCs can be characterized by particle size, morphology, entrapment efficiency, zeta potential and in vitro release behavior. Moreover, the crystallinity and melting behavior of the lipid are important to determine as these affect the release rate, drug loading, and EE (Roohinejad et al. 2018). X-ray spectroscopy and DSC are used to investigate lipid status.
Only one study investigated LF-loaded NLCs (Varela-Fernández et al. 2022). The context of the research was ocular drug delivery for keratoconus treatment. Entrapment efficiency and loading capacity was ~ 75% for 1 mg/ml LF. The in vitro release study demonstrated an initial burst release of 20% of total LF in the first hour followed by a controlled release where a cumulative ~ 50% of total LF was released after 24 h. The NLC-LF were stable, non-toxic and showed mucoadhesive properties. The study demonstrated the potential of topical ophthalmic delivery of NLC-LF.
Polymeric micro- and nanoparticles
Polymeric micro- and nanoparticles form a diverse group of compounds. Only 6 studies were found for lactoferrin delivery by polymeric particles. LF and gellan gum was combined through electrostatic complexation to enhance the antimicrobial properties of LF (Duarte et al. 2022). Fabrication of LF-gellan gum complexes was done by mixing vacuum-filtered stock solutions of LF and gellan gum at pH 4—the pH at which the greatest net charge difference between the biopolymers was observed. The LF-gellan gum complexes were characterized by zeta potential, isothermal titration calorimetry, FTIR, atomic force microscopy and minimum inhibitory concentration (MIC) assays to assess antimicrobial activity against S. aureus and E. coli. Duarte et al. (2022) found that LF-gellan gum complexes reduced the MIC for S. aureus compared to free LF, however the effect was reduced in tryptic soy broth, which contained higher concentrations of divalent cations—Fe2+, Mn2+, Zn2+, Cu2+ that competed with LF for anionic sites on the microbial membranes (Duarte et al. 2022). The study also reported that complexation to gellan gum reduced the flexibility of LF, which may limit its interaction with bacterial membranes. This may help explain why the MIC for E. coli was unaffected by LF-gellan gum. The findings from Duarte et al. (2022) suggest that LF-gellan gum complexes could be effective against S. aureus infections in vitro, however, further studies need to be done to investigate its effects in vivo.
López-Machado et al. (2021a, b) fabricated bLF-loaded polymeric nanoparticles (bLF-NPs) composed of poloxamer 188 (P188) and poly (lactic-co-glycolic acid) (PLGA). The bLF-NPs were fabricated by double emulsion and characterized by particle size, particle size distribution, zeta potential and EE. The optimum formulation achieved an EE of 56%. The bLF-NPs exhibited prolonged release of bLF with a cumulative 83.6% of bLF released after 48 h. P188 and PLGA were chosen as they could demonstrate improved permeability across corneal tissue, enhancing the anti-inflammatory effect of bLF. These polymers were also biocompatible and biodegradable and relatively large amounts of bLF could be loaded into these nanoparticles: concentrations of bLF of 8–11 mg/ml reached 50–60% encapsulation for P188 and at 19 mg/ml bLF, the maximum loading capacity was reached for PLGA particles. Moreover, the bLF-NPs could be sterilized with γ-irradiation with little effect on their physicochemical properties. The effect of these nanoparticles on bone tissue, bacteria or biofilms was not studied. However, these nanoparticles decreased the expression of inflammatory cytokines in the tear film to levels similar to free bLF, indicating that bLF encapsulated in these NPs retained its effect.
Two studies investigated the combination of bLF with beta-glucan (bG) (Kumar 2010; Kumar et al. 2013; Yang et al. 2020). Yang et al. mixed bLF and oat bG solutions at different proportions at 25 °C and at pH 5. Mixing of the two solutions was also carried out at 90 °C. The bLF-oat bG complexes were characterized by isothermal titration calorimetry (ITC), particle size, zeta potential, SEM, fluorescence spectroscopy, far-UV circular dichroism measurements, raman spectra collection and flow behaviour measurements. ITC showed that bLF and oat bG can bind to each other and suggests that the interaction is at least partly electrostatic. bLF is positively charged at pH 5, and oat bG is neutral or slightly negatively charged due to the presence of phosphate residues. Importantly, fluorescence spectroscopy showed that oat bG can change the structure of bLF. Turbidity and particle size was larger for complexes heated at 90 °C compared to 25 °C. This suggested the formation of larger biopolymer complexes at higher temperatures, involving aggregation and thermal denaturation of bLF in the presence of oat bG. Therefore, complexation of bLF with oat bG may result in the limitation of bLF’s properties, especially under elevated temperature conditions above 25 °C.
Hemant Kumar loaded bLF into barley bG microparticles (bLF-barley bG) using a cryo-milling technique to investigate its effect on osteoblasts and bone mineral density (Kumar 2010; Kumar et al. 2013). In vitro, bLF was released in a sustained manner from cryomilled barley bG. Initially, 25% burst bLF release was found and after 7 h, reached only 35%. Addition of Kollicoat increased the burst release to 57% and final bLF release after 7 h was 91%. In vivo, the study found that carriage of cryomilled bLF in barley bG increased the oral bioavailability of bLF in ovariectomized mice. However, bLF extracted from cryomilled bLF-barley bG complexes showed less activity on osteoblast proliferation compared to cryomilled free bLF. Importantly, complexation of bLF to barley bG did not increase bone mineral density to a greater extent compared to orally delivered free bLF. These results suggest that complexation of bLF to barley bG increases the oral bioavailability of bLF and preserves but does not enhance bLF’s bone-regenerating effects.
Kim et al. (2014) prepared poly(lactide-co-glycolide) (PLGA) microspheres coated with LF in order to study their effect on the osteogenic differentiation of rabbit adipose-derived stem cells (Fig. 4).
Fig. 4.
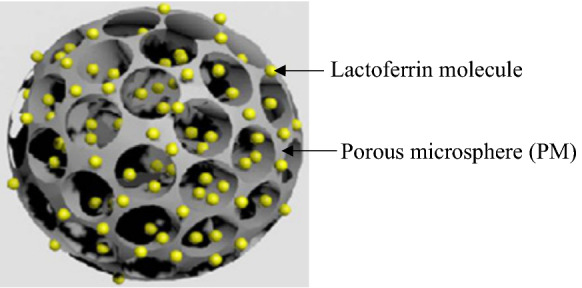
PM with adsorbed lactoferrin. Image used with permission from (Kim et al. 2014)
PMs were fabricated using a fluidic device with discontinuous and continuous phases (Kim et al. 2014). The discontinuous phase was a water-in-oil polymer emulsion composed of PLGA, polyvinyl alcohol (PVA) and gelatin in dichloromethane solution. The continuous phase was PVA solution. The discontinuous and continuous phases were mixed together at different flow rates through the fluidic device. The PMs were modified with negatively-charged heparin by immersion in Tris buffer. Then, LF was adsorbed on the surface of the PMs by combining Hep-PMs with LF in 2-(N-morpholino) ethanesulfonic acid (MES) buffer.
The PMs were characterized by SEM and X-ray photoelectron spectroscopy. In vitro release of LF into phosphate buffered saline was also measured. The release of LF was prolonged: ~ 47% of cumulative LF was released over 28 days. Furthermore, the study demonstrated that LF-impregnated PMs induced osteogenic differentiation of rabbit adipose-derived stem cells (rADSCs) by increasing ALP activity, calcium deposition, osteocalcin and osteopontin expressions compared with rADSCs grown in PMs without LF. In vivo studies will be needed to further determine the effects of LF-impregnated PMs.
Porous microspheres (PMs) offer two benefits for bone regeneration: they can be used as injectable scaffolds to repair irregularly-shaped bone defects during minimally invasive surgery; they can also contain and release many different drugs or proteins. PLGA has been used in orthopaedic implants and may be suitable for bone regeneration as it takes months to degrade in the body, approximating the rate of bone healing (Scholz 2009).
Görmez et al. (2015) prepared bLF-loaded gelatin microspheres (bLF-GM). The bLF-GM were fabricated by adding bLF in phosphate buffer to gelatin solution. Glutaraldehyde solution was added to harden the microspheres. bLF-GM was characterized by in vitro release of bLF: approximately 3 mg of bLF was released over 24 days (Görmez et al. 2015). Increasing the cross-linking density of the microspheres extended the duration of release. Görmez et al. (2015) found that 3 mg bLF-GM in combination with inorganic bovine bone promoted bone regeneration in bone defects surgically created around tooth implants in pigs. Compared to inorganic bovine bone alone, adding bLF-GM increased the percentage of hard tissue and newly formed bone and decreased the percentage of residual graft tissue.
Hydroxyapatite nanocrystals and micro-particles
Hydroxyapatite (HA) nanocrystals are a major inorganic constituent of bone tissue and are widely used as a bone graft material due to high biocompatibility and osteoconductivity (Murugan et al. 2010; Montesi et al. 2015a; Shi et al. 2017; Bastos et al. 2019). Synthetic biomimetic HA nanocrystals can be made to have a length of 100 nm, the width of 20–30 nm and a thickness of 3–6 nm, resembling the natural HA nanocrystals found in bone (Nocerino et al. 2014).
Nocerino et al. (2014) found that bLF-coated HA nanocrystals possessed concentration-dependent bacterial growth-inhibiting properties, including against S. aureus. bLF-HA was synthesized by precipitation of HA nanocrystals using (CH3COO)2Ca and H3PO4. bLF was then dissolved in HEPES buffer at pH 7.4 and was found to be strongly attracted to HA forming a monolayer protein coat around the nanocrystals. bLF-HA was characterized by FTIR and fourier transform Raman spectroscopy, which determined that the conformation of adsorbed bLF was only slightly altered compared to unadsorbed bLF. A downside to the bLF-HA particles was the slight cytotoxicity to THP-1 cells at concentrations used to inhibit the growth of bacteria (Nocerino et al. 2014).
HA can also be shaped in nanorod and microsphere forms, and LF can be adsorbed onto these particles (Shi et al. 2017). HA nanorods and HA microsphere powders were combined with LF in phosphate buffer solution at pH 7.4 at 37 °C for 24 h. The complex was washed twice with ultrapure water, recovered by centrifugation and freeze-dried. LF-HA nanorods and microspheres were characterized by N2 adsorption–desorption isotherms which determined that the particles were mesoporous (having pores of diameter 2–50 nm). Thermogravimetric analysis was used to determine the amount of LF protein attached to the HA. FTIR demonstrated that LF and HA interacted stably and that LF did not affect the conformation of HA. The study found that microspherical HA had higher biocompatibility compared to nanorod HA—this was attributed to the greater aggregation of the nanorods impairing nutrient and water absorption. The main study findings were that, compared to HA alone, HA-LF was more biocompatible toward MC3T3-E1 cells and HA-LF nanorods and microspheres stimulated greater cell proliferation of MC3T3-E1 (Shi et al. 2017). Importantly, microsphere HA-LF increased cell viability of MC3T3-E1 cells compared to free LF at 48 and 72 h (Shi et al. 2017).
Montesi et al. (2015a, b) fabricated HA-LF nanocrystals in a similar method as described by Nocerino et al. (2014). No LF was released from the HA surface for up to 14 days, indicating a strong affinity of LF for HA. It was found that HA and LF acted synergistically in MC3T3-E1 osteoblasts to trigger osteoblast viability, differentiation and bone matrix deposition. In contrast, osteoclast formation and activity was inhibited. These findings suggest that LF-adsorption onto HA can be used as a bone graft substitute, increasing the local concentration of LF, prolonging its residence time in the target tissue (Montesi et al. 2015a, b).
It has been reported that LF-HA particles may aggregate together and precipitate in an aqueous environment such as plasma, resulting in rapid clearance by the liver or toxicity to cells (Kim et al. 2016). Therefore, Kim et al. (2016) fabricated heparin-immobilized HA nanoparticles to deliver LF. Heparin’s negative charge was used to increase the electrostatic repulsion between HA particles and LF was conjugated to the heparin (Hep) coating the HA particles. Fabrication was a complex process involving the components HA, LF, dopamine, Hep, 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide (EDAC), N-hydroxysuccinimide (NHS), and 2-(N-morpholino) ethanesulfonic acid (MES) buffer.
LF-Hep-HA was characterized by measuring its particle size; zeta potential to determine if Hep had linked LF to HA particles; transmission electron microscopy for morphology; turbidity and precipitation studies to determine if LF-Hep-HA particles had aggregated. The study found that Hep immobilization onto HA nanoparticles prevented their aggregation and prolonged the release of LF over 4 weeks. LF-Hep-HA had low cytotoxicity and induced the osteogenic differentiation of rabbit adipose-derived stem cells. Further studies, perhaps using human adipose-derived stem cells, will be needed to determine the applicability for humans. These findings suggest the potential for LF-Hep-HA to be used as an injectable system to stimulate bone tissue regeneration (Kim et al. 2016).
Discussion
Nanoparticular drug carriers are an expanding research area as they can enhance existing treatments for diseases like infection and cancer. Nanoparticles, when targeted to specific tissues, provide a high local concentration of drug. This makes them highly suited to augmenting antimicrobial therapy. The human immune system is similarly able to create high local concentrations of antimicrobial peptides including LF around invading pathogens as part of the innate immune response (Vogel 2012). Nanoparticular carriers also prolong the release of the drug. When loaded with the right active molecule, nanocarriers can reduce the spread of antimicrobial resistance (Kalelkar et al. 2021).
The small size of nanoparticles also helps in biofilm penetration. Analysis of several studies has shown that large, highly positive or highly negatively charged lipid-based drug delivery systems penetrate biofilms poorly while a negative or near-neutral lipid nanoparticle facilitates greater biofilm penetration (Thorn et al. 2021). Lipid-based drug delivery systems include liposomes, SLNs and NLCs.
This review has introduced several nano and micro-particular carriers for LF. Liposomes are generally less stable than SLNs or NLCs, although a larger body of research exists around liposomes. Unlike SLNs and NLCs, liposomes can fuse with bacterial membranes, delivering active drug (Thorn et al. 2021; Shadvar et al. 2022). They can be made with rapeseed or milk-derived phospholipid, stigmasterol and hydrogenated phosphatidylcholine (Liu et al. 2013; Vergara and Shene 2019; Vergara et al. 2020) to improve stability and delay hydrolysis in the gastrointestinal environment. Use of these components is thought to improve stability as the fatty acid chains of the phospholipid are more saturated, hence the liposomal membrane is more rigid and less prone to leak drug (Roohinejad et al. 2018).
The cost of mass-producing liposomes is another barrier to their widespread use. Innovative techniques such as supercritical carbon dioxide need to be explored further (Hallan et al. 2022) and the effect of these processes on the structure of the drug molecule needs to be studied.
SLNs and NLCs are promising nanocarriers particularly in oral drug delivery. The use of saturated fatty acids like stearic acid improves their stability (Yao et al. 2014a). Preliminary studies involving Caco-2 cells have shown that SLN-bLF are taken up by gastrointestinal epithelium by an energy-dependent process (Yao et al. 2014a). Further research may involve oral delivery of SLN-LF to ovariectomized mice and comparing the skeletal composition with mice fed with a control diet. Ovariectomized mice are a model for post-menopausal osteoporosis. SLNs also have the potential to be implanted into bone defects via embedding in hydrogels (Wei et al. 2021).
Polymeric micro and nanoparticles represent a diverse group of compounds. Poloxamer and PLGA can preserve LF function and are able to load relatively high concentrations of bLF (López-Machado et al. 2021a). However, the biodegradation of these polymers requires careful consideration. PLGA takes months to degrade in the body. This may be an advantage if it is placed within bone tissue that is undergoing healing as it approximates the duration of the healing process (Scholz 2009). However, systemic administration of such polymers may be unsuitable due to their large size which impacts renal clearance (Wyss et al. 2020). The degradability of PLGA can be determined by several measurements (Hussein et al. 2013): (1) water uptake of the polymer—the greater the water uptake, the greater the degradability; (2) loss of mass of polymer over time; (3) change in pH of the degradation environment—the breakdown products of PLGA are acidic; (4) quantification of the acidic breakdown products of PLGA. Similar methods to determine poloxamer’s degradability can be used (Erlandsson 2002).
Despite these concerns, polymeric particles are non-toxic, biocompatible and versatile. They can be made to form porous microspheres (PMs) which prolong the release of drug. The study by Kim et al. (2014) showed that PMs containing LF could stimulate the osteogenic differentiation of rabbit adipose-derived stem cells. In vivo studies will be needed to confirm and quantify these bone-regenerating effects. It is envisioned that these PMs can be used in injectable scaffolds as part of minimally invasive surgery for bone diseases.
Certain polymers like beta glucan (bG) (Kumar 2010; Kumar et al. 2013; Yang et al. 2020) and gellan gum (Duarte et al. 2022) limit the conformational flexibility of LF. This does not necessarily lead to a reduction of efficacy of LF: complexation with gellan gum enhanced the bacteriostatic effect of LF in glucose-yeast-peptone broth (Duarte et al. 2022); complexation with barley bG increased the oral bioavailability and bone mineral density of an osteoporosis mouse model – however, the increase in bone mineral density was comparable to orally administered free bLF (Kumar 2010; Kumar et al. 2013).
Hydroxyapatite nanocrystals are biocompatible and well-established as a bone graft substitute (Murugan et al. 2010; Montesi et al. 2015a; Shi et al. 2017; Bastos et al. 2019). However, they may aggregate together (Shi et al. 2017) causing toxicity, hence may be unsuitable for systemic use.
Conclusion
In summary, different nano and microparticular drug carriers seem particularly suited to different delivery modes of LF for different therapies. Liposomes are promising oral, topical and intra-articular delivery carriers. SLNs and NLCs are promising oral delivery carriers and, if embedded within hydrogels, could be implanted into bone defects. Polymeric particles like beta glucan and gellan gum could deliver LF orally or parenterally. Other polymers like PLGA, P188 and gelatin are being investigated as a carrier for intraosseous delivery. Hydroxyapatite nanocrystals seem better suited for intraosseous delivery to affected bone tissue.
Acknowledgements
Disclosure
The authors have no relevant financial or non-financial interests to disclose.
Author contributions
JC and JW conceived the idea for the article. RO performed the literature search and data analysis. RO drafted the work. JC and JW critically revised the work.
Funding
The authors did not receive support from any organization for the submitted work.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abad I, Conesa C, Sánchez L. Development of encapsulation strategies and composite edible films to maintain lactoferrin bioactivity: a review. Materials. 2021 doi: 10.3390/MA14237358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adlerova L, Bartoskova A, Faldyna M. Lactoferrin: a review. Vet Med. 2008;53:457–468. doi: 10.17221/1978-VETMED. [DOI] [Google Scholar]
- Al-amin MD, Bellato F, Mastrotto F, et al. Dexamethasone loaded liposomes by thin-film hydration and microfluidic procedures: formulation challenges. Int J Mol Sci. 2020 doi: 10.3390/IJMS21051611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali AA (2015) Functionalised lipid Nanoparticles loaded with Paclitaxel for targeted release to ovarian cancer tissue. University of Auckland
- Ammons MC, Copié V. Mini-review: Lactoferrin: a bioinspired, anti-biofilm therapeutic. Biofouling. 2013;29:443–455. doi: 10.1080/08927014.2013.773317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anabousi S, Kleemann E, Bakowsky U, et al. Effect of PEGylation on the stability of liposomes during nebulisation and in lung surfactant. J Nanosci Nanotechnol. 2006;6:3010–3016. doi: 10.1166/JNN.2006.461. [DOI] [PubMed] [Google Scholar]
- Anali Bazán Henostroza M, Diniz Tavares G, Nishitani Yukuyama M, et al. Antibiotic-loaded lipid-based nanocarrier: a promising strategy to overcome bacterial infection. Int J Pharm. 2022 doi: 10.1016/J.IJPHARM.2022.121782. [DOI] [PubMed] [Google Scholar]
- Antoshin AA, Shpichka AI, Huang G, et al. Lactoferrin as a regenerative agent: the old-new panacea? Pharmacol Res. 2021;167:1043–6618. doi: 10.1016/j.phrs.2021.105564. [DOI] [PubMed] [Google Scholar]
- Avery TM, Boone RL, Lu J, et al. Analysis of antimicrobial and antibiofilm activity of human milk lactoferrin compared to bovine lactoferrin against multidrug resistant and susceptible Acinetobacter baumannii clinical isolates. ACS Infectious Diseases. 2021 doi: 10.1021/acsinfecdis.1c00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin A, Hjelde N, Goumalatsou C, Myers G. Oxford Handbook of Clinical Specialties. 10. Oxford: Oxford University Press; 2018. [Google Scholar]
- Bastos AR, da Silva LP, Maia FR, et al. Lactoferrin-hydroxyapatite containing spongy-like hydrogels for bone tissue engineering. Materials. 2019 doi: 10.3390/MA12132074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp CO, Gonias SL, Menapace DP, Pizzo SV. A new procedure for the synthesis of polyethylene glycol-protein adducts; effects on function, receptor recognition, and clearance of superoxide dismutase, lactoferrin, and α2-macroglobulin. Anal Biochem. 1983;131:25–33. doi: 10.1016/0003-2697(83)90131-8. [DOI] [PubMed] [Google Scholar]
- Berbari E, Baddour Larry M, Chen AF (2021) Prosthetic joint infection: epidemiology, microbiology, clinical manifestations, and diagnosis - UpToDate. https://www.uptodate.com/contents/prosthetic-joint-infection-epidemiology-microbiology-clinical-manifestations-and-diagnosis?search=prosthetic%20joint%20infection&source=search_result&selectedTitle=2~117&usage_type=default&display_rank=2. Accessed 23 Apr 2022
- Bhimani RS, Vendrov Y, Furmanski P. Influence of lactoferrin feeding and injection against systemic staphylococcal infections in mice. J Appl Microbiol. 1999;86:135–144. doi: 10.1046/J.1365-2672.1999.00644.X. [DOI] [PubMed] [Google Scholar]
- Brady RA, Leid JG, Calhoun JH, et al. Osteomyelitis and the role of biofilms in chronic infection. FEMS Immunol Med Microbiol. 2007 doi: 10.1111/j.1574-695X.2007.00357.x. [DOI] [PubMed] [Google Scholar]
- Buya AB, Witika BA, Bapolisi AM, et al. Application of lipid-based nanocarriers for antitubercular drug delivery: a review. Pharmaceutics. 2021 doi: 10.3390/PHARMACEUTICS13122041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campione E, Lanna C, Cosio T, et al. Lactoferrin against SARS-CoV-2: in vitro and in silico evidences. Front Pharmacol. 2021 doi: 10.3389/FPHAR.2021a.666600/FULL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campione E, Lanna C, Cosio T, et al. Lactoferrin as antiviral treatment in COVID-19 management: preliminary evidence. Int J Environ Res Public Health. 2021 doi: 10.3390/IJERPH182010985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Chai L, Li H, et al. Effect of bovine lactoferrin from iron-fortified formulas on diarrhea and respiratory tract infections of weaned infants in a randomized controlled trial. Nutrition. 2016;32:222–227. doi: 10.1016/j.nut.2015.08.010. [DOI] [PubMed] [Google Scholar]
- Chen S, Hanning S, Falconer J, et al. Recent advances in non-ionic surfactant vesicles (niosomes): fabrication, characterization, pharmaceutical and cosmetic applications. Eur J Pharm Biopharm. 2019;144:18–39. doi: 10.1016/J.EJPB.2019.08.015. [DOI] [PubMed] [Google Scholar]
- Cornish J, Naot D. Lactoferrin as an effector molecule in the skeleton. Biometals. 2010;23:425–430. doi: 10.1007/S10534-010-9320-6. [DOI] [PubMed] [Google Scholar]
- Cornish J, Callon KE, Naot D, et al. Lactoferrin Is a potent regulator of bone cell activity and increases bone formation in vivo. Endocrinology. 2004;145:4366–4374. doi: 10.1210/EN.2003-1307. [DOI] [PubMed] [Google Scholar]
- Ding W, Izumisawa T, Hattori Y, et al. Non-ionic surfactant modified cationic liposomes mediated gene transfection in vitro and in the mouse Lung. Biol Pharm Bull. 2009;32:311–315. doi: 10.1248/BPB.32.311. [DOI] [PubMed] [Google Scholar]
- Dix C, Wright O. bioavailability of a novel form of microencapsulated bovine lactoferrin and its effect on inflammatory markers and the gut microbiome: a pilot study. Nutrients. 2018 doi: 10.3390/nu10081115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- dos Santos Ramos MA, dos Santos KC, da Silva PB, et al. Nanotechnological strategies for systemic microbial infections treatment: a review. Int J Pharm. 2020;589:119780. doi: 10.1016/J.IJPHARM.2020.119780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte LGR, Alencar WMP, Iacuzio R, et al. Synthesis, characterization and application of antibacterial lactoferrin nanoparticles. Curr Res Food Sci. 2022;5:642–652. doi: 10.1016/J.CRFS.2022.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlandsson B. Stability-indicating changes in poloxamers: the degradation of ethylene oxide-propylene oxide block copolymers at 25 and 40 ℃. Polym Degrad Stab. 2002;78:571–575. doi: 10.1016/S0141-3910(02)00233-1. [DOI] [Google Scholar]
- Fan F, Shi P, Liu M, et al. Lactoferrin preserves bone homeostasis by regulating the RANKL/RANK/OPG pathway of osteoimmunology. Food Funct. 2018;9:2653–2660. doi: 10.1039/C8FO00303C. [DOI] [PubMed] [Google Scholar]
- Firestein GS, Guma M (2022) Pathogenesis of rheumatoid arthritis. In: UpToDate. https://www.uptodate.com/contents/pathogenesis-of-rheumatoid-arthritis?search=rheumatoid%20arthritis&topicRef=7516&source=see_link. Accessed 9 Sep 2022
- Fischer R, Debbabi H, Dubarry M, et al. Regulation of physiological and pathological Th1 and Th2 responses by lactoferrin. Biochem Cell Biol. 2006;84:303–311. doi: 10.1139/O06-058. [DOI] [PubMed] [Google Scholar]
- Furneri PM, Fuochi V, Pignatello R. Lipid-based nanosized delivery systems for fluoroquinolones: a review. Curr Pharm Des. 2017;23:6696–6704. doi: 10.2174/1381612823666171122110103. [DOI] [PubMed] [Google Scholar]
- González-Chávez S, Arevalo-Gallegos S, Rascon-Cruz Q. Lactoferrin: structure, function and applications. Int J Antimicrob Agents. 2009;33:301–302. doi: 10.1016/j.ijantimicag.2008.07.020. [DOI] [PubMed] [Google Scholar]
- Göppert TM, Müller RH. Plasma protein adsorption of tween 80-and poloxamer 188-stabilized solid lipid nanoparticles. J Drug Target. 2003;11:225–231. doi: 10.1080/10611860310001615956. [DOI] [PubMed] [Google Scholar]
- Gorantla S, Wadhwa G, Jain S, et al. (2021) Recent advances in nanocarriers for nutrient delivery. Drug Deliv and Transl Res. 2021;12(10):2359–2384. doi: 10.1007/S13346-021-01097-Z. [DOI] [PubMed] [Google Scholar]
- Görmez U, Kürkcü M, Benlidayi ME, et al. Effects of bovine lactoferrin in surgically created bone defects on bone regeneration around implants. J Oral Sci. 2015;57:7–15. doi: 10.2334/JOSNUSD.57.7. [DOI] [PubMed] [Google Scholar]
- Guan R, Ma J, Wu Y, et al. Development and characterization of lactoferrin nanoliposome: cellular uptake and stability. Nanoscale Res Lett. 2012 doi: 10.1186/1556-276X-7-679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gul Koca C, Yıldırım B, Ozmen O, et al. Effect of single-dose locally applied lactoferrin on autograft healing in peri-implant bone in rat models. Injury. 2022;53:858–867. doi: 10.1016/j.injury.2021.11.065. [DOI] [PubMed] [Google Scholar]
- Hallan SS, Amirian J, Brangule A, Bandere D. Lipid-based nano-sized cargos as a promising strategy in bone complications: a review. Nanomaterials. 2022;12:1146. doi: 10.3390/NANO12071146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer ND, Skaar EP. Molecular mechanisms of Staphylococcus aureus iron acquisition. Ann Rev Microbiol. 2011;65:129–147. doi: 10.1146/ANNUREV-MICRO-090110-102851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussein AS, Abdullah N, Ahmadun FR. In vitro degradation of poly (D, L-lactide-co-glycolide) nanoparticles loaded with linamarin. IET Nanobiotechnol. 2013;7:33–41. doi: 10.1049/IET-NBT.2012.0012. [DOI] [PubMed] [Google Scholar]
- Icriverzi M, Dinca V, Moisei M, et al. Lactoferrin in bone tissue regeneration. Curr Med Chem. 2019;27:838–853. doi: 10.2174/0929867326666190503121546. [DOI] [PubMed] [Google Scholar]
- Ishikado A, Imanaka H, Takeuchi T, et al. Liposomalization of lactoferrin enhanced it’s anti-inflammatory effects via oral administration. Biol Pharm Bull. 2005;28:1717–1721. doi: 10.1248/bpb.28.1717. [DOI] [PubMed] [Google Scholar]
- Janani K, Teja KV, Alam MK, et al. Efficacy of oregano essential oil extract in the inhibition of bacterial lipopolysaccharide (Lps)-induced osteoclastogenesis using raw 264.7 murine macrophage cell line—an in-vitro study. Separations. 2021 doi: 10.3390/SEPARATIONS8120240. [DOI] [Google Scholar]
- Jenssen H, Hancock REW. Antimicrobial properties of lactoferrin. Biochimie. 2009;91:19–29. doi: 10.1016/J.BIOCHI.2008.05.015. [DOI] [PubMed] [Google Scholar]
- Kalelkar PP, Riddick M. García AJ (2021) Biomaterial-based antimicrobial therapies for the treatment of bacterial infections. Nat Rev Mater. 2021;7(1):39–54. doi: 10.1038/S41578-021-00362-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawazoe A, Inubushi T, Miyauchi M, et al. Orally administered liposomal lactoferrin inhibits inflammation-related bone breakdown without interrupting orthodontic tooth movement. J Periodontol. 2013;84:1454–1462. doi: 10.1902/JOP.2012.120508. [DOI] [PubMed] [Google Scholar]
- Kilic E, Novoselova MV, Lim SH, et al. Formulation for oral delivery of lactoferrin based on bovine serum albumin and tannic acid multilayer microcapsules open. Sci Rep. 2017 doi: 10.1038/srep44159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SE, Lee DW, Yun YP, et al. Heparin-immobilized hydroxyapatite nanoparticles as a lactoferrin delivery system for improving osteogenic differentiation of adipose-derived stem cells. Biomed Mater. 2016;11:025004. doi: 10.1088/1748-6041/11/2/025004. [DOI] [PubMed] [Google Scholar]
- Kim SE, Yun YP, Shim KS, et al. Effect of lactoferrin-impregnated porous poly(lactide-co-glycolide) (PLGA) microspheres on osteogenic differentiation of rabbit adipose-derived stem cells (rADSCs) Colloids Surf B Biointerfaces. 2014;122:457–464. doi: 10.1016/J.COLSURFB.2014.06.057. [DOI] [PubMed] [Google Scholar]
- King JC, Cummings GE, Guo N, et al. A double-blind, placebo-controlled, pilot study of bovine lactoferrin supplementation in bottle-fed infants. J Pediatr Gastroenterol Nutr. 2007;44:245–251. doi: 10.1097/01.MPG.0000243435.54958.68. [DOI] [PubMed] [Google Scholar]
- Kowalczyk P, Kaczyńska K, Kleczkowska P, et al. The lactoferrin phenomenon—A miracle molecule. Molecules. 2022 doi: 10.3390/molecules27092941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogstad PA (2021) Hematogenous osteomyelitis in children: Epidemiology, pathogenesis, and microbiology - UpToDate. https://www.uptodate.com/contents/hematogenous-osteomyelitis-in-children-epidemiology-pathogenesis-and-microbiology?search=paediatric%20contiguous%20osteomyelitis&source=search_result&selectedTitle=4~150&usage_type=default&display_rank=4. Accessed 23 Apr 2022
- Kumar H. Development and evaluation of a b-glucan biopolymer formulation of lactoferrin produced using a novel cryomilling technique. Auckland: University of Auckland; 2010. [Google Scholar]
- Kumar H, Wen J, Shaw J, et al. Physiochemical characterization of b-glucan and in vitro release of lactoferrin from b-glucan microparticles. Curr Drug Deliv. 2013;10:713–721. doi: 10.2174/15672018113109990043. [DOI] [PubMed] [Google Scholar]
- Lalani T, Schmitt SK (2022) Nonvertebral osteomyelitis in adults: Clinical manifestations and diagnosis - UpToDate. https://www.uptodate.com/contents/nonvertebral-osteomyelitis-in-adults-clinical-manifestations-and-diagnosis?search=osteomyelitis&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1#H3897835854. Accessed 23 Apr 2022
- Liu W, Ye A, Liu W, et al. Stability during in vitro digestion of lactoferrin-loaded liposomes prepared from milk fat globule membrane-derived phospholipids. J Dairy Sci. 2013;96:2061–2070. doi: 10.3168/JDS.2012-6072. [DOI] [PubMed] [Google Scholar]
- Liu M. Development of novel solid lipid nanoparticles enriched hydrogels for topical delivery of L-Glutathione. Auckland: University of Auckland; 2019. [Google Scholar]
- López-Machado A, Díaz N, Cano A, et al. Development of topical eye-drops of lactoferrin-loaded biodegradable nanoparticles for the treatment of anterior segment inflammatory processes. Int J Pharm. 2021;609:121188. doi: 10.1016/J.IJPHARM.2021.121188. [DOI] [PubMed] [Google Scholar]
- López-Machado A, Díaz-Garrido N, Cano A, et al. Development of lactoferrin-loaded liposomes for the management of dry eye disease and ocular inflammation. Pharmaceutics. 2021 doi: 10.3390/PHARMACEUTICS13101698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters EA, Trombetta RP, de Mesy Bentley KL, et al. Evolving concepts in bone infection: redefining “biofilm”, “acute vs. chronic osteomyelitis”, “the immune proteome” and “local antibiotic therapy”. Bone Res. 2019 doi: 10.1038/S41413-019-0061-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi M, Hamishehkar H, McClements DJ, et al. Encapsulation of Spirulina protein hydrolysates in liposomes: Impact on antioxidant activity and gastrointestinal behavior. Food Chem. 2023;400:133973. doi: 10.1016/J.FOODCHEM.2022.133973. [DOI] [PubMed] [Google Scholar]
- Momodu II, Savaliya V (2022) Osteomyelitis - StatPearls - NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK532250/. Accessed 23 Apr 2022
- Montesi M, Panseri S, Iafisco M, et al. Coupling hydroxyapatite nanocrystals with lactoferrin as a promising strategy to fine regulate bone homeostasis. PLoS ONE. 2015 doi: 10.1371/journal.pone.0132633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montesi M, Panseri S, Iafisco M, et al. Effect of hydroxyapatite nanocrystals functionalized with lactoferrin in osteogenic differentiation of mesenchymal stem cells. J Biomed Mater Res A. 2015;103:224–234. doi: 10.1002/JBM.A.35170. [DOI] [PubMed] [Google Scholar]
- Moutinho CG, Matos CM, Teixeira JA, Balcão VM. Nanocarrier possibilities for functional targeting of bioactive peptides and proteins: state-of-the-art. J Drug Target. 2012;20:114–141. doi: 10.3109/1061186X.2011.628397. [DOI] [PubMed] [Google Scholar]
- Murugan R, Liao SS, Ramakrishna S, et al. Skeletal Regenerative Nanobiomaterials. In: Hussain NS, Santos JD, et al., editors. Biomaterials for bone, regenerative medicine. 1. Limited: Trans Tech Publications; 2010. pp. 1–35. [Google Scholar]
- Naot D, Grey A, Reid IR, Cornish J. Lactoferrin-A novel bone growth factor. Clin Med Res. 2004;3:93–101. doi: 10.3121/cmr.3.2.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naot D, Chhana A, Matthews BG, et al. Molecular mechanisms involved in the mitogenic effect of lactoferrin in osteoblasts. Bone. 2011 doi: 10.1016/j.bone.2011.04.002. [DOI] [PubMed] [Google Scholar]
- Naseri N, Valizadeh H, Zakeri-Milani P. Solid lipid nanoparticles and nanostructured lipid carriers: structure preparation and application. Adv Pharm Bull. 2015;5(3):305. doi: 10.15171/apb.2015.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nocerino N, Fulgione A, Iannaccone M, et al. Biological activity of lactoferrin-functionalized biomimetic hydroxyapatite nanocrystals. Int J Nanomedicine. 2014;9:1175–1184. doi: 10.2147/IJN.S55060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nojima Y, Suzuki Y, Iguchi K, et al. Development of poly(ethylene glycol) conjugated lactoferrin for oral administration. Bioconjug Chem. 2008;19:2253–2259. doi: 10.1021/BC800258V/ASSET/IMAGES/LARGE/BC-2008-00258V_0005.JPEG. [DOI] [PubMed] [Google Scholar]
- Nojima Y, Suzuki Y, Yoshida K, et al. Lactoferrin conjugated with 40-kDa branched poly(ethylene glycol) has an improved circulating half-life. Pharm Res. 2009;26:2125–2132. doi: 10.1007/S11095-009-9925-Z. [DOI] [PubMed] [Google Scholar]
- Onishi H. Lactoferrin delivery systems: approaches for its more effective use. Expert Opin Drug Deliv. 2011;8:1469–1479. doi: 10.1517/17425247.2011.615829. [DOI] [PubMed] [Google Scholar]
- Patel MR, San Martin-Gonzalez MF. Characterization of ergocalciferol loaded solid lipid nanoparticles. J Food Sci. 2012;77:N8–N13. doi: 10.1111/J.1750-3841.2011.02517.X. [DOI] [PubMed] [Google Scholar]
- Pereira Rosa L, Cristina da Silva F, Alves Nader S, et al. Antimicrobial photodynamic inactivation of Staphylococcus aureus biofilms in bone specimens using methylene blue, toluidine blue ortho and malachite green: an in vitro study. J Oral Biol. 2015 doi: 10.1016/j.archoralbio.2015.02.010. [DOI] [PubMed] [Google Scholar]
- Pignatello R, Leonardi A, Fuochi V, et al. A method for efficient loading of ciprofloxacin hydrochloride in cationic solid lipid nanoparticles: formulation and microbiological evaluation. Nanomaterials. 2018 doi: 10.3390/NANO8050304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieels J-P, Pizzo SV, Glasgow LR, et al. Hepatic receptor that specifically binds oligosaccharides containing fucosyl α 1 → 3 N-acetylglucosamine linkages. Proc Natl Acad Sci USA. 1978;75:2215–2219. doi: 10.1073/pnas.75.5.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roohinejad S, Greiner R, Oey I, Wen J (eds) (2018) Emulsion-based systems for delivery of food active compounds: Formation, application, health and safety. Wiley, New York
- Rosa L, Lepanto MS, Cutone A, Siciliano RA, Paesano R, Costi R, Musci G, Valenti P. Influence of oral administration mode on the efficacy of commercial bovine lactoferrin against iron and inflammatory homeostasis disorders. Biometals. 2020;33:159–168. doi: 10.1007/s10534-020-00236-2. [DOI] [PubMed] [Google Scholar]
- Rosa L, Tripepi G, Naldi E, et al. Ambulatory covid-19 patients treated with lactoferrin as a supplementary antiviral agent: a preliminary study. J Clin Med. 2021 doi: 10.3390/JCM10184276/S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayed YRS (2017) Formulation of solid lipid nanoparticles SLN using chitosan to form oral insulin - YouTube. https://www.youtube.com/watch?v=1xLWMzcQcf0. Accessed 8 May 2022
- Schmitt SK. Osteomyelitis. Infect Dis Clin North Am. 2017;31:325–338. doi: 10.1016/j.idc.2017.01.010. [DOI] [PubMed] [Google Scholar]
- Scholz C. The molecular structure of degradable polymers. In: Wuisman PI, Smit TH, editors. Degradable polymers for skeletal implants. Hauppauge: Nova Science Publishers; 2009. pp. 3–20. [Google Scholar]
- Shadvar P, Mirzaie A, Yazdani S. Fabrication and optimization of amoxicillin-loaded niosomes: an appropriate strategy to increase antimicrobial and anti-biofilm effects against multidrug-resistant Staphylococcus aureus strains. Drug Dev Ind Pharm. 2022 doi: 10.1080/03639045.2022.2027958/FORMAT/EPUB. [DOI] [PubMed] [Google Scholar]
- Sherman MP, Adamkin DH, Niklas V, et al. Randomized controlled trial of talactoferrin oral solution in preterm infants. J Pediatr. 2016;175:68–73. doi: 10.1016/j.jpeds.2016.04.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi P, Wang Q, Yu C, et al. Hydroxyapatite nanorod and microsphere functionalized with bioactive lactoferrin as a new biomaterial for enhancement bone regeneration. Colloids Surf B Biointerfaces. 2017;155:477–486. doi: 10.1016/J.COLSURFB.2017.04.042. [DOI] [PubMed] [Google Scholar]
- Shiga Y, Oshima Y, Kojima Y, et al. Recombinant human lactoferrin-Fc fusion with an improved plasma half-life. Eur J Pharm Sci. 2015;67:136–143. doi: 10.1016/J.EJPS.2014.11.005. [DOI] [PubMed] [Google Scholar]
- Shimizu H. Development of an enteric-coated lactoferrin tablet and its application. Biometals. 2004;17:343–347. doi: 10.1023/B:BIOM.0000027715.72746.03. [DOI] [PubMed] [Google Scholar]
- Sienkiewicz M, Jaśkiewicz A, Tarasiuk A, Fichna J. Lactoferrin: an overview of its main functions, immunomodulatory and antimicrobial role, and clinical significance. Crit Rev Food Sci Nutr. 2021 doi: 10.1080/10408398.2021.1895063. [DOI] [PubMed] [Google Scholar]
- Siqueiros-Cendón T, Arévalo-Gallegos S, Iglesias-Figueroa BF, et al. Immunomodulatory effects of lactoferrin. Acta Pharmacol Sin. 2014;35:557–566. doi: 10.1038/APS.2013.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MC, Crist RM, Clogston JD, McNeil SE. Zeta potential: a case study of cationic, anionic, and neutral liposomes. Anal Bioanal Chem. 2017;409:5779–5788. doi: 10.1007/S00216-017-0527-Z. [DOI] [PubMed] [Google Scholar]
- Takeuchi T, Kitagawa H, Harada E. Evidence of lactoferrin transportation into blood circulation from intestine via lymphatic pathway in adult rats. Exp Physiol. 2004;89:263–270. doi: 10.1113/EXPPHYSIOL.2003.026633. [DOI] [PubMed] [Google Scholar]
- Thorn CR, Thomas N, Boyd BJ, Prestidge CA. Nano-fats for bugs: the benefits of lipid nanoparticles for antimicrobial therapy. Drug Deliv Transl Res. 2021;11:1598–1624. doi: 10.1007/S13346-021-00921-W/FIGURES/12. [DOI] [PubMed] [Google Scholar]
- Tonguc-Altin K, Sandalli N, Duman G, et al. Development of novel formulations containing Lysozyme and Lactoferrin and evaluation of antibacterial effects on Mutans Streptococci and Lactobacilli. Arch Oral Biol. 2015 doi: 10.1016/j.archoralbio.2015.02.004. [DOI] [PubMed] [Google Scholar]
- Trif M, Guillen C, Vaughan DM, et al. Liposomes as possible carriers for lactoferrin in the local treatment of inflammatory diseases. Exp Biol Med. 2001;226:559–564. doi: 10.1177/153537020122600608. [DOI] [PubMed] [Google Scholar]
- Trif M, Guillen C, Vaughan DM, et al. Liposomes as possible carriers for lactoferrin in the local treatment of inflammatory diseases. Exp Biol Med. 2016;226:559–564. doi: 10.1177/153537020122600608. [DOI] [PubMed] [Google Scholar]
- Troost FJ, Steijns J, Saris WHM, Brummer RJM. Gastric digestion of bovine lactoferrin in vivo in adults. J Nutr. 2001;131:2101–2104. doi: 10.1093/JN/131.8.2101. [DOI] [PubMed] [Google Scholar]
- van Snick JL, Masson PL, Heremans JF. The involvement of lactoferrin in the hyposideremia of acute inflammation. J Exp Med. 1974;140:1068–1084. doi: 10.1084/jem.140.4.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Veen HA, Geerts MEJ, van Berkel PHC, Nuijens JH. Analytical cation-exchange chromatography to assess the identity, purity, and N-terminal integrity of human lactoferrin. Anal Biochem. 2002;309:60–66. doi: 10.1016/S0003-2697(02)00273-7. [DOI] [PubMed] [Google Scholar]
- Varela-Fernández R, García-Otero X, Díaz-Tomé V, et al. Lactoferrin-loaded nanostructured lipid carriers (NLCs) as a new formulation for optimized ocular drug delivery. Eur J Pharm Biopharm. 2022;172:144–156. doi: 10.1016/J.EJPB.2022.02.010. [DOI] [PubMed] [Google Scholar]
- Vergara D, Shene C. Encapsulation of lactoferrin into rapeseed phospholipids based liposomes: optimization and physicochemical characterization. J Food Eng. 2019;262:29–38. doi: 10.1016/J.JFOODENG.2019.05.012. [DOI] [Google Scholar]
- Vergara D, López O, Bustamante M, Shene C. An in vitro digestion study of encapsulated lactoferrin in rapeseed phospholipid–based liposomes. Food Chem. 2020;321:126717. doi: 10.1016/J.FOODCHEM.2020.126717. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Marshall JC, Dellinger RP, et al. Talactoferrin in severe sepsis: results from the phase II/III oral talactoferrin in severe sepsis trial. Crit Care Med. 2015;43:1832–1838. doi: 10.1097/CCM.0000000000001090. [DOI] [PubMed] [Google Scholar]
- Vogel HJ. Lactoferrin, a bird’s eye view. Biochem Cell Biol. 2012;90:233–245. doi: 10.1139/O2012-016. [DOI] [PubMed] [Google Scholar]
- Wang B, Timilsena YP, Blanch E, Adhikari B. Lactoferrin: structure, function, denaturation and digestion. Crit Rev in Food Sci Nutr. 2017;59:580–596. doi: 10.1080/10408398.2017.1381583. [DOI] [PubMed] [Google Scholar]
- Webb R, Wilson E, Voss L, et al (2022) Osteomyelitis. https://starship.org.nz/guidelines/osteomyelitis/. Accessed 24 Apr 2022
- Wei B, Wang W, Liu X, et al. Gelatin methacrylate hydrogel scaffold carrying resveratrol-loaded solid lipid nanoparticles for enhancement of osteogenic differentiation of BMSCs and effective bone regeneration. Regen Biomater. 2021;8:1–14. doi: 10.1093/RB/RBAB044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss PP, Lamichhane SP, Abed A, et al. Renal clearance of polymeric nanoparticles by mimicry of glycan surface of viruses. Biomaterials. 2020 doi: 10.1016/j.biomaterials.2019.119643. [DOI] [PubMed] [Google Scholar]
- Yamano E, Miyauchi M, Furusyo H, et al. Inhibitory effects of orally administrated liposomal bovine lactoferrin on the LPS-induced osteoclastogenesis. Lab Invest. 2010;90:1236–1246. doi: 10.1038/LABINVEST.2010.80. [DOI] [PubMed] [Google Scholar]
- Yanagisawa S, Nagasaki K, Chea C, et al. Oral administration of bovine lactoferrin suppresses the progression of rheumatoid arthritis in an SKG mouse model. PLoS ONE. 2022;17:e0263254–e0263254. doi: 10.1371/JOURNAL.PONE.0263254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Deng C, Xu L, et al. Protein-neutral polysaccharide nano- and micro-biopolymer complexes fabricated by lactoferrin and oat β-glucan: structural characteristics and molecular interaction mechanisms. Food Res Int. 2020;132:109111. doi: 10.1016/J.FOODRES.2020.109111. [DOI] [PubMed] [Google Scholar]
- Yao X. Development of a novel drug delivery system to enhance the oral bioavailability of lactoferrin. Auckland: University of Auckland; 2015. [Google Scholar]
- Yao X, Bunt C, Cornish J, et al. Improved RP-HPLC method for determination of bovine lactoferrin and its proteolytic degradation in simulated gastrointestinal fluids. Biomed Chromatogr. 2013;27:197–202. doi: 10.1002/BMC.2771. [DOI] [PubMed] [Google Scholar]
- Yao X, Bunt C, Cornish J, et al. Stability of bovine lactoferrin in luminal extracts and mucosal homogenates from rat intestine: a prelude to oral absorption. Chem Biol Drug Des. 2014;84:676–684. doi: 10.1111/cbdd.12360. [DOI] [PubMed] [Google Scholar]
- Yao X, Bunt C, Cornish J, et al. Preparation, optimization and characterization of bovine lactoferrin-loaded liposome and solid lipid particles modified by hydrophilic polymers using factorial design. Chem Biol Drug Des. 2014;83:560–575. doi: 10.1111/cbdd.12269. [DOI] [PubMed] [Google Scholar]
- Yao X, Bunt C, Cornish J, et al. Oral delivery of bovine lactoferrin using pectin- and chitosan-modified liposomes and solid lipid particles: improvement of stability of lactoferrin. Chem Biol Drug Des. 2015;86:466–475. doi: 10.1111/cbdd.12509. [DOI] [PubMed] [Google Scholar]


