Abstract
To counteract thrombosis, new safe and efficient antithrombotics are required. We herein report the design, synthesis, and biological activity of a series of amide-functionalized acylated 1,2,4-triazol-5-amines as selective inhibitors of blood coagulation factor XIIa and thrombin. The introduction of an amide moiety into the main scaffold of 3-aryl aminotriazoles added certain three-dimensional properties to synthesized compounds and allowed them to reach binding sites in FXIIa and thrombin previously unaddressed by non-functionalized 1,2,4-triazol-5-amines. Among synthesized compounds, one quinoxaline-derived aminotriazole bearing N-butylamide moiety inhibited FXIIa with the IC50 value of 28 nM, whereas the N-phenylamide-derived aminotriazole inhibited thrombin with the IC50 value of 41 nM. Performed mass-shift experiments and molecular modeling studies proved the covalent mechanism of FXIIa and thrombin inhibition by synthesized compounds. In plasma coagulation tests, developed aminotriazoles showed anticoagulant properties mainly affecting the intrinsic blood coagulation pathway, activation of which is associated with thrombosis but is negligible for hemostasis.
Keywords: anticoagulants, thrombosis, FXIIa, thrombin, covalent inhibitors, serine protease, blood coagulation
Thrombosis is a life-threatening pathology, which is associated with myocardial infarction, ischemic stroke, and venous thromboembolism.1 According to the recent report of the WHO, over the last 20 years, the ischemic heart disease and stroke remain the two top reasons of mortality worldwide and are now causing more deaths than ever before.2 This indicates that currently available measures of thrombosis prevention are insufficient. Therefore, the development of new safe and efficient antithrombotic drugs is of high clinical relevance.
Despite proven efficacy, currently available anticoagulants display certain drawbacks. For instance, heparins are injectable drugs which are mainly used in hospital settings. In contrast, vitamin K antagonists are orally available but demonstrate slow onset/offset of action and problematic drug–drug and drug–food interactions and also require frequent dose adjustments.8 Direct oral anticoagulants (DOACs) such as thrombin and FXa inhibitors (e.g., dabigatran (1) and rivaroxaban (2), Figure 1) demonstrate more predictable pharmacokinetics and rapid onset/offset of action. However, like all other available anticoagulants, DOACs cause major and potentially life-threatening side effects of internal bleeding.9−11 This side effect is hardly avoidable for conventional anticoagulants as they inhibit blood coagulation factors essential for both hemostasis and thrombosis. Therefore, new drugs exploiting novel mechanisms of action are required to counteract thrombosis without the risk of causing internal bleeding. This can be achieved by targeting alternative enzymes of the coagulation cascade, which are involved in pathological blood coagulation (thrombosis) but play a minor role in hemostasis.
Figure 1.
Examples of non-covalent (1, 2) and covalent (3–5 and 33b) small molecule inhibitors of plasma coagulation factors.3−5 Amide-functionalized aminotriazoles 5r and 33b were developed in this study.
Recently, two serine proteases, namely, FXIa and FXIIa, were validated as such drug targets fulfilling the desired criteria.12 Both enzymes belong to the intrinsic blood coagulation cascade, which in contrast to the extrinsic and common pathways plays a relatively little role in hemostasis but is involved in thrombosis.13 FXI and FXII deficiency protects animals from thrombosis, and inhibition of either of two enzymes reduces thrombus growth.14 FXIIa is particularly interesting as its deficiency does not impair hemostasis in animals and humans and FXII-deficient patients demonstrate a normal hemostatic ability.15,16 It is expected that FXIIa inhibition should be especially efficient in the prevention of so-called contact-mediated thrombosis. This type of thrombosis is caused by blood exposure to medical devices such as artificial heart valves, vascular catheters, dialysis membranes, and prosthetic joints, which could trigger FXII activation.14,17 The efficacy of FXIIa inhibition or FXII depletion was shown to provide thromboprotection in animal models of contact-mediated thrombosis.18−20 Moreover, apart from thrombosis prevention, FXIIa inhibition is promising for the treatment of (neuro)inflammation and hereditary angioedema, a life-threatening swelling disorder.21−23 Several mono- and bicyclic peptide-based compounds were reported to be potent FXIIa inhibitors (Figure 2).6,7 However, only a few small molecule inhibitors of FXIIa are known to date and none of them progressed into clinical trials.22,24,25
Figure 2.

Examples of monocyclic (A) and bicyclic (B) peptide-based FXIIa inhibitors.6,7
Apart from FXIIa and FXIa, it has been recently shown that the inhibition of thrombin (FIIa), a conventional antithrombotic drug target, might cause no bleeding complications when addressed with a specific inhibitor (e.g., aminopyrazole 3, Figure 1). Particularly, the clinical candidate VE-1902 (no exact structure was disclosed) was reported as a direct thrombin inhibitor with a covalent reversible mechanism of action.4,26 VE-1902 was shown to prevent thrombosis in animals causing practically no bleeding complications.26
Pursuing our own campaign on the development of covalent reversible FXIIa and/or thrombin inhibitors, we developed a series of acylated 1,2,4-triazol-5-amines and proved their in vitro anticoagulant properties and the ability to affect thrombin- and cancer cell-induced platelet aggregation.27,28 Despite promising enzyme inhibitory and anticoagulant properties, aminotriazoles (e.g., 4a,b, Figure 1) were less efficient than non-covalent anticoagulants dabigatran and rivaroxaban.27,28 Therefore, further structure optimization of this series of compounds is required to access more potent and selective FXIIa and/or thrombin inhibitors. In this regard, 1,2,4-triazol-5-amine scaffold represents an excellent synthon, synthetic modifications of which allow for the preparation of compounds of different complexities.29−31
Based on the previously proposed covalent binding mode of aminotriazole-based inhibitors in the active sites of FXIIa and thrombin27 (here exemplarily shown for FXIIa, Figure 3), unoccupied areas of the enzymes’ active site can be identified. In the directions of these areas (highlighted in yellow, Figure 3), the inhibitors should be extended to improve their binding affinity toward FXIIa and thrombin. We hypothesized that the aromatic residue in the 3-position of the 1,2,4-triazol-5-amine scaffold (e.g., inhibitor 4a, Figures 1 and 3) might be extended with an amide fragment to reach toward S1′ or S3–S4 substrate binding sites of the targeted enzymes. The introduction of an amide fragment might also improve compounds’ drug likeness by adding certain three-dimensional properties (e.g., decrease their planarity).
Figure 3.
Predicted model of covalent binding conformation of aminotriazole 4a (green stick model)27 in the active site of FXIIa (PDB: 6B74(5)). The residues are depicted as magenta stick models. Substrate-binding sites are labeled (S1–S4 and S1′–S2′). The surface is colored as follows: lipophilic regions are in orange, hydrophilic regions are in blue, and neutral regions are in white. Unoccupied areas of the FXIIa active site, which could be potentially addressed by the extended inhibitor, are highlighted in yellow and encircled with dashed red lines.
Herein, we report the design, synthesis, and biological activity of amide-functionalized acylated 1,2,4-triazol-5-amines, which showed covalent inhibition of FXIIa and/or thrombin and demonstrated anticoagulant properties.
Results and Discussion
Synthesis of Benzamide- and Propanamide-substituted Aminotriazoles 10, 13, and 14
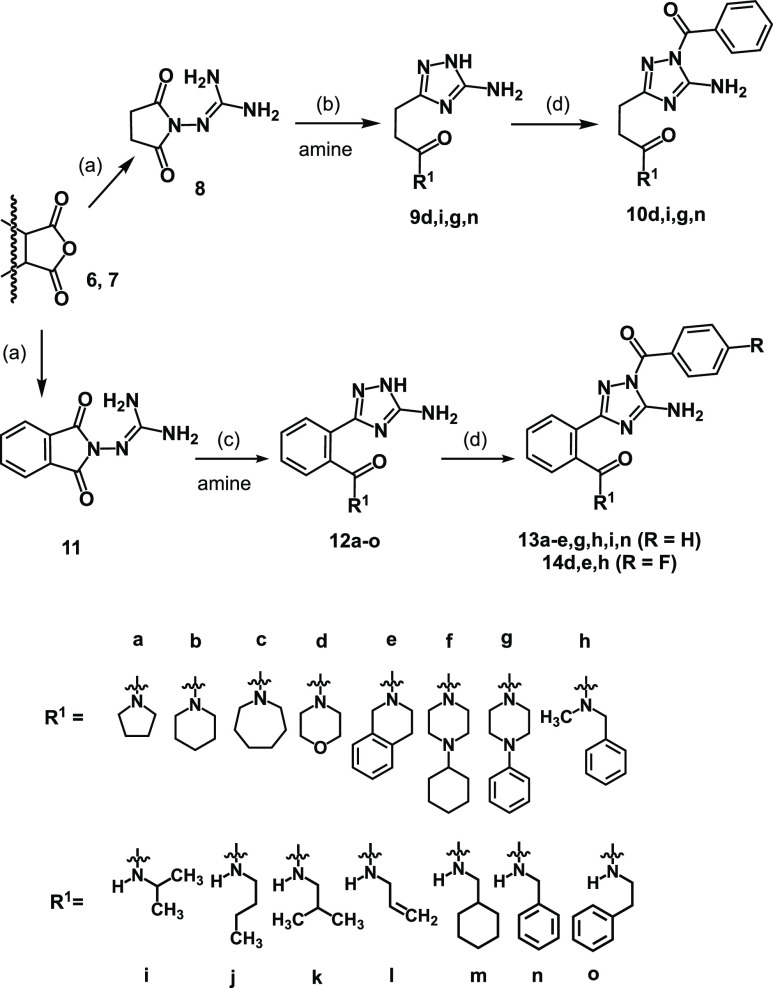
To address S1′ or S2–S4 substrate binding sites of FXIIa and/or thrombin, the aromatic residue in the 3-position of 1,2,4-triazol-5-amine scaffold (inhibitor 4a, Figure 1) should be extended by the introduction of an amide fragment, which could mimic the natural substrate’s peptide bond and possibly the hydrogen bond acceptor properties of the pyridyl nitrogen atom of 4a. This structural modification should allow for accessing aminotriazoles 13 (Scheme 1). However, the introduction of an amide moiety in a desired position of 3-aryl-1,2,4-triazol-5-amines is not a trivial task, especially when a broad series of compounds are to be generated to study structure–activity relationship (SAR). This is because the synthesis of 1,2,4-triazol-5-amine scaffold requires different conditions depending on the starting material structure.28 Therefore, a new general method allowing to access amide-functionalized aminotriazoles 13 required development. Retrosynthetic analysis of 13 showed that these compounds could be synthesized from phthalic anhydride, aminoguanidine, an amine, and an acid chloride (Scheme 1).
Scheme 1. Retrosynthetic Analysis of Aminotriazoles 13 Possessing an Amide Moiety.
Recently, a microwave-assisted synthesis of structurally related aminotriazoles 9 (Scheme 2) was reported.32 Although reported compounds 9 lack the pharmacophores required for the inhibition of FXIIa and thrombin, the synthetic method used for their preparation could be adapted for the synthesis of planned benzamides 13. At first, to have a broader picture of SAR in this series of compounds and probe the method, we synthesized four exemplary propanamides 10d,i,g,n (Scheme 2), the biological activity of which was compared to those of their rigidified aromatized analogues 13. For this purpose, succinic anhydride (6) was treated with aminoguanidine hydrochloride to afford N-guanidinosuccinimide 8, which was then subjected to nucleophilic ring opening with an amine, followed by the cyclocondensation step yielding 1,2,4-triazol-5-amines 9 (Scheme 2). Subsequent acylation of secondary amines 9 afforded benzoylated derivatives 10 (Scheme 2).
Scheme 2. Synthesis of N1-acylated 1,2,4-Triazol-5-amines 10, 13, and 14.
Succinic anhydride (6) and phthalic anhydride (7) were used as starting materials. (a) Aminoguanidine hydrochloride, 190 °C, neat, 2–4 h, 8 42%, 11 77%; (b) CH3CN, μW-irrad., 160 °C, 300 W, 30 min, 9d 43%, 9i 62%, 9g 70%, 9n 40%; (c) DMAP, CH3CN, microwave, 120 °C, 300 W, 30 min, 12a 46%, 12b 50%, 12c 63%, 12d 41%, 12e 33%, 12f 29%, 12g 56%, 12h 38%, 12i 11%, 12j 7%, 12k 8%, 12l 3%, 12m 4%, 12n 3%, 12o 5%; (d) acid chloride, pyridine/THF 2:1, 0 °C to r.t., 1–3 h, 10d 56%, 10i 43%, 10g 55%, 10n 59%, 13a 84%, 13b 60%, 13c 62%, 13d 70%, 13e 86%, 13g 44%, 13h 62%, 13i 76%, 13n 82%, 14d 76%, 14e 88%, 14h 84%.
As no similar method was reported for the synthesis of N-substituted benzamides 13, it had to be developed. For this, at first, N-guanidinophthalimide (11) was prepared in a dehydrative condensation reaction between phthalic anhydride (7) and aminoguanidine (Scheme 2). Then, multiple efforts were undertaken to optimize the next step, during which a nucleophilic attack of an amine opens the imide cycle of 11, followed by the intramolecular cyclocondensation reaction forming the 1,2,4-triazol-5-amine scaffold (Scheme 2). Selected entries of our optimization efforts of the model reaction between N-guanidinophthalimide (11) and piperidine are shown in Table 1 (full table is shown in the Supporting Information).
Table 1. Optimization of Reaction Conditions for the Microwave-assisted Synthesis of 12ba.
| entry | piperidine equiv | T °C | time, min | additive | isolated yield, % |
|---|---|---|---|---|---|
| 1 | 2 | 160 | 30 | 18b | |
| 2 | 2 | 160 | 30 | 40 | |
| 3 | 2 | 160 | 60 | 33 | |
| 4 | 4 | 160 | 30 | 33 | |
| 5 | 1 | 160 | 30 | 39 | |
| 6 | 1.5 | 160 | 30 | DMAP | 50 |
| 7 | 2 | 160 | 30 | DMAP | 40 |
| 8 | 1.2 | 120 | 30 | DMAP | 50 |
| 9 | 1.2 | 120 | 30 | DIPEA | 48 |
| 10 | 2 | reflux | 30 | 0 |
Reaction was performed using a Discover CEM microwave synthesizer, 1 mmol of 11 in 1 mL dry CH3CN. 12b was purified by flash-column chromatography.
Purified by recrystallization from EtOH/EtOAc/cyclohexane.
An initial attempt to synthesize aminotriazole 12b employing the microwave-assisted reaction conditions efficient for the synthesis of nonrigid propanamides 9 (isolated via filtration and recrystallization) resulted in a poor yield of 12b (18%, Table 1, entry 1). The reaction yield was significantly improved up to 40% (entry 2) upon product 12b purification via the flash-column chromatography. A prolonged reaction time (entry 3) as well as the variation of the amount of piperidine added to the reaction mixture (entries 4 and 5) negatively influenced the reaction yield. In contrast, an addition of DMAP or DIPEA not only improved the reaction yield up to 50% but also allowed to reduce the reaction temperature to 120 °C and the amount of piperidine to 1.2 equiv (Table 1, entries 6–9). Notably, a conventional reaction condition (reflux in CH3CN at 90 °C) yielded no desired product 12b (Table 1, entry 10), which might be related to the lower reaction temperature (due to the boiling point of CH3CN) insufficient to promote either the nucleophilic attack of an amine or the subsequent cyclocondensation step. Using the optimized conditions, a series of benzamide-functionalized 1,2,4-triazol-5-amines 12a–o were synthesized (Scheme 2). This approach allowed for the facile access to compounds bearing tertiary amides possessing aliphatic, cycloaliphatic, and aromatic substituents. The secondary amides, however, were obtained with lower yields. Finally, a regioselective acylation of 12 with benzoyl chloride or 4-fluorobenzoyl chloride afforded a series of acylated aminotriazoles 13 and 14 (Scheme 2). The introduced fluorine atom, on the one hand, might reduce the electron density on the carbonyl carbon atom of the acyl fragment, thereby affecting compounds’ biological activity. On the other hand, the fluorine atom introduction might improve compounds’ metabolic stability.
X-ray Crystal Structure of 1,2,4-Triazol-5-amine 14h
1,2,4-Triazol-5-amines are reported to exhibit annular tautomerism,32,33 which might complicate the acylation reaction. Theoretically, four possible acylation products could be formed, with three originating from the annular tautomers (N1, N2, and N3) and one from the acylation of the primary exocyclic amino group. The acylated products bearing an acyl fragment in an undesired position exhibit no FXIIa and thrombin inhibitory activity. Therefore, to unambiguously confirm the regioselective acylation of N1-atom and the successful introduction of an amide moiety, a single crystal of compound 14h was grown and analyzed using X-ray crystallography (Figure 4).
Figure 4.

X-ray crystal structure of FXIIa inhibitor N-acylated 1H-1,2,4-triazol-5-amine 14h displaying the thermal ellipsoids at the 50% probability level.
The crystal structure confirmed the position of the acyl moiety and showed that the amide fragment adds certain three-dimensional properties to the molecule. The amide moiety is out of the plane of the 3-phenyl-1,2,4-triazole residue. Also, the amide-substituted phenyl ring is not coplanar to the 1,2,4-triazole ring exhibiting an offset of 28°. Finally, the carbonyl carbon of the 4-fluorobenzoyl moiety is oriented in a way to form the intramolecular hydrogen bond with the exocyclic amino group of 14h, further shaping the overall structure of the molecule (Figure 4). Therefore, the introduction of an amide moiety indeed added to the three-dimensionality of the resulting molecules.
Serine Protease Inhibition by Acylated 1,2,4-Triazol-5-amines 10, 13, and 14 and Their Anticoagulant Activity
Synthesized series of amide-functionalized aminotriazoles 10, 13, and 14 were screened against two target enzymes FXIIa and thrombin as well as against three other physiologically relevant serine proteases including FXa, FXIa, and trypsin. Tests were performed utilizing fluorogenic substrates as reported previously.27,28 The results of the inhibitory activity are summarized in Table 2. As expected, acylated aminotriazoles 10 lacking the aromatic moiety showed only little ability to inhibit FXIIa and thrombin without affecting the other serine proteases. Their more rigid aromatic analogues 13 and 14 demonstrated slightly higher inhibitory activity against FXIIa and thrombin except for compound 13g compared to 10g (Table 2). Nevertheless, only N-isopropylamide 13i and N-benzylamide 13n showed submicromolar activity inhibiting FXIIa with the IC50 values of 0.7–0.8 μM. This indicates that benzamides 13 and 14 are significantly less active inhibitors than previously reported 3-pyridyl-substituted 1,2,4-triazol-5-amines lacking the amide residue but possessing a heteroatom in their aromatic ring (e.g., compounds 4a,b, Figure 1). This implies that the introduction of an amide group could not replace the heteroatom required for the successful inhibition of FXIIa and/or thrombin. The presence of an electron-poor aromatic system in 3-position of the triazole core seemed to be crucial, which is also in line with our previous observations.27 This phenomenon is most likely related to the covalent mechanism of action of acylated aminotriazoles, which largely depends on the effective charge on the carbonyl carbon atom interacting with the catalytic Ser195 of FXIIa and thrombin. Therefore, to get access to active inhibitors and to evaluate possible benefits of an amide moiety introduction, 1,2,4-triazol-5-amines possessing both a heteroaromatic substituent in 3-position of the triazole core and an amide moiety had to be synthesized. Also, it is notable that tested secondary amides (e.g., 13i and 13n) appeared to be more potent inhibitors compared to their counterparts possessing tertiary amide moiety. Therefore, it is more promising to focus on the libraries of aminotriazoles functionalized with secondary amide residues.
Table 2. Serine Protease Inhibition by Acylated 1,2,4-Triazol-5-amines 10, 13, and 14.
Measurements were performed in triplicate; the substrate concentration [S]0 = 25 μM; measured FXIIa Km = 167 ± 4 μM for Boc-Gln-Gly-Arg-AMC substrate; measured thrombin Km = 18 ± 1 μM for Boc-Val-Pro-Arg-AMC substrate. The Ki values could not be directly obtained from the Cheng–Prusoff equation in this case due to the enzyme–inhibitor covalent interaction.
In addition, selected acylated aminotriazoles of series 10, 13, and 14 as well as several non-acylated aminotriazoles 9 and 12 were screened for their ability to influence blood coagulation in vitro (Figure 5). As expected, being low-active FXIIa and thrombin inhibitors, tested aminotriazoles showed little to no ability to extend the blood coagulation time in activated partial thromboplastin time (aPTT) tests. Only secondary amide 13i, the most potent FXIIa inhibitor of this series of compounds (Table 2), prolonged the aPTT time 1.3-fold (Figure 5).
Figure 5.
In vitro anticoagulant activity (whole blood) of selected 1,2,4-triazol-5-amines tested at 300 μM compared to that of dabigatran (1) tested at 3 μM. The aPTT is shown in sec. The fold of aPTT increase compared to the effect of DMSO is shown under the diagram. Tests were performed at least in triplicate, and the average with standard deviation (SD) is given.
Synthesis of Pyrazine-2-carboxamide-substituted Aminotriazoles 5
As the heteroaromatic substituent in the 3-position of the aminotriazole core was found to be critical for the FXIIa and thrombin inhibition, we aimed to synthesize aminotriazoles 5 exhibiting a pyrazine scaffold substituted with an amide group. This scaffold is heteroaromatic and symmetrical, allowing the desired compounds 5 to be synthesized by the method developed for the synthesis of benzamides 12 (Scheme 2) but starting from 2,3-pyrazinedicarboxylic anhydride (15) instead of phthalic anhydride (Scheme 3).
Scheme 3. Synthesis of Acylated 1,2,4-Triazol-5-amines 18 and 5a–w.

(a) Aminoguanidine hydrochloride, AcOH, reflux, 1 h, 16 93%; (b) 1. Neat, 220 °C, 1 h; 2. NH3 (7 M in CH3OH), reflux, 1 h, 17 69%; (c) benzoic acid, DMAP, EDCI·HCl, DMF (dry), r.t., 4 h, 18 66%, 5a 71%, 5c 68%, 5d 69%, 5f 59%, 5g 44%, 5i 57%, 5j 82%, 5k 86%, 5i 80%, 5m 57%, 5n 86%, 5o 87%, 5p 74%, 5t 57%, 5u 86%, 5v 93%, 5w 54%; (d) KOH, H2O, reflux, 3 h, 19 71%; (e) BzCl, pyridine/THF 2:1, 0 °C to r.t., 7 h; (f) EtOH, r.t., 72 h, 20 61% (over 2 steps); (g) amine (neat or 4 M in MeOH), reflux, 24–96 h, 21a 92%, 21b 65%, 21c 81%, 21d 88%, 21e 72%, 21f 82%, 21g 80%, 21h 68%, 21i 82%, 21j 81%, 21k 63%, 21l 81%, 21m 85%, 21n 81%, 21o 54%, 21p 78%, 21q 53%, 21r 73%, 21s 36%, 21t 63%, 21u 63%, 21v 88%, 21w 76%; (h) BzCl, pyridine/THF 2:1, 0 °C to r.t., 1–3 h, 5b 24%, 5e 62%, 5h 60%, 5q 68%, 5r 58%, 5s 80%.
However, the fusion reaction between 15 and aminoguanidine hydrochloride failed to produce desired N-guanidinimide. Therefore, an alternative approach toward aminotriazoles 5 was pursued. For this, anhydride 15 reacted with aminoguanidine hydrochloride under milder conditions to give the key intermediate 16 (Scheme 3). Then, by controlling reaction conditions (temperature and base used) in the next step, either thermal decarboxylation and cyclocondensation reaction (one pot) or exclusively cyclocondensation reaction occurred to form aminotriazoles 17 and 19, respectively. Aminotriazole 17 was then selectively N1-acylated with benzoyl chloride to afford pyrazine-substituted derivative 18 lacking an amide moiety. The synthesis of this compound was required to compare its inhibitory properties with the activity of aminotriazoles 5a–w possessing an amide fragment (Scheme 3).
A relatively high reactivity of the triazole’s N1-atom prohibited the direct amide coupling reactions between carboxylate 19 and amines (cross-reactivity). Therefore, we considered that amides 21 can be obtained via the aminolysis of ester 20 (Scheme 3). To produce ester 20, carboxylic acid 19 was first esterified via a rather unusual method as standard esterification procedures failed. For this, 19 was treated with benzoyl chloride to form double acylated intermediate 19a, subsequent treatment of which with ethanol resulted in N1-atom deprotection as well as in the ester moiety formation. Ester 20 was then subjected to aminolysis reactions affording amides 21a–w in moderate to excellent yields, followed by their selective N1-acylation yielding amide-functionalized aminotriazoles 5a–w as final products (Scheme 3).
Serine Protease Inhibition by Acylated 1,2,4-Triazol-5-amines 5a–w and 18
Synthesized aminotriazoles 5a–w possessing a pyrazinyl moiety functionalized with an amide residue were tested against a series of serine proteases FXIIa, thrombin, FXa, trypsin, and FXIa (Table 3). Generally, the introduction of two heteroatoms into the aromatic ring (pyrazine) restored the compounds’ inhibitory activity toward FXIIa and thrombin, and pyrazineamides 5a–w in most of the cases appeared to be more potent inhibitors compared to benzamides 13 and 14 (Tables 2 and 3). The amide moiety structure significantly influenced the biological activity of the synthesized compounds affecting both the inhibitory potency and the selectivity profile.
Table 3. Serine Protease Inhibition by Acylated 1,2,4-Triazol-5-amines 5a–w.

Measurements were performed in triplicate; the substrate concentration [S]0 = 25 μM; measured FXIIa Km = 167 ± 4 μM for Boc-Gln-Gly-Arg-AMC substrate; measured thrombin Km = 18 ± 1 μM for Boc-Val-Pro-Arg-AMC substrate. The Ki values could not be directly obtained from the Cheng–Prusoff equation in this case due to the enzyme–inhibitor covalent interaction.
With respect to the FXIIa inhibition, the introduction of an unsubstituted amide moiety (5a) or an amide fragment with a short or bulky noncyclic alkane substituent (5b–d, g–i) led to compounds with a reduced ability to inhibit FXIIa (IC50FXIIa = 0.6–1.6 μM) when compared to the unsubstituted pyrazine derivative 18 (IC50FXIIa = 441 nM, Table 3). Among amides N-substituted with bulky cycloaliphatic substituents such as cyclohexyl, morpholinyl, and so forth (5l–s), only those possessing relatively small residues were somewhat more active than 18 (e.g., methylcyclopropyl-substituted amide 5l with an IC50FXIIa = 135 nM). Introduction of an aromatic amide moiety had ambiguous effects: N-phenylamide (5t) and N-phenylethylamide (5v) residues decreased the inhibitory activity toward FXIIa (2- to 3-fold), whereas N-benzylamide (5u) and N-thiophenylmethylamide (5w) fragments improved the FXIIa inhibition (e.g., 5w IC50FXIIa = 125 nM). Notably, possessing long and flexible aliphatic N-butylamide and N-pentylamide fragments, compounds 5e and 5f inhibited FXIIa with the IC50 values of 78 and 74 nM, respectively, being the most active FXIIa inhibitors in this series of compounds (Table 3). Moreover, aminotriazoles 5e and 5f did not affect the amidolytic activity of FXa, FXIa, and trypsin (IC50 > 5 μM) and also showed somewhat improved selectivity (ca. 3- to 4-fold) toward FXIIa over thrombin (Table 3).
With respect to thrombin inhibition, the introduction of an unsubstituted amide moiety into the structure of aminotriazole 18 had only a little effect on thrombin inhibition in terms of potency (5a IC50FIIa = 101 nM vs 18 IC50FIIa = 121 nM) but was beneficial in terms of selectivity. Thus, amide 5a showed a 10-fold lower IC50 value toward thrombin compared to FXIIa, whereas pyrazine derivative 18 was only 3.6-fold selective toward thrombin (Table 3). The introduction of an N-phenylethylamide moiety (5v) had a similar effect; it preserved the compound’s inhibitory activity toward thrombin while improving its selectivity. Most of other tested amides 5 possessing aliphatic, cycloaliphatic, and (hetero)aromatic residues at N-amide atom showed reduced ability to inhibit thrombin (IC50 varied 0.16–1.5 μM). Nevertheless, of this series of compounds, two amides were found to be active and selective inhibitors of thrombin. Thus, morpholine-substituted aminotriazole 5r and N-phenylamide 5t inhibited thrombin with IC50 values of 53 and 41 nM, respectively, being at the same time selective over other tested serine proteases including FXIIa (IC50 = 3.3 and 1.5 μM, respectively, Table 3).
Post-Screening Optimization
To further improve compounds’ inhibitory potency and selectivity profile, additional post-screening optimizations were attempted. For this, aminotriazole 21e bearing an N-butylamide moiety (the most promising moiety for the FXIIa inhibition, Table 3) was coupled with (S)-tetrahydronaphthalene-1-carboxylic-, 1-naphthoic-, and 2-iodophenylacetic acid giving acylated aminotriazoles 22–24, respectively (Scheme 4). These three acyl fragments have been previously reported to improve compounds’ selectivity and/or the inhibitory activity toward FXIIa.27,28 Additionally, a 6-aminonicotinoyl fragment was introduced into the structure of 21e as an arginine mimetic moiety (compound 34, Scheme 4). Similarly, aminotriazole 21r possessing a morpholine-amide fragment (the most beneficial for thrombin inhibition, Table 3) was acylated with pivaloyl chloride as pivaloyl residue is known to be beneficial for thrombin inhibition (compound 25, Scheme 4).26
Scheme 4. Synthesis of Acylated 1,2,4-Triazol-5-amines 22–25.
(a) (S)-1,2,3,4-tetrahydronaphthalene-1-carboxylic acid, DMAP, EDCI·HCl, DMF (dry), r.t., 4 h, 22 54%; (b) 1-naphthoic acid, DMAP, EDCI·HCl, DMF (dry), r.t., 4 h, 23 88%; (c) 2-iodophenylacetic acid, DMAP, EDCI·HCl, DMF (dry), r.t., 4 h, 24 74%; (d) pivaloyl chloride, pyridine/THF 2:1, 0 °C to r.t., 2 h, 25 69%; (e) 6-aminonicotinic acid, DMAP, EDCI·HCl, DMF (dry), r.t., 7 h, 34 47%.
Additionally, we performed the synthesis of quinoxaline-substituted aminotriazoles 33a–d possessing the N-butylamide moiety (Scheme 5). The synthesis of amides 33a–d was of particular interest as our previous studies showed that quinoxaline-substituted aminotriazoles are potent and selective FXIIa inhibitors.28 For the synthesis, tartaric acid (26) was esterified and oxidized to afford dioxosuccinate 26′, which was subjected to the cyclocondensation reaction with o-phenylenediamine yielding quinoxaline-2,3-dicarboxylate 27 (Scheme 5).34 Sequential hydrolysis and dehydration of 27 afforded anhydride 28,35 which was then reacted with aminoguanidine hydrochloride to yield the open-chain intermediate 29. Then, the aminoguanidine side chain of 29 was cyclized producing aminotriazole 30 bearing a free carboxylic acid moiety. Carboxylate 30 was esterified to give ester 31, which was then subjected to the aminolysis with n-butylamine yielding amide 32. Finally, N1-atom of aminotriazole 32 was acylated to afford final compounds 33a–d (Scheme 5).
Scheme 5. Synthesis of Acylated 1,2,4-Triazol-5-amines 33a–d.

(a) 1. EtOH, H2SO4, reflux, overnight; 2. NBS, CCl4, 76 °C, overnight; (b) AcOH, 125 °C, overnight, 27 35%; (c) 1. aq NaOH, reflux, 16 h, 91%; 2. Ac2O, neat, reflux, 3 h, 28 87%; (d) aminoguanidine hydrochloride, AcOH, reflux, 1 h, 29 85%; (e) KOH, H2O, reflux, 3 h, 30 84%; (f) 1. BzCl, pyridine/THF 2:1, 0 °C to r.t., 7 h; 2. EtOH, r.t., 72 h, 31 59%; (g) BuNH2, neat, reflux, 24 h, 32 80%; (h) acid chloride, pyridine/THF 1:7, 0 °C to r.t., 3 h, 33a 60%, 33b 99%; (i) (S)-1,2,3,4-tetrahydronaphthalene-1-carboxylic acid for 33c or 6-aminonicotinic acid for 33d, DMAP, EDCI·HCl, DMF (dry), r.t., 5–7 h, 33c 60%, 33d 99%.
Serine Protease Inhibition by Aminotriazoles 22–25, 33a–d, and 34
Acylated aminotriazoles 22–25, 33a–d, and 34 synthesized in the post-screening optimization step were tested for their ability to inhibit an extended list of eight serine proteases (Table 4). Their inhibitory properties were compared to those of compounds 5e and 5r. It has been found that the introduction of 1-naphthoyl moiety or 2-iodophenylacetic acid fragment (compounds 23 and 24, respectively) is beneficial in terms of FXIIa inhibitory activity. Aminotriazoles 23 and 24 inhibited FXIIa with IC50 = 43 and 57 nM, respectively, and demonstrated improved selectivity toward FXIIa over thrombin, when compared to their benzoylated counterpart 5e. The transition to quinoxaline-substituted aminotriazoles 33a–b further improved compounds’ selectivity preserving or even increasing their inhibitory potency toward FXIIa (Table 4). With an IC50 value of 28 nM, 1-naphthoylated aminotriazole 33b was the most potent and selective FXIIa inhibitor among quinoxaline derivatives. Compound 33b showed no inhibition of six tested serine proteases except for targeted FXIIa and chymotrypsin (Table 4). At that, 33b was about 20-fold selective toward FXIIa over chymotrypsin. Other structure modifications offered no benefits in terms of FXIIa and thrombin inhibition. Last, aminotriazole 5r found in the initial screening appeared to be a selective thrombin inhibitor (IC50 = 53 nM), affecting FXIIa and chymotrypsin in a micromolar range only.
Table 4. Serine Protease Inhibition by Acylated Aminotriazoles 22–25, 33a–d, and 34 Synthesized in the Post-screening Optimization.
| serine
protease IC50 ± SD (nM)a |
||||||||
|---|---|---|---|---|---|---|---|---|
| cmpd. | FXIIa | FIIa | FXa | FXIa | plasmin | PK | trypsin | chymotr. |
| 22 | 118 ± 4 | 303 ± 9 | >5000 | >5000 | >5000 | >5000 | >5000 | 259 ± 5 |
| 23 | 43 ± 5 | 655 ± 22 | >5000 | >5000 | >5000 | >5000 | >5000 | 375 ± 16 |
| 24 | 57 ± 1 | 1155 ± 70 | >5000 | >5000 | >5000 | >5000 | >5000 | 145 ± 9 |
| 25 | 160 ± 81 | >5000 | >5000 | >5000 | >5000 | >5000 | >5000 | >5000 |
| 33a | 42 ± 1 | >132,000 | >5000 | >5000 | >5000 | >5000 | >5000 | 857 ± 236 |
| 33b | 28 ± 4 | >132,000 | >5000 | >5000 | >5000 | >5000 | >5000 | 525 ± 30 |
| 33c | 55 ± 11 | >132,000 | >5000 | >5000 | >5000 | >5000 | >5000 | 153 ± 3 |
| 33d | 486 ± 88 | >5000 | >5000 | >5000 | 3866 ± 331 | |||
| 34 | 901 ± 94 | 440 ± 12 | >5000 | >5000 | 2363 ± 79 | |||
| 5e | 78 ± 16 | 364 ± 51 | >5000 | >5000 | >5000 | >5000 | >5000 | 896 ± 218 |
| 5r | 3260 ± 519 | 53 ± 12 | >5000 | >5000 | >5000 | >5000 | >5000 | 1148 ± 60 |
| dabig. (1) | >33,00027 | 6.4 ± 0.4 | >1000 | 2041 ± 36 | >5000 | >5000 | 59%@5 μM | >5000 |
| rivar. (2) | >33,00027 | 55%@33 μM | 0.7 ± 0.1 | >5000 | >5000 | >5000 | >5000 | >5000 |
Measurements were performed in triplicate; the substrate concentration [S]0 = 25 μM; measured FXIIa Km = 167 ± 4 μM for Boc-Gln-Gly-Arg-AMC substrate; measured thrombin Km = 18 ± 1 μM for Boc-Val-Pro-Arg-AMC substrate. The Ki values could not be directly obtained from the Cheng–Prusoff equation in this case due to the enzyme–inhibitor covalent interaction. PK—plasma kallikrein; chymotr.—chymotrypsin.
Anticoagulant Activity of Selected Amide-functionalized Acylated Aminotriazoles
Selected amide-functionalized acylated aminotriazoles, which showed FXIIa or thrombin inhibitory activity (Tables 3 and 4), were tested in two plasma coagulation tests, namely, aPTT and prothrombin time (PT). These two tests allow distinguishing whether the intrinsic or the extrinsic coagulation pathway is affected by a test compound. PT prolongation indicates that the inhibitor affects factors of the extrinsic and/or the common pathway, for example, FXa or thrombin. Simultaneous aPTT and PT prolongation also suggests the inhibition of coagulation factors of the extrinsic and/or the common pathway. However, when aPTT is extended and PT remains unaffected, this implies that the inhibitor addresses the intrinsic pathway’s coagulation factors, for example, FXIIa or FXIa.
As can be seen from Figure 6, 9 of 10 tested compounds significantly extended the plasma coagulation time in aPTT test when screened at 227 μM. Among them, N-butylamides 23 and 24 possessing 1-naphthoyl and 2-iodophenylacetic acid fragments, respectively, were the most active anticoagulants extending the plasma coagulation time (aPTT), 2.3- and 2.4-fold, respectively. Benzoylated N-butylamide 5e, N-pentylamide 5f, and morpholine-4-carboxamide 5r were slightly less active, prolonging aPTT 1.9- to 2.1-fold. Being mainly inhibitors of FXIIa, compounds 5e, 5f, 23, and 24 showed only a little influence on the PT (1.1- to 1.2-fold elongation). In contrast, N-phenylamide 5t and morpholine-4-carboxamide 5r extended not only aPTT but also PT (1.3-fold, both compounds) (Figure 6). This is associated with the ability of 5t and 5r to inhibit thrombin (IC50 = 41 and 53 nM, respectively), which is a part of the common coagulation pathway. Highly selective FXIIa inhibitors 33a–c exclusively prolonged aPTT (1.3- to 1.6-fold) having no influence on PT (Figure 6). It has been noted that being exposed to synthesized FXIIa inhibitors, normal plasma demonstrated a coagulability profile somewhat similar to FXII-deficient plasma, which is characterized by normal PT and extended aPTT (Figure 6). This interesting property of the synthesized acylated aminotriazoles suggests that they predominantly inhibit the intrinsic coagulation pathway with little to no influence on the extrinsic pathway. This contrasts with the conventional non-covalent anticoagulants such as dabigatran (1) and rivaroxaban (2), which significantly extended both PT (2.8- and 4.6-fold) and aPTT (3.3- and 2.5-fold). Therefore, the anticoagulation profile of synthesized aminotriazoles might be beneficial in terms of safety as anticoagulants capable of selective inhibition of coagulation factors involved in thrombosis (intrinsic pathway) without disruption of hemostasis (extrinsic pathway) should cause no bleeding complications.
Figure 6.
In vitro anticoagulant activity (plasma) of selected 1,2,4-triazol-5-amines tested at 227 μM compared to that of dabigatran (1) and rivaroxaban (2) tested at 2 μM. The aPTT and PT are shown in sec. The fold of aPTT and PT increase compared to the effect of DMSO is shown under the diagram. The aPTT for FXII-deficient plasma >300 s is also given for the comparison. Tests were performed at least in triplicate, and the average with SD is given.
Mechanism of FXIIa and Thrombin Inhibition
Mechanistically, the inhibition of FXIIa and thrombin by the acylated aminotriazoles may proceed via the steps arising from the catalytic mechanism of trypsin-like proteases36 and the inhibitors’ substrate-mimicking properties (Scheme 6). At first, an acylated aminotriazole (e.g., 23) may enter the active site of a serine protease to form the non-covalent Michaelis complex (A) stabilized by hydrogen bonds and hydrophobic interactions with the enzyme’s residues. This non-covalent binding positions the inhibitor’s carbonyl carbon in the proximity of the nucleophilic Ser195. Being deprotonated by His57, which is activated by Asp102, nucleophilic Oγ-atom of Ser195 attacks the inhibitor’s carbonyl carbon to form tetrahedral intermediate B (Scheme 6).
Scheme 6. Proposed Mechanism of Serine Protease Inhibition by the Synthesized Acylated Aminotriazoles.
An inhibitor, for example, 23, forms a non-covalent Michaelis complex A, which undergoes a nucleophilic attack by the enzyme’s catalytic Ser195 to form the tetrahedral intermediate B stabilized by the amino acids of the “oxyanion hole”. Complex B then collapses to produce acyl-enzyme complex C, an inhibited form of the enzyme. The enzyme recovers its catalytic ability upon complex C hydrolytic degradation.
The existence of otherwise unstable intermediate B (transition state) is possible due to its stabilization via hydrogen bonds formed between the backbone amides of the “oxyanion hole” (Ser195 and Gly193) and the inhibitor’s charged carbonyl oxygen (oxyanion). It was previously suggested that the tetrahedral structure of complex B allows for the hydrogen bond formation with Ser195 and Gly193, which is not possible for the Michaelis complex A bearing the trigonal carbonyl carbon.37,38 Next, intermediate B collapses and upon protonation by His57 releases C-terminus-like product 21e (deacylated aminotriazole) and a relatively stable acyl-enzyme complex C. Finally, His57 deprotonates a molecule of water, which hydrolyzes the acyl-enzyme complex C releasing an N-terminus-like product (1-naphthoic acid) and recovering the initial state of the enzyme. The enzyme remains inactivated for a lifetime of the acyl-enzyme complex C.
Owning to a very short half-life of the tetrahedral intermediate (B), its detection is challenging. Instead, a more stable complex C (Scheme 6), existing for minutes or even hours, could be detected and studied. To confirm that synthesized compounds trigger the acyl-enzyme complex C formation, we incubated native human FXIIa with two synthesized inhibitors 22 and 23. The enzyme–inhibitor mixtures were analyzed using size-exclusion chromatography (SEC) coupled with electrospray ionization mass spectrometry (ESI-MS) (Figure 7). The mass-shift experiments revealed that the initial mass of native FXIIa (29,498.2 Da, spectrum A) was shifted to 29,652.0 Da (spectrum B) and to 29,656.0 Da (spectrum C) upon the enzyme exposure to inhibitors 23 and 22, respectively. The mass shifts of Δm = 153.9 Da and Δm = 157.8 Da correspond to the adducts of the acyl moieties of 23 and 22 to FXIIa. These data confirm that synthesized acylated aminotriazoles covalently inhibit FXIIa forming a stable acyl-enzyme complex C like shown in Scheme 6.
Figure 7.
Deconvoluted SEC/ESI-TOF mass spectra of intact FXIIa (A) and the covalent complexes FXIIa-23′ (B) and FXIIa-22′ (C) formed after the FXIIa incubation (15 min) with inhibitors 23 and 22, respectively. Deconvoluted LC/ESI-qTOF mass spectra of intact thrombin (D) and the covalent complexes thrombin-5t′ (E) and thrombin-5r′ (F) formed after the thrombin incubation (15 min) with inhibitors 5t and 5r, respectively. The peaks of interest are labeled with the corresponding deconvoluted masses. Mass shifts of 153.9 Da (B) and 157.8 Da (C) were observed, which correspond to the inhibitors’ acyl moiety adduct to FXIIa, whereas mass shifts of 104.2 Da (E,F) correspond to the inhibitors’ acyl moiety adduct to thrombin. The schematic representation of FXIIa (red, A), thrombin (orange, D), and covalent complexes of FXIIa with inhibitors 22 (blue, C) and 23 (violet, B) as well as covalent complexes of thrombin with inhibitors 5t (green, E) and 5r (yellow, F) is also shown.
Similarly, to confirm that synthesized acylated aminotriazoles inhibit thrombin via acyl-enzyme complex C formation (Scheme 6), two benzoylated thrombin inhibitors 5t and 5r were studied in the mass-shift experiments (Figure 7D–F). The experiments showed that the initial mass of native thrombin (36,025.9 Da, spectrum D, Figure 7) was shifted to 36,130.1 Da (spectra E and F) upon the enzyme exposure to inhibitors 5t and 5r, respectively. The mass shift of Δm = 104.2 Da corresponds to the adducts of the acyl moiety of 5t and 5r to thrombin, confirming the covalent mechanism of thrombin inhibition.
It is also important to know whether the observed acylation of FXIIa and thrombin is reversible as the formation of completely irreversible complexes might be associated with adverse reactions; for example, it may elicit an immune response.39 Previously, in time-dependent mass-shift experiments, we demonstrated that the acyl-FXIIa complex bearing the β-naphthoyl moiety undergoes a hydrolytic degradation (deacylation) recovering the majority of native FXIIa 2 h after the removal of the inhibitor’s excess.27 The present study, however, revealed that the acyl-FXIIa complex stability depends on the acyl moiety structure. Thus, acyl-FXIIa complexes bearing benzoylated and α-naphthoylated Ser195, formed upon FXIIa exposed to inhibitors 5f and 23 (Tables 3 and 4), respectively, remained stable for more than 19 h after the removal of the inhibitors’ excess (Figures S1–S3 in the Supporting Information). On the other hand, acyl-thrombin complex exhibiting Ser195-O-benzoyl moiety transferred from inhibitor 5r (the same complex is shown in Figure 7E) was more susceptible to hydrolytic degradation. Thus, about 80% of native thrombin recovered after 9 h of incubation (the acyl-enzyme complex half-life was t1/2 ∼ 5 h, Figure 8). These data indicate that both the acyl moiety structure and the serine protease hydrolytic ability affect the stability of acyl-enzyme complex C (Scheme 6).
Figure 8.

Spontaneous recovery of thrombin (acyl-thrombin complex hydrolysis). Stacked deconvoluted LC/ESI-qTOF mass spectra of acyl-thrombin complex (thrombin-Ser195-O-benzoyl) recorded between 0 and 540 min of incubation are shown. To visualize the ratio between the acylated and the recovered thrombin, peak intensity was normalized to the peak of the acyl-thrombin complex. Between 0 and 60 min, only a peak of acyl-thrombin complex with a mass of 36,130.1470 Da was observed. After 300 min of incubation, a peak of recovered thrombin with a mass of 36,026.1619 Da was observed, and the amount of the recovered thrombin was increasing in the subsequent measurements (Δm = 104.0 Da indicates the benzoyl moiety cleavage). The acyl-thrombin complex half-life was t1/2 ∼ 5 h.
Binding Mode Study by Molecular Modeling
As short-living tetrahedral intermediate B is not detectable, we used computation modeling to predict its formation in the active site of FXIIa and thrombin. For this, FXIIa inhibitor 23 and thrombin inhibitor 5r were docked into the active site of FXIIa and thrombin, respectively, utilizing a covalent docking protocol (Figure 9A–F). The docking studies confirmed that both inhibitors 23 and 5r are capable of covalent bonding to the oxygen atom of catalytic Ser195, forming the corresponding tetrahedral intermediates in the active sites of FXIIa and thrombin. Apart from covalent interactions, formed tetrahedral intermediates are presumably stabilized by hydrogen bonds with the carbonyl oxygen atom of Cys191 and/or the backbone amides of the “oxyanion hole” (Gly193 and Ser195). In both FXIIa and thrombin, the aromatic part of the inhibitors’ acyl moiety projects toward the S1 substrate binding site. Here, at the entrance to S1 selectivity pocket of FXIIa (lipophilic area), the naphthoyl moiety of 23 forms lipophilic interactions with Ile213, Cys191, Gln192, and Trp215 (Figure 9A). Similarly, the benzoyl residue of 5r is involved in lipophilic interactions with Val213, Cys191, Ala190, and Trp215 at the entrance to the S1 pocket of thrombin (Figure 9B,C). FXIIa is characterized by more spacious S1′ and S2–S3 sites than thrombin.40 This allows inhibitor 23 to project its N-butylamide moiety in the direction of the S1′ binding site forming hydrogen bonds with Tyr99 (Figure 9A). Also, the triazole core and the pyrazine moiety of 23 might be involved in pi–pi interactions with His57 and Tyr99, respectively. Being restricted by narrower S1′ and S2 pockets of thrombin (especially by Tyr60A, Trp60D, and Lys60F), inhibitor 5r may adopt one of two conformations (Figure 9B,C). In both conformations, the pyrazine moiety of 5r forms pi–pi interactions with Trp60D (parallel displaced stacking) and Tyr60A (T-shaped stacking). The conformation of 5r shown in Figure 9B is additionally stabilized by hydrogen bonds between the compound’s primary amino group and Glu192 as well as between the compound’s carbonyl carbon atom and Gly216. The conformation of 5r in Figure 9C is flipped in the active site and stabilized by hydrogen bonds between the compound’s primary amino group and Gly216 as well as between His57 and the compound’s nitrogen atom of the triazole core. In this conformation, the morpholine-amide fragment of 5r projects toward the S3 binding site to form lipophilic interactions with Trp215 (Figure 9C).
Figure 9.
Calculated covalent binding conformation of inhibitor 23 (magenta stick model) in the active site of FXIIa (A—close-up view and D—overall structure with a molecular surface). Two predicted covalent binding conformations of inhibitor 5r (cyan stick models) in the active site of thrombin (FIIa) (B,C—close-up view and E,F—overall structure with a molecular surface). Amino acid residues are depicted as white stick models and are numbered according to the amino acid sequence of chymotrypsinogen residue numbering. Oxygen, nitrogen, and sulfur atoms are colored in red, blue, and yellow, respectively. Substrate-binding sites are labeled (S1–S3 and S1′). Hydrogen bonds are red lines. PDB ID used: 6B77/FXIIa5 and 6CYM/FIIa.4
Plasma Stability and Cytotoxicity
To the best of our knowledge, no literature data describing the plasma stability of acylated aminotriazoles targeting FXIIa or thrombin are available. Evaluation of stability in plasma, however, would be an important step toward further study of these compounds in animal models of thrombosis. To study the plasma stability of synthesized acylated aminotriazoles, selected compounds (5r, 23, and 33b) were incubated in non-activated citrated human plasma. Under the applied conditions, enzymes of the blood coagulation cascade remained as zymogens with inaccessible active sites, which prevented inhibitors from binding to them. Instead, compounds were exposed to the hydrolytic enzymes of plasma not associated with blood coagulation. To quantify the plasma stability, the residual anticoagulant activity of compounds was measured over different time intervals in the aPTT assay (Figure 10). We found that the anticoagulant properties of the compounds decreased significantly during the first 30 min of incubation. The plasma stability seemed to be structure-related. N-butylamide 23 possessing the 1-naphthoyl moiety appeared to be somewhat more stable in plasma compared to two other tested compounds. Nevertheless, the anticoagulant properties of all three compounds either completely disappeared or dropped to a low level after 60 min of preincubation in plasma (Figure 10).
Figure 10.
In vitro plasma stability of selected 1,2,4-triazol-5-amines. Compounds 5r, 23, and 33b were incubated at 37 °C in non-activated citrated human plasma. The residual anticoagulant activity (Y-axis, s) of compounds was measured over different time intervals (X-axis, min) as a measure of plasma stability as described in the Experimental Section for the aPTT assay. Tests were performed in triplicate, and the average with SD is given.
Some clinically used covalent enzyme inhibitors such as acetylsalicylic acid, rivastigmine, and some penicillins also acting as acylating agents are characterized by a very short plasma half-life. Synthesized aminotriazoles, however, are planned to be used as covalent inhibitors of FXIIa or thrombin, which under the physiological conditions exist as zymogens with inaccessible active sites. Thus, the inhibitor should stay intact for a reasonable period of time to be available when undesired activation of the target enzymes occurs to prevent thrombus formation. Therefore, further development of this series of compounds should be aimed at optimizing their plasma stability before they can be recommended for animal studies.
To further evaluate the safety profile of the synthesized acylated 1,2,4-triazol-5-amines, selected compounds 33b, 5r, 23, and 34 were assessed for their potential cytotoxicity against liver HepG2 cells and lung A549 cells (Figure 11). Performed tests demonstrated a relatively low cytotoxicity profile of the selected aminotriazoles. Even at the highest tested dose of 300 μM, none of the tested compounds reduced cell viability below 50%, which did not allow us to calculate the IC50 values.
Figure 11.

Cytotoxicity profile of synthesized FXIIa and thrombin inhibitors 33b, 5r, 23, and 34 tested in HepG2 and A549 cell lines. Camptothecin (2 μM) was used as a positive control and DMSO (1%) as a negative control. Tests were performed in triplicate, and the average with SD is given; *p < 0.05; **p < 0.01.
Conclusions
In this study, we disclosed the design, synthesis, and biological activity evaluation of a series of amide-functionalized acylated 1,2,4-triazol-5-amines, which selectively inhibit human FXIIa and thrombin. The introduction of an amide moiety into the main scaffold of 3-aryl aminotriazoles added certain three-dimensional properties to synthesized compounds (Figure 4) and allowed them to reach binding sites in FXIIa and thrombin previously unaddressed by non-functionalized 1,2,4-triazol-5-amines (Figure 9).
Performed SAR studies uncovered structural features required for successful FXIIa and thrombin inhibition (Figure 12). Thus, it has been shown that amide-functionalized aminotriazoles 10 lacking the aromatic moiety in the 3-position of their triazole core are only weak inhibitors of FXIIa and thrombin (Table 2). Also, their aromatic analogues 13 and 14 bearing 3-phenyl moiety demonstrated low inhibitory activity against targeted enzymes (Table 2). In contrast, aminotriazoles 5a–w possessing the 3-pyrazinyl moiety functionalized with an amide residue were potent FXIIa or thrombin inhibitors (Table 3). Among them, compounds 5e and 5f possessing N-butylamide and N-pentylamide fragments inhibited FXIIa with the IC50 values of 78 and 74 nM, respectively, whereas morpholinyl-substituted aminotriazole 5r and N-phenylamide 5t inhibited thrombin with the IC50 values of 53 and 41 nM, respectively. Subsequently performed post-screening optimization allowed accessing even more potent and selective FXIIa inhibitors. Thus, for instance, quinoxaline-derived aminotriazole 33b bearing N-butylamide moiety inhibited FXIIa with the IC50 value of 28 nM (Table 4). Performed mass-shift experiments and molecular modeling studies allowed us to confirm that synthesized compounds are covalent inhibitors of FXIIa and thrombin (Figures 7–9). These acylated aminotriazoles exhibit their inhibitory activity by targeting catalytic Ser195 in the active site of serine proteases (Scheme 6). The nucleophilic attack leads to the stable acyl-enzyme complex C formation (Scheme 6 and Figure 7). The enzyme remains inactivated for a lifetime of the acyl-enzyme complex. We also demonstrated that covalent inhibition of thrombin by synthesized compounds is reversible. Thus, thrombin benzoylated with one of the synthesized inhibitors underwent spontaneous recovery by about 80% after 9 h of incubation (acyl-enzyme complex half-life t1/2 ∼ 5 h, Figure 8). In aPTT and PT tests, synthesized compounds showed anticoagulant properties predominantly disrupting the intrinsic blood coagulation pathway, activation of which is associated with thrombosis but is negligible for hemostasis. Synthesized compounds also showed relatively low cytotoxicity against two cell lines. Overall, herein disclosed amide-functionalized acylated 1,2,4-triazol-5-amines may serve as a starting point for the development of new and potentially safe anticoagulants. Nevertheless, before recommending this series of compounds for animal studies, it is necessary to optimize their stability in plasma.
Figure 12.
Summary of amide-functionalized acylated 1,2,4-triazol-5-amine SAR.
Experimental Section
Chemistry, General
Unless otherwise mentioned, THF was dried with sodium/benzophenone and was freshly distilled before use. Thin-layer chromatography (TLC): silica gel 60 F254 plates (Merck). Flash chromatography: silica gel 60, 40–63 μm (Macherey-Nagel). Reversed-phase TLC: silica gel 60 RP-18 F254S plates (Merck). Automatic flash column chromatography: Isolera One (Biotage); brackets include eluent, cartridge-type. Melting point (m.p): melting point apparatus SMP 3 (Stuart Scientific), uncorrected. 1H NMR (400 MHz), 1H NMR (600 MHz), and 13C NMR (151 MHz): Agilent DD2 400 and 600 MHz spectrometers; chemical shifts (δ) are reported in ppm against the reference substance tetramethylsilane and calculated using the solvent residual peak of the undeuterated solvent. IR: IR Prestige-21 (Shimadzu). HRMS: MicrOTOF-QII (Bruker). HPLC method to determine the purity of compounds: equipment 1: pump: L-7100, degasser: L-7614, autosampler: L-7200, UV detector: L-7400, interface: D-7000, data transfer: D-line, data acquisition: HSMS software (all from LaChrom, Merck Hitachi); equipment 2: pump: LPG-3400SD, degasser: DG-1210, autosampler: ACC-3000T, UV detector: VWD-3400RS, interface: Dionex UltiMate 3000, data acquisition: Chromeleon 7 (Thermo Fisher Scientific); column: LiChrospher 60 RP-select B (5 μm), LiChroCART 250-4 mm cartridge; flow rate: 1.0 mL/min; injection volume: 5.0 μL; detection at λ = 210 nm; solvents: A: demineralized water with 0.05% (v/v) trifluoroacetic acid, B: acetonitrile with 0.05% (v/v) trifluoroacetic acid; gradient elution (% A): 0–4 min: 90%; 4–29 min: gradient from 90 to 0%; 29–31 min: 0%; 31–31.5 min: gradient from 0 to 90%; 31.5–40 min: 90%.
With the exception of compounds 10n (94.9%), 5s (94.3%), and 24 (94.0%), the purity of all test compounds was greater than 95%.
General Procedure A
If not explicitly described otherwise, respective 1,2,4-triazol-5-amine (1.00 equiv) was suspended in dry pyridine and dry THF mixture (2/1) at 0 °C. A solution of respective acid chloride (1.00–1.40 equiv) in dry THF (1 mL) was added to this suspension dropwise using a syringe pump (1 mL/h), and afterward, the suspension was stirred for 1 h at rt. The reaction mixture was quenched with H2O. If a precipitate was formed, it was filtered off, washed with water (3×), and dried in vacuo. Otherwise, the aqueous layer was extracted with EtOAc (3×), and the combined organic layers were dried (Na2SO4), filtered, and concentrated in vacuo. The residue was purified by flash column chromatography yielding N1-acylated 1,2,4-triazol-5-amines.
General Procedure B
If not explicitly described otherwise, respective 1,2,4-triazol-5-amine (1.00 equiv), carboxylic acid (1.00 equiv), and DMAP (2.00 equiv) were suspended in dry DMF (0.15 M) and stirred for 5 min. Then, EDCI·HCl (2.00 equiv) was added in portions and the mixture was stirred for 4 h at rt. After quenching with ice-cold H2O and stirring for 5 min, the resulting precipitate was filtered off, washed with water (3×), and dried in vacuo. If necessary, the product was purified by flash column chromatography.
3-(5-Amino-1-benzoyl-1H-1,2,4-triazol-3-yl)-1-morpholinopropan-1-one (10d)
According to general procedure A, aminotriazole 9d (80.0 mg, 355 μmol) was acylated using BzCl (41.3 μL, 355 μmol, 1.00 equiv) in dry pyridine/THF (2.80 mL/1.40 mL). Flash column chromatography (CH2Cl2/CH3OH = 1/0 → 9/1) yielded 10d as a colorless solid (65 mg, 197 μmol, 56%). TLC: Rf = 0.15 (CH2Cl2/CH3OH = 95/5). 1H NMR (600 MHz, DMSO-d6): δ (ppm) 2.63–2.72 (m, 4H, (CH2)2), 3.39–3.43 (m, 4H, 3/5-Hmorpholinyl), 3.48–3.54 (m, 4H, 2/6-Hmorpholinyl), 7.51–7.56 (m, 2H, 3/5-Hbenzoyl), 7.62–7.68 (m, 3H, 4-Hbenzoyl, NH2), 8.01–8.06 (m, 2H, 2/6-Hbenzoyl). 13C NMR (151 MHz, DMSO-d6): δ (ppm) 23.6 (1C, CH2), 29.2 (1C, CH2), 41.6 (1C, C-3/5morpholinyl), 45.3 (1C, C-3/5morpholinyl), 66.06 (1C, C-2/6morpholinyl), 66.08 (1C, C-2/6morpholinyl), 128.0 (2C, C-3/5benzoyl), 130.6 (2C, C-2/6benzoyl), 132.2 (1C, C-1benzoyl), 132.8 (1C, C-4benzoyl), 158.5 (1C, C-5triazole), 162.9 (1C, C-3triazole), 167.4 (1C, CONbenzoyl), 169.8 (1C, CONmorpholinyl). IR (neat): ν̃ [cm–1] 3525, 3425, 3101, 2912, 1697, 1624, 1546, 1446, 1365, 1327, 1219, 1099, 833, 698, 675. HRMS (APCI): m/z = 330.1561 calcd for [M + H]+; found, 330.1587. HPLC: tR = 13.9 min, purity: 97.4%.
3-(5-Amino-1-benzoyl-1H-1,2,4-triazol-3-yl)-N-isopropylpropanamide (10i)
According to general procedure A, aminotriazole 9i (80.0 mg, 406 μmol) was acylated using BzCl (47.1 μL, 406 μmol, 1.00 equiv) in dry pyridine/THF (3.20 mL/1.60 mL) and stirred for 1 h at 0 °C and then for 1.5 h at rt. Flash column chromatography (CH2Cl2/CH3OH = 1/0 → 9/1) yielded 10i as a colorless solid (52 mg, 173 μmol, 43%). TLC: Rf = 0.17 (CH2Cl2/CH3OH = 95/5). 1H NMR (600 MHz, DMSO-d6): δ (ppm) 1.01 (d, J = 7.6 Hz, 6H, (CH3)2), 2.37–2.41 (m, 2H, CH2), 2.63–2.67 (m, 2H, CH2), 3.77–3.84 (m, 1H, CH), 7.51–7.55 (m, 2H, 3/5-Hbenzoyl), 7.61–7.68 (m, 3H, 4-Hbenzoyl, NH2), 7.70 (d, J = 7.6 Hz, 1H, CONH), 8.04–8.07 (m, 2H, 2/6-Hbenzoyl). 13C NMR (151 MHz, DMSO-d6): δ (ppm) 22.4 (2C, (CH3)2), 24.1 (1C, CH2), 32.6 (1C, CH2), 40.2 (1C, CH), 128.0 (2C, C-3/5benzoyl), 130.6 (1C, C-2/6benzoyl), 132.2 (1C, C-1benzoyl), 132.8 (1C, C-4benzoyl), 158.5 (1C, C-5triazole), 162.9 (1C, C-3triazole), 167.4 (1C, CONbenzoyl), 169.8 (1C, CONH). IR (neat): ν̃ [cm–1] 3437, 3305, 2970, 1689, 1639, 1543, 1415, 1381, 1330, 1280, 1180, 1033, 925, 736. HRMS (APCI): m/z = 302.1612 calcd for [M + H]+; found, 302.1612. HPLC: tR = 14.8 min, purity: 95.1%.
3-(5-Amino-1-benzoyl-1H-1,2,4-triazol-3-yl)-1-(4-phenylpiperazin-1-yl)propan-1-one (10g)
According to general procedure A, aminotriazole 9g (100 mg, 333 μmol) was acylated using BzCl (38.7 μL, 183 μmol, 1.00 equiv) in dry pyridine/THF (2.60 mL/1.30 mL). Flash column chromatography (CH2Cl2/CH3OH = 1/0 → 9/1) yielded amide 10g as a colorless solid (74 mg, 183 μmol, 55%). TLC: Rf = 0.25 (CH2Cl2/CH3OH = 95/5). 1H NMR (600 MHz, DMSO-d6): δ (ppm) 2.70–2.74 (m, 4H, (CH2)2), 3.05–3.08 (m, 2H, 3/5-Hpiperazinyl), 3.08–3.11 (m, 2H, 3/5-Hpiperazinyl), 3.56–3.61 (m, 4H, 2/6-Hpiperazinyl), 6.79–6.83 (m, 1H, 4-Hphenyl), 6.91–6.95 (m, 2H, 2/6-Hphenyl), 7.21–7.25 (m, 2H, 3/5-Hphenyl), 7.47–7.53 (m, 2H, 3/5-Hbenzoyl), 7.59–7.63 (m, 1H, 4-Hbenzoyl), 7.65 (br s, 2H, NH2), 8.02–8.05 (m, 2H, 2/6-Hbenzoyl). 13C NMR (151 MHz, DMSO-d6): δ (ppm) 23.7 (1C, CH2), 29.3 (1C, CH2), 41.0 (1C, C-2/6piperazinyl), 44.6 (1C, C-2/6piperazinyl), 48.3 (1C, C-3/5piperazinyl), 48.6 (1C, C-3/5piperazinyl), 115.8 (2C, C-2/6phenyl), 119.3 (1C, C-4phenyl), 128.0 (2C, C-3/5benzoyl), 129.0 (2C, C-3/5phenyl), 130.6 (2C, C-2/6benzoyl), 132.2 (1C, C-1benzoyl), 132.8 (1C, C-4benzoyl), 150.8 (1C, C-1phenyl), 158.5 (1C, C-5triazole), 162.9 (1C, C-3triazole), 167.4 (1C, CONbenzoyl), 169.5 (1C, CONpiperazinyl). IR (neat): ν̃ [cm–1] 3444, 3271, 3209, 2831, 1693, 1620, 1597, 1535, 1500, 1334, 1230, 1018, 914, 752. HRMS (APCI): m/z = 405.2034 calcd for [M + H]+; found, 405.2058. HPLC: tR = 16.8 min, purity: 96.4%.
3-(5-Amino-1-benzoyl-1H-1,2,4-triazol-3-yl)-N-benzylpropanamide (10n)
According to general procedure A, aminotriazole 9n (80.0 mg, 326 μmol) was acylated using BzCl (37.9 μL, 326 μmol, 1.00 equiv) in dry pyridine/THF (2.5 mL/1.25 mL) and stirred first for 1 h at 0 °C and then for 2 h at rt. The solid was filtered off and washed with H2O (2×) to yield amide 10n as a colorless solid (67 mg, 192 μmol, 59%). TLC: Rf = 0.17 (CH2Cl2/CH3OH = 95/5). 1H NMR (600 MHz, DMSO-d6): δ (ppm) 2.51–2.54 (m, 2H, CH2), 2.68–2.73 (m, 2H, CH2), 4.26 (d, J = 6.0 Hz, 2H, (CH2)benzyl), 7.18–7.22 (m, 3H, 2/4/6-Hphenyl), 7.24–7.29 (m, 2H, 3/5-Hphenyl), 7.50–7.55 (m, 2H, 3/5-Hbenzoyl), 7.63–7.68 (m, 3H, 4-Hbenzoyl, NH2), 8.03–8.07 (m, 2H, 2/6-Hbenzoyl), 8.38 (t, J = 6.0 Hz, 1H, CONH). 13C NMR (151 MHz, DMSO-d6): δ (ppm) 24.1 (1C, CH2), 32.5 (1C, CH2), 42.0 (1C, CH2-benzyl), 126.6 (1C, C-4phenyl), 127.1 (2C, C-2/6phenyl), 128.0 (2C, C-3/5benzoyl), 128.2 (2C, C-3/5phenyl), 130.7 (2C, C-2/6benzoyl), 132.2 (1C, C-1benzoyl), 132.8 (1C, C-4benzoyl), 139.6 (1C, C-1phenyl), 158.6 (1C, C-5triazole), 162.8 (1C, C-3triazole), 167.4 (1C, CONbenzoyl), 170.9 (1C, CONH). IR (neat): ν̃ [cm–1] 3425, 3317, 3035, 2920, 1693, 1643, 1554, 1415, 1334, 1276, 1180, 929, 736, 694. HRMS (APCI): m/z = 350.1612 calcd for [M + H]+; found, 350.1629. HPLC: tR = 16.7 min, purity: 94.9%.
(2-(5-Amino-1-benzoyl-1H-1,2,4-triazol-3-yl)phenyl)(pyrrolidin-1-yl)methanone (13a)
According to general procedure A, aminotriazole 12a (35.4 mg, 138 μmol) was acylated using BzCl (16.0 μL, 138 μmol, 1.00 equiv) in dry pyridine/THF (1.00 mL/0.50 mL). Flash column chromatography (CH2Cl2/CH3OH = 1/0 → 9/1) yielded amide 13a as a colorless solid (42 mg, 116 μmol, 84%). TLC: Rf = 0.20 (CH2Cl2/CH3OH = 95/5). 1H NMR (600 MHz, DMSO-d6): δ (ppm) 1.47–1.61 (m, 4H, 3/4-Hpyrrolidinyl), 2.90–2.96 (m, 2H, 2/5-Hpyrrolidinyl), 3.15–3.22 (m, 2H, 2/5-Hpyrrolidinyl), 7.26–7.30 (m, 1H, 6-Hphenyl), 7.49–7.52 (m, 2H, 4/5-Hphenyl), 7.53–7.58 (m, 2H, 3/5-Hbenzoyl), 7.67–7.71 (m, 1H, 4-Hbenzoyl), 7.81 (br s, 2H, NH2), 7.97–8.01 (m, 1H, 3-Hphenyl), 8.04–8.07 (m, 2H, 2/6-Hbenzoyl). 13C NMR (151 MHz, DMSO-d6): δ (ppm) 23.8 (1C, C-3/4pyrrolidinyl), 25.2 (1C, C-3/4pyrrolidinyl), 44.8 (1C, C-2/5pyrrolidinyl), 47.4 (1C, C-2/5pyrrolidinyl), 126.1 (1C, C-1phenyl), 127.0 (1C, C-6phenyl), 128.1 (2C, C-3/5benzoyl), 128.5 (1C, C-5phenyl), 128.7 (1C, C-3phenyl), 130.1 (1C, C-4phenyl), 130.7 (2C, C-2/6benzoyl), 132.0 (1C, C-1benzoyl), 132.9 (1C, C-4benzoyl), 137.4 (1C, C-2phenyl), 158.6 (1C, C-5triazole), 158.9 (1C, C-3triazole), 167.6 (1C, CONbenzoyl), 168.0 (1C, CONpyrrolidinyl). IR (neat): ν̃ [cm–1] 3425, 3282, 3213, 2966, 2873, 1685, 1597, 1531, 1446, 1354, 1315, 1141, 960, 918, 771, 744, 678. HRMS (APCI): m/z = 362.1612 calcd for [M + H]+; found, 362.1642. HPLC: tR = 17.9 min, purity: 97.1%.
(2-(5-Amino-1-benzoyl-1H-1,2,4-triazol-3-yl)phenyl)(piperidin-1-yl)methanone (13b)
According to general procedure A, aminotriazole 12b (100 mg, 368 μmol) was acylated using BzCl (47.1 μL, 405 μmol, 1.10 equiv) in dry pyridine/THF (3.00 mL/1.50 mL). Flash column chromatography (CH2Cl2/CH3OH = 1/0 → 9/1) yielded amide 13b as a colorless solid (83 mg, 221 μmol, 60%). TLC: Rf = 0.18 (CH2Cl2/CH3OH = 95/5). 1H NMR (600 MHz, DMSO-d6): δ (ppm) 1.18–1.33 (m, 5H, 3/4/5-Hpiperidinyl), 1.46–1.54 (m, 1H, 4-Hpiperidinyl), 2.80–2.86 (m, 2H, 2/6-Hpiperidinyl), 3.00–3.06 (m, 1H, 2/6-Hpiperidinyl), 3.66–3.72 (m, 1H, 2/6-Hpiperidinyl), 7.24–7.27 (m, 1H, 6-Hphenyl), 7.49–7.52 (m, 2H, 4/5-Hphenyl), 7.55–7.59 (m, 2H, 3/5-Hbenzoyl), 7.68–7.71 (m, 1H, 4-Hbenzoyl), 7.82 (br s, 2H, NH2), 7.96–7.99 (m, 1H, 3-Hphenyl), 8.09–8.12 (m, 2H, 2/6-Hbenzoyl). 13C NMR (151 MHz, DMSO-d6): δ (ppm) 23.9 (1C, C-4pirperidinyl), 24.6 (1C, C-3/5pirperidinyl), 25.1 (1C, C-3/5pirperidinyl), 41.3 (1C, C-2/6pirperidinyl), 46.9 (1C, C-2/6pirperidinyl), 126.6 (1C, C-1phenyl), 126.8 (1C, C-6phenyl), 128.2 (2C, C-3/5benzoyl), 128.5 (1C, C-4phenyl), 128.6 (1C, C-3phenyl), 130.0 (1C, C-5phenyl), 131.0 (2C, C-2/6benzoyl), 131.8 (1C, C-1benzoyl), 133.0 (1C, C-4benzoyl), 136.5 (1C, C-2phenyl), 158.6 (1C, C-5triazole), 158.8 (1C, C-3triazole), 167.5 (1C, CONbenzoyl), 168.0 (1C, CONpirperidinyl). IR (neat): ν̃ [cm–1] 3402, 3109, 2939, 2850, 1681, 1604, 1531, 1446, 1354, 960, 709. HRMS (APCI): m/z = 376.1768 calcd for [M + H]+; found, 376.1768. HPLC: tR = 19.2 min, purity: 95.6%.
(2-(5-Amino-1-benzoyl-1H-1,2,4-triazol-3-yl)phenyl)(azepan-1-yl)methanone (13c)
According to general procedure A, aminotriazole 12c (100 mg, 350 μmol) was acylated using BzCl (40.7 μL, 350 μmol, 1.00 equiv) in dry pyridine/THF (2.70 mL/1.35 mL). Flash column chromatography (1. CH2Cl2/CH3OH = 1/0 → 9/1, 2. CH2Cl2/CH3OH = 1/0 → 94/6) yielded amide 13c as a colorless solid (85 mg, 218 μmol, 62%). TLC: Rf = 0.25 (CH2Cl2/CH3OH = 95/5). 1H NMR (600 MHz, DMSO-d6): δ (ppm) 1.32–1.58 (m, 8H, 3/4/5/6-Hazepanyl), 2.75–2.82 (m, 1H, 2/7-Hazepanyl), 2.89–2.96 (m, 1H, 2/7-Hazepanyl), 3.10–3.17 (m, 1H, 2/7-Hazepanyl), 3.67–3.73 (m, 1H, 2/7-Hazepanyl), 7.22–7.27 (m, 1H, 6-Hphenyl), 7.48–7.53 (m, 2H, 3/5-Hphenyl), 7.55–7.59 (m, 2H, 3/5-Hbenzoyl), 7.67–7.71 (m, 1H, 4-Hbenzoyl), 7.82 (br s, 2H, NH2), 7.97–8.00 (m, 1H, 3-Hphenyl), 8.06–8.10 (m, 2H, 2/6-Hbenzoyl). 13C NMR (151 MHz, DMSO-d6): δ (ppm) 26.3 (1C, C-3/4/5/6azepanyl), 26.8 (1C, C-3/4/5/6azepanyl), 27.0 (1C, C-3/4/5/6azepanyl), 28.2 (1C, C-3/4/5/6azepanyl), 44.2 (1C, C-2/7azepanyl), 47.9 (1C, C-2/7azepanyl), 126.3 (1C, C-1phenyl), 127.0 (1C, C-6phenyl), 128.1 (2C, C-3/5benzoyl), 128.3 (1C, C-5phenyl), 128.7 (1C, C-3phenyl), 129.9 (1C, C-4phenyl), 130.8 (2C, C-2/6benzoyl), 131.9 (1C, C-1benzoyl), 133.0 (1C, C-4benzoyl), 136.8 (1C, C-2phenyl), 158.6 (1C, C-5triazole), 158.9 (1C, C-3triazole), 167.5 (1C, CONbenzoyl), 169.3 (1C, CONazepanyl). IR (neat): ν̃ [cm–1] 3433, 2927, 2854, 1681, 1597, 1531, 1446, 1350, 1315, 1138, 960, 918, 744, 694. HRMS (APCI): m/z = 390.1925 calcd for [M + H]+; found, 390.1909. HPLC: tR = 19.6 min, purity: 96.4%.
(2-(5-Amino-1-benzoyl-1H-1,2,4-triazol-3-yl)phenyl)(morpholino)methanone (13d)
According to general procedure A, aminotriazole 12d (100 mg, 366 μmol) was acylated using BzCl (42.5 μL, 366 μmol, 1.00 equiv) in dry pyridine/THF (3.00 mL/1.50 mL). Flash column chromatography (CH2Cl2/CH3OH = 1/0 → 9/1) yielded amide 13d as a colorless solid (96 mg, 254 μmol, 70%). TLC: Rf = 0.20 (CH2Cl2/CH3OH = 95/5). 1H NMR (600 MHz, DMSO-d6): δ (ppm) 2.85–2.92 (m, 1H, 3/5-Hmorpholinyl), 2.96–3.07 (m, 2H, 3/5-Hmorpholinyl), 3.18–3.24 (m, 1H, 2/6-Hmorpholinyl), 3.25–3.31 (m, 2H, 2/6-Hmorpholinyl), 3.33–3.38 (m, 1H, 2/6-Hmorpholinyl), 3.51–3.57 (m, 1H, 3/5-Hmorpholinyl), 7.27–7.31 (m, 1H, 6-Hphenyl), 7.51–7.54 (m, 2H, 4/5-Hphenyl), 7.55–7.59 (m, 2H, 3/5-Hbenzoyl), 7.67–7.71 (m, 1H, 4-Hbenzoyl), 7.84 (br s, 2H, NH2), 8.00–8.02 (m, 1H, 3-Hphenyl), 8.09–8.11 (m, 2H, 2/6-Hbenzoyl). 13C NMR (151 MHz, DMSO-d6): δ (ppm) 41.0 (1C, C-3/5morpholinyl), 46.3 (1C, C-3/5morpholinyl), 65.3 (1C, C-2/6morpholinyl), 65.3 (1C, C-2/6morpholinyl), 126.5 (1C, C-1phenyl), 126.9 (1C, C-6phenyl), 128.0 (2C, C-3/5benzoyl), 128.4 (1C, C-3phenyl), 128.6 (1C, C-5phenyl), 130.0 (1C, C-4phenyl), 130.8 (2C, C-2/6benzoyl), 131.7 (1C, C-1benzoyl), 132.9 (1C, C-4benzoyl), 135.4 (1C, C-2phenyl), 158.44 (1C, C-5triazole), 158.46 (1C, C-3triazole), 167.4 (1C, CONbenzoyl), 168.4 (1C, CONmorpholine). IR (neat): ν̃ [cm–1] 3421, 3105, 2854, 1697, 1604, 1535, 1465, 1442, 1357, 1330, 1276, 1107, 960, 840, 736, 675. HRMS (APCI): m/z = 378.1561 calcd for [M + H]+; found, 378.1570. HPLC: tR = 16.2 min, purity: 98.8%.
(2-(5-Amino-1-benzoyl-1H-1,2,4-triazol-3-yl)phenyl)(3,4-dihydroisoquinolin-2(1H)-yl)methanone (13e)
According to general procedure A, aminotriazole 12e (45.0 mg, 141 μmol) was acylated using BzCl (16.4 μL, 141 μmol, 1.00 equiv) in dry pyridine/THF (1.00 mL/0.50 mL) and stirred first for 1 h at 0 °C and then for 2 h at rt. Flash column chromatography (CH2Cl2/CH3OH = 98/2) yielded amide 13e as a yellowish solid (51 mg, 120 μmol, 86%). TLC: Rf = 0.22 (CH2Cl2/CH3OH = 95/5). 1H NMR (600 MHz, DMSO-d6): δ (ppm) 2.41–2.48 (m, 0.6H, 1/3/4-Htetrahydroisoquonolinyl), 2.51–2.56 (m, 0.4H, 1/3/4-Htetrahydroisoquinolinyl*), 2.59–2.68 (m, 1H, 1/3/4-Htetrahydroisoquinolinyl), 3.07–3.14 (m, 0.6H, 1/3/4-Htetrahydroisoquinolinyl), 3.27–3.41 (m, 1H, 1/3/4-Htetrahydroisoquinolinyl), 3.67–3.73 (m, 0.4H, 1/3/4-Htetrahydroisoquinolinyl*), 4.02–4.18 (m, 1.4H, 1/3/4-Htetrahydroisoquinolinyl), 4.73–4.80 (m, 0.6H, 1/3/4-Htetrahydroisoquinolinyl), 6.77–6.79 (m, 0.4H, 5/6/7/8-Htetrahydroisoquinolinyl*), 6.95–6.99 (m, 0.4H, 5/6/7/8-Htetrahydroisoquinolinyl*), 7.01–7.05 (m, 1H, 5/6/7/8-Htetrahydroisoquinolinyl), 7.05–7.08 (m, 1H, 6-Hphenyl), 7.08–7.13 (m, 0.6H, 5/6/7/8-Htetrahydroisoquinolinyl), 7.13–7.18 (m, 0.6H, 5/6/7/8-Htetrahydroisoquinolinyl), 7.27–7.30 (m, 0.4H, 5/6/7/8-Htetrahydroisoquinolinyl*), 7.31–7.34 (m, 0.6H, 5/6/7/8-Htetrahydroisoquinolinyl), 7.39–7.43 (m, 1.2H, 3/5-Hbenzoyl), 7.43–7.47 (m, 0.8H, 3/5-Hbenzoyl*), 7.48–7.52 (m, 0.6H, 4-Hbenzoyl), 7.52–7.58 (m, 2H, 4/5-Hphenyl), 7.59–7.63 (m, 0.4H, 4-Hbenzoyl*), 7.68 (br s, 0.8H, NH2*), 7.81 (br s, 1.2H, NH2), 7.95–8.02 (m, 2.4H, 0.4 × 3-Hphenyl*, 2/6-Hbenzoyl), 8.02–8.05 (m, 0.6H, 0.6 × 3-Hphenyl). Ratio of rotamers is 6:4. The minor rotamer is marked with an asterisk (*). 13C NMR (151 MHz, DMSO-d6): δ (ppm) 27.3 (0.4C, C-1/3/4tetrahydroisoquinolinyl*), 28.4 (0.6C, C-1/3/4tetrahydroisoquinolinyl), 38.9 (0.4C, C-1/3/4tetrahydroisoquinolinyl*), 43.5 (0.6C, C-1/3/4tetrahydroisoquinolinyl), 43.7 (0.6C, C-1/3/4tetrahydroisoquinolinyl), 47.7 (0.4C, C-1/3/4tetrahydroisoquinolinyl*), 125.6, 125.7, 126.1, 126.2, 126.5, 126.6, 126.7, 126.9, 127.3, 127.9, 127.9, 128.4, 128.5, 128.6, 128.8, 128.8, 130.2, 130.6, 130.9, 131.8, 132.73 (0.6C, C-4benzoyl, C-4a/8atetrahydroisoquionolinyl), 132.74 (0.6C, C-4benzoyl, C-4a/8atetrahydroisoquionolinyl), 132.8 (0.4C, C-4benzoyl, C-4a/8atetrahydroisoquionolinyl*), 132.9 (0.4C, C-4benzoyl, C-4a/8atetrahydroisoquionolinyl*), 134.1 (0.6C, C-4a/8atetrahydroisoquionolinyl), 134.4 (0.4C, C-4a/8atetrahydroisoquionolinyl*), 136.0 (0.4C, C-2phenyl*), 136.1 (0.6C, C-2phenyl), 158.4 (0.4C, C-3/5triazole*), 158.57 (0.4C, C-3/5triazole*), 158.61 (0.6C, C-3/5triazole), 158.7 (0.6C, C-3/5triazole), 167.2 (0.4C, CONbenzoyl*), 167.6 (0.6C, CONbenzoyl), 168.4 (0.4C, CONtetrahydroisoquinolinyl*), 169.0 (0.6C, CONtetrahydroisoquinolinyl). The ratio of rotamers is 6:4. The minor rotamer is marked with an asterisk (*). IR (neat): ν̃ [cm–1] 3410, 3278, 3059, 3028, 1685, 1639, 1577, 1535, 1450, 1354, 1327, 1141, 960, 918, 736, 682, 671. HRMS (APCI): m/z = 424.1768 calcd for [M + H]+; found, 424.1769. HPLC: tR = 20.1 min, purity: 98.6%.
(2-(5-Amino-1-benzoyl-1H-1,2,4-triazol-3-yl)phenyl)(4-phenylpiperazin-1-yl)methanone (13g)
According to general procedure A, aminotriazole 12g (46.0 mg, 132 μmol) was acylated using BzCl (15.3 μL, 132 μmol, 1.00 equiv) in dry pyridine/THF (1.00 mL/0.50 mL). Flash column chromatography (CH2Cl2/CH3OH = 1/0 → 9/1) yielded amide 13g as a colorless solid (26 mg, 57 μmol, 44%). TLC: Rf = 0.29 (CH2Cl2/CH3OH = 95/5). 1H NMR (600 MHz, DMSO-d6): δ (ppm) 2.77–2.87 (m, 2H, 3/5-Hpiperazinyl), 2.89–2.98 (m, 2H, 3/5-Hpiperazinyl), 2.98–3.05 (m, 1H, 2/6-Hpiperazinyl), 3.09–3.18 (m, 2H, 2/6-Hpiperazinyl), 3.74–3.81 (m, 1H, 2/6-Hpiperazinyl), 6.76–6.80 (m, 1H, 4′-Hphenyl), 6.80–6.84 (m, 2H, 3′/5′-Hphenyl), 7.17–7.22 (m, 2H, 2′/6′-Hphenyl), 7.31–7.35 (m, 1H, 6-Hphenyl), 7.51–7.55 (m, 4H, 3/5-Hbenzoyl, 4/5-Hphenyl), 7.61–7.65 (m, 1H, 4-Hbenzoyl), 7.83 (br s, 2H, NH2), 8.02–8.04 (m, 1H, 3-Hphenyl), 8.06–8.09 (m, 2H, 2/6-Hbenzoyl). 13C NMR (151 MHz, DMSO-d6): δ (ppm) 40.6 (1C, C-2/6piperazinyl), 45.8 (1C, C-2/6piperazinyl), 47.6 (1C, C-3/5piperazinyl), 48.1 (1C, C-3/5piperazinyl), 115.8 (1C, C-3′/5′phenyl), 119.2 (1C, C-4′phenyl), 126.6 (1C, C-1phenyl), 127.1 (1C, C-6phenyl), 128.1 (2C, C-3/5benzoyl), 128.6 (1C, C-3phenyl), 128.8 (1C, C-4/5phenyl), 128.9 (2C, C-2′/6′phenyl), 130.1 (1C, C-4/5phenyl), 131.0 (2C, C-2/6benzoyl), 131.8 (1C, C-1benzoyl), 133.1 (1C, C-4benzoyl), 135.8 (1C, C-2phenyl), 150.6 (1C, C-1phenyl′), 158.59 (1C, C-5triazole), 158.63 (1C, C-3triazole), 167.5 (1C, CONbenzoyl), 168.4 (1C, CONpiperazinyl). IR (neat): ν̃ [cm–1] 3425, 3062, 2908, 2823, 1685, 1635, 1597, 1539, 1446, 1365, 1222, 1145, 1114, 960, 921, 771, 694. HRMS (APCI): m/z = 453.2034 calcd for [M + H]+; found, 453.2021. HPLC: tR = 19.2 min, purity: 98.4%.
2-(5-Amino-1-benzoyl-1H-1,2,4-triazol-3-yl)-N-benzyl-N-methylbenzamide (13h)
According to general procedure A, aminotriazole 12h (80.0 mg, 260 μmol) was acylated using BzCl (30.2 μL, 366 μmol, 1.00 equiv) in dry pyridine/THF (2.00 mL/1.00 mL). Flash column chromatography (1. CH2Cl2/CH3OH = 1/0 → 94/6, 2. CH2Cl2/CH3OH = 1/0 → 96/4) yielded amide 13h as a colorless solid (66 mg, 160 μmol, 62%). TLC: Rf = 0.28 (CH2Cl2/CH3OH = 95/5). 1H NMR (600 MHz, DMSO-d6): δ (ppm) 2.54 (s, 1.5H, CH3), 2.54 (s, 1.5H, CH3), 3.51 (br s, 0.5H, CH2), 4.00 (d, J = 16.0 Hz, 0.5H, CH2), 4.28 (d, J = 16.0 Hz, 0.5H, CH2), 5.02 (br s, 0.5H, CH2), 7.06–7.10 (m, 1H, 6-Hphenyl), 7.16–7.22 (m, 2.5H, 0.5 × 4-Hbenzyl, 2/6-Hbenzyl), 7.23–7.27 (m, 0.5H, 0.5 × 4-Hbenzyl), 7.30–7.36 (m, 2H, 3/5-Hbenzyl), 7.45–7.51 (m, 1H, 5-Hphenyl), 7.51–7.55 (m, 1H, 4-Hphenyl), 7.55–7.62 (m, 2H, 3/5-Hbenzoyl), 7.64–7.68 (m, 0.5H, 4-Hbenzoyl), 7.70–7.73 (m, 0.5H, 4-Hbenzoyl), 7.85 (br s, 1H, NH2), 7.86 (br s, 1H, NH2), 7.98–8.01 (m, 0.5H, 3-Hphenyl), 8.01–8.04 (m, 0.5H, 3-Hphenyl), 8.08–8.11 (m, 2H, 2/6-Hbenzoyl). Ratio of rotamers is 1:1. 13C NMR (151 MHz, DMSO-d6): δ (ppm) 31.5 (0.5C, CH3), 35.7 (0.5C, CH3), 49.0 (0.5C, CH2), 53.6 (0.5C, CH2), 126.5 (0.5C, C-1phenyl), 126.6 (0.5C, C-1phenyl), 126.9 (0.5C, C-6phenyl), 127.0 (2C, C-2/6benzyl), 127.2 (0.5C, C-6phenyl), 127.5 (1C, C-4benzyl), 128.2 (2C, C-3/5benzoyl), 128.4, 128.5, 128.69, 128.71, 128.73, 130.0 (1C, C-5phenyl), 130.2 (1C, C-4phenyl), 130.81 (1C, C-2/6benzoyl), 130.82 (1C, C-2/6benzoyl), 132.04 (0.5C, C-1benzoyl), 132.01 (0.5C, C-1benzoyl), 132.99 (0.5C, C-4benzoyl), 132.95 (0.5C, C-4benzoyl), 135.9 (0.5C, C-2phenyl), 136.2 (0.5C, C-2phenyl), 136.6 (0.5C, C-1benzyl), 137.4 (0.5C, C-1benzyl), 158.6 (0.5C, C-3/5triazole), 158.6 (0.5C, C-3/5triazole), 158.7 (0.5C, C-3/5triazole), 158.9 (0.5C, C-3/5triazole), 167.64 (0.5C, CONbenzoyl), 167.69 (0.5C, CONbenzoyl), 170.06 (0.5C, CONmethylbenzyl), 170.09 (0.5C, CONmethylbenzyl). Ratio of rotamers is 1:1. IR (neat): ν̃ [cm–1] 3437, 3059, 1685, 1600, 1531, 1446, 1396, 1350, 1315, 1141, 1103, 1060, 960, 921, 744, 694, 675. HRMS (APCI): m/z = 412.1768 calcd for [M + H]+; found, 412.1751. HPLC: tR = 20.0 min, purity: 96.8%.
2-(5-Amino-1-benzoyl-1H-1,2,4-triazol-3-yl)-N-isopropylbenzamide (13i)
According to general procedure A, aminotriazole 12i (34.0 mg, 139 μmol) was acylated using BzCl (16.1 μL, 138 μmol, 1.00 equiv) in dry pyridine/THF (1.00 mL/0.50 mL) and stirred first for 1 h at 0 °C and then for 2 h at rt. Flash column chromatography (CH2Cl2/CH3OH = 98/2) yielded amide 13i as a colorless solid (37 mg, 106 μmol, 76%). TLC: Rf = 0.27 (CH2Cl2/CH3OH = 95/5). 1H NMR (600 MHz, DMSO-d6): δ (ppm) 0.92 (d, J = 6.6 Hz, 6H, (CH3)2), 3.89–3.97 (m, 1H, CH), 7.34–7.36 (m, 1H, 6-Hphenyl), 7.47–7.52 (m, 2H, 4/5-Hphenyl), 7.52–7.55 (m, 2H, 3/5-Hbenzoyl), 7.66–7.70 (m, 1H, 4-Hbenzoyl), 7.77 (br s, 2H, NH2), 7.84–7.86 (m, 1H, 3-Hphenyl),7.95 (d, J = 7.9 Hz, 1H, CONH), 8.13–8.17 (m, 2H, 2/6-Hbenzoyl). 13C NMR (151 MHz, DMSO-d6): δ (ppm) 22.1 (2C, (CH3)2), 40.4 (1C, CH), 127.8 (1C, C-1phenyl), 128.0 (2C, C-3/5benzoyl), 128.1 (1C, C-6phenyl), 128.8 (1C, C-5phenyl), 129.0 (1C, C-3phenyl), 129.3 (1C, C-4phenyl), 131.0 (2C, C-2/6benzoyl), 131.8 (1C, C-1benzoyl), 133.1 (1C, C-4benzoyl), 138.0 (1C, C-2phenyl), 158.4 (1C, C-5triazole), 159.5 (1C, C-3triazole), 167.45 (1C, CONbenzoyl), 167.50 (1C, CONH). IR (neat): ν̃ [cm–1] 3394, 3321, 3059, 2978, 1689, 1620, 1600, 1539, 1492, 1354, 1319, 1138, 960, 925, 806, 786, 736, 694. HRMS (APCI): m/z = 350.1612 calcd for [M + H]+; found, 350.1624. HPLC: tR = 17.1 min, purity: 96.8%.
2-(5-Amino-1-benzoyl-1H-1,2,4-triazol-3-yl)-N-benzylbenzamide (13n)
According to general procedure A, aminotriazole 12n (75.0 mg, 256 μmol) was acylated using BzCl (29.7 μL, 256 μmol, 1.00 equiv) in dry pyridine/THF (2.00 mL/1.00 mL). Flash column chromatography (CH2Cl2/CH3OH = 1/0 → 9/1) yielded amide 13n as a colorless solid (83 mg, 209 μmol, 82%). TLC: Rf = 0.27 (CH2Cl2/CH3OH = 95/5). 1H NMR (600 MHz, DMSO-d6): δ (ppm) 4.30 (d, J = 6.0 Hz, 2H, CH2), 7.18–7.22 (m, 1H, 4-Hbenzyl), 7.22–7.29 (m, 4H, 2/3/5/6-Hbenzyl), 7.41–7.44 (m, 1H, 6-Hphenyl), 7.50–7.54 (m, 2H, 4/5-Hphenyl), 7.54–7.59 (m, 2H, 3/5-Hbenzoyl), 7.66–7.70 (m, 1H, 4-Hbenzoyl), 7.82 (br s, 2H, NH2), 7.87–7.90 (m, 1H, 3-Hphenyl), 8.14–8.17 (m, 2H, 2/6-Hbenzoyl), 8.70 (t, J = 6.0 Hz, 1H, CONH). 13C NMR (151 MHz, DMSO-d6): δ (ppm) 42.4 (1C, CH2), 126.6 (1C, C-4benzyl), 127.1 (2C, C-2/6benzyl), 127.8 (1C, C-1phenyl), 128.0 (1C, C-6phenyl), 128.1 (2C, C-3/5benzoyl), 128.2 (2C, C-3/5benzyl), 129.06 (1C, C-5phenyl), 129.09 (1C, C-3phenyl), 129.5 (1C, C-4phenyl), 131.1 (2C, C-2/6benzoyl), 131.8 (1C, C-1benzoyl), 133.1 (1C, C-4benzoyl), 137.5 (1C, C-2phenyl), 139.5 (1C, C-1benzyl), 158.5 (1C, C-5triazole), 159.4 (1C, C-3triazole), 167.4 (1C, CONbenzoyl), 168.7 (1C, CONH). IR (neat): ν̃ [cm–1] 3452, 3340, 3278, 3055, 1685, 1654, 1627, 1554, 1450, 1354, 1330, 1138 1080, 960, 918, 740, 698, 675. HRMS (APCI): m/z = 398.1612 calcd for [M + H]+; found, 398.1635. HPLC: tR = 18.5 min, purity: 97.4%.
(2-(5-Amino-1-(4-fluorobenzoyl)-1H-1,2,4-triazol-3-yl)phenyl)(morpholino)methanone (14d)
According to general procedure A, aminotriazole 12d (100 mg, 366 μmol) was acylated using 4-fluorobenzoyl chloride (43.2 μL, 366 μmol, 1.00 equiv) in dry pyridine/THF (3.00 mL/1.50 mL). Flash column chromatography (CH2Cl2/CH3OH = 1/0 → 9/1) yielded amide 14d as a colorless solid (110 mg, 278 μmol, 76%). TLC: Rf = 0.29 (CH2Cl2/CH3OH = 95/5). 1H NMR (600 MHz, DMSO-d6): δ (ppm) 2.86–2.92 (m, 1H, 3/5-Hmorpholinyl), 2.96–3.02 (m, 1H, 3/5-Hmorpholinyl), 3.06–3.12 (m, 1H, 3/5-Hmorpholinyl), 3.25–3.31 (m, 3H, 2/6-Hmorpholinyl), 3.37–3.43 (m, 1H, 2/6-Hmorpholinyl), 3.54–3.62 (m, 1H, 3/5-Hmorpholinyl), 7.28–7.31 (m, 1H, 6-Hphenyl), 7.38–7.42 (m, 2H, 3/5-Hfluorobenzoyl), 7.51–7.55 (m, 2H, 4/5-Hphenyl), 7.85 (br s, 2H, NH2), 8.00–8.03 (m, 1H, 3-Hphenyl), 8.21–8.25 (m, 2H, 2/6-Hfluorobenzoyl). 13C NMR (151 MHz, DMSO-d6): δ (ppm) 41.3 (1C, C-3/5morpholinyl), 46.5 (1C, C-3/5morpholinyl), 65.4 (1C, C-2/6morpholinyl), 65.5 (1C, C-2/6morpholinyl), 115.3 (d, J = 21.9 Hz, 2C, C-3/5fluorobenzoyl), 126.6 (1C, C-1phenyl), 127.1 (1C, C-6phenyl), 128.3 (d, J = 2.9 Hz, C1, C-1fluorobenzoyl), 128.5 (1C, C-3phenyl), 128.8 (1C, C-5phenyl), 130.2 (1C, C-4phenyl), 134.2 (d, J = 9.5 Hz, 2C, C-2/6fluorobenzoyl), 135.5 (1C, C-2phenyl), 158.61 (1C, C-5triazole), 158.64 (1C, C-3triazole), 164.8 (d, J = 252.5 Hz, 1C, C-4fluorobenzoyl), 166.2 (1C, CONfluorobenzoyl), 168.6 (1C, CONmorpohline). IR (neat): ν̃ [cm–1] 3421, 3109, 2858, 1681, 1597, 1535, 1462, 1438, 1357, 1327, 1234, 1111, 1014, 960, 925, 844, 736. HRMS (APCI): m/z = 396.1466 calcd for [M + H]+; found, 396.1489. HPLC: tR = 16.7 min, purity: 98.5%.
(2-(5-Amino-1-(4-fluorobenzoyl)-1H-1,2,4-triazol-3-yl)phenyl)(3,4-dihydroisoquinolin-2(1H)-yl)methanone (14e)
According to general procedure A, aminotriazole 12e (45 mg, 141 μmol) was acylated using 4-fluorobenzoyl chloride (16.7 μL, 141 μmol, 1.00 equiv) in dry pyridine/THF (1.0ß mL/0.50 mL) and stirred first for 1 h at 0 °C and then for 2 h at rt. Flash column chromatography (CH2Cl2/CH3OH = 98/2) yielded amide 14e as a yellowish solid (55 mg, 125 μmol, 88%). TLC: Rf = 0.22 (CH2Cl2/CH3OH = 95/5). 1H NMR (400 MHz, DMSO-d6): δ (ppm) 2.39–2.49 (m, 0.6H, 1/3/4-Htetrahydroisoquonolinyl), 2.51–2.71 (m, 1.6H, 1/3/4-Htetrahydroisoquonolinyl), 3.09–3.19 (m, 0.6H, 1/3/4-Htetrahydroisoquonolinyl), 3.23–3.30 (m, 0.4H, 1/3/4-Htetrahydroisoquonolinyl*), 3.40–3.50 (m, 0.4H, 1/3/4-Htetrahydroisoquonolinyl*), 3.66–3.75 (m, 0.4H, 1/3/4-Htetrahydroisoquonolinyl*), 4.08–4.22 (m, 0.8H, 1/3/4-Htetrahydroisoquonolinyl), 4.27 (d, J = 17.5 Hz, 0.6H, 1/3/4-Htetrahydroisoquonolinyl), 4.74 (d, J = 17.5 Hz, 0.6H, 1/3/4-Htetrahydroisoquonolinyl), 6.79–6.80 (m, 0.4H, 5/6/7/8tetrahydroisoquinolinyl*), 6.94–6.99 (m, 0.4H, 5/6/7/8tetrahydroisoquinolinyl*), 7.00–7.17 (m, 3H, 6-Hphenyl, 5/6/7/8tetrahydroisoquinolinyl), 7.17–7.28 (m, 2H, 3/5-Hfluorobenzoyl), 7.28–7.36 (m, 1.2H, 5/6/7/8tetrahydroisoquinolinyl), 7.51–7.57 (m, 2H, 4/5-Hphenyl), 7.68 (br s, 0.8H, NH2*), 7.81 (br s, 1.2H, NH2), 7.95–8.02 (m, 2.4H, 0.4 × 3-Hphenyl*, 2/6-Hfluorobenzoyl), 8.02–8.05 (m, 0.6H, 0.6 × 3-Hphenyl). Ratio of rotamers is 6:4. The minor rotamer is marked with an asterisk (*). 13C NMR (101 MHz, DMSO-d6): δ (ppm) 27.2 (0.4C, C-1/3/4tetrahydroisoquinolinyl), 28.3 (0.5C, C-1/3/4tetrahydroisoquinolinyl), 29.0 (0.4C, C-1/3/4tetrahydroisoquinolinyl), 43.5 (0.5C, C-1/3/4tetrahydroisoquinolinyl), 43.7 (0.6C, C-1/3/4tetrahydroisoquinolinyl), 47.7 (0.4C, C-1/3/4tetrahydroisoquinolinyl), 115.1 (d, J = 22.1 Hz, 1C, C-3/5fluorobenzoyl), 125.7, 126.0, 126.1, 126.2, 126.4, 126.5, 126.6, 126.9, 127.3, 128.3, 128.5, 128.6, 128.8, 128.9, 130.2, 132.7, 132.8, 133.8 (d, J = 9.5 Hz, 1C, C-2/6fluorobenzoyl), 134.0, 134.0, 134.1, 134.4, 136.0, 136.1, 158.4 (1C, C-3/5triazole), 158.6 (1C, C-3/5triazole), 163.4 (0.5C, C-4fluorobenzoyl), 166.2 (0.4C, CONfluorobenzoyl), 165.9 (0.6C, CONfluorobenzoyl), 168.4 (0.4C, CONtetrahydroisoquinolinyl), 169.0 (0.6C, CONtetrahydroisoquinolinyl). Ratio of rotamers is 6:4. The minor rotamer is marked with an asterisk (*). IR (neat): ν̃ [cm–1] 3398, 3194, 3062, 1685, 1581, 1446, 1350, 1319, 1253, 1226, 1161, 960, 925, 864, 748, 686. HRMS (APCI): m/z = 442.1674 calcd for [M + H]+; found, 442.1678. HPLC: tR = 20.4 min, purity: 96.5%.
2-(5-Amino-1-(4-fluorobenzoyl)-1H-1,2,4-triazol-3-yl)-N-benzyl-N-methylbenzamide (14h)
According to general procedure A, aminotriazole 12h (50.0 mg, 163 μmol) was acylated using 4-fluorobenzoyl chloride (19.2 μL, 163 μmol, 1.00 equiv) in dry pyridine/THF (1.5 mL/0.75 mL). Flash column chromatography (CH2Cl2/CH3OH = 1/0 → 9/1) yielded amide 14h as a colorless solid (59 mg, 137 μmol, 84%). TLC: Rf = 0.29 (CH2Cl2/CH3OH = 95/5). 1H NMR (600 MHz, DMSO-d6): δ (ppm) 2.53 (s, 1.5H, CH3), 2.62 (s, 1.5H, CH3), 3.69 (br s, 0.5H, CH2), 4.04 (d, J = 16.0 Hz, 0.5H, CH2), 4.27 (d, J = 16.0 Hz, 0.5H, CH2), 5.02 (br s, 0.5H, CH2), 7.08 (dd, J = 7.6/1.9 Hz, 1H, 6-Hphenyl), 7.16–7.23 (m, 2.5H, 0.5 × 4-Hbenzyl, 2/6-Hbenzyl), 7.23–7.23 (m, 0.5H, 0.5 × 4-Hbenzyl), 7.31–7.38 (m, 2H, 3/5-Hbenzyl), 7.38–7.46 (m, 2H, 3/5-Hfluorobenzoyl), 7.47–7.52 (m, 1H, 4-Hphenyl), 7.51–7.59 (m, 1H, 4-Hphenyl), 7.86 (br s, 1H, NH2), 7.87 (br s, 1H, NH2), 7.98–8.01 (m, 0.5H, 3-Hphenyl), 8.02–8.05 (m, 0.5H, 3-Hphenyl), 8.20–8.25 (m, 2H, 2/6-Hfluorobenzoyl). Ratio of rotamers is 1:1. 13C NMR (151 MHz, DMSO-d6): δ (ppm) 31.6 (s, 1.5H, CH3), 35.7 (s, 1.5H, CH3), 49.1 (s, 1.5H, CH3), 53.6 (s, 1.5H, CH3), 115.3 (d, J = 21.9 Hz, 2C, C-3/5fluorobenzoyl), 126.4 (0.5C, C-1phenyl), 126.5 (0.5C, C-1phenyl), 127.0, 127.0, 127.1, 127.1, 127.2, 127.5, 128.35, 128.42, 128.44, 128.64, 128.51, 128.72, 128.73, 128.74, 130.0 (1C, C-5phenyl), 130.2 (1C, C-4phenyl), 134.1 (d, J = 9.3 Hz, 2C, C-2/6fluorobenzyl), 135.9 (0.5C, C-2phenyl), 136.2 (0.5C, C-2phenyl), 136.6 (0.5C, C-1benzyl), 137.4 (0.5C, C-1benzyl), 158.6 (0.5C, C-3/5triazole), 158.7 (0.5C, C-3/5triazole), 158.7 (0.5C, C-3/5triazole), 158.9 (0.5C, C-3/5triazole), 164.75 (d, J = 252.0 Hz, 0.5C, C-4fluorobenzyl), 164.79 (d, J = 252.1 Hz, 0.5C, C-4fluorobenzoyl), 166.33 (0.5C, CON), 166.36 (0.5C, CON), 170.06 (0.5C, CONmethylbenzyl), 170.8 (0.5C, CONmethylbenzyl). Ratio of rotamers is 1:1. IR (neat): ν̃ [cm–1] 3437, 3109, 3043, 1689, 1627, 1597, 1543, 1404, 1357, 1315, 1230, 1161, 1103, 1064, 929, 860, 721. HRMS (APCI): m/z = 430.1674 calcd for [M + H]+; found, 430.1668. HPLC: tR = 20.3 min, purity: 97.2%.
(5-Amino-3-(pyrazin-2-yl)-1H-1,2,4-triazol-1-yl)(phenyl)methanone (18)
According to general procedure B, aminotriazole 17 (500 mg, 3.08 mmol) was acylated using benzoic acid (377 mg, 3.08 mmol), DMAP (753 mg, 6.17 mmol), and EDCI·HCl (1.18 g, 6.17 mmol) in dry DMF (20.0 mL). 18 was obtained as a colorless solid (542 mg, 2.04 mmol, 66%). TLC: Rf = 0.32 (CH2Cl2/CH3OH = 95/5). 1H NMR (600 MHz, DMSO-d6): δ (ppm) 7.58–7.61 (m, 2H, 3/5-Hbenzoyl), 7.67–7.72 (m, 1H, 4-Hbenzoyl), 7.95 (br s, 2H, NH2), 8.11–8.15 (m, 2H, 2/6-Hbenzoyl), 8.74 (d, J = 2.5 Hz, 1H, 5-Hpyrazinyl), 8.75 (dd, J = 2.5/1.5 Hz, 1H, 6-Hpyrazinyl), 9.18 (d, J = 1.5 Hz, 1H, 3-Hpyrazinyl). 13C NMR (151 MHz, DMSO-d6): δ (ppm) 128.2 (2C, C-3/5benzoyl), 130.8 (2C, C-2/6benzoyl), 131.9 (1C, C-1benzoyl), 133.1 (1C, C-4benzoyl), 143.4 (1C, C-3pyrazinyl), 144.6 (1C, C-2pyrazinyl), 144.7 (1C, C-6pyrazinyl), 145.6 (1C, C-5pyrazinyl), 157.6 (1C, C-3triazole), 159.1 (1C, C-5triazole), 167.8 (1C, CON). IR (neat): ν̃ [cm–1] 3460, 3271, 3062, 1693, 1639, 1600, 1543, 1450, 1361, 1327, 1149, 1014, 975, 848. HRMS (APCI): m/z = 267.0989 calcd for [M + H]+; found, 267.0972. HPLC: tR = 16.4 min, purity: 98.5%.
3-(5-Amino-1-benzoyl-1H-1,2,4-triazol-3-yl)pyrazine-2-carboxamide (5a)
According to general procedure B, aminotriazole 21a (70.0 mg, 341 μmol) was acylated using benzoic acid (41.7 mg, 341 μmol), DMAP (83.4 mg, 682 μmol), and EDCI·HCl (131 mg, 682 μmol) in dry DMF (2.2 mL). 5a was obtained as a colorless solid (75.0 mg, 242 μmol, 71%). TLC: Rf = 0.42 (CH2Cl2/CH3OH = 93/7). 1H NMR (600 MHz, DMSO-d6): δ (ppm) 7.58–7.63 (m, 2H, 3/5-Hbenzoyl), 7.67–7.71 (m, 1H, 4-Hbenzoyl), 7.74 (br s, 1H, CONH2), 7.86 (br s, 2H, NH2), 8.11–8.14 (m, 2H, 2/6-Hbenzoyl), 8.15 (br s, 1H, CONH2), 8.73 (d, J = 2.5 Hz, 1H, 6-Hpyrazinyl), 8.82 (d, J = 2.5 Hz, 1H, 5-Hpyrazinyl). 13C NMR (151 MHz, DMSO-d6): δ (ppm) 128.2 (2C, C-3/5benzoyl), 131.0 (2C, C-2/6benzoyl), 131.6 (1C, C-1benzoyl), 133.2 (1C, C-4benzoyl), 142.9 (1C, C-3pyrazinyl), 143.6 (1C, C-6pyrazinyl), 144.7 (1C, C-5pyrazinyl), 149.4 (1C, C-2pyrazinyl), 158.2 (1C, C-3triazole), 158.6 (1C, C-5triazole), 167.4 (1C, CONH2), 167.6 (1C, CONbenzoyl). IR (neat): ν̃ [cm–1] 3441, 3402, 3286, 1712, 1685, 1639, 1546, 1400, 1330, 1311, 1107, 975, 914, 756, 717. HRMS (APCI): m/z = 310.1047 calcd for [M + H]+; found, 310.1049. HPLC: tR = 11.6 min, purity: 96.9%.
3-(5-Amino-1-benzoyl-1H-1,2,4-triazol-3-yl)-N-methylpyrazine-2-carboxamide (5b)
According to general procedure A, aminotriazole 21b (50.0 mg, 228 μmol) was acylated using BzCl (26.5 μL, 228 μmol, 1.00 equiv) in dry pyridine/THF (2.1 mL/1.05 mL) and stirred first for 1 h at 0 °C and then for 1.5 h at rt. Flash column chromatography (CH2Cl2/CH3OH = 1/0 → 9/1) yielded 5b as a colorless solid (18 mg, 56 μmol, 24%). TLC: Rf = 0.38 (CH2Cl2/CH3OH = 93/7). 1H NMR (600 MHz, DMSO-d6): δ (ppm) 2.71 (d, J = 4.7 Hz, 3H, CH3), 7.54–7.58 (m, 2H, 3/5-Hbenzoyl), 7.67–7.71 (m, 1H, 4-Hbenzoyl), 7.86 (br s, 2H, NH2), 8.07–8.10 (m, 2H, 2/6-Hbenzoyl), 8.59 (q, J = 4.7 Hz, 1H, CONH), 8.73 (d, J = 2.5 Hz, 1H, 6-Hpyrazinyl), 8.83 (d, J = 2.5 Hz, 1H, 5-Hpyrazinyl). 13C NMR (151 MHz, DMSO-d6): δ (ppm) 25.9 (1C, CH3), 128.1 (2C, C-3/5benzoyl), 130.9 (2C, C-2/6benzoyl), 131.7 (1C, C-1benzoyl), 133.2 (1C, C-4benzoyl), 142.7 (1C, C-3pyrazinyl), 143.7 (1C, C-6pyrazinyl), 144.8 (1C, C-5pyrazinyl), 149.0 (1C, C-2pyrazinyl), 158.1 (1C, C-3triazole), 158.6 (1C, C-5triazole), 165.8 (1C, CONH), 167.6 (1C, CONbenzoyl). IR (neat): ν̃ [cm–1] 3456, 3271, 3093, 1685, 1651, 1442, 1354, 1327, 1161, 1111, 975, 921, 798, 748. HRMS (APCI): m/z = 324.1203 calcd for [M + H]+; found, 324.1193. HPLC: tR = 12.7 min, purity: 98.1%.
3-(5-Amino-1-benzoyl-1H-1,2,4-triazol-3-yl)-N-ethylpyrazine-2-carboxamide (5c)
According to general procedure B, aminotriazole 21c (80.0 mg, 343 μmol) was acylated using benzoic acid (41.9 mg, 343 μmol), DMAP (83.8 mg, 686 μmol), and EDCI·HCl (131 mg, 686 μmol) in dry DMF (2.2 mL). 5c was obtained as a colorless solid (79 mg, 234 μmol, 68%). TLC: Rf = 0.26 (CH2Cl2/CH3OH = 93/7). 1H NMR (400 MHz, DMSO-d6): δ (ppm) 1.00 (t, J = 7.2 Hz, 3H, CH3), 3.20 (qd, J = 7.2/5.5 Hz, 2H, CH2), 7.51–7.59 (m, 2H, 3/5-Hbenzoyl), 7.65–7.71 (m, 1H, 4-Hbenzoyl), 7.84 (br s, 2H, NH2), 8.06–8.13 (m, 2H, 2/6-Hbenzoyl), 8.60 (t, J = 5.6 Hz, 1H, CONH), 8.73 (d, J = 2.5 Hz, 1H, 6-Hpyrazinyl), 8.82 (d, J = 2.5 Hz, 1H, 5-Hpyrazinyl). 13C NMR (101 MHz, DMSO-d6): δ (ppm) 14.3 (1C, CH3), 33.7 (1C, CH2), 128.1 (2C, C-3/5benzoyl), 130.8 (2C, C-2/6benzoyl), 131.7 (1C, C-1benzoyl), 133.2 (1C, C-4benzoyl), 142.8 (1C, C-3pyrazinyl), 143.6 (1C, C-6pyrazinyl), 144.7 (1C, C-5pyrazinyl), 149.1 (1C, C-2pyrazinyl), 158.1 (1C, C-3triazole), 158.6 (1C, C-5triazole), 165.0 (1C, CONH), 167.6 (1C, CONbenzoyl). IR (neat): ν̃ [cm–1] 3456, 3278, 3109, 3062, 2974, 1689, 1654, 1631, 1346, 1323, 1184, 1114, 975, 918, 748. HRMS (APCI): m/z = 338.1360 calcd for [M + H]+; found, 338.1370. HPLC: tR = 13.8 min, purity: 99.2%.
3-(5-Amino-1-benzoyl-1H-1,2,4-triazol-3-yl)-N-propylpyrazine-2-carboxamide (5d)
According to general procedure B, aminotriazole 21d (85.0 mg, 344 μmol) was acylated using benzoic acid (42.0 mg, 344 μmol), DMAP (84.0 mg, 680 μmol), and EDCI·HCl (132 mg, 680 μmol) in dry DMF (2.2 mL). 5d was obtained as a colorless solid (83mg, 236 μmol, 69%). TLC: Rf = 0.23 (CH2Cl2/CH3OH = 93/7). 1H NMR (600 MHz, DMSO-d6): δ (ppm) 0.79 (t, J = 7.4 Hz, 3H, CH3), 1.38 (h, J = 7.3 Hz, 2H, 2-Hpropyl), 3.07–3.13 (m, 2H, 1-Hbutyl), 7.51–7.58 (m, 2H, 3/5-Hbenzoyl), 7.66–7.72 (m, 1H, 4-Hbenzoyl), 7.84 (br s, 2H, NH2), 8.07–8.12 (m, 2H, 2/6-Hbenzoyl), 8.59 (t, J = 5.8 Hz, 1H, CONH), 8.74 (d, J = 2.4 Hz, 1H, 6-Hpyrazinyl), 8.82 (d, J = 2.4 Hz, 1H, 5-Hpyrazinyl). 13C NMR (151 MHz, DMSO-d6): δ (ppm) 11.3 (1C, CH3), 22.0 (1C, C-2propyl), 40.6 (1C, C-1propyl), 128.1 (2C, C-3/5benzoyl), 130.9 (2C, C-2/6benzoyl), 131.7 (1C, C-1benzoyl), 133.2 (1C, C-41benzoyl), 142.8 (1C, C-3pyrazinyl), 143.7 (1C, C-6pyrazinyl), 144.7 (1C, C-5pyrazinyl), 149.2 (1C, C-2pyrazinyl), 158.1 (1C, C-3triazole), 158.6 (1C, C-5triazole), 165.2 (1C, CONH), 167.6 (1C, CONbenzoyl). IR (neat): ν̃ [cm–1] 3456, 3275, 3078, 3066, 2970, 1681, 1651, 1631, 1535, 1346, 1323, 1184, 975, 918, 764, 748. HRMS (APCI): m/z = 352.1516 calcd for [M + H]+; found, 352.1500. HPLC: tR = 15.1 min, purity: 98.0%.
3-(5-Amino-1-benzoyl-1H-1,2,4-triazol-3-yl)-N-butylpyrazine-2-carboxamide (5e)
According to general procedure A, aminotriazole 21e (50.0 mg, 143 μmol) was acylated using BzCl (25.1 μL, 216 μmol, 1.20 equiv) in dry pyridine/THF (1.50 mL/0.75 mL). 5e was obtained as a colorless solid (41 mg, 112 μmol, 62%). TLC: Rf = 0.45 (CH2Cl2/CH3OH = 93/7). 1H NMR (600 MH, DMSO-d6): δ (ppm) 0.82 (t, J = 7.4 Hz, 3H, CH3), 1.19–1.26 (m, 2H, 3-Hbutyl), 1.33–1.39 (m, 2H, 2-Hbutyl), 3.14 (td, J = 7.1/5.7 Hz, 2H, 1-Hbutyl), 7.52–7.57 (m, 2H, 3/5-Hbenzoyl), 7.67–7.71 (m, 1H, 4-Hbenzoyl), 7.85 (br s, 2H, NH2), 8.08–8.12 (m, 2H, 2/6-Hbenzoyl), 8.56 (t, J = 5.7 Hz, 1H, CONH), 8.74 (d, J = 2.5 Hz, 1H, 6-Hpyrazinyl), 8.82 (d, J = 2.5 Hz, 1H, 5-Hpyrazinyl). 13C NMR (151 MHz, DMSO-d6): δ (ppm) 13.6 (1C, CH3), 19.5 (1C, C-3butyl), 30.8 (1C, C-2butyl), 38.5 (1C, C-1butyl), 128.1 (2C, C-3/5benzoyl), 130.9 (2C, C-2/6benzoyl), 131.6 (1C, C-1benzoyl), 133.2 (1C, C-4benzoyl), 142.8 (1C, C-3pyrazinyl), 143.7 (1C, C-6pyrazinyl), 144.7 (1C, C-5pyrazinyl), 149.3 (1C, C-2pyrazinyl), 158.1 (1C, C-3triazole), 158.7 (1C, C-5triazole), 165.1 (1C, CONH), 167.5 (1C, CONbenzoyl). IR (neat): ν̃ [cm–1] 3456, 3267, 3074, 3062, 2958, 1685, 1651, 1635, 1535, 1446, 1354, 1327, 1315, 1184, 1111, 1072, 975, 921, 748. HRMS (APCI): m/z = 366.1673 calcd for [M + H]+; found, 366.1688. HPLC: tR = 16.4 min, purity: 99.2%.
3-(5-Amino-1-benzoyl-1H-1,2,4-triazol-3-yl)-N-pentylpyrazine-2-carboxamide (5f)
According to general procedure B, aminotriazole 21f (95.0 mg, 345 μmol) was acylated using benzoic acid (42.1 mg, 345 μmol), DMAP (84.3 mg, 690 μmol), and EDCI·HCl (132 mg, 690 μmol) in dry DMF (2.2 mL). 5f was obtained as a colorless solid (77 mg, 203 μmol, 59%). TLC: Rf = 0.27 (CH2Cl2/CH3OH = 93/7). 1H NMR (600 MHz, DMSO-d6): δ (ppm) 0.83 (t, J = 7.1 Hz, 3H, CH3), 1.14–1.27 (m, 4H, 3/4-Hpentyl), 1.34–1.40 (m, 2H, 2-Hpentyl), 3.13 (td, J = 7.3/5.8 Hz, 2H, 1-Hpentyl), 7.52–7.58 (m, 2H, 3/5-Hbenzoyl), 7.66–7.72 (m, 1H, 4-Hbenzoyl), 7.85 (br s, 2H, NH2), 8.09–8.14 (m, 2H, 2/6-Hbenzoyl), 8.56 (t, J = 5.7 Hz, 1H, CONH), 8.74 (d, J = 2.5 Hz, 1H, 6-Hpyrazinyl), 8.82 (d, J = 2.5 Hz, 1H, 5-Hpyrazinyl). 13C NMR (151 MHz, DMSO-d6): δ (ppm) 13.9 (1C, CH3), 21.8 (1C, C-3/4pentyl), 28.4 (1C, C-2pentyl), 28.6 (1C, C-3/4pentyl), 38.8 (1C, C-1pentyl), 128.1 (2C, C-3/5benzoyl), 130.9 (2C, C-2/5benzoyl), 131.6 (1C, C-1benzoyl), 133.2 (1C, C-4benzoyl), 142.7 (1C, C-3pyrazinyl), 143.7 (1C, C-6pyrazinyl), 144.7 (1C, C-5pyrazinyl), 149.3 (1C, C-2pyrazinyl), 158.1 (1C, C-3triazole), 158.7 (1C, C-5triazole), 165.1 (1C, CONH), 167.5 (1C, CONbenzoyl). IR (neat): ν̃ [cm–1] 3456, 3271, 3097, 3074, 2954, 2927, 2866 1685, 1651, 1539, 1354, 1327, 1184, 1111, 975, 921, 79, 748. HRMS (APCI): m/z = 380.1829 calcd for [M + H]+; found, 380.1832. HPLC: tR = 17.7 min, purity: 99.4%.
3-(5-Amino-1-benzoyl-1H-1,2,4-triazol-3-yl)-N-isopropylpyrazine-2-carboxamide (5g)
According to general procedure B, aminotriazole 21g (85.0 mg, 344 μmol) was acylated using benzoic acid (42.0 mg, 344 μmol), DMAP (84.0 mg, 688 μmol), and EDCI·HCl (132 mg, 688 μmol) in dry DMF (2.2 mL). Flash column chromatography (CH2Cl2/CH3OH = 1/0 → 92/8) yielded 5g as a colorless solid (53 mg, 151 μmol, 44%). TLC: Rf = 0.25 (CH2Cl2/CH3OH = 93/7). 1H NMR (600 MHz, DMSO-d6): δ (ppm) 1.00 (d, J = 6.6 Hz, 6H, (CH3)2), 3.90–4.00 (m, 1H, CH), 7.51–7.58 (m, 2H, 3/5-Hbenzoyl), 7.65–7.70 (m,1H, 4-Hbenzoyl), 7.83 (br s, 2H, NH2), 8.07–8.12 (m, 2H, 2/6-Hbenzoyl), 8.40 (d, J = 7.9 Hz, 1H, CONH), 8.73 (d, J = 2.4 Hz, 1H, 6-Hpyrazinyl), 8.82 (d, J = 2.5 Hz, 1H, 5-Hpyrazinyl). 13C NMR (151 MHz, DMSO-d6): δ (ppm) 22.0 (2C, (CH3)2), 40.7 (1C, CH), 128.1 (2C, C-3/5benzoyl), 130.8 (2C, C-2/6benzoyl), 131.7 (1C, C-1benzoyl), 133.2 (1C, C-4benzoyl), 142.7 (1C, C-3pyrazinyl), 143.7 (1C, C-6pyrazinyl), 144.6 (1C, C-5pyrazinyl), 149.3 (1C, C-2pyrazinyl), 158.1 (1C, C-3triazole), 158.6 (1C, C-5triazole), 164.3 (1C, CONH), 167.7 (1C, CONbenzoyl). IR (neat): ν̃ [cm–1] 3387, 3059, 2970, 1701, 1666, 1651, 1512, 1446, 1338, 1319, 1168, 1095, 979, 921, 759, 713. HRMS (APCI): m/z = 352.1516 calcd for [M + H]+; found, 352.1526. HPLC: tR = 14.8 min, purity: 98.9%.
3-(5-Amino-1-benzoyl-1H-1,2,4-triazol-3-yl)-N-(tert-butyl)pyrazine-2-carboxamide (5h)
According to general procedure A, aminotriazole 21h (50.0 mg, 191 μmol) was acylated using BzCl (22.2 μL, 191 μmol, 1.00 equiv) in dry pyridine/THF (1.80 mL/0.90 mL). Flash column chromatography (CH2Cl2/CH3OH = 1/0 → 92/8) yielded 5h as a colorless solid (42 mg, 115 μmol, 60%). TLC: Rf = 0.40 (CH2Cl2/CH3OH = 93/7). 1H NMR (600 MHz, DMSO-d6): δ (ppm) 1.18 (s, 9H, C(CH3)3), 7.51–7.55 (m, 2H, 3/5-Hbenzoyl), 7.65–7.70 (m, 1H, 4-Hbenzoyl), 7.81 (br s, 2H, NH2), 8.08 (br s, 1H, CONH), 8.08–8.11 (m, 2H, 2/6-Hbenzoyl), 8.70 (d, J = 2.4 Hz, 1H, 6-Hpyrazinyl), 8.79 (d, J = 2.5 Hz, 1H, 5-Hpyrazinyl). 13C NMR (151 MHz, DMSO-d6): δ (ppm) 28.1 (3C, C(CH3)3), 50.7 (1C, C(CH3)), 128.1 (2C, C-3/5benzoyl), 130.8 (2C, C-2/6benzoyl), 131.8 (1C, C-1benzoyl), 133.1 (1C, C-4benzoyl), 142.8 (1C, C-3pyrazinyl), 143.6 (1C, C-6pyrazinyl), 144.3 (1C, C-5pyrazinyl), 149.9 (1C, C-2pyrazinyl), 158.4 (1C, C-3triazole), 158.6 (1C, C-5triazole), 164.6 (1C, CONH), 167.7 (1C, CONbenzoyl). IR (neat): ν̃ [cm–1] 3398, 3379, 3059, 2970, 1693, 1674, 1651, 1523, 1446, 1354, 1319, 1180, 1157, 1099, 979, 925, 864, 806, 763. HRMS (APCI): m/z = 366.1673 calcd for [M + H]+; found, 366.1694. HPLC: tR = 16.3 min, purity: 99.5%.
3-(5-Amino-1-benzoyl-1H-1,2,4-triazol-3-yl)-N-isobutylpyrazine-2-carboxamide (5i)
According to general procedure B, aminotriazole 21i (90.0 mg, 344 μmol) was acylated using benzoic acid (42.1 mg, 344 μmol), DMAP (84.2 mg, 689 μmol), and EDCI·HCl (132 mg, 689 μmol) in dry DMF (2.2 mL). 5i was obtained as a colorless solid (72 mg, 197 μmol, 57%). TLC: Rf = 0.27 (CH2Cl2/CH3OH = 93/7). 1H NMR (600 MHz, DMSO-d6): δ (ppm) 0.79 (d, J = 6.7 Hz, 6H, (CH3)2), 1.63–1.73 (m, 1H, CH), 2.96 (t, J = 6.4 Hz, 2H, CH2), 7.52–7.56 (m, 2H, 3/5-Hbenzoyl), 7.66–7.72 (m, 1H, 4-Hbenzoyl), 7.84 (br s, 2H, NH2), 8.07–8.11 (m, 2H, 2/6-Hbenzoyl), 8.58 (t, J = 6.0 Hz, 1H, CONH), 8.74 (d, J = 2.5 Hz, 1H, 6-Hpyrazinyl), 8.82 (d, J = 2.5 Hz, 1H, 5-Hpyrazinyl). 13C NMR (151 MHz, DMSO-d6): δ (ppm) 20.0 (2C, (CH3)2), 27.9 (1C, CH), 46.4 (1C, CH2), 128.1 (2C, C-3/5benzoyl), 130.9 (2C, C-2/6benzoyl), 131.7 (1C, C-1benzoyl), 133.2 (1C, C-4benzoyl), 142.7 (1C, C-3pyrazinyl), 143.7 (1C, C-6pyrazinyl), 144.6 (1C, C-5pyrazinyl), 149.4 (1C, C-2pyrazinyl), 158.1 (1C, C-3triazole), 158.7 (1C, C-5triazole), 165.4 (1C, CONH), 167.6 (1C, CONbenzoyl). IR (neat): ν̃ [cm–1] 3456, 3267, 3074, 2962, 1685, 1651, 1635, 1539, 1446, 1350, 1327, 1188, 1111, 975, 921, 798, 748. HRMS (APCI): m/z = 366.1673 calcd for [M + H]+; found, 366.1673. HPLC: tR = 16.2 min, purity: 99.2%.
3-(5-Amino-1-benzoyl-1H-1,2,4-triazol-3-yl)-N-isopentylpyrazine-2-carboxamide (5j)
According to general procedure B, aminotriazole 21j (95.0 mg, 345 μmol) was acylated using benzoic acid (42.1 mg, 345 μmol), DMAP (84.3 mg, 690 μmol), and EDCI·HCl (132 mg, 690 μmol) in dry DMF (2.2 mL). 5j was obtained as a colorless solid (107 mg, 282 μmol, 82%). TLC: Rf = 0.42 (CH2Cl2/CH3OH = 93/7). 1H NMR (400 MHz, DMSO-d6): δ (ppm) 0.82 (d, J = 6.6 Hz, 6H, (CH3)2), 1.25–1.32 (m, 2H, 2-Hisopentyl), 1.46–1.60 (m, 1H, CH), 3.16 (dt, J = 8.0/5.9 Hz, 2H, 1-Hisopentyl), 7.51–7.58 (m, 2H, 3/5-Hbenzoyl), 7.64–7.73 (m, 1H, 4-Hbenzoyl), 7.85 (br s, 2H, NH2), 8.08–8.14 (m, 2H, 2/6-Hbenzoyl), 8.54 (t, J = 5.7 Hz, 1H, CONH), 8.74 (d, J = 2.5 Hz, 1H, 6-Hpyrazinyl), 8.82 (d, J = 2.5 Hz, 1H, 5-Hpyrazinyl). 13C NMR (101 MHz, DMSO-d6): δ (ppm) 22.3 (2C, (CH3)2), 25.1 (1C, CH), 37.1 (1C, C-1isopentyl), 37.6 (1C, C-2isopentyl), 128.1 (2C, C-3/5benzoyl), 130.9 (2C, C-2/6benzoyl), 131.6 (1C, C-1benzoyl), 133.2 (1C, C-4benzoyl), 142.8 (1C, C-3pyrazinyl), 143.7 (1C, C-6pyrazinyl), 144.6 (1C, C-5pyrazinyl), 149.2 (1C, C-2pyrazinyl), 158.1 (1C, C-3triazole), 158.7 (1C, C-5triazole), 165.1 (1C, CONH), 167.5 (1C, CONbenzoyl). IR (neat): ν̃ [cm–1] 3456, 3267, 3062, 2958, 1685, 1651, 1539, 1442, 1354, 1323, 1184, 1111, 975, 921, 748. HRMS (APCI): m/z = 380.1829 calcd for [M + H]+; found, 380.1845. HPLC: tR = 17.5 min, purity: 98.6%.
N-Allyl-3-(5-amino-1-benzoyl-1H-1,2,4-triazol-3-yl)pyrazine-2-carboxamide (5k)
According to general procedure B, aminotriazole 21k (84.0 mg, 343 μmol) was acylated using benzoic acid (41.8 mg, 343 μmol), DMAP (83.7 mg, 685 μmol), and EDCI·HCl (131 mg, 685 μmol) in dry DMF (2.2 mL). 5k was obtained as a colorless solid (101 mg, 295 μmol, 86%). TLC: Rf = 0.30 (CH2Cl2/CH3OH = 93/7). 1H NMR (600 MHz, DMSO-d6): δ (ppm) 3.77–3.82 (m, 2H, CH2), 4.97–5.02 (m, 1H, HB), 5.13–5.19 (m, 1H, HC), 5.76 (ddt, J = 17.2/10.4/5.2 Hz, 1H, HA), 7.53–7.58 (m, 2H, 3/5-Hbenzoyl), 7.67–7.71 (m, 1H, 4-Hbenzoyl), 7.85 (br s, 2H, NH2), 8.07–8.11 (m, 2H, 2/6-Hbenzoyl), 8.75 (d, J = 2.5 Hz, 1H, 6-Hpyrazinyl), 8.79 (t, J = 5.8 Hz, 1H, CONH), 8.84 (d, J = 2.5 Hz, 1H, 5-Hpyrazinyl). 13C NMR (151 MHz, DMSO-d6): δ (ppm) 41.2 (1C, CH2), 115.3 (1C, CHBHC), 128.1 (2C, C-3/5benzoyl), 130.9 (2C, C-2/6benzoyl), 131.7 (1C, C-1benzoyl), 133.2 (1C, C-4benzoyl), 134.7 (1C, CHA), 142.8 (1C, C-3pyrazinyl), 143.7 (1C, C-3pyrazinyl), 144.8 (1C, C-6pyrazinyl), 149.0 (1C, C-2pyrazinyl), 158.1 (1C, C-3triazole), 158.7 (1C, C-5triazole), 165.2 (1C, CONH), 167.6 (1C, CONbenzoyl). IR (neat): ν̃ [cm–1] 3460, 3263, 3174, 1689, 1654, 1631, 1531, 1354, 1323, 1184, 1107, 937, 921, 794, 748. HRMS (APCI): m/z = 350.1360 calcd for [M + H]+; found, 350.1365. HPLC: tR = 14.6 min, purity: 98.4%.
3-(5-Amino-1-benzoyl-1H-1,2,4-triazol-3-yl)-N-(cyclopropylmethyl)pyrazine-2-carboxamide (5l)
According to general procedure B, aminotriazole 21l (90.0 mg, 347 μmol) was acylated using benzoic acid (42.4 mg, 347 μmol), DMAP (85.0 mg, 694 μmol), and EDCI·HCl (133 mg, 694 μmol) in dry DMF (2.2 mL). 5l was obtained as a colorless solid (101 mg, 278 μmol, 80%). TLC: Rf = 0.28 (CH2Cl2/CH3OH = 93/7). 1H NMR (400 MHz, DMSO-d6): δ (ppm) 0.10–0.16 (m, 2H, (CH2)cyclopropyl), 0.28–0.41 (m, 2H, (CH2)cyclopropyl), 0.80–0.92 (m, 1H, CH), 3.04 (t, J = 6.3 Hz, 2H, CH2), 7.50–7.57 (m, 2H, 3/5-Hbenzoyl, 2H), 7.64–7.71 (m, 1H, 4-Hbenzoyl), 7.83 (br s, 2H, NH2), 8.04–8.11 (m, 2H, 2/6-Hbenzoyl), 8.66 (t, J = 5.8 Hz, 1H, CONH), 8.74 (d, J = 2.5 Hz, 1H, 6-Hpyrazinyl), 8.83 (d, J = 2.4 Hz, 1H, 5-Hpyrazinyl). 13C NMR (101 MHz, DMSO-d6): δ (ppm) 3.2 (2C, (CH2)cyclopropyl), 10.5 (1C, CH), 43.1 (1C, CH2), 128.1 (2C, C-3/5benzoyl), 130.1 (2C, C-2/6benzoyl), 131.7 (1C, C-1benzoyl), 133.2 (1C, C-4benzoyl), 142.8 (1C, C-3pyrazinyl), 143.7 (1C, C-6pyrazinyl), 144.7 (1C, C-5pyrazinyl), 149.1 (1C, C-2pyrazinyl), 158.1 (1C, C-3triazole), 158.6 (1C, C-5triazole), 165.0 (1C, CONH), 167.6 (1C, CONbenzoyl). IR (neat): ν̃ [cm–1] 3460, 3282, 3074, 2920, 1685, 1651, 1631, 1535, 1354, 1323, 1184, 1107, 975, 918, 748. HRMS (APCI): m/z = 364.1516 calcd for [M + H]+; found, 364.1532. HPLC: tR = 15.5 min, purity: 98.3%.
3-(5-Amino-1-benzoyl-1H-1,2,4-triazol-3-yl)-N-cyclopentylpyrazine-2-carboxamide (5m)
According to general procedure B, aminotriazole 21m (95.0 mg, 348 μmol) was acylated using benzoic acid (42.5 mg, 348 μmol), DMAP (84.9 mg, 695 μmol), and EDCI·HCl (132 mg, 695 μmol) in dry DMF (2.2 mL). 5m was obtained as a colorless solid (72 mg, 197 μmol, 57%). TLC: Rf = 0.33 (CH2Cl2/CH3OH = 93/7). 1H NMR (600 MHz, DMSO-d6): δ (ppm) 1.31–1.39 (m, 2H, 2/5-Hcyclopentyl), 1.40–1.46 (m, 2H, 3/4-Hcyclopentyl), 1.50–1.58 (m, 2H, 3/4-Hcyclopentyl), 1.63–1.71 (m, 2H, 2/5-Hcyclopentyl), 4.08 (m, 1H, 1-Hcyclopentyl), 7.51–7.61 (m, 2H, 3/5-Hbenzoyl), 7.67–7.71 (m, 1H, 4-Hbenzoyl), 7.84 (br s, 2H, NH2), 8.07–8.13 (m, 2H, 2/6-Hbenzoyl), 8.46 (d, J = 7.4 Hz, 1H, CONH), 8.72 (d, J = 2.5 Hz, 1H, 6-Hpyrazinyl), 8.81 (d, J = 2.5 Hz, 1H, 5-Hpyrazinyl). 13C NMR (151 MHz, DMSO-d6): δ (ppm) 23.4 (2C, C-3/4cyclopentyl), 31.8 (2C, C-2/6cyclopentyl), 50.6 (1C, C-1cyclopentyl), 128.1 (2C, C-3/5benzoyl), 130.9 (2C, C-2/6benzoyl), 131.7 (1C, C-1benzoyl), 133.2 (1C, C-4benzoyl), 142.6 (1C, C-3pyrazinyl), 143.7 (1C, C-6pyrazinyl), 144.5 (1C, C-5pyrazinyl), 149.5 (1C, C-2pyrazinyl), 158.1 (1C, C-3triazole), 158.6 (1C, C-5triazole), 164.8 (1C, CONH), 167.6 (1C, CONbenzoyl). IR (neat): ν̃ [cm–1] 3456, 3263, 3182, 3105, 2954, 2866, 1681, 1647, 1631, 1535, 1446, 1346, 1327, 1199, 1138, 1111, 975, 921, 790, 748. HRMS (APCI): m/z = 378.1673 calcd for [M + H]+; found, 378.1665. HPLC: tR = 16.3 min, purity: 97.8%.
3-(5-Amino-1-benzoyl-1H-1,2,4-triazol-3-yl)-N-cyclohexylpyrazine-2-carboxamide (5n)
According to general procedure B, aminotriazole 21n (100 mg, 348 μmol) was acylated using benzoic acid (42.5 mg, 348 μmol), DMAP (85.0 mg, 696 μmol), and EDCI·HCl (133 mg, 696 μmol) in dry DMF (2.2 mL). 5n was obtained as a colorless solid (117 mg, 299 μmol, 86%). TLC: Rf = 0.26 (CH2Cl2/CH3OH = 93/7). 1H NMR (600 MHz, DMSO-d6): δ (ppm) 0.99–1.11 (m, 3H, 1/2/6-Hcyclohexyl), 1.12–1.22 (m, 2H, 3/5-Hcyclohexyl), 1.47–1.54 (m, 1H, 4-Hcyclohexyl), 1.54–1.62 (m, 4H, 2/3/5/6-Hcyclohexyl), 3.55–3.64 (m, 1H, 1-Hcyclohexyl), 7.51–7.57 (m, 1H, 3/5-Hbenzoyl), 7.66–7.72 (m, 1H, 4-Hbenzoyl), 7.83 (br s, 2H, NH2), 8.06–8.11 (m, 2H, 2/6-Hbenzoyl), 8.36 (d, J = 8.1 Hz, 1H, CONH), 8.72 (d, J = 2.5 Hz, 1H, 6-Hpyrazinyl), 8.81 (d, J = 2.5 Hz, 1H, 5-Hpyrazinyl). 13C NMR (151 MHz, DMSO-d6): δ (ppm) 24.6 (2C, C-3/5cyclohexyl), 25.1 (1C, C-4cyclohexyl), 31.9 (2C, C-2/6cyclohexyl), 47.8 (1C, C-1cyclohexyl), 128.1 (2C, C-3/5benzoyl), 130.9 (2C, C-2/6benzoyl), 131.7 (1C, C-1benzoyl), 133.2 (1C, C-4benzoyl), 142.5 (1C, C-3pyrazinyl), 143.7 (1C, C-6pyrazinyl), 144.5 (1C, C-5pyrazinyl), 149.5 (1C, C-2pyrazinyl), 158.0 (1C, C-3triazole), 158.6 (1C, C-5triazole), 164.3 (1C, CONH), 167.6 (1C, CONbenzoyl). IR (neat): ν̃ [cm–1] 3456, 3267, 3074, 2927, 2846, 1681, 1647, 1535, 1446, 1327, 1311, 1184, 1111, 975, 918, 748. HRMS (APCI): m/z = 392.1829 calcd for [M + H]+; found, 392.1826. HPLC: tR = 17.3 min, purity: 99.6%.
3-(5-Amino-1-benzoyl-1H-1,2,4-triazol-3-yl)-N-cycloheptylpyrazine-2-carboxamide (5o)
According to general procedure B, aminotriazole 21o (105 mg, 348 μmol) was acylated using benzoic acid (42.6 mg, 348 μmol), DMAP (85.1 mg, 697 μmol), and EDCI·HCl (132 mg, 697 μmol) in dry DMF (2.2 mL). 5o was obtained as a colorless solid (123 mg, 303 μmol, 87%). TLC: Rf = 0.42 (CH2Cl2/CH3OH = 93/7). 1H NMR (600 MHz, DMSO-d6): δ (ppm) 1.24–1.31 (m, 2H, 3/4/5/6-Hcycloheptyl), 1.31–1.37 (m, 2H, 2/7-Hcycloheptyl), 1.46–1.56 (m, 2H, 3/4/5/6-Hcycloheptyl), 1.45–1.53 (m, 4H, 3/4/5/6-Hcycloheptyl), 1.61–1.73 (m, 2H, 2/7-Hcycloheptyl), 3.74–3.83 (m, 1H, 1-Hcycloheptyl), 7.51–7.58 (m, 2H, 3/5-Hbenzoyl), 7.66–7.72 (m, 1H, 4-Hbenzoyl), 7.84 (br s, 2H, NH2), 8.08–8.14 (m, 2H, 2/6-Hbenzoyl), 8.40 (d, J = 8.0 Hz, 1H, CONH), 8.72 (d, J = 2.5 Hz, 1H, 6-Hpyrazinyl), 8.81 (d, J = 2.4 Hz, 1H, 5-Hpyrazinyl). 13C NMR (151 MHz, DMSO-d6): δ (ppm) 23.7 (2C, C-3/4/5/6cycloheptyl), 27.6 (2C, C-3/4/5/6cycloheptyl), 33.8 (2C, C-2/7cycloheptyl), 50.0 (1C, C-1cycloheptyl), 128.1 (2C, C-3/5benzoyl), 130.9 (2C, C-2/6benzoyl), 131.6 (1C, C-1benzoyl), 133.2 (1C, C-4benzoyl), 142.5 (1C, C-3pyrazinyl), 143.7 (1C, C-6pyrazinyl), 144.5 (1C, C-5pyrazinyl), 149.6 (1C, C-2pyrazinyl), 158.1 (1C, C-3triazole), 158.7 (1C, C-5triazole), 164.0 (1C, CONH), 167.6 (1C, CONbenzoyl). IR (neat): ν̃ [cm–1] 3456, 3271, 3070, 2916, 2854, 1681, 1643, 1531, 1354, 1330, 1311, 1180, 975, 921, 794, 748. HRMS (APCI): m/z = 406.1986 calcd for [M + H]+; found, 406.2012. HPLC: tR = 18.3 min, purity: 95.8%.
3-(5-Amino-1-benzoyl-1H-1,2,4-triazol-3-yl)-N-(cyclohexylmethyl)pyrazine-2-carboxamide (5p)
According to general procedure B, aminotriazole 21p (105 mg, 348 μmol) was acylated using benzoic acid (42.6 mg, 348 μmol), DMAP (85.1 mg, 697 μmol), and EDCI·HCl (132 mg, 697 μmol) in dry DMF (2.2 mL). Flash column chromatography (CH2Cl2/CH3OH = 1/0 → 93/7) yielded 5p as a colorless solid (104 mg, 257 μmol, 74%). TLC: Rf = 0.27 (CH2Cl2/CH3OH = 93/7). 1H NMR (600 MHz, DMSO-d6): δ (ppm) 0.76–0.85 (m, 2H, 2/6-Hcyclohexyl), 1.04–1.15 (m, 3H, 3/4/5-Hcyclohexyl), 1.35–1.44 (m, 1H, 1-Hcyclohexyl), 1.53–1.64 (m, 5H, 2/3/4/5/6-Hcyclohexyl), 2.98 (dd, J = 7.0/5.9 Hz, 2H, CH2), 7.52–7.58 (m, 2H, 3/5-Hbenzoyl), 7.66–7.72 (m, 1H, 4-Hbenzoyl), 7.85 (br s, 2H, NH2), 8.10–8.13 (m, 2H, 2/6-Hbenzoyl), 8.54 (t, J = 5.9 Hz, 1H, NH), 8.74 (d, J = 2.5 Hz, 1H, 6-Hpyrazinyl), 8.82 (d, J = 2.4 Hz, 1H, 5-Hpyrazinyl). 13C NMR (151 MHz, DMSO-d6): δ (ppm) 25.3 (2C, C-3/5cyclohexyl), 26.0 (1C, C-4cyclohexyl), 30.3 (2C, C-2/6cyclohexyl), 37.1 (1C, C-1cyclohexyl), 45.0 (1C, CH2), 128.1 (2C, C-3/5benzoyl), 131.0 (2C, C-2/6benzoyl), 131.6 (1C, C-1benzoyl), 133.2 (1C, C-4benzoyl), 142.7 (1C, C-3pyrazinyl), 143.8 (1C, C-6pyrazinyl), 144.6 (1C, C-5pyrazinyl), 149.5 (1C, C-2pyrazinyl), 158.2 (1C, C-3triazole), 158.7 (1C, C-5triazole), 165.3 (1C, CONH), 167.5 (1C, CONbenzoyl). IR (neat): ν̃ [cm–1] 3456, 3267, 3074, 2920, 2850, 1681, 1651, 1535, 1346, 1327, 1315, 1184, 1111, 975, 921, 794, 748. HRMS (APCI): m/z = 406.1986 calcd for [M + H]+; found, 406.2017. HPLC: tR = 18.6 min, purity: 99.2%.
(3-(5-Amino-1-benzoyl-1H-1,2,4-triazol-3-yl)pyrazin-2-yl)(piperidin-1-yl)methanone (5q)
According to general procedure A, aminotriazole 21q (50.0 mg, 183 μmol) was acylated using BzCl (21.3 μL, 183 μmol, 1.00 equiv) in dry pyridine/THF (1.70 mL/0.85 mL). Flash column chromatography (1. CH/EtOAc = 1/0 → 2/8, 2. CH2Cl2/CH3OH = 1/0 → 93/7) yielded amide 5q as a colorless solid (47 mg, 125 μmol, 68%). TLC: Rf = 0.35 (CH2Cl2/CH3OH = 93/7). 1H NMR (600 MHz, DMSO-d6): δ (ppm) 1.28–1.34 (m, 4H, 3/4/5-Hpiperidinyl), 1.41–1.47 (m, 2H, 3/4/5-Hpiperidinyl), 2.98–3.02 (m, 2H, 2/6-Hpiperidinyl), 3.30–3.34 (d, 2H, 2/6-Hpiperidinyl), 7.56–7.59 (m, 2H, 3/5-Hbenzoyl), 7.68–7.71 (m, 1H, 4-Hbenzoyl), 7.88 (br s, 2H, NH2), 8.03–8.08 (m, 2H, 2/6-Hbenzoyl), 8.72 (d, J = 2.5 Hz, 1H, 6-Hpyrazinyl), 8.79 (d, J = 2.5 Hz, 1H, 5-Hpyrazinyl). 13C NMR (151 MHz, DMSO-d6): δ (ppm) 23.8 (1C, C-3/4/5piperidinyl), 24.5 (1C, C-3/4/5piperidinyl), 25.1 (1C, C-3/4/5piperidinyl), 41.4 (1C, C-2/6piperidinyl), 46.9 (1C, C-2/6piperidinyl), 128.2 (2C, C-3/5benzoyl), 130.7 (2C, C-2/6benzoyl), 131.8 (1C, C-1benzoyl), 133.1 (1C, C-4benzoyl), 141.5 (1C, C-3pyrazinyl), 144.2 (1C, C-5pyrazinyl), 144.3 (1C, C-6pyrazinyl), 149.7 (1C, C-6pyrazinyl), 157.5 (1C, C-3triazole), 158.8 (1C, C-5triazole), 164.9 (1C, CONpiperidinyl), 167.8 (1C, CONbenzoyl). IR (neat): ν̃ [cm–1] 3460, 3425, 3066, 2978, 2935, 1693, 1639, 1446, 1369, 1284, 1222, 1099, 937, 891, 856, 752. HRMS (APCI): m/z = 378.1673 calcd for [M + H]+; found, 378.1664. HPLC: tR = 16.6 min, purity: 97.7%.
(3-(5-Amino-1-benzoyl-1H-1,2,4-triazol-3-yl)pyrazin-2-yl)(morpholino)methanone (5r)
According to general procedure A, aminotriazole 21r (50.0 mg, 182 μmol) was acylated using BzCl (21.1 μL, 182 μmol, 1.00 equiv) in dry pyridine/THF (1.70 mL/0.85 mL). Flash column chromatography (CH2Cl2/CH3OH = 1/0 → 9/1) yielded amide 5r as a colorless solid (40 mg, 105 μmol, 58%). TLC: Rf = 0.24 (CH2Cl2/CH3OH = 93/7). 1H NMR (600 MHz, DMSO-d6): δ (ppm) 3.05–3.08 (m, 2H, 3/5-Hmorpholinyl), 3.37–3.41 (m, 6H, 2/3/5/6-Hmorpholinyl), 7.55–7.60 (m, 2H, 3/5-Hbenzoyl), 7.67–7.72 (m, 1H, 4-Hbenzoyl), 7.90 (br s, 2H, NH2), 8.04–8.06 (m, 2H, 2/6-Hbenzoyl), 8.74 (d, J = 2.5 Hz, 1H, 6-Hpyrazinyl), 8.81 (d, J = 2.4 Hz, 1H, 5-Hpyrazinyl). 13C NMR (151 MHz, DMSO-d6): δ (ppm) 41.2 (1C, C-3/5morpholinyl), 46.3 (1C, C-3/5morpholinyl), 65.3 (2C, C-2/6morpholinyl), 65.5 (1C, C-2/6morpholinyl), 128.2 (2C, C-3/5benzoyl), 130.7 (2C, C-2/6benzoyl), 131.8 (1C, C-1benzoyl), 133.1 (1C, C-4benzoyl), 141.5 (1C, C-3pyrazinyl), 144.4 (1C, C-6pyrazinyl), 144.5 (1C, C-5pyrazinyl), 148.9 (1C, C-2pyrazinyl), 157.3 (1C, C-3triazole), 158.8 (1C, C-5triazole), 165.4 (1C, CONmorpholinyl), 167.8 (1C, CONbenzoyl). IR (neat): ν̃ [cm–1] 3464, 3267, 3059, 2978, 2862, 1697, 1631, 1442, 1346, 1327, 1188, 1161, 1107, 1018, 921, 748, 709. HRMS (APCI): m/z = 380.1466 calcd for [M + H]+; found, 380.1467. HPLC: tR = 13.8 min, purity: 97.8%.
(3-(5-Amino-1-benzoyl-1H-1,2,4-triazol-3-yl)pyrazin-2-yl)(4-phenylpiperazin-1-yl)methanone (5s)
According to general procedure A, aminotriazole 21s (50.0 mg, 143 μmol) was acylated using BzCl (16.6 μL, 143 μmol, 1.00 equiv) in dry pyridine/THF (1.40 mL/0.7 mL). Flash column chromatography (CH2Cl2/CH3OH = 1/0 → 95/5) yielded amide 5s as a colorless solid (52 mg, 114 μmol, 80%). TLC: Rf = 0.34 (CH2Cl2/CH3OH = 93/7). 1H NMR (600 MHz, DMSO-d6): δ (ppm) 2.94–2.99 (m, 4H, 3/5-Hpiperazinyl), 3.18–3.22 (m, 2H, 2/6-Hpiperazinyl), 3.52–3.56 (m, 2H, 2/6-Hpiperazinyl), 6.78–6.82 (m, 1H, 4-Hphenyl), 6.84–6.87 (m, 2H, 2/6-Hphenyl), 7.19–7.23 (m, 2H, 3/5-Hphenyl), 7.50–7.54 (m, 2H, 3/5-Hbenzoyl), 7.57–7.61 (m, 1H, 4-Hbenzoyl), 7.88 (br s, 2H, NH2), 8.02–8.04 (m, 2H, 2/6-Hbenzoyl), 8.76 (d, J = 2.5 Hz, 1H, 6-Hpyrazinyl), 8.83 (d, J = 2.5 Hz, 1H, 5-Hpyrazinyl). 13C NMR (151 MHz, DMSO-d6): δ (ppm) 40.6 (1C, C-2/6piperazinyl), 45.7 (1C, C-2/6piperazinyl), 47.6 (1C, C-3/5piperazinyl), 48.1 (1C, C-3/5piperazinyl), 115.9 (2C, C-2/6phenyl), 119.3 (1C, C-4phenyl), 128.2 (2C, C-3/5benzoyl), 129.0 (2C, C-3/5phenyl), 130.7 (2C, C-2/6benzoyl), 131.7 (1C, C-1benzoyl), 133.1 (1C, C-4benzoyl), 141.4 (1C, C-3pyrazinyl), 144.4 (1C, C-6pyrazinyl), 144.5 (1C, C-5pyrazinyl), 149.1 (1C, C-2pyrazinyl), 150.6 (1C, C-1phenyl), 157.3 (1C, C-3triazole), 158.8 (1C, C-5triazole), 165.3 (1C, CONpiperazinyl), 167.8 (1C, CONbenzoyl). IR (neat): ν̃ [cm–1] 3444, 3417, 3055, 2970, 2831, 1685, 1639, 1597, 1442, 1427, 1307, 1269, 1230, 1157, 1111, 1010, 921, 759, 752. HRMS (APCI): m/z = 455.1938 calcd for [M + H]+; found, 455.1932. HPLC: tR = 17.5 min, purity: 94.3%.
3-(5-Amino-1-benzoyl-1H-1,2,4-triazol-3-yl)-N-phenylpyrazine-2-carboxamide (5t)
According to general procedure B, aminotriazole 21t (96.0 mg, 341 μmol) was acylated using benzoic acid (41.7 mg, 341 μmol), DMAP (83.4 mg, 683 μmol), and EDCI·HCl (131 mg, 683 μmol) in dry DMF (2.2 mL). Flash column chromatography (1. CH3OH/CH2Cl2 = 1/0 → 9/1, 2. CH3OH/CH2Cl2 = 1/0 → 94/6) yielded 5t as a colorless solid (75 mg, 195 μmol, 57%). TLC: Rf = 0.40 (CH2Cl2/CH3OH = 93/7). 1H NMR (600 MHz, DMSO-d6): δ (ppm) 7.02–7.06 (m, 2H, 3/5-Hbenzoyl), 7.13–7.17 (m, 1H, 4-Hphenyl), 7.35–7.39 (m, 1H, 3/5-Hphenyl), 7.43–7.47 (m, 1H, 4-Hbenzoyl), 7.71–7.74 (m, 2H, 2/6-Hphenyl), 7.90 (br s, 2H, NH2), 7.94–7.96 (m, 2H, 2/6-Hbenzoyl), 8.81 (d, J = 2.5 Hz, 1H, 6-Hpyrazinyl), 8.90 (d, J = 2.5 Hz, 1H, 5-Hpyrazinyl), 10.78 (br s, 1H, CONH). 13C NMR (151 MHz, DMSO-d6): δ (ppm) 119.5 (2C, C-2/6phenyl), 123.8 (1C, C-4phenyl), 127.8 (2C, C-3/5benzoyl), 128.9 (2C, C-3/5phenyl), 130.7 (2C, C-2/6benzoyl), 131.4 (1C, C-1benzoyl), 133.0 (1C, C-4benzoyl), 139.1 (1C, C-1phenyl), 142.6 (1C, C-3pyrazinyl), 143.7 (1C, C-6pyrazinyl),145.1 (1C, C-5pyrazinyl), 149.1 (1C, C-2pyrazinyl), 157.9 (1C, C-3triazole), 158.9 (1C, C-5triazole), 164.2 (1C, CONH), 167.5 (1C, CONbenzoyl). IR (neat): ν̃ [cm–1] 3375, 3070, 1701, 1678, 1535, 1446, 1350, 1319, 1145, 1091, 979, 925, 759, 717. HRMS (APCI): m/z = 386.1360 calcd for [M + H]+; found, 386.1369. HPLC: tR = 17.2 min, purity: 99.0%.
3-(5-Amino-1-benzoyl-1H-1,2,4-triazol-3-yl)-N-benzylpyrazine-2-carboxamide (5u)
According to general procedure B, aminotriazole 21u (101 mg, 342 μmol) was acylated using benzoic acid (41.8 mg, 342 μmol), DMAP (83.6 mg, 684 μmol), and EDCI·HCl (131 mg, 684 μmol) in dry DMF (2.2 mL). 5u was obtained as a colorless solid (117 mg, 293 μmol, 86%). TLC: Rf = 0.43 (CH2Cl2/CH3OH = 93/7). 1H NMR (600 MHz, DMSO-d6): δ (ppm) 4.38 (d, J = 6.0 Hz, 2H, CH2), 7.19–7.23 (m, 1H, 4-Hphenyl), 7.25–7.30 (m, 4H, 2/3/5/6-Hphenyl), 7.53–7.58 (m, 2H, 3/5-Hbenzoyl), 7.66–7.70 (m, 1H, 4-Hbenzoyl), 7.87 (br s, 2H, NH2), 8.06–8.10 (m, 2H, 2/6-Hbenzoyl), 8.76 (d, J = 2.4 Hz, 1H, 6-Hpyrazinyl), 8.85 (d, J = 2.4 Hz, 1H, 5-Hpyrazinyl), 9.15 (t, J = 6.1 Hz, 1H, CONH). 13C NMR (151 MHz, DMSO-d6): δ (ppm) 42.3 (1C, CH2), 126.8 (1C, C-4phenyl), 127.2 (2C, C-2/6phenyl, C-3/5phenyl), 128.1 (2C, C-3/5benzoyl), 128.2 (2C, C-2/6phenyl, C-3/5phenyl), 130.9 (2C, C-2/6benzoyl), 131.7 (1C, C-1benzoyl), 133.2 (1C, C-4benzoyl), 138.9 (1C, C-1phenyl), 142.8 (1C, C-3pyrazinyl), 143.8 (1C, C-6pyrazinyl), 144.9 (1C, C-5pyrazinyl), 149.0 (1C, C-2pyrazinyl), 158.1 (1C, C-3triazole), 158.7 (1C, C-5triazole), 165.3 (1C, CONH), 167.6 (1C, CONbenzoyl). IR (neat): ν̃ [cm–1] 3456, 3263, 3074, 1685, 1654, 1635, 1546, 1357, 1327, 1311, 1165, 1107, 975, 928, 725. HRMS (APCI): m/z = 400.1516 calcd for [M + H]+; found, 400.1554. HPLC: tR = 17.0 min, purity: 98.5%.
3-(5-Amino-1-benzoyl-1H-1,2,4-triazol-3-yl)-N-phenethylpyrazine-2-carboxamide (5v)
According to general procedure B, aminotriazole 21v (105 mg, 339 μmol) was acylated using benzoic acid (41.5 mg, 339 μmol), DMAP (82.9 mg, 679 μmol), and EDCI·HCl (130 mg, 679 μmol) in dry DMF (2.2 mL). 5v was obtained as a colorless solid (131 mg, 317 μmol, 93%). TLC: Rf = 0.43 (CH2Cl2/CH3OH = 93/7). 1H NMR (600 MHz, DMSO-d6): δ (ppm) 2.68–2.73 (m, 2H, NHCH2CH2), 3.34–3.39 (m, 2H, NHCH2CH2), 7.15–7.22 (m, 3H, C-2/4/6phenyl), 7.25–7.29 (m, 2H, C-3/5phenyl), 7.50–7.55 (m, 2H, C-3/5benzoyl), 7.63–7.67 (m, 1H, C-4benzoyl), 7.86 (br s, 2H, NH2), 8.07–8.11 (m, 2H, C-2/6benzoyl), 8.73 (t, J = 5.7 Hz, 1H, NH), 8.74 (d, J = 2.5 Hz, 1H, C-6pyrazinyl), 8.83 (d, J = 2.4 Hz, 1H, C-5pyrazinyl). 13C NMR (151 MHz, DMSO-d6): δ (ppm) 34.7 (1C, NHCH2CH2), 40.6 (1C, NHCH2CH2), 126.1 (1C, C-4phenyl), 128.1 (2C, C-3/5benzoyl), 128.3 (2C, C-3/5phenyl), 128.5 (2C, C-2/6phenyl), 130.9 (2C, C-2/6benzoyl), 131.7 (1C, C-1benzoyl), 133.2 (1C, C-4benzoyl), 139.3 (1C, C-4phenyl), 142.8 (1C, C-3pyrazinyl), 143.7 (1C, C-6pyrazinyl), 144.8 (1C, C-5benzoyl), 148.9 (1C, C-2pyrazinyl), 158.1 (1C, C-3triazole), 158.6 (1C, C-5triazole), 165.2 (1C, CONH), 167.6 (1C, CONbenzoyl). IR (neat): ν̃ [cm–1] 3456, 3282, 3062, 1681, 1651, 1631, 1535, 1446, 1350, 1323, 1188, 1111, 975, 921, 748. HRMS (APCI): m/z = 414.1673 calcd for [M + H]+; found, 414.1672. HPLC: tR = 17.5 min, purity: 99.2%.
3-(5-Amino-1-benzoyl-1H-1,2,4-triazol-3-yl)-N-(thiophen-2-ylmethyl)pyrazine-2-carboxamide (5w)
According to general procedure B, aminotriazole 21w (100 mg, 332 μmol) was acylated using benzoic acid (40.5 mg, 332 μmol), DMAP (81.1 mg, 664 μmol), and EDCI·HCl (127 mg, 664 μmol) in dry DMF (2.2 mL). 5w was obtained as a colorless solid (72 mg, 178 μmol, 54%). TLC: Rf = 0.26 (CH2Cl2/CH3OH = 93/7). 1H NMR (600 MHz, DMSO-d6): δ (ppm) 4.52 (d, J = 5.9 Hz, 2H, CH2), 6.92 (dd, J = 5.1/3.4 Hz, 1H, 4-Hthiophenyl), 6.96 (dd, J = 3.4/1.2 Hz, 1H, 3-Hthiophenyl), 7.36 (dd, J = 5.0/1.3 Hz, 1H, 5-Hthiophenyl), 7.54–7.59 (m, 2H, 3/5-Hbenzoyl), 7.66–7.72 (m, 1H, 4-Hbenzoyl), 7.86 (br s, 2H, NH2), 8.09 (m, 2H, 2/6-Hbenzoyl), 8.75 (d, J = 2.4 Hz, 1H, 6-Hpyrazinyl), 8.84 (d, J = 2.5 Hz, 1H, 5-Hpyrazinyl), 9.22 (t, J = 5.9 Hz, 1H, CONH). 13C NMR (151 MHz, DMSO-d6): δ (ppm) 37.5 8 (1C, CH2), 125.1 (1C, C-5thiophenyl), 125.6 (1C, C-3thiophenyl), 126.6 (1C, C-4thiophenyl), 128.1 (2C, C-3/5benzoyl), 130.9 (2C, C-2/6benzoyl), 131.7 (1C, C-1benzoyl), 133.2 (1C, C-4benzoyl), 141.5 (1C, C-1thiophenyl), 142.9 (1C, C-3pyrazinyl), 143.8 (1C, C-6pyrazinyl), 144.9 (1C, C-5pyrazinyl), 148.6 (1C, C-2pyrazinyl), 158.1 (1C, C-3triazole), 158.7 (1C, C-5triazole), 165.0 (1C, CONH), 167.6 (1C, CONbenzoyl). IR (neat): ν̃ [cm–1] 3456, 3251, 3059, 1685, 1658, 1639, 1535, 1446, 1354, 1327, 1107, 979, 798, 748. HRMS (APCI): m/z = 406.1081 calcd for [M + H]+; found, 406.1112. HPLC: tR = 16.6 min, purity: 98.9%.
(S)-3-(5-Amino-1-(1,2,3,4-tetrahydronaphthalene-1-carbonyl)-1H-1,2,4-triazol-3-yl)-N-butylpyrazine-2-carboxamide (22)
According to general procedure B, aminotriazole 21e (75.0 mg, 287 μmol) was acylated using (S)-1,2,3,4-tetrahydronaphthalene-1-carboxylic acid (50.6 mg, 287 μmol), DMAP (70.1 mg, 574 μmol), and EDCI·HCl (110 mg, 574 μmol) in dry DMF (1.8 mL). Flash column chromatography (CH2Cl2/CH3OH = 1/0 → 95/5) yielded amide 22 as a colorless solid (65 mg, 155 μmol, 54%). TLC: Rf = 0.26 (CH2Cl2/CH3OH = 93/7). 1H NMR (600 MHz, DMSO-d6): δ (ppm) 0.82 (t, J = 7.4 Hz, 3H, CH3), 1.20–1.30 (m, 2H, 3-Hbutyl), 1.39–1.47 (m, 2H, 2-Hbutyl), 1.69–1.76 (m, 1H, 3-Htetrahydronapthoyl), 1.86–1.93 (m, 1H, 3-Htetrahydronapthoyl), 2.03–2.09 (m, 1H, 2-Htetrahydronapthoyl), 2.10–2.17 (m, 1H, 2-Htetrahydronapthoyl), 2.72–2.84 (m, 2H, 4-Htetrahydronapthoyl), 3.13–3.23 (m, 2H, 1-Hbutyl), 4.94 (t, J = 6.3 Hz, 1H, 1-Htetrahydronapthoyl), 7.03–7.07 (m, 1H, 8-Htetrahydronapthoyl), 7.07–7.12 (m, 1H, 5/6/7-Htetrahydronapthoyl), 7.13–7.20 (m, 2H, 5/6/7-Htetrahydronapthoyl), 7.71 (br s, 2H, NH2), 8.64 (t, J = 5.7 Hz, 1H, NH), 8.75 (d, J = 2.4 Hz, 1H, 6-Hpyrazinyl), 8.84 (d, J = 2.5 Hz, 1H, 5-Hpyrazinyl). 13C NMR (151 MHz, DMSO-d6): δ (ppm) 13.6 (1C, CH3), 19.6 (1C, C-3butyl), 20.0 (1C, C-3tetrahydronapthoyl), 26.3 (1C, C-2tetrahydronapthoyl), 28.6 (1C, C-4tetrahydronapthoyl), 30.9 (1C, C-2butyl), 38.6 (1C, C-1butyl), 43.2 (1C, C-1tetrahydronapthoyl), 125.8 (1C, C-5/6/7/8tetrahydronapthoyl), 126.9 (1C, C-5/6/7/8tetrahydronapthoyl), 129.2 (1C, C-5/6/7/8tetrahydronapthoyl), 129.3 (1C, C-5/6/7/8tetrahydronapthoyl), 132.8 (1C, C-8atetrahydronapthoyl), 137.5 (1C, C-4atetrahydronapthoyl), 142.8 (1C, C-3pyrazinyl), 143.7 (1C, C-6pyrazinyl), 144.7 (1C, C-5pyrazinyl), 149.4 (1C, C-2pyrazinyl), 157.6 (1C, C-5triazole), 158.1 (1C, C-3triazole), 165.2 (1C, CONH), 175.5 (1C, CONtetrahydronapthoyl). IR (neat): ν̃ [cm–1] 3406, 3278, 3062, 2931, 1712, 1678, 1647, 1527, 1450, 1373, 1184, 1161, 1111, 987, 871, 748. HRMS (APCI): m/z = 420.2142 calcd for [M + H]+; found, 420.2150. HPLC: tR = 18.7 min, purity: 99.4%.
3-(1-(1-Naphthoyl)-5-amino-1H-1,2,4-triazol-3-yl)-N-butylpyrazine-2-carboxamide (23)
According to general procedure B, aminotriazole 21e (75.0 mg, 287 μmol) was acylated using 1-naphthoic acid (49.4 mg, 287 μmol), DMAP (70.1 mg, 574 μmol), and EDCI·HCl (110 mg, 574 μmol) in dry DMF (1.8 mL). 23 was obtained as a colorless solid (105 mg, 253 μmol, 88%). TLC: Rf = 0.26 (CH2Cl2/CH3OH = 93/7). 1H NMR (600 MHz, DMSO-d6): δ (ppm) 0.81 (t, J = 7.4 Hz, 3H, CH3), 1.12–1.20 (m, 2H, 3-Hbutyl), 1.23–1.30 (m, 2H, 2-Hbutyl), 2.94 (td, J = 7.1/5.7 Hz, 2H, 1-Hbutyl), 7.58–7.64 (m, 3H, 3/6/7-H1-napthoyl), 7.83–7.86 (m, 1H, 2-H1-napthoyl), 7.91–7.98 (m, 3H, NH2, 8-H1-napthoyl), 8.03–8.07 (m, 1H, 5-H1-napthoyl), 8.15–8.17 (m, 1H, 4-H1-napthoyl), 8.42 (t, J = 5.7 Hz, 1H, NH), 8.67 (d, J = 2.4 Hz, 1H, 6-Hpyrazinyl), 8.74 (d, J = 2.5 Hz, 1H, 5-Hpyrazinyl). 13C NMR (151 MHz, DMSO-d6): δ (ppm) 13.6 (1C, CH3), 19.5 (1C, C-3butyl), 30.7 (1C, C-2butyl), 38.4 (1C, C-1butyl), 124.5 (1C, C-3/81-napthoyl), 124.6 (1C, C-3/81-napthoyl), 126.5 (1C, C-6/71-napthoyl), 127.6 (1C, C-6/71-napthoyl), 128.1 (1C, C-21-napthoyl), 128.5 (1C, C-51-napthoyl), 129.5 (1C, C-8a1-napthoyl), 130.1 (1C, C-4a1-napthoyl), 131.5 (1C, C-41-napthoyl), 132.9 (1C, C-11-napthoyl), 142.9 (1C, C-3pyrazinyl), 143.7 (1C, C-6pyrazinyl), 144.6 (1C, C-5pyrazinyl), 149.1 (1C, C-2pyrazinyl), 158.2 (1C, C-5triazole), 158.4 (1C, C-3triazole), 164.8 (1C, CONH), 168.8 (1C, CON1-napthoyl). IR (neat): ν̃ [cm–1] 3456, 3275, 3101, 2958, 2870, 1701, 1643, 1554, 1446, 1342, 1311, 1192, 1053, 975, 910, 779. HRMS (APCI): m/z = 416.1829 calcd for [M + H]+; found, 416.1839. HPLC: tR = 18.1 min, purity: 95.1%.
3-(5-Amino-1-(2-(2-iodophenyl)acetyl)-1H-1,2,4-triazol-3-yl)-N-butylpyrazine-2-carboxamide (24)
According to general procedure B, aminotriazole 21e (75.0 mg, 287 μmol) was acylated using 2-(2-iodophenyl)acetic acid (75.2 mg, 287 μmol), DMAP (70.1 mg, 574 μmol), and EDCI·HCl (110 mg, 574 μmol) in dry DMF (1.8 mL). 24 was obtained as a colorless solid (107 mg, 212 μmol, 74%). TLC: Rf = 0.26 (CH2Cl2/CH3OH = 93/7). 1H NMR (600 MHz, DMSO-d6): δ (ppm) 0.84 (t, J = 7.4 Hz, 3H, CH3), 1.27–1.33 (m, 2H, 3-Hbutyl), 1.45–1.52 (m, 2H, 2-Hbutyl), 3.25 (td, J = 7.1/5.7 Hz, 2H, 1-Hbutyl), 4.50 (s, 2H, (CH2)iodophenylacetyl), 7.08 (td, J = 7.6/1.7 Hz, 1H, 4-Hiodophenyl), 7.41 (td, J = 7.5/1.3 Hz, 1H, 5-Hiodophenyl), 7.48 (dd, J = 7.6/1.7 Hz, 1H, 6-Hiodophenyl), 7.67 (br s, 2H, NH2), 7.89 (dd, J = 7.9/1.3 Hz, 1H, 4-Hiodophenyl), 8.63 (t, J = 5.7 Hz, 1H, NH), 8.75 (d, J = 2.4 Hz, 1H, 6-Hpyrazinyl), 8.84 (d, J = 2.4 Hz, 1H, 5-Hpyrazinyl). 13C NMR (151 MHz, DMSO-d6): δ (ppm) 13.7 (1C, CH3), 19.7 (1C, C-3butyl), 30.9 (1C, C-2butyl), 38.7 (1C, C-1butyl), 46.4 (1C, (CH2)iodophenylacetyl), 102.0 (1C, C-2iodophenyl), 128.4 (1C, C-5iodophenyl), 129.3 (1C, C-4iodophenyl), 131.9 (1C, C-6iodophenyl), 137.1 (1C, C-1iodophenyl), 138.8 (1C, C-3iodophenyl), 142.7 (1C, C-3pyrazinyl), 143.7 (1C, C-6pyrazinyl), 144.6 (1C, C-5pyrazinyl), 149.4 (1C, C-2pyrazinyl), 157.2 (1C, C-5triazole), 158.1 (1C, C-3triazole), 165.2 (1C, CONH), 170.5 (1C, CONiodophenylacetyl). IR (neat): ν̃ [cm–1] 3394, 3290, 3248, 3062, 2954, 2927, 1724, 1674, 1635, 1562, 1381, 1354, 1330, 1284, 1161, 1107, 1010, 999, 875, 752, 732. HRMS (APCI): m/z = 506.0796 calcd for [M + H]+; found, 506.0749. HPLC: tR = 18.4 min, purity: 94.0%.
1-(5-Amino-3-(3-(morpholine-4-carbonyl)pyrazin-2-yl)-1H-1,2,4-triazol-1-yl)-2,2-dimethylpropan-1-one (25)
According to general procedure A, aminotriazole 21r (50.0 mg, 182 μmol) was acylated using pivaloyl chloride (26.9 μL, 218 μmol, 1.20 equiv) in dry pyridine/THF (1.70 mL/0.85 mL). Flash column chromatography (CH2Cl2/CH3OH = 1/0 → 9/1) yielded amide 25 as a colorless solid (45 mg, 105 μmol, 69%). TLC: Rf = 0.34 (CH2Cl2/CH3OH = 93/7). 1H NMR (600 MHz, DMSO-d6): δ (ppm) 1.43 (s, 9H, C(CH3)3), 3.17–3.20 (m, 2H, 3/5-Hmorpholinyl), 3.51–3.54 (m, 2H, 2/6-Hmorpholinyl), 3.61–3.65 (m, 2H, 3/5-Hmorpholinyl), 3.68–3.72 (m, 2H, 2/6-Hmorpholinyl), 7.78 (br s, 2H, NH2), 8.75 (d, J = 2.4 Hz, 1H, 6-Hpyrazinyl), 8.83 (d, J = 2.4 Hz, 1H, 5-Hpyrazinyl). 13C NMR (151 MHz, DMSO-d6): δ (ppm) 26.4 (3C, C(CH3)3), 41.56 (1C, C(CH3)3, C-3/5morpholinyl), 41.60 (1C, C(CH3)3, C-3/5morpholinyl), 46.5 (1C, C-3/5morpholinyl), 65.7 (1C, C-2/6morpholinyl), 65.7 (1C, C-2/6morpholinyl), 141.6 (1C, C-3pyrazinyl), 144.2 (1C, C-6pyrazinyl), 144.6 (1C, C-5pyrazinyl), 148.7 (1C, C-2pyrazinyl), 156.3 (1C, C-3triazole), 158.8 (1C, C-5triazole), 165.5 (1C, CONmorpholinyl), 178.3 (1C, CONpivaloyl). IR (neat): ν̃ [cm–1] 3433, 3275, 3116, 2970, 2931, 1701, 1631, 1523, 1481, 1427, 1338, 1307, 1114, 1018, 975, 759. HRMS (APCI): m/z = 360.1779 calcd for [M + H]+; found, 360.1793. HPLC: tR = 14.0 min, purity: 97.9%.
3-(5-Amino-1-(6-aminonicotinoyl)-1H-1,2,4-triazol-3-yl)-N-butylpyrazine-2-carboxamide (34)
According to general procedure B, aminotriazole 21e (82 mg, 314 μmol) was acylated using 6-aminonicotinic acid (65 mg, 471 μmol, 1.50 equiv), DMAP (77 mg, 628 μmol, 2.00 equiv), and EDCI·HCl (120 mg, 628 μmol, 2.00 equiv) in dry DMF (8.0 mL). The mixture was stirred for 7 h. After addition of H2O, the aqueous layer was extracted with CH2Cl2 (3×). The combined organic layers were washed with saturated aqueous NH4Cl solution (2×), dried over Na2SO4, and filtered. The solvent was removed in vacuo to yield 34 as a colorless solid (56 mg, 147 μmol, 47%), which was used without further purification. TLC: Rf = 0.29 (CH2Cl2/CH3OH = 9/1). 1H NMR (600 MHz, DMSO, 299 K): δ (in ppm) 0.81 (t, J = 7.4 Hz, 3H, −CH3), 1.20–1.28 (m, 2H, 3-Hbutyl), 1.38 (tt, J = 7.7, 6.3 Hz, 2H, 2-Hbutyl), 3.20 (td, J = 7.1, 5.7 Hz, 2H, 1-Hbutyl), 6.49 (dd, J = 9.0, 0.7 Hz, 1H, 5-Hnicotinoyl), 7.07 (s, 2H, −NH2,nicotinoyl), 7.70 (s, 2H, −NH2,triazole), 8.22 (dd, J = 8.9, 2.5 Hz, 1H, 4-Hnicotinoyl), 8.56 (t, J = 5.7 Hz, 1H, −NH−), 8.73 (d, J = 2.5 Hz, 1H, 5/6-Hpyrazinyl), 8.82 (d, J = 2.5 Hz, 1H, 5/6-Hpyrazinyl), 8.89–8.93 (m, 1H, 2-Hnicotinoyl). 13C NMR (151 MHz, DMSO, 299 K): δ (in ppm) 13.6 (1C, −CH3), 19.5 (1C, 3-Cbutyl), 30.8 (1C, 2-Cbutyl), 38.5 (1C, 1-Cbutyl), 106.5 (1C, 5-Cnicotinoyl), 114.5 (1C, 3-Cnicotinoyl), 139.9 (1C, 4-Cnicotinoyl), 142.5 (1C, 2-Cpyrazinyl), 143.6 (1C, 5/6-Cpyraziyl), 144.6 (1C, 5/6-Cpyrazinyl), 149.5 (1C, 3-Cpyrazinyl), 154.3 (1C, 2-Cnicotinoyl), 157.5 (1C, 3/5-Ctriazole), 158.7 (1C, 3/5-Ctriazole), 162.5 (1C, 6-Cnicotinoyl), 165.4 (1C, C=Obutylamide), 165.5 (1C, C=Onicotinoyl). IR (neat): ν̃ [cm–1] 3449, 3279, 2955, 2870, 1370, 1647, 1555, 1447, 1350, 1223, 1188, 1015, 918, 845, 775, 698. HRMS (APCI): m/z = 382.1735 calcd for [M + H]+; found, 382.1764. qNMR (600 MHz, DMSO, 256 K): 97.4% (IC = TMB).
3-(5-Amino-1-benzoyl-1H-1,2,4-triazol-3-yl)-N-butylquinoxaline-2-carboxamide (33a)
According to general procedure A, triazole 32 (50 mg, 0.16 mmol, 1.00 equiv) was acylated using benzoyl chloride (20 μL, 0.18 mmol, 1.10 equiv) in dry THF/pyridine (8 mL/1 mL). The product was obtained as a colorless solid (40 mg, 0.10 mmol, 60%). TLC: Rf = 0.47 (CH2Cl2/CH3OH = 9/1). 1H NMR (600 MHz, DMSO, 299 K): δ (in ppm) 0.83 (t, J = 7.3 Hz, 3H, CH3), 1.20–1.27 (m, 2H, 3-Hbutyl), 1.35 (tt, J = 8.0/6.3 Hz, 2H, 2-Hbutyl), 3.13 (td, J = 7.2/5.7 Hz, 2H, 1-Hbutyl), 7.53–7.59 (m, 2H, 3/5-Hbenzoyl), 7.68–7.73 (m, 1H, 4-Hbenzoyl), 7.90 (br s, 2H, NH2), 7.95–8.00 (m, 2H, 6/7-Hquinoxalinyl), 8.13–8.17 (m, 2H, 2/6-Hbenzoyl), 8.17–8.23 (m, 2H, 5/8-Hquinoxalinyl), 8.66 (t, J = 5.7 Hz, 1H, CONH). 13C NMR (151 MHz, DMSO, 299 K): δ (in ppm) 13.6 (1C, CH3), 19.5 (1C, C-3butyl), 30.8 (1C, C-3butyl), 38.6 (1C, C-1butyl), 128.1 (2C, C-3/5benzoyl), 128.9 (1C, C-5/8quinoxalinyl), 129.1 (1C, C-5/8quinoxalinyl), 131.0 (2C, C-2/6benzoyl), 131.5 (1C, C-1benzoyl), 131.6 (1C, C-6/7quinoxalinyl), 131.8 (1C, C-6/7quinoxalinyl), 133.3 (1C, C-4benzoyl), 139.8 (1C, C-4a/8aquinoxalinyl), 140.8 (1C, C-4a/8aquinoxalinl), 142.6 (1C, C-3quinoxalinyl), 149.5 (1C, C-2quinoxalinyl), 158.1 (1C, C-3/5triazole), 158.7 (1C, C-3/5triazole), 165.6 (1C, CONH), 167.5 (1C, CONbenzoyl). IR (neat): ν̃ [cm–1] 3456, 3078, 2959, 1975, 1697, 1643, 1551, 1458, 1366, 1288,1188, 1123, 1030, 1011, 918, 791, 679. HRMS (APCI): m/z = 416.1830 calcd for [M + H]+; found, 416.1801. HPLC: tR = 18.2 min, purity: 99.5%.
3-(1-(1-Naphthoyl)-5-amino-1H-1,2,4-triazol-3-yl)-N-butylquinoxaline-2-carboxamide (33b)
According to general procedure A, triazole 32 (54 mg, 0.17 mmol, 1.00 equiv) was acylated using 1-naphthoyl chloride (30 μL, 0.19 mmol, 1.10 equiv) in dry THF/pyridine (8 mL/1 mL). The reaction mixture was poured into cold H2O and extracted with EtOAc (3×). The combined organic layers were dried over Na2SO4 and filtered, and the solvent was removed in vacuo. After flash column chromatography (CH2Cl2/CH3OH = 1/0 → 47/3), the product was obtained as a colorless solid (80 mg, 0.17 mmol, 99%). TLC: Rf = 0.42 (CH2Cl2/CH3OH = 9/1). 1H NMR (400 MHz, DMSO, 299 K): δ (in ppm) 0.79 (t, J = 7.2 Hz, 3H, CH3), 1.08–1.19 (m, 2H, 3-Hbutyl), 1.19–1.28 (m, 2H, 2-Hbutyl), 2.84 (td, J = 7.1/5.6 Hz, 2H, 1-Hbutyl), 7.59–7.67 (m, 3H, 3/6/7-H1-naphthoyl), 7.89–7.92 (m, 1H, 2-H1-naphthoyl), 7.92–7.96 (m, 2H, 6/7-Hquinoxalinyl), 7.97–8.02 (m, 3H, 5/8-H1-naphthoyl, NH2), 8.04–8.09 (m, 1H, 5/8-H1-naphthoyl), 8.10–8.17 (m, 2H, 5/8-Hquinoxalinyl), 8.18 (dt, J = 8.4, 1.1 Hz, 1H, 4-H1-naphthoyl), 8.49 (t, J = 5.7 Hz, 1H, NH). 13C NMR (101 MHz, DMSO, 299 K): δ (in ppm) 13.6 (1C, CH3), 19.5 (1C, C-3butyl), 30.6 (1C, C-2butyl), 38.4 (1C, C-1butyl), 124.5 (1C, C-5/81-naphthoyl), 124.6 (1C, C-3/6/71-naphthoyl), 126.5 (1C, C-3/6/71-naphthoyl), 127.6 (1C, C-3/6/71-naphthoyl), 128.4 (1C, C-21-naphthoyl), 128.5 (1C, C-5/81-naphthoyl), 128.8 (1C, C-5/8quinoxalinyl), 129.1 (1C, C-5/8quinoxalinyl), 129.6 (1C, C-8a1-naphthoyl), 129.9 (1C, C-1/4a1-naphthoyl), 131.5 (1C, C-6/7quinoxalinyl), 131.6 (1C, C-41-naphthoyl), 131.7 (1C, C-6/7quinoxalinyl), 132.9 (1C, C-1/4a1-naphthoyl), 139.8 (1C, C-4a/8aquinoxalinyl), 140.7 (1C, C-4a/8aquinoxalinyl), 142.8 (1C, C-3quinoxalinyl), 149.3 (1C, C-2quinoxalinyl), 158.3 (1C, C-3/5triazole), 158.4 (1C, C-3/5triazole), 165.2 (1C, CONH), 168.7 (1C, CONbenzoyl). IR (neat): ν̃ [cm–1] 3456, 3059, 2955, 1701, 1643, 1535, 1458, 1362, 1238, 1153, 980, 910, 845, 814, 799, 764, 613. HRMS (APCI): m/z = 466.1986 calcd for [M + H]+; found, 466.1957. HPLC: tR = 19.5 min, purity: 98.9%.
(S)-3-(5-amino-1-(1,2,3,4-tetrahydronaphthalene-1-carbonyl)-1H-1,2,4-triazol-3-yl)-N-butylquinoxaline-2-carboxamide (33c)
Triazole 32 (84 mg, 0.27 mmol, 1.00 equiv) was dissolved in dry DMF (9 mL) and cooled down to 0 °C. (S)-1,2,3,4-tetrahydronaphthoic acid (48 mg, 0.27 mmol, 1.00 equiv), EDCI·HCl (103 mg, 0.54 mmol, 2.00 equiv), and DMAP (66 mg, 0.54 mmol, 2.00 equiv) were added to the reaction mixture and stirred 1 h at 0 °C and 4 h at rt. The reaction mixture was poured into cold H2O and extracted with EtOAc (3×), the combined organic layers were dried over Na2SO4 and filtered, and the solvent was removed in vacuo. After purification via flash column chromatography (CH2Cl2/CH3OH = 1/0 → 19/1), product 33c was obtained as a colorless solid (75 mg, 0.16 mmol, 60%). TLC: Rf = 0.44 (CH2Cl2/CH3OH = 9/1). 1H NMR (400 MHz, DMSO, 299 K): δ (in ppm) 0.82 (t, J = 7.4 Hz, 3H, CH3), 1.23–1.35 (m, 2H, 3-Hbutyl), 1.42–1.54 (m, 2H, 2-Hbutyl), 1.68–1.81 (m, 1H, 3-Htetrahydronaphthoyl), 1.86–1.98 (m, 1H, 3-Htetrahydronaphthoyl), 2.04–2.24 (m, 2H, 2-Htetrahydronaphthoyl), 2.67–2.90 (m, 2H, 4-Htetrahydronaphthoyl), 3.15–3.32 (m, 2H, 2-Hbutyl), 4.99 (t, J = 6.4 Hz, 1H, 1-Htetrahydronaphthoyl), 7.05–7.22 (m, 4H, 5/6/7/8-Htetrahydronaphthoyl), 7.76 (br s, 2H, NH2), 7.95–8.03 (m, 2H, 6/7-Hquinoxalinyl), 8.16–8.26 (m, 2H, 5/8-Hquinoxalinyl), 8.77 (t, J = 5.6 Hz, 1H, CONH). 13C NMR (101 MHz, DMSO, 299 K): δ (in ppm) 13.6 (1C, CH3), 19.6 (1C, C-3butyl), 20.0 (1C, C-3tetrahydronaphthoyl), 26.4 (1C, C-2tetrahydronaphthoyl), 28.6 (1C, C-4tetrahydronaphthoyl), 30.9 (1C, C-2butyl), 38.8 (1C, C-1butyl), 43.2 (1C, C-1tetrahydronaphthoyl), 125.8 (1C, C-5/6/7/8tetrahydronaphthoyl), 126.9 (1C, C-5/6/7/8tetrahydronaphthoyl), 128.9 (1C, C-5/8quinoxalinyl), 129.2 (1C, C-5/6/7/8tetrahydronaphthoyl), 129.2 (1C, C-5/8quinoxalinyl), 129.3 (1C, C-5/6/7/8tetrahydronaphthoyl), 131.6 (1C, C-6/7quinoxalinyl), 131.8 (1C, C-6/7quinoxalinyl), 132.8 (1C, C-8atetrahydronaphthoyl), 137.5 (1C, C-4atetrahydonaphthoyl), 139.8 (1C, C-4a/8aquinoxalinyl), 140.8 (1C, C-4a/8aquinoxalinyl), 142.7 (1C, C-3quinoxalinyl), 149.6 (1C, C-2quinoxalinyl), 157.6 (1C, C-3/5triazole), 158.0 (1C, C-3/5triazole), 165.7 (1C, CONH), 175.5 (1C, CONtetrahydronaphthoyl). IR (neat): ν̃ [cm–1] 3456, 3279, 3032, 2951, 1709, 1655, 1454, 1373, 1304, 1277, 1238, 1103, 991, 945, 876, 818, 732, 690, 633. HRMS (APCI): m/z = 470.2299 calcd for [M + H]+; found, 470.2283. HPLC: tR = 10.2 min, purity: 96.5%.
3-(5-Amino-1-(6-aminonicotinoyl)-1H-1,2,4-triazol-3-yl)-N-butylquinoxaline-2-carboxamide (33d)
According to general procedure B, aminotriazole 32 (50 mg, 161 μmol) was acylated using 6-aminonicotinic acid (33 mg, 241 μmol, 1.50 equiv), DMAP (39 mg, 321 μmol, 2.00 equiv), and EDCI·HCl (62 mg, 321 μmol, 2.00 equiv) in dry DMF (6.0 mL). The mixture was stirred for 7 h. After addition of H2O, the formed precipitate was filtered off, washed with H2O, and dried in vacuo to yield 33d as a colorless solid (69 mg, 160 μmol, 99%), which was used without further purification. TLC: Rf = 0.50 (CH2Cl2/CH3OH = 9/1). 1H NMR (600 MHz, DMSO, 299 K): δ (in ppm) 0.81 (t, J = 7.4 Hz, 3H, −CH3), 1.25 (m, 2H, 3-Hbutyl), 1.39 (tt, J = 8.0, 6.2 Hz, 2H, 2-Hbutyl), 3.24 (td, J = 7.2, 5.7 Hz, 2H, 1-Hbutyl), 6.53 (dd, J = 8.9, 0.7 Hz, 1H, 5-Hnicotinoyl), 7.13 (br s, 2H, −NH2,triazole), 7.77 (br s, 2H, −NH2,nictotinoyl), 7.96–7.99 (m, 2H, 6/7-Hquinoxalinyl), 8.18 (ddd, J = 7.0, 4.1, 3.0 Hz, 1H, 5/8-Hquinoxalinyl), 8.20–8.24 (m, 1H, 5/8-Hquinoxalinyl), 8.26 (dd, J = 9.0, 2.5 Hz, 1H, 4-Hnicotinoyl), 8.69 (t, J = 5. Hz, 1H, −NH−), 8.95 (dd, J = 2.5, 0.7 Hz, 1H, 2-Hnicotinoyl). 13C NMR (151 MHz, DMSO, 299 K): δ (in ppm) 13.6 (1C, −CH3), 19.6 (1C, 3-Cbutyl), 30.9 (1C, 2-Cbutyl), 38.6 (1C, 1-Cbutyl), 106.6 (1C, 5-Cnicotinoyl), 114.4 (1C, 3-Cnicotinoyl), 128.8 (1C, 5/8-Cquinoxlinyl), 129.2 (1C, 5/8-Cquinoxalinyl), 131.5 (1C, 6/7-Cquinoxalinyl), 131.7 (1C, 6/7-Cquinoxalinyl), 139.9 (1C, 4a/8a-Cquinoxalinyl), 140.0 (1C, 4-Cquinoxalinyl), 140.8 (1C, 4a/8a-Cquinoxalinyl), 142.4 (1C, 2-Cquinoxalinyl), 149.7 (1C, 3-Cquinoxalinyl), 154.4 (1C, 2-Cnicotinoyl), 157.4 (1C, 3/5-Ctriazole), 158.7 (1C, 3/5-Ctriazole), 162.5 (1C, 6-Cnicotinoyl), 165.4 (1C, C=Onicotinoyl), 165.8 (1C, C=Obutylamide). IR (neat): ν̃ [cm–1] 3360, 2959, 2870, 1663, 1632, 1520, 1366, 1304, 1242, 1188, 1096, 964, 922, 641, 760, 671. HRMS (APCI): m/z = 432.1891 calcd for [M + H]+; found, 432.1934. HPLC: tR = 13.5 min, purity: 99.9%.
X-ray Diffraction
Data sets for compound 14h were collected with a D8 Venture Dual Source 100 CMOS diffractometer. Programs used: data collection: APEX3 V2016.1-0;41 cell refinement: SAINT V8.37A;41 data reduction: SAINT V8.37A;41 absorption correction: SADABS V2014/7;41 structure solution SHELXT-2015;42 structure refinement SHELXL-2015.43 R-values are given for observed reflections, and wR2 values are given for all reflections. CCDC-2165993 (14h) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre.44 X-ray crystal structure analysis of 14h is given in the Supporting Information.
FXIIa Inhibition Assay
The inhibitory activity of synthesized compounds against human coagulation factor XIIa was measured by quantifying the hydrolysis rate of the fluorogenic substrate as reported previously.27,28 Briefly, the activity was tested in the buffer (10 mM Tris-Cl, 150 mM NaCl, 10 mM MgCl2·6H2O, 1 mM CaCl2·2H2O, 0.1% w/v BSA, 0.01% v/v Triton-X100, pH = 7.4) utilizing clear flat-bottom, black poly-styrene 96-well plates. The enzyme (human β-FXIIa, HFXIIAB, >95% purity; Molecular Innovations, 2.5 nM—final concentration) and the fluorogenic substrate Boc-Gln-Gly-Arg-AMC (Pepta Nova, 25 μM—final concentration, Km = 167 μM) were used. Dilutions of test compounds ranging from 2 nM to 32 μM (final concentrations) in DMSO were prepared. The fluorogenic substrate solution was added into the wells, followed by the addition of 2 μL of test compound solution, and the reaction was triggered by addition of the enzyme solution (final testing volume—152 μL). In the case of blank (substrate + buffer) and control (substrate + enzyme) wells, 2 μL of DMSO was added instead of the test compounds’ solution. Fluorescence intensity was measured with a Microplate Reader Mithras LB 940 (Berthold Technologies, excitation at 355 nm, emission at 460 nm) for a period of 1 h with a read every minute. The reactions were performed at 25 °C. To derive 1 h IC50 values, endpoint RFU (single fluorescence reading after 1h) was used. Sigmoidal curves were prepared in GraphPad Prism software, and IC50 values were derived from the fitted curves.27,28
Thrombin (FIIa) Inhibition Assay
The inhibitory activity of synthesized compounds toward thrombin [human α-thrombin (active) protein, ABIN2127880, >95% purity; antibodies online, 0.25 nM—final concentration] was determined analogously to the described procedure for FXIIa. The fluorogenic substrate Boc-Val-Pro-Arg-AMC (Pepta Nova, 25 μM—final concentration, Km = 18 μM) was utilized.27,28
FXa Inhibition Assay
The inhibitory activity of synthesized compounds toward the coagulation factor Xa (human Factor Xa, HFXA, >95% purity; Molecular Innovations, 2.5 nM—final concentration) was determined analogous to the described procedure for FXIIa. The fluorogenic substrate Boc-Ile-Glu-Gly-Arg-AMC (Pepta Nova, 25 μM—final concentration) was utilized.27,28
FXIa Inhibition Assay
The inhibitory activity of synthesized compounds toward the coagulation factor XIa (human factor XIa, HFXIA, >95% purity; Molecular Innovations, 0.5 nM—final concentration) was determined analogous to the described procedure for FXIIa. The fluorogenic substrate Boc-Glu(OBzl)-Ala-Arg-AMC (Pepta Nova, 25 μM—final concentration) was utilized.
Plasmin Inhibition Assay
The inhibitory activity of synthesized compounds toward plasmin (human plasmin, >95% purity; Innovative Research, 10 nM—final concentration) was determined analogous to the described procedure for FXIIa. The fluorogenic substrate Boc-Val-Leu-Lys-AMC (Pepta Nova, 25 μM—final concentration) was utilized.
Plasma Kallikrein Inhibition Assay
The inhibitory activity of synthesized compounds toward plasma kallikrein (human plasma kallikrein, >94% purity; Innovative Research, 0.25 nM—final concentration) was determined analogous to the described procedure for FXIIa. The fluorogenic substrate Z-Phe-Lys-AMC (Pepta Nova, 25 μM—final concentration) was utilized.
Chymotrypsin Inhibition Assay
The inhibitory activity of synthesized compounds toward chymotrypsin (human chymotrypsin; >95% purity; Innovative Research, 0.5 nM—final concentration) was determined analogous to the described procedure for FXIIa. The fluorogenic substrate Suc-Ala-Ala-Pro-Phe-AMC (Sigma-Aldrich, 25 μM—final concentration) was utilized.
Trypsin Inhibition Assay
The inhibitory activity of synthesized compounds toward trypsin (porcine trypsin; Merck, 3.5 nM—final concentration) was determined analogous to the described procedure for FXIIa. The fluorogenic substrate Z-Gly-Gly-Arg-AMC (Sigma-Aldrich, 25 μM—final concentration) was utilized.27,28
In Vitro Coagulation Assays (aPTT and PT)
All measurements were performed using citrated (3.8%) human pooled plasma (Dunn Labortechnik GmbH, Germany) or citrated (3.8%) human whole blood (University Hospital Münster) on a semi-automated coagulometer (Thrombotimer-2, Behnk Elektronik, Germany) according to the manufacturer instructions as previously reported.27,28 For aPTT measurements, plasma or blood (100 μL) was placed into the incubation cuvettes of the instrument and incubated for 2 min at 37 °C. Then, the test compound solution (10 μL) or solvent (DMSO, 10 μL) was added with a pipette. After 1 min of incubation, 100 μL of prewarmed (37 °C) aPTT reagent (Convergent Technologies, Germany) was added and incubated for additional 2 min. The cuvettes were transferred to a measuring position, the coagulation was initiated by addition of 100 μL of CaCl2 solution (25 mM, prewarmed at 37 °C, Convergent Technologies, Germany), and the clotting time was recorded. For PT assays, plasma or blood (100 μL) was incubated for 2 min at 37 °C. Then, the test compound solution (10 μL) or solvent (DMSO, 10 μL) was added with a pipette. After 3 min of incubation, the cuvettes were transferred to a measuring position, the coagulation was initiated by addition of 100 μL of PT assay reagent already containing CaCl2 (prewarmed at 37 °C, Biolabo, France), and the clotting time was recorded.27,28
Analysis of the Covalent FXIIa–Inhibitor Complexes by SEC/ESI-MS
The analysis of covalent FXIIa–inhibitor complex was performed utilizing SEC/ESI-MS as reported previously.27,28 Briefly, 10 μL of the stock solution (37.67 μM) of human plasma β-FXIIa (HFXIIAB, >95% purity; Molecular Innovations) was diluted with 90 μL of purified water. An aliquot of 46.3 μL was mixed with 3.7 μL of the inhibitor solution (1 mM in DMSO) to prepare the enzyme–inhibitor solution (a molar ratio of 1:20), which was then analyzed via SEC/ESI-MS. SEC was performed using a MAbPac SEC-1 analytical column (150 × 4 mm, 5 μm particle size, 300 Å pore size) from Thermo Fisher Scientific (Bremen, DE). Mass spectrometric detection was carried out using a timsTOF time-of-flight mass spectrometer from Bruker Daltonics (Bremen, DE) equipped with an electrospray ionization interface. For chromatographic analysis of the sample solutions, isocratic elution was carried out using an aqueous ammonium acetate solution (10 mM, pH 6.8) as the mobile phase for 15 min. The enzyme–inhibitor complexes were analyzed in the positive ion mode using the following ESI-MS parameters: the mass range was set to m/z 1000–4000, nebulizer gas (nitrogen) was 1.2 bar, dry gas (nitrogen) was 9.0 L/min, the dry gas temperature was 200 °C, hexapole RF was 450 V, the pre puls storage time was 30 μs, and the transfer time was 100 μs. Deconvoluted mass spectra were obtained by averaging frames from 6.8 to 7.6 min retention time and deconvolution using the charge states +9 to +11 of the respective protein species using DataAnalysis 6.0 and the MaxEnt algorithm implemented in the software.
Analysis of the Covalent Thrombin–Inhibitor Complexes by LC/ESI-MS
Mass analysis of intact human thrombin [α-thrombin (active) protein, ABIN2127880, >95% purity; antibodies online] was performed using an UltiMate 3000 RS system (Thermo Fisher Scientific GmbH, Dreieich, Germany) connected to a maXis II UHR-qTOF mass spectrometer (Bruker Daltonik GmbH, Bremen, Germany) with a standard ESI source (Apollo, Bruker Daltonik GmbH, Bremen, Germany). After incubation (15 min) in the presence of the respective inhibitor [molar ratio thrombin (6.4 μM)/inhibitor (128 μM)—1:20], protein samples were either acidified to a final concentration of 0.5% of formic acid and 6.4 μM of protein or diluted to a final protein concentration of 64 nM and after a further incubation acidified to a final concentration of 0.5% of formic acid. The appropriate amount of protein solution (10 μL of the initial solution or 100 μL of the diluted solution) was loaded on a C4 column (Advance Bio RP-mAb C4, 2.1 mm × 50 mm, 3.5 μm, Agilent Technologies, Waldbronn, Germany) at a flow rate of 0.6 mL/min in 5% eluent B (eluent A: 0.1% formic acid in water; eluent B: 0.1% formic acid in acetonitrile). After a desalting period of 30 min at 5% B, a steep gradient was applied (5–60% B in 2 min), followed by 1 min at 60% B, 1 min from 60 to 100% B, 1 min at 100% B, and 3 min at 5% B. MS settings: capillary voltage 4500 V, end-plate offset −500 V, nebulizer 3.5 bar, dry gas 8.0 L/min, dry T = 200 °C, and mass range m/z 300–3000. Data were analyzed with DataAnalysis 4.4 (Bruker Daltonik GmbH, Bremen, Germany), and deconvolution was performed using the MaxEnt algorithm implemented in the software.
Thrombin–Inhibitor Covalent Complex Stability
2 μL of the stock solution of human α-thrombin (128 μM, ABIN2127880, >95% purity; antibodies online) was diluted with 37.436 μL of purified water. To this solution, inhibitor 5r solution (0.5 mM in DMSO) was added to prepare the enzyme–inhibitor solution (a molar ratio of 1:1.1). The enzyme–inhibitor solution was incubated for 15 min at room temperature to allow for the acyl-enzyme complex formation. Then, this solution was diluted with 3.960 mL of purified water (100-fold jump dilution). The acyl-enzyme complex stability was then analyzed using LC/ESI-MS method as indicated above (injection volume 100 μL). Samples were taken at 0, 10, 30, 60, 300, 360, 420, 480, and 540 min after the jump dilution.
Plasma Stability
50 μL of the test compound (2 mM in DMSO) was added to prewarmed (37 °C) 950 μL of citrated (3.8%) human pooled plasma (Dunn Labortechnik GmbH, Germany), and the mixture was gently mixed with a pipette for 1 min. The mixture was then incubated at 37 °C on a semi-automated coagulometer (Thrombotimer-2, Behnk Elektronik, Germany). The residual anticoagulant activity (in sec) of compounds was measured over different time intervals (0, 15, 30, 60, 90 min) as a measure of compounds’ plasma stability. For this, after the indicated time intervals, 100 μL of the compound–plasma mixture was withdrawn and analyzed in the aPTT test as described in the In Vitro Coagulation Assays section.
Cytotoxicity Studies
The cytotoxicity of the synthesized compounds was tested with the resazurin assay.27 Human liver carcinoma cells (HepG2, HB-8065) and lung adenocarcinoma cells (A549, CCL-185) were seeded with 10,000 cells/well (HepG2) and 10,000 cells/well (A549) in 96-well plates and incubated for 24 h. After replacing the medium by a serum-free medium, the cells were incubated for an additional 24 h. The synthesized compounds were applied in a concentration range of 0.5–300 μM. All compounds were dissolved in DMSO. The compounds were incubated for 24 h. Then, the standard resazurin solution of 10 μL was added to the cells and incubated for 1 h at 37 °C. The reduction of resazurin to resorufin was analyzed by screening the fluorescence at λ = 590 nm with a microplate reader (Infinite M200PRO, Tecan, Männendorf, Switzerland). Cytotoxicity assays were performed with three triplicates from three independent passages (n ≥ 9) for both cell lines. After subtraction of cell-free blank values, cellular viability was calculated as a test over control (T/C). The data are shown as the mean ± SD. Camptothecin (CPT, 2 μM) was used as a positive control. By analysis of variance (one-way ANOVA) and the Tukey post hoc test (*p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001), the concentration-dependent effects were analyzed.27
Molecular Modeling
Structures of small molecules were prepared using the LigPrep module of the Schrodinger suite (Schrodinger Release 2021-4: Schrodinger, LLC, New York, NY, 2021) employing the OPLS4 force field. The experimental structures of studied proteins were obtained from the RCSB database (PDB IDs: 6B77/FXIIa, 6CYM/thrombin). Structures were processed with the protein preparation wizard of Schrodinger Maestro suite 2021-4 (Schrödinger Release 2021-4) to optimize the hydrogen-bonding network, followed by constrained minimization (heavy-atom rmsd converge of 0.3 Å) to remove crystallographic artifacts. Covalent docking was carried out using the Schrodinger Covalent Docking procedure with Nucleophilic Addition to a Double Bond protocol. In both cases, active serine residues (195/FXIIa and 219/thrombin) were selected as attachment points and centers for Glide Grid.27 Input and output files are available upon request.
Acknowledgments
This project was supported by the Deutsche Forschungsgemeinschaft (DFG) grant (D.V.K.: KA 5558/1-1). The authors thank Dr. Jens Köhler and Claudia Thier for recording NMR spectra, Kirstin Lehmkuhl for performing HPLC analysis, and Prof. Dr. Henning Mootz for providing mass spectrometry resources.
Glossary
Abbreviations
- aPTT
activated partial thromboplastin time
- BzCl
benzoyl chloride
- DCM
dichloromethane
- DIPEA
N,N-diisopropylethylamine
- DMAP
4-dimethylaminopyridine
- DMF
dimethylformamide
- DMSO
dimethyl sulfoxide
- DOACs
direct oral anticoagulants
- EDCI·HCl
1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride
- ESI-MS
electrospray ionization mass spectrometry
- HRMS
high-resolution mass spectrometry
- PDB
protein data bank
- PT
prothrombin time
- RP
reversed phase
- SAR
structure–activity relationship
- SD
standard deviation
- SEC
size-exclusion chromatography
- THF
tetrahydrofuran
- TLC
thin-layer chromatography
- TOF
time-of-flight
- WHO
World Health Organization
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsptsci.2c00204.
1H and 13C NMR spectra for all synthesized compounds; experimental procedures and analytical data for compounds 8, 9d,g,i,n, 11, 12a–o, 16, 17, 19, 20, 21a–w, and 27–32; X-ray crystal structure analysis of 14h; and table with the optimization of reaction conditions for the microwave-assisted synthesis of 12b (PDF)
Molecular formula strings (XLS)
Author Contributions
L.I. and D.V.K. authors contributed equally. The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Notes
X-ray crystal structure of 14h (CCDC-2165993) can be obtained free of charge from the Cambridge Crystallographic Data Centre.44 Coordinates for the inhibitor 4a docked in FXIIa (PDB). Coordinates for inhibitor 5r docked (pose 1) in thrombin (PDB). Coordinates for inhibitor 5r docked (pose 2) in thrombin (PDB). Coordinates for inhibitor 23 docked in FXIIa (PDB). The authors will release the atomic coordinates upon article publication.
Supplementary Material
References
- Jackson S. P. Arterial thrombosis-insidious, unpredictable and deadly. Nat. Med. 2011, 17, 1423–1436. 10.1038/nm.2515. [DOI] [PubMed] [Google Scholar]
- World Health Organization Leading causes of death and disability 2000–2019: a visual summary. https://www.who.int/data/stories/leading-causes-of-death-and-disability-2000-2019-a-visual-summary (Feb 2, 2022).
- Stassen W.; Priepke J. M.; Ries H.; Hauel U. J.; Wienen N. In-vitro profile and ex-vivo anticoagulant activity of the direct thrombin inhibitor dabigatran and its orally active prodrug, dabigatran etexilate. Thromb. Haemostasis 2007, 98, 155–162. 10.1160/TH07-03-0183. [DOI] [PubMed] [Google Scholar]
- Sivaraja M.; Pozzi N.; Rienzo M.; Lin K.; Shiau T. P.; Clemens D. M.; Igoudin L.; Zalicki P.; Chang S. S.; Estiarte M. A.; Short K. M.; Williams D. C.; Datta A.; Di Cera E.; Kita D. B. Reversible covalent direct thrombin inhibitors. PLoS One 2018, 13, e0201377 10.1371/journal.pone.0201377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dementiev A.; Silva A.; Yee C.; Li Z.; Flavin M. T.; Sham H.; Partridge J. R. Structures of human plasma β-factor XIIa cocrystallized with potent inhibitors. Blood Adv. 2018, 2, 549–558. 10.1182/bloodadvances.2018016337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford D. J.; Duggan N. M.; Fry S. E.; Ripoll-Rozada J.; Agten S. M.; Liu W.; Corcilius L.; Hackeng T. M.; van Oerle R.; Spronk H. M. H.; Ashhurst A. S.; Mini Sasi V.; Kaczmarski J. A.; Jackson C. J.; Pereira P. J. B.; Passioura T.; Suga H.; Payne R. J. Potent cyclic peptide inhibitors of FXIIa discovered by mRNA display with genetic code reprogramming. J. Med. Chem. 2021, 64, 7853–7876. 10.1021/acs.jmedchem.1c00651. [DOI] [PubMed] [Google Scholar]
- Wilbs J.; Kong X. D.; Middendorp S. J.; Prince R.; Cooke A.; Demarest C. T.; Abdelhafez M. M.; Roberts K.; Umei N.; Gonschorek P.; Lamers C.; Deyle K.; Rieben R.; Cook K. E.; Angelillo-Scherrer A.; Heinis C. Cyclic peptide FXII inhibitor provides safe anticoagulation in a thrombosis model and in artificial lungs. Nat. Commun. 2020, 11, 3890. 10.1038/s41467-020-17648-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadhera R. K.; Russell C. E.; Piazza G. Cardiology patient page. Warfarin versus novel oral anticoagulants: how to choose?. Circulation 2014, 130, 191–193. 10.1161/circulationaha.114.010426. [DOI] [PubMed] [Google Scholar]
- Previtali E.; Bucciarelli P.; Passamonti S. M.; Martinelli I. Risk factors for venous and arterial thrombosis. Blood Transfus 2011, 9, 120. 10.2450/2010.0066-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoeb M.; Fang M. C. Assessing bleeding risk in patients taking anticoagulants. J. Thromb. Thrombolysis 2013, 35, 312–319. 10.1007/s11239-013-0899-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaherty M. L. Anticoagulant-associated intracerebral hemorrhage. Semin. Neurol. 2010, 30, 565–572. 10.1055/s-0030-1268866. [DOI] [PubMed] [Google Scholar]
- Cheng Q.; Tucker E. I.; Pine M. S.; Sisler I.; Matafonov A.; Sun M. F.; White-Adams T. C.; Smith S. A.; Hanson S. R.; McCarty O. J.; Renné T.; Gruber A.; Gailani D. A role for factor XIIa-mediated factor XI activation in thrombus formation in vivo. Blood 2010, 116, 3981–3989. 10.1182/blood-2010-02-270918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gailani D.; Renné T. The intrinsic pathway of coagulation: a target for treating thromboembolic disease?. J. Thromb. Haemostasis 2007, 5, 1106–1112. 10.1111/j.1538-7836.2007.02446.x. [DOI] [PubMed] [Google Scholar]
- Tillman B.; Gailani D. Inhibition of factors XI and XII for prevention of thrombosis induced by artificial surfaces. Semin. Thromb. Hemost. 2018, 44, 060–069. 10.1055/s-0037-1603937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenne E.; Nickel K. F.; Long A. T.; Fuchs T. A.; Stavrou E. X.; Stahl F. R.; Renné T. Factor XII: a novel target for safe prevention of thrombosis and inflammation. J. Intern. Med. 2015, 278, 571–585. 10.1111/joim.12430. [DOI] [PubMed] [Google Scholar]
- Renne T.; Pozgajova M.; Gruner S.; Schuh K.; Pauer H. U.; Burfeind P.; Gailani D.; Nieswandt B. Defective thrombus formation in mice lacking coagulation factor XII. J. Exp. Med. 2005, 202, 271–281. 10.1084/jem.20050664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohs T. C. L.; Lorentz C. U.; Johnson J.; Puy C.; Olson S. R.; Shatzel J. J.; Gailani D.; Hinds M. T.; Tucker E. I.; Gruber A.; McCarty O. J. T.; Wallisch M. Development of coagulation factor XII antibodies for inhibiting vascular device-related thrombosis. Cell. Mol. Bioeng. 2021, 14, 161–175. 10.1007/s12195-020-00657-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson M.; Rayzman V.; Nolte M. W.; Nickel K. F.; Björkqvist J.; Jämsä A.; Hardy M. P.; Fries M.; Schmidbauer S.; Hedenqvist P.; Broomé M.; Pragst I.; Dickneite G.; Wilson M. J.; Nash A. D.; Panousis C.; Renné T. A factor XIIa inhibitory antibody provides thromboprotection in extracorporeal circulation without increasing bleeding risk. Sci. Transl. Med. 2014, 6, 222ra17. 10.1126/scitranslmed.3006804. [DOI] [PubMed] [Google Scholar]
- Yau J. W.; Liao P.; Fredenburgh J. C.; Stafford A. R.; Revenko A. S.; Monia B. P.; Weitz J. I. Selective depletion of factor XI or factor XII with antisense oligonucleotides attenuates catheter thrombosis in rabbits. Blood 2014, 123, 2102–2107. 10.1182/blood-2013-12-540872. [DOI] [PubMed] [Google Scholar]
- Yau J. W.; Stafford A. R.; Liao P.; Fredenburgh J. C.; Roberts R.; Brash J. L.; Weitz J. I. Corn trypsin inhibitor coating attenuates the prothrombotic properties of catheters in vitro and in vivo. Acta Biomater. 2012, 8, 4092–4100. 10.1016/j.actbio.2012.07.019. [DOI] [PubMed] [Google Scholar]
- Cao H.; Biondo M.; Lioe H.; Busfield S.; Rayzman V.; Nieswandt B.; Bork K.; Harrison L. C.; Auyeung P.; Farkas H.; Csuka D.; Pelzing M.; Dower S.; Wilson M. J.; Nash A.; Nolte M. W.; Panousis C. Antibody-mediated inhibition of FXIIa blocks downstream bradykinin generation. J. Allergy Clin. Immunol. 2018, 142, 1355–1358. 10.1016/j.jaci.2018.06.014. [DOI] [PubMed] [Google Scholar]
- Kalinin D. V. Factor XII(a) inhibitors: a review of the patent literature. Expert Opin. Ther. Pat. 2021, 31, 1155–1176. 10.1080/13543776.2021.1945580. [DOI] [PubMed] [Google Scholar]
- Göbel K.; Pankratz S.; Asaridou C. M.; Herrmann A. M.; Bittner S.; Merker M.; Ruck T.; Glumm S.; Langhauser F.; Kraft P.; Krug T. F.; Breuer J.; Herold M.; Gross C. C.; Beckmann D.; Korb-Pap A.; Schuhmann M. K.; Kuerten S.; Mitroulis I.; Ruppert C.; Nolte M. W.; Panousis C.; Klotz L.; Kehrel B.; Korn T.; Langer H. F.; Pap T.; Nieswandt B.; Wiendl H.; Chavakis T.; Kleinschnitz C.; Meuth S. G. Blood coagulation factor XII drives adaptive immunity during neuroinflammation via CD87-mediated modulation of dendritic cells. Nat. Commun. 2016, 7, 11626. 10.1038/ncomms11626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davoine C.; Bouckaert C.; Fillet M.; Pochet L. Factor XII/XIIa inhibitors: Their discovery, development, and potential indications. Eur. J. Med. Chem. 2020, 208, 112753. 10.1016/j.ejmech.2020.112753. [DOI] [PubMed] [Google Scholar]
- Chen J. J. F.; Visco D. P. Jr. Identifying novel factor XIIa inhibitors with PCA-GA-SVM developed vHTS models. Eur. J. Med. Chem. 2017, 140, 31–41. 10.1016/j.ejmech.2017.08.056. [DOI] [PubMed] [Google Scholar]
- Sivaraja M.; Clemens D. M.; Sizikov S.; Dash S.; Xu C.; Rienzo M.; Yang B.; Ryan M.; Chattopadhyay M.; Igoudin L.; Chang S. S.; Keutzer S.; Zalicki P.; Estiarte M. A.; Shiau T. P.; Short K. M.; Williams D. C.; Datta A.; Pozzi N.; Di Cera E.; Gibson C. M.; Fox K. A. A.; Kita D. B. VE-1902-A direct thrombin inhibitor with reversible covalent mechanism of action shows efficacy with reduced bleeding in rodent models of thrombosis. Thromb. Res. 2020, 190, 112–121. 10.1016/j.thromres.2020.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korff M.; Imberg L.; Will J. M.; Bückreiß N.; Kalinina S. A.; Wenzel B. M.; Kastner G. A.; Daniliuc C. G.; Barth M.; Ovsepyan R. A.; Butov K. R.; Humpf H. U.; Lehr M.; Panteleev M. A.; Poso A.; Karst U.; Steinmetzer T.; Bendas G.; Kalinin D. V. Acylated 1H-1,2,4-Triazol-5-amines Targeting Human Coagulation Factor XIIa and Thrombin: Conventional and Microscale Synthesis, Anticoagulant Properties, and Mechanism of Action. J. Med. Chem. 2020, 63, 13159–13186. 10.1021/acs.jmedchem.0c01635. [DOI] [PubMed] [Google Scholar]
- Platte S.; Korff M.; Imberg L.; Balicioglu I.; Erbacher C.; Will J. M.; Daniliuc C. G.; Karst U.; Kalinin D. V. Microscale Parallel Synthesis of Acylated Aminotriazoles Enabling the Development of Factor XIIa and Thrombin Inhibitors. Chemmedchem 2021, 16, 3672–3690. 10.1002/cmdc.202100431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelli A.; Azzouni S.; Plais R.; Gaucher A.; Efrit M. L.; Prim D. Recent advances in the chemistry of 1,2,4-triazoles: Synthesis, reactivity and biological activities. Tetrahedron Lett. 2021, 86, 153518. 10.1016/j.tetlet.2021.153518. [DOI] [Google Scholar]
- Dolzhenko A. V.; Kalinina S. A.; Kalinin D. V. A novel multicomponent microwave-assisted synthesis of 5-aza-adenines. RSC Adv. 2013, 3, 15850–15855. 10.1039/c3ra41932k. [DOI] [Google Scholar]
- Aggarwal R.; Sumran G. An insight on medicinal attributes of 1,2,4-triazoles. Eur. J. Med. Chem. 2020, 205, 112652. 10.1016/j.ejmech.2020.112652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim F. P. L.; Tan L. Y.; Tiekink E. R. T.; Dolzhenko A. V. Synthesis of 3-(5-amino-1H-1,2,4-triazol-3-yl)propanamides and their tautomerism. RSC Adv. 2018, 8, 22351–22360. 10.1039/c8ra04576c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolzhenko A. V.; Pastorin G.; Dolzhenko A. V.; Chui W. K. An aqueous medium synthesis and tautomerism study of 3(5)-amino-1,2,4-triazoles. Tetrahedron Lett. 2009, 50, 2124–2128. 10.1016/j.tetlet.2009.02.172. [DOI] [Google Scholar]
- Gawrys P.; Marszalek T.; Bartnik E.; Kucinska M.; Ulanski J.; Zagorska M. Novel, Low-Cost, Highly soluble n-type semiconductors: tetraazaanthracene tetraesters. Org. Lett. 2011, 13, 6090–6093. 10.1021/ol2025789. [DOI] [PubMed] [Google Scholar]
- Okumura S.; Takeda Y.; Kiyokawa K.; Minakata S. Hypervalent iodine(III)-induced oxidative [4+2] annulation of o-phenylenediamines and electron-deficient alkynes: direct synthesis of quinoxalines from alkyne substrates under metal-free conditions. Chem. Commun. 2013, 49, 9266–9268. 10.1039/c3cc45469j. [DOI] [PubMed] [Google Scholar]
- Radisky E. S.; Lee J. M.; Lu C. J. K.; Koshland D. E. Insights into the serine protease mechanism from atomic resolution structures of trypsin reaction intermediates. Proc. Natl. Acad. Sci. U.S.A. 2006, 103, 6835–6840. 10.1073/pnas.0601910103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertus J. D.; Kraut J.; Alden R. A.; Birktoft J. J. Subtilisin. Stereochemical mechanism involving transition-state stabilization. Biochemistry 1972, 11, 4293–4303. 10.1021/bi00773a016. [DOI] [PubMed] [Google Scholar]
- Voet D.; Voet J. G.. Biochemistry; John Wiley & Sons, 2010; ISBN: 978-0-470-57095-1. [Google Scholar]
- Singh J.; Petter R. C.; Baillie T. A.; Whitty A. The resurgence of covalent drugs. Nat. Rev. Drug Discov. 2011, 10, 307–317. 10.1038/nrd3410. [DOI] [PubMed] [Google Scholar]
- Fischer P. M. Design of small-molecule active-site inhibitors of the S1A family proteases as procoagulant and anticoagulant drugs. J. Med. Chem. 2018, 61, 3799–3822. 10.1021/acs.jmedchem.7b00772. [DOI] [PubMed] [Google Scholar]
- APEX3 (2016), SAINT (2015) and SADABS (2015); Bruker AXS Inc.: Madison, Wisconsin, USA.
- Sheldrick G. M. SHELXT- Integrated space-group and crystal-structure determination. Acta Crystallogr., Sect. A: Found. Adv. 2015, 71, 3–8. 10.1107/s2053273314026370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldrick G. M. Crystal structure refinement withSHELXL. Acta Crystallogr., Sect. C: Struct. Chem. 2015, 71, 3–8. 10.1107/s2053229614024218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- www.ccdc.cam.ac.uk/data_request/cif (accessed November 17, 2022).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.