Abstract
High titers of anti-interferon-γ autoantibodies (AIGAs) are an important factor leading to persistent, relapsed, and refractory infections in HIV-negative hosts infected with Talaromyces marneffei (TM). We report 5 patients treated with pulses of high-dose intravenous cyclophosphamide (IVCY) who were followed for 2 years. Before IVCY therapy, all patients had multiple relapses, with a median (interquartile range [IQR]) of 2 (1–3) instances of relapse. The median serum AIGA titers (IQR) were 58 753 (41 203–89 605) ng/mL at diagnosis, 48 189.4 (15 537–83 375) ng/mL before IVCY therapy, and 10 721.2 (5637–13 245) ng/mL at the end of IVCY therapy (P < .05). After 3 months of follow-up, the median AIGA titers (IQR) rose gradually to 21 232.6 (9896–45 626) ng/mL, and to 37 464.2 (19 872–58 321) ng/mL at 24 months (P < .05). Five patients discontinued antimicrobial therapy within 3–12 months after completion of IVCY therapy, but only 1 patient had a relapse. In conclusion, pulses of short-term and high-dose IVCY can effectively reduce AIGA titers.
Keywords: anti-IFN-γ autoantibodies, intravenous cyclophosphamide therapy, Talaromyces marneffei
Anti-interferon (IFN)-γ autoantibodies (AIGAs) are anticytokine autoantibodies that are closely related to a variety of severe disseminated intracellular pathogenic infections, such as Talaromyces marneffei (TM), nontuberculous mycobacteria (NTM), and Salmonella [1–3]. In a previous study, we found that AIGAs were independent risk factors for TM infection in HIV-negative hosts and one of the most important immunodeficiency mechanisms in HIV-negative Talaromycosis marneffei (TSM) patients. Despite intensive antifungal therapy, >50% of patients have persistent or recurrent infections and experience multiple adverse drug effects [3–5]. This is related to the abnormal production of AIGAs, which can effectively block the IFN-γ signaling pathway, leading to a state of immunodeficiency. It has been reported that the use of anti-CD20 monoclonal antibody and cyclophosphamide to reduce AIGA titers can achieve good clinical efficacy in AIGA-associated NTM patients [6, 7]. However, rituximab is a high-cost drug that can only be used by a certain group of patients. In contrast, cyclophosphamide, which is less costly, has comparable efficacy to rituximab and has broader application prospects [8, 9]. Currently, there is no therapy available for AIGA-associated TM infection. Therefore, we report the use of immunotherapy with pulsed intravenous cyclophosphamide (IVCY) in 5 patients with AIGA-associated TM infection who had an ongoing increase in AIGAs and multiple relapses of TM infection.
METHODS
Patients and Methods
A prospective study was conducted among 5 HIV-negative patients over 18 years old at the First Affiliated Hospital of Guangxi Medical University who had been diagnosed with disseminated TSM and/or coinfection with other pathogens between 2018 and 2019. After a clear diagnosis of TSM, serum samples were tested for AIGAs for the first time and were identified as AIGA-positive, and the titers of AIGAs rose continuously. Before enrollment, all patients were regularly treated with at least 1 antifungal agent for TSM or drugs for other coinfections and had at least 1 relapse after improvement, with >6 months of antifungal therapy. No patients had other contraindications for cyclophosphamide before IVCY therapy, such as liver and kidney dysfunction, leucopenia, and pregnancy. After completion of IVCY therapy, 5 patients continued treatment for the primary infection and were followed up for 2 years.
This study was approved by the Faculty of Medicine at the First Affiliated Hospital of Guangxi Medical University (2021 [KY-E-262]). All patients provided written informed consent.
Treatment Regimens
Five enrolled patients were given pulsed IVCY at a dose of 0.8–1.0 mg/m2 every 3–4 weeks for 6 cycles until 6 months of therapy were completed. All patients received continuous antifungal or antimicrobial therapy for other pathogens.
Clinical Monitoring
Five patients received routine safety monitoring during IVCY therapy, including complete routine blood tests, routine urine tests, liver and kidney function chemistries, T-lymphocyte cell counts, quantitative immunoglobulin levels, and other biochemical indexes. AIGA titers were also monitored in all patients. Disease activity was evaluated by observing clinical signs and evidence of active infection on imaging examinations or pathogen pathology, culture, or smear. Treatment and clinical data were prospectively collected and documented.
Diagnostic Criteria for TM, NTM and Other Pathogens
Each patient fulfilled the diagnostic criteria for each disease. TM infection was diagnosed as follows: (1) Positive cultures for TM were characterized by dimorphic fungi that grew either as a mold at 25°C or as yeast at 37°C. (2) The yeast form of TM was confirmed by cytology and histopathology of tissues and secretions using Periodic Acid-Schiff (PAS) staining or Wright's stain, which revealed a characteristic morphology including a transverse septum [10]. NTM infection was diagnosed according to the guidelines of the 2007 American Thoracic Society (ATS)/Infectious Disease Society of America [10, 11]. Other pathogens were identified based on the positive culture of this pathogen from clinical specimens. TM and/or other pathogens were also identified in clinical specimens using metagenomics next-generation sequencing (mNGS) but still needed to meet the above criteria. Disseminated disease was defined as infection of at least 2 noncontiguous and sterile sites.
Definitions of Clinical Outcomes
(1) Cured (no recurrence of TM and/or NTM infection for at least 6 months after discontinuation of antifungal/anti-NTM therapy); (2) Improved (improvement in clinical symptoms, radiologic manifestations, laboratory tests after antifungal/anti-NTM treatment, but not cured). (3) persistent or relapsed infection (persistent infection: no improvement in clinical symptoms, radiologic manifestations, laboratory tests after 2 weeks of antifungal treatment/4 weeks of anti-NTM treatment; relapsed infection: improvement of clinical symptoms, radiologic manifestations, laboratory tests, negative pathogen detection after antifungal/anti-NTM effective treatment, followed by the reappearance of pathogen-associated infectious signs and/or positive pathogen testing).
Anti-IFN-γ Autoantibody Assay
AIGAs in the plasma were determined by enzyme-linked immunosorbent assay kits (USCN Life Science, Inc., Wuhan, China). To make this determination, first we added 100 µL each of standard, blank, and sample dilutions into appropriate wells and incubated them for 1 hour at 37°C. Then, we removed the liquid from each well and added 100 µL of detection reagent A. We incubated the wells for 1 hour at 37°C. Next, we used 1× wash solution, completely washed the wells 5 times, and removed any remaining wash buffer. Then, we added 90 µL of substrate solution to each well. These were incubated in the dark for 10–20 minutes at 37°C. Once the first 3 of the standard wells turned blue, we gently added 50 µL of stop solution to each well. After the liquid turned yellow, the color change was finally measured spectrophotometrically at a wavelength of 450 nm. The concentration of AIGAs in the sample was then determined by comparing the optical density of the sample to the standard curve. The positive titer value was determined to be 9583.21 ng/mL according to the methods used in our previous study [1].
CD4+ Cell and Peripheral Blood Mononuclear Cell Assay
Whole-blood samples from T. marneffei infection patients and healthy control subjects were collected in ethylene diaminetetraacetic acid-treated tubes and inert separation gel vacuum procoagulant collective tubes. Peripheral blood mononuclear cells (PBMCs) were separated by Lymphoprep (Stemcell Technologies, Canada) centrifugation. Briefly, fresh blood samples were mixed with an identical volume of phosphate buffer saline and were carefully placed on the surface of Lymphoprep separation medium. After centrifugation at 500×g for 20 minutes at 28°C, PBMCs were collected at the interphase and washed with phosphate buffer saline by centrifugation for 10 minutes at 300×g. Cells were stimulated in the presence of GolgiStop at 37°C in 5% CO2 for 5 hours. After stimulation, cells were surface-stained with an anti-CD4 monoclonal antibody (Percp-cy5.5; BD Pharmingen) for 30 minutes at 4°C. Intranuclear staining was performed with the use of anti-phosphor-signal transducer and activator of transcription 1 (STAT1; tyrosine 701) antibody (PE; BD Pharmingen). Data were collected with the use of FACS Can to flow cytometry (BD Biosciences) and analyzed with the use of FlowJo (Treestar).
Statistical Analysis
Data were analyzed using Statistical Package for the Social Sciences for Windows (SPSS), version 24. P values <.05 were considered statistically significant. Descriptive analysis was applied to demographic data. The Student t test and Fisher exact test were used to compare parameters between the 2 independent, unrelated patient groups.
RESULTS
Five HIV-negative patients with AIGA-associated TM infection were enrolled. The median age (interquartile range [IQR]) of the 5 patients (3 males and 2 females) was 53 (33–63) years. The underlying diseases or comorbidities included paroxysmal atrial fibrillation (n = 1), bronchial asthma (n = 1), autoimmune antibody abnormalities (n = 3); patient 2: (antinuclear antibody) ANA 1:100; patient 3: ANA 1:100, anticardiolipin antibody (+), anti-Ro-52 antibody (±); patient 4: ANA 1:320, anti-SSA antibody (+), anti-SSB antibody (+). All patients were diagnosed with disseminated TSM, with lymph node, lung, and bone involvement in 5 (5/5, 100%), skin involvement in 4 (4/5, 80%), and blood, liver, and spleen involvement in 2 patients (2/5, 40%). Four patients had NTM infection of the lung and bone or Salmonella infection of the blood and digestive tract (Table 1). All patients received antimicrobial treatment for the primary infection before, during, and after IVCY therapy, with a median duration (IQR) of 13 (12–14) months. All patients had multiple relapses, with a median (IQR) of 2 (1–3) relapses (Table 2).
Table 1.
Clinical Data Among Disseminated TM Patients Treated With IVCY
| Patient No. | Age/Sex | Organ Involvement | Underlying Diseases | Treatment Duration Before IVCY, mo | No. of Relapses Before IVCY Treatment | Other Opportunistic Infections/Organ |
|---|---|---|---|---|---|---|
| 1 | 63/M | Blood, lymph nodes, lung, liver, spleen, skin, multiple bones | … | 19 | 3 | M. chelonae: lung |
| 2 | 62/M | Lung, liver, multiple bones, lymph nodes | Atrial fibrillation ANA1:100 | 13 | 1 | … |
| 3 | 43/F | Lung, skin, blood, multiple bones, lymph nodes | Bronchial asthma Sweet's syndrome ANA1:100 anticardiolipin antibody (+), anti-Ro-52 antibody (±) | 12 | 3 |
M. abscessus: lung, bones Salmonella: blood, alimentary canal |
| 4 | 33/F | Lung, skin lymph nodes, multiple bones, liver, spleen | ANA 1:320, anti-SSA antibody (+), anti-SSB antibody (+) | 6 | 1 | M. abscessus: lung |
| 5 | 64/M | Lung, skin, lymph nodes, multiple bones, blood | … | 14 | 2 | Salmonella: blood |
Abbreviations: ANA, antinuclear antibody; IVCY, intravenous cyclophosphamide; NTM, nontuberculous mycobacteria; TM, Talaromyces marneffei.
Table 2.
Treatment and Outcome Among Disseminated TM Patients Treated With IVCY
| Patient No. | Parenteral Antibiotic Before IVCY | No. of IVCY Cycles/Total Dose/Duration | Parenteral Antibiotic During IVCY | Parenteral Antibiotic After IVCY | Follow-up After IVCY | Outcome |
|---|---|---|---|---|---|---|
| 1 | TM: AMB + voriconazole for 3 mo, changed to itraconazole for 3 mo, first relapse Then changed to voriconazole for 5 mo, second relapse Then AMB + voriconazole for 1 mo, changed to itraconazole for 4 mo, third relapse Then voriconazole for 3 mo, improved NTM: moxifloxacin + clarithromycin + ethambutol for 7 mo, improved |
6/5.8 g/5 mo | Voriconazole for 5 mo | TM: itraconazole for 6 mo NTM: moxifloxacin + clarithromycin for 12 mo |
2 y | Cured |
| 2 | TM: voriconazole for 8 mo, first relapse Then AMB for 1 mo, changed to voriconazole for 4 mo, improved |
6/5.6 g/5.5 mo | Voriconazole for 5.5 mo | Voriconazole for 3 mo | 2 y | Cured |
| 3 | TM: voriconazole for 2 mo, first relapse Then AMB for 1 mo, changed to itraconazole for 0.5 mo, second relapse Then AMB for 1 mo, changed to voriconazole for 4 mo, third relapse Then AMB for 0.5 mo, changed to voriconazole for 3 mo, improved NTM: moxifloxacin + clarithromycin + ethambutol for 4 mo, improved Salmonella: piperacillin/tazobactam + levofloxacin for 1 mo, cured |
6/5.4 g/5.5 mo | Voriconazole for 5.5 mo | TM: itraconazole 3 mo NTM: moxifloxacin + clarithromycin for 6 mo |
2 y | Relapsed with TM |
| 4 | TM: itraconazole for 2 mo, first relapse Then voriconazole for 2 mo, changed to itraconazole for 2 mo, improved NTM: moxifloxacin + clarithromycin + ethambutol for 4 mo, improved |
6/4.8 g/4.5 mo | Itraconazole for 4.5 mo | TM: itraconazole for 8 mo NTM: moxifloxacin + clarithromycin for 8 mo |
2 y | Cured |
| 5 | TM: voriconazole for 1 mo, changed to itraconazole for 1 mo, first relapse Then voriconazole for 3 mo, second relapse Then AMB for 1 mo, changed to voriconazole for 8 mo, improved Salmonella: imipenem for 0.5 mo, changed to piperacillin/tazobactam + levofloxacin for 0.5 mo, cured |
6/5.6 g/5 mo | Voriconazole for 5 mo | Voriconazole for 3 mo | 2 y | Cured |
Abbreviations: AMB, amphotericin B; IVCY, intravenous cyclophosphamide; NTM, nontuberculous mycobacteria; TM, Talaromyces marneffei.
Before the initiation of IVCY therapy, complete blood count examinations revealed that the median white blood cell count, neutrophil count, and absolute lymphocyte count (IQR) were 9.66 (6.58–10.03) × 109/L, 4.7 (6.7–7.79) × 109/L, and 2.32 (1.62–2.53) × 109/L, respectively. The median hemoglobin concentration, platelet count, and total bilirubin level (IQR) were 231 (126–276) g/L, 187 (132–283) × 1012/L, and 8.2 (7.6–10.1) µmol/L, respectively. Serum biochemical analysis showed a median serum albumin concentration and globulin concentration (IQR) of 36.3 (35.6–36.4) g/L and 37.4 (33.3–38.4) g/L, respectively. The median concentrations of aspartate aminotransferase, alanine aminotransferase, and creatinine (IQR) were 26 (20–31) U/L, 24 (23–36) U/L, and 68 (66–87) µmol/L, respectively. The median erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and procalcitonin (IQR) were 47 (37–49) mm/H, 32.5 (19.2–44.7), and 0.26 (0.19–0.55) ng/mL, respectively.
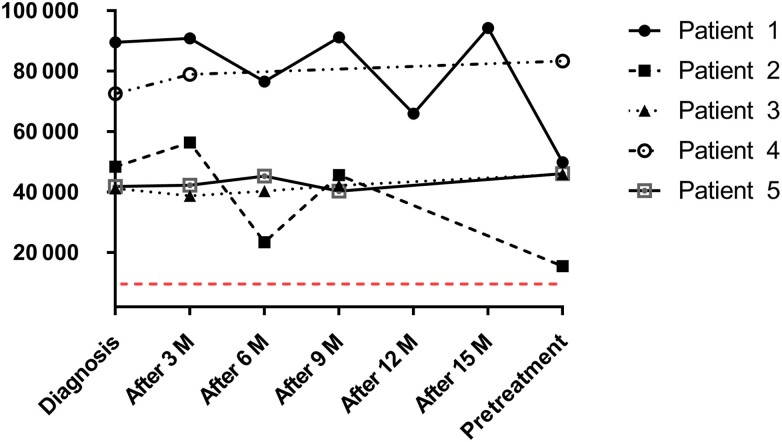
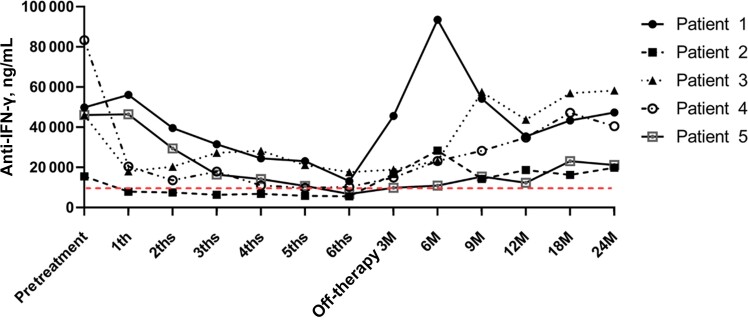
All patients received 6 pulses of IVCY and were treated with antimicrobial agents before and during IVCY therapy. AIGA titers were significantly elevated in 5 patients at diagnosis, with a median AIGA titer (IQR) of 48 450 (41 924–72 583) ng/mL. The median AIGA titer (IQR) of the 5 patients before the first pulse of IVCY was 46 124 (46 004–49 907) ng/mL. All patients’ duration from diagnosis to IVCV treatment was 13 (12–14) months; we detected the AIGA titers 3, 6, 9, 12, and 15 months after diagnosis. The AIGA titers of all patients before IVCY therapy were higher than the positive titer value (Table 3, Figure 1). Serological testing in all patients showed a significant decrease in AIGA titers after receiving IVCY therapy. The median (IQR) number of AIGA titers in serum after 4 months was 14 234 (11 029–24 564), and it was significantly different from 46 124 (46 004–49 907) ng/mL before pulse IVCY (P < .05). The median AIGA titer (IQR) after completion of the sixth pulse of IVCY was 10 320 (6784–13 245) ng/mL, and it was also significantly different from the number before pulse IVCY (P < .01). After 3 months of follow-up, the median (IQR) AIGA titers of all patients began to rise gradually to 16 738 (14 954–18 948) ng/mL and then to 40 532 (21 234–47 362) ng/mL at the end of 24 months of follow-up. The AIGA titers monitored after 12 months of follow-up were 34 912 (18 721–35 462), and it was significantly different from those after completion of the sixth IVCY therapy session (P < .05) (Table 4, Figure 2).
Table 3.
Anti-IFN-γ Autoantibody Titer Changes in Plasma From 5 Patients Before Treatment With IVCY (ng/mL)
| Patient No. | Treatment Duration Before IVCY, mo | AIGAs Titers at Diagnosis | After 3 mo | After 6 mo | After 9 mo | After 12 mo | After 15 mo | Pretreatment |
|---|---|---|---|---|---|---|---|---|
| 1 | 19 | 89 605 | 90 875 | 76 581 | 91 234 | 65 981 | 94 321 | 49 907 |
| 2 | 13 | 48 450 | 56 443 | 23 451 | 45 674 | … | … | 15 537 |
| 3 | 12 | 41 203 | 38 764 | 40 321 | 42 251 | … | … | 46 004 |
| 4 | 6 | 72 583 | 78 943 | … | … | … | … | 83 375 |
| 5 | 14 | 41 924 | 42 312 | 45 321 | 40 321 | … | … | 46 124 |
Abbreviations: AIGA, anti-interferon-γ autoantibody; IFN, interferon; IVCY, intravenous cyclophosphamide.
Figure 1.
Anti-IFN-γ autoantibody titer changes in plasma from 5 patients before treatment with IVCY: The AIGA titers of all patients before IVCY therapy were higher than the positive titer values. Abbreviations: AIGAs, anti-IFN-γ autoantibodies; IFN, interferon; IVCY, intravenous cyclophosphamide.
Table 4.
Anti-IFN-γ Autoantibody Titer Changes in Plasma From 5 Patients Treated With IVCY (ng/mL)
| Patient No. | AIGAs Titers at Pretreatment | Treatment Duration, mo | Post-treatment Duration, mo | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 3 | 6 | 9 | 12 | 18 | 24 | ||
| 1 | 49 907 | 56 085 | 39 636 | 31 554 | 24 564 | 23 101 | 13 245 | 45 626 | 93 520 | 54 211 | 35 462 | 43 412 | 47 362 |
| 2 | 15 537 | 7975 | 7551 | 6388.8 | 6873 | 5902 | 5637 | 16 738 | 28 342 | 14 321 | 18 721 | 16 251 | 19 872 |
| 3 | 46 004 | 18 056 | 20 387 | 27 295 | 28 299 | 21 234 | 17 620 | 18 949 | 23 303 | 57 510 | 43 767 | 56 973 | 58 321 |
| 4 | 83 375 | 20 461 | 13 615 | 17 986 | 11 029 | 9876 | 10 320 | 14 954 | 23 209 | 28 325 | 34 912 | 47 181 | 40 532 |
| 5 | 46 124 | 46 475 | 29 461 | 16 474 | 14 234 | 10 832 | 6784 | 9896 | 10 921 | 15 432 | 12 334 | 23 123 | 21 234 |
| IQR | 46 124 (46 004–49 907) | 20 461 (18 056–46 475) | 20 387 (13 615–29 461) | 17 986 (16 474–27 295) | 14 234 (11 029–24 564)* | 10 832 (9876–21 234) * | 10 320 (6784–13 245)** | 16 738 (14 954–18 949) | 23 303 (23 209–28 342) | 28 325 (15 432–54 211) | 34 912 (18 721–35 462) *** | 43 412 (23 123–47 181) *** | 40 532 (21 234–47 362) *** |
Compare with AIGA titers at pretreatment (*P < .05; **P < .01). Compare with AIGA titers at 6-month treatment duration (***P < .05).
Abbreviations: AIGA, anti-interferon-γ autoantibody; IQR, interquartile range; IVCY, intravenous cyclophosphamide.
Figure 2.
Anti-IFN-γ autoantibody titer changes in plasma from 5 patients treated with IVCV: There was a significant decrease in AIGA titers before and after receiving IVCY therapy. The AIGA titers in serum after 3, 4, 5, and 6 months were significantly different from those before pulse IVCY (P < .05). After 3 months of follow-up, the median AIGA titers of all patients began to rise gradually. The AIGA titers monitored after 12 months of follow-up were significantly different from those after completion of the sixth IVCY therapy session (P < .05). Abbreviations: AIGAs, anti-IFN-γ autoantibodies; IFN, interferon; IVCY, intravenous cyclophosphamide.
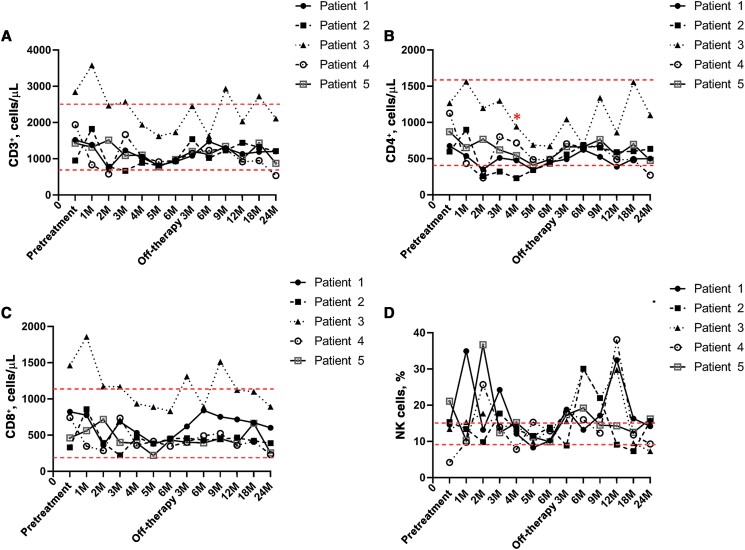
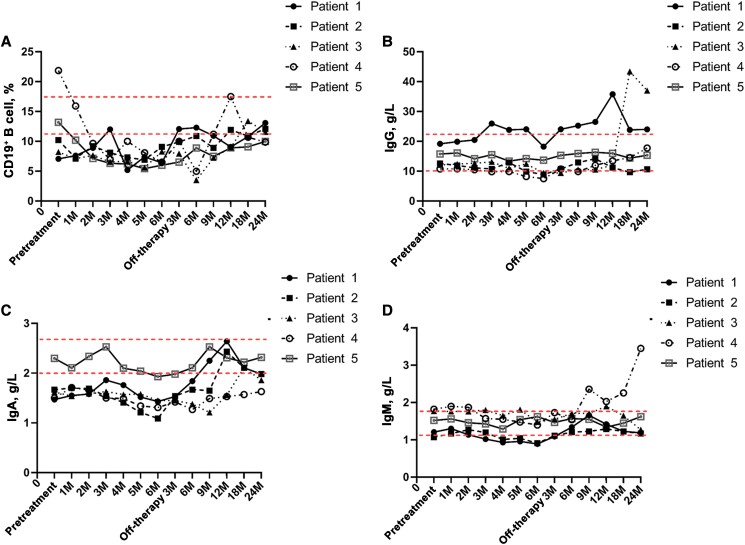
The T lymphocyte count, B lymphocyte count, and related basic laboratory parameters of all patients were documented. A lymphocyte subpopulation analysis showed that the median CD3+ T lymphocyte count (IQR) was 1522 (1432–1941) cells/µL before IVCY and decreased to 953 (932–982) cells/µL after completion of IVCY therapy. The median CD4+ T lymphocyte count (IQR) was 873 (676–1127)/µL before IVCY therapy and decreased to 486 (453–491)/µL after completion of IVCY therapy (P < .05). The median CD8+ T cell count (IQR) was 463 (744–824)/µL before IVCY therapy and then decreased to 443 (432–453)/µL after completion of IVCY therapy. The median proportion of the natural killer (NK) cell population (IQR) was 14.46% (13.44%–15.35%) before IVCY and then decreased to 12.9% (10.2%–13.3%) after completion of IVCY. All the relevant laboratory values of lymphocytes gradually increased after IVCY therapy (Table 5, Supplemental Table, Figure 3). The median proportion of CD19+ B cells (IQR) was 10.2% (8.22%–13.2%) before IVCY, which decreased to 6.5% (6.4%–8.3%) at completion of IVCY. Patients 1, 2, and 5 returned to normal levels 3 months after the end of IVCY, and patients 3 and 4 returned to normal levels 12 months after the end of IVCY. The median titers of serum immunoglobulin (Ig)G, IgA, and IgM were 12.61 (12.1–15.79) g/L, 1.62 (1.5–1.67) g/L, and 1.52 (1.21–1.77) g/L before treatment, respectively, and all decreased during pulsed IVCY therapy. All of these titers gradually increased after IVCY therapy (Table 6, Figure 4).
Table 5.
T Lymphocyte and Natural Killer Cell Changes in Plasma From 5 Patients Treated With IVCY
| … | Patient No. | Pretreatment | Treatment Duration, mo | Post-treatment Duration, mo | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 3 | 6 | 9 | 12 | 18 | 24 | |||
| CD3+ T lymphocyte count, /μL | 1 | 1522 | 1387 | 729 | 1231 | 1029 | 821 | 924 | 1084 | 1478 | 1297 | 1136 | 1191 | 1204 |
| 2 | 955 | 1829 | 777 | 671 | 891 | 811 | 932 | 1543 | 1021 | 1231 | 1443 | 1321 | 1211 | |
| 3 | 2845 | 3578 | 2467 | 2571 | 1941 | 1625 | 1731 | 2455 | 1625 | 2935 | 2030 | 2725 | 2111 | |
| 4 | 1941 | 834 | 582 | 1668 | 1044 | 917 | 953 | 1156 | 1234 | 1275 | 917 | 953 | 538 | |
| 5 | 1432 | 1321 | 1516 | 1093 | 1102 | 783 | 982 | 1201 | 1124 | 1342 | 994 | 1432 | 876 | |
| CD4+ T lymphocyte count, /μL | 1 | 676 | 538 | 354 | 510 | 478 | 342 | 453* | 493 | 623 | 525 | 389 | 498 | 501 |
| 2 | 597 | 900 | 259 | 321 | 231 | 342 | 432 | 558 | 689 | 652 | 590 | 602 | 634 | |
| 3 | 1268 | 1562 | 1197 | 1297 | 942 | 689 | 673 | 1043 | 689 | 1339 | 860 | 1558 | 1101 | |
| 4 | 1127 | 434 | 235 | 803 | 717 | 487 | 486 | 706 | 655 | 675 | 487 | 486 | 273 | |
| 5 | 873 | 653 | 768 | 621 | 542 | 412 | 491 | 665 | 662 | 764 | 543 | 698 | 478 | |
| CD4+ T lymphocyte cell, % | 1 | 34 | 28 | 38 | 31 | 36 | 30 | 39 | 35 | 32 | 30 | 24 | 31 | 31 |
| 2 | 47 | 39 | 23 | 37 | 15 | 31 | 36 | 26 | 38 | 35 | 30 | 35 | 38 | |
| 3 | 35 | 33 | 38 | 40 | 32 | 29 | 28 | 32 | 32 | 35 | 32 | 39 | 42 | |
| 4 | 42 | 42 | 30 | 38 | 41 | 32 | 34 | 41 | 38 | 38 | 41 | 40 | 37 | |
| 5 | 46 | 39 | 40 | 37 | 39 | 35 | 36 | 35 | 40 | 36 | 37 | 38 | 44 | |
| CD8+ T lymphocyte count, /μL | 1 | 824 | 776 | 361 | 686 | 532 | 381 | 443 | 621 | 843 | 754 | 717 | 672 | 602 |
| 2 | 332 | 859 | 393 | 220 | 473 | 384 | 453 | 459 | 432 | 444 | 465 | 421 | 390 | |
| 3 | 1463 | 1858 | 1172 | 1169 | 934 | 889 | 832 | 1311 | 889 | 1514 | 1124 | 1100 | 892 | |
| 4 | 744 | 348 | 287 | 735 | 360 | 415 | 348 | 402 | 492 | 524 | 360 | 415 | 231 | |
| 5 | 463 | 563 | 717 | 401 | 390 | 221 | 432 | 402 | 394 | 453 | 387 | 664 | 256 | |
| CD8+ T lymphocyte cell, % | 1 | 34 | 34 | 39 | 45 | 38 | 26 | 27 | 37 | 37 | 37 | 43 | 36 | 40 |
| 2 | 24 | 36 | 29 | 22 | 23 | 27 | 28 | 19 | 22 | 22 | 22 | 21 | 22 | |
| 3 | 31 | 31 | 27 | 25 | 28 | 34 | 28 | 23 | 24 | 21 | 25 | 20 | 22 | |
| 4 | 28 | 32 | 29 | 24 | 24 | 25 | 26 | 24 | 29 | 31 | 29 | 23 | 22 | |
| 5 | 22 | 32 | 27 | 26 | 25 | 18 | 33 | 23 | 25 | 23 | 28 | 26 | 19 | |
| Natural killer cell, % | 1 | 14.64 | 34.96 | 13.2 | 24.24 | 12.1 | 8.3 | 10.2 | 18.8 | 13.2 | 17.16 | 32.48 | 16.3 | 14.2 |
| 2 | 15.35 | 13.44 | 9.8 | 17.73 | 13.3 | 11.54 | 13.86 | 8.9 | 30.07 | 22.01 | 9.1 | 7.3 | 15.6 | |
| 3 | 13.44 | 15.35 | 17.73 | 13.86 | 14.75 | 9.8 | 13.3 | 15.6 | 30.07 | 22.01 | 29.74 | 9.53 | 7.3 | |
| 4 | 4.23 | 9.78 | 25.72 | 14 | 7.8 | 15.3 | 12.9 | 18 | 16.03 | 12.3 | 38.1 | 11.8 | 9.3 | |
| 5 | 21.1 | 10.3 | 36.71 | 12.4 | 15.2 | 11.2 | 9.93 | 17.4 | 19.2 | 14.5 | 14.3 | 12.5 | 16.21 | |
| Natural killer cell, /μL | 1 | 223 | 485 | 96 | 298 | 125 | 68 | 94 | 204 | 195 | 223 | 369 | 194 | 171 |
| 2 | 147 | 246 | 76 | 119 | 119 | 94 | 129 | 137 | 307 | 271 | 131 | 96 | 189 | |
| 3 | 382 | 549 | 437 | 356 | 286 | 159 | 230 | 383 | 489 | 646 | 604 | 260 | 154 | |
| 4 | 82 | 82 | 150 | 2342 | 81 | 140 | 123 | 208 | 198 | 157 | 349 | 112 | 50 | |
| 5 | 302 | 136 | 557 | 136 | 168 | 88 | 98 | 209 | 216 | 195 | 142 | 179 | 142 | |
Normal range: CD3+ T lymphocyte count: 690–254/μL; CD4+ T lymphocyte count: 410–1590/μL; CD4+ T lymphocyte cell: 30.1%–40.4%; CD8+ T lymphocyte count: 190–1140/μL; CD8+ T lymphocyte cell: 20.7%–29.4%; natural killer cell: 9%–15%.
Abbreviation: IVCY, intravenous cyclophosphamide.
*The 6-month CD4+ T lymphocyte count compared with pretreatment (*P < .05)
Figure 3.
T lymphocyte and natural killer cell changes in plasma from 5 patients treated with IVCV: CD3+ T lymphocyte count (A), CD4+ T lymphocyte count (P < .05) (B), CD8+ T lymphocyte count (C), and natural killer cell (D) decreased after completion of IVCY therapy. All the relevant laboratory values of lymphocytes gradually increased after IVCY therapy. Abbreviations: IVCY, intravenous cyclophosphamide; NK, natural killer.
Table 6.
B Cell and Serum Immunoglobulin Changes in 5 Patients With IVCY
| … | Patient No. | Pretreatment | Treatment Duration, mo | Post-treatment Duration, mo | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 3 | 6 | 9 | 12 | 18 | 24 | |||
| CD19+ B cell, % | 1 | 7.08 | 7.62 | 8.9 | 12 | 5.2 | 7.4 | 6.4 | 12.07 | 12.3 | 10.92 | 9.08 | 10.8 | 13.1 |
| 2 | 10.2 | 7.2 | 9.2 | 8.1 | 7.3 | 6.9 | 9.1 | 9.9 | 10.9 | 8.9 | 11.9 | 10.9 | 12.1 | |
| 3 | 8.22 | 7.12 | 7.66 | 6.55 | 6.68 | 5.62 | 8.3 | 7.95 | 3.52 | 7.23 | 8.99 | 13.4 | 11.6 | |
| 4 | 21.86 | 15.92 | 9.65 | 7 | 10 | 8.1 | 6.5 | 10 | 4.99 | 11.2 | 17.5 | 10.7 | 9.89 | |
| 5 | 13.2 | 10.2 | 7.13 | 6.32 | 6.22 | 5.45 | 6.01 | 6.54 | 8.88 | 7.49 | 8.9 | 9.09 | 9.98 | |
| CD19+ B cell, /μL | 1 | 108 | 106 | 65 | 148 | 54 | 61 | 59 | 131 | 182 | 142 | 103 | 129 | 158 |
| 2 | 97 | 132 | 72 | 54 | 65 | 56 | 85 | 153 | 111 | 110 | 172 | 144 | 147 | |
| 3 | 234 | 255 | 189 | 168 | 130 | 91 | 144 | 195 | 57 | 212 | 183 | 365 | 245 | |
| 4 | 424 | 133 | 56 | 118 | 104 | 74 | 62 | 116 | 62 | 143 | 160 | 102 | 53 | |
| 5 | 189 | 135 | 108 | 69 | 69 | 43 | 59 | 79 | 100 | 101 | 88 | 130 | 87 | |
| IgG, g/L | 1 | 19.2 | 19.9 | 20.47 | 25.97 | 23.85 | 24.05 | 18.21 | 24.05 | 25.26 | 26.53 | 35.81 | 23.85 | 24.04 |
| 2 | 12.61 | 11.93 | 11.14 | 10.75 | 12.9 | 9.9 | 8.9 | 10.75 | 12.9 | 14.2 | 11.2 | 9.7 | 10.7 | |
| 3 | 12.1 | 12.45 | 12.65 | 13.13 | 10.97 | 12.45 | 9.45 | 9.45 | 10.57 | 10.58 | 12.02 | 43.43 | 37.02 | |
| 4 | 10.77 | 10.81 | 10.54 | 9.86 | 9.89 | 8.21 | 7.54 | 10.73 | 9.9 | 11.98 | 13.46 | 14.58 | 17.79 | |
| 5 | 15.79 | 16.12 | 14.17 | 15.52 | 13.39 | 14.22 | 13.7 | 15.3 | 15.92 | 16.34 | 15.98 | 14.38 | 15.34 | |
| IgA, g/L | 1 | 1.47 | 1.55 | 1.58 | 1.86 | 1.76 | 1.52 | 1.43 | 1.53 | 1.84 | 2.25 | 2.64 | 2.11 | 1.98 |
| 2 | 1.67 | 1.7 | 1.69 | 1.54 | 1.41 | 1.21 | 1.09 | 1.54 | 1.67 | 1.65 | 2.43 | 2.11 | 1.98 | |
| 3 | 1.62 | 1.54 | 1.59 | 1.63 | 1.57 | 1.58 | 1.45 | 1.45 | 1.38 | 1.21 | 1.56 | 2.11 | 1.86 | |
| 4 | 1.5 | 1.71 | 1.66 | 1.5 | 1.49 | 1.34 | 1.31 | 1.42 | 1.27 | 1.49 | 1.53 | 1.57 | 1.63 | |
| 5 | 2.3 | 2.11 | 2.34 | 2.53 | 2.1 | 2.04 | 1.93 | 1.98 | 2.11 | 2.53 | 2.31 | 2.22 | 2.32 | |
| IgM, g/L | 1 | 1.21 | 1.3 | 1.14 | 1.02 | 0.93 | 0.96 | 0.89 | 1.09 | 1.34 | 1.66 | 1.42 | 1.23 | 1.19 |
| 2 | 1.07 | 1.17 | 1.27 | 1.2 | 1.01 | 1.04 | 0.91 | 1.11 | 1.21 | 1.23 | 1.29 | 1.22 | 1.17 | |
| 3 | 1.77 | 1.76 | 1.76 | 1.8 | 1.65 | 1.81 | 1.48 | 1.55 | 1.71 | 1.71 | 1.9 | 1.65 | 1.27 | |
| 4 | 1.82 | 1.9 | 1.87 | 1.57 | 1.55 | 1.48 | 1.4 | 1.73 | 1.54 | 2.36 | 2.03 | 2.26 | 3.45 | |
| 5 | 1.52 | 1.56 | 1.46 | 1.43 | 1.29 | 1.54 | 1.62 | 1.47 | 1.57 | 1.55 | 1.34 | 1.45 | 1.62 | |
Normal range: CD19+ B cell: 9.02%–14.1%; IgG: 8–18.0 g/L; IgA: 2.01–2.69 g/L; IgM: 0.84–1.32 g/L.
Abbreviations: Ig, immunoglobulin; IVCY, intravenous cyclophosphamide.
Figure 4.
B cell and serum immunoglobulin changes in 5 patients with IVCV: The CD19+ B cell count (A), serum IgG (B), IgA (C), and IgM (D) decreased after completion of IVCY therapy. All of these titers gradually increased after IVCY therapy. Abbreviations: Ig, immunoglobulin; IVCY, intravenous cyclophosphamide.
Patient 3 developed Sweet's syndrome 2 months after her diagnosis with TM infection and was ANA 1:100, anticardiolipin antibody (+), and anti-Ro-52 antibody (±). She was treated with prednisone at 30 mg/d, which was then reduced by 5 mg every 2–4 weeks to 5–10 mg for 9 months. The rash associated with Sweet's syndrome in this patient gradually subsided. Patient 4 was ANA 1:320, anti-SSA (+), and anti-SSB (+). She was treated with prednisone at 40 mg/d, which was reduced by 5 mg every 2 weeks to 5 mg for 14 months.
No significant adverse effects were observed among the 5 patients during the pulsed IVCY therapy. Patients 2 and 3 had anorexia the day after IVCY therapy and improved 2–3 days later. No one had nausea or vomiting. No patients had frequent urination, urgent urination, painful urination, hematuria, scanty urination, proteinuria, or liver function impairment during or after IVCY therapy. No patients had bone marrow suppression such as decreased white blood cells, decreased platelets, or anemia during or after IVCY therapy. All patients had no bladder cancer, skin cancer, myeloproliterative neoplasms, or myelodysplastic syndromes for 24 months after discontinuation of the 6 pulses of IVCY. After completion of 6 pulses of IVCY therapy, complete blood count examinations revealed that the median white blood cell count, neutrophil count, and absolute lymphocyte count (IQR) were 8.0 (7.66–8.84) × 109/L, 5.64 (5.03–5.7) × 109/L, and 1.12 (1.94–1.98) × 109/L, respectively. The median hemoglobin concentration and total bilirubin level (IQR) were 129 (109–136) g/L and 7.7 (7.3–10.4) µmol/L, respectively. Serum biochemical analysis showed a median serum albumin concentration and globulin concentration (IQR) of 38 (36.4–37.3) g/L and 31.8 (28.9–32.8) g/L, respectively. The median concentrations of aspartate aminotransferase, alanine aminotransferase, and creatinine (IQR) were 27 (21–28) U/L, 32 (22–35) U/L, and 78 (60–85) µmol/L, respectively. The median ESR, CRP concentration, and procalcitonin (IQR) were 27 (15–40) mm/H, 9.93 (2.59–15.94), and 0.066 (0.053–0.08) ng/mL, respectively. The autoantibody titer test showed decreased antibody titers or autoantibody negativity in 3 patients: patient 2: ANA (−); patient 3: ANA (−), anticardiolipin antibody (−); patient 4: ANA 1:100, anti-SSA (±), anti-SSB (+) (Table 7).
Table 7.
Clinical Data Before and After Completion of IVCY Treatment in 5 Patients
| … | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| … | Before | After | Before | After | Before | After | Before | After | Before | After |
| WBC, ×109/L | 10.56 | 8.84 | 10.03 | 5.51 | 9.66 | 9.51 | 6.58 | 7.66 | 9.85 | 8.0 |
| Neutrophil, ×109/L | 7.87 | 5.64 | 7.79 | 3.72 | 4.7 | 5.7 | 3.35 | 6.26 | 6.7 | 5.03 |
| Lymphocyte, ×109/L | 1.62 | 1.94 | 1.49 | 1.12 | 3.6 | 2.31 | 2.53 | 0.83 | 2.32 | 1.98 |
| Hemoglobin, g/L | 126 | 137 | 136 | 129 | 111 | 109 | 83 | 90.7 | 141 | 136 |
| Platelet, ×1012/L | 126 | 132 | 231 | 212 | 276 | 209 | 298 | 231 | 298 | 128 |
| CRP, mg/L | 105.7 | 15.94. | 32.5 | 2.59 | 16.2 | 28.7 | 19.2 | 2.1 | 44.7 | 9.93 |
| ESR, mm/H | 49 | 40 | 37 | 27 | 29 | 15 | 47 | 7 | 60 | 66 |
| Procalcitonin, ng/mL | 0.16 | 0.053. | 0.26 | 0.083 | 0.19 | 0.066 | 0.55 | 0.05 | 0.88 | 0.08 |
| Tbil, µmol/L | 11.9 | 13.3 | 7.6 | 7.7 | 8.2 | 7.3 | 10.1 | 10.4 | 4.3 | 4.5 |
| Albumin, g/L | 36.3 | 28.1 | 49.3 | 36.4 | 34.3 | 37.3 | 35.6 | 38 | 36.4 | 43.9 |
| Globulin, g/L | 45.8 | 44.4 | 33.3 | 28.9 | 37.4 | 31.8 | 24.1 | 24.8 | 38.4 | 32.8 |
| AST, U/L | 20 | 27 | 31 | 28 | 12 | 12 | 26 | 28 | 38 | 21 |
| ALT, U/L | 24 | 22 | 43 | 56 | 16 | 18 | 23 | 32 | 36 | 35 |
| Creatinine, µmol/L | 93 | 90 | 68 | 60 | 66 | 78 | 51 | 53 | 87 | 85 |
Normal range: WBC: 4–10 × 109/L; neutrophil: 2–6.5 × 109/L; lymphocyte: 1.1–3.2 × 109/L; hemoglobin: 135–175 g/L; platelet: 100–300 × 1012/L; CRP: <5 mg/L; ESR: 0–20 mm/H; procalcitonin: <0.05 ng/mL; Tbil: 3.4–20.5 µmol/L; albumin: 40–55 g/L; globulin: 20–40 g/L; AST: 15–45 U/L; ALT: 9–60 U/L; creatinine: 59–104 µmol/L.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; Tbil, total bilirubin; WBC, white blood cell.
Five patients continued to use antibiotics or antifungal agents to treat primary infections after completing IVCY therapy, with durations of 6, 3, 3, 8, and 3 months for antifungal treatment from patients 1–5 and of 12, 6, and 8 months for anti-NTM treatment for patients 1, 3, and 4, respectively. Patient 1 required itraconazole for maintenance treatment of TM infection until 6 months after IVCY discontinuation and a combination of moxifloxacin and clindamycin for maintenance treatment of NTM infection until 12 months after IVCY discontinuation. Patient 2 continued TM therapy with voriconazole until 3 months after completion of IVCY therapy. Patient 3 remained on TM treatment with itraconazole until 3 months after completion of IVCY and on NTM treatment with a combination of moxifloxacin and clindamycin until 6 months after completion of IVCY therapy. Patient 4 required itraconazole for maintenance therapy of TM infection until 8 months after IVCY discontinuation and a combination of moxifloxacin and clindamycin for maintenance therapy of NTM infection until 8 months after IVCY discontinuation. Patient 5 needed voriconazole to maintain treatment of TM infection until 3 months after completing IVCY therapy. All patients continued to be followed for 24 months after discontinuation of the 6 pulses of IVCY and achieved complete remission without relapse, except patient 3. Patient 3 had a relapsed TM infection at the 18th month of follow-up, and the median titers of AIGAs detected in the serum of the patient rose to 58 321 ng/mL.
DISCUSSION
Immunodeficiency in HIV-negative patients caused by AIGAs was defined as adult-onset immunodeficiency (AOID) in 2012. In 2019, the International Union of Immunological Societies Expert Committee updated the classification of innate/primary immunodeficiencies. Adult-onset immunodeficiency associated with interferon-γ autoantibody (IGA) belongs to the autoimmune phenotype of primary immunodeficiency [12]. AIGAs have been reported to be associated with severe disseminated NTM infection in previously healthy hosts [4, 5]. However, current studies have found that AIGAs are closely related to TM infection in HIV-negative hosts, especially in the epidemic area of TM in Southwest China. AIGAs are the most common form of immunodeficiency in HIV-negative hosts infected with TM. TSM patients with positive AIGAs are more likely to have relapsed, severe, and refractory infections and are more likely to have coinfections with other opportunistic pathogens [1, 5, 6].
Specific human leukocyte antigen class II haplotypes (HLA-DRB1 16:02 and HLA-DQB1 05:02) have been reported to be explicitly associated with AIGAs, especially in Southeast Asia. Furthermore, the production of this antibody might be related to the stimulation of pathogen infection [12, 13]. However, the relationship among the expression of HLA-DRB1 16:02 and HLA-DQB1 05:02, pathogenic species, and AIGA titers remains unclear. Studies have demonstrated that AIGAs target the binding activity of synthetic IFN-γ peptides (amino acid residues 121–131) in patients, including inhibition of IFN-γ-mediated immunocidal effects of macrophages against nontuberculous mycobacteria, and IFN-γ-regulated production of chemokines and cellular inflammatory factors is also blocked [14]. However, one investigator found that stimulating serum-intervened peripheral blood mononuclear cells from AIGA-positive patients using recombinant human IFN-γ (EE-IFN-γ) with a deletion in the P121–131 region of the C-terminus of IFN-γ EE-IFN-γ, while stimulating pSTAT1 and IL-12 production, restored only 40% of normal levels. Meanwhile, clinical attempts at immunomodulatory therapy using recombinant human IFN-γ to neutralize AIGAs did not improve patient prognosis [15, 16]. That AIGAs neutralize and block IFN-γ was just one of the reasons for immunodeficiency, and there are other more important immunomodulatory mechanisms of AIGAs that affect the impaired clearance of pathogens by the organism. Therefore, we suggested that not only the AIGA titer but also its combined neutralizing effect of the antibodies should be monitored for a more intuitive judgment in AIGA-positive patients infected with TM. AIGA measurement can use direct titers or IFN-γ neutralization. Measuring directly is more convenient and more widely accepted [1, 3, 6, 17]. But there is still no universally accepted standard for positivity of AIGA. In our study, all 5 patients had at least 1 relapse even with aggressive antifungal therapy. Patient 1 had 3 relapses during a 19-month course of TM infection. Four of the 5 patients had coinfections with other opportunistic pathogens, the most common of which was NTM infection. Before IVCY therapy, inflammatory markers were tested in 5 patients, showing an increase in ESR, CRP, procalcitonin, and white blood cell count. Moreover, high titers of AIGAs in serum also indicated that the patients remained in a state of persistent infection. Therefore, in the presence of high-titer AIGAs in serum, it is difficult to eradicate intracellular pathogens such as TM even with maintenance therapy with antimicrobials, and it is of great significance to start a therapeutic regimen that can effectively reduce AIGA titers to better control the infection in patients at this time.
A previous study reported that anti-CD20 monoclonal antibody therapy achieved good clinical efficacy in patients with positive AIGAs who were coinfected with refractory infections [5, 7, 18]. However, this high-cost treatment is unlikely to be considered in resource-limited settings and has not been proven to be effective in all patients in the literature. The use of an immunosuppressant with cyclophosphamide has been reported in the literature to be effective in reducing AIGA titers of patients with AIGA-associated NTM infection, but no clinical study data are available in patients with AIGA-associated TM infection. Cyclophosphamide is an alkylating agent that reduces the production of pathogenic autoantibodies by depleting B cells and affecting the differentiation of T cells. Currently, IVCY is used as a standard regimen for treatment, especially for for severe autoimmune and autoinflammatory diseases, such as systemic lupus erythematosus and antineutrophil cytoplasmic antibody-associated primary vasculitis syndrome [19, 20]. Previous studies have found no significant differences between 6 monthly pulses of IVCY and pulses of low-dose IVCY twice a month in the treatment of lupus nephritis [21, 22]. In our study, the serum AIGA titers of 5 patients decreased significantly during IVCY therapy, and the AIGA titers after the third pulse of IVCY were significantly different from those before IVCY. During pulsed IVCY therapy, the T-lymphocyte count, B-lymphocyte count, immunoglobulin concentration, and other related indicators decreased, but all increased after completion of IVCY therapy. Therefore, this was only a temporary decrease, and the T-lymphocyte count was still within the normal range during the decline. With the decline in AIGA titers, the related inhibitory function improved, which restored the body's immune killing function against TM infection. At this time, the combination of antifungal therapy increased eradication of intracellular TM, maybe completely eliminating intracellular pathogens and reducing recurrence. After the fourth pulse of IVCY, the AIGA titers of the 5 patients were relatively stable, with a slight decline. Moreover, considering the adverse side effects of cyclophosphamide, including suppression of T cells and B cells, severe nausea, vomiting, etc., we attempted to discontinue therapy after completing 6 pulses of IVCY and continued to use antibiotics for maintenance therapy, achieving good clinical prognoses.
The autoimmune antibodies of patients 2, 4, and 5 declined variously or even became negative at the end of therapy. Sweet's syndrome in patient 2 was also improved at the end of therapy. In previous studies, when patients infected with TM or NTM had positive AIGAs and Sweet's syndrome or ANA 1:320, glucocorticoids were added to the treatment but were not effective in decreasing the AIGA titers [23–25]. Five patients successively discontinued antimicrobial therapy and were then followed up for 24 months. After 3 months of follow-up, the AIGA titers of all patients started to increase gradually. There was a significant difference between AIGA titers after 12 months of follow-up and those after completing 6 pulses of IVCY therapy, which is consistent with prior reports. Five patients successively discontinued TM therapy between 3 and 9 months (median, 4.6 months) after completion of IVCY, and only patient 3 had a relapsed TM infection 18 months after the end of IVCY. Patients still had multiple relapses even with long-term aggressive TM therapy before receiving IVCY; comparatively, the course of TM therapy was significantly shortened after completion of IVCY therapy. We suggested that patients should remain on TM therapy for 6 months after IVCY discontinuation to reduce their physical and economic burdens.
There were some limitations to our study. First, we did not have a large patient population, and a control study was not conducted due to the small sample size. Second, there was no comparative study on the timing of starting IVCY and the course of IVCY, and further studies based on large samples are still needed to determine the timing of initiating IVCY therapy and its dosage, interval time, and course. Third, there is a lack of further basic immunological research to identify other potential antibody-producing cells to further explain the pathogenesis of the disease. Finally, CD14+ monocytes should be measured in these patients, as CD14+ monocytes have the highest responsiveness to IFN-γ. Cyclophosphamide can deplete T cells and affect the differentiation of T cells, and we have been studying the relationship between TM and CD4+ T cells, so we chose to pay attention to CD4+ cells in this study.
CONCLUSIONS
In conclusion, this study performed multisample and exploratory IVCY therapy for patients with AIGA-associated TM infection in the TM epidemic area of mainland China for the first time, and all patients were followed for a long period. In addition, this study demonstrated that pulses of short-term and high-dose IVCY could effectively reduce serum AIGA titers, caused no significant side effects, and may reduce TSM recurrence. Currently, the mechanism of AIGA production is unclear; thus, a pulse IVCY regimen has been shown to have certain clinical value for refractory TM infection at doses of 0.8–1.0 mg/m2 every 3–4 weeks for a total of 6 cycles, which may serve as a reference standard for the treatment of patients with AIGA-associated TM infections, especially in resource-limited countries.
Supplementary Material
Acknowledgments
Financial support. This work was supported by grants from the Natural Science Foundation of China (NSFC 82060364), the Science and Technology Department of Guangxi Zhuang Autonomous Foundation of Guangxi Key Research and Development Program (No. GuikeAB20238025), Guangxi Natural Science Foundation (NO. 2021GXNSFBA220064), the Shenzhen Science Technology Program (NO. JCYJ20210324115000002), and Futian Healthcare Research Project (No: FTWS2021004).
Author contributions. W.Z. and J.Z. contributed to the study conception and design. W.Z., G.F., and M.T. performed the study. S.T. and M.Y. performed data collection and analysis. W.Z. and M.T. contributed to writing and data interpretation. All authors agreed on the journal to which the article would be submitted. All authors reviewed and agreed on all versions of the article before submission, during revision, the final version accepted for publication, and any significant changes introduced at the proofing stage. All authors agreed to take responsibility and be accountable for the contents of the article.
Data sharing. All the data are fully available without restriction.
Ethical approval. This study was approved by the Faculty of Medicine at the First Affiliated Hospital of Guangxi Medical University (2021 [KY-E-262]). All patients provided written informed consent. The study was carried out in accordance with the principles of the Declaration of Helsinki. The first author vouches for the completeness and accuracy of the data and for the fidelity of the study to the protocol.
Consent for publication. Signed consent was obtained for the publication of the case details from the participants.
Contributor Information
Wen Zeng, Department of Respiratory and Critical Medicine, The Eighth Affiliated Hospital, Sun Yat-Sen University, Shenzhen, Guangdong, China; Department of Respiratory and Critical Medicine, The First Affiliated Hospital of Guangxi Medical University, Nanning, Guangxi, China.
Mengxin Tang, Department of Respiratory and Critical Medicine, The First Affiliated Hospital of Guangxi Medical University, Nanning, Guangxi, China.
Meiling Yang, Department of Respiratory and Critical Medicine, The First Affiliated Hospital of Guangxi Medical University, Nanning, Guangxi, China.
Gaoneng Fang, Department of Respiratory and Critical Medicine, The First Affiliated Hospital of Guangxi Medical University, Nanning, Guangxi, China.
Shudan Tang, Department of Respiratory and Critical Medicine, The First Affiliated Hospital of Guangxi Medical University, Nanning, Guangxi, China.
Jianquan Zhang, Department of Respiratory and Critical Medicine, The Eighth Affiliated Hospital, Sun Yat-Sen University, Shenzhen, Guangdong, China.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
References
- 1. Zeng W, Qiu Y, Tang S, Zhang J, Pan M, Zhong X. Characterization of anti-interferon-γ antibodies in HIV-negative patients infected with disseminated Talaromyces marneffei and cryptococcosis. Open Forum Infect Dis 2019; 6:XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hong GH, Ortega-Villa AM, Hunsberger S, et al. Natural history and evolution of anti-interferon-γ autoantibody-associated immunodeficiency syndrome in Thailand and the United States. Clin Infect Dis 2020; 71:53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Guo J, Ning XQ, Ding JY, et al. Anti-IFN-γ autoantibodies underlie disseminated Talaromyces marneffei infections. J Exp Med 2020; 217:e20190502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Qiu Y, Feng X, Zeng W, et al. Immunodeficiency disease spectrum in HIV-negative individuals with talaromycosis. J Clin Immunol 2021; 41:221–3. [DOI] [PubMed] [Google Scholar]
- 5. Chi CY, Lin CH, Ho MW, et al. Clinical manifestations, course, and outcome of patients with neutralizing anti-interferon-γ autoantibodies and disseminated nontuberculous mycobacterial infections. Medicine (Baltimore) 2016; 95:e3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Browne SK, Zaman R, Sampaio EP, et al. Anti-CD20 (rituximab) therapy for antiIFN-γ autoantibody-associated nontuberculous mycobacterial infection. Blood 2012; 119:3933–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Czaja CA, Merkel PA, Chan ED, et al. Rituximab as successful adjunct treatment in a patient with disseminated nontuberculous mycobacterial infection due to acquired anti-interferon-γ autoantibody. Clin Infect Dis 2014; 58:e115–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Laisuan W, Pisitkun P, Ngamjanyaporn P, Suangtamai T, Rotjanapan P. Prospective pilot study of cyclophosphamide as an adjunct treatment in patients with adult-onset immunodeficiency associated with anti-interferon-γ autoantibodies. Open Forum Infect Dis 2020; 7:XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chetchotisakd P, Anunnatsiri S, Nanagara R, Nithichanon A, Lertmemongkolchai G. Intravenous cyclophosphamide therapy for anti-IFN-gamma autoantibody-associated Mycobacterium abscessus infection. J Immunol Res 2018; 2018:6473629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hoenigl M, Strenger V, Buzina W, et al. European Organization for the Research and Treatment of Cancer/Mycoses Study Group (EORTC/MSG) host factors and invasive fungal infections in patients with haematological malignancies. J Antimicrob Chemother 2012; 67:2029–33. [DOI] [PubMed] [Google Scholar]
- 11. Nseir S, Grailles G, Soury-Lavergne A, Minacori F, Alves I, Durocher A. Accuracy of American Thoracic Society/Infectious Diseases Society of America criteria in predicting infection or colonization with multidrug-resistant bacteria at intensive-care unit admission. Clin Microbiol Infect 2010; 16:902–8. [DOI] [PubMed] [Google Scholar]
- 12. Tangye SG, Al-Herz W, Bousfiha A, et al. Human inborn errors of immunity: 2019 update on the classification from the International Union of Immunological Societies Expert Committee. J Clin Immunol 2020; 40:24–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chi CY, Chu CC, Liu JP, et al. Anti-IFN-γ autoantibodies in adults with disseminated nontuberculous mycobacterial infections are associated with HLA-DRB1*16:02 and HLA-DQB1*05:02 and the reactivation of latent varicella-zoster virus infection. Blood 2013; 121:1357–66. [DOI] [PubMed] [Google Scholar]
- 14. Krisnawati DI, Liu YC, Lee YJ, et al. Blockade effects of anti-interferon- (IFN-) γ autoantibodies on IFN-γ-regulated antimicrobial immunity. J Immunol Res 2019; 2019:1629258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lin CH, Chi CY, Shih HP, et al. Identification of a major epitope by anti-interferon-γ autoantibodies in patients with mycobacterial disease. Nat Med 2016; 22:994–1001. [DOI] [PubMed] [Google Scholar]
- 16. Su SS, Zhang SN, Ye JR, et al. Disseminated Talaromyces marneffei and Mycobacterium avium infection accompanied Sweet's syndrome in a patient with anti-interferon-γ autoantibodies: a case report. Infect Drug Resist 2019; 12:3189–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Browne SK, Burbelo PD, Chetchotisakd P, et al. Adult-onset immunodeficiency in Thailand and Taiwan. N Engl J Med 2012; 367:725–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Koizumi Y, Sakagami T, Nishiyama N, et al. Rituximab restores IFN-γ-STAT1 function and ameliorates disseminated Mycobacterium avium infection in a patient with anti-interferon-γ autoantibody. J Clin Immunol 2017; 37:644–9. [DOI] [PubMed] [Google Scholar]
- 19. Calguneri M, Ozbalkan Z, Ozturk MA, Apras S, Ertenli AI, Kiraz S. Intensified, intermittent, low-dose intravenous cyclophosphamide together with oral alternate-day steroid therapy in lupus nephritis (long-term outcome). Clin Rheumatol 2006; 25:782–8. [DOI] [PubMed] [Google Scholar]
- 20. Harper L, Morgan MD, Walsh M, et al. Pulse versus daily oral cyclophosphamide for induction of remission in ANCA-associated vasculitis: long-term follow-up. Ann Rheum Dis 2012; 71:955–60. [DOI] [PubMed] [Google Scholar]
- 21. Austin HA 3rd, Klippel JH, Balow JE, et al. Therapy of lupus nephritis. Controlled trial of prednisone and cytotoxic drugs. N Engl J Med 1986; 314:614–9. [DOI] [PubMed] [Google Scholar]
- 22. Houssiau FA, Vasconcelos C, D'Cruz D, et al. Immunosuppressive therapy in lupus nephritis: the Euro-Lupus Nephritis Trial, a randomized trial of low-dose versus high-dose intravenous cyclophosphamide. Arthritis Rheum 2002; 46:2121–31. [DOI] [PubMed] [Google Scholar]
- 23. Xu H, Liu D, He X, Zheng D, Deng Y. Sweet's syndrome associated with Talaromyces marneffei and Mycobacterium abscessus infection due to anti-interferon-gamma autoantibodies. Indian J Dermatol 2018; 63:428–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jutivorakool K, Sittiwattanawong P, Kantikosum K, et al. Skin manifestations in patients with adult-onset immunodeficiency due to anti-interferon-gamma autoantibody: a relationship with systemic infections. Acta Derm Venereol 2018; 98:742–7. [DOI] [PubMed] [Google Scholar]
- 25. Baerlecken N, Jacobs R, Stoll M, et al. Recurrent, multifocal Mycobacterium avium-intercellulare infection in a patient with interferon-gamma autoantibody. Clin Infect Dis 2009; 49:e76–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.