Abstract
Background
Large clinical trials on drugs for hospitalized coronavirus disease 2019 (COVID-19) patients have shown significant effects on mortality. There may be a discrepancy with the observed real-world effect. We describe the clinical characteristics and outcomes of hospitalized COVID-19 patients in the Netherlands during 4 pandemic waves and analyze the association of the newly introduced treatments with mortality, intensive care unit (ICU) admission, and discharge alive.
Methods
We conducted a nationwide retrospective analysis of hospitalized COVID-19 patients between February 27, 2020, and December 31, 2021. Patients were categorized into waves and into treatment groups (hydroxychloroquine, remdesivir, neutralizing severe acute respiratory syndrome coronavirus 2 monoclonal antibodies, corticosteroids, and interleukin [IL]-6 antagonists). Four types of Cox regression analyses were used: unadjusted, adjusted, propensity matched, and propensity weighted.
Results
Among 5643 patients from 11 hospitals, we observed a changing epidemiology during 4 pandemic waves, with a decrease in median age (67–64 years; P < .001), in in-hospital mortality on the ward (21%–15%; P < .001), and a trend in the ICU (24%–16%; P = .148). In ward patients, hydroxychloroquine was associated with increased mortality (1.54; 95% CI, 1.22–1.96), and remdesivir was associated with a higher rate of discharge alive within 29 days (1.16; 95% CI, 1.03–1.31). Corticosteroids were associated with a decrease in mortality (0.82; 95% CI, 0.69–0.96); the results of IL-6 antagonists were inconclusive. In patients directly admitted to the ICU, hydroxychloroquine, corticosteroids, and IL-6 antagonists were not associated with decreased mortality.
Conclusions
Both remdesivir and corticosteroids were associated with better outcomes in ward patients with COVID-19. Continuous evaluation of real-world treatment effects is needed.
Keywords: COVID-19, SARS-CoV-2, antiviral, epidemiology, immunosuppressive treatments
Since December 2019, coronavirus disease 2019 (COVID-19) has spread across the world, resulting in a global pandemic [1, 2]. The first case of a severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in the Netherlands was confirmed on February 27, 2020. Two and a half years later, >8 million cases have been registered nationwide. As of January 2022, 4 waves of COVID-19 patients have been identified in the Netherlands (March 2020–June 2020, July 2020–January 2021, February 2021–June 2021, and July 2021–January 2022), which have resulted in >20 000 deaths [3]. The clinical characteristics and outcomes of hospitalized COVID-19 patients have changed during the pandemic waves. Compared with the first, the later waves were less deadly, they involved younger patients with fewer comorbidities, and the disease presentation was less severe [4, 5]. This improvement is, at least partly, due to extensive testing [6] and the development of vaccines against SARS-CoV-2, both extremely effective at preventing severe disease [7]. A small minority of fully vaccinated persons still develop severe COVID-19, but when comparing vaccinated hospitalized COVID-19 patients with unvaccinated patients, they are older, have more comorbidities, and have a higher rate of immunosuppression [8].
During the rapid increase of hospitalizations for COVID-19, novel drug treatments have been tested and, if initial results were encouraging, rapidly implemented in everyday clinical practice through evidence-based guidelines [9–12]. Landmark randomized controlled trials studied antiviral agents (remdesivir [13], molnupiravir [14]), SARS-CoV-2-neutralizing monoclonal antibodies (mAbs; casirivimab/imdevimab [15] and sotrovimab [16]), and immunosuppressive drugs (dexamethasone [17], recombinant interleukin [IL]-1 [18], the IL-6-receptor antagonists tocilizumab and sarilumab [19]) among hospitalized COVID-19 patients [9–11]. Treatments have also been discarded after initial guideline recommendations, such as the use of broad-spectrum antibiotics for all admitted patients [20], lopinavir/ritonavir [21], and hydroxychloroquine [22].
For hospitalized patients with suspected or confirmed COVID-19, the Randomized Evaluation of COVID-19 Therapy (RECOVERY) trial, the global Randomized, Embedded, Multifactorial Adaptive Platform Trial for Community-Acquired Pneumonia (REMAP-CAP), the World Health Organization’s (WHO’s) Solidarity, and the National Institutes of Health (NIH)–initiated Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV) platform trials have been instrumental in identifying treatments that have an impact on mortality [11]. More specifically, the RECOVERY investigators reported that treatment with dexamethasone resulted in an absolute reduction in 28-day mortality of 12.1% among patients receiving invasive mechanical ventilation (IMV) compared with usual care and of 2.9% among those receiving oxygen without IMV [17], treatment with anti-IL-6-receptor antagonists resulted in an absolute reduction in 28-day mortality of 4% [19], and for casirivimab and imdevimab this reduction was 6% in seronegative patients [15]. However, there seems to be a discrepancy between the expected beneficial impact of the implementation of these novel treatment modalities on mortality and the observed real-world effect [23].
Despite all the aforementioned COVID-19 treatments, mortality among patients hospitalized for moderate to severe COVID-19 remains high [24, 25]. Therefore, we aim to describe and compare the clinical characteristics and outcomes for hospitalized COVID-19 patients in the Netherlands during the 4 pandemic waves. Furthermore, we aim to analyze the association of the newly introduced treatments for COVID-19 with in-hospital mortality, 12-week mortality, intensive care unit (ICU) admission, and discharged alive within 29 days.
METHODS
Study Design, Population, and Data Collection
This study was conducted using data from 2 observational cohort studies, that is, the Dutch National Intensive Care Evaluation (NICE) registry [26] and the COVIDPredict [27]. Nationwide aggregated data on hospitalization and outcome of all COVID-19 patients consecutively admitted to all hospitals in the Netherlands were extracted from the NICE registry. The COVIDPredict study is a retrospective observational cohort study including all patients with confirmed COVID-19 from 11 Dutch hospitals varying from peripheral hospitals to large teaching and academic medical centers across the Netherlands. Included patients were aged ≥18 years and hospitalized between February 27, 2020, and December 31, 2021, with confirmed COVID-19, defined as a positive SARS-CoV-2 polymerase chain reaction or a CORADS computed tomography (CT) thorax score >4 at admission [28]. All readmissions for COVID-19 within this time frame were also included. More details regarding patient selection and data collection can be found in the Supplementary Methods.
Patient Consent
The medical ethics committee of the Amsterdam University Medical Centers (Amsterdam UMC; 20.131) stated that no medical ethics approval was required for the NICE registry and approved the design of this work and an opt-out procedure (and waived the need for informed consent) for the COVIDPredict.
Outcomes
The primary outcome was in-hospital mortality. The secondary outcomes were 12-week mortality, ICU admission, and discharge alive within 29 days. Patients were categorized into waves based on date of hospitalization to describe the changing epidemiology during the pandemic in the Netherlands. The definition of the waves can be found in the Supplementary Methods. To analyze the treatment effects, patients were categorized into the following treatment groups: hydroxychloroquine, remdesivir, neutralizing SARS-CoV-2 mAbs, corticosteroids, and IL-6 antagonists. Patients were analyzed comparing patients who received the treatment of interest vs all those who did not. Because of the observational nature of our data, overlap of treatments was allowed. Further specification on the medications used can be found in the Supplementary Methods.
Statistical Analysis
Data distribution was analyzed using Shapiro-Wilk tests and histogram plots. Baseline characteristics, treatments, and outcomes of patients admitted per wave were compared using a 1-way analysis of variance for parametric data, a Kruskal-Wallis test for nonparametric data, and a chi-square test for categorical data, all unadjusted.
Patients admitted directly to the ward and patients admitted directly to the ICU were analyzed separately. Directly admitted to the ward was defined as admitted to the ward directly from the emergency room; directly admitted to the ICU was defined as admitted to the ICU on the day of admission. For the rate of discharge alive analysis, data for patients who died after 29 days were censored at day 29, as previously performed in a large randomized clinical trial executing similar analyses [29]. Patients who were transferred before 29 days were censored. Given the time-dependent nature of treatment exposure, a landmark analysis was used; patients who survived to the landmark time point of 2 days after admission were included. More details regarding this landmark analysis and imputation of missing data can be found in the Supplementary Methods. To analyze in-hospital mortality, 12-week mortality, ICU admission, and discharge alive within 29 days, 4 different Cox regression analyses were used: (1) unadjusted, (2) adjusted for confounding variables, (3) using propensity matching, and (4) using inverse probability weighting. The variables used as confounders in the adjusted Cox regression, the inverse probability weighting, and the propensity score–matching analyses and the methods used for the propensity score matching and inverse probability weighting can be found in the Supplementary Methods. A P value of <.05 was considered statistically significant.
RESULTS
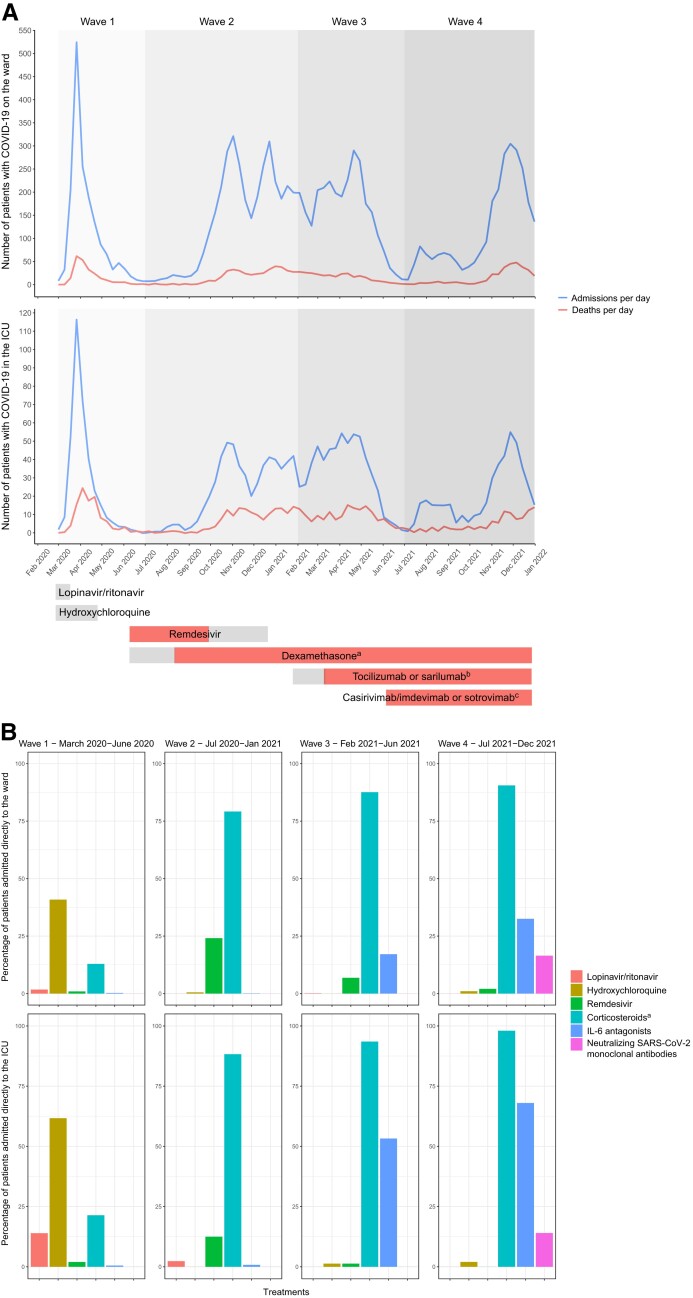
Nationwide aggregated data from the NICE registry showed that between February 27, 2020, and December 31, 2021, 89 110 patients with confirmed COVID-19 were admitted to the ward, and 16 590 patients, either directly or after deterioration, were admitted to the ICU in the Netherlands. Of these, 10 317 COVID-19 patients died on the ward and 4511 in the ICU. In this time period, 6 novel drug treatment modalities were implemented through national guidelines [10], either as standard or optional care for patients hospitalized for COVID-19: lopinavir/ritonavir, hydroxychloroquine, remdesivir, casirivimab/imdevimab or sotrovimab, dexamethasone, and tocilizumab or sarilumab (Figure 1A).
Figure 1.
Admissions, mortality, and treatments for COVID-19 in the first, second, third, and fourth waves in the Netherlands. A, Admissions, mortality, and novel antiviral and immunosuppressive treatments for COVID-19 in the first, second, third, and fourth waves in the Netherlands. Between February 27, 2020, and December 31, 2021, 89 110 patients with confirmed COVID-19 were admitted to the ward and 16 590 patients to the ICU in the Netherlands; 10 317 COVID-19 patients died on the ward and 4511 in the ICU. In this time period, 6 novel drug treatment modalities were implemented through national guidelines [10], either as standard or optional care for patients hospitalized because of COVID-19. Gray bars: included in national treatment guidelines as optional care; red bars: included as standard care. aDexamethasone was first only recommended for ICU patients, since September 29, 2020, also for ward patients. bIL-6 antagonists were first only recommended for ICU patients, since March 9, 2021, also for ward patients. cCasirivimab/imdevimab was recommended until December 23, 2021; sotrovimab was recommended since December 23, 2021. B, Administration of novel antiviral and immunosuppressive treatments for hospitalized COVID-19 in the first, second, third, and fourth waves in the Netherlands. The percentage of patients included in the COVIDPredict trial treated with the 6 novel drug treatment modalities implemented through national guidelines in the time period between February 27, 2020, and December 31, 2021. aIncluded dexamethasone, prednisolone, hydrocortisone, and methylprednisolone. Abbreviations: COVID-19, coronavirus disease 2019; ICU, intensive care unit; IL, interleukin; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Of the 8093 patients included in the COVIDPredict database, 5643 (69.7%) met our inclusion criteria; 5187 patients were directly admitted to the ward, and 456 patients were directly admitted to the ICU (see the Supplementary Results for more detailed information regarding the excluded patients). The overall median age of patients admitted to the ward for COVID-19 across all waves (interquartile range [IQR]) was 66 (56–77) years, and 3048 (59%) were males. Thirteen percent (n = 686) of the patients were admitted to the ICU, and 17% (n = 891) died during hospitalization (Table 1). For those patients directly admitted to the ICU across all waves, the median age (IQR) was 65 (57–72) years, and 340 (75%) were males. Twenty-six percent (n = 119) of patients died in the ICU, and 33% (n = 152) died during admission (Tables 1 and 2).
Table 1.
Characteristics and Outcomes of Patients Directly Admitted to the Ward
| … | All Patients | 1st Wave | 2nd Wave | 3rd Wave | 4th Wave | P |
|---|---|---|---|---|---|---|
| Number of patients admitted with COVID-19 | 5187 | 2458 | 1542 | 701 | 486 | |
| Dominant strain | … | Original (Wuhan) strain | Original (Wuhan) strain | Alpha | Delta | |
| Demographics | … | … | … | … | … | |
| Sex—male, No. (%) | 3048 (58.8) | 1499 (61.0) | 898 (58.2) | 404 (57.7) | 247 (50.9)a,b | <.001 |
| Age, median [IQR], y | 66.10 [56.00–77.00] | 68.00 [57.00–78.00] | 67.00 [57.00–77.00] | 63.00 [52.00–72.00]a,b | 65.00 [51.00–77.00]a,b | <.001 |
| Body mass index, median [IQR], kg/m2 | 27.47 [24.52–31.41] | 27.16 [24.39–30.83] | 27.51 [24.58–31.53] | 28.06 [24.85–32.44]a | 28.08 [24.66–32.72]a | .001 |
| Race, No. (%) | … | … | a | a | a,c | <.001 |
| Arab | 417 (9.7) | 171 (8.1) | 144 (11.3) | 56 (10.9) | 46 (11.4) | |
| Asian | 227 (5.3) | 98 (4.6) | 81 (6.4) | 37 (7.2) | 11 (2.7) | |
| Black | 428 (10.0) | 144 (6.8) | 155 (12.2) | 70 (13.7) | 59 (14.7) | |
| White | 3087 (71.8) | 1651 (78.2) | 840 (66.0) | 321 (62.7) | 275 (68.4) | |
| Other | 139 (3.2) | 47 (2.2) | 53 (4.2) | 28 (5.5) | 11 (2.7) | |
| Baseline characteristics | … | … | … | … | … | |
| Days since onset of symptoms, median [IQR] | 8.00 [5.00–11.00] | 7.00 [5.00–12.00] | 8.00 [5.00–11.00] | 9.00 [6.00–11.00]a,b | 7.00 [4.00–9.00]a,b,c | <.001 |
| Transfer from other hospital, No. (%) | 281 (5.4) | 49 (2.0) | 93 (6.0)a | 109 (15.5)a,b | 30 (6.2)a,c | <.001 |
| Do not resuscitate and/or intubate order, No. (%) | 1426 (31.0) | 722 (35.8) | 423 (28.9)a | 147 (21.7)a,b | 134 (30.0)c | <.001 |
| Vaccinated against SARS-CoV-2, No. (%) | 261 (9.2) | 0 (0.0) | 1 (0.1) | 56 (8.8)a,b | 204 (45.0)a,b,c | <.001 |
| Medical history | … | … | … | … | … | |
| Patients with comorbidities,d No. (%) | 3843 (83.7) | 1778 (83.2) | 1188 (85.4) | 493 (81.8) | 384 (83.7) | .170 |
| Number of comorbidities,d median [IQR] | 2.00 [1.00–3.00] | 2.00 [1.00–3.00] | 2.00 [1.00–3.00] | 2.00 [1.00–3.00]a,b | 2.00 [1.00–3.00] | .002 |
| Asthma or other chronic pulmonary disease, No. (%) | 1307 (25.4) | 632 (26.0) | 419 (27.3) | 160 (22.9) | 96 (19.8)a,b | .003 |
| Chronic cardiac disease, No. (%) | 1537 (29.9) | 750 (30.8) | 455 (29.8) | 180 (25.9) | 152 (31.3) | .081 |
| Chronic kidney disease, No. (%) | 672 (13.1) | 299 (12.3) | 214 (14.0) | 90 (12.9) | 69 (14.2) | .406 |
| Diabetes, No. (%) | 1058 (20.4) | 464 (18.9) | 360 (23.3)a | 138 (19.7) | 96 (19.8) | .007 |
| Hypertension, No. (%) | 2300 (44.7) | 1127 (46.1) | 701 (46.0) | 274 (39.3)a,b | 198 (41.0) | .003 |
| Malignancy, No. (%) | 385 (7.5) | 196 (8.1) | 118 (7.7) | 39 (5.6) | 32 (6.6) | .140 |
| Organ transplantation, No. (%) | 128 (2.5) | 32 (1.3) | 30 (2.0) | 36 (5.2)a,b | 30 (6.4)a,b | <.001 |
| Disease severity | … | … | … | … | … | |
| MEWS, median [IQR] | 2.00 [1.00–4.00] | 3.00 [1.00–4.00] | 2.00 [1.00–4.00] | 2.00 [1.00–3.00]a | 3.00 [2.00–4.00]c | <.001 |
| CT Severity Score, median [IQR] | 10.00 [6.00–14.00] | 8.00 [5.00–12.00] | 11.00 [8.00–15.00]a | 12.00 [10.00–16.00]a,b | 12.00 [9.00–15.00]a | <.001 |
| 4C Mortality Score, median [IQR] | 10.00 [7.00–13.00] | 10.00 [6.00–13.00] | 10.00 [7.00–13.00] | 9.00 [6.00–12.00]b | 10.00 [7.00–13.00]c | .002 |
| Outcomes | … | … | … | … | … | |
| Venous thromboembolism, No. (%) | 278 (5.4) | 133 (5.4) | 75 (4.9) | 43 (6.1) | 27 (5.6) | .654 |
| Length of hospital stay, median [IQR], d | 6.00 [3.00–11.00] | 5.00 [3.00–10.00] | 6.00 [3.00–10.00]a | 7.00 [4.00–12.00]a,b | 8.00 [4.00–14.00]a,b | <.001 |
| In-hospital mortality, No. (%) | 891 (17.2) | 510 (20.7) | 223 (14.5)a | 84 (12.0)a | 74 (15.2)a | <.001 |
| 12-wk mortality, No. (%) | 973 (18.8) | 550 (22.4) | 249 (16.1)a | 96 (13.7)a | 78 (16.0)a | <.001 |
| ICU admission, No. (%) | 686 (13.2) | 309 (12.6) | 172 (11.2) | 119 (17.0)a,b | 86 (17.7)a,b | <.001 |
| Mechanical ventilation, No. (%) | 497 (72.9) | 263 (85.7) | 112 (65.5)a | 74 (62.7)a | 48 (55.8)a | <.001 |
| Ventilator-free days, median [IQR] | 13.00 [0.00–22.00] | 8.00 [0.00–19.00] | 13.00 [0.00–23.00] | 20.00 [0.00–25.00]a | 19.50 [0.00–23.00]a | <.001 |
| Length of ICU stay, median [IQR], d | 8.00 [3.00–16.00] | 10.00 [5.00–19.00] | 7.00 [2.00–15.00]a | 7.00 [3.00–13.00]a | 5.00 [2.00–11.00]a | <.001 |
| ICU mortality, No. (%) | 167 (24.3) | 88 (28.5) | 42 (24.4) | 23 (19.3) | 14 (16.3) | .057 |
Abbreviations: CT, computed tomography; ICU, intensive care unit; MEWS, Modified Early Warning Score.
Significant vs first wave using a Dunn's test of multiple comparisons using rank sums for nonparametric continuous variables, a Tukey test for parametric continuous variables, and a Bonferroni correction for categorical variables.
Significant vs second wave using a Dunn's test of multiple comparisons using rank sums for nonparametric continuous variables, a Tukey test for parametric continuous variables, and a Bonferroni correction for categorical variables.
Significant vs third wave using a Dunn's test of multiple comparisons using rank sums for nonparametric continuous variables, a Tukey test for parametric continuous variables, and a Bonferroni correction for categorical variables.
Comorbidities include chronic cardiac disease, hypertension, chronic pulmonary disease, asthma, chronic kidney disease, liver disease, chronic neurologic disease, malignancy, chronic hematologic disease, HIV or AIDS, diabetes, rheumatic disorder, auto-immune disease, dementia, and obesity.
Table 2.
Characteristics and Outcomes of Patients Admitted Directly to the ICU
| … | All Patients | 1st Wave | 2nd Wave | 3rd Wave | 4th Wave | P |
|---|---|---|---|---|---|---|
| Number of patients admitted with COVID-19 | 456 | 201 | 128 | 77 | 50 | |
| Dominant strain | … | Original (Wuhan) strain | Original (Wuhan) strain | Alpha | Delta | |
| Demographic characteristics | … | … | … | … | … | |
| Sex—male, No. (%) | 340 (74.6) | 157 (78.1) | 93 (72.7) | 53 (68.8) | 37 (74.0) | .404 |
| Age, median [IQR], y | 65.00 [56.77–72.00] | 66.10 [59.00–73.00] | 64.50 [56.00–72.00] | 64.00 [52.00–72.00] | 62.00 [56.00–69.00] | .026 |
| Body mass index, median [IQR], kg/m2 | 28.04 [24.97–31.24] | 27.76 [25.17–29.93] | 27.26 [24.53–32.31] | 28.74 [25.56–33.33] | 29.06 [24.81–32.14] | .318 |
| Race, No. (%) | … | … | … | … | … | .207 |
| Arab | 40 (11.7) | 16 (10.0) | 13 (14.4) | 8 (15.7) | 3 (7.1) | |
| Asian | 27 (7.9) | 9 (5.6) | 7 (7.8) | 6 (11.8) | 5 (11.9) | |
| Black | 27 (7.9) | 10 (6.2) | 9 (10.0) | 2 (3.9) | 6 (14.3) | |
| White | 233 (67.9) | 119 (74.4) | 55 (61.1) | 31 (60.8) | 28 (66.7) | |
| Other | 16 (4.7) | 6 (3.8) | 6 (6.7) | 4 (7.8) | 0 (0.0) | |
| Baseline characteristics | … | … | … | … | … | |
| Days since onset of symptoms, median [IQR] | 8.00 [6.00–11.25] | 9.00 [7.00–13.00] | 8.00 [6.00–10.00] | 9.00 [6.00–12.00] | 8.50 [5.00–11.00] | .181 |
| Transfer from other hospital, No. (%) | 109 (23.9) | 43 (21.4) | 32 (25.0) | 28 (36.4) | 6 (12.0)c | .010 |
| Do not resuscitate and/or intubate order, No. (%) | 76 (18.2) | 35 (19.9) | 18 (15.0) | 14 (18.4) | 9 (19.6) | .748 |
| Vaccinated against SARS-CoV-2, No. (%) | 25 (9.2) | 0 (0.0) | 0 (0.0) | 7 (11.1)b | 18 (36.7)a,b,c | <.001 |
| Medical history | … | … | … | … | … | |
| Patients with comorbidities,d No. (%) | 336 (80.6) | 147 (82.6) | 97 (80.2) | 55 (79.7) | 37 (75.5) | .727 |
| Number of comorbidities,d median [IQR] | 2.00 [1.00–3.00] | 2.00 [1.00–3.00] | 2.00 [1.00–3.00] | 2.00 [1.00–3.00] | 2.00 [1.00–3.00] | .921 |
| Asthma or other chronic pulmonary disease, No. (%) | 110 (24.4) | 55 (27.8) | 27 (21.1) | 19 (25.7) | 9 (18.0) | .368 |
| Chronic cardiac disease, No. (%) | 117 (26.1) | 63 (32.0) | 23 (18.1)a | 20 (27.0) | 11 (22.0) | .042 |
| Chronic kidney disease, No. (%) | 44 (9.8) | 20 (10.2) | 12 (9.4) | 7 (9.3) | 5 (10.0) | .994 |
| Diabetes, No. (%) | 112 (24.6) | 50 (24.9) | 41 (32.0) | 14 (18.2) | 7 (14.0) | .036 |
| Hypertension, No. (%) | 208 (46.1) | 101 (51.0) | 55 (43.0) | 30 (40.0) | 22 (44.0) | .303 |
| Malignancy, No. (%) | 21 (4.7) | 10 (5.2) | 7 (5.6) | 3 (4.0) | 1 (2.0) | .756 |
| Organ transplantation, No. (%) | 5 (1.1) | 2 (1.0) | 2 (1.6) | 1 (1.3) | 0 (.0) | .844 |
| Disease severity | … | … | … | … | … | |
| MEWS, median [IQR] | 4.00 [3.00–6.00] | 4.00 [3.00–6.00] | 4.00 [3.00–5.00] | 4.00 [3.00–6.00] | 4.00 [2.00–6.00] | .582 |
| CT Severity Score, median [IQR] | 18.00 [14.75–22.00] | 17.00 [5.00–21.00] | 18.00 [15.00–21.00] | 20.00 [18.00–22.00] | 18.00 [15.00–23.00] | .078 |
| 4C Mortality Score, median [IQR] | 12.00 [10.00–14.00] | 12.00 [10.00–14.00] | 12.00 [9.00–14.00] | 11.00 [9.00–14.00] | 11.00 [10.00–13.00] | .218 |
| Clinical course data | … | … | … | … | … | |
| Venous thromboembolism, No. (%) | 80 (17.5) | 41 (20.4) | 22 (17.2) | 9 (11.7) | 8 (16.0) | .384 |
| Mechanical ventilation, No. (%) | 346 (76.4) | 168 (84.4) | 92 (72.4) | 54 (70.1)a | 32 (64.0)a | .003 |
| Ventilator-free days, median [IQR], d | 11.09 (10.61) | 9.18 (9.82) | 11.26 (11.22) | 15.20 (10.69)a | 13.45 (10.53) | .002 |
| Length of hospital stay, median [IQR], d | 15.00 [9.00–26.25] | 17.00 [9.00–31.00] | 14.00 [10.00–22.00] | 13.50 [9.00–23.00] | 21.00 [8.00–26.00] | .694 |
| Length of ICU stay, median [IQR], d | 9.00 [4.00–18.50] | 11.00 [5.00–23.00] | 8.00 [3.00–14.00] | 7.00 [3.00–13.00]a | 7.00 [3.00–13.00] | .004 |
| ICU mortality, No. (%) | 119 (26.1) | 60 (29.9) | 35 (27.3) | 16 (20.8) | 8 (16.0) | .148 |
| In-hospital mortality, No. (%) | 152 (33.3) | 79 (39.3) | 39 (30.5) | 18 (23.4) | 16 (32.0) | .067 |
| 12-wk mortality, No. (%) | 156 (34.2) | 80 (39.8) | 41 (32.0) | 18 (23.4) | 17 (34.0) | .069 |
Abbreviations: CT, computed tomography; ICU, intensive care unit; MEWS, Modified Early Warning Score.
Significant vs first wave using a Dunn's test of multiple comparisons using rank sums for nonparametric continuous variables, a Tukey test for parametric continuous variables, and a Bonferroni correction for categorical variables.
Significant vs second wave using a Dunn's test of multiple comparisons using rank sums for nonparametric continuous variables, a Tukey test for parametric continuous variables, and a Bonferroni correction for categorical variables.
Significant vs third wave using a Dunn's test of multiple comparisons using rank sums for nonparametric continuous variables, a Tukey test for parametric continuous variables, and a Bonferroni correction for categorical variables.
Comorbidities include chronic cardiac disease, hypertension, chronic pulmonary disease, asthma, chronic kidney disease, liver disease, chronic neurologic disease, malignancy, chronic hematologic disease, HIV or AIDS, diabetes, rheumatic disorder, auto-immune disease, dementia, and obesity.
Changing Epidemiology—Ward
The percentage of males decreased over the waves (from 61%, n = 1499, in wave 1 to 51%, n = 247, in wave 4; P < .001), as well as the median age (from 68 to 65 years; P < .001) (Table 1). The median Modified Early Warning Score (MEWS) on admission (IQR) was lower in wave 3 compared with the first wave (2 [1–3] compared with 3 [1–4]; P = .002). The crude in-hospital mortality of ward patients decreased from 21% (n = 510) in wave 1 to 15% (n = 74) in wave 4 (P < .001), while the crude ICU mortality in patients first admitted to the ward showed a positive trend (29%, n = 88, in wave 1 to 16%, n = 14, in wave 4; P = .057) (Table 1). Data regarding bloodstream infections (BSIs) can be found in the Supplementary Results and Supplementary Table 1.
With regards to the antiviral and immunosuppressive drugs administered, lopinavir/ritonavir and hydroxychloroquine were almost solely administered in wave 1 and remdesivir mostly in wave 2. Corticosteroids became standard treatment for hospitalized patients needing oxygen in wave 2, IL-6 antagonists in wave 3, and neutralizing SARS-CoV-2 mAbs were solely given in wave 4 (Figure 1B;Supplementary Figure 1). Overlap in treatments is specified in Supplementary Table 2. The most frequently occurring combinations were corticosteroids with remdesivir, IL-6 antagonists, or neutralizing SARS-CoV-2 mAbs.
Changing Epidemiology—ICU
For those patients admitted to the ICU, the median age decreased over time (from 66 years in wave 1 to 62 years in wave 4; P = .026) (Table 2). The crude ICU mortality showed a positive trend during the 4 waves (from 30%, n = 60, in wave 1 to 16%, n = 8, in wave 4; P = .148). More data on the changing epidemiology can be found in Figure 2B, Table 2, the Supplementary Results, and Supplementary Tables 3 and 4.
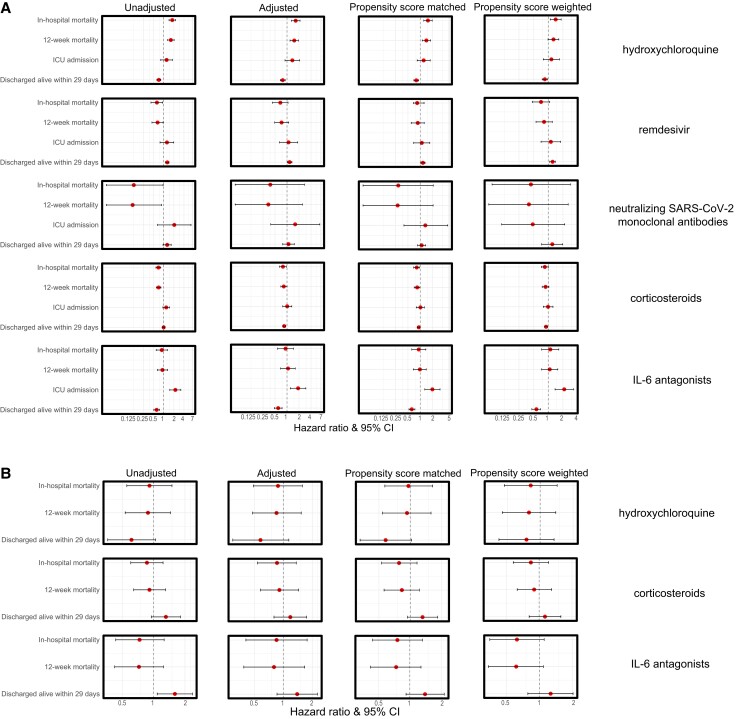
Figure 2.
Hazard ratios of treatment effects per treatment group. A, Patients admitted directly to the ward. B, Patients admitted directly to the ICU. Remdesivir and SARS-CoV-2-neutralizing monoclonal antibodies were not analyzed given the small sample size. Abbreviations: ICU, intensive care unit; IL, interleukin; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Association of Antivirals With Mortality—Ward
Over 80% of antiviral and/or immunosuppressive treatments were initiated within the first 2 days after hospital or ICU admission (Supplementary Figure 2). Therefore, landmarking at 2 days seemed the optimal time window for the analyses. Percentages of missing variables, information on propensity score matching and weighting, and the violation of the proportionality assumption can be found in the Supplementary Results, Supplementary Figures 3, 4, and 5, and Supplementary Table 5.
Ward patients treated with hydroxychloroquine in the first 2 days after admission showed an increased risk of mortality, with a hazard ratio (HR) of 1.65 (95% CI, 1.29–2.12) for in-hospital mortality and an HR of 1.52 (95% CI, 1.19–1.94) for 12-week mortality, and were at decreased risk for discharge alive, with an HR of 0.78 (95% CI, 0.68–0.91) in the propensity score–matched cohort. Propensity score weighting analysis showed similar results (Figure 2A; Supplementary Tables 6 and 7).
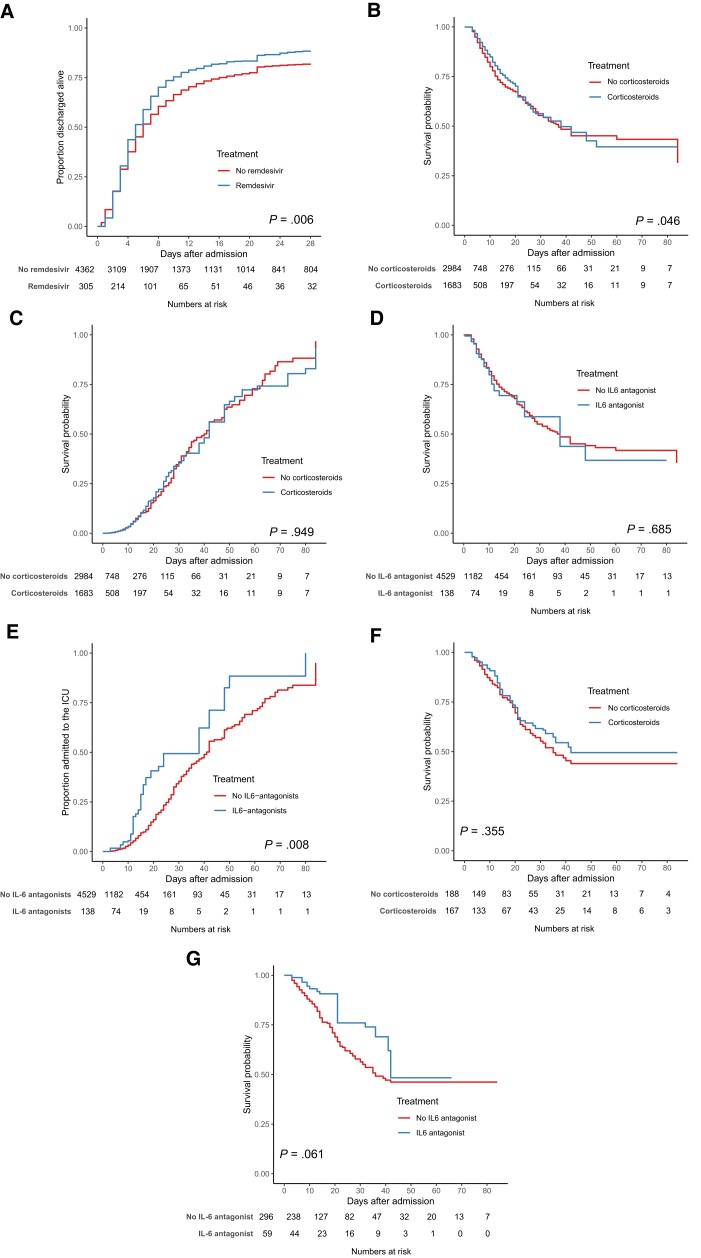
For remdesivir, the opposite association was seen. Patients treated with remdesivir showed decreased in-hospital mortality (9% vs 15%; P = .015) and increased rates of discharge alive (89% vs 82%; P = .011) using propensity score weighting; however, this was not the case in the propensity score–matched cohort (Supplementary Table 6). Patients treated with remdesivir were more likely to be discharged alive, with an HR of 1.16 (95% CI, 1.03–1.31) in the propensity score–matched cohort and 1.23 (95% CI, 1.07–1.40) in the propensity score–weighted cohort (Figures 2A and 3A; Supplementary Table 7).
Figure 3.
Effect of treatment in hospitalized COVID-19 patients of remdesivir (A), corticosteroids (B, C, F), and IL-6 antagonists (D, E, G) in patients directly admitted to the ward (A–E) and the ICU (F, G) in the propensity-weighted cohort. The treatment effect of remdesivir on discharge alive within 29 days is shown in patients directly admitted to the ward (A); the treatment effect of corticosteroids on in-hospital (B) and ICU admission (C) is shown in patients directly admitted to the ward, as well as the treatment effect of IL-6 antagonists on in-hospital (D) and ICU admission (E) in patients directly admitted to the ward. The treatment effect of corticosteroids (F) and IL-6 antagonists (G) is shown in patients directly admitted to the ICU. Abbreviations: COVID-19, coronavirus disease 2019; ICU, intensive care unit; IL, interleukin.
In patients treated with neutralizing SARS-CoV-2 mAbs, no significant association with mortality or being discharged alive was seen after adjusting for confounders. SARS-CoV-2 mAbs had a positive association with ICU admission in the propensity score–weighted cohort (2% vs 7%; P = .041) (Supplementary Table 6).
Association of Immunosuppressive Treatment With Mortality—Ward
Treatment with corticosteroids was significantly associated with lower in-hospital mortality and 12-week mortality and higher rates of discharge alive in the propensity score analyses (in-hospital mortality 12% vs 16%; P = .003; 12-week mortality 14% vs 17%; P = .004; and discharge alive within 29 days 85% vs 81%; P = .002). However, in the propensity score–weighted cohort, there was no significant difference (Figures 2A and 3B and C; Supplementary Table 6). Patients had a lower risk of in-hospital mortality (HR, 0.81; 95% CI, 0.69–0.96) and 12-week mortality (HR, 0.84; 95% CI, 0.71–0.98) in the propensity score–matched cohort (Figure 2A; Supplementary Table 7).
Patients treated with IL-6 antagonists showed increased 12-week mortality, ICU admission, and a lower number discharged alive in both the propensity score–matched cohort and the propensity score–weighted cohort (12-week mortality 26% vs 16%; P = .007; ICU admission 20% vs 6%; P < .001; and discharge alive within 29 days 72% vs 83% in the propensity score–weighted cohort; P = .005) (Figure 3D; Supplementary Table 6). Patients treated with IL-6 antagonists were at increased risk of ICU admission (HR, 1.98; 95% CI, 1.29–3.04; in the propensity score–matched cohort; HR, 2.13; 95% CI, 1.38–3.30; in the propensity score–weighted cohort) (Figure 2A and 3E; Supplementary Table 7).
Association of Antivirals and Immunosuppressive Treatment With Mortality—ICU
In patients directly admitted to the ICU who were treated with either hydroxychloroquine, corticosteroids, or IL-6 antagonists, no associations were seen with mortality or the rate of being discharged alive and use of any of these compounds. More data on the association of antivirals and immunosuppressive treatment with mortality can be found in Figure 3F and G, the Supplementary Results, and Supplementary Tables 8 and 9.
Since hydroxychloroquine is associated with a significant increase in mortality in patients directly admitted to the ward, we conducted a subanalysis excluding patients admitted in the first wave, as hydroxychloroquine was mostly administered in the first wave. In contrast to our main analyses, in patients admitted directly to the ICU in the second, third, and fourth waves, treatment with corticosteroids was associated with a significant decrease in in-hospital and 12-week mortality in the adjusted Cox regression (HR, 0.27; 95% CI, 0.09–0.75; HR, 0.33; 95% CI 0.11–0.89), this association was not seen in the propensity score–matched and propensity score–weighted analyses. See the Supplementary Results and Supplementary Figures 6 and 7 for more results.
DISCUSSION
In this observational cohort study of 5643 hospitalized COVID-19 patients, we observed a changing epidemiology during the 4 pandemic waves in the Netherlands. Over time, the percentage of males and the mean age of admitted patients decreased. While the in-hospital mortality of ward patients decreased over time, the in-hospital mortality of ICU patients did not change significantly. Using multiple Cox regression techniques, we found the following effects of the newly introduced COVID-19 drug treatments. First, hydroxychloroquine was associated with higher mortality and a lower rate of discharge alive. Second, remdesivir was positively associated in ward patients with the rate of being discharged alive within 29 days in all analyses. Third, in hospital SARS-CoV-2 monoclonal antibody treatment was associated with a lower rate of ICU admission but not mortality in the propensity score–weighted cohort of ward patients. Fourth, corticosteroid treatment was associated with mortality, and with higher rates of being discharged in the propensity score–matched cohort of patients directly admitted to the ward. Fifth, anti-IL-6 treatment in patients directly admitted to the ward was associated with increased mortality and ICU admission. Last, in patients directly admitted to the ICU who were treated with either hydroxychloroquine, corticosteroids, or IL-6 antagonists, no associations were seen with mortality or the rate of being discharged alive. As we investigated the real-life effectiveness of COVID-19 treatments, patients included in these analyses were often treated with >1 of the analyzed treatments.
Our findings on the changes in the patient characteristics of admitted patients and their outcomes during the consecutive waves of the pandemic are in line with several cohort studies performed in Italy, Spain, and the United States in which the second [4, 30, 31] and third waves [32, 33] were compared with the first. We now further expand this body of literature by including a fourth wave. We observed a nonsignificant decrease in ICU mortality during the 4 pandemic waves, possibly related to the sample size of our ICU cohort. In previously published data from the NICE including all COVID-19 patients admitted to the ICU in the Netherlands, a decreased mortality rate in the third wave compared with the first and second was indeed found [31]. Of interest, a recent study from South Africa found a different pattern of characteristics and outcomes in patients hospitalized with COVID-19 in the early phase of the fourth wave compared with earlier waves, with younger patients having fewer comorbidities, and a decrease in severity and mortality [5].
The increased mortality associated with hydroxychloroquine use is in line with earlier meta-analyses [22]. The same holds true for the effectiveness of remdesivir in nonventilated patients with COVID-19 requiring oxygen therapy [29, 34]. This is of interest, as the use of remdesivir was no longer advised in Dutch national guidelines after the negative interim analysis of the SOLIDARITY trial on remdesivir in admitted COVID-19 patients, published late 2020 [10, 35]. In contrast to earlier reports on the beneficial use of SARS-CoV-2 mAbs in (seronegative) hospitalized COVID-19 patients, we did not observe an association with mortality, although the sample size in this subanalysis was relatively low [11, 15].
The most recent Cochrane review assessing the treatment effect of systemic corticosteroids concluded that this treatment leads to slightly reduced all-cause mortality in people hospitalized for symptomatic COVID-19 with a risk ratio of 0.89 (95% CI, 0.80–1.00) [36], similar to our results in patients directly admitted to the ward. In our ICU cohort, anti-IL-6 treatment was not associated with decreased mortality. This is in accordance with a Bayesian reanalysis of a previous meta-analysis of 15 studies of hospitalized patients with COVID-19 treated with tocilizumab and corticosteroids, in which the use of oxygen only and noninvasive ventilation (NIV) was associated with a probability of a clinically meaningful mortality benefit from tocilizumab [37]. This study reported no convincing evidence for patients receiving IMV to benefit from tocilizumab [37].
The strengths of the present study are worth emphasizing. To our knowledge, this is the first time that the real-world effectiveness of the novel introduced treatments for COVID-19 have been studied this extensively. Another strength is the use of a landmark analysis in order to prevent bias; many cohort studies investigating treatment effect introduce biases, mainly selection and immortal time bias, leading to nonmeaningful results [23]. Lastly, as treatment for COVID-19 might be dependent on baseline characteristics linked to adverse outcomes like mortality, ICU admission, and a longer hospital stay, we used multiple Cox regression techniques to deal with this confounding and produce robust results. The outcome of ICU admission was most likely not influenced by a shortage of ICU beds. In the Netherlands, a critical shortage of ICU beds was a continuous threat, especially during the first wave [38], but did not reach the critical threshold upon which the national Intensive Care Triage Protocol had to be implemented. This study has several limitations. First, the changes in variants of the SARS-CoV-2 virus during our study period; even though we corrected for several baseline characteristics, the new genetic mutations could have had an influence on the outcomes and treatment effects. Second, the vaccination status of patients was only systematically collected after the SARS-CoV-2 vaccinations started to become widely available; therefore, we did not incorporate it into our analyses. Vaccines against SARS-CoV-2, all extremely effective at preventing severe disease and lowering mortality [7], have probably influenced the treatment effect. Unfortunately, this information was missing in our data. Third, even though several types of analyses were used, we were not able to match patients directly admitted to the ward treated with IL-6 antagonists with those not treated on admission, leading to a higher ICU admission rate in patients treated with IL-6 antagonists. Fourth, in patients directly admitted to the ward treated with neutralizing SARS-CoV-2 mAbs, the event rate was low, with only 1 deceased patient and 3 admitted to the ICU. Fifth, not all adjusted Cox regression analyses met the proportionality assumption. However, all analyses using propensity scores met this assumption; these are overall more favorable analyses than traditional regression analyses [39]. Several treatment changes over the pandemic waves were not analyzed here as our focus was on antiviral and immunosuppressive agents. In some hospitals in the Netherlands, higher prophylactic or therapeutic-dose low–molecular weight heparin was given in the ICU after a positive influence on mortality was published [40]. Furthermore, NIV and high-flow nasal oxygen therapy were more often used during later waves compared with IMV [31], reflected by more ICU admissions with lower intubation rates during the waves in our data.
Several clinicians and researchers have advocated for a more personalized approach in the treatment of COVID-19 patients [41]. The host response to SARS-CoV-2 is complex, with pathways that can be both beneficial and destructive. Immunomodulatory agents modifying these pathways can be effective for some patients, while for others they can ineffective or even harmful [11]. For example, the use of corticosteroids showed significant survival benefits in patients with the hyperinflammatory phenotype [42], and in ICU-admitted patients a beneficial effect of corticosteroids was seen in older and more severely ill patients while mortality was increased when administered within 7 days of onset [43]. Early administration of tocilizumab was associated with improvement in oxygenation in patients with high IL-6, while patients with low IL-6 treated with tocilizumab showed similar mortality rates as patients with high IL-6 not treated with tocilizumab [44]. In addition, targeted treatment with anakinra, an IL-1 receptor antagonist, reduced mortality in hospitalized patients at risk for unfavorable outcomes using baseline soluble urokinase plasminogen activator receptor (suPAR) as a biomarker [18]. This might be the future for more positive treatment effects in hospitalized COVID-19 patients. The variable treatment effects, depending on a patient’s host response and disease severity, might explain some of the contrasts from clinical studies. Personalized immunotherapy in COVID-19 needs to be further investigated through randomized clinical trials by looking into the most favorable biomarker-driven therapies [11]. Finally, we know from milder viral pneumonia such as influenza that steroids result in worse outcomes [45]. It can be questioned whether steroids are still beneficial in the milder COVID-19 variants as the clinical phenotype becomes more and more an influenza-like virus.
In summary, we observed a changing epidemiology during the 4 pandemic COVID-19 waves in the Netherlands, with younger patients per wave and lower in-hospital mortality for patients directly admitted to the ward. In our cohort of hospitalized patients, we only found positive associations of remdesivir and corticosteroids with the rate of being discharged alive within 29 days and, respectively, the in-hospital and 12-week mortality in patients directly admitted to the ward. Given the ongoing evolution of the SARS-CoV-2 virus with novel, clinically significant mutations appearing at a steady state during changing patient characteristics over time, it is essential to continuously re-evaluate the real-world effectiveness of newly introduced drugs to treat COVID-19.
Supplementary Material
Acknowledgments
We would like to thank all the members of the NICE and the COVIDPredict study group for the effort to collect patient data during the challenging situation of the pandemic.
The COVIDPredict Study Group. Brent Appelman, Center of Experimental and Molecular Medicine, Location Academic Medical Center, Amsterdam UMC, Amsterdam, the Netherlands. Michiel Schinkel, Center of Experimental and Molecular Medicine, Location Academic Medical Center, Amsterdam UMC, Amsterdam, the Netherlands. Martijn Beudel Department of Neurology, Amsterdam University Medical Centers, Amsterdam Neuroscience, University of Amsterdam, Amsterdam, the Netherlands, Paul Elbers, Department of Intensive Care, Amsterdam University Medical Centers, Amsterdam Neuroscience, University of Amsterdam, Amsterdam, the Netherlands. Ronald Henry, Department of Internal Medicine, Maastricht University Medical Center, Maastricht, the Netherlands. Esther K. Haspels-Hogervorst, Department of Intensive Care Medicine, Martini Ziekenhuis, Groningen, the Netherlands. Daisy Rusch, Research, Martini Ziekenhuis, Groningen, the Netherlands. Niels C. Gritters van den Oever, Department of Intensive Care, Treant Zorggroep, Hoogeveen, the Netherlands. Suat Simsek, Department of Internal Mediicine, Noordwest Hospital (NWZ), Alkmaar, the Netherlands. W. de Ruijter, Department of Intensive Care Medicine, Noordwest Hospital (NWZ), Alkmaar, the Netherlands. Frits H.M. van Osch, Department of Clinical Epidemiology, VieCuri Medical Centre, Venlo, the Netherlands, and NUTRIM, School for Nutrition and Translational Research in Metabolism, Maastricht University, Maastricht, the Netherlands. Joop P. van den Bergh, Department of Internal Medicine, VieCuri Medical Centre, Venlo, the Netherlands, and NUTRIM, School for Nutrition and Translational Research in Metabolism, Maastricht University, Maastricht, the Netherlands. Martijn D. de Kruif, Department of Pulmonary Medicine, Zuyderland Medical Centre Heerlen, Heerlen, the Netherlands. Renee Douma, Department of Internal Medicine, Flevoziekenhuis, Almere, Flevoland, the Netherlands. Lianne R. de Haan, Department of Internal Medicine, Flevoziekenhuis, Almere, Flevoland, the Netherlands. Hazra Moeniralam, Department of Internal Medicine, St. Antonius Hospital, Nieuwegein, the Netherlands. Kees Brinkman, Department of Internal Medicine, OLVG, Amsterdam, the Netherlands. N. Bokhizzou, Department of Internal Medicine, BovenIJ, Amsterdam, the Netherlands.
Financial support. This work was supported by the Talud Foundation (Stichting Talud) for the Amsterdam UMC Corona Research Fund, the Netherlands Organization for Scientific Research (VIDI grant number 91716475 to W.J.W.), the Netherlands Organisation for Health Research and Development (ZonMw; TURN-COVID grant number 10430142110001 to W.J.W.), and the H2020 EU ImmunoSep study (847422).
Disclaimer. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author contributions. M.S., B.A., H.P.S., W.J.W., and L.v.V. conceived and designed the study. M.S., B.A., D.D., N.K., R.S., A.V., and W.J.W. participated in data collection. M.S. and L.v.V. accessed and verified all the data. M.S., H.P.S., and L.v.V. performed statistical analysis. M.S., H.P.S., M.M., A.V., W.J.W., and L.v.V. participated in data analysis. M.S., W.J.W., and L.v.V. drafted the article. B.A., H.P.S., D.D., N.K., R.S., M.d.B., M.M., and A.V. participated in revision of the manuscript. All authors gave final approval for the version to be submitted. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Data sharing. The data that support the findings of this study are available from the corresponding author (M.S.) upon reasonable request.
Contributor Information
Marleen A Slim, Center for Experimental and Molecular Medicine, Amsterdam University Medical Centers—Location AMC, University of Amsterdam, Amsterdam Institute for Infection and Immunity, Amsterdam, the Netherlands; Department of Intensive Care, Amsterdam University Medical Centers—Location AMC, University of Amsterdam, Amsterdam Institute for Infection and Immunity, Amsterdam, the Netherlands.
Brent Appelman, Center for Experimental and Molecular Medicine, Amsterdam University Medical Centers—Location AMC, University of Amsterdam, Amsterdam Institute for Infection and Immunity, Amsterdam, the Netherlands.
Hessel Peters-Sengers, Center for Experimental and Molecular Medicine, Amsterdam University Medical Centers—Location AMC, University of Amsterdam, Amsterdam Institute for Infection and Immunity, Amsterdam, the Netherlands; Department of Epidemiology and Data Science, Amsterdam University Medical Centers—Location AMC, University of Amsterdam, Amsterdam Public Health Research Institute, Amsterdam, the Netherlands.
Dave A Dongelmans, Department of Intensive Care, Amsterdam University Medical Centers—Location AMC, University of Amsterdam, Amsterdam Institute for Infection and Immunity, Amsterdam, the Netherlands; National Intensive Care Evaluation (NICE) Foundation, Amsterdam, the Netherlands.
Nicolette F de Keizer, National Intensive Care Evaluation (NICE) Foundation, Amsterdam, the Netherlands; Department of Medical Informatics, Amsterdam University Medical Centers, University of Amsterdam—Location AMC, Amsterdam Public Health Research Institute, Amsterdam, the Netherlands.
Rogier P Schade, Department of Medical Microbiology and Infection Prevention, Amsterdam University Medical Centers—Location AMC, University of Amsterdam, Amsterdam, the Netherlands.
Mark G J de Boer, Department of Infectious Diseases and Department of Clinical Epidemiology, Leiden University Medical Center, Leiden, the Netherlands.
Marcella C A Müller, Department of Intensive Care, Amsterdam University Medical Centers—Location AMC, University of Amsterdam, Amsterdam Institute for Infection and Immunity, Amsterdam, the Netherlands.
Alexander P J Vlaar, Department of Intensive Care, Amsterdam University Medical Centers—Location AMC, University of Amsterdam, Amsterdam Institute for Infection and Immunity, Amsterdam, the Netherlands.
W Joost Wiersinga, Center for Experimental and Molecular Medicine, Amsterdam University Medical Centers—Location AMC, University of Amsterdam, Amsterdam Institute for Infection and Immunity, Amsterdam, the Netherlands; Division of Infectious Diseases, Department of Medicine, Amsterdam University Medical Centers—Location AMC, University of Amsterdam, Amsterdam, the Netherlands.
Lonneke A van Vught, Center for Experimental and Molecular Medicine, Amsterdam University Medical Centers—Location AMC, University of Amsterdam, Amsterdam Institute for Infection and Immunity, Amsterdam, the Netherlands; Department of Intensive Care, Amsterdam University Medical Centers—Location AMC, University of Amsterdam, Amsterdam Institute for Infection and Immunity, Amsterdam, the Netherlands.
NICE COVID-19 Research Consortium and the COVIDPredict study group:
Brent Appelman, Michiel Schinkel, Martijn Beudel, Ronald Henry, Esther K Haspels-Hogervorst, Daisy Rusch, Niels C Gritters van den Oever, Suat Simsek, W de Ruijter, Frits H M van Osch, Joop P van den Bergh, Martijn D de Kruif, Renee Douma, Lianne R de Haan, Hazra Moeniralam, Kees Brinkman, and N Bokhizzou
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
References
- 1. Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA 2020; 324:782–93. [DOI] [PubMed] [Google Scholar]
- 2. Barber RM, Sorensen RJD, Pigott DM, et al. Estimating global, regional, and national daily and cumulative infections with SARS-CoV-2 through Nov 14, 2021: a statistical analysis. Lancet 2022; 399:2351–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. RIVM . Coronadashboard. Secondary Coronadashboard. 2022. Available at: https://coronadashboard.rijksoverheid.nl/. Accessed August 8, 2022.
- 4. Hoogenboom WS, Pham A, Anand H, et al. Clinical characteristics of the first and second COVID-19 waves in the Bronx, New York: a retrospective cohort study. Lancet Reg Health Am 2021; 3:100041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Maslo C, Friedland R, Toubkin M, Laubscher A, Akaloo T, Kama B. Characteristics and outcomes of hospitalized patients in South Africa during the COVID-19 Omicron wave compared with previous waves. JAMA 2022; 327:583–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Di Bari M, Balzi D, Carreras G, Onder G. Extensive testing may reduce COVID-19 mortality: a lesson from Northern Italy. Front Med (Lausanne) 2020; 7:402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Feikin DR, Higdon MM, Abu-Raddad LJ, et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: results of a systematic review and meta-regression. Lancet 2022; 399:924–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brosh-Nissimov T, Orenbuch-Harroch E, Chowers M, et al. BNT162b2 vaccine breakthrough: clinical characteristics of 152 fully vaccinated hospitalized COVID-19 patients in Israel. Clin Microbiol Infect 2021; 27:1652–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. National Institutes of Health . Coronavirus disease 2019 (COVID-19) treatment guidelines. Secondary Coronavirus disease 2019 (COVID-19) treatment guidelines. 2020. Available at: https://www.covid19treatmentguidelines.nih.gov/. Accessed June 9, 2022. [PubMed]
- 10. Vollaard AM GM, van der Linden PD, Sinha B, de Boer M. Medicamenteuze Behandelopties bij Patiënten met COVID-19 (Infecties met SARS-CoV-2). Stichting Werkgroep Antibiotica Beleid (SWAB); 2021. Available at: https://swab.nl/nl/covid-19. Accessed June 9, 2022.
- 11. van de Veerdonk FL, Giamarellos-Bourboulis E, Pickkers P, et al. A guide to immunotherapy for COVID-19. Nat Med 2022; 28:39–50. [DOI] [PubMed] [Google Scholar]
- 12. Update to living WHO guideline on drugs for COVID-19. BMJ 2021; 374:n2219. [DOI] [PubMed] [Google Scholar]
- 13. Wang Y, Zhang D, Du G, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet 2020; 395:1569–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Arribas JR, Bhagani S, Lobo SM, et al. Randomized trial of molnupiravir or placebo in patients hospitalized with COVID-19. NEJM Evid. 2022; 1. [DOI] [PubMed] [Google Scholar]
- 15. Abani O, Abbas A, Abbas F, et al. Casirivimab and imdevimab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet 2022; 399:665–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gupta A, Gonzalez-Rojas Y, Juarez E, et al. Early treatment for COVID-19 with SARS-CoV-2 neutralizing antibody sotrovimab. N Engl J Med 2021; 385:1941–50. [DOI] [PubMed] [Google Scholar]
- 17. RECOVERY Collaborative Group, Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with COVID-19. N Engl J Med 2021; 384:693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kyriazopoulou E, Poulakou G, Milionis H, et al. Early treatment of COVID-19 with anakinra guided by soluble urokinase plasminogen receptor plasma levels: a double-blind, randomized controlled phase 3 trial. Nat Med 2021; 27:1752–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. RECOVERY Collaborative Group . Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet 2021; 397:1637–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sieswerda E, de Boer MGJ, Bonten MMJ, et al. Recommendations for antibacterial therapy in adults with COVID-19—an evidence based guideline. Clin Microbiol Infect 2021; 27:61–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Horby PW, Mafham M, Bell JL, et al. Lopinavir–ritonavir in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet 2020; 396:1345–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Axfors C, Schmitt AM, Janiaud P, et al. Mortality outcomes with hydroxychloroquine and chloroquine in COVID-19 from an international collaborative meta-analysis of randomized trials. Nat Commun 2021; 12:2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Renoux C, Azoulay L, Suissa S. Biases in evaluating the safety and effectiveness of drugs for the treatment of COVID-19: designing real-world evidence studies. Am J Epidemiol 2021; 190:1452–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Armstrong RA, Kane AD, Kursumovic E, Oglesby FC, Cook TM. Mortality in patients admitted to intensive care with COVID-19: an updated systematic review and meta-analysis of observational studies. Anaesthesia 2021; 76:537–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Carbonell R, Urgeles S, Rodriguez A, et al. Mortality comparison between the first and second/third waves among 3,795 critical COVID-19 patients with pneumonia admitted to the ICU: a multicentre retrospective cohort study. Lancet Reg Health Eur 2021; 11:100243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. van de Klundert N, Holman R, Dongelmans DA, de Keizer NF. Data resource profile: the Dutch National Intensive Care Evaluation (NICE) registry of admissions to adult intensive care units. Int J Epidemiol 2015; 44:1850–50. [DOI] [PubMed] [Google Scholar]
- 27. Peters EJ, Collard D, Van Assen S, et al. Outcomes of persons with coronavirus disease 2019 in hospitals with and without standard treatment with (hydroxy)chloroquine. Clin Microbiol Infect 2021; 27:264–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Prokop M, van Everdingen W, van Rees Vellinga T, et al. CO-RADS: a categorical CT assessment scheme for patients suspected of having COVID-19-definition and evaluation. Radiology 2020; 296:E97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of COVID-19—final report. N Engl J Med 2020; 383:1813–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bongiovanni M, Arienti R, Bini F, Bodini BD, Corbetta E, Gianturco L. Differences between the waves in Northern Italy: how the characteristics and the outcome of COVID-19 infected patients admitted to the emergency room have changed. J Infect 2021; 83:e32–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dongelmans DA, Termorshuizen F, Brinkman S, et al. Characteristics and outcome of COVID-19 patients admitted to the ICU: a nationwide cohort study on the comparison between the first and the consecutive upsurges of the second wave of the COVID-19 pandemic in the Netherlands. Ann Intensive Care 2022; 12:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Soriano V, de Mendoza C, Gomez-Gallego F, Corral O, Barreiro P. Third wave of COVID-19 in Madrid, Spain. Int J Infect Dis 2021; 107:212–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Giacomelli A, Ridolfo AL, Pezzati L, et al. Mortality rates among COVID-19 patients hospitalised during the first three waves of the epidemic in Milan, Italy: a prospective observational study. PLoS One 2022; 17:e0263548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lee TC, Murthy S, Del Corpo O, et al. Remdesivir for the treatment of COVID-19: a systematic review and meta-analysis. Clin Microbiol Infect 2022; 28:1203–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. WHO Solidarity Trial Consortium . Remdesivir and three other drugs for hospitalised patients with COVID-19: final results of the WHO solidarity randomised trial and updated meta-analyses. Lancet 2022; 399:1941–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wagner C, Griesel M, Mikolajewska A, et al. Systemic corticosteroids for the treatment of COVID-19. Cochrane Database Syst Rev 2021; 8:CD014963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Albuquerque AM, Tramujas L, Sewanan LR, Williams DR, Brophy JM. Mortality rates among hospitalized patients with COVID-19 infection treated with tocilizumab and corticosteroids: a Bayesian reanalysis of a previous meta-analysis. JAMA Netw Open 2022; 5:e220548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Verweij M, van de Vathorst S, Schermer M, Willems D, de Vries M. Ethical advice for an intensive care triage protocol in the COVID-19 pandemic: lessons learned from the Netherlands. Public Health Ethics 2020; 13:157–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Amoah J, Stuart EA, Cosgrove SE, et al. Comparing propensity score methods versus traditional regression analysis for the evaluation of observational data: a case study evaluating the treatment of gram-negative bloodstream infections. Clin Infect Dis 2020; 71:e497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Spyropoulos AC, Goldin M, Giannis D, et al. Efficacy and safety of therapeutic-dose heparin vs standard prophylactic or intermediate-dose heparins for thromboprophylaxis in high-risk hospitalized patients with COVID-19: the HEP-COVID randomized clinical trial. JAMA Intern Med 2021; 181:1612–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Amati F, Dela Cruz CS. One size does not fit all: moving towards a personalized approach for steroids in COVID-19. Chest 2021; 159:1693–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chen H, Xie J, Su N, et al. Corticosteroid therapy is associated with improved outcome in critically ill patients with COVID-19 with hyperinflammatory phenotype. Chest 2021; 159:1793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Torres A, Motos A, Cilloniz C, et al. Major candidate variables to guide personalised treatment with steroids in critically ill patients with COVID-19: CIBERESUCICOVID study. Intensive Care Med 2022; 48:850–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Galvan-Roman JM, Rodriguez-Garcia SC, Roy-Vallejo E, et al. IL-6 serum levels predict severity and response to tocilizumab in COVID-19: an observational study. J Allergy Clin Immunol 2021; 147:72–80 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhou Y, Fu X, Liu X, et al. Use of corticosteroids in influenza-associated acute respiratory distress syndrome and severe pneumonia: a systemic review and meta-analysis. Sci Rep 2020; 10:3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.