Abstract
In the first 2 years of the coronavirus disease 2019 pandemic, influenza transmission decreased substantially worldwide, meaning that health systems were not faced with simultaneous respiratory epidemics. In 2022, however, substantial influenza transmission returned to Nicaragua where it co-circulated with severe acute respiratory syndrome coronavirus 2, causing substantial disease burden.
Keywords: incidence rate, influenza, Nicaragua, SARS-CoV-2, vaccination
Early in the coronavirus disease 2019 (COVID-19) pandemic, influenza circulation collapsed globally, including in Nicaragua where only 5 cases of influenza (80% influenza B) were detected in 2021 [1, 2]. While concerns about the possibility of influenza and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) co-circulation were raised in the lead-up to the typical Northern Hemisphere influenza season in late 2020 and 2021, they did not materialize. Recently, however, substantial influenza transmission has returned to the Southern Hemisphere and tropical settings including Nicaragua (where prepandemic influenza transmission typically occurred between May and November and often with dual peaks), suggesting this reprieve is likely over [3]. While vaccine coverage during the early phase of the pandemic was higher than prepandemic levels, it decreased last season (2021–2022) [2, 4, 5]. Given the resurgence of influenza in 2022, this represents a worrying trend as the typical Northern Hemisphere influenza season approaches. Here we describe substantial influenza and SARS-CoV-2 co-circulation within a prospective, community-based household study in Managua, Nicaragua, and consider its implications for the looming fall/winter season in the Northern Hemisphere.
METHODS
The Household Influenza Cohort Study is an ongoing, community-based prospective cohort study in Managua, Nicaragua [6]. Participants presented to the study clinic upon the development of an acute illness and respiratory samples were collected from those meeting the testing criteria (fever/feverishness, conjunctivitis, rash, or loss of taste or smell). Respiratory samples were tested for influenza (using Centers for Disease Control and Prevention protocols) and SARS-CoV-2 by real time reverse-transcription polymerase chain reaction (RT-PCR) [7]. Samples were also collected from household members, regardless of symptoms, following the positive test (for influenza or SARS-CoV-2) of another household member [6].
Clinical Definitions
Illness severity was classified using symptom diaries and data from clinic visits [7]. Specifically, illnesses involving hospitalization, difficulty or rapid breathing, crepitus, chest wall indrawing, rhonchi, wheezing, and overall poor condition were classified as moderate/severe, while those with no symptoms or other symptom presentations were classified as mild/asymptomatic. Those requiring transfer to the hospital within 28 days of illness onset were classified as hospitalized. To assess whether the number of symptomatic influenza/SARS-CoV-2 coinfections we observed differed from the number we would expect (if circulation was independent), we pooled samples from the Household Influenza Cohort with those from the Nicaraguan Pediatric Influenza Cohort Study who met the same testing criteria for symptomatic illness. This was not done when calculating the incidence rate of coinfections to allow for comparability against single infections. Samples positive for both influenza and SARS-CoV-2 (via real-time RT-PCR) were considered coinfections.
Statistical Analysis
Incidence rates were calculated using a Poisson distribution [8] while the observed and expected number of coinfections were compared using the χ2 test. The attack rates for influenza A(H3N2) and SARS-CoV-2 were calculated among those participants who were enrolled during the entire study period (number of cases of each pathogen/total number of participants). To assess what these attack rates would look like in the United States (US), we standardized estimates using the 2022 World Population Prospects from the United Nations [9]. All statistical analyses were completed using R version 4.2.1 software.
Patient Consent
This study was approved by the institutional review boards at the University of Michigan and the Nicaraguan Ministry of Health. Informed consent and parental approval (for minors) was obtained for all participants and assent was obtained from all children aged ≥6 years.
RESULTS
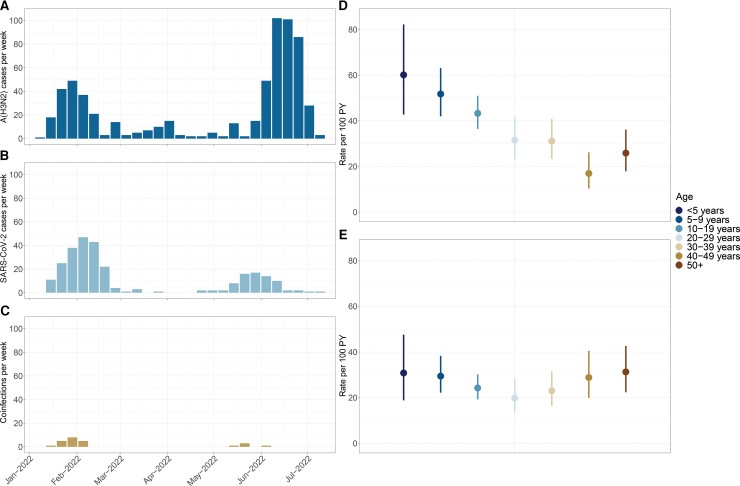
We examined influenza and SARS-CoV-2 infections and coinfections among 2117 participants (62.5% female) aged 0–89 years from 1 January to 20 July 2022. Overall, there were 433 influenza A(H3N2) infections (incidence rate of 37.6 per 100 person-years [PY]; 95% confidence interval [CI], 34.1–41.3), 296 SARS-CoV-2 infections (26.0 per 100 PY; 95% CI, 23.1–29.1), and 24 coinfections (2.6 per 100 PY; 95% CI, 1.7–3.9). SARS-CoV-2 infections occurred in 2 waves: the first (January–March) was dominated by BA.1, while the second (May–July) consisted largely of BA.2 (Supplementary Figure 1). Rates of influenza peaked among the youngest participants (aged <5 years) and steadily decreased thereafter. Rates of SARS-CoV-2 by age displayed a slight V-shaped trend (Figure 1). We observed no meaningful difference in incidence by sex (Supplementary Table 1). Similarly, we observed no difference in the age distribution between waves 1 and 2 for influenza (P = .24) or SARS-CoV-2 (P = .76). Looking at detections by household, 174 (40.1%) households experienced influenza A(H3N2), 105 (28.2%) had SARS-CoV-2, and 38 (10.7%) had both.
Figure 1.
Influenza and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in the cohort. A–C, Number of cases per week for influenza A(H3N2) (A), SARS-CoV-2 (B), and A(H3N2)/SARS-CoV-2 coinfections (C). D and E, Incidence rate (per 100 person-years [PY]) by age for influenza A(H3N2) and SARS-CoV-2, respectively.
Clinical Presentation and Severity
In total, 3 participants required hospitalization (2 with SARS-CoV-2, 1 with influenza A(H3N2) infections). No coinfected participants required hospitalization. A greater proportion of SARS-CoV-2 cases were classified as moderate/severe compared to influenza A(H3N2) (9.6% vs 4.2%, P = .004), despite the study population having a high level of hybrid immunity (prior infection and vaccination). However, no difference was observed among children (3.4% vs 5.5%, P = .4, Supplementary Table 3) nor when hospitalizations were compared (0.7% vs 0.2%, P = 1.0, Supplementary Table 3). While the most frequent symptom combinations were similar across infection types (fever and upper respiratory symptoms; Supplementary Figure 2), a greater proportion of SARS-CoV-2 infections presented with cough, myalgia, and arthralgia compared to influenza (Supplementary Table 2). However, a greater proportion of coinfected participants had fever compared to those with SARS-CoV-2 infections (P = .03).
Dual Burden
Influenza A(H3N2) and SARS-CoV-2 co-circulated for 22 of 29 (75.9%) of the study weeks. The influenza attack rate was 20.1% (95% CI, 18.4%–21.8%), whereas the attack rate of SARS-CoV-2 was 13.6% (95% CI, 12.2%–15.1%) (Table 1). When standardized to the age distribution of the US, which is older than Nicaragua and our cohort, we found similarly high attack rates, specifically 17.2% (95% CI, 14.0%–20.4%) for influenza and 14.3% (95% CI, 12.7%–16.0%) for SARS-CoV-2. In children aged 2–14 years, the attack rate of influenza was 26.8% (95% CI, 23.7%–29.9%) compared to an attack rate of 15.3% (95% CI, 12.7%–17.8%) for SARS-CoV-2. Indeed, when compared to prepandemic influenza seasons in the cohort (overall, 14.5 per 100 PY; range, 8.0–21.6) [10], the 2022 incidence rate to date, assuming no additional circulation, is substantially higher at 28.6 (95% CI, 25.0–32.5) per 100 PY. We observed approximately the expected number of symptomatic influenza/SARS-CoV-2 coinfections (P = .39; Supplementary Table 4).
Table 1.
Attack Rates of Influenza A(H3N2) and Severe Acute Respiratory Syndrome Coronavirus 2
| Infection | Attack Rate (Age 0–89 y) |
Attack Rate (Age 2–14 y) |
|---|---|---|
| This study | ||
| Influenza A(H3N2) | 20.1% (18.4–21.8) | 26.8% (23.7–29.9) |
| SARS-CoV-2 | 13.6% (12.2–15.1) | 15.3% (12.7–17.8) |
| 2009 influenza A(H1N1) pandemic [11] | … | 20.1% (18.8–21.4) |
Data are presented as attack rate (95% confidence interval).
Abbreviation: SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
DISCUSSION
Here we observed substantial simultaneous burden of influenza A(H3N2) and SARS-CoV-2 within a prospective, community-based household cohort in Managua, Nicaragua. Influenza and SARS-CoV-2 co-circulated for most of the study period and the number of coinfections was near what we would expect if the distribution of the pathogens were independent. This suggests limited viral interference and that the primary danger of co-circulation is high rates of single infections occurring concurrently. In fact, the estimated attack rate for influenza in children aged 2 to 14 years (26.8%) was higher than that seen in this population during the 2009 influenza A(H1N1) pandemic [11], and this was on top of a SARS-CoV-2 attack rate of 13.6%. Taken together, this represents a substantial overall burden on the health system. When standardized to the age distribution of the US, the influenza attack rate is slightly lower and SARS-CoV-2 is slightly higher, though the differences were not significant. It should also be noted that while influenza vaccination in the US decreased in 2021–2022, rates remain higher than in Nicaragua where efforts are focused primarily on pregnant women and young children [12]. SARS-CoV-2 seroprevalence also plays an important role. In Nicaragua, the majority of the population has previously been infected with SARS-CoV-2, and many have also been vaccinated [13]. Given the older age distribution in the US, we anticipate that similar levels of co-circulation may in fact lead to greater rates of illness and even with similar levels of severity, this would result in more severe disease.
The high attack rates in children are also concerning as they suggest the potential for substantial morbidity (including ambulatory healthcare visits, hospitalization, and even death) and further school disruptions. Furthermore, pediatric influenza vaccination coverage has steadily decreased since the start of the pandemic, even when adult vaccination coverage remained high. Additionally, though vaccines against SARS-CoV-2 have been approved for children in the US, vaccination coverage remains quite low among those aged <12 years. In fact, only 38% of 5- to 11-year-olds and 7% of children aged 6 months to 4 years have received at least 1 COVID-19 vaccine dose [14].
This study has several strengths. First, as this was a longitudinal, community-based study, we were able to calculate incidence rates of both SARS-CoV-2 and influenza in the population. Second, the study design involved testing asymptomatic participants following household activation, which improves the accuracy of these incidence measures by better capturing subclinical infections. Finally, studies have explored the burden and transmission of influenza in this community for >15 years, providing important context for these new estimates.
This study does have some limitations. While we failed to detect a difference in the number of observed and expected coinfections, the relatively small number (n = 48) precludes us from ruling out the possibility of viral interference. We also were unable to look at the potential for variant- or time-specific interaction. Additionally, we were only able to assess the number of symptomatic coinfections, so this likely represents an underestimate of the total coinfection burden (asymptomatic and symptomatic). Similarly, as a prospective, community-based study, we were underpowered to capture the most severe manifestations of both SARS-CoV-2 and influenza, which limits our ability to make severity-specific inferences. Though we believe the high incidence rates of influenza that we observed are suggestive of increased influenza susceptibility, we did not assess this directly. Finally, generalizing these findings to other populations should be done with appropriate consideration of differences in population-level immunity to both SARS-CoV-2 and influenza and the means through which the immunity was obtained (ie, infection and/or vaccination).
In this study we describe substantial concurrent circulation of influenza and SARS-CoV-2 within a prospective, community-based cohort. These findings suggest that increased susceptibility to influenza after low-circulation places populations at significant risk of having dual epidemics of influenza and SARS-CoV-2. That this is likely to be worse in populations with lower prior SARS-CoV-2 infection rates, further highlighting that vaccination against both SARS-CoV-2 and influenza is imperative this coming season.
Supplementary Material
Contributor Information
John T Kubale, ICPSR, University of Michigan, Ann Arbor, Michigan, USA.
Aaron M Frutos, School of Public Health, University of Michigan, Ann Arbor, Michigan, USA.
Angel Balmaseda, Centro Nacional de Diagnóstico y Referencia, Ministry of Health, Managua, Nicaragua; Sustainable Sciences Institute, Managua, Nicaragua.
Cristhiam Cerpas, Centro Nacional de Diagnóstico y Referencia, Ministry of Health, Managua, Nicaragua; Sustainable Sciences Institute, Managua, Nicaragua.
Saira Saborio, Centro Nacional de Diagnóstico y Referencia, Ministry of Health, Managua, Nicaragua; Sustainable Sciences Institute, Managua, Nicaragua.
Sergio Ojeda, Sustainable Sciences Institute, Managua, Nicaragua.
Carlos Barilla, Centro Nacional de Diagnóstico y Referencia, Ministry of Health, Managua, Nicaragua.
Nery Sanchez, Sustainable Sciences Institute, Managua, Nicaragua.
Gerald Vasquez, Centro Nacional de Diagnóstico y Referencia, Ministry of Health, Managua, Nicaragua.
Hanny Moreira, Centro Nacional de Diagnóstico y Referencia, Ministry of Health, Managua, Nicaragua.
Abigail Shotwell, School of Public Health, University of Michigan, Ann Arbor, Michigan, USA.
Alyssa Meyers, School of Public Health, University of Michigan, Ann Arbor, Michigan, USA.
Roger Lopez, Centro Nacional de Diagnóstico y Referencia, Ministry of Health, Managua, Nicaragua; Sustainable Sciences Institute, Managua, Nicaragua.
Miguel Plazaola, Sustainable Sciences Institute, Managua, Nicaragua.
Guillermina Kuan, Sustainable Sciences Institute, Managua, Nicaragua; Centro de Salud Sócrates Flores Vivas, Ministry of Health, Managua, Nicaragua.
Aubree Gordon, School of Public Health, University of Michigan, Ann Arbor, Michigan, USA.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank the study participants and their families, along with the study staff at Centro de Salud Sócrates Flores Vivas and the Centro Nacional de Diagnóstico y Referencia.
Financial support. This work was supported by the National Institute for Allergy and Infectious Diseases at the US National Institutes of Health (grant numbers R01 AI120997 and U01 AI144616 and contract number HHSN272201400006C).
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. Influenza laboratory surveillance information—virus detections by subtype reported to FluNet. https://www.who.int/tools/flunet. Accessed 2 August 2022.
- 2.Centers for Disease Control and Prevention. Influenza (flu)—past weekly surveillance reports. [. Accessed 19 August 2022.]. https://www.cdc.gov/flu/weekly/pastreports.htm
- 3.Australian Department of Health and Aged Care. Australian influenza surveillance report and activity updates—2022. [. Accessed 28 July 2022.]. https://www1.health.gov.au/internet/main/publishing.nsf/Content/cda-ozflu-2022.htm
- 4.Centers for Disease Control and Prevention. Influenza (flu)—coverage by season. [. Accessed 19 August 2022.]. https://www.cdc.gov/flu/fluvaxview/coverage-by-season.htm
- 5. Centers for Disease Control and Prevention. Weekly flu vaccination dashboard—data summary for the 2021–22 flu season. https://www.cdc.gov/flu/fluvaxview/dashboard/vaccination-dashboard.html. Accessed 7 September 2022.
- 6. Maier HE, Kuan G, Saborio S, et al. Clinical spectrum of SARS-CoV-2 infection and protection from symptomatic re-infection. Clin Infect Dis 2021; 75:e257–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chu DKW, Pan Y, Cheng SMS, et al. Molecular diagnosis of a novel coronavirus (2019-nCoV) causing an outbreak of pneumonia. Clin Chem 2020; 66:549–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ulm K. A simple method to calculate the confidence interval of a standardized mortality ratio (SMR). Am J Epidemiol 1990; 131:373–5. [DOI] [PubMed] [Google Scholar]
- 9. United Nations Department of Economic and Social Affairs, Population Division. World population prospects 2022. 2022. https://population.un.org/wpp/Download/Standard/Population/. Accessed 12 September 2022.
- 10. Maier HE, Kuan G, Gresh L, et al. The Nicaraguan Pediatric Influenza Cohort Study, 2011–2019: influenza incidence, seasonality, and transmission [manuscript published online ahead of print 26 May 2022]. Clin Infect Dis 2022. doi: 10.1093/cid/ciac420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gordon A, Saborío S, Videa E, et al. Clinical attack rate and presentation of pandemic H1N1 influenza versus seasonal influenza A and B in a pediatric cohort in Nicaragua. Clin Infect Dis 2010; 50:1462–7. [DOI] [PubMed] [Google Scholar]
- 12. Arriola CS, Vasconez N, Thompson MG, et al. Association of influenza vaccination during pregnancy with birth outcomes in Nicaragua. Vaccine 2017; 35:3056–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Maier HE, Balmaseda A, Saborio S, et al. Protection associated with previous SARS-CoV-2 infection in Nicaragua. N Engl J Med 2022; 387:568–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. American Academy of Pediatrics. Children and COVID-19 vaccination trends—summary of data publicly reported by the Centers for Disease Control and Prevention. 2022. https://www.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/children-and-covid-19-vaccination-trends/. Accessed 12 September 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.