Abstract
Reliable estimation of abundance is a prerequisite for a species’ conservation planning in human-dominated landscapes, especially if the species is elusive and involved in conflicts. As a means of population estimation, the importance of camera traps has been recognized globally, although estimating the abundance of unmarked, cryptic species has always been a challenge to conservation biologists. This study explores the use of the N-mixture model with three probability distributions, i.e., Poisson, negative binomial (NB) and zero-inflated Poisson (ZIP), to estimate the relative abundance of sloth bears (Melursus ursinus) based on a camera trapping exercise in Sanjay Tiger Reserve, Madhya Pradesh from December 2016 to April 2017. We used environmental and anthropogenic covariates to model the variation in the abundance of sloth bears. We also compared null model estimates (mean site abundance) obtained from the N-mixture model to those of the Royle-Nichols abundance-induced heterogeneity model (RN model) to assess the application of similar site-structured models. Models with Poisson distributions produced ecologically realistic and more precise estimates of mean site abundance (λ = 2.60 ± 0.64) compared with other distributions, despite the relatively high Akaike Information Criterion value. Area of mixed and sal forest, the photographic capture rate of humans and distance to the nearest village predicted a higher relative abundance of sloth bears. Mean site abundance estimates of sloth bears obtained from the N-mixture model (Poisson distribution) and the RN model were comparable, indicating the overall utility of these models in this field. However, density estimates of sloth bears based on spatially explicit methods are essential for evaluating the efficacy of the relatively more cost-effective N-mixture model. Compared to commonly used index/encounter-based methods, the N-mixture model equipped with knowledge on governing biotic and abiotic factors provides better relative abundance estimates for a species like the sloth bear. In the absence of absolute abundance estimates, the present study could be insightful for the long-term conservation and management of sloth bears.
Keywords: Sloth bear, Relative abundance, Camera trap, N-mixture model, Human-dominated landscape
Introduction
Knowledge of the abundance of a species is fundamental for ecological studies and a prerequisite for successful conservation planning and decision-making processes in the field of conservation biology (Kremen, Merenlender & Murphy, 1994; Gaston & Rodrigues, 2003; Nichols & Williams, 2006; McCarthy & Possingham, 2007). Reliable abundance estimates can also be crucial for identifying the biotic and abiotic factors that govern the population size (Meents et al., 1983). Inaccurate estimates or estimates based on empirical studies can lead to erroneous management decisions and undermine the effort to conserve the concerned species in its natural environment. However, estimating the abundance of cryptic species through the traditional capture-mark-recapture (CMR) method is labor-intensive and is not always logistically feasible. Exploration and subsequent implementation of cost-effective methodologies to monitor population abundance are thus required, given the limited resource availability in the field of wildlife conservation (Parker et al., 2011).
With the advent of science and technology, passive detectors, especially automated camera traps, have become indispensable in wildlife and conservation biology (O’Connell, Nichols & Karanth, 2011; Burton et al., 2015). However, despite the diverse applications of camera traps, their use has been confined to population estimation of individually identifiable or marked animals (Burton et al., 2015). Camera trap surveys provided successful population density estimates through spatial capture-recapture (SCR) models for marked animals (Karanth & Nichols, 1998; O’Connell, Nichols & Karanth, 2011). However, population estimation for unmarked or individually unidentifiable animals via camera trapping has posed challenges to ecologists and conservation biologists (Gilbert et al., 2021). Also, for unmarked species like Asian bears, population monitoring in a small area (<10,000 km2) requires spatially explicit methods (e.g., camera traps or genetics), but these studies remain restricted due to resource and logistical constraints (Proctor et al., 2022).
Photographic capture rates (number of individuals photo-captured/effort in terms of trap-nights) or relative abundance indices provide surrogates of population abundance (Carbone et al., 2001; O’Brien, Kinnaird & Wibisono, 2003; Bengsen et al., 2011). However, inferences drawn from these index-based surveys do not meet the implicit assumption of equal detection probability (Jennelle, Runge & MacKenzie, 2002; Harmsen et al., 2010; Sollmann et al., 2013; Burton et al., 2015). Rowcliffe et al. (2008) proposed the random encounter model (REM) to estimate density by incorporating information on animal movement, photographic capture rate and the detection zone of camera traps. Despite its unique and robust approach, the limited application of REM is due to the actual random deployment of camera traps and the inability to account for spatial variation of abundance. Also, the need to extrapolate density from the camera trap detection zone to the entire surveyed area, and the lack of knowledge regarding the movement of elusive species, further restricts its applicability (Gilbert et al., 2021). The application of SCR frameworks for unmarked individuals, otherwise known as unmarked spatial capture-recapture (USCR; Chandler & Andrew Royle, 2013), is another promising approach to estimating population density. However, its limited applicability is due to the underlying computationally intensive (Bayesian) framework (Royle et al., 2014; Gilbert et al., 2021), tendency to produce imprecise estimates (Augustine et al., 2019) and judicious selection of priors on the spatial scale of animal detection at camera traps (Sun, Fuller & Royle, 2014). On the other hand, site-structured models such as the Royle-Nichols abundance-induced heterogeneity model (RN model; Royle & Nichols, 2003) and the N-mixture model (Royle, 2004) have been widely used to draw inferences on abundance from detection-nondetection data and count data, respectively, based on spatially or temporally replicated surveys. In the recent past, the latter was more widely applied to estimate the abundance of terrestrial mammals from count data (Keever et al., 2017; Xiao et al., 2018; Kafley et al., 2019; Kidwai et al., 2019; Penjor et al., 2019; Searle et al., 2020). The major advantages of the N-mixture model are its cost-effectiveness, the ability to bypass individual identification, and the ability to account for spatial variation of abundance as a function of covariates. The N-mixture model typically requires the following assumptions: population closure at the site, equal probability of detection of all individuals at a site, no false-positive errors (i.e., misidentification or double-counting of individuals), and independent detection of individuals across the sampling units (Royle, 2004). However, N-mixture models are sensitive to assumption violations, especially false-positive errors, which are not commonly addressed (Kéry & Schaub, 2011). For free-ranging unmarked species, one individual would likely be photo-captured several times during the entire sampling occasion (O’Brien, Kinnaird & Wibisono, 2003), which can overestimate abundance (Link et al., 2018; Nakashima, 2020). However, relaxing the assumption of population closure at the site for free-ranging species, it is justifiable to interpret the true abundance of the site as “relative abundance,” which translates to the number of individuals utilizing a site at a given time (Kéry & Royle, 2015).
The sloth bear (Melursus ursinus), one of the four bear species found in the Indian subcontinent, is mainly confined to the isolated patches of forested habitat both inside and outside of the protected areas (PAs) (Sathyakumar et al., 2012; Dharaiya, Bargali & Sharp, 2016; Jhala, Qureshi & Nayak, 2020). Despite the species’ broad geographic distribution, especially across the moist and dry deciduous forests of the Western Ghats and central Indian landscape (Yoganand et al., 2006), information on population abundance based on a rigorous statistical framework is lacking (Dharaiya, Bargali & Sharp, 2016). However, the range-wide population abundance of sloth bears reportedly ranges between 10,000 and 20,000 (Garshelis, Joshi & Smith, 1999; Chauhan, 2006; Yoganand et al., 2006; Sathyakumar et al., 2012). None of these estimates was reliable enough to monitor the population trends because of the differential data collection methods and types of data used in the analyses (Sathyakumar et al., 2012; Dharaiya, Bargali & Sharp, 2016). The sloth bear is an omnivorous generalist, long-ranging ursid and often comes in conflict with humans (Rajpurohit & Krausman, 2000; Bargali, Akhtar & Chauhan, 2005; Garcia, Joshi & Dharaiya, 2016; Debata et al., 2017; Dhamorikar et al., 2017; Singh, Sonone & Dharaiya, 2018). The ever-increasing human population, subsequent habitat fragmentation and human-sloth bear conflicts are significant concerns for conserving this species. One recent study (Gomez et al., 2021) also indicated a steadily increasing trend of sloth bear mortality due to poaching. So far, only one study (Garshelis, Joshi & Smith, 1999) has attempted to make robust density estimates of the sloth bear, done in Royal Chitwan National Park (henceforth Chitwan), Nepal, based on a spatial mark-resight method. Reliable information on population abundance and identifying factors influencing abundance are crucial for the long-term conservation of sloth bears in human-dominated landscapes.
In this context, we explored the application of site-structured models with special reference to the N-mixture model (Royle, 2004) to quantify the relative abundance of sloth bears in Sanjay Tiger Reserve, Madhya Pradesh. The objectives of the present study were as follows: (1) to demonstrate the use of the N-mixture model in estimating the relative abundance of sloth bears and compare the mean site abundance estimates obtained from the N-mixture model to those of the RN model and (2) to identify the environmental and anthropogenic covariates that govern the relative abundance of sloth bears.
Materials and Methods
Research permissions and ethical considerations
Madhya Pradesh Forest Department (MPFD) issued all required permissions for our field surveys (Letter No: I/3129 dated 29/05/2014). Due to the non-invasive nature of sampling, we did not require ethical clearance for this study.
Study site
Sanjay Tiger Reserve (STR) is situated between 24.125122°N 81.546009°E (northwestern boundary) and 23.822641°N 82.175139°E (southeastern boundary) in the state of Madhya Pradesh in central India (Fig. 1). STR falls under the category “Central Highlands” as per the biogeographic classification of India (Rodgers & Panwar, 1988). The core and buffer areas of STR encompass 831.25 km2 and 812.58 km2, respectively. Furthermore, the core area of STR consists of two administrative parts, i.e., Sanjay National Park (SNP; 466.60 km2) and Dubri Wildlife Sanctuary (DWLS; 364.60 km2).
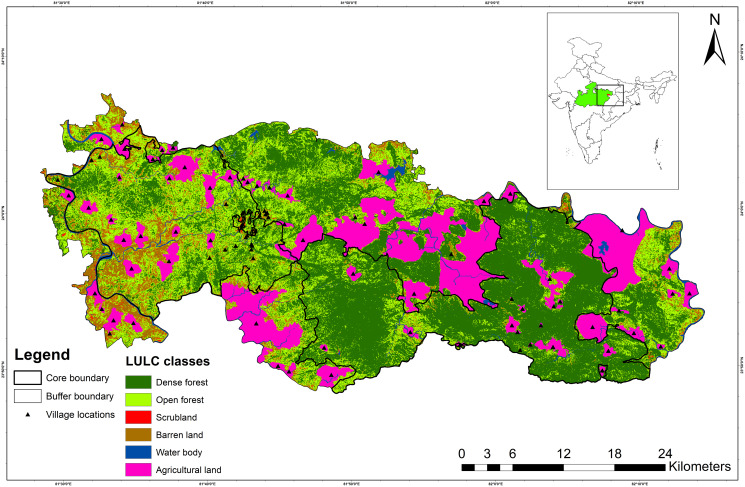
Figure 1. Map showing Sanjay Tiger Reserve with land use land cover (LULC) classes and location of villages; Inset shows the location of Sanjay Tiger Reserve in Madhya Pradesh, India.
The terrain of STR is rugged and undulating, with plateaus and gorges in the eastern part (SNP) and flat land in the western part (DWLS). STR has an elevation range between 425 and 732 m. The broad forest type of STR falls under sub-group 3C- “North Indian moist deciduous forests”, with subtype (C2) “Moist sal-bearing forest” with subdivision (2e) “Moist peninsular sal forest” (Champion & Seth, 1968). Sal (Shorea robusta) is dominant in SNP and DWLS, occupying about 80% of the entire area, followed by mixed, bamboo mixed, scrubland and grassland (Fig. S1). Apart from sal, other frequently found tree species include tendu (Diospyros melanoxylon), mahua (Madhuca longifolia), char (Buchanania cochinchinensis) and sedha (Lagerstroemia parviflora). STR receives a mean annual precipitation of 1,303 mm between June and September, and the annual temperature varies between 7.4 °C and 41.8 °C. Other than the sloth bear, tiger (Panthera tigris), leopard (Panthera pardus), striped hyena (Hyaena hyaena), Indian wolf (Canis lupus) and Asiatic wild dog (Cuon alpinus) are the major carnivores found in STR. Anthropogenic pressure is immense inside the core area of STR due to the presence of thirty-nine villages.
Camera trap survey
The camera trapping survey was conducted from December 2016 to April 2017 in the core area of STR. We divided the study area into 2 km × 2 km grids (henceforth, sites) following the All India Tiger Estimation protocol (Jhala, Qureshi & Gopal, 2015) and subsequently deployed camera traps in 143 locations (one at each site). We divided the core area into two blocks (SNP and DWLS), and camera traps were deployed in one block at a time due to the limited availability of camera traps. A pair of motion-triggered camera traps (Cuddeback C1 and Cuddeback Ambush) were deployed at each site opposite to one another to increase the detection (Pease, Nielsen & Holzmueller, 2016; O’Connor et al., 2017) at an approximate height of 30–40 cm above the ground. We placed the camera traps on forest roads, animal trails and dry riverbeds to maximize the photo-capture of sloth bears and other large carnivores (Karanth et al., 2011). The average distance between two adjacent camera trap locations was 2 km. We set 15 s time-lapse between two consecutive photographs. Cameras were active 24 h a day for an average trapping period of 42 days (range: 13–45 days) and checked once every 20–25 days to change batteries and memory cards. We collected the locations of camera traps by using Garmin e-Trex handheld GPS units (Garmin Inc., Olathe, KS, USA).
To avoid pseudoreplication, we considered two consecutive photographs of sloth bears or humans captured ≥30 min apart as independent events (O’Brien, Kinnaird & Wibisono, 2003; Xiao et al., 2018; Kafley et al., 2019). We set the temporal sampling unit (sampling occasion) as three days. We pooled the count of independent photographs of sloth bears from each site for each unit of three consecutive days, which yielded an encounter history of 15 sampling occasions. Reducing the sampling period by pooling data from a specific time (days) helped reduce overdispersion (Penjor et al., 2019).
Covariates
Local-scale spatial covariates
At each camera trap location, we measured habitat features within a 10 m-radius plot, with a focus on fruiting trees (≥2 m in height and ≥1 m girth at breast height), which comprise ≥1% of the diet of sloth bears (Ramesh et al., 2012; Rather et al., 2020). We also recorded the number of termite mounds and woody shrub species at every 10 and 5 m-radius plot, respectively. We calculated the photo-capture rate of humans (number of photographs of humans at each location/number of days each camera trap was active) as one of the surrogates of anthropogenic pressure at each site. Table 1 provides the details of local-scale covariates.
Table 1. List of covariates to model the relative abundance of sloth bears using the N-mixture model in Sanjay Tiger Reserve, Madhya Pradesh, India, during 2016–2017.
| A. Environmental covariates (with abbreviations) | Description | Range of values | Area |
|---|---|---|---|
| Land use land cover (LULC) classes | LULC was classified into six classes: dense forest, open forest, scrubland, barren land, agricultural land and water body. Area (in km2) of each class was calculated and extracted for each 12.40 km2 grid in Arc GIS 10.2. | 1–6 (Number of classes) | Dense forest-706.66 km2 Open forest-447.20 km2 Scrubland-41.81 km2 Barren land-66.77 km2 Agricultural land-303.25 km2 Water body-37.95 km2 |
| Forest type classes | Forest type map was classified into six classes: sal mixed, mixed, sal forest, bamboo mixed, grassland and non-forest. Area (in km2) of each class was calculated and extracted for each 12.40 km2 grid in Arc GIS 10.2. | 1–6 (Number of classes) | Sal mixed-538.06 km2 Mixed-457.54 km2 Sal forest-150.03 km2 Bamboo mixed-55.65 km2 Grassland-7.28 km2 Non-forest-390.02 km2 |
| Distance to water source (km) | Euclidean distance to nearest water source (natural or artificial) from the centroid of each 12.40 km2 grid was calculated using the Spatial analyst tool in Arc GIS 10.2. | 0–15 | - |
| Ruggedness index | Ruggedness index was generated for each 12.40 km2 grid, from DEM (Digital Elevation Model) by the roughness index tool in the 3D Analyst extension in Arc GIS 10.2. | 9.21–27.61 | - |
| Availability (or density) of fruiting trees (no./m2) | Number of fruiting trees was counted per 10 m-radius plot around each camera trap location and subsequently divided by the area (in m2) of each plot. | 0–0.09 | - |
| Density of shrubs (no./m2) | Number of woody shrubs was counted per 5 m-radius plot around each camera trap location and subsequently divided by the area (in m2) of each plot. | 0–1.39 | |
| No. of termite mounds | Number of termite mounds was counted per 10 m-radius plot around each camera trap location. | 0–4 | - |
| B. Anthropogenic covariates (with abbreviations) | |||
| Human photo-capture rate (no./total effort) | Number of total photographs of humans obtained from each camera trap was counted and divided by the total effort (trap-days or trap-nights) of each camera trap | 0–11.87 | - |
| Distance to village (km) | Euclidean distance to nearest village from the centroid of each 12.40 km2 grid was calculated using the Spatial analyst tool in Arc GIS 10.2. | 0–12 | - |
| Distance to metal road (km) | Euclidean distance to nearest metal road from the centroid of each 12.40 km2 grid was calculated using the Spatial analyst tool in Arc GIS 10.2. | 0–14 | - |
GIS-based covariates
We prepared the Land Use Land Cover (LULC) layer for STR by processing a 30 m spatial resolution Landsat-8 image (Image ID: LC81430432016297LGN00) dated 23 October 2016 in ERDAS IMAGINE 9.2 (Leica Geosystems, St. Gallen, Switzerland) following a standard hybrid image classification protocol (Ruppert et al., 1997). The image classification resulted in six classes, i.e., dense forest, open forest, scrubland, agricultural land, barren land, and water bodies. The average accuracy of the resultant classified image was 88.89%, and the overall Kappa coefficient was 0.85. To classify the forest types in STR, we processed a 30 m spatial resolution Landsat-8 image (Image ID: LC81430432018110LGN00) dated 2 May 2018, following the methodology mentioned above and with the support of 412 ground points. The classes included sal mixed, mixed, sal forest, bamboo mixed, grassland and non-forest. We categorized the different forest types following the broad classification by Champion & Seth (1968). Subsequently, we performed the accuracy assessment of the resultant classified image based on 392 ground points collected throughout the study area. This classified image’s average accuracy and overall Kappa coefficient were 64.30% and 0.45, respectively.
Other covariates used in the analyses were the terrain ruggedness index (RI; Riley, 1999) and the distances to the nearest water source, metal road and village (see Table 1 for details). All analyses were carried out in ERDAS IMAGINE 9.2 (Leica Geosystems, St. Gallen, Switzerland) and ArcGIS 10.2 (Environmental Systems Research Institute, Redlands, CA, USA).
Relative abundance model of sloth bear
We developed the N-mixture model (Royle, 2004; Kéry & Royle, 2015) to estimate the relative abundance of sloth bears in STR within 3.52 km × 3.52 km grids, as this represents the minimum home range (i.e., 12.40 km2) of sloth bear in central India (Yoganand, 2005). However, little is known about the home range size of the sloth bear in a human-dominated landscape, though studies elsewhere (Joshi, Garshelis & Smith, 1995; Ratnayeke, Manen & Padmalal, 2007) have indicated much smaller average home range estimates compared to those of the central Indian landscape (Yoganand, 2005). Since we deployed camera traps within smaller grids (2 km × 2 km), we used the data from all 1–4 camera traps falling inside each 3.52 km × 3.52 km grid and considered these as spatial replicates, following Xiao et al. (2018). We used the N-mixture model to estimate the relative abundance of sloth bears from independent photographic captures as a function of environmental and anthropogenic covariates (Table 1).
N-mixture models assume population closure (i.e., the number of individuals residing within a site is constant as no emigration or immigration occurs between sites). Due to the individually unidentifiable aspect and long-ranging behavior of the sloth bear, we believe that the closure assumption was likely to be violated. Hence, we relaxed the closure assumption by changing the interpretation from absolute abundance at a site to the number of individuals ever associated with a site during a given period (Kéry & Royle, 2015), otherwise known as relative abundance. We pooled independent detections of sloth bear for each site “i” from “R” sites where, i = 1, 2, 3,..., R during a sampling occasion of “T”, where t = 1, 2, 3,…, T. The observed count (yit) at “i” sites during “t” sampling occasions followed a binomial distribution, and the site abundance Ni followed a Poisson distribution (Kéry & Royle, 2015).
Due to the greater flexibility in modeling abundance with covariates and the ability to incorporate other effects (e.g., zero inflation, latent state), Poisson distribution is an integral part of the state process, i.e., abundance (Royle & Dorazio, 2008). On the other hand, binomial distribution accounts for imperfect detection or false-negative errors. The variation of observed counts (yit) is an effect of imperfect detection of the actual (unknown) abundance Ni and its variability among sites. In simple algebraic form, we expressed the two processes as:
State process: Ni ~ Poisson (λi); log (λi) = β0 + effects of covariates
Observation process: yit | Ni ~ Binomial (Ni, pit); logit (pit) = α0 + effects of covariates
where Ni is the latent abundance of site “i” (i = 1,…., R), λi is the mean abundance of site “i”, yit is the count of the species at site “i” and occasion “t” (t = 1,…., T), and pit is detection probability at site “i” and occasion “t”.
Studies have indicated that N-mixture models with Poisson and zero-inflated Poisson (ZIP) distributions can produce reliable relative abundance estimates if detection probability is modelled effectively with appropriate covariates (Barker et al., 2018; Kéry, 2018). We modelled the detection probability with the trapping period as we suspected that a more extended trapping period would positively influence the detection probability of sloth bears (O’Connor et al., 2017). We also used local-scale spatial covariates (and no GIS-based covariates) likely to affect the detection probability, following Hofmeester et al. (2019). The detection probability would be constant if none of the covariates influenced it (Kafley et al., 2019).
After determining the effect of covariates on the detection process, we modelled the relative abundance of sloth bears as a function of environmental (forest types, LULC types, RI, distance to nearest water source, availability of fruiting trees, shrub density and number of termite mounds) and anthropogenic (human photographic capture rate, distance to nearest village and metal road) covariates with three probability distributions, i.e., Poisson, ZIP and negative binomial (NB). Mean values of GIS-based environmental and anthropogenic covariates were calculated and extracted from each of the 12.40 km2 grids. For local-scale spatial covariates where the number of camera trap locations was more than one, we calculated the average values of each covariate for the 12.40 km2 grids. The selection of the most parsimonious models from the candidate model set followed the ascending Akaike Information Criterion (AIC) values (Burnham & Anderson, 2002). We reported uninformative parameters but drew final inferences only from the models within ≤2 ΔAIC units (Burnham & Anderson, 2002) with statistically significant (P < 0.05) parameters (Arnold, 2010). Model averaging was not considered in the case of a single top-ranked model or the presence of uninformative parameters in multiple top-ranked models (Arnold, 2010). However, due to the “good fit bad prediction dilemma” as mentioned by Kéry & Royle (2015), we further performed Goodness of Fit (GoF) tests with 1,000 iterations of bootstrapping for each of the most parsimonious models of corresponding distributions. We also calculated overdispersion parameters (ĉ) for the same models (Kéry & Royle, 2015). We proceeded with further residual diagnostics and mapping of residuals if none of them passed the GoF test (when P < 0.05) for the final checking of model adequacy (Kéry & Royle, 2015). The result was interpreted as an outcome of unstructured noise if no specific pattern of lack of fit was found (Kéry & Royle, 2015). Subsequently, we corrected it by inflating the predicted abundance’s range (confidence interval) (Johnson, Laake & Ver Hoef, 2010; Kéry & Royle, 2015). We standardized all covariates using Z-transformation before analyses, and subsequently, Pearson’s correlation tests were performed to avoid multi-collinearity. A pairwise Pearson’s correlation coefficient value of r≥ ± 0.7 was considered strongly correlated (Dormann et al., 2013) and was not modeled together, as we were interested in seeing the effect of each covariate on the relative abundance of sloth bears. All analyses were carried out using the packages “Unmarked” (Fiske & Chandler, 2011) and “AICmodavg” (Mazerolle, 2015) implemented in R (R Core Team, 2019).
We compared the mean site abundance estimates with estimates obtained from another frequently used site-structured model, the RN model (Royle & Nichols, 2003), to understand the performance of the N-mixture model. Since modeling the relative abundance of the sloth bear with both local-scale and GIS-based covariates by applying the RN model and subsequent comparison of the model performance (of both N-mixture and RN models) was beyond the scope of this paper, we restricted the comparison between the null models of the two frameworks mentioned above. We chose the same probability distribution for the RN model which was the most suitable for the N-mixture model. We used the program Presence (Version 2.13.11) (Hines, 2006) to estimate the mean site abundance of sloth bears by applying the RN model.
Results
Camera trap
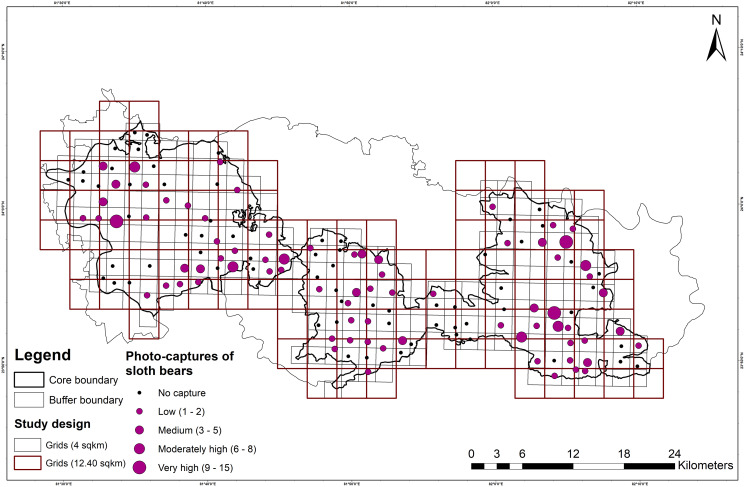
A total of 191 and 5,292 independent photographs of sloth bears and humans, respectively, were obtained from a total effort of 5,950 trap-nights (3,870 and 2,080 trap-nights in SNP and DWLS, respectively). Sloth bears were detected at 76 out of 143 (53.10%) camera trap locations (sites), and independent detections of sloth bear varied from 1 to 15 per site (Fig. 2). However, in the larger grids (12.40 km2), the presence of sloth bears was recorded at 51 out of 73 (70.0%) grids. In SNP, a higher number (120) of sloth bear detections was observed compared to 71 detections in DWLS.
Figure 2. Map showing the study design, followed in Sanjay Tiger Reserve, Madhya Pradesh, India.
It includes the larger sampling units, i.e., 12.40 km2 grids, fine-scale sampling units, i.e. 4 km2 grids and number of photo-captures of sloth bears.
Detection process
The density of fruiting trees significantly influenced the detection probability of sloth bears for Poisson, NB and ZIP distributions (Table 2). The estimated detection probability (p) of the best detection model (modelled with the density of fruiting trees) was found to be higher in Poisson (0.08 ± 0.01) than in ZIP (0.06 ± 0.02) and NB (0.01 ± 0.004) distributions (Table 2).
Table 2. Model parameter estimates (with constant abundance) for the detection probability (p) of sloth bear, obtained from the N-mixture model in Sanjay Tiger Reserve, Madhya Pradesh, India, during 2016–2017.
| Model description | β estimates | SE (Standard error) | P value | AIC | p | SE (Standard error) |
|---|---|---|---|---|---|---|
| Poisson | ||||||
| [p(fruit_density)λ(.)] | 0.27 | 0.11 | 0.01 (<0.05) | 1,020.22 | 0.08 | 0.01 |
| [p(shrub_density)λ(.)] | 0.16 | 0.14 | 0.25 (>0.05) | 1,025.15 | ||
| [p(termitemounds)λ(.)] | 0.09 | 0.11 | 0.42 (>0.05) | 1,025.85 | ||
| [p(trapdays_total)λ(.)] | 0.03 | 0.14 | 0.80 (>0.05) | 1,026.42 | ||
| NB | ||||||
| [p(fruit_density)λ(.)] | 0.28 | 0.14 | 0.04 (<0.05) | 991.86 | 0.01 | 0.004 |
| [p(shrub_density)λ(.)] | 0.09 | 0.17 | 0.57 (>0.05) | 995.44 | ||
| [p(termitemounds)λ(.)] | 0.08 | 0.15 | 0.59 (>0.05) | 995.47 | ||
| [p(trapdays_total)λ(.)] | 0.07 | 0.17 | 0.68 (>0.05) | 995.59 | ||
| ZIP | ||||||
| [p(fruit_density)λ(.)] | 0.29 | 0.11 | 0.007 (<0.05) | 1,017.04 | 0.06 | 0.02 |
| [p(shrub_density)λ(.)] | 0.16 | 0.13 | 0.22 (>0.05) | 1,022.56 | ||
| [p(termitemounds)λ(.)] | 0.10 | 0.11 | 0.377 (>0.05) | 1,023.30 | ||
| [p(trapdays_total)λ(.)] | −0.04 | 0.16 | 0.81 (>0.05) | 1,024.04 |
Note:
Three distributions [Poisson, negative binomial (NB) and zero-inflated Poisson (ZIP)] were used to model the detection probability of sloth bear; Covariates considered: fruit_density–density of fruiting trees per 10 m-radius plot around each camera trap location, shrub_density–density of shrubs per 5 m-radius plot around each camera trap location, termitemounds- number of termite mounds per 10 m-radius plot around each camera trap location, trapdays_total–total number of days or occasions for which camera trap (s) was active; AIC, Akaike Information Criterion; Detection probability estimates (p) were provided for the best-selected models of each distribution.
Relative abundance of sloth bears and effects of covariates
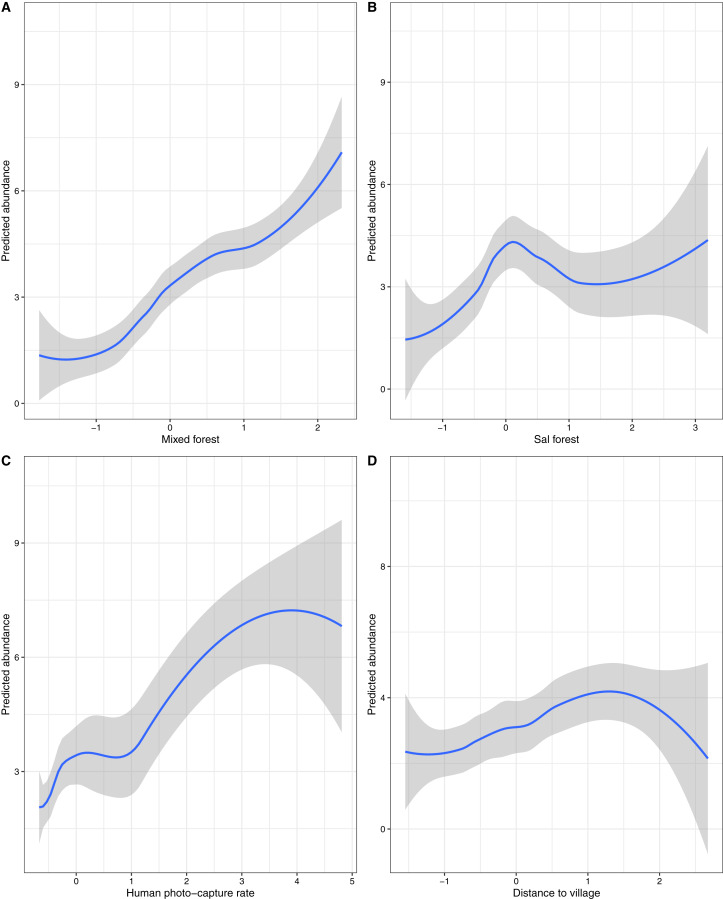
In top-ranked models, the relative abundance of sloth bears was significantly influenced by forest types (mixed and sal forest; environmental), distance to the nearest village (anthropogenic) and human photo-capture rate (anthropogenic) (Tables 3 and 4). In addition to these four, the most parsimonious models also included the LULC types (agricultural land, scrubland and water body); however, none of these covariates significantly influenced the relative abundance of sloth bears (Table 4). Models with NB distributions were the most parsimonious in terms of lower overall AIC values, followed by ZIP and Poisson models (Table 3). Due to uninformative parameters, we did not carry out model averaging for any top-ranked models. Areas of mixed and sal forest predicted a higher relative abundance of sloth bears (Table 4 and Fig. 3; also see Figs. S2 and S3). The distance to the nearest village and human photo-capture rate also had significant positive effects (Table 4 and Fig. 3; also see Figs. S2 and S3) on sloth bear abundance.
Table 3. Details of the model selection results of the N-mixture model for estimation of the relative abundance of sloth bears in Sanjay Tiger Reserve, Madhya Pradesh, India, during 2016–2017.
| Model description | nPars | AIC | ΔAIC | AICwt | Cumltv-wt |
|---|---|---|---|---|---|
| Poisson | |||||
| (p(fruit_density)λ(sal.forest+vildist+human_CR+mixed)) | 7 | 998.08 | 0.00 | 0.36 | 0.36 |
| (p(fruit_density)λ(sal.forest+vildist+human_CR+mixed+Agricultural.land)) | 8 | 999.52 | 1.44 | 0.17 | 0.53 |
| Null model (p(.)λ(.)) | 2 | 1,024.49 | 26.41 | 0.00 | 1.00 |
| NB | |||||
| (p(fruit_density)λ(sal.forest+vildist+human_CR+mixed)) | 8 | 983.00 | 0.00 | 0.11 | 0.11 |
| (p(fruit_density)λ(mixed +human_CR)) | 6 | 983.15 | 0.15 | 0.11 | 0.22 |
| (p(fruit_density)λ(mixed+human_CR+Agricultural. land)) | 7 | 983.43 | 0.43 | 0.09 | 0.31 |
| (p(fruit_density)λ(mixed+sal.forest+human_CR)) | 7 | 983.75 | 0.75 | 0.08 | 0.39 |
| (p(fruit_density)λ(mixed+vildist+human_CR)) | 7 | 983.81 | 0.81 | 0.08 | 0.47 |
| (p(fruit_density)λ(sal.forest+vildist+human_CR+mixed+Agricultural.land)) | 9 | 984.26 | 1.25 | 0.06 | 0.53 |
| (p(fruit_density)λ(mixed+Scrubland+human_CR)) | 7 | 984.60 | 1.59 | 0.05 | 0.58 |
| (p(fruit_density)λ(mixed+Agricultural.land)) | 6 | 984.71 | 1.70 | 0.05 | 0.63 |
| (p(fruit_density)λ(mixed+Water.body+human_CR)) | 7 | 984.96 | 1.96 | 0.04 | 0.67 |
| (p(fruit_density)λ(mixed+sal.forest)) | 6 | 984.99 | 1.99 | 0.04 | 0.71 |
| Null model (p(.)λ(.)) | 3 | 993.76 | 10.76 | 0.001 | 1.00 |
| ZIP | |||||
| (p(fruit_density)λ(sal.forest+vildist+human_CR+mixed)) | 8 | 995.09 | 0.00 | 0.36 | 0.36 |
| (p(fruit_density)λ(sal.forest+vildist+human_CR+mixed+Agricultural.land)) | 9 | 996.11 | 1.01 | 0.22 | 0.58 |
| Null model (p(.)λ(.)) | 3 | 1,022.10 | 27.00 | 0.00 | 1.00 |
Note:
Models include the most parsimonious models with the best-selected covariates and null models for the Poisson, negative binomial (NB) and zero-inflated Poisson (ZIP) distributions; Covariates considered: fruit_density- density of fruiting trees, mixed- area of mixed forest, sal.forest- area of sal forest, human_CR- photographic capture rate of humans, vildist–distance to the nearest village, Agricultural.land–area of agricultural land, Scrubland- area of scrubland and, Water.body–area of water body; Model selection was based on number of parameters (nPars), Akaike Information Criterion (AIC), the difference in AIC between best fit models (ΔAIC ≤ 2), AIC weight (AICwt) and cumulative AIC weight of models (Cumltv-wt); all models (including null models) are represented based on the lowest to the highest value of ΔAIC for each distribution.
Table 4. Parameter estimates of N-mixture models to determine the relative abundance of sloth bears in Sanjay Tiger Reserve, Madhya Pradesh, India, during 2016–2017.
| Model description | β estimates | SE (Standard error) | P value |
|---|---|---|---|
| Poisson | |||
| Intercept λ(.) | 0.95 | 0.25 | 0.0001 (<0.005) |
| λ(mixed) | 0.33 | 0.10 | 0.001 (<0.005) |
| λ(human_CR) | 0.27 | 0.08 | 0.0005 (<0.005) |
| λ(vildist) | 0.25 | 0.11 | 0.02 (<0.05) |
| λ(sal.forest) | 0.21 | 0.10 | 0.04 (<0.05) |
| λ(Agricultural.land) | −0.10 | 0.13 | 0.47 (>0.05) |
| NB | |||
| Intercept λ(.) | 2.38 | 0.35 | 1.54e−11 (<0.005) |
| λ(mixed) | 0.28 | 0.13 | 0.03 (<0.05) |
| λ(sal.forest) | 0.25 | 0.15 | 0.09 (>0.05) |
| λ(human_CR) | 0.24 | 0.12 | 0.04 (<0.05) |
| λ(vildist) | 0.23 | 0.14 | 0.09 (>0.05) |
| λ(Agricultural.land) | −0.21 | 0.16 | 0.19 (>0.05) |
| λ(Scrubland) | 0.14 | 0.18 | 0.45 (>0.05) |
| λ(Water.body) | −0.07 | 0.17 | 0.66 (>0.05) |
| ZIP | |||
| Intercept λ(.) | 1.40 | 0.38 | 0.0002 (<0.005) |
| λ(mixed) | 0.31 | 0.11 | 0.004 (<0.005) |
| λ(sal.forest) | 0.31 | 0.14 | 0.02 (<0.05) |
| λ(human_CR) | 0.27 | 0.07 | 0.0002 (<0.005) |
| λ(vildist) | 0.26 | 0.10 | 0.01 (<0.05) |
| λ(Agricultural.land) | −0.14 | 0.14 | 0.33 (>0.05) |
Note:
Estimates were derived from the Poisson, negative binomial (NB), and zero-inflated Poisson (ZIP) distributions for the mean site abundance (Lambda, λ) of sloth bears; Covariates considered: mixed- area of mixed forest, sal.forest–area of sal forest, human_CR–photographic capture rate of humans, vildist–distance to the nearest village, Agricultural.land–area of agricultural land, Scrubland- area of scrubland and, Water.body–area of water body.
Figure 3. Effects of environmental and anthropogenic covariates on the predicted relative abundance of sloth bears in Sanjay Tiger Reserve, Madhya Pradesh, India, 2016–2017.
Covariates considered: (A) Mixed forest, (B) sal forest, (C) human photo-capture rate and (D) distance to village. Prediction of relative abundance was based on the most parsimonious models of Poisson distribution; values of covariates were shown on a standardized scale.
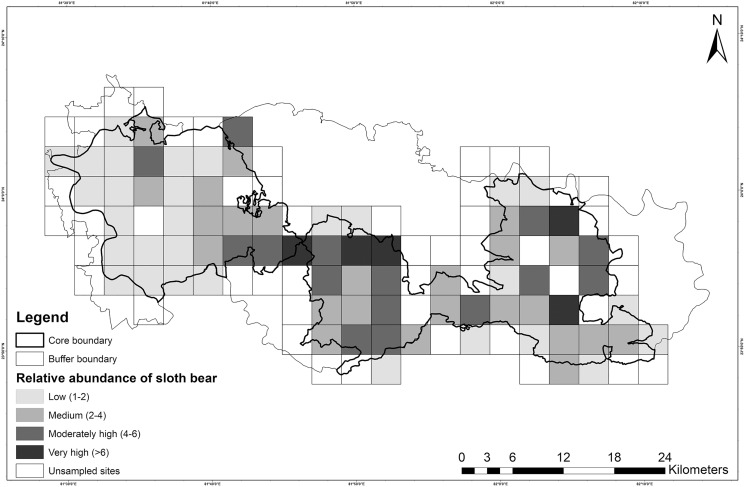
However, the models within ≤2 ΔAIC units for Poisson, ZIP and NB distributions did not pass the GoF test. Overdispersion parameter estimates (ĉ) revealed a marginal lack of fit, which did not appear to differ among Poisson (ĉ = 1.31), NB (ĉ = 1.19) and ZIP (ĉ = 1.28) distributions. Residual diagnostics and mapping of residuals did not show any specific pattern of lack of fit. Hence, this finding could be attributed to unstructured noise or overdispersion rather than a true lack of fit. The NB model (top-ranked) produced unusually high mean site abundance (λ = 10.80 ± 3.83) in comparison to mean site abundance estimates of ZIP (λ = 4.07 ± 1.56) and Poisson models (λ = 2.60 ± 0.64) (Table 5). Spatial variation of relative abundance produced by the Poisson model was less (1–11; see also Fig. 4) than those of the ZIP (1–14) and NB models (4–37).
Table 5. Mean site abundance (λ) of sloth bears and associated standard error (SE) in Sanjay Tiger Reserve, Madhya Pradesh, India, during 2016–2017.
| Model description | Mean site abundance (λ) ± Standard error (SE) |
|---|---|
| Poisson | |
| (p(fruit_density)λ(sal.forest+vildist+human_CR+mixed)) | 2.60 ± 0.64 |
| NB | |
| (p(fruit_density)λ(sal.forest+vildist+human_CR+mixed)) | 10.80 ± 3.83 |
| ZIP | |
| (p(fruit_density)λ(sal.forest+vildist+human_CR+mixed)) | 4.07 ± 1.56 |
Note:
Best-selected models were from three (Poisson, negative binomial (NB) and zero-inflated Poisson (ZIP)) distributions; Covariates considered: fruit_density–density of fruiting trees, mixed–area of mixed forest, sal.forest- area of sal forest, human_CR–photographic capture rate of humans and, vildist- distance to the nearest village.
Figure 4. Map showing the relative abundance of sloth bears in Sanjay Tiger Reserve, Madhya Pradesh, India, during 2016–2017; Estimates were based on the most parsimonious Poisson distribution model.
Comparing estimates with the RN model
The RN model (null model with Poisson distribution) produced a mean site abundance estimate of 1.62 ± 0.34, which was slightly lower than the estimate produced by the N-mixture model (2.05 ± 0.35). However, the mean site abundance estimate obtained from the N-mixture model was within the 95% confidence interval range (1.08–2.44) of the estimate produced by the RN model.
Discussion
Efficacy of the N-mixture model
We reported the first-ever estimates of the relative abundance of the sloth bear using the N-mixture model. We also identified associated factors governing the relative abundance by using count data obtained from the camera trap survey in STR. The N-mixture model can produce reliable abundance estimates if the data meet the assumptions of the N-mixture model while accounting for imperfect detection (Royle, 2004). Also, its ability to model spatial variation of abundance and detection probability as a function of relevant covariates makes its inference more robust and reliable than inferences drawn from conventional index/encounter rate-based surveys (Gilbert et al., 2021). A growing number of studies have assessed the population abundance (relative and absolute) of unmarked individuals by using the N-mixture model from camera trap data (Brodie & Giordano, 2013; Keever et al., 2017; Xiao et al., 2018; Kafley et al., 2019; Penjor et al., 2019) as well as employing other field methods (Belant et al., 2016; Ficetola et al., 2018; Kidwai et al., 2019; Searle et al., 2020). Estimating the abundance of unmarked animals from camera trap data has always been challenging for ecologists. For the past two decades, very few studies have actually assessed the population abundance of Asian bears employing statistically rigorous methods; instead, many of the studies have heavily relied upon sign index or interview-based distributions due to the relatively less expensive nature and ease of application in the field (Garshelis et al., 2022; Proctor et al., 2022). Information on sloth bear abundance and its governing factors is lacking throughout the species’ range (Dharaiya, Bargali & Sharp, 2016). Most of the previous studies (Eisenberg & Lockhart, 1972; Laurie & Seidensticker, 1977; Santiapillai & Santiapillai, 1990; Ratnayeke, Manen & Padmalal, 2007; Akhtar, Bargali & Chauhan, 2008) regarding the abundance of sloth bears produced empirical estimates, except for Garshelis, Joshi & Smith (1999), where the authors first estimated abundance from sound analytical methods in Chitwan. However, in the case of this wide-ranging bear species, site-structured models (i.e., the RN model and the N-mixture model) may be reliable for estimating the population trend with careful considerations of model assumptions and study design (Morin et al., 2022).
Moving forward, one should not interpret the abundance estimates obtained from site-structured models as a measure of absolute but relative abundance if the assumption of closure is likely to be violated (Barker et al., 2018; Link et al., 2018; Gilbert et al., 2021). In this context, mean abundance (lambda) from the N-mixture model reflects all individuals using the site (12.40 km2 grids in the present study) and not necessarily all individuals restricted to the site. Site abundance estimates obtained from the N-mixture models would be consistently larger than the actual abundance of free-ranging animals like sloth bears, as each site contains multiple overlapping territories of different individual sloth bears. In reality, the actual abundance of animals comes from a considerably larger geographic extent than the actual surveyed area, leading to an overestimation of the mean site abundance and total predicted abundance (Joseph et al., 2009). In the present study, the best-selected model (with NB distribution) in terms of AIC produced substantially larger and ecologically unrealistic abundance estimates of sloth bears compared to ZIP and Poisson models. Mean abundance estimates obtained from NB and ZIP models showed that a large number (~11 and ~4 for NB and ZIP models, respectively) of bears were utilizing each site with considerably low detection probability, which, in general, is unlikely for a species like the sloth bear. In the Indian sub-continent, the lack of studies focusing specifically on the density or abundance of sloth bears and other bear species in a scientifically rigorous framework poses difficulties for comparing our findings to other studies. We expected that for a species like sloth bear, an elusive and solitary ursid, count data obtained from camera traps would be zero-inflated. The NB model performs poorly with a zero-inflated count dataset or when the focal species is not frequently found or is truly absent from a large number of sites surveyed (Joseph et al., 2009). In the present study, NB and ZIP variants of N-mixture models produced lower detection probabilities than Poisson models. Due to low detection probability, N-mixture models can produce positively biased abundance estimates with less precision than other methods (Dénes, Silveira & Beissinger, 2015; Dennis, Morgan & Ridout, 2015). On the other hand, the N-mixture model with a Poisson distribution can handle datasets with excessive true zeros (sites where the species is truly absent), given that the species naturally occurs in low densities (Joseph et al., 2009). In our study, the Poisson model produced a comparatively low mean site abundance (2.60 ± 0.64) by modeling a substantial number (~30% of total sites surveyed) of unoccupied sites. We suggest interpreting the abundance estimates of sloth bears carefully with an ecologically realistic view rather than with strict adherence to the statistical properties of the models.
The mean site abundance of sloth bears produced from the N-mixture model (Poisson distribution) was comparable to estimates obtained from the RN model. Earlier studies have indicated that the RN model would not be a good choice when the focal species is common; in that case, the N-mixture model would be preferable (Dénes, Silveira & Beissinger, 2015; Kéry & Royle, 2015; Gilbert et al., 2021). The RN model reportedly produces imprecise estimates from camera trap-based surveys (covering comparatively small sampled areas), especially if the focal species is cryptic (Gupta et al., 2012; Kalle et al., 2014; Morin et al., 2022). However, there is evidence that the RN model can produce precise abundance estimates, enabling it to detect population trends (O’Brien et al., 2020). Estimates from the RN model can approximate the absolute density of the focal species if the study is conducted on a landscape scale in conjunction with an ecologically relevant grid size for camera trap deployment (Linden et al., 2017). On the other hand, simulation studies have shown that the N-mixture (Poisson and Binomial mixture) model may produce biased estimates of mean abundance in the context of false-positive detections (Nakashima, 2020). However, our findings from both models indicated the overall utility of site-structured models in estimating the relative abundance of elusive species like sloth bears. Although the number of camera trap locations/grid varied (1–4) in our study, the presence of >1 spatial replicates (camera trap locations) within most of the grids (>50%) might have improved the detection and robustness of mean site abundance estimates (Kolowski, Oley & McShea, 2021; Morin et al., 2022).
Applicability of other methods for unmarked population estimation
Due to the scarcity of spatially explicit studies, we could not directly compare relative abundance estimates of sloth bears with previous findings. Since the primary objective of this study was to understand the applicability of the N-mixture model, we considered other potential methods relevant to the abundance estimation of unmarked animals. However, it has also been found that abundance estimation of unmarked species like Asian bears through camera traps rarely produces precise estimates for conservation planning unless supported by ancillary information obtained from statistically rigorous methods and covering a large number of sampling sites (Morin et al., 2022). Apart from the site-structured models presented here, USCR (i.e., the spatial count (SC) model; Chandler & Andrew Royle, 2013) and its extension (i.e., spatial presence-absence (SPA) model; Ramsey, Caley & Robley, 2015) can also be applied to estimate the absolute density of cryptic species. The application of the SPA model has been well-demonstrated to estimate the density of small carnivores through camera traps (Chatterjee, Nigam & Habib, 2020a, 2020b). However, these models are computationally intensive (requiring Bayesian analysis) and sensitive to the appropriate choice of priors which could be challenging for a species like the sloth bear due to limited knowledge of its ranging pattern. Distance sampling through camera traps could also be a promising approach to estimating the density of unmarked animals (Howe et al., 2017), especially if the focal species is less abundant (Palencia et al., 2021). However, this method requires the random placement of camera traps which we could not implement, as this study followed the protocol of All India Tiger Estimation (Jhala, Qureshi & Gopal, 2015). Random placement of camera traps might result in substantially low detections of sloth bears, which may not be conducive for further analysis. The N-mixture model would be particularly suitable where finance and logistics are limiting factors. Moreover, if the study intends to model the spatial variation of abundance (relative or absolute), the N-mixture model is a better choice over other models, as long as it meets the objectives and the interpretation can be plausible.
Effects of environmental and anthropogenic covariates on relative abundance
In the present study, mixed and sal forests positively influenced the relative abundance of sloth bears. In STR, we calculated the density of termite mounds and fruiting trees, utilizing a 10 m-radius plot around each camera trap location. We recorded a high density (35/hectare) of termite mounds in mixed forest, followed by sal mixed and sal forest (27/hectare and 23/hectare, respectively). Similarly, the fruiting tree density was also higher (208/hectare) in the mixed forest in comparison to sal mixed (197/hectare) and sal forest (100/hectare). Due to fewer plots, we could not calculate meaningful density estimates of termite mounds and fruiting trees for the bamboo mixed forest and grasslands. In STR, a high number of fruiting trees and termite mounds in the mixed forest may explain the positive relationship between bear abundance and mixed forest. We found a similar observation from Panna National Park in central India, where the dense forest (comprised of miscellaneous tree species) had the highest densities of fruiting trees and prey insect colonies, with sloth bears preferring this habitat significantly (Yoganand, 2005). However, the limited number of surveyed plots (only at camera trap locations) to support our findings may not be enough to explain the effects of forest types and termite mounds on sloth bear abundance, for which more rigorous sampling is needed. In the North Bilaspur Forest Division, Akhtar, Bargali & Chauhan (2004) found that signs of sloth bears were more common in sal forest than in other forest types. We also found similar results in Chitwan but with distinct seasonal patterns (Garshelis, Joshi & Smith, 1999). The occurrence of bear signs (ground holes and mound holes) in sal forest was frequent in both landscapes, perhaps due to a relatively high number of termite mounds (Garshelis, Joshi & Smith, 1999; Akhtar, Bargali & Chauhan, 2004) and possibly the underground colonies of ants and termites. In the northern part of the Indian sub-continent, termite mounds are more abundant in sal forests (Chakraborty & Singh, 2020). However, underground colonies of termites and ants are difficult to quantify. Hence, we considered the number of termite mounds that are above-ground and visible as a surrogate for the availability of ants and termites (Akhtar, Bargali & Chauhan, 2004; Rather et al., 2020). We also could not find any significant relationship between the relative abundance of sloth bears and local-scale covariates, although the availability of fruiting trees positively influenced the detection probability of sloth bears. In Nepal’s Chitwan and Churia (a lowland forest outside the PAs) forests, the presence of fruiting trees and termite mounds positively influenced the sloth bear’s occupancy and detection probability (Paudel et al., 2022; Pokharel et al., 2022). Das et al. (2014) also recorded a similar observation in southern India during their occupancy-based study. However, we believe that our camera trap-based study design may not be appropriate for drawing inferences on such local-scale covariates, despite their proven importance to the sloth bear’s ecology. The ubiquitous nature of termites and ants in a relatively small area (Redford, 1987) may explain the insignificant relationship between bear abundance and termite mounds. The availability of fruiting trees and termite mounds at a particular point (10 m-radius plot, in our study) may not necessarily represent the entire sampling unit (12.40 km2 grids). Thus, it could explain the apparent insignificant contribution of fruiting tree density and termite mounds to the relative abundance of sloth bears.
Sloth bears generally avoid human disturbances (Santhanakrishnan et al., 2015; Puri et al., 2015; Paudel et al., 2022; Pokharel et al., 2022). However, they can also tolerate certain degree of human pressure or get habituated to living in disturbed and fragmented forests (Akhtar, Bargali & Chauhan, 2004; Prajapati, Koli & Sundar, 2021). In the present study, we found that the photographic capture rate of humans and distance to the nearest village positively correlated with relative abundance of sloth bears. However, based on the current study design, it is not known whether a causal relationship between these variables (relative abundance of sloth bear and human photographic capture rate) exists. Moreover, to our knowledge, no other studies have shown that any such ecological relationship prevails. We believe this finding may be purely attributed to the placement of camera traps on the forest roads and trails. Apart from sloth bears (and other carnivores), forest roads and trails are intensively used daily by local people living inside STR. We were interested in this relationship between human occurrence and sloth bear abundance as a matter of concern regarding the possible negative interactions between humans and sloth bears. However, to cope with extensive human pressure in STR, sloth bears have shown a fine-scale seasonal spatio-temporal segregation (Chaudhuri et al., 2022). On the other hand, sloth bears avoided areas close to villages in STR, which is in agreement with previous studies (Santhanakrishnan et al., 2015; Puri et al., 2015; Paudel et al., 2022; Pokharel et al., 2022). Such behavior could be due to the better availability of forage and shelter (day-resting den sites) in relatively undisturbed habitats.
Nevertheless, avoidance of human settlements does not necessarily curb the possibilities of conflict between humans and sloth bears, given the prevailing extensive anthropogenic pressure in STR. Sloth bears are also known to opportunistically raid crops and forage on fruits of Ziziphus mauritiana, Mangifera indica and Syzygium cumini found in the villages but less abundant inside the forest (Bargali, Akhtar & Chauhan, 2004; Palei, Debata & Sahu, 2020). Habituation to human settlements often leads to severe conflicts between humans and sloth bears (Bargali, Akhtar & Chauhan, 2005; Dhamorikar et al., 2017; Prajapati, Koli & Sundar, 2021), so there are significant conservation implications for this species in human-dominated landscapes.
Caveats and limitations
Our study has a few limitations and caveats. Firstly, the camera trap design was more oriented towards tiger movement, but also aimed at other co-predators. Secondly, we could not deploy camera traps intensively due to villages and agricultural fields, which resulted in an unequal number of camera traps (1–4) in each of the larger grids (12.40 km2). Such design may have affected the detection of sloth bears as they are presumed to raid crops and forage on fruits in the villages situated inside the core area of STR. Thirdly, we restricted the camera trap survey to the core area of STR and therefore, could not account for overall estimates of sloth bears’ relative abundance for the entire STR. Finally, we could not assess the seasonal variation of mean site abundance of sloth bears, as studies conducted elsewhere reported a distinct seasonal variation in sloth bear density (Garshelis, Joshi & Smith, 1999).
Conclusions
We demonstrated the application of the N-mixture model to estimate the relative abundance of sloth bears from camera trap data. Also, choosing an appropriate probability distribution is crucial to providing ecologically realistic estimates. The N-mixture and RN models with Poisson distribution could be suitable options for estimating the relative abundance of solitary, elusive carnivores, despite the better statistical properties of models with ZIP and NB distributions. However, limited knowledge of ranging patterns of sloth bears in human-dominated landscapes and substantial variation in home range estimates throughout the species’ distributional range could affect the choice of grid size (12.40 km2 grids in the current study) for the N-mixture model. If the goal is to detect the population changes over time, site-structured models are rarely effective, especially if knowledge of certain aspects of the species’ ecology (e.g., home range, population density) is limited (Morin et al., 2022). Future studies to monitor population changes of sloth bears should focus on the placement (i.e., spacing) of camera traps based on a more thorough understanding of their ranging patterns (Fuller et al., 2022). Also, a spatially extensive sampling area with several spatial replicates at each sampling unit (depending on the feasibility and size of the sampling unit) is required to assess the abundance with precision in the case of a low-density population of sloth bears. We highly recommend carrying out density estimation by applying a spatially explicit capture-recapture framework through genetics and comparing results with site-structured models (N-mixture and RN models) and other suitable camera trap-based methods (USCR, SPA, and distance sampling). However, we believe that, in the absence of absolute abundance estimates for an unmarked, habitat generalist, and conflict-prone species like the sloth bear, knowledge of an ecologically realistic relative abundance and associated factors governing the same are imperative for its long-term conservation in a human-dominated landscape.
Supplemental Information
Covariates considered: A) Mixed forest, (B) Sal forest, (C) Human photo-capture rate and (D) Distance to village. Prediction of relative abundance was based on the most parsimonious models of negative binomial (NB) distribution; Values of covariates were shown on a standardized scale.
Covariates considered: A) Mixed forest, (B) Sal forest, (C) Human photo-capture rate and (D) Distance to village. Prediction of relative abundance was based on the most parsimonious models of zero-inflated Poisson (ZIP) distribution; Values of covariates were shown on a standardized scale.
All covariates were standardized using the Z score.
Acknowledgments
We are grateful to Madhya Pradesh Forest Department (Mr. Narendra Kumar, Dr. Suhas Kumar, Mr. Jitendra Agarwal, Mr. R. K. Srivastava, Dr. Deleep Kumar and Mr. Vincent Rahim) for supporting this research by providing research permissions and logistics during fieldwork, and to the Wildlife Institute of India (Dr. V. B. Mathur, Dr. Dhananjai Mohan, Dr. Parag Nigam and Prof. Qamar Qureshi) for necessary institutional support. We sincerely thank Ms. Kamna Pokhariya and Ms. Poushali Hazra for their input in preparing spatial layers for analyses. We also thank Mr. Griffith Victor, Ms. Maithili Devadas and Ms. Snehaa Sundaram for helping us during the initial field data collection and data preparation. We are thankful to all our field assistants (Mr. Amritlal Yadav, Late Ramsakha Yadav, Mr. Rakesh Yadav, Mr. Rajkaran Yadav and Mr. Satendra Sahu) and the ground-level staff of Sanjay Tiger Reserve for their untiring work during data collection in the field. We greatly appreciate the timely logistics provided by the forest Range Officers (Mr. M. S. Marawi, Mr. V. B. S. Parihar, Mr. R. K. Jhariya and Mr. B. K. Tiwari) and the veterinary officer (Dr. Abhay Sengar) of Sanjay Tiger Reserve. Special thanks are due to all friends and colleagues at Wildlife Institute of India, especially Dr. Rahul De, Dr. Shrutarshi Paul, Ms. Meghna Bandyopadhyay, Mr. Dibyadeep Chatterjee, Ms. Tista Ghosh, and Dr. B. Navaneethan who have provided valuable suggestions during the preparation of this manuscript.
Funding Statement
This work was supported by the Madhya Pradesh Forest Department, Madhya Pradesh, India. There was no additional external funding received for this study. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare that they have no competing interests.
Author Contributions
Sankarshan Chaudhuri conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the article, and approved the final draft.
Rajasekar Rajaraman performed the experiments, analyzed the data, authored or reviewed drafts of the article, and approved the final draft.
Sankar Kalyanasundaram conceived and designed the experiments, authored or reviewed drafts of the article, and approved the final draft.
Sambandam Sathyakumar conceived and designed the experiments, authored or reviewed drafts of the article, and approved the final draft.
Ramesh Krishnamurthy conceived and designed the experiments, authored or reviewed drafts of the article, and approved the final draft.
Animal Ethics
The following information was supplied relating to ethical approvals (i.e., approving body and any reference numbers):
The work conducted here is using non-invasive sampling methods, i.e., deployment of camera traps in Sanjay Tiger Reserve with appropriate permissions. Due to the non-invasive nature of the work, no ethical approval is required.
Field Study Permissions
The following information was supplied relating to field study approvals (i.e., approving body and any reference numbers):
All required permissions for our field surveys were provided by the Madhya Pradesh Forest Department, Madhya Pradesh, India (Letter No: I/3129 dated 29/05/2014).
Data Availability
The following information was supplied regarding data availability:
The raw data is available in the Supplemental Files.
References
- Akhtar, Bargali & Chauhan (2004).Akhtar N, Bargali HS, Chauhan NPS. Sloth bear habitat use in disturbed and unprotected areas of Madhya Pradesh, India. Ursus. 2004;15:203–211. doi: 10.2192/1537-6176(2004)015<0203:SBHUID>2.0.CO;2. [DOI] [Google Scholar]
- Akhtar, Bargali & Chauhan (2008).Akhtar N, Bargali HS, Chauhan NPS. Distribution and population abundance of sloth bear (Melursus ursinus) in disturbed and unprotected habitat of North Bilaspur Forest Division, Chhattisgarh. Tigerpaper. 2008;35:15–21. [Google Scholar]
- Arnold (2010).Arnold TW. Uninformative parameters and model selection using Akaike’s Information Criterion. Journal of Wildlife Management. 2010;74(6):1175–1178. doi: 10.2193/2009-367. [DOI] [Google Scholar]
- Augustine et al. (2019).Augustine BC, Royle JA, Murphy SM, Chandler RB, Cox JJ, Kelly MJ. Spatial capture-recapture for categorically marked populations with an application to genetic capture-recapture. Ecosphere. 2019;10(4):e02627. doi: 10.1002/ecs2.2627. [DOI] [Google Scholar]
- Bargali, Akhtar & Chauhan (2004).Bargali HS, Akhtar N, Chauhan NPS. Feeding ecology of sloth bears in a disturbed area in central India. Ursus. 2004;15:212–217. doi: 10.2192/1537-6176(2004)015<0212:FEOSBI>2.0.CO;2. [DOI] [Google Scholar]
- Bargali, Akhtar & Chauhan (2005).Bargali HS, Akhtar N, Chauhan NPS. Characteristics of sloth bear attacks and human casualties in North Bilaspur Forest Division, Chhattisgarh, India. Ursus. 2005;16:263–267. doi: 10.2192/1537-6176(2005)016[0263:COSBAA]2.0.CO;2. [DOI] [Google Scholar]
- Barker et al. (2018).Barker RJ, Schofield MR, Link WA, Sauer JR. On the reliability of N-mixture models for count data. Biometrics. 2018;74:369–377. doi: 10.1111/biom.12734. [DOI] [PubMed] [Google Scholar]
- Belant et al. (2016).Belant JL, Bled F, Wilton CM, Fyumagwa R. Estimating lion abundance using N-mixture models for social species. Scientific Reports. 2016;6:1–9. doi: 10.1038/srep35920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengsen et al. (2011).Bengsen AJ, Leung LK, Lapidge SJ, Gordon IJ. Using a general index approach to analyze camera-trap abundance indices. The Journal of Wildlife Management. 2011;75:1222–1227. doi: 10.1002/jwmg.132. [DOI] [Google Scholar]
- Brodie & Giordano (2013).Brodie JF, Giordano A. Lack of trophic release with large mammal predators and prey in Borneo. Biological Conservation. 2013;163:58–67. doi: 10.1016/j.biocon.2013.01.003. [DOI] [Google Scholar]
- Burnham & Anderson (2002).Burnham KP, Anderson DR. A practical information-theoretic approach. New York: Springer; 2002. [Google Scholar]
- Burton et al. (2015).Burton AC, Neilson E, Moreira D, Ladle A, Steenweg R, Fisher JT, Bayne E, Boutin S. Wildlife camera trapping: A review and recommendations for linking surveys to ecological processes. Journal of Applied Ecology. 2015;52(3):675–685. doi: 10.1111/1365-2664.12432. [DOI] [Google Scholar]
- Carbone et al. (2001).Carbone C, Christie S, Conforti K, Coulson T, Franklin N, Ginsberg JR, Griffiths M, Holden J, Kawanishi K, Kinnaird M, Laidlaw R, Lynam A, Macdonald DW, Martyr D, McDougal C, Nath L, O’Brien T, Seidensticker J, Smith JLD, Sunquist M, Tilson R, Wan Shahruddin WN. The use of photographic rates to estimate densities of tigers and other cryptic mammals. Animal Conservation. 2001;4(1):75–79. doi: 10.1017/S1367943001001081. [DOI] [Google Scholar]
- Chakraborty & Singh (2020).Chakraborty JS, Singh S. Abundance, population density and spatial ecology of mound-building termites in moist tropical deciduous forests of northern India. Ecoscience. 2020 doi: 10.1080/11956860.2020.1772610. [DOI] [Google Scholar]
- Champion & Seth (1968).Champion HG, Seth SK. A revised survey of the forest types of India. Delhi: Manager of publications; 1968. [Google Scholar]
- Chandler & Andrew Royle (2013).Chandler RB, Andrew Royle J. Spatially explicit models for inference about density in unmarked or partially marked populations. Annals of Applied Statistics. 2013;7(2):936–954. doi: 10.1214/12-AOAS610. [DOI] [Google Scholar]
- Chatterjee, Nigam & Habib (2020a).Chatterjee N, Nigam P, Habib B. Population density and habitat use of two sympatric small cats in a central Indian reserve. PLOS ONE. 2020a;15(6):1–13. doi: 10.1371/journal.pone.0233569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee, Nigam & Habib (2020b).Chatterjee N, Nigam P, Habib B. Population estimate, habitat-use and activity patterns of the honey badger in a dry-deciduous forest of central India. Frontiers in Ecology and Evolution. 2020b;8:1–9. doi: 10.3389/fevo.2020.585256. [DOI] [Google Scholar]
- Chaudhuri et al. (2022).Chaudhuri S, Bandyopadhyay M, Rajaraman R, Kalyanasundaram S, Sathyakumar S, Krishnamurthy R. Spatio-temporal patterns and source-dispersion modelling towards sloth bear-human conflict management in central India. Frontiers in Conservation Science. 2022;3:1–15. doi: 10.3389/fcosc.2022.850309. [DOI] [Google Scholar]
- Chauhan (2006).Chauhan NS. The status of sloth bears in India; Understanding Asian Bears to Secure their Future, Japan Bear Network; 2006. pp. 26–34. Ibaraki, Japan. [Google Scholar]
- Das et al. (2014).Das S, Dutta S, Sen S, Jijumon AS, Babu S, Kumara HN, Singh M. Identifying regions for conservation of sloth bears through occupancy modelling in north-eastern Karnataka, India. Ursus. 2014;25(2):111–120. doi: 10.2192/URSUS-D-14-00008.1. [DOI] [Google Scholar]
- Debata et al. (2017).Debata S, Swain KK, Sahu HK, Palei HS. Human-sloth bear conflict in a human-dominated landscape of northern Odisha, India. Ursus. 2017;27(2):90–98. doi: 10.2192/URSUS-D-16-00007.1. [DOI] [Google Scholar]
- Dennis, Morgan & Ridout (2015).Dennis EB, Morgan BJT, Ridout MS. Computational aspects of N-mixture models. Biometrics. 2015;71(1):237–246. doi: 10.1111/biom.12246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhamorikar et al. (2017).Dhamorikar AH, Mehta P, Bargali H, Gore K. Characteristics of human-sloth bear (Melursus ursinus) encounters and the resulting human casualties in the Kanha-Pench corridor, Madhya Pradesh, India. PLOS ONE. 2017;12(4):1–18. doi: 10.1371/journal.pone.0176612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharaiya, Bargali & Sharp (2016).Dharaiya NA, Bargali H, Sharp T. Melursus ursinus. 2016;e.T13143A4:1–19. doi: 10.2305/IUCN.UK.2016-3.RLTS.T13143A45033815.en. The IUCN Red List of Threatened Species. [DOI] [Google Scholar]
- Dormann et al. (2013).Dormann CF, Elith J, Bacher S, Buchmann C, Carl G, Carré G, Marquéz JRG, Gruber B, Lafourcade B, Leitão PJ, Münkemüller T, Mcclean C, Osborne PE, Reineking B, Schröder B, Skidmore AK, Zurell D, Lautenbach S. Collinearity: a review of methods to deal with it and a simulation study evaluating their performance. Ecography. 2013;36(1):27–46. doi: 10.1111/j.1600-0587.2012.07348.x. [DOI] [Google Scholar]
- Dénes, Silveira & Beissinger (2015).Dénes FV, Silveira LF, Beissinger SR. Estimating abundance of unmarked animal populations: accounting for imperfect detection and other sources of zero inflation. Methods in Ecology and Evolution. 2015;6(5):543–556. doi: 10.1111/2041-210X.12333. [DOI] [Google Scholar]
- Eisenberg & Lockhart (1972).Eisenberg JF, Lockhart M. An ecological reconnaissance of Wilpattu National Park, Ceylon. Smithsonian Contributions to Zoology. 1972. https://repository.si.edu/bitstream/handle/10088/5167/SCtZ-0101-Lo_res.pdf?sequence=2 https://repository.si.edu/bitstream/handle/10088/5167/SCtZ-0101-Lo_res.pdf?sequence=2
- Ficetola et al. (2018).Ficetola GF, Barz B, Melotto A, Muraro M, Lunghi E, Caned C, Lo PE, Nanni V, Silva-rocha I, Urso A, Carretero MA, Salvi D, Scali S, Scarì G. N-mixture models reliably estimate the abundance of small vertebrates. Scientific Reports. 2018;8(1):1–8. doi: 10.1038/s41598-018-28432-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiske & Chandler (2011).Fiske IJ, Chandler RB. Unmarked: an R package for fitting hierarchical models of wildlife occurrence and abundance. Journal of Statistical Software. 2011;43(10):1–23. doi: 10.18637/jss.v043.i10. [DOI] [Google Scholar]
- Fuller et al. (2022).Fuller AK, Augustine BC, Morin DJ, Pigeon K, Boulanger J, Lee DC, Bisi F, Garshelis DL. The occupancy-abundance relationship and sampling designs using occupancy to monitor populations of Asian bears. Global Ecology and Conservation. 2022;35(7):e02075. doi: 10.1016/j.gecco.2022.e02075. [DOI] [Google Scholar]
- Garcia, Joshi & Dharaiya (2016).Garcia KC, Joshi HM, Dharaiya N. Assessment of human-sloth bear conflicts in North Gujarat, India. Ursus. 2016;27(1):5–10. doi: 10.2192/URSUS-D-15-00012.1. [DOI] [Google Scholar]
- Garshelis, Joshi & Smith (1999).Garshelis DL, Joshi AR, Smith JLD. Estimating density and relative abundance of sloth bears. Ursus. 1999;11:87–98. [Google Scholar]
- Garshelis et al. (2022).Garshelis DL, Pigeon K, Hwang M, Proctor M, McShea WJ, Fuller AK, Morin DJ. The need to step-up monitoring of Asian bears. Global Ecology and Conservation. 2022;35:e02087. doi: 10.1016/j.gecco.2022.e02087. [DOI] [Google Scholar]
- Gaston & Rodrigues (2003).Gaston KJ, Rodrigues ASL. Reserve selection in regions with poor biological data. Conservation Biology. 2003;17:188–195. doi: 10.1046/j.1523-1739.2003.01268.x. [DOI] [Google Scholar]
- Gilbert et al. (2021).Gilbert NA, Clare JDJ, Stenglein JL, Zuckerberg B. Abundance estimation of unmarked animals based on camera-trap data. Conservation Biology. 2021;35(1):88–100. doi: 10.1111/cobi.13517. [DOI] [PubMed] [Google Scholar]
- Gomez et al. (2021).Gomez L, Wright B, Shepherd CR, Joseph T. An analysis of the illegal bear trade in India. Global Ecology and Conservation. 2021;27(1):e01552. doi: 10.1016/j.gecco.2021.e01552. [DOI] [Google Scholar]
- Gupta et al. (2012).Gupta S, Mondal K, Sankar K, Qureshi Q. Abundance and habitat suitability model for ratel (Mellivora capensis) in Sariska Tiger Reserve, western India. Wildlife Biology in Practice. 2012;8(1):13–22. doi: 10.2461/wbp.2012.8.2. [DOI] [Google Scholar]
- Harmsen et al. (2010).Harmsen BJ, Foster RJ, Silver S, Ostro L, Doncaster CP. Differential use of trails by forest mammals and the implications for camera-trap studies: a case study from Belize. Biotropica. 2010;42(1):126–133. doi: 10.1111/j.1744-7429.2009.00544.x. [DOI] [Google Scholar]
- Hines (2006).Hines JE. PRESENCE- Software to estimate patch occupancy and related parameters. USGS-PWRC. 2006. http://www.mbr-pwrc.usgs.gov/software/presence.html http://www.mbr-pwrc.usgs.gov/software/presence.html
- Hofmeester et al. (2019).Hofmeester TR, Cromsigt JPGM, Odden J, Andrén H, Kindberg J, Linnell JDC. Framing pictures: a conceptual framework to identify and correct for biases in detection probability of camera traps enabling multi-species comparison. Ecology and Evolution. 2019;9(4):1–17. doi: 10.1002/ece3.4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe et al. (2017).Howe EJ, Buckland ST, Després-Einspenner ML, Kühl HS. Distance sampling with camera traps. Methods in Ecology and Evolution. 2017;8(11):1558–1565. doi: 10.1111/2041-210X.12790. [DOI] [Google Scholar]
- Jennelle, Runge & MacKenzie (2002).Jennelle CS, Runge MC, MacKenzie DI. The use of photographic rates to estimate densities of tigers and other cryptic mammals: a comment on misleading conclusions. Animal Conservation. 2002;5(2):119–120. doi: 10.1017/S1367943002002160. [DOI] [Google Scholar]
- Jhala, Qureshi & Gopal (2015).Jhala YV, Qureshi Q, Gopal R. The status of tigers, copredators & prey in India 2014. National Tiger Conservation Authority, New Delhi & Wildlife Institute of India, Dehradun. TR2015/021. 2015. https://wii.gov.in/images//images/documents/publications/ALL% 20INDIA%20TIGER%20ESTIMATION%202014%20Comprehensive%20Report_1. Pdf. [21 December 2020]. https://wii.gov.in/images//images/documents/publications/ALL% 20INDIA%20TIGER%20ESTIMATION%202014%20Comprehensive%20Report_1. Pdf
- Jhala, Qureshi & Nayak (2020).Jhala YV, Qureshi Q, Nayak AK. Status of tigers, copredators and prey in India, 2018. National Tiger Conservation Authority, Government of India, New Delhi, and Wildlife Institute of India, Dehradun. 2020. https://wii.gov.in/images//images/documents/publications/status_tigers_2018.pdf. [21 December 2020]. https://wii.gov.in/images//images/documents/publications/status_tigers_2018.pdf
- Johnson, Laake & Ver Hoef (2010).Johnson DS, Laake JL, Ver Hoef JM. A model-based approach for making ecological inference from distance sampling data. Biometrics. 2010;66(1):310–318. doi: 10.1111/j.1541-0420.2009.01265.x. [DOI] [PubMed] [Google Scholar]
- Joseph et al. (2009).Joseph LN, Elkin C, Martin TG, Possingham HP. Modeling abundance using N-mixture models: the importance of considering ecological mechanisms. Ecological Applications. 2009;19(3):631–642. doi: 10.1890/07-2107.1. [DOI] [PubMed] [Google Scholar]
- Joshi, Garshelis & Smith (1995).Joshi AR, Garshelis DL, Smith JLD. Home ranges of sloth bears in Nepal: implications for conservation. The Journal of Wildlife Management. 1995;59(2):204–214. doi: 10.2307/3808932. [DOI] [Google Scholar]
- Kafley et al. (2019).Kafley H, Lamichhane BR, Maharjan R, Thapaliya B, Bhattarai N, Khadka M, Gompper ME. Estimating prey abundance and distribution from camera trap data using binomial mixture models. European Journal of Wildlife Research. 2019;65(5):0–14. doi: 10.1007/s10344-019-1308-0. [DOI] [Google Scholar]
- Kalle et al. (2014).Kalle R, Ramesh T, Qureshi Q, Sankar K. Estimating seasonal abundance and habitat use of small carnivores in the Western Ghats using an occupancy approach. Journal of Tropical Ecology. 2014;30(5):469–480. doi: 10.1017/S0266467414000340. [DOI] [Google Scholar]
- Karanth et al. (2011).Karanth KU, Gopalaswamy AM, Kumar NS, Vaidyanathan S, Nichols JD, Mackenzie DI. Monitoring carnivore populations at the landscape scale: occupancy modelling of tigers from sign surveys. Journal of Applied Ecology. 2011;48(4):1048–1056. doi: 10.1111/j.1365-2664.2011.02002.x. [DOI] [Google Scholar]
- Karanth & Nichols (1998).Karanth KU, Nichols JD. Estimation of tiger densities in India using photographic captures and recaptures. Ecology. 1998;79:2852–2862. doi: 10.1890/0012-9658(1998)079[2852:EOTDII]2.0.CO;2. [DOI] [Google Scholar]
- Keever et al. (2017).Keever AC, Mcgowan CP, Ditchkoff SS, Acker PK. Efficacy of N-mixture models for surveying and monitoring white-tailed deer populations. Mammal Research. 2017;62(4):413–422. doi: 10.1007/s13364-017-0319-z. [DOI] [Google Scholar]
- Kéry (2018).Kéry M. Identifiability in N-mixture models: a large-scale screening test with bird data. Ecology. 2018;99:281–288. doi: 10.1002/ecy.2093. [DOI] [PubMed] [Google Scholar]
- Kéry & Royle (2015).Kéry M, Royle JA. Applied hierarchical modeling in ecology: analysis of distribution, abundance and species richness in R and BUGS: volume 1: prelude and static models. London: Academic Press; 2015. [Google Scholar]
- Kéry & Schaub (2011).Kéry M, Schaub M. Bayesian population analysis using WinBUGS: a hierarchical perspective. London: Academic Press; 2011. [Google Scholar]
- Kidwai et al. (2019).Kidwai Z, Jimenez J, Louw CJ, Nel HP, Marshal JP. Using N-mixture models to estimate abundance and temporal trends of black rhinoceros (Diceros bicornis L.) populations from aerial counts. Global Ecology and Conservation. 2019;19:e00687. doi: 10.1016/j.gecco.2019.e00687. [DOI] [Google Scholar]
- Kolowski, Oley & McShea (2021).Kolowski JM, Oley J, McShea WJ. High-density camera trap grid reveals lack of consistency in detection and capture rates across space and time. Ecosphere. 2021;12(2):e03350. doi: 10.1002/ecs2.3350. [DOI] [Google Scholar]
- Kremen, Merenlender & Murphy (1994).Kremen C, Merenlender AM, Murphy DD. Ecological monitoring: a vital need for integrated conservation and development programs in the tropics. Conservation Biology. 1994;8:388–397. doi: 10.1046/j.1523-1739.1994.08020388.x. [DOI] [Google Scholar]
- Laurie & Seidensticker (1977).Laurie A, Seidensticker J. Behavioural ecology of the sloth bear (Melursus ursinus) Journal of Zoology. 1977;182(2):187–204. doi: 10.1111/j.1469-7998.1977.tb04155.x. [DOI] [Google Scholar]
- Linden et al. (2017).Linden DW, Fuller AK, Royle JA, Hare MP. Examining the occupancy-density relationship for a low-density carnivore. Journal of Applied Ecology. 2017;54(6):2043–2052. doi: 10.1111/1365-2664.12883. [DOI] [Google Scholar]
- Link et al. (2018).Link WA, Schofield MR, Barker RJ, Sauer JR. On the robustness of N-mixture models. Ecology. 2018;99(7):1547–1551. doi: 10.1002/ecy.2362. [DOI] [PubMed] [Google Scholar]
- Mazerolle (2015).Mazerolle MJ. Estimating detectability and biological parameters of interest with the use of the R Environment. Journal of Herpetology. 2015;49(4):541–559. doi: 10.1670/14-075. [DOI] [Google Scholar]
- McCarthy & Possingham (2007).McCarthy MA, Possingham HP. Active adaptive management for conservation. Conservation Biology. 2007;21(4):956–963. doi: 10.1111/j.1523-1739.2007.00677.x. [DOI] [PubMed] [Google Scholar]
- Meents et al. (1983).Meents JK, Rice J, Anderson BW, Ohmart RD. Nonlinear relationships between birds and vegetation. Ecology. 1983;64(5):1022–1027. doi: 10.2307/1937809. [DOI] [Google Scholar]
- Morin et al. (2022).Morin DJ, Boulanger J, Bischof R, Lee DC, Ngoprasert D, Fuller AK, McLellan B, Steinmetz R, Sharma S, Garshelis D, Gopalaswamy A, Nawaz MA, Karanth U. Comparison of methods for estimating density and population trends for low-density Asian bears. Global Ecology and Conservation. 2022;35(1):e02058. doi: 10.1016/j.gecco.2022.e02058. [DOI] [Google Scholar]
- Nakashima (2020).Nakashima Y. Potentiality and limitations of N-mixture and Royle-Nichols models to estimate animal abundance based on noninstantaneous point surveys. Population Ecology. 2020;62(1):151–157. doi: 10.1002/1438-390X.12028. [DOI] [Google Scholar]
- Nichols & Williams (2006).Nichols JD, Williams BK. Monitoring for conservation. Trends in Ecology & Evolution. 2006;21:668–673. doi: 10.1016/j.tree.2006.08.007. [DOI] [PubMed] [Google Scholar]
- O’Brien et al. (2020).O’Brien TG, Ahumada J, Akampurila E, Beaudrot L, Boekee K, Brncic T, Hickey J, Jansen PA, Kayijamahe C, Moore J, Mugerwa B, Mulindahabi F, Ndoundou-Hockemba M, Niyigaba P, Nyiratuza M, Opepa CK, Rovero F, Uzabaho E, Strindberg S. Camera trapping reveals trends in forest duiker populations in African National Parks. Remote Sensing in Ecology and Conservation. 2020;6(2):168–180. doi: 10.1002/rse2.132. [DOI] [Google Scholar]
- O’Brien, Kinnaird & Wibisono (2003).O’Brien TG, Kinnaird MF, Wibisono HT. Crouching tigers, hidden prey: Sumatran tiger and prey populations in a tropical forest landscape. Animal Conservation. 2003;6(2):131–139. doi: 10.1017/S1367943003003172. [DOI] [Google Scholar]
- O’Connell, Nichols & Karanth (2011).O’Connell AF, Nichols JD, Karanth KU. Camera traps in animal ecology: methods and analyses. New York: Springer; 2011. [Google Scholar]
- O’Connor et al. (2017).O’Connor KM, Nathan LR, Liberati MR, Tingley MW, Vokoun JC, Rittenhouse TAG. Camera trap arrays improve detection probability of wildlife: investigating study design considerations using an empirical dataset. PLOS ONE. 2017;12(4):1–12. doi: 10.1371/journal.pone.0175684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palei, Debata & Sahu (2020).Palei HS, Debata S, Sahu HK. Diet of sloth bear in an agroforest landscape in eastern India. Agroforestry Systems. 2020;94(1):269–279. doi: 10.1007/s10457-019-00389-1. [DOI] [Google Scholar]
- Palencia et al. (2021).Palencia P, Rowcliffe JM, Vicente J, Acevedo P. Assessing the camera trap methodologies used to estimate density of unmarked populations. Journal of Applied Ecology. 2021;58(8):1583–1592. doi: 10.1111/1365-2664.13913. [DOI] [Google Scholar]
- Parker et al. (2011).Parker G, Sundaresan S, Chege G, O’Brien T. Using sample aerial surveys to estimate the abundance of the endangered Grevy’s zebra in northern Kenya. African Journal of Ecology. 2011;49(1):56–61. doi: 10.1111/j.1365-2028.2010.01232.x. [DOI] [Google Scholar]
- Paudel et al. (2022).Paudel RP, Kadariya R, Lamichhane BR, Subedi N, Sashika M, Shimozuru M, Tsubota T. Habitat occupancy of sloth bear Melursus ursinus in Chitwan National Park, Nepal. Ecology and Evolution. 2022;12(3):e8699. doi: 10.1002/ece3.8699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pease, Nielsen & Holzmueller (2016).Pease BS, Nielsen CK, Holzmueller EJ. Single-camera trap survey designs miss detections: impacts on estimates of occupancy and community metrics. PLOS ONE. 2016;11(11):1–14. doi: 10.1371/journal.pone.0166689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penjor et al. (2019).Penjor U, Tan CKW, Wangdi S, Macdonald DW. Understanding the environmental and anthropogenic correlates of tiger presence in a montane conservation landscape. Biological Conservation. 2019;238(9):108196. doi: 10.1016/j.biocon.2019.108196. [DOI] [Google Scholar]
- Pokharel et al. (2022).Pokharel M, Subba A, Rai D, Bhandari S, Ghimirey Y. Fine-scale ecological and anthropogenic variables predict the habitat use and detectability of sloth bears in the Churia habitat of east Nepal. Ecology and Evolution. 2022;12(1):e8512. doi: 10.1002/ece3.8512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prajapati, Koli & Sundar (2021).Prajapati U, Koli VK, Sundar KSG. Vulnerable sloth bears are attracted to human food waste: a novel situation in Mount Abu town, India. Oryx. 2021;55(5):1–9. doi: 10.1017/S0030605320000216. [DOI] [Google Scholar]
- Proctor et al. (2022).Proctor MF, Garshelis DL, Thatte P, Steinmetz R, Crudge B, McLellan BN, McShea WJ, Ngoprasert D, Nawaz MA, Te Wong S, Sharma S, Fuller AK, Dharaiya N, Pigeon KE, Fredriksson G, Wang D, Li S, Hwang M-H. Review of field methods for monitoring Asian bears. Global Ecology and Conservation. 2022;35(12):e02080. doi: 10.1016/j.gecco.2022.e02080. [DOI] [Google Scholar]
- Puri et al. (2015).Puri M, Srivathsa A, Karanth KK, Kumar NS, Karanth KU. Multiscale distribution models for conserving widespread species: the case of sloth bear Melursus ursinus in India. Diversity and Distributions. 2015;21(9):1–14. doi: 10.1111/ddi.12335. [DOI] [Google Scholar]
- R Core Team (2019).R Core Team . R: a language and environment for statistical computing. Vienna: The R Foundation for Statistical Computing; 2019. [Google Scholar]
- Rajpurohit & Krausman (2000).Rajpurohit KS, Krausman PR. Human-sloth-bear conflicts in Madhya Pradesh, India. Wildlife Society Bulletin. 2000;28:393–399. [Google Scholar]
- Ramesh et al. (2012).Ramesh T, Kalle R, Sankar K, Qureshi Q. Factors affecting habitat patch use by sloth bears in Mudumalai Tiger Reserve, Western Ghats, India. Ursus. 2012;23:78–85. [Google Scholar]
- Ramsey, Caley & Robley (2015).Ramsey DSL, Caley PA, Robley A. Estimating population density from presence-absence data using a spatially explicit model. Journal of Wildlife Management. 2015;79(3):491–499. doi: 10.1002/jwmg.851. [DOI] [Google Scholar]
- Rather et al. (2020).Rather TA, Tajdar S, Kumar S, Khan JA. Seasonal variation in the diet of sloth bears in Bandhavgarh Tiger Reserve, Madhya Pradesh, India. Ursus. 2020;2020(31e12):1–8. doi: 10.2192/URSUS-D-19-00013.2. [DOI] [Google Scholar]
- Ratnayeke, Manen & Padmalal (2007).Ratnayeke S, Manen FTV, Padmalal UKGK. Home ranges and habitat use of sloth bears Melursus ursinus inornatus in Wasgomuwa National Park, Sri Lanka. Wildlife Biology. 2007;13:272–284. doi: 10.2981/0909-6396(2007)13[272:HRAHUO]2.0.CO;2. [DOI] [Google Scholar]
- Redford (1987).Redford KH. Current Mammalogy. New York: Springer; 1987. Ants and termites as food: patterns of mammalian myrmecophagy; pp. 349–399. [Google Scholar]
- Riley (1999).Riley S. A terrain ruggedness index that quantifies topographic heterogeneity. Intermountain Journal of Sciences. 1999;5:23–27. [Google Scholar]
- Rodgers & Panwar (1988).Rodgers WA, Panwar HS. Planning a wildlife protected area network in India. Dehradun: Wildlife Institute of India; 1988. [Google Scholar]
- Rowcliffe et al. (2008).Rowcliffe MJ, Field J, Turvey ST, Carbone C. Estimating animal density using camera traps without the need for individual recognition. Journal of Applied Ecology. 2008;45(4):1228–1236. doi: 10.1111/j.1365-2664.2008.01473.x. [DOI] [Google Scholar]
- Royle (2004).Royle JA. N-Mixture models for estimating population size from spatially replicated counts. Biometrics. 2004;60(1):108–115. doi: 10.1111/j.0006-341X.2004.00142.x. [DOI] [PubMed] [Google Scholar]
- Royle et al. (2014).Royle JA, Chandler RB, Sollmann R, Gardner B. Spatial capture-recapture. London: Academic Press; 2014. [Google Scholar]
- Royle & Dorazio (2008).Royle JA, Dorazio RM. Hierarchical modeling and inference in ecology: the analysis of data from populations, metapopulations and communities. London: Academic Press; 2008. [Google Scholar]
- Royle & Nichols (2003).Royle JA, Nichols JD. Estimating abundance from repeated presence-absence data or point counts. Ecology. 2003;84(3):777–790. doi: 10.1890/0012-9658(2003)084[0777:EAFRPA]2.0.CO;2. [DOI] [Google Scholar]
- Ruppert et al. (1997).Ruppert GS, Schardt M, Balzuweit G, Hussain M. A hybrid classifier for remote sensing applications. International Journal of Neural Systems. 1997;8(01):63–68. doi: 10.1142/S0129065797000094. [DOI] [PubMed] [Google Scholar]
- Santhanakrishnan et al. (2015).Santhanakrishnan B, Thamizharasu K, Srinivas G, Kumara H. Linking critical patches of sloth bear Melursus ursinus for their conservation in Meghamalai hills, Western Ghats, India. Current Science. 2015;109:1492–1498. doi: 10.18520/v109/i8/1492-1498. [DOI] [Google Scholar]
- Santiapillai & Santiapillai (1990).Santiapillai A, Santiapillai C. Status, distribution and conservation of the sloth bear (Melurus ursinus) in Sri Lanka. Tigerpaper. 1990;17:13–15. [Google Scholar]
- Sathyakumar et al. (2012).Sathyakumar S, Kaul R, Ashraf NVK, Mookherjee A, Menon V. National bear conservation and welfare action plan. India: Wildlife Institute of India, and Wildlife Trust of India; 2012. [Google Scholar]
- Searle et al. (2020).Searle CE, Bauer DT, Kesch MK, Hunt JE, Mandisodza-Chikerema R, Flyman MV, Macdonald DW, Dickman AJ, Loveridge AJ. Drivers of leopard (Panthera pardus) habitat use and relative abundance in Africa’s largest transfrontier conservation area. Biological Conservation. 2020;248(3):1–9. doi: 10.1016/j.biocon.2020.108649. [DOI] [Google Scholar]
- Singh, Sonone & Dharaiya (2018).Singh N, Sonone S, Dharaiya N. Sloth bear attacks on humans in central India: implications for species conservation. Human-Wildlife Interactions. 2018;12:338–347. doi: 10.26077/2mgq-fs29. [DOI] [Google Scholar]
- Sollmann et al. (2013).Sollmann R, Gardner B, Chandler RB, Shindle DB, Onorato DP, Royle JA, O’Connell AF. Using multiple data sources provides density estimates for endangered Florida panther. Journal of Applied Ecology. 2013;50(4):961–968. doi: 10.1111/1365-2664.12098. [DOI] [Google Scholar]
- Sun, Fuller & Royle (2014).Sun CC, Fuller AK, Royle JA. Trap configuration and spacing influences parameter estimates in spatial capture-recapture models. PLOS ONE. 2014;9:e88025. doi: 10.1371/journal.pone.0088025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao et al. (2018).Xiao W, Hebblewhite M, Robinson H, Feng L, Zhou B, Mou P, Wang T, Ge J. Relationships between humans and ungulate prey shape Amur tiger occurrence in a core protected area along the Sino-Russian border. Ecology and Evolution. 2018;8(23):1–17. doi: 10.1002/ece3.4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoganand (2005).Yoganand K. Behavioural ecology of sloth bear (Melursus ursinus) in Panna National Park, Central India. 2005. PhD Thesis. Saurashtra University, Rajkot.
- Yoganand et al. (2006).Yoganand K, Rice CG, Johnsingh AJT, Seidensticker J. Is the sloth bear in India secure? A preliminary report on distribution, threats and conservation requirements. Journal of the Bombay Natural History Society. 2006;103:172–181. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Covariates considered: A) Mixed forest, (B) Sal forest, (C) Human photo-capture rate and (D) Distance to village. Prediction of relative abundance was based on the most parsimonious models of negative binomial (NB) distribution; Values of covariates were shown on a standardized scale.
Covariates considered: A) Mixed forest, (B) Sal forest, (C) Human photo-capture rate and (D) Distance to village. Prediction of relative abundance was based on the most parsimonious models of zero-inflated Poisson (ZIP) distribution; Values of covariates were shown on a standardized scale.
All covariates were standardized using the Z score.
Data Availability Statement
The following information was supplied regarding data availability:
The raw data is available in the Supplemental Files.