Abstract
Yersinia pseudotuberculosis, a gram-negative bacterium responsible for enteric and systemic infection in humans, produces a superantigenic toxin designated YPMa (Y. pseudotuberculosis-derived mitogen). To assess the role of YPMa in the pathogenesis of Y. pseudotuberculosis, we constructed a superantigen-deficient mutant and compared its virulence in a mouse model of infection to the virulence of the wild-type strain. Determination of the survival rate after intravenous (i.v.) bacterial inoculation of OF1 mice clearly showed that inactivation of ypmA, encoding YPMa, reduced the virulence of Y. pseudotuberculosis. Mice infected i.v. with 104 and 105 wild-type bacteria died within 9 days, whereas mice infected with the ypmA mutant survived 12 and 3 days longer, respectively. This decreased virulence of the ypmA mutant strain was not due to an impaired colonization of the spleen, liver, or lungs. In contrast to i.v. challenge, bacterial inoculation by the intragastric (i.g.) route did not reveal any difference in virulence between wild-type Y. pseudotuberculosis and the ypmA mutant since the 50% lethal doses were identical for both strains. Moreover, inactivation of ypmA gene did not affect the bacterial growth of Y. pseudotuberculosis in Peyer's patches, mesenteric lymph nodes (MLNs), and spleen after oral infection. Histological studies of spleen, liver, lungs, heart, Peyer's patches, and MLNs after i.v. or i.g. challenge with the wild type or the ypmA mutant did not reveal any feature that can be specifically related to YPMa. Our data show that the superantigenic toxin YPMa contributes to the virulence of Y. pseudotuberculosis in systemic infection in mice.
The gram-negative bacterium Yersinia pseudotuberculosis is widely spread in nature and is responsible for sporadic infection in many animal species (10). Humans are commonly infected after ingestion of food or water contaminated with excreta of infected animals. Y. pseudotuberculosis causes acute ileitis and mesenteric lymphadenitis, sometimes complicated by septicemia (10, 26), but is also responsible for the occurrence of postinfection complications such as reactive arthritis and erythema nodosum (10, 35, 45, 46). This microorganism has been suggested as one of the causative agents of Kawasaki syndrome, an acute, self-limited vasculitis affecting predominantly infants and young children (4, 25, 39). A few years ago, Y. pseudotuberculosis strains producing a mitogen activity were isolated from a mass outbreak in Japan (48) and from a patient with Kawasaki-like symptoms (1, 51). The substance, purified from bacterial lysates and exhibiting a mitogenic activity on human peripheral blood mononuclear cells (PBMC), was first designated YPM, for Y. pseudotuberculosis-derived mitogen (30), and then YPMa after the discovery of a variant (36). YPMa was characterized as a 14.5-kDa superantigenic toxin activating human T lymphocytes bearing T-cell receptors exhibiting the Vβ3, Vβ9, Vβ13.1, and Vβ13.2 variable regions (1, 48). The geographic distribution of the 456-bp superantigen-encoding gene ypmA is rather heterogeneous, being present in most Y. pseudotuberculosis strains from the Far East but only in about 20% of European clinical isolates (13, 52).
A recent study showed that 61% of patients acutely infected with Y. pseudotuberculosis, especially those with systemic complications, had elevated anti-YPM immunoglobulin G level in blood, thereby demonstrating the production of YPMa in vivo (2). Furthermore, Vβ3-bearing T cells were increased in patients during the acute phase of the disease (2). Additionally, Miyoshi-Akiyama et al. (32) found that purified YPMa was able to induce lethal shock in a murine experimental model, demonstrating the toxicity of the Y. pseudotuberculosis superantigenic toxin in vivo. The toxin was also found to alter in vitro epithelial function by reducing active ion transport and increasing epithelial permeability (16). Altogether, these data suggest a role of YPMa in the pathogenesis of Y. pseudotuberculosis infections, but experimental evidence necessary to confirm this hypothesis is lacking.
In this study, we constructed a superantigen-deficient mutant of Y. pseudotuberculosis and tested its virulence in a mouse experimental model of infection. Rodents have been extensively used as the animal model to study Yersinia infections since they develop a disease resembling yersiniosis in humans (22). We found that inactivation of ypmA reduced the virulence of Y. pseudotuberculosis after intravenous (i.v.) challenge but not after intragastric (i.g.) inoculation, demonstrating an exacerbated toxicity of YPMa-producing Y. pseudotuberculosis in systemic infection.
MATERIALS AND METHODS
Bacterial strains, growth conditions, and plasmids.
Bacterial strains and plasmids used in this study are listed in Table 1. Y. pseudotuberculosis AH was kindly provided by N. Takeda (Department of Pediatrics, Kurashiki Central Hospital, Okayama, Japan). Escherichia coli DH5α was used as the host for the pUC derivatives, and E. coli SY327λpir and SM10λpir were hosts for the suicide vector pCVD442. Y. pseudotuberculosis and E. coli strains were grown at 28 and 37°C, respectively, in Luria-Bertani (LB) broth or agar except where noted. Mating experiments were plated on M9 minimum medium agar (NaH2PO4, 33 mM; KH2PO4, 22 mM; NaCl, 8.5 mM; NH4Cl, 18 mM; MgSO4, 2 mM; CaCl2, 0.1 mM; thiamine, 0.3 μM; glucose, 5.5 mM; agar, 14 g/liter). Kanamycin and vancomycin were used at 50 μg/ml, and ampicillin was used at 100 μg/ml.
TABLE 1.
Bacterial strains and plasmids used in this work
| Strains and plasmids | Description | Source or reference |
|---|---|---|
| Strains | ||
| Y. pseudotuberculosis | ||
| AH | Isolate from a patient suffering from Kawasaki syndrome, pYV+ | Gift from N. Takeda |
| H194 | YPMa-deficient strain derived from Y. pseudotuberculosis AH, pYV+ | This study |
| E. coli | ||
| DH5α | supE Δlac U169 (φ80lacZΔM15) hsdR recA endA gyrA hri relA | 20 |
| SY327λpir | Δ(lac pro) argE(Am) recA rif nalA λpir | 29 |
| SM10λpir | thi thr leu sup tonA lacY recA::RP4-2-Tc::MuKm λpir | 40 |
| Plasmids | ||
| pUC18 | Cloning vector; Apr | 50 |
| pUC1318-KmII | 1.5-kb fragment from plasmid pJH1 of Enterococcus faecalis containing aph(3′)-IIIa gene cloned into pUC1318; Kmr | Gift from P. Trieu-Cuot |
| pCVD442 | Suicide vector containing the counterselectable marker sacB; Apr | 17 |
| pCCY10 | 6.5-kb HindIII fragment from genomic DNA of Y. pseudotuberculosis AH, containing the ypmA gene, cloned into pUC18 | This study |
| pCCY12 | 1.5-kb XbaI/BamHI fragment from pCCY10 cloned into pUC18, used for trans complementation of H194 | This study |
| pCCY17.1Δ | 3.5-kb KpnI fragment from pCCY10 cloned into pUC18; deletion of restriction sites from the polylinker | This study |
| pCS10 | 1,086-bp HindIII/BamHI fragment containing ypmA deleted from 434 bp, cloned into pCCY17.1Δ | This study |
| pCS20 | 1.5-kb PstI fragment from pUC1318-KmII containing the aph(3′)-IIIa gene, introduced into the unique PstI restriction site of pCS10 | This study |
| pCCY20 | 4.2-kb NarI/XbaI fragment from pCS20 cloned into pCVD442, used for allelic exchange | This study |
Southern hybridization.
Genomic DNA was extracted from bacterial cells as previously described (27) and digested by restriction endonucleases as recommended by the manufacturers (Life Technologies, Cergy Pontoise, France; Boehringer Mannheim France, Meylan, France). Restricted DNA fragments were separated by electrophoresis through a 0.8% agarose gel in Tris-borate-EDTA buffer and then transferred onto a nylon membrane (Hybond-N+; 0.45-μm pore size; Amersham Life Science, Buckinghamshire, England). Probes were generated by PCR amplification in the presence of digoxigenin-11-dUTP (Boehringer Mannheim France) and were purified on Spin-X columns (Corning Costar Corporation, Acton, Mass.). Hybridizations were carried out at 68°C in 5× SSC (0.75 M sodium chloride, 0.075 M sodium citrate [pH 7.0])–0.02% sodium dodecyl sulfate (SDS)–0.1% N-lauroyl sarcosine, 1% blocking reagent (Boehringer Mannheim France). After hybridization, filters were washed twice at room temperature for 15 min in 2× SSC–0.1% SDS and then twice at 68°C for 15 min in 0.1× SSC–0.1% SDS. Chemiluminescent detection of the digoxigenin-labeled probes was performed as instructed by the manufacturer (Boehringer Mannheim France). Nylon membranes were exposed with BioMax film (Eastman Kodak Company, Rochester, N.Y.) at room temperature.
Plasmid constructions.
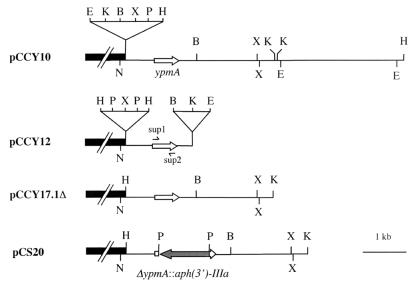
DNA hybridization of endonuclease-restricted genomic DNA from Y. pseudotuberculosis AH with a 418-bp ypmA-specific probe generated with sup1 (5′acacttttctctggagtagcg3′) and sup2 (5′acaggacatttcgtca3′) primers (Fig. 1) revealed the presence of ypmA on a 6.5-kb HindIII DNA fragment. This fragment was cloned into pUC18, and the recombinant plasmid was designated pCCY10 (Fig. 1). DNA sequencing of this HindIII fragment (i) confirmed the presence of the variant ypmA (24, 31) (accession no. D38523 and D38638) and (ii) defined the flanking regions of ypmA (data not shown). Plasmid pCCY12 consists of the 1.5-kb XbaI/BamHI fragment from pCCY10 inserted into pUC18 and was used for trans complementation of the ypmA mutant (Fig. 1). Plasmid pCCY17.1 corresponds to the 3.5-kb KpnI fragment from pCCY10 cloned into pUC18. To eliminate the PstI restriction site from the polylinker, pCCY17.1 was digested with HindIII and religated to itself to give pCCY17.1Δ which was used for the construction of the ypmA-deficient mutant (Fig. 1).
FIG. 1.
Restriction maps of pCCY10, pCCY12, pCCY17.1Δ, and pCS20. B, BamHI; E, EcoRI; H, HindIII; K, KpnI; N, NarI; P, PstI; X, XbaI. Solid black box, pUC18 vector; open arrow, ypmA; hatched arrow, aph(3′)-IIIa.
Inactivation of ypmA.
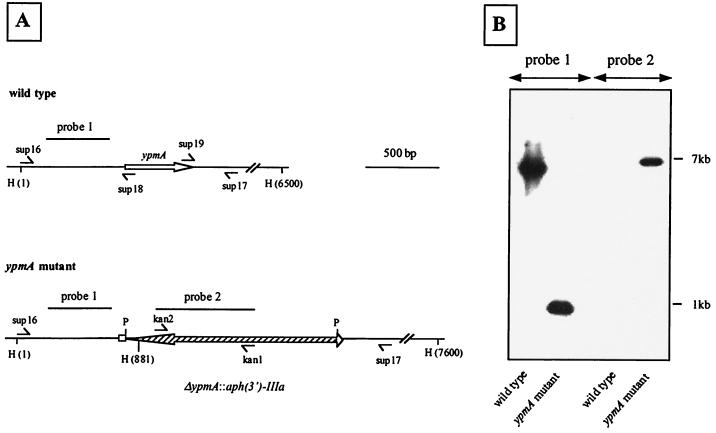
The ypmA gene was deleted by overlap extension using PCR (18, 23). Sup16 (5′gcggcaagctttgaagggttgtcacaattgcacct3′) and sup18 (5′acggactgcattggctgcagatttttcataactcaacctataaatat3′) primers yielded by PCR a 782-bp fragment encompassing the upstream and 5′ regions of ypmA, whereas sup19 (5′ctgcagccaatgcagtccgttgtcctgtgtgaaaaatatttaatggctc3′) and sup17 (5′aagtgggatccaggaggttcac3′) produced a 324-bp fragment covering the 3′ end of ypmA and its downstream region (Fig. 2A). Sup16 and sup17 contain the HindIII and BamHI restriction sites, respectively. Primers sup18 and sup19 were generated to obtain a 20-bp overlapping sequence containing a PstI restriction site located six nucleotides downstream of the guanine residue of the start codon of ypmA and 23 nucleotides upstream from the thymine residue of the stop codon of ypmA, respectively. Amplimers produced with sup16-sup18 and sup17-sup19 were purified, combined, annealed by their 20-bp overlapped region, and 3′ extended following the complementary strand. The resulting fusion was finally amplified with sup16 and sup17 primers to give a 1,086-bp fragment corresponding to a 434-bp deletion within ypmA with a PstI restriction site at the site of the deletion. Conditions for PCRs have been previously described (11, 18). The resulting fragment was digested with HindIII and BamHI and cloned into HindIII/BamHI-digested pCCY17.1Δ. The plasmid was designated pCS10. The kanamycin resistance-encoding gene [aph(3′)-IIIa] (47) was purified after digestion of pUC1318-KmII with PstI and then cloned into the unique PstI restriction site of pCS10 to give pCS20 (Fig. 1).
FIG. 2.
Construction of the Y. pseudotuberculosis superantigen-deficient mutant. (A) HindIII restriction map of ypmA and ΔypmA::aph(3′)-IIIa regions. ypmA and aph(3′)-IIIa are represented as open and hatched arrows, respectively. H, HindIII; P, PstI. (B) Southern blot of HindIII-digested DNA from wild-type Y. pseudotuberculosis AH and superantigen-deficient mutant H194 hybridized with probe 1 (451 bp), corresponding to the upstream region of ypmA, and probe 2 (673 bp), detecting the kanamycin resistance gene. For better clarity, the image was manipulated by Adobe Photoshop 5.0 software.
Construction of the superantigen-deficient Y. pseudotuberculosis.
The 4.2-kb NarI/XbaI fragment from pCS20 was purified, and its cohesive ends were filled with the Klenow fragment of DNA polymerase I as instructed by the manufacturer (Life Technologies). The resulting fragment was cloned into the suicide plasmid pCVD442 (17) digested with SmaI and transformed into E. coli SY327λpir. The construct was designated pCCY20 and was then transformed into E. coli SM10λpir for mating experiments.
Allelic exchange was carried out as previously described (7, 17), with minor modifications. Briefly, plasmid pCCY20 was transferred from E. coli SM10λpir(pCCY20) into wild-type Y. pseudotuberculosis AH by filter mating. Since auxotrophic E. coli SM10λpir does not grow on minimum medium, the first recombination event was selected on M9 minimum medium agar containing ampicillin. A single Yersinia colony was grown overnight in LB broth with kanamycin. The second recombination event was selected by plating an overnight culture on LB agar lacking sodium chloride and containing 10% sucrose in the presence of kanamycin. Incubation was performed at 30°C for 72 h. Sucrose- and kanamycin-resistant colonies resulting from the successful second recombination event were tested for sensitivity to ampicillin.
Genetic analysis of the ampicillin-sensitive strains was carried out by Southern hybridization using probes 1 and 2 (Fig. 2). Probe 1 (451 bp) located upstream of ypmA was generated with primers sup5 (5′ctcgggggattggttgtgga3′) and sup7 (5′ccttgggctccgatattgatc3′) and with pCCY10 as the template. The 673-bp aph(3′)-IIIa-specific probe (probe 2) (Fig. 2) was obtained by PCR with kan1 (5′ggaatgtctcctgctaagg3′) and kan2 (5′ggcttgatccccagtaag3′) primers, using pUC1318-KmII as a template. Phenotypic analyses of the recombinant strains, based on metabolic properties, were performed with the API20E identification system (bioMérieux, Lyon, France).
In vitro lymphocyte proliferation assay.
Lymphocyte proliferation tests were performed on human peripheral blood mononuclear cells (PBMC) as previously described (34). Briefly, PBMC were separated from healthy-donor whole blood by Ficoll-gradient centrifugation, and 106 cells were cultivated in Eagle medium supplemented with gentamicin (8 μg/ml), l-glutamine (2 mM), and inactivated calf serum (20%) in the presence of an overnight culture supernatant from Y. pseudotuberculosis. Incubation was carried out for 72 h at 37°C in a humidified atmosphere containing 5% CO2. Eighteen hours before the end of the culture, 1μCi of [3H]thymidine was added to the cells. The radioactivity was counted after lysis of the lymphocytes, and results were expressed as stimulation index, which represents the ratio of counts per minute from lymphocytes incubated with bacterial supernatant versus counts per minute from lymphocytes cultivated in the absence of mitogen.
Mouse experimental infection.
Six-week-old female outbred OF1 mice (Iffa Credo, L'Arbresle, France) were challenged either i.v. (0.3 ml of bacterial suspension in sterile phosphate-buffered saline [PBS]) or i.g. by using a gastric tube (0.2 ml of bacterial suspension in sterile distilled water). Bacterial inocula were prepared from overnight cultures in LB at 28°C. Cultures were centrifuged, and the bacterial pellets were washed once and resuspended in distilled water or PBS. Animals were kept in positive-pressure cabinets during experimentation, and mortality was monitored daily for 21 days after challenge. For each experimental infection, the presence of the virulence plasmid pYV was confirmed by PCR on bacterial thermolysates using YopH1 (5′catcgtcaggtatctcga3′) and YopH2 (5′caatcagttgcgcagtac3′) primers which are internal to yopH, a gene coding for a tyrosine phosphatase and located on pYV (6, 8, 14).
Bacterial growth in organs or tissues was assessed at different time points after challenge. Animals were sacrificed, and organs or tissues were aseptically removed and homogenized in PBS. Spleen, liver, and lungs were collected after i.v. challenge, whereas spleen, three Peyer's patches along 10-cm length of the ileum, and the entire mesenteric lymph node (MLN) chain were removed after i.g. challenge. Bacterial counts were obtained by spreading dilutions of organ or tissue homogenate on LB agar containing vancomycin, as Yersinia species are naturally resistant to this antibiotic.
Histological studies.
At days 2, 4, and 7 after i.v. challenge and at days 2, 4, 7, 11, and 21 after i.g. inoculation, one mouse from each group of animals was randomly chosen for histological examination. Specimens were fixed in 10% buffered formalin (spleen, heart, lungs, intestine, MLNs) or Bouin's fixative (liver) and embedded in paraffin. Sections were cut at 4-μm thickness from paraffin blocks and stained with hematoxylin and eosin for light microscopy.
RESULTS
Construction and characterization of the Y. pseudotuberculosis ypmA mutant.
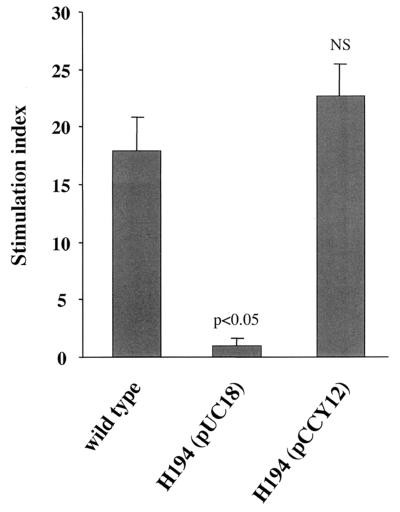
A superantigen-deficient strain was constructed from YPMa-producing Y. pseudotuberculosis AH. After cloning of ypmA and its flanking regions, a 434-bp internal deletion in ypmA was generated and replaced by a kanamycin resistance gene [aph(3′)-IIIa] (Fig. 2A). The construct was then introduced into the suicide vector pCVD442 to give pCCY20, which contains the sacB gene, a counterselectable marker allowing a positive selection for the second recombination event. Kanamycin- and ampicillin-resistant Y. pseudotuberculosis merodiploides were obtained by mating E. coli SM10λpir(pCCY20) with wild-type Y. pseudotuberculosis. One colony was selected and grown in the presence of kanamycin and plated on LB agar containing 10% sucrose and kanamycin. From 100 sucrose- and kanamycin-resistant clones, all were sensitive to ampicillin and one was randomly chosen for further characterization. The recombinant strain was phenotypically identical to the wild-type strain with regard to colony morphology on agar, in vitro growth rate at 28 and 37°C, and metabolic properties. The mutant strain was also genetically characterized. The oligonucleotide pairs kan1-sup16 and kan2-sup17 (Fig. 2A) both amplified by PCR a 1.6-kb fragment from DNA of the ypmA mutant strain, confirming the position of the kanamycin resistance gene (data not shown). DNA hybridization with probes 1 and 2 of HindIII-digested genomic DNA from AH and the mutant strain confirmed the single copy of the kanamycin resistance gene and its position in the genome (Fig. 2B). This isolate corresponding to a superantigen-deficient mutant from Y. pseudotuberculosis AH was designated H194. Complementation in trans of strain H194 with plasmid pCCY12, a pUC derivative containing the intact ypmA gene, restored the in vitro mitogen activity on PBMC (Fig. 3).
FIG. 3.
trans complementation of superantigen-deficient Y. pseudotuberculosis H194. pUC18 and plasmid pCCY12 containing the intact ypmA gene were introduced into superantigen-deficient Y. pseudotuberculosis H194 by electroporation. T-cell proliferation induced by overnight culture supernatants was measured by [3H]thymidine incorporation (34). Each column represents the average of three values ± SD. The nonparametric Mann-Whitney test was used for statistical evaluation of the results, the stimulation index from the wild-type strain being used as the reference. P = 0.05 was the limit of significance. NS, nonsignificant.
Virulence of the ypmA mutant after i.g. challenge.
To evaluate the effect of ypmA inactivation on virulence, OF1 mice were challenged with wild-type Y. pseudotuberculosis AH or with superantigen-deficient mutant H194 by the oral route, which is the natural mode of infection for Y. pseudotuberculosis. After inoculation with serial 10-fold dilutions of the wild type and H194, the 50% lethal dose (LD50) after 21 days was estimated at 109 for both strains, indicating a moderate virulence with regards to other Y. pseudotuberculosis strains (15, 33, 37, 42). The kinetics of animal killing over the 3 weeks were found to be similar for both wild-type and mutant strains (data not shown). These data showed that the presence of ypmA does not enhance the virulence of Y. pseudotuberculosis after i.g. challenge.
To estimate the translocation level through the intestinal barrier and the colonization of the lymphoid tissues by the wild type and H194 mutant, Yersinia counts in Peyer's patches, MLNs, and spleen were determined on days 2, 4, 7, 11, and 21 after i.g. challenge with 0.1 LD50 (108 bacteria). Colonization of organs and tissues with mutant H194 did not significantly differ from the parental strain AH: bacterial counts in the Peyer's patches reached a maximum at day 4 (105.66 ± 0.34 and 106.05 ± 0.46 CFU, respectively) and remained at a high level at day 21 (104.25 ± 2.23 and 104.36 ± 1.79 CFU, respectively). Yersinia counts peaked at day 4 in MLNs (104.12 ± 0.55 and 104.21 ± 0.81 CFU, respectively) and at day 7 in spleen (104.31 ± 1.45 and 105.55 ± 1.13 CFU, respectively) and then steadily decreased. Unlike in Peyer's patches, no bacteria were detected in spleen and MLNs of most animals by day 21. Histological studies of Peyer's patches, MLNs, and spleen at each time point revealed an early formation of abscesses composed of clusters of bacteria and polymorphonuclear neutrophils (data not shown). This histological study did not show any YPMa-specific lesions in the organs and tissues of infected mice and confirmed previous histological descriptions of Yersinia infections (22, 33, 41).
Virulence of ypmA mutant after i.v. challenge.
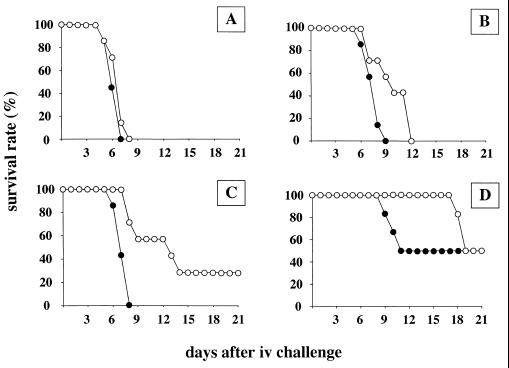
To further investigate the role of ypmA, we tested the virulence of wild-type and ypmA-deficient Y. pseudotuberculosis in systemic infection. OF1 mice were challenged i.v. with 10-fold serial dilution of inocula ranging from 103.3 to 106.3 bacteria, and the survival rate was monitored daily (Fig. 4). When mice were infected with the highest inoculum (106.3 bacteria), both strains were highly virulent since there were no survivors after day 8. Interestingly, the animals infected with a lower inoculum of H194 survived at a higher rate than mice infected with the wild-type strain. When challenged with 103.3 bacteria, mice infected with the ypmA mutant survived much longer (10 days) than the wild-type-infected animals; however, the survival rate reached 50% at day 21 for both strains (Fig. 4D), indicating that the systemic LD50s after 3 weeks were similar for the H194 mutant and wild-type Y. pseudotuberculosis. This kinetics of killing was reproduced in three separate experiments.
FIG. 4.
Virulence of wild-type and superantigen-deficient Y. pseudotuberculosis in mice. Wild-type Y. pseudotuberculosis (●) and its ypmA isogenic mutant H194 (○) were injected i.v. into OF1 mice. Groups of seven animals were challenged with 106.3 (A), 105.3 (B), 104.3 (C), or 103.3 (D) bacteria, and mortality was monitored over a 3-week period.
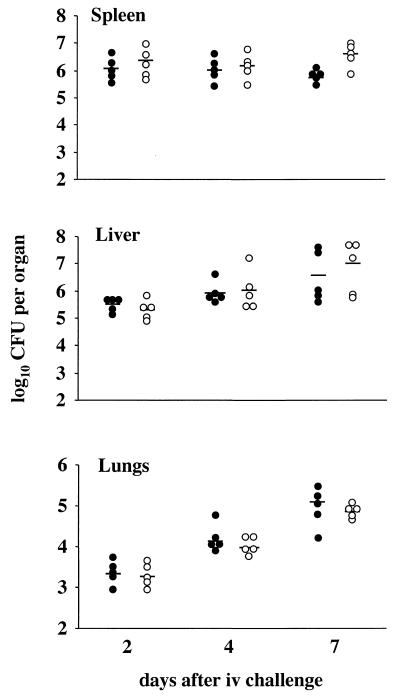
The difference in the kinetics of mortality between wild type and H194 could be attributed to a lower rate of multiplication of the mutant in vivo. To assess this question, we determined Yersinia counts in the spleen, liver, and lungs at days 2, 4, and 7 after i.v. challenge of OF1 mice with the wild type or ypmA mutant (103.4 bacteria). As illustrated in Fig. 5, similar bacterial counts were found in the organs of wild-type- and mutant-infected animals. High CFU numbers were recovered from the spleen early in the course of the infection (day 2), whereas in the liver and lungs, highest counts appeared only at day 7. The average weight of spleen at day 7 was more than 2.5 times the average weight at day 2 for both groups of mice (data not shown). This weight increase was attributed to a cellular recruitment induced by the infection. Histopathological manifestations in wild type or mutant i.v.-challenged mice were characterized by abscesses in the liver and the spleen composed of bacterial colonies and neutrophilic infiltration (days 2, 4, and 7) (data not shown). Histological studies of the heart did not reveal any alteration of the tissues. No histological features could be related to the presence of the YPMa superantigenic toxin. Altogether, the data from the experimental systemic infection showed that inactivation of ypmA did not affect tissue colonization by Y. pseudotuberculosis but increased the survival rate of mice, indicating that YPMa contributes to the virulence of Y. pseudotuberculosis in systemic infection.
FIG. 5.
Bacterial counts in spleen, liver, and lungs from mice infected i.v. with wild-type or YPMa-deficient Y. pseudotuberculosis H194. Groups of five OF1 mice were given 103.4 bacteria i.v., and animals were sacrificed at days 2, 4, and 7 after challenge. Each dot represents the number of CFU recovered per organ of one mouse infected with wild-type Y. pseudotuberculosis (●) or with the YPMa-deficient mutant (○). Detection limit was 102 CFU per organ. Horizontal bars indicate the mean CFU per organ.
DISCUSSION
In this study, we investigated the impact of the superantigenic toxin YPMa in the pathogenesis of Y. pseudotuberculosis. The effects of bacterial superantigens in vivo have mainly been studied by dosing animals with sometimes unphysiological amounts of purified proteins. Very few studies have used living microorganisms to investigate the role of superantigens in an experimental model (9, 38), and to our knowledge no study has demonstrated the role of a superantigenic molecule using isogenic mutants. Our experimental approach was to test the in vivo effect of YPMa in the context of other virulence factors of Yersinia (e.g., the Yop effectors [14]), using replicating Y. pseudotuberculosis rather than purified proteins. The use of living Yersinia also ensures the release of a physiological amount of superantigenic toxin over a long period of time. To construct the ypmA isogenic mutant, Y. pseudotuberculosis strain AH was used as parental strain because (i) it has a clinical relevance since it was isolated from a patient with Kawasaki syndrome, (ii) it contains the ypmA gene, which represents the most common variant found among the superantigen-producing Y. pseudotuberculosis (12, 36), and (iii) it produces in vitro a high mitogen activity compared to other superantigenic strains of Y. pseudotuberculosis (unpublished results).
Ueshiba et al. (49) observed that all Y. pseudotuberculosis isolates from systemic infections were superantigen-producing strains and that human Y. pseudotuberculosis systemic infections were characterized by symptoms (high fever, a scarlatiniform skin rash, desquamation, strawberry tongue) that resemble staphylococcal toxic shock syndrome or streptococcal scarlet fever, both systemic manifestations caused by superantigens. This comparison suggested that the superantigen of Y. pseudotuberculosis could be involved in the systemic type of infection. This hypothesis was strengthened by our experimental model which showed that the presence of YPMa aggravates the infection when Y. pseudotuberculosis follows the systemic route.
Since the toxic effect of the YPMa+ strain by the systemic route could not be attributed to a higher multiplication rate in animal organs, the exacerbated virulence of the wild type could be directly related to the production of YPMa. The mechanism by which YPMa induces a higher death rate is unclear but is probably multifactorial. The synergistic action of YPMa and LPS might provide a possible mechanism since superantigens are known to potentiate the toxicity of endotoxins (5, 43). A comparison of cytokine profiles and the characterization of T-cell populations recruited to the spleen after infection with the wild type or H194 should provide some insights into the role of YPMa when produced in vivo.
Intragastric infections did not reveal any superantigen-related toxicity in our experimental model: (i) the LD50 and mortality kinetics were identical for both wild-type and mutant Y. pseudotuberculosis and (ii) the bacterial counts recovered from organs and tissues indicated that YPMa did not affect the bacterial translocation across the intestinal barrier. Nevertheless, the role of YPMa after oral infection cannot be completely ruled out. Indeed, bacterial counts in Peyer's patches were still high after 21 days in both wild-type- and mutant-infected mice, whereas the numbers of bacteria in spleen and MLNs were low. The consequences of a long-term persistence of Y. pseudotuberculosis in Peyer's patches are unknown. Immunopathological disorders such as reactive arthritis, erythema nodosum, or vasculitis during human yersiniosis might occur several months after the enteric infection (45, 46). Hence, experimental infections for a longer period of time should be followed for the possible appearance of reactive arthritis or vasculitis. Furthermore, one cannot eliminate the hypothesis of a down-regulation of ypmA expression in the gut, which would explain the absence of toxicity of YPM+ Y. pseudotuberculosis in our i.g. infection model. Indeed, bacterial gene expression are often under a strict control of environmental stimuli (O2 level, pH, osmolarity, bacterial density, etc.) (19, 28, 44) and a variation of expression of ypmA, depending on the route of infection, should be considered to explain the difference in virulence between systemic and oral infections.
A variation of susceptibility of mice to Yersinia infection has been reported and was shown to be dependent on gamma interferon production (3, 21). Mouse strains other than outbred OF1 mice should now be tested to determine whether the toxicity of superantigen-producing Y. pseudotuberculosis is strain specific or whether it can be generalized to other mouse strains. It would also be interesting to evaluate the toxicity of YPM+ Y. pseudotuberculosis in relation to gamma interferon, an inflammatory cytokine frequently involved in response to superantigen.
In conclusion, we showed that YPMa is a virulence factor which exacerbates the toxicity of Y. pseudotuberculosis in systemic but not in gastric infection, even if the long-term effect of the presence of superantigen-producing Y. pseudotuberculosis in Peyer's patches remained to be evaluated. In light of this work, a reassessment of the systemic pathogenesis of Y. pseudotuberculosis, involving the superantigenic toxin YPMa, is now required to better understand human systemic yersiniosis.
ACKNOWLEDGMENTS
Christophe Carnoy was supported by a grant from the Centre Hospitalier Régional et Universitaire de Lille, by the Fondation pour La Recherche Médicale, and by the Région Nord-Pas de Calais. This work was partly supported by Institut IPSEN, Région Nord-Pas de Calais, and the European Regional Development Fund.
We gratefully thank Shamila Nair for reading the manuscript.
REFERENCES
- 1.Abe J, Takeda T, Watanabe Y, Nakao H, Kobayashi N, Leung D Y M, Kohsaka T. Evidence for superantigen production by Yersinia pseudotuberculosis. J Immunol. 1993;151:4183–4188. [PubMed] [Google Scholar]
- 2.Abe J, Onimaru M, Matsumoto S, Noma S, Baba K, Ito Y, Kohsaka T, Takeda T. Clinical role for a superantigen in Yersinia pseudotuberculosis infection. J Clin Investig. 1997;99:1823–1830. doi: 10.1172/JCI119349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Autenrieth I B, Beer M, Bohn E, Kaufmann S H E, Heesemann J. Immune responses to Yersinia enterocolitica in susceptible BALB/c and resistant C57BL/6 mice: an essential role for gamma interferon. Infect Immun. 1994;62:2590–2599. doi: 10.1128/iai.62.6.2590-2599.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baba K, Takeda N, Tanaka M. Cases of Yersinia pseudotuberculosis infection having diagnostic criteria of Kawasaki disease. Contrib Microbiol Immunol. 1991;12:292–296. [PubMed] [Google Scholar]
- 5.Blank C, Luz A, Bendigs S, Erdmann A, Wagner H, Heeg K. Superantigen and endotoxin synergize in the induction of lethal shock. Eur J Immunol. 1997;27:825–833. doi: 10.1002/eji.1830270405. [DOI] [PubMed] [Google Scholar]
- 6.Bliska J B, Guan K L, Dixon J E, Falkow S. Tyrosine phosphate hydrolysis of host proteins by an essential Yersinia virulence determinant. Proc Natl Acad Sci USA. 1991;88:1187–1191. doi: 10.1073/pnas.88.4.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blomfield I C, Vaughn V, Rest R F, Eisenstein B I. Allelic exchange in Escherichia coli using the Bacillus subtilis sacB gene and a temperature-sensitive pSC101 replicon. Mol Microbiol. 1991;5:1447–1457. doi: 10.1111/j.1365-2958.1991.tb00791.x. [DOI] [PubMed] [Google Scholar]
- 8.Bölin I, Wolf-Watz H. The plasmid-encoded Yop2b protein of Yersinia pseudotuberculosis is a virulence determinant regulated by calcium and temperature at the level of transcription. Mol Microbiol. 1988;2:237–245. doi: 10.1111/j.1365-2958.1988.tb00025.x. [DOI] [PubMed] [Google Scholar]
- 9.Bonventre P F, Heeg H, Cullen C, Lian C-J. Toxicity of recombinant toxic shock syndrome toxin 1 and mutant toxins produced by Staphylococcus aureus in a rabbit infection model of toxic shock syndrome. Infect Immun. 1993;61:793–799. doi: 10.1128/iai.61.3.793-799.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Butler T. Plague and other Yersinia infection. New York, N.Y: Plenum Press; 1983. [Google Scholar]
- 11.Carnoy C, Moseley S L. Mutational analysis of receptor binding mediated by the Dr family of Escherichia coli adhesins. Mol Microbiol. 1997;23:365–379. doi: 10.1046/j.1365-2958.1997.2231590.x. [DOI] [PubMed] [Google Scholar]
- 12.Carnoy C, Müller-Alouf H, Haentjens S, Simonet M. Polymorphism of ypm, Yersinia pseudotuberculosis superantigen encoding gene. Zentbl Bakteriol Suppl. 1998;29:397–398. [Google Scholar]
- 13.Carnoy C, Simonet M. Yersinia pseudotuberculosis superantigenic toxins. In: Alouf J E, Freer J H, editors. The comprehensive source book of bacterial protein toxins. 2nd ed. London, England: Academic Press; 1999. pp. 611–622. [Google Scholar]
- 14.Cornelis G R, Boland A, Boyd A P, Geuijen C, Iriarte M, Neyt C, Sory M-P, Stainier I. The virulence plasmid of Yersinia, an antihost genome. Microbiol Mol Biol Rev. 1998;62:1315–1352. doi: 10.1128/mmbr.62.4.1315-1352.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Almeida A M P, Guiyoule A, Guilvout I, Iteman I, Baranton G, Carniel E. Chromosomal irp2 gene in Yersinia: distribution, expression, deletion and impact on virulence. Microb Pathog. 1993;14:9–21. doi: 10.1006/mpat.1993.1002. [DOI] [PubMed] [Google Scholar]
- 16.Donnelly G A E, Lu J, Takeda T, McKay D M. Colonic epithelial physiology is altered in response to the bacterial superantigen Yersinia pseudotuberculosis mitogen. J Infect Dis. 1999;180:1590–1596. doi: 10.1086/315075. [DOI] [PubMed] [Google Scholar]
- 17.Donnenberg M S, Kaper J B. Construction of an eae deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector. Infect Immun. 1991;59:4310–4317. doi: 10.1128/iai.59.12.4310-4317.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donnenberg M S, Yu J, Kaper J B. A second chromosomal gene necessary for intimate attachment of enteropathogenic Escherichia coli to epithelial cells. J Bacteriol. 1993;175:4670–4680. doi: 10.1128/jb.175.15.4670-4680.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guiney D G. Regulation of bacterial virulence gene expression by the host environment. J Clin Investig. 1997;4:565–569. doi: 10.1172/JCI119196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983;166:557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 21.Hancock G E, Schaedler R W, MacDonald T T. Yersinia enterocolitica infection in resistant and susceptible strains of mice. Infect Immun. 1986;53:26–31. doi: 10.1128/iai.53.1.26-31.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heesemann J, Gaede K, Autenrieth I B. Experimental Yersinia enterocolitica infection in rodents: a model for human yersiniosis. APMIS. 1993;101:417–429. [PubMed] [Google Scholar]
- 23.Ho S N, Hunt H D, Horton R M, Pullen J K, Pease L R. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989;77:51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- 24.Ito Y, Abe J, Yoshino K-I, Takeda T, Kohsaka T. Sequence analysis of the gene for a novel superantigen produced by Yersinia pseudotuberculosis and expression of the recombinant protein. J Immunol. 1995;154:5896–5906. [PubMed] [Google Scholar]
- 25.Konishi N, Baba K, Abe J, Maruko T, Waki K, Takeda N, Tanaka M. A case of Kawasaki disease with coronary artery aneurysms documenting Yersinia pseudotuberculosis infection. Acta Paediatr. 1997;86:661–664. doi: 10.1111/j.1651-2227.1997.tb08952.x. [DOI] [PubMed] [Google Scholar]
- 26.Ljungberg P, Valtonen M, Harjola V P, Kaukoranta-Tolvanen S S, Vaara M. Report of four cases of Yersinia pseudotuberculosis septicemia and a literature review. Eur J Clin Microbiol Infect Dis. 1995;14:804–810. doi: 10.1007/BF01690998. [DOI] [PubMed] [Google Scholar]
- 27.Marmur J. A procedure for the isolation of desoxyribonucleic acid from microorganisms. J Mol Biol. 1961;3:208–218. [Google Scholar]
- 28.Mekalanos J J. Environmental signals controlling expression of virulence determinants in bacteria. J Bacteriol. 1992;174:1–7. doi: 10.1128/jb.174.1.1-7.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller V L, Mekalanos J J. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J Bacteriol. 1988;170:2575–2583. doi: 10.1128/jb.170.6.2575-2583.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miyoshi-Akiyama T, Imanischi K, Uchiyama T. Purification and partial characterization of a product from Yersinia pseudotuberculosis with the ability to activate human T cells. Infect Immun. 1993;61:3922–3927. doi: 10.1128/iai.61.9.3922-3927.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miyoshi-Akiyama T, Abe A, Kato H, Kawahara K, Narimatsu H, Uchiyama T. DNA sequencing of the gene encoding a bacterial superantigen, Yersinia pseudotuberculosis-derived mitogen (YPM), and characterization of the gene product, cloned YPM. J Immunol. 1995;154:5228–5234. [PubMed] [Google Scholar]
- 32.Miyoshi-Akiyama T, Fujimaki W, Yan X J, Yagi J, Imanishi K, Kato H, Tomonari K, Uchiyama T. Identification of murine T cells reactive with the bacterial superantigen Yersinia pseudotuberculosis-derived mitogen (YPM) and factors involved in YPM-induced toxicity in mice. Microbiol Immunol. 1997;41:345–352. doi: 10.1111/j.1348-0421.1997.tb01211.x. [DOI] [PubMed] [Google Scholar]
- 33.Monack D M, Mecsas J, Bouley D, Falkow S. Yersinia-induced apoptosis in vivo aids in the establishment of a systemic infection of mice. J Exp Med. 1998;188:2127–2137. doi: 10.1084/jem.188.11.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Müller-Alouf H, Geoffroy C, Geslin P, Bouvet A, Felten A, Günther E, Ozegowski J-H, Alouf J E. Streptococcal pyrogenic exotoxin A, streptolysin O, exoenzymes, serotype and biotype profiles of Streptococcus pyogenes isolates from patients with toxic shock syndrome and other severe infections. Zentbl Bakteriol. 1997;286:421–433. [PubMed] [Google Scholar]
- 35.Nakano T, Kawaguchi H, Nakao K, Maruyama T, Kamiya H, Sakurai M. Two outbreaks of Yersinia pseudotuberculosis 5a infection in Japan. Scand J Infect Dis. 1989;21:175–179. doi: 10.3109/00365548909039966. [DOI] [PubMed] [Google Scholar]
- 36.Ramamurthy T, Yoshino K-I, Abe J, Ikeda N, Takeda T. Purification, characterization and cloning of a novel variant of the superantigen Yersinia pseudotuberculosis-derived mitogen. FEBS Lett. 1997;413:174–176. doi: 10.1016/s0014-5793(97)00909-5. [DOI] [PubMed] [Google Scholar]
- 37.Riot B, Berche P, Simonet M. Urease is not involved in the virulence of Yersinia pseudotuberculosis in mice. Infect Immun. 1997;65:1985–1990. doi: 10.1128/iai.65.5.1985-1990.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rott O, Fleischer B. A superantigen as virulence factor in an acute bacterial infection. J Infect Dis. 1994;169:1142–1146. doi: 10.1093/infdis/169.5.1142. [DOI] [PubMed] [Google Scholar]
- 39.Sato K, Ouchi K, Taki M. Yersinia pseudotuberculosis infection in children, resembling Izumi fever and Kawasaki syndrome. Pediatr Infect Dis. 1983;2:123–126. doi: 10.1097/00006454-198303000-00011. [DOI] [PubMed] [Google Scholar]
- 40.Simon R, Priefer U, Pühler A. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram negative bacteria. Bio/Technology. 1983;1:784–791. [Google Scholar]
- 41.Simonet M, Richard S, Berche P. Electron microscopic evidence for in vivo extracellular localization of Yersinia pseudotuberculosis harboring the pYV plasmid. Infect Immun. 1990;58:841–845. doi: 10.1128/iai.58.3.841-845.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Simonet M, Fortineau N, Beretti J L, Berche P. Immunization with live aroA recombinant Salmonella typhimurium producing invasin inhibits intestinal translocation of Yersinia pseudotuberculosis. Infect Immun. 1994;62:863–867. doi: 10.1128/iai.62.3.863-867.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stiles B G, Bavari S, Krakauer T, Ulrich R G. Toxicity of staphylococcal enterotoxins potentiated by lipopolysaccharide: major histocompatibility complex class II molecule dependency and cytokine release. Infect Immun. 1993;61:5333–5338. doi: 10.1128/iai.61.12.5333-5338.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stock J B, Stock A M, Mottonen J M. Signal transduction in bacteria. Nature. 1990;344:395–400. doi: 10.1038/344395a0. [DOI] [PubMed] [Google Scholar]
- 45.Tertti R, Granfors K, Lehtonen O-P, Mertsola J, Mäkelä A-L, Välimäki I, Hänninen P, Toivanen A. An outbreak of Yersinia pseudotuberculosis infection. J Infect Dis. 1984;149:245–250. doi: 10.1093/infdis/149.2.245. [DOI] [PubMed] [Google Scholar]
- 46.Tertti R, Vuento R, Mikkola P, Granfors K, Mäkelä A-L, Toivanen A. Clinical manifestations of Yersinia pseudotuberculosis infection in children. Eur J Clin Microbiol Infect Dis. 1989;8:587–591. doi: 10.1007/BF01968134. [DOI] [PubMed] [Google Scholar]
- 47.Trieu-Cuot P, Courvalin P. Nucleotide sequence of the Streptococcus faecalis plasmid gene encoding the 3′5"-aminoglycoside phosphotransferase type III. Gene. 1983;23:331–341. doi: 10.1016/0378-1119(83)90022-7. [DOI] [PubMed] [Google Scholar]
- 48.Uchiyama T, Miyoshi-Akiyama T, Kato H, Fujimaki W, Imanishi K, Yan X-J. Superantigenic properties of a novel mitogenic substance produced by Yersinia pseudotuberculosis isolated from patients manifesting acute and systemic symptoms. J Immunol. 1993;151:4407–4413. [PubMed] [Google Scholar]
- 49.Ueshiba H, Kato H, Miyoshi-Akiyama T, Tsubokura M, Nagano T, Kaneko S, Uchiyama T. Analysis of the superantigen-producing ability of Yersinia pseudotuberculosis strains of various serotypes isolated from patients with systemic or gastroenteric infections, wildlife animals and natural environments. Zentbl Bakteriol. 1998;288:277–291. doi: 10.1016/s0934-8840(98)80051-0. [DOI] [PubMed] [Google Scholar]
- 50.Vieira J, Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982;19:259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- 51.Yoshino K-I, Abe J, Murata H, Takao T, Kohsaka T, Shimonishi Y, Takeda T. Purification and characterization of a novel superantigen produced by a clinical isolate of Yersinia pseudotuberculosis. FEBS Lett. 1994;356:141–144. doi: 10.1016/0014-5793(94)01249-0. [DOI] [PubMed] [Google Scholar]
- 52.Yoshino K-I, Ramamurthy T, Nair G B, Fukushima H, Ohtomo Y, Takeda N, Kaneko S, Takeda T. Geographical heterogeneity between Far East and Europe in prevalence of ypm gene encoding the novel superantigen among Yersinia pseudotuberculosis strains. J Clin Microbiol. 1995;33:3356–3358. doi: 10.1128/jcm.33.12.3356-3358.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]